User login
American Society of Neuroradiology (ASNR): Annual Meeting
Study suggests treating intracranial aneurysms larger than 5 mm
SAN DIEGO – Analyses of a single-center cohort of nearly 3,400 patients with unruptured aneurysms who underwent either treatment or observation suggest that aneurysms larger than 5 mm should be treated.
"Treatment, of course, has complications, and some patients deteriorate clinically. However, we find that unruptured aneurysm treatment-related complications leave no effect on function, as measured by modified Rankin Scale scores. On the other hand, once an aneurysm ruptures, there is a high incidence of death and disability. Based on that, we conclude unruptured aneurysms larger than 5 mm should be treated," Dr. Yuichi Murayama said at the annual meeting of the American Society of Neuroradiology.
In the cohort of 3,395 patients with unruptured intracranial aneurysms (UIAs), 28% were treated while the remaining 72% were managed conservatively and underwent biannual 3D computed tomography angiography (CTA). They were referred to Jikei University in Tokyo between January 2003 and December 2012.
Most UIAs were measured using 3D CTA because magnetic resonance angiography (MRA) was thought to be less accurate and more invasive. Patients were recommended for treatment (with either endovascular coiling or microsurgical clipping) if the aneurysm was larger than 5 mm and considered safely treatable, multiple aneurysms were present and one had previously ruptured, or there was a family history of subarachnoid hemorrhage. Endovascularly treated patients underwent MRA follow-up at 3, 6, and 12 months after treatment and then subsequently underwent annual MRA and magnetic resonance imaging (MRI) studies. Surgically treated patients had angiography at 12 months after treatment and then 3D CTA.
Almost 1,700 patients with UIAs who were not treated were followed over the 10-year period with CTA. Overall, 49 (2.9%) aneurysms ruptured, yielding an annual rupture rate of 0.8%/year. The average size of aneurysms in the treatment group was 7.8 mm, compared with 4.4 mm in the observation group. The frequency of treatment increased with size: 10% of small aneurysms (up to 4.9 mm in diameter), 50% of medium aneurysms (5.0-9.9 mm), and almost 100% of large (10.0-24.9 mm) and giant aneurysms (greater than 25 mm), reported Dr. Murayama, director of the center of endovascular surgery at Jikei University.
Rupture rates were 0.35%/year for small aneurysms, 2.2%/year for medium, 10.75%/year for large, and 50%/year for giant. Although the risk of rupture of small aneurysms is low, 17 small aneurysms ruptured within the observation period. Furthermore, Dr. Murayama said that while most ruptures occurred within the first 2 years of discovery, even apparently stable aneurysms might rupture after 2 years. The most common sites for aneurysm rupture were the anterior cerebral, middle cerebral, vertebral, and posterior communicating arteries.
About 200 additional patients were followed with MRA rather than CTA, Dr. Murayama said after the meeting. Ongoing analyses of the rupture rates in these patients may change the rupture rates for various aneurysm sizes, he said.
Dr. Murayama compared the results from his institution to the findings of another Japanese cohort in the UCAS study, which included 5,700 patients with almost 6,700 aneurysms enrolled from 283 medical centers (N. Engl. J. Med. 2012;366:2474-82). The annual risk of rupture for small aneurysms was 0.36%, very similar to that found by the Jikei group (0.35%). Similarly, the annual risk for 7- to 10-mm aneurysms was 1.7%, which was very similar to that found by the Jikei group (1.5%).
There was a more substantial difference between the two studies in the annual risk of rupture for aneurysms measuring 5-7 mm (0.5% in the UCAS cohort vs. 2.3% in the Jikei cohort). "For this size, the decision to treat is difficult because the risk of rupture is relatively low but treatment risk also exists. That is why, in our database, 50% of patients with 5- to 7-mm-sized aneurysms went to observation," Dr. Murayama said.
Using 3D CTA, Dr. Murayama addressed the question whether UIAs grow. In the Jikei cohort, 10% of aneurysms grew in size (defined as a change of 1 mm or more between the baseline measurement and follow-up). "If you see a change in morphology, treat without delay," he said.
Dr. Murayama reported no relevant financial relationships.
Treatment, complications, unruptured aneurysm treatment-related complications, Dr. Yuichi Murayama, American Society of Neuroradiology, biannual 3D computed tomography angiography (CTA),
SAN DIEGO – Analyses of a single-center cohort of nearly 3,400 patients with unruptured aneurysms who underwent either treatment or observation suggest that aneurysms larger than 5 mm should be treated.
"Treatment, of course, has complications, and some patients deteriorate clinically. However, we find that unruptured aneurysm treatment-related complications leave no effect on function, as measured by modified Rankin Scale scores. On the other hand, once an aneurysm ruptures, there is a high incidence of death and disability. Based on that, we conclude unruptured aneurysms larger than 5 mm should be treated," Dr. Yuichi Murayama said at the annual meeting of the American Society of Neuroradiology.
In the cohort of 3,395 patients with unruptured intracranial aneurysms (UIAs), 28% were treated while the remaining 72% were managed conservatively and underwent biannual 3D computed tomography angiography (CTA). They were referred to Jikei University in Tokyo between January 2003 and December 2012.
Most UIAs were measured using 3D CTA because magnetic resonance angiography (MRA) was thought to be less accurate and more invasive. Patients were recommended for treatment (with either endovascular coiling or microsurgical clipping) if the aneurysm was larger than 5 mm and considered safely treatable, multiple aneurysms were present and one had previously ruptured, or there was a family history of subarachnoid hemorrhage. Endovascularly treated patients underwent MRA follow-up at 3, 6, and 12 months after treatment and then subsequently underwent annual MRA and magnetic resonance imaging (MRI) studies. Surgically treated patients had angiography at 12 months after treatment and then 3D CTA.
Almost 1,700 patients with UIAs who were not treated were followed over the 10-year period with CTA. Overall, 49 (2.9%) aneurysms ruptured, yielding an annual rupture rate of 0.8%/year. The average size of aneurysms in the treatment group was 7.8 mm, compared with 4.4 mm in the observation group. The frequency of treatment increased with size: 10% of small aneurysms (up to 4.9 mm in diameter), 50% of medium aneurysms (5.0-9.9 mm), and almost 100% of large (10.0-24.9 mm) and giant aneurysms (greater than 25 mm), reported Dr. Murayama, director of the center of endovascular surgery at Jikei University.
Rupture rates were 0.35%/year for small aneurysms, 2.2%/year for medium, 10.75%/year for large, and 50%/year for giant. Although the risk of rupture of small aneurysms is low, 17 small aneurysms ruptured within the observation period. Furthermore, Dr. Murayama said that while most ruptures occurred within the first 2 years of discovery, even apparently stable aneurysms might rupture after 2 years. The most common sites for aneurysm rupture were the anterior cerebral, middle cerebral, vertebral, and posterior communicating arteries.
About 200 additional patients were followed with MRA rather than CTA, Dr. Murayama said after the meeting. Ongoing analyses of the rupture rates in these patients may change the rupture rates for various aneurysm sizes, he said.
Dr. Murayama compared the results from his institution to the findings of another Japanese cohort in the UCAS study, which included 5,700 patients with almost 6,700 aneurysms enrolled from 283 medical centers (N. Engl. J. Med. 2012;366:2474-82). The annual risk of rupture for small aneurysms was 0.36%, very similar to that found by the Jikei group (0.35%). Similarly, the annual risk for 7- to 10-mm aneurysms was 1.7%, which was very similar to that found by the Jikei group (1.5%).
There was a more substantial difference between the two studies in the annual risk of rupture for aneurysms measuring 5-7 mm (0.5% in the UCAS cohort vs. 2.3% in the Jikei cohort). "For this size, the decision to treat is difficult because the risk of rupture is relatively low but treatment risk also exists. That is why, in our database, 50% of patients with 5- to 7-mm-sized aneurysms went to observation," Dr. Murayama said.
Using 3D CTA, Dr. Murayama addressed the question whether UIAs grow. In the Jikei cohort, 10% of aneurysms grew in size (defined as a change of 1 mm or more between the baseline measurement and follow-up). "If you see a change in morphology, treat without delay," he said.
Dr. Murayama reported no relevant financial relationships.
SAN DIEGO – Analyses of a single-center cohort of nearly 3,400 patients with unruptured aneurysms who underwent either treatment or observation suggest that aneurysms larger than 5 mm should be treated.
"Treatment, of course, has complications, and some patients deteriorate clinically. However, we find that unruptured aneurysm treatment-related complications leave no effect on function, as measured by modified Rankin Scale scores. On the other hand, once an aneurysm ruptures, there is a high incidence of death and disability. Based on that, we conclude unruptured aneurysms larger than 5 mm should be treated," Dr. Yuichi Murayama said at the annual meeting of the American Society of Neuroradiology.
In the cohort of 3,395 patients with unruptured intracranial aneurysms (UIAs), 28% were treated while the remaining 72% were managed conservatively and underwent biannual 3D computed tomography angiography (CTA). They were referred to Jikei University in Tokyo between January 2003 and December 2012.
Most UIAs were measured using 3D CTA because magnetic resonance angiography (MRA) was thought to be less accurate and more invasive. Patients were recommended for treatment (with either endovascular coiling or microsurgical clipping) if the aneurysm was larger than 5 mm and considered safely treatable, multiple aneurysms were present and one had previously ruptured, or there was a family history of subarachnoid hemorrhage. Endovascularly treated patients underwent MRA follow-up at 3, 6, and 12 months after treatment and then subsequently underwent annual MRA and magnetic resonance imaging (MRI) studies. Surgically treated patients had angiography at 12 months after treatment and then 3D CTA.
Almost 1,700 patients with UIAs who were not treated were followed over the 10-year period with CTA. Overall, 49 (2.9%) aneurysms ruptured, yielding an annual rupture rate of 0.8%/year. The average size of aneurysms in the treatment group was 7.8 mm, compared with 4.4 mm in the observation group. The frequency of treatment increased with size: 10% of small aneurysms (up to 4.9 mm in diameter), 50% of medium aneurysms (5.0-9.9 mm), and almost 100% of large (10.0-24.9 mm) and giant aneurysms (greater than 25 mm), reported Dr. Murayama, director of the center of endovascular surgery at Jikei University.
Rupture rates were 0.35%/year for small aneurysms, 2.2%/year for medium, 10.75%/year for large, and 50%/year for giant. Although the risk of rupture of small aneurysms is low, 17 small aneurysms ruptured within the observation period. Furthermore, Dr. Murayama said that while most ruptures occurred within the first 2 years of discovery, even apparently stable aneurysms might rupture after 2 years. The most common sites for aneurysm rupture were the anterior cerebral, middle cerebral, vertebral, and posterior communicating arteries.
