User login
Diabetes, stroke linked to recurrent C. difficile
LAS VEGAS – Diabetes and stroke are risk factors for recurrent Clostridium difficile infection (CDI), with stroke patients at about 10 times the risk of recurrence.
The underlying cause for the association is a mystery, but one-sided paralysis is one possibility. “A lot of stroke patients may be hemiplegic, and they may be bedridden, so that may be a risk factor by itself. It’s something that may need to be studied in the future,” Alan Putrus, MD, a gastroenterology fellow at St. John Providence Hospital, Detroit, said in an interview.
CDI recurrence rates range from 5% to 47%, depending on the institution. Although risk factors of initial CDI have been well defined, few studies have looked at risk factors associated with recurrence.
In order to get at the question, the researchers conducted a study of 108 initial CDIs and 113 recurrences at two urban and one suburban hospital. Patients who experienced recurrence were matched 1:1 to age- and gender-matched controls with no recurrent CDI.
CDI recurrence rates were 16.5% and 15.9% in the two urban hospitals, and 14.9% in the suburban hospital.
Logistic regression revealed risk factors associated with CDI recurrence, including diabetes (odds ratio, 1.91; 95% confidence interval, 1.05-3.47; P = .04), stroke (OR, 9.73; 95% CI, 1.15-82.35; P = .04), exposure to proton pump inhibitors in the past 3 months (OR, 1.82; 95% CI, 1.03-3.23; P = .04), and admission to an intensive care unit in the past 3 months (OR, 1.95; 95% CI, 1.0-3.83; P = .04).
The results suggest that diabetes and especially stroke may be important risk factors for CDI recurrence, and their presence should prompt physicians to alter patient care accordingly, according to Dr. Putrus.
He stressed the importance of antibiotic stewardship. “Once you find a certain bug or pathogen, try to deescalate the antibiotics as soon as you can. If a patient is diabetic, controlling their blood sugar may also help,” Dr. Putrus said.
Finally, physicians should consider whether proton pump inhibitors are really necessary. Some patients start on PPIs but remain on the drugs long after symptoms have abated. “A lot of patients just have some discomfort in their abdomen, and they never stop taking it. They keep refilling it. So that’s a problem,” said Dr. Putrus.
Dr. Putrus has declared no conflicts of interest.
AGA Resource
AGA offers information to educate your patients about C. difficile and fecal microbiota transplant. Learn more at http://www.gastro.org/patient-care/conditions-diseases/clostridium-difficile-infection.
LAS VEGAS – Diabetes and stroke are risk factors for recurrent Clostridium difficile infection (CDI), with stroke patients at about 10 times the risk of recurrence.
The underlying cause for the association is a mystery, but one-sided paralysis is one possibility. “A lot of stroke patients may be hemiplegic, and they may be bedridden, so that may be a risk factor by itself. It’s something that may need to be studied in the future,” Alan Putrus, MD, a gastroenterology fellow at St. John Providence Hospital, Detroit, said in an interview.
CDI recurrence rates range from 5% to 47%, depending on the institution. Although risk factors of initial CDI have been well defined, few studies have looked at risk factors associated with recurrence.
In order to get at the question, the researchers conducted a study of 108 initial CDIs and 113 recurrences at two urban and one suburban hospital. Patients who experienced recurrence were matched 1:1 to age- and gender-matched controls with no recurrent CDI.
CDI recurrence rates were 16.5% and 15.9% in the two urban hospitals, and 14.9% in the suburban hospital.
Logistic regression revealed risk factors associated with CDI recurrence, including diabetes (odds ratio, 1.91; 95% confidence interval, 1.05-3.47; P = .04), stroke (OR, 9.73; 95% CI, 1.15-82.35; P = .04), exposure to proton pump inhibitors in the past 3 months (OR, 1.82; 95% CI, 1.03-3.23; P = .04), and admission to an intensive care unit in the past 3 months (OR, 1.95; 95% CI, 1.0-3.83; P = .04).
The results suggest that diabetes and especially stroke may be important risk factors for CDI recurrence, and their presence should prompt physicians to alter patient care accordingly, according to Dr. Putrus.
He stressed the importance of antibiotic stewardship. “Once you find a certain bug or pathogen, try to deescalate the antibiotics as soon as you can. If a patient is diabetic, controlling their blood sugar may also help,” Dr. Putrus said.
Finally, physicians should consider whether proton pump inhibitors are really necessary. Some patients start on PPIs but remain on the drugs long after symptoms have abated. “A lot of patients just have some discomfort in their abdomen, and they never stop taking it. They keep refilling it. So that’s a problem,” said Dr. Putrus.
Dr. Putrus has declared no conflicts of interest.
AGA Resource
AGA offers information to educate your patients about C. difficile and fecal microbiota transplant. Learn more at http://www.gastro.org/patient-care/conditions-diseases/clostridium-difficile-infection.
LAS VEGAS – Diabetes and stroke are risk factors for recurrent Clostridium difficile infection (CDI), with stroke patients at about 10 times the risk of recurrence.
The underlying cause for the association is a mystery, but one-sided paralysis is one possibility. “A lot of stroke patients may be hemiplegic, and they may be bedridden, so that may be a risk factor by itself. It’s something that may need to be studied in the future,” Alan Putrus, MD, a gastroenterology fellow at St. John Providence Hospital, Detroit, said in an interview.
CDI recurrence rates range from 5% to 47%, depending on the institution. Although risk factors of initial CDI have been well defined, few studies have looked at risk factors associated with recurrence.
In order to get at the question, the researchers conducted a study of 108 initial CDIs and 113 recurrences at two urban and one suburban hospital. Patients who experienced recurrence were matched 1:1 to age- and gender-matched controls with no recurrent CDI.
CDI recurrence rates were 16.5% and 15.9% in the two urban hospitals, and 14.9% in the suburban hospital.
Logistic regression revealed risk factors associated with CDI recurrence, including diabetes (odds ratio, 1.91; 95% confidence interval, 1.05-3.47; P = .04), stroke (OR, 9.73; 95% CI, 1.15-82.35; P = .04), exposure to proton pump inhibitors in the past 3 months (OR, 1.82; 95% CI, 1.03-3.23; P = .04), and admission to an intensive care unit in the past 3 months (OR, 1.95; 95% CI, 1.0-3.83; P = .04).
The results suggest that diabetes and especially stroke may be important risk factors for CDI recurrence, and their presence should prompt physicians to alter patient care accordingly, according to Dr. Putrus.
He stressed the importance of antibiotic stewardship. “Once you find a certain bug or pathogen, try to deescalate the antibiotics as soon as you can. If a patient is diabetic, controlling their blood sugar may also help,” Dr. Putrus said.
Finally, physicians should consider whether proton pump inhibitors are really necessary. Some patients start on PPIs but remain on the drugs long after symptoms have abated. “A lot of patients just have some discomfort in their abdomen, and they never stop taking it. They keep refilling it. So that’s a problem,” said Dr. Putrus.
Dr. Putrus has declared no conflicts of interest.
AGA Resource
AGA offers information to educate your patients about C. difficile and fecal microbiota transplant. Learn more at http://www.gastro.org/patient-care/conditions-diseases/clostridium-difficile-infection.
AT ACG 2016
Key clinical point:
Major finding: Diabetes, stroke, and a history of PPI use were all associated with higher risks of recurrence.
Data source: Case-control, retrospective study.
Disclosures: Dr. Putrus has declared no conflicts of interest.
Ozanimod has lasting effect on ulcerative colitis
LAS VEGAS – Ozanimod, an oral agent that selectively modulates the sphingosine 1-phosphate (S1P) 1 and 5 receptor, has a lasting effect on symptoms of ulcerative colitis, according to results from an open-label extension study.
The study extends the phase II TOUCHSTONE trial, in which patients with ulcerative colitis showed significant clinical improvement out to 32 weeks. The current study showed those improvements lasting out to at least 1 year, with about 80% of patients staying on the drug at the end of the study.
With other drug regimens, “the loss rates are at least 50% of the patients, so this is a remarkable level of durability over time,” William Sandborn, MD, chief of gastroenterology at the University of California at San Diego, said at the annual meeting of the American College of Gastroenterology. “With biologics, if you follow patients out for a year or 2, you see a fair amount of loss of response and some of that probably has to do with the formation of antidrug antibodies and immunogenicity,” Dr. Sandborn added.
In the original TOUCHSTONE study, 197 patients were randomized to placebo, ozanimod 0.5 mg, or ozanimod 1.0 mg, and followed out to week 32. Twenty-one percent of those in the 1.0-mg group achieved clinical remission, compared with 26% in the 0.5-mg group and 6% of those receiving placebo. Clinical response rates were 51%, 35%, and 20%, respectively.
In the open-label study, patients from all arms who did not respond to treatment after the induction phase, or relapsed during the maintenance phase, or completed the maintenance phase (170 patients in total) entered the open-label study with a dose of 1.0 mg ozanimod. At the time of the cut-off, patients in the extension study had been taking ozanimod for at least 1 year.
At the start of the extension study, the partial Mayo Score (pMS) for patients on placebo, ozanimod 0.5 mg, and 1.0 mg were 4.6, 4.5, and 3.3, respectively. All groups showed improvement in pMS by week 44 (1.7, 1.7, and 1.9, respectively)
At week 44, 90.9% of patients had little or no active disease (physician global assessment 0 or 1), 98.4% had little or no blood in their stools, and 84.7% had no blood in their stools.
