User login
Should the Pendulum Swing Back? More Transfers to the ICU After Implementing Ward-Based High-Flow Nasal Cannula Initiation Protocols for Bronchiolitis
As an appealing, physiologically plausible treatment, humidified oxygen delivery via high-flow nasal cannula (HFNC) has been rapidly adopted for the treatment of bronchiolitis despite weak evidence supporting its routine and early use in hypoxemic infants.1 Although HFNC use has been associated with decreased work of breathing and lower rates of progression to invasive ventilation in some studies, the one large trial published on the topic found no difference between early HFNC and standard oxygen therapy on length of stay in hospital, duration of oxygen therapy, or rates of intubation.2,3 No adequately powered studies have examined the effect of ward-based HFNC initiation on ICU transfer, an outcome that it is designed to prevent.
In this month’s issue of the Journal of Hospital Medicine, Coon et al examine the association between the implementation of ward-based HFNC initiation protocols and subsequent ICU transfer rates.4 Hospitals enrolled in the Pediatric Health Information System database were surveyed about their HFNC use and protocol implementation, with 41 (93% response rate) hospitals replying, 12 of which implemented ward-based HFNC initiation protocols during 2010 to 2016. Administrative data for bronchiolitis encounters were obtained with use of International Classification of Diseases, 9th and 10th Revisions, coding of children aged 3 to 24 months discharged during the respiratory seasons of the study period. The authors used an interrupted time series analysis to study the association between ward-based HFNC protocol initiation and several outcomes, revealing a small but significant increase in ICU transfers (absolute difference, 3.1%; 95% CI, 2.8%-3.4%) and ICU length of stay (absolute difference, 9.1 days per 100 patients; 95% CI 5.1-13.2), but not overall length of stay or use of mechanical ventilation. Modifications to the analysis that account for a learning period during the first season of implementation at each hospital, and for trends among nonadopting hospitals, did not substantially affect the findings.
The authors acknowledged many of the study’s limitations, including its retrospective design, presumption of bronchiolitis discharge code validity, restriction to tertiary care hospitals, and analysis of hospital-level rather than patient-level variables and outcomes. Because the data source does not capture patient-level HFNC use, the number and characteristics of patients receiving HFNC at the centers are unknown. It is also important to note that the 12 included protocols are quite heterogeneous, with differing exclusion criteria, maximum flow rates, and indications for ICU transfer. Given the rapid evolution of ward-based HFNC use for bronchiolitis, these protocols from 2010 to 2016 are already out of date. All of the protocols allowed much lower maximum flow rates (4-10 L/min) than would typically be expected today (usually 2 L/kg per minute, which translates to 10 L/min of flow for a 5-kg child or 20 L/min for a 10-kg child). Many also had time-based criteria prompting ICU transfer (eg, 24 hours without improvement) that are not typically included in more recent protocols. Few had instructions for weaning or discontinuation of HFNC.
In spite of the above limitations, the results of this large, multicenter study advance our understanding of the consequences of ward-based protocols for HFNC initiation. However, it is important to contextualize this work as an examination of the implementation of a technology to a broad population in a specific era, not necessarily a study of the effectiveness of the technology itself.
The pediatric hospital medicine community has long recognized the need for more evidence regarding HFNC use.5-7 Coon et al have highlighted possible unintended consequences, notably increased ICU use, that may be associated with ward-based HFNC implementation on a population basis. This finding mirrors evidence from a recent similarly designed study analyzing Canadian tertiary care centers implementing HFNC administration during 2009 to 2014, though not specifically limited to ward use.8
More recently there has been discussion of how we might deimplement ward-based HFNC protocols. Although it is increasingly clear that HFNC is not a panacea for bronchiolitis, there is not necessarily a problem with the technology; the problem that this study so clearly demonstrates is how we have applied it. We need pragmatic trials of HFNC protocols to understand what parameters should guide HFNC initiation as a rescue treatment; what oxygen and flow settings might prevent ICU transfer; how it should be used in populations that have been largely excluded from trials (ie, children with medical complexity); and how to optimally wean it. With that information we could construct evidence-based, utilitarian HFNC initiation and treatment protocols to maximize benefit and minimize harm and cost.
It is understandable that our desire to help patients has led us to hear the “siren’s call” for this therapy, and indeed we should work on putting some of the “horses back in the barn.”5,6 Until new evidence guides how to best use this technology, institutional practice guidelines for HFNC initiation in ward settings should target children for whom ICU transfer seems very likely (eg, having oxygen saturations not maintained on maximum low-flow oxygen therapy) so that HFNC is not used routinely and that we maximize its cost to benefit ratio. It is important to approach this shift in a thoughtful manner to prevent a pendulum swing to premature universal deimplementation.
1. Piper L, Stalets EL, Statile AM. Clinical practice update: high flow nasal cannula therapy for bronchiolitis outside the ICU in infants. J Hosp Med. 2019;14:E1-E3. https://doi.org/10.12788/jhm.3328.
2. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/nejmoa1714855.
3. Lin J, Zhang Y, Xiong L, Liu S, Gong C, Dai J. High-flow nasal cannula therapy for children with bronchiolitis: a systematic review and meta-analysis. Arch Dis Child. 2019;104(6):564-576. https://doi.org/10.1136/archdischild-2018-315846.
4. Coon ER, G. S, Brady PW. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3456.
5. de Benedictis FM. The Effectiveness of high-flow oxygen therapy and the fascinating song of the sirens. JAMA Pediatr. 2019;173(2):125-126. https://doi.org/10.1001/jamapediatrics.2018.3831.
6. Ralston SL. High-flow nasal cannula therapy for pediatric patients with bronchiolitis: time to put the horse back in the barn [online first]. JAMA Pediatr. 2020. https://doi.org/10.1001/jamapediatrics.2020.0040.
7. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2015-2862.
8. Garland H, Miller MR, Gunz AC, Lim RK. High-flow nasal cannula implementation has not reduced intubation rates for bronchiolitis in Canada [online first]. Paediatr Child Health. 2020. https://doi.org/10.1093/pch/pxaa023.
As an appealing, physiologically plausible treatment, humidified oxygen delivery via high-flow nasal cannula (HFNC) has been rapidly adopted for the treatment of bronchiolitis despite weak evidence supporting its routine and early use in hypoxemic infants.1 Although HFNC use has been associated with decreased work of breathing and lower rates of progression to invasive ventilation in some studies, the one large trial published on the topic found no difference between early HFNC and standard oxygen therapy on length of stay in hospital, duration of oxygen therapy, or rates of intubation.2,3 No adequately powered studies have examined the effect of ward-based HFNC initiation on ICU transfer, an outcome that it is designed to prevent.
In this month’s issue of the Journal of Hospital Medicine, Coon et al examine the association between the implementation of ward-based HFNC initiation protocols and subsequent ICU transfer rates.4 Hospitals enrolled in the Pediatric Health Information System database were surveyed about their HFNC use and protocol implementation, with 41 (93% response rate) hospitals replying, 12 of which implemented ward-based HFNC initiation protocols during 2010 to 2016. Administrative data for bronchiolitis encounters were obtained with use of International Classification of Diseases, 9th and 10th Revisions, coding of children aged 3 to 24 months discharged during the respiratory seasons of the study period. The authors used an interrupted time series analysis to study the association between ward-based HFNC protocol initiation and several outcomes, revealing a small but significant increase in ICU transfers (absolute difference, 3.1%; 95% CI, 2.8%-3.4%) and ICU length of stay (absolute difference, 9.1 days per 100 patients; 95% CI 5.1-13.2), but not overall length of stay or use of mechanical ventilation. Modifications to the analysis that account for a learning period during the first season of implementation at each hospital, and for trends among nonadopting hospitals, did not substantially affect the findings.
The authors acknowledged many of the study’s limitations, including its retrospective design, presumption of bronchiolitis discharge code validity, restriction to tertiary care hospitals, and analysis of hospital-level rather than patient-level variables and outcomes. Because the data source does not capture patient-level HFNC use, the number and characteristics of patients receiving HFNC at the centers are unknown. It is also important to note that the 12 included protocols are quite heterogeneous, with differing exclusion criteria, maximum flow rates, and indications for ICU transfer. Given the rapid evolution of ward-based HFNC use for bronchiolitis, these protocols from 2010 to 2016 are already out of date. All of the protocols allowed much lower maximum flow rates (4-10 L/min) than would typically be expected today (usually 2 L/kg per minute, which translates to 10 L/min of flow for a 5-kg child or 20 L/min for a 10-kg child). Many also had time-based criteria prompting ICU transfer (eg, 24 hours without improvement) that are not typically included in more recent protocols. Few had instructions for weaning or discontinuation of HFNC.
In spite of the above limitations, the results of this large, multicenter study advance our understanding of the consequences of ward-based protocols for HFNC initiation. However, it is important to contextualize this work as an examination of the implementation of a technology to a broad population in a specific era, not necessarily a study of the effectiveness of the technology itself.
The pediatric hospital medicine community has long recognized the need for more evidence regarding HFNC use.5-7 Coon et al have highlighted possible unintended consequences, notably increased ICU use, that may be associated with ward-based HFNC implementation on a population basis. This finding mirrors evidence from a recent similarly designed study analyzing Canadian tertiary care centers implementing HFNC administration during 2009 to 2014, though not specifically limited to ward use.8
More recently there has been discussion of how we might deimplement ward-based HFNC protocols. Although it is increasingly clear that HFNC is not a panacea for bronchiolitis, there is not necessarily a problem with the technology; the problem that this study so clearly demonstrates is how we have applied it. We need pragmatic trials of HFNC protocols to understand what parameters should guide HFNC initiation as a rescue treatment; what oxygen and flow settings might prevent ICU transfer; how it should be used in populations that have been largely excluded from trials (ie, children with medical complexity); and how to optimally wean it. With that information we could construct evidence-based, utilitarian HFNC initiation and treatment protocols to maximize benefit and minimize harm and cost.
It is understandable that our desire to help patients has led us to hear the “siren’s call” for this therapy, and indeed we should work on putting some of the “horses back in the barn.”5,6 Until new evidence guides how to best use this technology, institutional practice guidelines for HFNC initiation in ward settings should target children for whom ICU transfer seems very likely (eg, having oxygen saturations not maintained on maximum low-flow oxygen therapy) so that HFNC is not used routinely and that we maximize its cost to benefit ratio. It is important to approach this shift in a thoughtful manner to prevent a pendulum swing to premature universal deimplementation.
As an appealing, physiologically plausible treatment, humidified oxygen delivery via high-flow nasal cannula (HFNC) has been rapidly adopted for the treatment of bronchiolitis despite weak evidence supporting its routine and early use in hypoxemic infants.1 Although HFNC use has been associated with decreased work of breathing and lower rates of progression to invasive ventilation in some studies, the one large trial published on the topic found no difference between early HFNC and standard oxygen therapy on length of stay in hospital, duration of oxygen therapy, or rates of intubation.2,3 No adequately powered studies have examined the effect of ward-based HFNC initiation on ICU transfer, an outcome that it is designed to prevent.
In this month’s issue of the Journal of Hospital Medicine, Coon et al examine the association between the implementation of ward-based HFNC initiation protocols and subsequent ICU transfer rates.4 Hospitals enrolled in the Pediatric Health Information System database were surveyed about their HFNC use and protocol implementation, with 41 (93% response rate) hospitals replying, 12 of which implemented ward-based HFNC initiation protocols during 2010 to 2016. Administrative data for bronchiolitis encounters were obtained with use of International Classification of Diseases, 9th and 10th Revisions, coding of children aged 3 to 24 months discharged during the respiratory seasons of the study period. The authors used an interrupted time series analysis to study the association between ward-based HFNC protocol initiation and several outcomes, revealing a small but significant increase in ICU transfers (absolute difference, 3.1%; 95% CI, 2.8%-3.4%) and ICU length of stay (absolute difference, 9.1 days per 100 patients; 95% CI 5.1-13.2), but not overall length of stay or use of mechanical ventilation. Modifications to the analysis that account for a learning period during the first season of implementation at each hospital, and for trends among nonadopting hospitals, did not substantially affect the findings.
The authors acknowledged many of the study’s limitations, including its retrospective design, presumption of bronchiolitis discharge code validity, restriction to tertiary care hospitals, and analysis of hospital-level rather than patient-level variables and outcomes. Because the data source does not capture patient-level HFNC use, the number and characteristics of patients receiving HFNC at the centers are unknown. It is also important to note that the 12 included protocols are quite heterogeneous, with differing exclusion criteria, maximum flow rates, and indications for ICU transfer. Given the rapid evolution of ward-based HFNC use for bronchiolitis, these protocols from 2010 to 2016 are already out of date. All of the protocols allowed much lower maximum flow rates (4-10 L/min) than would typically be expected today (usually 2 L/kg per minute, which translates to 10 L/min of flow for a 5-kg child or 20 L/min for a 10-kg child). Many also had time-based criteria prompting ICU transfer (eg, 24 hours without improvement) that are not typically included in more recent protocols. Few had instructions for weaning or discontinuation of HFNC.
In spite of the above limitations, the results of this large, multicenter study advance our understanding of the consequences of ward-based protocols for HFNC initiation. However, it is important to contextualize this work as an examination of the implementation of a technology to a broad population in a specific era, not necessarily a study of the effectiveness of the technology itself.
The pediatric hospital medicine community has long recognized the need for more evidence regarding HFNC use.5-7 Coon et al have highlighted possible unintended consequences, notably increased ICU use, that may be associated with ward-based HFNC implementation on a population basis. This finding mirrors evidence from a recent similarly designed study analyzing Canadian tertiary care centers implementing HFNC administration during 2009 to 2014, though not specifically limited to ward use.8
More recently there has been discussion of how we might deimplement ward-based HFNC protocols. Although it is increasingly clear that HFNC is not a panacea for bronchiolitis, there is not necessarily a problem with the technology; the problem that this study so clearly demonstrates is how we have applied it. We need pragmatic trials of HFNC protocols to understand what parameters should guide HFNC initiation as a rescue treatment; what oxygen and flow settings might prevent ICU transfer; how it should be used in populations that have been largely excluded from trials (ie, children with medical complexity); and how to optimally wean it. With that information we could construct evidence-based, utilitarian HFNC initiation and treatment protocols to maximize benefit and minimize harm and cost.
It is understandable that our desire to help patients has led us to hear the “siren’s call” for this therapy, and indeed we should work on putting some of the “horses back in the barn.”5,6 Until new evidence guides how to best use this technology, institutional practice guidelines for HFNC initiation in ward settings should target children for whom ICU transfer seems very likely (eg, having oxygen saturations not maintained on maximum low-flow oxygen therapy) so that HFNC is not used routinely and that we maximize its cost to benefit ratio. It is important to approach this shift in a thoughtful manner to prevent a pendulum swing to premature universal deimplementation.
1. Piper L, Stalets EL, Statile AM. Clinical practice update: high flow nasal cannula therapy for bronchiolitis outside the ICU in infants. J Hosp Med. 2019;14:E1-E3. https://doi.org/10.12788/jhm.3328.
2. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/nejmoa1714855.
3. Lin J, Zhang Y, Xiong L, Liu S, Gong C, Dai J. High-flow nasal cannula therapy for children with bronchiolitis: a systematic review and meta-analysis. Arch Dis Child. 2019;104(6):564-576. https://doi.org/10.1136/archdischild-2018-315846.
4. Coon ER, G. S, Brady PW. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3456.
5. de Benedictis FM. The Effectiveness of high-flow oxygen therapy and the fascinating song of the sirens. JAMA Pediatr. 2019;173(2):125-126. https://doi.org/10.1001/jamapediatrics.2018.3831.
6. Ralston SL. High-flow nasal cannula therapy for pediatric patients with bronchiolitis: time to put the horse back in the barn [online first]. JAMA Pediatr. 2020. https://doi.org/10.1001/jamapediatrics.2020.0040.
7. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2015-2862.
8. Garland H, Miller MR, Gunz AC, Lim RK. High-flow nasal cannula implementation has not reduced intubation rates for bronchiolitis in Canada [online first]. Paediatr Child Health. 2020. https://doi.org/10.1093/pch/pxaa023.
1. Piper L, Stalets EL, Statile AM. Clinical practice update: high flow nasal cannula therapy for bronchiolitis outside the ICU in infants. J Hosp Med. 2019;14:E1-E3. https://doi.org/10.12788/jhm.3328.
2. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/nejmoa1714855.
3. Lin J, Zhang Y, Xiong L, Liu S, Gong C, Dai J. High-flow nasal cannula therapy for children with bronchiolitis: a systematic review and meta-analysis. Arch Dis Child. 2019;104(6):564-576. https://doi.org/10.1136/archdischild-2018-315846.
4. Coon ER, G. S, Brady PW. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3456.
5. de Benedictis FM. The Effectiveness of high-flow oxygen therapy and the fascinating song of the sirens. JAMA Pediatr. 2019;173(2):125-126. https://doi.org/10.1001/jamapediatrics.2018.3831.
6. Ralston SL. High-flow nasal cannula therapy for pediatric patients with bronchiolitis: time to put the horse back in the barn [online first]. JAMA Pediatr. 2020. https://doi.org/10.1001/jamapediatrics.2020.0040.
7. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2015-2862.
8. Garland H, Miller MR, Gunz AC, Lim RK. High-flow nasal cannula implementation has not reduced intubation rates for bronchiolitis in Canada [online first]. Paediatr Child Health. 2020. https://doi.org/10.1093/pch/pxaa023.
© 2020 Society of Hospital Medicine
LOS in Children With Medical Complexity
Children with medical complexity (CMC) have complex and chronic health conditions that often involve multiple organ systems and severely affect cognitive and physical functioning. Although the prevalence of CMC is low (1% of all children), they account for nearly one‐fifth of all pediatric admissions and one‐half of all hospital days and charges in the United States.[1] Over the last decade, CMC have had a particularly large and increasing impact in tertiary‐care children's hospitals.[1, 2] The Institute of Medicine has identified CMC as a priority population for a revised healthcare system.[3]
Medical homes, hospitals, health plans, states, federal agencies, and others are striving to reduce excessive hospital use in CMC because of its high cost.[4, 5, 6] Containing length of stay (LOS)an increasingly used indicator of the time sensitiveness and efficiency of hospital careis a common aim across these initiatives. CMC have longer hospitalizations than children without medical complexity. Speculated reasons for this are that CMC tend to have (1) higher severity of acute illnesses (eg, pneumonia, cellulitis), (2) prolonged recovery time in the hospital, and (3) higher risk of adverse events in the hospital. Moreover, hospital clinicians caring for CMC often find it difficult to determine discharge readiness, given that many CMC do not return to a completely healthy baseline.[7]
Little is known about long LOS in CMC, including which CMC have the highest risk of experiencing such stays and which stays might have the greatest opportunity to be shortened. Patient characteristics associated with prolonged length of stay have been studied extensively for many pediatric conditions (eg, asthma).[8, 9, 10, 11, 12, 13, 14] However, most of these studies excluded CMC. Therefore, the objectives of this study were to examine (1) the prevalence of long LOS in CMC, (2) patient characteristics associated with long LOS, and (3) hospital‐to‐hospital variation in prevalence of long LOS hospitalizations.
METHODS
Study Design and Data Source
This study is a multicenter, retrospective cohort analysis of the Pediatric Health Information System (PHIS). PHIS is an administrative database of 44 not for profit, tertiary care pediatric hospitals affiliated with the Children's Hospital Association (CHA) (Overland Park, KS). PHIS contains data regarding patient demographics, diagnoses, and procedures (with International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] codes), All‐Patient Refined Diagnostic Related Groups version 30 (APR‐DRGs) (3M Health Information Systems, Salt Lake City, UT), and service lines that aggregate the APR‐DRGs into 38 distinct groups. Data quality and reliability are assured through CHA and participating hospitals. In accordance with the policies of the Cincinnati Children's Hospital Medical Center Institutional Review Board, this study of deidentified data was not considered human subjects research.
Study Population
Inclusion Criteria
Children discharged following an observation or inpatient admission from a hospital participating in the PHIS database between January 1, 2013 and December 31, 2014 were eligible for inclusion if they were considered medically complex. Medical complexity was defined using Clinical Risk Groups (CRGs) version 1.8, developed by 3M Health Information Systems and the National Association of Children's Hospitals and Related Institutions. CRGs were used to assign each hospitalized patient to 1 of 9 mutually exclusive chronicity groups according to the presence, type, and severity of chronic conditions.[15, 16, 17, 18] Each patient's CRG designation was based on 2 years of previous hospital encounters.
