User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Practical, evidence-based, peer-reviewed solutions to common clinical problems in psychiatry
Adult ADHD: A sensible approach to diagnosis and treatment
Attention-deficit/hyperactivity disorder (ADHD) is common, with an estimated worldwide prevalence of 5.29% among children and adolescents and 2.5% among adults.1 DSM-5-TR classifies ADHD as a neurodevelopmental disorder, “a group of conditions with onset in the developmental period [that] typically manifest early in development, often before the child enters school.”2 Because of the expectation that ADHD symptoms emerge early in development, the diagnostic criteria specify that symptoms must have been present prior to age 12 to qualify as ADHD. However, recent years have shown a significant increase in the number of patients being diagnosed with ADHD for the first time in adulthood. One study found that the diagnosis of ADHD among adults in the United States doubled between 2007 and 2016.3
First-line treatment for ADHD is the stimulants methylphenidate and amphetamine/dextroamphetamine. In the United States, these medications are classified as Schedule II controlled substances, indicating a high risk for abuse. However, just as ADHD diagnoses among adults have increased, so have prescriptions for stimulants. For example, Olfson et al4 found that stimulant prescriptions among young adults increased by a factor of 10 between 1994 and 2009.
The increased prevalence of adult patients diagnosed with ADHD and taking stimulants frequently places clinicians in a position to consider the validity of existing diagnoses and evaluate new patients with ADHD-related concerns. In this article, we review some of the challenges associated with diagnosing ADHD in adults, discuss the risks of stimulant treatment, and present a practical approach to the diagnosis and treatment of ADHD in adults.
Challenges in diagnosis
DSM-5-TR diagnostic criteria for ADHD are summarized in Table 1. Establishing a diagnosis of adult ADHD can be challenging. As with many psychiatric conditions, symptoms of ADHD are highly subjective. Retrospectively diagnosing a developmental condition in adults is often biased by the patient’s current functioning.5 ADHD has a high heritability and adults may inquire about the diagnosis if their children are diagnosed with ADHD.6 Some experts have cautioned that clinicians must be careful in diagnosing ADHD in adults.7 Just as there are risks associated with underdiagnosing ADHD, there are risks associated with overdiagnosis. Overdiagnosis may medicalize normal variants in the population and lead to unnecessary treatment and a misappropriation of limited medical resources.8 Many false positive cases of late-onset ADHD may be attributable to nonimpairing cognitive fluctuations.9

Poor diagnostic practices can impede accuracy in establishing the presence or absence of ADHD. Unfortunately, methods of diagnosing adult ADHD have been shown to vary widely in terms of information sources, diagnostic instruments used, symptom threshold, and whether functional impairment is a requirement for diagnosis.10 A common practice in diagnosing adult ADHD involves asking patients to complete self-report questionnaires that list symptoms of ADHD, such as the Adult ADHD Self-Report Scale developed by the World Health Organization.11 However, self-reports of ADHD in adults are less reliable than informant reports, and some young adults without ADHD overreport symptoms.12,13 Symptom checklists are particularly susceptible to faking, which lessens their diagnostic value.14
The possibility of malingered symptoms of ADHD further increases the diagnostic difficulty. College students may be particularly susceptible to overreporting ADHD symptoms in order to obtain academic accommodations or stimulants in the hopes of improving school performance.15 One study found that 25% to 48% of college students self-referred for ADHD evaluations exaggerated their symptoms.16 In another study, 31% of adults failed the Word Memory Test, which suggests noncredible performance in their ADHD evaluation.17 College students can successfully feign ADHD symptoms in both self-reported symptoms and computer-based tests of attention.18 Harrison et al19 summarized many of these concerns in their 2007 study of ADHD malingering, noting the “almost perfect ability of the Faking group to choose items … that correspond to the DSM-IV symptoms, and to report these at levels even higher than persons with diagnosed ADHD.” They suggested “Clinicians should be suspicious of students or young adults presenting for a first-time diagnosis who rate themselves as being significantly symptomatic, yet have managed to achieve well in school and other life activities.”19
Another challenge in correctly diagnosing adult ADHD is identifying other conditions that may impair attention.20 Psychiatric conditions that may impair concentration include anxiety disorders, chronic stress, posttraumatic stress disorder, recent trauma, major depressive disorder (MDD), and bipolar disorder (BD). Undiagnosed learning disorders may present like ADHD. Focus can be negatively affected by sleep disorders such as sleep apnea, restless leg syndrome, or delayed sleep phase-onset disorder. Marijuana, cocaine, 3,4-methylenedioxy-methamphetamine (MDMA; “ecstasy”), caffeine, or prescription medications such as anticholinergics can also impair attention. Medical conditions that can present with attentional or executive functioning deficits include seizures, Lyme disease, HIV, encephalopathy, hypothyroidism, and “chemo brain.”21 Environmental factors such as age-related cognitive decline, sleep deprivation, inflammation, obesity, air pollution, chemical exposure, and excessive use of digital media may also produce symptoms similar to ADHD. Two studies of adult-onset ADHD concluded that 93% to 95% of cases were better explained by other conditions such as sleep disorders, substance use disorders, or another psychiatric disorder.22
Continue to: Risks associated with treatment
Risks associated with treatment
With or without an accurate ADHD diagnosis, prescribing stimulants presents certain risks (Table 223-40). One of the more well-known risks of stimulants is addiction or misuse.23 An estimated 5 million American adults misused prescription stimulants in 2016.24 Despite stimulants’ status as controlled substances, long-term concurrent use of stimulants with opioids is common among adults with ADHD.25 College students are particularly susceptible to misusing or diverting stimulants, often to improve their academic performance.26 At 1 university, 22% of students had misused stimulants in the past year.27 Prescribing short-acting stimulants (rather than extended-release formulations) increases the likelihood of misuse.28 Patients prescribed stimulants begin to receive requests to divert their medications to others as early as elementary school, and by college more than one-third of those taking stimulants have been asked to give, sell, or trade their medications.29 Diversion of stimulants by students with ADHD is prevalent, with 62% of patients engaging in diversion during their lifetime.15 Diverted stimulants can come from family members, black market sources, or deceived clinicians.30 Although students’ stimulant misuse/diversion often is academically motivated, nonmedical use of psychostimulants does not appear to have a statistically significant effect on improving grade point average.31 Despite a negligible impact on grades, most students who take stimulants identify their effect as strongly positive, producing a situation in which misusers of stimulants have little motivation to stop.32 While some patients might ask for a stimulant prescription with the rationale that liking the effects proves they have ADHD, this is inappropriate because most individuals like the effects of stimulant medications.33
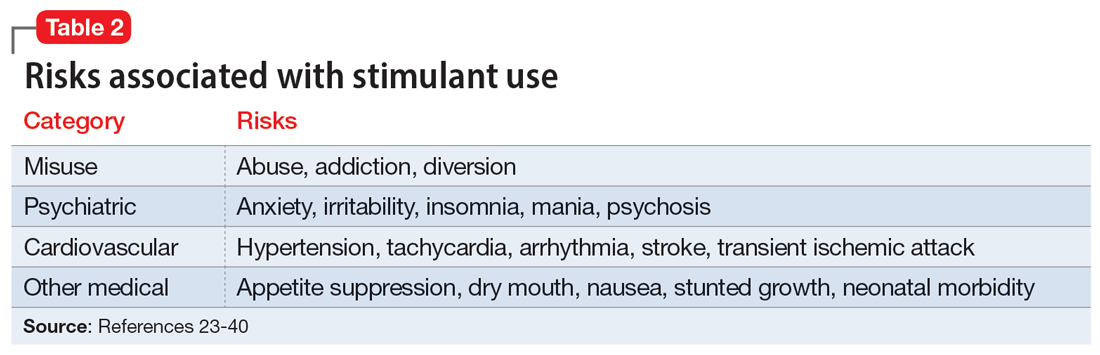
The use of stimulants increases the risk for several adverse psychiatric outcomes. Stimulants increase the risk of anxiety, so exercise caution when prescribing to patients with a comorbid anxiety disorder.34 Stimulants can also worsen irritability and insomnia, 2 issues common among patients with ADHD.32 Use of stimulant medications can trigger manic episodes. Viktorin et al35 found a >6-fold increase in manic episodes among patients with BD receiving methylphenidate monotherapy compared to those receiving a combination of methylphenidate and a mood stabilizer.35 The use of methylphenidate and amphetamine can lead to new-onset psychosis (or exacerbation of pre-existing psychotic illness); amphetamine use is associated with a higher risk of psychosis than methylphenidate.36
General medical adverse effects are also possible with stimulant use. Stimulants’ adverse effect profiles include appetite suppression, dry mouth, and nausea. Long-term use poses a risk for stunting growth in children.1 Using stimulants during pregnancy is associated with higher risk for neonatal morbidity, including preterm birth, CNS-related disorders, and seizures.37 Stimulants can raise blood pressure and increase heart rate. Serious cardiovascular events associated with stimulant use include ventricular arrhythmias, strokes, and transient ischemic attacks.38
Nonstimulant ADHD treatments are less risky than stimulants but still require monitoring for common adverse effects. Atomoxetine has been associated with sedation, growth retardation (in children), and in severe cases, liver injury or suicidal ideation.39 Bupropion (commonly used off-label for ADHD) can lower the seizure threshold and cause irritability, anorexia, and insomnia.39 Viloxazine, a newer agent, can cause hypertension, increased heart rate, nausea, drowsiness, headache, and insomnia.40
Sensible diagnosing
Given the challenges in accurately diagnosing ADHD in adults, we present a sensible approach to making the diagnosis (Table 3). The first step is to rule out other conditions that might better explain the patient’s symptoms. A thorough clinical interview (including a psychiatric review of symptoms) is the cornerstone of an initial diagnostic assessment. The use of validated screening questionnaires such as the Patient Health Questionnaire-9 and General Anxiety Disorder-7 may also provide information regarding psychiatric conditions that require additional evaluation.
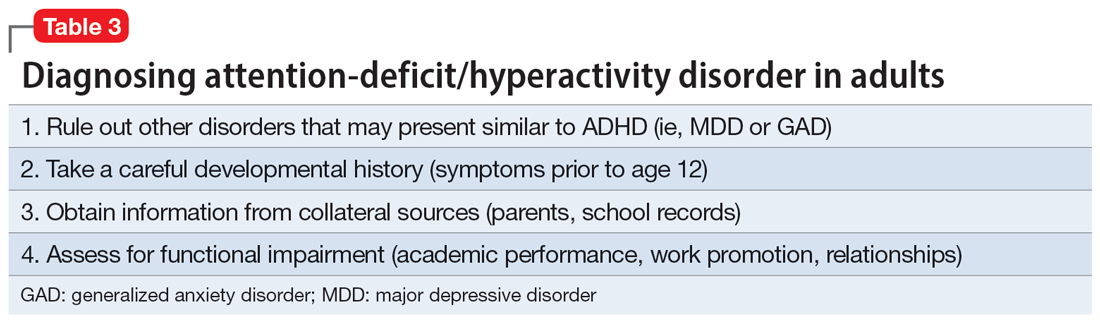
Continue to: Some of the most common conditions...
Some of the most common conditions we see mistaken for ADHD are MDD, generalized anxiety disorder (GAD), and BD. In DSM-5-TR, 1 of the diagnostic criteria for MDD is “diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).”41 Similarly, criteria for GAD include “difficulty concentrating.”42 DSM-5-TR also includes distractibility as one of the criteria for mania/hypomania. Table 420-22,41,42 lists other psychiatric, substance-related, medical, and environmental conditions that can produce ADHD-like symptoms. Referring to some medical and environmental explanations for inattention, Aiken22 pointed out, “Patients who suffer from these problems might ask their doctor for a stimulant, but none of those syndromes require a psychopharmacologic approach.” ADHD can be comorbid with other psychiatric conditions, so the presence of another psychiatric illness does not automatically rule out ADHD. If alternative psychiatric diagnoses have been identified, these can be discussed with the patient and treatment offered that targets the specified condition.
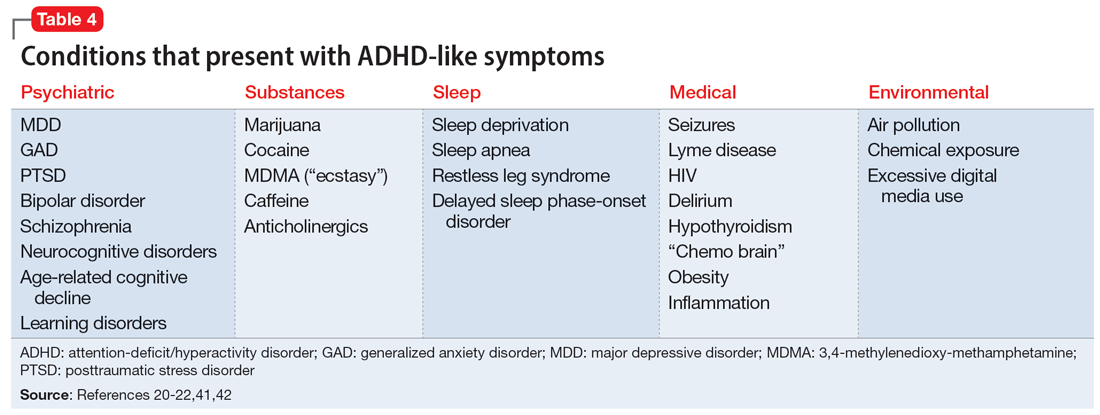
Once alternative explanations have been ruled out, focus on the patient’s developmental history. DSM-5-TR conceptualizes ADHD as a neurodevelopmental disorder, meaning it is expected to emerge early in life. Whereas previous editions of DSM specified that ADHD symptoms must be present before age 7, DSM-5 modified this age threshold to before age 12.1 This necessitates taking a careful life history in order to understand the presence or absence of symptoms at earlier developmental stages.5 ADHD should be verified by symptoms apparent in childhood and present across the lifespan.15
While this retrospective history is necessary, histories that rely on self-report alone are often unreliable. Collateral sources of information are generally more reliable when assessing for ADHD symptoms.13 Third-party sources can help confirm that any impairment is best attributed to ADHD rather than to another condition.15 Unfortunately, the difficulty of obtaining collateral information means it is often neglected, even in the literature.10 A parent is the ideal informant for gathering collateral information regarding a patient’s functioning in childhood.5 Suggested best practices also include obtaining collateral information from interviews with significant others, behavioral questionnaires completed by parents (for current and childhood symptoms), review of school records, and consideration of intellectual and achievement testing.43 If psychological testing is pursued, include validity testing to detect feigned symptoms.18,44
When evaluating for ADHD, assess not only for the presence of symptoms, but also if these symptoms produce significant functional impairment.13,15 Impairments in daily functioning can include impaired school participation, social participation, quality of relationships, family conflict, family activities, family functioning, and emotional functioning.45 Some symptoms may affect functioning in an adult’s life differently than they did during childhood, from missed work appointments to being late picking up kids from school. Research has shown that the correlation between the number of symptoms and functional impairment is weak, which means someone could experience all of the symptoms of ADHD without experiencing functional impairment.45 To make an accurate diagnosis, it is therefore important to clearly establish both the number of symptoms the patient is experiencing and whether these symptoms are clearly linked to functional impairments.10
Sensible treatment
Once a diagnosis of ADHD has been clearly established, clinicians need to consider how best to treat the condition (Table 5). Stimulants are generally considered first-line treatment for ADHD. In randomized clinical trials, they showed significant efficacy; for example, one study of 146 adults with ADHD found a 76% improvement with methylphenidate compared to 19% for the placebo group.46 Before starting a stimulant, certain comorbidities should be ruled out. If a patient has glaucoma or pheochromocytoma, they may first need treatment from or clearance by other specialists. Stimulants should likely be held in patients with hypertension, angina, or cardiovascular defects until receiving medical clearance. The risks of stimulants need to be discussed with female patients of childbearing age, weighing the benefits of treatment against the risks of medication use should the patient get pregnant. Patients with comorbid psychosis or uncontrolled bipolar illness should not receive stimulants due to the risk of exacerbation. Patients with active substance use disorders (SUDs) are generally not good candidates for stimulants because of the risk of misusing or diverting stimulants and the possibility that substance abuse may be causing their inattentive symptoms. Patients whose SUDs are in remission may cautiously be considered as candidates for stimulants. If patients misuse their prescribed stimulants, they should be switched to a nonstimulant medication such as atomoxetine, bupropion, guanfacine, or clonidine.47

Continue to: Once a patient is deemed...
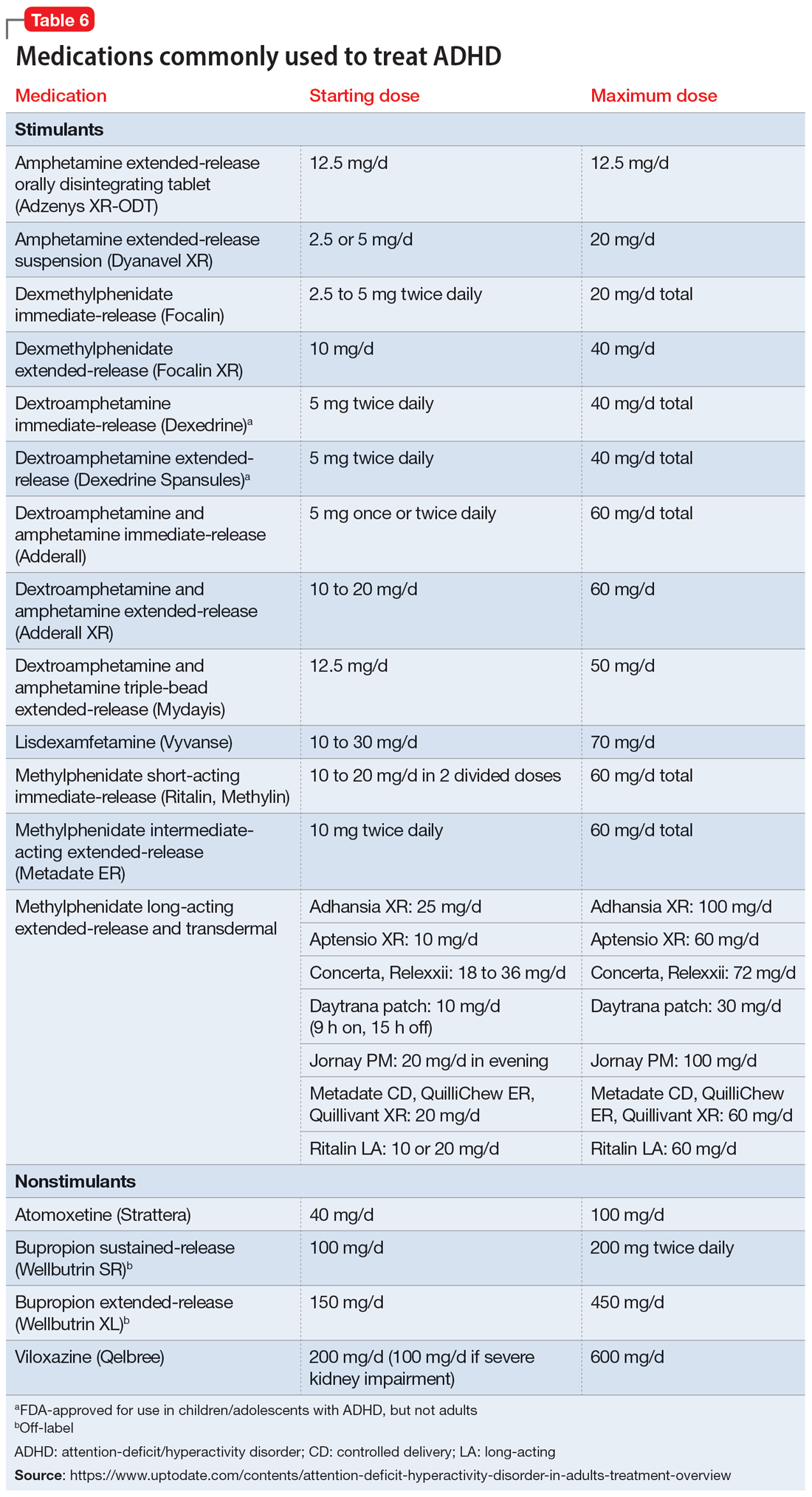
Once a patient is deemed to be a candidate for stimulants, clinicians need to choose between methylphenidate or amphetamine/dextroamphetamine formulations. Table 6 lists medications that are commonly prescribed to treat ADHD; unless otherwise noted, these are FDA-approved for this indication. As a general rule, for adults, long-acting stimulant formulations are preferred over short-acting formulations.28 Immediate-release stimulants are more prone to misuse or diversion compared to extended-release medications.29 Longer-acting formulations may also provide better full-day symptom control.48
In contrast to many other psychiatric medications, it may be beneficial to encourage periodically taking breaks or “medication holidays” from stimulants. Planned medication holidays for adults can involve intentionally not taking the medication over the weekend when the patient is not involved in work or school responsibilities. Such breaks have been shown to reduce adverse effects of stimulants (such as appetite suppression and insomnia) without significantly increasing ADHD symptoms.49 Short breaks can also help prevent medication tolerance and the subsequent need to increase doses.50 Medication holidays provide an opportunity to verify the ongoing benefits of the medication. It is advisable to periodically assess whether there is a continued need for stimulant treatment.51 If patients do not tolerate stimulants or have other contraindications, nonstimulants should be considered.
Lastly, no psychiatric patient should be treated with medication alone, and nonpharmacologic approaches should be incorporated as needed. Clear instructions, visual aids, nonverbal cues, frequent breaks to stand and stretch, schedules, normalizing failure as part of growth, and identifying triggers for emotional reactivity may help patients with ADHD.52 In a study of the academic performance of 92 college students taking medication for ADHD and 146 control students, treatment with stimulants alone did not eliminate the academic achievement deficit of those individuals with ADHD.53 Good study habits (even without stimulants) appeared more important in overcoming the achievement disparity of students with ADHD.53 Providing psychoeducation and training in concrete organization and planning skills have shown benefit.54 Practice of skills on a daily basis appears to be especially beneficial.55
Bottom Line
A sensible approach to diagnosing attention-deficit/hyperactivity disorder (ADHD) in adults includes ruling out other disorders that may present similar to ADHD, taking an appropriate developmental history, obtaining collateral information, and assessing for functional impairment. Sensible treatment involves ruling out comorbidities that stimulants could worsen, selecting extended-release stimulants, incorporating medication holidays, and using nonpharmacologic interventions.
Related Resources
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/ng87
- Substance Abuse and Mental Health Services Administration. Advisory: Prescription Stimulant Misuse Among Youth and Young Adults. https://store.samhsa.gov/product/prescription-stimulant-misuse-among-youth-young-adults/PEP21-06-01-003
Drug Brand Names
Amphetamine • Adzenys, Dyanavel, others
Atomoxetine • Strattera
Bupropion • Wellbutrin, Forfivo
Clonidine • Catapres, Kapvay
Dexmethylphenidate • Focalin
Dextroamphetamine • Dexedrine
Dextroamphetamine and amphetamine • Adderall, Mydayis
Guanfacine • Intuniv, Tenex
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin, others
Viloxazine • Qelbree
1. Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450-462.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:35.
3. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
4. Olfson M, Blanco C, Wang S, et al. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry. 2013;74(1):43-50.
5. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562-575.
7. Solanto MV. Child vs adult onset of attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(4):421.
8. Jummani RR, Hirsch E, Hirsch GS. Are we overdiagnosing and overtreating ADHD? Psychiatric Times. Published May 31, 2017. Accessed March 17, 2023. https://www.psychiatrictimes.com/view/are-we-overdiagnosing-and-overtreating-adhd
9. Sibley MH, Rohde LA, Swanson JM, et al; Multimodal Treatment Study of Children with ADHD (MTA) Cooperative Group. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149.
10. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165.
11. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527.
12. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry. 2016;73(7):655-656.
13. Sibley MH, Pelham WE, Molina BSG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052-1061.
14. Sollman MJ, Ranseen JD, Berry DT. Detection of feigned ADHD in college students. Psychol Assess. 2010;22(2):325-335.
15. Green AL, Rabiner DL. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9(3):559-568.
16. Sullivan BK, May K, Galbally L. Symptom exaggeration by college adults in attention-deficit hyperactivity disorder and learning disorder assessments. Appl Neuropsychol. 2007;14(3):189-207.
17. Suhr J, Hammers D, Dobbins-Buckland K, et al. The relationship of malingering test failure to self-reported symptoms and neuropsychological findings in adults referred for ADHD evaluation. Arch Clin Neuropsychol. 2008;23(5):521-530.
18. Lee Booksh R, Pella RD, Singh AN, et al. Ability of college students to simulate ADHD on objective measures of attention. J Atten Disord. 2010;13(4):325-338.
19. Harrison AG, Edwards MJ, Parker KC. Identifying students faking ADHD: preliminary findings and strategies for detection. Arch Clin Neuropsychol. 2007;22(5):577-588.
20. Lopez R, Micoulaud-Franchi JA, Galeria C, et al. Is adult-onset attention deficit/hyperactivity disorder frequent in clinical practice? Psychiatry Res. 2017;257:238-241.
21. Bhatia R. Rule out these causes of inattention before diagnosing ADHD. Current Psychiatry. 2016;15(10):32-33.
22. Aiken C. Adult-onset ADHD raises questions. Psychiatric Times. 2021;38(3):24.
23. Bjorn S, Weyandt LL. Issues pertaining to misuse of ADHD prescription medications. Psychiatric Times. 2018;35(9):17-19.
24. Compton WM, Han B, Blanco C, et al. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;175(8):741-755.
25. Wei YJ, Zhu Y, Liu W, et al. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw Open. 2018;1(4):e181152. doi:10.1001/jamanetworkopen.2018.1152
26. Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18(1):50-76.
27. Benson K, Woodlief DT, Flory K, et al. Is ADHD, independent of ODD, associated with whether and why college students misuse stimulant medication? Exp Clin Psychopharmacol. 2018;26(5):476-487.
28. Froehlich TE. ADHD medication adherence in college students-- a call to action for clinicians and researchers: commentary on “transition to college and adherence to prescribed attention deficit hyperactivity disorder medication.” J Dev Behav Pediatr. 2018;39(1):77-78.
29. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
30. Vrecko S. Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc Sci Med. 2015;131:297-304.
31. Munro BA, Weyandt LL, Marraccini ME, et al. The relationship between nonmedical use of prescription stimulants, executive functioning and academic outcomes. Addict Behav. 2017;65:250-257.
32. Rabiner DL, Anastopoulos AD, Costello EJ, et al. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord. 2009;13(3)259-270.
33. Tayag Y. Adult ADHD is the wild west of psychiatry. The Atlantic. Published April 14, 2023. Accessed May 3, 2023. https://www.theatlantic.com/health/archive/2023/04/adult-adhd-diagnosis-treatment-adderall-shortage/673719/
34. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270.
35. Viktorin A, Rydén E, Thase ME, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
36. Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019; 380(12):1128-1138.
37. Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140(6):e20170747. doi:10.1542/peds.2017-0747
38. Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10):e2130795. doi:10.1001/jamanetworkopen.2021.30795
39. Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48(3):240-248.
40. Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals; 2021.
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:183.
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:250.
43. DuPaul GJ, Weyandt LL, O’Dell SM, et al. College students with ADHD: current status and future directions. J Atten Disord. 2009;13(3):234-250.
44. Edmundson M, Berry DTR, Combs HL, et al. The effects of symptom information coaching on the feigning of adult ADHD. Psychol Assess. 2017;29(12):1429-1436.
45. Gordon M, Antshel K, Faraone S, et al. Symptoms versus impairment: the case for respecting DSM-IV’s criterion D. J Atten Disord. 2006;9(3):465-475.
46. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
47. Osser D, Awidi B. Treating adults with ADHD requires special considerations. Psychiatric News. Published August 30, 2018. Accessed March 17, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.pp8a1
48. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown, RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
49. Martins S, Tramontina S, Polanczyk G, et al. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14(2):195-206.
50. Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551-568.
51. Matthijssen AM, Dietrich A, Bierens M, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry. 2019;176(9):754-762.
52. Mason EJ, Joshi KG. Nonpharmacologic strategies for helping children with ADHD. Current Psychiatry. 2018;7(1):42,46.
53. Advokat C, Lane SM, Luo C. College students with and without ADHD: comparison of self-report of medication usage, study habits, and academic achievement. J Atten Disord. 2011;15(8):656-666.
54. Knouse LE, Cooper-Vince C, Sprich S, et al. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
55. Evans SW, Owens JS, Wymbs BT, et al. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157-198.
Attention-deficit/hyperactivity disorder (ADHD) is common, with an estimated worldwide prevalence of 5.29% among children and adolescents and 2.5% among adults.1 DSM-5-TR classifies ADHD as a neurodevelopmental disorder, “a group of conditions with onset in the developmental period [that] typically manifest early in development, often before the child enters school.”2 Because of the expectation that ADHD symptoms emerge early in development, the diagnostic criteria specify that symptoms must have been present prior to age 12 to qualify as ADHD. However, recent years have shown a significant increase in the number of patients being diagnosed with ADHD for the first time in adulthood. One study found that the diagnosis of ADHD among adults in the United States doubled between 2007 and 2016.3
First-line treatment for ADHD is the stimulants methylphenidate and amphetamine/dextroamphetamine. In the United States, these medications are classified as Schedule II controlled substances, indicating a high risk for abuse. However, just as ADHD diagnoses among adults have increased, so have prescriptions for stimulants. For example, Olfson et al4 found that stimulant prescriptions among young adults increased by a factor of 10 between 1994 and 2009.
The increased prevalence of adult patients diagnosed with ADHD and taking stimulants frequently places clinicians in a position to consider the validity of existing diagnoses and evaluate new patients with ADHD-related concerns. In this article, we review some of the challenges associated with diagnosing ADHD in adults, discuss the risks of stimulant treatment, and present a practical approach to the diagnosis and treatment of ADHD in adults.
Challenges in diagnosis
DSM-5-TR diagnostic criteria for ADHD are summarized in Table 1. Establishing a diagnosis of adult ADHD can be challenging. As with many psychiatric conditions, symptoms of ADHD are highly subjective. Retrospectively diagnosing a developmental condition in adults is often biased by the patient’s current functioning.5 ADHD has a high heritability and adults may inquire about the diagnosis if their children are diagnosed with ADHD.6 Some experts have cautioned that clinicians must be careful in diagnosing ADHD in adults.7 Just as there are risks associated with underdiagnosing ADHD, there are risks associated with overdiagnosis. Overdiagnosis may medicalize normal variants in the population and lead to unnecessary treatment and a misappropriation of limited medical resources.8 Many false positive cases of late-onset ADHD may be attributable to nonimpairing cognitive fluctuations.9

Poor diagnostic practices can impede accuracy in establishing the presence or absence of ADHD. Unfortunately, methods of diagnosing adult ADHD have been shown to vary widely in terms of information sources, diagnostic instruments used, symptom threshold, and whether functional impairment is a requirement for diagnosis.10 A common practice in diagnosing adult ADHD involves asking patients to complete self-report questionnaires that list symptoms of ADHD, such as the Adult ADHD Self-Report Scale developed by the World Health Organization.11 However, self-reports of ADHD in adults are less reliable than informant reports, and some young adults without ADHD overreport symptoms.12,13 Symptom checklists are particularly susceptible to faking, which lessens their diagnostic value.14
The possibility of malingered symptoms of ADHD further increases the diagnostic difficulty. College students may be particularly susceptible to overreporting ADHD symptoms in order to obtain academic accommodations or stimulants in the hopes of improving school performance.15 One study found that 25% to 48% of college students self-referred for ADHD evaluations exaggerated their symptoms.16 In another study, 31% of adults failed the Word Memory Test, which suggests noncredible performance in their ADHD evaluation.17 College students can successfully feign ADHD symptoms in both self-reported symptoms and computer-based tests of attention.18 Harrison et al19 summarized many of these concerns in their 2007 study of ADHD malingering, noting the “almost perfect ability of the Faking group to choose items … that correspond to the DSM-IV symptoms, and to report these at levels even higher than persons with diagnosed ADHD.” They suggested “Clinicians should be suspicious of students or young adults presenting for a first-time diagnosis who rate themselves as being significantly symptomatic, yet have managed to achieve well in school and other life activities.”19
Another challenge in correctly diagnosing adult ADHD is identifying other conditions that may impair attention.20 Psychiatric conditions that may impair concentration include anxiety disorders, chronic stress, posttraumatic stress disorder, recent trauma, major depressive disorder (MDD), and bipolar disorder (BD). Undiagnosed learning disorders may present like ADHD. Focus can be negatively affected by sleep disorders such as sleep apnea, restless leg syndrome, or delayed sleep phase-onset disorder. Marijuana, cocaine, 3,4-methylenedioxy-methamphetamine (MDMA; “ecstasy”), caffeine, or prescription medications such as anticholinergics can also impair attention. Medical conditions that can present with attentional or executive functioning deficits include seizures, Lyme disease, HIV, encephalopathy, hypothyroidism, and “chemo brain.”21 Environmental factors such as age-related cognitive decline, sleep deprivation, inflammation, obesity, air pollution, chemical exposure, and excessive use of digital media may also produce symptoms similar to ADHD. Two studies of adult-onset ADHD concluded that 93% to 95% of cases were better explained by other conditions such as sleep disorders, substance use disorders, or another psychiatric disorder.22
Continue to: Risks associated with treatment
Risks associated with treatment
With or without an accurate ADHD diagnosis, prescribing stimulants presents certain risks (Table 223-40). One of the more well-known risks of stimulants is addiction or misuse.23 An estimated 5 million American adults misused prescription stimulants in 2016.24 Despite stimulants’ status as controlled substances, long-term concurrent use of stimulants with opioids is common among adults with ADHD.25 College students are particularly susceptible to misusing or diverting stimulants, often to improve their academic performance.26 At 1 university, 22% of students had misused stimulants in the past year.27 Prescribing short-acting stimulants (rather than extended-release formulations) increases the likelihood of misuse.28 Patients prescribed stimulants begin to receive requests to divert their medications to others as early as elementary school, and by college more than one-third of those taking stimulants have been asked to give, sell, or trade their medications.29 Diversion of stimulants by students with ADHD is prevalent, with 62% of patients engaging in diversion during their lifetime.15 Diverted stimulants can come from family members, black market sources, or deceived clinicians.30 Although students’ stimulant misuse/diversion often is academically motivated, nonmedical use of psychostimulants does not appear to have a statistically significant effect on improving grade point average.31 Despite a negligible impact on grades, most students who take stimulants identify their effect as strongly positive, producing a situation in which misusers of stimulants have little motivation to stop.32 While some patients might ask for a stimulant prescription with the rationale that liking the effects proves they have ADHD, this is inappropriate because most individuals like the effects of stimulant medications.33

The use of stimulants increases the risk for several adverse psychiatric outcomes. Stimulants increase the risk of anxiety, so exercise caution when prescribing to patients with a comorbid anxiety disorder.34 Stimulants can also worsen irritability and insomnia, 2 issues common among patients with ADHD.32 Use of stimulant medications can trigger manic episodes. Viktorin et al35 found a >6-fold increase in manic episodes among patients with BD receiving methylphenidate monotherapy compared to those receiving a combination of methylphenidate and a mood stabilizer.35 The use of methylphenidate and amphetamine can lead to new-onset psychosis (or exacerbation of pre-existing psychotic illness); amphetamine use is associated with a higher risk of psychosis than methylphenidate.36
General medical adverse effects are also possible with stimulant use. Stimulants’ adverse effect profiles include appetite suppression, dry mouth, and nausea. Long-term use poses a risk for stunting growth in children.1 Using stimulants during pregnancy is associated with higher risk for neonatal morbidity, including preterm birth, CNS-related disorders, and seizures.37 Stimulants can raise blood pressure and increase heart rate. Serious cardiovascular events associated with stimulant use include ventricular arrhythmias, strokes, and transient ischemic attacks.38
Nonstimulant ADHD treatments are less risky than stimulants but still require monitoring for common adverse effects. Atomoxetine has been associated with sedation, growth retardation (in children), and in severe cases, liver injury or suicidal ideation.39 Bupropion (commonly used off-label for ADHD) can lower the seizure threshold and cause irritability, anorexia, and insomnia.39 Viloxazine, a newer agent, can cause hypertension, increased heart rate, nausea, drowsiness, headache, and insomnia.40
Sensible diagnosing
Given the challenges in accurately diagnosing ADHD in adults, we present a sensible approach to making the diagnosis (Table 3). The first step is to rule out other conditions that might better explain the patient’s symptoms. A thorough clinical interview (including a psychiatric review of symptoms) is the cornerstone of an initial diagnostic assessment. The use of validated screening questionnaires such as the Patient Health Questionnaire-9 and General Anxiety Disorder-7 may also provide information regarding psychiatric conditions that require additional evaluation.

Continue to: Some of the most common conditions...
Some of the most common conditions we see mistaken for ADHD are MDD, generalized anxiety disorder (GAD), and BD. In DSM-5-TR, 1 of the diagnostic criteria for MDD is “diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).”41 Similarly, criteria for GAD include “difficulty concentrating.”42 DSM-5-TR also includes distractibility as one of the criteria for mania/hypomania. Table 420-22,41,42 lists other psychiatric, substance-related, medical, and environmental conditions that can produce ADHD-like symptoms. Referring to some medical and environmental explanations for inattention, Aiken22 pointed out, “Patients who suffer from these problems might ask their doctor for a stimulant, but none of those syndromes require a psychopharmacologic approach.” ADHD can be comorbid with other psychiatric conditions, so the presence of another psychiatric illness does not automatically rule out ADHD. If alternative psychiatric diagnoses have been identified, these can be discussed with the patient and treatment offered that targets the specified condition.

Once alternative explanations have been ruled out, focus on the patient’s developmental history. DSM-5-TR conceptualizes ADHD as a neurodevelopmental disorder, meaning it is expected to emerge early in life. Whereas previous editions of DSM specified that ADHD symptoms must be present before age 7, DSM-5 modified this age threshold to before age 12.1 This necessitates taking a careful life history in order to understand the presence or absence of symptoms at earlier developmental stages.5 ADHD should be verified by symptoms apparent in childhood and present across the lifespan.15
While this retrospective history is necessary, histories that rely on self-report alone are often unreliable. Collateral sources of information are generally more reliable when assessing for ADHD symptoms.13 Third-party sources can help confirm that any impairment is best attributed to ADHD rather than to another condition.15 Unfortunately, the difficulty of obtaining collateral information means it is often neglected, even in the literature.10 A parent is the ideal informant for gathering collateral information regarding a patient’s functioning in childhood.5 Suggested best practices also include obtaining collateral information from interviews with significant others, behavioral questionnaires completed by parents (for current and childhood symptoms), review of school records, and consideration of intellectual and achievement testing.43 If psychological testing is pursued, include validity testing to detect feigned symptoms.18,44
When evaluating for ADHD, assess not only for the presence of symptoms, but also if these symptoms produce significant functional impairment.13,15 Impairments in daily functioning can include impaired school participation, social participation, quality of relationships, family conflict, family activities, family functioning, and emotional functioning.45 Some symptoms may affect functioning in an adult’s life differently than they did during childhood, from missed work appointments to being late picking up kids from school. Research has shown that the correlation between the number of symptoms and functional impairment is weak, which means someone could experience all of the symptoms of ADHD without experiencing functional impairment.45 To make an accurate diagnosis, it is therefore important to clearly establish both the number of symptoms the patient is experiencing and whether these symptoms are clearly linked to functional impairments.10
Sensible treatment
Once a diagnosis of ADHD has been clearly established, clinicians need to consider how best to treat the condition (Table 5). Stimulants are generally considered first-line treatment for ADHD. In randomized clinical trials, they showed significant efficacy; for example, one study of 146 adults with ADHD found a 76% improvement with methylphenidate compared to 19% for the placebo group.46 Before starting a stimulant, certain comorbidities should be ruled out. If a patient has glaucoma or pheochromocytoma, they may first need treatment from or clearance by other specialists. Stimulants should likely be held in patients with hypertension, angina, or cardiovascular defects until receiving medical clearance. The risks of stimulants need to be discussed with female patients of childbearing age, weighing the benefits of treatment against the risks of medication use should the patient get pregnant. Patients with comorbid psychosis or uncontrolled bipolar illness should not receive stimulants due to the risk of exacerbation. Patients with active substance use disorders (SUDs) are generally not good candidates for stimulants because of the risk of misusing or diverting stimulants and the possibility that substance abuse may be causing their inattentive symptoms. Patients whose SUDs are in remission may cautiously be considered as candidates for stimulants. If patients misuse their prescribed stimulants, they should be switched to a nonstimulant medication such as atomoxetine, bupropion, guanfacine, or clonidine.47

Continue to: Once a patient is deemed...
Once a patient is deemed to be a candidate for stimulants, clinicians need to choose between methylphenidate or amphetamine/dextroamphetamine formulations. Table 6 lists medications that are commonly prescribed to treat ADHD; unless otherwise noted, these are FDA-approved for this indication. As a general rule, for adults, long-acting stimulant formulations are preferred over short-acting formulations.28 Immediate-release stimulants are more prone to misuse or diversion compared to extended-release medications.29 Longer-acting formulations may also provide better full-day symptom control.48

In contrast to many other psychiatric medications, it may be beneficial to encourage periodically taking breaks or “medication holidays” from stimulants. Planned medication holidays for adults can involve intentionally not taking the medication over the weekend when the patient is not involved in work or school responsibilities. Such breaks have been shown to reduce adverse effects of stimulants (such as appetite suppression and insomnia) without significantly increasing ADHD symptoms.49 Short breaks can also help prevent medication tolerance and the subsequent need to increase doses.50 Medication holidays provide an opportunity to verify the ongoing benefits of the medication. It is advisable to periodically assess whether there is a continued need for stimulant treatment.51 If patients do not tolerate stimulants or have other contraindications, nonstimulants should be considered.
Lastly, no psychiatric patient should be treated with medication alone, and nonpharmacologic approaches should be incorporated as needed. Clear instructions, visual aids, nonverbal cues, frequent breaks to stand and stretch, schedules, normalizing failure as part of growth, and identifying triggers for emotional reactivity may help patients with ADHD.52 In a study of the academic performance of 92 college students taking medication for ADHD and 146 control students, treatment with stimulants alone did not eliminate the academic achievement deficit of those individuals with ADHD.53 Good study habits (even without stimulants) appeared more important in overcoming the achievement disparity of students with ADHD.53 Providing psychoeducation and training in concrete organization and planning skills have shown benefit.54 Practice of skills on a daily basis appears to be especially beneficial.55
Bottom Line
A sensible approach to diagnosing attention-deficit/hyperactivity disorder (ADHD) in adults includes ruling out other disorders that may present similar to ADHD, taking an appropriate developmental history, obtaining collateral information, and assessing for functional impairment. Sensible treatment involves ruling out comorbidities that stimulants could worsen, selecting extended-release stimulants, incorporating medication holidays, and using nonpharmacologic interventions.
Related Resources
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/ng87
- Substance Abuse and Mental Health Services Administration. Advisory: Prescription Stimulant Misuse Among Youth and Young Adults. https://store.samhsa.gov/product/prescription-stimulant-misuse-among-youth-young-adults/PEP21-06-01-003
Drug Brand Names
Amphetamine • Adzenys, Dyanavel, others
Atomoxetine • Strattera
Bupropion • Wellbutrin, Forfivo
Clonidine • Catapres, Kapvay
Dexmethylphenidate • Focalin
Dextroamphetamine • Dexedrine
Dextroamphetamine and amphetamine • Adderall, Mydayis
Guanfacine • Intuniv, Tenex
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin, others
Viloxazine • Qelbree
Attention-deficit/hyperactivity disorder (ADHD) is common, with an estimated worldwide prevalence of 5.29% among children and adolescents and 2.5% among adults.1 DSM-5-TR classifies ADHD as a neurodevelopmental disorder, “a group of conditions with onset in the developmental period [that] typically manifest early in development, often before the child enters school.”2 Because of the expectation that ADHD symptoms emerge early in development, the diagnostic criteria specify that symptoms must have been present prior to age 12 to qualify as ADHD. However, recent years have shown a significant increase in the number of patients being diagnosed with ADHD for the first time in adulthood. One study found that the diagnosis of ADHD among adults in the United States doubled between 2007 and 2016.3
First-line treatment for ADHD is the stimulants methylphenidate and amphetamine/dextroamphetamine. In the United States, these medications are classified as Schedule II controlled substances, indicating a high risk for abuse. However, just as ADHD diagnoses among adults have increased, so have prescriptions for stimulants. For example, Olfson et al4 found that stimulant prescriptions among young adults increased by a factor of 10 between 1994 and 2009.
The increased prevalence of adult patients diagnosed with ADHD and taking stimulants frequently places clinicians in a position to consider the validity of existing diagnoses and evaluate new patients with ADHD-related concerns. In this article, we review some of the challenges associated with diagnosing ADHD in adults, discuss the risks of stimulant treatment, and present a practical approach to the diagnosis and treatment of ADHD in adults.
Challenges in diagnosis
DSM-5-TR diagnostic criteria for ADHD are summarized in Table 1. Establishing a diagnosis of adult ADHD can be challenging. As with many psychiatric conditions, symptoms of ADHD are highly subjective. Retrospectively diagnosing a developmental condition in adults is often biased by the patient’s current functioning.5 ADHD has a high heritability and adults may inquire about the diagnosis if their children are diagnosed with ADHD.6 Some experts have cautioned that clinicians must be careful in diagnosing ADHD in adults.7 Just as there are risks associated with underdiagnosing ADHD, there are risks associated with overdiagnosis. Overdiagnosis may medicalize normal variants in the population and lead to unnecessary treatment and a misappropriation of limited medical resources.8 Many false positive cases of late-onset ADHD may be attributable to nonimpairing cognitive fluctuations.9

Poor diagnostic practices can impede accuracy in establishing the presence or absence of ADHD. Unfortunately, methods of diagnosing adult ADHD have been shown to vary widely in terms of information sources, diagnostic instruments used, symptom threshold, and whether functional impairment is a requirement for diagnosis.10 A common practice in diagnosing adult ADHD involves asking patients to complete self-report questionnaires that list symptoms of ADHD, such as the Adult ADHD Self-Report Scale developed by the World Health Organization.11 However, self-reports of ADHD in adults are less reliable than informant reports, and some young adults without ADHD overreport symptoms.12,13 Symptom checklists are particularly susceptible to faking, which lessens their diagnostic value.14
The possibility of malingered symptoms of ADHD further increases the diagnostic difficulty. College students may be particularly susceptible to overreporting ADHD symptoms in order to obtain academic accommodations or stimulants in the hopes of improving school performance.15 One study found that 25% to 48% of college students self-referred for ADHD evaluations exaggerated their symptoms.16 In another study, 31% of adults failed the Word Memory Test, which suggests noncredible performance in their ADHD evaluation.17 College students can successfully feign ADHD symptoms in both self-reported symptoms and computer-based tests of attention.18 Harrison et al19 summarized many of these concerns in their 2007 study of ADHD malingering, noting the “almost perfect ability of the Faking group to choose items … that correspond to the DSM-IV symptoms, and to report these at levels even higher than persons with diagnosed ADHD.” They suggested “Clinicians should be suspicious of students or young adults presenting for a first-time diagnosis who rate themselves as being significantly symptomatic, yet have managed to achieve well in school and other life activities.”19
Another challenge in correctly diagnosing adult ADHD is identifying other conditions that may impair attention.20 Psychiatric conditions that may impair concentration include anxiety disorders, chronic stress, posttraumatic stress disorder, recent trauma, major depressive disorder (MDD), and bipolar disorder (BD). Undiagnosed learning disorders may present like ADHD. Focus can be negatively affected by sleep disorders such as sleep apnea, restless leg syndrome, or delayed sleep phase-onset disorder. Marijuana, cocaine, 3,4-methylenedioxy-methamphetamine (MDMA; “ecstasy”), caffeine, or prescription medications such as anticholinergics can also impair attention. Medical conditions that can present with attentional or executive functioning deficits include seizures, Lyme disease, HIV, encephalopathy, hypothyroidism, and “chemo brain.”21 Environmental factors such as age-related cognitive decline, sleep deprivation, inflammation, obesity, air pollution, chemical exposure, and excessive use of digital media may also produce symptoms similar to ADHD. Two studies of adult-onset ADHD concluded that 93% to 95% of cases were better explained by other conditions such as sleep disorders, substance use disorders, or another psychiatric disorder.22
Continue to: Risks associated with treatment
Risks associated with treatment
With or without an accurate ADHD diagnosis, prescribing stimulants presents certain risks (Table 223-40). One of the more well-known risks of stimulants is addiction or misuse.23 An estimated 5 million American adults misused prescription stimulants in 2016.24 Despite stimulants’ status as controlled substances, long-term concurrent use of stimulants with opioids is common among adults with ADHD.25 College students are particularly susceptible to misusing or diverting stimulants, often to improve their academic performance.26 At 1 university, 22% of students had misused stimulants in the past year.27 Prescribing short-acting stimulants (rather than extended-release formulations) increases the likelihood of misuse.28 Patients prescribed stimulants begin to receive requests to divert their medications to others as early as elementary school, and by college more than one-third of those taking stimulants have been asked to give, sell, or trade their medications.29 Diversion of stimulants by students with ADHD is prevalent, with 62% of patients engaging in diversion during their lifetime.15 Diverted stimulants can come from family members, black market sources, or deceived clinicians.30 Although students’ stimulant misuse/diversion often is academically motivated, nonmedical use of psychostimulants does not appear to have a statistically significant effect on improving grade point average.31 Despite a negligible impact on grades, most students who take stimulants identify their effect as strongly positive, producing a situation in which misusers of stimulants have little motivation to stop.32 While some patients might ask for a stimulant prescription with the rationale that liking the effects proves they have ADHD, this is inappropriate because most individuals like the effects of stimulant medications.33

The use of stimulants increases the risk for several adverse psychiatric outcomes. Stimulants increase the risk of anxiety, so exercise caution when prescribing to patients with a comorbid anxiety disorder.34 Stimulants can also worsen irritability and insomnia, 2 issues common among patients with ADHD.32 Use of stimulant medications can trigger manic episodes. Viktorin et al35 found a >6-fold increase in manic episodes among patients with BD receiving methylphenidate monotherapy compared to those receiving a combination of methylphenidate and a mood stabilizer.35 The use of methylphenidate and amphetamine can lead to new-onset psychosis (or exacerbation of pre-existing psychotic illness); amphetamine use is associated with a higher risk of psychosis than methylphenidate.36
General medical adverse effects are also possible with stimulant use. Stimulants’ adverse effect profiles include appetite suppression, dry mouth, and nausea. Long-term use poses a risk for stunting growth in children.1 Using stimulants during pregnancy is associated with higher risk for neonatal morbidity, including preterm birth, CNS-related disorders, and seizures.37 Stimulants can raise blood pressure and increase heart rate. Serious cardiovascular events associated with stimulant use include ventricular arrhythmias, strokes, and transient ischemic attacks.38
Nonstimulant ADHD treatments are less risky than stimulants but still require monitoring for common adverse effects. Atomoxetine has been associated with sedation, growth retardation (in children), and in severe cases, liver injury or suicidal ideation.39 Bupropion (commonly used off-label for ADHD) can lower the seizure threshold and cause irritability, anorexia, and insomnia.39 Viloxazine, a newer agent, can cause hypertension, increased heart rate, nausea, drowsiness, headache, and insomnia.40
Sensible diagnosing
Given the challenges in accurately diagnosing ADHD in adults, we present a sensible approach to making the diagnosis (Table 3). The first step is to rule out other conditions that might better explain the patient’s symptoms. A thorough clinical interview (including a psychiatric review of symptoms) is the cornerstone of an initial diagnostic assessment. The use of validated screening questionnaires such as the Patient Health Questionnaire-9 and General Anxiety Disorder-7 may also provide information regarding psychiatric conditions that require additional evaluation.

Continue to: Some of the most common conditions...
Some of the most common conditions we see mistaken for ADHD are MDD, generalized anxiety disorder (GAD), and BD. In DSM-5-TR, 1 of the diagnostic criteria for MDD is “diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).”41 Similarly, criteria for GAD include “difficulty concentrating.”42 DSM-5-TR also includes distractibility as one of the criteria for mania/hypomania. Table 420-22,41,42 lists other psychiatric, substance-related, medical, and environmental conditions that can produce ADHD-like symptoms. Referring to some medical and environmental explanations for inattention, Aiken22 pointed out, “Patients who suffer from these problems might ask their doctor for a stimulant, but none of those syndromes require a psychopharmacologic approach.” ADHD can be comorbid with other psychiatric conditions, so the presence of another psychiatric illness does not automatically rule out ADHD. If alternative psychiatric diagnoses have been identified, these can be discussed with the patient and treatment offered that targets the specified condition.