Adverse events with a frequency higher than 2% included ulcerative colitis flare (5.9%), anemia (3.5%), upper respiratory tract infection (4.1%), nasal pharyngitis (3.5%), back pain (2.9%), arthralgia (2.4%), and headache (2.4%). The researchers noted some transient elevation of alanine aminotransferase or aspartate aminotransferase, but these elevations were temporary and reversed during ongoing treatment; 2.4% of patients experienced ALT and AST levels higher than three times the upper limit of normal, and all were asymptomatic.
Serious adverse events that occurred in two or more patients included anemia (1.2%) and ulcerative colitis flare (2.4%).
“This is a promising oral product with a similar mechanism of action to other lymphocyte trafficking agents,” said Stephen Hanauer, MD, medical director of the digestive health center at the Northwestern University, Chicago, who attended the session.
Ozanimod and other lymphocyte trafficking agents may offer a slightly different profile than some of the other drug classes, such as the anti–tumor necrosis factor agents, according to Dr. Hanauer, because the agents don’t affect lymphocytes already in the tissues. On the other hand, once the drug has acted, its effect may linger. “The time to effect may be a little slower, but the long-term effect seems to be as good or better as other mechanisms of action.”
The drug’s real place could be in early disease, Dr. Hanauer said. “If this is truly an effective and safe agent, the real positioning should be earlier in the disease, before patients are exposed to steroids and other immune suppressants, or biologics that have an infection risk.”
Celgene funded the study. Dr. Sandborn has received funding from Receptos and Celgene and consulted for both companies. Dr. Hanauer has consulted with Receptos, Celgene, Pfizer, Jansen, and AbbVie.
AGA Resource
AGA offers an IBD Clinical Service Line that provides tools to help you become more efficient, understand quality standards, and improve the process of care for patients. Learn more at http://www.gastro.org/patient-care/conditions-diseases/ibd
LAS VEGAS – Ozanimod, an oral agent that selectively modulates the sphingosine 1-phosphate (S1P) 1 and 5 receptor, has a lasting effect on symptoms of ulcerative colitis, according to results from an open-label extension study.
The study extends the phase II TOUCHSTONE trial, in which patients with ulcerative colitis showed significant clinical improvement out to 32 weeks. The current study showed those improvements lasting out to at least 1 year, with about 80% of patients staying on the drug at the end of the study.
With other drug regimens, “the loss rates are at least 50% of the patients, so this is a remarkable level of durability over time,” William Sandborn, MD, chief of gastroenterology at the University of California at San Diego, said at the annual meeting of the American College of Gastroenterology. “With biologics, if you follow patients out for a year or 2, you see a fair amount of loss of response and some of that probably has to do with the formation of antidrug antibodies and immunogenicity,” Dr. Sandborn added.
In the original TOUCHSTONE study, 197 patients were randomized to placebo, ozanimod 0.5 mg, or ozanimod 1.0 mg, and followed out to week 32. Twenty-one percent of those in the 1.0-mg group achieved clinical remission, compared with 26% in the 0.5-mg group and 6% of those receiving placebo. Clinical response rates were 51%, 35%, and 20%, respectively.
In the open-label study, patients from all arms who did not respond to treatment after the induction phase, or relapsed during the maintenance phase, or completed the maintenance phase (170 patients in total) entered the open-label study with a dose of 1.0 mg ozanimod. At the time of the cut-off, patients in the extension study had been taking ozanimod for at least 1 year.
At the start of the extension study, the partial Mayo Score (pMS) for patients on placebo, ozanimod 0.5 mg, and 1.0 mg were 4.6, 4.5, and 3.3, respectively. All groups showed improvement in pMS by week 44 (1.7, 1.7, and 1.9, respectively)
At week 44, 90.9% of patients had little or no active disease (physician global assessment 0 or 1), 98.4% had little or no blood in their stools, and 84.7% had no blood in their stools.
Adverse events with a frequency higher than 2% included ulcerative colitis flare (5.9%), anemia (3.5%), upper respiratory tract infection (4.1%), nasal pharyngitis (3.5%), back pain (2.9%), arthralgia (2.4%), and headache (2.4%). The researchers noted some transient elevation of alanine aminotransferase or aspartate aminotransferase, but these elevations were temporary and reversed during ongoing treatment; 2.4% of patients experienced ALT and AST levels higher than three times the upper limit of normal, and all were asymptomatic.
Serious adverse events that occurred in two or more patients included anemia (1.2%) and ulcerative colitis flare (2.4%).
“This is a promising oral product with a similar mechanism of action to other lymphocyte trafficking agents,” said Stephen Hanauer, MD, medical director of the digestive health center at the Northwestern University, Chicago, who attended the session.
Ozanimod and other lymphocyte trafficking agents may offer a slightly different profile than some of the other drug classes, such as the anti–tumor necrosis factor agents, according to Dr. Hanauer, because the agents don’t affect lymphocytes already in the tissues. On the other hand, once the drug has acted, its effect may linger. “The time to effect may be a little slower, but the long-term effect seems to be as good or better as other mechanisms of action.”
The drug’s real place could be in early disease, Dr. Hanauer said. “If this is truly an effective and safe agent, the real positioning should be earlier in the disease, before patients are exposed to steroids and other immune suppressants, or biologics that have an infection risk.”
Celgene funded the study. Dr. Sandborn has received funding from Receptos and Celgene and consulted for both companies. Dr. Hanauer has consulted with Receptos, Celgene, Pfizer, Jansen, and AbbVie.
AGA Resource
AGA offers an IBD Clinical Service Line that provides tools to help you become more efficient, understand quality standards, and improve the process of care for patients. Learn more at http://www.gastro.org/patient-care/conditions-diseases/ibd
LAS VEGAS – Ozanimod, an oral agent that selectively modulates the sphingosine 1-phosphate (S1P) 1 and 5 receptor, has a lasting effect on symptoms of ulcerative colitis, according to results from an open-label extension study.
The study extends the phase II TOUCHSTONE trial, in which patients with ulcerative colitis showed significant clinical improvement out to 32 weeks. The current study showed those improvements lasting out to at least 1 year, with about 80% of patients staying on the drug at the end of the study.
With other drug regimens, “the loss rates are at least 50% of the patients, so this is a remarkable level of durability over time,” William Sandborn, MD, chief of gastroenterology at the University of California at San Diego, said at the annual meeting of the American College of Gastroenterology. “With biologics, if you follow patients out for a year or 2, you see a fair amount of loss of response and some of that probably has to do with the formation of antidrug antibodies and immunogenicity,” Dr. Sandborn added.
In the original TOUCHSTONE study, 197 patients were randomized to placebo, ozanimod 0.5 mg, or ozanimod 1.0 mg, and followed out to week 32. Twenty-one percent of those in the 1.0-mg group achieved clinical remission, compared with 26% in the 0.5-mg group and 6% of those receiving placebo. Clinical response rates were 51%, 35%, and 20%, respectively.
In the open-label study, patients from all arms who did not respond to treatment after the induction phase, or relapsed during the maintenance phase, or completed the maintenance phase (170 patients in total) entered the open-label study with a dose of 1.0 mg ozanimod. At the time of the cut-off, patients in the extension study had been taking ozanimod for at least 1 year.
At the start of the extension study, the partial Mayo Score (pMS) for patients on placebo, ozanimod 0.5 mg, and 1.0 mg were 4.6, 4.5, and 3.3, respectively. All groups showed improvement in pMS by week 44 (1.7, 1.7, and 1.9, respectively)
At week 44, 90.9% of patients had little or no active disease (physician global assessment 0 or 1), 98.4% had little or no blood in their stools, and 84.7% had no blood in their stools.
Adverse events with a frequency higher than 2% included ulcerative colitis flare (5.9%), anemia (3.5%), upper respiratory tract infection (4.1%), nasal pharyngitis (3.5%), back pain (2.9%), arthralgia (2.4%), and headache (2.4%). The researchers noted some transient elevation of alanine aminotransferase or aspartate aminotransferase, but these elevations were temporary and reversed during ongoing treatment; 2.4% of patients experienced ALT and AST levels higher than three times the upper limit of normal, and all were asymptomatic.
Serious adverse events that occurred in two or more patients included anemia (1.2%) and ulcerative colitis flare (2.4%).
“This is a promising oral product with a similar mechanism of action to other lymphocyte trafficking agents,” said Stephen Hanauer, MD, medical director of the digestive health center at the Northwestern University, Chicago, who attended the session.
Ozanimod and other lymphocyte trafficking agents may offer a slightly different profile than some of the other drug classes, such as the anti–tumor necrosis factor agents, according to Dr. Hanauer, because the agents don’t affect lymphocytes already in the tissues. On the other hand, once the drug has acted, its effect may linger. “The time to effect may be a little slower, but the long-term effect seems to be as good or better as other mechanisms of action.”
The drug’s real place could be in early disease, Dr. Hanauer said. “If this is truly an effective and safe agent, the real positioning should be earlier in the disease, before patients are exposed to steroids and other immune suppressants, or biologics that have an infection risk.”
Celgene funded the study. Dr. Sandborn has received funding from Receptos and Celgene and consulted for both companies. Dr. Hanauer has consulted with Receptos, Celgene, Pfizer, Jansen, and AbbVie.