About 200 additional patients were followed with MRA rather than CTA, Dr. Murayama said after the meeting. Ongoing analyses of the rupture rates in these patients may change the rupture rates for various aneurysm sizes, he said.
Dr. Murayama compared the results from his institution to the findings of another Japanese cohort in the UCAS study, which included 5,700 patients with almost 6,700 aneurysms enrolled from 283 medical centers (N. Engl. J. Med. 2012;366:2474-82). The annual risk of rupture for small aneurysms was 0.36%, very similar to that found by the Jikei group (0.35%). Similarly, the annual risk for 7- to 10-mm aneurysms was 1.7%, which was very similar to that found by the Jikei group (1.5%).
There was a more substantial difference between the two studies in the annual risk of rupture for aneurysms measuring 5-7 mm (0.5% in the UCAS cohort vs. 2.3% in the Jikei cohort). "For this size, the decision to treat is difficult because the risk of rupture is relatively low but treatment risk also exists. That is why, in our database, 50% of patients with 5- to 7-mm-sized aneurysms went to observation," Dr. Murayama said.
Using 3D CTA, Dr. Murayama addressed the question whether UIAs grow. In the Jikei cohort, 10% of aneurysms grew in size (defined as a change of 1 mm or more between the baseline measurement and follow-up). "If you see a change in morphology, treat without delay," he said.
Dr. Murayama reported no relevant financial relationships.
Treatment, complications, unruptured aneurysm treatment-related complications, Dr. Yuichi Murayama, American Society of Neuroradiology, biannual 3D computed tomography angiography (CTA),
Treatment, complications, unruptured aneurysm treatment-related complications, Dr. Yuichi Murayama, American Society of Neuroradiology, biannual 3D computed tomography angiography (CTA),
AT THE ASNR ANNUAL MEETING
Major finding: During a 10-year period, ruptures occurred in 2.9% of intracranial aneurysms that were treated conservatively, with an annual rupture rate of 0.8%/year.
Data source: A single-center, prospective cohort study of 3,395 patients with unruptured intracranial aneurysms.
Disclosures: Dr. Murayama reported no relevant financial relationships.
Neurologist calls for shift in stroke paradigm
SAN DIEGO – Many of the guideline recommendations for intra-arterial catheter-directed treatment of acute ischemic stroke that were recently endorsed by neuroradiology and interventional neurology groups should be "revisited" to hasten time to revascularization to salvage more brain tissue following stroke, according to Dr. Jeffrey L. Saver.
While the multisociety guidelines endorsed by the American Society of Neuroradiology, the Society for Neurointerventional Surgery, and other professional groups recommend a door-to-revascularization time of 210 minutes (J. Vasc. Int. Radiol. 2013;24:151-63), Dr. Saver calls for 130 minutes and says this is an attainable goal in the age of stent retrievers.
"If you wait 3 hours and 30 minutes to get arteries unclogged, most of the affected brain is dead," Dr. Saver said at the annual meeting of the American Society of Neuroradiology. "That is playing Little League, and the ischemic brain is playing Major League."
Lessons from IV TPA show the dramatic effect of time in stroke outcomes, said Dr. Saver, director of the UCLA Comprehensive Stroke Center at the University of California, Los Angeles. In a study of more than 58,000 patients seen at 1,400 Get With the Guidelines stroke hospitals, he and his colleagues found that faster onset-to-treatment, in 15-minute increments, was associated with reduced in-hospital mortality (odds ratio, 0.96, P less than .001), reduced symptomatic intracranial hemorrhage (OR 0.96, P less than .001), increased achievement of independent ambulation at discharge (OR 1.04, P less than .001), and increased discharge to home (OR 1.03, P less than .001) (JAMA 2013;309:2480-8).
"In other words, for each 1,000 patients, accelerating TPA treatment by 15 minutes resulted in 18 more patients with improved ambulation at discharge, including 8 more who will ambulate fully independently, 13 more who will be discharged to a more independent environment, and 4 fewer patient deaths prior to discharge," he said.
Dr. Saver suggests paring down the endovascular time targets of the guidelines. For example, door-to-puncture time goals should be reduced from 120 minutes to 90 minutes, picture-to-puncture times from 95 to 65 minutes, puncture to first-pass from 45 to 20 minutes, and puncture to revascularization from 90 to 40 minutes.
One way to cut down time is to begin treatment before the patient arrives at the hospital. Dr. Saver described a German study comparing outcomes of initiating lytic therapy in a mobile stroke unit (with a neurologist and a CT scanner on board the ambulance) to lytic therapy given when the patient reached the emergency department. The average time from alarm to treatment was twice as long for those given hospital lysis, compared with those treated by the mobile units (73 minutes vs. 38 minutes), although no differences were found in efficacy or safety outcomes. (Lancet Neurol. 2012;11:397-404).
"This is a remarkable acceleration of care," Dr. Saver said. By putting head-only CT scanners into ambulances, the German groups have the capability of performing not only noncontrast CT, but also CT angiography or perfusion CT on site. Dr. Saver’s team is also investigating whether other treatments given in the field, such as neuroprotective agents or antihypertensives, might be beneficial.
Other strategies to shave time include emergency medical services prenotification, having a stroke tool kit in place, and rapid triage and stroke team notification. He also recommends having a single-call activation system of the stroke team. TPA can be premixed and should be easily accessible and stored in the emergency department and radiology suite. At UCLA, a visual decision aid outlining the pros and cons of TPA allows families to make rapid informed consent.
Rapid treatment times also depend upon access to specialized stroke care centers. In 2010, 47% of the U.S. population, or 147 million Americans, lived in jurisdictions that routed stroke patients to the nearest hospital, not a primary stroke center (PSC). "But we have reached a tipping point now that more than half of the U.S. population resides in a stroke-center domain," Dr. Saver said. Since the early 2000s, there has been a shift from an acute ischemic stroke treatment model that relied primarily on transport of patients to PSCs or stroke-ready hospitals where they could receive TPA and other acute therapies safely and efficiently. Nowadays, favor is given to a two-tier system of spokes (PSCs) and hubs (comprehensive stroke centers that offer advanced treatments for complex patients, staffed by stroke specialists). More than ever, rapid identification and evaluation of stroke patients and effective differential diversion assessments are critical in reducing door-to-needle or door-to-recanalization times.
According to Dr. Saver, in 2005, only one-quarter of patients were being treated based on best practice standards of door-to-IV TPA of 60 minutes or less, and that level remained virtually unchanged through 2009. More recently, that number has begun to inch up. "Nationally, we are moving the curve, but the best centers are not going for 60 minutes door-to-needle, they are going for 30 minutes," he said, noting that nationally the fastest door-to-needle times in the United States are being reported by Washington University in St. Louis (39 minutes). The world’s record is 20 minutes in Helsinki.
For a best practice example of rapid catheter-based reperfusion, Dr. Saver cited a recent report from the University of Calgary (J. Neurointervent. Surg. 2013;5[Suppl. 1]:i58-61) in which imaging to reperfusion time averaged 47 minutes. More than 80% of the 11 patients left the hospital with modified Rankin Scale scores of 1 or less.
Dr. Saver reported that UCLA receives compensation for his service as a consultant to Covidien, Grifols, and Lundbeck on the design and conduct of trials.
SAN DIEGO – Many of the guideline recommendations for intra-arterial catheter-directed treatment of acute ischemic stroke that were recently endorsed by neuroradiology and interventional neurology groups should be "revisited" to hasten time to revascularization to salvage more brain tissue following stroke, according to Dr. Jeffrey L. Saver.
While the multisociety guidelines endorsed by the American Society of Neuroradiology, the Society for Neurointerventional Surgery, and other professional groups recommend a door-to-revascularization time of 210 minutes (J. Vasc. Int. Radiol. 2013;24:151-63), Dr. Saver calls for 130 minutes and says this is an attainable goal in the age of stent retrievers.
"If you wait 3 hours and 30 minutes to get arteries unclogged, most of the affected brain is dead," Dr. Saver said at the annual meeting of the American Society of Neuroradiology. "That is playing Little League, and the ischemic brain is playing Major League."
Lessons from IV TPA show the dramatic effect of time in stroke outcomes, said Dr. Saver, director of the UCLA Comprehensive Stroke Center at the University of California, Los Angeles. In a study of more than 58,000 patients seen at 1,400 Get With the Guidelines stroke hospitals, he and his colleagues found that faster onset-to-treatment, in 15-minute increments, was associated with reduced in-hospital mortality (odds ratio, 0.96, P less than .001), reduced symptomatic intracranial hemorrhage (OR 0.96, P less than .001), increased achievement of independent ambulation at discharge (OR 1.04, P less than .001), and increased discharge to home (OR 1.03, P less than .001) (JAMA 2013;309:2480-8).
"In other words, for each 1,000 patients, accelerating TPA treatment by 15 minutes resulted in 18 more patients with improved ambulation at discharge, including 8 more who will ambulate fully independently, 13 more who will be discharged to a more independent environment, and 4 fewer patient deaths prior to discharge," he said.
Dr. Saver suggests paring down the endovascular time targets of the guidelines. For example, door-to-puncture time goals should be reduced from 120 minutes to 90 minutes, picture-to-puncture times from 95 to 65 minutes, puncture to first-pass from 45 to 20 minutes, and puncture to revascularization from 90 to 40 minutes.
One way to cut down time is to begin treatment before the patient arrives at the hospital. Dr. Saver described a German study comparing outcomes of initiating lytic therapy in a mobile stroke unit (with a neurologist and a CT scanner on board the ambulance) to lytic therapy given when the patient reached the emergency department. The average time from alarm to treatment was twice as long for those given hospital lysis, compared with those treated by the mobile units (73 minutes vs. 38 minutes), although no differences were found in efficacy or safety outcomes. (Lancet Neurol. 2012;11:397-404).
"This is a remarkable acceleration of care," Dr. Saver said. By putting head-only CT scanners into ambulances, the German groups have the capability of performing not only noncontrast CT, but also CT angiography or perfusion CT on site. Dr. Saver’s team is also investigating whether other treatments given in the field, such as neuroprotective agents or antihypertensives, might be beneficial.
Other strategies to shave time include emergency medical services prenotification, having a stroke tool kit in place, and rapid triage and stroke team notification. He also recommends having a single-call activation system of the stroke team. TPA can be premixed and should be easily accessible and stored in the emergency department and radiology suite. At UCLA, a visual decision aid outlining the pros and cons of TPA allows families to make rapid informed consent.
