User login
Opportunities for Stewardship in the Transition From Intravenous to Enteral Antibiotics in Hospitalized Pediatric Patients
Bacterial infections are a common reason for pediatric hospital admissions in the United States.1 Antibiotics are the mainstay of treatment, and whether to administer them intravenously (IV) or enterally is an important and, at times, challenging decision. Not all hospitalized patients with infections require IV antibiotics, and safe, effective early transitions to enteral therapy have been described for numerous infections.2-7 However, guidelines describing the ideal initial route of antibiotic administration and when to transition to oral therapy are lacking.5,7,8 This lack of high-quality evidence-based guidance may contribute to overuse of IV antibiotics for many hospitalized pediatric patients, even when safe and effective enteral options exist.9
Significant costs and harms are associated with the use of IV antibiotics. In particular, studies have demonstrated longer length of stay (LOS), increased costs, and worsened pain or anxiety related to complications (eg, phlebitis, extravasation injury, thrombosis, catheter-associated bloodstream infections) associated with IV antibiotics.3,4,10-13 Earlier transition to enteral therapy, however, can mitigate these increased risks and costs.
The Centers for Disease Control and Prevention lists the transition from IV to oral antibiotics as a key stewardship intervention for improving antibiotic use.14 The Infectious Diseases Society of America (IDSA) antibiotic stewardship program guidelines strongly recommend the timely conversion from IV to oral antibiotics, stating that efforts focusing on this transition should be integrated into routine practice.15 There are a few metrics in the literature to measure this intervention, but none is universally used, and a modified delphi process could not reach consensus on IV-to-oral transition metrics.16
Few studies describe the opportunity to transition to enteral antibiotics in hospitalized patients with common bacterial infections or explore variation across hospitals. It is critical to understand current practice of antibiotic administration in order to identify opportunities to optimize patient outcomes and promote high-value care. Furthermore, few studies have evaluated the feasibility of IV-to-oral transition metrics using an administrative database. Thus, the aims of this study were to (1) determine opportunities to transition from IV to enteral antibiotics for pediatric patients hospitalized with common bacterial infections based on their ability to tolerate other enteral medications, (2) describe variation in transition practices among children’s hospitals, and (3) evaluate the feasibility of novel IV-to-oral transition metrics using an administrative database to inform stewardship efforts.
METHODS
Study Design and Setting
This multicenter, retrospective cohort study used data from the Pediatric Health Information System (PHIS), an administrative and billing database containing encounter-level data from 52 tertiary care pediatric hospitals across the United States affiliated with the Children’s Hospital Association (Lenexa, Kansas). Hospitals submit encounter-level data, including demographics, medications, and diagnoses based on International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes. Data were de-identified at the time of submission, and data quality and reliability were assured by joint efforts between the Children’s Hospital Association and participating hospitals.
Study Population
This study included pediatric patients aged 60 days to 18 years who were hospitalized (inpatient or observation status) at one of the participating hospitals between January 1, 2017, and December 31, 2018, for one of the following seven common bacterial infections: community-acquired pneumonia (CAP), neck infection (superficial and deep), periorbital/orbital infection, urinary tract infection (UTI), osteomyelitis, septic arthritis, or skin and soft tissue infection (SSTI). The diagnosis cohorts were defined based on ICD-10-CM discharge diagnoses adapted from previous studies (Appendix Table 1).3,17-23 To define a cohort of generally healthy pediatric patients with an acute infection, we excluded patients hospitalized in the intensive care unit, patients with nonhome discharges, and patients with complex chronic conditions.24 We also excluded hospitals with incomplete data during the study period (n=1). The Institutional Review Board at Cincinnati Children’s Hospital Medical Center determined this study to be non–human-subjects research.
Outcomes
The primary outcomes were the number of opportunity days and the percent of days with opportunity to transition from IV to enteral therapy. Opportunity days, or days in which there was a potential opportunity to transition from IV to enteral antibiotics, were defined as days patients received only IV antibiotic doses and at least one enteral nonantibiotic medication, suggesting an ability to take enteral medications.13 We excluded days patients received IV antibiotics for which there was no enteral alternative (eg, vancomycin, Appendix Table 2). When measuring opportunity, to be conservative (ie, to underestimate rather than overestimate opportunity), we did not count as an opportunity day any day in which patients received both IV and enteral antibiotics. Percent opportunity, or the percent of days patients received antibiotics in which there was potential opportunity to transition from IV to enteral antibiotics, was defined as the number of opportunity days divided by number of inpatient days patients received enteral antibiotics or IV antibiotics with at least one enteral nonantibiotic medication (antibiotic days). Similar to opportunity days, antibiotic days excluded days patients were on IV antibiotics for which there was no enteral alternative. Based on our definition, a lower percent opportunity indicates that a hospital is using enteral antibiotics earlier during the hospitalization (earlier transition), while a higher percent opportunity represents later enteral antibiotic use (later transition).
Statistical Analysis
Demographic and clinical characteristics were summarized by diagnosis with descriptive statistics, including frequency with percentage, mean with standard deviation, and median with interquartile range (IQR). For each diagnosis, we evaluated aggregate opportunity days (sum of opportunity days among all hospitals), opportunity days per encounter, and aggregate percent opportunity using frequencies, mean with standard deviation, and percentages, respectively. We also calculated aggregate opportunity days for diagnosis-antibiotic combinations. To visually show variation in the percent opportunity across hospitals, we displayed the percent opportunity on a heat map, and evaluated percent opportunity across hospitals using chi-square tests. To compare the variability in the percent opportunity across and within hospitals, we used a generalized linear model with two fixed effects (hospital and diagnosis), and parsed the variability using the sum of squares. We performed a sensitivity analysis and excluded days that patients received antiemetic medications (eg, ondansetron, granisetron, prochlorperazine, promethazine), as these suggest potential intolerance of enteral medications. All statistical analyses were performed using SAS v.9.4 (SAS Institute Inc, Cary, North Carolina) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, California), and P values < .05 were considered statistically significant.
RESULTS
During the 2-year study period, 100,103 hospitalizations met our inclusion criteria across 51 hospitals and seven diagnosis categories (Table 1). Diagnosis cohorts ranged in size from 1,462 encounters for septic arthritis to 35,665 encounters for neck infections. Overall, we identified 88,522 aggregate opportunity days on which there was an opportunity to switch from IV to enteral treatment in the majority of participants (percent opportunity, 57%).
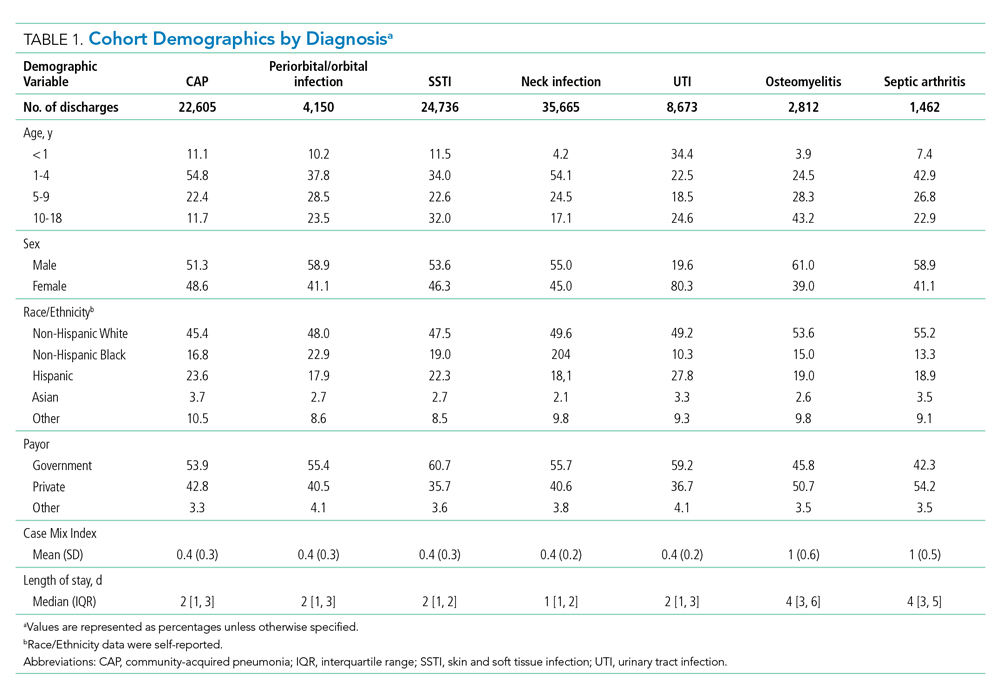
Opportunity by Diagnosis
The number of opportunity days (aggregate and mean per encounter) and percent opportunity varied by diagnosis (Table 2). The aggregate number of opportunity days ranged from 3,693 in patients with septic arthritis to 25,359 in patients with SSTI, and mean opportunity days per encounter ranged from 0.9 in CAP to 2.8 in septic arthritis. Percent opportunity was highest for septic arthritis at 72.7% and lowest for CAP at 39.7%.
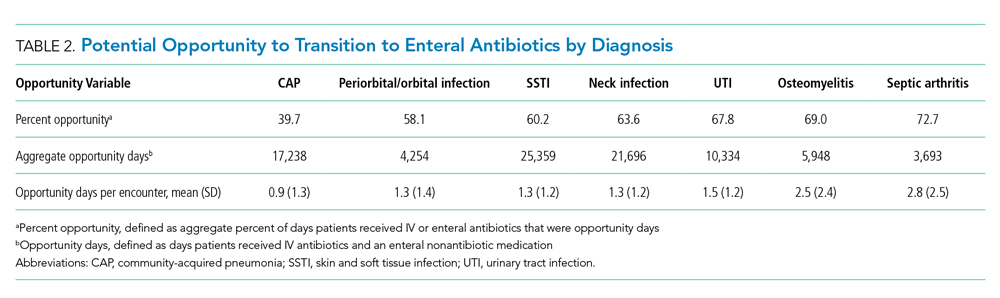
Variation in Opportunity Among Hospitals
The variation in the percent opportunity across hospitals was statistically significant for all diagnoses (Figure). Within hospitals, we observed similar practice patterns across diagnoses. For example, hospitals with a higher percent opportunity for one diagnosis tended to have higher percent opportunity for the other diagnoses (as noted in the top portion of the Figure), and those with lower percent opportunity for one diagnosis tended to also have lower percent opportunity for the other diagnoses studied (as noted in the bottom portion of the Figure). When evaluating variability in the percent opportunity, 45% of the variability was attributable to the hospital-effect and 35% to the diagnosis; the remainder was unexplained variability. Sensitivity analysis excluding days when patients received an antiemetic medication yielded no differences in our results.

Opportunity by Antibiotic
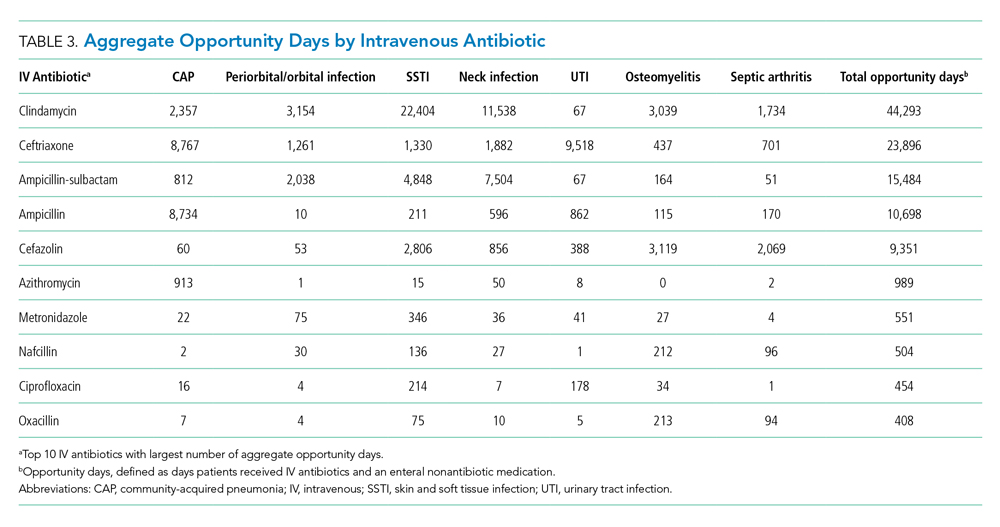
The aggregate number of opportunity days varied by antibiotic (Table 3). Intravenous antibiotics with the largest number of opportunity days included clindamycin (44,293), ceftriaxone (23,896), and ampicillin-sulbactam (15,484). Antibiotic-diagnosis combinations with the largest number of opportunity days for each diagnosis included ceftriaxone and ampicillin in CAP; clindamycin in cellulitis, SSTI, and neck infections; ceftriaxone in UTI; and cefazolin in osteomyelitis and septic arthritis.
DISCUSSION
In this multicenter study of pediatric patients hospitalized with common bacterial infections, there was the potential to transition from IV to enteral treatment in over half of the antibiotic days. The degree of opportunity varied by infection, antibiotic, and hospital. Antibiotics with a large aggregate number of opportunity days for enteral transition included clindamycin, which has excellent bioavailability; and ampicillin and ampicillin-sulbactam, which can achieve pharmacodynamic targets with oral equivalents.25-29 The across-hospital variation for a given diagnosis suggests that certain hospitals have strategies in place which permit an earlier transition to enteral antibiotics compared to other institutions in which there were likely missed opportunities to do so. This variability is likely due to limited evidence, emphasizing the need for robust studies to better understand the optimal initial antibiotic route and transition time. Our findings highlight the need for, and large potential impact of, stewardship efforts to promote earlier transition for specific drug targets. This study also demonstrates the feasibility of obtaining two metrics—percent opportunity and opportunity days—from administrative databases to inform stewardship efforts within and across hospitals.
Opportunity days and percent opportunity varied among diagnoses. The variation in aggregate opportunity days was largely a reflection of the number of encounters: Diagnoses such as SSTI, neck infections, and CAP had a large number of both aggregate opportunity days and encounters. The range of opportunity days per encounter (0.9-2.5) suggests potential missed opportunities to transition to enteral antibiotics across all diagnoses (Table 2). The higher opportunity days per encounter in osteomyelitis and septic arthritis may be related to longer LOS and higher percent opportunity. Percent opportunity likely varied among diagnoses due to differences in admission and discharge readiness criteria, diagnostic evaluation, frequency of antibiotic administration, and evidence on the optimal route of initial antibiotics and when to transition to oral formulations. For example, we hypothesize that certain diagnoses, such as osteomyelitis and septic arthritis, have admission and discharge readiness criteria directly tied to the perceived need for IV antibiotics, which may limit in-hospital days on enteral antibiotics and explain the high percent opportunity that we observed. The high percent opportunity seen in musculoskeletal infections also may be due to delays in initiating targeted treatment until culture results were available. Encounters for CAP had the lowest percent opportunity; we hypothesize that this is because admission and discharge readiness may be determined by factors other than the need for IV antibiotics (eg, need for supplemental oxygen), which may increase days on enteral antibiotics and lead to a lower percent opportunity.30
Urinary tract infection encounters had a high percent opportunity. As with musculoskeletal infection, this may be related to delays in initiating targeted treatment until culture results became available. Another reason for the high percent opportunity in UTI could be the common use of ceftriaxone, which, dosed every 24 hours, likely reduced the opportunity to transition to enteral antibiotics. There is strong evidence demonstrating no difference in outcomes based on antibiotic routes for UTI, and we would expect this to result in a low percent opportunity.2,31 While the observed high opportunity in UTI may relate to an initial unknown diagnosis or concern for systemic infection, this highlights potential opportunities for quality improvement initiatives to promote empiric oral antibiotics in clinically stable patients hospitalized with suspected UTI.
There was substantial variation in percent opportunity across hospitals for a given diagnosis, with less variation across diagnoses for a given hospital. Variation across hospitals but consistency within individual hospitals suggests that some hospitals may promote earlier transition from IV to enteral antibiotics as standard practice for all diagnoses, while other hospitals continue IV antibiotics for the entire hospitalization, highlighting potential missed opportunities at some institutions. While emerging data suggest that traditional long durations of IV antibiotics are not necessary for many infections, the limited evidence on the optimal time to switch to oral antibiotics may have influenced this variation.2-7 Many guidelines recommend initial IV antibiotics for hospitalized pediatric patients, but there are few studies comparing IV and enteral therapy.2,5,9 Limited evidence leaves significant room for hospital culture, antibiotic stewardship efforts, reimbursement considerations, and/or hospital workflow to influence transition timing and overall opportunity at individual hospitals.7,8,32-34 These findings emphasize the importance of research to identify optimal transition time and comparative effectiveness studies to evaluate whether initial IV antibiotics are truly needed for mild—and even severe—disease presentations. Since many patients are admitted for the perceived need for IV antibiotics, earlier use of enteral antibiotics could reduce rates of hospitalizations, LOS, healthcare costs, and resource utilization.
Antibiotics with a high number of opportunity days included clindamycin, ceftriaxone, ampicillin-sublactam, and ampicillin. Our findings are consistent with another study which found that most bioavailable drugs, including clindamycin, were administered via the IV route and accounted for a large number of antibiotic days.35 The Infectious Diseases Society of America recommends that hospitals promote earlier transition to oral formulations for highly bioavailable drugs.7 Given the high bioavailability of clindamycin, its common use in high-frequency encounters such as SSTI and neck infections, and the fact that it accounted for a large number of opportunity days, quality improvement initiatives promoting earlier transition to oral clindamycin could have a large impact across health systems.25,26 Additionally, although beta-lactam antibiotics such as amoxicillin and amoxicillin-sulbactam are not highly bioavailable, oral dosing can achieve sufficient serum concentrations to reach pharmacodynamic targets for common clinical indications; this could be an important quality improvement initiative.27-29 Several single-site studies have successfully implemented quality improvement initiatives to promote earlier IV-to-enteral transition, with resulting reductions in costs and no adverse events noted, highlighting the feasibility and impact of such efforts.13,36-38
This study also demonstrates the feasibility of collecting two metrics (percent opportunity and opportunity days) from administrative databases to inform IV-to-oral transition benchmarking and stewardship efforts. While there are several metrics in the literature for evaluating antibiotic transition (eg, days of IV or oral therapy, percentage of antibiotics given via the oral route, time to switch from IV to oral, and acceptance rate of suggested changes to antibiotic route), none are universally used or agreed upon.15,16,39 The opportunity metrics used in this study have several strengths, including the feasibility of obtaining them from existing databases and the ability to account for intake of other enteral medications; the latter is not evaluated in other metrics. These opportunity metrics can be used together to identify the percent of time in which there is opportunity to transition and total number of days to understand the full extent of potential opportunity for future interventions. As demonstrated in this study, these metrics can be measured by diagnosis, antibiotic, or diagnosis-antibiotic combination, and they can be used to evaluate stewardship efforts at a single institution over time or compare efforts across hospitals.
