User login
New cholesterol guidelines: Worth the wait?
On November 12, 2013, a joint task force for the American College of Cardiology and American Heart Association released new guidelines for treating high blood cholesterol to reduce the risk of atherosclerotic cardiovascular disease (ASCVD) in adults.1
This document arrives after several years of intense deliberation, 12 years after the third Adult Treatment Panel (ATP III) guidelines,2 and 8 years after an ATP III update recommending that low-density lipoprotein cholesterol (LDL-C) levels be lowered aggressively (to less than 70 mg/dL) as an option in patients at high risk.3 It represents a major shift in the approach to and management of high blood cholesterol and has sparked considerable controversy.
In the following commentary, we summarize the new guidelines and the philosophy employed by the task force in generating them. We will also examine some advantages and what we believe to be several shortcomings of the new guidelines. These latter points are illustrated through case examples.
IN RANDOMIZED CONTROLLED TRIALS WE TRUST
In collaboration with the National Heart, Lung, and Blood Institute of the National Institutes of Health, the American College of Cardiology and American Heart Association formed an expert panel task force in 2008.
The task force elected to use only evidence from randomized controlled trials, systematic reviews, and meta-analyses of randomized controlled trials (and only predefined outcomes of the trials, not post hoc analyses) in formulating its recommendations, with the goal of providing the strongest possible evidence.
The authors state that “By using [randomized controlled trial] data to identify those most likely to benefit [emphasis in original] from cholesterol-lowering statin therapy, the recommendations will be of value to primary care clinicians as well as specialists concerned with ASCVD prevention. Importantly, the recommendations were designed to be easy to use in the clinical setting, facilitating the implementation of a strategy of risk assessment and treatment focused on the prevention of ASCVD.”3 They also state the guidelines are meant to “inform clinical judgment, not replace it” and that clinician judgment in addition to discussion with patients remains vital.
During the deliberations, the National Heart, Lung, and Blood Institute removed itself from participating, stating its mission no longer included drafting new guidelines. Additionally, other initial members of the task force removed themselves because of disagreement and concerns about the direction of the new guidelines.
These guidelines, and their accompanying new cardiovascular risk calculator,4 were released without a preliminary period to allow for open discussion, comment, and critique by physicians outside the panel. No attempt was made to harmonize the guidelines with previous versions (eg, ATP III) or with current international guidelines.
WHAT’S NEW IN THE GUIDELINES?
The following are the major changes in the new guidelines for treating high blood cholesterol:
- Treatment goals for LDL-C and non-high-density lipoprotein cholesterol (non-HDL-C) are no longer recommended.
- High-intensity and moderate-intensity statin treatment is emphasized, and low-intensity statin therapy is nearly eliminated.
- “ASCVD” now includes stroke in addition to coronary heart disease and peripheral arterial disease.
- Four groups are targeted for treatment (see below).
- Nonstatin therapies have been markedly de-emphasized.
- No guidelines are provided for treating high triglyceride levels.
The new guidelines emphasize lifestyle modification as the foundation for reducing risk, regardless of cholesterol therapy. No recommendations are given for patients with New York Heart Association class II, III, or IV heart failure or for hemodialysis patients, because there were insufficient data from randomized controlled trials to support recommendations. Similarly, the guidelines apply only to people between the ages of 40 and 75 (risk calculator ages 40–79), because the authors believed there was not enough evidence from randomized controlled trials to allow development of guidelines outside of this age range.
FOUR MAJOR STATIN TREATMENT GROUPS
The new guidelines specify four groups that merit intensive or moderately intensive statin therapy (Table 1)1:
- People with clinical ASCVD
- People with LDL-C levels of 190 mg/dL or higher
- People with diabetes, age 40 to 75
- People without diabetes, age 40 to 75, with LDL-C levels 70–189 mg/dL, and a 10-year ASCVD risk of 7.5% or higher as determined by the new risk calculator4 (which also calculates the lifetime risk of ASCVD).
Below, we will address each of these four groups and provide case scenarios to consider. In general, our major disagreements with the new recommendations pertain to the first and fourth categories.
GROUP 1: PEOPLE WITH CLINICAL ASCVD
Advantages of the new guidelines
- They appropriately recommend statins in the highest tolerated doses as first-line treatment for this group at high risk.
- They designate all patients with ASCVD, including those with coronary, peripheral, and cerebrovascular disease, as a high-risk group.
- Without target LDL-C levels, treatment is simpler than before, requiring less monitoring of lipid levels. (This can also be seen as a limitation, as we discuss below.)
Limitations of the new guidelines
- They make follow-up LDL-C levels irrelevant, seeming to assume that there is no gradation in residual risk and, thus, no need to tailor therapy to the individual.
- Patients no longer have a goal to strive for or a way to monitor their progress.
- The guidelines ignore the pathophysiology of coronary artery disease and evidence of residual risk in patients on both moderate-intensity and high-intensity statin therapy.
- They also ignore the potential benefits of treating to lower LDL-C or non-HDL-C goals, thus eliminating consideration of multidrug therapy. They do not address patients with recurrent cardiovascular events already on maximal tolerated statin doses.
- They undermine the potential development and use of new therapies for dysplipidemia in patients with ASCVD.
Case 1: Is LDL-C 110 mg/dL low enough?
A 52-year-old African American man presents with newly discovered moderate coronary artery disease that is not severe enough to warrant stenting. He has no history of hypertension, diabetes mellitus, or smoking. His systolic blood pressure is 130 mm Hg, and his body mass index is 26 kg/m2. He exercises regularly and follows a low-cholesterol diet. He has the following fasting lipid values:
- Total cholesterol 290 mg/dL
- HDL-C 50 mg/dL
- Triglycerides 250 mg/dL
- Calculated LDL-C 190 mg/dL.
Two months later, after beginning atorvastatin 80 mg daily, meeting with a nutritionist, and redoubling his dietary efforts, his fasting lipid concentrations are:
- Total cholesterol 180 mg/dL
- HDL-C 55 mg/dL
- Triglycerides 75 mg/dL
- Calculated LDL-C 110 mg/dL.
Comment: Lack of LDL-C goals is a flaw
The new guidelines call for patients with known ASCVD, such as this patient, to receive intensive statin therapy—which he got.
However, once a patient is on therapy, the new guidelines do not encourage repeating the lipid panel other than to assess compliance. With intensive therapy, we expect a reduction in LDL-C of at least 50% (Table 1), but patient-to-patient differences in response to medications are common, and without repeat testing we would have no way of gauging this patient’s residual risk.
Further, the new guidelines emphasize the lack of hard outcome data supporting the addition of another lipid-lowering drug to a statin, although they do indicate that one can consider it. In a patient at high risk, such as this one, would you be comfortable with an LDL-C value of 110 mg/dL on maximum statin therapy? Would you consider adding a nonstatin drug?
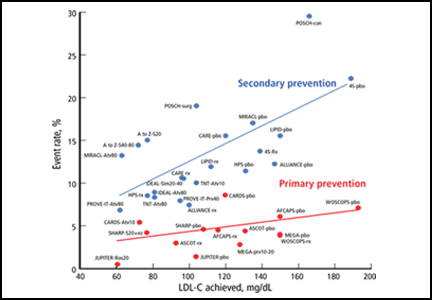
A preponderance of data shows that LDL plays a causal role in ASCVD development and adverse events. Genetic data show that the LDL particle and the LDL receptor pathway are mechanistically linked to ASCVD pathogenesis, with lifetime exposure as a critical determinant of risk.5,6 Moreover, randomized controlled trials of statins and other studies of cholesterol-lowering show a reproducible relationship between the LDL-C level achieved and absolute risk (Figure 1).7–24 We believe the totality of data constitutes a strong rationale for targeting LDL-C and establishing goals for lowering its levels. For these reasons, we believe that removing LDL-C goals is a fundamental flaw of the new guidelines.
The reason for the lack of data from randomized controlled trials demonstrating benefits of adding therapies to statins (when LDL-C is still high) or benefits of treating to specific goals is that no such trials have been performed. Even trials of nonpharmacologic means of lowering LDL-C, such as ileal bypass, which was used in the Program on the Surgical Control of the Hyperlipidemias trial,20 provide independent evidence that lowering LDL-C reduces the risk of ASCVD (Figure 1).
In addition, trials of nonstatin drugs, such as the Coronary Drug Project,25 which tested niacin, also showed outcome benefits. On the other hand, studies such as the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health26 and Treatment of HDL to Reduce the Incidence of Vascular Events27 trials did not show additional risk reduction when niacin was added to statin therapy. However, the study designs arguably had flaws, including requirement of aggressive LDL-lowering with statins, with LDL-C levels below 70 to 80 mg/dL before randomization.
Therefore, these trials do not tell us what to do for a patient on maximal intensive therapy who has recurrent ASCVD events or who, like our patient, has an LDL-C level higher than previous targets.
For this patient, we would recommend adding a second medication to further lower his LDL-C, but discussing with him the absence of proven benefit in clinical trials and the risks of side effects. At present, lacking LDL-C goals in the new guidelines, we are keeping with the ATP III goals to help guide therapeutic choices and individualize patient management.
GROUP 2: PEOPLE WITH LDL-C ≥ 190
Advantages of the new guidelines
- They state that these patients should receive statins in the highest tolerated doses, which is universally accepted.
Limitations of the new guidelines
- The new guidelines mention only that one “may consider” adding a second agent if LDL-C remains above 190 mg/dL after maximum-dose therapy. Patients with familial hypercholesterolemia or other severe forms of hypercholesterolemia typically end up on multidrug therapy to further reduce LDL-C. The absence of randomized controlled trial data in this setting to show an additive value of second and third lipid-lowering agents does not mean these agents do not provide benefit.
GROUP 3: DIABETES, AGE 40–75, LDL-C 70–189, NO CLINICAL ASCVD
Advantages of the new guidelines
- They call for aggressive treatment of people with diabetes, a group at high risk that derives significant benefit from statin therapy, as shown in randomized controlled trials.
Limitations of the new guidelines
- Although high-intensity statin therapy is indicated for this group, we believe that, using the new risk calculator, some patients may receive overly aggressive treatment, thus increasing the possibility of statin side effects.
- The guidelines do not address patients younger than 40 or older than 75.
- Diabetic patients have a high residual risk of ASCVD events, even on statin therapy. Yet the guidelines ignore the potential benefits of more aggressive LDL-lowering or non-LDL secondary targets for therapy.
Case 2: How low is too low?
A 63-year-old white woman, a nonsmoker with recently diagnosed diabetes, is seen by her primary care physician. She has hypertension, for which she takes lisinopril 5 mg daily. Her fasting lipid values are:
- Total cholesterol 160 mg/dL
- HDL-C 64 mg/dL
- Triglycerides 100 mg/dL
- Calculated LDL-C 76 mg/dL.
Her systolic blood pressure is 129 mm Hg, and based on the new risk calculator, her 10-year risk of cardiovascular disease is 10.2%. According to the new guidelines, she should be started on high-intensity statin treatment (Table 1).
Although this is an acceptable initial course of action, it necessitates close vigilance, since it may actually drive her LDL-C level too low. Randomized controlled trials have typically used an LDL-C concentration of less than or equal to 25 mg/dL as the safety cutoff. With a typical LDL-C reduction of at least 50% on high-intensity statins, our patient’s expected LDL-C level will likely be in the low 30s. We believe this would be a good outcome, provided that she tolerates the medication without adverse effects. However, responses to statins vary from patient to patient.
High-intensity statin therapy may not be necessary to reduce risk adequately in all patients who have diabetes without preexisting vascular disease. The Collaborative Atorvastatin Diabetes Study12 compared atorvastatin 10 mg vs placebo in people with type 2 diabetes, age 40 to 75, who had one or more cardiovascular risk factors but no signs or symptoms of preexisting ASCVD and who had only average or below-average cholesterol levels—precisely like this patient. The trial was terminated early because of a clear benefit (a 37% reduction in the composite end point of major adverse cardiovascular events) in the intervention group. For our patient, we believe an alternative and acceptable approach would be to begin moderate-intensity statin therapy (eg, with atorvastatin 10 mg) (Table 1).
Alternatively, in a patient with diabetes and previous atherosclerotic vascular disease or with a high 10-year risk and high LDL-C, limiting treatment to high-intensity statin therapy by itself may deny them the potential benefits of combination therapies and targeting to lower LDL-C levels or non-HDL-C secondary targets. Guidelines from the American Diabetes Association28 and the American Association of Clinical Endocrinologists29 continue to recommend an LDL-C goal of less than 70 mg/dL in patients at high risk, a non-HDL-C less than 100 mg/dL, an apolipoprotein B less than 80 mg/dL, and an LDL particle number less than 1,000 nmol/L.
GROUP 4: AGE 40–75, LDL-C 70–189, NO ASCVD, BUT 10-YEAR RISK ≥ 7.5%
Advantages of the new guidelines
- They may reduce ASCVD events for patients at higher risk.
- The risk calculator is easy to use and focuses on global risk, ie, all forms of ASCVD.
- The guidelines promote discussion of risks and benefits between patients and providers.
Limitations of the new guidelines
- The new risk calculator is controversial (see below).
- There is potential for overtreatment, particularly in older patients.
- There is potential for undertreatment, particularly in patients with an elevated LDL-C but whose 10-year risk is less than 7.5% because they are young.
- The guidelines do not address patients younger than 40 or older than 75.
- They do not take into account some traditional risk factors, such as family history, and nontraditional risk factors such as C-reactive protein as measured by ultrasensitive assays, lipoprotein(a), and apolipoprotein B.
Risk calculator controversy
The new risk calculator has aroused strong opinions on both sides of the aisle.
Shortly after the new guidelines were released, cardiologists Dr. Paul Ridker and Dr. Nancy Cook from Brigham and Women’s Hospital in Boston published analyses30 showing that the new risk calculator, which was based on older data from several large cohorts such as the Atherosclerosis Risk in Communities study,31 the Cardiovascular Health Study,32 the Coronary Artery Risk Development in Young Adults study,33 and the Framingham Heart Study,34,35 was inaccurate in other cohorts. Specifically, in more-recent cohorts (the Women’s Health Study,36 Physicians’ Health Study,37 and Women’s Health Initiative38), the new calculator overestimates the 10-year risk of ASCVD by 75% to 150%.30 Using the new calculator would make approximately 30 million more Americans eligible for statin treatment. The concern is that patients at lower risk would be treated and exposed to potential side effects of statin therapy.
In addition, the risk calculator relies heavily on age and sex and does not include other factors such as triglyceride level, family history, C-reactive protein, or lipoprotein(a). Importantly, and somewhat ironically given the otherwise absolute adherence to randomized controlled trial data for guideline development, the risk calculator has never been verified in prospective studies to adequately show that using it reduces ASCVD events.
Case 3: Overtreating a primary prevention patient
Based on the risk calculator, essentially any African American man in his early 60s with no other risk factors has a 10-year risk of ASCVD of 7.5% or higher and, according to the new guidelines, should receive at least moderate-intensity statin therapy.
