User login
Plaque rupture and thrombosis play central roles in the genesis of acute coronary syndrome. Aspirin has long been the preventive agent of choice. But dual antiplatelet therapy with aspirin plus clopidogrel (Plavix) is warranted in many patients to further reduce their risk of future cardiovascular events.
Although dual antiplatelet therapy is usually started by a subspecialist, the primary care physician is often the one who ensures that the patient remains compliant with it in the long term. A review of the seminal published data is helpful in understanding the rationale behind dual antiplatelet therapy and its risks and benefits.
In the mid-1990s, the thienopyridine ticlopidine (Ticlid) was found to significantly decrease the number of deaths, target-lesion revascularizations, and myocardial infarctions (MIs) in the 30 days following stent placement. 1 However, 2% to 3% of patients experienced neutropenia2 and thrombotic thrombocytopenic purpura with this drug,3 leading to the use of clopidogrel, another agent in the same class. Over the past decade, a large body of evidence has established the usefulness of clopidogrel in a number of clinical settings.
In this paper we review the current use of clopidogrel in ST-elevation MI, non-ST-elevation acute coronary syndromes, and percutaneous coronary intervention, and discuss the landmark trials that are the basis for the treatment guidelines published jointly by the American College of Cardiology (ACC) and the American Heart Association (AHA).4–6 We also briefly discuss the use of prasugrel (Effient), the newest antiplatelet agent to gain approval from the US Food and Drug Administration (FDA).
CLOPIDOGREL AS AN ALTERNATIVE TO ASPIRIN
Clopidogrel, a prodrug, is converted into its active form in the liver.7 It then irreversibly binds to the platelet P2Y12 receptor and inhibits adenosine diphosphate-induced platelet aggregation.
Those treated with clopidogrel had an annual risk of ischemic stroke, MI, or vascular death of 5.32%, compared with 5.83% in the aspirin group, for a statistically significant 8.7% relative risk reduction (P = .043). The observed frequency of neutropenia (neutrophils < 1.2 × 109/L) was 0.10% with clopidogrel vs 0.17% with aspirin. This study showed clopidogrel to be an effective alternative in patients who cannot tolerate aspirin.
CASE 1: ST-ELEVATION MI
A 57-year-old farmer in rural Ohio with a history of hypertension and hyperlipidemia presents to the local emergency department 45 minutes after the onset, while he was chopping wood, of dull, aching, substernal chest pain that radiates to his jaw. Electrocardiography reveals 2-mm ST-segment elevation in leads V1 through V6. He is treated with aspirin 162 mg, low-molecular-weight heparin, and tenecteplase.
What would be the value of starting dual antiplatelet therapy with clopidogrel in this patient?
Clopidogrel, aspirin, and fibrinolysis in ST-elevation MI
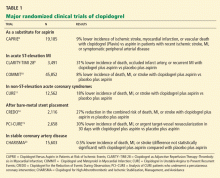
The CLARITY-TIMI 28 trial (Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis in Myocardial Infarction)9 included 3,491 patients (ages 18 to 75) from 319 international sites. All patients received a fibrinolytic agent, aspirin (162 mg to 325 mg on the first day and 75 mg to 162 mg thereafter), and heparin as part of standard care for acute ST-elevation MI (Table 1). Patients were randomized to receive a 300-mg loading dose of clopidogrel followed by 75 mg daily or placebo within 12 hours of onset of ST-elevation MI. The status of the infarct-related artery was ascertained by protocol-mandated coronary angiography 48 to 192 hours after starting the study medication. The primary end point was the composite of an occluded infarct-related artery on angiography, death from any cause prior to angiography, or recurrent MI prior to angiography.
Significantly fewer patients had an end point event in the clopidogrel group than in the placebo group, 15% vs 21.7% (P < .001), for a relative risk reduction of 31%. There was no significant increase in major or minor bleeding events.
Of note, the CLARITY-TIMI 28 patients were relatively young (average age 57 years) and at low cardiovascular risk (30-day mortality risk < 5%).
The COMMIT trial (Clopidogrel and Metoprolol in Myocardial Infarction)10 consisted of 45,852 patients with suspected acute MI admitted to 1,250 hospitals in China. Each patient received aspirin 162 mg daily plus either clopidogrel 75 mg daily (n = 22,961) or placebo (n = 22,891) for the duration of hospitalization (average 16 days) or 28 days, whichever came first.
The incidence of the primary composite end point of death, reinfarction, or stroke was significantly lower with clopidogrel than with placebo (9.2% vs 10.1%, P = .002). This was regardless of age (the average age was 61, and 26% of patients were older than 70), sex, time to presentation (67% presented within 12 hours), or reperfusion strategy (49% underwent fibrinolysis). The clopidogrel group did not have a significantly higher incidence of bleeding, but patients in this trial did not receive a loading dose of clopidogrel.
Comment. In view of the results of these trials, our 57-year-old patient should start clopidogrel early.
CASE 2: NON-ST-ELEVATION ACUTE CORONARY SYNDROME
A 65-year-old woman living independently with no significant medical history presents to the emergency room with 2 hours of waxing and waning substernal chest pain. Her blood pressure is 145/90 mm Hg, her heart rate is 95 beats per minute, and the results of her physical examination are unremarkable. Resting electrocardiography reveals 1.5-mm ST-segment depression in the inferior leads, and her troponin T level on admission is two times the upper limit of normal. She is given aspirin and is started on low-molecular-weight heparin and intravenous nitroglycerin.
What would be the value of starting clopidogrel in this patient?
Clopidogrel in non-ST-elevation acute coronary syndromes
The ACC/AHA guidelines strongly support starting clopidogrel in patients with non-ST-elevation acute coronary syndromes (Table 2).5
The CURE trial (Clopidogrel in Unstable Angina to Prevent Recurrent Events)11 provided the evidence for this recommendation. In this trial, 12,562 patients from 482 centers in 28 countries who presented within 24 hours of coronary symptoms, without ST elevation, were randomized to receive either clopidogrel (a 300-mg loading dose, followed by 75 mg daily) or placebo for 3 to 12 months (mean 9 months).
Significantly fewer patients in the clopidogrel group reached one of the end points of the composite primary outcome (cardiovascular death, nonfatal MI, or stroke): 9.3% vs 11.4%, 95% confidence interval (CI) 0.72–0.90, P < .001. Significantly fewer of them also suffered one of the secondary outcomes, ie, severe ischemia, heart failure, or need for revascularization.
