User login
Options for managing severe aortic stenosis: A case-based review
Surgical aortic valve replacement remains the gold standard treatment for symptomatic aortic valve stenosis in patients at low or moderate risk of surgical complications. But this is a disease of the elderly, many of whom are too frail or too sick to undergo surgery.
Now, patients who cannot undergo this surgery can be offered the less invasive option of transcatheter aortic valve replacement. Balloon valvuloplasty, sodium nitroprusside, and intra-aortic balloon counterpulsation can buy time for ill patients while more permanent mechanical interventions are being considered.
In this review, we will present several cases that highlight management choices for patients with severe aortic stenosis.
A PROGRESSIVE DISEASE OF THE ELDERLY
Aortic stenosis is the most common acquired valvular disease in the United States, and its incidence and prevalence are rising as the population ages. Epidemiologic studies suggest that 2% to 7% of all patients over age 65 have it.1,2
The natural history of the untreated disease is well established, with several case series showing an average decrease of 0.1 cm2 per year in aortic valve area and an increase of 7 mm Hg per year in the pressure gradient across the valve once the diagnosis is made.3,4 Development of angina, syncope, or heart failure is associated with adverse clinical outcomes, including death, and warrants prompt intervention with aortic valve replacement.5–7 Without intervention, the mortality rates reach as high as 75% in 3 years once symptoms develop.
Statins, bisphosphonates, and angiotensin-converting enzyme inhibitors have been used in attempts to slow or reverse the progression of aortic stenosis. However, studies of these drugs have had mixed results, and no definitive benefit has been shown.8–13 Surgical aortic valve replacement, on the other hand, normalizes the life expectancy of patients with aortic stenosis to that of age- and sex-matched controls and remains the gold standard therapy for patients who have symptoms.14
Traditionally, valve replacement has involved open heart surgery, since it requires direct visualization of the valve while the patient is on cardiopulmonary bypass. Unfortunately, many patients have multiple comorbid conditions and therefore are not candidates for open heart surgery. Options for these patients include aortic valvuloplasty and transcatheter aortic valve replacement. While there is considerable experience with aortic valvuloplasty, transcatheter aortic valve replacement is relatively new. In large randomized trials and registries, the transcatheter procedure has been shown to significantly improve long-term survival compared with medical management alone in inoperable patients and to have benefit similar to that of surgery in the high-risk population.15–17
CASE 1: SEVERE, SYMPTOMATIC STENOSIS IN A GOOD SURGICAL CANDIDATE
Mr. A, age 83, presents with shortness of breath and peripheral edema that have been worsening over the past several months. His pulse rate is 64 beats per minute and his blood pressure is 110/90 mm Hg. Auscultation reveals an absent aortic second heart sound with a late peaking systolic murmur that increases with expiration.
On echocardiography, his left ventricular ejection fraction is 55%, peak transaortic valve gradient 88 mm Hg, mean gradient 60 mm Hg, and effective valve area 0.6 cm2. He undergoes catheterization of the left side of his heart, which shows normal coronary arteries.
Mr. A also has hypertension and hyperlipidemia; his renal and pulmonary functions are normal.
How would you manage Mr. A’s aortic stenosis?
Symptomatic aortic stenosis leads to adverse clinical outcomes if managed medically without mechanical intervention,5–7 but patients who undergo aortic valve replacement have age-corrected postoperative survival rates that are nearly normal.14 Furthermore, thanks to improvements in surgical techniques and perioperative management, surgical mortality rates have decreased significantly in recent years and now range from 1% to 8%.18–20 The accumulated evidence showing clear superiority of a surgical approach over medical therapy has greatly simplified the therapeutic algorithm.21
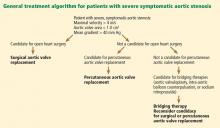
Consequently, the current guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) give surgery a class I indication (evidence or general agreement that the procedure is beneficial, useful, and effective) for symptomatic severe aortic stenosis (Figure 1). This level of recommendation also applies to patients who have severe but asymptomatic aortic stenosis who are undergoing other types of cardiac surgery and also to patients with severe aortic stenosis and left ventricular dysfunction (defined as an ejection fraction < 50%).21
Mr. A was referred for surgical aortic valve replacement, given its clear survival benefit.
CASE 2: SYMPTOMS AND LEFT VENTRICULAR DYSFUNCTION
Ms. B, age 79, has hypertension and hyperlipidemia and now presents to the outpatient department with worsening shortness of breath and chest discomfort. Electrocardiography shows significant left ventricular hypertrophy and abnormal repolarization. Left heart catheterization reveals mild nonobstructive coronary artery disease.
Echocardiography reveals an ejection fraction of 25%, severe left ventricular hypertrophy, and global hypokinesis. The aortic valve leaflets appear heavily calcified, with restricted motion. The peak and mean gradients across the aortic valve are 40 and 28 mm Hg, and the valve area is 0.8 cm2. Right heart catheterization shows a cardiac output of 3.1 L/min.
Does this patient’s aortic stenosis account for her clinical presentation?
Managing patients who have suspected severe aortic stenosis, left ventricular dysfunction, and low aortic valve gradients can be challenging. Although data for surgical intervention are not as robust for these patient subsets as for patients like Mr. A, several case series have suggested that survival in these patients is significantly better with surgery than with medical therapy alone.22–27
Specific factors predict whether patients with ventricular dysfunction and low gradients will benefit from aortic valve replacement. Dobutamine stress echocardiography is helpful in distinguishing true severe aortic stenosis from “pseudostenosis,” in which leaflet motion is restricted due to primary cardiomyopathy and low flow. Distinguishing between true aortic stenosis and pseudostenosis is of paramount value, as surgery is associated with improved long-term outcomes in patients with true aortic stenosis (even though they are at higher surgical risk), whereas those with pseudostenosis will not benefit from surgery.28–31
Infusion of dobutamine increases the flow across the aortic valve (if the left ventricle has contractile reserve; more on this below), and an increasing valve area with increasing doses of dobutamine is consistent with pseudostenosis. In this situation, treatment of the underlying cardiomyopathy is indicated as opposed to replacement of the aortic valve (Figure 2).
Contractile reserve is defined as an increase in stroke volume (> 20%), valvular gradient (> 10 mm Hg), or peak velocity (> 0.6 m/s) with peak dobutamine infusion. The presence of contractile reserve in patients with aortic stenosis identifies a high-risk group that benefits from aortic valve replacement (Figure 2).
Treatment of patients who have inadequate reserve is controversial. In the absence of contractile reserve, an adjunct imaging study such as computed tomography may be of value in detecting calcified valve leaflets, as the presence of calcium is associated with true aortic stenosis. Comorbid conditions should be taken into account as well, given the higher surgical risk in this patient subset, as aortic valve replacement in this already high-risk group of patients might be futile in some cases.
The ACC/AHA guidelines now give dobutamine stress echocardiography a class IIa indication (meaning the weight of the evidence or opinion is in favor of usefulness or efficacy) for determination of contractile reserve and valvular stenosis for patients with an ejection fraction of 30% or less or a mean gradient of 40 mm Hg or less.21
Ms. B underwent dobutamine stress echocardiography. It showed increases in ejection fraction, stroke volume, and transvalvular gradients, indicating that she did have contractile reserve and true severe aortic stenosis. Consequently, she was referred for surgical aortic valve replacement.
CASE 3: MODERATE STENOSIS AND THREE-VESSEL CORONARY ARTERY DISEASE
Mr. C, age 81, has hypertension and hyperlipidemia. He now presents to the emergency department with chest discomfort that began suddenly, awakening him from sleep. His presenting electrocardiogram shows nonspecific changes, and he is diagnosed with non-ST-elevation myocardial infarction. He undergoes left heart catheterization, which reveals severe three-vessel coronary artery disease.
Echocardiography reveals an ejection fraction of 55% and aortic stenosis, with an aortic valve area of 1.2 cm2, a peak gradient of 44 mm Hg, and a mean gradient of 28 mm Hg.
How would you manage his aortic stenosis?
Moderate aortic stenosis in a patient who needs surgery for severe triple-vessel coronary artery disease, other valve diseases, or aortic disease raises the question of whether aortic valve replacement should be performed in conjunction with these surgeries. Although these patients would not otherwise qualify for aortic valve replacement, the fact that they will undergo a procedure that will expose them to the risks associated with open heart surgery makes them reasonable candidates. Even if the patient does not need aortic valve replacement right now, aortic stenosis progresses at a predictable rate—the valve area decreases by a mean of 0.1 cm2/year and the gradients increase by 7 mm Hg/year. Therefore, clinical judgment should be exercised so that the patient will not need to undergo open heart surgery again in the near future.
The ACC/AHA guidelines recommend aortic valve replacement for patients with moderate aortic stenosis undergoing coronary artery bypass grafting or surgery on the aorta or other heart valves, giving it a class IIa indication.21 This recommendation is based on several retrospective case series that evaluated survival, the need for reoperation for aortic valve replacement, or both in patients undergoing coronary artery bypass grafting.32–35
No data exist, however, on adding aortic valve replacement to coronary artery bypass grafting in cases of mild aortic stenosis. As a result, it is controversial and carries a class IIb recommendation (meaning that its usefulness or efficacy is less well established). The ACC/AHA guidelines state that aortic valve replacement “may be considered” in patients undergoing coronary artery bypass grafting who have mild aortic stenosis (mean gradient < 30 mm Hg or jet velocity < 3 m/s) when there is evidence, such as moderate or severe valve calcification, that progression may be rapid (level of evidence C: based only on consensus opinion of experts, case studies or standard of care).21
Mr. C, who has moderate aortic stenosis, underwent aortic valve replacement in conjunction with three-vessel bypass grafting.
CASE 4: ASYMPTOMATIC BUT SEVERE STENOSIS
Mr. D, age 74, has hypertension, hyperlipidemia, and aortic stenosis. He now presents to the outpatient department for his annual echocardiogram to follow his aortic stenosis. He has a sedentary lifestyle but feels well performing activities of daily living. He denies dyspnea on exertion, chest pain, or syncope.
His echocardiogram reveals an effective aortic valve area of 0.7 cm2, peak gradient 90 mm Hg, and mean gradient 70 mm Hg. There is evidence of severe left ventricular hypertrophy, and the valve leaflets show bulky calcification and severe restriction. An echocardiogram performed at the same institution a year earlier revealed gradients of 60 and 40 mm Hg.
Blood is drawn for laboratory tests, including N-terminal pro-brain natriuretic peptide, which is 350 pg/mL (reference range for his age < 125 pg/mL). He is referred for a treadmill stress test, which elicits symptoms at a moderate activity level.
How would you manage his aortic stenosis?
Aortic valve replacement can be considered in patients who have asymptomatic but severe aortic stenosis with preserved left ventricular function (class IIb indication).21
Clinical assessment of asymptomatic aortic stenosis can be challenging, however, as patients may underreport their symptoms or decrease their activity levels to avoid symptoms. Exercise testing in such patients can elicit symptoms, unmask diminished exercise capacity, and help determine if they should be referred for surgery.36,37 Natriuretic peptide levels have been shown to correlate with the severity of aortic stenosis,38,39 and more importantly, to help predict symptom onset, cardiac death, and need for aortic valve replacement.40–42
Some patients with asymptomatic but severe aortic stenosis are at higher risk of morbidity and death. High-risk subsets include patients with rapid progression of aortic stenosis and those with critical aortic stenosis characterized by an aortic valve area less than 0.60 cm2, mean gradient greater than 60 mm Hg, and jet velocity greater than 5.0 m/s. It is reasonable to offer these patients surgery if their expected operative mortality risk is less than 1.0%.21
Mr. D has evidence of rapid progression as defined by an increase in aortic jet velocity of more than 0.3 m/s/year. He is at low surgical risk and was referred for elective aortic valve replacement.
CASE 5: TOO FRAIL FOR SURGERY
Mr. E, age 84, has severe aortic stenosis (valve area 0.6 cm2, peak and mean gradients of 88 and 56 mm Hg), coronary artery disease status post coronary artery bypass grafting, moderate chronic obstructive pulmonary disease (forced expiratory volume in 1 second 0.8 L), chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, hyperlipidemia, and diabetes mellitus. He has preserved left ventricular function. He presents to the outpatient department with worsening shortness of breath and peripheral edema over the past several months. Your impression is that he is very frail. How would you manage Mr. E’s aortic stenosis?
Advances in surgical techniques and perioperative management over the years have enabled higher-risk patients to undergo surgical aortic valve replacement with excellent out-comes.18–20,43 Yet many patients still cannot undergo surgery because their risk is too high. Patients ineligible for surgery have traditionally been treated medically—with poor out-comes—or with balloon aortic valvuloplasty to palliate symptoms.
Transcatheter aortic valve replacement, approved by the US Food and Drug Administration (FDA) in 2011, now provides another option for these patients. In this procedure, a bioprosthetic valve mounted on a metal frame is implanted over the native stenotic valve.
Currently, the only FDA-approved and commercially available valve in the United States is the Edwards SAPIEN valve, which has bovine pericardial tissue leaflets fixed to a balloon-expandable stainless steel frame (Figure 3). In the Placement of Aortic Transcatheter Valves (PARTNER) trial,15 patients who could not undergo surgery who underwent transcatheter replacement with this valve had a significantly better survival rate than patients treated medically.15,17 Use of this valve has also been compared against conventional surgical aortic valve replacement in high-risk patients and was found to have similar long-term outcomes (Figure 4).16 It was on the basis of this trial that this valve was granted approval for patients who cannot undergo surgery.
The standard of care for high-risk patients remains surgical aortic valve replacement, although it remains to be seen whether transcatheter replacement will be made available as well to patients eligible for surgery in the near future. There are currently no randomized data for transcatheter aortic valve replacement in patients at moderate to low surgical risk, and these patients should not be considered for this procedure.
Although the initial studies are encouraging for patients who cannot undergo surgery and who are at high risk without it, several issues and concerns remain. Importantly, the long-term durability of the transcatheter valve and longer-term outcomes remain unknown. Furthermore, the risk of vascular complications remains high (10% to 15%), dictating the need for careful patient selection. There are also concerns about the risks of stroke and of paravalvular aortic insufficiency. These issues are being investigated and addressed, however, and we hope that with increasing operator experience and improvements in the technique, outcomes will be improved.
Which approach for transcatheter aortic valve replacement?
There are several considerations in determining a patient’s eligibility for transcatheter aortic valve replacement.
Initially, these valves were placed by a transvenous, transseptal approach, but now retrograde placement through the femoral artery has become standard. In this procedure, the device is advanced retrograde from the femoral artery through the aorta and placed across the native aortic valve under fluoroscopic and echocardiographic guidance.
Patients who are not eligible for transfemoral placement because of severe atherosclerosis, tortuosity, or ectasia of the iliofemoral artery or aorta can still undergo percutaneous treatment with a transapical approach. This is a hybrid surgical-transcatheter approach in which the valve is delivered through a sheath placed by left ventricular apical puncture.17,44
A newer approach gaining popularity is the transaortic technique, in which the ascending aorta is accessed directly through a ministernotomy and the delivery sheath is placed with a direct puncture. Other approaches are through the axillary and subclavian arteries.
Other valves are under development
Several other valves are under development and will likely change the landscape of transcatheter aortic valve replacement with improving outcomes. Valves that are available in the United States are shown in Figure 3. The CoreValve, consisting of porcine pericardial leaflets mounted on a self-expanding nitinol stent, is currently being studied in a trial in the United States, and the manufacturer (Medtronic) will seek approval when results are complete in the near future.
Mr. E was initially referred for surgery, but when deemed to be unable to undergo surgery was found to be a good candidate for transcatheter aortic valve replacement.
CASE 6: LIFE-LIMITING COMORBID ILLNESS
Mr. F, age 77, has multiple problems: severe aortic stenosis (aortic valve area 0.6 cm2; peak and mean gradients of 92 and 59 mm Hg), stage IV pancreatic cancer, coronary artery disease status post coronary artery bypass grafting, chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, and hyperlipidemia. He presents to the outpatient department with shortness of breath at rest, orthopnea, effort intolerance, and peripheral edema over the past several months.
On physical examination rales in both lung bases can be heard. Left heart catheterization shows patent bypass grafts.
How would you manage Mr. F’s aortic stenosis?
Aortic valve replacement is not considered an option in patients with noncardiac illnesses and comorbidities that are life-limiting in the near term. Under these circumstances, aortic valvuloplasty can be offered as a means of palliating symptoms or, if the comorbid conditions can be modified, as a bridge to more definitive treatment with aortic valve replacement.
Since first described in 1986,45 percutaneous aortic valvuloplasty has been studied in several case series and registries, with consistent findings. Acutely, it increases the valve area and lessens the gradients across the valve, relieving symptoms. The risk of death during the procedure ranged from 3% to 13.5% in several case series, with a 30-day survival rate greater than 85%.46 However, the hemodynamic and symptomatic improvement is only short-term, as valve area and gradients gradually worsen within several months.47,48 Consequently, balloon valvuloplasty is considered a palliative approach.
Mr. F has a potentially life-limiting illness, ie, cancer, which would make him a candidate for aortic valvuloplasty rather than replacement. He can be referred for evaluation for this procedure in hopes of palliating his symptoms by relieving his dyspnea and improving his quality of life.
CASE 7: HEMODYNAMIC INSTABILITY
Mr. G, age 87, is scheduled for surgical aortic valve replacement because of severe aortic stenosis (valve area 0.5 cm2, peak and mean gradients 89 and 45 mm Hg) with an ejection fraction of 30%.
Two weeks before his scheduled surgery he presents to the emergency department with worsening fluid overload and increasing shortness of breath. His initial laboratory work shows new-onset renal failure, and he has signs of hypoperfusion on physical examination. He is transferred to the cardiac intensive care unit for further care.
How would you manage his aortic stenosis?
Patients with decompensated aortic stenosis and hemodynamic instability are at extreme risk during surgery. Medical stabilization beforehand may mitigate the risks associated with surgical or transcatheter aortic valve replacement. Aortic valvuloplasty, treatment with sodium nitroprusside, and support with intra-aortic balloon counterpulsation may help stabilize patients in this “low-output” setting.
Sodium nitroprusside has long been used in low-output states. By relaxing vascular smooth muscle, it leads to increased venous capacitance, decreasing preload and congestion. It also decreases systemic vascular resistance with a subsequent decrease in afterload, which in turn improves systolic emptying. Together, these effects reduce systolic and diastolic wall stress, lower myocardial oxygen consumption, and ultimately increase cardiac output.49,50
These theoretical benefits translate to clinical improvement and increased cardiac output, as shown in a case series of 25 patients with severe aortic stenosis and left ventricular systolic dysfunction (ejection fraction 35%) presenting in a low-output state in the absence of hypotension.51 These findings have led to a ACC/AHA recommendation for the use of sodium nitroprusside in patients who have severe aortic stenosis presenting in low-output state with decompensated heart failure.21
Intra-aortic balloon counterpulsation, introduced in 1968, has been used in several clinical settings, including acute coronary syndromes, intractable ventricular arrhythmias, and refractory heart failure, and for support of hemodynamics in the perioperative setting. Its role in managing ventricular septal rupture and acute mitral regurgitation is well established. It reliably reduces afterload and improves coronary perfusion, augmenting the cardiac output. This in turn leads to improved systemic perfusion, which can buy time for a critically ill patient during which the primary disease process is addressed.
Recently, a case series in which intraaortic balloon counterpulsation devices were placed in patients with severe aortic stenosis and cardiogenic shock showed findings similar to those with sodium nitroprusside infusion. Specifically, their use was associated with improved cardiac indices and filling pressures with a decrease in systemic vascular resistance. These changes have led to increased cardiac performance, resulting in better systemic perfusion.52 Thus, intra-aortic balloon counterpulsation can be an option for stabilizing patients with severe aortic stenosis and cardiogenic shock.
Mr. G was treated with sodium nitroprusside and intravenous diuretics. He achieved symptomatic relief and his renal function returned to baseline. He subsequently underwent aortic valve replacement during the hospitalization.
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009; 373:956–966.
- Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993; 21:1220–1225.
- Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol 1989; 13:545–550.
- Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997; 95:2262–2270.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J 1987; 8:471–483.
- Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J 1988; 9(suppl E):57–64.
- Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation 2001; 104:2205–2209.
- Cowell SJ, Newby DE, Prescott RJ, et al; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005; 352:2389–2397.
- Rossebø AB, Pedersen TR, Boman K, et al; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008; 359:1343–1356.
- Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 2007; 49:554–561.
- Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation 2004; 110:1291–1295.
- O’Brien KD, Probstfield JL, Caulked MT, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med 2005; 165:858–862.
- Lindblom D, Lindblom U, Qvist J, Lundström H. Long-term relative survival rates after heart valve replacement. J Am Coll Cardiol 1990; 15:566–573.
- Makkar RR, Fontana G P, Jilaihawi H, et al; PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012; 366:1696–1704.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Di Eusanio M, Fortuna D, Cristell D, et al; RERIC (Emilia Romagna Cardiac Surgery Registry) Investigators. Contemporary outcomes of conventional aortic valve replacement in 638 octogenarians: insights from an Italian Regional Cardiac Surgery Registry (RERIC). Eur J Cardiothorac Surg 2012; 41:1247–1252.
- Di Eusanio M, Fortuna D, De Palma R, et al. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg 2011; 141:940–947.
- Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of the Society of Thoracic Surgeons. Ann Thorac Surg 1999; 67:943–951.
- Bonow RO, Carabello BA, Chatterjee K, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52:e1–e142.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115:2856–2864.
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005; 91:1324–1329.
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997; 95:2395–2400.
- Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation 2000; 101:1940–1946.
- Pereira JJ, Lauer MS, Bashir M, et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 2002; 39:1356–1363.
- Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008; 86:1781–1789.
- Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol 2001; 37:2101–2107.
- Monin JL, Quéré J P, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108:319–324.
- Zuppiroli A, Mori F, Olivotto I, Castelli G, Favilli S, Dolara A. Therapeutic implications of contractile reserve elicited by dobutamine echocardiography in symptomatic, low-gradient aortic stenosis. Ital Heart J 2003; 4:264–270.
- Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865–1873.
- Ahmed AA, Graham AN, Lovell D, O’Kane HO. Management of mild to moderate aortic valve disease during coronary artery bypass grafting. Eur J Cardiothorac Surg 2003; 24:535–539.
- Verhoye J P, Merlicco F, Sami IM, et al. Aortic valve replacement for aortic stenosis after previous coronary artery bypass grafting: could early reoperation be prevented? J Heart Valve Dis 2006; 15:474–478.
- Hochrein J, Lucke JC, Harrison JK, et al. Mortality and need for reoperation in patients with mild-to-moderate asymptomatic aortic valve disease undergoing coronary artery bypass graft alone. Am Heart J 1999; 138:791–797.
- Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735–742.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Weber M, Hausen M, Arnold R, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide for conservatively and surgically treated patients with aortic valve stenosis. Heart 2006; 92:1639–1644.
- Gerber IL, Stewart RA, Legget ME, et al. Increased plasma natriuretic peptide levels refect symptom onset in aortic stenosis. Circulation 2003; 107:1884–1890.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lancellotti P, Moonen M, Magne J, et al. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol 2010; 105:383–388.
