User login
Disseminated Superficial Actinic Porokeratosis Treated With Ingenol Mebutate Gel 0.05%
Disseminated superficial actinic porokeratosis (DSAP) is a chronic condition characterized by numerous atrophic papules and patches with a distinctive peripheral keratotic ridge, typically found on sun-exposed areas.1,2 Treatment of DSAP is warranted not only for cosmetic and symptomatic benefits but also to prevent malignant transformation.3,4 Successful treatment of DSAP often is difficult and frequently requires the use of multiple modalities. Ingenol mebutate gel 0.05% is a topical medication primarily used for the treatment of actinic keratosis (AK) by inducing cell death.5 We report a case of DSAP treated effectively with ingenol mebutate gel 0.05%.
Case Report
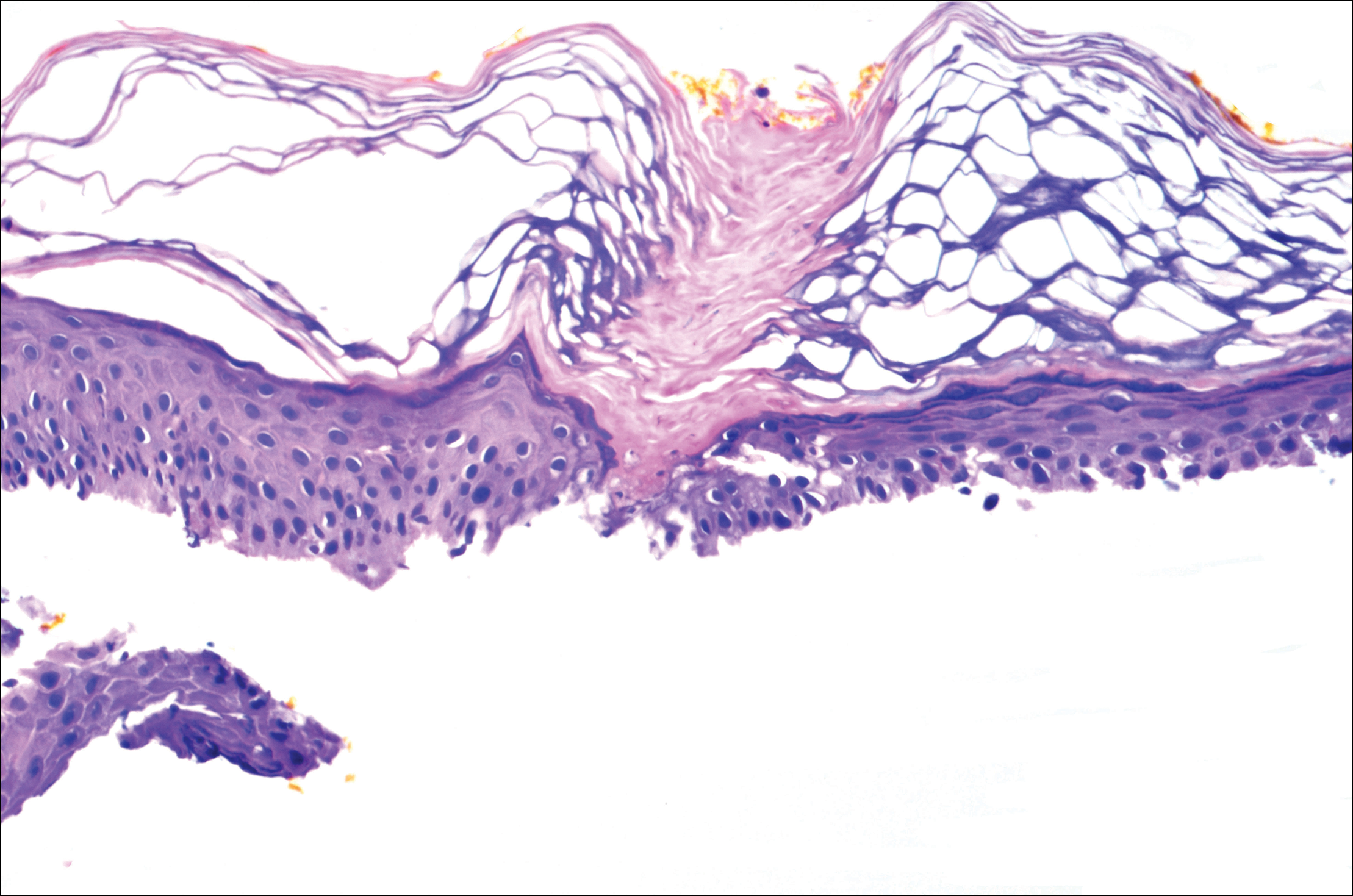
A 37-year-old woman was referred to the dermatology department for counseling for pseudoxanthoma elasticum (PXE), which had been proven on biopsy by an outside dermatologist 2 years prior. Physical examination revealed yellow papules on the neck that were characteristic of PXE, but no lesions were noted on the arms or legs. The only other cutaneous finding was a soft nodule on the right hip consistent with a lipoma. The patient returned to our institution 6 years later with lesions on both lower legs. She reported that these lesions had been present for 3 years and were exacerbated by sun exposure. On physical examination, multiple scattered, erythematous, annular, scaling papules and plaques were noted on the bilateral legs. A biopsy showed the histopathologic findings of DSAP (Figure 1). The patient had no family history of DSAP or PXE.
To determine the best treatment modality, we treated 4 test areas on both upper and lower legs: one with trichloroacetic acid (TCA), one with cryotherapy, one with imiquimod cream 5%, and one with tretinoin cream 0.1%. The patient returned 4 weeks later and showed modest response to TCA, cryotherapy, and tretinoin cream. Because cryotherapy was determined to be most effective, 20 more lesions were frozen at that visit. Over the next 2 years, the patient was treated with TCA, imiquimod cream 5%, and tretinoin cream 0.1%, but all ultimately proved ineffective for DSAP.
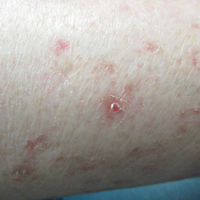
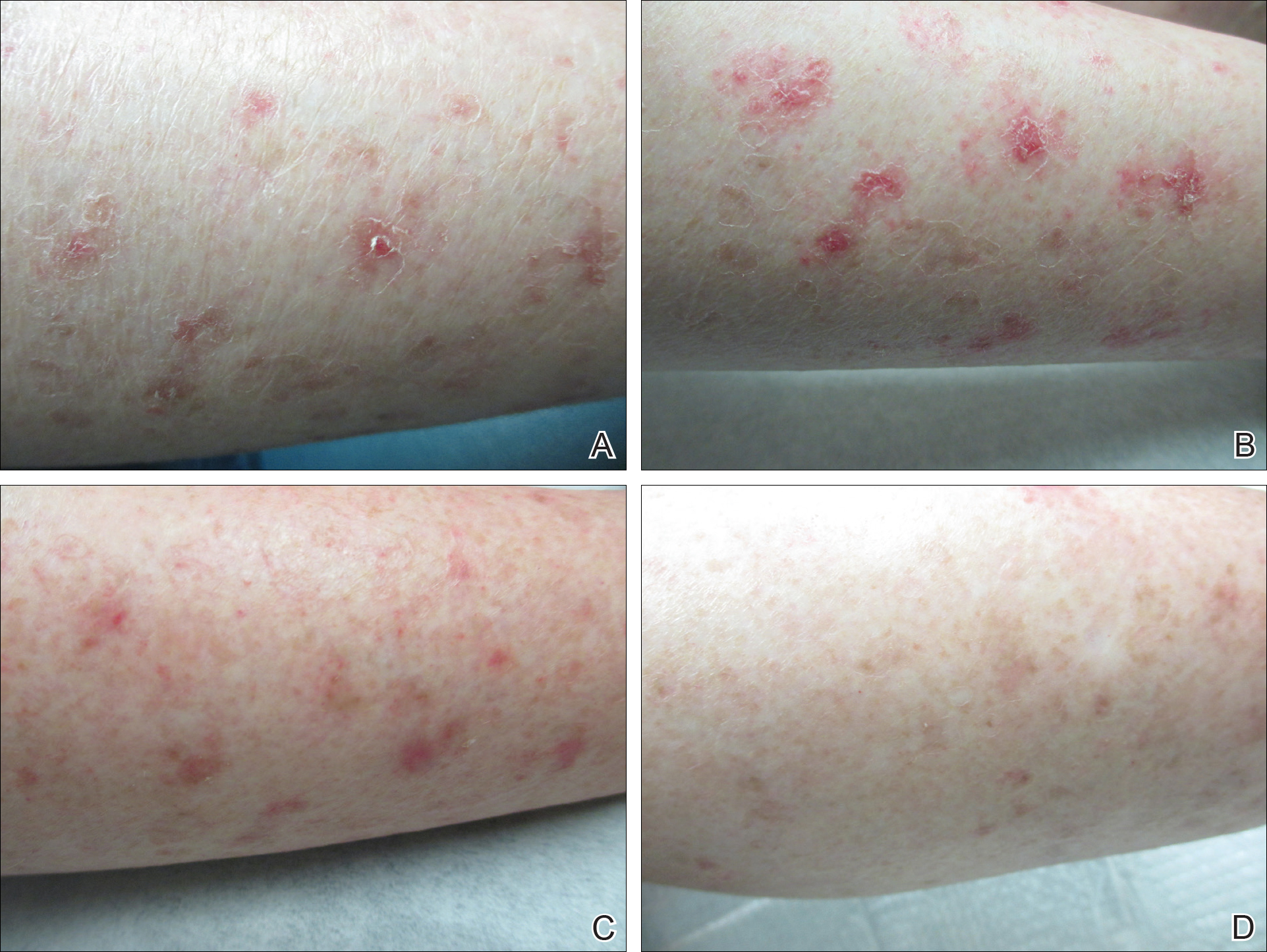
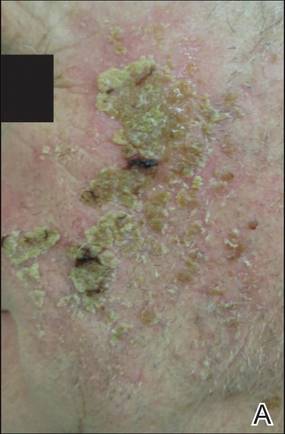
The patient returned 2 years after treatment failure (age 47 years) and was prescribed ingenol mebutate gel 0.05% for 2 days over an area of 25 cm2 on the right lower leg (Figure 2A). She returned for follow-up at days 3, 15, 30, and 60. At day 3, the patient developed an inflammatory response to the medication with moderate erythema and scaling of individual lesions. No vesiculation, pustulation, edema, or ulceration was exhibited (Figure 2B). At day 30, there was a marked reduction in scaling with some postinflammatory erythema (Figure 2C). At day 60, much of the erythema had faded and the scale remained notably reduced (Figure 2D).
