User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Practical, evidence-based, peer-reviewed solutions to common clinical problems in psychiatry
Adult ADHD: 6 studies of nonpharmacologic interventions
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
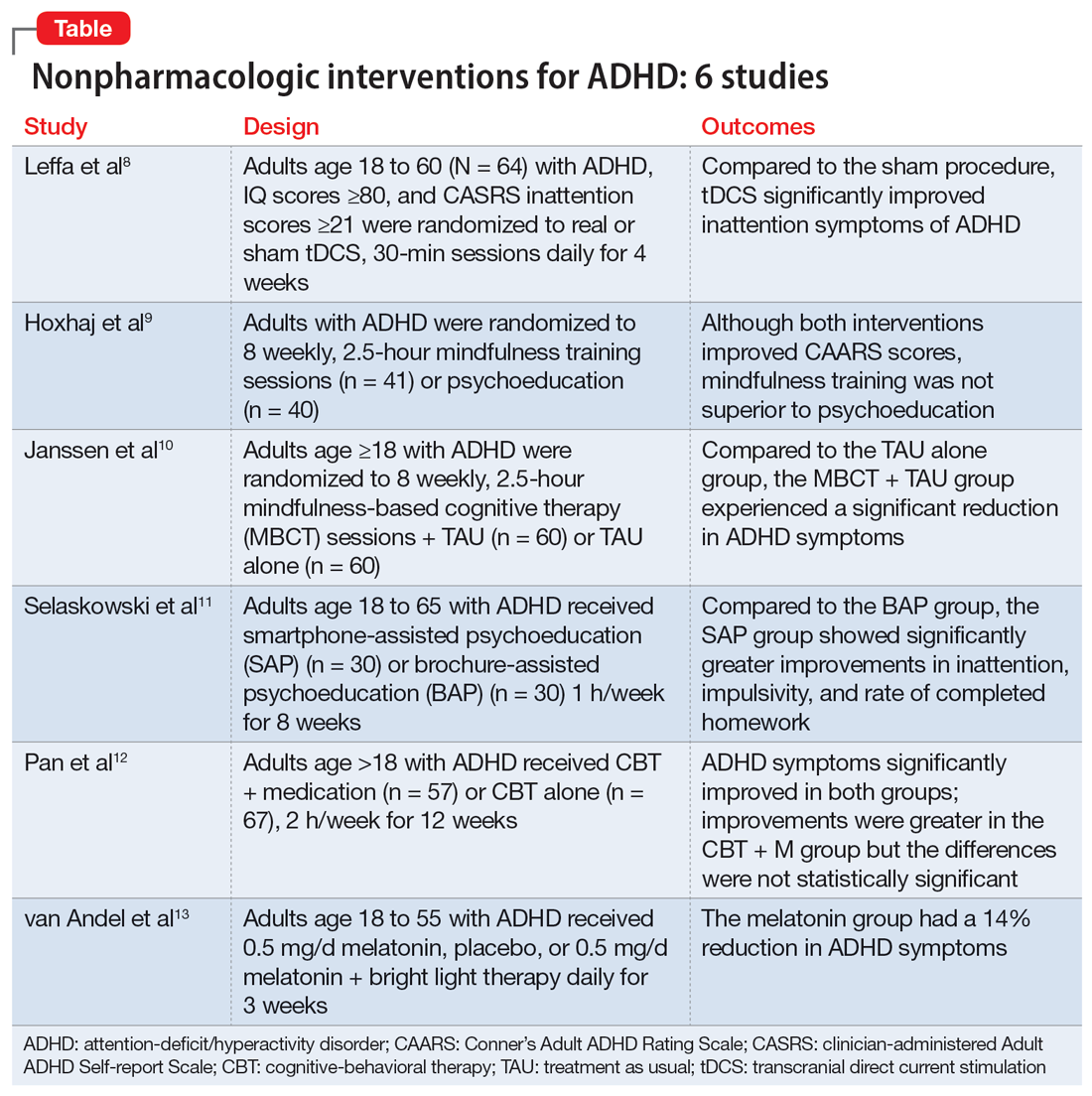
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).
1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).

1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).

1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
Auditory hallucinations in a patient who is hearing impaired
CASE New-onset auditory hallucinations
Ms. L, age 78, presents to our hospital with worsening anxiety due to auditory hallucinations. She has been hearing music, which she reports is worse at night and consists of songs, usually the song Jingle Bells, sometimes just melodies and other times with lyrics. Ms. L denies paranoia, visual hallucinations, or worsening mood.
Two weeks ago, Ms. L had visited another hospital, describing 5 days of right-side hearing loss accompanied by pain and burning in her ear and face, along with vesicular lesions in a dermatomal pattern extending into her auditory canal. During this visit, Ms. L’s complete blood count, urine culture, urine drug screen, electrolytes, liver panel, thyroid studies, and vitamin levels were unremarkable. A CT scan of her head showed no abnormalities.
Ms. L was diagnosed with Ramsay Hunt syndrome (herpes zoster oticus), which affects cranial nerves, because of physical examination findings with a dermatomal pattern of lesion distribution and associated pain. Ramsay Hunt syndrome can cause facial paralysis and hearing loss in the affected ear. She was discharged with prescriptions for prednisone 60 mg/d for 7 days and valacyclovir 1 g/d for 7 days and told to follow up with her primary care physician. During the present visit to our hospital, Ms. L’s home health nurse reports that she still has her entire bottles of valacyclovir and prednisone left. Ms. L also has left-side hearing loss that began 5 years ago and a history of recurrent major depressive disorder (MDD) and generalized anxiety disorder. Due to the recent onset of right-side hearing loss, her hearing impairment requires her to communicate via writing or via a voice-to-text app.
HISTORY Depressed and living alone
Ms. L was diagnosed with MDD more than 4 decades ago and has been receiving medication since then. She reports no prior psychiatric hospitalizations, suicide attempts, manic symptoms, or psychotic symptoms. For more than 20 years, she has seen a nurse practitioner, who had prescribed mirtazapine 30 mg/d for MDD, poor appetite, and sleep. Within the last 5 years, her nurse practitioner added risperidone 0.5 mg/d at night to augment the mirtazapine for tearfulness, irritability, and mood swings.
Ms. L’s medical history also includes hypertension and chronic obstructive pulmonary disease. She is a retired teacher and lives alone. She has a chore worker who visits her home for 1 hour 5 days a week to help with cleaning and lifting, and support from her son. Ms. L no longer drives and relies on others for transportation, but is able to manage her finances, activities of daily living, cooking, and walking without any assistance.
[polldaddy:12807642]
EVALUATION Identifying the cause of the music
Ms. L is alert and oriented to time and situation, her concentration is appropriate, and her recent and remote memories are preserved. A full cognitive screen is not performed, but she is able to spell WORLD forwards and backwards and adequately perform a serial 7s test. An examination of her ear does not reveal any open vesicular lesions or swelling, but she continues to report pain and tingling in the C7 dermatomal pattern. Her urine drug screen and infectious and autoimmune laboratory testing are unremarkable. She does not have electrolyte, renal function, or blood count abnormalities. An MRI of her brain that is performed to rule out intracranial pathology due to acute hearing loss shows no acute intracranial abnormalities, with some artifact effect due to motion. Because temporal lobe epilepsy can present with hallucinations,1 an EEG is performed to rule out seizure activity; it shows a normal wake pattern.
Psychiatry is consulted for management of the auditory hallucinations because Ms. L is distressed by hearing music. Ms. L is evaluated by Neurology and Otolaryngology. Neurology recommends a repeat brain MRI in the outpatient setting after seeing an artifact in the inpatient imaging, as well as follow-up with her primary care physician. Otolaryngology believes her symptoms are secondary to Ramsay Hunt syndrome with incomplete treatment, which is consistent with the initial diagnosis from her previous hospital visit, and recommends another course of oral corticosteroids, along with Audiology and Otolaryngology follow-up.
Continue to: The authors' observations
The authors’ observations
This is the first case we have seen detailing musical hallucinations (MH) secondary to Ramsay Hunt syndrome, although musical hallucinations have been associated with other etiologies of hearing loss. MH is a “release phenomenon” believed to be caused by deprivation of stimulation of the auditory cortex.2 They are categorized as complex auditory hallucinations made up of melodies and rhythms and may be present in up to 2.5% of patients with hearing impairment.1 The condition is mostly seen in older adults because this population is more likely to experience hearing loss. MH is more common among women (70% to 80% of cases) and is highly comorbid with psychiatric disorders such as schizophrenia, obsessive-compulsive disorder, or (as was the case for Ms. L) MDD.3 Hallucinations secondary to hearing loss may be more common in left-side hearing loss.4 In a 2005 study, Warner et al5 found religious music such as hymns or Christmas carols was most commonly heard, possibly due to repetitive past exposure.
There is no consensus on treatment for MH. Current treatment guidance comes from case reports and case series. Treatment is generally most successful when the etiology of the hallucination is both apparent and treatable, such as an infectious eitiology.3 In the case of MH due to hearing loss, hallucinations may improve following treatment with hearing aids or cochlear implants,1,3,6,7 which is what was advised for Ms. L. Table 17-9 outlines other possible measures for addressing musical hallucinations.
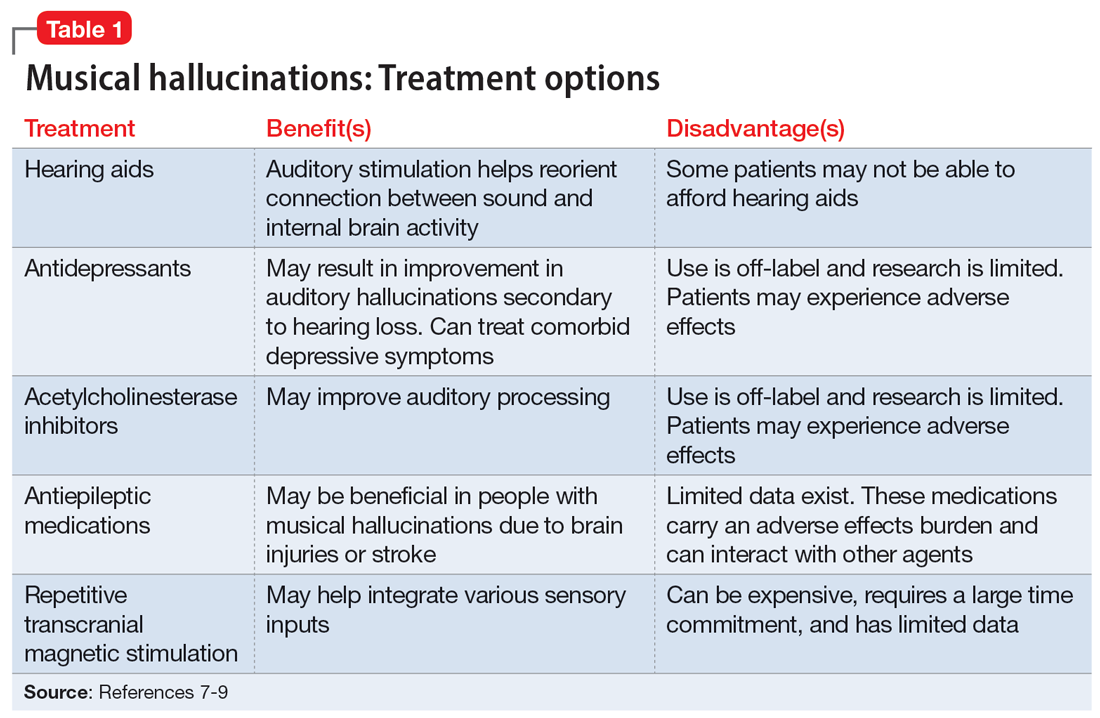
Anticholinesterases, antidepressants, and antiepileptics may provide some benefit.8 However, pharmacotherapy is generally less efficacious and can cause adverse effects, so environmental support and hearing aids may be a safer approach. No medications have been shown to completely cure MH.
TREATMENT Hearing loss management and follow-up
When speaking with the consulting psychiatry team, Ms. L reports her outpatient psychotropic regimen has been helpful. The team decides to continue mirtazapine 30 mg/d and risperidone 0.5 mg/d at night. We recommend that Ms. L discuss tapering off risperidone with her outpatient clinician if they feel it may be indicated to reduce the risk of adverse effects. The treatment team decides not to start corticosteroids due to the risk of steroid-induced psychotic symptoms. The team discusses hallucinations related to hearing loss with Ms. L and advise her to follow up with Audiology and Otolaryngology in the outpatient setting.
The authors’ observations
Approximately 40% of people age >60 struggle with hearing impairment4,9; this impacts their general quality of life and how clinicians communicate with such patients.10 People with hearing loss are more likely to develop feelings of social isolation, depression, and delirium (Table 28,10,11).11
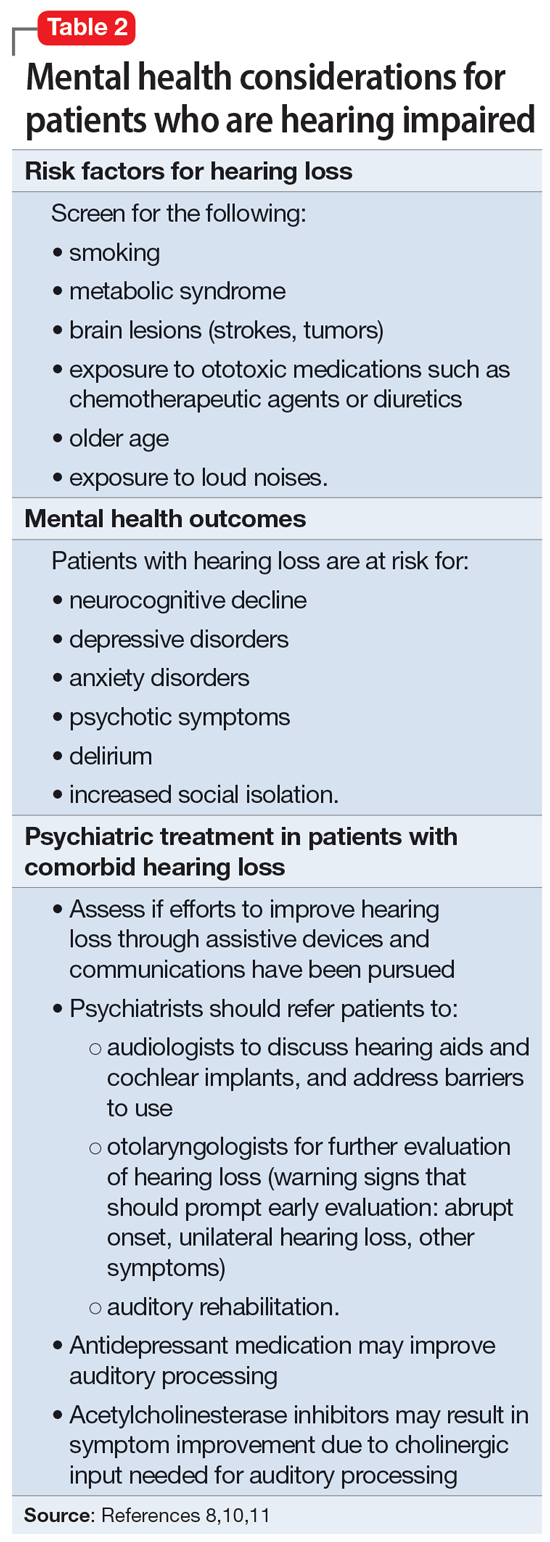
Risk factors for hearing loss include tobacco use, metabolic syndrome, exposure to loud noises, and exposure to certain ototoxic medications such as chemotherapeutic agents.11 As psychiatrists, it is important to identify patients who may be at risk for hearing loss and refer them to the appropriate medical professional. If hearing loss is new onset, refer the patient to an otolaryngologist for a full evaluation. Unilateral hearing loss should warrant further workup because this could be due to an acoustic neuroma.11
When providing care for a patient who uses a hearing aid, discuss adherence, barriers to adherence, and difficulties with adjusting the hearing aid. A referral to an audiologist may help patients address these barriers. Patients with hearing impairment or loss may benefit from auditory rehabilitation programs that provide communication strategies, ways to adapt to hearing loss, and information about different assistive options.11 Such programs are often run by audiologists or speech language pathologists and contain both counseling and group components.
Continue to: Is is critical for psychiatrists...
It is critical for psychiatrists to ensure appropriate communication with patients who are hearing impaired (Table 38-11). The use of assistive devices such as sound amplifiers, written messages, or family members to assist in communication is needed to prevent miscommunication.9-11
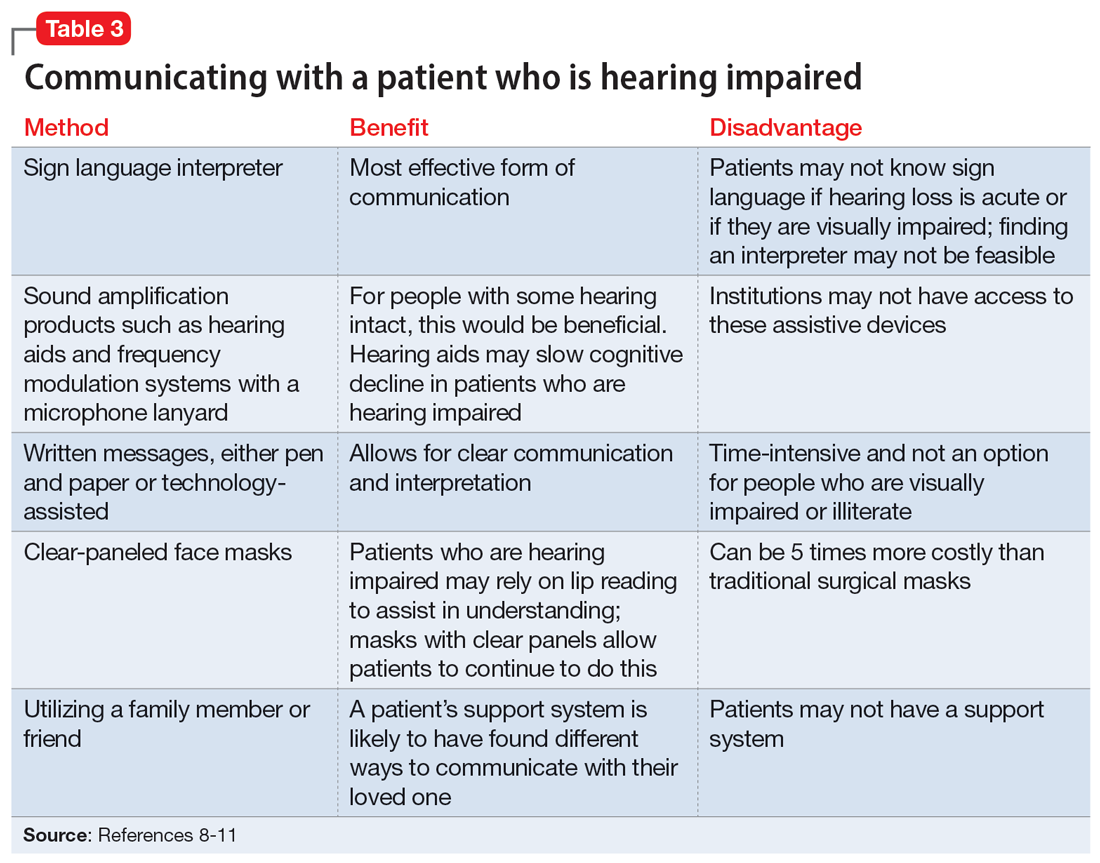
OUTCOME Lack of follow-up
A home health worker visits Ms. L, communicating with her using voice-to-text. Ms. L has not yet gone to her primary care physician, audiologist, or outpatient psychiatrist for follow-up because she needs to arrange transportation. Ms. L remains distressed by the music she is hearing, which is worse at night, along with her acute hearing loss.
Bottom Line
Hearing loss can predispose a person to psychiatric disorders and symptoms, including depression, delirium, and auditory hallucinations. Psychiatrists should strive to ensure clear communication with patients who are hearing impaired and should refer such patients to appropriate resources to improve outcomes.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- Sosland MD, Pinninti N. 5 ways to quiet auditory hallucinations. Current Psychiatry. 2005;4(4):110.
- Convery E, Keidser G, McLelland M, et al. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed E-Health. 2020;26(6):798-804. doi:10.1089/ tmj.2019.0109
Drug Brand Names
Mirtazapine • Remeron
Prednisone • Rayos
Risperidone • Risperdal
Valacyclovir • Valtrex
1. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. doi:10.1002/gps.618
2. Alvarez Perez P, Garcia-Antelo MJ, Rubio-Nazabal E. “Doctor, I hear music”: a brief review about musical hallucinations. Open Neurol J. 2017;11:11-14. doi:10.2174/1874205X01711010011
3. Sanchez TG, Rocha SCM, Knobel KAB, et al. Musical hallucination associated with hearing loss. Arq Neuropsiquiatr. 2011;69(2B):395-400. doi:10.1590/S0004-282X2011000300024
4. Teunisse RJ, Olde Rikkert MGM. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20(12):1075-1077. doi:10.1097/JGP.0b013e31823e31c4
5. Warner N, Aziz V. Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry. 2005;20(7):658-660. doi:10.1002/gps.1338
6. Low WK, Tham CA, D’Souza VD, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854-858. doi:10.1017/S0022215113001758
7. Brunner JP, Amedee RG. Musical hallucinations in a patient with presbycusis: a case report. Ochsner J. 2015;15(1):89-91.
8. Coebergh JAF, Lauw RF, Bots R, et al. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi:10.3389/fpsyg.2015.00814
9. Ten Hulzen RD, Fabry DA. Impact of hearing loss and universal face masking in the COVID-19 era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
10. Shukla A, Nieman CL, Price C, et al. Impact of hearing loss on patient-provider communication among hospitalized patients: a systematic review. Am J Med Qual. 2019;34(3):284-292. doi:10.1177/1062860618798926
11. Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi:10.1017/S0033291718003409
CASE New-onset auditory hallucinations
Ms. L, age 78, presents to our hospital with worsening anxiety due to auditory hallucinations. She has been hearing music, which she reports is worse at night and consists of songs, usually the song Jingle Bells, sometimes just melodies and other times with lyrics. Ms. L denies paranoia, visual hallucinations, or worsening mood.
Two weeks ago, Ms. L had visited another hospital, describing 5 days of right-side hearing loss accompanied by pain and burning in her ear and face, along with vesicular lesions in a dermatomal pattern extending into her auditory canal. During this visit, Ms. L’s complete blood count, urine culture, urine drug screen, electrolytes, liver panel, thyroid studies, and vitamin levels were unremarkable. A CT scan of her head showed no abnormalities.
Ms. L was diagnosed with Ramsay Hunt syndrome (herpes zoster oticus), which affects cranial nerves, because of physical examination findings with a dermatomal pattern of lesion distribution and associated pain. Ramsay Hunt syndrome can cause facial paralysis and hearing loss in the affected ear. She was discharged with prescriptions for prednisone 60 mg/d for 7 days and valacyclovir 1 g/d for 7 days and told to follow up with her primary care physician. During the present visit to our hospital, Ms. L’s home health nurse reports that she still has her entire bottles of valacyclovir and prednisone left. Ms. L also has left-side hearing loss that began 5 years ago and a history of recurrent major depressive disorder (MDD) and generalized anxiety disorder. Due to the recent onset of right-side hearing loss, her hearing impairment requires her to communicate via writing or via a voice-to-text app.
HISTORY Depressed and living alone
Ms. L was diagnosed with MDD more than 4 decades ago and has been receiving medication since then. She reports no prior psychiatric hospitalizations, suicide attempts, manic symptoms, or psychotic symptoms. For more than 20 years, she has seen a nurse practitioner, who had prescribed mirtazapine 30 mg/d for MDD, poor appetite, and sleep. Within the last 5 years, her nurse practitioner added risperidone 0.5 mg/d at night to augment the mirtazapine for tearfulness, irritability, and mood swings.
Ms. L’s medical history also includes hypertension and chronic obstructive pulmonary disease. She is a retired teacher and lives alone. She has a chore worker who visits her home for 1 hour 5 days a week to help with cleaning and lifting, and support from her son. Ms. L no longer drives and relies on others for transportation, but is able to manage her finances, activities of daily living, cooking, and walking without any assistance.
[polldaddy:12807642]
EVALUATION Identifying the cause of the music
Ms. L is alert and oriented to time and situation, her concentration is appropriate, and her recent and remote memories are preserved. A full cognitive screen is not performed, but she is able to spell WORLD forwards and backwards and adequately perform a serial 7s test. An examination of her ear does not reveal any open vesicular lesions or swelling, but she continues to report pain and tingling in the C7 dermatomal pattern. Her urine drug screen and infectious and autoimmune laboratory testing are unremarkable. She does not have electrolyte, renal function, or blood count abnormalities. An MRI of her brain that is performed to rule out intracranial pathology due to acute hearing loss shows no acute intracranial abnormalities, with some artifact effect due to motion. Because temporal lobe epilepsy can present with hallucinations,1 an EEG is performed to rule out seizure activity; it shows a normal wake pattern.
Psychiatry is consulted for management of the auditory hallucinations because Ms. L is distressed by hearing music. Ms. L is evaluated by Neurology and Otolaryngology. Neurology recommends a repeat brain MRI in the outpatient setting after seeing an artifact in the inpatient imaging, as well as follow-up with her primary care physician. Otolaryngology believes her symptoms are secondary to Ramsay Hunt syndrome with incomplete treatment, which is consistent with the initial diagnosis from her previous hospital visit, and recommends another course of oral corticosteroids, along with Audiology and Otolaryngology follow-up.
Continue to: The authors' observations
The authors’ observations
This is the first case we have seen detailing musical hallucinations (MH) secondary to Ramsay Hunt syndrome, although musical hallucinations have been associated with other etiologies of hearing loss. MH is a “release phenomenon” believed to be caused by deprivation of stimulation of the auditory cortex.2 They are categorized as complex auditory hallucinations made up of melodies and rhythms and may be present in up to 2.5% of patients with hearing impairment.1 The condition is mostly seen in older adults because this population is more likely to experience hearing loss. MH is more common among women (70% to 80% of cases) and is highly comorbid with psychiatric disorders such as schizophrenia, obsessive-compulsive disorder, or (as was the case for Ms. L) MDD.3 Hallucinations secondary to hearing loss may be more common in left-side hearing loss.4 In a 2005 study, Warner et al5 found religious music such as hymns or Christmas carols was most commonly heard, possibly due to repetitive past exposure.
There is no consensus on treatment for MH. Current treatment guidance comes from case reports and case series. Treatment is generally most successful when the etiology of the hallucination is both apparent and treatable, such as an infectious eitiology.3 In the case of MH due to hearing loss, hallucinations may improve following treatment with hearing aids or cochlear implants,1,3,6,7 which is what was advised for Ms. L. Table 17-9 outlines other possible measures for addressing musical hallucinations.