For example, consider a 64-year-old African American man whose systolic blood pressure is 129 mm Hg, who does not smoke, does not have diabetes, and does not have hypertension, and whose total cholesterol level is 180 mg/dL, HDL-C 70 mg/dL, triglycerides 130 mg/dL, and calculated LDL-C 84 mg/dL. His calculated 10-year risk is, surprisingly, 7.5%.
Alternatively, his twin brother is a two-pack-per-day smoker with untreated hypertension and systolic blood pressure 150 mm Hg, with fasting total cholesterol 153 mg/dL, HDL-C 70 mg/dL, triglycerides 60 mg/dL, and LDL-C 71 mg/dL. His calculated 10-year risk is 10.5%, so according to the new guidelines, he too should receive high-intensity statin therapy. Yet this patient clearly needs better blood pressure control and smoking cessation as his primary risk-reduction efforts, not a statin. While assessing global risk is important, a shortcoming of the new guidelines is that they can inappropriately lead to treating the risk score, not individualizing the treatment to the patient. Because of the errors inherent in the risk calculator, some experts have called for a temporary halt on implementing the new guidelines until the risk calculator can be further validated. In November 2013, the American Heart Association and the American College of Cardiology reaffirmed their support of the new guidelines and recommended that they be implemented as planned. As of the time this manuscript goes to print, there are no plans to halt implementation of the new guidelines.
Case 4: Undertreating a primary prevention patient
A 25-year-old white man with no medical history has a total cholesterol level of 310 mg/dL, HDL-C 50 mg/dL, triglycerides 400 mg/dL, and calculated LDL-C 180 mg/dL. He does not smoke or have hypertension or diabetes but has a strong family history of premature coronary disease (his father died of myocardial infarction at age 42). His body mass index is 25 kg/m2. Because he is less than 40 years old, the risk calculator does not apply to him.
If we assume he remains untreated and returns at age 40 with the same clinical factors and laboratory values, his calculated 10-year risk of an ASCVD event according to the new risk calculator will still be only 3.1%. Assuming his medical history remains unchanged as he continues to age, his 10-year risk would not reach 7.5% until he is 58. Would you feel comfortable waiting 33 years before starting statin therapy in this patient?
Waiting for dyslipidemic patients to reach middle age before starting LDL-C-lowering therapy is a failure of prevention. For practical reasons, there are no data from randomized controlled trials with hard outcomes in younger people. Nevertheless, a tenet of preventive cardiology is that cumulative exposure accelerates the “vascular age” ahead of the chronological age. This case illustrates why individualized recommendations guided by LDL-C goals as a target for therapy are needed. For this 25-year-old patient, we would recommend starting an intermediate- or high-potency statin.
Case 5: Rheumatoid arthritis
A 60-year-old postmenopausal white woman with severe rheumatoid arthritis presents for cholesterol evaluation. Her total cholesterol level is 235 mg/dL, HDL-C 50 mg/dL, and LDL-C 165 mg/dL. She does not smoke or have hypertension or diabetes. Her systolic blood pressure is 110 mm Hg. She has elevated C-reactive protein on an ultrasensitive assay and elevated lipoprotein(a).
Her calculated 10-year risk of ASCVD is 3.0%. Assuming her medical history remains the same, she would not reach a calculated 10-year risk of at least 7.5% until age 70. We suggest starting moderate- or high-dose statin therapy in this case, based on data (not from randomized controlled trials) showing an increased risk of ASCVD events in patients with rheumatologic disease, increased lipoprotein(a), and inflammatory markers like C-reactive protein. However, the current guidelines do not address this scenario, other than to suggest that clinician consideration can be given to other risk markers such as these, and that these findings should be discussed in detail with the patient. The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin trial16 showed a dramatic ASCVD risk reduction in just such patients (Figure 1).
APPLAUSE—AND RESERVATIONS
The newest guidelines for treating high blood cholesterol represent a monumental shift away from using target levels of LDL-C and non-HDL-C and toward a focus on statin intensity for patients in the four highest-risk groups.
We applaud the expert panel for its idealistic approach of using only data from randomized controlled trials, for placing more emphasis on higher-intensity statin treatment, for including stroke in the new definition of ASCVD, and for focusing more attention on treating diabetic patients more aggressively. Simplifying the guidelines is a noble goal. Emphasizing moderate-to-high-intensity statin therapy in patients at moderate-to-high risk should have substantial long-term public health benefits.
However, as we have shown in the case examples, there are significant limitations, and some patients can end up being overtreated, while others may be undertreated.
Guidelines need to be crafted by looking at all the evidence, including the pathophysiology of the disease process, not just data from randomized controlled trials. It is difficult to implement a guideline that on one hand used randomized controlled trials exclusively for recommendations, but on the other hand used an untested risk calculator to guide therapy. Randomized controlled trials are not available for every scenario.
Further, absence of randomized controlled trial data in a given scenario should not be interpreted as evidence of lack of benefit. An example of this is a primary-prevention patient under age 40 with elevated LDL-C below the 190 mg/dL cutoff who otherwise is healthy and without risk factors (eg, Case 4). By disregarding all evidence that is not from randomized controlled trials, the expert panel fails to account for the extensive pathophysiology of ASCVD, which often begins at a young age and takes decades to develop.5,6,39 An entire generation of patients who have not reached the age of inclusion in most randomized controlled trials with hard outcomes is excluded (unless the LDL-C level is very high), potentially setting back decades of progress in the field of prevention. Prevention only works if started. With childhood and young adult obesity sharply rising, we should not fail to address the under-40-year-old patient population in our guidelines.
Guidelines are designed to be expert opinion, not to dictate practice. Focusing on the individual patient instead of the general population at risk, the expert panel appropriately emphasizes the “importance of clinician judgment, weighing potential benefits, adverse effects, drug-drug interactions and patient preferences.” However, by excluding all data that do not come from randomized controlled trials, the panel neglects a very large base of knowledge and leaves many clinicians without as much expert opinion as we had hoped for.
LDL-C goals are important: they provide a scorecard to help the patient with lifestyle and dietary changes. They provide the health care provider guidance in making treatment decisions and focusing on treatment of a single patient, not a population. Moreover, if a patient has difficulty taking standard doses of statins because of side effects, the absence of LDL-C goals makes decision-making nearly impossible. We hope physicians will rely on LDL-C goals in such situations, falling back on the ATP III recommendations, although many patients may simply go untreated until they present with ASCVD or until they “age in” to a higher risk category.
We suggest caution in strict adherence to the new guidelines and instead urge physicians to consider a hybrid of the old guidelines (using the ATP III LDL-C goals) and the new ones (emphasizing global risk assessment and high-intensity statin treatment).
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- American Heart Association. 2013 Prevention guidelines tools. CV risk calculator. http://my.americanheart.org/professional/StatementsGuidelines/PreventionGuidelines/Prevention-Guidelines_UCM_457698_SubHomePage.jsp. Accessed December 10, 2013.
- Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29:431–438.
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009; 50(suppl):S172–S177.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- de Lemos JA, Blazing MA, Wiviott SD, et al; for the A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- Downs JR, Clearfield M, Weis S, et al; for the AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. Results of AFCAPS/TexCAPS. JAMA 1998; 279:1615–1622.
- Koren MJ, Hunninghake DB, on behalf of the ALLIANCE investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics. J Am Coll Cardiol 2004; 44:1772–1779.
- Sever PS, Dahlof B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; on behalf of the CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Sacks FM, Pfeffer MA, Moye LA, et al; for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335:1001–1009.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Pedersen TR, Faegeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Ridker PM, Danielson E, Fonseca FAH, et al; for the JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Nakamura H, Arakawa K, Itakura H, et al; for the MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin Japan (MEGA Study): a prospective rabndomised controlled trial. Lancet 2006; 368:1155–1163.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggreessive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia: report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med 1990; 323:946–955.
- Cannon CP, Braunwald E, McCabe CH, et al; for the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Shepherd J, Cobbe SM, Ford I, et al; for the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995; 333:1301–1308.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1989; 8:1245–1255.
- AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-Thrive Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract 2013; 19:536–557.
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013doi: 10.1016/S0140-6736(13)62388-0. [Epub ahead of print]
- The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989; 129:687–702.
- Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1:263–276.
- Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988; 41:1105–1116.
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham study. Ann N Y Acad Sci 1963; 107:539–556.
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979; 110:281–290.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belancer C, Buring JE, Cook N, et al; The Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321:129–135.
- Langer R, White E, Lewis C, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003; 13:S107–S121.
- Strong JP, Malcom GT, Oalmann MC, Wissler RW. The PDAY study: natural history, risk factors, and pathobiology. Ann N Y Acad Sci 1997; 811:226–235.
On November 12, 2013, a joint task force for the American College of Cardiology and American Heart Association released new guidelines for treating high blood cholesterol to reduce the risk of atherosclerotic cardiovascular disease (ASCVD) in adults.1
This document arrives after several years of intense deliberation, 12 years after the third Adult Treatment Panel (ATP III) guidelines,2 and 8 years after an ATP III update recommending that low-density lipoprotein cholesterol (LDL-C) levels be lowered aggressively (to less than 70 mg/dL) as an option in patients at high risk.3 It represents a major shift in the approach to and management of high blood cholesterol and has sparked considerable controversy.
In the following commentary, we summarize the new guidelines and the philosophy employed by the task force in generating them. We will also examine some advantages and what we believe to be several shortcomings of the new guidelines. These latter points are illustrated through case examples.
IN RANDOMIZED CONTROLLED TRIALS WE TRUST
In collaboration with the National Heart, Lung, and Blood Institute of the National Institutes of Health, the American College of Cardiology and American Heart Association formed an expert panel task force in 2008.
The task force elected to use only evidence from randomized controlled trials, systematic reviews, and meta-analyses of randomized controlled trials (and only predefined outcomes of the trials, not post hoc analyses) in formulating its recommendations, with the goal of providing the strongest possible evidence.
The authors state that “By using [randomized controlled trial] data to identify those most likely to benefit [emphasis in original] from cholesterol-lowering statin therapy, the recommendations will be of value to primary care clinicians as well as specialists concerned with ASCVD prevention. Importantly, the recommendations were designed to be easy to use in the clinical setting, facilitating the implementation of a strategy of risk assessment and treatment focused on the prevention of ASCVD.”3 They also state the guidelines are meant to “inform clinical judgment, not replace it” and that clinician judgment in addition to discussion with patients remains vital.
During the deliberations, the National Heart, Lung, and Blood Institute removed itself from participating, stating its mission no longer included drafting new guidelines. Additionally, other initial members of the task force removed themselves because of disagreement and concerns about the direction of the new guidelines.
These guidelines, and their accompanying new cardiovascular risk calculator,4 were released without a preliminary period to allow for open discussion, comment, and critique by physicians outside the panel. No attempt was made to harmonize the guidelines with previous versions (eg, ATP III) or with current international guidelines.
WHAT’S NEW IN THE GUIDELINES?
The following are the major changes in the new guidelines for treating high blood cholesterol:
- Treatment goals for LDL-C and non-high-density lipoprotein cholesterol (non-HDL-C) are no longer recommended.
- High-intensity and moderate-intensity statin treatment is emphasized, and low-intensity statin therapy is nearly eliminated.
- “ASCVD” now includes stroke in addition to coronary heart disease and peripheral arterial disease.
- Four groups are targeted for treatment (see below).
- Nonstatin therapies have been markedly de-emphasized.
- No guidelines are provided for treating high triglyceride levels.
The new guidelines emphasize lifestyle modification as the foundation for reducing risk, regardless of cholesterol therapy. No recommendations are given for patients with New York Heart Association class II, III, or IV heart failure or for hemodialysis patients, because there were insufficient data from randomized controlled trials to support recommendations. Similarly, the guidelines apply only to people between the ages of 40 and 75 (risk calculator ages 40–79), because the authors believed there was not enough evidence from randomized controlled trials to allow development of guidelines outside of this age range.
FOUR MAJOR STATIN TREATMENT GROUPS
The new guidelines specify four groups that merit intensive or moderately intensive statin therapy (Table 1)1:
- People with clinical ASCVD
- People with LDL-C levels of 190 mg/dL or higher
- People with diabetes, age 40 to 75
- People without diabetes, age 40 to 75, with LDL-C levels 70–189 mg/dL, and a 10-year ASCVD risk of 7.5% or higher as determined by the new risk calculator4 (which also calculates the lifetime risk of ASCVD).
Below, we will address each of these four groups and provide case scenarios to consider. In general, our major disagreements with the new recommendations pertain to the first and fourth categories.
GROUP 1: PEOPLE WITH CLINICAL ASCVD
Advantages of the new guidelines
- They appropriately recommend statins in the highest tolerated doses as first-line treatment for this group at high risk.
- They designate all patients with ASCVD, including those with coronary, peripheral, and cerebrovascular disease, as a high-risk group.
- Without target LDL-C levels, treatment is simpler than before, requiring less monitoring of lipid levels. (This can also be seen as a limitation, as we discuss below.)
Limitations of the new guidelines
- They make follow-up LDL-C levels irrelevant, seeming to assume that there is no gradation in residual risk and, thus, no need to tailor therapy to the individual.
- Patients no longer have a goal to strive for or a way to monitor their progress.
- The guidelines ignore the pathophysiology of coronary artery disease and evidence of residual risk in patients on both moderate-intensity and high-intensity statin therapy.
- They also ignore the potential benefits of treating to lower LDL-C or non-HDL-C goals, thus eliminating consideration of multidrug therapy. They do not address patients with recurrent cardiovascular events already on maximal tolerated statin doses.
- They undermine the potential development and use of new therapies for dysplipidemia in patients with ASCVD.
Case 1: Is LDL-C 110 mg/dL low enough?
A 52-year-old African American man presents with newly discovered moderate coronary artery disease that is not severe enough to warrant stenting. He has no history of hypertension, diabetes mellitus, or smoking. His systolic blood pressure is 130 mm Hg, and his body mass index is 26 kg/m2. He exercises regularly and follows a low-cholesterol diet. He has the following fasting lipid values:
- Total cholesterol 290 mg/dL
- HDL-C 50 mg/dL
- Triglycerides 250 mg/dL
- Calculated LDL-C 190 mg/dL.
Two months later, after beginning atorvastatin 80 mg daily, meeting with a nutritionist, and redoubling his dietary efforts, his fasting lipid concentrations are:
- Total cholesterol 180 mg/dL
- HDL-C 55 mg/dL
- Triglycerides 75 mg/dL
- Calculated LDL-C 110 mg/dL.
Comment: Lack of LDL-C goals is a flaw
The new guidelines call for patients with known ASCVD, such as this patient, to receive intensive statin therapy—which he got.
However, once a patient is on therapy, the new guidelines do not encourage repeating the lipid panel other than to assess compliance. With intensive therapy, we expect a reduction in LDL-C of at least 50% (Table 1), but patient-to-patient differences in response to medications are common, and without repeat testing we would have no way of gauging this patient’s residual risk.
