User login
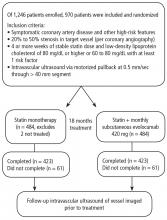
Results of the GLAGOV trial
Intravascular ultrasonography (IVUS) has been used for the past 20 years to measure atheromatous plaque in patients with coronary artery disease. The total volume of atherosclerosis in a coronary artery segment can be calculated using IVUS. A rotating transducer produces an image of a single, cross-sectional slice of the artery from which the atheroma area is calculated. A motorized device is used to withdraw the catheter, obtaining a series of cross-sectional slices at 1-mm intervals. The atheroma area for each slice is summated to obtain the total volume of atherosclerosis in the artery.
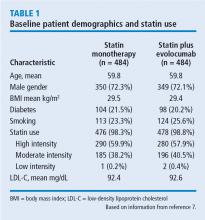
IVUS has demonstrated that statins slow the progression or even induce regression of coronary atherosclerosis in proportion to the degree of reduction in low-density lipoprotein cholesterol (LDL-C).1–4 No LDL-C-lowering therapy other than statins has shown regression of atherosclerosis in a trial using IVUS. The lowest LDL-C achieved in prior trials using statins was about 60 mg/dL.1,3 While this is very low, lower levels have not previously been explored.
Proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, a new class of drugs, are injectable, fully human monoclonal antibodies that inactivate the PCSK9 protein. PCSK9 inhibitors have been shown to lower LDL-C incrementally when added to statins, achieving very low LDL-C levels.5,6 However, no data exist describing the effect of low LDL-C levels reached using PCSK9 inhibitors on the progression of atherosclerosis.
THE GLAGOV TRIAL
RESULTS
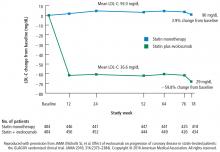
LDL-C levels
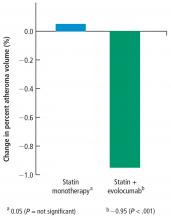
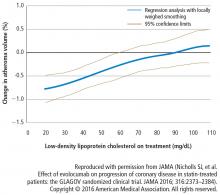
Change in percent atheroma volume
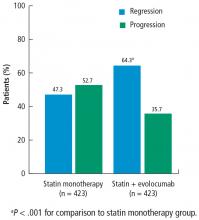
Total atheroma volume and percent of patients with atheroma regression
The secondary end point measuring the total atheroma volume in the coronaries showed no change in total volume of atherosclerotic plaque in the statin monotherapy group and a decrease in the statin plus evolocumab group.
Patients with LDL-C < 70 mg/dL
A subgroup of patients had a baseline LDL-C below 70 mg/dL, the lowest level recommended by guideline. Patients in this subgroup who received statin monotherapy remained at a mean LDL-C of 70 mg/dL whereas patients on statin plus evolocumab achieved a mean LDL-C of 24 mg/dL with a mean 2-week post-dosing trough level of 15 mg/dL, an unbelievably low level of LDL-C. In this subgroup, 81% of patients receiving statin plus evolocumab had atheroma regression, compared with 48% of patients in the statin monotherapy group. The percent of patients with atheroma regression in this subgroup of patients with low LDL-C at baseline was twice that seen in the larger study population (33% vs 17%), revealing profound levels of regression in patients treated with dual therapy.
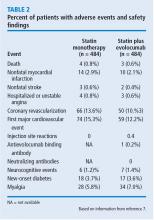
Safety
Limitations
Like all trials, this one has limitations. The population is very select: these are patients with clinically indicated angiogram, not a primary prevention population. Some study participants dropped out, which is always a limitation. And of course, this is a surrogate measure; it is a measure of disease activity, not a measure of morbidity and mortality. Morbidity and mortality data for this new class of drugs should be available in about a year, though this study suggests that those data will be favorable.
