User login
Endocrine Society: Annual Meeting (ENDO 2014)
VIDEO: Use late-night salivary cortisol to catch recurrent Cushing’s
CHICAGO – Late-night salivary cortisol exceeded normal limits in 10 women with recurrent Cushing’s disease a mean of 3.5 years after transsphenoidal surgery, but their urinary free cortisol remained in normal limits, according to a retrospective review from the Medical College of Wisconsin, Milwaukee.
That adds strength to the notion that late-night salivary cortisol (LNSC) catches recurrent Cushing’s that’s missed by urinary free cortisol, even though UFC remains a standard screening approach in some places.
The study is tiny and retrospective, but at the joint meeting of the International Congress of Endocrinology and the Endocrine Society, lead investigator Dr. Ty Carroll explained why the findings still matter, and also why two LNSC measurements are better than one.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Late-night salivary cortisol exceeded normal limits in 10 women with recurrent Cushing’s disease a mean of 3.5 years after transsphenoidal surgery, but their urinary free cortisol remained in normal limits, according to a retrospective review from the Medical College of Wisconsin, Milwaukee.
That adds strength to the notion that late-night salivary cortisol (LNSC) catches recurrent Cushing’s that’s missed by urinary free cortisol, even though UFC remains a standard screening approach in some places.
The study is tiny and retrospective, but at the joint meeting of the International Congress of Endocrinology and the Endocrine Society, lead investigator Dr. Ty Carroll explained why the findings still matter, and also why two LNSC measurements are better than one.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Late-night salivary cortisol exceeded normal limits in 10 women with recurrent Cushing’s disease a mean of 3.5 years after transsphenoidal surgery, but their urinary free cortisol remained in normal limits, according to a retrospective review from the Medical College of Wisconsin, Milwaukee.
That adds strength to the notion that late-night salivary cortisol (LNSC) catches recurrent Cushing’s that’s missed by urinary free cortisol, even though UFC remains a standard screening approach in some places.
The study is tiny and retrospective, but at the joint meeting of the International Congress of Endocrinology and the Endocrine Society, lead investigator Dr. Ty Carroll explained why the findings still matter, and also why two LNSC measurements are better than one.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ICE/ENDO 2014
VIDEO: Kisspeptin outperforms HCG for early miscarriage prediction
CHICAGO – A one-time measurement of plasma kisspeptin – a family of placental peptides also being studied for fertility treatment – better predicts miscarriage than does serial measurement of human chorionic gonadotropin (HCG), a widely used measure.
British researchers measured both in 993 asymptomatic women at approximately 11 weeks’ gestation, 50 of whom later miscarried. Plasma kisspeptin proved a more accurate predictor of miscarriage than HCG: the area under the receiver operating characteristic (ROC) curve for plasma kisspeptin was 0.899, compared with 0.775 for serum HCG. Plasma kisspeptin above 1,306 pmol/L was associated with a highly significant 87% reduced risk of miscarriage, even after adjusting for age, body mass index, gestational age, smoking, and blood pressure.
Lead investigator Dr. Ali Abbara of Imperial College London explained why that matters at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – A one-time measurement of plasma kisspeptin – a family of placental peptides also being studied for fertility treatment – better predicts miscarriage than does serial measurement of human chorionic gonadotropin (HCG), a widely used measure.
British researchers measured both in 993 asymptomatic women at approximately 11 weeks’ gestation, 50 of whom later miscarried. Plasma kisspeptin proved a more accurate predictor of miscarriage than HCG: the area under the receiver operating characteristic (ROC) curve for plasma kisspeptin was 0.899, compared with 0.775 for serum HCG. Plasma kisspeptin above 1,306 pmol/L was associated with a highly significant 87% reduced risk of miscarriage, even after adjusting for age, body mass index, gestational age, smoking, and blood pressure.
Lead investigator Dr. Ali Abbara of Imperial College London explained why that matters at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – A one-time measurement of plasma kisspeptin – a family of placental peptides also being studied for fertility treatment – better predicts miscarriage than does serial measurement of human chorionic gonadotropin (HCG), a widely used measure.
British researchers measured both in 993 asymptomatic women at approximately 11 weeks’ gestation, 50 of whom later miscarried. Plasma kisspeptin proved a more accurate predictor of miscarriage than HCG: the area under the receiver operating characteristic (ROC) curve for plasma kisspeptin was 0.899, compared with 0.775 for serum HCG. Plasma kisspeptin above 1,306 pmol/L was associated with a highly significant 87% reduced risk of miscarriage, even after adjusting for age, body mass index, gestational age, smoking, and blood pressure.
Lead investigator Dr. Ali Abbara of Imperial College London explained why that matters at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ICE/ENDO 2014
Denosumab's benefits persist through 8 years
CHICAGO – Postmenopausal women on denosumab for 8 years straight showed a continued near-linear increase in bone mineral density at the lumbar spine as well as persistent reduction of bone turnover markers and a sustained low incidence of new vertebral and nonvertebral fractures in the ongoing FREEDOM open-label extension study.
Moreover, no new safety signals have emerged during 5 years of additional denosumab (Prolia) on top of an initial 3 years occurring in the pivotal, phase III, randomized, double-blind, placebo-controlled FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) trial.
Importantly, the overall risk of adverse events has not increased over time, and rates of malignancies, serious infections, eczema, hypocalcemia, and other adverse events in denosumab-treated patients remain comparable to rates seen in placebo-treated controls in the earlier double-blind phase, Dr. E. Michael Lewiecki reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The benefit/risk profile for denosumab remains favorable," declared Dr. Lewiecki, director of the New Mexico Clinical Research and Osteoporosis Center, Albuquerque.
The FREEDOM open-label extension is designed to assess the efficacy and safety of up to 10 years of denosumab therapy. Dr. Lewiecki presented the latest update, based on 8 years of treatment, or 5 years for patients in the original placebo arm who later elected to crossover to denosumab upon completing the 3-year double-blind phase. His report included 2,243 women on denosumab at 60 mg by subcutaneous injection every 6 months continuously for 8 years and another 2,207 on the drug for 5 years.
Five cases of osteonecrosis of the jaw have occurred in the long-term therapy group, as well as three cases among those who crossed over to the drug. In addition, there has been one atypical femoral fracture during 8 consecutive years on denosumab and one case in the crossover group.
Impressively, lumbar spine bone mineral density (BMD) has increased by 18.4% over baseline with 8 years of denosumab and by 13.7% with 5 years of active therapy.
"The near-linear slope of increase is unchanged over the course of the study. A similar slope is seen in the crossover group," the endocrinologist observed.
Total hip BMD increased by 8.3% with 8 years of denosumab and 4.9% with 5 years of therapy.
In the pivotal phase III FREEDOM trial, 3 years of denosumab reduced the risk of vertebral fractures by 68% compared to placebo, hip fractures by 40%, and nonvertebral fractures by 20% (N. Engl. J. Med. 2009;361:756-65).
The yearly incidence of new vertebral fractures was 0.7%-1.1% in denosumab-treated patients during years 1-3 and 1.1%-1.3% annually in open-label years 4-8. The annual incidence of new nonvertebral fractures in the denosumab group was 2.1%-2.6% in the first 3 years of double-blind therapy and has trended lower since then: 1.5% in year 4, 1.2% in year 5, 1.8% in year 6, 1.6% in year 7, and 0.7% in year 8.
"There’s a suggestion of a further reduction in the risk of nonvertebral fractures out to the 8-year time point. It’ll be fascinating to look at the data for years 9 and 10 to see if that’s just a statistical aberrance or that very low risk of nonvertebral fractures persists," Dr. Lewiecki commented.
Levels of the bone turnover markers serum C-terminal telopeptide of type 1 collagen and procollagen type 1 N-terminal propeptide quickly dropped upon initiation of denosumab and have remained low through 8 years.
Asked about the mechanism underlying the continued linear increase in lumbar spine BMD seen through 8 years of denosumab, in sharp contrast to the bisphosphonates, where BMD plateaus, Dr. Lewiecki confessed that "Many of us are scratching our heads about this and trying to come up with an explanation."