- Langanay T, Flécher E, Fouquet O, et al. Aortic valve replacement in the elderly: the real life. Ann Thorac Surg 2012; 93:70–77.
- Christofferson RD, Kapadia SR, Rajagopal V, Tuzcu EM. Emerging transcatheter therapies for aortic and mitral disease. Heart 2009; 95:148–155.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Bernard Y, Etievent J, Mourand JL, et al. Long-term results of percutaneous aortic valvuloplasty compared with aortic valve replacement in patients more than 75 years old. J Am Coll Cardiol 1992; 20:796–801.
- Elkayam U, Janmohamed M, Habib M, Hatamizadeh P. Vasodilators in the management of acute heart failure. Crit Care Med 2008; 36(suppl 1):S95–S105.
- Popovic ZB, Khot UN, Novaro GM, et al. Effects of sodium nitroprusside in aortic stenosis associated with severe heart failure: pressure-volume loop analysis using a numerical model. Am J Physiol Heart Circ Physiol 2005; 288:H416–H423.
- Khot UN, Novaro GM, Popovic ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med 2003; 348:1756–1763.
- Aksoy O, Yousefzai R, Singh D, et al. Cardiogenic shock in the setting of severe aortic stenosis: role of intra-aortic balloon pump support. Heart 2011; 97:838–843.
Surgical aortic valve replacement remains the gold standard treatment for symptomatic aortic valve stenosis in patients at low or moderate risk of surgical complications. But this is a disease of the elderly, many of whom are too frail or too sick to undergo surgery.
Now, patients who cannot undergo this surgery can be offered the less invasive option of transcatheter aortic valve replacement. Balloon valvuloplasty, sodium nitroprusside, and intra-aortic balloon counterpulsation can buy time for ill patients while more permanent mechanical interventions are being considered.
In this review, we will present several cases that highlight management choices for patients with severe aortic stenosis.
A PROGRESSIVE DISEASE OF THE ELDERLY
Aortic stenosis is the most common acquired valvular disease in the United States, and its incidence and prevalence are rising as the population ages. Epidemiologic studies suggest that 2% to 7% of all patients over age 65 have it.1,2
The natural history of the untreated disease is well established, with several case series showing an average decrease of 0.1 cm2 per year in aortic valve area and an increase of 7 mm Hg per year in the pressure gradient across the valve once the diagnosis is made.3,4 Development of angina, syncope, or heart failure is associated with adverse clinical outcomes, including death, and warrants prompt intervention with aortic valve replacement.5–7 Without intervention, the mortality rates reach as high as 75% in 3 years once symptoms develop.
Statins, bisphosphonates, and angiotensin-converting enzyme inhibitors have been used in attempts to slow or reverse the progression of aortic stenosis. However, studies of these drugs have had mixed results, and no definitive benefit has been shown.8–13 Surgical aortic valve replacement, on the other hand, normalizes the life expectancy of patients with aortic stenosis to that of age- and sex-matched controls and remains the gold standard therapy for patients who have symptoms.14
Traditionally, valve replacement has involved open heart surgery, since it requires direct visualization of the valve while the patient is on cardiopulmonary bypass. Unfortunately, many patients have multiple comorbid conditions and therefore are not candidates for open heart surgery. Options for these patients include aortic valvuloplasty and transcatheter aortic valve replacement. While there is considerable experience with aortic valvuloplasty, transcatheter aortic valve replacement is relatively new. In large randomized trials and registries, the transcatheter procedure has been shown to significantly improve long-term survival compared with medical management alone in inoperable patients and to have benefit similar to that of surgery in the high-risk population.15–17
CASE 1: SEVERE, SYMPTOMATIC STENOSIS IN A GOOD SURGICAL CANDIDATE
Mr. A, age 83, presents with shortness of breath and peripheral edema that have been worsening over the past several months. His pulse rate is 64 beats per minute and his blood pressure is 110/90 mm Hg. Auscultation reveals an absent aortic second heart sound with a late peaking systolic murmur that increases with expiration.
On echocardiography, his left ventricular ejection fraction is 55%, peak transaortic valve gradient 88 mm Hg, mean gradient 60 mm Hg, and effective valve area 0.6 cm2. He undergoes catheterization of the left side of his heart, which shows normal coronary arteries.
Mr. A also has hypertension and hyperlipidemia; his renal and pulmonary functions are normal.
How would you manage Mr. A’s aortic stenosis?
Symptomatic aortic stenosis leads to adverse clinical outcomes if managed medically without mechanical intervention,5–7 but patients who undergo aortic valve replacement have age-corrected postoperative survival rates that are nearly normal.14 Furthermore, thanks to improvements in surgical techniques and perioperative management, surgical mortality rates have decreased significantly in recent years and now range from 1% to 8%.18–20 The accumulated evidence showing clear superiority of a surgical approach over medical therapy has greatly simplified the therapeutic algorithm.21
Consequently, the current guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) give surgery a class I indication (evidence or general agreement that the procedure is beneficial, useful, and effective) for symptomatic severe aortic stenosis (Figure 1). This level of recommendation also applies to patients who have severe but asymptomatic aortic stenosis who are undergoing other types of cardiac surgery and also to patients with severe aortic stenosis and left ventricular dysfunction (defined as an ejection fraction < 50%).21
Mr. A was referred for surgical aortic valve replacement, given its clear survival benefit.
CASE 2: SYMPTOMS AND LEFT VENTRICULAR DYSFUNCTION
Ms. B, age 79, has hypertension and hyperlipidemia and now presents to the outpatient department with worsening shortness of breath and chest discomfort. Electrocardiography shows significant left ventricular hypertrophy and abnormal repolarization. Left heart catheterization reveals mild nonobstructive coronary artery disease.
Echocardiography reveals an ejection fraction of 25%, severe left ventricular hypertrophy, and global hypokinesis. The aortic valve leaflets appear heavily calcified, with restricted motion. The peak and mean gradients across the aortic valve are 40 and 28 mm Hg, and the valve area is 0.8 cm2. Right heart catheterization shows a cardiac output of 3.1 L/min.
Does this patient’s aortic stenosis account for her clinical presentation?
Managing patients who have suspected severe aortic stenosis, left ventricular dysfunction, and low aortic valve gradients can be challenging. Although data for surgical intervention are not as robust for these patient subsets as for patients like Mr. A, several case series have suggested that survival in these patients is significantly better with surgery than with medical therapy alone.22–27
Specific factors predict whether patients with ventricular dysfunction and low gradients will benefit from aortic valve replacement. Dobutamine stress echocardiography is helpful in distinguishing true severe aortic stenosis from “pseudostenosis,” in which leaflet motion is restricted due to primary cardiomyopathy and low flow. Distinguishing between true aortic stenosis and pseudostenosis is of paramount value, as surgery is associated with improved long-term outcomes in patients with true aortic stenosis (even though they are at higher surgical risk), whereas those with pseudostenosis will not benefit from surgery.28–31
Infusion of dobutamine increases the flow across the aortic valve (if the left ventricle has contractile reserve; more on this below), and an increasing valve area with increasing doses of dobutamine is consistent with pseudostenosis. In this situation, treatment of the underlying cardiomyopathy is indicated as opposed to replacement of the aortic valve (Figure 2).
Contractile reserve is defined as an increase in stroke volume (> 20%), valvular gradient (> 10 mm Hg), or peak velocity (> 0.6 m/s) with peak dobutamine infusion. The presence of contractile reserve in patients with aortic stenosis identifies a high-risk group that benefits from aortic valve replacement (Figure 2).
Treatment of patients who have inadequate reserve is controversial. In the absence of contractile reserve, an adjunct imaging study such as computed tomography may be of value in detecting calcified valve leaflets, as the presence of calcium is associated with true aortic stenosis. Comorbid conditions should be taken into account as well, given the higher surgical risk in this patient subset, as aortic valve replacement in this already high-risk group of patients might be futile in some cases.
The ACC/AHA guidelines now give dobutamine stress echocardiography a class IIa indication (meaning the weight of the evidence or opinion is in favor of usefulness or efficacy) for determination of contractile reserve and valvular stenosis for patients with an ejection fraction of 30% or less or a mean gradient of 40 mm Hg or less.21
Ms. B underwent dobutamine stress echocardiography. It showed increases in ejection fraction, stroke volume, and transvalvular gradients, indicating that she did have contractile reserve and true severe aortic stenosis. Consequently, she was referred for surgical aortic valve replacement.
CASE 3: MODERATE STENOSIS AND THREE-VESSEL CORONARY ARTERY DISEASE
Mr. C, age 81, has hypertension and hyperlipidemia. He now presents to the emergency department with chest discomfort that began suddenly, awakening him from sleep. His presenting electrocardiogram shows nonspecific changes, and he is diagnosed with non-ST-elevation myocardial infarction. He undergoes left heart catheterization, which reveals severe three-vessel coronary artery disease.
Echocardiography reveals an ejection fraction of 55% and aortic stenosis, with an aortic valve area of 1.2 cm2, a peak gradient of 44 mm Hg, and a mean gradient of 28 mm Hg.
How would you manage his aortic stenosis?
Moderate aortic stenosis in a patient who needs surgery for severe triple-vessel coronary artery disease, other valve diseases, or aortic disease raises the question of whether aortic valve replacement should be performed in conjunction with these surgeries. Although these patients would not otherwise qualify for aortic valve replacement, the fact that they will undergo a procedure that will expose them to the risks associated with open heart surgery makes them reasonable candidates. Even if the patient does not need aortic valve replacement right now, aortic stenosis progresses at a predictable rate—the valve area decreases by a mean of 0.1 cm2/year and the gradients increase by 7 mm Hg/year. Therefore, clinical judgment should be exercised so that the patient will not need to undergo open heart surgery again in the near future.
The ACC/AHA guidelines recommend aortic valve replacement for patients with moderate aortic stenosis undergoing coronary artery bypass grafting or surgery on the aorta or other heart valves, giving it a class IIa indication.21 This recommendation is based on several retrospective case series that evaluated survival, the need for reoperation for aortic valve replacement, or both in patients undergoing coronary artery bypass grafting.32–35
No data exist, however, on adding aortic valve replacement to coronary artery bypass grafting in cases of mild aortic stenosis. As a result, it is controversial and carries a class IIb recommendation (meaning that its usefulness or efficacy is less well established). The ACC/AHA guidelines state that aortic valve replacement “may be considered” in patients undergoing coronary artery bypass grafting who have mild aortic stenosis (mean gradient < 30 mm Hg or jet velocity < 3 m/s) when there is evidence, such as moderate or severe valve calcification, that progression may be rapid (level of evidence C: based only on consensus opinion of experts, case studies or standard of care).21
Mr. C, who has moderate aortic stenosis, underwent aortic valve replacement in conjunction with three-vessel bypass grafting.
CASE 4: ASYMPTOMATIC BUT SEVERE STENOSIS
Mr. D, age 74, has hypertension, hyperlipidemia, and aortic stenosis. He now presents to the outpatient department for his annual echocardiogram to follow his aortic stenosis. He has a sedentary lifestyle but feels well performing activities of daily living. He denies dyspnea on exertion, chest pain, or syncope.
His echocardiogram reveals an effective aortic valve area of 0.7 cm2, peak gradient 90 mm Hg, and mean gradient 70 mm Hg. There is evidence of severe left ventricular hypertrophy, and the valve leaflets show bulky calcification and severe restriction. An echocardiogram performed at the same institution a year earlier revealed gradients of 60 and 40 mm Hg.
Blood is drawn for laboratory tests, including N-terminal pro-brain natriuretic peptide, which is 350 pg/mL (reference range for his age < 125 pg/mL). He is referred for a treadmill stress test, which elicits symptoms at a moderate activity level.
How would you manage his aortic stenosis?
Aortic valve replacement can be considered in patients who have asymptomatic but severe aortic stenosis with preserved left ventricular function (class IIb indication).21
Clinical assessment of asymptomatic aortic stenosis can be challenging, however, as patients may underreport their symptoms or decrease their activity levels to avoid symptoms. Exercise testing in such patients can elicit symptoms, unmask diminished exercise capacity, and help determine if they should be referred for surgery.36,37 Natriuretic peptide levels have been shown to correlate with the severity of aortic stenosis,38,39 and more importantly, to help predict symptom onset, cardiac death, and need for aortic valve replacement.40–42
Some patients with asymptomatic but severe aortic stenosis are at higher risk of morbidity and death. High-risk subsets include patients with rapid progression of aortic stenosis and those with critical aortic stenosis characterized by an aortic valve area less than 0.60 cm2, mean gradient greater than 60 mm Hg, and jet velocity greater than 5.0 m/s. It is reasonable to offer these patients surgery if their expected operative mortality risk is less than 1.0%.21
Mr. D has evidence of rapid progression as defined by an increase in aortic jet velocity of more than 0.3 m/s/year. He is at low surgical risk and was referred for elective aortic valve replacement.
CASE 5: TOO FRAIL FOR SURGERY
Mr. E, age 84, has severe aortic stenosis (valve area 0.6 cm2, peak and mean gradients of 88 and 56 mm Hg), coronary artery disease status post coronary artery bypass grafting, moderate chronic obstructive pulmonary disease (forced expiratory volume in 1 second 0.8 L), chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, hyperlipidemia, and diabetes mellitus. He has preserved left ventricular function. He presents to the outpatient department with worsening shortness of breath and peripheral edema over the past several months. Your impression is that he is very frail. How would you manage Mr. E’s aortic stenosis?
Advances in surgical techniques and perioperative management over the years have enabled higher-risk patients to undergo surgical aortic valve replacement with excellent out-comes.18–20,43 Yet many patients still cannot undergo surgery because their risk is too high. Patients ineligible for surgery have traditionally been treated medically—with poor out-comes—or with balloon aortic valvuloplasty to palliate symptoms.
Transcatheter aortic valve replacement, approved by the US Food and Drug Administration (FDA) in 2011, now provides another option for these patients. In this procedure, a bioprosthetic valve mounted on a metal frame is implanted over the native stenotic valve.
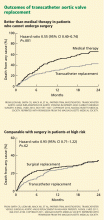
Currently, the only FDA-approved and commercially available valve in the United States is the Edwards SAPIEN valve, which has bovine pericardial tissue leaflets fixed to a balloon-expandable stainless steel frame (Figure 3). In the Placement of Aortic Transcatheter Valves (PARTNER) trial,15 patients who could not undergo surgery who underwent transcatheter replacement with this valve had a significantly better survival rate than patients treated medically.15,17 Use of this valve has also been compared against conventional surgical aortic valve replacement in high-risk patients and was found to have similar long-term outcomes (Figure 4).16 It was on the basis of this trial that this valve was granted approval for patients who cannot undergo surgery.
The standard of care for high-risk patients remains surgical aortic valve replacement, although it remains to be seen whether transcatheter replacement will be made available as well to patients eligible for surgery in the near future. There are currently no randomized data for transcatheter aortic valve replacement in patients at moderate to low surgical risk, and these patients should not be considered for this procedure.
Although the initial studies are encouraging for patients who cannot undergo surgery and who are at high risk without it, several issues and concerns remain. Importantly, the long-term durability of the transcatheter valve and longer-term outcomes remain unknown. Furthermore, the risk of vascular complications remains high (10% to 15%), dictating the need for careful patient selection. There are also concerns about the risks of stroke and of paravalvular aortic insufficiency. These issues are being investigated and addressed, however, and we hope that with increasing operator experience and improvements in the technique, outcomes will be improved.
Which approach for transcatheter aortic valve replacement?
There are several considerations in determining a patient’s eligibility for transcatheter aortic valve replacement.
Initially, these valves were placed by a transvenous, transseptal approach, but now retrograde placement through the femoral artery has become standard. In this procedure, the device is advanced retrograde from the femoral artery through the aorta and placed across the native aortic valve under fluoroscopic and echocardiographic guidance.
Patients who are not eligible for transfemoral placement because of severe atherosclerosis, tortuosity, or ectasia of the iliofemoral artery or aorta can still undergo percutaneous treatment with a transapical approach. This is a hybrid surgical-transcatheter approach in which the valve is delivered through a sheath placed by left ventricular apical puncture.17,44
A newer approach gaining popularity is the transaortic technique, in which the ascending aorta is accessed directly through a ministernotomy and the delivery sheath is placed with a direct puncture. Other approaches are through the axillary and subclavian arteries.
Other valves are under development
Several other valves are under development and will likely change the landscape of transcatheter aortic valve replacement with improving outcomes. Valves that are available in the United States are shown in Figure 3. The CoreValve, consisting of porcine pericardial leaflets mounted on a self-expanding nitinol stent, is currently being studied in a trial in the United States, and the manufacturer (Medtronic) will seek approval when results are complete in the near future.
Mr. E was initially referred for surgery, but when deemed to be unable to undergo surgery was found to be a good candidate for transcatheter aortic valve replacement.
CASE 6: LIFE-LIMITING COMORBID ILLNESS
Mr. F, age 77, has multiple problems: severe aortic stenosis (aortic valve area 0.6 cm2; peak and mean gradients of 92 and 59 mm Hg), stage IV pancreatic cancer, coronary artery disease status post coronary artery bypass grafting, chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, and hyperlipidemia. He presents to the outpatient department with shortness of breath at rest, orthopnea, effort intolerance, and peripheral edema over the past several months.
On physical examination rales in both lung bases can be heard. Left heart catheterization shows patent bypass grafts.
How would you manage Mr. F’s aortic stenosis?
Aortic valve replacement is not considered an option in patients with noncardiac illnesses and comorbidities that are life-limiting in the near term. Under these circumstances, aortic valvuloplasty can be offered as a means of palliating symptoms or, if the comorbid conditions can be modified, as a bridge to more definitive treatment with aortic valve replacement.
Since first described in 1986,45 percutaneous aortic valvuloplasty has been studied in several case series and registries, with consistent findings. Acutely, it increases the valve area and lessens the gradients across the valve, relieving symptoms. The risk of death during the procedure ranged from 3% to 13.5% in several case series, with a 30-day survival rate greater than 85%.46 However, the hemodynamic and symptomatic improvement is only short-term, as valve area and gradients gradually worsen within several months.47,48 Consequently, balloon valvuloplasty is considered a palliative approach.
Mr. F has a potentially life-limiting illness, ie, cancer, which would make him a candidate for aortic valvuloplasty rather than replacement. He can be referred for evaluation for this procedure in hopes of palliating his symptoms by relieving his dyspnea and improving his quality of life.
CASE 7: HEMODYNAMIC INSTABILITY
Mr. G, age 87, is scheduled for surgical aortic valve replacement because of severe aortic stenosis (valve area 0.5 cm2, peak and mean gradients 89 and 45 mm Hg) with an ejection fraction of 30%.
Two weeks before his scheduled surgery he presents to the emergency department with worsening fluid overload and increasing shortness of breath. His initial laboratory work shows new-onset renal failure, and he has signs of hypoperfusion on physical examination. He is transferred to the cardiac intensive care unit for further care.
How would you manage his aortic stenosis?
Patients with decompensated aortic stenosis and hemodynamic instability are at extreme risk during surgery. Medical stabilization beforehand may mitigate the risks associated with surgical or transcatheter aortic valve replacement. Aortic valvuloplasty, treatment with sodium nitroprusside, and support with intra-aortic balloon counterpulsation may help stabilize patients in this “low-output” setting.
Sodium nitroprusside has long been used in low-output states. By relaxing vascular smooth muscle, it leads to increased venous capacitance, decreasing preload and congestion. It also decreases systemic vascular resistance with a subsequent decrease in afterload, which in turn improves systolic emptying. Together, these effects reduce systolic and diastolic wall stress, lower myocardial oxygen consumption, and ultimately increase cardiac output.49,50
These theoretical benefits translate to clinical improvement and increased cardiac output, as shown in a case series of 25 patients with severe aortic stenosis and left ventricular systolic dysfunction (ejection fraction 35%) presenting in a low-output state in the absence of hypotension.51 These findings have led to a ACC/AHA recommendation for the use of sodium nitroprusside in patients who have severe aortic stenosis presenting in low-output state with decompensated heart failure.21
Intra-aortic balloon counterpulsation, introduced in 1968, has been used in several clinical settings, including acute coronary syndromes, intractable ventricular arrhythmias, and refractory heart failure, and for support of hemodynamics in the perioperative setting. Its role in managing ventricular septal rupture and acute mitral regurgitation is well established. It reliably reduces afterload and improves coronary perfusion, augmenting the cardiac output. This in turn leads to improved systemic perfusion, which can buy time for a critically ill patient during which the primary disease process is addressed.
Recently, a case series in which intraaortic balloon counterpulsation devices were placed in patients with severe aortic stenosis and cardiogenic shock showed findings similar to those with sodium nitroprusside infusion. Specifically, their use was associated with improved cardiac indices and filling pressures with a decrease in systemic vascular resistance. These changes have led to increased cardiac performance, resulting in better systemic perfusion.52 Thus, intra-aortic balloon counterpulsation can be an option for stabilizing patients with severe aortic stenosis and cardiogenic shock.
Mr. G was treated with sodium nitroprusside and intravenous diuretics. He achieved symptomatic relief and his renal function returned to baseline. He subsequently underwent aortic valve replacement during the hospitalization.
Surgical aortic valve replacement remains the gold standard treatment for symptomatic aortic valve stenosis in patients at low or moderate risk of surgical complications. But this is a disease of the elderly, many of whom are too frail or too sick to undergo surgery.
Now, patients who cannot undergo this surgery can be offered the less invasive option of transcatheter aortic valve replacement. Balloon valvuloplasty, sodium nitroprusside, and intra-aortic balloon counterpulsation can buy time for ill patients while more permanent mechanical interventions are being considered.
In this review, we will present several cases that highlight management choices for patients with severe aortic stenosis.
A PROGRESSIVE DISEASE OF THE ELDERLY
Aortic stenosis is the most common acquired valvular disease in the United States, and its incidence and prevalence are rising as the population ages. Epidemiologic studies suggest that 2% to 7% of all patients over age 65 have it.1,2
The natural history of the untreated disease is well established, with several case series showing an average decrease of 0.1 cm2 per year in aortic valve area and an increase of 7 mm Hg per year in the pressure gradient across the valve once the diagnosis is made.3,4 Development of angina, syncope, or heart failure is associated with adverse clinical outcomes, including death, and warrants prompt intervention with aortic valve replacement.5–7 Without intervention, the mortality rates reach as high as 75% in 3 years once symptoms develop.
Statins, bisphosphonates, and angiotensin-converting enzyme inhibitors have been used in attempts to slow or reverse the progression of aortic stenosis. However, studies of these drugs have had mixed results, and no definitive benefit has been shown.8–13 Surgical aortic valve replacement, on the other hand, normalizes the life expectancy of patients with aortic stenosis to that of age- and sex-matched controls and remains the gold standard therapy for patients who have symptoms.14
Traditionally, valve replacement has involved open heart surgery, since it requires direct visualization of the valve while the patient is on cardiopulmonary bypass. Unfortunately, many patients have multiple comorbid conditions and therefore are not candidates for open heart surgery. Options for these patients include aortic valvuloplasty and transcatheter aortic valve replacement. While there is considerable experience with aortic valvuloplasty, transcatheter aortic valve replacement is relatively new. In large randomized trials and registries, the transcatheter procedure has been shown to significantly improve long-term survival compared with medical management alone in inoperable patients and to have benefit similar to that of surgery in the high-risk population.15–17
CASE 1: SEVERE, SYMPTOMATIC STENOSIS IN A GOOD SURGICAL CANDIDATE
Mr. A, age 83, presents with shortness of breath and peripheral edema that have been worsening over the past several months. His pulse rate is 64 beats per minute and his blood pressure is 110/90 mm Hg. Auscultation reveals an absent aortic second heart sound with a late peaking systolic murmur that increases with expiration.
