User login
To the Editor:
Cutaneous aspergillosis mostly has been reported in immunosuppressed hosts and usually is caused by Aspergillus flavus or Aspergillus fumigatus. We report the occurrence of primary cutaneous aspergillosis (PCA) caused by a relatively rare species, Aspergillus nidulans, in a middle-aged patient without overt immunosuppression or history of trauma.
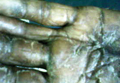
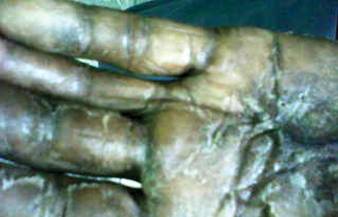
A 57-year-old woman was referred to the dermatology outpatient department for evaluation of a lesion on the right hand of 1 month's duration. On examination the lesion measured approximately 4×3 cm with central necrosis (Figure 1). Her medical history was unremarkable and routine laboratory test results were within reference range.

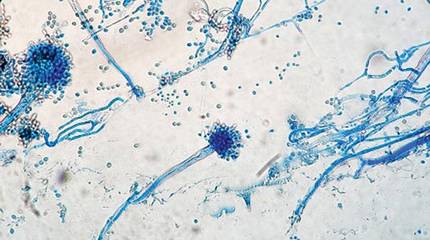
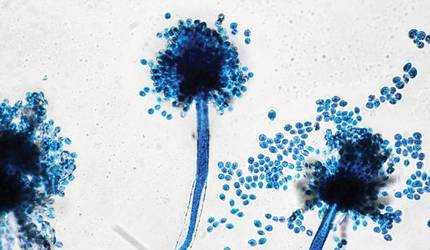
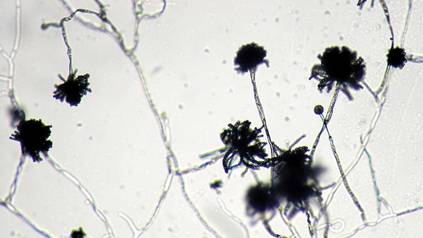
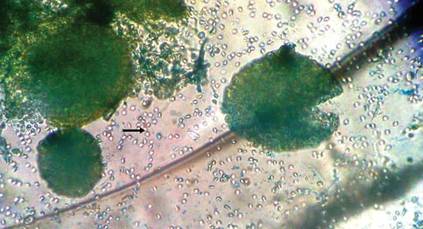
The patient was an agricultural worker with no history of trauma. Her history was unremarkable. A 20% potassium hydroxide mount of the tissue revealed septate, branched, hyaline hyphae. A soft, wooly, greenish brown growth was observed after 3 days of incubation on Sabouraud dextrose agar (Figure 2). No growth was observed on dermatophyte test medium. A lactophenol cotton blue mount revealed columnar conidial heads with brown, short, smooth-walled conidiophores (Figures 3–6). Vesicles were hemispheric and small (8–12 µm in diameter), with metulae and phialides occurring in the upper portion. Conidia were globose (3–4 µm) and rough. Based on these findings the fungus was identified as A nidulans. The patient did not respond to daily oral ketoconazole, and after 1 month of therapy the lesion did not regress. She was eventually treated with oral itraconazole and the lesion completely healed within 15 weeks.
An overwhelming majority of the cases of cutaneous aspergillosis have been reported either in immunocompromised hosts (ie, leukemia, cutaneous T-cell lymphoma, Hodgkin disease, human immunodeficiency virus/AIDS, solid-organ or hematopoietic stem cell transplant recipients) or in patients with contributing risk factors (ie, severe burns, diabetes mellitus, preterm or underweight neonates, elderly patients). Two outbreaks of this condition have been reported in neonatal intensive care units, with the source of contamination being linked to nonsterile disposable gloves, incubators, and humidity chambers.1,2 However, PCA is a relatively rare condition and often is associated with disruption of dermal integrity by trauma or maceration, followed by colonization of the wound by Aspergillus spores that are ubiquitously present in soil and decomposed vegetation.3-5 Our case was remarkable, as the patient was not immunosuppressed and did not have a history of trauma. However, we surmise that fungal inoculation might have inadvertently occurred through some trivial trauma sustained through her professional work.