Anticholinesterases, antidepressants, and antiepileptics may provide some benefit.8 However, pharmacotherapy is generally less efficacious and can cause adverse effects, so environmental support and hearing aids may be a safer approach. No medications have been shown to completely cure MH.
TREATMENT Hearing loss management and follow-up
When speaking with the consulting psychiatry team, Ms. L reports her outpatient psychotropic regimen has been helpful. The team decides to continue mirtazapine 30 mg/d and risperidone 0.5 mg/d at night. We recommend that Ms. L discuss tapering off risperidone with her outpatient clinician if they feel it may be indicated to reduce the risk of adverse effects. The treatment team decides not to start corticosteroids due to the risk of steroid-induced psychotic symptoms. The team discusses hallucinations related to hearing loss with Ms. L and advise her to follow up with Audiology and Otolaryngology in the outpatient setting.
The authors’ observations
Approximately 40% of people age >60 struggle with hearing impairment4,9; this impacts their general quality of life and how clinicians communicate with such patients.10 People with hearing loss are more likely to develop feelings of social isolation, depression, and delirium (Table 28,10,11).11

Risk factors for hearing loss include tobacco use, metabolic syndrome, exposure to loud noises, and exposure to certain ototoxic medications such as chemotherapeutic agents.11 As psychiatrists, it is important to identify patients who may be at risk for hearing loss and refer them to the appropriate medical professional. If hearing loss is new onset, refer the patient to an otolaryngologist for a full evaluation. Unilateral hearing loss should warrant further workup because this could be due to an acoustic neuroma.11
When providing care for a patient who uses a hearing aid, discuss adherence, barriers to adherence, and difficulties with adjusting the hearing aid. A referral to an audiologist may help patients address these barriers. Patients with hearing impairment or loss may benefit from auditory rehabilitation programs that provide communication strategies, ways to adapt to hearing loss, and information about different assistive options.11 Such programs are often run by audiologists or speech language pathologists and contain both counseling and group components.
Continue to: Is is critical for psychiatrists...
It is critical for psychiatrists to ensure appropriate communication with patients who are hearing impaired (Table 38-11). The use of assistive devices such as sound amplifiers, written messages, or family members to assist in communication is needed to prevent miscommunication.9-11

OUTCOME Lack of follow-up
A home health worker visits Ms. L, communicating with her using voice-to-text. Ms. L has not yet gone to her primary care physician, audiologist, or outpatient psychiatrist for follow-up because she needs to arrange transportation. Ms. L remains distressed by the music she is hearing, which is worse at night, along with her acute hearing loss.
Bottom Line
Hearing loss can predispose a person to psychiatric disorders and symptoms, including depression, delirium, and auditory hallucinations. Psychiatrists should strive to ensure clear communication with patients who are hearing impaired and should refer such patients to appropriate resources to improve outcomes.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- Sosland MD, Pinninti N. 5 ways to quiet auditory hallucinations. Current Psychiatry. 2005;4(4):110.
- Convery E, Keidser G, McLelland M, et al. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed E-Health. 2020;26(6):798-804. doi:10.1089/ tmj.2019.0109
Drug Brand Names
Mirtazapine • Remeron
Prednisone • Rayos
Risperidone • Risperdal
Valacyclovir • Valtrex
CASE New-onset auditory hallucinations
Ms. L, age 78, presents to our hospital with worsening anxiety due to auditory hallucinations. She has been hearing music, which she reports is worse at night and consists of songs, usually the song Jingle Bells, sometimes just melodies and other times with lyrics. Ms. L denies paranoia, visual hallucinations, or worsening mood.
Two weeks ago, Ms. L had visited another hospital, describing 5 days of right-side hearing loss accompanied by pain and burning in her ear and face, along with vesicular lesions in a dermatomal pattern extending into her auditory canal. During this visit, Ms. L’s complete blood count, urine culture, urine drug screen, electrolytes, liver panel, thyroid studies, and vitamin levels were unremarkable. A CT scan of her head showed no abnormalities.
Ms. L was diagnosed with Ramsay Hunt syndrome (herpes zoster oticus), which affects cranial nerves, because of physical examination findings with a dermatomal pattern of lesion distribution and associated pain. Ramsay Hunt syndrome can cause facial paralysis and hearing loss in the affected ear. She was discharged with prescriptions for prednisone 60 mg/d for 7 days and valacyclovir 1 g/d for 7 days and told to follow up with her primary care physician. During the present visit to our hospital, Ms. L’s home health nurse reports that she still has her entire bottles of valacyclovir and prednisone left. Ms. L also has left-side hearing loss that began 5 years ago and a history of recurrent major depressive disorder (MDD) and generalized anxiety disorder. Due to the recent onset of right-side hearing loss, her hearing impairment requires her to communicate via writing or via a voice-to-text app.
HISTORY Depressed and living alone
Ms. L was diagnosed with MDD more than 4 decades ago and has been receiving medication since then. She reports no prior psychiatric hospitalizations, suicide attempts, manic symptoms, or psychotic symptoms. For more than 20 years, she has seen a nurse practitioner, who had prescribed mirtazapine 30 mg/d for MDD, poor appetite, and sleep. Within the last 5 years, her nurse practitioner added risperidone 0.5 mg/d at night to augment the mirtazapine for tearfulness, irritability, and mood swings.
Ms. L’s medical history also includes hypertension and chronic obstructive pulmonary disease. She is a retired teacher and lives alone. She has a chore worker who visits her home for 1 hour 5 days a week to help with cleaning and lifting, and support from her son. Ms. L no longer drives and relies on others for transportation, but is able to manage her finances, activities of daily living, cooking, and walking without any assistance.
[polldaddy:12807642]
EVALUATION Identifying the cause of the music
Ms. L is alert and oriented to time and situation, her concentration is appropriate, and her recent and remote memories are preserved. A full cognitive screen is not performed, but she is able to spell WORLD forwards and backwards and adequately perform a serial 7s test. An examination of her ear does not reveal any open vesicular lesions or swelling, but she continues to report pain and tingling in the C7 dermatomal pattern. Her urine drug screen and infectious and autoimmune laboratory testing are unremarkable. She does not have electrolyte, renal function, or blood count abnormalities. An MRI of her brain that is performed to rule out intracranial pathology due to acute hearing loss shows no acute intracranial abnormalities, with some artifact effect due to motion. Because temporal lobe epilepsy can present with hallucinations,1 an EEG is performed to rule out seizure activity; it shows a normal wake pattern.
Psychiatry is consulted for management of the auditory hallucinations because Ms. L is distressed by hearing music. Ms. L is evaluated by Neurology and Otolaryngology. Neurology recommends a repeat brain MRI in the outpatient setting after seeing an artifact in the inpatient imaging, as well as follow-up with her primary care physician. Otolaryngology believes her symptoms are secondary to Ramsay Hunt syndrome with incomplete treatment, which is consistent with the initial diagnosis from her previous hospital visit, and recommends another course of oral corticosteroids, along with Audiology and Otolaryngology follow-up.
Continue to: The authors' observations
The authors’ observations
This is the first case we have seen detailing musical hallucinations (MH) secondary to Ramsay Hunt syndrome, although musical hallucinations have been associated with other etiologies of hearing loss. MH is a “release phenomenon” believed to be caused by deprivation of stimulation of the auditory cortex.2 They are categorized as complex auditory hallucinations made up of melodies and rhythms and may be present in up to 2.5% of patients with hearing impairment.1 The condition is mostly seen in older adults because this population is more likely to experience hearing loss. MH is more common among women (70% to 80% of cases) and is highly comorbid with psychiatric disorders such as schizophrenia, obsessive-compulsive disorder, or (as was the case for Ms. L) MDD.3 Hallucinations secondary to hearing loss may be more common in left-side hearing loss.4 In a 2005 study, Warner et al5 found religious music such as hymns or Christmas carols was most commonly heard, possibly due to repetitive past exposure.
There is no consensus on treatment for MH. Current treatment guidance comes from case reports and case series. Treatment is generally most successful when the etiology of the hallucination is both apparent and treatable, such as an infectious eitiology.3 In the case of MH due to hearing loss, hallucinations may improve following treatment with hearing aids or cochlear implants,1,3,6,7 which is what was advised for Ms. L. Table 17-9 outlines other possible measures for addressing musical hallucinations.

Anticholinesterases, antidepressants, and antiepileptics may provide some benefit.8 However, pharmacotherapy is generally less efficacious and can cause adverse effects, so environmental support and hearing aids may be a safer approach. No medications have been shown to completely cure MH.
TREATMENT Hearing loss management and follow-up
When speaking with the consulting psychiatry team, Ms. L reports her outpatient psychotropic regimen has been helpful. The team decides to continue mirtazapine 30 mg/d and risperidone 0.5 mg/d at night. We recommend that Ms. L discuss tapering off risperidone with her outpatient clinician if they feel it may be indicated to reduce the risk of adverse effects. The treatment team decides not to start corticosteroids due to the risk of steroid-induced psychotic symptoms. The team discusses hallucinations related to hearing loss with Ms. L and advise her to follow up with Audiology and Otolaryngology in the outpatient setting.
The authors’ observations
Approximately 40% of people age >60 struggle with hearing impairment4,9; this impacts their general quality of life and how clinicians communicate with such patients.10 People with hearing loss are more likely to develop feelings of social isolation, depression, and delirium (Table 28,10,11).11

Risk factors for hearing loss include tobacco use, metabolic syndrome, exposure to loud noises, and exposure to certain ototoxic medications such as chemotherapeutic agents.11 As psychiatrists, it is important to identify patients who may be at risk for hearing loss and refer them to the appropriate medical professional. If hearing loss is new onset, refer the patient to an otolaryngologist for a full evaluation. Unilateral hearing loss should warrant further workup because this could be due to an acoustic neuroma.11
When providing care for a patient who uses a hearing aid, discuss adherence, barriers to adherence, and difficulties with adjusting the hearing aid. A referral to an audiologist may help patients address these barriers. Patients with hearing impairment or loss may benefit from auditory rehabilitation programs that provide communication strategies, ways to adapt to hearing loss, and information about different assistive options.11 Such programs are often run by audiologists or speech language pathologists and contain both counseling and group components.
Continue to: Is is critical for psychiatrists...
It is critical for psychiatrists to ensure appropriate communication with patients who are hearing impaired (Table 38-11). The use of assistive devices such as sound amplifiers, written messages, or family members to assist in communication is needed to prevent miscommunication.9-11

OUTCOME Lack of follow-up
A home health worker visits Ms. L, communicating with her using voice-to-text. Ms. L has not yet gone to her primary care physician, audiologist, or outpatient psychiatrist for follow-up because she needs to arrange transportation. Ms. L remains distressed by the music she is hearing, which is worse at night, along with her acute hearing loss.
Bottom Line
Hearing loss can predispose a person to psychiatric disorders and symptoms, including depression, delirium, and auditory hallucinations. Psychiatrists should strive to ensure clear communication with patients who are hearing impaired and should refer such patients to appropriate resources to improve outcomes.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- Sosland MD, Pinninti N. 5 ways to quiet auditory hallucinations. Current Psychiatry. 2005;4(4):110.
- Convery E, Keidser G, McLelland M, et al. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed E-Health. 2020;26(6):798-804. doi:10.1089/ tmj.2019.0109
Drug Brand Names
Mirtazapine • Remeron
Prednisone • Rayos
Risperidone • Risperdal
Valacyclovir • Valtrex
1. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. doi:10.1002/gps.618
2. Alvarez Perez P, Garcia-Antelo MJ, Rubio-Nazabal E. “Doctor, I hear music”: a brief review about musical hallucinations. Open Neurol J. 2017;11:11-14. doi:10.2174/1874205X01711010011
3. Sanchez TG, Rocha SCM, Knobel KAB, et al. Musical hallucination associated with hearing loss. Arq Neuropsiquiatr. 2011;69(2B):395-400. doi:10.1590/S0004-282X2011000300024
4. Teunisse RJ, Olde Rikkert MGM. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20(12):1075-1077. doi:10.1097/JGP.0b013e31823e31c4
5. Warner N, Aziz V. Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry. 2005;20(7):658-660. doi:10.1002/gps.1338
6. Low WK, Tham CA, D’Souza VD, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854-858. doi:10.1017/S0022215113001758
7. Brunner JP, Amedee RG. Musical hallucinations in a patient with presbycusis: a case report. Ochsner J. 2015;15(1):89-91.
8. Coebergh JAF, Lauw RF, Bots R, et al. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi:10.3389/fpsyg.2015.00814
9. Ten Hulzen RD, Fabry DA. Impact of hearing loss and universal face masking in the COVID-19 era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
10. Shukla A, Nieman CL, Price C, et al. Impact of hearing loss on patient-provider communication among hospitalized patients: a systematic review. Am J Med Qual. 2019;34(3):284-292. doi:10.1177/1062860618798926
11. Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi:10.1017/S0033291718003409
1. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. doi:10.1002/gps.618
2. Alvarez Perez P, Garcia-Antelo MJ, Rubio-Nazabal E. “Doctor, I hear music”: a brief review about musical hallucinations. Open Neurol J. 2017;11:11-14. doi:10.2174/1874205X01711010011
3. Sanchez TG, Rocha SCM, Knobel KAB, et al. Musical hallucination associated with hearing loss. Arq Neuropsiquiatr. 2011;69(2B):395-400. doi:10.1590/S0004-282X2011000300024
4. Teunisse RJ, Olde Rikkert MGM. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20(12):1075-1077. doi:10.1097/JGP.0b013e31823e31c4
5. Warner N, Aziz V. Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry. 2005;20(7):658-660. doi:10.1002/gps.1338
6. Low WK, Tham CA, D’Souza VD, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854-858. doi:10.1017/S0022215113001758
7. Brunner JP, Amedee RG. Musical hallucinations in a patient with presbycusis: a case report. Ochsner J. 2015;15(1):89-91.
8. Coebergh JAF, Lauw RF, Bots R, et al. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi:10.3389/fpsyg.2015.00814
9. Ten Hulzen RD, Fabry DA. Impact of hearing loss and universal face masking in the COVID-19 era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
10. Shukla A, Nieman CL, Price C, et al. Impact of hearing loss on patient-provider communication among hospitalized patients: a systematic review. Am J Med Qual. 2019;34(3):284-292. doi:10.1177/1062860618798926
11. Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi:10.1017/S0033291718003409
Emotional blunting in patients taking antidepressants
When used to treat anxiety or depressive disorders, antidepressants can cause a variety of adverse effects, including emotional blunting. Emotional blunting has been described as emotional numbness, indifference, decreased responsiveness, or numbing. In a study of 669 patients who had been receiving antidepressants (selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], or other antidepressants), 46% said they had experienced emotional blunting.1 A 2019 study found that approximately one-third of patients with unipolar depression or bipolar depression stopped taking their antidepressant due to emotional blunting.2
Historically, there has been difficulty parsing out emotional blunting (a general decrease of all range of emotions) from anhedonia (a restriction of positive emotions). Additionally, some researchers have questioned if the blunting of emotions is part of depressive symptomatology. In a study of 38 adults, most felt able to differentiate emotional blunting due to antidepressants by considering the resolution of other depressive symptoms and timeline of onset.3
A significant limitation has been how clinicians measure or assess emotional blunting. The Oxford Depression Questionnaire (ODQ), previously known as the Oxford Questionnaire on the Emotional Side-effects of Antidepressants, was created based on a qualitative survey of patients who endorsed emotional blunting.4 A validated scale, the ODQ divides emotional blunting into 4 dimensions:
- general reduction in emotions
- reduction in positive emotions
- emotional detachment from others
- not caring.4
The sections of ODQ focus on exploring specific aspects of patients’ emotional experiences, comparing experiences in the past week to before the development of illness/emotional blunting, and patients’ opinions about antidepressants. Example statements from the ODQ (Table4) may help clinicians better understand and explore emotional blunting with their patients.

There are 2 leading theories behind the mechanism of emotional blunting on antidepressants, both focused on serotonin. The first theory offers that SSRIs alter frontal lobe activity through serotonergic effects. The second theory is focused on the downward effects of serotonin on dopamine in reward pathways.5
Treatment options: Limited evidence
Data on how to address antidepressant-induced emotional blunting are limited and based largely on case reports. One open-label study (N = 143) found that patients experiencing emotional blunting while taking SSRIs and SNRIs who were switched to vortioxetine had a statistically significant decrease in ODQ total score; 50% reported no emotional blunting.6 Options to address emotional blunting include decreasing the antidepressant dose, augmenting with or switching to another agent, or considering other treatments such as neuromodulation.5 Further research is necessary to clarify which intervention is best.
Clinicians will encounter emotional blunting in patients who are taking antidepressants. It is important to recognize and address these symptoms to help improve patients’ adherence and overall quality of life.
1. Goodwin GM, Price J, De Bodinat C, et al. Emotional blunting with antidepressant treatments: a survey among depressed patients. J Affect Disord. 2017;221:31-35.
2. Rosenblat JD, Simon GE, Sachs GS, et al. Treatment effectiveness and tolerability outcomes that are most important to individuals with bipolar and unipolar depression. J Affect Disord. 2019;243:116-120.
3. Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195(3):211-217.
4. Price J, Cole V, Doll H, et al. The Oxford Questionnaire on the Emotional Side-effects of Antidepressants (OQuESA): development, validity, reliability and sensitivity to change. J Affect Disord. 2012;140(1):66-74.
5. Ma H, Cai M, Wang H. Emotional blunting in patients with major depressive disorder: a brief non-systematic review of current research. Front Psychiatry. 2021;12:792960. doi:10.3389/fpsyt.2021.792960
6. Fagiolini A, Florea I, Loft H, et al. Effectiveness of vortioxetine on emotional blunting in patients with major depressive disorder with inadequate response to SSRI/SNRI treatment. J Affect Disord. 2021;283:472-479.
When used to treat anxiety or depressive disorders, antidepressants can cause a variety of adverse effects, including emotional blunting. Emotional blunting has been described as emotional numbness, indifference, decreased responsiveness, or numbing. In a study of 669 patients who had been receiving antidepressants (selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], or other antidepressants), 46% said they had experienced emotional blunting.1 A 2019 study found that approximately one-third of patients with unipolar depression or bipolar depression stopped taking their antidepressant due to emotional blunting.2
Historically, there has been difficulty parsing out emotional blunting (a general decrease of all range of emotions) from anhedonia (a restriction of positive emotions). Additionally, some researchers have questioned if the blunting of emotions is part of depressive symptomatology. In a study of 38 adults, most felt able to differentiate emotional blunting due to antidepressants by considering the resolution of other depressive symptoms and timeline of onset.3
A significant limitation has been how clinicians measure or assess emotional blunting. The Oxford Depression Questionnaire (ODQ), previously known as the Oxford Questionnaire on the Emotional Side-effects of Antidepressants, was created based on a qualitative survey of patients who endorsed emotional blunting.4 A validated scale, the ODQ divides emotional blunting into 4 dimensions:
- general reduction in emotions
- reduction in positive emotions
- emotional detachment from others
- not caring.4
The sections of ODQ focus on exploring specific aspects of patients’ emotional experiences, comparing experiences in the past week to before the development of illness/emotional blunting, and patients’ opinions about antidepressants. Example statements from the ODQ (Table4) may help clinicians better understand and explore emotional blunting with their patients.

There are 2 leading theories behind the mechanism of emotional blunting on antidepressants, both focused on serotonin. The first theory offers that SSRIs alter frontal lobe activity through serotonergic effects. The second theory is focused on the downward effects of serotonin on dopamine in reward pathways.5
Treatment options: Limited evidence
Data on how to address antidepressant-induced emotional blunting are limited and based largely on case reports. One open-label study (N = 143) found that patients experiencing emotional blunting while taking SSRIs and SNRIs who were switched to vortioxetine had a statistically significant decrease in ODQ total score; 50% reported no emotional blunting.6 Options to address emotional blunting include decreasing the antidepressant dose, augmenting with or switching to another agent, or considering other treatments such as neuromodulation.5 Further research is necessary to clarify which intervention is best.
Clinicians will encounter emotional blunting in patients who are taking antidepressants. It is important to recognize and address these symptoms to help improve patients’ adherence and overall quality of life.
When used to treat anxiety or depressive disorders, antidepressants can cause a variety of adverse effects, including emotional blunting. Emotional blunting has been described as emotional numbness, indifference, decreased responsiveness, or numbing. In a study of 669 patients who had been receiving antidepressants (selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], or other antidepressants), 46% said they had experienced emotional blunting.1 A 2019 study found that approximately one-third of patients with unipolar depression or bipolar depression stopped taking their antidepressant due to emotional blunting.2
Historically, there has been difficulty parsing out emotional blunting (a general decrease of all range of emotions) from anhedonia (a restriction of positive emotions). Additionally, some researchers have questioned if the blunting of emotions is part of depressive symptomatology. In a study of 38 adults, most felt able to differentiate emotional blunting due to antidepressants by considering the resolution of other depressive symptoms and timeline of onset.3
A significant limitation has been how clinicians measure or assess emotional blunting. The Oxford Depression Questionnaire (ODQ), previously known as the Oxford Questionnaire on the Emotional Side-effects of Antidepressants, was created based on a qualitative survey of patients who endorsed emotional blunting.4 A validated scale, the ODQ divides emotional blunting into 4 dimensions:
- general reduction in emotions
- reduction in positive emotions
- emotional detachment from others
- not caring.4
The sections of ODQ focus on exploring specific aspects of patients’ emotional experiences, comparing experiences in the past week to before the development of illness/emotional blunting, and patients’ opinions about antidepressants. Example statements from the ODQ (Table4) may help clinicians better understand and explore emotional blunting with their patients.