AGA Resource
AGA offers an IBD Clinical Service Line that provides tools to help you become more efficient, understand quality standards, and improve the process of care for patients. Learn more at http://www.gastro.org/patient-care/conditions-diseases/ibd
AT ACG 2016
Key clinical point:
Major finding: Ozanimod maintains efficacy in ulcerative colitis out to 1 year, with 90% of patients having little or no evidence of active disease.
Data source: Open-label extension study following a phase II clinical trial.
Disclosures: Celgene funded the study. Dr. Sandborn has received funding from Receptos and Celgene and consulted for both companies. Dr. Hanauer has consulted with Receptos, Celgene, Pfizer, Jansen, and AbbVie.
Treat ulcerative colitis ‘to target’
LAS VEGAS – In ulcerative colitis, symptoms aren’t everything. That’s the opinion of Stephen B. Hanauer, MD, medical director of Northwestern University’s Digestive Health Center, Chicago, who discussed the condition and the methods physicians use to assess patient improvement, at the annual meeting of the American College of Gastroenterology.
Many gastroenterologists accept patient reports of symptom improvement, but Dr. Hanauer advocates for a follow-up endoscopy, even when patients are making good progress.
Even in patients with improved symptoms, many endoscopic evaluations show inflammation and troubling histology that is in turn associated with worse quality of life and greater odds of hospitalizations and surgery (Dig Liver Dis. 2013 Dec;45:969-77).
So a better outcome is histological healing, characterized by the absence of neutrophils. A study presented at Digestive Disease Week® in 2015 (abstract 592) showed better relapse-free survival after histologic remission. “The deeper the remission that we achieve, the better the outcomes are going to be,” said Dr. Hanauer.
In fact, patients who report that they are asymptomatic may still be experiencing disease. “Many chronic patients accept a certain degree of symptoms as a new normal,” Dr. Hanauer said. Patients accept living with frequent nighttime awakenings to evacuate, or more frequent bowel movements. Further treatment could reduce or eliminate such problems. “There are subtle things you can improve upon, to convince them that the new normal is not the acceptable normal,” said Dr. Hanauer.
He suggests an endoscopy 3-12 months after patients report symptom abatement, although he admits that cost and other considerations can be barriers.
When an endoscopy is performed, it can lead to a conundrum if it shows residual inflammation despite a lack of symptoms. The physician might consider switching to a different class of drug, but that will usually raise cost and introduce new risks of adverse events. “We are doing that in some settings. For example, in postoperative Crohn’s disease, where patients have no active disease, we advocate prophylactically administering agents to prevent recurrence. So we are willing to do that in certain subsets of patients, but we have clinical trials to show the benefits of that,” said Dr. Hanauer. Unfortunately, there are no clinical trials demonstrating the benefits of such an approach in ulcerative colitis.
Biomarkers offer potential assistance for physicians who want to “treat to target,” as Dr. Hanauer put it, to achieve biological remission. Calprotectin – a protein secreted by neutrophils that mark the presence of these cells – are measurable in feces, and the levels correlate well with ulcerative colitis disease activity (Inflamm Bowel Dis. 2012 Nov;18:2011-7).
Dr. Hanauer’s advice to go beyond clinical remission is already occurring at the Food and Drug Administration, which no longer accepts clinical remission findings in testing new drugs for inflammatory bowel disease. “They are requiring patient-reported outcomes accompanied by endoscopic evidence of healing. I’m advocating what’s going on on a regulatory basis be applied on a clinical basis,” said Dr. Hanauer.
Dr. Hanauer had no relevant financial disclosures.
LAS VEGAS – In ulcerative colitis, symptoms aren’t everything. That’s the opinion of Stephen B. Hanauer, MD, medical director of Northwestern University’s Digestive Health Center, Chicago, who discussed the condition and the methods physicians use to assess patient improvement, at the annual meeting of the American College of Gastroenterology.
Many gastroenterologists accept patient reports of symptom improvement, but Dr. Hanauer advocates for a follow-up endoscopy, even when patients are making good progress.
Even in patients with improved symptoms, many endoscopic evaluations show inflammation and troubling histology that is in turn associated with worse quality of life and greater odds of hospitalizations and surgery (Dig Liver Dis. 2013 Dec;45:969-77).
So a better outcome is histological healing, characterized by the absence of neutrophils. A study presented at Digestive Disease Week® in 2015 (abstract 592) showed better relapse-free survival after histologic remission. “The deeper the remission that we achieve, the better the outcomes are going to be,” said Dr. Hanauer.
In fact, patients who report that they are asymptomatic may still be experiencing disease. “Many chronic patients accept a certain degree of symptoms as a new normal,” Dr. Hanauer said. Patients accept living with frequent nighttime awakenings to evacuate, or more frequent bowel movements. Further treatment could reduce or eliminate such problems. “There are subtle things you can improve upon, to convince them that the new normal is not the acceptable normal,” said Dr. Hanauer.
He suggests an endoscopy 3-12 months after patients report symptom abatement, although he admits that cost and other considerations can be barriers.
When an endoscopy is performed, it can lead to a conundrum if it shows residual inflammation despite a lack of symptoms. The physician might consider switching to a different class of drug, but that will usually raise cost and introduce new risks of adverse events. “We are doing that in some settings. For example, in postoperative Crohn’s disease, where patients have no active disease, we advocate prophylactically administering agents to prevent recurrence. So we are willing to do that in certain subsets of patients, but we have clinical trials to show the benefits of that,” said Dr. Hanauer. Unfortunately, there are no clinical trials demonstrating the benefits of such an approach in ulcerative colitis.
Biomarkers offer potential assistance for physicians who want to “treat to target,” as Dr. Hanauer put it, to achieve biological remission. Calprotectin – a protein secreted by neutrophils that mark the presence of these cells – are measurable in feces, and the levels correlate well with ulcerative colitis disease activity (Inflamm Bowel Dis. 2012 Nov;18:2011-7).
Dr. Hanauer’s advice to go beyond clinical remission is already occurring at the Food and Drug Administration, which no longer accepts clinical remission findings in testing new drugs for inflammatory bowel disease. “They are requiring patient-reported outcomes accompanied by endoscopic evidence of healing. I’m advocating what’s going on on a regulatory basis be applied on a clinical basis,” said Dr. Hanauer.
Dr. Hanauer had no relevant financial disclosures.
LAS VEGAS – In ulcerative colitis, symptoms aren’t everything. That’s the opinion of Stephen B. Hanauer, MD, medical director of Northwestern University’s Digestive Health Center, Chicago, who discussed the condition and the methods physicians use to assess patient improvement, at the annual meeting of the American College of Gastroenterology.
Many gastroenterologists accept patient reports of symptom improvement, but Dr. Hanauer advocates for a follow-up endoscopy, even when patients are making good progress.
Even in patients with improved symptoms, many endoscopic evaluations show inflammation and troubling histology that is in turn associated with worse quality of life and greater odds of hospitalizations and surgery (Dig Liver Dis. 2013 Dec;45:969-77).
So a better outcome is histological healing, characterized by the absence of neutrophils. A study presented at Digestive Disease Week® in 2015 (abstract 592) showed better relapse-free survival after histologic remission. “The deeper the remission that we achieve, the better the outcomes are going to be,” said Dr. Hanauer.
In fact, patients who report that they are asymptomatic may still be experiencing disease. “Many chronic patients accept a certain degree of symptoms as a new normal,” Dr. Hanauer said. Patients accept living with frequent nighttime awakenings to evacuate, or more frequent bowel movements. Further treatment could reduce or eliminate such problems. “There are subtle things you can improve upon, to convince them that the new normal is not the acceptable normal,” said Dr. Hanauer.
He suggests an endoscopy 3-12 months after patients report symptom abatement, although he admits that cost and other considerations can be barriers.
When an endoscopy is performed, it can lead to a conundrum if it shows residual inflammation despite a lack of symptoms. The physician might consider switching to a different class of drug, but that will usually raise cost and introduce new risks of adverse events. “We are doing that in some settings. For example, in postoperative Crohn’s disease, where patients have no active disease, we advocate prophylactically administering agents to prevent recurrence. So we are willing to do that in certain subsets of patients, but we have clinical trials to show the benefits of that,” said Dr. Hanauer. Unfortunately, there are no clinical trials demonstrating the benefits of such an approach in ulcerative colitis.
Biomarkers offer potential assistance for physicians who want to “treat to target,” as Dr. Hanauer put it, to achieve biological remission. Calprotectin – a protein secreted by neutrophils that mark the presence of these cells – are measurable in feces, and the levels correlate well with ulcerative colitis disease activity (Inflamm Bowel Dis. 2012 Nov;18:2011-7).
Dr. Hanauer’s advice to go beyond clinical remission is already occurring at the Food and Drug Administration, which no longer accepts clinical remission findings in testing new drugs for inflammatory bowel disease. “They are requiring patient-reported outcomes accompanied by endoscopic evidence of healing. I’m advocating what’s going on on a regulatory basis be applied on a clinical basis,” said Dr. Hanauer.
Dr. Hanauer had no relevant financial disclosures.
EXPERT ANALYSIS FROM ACG 2016
Diabetes, stroke linked to recurrent C. difficile
LAS VEGAS – Diabetes and stroke are risk factors for recurrent Clostridium difficile infection (CDI), with stroke patients at about 10 times the risk of recurrence.