Further, the new guidelines emphasize the lack of hard outcome data supporting the addition of another lipid-lowering drug to a statin, although they do indicate that one can consider it. In a patient at high risk, such as this one, would you be comfortable with an LDL-C value of 110 mg/dL on maximum statin therapy? Would you consider adding a nonstatin drug?
A preponderance of data shows that LDL plays a causal role in ASCVD development and adverse events. Genetic data show that the LDL particle and the LDL receptor pathway are mechanistically linked to ASCVD pathogenesis, with lifetime exposure as a critical determinant of risk.5,6 Moreover, randomized controlled trials of statins and other studies of cholesterol-lowering show a reproducible relationship between the LDL-C level achieved and absolute risk (Figure 1).7–24 We believe the totality of data constitutes a strong rationale for targeting LDL-C and establishing goals for lowering its levels. For these reasons, we believe that removing LDL-C goals is a fundamental flaw of the new guidelines.
The reason for the lack of data from randomized controlled trials demonstrating benefits of adding therapies to statins (when LDL-C is still high) or benefits of treating to specific goals is that no such trials have been performed. Even trials of nonpharmacologic means of lowering LDL-C, such as ileal bypass, which was used in the Program on the Surgical Control of the Hyperlipidemias trial,20 provide independent evidence that lowering LDL-C reduces the risk of ASCVD (Figure 1).
In addition, trials of nonstatin drugs, such as the Coronary Drug Project,25 which tested niacin, also showed outcome benefits. On the other hand, studies such as the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health26 and Treatment of HDL to Reduce the Incidence of Vascular Events27 trials did not show additional risk reduction when niacin was added to statin therapy. However, the study designs arguably had flaws, including requirement of aggressive LDL-lowering with statins, with LDL-C levels below 70 to 80 mg/dL before randomization.
Therefore, these trials do not tell us what to do for a patient on maximal intensive therapy who has recurrent ASCVD events or who, like our patient, has an LDL-C level higher than previous targets.
For this patient, we would recommend adding a second medication to further lower his LDL-C, but discussing with him the absence of proven benefit in clinical trials and the risks of side effects. At present, lacking LDL-C goals in the new guidelines, we are keeping with the ATP III goals to help guide therapeutic choices and individualize patient management.
GROUP 2: PEOPLE WITH LDL-C ≥ 190
Advantages of the new guidelines
- They state that these patients should receive statins in the highest tolerated doses, which is universally accepted.
Limitations of the new guidelines
- The new guidelines mention only that one “may consider” adding a second agent if LDL-C remains above 190 mg/dL after maximum-dose therapy. Patients with familial hypercholesterolemia or other severe forms of hypercholesterolemia typically end up on multidrug therapy to further reduce LDL-C. The absence of randomized controlled trial data in this setting to show an additive value of second and third lipid-lowering agents does not mean these agents do not provide benefit.
GROUP 3: DIABETES, AGE 40–75, LDL-C 70–189, NO CLINICAL ASCVD
Advantages of the new guidelines
- They call for aggressive treatment of people with diabetes, a group at high risk that derives significant benefit from statin therapy, as shown in randomized controlled trials.
Limitations of the new guidelines
- Although high-intensity statin therapy is indicated for this group, we believe that, using the new risk calculator, some patients may receive overly aggressive treatment, thus increasing the possibility of statin side effects.
- The guidelines do not address patients younger than 40 or older than 75.
- Diabetic patients have a high residual risk of ASCVD events, even on statin therapy. Yet the guidelines ignore the potential benefits of more aggressive LDL-lowering or non-LDL secondary targets for therapy.
Case 2: How low is too low?
A 63-year-old white woman, a nonsmoker with recently diagnosed diabetes, is seen by her primary care physician. She has hypertension, for which she takes lisinopril 5 mg daily. Her fasting lipid values are:
- Total cholesterol 160 mg/dL
- HDL-C 64 mg/dL
- Triglycerides 100 mg/dL
- Calculated LDL-C 76 mg/dL.
Her systolic blood pressure is 129 mm Hg, and based on the new risk calculator, her 10-year risk of cardiovascular disease is 10.2%. According to the new guidelines, she should be started on high-intensity statin treatment (Table 1).
Although this is an acceptable initial course of action, it necessitates close vigilance, since it may actually drive her LDL-C level too low. Randomized controlled trials have typically used an LDL-C concentration of less than or equal to 25 mg/dL as the safety cutoff. With a typical LDL-C reduction of at least 50% on high-intensity statins, our patient’s expected LDL-C level will likely be in the low 30s. We believe this would be a good outcome, provided that she tolerates the medication without adverse effects. However, responses to statins vary from patient to patient.
High-intensity statin therapy may not be necessary to reduce risk adequately in all patients who have diabetes without preexisting vascular disease. The Collaborative Atorvastatin Diabetes Study12 compared atorvastatin 10 mg vs placebo in people with type 2 diabetes, age 40 to 75, who had one or more cardiovascular risk factors but no signs or symptoms of preexisting ASCVD and who had only average or below-average cholesterol levels—precisely like this patient. The trial was terminated early because of a clear benefit (a 37% reduction in the composite end point of major adverse cardiovascular events) in the intervention group. For our patient, we believe an alternative and acceptable approach would be to begin moderate-intensity statin therapy (eg, with atorvastatin 10 mg) (Table 1).
Alternatively, in a patient with diabetes and previous atherosclerotic vascular disease or with a high 10-year risk and high LDL-C, limiting treatment to high-intensity statin therapy by itself may deny them the potential benefits of combination therapies and targeting to lower LDL-C levels or non-HDL-C secondary targets. Guidelines from the American Diabetes Association28 and the American Association of Clinical Endocrinologists29 continue to recommend an LDL-C goal of less than 70 mg/dL in patients at high risk, a non-HDL-C less than 100 mg/dL, an apolipoprotein B less than 80 mg/dL, and an LDL particle number less than 1,000 nmol/L.
GROUP 4: AGE 40–75, LDL-C 70–189, NO ASCVD, BUT 10-YEAR RISK ≥ 7.5%
Advantages of the new guidelines
- They may reduce ASCVD events for patients at higher risk.
- The risk calculator is easy to use and focuses on global risk, ie, all forms of ASCVD.
- The guidelines promote discussion of risks and benefits between patients and providers.
Limitations of the new guidelines
- The new risk calculator is controversial (see below).
- There is potential for overtreatment, particularly in older patients.
- There is potential for undertreatment, particularly in patients with an elevated LDL-C but whose 10-year risk is less than 7.5% because they are young.
- The guidelines do not address patients younger than 40 or older than 75.
- They do not take into account some traditional risk factors, such as family history, and nontraditional risk factors such as C-reactive protein as measured by ultrasensitive assays, lipoprotein(a), and apolipoprotein B.
Risk calculator controversy
The new risk calculator has aroused strong opinions on both sides of the aisle.
Shortly after the new guidelines were released, cardiologists Dr. Paul Ridker and Dr. Nancy Cook from Brigham and Women’s Hospital in Boston published analyses30 showing that the new risk calculator, which was based on older data from several large cohorts such as the Atherosclerosis Risk in Communities study,31 the Cardiovascular Health Study,32 the Coronary Artery Risk Development in Young Adults study,33 and the Framingham Heart Study,34,35 was inaccurate in other cohorts. Specifically, in more-recent cohorts (the Women’s Health Study,36 Physicians’ Health Study,37 and Women’s Health Initiative38), the new calculator overestimates the 10-year risk of ASCVD by 75% to 150%.30 Using the new calculator would make approximately 30 million more Americans eligible for statin treatment. The concern is that patients at lower risk would be treated and exposed to potential side effects of statin therapy.
In addition, the risk calculator relies heavily on age and sex and does not include other factors such as triglyceride level, family history, C-reactive protein, or lipoprotein(a). Importantly, and somewhat ironically given the otherwise absolute adherence to randomized controlled trial data for guideline development, the risk calculator has never been verified in prospective studies to adequately show that using it reduces ASCVD events.
Case 3: Overtreating a primary prevention patient
Based on the risk calculator, essentially any African American man in his early 60s with no other risk factors has a 10-year risk of ASCVD of 7.5% or higher and, according to the new guidelines, should receive at least moderate-intensity statin therapy.
For example, consider a 64-year-old African American man whose systolic blood pressure is 129 mm Hg, who does not smoke, does not have diabetes, and does not have hypertension, and whose total cholesterol level is 180 mg/dL, HDL-C 70 mg/dL, triglycerides 130 mg/dL, and calculated LDL-C 84 mg/dL. His calculated 10-year risk is, surprisingly, 7.5%.
Alternatively, his twin brother is a two-pack-per-day smoker with untreated hypertension and systolic blood pressure 150 mm Hg, with fasting total cholesterol 153 mg/dL, HDL-C 70 mg/dL, triglycerides 60 mg/dL, and LDL-C 71 mg/dL. His calculated 10-year risk is 10.5%, so according to the new guidelines, he too should receive high-intensity statin therapy. Yet this patient clearly needs better blood pressure control and smoking cessation as his primary risk-reduction efforts, not a statin. While assessing global risk is important, a shortcoming of the new guidelines is that they can inappropriately lead to treating the risk score, not individualizing the treatment to the patient. Because of the errors inherent in the risk calculator, some experts have called for a temporary halt on implementing the new guidelines until the risk calculator can be further validated. In November 2013, the American Heart Association and the American College of Cardiology reaffirmed their support of the new guidelines and recommended that they be implemented as planned. As of the time this manuscript goes to print, there are no plans to halt implementation of the new guidelines.
Case 4: Undertreating a primary prevention patient
A 25-year-old white man with no medical history has a total cholesterol level of 310 mg/dL, HDL-C 50 mg/dL, triglycerides 400 mg/dL, and calculated LDL-C 180 mg/dL. He does not smoke or have hypertension or diabetes but has a strong family history of premature coronary disease (his father died of myocardial infarction at age 42). His body mass index is 25 kg/m2. Because he is less than 40 years old, the risk calculator does not apply to him.
If we assume he remains untreated and returns at age 40 with the same clinical factors and laboratory values, his calculated 10-year risk of an ASCVD event according to the new risk calculator will still be only 3.1%. Assuming his medical history remains unchanged as he continues to age, his 10-year risk would not reach 7.5% until he is 58. Would you feel comfortable waiting 33 years before starting statin therapy in this patient?
Waiting for dyslipidemic patients to reach middle age before starting LDL-C-lowering therapy is a failure of prevention. For practical reasons, there are no data from randomized controlled trials with hard outcomes in younger people. Nevertheless, a tenet of preventive cardiology is that cumulative exposure accelerates the “vascular age” ahead of the chronological age. This case illustrates why individualized recommendations guided by LDL-C goals as a target for therapy are needed. For this 25-year-old patient, we would recommend starting an intermediate- or high-potency statin.
Case 5: Rheumatoid arthritis
A 60-year-old postmenopausal white woman with severe rheumatoid arthritis presents for cholesterol evaluation. Her total cholesterol level is 235 mg/dL, HDL-C 50 mg/dL, and LDL-C 165 mg/dL. She does not smoke or have hypertension or diabetes. Her systolic blood pressure is 110 mm Hg. She has elevated C-reactive protein on an ultrasensitive assay and elevated lipoprotein(a).
Her calculated 10-year risk of ASCVD is 3.0%. Assuming her medical history remains the same, she would not reach a calculated 10-year risk of at least 7.5% until age 70. We suggest starting moderate- or high-dose statin therapy in this case, based on data (not from randomized controlled trials) showing an increased risk of ASCVD events in patients with rheumatologic disease, increased lipoprotein(a), and inflammatory markers like C-reactive protein. However, the current guidelines do not address this scenario, other than to suggest that clinician consideration can be given to other risk markers such as these, and that these findings should be discussed in detail with the patient. The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin trial16 showed a dramatic ASCVD risk reduction in just such patients (Figure 1).
APPLAUSE—AND RESERVATIONS
The newest guidelines for treating high blood cholesterol represent a monumental shift away from using target levels of LDL-C and non-HDL-C and toward a focus on statin intensity for patients in the four highest-risk groups.
We applaud the expert panel for its idealistic approach of using only data from randomized controlled trials, for placing more emphasis on higher-intensity statin treatment, for including stroke in the new definition of ASCVD, and for focusing more attention on treating diabetic patients more aggressively. Simplifying the guidelines is a noble goal. Emphasizing moderate-to-high-intensity statin therapy in patients at moderate-to-high risk should have substantial long-term public health benefits.
However, as we have shown in the case examples, there are significant limitations, and some patients can end up being overtreated, while others may be undertreated.
Guidelines need to be crafted by looking at all the evidence, including the pathophysiology of the disease process, not just data from randomized controlled trials. It is difficult to implement a guideline that on one hand used randomized controlled trials exclusively for recommendations, but on the other hand used an untested risk calculator to guide therapy. Randomized controlled trials are not available for every scenario.
Further, absence of randomized controlled trial data in a given scenario should not be interpreted as evidence of lack of benefit. An example of this is a primary-prevention patient under age 40 with elevated LDL-C below the 190 mg/dL cutoff who otherwise is healthy and without risk factors (eg, Case 4). By disregarding all evidence that is not from randomized controlled trials, the expert panel fails to account for the extensive pathophysiology of ASCVD, which often begins at a young age and takes decades to develop.5,6,39 An entire generation of patients who have not reached the age of inclusion in most randomized controlled trials with hard outcomes is excluded (unless the LDL-C level is very high), potentially setting back decades of progress in the field of prevention. Prevention only works if started. With childhood and young adult obesity sharply rising, we should not fail to address the under-40-year-old patient population in our guidelines.
Guidelines are designed to be expert opinion, not to dictate practice. Focusing on the individual patient instead of the general population at risk, the expert panel appropriately emphasizes the “importance of clinician judgment, weighing potential benefits, adverse effects, drug-drug interactions and patient preferences.” However, by excluding all data that do not come from randomized controlled trials, the panel neglects a very large base of knowledge and leaves many clinicians without as much expert opinion as we had hoped for.
LDL-C goals are important: they provide a scorecard to help the patient with lifestyle and dietary changes. They provide the health care provider guidance in making treatment decisions and focusing on treatment of a single patient, not a population. Moreover, if a patient has difficulty taking standard doses of statins because of side effects, the absence of LDL-C goals makes decision-making nearly impossible. We hope physicians will rely on LDL-C goals in such situations, falling back on the ATP III recommendations, although many patients may simply go untreated until they present with ASCVD or until they “age in” to a higher risk category.
We suggest caution in strict adherence to the new guidelines and instead urge physicians to consider a hybrid of the old guidelines (using the ATP III LDL-C goals) and the new ones (emphasizing global risk assessment and high-intensity statin treatment).
On November 12, 2013, a joint task force for the American College of Cardiology and American Heart Association released new guidelines for treating high blood cholesterol to reduce the risk of atherosclerotic cardiovascular disease (ASCVD) in adults.1
This document arrives after several years of intense deliberation, 12 years after the third Adult Treatment Panel (ATP III) guidelines,2 and 8 years after an ATP III update recommending that low-density lipoprotein cholesterol (LDL-C) levels be lowered aggressively (to less than 70 mg/dL) as an option in patients at high risk.3 It represents a major shift in the approach to and management of high blood cholesterol and has sparked considerable controversy.