Rapid treatment times also depend upon access to specialized stroke care centers. In 2010, 47% of the U.S. population, or 147 million Americans, lived in jurisdictions that routed stroke patients to the nearest hospital, not a primary stroke center (PSC). "But we have reached a tipping point now that more than half of the U.S. population resides in a stroke-center domain," Dr. Saver said. Since the early 2000s, there has been a shift from an acute ischemic stroke treatment model that relied primarily on transport of patients to PSCs or stroke-ready hospitals where they could receive TPA and other acute therapies safely and efficiently. Nowadays, favor is given to a two-tier system of spokes (PSCs) and hubs (comprehensive stroke centers that offer advanced treatments for complex patients, staffed by stroke specialists). More than ever, rapid identification and evaluation of stroke patients and effective differential diversion assessments are critical in reducing door-to-needle or door-to-recanalization times.
According to Dr. Saver, in 2005, only one-quarter of patients were being treated based on best practice standards of door-to-IV TPA of 60 minutes or less, and that level remained virtually unchanged through 2009. More recently, that number has begun to inch up. "Nationally, we are moving the curve, but the best centers are not going for 60 minutes door-to-needle, they are going for 30 minutes," he said, noting that nationally the fastest door-to-needle times in the United States are being reported by Washington University in St. Louis (39 minutes). The world’s record is 20 minutes in Helsinki.
For a best practice example of rapid catheter-based reperfusion, Dr. Saver cited a recent report from the University of Calgary (J. Neurointervent. Surg. 2013;5[Suppl. 1]:i58-61) in which imaging to reperfusion time averaged 47 minutes. More than 80% of the 11 patients left the hospital with modified Rankin Scale scores of 1 or less.
Dr. Saver reported that UCLA receives compensation for his service as a consultant to Covidien, Grifols, and Lundbeck on the design and conduct of trials.
SAN DIEGO – Many of the guideline recommendations for intra-arterial catheter-directed treatment of acute ischemic stroke that were recently endorsed by neuroradiology and interventional neurology groups should be "revisited" to hasten time to revascularization to salvage more brain tissue following stroke, according to Dr. Jeffrey L. Saver.
While the multisociety guidelines endorsed by the American Society of Neuroradiology, the Society for Neurointerventional Surgery, and other professional groups recommend a door-to-revascularization time of 210 minutes (J. Vasc. Int. Radiol. 2013;24:151-63), Dr. Saver calls for 130 minutes and says this is an attainable goal in the age of stent retrievers.
"If you wait 3 hours and 30 minutes to get arteries unclogged, most of the affected brain is dead," Dr. Saver said at the annual meeting of the American Society of Neuroradiology. "That is playing Little League, and the ischemic brain is playing Major League."
Lessons from IV TPA show the dramatic effect of time in stroke outcomes, said Dr. Saver, director of the UCLA Comprehensive Stroke Center at the University of California, Los Angeles. In a study of more than 58,000 patients seen at 1,400 Get With the Guidelines stroke hospitals, he and his colleagues found that faster onset-to-treatment, in 15-minute increments, was associated with reduced in-hospital mortality (odds ratio, 0.96, P less than .001), reduced symptomatic intracranial hemorrhage (OR 0.96, P less than .001), increased achievement of independent ambulation at discharge (OR 1.04, P less than .001), and increased discharge to home (OR 1.03, P less than .001) (JAMA 2013;309:2480-8).
"In other words, for each 1,000 patients, accelerating TPA treatment by 15 minutes resulted in 18 more patients with improved ambulation at discharge, including 8 more who will ambulate fully independently, 13 more who will be discharged to a more independent environment, and 4 fewer patient deaths prior to discharge," he said.
Dr. Saver suggests paring down the endovascular time targets of the guidelines. For example, door-to-puncture time goals should be reduced from 120 minutes to 90 minutes, picture-to-puncture times from 95 to 65 minutes, puncture to first-pass from 45 to 20 minutes, and puncture to revascularization from 90 to 40 minutes.
One way to cut down time is to begin treatment before the patient arrives at the hospital. Dr. Saver described a German study comparing outcomes of initiating lytic therapy in a mobile stroke unit (with a neurologist and a CT scanner on board the ambulance) to lytic therapy given when the patient reached the emergency department. The average time from alarm to treatment was twice as long for those given hospital lysis, compared with those treated by the mobile units (73 minutes vs. 38 minutes), although no differences were found in efficacy or safety outcomes. (Lancet Neurol. 2012;11:397-404).
"This is a remarkable acceleration of care," Dr. Saver said. By putting head-only CT scanners into ambulances, the German groups have the capability of performing not only noncontrast CT, but also CT angiography or perfusion CT on site. Dr. Saver’s team is also investigating whether other treatments given in the field, such as neuroprotective agents or antihypertensives, might be beneficial.
Other strategies to shave time include emergency medical services prenotification, having a stroke tool kit in place, and rapid triage and stroke team notification. He also recommends having a single-call activation system of the stroke team. TPA can be premixed and should be easily accessible and stored in the emergency department and radiology suite. At UCLA, a visual decision aid outlining the pros and cons of TPA allows families to make rapid informed consent.
Rapid treatment times also depend upon access to specialized stroke care centers. In 2010, 47% of the U.S. population, or 147 million Americans, lived in jurisdictions that routed stroke patients to the nearest hospital, not a primary stroke center (PSC). "But we have reached a tipping point now that more than half of the U.S. population resides in a stroke-center domain," Dr. Saver said. Since the early 2000s, there has been a shift from an acute ischemic stroke treatment model that relied primarily on transport of patients to PSCs or stroke-ready hospitals where they could receive TPA and other acute therapies safely and efficiently. Nowadays, favor is given to a two-tier system of spokes (PSCs) and hubs (comprehensive stroke centers that offer advanced treatments for complex patients, staffed by stroke specialists). More than ever, rapid identification and evaluation of stroke patients and effective differential diversion assessments are critical in reducing door-to-needle or door-to-recanalization times.
According to Dr. Saver, in 2005, only one-quarter of patients were being treated based on best practice standards of door-to-IV TPA of 60 minutes or less, and that level remained virtually unchanged through 2009. More recently, that number has begun to inch up. "Nationally, we are moving the curve, but the best centers are not going for 60 minutes door-to-needle, they are going for 30 minutes," he said, noting that nationally the fastest door-to-needle times in the United States are being reported by Washington University in St. Louis (39 minutes). The world’s record is 20 minutes in Helsinki.
For a best practice example of rapid catheter-based reperfusion, Dr. Saver cited a recent report from the University of Calgary (J. Neurointervent. Surg. 2013;5[Suppl. 1]:i58-61) in which imaging to reperfusion time averaged 47 minutes. More than 80% of the 11 patients left the hospital with modified Rankin Scale scores of 1 or less.
Dr. Saver reported that UCLA receives compensation for his service as a consultant to Covidien, Grifols, and Lundbeck on the design and conduct of trials.
EXPERT ANALYSIS FROM THE ASNR ANNUAL MEETING
Percent insula infarction may help predict stroke treatment response
SAN DIEGO – The percentage of anterior insula that is infarcted at the time of admission in patients with acute middle cerebral artery occlusive stroke predicted infarct growth without significant revascularization in 74 consecutive patients who underwent intra-arterial therapy.
"We believe the insula is an overlooked biomarker. ... Although we have not proven it yet, we think it may do a better job than perfusion mismatch in stratifying patients who are likely to benefit from intra-arterial therapy [IAT] from patients unlikely to benefit," said Dr. Michael H. Lev, coauthor of the study and director of emergency neuroradiology and radiology at Massachusetts General Hospital’s Institute for Heart, Vascular, and Stroke Care in Boston.
Two neuroradiologists rated the admission diffusion-weighted imaging (DWI) scans of the patients according to percent anterior and posterior insula infarction using a 4-point scale (normal, less than 50%, greater than 50%, and 100%). (The anterior insula is approximately 25% of the whole insula.) Admission DWI and follow-up MRI core infarct volumes were segmented, and infarct growth was determined. Patients were stratified into those with good (TICI [thrombolysis in cerebral infarction] grade 2-3) vs. poor (TICI grade 0-1) recanalization.
No or poor recanalization occurred in 23 (31%) patients, according to Dr. Livia Morais, who presented the findings at the annual meeting of the American Society of Neuroradiology. In this group, the percent anterior insula infarct was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.43, P = .04). Predictors of final infarct volume were age, anterior/posterior/total insula percent infarct, and DWI lesion volume at admission (all P less than .05).
For the 69% with good recanalization, National Institutes of Health Stroke Scale score at admission was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.34, P = .02). Predictors of final infarct volume were percent anterior/posterior/total insula infarct and DWI lesion volume at admission (all P less than .05).
Patients who had less than 50% anterior insula infarct had significantly lower infarct volume (P less than .0001) and infarct growth (P less than .03), compared with those who had greater than 50% anterior insula infarct involvement.
One reason the investigators are focusing on the insula is that it is seen as a region of high ischemic vulnerability. "We speculate that anterior insula infarction may be a stronger surrogate for overall stroke severity than is DWI lesion volume because the unique vascular supply of this location reflects the combined effects of not only the degree of superior division MCA occlusion, but also the quality of collateral flow from both the anterior and inferior MCA divisions, as well as other pial collateral sources," said Dr. Morais, a neuroimaging fellow at Massachusetts General Hospital’s neurovascular laboratory.
This work is part of a body of research looking to identify treatment-relevant acute imaging targets, Dr. Lev said. "We are trying to break patients up into groups with high risk-to-benefit ratios and low risk-to-benefit ratios. Those who fall in-between, if they have no other exclusion criteria, can be treated." The results of this study indicate that patients who are IAT candidates with diffusion-weighted imaging lesion volume of less than 70 mL may be stratified into those with a high likelihood of benefitting from IAT based on the percent anterior insula involvement, he said.
In previous work, Dr. Lev showed that the insula was a very good predictor of aphasia recovery after infarction (Am. J. Neuroradiol. 2010;31:1661-8). The insula was also one of several predictors of motor improvement after stroke (Neurology 2012;78:1853-9). In another study, Dr. Lev said that infarction of the right insula and peri-insular regions was associated with the development of hospital-acquired pneumonia, which he attributed to the insula’s involvement with swallowing and immune modulation.
Dr. Morais said he had no relevant financial disclosures. Dr. Lev said he has received research funding from GE Healthcare.
SAN DIEGO – The percentage of anterior insula that is infarcted at the time of admission in patients with acute middle cerebral artery occlusive stroke predicted infarct growth without significant revascularization in 74 consecutive patients who underwent intra-arterial therapy.
"We believe the insula is an overlooked biomarker. ... Although we have not proven it yet, we think it may do a better job than perfusion mismatch in stratifying patients who are likely to benefit from intra-arterial therapy [IAT] from patients unlikely to benefit," said Dr. Michael H. Lev, coauthor of the study and director of emergency neuroradiology and radiology at Massachusetts General Hospital’s Institute for Heart, Vascular, and Stroke Care in Boston.