"I don’t know the reason why, but there are several hypotheses worth considering," he continued. "One is that the effects on cortical bone appear to be different with denosumab than with bisphosphonates. We see a reduction in cortical porosity and a larger improvement in bone mineral density at cortical skeletal sites. Secondly, there’s a possible parathyroid hormone effect that may play a role here: There is a larger and longer-lasting increase in parathyroid hormone with denosumab as compared with bisphosphonates. And finally, there’s animal data in monkeys showing ongoing bone modeling taking place with denosumab; it’s possible that may also play a role."
Denosumab is a fully human monoclonal antibody directed against the receptor activator of nuclear factor-kappa B (RANK) ligand.
The FREEDOM study is supported by Amgen. Dr. Lewiecki reported serving as a consultant to that company as well as to AgNovos Healthcare, Lilly, Merck, and Radius Health.
CHICAGO – Postmenopausal women on denosumab for 8 years straight showed a continued near-linear increase in bone mineral density at the lumbar spine as well as persistent reduction of bone turnover markers and a sustained low incidence of new vertebral and nonvertebral fractures in the ongoing FREEDOM open-label extension study.
Moreover, no new safety signals have emerged during 5 years of additional denosumab (Prolia) on top of an initial 3 years occurring in the pivotal, phase III, randomized, double-blind, placebo-controlled FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) trial.
Importantly, the overall risk of adverse events has not increased over time, and rates of malignancies, serious infections, eczema, hypocalcemia, and other adverse events in denosumab-treated patients remain comparable to rates seen in placebo-treated controls in the earlier double-blind phase, Dr. E. Michael Lewiecki reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The benefit/risk profile for denosumab remains favorable," declared Dr. Lewiecki, director of the New Mexico Clinical Research and Osteoporosis Center, Albuquerque.
The FREEDOM open-label extension is designed to assess the efficacy and safety of up to 10 years of denosumab therapy. Dr. Lewiecki presented the latest update, based on 8 years of treatment, or 5 years for patients in the original placebo arm who later elected to crossover to denosumab upon completing the 3-year double-blind phase. His report included 2,243 women on denosumab at 60 mg by subcutaneous injection every 6 months continuously for 8 years and another 2,207 on the drug for 5 years.
Five cases of osteonecrosis of the jaw have occurred in the long-term therapy group, as well as three cases among those who crossed over to the drug. In addition, there has been one atypical femoral fracture during 8 consecutive years on denosumab and one case in the crossover group.
Impressively, lumbar spine bone mineral density (BMD) has increased by 18.4% over baseline with 8 years of denosumab and by 13.7% with 5 years of active therapy.
"The near-linear slope of increase is unchanged over the course of the study. A similar slope is seen in the crossover group," the endocrinologist observed.
Total hip BMD increased by 8.3% with 8 years of denosumab and 4.9% with 5 years of therapy.
In the pivotal phase III FREEDOM trial, 3 years of denosumab reduced the risk of vertebral fractures by 68% compared to placebo, hip fractures by 40%, and nonvertebral fractures by 20% (N. Engl. J. Med. 2009;361:756-65).
The yearly incidence of new vertebral fractures was 0.7%-1.1% in denosumab-treated patients during years 1-3 and 1.1%-1.3% annually in open-label years 4-8. The annual incidence of new nonvertebral fractures in the denosumab group was 2.1%-2.6% in the first 3 years of double-blind therapy and has trended lower since then: 1.5% in year 4, 1.2% in year 5, 1.8% in year 6, 1.6% in year 7, and 0.7% in year 8.
"There’s a suggestion of a further reduction in the risk of nonvertebral fractures out to the 8-year time point. It’ll be fascinating to look at the data for years 9 and 10 to see if that’s just a statistical aberrance or that very low risk of nonvertebral fractures persists," Dr. Lewiecki commented.
Levels of the bone turnover markers serum C-terminal telopeptide of type 1 collagen and procollagen type 1 N-terminal propeptide quickly dropped upon initiation of denosumab and have remained low through 8 years.
Asked about the mechanism underlying the continued linear increase in lumbar spine BMD seen through 8 years of denosumab, in sharp contrast to the bisphosphonates, where BMD plateaus, Dr. Lewiecki confessed that "Many of us are scratching our heads about this and trying to come up with an explanation."
"I don’t know the reason why, but there are several hypotheses worth considering," he continued. "One is that the effects on cortical bone appear to be different with denosumab than with bisphosphonates. We see a reduction in cortical porosity and a larger improvement in bone mineral density at cortical skeletal sites. Secondly, there’s a possible parathyroid hormone effect that may play a role here: There is a larger and longer-lasting increase in parathyroid hormone with denosumab as compared with bisphosphonates. And finally, there’s animal data in monkeys showing ongoing bone modeling taking place with denosumab; it’s possible that may also play a role."
Denosumab is a fully human monoclonal antibody directed against the receptor activator of nuclear factor-kappa B (RANK) ligand.
The FREEDOM study is supported by Amgen. Dr. Lewiecki reported serving as a consultant to that company as well as to AgNovos Healthcare, Lilly, Merck, and Radius Health.
CHICAGO – Postmenopausal women on denosumab for 8 years straight showed a continued near-linear increase in bone mineral density at the lumbar spine as well as persistent reduction of bone turnover markers and a sustained low incidence of new vertebral and nonvertebral fractures in the ongoing FREEDOM open-label extension study.
Moreover, no new safety signals have emerged during 5 years of additional denosumab (Prolia) on top of an initial 3 years occurring in the pivotal, phase III, randomized, double-blind, placebo-controlled FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) trial.
Importantly, the overall risk of adverse events has not increased over time, and rates of malignancies, serious infections, eczema, hypocalcemia, and other adverse events in denosumab-treated patients remain comparable to rates seen in placebo-treated controls in the earlier double-blind phase, Dr. E. Michael Lewiecki reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The benefit/risk profile for denosumab remains favorable," declared Dr. Lewiecki, director of the New Mexico Clinical Research and Osteoporosis Center, Albuquerque.
The FREEDOM open-label extension is designed to assess the efficacy and safety of up to 10 years of denosumab therapy. Dr. Lewiecki presented the latest update, based on 8 years of treatment, or 5 years for patients in the original placebo arm who later elected to crossover to denosumab upon completing the 3-year double-blind phase. His report included 2,243 women on denosumab at 60 mg by subcutaneous injection every 6 months continuously for 8 years and another 2,207 on the drug for 5 years.
Five cases of osteonecrosis of the jaw have occurred in the long-term therapy group, as well as three cases among those who crossed over to the drug. In addition, there has been one atypical femoral fracture during 8 consecutive years on denosumab and one case in the crossover group.
Impressively, lumbar spine bone mineral density (BMD) has increased by 18.4% over baseline with 8 years of denosumab and by 13.7% with 5 years of active therapy.
"The near-linear slope of increase is unchanged over the course of the study. A similar slope is seen in the crossover group," the endocrinologist observed.
Total hip BMD increased by 8.3% with 8 years of denosumab and 4.9% with 5 years of therapy.
In the pivotal phase III FREEDOM trial, 3 years of denosumab reduced the risk of vertebral fractures by 68% compared to placebo, hip fractures by 40%, and nonvertebral fractures by 20% (N. Engl. J. Med. 2009;361:756-65).
The yearly incidence of new vertebral fractures was 0.7%-1.1% in denosumab-treated patients during years 1-3 and 1.1%-1.3% annually in open-label years 4-8. The annual incidence of new nonvertebral fractures in the denosumab group was 2.1%-2.6% in the first 3 years of double-blind therapy and has trended lower since then: 1.5% in year 4, 1.2% in year 5, 1.8% in year 6, 1.6% in year 7, and 0.7% in year 8.
"There’s a suggestion of a further reduction in the risk of nonvertebral fractures out to the 8-year time point. It’ll be fascinating to look at the data for years 9 and 10 to see if that’s just a statistical aberrance or that very low risk of nonvertebral fractures persists," Dr. Lewiecki commented.
Levels of the bone turnover markers serum C-terminal telopeptide of type 1 collagen and procollagen type 1 N-terminal propeptide quickly dropped upon initiation of denosumab and have remained low through 8 years.