Of concern was a higher rate of major bleeding in the clopidogrel group (3.7%) than in the placebo group (2.7%) without an excess of fatal bleeding. For every 1,000 patients treated with clopidogrel, 6 required a blood transfusion. Nevertheless, CURE proved that patients with non-ST-elevation acute coronary syndromes benefited from clopidogrel, regardless of whether they underwent percutaneous coronary intervention.
Comment. Our patient should receive clopidogrel and, if she has no significant bleeding, she should continue to take it for at least 12 months after discharge. It is important for the primary care physician to ensure compliance with this agent and not discontinue it on routine clinical follow-up.
CASE 3: BARE-METAL STENT PLACEMENT
A 62-year-old man with a history of hypertension, diabetes, and hyperlipidemia presents to his primary care physician’s office with stable-effort angina that is not responding to an excellent anti-ischemic regimen and is affecting his quality of life. He is referred for coronary angiography, which reveals 80% stenosis of the proximal left circumflex artery. He undergoes a percutaneous coronary intervention with placement of a bare-metal stent.
How long should he be on clopidogrel? And what if a drug-eluting stent had been placed instead of a bare-metal stent?
Dual therapy after bare-metal stent placement
Dual antiplatelet therapy with clopidogrel and aspirin is recommended in all patients receiving a stent (Table 2). The better safety and efficacy of clopidogrel compared with ticlopidine has been established in patients receiving a coronary artery stent,12,13 and clopidogrel’s favorable safety profile soon made it the thienopyridine of choice.
The CREDO trial (Clopidogrel for the Reduction of Events During Observation)14 randomized 2,116 patients undergoing an elective percutaneous coronary intervention (bare-metal stent placement only) to receive a 300-mg loading dose of clopidogrel 3 to 24 hours before the procedure, or placebo. All patients received 325 mg of aspirin. After the intervention, all patients received clopidogrel 75 mg daily and aspirin 325 mg daily through day 28. For day 29 through 12 months, those who had received the 300-mg preprocedural loading dose of clopidogrel continued with 75 mg daily, and those who had not received clopidogrel before the procedure received placebo.
No significant difference was seen in the primary outcome for those who received pretreatment with clopidogrel; however, in a subgroup analysis, those who received clopidogrel at least 6 hours before the percutaneous coronary intervention had a 38.6% relative risk reduction (Table 1). Long-term use of clopidogrel (ie, for 12 months) was associated with an overall relative reduction of 26.9% in the combined risk of death, MI, or stroke.
PCI-CURE, an analysis of 2,658 patients in the CURE trial with non-ST-elevation acute coronary syndrome who underwent PCI,15 yielded results similar to those of CREDO, with a 31% reduction in the rate of cardiovascular death or MI at 30 days and at 9 months. Of note, however, clopidogrel was given for a median of 6 days prior to the procedure.
Comment. The minimum suggested duration of clopidogrel treatment after placement of a bare-metal stent is 1 month. However, these trial results indicate that patients who are not at high risk of bleeding should take clopidogrel for at least 12 months.
Dual antiplatelet therapy with drug-eluting stents
Although rates of in-stent restenosis are clearly lower with drug-eluting stents than with bare-metal stents, the antiproliferative effect of drug-eluting stents may delay complete endothelialization of every strut. This may contribute to late (> 1 month after placement) or very late (> 1 year) thrombosis of the stent after clopidogrel is discontinued.16–18
In 2006, the FDA indicated that dual antiplatelet therapy was needed for 6 months with paclitaxel-eluting (Taxus) stents and 3 months with sirolimus-eluting (Cipher) stents. As reports of very late stent thrombosis began to appear in 2007, concern arose over the need to extend the duration of clopidogrel treatment.
Bavry et al19 quantified the incidence of late and very late stent thrombosis in a meta-analysis of 14 clinical trials that randomized patients to receive either a drug-eluting stent (paclitaxel or sirolimus) or a bare-metal stent.19 The incidence of stent thrombosis within 30 days in this analysis was similar for both groups—4.4 per 1,000 patients vs 5 per 1,000 (relative risk 0.89; 95% CI 0.46–1.75; P = .74). However, the rate of very late stent thrombosis was significantly higher in those receiving a drug-eluting stent vs a bare-metal stent—5 per 1,000 patients treated (relative risk 5.02, 95% CI 1.29–19.52; P = .02).
The results of this and other studies led the ACC and AHA to revise their joint guidelines to recommend thienopyridine treatment for at least 1 year for patients who receive a drug-eluting stent.6,20–22 In fact, many cardiologists consider indefinite dual antiplatelet therapy in patients with a drug-eluting stent to avoid very late in-stent thrombosis, especially in patients undergoing high-risk interventions such as placement of multiple stents, bifurcation lesions, and unprotected left main trunk interventions.
Thus, when faced with a patient with a recent coronary stent implantation, the primary care physician should be aware of the type of stent and the duration of therapy recommended by the interventional cardiologist. Also, in the absence of a pressing indication, elective surgery should be deferred for 1 year after placement of a drug-eluting stent, as this would necessitate stopping clopidogrel and would increase the risk of perioperative stent thrombosis, which is associated with high rates of morbidity and death.
CASE 4: HIGH-RISK CORONARY ARTERY DISEASE
A 67-year-old woman presents to your office to establish care. She has a history of diabetes and established coronary artery disease with two bare-metal stents placed 2 years ago. She is taking aspirin 81 mg.
What would be the value of adding clopidogrel to her regimen?
No indication for clopidogrel in chronic coronary artery disease
The CHARISMA trial (Clopidogrel for High Atherothrombotic and Ischemic Stabilization, Management, and Avoidance)23 randomized 15,603 patients with stable cardiovascular disease or multiple risk factors to receive either clopidogrel plus low-dose aspirin or placebo plus low-dose aspirin and followed them for a median of 28 months (Table 1).
The primary end point (a composite of MI, stroke, or death) was 6.8% with clopidogrel plus aspirin and 7.3% with aspirin alone, indicating no significant benefit with clopidogrel plus aspirin compared with aspirin alone in reducing the rate of MI, stroke, or cardiovascular death in patients with high-risk but stable atherothrombotic disease. A marginal statistical benefit with dual antiplatelet therapy was noted in the subgroup of patients with previously documented coronary, cerebrovascular, or peripheral vascular disease—6.9% with aspirin plus clopidogrel vs 7.9% with aspirin alone (relative risk 0.88; 95% CI 0.77–0.998; P = .046).