Once alternative explanations have been ruled out, focus on the patient’s developmental history. DSM-5-TR conceptualizes ADHD as a neurodevelopmental disorder, meaning it is expected to emerge early in life. Whereas previous editions of DSM specified that ADHD symptoms must be present before age 7, DSM-5 modified this age threshold to before age 12.1 This necessitates taking a careful life history in order to understand the presence or absence of symptoms at earlier developmental stages.5 ADHD should be verified by symptoms apparent in childhood and present across the lifespan.15
While this retrospective history is necessary, histories that rely on self-report alone are often unreliable. Collateral sources of information are generally more reliable when assessing for ADHD symptoms.13 Third-party sources can help confirm that any impairment is best attributed to ADHD rather than to another condition.15 Unfortunately, the difficulty of obtaining collateral information means it is often neglected, even in the literature.10 A parent is the ideal informant for gathering collateral information regarding a patient’s functioning in childhood.5 Suggested best practices also include obtaining collateral information from interviews with significant others, behavioral questionnaires completed by parents (for current and childhood symptoms), review of school records, and consideration of intellectual and achievement testing.43 If psychological testing is pursued, include validity testing to detect feigned symptoms.18,44
When evaluating for ADHD, assess not only for the presence of symptoms, but also if these symptoms produce significant functional impairment.13,15 Impairments in daily functioning can include impaired school participation, social participation, quality of relationships, family conflict, family activities, family functioning, and emotional functioning.45 Some symptoms may affect functioning in an adult’s life differently than they did during childhood, from missed work appointments to being late picking up kids from school. Research has shown that the correlation between the number of symptoms and functional impairment is weak, which means someone could experience all of the symptoms of ADHD without experiencing functional impairment.45 To make an accurate diagnosis, it is therefore important to clearly establish both the number of symptoms the patient is experiencing and whether these symptoms are clearly linked to functional impairments.10
Sensible treatment
Once a diagnosis of ADHD has been clearly established, clinicians need to consider how best to treat the condition (Table 5). Stimulants are generally considered first-line treatment for ADHD. In randomized clinical trials, they showed significant efficacy; for example, one study of 146 adults with ADHD found a 76% improvement with methylphenidate compared to 19% for the placebo group.46 Before starting a stimulant, certain comorbidities should be ruled out. If a patient has glaucoma or pheochromocytoma, they may first need treatment from or clearance by other specialists. Stimulants should likely be held in patients with hypertension, angina, or cardiovascular defects until receiving medical clearance. The risks of stimulants need to be discussed with female patients of childbearing age, weighing the benefits of treatment against the risks of medication use should the patient get pregnant. Patients with comorbid psychosis or uncontrolled bipolar illness should not receive stimulants due to the risk of exacerbation. Patients with active substance use disorders (SUDs) are generally not good candidates for stimulants because of the risk of misusing or diverting stimulants and the possibility that substance abuse may be causing their inattentive symptoms. Patients whose SUDs are in remission may cautiously be considered as candidates for stimulants. If patients misuse their prescribed stimulants, they should be switched to a nonstimulant medication such as atomoxetine, bupropion, guanfacine, or clonidine.47

Continue to: Once a patient is deemed...
Once a patient is deemed to be a candidate for stimulants, clinicians need to choose between methylphenidate or amphetamine/dextroamphetamine formulations. Table 6 lists medications that are commonly prescribed to treat ADHD; unless otherwise noted, these are FDA-approved for this indication. As a general rule, for adults, long-acting stimulant formulations are preferred over short-acting formulations.28 Immediate-release stimulants are more prone to misuse or diversion compared to extended-release medications.29 Longer-acting formulations may also provide better full-day symptom control.48

In contrast to many other psychiatric medications, it may be beneficial to encourage periodically taking breaks or “medication holidays” from stimulants. Planned medication holidays for adults can involve intentionally not taking the medication over the weekend when the patient is not involved in work or school responsibilities. Such breaks have been shown to reduce adverse effects of stimulants (such as appetite suppression and insomnia) without significantly increasing ADHD symptoms.49 Short breaks can also help prevent medication tolerance and the subsequent need to increase doses.50 Medication holidays provide an opportunity to verify the ongoing benefits of the medication. It is advisable to periodically assess whether there is a continued need for stimulant treatment.51 If patients do not tolerate stimulants or have other contraindications, nonstimulants should be considered.
Lastly, no psychiatric patient should be treated with medication alone, and nonpharmacologic approaches should be incorporated as needed. Clear instructions, visual aids, nonverbal cues, frequent breaks to stand and stretch, schedules, normalizing failure as part of growth, and identifying triggers for emotional reactivity may help patients with ADHD.52 In a study of the academic performance of 92 college students taking medication for ADHD and 146 control students, treatment with stimulants alone did not eliminate the academic achievement deficit of those individuals with ADHD.53 Good study habits (even without stimulants) appeared more important in overcoming the achievement disparity of students with ADHD.53 Providing psychoeducation and training in concrete organization and planning skills have shown benefit.54 Practice of skills on a daily basis appears to be especially beneficial.55
Bottom Line
A sensible approach to diagnosing attention-deficit/hyperactivity disorder (ADHD) in adults includes ruling out other disorders that may present similar to ADHD, taking an appropriate developmental history, obtaining collateral information, and assessing for functional impairment. Sensible treatment involves ruling out comorbidities that stimulants could worsen, selecting extended-release stimulants, incorporating medication holidays, and using nonpharmacologic interventions.
Related Resources
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/ng87
- Substance Abuse and Mental Health Services Administration. Advisory: Prescription Stimulant Misuse Among Youth and Young Adults. https://store.samhsa.gov/product/prescription-stimulant-misuse-among-youth-young-adults/PEP21-06-01-003
Drug Brand Names
Amphetamine • Adzenys, Dyanavel, others
Atomoxetine • Strattera
Bupropion • Wellbutrin, Forfivo
Clonidine • Catapres, Kapvay
Dexmethylphenidate • Focalin
Dextroamphetamine • Dexedrine
Dextroamphetamine and amphetamine • Adderall, Mydayis
Guanfacine • Intuniv, Tenex
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin, others
Viloxazine • Qelbree
1. Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450-462.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:35.
3. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
4. Olfson M, Blanco C, Wang S, et al. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry. 2013;74(1):43-50.
5. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562-575.
7. Solanto MV. Child vs adult onset of attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(4):421.
8. Jummani RR, Hirsch E, Hirsch GS. Are we overdiagnosing and overtreating ADHD? Psychiatric Times. Published May 31, 2017. Accessed March 17, 2023. https://www.psychiatrictimes.com/view/are-we-overdiagnosing-and-overtreating-adhd
9. Sibley MH, Rohde LA, Swanson JM, et al; Multimodal Treatment Study of Children with ADHD (MTA) Cooperative Group. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149.
10. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165.
11. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527.
12. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry. 2016;73(7):655-656.
13. Sibley MH, Pelham WE, Molina BSG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052-1061.
14. Sollman MJ, Ranseen JD, Berry DT. Detection of feigned ADHD in college students. Psychol Assess. 2010;22(2):325-335.
15. Green AL, Rabiner DL. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9(3):559-568.
16. Sullivan BK, May K, Galbally L. Symptom exaggeration by college adults in attention-deficit hyperactivity disorder and learning disorder assessments. Appl Neuropsychol. 2007;14(3):189-207.
17. Suhr J, Hammers D, Dobbins-Buckland K, et al. The relationship of malingering test failure to self-reported symptoms and neuropsychological findings in adults referred for ADHD evaluation. Arch Clin Neuropsychol. 2008;23(5):521-530.
18. Lee Booksh R, Pella RD, Singh AN, et al. Ability of college students to simulate ADHD on objective measures of attention. J Atten Disord. 2010;13(4):325-338.
19. Harrison AG, Edwards MJ, Parker KC. Identifying students faking ADHD: preliminary findings and strategies for detection. Arch Clin Neuropsychol. 2007;22(5):577-588.
20. Lopez R, Micoulaud-Franchi JA, Galeria C, et al. Is adult-onset attention deficit/hyperactivity disorder frequent in clinical practice? Psychiatry Res. 2017;257:238-241.
21. Bhatia R. Rule out these causes of inattention before diagnosing ADHD. Current Psychiatry. 2016;15(10):32-33.
22. Aiken C. Adult-onset ADHD raises questions. Psychiatric Times. 2021;38(3):24.
23. Bjorn S, Weyandt LL. Issues pertaining to misuse of ADHD prescription medications. Psychiatric Times. 2018;35(9):17-19.
24. Compton WM, Han B, Blanco C, et al. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;175(8):741-755.
25. Wei YJ, Zhu Y, Liu W, et al. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw Open. 2018;1(4):e181152. doi:10.1001/jamanetworkopen.2018.1152
26. Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18(1):50-76.
27. Benson K, Woodlief DT, Flory K, et al. Is ADHD, independent of ODD, associated with whether and why college students misuse stimulant medication? Exp Clin Psychopharmacol. 2018;26(5):476-487.
28. Froehlich TE. ADHD medication adherence in college students-- a call to action for clinicians and researchers: commentary on “transition to college and adherence to prescribed attention deficit hyperactivity disorder medication.” J Dev Behav Pediatr. 2018;39(1):77-78.
29. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
30. Vrecko S. Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc Sci Med. 2015;131:297-304.
31. Munro BA, Weyandt LL, Marraccini ME, et al. The relationship between nonmedical use of prescription stimulants, executive functioning and academic outcomes. Addict Behav. 2017;65:250-257.
32. Rabiner DL, Anastopoulos AD, Costello EJ, et al. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord. 2009;13(3)259-270.
33. Tayag Y. Adult ADHD is the wild west of psychiatry. The Atlantic. Published April 14, 2023. Accessed May 3, 2023. https://www.theatlantic.com/health/archive/2023/04/adult-adhd-diagnosis-treatment-adderall-shortage/673719/
34. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270.
35. Viktorin A, Rydén E, Thase ME, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
36. Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019; 380(12):1128-1138.
37. Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140(6):e20170747. doi:10.1542/peds.2017-0747
38. Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10):e2130795. doi:10.1001/jamanetworkopen.2021.30795
39. Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48(3):240-248.
40. Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals; 2021.
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:183.
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:250.
43. DuPaul GJ, Weyandt LL, O’Dell SM, et al. College students with ADHD: current status and future directions. J Atten Disord. 2009;13(3):234-250.
44. Edmundson M, Berry DTR, Combs HL, et al. The effects of symptom information coaching on the feigning of adult ADHD. Psychol Assess. 2017;29(12):1429-1436.
45. Gordon M, Antshel K, Faraone S, et al. Symptoms versus impairment: the case for respecting DSM-IV’s criterion D. J Atten Disord. 2006;9(3):465-475.
46. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
47. Osser D, Awidi B. Treating adults with ADHD requires special considerations. Psychiatric News. Published August 30, 2018. Accessed March 17, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.pp8a1
48. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown, RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
49. Martins S, Tramontina S, Polanczyk G, et al. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14(2):195-206.
50. Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551-568.
51. Matthijssen AM, Dietrich A, Bierens M, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry. 2019;176(9):754-762.
52. Mason EJ, Joshi KG. Nonpharmacologic strategies for helping children with ADHD. Current Psychiatry. 2018;7(1):42,46.
53. Advokat C, Lane SM, Luo C. College students with and without ADHD: comparison of self-report of medication usage, study habits, and academic achievement. J Atten Disord. 2011;15(8):656-666.
54. Knouse LE, Cooper-Vince C, Sprich S, et al. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
55. Evans SW, Owens JS, Wymbs BT, et al. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157-198.
1. Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450-462.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:35.
3. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
4. Olfson M, Blanco C, Wang S, et al. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry. 2013;74(1):43-50.
5. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562-575.
7. Solanto MV. Child vs adult onset of attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(4):421.
8. Jummani RR, Hirsch E, Hirsch GS. Are we overdiagnosing and overtreating ADHD? Psychiatric Times. Published May 31, 2017. Accessed March 17, 2023. https://www.psychiatrictimes.com/view/are-we-overdiagnosing-and-overtreating-adhd
9. Sibley MH, Rohde LA, Swanson JM, et al; Multimodal Treatment Study of Children with ADHD (MTA) Cooperative Group. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149.
10. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165.
11. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527.
12. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry. 2016;73(7):655-656.
13. Sibley MH, Pelham WE, Molina BSG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052-1061.
14. Sollman MJ, Ranseen JD, Berry DT. Detection of feigned ADHD in college students. Psychol Assess. 2010;22(2):325-335.
15. Green AL, Rabiner DL. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9(3):559-568.
16. Sullivan BK, May K, Galbally L. Symptom exaggeration by college adults in attention-deficit hyperactivity disorder and learning disorder assessments. Appl Neuropsychol. 2007;14(3):189-207.
17. Suhr J, Hammers D, Dobbins-Buckland K, et al. The relationship of malingering test failure to self-reported symptoms and neuropsychological findings in adults referred for ADHD evaluation. Arch Clin Neuropsychol. 2008;23(5):521-530.
18. Lee Booksh R, Pella RD, Singh AN, et al. Ability of college students to simulate ADHD on objective measures of attention. J Atten Disord. 2010;13(4):325-338.
19. Harrison AG, Edwards MJ, Parker KC. Identifying students faking ADHD: preliminary findings and strategies for detection. Arch Clin Neuropsychol. 2007;22(5):577-588.
20. Lopez R, Micoulaud-Franchi JA, Galeria C, et al. Is adult-onset attention deficit/hyperactivity disorder frequent in clinical practice? Psychiatry Res. 2017;257:238-241.
21. Bhatia R. Rule out these causes of inattention before diagnosing ADHD. Current Psychiatry. 2016;15(10):32-33.
22. Aiken C. Adult-onset ADHD raises questions. Psychiatric Times. 2021;38(3):24.
23. Bjorn S, Weyandt LL. Issues pertaining to misuse of ADHD prescription medications. Psychiatric Times. 2018;35(9):17-19.
24. Compton WM, Han B, Blanco C, et al. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;175(8):741-755.
25. Wei YJ, Zhu Y, Liu W, et al. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw Open. 2018;1(4):e181152. doi:10.1001/jamanetworkopen.2018.1152
26. Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18(1):50-76.
27. Benson K, Woodlief DT, Flory K, et al. Is ADHD, independent of ODD, associated with whether and why college students misuse stimulant medication? Exp Clin Psychopharmacol. 2018;26(5):476-487.
28. Froehlich TE. ADHD medication adherence in college students-- a call to action for clinicians and researchers: commentary on “transition to college and adherence to prescribed attention deficit hyperactivity disorder medication.” J Dev Behav Pediatr. 2018;39(1):77-78.
29. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
30. Vrecko S. Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc Sci Med. 2015;131:297-304.
31. Munro BA, Weyandt LL, Marraccini ME, et al. The relationship between nonmedical use of prescription stimulants, executive functioning and academic outcomes. Addict Behav. 2017;65:250-257.
32. Rabiner DL, Anastopoulos AD, Costello EJ, et al. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord. 2009;13(3)259-270.
33. Tayag Y. Adult ADHD is the wild west of psychiatry. The Atlantic. Published April 14, 2023. Accessed May 3, 2023. https://www.theatlantic.com/health/archive/2023/04/adult-adhd-diagnosis-treatment-adderall-shortage/673719/
34. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270.
35. Viktorin A, Rydén E, Thase ME, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
36. Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019; 380(12):1128-1138.
37. Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140(6):e20170747. doi:10.1542/peds.2017-0747
38. Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10):e2130795. doi:10.1001/jamanetworkopen.2021.30795
39. Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48(3):240-248.
40. Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals; 2021.
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:183.
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:250.
43. DuPaul GJ, Weyandt LL, O’Dell SM, et al. College students with ADHD: current status and future directions. J Atten Disord. 2009;13(3):234-250.
44. Edmundson M, Berry DTR, Combs HL, et al. The effects of symptom information coaching on the feigning of adult ADHD. Psychol Assess. 2017;29(12):1429-1436.
45. Gordon M, Antshel K, Faraone S, et al. Symptoms versus impairment: the case for respecting DSM-IV’s criterion D. J Atten Disord. 2006;9(3):465-475.
46. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
47. Osser D, Awidi B. Treating adults with ADHD requires special considerations. Psychiatric News. Published August 30, 2018. Accessed March 17, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.pp8a1
48. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown, RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
49. Martins S, Tramontina S, Polanczyk G, et al. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14(2):195-206.
50. Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551-568.
51. Matthijssen AM, Dietrich A, Bierens M, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry. 2019;176(9):754-762.
52. Mason EJ, Joshi KG. Nonpharmacologic strategies for helping children with ADHD. Current Psychiatry. 2018;7(1):42,46.
53. Advokat C, Lane SM, Luo C. College students with and without ADHD: comparison of self-report of medication usage, study habits, and academic achievement. J Atten Disord. 2011;15(8):656-666.
54. Knouse LE, Cooper-Vince C, Sprich S, et al. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
55. Evans SW, Owens JS, Wymbs BT, et al. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157-198.
Brain structural and cognitive changes during pregnancy
Pregnancy is unquestionably a major milestone in a woman’s life. During gestation, her body shape noticeably changes, but the invisible structural and cognitive changes in her brain are more striking. Some of those neurobiological changes are short-term, while others are long-lasting, well beyond delivery, and even into old age.
Physiological changes during pregnancy are extraordinary. The dramatic increases in estrogen, progesterone, and glucocorticoids help maintain pregnancy, ensure safe delivery of the baby, and trigger maternal behavior. However, other important changes also occur in the mother’s cardiac output, blood volume, renal function, respiratory output, and immune adaptations to accommodate the growth of the fetus. Gene expression also occurs to accomplish those changes, and there are lifelong repercussions from those drastic physiological changes.
During pregnancy, the brain is exposed to escalating levels of hormones released from the placenta, which the woman had never experienced. Those hormones regulate neuroplasticity, neuroinflammation, behavior, and cognition.
Structural brain changes1-6
Brain volume declines during pregnancy, reaching a nadir at the time of parturition. However, recovery occurs within 5 months after delivery. During the postpartum period, gray matter volume increases in the first 3 to 4 weeks, especially in areas involved in maternal behavior, including the amygdala, prefrontal cortex, and hypothalamus. Hippocampal gray matter decreases at 2 months postpartum compared to preconception levels, and reductions can still be observed up to 2 years following delivery. Gray matter reductions occur in multiple brain regions involved in social cognition, including the superior temporal gyrus, medial and inferior frontal cortex, fusiform areas, and hippocampus. Those changes correlate with positive maternal attachment. It is noteworthy that neural activity is highest in areas with reduced gray volume, so a decline in brain volume is associated with enhanced maternal attachment. Interestingly, those changes occur in fathers, too.
Childbearing improves stroke outcomes in middle age, but body weight will increase. The risk of Alzheimer’s disease increases with a higher number of gestations, but longevity is higher if the pregnancy occurs at an older age. Reproduction is also associated with shorter telomeres, which can elevate the risk of cancer, inflammation, diabetes, and dementia.
Cognitive changes7-10
The term “pregnancy brain” refers to cognitive changes during pregnancy and postpartum; these include decreased memory and concentration, absent-mindedness, heightened reactivity to threatening stimuli, and a decrease in motivation and executive functions. After delivery a mother has increased empathy (sometimes referred to as Theory of Mind) and greater activation in brain structures involved in empathy, including the paracingulate cortex, the posterior cingulate, and the insula. Also, the mirror neuron system becomes more activated in response to a woman’s own children compared to unfamiliar children. This incudes the ventral premotor cortex, the inferior frontal gyrus, and the posterior parietal cortex.
Certain forms of memory are impaired during pregnancy and early postpartum, including verbal free recall and working memory, as well as executive functions. Those are believed to correlate with glucocorticoids and estrogen levels.
Continue to: The following cognitive functions...
The following cognitive functions increase between the first and second trimester: verbal memory, attention, executive functions processing speed, verbal, and visuospatial abilities. Interestingly, mothers of a male fetus outperformed mothers of a female fetus on working memory and spatial ability.
Other changes11-16
- Cells from the fetus can traffic to the mother’s body and create microchimeric cells, which have short-term benefits (healing some of the other’s organs as stem cells do) but long-term downsides include future brain disorders such as Parkinson’s disease or Alzheimer’s disease, as well as autoimmune diseases and various types of cancer. The reverse also occurs with cells transferring from the mother to the fetus, persisting into infancy and childhood.
- Postpartum psychosis is associated with reductions in the volumes of the anterior cingulate, left parahippocampal gyrus, and superior temporal gyrus.
- A woman’s white matter increases during pregnancy compared to preconception. This is attributed to the high levels of prolactin, which proliferates oligodendrocytes, the glial cells that continuously manufacture myelin.
- The pituitary gland increases by 200% to 300% during pregnancy and returns to pre-pregnancy levels approximately 8 months following delivery. Prolactin also mediates the production of brain cells in the hippocampus (ie, neurogenesis).
- Sexual activity, even without pregnancy, increases neurogenesis. Plasma levels of prolactin increase significantly following an orgasm in both men and women, which indicates that sexual activity has beneficial brain effects.
- With pregnancy, the immune system shifts from proinflammatory to anti-inflammatory signaling. This protects the fetus from being attacked and rejected as foreign tissue. However, at the end of pregnancy, there is a “burst” of proinflammatory signaling, which serves as a major trigger to induce uterine contractions and initiate labor (to expel the foreign tissue).
- Brain levels of the anti-inflammatory cytokine interleukin-6 increase in the postpartum period, which represents a significant modification in the neuroimmune environment, and the maternal brain assumes an inflammatory-resistant state, which has cognitive and neuroplasticity implications. However, this neuroimmune dysregulation is implicated in postpartum depression and anxiety.
- Older females who were never pregnant or only had 1 pregnancy had better overall cognitive functioning than females who became pregnant at an young age.
- In animal studies, reproduction alleviates the negative effects of aging on several hippocampal functions, especially neurogenesis. Dendritic spine density in the CA1 region of the hippocampus is higher in pregnancy and early postpartum period compared to nulliparous females (based on animal studies).
Pregnancy is indispensable for the perpetuation of the species. Its hormonal, physiologic, neurobiological, and cognitive correlates are extensive. The cognitive changes in the postpartum period are designed by evolution to prepare a woman to care for her newborn and to ensure its survival. But the biological sequelae of pregnancy extend to the rest of a woman’s life and may predispose her to immune and brain disorders as she ages.
1. Barba-Müller E, Craddock S, Carmona S, et al. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch Womens Ment Health. 2019;22(2):289-299.
2. Pawluski JL, Hoekzema E, Leuner B, et al. Less can be more: fine tuning the maternal brain. Neurosci Biobehav Rev. 2022;133:104475. doi:10.1016/j.neubiorev.2021.11.045
3. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
4. Cárdenas EF, Kujawa A, Humphreys KL. Neurobiological changes during the peripartum period: implications for health and behavior. Soc Cogn Affect Neurosci. 2020;15(10):1097-1110.
5. Eid RS, Chaiton JA, Lieblich SE, et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1-17.
6. Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26(10):641-648.
7. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126(1):73-85.
8. Barda G, Mizrachi Y, Borokchovich I, et al. The effect of pregnancy on maternal cognition. Sci Rep. 2011;11(1)12187. doi:10.1038/s41598-021-91504-9
9. Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 2018;208(1):35-40.
10. Pownall M, Hutter RRC, Rockliffe L, et al. Memory and mood changes in pregnancy: a qualitative content analysis of women’s first-hand accounts. J Reprod Infant Psychol. 2023;41(5):516-527.
11. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
12. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi:10.1016/j.yfrne.2019.02.004
13. Haim A, Julian D, Albin-Brooks C, et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2017;59:67-78.
14. Benson JC, Malyuk DF, Madhavan A, et al. Pituitary volume changes in pregnancy and the post-partum period. Neuroradiol J. 2023. doi:10.1177/19714009231196470
15. Schepanski S, Chini M, Sternemann V, et al. Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat Commun. 2022;13(1):4571. doi:10.1038/s41467-022-32230-2
16. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26(2):201-209.
Pregnancy is unquestionably a major milestone in a woman’s life. During gestation, her body shape noticeably changes, but the invisible structural and cognitive changes in her brain are more striking. Some of those neurobiological changes are short-term, while others are long-lasting, well beyond delivery, and even into old age.
Physiological changes during pregnancy are extraordinary. The dramatic increases in estrogen, progesterone, and glucocorticoids help maintain pregnancy, ensure safe delivery of the baby, and trigger maternal behavior. However, other important changes also occur in the mother’s cardiac output, blood volume, renal function, respiratory output, and immune adaptations to accommodate the growth of the fetus. Gene expression also occurs to accomplish those changes, and there are lifelong repercussions from those drastic physiological changes.
During pregnancy, the brain is exposed to escalating levels of hormones released from the placenta, which the woman had never experienced. Those hormones regulate neuroplasticity, neuroinflammation, behavior, and cognition.
Structural brain changes1-6
Brain volume declines during pregnancy, reaching a nadir at the time of parturition. However, recovery occurs within 5 months after delivery. During the postpartum period, gray matter volume increases in the first 3 to 4 weeks, especially in areas involved in maternal behavior, including the amygdala, prefrontal cortex, and hypothalamus. Hippocampal gray matter decreases at 2 months postpartum compared to preconception levels, and reductions can still be observed up to 2 years following delivery. Gray matter reductions occur in multiple brain regions involved in social cognition, including the superior temporal gyrus, medial and inferior frontal cortex, fusiform areas, and hippocampus. Those changes correlate with positive maternal attachment. It is noteworthy that neural activity is highest in areas with reduced gray volume, so a decline in brain volume is associated with enhanced maternal attachment. Interestingly, those changes occur in fathers, too.
Childbearing improves stroke outcomes in middle age, but body weight will increase. The risk of Alzheimer’s disease increases with a higher number of gestations, but longevity is higher if the pregnancy occurs at an older age. Reproduction is also associated with shorter telomeres, which can elevate the risk of cancer, inflammation, diabetes, and dementia.
Cognitive changes7-10
The term “pregnancy brain” refers to cognitive changes during pregnancy and postpartum; these include decreased memory and concentration, absent-mindedness, heightened reactivity to threatening stimuli, and a decrease in motivation and executive functions. After delivery a mother has increased empathy (sometimes referred to as Theory of Mind) and greater activation in brain structures involved in empathy, including the paracingulate cortex, the posterior cingulate, and the insula. Also, the mirror neuron system becomes more activated in response to a woman’s own children compared to unfamiliar children. This incudes the ventral premotor cortex, the inferior frontal gyrus, and the posterior parietal cortex.
Certain forms of memory are impaired during pregnancy and early postpartum, including verbal free recall and working memory, as well as executive functions. Those are believed to correlate with glucocorticoids and estrogen levels.
Continue to: The following cognitive functions...
The following cognitive functions increase between the first and second trimester: verbal memory, attention, executive functions processing speed, verbal, and visuospatial abilities. Interestingly, mothers of a male fetus outperformed mothers of a female fetus on working memory and spatial ability.
Other changes11-16
- Cells from the fetus can traffic to the mother’s body and create microchimeric cells, which have short-term benefits (healing some of the other’s organs as stem cells do) but long-term downsides include future brain disorders such as Parkinson’s disease or Alzheimer’s disease, as well as autoimmune diseases and various types of cancer. The reverse also occurs with cells transferring from the mother to the fetus, persisting into infancy and childhood.
- Postpartum psychosis is associated with reductions in the volumes of the anterior cingulate, left parahippocampal gyrus, and superior temporal gyrus.
- A woman’s white matter increases during pregnancy compared to preconception. This is attributed to the high levels of prolactin, which proliferates oligodendrocytes, the glial cells that continuously manufacture myelin.
- The pituitary gland increases by 200% to 300% during pregnancy and returns to pre-pregnancy levels approximately 8 months following delivery. Prolactin also mediates the production of brain cells in the hippocampus (ie, neurogenesis).
- Sexual activity, even without pregnancy, increases neurogenesis. Plasma levels of prolactin increase significantly following an orgasm in both men and women, which indicates that sexual activity has beneficial brain effects.
- With pregnancy, the immune system shifts from proinflammatory to anti-inflammatory signaling. This protects the fetus from being attacked and rejected as foreign tissue. However, at the end of pregnancy, there is a “burst” of proinflammatory signaling, which serves as a major trigger to induce uterine contractions and initiate labor (to expel the foreign tissue).