As defined in prior studies and definitional frameworks of CMC,[1] patients belonging to CRG group 6 (significant chronic disease in 2 organ systems), CRG group 7 (dominant chronic disease in 3 organ systems), and CRG group 9 (catastrophic condition) were considered medically complex.[17, 19] Patients with malignancies (CRG group 8) were not included for analysis because they are a unique population with anticipated, long hospital stays. Patients with CRG group 5, representing those with chronic conditions affecting a single body system, were also not included because most do not have attributes consistent with medical complexity.
Exclusion Criteria
We used the APR‐DRG system, which leverages ICD‐9‐CM codes to identify the health problem most responsible for the hospitalization, to refine the study cohort. We excluded hospitalizations that were classified by the APR‐DRG system as neonatal, as we did not wish to focus on LOS in the neonatal intensive care unit (ICU) or for birth admissions. Similarly, hospitalizations for chemotherapy (APR‐DRG 693) or malignancy (identified with previously used ICD‐9‐CM codes)[20] were also excluded because long LOS is anticipated. We also excluded hospitalizations for medical rehabilitation (APR‐DRG 860).
Outcome Measures
The primary outcome measure was long LOS, defined as LOS 10 days. The cut point of LOS 10 days represents the 90th percentile of LOS for all children, with and without medical complexity, hospitalized during 2013 to 2014. LOS 10 days has previously been used as a threshold of long LOS.[21] For hospitalizations involving transfer at admission from another acute care facility, LOS was measured from the date of transfer. We also assessed hospitals' cost attributable to long LOS admissions.
Patient Demographics and Clinical Characteristics
We measured demographic characteristics including age, gender, race/ethnicity, insurance type, and distance traveled (the linear distance between the centroid of the patient's home ZIP code and the centroid of the hospital's ZIP code). Clinical characteristics included CRG classification, complex chronic condition (CCC), and dependence on medical technology. CCCs are defined as any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or 1 system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.[20] Medical technology included devices used to optimize the health and functioning of the child (eg, gastrostomy, tracheostomy, cerebrospinal fluid shunt).[22]
Hospitalization Characteristics
Characteristics of the hospitalization included transfer from an outside facility, ICU admission, surgical procedure (using surgical APR‐DRGs), and discharge disposition (home, skilled nursing facility, home health services, death, other). Cost of the hospitalization was estimated in the PHIS from charges using hospital and year‐specific ratios of cost to charge.
Statistical Analysis
Continuous data (eg, distance from hospital to home residence) were described with median and interquartile ranges (IQR) because they were not normally distributed. Categorical data (eg, type of chronic condition) were described with counts and frequencies. In bivariate analyses, demographic, clinical, and hospitalization characteristics were stratified by LOS (long LOS vs LOS <10 days), and compared using 2 statistics or Wilcoxon rank sum tests as appropriate.
We modeled the likelihood of experiencing a long LOS using generalized linear mixed effects models with a random hospital intercept and discharge‐level fixed effects for age, gender, payor, CCC type, ICU utilization, transfer status, a medical/surgical admission indicator derived from the APR‐DRG, and CRG assigned to each hospitalization. To examine hospital‐to‐hospital variability, we generated hospital risk‐adjusted rates of long LOS from these models. Similar models and hospital risk‐adjusted rates were built for a post hoc correlational analysis of 30‐day all cause readmission, where hospitals' rates and percent of long LOS were compared with a Pearson correlation coefficient. Also, for our multivariable models, we performed a sensitivity analysis using an alternative definition of long LOS as 4 days (the 75th percentile of LOS for all children, with and without medical complexity, hospitalized during 20132014). All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and P values <0.05 were considered statistically significant.
RESULTS
Study Population
There were 954,018 hospitalizations of 217,163 CMC at 44 children's hospitals included for analysis. Forty‐seven percent of hospitalizations were for females, 49.4% for non‐Hispanic whites, and 61.1% for children with government insurance. Fifteen percent (n = 142,082) had a long LOS of 10 days. The median (IQR) LOS of hospitalizations <10 days versus 10 days were 2 (IQR, 14) and 16 days (IQR, 1226), respectively. Long LOS hospitalizations accounted for 61.1% (3.7 million) hospital days and 61.8% ($13.7 billion) of total hospitalization costs for all CMC in the cohort (Table 1).
| Characteristic | Overall (n = 954,018) | Length of Stay | |
|---|---|---|---|
| <10 Days (n = 811,936) | 10 Days (n = 142,082) | ||
| |||
| Age at admission, y, % | |||
| <1 | 14.6 | 12.7 | 25.7 |
| 14 | 27.1 | 27.9 | 22.4 |
| 59 | 20.1 | 21.0 | 14.9 |
| 1018 | 33.6 | 34.0 | 31.7 |
| 18+ | 4.6 | 4.4 | 5.4 |
| Gender, % | |||
| Female | 47.0 | 46.9 | 47.5 |
| Race/ethnicity, % | |||
| Non‐Hispanic white | 49.4 | 49.4 | 49.4 |
| Non‐Hispanic black | 23.1 | 23.8 | 19.3 |
| Hispanic | 18.2 | 17.8 | 20.4 |
| Asian | 2.0 | 1.9 | 2.3 |
| Other | 7.4 | 7.1 | 8.6 |
| Complex chronic condition, % | |||
| Any | 79.5 | 77.3 | 91.8 |
| Technology assistance | 37.1 | 34.1 | 54.2 |
| Gastrointestinal | 30.0 | 27.2 | 45.9 |
| Neuromuscular | 28.2 | 27.7 | 30.9 |
| Cardiovascular | 16.8 | 14.5 | 29.9 |
| Respiratory | 14.1 | 11.5 | 29.4 |
| Congenital/genetic defect | 17.2 | 16.7 | 20.2 |
| Metabolic | 9.9 | 8.9 | 15.4 |
| Renal | 10.1 | 9.5 | 13.8 |
| Hematology/emmmunodeficiency | 11.7 | 12.0 | 10.0 |
| Neonatal | 3.8 | 3.1 | 7.7 |
| Transplantation | 4.5 | 4.2 | 6.7 |
| Clinical risk group, % | |||
| Chronic condition in 2 systems | 68.4 | 71.2 | 53.9 |
| Catastrophic chronic condition | 31.4 | 28.8 | 46.1 |
| Distance from hospital to home residence in miles, median [IQR] | 16.2 [7.440.4] | 15.8 [7.338.7] | 19.1 [8.552.6] |
| Transferred from outside hospital (%) | 6.5 | 5.3 | 13.6 |
| Admitted for surgery, % | 23.4 | 20.7 | 38.7 |
| Use of intensive care, % | 19.6 | 14.9 | 46.5 |
| Discharge disposition, % | |||
| Home | 91.2 | 92.9 | 81.4 |
| Home healthcare | 4.5 | 3.5 | 9.9 |
| Other | 2.9 | 2.6 | 4.5 |
| Postacute care facility | 1.1 | 0.8 | 3.1 |
| Died | 0.4 | 0.3 | 1.1 |
| Payor, % | |||
| Government | 61.1 | 60.6 | 63.5 |
| Private | 33.2 | 33.6 | 30.9 |
| Other | 5.7 | 5.7 | 5.7 |
| Hospital resource use | |||
| Median length of stay [IQR] | 3 [16] | 2 [14] | 16 [1226] |
| Median hospital cost [IQR] | $8,144 [$4,122$18,447] | $6,689 [$3,685$12,395] | $49,207 [$29,444$95,738] |
| Total hospital cost, $, billions | $22.2 | $8.5 | $13.7 |
Demographics and Clinical Characteristics of Children With and Without Long LOS
Compared with hospitalized CMC with LOS <10 days, a higher percentage of hospitalizations with LOS 10 days were CMC age <1 year (25.7% vs 12.7%, P < 0.001) and Hispanic (20.4% vs 17.8%, P < 0.001). CMC hospitalizations with a long LOS had a higher percentage of any CCC (91.8% vs 77.3%, P < 0.001); the most common CCCs were gastrointestinal (45.9%), neuromuscular (30.9%), and cardiovascular (29.9%). Hospitalizations of CMC with a long LOS had a higher percentage of a catastrophic chronic condition (46.1% vs 28.8%, P < 0.001) and technology dependence (46.1% vs 28.8%, P < 0.001) (Table 1).
Hospitalization Characteristics of Children With and Without Long LOS
Compared with hospitalizations of CMC with LOS <10 days, hospitalizations of CMC with a long LOS more often involved transfer in from another hospital at admission (13.6% vs 5.3%, P < 0.001). CMC hospital stays with a long LOS more often involved surgery (38.7% vs 20.7%, P < 0.001) and use of intensive care (46.5% vs 14.9%; P < 0.001). A higher percentage of CMC with long LOS were discharged with home health services (9.9% vs 3.5%; P < 0.001) (Table 1).
The most common admitting diagnoses and CCCs for hospitalizations of CMC with long LOS are presented in Table 2. The two most prevalent APR‐DRGs in CMC hospitalizations lasting 10 days or longer were cystic fibrosis (10.7%) and respiratory system disease with ventilator support (5.5%). The two most common chronic condition characteristics represented among long CMC hospitalizations were gastrointestinal devices (eg, gastrostomy tube) (39.7%) and heart and great vessel malformations (eg, tetralogy of Fallot) (12.8%). The 5 most common CCC subcategories, as listed in Table 2, account for nearly 100% of the patients with long LOS hospitalizations.
| |
| Most common reason for admission* | |
| Cystic fibrosis | 10.7% |
| Respiratory system diagnosis with ventilator support 96+ hours | 5.5% |
| Malfunction, reaction, and complication of cardiac or vascular device or procedure | 2.8% |
| Craniotomy except for trauma | 2.6% |
| Major small and large bowel procedures | 2.3% |
| Most common complex chronic condition | |
| Gastrointestinal devices | 39.7% |
| Heart and great vessel malformations | 12.8% |
| Cystic fibrosis | 12.5% |
| Dysrhythmias | 11.0% |
| Respiratory devices | 10.7% |
Multivariable Analysis of Characteristics Associated With Long LOS
In multivariable analysis, the highest likelihood of long LOS was experienced by children who received care in the ICU (odds ratio [OR]: 3.5, 95% confidence interval [CI]: 3.43.5), who had a respiratory CCC (OR: 2.7, 95% CI: 2.62.7), and who were transferred from another acute care hospital at admission (OR: 2.1, 95% CI: 2.0, 2.1). The likelihood of long LOS was also higher in children <1 year of age (OR: 1.2, 95% CI: 1.21.3), and Hispanic children (OR: 1.1, 95% CI 1.0‐1.10) (Table 3). Similar multivariable findings were observed in sensitivity analysis using the 75th percentile of LOS (4 days) as the model outcome.
| Characteristic | Odds Ratio (95% CI) of LOS 10 Days | P Value |
|---|---|---|
| ||
| Use of intensive care | 3.5 (3.4‐3.5) | <0.001 |
| Transfer from another acute‐care hospital | 2.1 (2.0‐2.1) | <0.001 |
| Procedure/surgery | 1.8 (1.8‐1.9) | <0.001 |
| Complex chronic condition | ||
| Respiratory | 2.7 (2.6‐2.7) | <0.001 |
| Gastrointestinal | 1.8 (1.8‐1.8) | <0.001 |
| Metabolic | 1.7 (1.7‐1.7) | <0.001 |
| Cardiovascular | 1.6 (1.5‐1.6) | <0.001 |
| Neonatal | 1.5 (1.5‐1.5) | <0.001 |
| Renal | 1.4 (1.4‐1.4) | <0.001 |
| Transplant | 1.4 (1.4‐1.4) | <0.001 |
| Hematology and immunodeficiency | 1.3 (1.3‐1.3) | <0.001 |
| Technology assistance | 1.1 (1.1, 1.1) | <0.001 |
| Neuromuscular | 0.9 (0.9‐0.9) | <0.001 |
| Congenital or genetic defect | 0.8 (0.8‐0.8) | <0.001 |
| Age at admission, y | ||
| <1 | 1.2 (1.2‐1.3) | <0.001 |
| 14 | 0.5 (0.5‐0.5) | <0.001 |
| 59 | 0.6 (0.6‐0.6) | <0.001 |
| 1018 | 0.9 (0.9‐0.9) | <0.001 |
| 18+ | Reference | |
| Male | 0.9 (0.9‐0.9) | <0.001 |
| Race/ethnicity | ||
| Non‐Hispanic black | 0.9 (0.9‐0.9) | <0.001 |
| Hispanic | 1.1 (1.0‐1.1) | <0.001 |
| Asian | 1.0 (1.0‐1.1) | 0.3 |
| Other | 1.1 (1.1‐1.1) | <0.001 |
| Non‐Hispanic white | Reference | |
| Payor | ||
| Private | 0.9 (0.8 0.9) | <0.001 |
| Other | 1.0 (1.0‐1.0) | 0.4 |
| Government | Reference | |
| Season | ||
| Spring | 1.0 (1.0 1.0) | <0.001 |
| Summer | 0.9 (0.9‐0.9) | <0.001 |
| Fall | 1.0 (0.9‐1.0) | <0.001 |
| Winter | Reference | |
Variation in the Prevalence of Long LOS Across Children's Hospitals
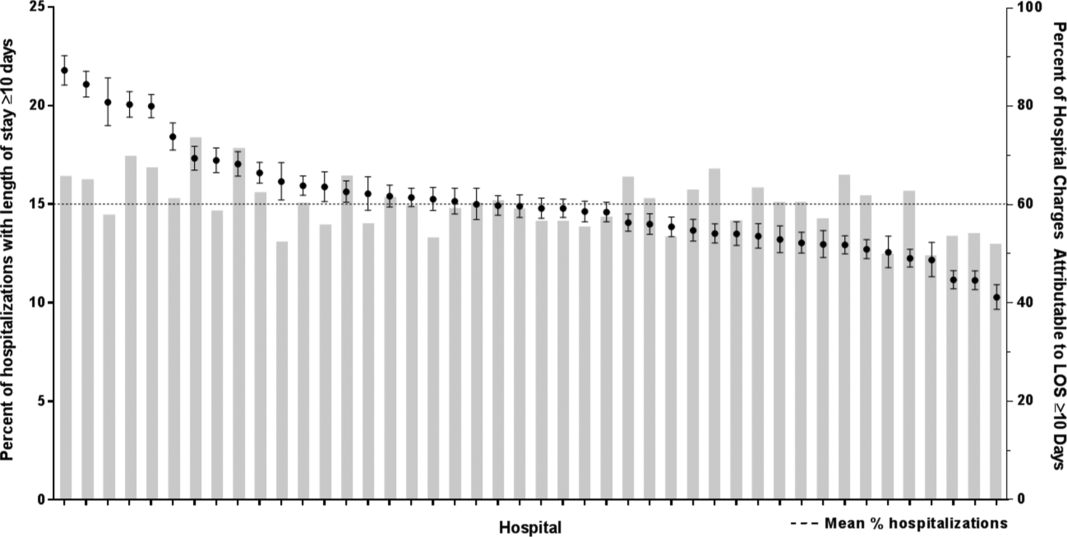
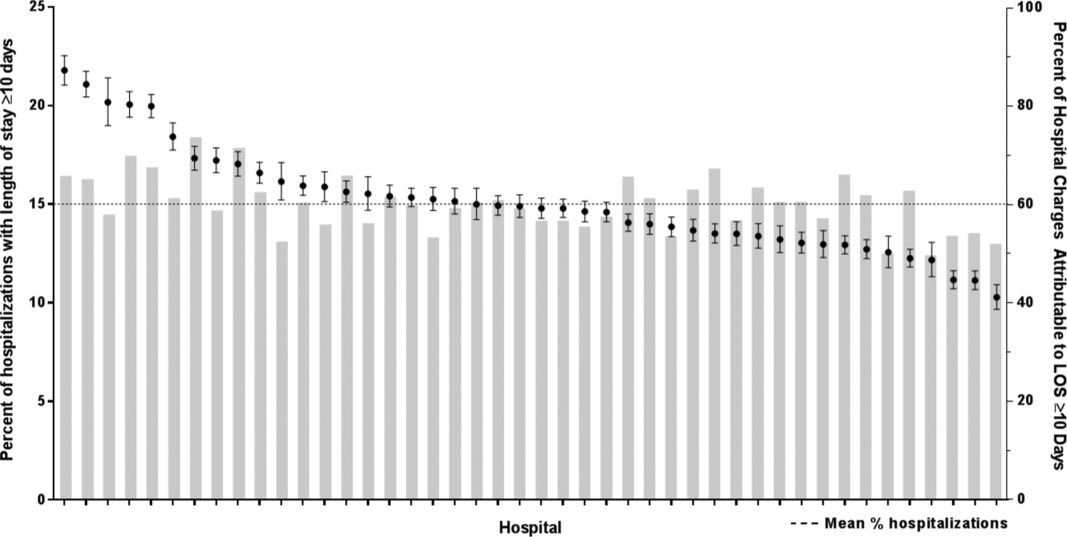
After controlling for demographic, clinical, and hospital characteristics associated with long LOS, there was significant (P < 0.001) variation in the prevalence of long LOS for CMC across children's hospitals in the cohort (range, 10.3%21.8%) (Figure 1). Twelve (27%) hospitals had a significantly (P < 0.001) higher prevalence of long LOS for their hospitalized CMC, compared to the mean. Eighteen (41%) had a significantly (P < 0.001) lower prevalence of long LOS for their hospitalized CMC. There was also significant variation across hospitals with respect to cost, with 49.7% to 73.7% of all hospital costs of CMC attributed to long LOS hospitalizations. Finally, there was indirect correlation with the prevalence of LOS across hospitals and the hospitals' 30‐day readmission rate ( = 0.3; P = 0.04). As the prevalence of long LOS increased, the readmission rate decreased.
DISCUSSION
The main findings from this study suggest that a small percentage of CMC experiencing long LOS account for the majority of hospital bed days and cost of all hospitalized CMC in children's hospitals. The likelihood of long LOS varies significantly by CMC's age, race/ethnicity, and payor as well as by type and number of chronic conditions. Among CMC with long LOS, the use of gastrointestinal devices such as gastrostomy tubes, as well as congenital heart disease, were highly prevalent. In multivariable analysis, the characteristics most strongly associated with LOS 10 days were use of the ICU, respiratory complex chronic condition, and transfer from another medical facility at admission. After adjusting for these factors, there was significant variation in the prevalence of LOS 10 days for CMC across children's hospitals.
Although it is well known that CMC as a whole have a major impact on resource use in children's hospitals, this study reveals that 15% of hospitalizations of CMC account for 62% of all hospital costs of CMC. That is, a small fraction of hospitalizations of CMC is largely responsible for the significant financial impact of hospital resource use. To date, most clinical efforts and policies striving to reduce hospital use in CMC have focused on avoiding readmissions or index hospital admissions entirely, rather than improving the efficiency of hospital care after admission occurs.[23, 24, 25, 26] In the adult population, the impact of long LOS on hospital costs has been recognized, and several Medicare incentive programs have focused on in‐hospital timeliness and efficiency. As a result, LOS in Medicare beneficiaries has decreased dramatically over the past 2 decades.[27, 28, 29, 30] Optimizing the efficiency of hospital care for CMC may be an important goal to pursue, especially with precedent set in the adult literature.
Perhaps the substantial variation across hospitals in the prevalence of long LOS in CMC indicates opportunity to improve the efficiency of their inpatient care. This variation was not due to differences across hospitals' case mix of CMC. Further investigation is needed to determine how much of it is due to differences in quality of care. Clinical practice guidelines for hospital treatment of common illnesses usually exclude CMC. In our clinical experience across 9 children's hospitals, we have experienced varying approaches to setting discharge goals (ie, parameters on how healthy the child needs to be to ensure a successful hospital discharge) for CMC.[31] When the goals are absent or not clearly articulated, they can contribute to a prolonged hospitalization. Some families of CMC report significant issues when working with pediatric hospital staff to assess their child's discharge readiness.[7, 32, 33] In addition, there is significant variation across states and regions in access to and quality of post‐discharge health services (eg, home nursing, postacute care, durable medical equipment).[34, 35] In some areas, many CMC are not actively involved with their primary care physician.[5] These issues might also influence the ability of some children's hospitals to efficiently discharge CMC to a safe and supportive post‐discharge environment. Further examination of hospital outliersthose with the lowest and highest percentage of CMC hospitalizations with long LOSmay reveal opportunities to identify and spread best practices.