Consequently, there is no compelling reason to start clopidogrel in this patient.
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel was recently approved by the FDA as antiplatelet treatment for patients with acute coronary syndromes planning to undergo a percutaneous coronary intervention.24 It has been shown to inhibit adenosine-diphosphate-induced platelet activation in a more consistent and effective manner than clopidogrel.25,26
Although both clopidogrel and prasugrel are prodrugs, 80% of absorbed clopidogrel is metabolized by esterases into inactive metabolites, and the availability of active metabolite can vary, as it is significantly influenced by polymorphisms in the cytochrome P450 system. 27 In contrast, prasugrel is not degraded by esterases, and its conversion to active metabolite by the cytochrome P450 system is not influenced by common genetic polymorphisms, particularly CYP2C19*2.
TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction) provided most of the evidence for the approval of prasugrel for clinical use.28,29 In this trial, a 60-mg loading dose of prasugrel followed by a daily maintenance dose of 10 mg was significantly superior to the current clopidogrel regimen in preventing death from cardiovascular causes, nonfatal MI, or nonfatal stroke during a study period of 15 months.28 Also observed was a 24% lower rate of MI, a 34% lower rate of urgent target-vessel revascularization, and a 52% lower rate of stent thrombosis.
These benefits, however, came at the cost of a significantly higher risk of major bleeding, including the potential for three excess fatal bleeding events for every 1,000 patients treated. Patients at highest risk at the dosages evaluated included the elderly (age 75 and older), patients who weigh less than 60 kg, and patients with a history of stroke or transient ischemic attack. Based on these results, we recommend caution with the use of prasugrel in these patient subsets.
Clinical use of prasugrel is likely to be highest in patients presenting with ST-elevation MI who are undergoing a primary percutaneous coronary intervention. There is currently no evidence from any randomized clinical trial to support the safety of prasugrel given in the emergency room or “upstream” in the setting of non-ST-elevation acute coronary syndromes.
Of note, patients with non-ST-elevation acute coronary syndromes in the TRITON trial were randomized only after angiographic definition. As a result, only 179 patients exposed to prasugrel were referred for coronary artery bypass surgery, but the rate of surgery-related major bleeding in this group was 13.4% (vs 3.2% in the clopidogrel group). Based on these data, prasugrel should be withheld for at least 1 week prior to any surgery.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Yeh SP, Hsueh EJ, Wu H, Wang YC. Ticlopidine-associated aplastic anemia. A case report and review of literature, Ann Hematol 1998; 76:87–90.
- Page Y, Tardy B, Zeni F, Comtet C, Terrana R, Bertrand JC. Thrombotic thrombocytopenic purpura related to ticlopidine. Lancet 1991; 337:774–776.
- Canadian Cardiovascular Society; Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007; 50:e1–e157.
- King SB, Smith SC, Hirshfeld JW, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:172–209.
- Kam PC, Nethery CM. The thienopyridine derivatives (platelet adenosine diphosphate receptor antagonists), pharmacology and clinical developments. Anaesthesia 2003; 58:28–35.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Sabatine MS, Cannon CP, Gibson M, et al; CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation, N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: a randomized placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Bhatt DL, Bertrand ME, Berger PB, et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J Am Coll Cardiol 2002; 39:9–14.
- Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH; CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS). Circulation 2000; 102:624–629.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Mehta SR, Yusuf S, Peters RJ, et a; Clopidogrel in Unstable Angina to Prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001; 358:527–533.
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007; 115:1051–1058.
- Kotani J, Awata M, Nanto S, et al. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol 2006; 47:2108–2111.
- Pfisterer M, Brunner-La Rocca HP, Buser PT, et al; BASKETLATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol 2006; 48:2584–2591.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med 2007; 356:1030–1039.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- US Food and Drug Administration. FDA Approves Effient to Reduce the Risk of Heart Attack in Angioplasty Patients. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm171497.htm. Accessed October 2, 2009.
- Jernber T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006; 27:1166–1173.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A TRITON-TIMI (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Card 2008; 51:2028–2033.
Plaque rupture and thrombosis play central roles in the genesis of acute coronary syndrome. Aspirin has long been the preventive agent of choice. But dual antiplatelet therapy with aspirin plus clopidogrel (Plavix) is warranted in many patients to further reduce their risk of future cardiovascular events.
Although dual antiplatelet therapy is usually started by a subspecialist, the primary care physician is often the one who ensures that the patient remains compliant with it in the long term. A review of the seminal published data is helpful in understanding the rationale behind dual antiplatelet therapy and its risks and benefits.
In the mid-1990s, the thienopyridine ticlopidine (Ticlid) was found to significantly decrease the number of deaths, target-lesion revascularizations, and myocardial infarctions (MIs) in the 30 days following stent placement. 1 However, 2% to 3% of patients experienced neutropenia2 and thrombotic thrombocytopenic purpura with this drug,3 leading to the use of clopidogrel, another agent in the same class. Over the past decade, a large body of evidence has established the usefulness of clopidogrel in a number of clinical settings.
In this paper we review the current use of clopidogrel in ST-elevation MI, non-ST-elevation acute coronary syndromes, and percutaneous coronary intervention, and discuss the landmark trials that are the basis for the treatment guidelines published jointly by the American College of Cardiology (ACC) and the American Heart Association (AHA).4–6 We also briefly discuss the use of prasugrel (Effient), the newest antiplatelet agent to gain approval from the US Food and Drug Administration (FDA).
CLOPIDOGREL AS AN ALTERNATIVE TO ASPIRIN
Clopidogrel, a prodrug, is converted into its active form in the liver.7 It then irreversibly binds to the platelet P2Y12 receptor and inhibits adenosine diphosphate-induced platelet aggregation.
Those treated with clopidogrel had an annual risk of ischemic stroke, MI, or vascular death of 5.32%, compared with 5.83% in the aspirin group, for a statistically significant 8.7% relative risk reduction (P = .043). The observed frequency of neutropenia (neutrophils < 1.2 × 109/L) was 0.10% with clopidogrel vs 0.17% with aspirin. This study showed clopidogrel to be an effective alternative in patients who cannot tolerate aspirin.
