User login
Results of the GLAGOV trial
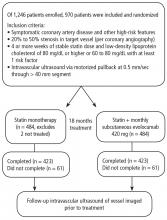
Intravascular ultrasonography (IVUS) has been used for the past 20 years to measure atheromatous plaque in patients with coronary artery disease. The total volume of atherosclerosis in a coronary artery segment can be calculated using IVUS. A rotating transducer produces an image of a single, cross-sectional slice of the artery from which the atheroma area is calculated. A motorized device is used to withdraw the catheter, obtaining a series of cross-sectional slices at 1-mm intervals. The atheroma area for each slice is summated to obtain the total volume of atherosclerosis in the artery.
IVUS has demonstrated that statins slow the progression or even induce regression of coronary atherosclerosis in proportion to the degree of reduction in low-density lipoprotein cholesterol (LDL-C).1–4 No LDL-C-lowering therapy other than statins has shown regression of atherosclerosis in a trial using IVUS. The lowest LDL-C achieved in prior trials using statins was about 60 mg/dL.1,3 While this is very low, lower levels have not previously been explored.
Proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, a new class of drugs, are injectable, fully human monoclonal antibodies that inactivate the PCSK9 protein. PCSK9 inhibitors have been shown to lower LDL-C incrementally when added to statins, achieving very low LDL-C levels.5,6 However, no data exist describing the effect of low LDL-C levels reached using PCSK9 inhibitors on the progression of atherosclerosis.
THE GLAGOV TRIAL
RESULTS
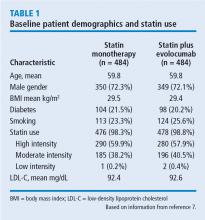
LDL-C levels
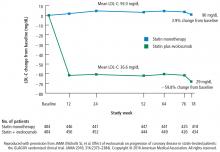
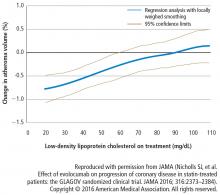
Change in percent atheroma volume
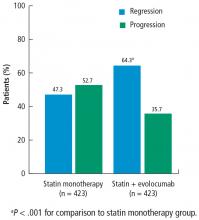
Total atheroma volume and percent of patients with atheroma regression
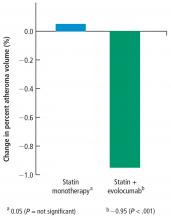
The secondary end point measuring the total atheroma volume in the coronaries showed no change in total volume of atherosclerotic plaque in the statin monotherapy group and a decrease in the statin plus evolocumab group.
Patients with LDL-C < 70 mg/dL
A subgroup of patients had a baseline LDL-C below 70 mg/dL, the lowest level recommended by guideline. Patients in this subgroup who received statin monotherapy remained at a mean LDL-C of 70 mg/dL whereas patients on statin plus evolocumab achieved a mean LDL-C of 24 mg/dL with a mean 2-week post-dosing trough level of 15 mg/dL, an unbelievably low level of LDL-C. In this subgroup, 81% of patients receiving statin plus evolocumab had atheroma regression, compared with 48% of patients in the statin monotherapy group. The percent of patients with atheroma regression in this subgroup of patients with low LDL-C at baseline was twice that seen in the larger study population (33% vs 17%), revealing profound levels of regression in patients treated with dual therapy.
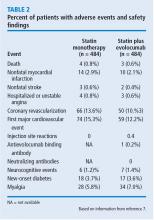
Safety
Limitations
Like all trials, this one has limitations. The population is very select: these are patients with clinically indicated angiogram, not a primary prevention population. Some study participants dropped out, which is always a limitation. And of course, this is a surrogate measure; it is a measure of disease activity, not a measure of morbidity and mortality. Morbidity and mortality data for this new class of drugs should be available in about a year, though this study suggests that those data will be favorable.
CONCLUSION
High LDL-C is universally accepted as a factor in the formation of arterial plaque and atherosclerosis. Statin therapy reduces LDL-C levels to slow or induce regression of coronary atherosclerosis in proportion to the magnitude of LDL-C reduction as measured by IVUS. However, the question of how far to reduce lipid levels has evolved over the last 4 decades. In the 1970s, a normal total cholesterol was < 300 mg/dL. More recent data that suggest optimal LDL-C levels for patients with coronary artery disease may be much lower than commonly achieved.
In this study, in patients with symptomatic coronary artery disease, treatment with statins and the addition of the PCSK9 inhibitor evolocumab achieved mean LDL-C levels of 36.6 mg/dL, produced atheroma regression with a mean change in PAV of about 1% (P < .001), induced regression in a greater percentage of patients, and showed incremental benefit for treatment of LDL-C down to as low as 20 mg/dL. The GLAGOV trial provides intriguing evidence that clinical benefits may extend to LDL-C levels as low as 20 mg/dL; however, the sample size of the trial was modest, providing limited power for safety assessments.
Since this presentation, the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial achieved a median LDL-C of 30 mg/dL and reduced risk of cardiovascular events in patients with atherosclerotic cardiovascular disease treated with evolocumab added to statin therapy.8 Additional large outcomes trials of PCSK9 inhibitors and their role in reducing LDL-C and regression of coronary atheroma and atherosclerosis are eagerly awaited.
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011; 365:2078–2087.
- Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007; 297: 499–508.
- Nissen SE, Nicholls SJ, Sipahi I, et al; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291:1071–1080.
- Robinson JG, Nedergaard BS, RogersWJ, et al; LAPLACE-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014; 311:1870–1882.
- Blom DJ, Hala T, Bolognese M, et al; DESCARTES Investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370:1809–1819.
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial. JAMA 2016; 316:2373–2384.
- Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376:1713–1722.
Intravascular ultrasonography (IVUS) has been used for the past 20 years to measure atheromatous plaque in patients with coronary artery disease. The total volume of atherosclerosis in a coronary artery segment can be calculated using IVUS. A rotating transducer produces an image of a single, cross-sectional slice of the artery from which the atheroma area is calculated. A motorized device is used to withdraw the catheter, obtaining a series of cross-sectional slices at 1-mm intervals. The atheroma area for each slice is summated to obtain the total volume of atherosclerosis in the artery.
IVUS has demonstrated that statins slow the progression or even induce regression of coronary atherosclerosis in proportion to the degree of reduction in low-density lipoprotein cholesterol (LDL-C).1–4 No LDL-C-lowering therapy other than statins has shown regression of atherosclerosis in a trial using IVUS. The lowest LDL-C achieved in prior trials using statins was about 60 mg/dL.1,3 While this is very low, lower levels have not previously been explored.
Proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, a new class of drugs, are injectable, fully human monoclonal antibodies that inactivate the PCSK9 protein. PCSK9 inhibitors have been shown to lower LDL-C incrementally when added to statins, achieving very low LDL-C levels.5,6 However, no data exist describing the effect of low LDL-C levels reached using PCSK9 inhibitors on the progression of atherosclerosis.
THE GLAGOV TRIAL
RESULTS
LDL-C levels
Change in percent atheroma volume
Total atheroma volume and percent of patients with atheroma regression
The secondary end point measuring the total atheroma volume in the coronaries showed no change in total volume of atherosclerotic plaque in the statin monotherapy group and a decrease in the statin plus evolocumab group.
Patients with LDL-C < 70 mg/dL
A subgroup of patients had a baseline LDL-C below 70 mg/dL, the lowest level recommended by guideline. Patients in this subgroup who received statin monotherapy remained at a mean LDL-C of 70 mg/dL whereas patients on statin plus evolocumab achieved a mean LDL-C of 24 mg/dL with a mean 2-week post-dosing trough level of 15 mg/dL, an unbelievably low level of LDL-C. In this subgroup, 81% of patients receiving statin plus evolocumab had atheroma regression, compared with 48% of patients in the statin monotherapy group. The percent of patients with atheroma regression in this subgroup of patients with low LDL-C at baseline was twice that seen in the larger study population (33% vs 17%), revealing profound levels of regression in patients treated with dual therapy.
Safety
Limitations
Like all trials, this one has limitations. The population is very select: these are patients with clinically indicated angiogram, not a primary prevention population. Some study participants dropped out, which is always a limitation. And of course, this is a surrogate measure; it is a measure of disease activity, not a measure of morbidity and mortality. Morbidity and mortality data for this new class of drugs should be available in about a year, though this study suggests that those data will be favorable.
CONCLUSION
High LDL-C is universally accepted as a factor in the formation of arterial plaque and atherosclerosis. Statin therapy reduces LDL-C levels to slow or induce regression of coronary atherosclerosis in proportion to the magnitude of LDL-C reduction as measured by IVUS. However, the question of how far to reduce lipid levels has evolved over the last 4 decades. In the 1970s, a normal total cholesterol was < 300 mg/dL. More recent data that suggest optimal LDL-C levels for patients with coronary artery disease may be much lower than commonly achieved.
In this study, in patients with symptomatic coronary artery disease, treatment with statins and the addition of the PCSK9 inhibitor evolocumab achieved mean LDL-C levels of 36.6 mg/dL, produced atheroma regression with a mean change in PAV of about 1% (P < .001), induced regression in a greater percentage of patients, and showed incremental benefit for treatment of LDL-C down to as low as 20 mg/dL. The GLAGOV trial provides intriguing evidence that clinical benefits may extend to LDL-C levels as low as 20 mg/dL; however, the sample size of the trial was modest, providing limited power for safety assessments.
Since this presentation, the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial achieved a median LDL-C of 30 mg/dL and reduced risk of cardiovascular events in patients with atherosclerotic cardiovascular disease treated with evolocumab added to statin therapy.8 Additional large outcomes trials of PCSK9 inhibitors and their role in reducing LDL-C and regression of coronary atheroma and atherosclerosis are eagerly awaited.
Intravascular ultrasonography (IVUS) has been used for the past 20 years to measure atheromatous plaque in patients with coronary artery disease. The total volume of atherosclerosis in a coronary artery segment can be calculated using IVUS. A rotating transducer produces an image of a single, cross-sectional slice of the artery from which the atheroma area is calculated. A motorized device is used to withdraw the catheter, obtaining a series of cross-sectional slices at 1-mm intervals. The atheroma area for each slice is summated to obtain the total volume of atherosclerosis in the artery.
IVUS has demonstrated that statins slow the progression or even induce regression of coronary atherosclerosis in proportion to the degree of reduction in low-density lipoprotein cholesterol (LDL-C).1–4 No LDL-C-lowering therapy other than statins has shown regression of atherosclerosis in a trial using IVUS. The lowest LDL-C achieved in prior trials using statins was about 60 mg/dL.1,3 While this is very low, lower levels have not previously been explored.
Proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, a new class of drugs, are injectable, fully human monoclonal antibodies that inactivate the PCSK9 protein. PCSK9 inhibitors have been shown to lower LDL-C incrementally when added to statins, achieving very low LDL-C levels.5,6 However, no data exist describing the effect of low LDL-C levels reached using PCSK9 inhibitors on the progression of atherosclerosis.
THE GLAGOV TRIAL
RESULTS
LDL-C levels
Change in percent atheroma volume
Total atheroma volume and percent of patients with atheroma regression
The secondary end point measuring the total atheroma volume in the coronaries showed no change in total volume of atherosclerotic plaque in the statin monotherapy group and a decrease in the statin plus evolocumab group.