- Brain levels of the anti-inflammatory cytokine interleukin-6 increase in the postpartum period, which represents a significant modification in the neuroimmune environment, and the maternal brain assumes an inflammatory-resistant state, which has cognitive and neuroplasticity implications. However, this neuroimmune dysregulation is implicated in postpartum depression and anxiety.
- Older females who were never pregnant or only had 1 pregnancy had better overall cognitive functioning than females who became pregnant at an young age.
- In animal studies, reproduction alleviates the negative effects of aging on several hippocampal functions, especially neurogenesis. Dendritic spine density in the CA1 region of the hippocampus is higher in pregnancy and early postpartum period compared to nulliparous females (based on animal studies).
Pregnancy is indispensable for the perpetuation of the species. Its hormonal, physiologic, neurobiological, and cognitive correlates are extensive. The cognitive changes in the postpartum period are designed by evolution to prepare a woman to care for her newborn and to ensure its survival. But the biological sequelae of pregnancy extend to the rest of a woman’s life and may predispose her to immune and brain disorders as she ages.
Pregnancy is unquestionably a major milestone in a woman’s life. During gestation, her body shape noticeably changes, but the invisible structural and cognitive changes in her brain are more striking. Some of those neurobiological changes are short-term, while others are long-lasting, well beyond delivery, and even into old age.
Physiological changes during pregnancy are extraordinary. The dramatic increases in estrogen, progesterone, and glucocorticoids help maintain pregnancy, ensure safe delivery of the baby, and trigger maternal behavior. However, other important changes also occur in the mother’s cardiac output, blood volume, renal function, respiratory output, and immune adaptations to accommodate the growth of the fetus. Gene expression also occurs to accomplish those changes, and there are lifelong repercussions from those drastic physiological changes.
During pregnancy, the brain is exposed to escalating levels of hormones released from the placenta, which the woman had never experienced. Those hormones regulate neuroplasticity, neuroinflammation, behavior, and cognition.
Structural brain changes1-6
Brain volume declines during pregnancy, reaching a nadir at the time of parturition. However, recovery occurs within 5 months after delivery. During the postpartum period, gray matter volume increases in the first 3 to 4 weeks, especially in areas involved in maternal behavior, including the amygdala, prefrontal cortex, and hypothalamus. Hippocampal gray matter decreases at 2 months postpartum compared to preconception levels, and reductions can still be observed up to 2 years following delivery. Gray matter reductions occur in multiple brain regions involved in social cognition, including the superior temporal gyrus, medial and inferior frontal cortex, fusiform areas, and hippocampus. Those changes correlate with positive maternal attachment. It is noteworthy that neural activity is highest in areas with reduced gray volume, so a decline in brain volume is associated with enhanced maternal attachment. Interestingly, those changes occur in fathers, too.
Childbearing improves stroke outcomes in middle age, but body weight will increase. The risk of Alzheimer’s disease increases with a higher number of gestations, but longevity is higher if the pregnancy occurs at an older age. Reproduction is also associated with shorter telomeres, which can elevate the risk of cancer, inflammation, diabetes, and dementia.
Cognitive changes7-10
The term “pregnancy brain” refers to cognitive changes during pregnancy and postpartum; these include decreased memory and concentration, absent-mindedness, heightened reactivity to threatening stimuli, and a decrease in motivation and executive functions. After delivery a mother has increased empathy (sometimes referred to as Theory of Mind) and greater activation in brain structures involved in empathy, including the paracingulate cortex, the posterior cingulate, and the insula. Also, the mirror neuron system becomes more activated in response to a woman’s own children compared to unfamiliar children. This incudes the ventral premotor cortex, the inferior frontal gyrus, and the posterior parietal cortex.
Certain forms of memory are impaired during pregnancy and early postpartum, including verbal free recall and working memory, as well as executive functions. Those are believed to correlate with glucocorticoids and estrogen levels.
Continue to: The following cognitive functions...
The following cognitive functions increase between the first and second trimester: verbal memory, attention, executive functions processing speed, verbal, and visuospatial abilities. Interestingly, mothers of a male fetus outperformed mothers of a female fetus on working memory and spatial ability.
Other changes11-16
- Cells from the fetus can traffic to the mother’s body and create microchimeric cells, which have short-term benefits (healing some of the other’s organs as stem cells do) but long-term downsides include future brain disorders such as Parkinson’s disease or Alzheimer’s disease, as well as autoimmune diseases and various types of cancer. The reverse also occurs with cells transferring from the mother to the fetus, persisting into infancy and childhood.
- Postpartum psychosis is associated with reductions in the volumes of the anterior cingulate, left parahippocampal gyrus, and superior temporal gyrus.
- A woman’s white matter increases during pregnancy compared to preconception. This is attributed to the high levels of prolactin, which proliferates oligodendrocytes, the glial cells that continuously manufacture myelin.
- The pituitary gland increases by 200% to 300% during pregnancy and returns to pre-pregnancy levels approximately 8 months following delivery. Prolactin also mediates the production of brain cells in the hippocampus (ie, neurogenesis).
- Sexual activity, even without pregnancy, increases neurogenesis. Plasma levels of prolactin increase significantly following an orgasm in both men and women, which indicates that sexual activity has beneficial brain effects.
- With pregnancy, the immune system shifts from proinflammatory to anti-inflammatory signaling. This protects the fetus from being attacked and rejected as foreign tissue. However, at the end of pregnancy, there is a “burst” of proinflammatory signaling, which serves as a major trigger to induce uterine contractions and initiate labor (to expel the foreign tissue).
- Brain levels of the anti-inflammatory cytokine interleukin-6 increase in the postpartum period, which represents a significant modification in the neuroimmune environment, and the maternal brain assumes an inflammatory-resistant state, which has cognitive and neuroplasticity implications. However, this neuroimmune dysregulation is implicated in postpartum depression and anxiety.
- Older females who were never pregnant or only had 1 pregnancy had better overall cognitive functioning than females who became pregnant at an young age.
- In animal studies, reproduction alleviates the negative effects of aging on several hippocampal functions, especially neurogenesis. Dendritic spine density in the CA1 region of the hippocampus is higher in pregnancy and early postpartum period compared to nulliparous females (based on animal studies).
Pregnancy is indispensable for the perpetuation of the species. Its hormonal, physiologic, neurobiological, and cognitive correlates are extensive. The cognitive changes in the postpartum period are designed by evolution to prepare a woman to care for her newborn and to ensure its survival. But the biological sequelae of pregnancy extend to the rest of a woman’s life and may predispose her to immune and brain disorders as she ages.
1. Barba-Müller E, Craddock S, Carmona S, et al. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch Womens Ment Health. 2019;22(2):289-299.
2. Pawluski JL, Hoekzema E, Leuner B, et al. Less can be more: fine tuning the maternal brain. Neurosci Biobehav Rev. 2022;133:104475. doi:10.1016/j.neubiorev.2021.11.045
3. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
4. Cárdenas EF, Kujawa A, Humphreys KL. Neurobiological changes during the peripartum period: implications for health and behavior. Soc Cogn Affect Neurosci. 2020;15(10):1097-1110.
5. Eid RS, Chaiton JA, Lieblich SE, et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1-17.
6. Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26(10):641-648.
7. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126(1):73-85.
8. Barda G, Mizrachi Y, Borokchovich I, et al. The effect of pregnancy on maternal cognition. Sci Rep. 2011;11(1)12187. doi:10.1038/s41598-021-91504-9
9. Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 2018;208(1):35-40.
10. Pownall M, Hutter RRC, Rockliffe L, et al. Memory and mood changes in pregnancy: a qualitative content analysis of women’s first-hand accounts. J Reprod Infant Psychol. 2023;41(5):516-527.
11. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
12. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi:10.1016/j.yfrne.2019.02.004
13. Haim A, Julian D, Albin-Brooks C, et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2017;59:67-78.
14. Benson JC, Malyuk DF, Madhavan A, et al. Pituitary volume changes in pregnancy and the post-partum period. Neuroradiol J. 2023. doi:10.1177/19714009231196470
15. Schepanski S, Chini M, Sternemann V, et al. Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat Commun. 2022;13(1):4571. doi:10.1038/s41467-022-32230-2
16. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26(2):201-209.
1. Barba-Müller E, Craddock S, Carmona S, et al. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch Womens Ment Health. 2019;22(2):289-299.
2. Pawluski JL, Hoekzema E, Leuner B, et al. Less can be more: fine tuning the maternal brain. Neurosci Biobehav Rev. 2022;133:104475. doi:10.1016/j.neubiorev.2021.11.045
3. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
4. Cárdenas EF, Kujawa A, Humphreys KL. Neurobiological changes during the peripartum period: implications for health and behavior. Soc Cogn Affect Neurosci. 2020;15(10):1097-1110.
5. Eid RS, Chaiton JA, Lieblich SE, et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1-17.
6. Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26(10):641-648.
7. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126(1):73-85.
8. Barda G, Mizrachi Y, Borokchovich I, et al. The effect of pregnancy on maternal cognition. Sci Rep. 2011;11(1)12187. doi:10.1038/s41598-021-91504-9
9. Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 2018;208(1):35-40.
10. Pownall M, Hutter RRC, Rockliffe L, et al. Memory and mood changes in pregnancy: a qualitative content analysis of women’s first-hand accounts. J Reprod Infant Psychol. 2023;41(5):516-527.
11. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
12. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi:10.1016/j.yfrne.2019.02.004
13. Haim A, Julian D, Albin-Brooks C, et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2017;59:67-78.
14. Benson JC, Malyuk DF, Madhavan A, et al. Pituitary volume changes in pregnancy and the post-partum period. Neuroradiol J. 2023. doi:10.1177/19714009231196470
15. Schepanski S, Chini M, Sternemann V, et al. Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat Commun. 2022;13(1):4571. doi:10.1038/s41467-022-32230-2
16. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26(2):201-209.
More on climate change and mental health, burnout among surgeons
More on climate change and mental health
Your recent editorial (“A toxic and fractured political system can breed angst and PTSD”
The article suggested that psychiatrists are unequivocally tasked with managing the psychological aftermath of climate-related disasters. However, it is crucial to acknowledge that this is an assumption and lacks empirical evidence. I concur with the authors’ recognition of the grave environmental concerns posed by pollution, but it is valid to question the extent to which these concerns are fueled by mass hysteria, exacerbated by articles such as this one. Climate change undoubtedly is a multifaceted issue at times exploited for political purposes. As a result, terms such as “climate change denialism” are warped expressions that polarize the public even further, hindering constructive dialogue. Rather than denying the issue at hand, I am advocating for environmentally friendly solutions that do not come at the cost of manipulating public sentiment for political gain.
Additionally, I would argue trauma often does not arise from climate change itself, but instead from the actions of misguided radical environmentalist policy that unwittingly can cause more harm than good. The devastating destruction in Maui is a case in point. The article focuses on climate change as a cause of nihilism in this country; however, there is serious need to explore broader sociological issues that underlie this sense of nihilism and lack of life meaning, especially in the young.
It is essential to engage in a balanced and evidence-based discussion regarding climate change and its potential mental health implications. While some concerns the authors raised are valid, it is equally important to avoid fomenting hysteria and consider alternative perspectives that may help bridge gaps in understanding and unite us in effectively addressing this global challenge.
Robert Barris, MD
Flushing, New York
I want to send my appreciation for publishing in the same issue your editorial “A toxic and fractured political system can breed angst and PTSD” and the article “Climate change and mental illness: What psychiatrists can do.” I believe the issues addressed are important and belong in the mainstream of current psychiatric discussion.
Regarding the differing views of optimists and pessimists, I agree that narrative is bound for destruction. Because of that, several months ago I decided to deliberately cultivate and maintain a sense of optimism while knowing the facts! I believe that stance is the only one that strategically can lead towards progress.
I also want to comment on the “religification” of politics. While I believe secular religions exist, I also believe what we are currently seeing in the United States is not the rise of secular religions, but instead an attempt to insert extreme religious beliefs into politics while using language to create the illusion that the Constitution’s barrier against the merging of church and state is not being breached. I don’t think we are seeing secular religion, but God-based religion masking as secular religion.
Michael A. Kalm, MD
Salt Lake City, Utah
More on physician burnout
I am writing in reference to “Burnout among surgeons: Lessons for psychiatrists” (
It would behoove institutions to teach methods to mitigate burnout starting with first-year medical students instead of waiting until the increased stress, workload, and responsibility of their intern year. Knowing there is a potential negative downstream effect on patient care, in addition to the negative personal and professional impact on surgeons, is significant. By taking the time to engage all medical students in confidential, affordable, accessible mental health care, institutions would not only decrease burnout in this population of physicians but decrease the likelihood of negative outcomes in patient care.
Elina Maymind, MD
Mt. Laurel, New Jersey
More on climate change and mental health
Your recent editorial (“A toxic and fractured political system can breed angst and PTSD”
The article suggested that psychiatrists are unequivocally tasked with managing the psychological aftermath of climate-related disasters. However, it is crucial to acknowledge that this is an assumption and lacks empirical evidence. I concur with the authors’ recognition of the grave environmental concerns posed by pollution, but it is valid to question the extent to which these concerns are fueled by mass hysteria, exacerbated by articles such as this one. Climate change undoubtedly is a multifaceted issue at times exploited for political purposes. As a result, terms such as “climate change denialism” are warped expressions that polarize the public even further, hindering constructive dialogue. Rather than denying the issue at hand, I am advocating for environmentally friendly solutions that do not come at the cost of manipulating public sentiment for political gain.
Additionally, I would argue trauma often does not arise from climate change itself, but instead from the actions of misguided radical environmentalist policy that unwittingly can cause more harm than good. The devastating destruction in Maui is a case in point. The article focuses on climate change as a cause of nihilism in this country; however, there is serious need to explore broader sociological issues that underlie this sense of nihilism and lack of life meaning, especially in the young.
It is essential to engage in a balanced and evidence-based discussion regarding climate change and its potential mental health implications. While some concerns the authors raised are valid, it is equally important to avoid fomenting hysteria and consider alternative perspectives that may help bridge gaps in understanding and unite us in effectively addressing this global challenge.
Robert Barris, MD
Flushing, New York
I want to send my appreciation for publishing in the same issue your editorial “A toxic and fractured political system can breed angst and PTSD” and the article “Climate change and mental illness: What psychiatrists can do.” I believe the issues addressed are important and belong in the mainstream of current psychiatric discussion.
Regarding the differing views of optimists and pessimists, I agree that narrative is bound for destruction. Because of that, several months ago I decided to deliberately cultivate and maintain a sense of optimism while knowing the facts! I believe that stance is the only one that strategically can lead towards progress.
I also want to comment on the “religification” of politics. While I believe secular religions exist, I also believe what we are currently seeing in the United States is not the rise of secular religions, but instead an attempt to insert extreme religious beliefs into politics while using language to create the illusion that the Constitution’s barrier against the merging of church and state is not being breached. I don’t think we are seeing secular religion, but God-based religion masking as secular religion.
Michael A. Kalm, MD
Salt Lake City, Utah
More on physician burnout
I am writing in reference to “Burnout among surgeons: Lessons for psychiatrists” (
It would behoove institutions to teach methods to mitigate burnout starting with first-year medical students instead of waiting until the increased stress, workload, and responsibility of their intern year. Knowing there is a potential negative downstream effect on patient care, in addition to the negative personal and professional impact on surgeons, is significant. By taking the time to engage all medical students in confidential, affordable, accessible mental health care, institutions would not only decrease burnout in this population of physicians but decrease the likelihood of negative outcomes in patient care.
Elina Maymind, MD
Mt. Laurel, New Jersey
More on climate change and mental health
Your recent editorial (“A toxic and fractured political system can breed angst and PTSD”
The article suggested that psychiatrists are unequivocally tasked with managing the psychological aftermath of climate-related disasters. However, it is crucial to acknowledge that this is an assumption and lacks empirical evidence. I concur with the authors’ recognition of the grave environmental concerns posed by pollution, but it is valid to question the extent to which these concerns are fueled by mass hysteria, exacerbated by articles such as this one. Climate change undoubtedly is a multifaceted issue at times exploited for political purposes. As a result, terms such as “climate change denialism” are warped expressions that polarize the public even further, hindering constructive dialogue. Rather than denying the issue at hand, I am advocating for environmentally friendly solutions that do not come at the cost of manipulating public sentiment for political gain.
Additionally, I would argue trauma often does not arise from climate change itself, but instead from the actions of misguided radical environmentalist policy that unwittingly can cause more harm than good. The devastating destruction in Maui is a case in point. The article focuses on climate change as a cause of nihilism in this country; however, there is serious need to explore broader sociological issues that underlie this sense of nihilism and lack of life meaning, especially in the young.
It is essential to engage in a balanced and evidence-based discussion regarding climate change and its potential mental health implications. While some concerns the authors raised are valid, it is equally important to avoid fomenting hysteria and consider alternative perspectives that may help bridge gaps in understanding and unite us in effectively addressing this global challenge.
Robert Barris, MD
Flushing, New York
I want to send my appreciation for publishing in the same issue your editorial “A toxic and fractured political system can breed angst and PTSD” and the article “Climate change and mental illness: What psychiatrists can do.” I believe the issues addressed are important and belong in the mainstream of current psychiatric discussion.
Regarding the differing views of optimists and pessimists, I agree that narrative is bound for destruction. Because of that, several months ago I decided to deliberately cultivate and maintain a sense of optimism while knowing the facts! I believe that stance is the only one that strategically can lead towards progress.
I also want to comment on the “religification” of politics. While I believe secular religions exist, I also believe what we are currently seeing in the United States is not the rise of secular religions, but instead an attempt to insert extreme religious beliefs into politics while using language to create the illusion that the Constitution’s barrier against the merging of church and state is not being breached. I don’t think we are seeing secular religion, but God-based religion masking as secular religion.
Michael A. Kalm, MD
Salt Lake City, Utah
More on physician burnout
I am writing in reference to “Burnout among surgeons: Lessons for psychiatrists” (
It would behoove institutions to teach methods to mitigate burnout starting with first-year medical students instead of waiting until the increased stress, workload, and responsibility of their intern year. Knowing there is a potential negative downstream effect on patient care, in addition to the negative personal and professional impact on surgeons, is significant. By taking the time to engage all medical students in confidential, affordable, accessible mental health care, institutions would not only decrease burnout in this population of physicians but decrease the likelihood of negative outcomes in patient care.
Elina Maymind, MD
Mt. Laurel, New Jersey
Managing psychotropic-induced hyperhidrosis
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.
The incidence of medication-induced hyperhidrosis is unknown,but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.
Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown,but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown,but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Supervising residents in an outpatient setting: 6 tips for success
The Accreditation Council for Graduate Medical Education (ACGME) requires supervision of residents “provides safe and effective care to patients; ensures each resident’s development of the skills, knowledge, and attitudes required to enter the unsupervised practice of medicine; and establishes a foundation for continued professional growth.”1 Beyond delineating supervision types (direct, indirect, or oversight), best practices for outpatient supervision are lacking, which perhaps contributes to challenges and discrepancies in experiences involving both trainees and supervisors.2 In this article, I provide 6 practical recommendations for supervisors to address and overcome these challenges.
1. Don’t skip orientation
Resist the pressure to jump directly into clinical care. Devote the first supervision session to learner orientation about expectations (eg, documentation and between-visit patient outreach), logistics (eg, electronic health record or absences), clinic workflow and processes (eg, no-shows or referrals), and team members. Provide this verbally and in writing; the former fosters additional discussion and prompts questions, while the latter serves as a useful reference.
2. Plan for the end at the beginning
Plan ahead for end-of-rotation issues (eg, transfers of care or clinician sign-out), particularly because handoffs are known patient safety risks.3 Provide a written sign-out template or example, set a deadline for the first draft, and ensure known verbal sign-out occurs to both you and any trainees coming into the rotation.
3. Facilitate self-identification of strengths, weaknesses, and goals
Individual learning plans (ILPs) are a fundamental component of adult learning theory, allowing for self-directed learning and ongoing assessment by trainee and supervisor. Complete the ILP together at the beginning of the rotation and regularly devote time to revisit and revise it. This process ensures targeted feedback, which will reduce the stress and potential surprises often associated with end-of-rotation evaluations.
4. Consider the homework you assign
Be intentional about assigned readings. Consider their frequency and length, highlight where you want learners to focus, provide self-reflection questions/prompts, and take time to discuss during supervision. If you use a structured curriculum, maintain flexibility so your trainees’ interests, topics arising in real-time clinical care, and relevant in-press articles can be included.
5. Use direct observation
Whenever possible, directly observe clinical care, particularly a patient’s intake. To reduce trainee (and patient) anxiety and preserve independence, state, “I’m the attending physician supervising Dr. (NAME), who will be your doctor. We provide feedback to trainees right up to graduation so I’m here to observe and will be quiet in the background.” While direct observation is associated with early learners and inpatient settings, it is also preferred by senior outpatient psychiatry residents4 and associated with positive educational and patient outcomes.5
6. Offer supplemental experiences
If feasible, offer additional interdisciplinary supervision (eg, social workers, psychologists, or peer support), scholarly opportunities (eg, case report collaboration or clinic talk), psychotherapy cases, or meeting with patients on your caseload (eg, patients with a rare diagnosis or unique presentation). These align with ACGME’s broad supervision requirements and offer much-appreciated individualized learning tailored to the trainee.
1. Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). Updated July 1, 2022. Accessed September 6, 2023. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Newman M, Ravindranath D, Figueroa S, et al. Perceptions of supervision in an outpatient psychiatry clinic. Acad Psychiatry. 2016;40(1):153-156. doi:10.1007/s40596-014-0191-y
3. The Joint Commission. Inadequate hand-off communication. Sentinel Event Alert. Issue 58. September 12, 2017. Accessed September 11, 2023. https://www.jointcommission.org/-/media/tjc/documents/resources/patient-safety-topics/sentinel-event/sea_58_hand_off_comms_9_6_17_final_(1).pdf
4. Tan LL, Kam CJW. How psychiatry residents perceive clinical teaching effectiveness with or without direct supervision. The Asia-Pacific Scholar. 2020;5(2):14-21.
5. Galanter CA, Nikolov R, Green N, et al. Direct supervision in outpatient psychiatric graduate medical education. Acad Psychiatry. 2016;40(1):157-163. doi:10.1007/s40596-014-0247-z
The Accreditation Council for Graduate Medical Education (ACGME) requires supervision of residents “provides safe and effective care to patients; ensures each resident’s development of the skills, knowledge, and attitudes required to enter the unsupervised practice of medicine; and establishes a foundation for continued professional growth.”1 Beyond delineating supervision types (direct, indirect, or oversight), best practices for outpatient supervision are lacking, which perhaps contributes to challenges and discrepancies in experiences involving both trainees and supervisors.2 In this article, I provide 6 practical recommendations for supervisors to address and overcome these challenges.
1. Don’t skip orientation
Resist the pressure to jump directly into clinical care. Devote the first supervision session to learner orientation about expectations (eg, documentation and between-visit patient outreach), logistics (eg, electronic health record or absences), clinic workflow and processes (eg, no-shows or referrals), and team members. Provide this verbally and in writing; the former fosters additional discussion and prompts questions, while the latter serves as a useful reference.
2. Plan for the end at the beginning
Plan ahead for end-of-rotation issues (eg, transfers of care or clinician sign-out), particularly because handoffs are known patient safety risks.3 Provide a written sign-out template or example, set a deadline for the first draft, and ensure known verbal sign-out occurs to both you and any trainees coming into the rotation.
3. Facilitate self-identification of strengths, weaknesses, and goals
Individual learning plans (ILPs) are a fundamental component of adult learning theory, allowing for self-directed learning and ongoing assessment by trainee and supervisor. Complete the ILP together at the beginning of the rotation and regularly devote time to revisit and revise it. This process ensures targeted feedback, which will reduce the stress and potential surprises often associated with end-of-rotation evaluations.
4. Consider the homework you assign
Be intentional about assigned readings. Consider their frequency and length, highlight where you want learners to focus, provide self-reflection questions/prompts, and take time to discuss during supervision. If you use a structured curriculum, maintain flexibility so your trainees’ interests, topics arising in real-time clinical care, and relevant in-press articles can be included.
5. Use direct observation
Whenever possible, directly observe clinical care, particularly a patient’s intake. To reduce trainee (and patient) anxiety and preserve independence, state, “I’m the attending physician supervising Dr. (NAME), who will be your doctor. We provide feedback to trainees right up to graduation so I’m here to observe and will be quiet in the background.” While direct observation is associated with early learners and inpatient settings, it is also preferred by senior outpatient psychiatry residents4 and associated with positive educational and patient outcomes.5
6. Offer supplemental experiences
If feasible, offer additional interdisciplinary supervision (eg, social workers, psychologists, or peer support), scholarly opportunities (eg, case report collaboration or clinic talk), psychotherapy cases, or meeting with patients on your caseload (eg, patients with a rare diagnosis or unique presentation). These align with ACGME’s broad supervision requirements and offer much-appreciated individualized learning tailored to the trainee.
The Accreditation Council for Graduate Medical Education (ACGME) requires supervision of residents “provides safe and effective care to patients; ensures each resident’s development of the skills, knowledge, and attitudes required to enter the unsupervised practice of medicine; and establishes a foundation for continued professional growth.”1 Beyond delineating supervision types (direct, indirect, or oversight), best practices for outpatient supervision are lacking, which perhaps contributes to challenges and discrepancies in experiences involving both trainees and supervisors.2 In this article, I provide 6 practical recommendations for supervisors to address and overcome these challenges.
1. Don’t skip orientation
Resist the pressure to jump directly into clinical care. Devote the first supervision session to learner orientation about expectations (eg, documentation and between-visit patient outreach), logistics (eg, electronic health record or absences), clinic workflow and processes (eg, no-shows or referrals), and team members. Provide this verbally and in writing; the former fosters additional discussion and prompts questions, while the latter serves as a useful reference.
2. Plan for the end at the beginning
Plan ahead for end-of-rotation issues (eg, transfers of care or clinician sign-out), particularly because handoffs are known patient safety risks.3 Provide a written sign-out template or example, set a deadline for the first draft, and ensure known verbal sign-out occurs to both you and any trainees coming into the rotation.
3. Facilitate self-identification of strengths, weaknesses, and goals
Individual learning plans (ILPs) are a fundamental component of adult learning theory, allowing for self-directed learning and ongoing assessment by trainee and supervisor. Complete the ILP together at the beginning of the rotation and regularly devote time to revisit and revise it. This process ensures targeted feedback, which will reduce the stress and potential surprises often associated with end-of-rotation evaluations.
4. Consider the homework you assign
Be intentional about assigned readings. Consider their frequency and length, highlight where you want learners to focus, provide self-reflection questions/prompts, and take time to discuss during supervision. If you use a structured curriculum, maintain flexibility so your trainees’ interests, topics arising in real-time clinical care, and relevant in-press articles can be included.