CASE 1: ST-ELEVATION MI
A 57-year-old farmer in rural Ohio with a history of hypertension and hyperlipidemia presents to the local emergency department 45 minutes after the onset, while he was chopping wood, of dull, aching, substernal chest pain that radiates to his jaw. Electrocardiography reveals 2-mm ST-segment elevation in leads V1 through V6. He is treated with aspirin 162 mg, low-molecular-weight heparin, and tenecteplase.
What would be the value of starting dual antiplatelet therapy with clopidogrel in this patient?
Clopidogrel, aspirin, and fibrinolysis in ST-elevation MI
The CLARITY-TIMI 28 trial (Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis in Myocardial Infarction)9 included 3,491 patients (ages 18 to 75) from 319 international sites. All patients received a fibrinolytic agent, aspirin (162 mg to 325 mg on the first day and 75 mg to 162 mg thereafter), and heparin as part of standard care for acute ST-elevation MI (Table 1). Patients were randomized to receive a 300-mg loading dose of clopidogrel followed by 75 mg daily or placebo within 12 hours of onset of ST-elevation MI. The status of the infarct-related artery was ascertained by protocol-mandated coronary angiography 48 to 192 hours after starting the study medication. The primary end point was the composite of an occluded infarct-related artery on angiography, death from any cause prior to angiography, or recurrent MI prior to angiography.
Significantly fewer patients had an end point event in the clopidogrel group than in the placebo group, 15% vs 21.7% (P < .001), for a relative risk reduction of 31%. There was no significant increase in major or minor bleeding events.
Of note, the CLARITY-TIMI 28 patients were relatively young (average age 57 years) and at low cardiovascular risk (30-day mortality risk < 5%).
The COMMIT trial (Clopidogrel and Metoprolol in Myocardial Infarction)10 consisted of 45,852 patients with suspected acute MI admitted to 1,250 hospitals in China. Each patient received aspirin 162 mg daily plus either clopidogrel 75 mg daily (n = 22,961) or placebo (n = 22,891) for the duration of hospitalization (average 16 days) or 28 days, whichever came first.
The incidence of the primary composite end point of death, reinfarction, or stroke was significantly lower with clopidogrel than with placebo (9.2% vs 10.1%, P = .002). This was regardless of age (the average age was 61, and 26% of patients were older than 70), sex, time to presentation (67% presented within 12 hours), or reperfusion strategy (49% underwent fibrinolysis). The clopidogrel group did not have a significantly higher incidence of bleeding, but patients in this trial did not receive a loading dose of clopidogrel.
Comment. In view of the results of these trials, our 57-year-old patient should start clopidogrel early.
CASE 2: NON-ST-ELEVATION ACUTE CORONARY SYNDROME
A 65-year-old woman living independently with no significant medical history presents to the emergency room with 2 hours of waxing and waning substernal chest pain. Her blood pressure is 145/90 mm Hg, her heart rate is 95 beats per minute, and the results of her physical examination are unremarkable. Resting electrocardiography reveals 1.5-mm ST-segment depression in the inferior leads, and her troponin T level on admission is two times the upper limit of normal. She is given aspirin and is started on low-molecular-weight heparin and intravenous nitroglycerin.
What would be the value of starting clopidogrel in this patient?
Clopidogrel in non-ST-elevation acute coronary syndromes
The ACC/AHA guidelines strongly support starting clopidogrel in patients with non-ST-elevation acute coronary syndromes (Table 2).5
The CURE trial (Clopidogrel in Unstable Angina to Prevent Recurrent Events)11 provided the evidence for this recommendation. In this trial, 12,562 patients from 482 centers in 28 countries who presented within 24 hours of coronary symptoms, without ST elevation, were randomized to receive either clopidogrel (a 300-mg loading dose, followed by 75 mg daily) or placebo for 3 to 12 months (mean 9 months).
Significantly fewer patients in the clopidogrel group reached one of the end points of the composite primary outcome (cardiovascular death, nonfatal MI, or stroke): 9.3% vs 11.4%, 95% confidence interval (CI) 0.72–0.90, P < .001. Significantly fewer of them also suffered one of the secondary outcomes, ie, severe ischemia, heart failure, or need for revascularization.
Of concern was a higher rate of major bleeding in the clopidogrel group (3.7%) than in the placebo group (2.7%) without an excess of fatal bleeding. For every 1,000 patients treated with clopidogrel, 6 required a blood transfusion. Nevertheless, CURE proved that patients with non-ST-elevation acute coronary syndromes benefited from clopidogrel, regardless of whether they underwent percutaneous coronary intervention.
Comment. Our patient should receive clopidogrel and, if she has no significant bleeding, she should continue to take it for at least 12 months after discharge. It is important for the primary care physician to ensure compliance with this agent and not discontinue it on routine clinical follow-up.
CASE 3: BARE-METAL STENT PLACEMENT
A 62-year-old man with a history of hypertension, diabetes, and hyperlipidemia presents to his primary care physician’s office with stable-effort angina that is not responding to an excellent anti-ischemic regimen and is affecting his quality of life. He is referred for coronary angiography, which reveals 80% stenosis of the proximal left circumflex artery. He undergoes a percutaneous coronary intervention with placement of a bare-metal stent.
How long should he be on clopidogrel? And what if a drug-eluting stent had been placed instead of a bare-metal stent?
Dual therapy after bare-metal stent placement
Dual antiplatelet therapy with clopidogrel and aspirin is recommended in all patients receiving a stent (Table 2). The better safety and efficacy of clopidogrel compared with ticlopidine has been established in patients receiving a coronary artery stent,12,13 and clopidogrel’s favorable safety profile soon made it the thienopyridine of choice.
The CREDO trial (Clopidogrel for the Reduction of Events During Observation)14 randomized 2,116 patients undergoing an elective percutaneous coronary intervention (bare-metal stent placement only) to receive a 300-mg loading dose of clopidogrel 3 to 24 hours before the procedure, or placebo. All patients received 325 mg of aspirin. After the intervention, all patients received clopidogrel 75 mg daily and aspirin 325 mg daily through day 28. For day 29 through 12 months, those who had received the 300-mg preprocedural loading dose of clopidogrel continued with 75 mg daily, and those who had not received clopidogrel before the procedure received placebo.
No significant difference was seen in the primary outcome for those who received pretreatment with clopidogrel; however, in a subgroup analysis, those who received clopidogrel at least 6 hours before the percutaneous coronary intervention had a 38.6% relative risk reduction (Table 1). Long-term use of clopidogrel (ie, for 12 months) was associated with an overall relative reduction of 26.9% in the combined risk of death, MI, or stroke.