Asked about the mechanism underlying the continued linear increase in lumbar spine BMD seen through 8 years of denosumab, in sharp contrast to the bisphosphonates, where BMD plateaus, Dr. Lewiecki confessed that "Many of us are scratching our heads about this and trying to come up with an explanation."
"I don’t know the reason why, but there are several hypotheses worth considering," he continued. "One is that the effects on cortical bone appear to be different with denosumab than with bisphosphonates. We see a reduction in cortical porosity and a larger improvement in bone mineral density at cortical skeletal sites. Secondly, there’s a possible parathyroid hormone effect that may play a role here: There is a larger and longer-lasting increase in parathyroid hormone with denosumab as compared with bisphosphonates. And finally, there’s animal data in monkeys showing ongoing bone modeling taking place with denosumab; it’s possible that may also play a role."
Denosumab is a fully human monoclonal antibody directed against the receptor activator of nuclear factor-kappa B (RANK) ligand.
The FREEDOM study is supported by Amgen. Dr. Lewiecki reported serving as a consultant to that company as well as to AgNovos Healthcare, Lilly, Merck, and Radius Health.
AT ICE/ENDO 2014
Key clinical point: An ongoing major study provides reassurance regarding the long-term safety and effectiveness of denosumab for postmenopausal osteoporosis.
Major finding: Eight years of denosumab was associated with sustained low fracture rates, steadily increasing bone mineral density, persistent reduction of bone turnover, and no increase in adverse events over time.
Data source: The FREEDOM trial open-label extension includes 4,450 women on denosumab for either 5 or 8 years and counting.
Disclosures: The study was sponsored by Amgen. The presenter serves as a consultant to the company.
Acromegaly: Look closely for sleep-disordered breathing
CHICAGO – Roughly 80% of patients with newly diagnosed and as-yet untreated acromegaly already have obstructive sleep apnea, according to the findings of what’s believed to be the largest-ever polysomnography study in such subjects.
Importantly, this study showed that the common practice of screening for sleep-disordered breathing with the Epworth Sleepiness Scale (ESS) and overnight oximetry to measure the desaturation index will greatly underdiagnose this important condition in patients with acromegaly. These are poor screening tools in this setting. They need to be supplemented by polysomnography, with the diagnosis of obstructive sleep apnea (OSA) hinging upon the finding of an abnormal apnea-hypopnea index, Dr. Andrew S. Powlson asserted at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Dr. Powlson of the University of Cambridge (U.K.), reported on 40 consecutive patients with newly diagnosed, treatment-naive acromegaly who were evaluated by the ESS, desaturation index, and polysomnography. The study’s purpose was to shed light on a controversy: Is sleep-disordered breathing an inherent feature of acromegaly or a secondary side effect of its treatment? The question takes on added clinical relevance in light of OSA’s known predisposition to premature cardiovascular and metabolic disease, motor vehicle accidents, and impaired quality of life.
Of the 40 patients, 31 (78%) met diagnostic criteria for OSA by polysomnography. The OSA was defined on the basis of the apnea-hypopnea index as mild in 12, moderate in 5, and severe in 14.
In contrast, the ESS performed dismally as a screening instrument: Only 12 of the 31 patients with OSA had an ESS score greater than 11, which is the standard threshold for further investigation. Moreover, measurement of the diffusion index during overnight oximetry identified only 21 patients as having OSA: 11 rated mild, 7 moderate, and 3 severe, as compared to 14 patients classified as having severe OSA by polysomnography.
Patients with acromegaly displayed an increased sleep arousal index and more periodic limb movements during sleep than in reference norms, which translates into marked disruption of the normal sleep cycle. Of note, however, polysomnography showed that sleep latency – that is, the time it takes to fall asleep – and total sleep time were normal in the acromegaly patients with OSA.
Instead, the predominant pattern was one of disrupted sleep architecture. Twenty-seven acromegaly patients spent longer than expected in stage-1 sleep, while the deeper sleep stages were dramatically diminished. Indeed, 26 patients had reduced stage-2 sleep, 31 had reduced REM sleep, and 26 had shortened slow wave sleep.
Dr. Powlson reported having no financial disclosures in connection with this study, conducted with institutional funds.
CHICAGO – Roughly 80% of patients with newly diagnosed and as-yet untreated acromegaly already have obstructive sleep apnea, according to the findings of what’s believed to be the largest-ever polysomnography study in such subjects.
Importantly, this study showed that the common practice of screening for sleep-disordered breathing with the Epworth Sleepiness Scale (ESS) and overnight oximetry to measure the desaturation index will greatly underdiagnose this important condition in patients with acromegaly. These are poor screening tools in this setting. They need to be supplemented by polysomnography, with the diagnosis of obstructive sleep apnea (OSA) hinging upon the finding of an abnormal apnea-hypopnea index, Dr. Andrew S. Powlson asserted at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Dr. Powlson of the University of Cambridge (U.K.), reported on 40 consecutive patients with newly diagnosed, treatment-naive acromegaly who were evaluated by the ESS, desaturation index, and polysomnography. The study’s purpose was to shed light on a controversy: Is sleep-disordered breathing an inherent feature of acromegaly or a secondary side effect of its treatment? The question takes on added clinical relevance in light of OSA’s known predisposition to premature cardiovascular and metabolic disease, motor vehicle accidents, and impaired quality of life.
Of the 40 patients, 31 (78%) met diagnostic criteria for OSA by polysomnography. The OSA was defined on the basis of the apnea-hypopnea index as mild in 12, moderate in 5, and severe in 14.
In contrast, the ESS performed dismally as a screening instrument: Only 12 of the 31 patients with OSA had an ESS score greater than 11, which is the standard threshold for further investigation. Moreover, measurement of the diffusion index during overnight oximetry identified only 21 patients as having OSA: 11 rated mild, 7 moderate, and 3 severe, as compared to 14 patients classified as having severe OSA by polysomnography.
Patients with acromegaly displayed an increased sleep arousal index and more periodic limb movements during sleep than in reference norms, which translates into marked disruption of the normal sleep cycle. Of note, however, polysomnography showed that sleep latency – that is, the time it takes to fall asleep – and total sleep time were normal in the acromegaly patients with OSA.
Instead, the predominant pattern was one of disrupted sleep architecture. Twenty-seven acromegaly patients spent longer than expected in stage-1 sleep, while the deeper sleep stages were dramatically diminished. Indeed, 26 patients had reduced stage-2 sleep, 31 had reduced REM sleep, and 26 had shortened slow wave sleep.
Dr. Powlson reported having no financial disclosures in connection with this study, conducted with institutional funds.
CHICAGO – Roughly 80% of patients with newly diagnosed and as-yet untreated acromegaly already have obstructive sleep apnea, according to the findings of what’s believed to be the largest-ever polysomnography study in such subjects.
Importantly, this study showed that the common practice of screening for sleep-disordered breathing with the Epworth Sleepiness Scale (ESS) and overnight oximetry to measure the desaturation index will greatly underdiagnose this important condition in patients with acromegaly. These are poor screening tools in this setting. They need to be supplemented by polysomnography, with the diagnosis of obstructive sleep apnea (OSA) hinging upon the finding of an abnormal apnea-hypopnea index, Dr. Andrew S. Powlson asserted at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Dr. Powlson of the University of Cambridge (U.K.), reported on 40 consecutive patients with newly diagnosed, treatment-naive acromegaly who were evaluated by the ESS, desaturation index, and polysomnography. The study’s purpose was to shed light on a controversy: Is sleep-disordered breathing an inherent feature of acromegaly or a secondary side effect of its treatment? The question takes on added clinical relevance in light of OSA’s known predisposition to premature cardiovascular and metabolic disease, motor vehicle accidents, and impaired quality of life.
Of the 40 patients, 31 (78%) met diagnostic criteria for OSA by polysomnography. The OSA was defined on the basis of the apnea-hypopnea index as mild in 12, moderate in 5, and severe in 14.