There are 2 leading theories behind the mechanism of emotional blunting on antidepressants, both focused on serotonin. The first theory offers that SSRIs alter frontal lobe activity through serotonergic effects. The second theory is focused on the downward effects of serotonin on dopamine in reward pathways.5
Treatment options: Limited evidence
Data on how to address antidepressant-induced emotional blunting are limited and based largely on case reports. One open-label study (N = 143) found that patients experiencing emotional blunting while taking SSRIs and SNRIs who were switched to vortioxetine had a statistically significant decrease in ODQ total score; 50% reported no emotional blunting.6 Options to address emotional blunting include decreasing the antidepressant dose, augmenting with or switching to another agent, or considering other treatments such as neuromodulation.5 Further research is necessary to clarify which intervention is best.
Clinicians will encounter emotional blunting in patients who are taking antidepressants. It is important to recognize and address these symptoms to help improve patients’ adherence and overall quality of life.
1. Goodwin GM, Price J, De Bodinat C, et al. Emotional blunting with antidepressant treatments: a survey among depressed patients. J Affect Disord. 2017;221:31-35.
2. Rosenblat JD, Simon GE, Sachs GS, et al. Treatment effectiveness and tolerability outcomes that are most important to individuals with bipolar and unipolar depression. J Affect Disord. 2019;243:116-120.
3. Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195(3):211-217.
4. Price J, Cole V, Doll H, et al. The Oxford Questionnaire on the Emotional Side-effects of Antidepressants (OQuESA): development, validity, reliability and sensitivity to change. J Affect Disord. 2012;140(1):66-74.
5. Ma H, Cai M, Wang H. Emotional blunting in patients with major depressive disorder: a brief non-systematic review of current research. Front Psychiatry. 2021;12:792960. doi:10.3389/fpsyt.2021.792960
6. Fagiolini A, Florea I, Loft H, et al. Effectiveness of vortioxetine on emotional blunting in patients with major depressive disorder with inadequate response to SSRI/SNRI treatment. J Affect Disord. 2021;283:472-479.
1. Goodwin GM, Price J, De Bodinat C, et al. Emotional blunting with antidepressant treatments: a survey among depressed patients. J Affect Disord. 2017;221:31-35.
2. Rosenblat JD, Simon GE, Sachs GS, et al. Treatment effectiveness and tolerability outcomes that are most important to individuals with bipolar and unipolar depression. J Affect Disord. 2019;243:116-120.
3. Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195(3):211-217.
4. Price J, Cole V, Doll H, et al. The Oxford Questionnaire on the Emotional Side-effects of Antidepressants (OQuESA): development, validity, reliability and sensitivity to change. J Affect Disord. 2012;140(1):66-74.
5. Ma H, Cai M, Wang H. Emotional blunting in patients with major depressive disorder: a brief non-systematic review of current research. Front Psychiatry. 2021;12:792960. doi:10.3389/fpsyt.2021.792960
6. Fagiolini A, Florea I, Loft H, et al. Effectiveness of vortioxetine on emotional blunting in patients with major depressive disorder with inadequate response to SSRI/SNRI treatment. J Affect Disord. 2021;283:472-479.
A street medicine view of tobacco use in patients with schizophrenia
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Throughout my psychiatric clerkship, I (JWF) participated in street medicine, the practice of providing care to patients (typically those who are homeless) at the location they currently reside, such as in a homeless encampment or community shelter. Our clinical team drove to locations that provided housing for patients diagnosed with schizophrenia, where we assisted with medications and blood draws. I remember pulling up the first day and seeing someone outside smoking a cigarette. I soon learned that many people living in such situations were smokers, and that among the substances they used, tobacco was the most common.
One patient said the cigarettes helped him manage the “voices in his head” as well as some of the adverse effects from medication, such as parkinsonism and akathisia. I asked my attending physician about this and she explained that for some patients, using tobacco was a way to mitigate the positive symptoms of schizophrenia and make the adverse effects of their therapy, particularly extrapyramidal symptoms (EPS), more bearable. By the end of my 2-week rotation, I was sure of a trend: our patients with schizophrenia smoked incessantly. Near the end of my rotation, I asked a patient, “Why do you smoke”? The patient looked at me, puzzled, and replied: “I just do.” This exchange only piqued my curiosity, and I could not help but wonder: what is the relationship between tobacco use and schizophrenia? How is tobacco use related to the pathophysiology of schizophrenia? Does tobacco use among patients with schizophrenia ameliorate aspects of their psychosis? Street medicine offered me a window into a biomedically intriguing question, and I wanted to learn more.
What smoking does for patients with schizophrenia
The high prevalence of smoking among patients with schizophrenia (50% to 88%) greatly exceeds the rates of smoking among patients with other psychiatric illnesses.1,2 The role of smoking in relation to schizophrenia and other psychoses is multidimensional, and evidence implicates smoking as a risk factor for schizophrenia.3,4
Two mechanisms may help explain tobacco use in patients with schizophrenia: reducing the adverse effects of antipsychotic medications and promoting neural transmission of dopamine. Second-generation antipsychotics (SGAs) are a first-line treatment, but they can produce EPS, metabolic dysregulation, and blood disorders such as hyponatremia and (rarely) agranulocytosis (1% with clozapine).5 Compared to those who are nonsmokers, patients with schizophrenia who smoke are more likely to experience more severe symptoms (eg, hallucinations and delusions) and less severe EPS.5,6 Research suggests that exposure to polycyclic aromatic hydrocarbons released during smoking induces cytochrome P450 1A2, an enzyme that metabolizes antipsychotic medications such as haloperidol, clozapine, and olanzapine. Increased metabolism results in lower serum concentrations of antipsychotics, lower efficacy, and more severe positive symptoms.5,6
Additionally, tobacco is an activator of nicotinic acetylcholine receptors (nAChR).6 When these receptors become activated, dopamine is released. Dopamine serves as a mediator of reward for nicotine use. In the context of schizophrenia, tobacco use opposes the mechanism of action of SGAs, which is to block neural transmission of dopamine.6 The etiology of EPS is related to the blockade of postsynaptic dopamine release in the striatum.6 By activating nAChR, smoking induces a downstream release of dopamine that can alleviate iatrogenic EPS by restoring neural transmission of dopamine.6 Nicotine may also modulate alpha-7 nicotinic receptor dysfunction, and improve the ability to filter out irrelevant environmental stimuli (impaired sensory gating), which can be overwhelming for patients with schizophrenia. It also can improve cognitive dysfunction and attention by inducing the release of dopamine in mesocortical pathways.7 The implications of this neural pathway are significant because smoking is significantly greater in tobacco users who are diagnosed with schizophrenia compared to tobacco users who lack a psychiatric diagnosis.6,7 Smoking may enhance dopaminergic neural transmission to a far greater extent in tobacco users with schizophrenia compared to tobacco users who do not develop schizophrenia, which suggests intrinsic differences at the neuronal level. Neural differences between tobacco users with or without schizophrenia may synergize with smoking in clinically and biologically meaningful ways. These pathways require further research to support or disprove these hypotheses.
Aside from the dopaminergic system, mechanisms influencing tobacco use among patients with schizophrenia may also be related to nicotine’s mild antidepressant effects. Evidence suggests a clinically meaningful association between nicotine dependence and mood disorders, and this association may be due to the antidepressant effects of nicotine.8-13 Patients with schizophrenia may experience respite from depressive symptoms through their tobacco use, eventually leading to nicotine dependence.
Continue to: Treatment of schizophrenia...
Treatment of schizophrenia involves multimodal management of a patient’s life, including reducing maladaptive habits that are harmful to health. Chronic smoking in patients with schizophrenia is associated not only with atherosclerosis and cardiovascular disease, but also with poor neurologic functioning, such as significant impairment in attention, working memory, learning, executive function, reasoning, problem-solving and speed of processing.14 One study found that in patients with schizophrenia, smoking increased the 20-year cardiovascular mortality risk by 86%.15
Despite challenges to abstinence, smoking cessation should be discussed with these patients, especially given the high prevalence of smoking among this vulnerable population. Bupropion and varenicline have been studied in the context of smoking cessation among patients with schizophrenia. Data on varenicline are mixed. Smokers with schizophrenia who received bupropion showed higher rates of abstinence from smoking compared to those who received placebo.16
As part of the biopsychosocial model of clinical care, sociodemographic factors must be considered in assessing the relationship between tobacco use and schizophrenia, because a large proportion of patients diagnosed with schizophrenia are members of underrepresented minority groups.17 A PubMed database search using keywords “African American” or “Black,” “tobacco,” and “schizophrenia” located only 12 studies, most of which lacked relevance to this question. Han et al18 is 1 of the few studies to investigate sociodemographic factors as they relate to tobacco use among adults with psychoses. Social determinants of health and other confounding variables also need defining to truly distinguish causation from correlation, especially regarding tobacco use and its association with other health risk behaviors.19
Without the street medicine component of the medical school training I received, the pattern of smoking among patients with schizophrenia may have remained invisible or insignificant to me, as tobacco use is not permitted in the inpatient and outpatient academic settings. This experience not only raised insightful questions, but also emphasized the clinical value of seeing patients within their living environment.
1. Patkar AA, Gopalakrishnan R, Lundy A, et al. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190(9):604-610. doi:10.1097/00005053-200209000-00005
2. Ding JB, Hu K. Cigarette smoking and schizophrenia: etiology, clinical, pharmacological, and treatment implications. Schizophr Res Treatment. 2021;2021:7698030. doi:10.1155/2021/7698030
3. Kendler KS, Lönn SL, Sundquist J, et al. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry. 2015;172(11):1092-1100. doi:10.1176/appi.ajp.2015.15010126
4. Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P T. 2014;39(9):638-645.
5. King M, Jones R, Petersen I, et al. Cigarette smoking as a risk factor for schizophrenia or all non-affective psychoses. Psychol Med. 2021;51(8):1373-1381. doi:10.1017/S0033291720000136
6. Sagud M, Mihaljevic Peles A, Pivac N, et al. Smoking in schizophrenia: recent findings about an old problem. Curr Opin Psychiatry. 2019;32(5):402-408. doi:10.1097/YCO.0000000000000529
7. Quigley H, MacCabe JH. The relationship between nicotine and psychosis. Ther Adv Psychopharmacol. 2019;9:2045125319859969. doi:10.1177/2045125319859969
8. Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66(1):79-85. doi:10.1016/s0091-3057(00)00205-7
9. Wang P, Abdin E, Asharani PV, et al. Nicotine dependence in patients with major depressive disorder and psychotic disorders and its relationship with quality of life. Int J Environ Res Public Health. 2021;18(24):13035. doi:10.3390/ijerph182413035
10. Popik P, Krawczyk M, Kos T, et al. Nicotine produces antidepressant-like actions: behavioral and neurochemical evidence. Eur J Pharmacol. 2005;515(1-3):128-133. doi:10.1016/j.ejphar.2005.04.009
11. Quattrocki E, Baird A, Yurgelun-Todd D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8(3):99-110.
12. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12. doi:10.4137/TUI.S32201
13. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185. doi:10.1146/annurev-publhealth-031816-044618
14. Coustals N, Martelli C, Brunet-Lecomte M, et al. Chronic smoking and cognition in patients with schizophrenia: a meta-analysis. Schizophr Res. 2020;222:113-121. doi:10.1016/j.schres.2020.03.071
15. Stolz PA, Wehring HJ, Liu F, et al. Effects of cigarette smoking and clozapine treatment on 20-year all-cause & cardiovascular mortality in schizophrenia. Psychiatr Q. 2019;90(2):351-359. doi:10.1007/s11126-018-9621-4
16. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2013(2):CD007253. doi:10.1002/14651858.CD007253.pub3
17. Heun-Johnson H, Menchine M, Axeen S, et al. Association between race/ethnicity and disparities in health care use before first-episode psychosis among privately insured young patients. JAMA Psychiatry. 2021;78(3):311-319. doi:10.1001/jamapsychiatry.2020.3995
18. Han B, Aung TW, Volkow ND, et al. Tobacco use, nicotine dependence, and cessation methods in us adults with psychosis. JAMA Netw Open. 2023;6(3):e234995. doi:10.1001/jamanetworkopen.2023.4995
19. Peltzer K, Pengpid S. Tobacco use and associated mental symptoms and health risk behaviours amongst individuals 15 years or older in South Africa. S Afr J Psychiatr. 2020;26:1499. doi:10.4102/sajpsychiatry.v26.i0.1499
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Throughout my psychiatric clerkship, I (JWF) participated in street medicine, the practice of providing care to patients (typically those who are homeless) at the location they currently reside, such as in a homeless encampment or community shelter. Our clinical team drove to locations that provided housing for patients diagnosed with schizophrenia, where we assisted with medications and blood draws. I remember pulling up the first day and seeing someone outside smoking a cigarette. I soon learned that many people living in such situations were smokers, and that among the substances they used, tobacco was the most common.
One patient said the cigarettes helped him manage the “voices in his head” as well as some of the adverse effects from medication, such as parkinsonism and akathisia. I asked my attending physician about this and she explained that for some patients, using tobacco was a way to mitigate the positive symptoms of schizophrenia and make the adverse effects of their therapy, particularly extrapyramidal symptoms (EPS), more bearable. By the end of my 2-week rotation, I was sure of a trend: our patients with schizophrenia smoked incessantly. Near the end of my rotation, I asked a patient, “Why do you smoke”? The patient looked at me, puzzled, and replied: “I just do.” This exchange only piqued my curiosity, and I could not help but wonder: what is the relationship between tobacco use and schizophrenia? How is tobacco use related to the pathophysiology of schizophrenia? Does tobacco use among patients with schizophrenia ameliorate aspects of their psychosis? Street medicine offered me a window into a biomedically intriguing question, and I wanted to learn more.
What smoking does for patients with schizophrenia
The high prevalence of smoking among patients with schizophrenia (50% to 88%) greatly exceeds the rates of smoking among patients with other psychiatric illnesses.1,2 The role of smoking in relation to schizophrenia and other psychoses is multidimensional, and evidence implicates smoking as a risk factor for schizophrenia.3,4
Two mechanisms may help explain tobacco use in patients with schizophrenia: reducing the adverse effects of antipsychotic medications and promoting neural transmission of dopamine. Second-generation antipsychotics (SGAs) are a first-line treatment, but they can produce EPS, metabolic dysregulation, and blood disorders such as hyponatremia and (rarely) agranulocytosis (1% with clozapine).5 Compared to those who are nonsmokers, patients with schizophrenia who smoke are more likely to experience more severe symptoms (eg, hallucinations and delusions) and less severe EPS.5,6 Research suggests that exposure to polycyclic aromatic hydrocarbons released during smoking induces cytochrome P450 1A2, an enzyme that metabolizes antipsychotic medications such as haloperidol, clozapine, and olanzapine. Increased metabolism results in lower serum concentrations of antipsychotics, lower efficacy, and more severe positive symptoms.5,6
Additionally, tobacco is an activator of nicotinic acetylcholine receptors (nAChR).6 When these receptors become activated, dopamine is released. Dopamine serves as a mediator of reward for nicotine use. In the context of schizophrenia, tobacco use opposes the mechanism of action of SGAs, which is to block neural transmission of dopamine.6 The etiology of EPS is related to the blockade of postsynaptic dopamine release in the striatum.6 By activating nAChR, smoking induces a downstream release of dopamine that can alleviate iatrogenic EPS by restoring neural transmission of dopamine.6 Nicotine may also modulate alpha-7 nicotinic receptor dysfunction, and improve the ability to filter out irrelevant environmental stimuli (impaired sensory gating), which can be overwhelming for patients with schizophrenia. It also can improve cognitive dysfunction and attention by inducing the release of dopamine in mesocortical pathways.7 The implications of this neural pathway are significant because smoking is significantly greater in tobacco users who are diagnosed with schizophrenia compared to tobacco users who lack a psychiatric diagnosis.6,7 Smoking may enhance dopaminergic neural transmission to a far greater extent in tobacco users with schizophrenia compared to tobacco users who do not develop schizophrenia, which suggests intrinsic differences at the neuronal level. Neural differences between tobacco users with or without schizophrenia may synergize with smoking in clinically and biologically meaningful ways. These pathways require further research to support or disprove these hypotheses.
Aside from the dopaminergic system, mechanisms influencing tobacco use among patients with schizophrenia may also be related to nicotine’s mild antidepressant effects. Evidence suggests a clinically meaningful association between nicotine dependence and mood disorders, and this association may be due to the antidepressant effects of nicotine.8-13 Patients with schizophrenia may experience respite from depressive symptoms through their tobacco use, eventually leading to nicotine dependence.
Continue to: Treatment of schizophrenia...
Treatment of schizophrenia involves multimodal management of a patient’s life, including reducing maladaptive habits that are harmful to health. Chronic smoking in patients with schizophrenia is associated not only with atherosclerosis and cardiovascular disease, but also with poor neurologic functioning, such as significant impairment in attention, working memory, learning, executive function, reasoning, problem-solving and speed of processing.14 One study found that in patients with schizophrenia, smoking increased the 20-year cardiovascular mortality risk by 86%.15
Despite challenges to abstinence, smoking cessation should be discussed with these patients, especially given the high prevalence of smoking among this vulnerable population. Bupropion and varenicline have been studied in the context of smoking cessation among patients with schizophrenia. Data on varenicline are mixed. Smokers with schizophrenia who received bupropion showed higher rates of abstinence from smoking compared to those who received placebo.16
As part of the biopsychosocial model of clinical care, sociodemographic factors must be considered in assessing the relationship between tobacco use and schizophrenia, because a large proportion of patients diagnosed with schizophrenia are members of underrepresented minority groups.17 A PubMed database search using keywords “African American” or “Black,” “tobacco,” and “schizophrenia” located only 12 studies, most of which lacked relevance to this question. Han et al18 is 1 of the few studies to investigate sociodemographic factors as they relate to tobacco use among adults with psychoses. Social determinants of health and other confounding variables also need defining to truly distinguish causation from correlation, especially regarding tobacco use and its association with other health risk behaviors.19
Without the street medicine component of the medical school training I received, the pattern of smoking among patients with schizophrenia may have remained invisible or insignificant to me, as tobacco use is not permitted in the inpatient and outpatient academic settings. This experience not only raised insightful questions, but also emphasized the clinical value of seeing patients within their living environment.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Throughout my psychiatric clerkship, I (JWF) participated in street medicine, the practice of providing care to patients (typically those who are homeless) at the location they currently reside, such as in a homeless encampment or community shelter. Our clinical team drove to locations that provided housing for patients diagnosed with schizophrenia, where we assisted with medications and blood draws. I remember pulling up the first day and seeing someone outside smoking a cigarette. I soon learned that many people living in such situations were smokers, and that among the substances they used, tobacco was the most common.
One patient said the cigarettes helped him manage the “voices in his head” as well as some of the adverse effects from medication, such as parkinsonism and akathisia. I asked my attending physician about this and she explained that for some patients, using tobacco was a way to mitigate the positive symptoms of schizophrenia and make the adverse effects of their therapy, particularly extrapyramidal symptoms (EPS), more bearable. By the end of my 2-week rotation, I was sure of a trend: our patients with schizophrenia smoked incessantly. Near the end of my rotation, I asked a patient, “Why do you smoke”? The patient looked at me, puzzled, and replied: “I just do.” This exchange only piqued my curiosity, and I could not help but wonder: what is the relationship between tobacco use and schizophrenia? How is tobacco use related to the pathophysiology of schizophrenia? Does tobacco use among patients with schizophrenia ameliorate aspects of their psychosis? Street medicine offered me a window into a biomedically intriguing question, and I wanted to learn more.
What smoking does for patients with schizophrenia
The high prevalence of smoking among patients with schizophrenia (50% to 88%) greatly exceeds the rates of smoking among patients with other psychiatric illnesses.1,2 The role of smoking in relation to schizophrenia and other psychoses is multidimensional, and evidence implicates smoking as a risk factor for schizophrenia.3,4
Two mechanisms may help explain tobacco use in patients with schizophrenia: reducing the adverse effects of antipsychotic medications and promoting neural transmission of dopamine. Second-generation antipsychotics (SGAs) are a first-line treatment, but they can produce EPS, metabolic dysregulation, and blood disorders such as hyponatremia and (rarely) agranulocytosis (1% with clozapine).5 Compared to those who are nonsmokers, patients with schizophrenia who smoke are more likely to experience more severe symptoms (eg, hallucinations and delusions) and less severe EPS.5,6 Research suggests that exposure to polycyclic aromatic hydrocarbons released during smoking induces cytochrome P450 1A2, an enzyme that metabolizes antipsychotic medications such as haloperidol, clozapine, and olanzapine. Increased metabolism results in lower serum concentrations of antipsychotics, lower efficacy, and more severe positive symptoms.5,6
Additionally, tobacco is an activator of nicotinic acetylcholine receptors (nAChR).6 When these receptors become activated, dopamine is released. Dopamine serves as a mediator of reward for nicotine use. In the context of schizophrenia, tobacco use opposes the mechanism of action of SGAs, which is to block neural transmission of dopamine.6 The etiology of EPS is related to the blockade of postsynaptic dopamine release in the striatum.6 By activating nAChR, smoking induces a downstream release of dopamine that can alleviate iatrogenic EPS by restoring neural transmission of dopamine.6 Nicotine may also modulate alpha-7 nicotinic receptor dysfunction, and improve the ability to filter out irrelevant environmental stimuli (impaired sensory gating), which can be overwhelming for patients with schizophrenia. It also can improve cognitive dysfunction and attention by inducing the release of dopamine in mesocortical pathways.7 The implications of this neural pathway are significant because smoking is significantly greater in tobacco users who are diagnosed with schizophrenia compared to tobacco users who lack a psychiatric diagnosis.6,7 Smoking may enhance dopaminergic neural transmission to a far greater extent in tobacco users with schizophrenia compared to tobacco users who do not develop schizophrenia, which suggests intrinsic differences at the neuronal level. Neural differences between tobacco users with or without schizophrenia may synergize with smoking in clinically and biologically meaningful ways. These pathways require further research to support or disprove these hypotheses.
Aside from the dopaminergic system, mechanisms influencing tobacco use among patients with schizophrenia may also be related to nicotine’s mild antidepressant effects. Evidence suggests a clinically meaningful association between nicotine dependence and mood disorders, and this association may be due to the antidepressant effects of nicotine.8-13 Patients with schizophrenia may experience respite from depressive symptoms through their tobacco use, eventually leading to nicotine dependence.
Continue to: Treatment of schizophrenia...
Treatment of schizophrenia involves multimodal management of a patient’s life, including reducing maladaptive habits that are harmful to health. Chronic smoking in patients with schizophrenia is associated not only with atherosclerosis and cardiovascular disease, but also with poor neurologic functioning, such as significant impairment in attention, working memory, learning, executive function, reasoning, problem-solving and speed of processing.14 One study found that in patients with schizophrenia, smoking increased the 20-year cardiovascular mortality risk by 86%.15
Despite challenges to abstinence, smoking cessation should be discussed with these patients, especially given the high prevalence of smoking among this vulnerable population. Bupropion and varenicline have been studied in the context of smoking cessation among patients with schizophrenia. Data on varenicline are mixed. Smokers with schizophrenia who received bupropion showed higher rates of abstinence from smoking compared to those who received placebo.16
As part of the biopsychosocial model of clinical care, sociodemographic factors must be considered in assessing the relationship between tobacco use and schizophrenia, because a large proportion of patients diagnosed with schizophrenia are members of underrepresented minority groups.17 A PubMed database search using keywords “African American” or “Black,” “tobacco,” and “schizophrenia” located only 12 studies, most of which lacked relevance to this question. Han et al18 is 1 of the few studies to investigate sociodemographic factors as they relate to tobacco use among adults with psychoses. Social determinants of health and other confounding variables also need defining to truly distinguish causation from correlation, especially regarding tobacco use and its association with other health risk behaviors.19
Without the street medicine component of the medical school training I received, the pattern of smoking among patients with schizophrenia may have remained invisible or insignificant to me, as tobacco use is not permitted in the inpatient and outpatient academic settings. This experience not only raised insightful questions, but also emphasized the clinical value of seeing patients within their living environment.
1. Patkar AA, Gopalakrishnan R, Lundy A, et al. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190(9):604-610. doi:10.1097/00005053-200209000-00005
2. Ding JB, Hu K. Cigarette smoking and schizophrenia: etiology, clinical, pharmacological, and treatment implications. Schizophr Res Treatment. 2021;2021:7698030. doi:10.1155/2021/7698030
3. Kendler KS, Lönn SL, Sundquist J, et al. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry. 2015;172(11):1092-1100. doi:10.1176/appi.ajp.2015.15010126
4. Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P T. 2014;39(9):638-645.
5. King M, Jones R, Petersen I, et al. Cigarette smoking as a risk factor for schizophrenia or all non-affective psychoses. Psychol Med. 2021;51(8):1373-1381. doi:10.1017/S0033291720000136
6. Sagud M, Mihaljevic Peles A, Pivac N, et al. Smoking in schizophrenia: recent findings about an old problem. Curr Opin Psychiatry. 2019;32(5):402-408. doi:10.1097/YCO.0000000000000529
7. Quigley H, MacCabe JH. The relationship between nicotine and psychosis. Ther Adv Psychopharmacol. 2019;9:2045125319859969. doi:10.1177/2045125319859969
8. Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66(1):79-85. doi:10.1016/s0091-3057(00)00205-7
9. Wang P, Abdin E, Asharani PV, et al. Nicotine dependence in patients with major depressive disorder and psychotic disorders and its relationship with quality of life. Int J Environ Res Public Health. 2021;18(24):13035. doi:10.3390/ijerph182413035
10. Popik P, Krawczyk M, Kos T, et al. Nicotine produces antidepressant-like actions: behavioral and neurochemical evidence. Eur J Pharmacol. 2005;515(1-3):128-133. doi:10.1016/j.ejphar.2005.04.009
11. Quattrocki E, Baird A, Yurgelun-Todd D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8(3):99-110.
12. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12. doi:10.4137/TUI.S32201
13. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185. doi:10.1146/annurev-publhealth-031816-044618
14. Coustals N, Martelli C, Brunet-Lecomte M, et al. Chronic smoking and cognition in patients with schizophrenia: a meta-analysis. Schizophr Res. 2020;222:113-121. doi:10.1016/j.schres.2020.03.071
15. Stolz PA, Wehring HJ, Liu F, et al. Effects of cigarette smoking and clozapine treatment on 20-year all-cause & cardiovascular mortality in schizophrenia. Psychiatr Q. 2019;90(2):351-359. doi:10.1007/s11126-018-9621-4
16. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2013(2):CD007253. doi:10.1002/14651858.CD007253.pub3
17. Heun-Johnson H, Menchine M, Axeen S, et al. Association between race/ethnicity and disparities in health care use before first-episode psychosis among privately insured young patients. JAMA Psychiatry. 2021;78(3):311-319. doi:10.1001/jamapsychiatry.2020.3995
18. Han B, Aung TW, Volkow ND, et al. Tobacco use, nicotine dependence, and cessation methods in us adults with psychosis. JAMA Netw Open. 2023;6(3):e234995. doi:10.1001/jamanetworkopen.2023.4995
19. Peltzer K, Pengpid S. Tobacco use and associated mental symptoms and health risk behaviours amongst individuals 15 years or older in South Africa. S Afr J Psychiatr. 2020;26:1499. doi:10.4102/sajpsychiatry.v26.i0.1499
1. Patkar AA, Gopalakrishnan R, Lundy A, et al. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190(9):604-610. doi:10.1097/00005053-200209000-00005
2. Ding JB, Hu K. Cigarette smoking and schizophrenia: etiology, clinical, pharmacological, and treatment implications. Schizophr Res Treatment. 2021;2021:7698030. doi:10.1155/2021/7698030
3. Kendler KS, Lönn SL, Sundquist J, et al. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry. 2015;172(11):1092-1100. doi:10.1176/appi.ajp.2015.15010126
4. Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P T. 2014;39(9):638-645.
5. King M, Jones R, Petersen I, et al. Cigarette smoking as a risk factor for schizophrenia or all non-affective psychoses. Psychol Med. 2021;51(8):1373-1381. doi:10.1017/S0033291720000136
6. Sagud M, Mihaljevic Peles A, Pivac N, et al. Smoking in schizophrenia: recent findings about an old problem. Curr Opin Psychiatry. 2019;32(5):402-408. doi:10.1097/YCO.0000000000000529
7. Quigley H, MacCabe JH. The relationship between nicotine and psychosis. Ther Adv Psychopharmacol. 2019;9:2045125319859969. doi:10.1177/2045125319859969
8. Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66(1):79-85. doi:10.1016/s0091-3057(00)00205-7
9. Wang P, Abdin E, Asharani PV, et al. Nicotine dependence in patients with major depressive disorder and psychotic disorders and its relationship with quality of life. Int J Environ Res Public Health. 2021;18(24):13035. doi:10.3390/ijerph182413035
10. Popik P, Krawczyk M, Kos T, et al. Nicotine produces antidepressant-like actions: behavioral and neurochemical evidence. Eur J Pharmacol. 2005;515(1-3):128-133. doi:10.1016/j.ejphar.2005.04.009
11. Quattrocki E, Baird A, Yurgelun-Todd D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8(3):99-110.
12. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12. doi:10.4137/TUI.S32201
13. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185. doi:10.1146/annurev-publhealth-031816-044618
14. Coustals N, Martelli C, Brunet-Lecomte M, et al. Chronic smoking and cognition in patients with schizophrenia: a meta-analysis. Schizophr Res. 2020;222:113-121. doi:10.1016/j.schres.2020.03.071
15. Stolz PA, Wehring HJ, Liu F, et al. Effects of cigarette smoking and clozapine treatment on 20-year all-cause & cardiovascular mortality in schizophrenia. Psychiatr Q. 2019;90(2):351-359. doi:10.1007/s11126-018-9621-4
16. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2013(2):CD007253. doi:10.1002/14651858.CD007253.pub3
17. Heun-Johnson H, Menchine M, Axeen S, et al. Association between race/ethnicity and disparities in health care use before first-episode psychosis among privately insured young patients. JAMA Psychiatry. 2021;78(3):311-319. doi:10.1001/jamapsychiatry.2020.3995
18. Han B, Aung TW, Volkow ND, et al. Tobacco use, nicotine dependence, and cessation methods in us adults with psychosis. JAMA Netw Open. 2023;6(3):e234995. doi:10.1001/jamanetworkopen.2023.4995
19. Peltzer K, Pengpid S. Tobacco use and associated mental symptoms and health risk behaviours amongst individuals 15 years or older in South Africa. S Afr J Psychiatr. 2020;26:1499. doi:10.4102/sajpsychiatry.v26.i0.1499
More on interventional psychiatry
Thank you very much to Drs. Vincent, Good, and El-Mallakh for their guest editorial on interventional psychiatry (“Interventional psychiatry: What are the next steps?”
The Clinical Transcranial Magnetic Stimulation Society (CTMSS) is well aware of these issues and is actively addressing them:
1. We have increased the number of PULSES courses—designed to serve as intensive, introductory courses on TMS—we provide, and increased the number of members on our PULSES team to address this. We have also increased the number of PULSES scholarships for psychiatry residents that cover the costs of the conference and materials.
2. We created a standing Resident Subcommittee of our Education Committee that is focused on psychiatry resident training. We realize not all psychiatric residency programs have active TMS programs or attendings who are trained in TMS. Last year we presented lectures aimed at introducing TMS to PGY-1 and PGY-2 psychiatry residents. These were recorded and are available for free on the CTMSS website (www.clinicaltmssociety.org).
3. The Resident Subcommittee presented the American Association of Directors of Psychiatric Residency Training with a curriculum submission that was accepted and will be available to all psychiatric residents across the country free of charge. (
4. The topic of resident/fellow training in all forms of neuromodulation was discussed during our monthly Grand Rounds webinar and at our annual meeting.
5. The issue of having a broader base of knowledge and training in neuromodulation was a topic at a recent Education Committee meeting, and this year we are adding lectures on electroconvulsive therapy and esketamine to our Grand Rounds webinars. Many CTMSS members are trained and knowledgeable in multiple neuromodulation modalities.
Continue to: 6. Many CTMSS members...
6. Many CTMSS members are involved in academic programs or are invited to training programs to teach psychiatric residents as guest lecturers.
7. The UK's Royal College of Psychiatrists has requested access to our prerecorded lectures, and CTMSS members are working on translating our lectures into Spanish.
Resident education is a key component of the main goals of the CTMSS. Our Board of Directors is fully committed to resident education and has directed the Education Committee to address it. We look forward to moving forward on educating psychiatric residents, with the hope of eventually engaging the ACGME to acknowledge TMS by name in the ACGME guidelines, provide residents with at least basic information on TMS, and clarify how competency in these therapies can be achieved.
Thank you very much to Drs. Vincent, Good, and El-Mallakh for their guest editorial on interventional psychiatry (“Interventional psychiatry: What are the next steps?”
The Clinical Transcranial Magnetic Stimulation Society (CTMSS) is well aware of these issues and is actively addressing them:
1. We have increased the number of PULSES courses—designed to serve as intensive, introductory courses on TMS—we provide, and increased the number of members on our PULSES team to address this. We have also increased the number of PULSES scholarships for psychiatry residents that cover the costs of the conference and materials.
2. We created a standing Resident Subcommittee of our Education Committee that is focused on psychiatry resident training. We realize not all psychiatric residency programs have active TMS programs or attendings who are trained in TMS. Last year we presented lectures aimed at introducing TMS to PGY-1 and PGY-2 psychiatry residents. These were recorded and are available for free on the CTMSS website (www.clinicaltmssociety.org).
3. The Resident Subcommittee presented the American Association of Directors of Psychiatric Residency Training with a curriculum submission that was accepted and will be available to all psychiatric residents across the country free of charge. (
4. The topic of resident/fellow training in all forms of neuromodulation was discussed during our monthly Grand Rounds webinar and at our annual meeting.
5. The issue of having a broader base of knowledge and training in neuromodulation was a topic at a recent Education Committee meeting, and this year we are adding lectures on electroconvulsive therapy and esketamine to our Grand Rounds webinars. Many CTMSS members are trained and knowledgeable in multiple neuromodulation modalities.
Continue to: 6. Many CTMSS members...
6. Many CTMSS members are involved in academic programs or are invited to training programs to teach psychiatric residents as guest lecturers.
7. The UK's Royal College of Psychiatrists has requested access to our prerecorded lectures, and CTMSS members are working on translating our lectures into Spanish.
Resident education is a key component of the main goals of the CTMSS. Our Board of Directors is fully committed to resident education and has directed the Education Committee to address it. We look forward to moving forward on educating psychiatric residents, with the hope of eventually engaging the ACGME to acknowledge TMS by name in the ACGME guidelines, provide residents with at least basic information on TMS, and clarify how competency in these therapies can be achieved.
Thank you very much to Drs. Vincent, Good, and El-Mallakh for their guest editorial on interventional psychiatry (“Interventional psychiatry: What are the next steps?”
The Clinical Transcranial Magnetic Stimulation Society (CTMSS) is well aware of these issues and is actively addressing them:
1. We have increased the number of PULSES courses—designed to serve as intensive, introductory courses on TMS—we provide, and increased the number of members on our PULSES team to address this. We have also increased the number of PULSES scholarships for psychiatry residents that cover the costs of the conference and materials.
2. We created a standing Resident Subcommittee of our Education Committee that is focused on psychiatry resident training. We realize not all psychiatric residency programs have active TMS programs or attendings who are trained in TMS. Last year we presented lectures aimed at introducing TMS to PGY-1 and PGY-2 psychiatry residents. These were recorded and are available for free on the CTMSS website (www.clinicaltmssociety.org).
3. The Resident Subcommittee presented the American Association of Directors of Psychiatric Residency Training with a curriculum submission that was accepted and will be available to all psychiatric residents across the country free of charge. (
4. The topic of resident/fellow training in all forms of neuromodulation was discussed during our monthly Grand Rounds webinar and at our annual meeting.
5. The issue of having a broader base of knowledge and training in neuromodulation was a topic at a recent Education Committee meeting, and this year we are adding lectures on electroconvulsive therapy and esketamine to our Grand Rounds webinars. Many CTMSS members are trained and knowledgeable in multiple neuromodulation modalities.
Continue to: 6. Many CTMSS members...
6. Many CTMSS members are involved in academic programs or are invited to training programs to teach psychiatric residents as guest lecturers.
7. The UK's Royal College of Psychiatrists has requested access to our prerecorded lectures, and CTMSS members are working on translating our lectures into Spanish.
Resident education is a key component of the main goals of the CTMSS. Our Board of Directors is fully committed to resident education and has directed the Education Committee to address it. We look forward to moving forward on educating psychiatric residents, with the hope of eventually engaging the ACGME to acknowledge TMS by name in the ACGME guidelines, provide residents with at least basic information on TMS, and clarify how competency in these therapies can be achieved.
Neuropsychiatric aspects of Parkinson’s disease: Practical considerations
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).
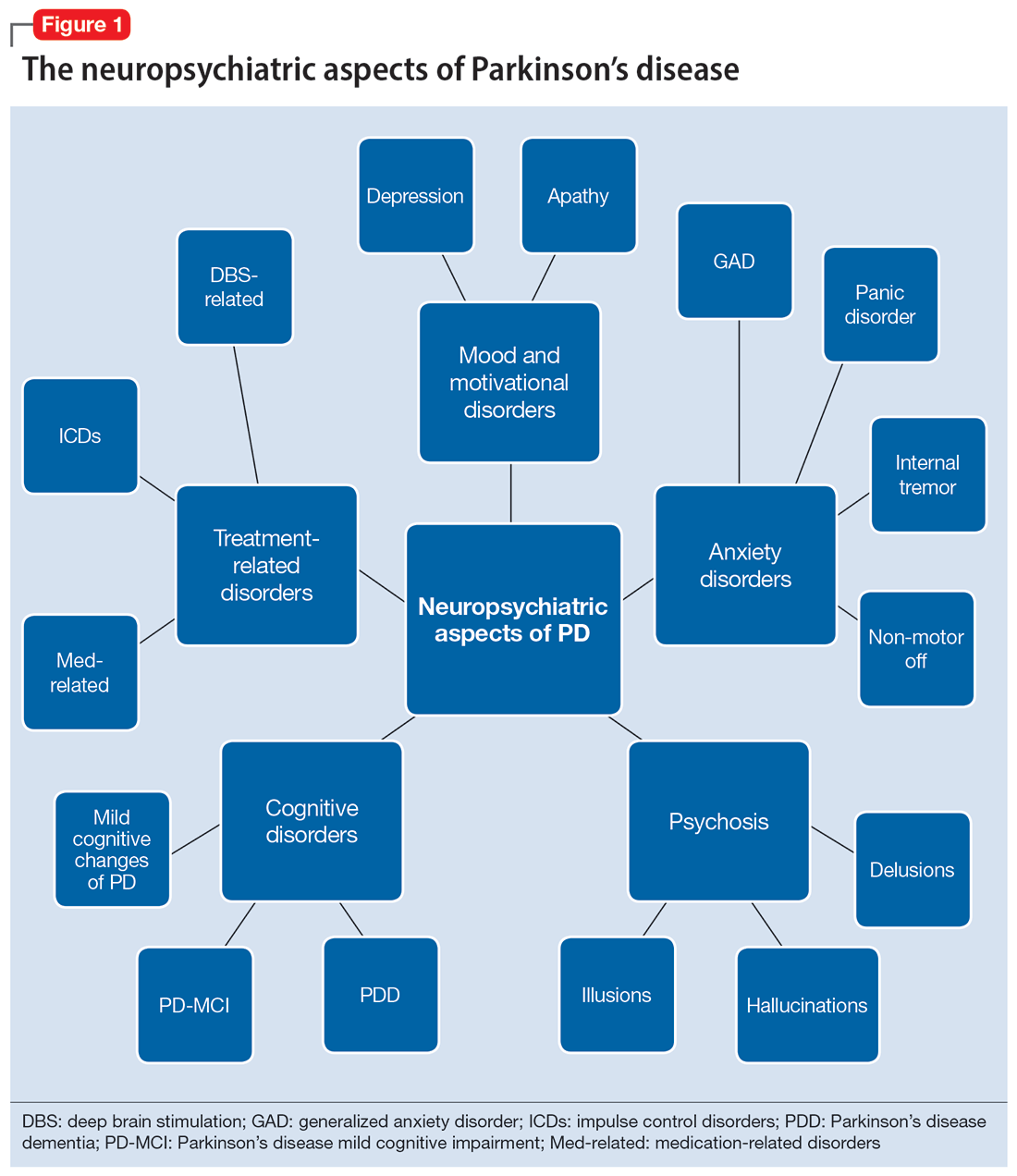
Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32
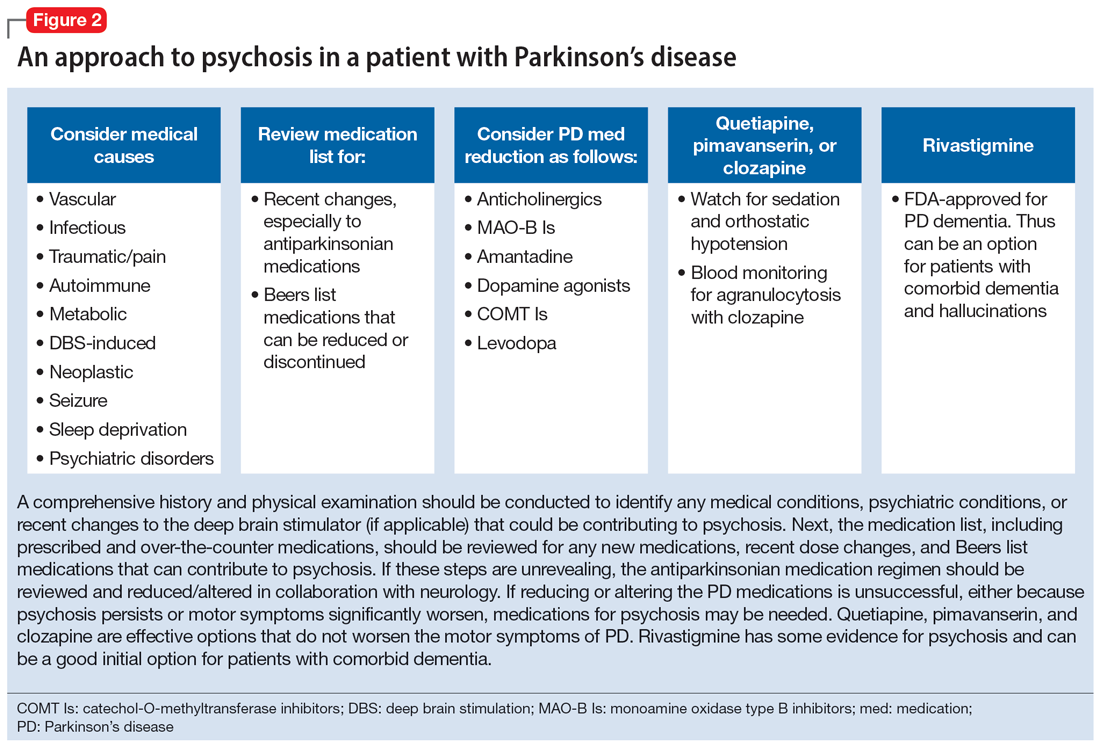
Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36
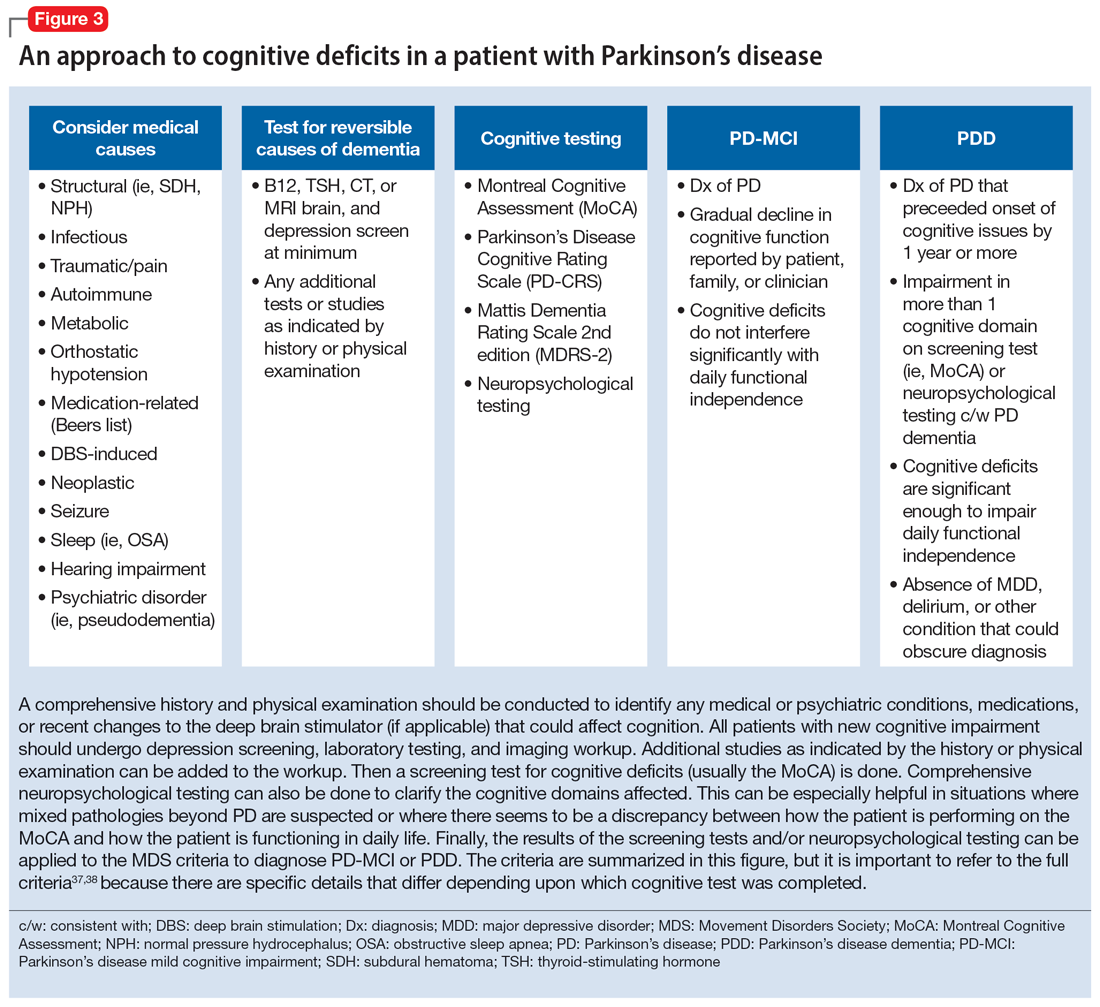
Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42
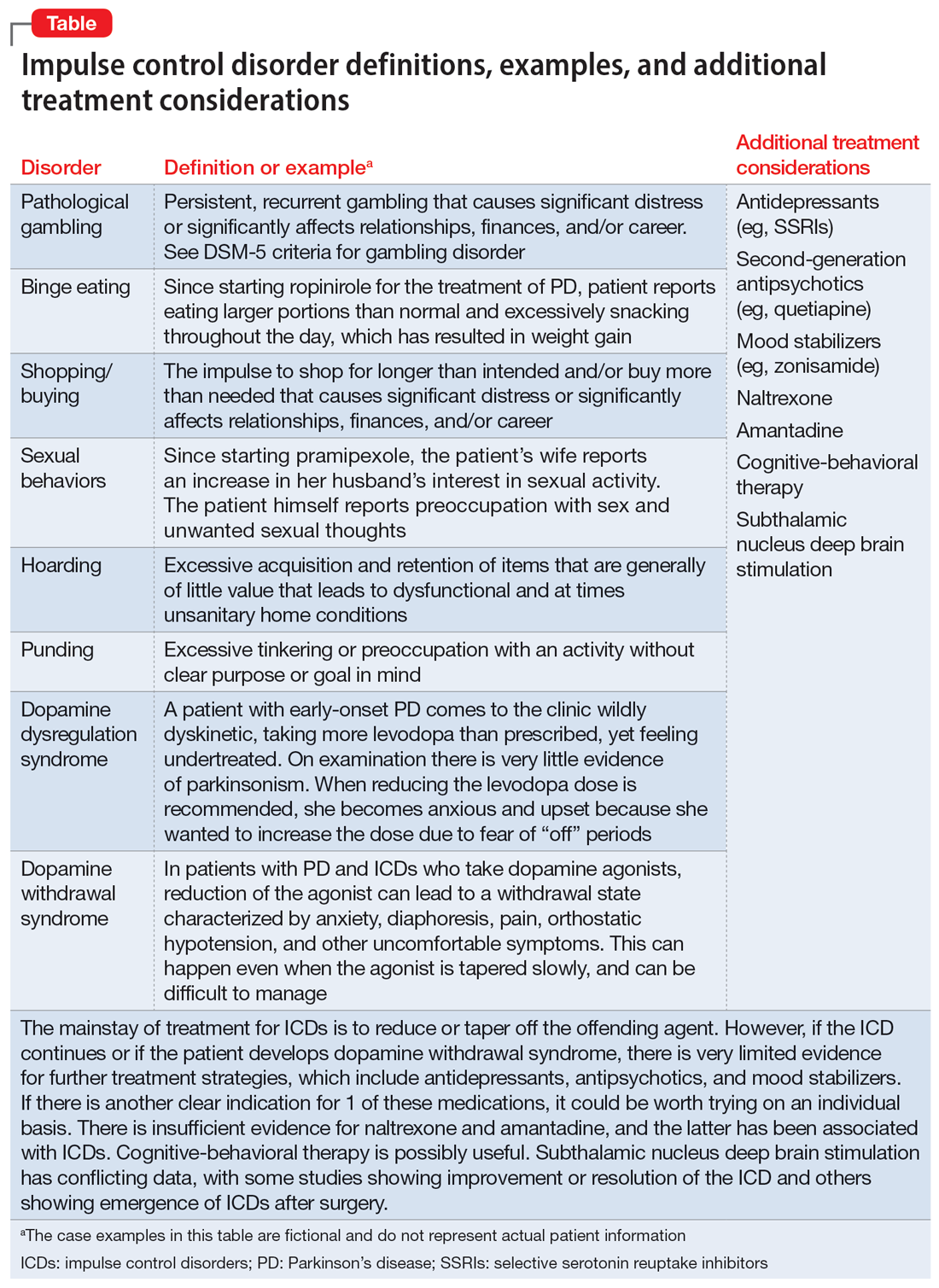
The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).

Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32

Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36

Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42

The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).

Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32

Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36

Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42

The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
ADHD in older adults: A closer look
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).
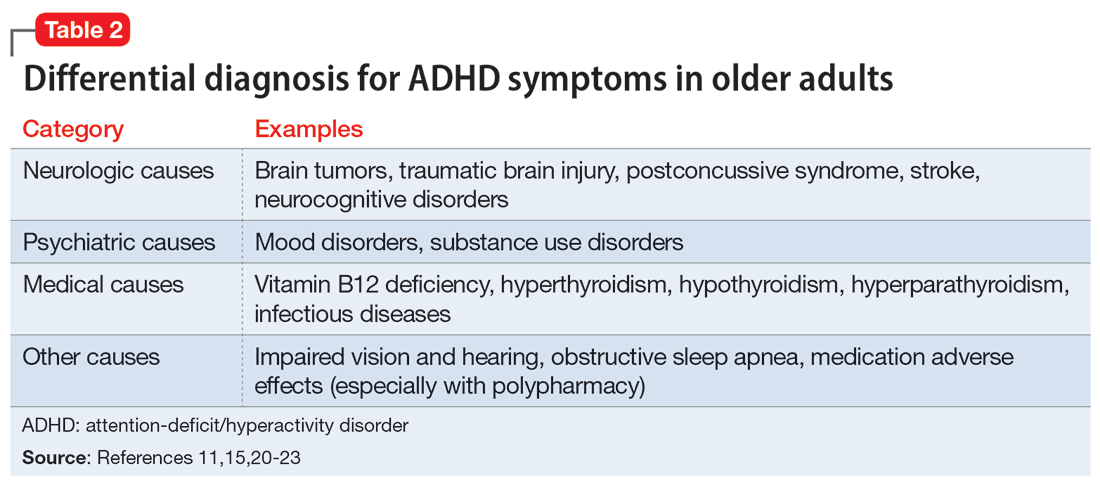
In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21
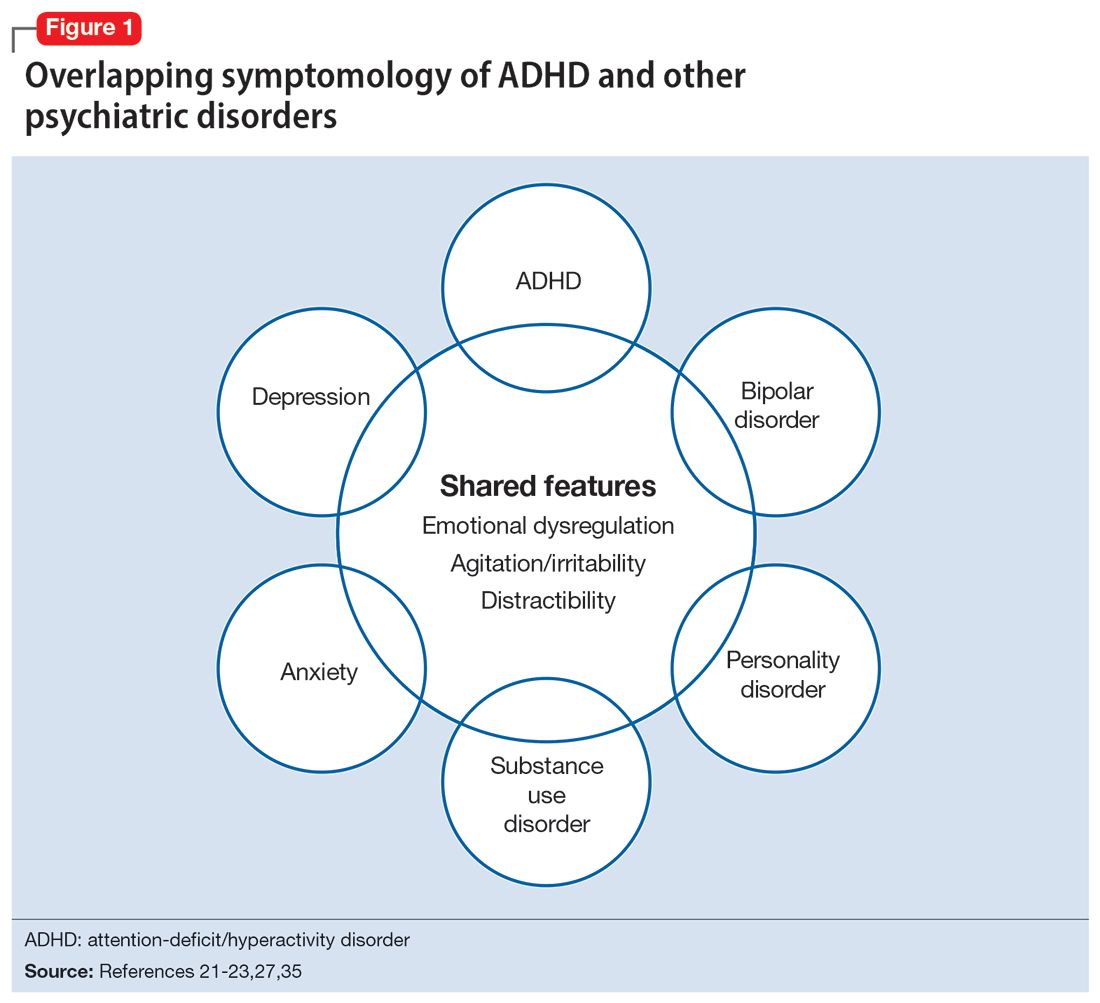
Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.
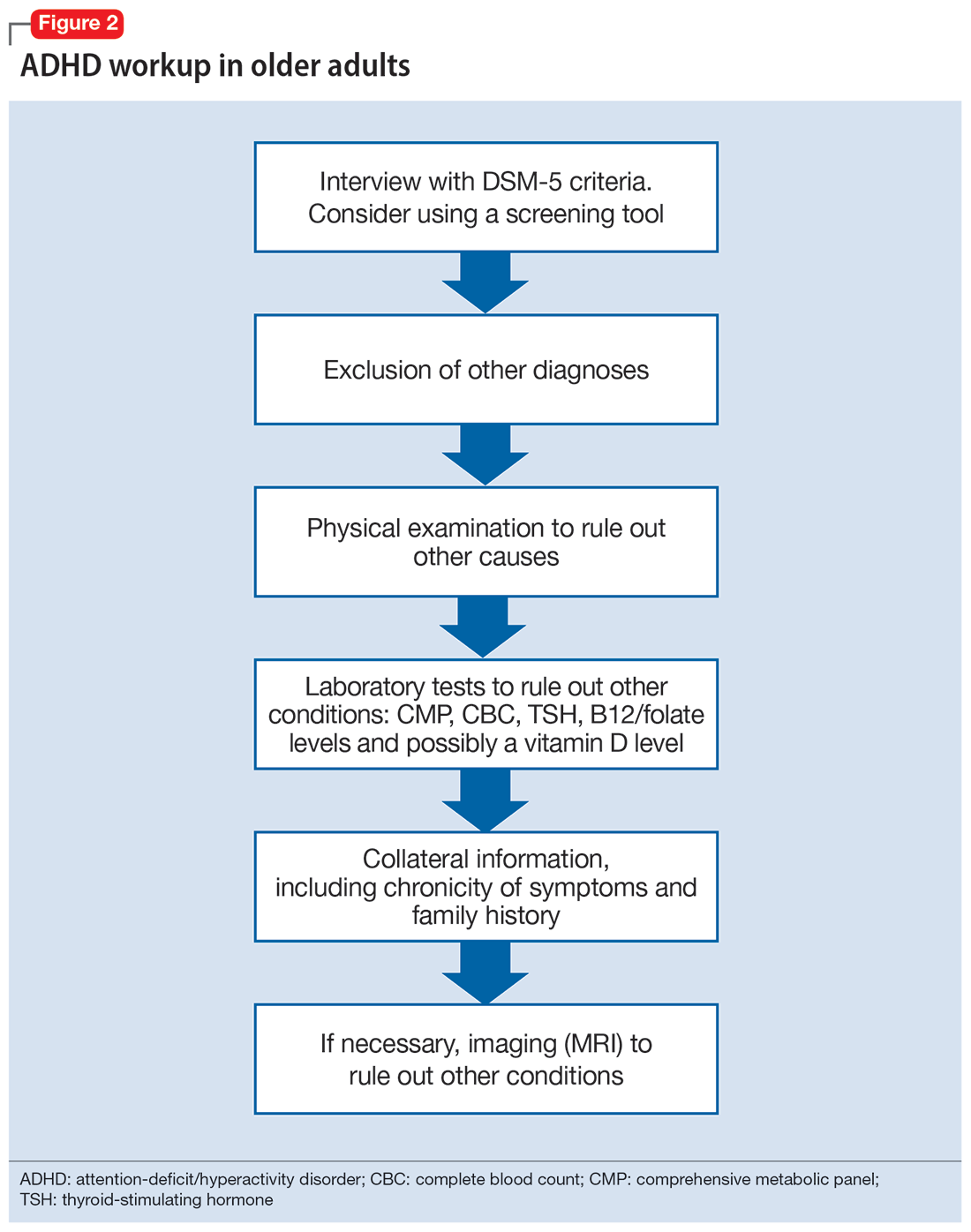
Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).