5. Use direct observation
Whenever possible, directly observe clinical care, particularly a patient’s intake. To reduce trainee (and patient) anxiety and preserve independence, state, “I’m the attending physician supervising Dr. (NAME), who will be your doctor. We provide feedback to trainees right up to graduation so I’m here to observe and will be quiet in the background.” While direct observation is associated with early learners and inpatient settings, it is also preferred by senior outpatient psychiatry residents4 and associated with positive educational and patient outcomes.5
6. Offer supplemental experiences
If feasible, offer additional interdisciplinary supervision (eg, social workers, psychologists, or peer support), scholarly opportunities (eg, case report collaboration or clinic talk), psychotherapy cases, or meeting with patients on your caseload (eg, patients with a rare diagnosis or unique presentation). These align with ACGME’s broad supervision requirements and offer much-appreciated individualized learning tailored to the trainee.
1. Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). Updated July 1, 2022. Accessed September 6, 2023. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Newman M, Ravindranath D, Figueroa S, et al. Perceptions of supervision in an outpatient psychiatry clinic. Acad Psychiatry. 2016;40(1):153-156. doi:10.1007/s40596-014-0191-y
3. The Joint Commission. Inadequate hand-off communication. Sentinel Event Alert. Issue 58. September 12, 2017. Accessed September 11, 2023. https://www.jointcommission.org/-/media/tjc/documents/resources/patient-safety-topics/sentinel-event/sea_58_hand_off_comms_9_6_17_final_(1).pdf
4. Tan LL, Kam CJW. How psychiatry residents perceive clinical teaching effectiveness with or without direct supervision. The Asia-Pacific Scholar. 2020;5(2):14-21.
5. Galanter CA, Nikolov R, Green N, et al. Direct supervision in outpatient psychiatric graduate medical education. Acad Psychiatry. 2016;40(1):157-163. doi:10.1007/s40596-014-0247-z
1. Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). Updated July 1, 2022. Accessed September 6, 2023. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Newman M, Ravindranath D, Figueroa S, et al. Perceptions of supervision in an outpatient psychiatry clinic. Acad Psychiatry. 2016;40(1):153-156. doi:10.1007/s40596-014-0191-y
3. The Joint Commission. Inadequate hand-off communication. Sentinel Event Alert. Issue 58. September 12, 2017. Accessed September 11, 2023. https://www.jointcommission.org/-/media/tjc/documents/resources/patient-safety-topics/sentinel-event/sea_58_hand_off_comms_9_6_17_final_(1).pdf
4. Tan LL, Kam CJW. How psychiatry residents perceive clinical teaching effectiveness with or without direct supervision. The Asia-Pacific Scholar. 2020;5(2):14-21.
5. Galanter CA, Nikolov R, Green N, et al. Direct supervision in outpatient psychiatric graduate medical education. Acad Psychiatry. 2016;40(1):157-163. doi:10.1007/s40596-014-0247-z
Interviewing a patient experiencing psychosis
Clinicians of all experience levels, particularly trainees, may struggle when interviewing an individual experiencing psychosis. Many clinicians feel unsure what to say when a patient expresses fixed beliefs that are not amenable to change despite conflicting evidence, or worry about inadvertently affirming these beliefs. Supporting and empathizing with a person experiencing psychosis while avoiding reinforcing delusional beliefs is an important skillset for clinicians to have. While there is no single “correct” approach to interviewing individuals with psychosis, key principles include:
1. Do not begin by challenging delusions
People experiencing delusions often feel strongly about the validity of their beliefs and find evidence to support them. Directly challenging these beliefs from the beginning may alienate them. Instead, explore with neutral questioning: “Can you tell me more about X?” “What did you notice that made you believe Y?” Later, when rapport is established, it may be appropriate to explore discrepancies that provide insight into their delusions, a technique used in cognitive-behavioral therapy for psychosis.
2. Validate the emotion, not the psychosis
Many interviewers worry that talking about a patient’s delusions or voices will inadvertently reinforce them. Instead of agreeing with the content, listen for and empathize with the emotion (which is often fear): “That sounds frightening.” If the emotion is unclear, ask: “How did you feel when that happened?” When unsure what to say, sometimes a neutral “mmm” conveys listening without reinforcing the psychosis.
3. Explicitly state emotions and intentions
People with psychosis may have difficulty processing others’ emotions and facial expressions.1 We recommend using verbal cues to assist them in recognizing emotions and intentions: “It makes me sad to hear how alone you felt,” or “I’m here to help you.” The interviewer may mildly “amplify” their facial expressions so that the person experiencing psychosis can more clearly identify the expressed emotion, though not all individuals with psychosis respond well to this.
4. Reflect the patient’s own words
We recommend using the patient’s exact (typically nonclinical) words in referring to their experiences to build rapport and a shared understanding of their subjective experience.2 Avoid introducing clinical jargon, such as “delusion” or “hallucination.” For example, the interviewer might follow a patient’s explanation of their experiences by asking: “You heard voices in the walls—what did they say?” If the patient uses clinical jargon, the interviewer should clarify their meaning: “When you say ‘paranoid,’ what does that mean to you?”
5. Be intentional with gestures and positioning
People with schizophrenia-spectrum disorders may have difficulty interpreting gestures and are more likely to perceive gestures as self-referential.1 We recommend minimizing gestures or using simple, neutral-to-positive movements appropriate to cultural context. For example, in the United States, hands with palms up in front of the body generally convey openness, whereas arms crossed over the chest may convey anger. We recommend that to avoid appearing confrontational, interviewers do not position themselves directly in front of the patient, instead positioning themselves at an angle. Consider mirroring patients’ gestures or postures to convey empathy and build rapport.3
1. Chapellier V, Pavlidou A, Maderthaner L, et al. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front Psychol. 2022;13:804093. doi:10.3389/fpsyg.2022.804093
2. Olson M, Seikkula J, Ziedonis D. The key elements of dialogic practice in Open Dialogue: fidelity criteria. University of Massachusetts Medical School. Published September 2, 2014. Accessed August 16, 2023. https://www.umassmed.edu/globalassets/psychiatry/open-dialogue/keyelementsv1.109022014.pdf
3. Raffard S, Salesse RN, Bortolon C, et al. Using mimicry of body movements by a virtual agent to increase synchronization behavior and rapport in individuals with schizophrenia. Sci Rep. 2018;8(1):17356. doi:10.1038/s41598-018-35813-6
Clinicians of all experience levels, particularly trainees, may struggle when interviewing an individual experiencing psychosis. Many clinicians feel unsure what to say when a patient expresses fixed beliefs that are not amenable to change despite conflicting evidence, or worry about inadvertently affirming these beliefs. Supporting and empathizing with a person experiencing psychosis while avoiding reinforcing delusional beliefs is an important skillset for clinicians to have. While there is no single “correct” approach to interviewing individuals with psychosis, key principles include:
1. Do not begin by challenging delusions
People experiencing delusions often feel strongly about the validity of their beliefs and find evidence to support them. Directly challenging these beliefs from the beginning may alienate them. Instead, explore with neutral questioning: “Can you tell me more about X?” “What did you notice that made you believe Y?” Later, when rapport is established, it may be appropriate to explore discrepancies that provide insight into their delusions, a technique used in cognitive-behavioral therapy for psychosis.
2. Validate the emotion, not the psychosis
Many interviewers worry that talking about a patient’s delusions or voices will inadvertently reinforce them. Instead of agreeing with the content, listen for and empathize with the emotion (which is often fear): “That sounds frightening.” If the emotion is unclear, ask: “How did you feel when that happened?” When unsure what to say, sometimes a neutral “mmm” conveys listening without reinforcing the psychosis.
3. Explicitly state emotions and intentions
People with psychosis may have difficulty processing others’ emotions and facial expressions.1 We recommend using verbal cues to assist them in recognizing emotions and intentions: “It makes me sad to hear how alone you felt,” or “I’m here to help you.” The interviewer may mildly “amplify” their facial expressions so that the person experiencing psychosis can more clearly identify the expressed emotion, though not all individuals with psychosis respond well to this.
4. Reflect the patient’s own words
We recommend using the patient’s exact (typically nonclinical) words in referring to their experiences to build rapport and a shared understanding of their subjective experience.2 Avoid introducing clinical jargon, such as “delusion” or “hallucination.” For example, the interviewer might follow a patient’s explanation of their experiences by asking: “You heard voices in the walls—what did they say?” If the patient uses clinical jargon, the interviewer should clarify their meaning: “When you say ‘paranoid,’ what does that mean to you?”
5. Be intentional with gestures and positioning
People with schizophrenia-spectrum disorders may have difficulty interpreting gestures and are more likely to perceive gestures as self-referential.1 We recommend minimizing gestures or using simple, neutral-to-positive movements appropriate to cultural context. For example, in the United States, hands with palms up in front of the body generally convey openness, whereas arms crossed over the chest may convey anger. We recommend that to avoid appearing confrontational, interviewers do not position themselves directly in front of the patient, instead positioning themselves at an angle. Consider mirroring patients’ gestures or postures to convey empathy and build rapport.3
Clinicians of all experience levels, particularly trainees, may struggle when interviewing an individual experiencing psychosis. Many clinicians feel unsure what to say when a patient expresses fixed beliefs that are not amenable to change despite conflicting evidence, or worry about inadvertently affirming these beliefs. Supporting and empathizing with a person experiencing psychosis while avoiding reinforcing delusional beliefs is an important skillset for clinicians to have. While there is no single “correct” approach to interviewing individuals with psychosis, key principles include:
1. Do not begin by challenging delusions
People experiencing delusions often feel strongly about the validity of their beliefs and find evidence to support them. Directly challenging these beliefs from the beginning may alienate them. Instead, explore with neutral questioning: “Can you tell me more about X?” “What did you notice that made you believe Y?” Later, when rapport is established, it may be appropriate to explore discrepancies that provide insight into their delusions, a technique used in cognitive-behavioral therapy for psychosis.
2. Validate the emotion, not the psychosis
Many interviewers worry that talking about a patient’s delusions or voices will inadvertently reinforce them. Instead of agreeing with the content, listen for and empathize with the emotion (which is often fear): “That sounds frightening.” If the emotion is unclear, ask: “How did you feel when that happened?” When unsure what to say, sometimes a neutral “mmm” conveys listening without reinforcing the psychosis.
3. Explicitly state emotions and intentions
People with psychosis may have difficulty processing others’ emotions and facial expressions.1 We recommend using verbal cues to assist them in recognizing emotions and intentions: “It makes me sad to hear how alone you felt,” or “I’m here to help you.” The interviewer may mildly “amplify” their facial expressions so that the person experiencing psychosis can more clearly identify the expressed emotion, though not all individuals with psychosis respond well to this.
4. Reflect the patient’s own words
We recommend using the patient’s exact (typically nonclinical) words in referring to their experiences to build rapport and a shared understanding of their subjective experience.2 Avoid introducing clinical jargon, such as “delusion” or “hallucination.” For example, the interviewer might follow a patient’s explanation of their experiences by asking: “You heard voices in the walls—what did they say?” If the patient uses clinical jargon, the interviewer should clarify their meaning: “When you say ‘paranoid,’ what does that mean to you?”
5. Be intentional with gestures and positioning
People with schizophrenia-spectrum disorders may have difficulty interpreting gestures and are more likely to perceive gestures as self-referential.1 We recommend minimizing gestures or using simple, neutral-to-positive movements appropriate to cultural context. For example, in the United States, hands with palms up in front of the body generally convey openness, whereas arms crossed over the chest may convey anger. We recommend that to avoid appearing confrontational, interviewers do not position themselves directly in front of the patient, instead positioning themselves at an angle. Consider mirroring patients’ gestures or postures to convey empathy and build rapport.3
1. Chapellier V, Pavlidou A, Maderthaner L, et al. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front Psychol. 2022;13:804093. doi:10.3389/fpsyg.2022.804093
2. Olson M, Seikkula J, Ziedonis D. The key elements of dialogic practice in Open Dialogue: fidelity criteria. University of Massachusetts Medical School. Published September 2, 2014. Accessed August 16, 2023. https://www.umassmed.edu/globalassets/psychiatry/open-dialogue/keyelementsv1.109022014.pdf
3. Raffard S, Salesse RN, Bortolon C, et al. Using mimicry of body movements by a virtual agent to increase synchronization behavior and rapport in individuals with schizophrenia. Sci Rep. 2018;8(1):17356. doi:10.1038/s41598-018-35813-6
1. Chapellier V, Pavlidou A, Maderthaner L, et al. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front Psychol. 2022;13:804093. doi:10.3389/fpsyg.2022.804093
2. Olson M, Seikkula J, Ziedonis D. The key elements of dialogic practice in Open Dialogue: fidelity criteria. University of Massachusetts Medical School. Published September 2, 2014. Accessed August 16, 2023. https://www.umassmed.edu/globalassets/psychiatry/open-dialogue/keyelementsv1.109022014.pdf
3. Raffard S, Salesse RN, Bortolon C, et al. Using mimicry of body movements by a virtual agent to increase synchronization behavior and rapport in individuals with schizophrenia. Sci Rep. 2018;8(1):17356. doi:10.1038/s41598-018-35813-6
The ‘borderlinization’ of our society and the mental health crisis
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
We appreciated Dr. Nasrallah’s recent editorial1 that implicated smartphones, social media, and video game addiction, combined with the pandemic, in causing default mode network (DMN) dysfunction. The United States Surgeon General’s May 2023 report echoed these concerns and recommended limiting the use of these platforms.2 While devices are accelerants on a raging fire of mental illness, we observe a more insidious etiology that kindled the flame long before the proliferation of social media use during the pandemic. I (MZP) call this the “borderlinization” of society.
Imagine living somewhere in America that time had forgotten, where youth did not use smartphones and social media or play video games, and throughout the pandemic, people continued to congregate and socialize. These are the religious enclaves throughout New York and New Jersey that we (MZP and RLP) serve. Yet if devices were predominantly to blame for the contemporary mental health crisis, we would not expect the growing mental health problems we encounter. So, what is going on?
Over the past decade, mental health awareness has permeated all institutions of education, media, business, and government, which has increased compassion for marginalized groups. Consequently, people who may have previously silently suffered have become encouraged and supported in seeking help. That is good news. The bad news is that we have also come to pathologize, label, and attempt to treat nearly all of life’s struggles, and have been exporting mental disease around the world.3 We are losing the sense of “normal” when more than one-half of all Americans will receive a DSM diagnosis in their lifetime.4
Traits of borderline personality disorder (BPD)—such as abandonment fears, unstable relationships, identity disturbance, affective instability, emptiness, anger, mistrust, and dissociation5—that previously were seen less often are now more commonplace among our patients. These patients’ therapists have “validated” their “victimization” of “microaggressions” such that they now require “trigger warnings,” “safe spaces,” and psychiatric “diagnosis and treatment” to be able to function “normally.” These developments have also positioned parents, educators, employers, and psychiatrists, who may share “power and privilege,” to “walk on eggshells” so as not to offend newfound hypersensitivities. Interestingly, the DMN may be a major, reversible driver in BPD,6 a possible final common pathway that is further impaired by devices starting to creep into our communities and amplify the dysfunction.
Beyond treating individual patients, we must consider mandating time away from devices to nourish our DMN. During a 25-hour period each week, we (MZP and RLP) unplug from all forms of work and electronics, remember the past, consider the future, reflect on self and others, connect with nature, meditate, and eat mindfully—all of which are DMN functions. We call it Shabbat, which people have observed for thousands of years to process the week before and rejuvenate for the week ahead. Excluding smartphones from school premises has also been helpful7 and could be implemented as a nationwide commitment to the developing brains of our youth. Finally, we need to look to our profession to promote resilience over dependence, distress tolerance over avoidance, and empathic communication over “cancellation” to help heal a divisive society.
1. Nasrallah HA. Is the contemporary mental health crisis among youth due to DMN disruption? Current Psychiatry. 2023;22(6):10-11,21. doi:10.12788/cp.0372
2. U.S. Department of Health and Human Services. Surgeon general issues new advisory about effects social media use has on youth mental health. May 23, 2023. Accessed June 4, 2023. https://www.hhs.gov/about/news/2023/05/23/surgeon-general-issues-new-advisory-about-effects-social-media-use-has-youth-mental-health.html
3. Watters E. Crazy Like Us: The Globalization of the American Psyche. Free Press; 2011.
4. Centers for Disease Control and Prevention. About mental health. April 25, 2023. Accessed June 4, 2023. https://www.cdc.gov/mentalhealth/learn/index.htm
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
6. Amiri S, Mirfazeli FS, Grafman J, et al. Alternation in functional connectivity within default mode network after psychodynamic psychotherapy in borderline personality disorder. Ann Gen Psychiatry. 2023;22(1):18. doi:10.1186/s12991-023-00449-y
7. Beland LP, Murphy R. Ill communication: technology, distraction & student performance. Labour Economics. 2016;41:61-76. doi:10.1016/j.labeco.2016.04.004
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
We appreciated Dr. Nasrallah’s recent editorial1 that implicated smartphones, social media, and video game addiction, combined with the pandemic, in causing default mode network (DMN) dysfunction. The United States Surgeon General’s May 2023 report echoed these concerns and recommended limiting the use of these platforms.2 While devices are accelerants on a raging fire of mental illness, we observe a more insidious etiology that kindled the flame long before the proliferation of social media use during the pandemic. I (MZP) call this the “borderlinization” of society.
Imagine living somewhere in America that time had forgotten, where youth did not use smartphones and social media or play video games, and throughout the pandemic, people continued to congregate and socialize. These are the religious enclaves throughout New York and New Jersey that we (MZP and RLP) serve. Yet if devices were predominantly to blame for the contemporary mental health crisis, we would not expect the growing mental health problems we encounter. So, what is going on?
Over the past decade, mental health awareness has permeated all institutions of education, media, business, and government, which has increased compassion for marginalized groups. Consequently, people who may have previously silently suffered have become encouraged and supported in seeking help. That is good news. The bad news is that we have also come to pathologize, label, and attempt to treat nearly all of life’s struggles, and have been exporting mental disease around the world.3 We are losing the sense of “normal” when more than one-half of all Americans will receive a DSM diagnosis in their lifetime.4
Traits of borderline personality disorder (BPD)—such as abandonment fears, unstable relationships, identity disturbance, affective instability, emptiness, anger, mistrust, and dissociation5—that previously were seen less often are now more commonplace among our patients. These patients’ therapists have “validated” their “victimization” of “microaggressions” such that they now require “trigger warnings,” “safe spaces,” and psychiatric “diagnosis and treatment” to be able to function “normally.” These developments have also positioned parents, educators, employers, and psychiatrists, who may share “power and privilege,” to “walk on eggshells” so as not to offend newfound hypersensitivities. Interestingly, the DMN may be a major, reversible driver in BPD,6 a possible final common pathway that is further impaired by devices starting to creep into our communities and amplify the dysfunction.
Beyond treating individual patients, we must consider mandating time away from devices to nourish our DMN. During a 25-hour period each week, we (MZP and RLP) unplug from all forms of work and electronics, remember the past, consider the future, reflect on self and others, connect with nature, meditate, and eat mindfully—all of which are DMN functions. We call it Shabbat, which people have observed for thousands of years to process the week before and rejuvenate for the week ahead. Excluding smartphones from school premises has also been helpful7 and could be implemented as a nationwide commitment to the developing brains of our youth. Finally, we need to look to our profession to promote resilience over dependence, distress tolerance over avoidance, and empathic communication over “cancellation” to help heal a divisive society.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
We appreciated Dr. Nasrallah’s recent editorial1 that implicated smartphones, social media, and video game addiction, combined with the pandemic, in causing default mode network (DMN) dysfunction. The United States Surgeon General’s May 2023 report echoed these concerns and recommended limiting the use of these platforms.2 While devices are accelerants on a raging fire of mental illness, we observe a more insidious etiology that kindled the flame long before the proliferation of social media use during the pandemic. I (MZP) call this the “borderlinization” of society.
Imagine living somewhere in America that time had forgotten, where youth did not use smartphones and social media or play video games, and throughout the pandemic, people continued to congregate and socialize. These are the religious enclaves throughout New York and New Jersey that we (MZP and RLP) serve. Yet if devices were predominantly to blame for the contemporary mental health crisis, we would not expect the growing mental health problems we encounter. So, what is going on?
Over the past decade, mental health awareness has permeated all institutions of education, media, business, and government, which has increased compassion for marginalized groups. Consequently, people who may have previously silently suffered have become encouraged and supported in seeking help. That is good news. The bad news is that we have also come to pathologize, label, and attempt to treat nearly all of life’s struggles, and have been exporting mental disease around the world.3 We are losing the sense of “normal” when more than one-half of all Americans will receive a DSM diagnosis in their lifetime.4
Traits of borderline personality disorder (BPD)—such as abandonment fears, unstable relationships, identity disturbance, affective instability, emptiness, anger, mistrust, and dissociation5—that previously were seen less often are now more commonplace among our patients. These patients’ therapists have “validated” their “victimization” of “microaggressions” such that they now require “trigger warnings,” “safe spaces,” and psychiatric “diagnosis and treatment” to be able to function “normally.” These developments have also positioned parents, educators, employers, and psychiatrists, who may share “power and privilege,” to “walk on eggshells” so as not to offend newfound hypersensitivities. Interestingly, the DMN may be a major, reversible driver in BPD,6 a possible final common pathway that is further impaired by devices starting to creep into our communities and amplify the dysfunction.
Beyond treating individual patients, we must consider mandating time away from devices to nourish our DMN. During a 25-hour period each week, we (MZP and RLP) unplug from all forms of work and electronics, remember the past, consider the future, reflect on self and others, connect with nature, meditate, and eat mindfully—all of which are DMN functions. We call it Shabbat, which people have observed for thousands of years to process the week before and rejuvenate for the week ahead. Excluding smartphones from school premises has also been helpful7 and could be implemented as a nationwide commitment to the developing brains of our youth. Finally, we need to look to our profession to promote resilience over dependence, distress tolerance over avoidance, and empathic communication over “cancellation” to help heal a divisive society.
1. Nasrallah HA. Is the contemporary mental health crisis among youth due to DMN disruption? Current Psychiatry. 2023;22(6):10-11,21. doi:10.12788/cp.0372
2. U.S. Department of Health and Human Services. Surgeon general issues new advisory about effects social media use has on youth mental health. May 23, 2023. Accessed June 4, 2023. https://www.hhs.gov/about/news/2023/05/23/surgeon-general-issues-new-advisory-about-effects-social-media-use-has-youth-mental-health.html
3. Watters E. Crazy Like Us: The Globalization of the American Psyche. Free Press; 2011.
4. Centers for Disease Control and Prevention. About mental health. April 25, 2023. Accessed June 4, 2023. https://www.cdc.gov/mentalhealth/learn/index.htm
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
6. Amiri S, Mirfazeli FS, Grafman J, et al. Alternation in functional connectivity within default mode network after psychodynamic psychotherapy in borderline personality disorder. Ann Gen Psychiatry. 2023;22(1):18. doi:10.1186/s12991-023-00449-y
7. Beland LP, Murphy R. Ill communication: technology, distraction & student performance. Labour Economics. 2016;41:61-76. doi:10.1016/j.labeco.2016.04.004
1. Nasrallah HA. Is the contemporary mental health crisis among youth due to DMN disruption? Current Psychiatry. 2023;22(6):10-11,21. doi:10.12788/cp.0372
2. U.S. Department of Health and Human Services. Surgeon general issues new advisory about effects social media use has on youth mental health. May 23, 2023. Accessed June 4, 2023. https://www.hhs.gov/about/news/2023/05/23/surgeon-general-issues-new-advisory-about-effects-social-media-use-has-youth-mental-health.html
3. Watters E. Crazy Like Us: The Globalization of the American Psyche. Free Press; 2011.
4. Centers for Disease Control and Prevention. About mental health. April 25, 2023. Accessed June 4, 2023. https://www.cdc.gov/mentalhealth/learn/index.htm
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
6. Amiri S, Mirfazeli FS, Grafman J, et al. Alternation in functional connectivity within default mode network after psychodynamic psychotherapy in borderline personality disorder. Ann Gen Psychiatry. 2023;22(1):18. doi:10.1186/s12991-023-00449-y
7. Beland LP, Murphy R. Ill communication: technology, distraction & student performance. Labour Economics. 2016;41:61-76. doi:10.1016/j.labeco.2016.04.004
Treating chronic insomnia: An alternating medication strategy
Patients with chronic insomnia that does not improve with nonpharmacologic techniques often develop tolerance to sedative medications (benzodiazepines) prescribed for nightly use. When nonbenzodiazepine medications are used, tachyphylaxis can develop and these medications no longer initiate or maintain sleep. Strategies that alternate between these 2 types of agents are simple to follow and may allow patients to maintain sensitivity to both types of medications. In this article, I review the types, causes, evaluation, and treatment of insomnia; describe an alternating medication strategy to help patients avoid developing tolerance/tachyphylaxis; and present 3 fictional case vignettes to illustrate this approach.
A common, troubling condition
Insomnia is a common problem among psychiatric patients. Approximately 30% to 50% of adults experience occasional, short-term (<3 months) insomnia, and 5% to 10% experience chronic (≥3 months) insomnia,1 with associated negative impacts on health and quality of life. Insomnia is sometimes primary and may have a hereditary component, but more often is associated with medical, neurologic, or psychiatric disorders.
Patterns of insomnia include difficulty falling asleep (initial or sleep-onset insomnia), remaining asleep (middle or sleep-maintenance insomnia), or falling back asleep after early awakening (late or sleep-offset insomnia). Sleep-onset insomnia correlates with high levels of anxiety and worrying, but once asleep, patients usually stay asleep. Sleep-maintenance problems involve multiple awakenings after falling asleep and taking hours to fall back to sleep. These patients experience inadequate sleep when they must wake up early for school or work. Early-awakening patients report feeling wide awake by 4 to 5
Caffeine is an important consideration for patients with sleep difficulties. Its use is widespread in much of the world, whether ingested as coffee, tea, in soft drinks, or in “energy” drinks that may contain as much as 200 mg of caffeine (twice the amount in a typical cup of brewed coffee). Caffeine may also be ingested as an ingredient of medications for headache or migraine. While some individuals maintain that they can fall asleep easily after drinking caffeinated coffee, many may not recognize the amount of caffeine they consume and its negative impact on sleep.2 Author Michael Pollan stopped use of all caffeine and reported on the surprising positive effect on his sleep.3
Patients with mood, anxiety, or psychotic disorders are likely to experience insomnia intermittently or chronically, and insomnia predisposes some individuals to develop mood and anxiety symptoms.4 Patients with insomnia often experience anxiety focused on a fear of not getting adequate sleep, which creates a vicious cycle in which hyperarousal associated with fear of not sleeping complicates other causes of insomnia. A patient’s chronotype (preference for the time of day in which they carry out activities vs sleeping) also may play a role in sleep difficulties (Box5).
Box
Chronotypes—the expression of circadian rhythmicity in an individual—have been studied extensively.5 Psychiatrists may encounter patients who sleep most of the day and stay awake at night, those who sleep up to 20 hours per day, and those who sleep <4 hours in 24 hours. Patients typically know which category they fall into. The early bird typically is awake by 6 or 7 am, remains alert through most of the day, and feels sleepy by 10 pm. The usual diurnal variation in cortisol, with peaks at 7 am and 7 pm and nadirs at 1 pm and 1 am, correspond with the early bird’s habits.