The demographic and clinical factors associated with long LOS in the present study, including age, ICU use, and transfer from another hospital, might help hospitals target which CMC have the greatest risk for experiencing long LOS. We found that infants age <1 year had longer LOS when compared with older children. Similar to our findings, younger‐aged children hospitalized with bronchiolitis have longer LOS.[36] Certainly, infants with medical complexity, in general, are a high‐acuity population with the potential for rapid clinical deterioration during an acute illness. Prolonged hospitalization for treatment and stabilization may be expected for many of them. Additional investigation is warranted to examine ICU use in CMC, and whether ICU admission or duration can be safely prevented or abbreviated. Opportunities to assess the quality of transfers into children's hospitals of CMC admitted to outside hospitals may be necessary. A study of pediatric burn patients reported that patients initially stabilized at a facility that was not a burn center and subsequently transferred to a burn center had a longer LOS than patients solely treated at a designated burn center.[37] Furthermore, events during transport itself may adversely impact the stability of an already fragile patient. Interventions to optimize the quality of care provided by transport teams have resulted in decreased LOS at the receiving hospital.[38]
This study's findings should be considered in the context of several limitations. Absent a gold‐standard definition of long LOS, we used the distribution of LOS across patients to inform our methods; LOS at the 90th percentile was selected as long. Although our sensitivity analysis using LOS at the 75th percentile produced similar findings, other cut points in LOS might be associated with different results. The study is not positioned to determine how much of the reported LOS was excessive, unnecessary, or preventable. The study findings may not generalize to types of hospitals not contained in PHIS (eg, nonchildren's hospitals and community hospitals). We did not focus on the impact of a new diagnosis (eg, new chronic illness) or acute in‐hospital event (eg, nosocomial infection) on prolonged LOS; future studies should investigate these clinical events with LOS.
PHIS does not contain information regarding characteristics that could influence LOS, including the children's social and familial attributes, transportation availability, home equipment needs, and local availability of postacute care facilities. Moreover, PHIS does not contain information about the hospital discharge procedures, process, or personnel across hospitals, which could influence LOS. Future studies on prolonged LOS should consider assessing this information. Because of the large sample size of hospitalizations included, the statistical power for the analyses was strong, rendering it possible that some findings that were statistically significant might have modest clinical significance (eg, relationship of Hispanic ethnicity with prolonged LOS). We could not determine why a positive correlation was not observed between hospitals' long LOS prevalence and their percentage of cost associated with long LOS; future studies should investigate the reasons for this finding.
Despite these limitations, the findings of the present study highlight the significance of long LOS in hospitalized CMC. These long hospitalizations account for a significant proportion of all hospital costs for this important population of children. The prevalence of long LOS for CMC varies considerably across children's hospitals, even after accounting for the case mix. Efforts to curtail hospital resource use and costs for CMC may benefit from focus on long LOS.
- , , , et al. Inpatient growth and resource use in 28 children's hospitals: a longitudinal, multi‐institutional study. JAMA Pediatr. 2013;167(2):170–177.
- , , , et al. Children with complex chronic conditions in inpatient hospital settings in the united states. Pediatrics. 2010;126(4):647–655.
- , . Meeting the health care needs of persons with disabilities. Milbank Q. 2002;80(2):381–391.
- , , , et al. Effect of an enhanced medical home on serious illness and cost of care among high‐risk children with chronic illness: a randomized clinical trial. JAMA. 2014;312(24):2640–2648.
- , , , et al. Children with medical complexity and Medicaid: spending and cost savings. Health Aff Proj Hope. 2014;33(12):2199–2206.
- Children's Hospital Association. CARE Award. Available at: https://www.childrenshospitals.org/Programs‐and‐Services/Quality‐Improvement‐and‐Measurement/CARE‐Award. Accessed December 18, 2015.
- , , , et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581.
- , , , et al. Weekend matters: Friday and Saturday admissions are associated with prolonged hospitalization of children. Clin Pediatr (Phila). 2013;52(9):875–878.
- , , , . Attributable cost and length of stay for central line‐associated bloodstream infections. Pediatrics. 2014;133(6):e1525–e1532.
- , , , et al. Effect of healthcare‐acquired infection on length of hospital stay and cost. Infect Control Hosp Epidemiol. 2007;28(3):280–292.
- , , , , . Hospital utilization and costs among children with influenza, 2003. Am J Prev Med. 2009;36(4):292–296.
- , , , . Charges and lengths of stay attributable to adverse patient‐care events using pediatric‐specific quality indicators: a multicenter study of freestanding children's hospitals. Pediatrics. 2008;121(6):e1653–e1659.
- , , , , . Variation in resource utilization for the management of uncomplicated community‐acquired pneumonia across community and children's hospitals. J Pediatr. 2014;165(3):585–591.
- , , , , . Variation and outcomes associated with direct hospital admission among children with pneumonia in the United States. JAMA Pediatr. 2014;168(9):829–836.
- , , , et al. Clinical Risk Groups (CRGs): a classification system for risk‐adjusted capitation‐based payment and health care management. Med Care. 2004;42(1):81–90.
- , , , et al. Identifying children with lifelong chronic conditions for care coordination by using hospital discharge data. Acad Pediatr. 2010;10(6):417–423.
- , , , , . Profile of medical charges for children by health status group and severity level in a Washington State Health Plan. Health Serv Res. 2004;39(1):73–89.
- , , , . Using medical billing data to evaluate chronically ill children over time. J Ambulatory Care Manage. 2006;29(4):283–290.
- , , , , , . Medical complexity and pediatric emergency department and inpatient utilization. Pediatrics. 2013;131(2):e559–e565.
- , , , , . Pediatric complex chronic conditions classification system version 2: updated for ICD‐10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199.
- . Analyzing intensive care unit length of stay data: problems and possible solutions. Crit Care Med. 1997;25(9):1594–1600.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- . Hospital readmissions and repeat emergency department visits among children with medical complexity: an integrative review. J Pediatr Nurs. 2013;28(4):316–339.
- , , , . Hospital readmission in children with complex chronic conditions discharged from subacute care. Hosp Pediatr. 2014;4(3):153–158.
- , , , et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6):e1628–e1647.
- , , , . Hospital readmissions for newly discharged pediatric home mechanical ventilation patients. Pediatr Pulmonol. 2012;47(4):409–414.
- , , , et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2008. JAMA. 2011;305(15):1560–1567.
- , , , et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147.
- U.S. Department of Health and Human Services. CMS Statistics 2013. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/CMS‐Statistics‐Reference‐Booklet/Downloads/CMS_Stats_2013_final.pdf. Published August 2013. Accessed October 6, 2015.
- Centers for Medicare and Medicaid Services. Evaluation of the premier hospital quality incentive demonstration. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Reports/downloads/Premier_ExecSum_2010.pdf. Published March 3, 2009. Accessed September 18, 2015.
- , , , et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955–962; quiz 965–966.
- , , , , . Parent and provider perspectives on pediatric readmissions: what can we learn about readiness for discharge? Hosp Pediatr. 2015;5(11):559–565.
- , . Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5(11):602–604.
- , , . Pediatric post‐acute hospital care: striving for identity and value. Hosp Pediatr. 2015;5(10):548–551.
- , , , et al. Pediatric hospital discharges to home health and postacute facility care: a national study. JAMA Pediatr. 2016;170(4):326–333.
- , , , et al. Bronchiolitis: clinical characteristics associated with hospitalization and length of stay. Pediatr Emerg Care. 2012;28(2):99–103.
- , , , , . The effect of transfers between health care facilities on costs and length of stay for pediatric burn patients. J Burn Care Res. 2015;36(1):178–183.
- , , , et al. Goal‐directed resuscitative interventions during pediatric interfacility transport. Crit Care Med. 2015;43(8):1692–1698.
Children with medical complexity (CMC) have complex and chronic health conditions that often involve multiple organ systems and severely affect cognitive and physical functioning. Although the prevalence of CMC is low (1% of all children), they account for nearly one‐fifth of all pediatric admissions and one‐half of all hospital days and charges in the United States.[1] Over the last decade, CMC have had a particularly large and increasing impact in tertiary‐care children's hospitals.[1, 2] The Institute of Medicine has identified CMC as a priority population for a revised healthcare system.[3]
Medical homes, hospitals, health plans, states, federal agencies, and others are striving to reduce excessive hospital use in CMC because of its high cost.[4, 5, 6] Containing length of stay (LOS)an increasingly used indicator of the time sensitiveness and efficiency of hospital careis a common aim across these initiatives. CMC have longer hospitalizations than children without medical complexity. Speculated reasons for this are that CMC tend to have (1) higher severity of acute illnesses (eg, pneumonia, cellulitis), (2) prolonged recovery time in the hospital, and (3) higher risk of adverse events in the hospital. Moreover, hospital clinicians caring for CMC often find it difficult to determine discharge readiness, given that many CMC do not return to a completely healthy baseline.[7]
Little is known about long LOS in CMC, including which CMC have the highest risk of experiencing such stays and which stays might have the greatest opportunity to be shortened. Patient characteristics associated with prolonged length of stay have been studied extensively for many pediatric conditions (eg, asthma).[8, 9, 10, 11, 12, 13, 14] However, most of these studies excluded CMC. Therefore, the objectives of this study were to examine (1) the prevalence of long LOS in CMC, (2) patient characteristics associated with long LOS, and (3) hospital‐to‐hospital variation in prevalence of long LOS hospitalizations.
METHODS
Study Design and Data Source
This study is a multicenter, retrospective cohort analysis of the Pediatric Health Information System (PHIS). PHIS is an administrative database of 44 not for profit, tertiary care pediatric hospitals affiliated with the Children's Hospital Association (CHA) (Overland Park, KS). PHIS contains data regarding patient demographics, diagnoses, and procedures (with International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] codes), All‐Patient Refined Diagnostic Related Groups version 30 (APR‐DRGs) (3M Health Information Systems, Salt Lake City, UT), and service lines that aggregate the APR‐DRGs into 38 distinct groups. Data quality and reliability are assured through CHA and participating hospitals. In accordance with the policies of the Cincinnati Children's Hospital Medical Center Institutional Review Board, this study of deidentified data was not considered human subjects research.
Study Population
Inclusion Criteria
Children discharged following an observation or inpatient admission from a hospital participating in the PHIS database between January 1, 2013 and December 31, 2014 were eligible for inclusion if they were considered medically complex. Medical complexity was defined using Clinical Risk Groups (CRGs) version 1.8, developed by 3M Health Information Systems and the National Association of Children's Hospitals and Related Institutions. CRGs were used to assign each hospitalized patient to 1 of 9 mutually exclusive chronicity groups according to the presence, type, and severity of chronic conditions.[15, 16, 17, 18] Each patient's CRG designation was based on 2 years of previous hospital encounters.
As defined in prior studies and definitional frameworks of CMC,[1] patients belonging to CRG group 6 (significant chronic disease in 2 organ systems), CRG group 7 (dominant chronic disease in 3 organ systems), and CRG group 9 (catastrophic condition) were considered medically complex.[17, 19] Patients with malignancies (CRG group 8) were not included for analysis because they are a unique population with anticipated, long hospital stays. Patients with CRG group 5, representing those with chronic conditions affecting a single body system, were also not included because most do not have attributes consistent with medical complexity.
Exclusion Criteria
We used the APR‐DRG system, which leverages ICD‐9‐CM codes to identify the health problem most responsible for the hospitalization, to refine the study cohort. We excluded hospitalizations that were classified by the APR‐DRG system as neonatal, as we did not wish to focus on LOS in the neonatal intensive care unit (ICU) or for birth admissions. Similarly, hospitalizations for chemotherapy (APR‐DRG 693) or malignancy (identified with previously used ICD‐9‐CM codes)[20] were also excluded because long LOS is anticipated. We also excluded hospitalizations for medical rehabilitation (APR‐DRG 860).
Outcome Measures
The primary outcome measure was long LOS, defined as LOS 10 days. The cut point of LOS 10 days represents the 90th percentile of LOS for all children, with and without medical complexity, hospitalized during 2013 to 2014. LOS 10 days has previously been used as a threshold of long LOS.[21] For hospitalizations involving transfer at admission from another acute care facility, LOS was measured from the date of transfer. We also assessed hospitals' cost attributable to long LOS admissions.
Patient Demographics and Clinical Characteristics
We measured demographic characteristics including age, gender, race/ethnicity, insurance type, and distance traveled (the linear distance between the centroid of the patient's home ZIP code and the centroid of the hospital's ZIP code). Clinical characteristics included CRG classification, complex chronic condition (CCC), and dependence on medical technology. CCCs are defined as any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or 1 system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.[20] Medical technology included devices used to optimize the health and functioning of the child (eg, gastrostomy, tracheostomy, cerebrospinal fluid shunt).[22]
Hospitalization Characteristics
Characteristics of the hospitalization included transfer from an outside facility, ICU admission, surgical procedure (using surgical APR‐DRGs), and discharge disposition (home, skilled nursing facility, home health services, death, other). Cost of the hospitalization was estimated in the PHIS from charges using hospital and year‐specific ratios of cost to charge.
Statistical Analysis
Continuous data (eg, distance from hospital to home residence) were described with median and interquartile ranges (IQR) because they were not normally distributed. Categorical data (eg, type of chronic condition) were described with counts and frequencies. In bivariate analyses, demographic, clinical, and hospitalization characteristics were stratified by LOS (long LOS vs LOS <10 days), and compared using 2 statistics or Wilcoxon rank sum tests as appropriate.
We modeled the likelihood of experiencing a long LOS using generalized linear mixed effects models with a random hospital intercept and discharge‐level fixed effects for age, gender, payor, CCC type, ICU utilization, transfer status, a medical/surgical admission indicator derived from the APR‐DRG, and CRG assigned to each hospitalization. To examine hospital‐to‐hospital variability, we generated hospital risk‐adjusted rates of long LOS from these models. Similar models and hospital risk‐adjusted rates were built for a post hoc correlational analysis of 30‐day all cause readmission, where hospitals' rates and percent of long LOS were compared with a Pearson correlation coefficient. Also, for our multivariable models, we performed a sensitivity analysis using an alternative definition of long LOS as 4 days (the 75th percentile of LOS for all children, with and without medical complexity, hospitalized during 20132014). All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and P values <0.05 were considered statistically significant.
RESULTS
Study Population
There were 954,018 hospitalizations of 217,163 CMC at 44 children's hospitals included for analysis. Forty‐seven percent of hospitalizations were for females, 49.4% for non‐Hispanic whites, and 61.1% for children with government insurance. Fifteen percent (n = 142,082) had a long LOS of 10 days. The median (IQR) LOS of hospitalizations <10 days versus 10 days were 2 (IQR, 14) and 16 days (IQR, 1226), respectively. Long LOS hospitalizations accounted for 61.1% (3.7 million) hospital days and 61.8% ($13.7 billion) of total hospitalization costs for all CMC in the cohort (Table 1).
| Characteristic | Overall (n = 954,018) | Length of Stay | |
|---|---|---|---|
| <10 Days (n = 811,936) | 10 Days (n = 142,082) | ||
| |||
| Age at admission, y, % | |||
| <1 | 14.6 | 12.7 | 25.7 |
| 14 | 27.1 | 27.9 | 22.4 |
| 59 | 20.1 | 21.0 | 14.9 |
| 1018 | 33.6 | 34.0 | 31.7 |
| 18+ | 4.6 | 4.4 | 5.4 |
| Gender, % | |||
| Female | 47.0 | 46.9 | 47.5 |
| Race/ethnicity, % | |||
| Non‐Hispanic white | 49.4 | 49.4 | 49.4 |
| Non‐Hispanic black | 23.1 | 23.8 | 19.3 |
| Hispanic | 18.2 | 17.8 | 20.4 |
| Asian | 2.0 | 1.9 | 2.3 |
| Other | 7.4 | 7.1 | 8.6 |
| Complex chronic condition, % | |||
| Any | 79.5 | 77.3 | 91.8 |
| Technology assistance | 37.1 | 34.1 | 54.2 |
| Gastrointestinal | 30.0 | 27.2 | 45.9 |
| Neuromuscular | 28.2 | 27.7 | 30.9 |
| Cardiovascular | 16.8 | 14.5 | 29.9 |
| Respiratory | 14.1 | 11.5 | 29.4 |
| Congenital/genetic defect | 17.2 | 16.7 | 20.2 |
| Metabolic | 9.9 | 8.9 | 15.4 |
| Renal | 10.1 | 9.5 | 13.8 |
| Hematology/emmmunodeficiency | 11.7 | 12.0 | 10.0 |
| Neonatal | 3.8 | 3.1 | 7.7 |
| Transplantation | 4.5 | 4.2 | 6.7 |
| Clinical risk group, % | |||
| Chronic condition in 2 systems | 68.4 | 71.2 | 53.9 |
| Catastrophic chronic condition | 31.4 | 28.8 | 46.1 |
| Distance from hospital to home residence in miles, median [IQR] | 16.2 [7.440.4] | 15.8 [7.338.7] | 19.1 [8.552.6] |
| Transferred from outside hospital (%) | 6.5 | 5.3 | 13.6 |
| Admitted for surgery, % | 23.4 | 20.7 | 38.7 |
| Use of intensive care, % | 19.6 | 14.9 | 46.5 |
| Discharge disposition, % | |||
| Home | 91.2 | 92.9 | 81.4 |
| Home healthcare | 4.5 | 3.5 | 9.9 |
| Other | 2.9 | 2.6 | 4.5 |
| Postacute care facility | 1.1 | 0.8 | 3.1 |
| Died | 0.4 | 0.3 | 1.1 |
| Payor, % | |||
| Government | 61.1 | 60.6 | 63.5 |
| Private | 33.2 | 33.6 | 30.9 |
| Other | 5.7 | 5.7 | 5.7 |
| Hospital resource use | |||
| Median length of stay [IQR] | 3 [16] | 2 [14] | 16 [1226] |
| Median hospital cost [IQR] | $8,144 [$4,122$18,447] | $6,689 [$3,685$12,395] | $49,207 [$29,444$95,738] |
| Total hospital cost, $, billions | $22.2 | $8.5 | $13.7 |
Demographics and Clinical Characteristics of Children With and Without Long LOS
Compared with hospitalized CMC with LOS <10 days, a higher percentage of hospitalizations with LOS 10 days were CMC age <1 year (25.7% vs 12.7%, P < 0.001) and Hispanic (20.4% vs 17.8%, P < 0.001). CMC hospitalizations with a long LOS had a higher percentage of any CCC (91.8% vs 77.3%, P < 0.001); the most common CCCs were gastrointestinal (45.9%), neuromuscular (30.9%), and cardiovascular (29.9%). Hospitalizations of CMC with a long LOS had a higher percentage of a catastrophic chronic condition (46.1% vs 28.8%, P < 0.001) and technology dependence (46.1% vs 28.8%, P < 0.001) (Table 1).
Hospitalization Characteristics of Children With and Without Long LOS
Compared with hospitalizations of CMC with LOS <10 days, hospitalizations of CMC with a long LOS more often involved transfer in from another hospital at admission (13.6% vs 5.3%, P < 0.001). CMC hospital stays with a long LOS more often involved surgery (38.7% vs 20.7%, P < 0.001) and use of intensive care (46.5% vs 14.9%; P < 0.001). A higher percentage of CMC with long LOS were discharged with home health services (9.9% vs 3.5%; P < 0.001) (Table 1).