In the following commentary, we summarize the new guidelines and the philosophy employed by the task force in generating them. We will also examine some advantages and what we believe to be several shortcomings of the new guidelines. These latter points are illustrated through case examples.
IN RANDOMIZED CONTROLLED TRIALS WE TRUST
In collaboration with the National Heart, Lung, and Blood Institute of the National Institutes of Health, the American College of Cardiology and American Heart Association formed an expert panel task force in 2008.
The task force elected to use only evidence from randomized controlled trials, systematic reviews, and meta-analyses of randomized controlled trials (and only predefined outcomes of the trials, not post hoc analyses) in formulating its recommendations, with the goal of providing the strongest possible evidence.
The authors state that “By using [randomized controlled trial] data to identify those most likely to benefit [emphasis in original] from cholesterol-lowering statin therapy, the recommendations will be of value to primary care clinicians as well as specialists concerned with ASCVD prevention. Importantly, the recommendations were designed to be easy to use in the clinical setting, facilitating the implementation of a strategy of risk assessment and treatment focused on the prevention of ASCVD.”3 They also state the guidelines are meant to “inform clinical judgment, not replace it” and that clinician judgment in addition to discussion with patients remains vital.
During the deliberations, the National Heart, Lung, and Blood Institute removed itself from participating, stating its mission no longer included drafting new guidelines. Additionally, other initial members of the task force removed themselves because of disagreement and concerns about the direction of the new guidelines.
These guidelines, and their accompanying new cardiovascular risk calculator,4 were released without a preliminary period to allow for open discussion, comment, and critique by physicians outside the panel. No attempt was made to harmonize the guidelines with previous versions (eg, ATP III) or with current international guidelines.
WHAT’S NEW IN THE GUIDELINES?
The following are the major changes in the new guidelines for treating high blood cholesterol:
- Treatment goals for LDL-C and non-high-density lipoprotein cholesterol (non-HDL-C) are no longer recommended.
- High-intensity and moderate-intensity statin treatment is emphasized, and low-intensity statin therapy is nearly eliminated.
- “ASCVD” now includes stroke in addition to coronary heart disease and peripheral arterial disease.
- Four groups are targeted for treatment (see below).
- Nonstatin therapies have been markedly de-emphasized.
- No guidelines are provided for treating high triglyceride levels.
The new guidelines emphasize lifestyle modification as the foundation for reducing risk, regardless of cholesterol therapy. No recommendations are given for patients with New York Heart Association class II, III, or IV heart failure or for hemodialysis patients, because there were insufficient data from randomized controlled trials to support recommendations. Similarly, the guidelines apply only to people between the ages of 40 and 75 (risk calculator ages 40–79), because the authors believed there was not enough evidence from randomized controlled trials to allow development of guidelines outside of this age range.
FOUR MAJOR STATIN TREATMENT GROUPS
The new guidelines specify four groups that merit intensive or moderately intensive statin therapy (Table 1)1:
- People with clinical ASCVD
- People with LDL-C levels of 190 mg/dL or higher
- People with diabetes, age 40 to 75
- People without diabetes, age 40 to 75, with LDL-C levels 70–189 mg/dL, and a 10-year ASCVD risk of 7.5% or higher as determined by the new risk calculator4 (which also calculates the lifetime risk of ASCVD).
Below, we will address each of these four groups and provide case scenarios to consider. In general, our major disagreements with the new recommendations pertain to the first and fourth categories.
GROUP 1: PEOPLE WITH CLINICAL ASCVD
Advantages of the new guidelines
- They appropriately recommend statins in the highest tolerated doses as first-line treatment for this group at high risk.
- They designate all patients with ASCVD, including those with coronary, peripheral, and cerebrovascular disease, as a high-risk group.
- Without target LDL-C levels, treatment is simpler than before, requiring less monitoring of lipid levels. (This can also be seen as a limitation, as we discuss below.)
Limitations of the new guidelines
- They make follow-up LDL-C levels irrelevant, seeming to assume that there is no gradation in residual risk and, thus, no need to tailor therapy to the individual.
- Patients no longer have a goal to strive for or a way to monitor their progress.
- The guidelines ignore the pathophysiology of coronary artery disease and evidence of residual risk in patients on both moderate-intensity and high-intensity statin therapy.
- They also ignore the potential benefits of treating to lower LDL-C or non-HDL-C goals, thus eliminating consideration of multidrug therapy. They do not address patients with recurrent cardiovascular events already on maximal tolerated statin doses.
- They undermine the potential development and use of new therapies for dysplipidemia in patients with ASCVD.
Case 1: Is LDL-C 110 mg/dL low enough?
A 52-year-old African American man presents with newly discovered moderate coronary artery disease that is not severe enough to warrant stenting. He has no history of hypertension, diabetes mellitus, or smoking. His systolic blood pressure is 130 mm Hg, and his body mass index is 26 kg/m2. He exercises regularly and follows a low-cholesterol diet. He has the following fasting lipid values:
- Total cholesterol 290 mg/dL
- HDL-C 50 mg/dL
- Triglycerides 250 mg/dL
- Calculated LDL-C 190 mg/dL.
Two months later, after beginning atorvastatin 80 mg daily, meeting with a nutritionist, and redoubling his dietary efforts, his fasting lipid concentrations are:
- Total cholesterol 180 mg/dL
- HDL-C 55 mg/dL
- Triglycerides 75 mg/dL
- Calculated LDL-C 110 mg/dL.
Comment: Lack of LDL-C goals is a flaw
The new guidelines call for patients with known ASCVD, such as this patient, to receive intensive statin therapy—which he got.
However, once a patient is on therapy, the new guidelines do not encourage repeating the lipid panel other than to assess compliance. With intensive therapy, we expect a reduction in LDL-C of at least 50% (Table 1), but patient-to-patient differences in response to medications are common, and without repeat testing we would have no way of gauging this patient’s residual risk.
Further, the new guidelines emphasize the lack of hard outcome data supporting the addition of another lipid-lowering drug to a statin, although they do indicate that one can consider it. In a patient at high risk, such as this one, would you be comfortable with an LDL-C value of 110 mg/dL on maximum statin therapy? Would you consider adding a nonstatin drug?
A preponderance of data shows that LDL plays a causal role in ASCVD development and adverse events. Genetic data show that the LDL particle and the LDL receptor pathway are mechanistically linked to ASCVD pathogenesis, with lifetime exposure as a critical determinant of risk.5,6 Moreover, randomized controlled trials of statins and other studies of cholesterol-lowering show a reproducible relationship between the LDL-C level achieved and absolute risk (Figure 1).7–24 We believe the totality of data constitutes a strong rationale for targeting LDL-C and establishing goals for lowering its levels. For these reasons, we believe that removing LDL-C goals is a fundamental flaw of the new guidelines.
The reason for the lack of data from randomized controlled trials demonstrating benefits of adding therapies to statins (when LDL-C is still high) or benefits of treating to specific goals is that no such trials have been performed. Even trials of nonpharmacologic means of lowering LDL-C, such as ileal bypass, which was used in the Program on the Surgical Control of the Hyperlipidemias trial,20 provide independent evidence that lowering LDL-C reduces the risk of ASCVD (Figure 1).
In addition, trials of nonstatin drugs, such as the Coronary Drug Project,25 which tested niacin, also showed outcome benefits. On the other hand, studies such as the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health26 and Treatment of HDL to Reduce the Incidence of Vascular Events27 trials did not show additional risk reduction when niacin was added to statin therapy. However, the study designs arguably had flaws, including requirement of aggressive LDL-lowering with statins, with LDL-C levels below 70 to 80 mg/dL before randomization.
Therefore, these trials do not tell us what to do for a patient on maximal intensive therapy who has recurrent ASCVD events or who, like our patient, has an LDL-C level higher than previous targets.
For this patient, we would recommend adding a second medication to further lower his LDL-C, but discussing with him the absence of proven benefit in clinical trials and the risks of side effects. At present, lacking LDL-C goals in the new guidelines, we are keeping with the ATP III goals to help guide therapeutic choices and individualize patient management.
GROUP 2: PEOPLE WITH LDL-C ≥ 190
Advantages of the new guidelines
- They state that these patients should receive statins in the highest tolerated doses, which is universally accepted.
Limitations of the new guidelines
- The new guidelines mention only that one “may consider” adding a second agent if LDL-C remains above 190 mg/dL after maximum-dose therapy. Patients with familial hypercholesterolemia or other severe forms of hypercholesterolemia typically end up on multidrug therapy to further reduce LDL-C. The absence of randomized controlled trial data in this setting to show an additive value of second and third lipid-lowering agents does not mean these agents do not provide benefit.
GROUP 3: DIABETES, AGE 40–75, LDL-C 70–189, NO CLINICAL ASCVD
Advantages of the new guidelines
- They call for aggressive treatment of people with diabetes, a group at high risk that derives significant benefit from statin therapy, as shown in randomized controlled trials.
Limitations of the new guidelines
- Although high-intensity statin therapy is indicated for this group, we believe that, using the new risk calculator, some patients may receive overly aggressive treatment, thus increasing the possibility of statin side effects.
- The guidelines do not address patients younger than 40 or older than 75.
- Diabetic patients have a high residual risk of ASCVD events, even on statin therapy. Yet the guidelines ignore the potential benefits of more aggressive LDL-lowering or non-LDL secondary targets for therapy.
Case 2: How low is too low?
A 63-year-old white woman, a nonsmoker with recently diagnosed diabetes, is seen by her primary care physician. She has hypertension, for which she takes lisinopril 5 mg daily. Her fasting lipid values are:
- Total cholesterol 160 mg/dL
- HDL-C 64 mg/dL
- Triglycerides 100 mg/dL
- Calculated LDL-C 76 mg/dL.
Her systolic blood pressure is 129 mm Hg, and based on the new risk calculator, her 10-year risk of cardiovascular disease is 10.2%. According to the new guidelines, she should be started on high-intensity statin treatment (Table 1).
Although this is an acceptable initial course of action, it necessitates close vigilance, since it may actually drive her LDL-C level too low. Randomized controlled trials have typically used an LDL-C concentration of less than or equal to 25 mg/dL as the safety cutoff. With a typical LDL-C reduction of at least 50% on high-intensity statins, our patient’s expected LDL-C level will likely be in the low 30s. We believe this would be a good outcome, provided that she tolerates the medication without adverse effects. However, responses to statins vary from patient to patient.
High-intensity statin therapy may not be necessary to reduce risk adequately in all patients who have diabetes without preexisting vascular disease. The Collaborative Atorvastatin Diabetes Study12 compared atorvastatin 10 mg vs placebo in people with type 2 diabetes, age 40 to 75, who had one or more cardiovascular risk factors but no signs or symptoms of preexisting ASCVD and who had only average or below-average cholesterol levels—precisely like this patient. The trial was terminated early because of a clear benefit (a 37% reduction in the composite end point of major adverse cardiovascular events) in the intervention group. For our patient, we believe an alternative and acceptable approach would be to begin moderate-intensity statin therapy (eg, with atorvastatin 10 mg) (Table 1).
Alternatively, in a patient with diabetes and previous atherosclerotic vascular disease or with a high 10-year risk and high LDL-C, limiting treatment to high-intensity statin therapy by itself may deny them the potential benefits of combination therapies and targeting to lower LDL-C levels or non-HDL-C secondary targets. Guidelines from the American Diabetes Association28 and the American Association of Clinical Endocrinologists29 continue to recommend an LDL-C goal of less than 70 mg/dL in patients at high risk, a non-HDL-C less than 100 mg/dL, an apolipoprotein B less than 80 mg/dL, and an LDL particle number less than 1,000 nmol/L.
GROUP 4: AGE 40–75, LDL-C 70–189, NO ASCVD, BUT 10-YEAR RISK ≥ 7.5%
Advantages of the new guidelines
- They may reduce ASCVD events for patients at higher risk.
- The risk calculator is easy to use and focuses on global risk, ie, all forms of ASCVD.
- The guidelines promote discussion of risks and benefits between patients and providers.
Limitations of the new guidelines
- The new risk calculator is controversial (see below).
- There is potential for overtreatment, particularly in older patients.
- There is potential for undertreatment, particularly in patients with an elevated LDL-C but whose 10-year risk is less than 7.5% because they are young.
- The guidelines do not address patients younger than 40 or older than 75.
- They do not take into account some traditional risk factors, such as family history, and nontraditional risk factors such as C-reactive protein as measured by ultrasensitive assays, lipoprotein(a), and apolipoprotein B.
Risk calculator controversy
The new risk calculator has aroused strong opinions on both sides of the aisle.
Shortly after the new guidelines were released, cardiologists Dr. Paul Ridker and Dr. Nancy Cook from Brigham and Women’s Hospital in Boston published analyses30 showing that the new risk calculator, which was based on older data from several large cohorts such as the Atherosclerosis Risk in Communities study,31 the Cardiovascular Health Study,32 the Coronary Artery Risk Development in Young Adults study,33 and the Framingham Heart Study,34,35 was inaccurate in other cohorts. Specifically, in more-recent cohorts (the Women’s Health Study,36 Physicians’ Health Study,37 and Women’s Health Initiative38), the new calculator overestimates the 10-year risk of ASCVD by 75% to 150%.30 Using the new calculator would make approximately 30 million more Americans eligible for statin treatment. The concern is that patients at lower risk would be treated and exposed to potential side effects of statin therapy.
In addition, the risk calculator relies heavily on age and sex and does not include other factors such as triglyceride level, family history, C-reactive protein, or lipoprotein(a). Importantly, and somewhat ironically given the otherwise absolute adherence to randomized controlled trial data for guideline development, the risk calculator has never been verified in prospective studies to adequately show that using it reduces ASCVD events.
Case 3: Overtreating a primary prevention patient
Based on the risk calculator, essentially any African American man in his early 60s with no other risk factors has a 10-year risk of ASCVD of 7.5% or higher and, according to the new guidelines, should receive at least moderate-intensity statin therapy.
For example, consider a 64-year-old African American man whose systolic blood pressure is 129 mm Hg, who does not smoke, does not have diabetes, and does not have hypertension, and whose total cholesterol level is 180 mg/dL, HDL-C 70 mg/dL, triglycerides 130 mg/dL, and calculated LDL-C 84 mg/dL. His calculated 10-year risk is, surprisingly, 7.5%.