These findings should be interpreted in the context of important limitations. First, we attempted to characterize potential opportunity to transition to enteral medications based on a patient’s ability to tolerate nonenteral medications. However, there are other factors that could limit the opportunity to transition that we could not account for with an administrative dataset, including the use of antibiotics prior to admission, disease progression, severity of illness, and malabsorptive concerns. Thus, though we may have overestimated the true opportunity to transition to enteral antibiotics, it is unlikely that this would account for all of the variation in transition times that we observed across hospitals. Second, while our study required patients to have one of seven types of infection, we did not exclude any additional infectious diagnoses (eg, concurrent bacteremia, Clostridioides difficile, otitis media) that could have driven the choice of antibiotic type and modality. Although emerging evidence is supporting earlier transitions to oral therapy, bacteremia is typically treated with IV antibiotics; this may have led to an overestimation of true opportunity.40 “Clostridioides” difficile and otitis media are typically treated with enteral therapy; concurrent infections such as these may have led to an underestimation of opportunity given the fact that, based on our definition, the days on which patients received both IV and enteral antibiotics were not counted as opportunity days. Third, because PHIS uses billing days to capture medication use, we were unable to distinguish transitions that occurred early in the day vs those that took place later in the day. This could have led to an underestimation of percent opportunity, particularly for diagnoses with a short LOS; it also likely led to an underestimation of the variability observed across hospitals. Fourth, because we used an administrative dataset, we are unable to understand reasoning behind transitioning time from IV to oral antibiotics, as well as provider, patient, and institutional level factors that influenced these decisions.
CONCLUSION
Children hospitalized with bacterial infections often receive IV antibiotics, and the timing of transition from IV to enteral antibiotics varies significantly across hospitals. Further research is needed to compare the effectiveness of IV and enteral antibiotics and better define criteria for transition to enteral therapy. We identified ample opportunities for quality improvement initiatives to promote earlier transition, which have the potential to reduce healthcare utilization and promote optimal patient-directed high-value care.
1. Keren R, Luan X, Localio R, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X
3. Keren R, Shah SS, Srivastava R, et al; for the Pediatric Research Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e201692. https://doi.org/10.1542/peds.2016-1692
5. Li HK, Agweyu A, English M, Bejon P. An unsupported preference for intravenous antibiotics. PLoS Med. 2015;12(5):e1001825. https://dx.doi.org/10.1371%2Fjournal.pmed.1001825
6. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393
7. Bradley JS, Byington CL, Shah SS, et al; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Management of community-acquired pneumonia (CAP) in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1542/peds.2011-2385
8. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8-S14. https://doi.org/10.1093/cid/cir363
9. Schroeder AR, Ralston SL. Intravenous antibiotic durations for common bacterial infections in children: when is enough? J Hosp Med. 2014;9(9):604-609. https://doi.org/10.1002/jhm.2239
10. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatric Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003
11. van Zanten AR, Engelfriet PM, van Dillen K, van Veen M, Nuijten MJ, Polderman KH. Importance of nondrug costs of intravenous antibiotic therapy. Crit Care. 2003;7(6):R184-R190. https://doi.org/10.1186/cc2388
12. Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117(4):1210-1215. https://doi.org/10.1542/peds.2005-1465
13. Girdwood SCT, Sellas MN, Courter JD, et al. Improving the transition of intravenous to enteral antibiotics in pediatric patients with pneumonia or skin and soft tissue infections. J Hosp Med. 2020;15(1):10-15. https://doi.org/10.12788/jhm.3253
14. Core Elements of Hospital Antibiotic Stewardship Programs. Centers for Disease Control and Prevention. Published 2019. Accessed May 30, 2020. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html
15. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. https://doi.org/10.1093/cid/ciw118
16. Science M, Timberlake K, Morris A, Read S, Le Saux N; Groupe Antibiothérapie en Pédiatrie Canada Alliance for Stewardship of Antimicrobials in Pediatrics (GAP Can ASAP). Quality metrics for antimicrobial stewardship programs. Pediatrics. 2019;143(4):e20182372. https://doi.org/10.1542/peds.2018-2372
17. Tchou MJ, Hall M, Shah SS, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Patterns of electrolyte testing at children’s hospitals for common inpatient diagnoses. Pediatrics. 2019;144(1):e20181644. https://doi.org/10.1542/peds.2018-1644
18. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
19. Desai S, Shah SS, Hall M, Richardson TE, Thomson JE; Pediatric Research in Inpatient Settings (PRIS) Network. Imaging strategies and outcomes in children hospitalized with cervical lymphadenitis. J Hosp Med. 2020;15(4):197-203. https://doi.org/10.12788/jhm.3333
20. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
21. Tieder JS, Hall M, Auger KA, et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics. 2011;128(2):323-330. https://doi.org/10.1542/peds.2010-2064
22. Singh JA, Yu S. The burden of septic arthritis on the U.S. inpatient care: a national study. PLoS One. 2017;12(8):e0182577. https://doi.org/10.1371/journal.pone.0182577
23. Foradori DM, Lopez MA, Hall M, et al. Invasive bacterial infections in infants younger than 60 days with skin and soft tissue infections. Pediatr Emerg Care. 2018. https://doi.org/10.1097/pec.0000000000001584
24. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
25. Arancibia A, Icarte A, González C, Morasso I. Dose-dependent bioavailability of amoxycillin. Int J Clin Pharmacol Ther Toxicol. 1988;26(6):300-303.
26. Grayson ML, Cosgrove S, Crowe S, et al. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs. 7th ed. CRC Press; 2018.
27. Downes KJ, Hahn A, Wiles J, Courter JD, Inks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in pediatrics’. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006
28. Gras-Le Guen C, Boscher C, Godon N, et al. Therapeutic amoxicillin levels achieved with oral administration in term neonates. Eur J Clin Pharmacol. 2007;63(7):657-662. https://doi.org/10.1007/s00228-007-0307-3
29. Sanchez Navarro A. New formulations of amoxicillin/clavulanic acid: a pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2005;44(11):1097-1115. https://doi.org/10.2165/00003088-200544110-00001
30. Fine MJ, Hough LJ, Medsger AR, et al. The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157(1):36-44. https://doi.org/10.1001/archinte.1997.00440220040006
31. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007(4):CD003237. https://doi.org/10.1002/14651858.cd003237.pub2
32. Nageswaran S, Woods CR, Benjamin DK Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25(8):695-699. https://doi.org/10.1097/01.inf.0000227820.36036.f1
33. Al-Nammari S, Roberton B, Ferguson C. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Should a child with preseptal periorbital cellulitis be treated with intravenous or oral antibiotics? Emerg Med J. 2007;24(2):128-129. https://doi.org/10.1136/emj.2006.045245
34. Vieira F, Allen SM, Stocks RMS, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483, vii. https://doi.org/10.1016/j.otc.2008.01.002
35. Smith M, Shah S, Kronman M, Patel S, Thurm C, Hersh AL. Route of administration for highly orally bioavailable antibiotics. Open Forum Infect Dis. 2017;4(Suppl 1):S498-S499. https://doi.org/10.1093/ofid/ofx163.1291
36. Brady PW, Brinkman WB, Simmons JM, et al. Oral antibiotics at discharge for children with acute osteomyelitis: a rapid cycle improvement project. BMJ Qual Saf. 2014;23(6):499-507. https://doi.org/10.1136/bmjqs-2013-002179
37. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3
38. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585
39. Public Health Ontario. Antimicrobial stewardship programs metric examples. Published 2017. Accessed June 1, 2020. https://www.publichealthontario.ca/-/media/documents/A/2017/asp-metrics-examples.pdf?la=en
40. Desai S, Aronson PL, Shabanova V, et al; Febrile Young Infant Research Collaborative. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
Bacterial infections are a common reason for pediatric hospital admissions in the United States.1 Antibiotics are the mainstay of treatment, and whether to administer them intravenously (IV) or enterally is an important and, at times, challenging decision. Not all hospitalized patients with infections require IV antibiotics, and safe, effective early transitions to enteral therapy have been described for numerous infections.2-7 However, guidelines describing the ideal initial route of antibiotic administration and when to transition to oral therapy are lacking.5,7,8 This lack of high-quality evidence-based guidance may contribute to overuse of IV antibiotics for many hospitalized pediatric patients, even when safe and effective enteral options exist.9
Significant costs and harms are associated with the use of IV antibiotics. In particular, studies have demonstrated longer length of stay (LOS), increased costs, and worsened pain or anxiety related to complications (eg, phlebitis, extravasation injury, thrombosis, catheter-associated bloodstream infections) associated with IV antibiotics.3,4,10-13 Earlier transition to enteral therapy, however, can mitigate these increased risks and costs.
The Centers for Disease Control and Prevention lists the transition from IV to oral antibiotics as a key stewardship intervention for improving antibiotic use.14 The Infectious Diseases Society of America (IDSA) antibiotic stewardship program guidelines strongly recommend the timely conversion from IV to oral antibiotics, stating that efforts focusing on this transition should be integrated into routine practice.15 There are a few metrics in the literature to measure this intervention, but none is universally used, and a modified delphi process could not reach consensus on IV-to-oral transition metrics.16
Few studies describe the opportunity to transition to enteral antibiotics in hospitalized patients with common bacterial infections or explore variation across hospitals. It is critical to understand current practice of antibiotic administration in order to identify opportunities to optimize patient outcomes and promote high-value care. Furthermore, few studies have evaluated the feasibility of IV-to-oral transition metrics using an administrative database. Thus, the aims of this study were to (1) determine opportunities to transition from IV to enteral antibiotics for pediatric patients hospitalized with common bacterial infections based on their ability to tolerate other enteral medications, (2) describe variation in transition practices among children’s hospitals, and (3) evaluate the feasibility of novel IV-to-oral transition metrics using an administrative database to inform stewardship efforts.
METHODS
Study Design and Setting
This multicenter, retrospective cohort study used data from the Pediatric Health Information System (PHIS), an administrative and billing database containing encounter-level data from 52 tertiary care pediatric hospitals across the United States affiliated with the Children’s Hospital Association (Lenexa, Kansas). Hospitals submit encounter-level data, including demographics, medications, and diagnoses based on International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes. Data were de-identified at the time of submission, and data quality and reliability were assured by joint efforts between the Children’s Hospital Association and participating hospitals.
Study Population
This study included pediatric patients aged 60 days to 18 years who were hospitalized (inpatient or observation status) at one of the participating hospitals between January 1, 2017, and December 31, 2018, for one of the following seven common bacterial infections: community-acquired pneumonia (CAP), neck infection (superficial and deep), periorbital/orbital infection, urinary tract infection (UTI), osteomyelitis, septic arthritis, or skin and soft tissue infection (SSTI). The diagnosis cohorts were defined based on ICD-10-CM discharge diagnoses adapted from previous studies (Appendix Table 1).3,17-23 To define a cohort of generally healthy pediatric patients with an acute infection, we excluded patients hospitalized in the intensive care unit, patients with nonhome discharges, and patients with complex chronic conditions.24 We also excluded hospitals with incomplete data during the study period (n=1). The Institutional Review Board at Cincinnati Children’s Hospital Medical Center determined this study to be non–human-subjects research.
Outcomes
The primary outcomes were the number of opportunity days and the percent of days with opportunity to transition from IV to enteral therapy. Opportunity days, or days in which there was a potential opportunity to transition from IV to enteral antibiotics, were defined as days patients received only IV antibiotic doses and at least one enteral nonantibiotic medication, suggesting an ability to take enteral medications.13 We excluded days patients received IV antibiotics for which there was no enteral alternative (eg, vancomycin, Appendix Table 2). When measuring opportunity, to be conservative (ie, to underestimate rather than overestimate opportunity), we did not count as an opportunity day any day in which patients received both IV and enteral antibiotics. Percent opportunity, or the percent of days patients received antibiotics in which there was potential opportunity to transition from IV to enteral antibiotics, was defined as the number of opportunity days divided by number of inpatient days patients received enteral antibiotics or IV antibiotics with at least one enteral nonantibiotic medication (antibiotic days). Similar to opportunity days, antibiotic days excluded days patients were on IV antibiotics for which there was no enteral alternative. Based on our definition, a lower percent opportunity indicates that a hospital is using enteral antibiotics earlier during the hospitalization (earlier transition), while a higher percent opportunity represents later enteral antibiotic use (later transition).
Statistical Analysis
Demographic and clinical characteristics were summarized by diagnosis with descriptive statistics, including frequency with percentage, mean with standard deviation, and median with interquartile range (IQR). For each diagnosis, we evaluated aggregate opportunity days (sum of opportunity days among all hospitals), opportunity days per encounter, and aggregate percent opportunity using frequencies, mean with standard deviation, and percentages, respectively. We also calculated aggregate opportunity days for diagnosis-antibiotic combinations. To visually show variation in the percent opportunity across hospitals, we displayed the percent opportunity on a heat map, and evaluated percent opportunity across hospitals using chi-square tests. To compare the variability in the percent opportunity across and within hospitals, we used a generalized linear model with two fixed effects (hospital and diagnosis), and parsed the variability using the sum of squares. We performed a sensitivity analysis and excluded days that patients received antiemetic medications (eg, ondansetron, granisetron, prochlorperazine, promethazine), as these suggest potential intolerance of enteral medications. All statistical analyses were performed using SAS v.9.4 (SAS Institute Inc, Cary, North Carolina) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, California), and P values < .05 were considered statistically significant.
RESULTS
During the 2-year study period, 100,103 hospitalizations met our inclusion criteria across 51 hospitals and seven diagnosis categories (Table 1). Diagnosis cohorts ranged in size from 1,462 encounters for septic arthritis to 35,665 encounters for neck infections. Overall, we identified 88,522 aggregate opportunity days on which there was an opportunity to switch from IV to enteral treatment in the majority of participants (percent opportunity, 57%).

Opportunity by Diagnosis
The number of opportunity days (aggregate and mean per encounter) and percent opportunity varied by diagnosis (Table 2). The aggregate number of opportunity days ranged from 3,693 in patients with septic arthritis to 25,359 in patients with SSTI, and mean opportunity days per encounter ranged from 0.9 in CAP to 2.8 in septic arthritis. Percent opportunity was highest for septic arthritis at 72.7% and lowest for CAP at 39.7%.

Variation in Opportunity Among Hospitals
The variation in the percent opportunity across hospitals was statistically significant for all diagnoses (Figure). Within hospitals, we observed similar practice patterns across diagnoses. For example, hospitals with a higher percent opportunity for one diagnosis tended to have higher percent opportunity for the other diagnoses (as noted in the top portion of the Figure), and those with lower percent opportunity for one diagnosis tended to also have lower percent opportunity for the other diagnoses studied (as noted in the bottom portion of the Figure). When evaluating variability in the percent opportunity, 45% of the variability was attributable to the hospital-effect and 35% to the diagnosis; the remainder was unexplained variability. Sensitivity analysis excluding days when patients received an antiemetic medication yielded no differences in our results.

Opportunity by Antibiotic
The aggregate number of opportunity days varied by antibiotic (Table 3). Intravenous antibiotics with the largest number of opportunity days included clindamycin (44,293), ceftriaxone (23,896), and ampicillin-sulbactam (15,484). Antibiotic-diagnosis combinations with the largest number of opportunity days for each diagnosis included ceftriaxone and ampicillin in CAP; clindamycin in cellulitis, SSTI, and neck infections; ceftriaxone in UTI; and cefazolin in osteomyelitis and septic arthritis.

DISCUSSION
In this multicenter study of pediatric patients hospitalized with common bacterial infections, there was the potential to transition from IV to enteral treatment in over half of the antibiotic days. The degree of opportunity varied by infection, antibiotic, and hospital. Antibiotics with a large aggregate number of opportunity days for enteral transition included clindamycin, which has excellent bioavailability; and ampicillin and ampicillin-sulbactam, which can achieve pharmacodynamic targets with oral equivalents.25-29 The across-hospital variation for a given diagnosis suggests that certain hospitals have strategies in place which permit an earlier transition to enteral antibiotics compared to other institutions in which there were likely missed opportunities to do so. This variability is likely due to limited evidence, emphasizing the need for robust studies to better understand the optimal initial antibiotic route and transition time. Our findings highlight the need for, and large potential impact of, stewardship efforts to promote earlier transition for specific drug targets. This study also demonstrates the feasibility of obtaining two metrics—percent opportunity and opportunity days—from administrative databases to inform stewardship efforts within and across hospitals.
Opportunity days and percent opportunity varied among diagnoses. The variation in aggregate opportunity days was largely a reflection of the number of encounters: Diagnoses such as SSTI, neck infections, and CAP had a large number of both aggregate opportunity days and encounters. The range of opportunity days per encounter (0.9-2.5) suggests potential missed opportunities to transition to enteral antibiotics across all diagnoses (Table 2). The higher opportunity days per encounter in osteomyelitis and septic arthritis may be related to longer LOS and higher percent opportunity. Percent opportunity likely varied among diagnoses due to differences in admission and discharge readiness criteria, diagnostic evaluation, frequency of antibiotic administration, and evidence on the optimal route of initial antibiotics and when to transition to oral formulations. For example, we hypothesize that certain diagnoses, such as osteomyelitis and septic arthritis, have admission and discharge readiness criteria directly tied to the perceived need for IV antibiotics, which may limit in-hospital days on enteral antibiotics and explain the high percent opportunity that we observed. The high percent opportunity seen in musculoskeletal infections also may be due to delays in initiating targeted treatment until culture results were available. Encounters for CAP had the lowest percent opportunity; we hypothesize that this is because admission and discharge readiness may be determined by factors other than the need for IV antibiotics (eg, need for supplemental oxygen), which may increase days on enteral antibiotics and lead to a lower percent opportunity.30
Urinary tract infection encounters had a high percent opportunity. As with musculoskeletal infection, this may be related to delays in initiating targeted treatment until culture results became available. Another reason for the high percent opportunity in UTI could be the common use of ceftriaxone, which, dosed every 24 hours, likely reduced the opportunity to transition to enteral antibiotics. There is strong evidence demonstrating no difference in outcomes based on antibiotic routes for UTI, and we would expect this to result in a low percent opportunity.2,31 While the observed high opportunity in UTI may relate to an initial unknown diagnosis or concern for systemic infection, this highlights potential opportunities for quality improvement initiatives to promote empiric oral antibiotics in clinically stable patients hospitalized with suspected UTI.
There was substantial variation in percent opportunity across hospitals for a given diagnosis, with less variation across diagnoses for a given hospital. Variation across hospitals but consistency within individual hospitals suggests that some hospitals may promote earlier transition from IV to enteral antibiotics as standard practice for all diagnoses, while other hospitals continue IV antibiotics for the entire hospitalization, highlighting potential missed opportunities at some institutions. While emerging data suggest that traditional long durations of IV antibiotics are not necessary for many infections, the limited evidence on the optimal time to switch to oral antibiotics may have influenced this variation.2-7 Many guidelines recommend initial IV antibiotics for hospitalized pediatric patients, but there are few studies comparing IV and enteral therapy.2,5,9 Limited evidence leaves significant room for hospital culture, antibiotic stewardship efforts, reimbursement considerations, and/or hospital workflow to influence transition timing and overall opportunity at individual hospitals.7,8,32-34 These findings emphasize the importance of research to identify optimal transition time and comparative effectiveness studies to evaluate whether initial IV antibiotics are truly needed for mild—and even severe—disease presentations. Since many patients are admitted for the perceived need for IV antibiotics, earlier use of enteral antibiotics could reduce rates of hospitalizations, LOS, healthcare costs, and resource utilization.