The most common admitting diagnoses and CCCs for hospitalizations of CMC with long LOS are presented in Table 2. The two most prevalent APR‐DRGs in CMC hospitalizations lasting 10 days or longer were cystic fibrosis (10.7%) and respiratory system disease with ventilator support (5.5%). The two most common chronic condition characteristics represented among long CMC hospitalizations were gastrointestinal devices (eg, gastrostomy tube) (39.7%) and heart and great vessel malformations (eg, tetralogy of Fallot) (12.8%). The 5 most common CCC subcategories, as listed in Table 2, account for nearly 100% of the patients with long LOS hospitalizations.
| |
| Most common reason for admission* | |
| Cystic fibrosis | 10.7% |
| Respiratory system diagnosis with ventilator support 96+ hours | 5.5% |
| Malfunction, reaction, and complication of cardiac or vascular device or procedure | 2.8% |
| Craniotomy except for trauma | 2.6% |
| Major small and large bowel procedures | 2.3% |
| Most common complex chronic condition | |
| Gastrointestinal devices | 39.7% |
| Heart and great vessel malformations | 12.8% |
| Cystic fibrosis | 12.5% |
| Dysrhythmias | 11.0% |
| Respiratory devices | 10.7% |
Multivariable Analysis of Characteristics Associated With Long LOS
In multivariable analysis, the highest likelihood of long LOS was experienced by children who received care in the ICU (odds ratio [OR]: 3.5, 95% confidence interval [CI]: 3.43.5), who had a respiratory CCC (OR: 2.7, 95% CI: 2.62.7), and who were transferred from another acute care hospital at admission (OR: 2.1, 95% CI: 2.0, 2.1). The likelihood of long LOS was also higher in children <1 year of age (OR: 1.2, 95% CI: 1.21.3), and Hispanic children (OR: 1.1, 95% CI 1.0‐1.10) (Table 3). Similar multivariable findings were observed in sensitivity analysis using the 75th percentile of LOS (4 days) as the model outcome.
| Characteristic | Odds Ratio (95% CI) of LOS 10 Days | P Value |
|---|---|---|
| ||
| Use of intensive care | 3.5 (3.4‐3.5) | <0.001 |
| Transfer from another acute‐care hospital | 2.1 (2.0‐2.1) | <0.001 |
| Procedure/surgery | 1.8 (1.8‐1.9) | <0.001 |
| Complex chronic condition | ||
| Respiratory | 2.7 (2.6‐2.7) | <0.001 |
| Gastrointestinal | 1.8 (1.8‐1.8) | <0.001 |
| Metabolic | 1.7 (1.7‐1.7) | <0.001 |
| Cardiovascular | 1.6 (1.5‐1.6) | <0.001 |
| Neonatal | 1.5 (1.5‐1.5) | <0.001 |
| Renal | 1.4 (1.4‐1.4) | <0.001 |
| Transplant | 1.4 (1.4‐1.4) | <0.001 |
| Hematology and immunodeficiency | 1.3 (1.3‐1.3) | <0.001 |
| Technology assistance | 1.1 (1.1, 1.1) | <0.001 |
| Neuromuscular | 0.9 (0.9‐0.9) | <0.001 |
| Congenital or genetic defect | 0.8 (0.8‐0.8) | <0.001 |
| Age at admission, y | ||
| <1 | 1.2 (1.2‐1.3) | <0.001 |
| 14 | 0.5 (0.5‐0.5) | <0.001 |
| 59 | 0.6 (0.6‐0.6) | <0.001 |
| 1018 | 0.9 (0.9‐0.9) | <0.001 |
| 18+ | Reference | |
| Male | 0.9 (0.9‐0.9) | <0.001 |
| Race/ethnicity | ||
| Non‐Hispanic black | 0.9 (0.9‐0.9) | <0.001 |
| Hispanic | 1.1 (1.0‐1.1) | <0.001 |
| Asian | 1.0 (1.0‐1.1) | 0.3 |
| Other | 1.1 (1.1‐1.1) | <0.001 |
| Non‐Hispanic white | Reference | |
| Payor | ||
| Private | 0.9 (0.8 0.9) | <0.001 |
| Other | 1.0 (1.0‐1.0) | 0.4 |
| Government | Reference | |
| Season | ||
| Spring | 1.0 (1.0 1.0) | <0.001 |
| Summer | 0.9 (0.9‐0.9) | <0.001 |
| Fall | 1.0 (0.9‐1.0) | <0.001 |
| Winter | Reference | |
Variation in the Prevalence of Long LOS Across Children's Hospitals
After controlling for demographic, clinical, and hospital characteristics associated with long LOS, there was significant (P < 0.001) variation in the prevalence of long LOS for CMC across children's hospitals in the cohort (range, 10.3%21.8%) (Figure 1). Twelve (27%) hospitals had a significantly (P < 0.001) higher prevalence of long LOS for their hospitalized CMC, compared to the mean. Eighteen (41%) had a significantly (P < 0.001) lower prevalence of long LOS for their hospitalized CMC. There was also significant variation across hospitals with respect to cost, with 49.7% to 73.7% of all hospital costs of CMC attributed to long LOS hospitalizations. Finally, there was indirect correlation with the prevalence of LOS across hospitals and the hospitals' 30‐day readmission rate ( = 0.3; P = 0.04). As the prevalence of long LOS increased, the readmission rate decreased.

DISCUSSION
The main findings from this study suggest that a small percentage of CMC experiencing long LOS account for the majority of hospital bed days and cost of all hospitalized CMC in children's hospitals. The likelihood of long LOS varies significantly by CMC's age, race/ethnicity, and payor as well as by type and number of chronic conditions. Among CMC with long LOS, the use of gastrointestinal devices such as gastrostomy tubes, as well as congenital heart disease, were highly prevalent. In multivariable analysis, the characteristics most strongly associated with LOS 10 days were use of the ICU, respiratory complex chronic condition, and transfer from another medical facility at admission. After adjusting for these factors, there was significant variation in the prevalence of LOS 10 days for CMC across children's hospitals.
Although it is well known that CMC as a whole have a major impact on resource use in children's hospitals, this study reveals that 15% of hospitalizations of CMC account for 62% of all hospital costs of CMC. That is, a small fraction of hospitalizations of CMC is largely responsible for the significant financial impact of hospital resource use. To date, most clinical efforts and policies striving to reduce hospital use in CMC have focused on avoiding readmissions or index hospital admissions entirely, rather than improving the efficiency of hospital care after admission occurs.[23, 24, 25, 26] In the adult population, the impact of long LOS on hospital costs has been recognized, and several Medicare incentive programs have focused on in‐hospital timeliness and efficiency. As a result, LOS in Medicare beneficiaries has decreased dramatically over the past 2 decades.[27, 28, 29, 30] Optimizing the efficiency of hospital care for CMC may be an important goal to pursue, especially with precedent set in the adult literature.
Perhaps the substantial variation across hospitals in the prevalence of long LOS in CMC indicates opportunity to improve the efficiency of their inpatient care. This variation was not due to differences across hospitals' case mix of CMC. Further investigation is needed to determine how much of it is due to differences in quality of care. Clinical practice guidelines for hospital treatment of common illnesses usually exclude CMC. In our clinical experience across 9 children's hospitals, we have experienced varying approaches to setting discharge goals (ie, parameters on how healthy the child needs to be to ensure a successful hospital discharge) for CMC.[31] When the goals are absent or not clearly articulated, they can contribute to a prolonged hospitalization. Some families of CMC report significant issues when working with pediatric hospital staff to assess their child's discharge readiness.[7, 32, 33] In addition, there is significant variation across states and regions in access to and quality of post‐discharge health services (eg, home nursing, postacute care, durable medical equipment).[34, 35] In some areas, many CMC are not actively involved with their primary care physician.[5] These issues might also influence the ability of some children's hospitals to efficiently discharge CMC to a safe and supportive post‐discharge environment. Further examination of hospital outliersthose with the lowest and highest percentage of CMC hospitalizations with long LOSmay reveal opportunities to identify and spread best practices.
The demographic and clinical factors associated with long LOS in the present study, including age, ICU use, and transfer from another hospital, might help hospitals target which CMC have the greatest risk for experiencing long LOS. We found that infants age <1 year had longer LOS when compared with older children. Similar to our findings, younger‐aged children hospitalized with bronchiolitis have longer LOS.[36] Certainly, infants with medical complexity, in general, are a high‐acuity population with the potential for rapid clinical deterioration during an acute illness. Prolonged hospitalization for treatment and stabilization may be expected for many of them. Additional investigation is warranted to examine ICU use in CMC, and whether ICU admission or duration can be safely prevented or abbreviated. Opportunities to assess the quality of transfers into children's hospitals of CMC admitted to outside hospitals may be necessary. A study of pediatric burn patients reported that patients initially stabilized at a facility that was not a burn center and subsequently transferred to a burn center had a longer LOS than patients solely treated at a designated burn center.[37] Furthermore, events during transport itself may adversely impact the stability of an already fragile patient. Interventions to optimize the quality of care provided by transport teams have resulted in decreased LOS at the receiving hospital.[38]
This study's findings should be considered in the context of several limitations. Absent a gold‐standard definition of long LOS, we used the distribution of LOS across patients to inform our methods; LOS at the 90th percentile was selected as long. Although our sensitivity analysis using LOS at the 75th percentile produced similar findings, other cut points in LOS might be associated with different results. The study is not positioned to determine how much of the reported LOS was excessive, unnecessary, or preventable. The study findings may not generalize to types of hospitals not contained in PHIS (eg, nonchildren's hospitals and community hospitals). We did not focus on the impact of a new diagnosis (eg, new chronic illness) or acute in‐hospital event (eg, nosocomial infection) on prolonged LOS; future studies should investigate these clinical events with LOS.
PHIS does not contain information regarding characteristics that could influence LOS, including the children's social and familial attributes, transportation availability, home equipment needs, and local availability of postacute care facilities. Moreover, PHIS does not contain information about the hospital discharge procedures, process, or personnel across hospitals, which could influence LOS. Future studies on prolonged LOS should consider assessing this information. Because of the large sample size of hospitalizations included, the statistical power for the analyses was strong, rendering it possible that some findings that were statistically significant might have modest clinical significance (eg, relationship of Hispanic ethnicity with prolonged LOS). We could not determine why a positive correlation was not observed between hospitals' long LOS prevalence and their percentage of cost associated with long LOS; future studies should investigate the reasons for this finding.
Despite these limitations, the findings of the present study highlight the significance of long LOS in hospitalized CMC. These long hospitalizations account for a significant proportion of all hospital costs for this important population of children. The prevalence of long LOS for CMC varies considerably across children's hospitals, even after accounting for the case mix. Efforts to curtail hospital resource use and costs for CMC may benefit from focus on long LOS.
Children with medical complexity (CMC) have complex and chronic health conditions that often involve multiple organ systems and severely affect cognitive and physical functioning. Although the prevalence of CMC is low (1% of all children), they account for nearly one‐fifth of all pediatric admissions and one‐half of all hospital days and charges in the United States.[1] Over the last decade, CMC have had a particularly large and increasing impact in tertiary‐care children's hospitals.[1, 2] The Institute of Medicine has identified CMC as a priority population for a revised healthcare system.[3]
Medical homes, hospitals, health plans, states, federal agencies, and others are striving to reduce excessive hospital use in CMC because of its high cost.[4, 5, 6] Containing length of stay (LOS)an increasingly used indicator of the time sensitiveness and efficiency of hospital careis a common aim across these initiatives. CMC have longer hospitalizations than children without medical complexity. Speculated reasons for this are that CMC tend to have (1) higher severity of acute illnesses (eg, pneumonia, cellulitis), (2) prolonged recovery time in the hospital, and (3) higher risk of adverse events in the hospital. Moreover, hospital clinicians caring for CMC often find it difficult to determine discharge readiness, given that many CMC do not return to a completely healthy baseline.[7]
Little is known about long LOS in CMC, including which CMC have the highest risk of experiencing such stays and which stays might have the greatest opportunity to be shortened. Patient characteristics associated with prolonged length of stay have been studied extensively for many pediatric conditions (eg, asthma).[8, 9, 10, 11, 12, 13, 14] However, most of these studies excluded CMC. Therefore, the objectives of this study were to examine (1) the prevalence of long LOS in CMC, (2) patient characteristics associated with long LOS, and (3) hospital‐to‐hospital variation in prevalence of long LOS hospitalizations.
METHODS
Study Design and Data Source
This study is a multicenter, retrospective cohort analysis of the Pediatric Health Information System (PHIS). PHIS is an administrative database of 44 not for profit, tertiary care pediatric hospitals affiliated with the Children's Hospital Association (CHA) (Overland Park, KS). PHIS contains data regarding patient demographics, diagnoses, and procedures (with International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] codes), All‐Patient Refined Diagnostic Related Groups version 30 (APR‐DRGs) (3M Health Information Systems, Salt Lake City, UT), and service lines that aggregate the APR‐DRGs into 38 distinct groups. Data quality and reliability are assured through CHA and participating hospitals. In accordance with the policies of the Cincinnati Children's Hospital Medical Center Institutional Review Board, this study of deidentified data was not considered human subjects research.
Study Population
Inclusion Criteria
Children discharged following an observation or inpatient admission from a hospital participating in the PHIS database between January 1, 2013 and December 31, 2014 were eligible for inclusion if they were considered medically complex. Medical complexity was defined using Clinical Risk Groups (CRGs) version 1.8, developed by 3M Health Information Systems and the National Association of Children's Hospitals and Related Institutions. CRGs were used to assign each hospitalized patient to 1 of 9 mutually exclusive chronicity groups according to the presence, type, and severity of chronic conditions.[15, 16, 17, 18] Each patient's CRG designation was based on 2 years of previous hospital encounters.
As defined in prior studies and definitional frameworks of CMC,[1] patients belonging to CRG group 6 (significant chronic disease in 2 organ systems), CRG group 7 (dominant chronic disease in 3 organ systems), and CRG group 9 (catastrophic condition) were considered medically complex.[17, 19] Patients with malignancies (CRG group 8) were not included for analysis because they are a unique population with anticipated, long hospital stays. Patients with CRG group 5, representing those with chronic conditions affecting a single body system, were also not included because most do not have attributes consistent with medical complexity.
Exclusion Criteria
We used the APR‐DRG system, which leverages ICD‐9‐CM codes to identify the health problem most responsible for the hospitalization, to refine the study cohort. We excluded hospitalizations that were classified by the APR‐DRG system as neonatal, as we did not wish to focus on LOS in the neonatal intensive care unit (ICU) or for birth admissions. Similarly, hospitalizations for chemotherapy (APR‐DRG 693) or malignancy (identified with previously used ICD‐9‐CM codes)[20] were also excluded because long LOS is anticipated. We also excluded hospitalizations for medical rehabilitation (APR‐DRG 860).
Outcome Measures
The primary outcome measure was long LOS, defined as LOS 10 days. The cut point of LOS 10 days represents the 90th percentile of LOS for all children, with and without medical complexity, hospitalized during 2013 to 2014. LOS 10 days has previously been used as a threshold of long LOS.[21] For hospitalizations involving transfer at admission from another acute care facility, LOS was measured from the date of transfer. We also assessed hospitals' cost attributable to long LOS admissions.
Patient Demographics and Clinical Characteristics
We measured demographic characteristics including age, gender, race/ethnicity, insurance type, and distance traveled (the linear distance between the centroid of the patient's home ZIP code and the centroid of the hospital's ZIP code). Clinical characteristics included CRG classification, complex chronic condition (CCC), and dependence on medical technology. CCCs are defined as any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or 1 system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.[20] Medical technology included devices used to optimize the health and functioning of the child (eg, gastrostomy, tracheostomy, cerebrospinal fluid shunt).[22]
Hospitalization Characteristics
Characteristics of the hospitalization included transfer from an outside facility, ICU admission, surgical procedure (using surgical APR‐DRGs), and discharge disposition (home, skilled nursing facility, home health services, death, other). Cost of the hospitalization was estimated in the PHIS from charges using hospital and year‐specific ratios of cost to charge.
Statistical Analysis
Continuous data (eg, distance from hospital to home residence) were described with median and interquartile ranges (IQR) because they were not normally distributed. Categorical data (eg, type of chronic condition) were described with counts and frequencies. In bivariate analyses, demographic, clinical, and hospitalization characteristics were stratified by LOS (long LOS vs LOS <10 days), and compared using 2 statistics or Wilcoxon rank sum tests as appropriate.
We modeled the likelihood of experiencing a long LOS using generalized linear mixed effects models with a random hospital intercept and discharge‐level fixed effects for age, gender, payor, CCC type, ICU utilization, transfer status, a medical/surgical admission indicator derived from the APR‐DRG, and CRG assigned to each hospitalization. To examine hospital‐to‐hospital variability, we generated hospital risk‐adjusted rates of long LOS from these models. Similar models and hospital risk‐adjusted rates were built for a post hoc correlational analysis of 30‐day all cause readmission, where hospitals' rates and percent of long LOS were compared with a Pearson correlation coefficient. Also, for our multivariable models, we performed a sensitivity analysis using an alternative definition of long LOS as 4 days (the 75th percentile of LOS for all children, with and without medical complexity, hospitalized during 20132014). All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and P values <0.05 were considered statistically significant.
RESULTS
Study Population
There were 954,018 hospitalizations of 217,163 CMC at 44 children's hospitals included for analysis. Forty‐seven percent of hospitalizations were for females, 49.4% for non‐Hispanic whites, and 61.1% for children with government insurance. Fifteen percent (n = 142,082) had a long LOS of 10 days. The median (IQR) LOS of hospitalizations <10 days versus 10 days were 2 (IQR, 14) and 16 days (IQR, 1226), respectively. Long LOS hospitalizations accounted for 61.1% (3.7 million) hospital days and 61.8% ($13.7 billion) of total hospitalization costs for all CMC in the cohort (Table 1).
| Characteristic | Overall (n = 954,018) | Length of Stay | |
|---|---|---|---|
| <10 Days (n = 811,936) | 10 Days (n = 142,082) | ||
| |||
| Age at admission, y, % | |||
| <1 | 14.6 | 12.7 | 25.7 |
| 14 | 27.1 | 27.9 | 22.4 |
| 59 | 20.1 | 21.0 | 14.9 |
| 1018 | 33.6 | 34.0 | 31.7 |
| 18+ | 4.6 | 4.4 | 5.4 |
| Gender, % | |||
| Female | 47.0 | 46.9 | 47.5 |
| Race/ethnicity, % | |||
| Non‐Hispanic white | 49.4 | 49.4 | 49.4 |
| Non‐Hispanic black | 23.1 | 23.8 | 19.3 |
| Hispanic | 18.2 | 17.8 | 20.4 |
| Asian | 2.0 | 1.9 | 2.3 |
| Other | 7.4 | 7.1 | 8.6 |
| Complex chronic condition, % | |||
| Any | 79.5 | 77.3 | 91.8 |
| Technology assistance | 37.1 | 34.1 | 54.2 |
| Gastrointestinal | 30.0 | 27.2 | 45.9 |
| Neuromuscular | 28.2 | 27.7 | 30.9 |
| Cardiovascular | 16.8 | 14.5 | 29.9 |
| Respiratory | 14.1 | 11.5 | 29.4 |
| Congenital/genetic defect | 17.2 | 16.7 | 20.2 |
| Metabolic | 9.9 | 8.9 | 15.4 |
| Renal | 10.1 | 9.5 | 13.8 |
| Hematology/emmmunodeficiency | 11.7 | 12.0 | 10.0 |
| Neonatal | 3.8 | 3.1 | 7.7 |
| Transplantation | 4.5 | 4.2 | 6.7 |
| Clinical risk group, % | |||
| Chronic condition in 2 systems | 68.4 | 71.2 | 53.9 |
| Catastrophic chronic condition | 31.4 | 28.8 | 46.1 |
| Distance from hospital to home residence in miles, median [IQR] | 16.2 [7.440.4] | 15.8 [7.338.7] | 19.1 [8.552.6] |
| Transferred from outside hospital (%) | 6.5 | 5.3 | 13.6 |
| Admitted for surgery, % | 23.4 | 20.7 | 38.7 |
| Use of intensive care, % | 19.6 | 14.9 | 46.5 |
| Discharge disposition, % | |||
| Home | 91.2 | 92.9 | 81.4 |
| Home healthcare | 4.5 | 3.5 | 9.9 |
| Other | 2.9 | 2.6 | 4.5 |
| Postacute care facility | 1.1 | 0.8 | 3.1 |
| Died | 0.4 | 0.3 | 1.1 |
| Payor, % | |||
| Government | 61.1 | 60.6 | 63.5 |
| Private | 33.2 | 33.6 | 30.9 |
| Other | 5.7 | 5.7 | 5.7 |
| Hospital resource use | |||
| Median length of stay [IQR] | 3 [16] | 2 [14] | 16 [1226] |
| Median hospital cost [IQR] | $8,144 [$4,122$18,447] | $6,689 [$3,685$12,395] | $49,207 [$29,444$95,738] |
| Total hospital cost, $, billions | $22.2 | $8.5 | $13.7 |
Demographics and Clinical Characteristics of Children With and Without Long LOS
Compared with hospitalized CMC with LOS <10 days, a higher percentage of hospitalizations with LOS 10 days were CMC age <1 year (25.7% vs 12.7%, P < 0.001) and Hispanic (20.4% vs 17.8%, P < 0.001). CMC hospitalizations with a long LOS had a higher percentage of any CCC (91.8% vs 77.3%, P < 0.001); the most common CCCs were gastrointestinal (45.9%), neuromuscular (30.9%), and cardiovascular (29.9%). Hospitalizations of CMC with a long LOS had a higher percentage of a catastrophic chronic condition (46.1% vs 28.8%, P < 0.001) and technology dependence (46.1% vs 28.8%, P < 0.001) (Table 1).