Alternatively, his twin brother is a two-pack-per-day smoker with untreated hypertension and systolic blood pressure 150 mm Hg, with fasting total cholesterol 153 mg/dL, HDL-C 70 mg/dL, triglycerides 60 mg/dL, and LDL-C 71 mg/dL. His calculated 10-year risk is 10.5%, so according to the new guidelines, he too should receive high-intensity statin therapy. Yet this patient clearly needs better blood pressure control and smoking cessation as his primary risk-reduction efforts, not a statin. While assessing global risk is important, a shortcoming of the new guidelines is that they can inappropriately lead to treating the risk score, not individualizing the treatment to the patient. Because of the errors inherent in the risk calculator, some experts have called for a temporary halt on implementing the new guidelines until the risk calculator can be further validated. In November 2013, the American Heart Association and the American College of Cardiology reaffirmed their support of the new guidelines and recommended that they be implemented as planned. As of the time this manuscript goes to print, there are no plans to halt implementation of the new guidelines.
Case 4: Undertreating a primary prevention patient
A 25-year-old white man with no medical history has a total cholesterol level of 310 mg/dL, HDL-C 50 mg/dL, triglycerides 400 mg/dL, and calculated LDL-C 180 mg/dL. He does not smoke or have hypertension or diabetes but has a strong family history of premature coronary disease (his father died of myocardial infarction at age 42). His body mass index is 25 kg/m2. Because he is less than 40 years old, the risk calculator does not apply to him.
If we assume he remains untreated and returns at age 40 with the same clinical factors and laboratory values, his calculated 10-year risk of an ASCVD event according to the new risk calculator will still be only 3.1%. Assuming his medical history remains unchanged as he continues to age, his 10-year risk would not reach 7.5% until he is 58. Would you feel comfortable waiting 33 years before starting statin therapy in this patient?
Waiting for dyslipidemic patients to reach middle age before starting LDL-C-lowering therapy is a failure of prevention. For practical reasons, there are no data from randomized controlled trials with hard outcomes in younger people. Nevertheless, a tenet of preventive cardiology is that cumulative exposure accelerates the “vascular age” ahead of the chronological age. This case illustrates why individualized recommendations guided by LDL-C goals as a target for therapy are needed. For this 25-year-old patient, we would recommend starting an intermediate- or high-potency statin.
Case 5: Rheumatoid arthritis
A 60-year-old postmenopausal white woman with severe rheumatoid arthritis presents for cholesterol evaluation. Her total cholesterol level is 235 mg/dL, HDL-C 50 mg/dL, and LDL-C 165 mg/dL. She does not smoke or have hypertension or diabetes. Her systolic blood pressure is 110 mm Hg. She has elevated C-reactive protein on an ultrasensitive assay and elevated lipoprotein(a).
Her calculated 10-year risk of ASCVD is 3.0%. Assuming her medical history remains the same, she would not reach a calculated 10-year risk of at least 7.5% until age 70. We suggest starting moderate- or high-dose statin therapy in this case, based on data (not from randomized controlled trials) showing an increased risk of ASCVD events in patients with rheumatologic disease, increased lipoprotein(a), and inflammatory markers like C-reactive protein. However, the current guidelines do not address this scenario, other than to suggest that clinician consideration can be given to other risk markers such as these, and that these findings should be discussed in detail with the patient. The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin trial16 showed a dramatic ASCVD risk reduction in just such patients (Figure 1).
APPLAUSE—AND RESERVATIONS
The newest guidelines for treating high blood cholesterol represent a monumental shift away from using target levels of LDL-C and non-HDL-C and toward a focus on statin intensity for patients in the four highest-risk groups.
We applaud the expert panel for its idealistic approach of using only data from randomized controlled trials, for placing more emphasis on higher-intensity statin treatment, for including stroke in the new definition of ASCVD, and for focusing more attention on treating diabetic patients more aggressively. Simplifying the guidelines is a noble goal. Emphasizing moderate-to-high-intensity statin therapy in patients at moderate-to-high risk should have substantial long-term public health benefits.
However, as we have shown in the case examples, there are significant limitations, and some patients can end up being overtreated, while others may be undertreated.
Guidelines need to be crafted by looking at all the evidence, including the pathophysiology of the disease process, not just data from randomized controlled trials. It is difficult to implement a guideline that on one hand used randomized controlled trials exclusively for recommendations, but on the other hand used an untested risk calculator to guide therapy. Randomized controlled trials are not available for every scenario.
Further, absence of randomized controlled trial data in a given scenario should not be interpreted as evidence of lack of benefit. An example of this is a primary-prevention patient under age 40 with elevated LDL-C below the 190 mg/dL cutoff who otherwise is healthy and without risk factors (eg, Case 4). By disregarding all evidence that is not from randomized controlled trials, the expert panel fails to account for the extensive pathophysiology of ASCVD, which often begins at a young age and takes decades to develop.5,6,39 An entire generation of patients who have not reached the age of inclusion in most randomized controlled trials with hard outcomes is excluded (unless the LDL-C level is very high), potentially setting back decades of progress in the field of prevention. Prevention only works if started. With childhood and young adult obesity sharply rising, we should not fail to address the under-40-year-old patient population in our guidelines.
Guidelines are designed to be expert opinion, not to dictate practice. Focusing on the individual patient instead of the general population at risk, the expert panel appropriately emphasizes the “importance of clinician judgment, weighing potential benefits, adverse effects, drug-drug interactions and patient preferences.” However, by excluding all data that do not come from randomized controlled trials, the panel neglects a very large base of knowledge and leaves many clinicians without as much expert opinion as we had hoped for.
LDL-C goals are important: they provide a scorecard to help the patient with lifestyle and dietary changes. They provide the health care provider guidance in making treatment decisions and focusing on treatment of a single patient, not a population. Moreover, if a patient has difficulty taking standard doses of statins because of side effects, the absence of LDL-C goals makes decision-making nearly impossible. We hope physicians will rely on LDL-C goals in such situations, falling back on the ATP III recommendations, although many patients may simply go untreated until they present with ASCVD or until they “age in” to a higher risk category.
We suggest caution in strict adherence to the new guidelines and instead urge physicians to consider a hybrid of the old guidelines (using the ATP III LDL-C goals) and the new ones (emphasizing global risk assessment and high-intensity statin treatment).
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- American Heart Association. 2013 Prevention guidelines tools. CV risk calculator. http://my.americanheart.org/professional/StatementsGuidelines/PreventionGuidelines/Prevention-Guidelines_UCM_457698_SubHomePage.jsp. Accessed December 10, 2013.
- Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29:431–438.
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009; 50(suppl):S172–S177.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- de Lemos JA, Blazing MA, Wiviott SD, et al; for the A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- Downs JR, Clearfield M, Weis S, et al; for the AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. Results of AFCAPS/TexCAPS. JAMA 1998; 279:1615–1622.
- Koren MJ, Hunninghake DB, on behalf of the ALLIANCE investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics. J Am Coll Cardiol 2004; 44:1772–1779.
- Sever PS, Dahlof B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; on behalf of the CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Sacks FM, Pfeffer MA, Moye LA, et al; for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335:1001–1009.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Pedersen TR, Faegeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Ridker PM, Danielson E, Fonseca FAH, et al; for the JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Nakamura H, Arakawa K, Itakura H, et al; for the MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin Japan (MEGA Study): a prospective rabndomised controlled trial. Lancet 2006; 368:1155–1163.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggreessive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia: report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med 1990; 323:946–955.
- Cannon CP, Braunwald E, McCabe CH, et al; for the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Shepherd J, Cobbe SM, Ford I, et al; for the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995; 333:1301–1308.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1989; 8:1245–1255.
- AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-Thrive Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract 2013; 19:536–557.
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013doi: 10.1016/S0140-6736(13)62388-0. [Epub ahead of print]
- The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989; 129:687–702.
- Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1:263–276.
- Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988; 41:1105–1116.
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham study. Ann N Y Acad Sci 1963; 107:539–556.
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979; 110:281–290.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belancer C, Buring JE, Cook N, et al; The Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321:129–135.
- Langer R, White E, Lewis C, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003; 13:S107–S121.
- Strong JP, Malcom GT, Oalmann MC, Wissler RW. The PDAY study: natural history, risk factors, and pathobiology. Ann N Y Acad Sci 1997; 811:226–235.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- American Heart Association. 2013 Prevention guidelines tools. CV risk calculator. http://my.americanheart.org/professional/StatementsGuidelines/PreventionGuidelines/Prevention-Guidelines_UCM_457698_SubHomePage.jsp. Accessed December 10, 2013.
- Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29:431–438.
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009; 50(suppl):S172–S177.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- de Lemos JA, Blazing MA, Wiviott SD, et al; for the A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- Downs JR, Clearfield M, Weis S, et al; for the AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. Results of AFCAPS/TexCAPS. JAMA 1998; 279:1615–1622.
- Koren MJ, Hunninghake DB, on behalf of the ALLIANCE investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics. J Am Coll Cardiol 2004; 44:1772–1779.
- Sever PS, Dahlof B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; on behalf of the CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Sacks FM, Pfeffer MA, Moye LA, et al; for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335:1001–1009.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Pedersen TR, Faegeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Ridker PM, Danielson E, Fonseca FAH, et al; for the JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Nakamura H, Arakawa K, Itakura H, et al; for the MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin Japan (MEGA Study): a prospective rabndomised controlled trial. Lancet 2006; 368:1155–1163.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggreessive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia: report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med 1990; 323:946–955.
- Cannon CP, Braunwald E, McCabe CH, et al; for the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Shepherd J, Cobbe SM, Ford I, et al; for the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995; 333:1301–1308.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1989; 8:1245–1255.
- AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-Thrive Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract 2013; 19:536–557.
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013doi: 10.1016/S0140-6736(13)62388-0. [Epub ahead of print]
- The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989; 129:687–702.
- Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1:263–276.
- Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988; 41:1105–1116.
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham study. Ann N Y Acad Sci 1963; 107:539–556.
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979; 110:281–290.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belancer C, Buring JE, Cook N, et al; The Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321:129–135.
- Langer R, White E, Lewis C, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003; 13:S107–S121.
- Strong JP, Malcom GT, Oalmann MC, Wissler RW. The PDAY study: natural history, risk factors, and pathobiology. Ann N Y Acad Sci 1997; 811:226–235.
Dual antiplatelet therapy in coronary artery disease: A case-based approach
Plaque rupture and thrombosis play central roles in the genesis of acute coronary syndrome. Aspirin has long been the preventive agent of choice. But dual antiplatelet therapy with aspirin plus clopidogrel (Plavix) is warranted in many patients to further reduce their risk of future cardiovascular events.
Although dual antiplatelet therapy is usually started by a subspecialist, the primary care physician is often the one who ensures that the patient remains compliant with it in the long term. A review of the seminal published data is helpful in understanding the rationale behind dual antiplatelet therapy and its risks and benefits.
In the mid-1990s, the thienopyridine ticlopidine (Ticlid) was found to significantly decrease the number of deaths, target-lesion revascularizations, and myocardial infarctions (MIs) in the 30 days following stent placement. 1 However, 2% to 3% of patients experienced neutropenia2 and thrombotic thrombocytopenic purpura with this drug,3 leading to the use of clopidogrel, another agent in the same class. Over the past decade, a large body of evidence has established the usefulness of clopidogrel in a number of clinical settings.
In this paper we review the current use of clopidogrel in ST-elevation MI, non-ST-elevation acute coronary syndromes, and percutaneous coronary intervention, and discuss the landmark trials that are the basis for the treatment guidelines published jointly by the American College of Cardiology (ACC) and the American Heart Association (AHA).4–6 We also briefly discuss the use of prasugrel (Effient), the newest antiplatelet agent to gain approval from the US Food and Drug Administration (FDA).
CLOPIDOGREL AS AN ALTERNATIVE TO ASPIRIN
Clopidogrel, a prodrug, is converted into its active form in the liver.7 It then irreversibly binds to the platelet P2Y12 receptor and inhibits adenosine diphosphate-induced platelet aggregation.
Those treated with clopidogrel had an annual risk of ischemic stroke, MI, or vascular death of 5.32%, compared with 5.83% in the aspirin group, for a statistically significant 8.7% relative risk reduction (P = .043). The observed frequency of neutropenia (neutrophils < 1.2 × 109/L) was 0.10% with clopidogrel vs 0.17% with aspirin. This study showed clopidogrel to be an effective alternative in patients who cannot tolerate aspirin.
CASE 1: ST-ELEVATION MI
A 57-year-old farmer in rural Ohio with a history of hypertension and hyperlipidemia presents to the local emergency department 45 minutes after the onset, while he was chopping wood, of dull, aching, substernal chest pain that radiates to his jaw. Electrocardiography reveals 2-mm ST-segment elevation in leads V1 through V6. He is treated with aspirin 162 mg, low-molecular-weight heparin, and tenecteplase.
What would be the value of starting dual antiplatelet therapy with clopidogrel in this patient?
Clopidogrel, aspirin, and fibrinolysis in ST-elevation MI
The CLARITY-TIMI 28 trial (Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis in Myocardial Infarction)9 included 3,491 patients (ages 18 to 75) from 319 international sites. All patients received a fibrinolytic agent, aspirin (162 mg to 325 mg on the first day and 75 mg to 162 mg thereafter), and heparin as part of standard care for acute ST-elevation MI (Table 1). Patients were randomized to receive a 300-mg loading dose of clopidogrel followed by 75 mg daily or placebo within 12 hours of onset of ST-elevation MI. The status of the infarct-related artery was ascertained by protocol-mandated coronary angiography 48 to 192 hours after starting the study medication. The primary end point was the composite of an occluded infarct-related artery on angiography, death from any cause prior to angiography, or recurrent MI prior to angiography.
Significantly fewer patients had an end point event in the clopidogrel group than in the placebo group, 15% vs 21.7% (P < .001), for a relative risk reduction of 31%. There was no significant increase in major or minor bleeding events.
Of note, the CLARITY-TIMI 28 patients were relatively young (average age 57 years) and at low cardiovascular risk (30-day mortality risk < 5%).
The COMMIT trial (Clopidogrel and Metoprolol in Myocardial Infarction)10 consisted of 45,852 patients with suspected acute MI admitted to 1,250 hospitals in China. Each patient received aspirin 162 mg daily plus either clopidogrel 75 mg daily (n = 22,961) or placebo (n = 22,891) for the duration of hospitalization (average 16 days) or 28 days, whichever came first.
The incidence of the primary composite end point of death, reinfarction, or stroke was significantly lower with clopidogrel than with placebo (9.2% vs 10.1%, P = .002). This was regardless of age (the average age was 61, and 26% of patients were older than 70), sex, time to presentation (67% presented within 12 hours), or reperfusion strategy (49% underwent fibrinolysis). The clopidogrel group did not have a significantly higher incidence of bleeding, but patients in this trial did not receive a loading dose of clopidogrel.
Comment. In view of the results of these trials, our 57-year-old patient should start clopidogrel early.
CASE 2: NON-ST-ELEVATION ACUTE CORONARY SYNDROME
A 65-year-old woman living independently with no significant medical history presents to the emergency room with 2 hours of waxing and waning substernal chest pain. Her blood pressure is 145/90 mm Hg, her heart rate is 95 beats per minute, and the results of her physical examination are unremarkable. Resting electrocardiography reveals 1.5-mm ST-segment depression in the inferior leads, and her troponin T level on admission is two times the upper limit of normal. She is given aspirin and is started on low-molecular-weight heparin and intravenous nitroglycerin.