On echocardiography, his left ventricular ejection fraction is 55%, peak transaortic valve gradient 88 mm Hg, mean gradient 60 mm Hg, and effective valve area 0.6 cm2. He undergoes catheterization of the left side of his heart, which shows normal coronary arteries.
Mr. A also has hypertension and hyperlipidemia; his renal and pulmonary functions are normal.
How would you manage Mr. A’s aortic stenosis?
Symptomatic aortic stenosis leads to adverse clinical outcomes if managed medically without mechanical intervention,5–7 but patients who undergo aortic valve replacement have age-corrected postoperative survival rates that are nearly normal.14 Furthermore, thanks to improvements in surgical techniques and perioperative management, surgical mortality rates have decreased significantly in recent years and now range from 1% to 8%.18–20 The accumulated evidence showing clear superiority of a surgical approach over medical therapy has greatly simplified the therapeutic algorithm.21
Consequently, the current guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) give surgery a class I indication (evidence or general agreement that the procedure is beneficial, useful, and effective) for symptomatic severe aortic stenosis (Figure 1). This level of recommendation also applies to patients who have severe but asymptomatic aortic stenosis who are undergoing other types of cardiac surgery and also to patients with severe aortic stenosis and left ventricular dysfunction (defined as an ejection fraction < 50%).21
Mr. A was referred for surgical aortic valve replacement, given its clear survival benefit.
CASE 2: SYMPTOMS AND LEFT VENTRICULAR DYSFUNCTION
Ms. B, age 79, has hypertension and hyperlipidemia and now presents to the outpatient department with worsening shortness of breath and chest discomfort. Electrocardiography shows significant left ventricular hypertrophy and abnormal repolarization. Left heart catheterization reveals mild nonobstructive coronary artery disease.
Echocardiography reveals an ejection fraction of 25%, severe left ventricular hypertrophy, and global hypokinesis. The aortic valve leaflets appear heavily calcified, with restricted motion. The peak and mean gradients across the aortic valve are 40 and 28 mm Hg, and the valve area is 0.8 cm2. Right heart catheterization shows a cardiac output of 3.1 L/min.
Does this patient’s aortic stenosis account for her clinical presentation?
Managing patients who have suspected severe aortic stenosis, left ventricular dysfunction, and low aortic valve gradients can be challenging. Although data for surgical intervention are not as robust for these patient subsets as for patients like Mr. A, several case series have suggested that survival in these patients is significantly better with surgery than with medical therapy alone.22–27
Specific factors predict whether patients with ventricular dysfunction and low gradients will benefit from aortic valve replacement. Dobutamine stress echocardiography is helpful in distinguishing true severe aortic stenosis from “pseudostenosis,” in which leaflet motion is restricted due to primary cardiomyopathy and low flow. Distinguishing between true aortic stenosis and pseudostenosis is of paramount value, as surgery is associated with improved long-term outcomes in patients with true aortic stenosis (even though they are at higher surgical risk), whereas those with pseudostenosis will not benefit from surgery.28–31
Infusion of dobutamine increases the flow across the aortic valve (if the left ventricle has contractile reserve; more on this below), and an increasing valve area with increasing doses of dobutamine is consistent with pseudostenosis. In this situation, treatment of the underlying cardiomyopathy is indicated as opposed to replacement of the aortic valve (Figure 2).
Contractile reserve is defined as an increase in stroke volume (> 20%), valvular gradient (> 10 mm Hg), or peak velocity (> 0.6 m/s) with peak dobutamine infusion. The presence of contractile reserve in patients with aortic stenosis identifies a high-risk group that benefits from aortic valve replacement (Figure 2).
Treatment of patients who have inadequate reserve is controversial. In the absence of contractile reserve, an adjunct imaging study such as computed tomography may be of value in detecting calcified valve leaflets, as the presence of calcium is associated with true aortic stenosis. Comorbid conditions should be taken into account as well, given the higher surgical risk in this patient subset, as aortic valve replacement in this already high-risk group of patients might be futile in some cases.
The ACC/AHA guidelines now give dobutamine stress echocardiography a class IIa indication (meaning the weight of the evidence or opinion is in favor of usefulness or efficacy) for determination of contractile reserve and valvular stenosis for patients with an ejection fraction of 30% or less or a mean gradient of 40 mm Hg or less.21
Ms. B underwent dobutamine stress echocardiography. It showed increases in ejection fraction, stroke volume, and transvalvular gradients, indicating that she did have contractile reserve and true severe aortic stenosis. Consequently, she was referred for surgical aortic valve replacement.
CASE 3: MODERATE STENOSIS AND THREE-VESSEL CORONARY ARTERY DISEASE
Mr. C, age 81, has hypertension and hyperlipidemia. He now presents to the emergency department with chest discomfort that began suddenly, awakening him from sleep. His presenting electrocardiogram shows nonspecific changes, and he is diagnosed with non-ST-elevation myocardial infarction. He undergoes left heart catheterization, which reveals severe three-vessel coronary artery disease.
Echocardiography reveals an ejection fraction of 55% and aortic stenosis, with an aortic valve area of 1.2 cm2, a peak gradient of 44 mm Hg, and a mean gradient of 28 mm Hg.
How would you manage his aortic stenosis?
Moderate aortic stenosis in a patient who needs surgery for severe triple-vessel coronary artery disease, other valve diseases, or aortic disease raises the question of whether aortic valve replacement should be performed in conjunction with these surgeries. Although these patients would not otherwise qualify for aortic valve replacement, the fact that they will undergo a procedure that will expose them to the risks associated with open heart surgery makes them reasonable candidates. Even if the patient does not need aortic valve replacement right now, aortic stenosis progresses at a predictable rate—the valve area decreases by a mean of 0.1 cm2/year and the gradients increase by 7 mm Hg/year. Therefore, clinical judgment should be exercised so that the patient will not need to undergo open heart surgery again in the near future.
The ACC/AHA guidelines recommend aortic valve replacement for patients with moderate aortic stenosis undergoing coronary artery bypass grafting or surgery on the aorta or other heart valves, giving it a class IIa indication.21 This recommendation is based on several retrospective case series that evaluated survival, the need for reoperation for aortic valve replacement, or both in patients undergoing coronary artery bypass grafting.32–35
No data exist, however, on adding aortic valve replacement to coronary artery bypass grafting in cases of mild aortic stenosis. As a result, it is controversial and carries a class IIb recommendation (meaning that its usefulness or efficacy is less well established). The ACC/AHA guidelines state that aortic valve replacement “may be considered” in patients undergoing coronary artery bypass grafting who have mild aortic stenosis (mean gradient < 30 mm Hg or jet velocity < 3 m/s) when there is evidence, such as moderate or severe valve calcification, that progression may be rapid (level of evidence C: based only on consensus opinion of experts, case studies or standard of care).21
Mr. C, who has moderate aortic stenosis, underwent aortic valve replacement in conjunction with three-vessel bypass grafting.
CASE 4: ASYMPTOMATIC BUT SEVERE STENOSIS
Mr. D, age 74, has hypertension, hyperlipidemia, and aortic stenosis. He now presents to the outpatient department for his annual echocardiogram to follow his aortic stenosis. He has a sedentary lifestyle but feels well performing activities of daily living. He denies dyspnea on exertion, chest pain, or syncope.
His echocardiogram reveals an effective aortic valve area of 0.7 cm2, peak gradient 90 mm Hg, and mean gradient 70 mm Hg. There is evidence of severe left ventricular hypertrophy, and the valve leaflets show bulky calcification and severe restriction. An echocardiogram performed at the same institution a year earlier revealed gradients of 60 and 40 mm Hg.
Blood is drawn for laboratory tests, including N-terminal pro-brain natriuretic peptide, which is 350 pg/mL (reference range for his age < 125 pg/mL). He is referred for a treadmill stress test, which elicits symptoms at a moderate activity level.
How would you manage his aortic stenosis?
Aortic valve replacement can be considered in patients who have asymptomatic but severe aortic stenosis with preserved left ventricular function (class IIb indication).21
Clinical assessment of asymptomatic aortic stenosis can be challenging, however, as patients may underreport their symptoms or decrease their activity levels to avoid symptoms. Exercise testing in such patients can elicit symptoms, unmask diminished exercise capacity, and help determine if they should be referred for surgery.36,37 Natriuretic peptide levels have been shown to correlate with the severity of aortic stenosis,38,39 and more importantly, to help predict symptom onset, cardiac death, and need for aortic valve replacement.40–42
Some patients with asymptomatic but severe aortic stenosis are at higher risk of morbidity and death. High-risk subsets include patients with rapid progression of aortic stenosis and those with critical aortic stenosis characterized by an aortic valve area less than 0.60 cm2, mean gradient greater than 60 mm Hg, and jet velocity greater than 5.0 m/s. It is reasonable to offer these patients surgery if their expected operative mortality risk is less than 1.0%.21
Mr. D has evidence of rapid progression as defined by an increase in aortic jet velocity of more than 0.3 m/s/year. He is at low surgical risk and was referred for elective aortic valve replacement.
CASE 5: TOO FRAIL FOR SURGERY
Mr. E, age 84, has severe aortic stenosis (valve area 0.6 cm2, peak and mean gradients of 88 and 56 mm Hg), coronary artery disease status post coronary artery bypass grafting, moderate chronic obstructive pulmonary disease (forced expiratory volume in 1 second 0.8 L), chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, hyperlipidemia, and diabetes mellitus. He has preserved left ventricular function. He presents to the outpatient department with worsening shortness of breath and peripheral edema over the past several months. Your impression is that he is very frail. How would you manage Mr. E’s aortic stenosis?
Advances in surgical techniques and perioperative management over the years have enabled higher-risk patients to undergo surgical aortic valve replacement with excellent out-comes.18–20,43 Yet many patients still cannot undergo surgery because their risk is too high. Patients ineligible for surgery have traditionally been treated medically—with poor out-comes—or with balloon aortic valvuloplasty to palliate symptoms.
Transcatheter aortic valve replacement, approved by the US Food and Drug Administration (FDA) in 2011, now provides another option for these patients. In this procedure, a bioprosthetic valve mounted on a metal frame is implanted over the native stenotic valve.
Currently, the only FDA-approved and commercially available valve in the United States is the Edwards SAPIEN valve, which has bovine pericardial tissue leaflets fixed to a balloon-expandable stainless steel frame (Figure 3). In the Placement of Aortic Transcatheter Valves (PARTNER) trial,15 patients who could not undergo surgery who underwent transcatheter replacement with this valve had a significantly better survival rate than patients treated medically.15,17 Use of this valve has also been compared against conventional surgical aortic valve replacement in high-risk patients and was found to have similar long-term outcomes (Figure 4).16 It was on the basis of this trial that this valve was granted approval for patients who cannot undergo surgery.
The standard of care for high-risk patients remains surgical aortic valve replacement, although it remains to be seen whether transcatheter replacement will be made available as well to patients eligible for surgery in the near future. There are currently no randomized data for transcatheter aortic valve replacement in patients at moderate to low surgical risk, and these patients should not be considered for this procedure.
Although the initial studies are encouraging for patients who cannot undergo surgery and who are at high risk without it, several issues and concerns remain. Importantly, the long-term durability of the transcatheter valve and longer-term outcomes remain unknown. Furthermore, the risk of vascular complications remains high (10% to 15%), dictating the need for careful patient selection. There are also concerns about the risks of stroke and of paravalvular aortic insufficiency. These issues are being investigated and addressed, however, and we hope that with increasing operator experience and improvements in the technique, outcomes will be improved.
Which approach for transcatheter aortic valve replacement?
There are several considerations in determining a patient’s eligibility for transcatheter aortic valve replacement.
Initially, these valves were placed by a transvenous, transseptal approach, but now retrograde placement through the femoral artery has become standard. In this procedure, the device is advanced retrograde from the femoral artery through the aorta and placed across the native aortic valve under fluoroscopic and echocardiographic guidance.
Patients who are not eligible for transfemoral placement because of severe atherosclerosis, tortuosity, or ectasia of the iliofemoral artery or aorta can still undergo percutaneous treatment with a transapical approach. This is a hybrid surgical-transcatheter approach in which the valve is delivered through a sheath placed by left ventricular apical puncture.17,44
A newer approach gaining popularity is the transaortic technique, in which the ascending aorta is accessed directly through a ministernotomy and the delivery sheath is placed with a direct puncture. Other approaches are through the axillary and subclavian arteries.
Other valves are under development
Several other valves are under development and will likely change the landscape of transcatheter aortic valve replacement with improving outcomes. Valves that are available in the United States are shown in Figure 3. The CoreValve, consisting of porcine pericardial leaflets mounted on a self-expanding nitinol stent, is currently being studied in a trial in the United States, and the manufacturer (Medtronic) will seek approval when results are complete in the near future.
Mr. E was initially referred for surgery, but when deemed to be unable to undergo surgery was found to be a good candidate for transcatheter aortic valve replacement.
CASE 6: LIFE-LIMITING COMORBID ILLNESS
Mr. F, age 77, has multiple problems: severe aortic stenosis (aortic valve area 0.6 cm2; peak and mean gradients of 92 and 59 mm Hg), stage IV pancreatic cancer, coronary artery disease status post coronary artery bypass grafting, chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, and hyperlipidemia. He presents to the outpatient department with shortness of breath at rest, orthopnea, effort intolerance, and peripheral edema over the past several months.
On physical examination rales in both lung bases can be heard. Left heart catheterization shows patent bypass grafts.
How would you manage Mr. F’s aortic stenosis?
Aortic valve replacement is not considered an option in patients with noncardiac illnesses and comorbidities that are life-limiting in the near term. Under these circumstances, aortic valvuloplasty can be offered as a means of palliating symptoms or, if the comorbid conditions can be modified, as a bridge to more definitive treatment with aortic valve replacement.
Since first described in 1986,45 percutaneous aortic valvuloplasty has been studied in several case series and registries, with consistent findings. Acutely, it increases the valve area and lessens the gradients across the valve, relieving symptoms. The risk of death during the procedure ranged from 3% to 13.5% in several case series, with a 30-day survival rate greater than 85%.46 However, the hemodynamic and symptomatic improvement is only short-term, as valve area and gradients gradually worsen within several months.47,48 Consequently, balloon valvuloplasty is considered a palliative approach.
Mr. F has a potentially life-limiting illness, ie, cancer, which would make him a candidate for aortic valvuloplasty rather than replacement. He can be referred for evaluation for this procedure in hopes of palliating his symptoms by relieving his dyspnea and improving his quality of life.
CASE 7: HEMODYNAMIC INSTABILITY
Mr. G, age 87, is scheduled for surgical aortic valve replacement because of severe aortic stenosis (valve area 0.5 cm2, peak and mean gradients 89 and 45 mm Hg) with an ejection fraction of 30%.
Two weeks before his scheduled surgery he presents to the emergency department with worsening fluid overload and increasing shortness of breath. His initial laboratory work shows new-onset renal failure, and he has signs of hypoperfusion on physical examination. He is transferred to the cardiac intensive care unit for further care.
How would you manage his aortic stenosis?
Patients with decompensated aortic stenosis and hemodynamic instability are at extreme risk during surgery. Medical stabilization beforehand may mitigate the risks associated with surgical or transcatheter aortic valve replacement. Aortic valvuloplasty, treatment with sodium nitroprusside, and support with intra-aortic balloon counterpulsation may help stabilize patients in this “low-output” setting.
Sodium nitroprusside has long been used in low-output states. By relaxing vascular smooth muscle, it leads to increased venous capacitance, decreasing preload and congestion. It also decreases systemic vascular resistance with a subsequent decrease in afterload, which in turn improves systolic emptying. Together, these effects reduce systolic and diastolic wall stress, lower myocardial oxygen consumption, and ultimately increase cardiac output.49,50
These theoretical benefits translate to clinical improvement and increased cardiac output, as shown in a case series of 25 patients with severe aortic stenosis and left ventricular systolic dysfunction (ejection fraction 35%) presenting in a low-output state in the absence of hypotension.51 These findings have led to a ACC/AHA recommendation for the use of sodium nitroprusside in patients who have severe aortic stenosis presenting in low-output state with decompensated heart failure.21
Intra-aortic balloon counterpulsation, introduced in 1968, has been used in several clinical settings, including acute coronary syndromes, intractable ventricular arrhythmias, and refractory heart failure, and for support of hemodynamics in the perioperative setting. Its role in managing ventricular septal rupture and acute mitral regurgitation is well established. It reliably reduces afterload and improves coronary perfusion, augmenting the cardiac output. This in turn leads to improved systemic perfusion, which can buy time for a critically ill patient during which the primary disease process is addressed.
Recently, a case series in which intraaortic balloon counterpulsation devices were placed in patients with severe aortic stenosis and cardiogenic shock showed findings similar to those with sodium nitroprusside infusion. Specifically, their use was associated with improved cardiac indices and filling pressures with a decrease in systemic vascular resistance. These changes have led to increased cardiac performance, resulting in better systemic perfusion.52 Thus, intra-aortic balloon counterpulsation can be an option for stabilizing patients with severe aortic stenosis and cardiogenic shock.
Mr. G was treated with sodium nitroprusside and intravenous diuretics. He achieved symptomatic relief and his renal function returned to baseline. He subsequently underwent aortic valve replacement during the hospitalization.
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009; 373:956–966.
- Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993; 21:1220–1225.
- Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol 1989; 13:545–550.
- Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997; 95:2262–2270.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J 1987; 8:471–483.
- Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J 1988; 9(suppl E):57–64.
- Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation 2001; 104:2205–2209.
- Cowell SJ, Newby DE, Prescott RJ, et al; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005; 352:2389–2397.
- Rossebø AB, Pedersen TR, Boman K, et al; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008; 359:1343–1356.
- Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 2007; 49:554–561.
- Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation 2004; 110:1291–1295.
- O’Brien KD, Probstfield JL, Caulked MT, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med 2005; 165:858–862.
- Lindblom D, Lindblom U, Qvist J, Lundström H. Long-term relative survival rates after heart valve replacement. J Am Coll Cardiol 1990; 15:566–573.
- Makkar RR, Fontana G P, Jilaihawi H, et al; PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012; 366:1696–1704.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Di Eusanio M, Fortuna D, Cristell D, et al; RERIC (Emilia Romagna Cardiac Surgery Registry) Investigators. Contemporary outcomes of conventional aortic valve replacement in 638 octogenarians: insights from an Italian Regional Cardiac Surgery Registry (RERIC). Eur J Cardiothorac Surg 2012; 41:1247–1252.
- Di Eusanio M, Fortuna D, De Palma R, et al. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg 2011; 141:940–947.
- Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of the Society of Thoracic Surgeons. Ann Thorac Surg 1999; 67:943–951.
- Bonow RO, Carabello BA, Chatterjee K, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52:e1–e142.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115:2856–2864.
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005; 91:1324–1329.
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997; 95:2395–2400.
- Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation 2000; 101:1940–1946.
- Pereira JJ, Lauer MS, Bashir M, et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 2002; 39:1356–1363.
- Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008; 86:1781–1789.
- Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol 2001; 37:2101–2107.
- Monin JL, Quéré J P, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108:319–324.
- Zuppiroli A, Mori F, Olivotto I, Castelli G, Favilli S, Dolara A. Therapeutic implications of contractile reserve elicited by dobutamine echocardiography in symptomatic, low-gradient aortic stenosis. Ital Heart J 2003; 4:264–270.
- Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865–1873.
- Ahmed AA, Graham AN, Lovell D, O’Kane HO. Management of mild to moderate aortic valve disease during coronary artery bypass grafting. Eur J Cardiothorac Surg 2003; 24:535–539.
- Verhoye J P, Merlicco F, Sami IM, et al. Aortic valve replacement for aortic stenosis after previous coronary artery bypass grafting: could early reoperation be prevented? J Heart Valve Dis 2006; 15:474–478.
- Hochrein J, Lucke JC, Harrison JK, et al. Mortality and need for reoperation in patients with mild-to-moderate asymptomatic aortic valve disease undergoing coronary artery bypass graft alone. Am Heart J 1999; 138:791–797.
- Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735–742.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Weber M, Hausen M, Arnold R, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide for conservatively and surgically treated patients with aortic valve stenosis. Heart 2006; 92:1639–1644.
- Gerber IL, Stewart RA, Legget ME, et al. Increased plasma natriuretic peptide levels refect symptom onset in aortic stenosis. Circulation 2003; 107:1884–1890.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lancellotti P, Moonen M, Magne J, et al. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol 2010; 105:383–388.
- Langanay T, Flécher E, Fouquet O, et al. Aortic valve replacement in the elderly: the real life. Ann Thorac Surg 2012; 93:70–77.
- Christofferson RD, Kapadia SR, Rajagopal V, Tuzcu EM. Emerging transcatheter therapies for aortic and mitral disease. Heart 2009; 95:148–155.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Bernard Y, Etievent J, Mourand JL, et al. Long-term results of percutaneous aortic valvuloplasty compared with aortic valve replacement in patients more than 75 years old. J Am Coll Cardiol 1992; 20:796–801.
- Elkayam U, Janmohamed M, Habib M, Hatamizadeh P. Vasodilators in the management of acute heart failure. Crit Care Med 2008; 36(suppl 1):S95–S105.
- Popovic ZB, Khot UN, Novaro GM, et al. Effects of sodium nitroprusside in aortic stenosis associated with severe heart failure: pressure-volume loop analysis using a numerical model. Am J Physiol Heart Circ Physiol 2005; 288:H416–H423.
- Khot UN, Novaro GM, Popovic ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med 2003; 348:1756–1763.
- Aksoy O, Yousefzai R, Singh D, et al. Cardiogenic shock in the setting of severe aortic stenosis: role of intra-aortic balloon pump support. Heart 2011; 97:838–843.
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009; 373:956–966.
- Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993; 21:1220–1225.
- Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol 1989; 13:545–550.
- Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997; 95:2262–2270.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J 1987; 8:471–483.
- Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J 1988; 9(suppl E):57–64.
- Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation 2001; 104:2205–2209.
- Cowell SJ, Newby DE, Prescott RJ, et al; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005; 352:2389–2397.
- Rossebø AB, Pedersen TR, Boman K, et al; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008; 359:1343–1356.
- Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 2007; 49:554–561.
- Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation 2004; 110:1291–1295.
- O’Brien KD, Probstfield JL, Caulked MT, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med 2005; 165:858–862.
- Lindblom D, Lindblom U, Qvist J, Lundström H. Long-term relative survival rates after heart valve replacement. J Am Coll Cardiol 1990; 15:566–573.