The 2 species that have most commonly been associated with PCA are A flavus and A fumigatus.6,7 There have been isolated reports of PCA caused by other organisms such as Aspergillus niger,8,9 Aspergillus terreus,10Aspergillus ustus,11 or Aspergillus calidoustus.12 In a report of a neutropenic 56-year-old patient suffering from acute myeloblastic leukemia, PCA developed in association with a double-lumen Hickman catheter after a period of prolonged hospitalization.13 A study by the National Institutes of Health (1976-1997) revealed 6 life-threatening cases of A nidulans infection in patients with chronic granulomatous disease.14
We did not perform antifungal susceptibility testing on the isolate in our patient. However, we observed disease that was refractory to ketoconazole therapy but successfully resolved with oral itraconazole. Antifungal susceptibility was noted in a large number of reported cases of Aspergillus infections that were resistant to conventional treatment, such as voriconazole, itraconazole, and amphotericin B.15 Thus antifungal susceptibility testing is necessary before starting treatment. There also have been reports of recurrence of cutaneous aspergillosis following incomplete and irregular treatment.16 Our case of PCA also failed to respond to ketoconazole therapy, thus stressing the need for thorough mycological characterization, including the determination of an antifungal susceptibility profile, for successful and complete management of this condition.
Acknowledgment
The authors would like to thank Arunaloke Chakraborti, MD, Chandigarh, India, for the help extended for identification of the fungus.
- Stock C, Veyrier M, Raberin H, et al. Severe cutaneous aspergillosis in a premature neonate linked to nonsterile disposable glove contamination [published online ahead of print August 31, 2011]? Am J Infect Control. 2012;40:465-467.
- Etienne KA, Subudhi CP, Chadwick PR, et al. Investigation of a cluster of cutaneous aspergillosis in a neonatal intensive care unit [published online ahead of print August 12, 2011]. J Hosp Infect. 2011;79:344-348.
- Isaac M. Cutaneous aspergillosis. Dermatol Clin. 1996;14:137-140.
- Cahill KM, Mofty AM, Kawaguchi TP. Primary cutaneous aspergillosis. Arch Dermatol. 1967;96:545-547.
- Carlile JR, Millet RE, Cho CT, et al. Primary cutaneous aspergillosis in a leukemic child. Arch Dermatol. 1978;114:78-80.
- John PU, Shadomy HJ. Deep fungal infections. In: Fitzpatrick TB, Eisen AZ, Wolff K, et al, eds. Dermatology in General Medicine. New York, NY: McGraw Hill; 1987:2266-2268.
- Chakrabarti A, Gupta V, Biswas G, et al. Primary cutaneous aspergillosis: our experience in 10 years. J Infect. 1998;37:24-27.
- Robinson A, Fien S, Grassi MA. Nonhealing scalp wound infected with Aspergillus niger in an elderly patient. Cutis. 2011;87:197-200.
- Thomas LM, Rand HK, Miller JL, et al. Primary cutaneous aspergillosis in a patient with a solid organ transplant: case report and review of the literature. Cutis. 2008;81:127-130.
- Yuanjie Z, Jingxia D, Hai W, et al. Primary cutaneous aspergillosis in a patient with cutaneous T-cell lymphoma [published online ahead of print October 22, 2008]. Mycoses. 2009;52:462-464.
- Krishnan-Natesan S, Chandrasekar PH, Manavathu EK, et al. Successful treatment of primary cutaneous Aspergillus ustus infection with surgical debridement and a combination of voriconazole and terbinafine [published online ahead of print October 7, 2008]. Diagn Microbiol Infect Dis. 2008;62:443-446.
- Sato Y, Suzino K, Suzuki A, et al. Case of primary cutaneous Aspergillus calidoustus infection caused by nerve block therapy [in Japanese]. Med Mycol J. 2011;52:239-244.
- Lucas GM, Tucker P, Merz WG. Primary cutaneous Aspergillus nidulans infection associated with a Hickman catheter in a patient with neutropenia. Clin Infect Dis. 1999;29:1594-1596.
- Segal BH, DeCarlo ES, Kwon-Chung KJ, et al. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore). 1998;77:345-354.
- Woodruff CA, Hebert AA. Neonatal primary cutaneous aspergillosis: case report and review of the literature. Pediatr Dermatol. 2002;19:439-444.
- Mohapatra S, Xess I, Swetha JV, et al. Primary cutaneous aspergillosis due to Aspergillus niger in an immunocompetent patient. Indian J Med Microbiol. 2009;27:367-370.
To the Editor:
Cutaneous aspergillosis mostly has been reported in immunosuppressed hosts and usually is caused by Aspergillus flavus or Aspergillus fumigatus. We report the occurrence of primary cutaneous aspergillosis (PCA) caused by a relatively rare species, Aspergillus nidulans, in a middle-aged patient without overt immunosuppression or history of trauma.