In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21

Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.

Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).

In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21

Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.

Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
Climate change and mental illness: What psychiatrists can do
“ Hope is engagement with the act of mapping our destinies.” 1
—Valerie Braithwaite
Why should psychiatrists care about climate change and try to mitigate its effects? First, we are tasked by society with managing the psychological and neuropsychiatric sequelae from disasters, which include climate change. The American Psychiatric Association’s position statement on climate change includes it as a legitimate focus for our specialty.2 Second, as physicians, we are morally obligated to do no harm. Since the health care sector contributes significantly to climate change (8.5% of national carbon emissions stem from health care) and causes demonstrable health impacts,3 managing these impacts and decarbonizing the health care industry is morally imperative.4 And third, psychiatric clinicians have transferrable skills that can address fears of climate change, challenge climate change denialism,5 motivate people to adopt more pro-environmental behaviors, and help communities not only endure the emotional impact of climate change but become more psychologically resilient.6
Most psychiatrists, however, did not receive formal training on climate change and the related field of disaster preparedness. For example, Harvard Medical School did not include a course on climate change in their medical student curriculum until 2023.7 In this article, we provide a basic framework of climate change and its impact on mental health, with particular focus on patients with serious mental illness (SMI). We offer concrete steps clinicians can take to prevent or mitigate harm from climate change for their patients, prepare for disasters at the level of individual patient encounters, and strengthen their clinics and communities. We also encourage clinicians to take active leadership roles in their professional organizations to be part of climate solutions, building on the trust patients continue to have in their physicians.8 Even if clinicians do not view climate change concerns under their conceived clinical care mandate, having a working knowledge about it is important because patients, paraprofessional staff, or medical trainees are likely to bring it up.9
Climate change and mental health
Climate change is harmful to human health, including mental health.10 It can impact mental health directly via its impact on brain function and neuropsychiatric sequelae, and indirectly via climate-related disasters leading to acute or chronic stress, losses, and displacement with psychiatric and psychological sequelae (Table 111-29).
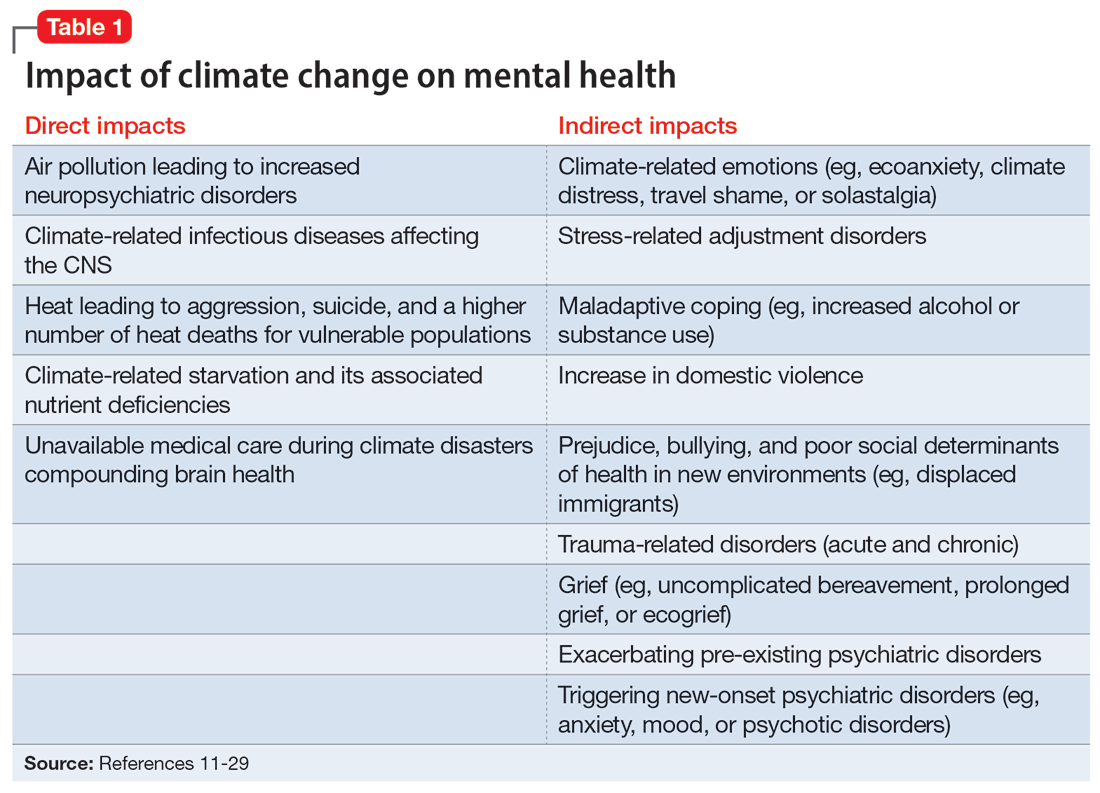
Direct impact
The effects of air pollution, heat, infections, and starvation are examples of how climate change directly impacts mental health. Air pollution and brain health are a concern for psychiatry, given the well-described effects of air deterioration on the developing brain.11 In animal models, airborne pollutants lead to widespread neuroinflammation and cell loss via a multitude of mechanisms.12 This is consistent with worse cognitive and behavioral functions across a wide range of cognitive domains seen in children exposed to pollution compared to those who grew up in environments with healthy air.13 Even low-level exposure to air pollution increases the risk for later onset of depression, suicide, and anxiety.14 Hippocampal atrophy observed in patients with first-episode psychosis may also be partially attributable to air pollution.15 An association between heat and suicide (and to a lesser extent, aggression) has also been reported.16
Worse physical health (eg, strokes) due to excessive heat can further compound mental health via elevated rates of depression. Data from the United States and Mexico show that for each degree Celsius increase in ambient temperature, suicide rates may increase by approximately 1%.17 A meta-analysis by Frangione et al18 similarly concluded that each degree Celsius increase results in an overall risk ratio of 1.016 (95% CI, 1.012 to 1.019) for deaths by suicide and suicide attempts. Additionally, global warming is shifting the endemic areas for many infectious agents, particularly vector-borne diseases,19 to regions in which they had hitherto been unknown, increasing the risk for future outbreaks and even pandemics.20 These infectious illnesses often carry neuropsychiatric morbidity, with seizures, encephalopathy with incomplete recovery, and psychiatric syndromes occurring in many cases. Crop failure can lead to starvation during pregnancy and childhood, which has wide-ranging consequences for brain development and later physical and psychological health in adults.21,22 Mothers affected by starvation also experience negative impacts on childbearing and childrearing.23
Indirect impact
Climate change’s indirect impact on mental health can stem from the stress of living through a disaster such as an extreme weather event; from losses, including the death of friends and family members; and from becoming temporarily displaced.24 Some climate change–driven disasters can be viewed as slow-moving, such as drought and the rising of sea levels, where displacement becomes permanent. Managing mass migration from internally or externally displaced people who must abandon their communities because of climate change will have significant repercussions for all societies.25 The term “climate refugee” is not (yet) included in the United Nations’ official definition of refugees; it defines refugees as individuals who have fled their countries because of war, violence, or persecution.26 These and other bureaucratic issues can come up when clinicians are trying to help migrants with immigration-related paperwork.
Continue to: As the inevitability of climate change...
As the inevitability of climate change sinks in, its long-term ramifications have introduced a new lexicon of psychological suffering related to the crisis.27 Common terms for such distress include ecoanxiety (fear of what is happening and will happen with climate change), ecogrief (sadness about the destruction of species and natural habitats), solastalgia28 (the nostalgia an individual feels for emotionally treasured landscapes that have changed), and terrafuria or ecorage (the reaction to betrayal and inaction by governments and leaders).29 Climate-related emotions can lead to pessimism about the future and a nihilistic outlook on an individual’s ability to effect change and have agency over their life’s outcomes.
The categories of direct and indirect impacts are not mutually exclusive. A child may be starving due to weather-related crop failure as the family is forced to move to another country, then have to contend with prejudice and bullying as an immigrant, and later become anxiously preoccupied with climate change and its ability to cause further distress.
Effect on individuals with serious mental illness
Patients with SMI are particularly vulnerable to the impact of climate change. They are less resilient to climate change–related events, such as heat waves or temporary displacement from flooding, both at the personal level due to illness factors (eg, negative symptoms or cognitive impairment) and at the community level due to social factors (eg, weaker social support or poverty).
Recognizing the increased vulnerability to heat waves and preparing for them is particularly important for patients with SMI because they are at an increased risk for heat-related illnesses.30 For example, patients may not appreciate the danger from heat and live in conditions that put them at risk (ie, not having air conditioning in their home or living alone). Their illness alone impairs heat regulation31; patients with depression and anxiety also dissipate heat less effectively.32,33 Additionally, many psychiatric medications, particularly antipsychotics, impair key mechanisms of heat dissipation.34,35 Antipsychotics render organisms more poikilothermic (susceptible to environmental temperature, like cold-blooded animals) and can be anticholinergic, which impedes sweating. A recent analysis of heat-related deaths during a period of extreme and prolonged heat in British Columbia in 2021 affirmed these concerns, reporting that patients with schizophrenia had the highest odds of death during this heat-related event.36
COVID-19 has shown that flexible models of care are needed to prevent disengagement from medical and psychiatric care37 and assure continued treatment with essential medications such as clozapine38 and long-acting injectable antipsychotics39 during periods of social change, as with climate change. While telehealth was critical during the COVID-19 pandemic40 and is here to stay, it alone may be insufficient given the digital divide (patients with SMI may be less likely to have access to or be proficient in the use of digital technologies). The pandemic has shown the importance of public health efforts, including benefits from targeted outreach, with regards to vaccinations for this patient group.41,42Table 2 summarizes things clinicians should consider when preparing patients with SMI for the effects of climate change.

Continue to: The psychiatrist's role
The psychiatrist’s role
There are many ways a psychiatrist can professionally get involved in addressing climate change. Table 343-53 outlines the 3 Ps of climate action (taking actions to mitigate the effects of climate change): personal, patient (and clinic), and political (advocacy).
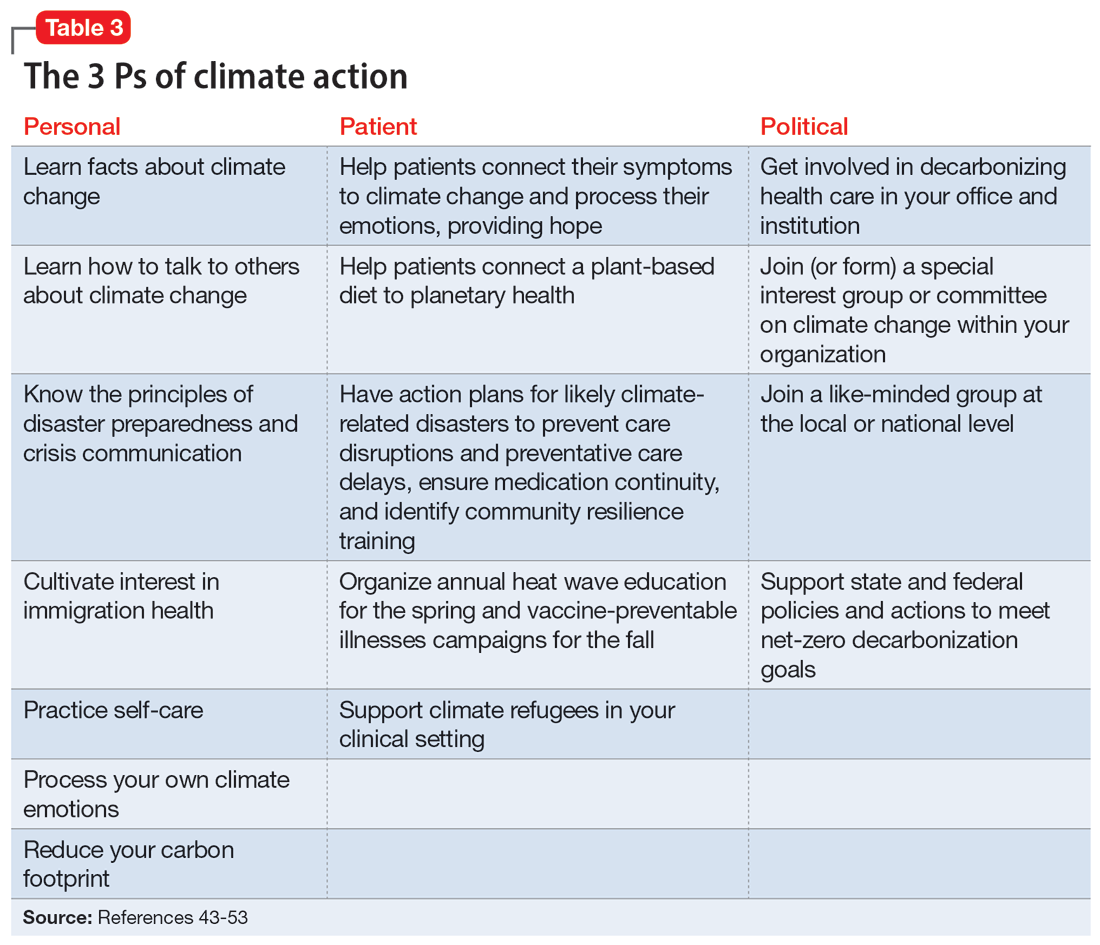
Personal
Even if clinicians believe climate change is important for their clinical work, they may still feel overwhelmed and unsure what to do in the context of competing responsibilities. A necessary first step is overcoming paralysis from the enormity of the problem, including the need to shift away from an expanding consumption model to environmental sustainability in a short period of time.
A good starting point is to get educated on the facts of climate change and how to discuss it in an office setting as well as in your personal life. A basic principle of climate change communication is that constructive hope (progress achieved despite everything) coupled with constructive doubt (the reality of the threat) can mobilize people towards action, whereas false hope or fatalistic doubt impedes action.43 The importance of optimal public health messaging cannot be overstated; well-meaning campaigns to change behavior can fail if they emphasize the wrong message. For example, in a study examining COVID-19 messaging in >80 countries, Dorison et al44 found that negatively framed messages mostly increased anxiety but had no benefit with regard to shifting people toward desired behaviors.
In addition, clinicians can learn how to confront climate disavowal and difficult emotions in themselves and even plan to shift to carbon neutrality, such as purchasing carbon offsets or green sources of energy and transportation. They may not be familiar with principles of disaster preparedness or crisis communication.46 Acquiring those professional skills may suggest next steps for action. Being familiar with the challenges and resources for immigrants, including individuals displaced due to climate change, may be necessary.47 Finally, to reduce the risk of burnout, it is important to practice self-care, including strategies to reduce feelings of being overwhelmed.
Patient
In clinical encounters, clinicians can be proactive in helping patients understand their climate-related anxieties around an uncertain future, including identifying barriers to climate action.48
Continue to: Clinics must prepare for disasters...
Clinics must prepare for disasters in their communities to prevent disruption of psychiatric care by having an action plan, including the provision of medications. Such action plans should be prioritized for the most likely scenarios in an individual’s setting (eg, heat waves, wildfires, hurricanes, or flooding).
It is important to educate clinic staff and include them in planning for emergencies, because an all-hands approach and buy-in from all team members is critical. Clinicians should review how patients would continue to receive services, particularly medications, in the event of a disaster. In some cases, providing a 90-day medication supply will suffice, while in others (eg, patients receiving long-acting antipsychotics or clozapine) more preparation is necessary. Some events are predictable and can be organized annually, such as clinicians becoming vaccine ambassadors and organizing vaccine campaigns every fall50; winter-related disaster preparation every fall; and heat wave education every spring (leaflets for patients, staff, and family members; review of safety of medications during heat waves). Plan for, monitor, and coordinate medical care and services for climate refugees and other populations that may otherwise delay medical care and impede illness prevention. Finally, support climate refugees, including connecting them to services or providing trauma-informed care.
Political
Some clinicians may feel compelled to become politically active to advocate for changes within the health care system. Two initiatives related to decarbonizing the health care sector are My Green Doctor51 and Health Care Without Harm,52 which offer help in shifting your office, clinic, or hospital towards carbon neutrality.
Climate change unevenly affects people and will continue to exacerbate inequalities in society, including individuals with mental illness.53 To work toward climate justice on behalf of their patients, clinicians could join (or form) climate committees of special interest groups in their professional organizations or setting. Joining like-minded groups working on climate change at the local or national level prevents an omission of a psychiatric voice and counteracts burnout. It is important to stay focused on the root causes of the problem during activism: doing something to reduce fossil fuel use is ultimately most important.54 The concrete goal of reaching the Paris 1.5-degree Celsius climate goal is a critical benchmark against which any other action can be measured.54
Planning for the future
Over the course of history, societies have always faced difficult periods in which they needed to rebuild after natural disasters or self-inflicted catastrophes such as terrorist attacks or wars. Since the advent of the nuclear age, people have lived under the existential threat of nuclear war. The Anthropocene is a proposed geological term that reflects the enormous and possibly disastrous impact human activity has had on our planet.55 While not yet formally adopted, this term has heuristic value, directing attention and reflection to our role and its now undisputed consequences. In the future, historians will debate if the scale of our current climate crisis has been different. It is, however, not controversial that humanity will be faced with the effects of climate change for the foreseeable future.10 Already, even “normal” weather events are fueled by energy in overcharged and altered weather systems due to global warming, leading to weather events ranging from droughts to floods and storms that are more severe, more frequent, and have longer-lasting effects on communities.56
Continue to: As physicians, we are tasked...
As physicians, we are tasked by society to create and maintain a health care system that addresses the needs of our patients and the communities in which they live. Increasingly, we are forced to contend with an addition to the traditional 5 phases of acute disaster management (prevention, mitigation, preparedness, response, and recovery) to manage prolonged or even parallel disasters, where a series of disasters occurs before the community has recovered and healed. We must grapple with a sense of an “extended period of insecurity and instability” (permacrisis) and must better prepare for and prevent the polycrisis (many simultaneous crises) or the metacrisis of our “age of turmoil”57 in which we must limit global warming, mitigate its damage, and increase community resilience to adapt.
Leading by personal example and providing hope may be what some patients need, as the reality of climate change contributes to the general uneasiness about the future and doomsday scenarios to which many fall victim. At the level of professional societies, many are calling for leadership, including from mental health organizations, to bolster the “social climate,” to help us strengthen our emotional resilience and social bonds to better withstand climate change together.58 It is becoming harder to justify standing on the sidelines,59 and it may be better for both our world and a clinician’s own sanity to be engaged in professional and private hopeful action1 to address climate change. Without ecological or planetary health, there can be no mental health.
Bottom Line
Clinicians can prepare their patients for climate-related disruptions and manage the impact climate change has on their mental health. Addressing climate change at clinical and political levels is consistent with the leadership roles and professional ethics clinicians face in daily practice.
Related Resources
- Lim C, MacLaurin S, Freudenreich O. Preparing patients with serious mental illness for extreme HEAT. Current Psychiatry. 2022;21(9):27-28. doi:10.12788/cp.0287
- My Green Doctor. https://mygreendoctor.org/
- The Climate Resilience for Frontline Clinics Toolkit from Americares. https://www.americares.org/what-we-do/community-health/climate-resilient-health-clinics
- Climate Psychiatry Alliance. https://www.climatepsychiatry.org/
Drug Brand Names
Clozapine • Clozaril
1. Kretz L. Hope in environmental philosophy. J Agricult Environ Ethics. 2013;26:925-944. doi:10.1007/s10806-012-9425-8
2. Ursano RJ, Morganstein JC, Cooper R. Position statement on mental health and climate change. American Psychiatric Association. March 2023. Accessed August 6, 2023. https://www.psychiatry.org/getattachment/0ce71f37-61a6-44d0-8fcd-c752b7e935fd/Position-Mental-Health-Climate-Change.pdf
3. Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
4. Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. health sector - a call to action. N Engl J Med. 2021;385(23):2117-2119. doi:10.1056/NEJMp2115675
5. Haase E, Augustinavicius JH, K. Climate change and psychiatry. In: Tasman A, Riba MB, Alarcón RD, et al, eds. Tasman’s Psychiatry. 5th ed. Springer; 2023.
6. Belkin G. Mental health and the global race to resilience. Psychiatr Times. 2023;40(3):26.
7. Hu SR, Yang JQ. Harvard Medical School will integrate climate change into M.D. curriculum. The Harvard Crimson. February 3, 2023. Accessed August 6, 2023. https://www.thecrimson.com/article/2023/2/3/hms-climate-curriculum/#:~:text=The%20new%20climate%20change%20curriculum,in%20arriving%20at%20climate%20solutions
8. Funk C, Gramlich J. Amid coronavirus threat, Americans generally have a high level of trust in medical doctors. Pew Research Center. March 13, 2020. Accessed August 6, 2023. https://www.pewresearch.org/fact-tank/2020/03/13/amid-coronavirus-threat-americans-generally-have-a-high-level-of-trust-in-medical-doctors/
9. Coverdale J, Balon R, Beresin EV, et al. Climate change: a call to action for the psychiatric profession. Acad Psychiatry. 2018;42(3):317-323. doi:10.1007/s40596-018-0885-7
10. Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2023. Accessed August 6, 2023. https://www.ipcc.ch/report/sixth-assessment-report-cycle/
11. Perera FP. Multiple threats to child health from fossil fuel combustion: impacts of air pollution and climate change. Environ Health Perspect. 2017;125(2):141-148. doi:10.1289/EHP299
12. Hahad O, Lelieveldz J, Birklein F, et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21(12):4306. doi:10.3390/ijms21124306
13. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational Neurosci. 2016;7(1):24-30. doi:10.1515/tnsci-2016-0005
14. Yang T, Wang J, Huang J, et al. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305-313. doi:10.1001/jamapsychiatry.2022.4812
15. Worthington MA, Petkova E, Freudenreich O, et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr Res. 2020;218:63-69. doi:10.1016/j.schres.2020.03.001
16. Dumont C, Haase E, Dolber T, et al. Climate change and risk of completed suicide. J Nerv Ment Dis. 2020;208(7):559-565. doi:10.1097/NMD.0000000000001162
17. Burke M, Gonzales F, Bayis P, et al. Higher temperatures increase suicide rates in the United States and Mexico. Nat Climate Change. 2018;8:723-729. doi:10.1038/s41558-018-0222-x
18. Frangione B, Villamizar LAR, Lang JJ, et al. Short-term changes in meteorological conditions and suicide: a systematic review and meta-analysis. Environ Res. 2022;207:112230. doi:10.1016/j.envres.2021.112230
19. Rocklov J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479-483. doi:10.1038/s41590-020-0648-y
20. Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-562. doi:10.1038/s41586-022-04788-w
21. Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141-145. doi:10.1016/j.maturitas.2011.06.017
22. Liu Y, Diao L, Xu L. The impact of childhood experience of starvations on the health of older adults: evidence from China. Int J Health Plann Manage. 2021;36(2):515-531. doi:10.1002/hpm.3099
23. Rothschild J, Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet. 2023;160(2):405-413. doi:10.1002/ijgo.14479
24. Cianconi P, Betro S, Janiri L. The impact of climate change on mental health: a systematic descriptive review. Frontiers Psychiatry. 2020;11:74. doi:10.3389/fpsyt.2020.00074
25. World Economic Forum. Climate refugees – the world’s forgotten victims. June 18, 2021. Accessed August 6, 2023. https://www.weforum.org/agenda/2021/06/climate-refugees-the-world-s-forgotten-victims
26. Climate Refugees. Accessed August 6, 2023. https://www.climate-refugees.org/why
27. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12(19):7836. doi:10.3390/su12197836
28. Galway LP, Beery T, Jones-Casey K, et al. Mapping the solastalgia literature: a scoping review study. Int J Environ Res Public Health. 2019;16(15):2662. doi:10.3390/ijerph16152662
29. Albrecht GA. Earth Emotions. New Words for a New World. Cornell University Press; 2019.
30. Sorensen C, Hess J. Treatment and prevention of heat-related illness. N Engl J Med. 2022;387(15):1404-1413. doi:10.1056/NEJMcp2210623
31. Chong TWH, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69(2-3):149-157. doi:10.1016/s0920-9964(03)00222-6
32. von Salis S, Ehlert U, Fischer S. Altered experienced thermoregulation in depression--no evidence for an effect of early life stress. Front Psychiatry. 2021;12:620656. doi:10.3389/fpsyt.2021.620656
33. Sarchiapone M, Gramaglia C, Iosue M, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: a systematic review and narrative synthesis. BMC Psychiatry. 2018;18(1):22. doi:10.1186/s12888-017-1551-4
34. Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry. 2007;22(6):335-338. doi:10.1016/j.eurpsy.2007.03.007
35. Ebi KL, Capon A, Berry P, et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698-708. doi:10.1016/S0140-6736(21)01208-3
36. Lee MJ, McLean KE, Kuo M, et al. Chronic diseases associated with mortality in British Columbia, Canada during the 2021 Western North America extreme heat event. Geohealth. 2023;7(3):e2022GH000729. doi:10.1029/2022GH000729
37. Busch AB, Huskamp HA, Raja P, et al. Disruptions in care for Medicare beneficiaries with severe mental illness during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2145677. doi:10.1001/jamanetworkopen.2021.45677
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223. doi:10.1503/jpn.200061
39. MacLaurin SA, Mulligan C, Van Alphen MU, et al. Optimal long-acting injectable antipsychotic management during COVID-19. J Clin Psychiatry. 2021;82(1): 20l13730. doi:10.4088/JCP.20l13730
40. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness. Psychiatr Serv. 2020;71(10):1078-1081. doi:10.1176/appi.ps.202000244
41. Van Alphen MU, Lim C, Freudenreich O. Mobile vaccine clinics for patients with serious mental illness and health care workers in outpatient mental health clinics. Psychiatr Serv. February 8, 2023. doi:10.1176/appi.ps.20220460
42. Lim C, Van Alphen MU, Maclaurin S, et al. Increasing COVID-19 vaccination rates among patients with serious mental illness: a pilot intervention study. Psychiatr Serv. 2022;73(11):1274-1277. doi:10.1176/appi.ps.202100702
43. Marlon JR, Bloodhart B, Ballew MT, et al. How hope and doubt affect climate change mobilization. Front Commun. May 21, 2019. doi:10.3389/fcomm.2019.00020
44. Dorison CA, Lerner JS, Heller BH, et al. In COVID-19 health messaging, loss framing increases anxiety with little-to-no concomitant benefits: experimental evidence from 84 countries. Affective Sci. 2022;3(3):577-602. doi:10.1007/s42761-022-00128-3
45. Maibach E. Increasing public awareness and facilitating behavior change: two guiding heuristics. George Mason University, Center for Climate Change Communication. September 2015. Accessed August 6, 2023. https://www.climatechangecommunication.org/wp-content/uploads/2018/06/Maibach-Two-hueristics-September-2015-revised.pdf
46. Koh KA, Raviola G, Stoddard FJ Jr. Psychiatry and crisis communication during COVID-19: a view from the trenches. Psychiatr Serv. 2021;72(5):615. doi:10.1176/appi.ps.202000912
47. Velez G, Adam B, Shadid O, et al. The clock is ticking: are we prepared for mass climate migration? Psychiatr News. March 24, 2023. Accessed August 6, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2023.04.4.3
48. Ingle HE, Mikulewicz M. Mental health and climate change: tackling invisible injustice. Lancet Planet Health. 2020;4:e128-e130. doi:10.1016/S2542-5196(20)30081-4
49. Shah UA, Merlo G. Personal and planetary health--the connection with dietary choices. JAMA. 2023;329(21):1823-1824. doi:10.1001/jama.2023.6118
50. Lim C, Van Alphen MU, Freudenreich O. Becoming vaccine ambassadors: a new role for psychiatrists. Current Psychiatry. 2021;20(8):10-11,17-21,26-28,38. doi:10.12788/cp.0155
51. My Green Doctor. Accessed August 6, 2023. https://mygreendoctor.org/
52. Healthcare Without Harm. Accessed August 6, 2023. https://noharm.org/
53. Levy BS, Patz JA. Climate change, human rights, and social justice. Ann Glob Health. 2015;81:310-322.
54. Intergovernmental Panel on Climate Change. Global warming of 1.5° C 2018. Accessed August 6, 2023. https://www.ipcc.ch/sr15/
55. Steffen W, Crutzen J, McNeill JR. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 2007;36(8):614-621. doi:10.1579/0044-7447(2007)36[614:taahno]2.0.co;2
56. American Meteorological Society. Explaining extreme events from a climate perspective. Accessed August 6, 2023. https://www.ametsoc.org/ams/index.cfm/publications/bulletin-of-the-american-meteorological-society-bams/explaining-extreme-events-from-a-climate-perspective/
57. Nierenberg AA. Coping in the age of turmoil. Psychiatr Ann. 2022;52(7):263. July 1, 2022. doi:10.3928/23258160-20220701-01
58. Belkin G. Leadership for the social climate. N Engl J Med. 2020;382(21):1975-1977. doi:10.1056/NEJMp2001507
59. Skinner JR. Doctors and climate change: first do no harm. J Paediatr Child Health. 2021;57(11):1754-1758. doi:10.1111/jpc.15658
“ Hope is engagement with the act of mapping our destinies.” 1
—Valerie Braithwaite
Why should psychiatrists care about climate change and try to mitigate its effects? First, we are tasked by society with managing the psychological and neuropsychiatric sequelae from disasters, which include climate change. The American Psychiatric Association’s position statement on climate change includes it as a legitimate focus for our specialty.2 Second, as physicians, we are morally obligated to do no harm. Since the health care sector contributes significantly to climate change (8.5% of national carbon emissions stem from health care) and causes demonstrable health impacts,3 managing these impacts and decarbonizing the health care industry is morally imperative.4 And third, psychiatric clinicians have transferrable skills that can address fears of climate change, challenge climate change denialism,5 motivate people to adopt more pro-environmental behaviors, and help communities not only endure the emotional impact of climate change but become more psychologically resilient.6
Most psychiatrists, however, did not receive formal training on climate change and the related field of disaster preparedness. For example, Harvard Medical School did not include a course on climate change in their medical student curriculum until 2023.7 In this article, we provide a basic framework of climate change and its impact on mental health, with particular focus on patients with serious mental illness (SMI). We offer concrete steps clinicians can take to prevent or mitigate harm from climate change for their patients, prepare for disasters at the level of individual patient encounters, and strengthen their clinics and communities. We also encourage clinicians to take active leadership roles in their professional organizations to be part of climate solutions, building on the trust patients continue to have in their physicians.8 Even if clinicians do not view climate change concerns under their conceived clinical care mandate, having a working knowledge about it is important because patients, paraprofessional staff, or medical trainees are likely to bring it up.9
Climate change and mental health
Climate change is harmful to human health, including mental health.10 It can impact mental health directly via its impact on brain function and neuropsychiatric sequelae, and indirectly via climate-related disasters leading to acute or chronic stress, losses, and displacement with psychiatric and psychological sequelae (Table 111-29).