CONCLUSION
High LDL-C is universally accepted as a factor in the formation of arterial plaque and atherosclerosis. Statin therapy reduces LDL-C levels to slow or induce regression of coronary atherosclerosis in proportion to the magnitude of LDL-C reduction as measured by IVUS. However, the question of how far to reduce lipid levels has evolved over the last 4 decades. In the 1970s, a normal total cholesterol was < 300 mg/dL. More recent data that suggest optimal LDL-C levels for patients with coronary artery disease may be much lower than commonly achieved.
In this study, in patients with symptomatic coronary artery disease, treatment with statins and the addition of the PCSK9 inhibitor evolocumab achieved mean LDL-C levels of 36.6 mg/dL, produced atheroma regression with a mean change in PAV of about 1% (P < .001), induced regression in a greater percentage of patients, and showed incremental benefit for treatment of LDL-C down to as low as 20 mg/dL. The GLAGOV trial provides intriguing evidence that clinical benefits may extend to LDL-C levels as low as 20 mg/dL; however, the sample size of the trial was modest, providing limited power for safety assessments.
Since this presentation, the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial achieved a median LDL-C of 30 mg/dL and reduced risk of cardiovascular events in patients with atherosclerotic cardiovascular disease treated with evolocumab added to statin therapy.8 Additional large outcomes trials of PCSK9 inhibitors and their role in reducing LDL-C and regression of coronary atheroma and atherosclerosis are eagerly awaited.
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011; 365:2078–2087.
- Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007; 297: 499–508.
- Nissen SE, Nicholls SJ, Sipahi I, et al; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291:1071–1080.
- Robinson JG, Nedergaard BS, RogersWJ, et al; LAPLACE-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014; 311:1870–1882.
- Blom DJ, Hala T, Bolognese M, et al; DESCARTES Investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370:1809–1819.
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial. JAMA 2016; 316:2373–2384.
- Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376:1713–1722.
Intravascular ultrasonography (IVUS) has been used for the past 20 years to measure atheromatous plaque in patients with coronary artery disease. The total volume of atherosclerosis in a coronary artery segment can be calculated using IVUS. A rotating transducer produces an image of a single, cross-sectional slice of the artery from which the atheroma area is calculated. A motorized device is used to withdraw the catheter, obtaining a series of cross-sectional slices at 1-mm intervals. The atheroma area for each slice is summated to obtain the total volume of atherosclerosis in the artery.
IVUS has demonstrated that statins slow the progression or even induce regression of coronary atherosclerosis in proportion to the degree of reduction in low-density lipoprotein cholesterol (LDL-C).1–4 No LDL-C-lowering therapy other than statins has shown regression of atherosclerosis in a trial using IVUS. The lowest LDL-C achieved in prior trials using statins was about 60 mg/dL.1,3 While this is very low, lower levels have not previously been explored.
Proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, a new class of drugs, are injectable, fully human monoclonal antibodies that inactivate the PCSK9 protein. PCSK9 inhibitors have been shown to lower LDL-C incrementally when added to statins, achieving very low LDL-C levels.5,6 However, no data exist describing the effect of low LDL-C levels reached using PCSK9 inhibitors on the progression of atherosclerosis.
THE GLAGOV TRIAL
RESULTS
LDL-C levels
Change in percent atheroma volume
Total atheroma volume and percent of patients with atheroma regression
The secondary end point measuring the total atheroma volume in the coronaries showed no change in total volume of atherosclerotic plaque in the statin monotherapy group and a decrease in the statin plus evolocumab group.
Patients with LDL-C < 70 mg/dL
A subgroup of patients had a baseline LDL-C below 70 mg/dL, the lowest level recommended by guideline. Patients in this subgroup who received statin monotherapy remained at a mean LDL-C of 70 mg/dL whereas patients on statin plus evolocumab achieved a mean LDL-C of 24 mg/dL with a mean 2-week post-dosing trough level of 15 mg/dL, an unbelievably low level of LDL-C. In this subgroup, 81% of patients receiving statin plus evolocumab had atheroma regression, compared with 48% of patients in the statin monotherapy group. The percent of patients with atheroma regression in this subgroup of patients with low LDL-C at baseline was twice that seen in the larger study population (33% vs 17%), revealing profound levels of regression in patients treated with dual therapy.
