User login
Systemic Targeted Treatments for Basal Cell Carcinoma
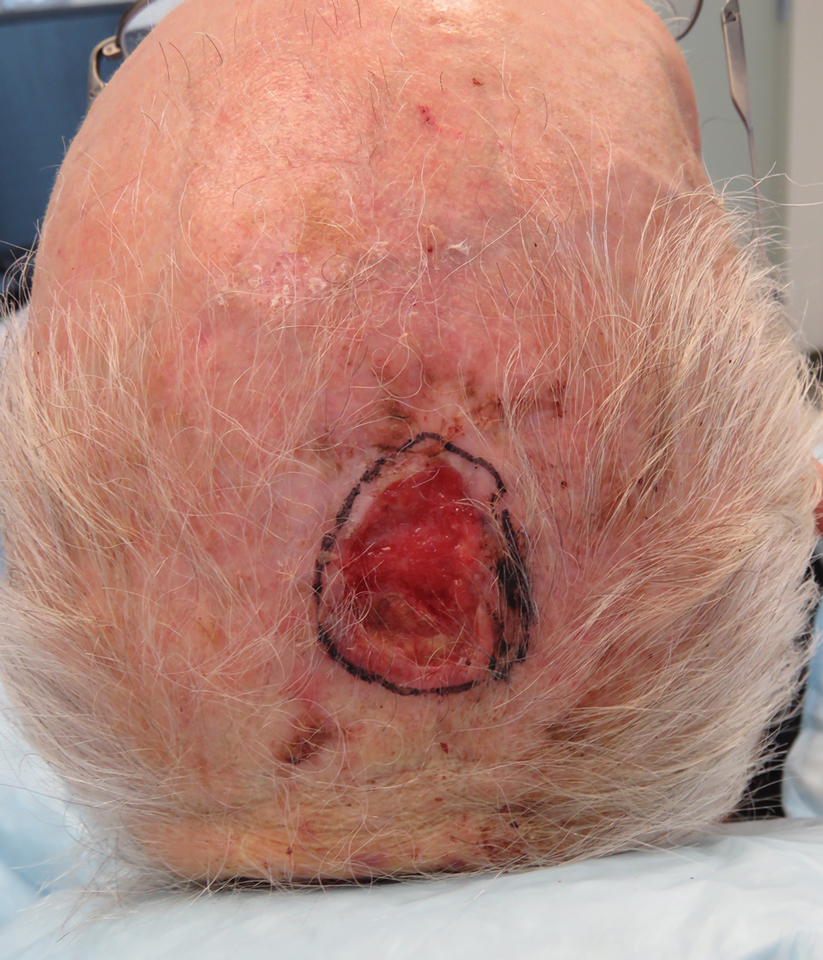
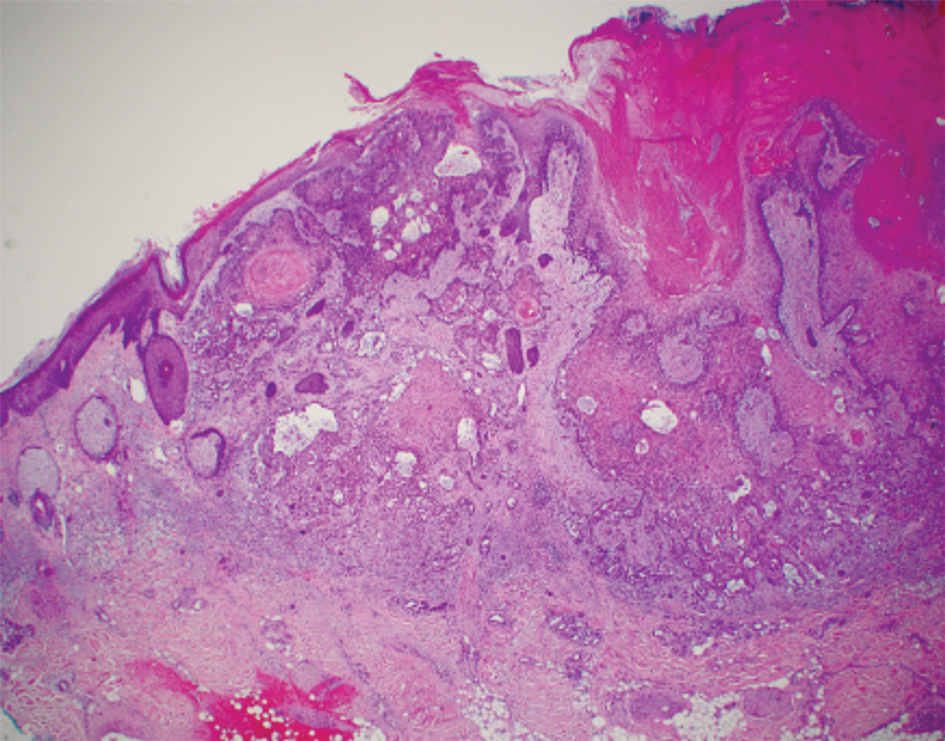
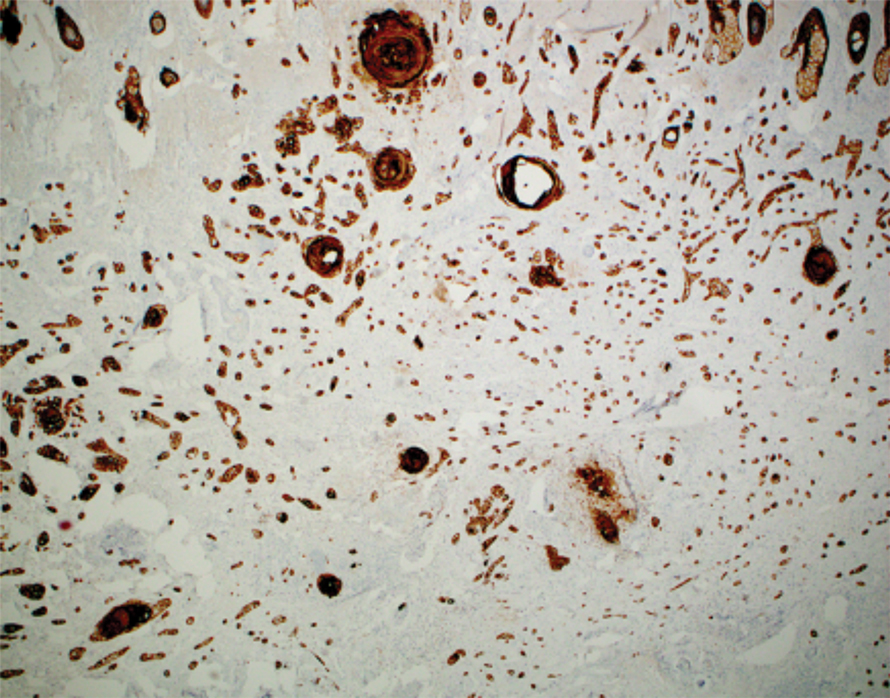
Basal cell carcinoma (BCC) is the most common keratinocyte carcinoma and affects more than 3 million individuals per year in the United States.1 Approximately 40% of patients diagnosed with BCC will develop another BCC within 5 years of the initial diagnosis.2 Most cases are successfully treated with surgical excision and occasionally topical therapy or radiotherapy. Despite the high cure rate with conventional treatments, BCC can recur and can cause substantial destruction of the surrounding tissue if left untreated.3-5 In some instances, BCC can even metastasize and lead to death.6 For patients who are poor candidates for surgical or topical treatment modalities because of locally advanced BCC (laBCC) or metastatic BCC (mBCC), systemic treatment may be indicated. Vismodegib, sonidegib, and cemiplimab are the only systemic medications approved by the US Food and Drug Administration (FDA) for the treatment of laBCC and/or mBCC. Vismodegib and sonidegib target the sonic hedgehog (SHH) signaling pathway that is abnormally activated in more than 90% of BCCs.7 Cemiplimab is an immune checkpoint inhibitor (ICI) that targets the programmed cell death protein 1 (PD-1) receptor.8 Herein, we review the clinical utility of these medications and their evolving roles in the treatment of BCC.
SHH Pathway Inhibitors
The SHH pathway is a key regulator of cell proliferation and differentiation during embryogenesis.7 During adulthood, SHH signaling decreases but still plays an important role in stem cell activation and in regulation of the hair follicle growth cycle.9,10 However, de novo mutations in the genes that comprise the SHH pathway can result in aberrant constitutive activation, leading to unrestricted cell proliferation. Genetic mutations resulting in activation of Smoothened (SMO), a G-protein–coupled receptor involved in the signal transduction and propagation of the SHH pathway, have been implicated in the pathogenesis of BCC. Inactivating mutations also are commonly observed in patched homolog 1, an upstream cell-surface protein that inhibits SMO.7 The mechanism by which vismodegib and sonidegib, 2 of the FDA-approved oral medications for the treatment of advanced BCC, block the SHH pathway is through the selective inhibition of SMO.7,11
Vismodegib first received FDA approval in 2012 for the treatment of laBCC and mBCC after initial results from the pivotal ERIVANCE phase 2 trial demonstrated an objective response rate (ORR) of 43% (27/63) and 30% (10/33) in patients with locally advanced and metastatic disease, respectively. In this single-arm study, all enrolled patients (63 with laBCC and 33 with mBCC) received 150 mg of oral vismodegib daily.12 Updated results at 39 months demonstrated improved ORRs of 60% (38/63) and 48% (16/33) for the laBCC and mBCC groups, respectively. A complete response (CR) and partial response (PR) were observed in 32% (n=20) and 29% (n=18) of patients with laBCC, respectively.13 These results have been confirmed in subsequent studies, including the large international open-label trial known as STEVIE, with ORRs of 68.5% for 1119 cases of laBCC and 37% for 96 cases of mBCC.14-17 The CR and PR rates were 33% and 35%, respectively, for the laBCC group. The CR and PR rates for the mBCC group were 5% and 32%, respectively.14
The FDA approval of sonidegib for laBCC—but not mBCC—occurred in 2015 after the pivotal BOLT randomized phase 2 trial demonstrated an initial ORR of 43% (18/42) for laBCC and 15% (2/13) for mBCC after administration of 200 mg of sonidegib daily.18 A final follow-up analysis at 42 months resulted in ORRs of 56% (37/66) and 8% (1/13) for the laBCC and mBCC groups, respectively.19 Additionally, improved efficacy was not observed in the 151 patients who were randomized to receive treatment with the higher 800-mg dose; however, they did experience a higher incidence of adverse events.18,19
Currently, the true clinical differences between vismodegib and sonidegib remain uncertain, as no head-to-head trials have been conducted. Moreover, direct comparison of the data from the ERIVANCE and BOLT trials is challenging owing to fundamental differences in methodologic design, including the criteria used to assess BCC severity. The ERIVANCE trial utilized the conventional Response Evaluation Criteria in Solid Tumors (RECIST), while BOLT used the rigorous modified RECIST. However, an expert consensus study attempted to compare the 2 trials by modifying the outcomes from BOLT with the former RECIST criteria. The expert group found that the 2 SHH inhibitors had comparable efficacy and adverse event profiles.20 Nevertheless, a recent meta-analysis found that although ORRs for laBCC were similar between the 2 drugs, the CR rate for vismodegib was 31% compared with 3% for sonidegib. Additionally, for mBCC, they reported the ORR of vismodegib to be 2.7 times higher than that of sonidegib (39% vs 15%).21
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors have successfully been utilized in the treatment of cutaneous squamous cell carcinoma (cSCC); however, their use for treating BCC has been limited until recently.22-25 In February 2021, cemiplimab became the first and only ICI approved for the treatment of laBCC and mBCC in patients who did not respond to or were intolerant to prior SHH inhibitor therapy.26 Cemiplimab—a human monoclonal antibody against the PD-1 receptor expressed on T cells—blocks its interaction with programmed cell death ligand 1 and programmed cell death ligand 2 present on tumor cells. The blockade of the PD-1 pathway releases the inhibition of the antitumor immune response and enables appropriate cytotoxic T-cell activity to occur.8
The FDA approval of cemiplimab for the treatment of advanced BCC was based on an open-label, multicenter, single-arm phase 2 trial (NCT03132636) evaluating 84 patients with laBCC refractory or intolerant to SHH inhibitor therapy.26 Patients received an intravenous infusion of cemiplimab 350 mg every 3 weeks for up to 93 weeks or until disease progression or unacceptable toxicity. An ORR of 31% (26/84) was observed with a CR and PR of 6% (5/84) and 25% (21/84), respectively. The median duration of follow-up was 15 months.26 Given the clinically meaningful results of this trial, investigating the efficacy of other PD-1 inhibitors, such as pembrolizumab and nivolumab, for treatment of advanced BCC may prove worthwhile.
Adverse Effects of Systemic Treatments
The 2 approved SHH inhibitors—vismodegib and sonidegib—appear to have similar side-effect profiles, with the most common adverse effects being muscle spasms, dysgeusia, alopecia, nausea, vomiting, diarrhea, weight loss, and fatigue.20,21,27 These side effects occur at high frequencies (>40%) for both SHH inhibitors and often lead to discontinuation of the medication.21 Rates of treatment discontinuation range from 15% to 50% on average.12-14,18 Fortunately, the majority of these adverse effects do not appear to increase in severity or frequency with prolonged use of these medications.14,16,28
Various conservative and pharmacologic measures can be implemented to help manage side effects. For muscle spasms, which are the most commonly reported adverse effect, supplementation with magnesium, transcutaneous electrical nerve stimulation, acupuncture, massages, stretching, and thermal compresses can potentially be beneficial.29 Calcium channel blockers also may be effective, as one small prospective cohort study reported a reduction in the frequency of muscle cramps with amlodipine 10 mg daily.30 For alopecia, which typically is reversible and caused by SHH inhibition of the normal hair cycle, minoxidil theoretically can help, as it reduces telogen arrest and extends the anagen growth phase.31,32 Although usually mild and self-limiting, management of dysgeusia, weight loss, and gastrointestinal upset often can be managed with dietary changes, such as smaller, more frequent meals.33,34 Finally, alternative dosing strategies and drug holidays have been employed to mitigate these side effects and increase drug tolerability.35,36 These are discussed in the Alternative Dosing section.
Given the essential role of the SHH pathway in embryologic development, SHH inhibitors carry a black box warning of embryofetal teratogenicity and are contraindicated in females who are pregnant or breastfeeding. For females of reproductive potential, verification of pregnancy status should be performed prior to initiating treatment with an SHH inhibitor. These patients should be counseled on the use of contraception during treatment and for at least 24 months and 20 months after cessation of vismodegib and sonidegib, respectively.27,37,38 Male patients, even after a vasectomy, should use barrier contraception during treatment and for at least 3 months and 8 months after the final dose of vismodegib and sonidegib, respectively.37,38
Laboratory abnormalities commonly associated with SHH inhibitors include elevated hepatic enzymes, particularly with vismodegib, and elevated creatine kinase levels, particularly with sonidegib.28,39 Other laboratory abnormalities that can occur include hypercholesterolemia, hypercreatininemia, hyperglycemia, and increased serum lipase levels.19,28 Although these laboratory abnormalities usually are asymptomatic and self-limiting, regular monitoring should be performed.
There also is concern that SHH inhibitors may induce the development of cSCC. A case-control study of 55 cases and 125 control patients found an increased risk for cSCC in those previously treated with vismodegib, with a hazard ratio of 8.12.40 However, a subsequent retrospective cohort study of 1675 patients with BCC failed to find any association with cSCC among those treated with vismodegib compared to those who received standard surgical therapy.41 Clinical data for sonidegib are lacking, but the BOLT trial found that cSCC occurred in 3 patients receiving treatment with the SHH inhibitor.18 Thus, further studies are needed to more thoroughly assess this concern. Close monitoring for cSCC may be warranted in patients prescribed SHH inhibitors at this time.
Cemiplimab has demonstrated an acceptable safety profile and is generally well tolerated. In the phase 2 trial of cemiplimab for cSCC, approximately 5% of patients discontinued treatment because of adverse effects. The most commonly reported side effects of cemiplimab were diarrhea (27%), fatigue (24%), nausea (17%), constipation (15%), and rash (15%).23 In the phase 2 trial for laBCC, grade 3 or 4 adverse events occurred in 48% of patients, with hypertension (5%) being the most common.26 Although rare, immune-mediated adverse reactions also can occur, given the mechanism of action of ICIs. These side effects, ranging in severity from mild to fatal, include pneumonitis, colitis, hepatitis, nephritis, myocarditis, and hypophysitis. Therefore, close monitoring for these immune-mediated reactions is critical, but most can be managed with corticosteroids or treatment interruption if they occur.42,43
No absolute contraindications exist for cemiplimab; however, extreme caution should be taken in immunosuppressed individuals, such as solid organ transplant recipients and those with chronic lymphocytic leukemia (CLL), as safety data are limited in these patients.44,45 Although small retrospective studies have reported reasonable tolerability in solid organ transplant recipients treated with ICIs, an allograft rejection rate of 41% was found in a meta-analysis of 64 patients.46-48 In CLL patients with keratinocyte carcinomas, ICIs have been safely used and have even demonstrated efficacy for CLL in some cases.49-52
Alternative Dosing
The side effects of SHH inhibitors have led to alternative dosing strategies to prevent medication discontinuation and improve adherence. In patients with basal cell nevus syndrome, multimonth drug holidays have been shown to increase drug tolerability without compromising efficacy.35,36 Weekly intermittent dosing regimens of vismodegib ranging from 1 week on followed by 1 to 3 weeks off demonstrated efficacy in a retrospective study of 7 patients with advanced BCC.53 All 7 patients experienced improvement in their BCCs, with 3 patients experiencing CR. Importantly, treatment-related adverse effects were mild and well tolerated, with no patients terminating the medication.53 Two other retrospective case series of patients with advanced BCC treated with vismodegib reported similar findings for those placed on an intermittent dosing schedule ranging from once every other day to once per week.54,55
In the large phase 2 randomized trial known as MIKIE, 2 different intermittent dosing regimens of 150 mg vismodegib daily for patients with multiple BCCs were found to have good activity and tolerability.56 The first group (n=116) received vismodegib for 12 weeks, then 3 rounds of 8 weeks of placebo, followed by 12 weeks of vismodegib; there was a 63% reduction in clinically evident BCCs after 73 weeks. The second group (n=113) received the medication for 24 weeks, then 3 rounds of 8 weeks of placebo, followed by 8 weeks of vismodegib; there was a 54% reduction at the end of 73 weeks.56 Subsequent analyses found improvements in health-related quality-of-life outcomes that were similar for both groups.57
Consequently, alternative dosing schedules appear to be a viable option for patients at risk of discontinuing treatment because of adverse effects, and current data support the recently approved recommendations of dose interruptions of up to 8 weeks to manage adverse effects in patients with laBCC or mBCC.58 Nevertheless, further clinical studies are required to determine the optimal intermittent dosing regimen for patients treated with SHH inhibitors.