Patients with LDL-C < 70 mg/dL
A subgroup of patients had a baseline LDL-C below 70 mg/dL, the lowest level recommended by guideline. Patients in this subgroup who received statin monotherapy remained at a mean LDL-C of 70 mg/dL whereas patients on statin plus evolocumab achieved a mean LDL-C of 24 mg/dL with a mean 2-week post-dosing trough level of 15 mg/dL, an unbelievably low level of LDL-C. In this subgroup, 81% of patients receiving statin plus evolocumab had atheroma regression, compared with 48% of patients in the statin monotherapy group. The percent of patients with atheroma regression in this subgroup of patients with low LDL-C at baseline was twice that seen in the larger study population (33% vs 17%), revealing profound levels of regression in patients treated with dual therapy.
Safety
Limitations
Like all trials, this one has limitations. The population is very select: these are patients with clinically indicated angiogram, not a primary prevention population. Some study participants dropped out, which is always a limitation. And of course, this is a surrogate measure; it is a measure of disease activity, not a measure of morbidity and mortality. Morbidity and mortality data for this new class of drugs should be available in about a year, though this study suggests that those data will be favorable.
CONCLUSION
High LDL-C is universally accepted as a factor in the formation of arterial plaque and atherosclerosis. Statin therapy reduces LDL-C levels to slow or induce regression of coronary atherosclerosis in proportion to the magnitude of LDL-C reduction as measured by IVUS. However, the question of how far to reduce lipid levels has evolved over the last 4 decades. In the 1970s, a normal total cholesterol was < 300 mg/dL. More recent data that suggest optimal LDL-C levels for patients with coronary artery disease may be much lower than commonly achieved.
In this study, in patients with symptomatic coronary artery disease, treatment with statins and the addition of the PCSK9 inhibitor evolocumab achieved mean LDL-C levels of 36.6 mg/dL, produced atheroma regression with a mean change in PAV of about 1% (P < .001), induced regression in a greater percentage of patients, and showed incremental benefit for treatment of LDL-C down to as low as 20 mg/dL. The GLAGOV trial provides intriguing evidence that clinical benefits may extend to LDL-C levels as low as 20 mg/dL; however, the sample size of the trial was modest, providing limited power for safety assessments.
Since this presentation, the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial achieved a median LDL-C of 30 mg/dL and reduced risk of cardiovascular events in patients with atherosclerotic cardiovascular disease treated with evolocumab added to statin therapy.8 Additional large outcomes trials of PCSK9 inhibitors and their role in reducing LDL-C and regression of coronary atheroma and atherosclerosis are eagerly awaited.
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011; 365:2078–2087.
- Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007; 297: 499–508.
- Nissen SE, Nicholls SJ, Sipahi I, et al; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291:1071–1080.
- Robinson JG, Nedergaard BS, RogersWJ, et al; LAPLACE-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014; 311:1870–1882.
- Blom DJ, Hala T, Bolognese M, et al; DESCARTES Investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370:1809–1819.
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial. JAMA 2016; 316:2373–2384.
- Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376:1713–1722.
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011; 365:2078–2087.
- Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007; 297: 499–508.
- Nissen SE, Nicholls SJ, Sipahi I, et al; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004; 291:1071–1080.
- Robinson JG, Nedergaard BS, RogersWJ, et al; LAPLACE-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014; 311:1870–1882.
- Blom DJ, Hala T, Bolognese M, et al; DESCARTES Investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370:1809–1819.
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial. JAMA 2016; 316:2373–2384.
- Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376:1713–1722.
KEY POINTS
- Statin therapy achieves regression of atherosclerosis in proportion to reductions in LDL-C.
- PCSK9 inhibitors are a new class of injectable human monoclonal antibodies shown to lower LDL-C when added to statin therapy.
- Treatment with statins plus the PCSK9 inhibitor, evolocumab, achieved mean LDL-C levels of 36.6 mg/dL, atheroma regression, and demonstrated clinical benefit for LDL-C as low as 20 mg/dL.
Niacin's effect on cardiovascular risk: Have we finally learned our lesson?
Randomized controlled trials have unequivocally shown that lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins reduces the rate of cardiovascular events.1–3 Yet many patients still have heart attacks even though they are on statins, so the search continues for other agents to lower cardiovascular risk.4
Niacin has been used for its lipid-modifying effects for more than 50 years. In addition to being the most potent agent for raising the level of high-density lipoprotein cholesterol (HDL-C), niacin decreases the atherogenic lipids triglyceride, LDL-C, and lipoprotein (a)5 and can be very effective in treating mixed dyslipidemias such as hypertriglyceridemia and low HDL-C. This is particularly important for the challenging patients seen in preventive cardiology clinics.
In 1986, before statins were available, the Coronary Drug Project6 showed that immediate-release forms of niacin lowered the rates of nonfatal myocardial infarction and long-term mortality. Later, imaging studies demonstrated that niacin slows progression of carotid intima-medial thickness and coronary atherosclerosis.7–9 Furthermore, meta-analyses of these studies suggest cardiovascular benefit for patients at high vascular risk.10
However, niacin is difficult to use in clinical practice. The near-ubiquitous experience of flushing has limited our ability to give doses high enough to modify plasma lipid levels and rates of clinical events.
To try to mitigate this side effect, investigators developed extended-release formulations and agents such as laropiprant, a chemical antagonist of the interaction between niacin and epidermal prostanoid receptors implicated as the mechanism behind flushing. Although these innovations do not eliminate flushing, they reduce it, and thus have prompted hopes of using niacin more widely in statin-treated patients. However, whether widespread use of niacin on a background of statin therapy would have an impact on cardiovascular events remained to be established.
WHAT WE HAVE LEARNED LATELY ABOUT NIACIN?
More-tolerable formulations of niacin prompted interest in its potential to lower the residual cardiovascular risk observed in statin-treated patients. Two large clinical trials attempted to determine its impact on cardiovascular events in the contemporary era.
The AIM-HIGH study
In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study,11 3,414 patients at high vascular risk with low HDL-C were treated with niacin or placebo. The trial was stopped early because of no evidence of clinical benefit with niacin and because of concern about an increased risk of stroke, a finding ultimately not observed on a complete review of the data.
I reviewed the limitations of this study earlier in this journal.12 The study was small, use of low-dose niacin was allowed in the placebo group, and physicians could treat high LDL-C as they saw fit during the study, so that more patients in the placebo group received high-dose statin therapy and ezetimibe. All of this likely limited the study’s ability to measure the clinical impact of niacin. As a result, this study was not a pure evaluation of the benefits of niacin vs placebo in addition to standard medical therapy. Hope remained that a much larger study with greater statistical power and a simpler design would provide a definitive answer.
HPS2–THRIVE
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), with more than 40,000 patients, was the largest cardiovascular outcomes trial of lipid-modifying therapy to date.13 Its purpose was to determine whether extended-release niacin plus the prostanoid receptor antagonist laropiprant would reduce the rate of cardiovascular events in patients with clinically established vascular disease.
Patients age 50 to 80 with a history of myocardial infarction, ischemic stroke, transient ischemic attack, peripheral arterial disease, or diabetes with other forms of coronary heart disease received a standardized LDL-C-lowering regimen with simvastatin 40 mg daily, with or without ezetimibe 10 mg daily, to achieve a total cholesterol target of 135 mg/dL or below. All were treated with extended-release niacin 2 g daily plus laropiprant 40 mg daily for 1 month to assess compliance. They were then randomized to treatment with extended-release niacin 2 g plus laropiprant 40 mg or placebo daily. At baseline, the mean lipid values were LDL-C 63 mg/dL, HDL-C 44 mg/dL, and triglyceride 125 mg/dL.
Before the end of the trial, the investigators reported a high rate of myopathy-related adverse events in the niacin group, particularly in Chinese patients.13 This contributed to a high dropout rate in the niacin group, in which one quarter of patients stopped taking the study drug.
During the study, niacin lowered the LDL-C level by a mean of 10 mg/dL, lowered triglycerides by 33 mg/dL, and raised HDL-C by 6 mg/dL. On the basis of previous observational studies and randomized clinical trials, the authors calculated that such lipid changes should translate to a 10% to 15% reduction in vascular events. However, no reduction was observed in the primary end point of major vascular events, which included nonfatal myocardial infarction, coronary death, any nonfatal or fatal stroke, and any arterial revascularization, including amputation. The rates were 15% in the placebo group vs 14.5% in the niacin group (P = .96).
A statistically significant 10% reduction in the rate of arterial revascularization was observed in the niacin group, perhaps consistent with earlier observations of an antiatherosclerotic effect.
Subgroup analyses, while always to be interpreted with caution, also provide some interesting findings for consideration. A significant interaction was observed between treatment and baseline LDL-C, with those in the highest LDL-C tertile (> 77 mg/dL) demonstrating a potential reduction in the primary end point with niacin treatment. In addition, a trend toward potential benefit with niacin in patients in Europe, but not in China, was also observed; however, this just failed to meet statistical significance.
HPS2-THRIVE provided important information about the safety of extended-release niacin in combination with laropiprant. The niacin group experienced higher rates not only of myopathy but also of diabetic complications, new diagnosis of diabetes, serious infections, and bleeding. Whether these observations were related to niacin or to laropiprant is unknown. In fact, recent reports suggest laropiprant has adverse effects that may have substantially reduced the potential benefits of niacin.
The overall conclusion of HPS2-THRIVE was that there was no widespread clinical benefit from the combination of niacin and laropiprant in statin-treated patients with vascular disease, and that there was a potential increase in adverse events. Accordingly, the combination treatment will not be integrated into clinical practice.
WHERE DO WE GO FROM HERE?
Despite their limitations, these two large trials suggest that niacin does not reduce cardiovascular risk in patients already receiving a statin.
Might some subgroups be more likely to benefit from niacin? The finding of potential benefit in patients with higher baseline LDL-C suggests this may be true. At baseline, the HPS2-THRIVE patients had very good LDL-C control and had HDL-C levels within the normal range, not necessarily reflecting the patients we see in daily practice, who require more effective reductions in vascular risk. Furthermore, failure of both fibrates and niacin to reduce risk may have reflected the attempt to study these agents in broad patient populations as opposed to focusing on specific cohorts, such as patients with mixed dyslipidemia, for which there is suggestion of benefit.14 It seems unlikely that such a study will be performed in a clinical setting in which niacin may be of greater utility. The experience of adverse events would appear to make that a certainty.
For now, niacin will remain useful in lipid clinics for managing refractory dyslipidemia. Specifically, its ability to lower triglyceride and lipoprotein (a) and to raise HDL-C will continue to be of interest in the clinical management of patients and in the formulation of treatment guidelines. Another reason to use it is to lower LDL-C in patients who cannot tolerate statins. However, there is currently no evidence from randomized controlled trials to support its broader use.
While registry information could provide some sense of real-world effects of niacin’s use, this is a suboptimal way to evaluate the potential efficacy of a therapy—randomized controlled trials are the gold standard. The major flaws of both of the large trials of niacin point out the need for thoughtful study design to avoid incorrectly dismissing potentially useful therapies. But for now, the renaissance of niacin as a means of lowering cardiovascular risk is only wishful thinking.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005; 46:1225–1228.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med 2012; 79:38–43.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875–1884.