Safety
Limitations
Like all trials, this one has limitations. The population is very select: these are patients with clinically indicated angiogram, not a primary prevention population. Some study participants dropped out, which is always a limitation. And of course, this is a surrogate measure; it is a measure of disease activity, not a measure of morbidity and mortality. Morbidity and mortality data for this new class of drugs should be available in about a year, though this study suggests that those data will be favorable.
CONCLUSION
High LDL-C is universally accepted as a factor in the formation of arterial plaque and atherosclerosis. Statin therapy reduces LDL-C levels to slow or induce regression of coronary atherosclerosis in proportion to the magnitude of LDL-C reduction as measured by IVUS. However, the question of how far to reduce lipid levels has evolved over the last 4 decades. In the 1970s, a normal total cholesterol was < 300 mg/dL. More recent data that suggest optimal LDL-C levels for patients with coronary artery disease may be much lower than commonly achieved.
In this study, in patients with symptomatic coronary artery disease, treatment with statins and the addition of the PCSK9 inhibitor evolocumab achieved mean LDL-C levels of 36.6 mg/dL, produced atheroma regression with a mean change in PAV of about 1% (P < .001), induced regression in a greater percentage of patients, and showed incremental benefit for treatment of LDL-C down to as low as 20 mg/dL. The GLAGOV trial provides intriguing evidence that clinical benefits may extend to LDL-C levels as low as 20 mg/dL; however, the sample size of the trial was modest, providing limited power for safety assessments.
Since this presentation, the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial achieved a median LDL-C of 30 mg/dL and reduced risk of cardiovascular events in patients with atherosclerotic cardiovascular disease treated with evolocumab added to statin therapy.8 Additional large outcomes trials of PCSK9 inhibitors and their role in reducing LDL-C and regression of coronary atheroma and atherosclerosis are eagerly awaited.
Intravascular ultrasonography (IVUS) has been used for the past 20 years to measure atheromatous plaque in patients with coronary artery disease. The total volume of atherosclerosis in a coronary artery segment can be calculated using IVUS. A rotating transducer produces an image of a single, cross-sectional slice of the artery from which the atheroma area is calculated. A motorized device is used to withdraw the catheter, obtaining a series of cross-sectional slices at 1-mm intervals. The atheroma area for each slice is summated to obtain the total volume of atherosclerosis in the artery.
IVUS has demonstrated that statins slow the progression or even induce regression of coronary atherosclerosis in proportion to the degree of reduction in low-density lipoprotein cholesterol (LDL-C).1–4 No LDL-C-lowering therapy other than statins has shown regression of atherosclerosis in a trial using IVUS. The lowest LDL-C achieved in prior trials using statins was about 60 mg/dL.1,3 While this is very low, lower levels have not previously been explored.
Proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, a new class of drugs, are injectable, fully human monoclonal antibodies that inactivate the PCSK9 protein. PCSK9 inhibitors have been shown to lower LDL-C incrementally when added to statins, achieving very low LDL-C levels.5,6 However, no data exist describing the effect of low LDL-C levels reached using PCSK9 inhibitors on the progression of atherosclerosis.
THE GLAGOV TRIAL
RESULTS
LDL-C levels
Change in percent atheroma volume
Total atheroma volume and percent of patients with atheroma regression
The secondary end point measuring the total atheroma volume in the coronaries showed no change in total volume of atherosclerotic plaque in the statin monotherapy group and a decrease in the statin plus evolocumab group.