Neoadjuvant Administration
Recently, vismodegib has been studied as a neoadjuvant therapy for BCC with promising results. Several small retrospective studies and case reports have documented successful treatment of both operable and inoperable periocular laBCC, with preservation of the eye in all patients.59-61 An open-label trial of 15 patients with advanced BCC who received neoadjuvant vismodegib for 3 to 6 months prior to surgical excision reported a mean reduction of 35% in the final surgical defect size, with no recurrence at 22 months.62,63 The latest and largest study performed was a phase 2 open-label trial known as VISMONEO, where 44 of 55 laBCC patients (80%) receiving neoadjuvant vismodegib for a mean duration of 6 months (range, 4–10 months) achieved the primary end point of tumor surgical downstaging.64 Of the 44 patients who had tumor downstaging, 27 (61%) experienced histologically proven CRs. Additionally, a 66% reduction in the average target lesion size was reported in this group compared to29% in the 11 patients who did not have tumor downstaging (P=.0002).64 Thus, SHH inhibitors may hold an important neoadjuvant role in the treatment of BCC by decreasing surgical defect size and allowing for surgical management of previously inoperable cases.
Synergism With Radiation
Preliminary data suggest SHH inhibitors may help potentiate the effects of radiation therapy for the treatment of BCC. Currently, the evidence primarily is limited to case studies, with several reports describing complete remission in patients with advanced BCCs who were considered unsuitable candidates for surgery. In these cases, vismodegib was administered either prior to or concurrently with radiation treatment.65-69 An in vitro study also documented the radiation-sensitizing effects of vismodegib in a BCC cell line.70 Recently, a phase 2 trial (ClinicalTrials.gov identifier NCT01835626) evaluating the concurrent use of vismodegib and radiotherapy for patients with advanced BCC was completed, but data has yet to be published.
Synergism With and Benefit of Antifungal Therapy
The antifungal drug itraconazole is a potent inhibitor of the SHH pathway and may have an adjunctive role in the treatment of BCC. Similar to vismodegib and sonidegib, itraconazole acts as a direct antagonist of SMO. However, it is thought to bind to a distinct site on SMO.71,72 An open-label, exploratory phase 2 trial of 19 patients with BCC found that oral itraconazole 200 to 400 mg daily decreased tumor proliferative index by 45% (P=.04), as measured by Ki-67; SHH activity by 65% (P=.03), as measured by GLI1 messenger RNA; and mean tumor area by 24%.73 In a case series of 5 patients with mBCC refractory to conventional SHH inhibitor therapy, combined treatment with itraconazole and arsenic trioxide resulted in stable disease and a 75% reduction in SHH activity (P<.001).74 One case report documented tumor regression leading to stable disease for 15 months in a patient with laBCC treated with itraconazole monotherapy due to being unable to afford vismodegib or sonidegib. However, within 2 months of treatment discontinuation, the lesion progressed considerably.75 The efficacy of a topical formulation of itraconazole also has been tested in an open-label, placebo-controlled phase 2 trial, but no benefit was observed.76
Posaconazole is a second-generation antifungal agent that may serve as a potential alternative to itraconazole.77 Although clinical data are lacking, a basic science study found that posaconazole could inhibit the growth of SHH-dependent BCC in vivo (in mice).78 Furthermore, posaconazole has demonstrated a better safety profile with fewer and more mild side effects than itraconazole and does not require dose adjustment for those with hepatic or renal failure.79,80 Thus, posaconazole may be a safer alternative to itraconazole for the treatment of BCC. Further clinical studies are needed to elucidate the potential synergistic effects of these antifungal agents with the 2 currently approved SHH inhibitors for the treatment of advanced BCC.
Drug Resistance
Treatment resistance to SHH inhibitors, though uncommon, is a growing concern. Acquired mutations in the SMO binding site or downstream mediators of the SHH pathway have been shown to confer resistance to vismodegib and sonidegib.72,81-83 In addition, it appears that there may be shared resistance among the drugs in this class. One study assessing the efficacy of sonidegib in 9 patients with laBCC resistant to vismodegib found that these patients also did not respond to sonidegib.84 Interestingly, 1 case report documented tumor regression of an intracranial BCC in a patient treated with sonidegib and itraconazole after failure with vismodegib.85 An in vitro study also found that itraconazole maintained SHH inhibitory activity for all drug-resistant SMO mutations that have been reported.72 Therefore, itraconazole monotherapy or combination therapy with a canonical SHH inhibitor may be considered for patients with recalcitrant BCC and warrants further investigation.
Taladegib is a newly developed SMO inhibitor that may serve as another promising alternative for patients who develop resistance to vismodegib or sonidegib. A phase 1 trial of taladegib for advanced BCC found an ORR of 69% (11/16) in the SHH inhibitor–naïve group and an ORR of 36% (11/32) in the group previously treated with a SHH inhibitor.86 Additionally, the safety profile and frequency of adverse effects appear to be similar to those associated with vismodegib and sonidegib.86,87 Unfortunately, no clinical trials evaluating taladegib for BCC are ongoing or in development at this time.
Recurrence
There appears to be a relatively high rate of recurrence for BCC patients who achieve a CR to SHH inhibitors. In a retrospective study of 116 laBCC patients who experienced a CR after vismodegib therapy, 54 patients (47%) relapsed at 36 months. Among the 54 patients that relapsed, 27 were re-treated with vismodegib, which resulted in an ORR of 85% (23/27), a CR rate of 37% (10/27), and a PR rate of 48% (13/27).88 Another retrospective study of 35 laBCC patients who relapsed after vismodegib treatment reported a 31% (11/35) clinical recurrence rate at 6-month follow-up.89 An observational retrospective study also assessed the efficacy of SHH inhibitor maintenance therapy for advanced BCC patients who achieved a CR.90 In the study, 27 (64%) patients received a maintenance dose of 150 mg vismodegib once per week for 1 year, while 15 (36%) patients decided not to take a maintenance dose following CR of their BCC. All patients who took the maintenance therapy did not experience clinical recurrence at 1-year follow-up, whereas 26% of patients not on the maintenance dose relapsed.90 Consequently, these results indicate that BCC recurrence is frequent after SHH inhibitor therapy and highlights the importance of close surveillance after CR is attained. Nevertheless, most patients still respond to treatment with SHH inhibitors after relapsing, and intermittent maintenance doses may be an effective means to reduce risk of recurrence.
Conclusion
Vismodegib and sonidegib are SHH inhibitors approved for the treatment of laBCC and mBCC. Cemiplimab is now also approved for patients who do not respond to SHH inhibitors or for whom SHH inhibitors are not tolerable. Although these systemic targeted therapies can lead to notable tumor shrinkage and even complete regression in some patients, recurrence is common, and adverse effects may limit their use. Drug resistance is an emerging issue that requires additional examination. Further clinical studies are needed to determine which patients are likely to respond to these targeted treatments.
Various intermittent and maintenance drug regimens should be evaluated for their potential to mitigate adverse effects and reduce risk of recurrence, respectively. The synergistic effects of these medications with other therapies as well as their neoadjuvant and adjuvant roles should be further investigated. For example, administration of an SHH inhibitor prior to surgical excision of a BCC may allow for a smaller surgical defect size, thereby improving cosmetic and functional outcomes. Moreover, these systemic targeted medications may allow for previously inoperable tumors to become amenable to surgical treatment.
Although SHH inhibitors and PD-1 inhibitors represent a major advancement in the field of oncodermatology, real-world efficacy and safety data in the upcoming years will be important for elucidating their true benefit for patients with BCC.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Kasumagic-Halilovic E, Hasic M, Ovcina-Kurtovic N. A clinical study of basal cell carcinoma. Med Arch. 2019;73:394-398.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e952-966.e952.
- Laga AC, Schaefer IM, Sholl LM, et al. Metastatic basal cell carcinoma. Am J Clin Pathol. 2019;152:706-717.
- Rimkus TK, Carpenter RL, Qasem S, et al. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel). 2016;8:22.
- Burova E, Hermann A, Waite J, et al. Characterization of the anti–PD-1 antibody REGN2810 and its antitumor activity in human PD-1 knock-in mice. Mol Cancer Ther. 2017;16:861-870.
- Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172-180.
- Paladini RD, Saleh J, Qian C, et al. Modulation of hair growth with small molecule agonists of the hedgehog signaling pathway. J Invest Dermatol. 2005;125:638-646.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Sekulic A, Migden MR, Basset-Seguin N, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Cozzani R, Del Aguila R, Carrizo M, et al. Efficacy and safety profile of vismodegib in a real-world setting cohort of patients with advanced basal cell carcinoma in Argentina. Int J Dermatol. 2020;59:627-632.
- Spallone G, Sollena P, Ventura A, et al. Efficacy and safety of vismodegib treatment in patients with advanced basal cell carcinoma and multiple comorbidities. Dermatol Ther. 2019;32:E13108.
- Fosko SW, Chu MB, Armbrecht E, et al. Efficacy, rate of tumor response, and safety of a short course (12-24 weeks) of oral vismodegib in various histologic subtypes (infiltrative, nodular, and superficial) of high-risk or locally advanced basal cell carcinoma, in an open-label, prospective case series clinical trial. J Am Acad Dermatol. 2020;82:946-954.
- Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716-728.
- Dummer R, Guminksi A, Gutzmer R, et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br J Dermatol. 2020;182:1369-1378.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Xie P, Lefrançois P. Efficacy, safety, and comparison of sonic hedgehog inhibitors in basal cell carcinomas: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79:1089-1100.e1017.
- Gentzler R, Hall R, Kunk PR, et al. Beyond melanoma: inhibiting the PD-1/PD-L1 pathway in solid tumors. Immunotherapy. 2016;8:583-600.
- Guminski AD, Lim AML, Khushalani NI, et al. Phase 2 study of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with metastatic cutaneous squamous cell carcinoma (mCSCC; group 1): 12-month follow-up [abstract]. J Clin Oncol. 2019;37(15 suppl):9526.
- Grob JJ, Gonzalez Mendoza R, Basset-Seguin N, et al. Pembrolizumab for recurrent/metastatic cutaneous squamous cell carcinoma (cSCC): efficacy and safety results from the phase II KEYNOTE-629 study [abstract]. Ann Oncol. 2019;30 (suppl 5):v908.
- Maubec E, Boubaya M, Petrow P, et al. Pembrolizumab as first-line therapy in patients with unresectable cutaneous squamous cell carcinoma (cSCC): phase 2 results from CARSKIN [abstract]. J Clin Oncol. 2019;37(15 suppl):9547.
- Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:848-857.
- Carpenter RL, Ray H. Safety and tolerability of sonic hedgehog pathway inhibitors in cancer. Drug Saf. 2019;42:263-279.
- Villani A, Fabbrocini G, Costa C, et al. Sonidegib: safety and efficacy in treatment of advanced basal cell carcinoma. Dermatol Ther (Heidelb). 2020;10:401-412.
- Wright A, Sluka KA. Nonpharmacological treatments for musculoskeletal pain. Clin J Pain. 2001;17:33-46.
- Ally MS, Tang JY, Lindgren J, et al. Effect of calcium channel blockade on vismodegib-induced muscle cramps. JAMA Dermatol. 2015;151:1132-1134.
- Yang X, Thai K-E. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil. Australas J Dermatol. 2016;57:e130-e132.
- Ferguson JS, Hannam S, Toholka R, et al. Hair loss and hedgehog inhibitors: a class effect? Br J Dermatol. 2015;173:262-264.
- Kumbargere Nagraj S, George RP, Shetty N, et al. Interventions for managing taste disturbances. Cochrane Database Syst Rev. 2017;12:CD010470.
- Jacobsen AA, Kydd AR, Strasswimmer J. Practical management of the adverse effects of hedgehog pathway inhibitor therapy for basal cell carcinoma. J Am Acad Dermatol. 2017;76:767-768.
- Ally MS, Tang JY, Joseph T, et al. The use of vismodegib to shrink keratocystic odontogenic tumors in patients with basal cell nevus syndrome. JAMA Dermatol. 2014;150:542-545.
- Yang X, Dinehart SM. Intermittent vismodegib therapy in basal cell nevus syndrome. JAMA Dermatol. 2016;152:223-224.
- Erivedge. Prescribing information. Genentech; 2015.
- Odomzo. Prescribing information. Novartis; 2015.
- Ventarola DJ, Silverstein DI. Vismodegib-associated hepatotoxicity: a potential side effect detected in postmarketing surveillance. J Am Acad Dermatol. 2014;71:397-398.
- Mohan SV, Chang J, Li S, et al. Increased risk of cutaneous squamous cell carcinoma after vismodegib therapy for basal cell carcinoma. JAMA Dermatol. 2016;152:527-532.
- Bhutani T, Abrouk M, Sima CS, et al. Risk of cutaneous squamous cell carcinoma after treatment of basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2017;77:713-718.
- Morgado M, Plácido A, Morgado S, et al. Management of the adverse effects of immune checkpoint inhibitors. Vaccines (Basel). 2020;8:575.
- Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563-580.
- Ntsethe A, Dludla PV, Nyambuya TM, et al. The impact of immune checkpoint inhibitors in patients with chronic lymphocytic leukemia (CLL): a protocol for a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99:E21167.
- Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904-1911.
- Tsung I, Worden FP, Fontana RJ. Safety and efficacy of checkpoint inhibitors in solid organ transplant recipients with cutaneous squamous cell carcinoma [abstract]. J Clin Oncol. 2020;38(15 suppl):E22014.
- Owoyemi I, Vaughan LE, Costello CM, et al. Clinical outcomes of solid organ transplant recipients with metastatic cancers who are treated with immune checkpoint inhibitors: a single-center analysis. Cancer. 2020;126:4780-4787.
- Kumar V, Shinagare AB, Rennke HG, et al. The safety and efficacy of checkpoint inhibitors in transplant recipients: a case series and systematic review of literature. Oncologist. 2020;25:505-514.
- Arenbergerova M, Fialova A, Arenberger P, et al. Killing two birds with one stone: response to pembrolizumab in a patient with metastatic melanoma and B-cell chronic lymphocytic leukaemia. J Eur Acad Dermatol Venereol. 2018;32:E72-E74.
- Archibald WJ, Meacham PJ, Williams AM, et al. Management of melanoma in patients with chronic lymphocytic leukemia. Leuk Res. 2018;71:43-46.
- Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129:3419-3427.
- Leiter U, Loquai C, Reinhardt L, et al. Immune checkpoint inhibition therapy for advanced skin cancer in patients with concomitant hematological malignancy: a retrospective multicenter DeCOG study of 84 patients. J Immunother Cancer. 2020;8:E000897.
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322.
- Woltsche N, Pichler N, Wolf I, et al. Managing adverse effects by dose reduction during routine treatment of locally advanced basal cell carcinoma with the hedgehog inhibitor vismodegib: a single centre experience. J Eur Acad Dermatol Venereol. 2019;33:E144-E145.
- Wong C, Poblete-Lopez C, Vidimos A. Comparison of daily dosing versus Monday through Friday dosing of vismodegib for locally advanced basal cell carcinoma and basal cell nevus syndrome: a retrospective case series. J Am Acad Dermatol. 2020;82:1539-1542.
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412.
- Schadendorf D, Hauschild A, Fosko S, et al. Quality-of-life analysis with intermittent vismodegib regimens in patients with multiple basal cell carcinomas: patient-reported outcomes from the MIKIE study. J Eur Acad Dermatol Venereol. 2020;34:E526-E529.
- Chanu P, Musib L, Wang X, et al. Vismodegib efficacy in advanced basal cell carcinoma maintained with 8-week dose interruptions: a model-based evaluation. J Invest Dermatol. 2021;141:930-933.
- Su MG, Potts LB, Tsai JH. Treatment of periocular basal cell carcinoma with neoadjuvant vismodegib. Am J Ophthalmol Case Rep. 2020;19:100755.
- González AR, Etchichury D, Gil ME, et al. Neoadjuvant vismodegib and Mohs micrographic surgery for locally advanced periocular basal cell carcinoma. Ophthalmic Plast Reconstr Surg. 2019;35:56-61.
- Sagiv O, Nagarajan P, Ferrarotto R, et al. Ocular preservation with neoadjuvant vismodegib in patients with locally advanced periocular basal cell carcinoma. Br J Ophthalmol. 2019;103:775-780.
- Ally MS, Aasi S, Wysong A, et al. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol. 2014;71:904-911.e1.
- Kwon GP, Ally MS, Bailey-Healy I, et al. Update to an open-label clinical trial of vismodegib as neoadjuvant before surgery for high-risk basal cell carcinoma (BCC). J Am Acad Dermatol. 2016;75:213-215.
- Mortier L, Bertrand N, Basset-Seguin N, et al. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: first results of a multicenter, open-label, phase 2 trial (VISMONEO study) [abstract]. J Clin Oncol. 2018;36(15 suppl):9509.