PCI-CURE, an analysis of 2,658 patients in the CURE trial with non-ST-elevation acute coronary syndrome who underwent PCI,15 yielded results similar to those of CREDO, with a 31% reduction in the rate of cardiovascular death or MI at 30 days and at 9 months. Of note, however, clopidogrel was given for a median of 6 days prior to the procedure.
Comment. The minimum suggested duration of clopidogrel treatment after placement of a bare-metal stent is 1 month. However, these trial results indicate that patients who are not at high risk of bleeding should take clopidogrel for at least 12 months.
Dual antiplatelet therapy with drug-eluting stents
Although rates of in-stent restenosis are clearly lower with drug-eluting stents than with bare-metal stents, the antiproliferative effect of drug-eluting stents may delay complete endothelialization of every strut. This may contribute to late (> 1 month after placement) or very late (> 1 year) thrombosis of the stent after clopidogrel is discontinued.16–18
In 2006, the FDA indicated that dual antiplatelet therapy was needed for 6 months with paclitaxel-eluting (Taxus) stents and 3 months with sirolimus-eluting (Cipher) stents. As reports of very late stent thrombosis began to appear in 2007, concern arose over the need to extend the duration of clopidogrel treatment.
Bavry et al19 quantified the incidence of late and very late stent thrombosis in a meta-analysis of 14 clinical trials that randomized patients to receive either a drug-eluting stent (paclitaxel or sirolimus) or a bare-metal stent.19 The incidence of stent thrombosis within 30 days in this analysis was similar for both groups—4.4 per 1,000 patients vs 5 per 1,000 (relative risk 0.89; 95% CI 0.46–1.75; P = .74). However, the rate of very late stent thrombosis was significantly higher in those receiving a drug-eluting stent vs a bare-metal stent—5 per 1,000 patients treated (relative risk 5.02, 95% CI 1.29–19.52; P = .02).
The results of this and other studies led the ACC and AHA to revise their joint guidelines to recommend thienopyridine treatment for at least 1 year for patients who receive a drug-eluting stent.6,20–22 In fact, many cardiologists consider indefinite dual antiplatelet therapy in patients with a drug-eluting stent to avoid very late in-stent thrombosis, especially in patients undergoing high-risk interventions such as placement of multiple stents, bifurcation lesions, and unprotected left main trunk interventions.
Thus, when faced with a patient with a recent coronary stent implantation, the primary care physician should be aware of the type of stent and the duration of therapy recommended by the interventional cardiologist. Also, in the absence of a pressing indication, elective surgery should be deferred for 1 year after placement of a drug-eluting stent, as this would necessitate stopping clopidogrel and would increase the risk of perioperative stent thrombosis, which is associated with high rates of morbidity and death.
CASE 4: HIGH-RISK CORONARY ARTERY DISEASE
A 67-year-old woman presents to your office to establish care. She has a history of diabetes and established coronary artery disease with two bare-metal stents placed 2 years ago. She is taking aspirin 81 mg.
What would be the value of adding clopidogrel to her regimen?
No indication for clopidogrel in chronic coronary artery disease
The CHARISMA trial (Clopidogrel for High Atherothrombotic and Ischemic Stabilization, Management, and Avoidance)23 randomized 15,603 patients with stable cardiovascular disease or multiple risk factors to receive either clopidogrel plus low-dose aspirin or placebo plus low-dose aspirin and followed them for a median of 28 months (Table 1).
The primary end point (a composite of MI, stroke, or death) was 6.8% with clopidogrel plus aspirin and 7.3% with aspirin alone, indicating no significant benefit with clopidogrel plus aspirin compared with aspirin alone in reducing the rate of MI, stroke, or cardiovascular death in patients with high-risk but stable atherothrombotic disease. A marginal statistical benefit with dual antiplatelet therapy was noted in the subgroup of patients with previously documented coronary, cerebrovascular, or peripheral vascular disease—6.9% with aspirin plus clopidogrel vs 7.9% with aspirin alone (relative risk 0.88; 95% CI 0.77–0.998; P = .046).
Consequently, there is no compelling reason to start clopidogrel in this patient.
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel was recently approved by the FDA as antiplatelet treatment for patients with acute coronary syndromes planning to undergo a percutaneous coronary intervention.24 It has been shown to inhibit adenosine-diphosphate-induced platelet activation in a more consistent and effective manner than clopidogrel.25,26
Although both clopidogrel and prasugrel are prodrugs, 80% of absorbed clopidogrel is metabolized by esterases into inactive metabolites, and the availability of active metabolite can vary, as it is significantly influenced by polymorphisms in the cytochrome P450 system. 27 In contrast, prasugrel is not degraded by esterases, and its conversion to active metabolite by the cytochrome P450 system is not influenced by common genetic polymorphisms, particularly CYP2C19*2.
TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction) provided most of the evidence for the approval of prasugrel for clinical use.28,29 In this trial, a 60-mg loading dose of prasugrel followed by a daily maintenance dose of 10 mg was significantly superior to the current clopidogrel regimen in preventing death from cardiovascular causes, nonfatal MI, or nonfatal stroke during a study period of 15 months.28 Also observed was a 24% lower rate of MI, a 34% lower rate of urgent target-vessel revascularization, and a 52% lower rate of stent thrombosis.
These benefits, however, came at the cost of a significantly higher risk of major bleeding, including the potential for three excess fatal bleeding events for every 1,000 patients treated. Patients at highest risk at the dosages evaluated included the elderly (age 75 and older), patients who weigh less than 60 kg, and patients with a history of stroke or transient ischemic attack. Based on these results, we recommend caution with the use of prasugrel in these patient subsets.
Clinical use of prasugrel is likely to be highest in patients presenting with ST-elevation MI who are undergoing a primary percutaneous coronary intervention. There is currently no evidence from any randomized clinical trial to support the safety of prasugrel given in the emergency room or “upstream” in the setting of non-ST-elevation acute coronary syndromes.
Of note, patients with non-ST-elevation acute coronary syndromes in the TRITON trial were randomized only after angiographic definition. As a result, only 179 patients exposed to prasugrel were referred for coronary artery bypass surgery, but the rate of surgery-related major bleeding in this group was 13.4% (vs 3.2% in the clopidogrel group). Based on these data, prasugrel should be withheld for at least 1 week prior to any surgery.