Randomized controlled trials have unequivocally shown that lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins reduces the rate of cardiovascular events.1–3 Yet many patients still have heart attacks even though they are on statins, so the search continues for other agents to lower cardiovascular risk.4
Niacin has been used for its lipid-modifying effects for more than 50 years. In addition to being the most potent agent for raising the level of high-density lipoprotein cholesterol (HDL-C), niacin decreases the atherogenic lipids triglyceride, LDL-C, and lipoprotein (a)5 and can be very effective in treating mixed dyslipidemias such as hypertriglyceridemia and low HDL-C. This is particularly important for the challenging patients seen in preventive cardiology clinics.
In 1986, before statins were available, the Coronary Drug Project6 showed that immediate-release forms of niacin lowered the rates of nonfatal myocardial infarction and long-term mortality. Later, imaging studies demonstrated that niacin slows progression of carotid intima-medial thickness and coronary atherosclerosis.7–9 Furthermore, meta-analyses of these studies suggest cardiovascular benefit for patients at high vascular risk.10
However, niacin is difficult to use in clinical practice. The near-ubiquitous experience of flushing has limited our ability to give doses high enough to modify plasma lipid levels and rates of clinical events.
To try to mitigate this side effect, investigators developed extended-release formulations and agents such as laropiprant, a chemical antagonist of the interaction between niacin and epidermal prostanoid receptors implicated as the mechanism behind flushing. Although these innovations do not eliminate flushing, they reduce it, and thus have prompted hopes of using niacin more widely in statin-treated patients. However, whether widespread use of niacin on a background of statin therapy would have an impact on cardiovascular events remained to be established.
WHAT WE HAVE LEARNED LATELY ABOUT NIACIN?
More-tolerable formulations of niacin prompted interest in its potential to lower the residual cardiovascular risk observed in statin-treated patients. Two large clinical trials attempted to determine its impact on cardiovascular events in the contemporary era.
The AIM-HIGH study
In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study,11 3,414 patients at high vascular risk with low HDL-C were treated with niacin or placebo. The trial was stopped early because of no evidence of clinical benefit with niacin and because of concern about an increased risk of stroke, a finding ultimately not observed on a complete review of the data.
I reviewed the limitations of this study earlier in this journal.12 The study was small, use of low-dose niacin was allowed in the placebo group, and physicians could treat high LDL-C as they saw fit during the study, so that more patients in the placebo group received high-dose statin therapy and ezetimibe. All of this likely limited the study’s ability to measure the clinical impact of niacin. As a result, this study was not a pure evaluation of the benefits of niacin vs placebo in addition to standard medical therapy. Hope remained that a much larger study with greater statistical power and a simpler design would provide a definitive answer.
HPS2–THRIVE
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), with more than 40,000 patients, was the largest cardiovascular outcomes trial of lipid-modifying therapy to date.13 Its purpose was to determine whether extended-release niacin plus the prostanoid receptor antagonist laropiprant would reduce the rate of cardiovascular events in patients with clinically established vascular disease.
Patients age 50 to 80 with a history of myocardial infarction, ischemic stroke, transient ischemic attack, peripheral arterial disease, or diabetes with other forms of coronary heart disease received a standardized LDL-C-lowering regimen with simvastatin 40 mg daily, with or without ezetimibe 10 mg daily, to achieve a total cholesterol target of 135 mg/dL or below. All were treated with extended-release niacin 2 g daily plus laropiprant 40 mg daily for 1 month to assess compliance. They were then randomized to treatment with extended-release niacin 2 g plus laropiprant 40 mg or placebo daily. At baseline, the mean lipid values were LDL-C 63 mg/dL, HDL-C 44 mg/dL, and triglyceride 125 mg/dL.
Before the end of the trial, the investigators reported a high rate of myopathy-related adverse events in the niacin group, particularly in Chinese patients.13 This contributed to a high dropout rate in the niacin group, in which one quarter of patients stopped taking the study drug.
During the study, niacin lowered the LDL-C level by a mean of 10 mg/dL, lowered triglycerides by 33 mg/dL, and raised HDL-C by 6 mg/dL. On the basis of previous observational studies and randomized clinical trials, the authors calculated that such lipid changes should translate to a 10% to 15% reduction in vascular events. However, no reduction was observed in the primary end point of major vascular events, which included nonfatal myocardial infarction, coronary death, any nonfatal or fatal stroke, and any arterial revascularization, including amputation. The rates were 15% in the placebo group vs 14.5% in the niacin group (P = .96).
A statistically significant 10% reduction in the rate of arterial revascularization was observed in the niacin group, perhaps consistent with earlier observations of an antiatherosclerotic effect.
Subgroup analyses, while always to be interpreted with caution, also provide some interesting findings for consideration. A significant interaction was observed between treatment and baseline LDL-C, with those in the highest LDL-C tertile (> 77 mg/dL) demonstrating a potential reduction in the primary end point with niacin treatment. In addition, a trend toward potential benefit with niacin in patients in Europe, but not in China, was also observed; however, this just failed to meet statistical significance.
HPS2-THRIVE provided important information about the safety of extended-release niacin in combination with laropiprant. The niacin group experienced higher rates not only of myopathy but also of diabetic complications, new diagnosis of diabetes, serious infections, and bleeding. Whether these observations were related to niacin or to laropiprant is unknown. In fact, recent reports suggest laropiprant has adverse effects that may have substantially reduced the potential benefits of niacin.
The overall conclusion of HPS2-THRIVE was that there was no widespread clinical benefit from the combination of niacin and laropiprant in statin-treated patients with vascular disease, and that there was a potential increase in adverse events. Accordingly, the combination treatment will not be integrated into clinical practice.
WHERE DO WE GO FROM HERE?
Despite their limitations, these two large trials suggest that niacin does not reduce cardiovascular risk in patients already receiving a statin.
Might some subgroups be more likely to benefit from niacin? The finding of potential benefit in patients with higher baseline LDL-C suggests this may be true. At baseline, the HPS2-THRIVE patients had very good LDL-C control and had HDL-C levels within the normal range, not necessarily reflecting the patients we see in daily practice, who require more effective reductions in vascular risk. Furthermore, failure of both fibrates and niacin to reduce risk may have reflected the attempt to study these agents in broad patient populations as opposed to focusing on specific cohorts, such as patients with mixed dyslipidemia, for which there is suggestion of benefit.14 It seems unlikely that such a study will be performed in a clinical setting in which niacin may be of greater utility. The experience of adverse events would appear to make that a certainty.
For now, niacin will remain useful in lipid clinics for managing refractory dyslipidemia. Specifically, its ability to lower triglyceride and lipoprotein (a) and to raise HDL-C will continue to be of interest in the clinical management of patients and in the formulation of treatment guidelines. Another reason to use it is to lower LDL-C in patients who cannot tolerate statins. However, there is currently no evidence from randomized controlled trials to support its broader use.
While registry information could provide some sense of real-world effects of niacin’s use, this is a suboptimal way to evaluate the potential efficacy of a therapy—randomized controlled trials are the gold standard. The major flaws of both of the large trials of niacin point out the need for thoughtful study design to avoid incorrectly dismissing potentially useful therapies. But for now, the renaissance of niacin as a means of lowering cardiovascular risk is only wishful thinking.
Randomized controlled trials have unequivocally shown that lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins reduces the rate of cardiovascular events.1–3 Yet many patients still have heart attacks even though they are on statins, so the search continues for other agents to lower cardiovascular risk.4
Niacin has been used for its lipid-modifying effects for more than 50 years. In addition to being the most potent agent for raising the level of high-density lipoprotein cholesterol (HDL-C), niacin decreases the atherogenic lipids triglyceride, LDL-C, and lipoprotein (a)5 and can be very effective in treating mixed dyslipidemias such as hypertriglyceridemia and low HDL-C. This is particularly important for the challenging patients seen in preventive cardiology clinics.
In 1986, before statins were available, the Coronary Drug Project6 showed that immediate-release forms of niacin lowered the rates of nonfatal myocardial infarction and long-term mortality. Later, imaging studies demonstrated that niacin slows progression of carotid intima-medial thickness and coronary atherosclerosis.7–9 Furthermore, meta-analyses of these studies suggest cardiovascular benefit for patients at high vascular risk.10
However, niacin is difficult to use in clinical practice. The near-ubiquitous experience of flushing has limited our ability to give doses high enough to modify plasma lipid levels and rates of clinical events.
To try to mitigate this side effect, investigators developed extended-release formulations and agents such as laropiprant, a chemical antagonist of the interaction between niacin and epidermal prostanoid receptors implicated as the mechanism behind flushing. Although these innovations do not eliminate flushing, they reduce it, and thus have prompted hopes of using niacin more widely in statin-treated patients. However, whether widespread use of niacin on a background of statin therapy would have an impact on cardiovascular events remained to be established.
WHAT WE HAVE LEARNED LATELY ABOUT NIACIN?
More-tolerable formulations of niacin prompted interest in its potential to lower the residual cardiovascular risk observed in statin-treated patients. Two large clinical trials attempted to determine its impact on cardiovascular events in the contemporary era.
The AIM-HIGH study
In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study,11 3,414 patients at high vascular risk with low HDL-C were treated with niacin or placebo. The trial was stopped early because of no evidence of clinical benefit with niacin and because of concern about an increased risk of stroke, a finding ultimately not observed on a complete review of the data.
I reviewed the limitations of this study earlier in this journal.12 The study was small, use of low-dose niacin was allowed in the placebo group, and physicians could treat high LDL-C as they saw fit during the study, so that more patients in the placebo group received high-dose statin therapy and ezetimibe. All of this likely limited the study’s ability to measure the clinical impact of niacin. As a result, this study was not a pure evaluation of the benefits of niacin vs placebo in addition to standard medical therapy. Hope remained that a much larger study with greater statistical power and a simpler design would provide a definitive answer.
HPS2–THRIVE
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), with more than 40,000 patients, was the largest cardiovascular outcomes trial of lipid-modifying therapy to date.13 Its purpose was to determine whether extended-release niacin plus the prostanoid receptor antagonist laropiprant would reduce the rate of cardiovascular events in patients with clinically established vascular disease.
Patients age 50 to 80 with a history of myocardial infarction, ischemic stroke, transient ischemic attack, peripheral arterial disease, or diabetes with other forms of coronary heart disease received a standardized LDL-C-lowering regimen with simvastatin 40 mg daily, with or without ezetimibe 10 mg daily, to achieve a total cholesterol target of 135 mg/dL or below. All were treated with extended-release niacin 2 g daily plus laropiprant 40 mg daily for 1 month to assess compliance. They were then randomized to treatment with extended-release niacin 2 g plus laropiprant 40 mg or placebo daily. At baseline, the mean lipid values were LDL-C 63 mg/dL, HDL-C 44 mg/dL, and triglyceride 125 mg/dL.
Before the end of the trial, the investigators reported a high rate of myopathy-related adverse events in the niacin group, particularly in Chinese patients.13 This contributed to a high dropout rate in the niacin group, in which one quarter of patients stopped taking the study drug.