What would be the value of starting clopidogrel in this patient?
Clopidogrel in non-ST-elevation acute coronary syndromes
The ACC/AHA guidelines strongly support starting clopidogrel in patients with non-ST-elevation acute coronary syndromes (Table 2).5
The CURE trial (Clopidogrel in Unstable Angina to Prevent Recurrent Events)11 provided the evidence for this recommendation. In this trial, 12,562 patients from 482 centers in 28 countries who presented within 24 hours of coronary symptoms, without ST elevation, were randomized to receive either clopidogrel (a 300-mg loading dose, followed by 75 mg daily) or placebo for 3 to 12 months (mean 9 months).
Significantly fewer patients in the clopidogrel group reached one of the end points of the composite primary outcome (cardiovascular death, nonfatal MI, or stroke): 9.3% vs 11.4%, 95% confidence interval (CI) 0.72–0.90, P < .001. Significantly fewer of them also suffered one of the secondary outcomes, ie, severe ischemia, heart failure, or need for revascularization.
Of concern was a higher rate of major bleeding in the clopidogrel group (3.7%) than in the placebo group (2.7%) without an excess of fatal bleeding. For every 1,000 patients treated with clopidogrel, 6 required a blood transfusion. Nevertheless, CURE proved that patients with non-ST-elevation acute coronary syndromes benefited from clopidogrel, regardless of whether they underwent percutaneous coronary intervention.
Comment. Our patient should receive clopidogrel and, if she has no significant bleeding, she should continue to take it for at least 12 months after discharge. It is important for the primary care physician to ensure compliance with this agent and not discontinue it on routine clinical follow-up.
CASE 3: BARE-METAL STENT PLACEMENT
A 62-year-old man with a history of hypertension, diabetes, and hyperlipidemia presents to his primary care physician’s office with stable-effort angina that is not responding to an excellent anti-ischemic regimen and is affecting his quality of life. He is referred for coronary angiography, which reveals 80% stenosis of the proximal left circumflex artery. He undergoes a percutaneous coronary intervention with placement of a bare-metal stent.
How long should he be on clopidogrel? And what if a drug-eluting stent had been placed instead of a bare-metal stent?
Dual therapy after bare-metal stent placement
Dual antiplatelet therapy with clopidogrel and aspirin is recommended in all patients receiving a stent (Table 2). The better safety and efficacy of clopidogrel compared with ticlopidine has been established in patients receiving a coronary artery stent,12,13 and clopidogrel’s favorable safety profile soon made it the thienopyridine of choice.
The CREDO trial (Clopidogrel for the Reduction of Events During Observation)14 randomized 2,116 patients undergoing an elective percutaneous coronary intervention (bare-metal stent placement only) to receive a 300-mg loading dose of clopidogrel 3 to 24 hours before the procedure, or placebo. All patients received 325 mg of aspirin. After the intervention, all patients received clopidogrel 75 mg daily and aspirin 325 mg daily through day 28. For day 29 through 12 months, those who had received the 300-mg preprocedural loading dose of clopidogrel continued with 75 mg daily, and those who had not received clopidogrel before the procedure received placebo.
No significant difference was seen in the primary outcome for those who received pretreatment with clopidogrel; however, in a subgroup analysis, those who received clopidogrel at least 6 hours before the percutaneous coronary intervention had a 38.6% relative risk reduction (Table 1). Long-term use of clopidogrel (ie, for 12 months) was associated with an overall relative reduction of 26.9% in the combined risk of death, MI, or stroke.
PCI-CURE, an analysis of 2,658 patients in the CURE trial with non-ST-elevation acute coronary syndrome who underwent PCI,15 yielded results similar to those of CREDO, with a 31% reduction in the rate of cardiovascular death or MI at 30 days and at 9 months. Of note, however, clopidogrel was given for a median of 6 days prior to the procedure.
Comment. The minimum suggested duration of clopidogrel treatment after placement of a bare-metal stent is 1 month. However, these trial results indicate that patients who are not at high risk of bleeding should take clopidogrel for at least 12 months.
Dual antiplatelet therapy with drug-eluting stents
Although rates of in-stent restenosis are clearly lower with drug-eluting stents than with bare-metal stents, the antiproliferative effect of drug-eluting stents may delay complete endothelialization of every strut. This may contribute to late (> 1 month after placement) or very late (> 1 year) thrombosis of the stent after clopidogrel is discontinued.16–18
In 2006, the FDA indicated that dual antiplatelet therapy was needed for 6 months with paclitaxel-eluting (Taxus) stents and 3 months with sirolimus-eluting (Cipher) stents. As reports of very late stent thrombosis began to appear in 2007, concern arose over the need to extend the duration of clopidogrel treatment.
Bavry et al19 quantified the incidence of late and very late stent thrombosis in a meta-analysis of 14 clinical trials that randomized patients to receive either a drug-eluting stent (paclitaxel or sirolimus) or a bare-metal stent.19 The incidence of stent thrombosis within 30 days in this analysis was similar for both groups—4.4 per 1,000 patients vs 5 per 1,000 (relative risk 0.89; 95% CI 0.46–1.75; P = .74). However, the rate of very late stent thrombosis was significantly higher in those receiving a drug-eluting stent vs a bare-metal stent—5 per 1,000 patients treated (relative risk 5.02, 95% CI 1.29–19.52; P = .02).
The results of this and other studies led the ACC and AHA to revise their joint guidelines to recommend thienopyridine treatment for at least 1 year for patients who receive a drug-eluting stent.6,20–22 In fact, many cardiologists consider indefinite dual antiplatelet therapy in patients with a drug-eluting stent to avoid very late in-stent thrombosis, especially in patients undergoing high-risk interventions such as placement of multiple stents, bifurcation lesions, and unprotected left main trunk interventions.
Thus, when faced with a patient with a recent coronary stent implantation, the primary care physician should be aware of the type of stent and the duration of therapy recommended by the interventional cardiologist. Also, in the absence of a pressing indication, elective surgery should be deferred for 1 year after placement of a drug-eluting stent, as this would necessitate stopping clopidogrel and would increase the risk of perioperative stent thrombosis, which is associated with high rates of morbidity and death.
CASE 4: HIGH-RISK CORONARY ARTERY DISEASE
A 67-year-old woman presents to your office to establish care. She has a history of diabetes and established coronary artery disease with two bare-metal stents placed 2 years ago. She is taking aspirin 81 mg.
What would be the value of adding clopidogrel to her regimen?
No indication for clopidogrel in chronic coronary artery disease
The CHARISMA trial (Clopidogrel for High Atherothrombotic and Ischemic Stabilization, Management, and Avoidance)23 randomized 15,603 patients with stable cardiovascular disease or multiple risk factors to receive either clopidogrel plus low-dose aspirin or placebo plus low-dose aspirin and followed them for a median of 28 months (Table 1).
The primary end point (a composite of MI, stroke, or death) was 6.8% with clopidogrel plus aspirin and 7.3% with aspirin alone, indicating no significant benefit with clopidogrel plus aspirin compared with aspirin alone in reducing the rate of MI, stroke, or cardiovascular death in patients with high-risk but stable atherothrombotic disease. A marginal statistical benefit with dual antiplatelet therapy was noted in the subgroup of patients with previously documented coronary, cerebrovascular, or peripheral vascular disease—6.9% with aspirin plus clopidogrel vs 7.9% with aspirin alone (relative risk 0.88; 95% CI 0.77–0.998; P = .046).
Consequently, there is no compelling reason to start clopidogrel in this patient.
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel was recently approved by the FDA as antiplatelet treatment for patients with acute coronary syndromes planning to undergo a percutaneous coronary intervention.24 It has been shown to inhibit adenosine-diphosphate-induced platelet activation in a more consistent and effective manner than clopidogrel.25,26
Although both clopidogrel and prasugrel are prodrugs, 80% of absorbed clopidogrel is metabolized by esterases into inactive metabolites, and the availability of active metabolite can vary, as it is significantly influenced by polymorphisms in the cytochrome P450 system. 27 In contrast, prasugrel is not degraded by esterases, and its conversion to active metabolite by the cytochrome P450 system is not influenced by common genetic polymorphisms, particularly CYP2C19*2.
TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction) provided most of the evidence for the approval of prasugrel for clinical use.28,29 In this trial, a 60-mg loading dose of prasugrel followed by a daily maintenance dose of 10 mg was significantly superior to the current clopidogrel regimen in preventing death from cardiovascular causes, nonfatal MI, or nonfatal stroke during a study period of 15 months.28 Also observed was a 24% lower rate of MI, a 34% lower rate of urgent target-vessel revascularization, and a 52% lower rate of stent thrombosis.
These benefits, however, came at the cost of a significantly higher risk of major bleeding, including the potential for three excess fatal bleeding events for every 1,000 patients treated. Patients at highest risk at the dosages evaluated included the elderly (age 75 and older), patients who weigh less than 60 kg, and patients with a history of stroke or transient ischemic attack. Based on these results, we recommend caution with the use of prasugrel in these patient subsets.
Clinical use of prasugrel is likely to be highest in patients presenting with ST-elevation MI who are undergoing a primary percutaneous coronary intervention. There is currently no evidence from any randomized clinical trial to support the safety of prasugrel given in the emergency room or “upstream” in the setting of non-ST-elevation acute coronary syndromes.
Of note, patients with non-ST-elevation acute coronary syndromes in the TRITON trial were randomized only after angiographic definition. As a result, only 179 patients exposed to prasugrel were referred for coronary artery bypass surgery, but the rate of surgery-related major bleeding in this group was 13.4% (vs 3.2% in the clopidogrel group). Based on these data, prasugrel should be withheld for at least 1 week prior to any surgery.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Yeh SP, Hsueh EJ, Wu H, Wang YC. Ticlopidine-associated aplastic anemia. A case report and review of literature, Ann Hematol 1998; 76:87–90.
- Page Y, Tardy B, Zeni F, Comtet C, Terrana R, Bertrand JC. Thrombotic thrombocytopenic purpura related to ticlopidine. Lancet 1991; 337:774–776.
- Canadian Cardiovascular Society; Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007; 50:e1–e157.
- King SB, Smith SC, Hirshfeld JW, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:172–209.
- Kam PC, Nethery CM. The thienopyridine derivatives (platelet adenosine diphosphate receptor antagonists), pharmacology and clinical developments. Anaesthesia 2003; 58:28–35.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Sabatine MS, Cannon CP, Gibson M, et al; CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation, N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: a randomized placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Bhatt DL, Bertrand ME, Berger PB, et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J Am Coll Cardiol 2002; 39:9–14.
- Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH; CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS). Circulation 2000; 102:624–629.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Mehta SR, Yusuf S, Peters RJ, et a; Clopidogrel in Unstable Angina to Prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001; 358:527–533.
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007; 115:1051–1058.
- Kotani J, Awata M, Nanto S, et al. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol 2006; 47:2108–2111.
- Pfisterer M, Brunner-La Rocca HP, Buser PT, et al; BASKETLATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol 2006; 48:2584–2591.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med 2007; 356:1030–1039.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- US Food and Drug Administration. FDA Approves Effient to Reduce the Risk of Heart Attack in Angioplasty Patients. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm171497.htm. Accessed October 2, 2009.
- Jernber T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006; 27:1166–1173.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A TRITON-TIMI (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Card 2008; 51:2028–2033.
Plaque rupture and thrombosis play central roles in the genesis of acute coronary syndrome. Aspirin has long been the preventive agent of choice. But dual antiplatelet therapy with aspirin plus clopidogrel (Plavix) is warranted in many patients to further reduce their risk of future cardiovascular events.
Although dual antiplatelet therapy is usually started by a subspecialist, the primary care physician is often the one who ensures that the patient remains compliant with it in the long term. A review of the seminal published data is helpful in understanding the rationale behind dual antiplatelet therapy and its risks and benefits.
In the mid-1990s, the thienopyridine ticlopidine (Ticlid) was found to significantly decrease the number of deaths, target-lesion revascularizations, and myocardial infarctions (MIs) in the 30 days following stent placement. 1 However, 2% to 3% of patients experienced neutropenia2 and thrombotic thrombocytopenic purpura with this drug,3 leading to the use of clopidogrel, another agent in the same class. Over the past decade, a large body of evidence has established the usefulness of clopidogrel in a number of clinical settings.
In this paper we review the current use of clopidogrel in ST-elevation MI, non-ST-elevation acute coronary syndromes, and percutaneous coronary intervention, and discuss the landmark trials that are the basis for the treatment guidelines published jointly by the American College of Cardiology (ACC) and the American Heart Association (AHA).4–6 We also briefly discuss the use of prasugrel (Effient), the newest antiplatelet agent to gain approval from the US Food and Drug Administration (FDA).
CLOPIDOGREL AS AN ALTERNATIVE TO ASPIRIN
Clopidogrel, a prodrug, is converted into its active form in the liver.7 It then irreversibly binds to the platelet P2Y12 receptor and inhibits adenosine diphosphate-induced platelet aggregation.
Those treated with clopidogrel had an annual risk of ischemic stroke, MI, or vascular death of 5.32%, compared with 5.83% in the aspirin group, for a statistically significant 8.7% relative risk reduction (P = .043). The observed frequency of neutropenia (neutrophils < 1.2 × 109/L) was 0.10% with clopidogrel vs 0.17% with aspirin. This study showed clopidogrel to be an effective alternative in patients who cannot tolerate aspirin.
CASE 1: ST-ELEVATION MI
A 57-year-old farmer in rural Ohio with a history of hypertension and hyperlipidemia presents to the local emergency department 45 minutes after the onset, while he was chopping wood, of dull, aching, substernal chest pain that radiates to his jaw. Electrocardiography reveals 2-mm ST-segment elevation in leads V1 through V6. He is treated with aspirin 162 mg, low-molecular-weight heparin, and tenecteplase.
What would be the value of starting dual antiplatelet therapy with clopidogrel in this patient?
Clopidogrel, aspirin, and fibrinolysis in ST-elevation MI
The CLARITY-TIMI 28 trial (Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis in Myocardial Infarction)9 included 3,491 patients (ages 18 to 75) from 319 international sites. All patients received a fibrinolytic agent, aspirin (162 mg to 325 mg on the first day and 75 mg to 162 mg thereafter), and heparin as part of standard care for acute ST-elevation MI (Table 1). Patients were randomized to receive a 300-mg loading dose of clopidogrel followed by 75 mg daily or placebo within 12 hours of onset of ST-elevation MI. The status of the infarct-related artery was ascertained by protocol-mandated coronary angiography 48 to 192 hours after starting the study medication. The primary end point was the composite of an occluded infarct-related artery on angiography, death from any cause prior to angiography, or recurrent MI prior to angiography.
Significantly fewer patients had an end point event in the clopidogrel group than in the placebo group, 15% vs 21.7% (P < .001), for a relative risk reduction of 31%. There was no significant increase in major or minor bleeding events.