In contrast, the ESS performed dismally as a screening instrument: Only 12 of the 31 patients with OSA had an ESS score greater than 11, which is the standard threshold for further investigation. Moreover, measurement of the diffusion index during overnight oximetry identified only 21 patients as having OSA: 11 rated mild, 7 moderate, and 3 severe, as compared to 14 patients classified as having severe OSA by polysomnography.
Patients with acromegaly displayed an increased sleep arousal index and more periodic limb movements during sleep than in reference norms, which translates into marked disruption of the normal sleep cycle. Of note, however, polysomnography showed that sleep latency – that is, the time it takes to fall asleep – and total sleep time were normal in the acromegaly patients with OSA.
Instead, the predominant pattern was one of disrupted sleep architecture. Twenty-seven acromegaly patients spent longer than expected in stage-1 sleep, while the deeper sleep stages were dramatically diminished. Indeed, 26 patients had reduced stage-2 sleep, 31 had reduced REM sleep, and 26 had shortened slow wave sleep.
Dr. Powlson reported having no financial disclosures in connection with this study, conducted with institutional funds.
AT ICE/ENDO 2014
Key clinical point: Reliance on the Epworth Sleepiness Scale and desaturation index on overnight oximetry to screen for OSA in patients with newly diagnosed acromegaly results in an unacceptable underdiagnosis rate.
Major finding: Thirty-one of 40 consecutive patients with de novo acromegaly had OSA on polysomnography.
Data source: Forty consecutive patients with newly diagnosed acromegaly underwent evaluation via the Epworth Sleepiness Scale, desaturation index, and polysomnography.
Disclosures: The presenter reported having no financial conflicts regarding this study, carried out with institutional funds.
New clinical practice guidelines on pheochromocytomas
CHICAGO – Genetic testing has jumped to the fore in the management of patients diagnosed as having a pheochromocytoma or paraganglioma, according to new clinical practice guidelines released by the Endocrine Society.
Indeed, the new guidelines call for genetic testing to be considered seriously in all patients with a proven pheochromocytoma or paraganglioma (PPGL), Dr. Jacques W. M. Lenders said in presenting highlights of the new guidelines at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"We recommend that all patients with PPGLs should be engaged in shared decision making for genetic testing. I don’t say that we should do genetic testing in everybody, but we should consider it and engage the patient in the final decision," said Dr. Lenders, who chaired the practice guidelines task force.
The strong emphasis on genetic testing arises from evidence that roughly one-third of all PPGLs are associated with germline mutations. Moreover, susceptibility mutations are present in 12% of patients with absolutely no suggestion of a positive family history. Some of these mutations – for example, those involving succinate dehydrogenase B (SDHB) – are associated with a high risk of metastasis and unfavorable prognosis. Thus, gene-testing results can have a major impact on patients with PPGL as well as their relatives.
Nonetheless, genetic testing in patients with PPGLs remains controversial.
"I must say, we on the guideline task force spent considerable time on what and how to do it," said Dr. Lenders, who is professor and deputy chair of internal medicine at Radboud University in Nijmegen, the Netherlands.
Since simultaneous testing for all the known culprit genes remains for now too expensive to be cost effective, the guidelines include a clinical feature–driven decisional algorithm designed to establish the priorities for genetic testing in a given patient with proven PPGL.
For example, patients with a metastatic PPGL should be tested for SDHB mutations, while those with a paraganglioma should undergo testing for succinate dehydrogenase mutations, according to the guidelines, published in full in concert with ICE/ENDO 2014 (J. Clin. Endocrinol. Metab. 2014;1915-42).
Dr. Lenders noted that PPGLs are uncommon tumors. It is estimated that 0.1%-1% of patients being treated for hypertension have pheochromocytomas, which are adrenal tumors resulting in excess production of epinephrine and norepinephrine. Symptoms can include paroxysmal severe headache, tachycardia, anxiety, and excessive sweating, along with tough-to-control hypertension.
While pheochromocytomas are typically benign, malignant transformation occurs in up to 17% of cases. And although a complete cure is often possible with timely therapy, the fact is that on average a 3-year delay transpires between symptomatic presentation and diagnosis of PPGL. Also, studies show that failure to appropriately follow up on a positive biochemical test is common in clinical practice; as a consequence, PPGLs are often overdiagnosed. For these reasons, Endocrine Society officials deemed PPGLs a priority area in need of practice guidelines.
In addition to routine consideration of genetic testing, other recommendations include:
• Diagnostic biochemical testing: Initial testing should include measurement of plasma free or urinary fractionated metanephrines, preferably using liquid chromatography with electrochemical or mass spectrometric laboratory methods. Immunoassays, although popular in Europe, haven’t yet been adequately validated. In measuring plasma metanephrines, the blood draw should be done with the patient in supine position, using reference standards established in the same position.
"False-positive test results are a major problem in daily clinical practice, and they outweigh by far the number of true-positive test results. That’s very important to realize," the endocrinologist said.
One common cause of false-positive test results are medications that trigger elevated metanephrine levels, according to guideline panelist Dr. William F. Young Jr., professor of medicine and chair of the department of endocrinology, diabetes, metabolism and nutrition at the Mayo Clinic, Rochester, Minn. The top three offending drugs in his experience are tricyclic antidepressants, antipsychotic agents, and levodopa. The guidelines list others, he added.
• Imaging: Once clear biochemical evidence of a PPGL is established, CT is preferred over MRI in order to locate the tumor because of its superior spatial resolution in the thorax, abdomen, and pelvis. 18F-fluorodeoxyglucose positron emission tomography/CT scanning is preferred over 123I-metaiodobenzylguanidine (MIBG) scintigraphy in patients with known metastatic PPGL. 123I-MIBG is best reserved for functional imaging in patients with metastatic PPGL who are being considered for radiotherapy using 131I-MIBG, in patients with an unusually large primary tumor, and in other special circumstances.
• Perioperative medical management: Preoperative blockade with an alpha-adrenergic–receptor blocker beginning 7-14 days before surgery is recommended together with a high-sodium diet and increased fluid intake as the best means of reducing the risk of perioperative cardiovascular problems.
• Surgery: Minimally invasive adrenalectomy is appropriate for most pheochromocytomas; open resection is best reserved for those tumors which are invasive or greater than 6 cm in size. The guidelines recommend open resection for paragangliomas, although laparoscopic surgery is described as reasonable for those which are small, noninvasive, and favorably located. Partial adrenalectomy is advised for patients with a hereditary pheochromocytoma and in other special circumstances.
• Team approach: Because PPGLs are uncommon, they are best managed by multidisciplinary teams at centers of expertise. That’s particularly important in nonstraightforward cases, such as those involving pregnancy, metastasis, diagnostic uncertainty, or surgical complexity, according to the guideline panelists.
All Endocrine Society clinical practice guidelines are funded by the society without any corporate support. Dr. Lenders reported having no financial conflicts.
CHICAGO – Genetic testing has jumped to the fore in the management of patients diagnosed as having a pheochromocytoma or paraganglioma, according to new clinical practice guidelines released by the Endocrine Society.
Indeed, the new guidelines call for genetic testing to be considered seriously in all patients with a proven pheochromocytoma or paraganglioma (PPGL), Dr. Jacques W. M. Lenders said in presenting highlights of the new guidelines at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"We recommend that all patients with PPGLs should be engaged in shared decision making for genetic testing. I don’t say that we should do genetic testing in everybody, but we should consider it and engage the patient in the final decision," said Dr. Lenders, who chaired the practice guidelines task force.
The strong emphasis on genetic testing arises from evidence that roughly one-third of all PPGLs are associated with germline mutations. Moreover, susceptibility mutations are present in 12% of patients with absolutely no suggestion of a positive family history. Some of these mutations – for example, those involving succinate dehydrogenase B (SDHB) – are associated with a high risk of metastasis and unfavorable prognosis. Thus, gene-testing results can have a major impact on patients with PPGL as well as their relatives.
Nonetheless, genetic testing in patients with PPGLs remains controversial.
"I must say, we on the guideline task force spent considerable time on what and how to do it," said Dr. Lenders, who is professor and deputy chair of internal medicine at Radboud University in Nijmegen, the Netherlands.