During the study, niacin lowered the LDL-C level by a mean of 10 mg/dL, lowered triglycerides by 33 mg/dL, and raised HDL-C by 6 mg/dL. On the basis of previous observational studies and randomized clinical trials, the authors calculated that such lipid changes should translate to a 10% to 15% reduction in vascular events. However, no reduction was observed in the primary end point of major vascular events, which included nonfatal myocardial infarction, coronary death, any nonfatal or fatal stroke, and any arterial revascularization, including amputation. The rates were 15% in the placebo group vs 14.5% in the niacin group (P = .96).
A statistically significant 10% reduction in the rate of arterial revascularization was observed in the niacin group, perhaps consistent with earlier observations of an antiatherosclerotic effect.
Subgroup analyses, while always to be interpreted with caution, also provide some interesting findings for consideration. A significant interaction was observed between treatment and baseline LDL-C, with those in the highest LDL-C tertile (> 77 mg/dL) demonstrating a potential reduction in the primary end point with niacin treatment. In addition, a trend toward potential benefit with niacin in patients in Europe, but not in China, was also observed; however, this just failed to meet statistical significance.
HPS2-THRIVE provided important information about the safety of extended-release niacin in combination with laropiprant. The niacin group experienced higher rates not only of myopathy but also of diabetic complications, new diagnosis of diabetes, serious infections, and bleeding. Whether these observations were related to niacin or to laropiprant is unknown. In fact, recent reports suggest laropiprant has adverse effects that may have substantially reduced the potential benefits of niacin.
The overall conclusion of HPS2-THRIVE was that there was no widespread clinical benefit from the combination of niacin and laropiprant in statin-treated patients with vascular disease, and that there was a potential increase in adverse events. Accordingly, the combination treatment will not be integrated into clinical practice.
WHERE DO WE GO FROM HERE?
Despite their limitations, these two large trials suggest that niacin does not reduce cardiovascular risk in patients already receiving a statin.
Might some subgroups be more likely to benefit from niacin? The finding of potential benefit in patients with higher baseline LDL-C suggests this may be true. At baseline, the HPS2-THRIVE patients had very good LDL-C control and had HDL-C levels within the normal range, not necessarily reflecting the patients we see in daily practice, who require more effective reductions in vascular risk. Furthermore, failure of both fibrates and niacin to reduce risk may have reflected the attempt to study these agents in broad patient populations as opposed to focusing on specific cohorts, such as patients with mixed dyslipidemia, for which there is suggestion of benefit.14 It seems unlikely that such a study will be performed in a clinical setting in which niacin may be of greater utility. The experience of adverse events would appear to make that a certainty.
For now, niacin will remain useful in lipid clinics for managing refractory dyslipidemia. Specifically, its ability to lower triglyceride and lipoprotein (a) and to raise HDL-C will continue to be of interest in the clinical management of patients and in the formulation of treatment guidelines. Another reason to use it is to lower LDL-C in patients who cannot tolerate statins. However, there is currently no evidence from randomized controlled trials to support its broader use.
While registry information could provide some sense of real-world effects of niacin’s use, this is a suboptimal way to evaluate the potential efficacy of a therapy—randomized controlled trials are the gold standard. The major flaws of both of the large trials of niacin point out the need for thoughtful study design to avoid incorrectly dismissing potentially useful therapies. But for now, the renaissance of niacin as a means of lowering cardiovascular risk is only wishful thinking.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005; 46:1225–1228.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med 2012; 79:38–43.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875–1884.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005; 46:1225–1228.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med 2012; 79:38–43.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875–1884.
Is niacin ineffective? Or did AIM-HIGH miss its target?
The recent publication of the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes)1 has thrown the use of niacin as a lipid-modifying therapy into question. The trial was stopped early because an interim analysis found that the patients who took extended-release niacin had no clinical benefit. In addition, it found a trend toward more ischemic strokes, though this finding was later found not to be statistically significant.
Complicating the interpretation, while both the treatment group and the control group in the study received statin therapy, the researchers attempted to keep low-density lipoprotein cholesterol (LDL-C) levels equal, meaning that patients in the control group received more intensive statin therapy than those in the treatment group. And the placebo that the control patients received was actually a low dose of niacin, to induce flushing and thus to blind study participants and their physicians to which drug they were taking.
In the article that follows, I will explore the background, design, findings, and implications of this key trial and try to untangle the many questions about how to interpret it.
LOWERING LDL-C REDUCES RISK, BUT DOES NOT ELIMINATE IT
Large randomized controlled trials have consistently shown that lowering the level of LDL-C reduces cardiovascular event rates by 25% to 45% both in people who are known to have coronary artery disease and in those who are not.2–4 As a result, guidelines for preventing cardiovascular disease have increasingly emphasized maintaining low LDL-C levels. This has led to a proliferation in the use of inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (statins) in patients at high cardiovascular risk.
However, these agents only reduce the risk—they do not eliminate it. Needed are additional therapies to complement existing LDL-C-lowering approaches to lower the cardiovascular risk even further.
Raising HDL-C: The next frontier
One such strategy for further lowering cardiovascular risk that has received considerable interest is to promote the biological activity of the “good” cholesterol.
Studies have consistently shown that the higher the plasma level of high-density lipoprotein cholesterol (HDL-C), the lower the risk of cardiovascular events, suggesting that raising HDL-C may be beneficial.5 Studies in animals with atherosclerosis show that raising HDL-C via genetic modification of the animal or direct infusion of the molecule has a favorable impact on both the size and the structure of experimental plaque.6,7
Accordingly, much activity has focused on developing new therapies that raise HDL-C more effectively than current ones.
Why niacin should protect the heart
For more than 50 years, niacin has been used to manage dyslipidemia.
In addition to raising HDL-C levels more effectively than any other agent available today, niacin also lowers the levels of LDL-C, triglycerides, and lipoprotein (a).8 Before statins were available, the Coronary Drug Project found that niacin reduced the rate of nonfatal myocardial infarction and the 15-year mortality rate.9 In addition, niacin has been shown to slow the progression of carotid intimal-medial thickness and coronary atherosclerosis, and even to reverse these processes in some trials.10–12
However, a number of issues remain about using niacin to prevent cardiovascular events. Nearly all patients who take it experience flushing, which limits its tolerability and, thus, our ability to titrate doses to levels needed for adequate lipid changes. While a number of modifications of niacin administration have been developed (eg, extended-release formulations and products that inhibit flushing), no large study has tested the clinical efficacy of these strategies. Furthermore, until AIM-HIGH, no large-scale trial had directly evaluated the impact of niacin therapy on a background of statin therapy.
AIM-HIGH STUDY DESIGN
The intent of the AIM-HIGH trial was to determine whether extended-release niacin (Niaspan) would reduce the risk of cardiovascular events when added to therapy with a statin—in this case, simvastatin (Zocor) supplemented with ezetimibe (Zetia).1
The trial was funded by the National Heart, Lung, and Blood Institute (NHLBI) and by Abbott Laboratories, which also supplied the extended-release niacin and the ezetimibe. Merck donated the simvastatin.
Patient characteristics
The patients were all at least 45 years of age with established, stable coronary heart disease, cerebrovascular or carotid arterial disease, or peripheral arterial disease. They also had to have low levels of HDL-C (< 40 mg/dL in men, < 50 mg/dL in women), elevated triglycerides (150–400 mg/dL), and LDL-C levels lower than 180 mg/dL if they were not taking a statin at entry.
The mean age of the patients was 64 years, 85% were men, and 92% were white. They had a high prevalence of cardiovascular risk factors: 34% had diabetes, 71% had hypertension, and 81% had metabolic syndrome. Nearly all (94%) of the patients were taking a statin at entry; 76% had been taking one for more than 1 year, and 40% had been taking one for more than 5 years.1
Simvastatin, ezetimibe, and either niacin or placebo
All lipid-modifying agents except statins and ezetimibe were stopped for least 4 weeks after enrollment.
All patients then entered a 4- to 8-week open-label period, during which they took simvastatin 40 mg daily and extended-release niacin starting at 500 mg and increased weekly up to 2,000 mg daily. Patients who could tolerate at least 1,500 mg daily were randomly assigned to treatment with either niacin 1,500 to 2,000 mg or matching placebo. Both groups continued to receive simvastatin. The placebo contained a small dose of immediate-release niacin (50 mg) in each tablet to induce flushing and to maintain blinding of treatment.
Given that niacin also lowers LDL-C, an algorithm was used to try to keep LDL-C levels roughly the same in both treatment groups. This involved adjusting the simvastatin dose and permitting the use of ezetimibe 10 mg to keep the LDL-C level between 40 and 80 mg/dL. Accordingly, participating physicians were told their patients’ LDL-C levels but were blinded to their HDL-C and triglyceride levels throughout the study.
Every 6 months, patients had a follow-up visit in the clinic, and midway through each 6-month interval they received a phone call from the investigators.1
AIM-HIGH end points
The primary end point was the composite of the first event of death due to coronary heart disease, nonfatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome, or symptom-driven revascularization of the coronary or cerebral arteries.
Secondary end points were:
- Death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, or hospitalization for acute coronary syndrome
- Death from coronary heart disease, nonfatal myocardial infarction, or ischemic stroke
- Death from cardiovascular causes.
Tertiary end points included:
- Death from any cause
- Individual components of the primary end point
- Prespecified subgroups according to sex, history or no history of diabetes, and presence or absence of the metabolic syndrome.1
All clinical events were adjudicated by a central committee.
STUDY HALTED EARLY
The study was planned to run for a mean of 4.6 years, during which 800 primary end point events were expected. With these numbers, the investigators calculated that the study had 85% power to detect a 25% reduction in the primary end point, at a one-sided alpha level of 0.025.
The plan called for an interim analysis when 50% of the anticipated events had occurred, with prespecified stopping boundaries based on either efficacy or futility. The boundary for lack of efficacy required an observed hazard ratio of at least 1.02 with a probability of less than .001.
In the interim analysis, after a median follow-up of only 3 years, the data and safety monitoring board recommended stopping the study early because the boundary for futility had been crossed and, unexpectedly, the rate of ischemic stroke was higher in the niacin-treated patients than in those receiving placebo.
MAJOR FINDINGS OF AIM-HIGH
Of 4,273 patients who began open-label treatment with niacin, 3,414 were randomized to treatment with niacin or placebo.1
HDL-C levels went up in both groups
At 2 years:
- HDL-C levels had increased by 25.0% (to 42 mg/dL) in the niacin group and by 9.8% (to 38 mg/dL) in the placebo group
- Triglycerides had decreased by 28.6% with niacin and by 8.1% with placebo
- LDL-C had decreased by 12.0% with niacin and by 5.5% with placebo.
Patients in the placebo group were more likely to have subsequently received the maximum dose of simvastatin, ie, 80 mg/day (24.7% vs 17.5%), and to have received ezetimibe (21.5% vs 9.5%). More patients in the niacin group required either dose reduction of the study drug (6.3% vs 3.4%) or drug discontinuation (25.4% vs 20.1%).1
No difference in the primary end point
There was no difference between the two treatment groups in the rate of the primary end point, which occurred in 282 (16.4%) of the 1,718 patients in the niacin group and 272 (16.2%) of the 1,696 patients in the placebo group (P = .79; hazard ratio 1.02, 95% confidence interval 0.87–1.21).1
However, more patients in the niacin group than in the placebo group who reached the primary end point did so by having a first ischemic stroke: 27 patients (1.6%) vs 15 patients (0.9%). Eight of these patients, all in the niacin group, had their stroke between 2 months and 4 years after they had stopped taking the study drug.