Comment
Disseminated superficial actinic porokeratosis is the most common subtype of porokeratosis, a keratinization disorder. There are 6 subtypes of porokeratosis identified in the literature: DSAP, disseminated superficial porokeratosis, classic porokeratosis of Mibelli, porokeratosis plantaris palmaris et disseminata, linear porokeratosis, and punctate porokeratosis.6 Disseminated superficial actinic porokeratosis has a female predominance (1.8:1 ratio)7 and generally appears in the third or fourth decades of life. Clonal proliferations of atypical keratinocytes have been implicated in the etiology of DSAP; however, the exact pathogenesis is unclear. Risk factors for DSAP include genetic susceptibility (eg, autosomal-dominant inheritance pattern), exposure to UV radiation, and drug-related immunosuppression or immunodeficiency.7 Other proposed etiologic risk factors include trauma and infection.8 Clinical diagnosis of DSAP is confirmed by the histological presence of a cornoid lamella (a thin column ofparakeratotic cells), a thinning epidermis, an absent or thinned granular cell layer, and a prominent dermal lymphocytic infiltrate.9,10
Disseminated superficial actinic porokeratosis clinically presents as small atrophic scaly papules and/or patches with raised peripheral ridges symmetrically dispersed on sun-exposed areas of the arms, legs, back, and shoulders. Although these lesions are extensive, they typically spare the mucous membranes, palms, and soles11; only a small percentage of cases report facial lesions,12 which often are asymptomatic but cosmetically bothersome. Additionally, approximately half of patients report symptoms of pruritus and/or stinging,13 thus treatment of DSAP is mainly indicated for symptomatic relief and cosmetic purposes. Malignant degeneration14,15 occurs in approximately 7.5% to 11% of porokeratosis cases,10,16 warranting treatment for preventative measures.
Management of DSAP is dependent on the extent of the disease and the level of concern for malignant transformation. Localized disease can be treated with cryotherapy, CO2 laser, and/or ablative techniques (eg, excision, curettage, dermabrasion) with variable degrees of success but high risk for scarring.1 More extensive disease requires treatment with topical retinoids, topical 5-fluorouracil, imiquimod cream 5%, diclofenac gel 3%, topical vitamin D3 analogues, and photodynamic therapy.1 Several other therapies have been reported in the literature with partial and/or complete success, including systemic retinoids (eg, acitretin), Q-switched ruby laser, Nd:YAG laser, fractional photothermolysis, Grenz rays, pulsed dye laser, fractional photothermolysis, topical corticosteroids, and fluor-hydroxy pulse peel.6 Although there is an extensive array of therapies for DSAP, treatment results are variable with mostly limited success. Successful treatment of DSAP is difficult and often requires the use of multiple modalities.
Ingenol mebutate is the active compound found in the sap of Euphorbia peplus used for the topical treatment of various skin conditions, including AKs.17 Ingenol mebutate gel 0.05% once daily for 2 days has been approved by the US Food and Drug Administration for the topical treatment of AKs. The mechanism of action of ingenol mebutate in AK therapy is not yet fully understood. In vivo and in vitro models have demonstrated both an induction of local lesion cell death and promotion of lesion-specific inflammatory response.18 When used in the treatment of AKs, ingenol mebutate gel 0.05% may cause a mild to moderate localized inflammatory response (eg, erythema, flaking/scaling, crusting, vesiculation/pustulation, erosion/ulceration, edema).
Our case is a rare report of successful treatment of DSAP with ingenol mebutate gel 0.05%. We found that treatment with ingenol mebutate gel 0.05% resulted in clinical improvement of DSAP lesions with minimal discomfort and good cosmetic response. This 2-day regimen is easy to use and patient friendly, improving medication compliance in such a cumbersome disease. We hope this case suggests that ingenol mebutate gel 0.05% could be a useful treatment alternative for DSAP, but future clinical studies should be conducted.
- Martin-Clavijo A, Kanelleas A, Vlachou C, et al. Porokeratoses. In: Lebwohl M, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease Comprehensive Therapeutic Strategies. 3rd ed. China: Elsevier Limited; 2010:584-586.
- Rouhani P, Fischer M, Meehan S, et al. Disseminated superficial actinic porokeratosis. Dermatology Online J. 2012;18:24.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536-538.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- O’Regan GM, Irvine AD. Porokeratosis. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Professional; 2012:442-446.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Brauer JA, Mandal R, Walters R, et al. Disseminated superficial porokeratosis. Dermatology Online J. 2010;16:20.
- Tallon B. Porokeratosis pathology. DermNet New Zealand website. http://www.dermnet.org.nz/pathology/porokeratosis-path.html. Updated December 2016. Accessed January 12, 2017.
- Skupsky H, Skupsky J, Goldenberg G. Disseminated superficial actinic porokeratosis: a treatment review [published online October 22, 2010]. J Dermatolog Treat. 2012;23:52-56.
- Spencer LV. Porokeratosis. UpToDate web site. https://eresources.library.mssm.edu:3285/contents/porokeratosis?source=search_result&search=porokeratosis&selectedTitle=1~22. Updated September 1, 2016. Accessed April 3, 2017.
- Sawyer R, Picou KA. Facial presentation of disseminated superficial actinic porokeratosis. Ear Nose Throat J. 1989;68:57-59.
- Schwarz T, Seiser A, Gschnait F. Disseminated superficial “actinic” porokeratosis. J Am Acad Dermatol. 1984;11(4, pt 2):724-730.
- Maubec E, Duvillard P, Margulis A, et al. Common skin cancers in porokeratosis. Br J Dermatol. 2005;152:1389-1391.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis [published online November 3, 2011]. Ann Dermatol. 2011;23:536-538.
- Kumari S, Mathur M. Disseminated superficial actinic porokeratosis. Nepal J Dermatol Venereol Leprol. 2010;9:22-24.
- Lebwohl M, Shumack S, Stein Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratosis. JAMA Dermatol. 2013;149:666-670.
- Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
Disseminated superficial actinic porokeratosis (DSAP) is a chronic condition characterized by numerous atrophic papules and patches with a distinctive peripheral keratotic ridge, typically found on sun-exposed areas.1,2 Treatment of DSAP is warranted not only for cosmetic and symptomatic benefits but also to prevent malignant transformation.3,4 Successful treatment of DSAP often is difficult and frequently requires the use of multiple modalities. Ingenol mebutate gel 0.05% is a topical medication primarily used for the treatment of actinic keratosis (AK) by inducing cell death.5 We report a case of DSAP treated effectively with ingenol mebutate gel 0.05%.