Plaque rupture and thrombosis play central roles in the genesis of acute coronary syndrome. Aspirin has long been the preventive agent of choice. But dual antiplatelet therapy with aspirin plus clopidogrel (Plavix) is warranted in many patients to further reduce their risk of future cardiovascular events.
Although dual antiplatelet therapy is usually started by a subspecialist, the primary care physician is often the one who ensures that the patient remains compliant with it in the long term. A review of the seminal published data is helpful in understanding the rationale behind dual antiplatelet therapy and its risks and benefits.
In the mid-1990s, the thienopyridine ticlopidine (Ticlid) was found to significantly decrease the number of deaths, target-lesion revascularizations, and myocardial infarctions (MIs) in the 30 days following stent placement. 1 However, 2% to 3% of patients experienced neutropenia2 and thrombotic thrombocytopenic purpura with this drug,3 leading to the use of clopidogrel, another agent in the same class. Over the past decade, a large body of evidence has established the usefulness of clopidogrel in a number of clinical settings.
In this paper we review the current use of clopidogrel in ST-elevation MI, non-ST-elevation acute coronary syndromes, and percutaneous coronary intervention, and discuss the landmark trials that are the basis for the treatment guidelines published jointly by the American College of Cardiology (ACC) and the American Heart Association (AHA).4–6 We also briefly discuss the use of prasugrel (Effient), the newest antiplatelet agent to gain approval from the US Food and Drug Administration (FDA).
CLOPIDOGREL AS AN ALTERNATIVE TO ASPIRIN
Clopidogrel, a prodrug, is converted into its active form in the liver.7 It then irreversibly binds to the platelet P2Y12 receptor and inhibits adenosine diphosphate-induced platelet aggregation.
Those treated with clopidogrel had an annual risk of ischemic stroke, MI, or vascular death of 5.32%, compared with 5.83% in the aspirin group, for a statistically significant 8.7% relative risk reduction (P = .043). The observed frequency of neutropenia (neutrophils < 1.2 × 109/L) was 0.10% with clopidogrel vs 0.17% with aspirin. This study showed clopidogrel to be an effective alternative in patients who cannot tolerate aspirin.
CASE 1: ST-ELEVATION MI
A 57-year-old farmer in rural Ohio with a history of hypertension and hyperlipidemia presents to the local emergency department 45 minutes after the onset, while he was chopping wood, of dull, aching, substernal chest pain that radiates to his jaw. Electrocardiography reveals 2-mm ST-segment elevation in leads V1 through V6. He is treated with aspirin 162 mg, low-molecular-weight heparin, and tenecteplase.
What would be the value of starting dual antiplatelet therapy with clopidogrel in this patient?
Clopidogrel, aspirin, and fibrinolysis in ST-elevation MI
The CLARITY-TIMI 28 trial (Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis in Myocardial Infarction)9 included 3,491 patients (ages 18 to 75) from 319 international sites. All patients received a fibrinolytic agent, aspirin (162 mg to 325 mg on the first day and 75 mg to 162 mg thereafter), and heparin as part of standard care for acute ST-elevation MI (Table 1). Patients were randomized to receive a 300-mg loading dose of clopidogrel followed by 75 mg daily or placebo within 12 hours of onset of ST-elevation MI. The status of the infarct-related artery was ascertained by protocol-mandated coronary angiography 48 to 192 hours after starting the study medication. The primary end point was the composite of an occluded infarct-related artery on angiography, death from any cause prior to angiography, or recurrent MI prior to angiography.
Significantly fewer patients had an end point event in the clopidogrel group than in the placebo group, 15% vs 21.7% (P < .001), for a relative risk reduction of 31%. There was no significant increase in major or minor bleeding events.
Of note, the CLARITY-TIMI 28 patients were relatively young (average age 57 years) and at low cardiovascular risk (30-day mortality risk < 5%).
The COMMIT trial (Clopidogrel and Metoprolol in Myocardial Infarction)10 consisted of 45,852 patients with suspected acute MI admitted to 1,250 hospitals in China. Each patient received aspirin 162 mg daily plus either clopidogrel 75 mg daily (n = 22,961) or placebo (n = 22,891) for the duration of hospitalization (average 16 days) or 28 days, whichever came first.
The incidence of the primary composite end point of death, reinfarction, or stroke was significantly lower with clopidogrel than with placebo (9.2% vs 10.1%, P = .002). This was regardless of age (the average age was 61, and 26% of patients were older than 70), sex, time to presentation (67% presented within 12 hours), or reperfusion strategy (49% underwent fibrinolysis). The clopidogrel group did not have a significantly higher incidence of bleeding, but patients in this trial did not receive a loading dose of clopidogrel.
Comment. In view of the results of these trials, our 57-year-old patient should start clopidogrel early.
CASE 2: NON-ST-ELEVATION ACUTE CORONARY SYNDROME
A 65-year-old woman living independently with no significant medical history presents to the emergency room with 2 hours of waxing and waning substernal chest pain. Her blood pressure is 145/90 mm Hg, her heart rate is 95 beats per minute, and the results of her physical examination are unremarkable. Resting electrocardiography reveals 1.5-mm ST-segment depression in the inferior leads, and her troponin T level on admission is two times the upper limit of normal. She is given aspirin and is started on low-molecular-weight heparin and intravenous nitroglycerin.
What would be the value of starting clopidogrel in this patient?
Clopidogrel in non-ST-elevation acute coronary syndromes
The ACC/AHA guidelines strongly support starting clopidogrel in patients with non-ST-elevation acute coronary syndromes (Table 2).5
The CURE trial (Clopidogrel in Unstable Angina to Prevent Recurrent Events)11 provided the evidence for this recommendation. In this trial, 12,562 patients from 482 centers in 28 countries who presented within 24 hours of coronary symptoms, without ST elevation, were randomized to receive either clopidogrel (a 300-mg loading dose, followed by 75 mg daily) or placebo for 3 to 12 months (mean 9 months).
Significantly fewer patients in the clopidogrel group reached one of the end points of the composite primary outcome (cardiovascular death, nonfatal MI, or stroke): 9.3% vs 11.4%, 95% confidence interval (CI) 0.72–0.90, P < .001. Significantly fewer of them also suffered one of the secondary outcomes, ie, severe ischemia, heart failure, or need for revascularization.