Antibiotics with a high number of opportunity days included clindamycin, ceftriaxone, ampicillin-sublactam, and ampicillin. Our findings are consistent with another study which found that most bioavailable drugs, including clindamycin, were administered via the IV route and accounted for a large number of antibiotic days.35 The Infectious Diseases Society of America recommends that hospitals promote earlier transition to oral formulations for highly bioavailable drugs.7 Given the high bioavailability of clindamycin, its common use in high-frequency encounters such as SSTI and neck infections, and the fact that it accounted for a large number of opportunity days, quality improvement initiatives promoting earlier transition to oral clindamycin could have a large impact across health systems.25,26 Additionally, although beta-lactam antibiotics such as amoxicillin and amoxicillin-sulbactam are not highly bioavailable, oral dosing can achieve sufficient serum concentrations to reach pharmacodynamic targets for common clinical indications; this could be an important quality improvement initiative.27-29 Several single-site studies have successfully implemented quality improvement initiatives to promote earlier IV-to-enteral transition, with resulting reductions in costs and no adverse events noted, highlighting the feasibility and impact of such efforts.13,36-38
This study also demonstrates the feasibility of collecting two metrics (percent opportunity and opportunity days) from administrative databases to inform IV-to-oral transition benchmarking and stewardship efforts. While there are several metrics in the literature for evaluating antibiotic transition (eg, days of IV or oral therapy, percentage of antibiotics given via the oral route, time to switch from IV to oral, and acceptance rate of suggested changes to antibiotic route), none are universally used or agreed upon.15,16,39 The opportunity metrics used in this study have several strengths, including the feasibility of obtaining them from existing databases and the ability to account for intake of other enteral medications; the latter is not evaluated in other metrics. These opportunity metrics can be used together to identify the percent of time in which there is opportunity to transition and total number of days to understand the full extent of potential opportunity for future interventions. As demonstrated in this study, these metrics can be measured by diagnosis, antibiotic, or diagnosis-antibiotic combination, and they can be used to evaluate stewardship efforts at a single institution over time or compare efforts across hospitals.
These findings should be interpreted in the context of important limitations. First, we attempted to characterize potential opportunity to transition to enteral medications based on a patient’s ability to tolerate nonenteral medications. However, there are other factors that could limit the opportunity to transition that we could not account for with an administrative dataset, including the use of antibiotics prior to admission, disease progression, severity of illness, and malabsorptive concerns. Thus, though we may have overestimated the true opportunity to transition to enteral antibiotics, it is unlikely that this would account for all of the variation in transition times that we observed across hospitals. Second, while our study required patients to have one of seven types of infection, we did not exclude any additional infectious diagnoses (eg, concurrent bacteremia, Clostridioides difficile, otitis media) that could have driven the choice of antibiotic type and modality. Although emerging evidence is supporting earlier transitions to oral therapy, bacteremia is typically treated with IV antibiotics; this may have led to an overestimation of true opportunity.40 “Clostridioides” difficile and otitis media are typically treated with enteral therapy; concurrent infections such as these may have led to an underestimation of opportunity given the fact that, based on our definition, the days on which patients received both IV and enteral antibiotics were not counted as opportunity days. Third, because PHIS uses billing days to capture medication use, we were unable to distinguish transitions that occurred early in the day vs those that took place later in the day. This could have led to an underestimation of percent opportunity, particularly for diagnoses with a short LOS; it also likely led to an underestimation of the variability observed across hospitals. Fourth, because we used an administrative dataset, we are unable to understand reasoning behind transitioning time from IV to oral antibiotics, as well as provider, patient, and institutional level factors that influenced these decisions.
CONCLUSION
Children hospitalized with bacterial infections often receive IV antibiotics, and the timing of transition from IV to enteral antibiotics varies significantly across hospitals. Further research is needed to compare the effectiveness of IV and enteral antibiotics and better define criteria for transition to enteral therapy. We identified ample opportunities for quality improvement initiatives to promote earlier transition, which have the potential to reduce healthcare utilization and promote optimal patient-directed high-value care.
Bacterial infections are a common reason for pediatric hospital admissions in the United States.1 Antibiotics are the mainstay of treatment, and whether to administer them intravenously (IV) or enterally is an important and, at times, challenging decision. Not all hospitalized patients with infections require IV antibiotics, and safe, effective early transitions to enteral therapy have been described for numerous infections.2-7 However, guidelines describing the ideal initial route of antibiotic administration and when to transition to oral therapy are lacking.5,7,8 This lack of high-quality evidence-based guidance may contribute to overuse of IV antibiotics for many hospitalized pediatric patients, even when safe and effective enteral options exist.9
Significant costs and harms are associated with the use of IV antibiotics. In particular, studies have demonstrated longer length of stay (LOS), increased costs, and worsened pain or anxiety related to complications (eg, phlebitis, extravasation injury, thrombosis, catheter-associated bloodstream infections) associated with IV antibiotics.3,4,10-13 Earlier transition to enteral therapy, however, can mitigate these increased risks and costs.
The Centers for Disease Control and Prevention lists the transition from IV to oral antibiotics as a key stewardship intervention for improving antibiotic use.14 The Infectious Diseases Society of America (IDSA) antibiotic stewardship program guidelines strongly recommend the timely conversion from IV to oral antibiotics, stating that efforts focusing on this transition should be integrated into routine practice.15 There are a few metrics in the literature to measure this intervention, but none is universally used, and a modified delphi process could not reach consensus on IV-to-oral transition metrics.16
Few studies describe the opportunity to transition to enteral antibiotics in hospitalized patients with common bacterial infections or explore variation across hospitals. It is critical to understand current practice of antibiotic administration in order to identify opportunities to optimize patient outcomes and promote high-value care. Furthermore, few studies have evaluated the feasibility of IV-to-oral transition metrics using an administrative database. Thus, the aims of this study were to (1) determine opportunities to transition from IV to enteral antibiotics for pediatric patients hospitalized with common bacterial infections based on their ability to tolerate other enteral medications, (2) describe variation in transition practices among children’s hospitals, and (3) evaluate the feasibility of novel IV-to-oral transition metrics using an administrative database to inform stewardship efforts.
METHODS
Study Design and Setting
This multicenter, retrospective cohort study used data from the Pediatric Health Information System (PHIS), an administrative and billing database containing encounter-level data from 52 tertiary care pediatric hospitals across the United States affiliated with the Children’s Hospital Association (Lenexa, Kansas). Hospitals submit encounter-level data, including demographics, medications, and diagnoses based on International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes. Data were de-identified at the time of submission, and data quality and reliability were assured by joint efforts between the Children’s Hospital Association and participating hospitals.
Study Population
This study included pediatric patients aged 60 days to 18 years who were hospitalized (inpatient or observation status) at one of the participating hospitals between January 1, 2017, and December 31, 2018, for one of the following seven common bacterial infections: community-acquired pneumonia (CAP), neck infection (superficial and deep), periorbital/orbital infection, urinary tract infection (UTI), osteomyelitis, septic arthritis, or skin and soft tissue infection (SSTI). The diagnosis cohorts were defined based on ICD-10-CM discharge diagnoses adapted from previous studies (Appendix Table 1).3,17-23 To define a cohort of generally healthy pediatric patients with an acute infection, we excluded patients hospitalized in the intensive care unit, patients with nonhome discharges, and patients with complex chronic conditions.24 We also excluded hospitals with incomplete data during the study period (n=1). The Institutional Review Board at Cincinnati Children’s Hospital Medical Center determined this study to be non–human-subjects research.
Outcomes
The primary outcomes were the number of opportunity days and the percent of days with opportunity to transition from IV to enteral therapy. Opportunity days, or days in which there was a potential opportunity to transition from IV to enteral antibiotics, were defined as days patients received only IV antibiotic doses and at least one enteral nonantibiotic medication, suggesting an ability to take enteral medications.13 We excluded days patients received IV antibiotics for which there was no enteral alternative (eg, vancomycin, Appendix Table 2). When measuring opportunity, to be conservative (ie, to underestimate rather than overestimate opportunity), we did not count as an opportunity day any day in which patients received both IV and enteral antibiotics. Percent opportunity, or the percent of days patients received antibiotics in which there was potential opportunity to transition from IV to enteral antibiotics, was defined as the number of opportunity days divided by number of inpatient days patients received enteral antibiotics or IV antibiotics with at least one enteral nonantibiotic medication (antibiotic days). Similar to opportunity days, antibiotic days excluded days patients were on IV antibiotics for which there was no enteral alternative. Based on our definition, a lower percent opportunity indicates that a hospital is using enteral antibiotics earlier during the hospitalization (earlier transition), while a higher percent opportunity represents later enteral antibiotic use (later transition).
Statistical Analysis
Demographic and clinical characteristics were summarized by diagnosis with descriptive statistics, including frequency with percentage, mean with standard deviation, and median with interquartile range (IQR). For each diagnosis, we evaluated aggregate opportunity days (sum of opportunity days among all hospitals), opportunity days per encounter, and aggregate percent opportunity using frequencies, mean with standard deviation, and percentages, respectively. We also calculated aggregate opportunity days for diagnosis-antibiotic combinations. To visually show variation in the percent opportunity across hospitals, we displayed the percent opportunity on a heat map, and evaluated percent opportunity across hospitals using chi-square tests. To compare the variability in the percent opportunity across and within hospitals, we used a generalized linear model with two fixed effects (hospital and diagnosis), and parsed the variability using the sum of squares. We performed a sensitivity analysis and excluded days that patients received antiemetic medications (eg, ondansetron, granisetron, prochlorperazine, promethazine), as these suggest potential intolerance of enteral medications. All statistical analyses were performed using SAS v.9.4 (SAS Institute Inc, Cary, North Carolina) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, California), and P values < .05 were considered statistically significant.
RESULTS
During the 2-year study period, 100,103 hospitalizations met our inclusion criteria across 51 hospitals and seven diagnosis categories (Table 1). Diagnosis cohorts ranged in size from 1,462 encounters for septic arthritis to 35,665 encounters for neck infections. Overall, we identified 88,522 aggregate opportunity days on which there was an opportunity to switch from IV to enteral treatment in the majority of participants (percent opportunity, 57%).

Opportunity by Diagnosis
The number of opportunity days (aggregate and mean per encounter) and percent opportunity varied by diagnosis (Table 2). The aggregate number of opportunity days ranged from 3,693 in patients with septic arthritis to 25,359 in patients with SSTI, and mean opportunity days per encounter ranged from 0.9 in CAP to 2.8 in septic arthritis. Percent opportunity was highest for septic arthritis at 72.7% and lowest for CAP at 39.7%.

Variation in Opportunity Among Hospitals
The variation in the percent opportunity across hospitals was statistically significant for all diagnoses (Figure). Within hospitals, we observed similar practice patterns across diagnoses. For example, hospitals with a higher percent opportunity for one diagnosis tended to have higher percent opportunity for the other diagnoses (as noted in the top portion of the Figure), and those with lower percent opportunity for one diagnosis tended to also have lower percent opportunity for the other diagnoses studied (as noted in the bottom portion of the Figure). When evaluating variability in the percent opportunity, 45% of the variability was attributable to the hospital-effect and 35% to the diagnosis; the remainder was unexplained variability. Sensitivity analysis excluding days when patients received an antiemetic medication yielded no differences in our results.

Opportunity by Antibiotic
The aggregate number of opportunity days varied by antibiotic (Table 3). Intravenous antibiotics with the largest number of opportunity days included clindamycin (44,293), ceftriaxone (23,896), and ampicillin-sulbactam (15,484). Antibiotic-diagnosis combinations with the largest number of opportunity days for each diagnosis included ceftriaxone and ampicillin in CAP; clindamycin in cellulitis, SSTI, and neck infections; ceftriaxone in UTI; and cefazolin in osteomyelitis and septic arthritis.

DISCUSSION
In this multicenter study of pediatric patients hospitalized with common bacterial infections, there was the potential to transition from IV to enteral treatment in over half of the antibiotic days. The degree of opportunity varied by infection, antibiotic, and hospital. Antibiotics with a large aggregate number of opportunity days for enteral transition included clindamycin, which has excellent bioavailability; and ampicillin and ampicillin-sulbactam, which can achieve pharmacodynamic targets with oral equivalents.25-29 The across-hospital variation for a given diagnosis suggests that certain hospitals have strategies in place which permit an earlier transition to enteral antibiotics compared to other institutions in which there were likely missed opportunities to do so. This variability is likely due to limited evidence, emphasizing the need for robust studies to better understand the optimal initial antibiotic route and transition time. Our findings highlight the need for, and large potential impact of, stewardship efforts to promote earlier transition for specific drug targets. This study also demonstrates the feasibility of obtaining two metrics—percent opportunity and opportunity days—from administrative databases to inform stewardship efforts within and across hospitals.
Opportunity days and percent opportunity varied among diagnoses. The variation in aggregate opportunity days was largely a reflection of the number of encounters: Diagnoses such as SSTI, neck infections, and CAP had a large number of both aggregate opportunity days and encounters. The range of opportunity days per encounter (0.9-2.5) suggests potential missed opportunities to transition to enteral antibiotics across all diagnoses (Table 2). The higher opportunity days per encounter in osteomyelitis and septic arthritis may be related to longer LOS and higher percent opportunity. Percent opportunity likely varied among diagnoses due to differences in admission and discharge readiness criteria, diagnostic evaluation, frequency of antibiotic administration, and evidence on the optimal route of initial antibiotics and when to transition to oral formulations. For example, we hypothesize that certain diagnoses, such as osteomyelitis and septic arthritis, have admission and discharge readiness criteria directly tied to the perceived need for IV antibiotics, which may limit in-hospital days on enteral antibiotics and explain the high percent opportunity that we observed. The high percent opportunity seen in musculoskeletal infections also may be due to delays in initiating targeted treatment until culture results were available. Encounters for CAP had the lowest percent opportunity; we hypothesize that this is because admission and discharge readiness may be determined by factors other than the need for IV antibiotics (eg, need for supplemental oxygen), which may increase days on enteral antibiotics and lead to a lower percent opportunity.30
Urinary tract infection encounters had a high percent opportunity. As with musculoskeletal infection, this may be related to delays in initiating targeted treatment until culture results became available. Another reason for the high percent opportunity in UTI could be the common use of ceftriaxone, which, dosed every 24 hours, likely reduced the opportunity to transition to enteral antibiotics. There is strong evidence demonstrating no difference in outcomes based on antibiotic routes for UTI, and we would expect this to result in a low percent opportunity.2,31 While the observed high opportunity in UTI may relate to an initial unknown diagnosis or concern for systemic infection, this highlights potential opportunities for quality improvement initiatives to promote empiric oral antibiotics in clinically stable patients hospitalized with suspected UTI.
There was substantial variation in percent opportunity across hospitals for a given diagnosis, with less variation across diagnoses for a given hospital. Variation across hospitals but consistency within individual hospitals suggests that some hospitals may promote earlier transition from IV to enteral antibiotics as standard practice for all diagnoses, while other hospitals continue IV antibiotics for the entire hospitalization, highlighting potential missed opportunities at some institutions. While emerging data suggest that traditional long durations of IV antibiotics are not necessary for many infections, the limited evidence on the optimal time to switch to oral antibiotics may have influenced this variation.2-7 Many guidelines recommend initial IV antibiotics for hospitalized pediatric patients, but there are few studies comparing IV and enteral therapy.2,5,9 Limited evidence leaves significant room for hospital culture, antibiotic stewardship efforts, reimbursement considerations, and/or hospital workflow to influence transition timing and overall opportunity at individual hospitals.7,8,32-34 These findings emphasize the importance of research to identify optimal transition time and comparative effectiveness studies to evaluate whether initial IV antibiotics are truly needed for mild—and even severe—disease presentations. Since many patients are admitted for the perceived need for IV antibiotics, earlier use of enteral antibiotics could reduce rates of hospitalizations, LOS, healthcare costs, and resource utilization.
Antibiotics with a high number of opportunity days included clindamycin, ceftriaxone, ampicillin-sublactam, and ampicillin. Our findings are consistent with another study which found that most bioavailable drugs, including clindamycin, were administered via the IV route and accounted for a large number of antibiotic days.35 The Infectious Diseases Society of America recommends that hospitals promote earlier transition to oral formulations for highly bioavailable drugs.7 Given the high bioavailability of clindamycin, its common use in high-frequency encounters such as SSTI and neck infections, and the fact that it accounted for a large number of opportunity days, quality improvement initiatives promoting earlier transition to oral clindamycin could have a large impact across health systems.25,26 Additionally, although beta-lactam antibiotics such as amoxicillin and amoxicillin-sulbactam are not highly bioavailable, oral dosing can achieve sufficient serum concentrations to reach pharmacodynamic targets for common clinical indications; this could be an important quality improvement initiative.27-29 Several single-site studies have successfully implemented quality improvement initiatives to promote earlier IV-to-enteral transition, with resulting reductions in costs and no adverse events noted, highlighting the feasibility and impact of such efforts.13,36-38
This study also demonstrates the feasibility of collecting two metrics (percent opportunity and opportunity days) from administrative databases to inform IV-to-oral transition benchmarking and stewardship efforts. While there are several metrics in the literature for evaluating antibiotic transition (eg, days of IV or oral therapy, percentage of antibiotics given via the oral route, time to switch from IV to oral, and acceptance rate of suggested changes to antibiotic route), none are universally used or agreed upon.15,16,39 The opportunity metrics used in this study have several strengths, including the feasibility of obtaining them from existing databases and the ability to account for intake of other enteral medications; the latter is not evaluated in other metrics. These opportunity metrics can be used together to identify the percent of time in which there is opportunity to transition and total number of days to understand the full extent of potential opportunity for future interventions. As demonstrated in this study, these metrics can be measured by diagnosis, antibiotic, or diagnosis-antibiotic combination, and they can be used to evaluate stewardship efforts at a single institution over time or compare efforts across hospitals.