Case Report
A 37-year-old woman was referred to the dermatology department for counseling for pseudoxanthoma elasticum (PXE), which had been proven on biopsy by an outside dermatologist 2 years prior. Physical examination revealed yellow papules on the neck that were characteristic of PXE, but no lesions were noted on the arms or legs. The only other cutaneous finding was a soft nodule on the right hip consistent with a lipoma. The patient returned to our institution 6 years later with lesions on both lower legs. She reported that these lesions had been present for 3 years and were exacerbated by sun exposure. On physical examination, multiple scattered, erythematous, annular, scaling papules and plaques were noted on the bilateral legs. A biopsy showed the histopathologic findings of DSAP (Figure 1). The patient had no family history of DSAP or PXE.

To determine the best treatment modality, we treated 4 test areas on both upper and lower legs: one with trichloroacetic acid (TCA), one with cryotherapy, one with imiquimod cream 5%, and one with tretinoin cream 0.1%. The patient returned 4 weeks later and showed modest response to TCA, cryotherapy, and tretinoin cream. Because cryotherapy was determined to be most effective, 20 more lesions were frozen at that visit. Over the next 2 years, the patient was treated with TCA, imiquimod cream 5%, and tretinoin cream 0.1%, but all ultimately proved ineffective for DSAP.
The patient returned 2 years after treatment failure (age 47 years) and was prescribed ingenol mebutate gel 0.05% for 2 days over an area of 25 cm2 on the right lower leg (Figure 2A). She returned for follow-up at days 3, 15, 30, and 60. At day 3, the patient developed an inflammatory response to the medication with moderate erythema and scaling of individual lesions. No vesiculation, pustulation, edema, or ulceration was exhibited (Figure 2B). At day 30, there was a marked reduction in scaling with some postinflammatory erythema (Figure 2C). At day 60, much of the erythema had faded and the scale remained notably reduced (Figure 2D).

Comment
Disseminated superficial actinic porokeratosis is the most common subtype of porokeratosis, a keratinization disorder. There are 6 subtypes of porokeratosis identified in the literature: DSAP, disseminated superficial porokeratosis, classic porokeratosis of Mibelli, porokeratosis plantaris palmaris et disseminata, linear porokeratosis, and punctate porokeratosis.6 Disseminated superficial actinic porokeratosis has a female predominance (1.8:1 ratio)7 and generally appears in the third or fourth decades of life. Clonal proliferations of atypical keratinocytes have been implicated in the etiology of DSAP; however, the exact pathogenesis is unclear. Risk factors for DSAP include genetic susceptibility (eg, autosomal-dominant inheritance pattern), exposure to UV radiation, and drug-related immunosuppression or immunodeficiency.7 Other proposed etiologic risk factors include trauma and infection.8 Clinical diagnosis of DSAP is confirmed by the histological presence of a cornoid lamella (a thin column ofparakeratotic cells), a thinning epidermis, an absent or thinned granular cell layer, and a prominent dermal lymphocytic infiltrate.9,10
Disseminated superficial actinic porokeratosis clinically presents as small atrophic scaly papules and/or patches with raised peripheral ridges symmetrically dispersed on sun-exposed areas of the arms, legs, back, and shoulders. Although these lesions are extensive, they typically spare the mucous membranes, palms, and soles11; only a small percentage of cases report facial lesions,12 which often are asymptomatic but cosmetically bothersome. Additionally, approximately half of patients report symptoms of pruritus and/or stinging,13 thus treatment of DSAP is mainly indicated for symptomatic relief and cosmetic purposes. Malignant degeneration14,15 occurs in approximately 7.5% to 11% of porokeratosis cases,10,16 warranting treatment for preventative measures.
Management of DSAP is dependent on the extent of the disease and the level of concern for malignant transformation. Localized disease can be treated with cryotherapy, CO2 laser, and/or ablative techniques (eg, excision, curettage, dermabrasion) with variable degrees of success but high risk for scarring.1 More extensive disease requires treatment with topical retinoids, topical 5-fluorouracil, imiquimod cream 5%, diclofenac gel 3%, topical vitamin D3 analogues, and photodynamic therapy.1 Several other therapies have been reported in the literature with partial and/or complete success, including systemic retinoids (eg, acitretin), Q-switched ruby laser, Nd:YAG laser, fractional photothermolysis, Grenz rays, pulsed dye laser, fractional photothermolysis, topical corticosteroids, and fluor-hydroxy pulse peel.6 Although there is an extensive array of therapies for DSAP, treatment results are variable with mostly limited success. Successful treatment of DSAP is difficult and often requires the use of multiple modalities.
Ingenol mebutate is the active compound found in the sap of Euphorbia peplus used for the topical treatment of various skin conditions, including AKs.17 Ingenol mebutate gel 0.05% once daily for 2 days has been approved by the US Food and Drug Administration for the topical treatment of AKs. The mechanism of action of ingenol mebutate in AK therapy is not yet fully understood. In vivo and in vitro models have demonstrated both an induction of local lesion cell death and promotion of lesion-specific inflammatory response.18 When used in the treatment of AKs, ingenol mebutate gel 0.05% may cause a mild to moderate localized inflammatory response (eg, erythema, flaking/scaling, crusting, vesiculation/pustulation, erosion/ulceration, edema).
Our case is a rare report of successful treatment of DSAP with ingenol mebutate gel 0.05%. We found that treatment with ingenol mebutate gel 0.05% resulted in clinical improvement of DSAP lesions with minimal discomfort and good cosmetic response. This 2-day regimen is easy to use and patient friendly, improving medication compliance in such a cumbersome disease. We hope this case suggests that ingenol mebutate gel 0.05% could be a useful treatment alternative for DSAP, but future clinical studies should be conducted.
Disseminated superficial actinic porokeratosis (DSAP) is a chronic condition characterized by numerous atrophic papules and patches with a distinctive peripheral keratotic ridge, typically found on sun-exposed areas.1,2 Treatment of DSAP is warranted not only for cosmetic and symptomatic benefits but also to prevent malignant transformation.3,4 Successful treatment of DSAP often is difficult and frequently requires the use of multiple modalities. Ingenol mebutate gel 0.05% is a topical medication primarily used for the treatment of actinic keratosis (AK) by inducing cell death.5 We report a case of DSAP treated effectively with ingenol mebutate gel 0.05%.
Case Report
A 37-year-old woman was referred to the dermatology department for counseling for pseudoxanthoma elasticum (PXE), which had been proven on biopsy by an outside dermatologist 2 years prior. Physical examination revealed yellow papules on the neck that were characteristic of PXE, but no lesions were noted on the arms or legs. The only other cutaneous finding was a soft nodule on the right hip consistent with a lipoma. The patient returned to our institution 6 years later with lesions on both lower legs. She reported that these lesions had been present for 3 years and were exacerbated by sun exposure. On physical examination, multiple scattered, erythematous, annular, scaling papules and plaques were noted on the bilateral legs. A biopsy showed the histopathologic findings of DSAP (Figure 1). The patient had no family history of DSAP or PXE.

To determine the best treatment modality, we treated 4 test areas on both upper and lower legs: one with trichloroacetic acid (TCA), one with cryotherapy, one with imiquimod cream 5%, and one with tretinoin cream 0.1%. The patient returned 4 weeks later and showed modest response to TCA, cryotherapy, and tretinoin cream. Because cryotherapy was determined to be most effective, 20 more lesions were frozen at that visit. Over the next 2 years, the patient was treated with TCA, imiquimod cream 5%, and tretinoin cream 0.1%, but all ultimately proved ineffective for DSAP.
The patient returned 2 years after treatment failure (age 47 years) and was prescribed ingenol mebutate gel 0.05% for 2 days over an area of 25 cm2 on the right lower leg (Figure 2A). She returned for follow-up at days 3, 15, 30, and 60. At day 3, the patient developed an inflammatory response to the medication with moderate erythema and scaling of individual lesions. No vesiculation, pustulation, edema, or ulceration was exhibited (Figure 2B). At day 30, there was a marked reduction in scaling with some postinflammatory erythema (Figure 2C). At day 60, much of the erythema had faded and the scale remained notably reduced (Figure 2D).