Of concern was a higher rate of major bleeding in the clopidogrel group (3.7%) than in the placebo group (2.7%) without an excess of fatal bleeding. For every 1,000 patients treated with clopidogrel, 6 required a blood transfusion. Nevertheless, CURE proved that patients with non-ST-elevation acute coronary syndromes benefited from clopidogrel, regardless of whether they underwent percutaneous coronary intervention.
Comment. Our patient should receive clopidogrel and, if she has no significant bleeding, she should continue to take it for at least 12 months after discharge. It is important for the primary care physician to ensure compliance with this agent and not discontinue it on routine clinical follow-up.
CASE 3: BARE-METAL STENT PLACEMENT
A 62-year-old man with a history of hypertension, diabetes, and hyperlipidemia presents to his primary care physician’s office with stable-effort angina that is not responding to an excellent anti-ischemic regimen and is affecting his quality of life. He is referred for coronary angiography, which reveals 80% stenosis of the proximal left circumflex artery. He undergoes a percutaneous coronary intervention with placement of a bare-metal stent.
How long should he be on clopidogrel? And what if a drug-eluting stent had been placed instead of a bare-metal stent?
Dual therapy after bare-metal stent placement
Dual antiplatelet therapy with clopidogrel and aspirin is recommended in all patients receiving a stent (Table 2). The better safety and efficacy of clopidogrel compared with ticlopidine has been established in patients receiving a coronary artery stent,12,13 and clopidogrel’s favorable safety profile soon made it the thienopyridine of choice.
The CREDO trial (Clopidogrel for the Reduction of Events During Observation)14 randomized 2,116 patients undergoing an elective percutaneous coronary intervention (bare-metal stent placement only) to receive a 300-mg loading dose of clopidogrel 3 to 24 hours before the procedure, or placebo. All patients received 325 mg of aspirin. After the intervention, all patients received clopidogrel 75 mg daily and aspirin 325 mg daily through day 28. For day 29 through 12 months, those who had received the 300-mg preprocedural loading dose of clopidogrel continued with 75 mg daily, and those who had not received clopidogrel before the procedure received placebo.
No significant difference was seen in the primary outcome for those who received pretreatment with clopidogrel; however, in a subgroup analysis, those who received clopidogrel at least 6 hours before the percutaneous coronary intervention had a 38.6% relative risk reduction (Table 1). Long-term use of clopidogrel (ie, for 12 months) was associated with an overall relative reduction of 26.9% in the combined risk of death, MI, or stroke.
PCI-CURE, an analysis of 2,658 patients in the CURE trial with non-ST-elevation acute coronary syndrome who underwent PCI,15 yielded results similar to those of CREDO, with a 31% reduction in the rate of cardiovascular death or MI at 30 days and at 9 months. Of note, however, clopidogrel was given for a median of 6 days prior to the procedure.
Comment. The minimum suggested duration of clopidogrel treatment after placement of a bare-metal stent is 1 month. However, these trial results indicate that patients who are not at high risk of bleeding should take clopidogrel for at least 12 months.
Dual antiplatelet therapy with drug-eluting stents
Although rates of in-stent restenosis are clearly lower with drug-eluting stents than with bare-metal stents, the antiproliferative effect of drug-eluting stents may delay complete endothelialization of every strut. This may contribute to late (> 1 month after placement) or very late (> 1 year) thrombosis of the stent after clopidogrel is discontinued.16–18
In 2006, the FDA indicated that dual antiplatelet therapy was needed for 6 months with paclitaxel-eluting (Taxus) stents and 3 months with sirolimus-eluting (Cipher) stents. As reports of very late stent thrombosis began to appear in 2007, concern arose over the need to extend the duration of clopidogrel treatment.
Bavry et al19 quantified the incidence of late and very late stent thrombosis in a meta-analysis of 14 clinical trials that randomized patients to receive either a drug-eluting stent (paclitaxel or sirolimus) or a bare-metal stent.19 The incidence of stent thrombosis within 30 days in this analysis was similar for both groups—4.4 per 1,000 patients vs 5 per 1,000 (relative risk 0.89; 95% CI 0.46–1.75; P = .74). However, the rate of very late stent thrombosis was significantly higher in those receiving a drug-eluting stent vs a bare-metal stent—5 per 1,000 patients treated (relative risk 5.02, 95% CI 1.29–19.52; P = .02).
The results of this and other studies led the ACC and AHA to revise their joint guidelines to recommend thienopyridine treatment for at least 1 year for patients who receive a drug-eluting stent.6,20–22 In fact, many cardiologists consider indefinite dual antiplatelet therapy in patients with a drug-eluting stent to avoid very late in-stent thrombosis, especially in patients undergoing high-risk interventions such as placement of multiple stents, bifurcation lesions, and unprotected left main trunk interventions.
Thus, when faced with a patient with a recent coronary stent implantation, the primary care physician should be aware of the type of stent and the duration of therapy recommended by the interventional cardiologist. Also, in the absence of a pressing indication, elective surgery should be deferred for 1 year after placement of a drug-eluting stent, as this would necessitate stopping clopidogrel and would increase the risk of perioperative stent thrombosis, which is associated with high rates of morbidity and death.
CASE 4: HIGH-RISK CORONARY ARTERY DISEASE
A 67-year-old woman presents to your office to establish care. She has a history of diabetes and established coronary artery disease with two bare-metal stents placed 2 years ago. She is taking aspirin 81 mg.
What would be the value of adding clopidogrel to her regimen?
No indication for clopidogrel in chronic coronary artery disease
The CHARISMA trial (Clopidogrel for High Atherothrombotic and Ischemic Stabilization, Management, and Avoidance)23 randomized 15,603 patients with stable cardiovascular disease or multiple risk factors to receive either clopidogrel plus low-dose aspirin or placebo plus low-dose aspirin and followed them for a median of 28 months (Table 1).
The primary end point (a composite of MI, stroke, or death) was 6.8% with clopidogrel plus aspirin and 7.3% with aspirin alone, indicating no significant benefit with clopidogrel plus aspirin compared with aspirin alone in reducing the rate of MI, stroke, or cardiovascular death in patients with high-risk but stable atherothrombotic disease. A marginal statistical benefit with dual antiplatelet therapy was noted in the subgroup of patients with previously documented coronary, cerebrovascular, or peripheral vascular disease—6.9% with aspirin plus clopidogrel vs 7.9% with aspirin alone (relative risk 0.88; 95% CI 0.77–0.998; P = .046).