Of note, the CLARITY-TIMI 28 patients were relatively young (average age 57 years) and at low cardiovascular risk (30-day mortality risk < 5%).
The COMMIT trial (Clopidogrel and Metoprolol in Myocardial Infarction)10 consisted of 45,852 patients with suspected acute MI admitted to 1,250 hospitals in China. Each patient received aspirin 162 mg daily plus either clopidogrel 75 mg daily (n = 22,961) or placebo (n = 22,891) for the duration of hospitalization (average 16 days) or 28 days, whichever came first.
The incidence of the primary composite end point of death, reinfarction, or stroke was significantly lower with clopidogrel than with placebo (9.2% vs 10.1%, P = .002). This was regardless of age (the average age was 61, and 26% of patients were older than 70), sex, time to presentation (67% presented within 12 hours), or reperfusion strategy (49% underwent fibrinolysis). The clopidogrel group did not have a significantly higher incidence of bleeding, but patients in this trial did not receive a loading dose of clopidogrel.
Comment. In view of the results of these trials, our 57-year-old patient should start clopidogrel early.
CASE 2: NON-ST-ELEVATION ACUTE CORONARY SYNDROME
A 65-year-old woman living independently with no significant medical history presents to the emergency room with 2 hours of waxing and waning substernal chest pain. Her blood pressure is 145/90 mm Hg, her heart rate is 95 beats per minute, and the results of her physical examination are unremarkable. Resting electrocardiography reveals 1.5-mm ST-segment depression in the inferior leads, and her troponin T level on admission is two times the upper limit of normal. She is given aspirin and is started on low-molecular-weight heparin and intravenous nitroglycerin.
What would be the value of starting clopidogrel in this patient?
Clopidogrel in non-ST-elevation acute coronary syndromes
The ACC/AHA guidelines strongly support starting clopidogrel in patients with non-ST-elevation acute coronary syndromes (Table 2).5
The CURE trial (Clopidogrel in Unstable Angina to Prevent Recurrent Events)11 provided the evidence for this recommendation. In this trial, 12,562 patients from 482 centers in 28 countries who presented within 24 hours of coronary symptoms, without ST elevation, were randomized to receive either clopidogrel (a 300-mg loading dose, followed by 75 mg daily) or placebo for 3 to 12 months (mean 9 months).
Significantly fewer patients in the clopidogrel group reached one of the end points of the composite primary outcome (cardiovascular death, nonfatal MI, or stroke): 9.3% vs 11.4%, 95% confidence interval (CI) 0.72–0.90, P < .001. Significantly fewer of them also suffered one of the secondary outcomes, ie, severe ischemia, heart failure, or need for revascularization.
Of concern was a higher rate of major bleeding in the clopidogrel group (3.7%) than in the placebo group (2.7%) without an excess of fatal bleeding. For every 1,000 patients treated with clopidogrel, 6 required a blood transfusion. Nevertheless, CURE proved that patients with non-ST-elevation acute coronary syndromes benefited from clopidogrel, regardless of whether they underwent percutaneous coronary intervention.
Comment. Our patient should receive clopidogrel and, if she has no significant bleeding, she should continue to take it for at least 12 months after discharge. It is important for the primary care physician to ensure compliance with this agent and not discontinue it on routine clinical follow-up.
CASE 3: BARE-METAL STENT PLACEMENT
A 62-year-old man with a history of hypertension, diabetes, and hyperlipidemia presents to his primary care physician’s office with stable-effort angina that is not responding to an excellent anti-ischemic regimen and is affecting his quality of life. He is referred for coronary angiography, which reveals 80% stenosis of the proximal left circumflex artery. He undergoes a percutaneous coronary intervention with placement of a bare-metal stent.
How long should he be on clopidogrel? And what if a drug-eluting stent had been placed instead of a bare-metal stent?
Dual therapy after bare-metal stent placement
Dual antiplatelet therapy with clopidogrel and aspirin is recommended in all patients receiving a stent (Table 2). The better safety and efficacy of clopidogrel compared with ticlopidine has been established in patients receiving a coronary artery stent,12,13 and clopidogrel’s favorable safety profile soon made it the thienopyridine of choice.
The CREDO trial (Clopidogrel for the Reduction of Events During Observation)14 randomized 2,116 patients undergoing an elective percutaneous coronary intervention (bare-metal stent placement only) to receive a 300-mg loading dose of clopidogrel 3 to 24 hours before the procedure, or placebo. All patients received 325 mg of aspirin. After the intervention, all patients received clopidogrel 75 mg daily and aspirin 325 mg daily through day 28. For day 29 through 12 months, those who had received the 300-mg preprocedural loading dose of clopidogrel continued with 75 mg daily, and those who had not received clopidogrel before the procedure received placebo.
No significant difference was seen in the primary outcome for those who received pretreatment with clopidogrel; however, in a subgroup analysis, those who received clopidogrel at least 6 hours before the percutaneous coronary intervention had a 38.6% relative risk reduction (Table 1). Long-term use of clopidogrel (ie, for 12 months) was associated with an overall relative reduction of 26.9% in the combined risk of death, MI, or stroke.
PCI-CURE, an analysis of 2,658 patients in the CURE trial with non-ST-elevation acute coronary syndrome who underwent PCI,15 yielded results similar to those of CREDO, with a 31% reduction in the rate of cardiovascular death or MI at 30 days and at 9 months. Of note, however, clopidogrel was given for a median of 6 days prior to the procedure.
Comment. The minimum suggested duration of clopidogrel treatment after placement of a bare-metal stent is 1 month. However, these trial results indicate that patients who are not at high risk of bleeding should take clopidogrel for at least 12 months.
Dual antiplatelet therapy with drug-eluting stents
Although rates of in-stent restenosis are clearly lower with drug-eluting stents than with bare-metal stents, the antiproliferative effect of drug-eluting stents may delay complete endothelialization of every strut. This may contribute to late (> 1 month after placement) or very late (> 1 year) thrombosis of the stent after clopidogrel is discontinued.16–18
In 2006, the FDA indicated that dual antiplatelet therapy was needed for 6 months with paclitaxel-eluting (Taxus) stents and 3 months with sirolimus-eluting (Cipher) stents. As reports of very late stent thrombosis began to appear in 2007, concern arose over the need to extend the duration of clopidogrel treatment.
Bavry et al19 quantified the incidence of late and very late stent thrombosis in a meta-analysis of 14 clinical trials that randomized patients to receive either a drug-eluting stent (paclitaxel or sirolimus) or a bare-metal stent.19 The incidence of stent thrombosis within 30 days in this analysis was similar for both groups—4.4 per 1,000 patients vs 5 per 1,000 (relative risk 0.89; 95% CI 0.46–1.75; P = .74). However, the rate of very late stent thrombosis was significantly higher in those receiving a drug-eluting stent vs a bare-metal stent—5 per 1,000 patients treated (relative risk 5.02, 95% CI 1.29–19.52; P = .02).
The results of this and other studies led the ACC and AHA to revise their joint guidelines to recommend thienopyridine treatment for at least 1 year for patients who receive a drug-eluting stent.6,20–22 In fact, many cardiologists consider indefinite dual antiplatelet therapy in patients with a drug-eluting stent to avoid very late in-stent thrombosis, especially in patients undergoing high-risk interventions such as placement of multiple stents, bifurcation lesions, and unprotected left main trunk interventions.
Thus, when faced with a patient with a recent coronary stent implantation, the primary care physician should be aware of the type of stent and the duration of therapy recommended by the interventional cardiologist. Also, in the absence of a pressing indication, elective surgery should be deferred for 1 year after placement of a drug-eluting stent, as this would necessitate stopping clopidogrel and would increase the risk of perioperative stent thrombosis, which is associated with high rates of morbidity and death.
CASE 4: HIGH-RISK CORONARY ARTERY DISEASE
A 67-year-old woman presents to your office to establish care. She has a history of diabetes and established coronary artery disease with two bare-metal stents placed 2 years ago. She is taking aspirin 81 mg.
What would be the value of adding clopidogrel to her regimen?
No indication for clopidogrel in chronic coronary artery disease
The CHARISMA trial (Clopidogrel for High Atherothrombotic and Ischemic Stabilization, Management, and Avoidance)23 randomized 15,603 patients with stable cardiovascular disease or multiple risk factors to receive either clopidogrel plus low-dose aspirin or placebo plus low-dose aspirin and followed them for a median of 28 months (Table 1).
The primary end point (a composite of MI, stroke, or death) was 6.8% with clopidogrel plus aspirin and 7.3% with aspirin alone, indicating no significant benefit with clopidogrel plus aspirin compared with aspirin alone in reducing the rate of MI, stroke, or cardiovascular death in patients with high-risk but stable atherothrombotic disease. A marginal statistical benefit with dual antiplatelet therapy was noted in the subgroup of patients with previously documented coronary, cerebrovascular, or peripheral vascular disease—6.9% with aspirin plus clopidogrel vs 7.9% with aspirin alone (relative risk 0.88; 95% CI 0.77–0.998; P = .046).
Consequently, there is no compelling reason to start clopidogrel in this patient.
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel was recently approved by the FDA as antiplatelet treatment for patients with acute coronary syndromes planning to undergo a percutaneous coronary intervention.24 It has been shown to inhibit adenosine-diphosphate-induced platelet activation in a more consistent and effective manner than clopidogrel.25,26
Although both clopidogrel and prasugrel are prodrugs, 80% of absorbed clopidogrel is metabolized by esterases into inactive metabolites, and the availability of active metabolite can vary, as it is significantly influenced by polymorphisms in the cytochrome P450 system. 27 In contrast, prasugrel is not degraded by esterases, and its conversion to active metabolite by the cytochrome P450 system is not influenced by common genetic polymorphisms, particularly CYP2C19*2.
TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction) provided most of the evidence for the approval of prasugrel for clinical use.28,29 In this trial, a 60-mg loading dose of prasugrel followed by a daily maintenance dose of 10 mg was significantly superior to the current clopidogrel regimen in preventing death from cardiovascular causes, nonfatal MI, or nonfatal stroke during a study period of 15 months.28 Also observed was a 24% lower rate of MI, a 34% lower rate of urgent target-vessel revascularization, and a 52% lower rate of stent thrombosis.
These benefits, however, came at the cost of a significantly higher risk of major bleeding, including the potential for three excess fatal bleeding events for every 1,000 patients treated. Patients at highest risk at the dosages evaluated included the elderly (age 75 and older), patients who weigh less than 60 kg, and patients with a history of stroke or transient ischemic attack. Based on these results, we recommend caution with the use of prasugrel in these patient subsets.
Clinical use of prasugrel is likely to be highest in patients presenting with ST-elevation MI who are undergoing a primary percutaneous coronary intervention. There is currently no evidence from any randomized clinical trial to support the safety of prasugrel given in the emergency room or “upstream” in the setting of non-ST-elevation acute coronary syndromes.
Of note, patients with non-ST-elevation acute coronary syndromes in the TRITON trial were randomized only after angiographic definition. As a result, only 179 patients exposed to prasugrel were referred for coronary artery bypass surgery, but the rate of surgery-related major bleeding in this group was 13.4% (vs 3.2% in the clopidogrel group). Based on these data, prasugrel should be withheld for at least 1 week prior to any surgery.
Plaque rupture and thrombosis play central roles in the genesis of acute coronary syndrome. Aspirin has long been the preventive agent of choice. But dual antiplatelet therapy with aspirin plus clopidogrel (Plavix) is warranted in many patients to further reduce their risk of future cardiovascular events.
Although dual antiplatelet therapy is usually started by a subspecialist, the primary care physician is often the one who ensures that the patient remains compliant with it in the long term. A review of the seminal published data is helpful in understanding the rationale behind dual antiplatelet therapy and its risks and benefits.
In the mid-1990s, the thienopyridine ticlopidine (Ticlid) was found to significantly decrease the number of deaths, target-lesion revascularizations, and myocardial infarctions (MIs) in the 30 days following stent placement. 1 However, 2% to 3% of patients experienced neutropenia2 and thrombotic thrombocytopenic purpura with this drug,3 leading to the use of clopidogrel, another agent in the same class. Over the past decade, a large body of evidence has established the usefulness of clopidogrel in a number of clinical settings.
In this paper we review the current use of clopidogrel in ST-elevation MI, non-ST-elevation acute coronary syndromes, and percutaneous coronary intervention, and discuss the landmark trials that are the basis for the treatment guidelines published jointly by the American College of Cardiology (ACC) and the American Heart Association (AHA).4–6 We also briefly discuss the use of prasugrel (Effient), the newest antiplatelet agent to gain approval from the US Food and Drug Administration (FDA).
CLOPIDOGREL AS AN ALTERNATIVE TO ASPIRIN
Clopidogrel, a prodrug, is converted into its active form in the liver.7 It then irreversibly binds to the platelet P2Y12 receptor and inhibits adenosine diphosphate-induced platelet aggregation.
Those treated with clopidogrel had an annual risk of ischemic stroke, MI, or vascular death of 5.32%, compared with 5.83% in the aspirin group, for a statistically significant 8.7% relative risk reduction (P = .043). The observed frequency of neutropenia (neutrophils < 1.2 × 109/L) was 0.10% with clopidogrel vs 0.17% with aspirin. This study showed clopidogrel to be an effective alternative in patients who cannot tolerate aspirin.
CASE 1: ST-ELEVATION MI
A 57-year-old farmer in rural Ohio with a history of hypertension and hyperlipidemia presents to the local emergency department 45 minutes after the onset, while he was chopping wood, of dull, aching, substernal chest pain that radiates to his jaw. Electrocardiography reveals 2-mm ST-segment elevation in leads V1 through V6. He is treated with aspirin 162 mg, low-molecular-weight heparin, and tenecteplase.
What would be the value of starting dual antiplatelet therapy with clopidogrel in this patient?
Clopidogrel, aspirin, and fibrinolysis in ST-elevation MI
The CLARITY-TIMI 28 trial (Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis in Myocardial Infarction)9 included 3,491 patients (ages 18 to 75) from 319 international sites. All patients received a fibrinolytic agent, aspirin (162 mg to 325 mg on the first day and 75 mg to 162 mg thereafter), and heparin as part of standard care for acute ST-elevation MI (Table 1). Patients were randomized to receive a 300-mg loading dose of clopidogrel followed by 75 mg daily or placebo within 12 hours of onset of ST-elevation MI. The status of the infarct-related artery was ascertained by protocol-mandated coronary angiography 48 to 192 hours after starting the study medication. The primary end point was the composite of an occluded infarct-related artery on angiography, death from any cause prior to angiography, or recurrent MI prior to angiography.
Significantly fewer patients had an end point event in the clopidogrel group than in the placebo group, 15% vs 21.7% (P < .001), for a relative risk reduction of 31%. There was no significant increase in major or minor bleeding events.
Of note, the CLARITY-TIMI 28 patients were relatively young (average age 57 years) and at low cardiovascular risk (30-day mortality risk < 5%).