- Makkar RR, Fontana G P, Jilaihawi H, et al; PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012; 366:1696–1704.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Di Eusanio M, Fortuna D, Cristell D, et al; RERIC (Emilia Romagna Cardiac Surgery Registry) Investigators. Contemporary outcomes of conventional aortic valve replacement in 638 octogenarians: insights from an Italian Regional Cardiac Surgery Registry (RERIC). Eur J Cardiothorac Surg 2012; 41:1247–1252.
- Di Eusanio M, Fortuna D, De Palma R, et al. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg 2011; 141:940–947.
- Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of the Society of Thoracic Surgeons. Ann Thorac Surg 1999; 67:943–951.
- Bonow RO, Carabello BA, Chatterjee K, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52:e1–e142.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115:2856–2864.
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005; 91:1324–1329.
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997; 95:2395–2400.
- Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation 2000; 101:1940–1946.
- Pereira JJ, Lauer MS, Bashir M, et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 2002; 39:1356–1363.
- Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008; 86:1781–1789.
- Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol 2001; 37:2101–2107.
- Monin JL, Quéré J P, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108:319–324.
- Zuppiroli A, Mori F, Olivotto I, Castelli G, Favilli S, Dolara A. Therapeutic implications of contractile reserve elicited by dobutamine echocardiography in symptomatic, low-gradient aortic stenosis. Ital Heart J 2003; 4:264–270.
- Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865–1873.
- Ahmed AA, Graham AN, Lovell D, O’Kane HO. Management of mild to moderate aortic valve disease during coronary artery bypass grafting. Eur J Cardiothorac Surg 2003; 24:535–539.
- Verhoye J P, Merlicco F, Sami IM, et al. Aortic valve replacement for aortic stenosis after previous coronary artery bypass grafting: could early reoperation be prevented? J Heart Valve Dis 2006; 15:474–478.
- Hochrein J, Lucke JC, Harrison JK, et al. Mortality and need for reoperation in patients with mild-to-moderate asymptomatic aortic valve disease undergoing coronary artery bypass graft alone. Am Heart J 1999; 138:791–797.
- Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735–742.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Weber M, Hausen M, Arnold R, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide for conservatively and surgically treated patients with aortic valve stenosis. Heart 2006; 92:1639–1644.
- Gerber IL, Stewart RA, Legget ME, et al. Increased plasma natriuretic peptide levels refect symptom onset in aortic stenosis. Circulation 2003; 107:1884–1890.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lancellotti P, Moonen M, Magne J, et al. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol 2010; 105:383–388.
- Langanay T, Flécher E, Fouquet O, et al. Aortic valve replacement in the elderly: the real life. Ann Thorac Surg 2012; 93:70–77.
- Christofferson RD, Kapadia SR, Rajagopal V, Tuzcu EM. Emerging transcatheter therapies for aortic and mitral disease. Heart 2009; 95:148–155.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Bernard Y, Etievent J, Mourand JL, et al. Long-term results of percutaneous aortic valvuloplasty compared with aortic valve replacement in patients more than 75 years old. J Am Coll Cardiol 1992; 20:796–801.
- Elkayam U, Janmohamed M, Habib M, Hatamizadeh P. Vasodilators in the management of acute heart failure. Crit Care Med 2008; 36(suppl 1):S95–S105.
- Popovic ZB, Khot UN, Novaro GM, et al. Effects of sodium nitroprusside in aortic stenosis associated with severe heart failure: pressure-volume loop analysis using a numerical model. Am J Physiol Heart Circ Physiol 2005; 288:H416–H423.
- Khot UN, Novaro GM, Popovic ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med 2003; 348:1756–1763.
- Aksoy O, Yousefzai R, Singh D, et al. Cardiogenic shock in the setting of severe aortic stenosis: role of intra-aortic balloon pump support. Heart 2011; 97:838–843.
KEY POINTS
- Calcific aortic stenosis is the most common acquired valvular disease, and its prevalence is increasing as the population ages.
- Patients who have symptoms should be referred for aortic valve replacement. Patients who are not candidates for open heart surgery may be eligible for transcatheter aortic valve replacement.
- For high-risk patients with multiple comorbidities, “bridging” therapies such as aortic valvuloplasty are an option.
- In patients with aortic stenosis who present with hemodynamic instability and circulatory collapse, time can be gained with the use of intravenous sodium nitroprusside (in the absence of hypotension) or intra-aortic balloon counterpulsation while more definitive treatment decisions are being made.
Hypothermia after cardiac arrest: Beneficial, but slow to be adopted
A 30-year-old man experienced an episode of syncope while at work. He fully recovered consciousness within 2 minutes, but the emergency services team was called. As he was being loaded into the ambulance he again lost consciousness, and he was noted to be in ventricular fibrillation. Advanced cardiac life support was immediately started and continued for 50 minutes before a hemodynamically stable spontaneous rhythm was obtained.
On arrival at the emergency department of the local hospital, he was intubated to protect his airway, as he was comatose. A 12-lead electrocardiogram showed ST-segment elevations in leads V1, V2, and V3 and a wide QRS complex with an rSR′ pattern, consistent with right bundle branch block.
Mild therapeutic hypothermia was initiated by infusing intravenous saline solution chilled to 4°C and by applying cooling blankets, and he was transferred to our hospital on an emergency basis for further management. Here, hypothermia was maintained using an intravenous cooling catheter.
HYPOTHERMIA: BENEFICIAL, BUT SLOW TO BE ADOPTED
Mild therapeutic hypothermia is a recommended therapeutic intervention for out-of-hospital cardiac arrest due to ventricular fibrillation. Nonetheless, first-responders, emergency-room staff, and intensive-care teams have been slow to adopt and integrate it into a comprehensive postresuscitation strategy. This article summarizes the evidence supporting this therapy and how it is performed.
PROPOSED MECHANISMS OF BENEFIT
Mild therapeutic hypothermia is thought to protect against anoxic brain injury in survivors of cardiac arrest via several mechanisms:
- Decreasing neuronal metabolism in the early stage of ischemic injury
- Decreasing glucose and oxygen consumption by the brain,1 which reduces supply-demand mismatch
- Decreasing the release of excitatory amino acids (eg, glutamate) that normally trigger cytotoxic cascades in the intermediate phase of injury2
- Reducing the production of harmful reactive oxygen species3
- Maintaining cellular pH4
- Reducing cell death5
- Slowing the breakdown of the blood-brain barrier that worsens cerebral edema.6
CLINICAL DATA SUPPORTING HYPOTHERMIA
There has been an interest in therapeutic hypothermia for several decades. In the 1950s, it was used in small numbers of cases in a variety of cardiac arrest situations.7,8 Interest was rekindled in the mid-1990s after a number of animal studies suggested it might be beneficial in prolonged cerebral ischemia and anoxia,9,10 and reports of case-series described its use in adults with out-of-hospital cardiac arrest.11,12
In October 2002, the International Liaison Committee on Resuscitation (ILCOR), made up of executive members of several organizations including the American Heart Association, recommended that “unconscious adult patients with spontaneous circulation after out-of-hospital cardiac arrest” should be cooled to 32°C to 34°C [89.6°F–93.2°F] for 12 to 24 hours “when the initial rhythm was ventricular fibrillation.”13
Two large randomized trials
This position statement was based largely on the results of two randomized clinical trials published simultaneously earlier in 2002.14,15 These two trials were important not only because they were the largest randomized trials of this therapy to that point, but also because they used meaningful, prospectively defined clinical end points: all-cause mortality and degree of cognitive preservation as assessed using the Glasgow-Pittsburgh Cerebral Performance Category (CPC) scale.
Bernard et al14 performed a randomized trial in four centers in Australia, assigning 77 patients either to a goal temperature of 32°C to 34°C or to normothermia for 12 hours, with all other resuscitative measures being the same in both groups. The primary outcome measured was survival to hospital discharge with sufficient neurologic function to be discharged to home or to a rehabilitation facility, ie, a CPC score of 1 or 2.
In the hypothermia group, 21 (49%) of the 43 patients survived and had an outcome that was considered “good” (ie, they were discharged home or to a rehabilitation facility), compared with 9 (26%) of the 34 patients in the normothermia group (unadjusted odds ratio 2.65, 95% confidence interval [CI] 1.02–6.88, P = .046). Proportionally fewer patients in the hypothermia group died—22 (51%) of 43 vs 23 (68%) of 34; however, the difference was not statistically significant (P = .145).
The Hypothermia After Cardiac Arrest Study Group15 screened 3,551 European patients who suffered out-of-hospital cardiac arrest15; 275 patients were randomized to mild therapeutic hypothermia or normothermia for 24 hours. The primary outcome was the percentage of patients who had a CPC score of 1 or 2 (vs 3 to 5) at 6 months, and the secondary outcome was the rate of death at 6 months.
At 6 months, 75 (55%) of the 136 patients in the hypothermia group had a CPC score of 1 or 2, compared with 54 (39%) of the 137 patients in the normothermia group (P = .009). The rate of death was also lower with hypothermia: 55% vs 41% (P = .02).
In both trials, patients were included only if their cardiac arrest was witnessed, if their initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, if circulation spontaneously returned within 60 minutes with standard basic and advanced cardiac life support protocols, and if they were still comatose on arrival at the hospital. They were excluded if they were over age 75, if they had suffered a cerebrovascular accident at the time of cardiac arrest, or if the arrest was caused by trauma or drug overdose. In addition, the European trial excluded patients who suffered another cardiac arrest after the initial return of spontaneous circulation but before cooling was started.
The standard of care
In view of the available clinical data, the 2002 ILCOR guidelines and a 2005 statement from the American Heart Association advocated mild therapeutic hypothermia for survivors of out-of-hospital ventricular tachycardia or fibrillation.16 Subsequently, this therapy has become more widely practiced and accepted as the standard of care among critical-care providers.
Of note, some public health officials and local governments are strongly promoting this treatment for survivors of cardiac arrest in the community.17 More and more of these groups are mandating that these patients be transported only to hospitals that have therapeutic hypothermia protocols in place, bypassing those not equipped to provide this treatment.18
INDICATIONS, CONTRAINDICATIONS, AND GRAY AREAS
What are the indications and contraindications to the use of hypothermia after out-of-hospital cardiac arrest? What are some of the “gray areas”?
Indications. This treatment is indicated for comatose adults who have had a witnessed cardiac arrest, whose initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, and whose circulation spontaneously returned in less than 60 minutes with basic and advanced cardiac life support. This carries a class I recommendation, level of evidence B, and was recently reinforced in the 2010 update to the American Heart Association guidelines for cardiopulmonary resuscitation.19
Absolute contraindications include hemorrhagic stroke (which must be proved by computed tomography) and cardiac arrest due to trauma (Table 2). Other major contraindications are a Glasgow Coma Scale score of 8 or higher before the initiation of mild therapeutic hypothermia, cardiac arrest due to drug overdose, and preexisting hypothermia (< 34°C) when first-responders arrive.
Relative contraindications include baseline coagulopathy and severe hypotension (mean atrial pressure < 60 mm Hg) that is not correctable by fluid infusion, vasopressors, or invasive hemodynamic support.
Gray areas. There are not enough data to make a firm recommendation about whether to apply mild therapeutic hypothermia if a witnessed cardiac arrest with ventricular fibrillation or ventricular tachycardia occurs in the hospital, but data from out-of-hospital cardiac arrest patients appear applicable for hospitalized patients.
The data are also quite limited and equivocal on its use for out-of-hospital cardiac arrest in patients whose initial cardiac rhythm is pulseless electrical activity or asystole,20,21 likely because of the competing risk of comorbidities and the resultant lower baseline survival rate in these patients.
Consequently, for in-hospital postarrest patients with any initial rhythm and for out-of-hospital cardiac arrest patients with rhythms other than ventricular tachycardia or ventricular fibrillation, the 2010 guideline recommendation on the use of mild therapeutic hypothermia is less enthusiastic (class IIb, level of evidence B).19
There are also few data on the use of mild therapeutic hypothermia in post-arrest patients in circulatory shock requiring vasopressors or intra-aortic balloon counterpulsation, largely limited to case series and comparisons with historical controls.22,23 Further investigation is clearly needed in these areas. Until then, it should be considered at the physician’s and the team’s discretion, on a case-by-case basis.
HYPOTHERMIA IN CASES OF VENTRICULAR FIBRILLATION AND ACUTE CORONARY SYNDROME
The value of coronary angiography after out-of-hospital cardiac arrest was first highlighted by Spaulding et al,24 who performed it urgently in 84 consecutive survivors of out-of-hospital cardiac arrest, 36 of whom had ST-segment elevation myocardial infarction (STEMI). Angiography uncovered an acute coronary occlusion in 40 (48%) of the 84 patients.
In this series, ST-segment elevation was a strong predictor of acute coronary occlusion (odds ratio 4.3; 95% CI 1.6–2.0; P = .004). However, 9 patients without chest pain or ST elevation were also found to have an occluded infarct-related artery. Successful angioplasty was an independent predictor of survival, highlighting the importance of an angiographic definition in this population.
These findings were recently confirmed in the larger Parisian Region Out of Hospital Cardiac Arrest (PROCAT) registry in 435 patients who had no obvious extracardiac cause of arrest, for whom successful culprit coronary angioplasty was associated with survival.25
Angioplasty comes first, but neither treatment need be delayed
Efforts to induce hypothermia must not be allowed to delay the door-to-balloon time of post-arrest patients in the setting of STEMI. The top priority is establishing patency of the infarct-related artery with a goal of salvaging ischemic myocardium and obtaining mechanical and electrical stabilization.
Fortunately, mild therapeutic hypothermia does not necessarily delay emergency revascularization if hypothermia protocols are well established. In fact, induction of mild therapeutic hypothermia prior to or on arrival at the catheterization laboratory has been shown to be feasible and safe.26,27
We believe that all centers performing primary percutaneous coronary intervention for STEMI should have immediate access to and expertise in mild therapeutic hypothermia. Regional planning and integration of STEMI and out-of-hospital cardiac arrest networks will ensure that most patients with STEMI have access to this treatment when it is indicated.
Does hypothermia help the heart? Does it increase bleeding?
Researchers have been interested in therapeutic hypothermia as a means of reducing myocardial infarct size,28,29 but clinical trials have not shown a clear-cut benefit in this regard. However, these investigations have also added to the evidence that antiplatelet and anticoagulation therapy in patients undergoing mild therapeutic hypothermia does not result in a statistically significant excess of major bleeding events, which is a potential concern.
Of note, these studies were neither powered nor specifically designed to evaluate for major bleeding as an end point. Therefore, these complications should still be carefully monitored for.
IS THERE AN OPTIMAL TIME TO BEGIN MILD THERAPEUTIC HYPOTHERMIA?
Experimental data suggest that mild therapeutic hypothermia should be started as soon as possible after a comprehensive clinical evaluation indicates the patient is eligible.30–33 However, clinical data are not robustly in favor of starting it before the patient reaches the hospital rather than on hospital arrival.
In a recent randomized trial in 2,334 survivors of out-of-hospital cardiac arrest, outcomes were no better if hypothermia was started by paramedics than if it was started on arrival at the hospital (47.5% vs 52.6% discharged to home or rehabilitation; 95% CI 0.70–1.17; P = .43).34
Earlier data from smaller studies had suggested that prehospital initiation of hypothermia (for example, using chilled intravenous saline infusions) in carefully selected patients with out-of-hospital cardiac arrest was safe and showed a nonsignificant trend toward better outcomes.20,35
The randomized controlled trials that showed hypothermia to be beneficial used very slow cooling methods; consequently, it is reasonable to allow up to 6 hours from initial presentation to first-responders to start it. There are, however, no conclusive data in humans for or against starting it later than 6 hours after presentation. Most experts believe that its potential neurologic and mortality benefits are largely lost if it is delayed more than 6 hours.
The overall message from these data seems to be that, in patients who survive cardiac arrest outside the hospital with ventricular tachycardia or fibrillation, mild therapeutic hypothermia is effective and safe and should be started as soon as possible after arrival at the hospital.
METHODS FOR INDUCING AND MAINTAINING HYPOTHERMIA
Cooling the patient
To cool the patient and keep him or her cold, caregivers have used ice packs placed around the head, groin, and axillae; intravenous infusion of saline maintained at 4°C (39°F); and cooling-air blankets. More recently, thermal wraps and intravascular cooling catheters have been used.36–38 The newer methods are more effective in rapidly bringing patients to the target temperature of 32 to 34°C (usually within 3 or 4 hours) and keeping them within this range, and they auto-adjust their output on the basis of measured core temperature.
The Pre ROSC Intranasal Cooling Effectiveness (PRINCE) trial demonstrated the safety and efficacy of nasopharyngeal cooling using a perfluorocarbon aerosol given via a nasopharyngeal cannula in patients with out-of-hospital cardiac arrest.39
Monitoring the core temperature
The patient’s core temperature is most commonly monitored with a probe in the esophagus, bladder, rectum, or pulmonary artery.40
Of these, the bladder and rectum are considered “intermediate” monitoring sites, as their temperatures tend to lag behind the core temperature. Furthermore, the bladder temperature can be significantly altered by the flow of urine, which can vary considerably during the cooling and rewarming process.
Esophageal temperature monitoring is relatively noninvasive and tends to reliably and accurately reflect core temperature as long as the probe is placed far enough down (about 45 cm from the nose in an average adult) that it is not affected by proximity to the trachea.
Pulmonary artery catheters are considered the gold standard for core temperature monitoring, but they pose risks such as bloodstream infection and large-vessel damage. In practice, many patients admitted to the coronary intensive care unit after out-of-hospital cardiac arrest require pulmonary artery catheterization anyway for other indications, and in these situations it is the preferred method of monitoring the core temperature.
However, no approach is ideal in terms of measuring the temperature in the critical end organs. Rather, core temperature monitoring serves as a guide to help ensure consistent clinical practice in attaining and maintaining mild therapeutic hypothermia.
Preventing shivering
To achieve and maintain the goal temperature, the body’s natural response to a decrease in core temperature—shivering—must be watched for and eliminated. A number of drugs may be used for this purpose.41
Paralytic drugs are used to reduce shivering; nursing staff must be trained to monitor for signs of occult shivering (eg, jaw vibration) and adjust the dose of paralytic drug accordingly. Since the patients are paralyzed, they must also receive continuous intravenous sedation.
Other commonly used drugs that decrease the hypothalamic drive to shiver include buspirone (BuSpar), a serotonin 5HT-1A partial agonist, and meperidine (Demerol), an opiate agonist of kappa and mu receptors.
Rewarming after 24 hours
Rewarming is conventionally started after 24 hours of mild therapeutic hypothermia, at a rate no greater than 0.5°C (1°F) per hour.
Because sedation is used during the hypothermia period of 24 hours, a washout period for these medications is necessary, and the neurologic prognosis of cardiac arrest patients who undergo mild therapeutic hypothermia cannot be adequately assessed until 72 hours after rewarming.
ADVERSE EFFECTS OF MILD THERAPEUTIC HYPOTHERMIA
Some of these effects are predictable. Decreasing the body temperature causes potassium to shift into the cells, and this same potassium will leave the intracellular space during the rewarming phase. For this reason, aggressive potassium repletion for mild hypokalemia (potassium levels of 3.0–3.5 mmol/L) during mild therapeutic hypothermia can result in dangerous hyperkalemia during rewarming and should generally be avoided.
As another example, the enzymes involved in coagulation are less effective at lower temperatures. Thus, if it occurs, active bleeding requiring transfusion warrants consideration of stopping the hypothermia.
Adverse effects should be watched for (eg, by checking electrolyte levels frequently, monitoring blood glucose, continuous electroencephalographic monitoring during the cooling phase, and avoiding placement of intracardiac catheters once the goal temperature is reached) and addressed as they happen. However, in a recent review of this subject42 the balance of evidence continued to indicate that the benefit of this treatment exceeds its risks.
OUR PATIENT RECOVERS
After 24 hours of therapeutic hypothermia, our patient was gradually rewarmed to a normal temperature, and sedation and paralysis were discontinued.
Analysis of his prearrest and postarrest 12-lead electrocardiograms revealed a type I Brugada pattern (coved ST elevation and negative T waves in V1, V2, and V3, caused by abnormal repolarization due to inherited mutations in SCN5A). Cardiac catheterization revealed normal coronary arteries, and MRI revealed no evidence of arrhythmogenic right ventricular cardiomyopathy or other structural abnormalities.
In the next 72 hours the patient was successfully extubated, and he gradually returned to full neurologic function. Before he went home a few days later, a single-lead cardioverter-defibrillator was implanted to prevent sudden cardiac death. All of his first-degree relatives were encouraged to undergo genetic screening for SCN5A mutations. The patient is currently back to his previous high level of functioning as a marketing manager, husband, and father of two young children.
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab 2003; 23:513–530.
- Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke 1996; 27:913–918.
- Thoresen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997; 8:3359–3362.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37(suppl 7):S186–S202.
- Yang D, Guo S, Zhang T, Li H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett 2009; 583:2500–2506.
- Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1994; 14:620–627.
- Benson DW, Williams GR, Spencer FC, Yates AJ. The use of hypothermia after cardiac arrest. Anesth Analg 1959; 38:423–428.
- Williams GR, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958; 148:462–468.
- Baker CJ, Onesti ST, Barth KN, Prestigiacomo CJ, Solomon RA. Hypothermic protection following middle cerebral artery occlusion in the rat. Surg Neurol 1991; 36:175–180.
- Ridenour TR, Warner DS, Todd MM, McAllister AC. Mild hypothermia reduces infarct size resulting from temporary but not permanent focal ischemia in rats. Stroke 1992; 23:733–738.
- Bernard SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med 1997; 30:146–153.
- Yanagawa Y, Ishihara S, Norio H, et al. Preliminary clinical outcome study of mild resuscitative hypothermia after out-of-hospital cardiopulmonary arrest. Resuscitation 1998; 39:61–66.
- Nolan JP, Morley PT, Vanden Hoek TL, et al; International Liaison Committee on Resuscitation. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation 2003; 108:118–121.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557–563.
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549–556.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005; 112(suppl 24):IV1–IV203.
- Hartocollis A. “City Pushes Cooling Therapy for Cardiac Arrest”. New York Times, December4th, 2008,A1. http://www.nytimes.com/2008/12/04/nyregion/04cool.html. Accessed May 31, , 2011.
- Nichol G, Aufderheide TP, Eigel B, et al; American Heart Association Emergency Cardiovascular Care Committee. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation 2010; 121:709–729.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122(suppl 3):S640–S56.
- Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001; 51:275–281.
- Kim F, Olsufka M, Longstreth WT, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064–3070.
- Hovdenes J, Laake JH, Aaberge L, Haugaa H, Bugge JF. Therapeutic hypothermia after out-of-hospital cardiac arrest: experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol Scand 2007; 51:137–142.
- Skulec R, Kovarnik T, Dostalova G, Kolar J, Linhart A. Induction of mild hypothermia in cardiac arrest survivors presenting with cardiogenic shock syndrome. Acta Anaesthesiol Scand 2008; 52:188–194.
- Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 1997; 336:1629–1633.
- Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv 2010; 3:200–207.
- Knafelj R, Radsel P, Ploj T, Noc M. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation 2007; 74:227–234.
- Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med 2008; 36:1780–1786.