Comment
Disseminated superficial actinic porokeratosis is the most common subtype of porokeratosis, a keratinization disorder. There are 6 subtypes of porokeratosis identified in the literature: DSAP, disseminated superficial porokeratosis, classic porokeratosis of Mibelli, porokeratosis plantaris palmaris et disseminata, linear porokeratosis, and punctate porokeratosis.6 Disseminated superficial actinic porokeratosis has a female predominance (1.8:1 ratio)7 and generally appears in the third or fourth decades of life. Clonal proliferations of atypical keratinocytes have been implicated in the etiology of DSAP; however, the exact pathogenesis is unclear. Risk factors for DSAP include genetic susceptibility (eg, autosomal-dominant inheritance pattern), exposure to UV radiation, and drug-related immunosuppression or immunodeficiency.7 Other proposed etiologic risk factors include trauma and infection.8 Clinical diagnosis of DSAP is confirmed by the histological presence of a cornoid lamella (a thin column ofparakeratotic cells), a thinning epidermis, an absent or thinned granular cell layer, and a prominent dermal lymphocytic infiltrate.9,10
Disseminated superficial actinic porokeratosis clinically presents as small atrophic scaly papules and/or patches with raised peripheral ridges symmetrically dispersed on sun-exposed areas of the arms, legs, back, and shoulders. Although these lesions are extensive, they typically spare the mucous membranes, palms, and soles11; only a small percentage of cases report facial lesions,12 which often are asymptomatic but cosmetically bothersome. Additionally, approximately half of patients report symptoms of pruritus and/or stinging,13 thus treatment of DSAP is mainly indicated for symptomatic relief and cosmetic purposes. Malignant degeneration14,15 occurs in approximately 7.5% to 11% of porokeratosis cases,10,16 warranting treatment for preventative measures.
Management of DSAP is dependent on the extent of the disease and the level of concern for malignant transformation. Localized disease can be treated with cryotherapy, CO2 laser, and/or ablative techniques (eg, excision, curettage, dermabrasion) with variable degrees of success but high risk for scarring.1 More extensive disease requires treatment with topical retinoids, topical 5-fluorouracil, imiquimod cream 5%, diclofenac gel 3%, topical vitamin D3 analogues, and photodynamic therapy.1 Several other therapies have been reported in the literature with partial and/or complete success, including systemic retinoids (eg, acitretin), Q-switched ruby laser, Nd:YAG laser, fractional photothermolysis, Grenz rays, pulsed dye laser, fractional photothermolysis, topical corticosteroids, and fluor-hydroxy pulse peel.6 Although there is an extensive array of therapies for DSAP, treatment results are variable with mostly limited success. Successful treatment of DSAP is difficult and often requires the use of multiple modalities.
Ingenol mebutate is the active compound found in the sap of Euphorbia peplus used for the topical treatment of various skin conditions, including AKs.17 Ingenol mebutate gel 0.05% once daily for 2 days has been approved by the US Food and Drug Administration for the topical treatment of AKs. The mechanism of action of ingenol mebutate in AK therapy is not yet fully understood. In vivo and in vitro models have demonstrated both an induction of local lesion cell death and promotion of lesion-specific inflammatory response.18 When used in the treatment of AKs, ingenol mebutate gel 0.05% may cause a mild to moderate localized inflammatory response (eg, erythema, flaking/scaling, crusting, vesiculation/pustulation, erosion/ulceration, edema).
Our case is a rare report of successful treatment of DSAP with ingenol mebutate gel 0.05%. We found that treatment with ingenol mebutate gel 0.05% resulted in clinical improvement of DSAP lesions with minimal discomfort and good cosmetic response. This 2-day regimen is easy to use and patient friendly, improving medication compliance in such a cumbersome disease. We hope this case suggests that ingenol mebutate gel 0.05% could be a useful treatment alternative for DSAP, but future clinical studies should be conducted.
- Martin-Clavijo A, Kanelleas A, Vlachou C, et al. Porokeratoses. In: Lebwohl M, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease Comprehensive Therapeutic Strategies. 3rd ed. China: Elsevier Limited; 2010:584-586.
- Rouhani P, Fischer M, Meehan S, et al. Disseminated superficial actinic porokeratosis. Dermatology Online J. 2012;18:24.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536-538.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- O’Regan GM, Irvine AD. Porokeratosis. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Professional; 2012:442-446.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Brauer JA, Mandal R, Walters R, et al. Disseminated superficial porokeratosis. Dermatology Online J. 2010;16:20.
- Tallon B. Porokeratosis pathology. DermNet New Zealand website. http://www.dermnet.org.nz/pathology/porokeratosis-path.html. Updated December 2016. Accessed January 12, 2017.
- Skupsky H, Skupsky J, Goldenberg G. Disseminated superficial actinic porokeratosis: a treatment review [published online October 22, 2010]. J Dermatolog Treat. 2012;23:52-56.
- Spencer LV. Porokeratosis. UpToDate web site. https://eresources.library.mssm.edu:3285/contents/porokeratosis?source=search_result&search=porokeratosis&selectedTitle=1~22. Updated September 1, 2016. Accessed April 3, 2017.
- Sawyer R, Picou KA. Facial presentation of disseminated superficial actinic porokeratosis. Ear Nose Throat J. 1989;68:57-59.
- Schwarz T, Seiser A, Gschnait F. Disseminated superficial “actinic” porokeratosis. J Am Acad Dermatol. 1984;11(4, pt 2):724-730.
- Maubec E, Duvillard P, Margulis A, et al. Common skin cancers in porokeratosis. Br J Dermatol. 2005;152:1389-1391.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis [published online November 3, 2011]. Ann Dermatol. 2011;23:536-538.
- Kumari S, Mathur M. Disseminated superficial actinic porokeratosis. Nepal J Dermatol Venereol Leprol. 2010;9:22-24.
- Lebwohl M, Shumack S, Stein Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratosis. JAMA Dermatol. 2013;149:666-670.
- Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
- Martin-Clavijo A, Kanelleas A, Vlachou C, et al. Porokeratoses. In: Lebwohl M, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease Comprehensive Therapeutic Strategies. 3rd ed. China: Elsevier Limited; 2010:584-586.
- Rouhani P, Fischer M, Meehan S, et al. Disseminated superficial actinic porokeratosis. Dermatology Online J. 2012;18:24.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536-538.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- O’Regan GM, Irvine AD. Porokeratosis. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Professional; 2012:442-446.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Brauer JA, Mandal R, Walters R, et al. Disseminated superficial porokeratosis. Dermatology Online J. 2010;16:20.
- Tallon B. Porokeratosis pathology. DermNet New Zealand website. http://www.dermnet.org.nz/pathology/porokeratosis-path.html. Updated December 2016. Accessed January 12, 2017.
- Skupsky H, Skupsky J, Goldenberg G. Disseminated superficial actinic porokeratosis: a treatment review [published online October 22, 2010]. J Dermatolog Treat. 2012;23:52-56.
- Spencer LV. Porokeratosis. UpToDate web site. https://eresources.library.mssm.edu:3285/contents/porokeratosis?source=search_result&search=porokeratosis&selectedTitle=1~22. Updated September 1, 2016. Accessed April 3, 2017.
- Sawyer R, Picou KA. Facial presentation of disseminated superficial actinic porokeratosis. Ear Nose Throat J. 1989;68:57-59.
- Schwarz T, Seiser A, Gschnait F. Disseminated superficial “actinic” porokeratosis. J Am Acad Dermatol. 1984;11(4, pt 2):724-730.
- Maubec E, Duvillard P, Margulis A, et al. Common skin cancers in porokeratosis. Br J Dermatol. 2005;152:1389-1391.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis [published online November 3, 2011]. Ann Dermatol. 2011;23:536-538.
- Kumari S, Mathur M. Disseminated superficial actinic porokeratosis. Nepal J Dermatol Venereol Leprol. 2010;9:22-24.
- Lebwohl M, Shumack S, Stein Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratosis. JAMA Dermatol. 2013;149:666-670.
- Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
Practice Points
- Disseminated superficial actinic porokeratosis (DSAP) is an uncommon skin condition consisting of multiple annular hyperkeratotic lesions on sun-exposed areas.
- Treatment of DSAP is necessary due to its potential for progression to malignancy.
- Consider ingenol mebutate gel 0.05% for the treatment of DSAP on the arms and legs.