- Strasswimmer JM. Potential synergy of radiation therapy with vismodegib for basal cell carcinoma. JAMA Dermatol. 2015;151:925-926.
- Gathings RM, Orscheln CS, Huang WW. Compassionate use of vismodegib and adjuvant radiotherapy in the treatment of multiple locally advanced and inoperable basal cell carcinomas and squamous cell carcinomas of the skin. J Am Acad Dermatol. 2014;70:E88-E89.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Janela-Lapert R, Dubray B, Duval-Modeste A, et al. Treatment of advanced basal cell carcinoma with vismodegib followed by radiotherapy [in French]. Ann Dermatol Venereol. 2020;147:780-782.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:2485.
- Kim J, Tang JY, Gong R, et al. Itraconazole, a commonly used antifungal that inhibits hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388-399.
- Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23-34.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Cia˛z˙yn´yska M, Narbutt J, Skibin´ska M, et al. Itraconazole—a new player in the therapy of advanced basal cell carcinoma: a case report. JCO Oncol Pract. 2020;16:837-838.
- Sohn GK, Kwon GP, Bailey-Healy I, et al. Topical itraconazole for the treatment of basal cell carcinoma in patients with basal cell nevus syndrome or high-frequency basal cell carcinomas: a phase 2, open-label, placebo-controlled trial. JAMA Dermatol. 2019;155:1078-1080.
- Lass-Flörl C. Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs. 2011;71:2405-2419.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Katragkou A, Tsikopoulou F, Roilides E, et al. Posaconazole: when and how? the clinician’s view. Mycoses. 2012;55:110-122.
- Raad II, Graybill JR, Bustamante AB, et al. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis. 2006;42:1726-1734.
- Atwood SX, Sarin KY, Whitson RJ, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27:342-353.
- Sun Q, Atzmony L, Zaki T, et al. Clues to primary vismodegib resistance lie in histology and genetics. J Clin Pathol. 2020;73:678-680.
- Verkouteren BJA, Wakkee M, van Geel M, et al. Molecular testing in metastatic basal cell carcinoma. J Am Acad Dermatol. 2021;85:1135-1142.
- Danial C, Sarin KY, Oro AE, et al. An investigator-initiated open-label trial of sonidegib in advanced basal cell carcinoma patients resistant to vismodegib. Clin Cancer Res. 2016;22:1325-1329.
- Yoon J, Apicelli AJ 3rd, Pavlopoulos TV. Intracranial regression of an advanced basal cell carcinoma using sonidegib and itraconazole after failure with vismodegib. JAAD Case Rep. 2017;4:10-12.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Ueno H, Kondo S, Yoshikawa S, et al. A phase I and pharmacokinetic study of taladegib, a Smoothened inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs. 2018;36:647-656.
- Herms F, Lambert J, Grob JJ, et al. Follow-up of patients with complete remission of locally advanced basal cell carcinoma after vismodegib discontinuation: a multicenter French study of 116 patients. J Clin Oncol. 2019;37:3275-3282.
- Villani A, Megna M, Fabbrocini G, et al. Long-term efficacy of vismodegib after its withdrawal and patients’ health-related quality of life using the Dermatology Life Quality Index (DLQI). Dermatol Ther (Heidelb). 2019;9:719-724.
- Scalvenzi M, Cappello M, Costa C, et al. Low-dose vismodegib as maintenance therapy after locally advanced basal cell carcinoma complete remission: high efficacy with minimal toxicity. Dermatol Ther (Heidelb). 2020;10:465-468.
Basal cell carcinoma (BCC) is the most common keratinocyte carcinoma and affects more than 3 million individuals per year in the United States.1 Approximately 40% of patients diagnosed with BCC will develop another BCC within 5 years of the initial diagnosis.2 Most cases are successfully treated with surgical excision and occasionally topical therapy or radiotherapy. Despite the high cure rate with conventional treatments, BCC can recur and can cause substantial destruction of the surrounding tissue if left untreated.3-5 In some instances, BCC can even metastasize and lead to death.6 For patients who are poor candidates for surgical or topical treatment modalities because of locally advanced BCC (laBCC) or metastatic BCC (mBCC), systemic treatment may be indicated. Vismodegib, sonidegib, and cemiplimab are the only systemic medications approved by the US Food and Drug Administration (FDA) for the treatment of laBCC and/or mBCC. Vismodegib and sonidegib target the sonic hedgehog (SHH) signaling pathway that is abnormally activated in more than 90% of BCCs.7 Cemiplimab is an immune checkpoint inhibitor (ICI) that targets the programmed cell death protein 1 (PD-1) receptor.8 Herein, we review the clinical utility of these medications and their evolving roles in the treatment of BCC.
SHH Pathway Inhibitors
The SHH pathway is a key regulator of cell proliferation and differentiation during embryogenesis.7 During adulthood, SHH signaling decreases but still plays an important role in stem cell activation and in regulation of the hair follicle growth cycle.9,10 However, de novo mutations in the genes that comprise the SHH pathway can result in aberrant constitutive activation, leading to unrestricted cell proliferation. Genetic mutations resulting in activation of Smoothened (SMO), a G-protein–coupled receptor involved in the signal transduction and propagation of the SHH pathway, have been implicated in the pathogenesis of BCC. Inactivating mutations also are commonly observed in patched homolog 1, an upstream cell-surface protein that inhibits SMO.7 The mechanism by which vismodegib and sonidegib, 2 of the FDA-approved oral medications for the treatment of advanced BCC, block the SHH pathway is through the selective inhibition of SMO.7,11
Vismodegib first received FDA approval in 2012 for the treatment of laBCC and mBCC after initial results from the pivotal ERIVANCE phase 2 trial demonstrated an objective response rate (ORR) of 43% (27/63) and 30% (10/33) in patients with locally advanced and metastatic disease, respectively. In this single-arm study, all enrolled patients (63 with laBCC and 33 with mBCC) received 150 mg of oral vismodegib daily.12 Updated results at 39 months demonstrated improved ORRs of 60% (38/63) and 48% (16/33) for the laBCC and mBCC groups, respectively. A complete response (CR) and partial response (PR) were observed in 32% (n=20) and 29% (n=18) of patients with laBCC, respectively.13 These results have been confirmed in subsequent studies, including the large international open-label trial known as STEVIE, with ORRs of 68.5% for 1119 cases of laBCC and 37% for 96 cases of mBCC.14-17 The CR and PR rates were 33% and 35%, respectively, for the laBCC group. The CR and PR rates for the mBCC group were 5% and 32%, respectively.14
The FDA approval of sonidegib for laBCC—but not mBCC—occurred in 2015 after the pivotal BOLT randomized phase 2 trial demonstrated an initial ORR of 43% (18/42) for laBCC and 15% (2/13) for mBCC after administration of 200 mg of sonidegib daily.18 A final follow-up analysis at 42 months resulted in ORRs of 56% (37/66) and 8% (1/13) for the laBCC and mBCC groups, respectively.19 Additionally, improved efficacy was not observed in the 151 patients who were randomized to receive treatment with the higher 800-mg dose; however, they did experience a higher incidence of adverse events.18,19
Currently, the true clinical differences between vismodegib and sonidegib remain uncertain, as no head-to-head trials have been conducted. Moreover, direct comparison of the data from the ERIVANCE and BOLT trials is challenging owing to fundamental differences in methodologic design, including the criteria used to assess BCC severity. The ERIVANCE trial utilized the conventional Response Evaluation Criteria in Solid Tumors (RECIST), while BOLT used the rigorous modified RECIST. However, an expert consensus study attempted to compare the 2 trials by modifying the outcomes from BOLT with the former RECIST criteria. The expert group found that the 2 SHH inhibitors had comparable efficacy and adverse event profiles.20 Nevertheless, a recent meta-analysis found that although ORRs for laBCC were similar between the 2 drugs, the CR rate for vismodegib was 31% compared with 3% for sonidegib. Additionally, for mBCC, they reported the ORR of vismodegib to be 2.7 times higher than that of sonidegib (39% vs 15%).21
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors have successfully been utilized in the treatment of cutaneous squamous cell carcinoma (cSCC); however, their use for treating BCC has been limited until recently.22-25 In February 2021, cemiplimab became the first and only ICI approved for the treatment of laBCC and mBCC in patients who did not respond to or were intolerant to prior SHH inhibitor therapy.26 Cemiplimab—a human monoclonal antibody against the PD-1 receptor expressed on T cells—blocks its interaction with programmed cell death ligand 1 and programmed cell death ligand 2 present on tumor cells. The blockade of the PD-1 pathway releases the inhibition of the antitumor immune response and enables appropriate cytotoxic T-cell activity to occur.8
The FDA approval of cemiplimab for the treatment of advanced BCC was based on an open-label, multicenter, single-arm phase 2 trial (NCT03132636) evaluating 84 patients with laBCC refractory or intolerant to SHH inhibitor therapy.26 Patients received an intravenous infusion of cemiplimab 350 mg every 3 weeks for up to 93 weeks or until disease progression or unacceptable toxicity. An ORR of 31% (26/84) was observed with a CR and PR of 6% (5/84) and 25% (21/84), respectively. The median duration of follow-up was 15 months.26 Given the clinically meaningful results of this trial, investigating the efficacy of other PD-1 inhibitors, such as pembrolizumab and nivolumab, for treatment of advanced BCC may prove worthwhile.
Adverse Effects of Systemic Treatments
The 2 approved SHH inhibitors—vismodegib and sonidegib—appear to have similar side-effect profiles, with the most common adverse effects being muscle spasms, dysgeusia, alopecia, nausea, vomiting, diarrhea, weight loss, and fatigue.20,21,27 These side effects occur at high frequencies (>40%) for both SHH inhibitors and often lead to discontinuation of the medication.21 Rates of treatment discontinuation range from 15% to 50% on average.12-14,18 Fortunately, the majority of these adverse effects do not appear to increase in severity or frequency with prolonged use of these medications.14,16,28
Various conservative and pharmacologic measures can be implemented to help manage side effects. For muscle spasms, which are the most commonly reported adverse effect, supplementation with magnesium, transcutaneous electrical nerve stimulation, acupuncture, massages, stretching, and thermal compresses can potentially be beneficial.29 Calcium channel blockers also may be effective, as one small prospective cohort study reported a reduction in the frequency of muscle cramps with amlodipine 10 mg daily.30 For alopecia, which typically is reversible and caused by SHH inhibition of the normal hair cycle, minoxidil theoretically can help, as it reduces telogen arrest and extends the anagen growth phase.31,32 Although usually mild and self-limiting, management of dysgeusia, weight loss, and gastrointestinal upset often can be managed with dietary changes, such as smaller, more frequent meals.33,34 Finally, alternative dosing strategies and drug holidays have been employed to mitigate these side effects and increase drug tolerability.35,36 These are discussed in the Alternative Dosing section.
Given the essential role of the SHH pathway in embryologic development, SHH inhibitors carry a black box warning of embryofetal teratogenicity and are contraindicated in females who are pregnant or breastfeeding. For females of reproductive potential, verification of pregnancy status should be performed prior to initiating treatment with an SHH inhibitor. These patients should be counseled on the use of contraception during treatment and for at least 24 months and 20 months after cessation of vismodegib and sonidegib, respectively.27,37,38 Male patients, even after a vasectomy, should use barrier contraception during treatment and for at least 3 months and 8 months after the final dose of vismodegib and sonidegib, respectively.37,38
Laboratory abnormalities commonly associated with SHH inhibitors include elevated hepatic enzymes, particularly with vismodegib, and elevated creatine kinase levels, particularly with sonidegib.28,39 Other laboratory abnormalities that can occur include hypercholesterolemia, hypercreatininemia, hyperglycemia, and increased serum lipase levels.19,28 Although these laboratory abnormalities usually are asymptomatic and self-limiting, regular monitoring should be performed.
There also is concern that SHH inhibitors may induce the development of cSCC. A case-control study of 55 cases and 125 control patients found an increased risk for cSCC in those previously treated with vismodegib, with a hazard ratio of 8.12.40 However, a subsequent retrospective cohort study of 1675 patients with BCC failed to find any association with cSCC among those treated with vismodegib compared to those who received standard surgical therapy.41 Clinical data for sonidegib are lacking, but the BOLT trial found that cSCC occurred in 3 patients receiving treatment with the SHH inhibitor.18 Thus, further studies are needed to more thoroughly assess this concern. Close monitoring for cSCC may be warranted in patients prescribed SHH inhibitors at this time.
Cemiplimab has demonstrated an acceptable safety profile and is generally well tolerated. In the phase 2 trial of cemiplimab for cSCC, approximately 5% of patients discontinued treatment because of adverse effects. The most commonly reported side effects of cemiplimab were diarrhea (27%), fatigue (24%), nausea (17%), constipation (15%), and rash (15%).23 In the phase 2 trial for laBCC, grade 3 or 4 adverse events occurred in 48% of patients, with hypertension (5%) being the most common.26 Although rare, immune-mediated adverse reactions also can occur, given the mechanism of action of ICIs. These side effects, ranging in severity from mild to fatal, include pneumonitis, colitis, hepatitis, nephritis, myocarditis, and hypophysitis. Therefore, close monitoring for these immune-mediated reactions is critical, but most can be managed with corticosteroids or treatment interruption if they occur.42,43
No absolute contraindications exist for cemiplimab; however, extreme caution should be taken in immunosuppressed individuals, such as solid organ transplant recipients and those with chronic lymphocytic leukemia (CLL), as safety data are limited in these patients.44,45 Although small retrospective studies have reported reasonable tolerability in solid organ transplant recipients treated with ICIs, an allograft rejection rate of 41% was found in a meta-analysis of 64 patients.46-48 In CLL patients with keratinocyte carcinomas, ICIs have been safely used and have even demonstrated efficacy for CLL in some cases.49-52
Alternative Dosing
The side effects of SHH inhibitors have led to alternative dosing strategies to prevent medication discontinuation and improve adherence. In patients with basal cell nevus syndrome, multimonth drug holidays have been shown to increase drug tolerability without compromising efficacy.35,36 Weekly intermittent dosing regimens of vismodegib ranging from 1 week on followed by 1 to 3 weeks off demonstrated efficacy in a retrospective study of 7 patients with advanced BCC.53 All 7 patients experienced improvement in their BCCs, with 3 patients experiencing CR. Importantly, treatment-related adverse effects were mild and well tolerated, with no patients terminating the medication.53 Two other retrospective case series of patients with advanced BCC treated with vismodegib reported similar findings for those placed on an intermittent dosing schedule ranging from once every other day to once per week.54,55
In the large phase 2 randomized trial known as MIKIE, 2 different intermittent dosing regimens of 150 mg vismodegib daily for patients with multiple BCCs were found to have good activity and tolerability.56 The first group (n=116) received vismodegib for 12 weeks, then 3 rounds of 8 weeks of placebo, followed by 12 weeks of vismodegib; there was a 63% reduction in clinically evident BCCs after 73 weeks. The second group (n=113) received the medication for 24 weeks, then 3 rounds of 8 weeks of placebo, followed by 8 weeks of vismodegib; there was a 54% reduction at the end of 73 weeks.56 Subsequent analyses found improvements in health-related quality-of-life outcomes that were similar for both groups.57
Consequently, alternative dosing schedules appear to be a viable option for patients at risk of discontinuing treatment because of adverse effects, and current data support the recently approved recommendations of dose interruptions of up to 8 weeks to manage adverse effects in patients with laBCC or mBCC.58 Nevertheless, further clinical studies are required to determine the optimal intermittent dosing regimen for patients treated with SHH inhibitors.
Neoadjuvant Administration
Recently, vismodegib has been studied as a neoadjuvant therapy for BCC with promising results. Several small retrospective studies and case reports have documented successful treatment of both operable and inoperable periocular laBCC, with preservation of the eye in all patients.59-61 An open-label trial of 15 patients with advanced BCC who received neoadjuvant vismodegib for 3 to 6 months prior to surgical excision reported a mean reduction of 35% in the final surgical defect size, with no recurrence at 22 months.62,63 The latest and largest study performed was a phase 2 open-label trial known as VISMONEO, where 44 of 55 laBCC patients (80%) receiving neoadjuvant vismodegib for a mean duration of 6 months (range, 4–10 months) achieved the primary end point of tumor surgical downstaging.64 Of the 44 patients who had tumor downstaging, 27 (61%) experienced histologically proven CRs. Additionally, a 66% reduction in the average target lesion size was reported in this group compared to29% in the 11 patients who did not have tumor downstaging (P=.0002).64 Thus, SHH inhibitors may hold an important neoadjuvant role in the treatment of BCC by decreasing surgical defect size and allowing for surgical management of previously inoperable cases.
Synergism With Radiation
Preliminary data suggest SHH inhibitors may help potentiate the effects of radiation therapy for the treatment of BCC. Currently, the evidence primarily is limited to case studies, with several reports describing complete remission in patients with advanced BCCs who were considered unsuitable candidates for surgery. In these cases, vismodegib was administered either prior to or concurrently with radiation treatment.65-69 An in vitro study also documented the radiation-sensitizing effects of vismodegib in a BCC cell line.70 Recently, a phase 2 trial (ClinicalTrials.gov identifier NCT01835626) evaluating the concurrent use of vismodegib and radiotherapy for patients with advanced BCC was completed, but data has yet to be published.