The underlying cause for the association is a mystery, but one-sided paralysis is one possibility. “A lot of stroke patients may be hemiplegic, and they may be bedridden, so that may be a risk factor by itself. It’s something that may need to be studied in the future,” Alan Putrus, MD, a gastroenterology fellow at St. John Providence Hospital, Detroit, said in an interview.
CDI recurrence rates range from 5% to 47%, depending on the institution. Although risk factors of initial CDI have been well defined, few studies have looked at risk factors associated with recurrence.
In order to get at the question, the researchers conducted a study of 108 initial CDIs and 113 recurrences at two urban and one suburban hospital. Patients who experienced recurrence were matched 1:1 to age- and gender-matched controls with no recurrent CDI.
CDI recurrence rates were 16.5% and 15.9% in the two urban hospitals, and 14.9% in the suburban hospital.
Logistic regression revealed risk factors associated with CDI recurrence, including diabetes (odds ratio, 1.91; 95% confidence interval, 1.05-3.47; P = .04), stroke (OR, 9.73; 95% CI, 1.15-82.35; P = .04), exposure to proton pump inhibitors in the past 3 months (OR, 1.82; 95% CI, 1.03-3.23; P = .04), and admission to an intensive care unit in the past 3 months (OR, 1.95; 95% CI, 1.0-3.83; P = .04).
The results suggest that diabetes and especially stroke may be important risk factors for CDI recurrence, and their presence should prompt physicians to alter patient care accordingly, according to Dr. Putrus.
He stressed the importance of antibiotic stewardship. “Once you find a certain bug or pathogen, try to deescalate the antibiotics as soon as you can. If a patient is diabetic, controlling their blood sugar may also help,” Dr. Putrus said.
Finally, physicians should consider whether proton pump inhibitors are really necessary. Some patients start on PPIs but remain on the drugs long after symptoms have abated. “A lot of patients just have some discomfort in their abdomen, and they never stop taking it. They keep refilling it. So that’s a problem,” said Dr. Putrus.
Dr. Putrus has declared no conflicts of interest.
LAS VEGAS – Diabetes and stroke are risk factors for recurrent Clostridium difficile infection (CDI), with stroke patients at about 10 times the risk of recurrence.
The underlying cause for the association is a mystery, but one-sided paralysis is one possibility. “A lot of stroke patients may be hemiplegic, and they may be bedridden, so that may be a risk factor by itself. It’s something that may need to be studied in the future,” Alan Putrus, MD, a gastroenterology fellow at St. John Providence Hospital, Detroit, said in an interview.
CDI recurrence rates range from 5% to 47%, depending on the institution. Although risk factors of initial CDI have been well defined, few studies have looked at risk factors associated with recurrence.
In order to get at the question, the researchers conducted a study of 108 initial CDIs and 113 recurrences at two urban and one suburban hospital. Patients who experienced recurrence were matched 1:1 to age- and gender-matched controls with no recurrent CDI.
CDI recurrence rates were 16.5% and 15.9% in the two urban hospitals, and 14.9% in the suburban hospital.
Logistic regression revealed risk factors associated with CDI recurrence, including diabetes (odds ratio, 1.91; 95% confidence interval, 1.05-3.47; P = .04), stroke (OR, 9.73; 95% CI, 1.15-82.35; P = .04), exposure to proton pump inhibitors in the past 3 months (OR, 1.82; 95% CI, 1.03-3.23; P = .04), and admission to an intensive care unit in the past 3 months (OR, 1.95; 95% CI, 1.0-3.83; P = .04).
The results suggest that diabetes and especially stroke may be important risk factors for CDI recurrence, and their presence should prompt physicians to alter patient care accordingly, according to Dr. Putrus.
He stressed the importance of antibiotic stewardship. “Once you find a certain bug or pathogen, try to deescalate the antibiotics as soon as you can. If a patient is diabetic, controlling their blood sugar may also help,” Dr. Putrus said.
Finally, physicians should consider whether proton pump inhibitors are really necessary. Some patients start on PPIs but remain on the drugs long after symptoms have abated. “A lot of patients just have some discomfort in their abdomen, and they never stop taking it. They keep refilling it. So that’s a problem,” said Dr. Putrus.
Dr. Putrus has declared no conflicts of interest.
LAS VEGAS – Diabetes and stroke are risk factors for recurrent Clostridium difficile infection (CDI), with stroke patients at about 10 times the risk of recurrence.
The underlying cause for the association is a mystery, but one-sided paralysis is one possibility. “A lot of stroke patients may be hemiplegic, and they may be bedridden, so that may be a risk factor by itself. It’s something that may need to be studied in the future,” Alan Putrus, MD, a gastroenterology fellow at St. John Providence Hospital, Detroit, said in an interview.
CDI recurrence rates range from 5% to 47%, depending on the institution. Although risk factors of initial CDI have been well defined, few studies have looked at risk factors associated with recurrence.
In order to get at the question, the researchers conducted a study of 108 initial CDIs and 113 recurrences at two urban and one suburban hospital. Patients who experienced recurrence were matched 1:1 to age- and gender-matched controls with no recurrent CDI.
CDI recurrence rates were 16.5% and 15.9% in the two urban hospitals, and 14.9% in the suburban hospital.
Logistic regression revealed risk factors associated with CDI recurrence, including diabetes (odds ratio, 1.91; 95% confidence interval, 1.05-3.47; P = .04), stroke (OR, 9.73; 95% CI, 1.15-82.35; P = .04), exposure to proton pump inhibitors in the past 3 months (OR, 1.82; 95% CI, 1.03-3.23; P = .04), and admission to an intensive care unit in the past 3 months (OR, 1.95; 95% CI, 1.0-3.83; P = .04).
The results suggest that diabetes and especially stroke may be important risk factors for CDI recurrence, and their presence should prompt physicians to alter patient care accordingly, according to Dr. Putrus.
He stressed the importance of antibiotic stewardship. “Once you find a certain bug or pathogen, try to deescalate the antibiotics as soon as you can. If a patient is diabetic, controlling their blood sugar may also help,” Dr. Putrus said.
Finally, physicians should consider whether proton pump inhibitors are really necessary. Some patients start on PPIs but remain on the drugs long after symptoms have abated. “A lot of patients just have some discomfort in their abdomen, and they never stop taking it. They keep refilling it. So that’s a problem,” said Dr. Putrus.
Dr. Putrus has declared no conflicts of interest.
AT ACG 2016
Key clinical point:
Major finding: Diabetes, stroke, and a history of PPI use were all associated with higher risks of recurrence.
Data source: Case-control, retrospective study.
Disclosures: Dr. Putrus has declared no conflicts of interest.
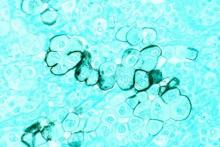
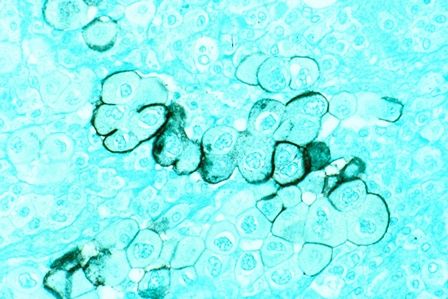
Biomarker identifies precancerous pancreatic cysts
LAS VEGAS – In fluid derived from pancreatic cysts, methylated DNA markers predict the presence of high-grade dysplasia (HGD) or cancer, and could help physicians decide whether to surgically remove cysts – a procedure that often has serious complications.
If validated in larger studies, the biomarkers have the potential to supplant the Fukuoka criteria that is currently used. “The markers could cause a paradigm shift in how we approach these lesions in our clinical practice,” Shounak Majumder, MD, a fellow at the Mayo Clinic in Rochester, Minn., said in an interview.
Less than 50% of cysts that are surgically resected turn out to be HGD or cancerous. “Having a cyst fluid marker could identify the patients that would benefit the most from surgery. If you’re going to go through a pancreatic resection, we’d rather give you the best chance of saying that we removed something that either has early cancer in it or will turn into cancer in the near future,” said Dr. Majumder.
The study looked at pancreatic cyst fluid from 83 cysts that had been surgically resected. The DNA samples were taken from the cyst fluid. Dr. Majumder believes that the cells shed from the cyst wall into the fluid. As a result, DNA from the fluid captures heterogeneity in the cyst more effectively than a biopsied sample.
The researchers found five methylated DNA markers that distinguished cancer or HGD from controls with areas under the ROC curve of 0.90 or higher. The top two (BMP3, EMX1) detected 93% of cases (95% CI, 66%-100%) at a specificity of 90% (95% CI, 80%-96%). Applied to eight cysts with intermediate-grade dysplasia, the biomarkers would have identified three at 95% specificity.
By comparison, the Fukuoka guidelines have 56% sensitivity and 73% specificity.
A limitation to the technique is that DNA cannot be extracted from all samples. About 5%-10% of pancreatic fluid samples are unusable, according to Somashekar Krishna, MD, MPH, assistant professor of medicine at the Ohio State University Medical Center, who attended the session. Dr. Krishna is conducting research combining endomicroscopy with molecular markers.
“We should have a foolproof system where if one fails, the other kicks in, and we have an answer for every patient. My opinion is that endomicroscopy has to be combined with molecular studies. I think combined we’ll have an excellent diagnostic yield,” Dr. Krishna said in an interview.
Dr. Majumder and Dr. Krishna have declared no conflicts of interest.
