User login
Developing a Patient- and Family-Centered Research Agenda for Hospital Medicine: The Improving Hospital Outcomes through Patient Engagement (i-HOPE) Study
Thirty-six million people are hospitalized annually in the United States,1 and a significant proportion of these patients are rehospitalized within 30 days.2 Gaps in hospital care are many and well documented, including high rates of adverse events, hospital-acquired conditions, and suboptimal care transitions.3-5 Despite significant efforts to improve the care of hospitalized patients and some incremental improvement in the safety of hospital care, hospital care remains suboptimal.6-9 Importantly, hospitalization remains a challenging and vulnerable time for patients and caregivers.
Despite research efforts to improve hospital care, there remains very little data regarding what patients, caregivers, and other stakeholders believe are the most important priorities for improving hospital care, experiences, and outcomes. Small studies described in brief reports provide limited insights into what aspects of hospital care are most important to patients and to their families.10-13 These small studies suggest that communication and the comfort of caregivers and of patient family members are important priorities, as are the provision of adequate sleeping arrangements, food choices, and psychosocial support. However, the limited nature of these studies precludes the possibility of larger conclusions regarding patient priorities.10-13
The evolution of patient-centered care has led to increasing efforts to engage, and partner, with patients, caregivers, and other stakeholders to obtain their input on healthcare, research, and improvement efforts.14 The guiding principle of this engagement is that patients and their caregivers are uniquely positioned to share their lived experiences of care and that their involvement ensures their voices are represented.15-17 Therefore to obtain greater insight into priority areas from the perspectives of patients, caregivers, and other healthcare stakeholders, we undertook a systematic engagement process to create a patient-partnered and stakeholder-partnered research agenda for improving the care of hospitalized adult patients.
METHODS
Guiding Frameworks for Study Methods
We used two established, validated methods to guide our collaborative, inclusive, and consultative approach to patient and stakeholder engagement and research prioritization:
- The Patient-Centered Outcomes Research Institute (PCORI) standards for formulating patient-centered research questions,18 which includes methods for stakeholder engagement that ensures the representativeness of engaged groups and dissemination of study results.18
- The James Lind Alliance (JLA) approach to “priority setting partnerships,” through which patients, caregivers, and clinicians partner to identify and prioritize unanswered questions.19
The Improving Hospital Outcomes through Patient Engagement (i-HOPE) study included eight stepwise phases to formulate and prioritize a set of patient-centered research questions to improve the care and experiences of hospitalized patients and their families.20 Our process is described below and summarized in Table 1.
Phases of Question Development
Phase 1: Steering Committee Formation
Nine clinical researchers, nine patients and/or caregivers, and two administrators from eight academic and community hospitals from across the United States formed a steering committee to participate in teleconferences every other week to manage all stages of the project including design, implementation, and dissemination. At the time of the project conceptualization, the researchers were a subgroup of the Society of Hospital Medicine Research Committee.21 Patient partners on the steering committee were identified from local patient and family advisory councils (PFACs) of the researchers’ institutions. Patients partners had previously participated in research or improvement initiatives with their hospitalist partners. Patient partners received stipends throughout the project in recognition of their participation and expertise. Included in the committee was a representative from the Society of Hospital Medicine (SHM)—our supporting and dissemination partner.
Phase 2: Stakeholder Identification
We created a list of potential stakeholder organizations to participate in the study based on the following:
- Organizations with which SHM has worked on initiatives related to the care of hospitalized adult patients
- Organizations with which steering committee members had worked
- Internet searches of organizations participating in similar PCORI-funded projects and of other professional societies that represented patients or providers who work in hospital or post-acute care settings
- Suggestions from stakeholders identified through the first two approaches as described above
We intended to have a broad representation of stakeholders to ensure diverse perspectives were included in the study. Stakeholder organizations included patient advocacy groups, providers, researchers, payers, policy makers and funding agencies.
Phase 3: Stakeholder Engagement and Awareness Training
Representatives from 39 stakeholder organizations who agreed to participate in the study were further orientated to the study rationale and methods via a series of interactive online webinars. This included reminding organziations that everyone’s input and perspective were valued and that we had a flat organization structure that ensured all stakeholders were equal.
Phase 4: Survey Development and Administration
We chose a survey approach to solicit input on identifying gaps in patient care and to generate research questions. The steering committee developed an online survey collaboratively with stakeholder organization representatives. We used survey pretesting with patient and researcher members from the steering committee. The goal of pretesting was to ensure accessibility and comprehension for all potential respondents, particularly patients and caregivers. The final survey asked respondents to record up to three questions that they thought would improve the care of hospitalized adult patients and their families. The specific wording of the survey is shown in the Figure and the entire survey in Appendix Document 1.
We chose three questions because that is the number of entries per participant that is recommended by JLA; it also minimizes responder burden.19 We asked respondents to identify the stakeholder group they represented (eg, patient, caregiver, healthcare provider, researcher) and for providers to identify where they primarily worked (eg, acute care hospital, post-acute care, advocacy group).
Survey Administration. We administered the survey electronically using Research Electronic Data Capture (REDCap), a secure web-based application used for collecting research data.22 Stakeholders were asked to disseminate the survey broadly using whatever methods that they felt was appropriate to their leadership or members.
Phase 5: Initial Question Categorization Using Qualitative Content Analysis
Six members of the steering committee independently performed qualitative content analysis to categorize all submitted questions.23,24 This analytic approach identifies, analyzes, and reports patterns within the data.23,24 We hypothesized that some of the submitted questions would relate to already-known problems with hospitalization. Therefore the steering committee developed an a priori codebook of 48 categories using common systems-based issues and diseases related to the care of hospitalized patients based on the hospitalist core competency topics developed by hospitalists and the SHM Education Committee,25 personal and clinical knowledge and experience related to the care of hospitalized adult patients, and published literature on the topic. These a priori categories and their definitions are shown in Appendix Document 2 and were the basis for our initial theory-driven (deductive) approach to data analysis.23
Once coding began, we identified 32 new and additional categories based on our review of the submitted questions, and these were the basis of our data-driven (inductive) approach to analysis.23 All proposed new codes and definitions were discussed with and approved by the entire steering committee prior to being added to the codebook (Appendix Document 2).
While coding categories were mutually exclusive, multiple codes could be attributed to a question depending on the content and meaning of a question. To ensure methodological rigor, reviewers met regularly via teleconference or communicated via email throughout the analysis to iteratively refine and define coding categories. All questions were reviewed independently, and then discussed, by at least two members of the analysis team. Any coding disparities were discussed and resolved by negotiated consensus.26 Analysis was conducted using Dedoose V8.0.35 (Sociocultural Research Consultants, Los Angeles, California).
Phase 6: Initial Question Identification Using Quantitative Content Analysis
Following thematic categorization, all steering committee members then reviewed each category to identify and quantify the most commonly submitted questions.27 A question was determined to be a commonly submitted question when it appeared at least 10 times.
Phase 7: Interim Priority Setting
We sent the list of the most commonly submitted questions (Appendix Document 3) to stakeholder organizations and patient partner networks for review and evaluation. Each organization was asked to engage with their constituents and leaders to collectively decide on which of these questions resonated and was most important. These preferences would then be used during the in-person meeting (Phase 8). We did not provide stakeholder organizations with information about how many times each question was submitted by respondents because we felt this could potentially bias their decision-making processes such that true importance and relevance would not obtained.
Phase 8: In-person Meeting for Final Question Prioritization and Refinement
Representatives from all 39 participating stakeholder organizations were invited to participate in a 2-day, in-person meeting to create a final prioritized list of questions to be used to guide patient-centered research seeking to improve the care of hospitalized adult patients and their caregivers. This meeting was attended by 43 stakeholders (26 stakeholder organization representatives and 17 steering committee members) from 37 unique stakeholder organizations. To facilitate the inclusiveness and to ensure a consensus-driven process, we used nominal group technique (NGT) to allow all of the meeting participants to discuss the list of prioritized questions in small groups.28 NGT allows participants to comprehend each other’s point of view to ensure no perpsectives are excluded.28 The NGT was followed by two rounds of individual voting. Stakeholders were then asked to frame their discussions and their votes based on the perspectives of their organizations or PFACs that they represent. The voting process required participants to make choices regarding the relative importance of all of the questions, which therefore makes the resulting list a true prioritized list. In the first round of voting, participants voted for up to five questions for inclusion on the prioritized list. Based on the distribution of votes, where one vote equals one point, each of the 36 questions was then ranked in order of the assigned points. The rank-ordering process resulted in a natural cut point or delineated point, resulting in the 11 questions considered to be the highest prioritized questions. Following this, a second round of voting took place with the same parameters as the first round and allowed us to rank order questions by order of importance and priority. Finally, during small and large group discussions, the original text of each question was edited, refined, and reformatted into questions that could drive a research agenda.
Ethical Oversight
This study was reviewed by the Institutional Review Board of the University of Texas Health Science Center at San Antonio and deemed not to be human subject research (UT Health San Antonio IRB Protocol Number: HSC20170058N).
RESULTS
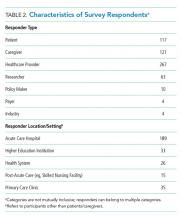
In total, 499 respondents from 39 unique stakeholder organizations responded to our survey. Respondents self-identified into multiple categorizes resulting in 267 healthcare providers, 244 patients and caregivers, and 63 researchers. Characteristics of respondents to the survey are shown in Table 2.
An overview of study results is shown in Table 1. Respondents submitted a total of 782 questions related to improving the care of hospitalized patients. These questions were categorized during thematic analysis into 70 distinct categories—52 that were health system related and 18 that were disease specific (Appendix 2). The most frequently used health system–related categories were related to discharge care transitions, medications, patient understanding, and patient-family-care team communication (Appendix 2).
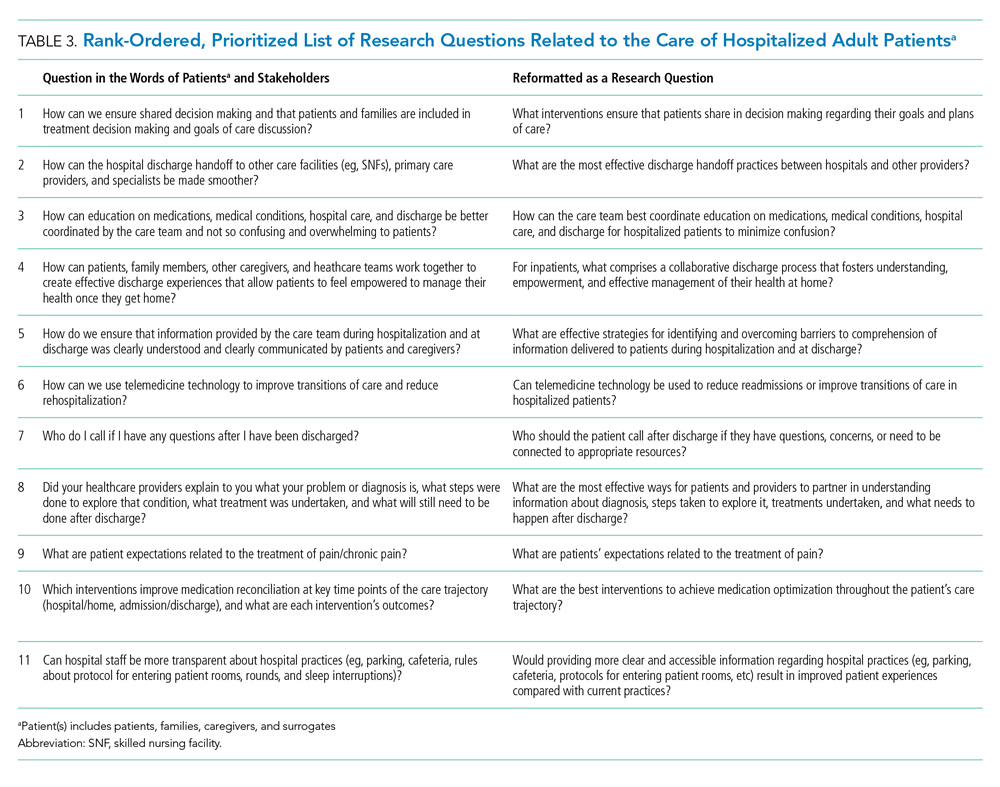
From these categories, 36 questions met our criteria for “commonly identified,” ie, submitted at least 10 times (Appendix Document 3). Notably, these 36 questions were derived from 67 different coding categories, of which 24 (36%) were a priori (theory-driven) categories23 created by the Steering Committee before analysis began and 43 (64%) categories were created as a result of this study’s stakeholder-engaged process and a data-driven approach23 to analysis (Appendix Document 3). These groups of questions were then presented during the 2-day, in-person meeting and reduced to a final 11 questions that were identified in rank order as top priorities (Table 3). The questions considered highest priority related to ensuring shared treatment and goals of care decision making, improving hospital discharge handoff to other care facilities and providers, and reducing the confusion related to education on medications, conditions, hospital care, and discharge.
DISCUSSION
Using a dynamic and collaborative stakeholder engagement process, we identified 11 questions prioritized in order of importance by patients, caregivers, and other healthcare stakeholders to improve the care of hospitalized adult patients. While some of the topics identified are already well-known topics in need of research and improvement, our findings frame these topics according to the perspectives of patients, caregivers, and stakeholders. This unique perspective adds a level of richness and nuance that provides insight into how to better address these topics and ultimately inform research and quality improvement efforts.
The question considered to be the highest priority area for future research and improvement surmised how it may be possible to implement interventions that engage patients in shared decision making. Shared decision making involves patients and their care team working together to make decisions about treatment, and other aspects of care based on sound clinical evidence that balances the risks and outcomes with patient preferences and values. Although considered critically important,29 a recent evaluation of shared decision making practices in over 250 inpatient encounters identified significant gaps in physicians’ abilities to perform key elements of a shared decision making approach and reinforced the need to identify what strategies can best promote widespread shared decision making.30 While there has been considerable effort to faciliate shared decision making in practice, there remains mixed evidence regarding the sustainability and impact of tools seeking to support shared decision making, such as decision aids, question prompt lists, and coaches.31 This suggests that new approaches to shared decision making may be required and likely explains why this question was rated as a top priority by stakeholders in the current study.
Respondents frequently framed their questions in terms of their lived experiences, providing stories and scenarios to illustrate the importance of the questions they submitted. This personal framing highlighted to us the need to think about improving care delivery from the end-user perspective. For example, respondents framed questions about care transitions not with regard to early appointments, instructions, or medication lists, but rather in terms of whom to call with questions or how best to reach their physician, nurse, or other identified provider. These perspectives suggest that strategies and approaches to improvement that start with patient and caregiver experiences, such as design thinking,32 may be important to continued efforts to improve hospital care. Additionally, the focus on the interpersonal aspects of care delivery highlights the need to focus on the patient-provider relationship and communication.
Questions submitted by respondents demonstrated a stark difference between “patient education” and “patient understanding,” which suggests that being provided with education or education materials regarding care did not necessarily lead to a clear patient understanding. The potential for lack of understanding was particularly prominent in the context of care plan development and during times of care transition—topics that were encompassed in 9 out of 11 of our prioritized research questions. This may suggest that approaches that improve the ability for healthcare providers to deliver information may not be sufficient to meet the needs of patients and caregivers. Rather, partnering to develop a shared understanding—whether about prognosis, medications, hospital, or discharge care plans—is critical. Improved communication practices are not an endpoint for information delivery, but rather a starting point leading to a shared understanding.
Several of the priority areas identified in our study reflect the immensely complex intersections among patients, caregivers, clinicians, and the healthcare delivery system. Addressing these gaps in order to reach the goal of ideal hospital care and an improved patient experience will likely require coordinated approaches and strong involvement and buy-in from multiple stakeholders including the voices of patients and caregivers. Creating patient-centered and stakeholder-driven research has been an increasing priority nationally.33 Yet to realize this, we must continue to understand the foundations and best practices of authentic stakeholder engagement so that it can be achieved in practice.34 We intend for this prioritized list of questions to galvanize funders, researchers, clinicians, professional societies, and patient and caregiver advocacy groups to work together to address these topics through the creation of new research evidence or the sustainable implementation of existing evidence.
Our findings provide a foundation for stakeholder groups to work in partnership to find research and improvement solutions to the problems identified. Our efforts demonstrate the value and importance of a systematic and broad engagement process to ensure that the voices of patients, caregivers, and other healthcare stakeholders are included in guiding hospital research and quality improvement efforts. This is highlighted by the fact our results of prioritized category areas for research were largely only uncovered following the creation of coding categories during the analysis process and were not captured using a priori catgeories that were expected by the steering committee.
The strengths of this study include our attempts to systematically identify and engage a wide range of perspectives in hospital medicine, including perspectives from patients and their caregivers. There are also acknowledged limitations in our study. While we included patients and PFACs from across the country, the opinions of the people we included may not be representative of all patients. Similarly, the perspectives of the other participants may not have completely represented their stakeholder organizations. While we attempted to include a broad range of organizations, there may be other relevant groups who were not represented in our sample.
In summary, our findings provide direction for the multiple stakeholders involved in improving hospital care. The results will allow the research community to focus on questions that are most important to patients, caregivers, and other stakeholders, reframing them in ways that are more relevant to patients’ lived experiences and that reflect the complexity of the issues. Our findings can also be used by healthcare providers and delivery organizations to target local improvement efforts. We hope that patients and caregivers will use our results to advocate for research and improvement in areas that matter the most to them. We hope that policy makers and funding agencies use our results to promote work in these areas and drive a national conversation about how to most effectively improve hospital care.
Acknowledgments
The authors would like to thank all patients, caregivers, and stakeholders who completed the survey. The authors also would like to acknowledge the organizations and individuals who participated in this study (see Appendix Document 4 for full list). At SHM, the authors would like to specifically thank Claudia Stahl, Jenna Goldstein, Kevin Vuernick, Dr Brad Sharpe, and Dr Larry Wellikson for their support.
Disclaimer
The statements presented in this publication are solely the responsibility of the authors and do not necessarily represent the views of the Department of Veterans Affairs, Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
1. American Hospital Association. 2019 American Hospital Association Hospital Statistics. Chicago, Illinois: American Hospital Association; 2019.
2. Alper E, O’Malley T, Greenwald J. UptoDate: Hospital discharge and readmission. https://www.uptodate.com/contents/hospital-discharge-and-readmission. Accessed August 8, 2019.
3. de Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: a systematic review. Qual Saf Heal Care. 2008;17(3):216-223. https://doi.org/10.1136/qshc.2007.023622.
4. Agency for Healthcare Research and Quality. Readmissions and Adverse Events After Discharge. https://psnet.ahrq.gov/primers/primer/11/Readmissions-and-Adverse-Events-After-Discharge. Accessed August 8, 2019.
5. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC; National Academies Press; 2001. https://doi.org/10.17226/10027.
6. Trivedi AN, Nsa W, Hausmann LRM, et al. Quality and equity of care in U.S. hospitals. N Engl J Med. 2014;371(24):2298-2308. https://doi.org/10.1056/NEJMsa1405003.
7. National Patient Safety Foundation. Free from Harm: Accelerating Patient Safety Improvement Fifteen Years after To Err Is Human. Boston: National Patient Safety Foundation; 2015.
8. Agency for Healthcare Research and Quality. AHRQ National Scorecard on Hospital-Acquired Conditions Updated Baseline Rates and Preliminary Results 2014–2017. https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/pfp/hacreport-2019.pdf. Accessed August 8, 2019.
9. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. https://doi.org/10.1002/jhm.2054.
10. Snyder HJ, Fletcher KE. The hospital experience through the patients’ eyes. J Patient Exp. 2019. https://doi.org/10.1177/2374373519843056.
11. Kebede S, Shihab HM, Berger ZD, Shah NG, Yeh H-C, Brotman DJ. Patients’ understanding of their hospitalizations and association with satisfaction. JAMA Intern Med. 2014;174(10):1698-1700. https://doi.org/10.1001/jamainternmed.2014.3765.
12. Shoeb M, Merel SE, Jackson MB, Anawalt BD. “Can we just stop and talk?” patients value verbal communication about discharge care plans. J Hosp Med. 2012;7(6):504-507. https://doi.org/10.1002/jhm.1937.
13. Neeman N, Quinn K, Shoeb M, Mourad M, Sehgal NL, Sliwka D. Postdischarge focus groups to improve the hospital experience. Am J Med Qual. 2013;28(6):536-538. https://doi.org/10.1177/1062860613488623.
14. Duffett L. Patient engagement: what partnering with patients in research is all about. Thromb Res. 2017;150:113-120. https://doi.org/10.1016/j.thromres.2016.10.029.
15. Pomey M, Hihat H, Khalifa M, Lebel P, Neron A, Dumez V. Patient partnership in quality improvement of healthcare services: patients’ inputs and challenges faced. Patient Exp J. 2015;2:29-42. https://doi.org/10.35680/2372-0247.1064.
16. Robbins M, Tufte J, Hsu C. Learning to “swim” with the experts: experiences of two patient co-investigators for a project funded by the Patient-Centered Outcomes Research Institute. Perm J. 2016;20(2):85-88. https://doi.org/10.7812/TPP/15-162.
17. Tai-Seale M, Sullivan G, Cheney A, Thomas K, Frosch D. The language of engagement: “aha!” moments from engaging patients and community partners in two pilot projects of the Patient-Centered Outcomes Research Institute. Perm J. 2016;20(2):89-92. https://doi.org/10.7812/TPP/15-123.
18. Patient-Centered Outcomes Research Institute (PCORI). PCORI Methodology Standards: Standards for Formulating Research Questions. https://www.pcori.org/research-results/about-our-research/research-methodology/pcori-methodology-standards#Formulating Research Questions. Accessed August 8, 2019.
19. James Lind Alliance. The James Lind Alliance Guidebook. Version 8. Southampton, England: James Lind Alliance; 2018.
20. Society of Hospital Medicine (SHM). Improving Hospital Outcomes through Patient Engagement: The i-HOPE Study. https://www.hospitalmedicine.org/clinical-topics/i-hope-study/. Accessed August 8, 2019.
21. Society of Hospital Medicine (SHM). Committees. https://www.hospitalmedicine.org/membership/committees/. Accessed August 8, 2019.
22. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
23. Schreier M. Qualitative content analysis in practice. Los Angeles, CA: SAGE Publications; 2012.
24. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x.
25. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine—2017 revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715.
26. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758-1772. https://doi.org/10.1111/j.1475-6773.2006.00684.x.
27. Coe K, Scacco JM. Content analysis, quantitative. Int Encycl Commun Res Methods. 2017:1-11. https://doi.org/10.1002/9781118901731.iecrm0045.
28. Centers for Disease Control and Prevention. Evaluation Briefs: Gaining Consensus Among Stakeholders Through the Nominal Group Technique. Atlanta, GA; 2018. https://www.cdc.gov/healthyyouth/evaluation/pdf/brief7.pdf. Accessed August 8, 2019.
29. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692. https://doi.org/10.1016/s0277-9536(96)00221-3.
30. Blankenburg R, Hilton JF, Yuan P, et al. Shared decision-making during inpatient rounds: opportunities for improvement in patient engagement and communication. J Hosp Med. 2018;13(7):453-461. https://doi.org/10.12788/jhm.2909.
31. Legare F, Adekpedjou R, Stacey D, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2018;7(7):CD006732. https://doi.org/10.1002/14651858.CD006732.pub4.
32. Roberts JP, Fisher TR, Trowbridge MJ, Bent C. A design thinking framework for healthcare management and innovation. Healthc (Amst). 2016;4(1):11-14. https://doi.org/10.1016/j.hjdsi.2015.12.002.
33. Selby JV, Beal AC, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA. 2012;307(15):1583-1584. https://doi.org/10.1001/jama.2012.500.
34. Harrison J, Auerbach A, Anderson W, et al. Patient stakeholder engagement in research: a narrative review to describe foundational principles and best practice activities. Health Expect. 2019;22(3):307-316. https://doi.org/10.1111/hex.12873.
Thirty-six million people are hospitalized annually in the United States,1 and a significant proportion of these patients are rehospitalized within 30 days.2 Gaps in hospital care are many and well documented, including high rates of adverse events, hospital-acquired conditions, and suboptimal care transitions.3-5 Despite significant efforts to improve the care of hospitalized patients and some incremental improvement in the safety of hospital care, hospital care remains suboptimal.6-9 Importantly, hospitalization remains a challenging and vulnerable time for patients and caregivers.
Despite research efforts to improve hospital care, there remains very little data regarding what patients, caregivers, and other stakeholders believe are the most important priorities for improving hospital care, experiences, and outcomes. Small studies described in brief reports provide limited insights into what aspects of hospital care are most important to patients and to their families.10-13 These small studies suggest that communication and the comfort of caregivers and of patient family members are important priorities, as are the provision of adequate sleeping arrangements, food choices, and psychosocial support. However, the limited nature of these studies precludes the possibility of larger conclusions regarding patient priorities.10-13
The evolution of patient-centered care has led to increasing efforts to engage, and partner, with patients, caregivers, and other stakeholders to obtain their input on healthcare, research, and improvement efforts.14 The guiding principle of this engagement is that patients and their caregivers are uniquely positioned to share their lived experiences of care and that their involvement ensures their voices are represented.15-17 Therefore to obtain greater insight into priority areas from the perspectives of patients, caregivers, and other healthcare stakeholders, we undertook a systematic engagement process to create a patient-partnered and stakeholder-partnered research agenda for improving the care of hospitalized adult patients.
METHODS
Guiding Frameworks for Study Methods
We used two established, validated methods to guide our collaborative, inclusive, and consultative approach to patient and stakeholder engagement and research prioritization:
- The Patient-Centered Outcomes Research Institute (PCORI) standards for formulating patient-centered research questions,18 which includes methods for stakeholder engagement that ensures the representativeness of engaged groups and dissemination of study results.18
- The James Lind Alliance (JLA) approach to “priority setting partnerships,” through which patients, caregivers, and clinicians partner to identify and prioritize unanswered questions.19
The Improving Hospital Outcomes through Patient Engagement (i-HOPE) study included eight stepwise phases to formulate and prioritize a set of patient-centered research questions to improve the care and experiences of hospitalized patients and their families.20 Our process is described below and summarized in Table 1.
Phases of Question Development
Phase 1: Steering Committee Formation
Nine clinical researchers, nine patients and/or caregivers, and two administrators from eight academic and community hospitals from across the United States formed a steering committee to participate in teleconferences every other week to manage all stages of the project including design, implementation, and dissemination. At the time of the project conceptualization, the researchers were a subgroup of the Society of Hospital Medicine Research Committee.21 Patient partners on the steering committee were identified from local patient and family advisory councils (PFACs) of the researchers’ institutions. Patients partners had previously participated in research or improvement initiatives with their hospitalist partners. Patient partners received stipends throughout the project in recognition of their participation and expertise. Included in the committee was a representative from the Society of Hospital Medicine (SHM)—our supporting and dissemination partner.
Phase 2: Stakeholder Identification
We created a list of potential stakeholder organizations to participate in the study based on the following:
- Organizations with which SHM has worked on initiatives related to the care of hospitalized adult patients
- Organizations with which steering committee members had worked
- Internet searches of organizations participating in similar PCORI-funded projects and of other professional societies that represented patients or providers who work in hospital or post-acute care settings
- Suggestions from stakeholders identified through the first two approaches as described above
We intended to have a broad representation of stakeholders to ensure diverse perspectives were included in the study. Stakeholder organizations included patient advocacy groups, providers, researchers, payers, policy makers and funding agencies.
Phase 3: Stakeholder Engagement and Awareness Training
Representatives from 39 stakeholder organizations who agreed to participate in the study were further orientated to the study rationale and methods via a series of interactive online webinars. This included reminding organziations that everyone’s input and perspective were valued and that we had a flat organization structure that ensured all stakeholders were equal.
Phase 4: Survey Development and Administration
We chose a survey approach to solicit input on identifying gaps in patient care and to generate research questions. The steering committee developed an online survey collaboratively with stakeholder organization representatives. We used survey pretesting with patient and researcher members from the steering committee. The goal of pretesting was to ensure accessibility and comprehension for all potential respondents, particularly patients and caregivers. The final survey asked respondents to record up to three questions that they thought would improve the care of hospitalized adult patients and their families. The specific wording of the survey is shown in the Figure and the entire survey in Appendix Document 1.
We chose three questions because that is the number of entries per participant that is recommended by JLA; it also minimizes responder burden.19 We asked respondents to identify the stakeholder group they represented (eg, patient, caregiver, healthcare provider, researcher) and for providers to identify where they primarily worked (eg, acute care hospital, post-acute care, advocacy group).
Survey Administration. We administered the survey electronically using Research Electronic Data Capture (REDCap), a secure web-based application used for collecting research data.22 Stakeholders were asked to disseminate the survey broadly using whatever methods that they felt was appropriate to their leadership or members.
Phase 5: Initial Question Categorization Using Qualitative Content Analysis
Six members of the steering committee independently performed qualitative content analysis to categorize all submitted questions.23,24 This analytic approach identifies, analyzes, and reports patterns within the data.23,24 We hypothesized that some of the submitted questions would relate to already-known problems with hospitalization. Therefore the steering committee developed an a priori codebook of 48 categories using common systems-based issues and diseases related to the care of hospitalized patients based on the hospitalist core competency topics developed by hospitalists and the SHM Education Committee,25 personal and clinical knowledge and experience related to the care of hospitalized adult patients, and published literature on the topic. These a priori categories and their definitions are shown in Appendix Document 2 and were the basis for our initial theory-driven (deductive) approach to data analysis.23
Once coding began, we identified 32 new and additional categories based on our review of the submitted questions, and these were the basis of our data-driven (inductive) approach to analysis.23 All proposed new codes and definitions were discussed with and approved by the entire steering committee prior to being added to the codebook (Appendix Document 2).
While coding categories were mutually exclusive, multiple codes could be attributed to a question depending on the content and meaning of a question. To ensure methodological rigor, reviewers met regularly via teleconference or communicated via email throughout the analysis to iteratively refine and define coding categories. All questions were reviewed independently, and then discussed, by at least two members of the analysis team. Any coding disparities were discussed and resolved by negotiated consensus.26 Analysis was conducted using Dedoose V8.0.35 (Sociocultural Research Consultants, Los Angeles, California).
Phase 6: Initial Question Identification Using Quantitative Content Analysis
Following thematic categorization, all steering committee members then reviewed each category to identify and quantify the most commonly submitted questions.27 A question was determined to be a commonly submitted question when it appeared at least 10 times.
Phase 7: Interim Priority Setting
We sent the list of the most commonly submitted questions (Appendix Document 3) to stakeholder organizations and patient partner networks for review and evaluation. Each organization was asked to engage with their constituents and leaders to collectively decide on which of these questions resonated and was most important. These preferences would then be used during the in-person meeting (Phase 8). We did not provide stakeholder organizations with information about how many times each question was submitted by respondents because we felt this could potentially bias their decision-making processes such that true importance and relevance would not obtained.
Phase 8: In-person Meeting for Final Question Prioritization and Refinement
Representatives from all 39 participating stakeholder organizations were invited to participate in a 2-day, in-person meeting to create a final prioritized list of questions to be used to guide patient-centered research seeking to improve the care of hospitalized adult patients and their caregivers. This meeting was attended by 43 stakeholders (26 stakeholder organization representatives and 17 steering committee members) from 37 unique stakeholder organizations. To facilitate the inclusiveness and to ensure a consensus-driven process, we used nominal group technique (NGT) to allow all of the meeting participants to discuss the list of prioritized questions in small groups.28 NGT allows participants to comprehend each other’s point of view to ensure no perpsectives are excluded.28 The NGT was followed by two rounds of individual voting. Stakeholders were then asked to frame their discussions and their votes based on the perspectives of their organizations or PFACs that they represent. The voting process required participants to make choices regarding the relative importance of all of the questions, which therefore makes the resulting list a true prioritized list. In the first round of voting, participants voted for up to five questions for inclusion on the prioritized list. Based on the distribution of votes, where one vote equals one point, each of the 36 questions was then ranked in order of the assigned points. The rank-ordering process resulted in a natural cut point or delineated point, resulting in the 11 questions considered to be the highest prioritized questions. Following this, a second round of voting took place with the same parameters as the first round and allowed us to rank order questions by order of importance and priority. Finally, during small and large group discussions, the original text of each question was edited, refined, and reformatted into questions that could drive a research agenda.
Ethical Oversight
This study was reviewed by the Institutional Review Board of the University of Texas Health Science Center at San Antonio and deemed not to be human subject research (UT Health San Antonio IRB Protocol Number: HSC20170058N).
RESULTS
In total, 499 respondents from 39 unique stakeholder organizations responded to our survey. Respondents self-identified into multiple categorizes resulting in 267 healthcare providers, 244 patients and caregivers, and 63 researchers. Characteristics of respondents to the survey are shown in Table 2.
An overview of study results is shown in Table 1. Respondents submitted a total of 782 questions related to improving the care of hospitalized patients. These questions were categorized during thematic analysis into 70 distinct categories—52 that were health system related and 18 that were disease specific (Appendix 2). The most frequently used health system–related categories were related to discharge care transitions, medications, patient understanding, and patient-family-care team communication (Appendix 2).
From these categories, 36 questions met our criteria for “commonly identified,” ie, submitted at least 10 times (Appendix Document 3). Notably, these 36 questions were derived from 67 different coding categories, of which 24 (36%) were a priori (theory-driven) categories23 created by the Steering Committee before analysis began and 43 (64%) categories were created as a result of this study’s stakeholder-engaged process and a data-driven approach23 to analysis (Appendix Document 3). These groups of questions were then presented during the 2-day, in-person meeting and reduced to a final 11 questions that were identified in rank order as top priorities (Table 3). The questions considered highest priority related to ensuring shared treatment and goals of care decision making, improving hospital discharge handoff to other care facilities and providers, and reducing the confusion related to education on medications, conditions, hospital care, and discharge.

DISCUSSION
Using a dynamic and collaborative stakeholder engagement process, we identified 11 questions prioritized in order of importance by patients, caregivers, and other healthcare stakeholders to improve the care of hospitalized adult patients. While some of the topics identified are already well-known topics in need of research and improvement, our findings frame these topics according to the perspectives of patients, caregivers, and stakeholders. This unique perspective adds a level of richness and nuance that provides insight into how to better address these topics and ultimately inform research and quality improvement efforts.
The question considered to be the highest priority area for future research and improvement surmised how it may be possible to implement interventions that engage patients in shared decision making. Shared decision making involves patients and their care team working together to make decisions about treatment, and other aspects of care based on sound clinical evidence that balances the risks and outcomes with patient preferences and values. Although considered critically important,29 a recent evaluation of shared decision making practices in over 250 inpatient encounters identified significant gaps in physicians’ abilities to perform key elements of a shared decision making approach and reinforced the need to identify what strategies can best promote widespread shared decision making.30 While there has been considerable effort to faciliate shared decision making in practice, there remains mixed evidence regarding the sustainability and impact of tools seeking to support shared decision making, such as decision aids, question prompt lists, and coaches.31 This suggests that new approaches to shared decision making may be required and likely explains why this question was rated as a top priority by stakeholders in the current study.
Respondents frequently framed their questions in terms of their lived experiences, providing stories and scenarios to illustrate the importance of the questions they submitted. This personal framing highlighted to us the need to think about improving care delivery from the end-user perspective. For example, respondents framed questions about care transitions not with regard to early appointments, instructions, or medication lists, but rather in terms of whom to call with questions or how best to reach their physician, nurse, or other identified provider. These perspectives suggest that strategies and approaches to improvement that start with patient and caregiver experiences, such as design thinking,32 may be important to continued efforts to improve hospital care. Additionally, the focus on the interpersonal aspects of care delivery highlights the need to focus on the patient-provider relationship and communication.
Questions submitted by respondents demonstrated a stark difference between “patient education” and “patient understanding,” which suggests that being provided with education or education materials regarding care did not necessarily lead to a clear patient understanding. The potential for lack of understanding was particularly prominent in the context of care plan development and during times of care transition—topics that were encompassed in 9 out of 11 of our prioritized research questions. This may suggest that approaches that improve the ability for healthcare providers to deliver information may not be sufficient to meet the needs of patients and caregivers. Rather, partnering to develop a shared understanding—whether about prognosis, medications, hospital, or discharge care plans—is critical. Improved communication practices are not an endpoint for information delivery, but rather a starting point leading to a shared understanding.
Several of the priority areas identified in our study reflect the immensely complex intersections among patients, caregivers, clinicians, and the healthcare delivery system. Addressing these gaps in order to reach the goal of ideal hospital care and an improved patient experience will likely require coordinated approaches and strong involvement and buy-in from multiple stakeholders including the voices of patients and caregivers. Creating patient-centered and stakeholder-driven research has been an increasing priority nationally.33 Yet to realize this, we must continue to understand the foundations and best practices of authentic stakeholder engagement so that it can be achieved in practice.34 We intend for this prioritized list of questions to galvanize funders, researchers, clinicians, professional societies, and patient and caregiver advocacy groups to work together to address these topics through the creation of new research evidence or the sustainable implementation of existing evidence.
Our findings provide a foundation for stakeholder groups to work in partnership to find research and improvement solutions to the problems identified. Our efforts demonstrate the value and importance of a systematic and broad engagement process to ensure that the voices of patients, caregivers, and other healthcare stakeholders are included in guiding hospital research and quality improvement efforts. This is highlighted by the fact our results of prioritized category areas for research were largely only uncovered following the creation of coding categories during the analysis process and were not captured using a priori catgeories that were expected by the steering committee.
The strengths of this study include our attempts to systematically identify and engage a wide range of perspectives in hospital medicine, including perspectives from patients and their caregivers. There are also acknowledged limitations in our study. While we included patients and PFACs from across the country, the opinions of the people we included may not be representative of all patients. Similarly, the perspectives of the other participants may not have completely represented their stakeholder organizations. While we attempted to include a broad range of organizations, there may be other relevant groups who were not represented in our sample.
In summary, our findings provide direction for the multiple stakeholders involved in improving hospital care. The results will allow the research community to focus on questions that are most important to patients, caregivers, and other stakeholders, reframing them in ways that are more relevant to patients’ lived experiences and that reflect the complexity of the issues. Our findings can also be used by healthcare providers and delivery organizations to target local improvement efforts. We hope that patients and caregivers will use our results to advocate for research and improvement in areas that matter the most to them. We hope that policy makers and funding agencies use our results to promote work in these areas and drive a national conversation about how to most effectively improve hospital care.
Acknowledgments
The authors would like to thank all patients, caregivers, and stakeholders who completed the survey. The authors also would like to acknowledge the organizations and individuals who participated in this study (see Appendix Document 4 for full list). At SHM, the authors would like to specifically thank Claudia Stahl, Jenna Goldstein, Kevin Vuernick, Dr Brad Sharpe, and Dr Larry Wellikson for their support.
Disclaimer
The statements presented in this publication are solely the responsibility of the authors and do not necessarily represent the views of the Department of Veterans Affairs, Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
Thirty-six million people are hospitalized annually in the United States,1 and a significant proportion of these patients are rehospitalized within 30 days.2 Gaps in hospital care are many and well documented, including high rates of adverse events, hospital-acquired conditions, and suboptimal care transitions.3-5 Despite significant efforts to improve the care of hospitalized patients and some incremental improvement in the safety of hospital care, hospital care remains suboptimal.6-9 Importantly, hospitalization remains a challenging and vulnerable time for patients and caregivers.
Despite research efforts to improve hospital care, there remains very little data regarding what patients, caregivers, and other stakeholders believe are the most important priorities for improving hospital care, experiences, and outcomes. Small studies described in brief reports provide limited insights into what aspects of hospital care are most important to patients and to their families.10-13 These small studies suggest that communication and the comfort of caregivers and of patient family members are important priorities, as are the provision of adequate sleeping arrangements, food choices, and psychosocial support. However, the limited nature of these studies precludes the possibility of larger conclusions regarding patient priorities.10-13
The evolution of patient-centered care has led to increasing efforts to engage, and partner, with patients, caregivers, and other stakeholders to obtain their input on healthcare, research, and improvement efforts.14 The guiding principle of this engagement is that patients and their caregivers are uniquely positioned to share their lived experiences of care and that their involvement ensures their voices are represented.15-17 Therefore to obtain greater insight into priority areas from the perspectives of patients, caregivers, and other healthcare stakeholders, we undertook a systematic engagement process to create a patient-partnered and stakeholder-partnered research agenda for improving the care of hospitalized adult patients.
METHODS
Guiding Frameworks for Study Methods
We used two established, validated methods to guide our collaborative, inclusive, and consultative approach to patient and stakeholder engagement and research prioritization:
- The Patient-Centered Outcomes Research Institute (PCORI) standards for formulating patient-centered research questions,18 which includes methods for stakeholder engagement that ensures the representativeness of engaged groups and dissemination of study results.18
- The James Lind Alliance (JLA) approach to “priority setting partnerships,” through which patients, caregivers, and clinicians partner to identify and prioritize unanswered questions.19
The Improving Hospital Outcomes through Patient Engagement (i-HOPE) study included eight stepwise phases to formulate and prioritize a set of patient-centered research questions to improve the care and experiences of hospitalized patients and their families.20 Our process is described below and summarized in Table 1.
Phases of Question Development
Phase 1: Steering Committee Formation
Nine clinical researchers, nine patients and/or caregivers, and two administrators from eight academic and community hospitals from across the United States formed a steering committee to participate in teleconferences every other week to manage all stages of the project including design, implementation, and dissemination. At the time of the project conceptualization, the researchers were a subgroup of the Society of Hospital Medicine Research Committee.21 Patient partners on the steering committee were identified from local patient and family advisory councils (PFACs) of the researchers’ institutions. Patients partners had previously participated in research or improvement initiatives with their hospitalist partners. Patient partners received stipends throughout the project in recognition of their participation and expertise. Included in the committee was a representative from the Society of Hospital Medicine (SHM)—our supporting and dissemination partner.
Phase 2: Stakeholder Identification
We created a list of potential stakeholder organizations to participate in the study based on the following:
- Organizations with which SHM has worked on initiatives related to the care of hospitalized adult patients
- Organizations with which steering committee members had worked
- Internet searches of organizations participating in similar PCORI-funded projects and of other professional societies that represented patients or providers who work in hospital or post-acute care settings
- Suggestions from stakeholders identified through the first two approaches as described above
We intended to have a broad representation of stakeholders to ensure diverse perspectives were included in the study. Stakeholder organizations included patient advocacy groups, providers, researchers, payers, policy makers and funding agencies.
Phase 3: Stakeholder Engagement and Awareness Training
Representatives from 39 stakeholder organizations who agreed to participate in the study were further orientated to the study rationale and methods via a series of interactive online webinars. This included reminding organziations that everyone’s input and perspective were valued and that we had a flat organization structure that ensured all stakeholders were equal.
Phase 4: Survey Development and Administration
We chose a survey approach to solicit input on identifying gaps in patient care and to generate research questions. The steering committee developed an online survey collaboratively with stakeholder organization representatives. We used survey pretesting with patient and researcher members from the steering committee. The goal of pretesting was to ensure accessibility and comprehension for all potential respondents, particularly patients and caregivers. The final survey asked respondents to record up to three questions that they thought would improve the care of hospitalized adult patients and their families. The specific wording of the survey is shown in the Figure and the entire survey in Appendix Document 1.
We chose three questions because that is the number of entries per participant that is recommended by JLA; it also minimizes responder burden.19 We asked respondents to identify the stakeholder group they represented (eg, patient, caregiver, healthcare provider, researcher) and for providers to identify where they primarily worked (eg, acute care hospital, post-acute care, advocacy group).
Survey Administration. We administered the survey electronically using Research Electronic Data Capture (REDCap), a secure web-based application used for collecting research data.22 Stakeholders were asked to disseminate the survey broadly using whatever methods that they felt was appropriate to their leadership or members.
Phase 5: Initial Question Categorization Using Qualitative Content Analysis
Six members of the steering committee independently performed qualitative content analysis to categorize all submitted questions.23,24 This analytic approach identifies, analyzes, and reports patterns within the data.23,24 We hypothesized that some of the submitted questions would relate to already-known problems with hospitalization. Therefore the steering committee developed an a priori codebook of 48 categories using common systems-based issues and diseases related to the care of hospitalized patients based on the hospitalist core competency topics developed by hospitalists and the SHM Education Committee,25 personal and clinical knowledge and experience related to the care of hospitalized adult patients, and published literature on the topic. These a priori categories and their definitions are shown in Appendix Document 2 and were the basis for our initial theory-driven (deductive) approach to data analysis.23
Once coding began, we identified 32 new and additional categories based on our review of the submitted questions, and these were the basis of our data-driven (inductive) approach to analysis.23 All proposed new codes and definitions were discussed with and approved by the entire steering committee prior to being added to the codebook (Appendix Document 2).
While coding categories were mutually exclusive, multiple codes could be attributed to a question depending on the content and meaning of a question. To ensure methodological rigor, reviewers met regularly via teleconference or communicated via email throughout the analysis to iteratively refine and define coding categories. All questions were reviewed independently, and then discussed, by at least two members of the analysis team. Any coding disparities were discussed and resolved by negotiated consensus.26 Analysis was conducted using Dedoose V8.0.35 (Sociocultural Research Consultants, Los Angeles, California).
Phase 6: Initial Question Identification Using Quantitative Content Analysis
Following thematic categorization, all steering committee members then reviewed each category to identify and quantify the most commonly submitted questions.27 A question was determined to be a commonly submitted question when it appeared at least 10 times.
Phase 7: Interim Priority Setting
We sent the list of the most commonly submitted questions (Appendix Document 3) to stakeholder organizations and patient partner networks for review and evaluation. Each organization was asked to engage with their constituents and leaders to collectively decide on which of these questions resonated and was most important. These preferences would then be used during the in-person meeting (Phase 8). We did not provide stakeholder organizations with information about how many times each question was submitted by respondents because we felt this could potentially bias their decision-making processes such that true importance and relevance would not obtained.
Phase 8: In-person Meeting for Final Question Prioritization and Refinement
Representatives from all 39 participating stakeholder organizations were invited to participate in a 2-day, in-person meeting to create a final prioritized list of questions to be used to guide patient-centered research seeking to improve the care of hospitalized adult patients and their caregivers. This meeting was attended by 43 stakeholders (26 stakeholder organization representatives and 17 steering committee members) from 37 unique stakeholder organizations. To facilitate the inclusiveness and to ensure a consensus-driven process, we used nominal group technique (NGT) to allow all of the meeting participants to discuss the list of prioritized questions in small groups.28 NGT allows participants to comprehend each other’s point of view to ensure no perpsectives are excluded.28 The NGT was followed by two rounds of individual voting. Stakeholders were then asked to frame their discussions and their votes based on the perspectives of their organizations or PFACs that they represent. The voting process required participants to make choices regarding the relative importance of all of the questions, which therefore makes the resulting list a true prioritized list. In the first round of voting, participants voted for up to five questions for inclusion on the prioritized list. Based on the distribution of votes, where one vote equals one point, each of the 36 questions was then ranked in order of the assigned points. The rank-ordering process resulted in a natural cut point or delineated point, resulting in the 11 questions considered to be the highest prioritized questions. Following this, a second round of voting took place with the same parameters as the first round and allowed us to rank order questions by order of importance and priority. Finally, during small and large group discussions, the original text of each question was edited, refined, and reformatted into questions that could drive a research agenda.
Ethical Oversight
This study was reviewed by the Institutional Review Board of the University of Texas Health Science Center at San Antonio and deemed not to be human subject research (UT Health San Antonio IRB Protocol Number: HSC20170058N).
RESULTS
In total, 499 respondents from 39 unique stakeholder organizations responded to our survey. Respondents self-identified into multiple categorizes resulting in 267 healthcare providers, 244 patients and caregivers, and 63 researchers. Characteristics of respondents to the survey are shown in Table 2.
An overview of study results is shown in Table 1. Respondents submitted a total of 782 questions related to improving the care of hospitalized patients. These questions were categorized during thematic analysis into 70 distinct categories—52 that were health system related and 18 that were disease specific (Appendix 2). The most frequently used health system–related categories were related to discharge care transitions, medications, patient understanding, and patient-family-care team communication (Appendix 2).
From these categories, 36 questions met our criteria for “commonly identified,” ie, submitted at least 10 times (Appendix Document 3). Notably, these 36 questions were derived from 67 different coding categories, of which 24 (36%) were a priori (theory-driven) categories23 created by the Steering Committee before analysis began and 43 (64%) categories were created as a result of this study’s stakeholder-engaged process and a data-driven approach23 to analysis (Appendix Document 3). These groups of questions were then presented during the 2-day, in-person meeting and reduced to a final 11 questions that were identified in rank order as top priorities (Table 3). The questions considered highest priority related to ensuring shared treatment and goals of care decision making, improving hospital discharge handoff to other care facilities and providers, and reducing the confusion related to education on medications, conditions, hospital care, and discharge.

DISCUSSION
Using a dynamic and collaborative stakeholder engagement process, we identified 11 questions prioritized in order of importance by patients, caregivers, and other healthcare stakeholders to improve the care of hospitalized adult patients. While some of the topics identified are already well-known topics in need of research and improvement, our findings frame these topics according to the perspectives of patients, caregivers, and stakeholders. This unique perspective adds a level of richness and nuance that provides insight into how to better address these topics and ultimately inform research and quality improvement efforts.
The question considered to be the highest priority area for future research and improvement surmised how it may be possible to implement interventions that engage patients in shared decision making. Shared decision making involves patients and their care team working together to make decisions about treatment, and other aspects of care based on sound clinical evidence that balances the risks and outcomes with patient preferences and values. Although considered critically important,29 a recent evaluation of shared decision making practices in over 250 inpatient encounters identified significant gaps in physicians’ abilities to perform key elements of a shared decision making approach and reinforced the need to identify what strategies can best promote widespread shared decision making.30 While there has been considerable effort to faciliate shared decision making in practice, there remains mixed evidence regarding the sustainability and impact of tools seeking to support shared decision making, such as decision aids, question prompt lists, and coaches.31 This suggests that new approaches to shared decision making may be required and likely explains why this question was rated as a top priority by stakeholders in the current study.
Respondents frequently framed their questions in terms of their lived experiences, providing stories and scenarios to illustrate the importance of the questions they submitted. This personal framing highlighted to us the need to think about improving care delivery from the end-user perspective. For example, respondents framed questions about care transitions not with regard to early appointments, instructions, or medication lists, but rather in terms of whom to call with questions or how best to reach their physician, nurse, or other identified provider. These perspectives suggest that strategies and approaches to improvement that start with patient and caregiver experiences, such as design thinking,32 may be important to continued efforts to improve hospital care. Additionally, the focus on the interpersonal aspects of care delivery highlights the need to focus on the patient-provider relationship and communication.
Questions submitted by respondents demonstrated a stark difference between “patient education” and “patient understanding,” which suggests that being provided with education or education materials regarding care did not necessarily lead to a clear patient understanding. The potential for lack of understanding was particularly prominent in the context of care plan development and during times of care transition—topics that were encompassed in 9 out of 11 of our prioritized research questions. This may suggest that approaches that improve the ability for healthcare providers to deliver information may not be sufficient to meet the needs of patients and caregivers. Rather, partnering to develop a shared understanding—whether about prognosis, medications, hospital, or discharge care plans—is critical. Improved communication practices are not an endpoint for information delivery, but rather a starting point leading to a shared understanding.
Several of the priority areas identified in our study reflect the immensely complex intersections among patients, caregivers, clinicians, and the healthcare delivery system. Addressing these gaps in order to reach the goal of ideal hospital care and an improved patient experience will likely require coordinated approaches and strong involvement and buy-in from multiple stakeholders including the voices of patients and caregivers. Creating patient-centered and stakeholder-driven research has been an increasing priority nationally.33 Yet to realize this, we must continue to understand the foundations and best practices of authentic stakeholder engagement so that it can be achieved in practice.34 We intend for this prioritized list of questions to galvanize funders, researchers, clinicians, professional societies, and patient and caregiver advocacy groups to work together to address these topics through the creation of new research evidence or the sustainable implementation of existing evidence.
Our findings provide a foundation for stakeholder groups to work in partnership to find research and improvement solutions to the problems identified. Our efforts demonstrate the value and importance of a systematic and broad engagement process to ensure that the voices of patients, caregivers, and other healthcare stakeholders are included in guiding hospital research and quality improvement efforts. This is highlighted by the fact our results of prioritized category areas for research were largely only uncovered following the creation of coding categories during the analysis process and were not captured using a priori catgeories that were expected by the steering committee.
The strengths of this study include our attempts to systematically identify and engage a wide range of perspectives in hospital medicine, including perspectives from patients and their caregivers. There are also acknowledged limitations in our study. While we included patients and PFACs from across the country, the opinions of the people we included may not be representative of all patients. Similarly, the perspectives of the other participants may not have completely represented their stakeholder organizations. While we attempted to include a broad range of organizations, there may be other relevant groups who were not represented in our sample.
In summary, our findings provide direction for the multiple stakeholders involved in improving hospital care. The results will allow the research community to focus on questions that are most important to patients, caregivers, and other stakeholders, reframing them in ways that are more relevant to patients’ lived experiences and that reflect the complexity of the issues. Our findings can also be used by healthcare providers and delivery organizations to target local improvement efforts. We hope that patients and caregivers will use our results to advocate for research and improvement in areas that matter the most to them. We hope that policy makers and funding agencies use our results to promote work in these areas and drive a national conversation about how to most effectively improve hospital care.
Acknowledgments
The authors would like to thank all patients, caregivers, and stakeholders who completed the survey. The authors also would like to acknowledge the organizations and individuals who participated in this study (see Appendix Document 4 for full list). At SHM, the authors would like to specifically thank Claudia Stahl, Jenna Goldstein, Kevin Vuernick, Dr Brad Sharpe, and Dr Larry Wellikson for their support.
Disclaimer
The statements presented in this publication are solely the responsibility of the authors and do not necessarily represent the views of the Department of Veterans Affairs, Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
1. American Hospital Association. 2019 American Hospital Association Hospital Statistics. Chicago, Illinois: American Hospital Association; 2019.
2. Alper E, O’Malley T, Greenwald J. UptoDate: Hospital discharge and readmission. https://www.uptodate.com/contents/hospital-discharge-and-readmission. Accessed August 8, 2019.
3. de Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: a systematic review. Qual Saf Heal Care. 2008;17(3):216-223. https://doi.org/10.1136/qshc.2007.023622.
4. Agency for Healthcare Research and Quality. Readmissions and Adverse Events After Discharge. https://psnet.ahrq.gov/primers/primer/11/Readmissions-and-Adverse-Events-After-Discharge. Accessed August 8, 2019.
5. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC; National Academies Press; 2001. https://doi.org/10.17226/10027.
6. Trivedi AN, Nsa W, Hausmann LRM, et al. Quality and equity of care in U.S. hospitals. N Engl J Med. 2014;371(24):2298-2308. https://doi.org/10.1056/NEJMsa1405003.
7. National Patient Safety Foundation. Free from Harm: Accelerating Patient Safety Improvement Fifteen Years after To Err Is Human. Boston: National Patient Safety Foundation; 2015.
8. Agency for Healthcare Research and Quality. AHRQ National Scorecard on Hospital-Acquired Conditions Updated Baseline Rates and Preliminary Results 2014–2017. https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/pfp/hacreport-2019.pdf. Accessed August 8, 2019.
9. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. https://doi.org/10.1002/jhm.2054.
10. Snyder HJ, Fletcher KE. The hospital experience through the patients’ eyes. J Patient Exp. 2019. https://doi.org/10.1177/2374373519843056.
11. Kebede S, Shihab HM, Berger ZD, Shah NG, Yeh H-C, Brotman DJ. Patients’ understanding of their hospitalizations and association with satisfaction. JAMA Intern Med. 2014;174(10):1698-1700. https://doi.org/10.1001/jamainternmed.2014.3765.
12. Shoeb M, Merel SE, Jackson MB, Anawalt BD. “Can we just stop and talk?” patients value verbal communication about discharge care plans. J Hosp Med. 2012;7(6):504-507. https://doi.org/10.1002/jhm.1937.
13. Neeman N, Quinn K, Shoeb M, Mourad M, Sehgal NL, Sliwka D. Postdischarge focus groups to improve the hospital experience. Am J Med Qual. 2013;28(6):536-538. https://doi.org/10.1177/1062860613488623.
14. Duffett L. Patient engagement: what partnering with patients in research is all about. Thromb Res. 2017;150:113-120. https://doi.org/10.1016/j.thromres.2016.10.029.
15. Pomey M, Hihat H, Khalifa M, Lebel P, Neron A, Dumez V. Patient partnership in quality improvement of healthcare services: patients’ inputs and challenges faced. Patient Exp J. 2015;2:29-42. https://doi.org/10.35680/2372-0247.1064.
16. Robbins M, Tufte J, Hsu C. Learning to “swim” with the experts: experiences of two patient co-investigators for a project funded by the Patient-Centered Outcomes Research Institute. Perm J. 2016;20(2):85-88. https://doi.org/10.7812/TPP/15-162.
17. Tai-Seale M, Sullivan G, Cheney A, Thomas K, Frosch D. The language of engagement: “aha!” moments from engaging patients and community partners in two pilot projects of the Patient-Centered Outcomes Research Institute. Perm J. 2016;20(2):89-92. https://doi.org/10.7812/TPP/15-123.
18. Patient-Centered Outcomes Research Institute (PCORI). PCORI Methodology Standards: Standards for Formulating Research Questions. https://www.pcori.org/research-results/about-our-research/research-methodology/pcori-methodology-standards#Formulating Research Questions. Accessed August 8, 2019.
19. James Lind Alliance. The James Lind Alliance Guidebook. Version 8. Southampton, England: James Lind Alliance; 2018.
20. Society of Hospital Medicine (SHM). Improving Hospital Outcomes through Patient Engagement: The i-HOPE Study. https://www.hospitalmedicine.org/clinical-topics/i-hope-study/. Accessed August 8, 2019.
21. Society of Hospital Medicine (SHM). Committees. https://www.hospitalmedicine.org/membership/committees/. Accessed August 8, 2019.
22. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
23. Schreier M. Qualitative content analysis in practice. Los Angeles, CA: SAGE Publications; 2012.
24. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x.
25. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine—2017 revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715.
26. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758-1772. https://doi.org/10.1111/j.1475-6773.2006.00684.x.
27. Coe K, Scacco JM. Content analysis, quantitative. Int Encycl Commun Res Methods. 2017:1-11. https://doi.org/10.1002/9781118901731.iecrm0045.
28. Centers for Disease Control and Prevention. Evaluation Briefs: Gaining Consensus Among Stakeholders Through the Nominal Group Technique. Atlanta, GA; 2018. https://www.cdc.gov/healthyyouth/evaluation/pdf/brief7.pdf. Accessed August 8, 2019.
29. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692. https://doi.org/10.1016/s0277-9536(96)00221-3.
30. Blankenburg R, Hilton JF, Yuan P, et al. Shared decision-making during inpatient rounds: opportunities for improvement in patient engagement and communication. J Hosp Med. 2018;13(7):453-461. https://doi.org/10.12788/jhm.2909.
31. Legare F, Adekpedjou R, Stacey D, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2018;7(7):CD006732. https://doi.org/10.1002/14651858.CD006732.pub4.
32. Roberts JP, Fisher TR, Trowbridge MJ, Bent C. A design thinking framework for healthcare management and innovation. Healthc (Amst). 2016;4(1):11-14. https://doi.org/10.1016/j.hjdsi.2015.12.002.
33. Selby JV, Beal AC, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA. 2012;307(15):1583-1584. https://doi.org/10.1001/jama.2012.500.
34. Harrison J, Auerbach A, Anderson W, et al. Patient stakeholder engagement in research: a narrative review to describe foundational principles and best practice activities. Health Expect. 2019;22(3):307-316. https://doi.org/10.1111/hex.12873.
1. American Hospital Association. 2019 American Hospital Association Hospital Statistics. Chicago, Illinois: American Hospital Association; 2019.
2. Alper E, O’Malley T, Greenwald J. UptoDate: Hospital discharge and readmission. https://www.uptodate.com/contents/hospital-discharge-and-readmission. Accessed August 8, 2019.
3. de Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: a systematic review. Qual Saf Heal Care. 2008;17(3):216-223. https://doi.org/10.1136/qshc.2007.023622.
4. Agency for Healthcare Research and Quality. Readmissions and Adverse Events After Discharge. https://psnet.ahrq.gov/primers/primer/11/Readmissions-and-Adverse-Events-After-Discharge. Accessed August 8, 2019.
5. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC; National Academies Press; 2001. https://doi.org/10.17226/10027.
6. Trivedi AN, Nsa W, Hausmann LRM, et al. Quality and equity of care in U.S. hospitals. N Engl J Med. 2014;371(24):2298-2308. https://doi.org/10.1056/NEJMsa1405003.
7. National Patient Safety Foundation. Free from Harm: Accelerating Patient Safety Improvement Fifteen Years after To Err Is Human. Boston: National Patient Safety Foundation; 2015.
8. Agency for Healthcare Research and Quality. AHRQ National Scorecard on Hospital-Acquired Conditions Updated Baseline Rates and Preliminary Results 2014–2017. https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/pfp/hacreport-2019.pdf. Accessed August 8, 2019.
9. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. https://doi.org/10.1002/jhm.2054.
10. Snyder HJ, Fletcher KE. The hospital experience through the patients’ eyes. J Patient Exp. 2019. https://doi.org/10.1177/2374373519843056.
11. Kebede S, Shihab HM, Berger ZD, Shah NG, Yeh H-C, Brotman DJ. Patients’ understanding of their hospitalizations and association with satisfaction. JAMA Intern Med. 2014;174(10):1698-1700. https://doi.org/10.1001/jamainternmed.2014.3765.
12. Shoeb M, Merel SE, Jackson MB, Anawalt BD. “Can we just stop and talk?” patients value verbal communication about discharge care plans. J Hosp Med. 2012;7(6):504-507. https://doi.org/10.1002/jhm.1937.
13. Neeman N, Quinn K, Shoeb M, Mourad M, Sehgal NL, Sliwka D. Postdischarge focus groups to improve the hospital experience. Am J Med Qual. 2013;28(6):536-538. https://doi.org/10.1177/1062860613488623.
14. Duffett L. Patient engagement: what partnering with patients in research is all about. Thromb Res. 2017;150:113-120. https://doi.org/10.1016/j.thromres.2016.10.029.
15. Pomey M, Hihat H, Khalifa M, Lebel P, Neron A, Dumez V. Patient partnership in quality improvement of healthcare services: patients’ inputs and challenges faced. Patient Exp J. 2015;2:29-42. https://doi.org/10.35680/2372-0247.1064.
16. Robbins M, Tufte J, Hsu C. Learning to “swim” with the experts: experiences of two patient co-investigators for a project funded by the Patient-Centered Outcomes Research Institute. Perm J. 2016;20(2):85-88. https://doi.org/10.7812/TPP/15-162.
17. Tai-Seale M, Sullivan G, Cheney A, Thomas K, Frosch D. The language of engagement: “aha!” moments from engaging patients and community partners in two pilot projects of the Patient-Centered Outcomes Research Institute. Perm J. 2016;20(2):89-92. https://doi.org/10.7812/TPP/15-123.
18. Patient-Centered Outcomes Research Institute (PCORI). PCORI Methodology Standards: Standards for Formulating Research Questions. https://www.pcori.org/research-results/about-our-research/research-methodology/pcori-methodology-standards#Formulating Research Questions. Accessed August 8, 2019.
19. James Lind Alliance. The James Lind Alliance Guidebook. Version 8. Southampton, England: James Lind Alliance; 2018.
20. Society of Hospital Medicine (SHM). Improving Hospital Outcomes through Patient Engagement: The i-HOPE Study. https://www.hospitalmedicine.org/clinical-topics/i-hope-study/. Accessed August 8, 2019.
21. Society of Hospital Medicine (SHM). Committees. https://www.hospitalmedicine.org/membership/committees/. Accessed August 8, 2019.
22. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
23. Schreier M. Qualitative content analysis in practice. Los Angeles, CA: SAGE Publications; 2012.
24. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x.
25. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine—2017 revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715.
26. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758-1772. https://doi.org/10.1111/j.1475-6773.2006.00684.x.
27. Coe K, Scacco JM. Content analysis, quantitative. Int Encycl Commun Res Methods. 2017:1-11. https://doi.org/10.1002/9781118901731.iecrm0045.
28. Centers for Disease Control and Prevention. Evaluation Briefs: Gaining Consensus Among Stakeholders Through the Nominal Group Technique. Atlanta, GA; 2018. https://www.cdc.gov/healthyyouth/evaluation/pdf/brief7.pdf. Accessed August 8, 2019.
29. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692. https://doi.org/10.1016/s0277-9536(96)00221-3.
30. Blankenburg R, Hilton JF, Yuan P, et al. Shared decision-making during inpatient rounds: opportunities for improvement in patient engagement and communication. J Hosp Med. 2018;13(7):453-461. https://doi.org/10.12788/jhm.2909.
31. Legare F, Adekpedjou R, Stacey D, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2018;7(7):CD006732. https://doi.org/10.1002/14651858.CD006732.pub4.
32. Roberts JP, Fisher TR, Trowbridge MJ, Bent C. A design thinking framework for healthcare management and innovation. Healthc (Amst). 2016;4(1):11-14. https://doi.org/10.1016/j.hjdsi.2015.12.002.
33. Selby JV, Beal AC, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA. 2012;307(15):1583-1584. https://doi.org/10.1001/jama.2012.500.
34. Harrison J, Auerbach A, Anderson W, et al. Patient stakeholder engagement in research: a narrative review to describe foundational principles and best practice activities. Health Expect. 2019;22(3):307-316. https://doi.org/10.1111/hex.12873.
© 2020 Society of Hospital Medicine
When Should an IVC Filter Be Used to Treat a DVT?
Case
A 67-year-old man with a history of hypertension presents with a swollen right lower extremity. An ultrasound reveals a DVT, and he is commenced on low-molecular-weight heparin and warfarin. Two days later, he develops slurred speech and right-sided weakness. A head CT reveals an intracranial hemorrhage. When should an inferior vena cava (IVC) filter be utilized for treatment of DVT?
Overview
It is estimated that 350,000 to 600,000 Americans develop a VTE each year.1 Patients with a DVT are at high risk of developing a pulmonary embolism (PE). In a multicenter study, nearly 40% of patients admitted with a DVT had evidence of a PE on ventilation perfusion scan.2 Treatment of a DVT is aimed at preventing the extension of the DVT and embolization.3 The American College of Chest Physicians (ACCP) recommends anticoagulation as the primary DVT treatment (Grade 1A).4 However, IVC filters might be considered when anticoagulation is contraindicated.
In 1868, Trousseau created the conceptual model of surgical interruption of the IVC to prevent PE. However, it wasn’t until 1959 by Bottini that the surgical interruption was successfully performed.5 The Mobin-Uddin filter was introduced in 1967 as the first mechanical IVC filter.6 IVC filters mechanically trap the DVT, preventing emboli from traveling into the pulmonary vasculature.7
There are two classes of IVC filters: permanent filters and removable filters. Removable filters include both temporary filters and retrievable filters. Temporary filters are attached to a catheter that exits the skin and therefore must be removed due to the risk of infection and embolization.7 Retrievable filters are similar in design to permanent filters but are designed to be removed. However, this must be done with caution, as neointimal hyperplasia can prevent removal or cause vessel wall damage upon removal.8
IVC filters are inserted into the vena cava percutaneously via the femoral or jugular approach under fluoroscopy or ultrasound guidance (see Figure 1, p. 16). The filters typically are placed infrarenally, unless there is an indication for a suprarenal filter (e.g., renal vein thrombosis or IVC thrombus extending above the renal veins).7 Complete IVC thrombosis is an absolute contraindication to IVC filter placement, and the relative contraindications include significant coagulopathy and bacteremia.9
The incidence of complications related to IVC filter placement is 4% to 11%. Complications include:
- Insertion-site thrombosis;
- IVC thrombosis;
- Recurrent DVT postphlebitic syndrome;
- Filter migration;
- Erosion of the filter through the vessel wall; and
- Vena caval obstruction.10
A review of the National Hospital Discharge Survey database for trends in IVC filter use in the U.S. found a dramatic increase in the use of IVC filters from 1979 to 1999—to 49,000 patients from 2,000 patients with IVC filters in place. The indications for IVC filter use vary such that it is imperative there are well-designed trials and guidelines to guide appropriate use.11
The Evidence
The 2008 ACCP guidelines on VTE management follow a grading system that classifies recommendations as Grade 1 (strong) or Grade 2 (weak), and classifies the quality of evidence as A (high), B (moderate), or C (low).12 The ACCP guidelines’ recommended first-line treatment for a confirmed DVT is anticoagulation with subcutaneous low-molecular-weight heparin, intravenous unfractionated heparin, monitored subcutaneous heparin, fixed-dose subcutaneous unfractionated heparin, or subcutaneous fondaparinux (all Grade 1A recommendations). The ACCP recommends against the routine use of an IVC filter in addition to anticoagulants (Grade 1A). However, for patients with acute proximal DVT, if anticoagulant therapy is not possible because of the risk of bleeding, IVC filter placement is recommended (Grade 1C). If a patient requires an IVC filter for treatment of an acute DVT as an alternative to anticoagulation, it is recommended to start anticoagulant therapy once the risk of bleeding resolves (Grade 1C).4
The 2008 ACCP guidelines for IVC filter use have a few important changes from the 2004 version. First, the IVC filter placement recommendation for patients with contraindications to anticoagulation was strengthened from Grade 2C to Grade 1C. Second, the 2008 guidelines omitted the early recommendation of IVC filter use for recurrent VTE, despite adequate anticoagulation (Grade 2C).13
Only one randomized study has evaluated the efficacy of IVC filters. All other studies of IVC filters are retrospective or prospective case series.
The PREPIC study randomized 400 patients with proximal DVT considered to be at high risk for PE to receive either an IVC filter or no IVC filter. Additionally, patients were randomized to receive enoxaparin or unfractionated heparin as a bridge to warfarin therapy, which was continued for at least three months. The primary endpoints were recurrent DVT, PE, major bleeding, or death. The patients were followed up at day 12, two years, and then annually up to eight years following randomization.14 At day 12, there were fewer PEs in the group that received filters (OR 0.22, 95% CI, 0.05-0.90). However, at year two, there was no significant difference in PE development in the filter group compared with the no-filter group (OR 0.50, 95% CI, 0.19-1.33).
Additionally, at year two, the filter group was more likely to develop recurrent DVT (OR 1.87, 95% CI, 1.10-3.20). At year eight, there was a significant reduction in the number of PEs in the filter group versus the no-filter group (6.2% vs.15.1%, P=0.008). However, at eight-year followup, IVC filter use was associated with increased DVT (35.7% vs. 27.5%, P=0.042). There was no difference in mortality between the two groups.
In summary, the use of IVC filters was associated with decreased incidence of PE at eight years, offset by higher rates of recurrent DVT and no overall mortality benefit.14,15 Importantly, the indications for IVC filter use in this study differ from the current ACCP guidelines; all patients were given concomitant anticoagulation for at least three months, which might not be possible in patients for whom the ACCP recommends IVC filters.
There are no randomized studies to compare the efficacy of permanent IVC filters and retrievable filters for PE prevention. A retrospective study comparing the clinical effectiveness of the two filter types reported no difference in the rates of symptomatic PE (permanent filter 4% vs. retrievable filter 4.7%, P=0.67) or DVT (11.3% vs. 12.6%, P=0.59). In addition, the frequency of symptomatic IVC thrombosis was similar (1.1% vs. 0.5%, p=0.39).16 A paper reviewing the efficacy of IVC filters reported that permanent filters were associated with a 0%-6.2% rate of PE versus a 0%-1.9% rate with retrievable filters.7 Notably, these studies were not randomized controlled trials—rather, case series—and the indications for IVC filters were not necessarily those currently recommended by the ACCP.
Due to the long-term complications of permanent IVC filters, it is suggested that a retrievable IVC filter be used for patients with temporary contraindications to anticoagulation.17 Comerata et al created a clinical decision-making tool for picking the type of filter to employ. If the duration of contraindication to anticoagulation is short or uncertain, a retrievable filter is recommended.18 Table 1 (p. 15) outlines the recommendations for IVC filter placement.
There are no randomized controlled trials to guide the use of concomitant anticoagulation after filter insertion, although this intervention may be beneficial to prevent DVT propagation, recurrence, or IVC filter thrombosis.5 A meta-analysis of 14 studies evaluating the rates of VTE after IVC filter placement demonstrated a non-statistically significant trend toward fewer VTE events in the patients with an IVC filter and concomitant anticoagulation in comparison with those who solely had an IVC filter (OR 0.64, 95% CI, 0.35-1.2). The duration and degree of anticoagulation was not presented in all of the studies in the meta-analysis, therefore limiting the analysis.19
In addition to the ACCP guidelines, there have been other proposed indications for IVC filter use, including recurrent VTE despite anticoagulation, chronic recurrent PE with pulmonary hypertension, extensive free-floating iliofemoral thrombus, and thrombolysis of ilio-caval thrombus.20 The ACCP guidelines do not specifically address these individual indications, and at this time there are no randomized controlled trials to guide IVC filter use in these cases.
Back to the Case
Our patient developed a significant complication from anticoagulation. Current ACCP guidelines recommend an IVC filter if anticoagulant therapy is contraindicated (Grade 1C). The anticoagulation was discontinued and a retrievable IVC filter was placed. Once a patient no longer has a contraindication for anticoagulation, the ACCP recommends restarting a conventional course of anticoagulation. Thus, once the patient can tolerate anticoagulation, consideration will be given to removal of the retrievable filter.
Bottom Line
An IVC filter should be considered in patients with a DVT who have a contraindication to anticoagulation. Other indications for IVC filter use are not supported by the current literature. TH
Drs. Bhogal and Eid are hospitalist fellows and instructors at Johns Hopkins Bayview Medical Center in Baltimore. Dr. Kantsiper is a hospitalist and assistant professor at Bayview Medical Center.
References
- The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. U.S. Department of Health & Human Services Web site. Available at: www.surgeongeneral.gov/topics/deepvein/. Accessed Jan. 25, 2010.
- Moser KM, Fedullo PR, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271(3):223-225.
- Bates SM, Ginsberg JS. Treatment of deep vein thrombosis. N Engl J Med. 2004;351:268-277.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians. Antithrombotic therapy for venous theomboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S.
- Becker DM, Philbrick JT, Selby JB. Inferior vena cava filters. Indications, safety, effectiveness. Arch Intern Med. 1992;152(10):1985-1994.
- Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000;95(12):3669-3677.
- Chung J, Owen RJ. Using inferior vena cava filters to prevent pulmonary embolism. Can Fam Physician. 2008;54(1):49-55.
- Ku GH. Billett HH. Long lives, short indications. The case for removable inferior cava filters. Thromb Haemost. 2005;93(1):17-22.
- Stavropoulos WS. Inferior vena cava filters. Tech Vasc Interv Radiol. 2004;7(2):91-95.
- Crowther MA. Inferior vena cava filters in the management of venous thromboembolism. Am J Med. 2007;120(10 Suppl 2):S13–S17.
- Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541-1545.
- Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129(1):174-181.
- Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):401S-428S.
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7):409-415.
- Decousus H, Barral F, Buchmuller-Cordier A, et al. Participating centers eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC randomization croup. Circulation. 2005;112:416-422.
- Kim HS, Young MJ, Narayan AK, Liddell RP, Streiff MB. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol. 2008:19(3):393-399.
- Houman Fekrazad M, Lopes RD, Stashenko GJ, Alexander JH, Garcia D. Treatment of venous thromboembolism: guidelines translated for the clinician. J Thromb Thrombolysis. 2009; 28(3):270–275.
- Comerota AJ. Retrievable IVC filters: a decision matrix for appropriate utilization. Perspect Vasc Surg Endovasc Ther. 2006;18(1):11-17.
- Ray CE Jr, Prochazka A. The need for anticoagulation following inferior vena cava filter placement: systematic review. Cardiovasc Intervent Radiol. 2008; 31(2):316-324.
- Hajduk B, Tomkowski WZ, Malek G, Davidson BL. Vena cava filter occlusion and venous thromboembolism risk in persistently anticoagulated patients: A prospective, observational cohort study. Chest. 2009.
Case
A 67-year-old man with a history of hypertension presents with a swollen right lower extremity. An ultrasound reveals a DVT, and he is commenced on low-molecular-weight heparin and warfarin. Two days later, he develops slurred speech and right-sided weakness. A head CT reveals an intracranial hemorrhage. When should an inferior vena cava (IVC) filter be utilized for treatment of DVT?
Overview
It is estimated that 350,000 to 600,000 Americans develop a VTE each year.1 Patients with a DVT are at high risk of developing a pulmonary embolism (PE). In a multicenter study, nearly 40% of patients admitted with a DVT had evidence of a PE on ventilation perfusion scan.2 Treatment of a DVT is aimed at preventing the extension of the DVT and embolization.3 The American College of Chest Physicians (ACCP) recommends anticoagulation as the primary DVT treatment (Grade 1A).4 However, IVC filters might be considered when anticoagulation is contraindicated.
In 1868, Trousseau created the conceptual model of surgical interruption of the IVC to prevent PE. However, it wasn’t until 1959 by Bottini that the surgical interruption was successfully performed.5 The Mobin-Uddin filter was introduced in 1967 as the first mechanical IVC filter.6 IVC filters mechanically trap the DVT, preventing emboli from traveling into the pulmonary vasculature.7
There are two classes of IVC filters: permanent filters and removable filters. Removable filters include both temporary filters and retrievable filters. Temporary filters are attached to a catheter that exits the skin and therefore must be removed due to the risk of infection and embolization.7 Retrievable filters are similar in design to permanent filters but are designed to be removed. However, this must be done with caution, as neointimal hyperplasia can prevent removal or cause vessel wall damage upon removal.8
IVC filters are inserted into the vena cava percutaneously via the femoral or jugular approach under fluoroscopy or ultrasound guidance (see Figure 1, p. 16). The filters typically are placed infrarenally, unless there is an indication for a suprarenal filter (e.g., renal vein thrombosis or IVC thrombus extending above the renal veins).7 Complete IVC thrombosis is an absolute contraindication to IVC filter placement, and the relative contraindications include significant coagulopathy and bacteremia.9
The incidence of complications related to IVC filter placement is 4% to 11%. Complications include:
- Insertion-site thrombosis;
- IVC thrombosis;
- Recurrent DVT postphlebitic syndrome;
- Filter migration;
- Erosion of the filter through the vessel wall; and
- Vena caval obstruction.10
A review of the National Hospital Discharge Survey database for trends in IVC filter use in the U.S. found a dramatic increase in the use of IVC filters from 1979 to 1999—to 49,000 patients from 2,000 patients with IVC filters in place. The indications for IVC filter use vary such that it is imperative there are well-designed trials and guidelines to guide appropriate use.11
The Evidence
The 2008 ACCP guidelines on VTE management follow a grading system that classifies recommendations as Grade 1 (strong) or Grade 2 (weak), and classifies the quality of evidence as A (high), B (moderate), or C (low).12 The ACCP guidelines’ recommended first-line treatment for a confirmed DVT is anticoagulation with subcutaneous low-molecular-weight heparin, intravenous unfractionated heparin, monitored subcutaneous heparin, fixed-dose subcutaneous unfractionated heparin, or subcutaneous fondaparinux (all Grade 1A recommendations). The ACCP recommends against the routine use of an IVC filter in addition to anticoagulants (Grade 1A). However, for patients with acute proximal DVT, if anticoagulant therapy is not possible because of the risk of bleeding, IVC filter placement is recommended (Grade 1C). If a patient requires an IVC filter for treatment of an acute DVT as an alternative to anticoagulation, it is recommended to start anticoagulant therapy once the risk of bleeding resolves (Grade 1C).4
The 2008 ACCP guidelines for IVC filter use have a few important changes from the 2004 version. First, the IVC filter placement recommendation for patients with contraindications to anticoagulation was strengthened from Grade 2C to Grade 1C. Second, the 2008 guidelines omitted the early recommendation of IVC filter use for recurrent VTE, despite adequate anticoagulation (Grade 2C).13
Only one randomized study has evaluated the efficacy of IVC filters. All other studies of IVC filters are retrospective or prospective case series.
The PREPIC study randomized 400 patients with proximal DVT considered to be at high risk for PE to receive either an IVC filter or no IVC filter. Additionally, patients were randomized to receive enoxaparin or unfractionated heparin as a bridge to warfarin therapy, which was continued for at least three months. The primary endpoints were recurrent DVT, PE, major bleeding, or death. The patients were followed up at day 12, two years, and then annually up to eight years following randomization.14 At day 12, there were fewer PEs in the group that received filters (OR 0.22, 95% CI, 0.05-0.90). However, at year two, there was no significant difference in PE development in the filter group compared with the no-filter group (OR 0.50, 95% CI, 0.19-1.33).
Additionally, at year two, the filter group was more likely to develop recurrent DVT (OR 1.87, 95% CI, 1.10-3.20). At year eight, there was a significant reduction in the number of PEs in the filter group versus the no-filter group (6.2% vs.15.1%, P=0.008). However, at eight-year followup, IVC filter use was associated with increased DVT (35.7% vs. 27.5%, P=0.042). There was no difference in mortality between the two groups.
In summary, the use of IVC filters was associated with decreased incidence of PE at eight years, offset by higher rates of recurrent DVT and no overall mortality benefit.14,15 Importantly, the indications for IVC filter use in this study differ from the current ACCP guidelines; all patients were given concomitant anticoagulation for at least three months, which might not be possible in patients for whom the ACCP recommends IVC filters.
There are no randomized studies to compare the efficacy of permanent IVC filters and retrievable filters for PE prevention. A retrospective study comparing the clinical effectiveness of the two filter types reported no difference in the rates of symptomatic PE (permanent filter 4% vs. retrievable filter 4.7%, P=0.67) or DVT (11.3% vs. 12.6%, P=0.59). In addition, the frequency of symptomatic IVC thrombosis was similar (1.1% vs. 0.5%, p=0.39).16 A paper reviewing the efficacy of IVC filters reported that permanent filters were associated with a 0%-6.2% rate of PE versus a 0%-1.9% rate with retrievable filters.7 Notably, these studies were not randomized controlled trials—rather, case series—and the indications for IVC filters were not necessarily those currently recommended by the ACCP.
Due to the long-term complications of permanent IVC filters, it is suggested that a retrievable IVC filter be used for patients with temporary contraindications to anticoagulation.17 Comerata et al created a clinical decision-making tool for picking the type of filter to employ. If the duration of contraindication to anticoagulation is short or uncertain, a retrievable filter is recommended.18 Table 1 (p. 15) outlines the recommendations for IVC filter placement.
There are no randomized controlled trials to guide the use of concomitant anticoagulation after filter insertion, although this intervention may be beneficial to prevent DVT propagation, recurrence, or IVC filter thrombosis.5 A meta-analysis of 14 studies evaluating the rates of VTE after IVC filter placement demonstrated a non-statistically significant trend toward fewer VTE events in the patients with an IVC filter and concomitant anticoagulation in comparison with those who solely had an IVC filter (OR 0.64, 95% CI, 0.35-1.2). The duration and degree of anticoagulation was not presented in all of the studies in the meta-analysis, therefore limiting the analysis.19
In addition to the ACCP guidelines, there have been other proposed indications for IVC filter use, including recurrent VTE despite anticoagulation, chronic recurrent PE with pulmonary hypertension, extensive free-floating iliofemoral thrombus, and thrombolysis of ilio-caval thrombus.20 The ACCP guidelines do not specifically address these individual indications, and at this time there are no randomized controlled trials to guide IVC filter use in these cases.
Back to the Case
Our patient developed a significant complication from anticoagulation. Current ACCP guidelines recommend an IVC filter if anticoagulant therapy is contraindicated (Grade 1C). The anticoagulation was discontinued and a retrievable IVC filter was placed. Once a patient no longer has a contraindication for anticoagulation, the ACCP recommends restarting a conventional course of anticoagulation. Thus, once the patient can tolerate anticoagulation, consideration will be given to removal of the retrievable filter.
Bottom Line
An IVC filter should be considered in patients with a DVT who have a contraindication to anticoagulation. Other indications for IVC filter use are not supported by the current literature. TH
Drs. Bhogal and Eid are hospitalist fellows and instructors at Johns Hopkins Bayview Medical Center in Baltimore. Dr. Kantsiper is a hospitalist and assistant professor at Bayview Medical Center.
References
- The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. U.S. Department of Health & Human Services Web site. Available at: www.surgeongeneral.gov/topics/deepvein/. Accessed Jan. 25, 2010.
- Moser KM, Fedullo PR, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271(3):223-225.
- Bates SM, Ginsberg JS. Treatment of deep vein thrombosis. N Engl J Med. 2004;351:268-277.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians. Antithrombotic therapy for venous theomboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S.
- Becker DM, Philbrick JT, Selby JB. Inferior vena cava filters. Indications, safety, effectiveness. Arch Intern Med. 1992;152(10):1985-1994.
- Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000;95(12):3669-3677.
- Chung J, Owen RJ. Using inferior vena cava filters to prevent pulmonary embolism. Can Fam Physician. 2008;54(1):49-55.
- Ku GH. Billett HH. Long lives, short indications. The case for removable inferior cava filters. Thromb Haemost. 2005;93(1):17-22.
- Stavropoulos WS. Inferior vena cava filters. Tech Vasc Interv Radiol. 2004;7(2):91-95.
- Crowther MA. Inferior vena cava filters in the management of venous thromboembolism. Am J Med. 2007;120(10 Suppl 2):S13–S17.
- Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541-1545.
- Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129(1):174-181.
- Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):401S-428S.
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7):409-415.
- Decousus H, Barral F, Buchmuller-Cordier A, et al. Participating centers eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC randomization croup. Circulation. 2005;112:416-422.
- Kim HS, Young MJ, Narayan AK, Liddell RP, Streiff MB. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol. 2008:19(3):393-399.
- Houman Fekrazad M, Lopes RD, Stashenko GJ, Alexander JH, Garcia D. Treatment of venous thromboembolism: guidelines translated for the clinician. J Thromb Thrombolysis. 2009; 28(3):270–275.
- Comerota AJ. Retrievable IVC filters: a decision matrix for appropriate utilization. Perspect Vasc Surg Endovasc Ther. 2006;18(1):11-17.
- Ray CE Jr, Prochazka A. The need for anticoagulation following inferior vena cava filter placement: systematic review. Cardiovasc Intervent Radiol. 2008; 31(2):316-324.
- Hajduk B, Tomkowski WZ, Malek G, Davidson BL. Vena cava filter occlusion and venous thromboembolism risk in persistently anticoagulated patients: A prospective, observational cohort study. Chest. 2009.
Case
A 67-year-old man with a history of hypertension presents with a swollen right lower extremity. An ultrasound reveals a DVT, and he is commenced on low-molecular-weight heparin and warfarin. Two days later, he develops slurred speech and right-sided weakness. A head CT reveals an intracranial hemorrhage. When should an inferior vena cava (IVC) filter be utilized for treatment of DVT?
Overview
It is estimated that 350,000 to 600,000 Americans develop a VTE each year.1 Patients with a DVT are at high risk of developing a pulmonary embolism (PE). In a multicenter study, nearly 40% of patients admitted with a DVT had evidence of a PE on ventilation perfusion scan.2 Treatment of a DVT is aimed at preventing the extension of the DVT and embolization.3 The American College of Chest Physicians (ACCP) recommends anticoagulation as the primary DVT treatment (Grade 1A).4 However, IVC filters might be considered when anticoagulation is contraindicated.
In 1868, Trousseau created the conceptual model of surgical interruption of the IVC to prevent PE. However, it wasn’t until 1959 by Bottini that the surgical interruption was successfully performed.5 The Mobin-Uddin filter was introduced in 1967 as the first mechanical IVC filter.6 IVC filters mechanically trap the DVT, preventing emboli from traveling into the pulmonary vasculature.7
There are two classes of IVC filters: permanent filters and removable filters. Removable filters include both temporary filters and retrievable filters. Temporary filters are attached to a catheter that exits the skin and therefore must be removed due to the risk of infection and embolization.7 Retrievable filters are similar in design to permanent filters but are designed to be removed. However, this must be done with caution, as neointimal hyperplasia can prevent removal or cause vessel wall damage upon removal.8
IVC filters are inserted into the vena cava percutaneously via the femoral or jugular approach under fluoroscopy or ultrasound guidance (see Figure 1, p. 16). The filters typically are placed infrarenally, unless there is an indication for a suprarenal filter (e.g., renal vein thrombosis or IVC thrombus extending above the renal veins).7 Complete IVC thrombosis is an absolute contraindication to IVC filter placement, and the relative contraindications include significant coagulopathy and bacteremia.9
The incidence of complications related to IVC filter placement is 4% to 11%. Complications include:
- Insertion-site thrombosis;
- IVC thrombosis;
- Recurrent DVT postphlebitic syndrome;
- Filter migration;
- Erosion of the filter through the vessel wall; and
- Vena caval obstruction.10
A review of the National Hospital Discharge Survey database for trends in IVC filter use in the U.S. found a dramatic increase in the use of IVC filters from 1979 to 1999—to 49,000 patients from 2,000 patients with IVC filters in place. The indications for IVC filter use vary such that it is imperative there are well-designed trials and guidelines to guide appropriate use.11
The Evidence
The 2008 ACCP guidelines on VTE management follow a grading system that classifies recommendations as Grade 1 (strong) or Grade 2 (weak), and classifies the quality of evidence as A (high), B (moderate), or C (low).12 The ACCP guidelines’ recommended first-line treatment for a confirmed DVT is anticoagulation with subcutaneous low-molecular-weight heparin, intravenous unfractionated heparin, monitored subcutaneous heparin, fixed-dose subcutaneous unfractionated heparin, or subcutaneous fondaparinux (all Grade 1A recommendations). The ACCP recommends against the routine use of an IVC filter in addition to anticoagulants (Grade 1A). However, for patients with acute proximal DVT, if anticoagulant therapy is not possible because of the risk of bleeding, IVC filter placement is recommended (Grade 1C). If a patient requires an IVC filter for treatment of an acute DVT as an alternative to anticoagulation, it is recommended to start anticoagulant therapy once the risk of bleeding resolves (Grade 1C).4
The 2008 ACCP guidelines for IVC filter use have a few important changes from the 2004 version. First, the IVC filter placement recommendation for patients with contraindications to anticoagulation was strengthened from Grade 2C to Grade 1C. Second, the 2008 guidelines omitted the early recommendation of IVC filter use for recurrent VTE, despite adequate anticoagulation (Grade 2C).13
Only one randomized study has evaluated the efficacy of IVC filters. All other studies of IVC filters are retrospective or prospective case series.
The PREPIC study randomized 400 patients with proximal DVT considered to be at high risk for PE to receive either an IVC filter or no IVC filter. Additionally, patients were randomized to receive enoxaparin or unfractionated heparin as a bridge to warfarin therapy, which was continued for at least three months. The primary endpoints were recurrent DVT, PE, major bleeding, or death. The patients were followed up at day 12, two years, and then annually up to eight years following randomization.14 At day 12, there were fewer PEs in the group that received filters (OR 0.22, 95% CI, 0.05-0.90). However, at year two, there was no significant difference in PE development in the filter group compared with the no-filter group (OR 0.50, 95% CI, 0.19-1.33).
Additionally, at year two, the filter group was more likely to develop recurrent DVT (OR 1.87, 95% CI, 1.10-3.20). At year eight, there was a significant reduction in the number of PEs in the filter group versus the no-filter group (6.2% vs.15.1%, P=0.008). However, at eight-year followup, IVC filter use was associated with increased DVT (35.7% vs. 27.5%, P=0.042). There was no difference in mortality between the two groups.
In summary, the use of IVC filters was associated with decreased incidence of PE at eight years, offset by higher rates of recurrent DVT and no overall mortality benefit.14,15 Importantly, the indications for IVC filter use in this study differ from the current ACCP guidelines; all patients were given concomitant anticoagulation for at least three months, which might not be possible in patients for whom the ACCP recommends IVC filters.
There are no randomized studies to compare the efficacy of permanent IVC filters and retrievable filters for PE prevention. A retrospective study comparing the clinical effectiveness of the two filter types reported no difference in the rates of symptomatic PE (permanent filter 4% vs. retrievable filter 4.7%, P=0.67) or DVT (11.3% vs. 12.6%, P=0.59). In addition, the frequency of symptomatic IVC thrombosis was similar (1.1% vs. 0.5%, p=0.39).16 A paper reviewing the efficacy of IVC filters reported that permanent filters were associated with a 0%-6.2% rate of PE versus a 0%-1.9% rate with retrievable filters.7 Notably, these studies were not randomized controlled trials—rather, case series—and the indications for IVC filters were not necessarily those currently recommended by the ACCP.
Due to the long-term complications of permanent IVC filters, it is suggested that a retrievable IVC filter be used for patients with temporary contraindications to anticoagulation.17 Comerata et al created a clinical decision-making tool for picking the type of filter to employ. If the duration of contraindication to anticoagulation is short or uncertain, a retrievable filter is recommended.18 Table 1 (p. 15) outlines the recommendations for IVC filter placement.
There are no randomized controlled trials to guide the use of concomitant anticoagulation after filter insertion, although this intervention may be beneficial to prevent DVT propagation, recurrence, or IVC filter thrombosis.5 A meta-analysis of 14 studies evaluating the rates of VTE after IVC filter placement demonstrated a non-statistically significant trend toward fewer VTE events in the patients with an IVC filter and concomitant anticoagulation in comparison with those who solely had an IVC filter (OR 0.64, 95% CI, 0.35-1.2). The duration and degree of anticoagulation was not presented in all of the studies in the meta-analysis, therefore limiting the analysis.19
In addition to the ACCP guidelines, there have been other proposed indications for IVC filter use, including recurrent VTE despite anticoagulation, chronic recurrent PE with pulmonary hypertension, extensive free-floating iliofemoral thrombus, and thrombolysis of ilio-caval thrombus.20 The ACCP guidelines do not specifically address these individual indications, and at this time there are no randomized controlled trials to guide IVC filter use in these cases.
Back to the Case
Our patient developed a significant complication from anticoagulation. Current ACCP guidelines recommend an IVC filter if anticoagulant therapy is contraindicated (Grade 1C). The anticoagulation was discontinued and a retrievable IVC filter was placed. Once a patient no longer has a contraindication for anticoagulation, the ACCP recommends restarting a conventional course of anticoagulation. Thus, once the patient can tolerate anticoagulation, consideration will be given to removal of the retrievable filter.
Bottom Line
An IVC filter should be considered in patients with a DVT who have a contraindication to anticoagulation. Other indications for IVC filter use are not supported by the current literature. TH
Drs. Bhogal and Eid are hospitalist fellows and instructors at Johns Hopkins Bayview Medical Center in Baltimore. Dr. Kantsiper is a hospitalist and assistant professor at Bayview Medical Center.
References
- The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. U.S. Department of Health & Human Services Web site. Available at: www.surgeongeneral.gov/topics/deepvein/. Accessed Jan. 25, 2010.
- Moser KM, Fedullo PR, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271(3):223-225.
- Bates SM, Ginsberg JS. Treatment of deep vein thrombosis. N Engl J Med. 2004;351:268-277.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians. Antithrombotic therapy for venous theomboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S.
- Becker DM, Philbrick JT, Selby JB. Inferior vena cava filters. Indications, safety, effectiveness. Arch Intern Med. 1992;152(10):1985-1994.
- Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000;95(12):3669-3677.
- Chung J, Owen RJ. Using inferior vena cava filters to prevent pulmonary embolism. Can Fam Physician. 2008;54(1):49-55.
- Ku GH. Billett HH. Long lives, short indications. The case for removable inferior cava filters. Thromb Haemost. 2005;93(1):17-22.
- Stavropoulos WS. Inferior vena cava filters. Tech Vasc Interv Radiol. 2004;7(2):91-95.
- Crowther MA. Inferior vena cava filters in the management of venous thromboembolism. Am J Med. 2007;120(10 Suppl 2):S13–S17.
- Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541-1545.
- Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129(1):174-181.
- Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):401S-428S.
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7):409-415.
- Decousus H, Barral F, Buchmuller-Cordier A, et al. Participating centers eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC randomization croup. Circulation. 2005;112:416-422.
- Kim HS, Young MJ, Narayan AK, Liddell RP, Streiff MB. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol. 2008:19(3):393-399.
- Houman Fekrazad M, Lopes RD, Stashenko GJ, Alexander JH, Garcia D. Treatment of venous thromboembolism: guidelines translated for the clinician. J Thromb Thrombolysis. 2009; 28(3):270–275.
- Comerota AJ. Retrievable IVC filters: a decision matrix for appropriate utilization. Perspect Vasc Surg Endovasc Ther. 2006;18(1):11-17.
- Ray CE Jr, Prochazka A. The need for anticoagulation following inferior vena cava filter placement: systematic review. Cardiovasc Intervent Radiol. 2008; 31(2):316-324.
- Hajduk B, Tomkowski WZ, Malek G, Davidson BL. Vena cava filter occlusion and venous thromboembolism risk in persistently anticoagulated patients: A prospective, observational cohort study. Chest. 2009.
In the Literature
In This Edition
Literature at a Glance: A guide to this month’s studies
- PPI use with clopidrogrel in ACS.
- Chlorhexidine sponge use reduces line infections.
- Extended thienopyridine use does not benefit DES patients.
- CABG is revascularization choice for severe CAD.
- Pre-treated CVC use reduces bloodstream infections.
- Hospitalist use grows in U.S.
- Sepsis order set improves outcomes.
- Admission day predicts acute PE mortality.
PPI Use with Clopidogrel in Acute Coronary Syndrome Is Associated with Readmissions and Mortality
Clinical question: Does concomitant use of clopidogrel and a proton pump inhibitor (PPI) following hospitalization for acute coronary syndrome (ACS) lead to adverse outcomes?
Background: Prophylactic PPIs often are prescribed with clopidogrel to reduce the risk of gastrointestinal bleeding. Mechanistic studies have shown that omeprazole decreases the platelet-inhibitory effect of clopidogrel, raising concerns that PPIs might interfere with clopidogrel’s beneficial effects. The clinical significance of this finding is unknown.
Study design: Retrospective cohort study.
Setting: 127 VA hospitals.
Synopsis: Investigators used data from the Cardiac Care Follow-up Clinical Study and VA pharmacy records to examine 8,205 male veterans who were hospitalized for ACS and treated with clopidogrel. Patients who filled prescriptions for both clopidogrel and a PPI were at significantly higher risk for death or readmission with ACS compared with those who filled prescriptions for clopidogrel only (adjusted odds ratio, 1.25; 95% confidence interval, 1.11-1.41). Patients who filled prescriptions for PPIs alone had similar risk for adverse events as those who took neither medication.
Subanalyses found similarly increased risk among patients prescribed omeprazole and rabeprazole, but those taking lanzoprazole and pantoprazole were not examined due to the small sample size. Although causality cannot be inferred from this observational study, and the risk associated with combined clopidigrel and PPI use appeared small, alternatives for gastric acid reduction exist. Thus, it may be prudent to restrict PPI use to patients who have a clear indication for their use until more definitive clinical trials can be conducted.
Bottom line: Among patients who are treated with clopidogrel for ACS, PPIs should be reserved for patients with a clear indication for gastric acid reduction and who cannot use alternative therapies.
Citation: Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301(9):937-944.
Chlorhexidine-Impregnated Sponge Use Reduces Line Infections
Clinical question: Does dressing vascular catheters with chlorhexidine gluconate-impregnated sponges (CHGIS) reduce rates of catheter-related infections, and are dressing changes every seven days inferior to every three days?
Background: Process improvement strategies—including educating providers, strictly adhering to sterile technique, and promptly removing unnecessary catheters—greatly decrease catheter-related infections. It is unclear if CHGIS dressings offer additional benefit. Also uncertain is whether weekly dressing changes are as safe as changing dressings every three days.
Study design: A 2x2 factorial, assessor-blinded, randomized controlled trial.
Setting: ICUs in three university hospitals and two general hospitals in France.
Synopsis: 1,636 French adults expected to require arterial and central venous catheters for >48 hours were randomly assigned to one of four groups. Each group received either CHGIS dressings or standard dressings, and each group had dressing changes every three or seven days. Dressings were changed sooner if soiled or nonadherent. CHGIS dressings were associated with fewer catheter-related infections than standard dressings (0.6 vs. 1.4 infections per 1,000 catheter days; P=0.03). No significant difference in rates of catheter colonization existed between the three-day and seven-day dressing change strategies (10.4 vs. 11 events per 1,000 catheter days, P>0.05).
Although microbiology assessors were blinded to patients’ status, the ICU staff was not, potentially creating experimenter bias. Approximately 30% of the venous catheters and 40% of the arterial catheters were in a femoral site. Secondary analyses found higher rates of severe dermatitis among patients with CHGIS dressings but no difference in minimal bactericidal concentration (MBC) or colonizing organisms. Preliminary calculations suggested CHGIS dressings could be cost-effective.
Bottom line: Among critically ill adults, CHGIS catheter dressings may marginally reduce catheter-related infection rates, but further evaluation is needed before this technology can be adopted widely.
Citation: Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically-ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231-1241.
Thienopyridine Use Six Months after Sirolimus-Eluting Stent Implant-ation Offers No Benefit
Clinical question: What are the relative contributions of aspirin and thienopyridine on preventing stent thrombosis in patients with sirolimus-eluting stents?
Background: There are no randomized clinical trials addressing the optimal duration, or the risks associated with discontinuation, of dual-antiplatelet therapy after drug-eluting stent (DES) implantation. Nevertheless, many patients continue to be maintained on dual-antiplatelet therapy beyond one year of their index DES implantation.
Study design: Prospective multicenter observational study.
Setting: Hospitals in Japan.
Synopsis: This study observed 10,778 Japanese patients undergoing sirolimus-eluting stent implantation. Patients discontinuing both thienopyridine and aspirin had a significantly higher rate of stent thrombosis than those who continued both medications for up to 18 months. However, discontinuation of thienopyridine alone was not associated with an excess risk of stent thrombosis. Additionally, a landmark analysis of patients who were free of events at six months showed rates of death for myocardial infarction (MI) at 24 months were 4.1% for patients taking thienopyridine and 4.1% for patients not taking thienopyridine (P=0.99). Ticlodipine was the thienopyridine used by more than 95% of patients.
Hospitalists should be aware that the role thienopyridine therapy plays in reducing stent thrombosis beyond one month after implantation has not been well addressed.
Bottom line: Discontinuation of thienopyridine therapy after six months while maintaining aspirin therapy is not associated with increased risk of stent thrombosis in patients with sirolimus-eluting stents.
Citation: Kimura T, Morimoto T, Nakagawa Y, et al. Antiplatelet therapy and stent thrombosis after sirolimus-eluting stent implantation. Circulation. 2009;119(7):987-995.
Compared with PCI, CABG Results in Lower Rates of Major Adverse Events in Severe CAD Patients
Clinical question: What is the optimal revascularization strategy for previously untreated severe coronary artery disease (CAD)?
Background: Coronary artery bypass grafting (CABG) is the treatment of choice in three-vessel and left-main CAD. However, percutaneous coronary intervention (PCI) with drug-eluting stents often is utilized despite the lack of adequately powered randomized trials.
Study design: Prospective multicenter randomized clinical trial.
Setting: 85 hospitals in Europe and the U.S.
Synopsis: 1,800 patients with an average age of 65 and previously untreated three-vessel or left-main CAD amenable to therapy with both PCI and CABG were randomized to CABG or PCI. The primary combined endpoint was a major adverse cardiac or cerebrovascular event, defined as death, stroke, MI, or repeat revascularization. PCI was associated with a significantly higher rate of major adverse cardiac or cerebrovascular events, due mostly to a higher rate of repeat revascularization (13.5% vs. 5.9%, P<0.001). At 12 months, the two groups had similar rates of death from any cause or MI, and similar rates of the combined endpoint of death from any cause, stroke, or MI; however, the rate of stroke was 1.6% higher in the CABG group.
Hospitalists should continue to favor CABG over PCI but give consideration to the risks involved with such an intervention.
Bottom line: CABG remains the revascularization choice in patients with severe CAD.
Citation: Serruys PW, Morice MC, Kappetein AP, et al. Percuta-neous coronary intervention versus coronary artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961-972.
Pre-Medicated Central Venous Catheters Reduce Risk of Catheter-Related Bloodstream Infections
Clinical question: Does pre-treating central venous catheters with anti-infective agents prevent catheter-related bloodstream infections?
Background: Use of central venous catheters (CVC) is associated with catheter-related bloodstream infection (CRBSI), with CRBSI-related mortality rates as high as 25%. Previous reviews have indicated that CVCs coated or impregnated with anti-infectives may reduce CRBSI incidence. This review integrates new trial data with information from prior reviews.
Study design: Meta-analysis of 27 randomized controlled trials.
Setting: Meta-analysis.
Synopsis: The authors report CVCs pre-treated with anti-infectives (AI-CVCs) are clinically effective in reducing the risk of CRBSI. The odds of having a CRBSI with a treated CVC versus an untreated CVC are 0.49 to 1 (95% CI, 0.37–0.64, 27 studies, fixed effects). The study also finds the use of AI-CVCs might provide a large cost savings in Great Britain. Because the findings are based on a meta-analysis, they are limited by the quality, context, and consistency of the original studies. The authors note that many of the studies had unsatisfactory descriptions of methodology. The current study is unable to separate the risk reduction attributable to AI-CVC versus that attributable to other infection control practices. Also, original data is insufficient to assess the benefits of AI-CVCs placed for longer than 12 days.
To summarize, AI-CVCs may present a means to reduce CRBSI, but more investigation of its role within infection control protocols is needed, as is investigation of longer duration of treatment.
Bottom line: Central venous catheters pre-treated with anti-infectives significantly reduce catheter-related bloodstream infections.
Citation: Hockenhull JC, Dwan KM, Smith GW, et al. The clinical effectiveness of central venous catheters treated with anti-infective agents in preventing catheter-related bloodstream infections: a systematic review. Crit Care Med. 2009;37(2):702-712.
Fivefold Increase in Hospitalists in the U.S. from 1995 to 2006
Clinical question: What is the growth rate of hospitalists and hospitalist-provided care?
Background: Survey data has shown a sharp increase in the number of hospitalists, but until now there have not been any national or population-based data on the growth of hospitalist care.
Study design: Descriptive analysis.
Setting: Medicare-enrolled patients.
Synopsis: The study is based on national Medicare data from 2.1 million admissions involving 990,785 patients in 5,800 hospitals and 120,226 general internists. It represents 5% of inpatient Medicare claims generated by general internists. The authors define “hospitalist” as a general internist who generates >90% of his or her claims from the care of hospitalized patients.
U.S. hospitals have seen substantial growth in hospitalists over the period examined. The nation saw a 500% increase in the number of general-internist hospitalists, and a 28% increase (to 37.1% in 2006 from 9.1% in 1995) in the number of Medicare patients who received care from a hospitalist. The odds that a hospitalized Medicare patient received care from a hospitalist increased 29.2% per year from 1997 to 2006. The percentage of hospitals with at least three hospitalists rose to 47.1% in 2006 from 11.6% in 1995.
This analysis might actually have underestimated HM’s growth. Analysis of Medicare claims does not identify pediatric hospitalists and hospitalists who work exclusively within HMOs. This analysis also did not include family practitioners or internal-medicine subspecialists who are hospitalists.
Bottom line: Medicare claims data confirm survey data findings: Hospitalists and hospitalist care has grown sharply over the last decade.
Citation: Kuo YF, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360:1102-1012.
Standardized Order Set for Bacteremic Sepsis Improves LOS and Mortality
Clinical question: Does a standardized order set for bacteremic sepsis impact patient management and outcomes?
Background: Prompt cardiovascular resuscitation and appropriate antibiotics decrease morbidity and mortality in bacteremic sepsis. This study examined whether hospitalwide, standardized sepsis order set improved management and outcomes.
Study design: Retrospective, before-and-after study design.
Setting: 1,200-bed academic medical center.
Synopsis: Two hundred patients with bacteremic severe sepsis were randomly selected from 18 months before the order set was introduced, and 200 were selected from 18 months after the order set was introduced. Primary outcomes measured were quantity of fluid administered and appropriate initial antibiotics. Secondary outcomes measured were hospital mortality and length of stay. Patients in the “after” group received more intravenous fluid (1627±1862 ml vs. 2054±2237 ml, P=.04), more appropriate antibiotics (53.0% vs. 65.5%, P=.01), had shorter hospital stays (28.7±30.1 days vs. 22.4±20.9 days, P=.02), and decreased in-house mortality (55.0% vs. 39.5%, P =<0.01).
The retrospective design of the study limited its ability to determine causal relationship. Extensive education may have contributed to the change (Hawthorne effect). Management in the ICU and ED, not the hospital wards, was the primary reason for mortality difference.
Bottom line: A standardized order set for bacteremic sepsis was associated with increased compliance with evidence-based treatment and improved outcomes. Hospitalists should promptly treat bacteremic sepsis with appropriate fluid resuscitation and antibiotics.
Citation: Thiel SW, Asghar MF, Micek ST, Reichley RM, Doherty JA, Kollef MH. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med. 2009;37(3):819-824.
Admission Day of the Week Predicts Mortality in Patients with Acute Pulmonary Embolus
Clinical question: Do weekend pulmonary embolus (PE) admissions have worse outcomes than weekday admissions?
Background: Studies of patients with acute cardiovascular diagnoses (e.g., stroke, cardiac arrest) have shown higher short-term mortality and longer length of stay (LOS) for weekend versus weekday admissions. PE diagnosis is complex, requiring timely testing and experienced staff who are sometimes unavailable on weekends. Optimal anticoagulation therapy also depends on provider skill.
Study design: Retrospective observational study.
Setting: 186 private Pennsylvania hospitals, January 2000 through November 2002.
Synopsis: Using the Pennsylvania Health Care Cost Containment Council database, the authors reviewed 15,531 records of patients with a primary or secondary PE diagnosis code. The primary outcome was all-cause mortality over 30 days; LOS was the secondary outcome.
Weekend admissions in the highest severity of illness risk class had higher 30-day mortality than weekday admissions. Weekend admissions were significantly more likely than weekday admissions to be clinically unstable and to have abnormal lab parameters. Adjusted for severity of illness risk class, overall mortality was 1.4% higher for weekend versus weekday admissions. All excess mortality came from the sickest group of patients. LOS did not differ.
Less-experienced caregivers or delayed diagnostic testing may play a role in poor outcomes. Patients admitted on weekends might receive delayed care from the first onset of symptoms. This is important because timely therapy has been shown to influence outcomes in acute PE. Reasons for these observed differences should be explored further to help provide more consistent PE management, regardless of admission day.
Bottom line: The sickest patients with PE admitted on weekends experienced small but significantly greater 30-day mortality compared with those admitted on weekdays.
Citation: Aujesky D, Jimenez D, Mor M, Geng M, Fine M, Ibrahim S. Weekend versus weekday admission and mortality after acute pulmonary embolism. Circulation. 2009;119:962-968. TH
In This Edition
Literature at a Glance: A guide to this month’s studies
- PPI use with clopidrogrel in ACS.
- Chlorhexidine sponge use reduces line infections.
- Extended thienopyridine use does not benefit DES patients.
- CABG is revascularization choice for severe CAD.
- Pre-treated CVC use reduces bloodstream infections.
- Hospitalist use grows in U.S.
- Sepsis order set improves outcomes.
- Admission day predicts acute PE mortality.
PPI Use with Clopidogrel in Acute Coronary Syndrome Is Associated with Readmissions and Mortality
Clinical question: Does concomitant use of clopidogrel and a proton pump inhibitor (PPI) following hospitalization for acute coronary syndrome (ACS) lead to adverse outcomes?
Background: Prophylactic PPIs often are prescribed with clopidogrel to reduce the risk of gastrointestinal bleeding. Mechanistic studies have shown that omeprazole decreases the platelet-inhibitory effect of clopidogrel, raising concerns that PPIs might interfere with clopidogrel’s beneficial effects. The clinical significance of this finding is unknown.
Study design: Retrospective cohort study.
Setting: 127 VA hospitals.
Synopsis: Investigators used data from the Cardiac Care Follow-up Clinical Study and VA pharmacy records to examine 8,205 male veterans who were hospitalized for ACS and treated with clopidogrel. Patients who filled prescriptions for both clopidogrel and a PPI were at significantly higher risk for death or readmission with ACS compared with those who filled prescriptions for clopidogrel only (adjusted odds ratio, 1.25; 95% confidence interval, 1.11-1.41). Patients who filled prescriptions for PPIs alone had similar risk for adverse events as those who took neither medication.
Subanalyses found similarly increased risk among patients prescribed omeprazole and rabeprazole, but those taking lanzoprazole and pantoprazole were not examined due to the small sample size. Although causality cannot be inferred from this observational study, and the risk associated with combined clopidigrel and PPI use appeared small, alternatives for gastric acid reduction exist. Thus, it may be prudent to restrict PPI use to patients who have a clear indication for their use until more definitive clinical trials can be conducted.
Bottom line: Among patients who are treated with clopidogrel for ACS, PPIs should be reserved for patients with a clear indication for gastric acid reduction and who cannot use alternative therapies.
Citation: Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301(9):937-944.
Chlorhexidine-Impregnated Sponge Use Reduces Line Infections
Clinical question: Does dressing vascular catheters with chlorhexidine gluconate-impregnated sponges (CHGIS) reduce rates of catheter-related infections, and are dressing changes every seven days inferior to every three days?
Background: Process improvement strategies—including educating providers, strictly adhering to sterile technique, and promptly removing unnecessary catheters—greatly decrease catheter-related infections. It is unclear if CHGIS dressings offer additional benefit. Also uncertain is whether weekly dressing changes are as safe as changing dressings every three days.
Study design: A 2x2 factorial, assessor-blinded, randomized controlled trial.
Setting: ICUs in three university hospitals and two general hospitals in France.
Synopsis: 1,636 French adults expected to require arterial and central venous catheters for >48 hours were randomly assigned to one of four groups. Each group received either CHGIS dressings or standard dressings, and each group had dressing changes every three or seven days. Dressings were changed sooner if soiled or nonadherent. CHGIS dressings were associated with fewer catheter-related infections than standard dressings (0.6 vs. 1.4 infections per 1,000 catheter days; P=0.03). No significant difference in rates of catheter colonization existed between the three-day and seven-day dressing change strategies (10.4 vs. 11 events per 1,000 catheter days, P>0.05).
Although microbiology assessors were blinded to patients’ status, the ICU staff was not, potentially creating experimenter bias. Approximately 30% of the venous catheters and 40% of the arterial catheters were in a femoral site. Secondary analyses found higher rates of severe dermatitis among patients with CHGIS dressings but no difference in minimal bactericidal concentration (MBC) or colonizing organisms. Preliminary calculations suggested CHGIS dressings could be cost-effective.
Bottom line: Among critically ill adults, CHGIS catheter dressings may marginally reduce catheter-related infection rates, but further evaluation is needed before this technology can be adopted widely.
Citation: Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically-ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231-1241.
Thienopyridine Use Six Months after Sirolimus-Eluting Stent Implant-ation Offers No Benefit
Clinical question: What are the relative contributions of aspirin and thienopyridine on preventing stent thrombosis in patients with sirolimus-eluting stents?
Background: There are no randomized clinical trials addressing the optimal duration, or the risks associated with discontinuation, of dual-antiplatelet therapy after drug-eluting stent (DES) implantation. Nevertheless, many patients continue to be maintained on dual-antiplatelet therapy beyond one year of their index DES implantation.
Study design: Prospective multicenter observational study.
Setting: Hospitals in Japan.
Synopsis: This study observed 10,778 Japanese patients undergoing sirolimus-eluting stent implantation. Patients discontinuing both thienopyridine and aspirin had a significantly higher rate of stent thrombosis than those who continued both medications for up to 18 months. However, discontinuation of thienopyridine alone was not associated with an excess risk of stent thrombosis. Additionally, a landmark analysis of patients who were free of events at six months showed rates of death for myocardial infarction (MI) at 24 months were 4.1% for patients taking thienopyridine and 4.1% for patients not taking thienopyridine (P=0.99). Ticlodipine was the thienopyridine used by more than 95% of patients.
Hospitalists should be aware that the role thienopyridine therapy plays in reducing stent thrombosis beyond one month after implantation has not been well addressed.
Bottom line: Discontinuation of thienopyridine therapy after six months while maintaining aspirin therapy is not associated with increased risk of stent thrombosis in patients with sirolimus-eluting stents.
Citation: Kimura T, Morimoto T, Nakagawa Y, et al. Antiplatelet therapy and stent thrombosis after sirolimus-eluting stent implantation. Circulation. 2009;119(7):987-995.
Compared with PCI, CABG Results in Lower Rates of Major Adverse Events in Severe CAD Patients
Clinical question: What is the optimal revascularization strategy for previously untreated severe coronary artery disease (CAD)?
Background: Coronary artery bypass grafting (CABG) is the treatment of choice in three-vessel and left-main CAD. However, percutaneous coronary intervention (PCI) with drug-eluting stents often is utilized despite the lack of adequately powered randomized trials.
Study design: Prospective multicenter randomized clinical trial.
Setting: 85 hospitals in Europe and the U.S.
Synopsis: 1,800 patients with an average age of 65 and previously untreated three-vessel or left-main CAD amenable to therapy with both PCI and CABG were randomized to CABG or PCI. The primary combined endpoint was a major adverse cardiac or cerebrovascular event, defined as death, stroke, MI, or repeat revascularization. PCI was associated with a significantly higher rate of major adverse cardiac or cerebrovascular events, due mostly to a higher rate of repeat revascularization (13.5% vs. 5.9%, P<0.001). At 12 months, the two groups had similar rates of death from any cause or MI, and similar rates of the combined endpoint of death from any cause, stroke, or MI; however, the rate of stroke was 1.6% higher in the CABG group.
Hospitalists should continue to favor CABG over PCI but give consideration to the risks involved with such an intervention.
Bottom line: CABG remains the revascularization choice in patients with severe CAD.
Citation: Serruys PW, Morice MC, Kappetein AP, et al. Percuta-neous coronary intervention versus coronary artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961-972.
Pre-Medicated Central Venous Catheters Reduce Risk of Catheter-Related Bloodstream Infections
Clinical question: Does pre-treating central venous catheters with anti-infective agents prevent catheter-related bloodstream infections?
Background: Use of central venous catheters (CVC) is associated with catheter-related bloodstream infection (CRBSI), with CRBSI-related mortality rates as high as 25%. Previous reviews have indicated that CVCs coated or impregnated with anti-infectives may reduce CRBSI incidence. This review integrates new trial data with information from prior reviews.
Study design: Meta-analysis of 27 randomized controlled trials.
Setting: Meta-analysis.
Synopsis: The authors report CVCs pre-treated with anti-infectives (AI-CVCs) are clinically effective in reducing the risk of CRBSI. The odds of having a CRBSI with a treated CVC versus an untreated CVC are 0.49 to 1 (95% CI, 0.37–0.64, 27 studies, fixed effects). The study also finds the use of AI-CVCs might provide a large cost savings in Great Britain. Because the findings are based on a meta-analysis, they are limited by the quality, context, and consistency of the original studies. The authors note that many of the studies had unsatisfactory descriptions of methodology. The current study is unable to separate the risk reduction attributable to AI-CVC versus that attributable to other infection control practices. Also, original data is insufficient to assess the benefits of AI-CVCs placed for longer than 12 days.
To summarize, AI-CVCs may present a means to reduce CRBSI, but more investigation of its role within infection control protocols is needed, as is investigation of longer duration of treatment.
Bottom line: Central venous catheters pre-treated with anti-infectives significantly reduce catheter-related bloodstream infections.
Citation: Hockenhull JC, Dwan KM, Smith GW, et al. The clinical effectiveness of central venous catheters treated with anti-infective agents in preventing catheter-related bloodstream infections: a systematic review. Crit Care Med. 2009;37(2):702-712.
Fivefold Increase in Hospitalists in the U.S. from 1995 to 2006
Clinical question: What is the growth rate of hospitalists and hospitalist-provided care?
Background: Survey data has shown a sharp increase in the number of hospitalists, but until now there have not been any national or population-based data on the growth of hospitalist care.
Study design: Descriptive analysis.
Setting: Medicare-enrolled patients.
Synopsis: The study is based on national Medicare data from 2.1 million admissions involving 990,785 patients in 5,800 hospitals and 120,226 general internists. It represents 5% of inpatient Medicare claims generated by general internists. The authors define “hospitalist” as a general internist who generates >90% of his or her claims from the care of hospitalized patients.
U.S. hospitals have seen substantial growth in hospitalists over the period examined. The nation saw a 500% increase in the number of general-internist hospitalists, and a 28% increase (to 37.1% in 2006 from 9.1% in 1995) in the number of Medicare patients who received care from a hospitalist. The odds that a hospitalized Medicare patient received care from a hospitalist increased 29.2% per year from 1997 to 2006. The percentage of hospitals with at least three hospitalists rose to 47.1% in 2006 from 11.6% in 1995.
This analysis might actually have underestimated HM’s growth. Analysis of Medicare claims does not identify pediatric hospitalists and hospitalists who work exclusively within HMOs. This analysis also did not include family practitioners or internal-medicine subspecialists who are hospitalists.
Bottom line: Medicare claims data confirm survey data findings: Hospitalists and hospitalist care has grown sharply over the last decade.
Citation: Kuo YF, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360:1102-1012.
Standardized Order Set for Bacteremic Sepsis Improves LOS and Mortality
Clinical question: Does a standardized order set for bacteremic sepsis impact patient management and outcomes?
Background: Prompt cardiovascular resuscitation and appropriate antibiotics decrease morbidity and mortality in bacteremic sepsis. This study examined whether hospitalwide, standardized sepsis order set improved management and outcomes.
Study design: Retrospective, before-and-after study design.
Setting: 1,200-bed academic medical center.
Synopsis: Two hundred patients with bacteremic severe sepsis were randomly selected from 18 months before the order set was introduced, and 200 were selected from 18 months after the order set was introduced. Primary outcomes measured were quantity of fluid administered and appropriate initial antibiotics. Secondary outcomes measured were hospital mortality and length of stay. Patients in the “after” group received more intravenous fluid (1627±1862 ml vs. 2054±2237 ml, P=.04), more appropriate antibiotics (53.0% vs. 65.5%, P=.01), had shorter hospital stays (28.7±30.1 days vs. 22.4±20.9 days, P=.02), and decreased in-house mortality (55.0% vs. 39.5%, P =<0.01).
The retrospective design of the study limited its ability to determine causal relationship. Extensive education may have contributed to the change (Hawthorne effect). Management in the ICU and ED, not the hospital wards, was the primary reason for mortality difference.
Bottom line: A standardized order set for bacteremic sepsis was associated with increased compliance with evidence-based treatment and improved outcomes. Hospitalists should promptly treat bacteremic sepsis with appropriate fluid resuscitation and antibiotics.
Citation: Thiel SW, Asghar MF, Micek ST, Reichley RM, Doherty JA, Kollef MH. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med. 2009;37(3):819-824.
Admission Day of the Week Predicts Mortality in Patients with Acute Pulmonary Embolus
Clinical question: Do weekend pulmonary embolus (PE) admissions have worse outcomes than weekday admissions?
Background: Studies of patients with acute cardiovascular diagnoses (e.g., stroke, cardiac arrest) have shown higher short-term mortality and longer length of stay (LOS) for weekend versus weekday admissions. PE diagnosis is complex, requiring timely testing and experienced staff who are sometimes unavailable on weekends. Optimal anticoagulation therapy also depends on provider skill.
Study design: Retrospective observational study.
Setting: 186 private Pennsylvania hospitals, January 2000 through November 2002.
Synopsis: Using the Pennsylvania Health Care Cost Containment Council database, the authors reviewed 15,531 records of patients with a primary or secondary PE diagnosis code. The primary outcome was all-cause mortality over 30 days; LOS was the secondary outcome.
Weekend admissions in the highest severity of illness risk class had higher 30-day mortality than weekday admissions. Weekend admissions were significantly more likely than weekday admissions to be clinically unstable and to have abnormal lab parameters. Adjusted for severity of illness risk class, overall mortality was 1.4% higher for weekend versus weekday admissions. All excess mortality came from the sickest group of patients. LOS did not differ.
Less-experienced caregivers or delayed diagnostic testing may play a role in poor outcomes. Patients admitted on weekends might receive delayed care from the first onset of symptoms. This is important because timely therapy has been shown to influence outcomes in acute PE. Reasons for these observed differences should be explored further to help provide more consistent PE management, regardless of admission day.
Bottom line: The sickest patients with PE admitted on weekends experienced small but significantly greater 30-day mortality compared with those admitted on weekdays.
Citation: Aujesky D, Jimenez D, Mor M, Geng M, Fine M, Ibrahim S. Weekend versus weekday admission and mortality after acute pulmonary embolism. Circulation. 2009;119:962-968. TH
In This Edition
Literature at a Glance: A guide to this month’s studies
- PPI use with clopidrogrel in ACS.
- Chlorhexidine sponge use reduces line infections.
- Extended thienopyridine use does not benefit DES patients.
- CABG is revascularization choice for severe CAD.
- Pre-treated CVC use reduces bloodstream infections.
- Hospitalist use grows in U.S.
- Sepsis order set improves outcomes.
- Admission day predicts acute PE mortality.
PPI Use with Clopidogrel in Acute Coronary Syndrome Is Associated with Readmissions and Mortality
Clinical question: Does concomitant use of clopidogrel and a proton pump inhibitor (PPI) following hospitalization for acute coronary syndrome (ACS) lead to adverse outcomes?
Background: Prophylactic PPIs often are prescribed with clopidogrel to reduce the risk of gastrointestinal bleeding. Mechanistic studies have shown that omeprazole decreases the platelet-inhibitory effect of clopidogrel, raising concerns that PPIs might interfere with clopidogrel’s beneficial effects. The clinical significance of this finding is unknown.
Study design: Retrospective cohort study.
Setting: 127 VA hospitals.
Synopsis: Investigators used data from the Cardiac Care Follow-up Clinical Study and VA pharmacy records to examine 8,205 male veterans who were hospitalized for ACS and treated with clopidogrel. Patients who filled prescriptions for both clopidogrel and a PPI were at significantly higher risk for death or readmission with ACS compared with those who filled prescriptions for clopidogrel only (adjusted odds ratio, 1.25; 95% confidence interval, 1.11-1.41). Patients who filled prescriptions for PPIs alone had similar risk for adverse events as those who took neither medication.
Subanalyses found similarly increased risk among patients prescribed omeprazole and rabeprazole, but those taking lanzoprazole and pantoprazole were not examined due to the small sample size. Although causality cannot be inferred from this observational study, and the risk associated with combined clopidigrel and PPI use appeared small, alternatives for gastric acid reduction exist. Thus, it may be prudent to restrict PPI use to patients who have a clear indication for their use until more definitive clinical trials can be conducted.
Bottom line: Among patients who are treated with clopidogrel for ACS, PPIs should be reserved for patients with a clear indication for gastric acid reduction and who cannot use alternative therapies.
Citation: Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301(9):937-944.
Chlorhexidine-Impregnated Sponge Use Reduces Line Infections
Clinical question: Does dressing vascular catheters with chlorhexidine gluconate-impregnated sponges (CHGIS) reduce rates of catheter-related infections, and are dressing changes every seven days inferior to every three days?
Background: Process improvement strategies—including educating providers, strictly adhering to sterile technique, and promptly removing unnecessary catheters—greatly decrease catheter-related infections. It is unclear if CHGIS dressings offer additional benefit. Also uncertain is whether weekly dressing changes are as safe as changing dressings every three days.
Study design: A 2x2 factorial, assessor-blinded, randomized controlled trial.
Setting: ICUs in three university hospitals and two general hospitals in France.
Synopsis: 1,636 French adults expected to require arterial and central venous catheters for >48 hours were randomly assigned to one of four groups. Each group received either CHGIS dressings or standard dressings, and each group had dressing changes every three or seven days. Dressings were changed sooner if soiled or nonadherent. CHGIS dressings were associated with fewer catheter-related infections than standard dressings (0.6 vs. 1.4 infections per 1,000 catheter days; P=0.03). No significant difference in rates of catheter colonization existed between the three-day and seven-day dressing change strategies (10.4 vs. 11 events per 1,000 catheter days, P>0.05).
Although microbiology assessors were blinded to patients’ status, the ICU staff was not, potentially creating experimenter bias. Approximately 30% of the venous catheters and 40% of the arterial catheters were in a femoral site. Secondary analyses found higher rates of severe dermatitis among patients with CHGIS dressings but no difference in minimal bactericidal concentration (MBC) or colonizing organisms. Preliminary calculations suggested CHGIS dressings could be cost-effective.
Bottom line: Among critically ill adults, CHGIS catheter dressings may marginally reduce catheter-related infection rates, but further evaluation is needed before this technology can be adopted widely.
Citation: Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically-ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231-1241.
Thienopyridine Use Six Months after Sirolimus-Eluting Stent Implant-ation Offers No Benefit
Clinical question: What are the relative contributions of aspirin and thienopyridine on preventing stent thrombosis in patients with sirolimus-eluting stents?
Background: There are no randomized clinical trials addressing the optimal duration, or the risks associated with discontinuation, of dual-antiplatelet therapy after drug-eluting stent (DES) implantation. Nevertheless, many patients continue to be maintained on dual-antiplatelet therapy beyond one year of their index DES implantation.
Study design: Prospective multicenter observational study.
Setting: Hospitals in Japan.
Synopsis: This study observed 10,778 Japanese patients undergoing sirolimus-eluting stent implantation. Patients discontinuing both thienopyridine and aspirin had a significantly higher rate of stent thrombosis than those who continued both medications for up to 18 months. However, discontinuation of thienopyridine alone was not associated with an excess risk of stent thrombosis. Additionally, a landmark analysis of patients who were free of events at six months showed rates of death for myocardial infarction (MI) at 24 months were 4.1% for patients taking thienopyridine and 4.1% for patients not taking thienopyridine (P=0.99). Ticlodipine was the thienopyridine used by more than 95% of patients.
Hospitalists should be aware that the role thienopyridine therapy plays in reducing stent thrombosis beyond one month after implantation has not been well addressed.
Bottom line: Discontinuation of thienopyridine therapy after six months while maintaining aspirin therapy is not associated with increased risk of stent thrombosis in patients with sirolimus-eluting stents.
Citation: Kimura T, Morimoto T, Nakagawa Y, et al. Antiplatelet therapy and stent thrombosis after sirolimus-eluting stent implantation. Circulation. 2009;119(7):987-995.
Compared with PCI, CABG Results in Lower Rates of Major Adverse Events in Severe CAD Patients
Clinical question: What is the optimal revascularization strategy for previously untreated severe coronary artery disease (CAD)?
Background: Coronary artery bypass grafting (CABG) is the treatment of choice in three-vessel and left-main CAD. However, percutaneous coronary intervention (PCI) with drug-eluting stents often is utilized despite the lack of adequately powered randomized trials.
Study design: Prospective multicenter randomized clinical trial.
Setting: 85 hospitals in Europe and the U.S.
Synopsis: 1,800 patients with an average age of 65 and previously untreated three-vessel or left-main CAD amenable to therapy with both PCI and CABG were randomized to CABG or PCI. The primary combined endpoint was a major adverse cardiac or cerebrovascular event, defined as death, stroke, MI, or repeat revascularization. PCI was associated with a significantly higher rate of major adverse cardiac or cerebrovascular events, due mostly to a higher rate of repeat revascularization (13.5% vs. 5.9%, P<0.001). At 12 months, the two groups had similar rates of death from any cause or MI, and similar rates of the combined endpoint of death from any cause, stroke, or MI; however, the rate of stroke was 1.6% higher in the CABG group.
Hospitalists should continue to favor CABG over PCI but give consideration to the risks involved with such an intervention.
Bottom line: CABG remains the revascularization choice in patients with severe CAD.
Citation: Serruys PW, Morice MC, Kappetein AP, et al. Percuta-neous coronary intervention versus coronary artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961-972.
Pre-Medicated Central Venous Catheters Reduce Risk of Catheter-Related Bloodstream Infections
Clinical question: Does pre-treating central venous catheters with anti-infective agents prevent catheter-related bloodstream infections?
Background: Use of central venous catheters (CVC) is associated with catheter-related bloodstream infection (CRBSI), with CRBSI-related mortality rates as high as 25%. Previous reviews have indicated that CVCs coated or impregnated with anti-infectives may reduce CRBSI incidence. This review integrates new trial data with information from prior reviews.
Study design: Meta-analysis of 27 randomized controlled trials.
Setting: Meta-analysis.
Synopsis: The authors report CVCs pre-treated with anti-infectives (AI-CVCs) are clinically effective in reducing the risk of CRBSI. The odds of having a CRBSI with a treated CVC versus an untreated CVC are 0.49 to 1 (95% CI, 0.37–0.64, 27 studies, fixed effects). The study also finds the use of AI-CVCs might provide a large cost savings in Great Britain. Because the findings are based on a meta-analysis, they are limited by the quality, context, and consistency of the original studies. The authors note that many of the studies had unsatisfactory descriptions of methodology. The current study is unable to separate the risk reduction attributable to AI-CVC versus that attributable to other infection control practices. Also, original data is insufficient to assess the benefits of AI-CVCs placed for longer than 12 days.
To summarize, AI-CVCs may present a means to reduce CRBSI, but more investigation of its role within infection control protocols is needed, as is investigation of longer duration of treatment.
Bottom line: Central venous catheters pre-treated with anti-infectives significantly reduce catheter-related bloodstream infections.
Citation: Hockenhull JC, Dwan KM, Smith GW, et al. The clinical effectiveness of central venous catheters treated with anti-infective agents in preventing catheter-related bloodstream infections: a systematic review. Crit Care Med. 2009;37(2):702-712.
Fivefold Increase in Hospitalists in the U.S. from 1995 to 2006
Clinical question: What is the growth rate of hospitalists and hospitalist-provided care?
Background: Survey data has shown a sharp increase in the number of hospitalists, but until now there have not been any national or population-based data on the growth of hospitalist care.
Study design: Descriptive analysis.
Setting: Medicare-enrolled patients.
Synopsis: The study is based on national Medicare data from 2.1 million admissions involving 990,785 patients in 5,800 hospitals and 120,226 general internists. It represents 5% of inpatient Medicare claims generated by general internists. The authors define “hospitalist” as a general internist who generates >90% of his or her claims from the care of hospitalized patients.
U.S. hospitals have seen substantial growth in hospitalists over the period examined. The nation saw a 500% increase in the number of general-internist hospitalists, and a 28% increase (to 37.1% in 2006 from 9.1% in 1995) in the number of Medicare patients who received care from a hospitalist. The odds that a hospitalized Medicare patient received care from a hospitalist increased 29.2% per year from 1997 to 2006. The percentage of hospitals with at least three hospitalists rose to 47.1% in 2006 from 11.6% in 1995.
This analysis might actually have underestimated HM’s growth. Analysis of Medicare claims does not identify pediatric hospitalists and hospitalists who work exclusively within HMOs. This analysis also did not include family practitioners or internal-medicine subspecialists who are hospitalists.
Bottom line: Medicare claims data confirm survey data findings: Hospitalists and hospitalist care has grown sharply over the last decade.
Citation: Kuo YF, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360:1102-1012.
Standardized Order Set for Bacteremic Sepsis Improves LOS and Mortality
Clinical question: Does a standardized order set for bacteremic sepsis impact patient management and outcomes?
Background: Prompt cardiovascular resuscitation and appropriate antibiotics decrease morbidity and mortality in bacteremic sepsis. This study examined whether hospitalwide, standardized sepsis order set improved management and outcomes.
Study design: Retrospective, before-and-after study design.
Setting: 1,200-bed academic medical center.
Synopsis: Two hundred patients with bacteremic severe sepsis were randomly selected from 18 months before the order set was introduced, and 200 were selected from 18 months after the order set was introduced. Primary outcomes measured were quantity of fluid administered and appropriate initial antibiotics. Secondary outcomes measured were hospital mortality and length of stay. Patients in the “after” group received more intravenous fluid (1627±1862 ml vs. 2054±2237 ml, P=.04), more appropriate antibiotics (53.0% vs. 65.5%, P=.01), had shorter hospital stays (28.7±30.1 days vs. 22.4±20.9 days, P=.02), and decreased in-house mortality (55.0% vs. 39.5%, P =<0.01).
The retrospective design of the study limited its ability to determine causal relationship. Extensive education may have contributed to the change (Hawthorne effect). Management in the ICU and ED, not the hospital wards, was the primary reason for mortality difference.
Bottom line: A standardized order set for bacteremic sepsis was associated with increased compliance with evidence-based treatment and improved outcomes. Hospitalists should promptly treat bacteremic sepsis with appropriate fluid resuscitation and antibiotics.
Citation: Thiel SW, Asghar MF, Micek ST, Reichley RM, Doherty JA, Kollef MH. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med. 2009;37(3):819-824.
Admission Day of the Week Predicts Mortality in Patients with Acute Pulmonary Embolus
Clinical question: Do weekend pulmonary embolus (PE) admissions have worse outcomes than weekday admissions?
Background: Studies of patients with acute cardiovascular diagnoses (e.g., stroke, cardiac arrest) have shown higher short-term mortality and longer length of stay (LOS) for weekend versus weekday admissions. PE diagnosis is complex, requiring timely testing and experienced staff who are sometimes unavailable on weekends. Optimal anticoagulation therapy also depends on provider skill.
Study design: Retrospective observational study.
Setting: 186 private Pennsylvania hospitals, January 2000 through November 2002.
Synopsis: Using the Pennsylvania Health Care Cost Containment Council database, the authors reviewed 15,531 records of patients with a primary or secondary PE diagnosis code. The primary outcome was all-cause mortality over 30 days; LOS was the secondary outcome.
Weekend admissions in the highest severity of illness risk class had higher 30-day mortality than weekday admissions. Weekend admissions were significantly more likely than weekday admissions to be clinically unstable and to have abnormal lab parameters. Adjusted for severity of illness risk class, overall mortality was 1.4% higher for weekend versus weekday admissions. All excess mortality came from the sickest group of patients. LOS did not differ.
Less-experienced caregivers or delayed diagnostic testing may play a role in poor outcomes. Patients admitted on weekends might receive delayed care from the first onset of symptoms. This is important because timely therapy has been shown to influence outcomes in acute PE. Reasons for these observed differences should be explored further to help provide more consistent PE management, regardless of admission day.
Bottom line: The sickest patients with PE admitted on weekends experienced small but significantly greater 30-day mortality compared with those admitted on weekdays.
Citation: Aujesky D, Jimenez D, Mor M, Geng M, Fine M, Ibrahim S. Weekend versus weekday admission and mortality after acute pulmonary embolism. Circulation. 2009;119:962-968. TH