Synergism With and Benefit of Antifungal Therapy
The antifungal drug itraconazole is a potent inhibitor of the SHH pathway and may have an adjunctive role in the treatment of BCC. Similar to vismodegib and sonidegib, itraconazole acts as a direct antagonist of SMO. However, it is thought to bind to a distinct site on SMO.71,72 An open-label, exploratory phase 2 trial of 19 patients with BCC found that oral itraconazole 200 to 400 mg daily decreased tumor proliferative index by 45% (P=.04), as measured by Ki-67; SHH activity by 65% (P=.03), as measured by GLI1 messenger RNA; and mean tumor area by 24%.73 In a case series of 5 patients with mBCC refractory to conventional SHH inhibitor therapy, combined treatment with itraconazole and arsenic trioxide resulted in stable disease and a 75% reduction in SHH activity (P<.001).74 One case report documented tumor regression leading to stable disease for 15 months in a patient with laBCC treated with itraconazole monotherapy due to being unable to afford vismodegib or sonidegib. However, within 2 months of treatment discontinuation, the lesion progressed considerably.75 The efficacy of a topical formulation of itraconazole also has been tested in an open-label, placebo-controlled phase 2 trial, but no benefit was observed.76
Posaconazole is a second-generation antifungal agent that may serve as a potential alternative to itraconazole.77 Although clinical data are lacking, a basic science study found that posaconazole could inhibit the growth of SHH-dependent BCC in vivo (in mice).78 Furthermore, posaconazole has demonstrated a better safety profile with fewer and more mild side effects than itraconazole and does not require dose adjustment for those with hepatic or renal failure.79,80 Thus, posaconazole may be a safer alternative to itraconazole for the treatment of BCC. Further clinical studies are needed to elucidate the potential synergistic effects of these antifungal agents with the 2 currently approved SHH inhibitors for the treatment of advanced BCC.
Drug Resistance
Treatment resistance to SHH inhibitors, though uncommon, is a growing concern. Acquired mutations in the SMO binding site or downstream mediators of the SHH pathway have been shown to confer resistance to vismodegib and sonidegib.72,81-83 In addition, it appears that there may be shared resistance among the drugs in this class. One study assessing the efficacy of sonidegib in 9 patients with laBCC resistant to vismodegib found that these patients also did not respond to sonidegib.84 Interestingly, 1 case report documented tumor regression of an intracranial BCC in a patient treated with sonidegib and itraconazole after failure with vismodegib.85 An in vitro study also found that itraconazole maintained SHH inhibitory activity for all drug-resistant SMO mutations that have been reported.72 Therefore, itraconazole monotherapy or combination therapy with a canonical SHH inhibitor may be considered for patients with recalcitrant BCC and warrants further investigation.
Taladegib is a newly developed SMO inhibitor that may serve as another promising alternative for patients who develop resistance to vismodegib or sonidegib. A phase 1 trial of taladegib for advanced BCC found an ORR of 69% (11/16) in the SHH inhibitor–naïve group and an ORR of 36% (11/32) in the group previously treated with a SHH inhibitor.86 Additionally, the safety profile and frequency of adverse effects appear to be similar to those associated with vismodegib and sonidegib.86,87 Unfortunately, no clinical trials evaluating taladegib for BCC are ongoing or in development at this time.
Recurrence
There appears to be a relatively high rate of recurrence for BCC patients who achieve a CR to SHH inhibitors. In a retrospective study of 116 laBCC patients who experienced a CR after vismodegib therapy, 54 patients (47%) relapsed at 36 months. Among the 54 patients that relapsed, 27 were re-treated with vismodegib, which resulted in an ORR of 85% (23/27), a CR rate of 37% (10/27), and a PR rate of 48% (13/27).88 Another retrospective study of 35 laBCC patients who relapsed after vismodegib treatment reported a 31% (11/35) clinical recurrence rate at 6-month follow-up.89 An observational retrospective study also assessed the efficacy of SHH inhibitor maintenance therapy for advanced BCC patients who achieved a CR.90 In the study, 27 (64%) patients received a maintenance dose of 150 mg vismodegib once per week for 1 year, while 15 (36%) patients decided not to take a maintenance dose following CR of their BCC. All patients who took the maintenance therapy did not experience clinical recurrence at 1-year follow-up, whereas 26% of patients not on the maintenance dose relapsed.90 Consequently, these results indicate that BCC recurrence is frequent after SHH inhibitor therapy and highlights the importance of close surveillance after CR is attained. Nevertheless, most patients still respond to treatment with SHH inhibitors after relapsing, and intermittent maintenance doses may be an effective means to reduce risk of recurrence.
Conclusion
Vismodegib and sonidegib are SHH inhibitors approved for the treatment of laBCC and mBCC. Cemiplimab is now also approved for patients who do not respond to SHH inhibitors or for whom SHH inhibitors are not tolerable. Although these systemic targeted therapies can lead to notable tumor shrinkage and even complete regression in some patients, recurrence is common, and adverse effects may limit their use. Drug resistance is an emerging issue that requires additional examination. Further clinical studies are needed to determine which patients are likely to respond to these targeted treatments.
Various intermittent and maintenance drug regimens should be evaluated for their potential to mitigate adverse effects and reduce risk of recurrence, respectively. The synergistic effects of these medications with other therapies as well as their neoadjuvant and adjuvant roles should be further investigated. For example, administration of an SHH inhibitor prior to surgical excision of a BCC may allow for a smaller surgical defect size, thereby improving cosmetic and functional outcomes. Moreover, these systemic targeted medications may allow for previously inoperable tumors to become amenable to surgical treatment.
Although SHH inhibitors and PD-1 inhibitors represent a major advancement in the field of oncodermatology, real-world efficacy and safety data in the upcoming years will be important for elucidating their true benefit for patients with BCC.
Basal cell carcinoma (BCC) is the most common keratinocyte carcinoma and affects more than 3 million individuals per year in the United States.1 Approximately 40% of patients diagnosed with BCC will develop another BCC within 5 years of the initial diagnosis.2 Most cases are successfully treated with surgical excision and occasionally topical therapy or radiotherapy. Despite the high cure rate with conventional treatments, BCC can recur and can cause substantial destruction of the surrounding tissue if left untreated.3-5 In some instances, BCC can even metastasize and lead to death.6 For patients who are poor candidates for surgical or topical treatment modalities because of locally advanced BCC (laBCC) or metastatic BCC (mBCC), systemic treatment may be indicated. Vismodegib, sonidegib, and cemiplimab are the only systemic medications approved by the US Food and Drug Administration (FDA) for the treatment of laBCC and/or mBCC. Vismodegib and sonidegib target the sonic hedgehog (SHH) signaling pathway that is abnormally activated in more than 90% of BCCs.7 Cemiplimab is an immune checkpoint inhibitor (ICI) that targets the programmed cell death protein 1 (PD-1) receptor.8 Herein, we review the clinical utility of these medications and their evolving roles in the treatment of BCC.
SHH Pathway Inhibitors
The SHH pathway is a key regulator of cell proliferation and differentiation during embryogenesis.7 During adulthood, SHH signaling decreases but still plays an important role in stem cell activation and in regulation of the hair follicle growth cycle.9,10 However, de novo mutations in the genes that comprise the SHH pathway can result in aberrant constitutive activation, leading to unrestricted cell proliferation. Genetic mutations resulting in activation of Smoothened (SMO), a G-protein–coupled receptor involved in the signal transduction and propagation of the SHH pathway, have been implicated in the pathogenesis of BCC. Inactivating mutations also are commonly observed in patched homolog 1, an upstream cell-surface protein that inhibits SMO.7 The mechanism by which vismodegib and sonidegib, 2 of the FDA-approved oral medications for the treatment of advanced BCC, block the SHH pathway is through the selective inhibition of SMO.7,11
Vismodegib first received FDA approval in 2012 for the treatment of laBCC and mBCC after initial results from the pivotal ERIVANCE phase 2 trial demonstrated an objective response rate (ORR) of 43% (27/63) and 30% (10/33) in patients with locally advanced and metastatic disease, respectively. In this single-arm study, all enrolled patients (63 with laBCC and 33 with mBCC) received 150 mg of oral vismodegib daily.12 Updated results at 39 months demonstrated improved ORRs of 60% (38/63) and 48% (16/33) for the laBCC and mBCC groups, respectively. A complete response (CR) and partial response (PR) were observed in 32% (n=20) and 29% (n=18) of patients with laBCC, respectively.13 These results have been confirmed in subsequent studies, including the large international open-label trial known as STEVIE, with ORRs of 68.5% for 1119 cases of laBCC and 37% for 96 cases of mBCC.14-17 The CR and PR rates were 33% and 35%, respectively, for the laBCC group. The CR and PR rates for the mBCC group were 5% and 32%, respectively.14
The FDA approval of sonidegib for laBCC—but not mBCC—occurred in 2015 after the pivotal BOLT randomized phase 2 trial demonstrated an initial ORR of 43% (18/42) for laBCC and 15% (2/13) for mBCC after administration of 200 mg of sonidegib daily.18 A final follow-up analysis at 42 months resulted in ORRs of 56% (37/66) and 8% (1/13) for the laBCC and mBCC groups, respectively.19 Additionally, improved efficacy was not observed in the 151 patients who were randomized to receive treatment with the higher 800-mg dose; however, they did experience a higher incidence of adverse events.18,19
Currently, the true clinical differences between vismodegib and sonidegib remain uncertain, as no head-to-head trials have been conducted. Moreover, direct comparison of the data from the ERIVANCE and BOLT trials is challenging owing to fundamental differences in methodologic design, including the criteria used to assess BCC severity. The ERIVANCE trial utilized the conventional Response Evaluation Criteria in Solid Tumors (RECIST), while BOLT used the rigorous modified RECIST. However, an expert consensus study attempted to compare the 2 trials by modifying the outcomes from BOLT with the former RECIST criteria. The expert group found that the 2 SHH inhibitors had comparable efficacy and adverse event profiles.20 Nevertheless, a recent meta-analysis found that although ORRs for laBCC were similar between the 2 drugs, the CR rate for vismodegib was 31% compared with 3% for sonidegib. Additionally, for mBCC, they reported the ORR of vismodegib to be 2.7 times higher than that of sonidegib (39% vs 15%).21
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors have successfully been utilized in the treatment of cutaneous squamous cell carcinoma (cSCC); however, their use for treating BCC has been limited until recently.22-25 In February 2021, cemiplimab became the first and only ICI approved for the treatment of laBCC and mBCC in patients who did not respond to or were intolerant to prior SHH inhibitor therapy.26 Cemiplimab—a human monoclonal antibody against the PD-1 receptor expressed on T cells—blocks its interaction with programmed cell death ligand 1 and programmed cell death ligand 2 present on tumor cells. The blockade of the PD-1 pathway releases the inhibition of the antitumor immune response and enables appropriate cytotoxic T-cell activity to occur.8
The FDA approval of cemiplimab for the treatment of advanced BCC was based on an open-label, multicenter, single-arm phase 2 trial (NCT03132636) evaluating 84 patients with laBCC refractory or intolerant to SHH inhibitor therapy.26 Patients received an intravenous infusion of cemiplimab 350 mg every 3 weeks for up to 93 weeks or until disease progression or unacceptable toxicity. An ORR of 31% (26/84) was observed with a CR and PR of 6% (5/84) and 25% (21/84), respectively. The median duration of follow-up was 15 months.26 Given the clinically meaningful results of this trial, investigating the efficacy of other PD-1 inhibitors, such as pembrolizumab and nivolumab, for treatment of advanced BCC may prove worthwhile.
Adverse Effects of Systemic Treatments
The 2 approved SHH inhibitors—vismodegib and sonidegib—appear to have similar side-effect profiles, with the most common adverse effects being muscle spasms, dysgeusia, alopecia, nausea, vomiting, diarrhea, weight loss, and fatigue.20,21,27 These side effects occur at high frequencies (>40%) for both SHH inhibitors and often lead to discontinuation of the medication.21 Rates of treatment discontinuation range from 15% to 50% on average.12-14,18 Fortunately, the majority of these adverse effects do not appear to increase in severity or frequency with prolonged use of these medications.14,16,28
Various conservative and pharmacologic measures can be implemented to help manage side effects. For muscle spasms, which are the most commonly reported adverse effect, supplementation with magnesium, transcutaneous electrical nerve stimulation, acupuncture, massages, stretching, and thermal compresses can potentially be beneficial.29 Calcium channel blockers also may be effective, as one small prospective cohort study reported a reduction in the frequency of muscle cramps with amlodipine 10 mg daily.30 For alopecia, which typically is reversible and caused by SHH inhibition of the normal hair cycle, minoxidil theoretically can help, as it reduces telogen arrest and extends the anagen growth phase.31,32 Although usually mild and self-limiting, management of dysgeusia, weight loss, and gastrointestinal upset often can be managed with dietary changes, such as smaller, more frequent meals.33,34 Finally, alternative dosing strategies and drug holidays have been employed to mitigate these side effects and increase drug tolerability.35,36 These are discussed in the Alternative Dosing section.
Given the essential role of the SHH pathway in embryologic development, SHH inhibitors carry a black box warning of embryofetal teratogenicity and are contraindicated in females who are pregnant or breastfeeding. For females of reproductive potential, verification of pregnancy status should be performed prior to initiating treatment with an SHH inhibitor. These patients should be counseled on the use of contraception during treatment and for at least 24 months and 20 months after cessation of vismodegib and sonidegib, respectively.27,37,38 Male patients, even after a vasectomy, should use barrier contraception during treatment and for at least 3 months and 8 months after the final dose of vismodegib and sonidegib, respectively.37,38
Laboratory abnormalities commonly associated with SHH inhibitors include elevated hepatic enzymes, particularly with vismodegib, and elevated creatine kinase levels, particularly with sonidegib.28,39 Other laboratory abnormalities that can occur include hypercholesterolemia, hypercreatininemia, hyperglycemia, and increased serum lipase levels.19,28 Although these laboratory abnormalities usually are asymptomatic and self-limiting, regular monitoring should be performed.
There also is concern that SHH inhibitors may induce the development of cSCC. A case-control study of 55 cases and 125 control patients found an increased risk for cSCC in those previously treated with vismodegib, with a hazard ratio of 8.12.40 However, a subsequent retrospective cohort study of 1675 patients with BCC failed to find any association with cSCC among those treated with vismodegib compared to those who received standard surgical therapy.41 Clinical data for sonidegib are lacking, but the BOLT trial found that cSCC occurred in 3 patients receiving treatment with the SHH inhibitor.18 Thus, further studies are needed to more thoroughly assess this concern. Close monitoring for cSCC may be warranted in patients prescribed SHH inhibitors at this time.
Cemiplimab has demonstrated an acceptable safety profile and is generally well tolerated. In the phase 2 trial of cemiplimab for cSCC, approximately 5% of patients discontinued treatment because of adverse effects. The most commonly reported side effects of cemiplimab were diarrhea (27%), fatigue (24%), nausea (17%), constipation (15%), and rash (15%).23 In the phase 2 trial for laBCC, grade 3 or 4 adverse events occurred in 48% of patients, with hypertension (5%) being the most common.26 Although rare, immune-mediated adverse reactions also can occur, given the mechanism of action of ICIs. These side effects, ranging in severity from mild to fatal, include pneumonitis, colitis, hepatitis, nephritis, myocarditis, and hypophysitis. Therefore, close monitoring for these immune-mediated reactions is critical, but most can be managed with corticosteroids or treatment interruption if they occur.42,43
No absolute contraindications exist for cemiplimab; however, extreme caution should be taken in immunosuppressed individuals, such as solid organ transplant recipients and those with chronic lymphocytic leukemia (CLL), as safety data are limited in these patients.44,45 Although small retrospective studies have reported reasonable tolerability in solid organ transplant recipients treated with ICIs, an allograft rejection rate of 41% was found in a meta-analysis of 64 patients.46-48 In CLL patients with keratinocyte carcinomas, ICIs have been safely used and have even demonstrated efficacy for CLL in some cases.49-52
Alternative Dosing
The side effects of SHH inhibitors have led to alternative dosing strategies to prevent medication discontinuation and improve adherence. In patients with basal cell nevus syndrome, multimonth drug holidays have been shown to increase drug tolerability without compromising efficacy.35,36 Weekly intermittent dosing regimens of vismodegib ranging from 1 week on followed by 1 to 3 weeks off demonstrated efficacy in a retrospective study of 7 patients with advanced BCC.53 All 7 patients experienced improvement in their BCCs, with 3 patients experiencing CR. Importantly, treatment-related adverse effects were mild and well tolerated, with no patients terminating the medication.53 Two other retrospective case series of patients with advanced BCC treated with vismodegib reported similar findings for those placed on an intermittent dosing schedule ranging from once every other day to once per week.54,55
In the large phase 2 randomized trial known as MIKIE, 2 different intermittent dosing regimens of 150 mg vismodegib daily for patients with multiple BCCs were found to have good activity and tolerability.56 The first group (n=116) received vismodegib for 12 weeks, then 3 rounds of 8 weeks of placebo, followed by 12 weeks of vismodegib; there was a 63% reduction in clinically evident BCCs after 73 weeks. The second group (n=113) received the medication for 24 weeks, then 3 rounds of 8 weeks of placebo, followed by 8 weeks of vismodegib; there was a 54% reduction at the end of 73 weeks.56 Subsequent analyses found improvements in health-related quality-of-life outcomes that were similar for both groups.57
Consequently, alternative dosing schedules appear to be a viable option for patients at risk of discontinuing treatment because of adverse effects, and current data support the recently approved recommendations of dose interruptions of up to 8 weeks to manage adverse effects in patients with laBCC or mBCC.58 Nevertheless, further clinical studies are required to determine the optimal intermittent dosing regimen for patients treated with SHH inhibitors.
Neoadjuvant Administration
Recently, vismodegib has been studied as a neoadjuvant therapy for BCC with promising results. Several small retrospective studies and case reports have documented successful treatment of both operable and inoperable periocular laBCC, with preservation of the eye in all patients.59-61 An open-label trial of 15 patients with advanced BCC who received neoadjuvant vismodegib for 3 to 6 months prior to surgical excision reported a mean reduction of 35% in the final surgical defect size, with no recurrence at 22 months.62,63 The latest and largest study performed was a phase 2 open-label trial known as VISMONEO, where 44 of 55 laBCC patients (80%) receiving neoadjuvant vismodegib for a mean duration of 6 months (range, 4–10 months) achieved the primary end point of tumor surgical downstaging.64 Of the 44 patients who had tumor downstaging, 27 (61%) experienced histologically proven CRs. Additionally, a 66% reduction in the average target lesion size was reported in this group compared to29% in the 11 patients who did not have tumor downstaging (P=.0002).64 Thus, SHH inhibitors may hold an important neoadjuvant role in the treatment of BCC by decreasing surgical defect size and allowing for surgical management of previously inoperable cases.