Since simultaneous testing for all the known culprit genes remains for now too expensive to be cost effective, the guidelines include a clinical feature–driven decisional algorithm designed to establish the priorities for genetic testing in a given patient with proven PPGL.
For example, patients with a metastatic PPGL should be tested for SDHB mutations, while those with a paraganglioma should undergo testing for succinate dehydrogenase mutations, according to the guidelines, published in full in concert with ICE/ENDO 2014 (J. Clin. Endocrinol. Metab. 2014;1915-42).
Dr. Lenders noted that PPGLs are uncommon tumors. It is estimated that 0.1%-1% of patients being treated for hypertension have pheochromocytomas, which are adrenal tumors resulting in excess production of epinephrine and norepinephrine. Symptoms can include paroxysmal severe headache, tachycardia, anxiety, and excessive sweating, along with tough-to-control hypertension.
While pheochromocytomas are typically benign, malignant transformation occurs in up to 17% of cases. And although a complete cure is often possible with timely therapy, the fact is that on average a 3-year delay transpires between symptomatic presentation and diagnosis of PPGL. Also, studies show that failure to appropriately follow up on a positive biochemical test is common in clinical practice; as a consequence, PPGLs are often overdiagnosed. For these reasons, Endocrine Society officials deemed PPGLs a priority area in need of practice guidelines.
In addition to routine consideration of genetic testing, other recommendations include:
• Diagnostic biochemical testing: Initial testing should include measurement of plasma free or urinary fractionated metanephrines, preferably using liquid chromatography with electrochemical or mass spectrometric laboratory methods. Immunoassays, although popular in Europe, haven’t yet been adequately validated. In measuring plasma metanephrines, the blood draw should be done with the patient in supine position, using reference standards established in the same position.
"False-positive test results are a major problem in daily clinical practice, and they outweigh by far the number of true-positive test results. That’s very important to realize," the endocrinologist said.
One common cause of false-positive test results are medications that trigger elevated metanephrine levels, according to guideline panelist Dr. William F. Young Jr., professor of medicine and chair of the department of endocrinology, diabetes, metabolism and nutrition at the Mayo Clinic, Rochester, Minn. The top three offending drugs in his experience are tricyclic antidepressants, antipsychotic agents, and levodopa. The guidelines list others, he added.
• Imaging: Once clear biochemical evidence of a PPGL is established, CT is preferred over MRI in order to locate the tumor because of its superior spatial resolution in the thorax, abdomen, and pelvis. 18F-fluorodeoxyglucose positron emission tomography/CT scanning is preferred over 123I-metaiodobenzylguanidine (MIBG) scintigraphy in patients with known metastatic PPGL. 123I-MIBG is best reserved for functional imaging in patients with metastatic PPGL who are being considered for radiotherapy using 131I-MIBG, in patients with an unusually large primary tumor, and in other special circumstances.
• Perioperative medical management: Preoperative blockade with an alpha-adrenergic–receptor blocker beginning 7-14 days before surgery is recommended together with a high-sodium diet and increased fluid intake as the best means of reducing the risk of perioperative cardiovascular problems.
• Surgery: Minimally invasive adrenalectomy is appropriate for most pheochromocytomas; open resection is best reserved for those tumors which are invasive or greater than 6 cm in size. The guidelines recommend open resection for paragangliomas, although laparoscopic surgery is described as reasonable for those which are small, noninvasive, and favorably located. Partial adrenalectomy is advised for patients with a hereditary pheochromocytoma and in other special circumstances.
• Team approach: Because PPGLs are uncommon, they are best managed by multidisciplinary teams at centers of expertise. That’s particularly important in nonstraightforward cases, such as those involving pregnancy, metastasis, diagnostic uncertainty, or surgical complexity, according to the guideline panelists.
All Endocrine Society clinical practice guidelines are funded by the society without any corporate support. Dr. Lenders reported having no financial conflicts.
CHICAGO – Genetic testing has jumped to the fore in the management of patients diagnosed as having a pheochromocytoma or paraganglioma, according to new clinical practice guidelines released by the Endocrine Society.
Indeed, the new guidelines call for genetic testing to be considered seriously in all patients with a proven pheochromocytoma or paraganglioma (PPGL), Dr. Jacques W. M. Lenders said in presenting highlights of the new guidelines at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"We recommend that all patients with PPGLs should be engaged in shared decision making for genetic testing. I don’t say that we should do genetic testing in everybody, but we should consider it and engage the patient in the final decision," said Dr. Lenders, who chaired the practice guidelines task force.
The strong emphasis on genetic testing arises from evidence that roughly one-third of all PPGLs are associated with germline mutations. Moreover, susceptibility mutations are present in 12% of patients with absolutely no suggestion of a positive family history. Some of these mutations – for example, those involving succinate dehydrogenase B (SDHB) – are associated with a high risk of metastasis and unfavorable prognosis. Thus, gene-testing results can have a major impact on patients with PPGL as well as their relatives.
Nonetheless, genetic testing in patients with PPGLs remains controversial.
"I must say, we on the guideline task force spent considerable time on what and how to do it," said Dr. Lenders, who is professor and deputy chair of internal medicine at Radboud University in Nijmegen, the Netherlands.
Since simultaneous testing for all the known culprit genes remains for now too expensive to be cost effective, the guidelines include a clinical feature–driven decisional algorithm designed to establish the priorities for genetic testing in a given patient with proven PPGL.
For example, patients with a metastatic PPGL should be tested for SDHB mutations, while those with a paraganglioma should undergo testing for succinate dehydrogenase mutations, according to the guidelines, published in full in concert with ICE/ENDO 2014 (J. Clin. Endocrinol. Metab. 2014;1915-42).
Dr. Lenders noted that PPGLs are uncommon tumors. It is estimated that 0.1%-1% of patients being treated for hypertension have pheochromocytomas, which are adrenal tumors resulting in excess production of epinephrine and norepinephrine. Symptoms can include paroxysmal severe headache, tachycardia, anxiety, and excessive sweating, along with tough-to-control hypertension.
While pheochromocytomas are typically benign, malignant transformation occurs in up to 17% of cases. And although a complete cure is often possible with timely therapy, the fact is that on average a 3-year delay transpires between symptomatic presentation and diagnosis of PPGL. Also, studies show that failure to appropriately follow up on a positive biochemical test is common in clinical practice; as a consequence, PPGLs are often overdiagnosed. For these reasons, Endocrine Society officials deemed PPGLs a priority area in need of practice guidelines.
In addition to routine consideration of genetic testing, other recommendations include:
• Diagnostic biochemical testing: Initial testing should include measurement of plasma free or urinary fractionated metanephrines, preferably using liquid chromatography with electrochemical or mass spectrometric laboratory methods. Immunoassays, although popular in Europe, haven’t yet been adequately validated. In measuring plasma metanephrines, the blood draw should be done with the patient in supine position, using reference standards established in the same position.
"False-positive test results are a major problem in daily clinical practice, and they outweigh by far the number of true-positive test results. That’s very important to realize," the endocrinologist said.
One common cause of false-positive test results are medications that trigger elevated metanephrine levels, according to guideline panelist Dr. William F. Young Jr., professor of medicine and chair of the department of endocrinology, diabetes, metabolism and nutrition at the Mayo Clinic, Rochester, Minn. The top three offending drugs in his experience are tricyclic antidepressants, antipsychotic agents, and levodopa. The guidelines list others, he added.
• Imaging: Once clear biochemical evidence of a PPGL is established, CT is preferred over MRI in order to locate the tumor because of its superior spatial resolution in the thorax, abdomen, and pelvis. 18F-fluorodeoxyglucose positron emission tomography/CT scanning is preferred over 123I-metaiodobenzylguanidine (MIBG) scintigraphy in patients with known metastatic PPGL. 123I-MIBG is best reserved for functional imaging in patients with metastatic PPGL who are being considered for radiotherapy using 131I-MIBG, in patients with an unusually large primary tumor, and in other special circumstances.
• Perioperative medical management: Preoperative blockade with an alpha-adrenergic–receptor blocker beginning 7-14 days before surgery is recommended together with a high-sodium diet and increased fluid intake as the best means of reducing the risk of perioperative cardiovascular problems.