Two neuroradiologists rated the admission diffusion-weighted imaging (DWI) scans of the patients according to percent anterior and posterior insula infarction using a 4-point scale (normal, less than 50%, greater than 50%, and 100%). (The anterior insula is approximately 25% of the whole insula.) Admission DWI and follow-up MRI core infarct volumes were segmented, and infarct growth was determined. Patients were stratified into those with good (TICI [thrombolysis in cerebral infarction] grade 2-3) vs. poor (TICI grade 0-1) recanalization.
No or poor recanalization occurred in 23 (31%) patients, according to Dr. Livia Morais, who presented the findings at the annual meeting of the American Society of Neuroradiology. In this group, the percent anterior insula infarct was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.43, P = .04). Predictors of final infarct volume were age, anterior/posterior/total insula percent infarct, and DWI lesion volume at admission (all P less than .05).
For the 69% with good recanalization, National Institutes of Health Stroke Scale score at admission was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.34, P = .02). Predictors of final infarct volume were percent anterior/posterior/total insula infarct and DWI lesion volume at admission (all P less than .05).
Patients who had less than 50% anterior insula infarct had significantly lower infarct volume (P less than .0001) and infarct growth (P less than .03), compared with those who had greater than 50% anterior insula infarct involvement.
One reason the investigators are focusing on the insula is that it is seen as a region of high ischemic vulnerability. "We speculate that anterior insula infarction may be a stronger surrogate for overall stroke severity than is DWI lesion volume because the unique vascular supply of this location reflects the combined effects of not only the degree of superior division MCA occlusion, but also the quality of collateral flow from both the anterior and inferior MCA divisions, as well as other pial collateral sources," said Dr. Morais, a neuroimaging fellow at Massachusetts General Hospital’s neurovascular laboratory.
This work is part of a body of research looking to identify treatment-relevant acute imaging targets, Dr. Lev said. "We are trying to break patients up into groups with high risk-to-benefit ratios and low risk-to-benefit ratios. Those who fall in-between, if they have no other exclusion criteria, can be treated." The results of this study indicate that patients who are IAT candidates with diffusion-weighted imaging lesion volume of less than 70 mL may be stratified into those with a high likelihood of benefitting from IAT based on the percent anterior insula involvement, he said.
In previous work, Dr. Lev showed that the insula was a very good predictor of aphasia recovery after infarction (Am. J. Neuroradiol. 2010;31:1661-8). The insula was also one of several predictors of motor improvement after stroke (Neurology 2012;78:1853-9). In another study, Dr. Lev said that infarction of the right insula and peri-insular regions was associated with the development of hospital-acquired pneumonia, which he attributed to the insula’s involvement with swallowing and immune modulation.
Dr. Morais said he had no relevant financial disclosures. Dr. Lev said he has received research funding from GE Healthcare.
SAN DIEGO – The percentage of anterior insula that is infarcted at the time of admission in patients with acute middle cerebral artery occlusive stroke predicted infarct growth without significant revascularization in 74 consecutive patients who underwent intra-arterial therapy.
"We believe the insula is an overlooked biomarker. ... Although we have not proven it yet, we think it may do a better job than perfusion mismatch in stratifying patients who are likely to benefit from intra-arterial therapy [IAT] from patients unlikely to benefit," said Dr. Michael H. Lev, coauthor of the study and director of emergency neuroradiology and radiology at Massachusetts General Hospital’s Institute for Heart, Vascular, and Stroke Care in Boston.
Two neuroradiologists rated the admission diffusion-weighted imaging (DWI) scans of the patients according to percent anterior and posterior insula infarction using a 4-point scale (normal, less than 50%, greater than 50%, and 100%). (The anterior insula is approximately 25% of the whole insula.) Admission DWI and follow-up MRI core infarct volumes were segmented, and infarct growth was determined. Patients were stratified into those with good (TICI [thrombolysis in cerebral infarction] grade 2-3) vs. poor (TICI grade 0-1) recanalization.
No or poor recanalization occurred in 23 (31%) patients, according to Dr. Livia Morais, who presented the findings at the annual meeting of the American Society of Neuroradiology. In this group, the percent anterior insula infarct was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.43, P = .04). Predictors of final infarct volume were age, anterior/posterior/total insula percent infarct, and DWI lesion volume at admission (all P less than .05).
For the 69% with good recanalization, National Institutes of Health Stroke Scale score at admission was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.34, P = .02). Predictors of final infarct volume were percent anterior/posterior/total insula infarct and DWI lesion volume at admission (all P less than .05).
Patients who had less than 50% anterior insula infarct had significantly lower infarct volume (P less than .0001) and infarct growth (P less than .03), compared with those who had greater than 50% anterior insula infarct involvement.
One reason the investigators are focusing on the insula is that it is seen as a region of high ischemic vulnerability. "We speculate that anterior insula infarction may be a stronger surrogate for overall stroke severity than is DWI lesion volume because the unique vascular supply of this location reflects the combined effects of not only the degree of superior division MCA occlusion, but also the quality of collateral flow from both the anterior and inferior MCA divisions, as well as other pial collateral sources," said Dr. Morais, a neuroimaging fellow at Massachusetts General Hospital’s neurovascular laboratory.
This work is part of a body of research looking to identify treatment-relevant acute imaging targets, Dr. Lev said. "We are trying to break patients up into groups with high risk-to-benefit ratios and low risk-to-benefit ratios. Those who fall in-between, if they have no other exclusion criteria, can be treated." The results of this study indicate that patients who are IAT candidates with diffusion-weighted imaging lesion volume of less than 70 mL may be stratified into those with a high likelihood of benefitting from IAT based on the percent anterior insula involvement, he said.
In previous work, Dr. Lev showed that the insula was a very good predictor of aphasia recovery after infarction (Am. J. Neuroradiol. 2010;31:1661-8). The insula was also one of several predictors of motor improvement after stroke (Neurology 2012;78:1853-9). In another study, Dr. Lev said that infarction of the right insula and peri-insular regions was associated with the development of hospital-acquired pneumonia, which he attributed to the insula’s involvement with swallowing and immune modulation.
Dr. Morais said he had no relevant financial disclosures. Dr. Lev said he has received research funding from GE Healthcare.
AT THE ASNR ANNUAL MEETING
Major finding: The percent anterior insula infarct at admission was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.43, P = .04) in 23 patients who had no or poor recanalization following intra-arterial therapy for acute infarction of the middle cerebral artery.
Data source: A prospective study of 74 consecutive patients with acute middle cerebral artery occlusive stroke who underwent intra-arterial therapy.
Disclosures: Dr. Morais said he had no relevant financial disclosures. Dr. Lev said he has received research funding from GE Healthcare.
Stroke outcomes poorer when criteria precluded endovascular therapy
SAN DIEGO – Acute ischemic stroke patients who didn’t meet criteria for endovascular intervention were significantly less likely than treated patients to be discharged to home and half as likely to achieve good functional outcomes at 90 days, according to a retrospective study.
More than one-third of the patients excluded from endovascular intervention, however, went on to achieve functional independence at 3 months.
The results suggest that endovascular intervention selection strategies may need to be revamped and made more inclusive, Dr. Ali Shaibani said at the annual meeting of the American Society of Neuroradiology.
"While a good number of studies have been dedicated to investigating the outcomes of AIS patients who undergo endovascular intervention, outcomes have not been well studied in those who are deemed ineligible for endovascular intervention," said Dr. Shaibani, associate professor in radiology and neurological surgery at Northwestern University, Chicago.
In the retrospective study, investigators reviewed the charts of all 110 acute ischemic stroke (AIS) patients who underwent perfusion imaging from February 2010 to August 2012. The inclusion criteria were symptom onset-to-presentation time of 8 hours or less, anterior circulation large-vessel occlusion (either of the middle cerebral artery (MCA) or internal carotid artery (ICA)) as determined by CT/MR angiography, and a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 8.
Patients who were selected for endovascular treatment had the following perfusion imaging profiles: cerebral blood volume/diffusion weighted imaging (CBV/DWI) infarct core less than 1/3 MCA territory and mismatch of the ischemic penumbra more than 20% of the infarct core.
"While a good number of studies have been dedicated to investigating the outcomes of AIS patients who undergo endovascular intervention, outcomes have not been well studied in those who are deemed ineligible for endovascular intervention."
Less than half (43.6%) of the patients were found to be eligible for endovascular treatment. Patients who were not selected were significantly older (81 years vs. 74 years, P = .04) and had more risk factors (53.2% vs. 29.2%, P = .04), such as hyperlipidemia (83.9% vs. 25.0%, P = .03), than those selected for treatment. Patients not selected for treatment were less likely to be hypertensive (3.2% vs. 14.6%, P = .05). No significant differences between groups were found for atrial fibrillation or diabetes mellitus.
Patients not selected for endovascular therapy also were more likely to have received intravenous tissue plasminogen activator (TPA) than those selected (64.5% vs. 41.7%, P = .04), and they presented to the hospital significantly earlier (166 min. vs. 250 min., P = .03). That may reflect the finding that 20% of those selected for endovascular therapy transferred from other medical facilities, while none of the nonselected patients were transfers (P = .03).
Despite earlier time to presentation and greater receipt of intravenous TPA, patients who weren’t selected for endovascular intervention were discharged to home at almost one-sixth the rate of selected patients (3.2% vs. 18.8%, P = .001). There were no significant differences in baseline NIHSS scores between nonselected and selected groups (19.35 vs. 18.67), but selected patients had better NIHSS scores at discharge (13.43 vs. 9.8, P = .02).
Although selected patients were also twice as likely (66.7% vs. 35.7%) to have good functional outcomes at 90 days (as defined by modified Rankin Scale scores of 0-2), Dr. Shaibani pointed out that nearly one-third of nonintervention patients did achieve functional independence.
"This suggests the need to refine patient selection strategies for [intra-arterial] intervention and to be more inclusive," said Dr. Shaibani. He suggested reconsidering the importance of other factors besides perfusion imaging criteria, such as the location of the occlusion in the MCA and pre-existing modified Rankin Scale scores.
Dr. Shaibani had no relevant financial relationships.
SAN DIEGO – Acute ischemic stroke patients who didn’t meet criteria for endovascular intervention were significantly less likely than treated patients to be discharged to home and half as likely to achieve good functional outcomes at 90 days, according to a retrospective study.
More than one-third of the patients excluded from endovascular intervention, however, went on to achieve functional independence at 3 months.
The results suggest that endovascular intervention selection strategies may need to be revamped and made more inclusive, Dr. Ali Shaibani said at the annual meeting of the American Society of Neuroradiology.
"While a good number of studies have been dedicated to investigating the outcomes of AIS patients who undergo endovascular intervention, outcomes have not been well studied in those who are deemed ineligible for endovascular intervention," said Dr. Shaibani, associate professor in radiology and neurological surgery at Northwestern University, Chicago.