Further analysis that included all ischemic strokes revealed the same trend: 29 vs 18 patients (P = .11).1
No benefit was observed for niacin-treated patients in terms of any of the secondary or tertiary end points.
Subgroup analysis revealed no evidence of statistical heterogeneity: ie, niacin seemed to lack efficacy in all the prespecified subgroups studied (age 65 and older vs younger, men vs women, and those with or without diabetes, metabolic syndrome, prior myocardial infarction, or statin use at entry).
In general, niacin was well tolerated in the active-treatment group, with a low incidence of liver and muscle abnormalities.
PUTTING AIM-HIGH IN CONTEXT
How should practicing clinicians interpret these outcomes?
Ever since the NHLBI reported (in an urgent press release) that it was stopping the study early due to futility and a potential excess of strokes,13 there has been considerable debate as to which factors contributed to these outcomes. In the wake of the publication of more detailed information about the trial,1 this debate is likely to continue.
The AIM-HIGH results can be interpreted in several ways:
- Perhaps niacin is no good as a preventive agent
- Perhaps raising HDL-C is flawed as a preventive strategy
- Perhaps AIM-HIGH had methodologic flaws, such as looking at the wrong patient cohort or using a treatment protocol that set itself up for failure
- Perhaps statins are so good that, once you prescribe one, anything else you give provides no additional benefit.
Which of these is correct?
Is niacin no good?
In its most simple form, AIM-HIGH has always been seen as a clinical trial of niacin. While the early trials of immediate-release niacin were encouraging in terms of its effects on lipids, atherosclerotic plaque, and cardiovascular outcomes, using it in clinical practice has always been challenging, largely because many patients cannot tolerate it in doses high enough to be effective. A number of developments have improved niacin’s tolerability, but its clinical impact in the statin era has not been evaluated.
Niacin’s lack of efficacy in this trial will ultimately be viewed as a failure of the drug itself, but is this the case?
AIM-HIGH was not simply a direct comparison of niacin vs placebo on top of standard medical practice. The investigators recognized that niacin has additional effects—in particular, lowering levels of atherogenic lipids—and they attempted to control for these effects by titrating the other LDL-C-lowering therapies during the study. As a result, the trial was actually a comparison between niacin plus low-dose simvastatin on the one hand, and placebo plus high-dose simvastatin (and, more often, also ezetimibe) on the other.
Furthermore, the placebo-treated patients received small doses of immediate-release niacin to induce flushing and maintain blinding. It is therefore hard to conclude that this clinical trial was a direct evaluation of the impact of niacin.
In contrast, the Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) study is currently evaluating extended-release niacin in combination with laropiprant, a prostaglandin receptor antagonist, vs placebo in more than 24,000 statin-treated patients.14 Without any in-trial titration of lipids, this study provides a more direct comparison of the effects of niacin in the statin era.
Niacin continues to attract interest, largely because it can raise HDL-C by 20% to 30% when given at doses of 1,500 mg or more. Also, consistent observations from population studies of an inverse relationship between HDL-C levels and cardiovascular risk5 have stimulated interest in developing novel agents that substantially raise HDL-C.
Is raising HDL-C a flawed strategy?
The failure of HDL-C-raising therapies in clinical trials15,16 has fueled concern that HDL may not be the magic elixir that many have sought. Given that niacin is the most effective HDL-C-raising agent currently available, its lack of efficacy in AIM-HIGH could be perceived as another nail in the coffin of the hypothesis that raising the HDL-C level with pharmacologic agents is beneficial.
AIM-HIGH was designed to examine the effects of raising HDL-C. To this end, it was performed exclusively in patients with low HDL-C levels, and the investigators tried to isolate the potential effects of raising HDL-C by equalizing the LDL-C levels in the treatment groups.
However, the HDL-C changes observed in AIM-HIGH are likely to have undermined the study objective. While niacin predictably increased HDL-C levels by 25%, an unexpected increase in HDL-C of 9.8% in the placebo-treated patients resulted in a difference in achieved HDL-C levels of only 4 mg/dL between the groups. This was far less than anticipated, and it likely had a major impact on an already underpowered study.
AIM-HIGH was designed to have 85% power to demonstrate a 25% reduction in clinical events, which was an optimistic estimate. On the basis of population studies, a difference of 4 mg/dL in HDL-C would be anticipated to result in no more than a 10% lower rate of clinical events, far beyond AIM-HIGH’s limit of detection.
The reasons for the increase in HDL-C in the placebo group are unknown, but they likely reflect the use of higher doses of simvastatin, some regression to the mean, and, possibly, the small doses of immediate-release niacin that the placebo contained. (Contrary to the belief of the investigators, there have been some reports of lipid changes with such doses,17 which may have contributed to the observed HDL-C-raising.)
Given that the HDL-C difference between the groups was relatively small and that niacin has additional effects beyond raising HDL-C and lowering LDL-C, it is unlikely that the futility of AIM-HIGH reflects a major indictment of HDL-C-raising. For the time being, the jury is still out on this question.
Was AIM-HIGH methodologically flawed?
A number of methodologic issues may have affected AIM-HIGH’s ability to adequately address its objectives.
The wrong cohort? In planning a study such as AIM-HIGH, the need for a relatively small sample size and the need to detect the greatest relative risk reduction with niacin would require enrollment of patients at the highest risk of cardiovascular events despite the use of statins. These needs were satisfied by only including patients who had atherosclerotic cardiovascular disease and low HDL-C levels. The inclusion of patients with low levels of HDL-C was also expected to promote greater increases in this lipid, and potentially event reduction, with niacin.
But no benefit was observed. It remains to be determined whether the inclusion of a high proportion of patients with the metabolic syndrome adversely affected the ability to detect a benefit with niacin. While post hoc analyses of studies of carotid intimal-medial thickness demonstrated no relationship between raising HDL-C with niacin and slowing of disease progression in patients with the metabolic syndrome,18 it remains to be determined whether this would translate to any effect on cardiovascular event rates.
Inadequate statistical power? An underpowered study would leave very little room for error, a pertinent point given the variability in therapeutic response in both actively treated and placebo-treated patients typically encountered in clinical trials. Giving low doses of immediate-release niacin and titrating the simvastatin dose to control LDL-C, resulting in imbalances in lipid-modifying therapies, represent additional flaws in the study design.
Stopped too soon? The early cessation of the study was somewhat questionable. The study crossed the prespecified boundary for lack of efficacy at the time of the interim analysis, and initial review by the data and safety monitoring board suggested an excess rate of ischemic stroke with niacin. The inclusion of this latter finding in the press release prompted considerable speculation regarding potential mechanisms and also concern among patients currently taking niacin. The subsequent finding that this signal was not statistically significant serves as an important warning for those conducting clinical trials not to prematurely overstate preliminary observations.
The implications for agents used in clinical practice are considerable: negative findings should not be overemphasized without robust evidence.
Do statins make everything else irrelevant?
The final factor to consider is the relative modifiability of residual clinical risk in statin-treated patients.
While residual risk is often cited as the reason to develop new antiatherosclerotic therapies, it is unknown how many of these ongoing events can be prevented. Several nonmodifiable factors such as age and concomitant disease are likely to contribute to these clinical events, which may limit our ability to further reduce event rates in patients who have already achieved low LDL-C levels with statin therapy. This may underscore the observation that no major clinical trial has demonstrated clinical benefit of an antiatherosclerotic agent on top of background medical care that included statins.
The finding that atherosclerosis continues to progress in many patients even though they take statins in high doses or achieve low LDL-C levels suggests that there is still room for improvement.
WHAT FUTURE FOR NIACIN?
So what does the future hold for niacin? The ongoing HPS2-THRIVE study provides another opportunity to evaluate the potential clinical efficacy of niacin in statin-treated patients. For now, we must wait for the results of this study.
In the meantime, it would seem reasonable to continue treatment with niacin in patients who need it for its multiple lipid-modifying effects. Whether clinicians will be less likely to initiate niacin therapy until there is clear evidence of clinical benefit remains uncertain. As for HDL-C, it remains to be determined whether any therapy targeting either quantitative or qualitative changes will be beneficial.
Over the last 3 decades, clinical trials have provided important insights into the prevention of cardiovascular events and have had a profound impact on clinical practice. Such studies simply evaluate whether one strategy is better or worse than the existing standard of care. They do not provide mechanistic insights, and when attempts have been made to address mechanisms in the study design, the trial, as in the case of AIM-HIGH, leaves more questions than answers.
Future trials will provide more clarity as to the optimal way to treat patients, but they must be based on a robust design that permits the study question to be adequately addressed.
- The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977; 62:707–714.
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 1991; 353:265–267.
- Nicholls SJ, Cutri B, Worthley SG, et al. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol 2005; 25:2416–2421.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao X-Q, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- US Department of Health and Human Services. NIH stops clinical trial on combination cholesterol treatment. http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2792. Accessed November 30, 2011.
- Brown BG, Zhao XQ. Nicotinic acid, alone and in combinations, for reduction of cardiovascular risk. Am J Cardiol 2008; 101:58B–62B.
- Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Luria MH, Sapoznikov D. Raising HDL cholesterol with low-dose nicotinic acid and bezafibrate: preliminary experience. Postgrad Med J 1993; 69:296–299.
- Taylor AJ, Zhu D, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Relationship between glycemic status and progression of carotid intima-media thickness during treatment with combined statin and extended-release niacin in ARBITER 2. Vasc Health Risk Manag 2007; 3:159–164.
The recent publication of the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes)1 has thrown the use of niacin as a lipid-modifying therapy into question. The trial was stopped early because an interim analysis found that the patients who took extended-release niacin had no clinical benefit. In addition, it found a trend toward more ischemic strokes, though this finding was later found not to be statistically significant.
Complicating the interpretation, while both the treatment group and the control group in the study received statin therapy, the researchers attempted to keep low-density lipoprotein cholesterol (LDL-C) levels equal, meaning that patients in the control group received more intensive statin therapy than those in the treatment group. And the placebo that the control patients received was actually a low dose of niacin, to induce flushing and thus to blind study participants and their physicians to which drug they were taking.
In the article that follows, I will explore the background, design, findings, and implications of this key trial and try to untangle the many questions about how to interpret it.
LOWERING LDL-C REDUCES RISK, BUT DOES NOT ELIMINATE IT
Large randomized controlled trials have consistently shown that lowering the level of LDL-C reduces cardiovascular event rates by 25% to 45% both in people who are known to have coronary artery disease and in those who are not.2–4 As a result, guidelines for preventing cardiovascular disease have increasingly emphasized maintaining low LDL-C levels. This has led to a proliferation in the use of inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (statins) in patients at high cardiovascular risk.
However, these agents only reduce the risk—they do not eliminate it. Needed are additional therapies to complement existing LDL-C-lowering approaches to lower the cardiovascular risk even further.
Raising HDL-C: The next frontier
One such strategy for further lowering cardiovascular risk that has received considerable interest is to promote the biological activity of the “good” cholesterol.
Studies have consistently shown that the higher the plasma level of high-density lipoprotein cholesterol (HDL-C), the lower the risk of cardiovascular events, suggesting that raising HDL-C may be beneficial.5 Studies in animals with atherosclerosis show that raising HDL-C via genetic modification of the animal or direct infusion of the molecule has a favorable impact on both the size and the structure of experimental plaque.6,7
Accordingly, much activity has focused on developing new therapies that raise HDL-C more effectively than current ones.