A 57-year-old woman was referred to the dermatology outpatient department for evaluation of a lesion on the right hand of 1 month's duration. On examination the lesion measured approximately 4×3 cm with central necrosis (Figure 1). Her medical history was unremarkable and routine laboratory test results were within reference range.


The patient was an agricultural worker with no history of trauma. Her history was unremarkable. A 20% potassium hydroxide mount of the tissue revealed septate, branched, hyaline hyphae. A soft, wooly, greenish brown growth was observed after 3 days of incubation on Sabouraud dextrose agar (Figure 2). No growth was observed on dermatophyte test medium. A lactophenol cotton blue mount revealed columnar conidial heads with brown, short, smooth-walled conidiophores (Figures 3–6). Vesicles were hemispheric and small (8–12 µm in diameter), with metulae and phialides occurring in the upper portion. Conidia were globose (3–4 µm) and rough. Based on these findings the fungus was identified as A nidulans. The patient did not respond to daily oral ketoconazole, and after 1 month of therapy the lesion did not regress. She was eventually treated with oral itraconazole and the lesion completely healed within 15 weeks.




An overwhelming majority of the cases of cutaneous aspergillosis have been reported either in immunocompromised hosts (ie, leukemia, cutaneous T-cell lymphoma, Hodgkin disease, human immunodeficiency virus/AIDS, solid-organ or hematopoietic stem cell transplant recipients) or in patients with contributing risk factors (ie, severe burns, diabetes mellitus, preterm or underweight neonates, elderly patients). Two outbreaks of this condition have been reported in neonatal intensive care units, with the source of contamination being linked to nonsterile disposable gloves, incubators, and humidity chambers.1,2 However, PCA is a relatively rare condition and often is associated with disruption of dermal integrity by trauma or maceration, followed by colonization of the wound by Aspergillus spores that are ubiquitously present in soil and decomposed vegetation.3-5 Our case was remarkable, as the patient was not immunosuppressed and did not have a history of trauma. However, we surmise that fungal inoculation might have inadvertently occurred through some trivial trauma sustained through her professional work.
The 2 species that have most commonly been associated with PCA are A flavus and A fumigatus.6,7 There have been isolated reports of PCA caused by other organisms such as Aspergillus niger,8,9 Aspergillus terreus,10Aspergillus ustus,11 or Aspergillus calidoustus.12 In a report of a neutropenic 56-year-old patient suffering from acute myeloblastic leukemia, PCA developed in association with a double-lumen Hickman catheter after a period of prolonged hospitalization.13 A study by the National Institutes of Health (1976-1997) revealed 6 life-threatening cases of A nidulans infection in patients with chronic granulomatous disease.14
We did not perform antifungal susceptibility testing on the isolate in our patient. However, we observed disease that was refractory to ketoconazole therapy but successfully resolved with oral itraconazole. Antifungal susceptibility was noted in a large number of reported cases of Aspergillus infections that were resistant to conventional treatment, such as voriconazole, itraconazole, and amphotericin B.15 Thus antifungal susceptibility testing is necessary before starting treatment. There also have been reports of recurrence of cutaneous aspergillosis following incomplete and irregular treatment.16 Our case of PCA also failed to respond to ketoconazole therapy, thus stressing the need for thorough mycological characterization, including the determination of an antifungal susceptibility profile, for successful and complete management of this condition.
Acknowledgment
The authors would like to thank Arunaloke Chakraborti, MD, Chandigarh, India, for the help extended for identification of the fungus.
To the Editor:
Cutaneous aspergillosis mostly has been reported in immunosuppressed hosts and usually is caused by Aspergillus flavus or Aspergillus fumigatus. We report the occurrence of primary cutaneous aspergillosis (PCA) caused by a relatively rare species, Aspergillus nidulans, in a middle-aged patient without overt immunosuppression or history of trauma.
A 57-year-old woman was referred to the dermatology outpatient department for evaluation of a lesion on the right hand of 1 month's duration. On examination the lesion measured approximately 4×3 cm with central necrosis (Figure 1). Her medical history was unremarkable and routine laboratory test results were within reference range.