Direct impact
The effects of air pollution, heat, infections, and starvation are examples of how climate change directly impacts mental health. Air pollution and brain health are a concern for psychiatry, given the well-described effects of air deterioration on the developing brain.11 In animal models, airborne pollutants lead to widespread neuroinflammation and cell loss via a multitude of mechanisms.12 This is consistent with worse cognitive and behavioral functions across a wide range of cognitive domains seen in children exposed to pollution compared to those who grew up in environments with healthy air.13 Even low-level exposure to air pollution increases the risk for later onset of depression, suicide, and anxiety.14 Hippocampal atrophy observed in patients with first-episode psychosis may also be partially attributable to air pollution.15 An association between heat and suicide (and to a lesser extent, aggression) has also been reported.16
Worse physical health (eg, strokes) due to excessive heat can further compound mental health via elevated rates of depression. Data from the United States and Mexico show that for each degree Celsius increase in ambient temperature, suicide rates may increase by approximately 1%.17 A meta-analysis by Frangione et al18 similarly concluded that each degree Celsius increase results in an overall risk ratio of 1.016 (95% CI, 1.012 to 1.019) for deaths by suicide and suicide attempts. Additionally, global warming is shifting the endemic areas for many infectious agents, particularly vector-borne diseases,19 to regions in which they had hitherto been unknown, increasing the risk for future outbreaks and even pandemics.20 These infectious illnesses often carry neuropsychiatric morbidity, with seizures, encephalopathy with incomplete recovery, and psychiatric syndromes occurring in many cases. Crop failure can lead to starvation during pregnancy and childhood, which has wide-ranging consequences for brain development and later physical and psychological health in adults.21,22 Mothers affected by starvation also experience negative impacts on childbearing and childrearing.23
Indirect impact
Climate change’s indirect impact on mental health can stem from the stress of living through a disaster such as an extreme weather event; from losses, including the death of friends and family members; and from becoming temporarily displaced.24 Some climate change–driven disasters can be viewed as slow-moving, such as drought and the rising of sea levels, where displacement becomes permanent. Managing mass migration from internally or externally displaced people who must abandon their communities because of climate change will have significant repercussions for all societies.25 The term “climate refugee” is not (yet) included in the United Nations’ official definition of refugees; it defines refugees as individuals who have fled their countries because of war, violence, or persecution.26 These and other bureaucratic issues can come up when clinicians are trying to help migrants with immigration-related paperwork.
Continue to: As the inevitability of climate change...
As the inevitability of climate change sinks in, its long-term ramifications have introduced a new lexicon of psychological suffering related to the crisis.27 Common terms for such distress include ecoanxiety (fear of what is happening and will happen with climate change), ecogrief (sadness about the destruction of species and natural habitats), solastalgia28 (the nostalgia an individual feels for emotionally treasured landscapes that have changed), and terrafuria or ecorage (the reaction to betrayal and inaction by governments and leaders).29 Climate-related emotions can lead to pessimism about the future and a nihilistic outlook on an individual’s ability to effect change and have agency over their life’s outcomes.
The categories of direct and indirect impacts are not mutually exclusive. A child may be starving due to weather-related crop failure as the family is forced to move to another country, then have to contend with prejudice and bullying as an immigrant, and later become anxiously preoccupied with climate change and its ability to cause further distress.
Effect on individuals with serious mental illness
Patients with SMI are particularly vulnerable to the impact of climate change. They are less resilient to climate change–related events, such as heat waves or temporary displacement from flooding, both at the personal level due to illness factors (eg, negative symptoms or cognitive impairment) and at the community level due to social factors (eg, weaker social support or poverty).
Recognizing the increased vulnerability to heat waves and preparing for them is particularly important for patients with SMI because they are at an increased risk for heat-related illnesses.30 For example, patients may not appreciate the danger from heat and live in conditions that put them at risk (ie, not having air conditioning in their home or living alone). Their illness alone impairs heat regulation31; patients with depression and anxiety also dissipate heat less effectively.32,33 Additionally, many psychiatric medications, particularly antipsychotics, impair key mechanisms of heat dissipation.34,35 Antipsychotics render organisms more poikilothermic (susceptible to environmental temperature, like cold-blooded animals) and can be anticholinergic, which impedes sweating. A recent analysis of heat-related deaths during a period of extreme and prolonged heat in British Columbia in 2021 affirmed these concerns, reporting that patients with schizophrenia had the highest odds of death during this heat-related event.36
COVID-19 has shown that flexible models of care are needed to prevent disengagement from medical and psychiatric care37 and assure continued treatment with essential medications such as clozapine38 and long-acting injectable antipsychotics39 during periods of social change, as with climate change. While telehealth was critical during the COVID-19 pandemic40 and is here to stay, it alone may be insufficient given the digital divide (patients with SMI may be less likely to have access to or be proficient in the use of digital technologies). The pandemic has shown the importance of public health efforts, including benefits from targeted outreach, with regards to vaccinations for this patient group.41,42Table 2 summarizes things clinicians should consider when preparing patients with SMI for the effects of climate change.

Continue to: The psychiatrist's role
The psychiatrist’s role
There are many ways a psychiatrist can professionally get involved in addressing climate change. Table 343-53 outlines the 3 Ps of climate action (taking actions to mitigate the effects of climate change): personal, patient (and clinic), and political (advocacy).

Personal
Even if clinicians believe climate change is important for their clinical work, they may still feel overwhelmed and unsure what to do in the context of competing responsibilities. A necessary first step is overcoming paralysis from the enormity of the problem, including the need to shift away from an expanding consumption model to environmental sustainability in a short period of time.
A good starting point is to get educated on the facts of climate change and how to discuss it in an office setting as well as in your personal life. A basic principle of climate change communication is that constructive hope (progress achieved despite everything) coupled with constructive doubt (the reality of the threat) can mobilize people towards action, whereas false hope or fatalistic doubt impedes action.43 The importance of optimal public health messaging cannot be overstated; well-meaning campaigns to change behavior can fail if they emphasize the wrong message. For example, in a study examining COVID-19 messaging in >80 countries, Dorison et al44 found that negatively framed messages mostly increased anxiety but had no benefit with regard to shifting people toward desired behaviors.
In addition, clinicians can learn how to confront climate disavowal and difficult emotions in themselves and even plan to shift to carbon neutrality, such as purchasing carbon offsets or green sources of energy and transportation. They may not be familiar with principles of disaster preparedness or crisis communication.46 Acquiring those professional skills may suggest next steps for action. Being familiar with the challenges and resources for immigrants, including individuals displaced due to climate change, may be necessary.47 Finally, to reduce the risk of burnout, it is important to practice self-care, including strategies to reduce feelings of being overwhelmed.
Patient
In clinical encounters, clinicians can be proactive in helping patients understand their climate-related anxieties around an uncertain future, including identifying barriers to climate action.48
Continue to: Clinics must prepare for disasters...
Clinics must prepare for disasters in their communities to prevent disruption of psychiatric care by having an action plan, including the provision of medications. Such action plans should be prioritized for the most likely scenarios in an individual’s setting (eg, heat waves, wildfires, hurricanes, or flooding).
It is important to educate clinic staff and include them in planning for emergencies, because an all-hands approach and buy-in from all team members is critical. Clinicians should review how patients would continue to receive services, particularly medications, in the event of a disaster. In some cases, providing a 90-day medication supply will suffice, while in others (eg, patients receiving long-acting antipsychotics or clozapine) more preparation is necessary. Some events are predictable and can be organized annually, such as clinicians becoming vaccine ambassadors and organizing vaccine campaigns every fall50; winter-related disaster preparation every fall; and heat wave education every spring (leaflets for patients, staff, and family members; review of safety of medications during heat waves). Plan for, monitor, and coordinate medical care and services for climate refugees and other populations that may otherwise delay medical care and impede illness prevention. Finally, support climate refugees, including connecting them to services or providing trauma-informed care.
Political
Some clinicians may feel compelled to become politically active to advocate for changes within the health care system. Two initiatives related to decarbonizing the health care sector are My Green Doctor51 and Health Care Without Harm,52 which offer help in shifting your office, clinic, or hospital towards carbon neutrality.
Climate change unevenly affects people and will continue to exacerbate inequalities in society, including individuals with mental illness.53 To work toward climate justice on behalf of their patients, clinicians could join (or form) climate committees of special interest groups in their professional organizations or setting. Joining like-minded groups working on climate change at the local or national level prevents an omission of a psychiatric voice and counteracts burnout. It is important to stay focused on the root causes of the problem during activism: doing something to reduce fossil fuel use is ultimately most important.54 The concrete goal of reaching the Paris 1.5-degree Celsius climate goal is a critical benchmark against which any other action can be measured.54
Planning for the future
Over the course of history, societies have always faced difficult periods in which they needed to rebuild after natural disasters or self-inflicted catastrophes such as terrorist attacks or wars. Since the advent of the nuclear age, people have lived under the existential threat of nuclear war. The Anthropocene is a proposed geological term that reflects the enormous and possibly disastrous impact human activity has had on our planet.55 While not yet formally adopted, this term has heuristic value, directing attention and reflection to our role and its now undisputed consequences. In the future, historians will debate if the scale of our current climate crisis has been different. It is, however, not controversial that humanity will be faced with the effects of climate change for the foreseeable future.10 Already, even “normal” weather events are fueled by energy in overcharged and altered weather systems due to global warming, leading to weather events ranging from droughts to floods and storms that are more severe, more frequent, and have longer-lasting effects on communities.56
Continue to: As physicians, we are tasked...
As physicians, we are tasked by society to create and maintain a health care system that addresses the needs of our patients and the communities in which they live. Increasingly, we are forced to contend with an addition to the traditional 5 phases of acute disaster management (prevention, mitigation, preparedness, response, and recovery) to manage prolonged or even parallel disasters, where a series of disasters occurs before the community has recovered and healed. We must grapple with a sense of an “extended period of insecurity and instability” (permacrisis) and must better prepare for and prevent the polycrisis (many simultaneous crises) or the metacrisis of our “age of turmoil”57 in which we must limit global warming, mitigate its damage, and increase community resilience to adapt.
Leading by personal example and providing hope may be what some patients need, as the reality of climate change contributes to the general uneasiness about the future and doomsday scenarios to which many fall victim. At the level of professional societies, many are calling for leadership, including from mental health organizations, to bolster the “social climate,” to help us strengthen our emotional resilience and social bonds to better withstand climate change together.58 It is becoming harder to justify standing on the sidelines,59 and it may be better for both our world and a clinician’s own sanity to be engaged in professional and private hopeful action1 to address climate change. Without ecological or planetary health, there can be no mental health.
Bottom Line
Clinicians can prepare their patients for climate-related disruptions and manage the impact climate change has on their mental health. Addressing climate change at clinical and political levels is consistent with the leadership roles and professional ethics clinicians face in daily practice.
Related Resources
- Lim C, MacLaurin S, Freudenreich O. Preparing patients with serious mental illness for extreme HEAT. Current Psychiatry. 2022;21(9):27-28. doi:10.12788/cp.0287
- My Green Doctor. https://mygreendoctor.org/
- The Climate Resilience for Frontline Clinics Toolkit from Americares. https://www.americares.org/what-we-do/community-health/climate-resilient-health-clinics
- Climate Psychiatry Alliance. https://www.climatepsychiatry.org/
Drug Brand Names
Clozapine • Clozaril
“ Hope is engagement with the act of mapping our destinies.” 1
—Valerie Braithwaite
Why should psychiatrists care about climate change and try to mitigate its effects? First, we are tasked by society with managing the psychological and neuropsychiatric sequelae from disasters, which include climate change. The American Psychiatric Association’s position statement on climate change includes it as a legitimate focus for our specialty.2 Second, as physicians, we are morally obligated to do no harm. Since the health care sector contributes significantly to climate change (8.5% of national carbon emissions stem from health care) and causes demonstrable health impacts,3 managing these impacts and decarbonizing the health care industry is morally imperative.4 And third, psychiatric clinicians have transferrable skills that can address fears of climate change, challenge climate change denialism,5 motivate people to adopt more pro-environmental behaviors, and help communities not only endure the emotional impact of climate change but become more psychologically resilient.6
Most psychiatrists, however, did not receive formal training on climate change and the related field of disaster preparedness. For example, Harvard Medical School did not include a course on climate change in their medical student curriculum until 2023.7 In this article, we provide a basic framework of climate change and its impact on mental health, with particular focus on patients with serious mental illness (SMI). We offer concrete steps clinicians can take to prevent or mitigate harm from climate change for their patients, prepare for disasters at the level of individual patient encounters, and strengthen their clinics and communities. We also encourage clinicians to take active leadership roles in their professional organizations to be part of climate solutions, building on the trust patients continue to have in their physicians.8 Even if clinicians do not view climate change concerns under their conceived clinical care mandate, having a working knowledge about it is important because patients, paraprofessional staff, or medical trainees are likely to bring it up.9
Climate change and mental health
Climate change is harmful to human health, including mental health.10 It can impact mental health directly via its impact on brain function and neuropsychiatric sequelae, and indirectly via climate-related disasters leading to acute or chronic stress, losses, and displacement with psychiatric and psychological sequelae (Table 111-29).

Direct impact
The effects of air pollution, heat, infections, and starvation are examples of how climate change directly impacts mental health. Air pollution and brain health are a concern for psychiatry, given the well-described effects of air deterioration on the developing brain.11 In animal models, airborne pollutants lead to widespread neuroinflammation and cell loss via a multitude of mechanisms.12 This is consistent with worse cognitive and behavioral functions across a wide range of cognitive domains seen in children exposed to pollution compared to those who grew up in environments with healthy air.13 Even low-level exposure to air pollution increases the risk for later onset of depression, suicide, and anxiety.14 Hippocampal atrophy observed in patients with first-episode psychosis may also be partially attributable to air pollution.15 An association between heat and suicide (and to a lesser extent, aggression) has also been reported.16
Worse physical health (eg, strokes) due to excessive heat can further compound mental health via elevated rates of depression. Data from the United States and Mexico show that for each degree Celsius increase in ambient temperature, suicide rates may increase by approximately 1%.17 A meta-analysis by Frangione et al18 similarly concluded that each degree Celsius increase results in an overall risk ratio of 1.016 (95% CI, 1.012 to 1.019) for deaths by suicide and suicide attempts. Additionally, global warming is shifting the endemic areas for many infectious agents, particularly vector-borne diseases,19 to regions in which they had hitherto been unknown, increasing the risk for future outbreaks and even pandemics.20 These infectious illnesses often carry neuropsychiatric morbidity, with seizures, encephalopathy with incomplete recovery, and psychiatric syndromes occurring in many cases. Crop failure can lead to starvation during pregnancy and childhood, which has wide-ranging consequences for brain development and later physical and psychological health in adults.21,22 Mothers affected by starvation also experience negative impacts on childbearing and childrearing.23
Indirect impact
Climate change’s indirect impact on mental health can stem from the stress of living through a disaster such as an extreme weather event; from losses, including the death of friends and family members; and from becoming temporarily displaced.24 Some climate change–driven disasters can be viewed as slow-moving, such as drought and the rising of sea levels, where displacement becomes permanent. Managing mass migration from internally or externally displaced people who must abandon their communities because of climate change will have significant repercussions for all societies.25 The term “climate refugee” is not (yet) included in the United Nations’ official definition of refugees; it defines refugees as individuals who have fled their countries because of war, violence, or persecution.26 These and other bureaucratic issues can come up when clinicians are trying to help migrants with immigration-related paperwork.
Continue to: As the inevitability of climate change...
As the inevitability of climate change sinks in, its long-term ramifications have introduced a new lexicon of psychological suffering related to the crisis.27 Common terms for such distress include ecoanxiety (fear of what is happening and will happen with climate change), ecogrief (sadness about the destruction of species and natural habitats), solastalgia28 (the nostalgia an individual feels for emotionally treasured landscapes that have changed), and terrafuria or ecorage (the reaction to betrayal and inaction by governments and leaders).29 Climate-related emotions can lead to pessimism about the future and a nihilistic outlook on an individual’s ability to effect change and have agency over their life’s outcomes.
The categories of direct and indirect impacts are not mutually exclusive. A child may be starving due to weather-related crop failure as the family is forced to move to another country, then have to contend with prejudice and bullying as an immigrant, and later become anxiously preoccupied with climate change and its ability to cause further distress.
Effect on individuals with serious mental illness
Patients with SMI are particularly vulnerable to the impact of climate change. They are less resilient to climate change–related events, such as heat waves or temporary displacement from flooding, both at the personal level due to illness factors (eg, negative symptoms or cognitive impairment) and at the community level due to social factors (eg, weaker social support or poverty).
Recognizing the increased vulnerability to heat waves and preparing for them is particularly important for patients with SMI because they are at an increased risk for heat-related illnesses.30 For example, patients may not appreciate the danger from heat and live in conditions that put them at risk (ie, not having air conditioning in their home or living alone). Their illness alone impairs heat regulation31; patients with depression and anxiety also dissipate heat less effectively.32,33 Additionally, many psychiatric medications, particularly antipsychotics, impair key mechanisms of heat dissipation.34,35 Antipsychotics render organisms more poikilothermic (susceptible to environmental temperature, like cold-blooded animals) and can be anticholinergic, which impedes sweating. A recent analysis of heat-related deaths during a period of extreme and prolonged heat in British Columbia in 2021 affirmed these concerns, reporting that patients with schizophrenia had the highest odds of death during this heat-related event.36
COVID-19 has shown that flexible models of care are needed to prevent disengagement from medical and psychiatric care37 and assure continued treatment with essential medications such as clozapine38 and long-acting injectable antipsychotics39 during periods of social change, as with climate change. While telehealth was critical during the COVID-19 pandemic40 and is here to stay, it alone may be insufficient given the digital divide (patients with SMI may be less likely to have access to or be proficient in the use of digital technologies). The pandemic has shown the importance of public health efforts, including benefits from targeted outreach, with regards to vaccinations for this patient group.41,42Table 2 summarizes things clinicians should consider when preparing patients with SMI for the effects of climate change.

Continue to: The psychiatrist's role
The psychiatrist’s role
There are many ways a psychiatrist can professionally get involved in addressing climate change. Table 343-53 outlines the 3 Ps of climate action (taking actions to mitigate the effects of climate change): personal, patient (and clinic), and political (advocacy).