Why niacin should protect the heart
For more than 50 years, niacin has been used to manage dyslipidemia.
In addition to raising HDL-C levels more effectively than any other agent available today, niacin also lowers the levels of LDL-C, triglycerides, and lipoprotein (a).8 Before statins were available, the Coronary Drug Project found that niacin reduced the rate of nonfatal myocardial infarction and the 15-year mortality rate.9 In addition, niacin has been shown to slow the progression of carotid intimal-medial thickness and coronary atherosclerosis, and even to reverse these processes in some trials.10–12
However, a number of issues remain about using niacin to prevent cardiovascular events. Nearly all patients who take it experience flushing, which limits its tolerability and, thus, our ability to titrate doses to levels needed for adequate lipid changes. While a number of modifications of niacin administration have been developed (eg, extended-release formulations and products that inhibit flushing), no large study has tested the clinical efficacy of these strategies. Furthermore, until AIM-HIGH, no large-scale trial had directly evaluated the impact of niacin therapy on a background of statin therapy.
AIM-HIGH STUDY DESIGN
The intent of the AIM-HIGH trial was to determine whether extended-release niacin (Niaspan) would reduce the risk of cardiovascular events when added to therapy with a statin—in this case, simvastatin (Zocor) supplemented with ezetimibe (Zetia).1
The trial was funded by the National Heart, Lung, and Blood Institute (NHLBI) and by Abbott Laboratories, which also supplied the extended-release niacin and the ezetimibe. Merck donated the simvastatin.
Patient characteristics
The patients were all at least 45 years of age with established, stable coronary heart disease, cerebrovascular or carotid arterial disease, or peripheral arterial disease. They also had to have low levels of HDL-C (< 40 mg/dL in men, < 50 mg/dL in women), elevated triglycerides (150–400 mg/dL), and LDL-C levels lower than 180 mg/dL if they were not taking a statin at entry.
The mean age of the patients was 64 years, 85% were men, and 92% were white. They had a high prevalence of cardiovascular risk factors: 34% had diabetes, 71% had hypertension, and 81% had metabolic syndrome. Nearly all (94%) of the patients were taking a statin at entry; 76% had been taking one for more than 1 year, and 40% had been taking one for more than 5 years.1
Simvastatin, ezetimibe, and either niacin or placebo
All lipid-modifying agents except statins and ezetimibe were stopped for least 4 weeks after enrollment.
All patients then entered a 4- to 8-week open-label period, during which they took simvastatin 40 mg daily and extended-release niacin starting at 500 mg and increased weekly up to 2,000 mg daily. Patients who could tolerate at least 1,500 mg daily were randomly assigned to treatment with either niacin 1,500 to 2,000 mg or matching placebo. Both groups continued to receive simvastatin. The placebo contained a small dose of immediate-release niacin (50 mg) in each tablet to induce flushing and to maintain blinding of treatment.
Given that niacin also lowers LDL-C, an algorithm was used to try to keep LDL-C levels roughly the same in both treatment groups. This involved adjusting the simvastatin dose and permitting the use of ezetimibe 10 mg to keep the LDL-C level between 40 and 80 mg/dL. Accordingly, participating physicians were told their patients’ LDL-C levels but were blinded to their HDL-C and triglyceride levels throughout the study.
Every 6 months, patients had a follow-up visit in the clinic, and midway through each 6-month interval they received a phone call from the investigators.1
AIM-HIGH end points
The primary end point was the composite of the first event of death due to coronary heart disease, nonfatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome, or symptom-driven revascularization of the coronary or cerebral arteries.
Secondary end points were:
- Death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, or hospitalization for acute coronary syndrome
- Death from coronary heart disease, nonfatal myocardial infarction, or ischemic stroke
- Death from cardiovascular causes.
Tertiary end points included:
- Death from any cause
- Individual components of the primary end point
- Prespecified subgroups according to sex, history or no history of diabetes, and presence or absence of the metabolic syndrome.1
All clinical events were adjudicated by a central committee.
STUDY HALTED EARLY
The study was planned to run for a mean of 4.6 years, during which 800 primary end point events were expected. With these numbers, the investigators calculated that the study had 85% power to detect a 25% reduction in the primary end point, at a one-sided alpha level of 0.025.
The plan called for an interim analysis when 50% of the anticipated events had occurred, with prespecified stopping boundaries based on either efficacy or futility. The boundary for lack of efficacy required an observed hazard ratio of at least 1.02 with a probability of less than .001.
In the interim analysis, after a median follow-up of only 3 years, the data and safety monitoring board recommended stopping the study early because the boundary for futility had been crossed and, unexpectedly, the rate of ischemic stroke was higher in the niacin-treated patients than in those receiving placebo.
MAJOR FINDINGS OF AIM-HIGH
Of 4,273 patients who began open-label treatment with niacin, 3,414 were randomized to treatment with niacin or placebo.1
HDL-C levels went up in both groups
At 2 years:
- HDL-C levels had increased by 25.0% (to 42 mg/dL) in the niacin group and by 9.8% (to 38 mg/dL) in the placebo group
- Triglycerides had decreased by 28.6% with niacin and by 8.1% with placebo
- LDL-C had decreased by 12.0% with niacin and by 5.5% with placebo.
Patients in the placebo group were more likely to have subsequently received the maximum dose of simvastatin, ie, 80 mg/day (24.7% vs 17.5%), and to have received ezetimibe (21.5% vs 9.5%). More patients in the niacin group required either dose reduction of the study drug (6.3% vs 3.4%) or drug discontinuation (25.4% vs 20.1%).1
No difference in the primary end point
There was no difference between the two treatment groups in the rate of the primary end point, which occurred in 282 (16.4%) of the 1,718 patients in the niacin group and 272 (16.2%) of the 1,696 patients in the placebo group (P = .79; hazard ratio 1.02, 95% confidence interval 0.87–1.21).1
However, more patients in the niacin group than in the placebo group who reached the primary end point did so by having a first ischemic stroke: 27 patients (1.6%) vs 15 patients (0.9%). Eight of these patients, all in the niacin group, had their stroke between 2 months and 4 years after they had stopped taking the study drug.
Further analysis that included all ischemic strokes revealed the same trend: 29 vs 18 patients (P = .11).1
No benefit was observed for niacin-treated patients in terms of any of the secondary or tertiary end points.
Subgroup analysis revealed no evidence of statistical heterogeneity: ie, niacin seemed to lack efficacy in all the prespecified subgroups studied (age 65 and older vs younger, men vs women, and those with or without diabetes, metabolic syndrome, prior myocardial infarction, or statin use at entry).
In general, niacin was well tolerated in the active-treatment group, with a low incidence of liver and muscle abnormalities.
PUTTING AIM-HIGH IN CONTEXT
How should practicing clinicians interpret these outcomes?
Ever since the NHLBI reported (in an urgent press release) that it was stopping the study early due to futility and a potential excess of strokes,13 there has been considerable debate as to which factors contributed to these outcomes. In the wake of the publication of more detailed information about the trial,1 this debate is likely to continue.
The AIM-HIGH results can be interpreted in several ways:
- Perhaps niacin is no good as a preventive agent
- Perhaps raising HDL-C is flawed as a preventive strategy
- Perhaps AIM-HIGH had methodologic flaws, such as looking at the wrong patient cohort or using a treatment protocol that set itself up for failure
- Perhaps statins are so good that, once you prescribe one, anything else you give provides no additional benefit.
Which of these is correct?
Is niacin no good?
In its most simple form, AIM-HIGH has always been seen as a clinical trial of niacin. While the early trials of immediate-release niacin were encouraging in terms of its effects on lipids, atherosclerotic plaque, and cardiovascular outcomes, using it in clinical practice has always been challenging, largely because many patients cannot tolerate it in doses high enough to be effective. A number of developments have improved niacin’s tolerability, but its clinical impact in the statin era has not been evaluated.
Niacin’s lack of efficacy in this trial will ultimately be viewed as a failure of the drug itself, but is this the case?
AIM-HIGH was not simply a direct comparison of niacin vs placebo on top of standard medical practice. The investigators recognized that niacin has additional effects—in particular, lowering levels of atherogenic lipids—and they attempted to control for these effects by titrating the other LDL-C-lowering therapies during the study. As a result, the trial was actually a comparison between niacin plus low-dose simvastatin on the one hand, and placebo plus high-dose simvastatin (and, more often, also ezetimibe) on the other.
Furthermore, the placebo-treated patients received small doses of immediate-release niacin to induce flushing and maintain blinding. It is therefore hard to conclude that this clinical trial was a direct evaluation of the impact of niacin.
In contrast, the Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) study is currently evaluating extended-release niacin in combination with laropiprant, a prostaglandin receptor antagonist, vs placebo in more than 24,000 statin-treated patients.14 Without any in-trial titration of lipids, this study provides a more direct comparison of the effects of niacin in the statin era.
Niacin continues to attract interest, largely because it can raise HDL-C by 20% to 30% when given at doses of 1,500 mg or more. Also, consistent observations from population studies of an inverse relationship between HDL-C levels and cardiovascular risk5 have stimulated interest in developing novel agents that substantially raise HDL-C.
Is raising HDL-C a flawed strategy?
The failure of HDL-C-raising therapies in clinical trials15,16 has fueled concern that HDL may not be the magic elixir that many have sought. Given that niacin is the most effective HDL-C-raising agent currently available, its lack of efficacy in AIM-HIGH could be perceived as another nail in the coffin of the hypothesis that raising the HDL-C level with pharmacologic agents is beneficial.
AIM-HIGH was designed to examine the effects of raising HDL-C. To this end, it was performed exclusively in patients with low HDL-C levels, and the investigators tried to isolate the potential effects of raising HDL-C by equalizing the LDL-C levels in the treatment groups.
However, the HDL-C changes observed in AIM-HIGH are likely to have undermined the study objective. While niacin predictably increased HDL-C levels by 25%, an unexpected increase in HDL-C of 9.8% in the placebo-treated patients resulted in a difference in achieved HDL-C levels of only 4 mg/dL between the groups. This was far less than anticipated, and it likely had a major impact on an already underpowered study.
AIM-HIGH was designed to have 85% power to demonstrate a 25% reduction in clinical events, which was an optimistic estimate. On the basis of population studies, a difference of 4 mg/dL in HDL-C would be anticipated to result in no more than a 10% lower rate of clinical events, far beyond AIM-HIGH’s limit of detection.
The reasons for the increase in HDL-C in the placebo group are unknown, but they likely reflect the use of higher doses of simvastatin, some regression to the mean, and, possibly, the small doses of immediate-release niacin that the placebo contained. (Contrary to the belief of the investigators, there have been some reports of lipid changes with such doses,17 which may have contributed to the observed HDL-C-raising.)
Given that the HDL-C difference between the groups was relatively small and that niacin has additional effects beyond raising HDL-C and lowering LDL-C, it is unlikely that the futility of AIM-HIGH reflects a major indictment of HDL-C-raising. For the time being, the jury is still out on this question.
Was AIM-HIGH methodologically flawed?
A number of methodologic issues may have affected AIM-HIGH’s ability to adequately address its objectives.