LAS VEGAS – In fluid derived from pancreatic cysts, methylated DNA markers predict the presence of high-grade dysplasia (HGD) or cancer, and could help physicians decide whether to surgically remove cysts – a procedure that often has serious complications.
If validated in larger studies, the biomarkers have the potential to supplant the Fukuoka criteria that is currently used. “The markers could cause a paradigm shift in how we approach these lesions in our clinical practice,” Shounak Majumder, MD, a fellow at the Mayo Clinic in Rochester, Minn., said in an interview.
Less than 50% of cysts that are surgically resected turn out to be HGD or cancerous. “Having a cyst fluid marker could identify the patients that would benefit the most from surgery. If you’re going to go through a pancreatic resection, we’d rather give you the best chance of saying that we removed something that either has early cancer in it or will turn into cancer in the near future,” said Dr. Majumder.
The study looked at pancreatic cyst fluid from 83 cysts that had been surgically resected. The DNA samples were taken from the cyst fluid. Dr. Majumder believes that the cells shed from the cyst wall into the fluid. As a result, DNA from the fluid captures heterogeneity in the cyst more effectively than a biopsied sample.
The researchers found five methylated DNA markers that distinguished cancer or HGD from controls with areas under the ROC curve of 0.90 or higher. The top two (BMP3, EMX1) detected 93% of cases (95% CI, 66%-100%) at a specificity of 90% (95% CI, 80%-96%). Applied to eight cysts with intermediate-grade dysplasia, the biomarkers would have identified three at 95% specificity.
By comparison, the Fukuoka guidelines have 56% sensitivity and 73% specificity.
A limitation to the technique is that DNA cannot be extracted from all samples. About 5%-10% of pancreatic fluid samples are unusable, according to Somashekar Krishna, MD, MPH, assistant professor of medicine at the Ohio State University Medical Center, who attended the session. Dr. Krishna is conducting research combining endomicroscopy with molecular markers.
“We should have a foolproof system where if one fails, the other kicks in, and we have an answer for every patient. My opinion is that endomicroscopy has to be combined with molecular studies. I think combined we’ll have an excellent diagnostic yield,” Dr. Krishna said in an interview.
Dr. Majumder and Dr. Krishna have declared no conflicts of interest.
LAS VEGAS – In fluid derived from pancreatic cysts, methylated DNA markers predict the presence of high-grade dysplasia (HGD) or cancer, and could help physicians decide whether to surgically remove cysts – a procedure that often has serious complications.
If validated in larger studies, the biomarkers have the potential to supplant the Fukuoka criteria that is currently used. “The markers could cause a paradigm shift in how we approach these lesions in our clinical practice,” Shounak Majumder, MD, a fellow at the Mayo Clinic in Rochester, Minn., said in an interview.
Less than 50% of cysts that are surgically resected turn out to be HGD or cancerous. “Having a cyst fluid marker could identify the patients that would benefit the most from surgery. If you’re going to go through a pancreatic resection, we’d rather give you the best chance of saying that we removed something that either has early cancer in it or will turn into cancer in the near future,” said Dr. Majumder.
The study looked at pancreatic cyst fluid from 83 cysts that had been surgically resected. The DNA samples were taken from the cyst fluid. Dr. Majumder believes that the cells shed from the cyst wall into the fluid. As a result, DNA from the fluid captures heterogeneity in the cyst more effectively than a biopsied sample.
The researchers found five methylated DNA markers that distinguished cancer or HGD from controls with areas under the ROC curve of 0.90 or higher. The top two (BMP3, EMX1) detected 93% of cases (95% CI, 66%-100%) at a specificity of 90% (95% CI, 80%-96%). Applied to eight cysts with intermediate-grade dysplasia, the biomarkers would have identified three at 95% specificity.
By comparison, the Fukuoka guidelines have 56% sensitivity and 73% specificity.
A limitation to the technique is that DNA cannot be extracted from all samples. About 5%-10% of pancreatic fluid samples are unusable, according to Somashekar Krishna, MD, MPH, assistant professor of medicine at the Ohio State University Medical Center, who attended the session. Dr. Krishna is conducting research combining endomicroscopy with molecular markers.
“We should have a foolproof system where if one fails, the other kicks in, and we have an answer for every patient. My opinion is that endomicroscopy has to be combined with molecular studies. I think combined we’ll have an excellent diagnostic yield,” Dr. Krishna said in an interview.
Dr. Majumder and Dr. Krishna have declared no conflicts of interest.
AT ACG 2016
Key clinical point:
Major finding: DNA markers isolated from pancreatic fluid predicted cancer or high-grade dysplasia with 90% specificity and 93% sensitivity.
Data source: Pilot study, retrospective analysis.
Disclosures: Dr. Majumder and Dr. Krishna have declared no conflicts of interest.
Automated colonoscopy risk classification for veterans
LAS VEGAS – A new scoring system for military veterans estimates risk of advanced colorectal neoplasia based on a list of lifestyle and clinical factors, and could help prioritize patients for screening colonoscopy. The study used natural-language processing to analyze patient medical records from the VA health care system.
“Those who were at very low risk had no colon cancer, and their risk of advanced polyps was pretty low, while those in the high-risk group should probably give thought to having a colonoscopy,” Thomas F. Imperiale, MD, a gastroenterologist and professor of medicine at the Indiana University School of Medicine, Indianapolis, said in an interview. Dr. Imperiale presented the results at the annual meeting of the American College of Gastroenterology.
When asked during the Q&A session if he could identify a subset of patients who could skip colonoscopies altogether, Dr. Imperiale said that he could not. “Prediction is possible, and if those predictions stand up in validation, they may be used to at least provide veterans with a choice, and perhaps provide the health care system a choice about how they screen their patients,” he said.
The researchers identified 66,725 veterans who underwent a diagnostic or screening colonoscopy between 2002 and 2009 at one of 14 VA medical centers. They used natural language processing to identify the most advanced finding and the location within the colorectum.
The rate of advanced neoplasia was 8.8%, and the rate of colorectal cancer (CRC) was 1.2%. Independent risk factors included age, sex, tobacco use, and exposure to COX-1 and COX-2 nonsteroidal anti-inflammatory drugs. The researchers defined four risk categories, low to high, with advanced neoplasia risks of 2.9% (CRC risk, 0%), 5.3% (CRC risk, 0.5%), 8.5% (CRC risk, 0.9%), and 11.4% (CRC risk, 2.1%). For advanced neoplasia, the goodness-of-fit P value was 1.00 and the c statistic was 0.58. For CRC, these values were 1.00 and 0.64, respectively.
Dr. Imperiale noted that the results remain relatively crude, and that the field of natural-language processing continues to evolve. In fact, software available today outperforms that used in the study. “It’s a field that’s moving quickly,” said Dr. Imperiale.
The results could help prioritize patients for colonoscopies, according to Victor Levy, MD, a senior attending physician at Mary Imogene Bassett Healthcare, Cooperstown, N.Y., who attended the presentation. And the study represents good news for the field overall. “Big data analytics has been accepted in other industries, and it’s now finally beginning to exert its effects in health care,” Dr. Levy said in an interview.
The results don’t generalize to other groups, because veterans have unique covariables and confounding factors. A new model would have to be developed for other populations.
Dr. Imperiale and Dr. Levy have no disclosures.
*Updated on 10/27/16
LAS VEGAS – A new scoring system for military veterans estimates risk of advanced colorectal neoplasia based on a list of lifestyle and clinical factors, and could help prioritize patients for screening colonoscopy. The study used natural-language processing to analyze patient medical records from the VA health care system.
“Those who were at very low risk had no colon cancer, and their risk of advanced polyps was pretty low, while those in the high-risk group should probably give thought to having a colonoscopy,” Thomas F. Imperiale, MD, a gastroenterologist and professor of medicine at the Indiana University School of Medicine, Indianapolis, said in an interview. Dr. Imperiale presented the results at the annual meeting of the American College of Gastroenterology.
When asked during the Q&A session if he could identify a subset of patients who could skip colonoscopies altogether, Dr. Imperiale said that he could not. “Prediction is possible, and if those predictions stand up in validation, they may be used to at least provide veterans with a choice, and perhaps provide the health care system a choice about how they screen their patients,” he said.
The researchers identified 66,725 veterans who underwent a diagnostic or screening colonoscopy between 2002 and 2009 at one of 14 VA medical centers. They used natural language processing to identify the most advanced finding and the location within the colorectum.
The rate of advanced neoplasia was 8.8%, and the rate of colorectal cancer (CRC) was 1.2%. Independent risk factors included age, sex, tobacco use, and exposure to COX-1 and COX-2 nonsteroidal anti-inflammatory drugs. The researchers defined four risk categories, low to high, with advanced neoplasia risks of 2.9% (CRC risk, 0%), 5.3% (CRC risk, 0.5%), 8.5% (CRC risk, 0.9%), and 11.4% (CRC risk, 2.1%). For advanced neoplasia, the goodness-of-fit P value was 1.00 and the c statistic was 0.58. For CRC, these values were 1.00 and 0.64, respectively.
Dr. Imperiale noted that the results remain relatively crude, and that the field of natural-language processing continues to evolve. In fact, software available today outperforms that used in the study. “It’s a field that’s moving quickly,” said Dr. Imperiale.
The results could help prioritize patients for colonoscopies, according to Victor Levy, MD, a senior attending physician at Mary Imogene Bassett Healthcare, Cooperstown, N.Y., who attended the presentation. And the study represents good news for the field overall. “Big data analytics has been accepted in other industries, and it’s now finally beginning to exert its effects in health care,” Dr. Levy said in an interview.