The patient was an agricultural worker with no history of trauma. Her history was unremarkable. A 20% potassium hydroxide mount of the tissue revealed septate, branched, hyaline hyphae. A soft, wooly, greenish brown growth was observed after 3 days of incubation on Sabouraud dextrose agar (Figure 2). No growth was observed on dermatophyte test medium. A lactophenol cotton blue mount revealed columnar conidial heads with brown, short, smooth-walled conidiophores (Figures 3–6). Vesicles were hemispheric and small (8–12 µm in diameter), with metulae and phialides occurring in the upper portion. Conidia were globose (3–4 µm) and rough. Based on these findings the fungus was identified as A nidulans. The patient did not respond to daily oral ketoconazole, and after 1 month of therapy the lesion did not regress. She was eventually treated with oral itraconazole and the lesion completely healed within 15 weeks.




An overwhelming majority of the cases of cutaneous aspergillosis have been reported either in immunocompromised hosts (ie, leukemia, cutaneous T-cell lymphoma, Hodgkin disease, human immunodeficiency virus/AIDS, solid-organ or hematopoietic stem cell transplant recipients) or in patients with contributing risk factors (ie, severe burns, diabetes mellitus, preterm or underweight neonates, elderly patients). Two outbreaks of this condition have been reported in neonatal intensive care units, with the source of contamination being linked to nonsterile disposable gloves, incubators, and humidity chambers.1,2 However, PCA is a relatively rare condition and often is associated with disruption of dermal integrity by trauma or maceration, followed by colonization of the wound by Aspergillus spores that are ubiquitously present in soil and decomposed vegetation.3-5 Our case was remarkable, as the patient was not immunosuppressed and did not have a history of trauma. However, we surmise that fungal inoculation might have inadvertently occurred through some trivial trauma sustained through her professional work.
The 2 species that have most commonly been associated with PCA are A flavus and A fumigatus.6,7 There have been isolated reports of PCA caused by other organisms such as Aspergillus niger,8,9 Aspergillus terreus,10Aspergillus ustus,11 or Aspergillus calidoustus.12 In a report of a neutropenic 56-year-old patient suffering from acute myeloblastic leukemia, PCA developed in association with a double-lumen Hickman catheter after a period of prolonged hospitalization.13 A study by the National Institutes of Health (1976-1997) revealed 6 life-threatening cases of A nidulans infection in patients with chronic granulomatous disease.14
We did not perform antifungal susceptibility testing on the isolate in our patient. However, we observed disease that was refractory to ketoconazole therapy but successfully resolved with oral itraconazole. Antifungal susceptibility was noted in a large number of reported cases of Aspergillus infections that were resistant to conventional treatment, such as voriconazole, itraconazole, and amphotericin B.15 Thus antifungal susceptibility testing is necessary before starting treatment. There also have been reports of recurrence of cutaneous aspergillosis following incomplete and irregular treatment.16 Our case of PCA also failed to respond to ketoconazole therapy, thus stressing the need for thorough mycological characterization, including the determination of an antifungal susceptibility profile, for successful and complete management of this condition.
Acknowledgment
The authors would like to thank Arunaloke Chakraborti, MD, Chandigarh, India, for the help extended for identification of the fungus.
- Stock C, Veyrier M, Raberin H, et al. Severe cutaneous aspergillosis in a premature neonate linked to nonsterile disposable glove contamination [published online ahead of print August 31, 2011]? Am J Infect Control. 2012;40:465-467.
- Etienne KA, Subudhi CP, Chadwick PR, et al. Investigation of a cluster of cutaneous aspergillosis in a neonatal intensive care unit [published online ahead of print August 12, 2011]. J Hosp Infect. 2011;79:344-348.
- Isaac M. Cutaneous aspergillosis. Dermatol Clin. 1996;14:137-140.
- Cahill KM, Mofty AM, Kawaguchi TP. Primary cutaneous aspergillosis. Arch Dermatol. 1967;96:545-547.
- Carlile JR, Millet RE, Cho CT, et al. Primary cutaneous aspergillosis in a leukemic child. Arch Dermatol. 1978;114:78-80.
- John PU, Shadomy HJ. Deep fungal infections. In: Fitzpatrick TB, Eisen AZ, Wolff K, et al, eds. Dermatology in General Medicine. New York, NY: McGraw Hill; 1987:2266-2268.
- Chakrabarti A, Gupta V, Biswas G, et al. Primary cutaneous aspergillosis: our experience in 10 years. J Infect. 1998;37:24-27.
- Robinson A, Fien S, Grassi MA. Nonhealing scalp wound infected with Aspergillus niger in an elderly patient. Cutis. 2011;87:197-200.
- Thomas LM, Rand HK, Miller JL, et al. Primary cutaneous aspergillosis in a patient with a solid organ transplant: case report and review of the literature. Cutis. 2008;81:127-130.