These findings should be interpreted in the context of important limitations. First, we attempted to characterize potential opportunity to transition to enteral medications based on a patient’s ability to tolerate nonenteral medications. However, there are other factors that could limit the opportunity to transition that we could not account for with an administrative dataset, including the use of antibiotics prior to admission, disease progression, severity of illness, and malabsorptive concerns. Thus, though we may have overestimated the true opportunity to transition to enteral antibiotics, it is unlikely that this would account for all of the variation in transition times that we observed across hospitals. Second, while our study required patients to have one of seven types of infection, we did not exclude any additional infectious diagnoses (eg, concurrent bacteremia, Clostridioides difficile, otitis media) that could have driven the choice of antibiotic type and modality. Although emerging evidence is supporting earlier transitions to oral therapy, bacteremia is typically treated with IV antibiotics; this may have led to an overestimation of true opportunity.40 “Clostridioides” difficile and otitis media are typically treated with enteral therapy; concurrent infections such as these may have led to an underestimation of opportunity given the fact that, based on our definition, the days on which patients received both IV and enteral antibiotics were not counted as opportunity days. Third, because PHIS uses billing days to capture medication use, we were unable to distinguish transitions that occurred early in the day vs those that took place later in the day. This could have led to an underestimation of percent opportunity, particularly for diagnoses with a short LOS; it also likely led to an underestimation of the variability observed across hospitals. Fourth, because we used an administrative dataset, we are unable to understand reasoning behind transitioning time from IV to oral antibiotics, as well as provider, patient, and institutional level factors that influenced these decisions.
CONCLUSION
Children hospitalized with bacterial infections often receive IV antibiotics, and the timing of transition from IV to enteral antibiotics varies significantly across hospitals. Further research is needed to compare the effectiveness of IV and enteral antibiotics and better define criteria for transition to enteral therapy. We identified ample opportunities for quality improvement initiatives to promote earlier transition, which have the potential to reduce healthcare utilization and promote optimal patient-directed high-value care.
1. Keren R, Luan X, Localio R, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X
3. Keren R, Shah SS, Srivastava R, et al; for the Pediatric Research Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e201692. https://doi.org/10.1542/peds.2016-1692
5. Li HK, Agweyu A, English M, Bejon P. An unsupported preference for intravenous antibiotics. PLoS Med. 2015;12(5):e1001825. https://dx.doi.org/10.1371%2Fjournal.pmed.1001825
6. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393
7. Bradley JS, Byington CL, Shah SS, et al; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Management of community-acquired pneumonia (CAP) in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1542/peds.2011-2385
8. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8-S14. https://doi.org/10.1093/cid/cir363
9. Schroeder AR, Ralston SL. Intravenous antibiotic durations for common bacterial infections in children: when is enough? J Hosp Med. 2014;9(9):604-609. https://doi.org/10.1002/jhm.2239
10. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatric Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003
11. van Zanten AR, Engelfriet PM, van Dillen K, van Veen M, Nuijten MJ, Polderman KH. Importance of nondrug costs of intravenous antibiotic therapy. Crit Care. 2003;7(6):R184-R190. https://doi.org/10.1186/cc2388
12. Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117(4):1210-1215. https://doi.org/10.1542/peds.2005-1465
13. Girdwood SCT, Sellas MN, Courter JD, et al. Improving the transition of intravenous to enteral antibiotics in pediatric patients with pneumonia or skin and soft tissue infections. J Hosp Med. 2020;15(1):10-15. https://doi.org/10.12788/jhm.3253
14. Core Elements of Hospital Antibiotic Stewardship Programs. Centers for Disease Control and Prevention. Published 2019. Accessed May 30, 2020. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html
15. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. https://doi.org/10.1093/cid/ciw118
16. Science M, Timberlake K, Morris A, Read S, Le Saux N; Groupe Antibiothérapie en Pédiatrie Canada Alliance for Stewardship of Antimicrobials in Pediatrics (GAP Can ASAP). Quality metrics for antimicrobial stewardship programs. Pediatrics. 2019;143(4):e20182372. https://doi.org/10.1542/peds.2018-2372
17. Tchou MJ, Hall M, Shah SS, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Patterns of electrolyte testing at children’s hospitals for common inpatient diagnoses. Pediatrics. 2019;144(1):e20181644. https://doi.org/10.1542/peds.2018-1644
18. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
19. Desai S, Shah SS, Hall M, Richardson TE, Thomson JE; Pediatric Research in Inpatient Settings (PRIS) Network. Imaging strategies and outcomes in children hospitalized with cervical lymphadenitis. J Hosp Med. 2020;15(4):197-203. https://doi.org/10.12788/jhm.3333
20. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
21. Tieder JS, Hall M, Auger KA, et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics. 2011;128(2):323-330. https://doi.org/10.1542/peds.2010-2064
22. Singh JA, Yu S. The burden of septic arthritis on the U.S. inpatient care: a national study. PLoS One. 2017;12(8):e0182577. https://doi.org/10.1371/journal.pone.0182577
23. Foradori DM, Lopez MA, Hall M, et al. Invasive bacterial infections in infants younger than 60 days with skin and soft tissue infections. Pediatr Emerg Care. 2018. https://doi.org/10.1097/pec.0000000000001584
24. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
25. Arancibia A, Icarte A, González C, Morasso I. Dose-dependent bioavailability of amoxycillin. Int J Clin Pharmacol Ther Toxicol. 1988;26(6):300-303.
26. Grayson ML, Cosgrove S, Crowe S, et al. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs. 7th ed. CRC Press; 2018.
27. Downes KJ, Hahn A, Wiles J, Courter JD, Inks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in pediatrics’. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006
28. Gras-Le Guen C, Boscher C, Godon N, et al. Therapeutic amoxicillin levels achieved with oral administration in term neonates. Eur J Clin Pharmacol. 2007;63(7):657-662. https://doi.org/10.1007/s00228-007-0307-3
29. Sanchez Navarro A. New formulations of amoxicillin/clavulanic acid: a pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2005;44(11):1097-1115. https://doi.org/10.2165/00003088-200544110-00001
30. Fine MJ, Hough LJ, Medsger AR, et al. The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157(1):36-44. https://doi.org/10.1001/archinte.1997.00440220040006
31. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007(4):CD003237. https://doi.org/10.1002/14651858.cd003237.pub2
32. Nageswaran S, Woods CR, Benjamin DK Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25(8):695-699. https://doi.org/10.1097/01.inf.0000227820.36036.f1
33. Al-Nammari S, Roberton B, Ferguson C. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Should a child with preseptal periorbital cellulitis be treated with intravenous or oral antibiotics? Emerg Med J. 2007;24(2):128-129. https://doi.org/10.1136/emj.2006.045245
34. Vieira F, Allen SM, Stocks RMS, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483, vii. https://doi.org/10.1016/j.otc.2008.01.002
35. Smith M, Shah S, Kronman M, Patel S, Thurm C, Hersh AL. Route of administration for highly orally bioavailable antibiotics. Open Forum Infect Dis. 2017;4(Suppl 1):S498-S499. https://doi.org/10.1093/ofid/ofx163.1291
36. Brady PW, Brinkman WB, Simmons JM, et al. Oral antibiotics at discharge for children with acute osteomyelitis: a rapid cycle improvement project. BMJ Qual Saf. 2014;23(6):499-507. https://doi.org/10.1136/bmjqs-2013-002179
37. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3
38. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585
39. Public Health Ontario. Antimicrobial stewardship programs metric examples. Published 2017. Accessed June 1, 2020. https://www.publichealthontario.ca/-/media/documents/A/2017/asp-metrics-examples.pdf?la=en
40. Desai S, Aronson PL, Shabanova V, et al; Febrile Young Infant Research Collaborative. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
1. Keren R, Luan X, Localio R, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X
3. Keren R, Shah SS, Srivastava R, et al; for the Pediatric Research Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e201692. https://doi.org/10.1542/peds.2016-1692
5. Li HK, Agweyu A, English M, Bejon P. An unsupported preference for intravenous antibiotics. PLoS Med. 2015;12(5):e1001825. https://dx.doi.org/10.1371%2Fjournal.pmed.1001825
6. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393
7. Bradley JS, Byington CL, Shah SS, et al; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Management of community-acquired pneumonia (CAP) in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1542/peds.2011-2385
8. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8-S14. https://doi.org/10.1093/cid/cir363
9. Schroeder AR, Ralston SL. Intravenous antibiotic durations for common bacterial infections in children: when is enough? J Hosp Med. 2014;9(9):604-609. https://doi.org/10.1002/jhm.2239
10. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatric Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003
11. van Zanten AR, Engelfriet PM, van Dillen K, van Veen M, Nuijten MJ, Polderman KH. Importance of nondrug costs of intravenous antibiotic therapy. Crit Care. 2003;7(6):R184-R190. https://doi.org/10.1186/cc2388
12. Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117(4):1210-1215. https://doi.org/10.1542/peds.2005-1465
13. Girdwood SCT, Sellas MN, Courter JD, et al. Improving the transition of intravenous to enteral antibiotics in pediatric patients with pneumonia or skin and soft tissue infections. J Hosp Med. 2020;15(1):10-15. https://doi.org/10.12788/jhm.3253
14. Core Elements of Hospital Antibiotic Stewardship Programs. Centers for Disease Control and Prevention. Published 2019. Accessed May 30, 2020. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html
15. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. https://doi.org/10.1093/cid/ciw118
16. Science M, Timberlake K, Morris A, Read S, Le Saux N; Groupe Antibiothérapie en Pédiatrie Canada Alliance for Stewardship of Antimicrobials in Pediatrics (GAP Can ASAP). Quality metrics for antimicrobial stewardship programs. Pediatrics. 2019;143(4):e20182372. https://doi.org/10.1542/peds.2018-2372
17. Tchou MJ, Hall M, Shah SS, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Patterns of electrolyte testing at children’s hospitals for common inpatient diagnoses. Pediatrics. 2019;144(1):e20181644. https://doi.org/10.1542/peds.2018-1644
18. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
19. Desai S, Shah SS, Hall M, Richardson TE, Thomson JE; Pediatric Research in Inpatient Settings (PRIS) Network. Imaging strategies and outcomes in children hospitalized with cervical lymphadenitis. J Hosp Med. 2020;15(4):197-203. https://doi.org/10.12788/jhm.3333
20. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
21. Tieder JS, Hall M, Auger KA, et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics. 2011;128(2):323-330. https://doi.org/10.1542/peds.2010-2064
22. Singh JA, Yu S. The burden of septic arthritis on the U.S. inpatient care: a national study. PLoS One. 2017;12(8):e0182577. https://doi.org/10.1371/journal.pone.0182577
23. Foradori DM, Lopez MA, Hall M, et al. Invasive bacterial infections in infants younger than 60 days with skin and soft tissue infections. Pediatr Emerg Care. 2018. https://doi.org/10.1097/pec.0000000000001584
24. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
25. Arancibia A, Icarte A, González C, Morasso I. Dose-dependent bioavailability of amoxycillin. Int J Clin Pharmacol Ther Toxicol. 1988;26(6):300-303.
26. Grayson ML, Cosgrove S, Crowe S, et al. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs. 7th ed. CRC Press; 2018.
27. Downes KJ, Hahn A, Wiles J, Courter JD, Inks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in pediatrics’. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006
28. Gras-Le Guen C, Boscher C, Godon N, et al. Therapeutic amoxicillin levels achieved with oral administration in term neonates. Eur J Clin Pharmacol. 2007;63(7):657-662. https://doi.org/10.1007/s00228-007-0307-3
29. Sanchez Navarro A. New formulations of amoxicillin/clavulanic acid: a pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2005;44(11):1097-1115. https://doi.org/10.2165/00003088-200544110-00001
30. Fine MJ, Hough LJ, Medsger AR, et al. The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157(1):36-44. https://doi.org/10.1001/archinte.1997.00440220040006
31. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007(4):CD003237. https://doi.org/10.1002/14651858.cd003237.pub2
32. Nageswaran S, Woods CR, Benjamin DK Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25(8):695-699. https://doi.org/10.1097/01.inf.0000227820.36036.f1
33. Al-Nammari S, Roberton B, Ferguson C. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Should a child with preseptal periorbital cellulitis be treated with intravenous or oral antibiotics? Emerg Med J. 2007;24(2):128-129. https://doi.org/10.1136/emj.2006.045245
34. Vieira F, Allen SM, Stocks RMS, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483, vii. https://doi.org/10.1016/j.otc.2008.01.002
35. Smith M, Shah S, Kronman M, Patel S, Thurm C, Hersh AL. Route of administration for highly orally bioavailable antibiotics. Open Forum Infect Dis. 2017;4(Suppl 1):S498-S499. https://doi.org/10.1093/ofid/ofx163.1291
36. Brady PW, Brinkman WB, Simmons JM, et al. Oral antibiotics at discharge for children with acute osteomyelitis: a rapid cycle improvement project. BMJ Qual Saf. 2014;23(6):499-507. https://doi.org/10.1136/bmjqs-2013-002179
37. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3
38. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585
39. Public Health Ontario. Antimicrobial stewardship programs metric examples. Published 2017. Accessed June 1, 2020. https://www.publichealthontario.ca/-/media/documents/A/2017/asp-metrics-examples.pdf?la=en
40. Desai S, Aronson PL, Shabanova V, et al; Febrile Young Infant Research Collaborative. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Therapeutic Monitoring of Vancomycin
Vancomycin, a glycopeptide antibiotic, has been used for decades, yet knowledge gaps remain regarding the most appropriate dosing approach to optimize therapeutic effect while avoiding adverse effects in all patient populations. A committee composed of members of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists reviewed data available since publication of the original 2009 vancomycin dosing guidelines to provide new recommendations regarding vancomycin dosing and serum concentration–monitoring in the empiric treatment of presumed or confirmed methicillin-resistant Staphylococcus aureus (MRSA) infections.1
The new guidelines provide 25 recommendations encompassing the following topics: vancomycin dosing and monitoring in adult, pediatric, and neonate care; vancomycin minimum inhibitory concentration (MIC) susceptibility testing; continuous infusion vs intermittent infusion; loading doses; dosing in obesity; and dosing in patients on hemodialysis and continuous renal replacement therapy. Because hospitalists in pediatric and adult care frequently prescribe vancomycin for empiric and targeted treatment of serious infections, they have a vested interest in ensuring optimal vancomycin outcomes (ie, best efficacy with least toxicity) with use of therapeutic drug monitoring and personalized dosing of vancomycin. Thus, it is important for hospitalists to be aware of the updated guideline and pivotal changes regarding therapeutic drug monitoring. In this guideline review, we will focus on the major differences from the 2009 guideline, specifically regarding therapeutic monitoring in adults and children.
The guideline includes pharmacology language and terminology with which many clinicians may not be familiar. To better understand the rationale for the guideline changes, a few concepts will be reviewed. Overall, antibiotics are dosed based on preclinical studies to determine the needed drug exposure for optimal efficacy. β-Lactams, for example, are optimally dosed with longer drug exposure time above the MIC of the infectious organism. Alternatively, area under the concentration time curve (AUC) describes the efficacy and toxicity of many other antibiotics. Since AUC is derived from products of concentration (mg/L) and time (hours), the units are often mg × h/L. For vancomycin, both drug exposure (ie, AUC) and organism susceptibility (ie, MIC) are incorporated to determine optimal drug exposure, with the ratio of AUC to MIC being the ideal marker. Therapeutic drug monitoring of vancomycin has classically been conducted with trough concentration monitoring, but with the updated guideline, there will be a transition to AUC monitoring that will affect patient care and experience.
KEY RECOMMENDATIONS FOR HOSPITALISTS TREATING ADULTS
The following is a summary of recommendations 1 to 6:
- In adults, the optimal drug exposure for vancomycin should be an AUC to MIC ratio of 400 to 600 for MRSA, with the assumption of MIC to be 1 mg/L (evidence quality: A-II).
- The preferred method to monitor AUC is with a clinical statistical software that uses two blood samples (1 to 2 hours after completion of infusion and at the end of a dosing interval [ie, trough]) (evidence quality: A-II).
- An alternative approach would be to use first-order pharmacokinetic equations at steady state with a peak and trough (evidence quality: A-II).
- These approaches replace the previously recommended trough-only monitoring. AUC-targeted exposure should generally be achieved within 48 hours; severity of infection does not justify higher AUC goals. Once the goal AUC is achieved, once-weekly monitoring is recommended for hemodynamically stable patients, but more frequent or daily monitoring is advised in patients at high risk of nephrotoxicity or who are hemodynamically unstable (evidence quality: B-II).
The currently accepted optimal drug exposure for vancomycin is an AUC to MIC ratio of 400 to 600 to maximize efficacy and minimize nephrotoxicity.2 Due to clinical inconvenience of performing AUC-based monitoring for vancomycin in the past, previous guidelines recommended using trough concentrations as a surrogate marker for an AUC to MIC ratio, with the goal trough being 15 to 20 mg/L for serious MRSA infections.3 However, trough values may not correlate well with AUC. For example, a trough of 15 mg/L may represent an AUC ranging from 400 to 1000 mg × h/L over 24 hours. Without knowing an accurate AUC, there is risk for ineffective bactericidal activity with low AUCs or nephrotoxicity with high AUCs. Compared with trough-only monitoring, AUC-guided dosing is associated with decreased risk of acute kidney injury.4,5 Therefore, the recommendation to transition to two-sample collection with a peak and trough was included.
Software programs are now readily available to compute the AUC and work best with peak and trough values rather than a single trough value because computing with two concentrations will rely more on specific patient data than it does on previously published vancomycin models. Trough-only monitoring (and without the support of clinical software) may still be possible when the exposures needed are further from the toxic range. To this end, trough-only monitoring may be reasonable when infections are not MRSA and are less invasive (eg, cellulitis) since the guideline found insufficient evidence for AUC monitoring in these scenarios. While specific targets are not provided, a plethora of historical literature demonstrated low kidney injury rates when troughs were maintained between 5 to 10 mg/L.
KEY RECOMMENDATIONS FOR PEDIATRIC HOSPITALISTS
The following is a summary of recommendations 18 to 20:
- In pediatric care, based on a target AUC to MIC ratio of 400 to 600 with the assumption of MIC to be 1 mg/L, initial vancomycin dosage for MRSA is as follows (evidence quality: A-II) :
- 60 to 80 mg/kg per day, divided into four doses, each given 6 hours apart, for children 3 months and older but younger than 12 years
- 60 to 70 mg/kg per day, divided into four doses, each given 6 hours apart, for children 12 years and older
- As recommended in adults, use of a statistical software program to measure AUC is the optimal approach in pediatric care because it can account for age, weight, and renal function, which should be monitored closely. Monitoring should begin within 48 hours of therapy. Vancomycin AUC and trough concentrations should be less than 800 µg × h/mL over 24 hours and 15 µg/mL, respectively, to minimize acute kidney injury (evidence quality: A-II).
All the recommendations for pediatrics are new for the updated guideline. Pediatric data to support these recommendations are fewer in comparison with adult literature. Given MRSA infections are felt to be similar in adults and children, many pediatric recommendations are extrapolated from adult data and recommendations. The strongest level of evidence in children is the association of acute kidney injury with higher vancomycin exposure, especially with troughs exceeding 15 to 20 mg/L.6 In addition, one pediatric study found an AUC exposure of greater than 800 mg × h/L over 24 hours was strongly associated with risk for acute kidney injury.7 These findings suggest that high vancomycin exposure correlates with nephrotoxicity, so with AUC monitoring, the goal exposure should be less than 800 mg × hr/L over 24 hours.
Only one study has evaluated statistical software and prediction of AUC in pediatrics.8 A two-concentration approach (peak and trough) outperformed trough-only monitoring for accuracy and precision in determining AUC. While limited to one study, the results are similar to the studies completed in adults, thereby leading to the recommendation of the two-concentration technique in children.
