User login
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
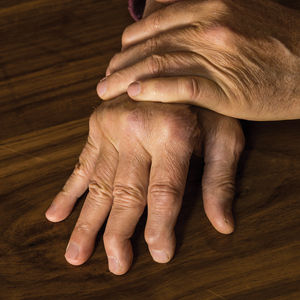
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.