In the retrospective study, investigators reviewed the charts of all 110 acute ischemic stroke (AIS) patients who underwent perfusion imaging from February 2010 to August 2012. The inclusion criteria were symptom onset-to-presentation time of 8 hours or less, anterior circulation large-vessel occlusion (either of the middle cerebral artery (MCA) or internal carotid artery (ICA)) as determined by CT/MR angiography, and a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 8.
Patients who were selected for endovascular treatment had the following perfusion imaging profiles: cerebral blood volume/diffusion weighted imaging (CBV/DWI) infarct core less than 1/3 MCA territory and mismatch of the ischemic penumbra more than 20% of the infarct core.
"While a good number of studies have been dedicated to investigating the outcomes of AIS patients who undergo endovascular intervention, outcomes have not been well studied in those who are deemed ineligible for endovascular intervention."
Less than half (43.6%) of the patients were found to be eligible for endovascular treatment. Patients who were not selected were significantly older (81 years vs. 74 years, P = .04) and had more risk factors (53.2% vs. 29.2%, P = .04), such as hyperlipidemia (83.9% vs. 25.0%, P = .03), than those selected for treatment. Patients not selected for treatment were less likely to be hypertensive (3.2% vs. 14.6%, P = .05). No significant differences between groups were found for atrial fibrillation or diabetes mellitus.
Patients not selected for endovascular therapy also were more likely to have received intravenous tissue plasminogen activator (TPA) than those selected (64.5% vs. 41.7%, P = .04), and they presented to the hospital significantly earlier (166 min. vs. 250 min., P = .03). That may reflect the finding that 20% of those selected for endovascular therapy transferred from other medical facilities, while none of the nonselected patients were transfers (P = .03).
Despite earlier time to presentation and greater receipt of intravenous TPA, patients who weren’t selected for endovascular intervention were discharged to home at almost one-sixth the rate of selected patients (3.2% vs. 18.8%, P = .001). There were no significant differences in baseline NIHSS scores between nonselected and selected groups (19.35 vs. 18.67), but selected patients had better NIHSS scores at discharge (13.43 vs. 9.8, P = .02).
Although selected patients were also twice as likely (66.7% vs. 35.7%) to have good functional outcomes at 90 days (as defined by modified Rankin Scale scores of 0-2), Dr. Shaibani pointed out that nearly one-third of nonintervention patients did achieve functional independence.
"This suggests the need to refine patient selection strategies for [intra-arterial] intervention and to be more inclusive," said Dr. Shaibani. He suggested reconsidering the importance of other factors besides perfusion imaging criteria, such as the location of the occlusion in the MCA and pre-existing modified Rankin Scale scores.
Dr. Shaibani had no relevant financial relationships.
SAN DIEGO – Acute ischemic stroke patients who didn’t meet criteria for endovascular intervention were significantly less likely than treated patients to be discharged to home and half as likely to achieve good functional outcomes at 90 days, according to a retrospective study.
More than one-third of the patients excluded from endovascular intervention, however, went on to achieve functional independence at 3 months.
The results suggest that endovascular intervention selection strategies may need to be revamped and made more inclusive, Dr. Ali Shaibani said at the annual meeting of the American Society of Neuroradiology.
"While a good number of studies have been dedicated to investigating the outcomes of AIS patients who undergo endovascular intervention, outcomes have not been well studied in those who are deemed ineligible for endovascular intervention," said Dr. Shaibani, associate professor in radiology and neurological surgery at Northwestern University, Chicago.
In the retrospective study, investigators reviewed the charts of all 110 acute ischemic stroke (AIS) patients who underwent perfusion imaging from February 2010 to August 2012. The inclusion criteria were symptom onset-to-presentation time of 8 hours or less, anterior circulation large-vessel occlusion (either of the middle cerebral artery (MCA) or internal carotid artery (ICA)) as determined by CT/MR angiography, and a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 8.
Patients who were selected for endovascular treatment had the following perfusion imaging profiles: cerebral blood volume/diffusion weighted imaging (CBV/DWI) infarct core less than 1/3 MCA territory and mismatch of the ischemic penumbra more than 20% of the infarct core.
"While a good number of studies have been dedicated to investigating the outcomes of AIS patients who undergo endovascular intervention, outcomes have not been well studied in those who are deemed ineligible for endovascular intervention."
Less than half (43.6%) of the patients were found to be eligible for endovascular treatment. Patients who were not selected were significantly older (81 years vs. 74 years, P = .04) and had more risk factors (53.2% vs. 29.2%, P = .04), such as hyperlipidemia (83.9% vs. 25.0%, P = .03), than those selected for treatment. Patients not selected for treatment were less likely to be hypertensive (3.2% vs. 14.6%, P = .05). No significant differences between groups were found for atrial fibrillation or diabetes mellitus.
Patients not selected for endovascular therapy also were more likely to have received intravenous tissue plasminogen activator (TPA) than those selected (64.5% vs. 41.7%, P = .04), and they presented to the hospital significantly earlier (166 min. vs. 250 min., P = .03). That may reflect the finding that 20% of those selected for endovascular therapy transferred from other medical facilities, while none of the nonselected patients were transfers (P = .03).
Despite earlier time to presentation and greater receipt of intravenous TPA, patients who weren’t selected for endovascular intervention were discharged to home at almost one-sixth the rate of selected patients (3.2% vs. 18.8%, P = .001). There were no significant differences in baseline NIHSS scores between nonselected and selected groups (19.35 vs. 18.67), but selected patients had better NIHSS scores at discharge (13.43 vs. 9.8, P = .02).
Although selected patients were also twice as likely (66.7% vs. 35.7%) to have good functional outcomes at 90 days (as defined by modified Rankin Scale scores of 0-2), Dr. Shaibani pointed out that nearly one-third of nonintervention patients did achieve functional independence.
"This suggests the need to refine patient selection strategies for [intra-arterial] intervention and to be more inclusive," said Dr. Shaibani. He suggested reconsidering the importance of other factors besides perfusion imaging criteria, such as the location of the occlusion in the MCA and pre-existing modified Rankin Scale scores.
Dr. Shaibani had no relevant financial relationships.
AT THE ASNR ANNUAL MEETING
Major finding: Acute ischemic stroke patients excluded from endovascular intervention because they failed to meet perfusion imaging criteria were discharged home at nearly one-sixth the rate of treated patients, and they were half as likely to have good functional outcomes at 90 days. However, one-third of those excluded from intervention were able to achieve functional independence at 3 months.
Data source: Retrospective study of 110 patients.
Disclosures: Dr. Shaibani had no relevant financial relationships.
ICU stay may be unnecessary after elective endovascular aneurysm treatment
SAN DIEGO – In a retrospective review of almost 700 patients who underwent elective endovascular aneurysm treatment, 4% experienced postoperative complications, mostly within the first 4 hours post procedure. Almost half of the complications were groin hematomas or retroperitoneal hematomas that extended the length of inpatient stay but required no further treatment. The results suggest that patients undergoing endovascular aneurysm treatment may be transferred to a less resource-intensive environment once they have been followed closely for 4 hours, according to Bhuvic Patel, who presented the findings at the annual meeting of the American Society of Neuroradiology.
The 687 patients had unruptured intracranial aneurysms and underwent elective endovascular treatment from March 2002 to June 2012. Most patients underwent coiling alone (329) or stent-assisted coiling (242), although other patients underwent balloon-assisted coiling, Onyx HD 500 occlusion, or treatment with a pipeline embolization device with or without coiling. Nine patients experienced a complication during the procedure and were excluded from further analysis. After the procedure, patients were monitored for at least 24 hours in a neurologic intensive care unit or postanesthesia care unit.
In total, 4% had postprocedural complications (27/678). These included three intracerebral hemorrhages, six ischemic strokes, four cardiac events, five retroperitoneal hematomas, and nine groin hematomas.
Looking at the timing of the complications, 74% were detected within the first 4 hours following the procedure. These included one hemorrhage, four ischemic strokes, all four of the cardiac events, all nine of the hematomas, and two retroperitoneal hematomas.
"As you get further away from the procedures, fewer events were detected," said Mr. Patel, who is currently a medical student at the Washington University School of Medicine, St. Louis. Four complications (14.8%) were noted between 4 and 12 hours post procedure, one (3.7%) between 12 and 24 hours, and two (7.4%) more than 24 hours post procedure.
Of the two hemorrhages that were discovered 4 hours or more post procedure, both were diagnosed by head CT after patients complained of headache and neither resulted in any permanent significant deficit. For two patients who had ischemic stroke detected by MRI after 4 hours, deficits were considered minor although one patient was discharged to a skilled nursing facility.
"The complications that were detected more than 4 hours post procedure could all have been managed in a floor setting. We think it is reasonable for patients to be monitored in a postoperative intensive care setting for the first 4 hours following routine endovascular aneurysm treatment and then be transferred to be floor bed. This can translate to a lot of cost savings," says Mr. Patel.
Dr. Patel has no relevant financial disclosures.
SAN DIEGO – In a retrospective review of almost 700 patients who underwent elective endovascular aneurysm treatment, 4% experienced postoperative complications, mostly within the first 4 hours post procedure. Almost half of the complications were groin hematomas or retroperitoneal hematomas that extended the length of inpatient stay but required no further treatment. The results suggest that patients undergoing endovascular aneurysm treatment may be transferred to a less resource-intensive environment once they have been followed closely for 4 hours, according to Bhuvic Patel, who presented the findings at the annual meeting of the American Society of Neuroradiology.
The 687 patients had unruptured intracranial aneurysms and underwent elective endovascular treatment from March 2002 to June 2012. Most patients underwent coiling alone (329) or stent-assisted coiling (242), although other patients underwent balloon-assisted coiling, Onyx HD 500 occlusion, or treatment with a pipeline embolization device with or without coiling. Nine patients experienced a complication during the procedure and were excluded from further analysis. After the procedure, patients were monitored for at least 24 hours in a neurologic intensive care unit or postanesthesia care unit.
In total, 4% had postprocedural complications (27/678). These included three intracerebral hemorrhages, six ischemic strokes, four cardiac events, five retroperitoneal hematomas, and nine groin hematomas.
Looking at the timing of the complications, 74% were detected within the first 4 hours following the procedure. These included one hemorrhage, four ischemic strokes, all four of the cardiac events, all nine of the hematomas, and two retroperitoneal hematomas.
"As you get further away from the procedures, fewer events were detected," said Mr. Patel, who is currently a medical student at the Washington University School of Medicine, St. Louis. Four complications (14.8%) were noted between 4 and 12 hours post procedure, one (3.7%) between 12 and 24 hours, and two (7.4%) more than 24 hours post procedure.