- Yuanjie Z, Jingxia D, Hai W, et al. Primary cutaneous aspergillosis in a patient with cutaneous T-cell lymphoma [published online ahead of print October 22, 2008]. Mycoses. 2009;52:462-464.
- Krishnan-Natesan S, Chandrasekar PH, Manavathu EK, et al. Successful treatment of primary cutaneous Aspergillus ustus infection with surgical debridement and a combination of voriconazole and terbinafine [published online ahead of print October 7, 2008]. Diagn Microbiol Infect Dis. 2008;62:443-446.
- Sato Y, Suzino K, Suzuki A, et al. Case of primary cutaneous Aspergillus calidoustus infection caused by nerve block therapy [in Japanese]. Med Mycol J. 2011;52:239-244.
- Lucas GM, Tucker P, Merz WG. Primary cutaneous Aspergillus nidulans infection associated with a Hickman catheter in a patient with neutropenia. Clin Infect Dis. 1999;29:1594-1596.
- Segal BH, DeCarlo ES, Kwon-Chung KJ, et al. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore). 1998;77:345-354.
- Woodruff CA, Hebert AA. Neonatal primary cutaneous aspergillosis: case report and review of the literature. Pediatr Dermatol. 2002;19:439-444.
- Mohapatra S, Xess I, Swetha JV, et al. Primary cutaneous aspergillosis due to Aspergillus niger in an immunocompetent patient. Indian J Med Microbiol. 2009;27:367-370.
- Stock C, Veyrier M, Raberin H, et al. Severe cutaneous aspergillosis in a premature neonate linked to nonsterile disposable glove contamination [published online ahead of print August 31, 2011]? Am J Infect Control. 2012;40:465-467.
- Etienne KA, Subudhi CP, Chadwick PR, et al. Investigation of a cluster of cutaneous aspergillosis in a neonatal intensive care unit [published online ahead of print August 12, 2011]. J Hosp Infect. 2011;79:344-348.
- Isaac M. Cutaneous aspergillosis. Dermatol Clin. 1996;14:137-140.
- Cahill KM, Mofty AM, Kawaguchi TP. Primary cutaneous aspergillosis. Arch Dermatol. 1967;96:545-547.
- Carlile JR, Millet RE, Cho CT, et al. Primary cutaneous aspergillosis in a leukemic child. Arch Dermatol. 1978;114:78-80.
- John PU, Shadomy HJ. Deep fungal infections. In: Fitzpatrick TB, Eisen AZ, Wolff K, et al, eds. Dermatology in General Medicine. New York, NY: McGraw Hill; 1987:2266-2268.
- Chakrabarti A, Gupta V, Biswas G, et al. Primary cutaneous aspergillosis: our experience in 10 years. J Infect. 1998;37:24-27.
- Robinson A, Fien S, Grassi MA. Nonhealing scalp wound infected with Aspergillus niger in an elderly patient. Cutis. 2011;87:197-200.
- Thomas LM, Rand HK, Miller JL, et al. Primary cutaneous aspergillosis in a patient with a solid organ transplant: case report and review of the literature. Cutis. 2008;81:127-130.
- Yuanjie Z, Jingxia D, Hai W, et al. Primary cutaneous aspergillosis in a patient with cutaneous T-cell lymphoma [published online ahead of print October 22, 2008]. Mycoses. 2009;52:462-464.
- Krishnan-Natesan S, Chandrasekar PH, Manavathu EK, et al. Successful treatment of primary cutaneous Aspergillus ustus infection with surgical debridement and a combination of voriconazole and terbinafine [published online ahead of print October 7, 2008]. Diagn Microbiol Infect Dis. 2008;62:443-446.
- Sato Y, Suzino K, Suzuki A, et al. Case of primary cutaneous Aspergillus calidoustus infection caused by nerve block therapy [in Japanese]. Med Mycol J. 2011;52:239-244.
- Lucas GM, Tucker P, Merz WG. Primary cutaneous Aspergillus nidulans infection associated with a Hickman catheter in a patient with neutropenia. Clin Infect Dis. 1999;29:1594-1596.
- Segal BH, DeCarlo ES, Kwon-Chung KJ, et al. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore). 1998;77:345-354.
- Woodruff CA, Hebert AA. Neonatal primary cutaneous aspergillosis: case report and review of the literature. Pediatr Dermatol. 2002;19:439-444.
- Mohapatra S, Xess I, Swetha JV, et al. Primary cutaneous aspergillosis due to Aspergillus niger in an immunocompetent patient. Indian J Med Microbiol. 2009;27:367-370.