• Surgery: Minimally invasive adrenalectomy is appropriate for most pheochromocytomas; open resection is best reserved for those tumors which are invasive or greater than 6 cm in size. The guidelines recommend open resection for paragangliomas, although laparoscopic surgery is described as reasonable for those which are small, noninvasive, and favorably located. Partial adrenalectomy is advised for patients with a hereditary pheochromocytoma and in other special circumstances.
• Team approach: Because PPGLs are uncommon, they are best managed by multidisciplinary teams at centers of expertise. That’s particularly important in nonstraightforward cases, such as those involving pregnancy, metastasis, diagnostic uncertainty, or surgical complexity, according to the guideline panelists.
All Endocrine Society clinical practice guidelines are funded by the society without any corporate support. Dr. Lenders reported having no financial conflicts.
AT ICE/ENDO 2014
Soy supplements boost low testosterone in diabetic men
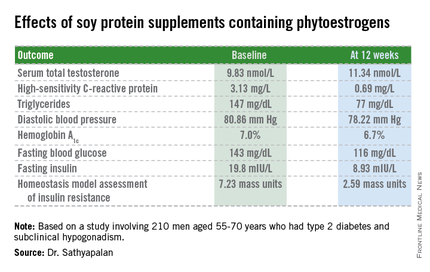
CHICAGO – Soy protein bars containing phytoestrogens raised borderline low serum total testosterone levels while improving cardiovascular risk factors and glycemic control in older type 2 diabetic men with compensated hypogonadism.
The randomized trial was conducted because of theoretic safety concerns surrounding consumption of soy supplements by this population. Soy contains phytoestrogens, natural estrogens that might have a testosterone-lowering effect that would be particularly unwelcome in older men with type 2 diabetes, because low testosterone is associated with increased cardiovascular risk.
Thus, there was the possibility that the established cardiovascular and type 2 diabetic preventive benefits of frequent soy intake might be negated by a concomitant testosterone-lowering effect in men already at increased cardiovascular risk with low testosterone, Dr. Thozhukat Sathyapalan explained at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Those concerns were shown to be groundless in the 12-week, double-blind, randomized clinical trial, reported Dr. Sathyapalan, an endocrinologist at the University of Hull (U.K.).
The study included 210 men with type 2 diabetes aged 55-70 years with subclinical hypogonadism as defined by a serum total testosterone of 12 nmol/L or less but normal gonadotrophin levels. They were randomized to 3 months of daily consumption of two cereal bars, each containing 30 g of soy protein, with the bars eaten by one group containing 66 mg each of soy phytoestrogens, while the other group’s soy bars were phytoestrogen free. That level of soy phytoestrogen consumption – 132 mg/day – is equivalent to intake in a typical Asian diet.
The primary outcome was change in serum total testosterone over the course of 3 months. It rose in both groups. Diastolic blood pressure fell to a similar extent in both study arms, while systolic pressure was unchanged over time. Endothelial function as assessed by the reactive hyperemia index improved in the soy-plus-phytoestrogens group and worsened in the soy-only group. Improvements in multiple other markers of cardiovascular risk and glycemic control were restricted to the soy-with-phytoestrogens group. Body weight remained steady in both groups throughout the study period.
The study was funded by the U.K. Food Standards Agency, which regulates food safety. Dr. Sathyapalan reported having no financial conflicts.
CHICAGO – Soy protein bars containing phytoestrogens raised borderline low serum total testosterone levels while improving cardiovascular risk factors and glycemic control in older type 2 diabetic men with compensated hypogonadism.
The randomized trial was conducted because of theoretic safety concerns surrounding consumption of soy supplements by this population. Soy contains phytoestrogens, natural estrogens that might have a testosterone-lowering effect that would be particularly unwelcome in older men with type 2 diabetes, because low testosterone is associated with increased cardiovascular risk.

Thus, there was the possibility that the established cardiovascular and type 2 diabetic preventive benefits of frequent soy intake might be negated by a concomitant testosterone-lowering effect in men already at increased cardiovascular risk with low testosterone, Dr. Thozhukat Sathyapalan explained at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Those concerns were shown to be groundless in the 12-week, double-blind, randomized clinical trial, reported Dr. Sathyapalan, an endocrinologist at the University of Hull (U.K.).
The study included 210 men with type 2 diabetes aged 55-70 years with subclinical hypogonadism as defined by a serum total testosterone of 12 nmol/L or less but normal gonadotrophin levels. They were randomized to 3 months of daily consumption of two cereal bars, each containing 30 g of soy protein, with the bars eaten by one group containing 66 mg each of soy phytoestrogens, while the other group’s soy bars were phytoestrogen free. That level of soy phytoestrogen consumption – 132 mg/day – is equivalent to intake in a typical Asian diet.
The primary outcome was change in serum total testosterone over the course of 3 months. It rose in both groups. Diastolic blood pressure fell to a similar extent in both study arms, while systolic pressure was unchanged over time. Endothelial function as assessed by the reactive hyperemia index improved in the soy-plus-phytoestrogens group and worsened in the soy-only group. Improvements in multiple other markers of cardiovascular risk and glycemic control were restricted to the soy-with-phytoestrogens group. Body weight remained steady in both groups throughout the study period.
The study was funded by the U.K. Food Standards Agency, which regulates food safety. Dr. Sathyapalan reported having no financial conflicts.
CHICAGO – Soy protein bars containing phytoestrogens raised borderline low serum total testosterone levels while improving cardiovascular risk factors and glycemic control in older type 2 diabetic men with compensated hypogonadism.
The randomized trial was conducted because of theoretic safety concerns surrounding consumption of soy supplements by this population. Soy contains phytoestrogens, natural estrogens that might have a testosterone-lowering effect that would be particularly unwelcome in older men with type 2 diabetes, because low testosterone is associated with increased cardiovascular risk.

Thus, there was the possibility that the established cardiovascular and type 2 diabetic preventive benefits of frequent soy intake might be negated by a concomitant testosterone-lowering effect in men already at increased cardiovascular risk with low testosterone, Dr. Thozhukat Sathyapalan explained at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Those concerns were shown to be groundless in the 12-week, double-blind, randomized clinical trial, reported Dr. Sathyapalan, an endocrinologist at the University of Hull (U.K.).
The study included 210 men with type 2 diabetes aged 55-70 years with subclinical hypogonadism as defined by a serum total testosterone of 12 nmol/L or less but normal gonadotrophin levels. They were randomized to 3 months of daily consumption of two cereal bars, each containing 30 g of soy protein, with the bars eaten by one group containing 66 mg each of soy phytoestrogens, while the other group’s soy bars were phytoestrogen free. That level of soy phytoestrogen consumption – 132 mg/day – is equivalent to intake in a typical Asian diet.
The primary outcome was change in serum total testosterone over the course of 3 months. It rose in both groups. Diastolic blood pressure fell to a similar extent in both study arms, while systolic pressure was unchanged over time. Endothelial function as assessed by the reactive hyperemia index improved in the soy-plus-phytoestrogens group and worsened in the soy-only group. Improvements in multiple other markers of cardiovascular risk and glycemic control were restricted to the soy-with-phytoestrogens group. Body weight remained steady in both groups throughout the study period.
The study was funded by the U.K. Food Standards Agency, which regulates food safety. Dr. Sathyapalan reported having no financial conflicts.
AT ICE/ENDO 2014
Key clinical point: Soy supplements containing phytoestrogens in amounts equivalent to a typical Asian diet don’t reduce testosterone levels in older type 2 diabetic men with subclinical hypogonadism; instead, they increase testosterone.
Major finding: Eating cereal bars containing soy protein with soy phytoestrogens resulted in increased serum total testosterone, lowered cardiovascular risk factors, and improved metabolic control in older type 2 diabetic men with borderline low testosterone.
Data source: A 12-week, randomized, double-blind clinical trial of 210 men aged 55-70.
Disclosures: The U.K. Food Standards Agency funded the study. The presenter reported having no conflicts of interest.