Reduced Degree of Irritation During a Second Cycle of Ingenol Mebutate Gel 0.015% for the Treatment of Actinic Keratosis
Actinic keratoses (AKs) are common skin lesions resulting from cumulative exposure to UV radiation and are associated with an increased risk for invasive squamous cell carcinoma1; therefore, diagnosis and treatment are important.2 Individual AKs are most frequently treated with cryosurgery, while topical agents including ingenol mebutate gel are used as field treatments on areas of confluent AKs of sun-damaged skin.2,3 Studies have shown that rates of complete clearance with topical therapy can be improved with more than a single treatment course.4-6
Although the mechanisms of action of ingenol mebutate on AKs are not fully understood, studies indicate that it induces cell death in proliferating keratinocytes, which suggests that it may act preferentially on AKs and not on healthy skin.7 The field treatment of AKs of the face and scalp using ingenol mebutate gel 0.015% involves a 3-day regimen,8 and clearance rates are similar to those observed with topical agents that are used for longer periods of time.3,9,10 Local skin reactions (LSRs) associated with application of ingenol mebutate gel 0.015% on the face and scalp generally are mild to moderate in intensity and resolve after 2 weeks without sequelae.3
The presumption that the cytotoxic actions of ingenol mebutate affect proliferating keratinocytes preferentially was the basis for this study. We hypothesized that application of a second sequential cycle of ingenol mebutate during AK treatment should produce lower LSR scores than the first application cycle due to the specific elimination of transformed keratinocytes from the treatment area. This open-label study compared the intensity of LSRs during 2 sequential cycles of treatment on the same site of the face or scalp using ingenol mebutate gel 0.015%.
Methods
Study Population
Eligible participants were adults with 4 to 8 clinically typical, visible, nonhypertrophic AKs in a 25-cm2 contiguous area of the face or scalp. Inclusion and exclusion criteria were the same as in the pivotal studies.3 The study was approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (New York, New York). Enrollment took place from March 2013 to August 2013.
Study Design and Assessments
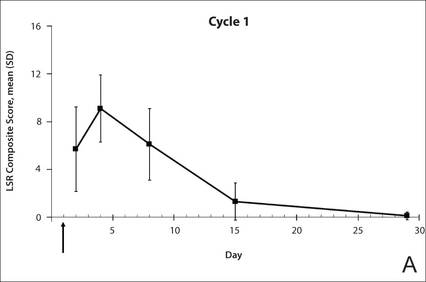
All participants were treated with 2 sequential 4-week cycles of ingenol mebutate gel 0.015% applied once daily for 3 consecutive days starting on the first day of each cycle (day 1 and day 29). Participants were evaluated at 11 visits (days 1, 2, 4, 8, 15, 29, 30, 32, 36, 43, and 56) during the 56-day study period (Figure 1). Eligibility, demographics, and medical history were assessed at day 1, and concomitant medications and adverse events (AEs) were evaluated at all visits. Using standardized photographic guides, 6 individual LSRs—erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were assessed on a scale of 0 (none) to 4 (severe), with higher numbers indicating more severe reactions. For each participant, a composite score was calculated as the sum of the individual LSR scores.3 Throughout the study, 3 qualified evaluators assessed AK lesion count and graded the LSRs. The same evaluator assessed both treatment courses for each participant for the majority of assessments.
 |

|
The primary end point of the study was to evaluate the degree of irritation in each of the 2 sequential cycles of ingenol mebutate treatment by assessing the mean area under the curve (AUC) of the composite LSR score over time following each of the 2 applications. Actinic keratoses were counted at baseline and at the end of each treatment cycle. The paired t test was used to compare AUCs of the composite LSR scores of the 2 cycles and to compare the changes in lesion counts from baseline to day 29 and from baseline to day 56. The complete clearance rates (number of participants with no AKs) at the end of cycles 1 and 2 were compared using a logistic regression model. Participant-perceived irritation and treatment satisfaction were evaluated using a 0 to 100 visual analog scale (VAS), with higher numbers indicating greater irritation and higher satisfaction. Participant-reported scores were summarized.
Results
Participant Characteristics
A total of 20 participants were enrolled in the study. At the completion of the study, 2 participants withdrew consent but allowed use of data from their completed assessments. Consequently, a total of 18 patients completed the entire study. The mean age was 75.35 years (median, 77.5 years; age range, 49–87 years). Most of the participants (15/20 [75%]) were men. All participants were white, and 2 were of Hispanic ethnicity. Of the 20 participants, 19 (95%) were Fitzpatrick skin type II, and 1 (5%) was Fitzpatrick skin type I. Most of the participants (16/20 [80%]) received treatment of lesions on the face. With the exception of 2 (10%) participants, all had received prior treatment of AKs, including cryosurgery (16/20 [80%]), imiquimod (5/20 [25%]), fluorouracil (2/20 [10%]), diclofenac (2/20 [10%]), and photodynamic therapy (2/20 [10%]); 8 (40%) participants had received more than 1 type of treatment.
LSRs in Cycles 1 and 2
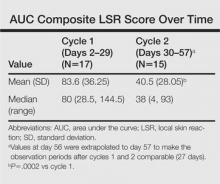
The time course for the development and resolution of LSRs during both treatment cycles was similar. Local skin reactions were evident on day 2 in each cycle, peaked at 3 days after the application of the first dose, declined rapidly by the 15th day of the cycle, and returned to baseline by the end of each 4-week cycle (Figure 1). The mean (standard deviation [SD]) composite LSR score at 3 days after application of the first dose was higher in cycle 1 than in cycle 2 (9.1 [2.83] vs 5.0 [3.24])(Figure 1). The composite LSR score assessed over time based on the mean (SD) AUC was significantly lower in cycle 2 than in cycle 1 (40.5 [28.05] vs 83.6 [36.25])(P=.0002)(Table). Statistical differences in scores for individual reactions between the 2 cycles were not determined because of the risk for a spurious indication of significance from multiple comparisons in such a limited patient sample.
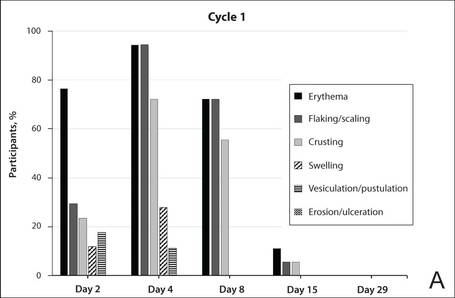
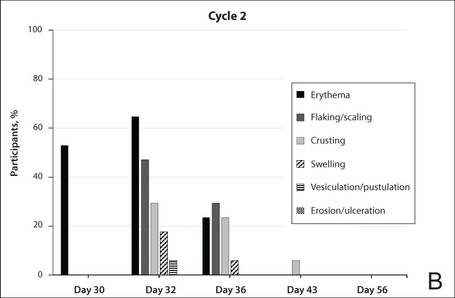
The percentage of participants who had a score greater than 1 for any of the 6 components of the LSR assessment was lower in cycle 2 than in cycle 1 at all of the assessed time points (Figure 2). In both cycles, the percentage of participants with an LSR score greater than 1 was highest 3 days after the application of the first dose in the cycle (day 4 or day 32, respectively). Erythema, flaking/scaling, and crusting were the most freq-uently observed reactions. At day 29, there were no participants with an LSR score greater than 1 in any of the 6 components. At day 29 and day 56, 94% (17/18) and 100% (18/18) of participants, respectively, had a score of 0 for all reactions.
 |
 |
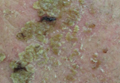
The photographs in Figure 3, taken 7 days after the application of the first dose of ingenol mebutate gel 0.015% in each cycle of treatment of AK lesions on the face, show that there was less flaking/scaling and crusting in cycle 2 than in cycle 1. A review of participant photographs from the third treatment day of each cycle showed that the areas of erythema were the same in both cycles. The other 5 LSRs—flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were observed in different areas of the treated field in the 2 cycles when applicable.
Adverse Events
The few AEs that were reported were considered to be mild in severity. The AEs included application-site pain (n=5), application-site pruritus (n=3), and nasopharyngitis (n=1). No serious AEs were reported. After the first treatment cycle, 1 participant experienced hypopigmentation at the treatment site that persisted as faint hypopigmentation at the last study visit (day 56).
AK Lesion Count
The lesion count in all participants at baseline ranged from 4 to 8, with a mean (SD) of 5.9 (1.55). Mean lesion count was substantially reduced at the end of cycle 1 (0.9 [1.39]) and cycle 2 (0.3 [0.57]). The change in lesion count from baseline to day 56 was greater than the change from baseline to day 29 (-5.7 [1.61] vs -5.0 [1.57])(P=.0137). Complete clearance at day 29 and day 56 was achieved in 55.6% (10/18) and 77.8% (14/18) of participants, respectively. The difference in the clearance rate between day 29 and day 56 did not reach statistical significance, most likely due to the small sample size.
Participant-Reported Outcomes
 |
 |
Visual analog scale scores for participant-perceived irritation were less than 50 on a scale of 0 to 100 during both application cycles. At 1 day and 3 days after application of the first dose of ingenol mebutate gel 0.015% in cycle 1, the mean (SD) VAS scores for irritation were 31.8 (37.06) and 37.9 (30.77), respectively. At the same time points in cycle 2, VAS scores were 44.2 (32.45) and 49.6 (26.90), respectively. No information was available regarding resolution of participant-perceived irritation, as irritation data were not collected after day 4 of each treatment cycle; therefore, P values were not determined. Participant satisfaction with treatment was high and nearly the same at the end of cycles 1 and 2 (VAS scores: 83.7 [12.73] and 83.8 [20.46], respectively).