The COMMIT trial (Clopidogrel and Metoprolol in Myocardial Infarction)10 consisted of 45,852 patients with suspected acute MI admitted to 1,250 hospitals in China. Each patient received aspirin 162 mg daily plus either clopidogrel 75 mg daily (n = 22,961) or placebo (n = 22,891) for the duration of hospitalization (average 16 days) or 28 days, whichever came first.
The incidence of the primary composite end point of death, reinfarction, or stroke was significantly lower with clopidogrel than with placebo (9.2% vs 10.1%, P = .002). This was regardless of age (the average age was 61, and 26% of patients were older than 70), sex, time to presentation (67% presented within 12 hours), or reperfusion strategy (49% underwent fibrinolysis). The clopidogrel group did not have a significantly higher incidence of bleeding, but patients in this trial did not receive a loading dose of clopidogrel.
Comment. In view of the results of these trials, our 57-year-old patient should start clopidogrel early.
CASE 2: NON-ST-ELEVATION ACUTE CORONARY SYNDROME
A 65-year-old woman living independently with no significant medical history presents to the emergency room with 2 hours of waxing and waning substernal chest pain. Her blood pressure is 145/90 mm Hg, her heart rate is 95 beats per minute, and the results of her physical examination are unremarkable. Resting electrocardiography reveals 1.5-mm ST-segment depression in the inferior leads, and her troponin T level on admission is two times the upper limit of normal. She is given aspirin and is started on low-molecular-weight heparin and intravenous nitroglycerin.
What would be the value of starting clopidogrel in this patient?
Clopidogrel in non-ST-elevation acute coronary syndromes
The ACC/AHA guidelines strongly support starting clopidogrel in patients with non-ST-elevation acute coronary syndromes (Table 2).5
The CURE trial (Clopidogrel in Unstable Angina to Prevent Recurrent Events)11 provided the evidence for this recommendation. In this trial, 12,562 patients from 482 centers in 28 countries who presented within 24 hours of coronary symptoms, without ST elevation, were randomized to receive either clopidogrel (a 300-mg loading dose, followed by 75 mg daily) or placebo for 3 to 12 months (mean 9 months).
Significantly fewer patients in the clopidogrel group reached one of the end points of the composite primary outcome (cardiovascular death, nonfatal MI, or stroke): 9.3% vs 11.4%, 95% confidence interval (CI) 0.72–0.90, P < .001. Significantly fewer of them also suffered one of the secondary outcomes, ie, severe ischemia, heart failure, or need for revascularization.
Of concern was a higher rate of major bleeding in the clopidogrel group (3.7%) than in the placebo group (2.7%) without an excess of fatal bleeding. For every 1,000 patients treated with clopidogrel, 6 required a blood transfusion. Nevertheless, CURE proved that patients with non-ST-elevation acute coronary syndromes benefited from clopidogrel, regardless of whether they underwent percutaneous coronary intervention.
Comment. Our patient should receive clopidogrel and, if she has no significant bleeding, she should continue to take it for at least 12 months after discharge. It is important for the primary care physician to ensure compliance with this agent and not discontinue it on routine clinical follow-up.
CASE 3: BARE-METAL STENT PLACEMENT
A 62-year-old man with a history of hypertension, diabetes, and hyperlipidemia presents to his primary care physician’s office with stable-effort angina that is not responding to an excellent anti-ischemic regimen and is affecting his quality of life. He is referred for coronary angiography, which reveals 80% stenosis of the proximal left circumflex artery. He undergoes a percutaneous coronary intervention with placement of a bare-metal stent.
How long should he be on clopidogrel? And what if a drug-eluting stent had been placed instead of a bare-metal stent?
Dual therapy after bare-metal stent placement
Dual antiplatelet therapy with clopidogrel and aspirin is recommended in all patients receiving a stent (Table 2). The better safety and efficacy of clopidogrel compared with ticlopidine has been established in patients receiving a coronary artery stent,12,13 and clopidogrel’s favorable safety profile soon made it the thienopyridine of choice.
The CREDO trial (Clopidogrel for the Reduction of Events During Observation)14 randomized 2,116 patients undergoing an elective percutaneous coronary intervention (bare-metal stent placement only) to receive a 300-mg loading dose of clopidogrel 3 to 24 hours before the procedure, or placebo. All patients received 325 mg of aspirin. After the intervention, all patients received clopidogrel 75 mg daily and aspirin 325 mg daily through day 28. For day 29 through 12 months, those who had received the 300-mg preprocedural loading dose of clopidogrel continued with 75 mg daily, and those who had not received clopidogrel before the procedure received placebo.
No significant difference was seen in the primary outcome for those who received pretreatment with clopidogrel; however, in a subgroup analysis, those who received clopidogrel at least 6 hours before the percutaneous coronary intervention had a 38.6% relative risk reduction (Table 1). Long-term use of clopidogrel (ie, for 12 months) was associated with an overall relative reduction of 26.9% in the combined risk of death, MI, or stroke.
PCI-CURE, an analysis of 2,658 patients in the CURE trial with non-ST-elevation acute coronary syndrome who underwent PCI,15 yielded results similar to those of CREDO, with a 31% reduction in the rate of cardiovascular death or MI at 30 days and at 9 months. Of note, however, clopidogrel was given for a median of 6 days prior to the procedure.
Comment. The minimum suggested duration of clopidogrel treatment after placement of a bare-metal stent is 1 month. However, these trial results indicate that patients who are not at high risk of bleeding should take clopidogrel for at least 12 months.
Dual antiplatelet therapy with drug-eluting stents
Although rates of in-stent restenosis are clearly lower with drug-eluting stents than with bare-metal stents, the antiproliferative effect of drug-eluting stents may delay complete endothelialization of every strut. This may contribute to late (> 1 month after placement) or very late (> 1 year) thrombosis of the stent after clopidogrel is discontinued.16–18
In 2006, the FDA indicated that dual antiplatelet therapy was needed for 6 months with paclitaxel-eluting (Taxus) stents and 3 months with sirolimus-eluting (Cipher) stents. As reports of very late stent thrombosis began to appear in 2007, concern arose over the need to extend the duration of clopidogrel treatment.
Bavry et al19 quantified the incidence of late and very late stent thrombosis in a meta-analysis of 14 clinical trials that randomized patients to receive either a drug-eluting stent (paclitaxel or sirolimus) or a bare-metal stent.19 The incidence of stent thrombosis within 30 days in this analysis was similar for both groups—4.4 per 1,000 patients vs 5 per 1,000 (relative risk 0.89; 95% CI 0.46–1.75; P = .74). However, the rate of very late stent thrombosis was significantly higher in those receiving a drug-eluting stent vs a bare-metal stent—5 per 1,000 patients treated (relative risk 5.02, 95% CI 1.29–19.52; P = .02).
The results of this and other studies led the ACC and AHA to revise their joint guidelines to recommend thienopyridine treatment for at least 1 year for patients who receive a drug-eluting stent.6,20–22 In fact, many cardiologists consider indefinite dual antiplatelet therapy in patients with a drug-eluting stent to avoid very late in-stent thrombosis, especially in patients undergoing high-risk interventions such as placement of multiple stents, bifurcation lesions, and unprotected left main trunk interventions.
Thus, when faced with a patient with a recent coronary stent implantation, the primary care physician should be aware of the type of stent and the duration of therapy recommended by the interventional cardiologist. Also, in the absence of a pressing indication, elective surgery should be deferred for 1 year after placement of a drug-eluting stent, as this would necessitate stopping clopidogrel and would increase the risk of perioperative stent thrombosis, which is associated with high rates of morbidity and death.
CASE 4: HIGH-RISK CORONARY ARTERY DISEASE
A 67-year-old woman presents to your office to establish care. She has a history of diabetes and established coronary artery disease with two bare-metal stents placed 2 years ago. She is taking aspirin 81 mg.
What would be the value of adding clopidogrel to her regimen?
No indication for clopidogrel in chronic coronary artery disease
The CHARISMA trial (Clopidogrel for High Atherothrombotic and Ischemic Stabilization, Management, and Avoidance)23 randomized 15,603 patients with stable cardiovascular disease or multiple risk factors to receive either clopidogrel plus low-dose aspirin or placebo plus low-dose aspirin and followed them for a median of 28 months (Table 1).
The primary end point (a composite of MI, stroke, or death) was 6.8% with clopidogrel plus aspirin and 7.3% with aspirin alone, indicating no significant benefit with clopidogrel plus aspirin compared with aspirin alone in reducing the rate of MI, stroke, or cardiovascular death in patients with high-risk but stable atherothrombotic disease. A marginal statistical benefit with dual antiplatelet therapy was noted in the subgroup of patients with previously documented coronary, cerebrovascular, or peripheral vascular disease—6.9% with aspirin plus clopidogrel vs 7.9% with aspirin alone (relative risk 0.88; 95% CI 0.77–0.998; P = .046).
Consequently, there is no compelling reason to start clopidogrel in this patient.
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel was recently approved by the FDA as antiplatelet treatment for patients with acute coronary syndromes planning to undergo a percutaneous coronary intervention.24 It has been shown to inhibit adenosine-diphosphate-induced platelet activation in a more consistent and effective manner than clopidogrel.25,26
Although both clopidogrel and prasugrel are prodrugs, 80% of absorbed clopidogrel is metabolized by esterases into inactive metabolites, and the availability of active metabolite can vary, as it is significantly influenced by polymorphisms in the cytochrome P450 system. 27 In contrast, prasugrel is not degraded by esterases, and its conversion to active metabolite by the cytochrome P450 system is not influenced by common genetic polymorphisms, particularly CYP2C19*2.
TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction) provided most of the evidence for the approval of prasugrel for clinical use.28,29 In this trial, a 60-mg loading dose of prasugrel followed by a daily maintenance dose of 10 mg was significantly superior to the current clopidogrel regimen in preventing death from cardiovascular causes, nonfatal MI, or nonfatal stroke during a study period of 15 months.28 Also observed was a 24% lower rate of MI, a 34% lower rate of urgent target-vessel revascularization, and a 52% lower rate of stent thrombosis.
These benefits, however, came at the cost of a significantly higher risk of major bleeding, including the potential for three excess fatal bleeding events for every 1,000 patients treated. Patients at highest risk at the dosages evaluated included the elderly (age 75 and older), patients who weigh less than 60 kg, and patients with a history of stroke or transient ischemic attack. Based on these results, we recommend caution with the use of prasugrel in these patient subsets.
Clinical use of prasugrel is likely to be highest in patients presenting with ST-elevation MI who are undergoing a primary percutaneous coronary intervention. There is currently no evidence from any randomized clinical trial to support the safety of prasugrel given in the emergency room or “upstream” in the setting of non-ST-elevation acute coronary syndromes.
Of note, patients with non-ST-elevation acute coronary syndromes in the TRITON trial were randomized only after angiographic definition. As a result, only 179 patients exposed to prasugrel were referred for coronary artery bypass surgery, but the rate of surgery-related major bleeding in this group was 13.4% (vs 3.2% in the clopidogrel group). Based on these data, prasugrel should be withheld for at least 1 week prior to any surgery.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Yeh SP, Hsueh EJ, Wu H, Wang YC. Ticlopidine-associated aplastic anemia. A case report and review of literature, Ann Hematol 1998; 76:87–90.
- Page Y, Tardy B, Zeni F, Comtet C, Terrana R, Bertrand JC. Thrombotic thrombocytopenic purpura related to ticlopidine. Lancet 1991; 337:774–776.
- Canadian Cardiovascular Society; Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007; 50:e1–e157.
- King SB, Smith SC, Hirshfeld JW, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:172–209.
- Kam PC, Nethery CM. The thienopyridine derivatives (platelet adenosine diphosphate receptor antagonists), pharmacology and clinical developments. Anaesthesia 2003; 58:28–35.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Sabatine MS, Cannon CP, Gibson M, et al; CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation, N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: a randomized placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Bhatt DL, Bertrand ME, Berger PB, et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J Am Coll Cardiol 2002; 39:9–14.
- Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH; CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS). Circulation 2000; 102:624–629.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Mehta SR, Yusuf S, Peters RJ, et a; Clopidogrel in Unstable Angina to Prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001; 358:527–533.
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007; 115:1051–1058.
- Kotani J, Awata M, Nanto S, et al. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol 2006; 47:2108–2111.
- Pfisterer M, Brunner-La Rocca HP, Buser PT, et al; BASKETLATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol 2006; 48:2584–2591.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med 2007; 356:1030–1039.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- US Food and Drug Administration. FDA Approves Effient to Reduce the Risk of Heart Attack in Angioplasty Patients. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm171497.htm. Accessed October 2, 2009.
- Jernber T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006; 27:1166–1173.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A TRITON-TIMI (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Card 2008; 51:2028–2033.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Yeh SP, Hsueh EJ, Wu H, Wang YC. Ticlopidine-associated aplastic anemia. A case report and review of literature, Ann Hematol 1998; 76:87–90.
- Page Y, Tardy B, Zeni F, Comtet C, Terrana R, Bertrand JC. Thrombotic thrombocytopenic purpura related to ticlopidine. Lancet 1991; 337:774–776.
- Canadian Cardiovascular Society; Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007; 50:e1–e157.
- King SB, Smith SC, Hirshfeld JW, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:172–209.
- Kam PC, Nethery CM. The thienopyridine derivatives (platelet adenosine diphosphate receptor antagonists), pharmacology and clinical developments. Anaesthesia 2003; 58:28–35.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Sabatine MS, Cannon CP, Gibson M, et al; CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation, N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: a randomized placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Bhatt DL, Bertrand ME, Berger PB, et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J Am Coll Cardiol 2002; 39:9–14.
- Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH; CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: The Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS). Circulation 2000; 102:624–629.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Mehta SR, Yusuf S, Peters RJ, et a; Clopidogrel in Unstable Angina to Prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001; 358:527–533.
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007; 115:1051–1058.
- Kotani J, Awata M, Nanto S, et al. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol 2006; 47:2108–2111.
- Pfisterer M, Brunner-La Rocca HP, Buser PT, et al; BASKETLATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol 2006; 48:2584–2591.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007; 356:998–1008.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med 2007; 356:1030–1039.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- US Food and Drug Administration. FDA Approves Effient to Reduce the Risk of Heart Attack in Angioplasty Patients. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm171497.htm. Accessed October 2, 2009.
- Jernber T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006; 27:1166–1173.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A TRITON-TIMI (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Card 2008; 51:2028–2033.
KEY POINTS
- Dual antiplatelet therapy is recommended after ST-elevation MI or non-ST-elevation acute coronary syndromes, with aspirin indefinitely and clopidogrel for up to 1 year.
- Dual antiplatelet therapy is recommended for at least 1 month after placement of a bare-metal stent and for at least 1 year (or possibly indefinitely) after placement of a drug-eluting stent.
- There is no compelling indication for clopidogrel in patients with chronic coronary artery disease.
- Compared with clopidogrel, prasugrel (Effient) is associated with lower rates of MI, urgent target-vessel revascularization, and in-stent thrombosis, but at the cost of a higher risk of major bleeding.