Hypothyroid patients may need to surrender the car keys
CHICAGO – Hypothyroid patients exhibit objective cognitive deficits and motor slowing rendering them unsafe to operate a motor vehicle.
That’s the key take-home message from a longitudinal study in which 32 patients with thyroid cancer completed an extensive battery of neurocognitive and psychological tests as well as measured performance on a driving simulator at three time points: while euthyroid, again while temporarily hypothyroid as part of their cancer therapy and assessment, and finally while once again euthyroid after restoration of thyroid hormone therapy.
"These findings provide objective evidence warranting admonitions against operating motor vehicles for hypothyroid patients and confidence in removing such stipulations upon restoration of a euthyroid state," Dr. Kenneth B. Ain said at the joint meeting of the International Congress of Endocrinology and the Endocrine Society*.
In his own clinical practice he has long included a boxed warning against driving while hypothyroid on all of his written instructions to patients. But most physicians don’t warn their hypothyroid patients that they are driving impaired, nor do the joint practice guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists address the issue. That’s largely because there hasn’t been objective, quantitative evidence to provide firm support for such cautionary admonitions – until now, observed Dr. Ain, professor of medicine and director of the thyroid oncology program at the University of Kentucky, Lexington.
While hypothyroid, study participants experienced an 8.5% increase in braking time on a driving simulator. That’s equivalent to the degree of impairment other investigators have shown to be associated with a blood alcohol level of 82 mg/dL, which is above the legal driving limit in the United States.
"Once our study is published, a patient who is involved in an auto accident [in which] there is death or significant harm could be considered an impaired patient. And if physicians do not warn the patient of this risk, they would be considered an agent of harm. They could be liable for the consequences, the same as a neurologist who doesn’t warn a patient with a grand mal seizure disorder not to drive," Dr. Ain said.
In an interview, he noted that thyroid cancer patients undergoing thyroid hormone depletion temporarily as part of their treatment are merely a small fraction of the total impaired hypothyroid driver population. Investigators with the Framingham Heart Study have reported that 4.4% of individuals above age 60 are hypothyroid. Many of these individuals remain undiagnosed or undertreated. Plus, the noncompliance rate with levothyroxine therapy has been estimated at 17%-32%.
Moreover, once a patient is newly diagnosed as being profoundly hypothyroid and receives a prescription for thyroid hormone replacement, there is a lag time involved in achieving a euthyroid state. The half-life of levothyroxine is 1 week. It takes 6-8 weeks to reach a steady state. Probably at least 2 weeks of therapy are required before there is any improvement in the neurologic impairments documented in this study, Dr. Ain speculated.
"We’re really talking here about a public health problem, one that requires a public health response and acknowledgment that this is a danger," according to the endocrinologist.
Testing during the hypothyroid phase of the study showed significant declines in measures of executive function and information-processing speed. Fine motor performance of the hands was slowed by 13%. Mean scores on the Beck Depression Inventory deteriorated from 7.9 while euthyroid to 18.9 while hypothyroid, consistent with mild bordering on moderate depression; this depression was characterized by vegetative symptoms and altered mood, but without the impaired self-esteem and sense of guilt often characteristic of other forms of depression.
Dr. Ain reported receiving a research grant from Genzyme, which funded this study.
*Correction, 7/1/2014: An earlier version of this article misstated the name of the International Congress of Endocrinology.
CHICAGO – Hypothyroid patients exhibit objective cognitive deficits and motor slowing rendering them unsafe to operate a motor vehicle.
That’s the key take-home message from a longitudinal study in which 32 patients with thyroid cancer completed an extensive battery of neurocognitive and psychological tests as well as measured performance on a driving simulator at three time points: while euthyroid, again while temporarily hypothyroid as part of their cancer therapy and assessment, and finally while once again euthyroid after restoration of thyroid hormone therapy.
"These findings provide objective evidence warranting admonitions against operating motor vehicles for hypothyroid patients and confidence in removing such stipulations upon restoration of a euthyroid state," Dr. Kenneth B. Ain said at the joint meeting of the International Congress of Endocrinology and the Endocrine Society*.
In his own clinical practice he has long included a boxed warning against driving while hypothyroid on all of his written instructions to patients. But most physicians don’t warn their hypothyroid patients that they are driving impaired, nor do the joint practice guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists address the issue. That’s largely because there hasn’t been objective, quantitative evidence to provide firm support for such cautionary admonitions – until now, observed Dr. Ain, professor of medicine and director of the thyroid oncology program at the University of Kentucky, Lexington.
While hypothyroid, study participants experienced an 8.5% increase in braking time on a driving simulator. That’s equivalent to the degree of impairment other investigators have shown to be associated with a blood alcohol level of 82 mg/dL, which is above the legal driving limit in the United States.
"Once our study is published, a patient who is involved in an auto accident [in which] there is death or significant harm could be considered an impaired patient. And if physicians do not warn the patient of this risk, they would be considered an agent of harm. They could be liable for the consequences, the same as a neurologist who doesn’t warn a patient with a grand mal seizure disorder not to drive," Dr. Ain said.
In an interview, he noted that thyroid cancer patients undergoing thyroid hormone depletion temporarily as part of their treatment are merely a small fraction of the total impaired hypothyroid driver population. Investigators with the Framingham Heart Study have reported that 4.4% of individuals above age 60 are hypothyroid. Many of these individuals remain undiagnosed or undertreated. Plus, the noncompliance rate with levothyroxine therapy has been estimated at 17%-32%.
Moreover, once a patient is newly diagnosed as being profoundly hypothyroid and receives a prescription for thyroid hormone replacement, there is a lag time involved in achieving a euthyroid state. The half-life of levothyroxine is 1 week. It takes 6-8 weeks to reach a steady state. Probably at least 2 weeks of therapy are required before there is any improvement in the neurologic impairments documented in this study, Dr. Ain speculated.
"We’re really talking here about a public health problem, one that requires a public health response and acknowledgment that this is a danger," according to the endocrinologist.
Testing during the hypothyroid phase of the study showed significant declines in measures of executive function and information-processing speed. Fine motor performance of the hands was slowed by 13%. Mean scores on the Beck Depression Inventory deteriorated from 7.9 while euthyroid to 18.9 while hypothyroid, consistent with mild bordering on moderate depression; this depression was characterized by vegetative symptoms and altered mood, but without the impaired self-esteem and sense of guilt often characteristic of other forms of depression.
Dr. Ain reported receiving a research grant from Genzyme, which funded this study.
*Correction, 7/1/2014: An earlier version of this article misstated the name of the International Congress of Endocrinology.
CHICAGO – Hypothyroid patients exhibit objective cognitive deficits and motor slowing rendering them unsafe to operate a motor vehicle.
That’s the key take-home message from a longitudinal study in which 32 patients with thyroid cancer completed an extensive battery of neurocognitive and psychological tests as well as measured performance on a driving simulator at three time points: while euthyroid, again while temporarily hypothyroid as part of their cancer therapy and assessment, and finally while once again euthyroid after restoration of thyroid hormone therapy.
"These findings provide objective evidence warranting admonitions against operating motor vehicles for hypothyroid patients and confidence in removing such stipulations upon restoration of a euthyroid state," Dr. Kenneth B. Ain said at the joint meeting of the International Congress of Endocrinology and the Endocrine Society*.
In his own clinical practice he has long included a boxed warning against driving while hypothyroid on all of his written instructions to patients. But most physicians don’t warn their hypothyroid patients that they are driving impaired, nor do the joint practice guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists address the issue. That’s largely because there hasn’t been objective, quantitative evidence to provide firm support for such cautionary admonitions – until now, observed Dr. Ain, professor of medicine and director of the thyroid oncology program at the University of Kentucky, Lexington.
While hypothyroid, study participants experienced an 8.5% increase in braking time on a driving simulator. That’s equivalent to the degree of impairment other investigators have shown to be associated with a blood alcohol level of 82 mg/dL, which is above the legal driving limit in the United States.
"Once our study is published, a patient who is involved in an auto accident [in which] there is death or significant harm could be considered an impaired patient. And if physicians do not warn the patient of this risk, they would be considered an agent of harm. They could be liable for the consequences, the same as a neurologist who doesn’t warn a patient with a grand mal seizure disorder not to drive," Dr. Ain said.