- O’Neill WW, on behalf of the COOL-MI Investigators. Cooling as an adjunct to primary PCI for myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Grines CL, on behalf of the ICE-IT Investigators. Intravascular cooling adjunctive to percutaneous coronary intervention for acute myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Weil MH, Gazmuri RJ. Hypothermia after cardiac arrest. Crit Care Med 1991; 19:315.
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004; 109:2786–2791.
- Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 2008; 77:242–249.
- Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008; 76:431–442.
- Bernard SA, Smith K, Cameron P, et al; Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation 2010; 122:737–742.
- Bruel C, Parienti JJ, Marie W, et al. Mild hypothermia during advanced life support: a preliminary study in out-of-hospital cardiac arrest. Crit Care 2008; 12:R31.
- Pichon N, Amiel JB, François B, Dugard A, Etchecopar C, Vignon P. Efficacy of and tolerance to mild induced hypothermia after out-of-hospital cardiac arrest using an endovascular cooling system. Crit Care 2007; 11:R71.
- Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol 2009; 133:223–228.
- Heard KJ, Peberdy MA, Sayre MR, et al. A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation 2010; 81:9–14.
- Castrén M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010; 122:729–736.
- Insler SR, Sessler DI. Perioperative thermoregulation and temperature monitoring. Anesthesiol Clin 2006; 24:823–837.
- Weant KA, Martin JE, Humphries RL, Cook AM. Pharmacologic options for reducing the shivering response to therapeutic hypothermia. Pharmacotherapy 2010; 30:830–841.
- Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 2010; 363:1256–1264.
A 30-year-old man experienced an episode of syncope while at work. He fully recovered consciousness within 2 minutes, but the emergency services team was called. As he was being loaded into the ambulance he again lost consciousness, and he was noted to be in ventricular fibrillation. Advanced cardiac life support was immediately started and continued for 50 minutes before a hemodynamically stable spontaneous rhythm was obtained.
On arrival at the emergency department of the local hospital, he was intubated to protect his airway, as he was comatose. A 12-lead electrocardiogram showed ST-segment elevations in leads V1, V2, and V3 and a wide QRS complex with an rSR′ pattern, consistent with right bundle branch block.
Mild therapeutic hypothermia was initiated by infusing intravenous saline solution chilled to 4°C and by applying cooling blankets, and he was transferred to our hospital on an emergency basis for further management. Here, hypothermia was maintained using an intravenous cooling catheter.
HYPOTHERMIA: BENEFICIAL, BUT SLOW TO BE ADOPTED
Mild therapeutic hypothermia is a recommended therapeutic intervention for out-of-hospital cardiac arrest due to ventricular fibrillation. Nonetheless, first-responders, emergency-room staff, and intensive-care teams have been slow to adopt and integrate it into a comprehensive postresuscitation strategy. This article summarizes the evidence supporting this therapy and how it is performed.
PROPOSED MECHANISMS OF BENEFIT
Mild therapeutic hypothermia is thought to protect against anoxic brain injury in survivors of cardiac arrest via several mechanisms:
- Decreasing neuronal metabolism in the early stage of ischemic injury
- Decreasing glucose and oxygen consumption by the brain,1 which reduces supply-demand mismatch
- Decreasing the release of excitatory amino acids (eg, glutamate) that normally trigger cytotoxic cascades in the intermediate phase of injury2
- Reducing the production of harmful reactive oxygen species3
- Maintaining cellular pH4
- Reducing cell death5
- Slowing the breakdown of the blood-brain barrier that worsens cerebral edema.6
CLINICAL DATA SUPPORTING HYPOTHERMIA
There has been an interest in therapeutic hypothermia for several decades. In the 1950s, it was used in small numbers of cases in a variety of cardiac arrest situations.7,8 Interest was rekindled in the mid-1990s after a number of animal studies suggested it might be beneficial in prolonged cerebral ischemia and anoxia,9,10 and reports of case-series described its use in adults with out-of-hospital cardiac arrest.11,12
In October 2002, the International Liaison Committee on Resuscitation (ILCOR), made up of executive members of several organizations including the American Heart Association, recommended that “unconscious adult patients with spontaneous circulation after out-of-hospital cardiac arrest” should be cooled to 32°C to 34°C [89.6°F–93.2°F] for 12 to 24 hours “when the initial rhythm was ventricular fibrillation.”13
Two large randomized trials
This position statement was based largely on the results of two randomized clinical trials published simultaneously earlier in 2002.14,15 These two trials were important not only because they were the largest randomized trials of this therapy to that point, but also because they used meaningful, prospectively defined clinical end points: all-cause mortality and degree of cognitive preservation as assessed using the Glasgow-Pittsburgh Cerebral Performance Category (CPC) scale.
Bernard et al14 performed a randomized trial in four centers in Australia, assigning 77 patients either to a goal temperature of 32°C to 34°C or to normothermia for 12 hours, with all other resuscitative measures being the same in both groups. The primary outcome measured was survival to hospital discharge with sufficient neurologic function to be discharged to home or to a rehabilitation facility, ie, a CPC score of 1 or 2.
In the hypothermia group, 21 (49%) of the 43 patients survived and had an outcome that was considered “good” (ie, they were discharged home or to a rehabilitation facility), compared with 9 (26%) of the 34 patients in the normothermia group (unadjusted odds ratio 2.65, 95% confidence interval [CI] 1.02–6.88, P = .046). Proportionally fewer patients in the hypothermia group died—22 (51%) of 43 vs 23 (68%) of 34; however, the difference was not statistically significant (P = .145).
The Hypothermia After Cardiac Arrest Study Group15 screened 3,551 European patients who suffered out-of-hospital cardiac arrest15; 275 patients were randomized to mild therapeutic hypothermia or normothermia for 24 hours. The primary outcome was the percentage of patients who had a CPC score of 1 or 2 (vs 3 to 5) at 6 months, and the secondary outcome was the rate of death at 6 months.
At 6 months, 75 (55%) of the 136 patients in the hypothermia group had a CPC score of 1 or 2, compared with 54 (39%) of the 137 patients in the normothermia group (P = .009). The rate of death was also lower with hypothermia: 55% vs 41% (P = .02).
In both trials, patients were included only if their cardiac arrest was witnessed, if their initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, if circulation spontaneously returned within 60 minutes with standard basic and advanced cardiac life support protocols, and if they were still comatose on arrival at the hospital. They were excluded if they were over age 75, if they had suffered a cerebrovascular accident at the time of cardiac arrest, or if the arrest was caused by trauma or drug overdose. In addition, the European trial excluded patients who suffered another cardiac arrest after the initial return of spontaneous circulation but before cooling was started.
The standard of care
In view of the available clinical data, the 2002 ILCOR guidelines and a 2005 statement from the American Heart Association advocated mild therapeutic hypothermia for survivors of out-of-hospital ventricular tachycardia or fibrillation.16 Subsequently, this therapy has become more widely practiced and accepted as the standard of care among critical-care providers.
Of note, some public health officials and local governments are strongly promoting this treatment for survivors of cardiac arrest in the community.17 More and more of these groups are mandating that these patients be transported only to hospitals that have therapeutic hypothermia protocols in place, bypassing those not equipped to provide this treatment.18
INDICATIONS, CONTRAINDICATIONS, AND GRAY AREAS
What are the indications and contraindications to the use of hypothermia after out-of-hospital cardiac arrest? What are some of the “gray areas”?
Indications. This treatment is indicated for comatose adults who have had a witnessed cardiac arrest, whose initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, and whose circulation spontaneously returned in less than 60 minutes with basic and advanced cardiac life support. This carries a class I recommendation, level of evidence B, and was recently reinforced in the 2010 update to the American Heart Association guidelines for cardiopulmonary resuscitation.19
Absolute contraindications include hemorrhagic stroke (which must be proved by computed tomography) and cardiac arrest due to trauma (Table 2). Other major contraindications are a Glasgow Coma Scale score of 8 or higher before the initiation of mild therapeutic hypothermia, cardiac arrest due to drug overdose, and preexisting hypothermia (< 34°C) when first-responders arrive.
Relative contraindications include baseline coagulopathy and severe hypotension (mean atrial pressure < 60 mm Hg) that is not correctable by fluid infusion, vasopressors, or invasive hemodynamic support.
Gray areas. There are not enough data to make a firm recommendation about whether to apply mild therapeutic hypothermia if a witnessed cardiac arrest with ventricular fibrillation or ventricular tachycardia occurs in the hospital, but data from out-of-hospital cardiac arrest patients appear applicable for hospitalized patients.
The data are also quite limited and equivocal on its use for out-of-hospital cardiac arrest in patients whose initial cardiac rhythm is pulseless electrical activity or asystole,20,21 likely because of the competing risk of comorbidities and the resultant lower baseline survival rate in these patients.
Consequently, for in-hospital postarrest patients with any initial rhythm and for out-of-hospital cardiac arrest patients with rhythms other than ventricular tachycardia or ventricular fibrillation, the 2010 guideline recommendation on the use of mild therapeutic hypothermia is less enthusiastic (class IIb, level of evidence B).19
There are also few data on the use of mild therapeutic hypothermia in post-arrest patients in circulatory shock requiring vasopressors or intra-aortic balloon counterpulsation, largely limited to case series and comparisons with historical controls.22,23 Further investigation is clearly needed in these areas. Until then, it should be considered at the physician’s and the team’s discretion, on a case-by-case basis.
HYPOTHERMIA IN CASES OF VENTRICULAR FIBRILLATION AND ACUTE CORONARY SYNDROME
The value of coronary angiography after out-of-hospital cardiac arrest was first highlighted by Spaulding et al,24 who performed it urgently in 84 consecutive survivors of out-of-hospital cardiac arrest, 36 of whom had ST-segment elevation myocardial infarction (STEMI). Angiography uncovered an acute coronary occlusion in 40 (48%) of the 84 patients.
In this series, ST-segment elevation was a strong predictor of acute coronary occlusion (odds ratio 4.3; 95% CI 1.6–2.0; P = .004). However, 9 patients without chest pain or ST elevation were also found to have an occluded infarct-related artery. Successful angioplasty was an independent predictor of survival, highlighting the importance of an angiographic definition in this population.
These findings were recently confirmed in the larger Parisian Region Out of Hospital Cardiac Arrest (PROCAT) registry in 435 patients who had no obvious extracardiac cause of arrest, for whom successful culprit coronary angioplasty was associated with survival.25
Angioplasty comes first, but neither treatment need be delayed
Efforts to induce hypothermia must not be allowed to delay the door-to-balloon time of post-arrest patients in the setting of STEMI. The top priority is establishing patency of the infarct-related artery with a goal of salvaging ischemic myocardium and obtaining mechanical and electrical stabilization.
Fortunately, mild therapeutic hypothermia does not necessarily delay emergency revascularization if hypothermia protocols are well established. In fact, induction of mild therapeutic hypothermia prior to or on arrival at the catheterization laboratory has been shown to be feasible and safe.26,27
We believe that all centers performing primary percutaneous coronary intervention for STEMI should have immediate access to and expertise in mild therapeutic hypothermia. Regional planning and integration of STEMI and out-of-hospital cardiac arrest networks will ensure that most patients with STEMI have access to this treatment when it is indicated.
Does hypothermia help the heart? Does it increase bleeding?
Researchers have been interested in therapeutic hypothermia as a means of reducing myocardial infarct size,28,29 but clinical trials have not shown a clear-cut benefit in this regard. However, these investigations have also added to the evidence that antiplatelet and anticoagulation therapy in patients undergoing mild therapeutic hypothermia does not result in a statistically significant excess of major bleeding events, which is a potential concern.
Of note, these studies were neither powered nor specifically designed to evaluate for major bleeding as an end point. Therefore, these complications should still be carefully monitored for.
IS THERE AN OPTIMAL TIME TO BEGIN MILD THERAPEUTIC HYPOTHERMIA?
Experimental data suggest that mild therapeutic hypothermia should be started as soon as possible after a comprehensive clinical evaluation indicates the patient is eligible.30–33 However, clinical data are not robustly in favor of starting it before the patient reaches the hospital rather than on hospital arrival.
In a recent randomized trial in 2,334 survivors of out-of-hospital cardiac arrest, outcomes were no better if hypothermia was started by paramedics than if it was started on arrival at the hospital (47.5% vs 52.6% discharged to home or rehabilitation; 95% CI 0.70–1.17; P = .43).34
Earlier data from smaller studies had suggested that prehospital initiation of hypothermia (for example, using chilled intravenous saline infusions) in carefully selected patients with out-of-hospital cardiac arrest was safe and showed a nonsignificant trend toward better outcomes.20,35
The randomized controlled trials that showed hypothermia to be beneficial used very slow cooling methods; consequently, it is reasonable to allow up to 6 hours from initial presentation to first-responders to start it. There are, however, no conclusive data in humans for or against starting it later than 6 hours after presentation. Most experts believe that its potential neurologic and mortality benefits are largely lost if it is delayed more than 6 hours.
The overall message from these data seems to be that, in patients who survive cardiac arrest outside the hospital with ventricular tachycardia or fibrillation, mild therapeutic hypothermia is effective and safe and should be started as soon as possible after arrival at the hospital.
METHODS FOR INDUCING AND MAINTAINING HYPOTHERMIA
Cooling the patient
To cool the patient and keep him or her cold, caregivers have used ice packs placed around the head, groin, and axillae; intravenous infusion of saline maintained at 4°C (39°F); and cooling-air blankets. More recently, thermal wraps and intravascular cooling catheters have been used.36–38 The newer methods are more effective in rapidly bringing patients to the target temperature of 32 to 34°C (usually within 3 or 4 hours) and keeping them within this range, and they auto-adjust their output on the basis of measured core temperature.
The Pre ROSC Intranasal Cooling Effectiveness (PRINCE) trial demonstrated the safety and efficacy of nasopharyngeal cooling using a perfluorocarbon aerosol given via a nasopharyngeal cannula in patients with out-of-hospital cardiac arrest.39
Monitoring the core temperature
The patient’s core temperature is most commonly monitored with a probe in the esophagus, bladder, rectum, or pulmonary artery.40
Of these, the bladder and rectum are considered “intermediate” monitoring sites, as their temperatures tend to lag behind the core temperature. Furthermore, the bladder temperature can be significantly altered by the flow of urine, which can vary considerably during the cooling and rewarming process.
Esophageal temperature monitoring is relatively noninvasive and tends to reliably and accurately reflect core temperature as long as the probe is placed far enough down (about 45 cm from the nose in an average adult) that it is not affected by proximity to the trachea.
Pulmonary artery catheters are considered the gold standard for core temperature monitoring, but they pose risks such as bloodstream infection and large-vessel damage. In practice, many patients admitted to the coronary intensive care unit after out-of-hospital cardiac arrest require pulmonary artery catheterization anyway for other indications, and in these situations it is the preferred method of monitoring the core temperature.
However, no approach is ideal in terms of measuring the temperature in the critical end organs. Rather, core temperature monitoring serves as a guide to help ensure consistent clinical practice in attaining and maintaining mild therapeutic hypothermia.
Preventing shivering
To achieve and maintain the goal temperature, the body’s natural response to a decrease in core temperature—shivering—must be watched for and eliminated. A number of drugs may be used for this purpose.41
Paralytic drugs are used to reduce shivering; nursing staff must be trained to monitor for signs of occult shivering (eg, jaw vibration) and adjust the dose of paralytic drug accordingly. Since the patients are paralyzed, they must also receive continuous intravenous sedation.
Other commonly used drugs that decrease the hypothalamic drive to shiver include buspirone (BuSpar), a serotonin 5HT-1A partial agonist, and meperidine (Demerol), an opiate agonist of kappa and mu receptors.
Rewarming after 24 hours
Rewarming is conventionally started after 24 hours of mild therapeutic hypothermia, at a rate no greater than 0.5°C (1°F) per hour.
Because sedation is used during the hypothermia period of 24 hours, a washout period for these medications is necessary, and the neurologic prognosis of cardiac arrest patients who undergo mild therapeutic hypothermia cannot be adequately assessed until 72 hours after rewarming.
ADVERSE EFFECTS OF MILD THERAPEUTIC HYPOTHERMIA
Some of these effects are predictable. Decreasing the body temperature causes potassium to shift into the cells, and this same potassium will leave the intracellular space during the rewarming phase. For this reason, aggressive potassium repletion for mild hypokalemia (potassium levels of 3.0–3.5 mmol/L) during mild therapeutic hypothermia can result in dangerous hyperkalemia during rewarming and should generally be avoided.
As another example, the enzymes involved in coagulation are less effective at lower temperatures. Thus, if it occurs, active bleeding requiring transfusion warrants consideration of stopping the hypothermia.
Adverse effects should be watched for (eg, by checking electrolyte levels frequently, monitoring blood glucose, continuous electroencephalographic monitoring during the cooling phase, and avoiding placement of intracardiac catheters once the goal temperature is reached) and addressed as they happen. However, in a recent review of this subject42 the balance of evidence continued to indicate that the benefit of this treatment exceeds its risks.
OUR PATIENT RECOVERS
After 24 hours of therapeutic hypothermia, our patient was gradually rewarmed to a normal temperature, and sedation and paralysis were discontinued.
Analysis of his prearrest and postarrest 12-lead electrocardiograms revealed a type I Brugada pattern (coved ST elevation and negative T waves in V1, V2, and V3, caused by abnormal repolarization due to inherited mutations in SCN5A). Cardiac catheterization revealed normal coronary arteries, and MRI revealed no evidence of arrhythmogenic right ventricular cardiomyopathy or other structural abnormalities.
In the next 72 hours the patient was successfully extubated, and he gradually returned to full neurologic function. Before he went home a few days later, a single-lead cardioverter-defibrillator was implanted to prevent sudden cardiac death. All of his first-degree relatives were encouraged to undergo genetic screening for SCN5A mutations. The patient is currently back to his previous high level of functioning as a marketing manager, husband, and father of two young children.
A 30-year-old man experienced an episode of syncope while at work. He fully recovered consciousness within 2 minutes, but the emergency services team was called. As he was being loaded into the ambulance he again lost consciousness, and he was noted to be in ventricular fibrillation. Advanced cardiac life support was immediately started and continued for 50 minutes before a hemodynamically stable spontaneous rhythm was obtained.
On arrival at the emergency department of the local hospital, he was intubated to protect his airway, as he was comatose. A 12-lead electrocardiogram showed ST-segment elevations in leads V1, V2, and V3 and a wide QRS complex with an rSR′ pattern, consistent with right bundle branch block.
Mild therapeutic hypothermia was initiated by infusing intravenous saline solution chilled to 4°C and by applying cooling blankets, and he was transferred to our hospital on an emergency basis for further management. Here, hypothermia was maintained using an intravenous cooling catheter.
HYPOTHERMIA: BENEFICIAL, BUT SLOW TO BE ADOPTED
Mild therapeutic hypothermia is a recommended therapeutic intervention for out-of-hospital cardiac arrest due to ventricular fibrillation. Nonetheless, first-responders, emergency-room staff, and intensive-care teams have been slow to adopt and integrate it into a comprehensive postresuscitation strategy. This article summarizes the evidence supporting this therapy and how it is performed.
PROPOSED MECHANISMS OF BENEFIT
Mild therapeutic hypothermia is thought to protect against anoxic brain injury in survivors of cardiac arrest via several mechanisms:
- Decreasing neuronal metabolism in the early stage of ischemic injury
- Decreasing glucose and oxygen consumption by the brain,1 which reduces supply-demand mismatch
- Decreasing the release of excitatory amino acids (eg, glutamate) that normally trigger cytotoxic cascades in the intermediate phase of injury2
- Reducing the production of harmful reactive oxygen species3
- Maintaining cellular pH4
- Reducing cell death5
- Slowing the breakdown of the blood-brain barrier that worsens cerebral edema.6
CLINICAL DATA SUPPORTING HYPOTHERMIA
There has been an interest in therapeutic hypothermia for several decades. In the 1950s, it was used in small numbers of cases in a variety of cardiac arrest situations.7,8 Interest was rekindled in the mid-1990s after a number of animal studies suggested it might be beneficial in prolonged cerebral ischemia and anoxia,9,10 and reports of case-series described its use in adults with out-of-hospital cardiac arrest.11,12
In October 2002, the International Liaison Committee on Resuscitation (ILCOR), made up of executive members of several organizations including the American Heart Association, recommended that “unconscious adult patients with spontaneous circulation after out-of-hospital cardiac arrest” should be cooled to 32°C to 34°C [89.6°F–93.2°F] for 12 to 24 hours “when the initial rhythm was ventricular fibrillation.”13
Two large randomized trials
This position statement was based largely on the results of two randomized clinical trials published simultaneously earlier in 2002.14,15 These two trials were important not only because they were the largest randomized trials of this therapy to that point, but also because they used meaningful, prospectively defined clinical end points: all-cause mortality and degree of cognitive preservation as assessed using the Glasgow-Pittsburgh Cerebral Performance Category (CPC) scale.
Bernard et al14 performed a randomized trial in four centers in Australia, assigning 77 patients either to a goal temperature of 32°C to 34°C or to normothermia for 12 hours, with all other resuscitative measures being the same in both groups. The primary outcome measured was survival to hospital discharge with sufficient neurologic function to be discharged to home or to a rehabilitation facility, ie, a CPC score of 1 or 2.
In the hypothermia group, 21 (49%) of the 43 patients survived and had an outcome that was considered “good” (ie, they were discharged home or to a rehabilitation facility), compared with 9 (26%) of the 34 patients in the normothermia group (unadjusted odds ratio 2.65, 95% confidence interval [CI] 1.02–6.88, P = .046). Proportionally fewer patients in the hypothermia group died—22 (51%) of 43 vs 23 (68%) of 34; however, the difference was not statistically significant (P = .145).
The Hypothermia After Cardiac Arrest Study Group15 screened 3,551 European patients who suffered out-of-hospital cardiac arrest15; 275 patients were randomized to mild therapeutic hypothermia or normothermia for 24 hours. The primary outcome was the percentage of patients who had a CPC score of 1 or 2 (vs 3 to 5) at 6 months, and the secondary outcome was the rate of death at 6 months.
At 6 months, 75 (55%) of the 136 patients in the hypothermia group had a CPC score of 1 or 2, compared with 54 (39%) of the 137 patients in the normothermia group (P = .009). The rate of death was also lower with hypothermia: 55% vs 41% (P = .02).
In both trials, patients were included only if their cardiac arrest was witnessed, if their initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, if circulation spontaneously returned within 60 minutes with standard basic and advanced cardiac life support protocols, and if they were still comatose on arrival at the hospital. They were excluded if they were over age 75, if they had suffered a cerebrovascular accident at the time of cardiac arrest, or if the arrest was caused by trauma or drug overdose. In addition, the European trial excluded patients who suffered another cardiac arrest after the initial return of spontaneous circulation but before cooling was started.
The standard of care
In view of the available clinical data, the 2002 ILCOR guidelines and a 2005 statement from the American Heart Association advocated mild therapeutic hypothermia for survivors of out-of-hospital ventricular tachycardia or fibrillation.16 Subsequently, this therapy has become more widely practiced and accepted as the standard of care among critical-care providers.