Hospitalization Characteristics of Children With and Without Long LOS
Compared with hospitalizations of CMC with LOS <10 days, hospitalizations of CMC with a long LOS more often involved transfer in from another hospital at admission (13.6% vs 5.3%, P < 0.001). CMC hospital stays with a long LOS more often involved surgery (38.7% vs 20.7%, P < 0.001) and use of intensive care (46.5% vs 14.9%; P < 0.001). A higher percentage of CMC with long LOS were discharged with home health services (9.9% vs 3.5%; P < 0.001) (Table 1).
The most common admitting diagnoses and CCCs for hospitalizations of CMC with long LOS are presented in Table 2. The two most prevalent APR‐DRGs in CMC hospitalizations lasting 10 days or longer were cystic fibrosis (10.7%) and respiratory system disease with ventilator support (5.5%). The two most common chronic condition characteristics represented among long CMC hospitalizations were gastrointestinal devices (eg, gastrostomy tube) (39.7%) and heart and great vessel malformations (eg, tetralogy of Fallot) (12.8%). The 5 most common CCC subcategories, as listed in Table 2, account for nearly 100% of the patients with long LOS hospitalizations.
| |
| Most common reason for admission* | |
| Cystic fibrosis | 10.7% |
| Respiratory system diagnosis with ventilator support 96+ hours | 5.5% |
| Malfunction, reaction, and complication of cardiac or vascular device or procedure | 2.8% |
| Craniotomy except for trauma | 2.6% |
| Major small and large bowel procedures | 2.3% |
| Most common complex chronic condition | |
| Gastrointestinal devices | 39.7% |
| Heart and great vessel malformations | 12.8% |
| Cystic fibrosis | 12.5% |
| Dysrhythmias | 11.0% |
| Respiratory devices | 10.7% |
Multivariable Analysis of Characteristics Associated With Long LOS
In multivariable analysis, the highest likelihood of long LOS was experienced by children who received care in the ICU (odds ratio [OR]: 3.5, 95% confidence interval [CI]: 3.43.5), who had a respiratory CCC (OR: 2.7, 95% CI: 2.62.7), and who were transferred from another acute care hospital at admission (OR: 2.1, 95% CI: 2.0, 2.1). The likelihood of long LOS was also higher in children <1 year of age (OR: 1.2, 95% CI: 1.21.3), and Hispanic children (OR: 1.1, 95% CI 1.0‐1.10) (Table 3). Similar multivariable findings were observed in sensitivity analysis using the 75th percentile of LOS (4 days) as the model outcome.
| Characteristic | Odds Ratio (95% CI) of LOS 10 Days | P Value |
|---|---|---|
| ||
| Use of intensive care | 3.5 (3.4‐3.5) | <0.001 |
| Transfer from another acute‐care hospital | 2.1 (2.0‐2.1) | <0.001 |
| Procedure/surgery | 1.8 (1.8‐1.9) | <0.001 |
| Complex chronic condition | ||
| Respiratory | 2.7 (2.6‐2.7) | <0.001 |
| Gastrointestinal | 1.8 (1.8‐1.8) | <0.001 |
| Metabolic | 1.7 (1.7‐1.7) | <0.001 |
| Cardiovascular | 1.6 (1.5‐1.6) | <0.001 |
| Neonatal | 1.5 (1.5‐1.5) | <0.001 |
| Renal | 1.4 (1.4‐1.4) | <0.001 |
| Transplant | 1.4 (1.4‐1.4) | <0.001 |
| Hematology and immunodeficiency | 1.3 (1.3‐1.3) | <0.001 |
| Technology assistance | 1.1 (1.1, 1.1) | <0.001 |
| Neuromuscular | 0.9 (0.9‐0.9) | <0.001 |
| Congenital or genetic defect | 0.8 (0.8‐0.8) | <0.001 |
| Age at admission, y | ||
| <1 | 1.2 (1.2‐1.3) | <0.001 |
| 14 | 0.5 (0.5‐0.5) | <0.001 |
| 59 | 0.6 (0.6‐0.6) | <0.001 |
| 1018 | 0.9 (0.9‐0.9) | <0.001 |
| 18+ | Reference | |
| Male | 0.9 (0.9‐0.9) | <0.001 |
| Race/ethnicity | ||
| Non‐Hispanic black | 0.9 (0.9‐0.9) | <0.001 |
| Hispanic | 1.1 (1.0‐1.1) | <0.001 |
| Asian | 1.0 (1.0‐1.1) | 0.3 |
| Other | 1.1 (1.1‐1.1) | <0.001 |
| Non‐Hispanic white | Reference | |
| Payor | ||
| Private | 0.9 (0.8 0.9) | <0.001 |
| Other | 1.0 (1.0‐1.0) | 0.4 |
| Government | Reference | |
| Season | ||
| Spring | 1.0 (1.0 1.0) | <0.001 |
| Summer | 0.9 (0.9‐0.9) | <0.001 |
| Fall | 1.0 (0.9‐1.0) | <0.001 |
| Winter | Reference | |
Variation in the Prevalence of Long LOS Across Children's Hospitals
After controlling for demographic, clinical, and hospital characteristics associated with long LOS, there was significant (P < 0.001) variation in the prevalence of long LOS for CMC across children's hospitals in the cohort (range, 10.3%21.8%) (Figure 1). Twelve (27%) hospitals had a significantly (P < 0.001) higher prevalence of long LOS for their hospitalized CMC, compared to the mean. Eighteen (41%) had a significantly (P < 0.001) lower prevalence of long LOS for their hospitalized CMC. There was also significant variation across hospitals with respect to cost, with 49.7% to 73.7% of all hospital costs of CMC attributed to long LOS hospitalizations. Finally, there was indirect correlation with the prevalence of LOS across hospitals and the hospitals' 30‐day readmission rate ( = 0.3; P = 0.04). As the prevalence of long LOS increased, the readmission rate decreased.

DISCUSSION
The main findings from this study suggest that a small percentage of CMC experiencing long LOS account for the majority of hospital bed days and cost of all hospitalized CMC in children's hospitals. The likelihood of long LOS varies significantly by CMC's age, race/ethnicity, and payor as well as by type and number of chronic conditions. Among CMC with long LOS, the use of gastrointestinal devices such as gastrostomy tubes, as well as congenital heart disease, were highly prevalent. In multivariable analysis, the characteristics most strongly associated with LOS 10 days were use of the ICU, respiratory complex chronic condition, and transfer from another medical facility at admission. After adjusting for these factors, there was significant variation in the prevalence of LOS 10 days for CMC across children's hospitals.
Although it is well known that CMC as a whole have a major impact on resource use in children's hospitals, this study reveals that 15% of hospitalizations of CMC account for 62% of all hospital costs of CMC. That is, a small fraction of hospitalizations of CMC is largely responsible for the significant financial impact of hospital resource use. To date, most clinical efforts and policies striving to reduce hospital use in CMC have focused on avoiding readmissions or index hospital admissions entirely, rather than improving the efficiency of hospital care after admission occurs.[23, 24, 25, 26] In the adult population, the impact of long LOS on hospital costs has been recognized, and several Medicare incentive programs have focused on in‐hospital timeliness and efficiency. As a result, LOS in Medicare beneficiaries has decreased dramatically over the past 2 decades.[27, 28, 29, 30] Optimizing the efficiency of hospital care for CMC may be an important goal to pursue, especially with precedent set in the adult literature.
Perhaps the substantial variation across hospitals in the prevalence of long LOS in CMC indicates opportunity to improve the efficiency of their inpatient care. This variation was not due to differences across hospitals' case mix of CMC. Further investigation is needed to determine how much of it is due to differences in quality of care. Clinical practice guidelines for hospital treatment of common illnesses usually exclude CMC. In our clinical experience across 9 children's hospitals, we have experienced varying approaches to setting discharge goals (ie, parameters on how healthy the child needs to be to ensure a successful hospital discharge) for CMC.[31] When the goals are absent or not clearly articulated, they can contribute to a prolonged hospitalization. Some families of CMC report significant issues when working with pediatric hospital staff to assess their child's discharge readiness.[7, 32, 33] In addition, there is significant variation across states and regions in access to and quality of post‐discharge health services (eg, home nursing, postacute care, durable medical equipment).[34, 35] In some areas, many CMC are not actively involved with their primary care physician.[5] These issues might also influence the ability of some children's hospitals to efficiently discharge CMC to a safe and supportive post‐discharge environment. Further examination of hospital outliersthose with the lowest and highest percentage of CMC hospitalizations with long LOSmay reveal opportunities to identify and spread best practices.
The demographic and clinical factors associated with long LOS in the present study, including age, ICU use, and transfer from another hospital, might help hospitals target which CMC have the greatest risk for experiencing long LOS. We found that infants age <1 year had longer LOS when compared with older children. Similar to our findings, younger‐aged children hospitalized with bronchiolitis have longer LOS.[36] Certainly, infants with medical complexity, in general, are a high‐acuity population with the potential for rapid clinical deterioration during an acute illness. Prolonged hospitalization for treatment and stabilization may be expected for many of them. Additional investigation is warranted to examine ICU use in CMC, and whether ICU admission or duration can be safely prevented or abbreviated. Opportunities to assess the quality of transfers into children's hospitals of CMC admitted to outside hospitals may be necessary. A study of pediatric burn patients reported that patients initially stabilized at a facility that was not a burn center and subsequently transferred to a burn center had a longer LOS than patients solely treated at a designated burn center.[37] Furthermore, events during transport itself may adversely impact the stability of an already fragile patient. Interventions to optimize the quality of care provided by transport teams have resulted in decreased LOS at the receiving hospital.[38]
This study's findings should be considered in the context of several limitations. Absent a gold‐standard definition of long LOS, we used the distribution of LOS across patients to inform our methods; LOS at the 90th percentile was selected as long. Although our sensitivity analysis using LOS at the 75th percentile produced similar findings, other cut points in LOS might be associated with different results. The study is not positioned to determine how much of the reported LOS was excessive, unnecessary, or preventable. The study findings may not generalize to types of hospitals not contained in PHIS (eg, nonchildren's hospitals and community hospitals). We did not focus on the impact of a new diagnosis (eg, new chronic illness) or acute in‐hospital event (eg, nosocomial infection) on prolonged LOS; future studies should investigate these clinical events with LOS.
PHIS does not contain information regarding characteristics that could influence LOS, including the children's social and familial attributes, transportation availability, home equipment needs, and local availability of postacute care facilities. Moreover, PHIS does not contain information about the hospital discharge procedures, process, or personnel across hospitals, which could influence LOS. Future studies on prolonged LOS should consider assessing this information. Because of the large sample size of hospitalizations included, the statistical power for the analyses was strong, rendering it possible that some findings that were statistically significant might have modest clinical significance (eg, relationship of Hispanic ethnicity with prolonged LOS). We could not determine why a positive correlation was not observed between hospitals' long LOS prevalence and their percentage of cost associated with long LOS; future studies should investigate the reasons for this finding.
Despite these limitations, the findings of the present study highlight the significance of long LOS in hospitalized CMC. These long hospitalizations account for a significant proportion of all hospital costs for this important population of children. The prevalence of long LOS for CMC varies considerably across children's hospitals, even after accounting for the case mix. Efforts to curtail hospital resource use and costs for CMC may benefit from focus on long LOS.
- , , , et al. Inpatient growth and resource use in 28 children's hospitals: a longitudinal, multi‐institutional study. JAMA Pediatr. 2013;167(2):170–177.
- , , , et al. Children with complex chronic conditions in inpatient hospital settings in the united states. Pediatrics. 2010;126(4):647–655.
- , . Meeting the health care needs of persons with disabilities. Milbank Q. 2002;80(2):381–391.
- , , , et al. Effect of an enhanced medical home on serious illness and cost of care among high‐risk children with chronic illness: a randomized clinical trial. JAMA. 2014;312(24):2640–2648.
- , , , et al. Children with medical complexity and Medicaid: spending and cost savings. Health Aff Proj Hope. 2014;33(12):2199–2206.
- Children's Hospital Association. CARE Award. Available at: https://www.childrenshospitals.org/Programs‐and‐Services/Quality‐Improvement‐and‐Measurement/CARE‐Award. Accessed December 18, 2015.
- , , , et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581.
- , , , et al. Weekend matters: Friday and Saturday admissions are associated with prolonged hospitalization of children. Clin Pediatr (Phila). 2013;52(9):875–878.
- , , , . Attributable cost and length of stay for central line‐associated bloodstream infections. Pediatrics. 2014;133(6):e1525–e1532.
- , , , et al. Effect of healthcare‐acquired infection on length of hospital stay and cost. Infect Control Hosp Epidemiol. 2007;28(3):280–292.
- , , , , . Hospital utilization and costs among children with influenza, 2003. Am J Prev Med. 2009;36(4):292–296.
- , , , . Charges and lengths of stay attributable to adverse patient‐care events using pediatric‐specific quality indicators: a multicenter study of freestanding children's hospitals. Pediatrics. 2008;121(6):e1653–e1659.
- , , , , . Variation in resource utilization for the management of uncomplicated community‐acquired pneumonia across community and children's hospitals. J Pediatr. 2014;165(3):585–591.
- , , , , . Variation and outcomes associated with direct hospital admission among children with pneumonia in the United States. JAMA Pediatr. 2014;168(9):829–836.
- , , , et al. Clinical Risk Groups (CRGs): a classification system for risk‐adjusted capitation‐based payment and health care management. Med Care. 2004;42(1):81–90.
- , , , et al. Identifying children with lifelong chronic conditions for care coordination by using hospital discharge data. Acad Pediatr. 2010;10(6):417–423.
- , , , , . Profile of medical charges for children by health status group and severity level in a Washington State Health Plan. Health Serv Res. 2004;39(1):73–89.
- , , , . Using medical billing data to evaluate chronically ill children over time. J Ambulatory Care Manage. 2006;29(4):283–290.
- , , , , , . Medical complexity and pediatric emergency department and inpatient utilization. Pediatrics. 2013;131(2):e559–e565.
- , , , , . Pediatric complex chronic conditions classification system version 2: updated for ICD‐10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199.
- . Analyzing intensive care unit length of stay data: problems and possible solutions. Crit Care Med. 1997;25(9):1594–1600.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- . Hospital readmissions and repeat emergency department visits among children with medical complexity: an integrative review. J Pediatr Nurs. 2013;28(4):316–339.
- , , , . Hospital readmission in children with complex chronic conditions discharged from subacute care. Hosp Pediatr. 2014;4(3):153–158.
- , , , et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6):e1628–e1647.
- , , , . Hospital readmissions for newly discharged pediatric home mechanical ventilation patients. Pediatr Pulmonol. 2012;47(4):409–414.
- , , , et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2008. JAMA. 2011;305(15):1560–1567.
- , , , et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147.
- U.S. Department of Health and Human Services. CMS Statistics 2013. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/CMS‐Statistics‐Reference‐Booklet/Downloads/CMS_Stats_2013_final.pdf. Published August 2013. Accessed October 6, 2015.
- Centers for Medicare and Medicaid Services. Evaluation of the premier hospital quality incentive demonstration. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Reports/downloads/Premier_ExecSum_2010.pdf. Published March 3, 2009. Accessed September 18, 2015.
- , , , et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955–962; quiz 965–966.
- , , , , . Parent and provider perspectives on pediatric readmissions: what can we learn about readiness for discharge? Hosp Pediatr. 2015;5(11):559–565.
- , . Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5(11):602–604.
- , , . Pediatric post‐acute hospital care: striving for identity and value. Hosp Pediatr. 2015;5(10):548–551.
- , , , et al. Pediatric hospital discharges to home health and postacute facility care: a national study. JAMA Pediatr. 2016;170(4):326–333.
- , , , et al. Bronchiolitis: clinical characteristics associated with hospitalization and length of stay. Pediatr Emerg Care. 2012;28(2):99–103.
- , , , , . The effect of transfers between health care facilities on costs and length of stay for pediatric burn patients. J Burn Care Res. 2015;36(1):178–183.
- , , , et al. Goal‐directed resuscitative interventions during pediatric interfacility transport. Crit Care Med. 2015;43(8):1692–1698.
- , , , et al. Inpatient growth and resource use in 28 children's hospitals: a longitudinal, multi‐institutional study. JAMA Pediatr. 2013;167(2):170–177.
- , , , et al. Children with complex chronic conditions in inpatient hospital settings in the united states. Pediatrics. 2010;126(4):647–655.
- , . Meeting the health care needs of persons with disabilities. Milbank Q. 2002;80(2):381–391.
- , , , et al. Effect of an enhanced medical home on serious illness and cost of care among high‐risk children with chronic illness: a randomized clinical trial. JAMA. 2014;312(24):2640–2648.
- , , , et al. Children with medical complexity and Medicaid: spending and cost savings. Health Aff Proj Hope. 2014;33(12):2199–2206.
- Children's Hospital Association. CARE Award. Available at: https://www.childrenshospitals.org/Programs‐and‐Services/Quality‐Improvement‐and‐Measurement/CARE‐Award. Accessed December 18, 2015.
- , , , et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581.
- , , , et al. Weekend matters: Friday and Saturday admissions are associated with prolonged hospitalization of children. Clin Pediatr (Phila). 2013;52(9):875–878.
- , , , . Attributable cost and length of stay for central line‐associated bloodstream infections. Pediatrics. 2014;133(6):e1525–e1532.
- , , , et al. Effect of healthcare‐acquired infection on length of hospital stay and cost. Infect Control Hosp Epidemiol. 2007;28(3):280–292.
- , , , , . Hospital utilization and costs among children with influenza, 2003. Am J Prev Med. 2009;36(4):292–296.
- , , , . Charges and lengths of stay attributable to adverse patient‐care events using pediatric‐specific quality indicators: a multicenter study of freestanding children's hospitals. Pediatrics. 2008;121(6):e1653–e1659.
- , , , , . Variation in resource utilization for the management of uncomplicated community‐acquired pneumonia across community and children's hospitals. J Pediatr. 2014;165(3):585–591.
- , , , , . Variation and outcomes associated with direct hospital admission among children with pneumonia in the United States. JAMA Pediatr. 2014;168(9):829–836.
- , , , et al. Clinical Risk Groups (CRGs): a classification system for risk‐adjusted capitation‐based payment and health care management. Med Care. 2004;42(1):81–90.
- , , , et al. Identifying children with lifelong chronic conditions for care coordination by using hospital discharge data. Acad Pediatr. 2010;10(6):417–423.
- , , , , . Profile of medical charges for children by health status group and severity level in a Washington State Health Plan. Health Serv Res. 2004;39(1):73–89.
- , , , . Using medical billing data to evaluate chronically ill children over time. J Ambulatory Care Manage. 2006;29(4):283–290.
- , , , , , . Medical complexity and pediatric emergency department and inpatient utilization. Pediatrics. 2013;131(2):e559–e565.
- , , , , . Pediatric complex chronic conditions classification system version 2: updated for ICD‐10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199.
- . Analyzing intensive care unit length of stay data: problems and possible solutions. Crit Care Med. 1997;25(9):1594–1600.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- . Hospital readmissions and repeat emergency department visits among children with medical complexity: an integrative review. J Pediatr Nurs. 2013;28(4):316–339.
- , , , . Hospital readmission in children with complex chronic conditions discharged from subacute care. Hosp Pediatr. 2014;4(3):153–158.
- , , , et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics. 2014;134(6):e1628–e1647.
- , , , . Hospital readmissions for newly discharged pediatric home mechanical ventilation patients. Pediatr Pulmonol. 2012;47(4):409–414.
- , , , et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2008. JAMA. 2011;305(15):1560–1567.
- , , , et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147.