Comment
Our findings show that a second course of treatment with ingenol mebutate gel 0.015% on the same site on the face or scalp produced a less intense inflammatory reaction than the first course of treatment. Composite LSR scores at each time point after the start of treatment were lower in cycle 2 than in cycle 1. The percentage of participants who demonstrated a severity score greater than 1 for any of the 6 components of the LSR assessment also was lower at time points in cycle 2 than in cycle 1. These results are consistent with the hypothesis that the activity of ingenol mebutate includes a mechanism that specifically targets transformed keratinocytes, which are reduced by the start of a second cycle of treatment.
The mechanism for the clinical efficacy of ingenol mebutate has not been fully described. Studies in preclinical models suggest at least 2 components, including direct cytotoxic effects on tumor cells and a localized inflammatory reaction that includes protein kinase C activation.11 Ingenol mebutate preferentially induces death in tumor cells and in proliferating undifferentiated keratinocytes.7,12 Cell death and protein kinase C activation lead to an inflammatory response dominated by neutrophils and other immunocompetent cells that add to the destruction of transformed cells.11
The reduced inflammatory response observed in participants during the second cycle of treatment in this study is consistent with the theory of a preferential action on transformed keratinocytes by ingenol mebutate. Once transformed keratinocytes are substantially cleared in cycle 1, fewer target cells remain, and therefore the inflammatory response is less intense in cycle 2. If ingenol mebutate were uniformly cytotoxic and inflammatory to all cells, the LSR scores in both cycles would be expected to be similar.
Assessment of participant-perceived irritation supplemented the measurement of the 6 visible manifestations of inflammation over each 4-week cycle. Participant-perceived irritation was recorded early in the cycles at 1 and 3 days after the first dose. Although it is difficult to standardize patient perceptions, VAS scores for irritation in cycle 2 were higher than those reported in cycle 1, which suggests an increased perception of irritation. The clinical relevance of this perception is not certain and may be due to the small number of participants and/or the time interval between the 2 treatment courses.
The results of this study were limited by the small patient sample. Additionally, LSR assessments were limited by the quality of the photographs. However, LSRs and AK clearance rates were similar to the pooled findings seen in the phase 3 studies of ingenol mebutate.3 Adverse events were predominantly conditions that occurred at the application site, as in phase 3 studies.3 Similarly, the time course of LSR development and resolution followed the same pattern as in those trials. The peak composite LSR score for the face and scalp was approximately 9 in both the present study (cycle 1) and in the pooled phase 3 studies.3
Conclusion
Ingenol mebutate gel 0.015% may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over the course of 2 treatment cycles. Patients may experience fewer LSRs on reapplication of ingenol mebutate to a previously treated site.
Acknowledgment
Editorial support was provided by Tanya MacNeil, PhD, of p-value communications, LLC, Cedar Knolls, New Jersey.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
2. Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? part 1: overview and investigational topical agents. Cutis. 2012;89:241-250.
3. Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
4. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157:133-141.
5. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57:265-268.
6. Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2:20-28.
7. Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
8. Picato gel 0.015%, 0.05% [package insert]. Parsippany, NJ: LEO Pharma; 2013.
9. Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
10. Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62:582-590.
11. Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123-8132.
12. Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
Actinic keratoses (AKs) are common skin lesions resulting from cumulative exposure to UV radiation and are associated with an increased risk for invasive squamous cell carcinoma1; therefore, diagnosis and treatment are important.2 Individual AKs are most frequently treated with cryosurgery, while topical agents including ingenol mebutate gel are used as field treatments on areas of confluent AKs of sun-damaged skin.2,3 Studies have shown that rates of complete clearance with topical therapy can be improved with more than a single treatment course.4-6
Although the mechanisms of action of ingenol mebutate on AKs are not fully understood, studies indicate that it induces cell death in proliferating keratinocytes, which suggests that it may act preferentially on AKs and not on healthy skin.7 The field treatment of AKs of the face and scalp using ingenol mebutate gel 0.015% involves a 3-day regimen,8 and clearance rates are similar to those observed with topical agents that are used for longer periods of time.3,9,10 Local skin reactions (LSRs) associated with application of ingenol mebutate gel 0.015% on the face and scalp generally are mild to moderate in intensity and resolve after 2 weeks without sequelae.3
The presumption that the cytotoxic actions of ingenol mebutate affect proliferating keratinocytes preferentially was the basis for this study. We hypothesized that application of a second sequential cycle of ingenol mebutate during AK treatment should produce lower LSR scores than the first application cycle due to the specific elimination of transformed keratinocytes from the treatment area. This open-label study compared the intensity of LSRs during 2 sequential cycles of treatment on the same site of the face or scalp using ingenol mebutate gel 0.015%.
Methods
Study Population
Eligible participants were adults with 4 to 8 clinically typical, visible, nonhypertrophic AKs in a 25-cm2 contiguous area of the face or scalp. Inclusion and exclusion criteria were the same as in the pivotal studies.3 The study was approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (New York, New York). Enrollment took place from March 2013 to August 2013.
Study Design and Assessments
All participants were treated with 2 sequential 4-week cycles of ingenol mebutate gel 0.015% applied once daily for 3 consecutive days starting on the first day of each cycle (day 1 and day 29). Participants were evaluated at 11 visits (days 1, 2, 4, 8, 15, 29, 30, 32, 36, 43, and 56) during the 56-day study period (Figure 1). Eligibility, demographics, and medical history were assessed at day 1, and concomitant medications and adverse events (AEs) were evaluated at all visits. Using standardized photographic guides, 6 individual LSRs—erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were assessed on a scale of 0 (none) to 4 (severe), with higher numbers indicating more severe reactions. For each participant, a composite score was calculated as the sum of the individual LSR scores.3 Throughout the study, 3 qualified evaluators assessed AK lesion count and graded the LSRs. The same evaluator assessed both treatment courses for each participant for the majority of assessments.
 |

|
The primary end point of the study was to evaluate the degree of irritation in each of the 2 sequential cycles of ingenol mebutate treatment by assessing the mean area under the curve (AUC) of the composite LSR score over time following each of the 2 applications. Actinic keratoses were counted at baseline and at the end of each treatment cycle. The paired t test was used to compare AUCs of the composite LSR scores of the 2 cycles and to compare the changes in lesion counts from baseline to day 29 and from baseline to day 56. The complete clearance rates (number of participants with no AKs) at the end of cycles 1 and 2 were compared using a logistic regression model. Participant-perceived irritation and treatment satisfaction were evaluated using a 0 to 100 visual analog scale (VAS), with higher numbers indicating greater irritation and higher satisfaction. Participant-reported scores were summarized.
Results
Participant Characteristics
A total of 20 participants were enrolled in the study. At the completion of the study, 2 participants withdrew consent but allowed use of data from their completed assessments. Consequently, a total of 18 patients completed the entire study. The mean age was 75.35 years (median, 77.5 years; age range, 49–87 years). Most of the participants (15/20 [75%]) were men. All participants were white, and 2 were of Hispanic ethnicity. Of the 20 participants, 19 (95%) were Fitzpatrick skin type II, and 1 (5%) was Fitzpatrick skin type I. Most of the participants (16/20 [80%]) received treatment of lesions on the face. With the exception of 2 (10%) participants, all had received prior treatment of AKs, including cryosurgery (16/20 [80%]), imiquimod (5/20 [25%]), fluorouracil (2/20 [10%]), diclofenac (2/20 [10%]), and photodynamic therapy (2/20 [10%]); 8 (40%) participants had received more than 1 type of treatment.
LSRs in Cycles 1 and 2
The time course for the development and resolution of LSRs during both treatment cycles was similar. Local skin reactions were evident on day 2 in each cycle, peaked at 3 days after the application of the first dose, declined rapidly by the 15th day of the cycle, and returned to baseline by the end of each 4-week cycle (Figure 1). The mean (standard deviation [SD]) composite LSR score at 3 days after application of the first dose was higher in cycle 1 than in cycle 2 (9.1 [2.83] vs 5.0 [3.24])(Figure 1). The composite LSR score assessed over time based on the mean (SD) AUC was significantly lower in cycle 2 than in cycle 1 (40.5 [28.05] vs 83.6 [36.25])(P=.0002)(Table). Statistical differences in scores for individual reactions between the 2 cycles were not determined because of the risk for a spurious indication of significance from multiple comparisons in such a limited patient sample.