The wrong cohort? In planning a study such as AIM-HIGH, the need for a relatively small sample size and the need to detect the greatest relative risk reduction with niacin would require enrollment of patients at the highest risk of cardiovascular events despite the use of statins. These needs were satisfied by only including patients who had atherosclerotic cardiovascular disease and low HDL-C levels. The inclusion of patients with low levels of HDL-C was also expected to promote greater increases in this lipid, and potentially event reduction, with niacin.
But no benefit was observed. It remains to be determined whether the inclusion of a high proportion of patients with the metabolic syndrome adversely affected the ability to detect a benefit with niacin. While post hoc analyses of studies of carotid intimal-medial thickness demonstrated no relationship between raising HDL-C with niacin and slowing of disease progression in patients with the metabolic syndrome,18 it remains to be determined whether this would translate to any effect on cardiovascular event rates.
Inadequate statistical power? An underpowered study would leave very little room for error, a pertinent point given the variability in therapeutic response in both actively treated and placebo-treated patients typically encountered in clinical trials. Giving low doses of immediate-release niacin and titrating the simvastatin dose to control LDL-C, resulting in imbalances in lipid-modifying therapies, represent additional flaws in the study design.
Stopped too soon? The early cessation of the study was somewhat questionable. The study crossed the prespecified boundary for lack of efficacy at the time of the interim analysis, and initial review by the data and safety monitoring board suggested an excess rate of ischemic stroke with niacin. The inclusion of this latter finding in the press release prompted considerable speculation regarding potential mechanisms and also concern among patients currently taking niacin. The subsequent finding that this signal was not statistically significant serves as an important warning for those conducting clinical trials not to prematurely overstate preliminary observations.
The implications for agents used in clinical practice are considerable: negative findings should not be overemphasized without robust evidence.
Do statins make everything else irrelevant?
The final factor to consider is the relative modifiability of residual clinical risk in statin-treated patients.
While residual risk is often cited as the reason to develop new antiatherosclerotic therapies, it is unknown how many of these ongoing events can be prevented. Several nonmodifiable factors such as age and concomitant disease are likely to contribute to these clinical events, which may limit our ability to further reduce event rates in patients who have already achieved low LDL-C levels with statin therapy. This may underscore the observation that no major clinical trial has demonstrated clinical benefit of an antiatherosclerotic agent on top of background medical care that included statins.
The finding that atherosclerosis continues to progress in many patients even though they take statins in high doses or achieve low LDL-C levels suggests that there is still room for improvement.
WHAT FUTURE FOR NIACIN?
So what does the future hold for niacin? The ongoing HPS2-THRIVE study provides another opportunity to evaluate the potential clinical efficacy of niacin in statin-treated patients. For now, we must wait for the results of this study.
In the meantime, it would seem reasonable to continue treatment with niacin in patients who need it for its multiple lipid-modifying effects. Whether clinicians will be less likely to initiate niacin therapy until there is clear evidence of clinical benefit remains uncertain. As for HDL-C, it remains to be determined whether any therapy targeting either quantitative or qualitative changes will be beneficial.
Over the last 3 decades, clinical trials have provided important insights into the prevention of cardiovascular events and have had a profound impact on clinical practice. Such studies simply evaluate whether one strategy is better or worse than the existing standard of care. They do not provide mechanistic insights, and when attempts have been made to address mechanisms in the study design, the trial, as in the case of AIM-HIGH, leaves more questions than answers.
Future trials will provide more clarity as to the optimal way to treat patients, but they must be based on a robust design that permits the study question to be adequately addressed.
The recent publication of the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes)1 has thrown the use of niacin as a lipid-modifying therapy into question. The trial was stopped early because an interim analysis found that the patients who took extended-release niacin had no clinical benefit. In addition, it found a trend toward more ischemic strokes, though this finding was later found not to be statistically significant.
Complicating the interpretation, while both the treatment group and the control group in the study received statin therapy, the researchers attempted to keep low-density lipoprotein cholesterol (LDL-C) levels equal, meaning that patients in the control group received more intensive statin therapy than those in the treatment group. And the placebo that the control patients received was actually a low dose of niacin, to induce flushing and thus to blind study participants and their physicians to which drug they were taking.
In the article that follows, I will explore the background, design, findings, and implications of this key trial and try to untangle the many questions about how to interpret it.
LOWERING LDL-C REDUCES RISK, BUT DOES NOT ELIMINATE IT
Large randomized controlled trials have consistently shown that lowering the level of LDL-C reduces cardiovascular event rates by 25% to 45% both in people who are known to have coronary artery disease and in those who are not.2–4 As a result, guidelines for preventing cardiovascular disease have increasingly emphasized maintaining low LDL-C levels. This has led to a proliferation in the use of inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (statins) in patients at high cardiovascular risk.
However, these agents only reduce the risk—they do not eliminate it. Needed are additional therapies to complement existing LDL-C-lowering approaches to lower the cardiovascular risk even further.
Raising HDL-C: The next frontier
One such strategy for further lowering cardiovascular risk that has received considerable interest is to promote the biological activity of the “good” cholesterol.
Studies have consistently shown that the higher the plasma level of high-density lipoprotein cholesterol (HDL-C), the lower the risk of cardiovascular events, suggesting that raising HDL-C may be beneficial.5 Studies in animals with atherosclerosis show that raising HDL-C via genetic modification of the animal or direct infusion of the molecule has a favorable impact on both the size and the structure of experimental plaque.6,7
Accordingly, much activity has focused on developing new therapies that raise HDL-C more effectively than current ones.
Why niacin should protect the heart
For more than 50 years, niacin has been used to manage dyslipidemia.
In addition to raising HDL-C levels more effectively than any other agent available today, niacin also lowers the levels of LDL-C, triglycerides, and lipoprotein (a).8 Before statins were available, the Coronary Drug Project found that niacin reduced the rate of nonfatal myocardial infarction and the 15-year mortality rate.9 In addition, niacin has been shown to slow the progression of carotid intimal-medial thickness and coronary atherosclerosis, and even to reverse these processes in some trials.10–12
However, a number of issues remain about using niacin to prevent cardiovascular events. Nearly all patients who take it experience flushing, which limits its tolerability and, thus, our ability to titrate doses to levels needed for adequate lipid changes. While a number of modifications of niacin administration have been developed (eg, extended-release formulations and products that inhibit flushing), no large study has tested the clinical efficacy of these strategies. Furthermore, until AIM-HIGH, no large-scale trial had directly evaluated the impact of niacin therapy on a background of statin therapy.
AIM-HIGH STUDY DESIGN
The intent of the AIM-HIGH trial was to determine whether extended-release niacin (Niaspan) would reduce the risk of cardiovascular events when added to therapy with a statin—in this case, simvastatin (Zocor) supplemented with ezetimibe (Zetia).1
The trial was funded by the National Heart, Lung, and Blood Institute (NHLBI) and by Abbott Laboratories, which also supplied the extended-release niacin and the ezetimibe. Merck donated the simvastatin.
Patient characteristics
The patients were all at least 45 years of age with established, stable coronary heart disease, cerebrovascular or carotid arterial disease, or peripheral arterial disease. They also had to have low levels of HDL-C (< 40 mg/dL in men, < 50 mg/dL in women), elevated triglycerides (150–400 mg/dL), and LDL-C levels lower than 180 mg/dL if they were not taking a statin at entry.
The mean age of the patients was 64 years, 85% were men, and 92% were white. They had a high prevalence of cardiovascular risk factors: 34% had diabetes, 71% had hypertension, and 81% had metabolic syndrome. Nearly all (94%) of the patients were taking a statin at entry; 76% had been taking one for more than 1 year, and 40% had been taking one for more than 5 years.1
Simvastatin, ezetimibe, and either niacin or placebo
All lipid-modifying agents except statins and ezetimibe were stopped for least 4 weeks after enrollment.
All patients then entered a 4- to 8-week open-label period, during which they took simvastatin 40 mg daily and extended-release niacin starting at 500 mg and increased weekly up to 2,000 mg daily. Patients who could tolerate at least 1,500 mg daily were randomly assigned to treatment with either niacin 1,500 to 2,000 mg or matching placebo. Both groups continued to receive simvastatin. The placebo contained a small dose of immediate-release niacin (50 mg) in each tablet to induce flushing and to maintain blinding of treatment.
Given that niacin also lowers LDL-C, an algorithm was used to try to keep LDL-C levels roughly the same in both treatment groups. This involved adjusting the simvastatin dose and permitting the use of ezetimibe 10 mg to keep the LDL-C level between 40 and 80 mg/dL. Accordingly, participating physicians were told their patients’ LDL-C levels but were blinded to their HDL-C and triglyceride levels throughout the study.
Every 6 months, patients had a follow-up visit in the clinic, and midway through each 6-month interval they received a phone call from the investigators.1
AIM-HIGH end points
The primary end point was the composite of the first event of death due to coronary heart disease, nonfatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome, or symptom-driven revascularization of the coronary or cerebral arteries.
Secondary end points were:
- Death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, or hospitalization for acute coronary syndrome
- Death from coronary heart disease, nonfatal myocardial infarction, or ischemic stroke
- Death from cardiovascular causes.
Tertiary end points included:
- Death from any cause
- Individual components of the primary end point
- Prespecified subgroups according to sex, history or no history of diabetes, and presence or absence of the metabolic syndrome.1
All clinical events were adjudicated by a central committee.
STUDY HALTED EARLY
The study was planned to run for a mean of 4.6 years, during which 800 primary end point events were expected. With these numbers, the investigators calculated that the study had 85% power to detect a 25% reduction in the primary end point, at a one-sided alpha level of 0.025.
The plan called for an interim analysis when 50% of the anticipated events had occurred, with prespecified stopping boundaries based on either efficacy or futility. The boundary for lack of efficacy required an observed hazard ratio of at least 1.02 with a probability of less than .001.
In the interim analysis, after a median follow-up of only 3 years, the data and safety monitoring board recommended stopping the study early because the boundary for futility had been crossed and, unexpectedly, the rate of ischemic stroke was higher in the niacin-treated patients than in those receiving placebo.
MAJOR FINDINGS OF AIM-HIGH
Of 4,273 patients who began open-label treatment with niacin, 3,414 were randomized to treatment with niacin or placebo.1
HDL-C levels went up in both groups
At 2 years:
- HDL-C levels had increased by 25.0% (to 42 mg/dL) in the niacin group and by 9.8% (to 38 mg/dL) in the placebo group
- Triglycerides had decreased by 28.6% with niacin and by 8.1% with placebo
- LDL-C had decreased by 12.0% with niacin and by 5.5% with placebo.
Patients in the placebo group were more likely to have subsequently received the maximum dose of simvastatin, ie, 80 mg/day (24.7% vs 17.5%), and to have received ezetimibe (21.5% vs 9.5%). More patients in the niacin group required either dose reduction of the study drug (6.3% vs 3.4%) or drug discontinuation (25.4% vs 20.1%).1
No difference in the primary end point
There was no difference between the two treatment groups in the rate of the primary end point, which occurred in 282 (16.4%) of the 1,718 patients in the niacin group and 272 (16.2%) of the 1,696 patients in the placebo group (P = .79; hazard ratio 1.02, 95% confidence interval 0.87–1.21).1
However, more patients in the niacin group than in the placebo group who reached the primary end point did so by having a first ischemic stroke: 27 patients (1.6%) vs 15 patients (0.9%). Eight of these patients, all in the niacin group, had their stroke between 2 months and 4 years after they had stopped taking the study drug.