Patients with LDL-C < 70 mg/dL
A subgroup of patients had a baseline LDL-C below 70 mg/dL, the lowest level recommended by guideline. Patients in this subgroup who received statin monotherapy remained at a mean LDL-C of 70 mg/dL whereas patients on statin plus evolocumab achieved a mean LDL-C of 24 mg/dL with a mean 2-week post-dosing trough level of 15 mg/dL, an unbelievably low level of LDL-C. In this subgroup, 81% of patients receiving statin plus evolocumab had atheroma regression, compared with 48% of patients in the statin monotherapy group. The percent of patients with atheroma regression in this subgroup of patients with low LDL-C at baseline was twice that seen in the larger study population (33% vs 17%), revealing profound levels of regression in patients treated with dual therapy.
Safety
Limitations
Like all trials, this one has limitations. The population is very select: these are patients with clinically indicated angiogram, not a primary prevention population. Some study participants dropped out, which is always a limitation. And of course, this is a surrogate measure; it is a measure of disease activity, not a measure of morbidity and mortality. Morbidity and mortality data for this new class of drugs should be available in about a year, though this study suggests that those data will be favorable.
CONCLUSION
High LDL-C is universally accepted as a factor in the formation of arterial plaque and atherosclerosis. Statin therapy reduces LDL-C levels to slow or induce regression of coronary atherosclerosis in proportion to the magnitude of LDL-C reduction as measured by IVUS. However, the question of how far to reduce lipid levels has evolved over the last 4 decades. In the 1970s, a normal total cholesterol was < 300 mg/dL. More recent data that suggest optimal LDL-C levels for patients with coronary artery disease may be much lower than commonly achieved.
In this study, in patients with symptomatic coronary artery disease, treatment with statins and the addition of the PCSK9 inhibitor evolocumab achieved mean LDL-C levels of 36.6 mg/dL, produced atheroma regression with a mean change in PAV of about 1% (P < .001), induced regression in a greater percentage of patients, and showed incremental benefit for treatment of LDL-C down to as low as 20 mg/dL. The GLAGOV trial provides intriguing evidence that clinical benefits may extend to LDL-C levels as low as 20 mg/dL; however, the sample size of the trial was modest, providing limited power for safety assessments.
Since this presentation, the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial achieved a median LDL-C of 30 mg/dL and reduced risk of cardiovascular events in patients with atherosclerotic cardiovascular disease treated with evolocumab added to statin therapy.8 Additional large outcomes trials of PCSK9 inhibitors and their role in reducing LDL-C and regression of coronary atheroma and atherosclerosis are eagerly awaited.
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011; 365:2078–2087.
- Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007; 297: 499–508.
- Nissen SE, Nicholls SJ, Sipahi I, et al; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291:1071–1080.
- Robinson JG, Nedergaard BS, RogersWJ, et al; LAPLACE-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014; 311:1870–1882.
- Blom DJ, Hala T, Bolognese M, et al; DESCARTES Investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370:1809–1819.
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial. JAMA 2016; 316:2373–2384.
- Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376:1713–1722.
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011; 365:2078–2087.
- Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007; 297: 499–508.
- Nissen SE, Nicholls SJ, Sipahi I, et al; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291:1071–1080.
- Robinson JG, Nedergaard BS, RogersWJ, et al; LAPLACE-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014; 311:1870–1882.
- Blom DJ, Hala T, Bolognese M, et al; DESCARTES Investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370:1809–1819.
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial. JAMA 2016; 316:2373–2384.
- Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376:1713–1722.
KEY POINTS
- Statin therapy achieves regression of atherosclerosis in proportion to reductions in LDL-C.
- PCSK9 inhibitors are a new class of injectable human monoclonal antibodies shown to lower LDL-C when added to statin therapy.
- Treatment with statins plus the PCSK9 inhibitor, evolocumab, achieved mean LDL-C levels of 36.6 mg/dL, atheroma regression, and demonstrated clinical benefit for LDL-C as low as 20 mg/dL.