Of the two hemorrhages that were discovered 4 hours or more post procedure, both were diagnosed by head CT after patients complained of headache and neither resulted in any permanent significant deficit. For two patients who had ischemic stroke detected by MRI after 4 hours, deficits were considered minor although one patient was discharged to a skilled nursing facility.
"The complications that were detected more than 4 hours post procedure could all have been managed in a floor setting. We think it is reasonable for patients to be monitored in a postoperative intensive care setting for the first 4 hours following routine endovascular aneurysm treatment and then be transferred to be floor bed. This can translate to a lot of cost savings," says Mr. Patel.
Dr. Patel has no relevant financial disclosures.
SAN DIEGO – In a retrospective review of almost 700 patients who underwent elective endovascular aneurysm treatment, 4% experienced postoperative complications, mostly within the first 4 hours post procedure. Almost half of the complications were groin hematomas or retroperitoneal hematomas that extended the length of inpatient stay but required no further treatment. The results suggest that patients undergoing endovascular aneurysm treatment may be transferred to a less resource-intensive environment once they have been followed closely for 4 hours, according to Bhuvic Patel, who presented the findings at the annual meeting of the American Society of Neuroradiology.
The 687 patients had unruptured intracranial aneurysms and underwent elective endovascular treatment from March 2002 to June 2012. Most patients underwent coiling alone (329) or stent-assisted coiling (242), although other patients underwent balloon-assisted coiling, Onyx HD 500 occlusion, or treatment with a pipeline embolization device with or without coiling. Nine patients experienced a complication during the procedure and were excluded from further analysis. After the procedure, patients were monitored for at least 24 hours in a neurologic intensive care unit or postanesthesia care unit.
In total, 4% had postprocedural complications (27/678). These included three intracerebral hemorrhages, six ischemic strokes, four cardiac events, five retroperitoneal hematomas, and nine groin hematomas.
Looking at the timing of the complications, 74% were detected within the first 4 hours following the procedure. These included one hemorrhage, four ischemic strokes, all four of the cardiac events, all nine of the hematomas, and two retroperitoneal hematomas.
"As you get further away from the procedures, fewer events were detected," said Mr. Patel, who is currently a medical student at the Washington University School of Medicine, St. Louis. Four complications (14.8%) were noted between 4 and 12 hours post procedure, one (3.7%) between 12 and 24 hours, and two (7.4%) more than 24 hours post procedure.
Of the two hemorrhages that were discovered 4 hours or more post procedure, both were diagnosed by head CT after patients complained of headache and neither resulted in any permanent significant deficit. For two patients who had ischemic stroke detected by MRI after 4 hours, deficits were considered minor although one patient was discharged to a skilled nursing facility.
"The complications that were detected more than 4 hours post procedure could all have been managed in a floor setting. We think it is reasonable for patients to be monitored in a postoperative intensive care setting for the first 4 hours following routine endovascular aneurysm treatment and then be transferred to be floor bed. This can translate to a lot of cost savings," says Mr. Patel.
Dr. Patel has no relevant financial disclosures.
AT THE ASNR ANNUAL MEETING
Major finding: Only 4% of 687 patients undergoing routine endovascular aneurysm treatment experienced postoperative complications and almost three-quarters of these complications occurred within 4 hours of the procedure.
Data source: Retrospective review.
Disclosures: Dr. Patel has no relevant financial disclosures.
Stent-retriever outcomes good in stroke patients with 'T' occlusions
SAN DIEGO – Stent retrievers were associated with higher rates of recanalization, shorter recanalization times, and better outcomes than multimodality recanalization strategies, based on a retrospective, single-center chart review of 60 acute stroke patients with a substantial proportion of carotid "T" occlusions.
The study, presented at the annual meeting of the American Society of Neuroradiology, included 23 patients treated primarily with stent retrievers (Solitaire AB neurovascular remodeling device) and 37 treated with other modalities.
The multimodality group received intra-arterial tissue plasminogen activator therapy (10), mechanical thromboaspiration (18), angioplasty (6), earlier-generation mechanical retriever devices (2), and stent only (1). The two groups were similar in age, gender, intravenous tissue plasminogen activator use (65% and 68%), and stroke risk factors.
Terminal carotid "T" occlusions, which generally respond poorly to thrombolysis, were present in 12 of the 23 patients in the stent-retrieval group and in 12 of the 37 patients in the multimodality cohort, said Dr. Cheemun Lum, a neurointerventional radiologist at the Ottawa Hospital, University of Ottawa.
The stent-retriever group had higher recanalization rates than the multimodality group (87% vs. 51%, P = .005). There was no significant difference in median time to recanalization between groups (68 minutes vs. 79 minutes).
Mortality was eight-fold higher in the multimodality group than in the stent-retriever group (32% vs. 4%, P = .011). At 30-day follow-up, 61% of the stent retriever group had good outcomes, defined as a Modified Rankin Scale score of 2 or less. When the outcomes definition was expanded to include a 10-point reduction in score on the National Institutes of Health Stroke Scale, 78% of the stent-retriever group had good outcomes at 30 days, compared with 43% in the multimodality group (P = .008).
Restricting the analysis to patients with terminal carotid "T" occlusions, recanalization rates were higher in the stent-retriever group (83% vs. 38%, P = .012) and recanalization times were shorter (69 minutes vs. 191 minutes, P = .002). Three times as many patients with terminal carotid "T" occlusions in the stent-retriever group had good outcomes as did those undergoing older techniques (67% vs. 22%, P = .04).
Dr. Lum said that he had no financial disclosures.
SAN DIEGO – Stent retrievers were associated with higher rates of recanalization, shorter recanalization times, and better outcomes than multimodality recanalization strategies, based on a retrospective, single-center chart review of 60 acute stroke patients with a substantial proportion of carotid "T" occlusions.
The study, presented at the annual meeting of the American Society of Neuroradiology, included 23 patients treated primarily with stent retrievers (Solitaire AB neurovascular remodeling device) and 37 treated with other modalities.
The multimodality group received intra-arterial tissue plasminogen activator therapy (10), mechanical thromboaspiration (18), angioplasty (6), earlier-generation mechanical retriever devices (2), and stent only (1). The two groups were similar in age, gender, intravenous tissue plasminogen activator use (65% and 68%), and stroke risk factors.
Terminal carotid "T" occlusions, which generally respond poorly to thrombolysis, were present in 12 of the 23 patients in the stent-retrieval group and in 12 of the 37 patients in the multimodality cohort, said Dr. Cheemun Lum, a neurointerventional radiologist at the Ottawa Hospital, University of Ottawa.
The stent-retriever group had higher recanalization rates than the multimodality group (87% vs. 51%, P = .005). There was no significant difference in median time to recanalization between groups (68 minutes vs. 79 minutes).
Mortality was eight-fold higher in the multimodality group than in the stent-retriever group (32% vs. 4%, P = .011). At 30-day follow-up, 61% of the stent retriever group had good outcomes, defined as a Modified Rankin Scale score of 2 or less. When the outcomes definition was expanded to include a 10-point reduction in score on the National Institutes of Health Stroke Scale, 78% of the stent-retriever group had good outcomes at 30 days, compared with 43% in the multimodality group (P = .008).
Restricting the analysis to patients with terminal carotid "T" occlusions, recanalization rates were higher in the stent-retriever group (83% vs. 38%, P = .012) and recanalization times were shorter (69 minutes vs. 191 minutes, P = .002). Three times as many patients with terminal carotid "T" occlusions in the stent-retriever group had good outcomes as did those undergoing older techniques (67% vs. 22%, P = .04).
Dr. Lum said that he had no financial disclosures.
SAN DIEGO – Stent retrievers were associated with higher rates of recanalization, shorter recanalization times, and better outcomes than multimodality recanalization strategies, based on a retrospective, single-center chart review of 60 acute stroke patients with a substantial proportion of carotid "T" occlusions.
The study, presented at the annual meeting of the American Society of Neuroradiology, included 23 patients treated primarily with stent retrievers (Solitaire AB neurovascular remodeling device) and 37 treated with other modalities.
The multimodality group received intra-arterial tissue plasminogen activator therapy (10), mechanical thromboaspiration (18), angioplasty (6), earlier-generation mechanical retriever devices (2), and stent only (1). The two groups were similar in age, gender, intravenous tissue plasminogen activator use (65% and 68%), and stroke risk factors.
Terminal carotid "T" occlusions, which generally respond poorly to thrombolysis, were present in 12 of the 23 patients in the stent-retrieval group and in 12 of the 37 patients in the multimodality cohort, said Dr. Cheemun Lum, a neurointerventional radiologist at the Ottawa Hospital, University of Ottawa.
The stent-retriever group had higher recanalization rates than the multimodality group (87% vs. 51%, P = .005). There was no significant difference in median time to recanalization between groups (68 minutes vs. 79 minutes).
Mortality was eight-fold higher in the multimodality group than in the stent-retriever group (32% vs. 4%, P = .011). At 30-day follow-up, 61% of the stent retriever group had good outcomes, defined as a Modified Rankin Scale score of 2 or less. When the outcomes definition was expanded to include a 10-point reduction in score on the National Institutes of Health Stroke Scale, 78% of the stent-retriever group had good outcomes at 30 days, compared with 43% in the multimodality group (P = .008).
Restricting the analysis to patients with terminal carotid "T" occlusions, recanalization rates were higher in the stent-retriever group (83% vs. 38%, P = .012) and recanalization times were shorter (69 minutes vs. 191 minutes, P = .002). Three times as many patients with terminal carotid "T" occlusions in the stent-retriever group had good outcomes as did those undergoing older techniques (67% vs. 22%, P = .04).
Dr. Lum said that he had no financial disclosures.
AT THE ASNR ANNUAL MEETING
Major finding: Recanalization rates were higher in the stent-retriever group (83% vs. 38%, P = .012) when the analysis was restricted to patients with terminal carotid "T" occlusions.
Data source: Single-center chart review of 60 acute stroke patients.
Disclosures: Dr. Lum said that he had no financial disclosures.
Imaging algorithm may improve stroke treatment selection
SAN DIEGO – A Massachusetts General Hospital neuroimaging algorithm that is used to help in the selection of appropriate treatment for patients with severe ischemic strokes caused by anterior circulation occlusions led to significant improvements in mortality and outcomes and a decrease in the number of stroke interventions after it was implemented at the Cleveland Clinic, according to Dr. Ramon Gilberto Gonzalez.
With a few exceptions, the algorithm does not use perfusion imaging, either by MRI or CT, in the assessment of anterior circulation occlusion (ACO) patients for intravenous tissue plasminogen activator (TPA) or endovascular therapy.
"One of the challenges in imaging stroke is that it is very heterogeneous. You don’t know what is going to show up in your emergency department. It is important to formulate an imaging program that can optimize all the information you get from all patients, whether they need intra-arterial therapy or just watchful waiting. A system is needed that is efficient for all patients, and that is a challenge," Dr. Gonzalez said at the annual meeting of the American Society of Neuroradiology. He is lead author of the paper describing the algorithm and director of the neuroradiology division at Massachusetts General Hospital (MGH), Boston.