Synergism With Radiation
Preliminary data suggest SHH inhibitors may help potentiate the effects of radiation therapy for the treatment of BCC. Currently, the evidence primarily is limited to case studies, with several reports describing complete remission in patients with advanced BCCs who were considered unsuitable candidates for surgery. In these cases, vismodegib was administered either prior to or concurrently with radiation treatment.65-69 An in vitro study also documented the radiation-sensitizing effects of vismodegib in a BCC cell line.70 Recently, a phase 2 trial (ClinicalTrials.gov identifier NCT01835626) evaluating the concurrent use of vismodegib and radiotherapy for patients with advanced BCC was completed, but data has yet to be published.
Synergism With and Benefit of Antifungal Therapy
The antifungal drug itraconazole is a potent inhibitor of the SHH pathway and may have an adjunctive role in the treatment of BCC. Similar to vismodegib and sonidegib, itraconazole acts as a direct antagonist of SMO. However, it is thought to bind to a distinct site on SMO.71,72 An open-label, exploratory phase 2 trial of 19 patients with BCC found that oral itraconazole 200 to 400 mg daily decreased tumor proliferative index by 45% (P=.04), as measured by Ki-67; SHH activity by 65% (P=.03), as measured by GLI1 messenger RNA; and mean tumor area by 24%.73 In a case series of 5 patients with mBCC refractory to conventional SHH inhibitor therapy, combined treatment with itraconazole and arsenic trioxide resulted in stable disease and a 75% reduction in SHH activity (P<.001).74 One case report documented tumor regression leading to stable disease for 15 months in a patient with laBCC treated with itraconazole monotherapy due to being unable to afford vismodegib or sonidegib. However, within 2 months of treatment discontinuation, the lesion progressed considerably.75 The efficacy of a topical formulation of itraconazole also has been tested in an open-label, placebo-controlled phase 2 trial, but no benefit was observed.76
Posaconazole is a second-generation antifungal agent that may serve as a potential alternative to itraconazole.77 Although clinical data are lacking, a basic science study found that posaconazole could inhibit the growth of SHH-dependent BCC in vivo (in mice).78 Furthermore, posaconazole has demonstrated a better safety profile with fewer and more mild side effects than itraconazole and does not require dose adjustment for those with hepatic or renal failure.79,80 Thus, posaconazole may be a safer alternative to itraconazole for the treatment of BCC. Further clinical studies are needed to elucidate the potential synergistic effects of these antifungal agents with the 2 currently approved SHH inhibitors for the treatment of advanced BCC.
Drug Resistance
Treatment resistance to SHH inhibitors, though uncommon, is a growing concern. Acquired mutations in the SMO binding site or downstream mediators of the SHH pathway have been shown to confer resistance to vismodegib and sonidegib.72,81-83 In addition, it appears that there may be shared resistance among the drugs in this class. One study assessing the efficacy of sonidegib in 9 patients with laBCC resistant to vismodegib found that these patients also did not respond to sonidegib.84 Interestingly, 1 case report documented tumor regression of an intracranial BCC in a patient treated with sonidegib and itraconazole after failure with vismodegib.85 An in vitro study also found that itraconazole maintained SHH inhibitory activity for all drug-resistant SMO mutations that have been reported.72 Therefore, itraconazole monotherapy or combination therapy with a canonical SHH inhibitor may be considered for patients with recalcitrant BCC and warrants further investigation.
Taladegib is a newly developed SMO inhibitor that may serve as another promising alternative for patients who develop resistance to vismodegib or sonidegib. A phase 1 trial of taladegib for advanced BCC found an ORR of 69% (11/16) in the SHH inhibitor–naïve group and an ORR of 36% (11/32) in the group previously treated with a SHH inhibitor.86 Additionally, the safety profile and frequency of adverse effects appear to be similar to those associated with vismodegib and sonidegib.86,87 Unfortunately, no clinical trials evaluating taladegib for BCC are ongoing or in development at this time.
Recurrence
There appears to be a relatively high rate of recurrence for BCC patients who achieve a CR to SHH inhibitors. In a retrospective study of 116 laBCC patients who experienced a CR after vismodegib therapy, 54 patients (47%) relapsed at 36 months. Among the 54 patients that relapsed, 27 were re-treated with vismodegib, which resulted in an ORR of 85% (23/27), a CR rate of 37% (10/27), and a PR rate of 48% (13/27).88 Another retrospective study of 35 laBCC patients who relapsed after vismodegib treatment reported a 31% (11/35) clinical recurrence rate at 6-month follow-up.89 An observational retrospective study also assessed the efficacy of SHH inhibitor maintenance therapy for advanced BCC patients who achieved a CR.90 In the study, 27 (64%) patients received a maintenance dose of 150 mg vismodegib once per week for 1 year, while 15 (36%) patients decided not to take a maintenance dose following CR of their BCC. All patients who took the maintenance therapy did not experience clinical recurrence at 1-year follow-up, whereas 26% of patients not on the maintenance dose relapsed.90 Consequently, these results indicate that BCC recurrence is frequent after SHH inhibitor therapy and highlights the importance of close surveillance after CR is attained. Nevertheless, most patients still respond to treatment with SHH inhibitors after relapsing, and intermittent maintenance doses may be an effective means to reduce risk of recurrence.
Conclusion
Vismodegib and sonidegib are SHH inhibitors approved for the treatment of laBCC and mBCC. Cemiplimab is now also approved for patients who do not respond to SHH inhibitors or for whom SHH inhibitors are not tolerable. Although these systemic targeted therapies can lead to notable tumor shrinkage and even complete regression in some patients, recurrence is common, and adverse effects may limit their use. Drug resistance is an emerging issue that requires additional examination. Further clinical studies are needed to determine which patients are likely to respond to these targeted treatments.
Various intermittent and maintenance drug regimens should be evaluated for their potential to mitigate adverse effects and reduce risk of recurrence, respectively. The synergistic effects of these medications with other therapies as well as their neoadjuvant and adjuvant roles should be further investigated. For example, administration of an SHH inhibitor prior to surgical excision of a BCC may allow for a smaller surgical defect size, thereby improving cosmetic and functional outcomes. Moreover, these systemic targeted medications may allow for previously inoperable tumors to become amenable to surgical treatment.
Although SHH inhibitors and PD-1 inhibitors represent a major advancement in the field of oncodermatology, real-world efficacy and safety data in the upcoming years will be important for elucidating their true benefit for patients with BCC.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Kasumagic-Halilovic E, Hasic M, Ovcina-Kurtovic N. A clinical study of basal cell carcinoma. Med Arch. 2019;73:394-398.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e952-966.e952.
- Laga AC, Schaefer IM, Sholl LM, et al. Metastatic basal cell carcinoma. Am J Clin Pathol. 2019;152:706-717.
- Rimkus TK, Carpenter RL, Qasem S, et al. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel). 2016;8:22.
- Burova E, Hermann A, Waite J, et al. Characterization of the anti–PD-1 antibody REGN2810 and its antitumor activity in human PD-1 knock-in mice. Mol Cancer Ther. 2017;16:861-870.
- Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172-180.
- Paladini RD, Saleh J, Qian C, et al. Modulation of hair growth with small molecule agonists of the hedgehog signaling pathway. J Invest Dermatol. 2005;125:638-646.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Sekulic A, Migden MR, Basset-Seguin N, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Cozzani R, Del Aguila R, Carrizo M, et al. Efficacy and safety profile of vismodegib in a real-world setting cohort of patients with advanced basal cell carcinoma in Argentina. Int J Dermatol. 2020;59:627-632.
- Spallone G, Sollena P, Ventura A, et al. Efficacy and safety of vismodegib treatment in patients with advanced basal cell carcinoma and multiple comorbidities. Dermatol Ther. 2019;32:E13108.
- Fosko SW, Chu MB, Armbrecht E, et al. Efficacy, rate of tumor response, and safety of a short course (12-24 weeks) of oral vismodegib in various histologic subtypes (infiltrative, nodular, and superficial) of high-risk or locally advanced basal cell carcinoma, in an open-label, prospective case series clinical trial. J Am Acad Dermatol. 2020;82:946-954.
- Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716-728.
- Dummer R, Guminksi A, Gutzmer R, et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br J Dermatol. 2020;182:1369-1378.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Xie P, Lefrançois P. Efficacy, safety, and comparison of sonic hedgehog inhibitors in basal cell carcinomas: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79:1089-1100.e1017.
- Gentzler R, Hall R, Kunk PR, et al. Beyond melanoma: inhibiting the PD-1/PD-L1 pathway in solid tumors. Immunotherapy. 2016;8:583-600.
- Guminski AD, Lim AML, Khushalani NI, et al. Phase 2 study of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with metastatic cutaneous squamous cell carcinoma (mCSCC; group 1): 12-month follow-up [abstract]. J Clin Oncol. 2019;37(15 suppl):9526.
- Grob JJ, Gonzalez Mendoza R, Basset-Seguin N, et al. Pembrolizumab for recurrent/metastatic cutaneous squamous cell carcinoma (cSCC): efficacy and safety results from the phase II KEYNOTE-629 study [abstract]. Ann Oncol. 2019;30 (suppl 5):v908.
- Maubec E, Boubaya M, Petrow P, et al. Pembrolizumab as first-line therapy in patients with unresectable cutaneous squamous cell carcinoma (cSCC): phase 2 results from CARSKIN [abstract]. J Clin Oncol. 2019;37(15 suppl):9547.
- Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:848-857.
- Carpenter RL, Ray H. Safety and tolerability of sonic hedgehog pathway inhibitors in cancer. Drug Saf. 2019;42:263-279.
- Villani A, Fabbrocini G, Costa C, et al. Sonidegib: safety and efficacy in treatment of advanced basal cell carcinoma. Dermatol Ther (Heidelb). 2020;10:401-412.
- Wright A, Sluka KA. Nonpharmacological treatments for musculoskeletal pain. Clin J Pain. 2001;17:33-46.
- Ally MS, Tang JY, Lindgren J, et al. Effect of calcium channel blockade on vismodegib-induced muscle cramps. JAMA Dermatol. 2015;151:1132-1134.
- Yang X, Thai K-E. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil. Australas J Dermatol. 2016;57:e130-e132.
- Ferguson JS, Hannam S, Toholka R, et al. Hair loss and hedgehog inhibitors: a class effect? Br J Dermatol. 2015;173:262-264.
- Kumbargere Nagraj S, George RP, Shetty N, et al. Interventions for managing taste disturbances. Cochrane Database Syst Rev. 2017;12:CD010470.
- Jacobsen AA, Kydd AR, Strasswimmer J. Practical management of the adverse effects of hedgehog pathway inhibitor therapy for basal cell carcinoma. J Am Acad Dermatol. 2017;76:767-768.
- Ally MS, Tang JY, Joseph T, et al. The use of vismodegib to shrink keratocystic odontogenic tumors in patients with basal cell nevus syndrome. JAMA Dermatol. 2014;150:542-545.
- Yang X, Dinehart SM. Intermittent vismodegib therapy in basal cell nevus syndrome. JAMA Dermatol. 2016;152:223-224.
- Erivedge. Prescribing information. Genentech; 2015.
- Odomzo. Prescribing information. Novartis; 2015.
- Ventarola DJ, Silverstein DI. Vismodegib-associated hepatotoxicity: a potential side effect detected in postmarketing surveillance. J Am Acad Dermatol. 2014;71:397-398.
- Mohan SV, Chang J, Li S, et al. Increased risk of cutaneous squamous cell carcinoma after vismodegib therapy for basal cell carcinoma. JAMA Dermatol. 2016;152:527-532.
- Bhutani T, Abrouk M, Sima CS, et al. Risk of cutaneous squamous cell carcinoma after treatment of basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2017;77:713-718.
- Morgado M, Plácido A, Morgado S, et al. Management of the adverse effects of immune checkpoint inhibitors. Vaccines (Basel). 2020;8:575.
- Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563-580.
- Ntsethe A, Dludla PV, Nyambuya TM, et al. The impact of immune checkpoint inhibitors in patients with chronic lymphocytic leukemia (CLL): a protocol for a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99:E21167.
- Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904-1911.
- Tsung I, Worden FP, Fontana RJ. Safety and efficacy of checkpoint inhibitors in solid organ transplant recipients with cutaneous squamous cell carcinoma [abstract]. J Clin Oncol. 2020;38(15 suppl):E22014.
- Owoyemi I, Vaughan LE, Costello CM, et al. Clinical outcomes of solid organ transplant recipients with metastatic cancers who are treated with immune checkpoint inhibitors: a single-center analysis. Cancer. 2020;126:4780-4787.
- Kumar V, Shinagare AB, Rennke HG, et al. The safety and efficacy of checkpoint inhibitors in transplant recipients: a case series and systematic review of literature. Oncologist. 2020;25:505-514.
- Arenbergerova M, Fialova A, Arenberger P, et al. Killing two birds with one stone: response to pembrolizumab in a patient with metastatic melanoma and B-cell chronic lymphocytic leukaemia. J Eur Acad Dermatol Venereol. 2018;32:E72-E74.
- Archibald WJ, Meacham PJ, Williams AM, et al. Management of melanoma in patients with chronic lymphocytic leukemia. Leuk Res. 2018;71:43-46.
- Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129:3419-3427.
- Leiter U, Loquai C, Reinhardt L, et al. Immune checkpoint inhibition therapy for advanced skin cancer in patients with concomitant hematological malignancy: a retrospective multicenter DeCOG study of 84 patients. J Immunother Cancer. 2020;8:E000897.
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322.
- Woltsche N, Pichler N, Wolf I, et al. Managing adverse effects by dose reduction during routine treatment of locally advanced basal cell carcinoma with the hedgehog inhibitor vismodegib: a single centre experience. J Eur Acad Dermatol Venereol. 2019;33:E144-E145.
- Wong C, Poblete-Lopez C, Vidimos A. Comparison of daily dosing versus Monday through Friday dosing of vismodegib for locally advanced basal cell carcinoma and basal cell nevus syndrome: a retrospective case series. J Am Acad Dermatol. 2020;82:1539-1542.
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412.
- Schadendorf D, Hauschild A, Fosko S, et al. Quality-of-life analysis with intermittent vismodegib regimens in patients with multiple basal cell carcinomas: patient-reported outcomes from the MIKIE study. J Eur Acad Dermatol Venereol. 2020;34:E526-E529.
- Chanu P, Musib L, Wang X, et al. Vismodegib efficacy in advanced basal cell carcinoma maintained with 8-week dose interruptions: a model-based evaluation. J Invest Dermatol. 2021;141:930-933.
- Su MG, Potts LB, Tsai JH. Treatment of periocular basal cell carcinoma with neoadjuvant vismodegib. Am J Ophthalmol Case Rep. 2020;19:100755.
- González AR, Etchichury D, Gil ME, et al. Neoadjuvant vismodegib and Mohs micrographic surgery for locally advanced periocular basal cell carcinoma. Ophthalmic Plast Reconstr Surg. 2019;35:56-61.
- Sagiv O, Nagarajan P, Ferrarotto R, et al. Ocular preservation with neoadjuvant vismodegib in patients with locally advanced periocular basal cell carcinoma. Br J Ophthalmol. 2019;103:775-780.
- Ally MS, Aasi S, Wysong A, et al. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol. 2014;71:904-911.e1.
- Kwon GP, Ally MS, Bailey-Healy I, et al. Update to an open-label clinical trial of vismodegib as neoadjuvant before surgery for high-risk basal cell carcinoma (BCC). J Am Acad Dermatol. 2016;75:213-215.
- Mortier L, Bertrand N, Basset-Seguin N, et al. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: first results of a multicenter, open-label, phase 2 trial (VISMONEO study) [abstract]. J Clin Oncol. 2018;36(15 suppl):9509.
- Strasswimmer JM. Potential synergy of radiation therapy with vismodegib for basal cell carcinoma. JAMA Dermatol. 2015;151:925-926.
- Gathings RM, Orscheln CS, Huang WW. Compassionate use of vismodegib and adjuvant radiotherapy in the treatment of multiple locally advanced and inoperable basal cell carcinomas and squamous cell carcinomas of the skin. J Am Acad Dermatol. 2014;70:E88-E89.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Janela-Lapert R, Dubray B, Duval-Modeste A, et al. Treatment of advanced basal cell carcinoma with vismodegib followed by radiotherapy [in French]. Ann Dermatol Venereol. 2020;147:780-782.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:2485.
- Kim J, Tang JY, Gong R, et al. Itraconazole, a commonly used antifungal that inhibits hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388-399.
- Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23-34.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Cia˛z˙yn´yska M, Narbutt J, Skibin´ska M, et al. Itraconazole—a new player in the therapy of advanced basal cell carcinoma: a case report. JCO Oncol Pract. 2020;16:837-838.
- Sohn GK, Kwon GP, Bailey-Healy I, et al. Topical itraconazole for the treatment of basal cell carcinoma in patients with basal cell nevus syndrome or high-frequency basal cell carcinomas: a phase 2, open-label, placebo-controlled trial. JAMA Dermatol. 2019;155:1078-1080.
- Lass-Flörl C. Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs. 2011;71:2405-2419.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Katragkou A, Tsikopoulou F, Roilides E, et al. Posaconazole: when and how? the clinician’s view. Mycoses. 2012;55:110-122.
- Raad II, Graybill JR, Bustamante AB, et al. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis. 2006;42:1726-1734.