Prospective outcome data are lacking, but multiple retrospective studies have examined S aureus bacteremia in children. Thus far, there have been no studies that have determined the optimal vancomycin exposure required for successful outcomes.9,10 The proven risks of toxicity are the primary driver for the pediatric guideline change with the outcomes extrapolated from adult data.
CRITIQUE
Methods in Preparing Guideline
The main strength of the guideline is that the committee was represented by multiple organizations, which created a multidisciplinary panel of pharmacists and infectious disease physicians with clinical and research expertise in vancomycin dosing. Evidence was graded using an adaptation from the Canadian Task Force on the Periodic Health Examination.11 The draft was peer-reviewed by the society organizations and allowed for comments, suggestions, and recommendations.
Sources of Potential Conflict of Interest or Bias
Disclosures of all authors were reported and identified in the guideline. While many members are involved with pharmaceutical companies through research or speakers’ roles, vancomycin, a generic drug, should have minimal conflicts of interest or bias from this involvement.
Generalizability
Implementation of vancomycin AUC dosing will be hospital dependent due to the implementation-related increase in human resources and the cost of clinical software; many hospital systems do not already have the software integrated into their clinical practice. Local guidelines will have to be developed to help clinicians determine which clinical situations require AUC-based dosing vs trough-only monitoring. Pharmacists at many hospitals are primarily responsible for vancomycin monitoring and provide dosing recommendations to physicians. Depending on a hospital system’s decision, the workload to determine the optimal vancomycin dose may increase, and it will be important to have close collaboration between hospitalists, pharmacists, and infectious diseases clinicians to appropriately educate clinicians who might be required to dose/monitor vancomycin. One potential way to decrease the burden of monitoring with two concentrations is to use specialized software that can perform complex assessments with only a single concentration. These software applications will still require serious collaboration of the aforementioned practitioners to implement. The variation in guideline adoption will likely be even more significant in pediatrics because the literature is extrapolated and the increased blood draws can be more problematic in pediatric patients.
Furthermore, clinicians should understand the dosing guideline is specifically addressing treatment of MRSA infections and extrapolation to other organisms such as coagulase-negative staphylococcal or methicillin-susceptible S aureus infections should be cautioned. Another caveat to note is that, when the MRSA isolate has an MIC of 2 mg/L or higher, these infections are associated with poor outcomes when vancomycin is used and alternative agents are recommended.
AREAS IN NEED OF FUTURE STUDY
Research gaps still remain with appropriate vancomycin drug exposure. In pediatrics, determining the appropriate AUC target will be important given that current recommendations extrapolate from adult data. Future studies can focus on prospective outcome data in both pediatric and adult patients for infections outside of bacteremia or pneumonia, notably central nervous system and osteomyelitis infections. Thresholds for kidney injury will need to be more clearly defined for both adult and pediatric patients. There should also be research emphasis on the appropriate dosing for other non-MRSA invasive infections, notably coagulase-negative staphylococcal infections.
Disclosures
Dr Scheetz reported personal fees for consulting for Achaogen, SIGA technologies, and for serving on an advisory board for Paratek; grants from Merck and Co, Allecra, Nevakar, and SuperTrans Medical; personal fees from Hall, Booth, Smith, PC, and Chambless, Higdon, Richardson, Katz & Griggs, LLP, for consulting and expert testimony, outside the submitted work. In addition, Dr. Scheetz has patent US 2019 / 0099500 A1 pending. Dr Murphy reported having received fees from Becton Dickinson for participation to review IDSA guidelines on gastroenteritis. Dr Tang Girdwood has nothing to disclose.
Funding
Dr Murphy and Dr Tang Girdwood are supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-09). Dr Tang Girdwood is also supported by the Cincinnati Children’s Hospital Medical Center Arnold W Strauss Fellow Award and Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award. Dr Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award (R21AI149026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. https://doi.org/10.1093/ajhp/zxaa036
2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. https://doi.org/10.1371/journal.pone.0146224
3. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. https://doi.org/10.2146/ajhp080434
4. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. https://doi.org/10.1128/aac.01293-17
5. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. https://doi.org/10.1128/aac.02042-17
6. Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(7):654-661. https://doi.org/10.1097/inf.0000000000001882
7. Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-e116. https://doi.org/10.1093/jpids/piu110
8. Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Ther Drug Monit. 2014;36(4):510-518. https://doi.org/10.1097/ftd.0000000000000039
9. Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619-625. https://doi.org/10.1097/ftd.0000000000000190
10. McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263-268. https://doi.org/10.1097/inf.0000000000000991
11. The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121(9):1193-1254.
Vancomycin, a glycopeptide antibiotic, has been used for decades, yet knowledge gaps remain regarding the most appropriate dosing approach to optimize therapeutic effect while avoiding adverse effects in all patient populations. A committee composed of members of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists reviewed data available since publication of the original 2009 vancomycin dosing guidelines to provide new recommendations regarding vancomycin dosing and serum concentration–monitoring in the empiric treatment of presumed or confirmed methicillin-resistant Staphylococcus aureus (MRSA) infections.1
The new guidelines provide 25 recommendations encompassing the following topics: vancomycin dosing and monitoring in adult, pediatric, and neonate care; vancomycin minimum inhibitory concentration (MIC) susceptibility testing; continuous infusion vs intermittent infusion; loading doses; dosing in obesity; and dosing in patients on hemodialysis and continuous renal replacement therapy. Because hospitalists in pediatric and adult care frequently prescribe vancomycin for empiric and targeted treatment of serious infections, they have a vested interest in ensuring optimal vancomycin outcomes (ie, best efficacy with least toxicity) with use of therapeutic drug monitoring and personalized dosing of vancomycin. Thus, it is important for hospitalists to be aware of the updated guideline and pivotal changes regarding therapeutic drug monitoring. In this guideline review, we will focus on the major differences from the 2009 guideline, specifically regarding therapeutic monitoring in adults and children.
The guideline includes pharmacology language and terminology with which many clinicians may not be familiar. To better understand the rationale for the guideline changes, a few concepts will be reviewed. Overall, antibiotics are dosed based on preclinical studies to determine the needed drug exposure for optimal efficacy. β-Lactams, for example, are optimally dosed with longer drug exposure time above the MIC of the infectious organism. Alternatively, area under the concentration time curve (AUC) describes the efficacy and toxicity of many other antibiotics. Since AUC is derived from products of concentration (mg/L) and time (hours), the units are often mg × h/L. For vancomycin, both drug exposure (ie, AUC) and organism susceptibility (ie, MIC) are incorporated to determine optimal drug exposure, with the ratio of AUC to MIC being the ideal marker. Therapeutic drug monitoring of vancomycin has classically been conducted with trough concentration monitoring, but with the updated guideline, there will be a transition to AUC monitoring that will affect patient care and experience.
KEY RECOMMENDATIONS FOR HOSPITALISTS TREATING ADULTS
The following is a summary of recommendations 1 to 6:
- In adults, the optimal drug exposure for vancomycin should be an AUC to MIC ratio of 400 to 600 for MRSA, with the assumption of MIC to be 1 mg/L (evidence quality: A-II).
- The preferred method to monitor AUC is with a clinical statistical software that uses two blood samples (1 to 2 hours after completion of infusion and at the end of a dosing interval [ie, trough]) (evidence quality: A-II).
- An alternative approach would be to use first-order pharmacokinetic equations at steady state with a peak and trough (evidence quality: A-II).
- These approaches replace the previously recommended trough-only monitoring. AUC-targeted exposure should generally be achieved within 48 hours; severity of infection does not justify higher AUC goals. Once the goal AUC is achieved, once-weekly monitoring is recommended for hemodynamically stable patients, but more frequent or daily monitoring is advised in patients at high risk of nephrotoxicity or who are hemodynamically unstable (evidence quality: B-II).
The currently accepted optimal drug exposure for vancomycin is an AUC to MIC ratio of 400 to 600 to maximize efficacy and minimize nephrotoxicity.2 Due to clinical inconvenience of performing AUC-based monitoring for vancomycin in the past, previous guidelines recommended using trough concentrations as a surrogate marker for an AUC to MIC ratio, with the goal trough being 15 to 20 mg/L for serious MRSA infections.3 However, trough values may not correlate well with AUC. For example, a trough of 15 mg/L may represent an AUC ranging from 400 to 1000 mg × h/L over 24 hours. Without knowing an accurate AUC, there is risk for ineffective bactericidal activity with low AUCs or nephrotoxicity with high AUCs. Compared with trough-only monitoring, AUC-guided dosing is associated with decreased risk of acute kidney injury.4,5 Therefore, the recommendation to transition to two-sample collection with a peak and trough was included.
Software programs are now readily available to compute the AUC and work best with peak and trough values rather than a single trough value because computing with two concentrations will rely more on specific patient data than it does on previously published vancomycin models. Trough-only monitoring (and without the support of clinical software) may still be possible when the exposures needed are further from the toxic range. To this end, trough-only monitoring may be reasonable when infections are not MRSA and are less invasive (eg, cellulitis) since the guideline found insufficient evidence for AUC monitoring in these scenarios. While specific targets are not provided, a plethora of historical literature demonstrated low kidney injury rates when troughs were maintained between 5 to 10 mg/L.
KEY RECOMMENDATIONS FOR PEDIATRIC HOSPITALISTS
The following is a summary of recommendations 18 to 20:
- In pediatric care, based on a target AUC to MIC ratio of 400 to 600 with the assumption of MIC to be 1 mg/L, initial vancomycin dosage for MRSA is as follows (evidence quality: A-II) :
- 60 to 80 mg/kg per day, divided into four doses, each given 6 hours apart, for children 3 months and older but younger than 12 years
- 60 to 70 mg/kg per day, divided into four doses, each given 6 hours apart, for children 12 years and older
- As recommended in adults, use of a statistical software program to measure AUC is the optimal approach in pediatric care because it can account for age, weight, and renal function, which should be monitored closely. Monitoring should begin within 48 hours of therapy. Vancomycin AUC and trough concentrations should be less than 800 µg × h/mL over 24 hours and 15 µg/mL, respectively, to minimize acute kidney injury (evidence quality: A-II).
All the recommendations for pediatrics are new for the updated guideline. Pediatric data to support these recommendations are fewer in comparison with adult literature. Given MRSA infections are felt to be similar in adults and children, many pediatric recommendations are extrapolated from adult data and recommendations. The strongest level of evidence in children is the association of acute kidney injury with higher vancomycin exposure, especially with troughs exceeding 15 to 20 mg/L.6 In addition, one pediatric study found an AUC exposure of greater than 800 mg × h/L over 24 hours was strongly associated with risk for acute kidney injury.7 These findings suggest that high vancomycin exposure correlates with nephrotoxicity, so with AUC monitoring, the goal exposure should be less than 800 mg × hr/L over 24 hours.
Only one study has evaluated statistical software and prediction of AUC in pediatrics.8 A two-concentration approach (peak and trough) outperformed trough-only monitoring for accuracy and precision in determining AUC. While limited to one study, the results are similar to the studies completed in adults, thereby leading to the recommendation of the two-concentration technique in children.
Prospective outcome data are lacking, but multiple retrospective studies have examined S aureus bacteremia in children. Thus far, there have been no studies that have determined the optimal vancomycin exposure required for successful outcomes.9,10 The proven risks of toxicity are the primary driver for the pediatric guideline change with the outcomes extrapolated from adult data.
CRITIQUE
Methods in Preparing Guideline
The main strength of the guideline is that the committee was represented by multiple organizations, which created a multidisciplinary panel of pharmacists and infectious disease physicians with clinical and research expertise in vancomycin dosing. Evidence was graded using an adaptation from the Canadian Task Force on the Periodic Health Examination.11 The draft was peer-reviewed by the society organizations and allowed for comments, suggestions, and recommendations.
Sources of Potential Conflict of Interest or Bias
Disclosures of all authors were reported and identified in the guideline. While many members are involved with pharmaceutical companies through research or speakers’ roles, vancomycin, a generic drug, should have minimal conflicts of interest or bias from this involvement.
Generalizability
Implementation of vancomycin AUC dosing will be hospital dependent due to the implementation-related increase in human resources and the cost of clinical software; many hospital systems do not already have the software integrated into their clinical practice. Local guidelines will have to be developed to help clinicians determine which clinical situations require AUC-based dosing vs trough-only monitoring. Pharmacists at many hospitals are primarily responsible for vancomycin monitoring and provide dosing recommendations to physicians. Depending on a hospital system’s decision, the workload to determine the optimal vancomycin dose may increase, and it will be important to have close collaboration between hospitalists, pharmacists, and infectious diseases clinicians to appropriately educate clinicians who might be required to dose/monitor vancomycin. One potential way to decrease the burden of monitoring with two concentrations is to use specialized software that can perform complex assessments with only a single concentration. These software applications will still require serious collaboration of the aforementioned practitioners to implement. The variation in guideline adoption will likely be even more significant in pediatrics because the literature is extrapolated and the increased blood draws can be more problematic in pediatric patients.
Furthermore, clinicians should understand the dosing guideline is specifically addressing treatment of MRSA infections and extrapolation to other organisms such as coagulase-negative staphylococcal or methicillin-susceptible S aureus infections should be cautioned. Another caveat to note is that, when the MRSA isolate has an MIC of 2 mg/L or higher, these infections are associated with poor outcomes when vancomycin is used and alternative agents are recommended.
AREAS IN NEED OF FUTURE STUDY
Research gaps still remain with appropriate vancomycin drug exposure. In pediatrics, determining the appropriate AUC target will be important given that current recommendations extrapolate from adult data. Future studies can focus on prospective outcome data in both pediatric and adult patients for infections outside of bacteremia or pneumonia, notably central nervous system and osteomyelitis infections. Thresholds for kidney injury will need to be more clearly defined for both adult and pediatric patients. There should also be research emphasis on the appropriate dosing for other non-MRSA invasive infections, notably coagulase-negative staphylococcal infections.
Disclosures
Dr Scheetz reported personal fees for consulting for Achaogen, SIGA technologies, and for serving on an advisory board for Paratek; grants from Merck and Co, Allecra, Nevakar, and SuperTrans Medical; personal fees from Hall, Booth, Smith, PC, and Chambless, Higdon, Richardson, Katz & Griggs, LLP, for consulting and expert testimony, outside the submitted work. In addition, Dr. Scheetz has patent US 2019 / 0099500 A1 pending. Dr Murphy reported having received fees from Becton Dickinson for participation to review IDSA guidelines on gastroenteritis. Dr Tang Girdwood has nothing to disclose.
Funding
Dr Murphy and Dr Tang Girdwood are supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-09). Dr Tang Girdwood is also supported by the Cincinnati Children’s Hospital Medical Center Arnold W Strauss Fellow Award and Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award. Dr Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award (R21AI149026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Vancomycin, a glycopeptide antibiotic, has been used for decades, yet knowledge gaps remain regarding the most appropriate dosing approach to optimize therapeutic effect while avoiding adverse effects in all patient populations. A committee composed of members of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists reviewed data available since publication of the original 2009 vancomycin dosing guidelines to provide new recommendations regarding vancomycin dosing and serum concentration–monitoring in the empiric treatment of presumed or confirmed methicillin-resistant Staphylococcus aureus (MRSA) infections.1
The new guidelines provide 25 recommendations encompassing the following topics: vancomycin dosing and monitoring in adult, pediatric, and neonate care; vancomycin minimum inhibitory concentration (MIC) susceptibility testing; continuous infusion vs intermittent infusion; loading doses; dosing in obesity; and dosing in patients on hemodialysis and continuous renal replacement therapy. Because hospitalists in pediatric and adult care frequently prescribe vancomycin for empiric and targeted treatment of serious infections, they have a vested interest in ensuring optimal vancomycin outcomes (ie, best efficacy with least toxicity) with use of therapeutic drug monitoring and personalized dosing of vancomycin. Thus, it is important for hospitalists to be aware of the updated guideline and pivotal changes regarding therapeutic drug monitoring. In this guideline review, we will focus on the major differences from the 2009 guideline, specifically regarding therapeutic monitoring in adults and children.
The guideline includes pharmacology language and terminology with which many clinicians may not be familiar. To better understand the rationale for the guideline changes, a few concepts will be reviewed. Overall, antibiotics are dosed based on preclinical studies to determine the needed drug exposure for optimal efficacy. β-Lactams, for example, are optimally dosed with longer drug exposure time above the MIC of the infectious organism. Alternatively, area under the concentration time curve (AUC) describes the efficacy and toxicity of many other antibiotics. Since AUC is derived from products of concentration (mg/L) and time (hours), the units are often mg × h/L. For vancomycin, both drug exposure (ie, AUC) and organism susceptibility (ie, MIC) are incorporated to determine optimal drug exposure, with the ratio of AUC to MIC being the ideal marker. Therapeutic drug monitoring of vancomycin has classically been conducted with trough concentration monitoring, but with the updated guideline, there will be a transition to AUC monitoring that will affect patient care and experience.
KEY RECOMMENDATIONS FOR HOSPITALISTS TREATING ADULTS
The following is a summary of recommendations 1 to 6:
- In adults, the optimal drug exposure for vancomycin should be an AUC to MIC ratio of 400 to 600 for MRSA, with the assumption of MIC to be 1 mg/L (evidence quality: A-II).
- The preferred method to monitor AUC is with a clinical statistical software that uses two blood samples (1 to 2 hours after completion of infusion and at the end of a dosing interval [ie, trough]) (evidence quality: A-II).
- An alternative approach would be to use first-order pharmacokinetic equations at steady state with a peak and trough (evidence quality: A-II).
- These approaches replace the previously recommended trough-only monitoring. AUC-targeted exposure should generally be achieved within 48 hours; severity of infection does not justify higher AUC goals. Once the goal AUC is achieved, once-weekly monitoring is recommended for hemodynamically stable patients, but more frequent or daily monitoring is advised in patients at high risk of nephrotoxicity or who are hemodynamically unstable (evidence quality: B-II).
The currently accepted optimal drug exposure for vancomycin is an AUC to MIC ratio of 400 to 600 to maximize efficacy and minimize nephrotoxicity.2 Due to clinical inconvenience of performing AUC-based monitoring for vancomycin in the past, previous guidelines recommended using trough concentrations as a surrogate marker for an AUC to MIC ratio, with the goal trough being 15 to 20 mg/L for serious MRSA infections.3 However, trough values may not correlate well with AUC. For example, a trough of 15 mg/L may represent an AUC ranging from 400 to 1000 mg × h/L over 24 hours. Without knowing an accurate AUC, there is risk for ineffective bactericidal activity with low AUCs or nephrotoxicity with high AUCs. Compared with trough-only monitoring, AUC-guided dosing is associated with decreased risk of acute kidney injury.4,5 Therefore, the recommendation to transition to two-sample collection with a peak and trough was included.