The algorithm was developed from both experience and evidence, Dr. Gonzalez explained. Individual neuroradiology and neurology faculty from MGH presented the best evidence from the literature and clinical experience regarding imaging methods and the National Institutes of Health Stroke Scale (NIHSS). Other faculty and fellows who heard the presentations met to weigh the evidence and make recommendations. The methods were rated on such metrics as sensitivity and specificity, value for patient care, usability in the acute setting, work flow, repeatability, reliability, and clinical efficacy.
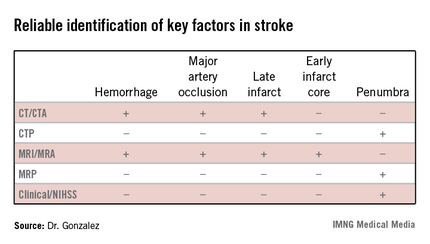
The algorithm reflected how well different imaging methods provided information on key factors in stroke. "In addition to time and hemorrhage, we must consider how much of the brain has already died [the core] and how much of the brain is likely to die if you do not do anything to help" the penumbra. Each imaging method has benefits and weaknesses for different stroke parameters, Dr. Gonzalez said. (See table.) CT/CT angiography (CTA) and MRI/MR angiography (MRA) were found to be useful for demonstrating hemorrhage, major artery occlusion, and late infarct, while only MRI/MRA helped visualize the early infarct core.
In the algorithm, all patients presenting with a stroke syndrome (possible hemorrhage or large infarct) receive a neurological exam, including the NIHSS. "The single most important parameter is the neurological exam," according to Dr. Gonzalez, which he said can determine an overall size of the penumbra and core combined. "The neurologic exam is just as good as CTP [CT perfusion] or MRP [MRI perfusion]."
According to the stroke imaging algorithm (J. Neurointervent. Surg. 2013;5:i7-12), the first imaging study should be a noncontrast CT (NCCT), followed by a CTA if a proximal occlusion is accessible and the patient is eligible for MRI. If the NCCT does not demonstrate a hemorrhage or large hypodensity, and the patient is within the time window, TPA is prepared while the CTA is performed, and the infusion is started. If the patient has a distal internal carotid artery and/or proximal middle cerebral artery occlusion, he will undergo diffusion-weighted imaging (DWI). Patients with DWI lesions less than 70 mL in volume are sent for intra-arterial therapy as long as they meet clinical and medical criteria. "DWI is really the only method we have to determine the core volume with sufficient precision to be able to make good clinical decisions," Dr. Gonzalez said.
Perfusion CT and perfusion MR are not part of the recommended imaging workup, with some exceptions. "For many years, radiologists thought you could substitute DWI with CT perfusion. We came to the conclusion that you cannot," Dr. Gonzalez said. The reviewing panel found that there was no or poor evidence that CTP could be used for early estimation of the infarct core or penumbra, and had no proven role in selecting ACO patients for IV thrombolysis or endovascular therapy. Similarly, they found the evidence indicated that MRP had no proven role in selecting ACO patients for endovascular therapy. Perfusion imaging may be appropriate if patients cannot be scanned by an MRI or are not otherwise eligible for intra-arterial therapy, or if perfusion data are needed for another reason.
After adoption of the algorithm, the number of CT perfusion exams performed at MGH on stroke patients dropped from 40-50 per month to about 10 per month. No discernible effects on the rate of good outcomes (P = .67) or median modified Rankin Scale score (P = .85) were found.
A little more than a year of testing at the Cleveland Clinic showed that the algorithm resulted in a significant reduction in mortality and improvement of modified Rankin Scale scores independent of the treatment received. Interestingly, the number of interventions for stroke decreased by 40% as patient selection through imaging targeted patients more likely to benefit and excluded those not suitable for intra-arterial therapy (Stroke 2012;43:A161).
Dr. Gonzalez reported having no relevant financial disclosures.
SAN DIEGO – A Massachusetts General Hospital neuroimaging algorithm that is used to help in the selection of appropriate treatment for patients with severe ischemic strokes caused by anterior circulation occlusions led to significant improvements in mortality and outcomes and a decrease in the number of stroke interventions after it was implemented at the Cleveland Clinic, according to Dr. Ramon Gilberto Gonzalez.
With a few exceptions, the algorithm does not use perfusion imaging, either by MRI or CT, in the assessment of anterior circulation occlusion (ACO) patients for intravenous tissue plasminogen activator (TPA) or endovascular therapy.

"One of the challenges in imaging stroke is that it is very heterogeneous. You don’t know what is going to show up in your emergency department. It is important to formulate an imaging program that can optimize all the information you get from all patients, whether they need intra-arterial therapy or just watchful waiting. A system is needed that is efficient for all patients, and that is a challenge," Dr. Gonzalez said at the annual meeting of the American Society of Neuroradiology. He is lead author of the paper describing the algorithm and director of the neuroradiology division at Massachusetts General Hospital (MGH), Boston.
The algorithm was developed from both experience and evidence, Dr. Gonzalez explained. Individual neuroradiology and neurology faculty from MGH presented the best evidence from the literature and clinical experience regarding imaging methods and the National Institutes of Health Stroke Scale (NIHSS). Other faculty and fellows who heard the presentations met to weigh the evidence and make recommendations. The methods were rated on such metrics as sensitivity and specificity, value for patient care, usability in the acute setting, work flow, repeatability, reliability, and clinical efficacy.
The algorithm reflected how well different imaging methods provided information on key factors in stroke. "In addition to time and hemorrhage, we must consider how much of the brain has already died [the core] and how much of the brain is likely to die if you do not do anything to help" the penumbra. Each imaging method has benefits and weaknesses for different stroke parameters, Dr. Gonzalez said. (See table.) CT/CT angiography (CTA) and MRI/MR angiography (MRA) were found to be useful for demonstrating hemorrhage, major artery occlusion, and late infarct, while only MRI/MRA helped visualize the early infarct core.
In the algorithm, all patients presenting with a stroke syndrome (possible hemorrhage or large infarct) receive a neurological exam, including the NIHSS. "The single most important parameter is the neurological exam," according to Dr. Gonzalez, which he said can determine an overall size of the penumbra and core combined. "The neurologic exam is just as good as CTP [CT perfusion] or MRP [MRI perfusion]."
According to the stroke imaging algorithm (J. Neurointervent. Surg. 2013;5:i7-12), the first imaging study should be a noncontrast CT (NCCT), followed by a CTA if a proximal occlusion is accessible and the patient is eligible for MRI. If the NCCT does not demonstrate a hemorrhage or large hypodensity, and the patient is within the time window, TPA is prepared while the CTA is performed, and the infusion is started. If the patient has a distal internal carotid artery and/or proximal middle cerebral artery occlusion, he will undergo diffusion-weighted imaging (DWI). Patients with DWI lesions less than 70 mL in volume are sent for intra-arterial therapy as long as they meet clinical and medical criteria. "DWI is really the only method we have to determine the core volume with sufficient precision to be able to make good clinical decisions," Dr. Gonzalez said.
Perfusion CT and perfusion MR are not part of the recommended imaging workup, with some exceptions. "For many years, radiologists thought you could substitute DWI with CT perfusion. We came to the conclusion that you cannot," Dr. Gonzalez said. The reviewing panel found that there was no or poor evidence that CTP could be used for early estimation of the infarct core or penumbra, and had no proven role in selecting ACO patients for IV thrombolysis or endovascular therapy. Similarly, they found the evidence indicated that MRP had no proven role in selecting ACO patients for endovascular therapy. Perfusion imaging may be appropriate if patients cannot be scanned by an MRI or are not otherwise eligible for intra-arterial therapy, or if perfusion data are needed for another reason.
After adoption of the algorithm, the number of CT perfusion exams performed at MGH on stroke patients dropped from 40-50 per month to about 10 per month. No discernible effects on the rate of good outcomes (P = .67) or median modified Rankin Scale score (P = .85) were found.
A little more than a year of testing at the Cleveland Clinic showed that the algorithm resulted in a significant reduction in mortality and improvement of modified Rankin Scale scores independent of the treatment received. Interestingly, the number of interventions for stroke decreased by 40% as patient selection through imaging targeted patients more likely to benefit and excluded those not suitable for intra-arterial therapy (Stroke 2012;43:A161).
Dr. Gonzalez reported having no relevant financial disclosures.
SAN DIEGO – A Massachusetts General Hospital neuroimaging algorithm that is used to help in the selection of appropriate treatment for patients with severe ischemic strokes caused by anterior circulation occlusions led to significant improvements in mortality and outcomes and a decrease in the number of stroke interventions after it was implemented at the Cleveland Clinic, according to Dr. Ramon Gilberto Gonzalez.
With a few exceptions, the algorithm does not use perfusion imaging, either by MRI or CT, in the assessment of anterior circulation occlusion (ACO) patients for intravenous tissue plasminogen activator (TPA) or endovascular therapy.

"One of the challenges in imaging stroke is that it is very heterogeneous. You don’t know what is going to show up in your emergency department. It is important to formulate an imaging program that can optimize all the information you get from all patients, whether they need intra-arterial therapy or just watchful waiting. A system is needed that is efficient for all patients, and that is a challenge," Dr. Gonzalez said at the annual meeting of the American Society of Neuroradiology. He is lead author of the paper describing the algorithm and director of the neuroradiology division at Massachusetts General Hospital (MGH), Boston.
The algorithm was developed from both experience and evidence, Dr. Gonzalez explained. Individual neuroradiology and neurology faculty from MGH presented the best evidence from the literature and clinical experience regarding imaging methods and the National Institutes of Health Stroke Scale (NIHSS). Other faculty and fellows who heard the presentations met to weigh the evidence and make recommendations. The methods were rated on such metrics as sensitivity and specificity, value for patient care, usability in the acute setting, work flow, repeatability, reliability, and clinical efficacy.
The algorithm reflected how well different imaging methods provided information on key factors in stroke. "In addition to time and hemorrhage, we must consider how much of the brain has already died [the core] and how much of the brain is likely to die if you do not do anything to help" the penumbra. Each imaging method has benefits and weaknesses for different stroke parameters, Dr. Gonzalez said. (See table.) CT/CT angiography (CTA) and MRI/MR angiography (MRA) were found to be useful for demonstrating hemorrhage, major artery occlusion, and late infarct, while only MRI/MRA helped visualize the early infarct core.
In the algorithm, all patients presenting with a stroke syndrome (possible hemorrhage or large infarct) receive a neurological exam, including the NIHSS. "The single most important parameter is the neurological exam," according to Dr. Gonzalez, which he said can determine an overall size of the penumbra and core combined. "The neurologic exam is just as good as CTP [CT perfusion] or MRP [MRI perfusion]."