- U.S. Department of Health and Human Services. CMS Statistics 2013. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/CMS‐Statistics‐Reference‐Booklet/Downloads/CMS_Stats_2013_final.pdf. Published August 2013. Accessed October 6, 2015.
- Centers for Medicare and Medicaid Services. Evaluation of the premier hospital quality incentive demonstration. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Reports/downloads/Premier_ExecSum_2010.pdf. Published March 3, 2009. Accessed September 18, 2015.
- , , , et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955–962; quiz 965–966.
- , , , , . Parent and provider perspectives on pediatric readmissions: what can we learn about readiness for discharge? Hosp Pediatr. 2015;5(11):559–565.
- , . Preventing readmissions in children: how do we do that? Hosp Pediatr. 2015;5(11):602–604.
- , , . Pediatric post‐acute hospital care: striving for identity and value. Hosp Pediatr. 2015;5(10):548–551.
- , , , et al. Pediatric hospital discharges to home health and postacute facility care: a national study. JAMA Pediatr. 2016;170(4):326–333.
- , , , et al. Bronchiolitis: clinical characteristics associated with hospitalization and length of stay. Pediatr Emerg Care. 2012;28(2):99–103.
- , , , , . The effect of transfers between health care facilities on costs and length of stay for pediatric burn patients. J Burn Care Res. 2015;36(1):178–183.
- , , , et al. Goal‐directed resuscitative interventions during pediatric interfacility transport. Crit Care Med. 2015;43(8):1692–1698.
Pediatric Inpatient Guidelines Quality
Researchers from the Pediatric Research in Inpatient Settings (PRIS) network, an open pediatric hospitalist research network,[1] have identified inpatient pediatric medical and surgical conditions considered high priority for quality improvement (QI) initiatives and/or comparative effectiveness research based on prevalence, cost, and interhospital variation in resource utilization.[2] One approach for improving the quality of care within hospitals is to operationalize evidence‐based guidelines into practice.[3] Although guidelines may be used by individual clinicians, systematic adoption by hospitals into clinical workflow has the potential to influence providers to adhere to evidence‐based care, reduce unwarranted variation, and ultimately improve patient outcomes.[3, 4, 5, 6]
There are critical appraisal tools to measure the methodological quality, as defined by the Institute of Medicine (IOM) and others in their guidelines.[7, 8, 9, 10, 11, 12] One such validated tool is the AGREE II instrument, created by the AGREE (Appraisal of Guidelines for REsearch and Evaluation) collaboration.[13, 14] It defines methodological quality as the confidence that the biases linked to the rigor of development, presentation, and applicability of a clinical practice guideline have been minimized and that each step of the development process is clearly reported.[13]
The objective of our study was to rate the methodological quality of national guidelines for 20 of the PRIS priority pediatric inpatient conditions.[2] Our intent in pursuing this project was 2‐fold: first, to inform pediatric inpatient QI initiatives, and second, to call out priority pediatric inpatient conditions for which high methodological‐quality guidelines are currently lacking.
METHODS
The study methods involved (1) prioritizing pediatric inpatient conditions, (2) identifying national guidelines for the priority conditions, and (3) rating the methodological quality of available guidelines. This study was considered nonhuman‐subject research (A. Johnson, personal e‐mail communication, November 14, 2012), and the original prioritization study was deemed exempt from review by the institutional review board of the Children's Hospital of Philadelphia under 45 CFR 46.102(f).[2]
Prioritizing Pediatric Inpatient Conditions
Methods for developing the prioritization list are published elsewhere in detail and briefly described here.[1] An International Classification of Diseases, 9th Revision, Clinical Modification‐based clinical condition grouper was created for primary discharge diagnosis codes for inpatient, ambulatory surgery, and observation unit encounters accounting for either 80% of all encounters or 80% of all charges for over 3.4 million discharges from 2004 to 2009 for 38 children's hospitals in the Pediatric Health Information Systems (PHIS) database, which includes administrative and billing data.[15] A standardized cost master index was created to assign the same unit cost for each billable item (calculated as the median of median hospital unit costs) to allow for comparisons of resource utilization across hospitals (eg, the cost of a chest x‐ray was set to be the same across all hospitals in 2009 dollars). Total hospital costs were then recalculated for every admission by multiplying the standardized cost master index by the number of units for each item in the hospital bill, and then summing the standardized costs of each line item in every bill. Conditions were ranked based on prevalence and total cost across all hospitals in the study period. The variation in standardized costs across hospitals for each condition was determined.
For the current study, conditions were considered if they had a top 20 prevalence rank, a top 20 cost rank, high variation (intraclass correlation coefficient >0.1) in standardized costs across hospitals, a minimum number of PHIS hospitals with annualized overexpenditures (using the standardized cost master) of at least $50,000 when compared to the mean, or a minimum median of 200 cases per hospital over the 6‐year study period to assure sufficient hospital volume for future interventions. This resulted in 29 conditions; the selected 20 conditions matched the top 20 prevalence rank (see Supporting Information, Table 1, in the online version of this article).[2]
| Condition by PRIS Priority Rank | Guidelines Meeting Inclusion Criteriaa | Guidelines Citation | Mean Overall Reviewer Methodological Quality Rating (Rater 1, Rater 2)b | Recommended for Use in the Pediatric Inpatient Setting, Mean (Rater 1, Rater 2)c | Weighted Kappa(95% Confidence Interval) |
|---|---|---|---|---|---|
| |||||
| Otitis media, unspecified, s | 1 | American Academy of Family Physicians; American Academy of Otolaryngology‐Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Clinical Practice Guidelines: Otitis media with effusion. Pediatrics. 2004 May;113(5):1412‐29. | 6 (6, 6) | 3 (3, 3) | 0.76 (0.490.93) |
| Hypertrophy of tonsils and adenoids, s | 1 | Baugh RF et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1S30. | 6.5 (7, 6) | 3 (3, 3) | 0.49 (0.050.81) |
| Asthma, m | 1 | National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma‐full report 2007. Pages 1‐440. Available at: | 7 (7, 7) | 3 (3, 3) | 0.62 (0.210.87) |
| Bronchiolitis, m | 1 | American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:17741793. | 6.5 (6, 7) | 3 (3, 3) | 0.95 (0.871.00) |
| Pneumonia, m | 1 | Bradley JS et al.The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25e76. | 6 (6, 6) | 3 (3, 3) | 0.82 (0.640.96) |
| Dental caries, s | 1 | American Academy on Pediatric Dentistry Clinical Affairs CommitteePulp Therapy Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;30:170174. | 3 (3, 3) | 1.5 (1, 2) | 0.51 (0.140.83) |
| Chemotherapy, m | 0 | ||||
| Cellulitis, m | 1 | Stevens DL et al. Practice guidelines for the diagnosis and management of skin and softtissue infections. Clin Infect Dis. 2005;41:13731406. | 4.5 (4, 5) | 2.5 (2, 3) | 0.52 (0.150.79) |
| Inguinal hernia, s | 0 | ||||
| Gastroesophageal reflux and esophagitis, m, s | 2 | Vandenplas Y et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of NASPGHAN and ESPGHAN. J Pediatr Gastroenterol Nutr. 2009;49(4):498547. | 5 (5, 5) | 3 (3, 3) | 0.69 (0.450.87) |
| Furuta GT et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:13421363. | 5 (5, 5) | 2.5 (2, 3) | 0.93 (0.850.98) | ||
| Dehydration, m | 0 | ||||
| Redundant prepuce and phimosis, s | 1 | American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012;130(3):e756e785. | 6 (6, 6) | 3 (3, 3) | 0.66 (0.250.89) |
| Abdominal pain, m | 0 | ||||
| Other convulsions, m | 0 | ||||
| Urinary tract infection, m | 1 | Roberts KB et al. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595610. | 5.5 (5, 6) | 2.5 (2, 3) | 0.62 (0.230.84) |
| Acute appendicitis without peritonitis, s | 1 | Solomkin JS et al. Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133164. | 4.5 (5, 4) | 2.5 (3, 2) | 0.37 (0.110.81) |
| Eso‐ exo‐ hetero‐, and hypertropia, s | 0 | ||||
| Fever, m | 0 | ||||
| Seizures with and without intractable epilepsy, m | 3 | Brophy GM et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:323. | 5 (5, 5) | 3 (3, 3) | 0.95 (0.870.99) |
| Hirtz D et al. Practice parameter: treatment of the child with a first unprovoked seizure: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2003;60:166175. | 5 (5, 5) | 2.5 (2, 3) | 0.73 (0.410.94) | ||
| Riviello JJ Jr et al. Practice parameter: diagnostic assessment of the child with status epilepticus (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2006;67:15421550. | 5 (4, 6) | 2.5 (2, 3) | 0.80 (0.630.94) | ||
| Sickle cell disease with crisis, m | 2 | Section on Hematology/Oncology Committee on Genetics; American Academy of Pediatrics. Health supervision for children with sickle cell disease. Pediatrics. 2002;109:526535. | 3.5 (3, 4) | 1.5 (1, 2) | 0.92 (0.800.98) |
| National Heart, Lung, and Blood Institute, National Institutes of Health. The management of sickle cell disease. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Available at: | 4 (4, 4) | 2.5 (2, 3) | 0.91 (0.800.97) | ||
Identifying National Guidelines
We developed a search protocol (see Supporting Information, Table 2, in the online version of this article) using condition‐specific keywords and the following criteria: guideline, pediatric, 2002 to 2012. A medical librarian (E.E.) used the protocol to search PubMed, National Guidelines Clearing House, and the American Academy of Pediatrics website for guidelines for the 20 selected conditions.
We limited our study to US national guidelines published or updated from 2002 to 2012 to be most relevant to the 38 US children's hospitals in the original study. Guidelines had to address either medical or surgical or both types of inpatient management for the condition, depending on how the condition was categorized on the PRIS prioritization list. For example, to target inpatient issues, otitis media was treated as a surgical condition when the prioritization list was created, therefore guidelines included in our study needed to address surgical management (ie, myringotomy or tympanostomy tubes).[2] Guidelines specific to 1 organism, test, or treatment were a priori excluded, as they would not map well to the prioritization list, and would be difficult to interpret. Guidelines focusing exclusively on condition prevention were also excluded. Guidelines with a broad subject matter (eg, abdominal infection) or unclear age were included if they contained a significant focus on the condition of interest (eg, appendicitis without peritonitis), such that the course of pediatric inpatient care was described for that condition. Retracted or outdated (superseded by a more current version) guidelines were excluded.
An investigator (G.H.) reviewed potentially relevant results from the librarian's search. For example, the search for tonsillectomy guidelines retrieved a guideline on the use of polysomnography prior to tonsillectomy in children but did not cover the inpatient management or tonsillectomy procedure.[16] This guideline was excluded from our study, as it focused on a specific test and did not discuss surgical management of the condition.
Rating Methodological Quality of Guidelines
Methodological quality of guidelines was rated with the AGREE II tool by 2 investigators (G.H. and K.N.).[13, 17] This tool has 2 overall guideline assessments and 23 subcomponents within 6 domains, reflecting many of the IOM's recommendations for methodological quality in guidelines: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence.[8, 17]
The AGREE II tool rates each of the 23 subcomponent questions using a 7‐point scale (1=strongly disagree7=strongly agree). We followed the AGREE II user's manual suggestion in rating subcomponents as 1, indicating an absence of information for that question if the question was not addressed within the guideline.[13] The AGREE II user's manual describes the option of creating standardized domain scores; however, as the objective of our study was to assess the overall methodological quality of the guideline and not to highlight particular areas of strengths/weaknesses in the domains, we elected to present raw scores only.[13]
For the overall guideline rating item 1 (Rate the overall quality of this guideline.) the AGREE II tool instructs that a score of 1 indicates lowest possible quality and 7 indicates highest possible quality.[13] As these score anchors are far apart with no guide for interpretation of intermediate results, we modified the descriptive terms on the tool to define scores <3 as low quality, scores 3 to 5 as moderate quality, and scores >5 as high quality to allow for easier interpretation of our results. We also modified the final overall recommendation score (on a 3‐point scale) from I would recommend this guideline for use to I would recommend this guideline for use in the pediatric inpatient setting.[13, 17] A score of 1 indicated to not recommend, 2 indicated to recommend with modifications, and 3 indicated to recommend without modification.
Significant discrepancies (>2‐point difference on overall rating) between the 2 raters were to be settled by consensus scoring by 3 senior investigators blinded to previous reviews, using a modified Delphi technique.[18]
Inter‐rater reliability was measured using a weighted kappa coefficient and reported using a bootstrapped method with 95% confidence intervals. Interpretation of kappa is such that 0 is the amount of agreement that would be expected by chance, and 1 is perfect agreement, with previous researchers stating scores >0.81 indicate almost perfect agreement.[19]
RESULTS
The librarian's search retrieved 2869 potential results (Figure 1). Seventeen guidelines met inclusion criteria for 13 of the 20 priority conditions. Seven conditions did not have national guidelines meeting inclusion criteria. Table 1 displays the 20 medical and surgical conditions on the modified PRIS prioritization list, including overall guideline scoring, recommendation scores, and kappa results for each guideline. The highest methodological‐quality guidelines were for asthma,[20] tonsillectomy,[21] and bronchiolitis[22] (mean overall rating 7, 6.5, and 6.5, respectively). The lowest methodological‐quality guidelines were for 2 sickle cell disease guidelines[23, 24] and 1 dental caries guideline[25] (mean overall rating 4, 3.5, and 3, respectively). Seven guidelines were rated as high overall quality, and 10 guidelines were rated as moderate overall quality. Eight of the 17 guidelines[20, 21, 22, 26, 27, 28, 29, 30] were recommended for use in the pediatric inpatient setting without modification by both reviewers. Two guidelines (for dental caries[25] and sickle cell[23]) were not recommended for use by 1 reviewer.
As an example of scoring, a national guideline for asthma had high overall scores (7 from each reviewer) and high scores across most AGREE II subcomponents. The guideline was found by both reviewers to be systematic in describing guideline development with clearly stated recommendations linked to the available evidence (including strengths and limitations) and implementation considerations.[20] Conversely, a national guideline for sickle cell disease had moderate overall scores (scores of 3 and 4) and low‐moderate scores across the majority of the subcomponent items.[23] The reviewers believe that this guideline would have been strengthened by increased transparency in guideline development, discussion of the evidence surrounding recommendations, and discussion of implementation factors. A table with detailed scoring of each guideline is available (see Supporting Information, Table 3, in the online version of this article).
Agreement between the 2 raters was almost perfect,[19] with an overall boot‐strapped weighted kappa of 0.83 (95% confidence interval 0.780.87) across 850 scores. There were no discrepancies between reviewers in overall scoring requiring consensus scoring.
DISCUSSION
Using a modified version of a published prioritization list for inpatient pediatric conditions, we found national guidelines for 13 of 20 conditions with high prevalence, cost, and interhospital variation in resource utilization. Seven conditions had no national guidelines published within the past 10 years applicable for use in the pediatric inpatient setting. Of 17 guidelines for 13 conditions, 10 had moderate and 7 had high methodological quality.
Our findings add to the literature describing methodological quality of guidelines. Many publications focus on the methodological quality of guidelines as a group and use a standardized instrument (eg, the AGREE II tool) to rate within domains (eg, domain 1: scope and purpose) across guidelines in an effort to encourage improvement in developing and reporting in guidelines.[31, 32] Our study differs in that we chose to focus on the overall quality rating of individual guidelines for specific prioritized conditions to allow hospitals to guide QI initiatives. One study that had a similar aim to ours surveyed Dutch pediatricians to select priority conditions and used the AGREE II tool to rate 17 guidelines, recommending 14 for use in the Netherlands.[33]
Identifying high methodological‐quality guidelines is only 1 in a series of steps prior to successful guideline implementation in hospitals. Other aspects of guidelines, including the strength of the evidence (eg, from randomized controlled trials) and subsequent force and clarity (eg, use of must instead of consider) of recommendations, may affect clinician or patient adherence, work processes, and ultimately patient outcomes. Strong evidence should translate into forceful and clear recommendations. Authors with the Yale Guideline Recommendation Corpus describe significant variation in reporting of guideline recommendations, and further studies have shown that the force and clarity of a recommendation is associated with adherence rates.[34, 35, 36, 37] Unfortunately, current guideline appraisal tools lack the means to score the strength of evidence, and force and clarity of recommendations.[10]
Implementation science demonstrates that there are many important factors in translating best practice into improvements in clinical care. In addition to implementation considerations such as adherence, patient preferences, and work processes, variability in methodological quality, strength of evidence, and force and clarity of recommendations may be additional reasons why evidence for the impact of guidelines on patient outcomes remains mixed in the literature.[38] One recent study found that adherence to antibiotics recommended within a national pediatric community‐acquired pneumonia guideline, which had a high methodological‐quality score in our study, did not change hospital length of stay or readmissions.[29, 39] There are several possible interpretations for this. Recommendations may not have been based upon strong evidence, research methodology assessing how adherence to recommendations impacts patient outcomes may have been limited, or the outcomes measured in current studies (such as readmission) are not the outcomes that may be improved by adherence to these recommendations (such as decreasing antimicrobial resistance). These are important considerations when hospitals are incorporating recommendations from guidelines into practice. Hospitals should assess the multiple aspects of guidelines, including methodological quality, which our study helps to identify, strength of evidence, and force and clarity of recommendations, as well as adherence, patient preferences, work processes, and key outcome measures when implementing guidelines into clinical practice. A study utilizing a robust QI methodology demonstrated that clinician adherence to several elements in an asthma guideline, which also had a high methodological‐quality score in our study, led to a significant decrease in 6‐month hospital and emergency department readmission for asthma.[6, 20]
Our study also highlights that several pediatric conditions with high prevalence, cost, and interhospital resource utilization variation lack recent national pediatric guidelines applicable to the inpatient setting. If strong evidence exists for these priority conditions, professional societies should create high methodological‐quality guidelines with strong and clear recommendations. If evidence is lacking for these priority conditions, then investigators should focus on generating research in these areas.
There are several limitations to this study. The AGREE II tool does not have a mechanism to measure the strength of evidence used in a guideline. Methodological quality of a guideline alone may not translate into improved outcomes. Conditions may have national guidelines published before 2002, institution‐specific or international guidelines, or adult guidelines that might be amenable to use in the pediatric inpatient setting but were not included in this study. Several conditions on the prioritization list are broad in nature (eg, dehydration) and may not be amenable to the creation of guidelines. Other conditions on the prioritization list (eg, chemotherapy or cellulitis) may have useful guidelines within the context of specific conditions (eg, acute lymphoblastic leukemia) or for specific organisms (eg, methicillin‐resistant Staphylococcus aureus). We elected to exclude these narrower guidelines to focus on broad and comprehensive guidelines applicable to a wider range of clinical situations. Additionally, although use of a validated tool attempts to objectively guide ratings, the rating of quality is to some degree subjective. Finally, our study used a previously published prioritization list using data from children's hospitals, and the list likely under‐represents conditions commonly managed in community hospitals (eg, hyperbilirubinemia).[2] Exclusion of these conditions was not reflective of importance or quality of available national guidelines.
CONCLUSIONS
Our study adds to recent publications on the need to prioritize conditions for QI in children's hospitals. We identified a group of moderate to high methodological‐quality national guidelines for pediatric inpatient conditions with high prevalence, cost, and variation in interhospital resource utilization. Not all prioritized conditions have national high methodological‐quality guidelines available. Hospitals should prioritize conditions with high methodological‐quality guidelines to allocate resources for QI initiatives. Professional societies should focus their efforts on producing methodologically sound guidelines for prioritized conditions currently lacking high‐quality guidelines if sufficient evidence exists.
Acknowledgements
The authors thank Christopher G. Maloney, MD, PhD, for critical review of the manuscript, and Gregory J. Stoddard, MS, for statistical support. Mr. Stoddard's work is supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant 8UL1TR000105 (formerly UL1RR025764).
Disclosures
Sanjay Mahant, Ron Keren, and Raj Srivastava are all Executive Council members of the Pediatric Research in Inpatient Settings (PRIS) Network. PRIS, Sanjay Mahant, Ron Keren, and Raj Srivastava are all supported by grants from the Children's Hospital Association. Sanjay Mahant is also supported by research grants from the Canadian Institute of Health Research and Physician Services Incorporated. Ron Keren and Raj Srivastava also serve as medical legal consultants. The remaining authors have no financial relationships relevant to this article to disclose.