Consequently, there is no compelling reason to start clopidogrel in this patient.
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel was recently approved by the FDA as antiplatelet treatment for patients with acute coronary syndromes planning to undergo a percutaneous coronary intervention.24 It has been shown to inhibit adenosine-diphosphate-induced platelet activation in a more consistent and effective manner than clopidogrel.25,26
Although both clopidogrel and prasugrel are prodrugs, 80% of absorbed clopidogrel is metabolized by esterases into inactive metabolites, and the availability of active metabolite can vary, as it is significantly influenced by polymorphisms in the cytochrome P450 system. 27 In contrast, prasugrel is not degraded by esterases, and its conversion to active metabolite by the cytochrome P450 system is not influenced by common genetic polymorphisms, particularly CYP2C19*2.
TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction) provided most of the evidence for the approval of prasugrel for clinical use.28,29 In this trial, a 60-mg loading dose of prasugrel followed by a daily maintenance dose of 10 mg was significantly superior to the current clopidogrel regimen in preventing death from cardiovascular causes, nonfatal MI, or nonfatal stroke during a study period of 15 months.28 Also observed was a 24% lower rate of MI, a 34% lower rate of urgent target-vessel revascularization, and a 52% lower rate of stent thrombosis.
These benefits, however, came at the cost of a significantly higher risk of major bleeding, including the potential for three excess fatal bleeding events for every 1,000 patients treated. Patients at highest risk at the dosages evaluated included the elderly (age 75 and older), patients who weigh less than 60 kg, and patients with a history of stroke or transient ischemic attack. Based on these results, we recommend caution with the use of prasugrel in these patient subsets.
Clinical use of prasugrel is likely to be highest in patients presenting with ST-elevation MI who are undergoing a primary percutaneous coronary intervention. There is currently no evidence from any randomized clinical trial to support the safety of prasugrel given in the emergency room or “upstream” in the setting of non-ST-elevation acute coronary syndromes.
Of note, patients with non-ST-elevation acute coronary syndromes in the TRITON trial were randomized only after angiographic definition. As a result, only 179 patients exposed to prasugrel were referred for coronary artery bypass surgery, but the rate of surgery-related major bleeding in this group was 13.4% (vs 3.2% in the clopidogrel group). Based on these data, prasugrel should be withheld for at least 1 week prior to any surgery.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Yeh SP, Hsueh EJ, Wu H, Wang YC. Ticlopidine-associated aplastic anemia. A case report and review of literature, Ann Hematol 1998; 76:87–90.
- Page Y, Tardy B, Zeni F, Comtet C, Terrana R, Bertrand JC. Thrombotic thrombocytopenic purpura related to ticlopidine. Lancet 1991; 337:774–776.
- Canadian Cardiovascular Society; Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007; 50:e1–e157.
- King SB, Smith SC, Hirshfeld JW, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:172–209.
- Kam PC, Nethery CM. The thienopyridine derivatives (platelet adenosine diphosphate receptor antagonists), pharmacology and clinical developments. Anaesthesia 2003; 58:28–35.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Sabatine MS, Cannon CP, Gibson M, et al; CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation, N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: a randomized placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Bhatt DL, Bertrand ME, Berger PB, et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J Am Coll Cardiol 2002; 39:9–14.
- Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH; CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS). Circulation 2000; 102:624–629.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Mehta SR, Yusuf S, Peters RJ, et a; Clopidogrel in Unstable Angina to Prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001; 358:527–533.
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007; 115:1051–1058.
- Kotani J, Awata M, Nanto S, et al. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol 2006; 47:2108–2111.
- Pfisterer M, Brunner-La Rocca HP, Buser PT, et al; BASKETLATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol 2006; 48:2584–2591.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med 2007; 356:1030–1039.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- US Food and Drug Administration. FDA Approves Effient to Reduce the Risk of Heart Attack in Angioplasty Patients. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm171497.htm. Accessed October 2, 2009.
- Jernber T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006; 27:1166–1173.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A TRITON-TIMI (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Card 2008; 51:2028–2033.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Yeh SP, Hsueh EJ, Wu H, Wang YC. Ticlopidine-associated aplastic anemia. A case report and review of literature, Ann Hematol 1998; 76:87–90.
- Page Y, Tardy B, Zeni F, Comtet C, Terrana R, Bertrand JC. Thrombotic thrombocytopenic purpura related to ticlopidine. Lancet 1991; 337:774–776.
- Canadian Cardiovascular Society; Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007; 50:e1–e157.
- King SB, Smith SC, Hirshfeld JW, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:172–209.
- Kam PC, Nethery CM. The thienopyridine derivatives (platelet adenosine diphosphate receptor antagonists), pharmacology and clinical developments. Anaesthesia 2003; 58:28–35.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Sabatine MS, Cannon CP, Gibson M, et al; CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation, N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: a randomized placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Bhatt DL, Bertrand ME, Berger PB, et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J Am Coll Cardiol 2002; 39:9–14.
- Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH; CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS). Circulation 2000; 102:624–629.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Mehta SR, Yusuf S, Peters RJ, et a; Clopidogrel in Unstable Angina to Prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001; 358:527–533.
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007; 115:1051–1058.
- Kotani J, Awata M, Nanto S, et al. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol 2006; 47:2108–2111.
- Pfisterer M, Brunner-La Rocca HP, Buser PT, et al; BASKETLATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol 2006; 48:2584–2591.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med 2007; 356:1030–1039.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- US Food and Drug Administration. FDA Approves Effient to Reduce the Risk of Heart Attack in Angioplasty Patients. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm171497.htm. Accessed October 2, 2009.
- Jernber T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006; 27:1166–1173.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A TRITON-TIMI (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Card 2008; 51:2028–2033.
KEY POINTS
- Dual antiplatelet therapy is recommended after ST-elevation MI or non-ST-elevation acute coronary syndromes, with aspirin indefinitely and clopidogrel for up to 1 year.
- Dual antiplatelet therapy is recommended for at least 1 month after placement of a bare-metal stent and for at least 1 year (or possibly indefinitely) after placement of a drug-eluting stent.
- There is no compelling indication for clopidogrel in patients with chronic coronary artery disease.
- Compared with clopidogrel, prasugrel (Effient) is associated with lower rates of MI, urgent target-vessel revascularization, and in-stent thrombosis, but at the cost of a higher risk of major bleeding.