Night owls typically report feeling exhausted and irritable in the early morning; prefer to sleep past noon; feel energized around dark, when they can do their best studying, concentrating, etc; and do not feel sleepy until early morning. While this night owl pattern is a natural variation and not necessarily associated with psychiatric illness, patients with mood disorders frequently have chaotic sleep patterns that may not conform to a pattern. Night owls maintain the same diurnal pattern of cortisol secretion as early birds.
Certain medications may contribute to insomnia, particularly stimulants. It is important to understand and explain to patients the time frame during which immediate-release or extended-release (ER) stimulants are active, which varies in individuals depending on liver enzyme activity. Other commonly used psychotropic medications—including bupropion, modafinil, armodafinil, atomoxetine, amphetamine salts, and methylphenidate—may interfere with sleep if used later in the day.6
Patients typically do not mention their use of alcohol and/or marijuana unless asked. Those who are binge drinkers or alcohol-dependent may expect alcohol to help them fall asleep, but usually find their sleep is disrupted and difficult to maintain. Patients may use marijuana to help them sleep, particularly marijuana high in tetrahydrocannabinol (THC). While it may help with sleep initiation, THC can disrupt sleep maintenance. Cannabidiol does not have intrinsic sedating effects and may even interfere with sleep.7,8
Continue to: Women may be more likely...
Women may be more likely than men to experience insomnia.9 The onset of menopause can bring hot flashes that interfere with sleep.
Women with a history of mood disorders are more likely to have a history of premenstrual dysphoric disorder, postpartum depression, and unusual responses to oral contraceptives.10 These women are more likely to report problems with mood, energy, and sleep at perimenopause. Treatment with estrogen replacement may be an option for women without risk factors, such as clotting disorders, smoking history, or a personal or family history of breast or uterine cancer. For many who are not candidates for or who refuse estrogen replacement, use of a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor at low doses may help with vasomotor symptoms but not with insomnia.
Insomnia symptoms typically increase with age.11 When sleep is adequate early in life but becomes a problem in midlife, an individual’s eating habits, obesity, and lack of exercise may be contributing factors. The typical American diet includes highly refined carbohydrates with excess salt; such foods are often readily available to the exclusion of healthy options. Overweight and obese patients may insist they eat a healthy diet with 3 meals per day, but a careful history often uncovers nighttime binge eating. Nighttime binge eating is rarely reported. This not only maintains obesity, but also interferes with sleep, since patients stay up late to avoid discovery by family members.12 This lack of sleep can lead to an endless loop because insufficient sleep is a risk factor for obesity.13
Evaluating sleep difficulties
New patient evaluations should include a careful history beginning with childhood, including personal early childhood history and family psychiatric history. Patients often report the onset of sleep difficulty and anxiety during childhood, which should raise further questions about aspects of mood regulation from early life such as concentration, energy, motivation, appetite, and academic performance. While many children and adolescents are diagnosed with attention-deficit/hyperactivity disorder due to concentration problems that cause difficulties at school, be aware this might be part of a syndrome related to mood regulation.14 Unexpected responses to an SSRI—such as agitation, euphoria, or an immediate response with the first dose—should also raise suspicion of a mood disorder. Once the underlying mood disorder is stabilized, many patients report improved sleep.15
If a patient reports having difficulty falling and remaining asleep but is not sure if there is a pattern, keeping a sleep diary can help. Further questioning may uncover the cause. Does the patient have spontaneous jerks of lower extremities (restless leg syndrome) that interfere with falling asleep or wake them up? Have they noticed problems with dreams/nightmares that wake them, which could be associated with posttraumatic stress, anxiety, or depression? Have they been told by a partner that they act out dreams or are seemingly awake but not responsive, which could point to REM sleep behavior disorder or early Parkinson’s disease? Referral to a sleep laboratory and a neurologist can help establish the correct diagnosis and point to appropriate treatment.16-18
Treatment options
Several cognitive-behavioral techniques, including cognitive-behavioral therapy for insomnia (CBT-I), yogic breathing, progressive relaxation, mindfulness meditation, and sleep hygiene techniques may help considerably,19,20 but insomnia often remains difficult to treat. Pharmacotherapy is not necessarily more effective than nonpharmacologic approaches. Both options require the patient to take initiative to either find nonpharmacologic approaches or discuss the problem with a physician and agree to take medication.21 A trial comparing CBT-I to sedatives or the combination of CBT-I plus sedatives found higher rates of sleep with CBT-I for 3 months, after which improvement fluctuated; the combination showed sustained improvement for the entire 6-month trial.22 CBT-I has also been shown to be as effective with patients who do not have psychiatric illness as for those who are depressed, anxious, or stressed.23 However, behavioral techniques that require regular practice may be difficult for individuals to maintain, particularly when they are depressed or anxious.
Continue to: Clinicians should understand...
Clinicians should understand the distinctions among the various types of pharmacotherapy for insomnia. Sedative-hypnotics include medications with varying half-lives and metabolic pathways. Short-acting benzodiazepines such as triazolam or alprazolam and the “z-drugs” zolpidem or zaleplon may help initiate sleep in patients with sleep-onset insomnia. Longer-acting benzodiazepines such as diazepam, clonazepam, or temazepam and the z-drug eszopiclone may also help with sleep maintenance.23 Based on my clinical experience, individual patients may respond better to 1 type of medication over another, or even to different agents within the same class of sedative-hypnotics.
Some clinicians prescribe nonbenzodiazepine medications for sleep, such as doxepin (which is FDA-approved for treating insomnia) or off-label trazodone, mirtazapine, or quetiapine. Their antihistaminic properties confer sedating effects. Virtually all over-the-counter (OTC) medications for insomnia are antihistaminic. These OTC medications are not designed to treat insomnia, and the optimal dosage to maintain sleep without daytime sedation must be determined by trial and error. Sedating nonbenzodiazepine medications may be slowly absorbed if taken at bedtime (depending on whether they are taken with or without food) and cause daytime sedation and cognitive slowness in patients with sleep-onset and maintenance insomnia who must wake up early. Starting trazodone at 50 to 75 mg may cause slow metabolizers to wake up with considerable sedation, while fast metabolizers might never feel soundly asleep.24
Patients with mood and anxiety disorders that complicate insomnia are often prescribed second-generation antipsychotics such as quetiapine, lurasidone, or olanzapine, which are sedating as well as mood-stabilizing. These approaches require careful attention to titrating doses and timing their use.
Problems with pharmacotherapy
When either benzodiazepines or nonbenzodiazepine medications are used on a long-standing, nightly basis, they often stop working well. It is not unusual that after days to weeks of taking a benzodiazepine, patients find they no longer stay asleep but can’t fall asleep if they don’t take them. Once tolerance develops, the individual experiences pharmacologic withdrawal with an inability to fall asleep or stay asleep. The medication becomes necessary but ineffective, and many patients increase their use to higher doses to fall asleep, and sometimes in early morning to maintain sleep. This leads to negative effects on cognition, coordination/balance, and mood during the day, especially in older patients.
Nonbenzodiazepine sedating medications do not lead to pharmacologic tolerance but do lead to tachyphylaxis as the CNS attempts to downregulate sedation to keep the organism safe. For some patients, this happens quickly, within a matter of days.25 Others increase doses to stay asleep. For example, a patient with a starting dose of trazodone 75 mg/d might increase the dosage to 300 mg/d. While trazodone is approved in doses of 300 to 600 mg as an antidepressant, it is preferable to keep doses lower when used only for sedation.
Continue to: An alternating medication strategy
An alternating medication strategy
Alternating between medications from different classes can help patients avoid developing tolerance with benzodiazepines or tachyphylaxis as occurs with antihistaminic medications. It can be effective for patients with primary insomnia as well as for those whose sleep problems are associated with mood or anxiety disorders. Patients typically maintain sensitivity to any form of pharmacologic sedation for several nights without loss of effect but need to take a break to maintain the sedation effect. For example, in 1 case study, a 30-year-old female who rapidly developed tachyphylaxis to the sedative action of mirtazapine experienced a return of the medication’s sedative effects after taking a 3-day break.25
To initiate an alternating strategy, the clinician must first help the patient establish a sedating dose of 2 medications from different classes, such as trazodone and zolpidem, and then instruct the patient to use each for 2 to 3 consecutive nights on an alternating basis. Patients can use calendars or pillboxes to avoid confusion about which medication to take on a given night. In many cases, this approach can work indefinitely.
The following 3 case vignettes illustrate how this alternating medication strategy can work.
CASE 1
Mr. B, age 58, is a married salesman whose territory includes 3 states. He drives from client to client from Monday through Thursday each week, staying overnight in hotels. He is comfortable talking to clients, has a close and supportive relationship with his wife, and enjoys socializing with friends. Mr. B has a high level of trait anxiety and perfectionism and is proud of his sales record throughout his career, but this leads to insomnia during his nights on the road, and often on Sunday night as he starts anticipating the week ahead. Mr. B denies having a depressed mood or cognitive problems. When on vacation with his wife he has no trouble sleeping. He has no psychiatric family history or any substantial medical problems. He simply wishes that he could sleep on work nights.
We set up an alternating medication approach. Mr. B takes trazodone 100 mg on the first night and 150 mg on the second and third nights. He then takes triazolam 0.25 mg for 2 nights; previously, he had found that zolpidem did not work as well for maintaining sleep. He can sleep adequately for the 2 weekend nights, then restarts the alternating pattern. Mr. B has done well with this regimen for >10 years.
Continue to: CASE 2
CASE 2
Ms. C, age 60, is widowed and has a successful career as a corporate attorney. She has been anxious since early childhood and has had trouble falling asleep for much of her life. Once she falls asleep on her sofa—often between 1 and 2
Ms. C denies having depression, but experienced appropriate grief related to her husband’s illness and death from metastatic cancer 3 years ago. At the time, her internist prescribed escitalopram and zolpidem; escitalopram caused greater agitation and distress, so she stopped it after 10 days. Zolpidem 10 mg/d allowed her to sleep but she worried about taking it because her mother had long-standing sedative dependence. Ms. C lives alone, but her adult children live nearby, and she has a strong support system that includes colleagues at her firm, friends at her book club, and a support group for partners of cancer patients.
Ms. C tries trazodone, starting with 50 mg, but reports feeling agitated rather than sleepy and has cognitive fogginess in the morning. She is switched to quetiapine 50 mg, which she tolerates well and allows her to sleep soundly. To avoid developing tachyphylaxis with quetiapine, she takes eszopiclone 3 mg for 2 nights, alternating with quetiapine for 3 nights. This strategy allows her to reliably fall asleep by 11
CASE 3
Ms. D, age 55, is married with a long-standing diagnosis of generalized anxiety disorder (GAD), panic disorder, and depression so severe she is unable to work as a preschool teacher. She notes that past clinicians have prescribed a wide array of antidepressants and benzodiazepines but she remains anxious, agitated, and unable to sleep. She worries constantly about running out of benzodiazepines, which are “the only medication that helps me.” At the time of evaluation, her medications are venlafaxine ER 150 mg/d, lorazepam 1 mg 3 times daily and 2 mg at bedtime, and buspirone 15 mg 3 times daily, which she admits to not taking. She is overweight and does not exercise. She spends her days snacking and watching television. She can’t settle down enough to read and feels overwhelmed most of the time. Her adult children won’t allow her to babysit their young children because she dozes during the day.
Ms. D has a strong family history of psychiatric illness, including a father with bipolar I disorder and alcohol use disorder and a sister with schizoaffective disorder. Ms. D has never felt overtly manic, but has spent most of her life feeling depressed, anxious, and hopeless, and at times she has wished she was dead. She has had poor responses to many antidepressants, with transient euphoria followed by more anxiety.
Continue to: Rather than major depressive disorder...
Rather than major depressive disorder or GAD, Ms. D’s symptoms better meet the criteria for bipolar II disorder. She agrees to a slow taper of venlafaxine and a slow increase of divalproex, starting with 125 mg each evening. While taking venlafaxine 75 mg/d and divalproex 375 mg/d, she experiences distinct improvement in anxiety and agitation, which further improve after venlafaxine is stopped and divalproex is increased to 750 mg in the evening. She finds that she forgets daytime doses of lorazepam but depends on it to fall asleep. While taking quetiapine 50 mg and lorazepam 1 mg at bedtime, Ms. D reports sleeping soundly and feeling alert in the morning. Over several weeks, she tapers lorazepam slowly by 0.5 mg every 2 weeks. She finds she needs a higher dose of quetiapine to stay asleep, eventually requiring 400 mg each night. Ms. D says overall she feels better but is distressed because she has gained 25 lbs since starting divalproex and quetiapine.
To avoid further increases in quetiapine and maintain its sedating effect, Ms. D is switched to an alternating schedule of clonazepam 1.5 mg for 2 nights and quetiapine 300 mg for 3 nights. She agrees to begin exercising by walking in her neighborhood daily, and gradually increases this to 1 hour per day. After starting to exercise regularly, she finds she is oversedated by quetiapine at night, so she is gradually decreased to a dose of 150 mg, while still alternating with clonazepam 1.5 mg. Ms. D loses most of the weight she had gained and begins volunteering as a reading coach in the elementary school in her neighborhood.
Bottom Line
Patients with chronic insomnia can often maintain adequate sedation without developing tolerance to benzodiazepines or tachyphylaxis with nonsedating agents by using 2 sleep medications that have different mechanisms of action on an alternating schedule.
Related Resources
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2): 307-349. doi:10.5664/jcsm.6470
- Muppavarapu K, Muthukanagaraj M, Saeed SA. Cognitive-behavioral therapy for insomnia: a review of 8 studies. Current Psychiatry. 2020;19(9):40-46. doi:10.12788/cp.0040
Drug Brand Names
Alprazolam • Xanax
Armodafinil • Nuvigil
Atomoxetine • Strattera
Bupropion • Wellbutrin
Clonazepam • Klonopin
Diazepam • Valium
Divalproex • Depakote
Doxepin • Sinequan
Escitalopram • Lexapro
Eszopiclone • Lunesta
Lorazepam • Ativan
Lurasidone • Latuda
Methylphenidate • Concerta
Mirtazapine • Remeron
Modafinil • Provigil
Olanzapine • Zyprexa
Quetiapine • Seroquel
Temazepam • Restoril
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Zaleplon • Sonata
Zolpidem • Ambien
1. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
2. Drake C, Roehrs T, Shambroom J, et al. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9(11):1195-1200.
3. Pollan M. Caffeine: How Coffee and Tea Created the Modern World. 2023; Audible Audiobooks.
4. Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021:17:2549-2566.
5. Vitale JA, Roveda E, Montaruli A, et al. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405-415.
6. Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509-517.
7. Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19(4):23.
8. Monti JM, Pandi-Perumal SR. Clinical management of sleep and sleep disorders with cannabis and cannabinoids: implications to practicing psychiatrists. Clin Neuropharmacol. 2022;45(2):27-31.
9. Dockray S, Steptoe A. Chronotype and diurnal cortisol profile in working women: differences between work and leisure days. Psychoneuroendocrinology. 2011;36(5):649-655.
10. Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(5 Suppl):S102-S108.
11. Rosenberg RP, Krystal AD. Diagnosing and treating insomnia in adults and older adults. J Clin Psychiatry. 2021;82(6):59-66.
12. Stunkard A. Eating disorders and obesity. Psychiatr Clin North Am. 2011; 34(4):765-771.
13. Crönlein T. Insomnia and obesity. Curr Opin Psychiatry. 2016;29(6):409-412.
14. Gillberg C, Gillberg IC, Rasmussen P, et al. Co-existing disorders in ADHD -- implications for diagnosis and intervention. Eur Child Adolesc Psychiatry. 2004; 1(Suppl 1):i80-i92.
15. Goldberg JF, Nierenberg AA, Iosifescu DV. Wrestling with antidepressant use in bipolar disorder: the ongoing debate. J Clin Psychiatry. 2021;82(1):19. doi:10.4088/JCP.19ac13181
16. Baltzan M, Yao C, Rizzo D, et al. Dream enactment behavior: review for the clinician. J Clin Sleep Med. 2020;16(11):1949-1969.
17. Barone DA. Dream enactment behavior—a real nightmare: a review of post-traumatic stress disorder, REM sleep behavior disorder, and trauma-associated sleep disorder. J Clin Sleep Med. 2020;16(11):1943-1948.
18. Figorilli M, Meloni M, Lanza G, et al. Considering REM sleep behavior disorder in the management of Parkinson’s disease. Nat Sci Sleep. 2023;15:333-352.
19. Rios P, Cardoso R, Morra D, et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281-297.
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139.
21. Lu M, Zhang Y, Zhang J, et al. Comparative effectiveness of digital cognitive behavioral therapy vs. medication therapy among patients with insomnia. JAMA Network Open. 2023;6(4):e237597.
22. Sweetman A, McEvoy RD, Catcheside PG, et al. Effect of depression, anxiety, and stress symptoms on response to cognitive behavioral therapy for insomnia in patients with comorbid insomnia and sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2021;17(3):545-554.
23. O’Brien CP. Benzodiazepine use, abuse and dependence. J Clin Psychiatry. 2005;66(Suppl 2):28-33.
24. Wichniak A, Wierzbicka AE, Jarema M. Treatment of insomnia - effect of trazodone and hypnotics on sleep. Psychiatr Pol. 2021;55(4):743-755.
25. Papazisis G, Siafis S, Tzachanis D. Tachyphylaxis to the sedative action of mirtazapine. Am J Case Rep. 2018;19:410-412.
Patients with chronic insomnia that does not improve with nonpharmacologic techniques often develop tolerance to sedative medications (benzodiazepines) prescribed for nightly use. When nonbenzodiazepine medications are used, tachyphylaxis can develop and these medications no longer initiate or maintain sleep. Strategies that alternate between these 2 types of agents are simple to follow and may allow patients to maintain sensitivity to both types of medications. In this article, I review the types, causes, evaluation, and treatment of insomnia; describe an alternating medication strategy to help patients avoid developing tolerance/tachyphylaxis; and present 3 fictional case vignettes to illustrate this approach.
A common, troubling condition
Insomnia is a common problem among psychiatric patients. Approximately 30% to 50% of adults experience occasional, short-term (<3 months) insomnia, and 5% to 10% experience chronic (≥3 months) insomnia,1 with associated negative impacts on health and quality of life. Insomnia is sometimes primary and may have a hereditary component, but more often is associated with medical, neurologic, or psychiatric disorders.
Patterns of insomnia include difficulty falling asleep (initial or sleep-onset insomnia), remaining asleep (middle or sleep-maintenance insomnia), or falling back asleep after early awakening (late or sleep-offset insomnia). Sleep-onset insomnia correlates with high levels of anxiety and worrying, but once asleep, patients usually stay asleep. Sleep-maintenance problems involve multiple awakenings after falling asleep and taking hours to fall back to sleep. These patients experience inadequate sleep when they must wake up early for school or work. Early-awakening patients report feeling wide awake by 4 to 5
Caffeine is an important consideration for patients with sleep difficulties. Its use is widespread in much of the world, whether ingested as coffee, tea, in soft drinks, or in “energy” drinks that may contain as much as 200 mg of caffeine (twice the amount in a typical cup of brewed coffee). Caffeine may also be ingested as an ingredient of medications for headache or migraine. While some individuals maintain that they can fall asleep easily after drinking caffeinated coffee, many may not recognize the amount of caffeine they consume and its negative impact on sleep.2 Author Michael Pollan stopped use of all caffeine and reported on the surprising positive effect on his sleep.3
Patients with mood, anxiety, or psychotic disorders are likely to experience insomnia intermittently or chronically, and insomnia predisposes some individuals to develop mood and anxiety symptoms.4 Patients with insomnia often experience anxiety focused on a fear of not getting adequate sleep, which creates a vicious cycle in which hyperarousal associated with fear of not sleeping complicates other causes of insomnia. A patient’s chronotype (preference for the time of day in which they carry out activities vs sleeping) also may play a role in sleep difficulties (Box5).
Box
Chronotypes—the expression of circadian rhythmicity in an individual—have been studied extensively.5 Psychiatrists may encounter patients who sleep most of the day and stay awake at night, those who sleep up to 20 hours per day, and those who sleep <4 hours in 24 hours. Patients typically know which category they fall into. The early bird typically is awake by 6 or 7 am, remains alert through most of the day, and feels sleepy by 10 pm. The usual diurnal variation in cortisol, with peaks at 7 am and 7 pm and nadirs at 1 pm and 1 am, correspond with the early bird’s habits.
Night owls typically report feeling exhausted and irritable in the early morning; prefer to sleep past noon; feel energized around dark, when they can do their best studying, concentrating, etc; and do not feel sleepy until early morning. While this night owl pattern is a natural variation and not necessarily associated with psychiatric illness, patients with mood disorders frequently have chaotic sleep patterns that may not conform to a pattern. Night owls maintain the same diurnal pattern of cortisol secretion as early birds.
Certain medications may contribute to insomnia, particularly stimulants. It is important to understand and explain to patients the time frame during which immediate-release or extended-release (ER) stimulants are active, which varies in individuals depending on liver enzyme activity. Other commonly used psychotropic medications—including bupropion, modafinil, armodafinil, atomoxetine, amphetamine salts, and methylphenidate—may interfere with sleep if used later in the day.6
Patients typically do not mention their use of alcohol and/or marijuana unless asked. Those who are binge drinkers or alcohol-dependent may expect alcohol to help them fall asleep, but usually find their sleep is disrupted and difficult to maintain. Patients may use marijuana to help them sleep, particularly marijuana high in tetrahydrocannabinol (THC). While it may help with sleep initiation, THC can disrupt sleep maintenance. Cannabidiol does not have intrinsic sedating effects and may even interfere with sleep.7,8
Continue to: Women may be more likely...
Women may be more likely than men to experience insomnia.9 The onset of menopause can bring hot flashes that interfere with sleep.
Women with a history of mood disorders are more likely to have a history of premenstrual dysphoric disorder, postpartum depression, and unusual responses to oral contraceptives.10 These women are more likely to report problems with mood, energy, and sleep at perimenopause. Treatment with estrogen replacement may be an option for women without risk factors, such as clotting disorders, smoking history, or a personal or family history of breast or uterine cancer. For many who are not candidates for or who refuse estrogen replacement, use of a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor at low doses may help with vasomotor symptoms but not with insomnia.
Insomnia symptoms typically increase with age.11 When sleep is adequate early in life but becomes a problem in midlife, an individual’s eating habits, obesity, and lack of exercise may be contributing factors. The typical American diet includes highly refined carbohydrates with excess salt; such foods are often readily available to the exclusion of healthy options. Overweight and obese patients may insist they eat a healthy diet with 3 meals per day, but a careful history often uncovers nighttime binge eating. Nighttime binge eating is rarely reported. This not only maintains obesity, but also interferes with sleep, since patients stay up late to avoid discovery by family members.12 This lack of sleep can lead to an endless loop because insufficient sleep is a risk factor for obesity.13
Evaluating sleep difficulties
New patient evaluations should include a careful history beginning with childhood, including personal early childhood history and family psychiatric history. Patients often report the onset of sleep difficulty and anxiety during childhood, which should raise further questions about aspects of mood regulation from early life such as concentration, energy, motivation, appetite, and academic performance. While many children and adolescents are diagnosed with attention-deficit/hyperactivity disorder due to concentration problems that cause difficulties at school, be aware this might be part of a syndrome related to mood regulation.14 Unexpected responses to an SSRI—such as agitation, euphoria, or an immediate response with the first dose—should also raise suspicion of a mood disorder. Once the underlying mood disorder is stabilized, many patients report improved sleep.15
If a patient reports having difficulty falling and remaining asleep but is not sure if there is a pattern, keeping a sleep diary can help. Further questioning may uncover the cause. Does the patient have spontaneous jerks of lower extremities (restless leg syndrome) that interfere with falling asleep or wake them up? Have they noticed problems with dreams/nightmares that wake them, which could be associated with posttraumatic stress, anxiety, or depression? Have they been told by a partner that they act out dreams or are seemingly awake but not responsive, which could point to REM sleep behavior disorder or early Parkinson’s disease? Referral to a sleep laboratory and a neurologist can help establish the correct diagnosis and point to appropriate treatment.16-18
Treatment options
Several cognitive-behavioral techniques, including cognitive-behavioral therapy for insomnia (CBT-I), yogic breathing, progressive relaxation, mindfulness meditation, and sleep hygiene techniques may help considerably,19,20 but insomnia often remains difficult to treat. Pharmacotherapy is not necessarily more effective than nonpharmacologic approaches. Both options require the patient to take initiative to either find nonpharmacologic approaches or discuss the problem with a physician and agree to take medication.21 A trial comparing CBT-I to sedatives or the combination of CBT-I plus sedatives found higher rates of sleep with CBT-I for 3 months, after which improvement fluctuated; the combination showed sustained improvement for the entire 6-month trial.22 CBT-I has also been shown to be as effective with patients who do not have psychiatric illness as for those who are depressed, anxious, or stressed.23 However, behavioral techniques that require regular practice may be difficult for individuals to maintain, particularly when they are depressed or anxious.
Continue to: Clinicians should understand...
Clinicians should understand the distinctions among the various types of pharmacotherapy for insomnia. Sedative-hypnotics include medications with varying half-lives and metabolic pathways. Short-acting benzodiazepines such as triazolam or alprazolam and the “z-drugs” zolpidem or zaleplon may help initiate sleep in patients with sleep-onset insomnia. Longer-acting benzodiazepines such as diazepam, clonazepam, or temazepam and the z-drug eszopiclone may also help with sleep maintenance.23 Based on my clinical experience, individual patients may respond better to 1 type of medication over another, or even to different agents within the same class of sedative-hypnotics.
Some clinicians prescribe nonbenzodiazepine medications for sleep, such as doxepin (which is FDA-approved for treating insomnia) or off-label trazodone, mirtazapine, or quetiapine. Their antihistaminic properties confer sedating effects. Virtually all over-the-counter (OTC) medications for insomnia are antihistaminic. These OTC medications are not designed to treat insomnia, and the optimal dosage to maintain sleep without daytime sedation must be determined by trial and error. Sedating nonbenzodiazepine medications may be slowly absorbed if taken at bedtime (depending on whether they are taken with or without food) and cause daytime sedation and cognitive slowness in patients with sleep-onset and maintenance insomnia who must wake up early. Starting trazodone at 50 to 75 mg may cause slow metabolizers to wake up with considerable sedation, while fast metabolizers might never feel soundly asleep.24
Patients with mood and anxiety disorders that complicate insomnia are often prescribed second-generation antipsychotics such as quetiapine, lurasidone, or olanzapine, which are sedating as well as mood-stabilizing. These approaches require careful attention to titrating doses and timing their use.
Problems with pharmacotherapy
When either benzodiazepines or nonbenzodiazepine medications are used on a long-standing, nightly basis, they often stop working well. It is not unusual that after days to weeks of taking a benzodiazepine, patients find they no longer stay asleep but can’t fall asleep if they don’t take them. Once tolerance develops, the individual experiences pharmacologic withdrawal with an inability to fall asleep or stay asleep. The medication becomes necessary but ineffective, and many patients increase their use to higher doses to fall asleep, and sometimes in early morning to maintain sleep. This leads to negative effects on cognition, coordination/balance, and mood during the day, especially in older patients.