Software programs are now readily available to compute the AUC and work best with peak and trough values rather than a single trough value because computing with two concentrations will rely more on specific patient data than it does on previously published vancomycin models. Trough-only monitoring (and without the support of clinical software) may still be possible when the exposures needed are further from the toxic range. To this end, trough-only monitoring may be reasonable when infections are not MRSA and are less invasive (eg, cellulitis) since the guideline found insufficient evidence for AUC monitoring in these scenarios. While specific targets are not provided, a plethora of historical literature demonstrated low kidney injury rates when troughs were maintained between 5 to 10 mg/L.
KEY RECOMMENDATIONS FOR PEDIATRIC HOSPITALISTS
The following is a summary of recommendations 18 to 20:
- In pediatric care, based on a target AUC to MIC ratio of 400 to 600 with the assumption of MIC to be 1 mg/L, initial vancomycin dosage for MRSA is as follows (evidence quality: A-II) :
- 60 to 80 mg/kg per day, divided into four doses, each given 6 hours apart, for children 3 months and older but younger than 12 years
- 60 to 70 mg/kg per day, divided into four doses, each given 6 hours apart, for children 12 years and older
- As recommended in adults, use of a statistical software program to measure AUC is the optimal approach in pediatric care because it can account for age, weight, and renal function, which should be monitored closely. Monitoring should begin within 48 hours of therapy. Vancomycin AUC and trough concentrations should be less than 800 µg × h/mL over 24 hours and 15 µg/mL, respectively, to minimize acute kidney injury (evidence quality: A-II).
All the recommendations for pediatrics are new for the updated guideline. Pediatric data to support these recommendations are fewer in comparison with adult literature. Given MRSA infections are felt to be similar in adults and children, many pediatric recommendations are extrapolated from adult data and recommendations. The strongest level of evidence in children is the association of acute kidney injury with higher vancomycin exposure, especially with troughs exceeding 15 to 20 mg/L.6 In addition, one pediatric study found an AUC exposure of greater than 800 mg × h/L over 24 hours was strongly associated with risk for acute kidney injury.7 These findings suggest that high vancomycin exposure correlates with nephrotoxicity, so with AUC monitoring, the goal exposure should be less than 800 mg × hr/L over 24 hours.
Only one study has evaluated statistical software and prediction of AUC in pediatrics.8 A two-concentration approach (peak and trough) outperformed trough-only monitoring for accuracy and precision in determining AUC. While limited to one study, the results are similar to the studies completed in adults, thereby leading to the recommendation of the two-concentration technique in children.
Prospective outcome data are lacking, but multiple retrospective studies have examined S aureus bacteremia in children. Thus far, there have been no studies that have determined the optimal vancomycin exposure required for successful outcomes.9,10 The proven risks of toxicity are the primary driver for the pediatric guideline change with the outcomes extrapolated from adult data.
CRITIQUE
Methods in Preparing Guideline
The main strength of the guideline is that the committee was represented by multiple organizations, which created a multidisciplinary panel of pharmacists and infectious disease physicians with clinical and research expertise in vancomycin dosing. Evidence was graded using an adaptation from the Canadian Task Force on the Periodic Health Examination.11 The draft was peer-reviewed by the society organizations and allowed for comments, suggestions, and recommendations.
Sources of Potential Conflict of Interest or Bias
Disclosures of all authors were reported and identified in the guideline. While many members are involved with pharmaceutical companies through research or speakers’ roles, vancomycin, a generic drug, should have minimal conflicts of interest or bias from this involvement.
Generalizability
Implementation of vancomycin AUC dosing will be hospital dependent due to the implementation-related increase in human resources and the cost of clinical software; many hospital systems do not already have the software integrated into their clinical practice. Local guidelines will have to be developed to help clinicians determine which clinical situations require AUC-based dosing vs trough-only monitoring. Pharmacists at many hospitals are primarily responsible for vancomycin monitoring and provide dosing recommendations to physicians. Depending on a hospital system’s decision, the workload to determine the optimal vancomycin dose may increase, and it will be important to have close collaboration between hospitalists, pharmacists, and infectious diseases clinicians to appropriately educate clinicians who might be required to dose/monitor vancomycin. One potential way to decrease the burden of monitoring with two concentrations is to use specialized software that can perform complex assessments with only a single concentration. These software applications will still require serious collaboration of the aforementioned practitioners to implement. The variation in guideline adoption will likely be even more significant in pediatrics because the literature is extrapolated and the increased blood draws can be more problematic in pediatric patients.
Furthermore, clinicians should understand the dosing guideline is specifically addressing treatment of MRSA infections and extrapolation to other organisms such as coagulase-negative staphylococcal or methicillin-susceptible S aureus infections should be cautioned. Another caveat to note is that, when the MRSA isolate has an MIC of 2 mg/L or higher, these infections are associated with poor outcomes when vancomycin is used and alternative agents are recommended.
AREAS IN NEED OF FUTURE STUDY
Research gaps still remain with appropriate vancomycin drug exposure. In pediatrics, determining the appropriate AUC target will be important given that current recommendations extrapolate from adult data. Future studies can focus on prospective outcome data in both pediatric and adult patients for infections outside of bacteremia or pneumonia, notably central nervous system and osteomyelitis infections. Thresholds for kidney injury will need to be more clearly defined for both adult and pediatric patients. There should also be research emphasis on the appropriate dosing for other non-MRSA invasive infections, notably coagulase-negative staphylococcal infections.
Disclosures
Dr Scheetz reported personal fees for consulting for Achaogen, SIGA technologies, and for serving on an advisory board for Paratek; grants from Merck and Co, Allecra, Nevakar, and SuperTrans Medical; personal fees from Hall, Booth, Smith, PC, and Chambless, Higdon, Richardson, Katz & Griggs, LLP, for consulting and expert testimony, outside the submitted work. In addition, Dr. Scheetz has patent US 2019 / 0099500 A1 pending. Dr Murphy reported having received fees from Becton Dickinson for participation to review IDSA guidelines on gastroenteritis. Dr Tang Girdwood has nothing to disclose.
Funding
Dr Murphy and Dr Tang Girdwood are supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-09). Dr Tang Girdwood is also supported by the Cincinnati Children’s Hospital Medical Center Arnold W Strauss Fellow Award and Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award. Dr Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award (R21AI149026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. https://doi.org/10.1093/ajhp/zxaa036
2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. https://doi.org/10.1371/journal.pone.0146224
3. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. https://doi.org/10.2146/ajhp080434
4. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. https://doi.org/10.1128/aac.01293-17
5. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. https://doi.org/10.1128/aac.02042-17
6. Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(7):654-661. https://doi.org/10.1097/inf.0000000000001882
7. Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-e116. https://doi.org/10.1093/jpids/piu110
8. Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Ther Drug Monit. 2014;36(4):510-518. https://doi.org/10.1097/ftd.0000000000000039
9. Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619-625. https://doi.org/10.1097/ftd.0000000000000190
10. McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263-268. https://doi.org/10.1097/inf.0000000000000991
11. The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121(9):1193-1254.
1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. https://doi.org/10.1093/ajhp/zxaa036
2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. https://doi.org/10.1371/journal.pone.0146224
3. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. https://doi.org/10.2146/ajhp080434
4. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. https://doi.org/10.1128/aac.01293-17
5. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. https://doi.org/10.1128/aac.02042-17
6. Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(7):654-661. https://doi.org/10.1097/inf.0000000000001882
7. Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-e116. https://doi.org/10.1093/jpids/piu110
8. Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Ther Drug Monit. 2014;36(4):510-518. https://doi.org/10.1097/ftd.0000000000000039
9. Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619-625. https://doi.org/10.1097/ftd.0000000000000190
10. McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263-268. https://doi.org/10.1097/inf.0000000000000991
11. The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121(9):1193-1254.
© 2020 Society of Hospital Medicine
Methodologic Progress Note: Opportunistic Sampling for Pharmacology Studies in Hospitalized Children
Challenges in conducting and completing studies of drugs in vulnerable populations, such as hospitalized children, include weak study designs and lack of sufficient sample sizes to achieve adequate power.1 Limitations in the amount of blood that can be safely drawn in children and low parental consent rates due to concerns for anemia or pain, if venipuncture is required, lead to an insufficient number of patients enrolled in traditional clinical studies.2 Thus, sample size targets are often not met. Recognizing the limited pediatric data for many drugs routinely prescribed off-label in children, the Food and Drug Administration implemented the Best Pharmaceuticals Children Act and the Pediatric Research Equity Act (PREA) in 2003; these legislative acts require clinical studies to assess the safety and efficacy of new drugs in children.1 While studies conducted under these acts have provided important information for the clinical care of children, only one-third of mandatory pediatric postmarketing studies of the 114 new drugs and new indications subject to PREA requirements between 2007 and 2014 had been completed within seven years.3
Despite the challenges in conducting studies of drugs in children, robust pediatric data must be generated in children, especially in those with medical complexity or with chronic medical diseases and who have significant risk of experiencing adverse drug events. Data in adults cannot simply be extrapolated to children. In addition, studies from healthy children may not apply to hospitalized pediatric patients because of significant physiologic changes that occur in children who are ill enough to be hospitalized. Opportunistic sampling can provide robust drug disposition data and overcome some of the challenges encountered by traditional drug studies. In this Methodologic Progress Note, we describe the utility of opportunistic sampling as a research tool for hospitalists, in partnership with clinical pharmacologists, to study drug pharmacokinetics (PK) in hospitalized children.
OPPORTUNISTIC SAMPLING DEFINED
Opportunistic sampling relies on the use of blood samples that are ordered for clinical purposes, and its use is endorsed by the Pediatric Trials Network.2,5 Opportunistic sampling approaches have two types: sparse sampling and scavenged or remnant sampling. In sparse opportunistic sampling, additional blood is obtained at the same time clinical samples are ordered, avoiding the need for additional punctures.5 This approach requires bedside personnel to obtain additional blood that is sent to the research team for further processing. Scavenged sampling relies on leftover residual blood from clinical samples.2,5 After the clinical laboratory performs the laboratory test ordered by the clinical team, the research team can scavenge residual blood for measurement of drug concentrations. When drug concentrations are measured from multiple patients, clinical pharmacologists can perform population PK modeling to characterize the pharmacokinetics of the drug and its variability within the population level.
The Figure shows how scavenged samples from hypothetical patients could be used to generate a PK curve. In this example, three patients are admitted for osteomyelitis and treated with the same antibiotic administered every eight hours, as shown by the theoretical concentration versus time profiles in the top panel. To determine the effectiveness of treatment and timing of transition to enteral antibiotics, the clinical team orders C-reactive protein (CRP) approximately every 48 hours for each patient. Each patient has his/her first dose of antibiotic at a different time of day depending on the time of admission or surgical drainage. Therefore, the timing of the blood draw with respect to the most recent antibiotic dose varies between patients even if blood draws are ordered at the same time for all patients (ie, 4
Opportunistic sampling has advantages over traditional intensive PK studies that often require multiple blood draws (typically >8-10) within one dosing interval to adequately describe the phases of absorption, distribution, metabolism, and elimination. The number of vascular punctures can be painful if blood cannot be drawn from existing vascular access, and the large amount of blood (sometimes >1 mL/sample) required for these studies can be impractical, burdensome, and even dangerous in young children and neonates. Scavenged sampling reduces the risk of anemia because no additional blood is drawn beyond what is obtained for clinical purposes, and it does not disrupt nursing workflow or add to nursing workload. Approval to use scavenged blood requires approval from the institutional review board. At some institutions, the consent to treat form on admission may address the use of scavenged samples and therefore allow for waiver of consent. In addition, the consent process may occur retroactively after samples are collected. These methods lead to increased enrollment.
Limitations in this approach are that drugs may degrade over time in whole blood or processed samples. Therefore, the process by which the clinical laboratory stores the residual blood after clinical tests must be understood, and the stability of the drug or metabolite of interest in blood or plasma over time must be ensured. In addition, residual blood may not be present after a clinical test, and recording times of the drug administration and lab draws may be inaccurate.2,5
APPLICATIONS OF OPPORTUNISTIC SAMPLING IN CLINICAL PHARMACOLOGY RESEARCH
/section>Opportunistic sampling has been successfully used to study a variety of drugs in different pediatric populations but has been primarily used in neonates. The multicenter Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care trial has utilized this approach to evaluate the PK of over 30 drugs.5 Several antimicrobials have been studied through opportunistic sampling, including those frequently used in pediatric hospital medicine, such as ampicillin6 and clindamycin.7
This sampling approach may be most beneficial in studying select patients. Obese patients, who are often excluded in pediatric drug trials, have been previously included in opportunistic drug studies.8 The utility of opportunistic sampling to study antimicrobials, morphine and cardiac drugs has been demonstrated in neonates, both preterm and term, in whom additional blood draws can be challenging because of low total blood volume and limited vascular access.6,7,9-12
Although the frequency of blood draws from patients admitted to pediatric hospital medicine services is generally lower than that for patients on other subspecialty services, such as critical care, we can capitalize on the high volume of patients with common diagnoses (eg, pneumonia, skin, and soft tissue infections) who are admitted to hospital medicine. Using opportunistic sampling, we can study the PK of drugs frequently used in hospital medicine, such as antibiotics, antiepileptic drugs, steroids, and pain medications. In addition, we can measure drug concentrations to study the effects of route administration, oral versus enteric tube versus intravenous, to guide not only the dosing but also the timing of transition to enteral medications. Finally, we can study drugs that are commonly used in adult and pediatric patient populations cared for by hospitalists but who are often excluded from clinical drug trials, such as patients with medical complexity, patients with medical devices (eg, nervous system shunts and tracheostomies), patients taking concomitant medications, or patients on extracorporeal devices such as dialysis, to validate drug regimens.
CONCLUSION
Generating robust pediatric clinical pharmacology data has many inherent challenges because of the vulnerability of children. However, their vulnerability requires that medications be studied thoroughly in children to ensure their safety and effectiveness. Opportunistic sampling allows for rigorous studies to be conducted with adequate sample sizes while minimizing the risk of pain, anemia, and other adverse events related to clinical drug trials. Pediatric hospitalists should consider this approach to advance their knowledge of commonly used drugs that have not been adequately studied in hospitalized children and can expand the use of opportunistic sampling to study other aspects of disease, such as diagnostic or prognostic biomarkers.
1. Field MJ, Boat TF, eds. Safe and Effective Medicines for Children: Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, DC, USA: National Academies Press; 2012.
2. Laughon MM, Benjamin DK, Jr., Capparelli EV, et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4(5):643-652. https://doi.org/10.1586/ecp.11.43.
3. Hwang TJ, Orenstein L, Kesselheim AS, Bourgeois FT. Completion rate and reporting of mandatory pediatric postmarketing studies under the US Pediatric Research Equity Act. JAMA Pediatr. 2018;173(1):68-74. https://doi.org/10.1001/jamapediatrics.2018.3416.
4. Rieder M. Adverse drug reactions in children: pediatric pharmacy and drug safety. J Pediatr Pharmacol Ther. 2019;24(1):4-9. https://doi.org/10.5863/1551-6776-24.1.4.
5. Balevic SJ, Cohen-Wolkowiez M. Innovative study designs optimizing clinical pharmacology research in infants and children. J Clin Pharmacol. 2018;58(10):S58-S72. https://doi.org/10.1002/jcph.1053.
6. Tremoulet A, Le J, Poindexter B, et al. Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design. Antimicrob Agents Chemother. 2014;58(6):3013-3020. https://doi.org/10.1128/AAC.02374-13.
7. Gonzalez D, Melloni C, Yogev R, et al. Use of opportunistic clinical data and a population pharmacokinetic model to support dosing of clindamycin for premature infants to adolescents. Clin Pharmacol Ther. 2014;96(4):429-437. https://doi.org/10.1038/clpt.2014.134.
8. Smith MJ, Gonzalez D, Goldman JL, et al. Pharmacokinetics of clindamycin in obese and nonobese children. Antimicrob Agents Chemother. 2017;61(4). https://doi.org/10.1128/AAC.02014-16.
9. Leroux S, Turner MA, Guellec CB, et al. Pharmacokinetic studies in neonates: The utility of an opportunistic sampling design. Clin Pharmacokinet. 2015;54(12):1273-1285. https://doi.org/10.1007/s40262-015-0291-1.
10. Dallefeld SH, Atz AM, Yogev R, et al. A pharmacokinetic model for amiodarone in infants developed from an opportunistic sampling trial and published literature data. J Pharmacokinet Pharmacodyn. 2018;45(3):419-430. https://doi.org/10.1007/s10928-018-9576-y.
11. Thakkar N, Gonzalez D, Cohen-Wolkowiez M, et al. An opportunistic study evaluating pharmacokinetics of sildenafil for the treatment of pulmonary hypertension in infants. J Perinatol. 2016;36(9):744-747. https://doi.org/10.1038/jp.2016.79.
12. Euteneuer JC, Mizuno T, Fukuda T, Zhao J, Setchell KD, Vinks AA. Large variability in morphine concentrations in critically ill neonates receiving standard of care postoperative pain-management. Clin Pharmacol Ther. 2018;103:S45-S45.
Challenges in conducting and completing studies of drugs in vulnerable populations, such as hospitalized children, include weak study designs and lack of sufficient sample sizes to achieve adequate power.1 Limitations in the amount of blood that can be safely drawn in children and low parental consent rates due to concerns for anemia or pain, if venipuncture is required, lead to an insufficient number of patients enrolled in traditional clinical studies.2 Thus, sample size targets are often not met. Recognizing the limited pediatric data for many drugs routinely prescribed off-label in children, the Food and Drug Administration implemented the Best Pharmaceuticals Children Act and the Pediatric Research Equity Act (PREA) in 2003; these legislative acts require clinical studies to assess the safety and efficacy of new drugs in children.1 While studies conducted under these acts have provided important information for the clinical care of children, only one-third of mandatory pediatric postmarketing studies of the 114 new drugs and new indications subject to PREA requirements between 2007 and 2014 had been completed within seven years.3
Despite the challenges in conducting studies of drugs in children, robust pediatric data must be generated in children, especially in those with medical complexity or with chronic medical diseases and who have significant risk of experiencing adverse drug events. Data in adults cannot simply be extrapolated to children. In addition, studies from healthy children may not apply to hospitalized pediatric patients because of significant physiologic changes that occur in children who are ill enough to be hospitalized. Opportunistic sampling can provide robust drug disposition data and overcome some of the challenges encountered by traditional drug studies. In this Methodologic Progress Note, we describe the utility of opportunistic sampling as a research tool for hospitalists, in partnership with clinical pharmacologists, to study drug pharmacokinetics (PK) in hospitalized children.
OPPORTUNISTIC SAMPLING DEFINED
Opportunistic sampling relies on the use of blood samples that are ordered for clinical purposes, and its use is endorsed by the Pediatric Trials Network.2,5 Opportunistic sampling approaches have two types: sparse sampling and scavenged or remnant sampling. In sparse opportunistic sampling, additional blood is obtained at the same time clinical samples are ordered, avoiding the need for additional punctures.5 This approach requires bedside personnel to obtain additional blood that is sent to the research team for further processing. Scavenged sampling relies on leftover residual blood from clinical samples.2,5 After the clinical laboratory performs the laboratory test ordered by the clinical team, the research team can scavenge residual blood for measurement of drug concentrations. When drug concentrations are measured from multiple patients, clinical pharmacologists can perform population PK modeling to characterize the pharmacokinetics of the drug and its variability within the population level.