Further analysis that included all ischemic strokes revealed the same trend: 29 vs 18 patients (P = .11).1
No benefit was observed for niacin-treated patients in terms of any of the secondary or tertiary end points.
Subgroup analysis revealed no evidence of statistical heterogeneity: ie, niacin seemed to lack efficacy in all the prespecified subgroups studied (age 65 and older vs younger, men vs women, and those with or without diabetes, metabolic syndrome, prior myocardial infarction, or statin use at entry).
In general, niacin was well tolerated in the active-treatment group, with a low incidence of liver and muscle abnormalities.
PUTTING AIM-HIGH IN CONTEXT
How should practicing clinicians interpret these outcomes?
Ever since the NHLBI reported (in an urgent press release) that it was stopping the study early due to futility and a potential excess of strokes,13 there has been considerable debate as to which factors contributed to these outcomes. In the wake of the publication of more detailed information about the trial,1 this debate is likely to continue.
The AIM-HIGH results can be interpreted in several ways:
- Perhaps niacin is no good as a preventive agent
- Perhaps raising HDL-C is flawed as a preventive strategy
- Perhaps AIM-HIGH had methodologic flaws, such as looking at the wrong patient cohort or using a treatment protocol that set itself up for failure
- Perhaps statins are so good that, once you prescribe one, anything else you give provides no additional benefit.
Which of these is correct?
Is niacin no good?
In its most simple form, AIM-HIGH has always been seen as a clinical trial of niacin. While the early trials of immediate-release niacin were encouraging in terms of its effects on lipids, atherosclerotic plaque, and cardiovascular outcomes, using it in clinical practice has always been challenging, largely because many patients cannot tolerate it in doses high enough to be effective. A number of developments have improved niacin’s tolerability, but its clinical impact in the statin era has not been evaluated.
Niacin’s lack of efficacy in this trial will ultimately be viewed as a failure of the drug itself, but is this the case?
AIM-HIGH was not simply a direct comparison of niacin vs placebo on top of standard medical practice. The investigators recognized that niacin has additional effects—in particular, lowering levels of atherogenic lipids—and they attempted to control for these effects by titrating the other LDL-C-lowering therapies during the study. As a result, the trial was actually a comparison between niacin plus low-dose simvastatin on the one hand, and placebo plus high-dose simvastatin (and, more often, also ezetimibe) on the other.
Furthermore, the placebo-treated patients received small doses of immediate-release niacin to induce flushing and maintain blinding. It is therefore hard to conclude that this clinical trial was a direct evaluation of the impact of niacin.
In contrast, the Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) study is currently evaluating extended-release niacin in combination with laropiprant, a prostaglandin receptor antagonist, vs placebo in more than 24,000 statin-treated patients.14 Without any in-trial titration of lipids, this study provides a more direct comparison of the effects of niacin in the statin era.
Niacin continues to attract interest, largely because it can raise HDL-C by 20% to 30% when given at doses of 1,500 mg or more. Also, consistent observations from population studies of an inverse relationship between HDL-C levels and cardiovascular risk5 have stimulated interest in developing novel agents that substantially raise HDL-C.
Is raising HDL-C a flawed strategy?
The failure of HDL-C-raising therapies in clinical trials15,16 has fueled concern that HDL may not be the magic elixir that many have sought. Given that niacin is the most effective HDL-C-raising agent currently available, its lack of efficacy in AIM-HIGH could be perceived as another nail in the coffin of the hypothesis that raising the HDL-C level with pharmacologic agents is beneficial.
AIM-HIGH was designed to examine the effects of raising HDL-C. To this end, it was performed exclusively in patients with low HDL-C levels, and the investigators tried to isolate the potential effects of raising HDL-C by equalizing the LDL-C levels in the treatment groups.
However, the HDL-C changes observed in AIM-HIGH are likely to have undermined the study objective. While niacin predictably increased HDL-C levels by 25%, an unexpected increase in HDL-C of 9.8% in the placebo-treated patients resulted in a difference in achieved HDL-C levels of only 4 mg/dL between the groups. This was far less than anticipated, and it likely had a major impact on an already underpowered study.
AIM-HIGH was designed to have 85% power to demonstrate a 25% reduction in clinical events, which was an optimistic estimate. On the basis of population studies, a difference of 4 mg/dL in HDL-C would be anticipated to result in no more than a 10% lower rate of clinical events, far beyond AIM-HIGH’s limit of detection.
The reasons for the increase in HDL-C in the placebo group are unknown, but they likely reflect the use of higher doses of simvastatin, some regression to the mean, and, possibly, the small doses of immediate-release niacin that the placebo contained. (Contrary to the belief of the investigators, there have been some reports of lipid changes with such doses,17 which may have contributed to the observed HDL-C-raising.)
Given that the HDL-C difference between the groups was relatively small and that niacin has additional effects beyond raising HDL-C and lowering LDL-C, it is unlikely that the futility of AIM-HIGH reflects a major indictment of HDL-C-raising. For the time being, the jury is still out on this question.
Was AIM-HIGH methodologically flawed?
A number of methodologic issues may have affected AIM-HIGH’s ability to adequately address its objectives.
The wrong cohort? In planning a study such as AIM-HIGH, the need for a relatively small sample size and the need to detect the greatest relative risk reduction with niacin would require enrollment of patients at the highest risk of cardiovascular events despite the use of statins. These needs were satisfied by only including patients who had atherosclerotic cardiovascular disease and low HDL-C levels. The inclusion of patients with low levels of HDL-C was also expected to promote greater increases in this lipid, and potentially event reduction, with niacin.
But no benefit was observed. It remains to be determined whether the inclusion of a high proportion of patients with the metabolic syndrome adversely affected the ability to detect a benefit with niacin. While post hoc analyses of studies of carotid intimal-medial thickness demonstrated no relationship between raising HDL-C with niacin and slowing of disease progression in patients with the metabolic syndrome,18 it remains to be determined whether this would translate to any effect on cardiovascular event rates.
Inadequate statistical power? An underpowered study would leave very little room for error, a pertinent point given the variability in therapeutic response in both actively treated and placebo-treated patients typically encountered in clinical trials. Giving low doses of immediate-release niacin and titrating the simvastatin dose to control LDL-C, resulting in imbalances in lipid-modifying therapies, represent additional flaws in the study design.
Stopped too soon? The early cessation of the study was somewhat questionable. The study crossed the prespecified boundary for lack of efficacy at the time of the interim analysis, and initial review by the data and safety monitoring board suggested an excess rate of ischemic stroke with niacin. The inclusion of this latter finding in the press release prompted considerable speculation regarding potential mechanisms and also concern among patients currently taking niacin. The subsequent finding that this signal was not statistically significant serves as an important warning for those conducting clinical trials not to prematurely overstate preliminary observations.
The implications for agents used in clinical practice are considerable: negative findings should not be overemphasized without robust evidence.
Do statins make everything else irrelevant?
The final factor to consider is the relative modifiability of residual clinical risk in statin-treated patients.
While residual risk is often cited as the reason to develop new antiatherosclerotic therapies, it is unknown how many of these ongoing events can be prevented. Several nonmodifiable factors such as age and concomitant disease are likely to contribute to these clinical events, which may limit our ability to further reduce event rates in patients who have already achieved low LDL-C levels with statin therapy. This may underscore the observation that no major clinical trial has demonstrated clinical benefit of an antiatherosclerotic agent on top of background medical care that included statins.
The finding that atherosclerosis continues to progress in many patients even though they take statins in high doses or achieve low LDL-C levels suggests that there is still room for improvement.
WHAT FUTURE FOR NIACIN?
So what does the future hold for niacin? The ongoing HPS2-THRIVE study provides another opportunity to evaluate the potential clinical efficacy of niacin in statin-treated patients. For now, we must wait for the results of this study.
In the meantime, it would seem reasonable to continue treatment with niacin in patients who need it for its multiple lipid-modifying effects. Whether clinicians will be less likely to initiate niacin therapy until there is clear evidence of clinical benefit remains uncertain. As for HDL-C, it remains to be determined whether any therapy targeting either quantitative or qualitative changes will be beneficial.
Over the last 3 decades, clinical trials have provided important insights into the prevention of cardiovascular events and have had a profound impact on clinical practice. Such studies simply evaluate whether one strategy is better or worse than the existing standard of care. They do not provide mechanistic insights, and when attempts have been made to address mechanisms in the study design, the trial, as in the case of AIM-HIGH, leaves more questions than answers.
Future trials will provide more clarity as to the optimal way to treat patients, but they must be based on a robust design that permits the study question to be adequately addressed.
- The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977; 62:707–714.
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 1991; 353:265–267.
- Nicholls SJ, Cutri B, Worthley SG, et al. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol 2005; 25:2416–2421.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao X-Q, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- US Department of Health and Human Services. NIH stops clinical trial on combination cholesterol treatment. http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2792. Accessed November 30, 2011.
- Brown BG, Zhao XQ. Nicotinic acid, alone and in combinations, for reduction of cardiovascular risk. Am J Cardiol 2008; 101:58B–62B.
- Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Luria MH, Sapoznikov D. Raising HDL cholesterol with low-dose nicotinic acid and bezafibrate: preliminary experience. Postgrad Med J 1993; 69:296–299.
- Taylor AJ, Zhu D, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Relationship between glycemic status and progression of carotid intima-media thickness during treatment with combined statin and extended-release niacin in ARBITER 2. Vasc Health Risk Manag 2007; 3:159–164.
- The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977; 62:707–714.
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 1991; 353:265–267.
- Nicholls SJ, Cutri B, Worthley SG, et al. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol 2005; 25:2416–2421.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao X-Q, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- US Department of Health and Human Services. NIH stops clinical trial on combination cholesterol treatment. http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2792. Accessed November 30, 2011.
- Brown BG, Zhao XQ. Nicotinic acid, alone and in combinations, for reduction of cardiovascular risk. Am J Cardiol 2008; 101:58B–62B.
- Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Luria MH, Sapoznikov D. Raising HDL cholesterol with low-dose nicotinic acid and bezafibrate: preliminary experience. Postgrad Med J 1993; 69:296–299.
- Taylor AJ, Zhu D, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Relationship between glycemic status and progression of carotid intima-media thickness during treatment with combined statin and extended-release niacin in ARBITER 2. Vasc Health Risk Manag 2007; 3:159–164.
KEY POINTS
- The study was stopped early because of the concerns raised by the interim analysis.
- The AIM-HIGH results can be interpreted in several ways: perhaps niacin is no good as a preventive agent; perhaps raising levels of high-density lipoprotein cholesterol (HDL-C) is flawed as a preventive strategy; perhaps AIM-HIGH had methodologic flaws; or perhaps statins are so good that, once you prescribe one, anything else you do will not make much of a difference.
- It seems reasonable to continue niacin treatment in patients who need its multiple lipid-modifying effects. It is uncertain if clinicians will be less likely to prescribe niacin therapy until we have clear evidence of clinical benefit. As for HDL-C, it remains to be determined whether any therapy targeting quantitative or qualitative changes will be beneficial.