The results don’t generalize to other groups, because veterans have unique covariables and confounding factors. A new model would have to be developed for other populations.
Dr. Imperiale and Dr. Levy have no disclosures.
*Updated on 10/27/16
LAS VEGAS – A new scoring system for military veterans estimates risk of advanced colorectal neoplasia based on a list of lifestyle and clinical factors, and could help prioritize patients for screening colonoscopy. The study used natural-language processing to analyze patient medical records from the VA health care system.
“Those who were at very low risk had no colon cancer, and their risk of advanced polyps was pretty low, while those in the high-risk group should probably give thought to having a colonoscopy,” Thomas F. Imperiale, MD, a gastroenterologist and professor of medicine at the Indiana University School of Medicine, Indianapolis, said in an interview. Dr. Imperiale presented the results at the annual meeting of the American College of Gastroenterology.
When asked during the Q&A session if he could identify a subset of patients who could skip colonoscopies altogether, Dr. Imperiale said that he could not. “Prediction is possible, and if those predictions stand up in validation, they may be used to at least provide veterans with a choice, and perhaps provide the health care system a choice about how they screen their patients,” he said.
The researchers identified 66,725 veterans who underwent a diagnostic or screening colonoscopy between 2002 and 2009 at one of 14 VA medical centers. They used natural language processing to identify the most advanced finding and the location within the colorectum.
The rate of advanced neoplasia was 8.8%, and the rate of colorectal cancer (CRC) was 1.2%. Independent risk factors included age, sex, tobacco use, and exposure to COX-1 and COX-2 nonsteroidal anti-inflammatory drugs. The researchers defined four risk categories, low to high, with advanced neoplasia risks of 2.9% (CRC risk, 0%), 5.3% (CRC risk, 0.5%), 8.5% (CRC risk, 0.9%), and 11.4% (CRC risk, 2.1%). For advanced neoplasia, the goodness-of-fit P value was 1.00 and the c statistic was 0.58. For CRC, these values were 1.00 and 0.64, respectively.
Dr. Imperiale noted that the results remain relatively crude, and that the field of natural-language processing continues to evolve. In fact, software available today outperforms that used in the study. “It’s a field that’s moving quickly,” said Dr. Imperiale.
The results could help prioritize patients for colonoscopies, according to Victor Levy, MD, a senior attending physician at Mary Imogene Bassett Healthcare, Cooperstown, N.Y., who attended the presentation. And the study represents good news for the field overall. “Big data analytics has been accepted in other industries, and it’s now finally beginning to exert its effects in health care,” Dr. Levy said in an interview.
The results don’t generalize to other groups, because veterans have unique covariables and confounding factors. A new model would have to be developed for other populations.
Dr. Imperiale and Dr. Levy have no disclosures.
*Updated on 10/27/16
AT ACG 2016
Key clinical point:
Major finding: Patients in the lowest-risk group were predicted to have no colorectal cancers and to be at low risk for other advanced neoplasia. The high-risk patient group had higher predicted rates of advanced neoplasia and may be candidates for more aggressive screening.
Data source: Retrospective analysis of electronic medical records.
Disclosures: Dr. Imperiale and Dr. Levy have no disclosures.
IBD medical homes dramatically cut ED visits, hospitalizations
LAS VEGAS – A collaborative care approach to patients with inflammatory bowel disease (IBD) dramatically reduced emergency department visits and hospitalizations and also boosted quality of life.
The IBD patient-centered medical home employs open-access scheduling, remote monitoring, and telemedicine. A patient’s team includes a social worker, a nurse practitioner, a dietitian, a psychiatrist, and other specialists. The gastroenterologist acts as a primary care physician, according to Miguel Regueiro, MD, AGAF, medical director of the IBD Center at the University of Pittsburgh Medical Center, who presented the outcomes at the annual meeting of the American College of Gastroenterology.
Patients were eligible if at least 25% of their health care expenditures in the prior year were related to Crohn’s disease or ulcerative colitis.
The team enrolled 308 patients in the first year, with 290 remaining by the end of the year. Quality of life, as measured by the Short Inflammatory Bowel Disease Questionnaire, rose from a mean score of 7.1 to 9.1 points by the end of the study. There was a 51.9% decrease in ED visits (322 to 155), and a 53.1% reduction in hospitalizations (160 to 75), compared with the previous year.
Among patients who had been in the program for at least 6 months, ED visits dropped by 47% (197 to 116; P = .001) and hospitalizations dropped by 44% (100 to 56; P less than .005).
Physicians must also rise to the challenge. “The majority of gastroenterologists wouldn’t want to do this because it puts responsibility on them for the whole patient rather than just the disease,” said Dr. Regueiro.
But that discomfort shouldn’t sway them from making necessary changes. Rising health care costs could force specialists into new roles. “The way I look at it, if we don’t do something like this, the insurance companies will tell us what to do,” said Dr. Regueiro.
Dr. Regueiro is a member of advisory boards for AbbVie, Jansen, Takeda, UCB, and Pfizer. Dr. Quigley reported no conflicts of interest.
LAS VEGAS – A collaborative care approach to patients with inflammatory bowel disease (IBD) dramatically reduced emergency department visits and hospitalizations and also boosted quality of life.
The IBD patient-centered medical home employs open-access scheduling, remote monitoring, and telemedicine. A patient’s team includes a social worker, a nurse practitioner, a dietitian, a psychiatrist, and other specialists. The gastroenterologist acts as a primary care physician, according to Miguel Regueiro, MD, AGAF, medical director of the IBD Center at the University of Pittsburgh Medical Center, who presented the outcomes at the annual meeting of the American College of Gastroenterology.
Patients were eligible if at least 25% of their health care expenditures in the prior year were related to Crohn’s disease or ulcerative colitis.
The team enrolled 308 patients in the first year, with 290 remaining by the end of the year. Quality of life, as measured by the Short Inflammatory Bowel Disease Questionnaire, rose from a mean score of 7.1 to 9.1 points by the end of the study. There was a 51.9% decrease in ED visits (322 to 155), and a 53.1% reduction in hospitalizations (160 to 75), compared with the previous year.
Among patients who had been in the program for at least 6 months, ED visits dropped by 47% (197 to 116; P = .001) and hospitalizations dropped by 44% (100 to 56; P less than .005).
Physicians must also rise to the challenge. “The majority of gastroenterologists wouldn’t want to do this because it puts responsibility on them for the whole patient rather than just the disease,” said Dr. Regueiro.
But that discomfort shouldn’t sway them from making necessary changes. Rising health care costs could force specialists into new roles. “The way I look at it, if we don’t do something like this, the insurance companies will tell us what to do,” said Dr. Regueiro.
Dr. Regueiro is a member of advisory boards for AbbVie, Jansen, Takeda, UCB, and Pfizer. Dr. Quigley reported no conflicts of interest.
LAS VEGAS – A collaborative care approach to patients with inflammatory bowel disease (IBD) dramatically reduced emergency department visits and hospitalizations and also boosted quality of life.
The IBD patient-centered medical home employs open-access scheduling, remote monitoring, and telemedicine. A patient’s team includes a social worker, a nurse practitioner, a dietitian, a psychiatrist, and other specialists. The gastroenterologist acts as a primary care physician, according to Miguel Regueiro, MD, AGAF, medical director of the IBD Center at the University of Pittsburgh Medical Center, who presented the outcomes at the annual meeting of the American College of Gastroenterology.
Patients were eligible if at least 25% of their health care expenditures in the prior year were related to Crohn’s disease or ulcerative colitis.
The team enrolled 308 patients in the first year, with 290 remaining by the end of the year. Quality of life, as measured by the Short Inflammatory Bowel Disease Questionnaire, rose from a mean score of 7.1 to 9.1 points by the end of the study. There was a 51.9% decrease in ED visits (322 to 155), and a 53.1% reduction in hospitalizations (160 to 75), compared with the previous year.
Among patients who had been in the program for at least 6 months, ED visits dropped by 47% (197 to 116; P = .001) and hospitalizations dropped by 44% (100 to 56; P less than .005).
Physicians must also rise to the challenge. “The majority of gastroenterologists wouldn’t want to do this because it puts responsibility on them for the whole patient rather than just the disease,” said Dr. Regueiro.
But that discomfort shouldn’t sway them from making necessary changes. Rising health care costs could force specialists into new roles. “The way I look at it, if we don’t do something like this, the insurance companies will tell us what to do,” said Dr. Regueiro.
Dr. Regueiro is a member of advisory boards for AbbVie, Jansen, Takeda, UCB, and Pfizer. Dr. Quigley reported no conflicts of interest.
AT ACG 2016
Key clinical point:
Major finding: ED visits were cut by 51.9% and hospitalizations by 53.1% compared to the previous year.
Data source: Observational study.
Disclosures: Dr. Regueiro is a member of advisory boards for AbbVie, Jansen, Takeda, UCB, and Pfizer. Dr. Quigley reported no conflicts of interest.
Ozanimod has lasting effect on ulcerative colitis
LAS VEGAS – Ozanimod, an oral agent that selectively modulates the sphingosine 1-phosphate (S1P) 1 and 5 receptor, has a lasting effect on symptoms of ulcerative colitis, according to results from an open-label extension study.