The Figure shows how scavenged samples from hypothetical patients could be used to generate a PK curve. In this example, three patients are admitted for osteomyelitis and treated with the same antibiotic administered every eight hours, as shown by the theoretical concentration versus time profiles in the top panel. To determine the effectiveness of treatment and timing of transition to enteral antibiotics, the clinical team orders C-reactive protein (CRP) approximately every 48 hours for each patient. Each patient has his/her first dose of antibiotic at a different time of day depending on the time of admission or surgical drainage. Therefore, the timing of the blood draw with respect to the most recent antibiotic dose varies between patients even if blood draws are ordered at the same time for all patients (ie, 4
Opportunistic sampling has advantages over traditional intensive PK studies that often require multiple blood draws (typically >8-10) within one dosing interval to adequately describe the phases of absorption, distribution, metabolism, and elimination. The number of vascular punctures can be painful if blood cannot be drawn from existing vascular access, and the large amount of blood (sometimes >1 mL/sample) required for these studies can be impractical, burdensome, and even dangerous in young children and neonates. Scavenged sampling reduces the risk of anemia because no additional blood is drawn beyond what is obtained for clinical purposes, and it does not disrupt nursing workflow or add to nursing workload. Approval to use scavenged blood requires approval from the institutional review board. At some institutions, the consent to treat form on admission may address the use of scavenged samples and therefore allow for waiver of consent. In addition, the consent process may occur retroactively after samples are collected. These methods lead to increased enrollment.
Limitations in this approach are that drugs may degrade over time in whole blood or processed samples. Therefore, the process by which the clinical laboratory stores the residual blood after clinical tests must be understood, and the stability of the drug or metabolite of interest in blood or plasma over time must be ensured. In addition, residual blood may not be present after a clinical test, and recording times of the drug administration and lab draws may be inaccurate.2,5
APPLICATIONS OF OPPORTUNISTIC SAMPLING IN CLINICAL PHARMACOLOGY RESEARCH
/section>Opportunistic sampling has been successfully used to study a variety of drugs in different pediatric populations but has been primarily used in neonates. The multicenter Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care trial has utilized this approach to evaluate the PK of over 30 drugs.5 Several antimicrobials have been studied through opportunistic sampling, including those frequently used in pediatric hospital medicine, such as ampicillin6 and clindamycin.7
This sampling approach may be most beneficial in studying select patients. Obese patients, who are often excluded in pediatric drug trials, have been previously included in opportunistic drug studies.8 The utility of opportunistic sampling to study antimicrobials, morphine and cardiac drugs has been demonstrated in neonates, both preterm and term, in whom additional blood draws can be challenging because of low total blood volume and limited vascular access.6,7,9-12
Although the frequency of blood draws from patients admitted to pediatric hospital medicine services is generally lower than that for patients on other subspecialty services, such as critical care, we can capitalize on the high volume of patients with common diagnoses (eg, pneumonia, skin, and soft tissue infections) who are admitted to hospital medicine. Using opportunistic sampling, we can study the PK of drugs frequently used in hospital medicine, such as antibiotics, antiepileptic drugs, steroids, and pain medications. In addition, we can measure drug concentrations to study the effects of route administration, oral versus enteric tube versus intravenous, to guide not only the dosing but also the timing of transition to enteral medications. Finally, we can study drugs that are commonly used in adult and pediatric patient populations cared for by hospitalists but who are often excluded from clinical drug trials, such as patients with medical complexity, patients with medical devices (eg, nervous system shunts and tracheostomies), patients taking concomitant medications, or patients on extracorporeal devices such as dialysis, to validate drug regimens.
CONCLUSION
Generating robust pediatric clinical pharmacology data has many inherent challenges because of the vulnerability of children. However, their vulnerability requires that medications be studied thoroughly in children to ensure their safety and effectiveness. Opportunistic sampling allows for rigorous studies to be conducted with adequate sample sizes while minimizing the risk of pain, anemia, and other adverse events related to clinical drug trials. Pediatric hospitalists should consider this approach to advance their knowledge of commonly used drugs that have not been adequately studied in hospitalized children and can expand the use of opportunistic sampling to study other aspects of disease, such as diagnostic or prognostic biomarkers.
Challenges in conducting and completing studies of drugs in vulnerable populations, such as hospitalized children, include weak study designs and lack of sufficient sample sizes to achieve adequate power.1 Limitations in the amount of blood that can be safely drawn in children and low parental consent rates due to concerns for anemia or pain, if venipuncture is required, lead to an insufficient number of patients enrolled in traditional clinical studies.2 Thus, sample size targets are often not met. Recognizing the limited pediatric data for many drugs routinely prescribed off-label in children, the Food and Drug Administration implemented the Best Pharmaceuticals Children Act and the Pediatric Research Equity Act (PREA) in 2003; these legislative acts require clinical studies to assess the safety and efficacy of new drugs in children.1 While studies conducted under these acts have provided important information for the clinical care of children, only one-third of mandatory pediatric postmarketing studies of the 114 new drugs and new indications subject to PREA requirements between 2007 and 2014 had been completed within seven years.3
Despite the challenges in conducting studies of drugs in children, robust pediatric data must be generated in children, especially in those with medical complexity or with chronic medical diseases and who have significant risk of experiencing adverse drug events. Data in adults cannot simply be extrapolated to children. In addition, studies from healthy children may not apply to hospitalized pediatric patients because of significant physiologic changes that occur in children who are ill enough to be hospitalized. Opportunistic sampling can provide robust drug disposition data and overcome some of the challenges encountered by traditional drug studies. In this Methodologic Progress Note, we describe the utility of opportunistic sampling as a research tool for hospitalists, in partnership with clinical pharmacologists, to study drug pharmacokinetics (PK) in hospitalized children.
OPPORTUNISTIC SAMPLING DEFINED
Opportunistic sampling relies on the use of blood samples that are ordered for clinical purposes, and its use is endorsed by the Pediatric Trials Network.2,5 Opportunistic sampling approaches have two types: sparse sampling and scavenged or remnant sampling. In sparse opportunistic sampling, additional blood is obtained at the same time clinical samples are ordered, avoiding the need for additional punctures.5 This approach requires bedside personnel to obtain additional blood that is sent to the research team for further processing. Scavenged sampling relies on leftover residual blood from clinical samples.2,5 After the clinical laboratory performs the laboratory test ordered by the clinical team, the research team can scavenge residual blood for measurement of drug concentrations. When drug concentrations are measured from multiple patients, clinical pharmacologists can perform population PK modeling to characterize the pharmacokinetics of the drug and its variability within the population level.
The Figure shows how scavenged samples from hypothetical patients could be used to generate a PK curve. In this example, three patients are admitted for osteomyelitis and treated with the same antibiotic administered every eight hours, as shown by the theoretical concentration versus time profiles in the top panel. To determine the effectiveness of treatment and timing of transition to enteral antibiotics, the clinical team orders C-reactive protein (CRP) approximately every 48 hours for each patient. Each patient has his/her first dose of antibiotic at a different time of day depending on the time of admission or surgical drainage. Therefore, the timing of the blood draw with respect to the most recent antibiotic dose varies between patients even if blood draws are ordered at the same time for all patients (ie, 4
Opportunistic sampling has advantages over traditional intensive PK studies that often require multiple blood draws (typically >8-10) within one dosing interval to adequately describe the phases of absorption, distribution, metabolism, and elimination. The number of vascular punctures can be painful if blood cannot be drawn from existing vascular access, and the large amount of blood (sometimes >1 mL/sample) required for these studies can be impractical, burdensome, and even dangerous in young children and neonates. Scavenged sampling reduces the risk of anemia because no additional blood is drawn beyond what is obtained for clinical purposes, and it does not disrupt nursing workflow or add to nursing workload. Approval to use scavenged blood requires approval from the institutional review board. At some institutions, the consent to treat form on admission may address the use of scavenged samples and therefore allow for waiver of consent. In addition, the consent process may occur retroactively after samples are collected. These methods lead to increased enrollment.
Limitations in this approach are that drugs may degrade over time in whole blood or processed samples. Therefore, the process by which the clinical laboratory stores the residual blood after clinical tests must be understood, and the stability of the drug or metabolite of interest in blood or plasma over time must be ensured. In addition, residual blood may not be present after a clinical test, and recording times of the drug administration and lab draws may be inaccurate.2,5
APPLICATIONS OF OPPORTUNISTIC SAMPLING IN CLINICAL PHARMACOLOGY RESEARCH
/section>Opportunistic sampling has been successfully used to study a variety of drugs in different pediatric populations but has been primarily used in neonates. The multicenter Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care trial has utilized this approach to evaluate the PK of over 30 drugs.5 Several antimicrobials have been studied through opportunistic sampling, including those frequently used in pediatric hospital medicine, such as ampicillin6 and clindamycin.7
This sampling approach may be most beneficial in studying select patients. Obese patients, who are often excluded in pediatric drug trials, have been previously included in opportunistic drug studies.8 The utility of opportunistic sampling to study antimicrobials, morphine and cardiac drugs has been demonstrated in neonates, both preterm and term, in whom additional blood draws can be challenging because of low total blood volume and limited vascular access.6,7,9-12
Although the frequency of blood draws from patients admitted to pediatric hospital medicine services is generally lower than that for patients on other subspecialty services, such as critical care, we can capitalize on the high volume of patients with common diagnoses (eg, pneumonia, skin, and soft tissue infections) who are admitted to hospital medicine. Using opportunistic sampling, we can study the PK of drugs frequently used in hospital medicine, such as antibiotics, antiepileptic drugs, steroids, and pain medications. In addition, we can measure drug concentrations to study the effects of route administration, oral versus enteric tube versus intravenous, to guide not only the dosing but also the timing of transition to enteral medications. Finally, we can study drugs that are commonly used in adult and pediatric patient populations cared for by hospitalists but who are often excluded from clinical drug trials, such as patients with medical complexity, patients with medical devices (eg, nervous system shunts and tracheostomies), patients taking concomitant medications, or patients on extracorporeal devices such as dialysis, to validate drug regimens.
CONCLUSION
Generating robust pediatric clinical pharmacology data has many inherent challenges because of the vulnerability of children. However, their vulnerability requires that medications be studied thoroughly in children to ensure their safety and effectiveness. Opportunistic sampling allows for rigorous studies to be conducted with adequate sample sizes while minimizing the risk of pain, anemia, and other adverse events related to clinical drug trials. Pediatric hospitalists should consider this approach to advance their knowledge of commonly used drugs that have not been adequately studied in hospitalized children and can expand the use of opportunistic sampling to study other aspects of disease, such as diagnostic or prognostic biomarkers.
1. Field MJ, Boat TF, eds. Safe and Effective Medicines for Children: Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, DC, USA: National Academies Press; 2012.
2. Laughon MM, Benjamin DK, Jr., Capparelli EV, et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4(5):643-652. https://doi.org/10.1586/ecp.11.43.
3. Hwang TJ, Orenstein L, Kesselheim AS, Bourgeois FT. Completion rate and reporting of mandatory pediatric postmarketing studies under the US Pediatric Research Equity Act. JAMA Pediatr. 2018;173(1):68-74. https://doi.org/10.1001/jamapediatrics.2018.3416.
4. Rieder M. Adverse drug reactions in children: pediatric pharmacy and drug safety. J Pediatr Pharmacol Ther. 2019;24(1):4-9. https://doi.org/10.5863/1551-6776-24.1.4.
5. Balevic SJ, Cohen-Wolkowiez M. Innovative study designs optimizing clinical pharmacology research in infants and children. J Clin Pharmacol. 2018;58(10):S58-S72. https://doi.org/10.1002/jcph.1053.
6. Tremoulet A, Le J, Poindexter B, et al. Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design. Antimicrob Agents Chemother. 2014;58(6):3013-3020. https://doi.org/10.1128/AAC.02374-13.
7. Gonzalez D, Melloni C, Yogev R, et al. Use of opportunistic clinical data and a population pharmacokinetic model to support dosing of clindamycin for premature infants to adolescents. Clin Pharmacol Ther. 2014;96(4):429-437. https://doi.org/10.1038/clpt.2014.134.
8. Smith MJ, Gonzalez D, Goldman JL, et al. Pharmacokinetics of clindamycin in obese and nonobese children. Antimicrob Agents Chemother. 2017;61(4). https://doi.org/10.1128/AAC.02014-16.
9. Leroux S, Turner MA, Guellec CB, et al. Pharmacokinetic studies in neonates: The utility of an opportunistic sampling design. Clin Pharmacokinet. 2015;54(12):1273-1285. https://doi.org/10.1007/s40262-015-0291-1.
10. Dallefeld SH, Atz AM, Yogev R, et al. A pharmacokinetic model for amiodarone in infants developed from an opportunistic sampling trial and published literature data. J Pharmacokinet Pharmacodyn. 2018;45(3):419-430. https://doi.org/10.1007/s10928-018-9576-y.
11. Thakkar N, Gonzalez D, Cohen-Wolkowiez M, et al. An opportunistic study evaluating pharmacokinetics of sildenafil for the treatment of pulmonary hypertension in infants. J Perinatol. 2016;36(9):744-747. https://doi.org/10.1038/jp.2016.79.
12. Euteneuer JC, Mizuno T, Fukuda T, Zhao J, Setchell KD, Vinks AA. Large variability in morphine concentrations in critically ill neonates receiving standard of care postoperative pain-management. Clin Pharmacol Ther. 2018;103:S45-S45.
1. Field MJ, Boat TF, eds. Safe and Effective Medicines for Children: Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, DC, USA: National Academies Press; 2012.
2. Laughon MM, Benjamin DK, Jr., Capparelli EV, et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4(5):643-652. https://doi.org/10.1586/ecp.11.43.
3. Hwang TJ, Orenstein L, Kesselheim AS, Bourgeois FT. Completion rate and reporting of mandatory pediatric postmarketing studies under the US Pediatric Research Equity Act. JAMA Pediatr. 2018;173(1):68-74. https://doi.org/10.1001/jamapediatrics.2018.3416.
4. Rieder M. Adverse drug reactions in children: pediatric pharmacy and drug safety. J Pediatr Pharmacol Ther. 2019;24(1):4-9. https://doi.org/10.5863/1551-6776-24.1.4.
5. Balevic SJ, Cohen-Wolkowiez M. Innovative study designs optimizing clinical pharmacology research in infants and children. J Clin Pharmacol. 2018;58(10):S58-S72. https://doi.org/10.1002/jcph.1053.
6. Tremoulet A, Le J, Poindexter B, et al. Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design. Antimicrob Agents Chemother. 2014;58(6):3013-3020. https://doi.org/10.1128/AAC.02374-13.
7. Gonzalez D, Melloni C, Yogev R, et al. Use of opportunistic clinical data and a population pharmacokinetic model to support dosing of clindamycin for premature infants to adolescents. Clin Pharmacol Ther. 2014;96(4):429-437. https://doi.org/10.1038/clpt.2014.134.
8. Smith MJ, Gonzalez D, Goldman JL, et al. Pharmacokinetics of clindamycin in obese and nonobese children. Antimicrob Agents Chemother. 2017;61(4). https://doi.org/10.1128/AAC.02014-16.
9. Leroux S, Turner MA, Guellec CB, et al. Pharmacokinetic studies in neonates: The utility of an opportunistic sampling design. Clin Pharmacokinet. 2015;54(12):1273-1285. https://doi.org/10.1007/s40262-015-0291-1.
10. Dallefeld SH, Atz AM, Yogev R, et al. A pharmacokinetic model for amiodarone in infants developed from an opportunistic sampling trial and published literature data. J Pharmacokinet Pharmacodyn. 2018;45(3):419-430. https://doi.org/10.1007/s10928-018-9576-y.
11. Thakkar N, Gonzalez D, Cohen-Wolkowiez M, et al. An opportunistic study evaluating pharmacokinetics of sildenafil for the treatment of pulmonary hypertension in infants. J Perinatol. 2016;36(9):744-747. https://doi.org/10.1038/jp.2016.79.
12. Euteneuer JC, Mizuno T, Fukuda T, Zhao J, Setchell KD, Vinks AA. Large variability in morphine concentrations in critically ill neonates receiving standard of care postoperative pain-management. Clin Pharmacol Ther. 2018;103:S45-S45.
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Maintenance Intravenous Fluids in Infants and Children
Hospitalized children with inadequate fluid intake are often administered maintenance intravenous fluids (IVFs) to support metabolic needs and sensible losses. Historically, hypotonic IVFs have been the standard, based on theoretical water and electrolyte requirements for estimated energy expenditure.1 However, when combined with increased levels of arginine vasopressin (AVP) seen in acutely ill children which impairs free-water excretion,2 hypotonic IVF can resu
KEY RECOMMENDATION FOR HOSPITALISTS
Patients between the ages of 28 days and 18 years should receive isotonic solutions with appropriate potassium chloride and dextrose for maintenance IVFs (evidence quality: high; recommendation strength: strong)
Isotonic fluids, such as 0.9% NaCl (normal saline), Hartmann solution and PlasmaLyte, contain a sodium concentration similar to that of plasma (135-144 mEq/L). Lactated Ringer solution (LR) is near-isotonic (sodium 130 mEq/L), but was not used in any of the reviewed studies and therefore not included in the recommendation. Excluded are patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, severe burns, or patients in the neonatal intensive care unit.
The primary benefit of the AAP recommendation is the reduced risk of iatrogenic hyponatremia and its associated sequelae, including complications or impact on cost of care. The number needed to treat with isotonic fluids was 7.5 to prevent any hyponatremia and 27.8 to prevent moderate hyponatremia (<130 mEq/L). Increases in readmission rates, length of stay, and cost of hospitalization have been reported in a recent meta-analysis reviewing the economic burden of hyponatremia in both adults and children.4
Potential harms from the use of isotonic fluids include hypernatremia, hyperchloremic metabolic acidosis, and fluid overload, although available data have not demonstrated an increased risk of these complications. In light of a recent normal saline (NS) shortage in the United States, limited availability is also a consideration. Plasmalyte is more costly than NS and is currently incompatible with the addition of dextrose.
CRITIQUE
Methods in Preparing Guideline
The guideline development committee included broad representation by pediatric experts in primary care, hospital medicine, emergency medicine, critical care medicine, nephrology, anesthesiology, surgery and quality improvement, as well as a guideline methodologist/informatician and epidemiologist.
Search strategies from recently published systematic reviews of clinical trials comparing isotonic with hypotonic maintenance IVFs were used to identify studies eligible for inclusion. A total of 17 studies with 2,455 total patients were initially identified and included. One additional study meeting inclusion criteria was found after the committee convened and excluded from the guideline.5 Three reviewers from the subcommittee performed a structured critical appraisal of each article. The methods of each trial were assessed for risk-of-bias in multiple domains, including randomization, allocation concealment, performance, detection, attrition and reporting. Forest plots were generated using random-effects models and Mantel-Haenzel statistics with the outcome of hyponatremia. The guideline underwent review by various stakeholders including AAP councils, committees, and sections, and individuals considered experts in the field.