- Atwood SX, Sarin KY, Whitson RJ, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27:342-353.
- Sun Q, Atzmony L, Zaki T, et al. Clues to primary vismodegib resistance lie in histology and genetics. J Clin Pathol. 2020;73:678-680.
- Verkouteren BJA, Wakkee M, van Geel M, et al. Molecular testing in metastatic basal cell carcinoma. J Am Acad Dermatol. 2021;85:1135-1142.
- Danial C, Sarin KY, Oro AE, et al. An investigator-initiated open-label trial of sonidegib in advanced basal cell carcinoma patients resistant to vismodegib. Clin Cancer Res. 2016;22:1325-1329.
- Yoon J, Apicelli AJ 3rd, Pavlopoulos TV. Intracranial regression of an advanced basal cell carcinoma using sonidegib and itraconazole after failure with vismodegib. JAAD Case Rep. 2017;4:10-12.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Ueno H, Kondo S, Yoshikawa S, et al. A phase I and pharmacokinetic study of taladegib, a Smoothened inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs. 2018;36:647-656.
- Herms F, Lambert J, Grob JJ, et al. Follow-up of patients with complete remission of locally advanced basal cell carcinoma after vismodegib discontinuation: a multicenter French study of 116 patients. J Clin Oncol. 2019;37:3275-3282.
- Villani A, Megna M, Fabbrocini G, et al. Long-term efficacy of vismodegib after its withdrawal and patients’ health-related quality of life using the Dermatology Life Quality Index (DLQI). Dermatol Ther (Heidelb). 2019;9:719-724.
- Scalvenzi M, Cappello M, Costa C, et al. Low-dose vismodegib as maintenance therapy after locally advanced basal cell carcinoma complete remission: high efficacy with minimal toxicity. Dermatol Ther (Heidelb). 2020;10:465-468.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Kasumagic-Halilovic E, Hasic M, Ovcina-Kurtovic N. A clinical study of basal cell carcinoma. Med Arch. 2019;73:394-398.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e952-966.e952.
- Laga AC, Schaefer IM, Sholl LM, et al. Metastatic basal cell carcinoma. Am J Clin Pathol. 2019;152:706-717.
- Rimkus TK, Carpenter RL, Qasem S, et al. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel). 2016;8:22.
- Burova E, Hermann A, Waite J, et al. Characterization of the anti–PD-1 antibody REGN2810 and its antitumor activity in human PD-1 knock-in mice. Mol Cancer Ther. 2017;16:861-870.
- Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172-180.
- Paladini RD, Saleh J, Qian C, et al. Modulation of hair growth with small molecule agonists of the hedgehog signaling pathway. J Invest Dermatol. 2005;125:638-646.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Sekulic A, Migden MR, Basset-Seguin N, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Cozzani R, Del Aguila R, Carrizo M, et al. Efficacy and safety profile of vismodegib in a real-world setting cohort of patients with advanced basal cell carcinoma in Argentina. Int J Dermatol. 2020;59:627-632.
- Spallone G, Sollena P, Ventura A, et al. Efficacy and safety of vismodegib treatment in patients with advanced basal cell carcinoma and multiple comorbidities. Dermatol Ther. 2019;32:E13108.
- Fosko SW, Chu MB, Armbrecht E, et al. Efficacy, rate of tumor response, and safety of a short course (12-24 weeks) of oral vismodegib in various histologic subtypes (infiltrative, nodular, and superficial) of high-risk or locally advanced basal cell carcinoma, in an open-label, prospective case series clinical trial. J Am Acad Dermatol. 2020;82:946-954.
- Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716-728.
- Dummer R, Guminksi A, Gutzmer R, et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br J Dermatol. 2020;182:1369-1378.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Xie P, Lefrançois P. Efficacy, safety, and comparison of sonic hedgehog inhibitors in basal cell carcinomas: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79:1089-1100.e1017.
- Gentzler R, Hall R, Kunk PR, et al. Beyond melanoma: inhibiting the PD-1/PD-L1 pathway in solid tumors. Immunotherapy. 2016;8:583-600.
- Guminski AD, Lim AML, Khushalani NI, et al. Phase 2 study of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with metastatic cutaneous squamous cell carcinoma (mCSCC; group 1): 12-month follow-up [abstract]. J Clin Oncol. 2019;37(15 suppl):9526.
- Grob JJ, Gonzalez Mendoza R, Basset-Seguin N, et al. Pembrolizumab for recurrent/metastatic cutaneous squamous cell carcinoma (cSCC): efficacy and safety results from the phase II KEYNOTE-629 study [abstract]. Ann Oncol. 2019;30 (suppl 5):v908.
- Maubec E, Boubaya M, Petrow P, et al. Pembrolizumab as first-line therapy in patients with unresectable cutaneous squamous cell carcinoma (cSCC): phase 2 results from CARSKIN [abstract]. J Clin Oncol. 2019;37(15 suppl):9547.
- Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:848-857.
- Carpenter RL, Ray H. Safety and tolerability of sonic hedgehog pathway inhibitors in cancer. Drug Saf. 2019;42:263-279.
- Villani A, Fabbrocini G, Costa C, et al. Sonidegib: safety and efficacy in treatment of advanced basal cell carcinoma. Dermatol Ther (Heidelb). 2020;10:401-412.
- Wright A, Sluka KA. Nonpharmacological treatments for musculoskeletal pain. Clin J Pain. 2001;17:33-46.
- Ally MS, Tang JY, Lindgren J, et al. Effect of calcium channel blockade on vismodegib-induced muscle cramps. JAMA Dermatol. 2015;151:1132-1134.
- Yang X, Thai K-E. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil. Australas J Dermatol. 2016;57:e130-e132.
- Ferguson JS, Hannam S, Toholka R, et al. Hair loss and hedgehog inhibitors: a class effect? Br J Dermatol. 2015;173:262-264.
- Kumbargere Nagraj S, George RP, Shetty N, et al. Interventions for managing taste disturbances. Cochrane Database Syst Rev. 2017;12:CD010470.
- Jacobsen AA, Kydd AR, Strasswimmer J. Practical management of the adverse effects of hedgehog pathway inhibitor therapy for basal cell carcinoma. J Am Acad Dermatol. 2017;76:767-768.
- Ally MS, Tang JY, Joseph T, et al. The use of vismodegib to shrink keratocystic odontogenic tumors in patients with basal cell nevus syndrome. JAMA Dermatol. 2014;150:542-545.
- Yang X, Dinehart SM. Intermittent vismodegib therapy in basal cell nevus syndrome. JAMA Dermatol. 2016;152:223-224.
- Erivedge. Prescribing information. Genentech; 2015.
- Odomzo. Prescribing information. Novartis; 2015.
- Ventarola DJ, Silverstein DI. Vismodegib-associated hepatotoxicity: a potential side effect detected in postmarketing surveillance. J Am Acad Dermatol. 2014;71:397-398.
- Mohan SV, Chang J, Li S, et al. Increased risk of cutaneous squamous cell carcinoma after vismodegib therapy for basal cell carcinoma. JAMA Dermatol. 2016;152:527-532.
- Bhutani T, Abrouk M, Sima CS, et al. Risk of cutaneous squamous cell carcinoma after treatment of basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2017;77:713-718.
- Morgado M, Plácido A, Morgado S, et al. Management of the adverse effects of immune checkpoint inhibitors. Vaccines (Basel). 2020;8:575.
- Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563-580.
- Ntsethe A, Dludla PV, Nyambuya TM, et al. The impact of immune checkpoint inhibitors in patients with chronic lymphocytic leukemia (CLL): a protocol for a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99:E21167.
- Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904-1911.
- Tsung I, Worden FP, Fontana RJ. Safety and efficacy of checkpoint inhibitors in solid organ transplant recipients with cutaneous squamous cell carcinoma [abstract]. J Clin Oncol. 2020;38(15 suppl):E22014.
- Owoyemi I, Vaughan LE, Costello CM, et al. Clinical outcomes of solid organ transplant recipients with metastatic cancers who are treated with immune checkpoint inhibitors: a single-center analysis. Cancer. 2020;126:4780-4787.
- Kumar V, Shinagare AB, Rennke HG, et al. The safety and efficacy of checkpoint inhibitors in transplant recipients: a case series and systematic review of literature. Oncologist. 2020;25:505-514.
- Arenbergerova M, Fialova A, Arenberger P, et al. Killing two birds with one stone: response to pembrolizumab in a patient with metastatic melanoma and B-cell chronic lymphocytic leukaemia. J Eur Acad Dermatol Venereol. 2018;32:E72-E74.
- Archibald WJ, Meacham PJ, Williams AM, et al. Management of melanoma in patients with chronic lymphocytic leukemia. Leuk Res. 2018;71:43-46.
- Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129:3419-3427.
- Leiter U, Loquai C, Reinhardt L, et al. Immune checkpoint inhibition therapy for advanced skin cancer in patients with concomitant hematological malignancy: a retrospective multicenter DeCOG study of 84 patients. J Immunother Cancer. 2020;8:E000897.
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322.
- Woltsche N, Pichler N, Wolf I, et al. Managing adverse effects by dose reduction during routine treatment of locally advanced basal cell carcinoma with the hedgehog inhibitor vismodegib: a single centre experience. J Eur Acad Dermatol Venereol. 2019;33:E144-E145.
- Wong C, Poblete-Lopez C, Vidimos A. Comparison of daily dosing versus Monday through Friday dosing of vismodegib for locally advanced basal cell carcinoma and basal cell nevus syndrome: a retrospective case series. J Am Acad Dermatol. 2020;82:1539-1542.
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412.
- Schadendorf D, Hauschild A, Fosko S, et al. Quality-of-life analysis with intermittent vismodegib regimens in patients with multiple basal cell carcinomas: patient-reported outcomes from the MIKIE study. J Eur Acad Dermatol Venereol. 2020;34:E526-E529.
- Chanu P, Musib L, Wang X, et al. Vismodegib efficacy in advanced basal cell carcinoma maintained with 8-week dose interruptions: a model-based evaluation. J Invest Dermatol. 2021;141:930-933.
- Su MG, Potts LB, Tsai JH. Treatment of periocular basal cell carcinoma with neoadjuvant vismodegib. Am J Ophthalmol Case Rep. 2020;19:100755.
- González AR, Etchichury D, Gil ME, et al. Neoadjuvant vismodegib and Mohs micrographic surgery for locally advanced periocular basal cell carcinoma. Ophthalmic Plast Reconstr Surg. 2019;35:56-61.
- Sagiv O, Nagarajan P, Ferrarotto R, et al. Ocular preservation with neoadjuvant vismodegib in patients with locally advanced periocular basal cell carcinoma. Br J Ophthalmol. 2019;103:775-780.
- Ally MS, Aasi S, Wysong A, et al. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol. 2014;71:904-911.e1.
- Kwon GP, Ally MS, Bailey-Healy I, et al. Update to an open-label clinical trial of vismodegib as neoadjuvant before surgery for high-risk basal cell carcinoma (BCC). J Am Acad Dermatol. 2016;75:213-215.
- Mortier L, Bertrand N, Basset-Seguin N, et al. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: first results of a multicenter, open-label, phase 2 trial (VISMONEO study) [abstract]. J Clin Oncol. 2018;36(15 suppl):9509.
- Strasswimmer JM. Potential synergy of radiation therapy with vismodegib for basal cell carcinoma. JAMA Dermatol. 2015;151:925-926.
- Gathings RM, Orscheln CS, Huang WW. Compassionate use of vismodegib and adjuvant radiotherapy in the treatment of multiple locally advanced and inoperable basal cell carcinomas and squamous cell carcinomas of the skin. J Am Acad Dermatol. 2014;70:E88-E89.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Janela-Lapert R, Dubray B, Duval-Modeste A, et al. Treatment of advanced basal cell carcinoma with vismodegib followed by radiotherapy [in French]. Ann Dermatol Venereol. 2020;147:780-782.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:2485.
- Kim J, Tang JY, Gong R, et al. Itraconazole, a commonly used antifungal that inhibits hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388-399.
- Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23-34.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Cia˛z˙yn´yska M, Narbutt J, Skibin´ska M, et al. Itraconazole—a new player in the therapy of advanced basal cell carcinoma: a case report. JCO Oncol Pract. 2020;16:837-838.
- Sohn GK, Kwon GP, Bailey-Healy I, et al. Topical itraconazole for the treatment of basal cell carcinoma in patients with basal cell nevus syndrome or high-frequency basal cell carcinomas: a phase 2, open-label, placebo-controlled trial. JAMA Dermatol. 2019;155:1078-1080.
- Lass-Flörl C. Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs. 2011;71:2405-2419.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Katragkou A, Tsikopoulou F, Roilides E, et al. Posaconazole: when and how? the clinician’s view. Mycoses. 2012;55:110-122.
- Raad II, Graybill JR, Bustamante AB, et al. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis. 2006;42:1726-1734.
- Atwood SX, Sarin KY, Whitson RJ, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27:342-353.
- Sun Q, Atzmony L, Zaki T, et al. Clues to primary vismodegib resistance lie in histology and genetics. J Clin Pathol. 2020;73:678-680.
- Verkouteren BJA, Wakkee M, van Geel M, et al. Molecular testing in metastatic basal cell carcinoma. J Am Acad Dermatol. 2021;85:1135-1142.
- Danial C, Sarin KY, Oro AE, et al. An investigator-initiated open-label trial of sonidegib in advanced basal cell carcinoma patients resistant to vismodegib. Clin Cancer Res. 2016;22:1325-1329.
- Yoon J, Apicelli AJ 3rd, Pavlopoulos TV. Intracranial regression of an advanced basal cell carcinoma using sonidegib and itraconazole after failure with vismodegib. JAAD Case Rep. 2017;4:10-12.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Ueno H, Kondo S, Yoshikawa S, et al. A phase I and pharmacokinetic study of taladegib, a Smoothened inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs. 2018;36:647-656.
- Herms F, Lambert J, Grob JJ, et al. Follow-up of patients with complete remission of locally advanced basal cell carcinoma after vismodegib discontinuation: a multicenter French study of 116 patients. J Clin Oncol. 2019;37:3275-3282.
- Villani A, Megna M, Fabbrocini G, et al. Long-term efficacy of vismodegib after its withdrawal and patients’ health-related quality of life using the Dermatology Life Quality Index (DLQI). Dermatol Ther (Heidelb). 2019;9:719-724.
- Scalvenzi M, Cappello M, Costa C, et al. Low-dose vismodegib as maintenance therapy after locally advanced basal cell carcinoma complete remission: high efficacy with minimal toxicity. Dermatol Ther (Heidelb). 2020;10:465-468.
Practice Points
- The sonic hedgehog (SHH) inhibitors vismodegib and sonidegib currently are the only 2 oral medications approved by the US Food and Drug Administration for the first-line treatment of locally advanced basal cell carcinoma (BCC). Vismodegib also is approved for metastatic BCC.
- Cemiplimab, a programmed cell death protein 1 inhibitor, is now an approved treatment for patients with advanced BCC refractory or intolerant to SHH inhibitor therapy.
- Adverse effects of SHH inhibitors, most commonly muscle spasms, often lead to treatment discontinuation, but intermittent dosing regimens can be used to increase tolerability and adherence.
- Combining SHH inhibitors with radiotherapy or antifungal therapy as well as maintenance dosing strategies may help reduce the risk of recurrence.
- Neoadjuvant administration of a SHH inhibitor may enable surgical excision of previously inoperable cases through tumor shrinkage.
Squamoid Eccrine Ductal Carcinoma
Squamoid eccrine ductal carcinoma (SEDC) is an aggressive underrecognized cutaneous malignancy of unknown etiology.1 It is most likely to occur in sun-exposed areas of the body, most commonly the head and neck. Risk factors include male sex, increased age, and chronic immunosuppression.1-4 Current reports suggest that SEDC is likely a high-grade subtype of squamous cell carcinoma (SCC) with a high risk for local recurrence (25%) and metastasis (13%).1,3,5,6 There are as few as 56 cases of SEDC reported in the literature; however, the number of cases may be closer to 100 due to SEDC being classified as either adenosquamous carcinoma of the skin or ductal eccrine carcinoma with squamous differentiation.1
Clinically, SEDC mimics keratinocyte carcinomas. Histologically, SEDC is biphasic, with a superficial portion resembling well-differentiated SCC and a deeply invasive portion having infiltrative irregular cords with ductal differentiation. Perineural invasion (PNI) frequently is present. Multiple connections to the overlying epidermis also can be seen, serving as a subtle clue to the diagnosis on broad superficial specimens.1-3 Due to superficial sampling, approximately 50% of reported cases are misdiagnosed as SCC during the initial biopsy.4 The diagnosis of SEDC often is made during complete excision when deeper tissue is sampled. Establishing an accurate diagnosis is important given the more aggressive nature of SEDC compared with SCC and its proclivity for PNI.1,3,6 The purpose of this review is to increase awareness of this underrecognized entity and describe the histologic findings that help distinguish SEDC from SCC.
Patient Chart Review
We reviewed chart notes as well as frozen and formalin-fixed paraffin-embedded tissue sections from all 5 patients diagnosed with SEDC at a single institution between November 2018 and May 2020. The mean age of patients was 81 years, and 4 were male. Four of the patients presented for MMS with a preoperative diagnosis of SCC per the original biopsy results. Only 1 patient had a preoperative diagnosis of SEDC. The details of each case are recorded in the Table. All tumors were greater than 2 cm in diameter on initial presentation, were located on the head, and clinically resembled keratinocyte carcinoma with either a nodular or plaquelike appearance (Figure 1).
Intraoperative histologic examination of the excised tissue revealed a biphasic pattern consisting of superficial SCC features overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation in all 5 patients (Figures 2–4). Immunohistochemical staining with cytokeratin AE1/AE3 revealed thin strands of carcinoma in the mid to deeper dermis with squamous differentiation and eccrine ductal differentiation (Figure 5), thus confirming the diagnosis in all 5 patients.