The percentage of participants who had a score greater than 1 for any of the 6 components of the LSR assessment was lower in cycle 2 than in cycle 1 at all of the assessed time points (Figure 2). In both cycles, the percentage of participants with an LSR score greater than 1 was highest 3 days after the application of the first dose in the cycle (day 4 or day 32, respectively). Erythema, flaking/scaling, and crusting were the most freq-uently observed reactions. At day 29, there were no participants with an LSR score greater than 1 in any of the 6 components. At day 29 and day 56, 94% (17/18) and 100% (18/18) of participants, respectively, had a score of 0 for all reactions.
 |
 |
The photographs in Figure 3, taken 7 days after the application of the first dose of ingenol mebutate gel 0.015% in each cycle of treatment of AK lesions on the face, show that there was less flaking/scaling and crusting in cycle 2 than in cycle 1. A review of participant photographs from the third treatment day of each cycle showed that the areas of erythema were the same in both cycles. The other 5 LSRs—flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were observed in different areas of the treated field in the 2 cycles when applicable.
Adverse Events
The few AEs that were reported were considered to be mild in severity. The AEs included application-site pain (n=5), application-site pruritus (n=3), and nasopharyngitis (n=1). No serious AEs were reported. After the first treatment cycle, 1 participant experienced hypopigmentation at the treatment site that persisted as faint hypopigmentation at the last study visit (day 56).
AK Lesion Count
The lesion count in all participants at baseline ranged from 4 to 8, with a mean (SD) of 5.9 (1.55). Mean lesion count was substantially reduced at the end of cycle 1 (0.9 [1.39]) and cycle 2 (0.3 [0.57]). The change in lesion count from baseline to day 56 was greater than the change from baseline to day 29 (-5.7 [1.61] vs -5.0 [1.57])(P=.0137). Complete clearance at day 29 and day 56 was achieved in 55.6% (10/18) and 77.8% (14/18) of participants, respectively. The difference in the clearance rate between day 29 and day 56 did not reach statistical significance, most likely due to the small sample size.
Participant-Reported Outcomes
 |
 |
Visual analog scale scores for participant-perceived irritation were less than 50 on a scale of 0 to 100 during both application cycles. At 1 day and 3 days after application of the first dose of ingenol mebutate gel 0.015% in cycle 1, the mean (SD) VAS scores for irritation were 31.8 (37.06) and 37.9 (30.77), respectively. At the same time points in cycle 2, VAS scores were 44.2 (32.45) and 49.6 (26.90), respectively. No information was available regarding resolution of participant-perceived irritation, as irritation data were not collected after day 4 of each treatment cycle; therefore, P values were not determined. Participant satisfaction with treatment was high and nearly the same at the end of cycles 1 and 2 (VAS scores: 83.7 [12.73] and 83.8 [20.46], respectively).
Comment
Our findings show that a second course of treatment with ingenol mebutate gel 0.015% on the same site on the face or scalp produced a less intense inflammatory reaction than the first course of treatment. Composite LSR scores at each time point after the start of treatment were lower in cycle 2 than in cycle 1. The percentage of participants who demonstrated a severity score greater than 1 for any of the 6 components of the LSR assessment also was lower at time points in cycle 2 than in cycle 1. These results are consistent with the hypothesis that the activity of ingenol mebutate includes a mechanism that specifically targets transformed keratinocytes, which are reduced by the start of a second cycle of treatment.
The mechanism for the clinical efficacy of ingenol mebutate has not been fully described. Studies in preclinical models suggest at least 2 components, including direct cytotoxic effects on tumor cells and a localized inflammatory reaction that includes protein kinase C activation.11 Ingenol mebutate preferentially induces death in tumor cells and in proliferating undifferentiated keratinocytes.7,12 Cell death and protein kinase C activation lead to an inflammatory response dominated by neutrophils and other immunocompetent cells that add to the destruction of transformed cells.11
The reduced inflammatory response observed in participants during the second cycle of treatment in this study is consistent with the theory of a preferential action on transformed keratinocytes by ingenol mebutate. Once transformed keratinocytes are substantially cleared in cycle 1, fewer target cells remain, and therefore the inflammatory response is less intense in cycle 2. If ingenol mebutate were uniformly cytotoxic and inflammatory to all cells, the LSR scores in both cycles would be expected to be similar.
Assessment of participant-perceived irritation supplemented the measurement of the 6 visible manifestations of inflammation over each 4-week cycle. Participant-perceived irritation was recorded early in the cycles at 1 and 3 days after the first dose. Although it is difficult to standardize patient perceptions, VAS scores for irritation in cycle 2 were higher than those reported in cycle 1, which suggests an increased perception of irritation. The clinical relevance of this perception is not certain and may be due to the small number of participants and/or the time interval between the 2 treatment courses.
The results of this study were limited by the small patient sample. Additionally, LSR assessments were limited by the quality of the photographs. However, LSRs and AK clearance rates were similar to the pooled findings seen in the phase 3 studies of ingenol mebutate.3 Adverse events were predominantly conditions that occurred at the application site, as in phase 3 studies.3 Similarly, the time course of LSR development and resolution followed the same pattern as in those trials. The peak composite LSR score for the face and scalp was approximately 9 in both the present study (cycle 1) and in the pooled phase 3 studies.3
Conclusion
Ingenol mebutate gel 0.015% may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over the course of 2 treatment cycles. Patients may experience fewer LSRs on reapplication of ingenol mebutate to a previously treated site.
Acknowledgment
Editorial support was provided by Tanya MacNeil, PhD, of p-value communications, LLC, Cedar Knolls, New Jersey.
Actinic keratoses (AKs) are common skin lesions resulting from cumulative exposure to UV radiation and are associated with an increased risk for invasive squamous cell carcinoma1; therefore, diagnosis and treatment are important.2 Individual AKs are most frequently treated with cryosurgery, while topical agents including ingenol mebutate gel are used as field treatments on areas of confluent AKs of sun-damaged skin.2,3 Studies have shown that rates of complete clearance with topical therapy can be improved with more than a single treatment course.4-6
Although the mechanisms of action of ingenol mebutate on AKs are not fully understood, studies indicate that it induces cell death in proliferating keratinocytes, which suggests that it may act preferentially on AKs and not on healthy skin.7 The field treatment of AKs of the face and scalp using ingenol mebutate gel 0.015% involves a 3-day regimen,8 and clearance rates are similar to those observed with topical agents that are used for longer periods of time.3,9,10 Local skin reactions (LSRs) associated with application of ingenol mebutate gel 0.015% on the face and scalp generally are mild to moderate in intensity and resolve after 2 weeks without sequelae.3
The presumption that the cytotoxic actions of ingenol mebutate affect proliferating keratinocytes preferentially was the basis for this study. We hypothesized that application of a second sequential cycle of ingenol mebutate during AK treatment should produce lower LSR scores than the first application cycle due to the specific elimination of transformed keratinocytes from the treatment area. This open-label study compared the intensity of LSRs during 2 sequential cycles of treatment on the same site of the face or scalp using ingenol mebutate gel 0.015%.
Methods
Study Population
Eligible participants were adults with 4 to 8 clinically typical, visible, nonhypertrophic AKs in a 25-cm2 contiguous area of the face or scalp. Inclusion and exclusion criteria were the same as in the pivotal studies.3 The study was approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (New York, New York). Enrollment took place from March 2013 to August 2013.
Study Design and Assessments
All participants were treated with 2 sequential 4-week cycles of ingenol mebutate gel 0.015% applied once daily for 3 consecutive days starting on the first day of each cycle (day 1 and day 29). Participants were evaluated at 11 visits (days 1, 2, 4, 8, 15, 29, 30, 32, 36, 43, and 56) during the 56-day study period (Figure 1). Eligibility, demographics, and medical history were assessed at day 1, and concomitant medications and adverse events (AEs) were evaluated at all visits. Using standardized photographic guides, 6 individual LSRs—erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were assessed on a scale of 0 (none) to 4 (severe), with higher numbers indicating more severe reactions. For each participant, a composite score was calculated as the sum of the individual LSR scores.3 Throughout the study, 3 qualified evaluators assessed AK lesion count and graded the LSRs. The same evaluator assessed both treatment courses for each participant for the majority of assessments.
 |

|
The primary end point of the study was to evaluate the degree of irritation in each of the 2 sequential cycles of ingenol mebutate treatment by assessing the mean area under the curve (AUC) of the composite LSR score over time following each of the 2 applications. Actinic keratoses were counted at baseline and at the end of each treatment cycle. The paired t test was used to compare AUCs of the composite LSR scores of the 2 cycles and to compare the changes in lesion counts from baseline to day 29 and from baseline to day 56. The complete clearance rates (number of participants with no AKs) at the end of cycles 1 and 2 were compared using a logistic regression model. Participant-perceived irritation and treatment satisfaction were evaluated using a 0 to 100 visual analog scale (VAS), with higher numbers indicating greater irritation and higher satisfaction. Participant-reported scores were summarized.