Nonbenzodiazepine sedating medications do not lead to pharmacologic tolerance but do lead to tachyphylaxis as the CNS attempts to downregulate sedation to keep the organism safe. For some patients, this happens quickly, within a matter of days.25 Others increase doses to stay asleep. For example, a patient with a starting dose of trazodone 75 mg/d might increase the dosage to 300 mg/d. While trazodone is approved in doses of 300 to 600 mg as an antidepressant, it is preferable to keep doses lower when used only for sedation.
Continue to: An alternating medication strategy
An alternating medication strategy
Alternating between medications from different classes can help patients avoid developing tolerance with benzodiazepines or tachyphylaxis as occurs with antihistaminic medications. It can be effective for patients with primary insomnia as well as for those whose sleep problems are associated with mood or anxiety disorders. Patients typically maintain sensitivity to any form of pharmacologic sedation for several nights without loss of effect but need to take a break to maintain the sedation effect. For example, in 1 case study, a 30-year-old female who rapidly developed tachyphylaxis to the sedative action of mirtazapine experienced a return of the medication’s sedative effects after taking a 3-day break.25
To initiate an alternating strategy, the clinician must first help the patient establish a sedating dose of 2 medications from different classes, such as trazodone and zolpidem, and then instruct the patient to use each for 2 to 3 consecutive nights on an alternating basis. Patients can use calendars or pillboxes to avoid confusion about which medication to take on a given night. In many cases, this approach can work indefinitely.
The following 3 case vignettes illustrate how this alternating medication strategy can work.
CASE 1
Mr. B, age 58, is a married salesman whose territory includes 3 states. He drives from client to client from Monday through Thursday each week, staying overnight in hotels. He is comfortable talking to clients, has a close and supportive relationship with his wife, and enjoys socializing with friends. Mr. B has a high level of trait anxiety and perfectionism and is proud of his sales record throughout his career, but this leads to insomnia during his nights on the road, and often on Sunday night as he starts anticipating the week ahead. Mr. B denies having a depressed mood or cognitive problems. When on vacation with his wife he has no trouble sleeping. He has no psychiatric family history or any substantial medical problems. He simply wishes that he could sleep on work nights.
We set up an alternating medication approach. Mr. B takes trazodone 100 mg on the first night and 150 mg on the second and third nights. He then takes triazolam 0.25 mg for 2 nights; previously, he had found that zolpidem did not work as well for maintaining sleep. He can sleep adequately for the 2 weekend nights, then restarts the alternating pattern. Mr. B has done well with this regimen for >10 years.
Continue to: CASE 2
CASE 2
Ms. C, age 60, is widowed and has a successful career as a corporate attorney. She has been anxious since early childhood and has had trouble falling asleep for much of her life. Once she falls asleep on her sofa—often between 1 and 2
Ms. C denies having depression, but experienced appropriate grief related to her husband’s illness and death from metastatic cancer 3 years ago. At the time, her internist prescribed escitalopram and zolpidem; escitalopram caused greater agitation and distress, so she stopped it after 10 days. Zolpidem 10 mg/d allowed her to sleep but she worried about taking it because her mother had long-standing sedative dependence. Ms. C lives alone, but her adult children live nearby, and she has a strong support system that includes colleagues at her firm, friends at her book club, and a support group for partners of cancer patients.
Ms. C tries trazodone, starting with 50 mg, but reports feeling agitated rather than sleepy and has cognitive fogginess in the morning. She is switched to quetiapine 50 mg, which she tolerates well and allows her to sleep soundly. To avoid developing tachyphylaxis with quetiapine, she takes eszopiclone 3 mg for 2 nights, alternating with quetiapine for 3 nights. This strategy allows her to reliably fall asleep by 11
CASE 3
Ms. D, age 55, is married with a long-standing diagnosis of generalized anxiety disorder (GAD), panic disorder, and depression so severe she is unable to work as a preschool teacher. She notes that past clinicians have prescribed a wide array of antidepressants and benzodiazepines but she remains anxious, agitated, and unable to sleep. She worries constantly about running out of benzodiazepines, which are “the only medication that helps me.” At the time of evaluation, her medications are venlafaxine ER 150 mg/d, lorazepam 1 mg 3 times daily and 2 mg at bedtime, and buspirone 15 mg 3 times daily, which she admits to not taking. She is overweight and does not exercise. She spends her days snacking and watching television. She can’t settle down enough to read and feels overwhelmed most of the time. Her adult children won’t allow her to babysit their young children because she dozes during the day.
Ms. D has a strong family history of psychiatric illness, including a father with bipolar I disorder and alcohol use disorder and a sister with schizoaffective disorder. Ms. D has never felt overtly manic, but has spent most of her life feeling depressed, anxious, and hopeless, and at times she has wished she was dead. She has had poor responses to many antidepressants, with transient euphoria followed by more anxiety.
Continue to: Rather than major depressive disorder...
Rather than major depressive disorder or GAD, Ms. D’s symptoms better meet the criteria for bipolar II disorder. She agrees to a slow taper of venlafaxine and a slow increase of divalproex, starting with 125 mg each evening. While taking venlafaxine 75 mg/d and divalproex 375 mg/d, she experiences distinct improvement in anxiety and agitation, which further improve after venlafaxine is stopped and divalproex is increased to 750 mg in the evening. She finds that she forgets daytime doses of lorazepam but depends on it to fall asleep. While taking quetiapine 50 mg and lorazepam 1 mg at bedtime, Ms. D reports sleeping soundly and feeling alert in the morning. Over several weeks, she tapers lorazepam slowly by 0.5 mg every 2 weeks. She finds she needs a higher dose of quetiapine to stay asleep, eventually requiring 400 mg each night. Ms. D says overall she feels better but is distressed because she has gained 25 lbs since starting divalproex and quetiapine.
To avoid further increases in quetiapine and maintain its sedating effect, Ms. D is switched to an alternating schedule of clonazepam 1.5 mg for 2 nights and quetiapine 300 mg for 3 nights. She agrees to begin exercising by walking in her neighborhood daily, and gradually increases this to 1 hour per day. After starting to exercise regularly, she finds she is oversedated by quetiapine at night, so she is gradually decreased to a dose of 150 mg, while still alternating with clonazepam 1.5 mg. Ms. D loses most of the weight she had gained and begins volunteering as a reading coach in the elementary school in her neighborhood.
Bottom Line
Patients with chronic insomnia can often maintain adequate sedation without developing tolerance to benzodiazepines or tachyphylaxis with nonsedating agents by using 2 sleep medications that have different mechanisms of action on an alternating schedule.
Related Resources
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2): 307-349. doi:10.5664/jcsm.6470
- Muppavarapu K, Muthukanagaraj M, Saeed SA. Cognitive-behavioral therapy for insomnia: a review of 8 studies. Current Psychiatry. 2020;19(9):40-46. doi:10.12788/cp.0040
Drug Brand Names
Alprazolam • Xanax
Armodafinil • Nuvigil
Atomoxetine • Strattera
Bupropion • Wellbutrin
Clonazepam • Klonopin
Diazepam • Valium
Divalproex • Depakote
Doxepin • Sinequan
Escitalopram • Lexapro
Eszopiclone • Lunesta
Lorazepam • Ativan
Lurasidone • Latuda
Methylphenidate • Concerta
Mirtazapine • Remeron
Modafinil • Provigil
Olanzapine • Zyprexa
Quetiapine • Seroquel
Temazepam • Restoril
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Zaleplon • Sonata
Zolpidem • Ambien
Patients with chronic insomnia that does not improve with nonpharmacologic techniques often develop tolerance to sedative medications (benzodiazepines) prescribed for nightly use. When nonbenzodiazepine medications are used, tachyphylaxis can develop and these medications no longer initiate or maintain sleep. Strategies that alternate between these 2 types of agents are simple to follow and may allow patients to maintain sensitivity to both types of medications. In this article, I review the types, causes, evaluation, and treatment of insomnia; describe an alternating medication strategy to help patients avoid developing tolerance/tachyphylaxis; and present 3 fictional case vignettes to illustrate this approach.
A common, troubling condition
Insomnia is a common problem among psychiatric patients. Approximately 30% to 50% of adults experience occasional, short-term (<3 months) insomnia, and 5% to 10% experience chronic (≥3 months) insomnia,1 with associated negative impacts on health and quality of life. Insomnia is sometimes primary and may have a hereditary component, but more often is associated with medical, neurologic, or psychiatric disorders.
Patterns of insomnia include difficulty falling asleep (initial or sleep-onset insomnia), remaining asleep (middle or sleep-maintenance insomnia), or falling back asleep after early awakening (late or sleep-offset insomnia). Sleep-onset insomnia correlates with high levels of anxiety and worrying, but once asleep, patients usually stay asleep. Sleep-maintenance problems involve multiple awakenings after falling asleep and taking hours to fall back to sleep. These patients experience inadequate sleep when they must wake up early for school or work. Early-awakening patients report feeling wide awake by 4 to 5
Caffeine is an important consideration for patients with sleep difficulties. Its use is widespread in much of the world, whether ingested as coffee, tea, in soft drinks, or in “energy” drinks that may contain as much as 200 mg of caffeine (twice the amount in a typical cup of brewed coffee). Caffeine may also be ingested as an ingredient of medications for headache or migraine. While some individuals maintain that they can fall asleep easily after drinking caffeinated coffee, many may not recognize the amount of caffeine they consume and its negative impact on sleep.2 Author Michael Pollan stopped use of all caffeine and reported on the surprising positive effect on his sleep.3
Patients with mood, anxiety, or psychotic disorders are likely to experience insomnia intermittently or chronically, and insomnia predisposes some individuals to develop mood and anxiety symptoms.4 Patients with insomnia often experience anxiety focused on a fear of not getting adequate sleep, which creates a vicious cycle in which hyperarousal associated with fear of not sleeping complicates other causes of insomnia. A patient’s chronotype (preference for the time of day in which they carry out activities vs sleeping) also may play a role in sleep difficulties (Box5).
Box
Chronotypes—the expression of circadian rhythmicity in an individual—have been studied extensively.5 Psychiatrists may encounter patients who sleep most of the day and stay awake at night, those who sleep up to 20 hours per day, and those who sleep <4 hours in 24 hours. Patients typically know which category they fall into. The early bird typically is awake by 6 or 7 am, remains alert through most of the day, and feels sleepy by 10 pm. The usual diurnal variation in cortisol, with peaks at 7 am and 7 pm and nadirs at 1 pm and 1 am, correspond with the early bird’s habits.
Night owls typically report feeling exhausted and irritable in the early morning; prefer to sleep past noon; feel energized around dark, when they can do their best studying, concentrating, etc; and do not feel sleepy until early morning. While this night owl pattern is a natural variation and not necessarily associated with psychiatric illness, patients with mood disorders frequently have chaotic sleep patterns that may not conform to a pattern. Night owls maintain the same diurnal pattern of cortisol secretion as early birds.
Certain medications may contribute to insomnia, particularly stimulants. It is important to understand and explain to patients the time frame during which immediate-release or extended-release (ER) stimulants are active, which varies in individuals depending on liver enzyme activity. Other commonly used psychotropic medications—including bupropion, modafinil, armodafinil, atomoxetine, amphetamine salts, and methylphenidate—may interfere with sleep if used later in the day.6
Patients typically do not mention their use of alcohol and/or marijuana unless asked. Those who are binge drinkers or alcohol-dependent may expect alcohol to help them fall asleep, but usually find their sleep is disrupted and difficult to maintain. Patients may use marijuana to help them sleep, particularly marijuana high in tetrahydrocannabinol (THC). While it may help with sleep initiation, THC can disrupt sleep maintenance. Cannabidiol does not have intrinsic sedating effects and may even interfere with sleep.7,8
Continue to: Women may be more likely...
Women may be more likely than men to experience insomnia.9 The onset of menopause can bring hot flashes that interfere with sleep.
Women with a history of mood disorders are more likely to have a history of premenstrual dysphoric disorder, postpartum depression, and unusual responses to oral contraceptives.10 These women are more likely to report problems with mood, energy, and sleep at perimenopause. Treatment with estrogen replacement may be an option for women without risk factors, such as clotting disorders, smoking history, or a personal or family history of breast or uterine cancer. For many who are not candidates for or who refuse estrogen replacement, use of a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor at low doses may help with vasomotor symptoms but not with insomnia.
Insomnia symptoms typically increase with age.11 When sleep is adequate early in life but becomes a problem in midlife, an individual’s eating habits, obesity, and lack of exercise may be contributing factors. The typical American diet includes highly refined carbohydrates with excess salt; such foods are often readily available to the exclusion of healthy options. Overweight and obese patients may insist they eat a healthy diet with 3 meals per day, but a careful history often uncovers nighttime binge eating. Nighttime binge eating is rarely reported. This not only maintains obesity, but also interferes with sleep, since patients stay up late to avoid discovery by family members.12 This lack of sleep can lead to an endless loop because insufficient sleep is a risk factor for obesity.13
Evaluating sleep difficulties
New patient evaluations should include a careful history beginning with childhood, including personal early childhood history and family psychiatric history. Patients often report the onset of sleep difficulty and anxiety during childhood, which should raise further questions about aspects of mood regulation from early life such as concentration, energy, motivation, appetite, and academic performance. While many children and adolescents are diagnosed with attention-deficit/hyperactivity disorder due to concentration problems that cause difficulties at school, be aware this might be part of a syndrome related to mood regulation.14 Unexpected responses to an SSRI—such as agitation, euphoria, or an immediate response with the first dose—should also raise suspicion of a mood disorder. Once the underlying mood disorder is stabilized, many patients report improved sleep.15
If a patient reports having difficulty falling and remaining asleep but is not sure if there is a pattern, keeping a sleep diary can help. Further questioning may uncover the cause. Does the patient have spontaneous jerks of lower extremities (restless leg syndrome) that interfere with falling asleep or wake them up? Have they noticed problems with dreams/nightmares that wake them, which could be associated with posttraumatic stress, anxiety, or depression? Have they been told by a partner that they act out dreams or are seemingly awake but not responsive, which could point to REM sleep behavior disorder or early Parkinson’s disease? Referral to a sleep laboratory and a neurologist can help establish the correct diagnosis and point to appropriate treatment.16-18
Treatment options
Several cognitive-behavioral techniques, including cognitive-behavioral therapy for insomnia (CBT-I), yogic breathing, progressive relaxation, mindfulness meditation, and sleep hygiene techniques may help considerably,19,20 but insomnia often remains difficult to treat. Pharmacotherapy is not necessarily more effective than nonpharmacologic approaches. Both options require the patient to take initiative to either find nonpharmacologic approaches or discuss the problem with a physician and agree to take medication.21 A trial comparing CBT-I to sedatives or the combination of CBT-I plus sedatives found higher rates of sleep with CBT-I for 3 months, after which improvement fluctuated; the combination showed sustained improvement for the entire 6-month trial.22 CBT-I has also been shown to be as effective with patients who do not have psychiatric illness as for those who are depressed, anxious, or stressed.23 However, behavioral techniques that require regular practice may be difficult for individuals to maintain, particularly when they are depressed or anxious.
Continue to: Clinicians should understand...
Clinicians should understand the distinctions among the various types of pharmacotherapy for insomnia. Sedative-hypnotics include medications with varying half-lives and metabolic pathways. Short-acting benzodiazepines such as triazolam or alprazolam and the “z-drugs” zolpidem or zaleplon may help initiate sleep in patients with sleep-onset insomnia. Longer-acting benzodiazepines such as diazepam, clonazepam, or temazepam and the z-drug eszopiclone may also help with sleep maintenance.23 Based on my clinical experience, individual patients may respond better to 1 type of medication over another, or even to different agents within the same class of sedative-hypnotics.
Some clinicians prescribe nonbenzodiazepine medications for sleep, such as doxepin (which is FDA-approved for treating insomnia) or off-label trazodone, mirtazapine, or quetiapine. Their antihistaminic properties confer sedating effects. Virtually all over-the-counter (OTC) medications for insomnia are antihistaminic. These OTC medications are not designed to treat insomnia, and the optimal dosage to maintain sleep without daytime sedation must be determined by trial and error. Sedating nonbenzodiazepine medications may be slowly absorbed if taken at bedtime (depending on whether they are taken with or without food) and cause daytime sedation and cognitive slowness in patients with sleep-onset and maintenance insomnia who must wake up early. Starting trazodone at 50 to 75 mg may cause slow metabolizers to wake up with considerable sedation, while fast metabolizers might never feel soundly asleep.24
Patients with mood and anxiety disorders that complicate insomnia are often prescribed second-generation antipsychotics such as quetiapine, lurasidone, or olanzapine, which are sedating as well as mood-stabilizing. These approaches require careful attention to titrating doses and timing their use.
Problems with pharmacotherapy
When either benzodiazepines or nonbenzodiazepine medications are used on a long-standing, nightly basis, they often stop working well. It is not unusual that after days to weeks of taking a benzodiazepine, patients find they no longer stay asleep but can’t fall asleep if they don’t take them. Once tolerance develops, the individual experiences pharmacologic withdrawal with an inability to fall asleep or stay asleep. The medication becomes necessary but ineffective, and many patients increase their use to higher doses to fall asleep, and sometimes in early morning to maintain sleep. This leads to negative effects on cognition, coordination/balance, and mood during the day, especially in older patients.
Nonbenzodiazepine sedating medications do not lead to pharmacologic tolerance but do lead to tachyphylaxis as the CNS attempts to downregulate sedation to keep the organism safe. For some patients, this happens quickly, within a matter of days.25 Others increase doses to stay asleep. For example, a patient with a starting dose of trazodone 75 mg/d might increase the dosage to 300 mg/d. While trazodone is approved in doses of 300 to 600 mg as an antidepressant, it is preferable to keep doses lower when used only for sedation.
Continue to: An alternating medication strategy
An alternating medication strategy
Alternating between medications from different classes can help patients avoid developing tolerance with benzodiazepines or tachyphylaxis as occurs with antihistaminic medications. It can be effective for patients with primary insomnia as well as for those whose sleep problems are associated with mood or anxiety disorders. Patients typically maintain sensitivity to any form of pharmacologic sedation for several nights without loss of effect but need to take a break to maintain the sedation effect. For example, in 1 case study, a 30-year-old female who rapidly developed tachyphylaxis to the sedative action of mirtazapine experienced a return of the medication’s sedative effects after taking a 3-day break.25
To initiate an alternating strategy, the clinician must first help the patient establish a sedating dose of 2 medications from different classes, such as trazodone and zolpidem, and then instruct the patient to use each for 2 to 3 consecutive nights on an alternating basis. Patients can use calendars or pillboxes to avoid confusion about which medication to take on a given night. In many cases, this approach can work indefinitely.
The following 3 case vignettes illustrate how this alternating medication strategy can work.
CASE 1
Mr. B, age 58, is a married salesman whose territory includes 3 states. He drives from client to client from Monday through Thursday each week, staying overnight in hotels. He is comfortable talking to clients, has a close and supportive relationship with his wife, and enjoys socializing with friends. Mr. B has a high level of trait anxiety and perfectionism and is proud of his sales record throughout his career, but this leads to insomnia during his nights on the road, and often on Sunday night as he starts anticipating the week ahead. Mr. B denies having a depressed mood or cognitive problems. When on vacation with his wife he has no trouble sleeping. He has no psychiatric family history or any substantial medical problems. He simply wishes that he could sleep on work nights.
We set up an alternating medication approach. Mr. B takes trazodone 100 mg on the first night and 150 mg on the second and third nights. He then takes triazolam 0.25 mg for 2 nights; previously, he had found that zolpidem did not work as well for maintaining sleep. He can sleep adequately for the 2 weekend nights, then restarts the alternating pattern. Mr. B has done well with this regimen for >10 years.
Continue to: CASE 2
CASE 2
Ms. C, age 60, is widowed and has a successful career as a corporate attorney. She has been anxious since early childhood and has had trouble falling asleep for much of her life. Once she falls asleep on her sofa—often between 1 and 2
Ms. C denies having depression, but experienced appropriate grief related to her husband’s illness and death from metastatic cancer 3 years ago. At the time, her internist prescribed escitalopram and zolpidem; escitalopram caused greater agitation and distress, so she stopped it after 10 days. Zolpidem 10 mg/d allowed her to sleep but she worried about taking it because her mother had long-standing sedative dependence. Ms. C lives alone, but her adult children live nearby, and she has a strong support system that includes colleagues at her firm, friends at her book club, and a support group for partners of cancer patients.
Ms. C tries trazodone, starting with 50 mg, but reports feeling agitated rather than sleepy and has cognitive fogginess in the morning. She is switched to quetiapine 50 mg, which she tolerates well and allows her to sleep soundly. To avoid developing tachyphylaxis with quetiapine, she takes eszopiclone 3 mg for 2 nights, alternating with quetiapine for 3 nights. This strategy allows her to reliably fall asleep by 11
CASE 3
Ms. D, age 55, is married with a long-standing diagnosis of generalized anxiety disorder (GAD), panic disorder, and depression so severe she is unable to work as a preschool teacher. She notes that past clinicians have prescribed a wide array of antidepressants and benzodiazepines but she remains anxious, agitated, and unable to sleep. She worries constantly about running out of benzodiazepines, which are “the only medication that helps me.” At the time of evaluation, her medications are venlafaxine ER 150 mg/d, lorazepam 1 mg 3 times daily and 2 mg at bedtime, and buspirone 15 mg 3 times daily, which she admits to not taking. She is overweight and does not exercise. She spends her days snacking and watching television. She can’t settle down enough to read and feels overwhelmed most of the time. Her adult children won’t allow her to babysit their young children because she dozes during the day.
Ms. D has a strong family history of psychiatric illness, including a father with bipolar I disorder and alcohol use disorder and a sister with schizoaffective disorder. Ms. D has never felt overtly manic, but has spent most of her life feeling depressed, anxious, and hopeless, and at times she has wished she was dead. She has had poor responses to many antidepressants, with transient euphoria followed by more anxiety.
Continue to: Rather than major depressive disorder...
Rather than major depressive disorder or GAD, Ms. D’s symptoms better meet the criteria for bipolar II disorder. She agrees to a slow taper of venlafaxine and a slow increase of divalproex, starting with 125 mg each evening. While taking venlafaxine 75 mg/d and divalproex 375 mg/d, she experiences distinct improvement in anxiety and agitation, which further improve after venlafaxine is stopped and divalproex is increased to 750 mg in the evening. She finds that she forgets daytime doses of lorazepam but depends on it to fall asleep. While taking quetiapine 50 mg and lorazepam 1 mg at bedtime, Ms. D reports sleeping soundly and feeling alert in the morning. Over several weeks, she tapers lorazepam slowly by 0.5 mg every 2 weeks. She finds she needs a higher dose of quetiapine to stay asleep, eventually requiring 400 mg each night. Ms. D says overall she feels better but is distressed because she has gained 25 lbs since starting divalproex and quetiapine.
To avoid further increases in quetiapine and maintain its sedating effect, Ms. D is switched to an alternating schedule of clonazepam 1.5 mg for 2 nights and quetiapine 300 mg for 3 nights. She agrees to begin exercising by walking in her neighborhood daily, and gradually increases this to 1 hour per day. After starting to exercise regularly, she finds she is oversedated by quetiapine at night, so she is gradually decreased to a dose of 150 mg, while still alternating with clonazepam 1.5 mg. Ms. D loses most of the weight she had gained and begins volunteering as a reading coach in the elementary school in her neighborhood.
Bottom Line
Patients with chronic insomnia can often maintain adequate sedation without developing tolerance to benzodiazepines or tachyphylaxis with nonsedating agents by using 2 sleep medications that have different mechanisms of action on an alternating schedule.
Related Resources
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2): 307-349. doi:10.5664/jcsm.6470
- Muppavarapu K, Muthukanagaraj M, Saeed SA. Cognitive-behavioral therapy for insomnia: a review of 8 studies. Current Psychiatry. 2020;19(9):40-46. doi:10.12788/cp.0040
Drug Brand Names
Alprazolam • Xanax
Armodafinil • Nuvigil
Atomoxetine • Strattera
Bupropion • Wellbutrin
Clonazepam • Klonopin
Diazepam • Valium
Divalproex • Depakote
Doxepin • Sinequan
Escitalopram • Lexapro
Eszopiclone • Lunesta
Lorazepam • Ativan
Lurasidone • Latuda
Methylphenidate • Concerta
Mirtazapine • Remeron
Modafinil • Provigil
Olanzapine • Zyprexa
Quetiapine • Seroquel
Temazepam • Restoril
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Zaleplon • Sonata
Zolpidem • Ambien
1. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
2. Drake C, Roehrs T, Shambroom J, et al. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9(11):1195-1200.
3. Pollan M. Caffeine: How Coffee and Tea Created the Modern World. 2023; Audible Audiobooks.
4. Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021:17:2549-2566.
5. Vitale JA, Roveda E, Montaruli A, et al. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405-415.
6. Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509-517.
7. Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19(4):23.
8. Monti JM, Pandi-Perumal SR. Clinical management of sleep and sleep disorders with cannabis and cannabinoids: implications to practicing psychiatrists. Clin Neuropharmacol. 2022;45(2):27-31.
9. Dockray S, Steptoe A. Chronotype and diurnal cortisol profile in working women: differences between work and leisure days. Psychoneuroendocrinology. 2011;36(5):649-655.
10. Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(5 Suppl):S102-S108.
11. Rosenberg RP, Krystal AD. Diagnosing and treating insomnia in adults and older adults. J Clin Psychiatry. 2021;82(6):59-66.
12. Stunkard A. Eating disorders and obesity. Psychiatr Clin North Am. 2011; 34(4):765-771.
13. Crönlein T. Insomnia and obesity. Curr Opin Psychiatry. 2016;29(6):409-412.
14. Gillberg C, Gillberg IC, Rasmussen P, et al. Co-existing disorders in ADHD -- implications for diagnosis and intervention. Eur Child Adolesc Psychiatry. 2004; 1(Suppl 1):i80-i92.
15. Goldberg JF, Nierenberg AA, Iosifescu DV. Wrestling with antidepressant use in bipolar disorder: the ongoing debate. J Clin Psychiatry. 2021;82(1):19. doi:10.4088/JCP.19ac13181
16. Baltzan M, Yao C, Rizzo D, et al. Dream enactment behavior: review for the clinician. J Clin Sleep Med. 2020;16(11):1949-1969.
17. Barone DA. Dream enactment behavior—a real nightmare: a review of post-traumatic stress disorder, REM sleep behavior disorder, and trauma-associated sleep disorder. J Clin Sleep Med. 2020;16(11):1943-1948.
18. Figorilli M, Meloni M, Lanza G, et al. Considering REM sleep behavior disorder in the management of Parkinson’s disease. Nat Sci Sleep. 2023;15:333-352.
19. Rios P, Cardoso R, Morra D, et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281-297.
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139.
21. Lu M, Zhang Y, Zhang J, et al. Comparative effectiveness of digital cognitive behavioral therapy vs. medication therapy among patients with insomnia. JAMA Network Open. 2023;6(4):e237597.