The median depth of tumor invasion was 4.1 mm (range, 2.2–5.45 mm). Ulceration was seen in 3 of the patients, and PNI of large-caliber nerves was observed in all 5 patients. A connection with the overlying epidermis was present in all 5 patients. All 5 patients required more than 1 Mohs stage for complete tumor clearance (Table).
In 4 of the patients, nodal imaging performed at the time of diagnosis revealed no evidence of metastasis. Two patients received adjuvant radiation therapy, and none demonstrated evidence of recurrence. The mean follow-up time was 11 months (range, 6.5–18 months) for the 4 cases with available follow-up data (Table).
Literature Review
A PubMed review of the literature using the search term squamoid eccrine ductal carcinoma resulted in 28 articles, 19 of which were included in the review based on inclusion criteria (original articles available in English, in full text, and pertained to SEDC). Our review yielded 56 cases of SEDC.1-19 The mean age of patients with SEDC was 72 years. The number of male and female cases was 52% (29/56) and 48% (27/56), respectively. The most common location of SEDC was on the head or neck (71% [40/56]), followed by the extremities (19% [11/56]). Immunosuppression was noted in 9% (5/56) of cases. Wide local excision was the most commonly employed treatment modality (91% [51/56]), with MMS being used in 4 patients (7%). Adjuvant radiation was reported in 5% (3/56) of cases. Perineural invasion was reported in 34% (19/56) of cases. Recurrence was seen in 23% (13/56) of cases, with a mean time to recurrence of 10.4 months. Metastasis to regional lymph nodes was observed in 13% (7/56) of cases, with 7% (4/56) of those cases having distant metastases.
Comment
Squamoid eccrine ductal carcinoma was successfully treated with MMS in all 5 of the patients we reviewed. Recognition of a distinct biphasic pattern consisting of squamous differentiation superficially with epidermal connection overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation should lead to consideration of this diagnosis. A thorough inspection for PNI also should be performed, as this finding was present in all of 5 cases and in 34% of reported cases in our literature review.
The differential diagnosis for SEDC includes SCC, metastatic adenocarcinoma with squamoid features, and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma (MAC), and porocarcinoma with squamous differentiation. The combination of histologic features with the immunoexpression profile of carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), cytokeratin (CK) 5/6, and p63 can effectively exclude the other entities in the differential and confirm the diagnosis of SEDC.1,3,4 While the diagnosis of SEDC relies on the specific histologic features of multiple surface attachments and superficial squamoid changes with deep ductular elements, immunohistochemistry can nonetheless be adjunctive in difficult cases. Positive immunohistochemical staining for CEA and EMA can help to highlight and delineate true glandular elements, whereas CK5/6 highlights the overall contour of the tumor, displaying more clearly the multiple epidermal attachments and the subtle infiltrative nature of the deeper components of invasive cords and ducts. In addition, the combination of CK5/6 and p63 positivity supports the primary cutaneous nature of the lesion rather than metastatic adenocarcinoma.13,20 Other markers of eccrine secretory coils, such as CK7, CAM5.2, and S100, also are sometimes used for confirmation, some of which can aid in distinction from noneccrine sweat gland differentiation, as CK7 and CAM5.2 are negative in both luminal and basal cells of the dermal duct while being positive within the secretory coil, and S100 protein is expressed within eccrine secretory coil but negative within the apocrine sweat glands.2,4,21
The clinical findings from our chart review corroborated those reported in the literature. The mean age of SEDC in the 5 patients we reviewed was 81 years, and all cases presented on the head, consistent with the findings observed in the literature. Although 4 of our cases were male, there may not be a difference in risk based on sex as previously thought.1 Our literature review revealed an almost equivalent percentage of male and female cases, with 52% being male.
Immunosuppression has been associated with an increased risk for SEDC. Our literature review revealed that approximately 9% (5/56) of cases occurred in immunosuppressed individuals. Two of these reported cases were in the setting of underlying chronic lymphocytic leukemia, 2 in individuals with a history of organ transplant, and 1 treated with azathioprine for myasthenia gravis.2,4,10,12,13 Our chart review supported this correlation, as all 5 patients had a medical history potentially consistent with being in an immunocompromised state (Table). Notably, patient 5 represents a unique case of SEDC occurring in the setting of HIV. The patient had HIV for 33 years, with his most recent CD4+ count of 794 mm3 and HIV-1 RNA load of 35 copies/mL. Given that HIV-positive individuals may have more than a 2-fold increased risk of SCC, a greater degree of suspicion for SEDC should be maintained for these patients.22,23
The etiology of SEDC is controversial but is thought to be either an SCC arising from eccrine glands or a variant of eccrine carcinoma with extensive squamoid differentiation.4,6,13,14,17,24 While SEDC certainly appears to share the proclivity for PNI with the malignant eccrine tumor MAC, it is simultaneously quite distinct, demonstrating nuclear pleomorphism and mitotic activity, both of which are lacking in the bland nature of MACs.12,25
The exact prevalence of SEDC is difficult to ascertain because of its frequent misdiagnosis and variable nomenclature used within the literature. Most reported cases of SEDC are mistakenly diagnosed as SCC on the initial shave or punch biopsy because of superficial sampling. This also was the case in 4 of the patients we reviewed. In addition, there are reported cases of SEDC that were referred to by the investigators as cutaneous adenosquamous carcinoma (cASC), among other descriptors, such as ductal eccrine carcinoma with squamous differentiation, adnexal carcinoma with squamous and ductal differentiation, and syringoid eccrine carcinoma.26-32 While the World Health Organization classifies SEDC as a distinct variant of cASC, which is a rare variant of SCC in itself, the 2 can be differentiated. Despite the similar clinical and histologic features shared between cASC and SEDC, the neoplastic aggregates in SEDC exhibit ductal differentiation containing lumina positive for CEA and EMA.4 Overall, we favor the term squamoid eccrine ductal carcinoma, as there has recently been more uniformity for the designation of this disease entity as such.
It is unclear whether the high incidence of local recurrence (23% [13/56]) of SEDC reported in the literature is related to the treatment modality employed (ie, wide local excision) or due to the innate aggressiveness of SEDC.1,3,5 The literature has shown that MMS has lower recurrence rates than other treatments at 5-year follow-up for SCC (3.1%–5%) and eccrine carcinomas (0%–5%).33,34 Although studies assessing tumor behavior or comparing treatment modalities are limited because of the rarity and underrecognition of SEDC, MMS has been used several times for SEDC with only 1 recurrence reported.4,13,17,24 Given that all 5 of the patients we reviewed required more than 1 Mohs stage for complete tumor clearance and none demonstrated evidence of recurrence or metastasis (Table), we recommend MMS as the treatment of choice for SEDC.
Conclusion
Squamoid eccrine ductal carcinoma is a rare but likely underdiagnosed cutaneous tumor of uncertain etiology. Because of its propensity for recurrence and metastasis, excision of SEDC with complete circumferential peripheral and deep margin assessment with close follow-up is recommended.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Jacob J, Kugelman L. Squamoid eccrine ductal carcinoma. Cutis. 2018;101:378-380, 385.
- Yim S, Lee YH, Chae SW, et al. Squamoid eccrine ductal carcinoma of the ear helix. Clin Case Rep. 2019;7:1409-1411.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Jung YH, Jo HJ, Kang MS. Squamoid eccrine ductal carcinoma of the scalp. Korean J Pathol. 2012;46:278-281.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;91:799-802.
- Phan K, Kim L, Lim P, et al. A case report of temple squamoid eccrine ductal carcinoma: a diagnostic challenge beneath the tip of the iceberg. Dermatol Ther. 2020;33:E13213.
- McKissack SS, Wohltmann W, Dalton SR, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Lobo-Jardim MM, Souza BdCE, Kakizaki P, et al. Dermoscopy of squamoid eccrine ductal carcinoma: an aid for early diagnosis. An Bras Dermatol. 2018;93:893-895.
- Chan H, Howard V, Moir D, et al. Squamoid eccrine ductal carcinoma of the scalp. Aust J Dermatol. 2016;57:E117-E119.
- Wang B, Jarell AD, Bingham JL, et al. PET/CT imaging of squamoid eccrine ductal carcinoma. Clin Nucl Med. 2015;40:322-324.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. J Clin Aesthet Dermatol. 2013;6:33-36.
- Pusiol T, Morichetti D, Zorzi MG, et al. Squamoid eccrine ductal carcinoma: inappropriate diagnosis. Dermatol Surg. 2011;37:1819-1820.
- Kavand S, Cassarino DS. “Squamoid eccrine ductal carcinoma”: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Wasserman DI, Sack J, Gonzalez-Serva A, et al. Sentinel lymph node biopsy for a squamoid eccrine carcinoma with lymphatic invasion. Dermatol Surg. 2007;33:1126-1129.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Herrero J, Monteagudo C, Jorda E, et al. Squamoid eccrine ductal carcinoma. Histopathology. 1998;32:478-480.
- Wong TY, Suster S, Mihm MC. Squamoid eccrine ductal carcinoma. Histopathology. 1997;30:288-293.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 4th ed. Elsevier/Saunders; 2014.
- Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350-360.
- Asgari MM, Ray GT, Quesenberry CP Jr, et al. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892-896.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207.
- Kazakov DV. Cutaneous Adnexal Tumors. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012.
- Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
- Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
- Ko CJ, Leffell DJ, McNiff JM. Adenosquamous carcinoma: a report of nine cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
- Patel V, Squires SM, Liu DY, et al. Cutaneous adenosquamous carcinoma: a rare neoplasm with biphasic differentiation. Cutis. 2014;94:231-233.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Sidiropoulos M, Sade S, Al-Habeeb A, et al. Syringoid eccrine carcinoma: a clinicopathological and immunohistochemical study of four cases. J Clin Pathol. 2011;64:788-792.
- Azorín D, López-Ríos F, Ballestín C, et al. Primary cutaneous adenosquamous carcinoma: a case report and review of the literature. J Cutan Pathol. 2001;28:542-545.
- Wildemore JK, Lee JB, Humphreys TR. Mohs surgery for malignant eccrine neoplasms. Dermatol Surg. 2004;30(12 pt 2):1574-1579.
- Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33-57.
Squamoid eccrine ductal carcinoma (SEDC) is an aggressive underrecognized cutaneous malignancy of unknown etiology.1 It is most likely to occur in sun-exposed areas of the body, most commonly the head and neck. Risk factors include male sex, increased age, and chronic immunosuppression.1-4 Current reports suggest that SEDC is likely a high-grade subtype of squamous cell carcinoma (SCC) with a high risk for local recurrence (25%) and metastasis (13%).1,3,5,6 There are as few as 56 cases of SEDC reported in the literature; however, the number of cases may be closer to 100 due to SEDC being classified as either adenosquamous carcinoma of the skin or ductal eccrine carcinoma with squamous differentiation.1
Clinically, SEDC mimics keratinocyte carcinomas. Histologically, SEDC is biphasic, with a superficial portion resembling well-differentiated SCC and a deeply invasive portion having infiltrative irregular cords with ductal differentiation. Perineural invasion (PNI) frequently is present. Multiple connections to the overlying epidermis also can be seen, serving as a subtle clue to the diagnosis on broad superficial specimens.1-3 Due to superficial sampling, approximately 50% of reported cases are misdiagnosed as SCC during the initial biopsy.4 The diagnosis of SEDC often is made during complete excision when deeper tissue is sampled. Establishing an accurate diagnosis is important given the more aggressive nature of SEDC compared with SCC and its proclivity for PNI.1,3,6 The purpose of this review is to increase awareness of this underrecognized entity and describe the histologic findings that help distinguish SEDC from SCC.
Patient Chart Review
We reviewed chart notes as well as frozen and formalin-fixed paraffin-embedded tissue sections from all 5 patients diagnosed with SEDC at a single institution between November 2018 and May 2020. The mean age of patients was 81 years, and 4 were male. Four of the patients presented for MMS with a preoperative diagnosis of SCC per the original biopsy results. Only 1 patient had a preoperative diagnosis of SEDC. The details of each case are recorded in the Table. All tumors were greater than 2 cm in diameter on initial presentation, were located on the head, and clinically resembled keratinocyte carcinoma with either a nodular or plaquelike appearance (Figure 1).
Intraoperative histologic examination of the excised tissue revealed a biphasic pattern consisting of superficial SCC features overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation in all 5 patients (Figures 2–4). Immunohistochemical staining with cytokeratin AE1/AE3 revealed thin strands of carcinoma in the mid to deeper dermis with squamous differentiation and eccrine ductal differentiation (Figure 5), thus confirming the diagnosis in all 5 patients.
The median depth of tumor invasion was 4.1 mm (range, 2.2–5.45 mm). Ulceration was seen in 3 of the patients, and PNI of large-caliber nerves was observed in all 5 patients. A connection with the overlying epidermis was present in all 5 patients. All 5 patients required more than 1 Mohs stage for complete tumor clearance (Table).
In 4 of the patients, nodal imaging performed at the time of diagnosis revealed no evidence of metastasis. Two patients received adjuvant radiation therapy, and none demonstrated evidence of recurrence. The mean follow-up time was 11 months (range, 6.5–18 months) for the 4 cases with available follow-up data (Table).
Literature Review
A PubMed review of the literature using the search term squamoid eccrine ductal carcinoma resulted in 28 articles, 19 of which were included in the review based on inclusion criteria (original articles available in English, in full text, and pertained to SEDC). Our review yielded 56 cases of SEDC.1-19 The mean age of patients with SEDC was 72 years. The number of male and female cases was 52% (29/56) and 48% (27/56), respectively. The most common location of SEDC was on the head or neck (71% [40/56]), followed by the extremities (19% [11/56]). Immunosuppression was noted in 9% (5/56) of cases. Wide local excision was the most commonly employed treatment modality (91% [51/56]), with MMS being used in 4 patients (7%). Adjuvant radiation was reported in 5% (3/56) of cases. Perineural invasion was reported in 34% (19/56) of cases. Recurrence was seen in 23% (13/56) of cases, with a mean time to recurrence of 10.4 months. Metastasis to regional lymph nodes was observed in 13% (7/56) of cases, with 7% (4/56) of those cases having distant metastases.
Comment
Squamoid eccrine ductal carcinoma was successfully treated with MMS in all 5 of the patients we reviewed. Recognition of a distinct biphasic pattern consisting of squamous differentiation superficially with epidermal connection overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation should lead to consideration of this diagnosis. A thorough inspection for PNI also should be performed, as this finding was present in all of 5 cases and in 34% of reported cases in our literature review.
The differential diagnosis for SEDC includes SCC, metastatic adenocarcinoma with squamoid features, and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma (MAC), and porocarcinoma with squamous differentiation. The combination of histologic features with the immunoexpression profile of carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), cytokeratin (CK) 5/6, and p63 can effectively exclude the other entities in the differential and confirm the diagnosis of SEDC.1,3,4 While the diagnosis of SEDC relies on the specific histologic features of multiple surface attachments and superficial squamoid changes with deep ductular elements, immunohistochemistry can nonetheless be adjunctive in difficult cases. Positive immunohistochemical staining for CEA and EMA can help to highlight and delineate true glandular elements, whereas CK5/6 highlights the overall contour of the tumor, displaying more clearly the multiple epidermal attachments and the subtle infiltrative nature of the deeper components of invasive cords and ducts. In addition, the combination of CK5/6 and p63 positivity supports the primary cutaneous nature of the lesion rather than metastatic adenocarcinoma.13,20 Other markers of eccrine secretory coils, such as CK7, CAM5.2, and S100, also are sometimes used for confirmation, some of which can aid in distinction from noneccrine sweat gland differentiation, as CK7 and CAM5.2 are negative in both luminal and basal cells of the dermal duct while being positive within the secretory coil, and S100 protein is expressed within eccrine secretory coil but negative within the apocrine sweat glands.2,4,21
The clinical findings from our chart review corroborated those reported in the literature. The mean age of SEDC in the 5 patients we reviewed was 81 years, and all cases presented on the head, consistent with the findings observed in the literature. Although 4 of our cases were male, there may not be a difference in risk based on sex as previously thought.1 Our literature review revealed an almost equivalent percentage of male and female cases, with 52% being male.
Immunosuppression has been associated with an increased risk for SEDC. Our literature review revealed that approximately 9% (5/56) of cases occurred in immunosuppressed individuals. Two of these reported cases were in the setting of underlying chronic lymphocytic leukemia, 2 in individuals with a history of organ transplant, and 1 treated with azathioprine for myasthenia gravis.2,4,10,12,13 Our chart review supported this correlation, as all 5 patients had a medical history potentially consistent with being in an immunocompromised state (Table). Notably, patient 5 represents a unique case of SEDC occurring in the setting of HIV. The patient had HIV for 33 years, with his most recent CD4+ count of 794 mm3 and HIV-1 RNA load of 35 copies/mL. Given that HIV-positive individuals may have more than a 2-fold increased risk of SCC, a greater degree of suspicion for SEDC should be maintained for these patients.22,23
The etiology of SEDC is controversial but is thought to be either an SCC arising from eccrine glands or a variant of eccrine carcinoma with extensive squamoid differentiation.4,6,13,14,17,24 While SEDC certainly appears to share the proclivity for PNI with the malignant eccrine tumor MAC, it is simultaneously quite distinct, demonstrating nuclear pleomorphism and mitotic activity, both of which are lacking in the bland nature of MACs.12,25
The exact prevalence of SEDC is difficult to ascertain because of its frequent misdiagnosis and variable nomenclature used within the literature. Most reported cases of SEDC are mistakenly diagnosed as SCC on the initial shave or punch biopsy because of superficial sampling. This also was the case in 4 of the patients we reviewed. In addition, there are reported cases of SEDC that were referred to by the investigators as cutaneous adenosquamous carcinoma (cASC), among other descriptors, such as ductal eccrine carcinoma with squamous differentiation, adnexal carcinoma with squamous and ductal differentiation, and syringoid eccrine carcinoma.26-32 While the World Health Organization classifies SEDC as a distinct variant of cASC, which is a rare variant of SCC in itself, the 2 can be differentiated. Despite the similar clinical and histologic features shared between cASC and SEDC, the neoplastic aggregates in SEDC exhibit ductal differentiation containing lumina positive for CEA and EMA.4 Overall, we favor the term squamoid eccrine ductal carcinoma, as there has recently been more uniformity for the designation of this disease entity as such.