- , . Development of the Pediatric Research in Inpatient Settings (PRIS) Network: lessons learned. J Hosp Med. 2012;7(8):661–664.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1–10.
- , . How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185–1191.
- . Making it easy to do it right. N Engl J Med. 2001;345(13):991–993.
- , , , , . Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318(7182):527–530.
- , , , et al. The Joint Commission Children's Asthma Care quality measures and asthma readmissions. Pediatrics. 2012;130(3):482–491.
- , , , et al. Toward transparent clinical policies. Pediatrics. 2008;121(3):643–646.
- Field MJ, Lohr KN, eds.; Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
- , , , , . Development and application of a generic methodology to assess the quality of clinical guidelines. Int J Qual Health Care. 1999;11(1):21–28.
- , , , , . A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int J Qual Health Care. 2005;17(3):235–242.
- , , . How to decide whether a clinical practice guideline is trustworthy. JAMA. 2013;309(2):139–140.
- Field MJ, Lohr KN, eds.; Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Guidelines for Clinical Practice: From Development to Use. Washington, DC: National Academies Press; 1992.
- The AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12:18–23.
- . AGREE II‐improving the quality of clinical care. Lancet. 2010;376(9747):1128–1129.
- , , , . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055.
- , , , et al. Clinical practice guideline: polysomnography for sleep‐disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1 suppl):S1–S15.
- , , , et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–E842.
- , . The Delphi method as a research tool: an example, design considerations and applications. Inform Manag. 2004;42(1):15–29.
- , . The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174.
- National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma‐full report 2007. Pages 1–440. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. Accessed on 24 August 2012.
- , , , et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1–S30.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- Health supervision for children with sickle cell disease. Pediatrics. 2002;109(3):526–535.
- National Heart, Lung, and Blood Institute, National Institutes of Health. The management of sickle cell disease. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Available at: http://www.nhlbi.nih.gov/health/prof/blood/sickle/sc_mngt.pdf. Revised June 2002. Accessed on 13 October 2012.
- American Academy on Pediatric Dentistry Clinical Affairs Committee–Pulp Therapy Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;30:170–174.
- , , , et al.; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23.
- American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012;130(3):e756–e785.
- , , , et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498–547.
- , , , et al. The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76.
- American Academy of Family Physicians; American Academy of Otolaryngology‐Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Clinical Practice Guidelines: Otitis media with effusion. Pediatrics. 2004 May;113(5):1412–29.
- , , . Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer‐reviewed medical literature. JAMA. 1999;281(20):1900–1905.
- , , , . Quality of reporting and evidence in American Academy of Pediatrics guidelines. Pediatrics. 2013;131(4):732–738.
- , , . Quality of evidence‐based pediatric guidelines. Pediatrics. 2005;115(5):1378–1391.
- , , . The Yale Guideline Recommendation Corpus: a representative sample of the knowledge content of guidelines. Int J Med Inform. 2009;78(5):354–363.
- , , . How often is strength of recommendation indicated in guidelines? Analysis of the Yale Guideline Recommendation Corpus. AMIA Annu Symp Proc. 2008:984.
- , , . Clinical practice guideline development manual, third edition: a quality‐driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2013;148(1 suppl):S1–S55.
- , , , , , . Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ. 1998;317(7162):858–861.
- , , , et al. Toward evidence‐based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966–1998. J Gen Intern Med. 2006;21(suppl 2):S14–S20.
- , , , . Effectiveness of antimicrobial guidelines for community‐acquired pneumonia in children. Pediatrics. 2012;129(5):e1326–e1333.
Researchers from the Pediatric Research in Inpatient Settings (PRIS) network, an open pediatric hospitalist research network,[1] have identified inpatient pediatric medical and surgical conditions considered high priority for quality improvement (QI) initiatives and/or comparative effectiveness research based on prevalence, cost, and interhospital variation in resource utilization.[2] One approach for improving the quality of care within hospitals is to operationalize evidence‐based guidelines into practice.[3] Although guidelines may be used by individual clinicians, systematic adoption by hospitals into clinical workflow has the potential to influence providers to adhere to evidence‐based care, reduce unwarranted variation, and ultimately improve patient outcomes.[3, 4, 5, 6]
There are critical appraisal tools to measure the methodological quality, as defined by the Institute of Medicine (IOM) and others in their guidelines.[7, 8, 9, 10, 11, 12] One such validated tool is the AGREE II instrument, created by the AGREE (Appraisal of Guidelines for REsearch and Evaluation) collaboration.[13, 14] It defines methodological quality as the confidence that the biases linked to the rigor of development, presentation, and applicability of a clinical practice guideline have been minimized and that each step of the development process is clearly reported.[13]
The objective of our study was to rate the methodological quality of national guidelines for 20 of the PRIS priority pediatric inpatient conditions.[2] Our intent in pursuing this project was 2‐fold: first, to inform pediatric inpatient QI initiatives, and second, to call out priority pediatric inpatient conditions for which high methodological‐quality guidelines are currently lacking.
METHODS
The study methods involved (1) prioritizing pediatric inpatient conditions, (2) identifying national guidelines for the priority conditions, and (3) rating the methodological quality of available guidelines. This study was considered nonhuman‐subject research (A. Johnson, personal e‐mail communication, November 14, 2012), and the original prioritization study was deemed exempt from review by the institutional review board of the Children's Hospital of Philadelphia under 45 CFR 46.102(f).[2]
Prioritizing Pediatric Inpatient Conditions
Methods for developing the prioritization list are published elsewhere in detail and briefly described here.[1] An International Classification of Diseases, 9th Revision, Clinical Modification‐based clinical condition grouper was created for primary discharge diagnosis codes for inpatient, ambulatory surgery, and observation unit encounters accounting for either 80% of all encounters or 80% of all charges for over 3.4 million discharges from 2004 to 2009 for 38 children's hospitals in the Pediatric Health Information Systems (PHIS) database, which includes administrative and billing data.[15] A standardized cost master index was created to assign the same unit cost for each billable item (calculated as the median of median hospital unit costs) to allow for comparisons of resource utilization across hospitals (eg, the cost of a chest x‐ray was set to be the same across all hospitals in 2009 dollars). Total hospital costs were then recalculated for every admission by multiplying the standardized cost master index by the number of units for each item in the hospital bill, and then summing the standardized costs of each line item in every bill. Conditions were ranked based on prevalence and total cost across all hospitals in the study period. The variation in standardized costs across hospitals for each condition was determined.
For the current study, conditions were considered if they had a top 20 prevalence rank, a top 20 cost rank, high variation (intraclass correlation coefficient >0.1) in standardized costs across hospitals, a minimum number of PHIS hospitals with annualized overexpenditures (using the standardized cost master) of at least $50,000 when compared to the mean, or a minimum median of 200 cases per hospital over the 6‐year study period to assure sufficient hospital volume for future interventions. This resulted in 29 conditions; the selected 20 conditions matched the top 20 prevalence rank (see Supporting Information, Table 1, in the online version of this article).[2]
| Condition by PRIS Priority Rank | Guidelines Meeting Inclusion Criteriaa | Guidelines Citation | Mean Overall Reviewer Methodological Quality Rating (Rater 1, Rater 2)b | Recommended for Use in the Pediatric Inpatient Setting, Mean (Rater 1, Rater 2)c | Weighted Kappa(95% Confidence Interval) |
|---|---|---|---|---|---|
| |||||
| Otitis media, unspecified, s | 1 | American Academy of Family Physicians; American Academy of Otolaryngology‐Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Clinical Practice Guidelines: Otitis media with effusion. Pediatrics. 2004 May;113(5):1412‐29. | 6 (6, 6) | 3 (3, 3) | 0.76 (0.490.93) |
| Hypertrophy of tonsils and adenoids, s | 1 | Baugh RF et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1S30. | 6.5 (7, 6) | 3 (3, 3) | 0.49 (0.050.81) |
| Asthma, m | 1 | National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma‐full report 2007. Pages 1‐440. Available at: | 7 (7, 7) | 3 (3, 3) | 0.62 (0.210.87) |
| Bronchiolitis, m | 1 | American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:17741793. | 6.5 (6, 7) | 3 (3, 3) | 0.95 (0.871.00) |
| Pneumonia, m | 1 | Bradley JS et al.The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25e76. | 6 (6, 6) | 3 (3, 3) | 0.82 (0.640.96) |
| Dental caries, s | 1 | American Academy on Pediatric Dentistry Clinical Affairs CommitteePulp Therapy Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;30:170174. | 3 (3, 3) | 1.5 (1, 2) | 0.51 (0.140.83) |
| Chemotherapy, m | 0 | ||||
| Cellulitis, m | 1 | Stevens DL et al. Practice guidelines for the diagnosis and management of skin and softtissue infections. Clin Infect Dis. 2005;41:13731406. | 4.5 (4, 5) | 2.5 (2, 3) | 0.52 (0.150.79) |
| Inguinal hernia, s | 0 | ||||
| Gastroesophageal reflux and esophagitis, m, s | 2 | Vandenplas Y et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of NASPGHAN and ESPGHAN. J Pediatr Gastroenterol Nutr. 2009;49(4):498547. | 5 (5, 5) | 3 (3, 3) | 0.69 (0.450.87) |
| Furuta GT et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:13421363. | 5 (5, 5) | 2.5 (2, 3) | 0.93 (0.850.98) | ||
| Dehydration, m | 0 | ||||
| Redundant prepuce and phimosis, s | 1 | American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012;130(3):e756e785. | 6 (6, 6) | 3 (3, 3) | 0.66 (0.250.89) |
| Abdominal pain, m | 0 | ||||
| Other convulsions, m | 0 | ||||
| Urinary tract infection, m | 1 | Roberts KB et al. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595610. | 5.5 (5, 6) | 2.5 (2, 3) | 0.62 (0.230.84) |
| Acute appendicitis without peritonitis, s | 1 | Solomkin JS et al. Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133164. | 4.5 (5, 4) | 2.5 (3, 2) | 0.37 (0.110.81) |
| Eso‐ exo‐ hetero‐, and hypertropia, s | 0 | ||||
| Fever, m | 0 | ||||
| Seizures with and without intractable epilepsy, m | 3 | Brophy GM et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:323. | 5 (5, 5) | 3 (3, 3) | 0.95 (0.870.99) |
| Hirtz D et al. Practice parameter: treatment of the child with a first unprovoked seizure: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2003;60:166175. | 5 (5, 5) | 2.5 (2, 3) | 0.73 (0.410.94) | ||
| Riviello JJ Jr et al. Practice parameter: diagnostic assessment of the child with status epilepticus (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2006;67:15421550. | 5 (4, 6) | 2.5 (2, 3) | 0.80 (0.630.94) | ||
| Sickle cell disease with crisis, m | 2 | Section on Hematology/Oncology Committee on Genetics; American Academy of Pediatrics. Health supervision for children with sickle cell disease. Pediatrics. 2002;109:526535. | 3.5 (3, 4) | 1.5 (1, 2) | 0.92 (0.800.98) |
| National Heart, Lung, and Blood Institute, National Institutes of Health. The management of sickle cell disease. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Available at: | 4 (4, 4) | 2.5 (2, 3) | 0.91 (0.800.97) | ||
Identifying National Guidelines
We developed a search protocol (see Supporting Information, Table 2, in the online version of this article) using condition‐specific keywords and the following criteria: guideline, pediatric, 2002 to 2012. A medical librarian (E.E.) used the protocol to search PubMed, National Guidelines Clearing House, and the American Academy of Pediatrics website for guidelines for the 20 selected conditions.
We limited our study to US national guidelines published or updated from 2002 to 2012 to be most relevant to the 38 US children's hospitals in the original study. Guidelines had to address either medical or surgical or both types of inpatient management for the condition, depending on how the condition was categorized on the PRIS prioritization list. For example, to target inpatient issues, otitis media was treated as a surgical condition when the prioritization list was created, therefore guidelines included in our study needed to address surgical management (ie, myringotomy or tympanostomy tubes).[2] Guidelines specific to 1 organism, test, or treatment were a priori excluded, as they would not map well to the prioritization list, and would be difficult to interpret. Guidelines focusing exclusively on condition prevention were also excluded. Guidelines with a broad subject matter (eg, abdominal infection) or unclear age were included if they contained a significant focus on the condition of interest (eg, appendicitis without peritonitis), such that the course of pediatric inpatient care was described for that condition. Retracted or outdated (superseded by a more current version) guidelines were excluded.
An investigator (G.H.) reviewed potentially relevant results from the librarian's search. For example, the search for tonsillectomy guidelines retrieved a guideline on the use of polysomnography prior to tonsillectomy in children but did not cover the inpatient management or tonsillectomy procedure.[16] This guideline was excluded from our study, as it focused on a specific test and did not discuss surgical management of the condition.
Rating Methodological Quality of Guidelines
Methodological quality of guidelines was rated with the AGREE II tool by 2 investigators (G.H. and K.N.).[13, 17] This tool has 2 overall guideline assessments and 23 subcomponents within 6 domains, reflecting many of the IOM's recommendations for methodological quality in guidelines: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence.[8, 17]
The AGREE II tool rates each of the 23 subcomponent questions using a 7‐point scale (1=strongly disagree7=strongly agree). We followed the AGREE II user's manual suggestion in rating subcomponents as 1, indicating an absence of information for that question if the question was not addressed within the guideline.[13] The AGREE II user's manual describes the option of creating standardized domain scores; however, as the objective of our study was to assess the overall methodological quality of the guideline and not to highlight particular areas of strengths/weaknesses in the domains, we elected to present raw scores only.[13]
For the overall guideline rating item 1 (Rate the overall quality of this guideline.) the AGREE II tool instructs that a score of 1 indicates lowest possible quality and 7 indicates highest possible quality.[13] As these score anchors are far apart with no guide for interpretation of intermediate results, we modified the descriptive terms on the tool to define scores <3 as low quality, scores 3 to 5 as moderate quality, and scores >5 as high quality to allow for easier interpretation of our results. We also modified the final overall recommendation score (on a 3‐point scale) from I would recommend this guideline for use to I would recommend this guideline for use in the pediatric inpatient setting.[13, 17] A score of 1 indicated to not recommend, 2 indicated to recommend with modifications, and 3 indicated to recommend without modification.
Significant discrepancies (>2‐point difference on overall rating) between the 2 raters were to be settled by consensus scoring by 3 senior investigators blinded to previous reviews, using a modified Delphi technique.[18]
Inter‐rater reliability was measured using a weighted kappa coefficient and reported using a bootstrapped method with 95% confidence intervals. Interpretation of kappa is such that 0 is the amount of agreement that would be expected by chance, and 1 is perfect agreement, with previous researchers stating scores >0.81 indicate almost perfect agreement.[19]
RESULTS
The librarian's search retrieved 2869 potential results (Figure 1). Seventeen guidelines met inclusion criteria for 13 of the 20 priority conditions. Seven conditions did not have national guidelines meeting inclusion criteria. Table 1 displays the 20 medical and surgical conditions on the modified PRIS prioritization list, including overall guideline scoring, recommendation scores, and kappa results for each guideline. The highest methodological‐quality guidelines were for asthma,[20] tonsillectomy,[21] and bronchiolitis[22] (mean overall rating 7, 6.5, and 6.5, respectively). The lowest methodological‐quality guidelines were for 2 sickle cell disease guidelines[23, 24] and 1 dental caries guideline[25] (mean overall rating 4, 3.5, and 3, respectively). Seven guidelines were rated as high overall quality, and 10 guidelines were rated as moderate overall quality. Eight of the 17 guidelines[20, 21, 22, 26, 27, 28, 29, 30] were recommended for use in the pediatric inpatient setting without modification by both reviewers. Two guidelines (for dental caries[25] and sickle cell[23]) were not recommended for use by 1 reviewer.

As an example of scoring, a national guideline for asthma had high overall scores (7 from each reviewer) and high scores across most AGREE II subcomponents. The guideline was found by both reviewers to be systematic in describing guideline development with clearly stated recommendations linked to the available evidence (including strengths and limitations) and implementation considerations.[20] Conversely, a national guideline for sickle cell disease had moderate overall scores (scores of 3 and 4) and low‐moderate scores across the majority of the subcomponent items.[23] The reviewers believe that this guideline would have been strengthened by increased transparency in guideline development, discussion of the evidence surrounding recommendations, and discussion of implementation factors. A table with detailed scoring of each guideline is available (see Supporting Information, Table 3, in the online version of this article).
Agreement between the 2 raters was almost perfect,[19] with an overall boot‐strapped weighted kappa of 0.83 (95% confidence interval 0.780.87) across 850 scores. There were no discrepancies between reviewers in overall scoring requiring consensus scoring.
DISCUSSION
Using a modified version of a published prioritization list for inpatient pediatric conditions, we found national guidelines for 13 of 20 conditions with high prevalence, cost, and interhospital variation in resource utilization. Seven conditions had no national guidelines published within the past 10 years applicable for use in the pediatric inpatient setting. Of 17 guidelines for 13 conditions, 10 had moderate and 7 had high methodological quality.
Our findings add to the literature describing methodological quality of guidelines. Many publications focus on the methodological quality of guidelines as a group and use a standardized instrument (eg, the AGREE II tool) to rate within domains (eg, domain 1: scope and purpose) across guidelines in an effort to encourage improvement in developing and reporting in guidelines.[31, 32] Our study differs in that we chose to focus on the overall quality rating of individual guidelines for specific prioritized conditions to allow hospitals to guide QI initiatives. One study that had a similar aim to ours surveyed Dutch pediatricians to select priority conditions and used the AGREE II tool to rate 17 guidelines, recommending 14 for use in the Netherlands.[33]
Identifying high methodological‐quality guidelines is only 1 in a series of steps prior to successful guideline implementation in hospitals. Other aspects of guidelines, including the strength of the evidence (eg, from randomized controlled trials) and subsequent force and clarity (eg, use of must instead of consider) of recommendations, may affect clinician or patient adherence, work processes, and ultimately patient outcomes. Strong evidence should translate into forceful and clear recommendations. Authors with the Yale Guideline Recommendation Corpus describe significant variation in reporting of guideline recommendations, and further studies have shown that the force and clarity of a recommendation is associated with adherence rates.[34, 35, 36, 37] Unfortunately, current guideline appraisal tools lack the means to score the strength of evidence, and force and clarity of recommendations.[10]
Implementation science demonstrates that there are many important factors in translating best practice into improvements in clinical care. In addition to implementation considerations such as adherence, patient preferences, and work processes, variability in methodological quality, strength of evidence, and force and clarity of recommendations may be additional reasons why evidence for the impact of guidelines on patient outcomes remains mixed in the literature.[38] One recent study found that adherence to antibiotics recommended within a national pediatric community‐acquired pneumonia guideline, which had a high methodological‐quality score in our study, did not change hospital length of stay or readmissions.[29, 39] There are several possible interpretations for this. Recommendations may not have been based upon strong evidence, research methodology assessing how adherence to recommendations impacts patient outcomes may have been limited, or the outcomes measured in current studies (such as readmission) are not the outcomes that may be improved by adherence to these recommendations (such as decreasing antimicrobial resistance). These are important considerations when hospitals are incorporating recommendations from guidelines into practice. Hospitals should assess the multiple aspects of guidelines, including methodological quality, which our study helps to identify, strength of evidence, and force and clarity of recommendations, as well as adherence, patient preferences, work processes, and key outcome measures when implementing guidelines into clinical practice. A study utilizing a robust QI methodology demonstrated that clinician adherence to several elements in an asthma guideline, which also had a high methodological‐quality score in our study, led to a significant decrease in 6‐month hospital and emergency department readmission for asthma.[6, 20]
Our study also highlights that several pediatric conditions with high prevalence, cost, and interhospital resource utilization variation lack recent national pediatric guidelines applicable to the inpatient setting. If strong evidence exists for these priority conditions, professional societies should create high methodological‐quality guidelines with strong and clear recommendations. If evidence is lacking for these priority conditions, then investigators should focus on generating research in these areas.