22. Sweetman A, McEvoy RD, Catcheside PG, et al. Effect of depression, anxiety, and stress symptoms on response to cognitive behavioral therapy for insomnia in patients with comorbid insomnia and sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2021;17(3):545-554.
23. O’Brien CP. Benzodiazepine use, abuse and dependence. J Clin Psychiatry. 2005;66(Suppl 2):28-33.
24. Wichniak A, Wierzbicka AE, Jarema M. Treatment of insomnia - effect of trazodone and hypnotics on sleep. Psychiatr Pol. 2021;55(4):743-755.
25. Papazisis G, Siafis S, Tzachanis D. Tachyphylaxis to the sedative action of mirtazapine. Am J Case Rep. 2018;19:410-412.
1. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
2. Drake C, Roehrs T, Shambroom J, et al. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9(11):1195-1200.
3. Pollan M. Caffeine: How Coffee and Tea Created the Modern World. 2023; Audible Audiobooks.
4. Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021:17:2549-2566.
5. Vitale JA, Roveda E, Montaruli A, et al. Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405-415.
6. Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509-517.
7. Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19(4):23.
8. Monti JM, Pandi-Perumal SR. Clinical management of sleep and sleep disorders with cannabis and cannabinoids: implications to practicing psychiatrists. Clin Neuropharmacol. 2022;45(2):27-31.
9. Dockray S, Steptoe A. Chronotype and diurnal cortisol profile in working women: differences between work and leisure days. Psychoneuroendocrinology. 2011;36(5):649-655.
10. Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(5 Suppl):S102-S108.
11. Rosenberg RP, Krystal AD. Diagnosing and treating insomnia in adults and older adults. J Clin Psychiatry. 2021;82(6):59-66.
12. Stunkard A. Eating disorders and obesity. Psychiatr Clin North Am. 2011; 34(4):765-771.
13. Crönlein T. Insomnia and obesity. Curr Opin Psychiatry. 2016;29(6):409-412.
14. Gillberg C, Gillberg IC, Rasmussen P, et al. Co-existing disorders in ADHD -- implications for diagnosis and intervention. Eur Child Adolesc Psychiatry. 2004; 1(Suppl 1):i80-i92.
15. Goldberg JF, Nierenberg AA, Iosifescu DV. Wrestling with antidepressant use in bipolar disorder: the ongoing debate. J Clin Psychiatry. 2021;82(1):19. doi:10.4088/JCP.19ac13181
16. Baltzan M, Yao C, Rizzo D, et al. Dream enactment behavior: review for the clinician. J Clin Sleep Med. 2020;16(11):1949-1969.
17. Barone DA. Dream enactment behavior—a real nightmare: a review of post-traumatic stress disorder, REM sleep behavior disorder, and trauma-associated sleep disorder. J Clin Sleep Med. 2020;16(11):1943-1948.
18. Figorilli M, Meloni M, Lanza G, et al. Considering REM sleep behavior disorder in the management of Parkinson’s disease. Nat Sci Sleep. 2023;15:333-352.
19. Rios P, Cardoso R, Morra D, et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281-297.
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139.
21. Lu M, Zhang Y, Zhang J, et al. Comparative effectiveness of digital cognitive behavioral therapy vs. medication therapy among patients with insomnia. JAMA Network Open. 2023;6(4):e237597.
22. Sweetman A, McEvoy RD, Catcheside PG, et al. Effect of depression, anxiety, and stress symptoms on response to cognitive behavioral therapy for insomnia in patients with comorbid insomnia and sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2021;17(3):545-554.
23. O’Brien CP. Benzodiazepine use, abuse and dependence. J Clin Psychiatry. 2005;66(Suppl 2):28-33.
24. Wichniak A, Wierzbicka AE, Jarema M. Treatment of insomnia - effect of trazodone and hypnotics on sleep. Psychiatr Pol. 2021;55(4):743-755.
25. Papazisis G, Siafis S, Tzachanis D. Tachyphylaxis to the sedative action of mirtazapine. Am J Case Rep. 2018;19:410-412.
The pandemic has permanently changed us, and its biopsychosocial sequelae linger…
Good riddance COVID-19 pandemic? Alas, that’s wishful thinking.
Many assume the pandemic is in our rearview mirror, but its biological, psychological, and social impacts continue to unfold. Its repercussions are etched into our brain, mind, emotions, behaviors, cognition, and outlook on life. Welcome to Pandemic 2.0.
Think of people who survive a heart attack. They experience multiple changes. Their initial ephemeral thrill of beating death is rapidly tempered with anxiety and worry about a future myocardial infarction and health issues in general. They become more risk-averse and more prone to dysphoria, irritability, and impatience. These individuals adopt a healthy lifestyle (diet and exercise), which they had neglected before. They develop more disciplined personality traits, feel a greater appreciation for being alive, and develop a closer affinity to family and friends. Simple things they had overlooked become more meaningful. They reevaluate their life goals, including career vs personal fulfilment. Some may overindulge in pleasurable activities in case their heart fails again. Some of those changes may be abrupt or transient, while others may become permanent features of their lives. And some may seek psychotherapy, which they may never have considered before.
The pandemic is the equivalent of a “societal cardiac arrest.” Its immediate impact was devastating. Bustling cities suddenly became ghost towns. Schools were closed, and children were locked at home with their parents, who were laid off. Businesses shut down; the economy tanked. Anxiety about being infected and dying skyrocketed, triggering a universal acute stress reaction that worsened the mental health of the population, but especially of the millions with preexisting psychiatric disorders. Routine medical and dental care stopped. Television and social media disseminated alarming updates about massive intensive care unit admissions and morgues overflowing with corpses of COVID-19 victims. Posttraumatic stress disorder (PTSD) was brewing across the nation as everyone faced this life-threatening pandemic.
The warp-speed development of vaccines for COVID-19 was equivalent to a defibrillator for the societal asystole, but the turmoil continued among the frazzled population. Some refused the vaccine due to conspiracy theories about their dangerous adverse effects. Employees in the private sector, state and federal government, and even the military who refused the mandatory vaccination lost their jobs. Controversy about shuttering schools and depriving children of face-to-face learning and socializing prompted some states to keep schools open, in contrast to most other states. Anger escalated about wearing masks, social distancing, and avoiding gatherings such as at restaurants or houses of worship. Cynicism and mistrust sprouted about the competence and reliability of health “experts” due to some conflicting signals, precluding wide adherence to medical advice.
The lingering effects of the COVID-19 pandemic
Those were the immediate repercussions of the pandemic. But what are its lingering effects? The sequelae extend across 1) the health care system; 2) the mental and emotional wellness of the population; 3) education; 4) work culture; 5) the economy; 6) societal operations; 7) technological and digital transformations; 8) mistrust in various societal institutions; 9) lack of confidence in medical information; and 10) preparedness for another pandemic due to a new strain.
As all psychiatrists know, the demand for mental health services continues to surge well after the pandemic has subsided, straining access to outpatient and inpatient care. Multiple lines of evidence confirm a deterioration in the long-term psychological well-being of children and adolescents because of lockdowns, social isolation, and anxiety about their own health and the health of their loved ones, leading to a serious rise in depression and suicidal behavior.1-3
Contunue to: Adults who survived pandemic...
Adults who survived the pandemic experienced grief during 2 very stressful years, with no peace of mind or “normal living.” Many began to contemplate the meaning of life and reevaluate the future, waxing more philosophical and embarking on “personal archeology.” The fragility of life suddenly became a ubiquitous epiphany that changed people’s habits. Working from home, which was necessary during the pandemic, became a preferred option for many, and home became an emotional refuge, not just a physical, brick-and-mortar refuge. Millions decided to quit working altogether (the “great resignation”).
Sexual activity declined precipitously during the pandemic for singles (French kissing became “the kiss of death”) but intercourse increased among couples, eventuating in a significant rise in births after the pandemic (a baby boomlet). Sexual interest among college students declined after the pandemic, which may be either due to fear of getting infected or a sublimation of libido to invest the energy in other, less risky activities.
At the societal level, the pandemic’s sequelae included a major shift to virtual communications, not just in health care (telepsychiatry and telemedicine) but also in business. Technology saved the day during the nadir of the pandemic by enabling psychiatrists and psychotherapists to treat their patients remotely. This was not technologically feasible during the past century’s influenza pandemics (1918, 1957, and 1968).
The intellectual and social development of an entire generation of children was stunted due to the COVID-19 pandemic. Consequences will continue to emerge in the years to come and may have ripple effects on this generation’s functioning. This may have particularly affected children of lower socioeconomic status, whose families cannot afford private schools and who are in dire need of good education to put them on the path of upward mobility.
As for adults who did not get infected by COVID-19, they suffered in 2 ways. First, they experienced a certain degree of brain atrophy, which is known to occur in chronic stress. This is attributed to persistent hypercortisolemia, which is toxic to the hippocampus. PTSD is well known to be associated with hippocampal atrophy.4 Additionally, a significant proportion of adults who contracted the COVID-19 virus and “recovered” were subsequently diagnosed with “long COVID,” with multiple neuropsychiatric symptoms, including psychosis, mania, depression, and panic attacks, as well as memory impairment and loss of the senses of smell and taste. For these individuals, the pandemic has not subsided; they will carry its neuropsychiatric scars for a long time.
Continue to: Economically, the pandemic...
Economically, the pandemic caused a horrific economic setback in its acute phase, which prompted the government to spend trillions to support the unemployed as well as blighted businesses. The economic sequalae of deficit spending of unprecedented proportions due to the pandemic triggered painful inflation that is ongoing. Interestingly, the numerical terms “billion” and “trillion” lost their loftiness as very huge numbers. Few people realize that counting to a billion (at one number per second) would take 31.7 years, while counting to a trillion would take 31,700 years! The inflationary impact of spending $6 trillion (which would take almost 200,000 years to count) becomes mathematically jarring. And despite the heroic measures to support the economy, some business perished, although others were created, changing the human architecture of the economy.
The pandemic drastically suppressed the “hunting and gathering” instinct of humans and demolished the fabled concept of work ethic. The “great resignation,” coupled with a desire to work from home on a mass scale, led to a glut of vacant office space in many large cities, lowering the value of commercial real estate. Following the pandemic, there was an uptick in moving away from urban areas, reflecting a creative destruction and reversal of a decades-long trend to gravitate to cities to work or live.
There was also political fallout from the pandemic. Staying at home is conducive to overdosing on television and social media, leading to an intensification and ossification of political hyperpartisanship and the further displacement of religious beliefs by passionately entrenched political beliefs. This continues to have seismic effects on political stability and harmony in our country. The pandemic may have instigated new models of national voting, which triggered further political friction.
Other examples of the pandemic’s aftereffects include a shortage of lifeguards and truck drivers, replacing the traditional handshake with a first bump, and increased spending on pleasurable activities (reminiscent of the Roaring 20s following the 1918 influenza pandemic), which may reflect an instinct to “live it up” before another deadly pandemic occurs.
Ironically, as I was finishing writing this article in early September 2023, the government announced that COVID-19 cases were again rising and a new vaccine was available for the new viral “strain.”
Here we go again: as the French saying goes: plus ça change, plus c’est la même chose…
1. Chavira DA, Ponting C, Ramos G. The impact of COVID-19 on child and adolescent mental health and treatment considerations. Behav Res Ther. 2022;157:104169. doi:10.1016/j.brat.2022.104169
2. Panchal U, Salazar de Pablo G, Franco M, et al. The impact of COVID-19 lockdown on child and adolescent mental health: systematic review. Eur Child Adolesc Psychiatry. 2023;32:1151-1177.
3. Mazrekaj D, De Witte K. The impact of school closures on learning and mental health of children: lessons from the COVID-19 pandemic. Perspectives on Psychological Science. 2023. https://doi.org/10.1177/17456916231181108
4. Logue MW, van Rooij SJH, Dennis EL, et al. A smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83(3):244-253.
Good riddance COVID-19 pandemic? Alas, that’s wishful thinking.
Many assume the pandemic is in our rearview mirror, but its biological, psychological, and social impacts continue to unfold. Its repercussions are etched into our brain, mind, emotions, behaviors, cognition, and outlook on life. Welcome to Pandemic 2.0.
Think of people who survive a heart attack. They experience multiple changes. Their initial ephemeral thrill of beating death is rapidly tempered with anxiety and worry about a future myocardial infarction and health issues in general. They become more risk-averse and more prone to dysphoria, irritability, and impatience. These individuals adopt a healthy lifestyle (diet and exercise), which they had neglected before. They develop more disciplined personality traits, feel a greater appreciation for being alive, and develop a closer affinity to family and friends. Simple things they had overlooked become more meaningful. They reevaluate their life goals, including career vs personal fulfilment. Some may overindulge in pleasurable activities in case their heart fails again. Some of those changes may be abrupt or transient, while others may become permanent features of their lives. And some may seek psychotherapy, which they may never have considered before.
The pandemic is the equivalent of a “societal cardiac arrest.” Its immediate impact was devastating. Bustling cities suddenly became ghost towns. Schools were closed, and children were locked at home with their parents, who were laid off. Businesses shut down; the economy tanked. Anxiety about being infected and dying skyrocketed, triggering a universal acute stress reaction that worsened the mental health of the population, but especially of the millions with preexisting psychiatric disorders. Routine medical and dental care stopped. Television and social media disseminated alarming updates about massive intensive care unit admissions and morgues overflowing with corpses of COVID-19 victims. Posttraumatic stress disorder (PTSD) was brewing across the nation as everyone faced this life-threatening pandemic.
The warp-speed development of vaccines for COVID-19 was equivalent to a defibrillator for the societal asystole, but the turmoil continued among the frazzled population. Some refused the vaccine due to conspiracy theories about their dangerous adverse effects. Employees in the private sector, state and federal government, and even the military who refused the mandatory vaccination lost their jobs. Controversy about shuttering schools and depriving children of face-to-face learning and socializing prompted some states to keep schools open, in contrast to most other states. Anger escalated about wearing masks, social distancing, and avoiding gatherings such as at restaurants or houses of worship. Cynicism and mistrust sprouted about the competence and reliability of health “experts” due to some conflicting signals, precluding wide adherence to medical advice.
The lingering effects of the COVID-19 pandemic
Those were the immediate repercussions of the pandemic. But what are its lingering effects? The sequelae extend across 1) the health care system; 2) the mental and emotional wellness of the population; 3) education; 4) work culture; 5) the economy; 6) societal operations; 7) technological and digital transformations; 8) mistrust in various societal institutions; 9) lack of confidence in medical information; and 10) preparedness for another pandemic due to a new strain.
As all psychiatrists know, the demand for mental health services continues to surge well after the pandemic has subsided, straining access to outpatient and inpatient care. Multiple lines of evidence confirm a deterioration in the long-term psychological well-being of children and adolescents because of lockdowns, social isolation, and anxiety about their own health and the health of their loved ones, leading to a serious rise in depression and suicidal behavior.1-3
Contunue to: Adults who survived pandemic...
Adults who survived the pandemic experienced grief during 2 very stressful years, with no peace of mind or “normal living.” Many began to contemplate the meaning of life and reevaluate the future, waxing more philosophical and embarking on “personal archeology.” The fragility of life suddenly became a ubiquitous epiphany that changed people’s habits. Working from home, which was necessary during the pandemic, became a preferred option for many, and home became an emotional refuge, not just a physical, brick-and-mortar refuge. Millions decided to quit working altogether (the “great resignation”).
Sexual activity declined precipitously during the pandemic for singles (French kissing became “the kiss of death”) but intercourse increased among couples, eventuating in a significant rise in births after the pandemic (a baby boomlet). Sexual interest among college students declined after the pandemic, which may be either due to fear of getting infected or a sublimation of libido to invest the energy in other, less risky activities.
At the societal level, the pandemic’s sequelae included a major shift to virtual communications, not just in health care (telepsychiatry and telemedicine) but also in business. Technology saved the day during the nadir of the pandemic by enabling psychiatrists and psychotherapists to treat their patients remotely. This was not technologically feasible during the past century’s influenza pandemics (1918, 1957, and 1968).
The intellectual and social development of an entire generation of children was stunted due to the COVID-19 pandemic. Consequences will continue to emerge in the years to come and may have ripple effects on this generation’s functioning. This may have particularly affected children of lower socioeconomic status, whose families cannot afford private schools and who are in dire need of good education to put them on the path of upward mobility.
As for adults who did not get infected by COVID-19, they suffered in 2 ways. First, they experienced a certain degree of brain atrophy, which is known to occur in chronic stress. This is attributed to persistent hypercortisolemia, which is toxic to the hippocampus. PTSD is well known to be associated with hippocampal atrophy.4 Additionally, a significant proportion of adults who contracted the COVID-19 virus and “recovered” were subsequently diagnosed with “long COVID,” with multiple neuropsychiatric symptoms, including psychosis, mania, depression, and panic attacks, as well as memory impairment and loss of the senses of smell and taste. For these individuals, the pandemic has not subsided; they will carry its neuropsychiatric scars for a long time.
Continue to: Economically, the pandemic...
Economically, the pandemic caused a horrific economic setback in its acute phase, which prompted the government to spend trillions to support the unemployed as well as blighted businesses. The economic sequalae of deficit spending of unprecedented proportions due to the pandemic triggered painful inflation that is ongoing. Interestingly, the numerical terms “billion” and “trillion” lost their loftiness as very huge numbers. Few people realize that counting to a billion (at one number per second) would take 31.7 years, while counting to a trillion would take 31,700 years! The inflationary impact of spending $6 trillion (which would take almost 200,000 years to count) becomes mathematically jarring. And despite the heroic measures to support the economy, some business perished, although others were created, changing the human architecture of the economy.
The pandemic drastically suppressed the “hunting and gathering” instinct of humans and demolished the fabled concept of work ethic. The “great resignation,” coupled with a desire to work from home on a mass scale, led to a glut of vacant office space in many large cities, lowering the value of commercial real estate. Following the pandemic, there was an uptick in moving away from urban areas, reflecting a creative destruction and reversal of a decades-long trend to gravitate to cities to work or live.
There was also political fallout from the pandemic. Staying at home is conducive to overdosing on television and social media, leading to an intensification and ossification of political hyperpartisanship and the further displacement of religious beliefs by passionately entrenched political beliefs. This continues to have seismic effects on political stability and harmony in our country. The pandemic may have instigated new models of national voting, which triggered further political friction.
Other examples of the pandemic’s aftereffects include a shortage of lifeguards and truck drivers, replacing the traditional handshake with a first bump, and increased spending on pleasurable activities (reminiscent of the Roaring 20s following the 1918 influenza pandemic), which may reflect an instinct to “live it up” before another deadly pandemic occurs.
Ironically, as I was finishing writing this article in early September 2023, the government announced that COVID-19 cases were again rising and a new vaccine was available for the new viral “strain.”
Here we go again: as the French saying goes: plus ça change, plus c’est la même chose…
Good riddance COVID-19 pandemic? Alas, that’s wishful thinking.
Many assume the pandemic is in our rearview mirror, but its biological, psychological, and social impacts continue to unfold. Its repercussions are etched into our brain, mind, emotions, behaviors, cognition, and outlook on life. Welcome to Pandemic 2.0.
Think of people who survive a heart attack. They experience multiple changes. Their initial ephemeral thrill of beating death is rapidly tempered with anxiety and worry about a future myocardial infarction and health issues in general. They become more risk-averse and more prone to dysphoria, irritability, and impatience. These individuals adopt a healthy lifestyle (diet and exercise), which they had neglected before. They develop more disciplined personality traits, feel a greater appreciation for being alive, and develop a closer affinity to family and friends. Simple things they had overlooked become more meaningful. They reevaluate their life goals, including career vs personal fulfilment. Some may overindulge in pleasurable activities in case their heart fails again. Some of those changes may be abrupt or transient, while others may become permanent features of their lives. And some may seek psychotherapy, which they may never have considered before.
The pandemic is the equivalent of a “societal cardiac arrest.” Its immediate impact was devastating. Bustling cities suddenly became ghost towns. Schools were closed, and children were locked at home with their parents, who were laid off. Businesses shut down; the economy tanked. Anxiety about being infected and dying skyrocketed, triggering a universal acute stress reaction that worsened the mental health of the population, but especially of the millions with preexisting psychiatric disorders. Routine medical and dental care stopped. Television and social media disseminated alarming updates about massive intensive care unit admissions and morgues overflowing with corpses of COVID-19 victims. Posttraumatic stress disorder (PTSD) was brewing across the nation as everyone faced this life-threatening pandemic.
The warp-speed development of vaccines for COVID-19 was equivalent to a defibrillator for the societal asystole, but the turmoil continued among the frazzled population. Some refused the vaccine due to conspiracy theories about their dangerous adverse effects. Employees in the private sector, state and federal government, and even the military who refused the mandatory vaccination lost their jobs. Controversy about shuttering schools and depriving children of face-to-face learning and socializing prompted some states to keep schools open, in contrast to most other states. Anger escalated about wearing masks, social distancing, and avoiding gatherings such as at restaurants or houses of worship. Cynicism and mistrust sprouted about the competence and reliability of health “experts” due to some conflicting signals, precluding wide adherence to medical advice.
The lingering effects of the COVID-19 pandemic
Those were the immediate repercussions of the pandemic. But what are its lingering effects? The sequelae extend across 1) the health care system; 2) the mental and emotional wellness of the population; 3) education; 4) work culture; 5) the economy; 6) societal operations; 7) technological and digital transformations; 8) mistrust in various societal institutions; 9) lack of confidence in medical information; and 10) preparedness for another pandemic due to a new strain.
As all psychiatrists know, the demand for mental health services continues to surge well after the pandemic has subsided, straining access to outpatient and inpatient care. Multiple lines of evidence confirm a deterioration in the long-term psychological well-being of children and adolescents because of lockdowns, social isolation, and anxiety about their own health and the health of their loved ones, leading to a serious rise in depression and suicidal behavior.1-3
Contunue to: Adults who survived pandemic...
Adults who survived the pandemic experienced grief during 2 very stressful years, with no peace of mind or “normal living.” Many began to contemplate the meaning of life and reevaluate the future, waxing more philosophical and embarking on “personal archeology.” The fragility of life suddenly became a ubiquitous epiphany that changed people’s habits. Working from home, which was necessary during the pandemic, became a preferred option for many, and home became an emotional refuge, not just a physical, brick-and-mortar refuge. Millions decided to quit working altogether (the “great resignation”).
Sexual activity declined precipitously during the pandemic for singles (French kissing became “the kiss of death”) but intercourse increased among couples, eventuating in a significant rise in births after the pandemic (a baby boomlet). Sexual interest among college students declined after the pandemic, which may be either due to fear of getting infected or a sublimation of libido to invest the energy in other, less risky activities.
At the societal level, the pandemic’s sequelae included a major shift to virtual communications, not just in health care (telepsychiatry and telemedicine) but also in business. Technology saved the day during the nadir of the pandemic by enabling psychiatrists and psychotherapists to treat their patients remotely. This was not technologically feasible during the past century’s influenza pandemics (1918, 1957, and 1968).
The intellectual and social development of an entire generation of children was stunted due to the COVID-19 pandemic. Consequences will continue to emerge in the years to come and may have ripple effects on this generation’s functioning. This may have particularly affected children of lower socioeconomic status, whose families cannot afford private schools and who are in dire need of good education to put them on the path of upward mobility.
As for adults who did not get infected by COVID-19, they suffered in 2 ways. First, they experienced a certain degree of brain atrophy, which is known to occur in chronic stress. This is attributed to persistent hypercortisolemia, which is toxic to the hippocampus. PTSD is well known to be associated with hippocampal atrophy.4 Additionally, a significant proportion of adults who contracted the COVID-19 virus and “recovered” were subsequently diagnosed with “long COVID,” with multiple neuropsychiatric symptoms, including psychosis, mania, depression, and panic attacks, as well as memory impairment and loss of the senses of smell and taste. For these individuals, the pandemic has not subsided; they will carry its neuropsychiatric scars for a long time.
Continue to: Economically, the pandemic...
Economically, the pandemic caused a horrific economic setback in its acute phase, which prompted the government to spend trillions to support the unemployed as well as blighted businesses. The economic sequalae of deficit spending of unprecedented proportions due to the pandemic triggered painful inflation that is ongoing. Interestingly, the numerical terms “billion” and “trillion” lost their loftiness as very huge numbers. Few people realize that counting to a billion (at one number per second) would take 31.7 years, while counting to a trillion would take 31,700 years! The inflationary impact of spending $6 trillion (which would take almost 200,000 years to count) becomes mathematically jarring. And despite the heroic measures to support the economy, some business perished, although others were created, changing the human architecture of the economy.
The pandemic drastically suppressed the “hunting and gathering” instinct of humans and demolished the fabled concept of work ethic. The “great resignation,” coupled with a desire to work from home on a mass scale, led to a glut of vacant office space in many large cities, lowering the value of commercial real estate. Following the pandemic, there was an uptick in moving away from urban areas, reflecting a creative destruction and reversal of a decades-long trend to gravitate to cities to work or live.
There was also political fallout from the pandemic. Staying at home is conducive to overdosing on television and social media, leading to an intensification and ossification of political hyperpartisanship and the further displacement of religious beliefs by passionately entrenched political beliefs. This continues to have seismic effects on political stability and harmony in our country. The pandemic may have instigated new models of national voting, which triggered further political friction.
Other examples of the pandemic’s aftereffects include a shortage of lifeguards and truck drivers, replacing the traditional handshake with a first bump, and increased spending on pleasurable activities (reminiscent of the Roaring 20s following the 1918 influenza pandemic), which may reflect an instinct to “live it up” before another deadly pandemic occurs.
Ironically, as I was finishing writing this article in early September 2023, the government announced that COVID-19 cases were again rising and a new vaccine was available for the new viral “strain.”
Here we go again: as the French saying goes: plus ça change, plus c’est la même chose…
1. Chavira DA, Ponting C, Ramos G. The impact of COVID-19 on child and adolescent mental health and treatment considerations. Behav Res Ther. 2022;157:104169. doi:10.1016/j.brat.2022.104169
2. Panchal U, Salazar de Pablo G, Franco M, et al. The impact of COVID-19 lockdown on child and adolescent mental health: systematic review. Eur Child Adolesc Psychiatry. 2023;32:1151-1177.
3. Mazrekaj D, De Witte K. The impact of school closures on learning and mental health of children: lessons from the COVID-19 pandemic. Perspectives on Psychological Science. 2023. https://doi.org/10.1177/17456916231181108
4. Logue MW, van Rooij SJH, Dennis EL, et al. A smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83(3):244-253.
1. Chavira DA, Ponting C, Ramos G. The impact of COVID-19 on child and adolescent mental health and treatment considerations. Behav Res Ther. 2022;157:104169. doi:10.1016/j.brat.2022.104169
2. Panchal U, Salazar de Pablo G, Franco M, et al. The impact of COVID-19 lockdown on child and adolescent mental health: systematic review. Eur Child Adolesc Psychiatry. 2023;32:1151-1177.
3. Mazrekaj D, De Witte K. The impact of school closures on learning and mental health of children: lessons from the COVID-19 pandemic. Perspectives on Psychological Science. 2023. https://doi.org/10.1177/17456916231181108
4. Logue MW, van Rooij SJH, Dennis EL, et al. A smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83(3):244-253.