According to the stroke imaging algorithm (J. Neurointervent. Surg. 2013;5:i7-12), the first imaging study should be a noncontrast CT (NCCT), followed by a CTA if a proximal occlusion is accessible and the patient is eligible for MRI. If the NCCT does not demonstrate a hemorrhage or large hypodensity, and the patient is within the time window, TPA is prepared while the CTA is performed, and the infusion is started. If the patient has a distal internal carotid artery and/or proximal middle cerebral artery occlusion, he will undergo diffusion-weighted imaging (DWI). Patients with DWI lesions less than 70 mL in volume are sent for intra-arterial therapy as long as they meet clinical and medical criteria. "DWI is really the only method we have to determine the core volume with sufficient precision to be able to make good clinical decisions," Dr. Gonzalez said.
Perfusion CT and perfusion MR are not part of the recommended imaging workup, with some exceptions. "For many years, radiologists thought you could substitute DWI with CT perfusion. We came to the conclusion that you cannot," Dr. Gonzalez said. The reviewing panel found that there was no or poor evidence that CTP could be used for early estimation of the infarct core or penumbra, and had no proven role in selecting ACO patients for IV thrombolysis or endovascular therapy. Similarly, they found the evidence indicated that MRP had no proven role in selecting ACO patients for endovascular therapy. Perfusion imaging may be appropriate if patients cannot be scanned by an MRI or are not otherwise eligible for intra-arterial therapy, or if perfusion data are needed for another reason.
After adoption of the algorithm, the number of CT perfusion exams performed at MGH on stroke patients dropped from 40-50 per month to about 10 per month. No discernible effects on the rate of good outcomes (P = .67) or median modified Rankin Scale score (P = .85) were found.
A little more than a year of testing at the Cleveland Clinic showed that the algorithm resulted in a significant reduction in mortality and improvement of modified Rankin Scale scores independent of the treatment received. Interestingly, the number of interventions for stroke decreased by 40% as patient selection through imaging targeted patients more likely to benefit and excluded those not suitable for intra-arterial therapy (Stroke 2012;43:A161).
Dr. Gonzalez reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ASNR ANNUAL MEETING
Transfers may have worse ischemic stroke outcomes
SAN DIEGO – Acute ischemic stroke patients who required transfer to a comprehensive stroke center in order to receive intra-arterial therapy were significantly more likely to have worse functional outcomes at 90 days than were patients who presented directly to such centers in a retrospective analysis of 116 patients.
The poor outcome of the transferred patients was independent of baseline risk factors, stroke severity, time to intra-arterial therapy, and whether the process was a success or the patient had complications, according to Dr. Ali Shaibani, who presented the findings at the annual meeting of the American Society of Neuroradiology.
"The time to intervention is critical for AIS [acute ischemic stroke] patients who are candidates for intra-arterial therapy. Access to endovascular therapy is often limited to comprehensive stroke centers. An increasing number of institutions are transferring AIS patients for therapy. The outcome for this subset of patients who are being transferred has not been studied well," said Dr. Shaibani of the departments of radiology and neurologic surgery at Northwestern University, Chicago.
This retrospective analysis analyzed 116 AIS patients deemed eligible for intra-arterial therapy who were seen at four Chicago-area medical centers. More than half (58.6%) were transferred from outside institutions.
Dr. Shaibani and his colleagues found that transfer patients tended to be younger than nontransfers (59 years vs. 69 years, P = .002) and were less likely to have had a history of prior stroke (3% vs. 22%, P = .002) or cardiac problems (18% vs. 37%, P = .040). Transfer patients had worse National Institutes of Health Stroke Scale scores at baseline (20 vs. 17, P = .005) and were more likely to have internal carotid artery occlusions (45.6% vs. 22.9%, P = .012). No differences between groups were found for THRIVE (Totaled Health Risks in Vascular Events) scores, a clinical scoring system designed to help clinicians predict a patient’s chances of achieving a good outcome after AIS.
At 90 days after intra-arterial therapy, only 16% of transfer patients had a good functional outcome, as defined by a modified Rankin Scale score of 0-2. Significantly more nontransferred patients (60%) had a good outcome (P less than .001). In a multivariate analysis, transfer status was an independent predictor of poor functional outcome (adjusted odds ratio = 0.05; 95% confidence interval, 0.011-0.222), after the findings were adjusted for relevant covariates such as baseline risk factors, stroke severity, time to intra-arterial therapy, or procedural success or complications. No differences between groups were found for median symptom onset to groin puncture times, rates of successful recanalization (defined as thrombolysis in cerebral infarction grade 2b or higher), or the presence of symptomatic intracranial hemorrhage.
Dr. Shaibani said it was not clear what factors were contributing to the findings. He said future work should explore the influence of baseline/final infarct volume, premorbid functional status, and poststroke care.
Dr. Shaibani reported that he had no relevant financial disclosures.
SAN DIEGO – Acute ischemic stroke patients who required transfer to a comprehensive stroke center in order to receive intra-arterial therapy were significantly more likely to have worse functional outcomes at 90 days than were patients who presented directly to such centers in a retrospective analysis of 116 patients.
The poor outcome of the transferred patients was independent of baseline risk factors, stroke severity, time to intra-arterial therapy, and whether the process was a success or the patient had complications, according to Dr. Ali Shaibani, who presented the findings at the annual meeting of the American Society of Neuroradiology.
"The time to intervention is critical for AIS [acute ischemic stroke] patients who are candidates for intra-arterial therapy. Access to endovascular therapy is often limited to comprehensive stroke centers. An increasing number of institutions are transferring AIS patients for therapy. The outcome for this subset of patients who are being transferred has not been studied well," said Dr. Shaibani of the departments of radiology and neurologic surgery at Northwestern University, Chicago.
This retrospective analysis analyzed 116 AIS patients deemed eligible for intra-arterial therapy who were seen at four Chicago-area medical centers. More than half (58.6%) were transferred from outside institutions.
Dr. Shaibani and his colleagues found that transfer patients tended to be younger than nontransfers (59 years vs. 69 years, P = .002) and were less likely to have had a history of prior stroke (3% vs. 22%, P = .002) or cardiac problems (18% vs. 37%, P = .040). Transfer patients had worse National Institutes of Health Stroke Scale scores at baseline (20 vs. 17, P = .005) and were more likely to have internal carotid artery occlusions (45.6% vs. 22.9%, P = .012). No differences between groups were found for THRIVE (Totaled Health Risks in Vascular Events) scores, a clinical scoring system designed to help clinicians predict a patient’s chances of achieving a good outcome after AIS.
At 90 days after intra-arterial therapy, only 16% of transfer patients had a good functional outcome, as defined by a modified Rankin Scale score of 0-2. Significantly more nontransferred patients (60%) had a good outcome (P less than .001). In a multivariate analysis, transfer status was an independent predictor of poor functional outcome (adjusted odds ratio = 0.05; 95% confidence interval, 0.011-0.222), after the findings were adjusted for relevant covariates such as baseline risk factors, stroke severity, time to intra-arterial therapy, or procedural success or complications. No differences between groups were found for median symptom onset to groin puncture times, rates of successful recanalization (defined as thrombolysis in cerebral infarction grade 2b or higher), or the presence of symptomatic intracranial hemorrhage.
Dr. Shaibani said it was not clear what factors were contributing to the findings. He said future work should explore the influence of baseline/final infarct volume, premorbid functional status, and poststroke care.
Dr. Shaibani reported that he had no relevant financial disclosures.
SAN DIEGO – Acute ischemic stroke patients who required transfer to a comprehensive stroke center in order to receive intra-arterial therapy were significantly more likely to have worse functional outcomes at 90 days than were patients who presented directly to such centers in a retrospective analysis of 116 patients.
The poor outcome of the transferred patients was independent of baseline risk factors, stroke severity, time to intra-arterial therapy, and whether the process was a success or the patient had complications, according to Dr. Ali Shaibani, who presented the findings at the annual meeting of the American Society of Neuroradiology.
"The time to intervention is critical for AIS [acute ischemic stroke] patients who are candidates for intra-arterial therapy. Access to endovascular therapy is often limited to comprehensive stroke centers. An increasing number of institutions are transferring AIS patients for therapy. The outcome for this subset of patients who are being transferred has not been studied well," said Dr. Shaibani of the departments of radiology and neurologic surgery at Northwestern University, Chicago.
This retrospective analysis analyzed 116 AIS patients deemed eligible for intra-arterial therapy who were seen at four Chicago-area medical centers. More than half (58.6%) were transferred from outside institutions.
Dr. Shaibani and his colleagues found that transfer patients tended to be younger than nontransfers (59 years vs. 69 years, P = .002) and were less likely to have had a history of prior stroke (3% vs. 22%, P = .002) or cardiac problems (18% vs. 37%, P = .040). Transfer patients had worse National Institutes of Health Stroke Scale scores at baseline (20 vs. 17, P = .005) and were more likely to have internal carotid artery occlusions (45.6% vs. 22.9%, P = .012). No differences between groups were found for THRIVE (Totaled Health Risks in Vascular Events) scores, a clinical scoring system designed to help clinicians predict a patient’s chances of achieving a good outcome after AIS.
At 90 days after intra-arterial therapy, only 16% of transfer patients had a good functional outcome, as defined by a modified Rankin Scale score of 0-2. Significantly more nontransferred patients (60%) had a good outcome (P less than .001). In a multivariate analysis, transfer status was an independent predictor of poor functional outcome (adjusted odds ratio = 0.05; 95% confidence interval, 0.011-0.222), after the findings were adjusted for relevant covariates such as baseline risk factors, stroke severity, time to intra-arterial therapy, or procedural success or complications. No differences between groups were found for median symptom onset to groin puncture times, rates of successful recanalization (defined as thrombolysis in cerebral infarction grade 2b or higher), or the presence of symptomatic intracranial hemorrhage.
Dr. Shaibani said it was not clear what factors were contributing to the findings. He said future work should explore the influence of baseline/final infarct volume, premorbid functional status, and poststroke care.
Dr. Shaibani reported that he had no relevant financial disclosures.
AT THE ASNR ANNUAL MEETING
Major finding: Significantly fewer patients who were transferred from an outside institution to a comprehensive stroke center had good functional outcomes at 90 days than did nontransferred patients (modified Rankin Scale score of 0-2; 16% vs. 60%, respectively), independent of baseline risk factors, stroke severity, time to intra-arterial therapy, and procedural success/complications.
Data source: A retrospective study of 116 patients with acute ischemic stroke who were eligible for intra-arterial therapy.
Disclosures: Dr. Shaibani said he had no relevant financial disclosures.