Of note, some public health officials and local governments are strongly promoting this treatment for survivors of cardiac arrest in the community.17 More and more of these groups are mandating that these patients be transported only to hospitals that have therapeutic hypothermia protocols in place, bypassing those not equipped to provide this treatment.18
INDICATIONS, CONTRAINDICATIONS, AND GRAY AREAS
What are the indications and contraindications to the use of hypothermia after out-of-hospital cardiac arrest? What are some of the “gray areas”?
Indications. This treatment is indicated for comatose adults who have had a witnessed cardiac arrest, whose initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, and whose circulation spontaneously returned in less than 60 minutes with basic and advanced cardiac life support. This carries a class I recommendation, level of evidence B, and was recently reinforced in the 2010 update to the American Heart Association guidelines for cardiopulmonary resuscitation.19
Absolute contraindications include hemorrhagic stroke (which must be proved by computed tomography) and cardiac arrest due to trauma (Table 2). Other major contraindications are a Glasgow Coma Scale score of 8 or higher before the initiation of mild therapeutic hypothermia, cardiac arrest due to drug overdose, and preexisting hypothermia (< 34°C) when first-responders arrive.
Relative contraindications include baseline coagulopathy and severe hypotension (mean atrial pressure < 60 mm Hg) that is not correctable by fluid infusion, vasopressors, or invasive hemodynamic support.
Gray areas. There are not enough data to make a firm recommendation about whether to apply mild therapeutic hypothermia if a witnessed cardiac arrest with ventricular fibrillation or ventricular tachycardia occurs in the hospital, but data from out-of-hospital cardiac arrest patients appear applicable for hospitalized patients.
The data are also quite limited and equivocal on its use for out-of-hospital cardiac arrest in patients whose initial cardiac rhythm is pulseless electrical activity or asystole,20,21 likely because of the competing risk of comorbidities and the resultant lower baseline survival rate in these patients.
Consequently, for in-hospital postarrest patients with any initial rhythm and for out-of-hospital cardiac arrest patients with rhythms other than ventricular tachycardia or ventricular fibrillation, the 2010 guideline recommendation on the use of mild therapeutic hypothermia is less enthusiastic (class IIb, level of evidence B).19
There are also few data on the use of mild therapeutic hypothermia in post-arrest patients in circulatory shock requiring vasopressors or intra-aortic balloon counterpulsation, largely limited to case series and comparisons with historical controls.22,23 Further investigation is clearly needed in these areas. Until then, it should be considered at the physician’s and the team’s discretion, on a case-by-case basis.
HYPOTHERMIA IN CASES OF VENTRICULAR FIBRILLATION AND ACUTE CORONARY SYNDROME
The value of coronary angiography after out-of-hospital cardiac arrest was first highlighted by Spaulding et al,24 who performed it urgently in 84 consecutive survivors of out-of-hospital cardiac arrest, 36 of whom had ST-segment elevation myocardial infarction (STEMI). Angiography uncovered an acute coronary occlusion in 40 (48%) of the 84 patients.
In this series, ST-segment elevation was a strong predictor of acute coronary occlusion (odds ratio 4.3; 95% CI 1.6–2.0; P = .004). However, 9 patients without chest pain or ST elevation were also found to have an occluded infarct-related artery. Successful angioplasty was an independent predictor of survival, highlighting the importance of an angiographic definition in this population.
These findings were recently confirmed in the larger Parisian Region Out of Hospital Cardiac Arrest (PROCAT) registry in 435 patients who had no obvious extracardiac cause of arrest, for whom successful culprit coronary angioplasty was associated with survival.25
Angioplasty comes first, but neither treatment need be delayed
Efforts to induce hypothermia must not be allowed to delay the door-to-balloon time of post-arrest patients in the setting of STEMI. The top priority is establishing patency of the infarct-related artery with a goal of salvaging ischemic myocardium and obtaining mechanical and electrical stabilization.
Fortunately, mild therapeutic hypothermia does not necessarily delay emergency revascularization if hypothermia protocols are well established. In fact, induction of mild therapeutic hypothermia prior to or on arrival at the catheterization laboratory has been shown to be feasible and safe.26,27
We believe that all centers performing primary percutaneous coronary intervention for STEMI should have immediate access to and expertise in mild therapeutic hypothermia. Regional planning and integration of STEMI and out-of-hospital cardiac arrest networks will ensure that most patients with STEMI have access to this treatment when it is indicated.
Does hypothermia help the heart? Does it increase bleeding?
Researchers have been interested in therapeutic hypothermia as a means of reducing myocardial infarct size,28,29 but clinical trials have not shown a clear-cut benefit in this regard. However, these investigations have also added to the evidence that antiplatelet and anticoagulation therapy in patients undergoing mild therapeutic hypothermia does not result in a statistically significant excess of major bleeding events, which is a potential concern.
Of note, these studies were neither powered nor specifically designed to evaluate for major bleeding as an end point. Therefore, these complications should still be carefully monitored for.
IS THERE AN OPTIMAL TIME TO BEGIN MILD THERAPEUTIC HYPOTHERMIA?
Experimental data suggest that mild therapeutic hypothermia should be started as soon as possible after a comprehensive clinical evaluation indicates the patient is eligible.30–33 However, clinical data are not robustly in favor of starting it before the patient reaches the hospital rather than on hospital arrival.
In a recent randomized trial in 2,334 survivors of out-of-hospital cardiac arrest, outcomes were no better if hypothermia was started by paramedics than if it was started on arrival at the hospital (47.5% vs 52.6% discharged to home or rehabilitation; 95% CI 0.70–1.17; P = .43).34
Earlier data from smaller studies had suggested that prehospital initiation of hypothermia (for example, using chilled intravenous saline infusions) in carefully selected patients with out-of-hospital cardiac arrest was safe and showed a nonsignificant trend toward better outcomes.20,35
The randomized controlled trials that showed hypothermia to be beneficial used very slow cooling methods; consequently, it is reasonable to allow up to 6 hours from initial presentation to first-responders to start it. There are, however, no conclusive data in humans for or against starting it later than 6 hours after presentation. Most experts believe that its potential neurologic and mortality benefits are largely lost if it is delayed more than 6 hours.
The overall message from these data seems to be that, in patients who survive cardiac arrest outside the hospital with ventricular tachycardia or fibrillation, mild therapeutic hypothermia is effective and safe and should be started as soon as possible after arrival at the hospital.
METHODS FOR INDUCING AND MAINTAINING HYPOTHERMIA
Cooling the patient
To cool the patient and keep him or her cold, caregivers have used ice packs placed around the head, groin, and axillae; intravenous infusion of saline maintained at 4°C (39°F); and cooling-air blankets. More recently, thermal wraps and intravascular cooling catheters have been used.36–38 The newer methods are more effective in rapidly bringing patients to the target temperature of 32 to 34°C (usually within 3 or 4 hours) and keeping them within this range, and they auto-adjust their output on the basis of measured core temperature.
The Pre ROSC Intranasal Cooling Effectiveness (PRINCE) trial demonstrated the safety and efficacy of nasopharyngeal cooling using a perfluorocarbon aerosol given via a nasopharyngeal cannula in patients with out-of-hospital cardiac arrest.39
Monitoring the core temperature
The patient’s core temperature is most commonly monitored with a probe in the esophagus, bladder, rectum, or pulmonary artery.40
Of these, the bladder and rectum are considered “intermediate” monitoring sites, as their temperatures tend to lag behind the core temperature. Furthermore, the bladder temperature can be significantly altered by the flow of urine, which can vary considerably during the cooling and rewarming process.
Esophageal temperature monitoring is relatively noninvasive and tends to reliably and accurately reflect core temperature as long as the probe is placed far enough down (about 45 cm from the nose in an average adult) that it is not affected by proximity to the trachea.
Pulmonary artery catheters are considered the gold standard for core temperature monitoring, but they pose risks such as bloodstream infection and large-vessel damage. In practice, many patients admitted to the coronary intensive care unit after out-of-hospital cardiac arrest require pulmonary artery catheterization anyway for other indications, and in these situations it is the preferred method of monitoring the core temperature.
However, no approach is ideal in terms of measuring the temperature in the critical end organs. Rather, core temperature monitoring serves as a guide to help ensure consistent clinical practice in attaining and maintaining mild therapeutic hypothermia.
Preventing shivering
To achieve and maintain the goal temperature, the body’s natural response to a decrease in core temperature—shivering—must be watched for and eliminated. A number of drugs may be used for this purpose.41
Paralytic drugs are used to reduce shivering; nursing staff must be trained to monitor for signs of occult shivering (eg, jaw vibration) and adjust the dose of paralytic drug accordingly. Since the patients are paralyzed, they must also receive continuous intravenous sedation.
Other commonly used drugs that decrease the hypothalamic drive to shiver include buspirone (BuSpar), a serotonin 5HT-1A partial agonist, and meperidine (Demerol), an opiate agonist of kappa and mu receptors.
Rewarming after 24 hours
Rewarming is conventionally started after 24 hours of mild therapeutic hypothermia, at a rate no greater than 0.5°C (1°F) per hour.
Because sedation is used during the hypothermia period of 24 hours, a washout period for these medications is necessary, and the neurologic prognosis of cardiac arrest patients who undergo mild therapeutic hypothermia cannot be adequately assessed until 72 hours after rewarming.
ADVERSE EFFECTS OF MILD THERAPEUTIC HYPOTHERMIA
Some of these effects are predictable. Decreasing the body temperature causes potassium to shift into the cells, and this same potassium will leave the intracellular space during the rewarming phase. For this reason, aggressive potassium repletion for mild hypokalemia (potassium levels of 3.0–3.5 mmol/L) during mild therapeutic hypothermia can result in dangerous hyperkalemia during rewarming and should generally be avoided.
As another example, the enzymes involved in coagulation are less effective at lower temperatures. Thus, if it occurs, active bleeding requiring transfusion warrants consideration of stopping the hypothermia.
Adverse effects should be watched for (eg, by checking electrolyte levels frequently, monitoring blood glucose, continuous electroencephalographic monitoring during the cooling phase, and avoiding placement of intracardiac catheters once the goal temperature is reached) and addressed as they happen. However, in a recent review of this subject42 the balance of evidence continued to indicate that the benefit of this treatment exceeds its risks.
OUR PATIENT RECOVERS
After 24 hours of therapeutic hypothermia, our patient was gradually rewarmed to a normal temperature, and sedation and paralysis were discontinued.
Analysis of his prearrest and postarrest 12-lead electrocardiograms revealed a type I Brugada pattern (coved ST elevation and negative T waves in V1, V2, and V3, caused by abnormal repolarization due to inherited mutations in SCN5A). Cardiac catheterization revealed normal coronary arteries, and MRI revealed no evidence of arrhythmogenic right ventricular cardiomyopathy or other structural abnormalities.
In the next 72 hours the patient was successfully extubated, and he gradually returned to full neurologic function. Before he went home a few days later, a single-lead cardioverter-defibrillator was implanted to prevent sudden cardiac death. All of his first-degree relatives were encouraged to undergo genetic screening for SCN5A mutations. The patient is currently back to his previous high level of functioning as a marketing manager, husband, and father of two young children.
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab 2003; 23:513–530.
- Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke 1996; 27:913–918.
- Thoresen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997; 8:3359–3362.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37(suppl 7):S186–S202.
- Yang D, Guo S, Zhang T, Li H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett 2009; 583:2500–2506.
- Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1994; 14:620–627.
- Benson DW, Williams GR, Spencer FC, Yates AJ. The use of hypothermia after cardiac arrest. Anesth Analg 1959; 38:423–428.
- Williams GR, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958; 148:462–468.
- Baker CJ, Onesti ST, Barth KN, Prestigiacomo CJ, Solomon RA. Hypothermic protection following middle cerebral artery occlusion in the rat. Surg Neurol 1991; 36:175–180.
- Ridenour TR, Warner DS, Todd MM, McAllister AC. Mild hypothermia reduces infarct size resulting from temporary but not permanent focal ischemia in rats. Stroke 1992; 23:733–738.
- Bernard SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med 1997; 30:146–153.
- Yanagawa Y, Ishihara S, Norio H, et al. Preliminary clinical outcome study of mild resuscitative hypothermia after out-of-hospital cardiopulmonary arrest. Resuscitation 1998; 39:61–66.
- Nolan JP, Morley PT, Vanden Hoek TL, et al; International Liaison Committee on Resuscitation. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation 2003; 108:118–121.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557–563.
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549–556.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005; 112(suppl 24):IV1–IV203.
- Hartocollis A. “City Pushes Cooling Therapy for Cardiac Arrest”. New York Times, December4th, 2008,A1. http://www.nytimes.com/2008/12/04/nyregion/04cool.html. Accessed May 31, , 2011.
- Nichol G, Aufderheide TP, Eigel B, et al; American Heart Association Emergency Cardiovascular Care Committee. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation 2010; 121:709–729.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122(suppl 3):S640–S56.
- Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001; 51:275–281.
- Kim F, Olsufka M, Longstreth WT, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064–3070.
- Hovdenes J, Laake JH, Aaberge L, Haugaa H, Bugge JF. Therapeutic hypothermia after out-of-hospital cardiac arrest: experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol Scand 2007; 51:137–142.
- Skulec R, Kovarnik T, Dostalova G, Kolar J, Linhart A. Induction of mild hypothermia in cardiac arrest survivors presenting with cardiogenic shock syndrome. Acta Anaesthesiol Scand 2008; 52:188–194.
- Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 1997; 336:1629–1633.
- Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv 2010; 3:200–207.
- Knafelj R, Radsel P, Ploj T, Noc M. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation 2007; 74:227–234.
- Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med 2008; 36:1780–1786.
- O’Neill WW, on behalf of the COOL-MI Investigators. Cooling as an adjunct to primary PCI for myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Grines CL, on behalf of the ICE-IT Investigators. Intravascular cooling adjunctive to percutaneous coronary intervention for acute myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Weil MH, Gazmuri RJ. Hypothermia after cardiac arrest. Crit Care Med 1991; 19:315.
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004; 109:2786–2791.
- Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 2008; 77:242–249.
- Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008; 76:431–442.
- Bernard SA, Smith K, Cameron P, et al; Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation 2010; 122:737–742.
- Bruel C, Parienti JJ, Marie W, et al. Mild hypothermia during advanced life support: a preliminary study in out-of-hospital cardiac arrest. Crit Care 2008; 12:R31.
- Pichon N, Amiel JB, François B, Dugard A, Etchecopar C, Vignon P. Efficacy of and tolerance to mild induced hypothermia after out-of-hospital cardiac arrest using an endovascular cooling system. Crit Care 2007; 11:R71.
- Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol 2009; 133:223–228.
- Heard KJ, Peberdy MA, Sayre MR, et al. A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation 2010; 81:9–14.
- Castrén M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010; 122:729–736.
- Insler SR, Sessler DI. Perioperative thermoregulation and temperature monitoring. Anesthesiol Clin 2006; 24:823–837.
- Weant KA, Martin JE, Humphries RL, Cook AM. Pharmacologic options for reducing the shivering response to therapeutic hypothermia. Pharmacotherapy 2010; 30:830–841.
- Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 2010; 363:1256–1264.
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab 2003; 23:513–530.
- Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke 1996; 27:913–918.
- Thoresen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997; 8:3359–3362.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37(suppl 7):S186–S202.
- Yang D, Guo S, Zhang T, Li H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett 2009; 583:2500–2506.
- Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1994; 14:620–627.
- Benson DW, Williams GR, Spencer FC, Yates AJ. The use of hypothermia after cardiac arrest. Anesth Analg 1959; 38:423–428.
- Williams GR, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958; 148:462–468.
- Baker CJ, Onesti ST, Barth KN, Prestigiacomo CJ, Solomon RA. Hypothermic protection following middle cerebral artery occlusion in the rat. Surg Neurol 1991; 36:175–180.
- Ridenour TR, Warner DS, Todd MM, McAllister AC. Mild hypothermia reduces infarct size resulting from temporary but not permanent focal ischemia in rats. Stroke 1992; 23:733–738.
- Bernard SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med 1997; 30:146–153.
- Yanagawa Y, Ishihara S, Norio H, et al. Preliminary clinical outcome study of mild resuscitative hypothermia after out-of-hospital cardiopulmonary arrest. Resuscitation 1998; 39:61–66.
- Nolan JP, Morley PT, Vanden Hoek TL, et al; International Liaison Committee on Resuscitation. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation 2003; 108:118–121.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557–563.
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549–556.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005; 112(suppl 24):IV1–IV203.
- Hartocollis A. “City Pushes Cooling Therapy for Cardiac Arrest”. New York Times, December4th, 2008,A1. http://www.nytimes.com/2008/12/04/nyregion/04cool.html. Accessed May 31, , 2011.
- Nichol G, Aufderheide TP, Eigel B, et al; American Heart Association Emergency Cardiovascular Care Committee. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation 2010; 121:709–729.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122(suppl 3):S640–S56.
- Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001; 51:275–281.
- Kim F, Olsufka M, Longstreth WT, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064–3070.
- Hovdenes J, Laake JH, Aaberge L, Haugaa H, Bugge JF. Therapeutic hypothermia after out-of-hospital cardiac arrest: experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol Scand 2007; 51:137–142.
- Skulec R, Kovarnik T, Dostalova G, Kolar J, Linhart A. Induction of mild hypothermia in cardiac arrest survivors presenting with cardiogenic shock syndrome. Acta Anaesthesiol Scand 2008; 52:188–194.
- Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 1997; 336:1629–1633.
- Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv 2010; 3:200–207.
- Knafelj R, Radsel P, Ploj T, Noc M. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation 2007; 74:227–234.
- Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med 2008; 36:1780–1786.
- O’Neill WW, on behalf of the COOL-MI Investigators. Cooling as an adjunct to primary PCI for myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Grines CL, on behalf of the ICE-IT Investigators. Intravascular cooling adjunctive to percutaneous coronary intervention for acute myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Weil MH, Gazmuri RJ. Hypothermia after cardiac arrest. Crit Care Med 1991; 19:315.
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004; 109:2786–2791.
- Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 2008; 77:242–249.
- Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008; 76:431–442.
- Bernard SA, Smith K, Cameron P, et al; Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation 2010; 122:737–742.
- Bruel C, Parienti JJ, Marie W, et al. Mild hypothermia during advanced life support: a preliminary study in out-of-hospital cardiac arrest. Crit Care 2008; 12:R31.
- Pichon N, Amiel JB, François B, Dugard A, Etchecopar C, Vignon P. Efficacy of and tolerance to mild induced hypothermia after out-of-hospital cardiac arrest using an endovascular cooling system. Crit Care 2007; 11:R71.
- Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol 2009; 133:223–228.
- Heard KJ, Peberdy MA, Sayre MR, et al. A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation 2010; 81:9–14.
- Castrén M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010; 122:729–736.
- Insler SR, Sessler DI. Perioperative thermoregulation and temperature monitoring. Anesthesiol Clin 2006; 24:823–837.
- Weant KA, Martin JE, Humphries RL, Cook AM. Pharmacologic options for reducing the shivering response to therapeutic hypothermia. Pharmacotherapy 2010; 30:830–841.
- Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 2010; 363:1256–1264.
KEY POINTS
- This treatment is indicated for comatose adult patients who have had a witnessed cardiac arrest, whose initial cardiac rhythm is ventricular fibrillation or pulseless ventricular tachycardia, and who have return of spontaneous circulation with basic and advanced cardiac life support.
- Contraindications include hemorrhagic stroke, a Glasgow Coma Scale score of 8 or higher, cardiac arrest due to drug overdose, and preexisting hypothermia. Relative contraindications include baseline coagulopathy and severe hypotension (mean arterial pressure < 60 mm Hg) that is not correctable by fluid infusion, vasopressors, or invasive hemodynamic support.
- Adverse effects have included hypokalemia, bradyarrhythmia, ventricular tachycardia, hypotension, seizures, hyperglycemia, a transient decrease in the glomerular filtration rate, abnormal coagulation studies, and an increased incidence of pneumonia and sepsis.
Dual antiplatelet therapy in coronary artery disease: A case-based approach
Plaque rupture and thrombosis play central roles in the genesis of acute coronary syndrome. Aspirin has long been the preventive agent of choice. But dual antiplatelet therapy with aspirin plus clopidogrel (Plavix) is warranted in many patients to further reduce their risk of future cardiovascular events.
Although dual antiplatelet therapy is usually started by a subspecialist, the primary care physician is often the one who ensures that the patient remains compliant with it in the long term. A review of the seminal published data is helpful in understanding the rationale behind dual antiplatelet therapy and its risks and benefits.
In the mid-1990s, the thienopyridine ticlopidine (Ticlid) was found to significantly decrease the number of deaths, target-lesion revascularizations, and myocardial infarctions (MIs) in the 30 days following stent placement. 1 However, 2% to 3% of patients experienced neutropenia2 and thrombotic thrombocytopenic purpura with this drug,3 leading to the use of clopidogrel, another agent in the same class. Over the past decade, a large body of evidence has established the usefulness of clopidogrel in a number of clinical settings.
In this paper we review the current use of clopidogrel in ST-elevation MI, non-ST-elevation acute coronary syndromes, and percutaneous coronary intervention, and discuss the landmark trials that are the basis for the treatment guidelines published jointly by the American College of Cardiology (ACC) and the American Heart Association (AHA).4–6 We also briefly discuss the use of prasugrel (Effient), the newest antiplatelet agent to gain approval from the US Food and Drug Administration (FDA).
CLOPIDOGREL AS AN ALTERNATIVE TO ASPIRIN
Clopidogrel, a prodrug, is converted into its active form in the liver.7 It then irreversibly binds to the platelet P2Y12 receptor and inhibits adenosine diphosphate-induced platelet aggregation.
Those treated with clopidogrel had an annual risk of ischemic stroke, MI, or vascular death of 5.32%, compared with 5.83% in the aspirin group, for a statistically significant 8.7% relative risk reduction (P = .043). The observed frequency of neutropenia (neutrophils < 1.2 × 109/L) was 0.10% with clopidogrel vs 0.17% with aspirin. This study showed clopidogrel to be an effective alternative in patients who cannot tolerate aspirin.
CASE 1: ST-ELEVATION MI
A 57-year-old farmer in rural Ohio with a history of hypertension and hyperlipidemia presents to the local emergency department 45 minutes after the onset, while he was chopping wood, of dull, aching, substernal chest pain that radiates to his jaw. Electrocardiography reveals 2-mm ST-segment elevation in leads V1 through V6. He is treated with aspirin 162 mg, low-molecular-weight heparin, and tenecteplase.
What would be the value of starting dual antiplatelet therapy with clopidogrel in this patient?
Clopidogrel, aspirin, and fibrinolysis in ST-elevation MI
The CLARITY-TIMI 28 trial (Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis in Myocardial Infarction)9 included 3,491 patients (ages 18 to 75) from 319 international sites. All patients received a fibrinolytic agent, aspirin (162 mg to 325 mg on the first day and 75 mg to 162 mg thereafter), and heparin as part of standard care for acute ST-elevation MI (Table 1). Patients were randomized to receive a 300-mg loading dose of clopidogrel followed by 75 mg daily or placebo within 12 hours of onset of ST-elevation MI. The status of the infarct-related artery was ascertained by protocol-mandated coronary angiography 48 to 192 hours after starting the study medication. The primary end point was the composite of an occluded infarct-related artery on angiography, death from any cause prior to angiography, or recurrent MI prior to angiography.