Personal
Even if clinicians believe climate change is important for their clinical work, they may still feel overwhelmed and unsure what to do in the context of competing responsibilities. A necessary first step is overcoming paralysis from the enormity of the problem, including the need to shift away from an expanding consumption model to environmental sustainability in a short period of time.
A good starting point is to get educated on the facts of climate change and how to discuss it in an office setting as well as in your personal life. A basic principle of climate change communication is that constructive hope (progress achieved despite everything) coupled with constructive doubt (the reality of the threat) can mobilize people towards action, whereas false hope or fatalistic doubt impedes action.43 The importance of optimal public health messaging cannot be overstated; well-meaning campaigns to change behavior can fail if they emphasize the wrong message. For example, in a study examining COVID-19 messaging in >80 countries, Dorison et al44 found that negatively framed messages mostly increased anxiety but had no benefit with regard to shifting people toward desired behaviors.
In addition, clinicians can learn how to confront climate disavowal and difficult emotions in themselves and even plan to shift to carbon neutrality, such as purchasing carbon offsets or green sources of energy and transportation. They may not be familiar with principles of disaster preparedness or crisis communication.46 Acquiring those professional skills may suggest next steps for action. Being familiar with the challenges and resources for immigrants, including individuals displaced due to climate change, may be necessary.47 Finally, to reduce the risk of burnout, it is important to practice self-care, including strategies to reduce feelings of being overwhelmed.
Patient
In clinical encounters, clinicians can be proactive in helping patients understand their climate-related anxieties around an uncertain future, including identifying barriers to climate action.48
Continue to: Clinics must prepare for disasters...
Clinics must prepare for disasters in their communities to prevent disruption of psychiatric care by having an action plan, including the provision of medications. Such action plans should be prioritized for the most likely scenarios in an individual’s setting (eg, heat waves, wildfires, hurricanes, or flooding).
It is important to educate clinic staff and include them in planning for emergencies, because an all-hands approach and buy-in from all team members is critical. Clinicians should review how patients would continue to receive services, particularly medications, in the event of a disaster. In some cases, providing a 90-day medication supply will suffice, while in others (eg, patients receiving long-acting antipsychotics or clozapine) more preparation is necessary. Some events are predictable and can be organized annually, such as clinicians becoming vaccine ambassadors and organizing vaccine campaigns every fall50; winter-related disaster preparation every fall; and heat wave education every spring (leaflets for patients, staff, and family members; review of safety of medications during heat waves). Plan for, monitor, and coordinate medical care and services for climate refugees and other populations that may otherwise delay medical care and impede illness prevention. Finally, support climate refugees, including connecting them to services or providing trauma-informed care.
Political
Some clinicians may feel compelled to become politically active to advocate for changes within the health care system. Two initiatives related to decarbonizing the health care sector are My Green Doctor51 and Health Care Without Harm,52 which offer help in shifting your office, clinic, or hospital towards carbon neutrality.
Climate change unevenly affects people and will continue to exacerbate inequalities in society, including individuals with mental illness.53 To work toward climate justice on behalf of their patients, clinicians could join (or form) climate committees of special interest groups in their professional organizations or setting. Joining like-minded groups working on climate change at the local or national level prevents an omission of a psychiatric voice and counteracts burnout. It is important to stay focused on the root causes of the problem during activism: doing something to reduce fossil fuel use is ultimately most important.54 The concrete goal of reaching the Paris 1.5-degree Celsius climate goal is a critical benchmark against which any other action can be measured.54
Planning for the future
Over the course of history, societies have always faced difficult periods in which they needed to rebuild after natural disasters or self-inflicted catastrophes such as terrorist attacks or wars. Since the advent of the nuclear age, people have lived under the existential threat of nuclear war. The Anthropocene is a proposed geological term that reflects the enormous and possibly disastrous impact human activity has had on our planet.55 While not yet formally adopted, this term has heuristic value, directing attention and reflection to our role and its now undisputed consequences. In the future, historians will debate if the scale of our current climate crisis has been different. It is, however, not controversial that humanity will be faced with the effects of climate change for the foreseeable future.10 Already, even “normal” weather events are fueled by energy in overcharged and altered weather systems due to global warming, leading to weather events ranging from droughts to floods and storms that are more severe, more frequent, and have longer-lasting effects on communities.56
Continue to: As physicians, we are tasked...
As physicians, we are tasked by society to create and maintain a health care system that addresses the needs of our patients and the communities in which they live. Increasingly, we are forced to contend with an addition to the traditional 5 phases of acute disaster management (prevention, mitigation, preparedness, response, and recovery) to manage prolonged or even parallel disasters, where a series of disasters occurs before the community has recovered and healed. We must grapple with a sense of an “extended period of insecurity and instability” (permacrisis) and must better prepare for and prevent the polycrisis (many simultaneous crises) or the metacrisis of our “age of turmoil”57 in which we must limit global warming, mitigate its damage, and increase community resilience to adapt.
Leading by personal example and providing hope may be what some patients need, as the reality of climate change contributes to the general uneasiness about the future and doomsday scenarios to which many fall victim. At the level of professional societies, many are calling for leadership, including from mental health organizations, to bolster the “social climate,” to help us strengthen our emotional resilience and social bonds to better withstand climate change together.58 It is becoming harder to justify standing on the sidelines,59 and it may be better for both our world and a clinician’s own sanity to be engaged in professional and private hopeful action1 to address climate change. Without ecological or planetary health, there can be no mental health.
Bottom Line
Clinicians can prepare their patients for climate-related disruptions and manage the impact climate change has on their mental health. Addressing climate change at clinical and political levels is consistent with the leadership roles and professional ethics clinicians face in daily practice.
Related Resources
- Lim C, MacLaurin S, Freudenreich O. Preparing patients with serious mental illness for extreme HEAT. Current Psychiatry. 2022;21(9):27-28. doi:10.12788/cp.0287
- My Green Doctor. https://mygreendoctor.org/
- The Climate Resilience for Frontline Clinics Toolkit from Americares. https://www.americares.org/what-we-do/community-health/climate-resilient-health-clinics
- Climate Psychiatry Alliance. https://www.climatepsychiatry.org/
Drug Brand Names
Clozapine • Clozaril
1. Kretz L. Hope in environmental philosophy. J Agricult Environ Ethics. 2013;26:925-944. doi:10.1007/s10806-012-9425-8
2. Ursano RJ, Morganstein JC, Cooper R. Position statement on mental health and climate change. American Psychiatric Association. March 2023. Accessed August 6, 2023. https://www.psychiatry.org/getattachment/0ce71f37-61a6-44d0-8fcd-c752b7e935fd/Position-Mental-Health-Climate-Change.pdf
3. Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
4. Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. health sector - a call to action. N Engl J Med. 2021;385(23):2117-2119. doi:10.1056/NEJMp2115675
5. Haase E, Augustinavicius JH, K. Climate change and psychiatry. In: Tasman A, Riba MB, Alarcón RD, et al, eds. Tasman’s Psychiatry. 5th ed. Springer; 2023.
6. Belkin G. Mental health and the global race to resilience. Psychiatr Times. 2023;40(3):26.
7. Hu SR, Yang JQ. Harvard Medical School will integrate climate change into M.D. curriculum. The Harvard Crimson. February 3, 2023. Accessed August 6, 2023. https://www.thecrimson.com/article/2023/2/3/hms-climate-curriculum/#:~:text=The%20new%20climate%20change%20curriculum,in%20arriving%20at%20climate%20solutions
8. Funk C, Gramlich J. Amid coronavirus threat, Americans generally have a high level of trust in medical doctors. Pew Research Center. March 13, 2020. Accessed August 6, 2023. https://www.pewresearch.org/fact-tank/2020/03/13/amid-coronavirus-threat-americans-generally-have-a-high-level-of-trust-in-medical-doctors/
9. Coverdale J, Balon R, Beresin EV, et al. Climate change: a call to action for the psychiatric profession. Acad Psychiatry. 2018;42(3):317-323. doi:10.1007/s40596-018-0885-7
10. Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2023. Accessed August 6, 2023. https://www.ipcc.ch/report/sixth-assessment-report-cycle/
11. Perera FP. Multiple threats to child health from fossil fuel combustion: impacts of air pollution and climate change. Environ Health Perspect. 2017;125(2):141-148. doi:10.1289/EHP299
12. Hahad O, Lelieveldz J, Birklein F, et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21(12):4306. doi:10.3390/ijms21124306
13. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational Neurosci. 2016;7(1):24-30. doi:10.1515/tnsci-2016-0005
14. Yang T, Wang J, Huang J, et al. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305-313. doi:10.1001/jamapsychiatry.2022.4812
15. Worthington MA, Petkova E, Freudenreich O, et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr Res. 2020;218:63-69. doi:10.1016/j.schres.2020.03.001
16. Dumont C, Haase E, Dolber T, et al. Climate change and risk of completed suicide. J Nerv Ment Dis. 2020;208(7):559-565. doi:10.1097/NMD.0000000000001162
17. Burke M, Gonzales F, Bayis P, et al. Higher temperatures increase suicide rates in the United States and Mexico. Nat Climate Change. 2018;8:723-729. doi:10.1038/s41558-018-0222-x
18. Frangione B, Villamizar LAR, Lang JJ, et al. Short-term changes in meteorological conditions and suicide: a systematic review and meta-analysis. Environ Res. 2022;207:112230. doi:10.1016/j.envres.2021.112230
19. Rocklov J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479-483. doi:10.1038/s41590-020-0648-y
20. Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-562. doi:10.1038/s41586-022-04788-w
21. Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141-145. doi:10.1016/j.maturitas.2011.06.017
22. Liu Y, Diao L, Xu L. The impact of childhood experience of starvations on the health of older adults: evidence from China. Int J Health Plann Manage. 2021;36(2):515-531. doi:10.1002/hpm.3099
23. Rothschild J, Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet. 2023;160(2):405-413. doi:10.1002/ijgo.14479
24. Cianconi P, Betro S, Janiri L. The impact of climate change on mental health: a systematic descriptive review. Frontiers Psychiatry. 2020;11:74. doi:10.3389/fpsyt.2020.00074
25. World Economic Forum. Climate refugees – the world’s forgotten victims. June 18, 2021. Accessed August 6, 2023. https://www.weforum.org/agenda/2021/06/climate-refugees-the-world-s-forgotten-victims
26. Climate Refugees. Accessed August 6, 2023. https://www.climate-refugees.org/why
27. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12(19):7836. doi:10.3390/su12197836
28. Galway LP, Beery T, Jones-Casey K, et al. Mapping the solastalgia literature: a scoping review study. Int J Environ Res Public Health. 2019;16(15):2662. doi:10.3390/ijerph16152662
29. Albrecht GA. Earth Emotions. New Words for a New World. Cornell University Press; 2019.
30. Sorensen C, Hess J. Treatment and prevention of heat-related illness. N Engl J Med. 2022;387(15):1404-1413. doi:10.1056/NEJMcp2210623
31. Chong TWH, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69(2-3):149-157. doi:10.1016/s0920-9964(03)00222-6
32. von Salis S, Ehlert U, Fischer S. Altered experienced thermoregulation in depression--no evidence for an effect of early life stress. Front Psychiatry. 2021;12:620656. doi:10.3389/fpsyt.2021.620656
33. Sarchiapone M, Gramaglia C, Iosue M, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: a systematic review and narrative synthesis. BMC Psychiatry. 2018;18(1):22. doi:10.1186/s12888-017-1551-4
34. Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry. 2007;22(6):335-338. doi:10.1016/j.eurpsy.2007.03.007
35. Ebi KL, Capon A, Berry P, et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698-708. doi:10.1016/S0140-6736(21)01208-3
36. Lee MJ, McLean KE, Kuo M, et al. Chronic diseases associated with mortality in British Columbia, Canada during the 2021 Western North America extreme heat event. Geohealth. 2023;7(3):e2022GH000729. doi:10.1029/2022GH000729
37. Busch AB, Huskamp HA, Raja P, et al. Disruptions in care for Medicare beneficiaries with severe mental illness during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2145677. doi:10.1001/jamanetworkopen.2021.45677
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223. doi:10.1503/jpn.200061
39. MacLaurin SA, Mulligan C, Van Alphen MU, et al. Optimal long-acting injectable antipsychotic management during COVID-19. J Clin Psychiatry. 2021;82(1): 20l13730. doi:10.4088/JCP.20l13730
40. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness. Psychiatr Serv. 2020;71(10):1078-1081. doi:10.1176/appi.ps.202000244
41. Van Alphen MU, Lim C, Freudenreich O. Mobile vaccine clinics for patients with serious mental illness and health care workers in outpatient mental health clinics. Psychiatr Serv. February 8, 2023. doi:10.1176/appi.ps.20220460
42. Lim C, Van Alphen MU, Maclaurin S, et al. Increasing COVID-19 vaccination rates among patients with serious mental illness: a pilot intervention study. Psychiatr Serv. 2022;73(11):1274-1277. doi:10.1176/appi.ps.202100702
43. Marlon JR, Bloodhart B, Ballew MT, et al. How hope and doubt affect climate change mobilization. Front Commun. May 21, 2019. doi:10.3389/fcomm.2019.00020
44. Dorison CA, Lerner JS, Heller BH, et al. In COVID-19 health messaging, loss framing increases anxiety with little-to-no concomitant benefits: experimental evidence from 84 countries. Affective Sci. 2022;3(3):577-602. doi:10.1007/s42761-022-00128-3
45. Maibach E. Increasing public awareness and facilitating behavior change: two guiding heuristics. George Mason University, Center for Climate Change Communication. September 2015. Accessed August 6, 2023. https://www.climatechangecommunication.org/wp-content/uploads/2018/06/Maibach-Two-hueristics-September-2015-revised.pdf
46. Koh KA, Raviola G, Stoddard FJ Jr. Psychiatry and crisis communication during COVID-19: a view from the trenches. Psychiatr Serv. 2021;72(5):615. doi:10.1176/appi.ps.202000912
47. Velez G, Adam B, Shadid O, et al. The clock is ticking: are we prepared for mass climate migration? Psychiatr News. March 24, 2023. Accessed August 6, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2023.04.4.3
48. Ingle HE, Mikulewicz M. Mental health and climate change: tackling invisible injustice. Lancet Planet Health. 2020;4:e128-e130. doi:10.1016/S2542-5196(20)30081-4
49. Shah UA, Merlo G. Personal and planetary health--the connection with dietary choices. JAMA. 2023;329(21):1823-1824. doi:10.1001/jama.2023.6118
50. Lim C, Van Alphen MU, Freudenreich O. Becoming vaccine ambassadors: a new role for psychiatrists. Current Psychiatry. 2021;20(8):10-11,17-21,26-28,38. doi:10.12788/cp.0155
51. My Green Doctor. Accessed August 6, 2023. https://mygreendoctor.org/
52. Healthcare Without Harm. Accessed August 6, 2023. https://noharm.org/
53. Levy BS, Patz JA. Climate change, human rights, and social justice. Ann Glob Health. 2015;81:310-322.
54. Intergovernmental Panel on Climate Change. Global warming of 1.5° C 2018. Accessed August 6, 2023. https://www.ipcc.ch/sr15/
55. Steffen W, Crutzen J, McNeill JR. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 2007;36(8):614-621. doi:10.1579/0044-7447(2007)36[614:taahno]2.0.co;2
56. American Meteorological Society. Explaining extreme events from a climate perspective. Accessed August 6, 2023. https://www.ametsoc.org/ams/index.cfm/publications/bulletin-of-the-american-meteorological-society-bams/explaining-extreme-events-from-a-climate-perspective/
57. Nierenberg AA. Coping in the age of turmoil. Psychiatr Ann. 2022;52(7):263. July 1, 2022. doi:10.3928/23258160-20220701-01
58. Belkin G. Leadership for the social climate. N Engl J Med. 2020;382(21):1975-1977. doi:10.1056/NEJMp2001507
59. Skinner JR. Doctors and climate change: first do no harm. J Paediatr Child Health. 2021;57(11):1754-1758. doi:10.1111/jpc.15658
1. Kretz L. Hope in environmental philosophy. J Agricult Environ Ethics. 2013;26:925-944. doi:10.1007/s10806-012-9425-8
2. Ursano RJ, Morganstein JC, Cooper R. Position statement on mental health and climate change. American Psychiatric Association. March 2023. Accessed August 6, 2023. https://www.psychiatry.org/getattachment/0ce71f37-61a6-44d0-8fcd-c752b7e935fd/Position-Mental-Health-Climate-Change.pdf
3. Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
4. Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. health sector - a call to action. N Engl J Med. 2021;385(23):2117-2119. doi:10.1056/NEJMp2115675
5. Haase E, Augustinavicius JH, K. Climate change and psychiatry. In: Tasman A, Riba MB, Alarcón RD, et al, eds. Tasman’s Psychiatry. 5th ed. Springer; 2023.
6. Belkin G. Mental health and the global race to resilience. Psychiatr Times. 2023;40(3):26.
7. Hu SR, Yang JQ. Harvard Medical School will integrate climate change into M.D. curriculum. The Harvard Crimson. February 3, 2023. Accessed August 6, 2023. https://www.thecrimson.com/article/2023/2/3/hms-climate-curriculum/#:~:text=The%20new%20climate%20change%20curriculum,in%20arriving%20at%20climate%20solutions
8. Funk C, Gramlich J. Amid coronavirus threat, Americans generally have a high level of trust in medical doctors. Pew Research Center. March 13, 2020. Accessed August 6, 2023. https://www.pewresearch.org/fact-tank/2020/03/13/amid-coronavirus-threat-americans-generally-have-a-high-level-of-trust-in-medical-doctors/
9. Coverdale J, Balon R, Beresin EV, et al. Climate change: a call to action for the psychiatric profession. Acad Psychiatry. 2018;42(3):317-323. doi:10.1007/s40596-018-0885-7
10. Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2023. Accessed August 6, 2023. https://www.ipcc.ch/report/sixth-assessment-report-cycle/
11. Perera FP. Multiple threats to child health from fossil fuel combustion: impacts of air pollution and climate change. Environ Health Perspect. 2017;125(2):141-148. doi:10.1289/EHP299
12. Hahad O, Lelieveldz J, Birklein F, et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21(12):4306. doi:10.3390/ijms21124306
13. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational Neurosci. 2016;7(1):24-30. doi:10.1515/tnsci-2016-0005
14. Yang T, Wang J, Huang J, et al. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305-313. doi:10.1001/jamapsychiatry.2022.4812
15. Worthington MA, Petkova E, Freudenreich O, et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr Res. 2020;218:63-69. doi:10.1016/j.schres.2020.03.001
16. Dumont C, Haase E, Dolber T, et al. Climate change and risk of completed suicide. J Nerv Ment Dis. 2020;208(7):559-565. doi:10.1097/NMD.0000000000001162
17. Burke M, Gonzales F, Bayis P, et al. Higher temperatures increase suicide rates in the United States and Mexico. Nat Climate Change. 2018;8:723-729. doi:10.1038/s41558-018-0222-x
18. Frangione B, Villamizar LAR, Lang JJ, et al. Short-term changes in meteorological conditions and suicide: a systematic review and meta-analysis. Environ Res. 2022;207:112230. doi:10.1016/j.envres.2021.112230
19. Rocklov J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479-483. doi:10.1038/s41590-020-0648-y
20. Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-562. doi:10.1038/s41586-022-04788-w
21. Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141-145. doi:10.1016/j.maturitas.2011.06.017
22. Liu Y, Diao L, Xu L. The impact of childhood experience of starvations on the health of older adults: evidence from China. Int J Health Plann Manage. 2021;36(2):515-531. doi:10.1002/hpm.3099
23. Rothschild J, Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet. 2023;160(2):405-413. doi:10.1002/ijgo.14479
24. Cianconi P, Betro S, Janiri L. The impact of climate change on mental health: a systematic descriptive review. Frontiers Psychiatry. 2020;11:74. doi:10.3389/fpsyt.2020.00074
25. World Economic Forum. Climate refugees – the world’s forgotten victims. June 18, 2021. Accessed August 6, 2023. https://www.weforum.org/agenda/2021/06/climate-refugees-the-world-s-forgotten-victims
26. Climate Refugees. Accessed August 6, 2023. https://www.climate-refugees.org/why
27. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12(19):7836. doi:10.3390/su12197836
28. Galway LP, Beery T, Jones-Casey K, et al. Mapping the solastalgia literature: a scoping review study. Int J Environ Res Public Health. 2019;16(15):2662. doi:10.3390/ijerph16152662
29. Albrecht GA. Earth Emotions. New Words for a New World. Cornell University Press; 2019.
30. Sorensen C, Hess J. Treatment and prevention of heat-related illness. N Engl J Med. 2022;387(15):1404-1413. doi:10.1056/NEJMcp2210623
31. Chong TWH, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69(2-3):149-157. doi:10.1016/s0920-9964(03)00222-6
32. von Salis S, Ehlert U, Fischer S. Altered experienced thermoregulation in depression--no evidence for an effect of early life stress. Front Psychiatry. 2021;12:620656. doi:10.3389/fpsyt.2021.620656
33. Sarchiapone M, Gramaglia C, Iosue M, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: a systematic review and narrative synthesis. BMC Psychiatry. 2018;18(1):22. doi:10.1186/s12888-017-1551-4
34. Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry. 2007;22(6):335-338. doi:10.1016/j.eurpsy.2007.03.007
35. Ebi KL, Capon A, Berry P, et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698-708. doi:10.1016/S0140-6736(21)01208-3
36. Lee MJ, McLean KE, Kuo M, et al. Chronic diseases associated with mortality in British Columbia, Canada during the 2021 Western North America extreme heat event. Geohealth. 2023;7(3):e2022GH000729. doi:10.1029/2022GH000729
37. Busch AB, Huskamp HA, Raja P, et al. Disruptions in care for Medicare beneficiaries with severe mental illness during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2145677. doi:10.1001/jamanetworkopen.2021.45677
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223. doi:10.1503/jpn.200061
39. MacLaurin SA, Mulligan C, Van Alphen MU, et al. Optimal long-acting injectable antipsychotic management during COVID-19. J Clin Psychiatry. 2021;82(1): 20l13730. doi:10.4088/JCP.20l13730
40. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness. Psychiatr Serv. 2020;71(10):1078-1081. doi:10.1176/appi.ps.202000244
41. Van Alphen MU, Lim C, Freudenreich O. Mobile vaccine clinics for patients with serious mental illness and health care workers in outpatient mental health clinics. Psychiatr Serv. February 8, 2023. doi:10.1176/appi.ps.20220460
42. Lim C, Van Alphen MU, Maclaurin S, et al. Increasing COVID-19 vaccination rates among patients with serious mental illness: a pilot intervention study. Psychiatr Serv. 2022;73(11):1274-1277. doi:10.1176/appi.ps.202100702
43. Marlon JR, Bloodhart B, Ballew MT, et al. How hope and doubt affect climate change mobilization. Front Commun. May 21, 2019. doi:10.3389/fcomm.2019.00020
44. Dorison CA, Lerner JS, Heller BH, et al. In COVID-19 health messaging, loss framing increases anxiety with little-to-no concomitant benefits: experimental evidence from 84 countries. Affective Sci. 2022;3(3):577-602. doi:10.1007/s42761-022-00128-3
45. Maibach E. Increasing public awareness and facilitating behavior change: two guiding heuristics. George Mason University, Center for Climate Change Communication. September 2015. Accessed August 6, 2023. https://www.climatechangecommunication.org/wp-content/uploads/2018/06/Maibach-Two-hueristics-September-2015-revised.pdf
46. Koh KA, Raviola G, Stoddard FJ Jr. Psychiatry and crisis communication during COVID-19: a view from the trenches. Psychiatr Serv. 2021;72(5):615. doi:10.1176/appi.ps.202000912
47. Velez G, Adam B, Shadid O, et al. The clock is ticking: are we prepared for mass climate migration? Psychiatr News. March 24, 2023. Accessed August 6, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2023.04.4.3
48. Ingle HE, Mikulewicz M. Mental health and climate change: tackling invisible injustice. Lancet Planet Health. 2020;4:e128-e130. doi:10.1016/S2542-5196(20)30081-4
49. Shah UA, Merlo G. Personal and planetary health--the connection with dietary choices. JAMA. 2023;329(21):1823-1824. doi:10.1001/jama.2023.6118
50. Lim C, Van Alphen MU, Freudenreich O. Becoming vaccine ambassadors: a new role for psychiatrists. Current Psychiatry. 2021;20(8):10-11,17-21,26-28,38. doi:10.12788/cp.0155
51. My Green Doctor. Accessed August 6, 2023. https://mygreendoctor.org/
52. Healthcare Without Harm. Accessed August 6, 2023. https://noharm.org/
53. Levy BS, Patz JA. Climate change, human rights, and social justice. Ann Glob Health. 2015;81:310-322.
54. Intergovernmental Panel on Climate Change. Global warming of 1.5° C 2018. Accessed August 6, 2023. https://www.ipcc.ch/sr15/
55. Steffen W, Crutzen J, McNeill JR. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 2007;36(8):614-621. doi:10.1579/0044-7447(2007)36[614:taahno]2.0.co;2
56. American Meteorological Society. Explaining extreme events from a climate perspective. Accessed August 6, 2023. https://www.ametsoc.org/ams/index.cfm/publications/bulletin-of-the-american-meteorological-society-bams/explaining-extreme-events-from-a-climate-perspective/
57. Nierenberg AA. Coping in the age of turmoil. Psychiatr Ann. 2022;52(7):263. July 1, 2022. doi:10.3928/23258160-20220701-01
58. Belkin G. Leadership for the social climate. N Engl J Med. 2020;382(21):1975-1977. doi:10.1056/NEJMp2001507
59. Skinner JR. Doctors and climate change: first do no harm. J Paediatr Child Health. 2021;57(11):1754-1758. doi:10.1111/jpc.15658
A toxic and fractured political system can breed angst and PTSD
As psychiatrists know, many of our severely traumatized adult patients were victims of abuse during childhood. We routinely ask every new patient about physical, emotional, or sexual abuse when they were growing up because of the well-established, serious neurobiological and mental repercussions.1,2
Perhaps one of the worst experiences for a child is to witness bitterly adversarial parents (their vital role models) who argue viciously, despise each other, and hurl insults (and even punches) at each other. Such a chronically and emotionally traumatic upbringing can haunt kids well into adulthood, disrupting their hypothalamic-pituitary-adrenal axis and triggering anxiety, depression, and even psychosis due to epigenetic changes that ultimately lead to abnormal brain development.3
It often feels that the governance of our country, or the national “political family,” is seriously fractured like a hopelessly dysfunctional family. Could that be negatively impacting the mental health of the citizenry? Having 2 antagonistic political parties expressing visceral hatred and undisguised contempt for each other 24/7 (thanks to the enabling era of cable TV, the internet, and social media) has transformed each party’s fanatic followers from fellow citizens to ideological combatants. In this poisonous societal zeitgeist of bidirectional acrimony and mutual detestation, the opposing parties and their “intellectual militias” label each other as “extremists” or “radicals.” They become completely blind to any redeeming social value in the ideas or principles of their political opponents. They spend enormous time and energy on undermining each other instead of attending to the myriad vital issues involved in the governance of a massive and complex country.
Winston Churchill said, “Democracy is the worst form of government, except for all the others that have been tried.”4 The current toxic cloud of intense “hyperpartisanship” is emblematic of the dark Machiavellian side of democracy. But those who lament the current distorted version of democracy should contemplate living in a dictatorship or totalitarian regime, where a despot would execute any dissenter or invade and destroy an adjacent country at a whim.
Churchill made that statement in 1947. The internet, social media, and smartphones were science fiction back then. Those technological advances have added fuel to the political process and significantly stoked the flames of hyperpartisanship. It’s now democracy on steroids, where freedom of expression goes to extremes, highlighting the warts and pitfalls of the democratic system. Political rivals can now communicate their ferocious disagreements to millions of their disciples instantaneously, triggering immediate rebuttals and counterattacks by their adversaries. This “virtual guerilla warfare” is mentally and emotionally exhausting to all involved, especially to the subset of neutral bystanders who are unaffiliated with either political party, which, due to the “religification” of politics, have become like secular religions.5 Chronic, unremitting, inescapable stress is a sure pathway for anxiety, depression, posttraumatic stress disorder, and even brain atrophy.
Optimists may point out that the United States has weathered and emerged stronger from many serious traumas, including the Civil War (with its lethal divisiveness), World War I, the deadly 1918 influenza pandemic, the Great Depression, Pearl Harbor, World War II, the Cold War, the Vietnam War, the Watergate political scandal, the 9/11 terrorist attacks, the banking collapse and recession, and most recently the COVID-19 pandemic, which brought society to a standstill and induced so much anxiety and uncertainty.
On the other hand, pessimists would insist those sequential crises left indelible scars that cumulatively altered the mindset of political rivals, predisposing them to extreme views of each other. Alienation inevitably leads to fanaticism. It’s perplexing but fascinating how the fierce bidirectional missives of weaponized ideas can be as virulent and destructive as a traditional physical war. Perhaps in this era, the pen is mightier than the sword after all.
Continue to: From a psychiatric perspective...
From a psychiatric perspective, the intransigent groupthink of political partisanship eerily resembles folie en masse, a psychiatric syndrome for which there is no established treatment. It has become a serious threat to our modern democracy. So I decided to ask the “know-it-all” artificial intelligence ChatGPT, whom I previously had “invited” to write a “guest editorial” about myths surrounding psychiatry,6 to answer 3 burning questions:
1. Is there toxic hyperpartisanship in the USA today? (Box 1)
Box 1
Yes, there is toxic hyperpartisanship in the United States today. Political polarization has been on the rise for several decades, and in recent years, it has become increasingly toxic and divisive. This toxic hyperpartisanship has contributed to a breakdown in civil discourse and a lack of cooperation and compromise in government.
The toxic hyperpartisanship is evident in many aspects of American society, including politics, media, and social media. Political leaders often engage in divisive rhetoric and focus on winning at all costs, rather than working towards solutions that benefit the country as a whole. Media outlets often present news in a partisan manner, further fueling political polarization. Social media platforms have also played a role in exacerbating toxic hyperpartisanship by allowing the spread of misinformation and creating echo chambers where people are only exposed to like-minded viewpoints.