A strength of the guideline is the high quality of the evidence and the consistent findings. All of the included studies were randomized clinical trials and the number of included patients was large. Of the 17 included studies, 16 reported a risk ratio favoring isotonic fluids over hypotonic fluids in the prevention of developing hyponatremia; the results of the study that favored hypotonic fluids were not statistically significant on their own. A sensitivity analysis was performed to exclude one study with a 20% weight, determined by multiple factors such as sample size, confidence interval, and an unusually high rate of hyponatremia in the isotonic and hypotonic fluids groups (33.3 % and 70%, respectively).6 After exclusion, there was no change in the overall estimated risk in hypotonic fluids leading to hyponatremia. Only one trial had two sources of high risk of bias (allocation concealment, attrition) and the remaining had only low or unclear risk of biases in the various domains.
The study that was excluded due to its late identification similarly shows increased risk of hyponatremia in groups administered hypotonic fluids (risk ratio 6.5-8.5), and would likely not affect the estimated risk.5
Despite differences in types of patients enrolled, rate of administered fluids, type of IVF, frequency of lab testing, and study duration, the I2 (degree of heterogeneity) of the forest plot of all included studies remained low at 14% and the increased risk of hyponatremia from hypotonic fluids remained consistent.
Due to study design differences, a limitation of the guideline is that no recommendation is made regarding the type of isotonic fluids and the rate of IVF administration. Additionally, due to the low frequency of clinically significant sequelae of hyponatremia, such as hyponatremic encephalopathy, it remains uncertain how many patients would need to be treated with isotonic fluids to prevent a rare but potentially devastating event.
Sources of Potential Conflict of Interest or Bias
The guideline was developed and funded by the AAP. A formal conflict of interest management policy was followed, and subcommittee members had no conflicts of interests or financial relationships relevant to the guideline to disclose.
Generalizability
Given the large number of patients included in the studies and heterogeneity of the population included, the recommendation applies to most patients cared for by pediatric hospitalists. Several patient exclusions relevant to the pediatric hospitalist deserve mention: neonates, kidney disease, and voluminous diarrhea. Neonates under the age of 28 days, including febrile neonates, are excluded from the guideline because of the immature concentrating abilities of neonatal kidneys. Patients with renal impairment were excluded from the guideline recommendation because several studies excluded patients with kidney disease. Hospitalists often care for children who sustain prerenal acute kidney injury from severe dehydration. In this condition, the kidney conserves water through the release of AVP. While an excluded population, these patients would be even more susceptible to develop hyponatremia if administered hypotonic fluids. Patients with “voluminous diarrhea” are excluded from the guideline because those with gastroenteritis with ongoing losses may require IVFs at rates higher than maintenance, and are particularly vulnerable to electrolyte derangements. The guideline, however, does not define voluminous diarrhea, leaving it to the discretion of the treating clinician.
Finally, it is critical to mention that IVF should be considered a therapy to be judiciously used, and discontinued when possible. While the guideline addresses the choice of fluid composition, alternatives to orally or enterally hydrate a patient are always preferred.
AREAS IN NEED OF FUTURE STUDY
While the guideline strongly recommends isotonic fluids for maintenance therapy, the choice of isotonic fluid remains with the clinician. Most included studies used NS for their isotonic groups, but Hartmann’s solution and Plasmalyte were represented in a few studies. LR, one of the more widely used balanced solutions, though slightly hypotonic (130 mEq/L), was not studied. The exclusion of LR from the included studies is unfortunate, as the benefit of balanced solutions compared to NS after significant fluid resuscitation has been shown in the setting of severe sepsis and shock.7 Hyperchloremic metabolic acidosis after fluid resuscitation with NS has raised concern about continuing NS as maintenance fluid and possibly worsening acidosis or hyperchloremia and its adverse effects.8 Further studies on the potential benefit of LR as maintenance fluid, or the potential harms of unbalanced solutions as maintenance fluids in the setting of significant resuscitation are needed.
Disclosures
The authors have nothing to disclose.
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832. PubMed
2. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.12788/jhm.3177 PubMed
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6). doi: 10.12788/jhm.3177 PubMed
4. Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med. 2016;129(8):823-835 e824. doi: 10.12788/jhm.3177 PubMed
5. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82(1):13-18. doi: 10.12788/jhm.3177 PubMed
6. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: a randomized controlled trial. Indian Pediatr. 2014;51(12):969-974. PubMed
7. Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177-1183. doi: 10.1097/CCM.0000000000002365 PubMed
8. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155-160. doi: 10.1097/PCC.0000000000001401. PubMed
Hospitalized children with inadequate fluid intake are often administered maintenance intravenous fluids (IVFs) to support metabolic needs and sensible losses. Historically, hypotonic IVFs have been the standard, based on theoretical water and electrolyte requirements for estimated energy expenditure.1 However, when combined with increased levels of arginine vasopressin (AVP) seen in acutely ill children which impairs free-water excretion,2 hypotonic IVF can resu
KEY RECOMMENDATION FOR HOSPITALISTS
Patients between the ages of 28 days and 18 years should receive isotonic solutions with appropriate potassium chloride and dextrose for maintenance IVFs (evidence quality: high; recommendation strength: strong)
Isotonic fluids, such as 0.9% NaCl (normal saline), Hartmann solution and PlasmaLyte, contain a sodium concentration similar to that of plasma (135-144 mEq/L). Lactated Ringer solution (LR) is near-isotonic (sodium 130 mEq/L), but was not used in any of the reviewed studies and therefore not included in the recommendation. Excluded are patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, severe burns, or patients in the neonatal intensive care unit.
The primary benefit of the AAP recommendation is the reduced risk of iatrogenic hyponatremia and its associated sequelae, including complications or impact on cost of care. The number needed to treat with isotonic fluids was 7.5 to prevent any hyponatremia and 27.8 to prevent moderate hyponatremia (<130 mEq/L). Increases in readmission rates, length of stay, and cost of hospitalization have been reported in a recent meta-analysis reviewing the economic burden of hyponatremia in both adults and children.4
Potential harms from the use of isotonic fluids include hypernatremia, hyperchloremic metabolic acidosis, and fluid overload, although available data have not demonstrated an increased risk of these complications. In light of a recent normal saline (NS) shortage in the United States, limited availability is also a consideration. Plasmalyte is more costly than NS and is currently incompatible with the addition of dextrose.
CRITIQUE
Methods in Preparing Guideline
The guideline development committee included broad representation by pediatric experts in primary care, hospital medicine, emergency medicine, critical care medicine, nephrology, anesthesiology, surgery and quality improvement, as well as a guideline methodologist/informatician and epidemiologist.
Search strategies from recently published systematic reviews of clinical trials comparing isotonic with hypotonic maintenance IVFs were used to identify studies eligible for inclusion. A total of 17 studies with 2,455 total patients were initially identified and included. One additional study meeting inclusion criteria was found after the committee convened and excluded from the guideline.5 Three reviewers from the subcommittee performed a structured critical appraisal of each article. The methods of each trial were assessed for risk-of-bias in multiple domains, including randomization, allocation concealment, performance, detection, attrition and reporting. Forest plots were generated using random-effects models and Mantel-Haenzel statistics with the outcome of hyponatremia. The guideline underwent review by various stakeholders including AAP councils, committees, and sections, and individuals considered experts in the field.
A strength of the guideline is the high quality of the evidence and the consistent findings. All of the included studies were randomized clinical trials and the number of included patients was large. Of the 17 included studies, 16 reported a risk ratio favoring isotonic fluids over hypotonic fluids in the prevention of developing hyponatremia; the results of the study that favored hypotonic fluids were not statistically significant on their own. A sensitivity analysis was performed to exclude one study with a 20% weight, determined by multiple factors such as sample size, confidence interval, and an unusually high rate of hyponatremia in the isotonic and hypotonic fluids groups (33.3 % and 70%, respectively).6 After exclusion, there was no change in the overall estimated risk in hypotonic fluids leading to hyponatremia. Only one trial had two sources of high risk of bias (allocation concealment, attrition) and the remaining had only low or unclear risk of biases in the various domains.
The study that was excluded due to its late identification similarly shows increased risk of hyponatremia in groups administered hypotonic fluids (risk ratio 6.5-8.5), and would likely not affect the estimated risk.5
Despite differences in types of patients enrolled, rate of administered fluids, type of IVF, frequency of lab testing, and study duration, the I2 (degree of heterogeneity) of the forest plot of all included studies remained low at 14% and the increased risk of hyponatremia from hypotonic fluids remained consistent.
Due to study design differences, a limitation of the guideline is that no recommendation is made regarding the type of isotonic fluids and the rate of IVF administration. Additionally, due to the low frequency of clinically significant sequelae of hyponatremia, such as hyponatremic encephalopathy, it remains uncertain how many patients would need to be treated with isotonic fluids to prevent a rare but potentially devastating event.
Sources of Potential Conflict of Interest or Bias
The guideline was developed and funded by the AAP. A formal conflict of interest management policy was followed, and subcommittee members had no conflicts of interests or financial relationships relevant to the guideline to disclose.
Generalizability
Given the large number of patients included in the studies and heterogeneity of the population included, the recommendation applies to most patients cared for by pediatric hospitalists. Several patient exclusions relevant to the pediatric hospitalist deserve mention: neonates, kidney disease, and voluminous diarrhea. Neonates under the age of 28 days, including febrile neonates, are excluded from the guideline because of the immature concentrating abilities of neonatal kidneys. Patients with renal impairment were excluded from the guideline recommendation because several studies excluded patients with kidney disease. Hospitalists often care for children who sustain prerenal acute kidney injury from severe dehydration. In this condition, the kidney conserves water through the release of AVP. While an excluded population, these patients would be even more susceptible to develop hyponatremia if administered hypotonic fluids. Patients with “voluminous diarrhea” are excluded from the guideline because those with gastroenteritis with ongoing losses may require IVFs at rates higher than maintenance, and are particularly vulnerable to electrolyte derangements. The guideline, however, does not define voluminous diarrhea, leaving it to the discretion of the treating clinician.
Finally, it is critical to mention that IVF should be considered a therapy to be judiciously used, and discontinued when possible. While the guideline addresses the choice of fluid composition, alternatives to orally or enterally hydrate a patient are always preferred.
AREAS IN NEED OF FUTURE STUDY
While the guideline strongly recommends isotonic fluids for maintenance therapy, the choice of isotonic fluid remains with the clinician. Most included studies used NS for their isotonic groups, but Hartmann’s solution and Plasmalyte were represented in a few studies. LR, one of the more widely used balanced solutions, though slightly hypotonic (130 mEq/L), was not studied. The exclusion of LR from the included studies is unfortunate, as the benefit of balanced solutions compared to NS after significant fluid resuscitation has been shown in the setting of severe sepsis and shock.7 Hyperchloremic metabolic acidosis after fluid resuscitation with NS has raised concern about continuing NS as maintenance fluid and possibly worsening acidosis or hyperchloremia and its adverse effects.8 Further studies on the potential benefit of LR as maintenance fluid, or the potential harms of unbalanced solutions as maintenance fluids in the setting of significant resuscitation are needed.
Disclosures
The authors have nothing to disclose.
Hospitalized children with inadequate fluid intake are often administered maintenance intravenous fluids (IVFs) to support metabolic needs and sensible losses. Historically, hypotonic IVFs have been the standard, based on theoretical water and electrolyte requirements for estimated energy expenditure.1 However, when combined with increased levels of arginine vasopressin (AVP) seen in acutely ill children which impairs free-water excretion,2 hypotonic IVF can resu
KEY RECOMMENDATION FOR HOSPITALISTS
Patients between the ages of 28 days and 18 years should receive isotonic solutions with appropriate potassium chloride and dextrose for maintenance IVFs (evidence quality: high; recommendation strength: strong)
Isotonic fluids, such as 0.9% NaCl (normal saline), Hartmann solution and PlasmaLyte, contain a sodium concentration similar to that of plasma (135-144 mEq/L). Lactated Ringer solution (LR) is near-isotonic (sodium 130 mEq/L), but was not used in any of the reviewed studies and therefore not included in the recommendation. Excluded are patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, severe burns, or patients in the neonatal intensive care unit.
The primary benefit of the AAP recommendation is the reduced risk of iatrogenic hyponatremia and its associated sequelae, including complications or impact on cost of care. The number needed to treat with isotonic fluids was 7.5 to prevent any hyponatremia and 27.8 to prevent moderate hyponatremia (<130 mEq/L). Increases in readmission rates, length of stay, and cost of hospitalization have been reported in a recent meta-analysis reviewing the economic burden of hyponatremia in both adults and children.4
Potential harms from the use of isotonic fluids include hypernatremia, hyperchloremic metabolic acidosis, and fluid overload, although available data have not demonstrated an increased risk of these complications. In light of a recent normal saline (NS) shortage in the United States, limited availability is also a consideration. Plasmalyte is more costly than NS and is currently incompatible with the addition of dextrose.
CRITIQUE
Methods in Preparing Guideline
The guideline development committee included broad representation by pediatric experts in primary care, hospital medicine, emergency medicine, critical care medicine, nephrology, anesthesiology, surgery and quality improvement, as well as a guideline methodologist/informatician and epidemiologist.
Search strategies from recently published systematic reviews of clinical trials comparing isotonic with hypotonic maintenance IVFs were used to identify studies eligible for inclusion. A total of 17 studies with 2,455 total patients were initially identified and included. One additional study meeting inclusion criteria was found after the committee convened and excluded from the guideline.5 Three reviewers from the subcommittee performed a structured critical appraisal of each article. The methods of each trial were assessed for risk-of-bias in multiple domains, including randomization, allocation concealment, performance, detection, attrition and reporting. Forest plots were generated using random-effects models and Mantel-Haenzel statistics with the outcome of hyponatremia. The guideline underwent review by various stakeholders including AAP councils, committees, and sections, and individuals considered experts in the field.
A strength of the guideline is the high quality of the evidence and the consistent findings. All of the included studies were randomized clinical trials and the number of included patients was large. Of the 17 included studies, 16 reported a risk ratio favoring isotonic fluids over hypotonic fluids in the prevention of developing hyponatremia; the results of the study that favored hypotonic fluids were not statistically significant on their own. A sensitivity analysis was performed to exclude one study with a 20% weight, determined by multiple factors such as sample size, confidence interval, and an unusually high rate of hyponatremia in the isotonic and hypotonic fluids groups (33.3 % and 70%, respectively).6 After exclusion, there was no change in the overall estimated risk in hypotonic fluids leading to hyponatremia. Only one trial had two sources of high risk of bias (allocation concealment, attrition) and the remaining had only low or unclear risk of biases in the various domains.
The study that was excluded due to its late identification similarly shows increased risk of hyponatremia in groups administered hypotonic fluids (risk ratio 6.5-8.5), and would likely not affect the estimated risk.5
Despite differences in types of patients enrolled, rate of administered fluids, type of IVF, frequency of lab testing, and study duration, the I2 (degree of heterogeneity) of the forest plot of all included studies remained low at 14% and the increased risk of hyponatremia from hypotonic fluids remained consistent.
Due to study design differences, a limitation of the guideline is that no recommendation is made regarding the type of isotonic fluids and the rate of IVF administration. Additionally, due to the low frequency of clinically significant sequelae of hyponatremia, such as hyponatremic encephalopathy, it remains uncertain how many patients would need to be treated with isotonic fluids to prevent a rare but potentially devastating event.
Sources of Potential Conflict of Interest or Bias
The guideline was developed and funded by the AAP. A formal conflict of interest management policy was followed, and subcommittee members had no conflicts of interests or financial relationships relevant to the guideline to disclose.
Generalizability
Given the large number of patients included in the studies and heterogeneity of the population included, the recommendation applies to most patients cared for by pediatric hospitalists. Several patient exclusions relevant to the pediatric hospitalist deserve mention: neonates, kidney disease, and voluminous diarrhea. Neonates under the age of 28 days, including febrile neonates, are excluded from the guideline because of the immature concentrating abilities of neonatal kidneys. Patients with renal impairment were excluded from the guideline recommendation because several studies excluded patients with kidney disease. Hospitalists often care for children who sustain prerenal acute kidney injury from severe dehydration. In this condition, the kidney conserves water through the release of AVP. While an excluded population, these patients would be even more susceptible to develop hyponatremia if administered hypotonic fluids. Patients with “voluminous diarrhea” are excluded from the guideline because those with gastroenteritis with ongoing losses may require IVFs at rates higher than maintenance, and are particularly vulnerable to electrolyte derangements. The guideline, however, does not define voluminous diarrhea, leaving it to the discretion of the treating clinician.
Finally, it is critical to mention that IVF should be considered a therapy to be judiciously used, and discontinued when possible. While the guideline addresses the choice of fluid composition, alternatives to orally or enterally hydrate a patient are always preferred.
AREAS IN NEED OF FUTURE STUDY
While the guideline strongly recommends isotonic fluids for maintenance therapy, the choice of isotonic fluid remains with the clinician. Most included studies used NS for their isotonic groups, but Hartmann’s solution and Plasmalyte were represented in a few studies. LR, one of the more widely used balanced solutions, though slightly hypotonic (130 mEq/L), was not studied. The exclusion of LR from the included studies is unfortunate, as the benefit of balanced solutions compared to NS after significant fluid resuscitation has been shown in the setting of severe sepsis and shock.7 Hyperchloremic metabolic acidosis after fluid resuscitation with NS has raised concern about continuing NS as maintenance fluid and possibly worsening acidosis or hyperchloremia and its adverse effects.8 Further studies on the potential benefit of LR as maintenance fluid, or the potential harms of unbalanced solutions as maintenance fluids in the setting of significant resuscitation are needed.
Disclosures
The authors have nothing to disclose.
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832. PubMed
2. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.12788/jhm.3177 PubMed
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6). doi: 10.12788/jhm.3177 PubMed
4. Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med. 2016;129(8):823-835 e824. doi: 10.12788/jhm.3177 PubMed
5. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82(1):13-18. doi: 10.12788/jhm.3177 PubMed
6. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: a randomized controlled trial. Indian Pediatr. 2014;51(12):969-974. PubMed
7. Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177-1183. doi: 10.1097/CCM.0000000000002365 PubMed
8. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155-160. doi: 10.1097/PCC.0000000000001401. PubMed
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832. PubMed
2. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.12788/jhm.3177 PubMed
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6). doi: 10.12788/jhm.3177 PubMed
4. Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med. 2016;129(8):823-835 e824. doi: 10.12788/jhm.3177 PubMed
5. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82(1):13-18. doi: 10.12788/jhm.3177 PubMed
6. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: a randomized controlled trial. Indian Pediatr. 2014;51(12):969-974. PubMed
7. Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177-1183. doi: 10.1097/CCM.0000000000002365 PubMed
8. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155-160. doi: 10.1097/PCC.0000000000001401. PubMed
© 2019 Society of Hospital Medicine