It is unclear whether the high incidence of local recurrence (23% [13/56]) of SEDC reported in the literature is related to the treatment modality employed (ie, wide local excision) or due to the innate aggressiveness of SEDC.1,3,5 The literature has shown that MMS has lower recurrence rates than other treatments at 5-year follow-up for SCC (3.1%–5%) and eccrine carcinomas (0%–5%).33,34 Although studies assessing tumor behavior or comparing treatment modalities are limited because of the rarity and underrecognition of SEDC, MMS has been used several times for SEDC with only 1 recurrence reported.4,13,17,24 Given that all 5 of the patients we reviewed required more than 1 Mohs stage for complete tumor clearance and none demonstrated evidence of recurrence or metastasis (Table), we recommend MMS as the treatment of choice for SEDC.
Conclusion
Squamoid eccrine ductal carcinoma is a rare but likely underdiagnosed cutaneous tumor of uncertain etiology. Because of its propensity for recurrence and metastasis, excision of SEDC with complete circumferential peripheral and deep margin assessment with close follow-up is recommended.
Squamoid eccrine ductal carcinoma (SEDC) is an aggressive underrecognized cutaneous malignancy of unknown etiology.1 It is most likely to occur in sun-exposed areas of the body, most commonly the head and neck. Risk factors include male sex, increased age, and chronic immunosuppression.1-4 Current reports suggest that SEDC is likely a high-grade subtype of squamous cell carcinoma (SCC) with a high risk for local recurrence (25%) and metastasis (13%).1,3,5,6 There are as few as 56 cases of SEDC reported in the literature; however, the number of cases may be closer to 100 due to SEDC being classified as either adenosquamous carcinoma of the skin or ductal eccrine carcinoma with squamous differentiation.1
Clinically, SEDC mimics keratinocyte carcinomas. Histologically, SEDC is biphasic, with a superficial portion resembling well-differentiated SCC and a deeply invasive portion having infiltrative irregular cords with ductal differentiation. Perineural invasion (PNI) frequently is present. Multiple connections to the overlying epidermis also can be seen, serving as a subtle clue to the diagnosis on broad superficial specimens.1-3 Due to superficial sampling, approximately 50% of reported cases are misdiagnosed as SCC during the initial biopsy.4 The diagnosis of SEDC often is made during complete excision when deeper tissue is sampled. Establishing an accurate diagnosis is important given the more aggressive nature of SEDC compared with SCC and its proclivity for PNI.1,3,6 The purpose of this review is to increase awareness of this underrecognized entity and describe the histologic findings that help distinguish SEDC from SCC.
Patient Chart Review
We reviewed chart notes as well as frozen and formalin-fixed paraffin-embedded tissue sections from all 5 patients diagnosed with SEDC at a single institution between November 2018 and May 2020. The mean age of patients was 81 years, and 4 were male. Four of the patients presented for MMS with a preoperative diagnosis of SCC per the original biopsy results. Only 1 patient had a preoperative diagnosis of SEDC. The details of each case are recorded in the Table. All tumors were greater than 2 cm in diameter on initial presentation, were located on the head, and clinically resembled keratinocyte carcinoma with either a nodular or plaquelike appearance (Figure 1).
Intraoperative histologic examination of the excised tissue revealed a biphasic pattern consisting of superficial SCC features overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation in all 5 patients (Figures 2–4). Immunohistochemical staining with cytokeratin AE1/AE3 revealed thin strands of carcinoma in the mid to deeper dermis with squamous differentiation and eccrine ductal differentiation (Figure 5), thus confirming the diagnosis in all 5 patients.
The median depth of tumor invasion was 4.1 mm (range, 2.2–5.45 mm). Ulceration was seen in 3 of the patients, and PNI of large-caliber nerves was observed in all 5 patients. A connection with the overlying epidermis was present in all 5 patients. All 5 patients required more than 1 Mohs stage for complete tumor clearance (Table).
In 4 of the patients, nodal imaging performed at the time of diagnosis revealed no evidence of metastasis. Two patients received adjuvant radiation therapy, and none demonstrated evidence of recurrence. The mean follow-up time was 11 months (range, 6.5–18 months) for the 4 cases with available follow-up data (Table).
Literature Review
A PubMed review of the literature using the search term squamoid eccrine ductal carcinoma resulted in 28 articles, 19 of which were included in the review based on inclusion criteria (original articles available in English, in full text, and pertained to SEDC). Our review yielded 56 cases of SEDC.1-19 The mean age of patients with SEDC was 72 years. The number of male and female cases was 52% (29/56) and 48% (27/56), respectively. The most common location of SEDC was on the head or neck (71% [40/56]), followed by the extremities (19% [11/56]). Immunosuppression was noted in 9% (5/56) of cases. Wide local excision was the most commonly employed treatment modality (91% [51/56]), with MMS being used in 4 patients (7%). Adjuvant radiation was reported in 5% (3/56) of cases. Perineural invasion was reported in 34% (19/56) of cases. Recurrence was seen in 23% (13/56) of cases, with a mean time to recurrence of 10.4 months. Metastasis to regional lymph nodes was observed in 13% (7/56) of cases, with 7% (4/56) of those cases having distant metastases.
Comment
Squamoid eccrine ductal carcinoma was successfully treated with MMS in all 5 of the patients we reviewed. Recognition of a distinct biphasic pattern consisting of squamous differentiation superficially with epidermal connection overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation should lead to consideration of this diagnosis. A thorough inspection for PNI also should be performed, as this finding was present in all of 5 cases and in 34% of reported cases in our literature review.
The differential diagnosis for SEDC includes SCC, metastatic adenocarcinoma with squamoid features, and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma (MAC), and porocarcinoma with squamous differentiation. The combination of histologic features with the immunoexpression profile of carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), cytokeratin (CK) 5/6, and p63 can effectively exclude the other entities in the differential and confirm the diagnosis of SEDC.1,3,4 While the diagnosis of SEDC relies on the specific histologic features of multiple surface attachments and superficial squamoid changes with deep ductular elements, immunohistochemistry can nonetheless be adjunctive in difficult cases. Positive immunohistochemical staining for CEA and EMA can help to highlight and delineate true glandular elements, whereas CK5/6 highlights the overall contour of the tumor, displaying more clearly the multiple epidermal attachments and the subtle infiltrative nature of the deeper components of invasive cords and ducts. In addition, the combination of CK5/6 and p63 positivity supports the primary cutaneous nature of the lesion rather than metastatic adenocarcinoma.13,20 Other markers of eccrine secretory coils, such as CK7, CAM5.2, and S100, also are sometimes used for confirmation, some of which can aid in distinction from noneccrine sweat gland differentiation, as CK7 and CAM5.2 are negative in both luminal and basal cells of the dermal duct while being positive within the secretory coil, and S100 protein is expressed within eccrine secretory coil but negative within the apocrine sweat glands.2,4,21
The clinical findings from our chart review corroborated those reported in the literature. The mean age of SEDC in the 5 patients we reviewed was 81 years, and all cases presented on the head, consistent with the findings observed in the literature. Although 4 of our cases were male, there may not be a difference in risk based on sex as previously thought.1 Our literature review revealed an almost equivalent percentage of male and female cases, with 52% being male.
Immunosuppression has been associated with an increased risk for SEDC. Our literature review revealed that approximately 9% (5/56) of cases occurred in immunosuppressed individuals. Two of these reported cases were in the setting of underlying chronic lymphocytic leukemia, 2 in individuals with a history of organ transplant, and 1 treated with azathioprine for myasthenia gravis.2,4,10,12,13 Our chart review supported this correlation, as all 5 patients had a medical history potentially consistent with being in an immunocompromised state (Table). Notably, patient 5 represents a unique case of SEDC occurring in the setting of HIV. The patient had HIV for 33 years, with his most recent CD4+ count of 794 mm3 and HIV-1 RNA load of 35 copies/mL. Given that HIV-positive individuals may have more than a 2-fold increased risk of SCC, a greater degree of suspicion for SEDC should be maintained for these patients.22,23
The etiology of SEDC is controversial but is thought to be either an SCC arising from eccrine glands or a variant of eccrine carcinoma with extensive squamoid differentiation.4,6,13,14,17,24 While SEDC certainly appears to share the proclivity for PNI with the malignant eccrine tumor MAC, it is simultaneously quite distinct, demonstrating nuclear pleomorphism and mitotic activity, both of which are lacking in the bland nature of MACs.12,25
The exact prevalence of SEDC is difficult to ascertain because of its frequent misdiagnosis and variable nomenclature used within the literature. Most reported cases of SEDC are mistakenly diagnosed as SCC on the initial shave or punch biopsy because of superficial sampling. This also was the case in 4 of the patients we reviewed. In addition, there are reported cases of SEDC that were referred to by the investigators as cutaneous adenosquamous carcinoma (cASC), among other descriptors, such as ductal eccrine carcinoma with squamous differentiation, adnexal carcinoma with squamous and ductal differentiation, and syringoid eccrine carcinoma.26-32 While the World Health Organization classifies SEDC as a distinct variant of cASC, which is a rare variant of SCC in itself, the 2 can be differentiated. Despite the similar clinical and histologic features shared between cASC and SEDC, the neoplastic aggregates in SEDC exhibit ductal differentiation containing lumina positive for CEA and EMA.4 Overall, we favor the term squamoid eccrine ductal carcinoma, as there has recently been more uniformity for the designation of this disease entity as such.
It is unclear whether the high incidence of local recurrence (23% [13/56]) of SEDC reported in the literature is related to the treatment modality employed (ie, wide local excision) or due to the innate aggressiveness of SEDC.1,3,5 The literature has shown that MMS has lower recurrence rates than other treatments at 5-year follow-up for SCC (3.1%–5%) and eccrine carcinomas (0%–5%).33,34 Although studies assessing tumor behavior or comparing treatment modalities are limited because of the rarity and underrecognition of SEDC, MMS has been used several times for SEDC with only 1 recurrence reported.4,13,17,24 Given that all 5 of the patients we reviewed required more than 1 Mohs stage for complete tumor clearance and none demonstrated evidence of recurrence or metastasis (Table), we recommend MMS as the treatment of choice for SEDC.
Conclusion
Squamoid eccrine ductal carcinoma is a rare but likely underdiagnosed cutaneous tumor of uncertain etiology. Because of its propensity for recurrence and metastasis, excision of SEDC with complete circumferential peripheral and deep margin assessment with close follow-up is recommended.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Jacob J, Kugelman L. Squamoid eccrine ductal carcinoma. Cutis. 2018;101:378-380, 385.
- Yim S, Lee YH, Chae SW, et al. Squamoid eccrine ductal carcinoma of the ear helix. Clin Case Rep. 2019;7:1409-1411.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Jung YH, Jo HJ, Kang MS. Squamoid eccrine ductal carcinoma of the scalp. Korean J Pathol. 2012;46:278-281.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;91:799-802.
- Phan K, Kim L, Lim P, et al. A case report of temple squamoid eccrine ductal carcinoma: a diagnostic challenge beneath the tip of the iceberg. Dermatol Ther. 2020;33:E13213.
- McKissack SS, Wohltmann W, Dalton SR, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Lobo-Jardim MM, Souza BdCE, Kakizaki P, et al. Dermoscopy of squamoid eccrine ductal carcinoma: an aid for early diagnosis. An Bras Dermatol. 2018;93:893-895.
- Chan H, Howard V, Moir D, et al. Squamoid eccrine ductal carcinoma of the scalp. Aust J Dermatol. 2016;57:E117-E119.
- Wang B, Jarell AD, Bingham JL, et al. PET/CT imaging of squamoid eccrine ductal carcinoma. Clin Nucl Med. 2015;40:322-324.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. J Clin Aesthet Dermatol. 2013;6:33-36.
- Pusiol T, Morichetti D, Zorzi MG, et al. Squamoid eccrine ductal carcinoma: inappropriate diagnosis. Dermatol Surg. 2011;37:1819-1820.
- Kavand S, Cassarino DS. “Squamoid eccrine ductal carcinoma”: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Wasserman DI, Sack J, Gonzalez-Serva A, et al. Sentinel lymph node biopsy for a squamoid eccrine carcinoma with lymphatic invasion. Dermatol Surg. 2007;33:1126-1129.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Herrero J, Monteagudo C, Jorda E, et al. Squamoid eccrine ductal carcinoma. Histopathology. 1998;32:478-480.
- Wong TY, Suster S, Mihm MC. Squamoid eccrine ductal carcinoma. Histopathology. 1997;30:288-293.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 4th ed. Elsevier/Saunders; 2014.
- Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350-360.
- Asgari MM, Ray GT, Quesenberry CP Jr, et al. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892-896.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207.
- Kazakov DV. Cutaneous Adnexal Tumors. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012.
- Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
- Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
- Ko CJ, Leffell DJ, McNiff JM. Adenosquamous carcinoma: a report of nine cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
- Patel V, Squires SM, Liu DY, et al. Cutaneous adenosquamous carcinoma: a rare neoplasm with biphasic differentiation. Cutis. 2014;94:231-233.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Sidiropoulos M, Sade S, Al-Habeeb A, et al. Syringoid eccrine carcinoma: a clinicopathological and immunohistochemical study of four cases. J Clin Pathol. 2011;64:788-792.
- Azorín D, López-Ríos F, Ballestín C, et al. Primary cutaneous adenosquamous carcinoma: a case report and review of the literature. J Cutan Pathol. 2001;28:542-545.
- Wildemore JK, Lee JB, Humphreys TR. Mohs surgery for malignant eccrine neoplasms. Dermatol Surg. 2004;30(12 pt 2):1574-1579.
- Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33-57.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Jacob J, Kugelman L. Squamoid eccrine ductal carcinoma. Cutis. 2018;101:378-380, 385.
- Yim S, Lee YH, Chae SW, et al. Squamoid eccrine ductal carcinoma of the ear helix. Clin Case Rep. 2019;7:1409-1411.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Jung YH, Jo HJ, Kang MS. Squamoid eccrine ductal carcinoma of the scalp. Korean J Pathol. 2012;46:278-281.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;91:799-802.
- Phan K, Kim L, Lim P, et al. A case report of temple squamoid eccrine ductal carcinoma: a diagnostic challenge beneath the tip of the iceberg. Dermatol Ther. 2020;33:E13213.
- McKissack SS, Wohltmann W, Dalton SR, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Lobo-Jardim MM, Souza BdCE, Kakizaki P, et al. Dermoscopy of squamoid eccrine ductal carcinoma: an aid for early diagnosis. An Bras Dermatol. 2018;93:893-895.
- Chan H, Howard V, Moir D, et al. Squamoid eccrine ductal carcinoma of the scalp. Aust J Dermatol. 2016;57:E117-E119.
- Wang B, Jarell AD, Bingham JL, et al. PET/CT imaging of squamoid eccrine ductal carcinoma. Clin Nucl Med. 2015;40:322-324.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. J Clin Aesthet Dermatol. 2013;6:33-36.
- Pusiol T, Morichetti D, Zorzi MG, et al. Squamoid eccrine ductal carcinoma: inappropriate diagnosis. Dermatol Surg. 2011;37:1819-1820.
- Kavand S, Cassarino DS. “Squamoid eccrine ductal carcinoma”: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Wasserman DI, Sack J, Gonzalez-Serva A, et al. Sentinel lymph node biopsy for a squamoid eccrine carcinoma with lymphatic invasion. Dermatol Surg. 2007;33:1126-1129.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Herrero J, Monteagudo C, Jorda E, et al. Squamoid eccrine ductal carcinoma. Histopathology. 1998;32:478-480.
- Wong TY, Suster S, Mihm MC. Squamoid eccrine ductal carcinoma. Histopathology. 1997;30:288-293.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 4th ed. Elsevier/Saunders; 2014.
- Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350-360.
- Asgari MM, Ray GT, Quesenberry CP Jr, et al. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892-896.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207.
- Kazakov DV. Cutaneous Adnexal Tumors. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012.
- Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
- Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
- Ko CJ, Leffell DJ, McNiff JM. Adenosquamous carcinoma: a report of nine cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
- Patel V, Squires SM, Liu DY, et al. Cutaneous adenosquamous carcinoma: a rare neoplasm with biphasic differentiation. Cutis. 2014;94:231-233.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Sidiropoulos M, Sade S, Al-Habeeb A, et al. Syringoid eccrine carcinoma: a clinicopathological and immunohistochemical study of four cases. J Clin Pathol. 2011;64:788-792.
- Azorín D, López-Ríos F, Ballestín C, et al. Primary cutaneous adenosquamous carcinoma: a case report and review of the literature. J Cutan Pathol. 2001;28:542-545.
- Wildemore JK, Lee JB, Humphreys TR. Mohs surgery for malignant eccrine neoplasms. Dermatol Surg. 2004;30(12 pt 2):1574-1579.
- Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33-57.
PRACTICE POINTS
- Squamoid eccrine ductal carcinoma is an aggressive underrecognized cutaneous malignancy that often is misdiagnosed as squamous cell carcinoma (SCC) during initial biopsy.
- Squamoid eccrine ductal carcinoma has a biphasic histologic appearance with a superficial portion resembling well-differentiated SCC and a deeply invasive portion comprised of infiltrative irregular cords with ductal differentiation.
- Excision with complete circumferential peripheral and deep margin assessment with close follow-up is recommended for these patients because of the high risk for recurrence and metastasis.