There are several limitations to this study. The AGREE II tool does not have a mechanism to measure the strength of evidence used in a guideline. Methodological quality of a guideline alone may not translate into improved outcomes. Conditions may have national guidelines published before 2002, institution‐specific or international guidelines, or adult guidelines that might be amenable to use in the pediatric inpatient setting but were not included in this study. Several conditions on the prioritization list are broad in nature (eg, dehydration) and may not be amenable to the creation of guidelines. Other conditions on the prioritization list (eg, chemotherapy or cellulitis) may have useful guidelines within the context of specific conditions (eg, acute lymphoblastic leukemia) or for specific organisms (eg, methicillin‐resistant Staphylococcus aureus). We elected to exclude these narrower guidelines to focus on broad and comprehensive guidelines applicable to a wider range of clinical situations. Additionally, although use of a validated tool attempts to objectively guide ratings, the rating of quality is to some degree subjective. Finally, our study used a previously published prioritization list using data from children's hospitals, and the list likely under‐represents conditions commonly managed in community hospitals (eg, hyperbilirubinemia).[2] Exclusion of these conditions was not reflective of importance or quality of available national guidelines.
CONCLUSIONS
Our study adds to recent publications on the need to prioritize conditions for QI in children's hospitals. We identified a group of moderate to high methodological‐quality national guidelines for pediatric inpatient conditions with high prevalence, cost, and variation in interhospital resource utilization. Not all prioritized conditions have national high methodological‐quality guidelines available. Hospitals should prioritize conditions with high methodological‐quality guidelines to allocate resources for QI initiatives. Professional societies should focus their efforts on producing methodologically sound guidelines for prioritized conditions currently lacking high‐quality guidelines if sufficient evidence exists.
Acknowledgements
The authors thank Christopher G. Maloney, MD, PhD, for critical review of the manuscript, and Gregory J. Stoddard, MS, for statistical support. Mr. Stoddard's work is supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant 8UL1TR000105 (formerly UL1RR025764).
Disclosures
Sanjay Mahant, Ron Keren, and Raj Srivastava are all Executive Council members of the Pediatric Research in Inpatient Settings (PRIS) Network. PRIS, Sanjay Mahant, Ron Keren, and Raj Srivastava are all supported by grants from the Children's Hospital Association. Sanjay Mahant is also supported by research grants from the Canadian Institute of Health Research and Physician Services Incorporated. Ron Keren and Raj Srivastava also serve as medical legal consultants. The remaining authors have no financial relationships relevant to this article to disclose.
Researchers from the Pediatric Research in Inpatient Settings (PRIS) network, an open pediatric hospitalist research network,[1] have identified inpatient pediatric medical and surgical conditions considered high priority for quality improvement (QI) initiatives and/or comparative effectiveness research based on prevalence, cost, and interhospital variation in resource utilization.[2] One approach for improving the quality of care within hospitals is to operationalize evidence‐based guidelines into practice.[3] Although guidelines may be used by individual clinicians, systematic adoption by hospitals into clinical workflow has the potential to influence providers to adhere to evidence‐based care, reduce unwarranted variation, and ultimately improve patient outcomes.[3, 4, 5, 6]
There are critical appraisal tools to measure the methodological quality, as defined by the Institute of Medicine (IOM) and others in their guidelines.[7, 8, 9, 10, 11, 12] One such validated tool is the AGREE II instrument, created by the AGREE (Appraisal of Guidelines for REsearch and Evaluation) collaboration.[13, 14] It defines methodological quality as the confidence that the biases linked to the rigor of development, presentation, and applicability of a clinical practice guideline have been minimized and that each step of the development process is clearly reported.[13]
The objective of our study was to rate the methodological quality of national guidelines for 20 of the PRIS priority pediatric inpatient conditions.[2] Our intent in pursuing this project was 2‐fold: first, to inform pediatric inpatient QI initiatives, and second, to call out priority pediatric inpatient conditions for which high methodological‐quality guidelines are currently lacking.
METHODS
The study methods involved (1) prioritizing pediatric inpatient conditions, (2) identifying national guidelines for the priority conditions, and (3) rating the methodological quality of available guidelines. This study was considered nonhuman‐subject research (A. Johnson, personal e‐mail communication, November 14, 2012), and the original prioritization study was deemed exempt from review by the institutional review board of the Children's Hospital of Philadelphia under 45 CFR 46.102(f).[2]
Prioritizing Pediatric Inpatient Conditions
Methods for developing the prioritization list are published elsewhere in detail and briefly described here.[1] An International Classification of Diseases, 9th Revision, Clinical Modification‐based clinical condition grouper was created for primary discharge diagnosis codes for inpatient, ambulatory surgery, and observation unit encounters accounting for either 80% of all encounters or 80% of all charges for over 3.4 million discharges from 2004 to 2009 for 38 children's hospitals in the Pediatric Health Information Systems (PHIS) database, which includes administrative and billing data.[15] A standardized cost master index was created to assign the same unit cost for each billable item (calculated as the median of median hospital unit costs) to allow for comparisons of resource utilization across hospitals (eg, the cost of a chest x‐ray was set to be the same across all hospitals in 2009 dollars). Total hospital costs were then recalculated for every admission by multiplying the standardized cost master index by the number of units for each item in the hospital bill, and then summing the standardized costs of each line item in every bill. Conditions were ranked based on prevalence and total cost across all hospitals in the study period. The variation in standardized costs across hospitals for each condition was determined.
For the current study, conditions were considered if they had a top 20 prevalence rank, a top 20 cost rank, high variation (intraclass correlation coefficient >0.1) in standardized costs across hospitals, a minimum number of PHIS hospitals with annualized overexpenditures (using the standardized cost master) of at least $50,000 when compared to the mean, or a minimum median of 200 cases per hospital over the 6‐year study period to assure sufficient hospital volume for future interventions. This resulted in 29 conditions; the selected 20 conditions matched the top 20 prevalence rank (see Supporting Information, Table 1, in the online version of this article).[2]
| Condition by PRIS Priority Rank | Guidelines Meeting Inclusion Criteriaa | Guidelines Citation | Mean Overall Reviewer Methodological Quality Rating (Rater 1, Rater 2)b | Recommended for Use in the Pediatric Inpatient Setting, Mean (Rater 1, Rater 2)c | Weighted Kappa(95% Confidence Interval) |
|---|---|---|---|---|---|
| |||||
| Otitis media, unspecified, s | 1 | American Academy of Family Physicians; American Academy of Otolaryngology‐Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Clinical Practice Guidelines: Otitis media with effusion. Pediatrics. 2004 May;113(5):1412‐29. | 6 (6, 6) | 3 (3, 3) | 0.76 (0.490.93) |
| Hypertrophy of tonsils and adenoids, s | 1 | Baugh RF et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1S30. | 6.5 (7, 6) | 3 (3, 3) | 0.49 (0.050.81) |
| Asthma, m | 1 | National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma‐full report 2007. Pages 1‐440. Available at: | 7 (7, 7) | 3 (3, 3) | 0.62 (0.210.87) |
| Bronchiolitis, m | 1 | American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:17741793. | 6.5 (6, 7) | 3 (3, 3) | 0.95 (0.871.00) |
| Pneumonia, m | 1 | Bradley JS et al.The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25e76. | 6 (6, 6) | 3 (3, 3) | 0.82 (0.640.96) |
| Dental caries, s | 1 | American Academy on Pediatric Dentistry Clinical Affairs CommitteePulp Therapy Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;30:170174. | 3 (3, 3) | 1.5 (1, 2) | 0.51 (0.140.83) |
| Chemotherapy, m | 0 | ||||
| Cellulitis, m | 1 | Stevens DL et al. Practice guidelines for the diagnosis and management of skin and softtissue infections. Clin Infect Dis. 2005;41:13731406. | 4.5 (4, 5) | 2.5 (2, 3) | 0.52 (0.150.79) |
| Inguinal hernia, s | 0 | ||||
| Gastroesophageal reflux and esophagitis, m, s | 2 | Vandenplas Y et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of NASPGHAN and ESPGHAN. J Pediatr Gastroenterol Nutr. 2009;49(4):498547. | 5 (5, 5) | 3 (3, 3) | 0.69 (0.450.87) |
| Furuta GT et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:13421363. | 5 (5, 5) | 2.5 (2, 3) | 0.93 (0.850.98) | ||
| Dehydration, m | 0 | ||||
| Redundant prepuce and phimosis, s | 1 | American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012;130(3):e756e785. | 6 (6, 6) | 3 (3, 3) | 0.66 (0.250.89) |
| Abdominal pain, m | 0 | ||||
| Other convulsions, m | 0 | ||||
| Urinary tract infection, m | 1 | Roberts KB et al. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595610. | 5.5 (5, 6) | 2.5 (2, 3) | 0.62 (0.230.84) |
| Acute appendicitis without peritonitis, s | 1 | Solomkin JS et al. Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133164. | 4.5 (5, 4) | 2.5 (3, 2) | 0.37 (0.110.81) |
| Eso‐ exo‐ hetero‐, and hypertropia, s | 0 | ||||
| Fever, m | 0 | ||||
| Seizures with and without intractable epilepsy, m | 3 | Brophy GM et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:323. | 5 (5, 5) | 3 (3, 3) | 0.95 (0.870.99) |
| Hirtz D et al. Practice parameter: treatment of the child with a first unprovoked seizure: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2003;60:166175. | 5 (5, 5) | 2.5 (2, 3) | 0.73 (0.410.94) | ||
| Riviello JJ Jr et al. Practice parameter: diagnostic assessment of the child with status epilepticus (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2006;67:15421550. | 5 (4, 6) | 2.5 (2, 3) | 0.80 (0.630.94) | ||
| Sickle cell disease with crisis, m | 2 | Section on Hematology/Oncology Committee on Genetics; American Academy of Pediatrics. Health supervision for children with sickle cell disease. Pediatrics. 2002;109:526535. | 3.5 (3, 4) | 1.5 (1, 2) | 0.92 (0.800.98) |
| National Heart, Lung, and Blood Institute, National Institutes of Health. The management of sickle cell disease. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Available at: | 4 (4, 4) | 2.5 (2, 3) | 0.91 (0.800.97) | ||
Identifying National Guidelines
We developed a search protocol (see Supporting Information, Table 2, in the online version of this article) using condition‐specific keywords and the following criteria: guideline, pediatric, 2002 to 2012. A medical librarian (E.E.) used the protocol to search PubMed, National Guidelines Clearing House, and the American Academy of Pediatrics website for guidelines for the 20 selected conditions.
We limited our study to US national guidelines published or updated from 2002 to 2012 to be most relevant to the 38 US children's hospitals in the original study. Guidelines had to address either medical or surgical or both types of inpatient management for the condition, depending on how the condition was categorized on the PRIS prioritization list. For example, to target inpatient issues, otitis media was treated as a surgical condition when the prioritization list was created, therefore guidelines included in our study needed to address surgical management (ie, myringotomy or tympanostomy tubes).[2] Guidelines specific to 1 organism, test, or treatment were a priori excluded, as they would not map well to the prioritization list, and would be difficult to interpret. Guidelines focusing exclusively on condition prevention were also excluded. Guidelines with a broad subject matter (eg, abdominal infection) or unclear age were included if they contained a significant focus on the condition of interest (eg, appendicitis without peritonitis), such that the course of pediatric inpatient care was described for that condition. Retracted or outdated (superseded by a more current version) guidelines were excluded.
An investigator (G.H.) reviewed potentially relevant results from the librarian's search. For example, the search for tonsillectomy guidelines retrieved a guideline on the use of polysomnography prior to tonsillectomy in children but did not cover the inpatient management or tonsillectomy procedure.[16] This guideline was excluded from our study, as it focused on a specific test and did not discuss surgical management of the condition.
Rating Methodological Quality of Guidelines
Methodological quality of guidelines was rated with the AGREE II tool by 2 investigators (G.H. and K.N.).[13, 17] This tool has 2 overall guideline assessments and 23 subcomponents within 6 domains, reflecting many of the IOM's recommendations for methodological quality in guidelines: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence.[8, 17]
The AGREE II tool rates each of the 23 subcomponent questions using a 7‐point scale (1=strongly disagree7=strongly agree). We followed the AGREE II user's manual suggestion in rating subcomponents as 1, indicating an absence of information for that question if the question was not addressed within the guideline.[13] The AGREE II user's manual describes the option of creating standardized domain scores; however, as the objective of our study was to assess the overall methodological quality of the guideline and not to highlight particular areas of strengths/weaknesses in the domains, we elected to present raw scores only.[13]
For the overall guideline rating item 1 (Rate the overall quality of this guideline.) the AGREE II tool instructs that a score of 1 indicates lowest possible quality and 7 indicates highest possible quality.[13] As these score anchors are far apart with no guide for interpretation of intermediate results, we modified the descriptive terms on the tool to define scores <3 as low quality, scores 3 to 5 as moderate quality, and scores >5 as high quality to allow for easier interpretation of our results. We also modified the final overall recommendation score (on a 3‐point scale) from I would recommend this guideline for use to I would recommend this guideline for use in the pediatric inpatient setting.[13, 17] A score of 1 indicated to not recommend, 2 indicated to recommend with modifications, and 3 indicated to recommend without modification.
Significant discrepancies (>2‐point difference on overall rating) between the 2 raters were to be settled by consensus scoring by 3 senior investigators blinded to previous reviews, using a modified Delphi technique.[18]
Inter‐rater reliability was measured using a weighted kappa coefficient and reported using a bootstrapped method with 95% confidence intervals. Interpretation of kappa is such that 0 is the amount of agreement that would be expected by chance, and 1 is perfect agreement, with previous researchers stating scores >0.81 indicate almost perfect agreement.[19]
RESULTS
The librarian's search retrieved 2869 potential results (Figure 1). Seventeen guidelines met inclusion criteria for 13 of the 20 priority conditions. Seven conditions did not have national guidelines meeting inclusion criteria. Table 1 displays the 20 medical and surgical conditions on the modified PRIS prioritization list, including overall guideline scoring, recommendation scores, and kappa results for each guideline. The highest methodological‐quality guidelines were for asthma,[20] tonsillectomy,[21] and bronchiolitis[22] (mean overall rating 7, 6.5, and 6.5, respectively). The lowest methodological‐quality guidelines were for 2 sickle cell disease guidelines[23, 24] and 1 dental caries guideline[25] (mean overall rating 4, 3.5, and 3, respectively). Seven guidelines were rated as high overall quality, and 10 guidelines were rated as moderate overall quality. Eight of the 17 guidelines[20, 21, 22, 26, 27, 28, 29, 30] were recommended for use in the pediatric inpatient setting without modification by both reviewers. Two guidelines (for dental caries[25] and sickle cell[23]) were not recommended for use by 1 reviewer.

As an example of scoring, a national guideline for asthma had high overall scores (7 from each reviewer) and high scores across most AGREE II subcomponents. The guideline was found by both reviewers to be systematic in describing guideline development with clearly stated recommendations linked to the available evidence (including strengths and limitations) and implementation considerations.[20] Conversely, a national guideline for sickle cell disease had moderate overall scores (scores of 3 and 4) and low‐moderate scores across the majority of the subcomponent items.[23] The reviewers believe that this guideline would have been strengthened by increased transparency in guideline development, discussion of the evidence surrounding recommendations, and discussion of implementation factors. A table with detailed scoring of each guideline is available (see Supporting Information, Table 3, in the online version of this article).
Agreement between the 2 raters was almost perfect,[19] with an overall boot‐strapped weighted kappa of 0.83 (95% confidence interval 0.780.87) across 850 scores. There were no discrepancies between reviewers in overall scoring requiring consensus scoring.
DISCUSSION
Using a modified version of a published prioritization list for inpatient pediatric conditions, we found national guidelines for 13 of 20 conditions with high prevalence, cost, and interhospital variation in resource utilization. Seven conditions had no national guidelines published within the past 10 years applicable for use in the pediatric inpatient setting. Of 17 guidelines for 13 conditions, 10 had moderate and 7 had high methodological quality.
Our findings add to the literature describing methodological quality of guidelines. Many publications focus on the methodological quality of guidelines as a group and use a standardized instrument (eg, the AGREE II tool) to rate within domains (eg, domain 1: scope and purpose) across guidelines in an effort to encourage improvement in developing and reporting in guidelines.[31, 32] Our study differs in that we chose to focus on the overall quality rating of individual guidelines for specific prioritized conditions to allow hospitals to guide QI initiatives. One study that had a similar aim to ours surveyed Dutch pediatricians to select priority conditions and used the AGREE II tool to rate 17 guidelines, recommending 14 for use in the Netherlands.[33]
Identifying high methodological‐quality guidelines is only 1 in a series of steps prior to successful guideline implementation in hospitals. Other aspects of guidelines, including the strength of the evidence (eg, from randomized controlled trials) and subsequent force and clarity (eg, use of must instead of consider) of recommendations, may affect clinician or patient adherence, work processes, and ultimately patient outcomes. Strong evidence should translate into forceful and clear recommendations. Authors with the Yale Guideline Recommendation Corpus describe significant variation in reporting of guideline recommendations, and further studies have shown that the force and clarity of a recommendation is associated with adherence rates.[34, 35, 36, 37] Unfortunately, current guideline appraisal tools lack the means to score the strength of evidence, and force and clarity of recommendations.[10]
Implementation science demonstrates that there are many important factors in translating best practice into improvements in clinical care. In addition to implementation considerations such as adherence, patient preferences, and work processes, variability in methodological quality, strength of evidence, and force and clarity of recommendations may be additional reasons why evidence for the impact of guidelines on patient outcomes remains mixed in the literature.[38] One recent study found that adherence to antibiotics recommended within a national pediatric community‐acquired pneumonia guideline, which had a high methodological‐quality score in our study, did not change hospital length of stay or readmissions.[29, 39] There are several possible interpretations for this. Recommendations may not have been based upon strong evidence, research methodology assessing how adherence to recommendations impacts patient outcomes may have been limited, or the outcomes measured in current studies (such as readmission) are not the outcomes that may be improved by adherence to these recommendations (such as decreasing antimicrobial resistance). These are important considerations when hospitals are incorporating recommendations from guidelines into practice. Hospitals should assess the multiple aspects of guidelines, including methodological quality, which our study helps to identify, strength of evidence, and force and clarity of recommendations, as well as adherence, patient preferences, work processes, and key outcome measures when implementing guidelines into clinical practice. A study utilizing a robust QI methodology demonstrated that clinician adherence to several elements in an asthma guideline, which also had a high methodological‐quality score in our study, led to a significant decrease in 6‐month hospital and emergency department readmission for asthma.[6, 20]
Our study also highlights that several pediatric conditions with high prevalence, cost, and interhospital resource utilization variation lack recent national pediatric guidelines applicable to the inpatient setting. If strong evidence exists for these priority conditions, professional societies should create high methodological‐quality guidelines with strong and clear recommendations. If evidence is lacking for these priority conditions, then investigators should focus on generating research in these areas.
There are several limitations to this study. The AGREE II tool does not have a mechanism to measure the strength of evidence used in a guideline. Methodological quality of a guideline alone may not translate into improved outcomes. Conditions may have national guidelines published before 2002, institution‐specific or international guidelines, or adult guidelines that might be amenable to use in the pediatric inpatient setting but were not included in this study. Several conditions on the prioritization list are broad in nature (eg, dehydration) and may not be amenable to the creation of guidelines. Other conditions on the prioritization list (eg, chemotherapy or cellulitis) may have useful guidelines within the context of specific conditions (eg, acute lymphoblastic leukemia) or for specific organisms (eg, methicillin‐resistant Staphylococcus aureus). We elected to exclude these narrower guidelines to focus on broad and comprehensive guidelines applicable to a wider range of clinical situations. Additionally, although use of a validated tool attempts to objectively guide ratings, the rating of quality is to some degree subjective. Finally, our study used a previously published prioritization list using data from children's hospitals, and the list likely under‐represents conditions commonly managed in community hospitals (eg, hyperbilirubinemia).[2] Exclusion of these conditions was not reflective of importance or quality of available national guidelines.
CONCLUSIONS
Our study adds to recent publications on the need to prioritize conditions for QI in children's hospitals. We identified a group of moderate to high methodological‐quality national guidelines for pediatric inpatient conditions with high prevalence, cost, and variation in interhospital resource utilization. Not all prioritized conditions have national high methodological‐quality guidelines available. Hospitals should prioritize conditions with high methodological‐quality guidelines to allocate resources for QI initiatives. Professional societies should focus their efforts on producing methodologically sound guidelines for prioritized conditions currently lacking high‐quality guidelines if sufficient evidence exists.
Acknowledgements
The authors thank Christopher G. Maloney, MD, PhD, for critical review of the manuscript, and Gregory J. Stoddard, MS, for statistical support. Mr. Stoddard's work is supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant 8UL1TR000105 (formerly UL1RR025764).
Disclosures
Sanjay Mahant, