User login
Nosocomial Pneumonia
(This chapter has been reprinted with permission from Williams MV, Hayward R: Comprehensive Hospital Medicine, 1st edition. Philadelphia, WB Saunders, in press.)
Background
Nosocomial pneumonia (NP) is the leading cause of mortality among patients who die from hospital-acquired infections. Defined as pneumonia occurring 48 hours or more after hospital admission, NP also includes the subset of ventilator-associated pneumonia (VAP), defined as pneumonia developing 48 to 72 hours after initiation of mechanical ventilation. The incidence of NP is between 5 and 15 cases per 1000 hospital admissions. Healthcare-associated pneumonia (HCAP), part of the continuum of NP, describes an increasingly common proportion of pneumonia developing outside the hospital (Table I) (1). Typically afflicting people in a nursing home or assisted living setting, these patients are at risk for antibiotic-resistant-organisms and should be approached similarly to cases of nosocomial pneumonia rather than community-acquired pneumonia. Most of the data informing our diagnostic and treatment decisions about NP come from studies performed in mechanically ventilated patients and are extrapolated to make recommendations for non-ventilated patients.
Mortality attributable to NP is debated, but may be as high as 30%. The presence of nosocomial pneumonia increases hospital length of stay an average of 7–10 days, and in the case of VAP, is estimated to cost between $10,000 and $40,000 per case (2).
Assessment
Clinical Presentation
Signs and Symptoms
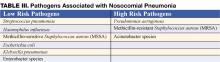
Nosocomial pneumonia is usually diagnosed based on clinical grounds. Typical symptoms and signs consist of fever, cough with sputum, and shortness of breath in the setting of hypoxia and a new infiltrate on chest radiograph (CXR). In the elderly, signs may be more subtle and delirium, fever, or leukocytosis in the absence of cough should trigger its consideration. The likelihood of NP increases among patients with risk factors for microaspiration, oropharyngeal colonization, or overgrowth of resistant organisms (Table II) (3).
Differential Diagnosis
Prior to settling on a diagnosis of NP, alternative causes of fever, hypoxia, and pulmonary infiltrates should be considered. Most commonly, these include pulmonary embolus, pulmonary edema, or atelectasis. Alternative infectious sources, such as urinary tract, skin and soft-tissue infections, and device-related infections (i.e., central venous catheters) are common in hospitalized patients and should be ruled out before diagnosing nosocomial pneumonia.
Diagnosis
Diagnostic strategies for NP seek to confirm the diagnosis and identify an etiologic pathogen, thus allowing timely, effective, and streamlined antibiotic therapy. Unfortunately, no consensus exists on the best approach to diagnosing nosocomial pneumonia. After obtaining a complete blood count and blood cultures, you can choose between a clinical or microbiologic diagnostic approach to diagnosis. A clinical diagnosis relies on a new or progressive radiographic infiltrate along with signs of infection such as fever, leukocytosis, or purulent sputum. Clinical diagnosis is sensitive, but is likely to lead to antibiotic overuse. The microbiologic approach requires sampling of secretions from the respiratory tract and may reduce inappropriate antibiotic use, but takes longer and may not be available in all hospitals.
Preferred Studies
The microbiologic approach to diagnosis relies on the use of quantitative or semi-quantitative cultures to create thresholds for antibiotic treatment. Bacterial cultures that demonstrate a level of growth above the thresholds described below warrant treatment, while those below it should trigger withholding or discontinuation of antibiotics.
Bronchoscopic Approaches: Bronchoalveolar lavage (BAL) with a cutoff of 10 (4) organisms/mL or protected specimen brush (PSB) with a cutoff of 10 (3) organisms/mL are felt to be the most specific diagnostic tests when performed prior to initiating antibiotics, or prior to changing antibiotics if a patient is already receiving them. In clinically stable patients, antibiotics can be safely discontinued if bacterial growth falls below the thresholds. If cultures are positive, antibiotic therapy should be tailored to target the organism identified. The bronchoscopic approach is favored in patients who are mechanically ventilated, develop their pneumonia late in the hospital stay (>5–7 days), are at risk for unusual pathogens, are failing therapy or suspected of having an alternative diagnosis.
Non-Bronchoscopic Approaches: Qualitative endotracheal aspirates (ETA) have been shown to be quite sensitive in ventilated patients, regularly identify organisms that may be subsequently found by BAL or PSB, and if negative, should result in withholding antibiotics. Quantitative endotracheal aspirates with a cutoff of 10 (6) organisms/mL are often encouraged to reduce antibiotic overuse, but results should be interpreted cautiously as they only have a sensitivity and specificity of about 75% (1). Consideration should be given to withholding antibiotics in a clinically stable patient with a negative quantitative ETA if antibiotics have not been changed in the preceding 72 hours. Many ICUs have begun to perform blinded sampling of lower respiratory tract secretions with suction catheters (blind PSB, blind mini-BAL). These techniques can be performed at all hours by trained respiratory therapists or nurses, provide culture data similar to that of bronchoscopy, and may be safer and less costly than bronchoscopy. In general, non-bronchoscopic techniques are preferred in patients who are not mechanically ventilated. Sputum sampling, while easy to obtain, has not been well studied in NP. However, in patients in whom bronchoscopic or other non-bronchoscopic techniques are not feasible, sputum sampling may be performed to identify potentially resistant organisms and help tailor therapy.
Alternative Options
Clinical Pulmonary Infection Score—Combining Clinical and Microbiologic Approaches
The clinical diagnosis of nosocomial pneumonia (new infiltrate + fever, leukocytosis, or purulent sputum) likely leads to antibiotic overuse, yet pursuing a bronchoscopic diagnosis is invasive, costly, and requires technical expertise. The quantitative ETA, blind PSB, and blind BAL discussed above are examples of some compromises that avoid the need for bronchoscopy, yet add microbiologic data in an attempt to prevent excess antibiotic therapy. Formally combining diagnostic approaches (clinical + microbiologic) may also be useful. One such option is the use of the clinical pulmonary infection score (CPIS), which combines clinical, radiographic, physiological, and microbiologic data into a numerical result. Scores >6 have been shown to correlate well with quantitative BAL (4). More recent studies, however, have suggested a lower specificity which could still result in antibiotic overuse, but this approach remains more accurate than a general clinical approach. Using the CPIS serially at the time NP is suspected and again at 72 hours may be more useful. Patients with an initial low clinical suspicion for pneumonia (CPIS of 6 or less) could have antibiotics safely discontinued at 72 hours if the CPIS remains low (5). Such a strategy may be useful in settings where more sophisticated diagnostic modalities are not available.
Multiple studies of biological markers of infection have attempted to find a non-invasive, rapid, accurate means of determining who needs antibiotics for presumed NP. Unfortunately, the results have largely been disappointing. More recently, measurement of a soluble triggering receptor expressed on myeloid cells (sTREM-1) that is upregulated in the setting of infection has been shown to improve our ability to diagnose NP accurately. Measurement of sTREM-1 was 98% sensitive and 90% specific for the diagnosis of pneumonia in mechanically ventilated patients (6). While promising, more data is needed before this test can be recommended for routine use.
Management
Initial Treatment
Early initiation of adequate empiric antibiotic therapy (i.e., the antibiotics administered are shown to be active against all organisms isolated) is associated with improved survival compared with initial inadequate therapy (1,7). Antibiotics should be started immediately after obtaining blood and sputum samples for culture and should not be withheld in the event of delay in diagnostic testing. The need to choose antibiotics quickly and expeditiously drives the use of broad spectrum antibiotics. In an effort to avoid unnecessary overuse of broad spectrum antibiotics, therapy should be based on risk for multidrug-resistant (MDR) pathogens. Identifying patients at low risk for MDR pathogens by clinical criteria allows for more narrow, but effective, antibiotic therapy. Low risk patients include those who develop their pneumonia early in the hospitalization (<5–7 days), are not immunocompromised, have not had prior broad spectrum antibiotics, and do not have risk factors for HCAP (Table I) (1,7). In these patients antibiotics should target common community-acquired organisms (Table III–low risk pathogens). Appropriate initial antibiotic therapy could include a third generation cephalosporin or a beta-lactam/beta-lactamase inhibitor. In some communities or hospital wards the incidence of methicillin-resistance among Staphylococcus aureus isolates (MRSA) may be high enough to warrant initial empiric therapy with vancomycin or linezolid.
Unfortunately, today’s increasingly complex hospitalized patients are unlikely to be “low risk,” especially in intensive care units.
Patients not meeting low risk criteria are considered to be at high risk for MDR pathogens (Table III–high risk pathogens). Initial empiric therapy needs to be broad and should include one antipseudomonal agent (cefepime or imipenem or beta-lactam/beta-lactamase inhibitor) plus a fluoroquinolone or aminoglycoside plus vancomycin or linezolid. The specific initial empiric therapy should be dictated by local resistance patterns, cost, and availability of preferred agents. When such broad spectrum therapy is initiated, it becomes imperative that antibiotics are “de-escalated” to limit antibiotic overuse. De-escalation therapy focuses on narrowing the antibiotic spectrum based on culture results, and limiting the overall duration of therapy. Hospitalists should aim to accomplish such de-escalation within 48–72 hours of initiating broad-spectrum antibiotics.
Subsequent Treatment
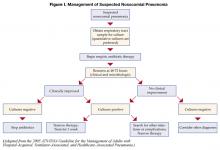
Patients started on initial empiric antibiotic therapy for presumed nosocomial pneumonia should be reassessed at 48–72 hours. Specifically, cultures should be checked and the clinical response to treatment evaluated. Figure I describes an algorithm for guiding treatment (1). In patients who are clinically stable and have negative lower respiratory tract cultures, antibiotics can be stopped. Patients with positive cultures should have antibiotics tailored, or “de-escalated” based on the organisms identified. In general, the most narrow spectrum antibiotic that is active against the bacteria isolated should be used. The use of combination therapy for gram negative organisms (two or more antibiotics active against a bacterial isolate) is widely practiced to achieve synergy, or prevent the development of resistance. However, in the absence of neutropenia, combination therapy has not been shown to be superior to monotherapyy (8), and monotherapy is preferred. The isolation of MRSA from a respiratory sample should also result in use of monotherapy. While some studies have suggested that linezolid may be superior to vancomycin for MRSA pneumonia, this finding needs validation in prospective studies.
A second component of de-escalation is shortening the total duration of therapy. The CPIS may be used to shorten the duration of therapy in patients at low risk for pneumonia. Investigators at a Veterans Affairs medical center randomized patients suspected of having NP, but who had a CPIS score < 6, to either treatment for 10–21 days, or short course therapy. Patients receiving short course therapy were reassessed at day 3, and if their CPIS score remained < 6, antibiotics were stopped (5). The short course therapy group had no difference in mortality when compared to the standard treatment group, but had less antibiotic use, shorter ICU stays, and was less likely to develop a superinfection or infection with a resistant organism. If the CPIS is not used, or if patients are felt to be at higher risk or convincingly demonstrated to have NP, a shorter course of therapy may still be preferred. A large randomized trial showed that 8 days of antibiotic therapy for patients with VAP resulted in similar clinical outcomes when compared to 15 days of therapy. Additionally, shorter duration antibiotic therapy was associated with lower likelihood of developing subsequent infections with multi-resistant pathogens. A subset of patients in the 8 day treatment group infected with non-fermenting gram negative bacilli (e.g., Pseudomonas aeruginosa) did have a higher pulmonary infection recurrence rate, but due to aggressive surveillance, this did not translate into a higher mortality risk in this subset of patients (9).
In summary, treatment of patients with suspected NP starts with immediate initiation of antibiotics and collection of respiratory secretions. While low risk patients can receive narrower spectrum therapy, most patients will require broad initial empiric therapy. The antibiotic regimen, however, should be narrowed at 48–72 hours based on microbiological results if the patient is improving. Overall treatment duration of 1 week is safe and effective with less chance of promoting growth of resistant organisms. In the subset of patients with pseudomonal infections, treatment of 1 week duration should be followed by active surveillance for recurrence, or alternatively, treatment can be extended to two weeks.
Prognosis
Once treatment for NP is initiated, clinical improvement is usually seen by 48–72 hours. There is little support for following either microbiologic response (clearance of positive cultures) or the response by chest radiography. The chest radiograph often lags behind the clinical response, however, a markedly worsening CXR (>50% increase in infiltrate) within the first 48 hours may indicate treatment failure. Clinical resolution as measured by temperature, white blood cell count, and oxygenation usually occurs by 6–7 days (10). Failure of oxygenation to improve by 72 hours has been shown to be predictive of treatment failure.
The overall mortality in patients with NP is as high as 30–70%, largely due to severe comorbid disease in the at risk population. Higher mortality rates are seen in patients with VAP and resistant organisms. The mortality attributable to the episode of NP is about 30%, and can be reduced to <15% with appropriate antibiotic therapy (1).
Prevention
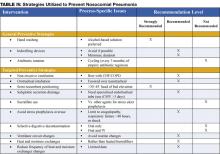
Preventive strategies are either directed at reducing the overall incidence of infectious complications in hospitalized patients, or they are specifically targeted at reducing the incidence of nosocomial pneumonia (3). The majority of the data supporting preventive strategies is limited to patients in the ICU, and in particular, patients receiving mechanical ventilation. However, many of the preventive principles can be extrapolated to the non-ICU population. The preventive strategies are highlighted in Table IV (page 18).
General Preventive Strategies
General preventive strategies aim to avoid contamination of patients with antimicrobial resistant organisms that exist in hospitals, or mitigating the emergence of antimicrobial resistant organisms in the first place. Preventing iatrogenic spread of resistant organisms depends on careful hand hygiene. Hand washing before and after patient contact reduces the incidence of nosocomial infection. Alcohol-based hand rinses placed at the bedside may actually be superior to soap and water, and in addition, improve compliance with hand hygiene.
Minimizing the use of indwelling devices (central lines, urinary catheters) also reduces the emergence of resistant organisms. When these devices are necessary, focusing on their timely removal is critical. The control of antibiotic use has been central to many preventive strategies. Prolonged or unnecessary use of broad-spectrum antibiotics is strongly associated with development and colonization of resistant organisms. Strategies that focus on aggressive antibiotic de-escalation (described above) are a key preventive tool. Some institutions have had success with antibiotic restriction or rotation, but long term data on the effectiveness of these techniques are lacking.
Targeted Preventive Strategies
Preventive strategies to lower the incidence of NP focus on reducing risk factors for oropharyngeal or gastric colonization and subsequent aspiration of contaminated oropharyngeal or gastric secretions (1,3,7,11).
Endotracheal intubation is one of the most important risk factors for NP in patients requiring ventilatory support. The use of non-invasive ventilation (NIV) or positive pressure mask ventilation in selected groups of patients has been effective in preventing nosocomial pneumonia. Non-invasive ventilation has been most successful in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) and pulmonary edema secondary to congestive heart failure (CHF) and should be considered in appropriately selected patients. When intubation is required the use of nasotracheal intubation should be avoided due higher rates of NP when compared to orotracheal intubation.
Supine positioning may contribute to the development of NP, likely due to an increased risk of gastric reflux and subsequent aspiration. Studies of semi-recumbent positioning (elevation of the head of the bed >45 degrees) have shown less reflux, less aspiration, and in one recent randomized control trial, a significant reduction in the rate of VAP (12). Elevation of the head of the bed is clearly indicated in mechanically ventilated patients and is also likely to benefit all patients at risk for aspiration and subsequent NP, although this technique has not been well studied in non-ventilated patients.
Subglottic secretion drainage (SSD) involves the removal of pooled secretions above the cuff of a specialized endotracheal tube that might otherwise leak into the lung. A meta-analysis of five studies evaluating this new technology showed significant reductions in the incidence of VAP. The use of SSD should be considered for use in patients requiring more than 3 days of mechanical ventilation (13).
Medications used for stress ulcer prophylaxis that increase gastric pH-such as H2 antagonists and antacids-allow for colonization of the upper gastrointestinal tract by potentially pathogenic organisms and therefore increase the risk for NP. The use of sucralfate instead of H2 antagonists is felt to lead to less alkalinization of the stomach and less bacterial overgrowth. The ability of sucralfate to prevent nosocomial pneumonia, however, has not been well demonstrated and its routine use is not recommended (14). Instead, efforts should be targeted at limiting use of stress ulcer prophylaxis to populations at high risk for clinically significant bleeding, namely patients with coagulopathy and prolonged ventilatory failure. Most patients who are not in the ICU should not receive stress ulcer prophylaxis. The risk of NP related to use of proton pump inhibitors has not been well studied.
Selective digestive decontamination (SDD) involves sterilization of the oropharynx and gastrointestinal tract in mechanically ventilated patients in order to prevent aspiration of large numbers of potentially pathogenic organisms and subsequent VAP. Most evaluations of SDD have involved oral (and sometimes gastric) application of topical polymixin, aminoglycoside, and amphotericin. In many cases, short courses of IV antibiotics have been added. At least 10 meta-analyses have shown a reduction in the risk of VAP with the use of SDD. The addition of IV antibiotics may also provide a mortality benefit. However, the long-term risk for emergence of resistant organisms, and insufficient data on the cost-effectiveness of SDD prevent its recommendation for routine use (14).
There are several preventive strategies targeted at reducing aspiration of contaminants in ventilator circuits, filters, and tubing. Recommended strategies, listed in Table III, page 16, include avoidance of routine ventilator circuit changes (change the tubing only when visibly contaminated or for a new patient), use of heat and moisture exchangers rather than heated humidifiers, and reduction in the frequency of changes of the heat and moisture exchangers (1,11,14).
Discharge/Follow-up Plans
Patients should be followed in the hospital until it is clear they are responding to therapy and clinically improving. There has been limited evaluation of strategies to rapidly transition patients to oral therapy. However, if patients are improving, are tolerating oral therapy, have a functional GI tract, and have an organism isolated that is sensitive to available oral antibiotics, the switch to oral therapy can be made. If no organism is isolated, but a patient definitely was felt to have NP, the oral antibiotics selected should have the same spectrum of activity as the previously administered IV antibiotics. In many cases, patients will have an infection with an organism that is only susceptible to IV antibiotics. These patients are likely to be ill enough to complete a full one week IV course in the hospital, but if they have no active co-morbid illness and have improved, they can have a PICC line placed (or other long-term IV access) and receive the remainder of their therapy at home or in another lower acuity setting.
In all patients who develop NP, reversible causes of aspiration should be sought, and in cases where multidrug-resistant organisms are isolated, this should be reported to any facility to which a patient is being transferred or to the primary care physician or home nurse who will assume care after discharge.
References
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
- Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31:1312-7.
- Flanders SA, Collard HR, Saint S. Preventing Nosocomial Pneumonia. In: Lautenbach E, Woeltje K, eds. The Society for Healthcare Epidemiology of America: Practical Handbook for Healthcare Epidemiologists. Thorofare, NJ: Slack, 2004:69-78.
- Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121-9.
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505-11.
- Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451-8.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867-903.
- Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and metaanalysis of randomised trials. BMJ. 2004;328:668.
- Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588-98.
- Dennesen PJ, van der Ven AJ, Kessels AG, Ramsay G, Bonten MJ. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med. 2001;163:1371-5.
- Collard HR, Saint S, Matthay MA. Prevention of ventilator-associated pneumonia: an evidence-based systematic review. Ann Intern Med. 2003;138:494-501.
- Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851-8.
- Dezfulian C, Shojania K, Collard HR, Kim HM, Matthay MA, Saint S. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a metaanalysis. Am J Med. 2005;118:11-8.
- Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141:305-13.
(This chapter has been reprinted with permission from Williams MV, Hayward R: Comprehensive Hospital Medicine, 1st edition. Philadelphia, WB Saunders, in press.)
Background
Nosocomial pneumonia (NP) is the leading cause of mortality among patients who die from hospital-acquired infections. Defined as pneumonia occurring 48 hours or more after hospital admission, NP also includes the subset of ventilator-associated pneumonia (VAP), defined as pneumonia developing 48 to 72 hours after initiation of mechanical ventilation. The incidence of NP is between 5 and 15 cases per 1000 hospital admissions. Healthcare-associated pneumonia (HCAP), part of the continuum of NP, describes an increasingly common proportion of pneumonia developing outside the hospital (Table I) (1). Typically afflicting people in a nursing home or assisted living setting, these patients are at risk for antibiotic-resistant-organisms and should be approached similarly to cases of nosocomial pneumonia rather than community-acquired pneumonia. Most of the data informing our diagnostic and treatment decisions about NP come from studies performed in mechanically ventilated patients and are extrapolated to make recommendations for non-ventilated patients.
Mortality attributable to NP is debated, but may be as high as 30%. The presence of nosocomial pneumonia increases hospital length of stay an average of 7–10 days, and in the case of VAP, is estimated to cost between $10,000 and $40,000 per case (2).
Assessment
Clinical Presentation
Signs and Symptoms
Nosocomial pneumonia is usually diagnosed based on clinical grounds. Typical symptoms and signs consist of fever, cough with sputum, and shortness of breath in the setting of hypoxia and a new infiltrate on chest radiograph (CXR). In the elderly, signs may be more subtle and delirium, fever, or leukocytosis in the absence of cough should trigger its consideration. The likelihood of NP increases among patients with risk factors for microaspiration, oropharyngeal colonization, or overgrowth of resistant organisms (Table II) (3).
Differential Diagnosis
Prior to settling on a diagnosis of NP, alternative causes of fever, hypoxia, and pulmonary infiltrates should be considered. Most commonly, these include pulmonary embolus, pulmonary edema, or atelectasis. Alternative infectious sources, such as urinary tract, skin and soft-tissue infections, and device-related infections (i.e., central venous catheters) are common in hospitalized patients and should be ruled out before diagnosing nosocomial pneumonia.
Diagnosis
Diagnostic strategies for NP seek to confirm the diagnosis and identify an etiologic pathogen, thus allowing timely, effective, and streamlined antibiotic therapy. Unfortunately, no consensus exists on the best approach to diagnosing nosocomial pneumonia. After obtaining a complete blood count and blood cultures, you can choose between a clinical or microbiologic diagnostic approach to diagnosis. A clinical diagnosis relies on a new or progressive radiographic infiltrate along with signs of infection such as fever, leukocytosis, or purulent sputum. Clinical diagnosis is sensitive, but is likely to lead to antibiotic overuse. The microbiologic approach requires sampling of secretions from the respiratory tract and may reduce inappropriate antibiotic use, but takes longer and may not be available in all hospitals.
Preferred Studies
The microbiologic approach to diagnosis relies on the use of quantitative or semi-quantitative cultures to create thresholds for antibiotic treatment. Bacterial cultures that demonstrate a level of growth above the thresholds described below warrant treatment, while those below it should trigger withholding or discontinuation of antibiotics.
Bronchoscopic Approaches: Bronchoalveolar lavage (BAL) with a cutoff of 10 (4) organisms/mL or protected specimen brush (PSB) with a cutoff of 10 (3) organisms/mL are felt to be the most specific diagnostic tests when performed prior to initiating antibiotics, or prior to changing antibiotics if a patient is already receiving them. In clinically stable patients, antibiotics can be safely discontinued if bacterial growth falls below the thresholds. If cultures are positive, antibiotic therapy should be tailored to target the organism identified. The bronchoscopic approach is favored in patients who are mechanically ventilated, develop their pneumonia late in the hospital stay (>5–7 days), are at risk for unusual pathogens, are failing therapy or suspected of having an alternative diagnosis.
Non-Bronchoscopic Approaches: Qualitative endotracheal aspirates (ETA) have been shown to be quite sensitive in ventilated patients, regularly identify organisms that may be subsequently found by BAL or PSB, and if negative, should result in withholding antibiotics. Quantitative endotracheal aspirates with a cutoff of 10 (6) organisms/mL are often encouraged to reduce antibiotic overuse, but results should be interpreted cautiously as they only have a sensitivity and specificity of about 75% (1). Consideration should be given to withholding antibiotics in a clinically stable patient with a negative quantitative ETA if antibiotics have not been changed in the preceding 72 hours. Many ICUs have begun to perform blinded sampling of lower respiratory tract secretions with suction catheters (blind PSB, blind mini-BAL). These techniques can be performed at all hours by trained respiratory therapists or nurses, provide culture data similar to that of bronchoscopy, and may be safer and less costly than bronchoscopy. In general, non-bronchoscopic techniques are preferred in patients who are not mechanically ventilated. Sputum sampling, while easy to obtain, has not been well studied in NP. However, in patients in whom bronchoscopic or other non-bronchoscopic techniques are not feasible, sputum sampling may be performed to identify potentially resistant organisms and help tailor therapy.
Alternative Options
Clinical Pulmonary Infection Score—Combining Clinical and Microbiologic Approaches
The clinical diagnosis of nosocomial pneumonia (new infiltrate + fever, leukocytosis, or purulent sputum) likely leads to antibiotic overuse, yet pursuing a bronchoscopic diagnosis is invasive, costly, and requires technical expertise. The quantitative ETA, blind PSB, and blind BAL discussed above are examples of some compromises that avoid the need for bronchoscopy, yet add microbiologic data in an attempt to prevent excess antibiotic therapy. Formally combining diagnostic approaches (clinical + microbiologic) may also be useful. One such option is the use of the clinical pulmonary infection score (CPIS), which combines clinical, radiographic, physiological, and microbiologic data into a numerical result. Scores >6 have been shown to correlate well with quantitative BAL (4). More recent studies, however, have suggested a lower specificity which could still result in antibiotic overuse, but this approach remains more accurate than a general clinical approach. Using the CPIS serially at the time NP is suspected and again at 72 hours may be more useful. Patients with an initial low clinical suspicion for pneumonia (CPIS of 6 or less) could have antibiotics safely discontinued at 72 hours if the CPIS remains low (5). Such a strategy may be useful in settings where more sophisticated diagnostic modalities are not available.
Multiple studies of biological markers of infection have attempted to find a non-invasive, rapid, accurate means of determining who needs antibiotics for presumed NP. Unfortunately, the results have largely been disappointing. More recently, measurement of a soluble triggering receptor expressed on myeloid cells (sTREM-1) that is upregulated in the setting of infection has been shown to improve our ability to diagnose NP accurately. Measurement of sTREM-1 was 98% sensitive and 90% specific for the diagnosis of pneumonia in mechanically ventilated patients (6). While promising, more data is needed before this test can be recommended for routine use.
Management
Initial Treatment
Early initiation of adequate empiric antibiotic therapy (i.e., the antibiotics administered are shown to be active against all organisms isolated) is associated with improved survival compared with initial inadequate therapy (1,7). Antibiotics should be started immediately after obtaining blood and sputum samples for culture and should not be withheld in the event of delay in diagnostic testing. The need to choose antibiotics quickly and expeditiously drives the use of broad spectrum antibiotics. In an effort to avoid unnecessary overuse of broad spectrum antibiotics, therapy should be based on risk for multidrug-resistant (MDR) pathogens. Identifying patients at low risk for MDR pathogens by clinical criteria allows for more narrow, but effective, antibiotic therapy. Low risk patients include those who develop their pneumonia early in the hospitalization (<5–7 days), are not immunocompromised, have not had prior broad spectrum antibiotics, and do not have risk factors for HCAP (Table I) (1,7). In these patients antibiotics should target common community-acquired organisms (Table III–low risk pathogens). Appropriate initial antibiotic therapy could include a third generation cephalosporin or a beta-lactam/beta-lactamase inhibitor. In some communities or hospital wards the incidence of methicillin-resistance among Staphylococcus aureus isolates (MRSA) may be high enough to warrant initial empiric therapy with vancomycin or linezolid.
Unfortunately, today’s increasingly complex hospitalized patients are unlikely to be “low risk,” especially in intensive care units.
Patients not meeting low risk criteria are considered to be at high risk for MDR pathogens (Table III–high risk pathogens). Initial empiric therapy needs to be broad and should include one antipseudomonal agent (cefepime or imipenem or beta-lactam/beta-lactamase inhibitor) plus a fluoroquinolone or aminoglycoside plus vancomycin or linezolid. The specific initial empiric therapy should be dictated by local resistance patterns, cost, and availability of preferred agents. When such broad spectrum therapy is initiated, it becomes imperative that antibiotics are “de-escalated” to limit antibiotic overuse. De-escalation therapy focuses on narrowing the antibiotic spectrum based on culture results, and limiting the overall duration of therapy. Hospitalists should aim to accomplish such de-escalation within 48–72 hours of initiating broad-spectrum antibiotics.
Subsequent Treatment
Patients started on initial empiric antibiotic therapy for presumed nosocomial pneumonia should be reassessed at 48–72 hours. Specifically, cultures should be checked and the clinical response to treatment evaluated. Figure I describes an algorithm for guiding treatment (1). In patients who are clinically stable and have negative lower respiratory tract cultures, antibiotics can be stopped. Patients with positive cultures should have antibiotics tailored, or “de-escalated” based on the organisms identified. In general, the most narrow spectrum antibiotic that is active against the bacteria isolated should be used. The use of combination therapy for gram negative organisms (two or more antibiotics active against a bacterial isolate) is widely practiced to achieve synergy, or prevent the development of resistance. However, in the absence of neutropenia, combination therapy has not been shown to be superior to monotherapyy (8), and monotherapy is preferred. The isolation of MRSA from a respiratory sample should also result in use of monotherapy. While some studies have suggested that linezolid may be superior to vancomycin for MRSA pneumonia, this finding needs validation in prospective studies.
A second component of de-escalation is shortening the total duration of therapy. The CPIS may be used to shorten the duration of therapy in patients at low risk for pneumonia. Investigators at a Veterans Affairs medical center randomized patients suspected of having NP, but who had a CPIS score < 6, to either treatment for 10–21 days, or short course therapy. Patients receiving short course therapy were reassessed at day 3, and if their CPIS score remained < 6, antibiotics were stopped (5). The short course therapy group had no difference in mortality when compared to the standard treatment group, but had less antibiotic use, shorter ICU stays, and was less likely to develop a superinfection or infection with a resistant organism. If the CPIS is not used, or if patients are felt to be at higher risk or convincingly demonstrated to have NP, a shorter course of therapy may still be preferred. A large randomized trial showed that 8 days of antibiotic therapy for patients with VAP resulted in similar clinical outcomes when compared to 15 days of therapy. Additionally, shorter duration antibiotic therapy was associated with lower likelihood of developing subsequent infections with multi-resistant pathogens. A subset of patients in the 8 day treatment group infected with non-fermenting gram negative bacilli (e.g., Pseudomonas aeruginosa) did have a higher pulmonary infection recurrence rate, but due to aggressive surveillance, this did not translate into a higher mortality risk in this subset of patients (9).
In summary, treatment of patients with suspected NP starts with immediate initiation of antibiotics and collection of respiratory secretions. While low risk patients can receive narrower spectrum therapy, most patients will require broad initial empiric therapy. The antibiotic regimen, however, should be narrowed at 48–72 hours based on microbiological results if the patient is improving. Overall treatment duration of 1 week is safe and effective with less chance of promoting growth of resistant organisms. In the subset of patients with pseudomonal infections, treatment of 1 week duration should be followed by active surveillance for recurrence, or alternatively, treatment can be extended to two weeks.
Prognosis
Once treatment for NP is initiated, clinical improvement is usually seen by 48–72 hours. There is little support for following either microbiologic response (clearance of positive cultures) or the response by chest radiography. The chest radiograph often lags behind the clinical response, however, a markedly worsening CXR (>50% increase in infiltrate) within the first 48 hours may indicate treatment failure. Clinical resolution as measured by temperature, white blood cell count, and oxygenation usually occurs by 6–7 days (10). Failure of oxygenation to improve by 72 hours has been shown to be predictive of treatment failure.
The overall mortality in patients with NP is as high as 30–70%, largely due to severe comorbid disease in the at risk population. Higher mortality rates are seen in patients with VAP and resistant organisms. The mortality attributable to the episode of NP is about 30%, and can be reduced to <15% with appropriate antibiotic therapy (1).
Prevention
Preventive strategies are either directed at reducing the overall incidence of infectious complications in hospitalized patients, or they are specifically targeted at reducing the incidence of nosocomial pneumonia (3). The majority of the data supporting preventive strategies is limited to patients in the ICU, and in particular, patients receiving mechanical ventilation. However, many of the preventive principles can be extrapolated to the non-ICU population. The preventive strategies are highlighted in Table IV (page 18).
General Preventive Strategies
General preventive strategies aim to avoid contamination of patients with antimicrobial resistant organisms that exist in hospitals, or mitigating the emergence of antimicrobial resistant organisms in the first place. Preventing iatrogenic spread of resistant organisms depends on careful hand hygiene. Hand washing before and after patient contact reduces the incidence of nosocomial infection. Alcohol-based hand rinses placed at the bedside may actually be superior to soap and water, and in addition, improve compliance with hand hygiene.
Minimizing the use of indwelling devices (central lines, urinary catheters) also reduces the emergence of resistant organisms. When these devices are necessary, focusing on their timely removal is critical. The control of antibiotic use has been central to many preventive strategies. Prolonged or unnecessary use of broad-spectrum antibiotics is strongly associated with development and colonization of resistant organisms. Strategies that focus on aggressive antibiotic de-escalation (described above) are a key preventive tool. Some institutions have had success with antibiotic restriction or rotation, but long term data on the effectiveness of these techniques are lacking.
Targeted Preventive Strategies
Preventive strategies to lower the incidence of NP focus on reducing risk factors for oropharyngeal or gastric colonization and subsequent aspiration of contaminated oropharyngeal or gastric secretions (1,3,7,11).
Endotracheal intubation is one of the most important risk factors for NP in patients requiring ventilatory support. The use of non-invasive ventilation (NIV) or positive pressure mask ventilation in selected groups of patients has been effective in preventing nosocomial pneumonia. Non-invasive ventilation has been most successful in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) and pulmonary edema secondary to congestive heart failure (CHF) and should be considered in appropriately selected patients. When intubation is required the use of nasotracheal intubation should be avoided due higher rates of NP when compared to orotracheal intubation.
Supine positioning may contribute to the development of NP, likely due to an increased risk of gastric reflux and subsequent aspiration. Studies of semi-recumbent positioning (elevation of the head of the bed >45 degrees) have shown less reflux, less aspiration, and in one recent randomized control trial, a significant reduction in the rate of VAP (12). Elevation of the head of the bed is clearly indicated in mechanically ventilated patients and is also likely to benefit all patients at risk for aspiration and subsequent NP, although this technique has not been well studied in non-ventilated patients.
Subglottic secretion drainage (SSD) involves the removal of pooled secretions above the cuff of a specialized endotracheal tube that might otherwise leak into the lung. A meta-analysis of five studies evaluating this new technology showed significant reductions in the incidence of VAP. The use of SSD should be considered for use in patients requiring more than 3 days of mechanical ventilation (13).
Medications used for stress ulcer prophylaxis that increase gastric pH-such as H2 antagonists and antacids-allow for colonization of the upper gastrointestinal tract by potentially pathogenic organisms and therefore increase the risk for NP. The use of sucralfate instead of H2 antagonists is felt to lead to less alkalinization of the stomach and less bacterial overgrowth. The ability of sucralfate to prevent nosocomial pneumonia, however, has not been well demonstrated and its routine use is not recommended (14). Instead, efforts should be targeted at limiting use of stress ulcer prophylaxis to populations at high risk for clinically significant bleeding, namely patients with coagulopathy and prolonged ventilatory failure. Most patients who are not in the ICU should not receive stress ulcer prophylaxis. The risk of NP related to use of proton pump inhibitors has not been well studied.
Selective digestive decontamination (SDD) involves sterilization of the oropharynx and gastrointestinal tract in mechanically ventilated patients in order to prevent aspiration of large numbers of potentially pathogenic organisms and subsequent VAP. Most evaluations of SDD have involved oral (and sometimes gastric) application of topical polymixin, aminoglycoside, and amphotericin. In many cases, short courses of IV antibiotics have been added. At least 10 meta-analyses have shown a reduction in the risk of VAP with the use of SDD. The addition of IV antibiotics may also provide a mortality benefit. However, the long-term risk for emergence of resistant organisms, and insufficient data on the cost-effectiveness of SDD prevent its recommendation for routine use (14).
There are several preventive strategies targeted at reducing aspiration of contaminants in ventilator circuits, filters, and tubing. Recommended strategies, listed in Table III, page 16, include avoidance of routine ventilator circuit changes (change the tubing only when visibly contaminated or for a new patient), use of heat and moisture exchangers rather than heated humidifiers, and reduction in the frequency of changes of the heat and moisture exchangers (1,11,14).
Discharge/Follow-up Plans
Patients should be followed in the hospital until it is clear they are responding to therapy and clinically improving. There has been limited evaluation of strategies to rapidly transition patients to oral therapy. However, if patients are improving, are tolerating oral therapy, have a functional GI tract, and have an organism isolated that is sensitive to available oral antibiotics, the switch to oral therapy can be made. If no organism is isolated, but a patient definitely was felt to have NP, the oral antibiotics selected should have the same spectrum of activity as the previously administered IV antibiotics. In many cases, patients will have an infection with an organism that is only susceptible to IV antibiotics. These patients are likely to be ill enough to complete a full one week IV course in the hospital, but if they have no active co-morbid illness and have improved, they can have a PICC line placed (or other long-term IV access) and receive the remainder of their therapy at home or in another lower acuity setting.
In all patients who develop NP, reversible causes of aspiration should be sought, and in cases where multidrug-resistant organisms are isolated, this should be reported to any facility to which a patient is being transferred or to the primary care physician or home nurse who will assume care after discharge.
References
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
- Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31:1312-7.
- Flanders SA, Collard HR, Saint S. Preventing Nosocomial Pneumonia. In: Lautenbach E, Woeltje K, eds. The Society for Healthcare Epidemiology of America: Practical Handbook for Healthcare Epidemiologists. Thorofare, NJ: Slack, 2004:69-78.
- Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121-9.
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505-11.
- Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451-8.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867-903.
- Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and metaanalysis of randomised trials. BMJ. 2004;328:668.
- Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588-98.
- Dennesen PJ, van der Ven AJ, Kessels AG, Ramsay G, Bonten MJ. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med. 2001;163:1371-5.
- Collard HR, Saint S, Matthay MA. Prevention of ventilator-associated pneumonia: an evidence-based systematic review. Ann Intern Med. 2003;138:494-501.
- Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851-8.
- Dezfulian C, Shojania K, Collard HR, Kim HM, Matthay MA, Saint S. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a metaanalysis. Am J Med. 2005;118:11-8.
- Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141:305-13.
(This chapter has been reprinted with permission from Williams MV, Hayward R: Comprehensive Hospital Medicine, 1st edition. Philadelphia, WB Saunders, in press.)
Background
Nosocomial pneumonia (NP) is the leading cause of mortality among patients who die from hospital-acquired infections. Defined as pneumonia occurring 48 hours or more after hospital admission, NP also includes the subset of ventilator-associated pneumonia (VAP), defined as pneumonia developing 48 to 72 hours after initiation of mechanical ventilation. The incidence of NP is between 5 and 15 cases per 1000 hospital admissions. Healthcare-associated pneumonia (HCAP), part of the continuum of NP, describes an increasingly common proportion of pneumonia developing outside the hospital (Table I) (1). Typically afflicting people in a nursing home or assisted living setting, these patients are at risk for antibiotic-resistant-organisms and should be approached similarly to cases of nosocomial pneumonia rather than community-acquired pneumonia. Most of the data informing our diagnostic and treatment decisions about NP come from studies performed in mechanically ventilated patients and are extrapolated to make recommendations for non-ventilated patients.
Mortality attributable to NP is debated, but may be as high as 30%. The presence of nosocomial pneumonia increases hospital length of stay an average of 7–10 days, and in the case of VAP, is estimated to cost between $10,000 and $40,000 per case (2).
Assessment
Clinical Presentation
Signs and Symptoms
Nosocomial pneumonia is usually diagnosed based on clinical grounds. Typical symptoms and signs consist of fever, cough with sputum, and shortness of breath in the setting of hypoxia and a new infiltrate on chest radiograph (CXR). In the elderly, signs may be more subtle and delirium, fever, or leukocytosis in the absence of cough should trigger its consideration. The likelihood of NP increases among patients with risk factors for microaspiration, oropharyngeal colonization, or overgrowth of resistant organisms (Table II) (3).
Differential Diagnosis
Prior to settling on a diagnosis of NP, alternative causes of fever, hypoxia, and pulmonary infiltrates should be considered. Most commonly, these include pulmonary embolus, pulmonary edema, or atelectasis. Alternative infectious sources, such as urinary tract, skin and soft-tissue infections, and device-related infections (i.e., central venous catheters) are common in hospitalized patients and should be ruled out before diagnosing nosocomial pneumonia.
Diagnosis
Diagnostic strategies for NP seek to confirm the diagnosis and identify an etiologic pathogen, thus allowing timely, effective, and streamlined antibiotic therapy. Unfortunately, no consensus exists on the best approach to diagnosing nosocomial pneumonia. After obtaining a complete blood count and blood cultures, you can choose between a clinical or microbiologic diagnostic approach to diagnosis. A clinical diagnosis relies on a new or progressive radiographic infiltrate along with signs of infection such as fever, leukocytosis, or purulent sputum. Clinical diagnosis is sensitive, but is likely to lead to antibiotic overuse. The microbiologic approach requires sampling of secretions from the respiratory tract and may reduce inappropriate antibiotic use, but takes longer and may not be available in all hospitals.
Preferred Studies
The microbiologic approach to diagnosis relies on the use of quantitative or semi-quantitative cultures to create thresholds for antibiotic treatment. Bacterial cultures that demonstrate a level of growth above the thresholds described below warrant treatment, while those below it should trigger withholding or discontinuation of antibiotics.
Bronchoscopic Approaches: Bronchoalveolar lavage (BAL) with a cutoff of 10 (4) organisms/mL or protected specimen brush (PSB) with a cutoff of 10 (3) organisms/mL are felt to be the most specific diagnostic tests when performed prior to initiating antibiotics, or prior to changing antibiotics if a patient is already receiving them. In clinically stable patients, antibiotics can be safely discontinued if bacterial growth falls below the thresholds. If cultures are positive, antibiotic therapy should be tailored to target the organism identified. The bronchoscopic approach is favored in patients who are mechanically ventilated, develop their pneumonia late in the hospital stay (>5–7 days), are at risk for unusual pathogens, are failing therapy or suspected of having an alternative diagnosis.
Non-Bronchoscopic Approaches: Qualitative endotracheal aspirates (ETA) have been shown to be quite sensitive in ventilated patients, regularly identify organisms that may be subsequently found by BAL or PSB, and if negative, should result in withholding antibiotics. Quantitative endotracheal aspirates with a cutoff of 10 (6) organisms/mL are often encouraged to reduce antibiotic overuse, but results should be interpreted cautiously as they only have a sensitivity and specificity of about 75% (1). Consideration should be given to withholding antibiotics in a clinically stable patient with a negative quantitative ETA if antibiotics have not been changed in the preceding 72 hours. Many ICUs have begun to perform blinded sampling of lower respiratory tract secretions with suction catheters (blind PSB, blind mini-BAL). These techniques can be performed at all hours by trained respiratory therapists or nurses, provide culture data similar to that of bronchoscopy, and may be safer and less costly than bronchoscopy. In general, non-bronchoscopic techniques are preferred in patients who are not mechanically ventilated. Sputum sampling, while easy to obtain, has not been well studied in NP. However, in patients in whom bronchoscopic or other non-bronchoscopic techniques are not feasible, sputum sampling may be performed to identify potentially resistant organisms and help tailor therapy.
Alternative Options
Clinical Pulmonary Infection Score—Combining Clinical and Microbiologic Approaches
The clinical diagnosis of nosocomial pneumonia (new infiltrate + fever, leukocytosis, or purulent sputum) likely leads to antibiotic overuse, yet pursuing a bronchoscopic diagnosis is invasive, costly, and requires technical expertise. The quantitative ETA, blind PSB, and blind BAL discussed above are examples of some compromises that avoid the need for bronchoscopy, yet add microbiologic data in an attempt to prevent excess antibiotic therapy. Formally combining diagnostic approaches (clinical + microbiologic) may also be useful. One such option is the use of the clinical pulmonary infection score (CPIS), which combines clinical, radiographic, physiological, and microbiologic data into a numerical result. Scores >6 have been shown to correlate well with quantitative BAL (4). More recent studies, however, have suggested a lower specificity which could still result in antibiotic overuse, but this approach remains more accurate than a general clinical approach. Using the CPIS serially at the time NP is suspected and again at 72 hours may be more useful. Patients with an initial low clinical suspicion for pneumonia (CPIS of 6 or less) could have antibiotics safely discontinued at 72 hours if the CPIS remains low (5). Such a strategy may be useful in settings where more sophisticated diagnostic modalities are not available.
Multiple studies of biological markers of infection have attempted to find a non-invasive, rapid, accurate means of determining who needs antibiotics for presumed NP. Unfortunately, the results have largely been disappointing. More recently, measurement of a soluble triggering receptor expressed on myeloid cells (sTREM-1) that is upregulated in the setting of infection has been shown to improve our ability to diagnose NP accurately. Measurement of sTREM-1 was 98% sensitive and 90% specific for the diagnosis of pneumonia in mechanically ventilated patients (6). While promising, more data is needed before this test can be recommended for routine use.
Management
Initial Treatment
Early initiation of adequate empiric antibiotic therapy (i.e., the antibiotics administered are shown to be active against all organisms isolated) is associated with improved survival compared with initial inadequate therapy (1,7). Antibiotics should be started immediately after obtaining blood and sputum samples for culture and should not be withheld in the event of delay in diagnostic testing. The need to choose antibiotics quickly and expeditiously drives the use of broad spectrum antibiotics. In an effort to avoid unnecessary overuse of broad spectrum antibiotics, therapy should be based on risk for multidrug-resistant (MDR) pathogens. Identifying patients at low risk for MDR pathogens by clinical criteria allows for more narrow, but effective, antibiotic therapy. Low risk patients include those who develop their pneumonia early in the hospitalization (<5–7 days), are not immunocompromised, have not had prior broad spectrum antibiotics, and do not have risk factors for HCAP (Table I) (1,7). In these patients antibiotics should target common community-acquired organisms (Table III–low risk pathogens). Appropriate initial antibiotic therapy could include a third generation cephalosporin or a beta-lactam/beta-lactamase inhibitor. In some communities or hospital wards the incidence of methicillin-resistance among Staphylococcus aureus isolates (MRSA) may be high enough to warrant initial empiric therapy with vancomycin or linezolid.
Unfortunately, today’s increasingly complex hospitalized patients are unlikely to be “low risk,” especially in intensive care units.
Patients not meeting low risk criteria are considered to be at high risk for MDR pathogens (Table III–high risk pathogens). Initial empiric therapy needs to be broad and should include one antipseudomonal agent (cefepime or imipenem or beta-lactam/beta-lactamase inhibitor) plus a fluoroquinolone or aminoglycoside plus vancomycin or linezolid. The specific initial empiric therapy should be dictated by local resistance patterns, cost, and availability of preferred agents. When such broad spectrum therapy is initiated, it becomes imperative that antibiotics are “de-escalated” to limit antibiotic overuse. De-escalation therapy focuses on narrowing the antibiotic spectrum based on culture results, and limiting the overall duration of therapy. Hospitalists should aim to accomplish such de-escalation within 48–72 hours of initiating broad-spectrum antibiotics.
Subsequent Treatment
Patients started on initial empiric antibiotic therapy for presumed nosocomial pneumonia should be reassessed at 48–72 hours. Specifically, cultures should be checked and the clinical response to treatment evaluated. Figure I describes an algorithm for guiding treatment (1). In patients who are clinically stable and have negative lower respiratory tract cultures, antibiotics can be stopped. Patients with positive cultures should have antibiotics tailored, or “de-escalated” based on the organisms identified. In general, the most narrow spectrum antibiotic that is active against the bacteria isolated should be used. The use of combination therapy for gram negative organisms (two or more antibiotics active against a bacterial isolate) is widely practiced to achieve synergy, or prevent the development of resistance. However, in the absence of neutropenia, combination therapy has not been shown to be superior to monotherapyy (8), and monotherapy is preferred. The isolation of MRSA from a respiratory sample should also result in use of monotherapy. While some studies have suggested that linezolid may be superior to vancomycin for MRSA pneumonia, this finding needs validation in prospective studies.
A second component of de-escalation is shortening the total duration of therapy. The CPIS may be used to shorten the duration of therapy in patients at low risk for pneumonia. Investigators at a Veterans Affairs medical center randomized patients suspected of having NP, but who had a CPIS score < 6, to either treatment for 10–21 days, or short course therapy. Patients receiving short course therapy were reassessed at day 3, and if their CPIS score remained < 6, antibiotics were stopped (5). The short course therapy group had no difference in mortality when compared to the standard treatment group, but had less antibiotic use, shorter ICU stays, and was less likely to develop a superinfection or infection with a resistant organism. If the CPIS is not used, or if patients are felt to be at higher risk or convincingly demonstrated to have NP, a shorter course of therapy may still be preferred. A large randomized trial showed that 8 days of antibiotic therapy for patients with VAP resulted in similar clinical outcomes when compared to 15 days of therapy. Additionally, shorter duration antibiotic therapy was associated with lower likelihood of developing subsequent infections with multi-resistant pathogens. A subset of patients in the 8 day treatment group infected with non-fermenting gram negative bacilli (e.g., Pseudomonas aeruginosa) did have a higher pulmonary infection recurrence rate, but due to aggressive surveillance, this did not translate into a higher mortality risk in this subset of patients (9).
In summary, treatment of patients with suspected NP starts with immediate initiation of antibiotics and collection of respiratory secretions. While low risk patients can receive narrower spectrum therapy, most patients will require broad initial empiric therapy. The antibiotic regimen, however, should be narrowed at 48–72 hours based on microbiological results if the patient is improving. Overall treatment duration of 1 week is safe and effective with less chance of promoting growth of resistant organisms. In the subset of patients with pseudomonal infections, treatment of 1 week duration should be followed by active surveillance for recurrence, or alternatively, treatment can be extended to two weeks.
Prognosis
Once treatment for NP is initiated, clinical improvement is usually seen by 48–72 hours. There is little support for following either microbiologic response (clearance of positive cultures) or the response by chest radiography. The chest radiograph often lags behind the clinical response, however, a markedly worsening CXR (>50% increase in infiltrate) within the first 48 hours may indicate treatment failure. Clinical resolution as measured by temperature, white blood cell count, and oxygenation usually occurs by 6–7 days (10). Failure of oxygenation to improve by 72 hours has been shown to be predictive of treatment failure.
The overall mortality in patients with NP is as high as 30–70%, largely due to severe comorbid disease in the at risk population. Higher mortality rates are seen in patients with VAP and resistant organisms. The mortality attributable to the episode of NP is about 30%, and can be reduced to <15% with appropriate antibiotic therapy (1).
Prevention
Preventive strategies are either directed at reducing the overall incidence of infectious complications in hospitalized patients, or they are specifically targeted at reducing the incidence of nosocomial pneumonia (3). The majority of the data supporting preventive strategies is limited to patients in the ICU, and in particular, patients receiving mechanical ventilation. However, many of the preventive principles can be extrapolated to the non-ICU population. The preventive strategies are highlighted in Table IV (page 18).
General Preventive Strategies
General preventive strategies aim to avoid contamination of patients with antimicrobial resistant organisms that exist in hospitals, or mitigating the emergence of antimicrobial resistant organisms in the first place. Preventing iatrogenic spread of resistant organisms depends on careful hand hygiene. Hand washing before and after patient contact reduces the incidence of nosocomial infection. Alcohol-based hand rinses placed at the bedside may actually be superior to soap and water, and in addition, improve compliance with hand hygiene.
Minimizing the use of indwelling devices (central lines, urinary catheters) also reduces the emergence of resistant organisms. When these devices are necessary, focusing on their timely removal is critical. The control of antibiotic use has been central to many preventive strategies. Prolonged or unnecessary use of broad-spectrum antibiotics is strongly associated with development and colonization of resistant organisms. Strategies that focus on aggressive antibiotic de-escalation (described above) are a key preventive tool. Some institutions have had success with antibiotic restriction or rotation, but long term data on the effectiveness of these techniques are lacking.
Targeted Preventive Strategies
Preventive strategies to lower the incidence of NP focus on reducing risk factors for oropharyngeal or gastric colonization and subsequent aspiration of contaminated oropharyngeal or gastric secretions (1,3,7,11).
Endotracheal intubation is one of the most important risk factors for NP in patients requiring ventilatory support. The use of non-invasive ventilation (NIV) or positive pressure mask ventilation in selected groups of patients has been effective in preventing nosocomial pneumonia. Non-invasive ventilation has been most successful in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) and pulmonary edema secondary to congestive heart failure (CHF) and should be considered in appropriately selected patients. When intubation is required the use of nasotracheal intubation should be avoided due higher rates of NP when compared to orotracheal intubation.
Supine positioning may contribute to the development of NP, likely due to an increased risk of gastric reflux and subsequent aspiration. Studies of semi-recumbent positioning (elevation of the head of the bed >45 degrees) have shown less reflux, less aspiration, and in one recent randomized control trial, a significant reduction in the rate of VAP (12). Elevation of the head of the bed is clearly indicated in mechanically ventilated patients and is also likely to benefit all patients at risk for aspiration and subsequent NP, although this technique has not been well studied in non-ventilated patients.
Subglottic secretion drainage (SSD) involves the removal of pooled secretions above the cuff of a specialized endotracheal tube that might otherwise leak into the lung. A meta-analysis of five studies evaluating this new technology showed significant reductions in the incidence of VAP. The use of SSD should be considered for use in patients requiring more than 3 days of mechanical ventilation (13).
Medications used for stress ulcer prophylaxis that increase gastric pH-such as H2 antagonists and antacids-allow for colonization of the upper gastrointestinal tract by potentially pathogenic organisms and therefore increase the risk for NP. The use of sucralfate instead of H2 antagonists is felt to lead to less alkalinization of the stomach and less bacterial overgrowth. The ability of sucralfate to prevent nosocomial pneumonia, however, has not been well demonstrated and its routine use is not recommended (14). Instead, efforts should be targeted at limiting use of stress ulcer prophylaxis to populations at high risk for clinically significant bleeding, namely patients with coagulopathy and prolonged ventilatory failure. Most patients who are not in the ICU should not receive stress ulcer prophylaxis. The risk of NP related to use of proton pump inhibitors has not been well studied.
Selective digestive decontamination (SDD) involves sterilization of the oropharynx and gastrointestinal tract in mechanically ventilated patients in order to prevent aspiration of large numbers of potentially pathogenic organisms and subsequent VAP. Most evaluations of SDD have involved oral (and sometimes gastric) application of topical polymixin, aminoglycoside, and amphotericin. In many cases, short courses of IV antibiotics have been added. At least 10 meta-analyses have shown a reduction in the risk of VAP with the use of SDD. The addition of IV antibiotics may also provide a mortality benefit. However, the long-term risk for emergence of resistant organisms, and insufficient data on the cost-effectiveness of SDD prevent its recommendation for routine use (14).
There are several preventive strategies targeted at reducing aspiration of contaminants in ventilator circuits, filters, and tubing. Recommended strategies, listed in Table III, page 16, include avoidance of routine ventilator circuit changes (change the tubing only when visibly contaminated or for a new patient), use of heat and moisture exchangers rather than heated humidifiers, and reduction in the frequency of changes of the heat and moisture exchangers (1,11,14).
Discharge/Follow-up Plans
Patients should be followed in the hospital until it is clear they are responding to therapy and clinically improving. There has been limited evaluation of strategies to rapidly transition patients to oral therapy. However, if patients are improving, are tolerating oral therapy, have a functional GI tract, and have an organism isolated that is sensitive to available oral antibiotics, the switch to oral therapy can be made. If no organism is isolated, but a patient definitely was felt to have NP, the oral antibiotics selected should have the same spectrum of activity as the previously administered IV antibiotics. In many cases, patients will have an infection with an organism that is only susceptible to IV antibiotics. These patients are likely to be ill enough to complete a full one week IV course in the hospital, but if they have no active co-morbid illness and have improved, they can have a PICC line placed (or other long-term IV access) and receive the remainder of their therapy at home or in another lower acuity setting.
In all patients who develop NP, reversible causes of aspiration should be sought, and in cases where multidrug-resistant organisms are isolated, this should be reported to any facility to which a patient is being transferred or to the primary care physician or home nurse who will assume care after discharge.
References
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
- Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31:1312-7.
- Flanders SA, Collard HR, Saint S. Preventing Nosocomial Pneumonia. In: Lautenbach E, Woeltje K, eds. The Society for Healthcare Epidemiology of America: Practical Handbook for Healthcare Epidemiologists. Thorofare, NJ: Slack, 2004:69-78.
- Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121-9.
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505-11.
- Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451-8.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867-903.
- Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and metaanalysis of randomised trials. BMJ. 2004;328:668.
- Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588-98.
- Dennesen PJ, van der Ven AJ, Kessels AG, Ramsay G, Bonten MJ. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med. 2001;163:1371-5.
- Collard HR, Saint S, Matthay MA. Prevention of ventilator-associated pneumonia: an evidence-based systematic review. Ann Intern Med. 2003;138:494-501.
- Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851-8.
- Dezfulian C, Shojania K, Collard HR, Kim HM, Matthay MA, Saint S. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a metaanalysis. Am J Med. 2005;118:11-8.
- Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141:305-13.