The study extends the phase II TOUCHSTONE trial, in which patients with ulcerative colitis showed significant clinical improvement out to 32 weeks. The current study showed those improvements lasting out to at least 1 year, with about 80% of patients staying on the drug at the end of the study.
With other drug regimens, “the loss rates are at least 50% of the patients, so this is a remarkable level of durability over time,” William Sandborn, MD, chief of gastroenterology at the University of California at San Diego, said at the annual meeting of the American College of Gastroenterology. “With biologics, if you follow patients out for a year or 2, you see a fair amount of loss of response and some of that probably has to do with the formation of antidrug antibodies and immunogenicity,” Dr. Sandborn added.
In the original TOUCHSTONE study, 197 patients were randomized to placebo, ozanimod 0.5 mg, or ozanimod 1.0 mg, and followed out to week 32. Twenty-one percent of those in the 1.0-mg group achieved clinical remission, compared with 26% in the 0.5-mg group and 6% of those receiving placebo. Clinical response rates were 51%, 35%, and 20%, respectively.
In the open-label study, patients from all arms who did not respond to treatment after the induction phase, or relapsed during the maintenance phase, or completed the maintenance phase (170 patients in total) entered the open-label study with a dose of 1.0 mg ozanimod. At the time of the cut-off, patients in the extension study had been taking ozanimod for at least 1 year.
At the start of the extension study, the partial Mayo Score (pMS) for patients on placebo, ozanimod 0.5 mg, and 1.0 mg were 4.6, 4.5, and 3.3, respectively. All groups showed improvement in pMS by week 44 (1.7, 1.7, and 1.9, respectively)
At week 44, 90.9% of patients had little or no active disease (physician global assessment 0 or 1), 98.4% had little or no blood in their stools, and 84.7% had no blood in their stools.
Adverse events with a frequency higher than 2% included ulcerative colitis flare (5.9%), anemia (3.5%), upper respiratory tract infection (4.1%), nasal pharyngitis (3.5%), back pain (2.9%), arthralgia (2.4%), and headache (2.4%). The researchers noted some transient elevation of alanine aminotransferase or aspartate aminotransferase, but these elevations were temporary and reversed during ongoing treatment; 2.4% of patients experienced ALT and AST levels higher than three times the upper limit of normal, and all were asymptomatic.
Serious adverse events that occurred in two or more patients included anemia (1.2%) and ulcerative colitis flare (2.4%).
“This is a promising oral product with a similar mechanism of action to other lymphocyte trafficking agents,” said Stephen Hanauer, MD, medical director of the digestive health center at the Northwestern University, Chicago, who attended the session.
Ozanimod and other lymphocyte trafficking agents may offer a slightly different profile than some of the other drug classes, such as the anti–tumor necrosis factor agents, according to Dr. Hanauer, because the agents don’t affect lymphocytes already in the tissues. On the other hand, once the drug has acted, its effect may linger. “The time to effect may be a little slower, but the long-term effect seems to be as good or better as other mechanisms of action.”
The drug’s real place could be in early disease, Dr. Hanauer said. “If this is truly an effective and safe agent, the real positioning should be earlier in the disease, before patients are exposed to steroids and other immune suppressants, or biologics that have an infection risk.”
Celgene funded the study. Dr. Sandborn has received funding from Receptos and Celgene and consulted for both companies. Dr. Hanauer has consulted with Receptos, Celgene, Pfizer, Jansen, and AbbVie.
LAS VEGAS – Ozanimod, an oral agent that selectively modulates the sphingosine 1-phosphate (S1P) 1 and 5 receptor, has a lasting effect on symptoms of ulcerative colitis, according to results from an open-label extension study.
The study extends the phase II TOUCHSTONE trial, in which patients with ulcerative colitis showed significant clinical improvement out to 32 weeks. The current study showed those improvements lasting out to at least 1 year, with about 80% of patients staying on the drug at the end of the study.
With other drug regimens, “the loss rates are at least 50% of the patients, so this is a remarkable level of durability over time,” William Sandborn, MD, chief of gastroenterology at the University of California at San Diego, said at the annual meeting of the American College of Gastroenterology. “With biologics, if you follow patients out for a year or 2, you see a fair amount of loss of response and some of that probably has to do with the formation of antidrug antibodies and immunogenicity,” Dr. Sandborn added.
In the original TOUCHSTONE study, 197 patients were randomized to placebo, ozanimod 0.5 mg, or ozanimod 1.0 mg, and followed out to week 32. Twenty-one percent of those in the 1.0-mg group achieved clinical remission, compared with 26% in the 0.5-mg group and 6% of those receiving placebo. Clinical response rates were 51%, 35%, and 20%, respectively.
In the open-label study, patients from all arms who did not respond to treatment after the induction phase, or relapsed during the maintenance phase, or completed the maintenance phase (170 patients in total) entered the open-label study with a dose of 1.0 mg ozanimod. At the time of the cut-off, patients in the extension study had been taking ozanimod for at least 1 year.
At the start of the extension study, the partial Mayo Score (pMS) for patients on placebo, ozanimod 0.5 mg, and 1.0 mg were 4.6, 4.5, and 3.3, respectively. All groups showed improvement in pMS by week 44 (1.7, 1.7, and 1.9, respectively)
At week 44, 90.9% of patients had little or no active disease (physician global assessment 0 or 1), 98.4% had little or no blood in their stools, and 84.7% had no blood in their stools.
Adverse events with a frequency higher than 2% included ulcerative colitis flare (5.9%), anemia (3.5%), upper respiratory tract infection (4.1%), nasal pharyngitis (3.5%), back pain (2.9%), arthralgia (2.4%), and headache (2.4%). The researchers noted some transient elevation of alanine aminotransferase or aspartate aminotransferase, but these elevations were temporary and reversed during ongoing treatment; 2.4% of patients experienced ALT and AST levels higher than three times the upper limit of normal, and all were asymptomatic.
Serious adverse events that occurred in two or more patients included anemia (1.2%) and ulcerative colitis flare (2.4%).
“This is a promising oral product with a similar mechanism of action to other lymphocyte trafficking agents,” said Stephen Hanauer, MD, medical director of the digestive health center at the Northwestern University, Chicago, who attended the session.
Ozanimod and other lymphocyte trafficking agents may offer a slightly different profile than some of the other drug classes, such as the anti–tumor necrosis factor agents, according to Dr. Hanauer, because the agents don’t affect lymphocytes already in the tissues. On the other hand, once the drug has acted, its effect may linger. “The time to effect may be a little slower, but the long-term effect seems to be as good or better as other mechanisms of action.”
The drug’s real place could be in early disease, Dr. Hanauer said. “If this is truly an effective and safe agent, the real positioning should be earlier in the disease, before patients are exposed to steroids and other immune suppressants, or biologics that have an infection risk.”
Celgene funded the study. Dr. Sandborn has received funding from Receptos and Celgene and consulted for both companies. Dr. Hanauer has consulted with Receptos, Celgene, Pfizer, Jansen, and AbbVie.
LAS VEGAS – Ozanimod, an oral agent that selectively modulates the sphingosine 1-phosphate (S1P) 1 and 5 receptor, has a lasting effect on symptoms of ulcerative colitis, according to results from an open-label extension study.
The study extends the phase II TOUCHSTONE trial, in which patients with ulcerative colitis showed significant clinical improvement out to 32 weeks. The current study showed those improvements lasting out to at least 1 year, with about 80% of patients staying on the drug at the end of the study.
With other drug regimens, “the loss rates are at least 50% of the patients, so this is a remarkable level of durability over time,” William Sandborn, MD, chief of gastroenterology at the University of California at San Diego, said at the annual meeting of the American College of Gastroenterology. “With biologics, if you follow patients out for a year or 2, you see a fair amount of loss of response and some of that probably has to do with the formation of antidrug antibodies and immunogenicity,” Dr. Sandborn added.
In the original TOUCHSTONE study, 197 patients were randomized to placebo, ozanimod 0.5 mg, or ozanimod 1.0 mg, and followed out to week 32. Twenty-one percent of those in the 1.0-mg group achieved clinical remission, compared with 26% in the 0.5-mg group and 6% of those receiving placebo. Clinical response rates were 51%, 35%, and 20%, respectively.
In the open-label study, patients from all arms who did not respond to treatment after the induction phase, or relapsed during the maintenance phase, or completed the maintenance phase (170 patients in total) entered the open-label study with a dose of 1.0 mg ozanimod. At the time of the cut-off, patients in the extension study had been taking ozanimod for at least 1 year.
At the start of the extension study, the partial Mayo Score (pMS) for patients on placebo, ozanimod 0.5 mg, and 1.0 mg were 4.6, 4.5, and 3.3, respectively. All groups showed improvement in pMS by week 44 (1.7, 1.7, and 1.9, respectively)
At week 44, 90.9% of patients had little or no active disease (physician global assessment 0 or 1), 98.4% had little or no blood in their stools, and 84.7% had no blood in their stools.
Adverse events with a frequency higher than 2% included ulcerative colitis flare (5.9%), anemia (3.5%), upper respiratory tract infection (4.1%), nasal pharyngitis (3.5%), back pain (2.9%), arthralgia (2.4%), and headache (2.4%). The researchers noted some transient elevation of alanine aminotransferase or aspartate aminotransferase, but these elevations were temporary and reversed during ongoing treatment; 2.4% of patients experienced ALT and AST levels higher than three times the upper limit of normal, and all were asymptomatic.