Significantly fewer patients had an end point event in the clopidogrel group than in the placebo group, 15% vs 21.7% (P < .001), for a relative risk reduction of 31%. There was no significant increase in major or minor bleeding events.
Of note, the CLARITY-TIMI 28 patients were relatively young (average age 57 years) and at low cardiovascular risk (30-day mortality risk < 5%).
The COMMIT trial (Clopidogrel and Metoprolol in Myocardial Infarction)10 consisted of 45,852 patients with suspected acute MI admitted to 1,250 hospitals in China. Each patient received aspirin 162 mg daily plus either clopidogrel 75 mg daily (n = 22,961) or placebo (n = 22,891) for the duration of hospitalization (average 16 days) or 28 days, whichever came first.
The incidence of the primary composite end point of death, reinfarction, or stroke was significantly lower with clopidogrel than with placebo (9.2% vs 10.1%, P = .002). This was regardless of age (the average age was 61, and 26% of patients were older than 70), sex, time to presentation (67% presented within 12 hours), or reperfusion strategy (49% underwent fibrinolysis). The clopidogrel group did not have a significantly higher incidence of bleeding, but patients in this trial did not receive a loading dose of clopidogrel.
Comment. In view of the results of these trials, our 57-year-old patient should start clopidogrel early.
CASE 2: NON-ST-ELEVATION ACUTE CORONARY SYNDROME
A 65-year-old woman living independently with no significant medical history presents to the emergency room with 2 hours of waxing and waning substernal chest pain. Her blood pressure is 145/90 mm Hg, her heart rate is 95 beats per minute, and the results of her physical examination are unremarkable. Resting electrocardiography reveals 1.5-mm ST-segment depression in the inferior leads, and her troponin T level on admission is two times the upper limit of normal. She is given aspirin and is started on low-molecular-weight heparin and intravenous nitroglycerin.
What would be the value of starting clopidogrel in this patient?
Clopidogrel in non-ST-elevation acute coronary syndromes
The ACC/AHA guidelines strongly support starting clopidogrel in patients with non-ST-elevation acute coronary syndromes (Table 2).5
The CURE trial (Clopidogrel in Unstable Angina to Prevent Recurrent Events)11 provided the evidence for this recommendation. In this trial, 12,562 patients from 482 centers in 28 countries who presented within 24 hours of coronary symptoms, without ST elevation, were randomized to receive either clopidogrel (a 300-mg loading dose, followed by 75 mg daily) or placebo for 3 to 12 months (mean 9 months).
Significantly fewer patients in the clopidogrel group reached one of the end points of the composite primary outcome (cardiovascular death, nonfatal MI, or stroke): 9.3% vs 11.4%, 95% confidence interval (CI) 0.72–0.90, P < .001. Significantly fewer of them also suffered one of the secondary outcomes, ie, severe ischemia, heart failure, or need for revascularization.
Of concern was a higher rate of major bleeding in the clopidogrel group (3.7%) than in the placebo group (2.7%) without an excess of fatal bleeding. For every 1,000 patients treated with clopidogrel, 6 required a blood transfusion. Nevertheless, CURE proved that patients with non-ST-elevation acute coronary syndromes benefited from clopidogrel, regardless of whether they underwent percutaneous coronary intervention.
Comment. Our patient should receive clopidogrel and, if she has no significant bleeding, she should continue to take it for at least 12 months after discharge. It is important for the primary care physician to ensure compliance with this agent and not discontinue it on routine clinical follow-up.
CASE 3: BARE-METAL STENT PLACEMENT
A 62-year-old man with a history of hypertension, diabetes, and hyperlipidemia presents to his primary care physician’s office with stable-effort angina that is not responding to an excellent anti-ischemic regimen and is affecting his quality of life. He is referred for coronary angiography, which reveals 80% stenosis of the proximal left circumflex artery. He undergoes a percutaneous coronary intervention with placement of a bare-metal stent.
How long should he be on clopidogrel? And what if a drug-eluting stent had been placed instead of a bare-metal stent?
Dual therapy after bare-metal stent placement
Dual antiplatelet therapy with clopidogrel and aspirin is recommended in all patients receiving a stent (Table 2). The better safety and efficacy of clopidogrel compared with ticlopidine has been established in patients receiving a coronary artery stent,12,13 and clopidogrel’s favorable safety profile soon made it the thienopyridine of choice.
The CREDO trial (Clopidogrel for the Reduction of Events During Observation)14 randomized 2,116 patients undergoing an elective percutaneous coronary intervention (bare-metal stent placement only) to receive a 300-mg loading dose of clopidogrel 3 to 24 hours before the procedure, or placebo. All patients received 325 mg of aspirin. After the intervention, all patients received clopidogrel 75 mg daily and aspirin 325 mg daily through day 28. For day 29 through 12 months, those who had received the 300-mg preprocedural loading dose of clopidogrel continued with 75 mg daily, and those who had not received clopidogrel before the procedure received placebo.
No significant difference was seen in the primary outcome for those who received pretreatment with clopidogrel; however, in a subgroup analysis, those who received clopidogrel at least 6 hours before the percutaneous coronary intervention had a 38.6% relative risk reduction (Table 1). Long-term use of clopidogrel (ie, for 12 months) was associated with an overall relative reduction of 26.9% in the combined risk of death, MI, or stroke.
PCI-CURE, an analysis of 2,658 patients in the CURE trial with non-ST-elevation acute coronary syndrome who underwent PCI,15 yielded results similar to those of CREDO, with a 31% reduction in the rate of cardiovascular death or MI at 30 days and at 9 months. Of note, however, clopidogrel was given for a median of 6 days prior to the procedure.
Comment. The minimum suggested duration of clopidogrel treatment after placement of a bare-metal stent is 1 month. However, these trial results indicate that patients who are not at high risk of bleeding should take clopidogrel for at least 12 months.
Dual antiplatelet therapy with drug-eluting stents
Although rates of in-stent restenosis are clearly lower with drug-eluting stents than with bare-metal stents, the antiproliferative effect of drug-eluting stents may delay complete endothelialization of every strut. This may contribute to late (> 1 month after placement) or very late (> 1 year) thrombosis of the stent after clopidogrel is discontinued.16–18
In 2006, the FDA indicated that dual antiplatelet therapy was needed for 6 months with paclitaxel-eluting (Taxus) stents and 3 months with sirolimus-eluting (Cipher) stents. As reports of very late stent thrombosis began to appear in 2007, concern arose over the need to extend the duration of clopidogrel treatment.
Bavry et al19 quantified the incidence of late and very late stent thrombosis in a meta-analysis of 14 clinical trials that randomized patients to receive either a drug-eluting stent (paclitaxel or sirolimus) or a bare-metal stent.19 The incidence of stent thrombosis within 30 days in this analysis was similar for both groups—4.4 per 1,000 patients vs 5 per 1,000 (relative risk 0.89; 95% CI 0.46–1.75; P = .74). However, the rate of very late stent thrombosis was significantly higher in those receiving a drug-eluting stent vs a bare-metal stent—5 per 1,000 patients treated (relative risk 5.02, 95% CI 1.29–19.52; P = .02).
The results of this and other studies led the ACC and AHA to revise their joint guidelines to recommend thienopyridine treatment for at least 1 year for patients who receive a drug-eluting stent.6,20–22 In fact, many cardiologists consider indefinite dual antiplatelet therapy in patients with a drug-eluting stent to avoid very late in-stent thrombosis, especially in patients undergoing high-risk interventions such as placement of multiple stents, bifurcation lesions, and unprotected left main trunk interventions.
Thus, when faced with a patient with a recent coronary stent implantation, the primary care physician should be aware of the type of stent and the duration of therapy recommended by the interventional cardiologist. Also, in the absence of a pressing indication, elective surgery should be deferred for 1 year after placement of a drug-eluting stent, as this would necessitate stopping clopidogrel and would increase the risk of perioperative stent thrombosis, which is associated with high rates of morbidity and death.
CASE 4: HIGH-RISK CORONARY ARTERY DISEASE
A 67-year-old woman presents to your office to establish care. She has a history of diabetes and established coronary artery disease with two bare-metal stents placed 2 years ago. She is taking aspirin 81 mg.
What would be the value of adding clopidogrel to her regimen?
No indication for clopidogrel in chronic coronary artery disease
The CHARISMA trial (Clopidogrel for High Atherothrombotic and Ischemic Stabilization, Management, and Avoidance)23 randomized 15,603 patients with stable cardiovascular disease or multiple risk factors to receive either clopidogrel plus low-dose aspirin or placebo plus low-dose aspirin and followed them for a median of 28 months (Table 1).
The primary end point (a composite of MI, stroke, or death) was 6.8% with clopidogrel plus aspirin and 7.3% with aspirin alone, indicating no significant benefit with clopidogrel plus aspirin compared with aspirin alone in reducing the rate of MI, stroke, or cardiovascular death in patients with high-risk but stable atherothrombotic disease. A marginal statistical benefit with dual antiplatelet therapy was noted in the subgroup of patients with previously documented coronary, cerebrovascular, or peripheral vascular disease—6.9% with aspirin plus clopidogrel vs 7.9% with aspirin alone (relative risk 0.88; 95% CI 0.77–0.998; P = .046).
Consequently, there is no compelling reason to start clopidogrel in this patient.
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel was recently approved by the FDA as antiplatelet treatment for patients with acute coronary syndromes planning to undergo a percutaneous coronary intervention.24 It has been shown to inhibit adenosine-diphosphate-induced platelet activation in a more consistent and effective manner than clopidogrel.25,26
Although both clopidogrel and prasugrel are prodrugs, 80% of absorbed clopidogrel is metabolized by esterases into inactive metabolites, and the availability of active metabolite can vary, as it is significantly influenced by polymorphisms in the cytochrome P450 system. 27 In contrast, prasugrel is not degraded by esterases, and its conversion to active metabolite by the cytochrome P450 system is not influenced by common genetic polymorphisms, particularly CYP2C19*2.
TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction) provided most of the evidence for the approval of prasugrel for clinical use.28,29 In this trial, a 60-mg loading dose of prasugrel followed by a daily maintenance dose of 10 mg was significantly superior to the current clopidogrel regimen in preventing death from cardiovascular causes, nonfatal MI, or nonfatal stroke during a study period of 15 months.28 Also observed was a 24% lower rate of MI, a 34% lower rate of urgent target-vessel revascularization, and a 52% lower rate of stent thrombosis.
These benefits, however, came at the cost of a significantly higher risk of major bleeding, including the potential for three excess fatal bleeding events for every 1,000 patients treated. Patients at highest risk at the dosages evaluated included the elderly (age 75 and older), patients who weigh less than 60 kg, and patients with a history of stroke or transient ischemic attack. Based on these results, we recommend caution with the use of prasugrel in these patient subsets.
Clinical use of prasugrel is likely to be highest in patients presenting with ST-elevation MI who are undergoing a primary percutaneous coronary intervention. There is currently no evidence from any randomized clinical trial to support the safety of prasugrel given in the emergency room or “upstream” in the setting of non-ST-elevation acute coronary syndromes.
Of note, patients with non-ST-elevation acute coronary syndromes in the TRITON trial were randomized only after angiographic definition. As a result, only 179 patients exposed to prasugrel were referred for coronary artery bypass surgery, but the rate of surgery-related major bleeding in this group was 13.4% (vs 3.2% in the clopidogrel group). Based on these data, prasugrel should be withheld for at least 1 week prior to any surgery.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Yeh SP, Hsueh EJ, Wu H, Wang YC. Ticlopidine-associated aplastic anemia. A case report and review of literature, Ann Hematol 1998; 76:87–90.
- Page Y, Tardy B, Zeni F, Comtet C, Terrana R, Bertrand JC. Thrombotic thrombocytopenic purpura related to ticlopidine. Lancet 1991; 337:774–776.
- Canadian Cardiovascular Society; Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007; 50:e1–e157.
- King SB, Smith SC, Hirshfeld JW, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:172–209.
- Kam PC, Nethery CM. The thienopyridine derivatives (platelet adenosine diphosphate receptor antagonists), pharmacology and clinical developments. Anaesthesia 2003; 58:28–35.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Sabatine MS, Cannon CP, Gibson M, et al; CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation, N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: a randomized placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Bhatt DL, Bertrand ME, Berger PB, et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J Am Coll Cardiol 2002; 39:9–14.
- Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH; CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS). Circulation 2000; 102:624–629.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Mehta SR, Yusuf S, Peters RJ, et a; Clopidogrel in Unstable Angina to Prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001; 358:527–533.
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007; 115:1051–1058.
- Kotani J, Awata M, Nanto S, et al. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol 2006; 47:2108–2111.
- Pfisterer M, Brunner-La Rocca HP, Buser PT, et al; BASKETLATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol 2006; 48:2584–2591.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med 2007; 356:1030–1039.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- US Food and Drug Administration. FDA Approves Effient to Reduce the Risk of Heart Attack in Angioplasty Patients. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm171497.htm. Accessed October 2, 2009.
- Jernber T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006; 27:1166–1173.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A TRITON-TIMI (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Card 2008; 51:2028–2033.
Plaque rupture and thrombosis play central roles in the genesis of acute coronary syndrome. Aspirin has long been the preventive agent of choice. But dual antiplatelet therapy with aspirin plus clopidogrel (Plavix) is warranted in many patients to further reduce their risk of future cardiovascular events.
Although dual antiplatelet therapy is usually started by a subspecialist, the primary care physician is often the one who ensures that the patient remains compliant with it in the long term. A review of the seminal published data is helpful in understanding the rationale behind dual antiplatelet therapy and its risks and benefits.
In the mid-1990s, the thienopyridine ticlopidine (Ticlid) was found to significantly decrease the number of deaths, target-lesion revascularizations, and myocardial infarctions (MIs) in the 30 days following stent placement. 1 However, 2% to 3% of patients experienced neutropenia2 and thrombotic thrombocytopenic purpura with this drug,3 leading to the use of clopidogrel, another agent in the same class. Over the past decade, a large body of evidence has established the usefulness of clopidogrel in a number of clinical settings.
In this paper we review the current use of clopidogrel in ST-elevation MI, non-ST-elevation acute coronary syndromes, and percutaneous coronary intervention, and discuss the landmark trials that are the basis for the treatment guidelines published jointly by the American College of Cardiology (ACC) and the American Heart Association (AHA).4–6 We also briefly discuss the use of prasugrel (Effient), the newest antiplatelet agent to gain approval from the US Food and Drug Administration (FDA).
CLOPIDOGREL AS AN ALTERNATIVE TO ASPIRIN
Clopidogrel, a prodrug, is converted into its active form in the liver.7 It then irreversibly binds to the platelet P2Y12 receptor and inhibits adenosine diphosphate-induced platelet aggregation.
Those treated with clopidogrel had an annual risk of ischemic stroke, MI, or vascular death of 5.32%, compared with 5.83% in the aspirin group, for a statistically significant 8.7% relative risk reduction (P = .043). The observed frequency of neutropenia (neutrophils < 1.2 × 109/L) was 0.10% with clopidogrel vs 0.17% with aspirin. This study showed clopidogrel to be an effective alternative in patients who cannot tolerate aspirin.
CASE 1: ST-ELEVATION MI
A 57-year-old farmer in rural Ohio with a history of hypertension and hyperlipidemia presents to the local emergency department 45 minutes after the onset, while he was chopping wood, of dull, aching, substernal chest pain that radiates to his jaw. Electrocardiography reveals 2-mm ST-segment elevation in leads V1 through V6. He is treated with aspirin 162 mg, low-molecular-weight heparin, and tenecteplase.
What would be the value of starting dual antiplatelet therapy with clopidogrel in this patient?
Clopidogrel, aspirin, and fibrinolysis in ST-elevation MI
The CLARITY-TIMI 28 trial (Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis in Myocardial Infarction)9 included 3,491 patients (ages 18 to 75) from 319 international sites. All patients received a fibrinolytic agent, aspirin (162 mg to 325 mg on the first day and 75 mg to 162 mg thereafter), and heparin as part of standard care for acute ST-elevation MI (Table 1). Patients were randomized to receive a 300-mg loading dose of clopidogrel followed by 75 mg daily or placebo within 12 hours of onset of ST-elevation MI. The status of the infarct-related artery was ascertained by protocol-mandated coronary angiography 48 to 192 hours after starting the study medication. The primary end point was the composite of an occluded infarct-related artery on angiography, death from any cause prior to angiography, or recurrent MI prior to angiography.
Significantly fewer patients had an end point event in the clopidogrel group than in the placebo group, 15% vs 21.7% (P < .001), for a relative risk reduction of 31%. There was no significant increase in major or minor bleeding events.
Of note, the CLARITY-TIMI 28 patients were relatively young (average age 57 years) and at low cardiovascular risk (30-day mortality risk < 5%).
The COMMIT trial (Clopidogrel and Metoprolol in Myocardial Infarction)10 consisted of 45,852 patients with suspected acute MI admitted to 1,250 hospitals in China. Each patient received aspirin 162 mg daily plus either clopidogrel 75 mg daily (n = 22,961) or placebo (n = 22,891) for the duration of hospitalization (average 16 days) or 28 days, whichever came first.
The incidence of the primary composite end point of death, reinfarction, or stroke was significantly lower with clopidogrel than with placebo (9.2% vs 10.1%, P = .002). This was regardless of age (the average age was 61, and 26% of patients were older than 70), sex, time to presentation (67% presented within 12 hours), or reperfusion strategy (49% underwent fibrinolysis). The clopidogrel group did not have a significantly higher incidence of bleeding, but patients in this trial did not receive a loading dose of clopidogrel.
Comment. In view of the results of these trials, our 57-year-old patient should start clopidogrel early.
CASE 2: NON-ST-ELEVATION ACUTE CORONARY SYNDROME
A 65-year-old woman living independently with no significant medical history presents to the emergency room with 2 hours of waxing and waning substernal chest pain. Her blood pressure is 145/90 mm Hg, her heart rate is 95 beats per minute, and the results of her physical examination are unremarkable. Resting electrocardiography reveals 1.5-mm ST-segment depression in the inferior leads, and her troponin T level on admission is two times the upper limit of normal. She is given aspirin and is started on low-molecular-weight heparin and intravenous nitroglycerin.
What would be the value of starting clopidogrel in this patient?
Clopidogrel in non-ST-elevation acute coronary syndromes
The ACC/AHA guidelines strongly support starting clopidogrel in patients with non-ST-elevation acute coronary syndromes (Table 2).5
The CURE trial (Clopidogrel in Unstable Angina to Prevent Recurrent Events)11 provided the evidence for this recommendation. In this trial, 12,562 patients from 482 centers in 28 countries who presented within 24 hours of coronary symptoms, without ST elevation, were randomized to receive either clopidogrel (a 300-mg loading dose, followed by 75 mg daily) or placebo for 3 to 12 months (mean 9 months).
Significantly fewer patients in the clopidogrel group reached one of the end points of the composite primary outcome (cardiovascular death, nonfatal MI, or stroke): 9.3% vs 11.4%, 95% confidence interval (CI) 0.72–0.90, P < .001. Significantly fewer of them also suffered one of the secondary outcomes, ie, severe ischemia, heart failure, or need for revascularization.
Of concern was a higher rate of major bleeding in the clopidogrel group (3.7%) than in the placebo group (2.7%) without an excess of fatal bleeding. For every 1,000 patients treated with clopidogrel, 6 required a blood transfusion. Nevertheless, CURE proved that patients with non-ST-elevation acute coronary syndromes benefited from clopidogrel, regardless of whether they underwent percutaneous coronary intervention.
Comment. Our patient should receive clopidogrel and, if she has no significant bleeding, she should continue to take it for at least 12 months after discharge. It is important for the primary care physician to ensure compliance with this agent and not discontinue it on routine clinical follow-up.
CASE 3: BARE-METAL STENT PLACEMENT
A 62-year-old man with a history of hypertension, diabetes, and hyperlipidemia presents to his primary care physician’s office with stable-effort angina that is not responding to an excellent anti-ischemic regimen and is affecting his quality of life. He is referred for coronary angiography, which reveals 80% stenosis of the proximal left circumflex artery. He undergoes a percutaneous coronary intervention with placement of a bare-metal stent.
How long should he be on clopidogrel? And what if a drug-eluting stent had been placed instead of a bare-metal stent?
Dual therapy after bare-metal stent placement
Dual antiplatelet therapy with clopidogrel and aspirin is recommended in all patients receiving a stent (Table 2). The better safety and efficacy of clopidogrel compared with ticlopidine has been established in patients receiving a coronary artery stent,12,13 and clopidogrel’s favorable safety profile soon made it the thienopyridine of choice.
The CREDO trial (Clopidogrel for the Reduction of Events During Observation)14 randomized 2,116 patients undergoing an elective percutaneous coronary intervention (bare-metal stent placement only) to receive a 300-mg loading dose of clopidogrel 3 to 24 hours before the procedure, or placebo. All patients received 325 mg of aspirin. After the intervention, all patients received clopidogrel 75 mg daily and aspirin 325 mg daily through day 28. For day 29 through 12 months, those who had received the 300-mg preprocedural loading dose of clopidogrel continued with 75 mg daily, and those who had not received clopidogrel before the procedure received placebo.
No significant difference was seen in the primary outcome for those who received pretreatment with clopidogrel; however, in a subgroup analysis, those who received clopidogrel at least 6 hours before the percutaneous coronary intervention had a 38.6% relative risk reduction (Table 1). Long-term use of clopidogrel (ie, for 12 months) was associated with an overall relative reduction of 26.9% in the combined risk of death, MI, or stroke.
PCI-CURE, an analysis of 2,658 patients in the CURE trial with non-ST-elevation acute coronary syndrome who underwent PCI,15 yielded results similar to those of CREDO, with a 31% reduction in the rate of cardiovascular death or MI at 30 days and at 9 months. Of note, however, clopidogrel was given for a median of 6 days prior to the procedure.
Comment. The minimum suggested duration of clopidogrel treatment after placement of a bare-metal stent is 1 month. However, these trial results indicate that patients who are not at high risk of bleeding should take clopidogrel for at least 12 months.
Dual antiplatelet therapy with drug-eluting stents
Although rates of in-stent restenosis are clearly lower with drug-eluting stents than with bare-metal stents, the antiproliferative effect of drug-eluting stents may delay complete endothelialization of every strut. This may contribute to late (> 1 month after placement) or very late (> 1 year) thrombosis of the stent after clopidogrel is discontinued.16–18
In 2006, the FDA indicated that dual antiplatelet therapy was needed for 6 months with paclitaxel-eluting (Taxus) stents and 3 months with sirolimus-eluting (Cipher) stents. As reports of very late stent thrombosis began to appear in 2007, concern arose over the need to extend the duration of clopidogrel treatment.
Bavry et al19 quantified the incidence of late and very late stent thrombosis in a meta-analysis of 14 clinical trials that randomized patients to receive either a drug-eluting stent (paclitaxel or sirolimus) or a bare-metal stent.19 The incidence of stent thrombosis within 30 days in this analysis was similar for both groups—4.4 per 1,000 patients vs 5 per 1,000 (relative risk 0.89; 95% CI 0.46–1.75; P = .74). However, the rate of very late stent thrombosis was significantly higher in those receiving a drug-eluting stent vs a bare-metal stent—5 per 1,000 patients treated (relative risk 5.02, 95% CI 1.29–19.52; P = .02).
The results of this and other studies led the ACC and AHA to revise their joint guidelines to recommend thienopyridine treatment for at least 1 year for patients who receive a drug-eluting stent.6,20–22 In fact, many cardiologists consider indefinite dual antiplatelet therapy in patients with a drug-eluting stent to avoid very late in-stent thrombosis, especially in patients undergoing high-risk interventions such as placement of multiple stents, bifurcation lesions, and unprotected left main trunk interventions.
Thus, when faced with a patient with a recent coronary stent implantation, the primary care physician should be aware of the type of stent and the duration of therapy recommended by the interventional cardiologist. Also, in the absence of a pressing indication, elective surgery should be deferred for 1 year after placement of a drug-eluting stent, as this would necessitate stopping clopidogrel and would increase the risk of perioperative stent thrombosis, which is associated with high rates of morbidity and death.
CASE 4: HIGH-RISK CORONARY ARTERY DISEASE
A 67-year-old woman presents to your office to establish care. She has a history of diabetes and established coronary artery disease with two bare-metal stents placed 2 years ago. She is taking aspirin 81 mg.
What would be the value of adding clopidogrel to her regimen?
No indication for clopidogrel in chronic coronary artery disease
The CHARISMA trial (Clopidogrel for High Atherothrombotic and Ischemic Stabilization, Management, and Avoidance)23 randomized 15,603 patients with stable cardiovascular disease or multiple risk factors to receive either clopidogrel plus low-dose aspirin or placebo plus low-dose aspirin and followed them for a median of 28 months (Table 1).
The primary end point (a composite of MI, stroke, or death) was 6.8% with clopidogrel plus aspirin and 7.3% with aspirin alone, indicating no significant benefit with clopidogrel plus aspirin compared with aspirin alone in reducing the rate of MI, stroke, or cardiovascular death in patients with high-risk but stable atherothrombotic disease. A marginal statistical benefit with dual antiplatelet therapy was noted in the subgroup of patients with previously documented coronary, cerebrovascular, or peripheral vascular disease—6.9% with aspirin plus clopidogrel vs 7.9% with aspirin alone (relative risk 0.88; 95% CI 0.77–0.998; P = .046).
Consequently, there is no compelling reason to start clopidogrel in this patient.
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel was recently approved by the FDA as antiplatelet treatment for patients with acute coronary syndromes planning to undergo a percutaneous coronary intervention.24 It has been shown to inhibit adenosine-diphosphate-induced platelet activation in a more consistent and effective manner than clopidogrel.25,26
Although both clopidogrel and prasugrel are prodrugs, 80% of absorbed clopidogrel is metabolized by esterases into inactive metabolites, and the availability of active metabolite can vary, as it is significantly influenced by polymorphisms in the cytochrome P450 system. 27 In contrast, prasugrel is not degraded by esterases, and its conversion to active metabolite by the cytochrome P450 system is not influenced by common genetic polymorphisms, particularly CYP2C19*2.
TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction) provided most of the evidence for the approval of prasugrel for clinical use.28,29 In this trial, a 60-mg loading dose of prasugrel followed by a daily maintenance dose of 10 mg was significantly superior to the current clopidogrel regimen in preventing death from cardiovascular causes, nonfatal MI, or nonfatal stroke during a study period of 15 months.28 Also observed was a 24% lower rate of MI, a 34% lower rate of urgent target-vessel revascularization, and a 52% lower rate of stent thrombosis.
These benefits, however, came at the cost of a significantly higher risk of major bleeding, including the potential for three excess fatal bleeding events for every 1,000 patients treated. Patients at highest risk at the dosages evaluated included the elderly (age 75 and older), patients who weigh less than 60 kg, and patients with a history of stroke or transient ischemic attack. Based on these results, we recommend caution with the use of prasugrel in these patient subsets.
Clinical use of prasugrel is likely to be highest in patients presenting with ST-elevation MI who are undergoing a primary percutaneous coronary intervention. There is currently no evidence from any randomized clinical trial to support the safety of prasugrel given in the emergency room or “upstream” in the setting of non-ST-elevation acute coronary syndromes.
Of note, patients with non-ST-elevation acute coronary syndromes in the TRITON trial were randomized only after angiographic definition. As a result, only 179 patients exposed to prasugrel were referred for coronary artery bypass surgery, but the rate of surgery-related major bleeding in this group was 13.4% (vs 3.2% in the clopidogrel group). Based on these data, prasugrel should be withheld for at least 1 week prior to any surgery.
Plaque rupture and thrombosis play central roles in the genesis of acute coronary syndrome. Aspirin has long been the preventive agent of choice. But dual antiplatelet therapy with aspirin plus clopidogrel (Plavix) is warranted in many patients to further reduce their risk of future cardiovascular events.
Although dual antiplatelet therapy is usually started by a subspecialist, the primary care physician is often the one who ensures that the patient remains compliant with it in the long term. A review of the seminal published data is helpful in understanding the rationale behind dual antiplatelet therapy and its risks and benefits.
In the mid-1990s, the thienopyridine ticlopidine (Ticlid) was found to significantly decrease the number of deaths, target-lesion revascularizations, and myocardial infarctions (MIs) in the 30 days following stent placement. 1 However, 2% to 3% of patients experienced neutropenia2 and thrombotic thrombocytopenic purpura with this drug,3 leading to the use of clopidogrel, another agent in the same class. Over the past decade, a large body of evidence has established the usefulness of clopidogrel in a number of clinical settings.
In this paper we review the current use of clopidogrel in ST-elevation MI, non-ST-elevation acute coronary syndromes, and percutaneous coronary intervention, and discuss the landmark trials that are the basis for the treatment guidelines published jointly by the American College of Cardiology (ACC) and the American Heart Association (AHA).4–6 We also briefly discuss the use of prasugrel (Effient), the newest antiplatelet agent to gain approval from the US Food and Drug Administration (FDA).
CLOPIDOGREL AS AN ALTERNATIVE TO ASPIRIN
Clopidogrel, a prodrug, is converted into its active form in the liver.7 It then irreversibly binds to the platelet P2Y12 receptor and inhibits adenosine diphosphate-induced platelet aggregation.
Those treated with clopidogrel had an annual risk of ischemic stroke, MI, or vascular death of 5.32%, compared with 5.83% in the aspirin group, for a statistically significant 8.7% relative risk reduction (P = .043). The observed frequency of neutropenia (neutrophils < 1.2 × 109/L) was 0.10% with clopidogrel vs 0.17% with aspirin. This study showed clopidogrel to be an effective alternative in patients who cannot tolerate aspirin.
CASE 1: ST-ELEVATION MI
A 57-year-old farmer in rural Ohio with a history of hypertension and hyperlipidemia presents to the local emergency department 45 minutes after the onset, while he was chopping wood, of dull, aching, substernal chest pain that radiates to his jaw. Electrocardiography reveals 2-mm ST-segment elevation in leads V1 through V6. He is treated with aspirin 162 mg, low-molecular-weight heparin, and tenecteplase.
What would be the value of starting dual antiplatelet therapy with clopidogrel in this patient?
Clopidogrel, aspirin, and fibrinolysis in ST-elevation MI
The CLARITY-TIMI 28 trial (Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis in Myocardial Infarction)9 included 3,491 patients (ages 18 to 75) from 319 international sites. All patients received a fibrinolytic agent, aspirin (162 mg to 325 mg on the first day and 75 mg to 162 mg thereafter), and heparin as part of standard care for acute ST-elevation MI (Table 1). Patients were randomized to receive a 300-mg loading dose of clopidogrel followed by 75 mg daily or placebo within 12 hours of onset of ST-elevation MI. The status of the infarct-related artery was ascertained by protocol-mandated coronary angiography 48 to 192 hours after starting the study medication. The primary end point was the composite of an occluded infarct-related artery on angiography, death from any cause prior to angiography, or recurrent MI prior to angiography.
Significantly fewer patients had an end point event in the clopidogrel group than in the placebo group, 15% vs 21.7% (P < .001), for a relative risk reduction of 31%. There was no significant increase in major or minor bleeding events.
Of note, the CLARITY-TIMI 28 patients were relatively young (average age 57 years) and at low cardiovascular risk (30-day mortality risk < 5%).
The COMMIT trial (Clopidogrel and Metoprolol in Myocardial Infarction)10 consisted of 45,852 patients with suspected acute MI admitted to 1,250 hospitals in China. Each patient received aspirin 162 mg daily plus either clopidogrel 75 mg daily (n = 22,961) or placebo (n = 22,891) for the duration of hospitalization (average 16 days) or 28 days, whichever came first.
The incidence of the primary composite end point of death, reinfarction, or stroke was significantly lower with clopidogrel than with placebo (9.2% vs 10.1%, P = .002). This was regardless of age (the average age was 61, and 26% of patients were older than 70), sex, time to presentation (67% presented within 12 hours), or reperfusion strategy (49% underwent fibrinolysis). The clopidogrel group did not have a significantly higher incidence of bleeding, but patients in this trial did not receive a loading dose of clopidogrel.
Comment. In view of the results of these trials, our 57-year-old patient should start clopidogrel early.
CASE 2: NON-ST-ELEVATION ACUTE CORONARY SYNDROME
A 65-year-old woman living independently with no significant medical history presents to the emergency room with 2 hours of waxing and waning substernal chest pain. Her blood pressure is 145/90 mm Hg, her heart rate is 95 beats per minute, and the results of her physical examination are unremarkable. Resting electrocardiography reveals 1.5-mm ST-segment depression in the inferior leads, and her troponin T level on admission is two times the upper limit of normal. She is given aspirin and is started on low-molecular-weight heparin and intravenous nitroglycerin.
What would be the value of starting clopidogrel in this patient?
Clopidogrel in non-ST-elevation acute coronary syndromes
The ACC/AHA guidelines strongly support starting clopidogrel in patients with non-ST-elevation acute coronary syndromes (Table 2).5
The CURE trial (Clopidogrel in Unstable Angina to Prevent Recurrent Events)11 provided the evidence for this recommendation. In this trial, 12,562 patients from 482 centers in 28 countries who presented within 24 hours of coronary symptoms, without ST elevation, were randomized to receive either clopidogrel (a 300-mg loading dose, followed by 75 mg daily) or placebo for 3 to 12 months (mean 9 months).
Significantly fewer patients in the clopidogrel group reached one of the end points of the composite primary outcome (cardiovascular death, nonfatal MI, or stroke): 9.3% vs 11.4%, 95% confidence interval (CI) 0.72–0.90, P < .001. Significantly fewer of them also suffered one of the secondary outcomes, ie, severe ischemia, heart failure, or need for revascularization.
Of concern was a higher rate of major bleeding in the clopidogrel group (3.7%) than in the placebo group (2.7%) without an excess of fatal bleeding. For every 1,000 patients treated with clopidogrel, 6 required a blood transfusion. Nevertheless, CURE proved that patients with non-ST-elevation acute coronary syndromes benefited from clopidogrel, regardless of whether they underwent percutaneous coronary intervention.
Comment. Our patient should receive clopidogrel and, if she has no significant bleeding, she should continue to take it for at least 12 months after discharge. It is important for the primary care physician to ensure compliance with this agent and not discontinue it on routine clinical follow-up.
CASE 3: BARE-METAL STENT PLACEMENT
A 62-year-old man with a history of hypertension, diabetes, and hyperlipidemia presents to his primary care physician’s office with stable-effort angina that is not responding to an excellent anti-ischemic regimen and is affecting his quality of life. He is referred for coronary angiography, which reveals 80% stenosis of the proximal left circumflex artery. He undergoes a percutaneous coronary intervention with placement of a bare-metal stent.
How long should he be on clopidogrel? And what if a drug-eluting stent had been placed instead of a bare-metal stent?
Dual therapy after bare-metal stent placement
Dual antiplatelet therapy with clopidogrel and aspirin is recommended in all patients receiving a stent (Table 2). The better safety and efficacy of clopidogrel compared with ticlopidine has been established in patients receiving a coronary artery stent,12,13 and clopidogrel’s favorable safety profile soon made it the thienopyridine of choice.
The CREDO trial (Clopidogrel for the Reduction of Events During Observation)14 randomized 2,116 patients undergoing an elective percutaneous coronary intervention (bare-metal stent placement only) to receive a 300-mg loading dose of clopidogrel 3 to 24 hours before the procedure, or placebo. All patients received 325 mg of aspirin. After the intervention, all patients received clopidogrel 75 mg daily and aspirin 325 mg daily through day 28. For day 29 through 12 months, those who had received the 300-mg preprocedural loading dose of clopidogrel continued with 75 mg daily, and those who had not received clopidogrel before the procedure received placebo.
No significant difference was seen in the primary outcome for those who received pretreatment with clopidogrel; however, in a subgroup analysis, those who received clopidogrel at least 6 hours before the percutaneous coronary intervention had a 38.6% relative risk reduction (Table 1). Long-term use of clopidogrel (ie, for 12 months) was associated with an overall relative reduction of 26.9% in the combined risk of death, MI, or stroke.
PCI-CURE, an analysis of 2,658 patients in the CURE trial with non-ST-elevation acute coronary syndrome who underwent PCI,15 yielded results similar to those of CREDO, with a 31% reduction in the rate of cardiovascular death or MI at 30 days and at 9 months. Of note, however, clopidogrel was given for a median of 6 days prior to the procedure.
Comment. The minimum suggested duration of clopidogrel treatment after placement of a bare-metal stent is 1 month. However, these trial results indicate that patients who are not at high risk of bleeding should take clopidogrel for at least 12 months.
Dual antiplatelet therapy with drug-eluting stents
Although rates of in-stent restenosis are clearly lower with drug-eluting stents than with bare-metal stents, the antiproliferative effect of drug-eluting stents may delay complete endothelialization of every strut. This may contribute to late (> 1 month after placement) or very late (> 1 year) thrombosis of the stent after clopidogrel is discontinued.16–18
In 2006, the FDA indicated that dual antiplatelet therapy was needed for 6 months with paclitaxel-eluting (Taxus) stents and 3 months with sirolimus-eluting (Cipher) stents. As reports of very late stent thrombosis began to appear in 2007, concern arose over the need to extend the duration of clopidogrel treatment.
Bavry et al19 quantified the incidence of late and very late stent thrombosis in a meta-analysis of 14 clinical trials that randomized patients to receive either a drug-eluting stent (paclitaxel or sirolimus) or a bare-metal stent.19 The incidence of stent thrombosis within 30 days in this analysis was similar for both groups—4.4 per 1,000 patients vs 5 per 1,000 (relative risk 0.89; 95% CI 0.46–1.75; P = .74). However, the rate of very late stent thrombosis was significantly higher in those receiving a drug-eluting stent vs a bare-metal stent—5 per 1,000 patients treated (relative risk 5.02, 95% CI 1.29–19.52; P = .02).
The results of this and other studies led the ACC and AHA to revise their joint guidelines to recommend thienopyridine treatment for at least 1 year for patients who receive a drug-eluting stent.6,20–22 In fact, many cardiologists consider indefinite dual antiplatelet therapy in patients with a drug-eluting stent to avoid very late in-stent thrombosis, especially in patients undergoing high-risk interventions such as placement of multiple stents, bifurcation lesions, and unprotected left main trunk interventions.
Thus, when faced with a patient with a recent coronary stent implantation, the primary care physician should be aware of the type of stent and the duration of therapy recommended by the interventional cardiologist. Also, in the absence of a pressing indication, elective surgery should be deferred for 1 year after placement of a drug-eluting stent, as this would necessitate stopping clopidogrel and would increase the risk of perioperative stent thrombosis, which is associated with high rates of morbidity and death.
CASE 4: HIGH-RISK CORONARY ARTERY DISEASE
A 67-year-old woman presents to your office to establish care. She has a history of diabetes and established coronary artery disease with two bare-metal stents placed 2 years ago. She is taking aspirin 81 mg.
What would be the value of adding clopidogrel to her regimen?
No indication for clopidogrel in chronic coronary artery disease
The CHARISMA trial (Clopidogrel for High Atherothrombotic and Ischemic Stabilization, Management, and Avoidance)23 randomized 15,603 patients with stable cardiovascular disease or multiple risk factors to receive either clopidogrel plus low-dose aspirin or placebo plus low-dose aspirin and followed them for a median of 28 months (Table 1).
The primary end point (a composite of MI, stroke, or death) was 6.8% with clopidogrel plus aspirin and 7.3% with aspirin alone, indicating no significant benefit with clopidogrel plus aspirin compared with aspirin alone in reducing the rate of MI, stroke, or cardiovascular death in patients with high-risk but stable atherothrombotic disease. A marginal statistical benefit with dual antiplatelet therapy was noted in the subgroup of patients with previously documented coronary, cerebrovascular, or peripheral vascular disease—6.9% with aspirin plus clopidogrel vs 7.9% with aspirin alone (relative risk 0.88; 95% CI 0.77–0.998; P = .046).
Consequently, there is no compelling reason to start clopidogrel in this patient.
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel was recently approved by the FDA as antiplatelet treatment for patients with acute coronary syndromes planning to undergo a percutaneous coronary intervention.24 It has been shown to inhibit adenosine-diphosphate-induced platelet activation in a more consistent and effective manner than clopidogrel.25,26
Although both clopidogrel and prasugrel are prodrugs, 80% of absorbed clopidogrel is metabolized by esterases into inactive metabolites, and the availability of active metabolite can vary, as it is significantly influenced by polymorphisms in the cytochrome P450 system. 27 In contrast, prasugrel is not degraded by esterases, and its conversion to active metabolite by the cytochrome P450 system is not influenced by common genetic polymorphisms, particularly CYP2C19*2.
TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction) provided most of the evidence for the approval of prasugrel for clinical use.28,29 In this trial, a 60-mg loading dose of prasugrel followed by a daily maintenance dose of 10 mg was significantly superior to the current clopidogrel regimen in preventing death from cardiovascular causes, nonfatal MI, or nonfatal stroke during a study period of 15 months.28 Also observed was a 24% lower rate of MI, a 34% lower rate of urgent target-vessel revascularization, and a 52% lower rate of stent thrombosis.
These benefits, however, came at the cost of a significantly higher risk of major bleeding, including the potential for three excess fatal bleeding events for every 1,000 patients treated. Patients at highest risk at the dosages evaluated included the elderly (age 75 and older), patients who weigh less than 60 kg, and patients with a history of stroke or transient ischemic attack. Based on these results, we recommend caution with the use of prasugrel in these patient subsets.
Clinical use of prasugrel is likely to be highest in patients presenting with ST-elevation MI who are undergoing a primary percutaneous coronary intervention. There is currently no evidence from any randomized clinical trial to support the safety of prasugrel given in the emergency room or “upstream” in the setting of non-ST-elevation acute coronary syndromes.
Of note, patients with non-ST-elevation acute coronary syndromes in the TRITON trial were randomized only after angiographic definition. As a result, only 179 patients exposed to prasugrel were referred for coronary artery bypass surgery, but the rate of surgery-related major bleeding in this group was 13.4% (vs 3.2% in the clopidogrel group). Based on these data, prasugrel should be withheld for at least 1 week prior to any surgery.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Yeh SP, Hsueh EJ, Wu H, Wang YC. Ticlopidine-associated aplastic anemia. A case report and review of literature, Ann Hematol 1998; 76:87–90.
- Page Y, Tardy B, Zeni F, Comtet C, Terrana R, Bertrand JC. Thrombotic thrombocytopenic purpura related to ticlopidine. Lancet 1991; 337:774–776.
- Canadian Cardiovascular Society; Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007; 50:e1–e157.
- King SB, Smith SC, Hirshfeld JW, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:172–209.
- Kam PC, Nethery CM. The thienopyridine derivatives (platelet adenosine diphosphate receptor antagonists), pharmacology and clinical developments. Anaesthesia 2003; 58:28–35.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Sabatine MS, Cannon CP, Gibson M, et al; CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation, N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: a randomized placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Bhatt DL, Bertrand ME, Berger PB, et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J Am Coll Cardiol 2002; 39:9–14.
- Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH; CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS). Circulation 2000; 102:624–629.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Mehta SR, Yusuf S, Peters RJ, et a; Clopidogrel in Unstable Angina to Prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001; 358:527–533.
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007; 115:1051–1058.
- Kotani J, Awata M, Nanto S, et al. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol 2006; 47:2108–2111.
- Pfisterer M, Brunner-La Rocca HP, Buser PT, et al; BASKETLATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol 2006; 48:2584–2591.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med 2007; 356:1030–1039.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- US Food and Drug Administration. FDA Approves Effient to Reduce the Risk of Heart Attack in Angioplasty Patients. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm171497.htm. Accessed October 2, 2009.
- Jernber T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006; 27:1166–1173.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A TRITON-TIMI (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Card 2008; 51:2028–2033.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Yeh SP, Hsueh EJ, Wu H, Wang YC. Ticlopidine-associated aplastic anemia. A case report and review of literature, Ann Hematol 1998; 76:87–90.
- Page Y, Tardy B, Zeni F, Comtet C, Terrana R, Bertrand JC. Thrombotic thrombocytopenic purpura related to ticlopidine. Lancet 1991; 337:774–776.
- Canadian Cardiovascular Society; Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007; 50:e1–e157.
- King SB, Smith SC, Hirshfeld JW, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:172–209.
- Kam PC, Nethery CM. The thienopyridine derivatives (platelet adenosine diphosphate receptor antagonists), pharmacology and clinical developments. Anaesthesia 2003; 58:28–35.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Sabatine MS, Cannon CP, Gibson M, et al; CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation, N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: a randomized placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Bhatt DL, Bertrand ME, Berger PB, et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J Am Coll Cardiol 2002; 39:9–14.
- Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH; CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS). Circulation 2000; 102:624–629.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Mehta SR, Yusuf S, Peters RJ, et a; Clopidogrel in Unstable Angina to Prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001; 358:527–533.
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007; 115:1051–1058.
- Kotani J, Awata M, Nanto S, et al. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol 2006; 47:2108–2111.
- Pfisterer M, Brunner-La Rocca HP, Buser PT, et al; BASKETLATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol 2006; 48:2584–2591.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med 2007; 356:1030–1039.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- US Food and Drug Administration. FDA Approves Effient to Reduce the Risk of Heart Attack in Angioplasty Patients. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm171497.htm. Accessed October 2, 2009.
- Jernber T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006; 27:1166–1173.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A TRITON-TIMI (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Card 2008; 51:2028–2033.
KEY POINTS
- Dual antiplatelet therapy is recommended after ST-elevation MI or non-ST-elevation acute coronary syndromes, with aspirin indefinitely and clopidogrel for up to 1 year.
- Dual antiplatelet therapy is recommended for at least 1 month after placement of a bare-metal stent and for at least 1 year (or possibly indefinitely) after placement of a drug-eluting stent.
- There is no compelling indication for clopidogrel in patients with chronic coronary artery disease.
- Compared with clopidogrel, prasugrel (Effient) is associated with lower rates of MI, urgent target-vessel revascularization, and in-stent thrombosis, but at the cost of a higher risk of major bleeding.