In an interview, he noted that thyroid cancer patients undergoing thyroid hormone depletion temporarily as part of their treatment are merely a small fraction of the total impaired hypothyroid driver population. Investigators with the Framingham Heart Study have reported that 4.4% of individuals above age 60 are hypothyroid. Many of these individuals remain undiagnosed or undertreated. Plus, the noncompliance rate with levothyroxine therapy has been estimated at 17%-32%.
Moreover, once a patient is newly diagnosed as being profoundly hypothyroid and receives a prescription for thyroid hormone replacement, there is a lag time involved in achieving a euthyroid state. The half-life of levothyroxine is 1 week. It takes 6-8 weeks to reach a steady state. Probably at least 2 weeks of therapy are required before there is any improvement in the neurologic impairments documented in this study, Dr. Ain speculated.
"We’re really talking here about a public health problem, one that requires a public health response and acknowledgment that this is a danger," according to the endocrinologist.
Testing during the hypothyroid phase of the study showed significant declines in measures of executive function and information-processing speed. Fine motor performance of the hands was slowed by 13%. Mean scores on the Beck Depression Inventory deteriorated from 7.9 while euthyroid to 18.9 while hypothyroid, consistent with mild bordering on moderate depression; this depression was characterized by vegetative symptoms and altered mood, but without the impaired self-esteem and sense of guilt often characteristic of other forms of depression.
Dr. Ain reported receiving a research grant from Genzyme, which funded this study.
*Correction, 7/1/2014: An earlier version of this article misstated the name of the International Congress of Endocrinology.
AT ICE/ENDO 2014
Key clinical point: Hypothyroid patients are driving impaired. Physicians need to document, firmly cautioning them to that effect, or face possible liability concerns in the event of a serious motor vehicle accident.
Major finding: Hypothyroid patients showed an increased automobile braking time on a driving simulator that was equivalent to having a blood alcohol level above the U.S. legal driving limit.
Data source: A longitudinal study in which 32 thyroid cancer patients served as their own controls. They completed an extensive neurocognitive test battery and driving simulator performance test while euthyroid, then while temporarily hypothyroid as part of their cancer-treatment regimen, and once again after restoration to the euthyroid state.
Disclosures: Dr. Ain reported receiving a research grant from Genzyme, which funded this study.
Weight loss: Most obese adults aren’t even trying
CHICAGO – Nearly 60% of obese adults aren’t currently taking any steps to lose weight, according to a large national survey.
"That’s a surprisingly high figure. It suggests a dire need to better educate the public about the health consequences of obesity and the importance of addressing the problem with their doctors," Z. Jason Wang, Ph.D., said at the joint meeting of the International Congress of Endocrinology and the Endocrine Society*.
Moreover, of the minority of obese U.S. adults who report they actually are trying to lose weight, only 1 in 20 is taking prescription weight loss medication or has resorted to bariatric surgery. The rest are using what Dr. Wang categorized as self-modification methods: diet, exercise, OTC weight loss agents, structured weight management programs, and/or nutritional supplements.
Patient satisfaction was much higher among those using surgery or prescription medications. Thirty-nine percent of them reported being extremely or very satisfied with their weight loss method, compared with just 20% using only self-modification methods.
"This finding may mean that diet and exercise alone just don’t work for a lot of people," said Dr. Wang, director of health economics and outcomes research at Eisai in Woodcliff Lake, N.J.
He presented an analysis of data obtained from 22,927 obese adult participants in the 2012 National Health and Wellness Survey, an annual Internet-based survey which samples a demographically representative slice of the adult U.S. population.
Fully 59% of the obese respondents indicated they aren’t taking any action in an effort to lose weight. A mere 2% reported taking prescription weight loss medication or having bariatric surgery. Another 39% were relying on self-modification methods.
The National Health and Wellness Survey is conducted by Kantar Health, a health care industry consulting company. The analysis was supported by Eisai. The presenter is an Eisai employee.
*Correction, 7/1/2014: An earlier version of this article misstated the name of the International Congress of Endocrinology.
CHICAGO – Nearly 60% of obese adults aren’t currently taking any steps to lose weight, according to a large national survey.
"That’s a surprisingly high figure. It suggests a dire need to better educate the public about the health consequences of obesity and the importance of addressing the problem with their doctors," Z. Jason Wang, Ph.D., said at the joint meeting of the International Congress of Endocrinology and the Endocrine Society*.
Moreover, of the minority of obese U.S. adults who report they actually are trying to lose weight, only 1 in 20 is taking prescription weight loss medication or has resorted to bariatric surgery. The rest are using what Dr. Wang categorized as self-modification methods: diet, exercise, OTC weight loss agents, structured weight management programs, and/or nutritional supplements.
Patient satisfaction was much higher among those using surgery or prescription medications. Thirty-nine percent of them reported being extremely or very satisfied with their weight loss method, compared with just 20% using only self-modification methods.
"This finding may mean that diet and exercise alone just don’t work for a lot of people," said Dr. Wang, director of health economics and outcomes research at Eisai in Woodcliff Lake, N.J.
He presented an analysis of data obtained from 22,927 obese adult participants in the 2012 National Health and Wellness Survey, an annual Internet-based survey which samples a demographically representative slice of the adult U.S. population.
Fully 59% of the obese respondents indicated they aren’t taking any action in an effort to lose weight. A mere 2% reported taking prescription weight loss medication or having bariatric surgery. Another 39% were relying on self-modification methods.
The National Health and Wellness Survey is conducted by Kantar Health, a health care industry consulting company. The analysis was supported by Eisai. The presenter is an Eisai employee.
*Correction, 7/1/2014: An earlier version of this article misstated the name of the International Congress of Endocrinology.
CHICAGO – Nearly 60% of obese adults aren’t currently taking any steps to lose weight, according to a large national survey.
"That’s a surprisingly high figure. It suggests a dire need to better educate the public about the health consequences of obesity and the importance of addressing the problem with their doctors," Z. Jason Wang, Ph.D., said at the joint meeting of the International Congress of Endocrinology and the Endocrine Society*.
Moreover, of the minority of obese U.S. adults who report they actually are trying to lose weight, only 1 in 20 is taking prescription weight loss medication or has resorted to bariatric surgery. The rest are using what Dr. Wang categorized as self-modification methods: diet, exercise, OTC weight loss agents, structured weight management programs, and/or nutritional supplements.
Patient satisfaction was much higher among those using surgery or prescription medications. Thirty-nine percent of them reported being extremely or very satisfied with their weight loss method, compared with just 20% using only self-modification methods.
"This finding may mean that diet and exercise alone just don’t work for a lot of people," said Dr. Wang, director of health economics and outcomes research at Eisai in Woodcliff Lake, N.J.
He presented an analysis of data obtained from 22,927 obese adult participants in the 2012 National Health and Wellness Survey, an annual Internet-based survey which samples a demographically representative slice of the adult U.S. population.
Fully 59% of the obese respondents indicated they aren’t taking any action in an effort to lose weight. A mere 2% reported taking prescription weight loss medication or having bariatric surgery. Another 39% were relying on self-modification methods.
The National Health and Wellness Survey is conducted by Kantar Health, a health care industry consulting company. The analysis was supported by Eisai. The presenter is an Eisai employee.
*Correction, 7/1/2014: An earlier version of this article misstated the name of the International Congress of Endocrinology.
AT ICE/ENDO 2014
Key clinical point: Most obese adults aren’t making any effort to lose weight.
Major finding: Among obese adults who are taking action to lose weight, just 1 in 20 is on prescription weight loss medication or has undergone bariatric surgery. Of that group, 39% indicated they are extremely or very satisfied with their weight loss approach, compared with just 20% of those using other methods, including diet, exercise, OTC drugs, and weight management programs.
Data source: This analysis included nearly 23,000 adults with a body mass index of 30 kg/m2 or more who participated in the 2012 National Health and Wellness Survey.
Disclosures: The survey was conducted by Kantar Health. The analysis was supported by Eisai. The presenter is an Eisai employee.