Results
Participant Characteristics
A total of 20 participants were enrolled in the study. At the completion of the study, 2 participants withdrew consent but allowed use of data from their completed assessments. Consequently, a total of 18 patients completed the entire study. The mean age was 75.35 years (median, 77.5 years; age range, 49–87 years). Most of the participants (15/20 [75%]) were men. All participants were white, and 2 were of Hispanic ethnicity. Of the 20 participants, 19 (95%) were Fitzpatrick skin type II, and 1 (5%) was Fitzpatrick skin type I. Most of the participants (16/20 [80%]) received treatment of lesions on the face. With the exception of 2 (10%) participants, all had received prior treatment of AKs, including cryosurgery (16/20 [80%]), imiquimod (5/20 [25%]), fluorouracil (2/20 [10%]), diclofenac (2/20 [10%]), and photodynamic therapy (2/20 [10%]); 8 (40%) participants had received more than 1 type of treatment.
LSRs in Cycles 1 and 2
The time course for the development and resolution of LSRs during both treatment cycles was similar. Local skin reactions were evident on day 2 in each cycle, peaked at 3 days after the application of the first dose, declined rapidly by the 15th day of the cycle, and returned to baseline by the end of each 4-week cycle (Figure 1). The mean (standard deviation [SD]) composite LSR score at 3 days after application of the first dose was higher in cycle 1 than in cycle 2 (9.1 [2.83] vs 5.0 [3.24])(Figure 1). The composite LSR score assessed over time based on the mean (SD) AUC was significantly lower in cycle 2 than in cycle 1 (40.5 [28.05] vs 83.6 [36.25])(P=.0002)(Table). Statistical differences in scores for individual reactions between the 2 cycles were not determined because of the risk for a spurious indication of significance from multiple comparisons in such a limited patient sample.
The percentage of participants who had a score greater than 1 for any of the 6 components of the LSR assessment was lower in cycle 2 than in cycle 1 at all of the assessed time points (Figure 2). In both cycles, the percentage of participants with an LSR score greater than 1 was highest 3 days after the application of the first dose in the cycle (day 4 or day 32, respectively). Erythema, flaking/scaling, and crusting were the most freq-uently observed reactions. At day 29, there were no participants with an LSR score greater than 1 in any of the 6 components. At day 29 and day 56, 94% (17/18) and 100% (18/18) of participants, respectively, had a score of 0 for all reactions.
 |
 |
The photographs in Figure 3, taken 7 days after the application of the first dose of ingenol mebutate gel 0.015% in each cycle of treatment of AK lesions on the face, show that there was less flaking/scaling and crusting in cycle 2 than in cycle 1. A review of participant photographs from the third treatment day of each cycle showed that the areas of erythema were the same in both cycles. The other 5 LSRs—flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were observed in different areas of the treated field in the 2 cycles when applicable.
Adverse Events
The few AEs that were reported were considered to be mild in severity. The AEs included application-site pain (n=5), application-site pruritus (n=3), and nasopharyngitis (n=1). No serious AEs were reported. After the first treatment cycle, 1 participant experienced hypopigmentation at the treatment site that persisted as faint hypopigmentation at the last study visit (day 56).
AK Lesion Count
The lesion count in all participants at baseline ranged from 4 to 8, with a mean (SD) of 5.9 (1.55). Mean lesion count was substantially reduced at the end of cycle 1 (0.9 [1.39]) and cycle 2 (0.3 [0.57]). The change in lesion count from baseline to day 56 was greater than the change from baseline to day 29 (-5.7 [1.61] vs -5.0 [1.57])(P=.0137). Complete clearance at day 29 and day 56 was achieved in 55.6% (10/18) and 77.8% (14/18) of participants, respectively. The difference in the clearance rate between day 29 and day 56 did not reach statistical significance, most likely due to the small sample size.
Participant-Reported Outcomes
 |
 |
Visual analog scale scores for participant-perceived irritation were less than 50 on a scale of 0 to 100 during both application cycles. At 1 day and 3 days after application of the first dose of ingenol mebutate gel 0.015% in cycle 1, the mean (SD) VAS scores for irritation were 31.8 (37.06) and 37.9 (30.77), respectively. At the same time points in cycle 2, VAS scores were 44.2 (32.45) and 49.6 (26.90), respectively. No information was available regarding resolution of participant-perceived irritation, as irritation data were not collected after day 4 of each treatment cycle; therefore, P values were not determined. Participant satisfaction with treatment was high and nearly the same at the end of cycles 1 and 2 (VAS scores: 83.7 [12.73] and 83.8 [20.46], respectively).
Comment
Our findings show that a second course of treatment with ingenol mebutate gel 0.015% on the same site on the face or scalp produced a less intense inflammatory reaction than the first course of treatment. Composite LSR scores at each time point after the start of treatment were lower in cycle 2 than in cycle 1. The percentage of participants who demonstrated a severity score greater than 1 for any of the 6 components of the LSR assessment also was lower at time points in cycle 2 than in cycle 1. These results are consistent with the hypothesis that the activity of ingenol mebutate includes a mechanism that specifically targets transformed keratinocytes, which are reduced by the start of a second cycle of treatment.
The mechanism for the clinical efficacy of ingenol mebutate has not been fully described. Studies in preclinical models suggest at least 2 components, including direct cytotoxic effects on tumor cells and a localized inflammatory reaction that includes protein kinase C activation.11 Ingenol mebutate preferentially induces death in tumor cells and in proliferating undifferentiated keratinocytes.7,12 Cell death and protein kinase C activation lead to an inflammatory response dominated by neutrophils and other immunocompetent cells that add to the destruction of transformed cells.11
The reduced inflammatory response observed in participants during the second cycle of treatment in this study is consistent with the theory of a preferential action on transformed keratinocytes by ingenol mebutate. Once transformed keratinocytes are substantially cleared in cycle 1, fewer target cells remain, and therefore the inflammatory response is less intense in cycle 2. If ingenol mebutate were uniformly cytotoxic and inflammatory to all cells, the LSR scores in both cycles would be expected to be similar.
Assessment of participant-perceived irritation supplemented the measurement of the 6 visible manifestations of inflammation over each 4-week cycle. Participant-perceived irritation was recorded early in the cycles at 1 and 3 days after the first dose. Although it is difficult to standardize patient perceptions, VAS scores for irritation in cycle 2 were higher than those reported in cycle 1, which suggests an increased perception of irritation. The clinical relevance of this perception is not certain and may be due to the small number of participants and/or the time interval between the 2 treatment courses.
The results of this study were limited by the small patient sample. Additionally, LSR assessments were limited by the quality of the photographs. However, LSRs and AK clearance rates were similar to the pooled findings seen in the phase 3 studies of ingenol mebutate.3 Adverse events were predominantly conditions that occurred at the application site, as in phase 3 studies.3 Similarly, the time course of LSR development and resolution followed the same pattern as in those trials. The peak composite LSR score for the face and scalp was approximately 9 in both the present study (cycle 1) and in the pooled phase 3 studies.3
Conclusion
Ingenol mebutate gel 0.015% may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over the course of 2 treatment cycles. Patients may experience fewer LSRs on reapplication of ingenol mebutate to a previously treated site.
Acknowledgment
Editorial support was provided by Tanya MacNeil, PhD, of p-value communications, LLC, Cedar Knolls, New Jersey.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
2. Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? part 1: overview and investigational topical agents. Cutis. 2012;89:241-250.
3. Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
4. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157:133-141.
5. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57:265-268.
6. Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2:20-28.
7. Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
8. Picato gel 0.015%, 0.05% [package insert]. Parsippany, NJ: LEO Pharma; 2013.
9. Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
10. Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62:582-590.
11. Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123-8132.
12. Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
2. Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? part 1: overview and investigational topical agents. Cutis. 2012;89:241-250.
3. Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
4. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157:133-141.
5. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57:265-268.
6. Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2:20-28.
7. Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
8. Picato gel 0.015%, 0.05% [package insert]. Parsippany, NJ: LEO Pharma; 2013.
9. Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
10. Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62:582-590.
11. Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123-8132.
12. Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
Practice Points
- Reapplication of ingenol mebutate gel 0.015% to the same treatment area on the face or scalp produced a less intense inflammatory reaction than the first treatment course.
- Ingenol mebutate may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over 2 treatment cycles.
- Almost all patients were either clear or almost clear of actinic keratosis lesions by 4 weeks following the second application of ingenol mebutate.