The consequences of toxic hyperpartisanship are significant, as it can hinder progress on important issues and erode trust in institutions and government. It also makes it difficult for people to engage in productive dialogue and find common ground, leading to a lack of cooperation and compromise.
It is important for individuals to be aware of the impact of toxic hyperpartisanship and work towards finding ways to bridge the political divide and engage in constructive dialogue.
2. How can severe hyperpartisanship be corrected? (Supplemental Box 1)
Supplemental Box 1
Severe hyperpartisanship, characterized by intense and entrenched divisions between political parties, can be a challenging issue to address, but there are several steps that can be taken to work towards correcting it. Here are some potential approaches:
1. Promote civil discourse: Encourage respectful and constructive conversations between individuals with differing political views. This can be achieved through education programs, media campaigns, and initiatives to foster open dialogue.
2. Electoral reforms: Consider implementing electoral reforms that can reduce the influence of extreme partisanship. For example, ranked-choice voting or nonpartisan primaries may encourage candidates to appeal to a broader base of voters rather than just their party’s base.
3. Campaign finance reform: Limit the influence of money in politics, as it can exacerbate hyperpartisanship by allowing wealthy donors to have undue influence over candidates and parties.
4. Independent redistricting: Ensure that electoral district boundaries are drawn in a fair and nonpartisan manner. Gerrymandering, the manipulation of district lines to favor one party, can contribute to hyperpartisanship by creating “safe” districts where extreme candidates are more likely to be elected.
5. Promote media literacy: Encourage media literacy education to help citizens critically evaluate information sources and avoid echo chambers. A well-informed electorate is less susceptible to extreme partisanship.
6. Encourage bipartisan collaboration: Highlight and support instances of successful bipartisan collaboration. Elected officials who work across the aisle can serve as positive examples and encourage others to follow suit.
7. Address economic inequality: Tackling economic disparities can alleviate some of the underlying factors that contribute to polarization. When people feel economically secure, they may be more willing to seek common ground.
8. Civics education: Improve civics education in schools to foster a deeper understanding of democratic values, the political process, and the importance of civic engagement.
9. Leadership example: Encourage political leaders to model cooperation and compromise rather than relying on divisive rhetoric. Voters often take cues from their leaders’ behavior.
10. Promote third parties: Support and encourage the emergence of viable thirdparty candidates. A more diverse political landscape can provide alternatives to extreme partisanship.
11. Focus on common ground: Identify and prioritize issues where there is potential for bipartisan consensus and progress. Building success on these issues can create momentum for further cooperation.
12. Encourage grassroots activism: Support grassroots movements that seek to bridge divides and foster understanding between individuals and communities with different political perspectives.
It’s essential to recognize that addressing severe hyperpartisanship is a complex and long-term process that requires collective effort from citizens, political leaders, educators, and institutions. It won’t be achieved overnight, but through sustained and committed actions, progress can be made toward a more collaborative and less divisive political climate.
3. What can cause the collapse of a democracy? (Supplemental Box 2).
Supplemental Box 2
The collapse of a democracy can be a complex and multifaceted process, influenced by various factors and circumstances. Some of the common causes that can lead to the erosion or breakdown of a democratic system include:
1. Authoritarianism and power concentration: When leaders or institutions become increasingly authoritarian, centralizing power and weakening checks and balances, it can undermine democratic principles.
2. Corruption and lack of accountability: Rampant corruption among political leaders and institutions can erode public trust in the democratic system, leading to disillusionment and disengagement.
3. Economic instability: Prolonged economic crises or severe inequality can lead to social unrest, increasing the appeal of populist or extremist movements that may challenge democratic norms.
4. Weakening of rule of law: If the rule of law is weakened, and there is a lack of respect for judicial independence and constitutional principles, democratic institutions can become vulnerable to manipulation.
5. Erosion of civil liberties: Suppression of freedom of speech, media, assembly, and association can limit citizens’ ability to express their opinions and participate in the democratic process.
6. Polarization and divisive politics: Extreme political polarization and a breakdown of civil discourse can hinder cooperation and compromise essential for a functioning democracy.
7. External interference: Foreign intervention, propaganda, or cyberattacks can influence electoral processes and undermine democratic institutions.
8. Rise of populism and nationalism: Populist movements that challenge democratic norms, demonize minorities, or advocate for a strongman leadership can pose a threat to democracy.
9. Demographic and social shifts: Demographic changes and social tensions can exacerbate political divisions and weaken the fabric of democratic societies.
10. Lack of civic education and participation: A lack of civic education and low voter turnout can lead to reduced civic engagement and undermine the democratic process.
It is crucial to understand that no democracy is immune to these challenges, and the strength of a democratic system depends on the vigilance and engagement of its citizens, the integrity of its institutions, and the commitment to upholding democratic values. Safeguarding and strengthening democracy requires ongoing efforts to address these issues and protect the principles that underpin democratic governance.
Judge for yourself, but I believe the ChatGPT responses were spot-on.
1. Lippard ETC, Nemeroff CB. The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry. 2023;180(8):548-564.
2. Nemeroff CB. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron. 2016;89(5):892-909.
3. Zhang ZZ, Moeckel C, Mustafa M, et al. The association of epigenetic age acceleration and depressive and anxiety symptom severity among children recently exposed to substantiated maltreatment. J Psychiatr Res. 2023;165:7-13.
4. International Churchill Society. The worst form of government. Accessed August 8, 2023. https://winstonchurchill.org/resources/quotes/the-worst-form-of-government/
5. Nasrallah HA. From ideology to articles of faith: the ‘religification’ of political beliefs. Current Psychiatry. 2021;20(7):4-5,19.
6. Nasrallah HA. A ‘guest editorial’ … generated by ChatGPT? Current Psychiatry. 2023;22(4):22:6-7.
As psychiatrists know, many of our severely traumatized adult patients were victims of abuse during childhood. We routinely ask every new patient about physical, emotional, or sexual abuse when they were growing up because of the well-established, serious neurobiological and mental repercussions.1,2
Perhaps one of the worst experiences for a child is to witness bitterly adversarial parents (their vital role models) who argue viciously, despise each other, and hurl insults (and even punches) at each other. Such a chronically and emotionally traumatic upbringing can haunt kids well into adulthood, disrupting their hypothalamic-pituitary-adrenal axis and triggering anxiety, depression, and even psychosis due to epigenetic changes that ultimately lead to abnormal brain development.3
It often feels that the governance of our country, or the national “political family,” is seriously fractured like a hopelessly dysfunctional family. Could that be negatively impacting the mental health of the citizenry? Having 2 antagonistic political parties expressing visceral hatred and undisguised contempt for each other 24/7 (thanks to the enabling era of cable TV, the internet, and social media) has transformed each party’s fanatic followers from fellow citizens to ideological combatants. In this poisonous societal zeitgeist of bidirectional acrimony and mutual detestation, the opposing parties and their “intellectual militias” label each other as “extremists” or “radicals.” They become completely blind to any redeeming social value in the ideas or principles of their political opponents. They spend enormous time and energy on undermining each other instead of attending to the myriad vital issues involved in the governance of a massive and complex country.
Winston Churchill said, “Democracy is the worst form of government, except for all the others that have been tried.”4 The current toxic cloud of intense “hyperpartisanship” is emblematic of the dark Machiavellian side of democracy. But those who lament the current distorted version of democracy should contemplate living in a dictatorship or totalitarian regime, where a despot would execute any dissenter or invade and destroy an adjacent country at a whim.
Churchill made that statement in 1947. The internet, social media, and smartphones were science fiction back then. Those technological advances have added fuel to the political process and significantly stoked the flames of hyperpartisanship. It’s now democracy on steroids, where freedom of expression goes to extremes, highlighting the warts and pitfalls of the democratic system. Political rivals can now communicate their ferocious disagreements to millions of their disciples instantaneously, triggering immediate rebuttals and counterattacks by their adversaries. This “virtual guerilla warfare” is mentally and emotionally exhausting to all involved, especially to the subset of neutral bystanders who are unaffiliated with either political party, which, due to the “religification” of politics, have become like secular religions.5 Chronic, unremitting, inescapable stress is a sure pathway for anxiety, depression, posttraumatic stress disorder, and even brain atrophy.
Optimists may point out that the United States has weathered and emerged stronger from many serious traumas, including the Civil War (with its lethal divisiveness), World War I, the deadly 1918 influenza pandemic, the Great Depression, Pearl Harbor, World War II, the Cold War, the Vietnam War, the Watergate political scandal, the 9/11 terrorist attacks, the banking collapse and recession, and most recently the COVID-19 pandemic, which brought society to a standstill and induced so much anxiety and uncertainty.
On the other hand, pessimists would insist those sequential crises left indelible scars that cumulatively altered the mindset of political rivals, predisposing them to extreme views of each other. Alienation inevitably leads to fanaticism. It’s perplexing but fascinating how the fierce bidirectional missives of weaponized ideas can be as virulent and destructive as a traditional physical war. Perhaps in this era, the pen is mightier than the sword after all.
Continue to: From a psychiatric perspective...
From a psychiatric perspective, the intransigent groupthink of political partisanship eerily resembles folie en masse, a psychiatric syndrome for which there is no established treatment. It has become a serious threat to our modern democracy. So I decided to ask the “know-it-all” artificial intelligence ChatGPT, whom I previously had “invited” to write a “guest editorial” about myths surrounding psychiatry,6 to answer 3 burning questions:
1. Is there toxic hyperpartisanship in the USA today? (Box 1)
Box 1
Yes, there is toxic hyperpartisanship in the United States today. Political polarization has been on the rise for several decades, and in recent years, it has become increasingly toxic and divisive. This toxic hyperpartisanship has contributed to a breakdown in civil discourse and a lack of cooperation and compromise in government.
The toxic hyperpartisanship is evident in many aspects of American society, including politics, media, and social media. Political leaders often engage in divisive rhetoric and focus on winning at all costs, rather than working towards solutions that benefit the country as a whole. Media outlets often present news in a partisan manner, further fueling political polarization. Social media platforms have also played a role in exacerbating toxic hyperpartisanship by allowing the spread of misinformation and creating echo chambers where people are only exposed to like-minded viewpoints.
The consequences of toxic hyperpartisanship are significant, as it can hinder progress on important issues and erode trust in institutions and government. It also makes it difficult for people to engage in productive dialogue and find common ground, leading to a lack of cooperation and compromise.
It is important for individuals to be aware of the impact of toxic hyperpartisanship and work towards finding ways to bridge the political divide and engage in constructive dialogue.
2. How can severe hyperpartisanship be corrected? (Supplemental Box 1)
Supplemental Box 1
Severe hyperpartisanship, characterized by intense and entrenched divisions between political parties, can be a challenging issue to address, but there are several steps that can be taken to work towards correcting it. Here are some potential approaches:
1. Promote civil discourse: Encourage respectful and constructive conversations between individuals with differing political views. This can be achieved through education programs, media campaigns, and initiatives to foster open dialogue.
2. Electoral reforms: Consider implementing electoral reforms that can reduce the influence of extreme partisanship. For example, ranked-choice voting or nonpartisan primaries may encourage candidates to appeal to a broader base of voters rather than just their party’s base.
3. Campaign finance reform: Limit the influence of money in politics, as it can exacerbate hyperpartisanship by allowing wealthy donors to have undue influence over candidates and parties.
4. Independent redistricting: Ensure that electoral district boundaries are drawn in a fair and nonpartisan manner. Gerrymandering, the manipulation of district lines to favor one party, can contribute to hyperpartisanship by creating “safe” districts where extreme candidates are more likely to be elected.
5. Promote media literacy: Encourage media literacy education to help citizens critically evaluate information sources and avoid echo chambers. A well-informed electorate is less susceptible to extreme partisanship.
6. Encourage bipartisan collaboration: Highlight and support instances of successful bipartisan collaboration. Elected officials who work across the aisle can serve as positive examples and encourage others to follow suit.
7. Address economic inequality: Tackling economic disparities can alleviate some of the underlying factors that contribute to polarization. When people feel economically secure, they may be more willing to seek common ground.
8. Civics education: Improve civics education in schools to foster a deeper understanding of democratic values, the political process, and the importance of civic engagement.
9. Leadership example: Encourage political leaders to model cooperation and compromise rather than relying on divisive rhetoric. Voters often take cues from their leaders’ behavior.
10. Promote third parties: Support and encourage the emergence of viable thirdparty candidates. A more diverse political landscape can provide alternatives to extreme partisanship.
11. Focus on common ground: Identify and prioritize issues where there is potential for bipartisan consensus and progress. Building success on these issues can create momentum for further cooperation.
12. Encourage grassroots activism: Support grassroots movements that seek to bridge divides and foster understanding between individuals and communities with different political perspectives.
It’s essential to recognize that addressing severe hyperpartisanship is a complex and long-term process that requires collective effort from citizens, political leaders, educators, and institutions. It won’t be achieved overnight, but through sustained and committed actions, progress can be made toward a more collaborative and less divisive political climate.
3. What can cause the collapse of a democracy? (Supplemental Box 2).
Supplemental Box 2
The collapse of a democracy can be a complex and multifaceted process, influenced by various factors and circumstances. Some of the common causes that can lead to the erosion or breakdown of a democratic system include:
1. Authoritarianism and power concentration: When leaders or institutions become increasingly authoritarian, centralizing power and weakening checks and balances, it can undermine democratic principles.
2. Corruption and lack of accountability: Rampant corruption among political leaders and institutions can erode public trust in the democratic system, leading to disillusionment and disengagement.
3. Economic instability: Prolonged economic crises or severe inequality can lead to social unrest, increasing the appeal of populist or extremist movements that may challenge democratic norms.
4. Weakening of rule of law: If the rule of law is weakened, and there is a lack of respect for judicial independence and constitutional principles, democratic institutions can become vulnerable to manipulation.
5. Erosion of civil liberties: Suppression of freedom of speech, media, assembly, and association can limit citizens’ ability to express their opinions and participate in the democratic process.
6. Polarization and divisive politics: Extreme political polarization and a breakdown of civil discourse can hinder cooperation and compromise essential for a functioning democracy.
7. External interference: Foreign intervention, propaganda, or cyberattacks can influence electoral processes and undermine democratic institutions.
8. Rise of populism and nationalism: Populist movements that challenge democratic norms, demonize minorities, or advocate for a strongman leadership can pose a threat to democracy.
9. Demographic and social shifts: Demographic changes and social tensions can exacerbate political divisions and weaken the fabric of democratic societies.
10. Lack of civic education and participation: A lack of civic education and low voter turnout can lead to reduced civic engagement and undermine the democratic process.
It is crucial to understand that no democracy is immune to these challenges, and the strength of a democratic system depends on the vigilance and engagement of its citizens, the integrity of its institutions, and the commitment to upholding democratic values. Safeguarding and strengthening democracy requires ongoing efforts to address these issues and protect the principles that underpin democratic governance.
Judge for yourself, but I believe the ChatGPT responses were spot-on.
As psychiatrists know, many of our severely traumatized adult patients were victims of abuse during childhood. We routinely ask every new patient about physical, emotional, or sexual abuse when they were growing up because of the well-established, serious neurobiological and mental repercussions.1,2
Perhaps one of the worst experiences for a child is to witness bitterly adversarial parents (their vital role models) who argue viciously, despise each other, and hurl insults (and even punches) at each other. Such a chronically and emotionally traumatic upbringing can haunt kids well into adulthood, disrupting their hypothalamic-pituitary-adrenal axis and triggering anxiety, depression, and even psychosis due to epigenetic changes that ultimately lead to abnormal brain development.3
It often feels that the governance of our country, or the national “political family,” is seriously fractured like a hopelessly dysfunctional family. Could that be negatively impacting the mental health of the citizenry? Having 2 antagonistic political parties expressing visceral hatred and undisguised contempt for each other 24/7 (thanks to the enabling era of cable TV, the internet, and social media) has transformed each party’s fanatic followers from fellow citizens to ideological combatants. In this poisonous societal zeitgeist of bidirectional acrimony and mutual detestation, the opposing parties and their “intellectual militias” label each other as “extremists” or “radicals.” They become completely blind to any redeeming social value in the ideas or principles of their political opponents. They spend enormous time and energy on undermining each other instead of attending to the myriad vital issues involved in the governance of a massive and complex country.
Winston Churchill said, “Democracy is the worst form of government, except for all the others that have been tried.”4 The current toxic cloud of intense “hyperpartisanship” is emblematic of the dark Machiavellian side of democracy. But those who lament the current distorted version of democracy should contemplate living in a dictatorship or totalitarian regime, where a despot would execute any dissenter or invade and destroy an adjacent country at a whim.
Churchill made that statement in 1947. The internet, social media, and smartphones were science fiction back then. Those technological advances have added fuel to the political process and significantly stoked the flames of hyperpartisanship. It’s now democracy on steroids, where freedom of expression goes to extremes, highlighting the warts and pitfalls of the democratic system. Political rivals can now communicate their ferocious disagreements to millions of their disciples instantaneously, triggering immediate rebuttals and counterattacks by their adversaries. This “virtual guerilla warfare” is mentally and emotionally exhausting to all involved, especially to the subset of neutral bystanders who are unaffiliated with either political party, which, due to the “religification” of politics, have become like secular religions.5 Chronic, unremitting, inescapable stress is a sure pathway for anxiety, depression, posttraumatic stress disorder, and even brain atrophy.
Optimists may point out that the United States has weathered and emerged stronger from many serious traumas, including the Civil War (with its lethal divisiveness), World War I, the deadly 1918 influenza pandemic, the Great Depression, Pearl Harbor, World War II, the Cold War, the Vietnam War, the Watergate political scandal, the 9/11 terrorist attacks, the banking collapse and recession, and most recently the COVID-19 pandemic, which brought society to a standstill and induced so much anxiety and uncertainty.
On the other hand, pessimists would insist those sequential crises left indelible scars that cumulatively altered the mindset of political rivals, predisposing them to extreme views of each other. Alienation inevitably leads to fanaticism. It’s perplexing but fascinating how the fierce bidirectional missives of weaponized ideas can be as virulent and destructive as a traditional physical war. Perhaps in this era, the pen is mightier than the sword after all.
Continue to: From a psychiatric perspective...
From a psychiatric perspective, the intransigent groupthink of political partisanship eerily resembles folie en masse, a psychiatric syndrome for which there is no established treatment. It has become a serious threat to our modern democracy. So I decided to ask the “know-it-all” artificial intelligence ChatGPT, whom I previously had “invited” to write a “guest editorial” about myths surrounding psychiatry,6 to answer 3 burning questions:
1. Is there toxic hyperpartisanship in the USA today? (Box 1)
Box 1
Yes, there is toxic hyperpartisanship in the United States today. Political polarization has been on the rise for several decades, and in recent years, it has become increasingly toxic and divisive. This toxic hyperpartisanship has contributed to a breakdown in civil discourse and a lack of cooperation and compromise in government.
The toxic hyperpartisanship is evident in many aspects of American society, including politics, media, and social media. Political leaders often engage in divisive rhetoric and focus on winning at all costs, rather than working towards solutions that benefit the country as a whole. Media outlets often present news in a partisan manner, further fueling political polarization. Social media platforms have also played a role in exacerbating toxic hyperpartisanship by allowing the spread of misinformation and creating echo chambers where people are only exposed to like-minded viewpoints.
The consequences of toxic hyperpartisanship are significant, as it can hinder progress on important issues and erode trust in institutions and government. It also makes it difficult for people to engage in productive dialogue and find common ground, leading to a lack of cooperation and compromise.
It is important for individuals to be aware of the impact of toxic hyperpartisanship and work towards finding ways to bridge the political divide and engage in constructive dialogue.
2. How can severe hyperpartisanship be corrected? (Supplemental Box 1)
Supplemental Box 1
Severe hyperpartisanship, characterized by intense and entrenched divisions between political parties, can be a challenging issue to address, but there are several steps that can be taken to work towards correcting it. Here are some potential approaches:
1. Promote civil discourse: Encourage respectful and constructive conversations between individuals with differing political views. This can be achieved through education programs, media campaigns, and initiatives to foster open dialogue.
2. Electoral reforms: Consider implementing electoral reforms that can reduce the influence of extreme partisanship. For example, ranked-choice voting or nonpartisan primaries may encourage candidates to appeal to a broader base of voters rather than just their party’s base.
3. Campaign finance reform: Limit the influence of money in politics, as it can exacerbate hyperpartisanship by allowing wealthy donors to have undue influence over candidates and parties.
4. Independent redistricting: Ensure that electoral district boundaries are drawn in a fair and nonpartisan manner. Gerrymandering, the manipulation of district lines to favor one party, can contribute to hyperpartisanship by creating “safe” districts where extreme candidates are more likely to be elected.
5. Promote media literacy: Encourage media literacy education to help citizens critically evaluate information sources and avoid echo chambers. A well-informed electorate is less susceptible to extreme partisanship.
6. Encourage bipartisan collaboration: Highlight and support instances of successful bipartisan collaboration. Elected officials who work across the aisle can serve as positive examples and encourage others to follow suit.
7. Address economic inequality: Tackling economic disparities can alleviate some of the underlying factors that contribute to polarization. When people feel economically secure, they may be more willing to seek common ground.
8. Civics education: Improve civics education in schools to foster a deeper understanding of democratic values, the political process, and the importance of civic engagement.
9. Leadership example: Encourage political leaders to model cooperation and compromise rather than relying on divisive rhetoric. Voters often take cues from their leaders’ behavior.
10. Promote third parties: Support and encourage the emergence of viable thirdparty candidates. A more diverse political landscape can provide alternatives to extreme partisanship.
11. Focus on common ground: Identify and prioritize issues where there is potential for bipartisan consensus and progress. Building success on these issues can create momentum for further cooperation.
12. Encourage grassroots activism: Support grassroots movements that seek to bridge divides and foster understanding between individuals and communities with different political perspectives.
It’s essential to recognize that addressing severe hyperpartisanship is a complex and long-term process that requires collective effort from citizens, political leaders, educators, and institutions. It won’t be achieved overnight, but through sustained and committed actions, progress can be made toward a more collaborative and less divisive political climate.
3. What can cause the collapse of a democracy? (Supplemental Box 2).
Supplemental Box 2
The collapse of a democracy can be a complex and multifaceted process, influenced by various factors and circumstances. Some of the common causes that can lead to the erosion or breakdown of a democratic system include:
1. Authoritarianism and power concentration: When leaders or institutions become increasingly authoritarian, centralizing power and weakening checks and balances, it can undermine democratic principles.
2. Corruption and lack of accountability: Rampant corruption among political leaders and institutions can erode public trust in the democratic system, leading to disillusionment and disengagement.
3. Economic instability: Prolonged economic crises or severe inequality can lead to social unrest, increasing the appeal of populist or extremist movements that may challenge democratic norms.
4. Weakening of rule of law: If the rule of law is weakened, and there is a lack of respect for judicial independence and constitutional principles, democratic institutions can become vulnerable to manipulation.
5. Erosion of civil liberties: Suppression of freedom of speech, media, assembly, and association can limit citizens’ ability to express their opinions and participate in the democratic process.
6. Polarization and divisive politics: Extreme political polarization and a breakdown of civil discourse can hinder cooperation and compromise essential for a functioning democracy.
7. External interference: Foreign intervention, propaganda, or cyberattacks can influence electoral processes and undermine democratic institutions.
8. Rise of populism and nationalism: Populist movements that challenge democratic norms, demonize minorities, or advocate for a strongman leadership can pose a threat to democracy.
9. Demographic and social shifts: Demographic changes and social tensions can exacerbate political divisions and weaken the fabric of democratic societies.
10. Lack of civic education and participation: A lack of civic education and low voter turnout can lead to reduced civic engagement and undermine the democratic process.
It is crucial to understand that no democracy is immune to these challenges, and the strength of a democratic system depends on the vigilance and engagement of its citizens, the integrity of its institutions, and the commitment to upholding democratic values. Safeguarding and strengthening democracy requires ongoing efforts to address these issues and protect the principles that underpin democratic governance.
Judge for yourself, but I believe the ChatGPT responses were spot-on.
1. Lippard ETC, Nemeroff CB. The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry. 2023;180(8):548-564.
2. Nemeroff CB. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron. 2016;89(5):892-909.
3. Zhang ZZ, Moeckel C, Mustafa M, et al. The association of epigenetic age acceleration and depressive and anxiety symptom severity among children recently exposed to substantiated maltreatment. J Psychiatr Res. 2023;165:7-13.
4. International Churchill Society. The worst form of government. Accessed August 8, 2023. https://winstonchurchill.org/resources/quotes/the-worst-form-of-government/
5. Nasrallah HA. From ideology to articles of faith: the ‘religification’ of political beliefs. Current Psychiatry. 2021;20(7):4-5,19.
6. Nasrallah HA. A ‘guest editorial’ … generated by ChatGPT? Current Psychiatry. 2023;22(4):22:6-7.
1. Lippard ETC, Nemeroff CB. The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry. 2023;180(8):548-564.
2. Nemeroff CB. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron. 2016;89(5):892-909.
3. Zhang ZZ, Moeckel C, Mustafa M, et al. The association of epigenetic age acceleration and depressive and anxiety symptom severity among children recently exposed to substantiated maltreatment. J Psychiatr Res. 2023;165:7-13.
4. International Churchill Society. The worst form of government. Accessed August 8, 2023. https://winstonchurchill.org/resources/quotes/the-worst-form-of-government/
5. Nasrallah HA. From ideology to articles of faith: the ‘religification’ of political beliefs. Current Psychiatry. 2021;20(7):4-5,19.
6. Nasrallah HA. A ‘guest editorial’ … generated by ChatGPT? Current Psychiatry. 2023;22(4):22:6-7.