Serious adverse events that occurred in two or more patients included anemia (1.2%) and ulcerative colitis flare (2.4%).
“This is a promising oral product with a similar mechanism of action to other lymphocyte trafficking agents,” said Stephen Hanauer, MD, medical director of the digestive health center at the Northwestern University, Chicago, who attended the session.
Ozanimod and other lymphocyte trafficking agents may offer a slightly different profile than some of the other drug classes, such as the anti–tumor necrosis factor agents, according to Dr. Hanauer, because the agents don’t affect lymphocytes already in the tissues. On the other hand, once the drug has acted, its effect may linger. “The time to effect may be a little slower, but the long-term effect seems to be as good or better as other mechanisms of action.”
The drug’s real place could be in early disease, Dr. Hanauer said. “If this is truly an effective and safe agent, the real positioning should be earlier in the disease, before patients are exposed to steroids and other immune suppressants, or biologics that have an infection risk.”
Celgene funded the study. Dr. Sandborn has received funding from Receptos and Celgene and consulted for both companies. Dr. Hanauer has consulted with Receptos, Celgene, Pfizer, Jansen, and AbbVie.
AT ACG 2016
Key clinical point:
Major finding: Ozanimod maintains efficacy in ulcerative colitis out to 1 year, with 90% of patients having little or no evidence of active disease.
Data source: Open-label extension study following a phase II clinical trial.
Disclosures: Celgene funded the study. Dr. Sandborn has received funding from Receptos and Celgene and consulted for both companies. Dr. Hanauer has consulted with Receptos, Celgene, Pfizer, Jansen, and AbbVie.
Pregabalin reduces pain in IBS patients
LAS VEGAS – Pregabalin reduced abdominal pain in patients with irritable bowel syndrome (IBS) and moderate to severe abdominal pain, according to a study presented at the annual meeting of the American College of Gastroenterology.
Antispasmodics and neuromodulators are commonly used to treat such patients, but a significant number don’t respond to these agents, and opioids carry risks of addiction.
The drug makes sense for IBS patients experiencing significant pain, according to Yuri Saito, MD, of the department of medicine and a consultant in the division of gastroenterology at the Mayo Clinic. Rochester, Minn., who presented the research. She noted that pregabalin is approved by the Food and Drug Administration for fibromyalgia, which occurs in many IBS patients. IBS patients also experience frequent anxiety, which can exacerbate symptoms. Pregabalin is not approved for anxiety, but is often prescribed off label. “We thought there were multiple reasons why pregabalin would potentially be effective in IBS,” Dr. Saito said in an interview.
Patients taking pregabalin (n = 41) had lower Bowel Symptom Scale (BSS) pain scores than did placebo (n = 44; 25 vs. 42; P =.008) and lower overall BSS severity scores at weeks 9-12 (26 vs. 42; P =.009). BSS diarrhea scores were lower in pregabalin (17 vs. 32; P = .049), as were bloating BSS scores (29 vs. 44; P =.016).
The study focused on patients with moderate to severe pain, who had experienced three or more pain attacks in a month, and at least one attack during a 2-week screening period. The pregabalin dosage began at 75 mg twice per day and was ramped up to 225 mg twice per day. That dosage was maintained from day 7 through week 12.
Somewhat disappointingly, the researchers found no difference in quality of life measures, but the presence of fibromyalgia may have complicated those measures, Dr. Gerson said.
Thirty-two percent of subjects in the pregabalin arm experienced dizziness, compared with 5% in the placebo group (P =.01). Other side effects included blurred vision (15% vs. 2%; P =.05) and feeling high or tipsy (10% vs. 0%; P =.05).
The results are encouraging and provide an additional treatment option. “I think it’s probably useful, but mainly in patients with diarrhea-prominent IBS,” said Dr. Gerson.
Dr. Saito was more effusive: “The take-home message is that, for patients with moderate to severe pain who have not responded to antispasmodics or other neuromodulators, Pregabalin may be useful as an alternate modality.”
Dr. Saito is an adviser or board member with Commonwealth Labs, Salix, and Synergy. Dr. Gerson is on Allergan’s speakers bureau.
LAS VEGAS – Pregabalin reduced abdominal pain in patients with irritable bowel syndrome (IBS) and moderate to severe abdominal pain, according to a study presented at the annual meeting of the American College of Gastroenterology.
Antispasmodics and neuromodulators are commonly used to treat such patients, but a significant number don’t respond to these agents, and opioids carry risks of addiction.
The drug makes sense for IBS patients experiencing significant pain, according to Yuri Saito, MD, of the department of medicine and a consultant in the division of gastroenterology at the Mayo Clinic. Rochester, Minn., who presented the research. She noted that pregabalin is approved by the Food and Drug Administration for fibromyalgia, which occurs in many IBS patients. IBS patients also experience frequent anxiety, which can exacerbate symptoms. Pregabalin is not approved for anxiety, but is often prescribed off label. “We thought there were multiple reasons why pregabalin would potentially be effective in IBS,” Dr. Saito said in an interview.
Patients taking pregabalin (n = 41) had lower Bowel Symptom Scale (BSS) pain scores than did placebo (n = 44; 25 vs. 42; P =.008) and lower overall BSS severity scores at weeks 9-12 (26 vs. 42; P =.009). BSS diarrhea scores were lower in pregabalin (17 vs. 32; P = .049), as were bloating BSS scores (29 vs. 44; P =.016).
The study focused on patients with moderate to severe pain, who had experienced three or more pain attacks in a month, and at least one attack during a 2-week screening period. The pregabalin dosage began at 75 mg twice per day and was ramped up to 225 mg twice per day. That dosage was maintained from day 7 through week 12.
Somewhat disappointingly, the researchers found no difference in quality of life measures, but the presence of fibromyalgia may have complicated those measures, Dr. Gerson said.
Thirty-two percent of subjects in the pregabalin arm experienced dizziness, compared with 5% in the placebo group (P =.01). Other side effects included blurred vision (15% vs. 2%; P =.05) and feeling high or tipsy (10% vs. 0%; P =.05).
The results are encouraging and provide an additional treatment option. “I think it’s probably useful, but mainly in patients with diarrhea-prominent IBS,” said Dr. Gerson.
Dr. Saito was more effusive: “The take-home message is that, for patients with moderate to severe pain who have not responded to antispasmodics or other neuromodulators, Pregabalin may be useful as an alternate modality.”
Dr. Saito is an adviser or board member with Commonwealth Labs, Salix, and Synergy. Dr. Gerson is on Allergan’s speakers bureau.
LAS VEGAS – Pregabalin reduced abdominal pain in patients with irritable bowel syndrome (IBS) and moderate to severe abdominal pain, according to a study presented at the annual meeting of the American College of Gastroenterology.
Antispasmodics and neuromodulators are commonly used to treat such patients, but a significant number don’t respond to these agents, and opioids carry risks of addiction.
The drug makes sense for IBS patients experiencing significant pain, according to Yuri Saito, MD, of the department of medicine and a consultant in the division of gastroenterology at the Mayo Clinic. Rochester, Minn., who presented the research. She noted that pregabalin is approved by the Food and Drug Administration for fibromyalgia, which occurs in many IBS patients. IBS patients also experience frequent anxiety, which can exacerbate symptoms. Pregabalin is not approved for anxiety, but is often prescribed off label. “We thought there were multiple reasons why pregabalin would potentially be effective in IBS,” Dr. Saito said in an interview.
Patients taking pregabalin (n = 41) had lower Bowel Symptom Scale (BSS) pain scores than did placebo (n = 44; 25 vs. 42; P =.008) and lower overall BSS severity scores at weeks 9-12 (26 vs. 42; P =.009). BSS diarrhea scores were lower in pregabalin (17 vs. 32; P = .049), as were bloating BSS scores (29 vs. 44; P =.016).
The study focused on patients with moderate to severe pain, who had experienced three or more pain attacks in a month, and at least one attack during a 2-week screening period. The pregabalin dosage began at 75 mg twice per day and was ramped up to 225 mg twice per day. That dosage was maintained from day 7 through week 12.
Somewhat disappointingly, the researchers found no difference in quality of life measures, but the presence of fibromyalgia may have complicated those measures, Dr. Gerson said.
Thirty-two percent of subjects in the pregabalin arm experienced dizziness, compared with 5% in the placebo group (P =.01). Other side effects included blurred vision (15% vs. 2%; P =.05) and feeling high or tipsy (10% vs. 0%; P =.05).
The results are encouraging and provide an additional treatment option. “I think it’s probably useful, but mainly in patients with diarrhea-prominent IBS,” said Dr. Gerson.
Dr. Saito was more effusive: “The take-home message is that, for patients with moderate to severe pain who have not responded to antispasmodics or other neuromodulators, Pregabalin may be useful as an alternate modality.”
Dr. Saito is an adviser or board member with Commonwealth Labs, Salix, and Synergy. Dr. Gerson is on Allergan’s speakers bureau.
AT ACG 2016
Key clinical point:
Major finding: In a pilot study, pregabalin reduced pain scores and diarrhea in patients with IBS and moderate to severe abdominal pain.
Data source: A randomized, placebo controlled clinical trial.
Disclosures: The study was funded by Pfizer. Dr. Saito is an adviser or board member with Commonwealth Labs, Salix, and Synergy. Dr. Gerson is on Allergan’s speakers bureau.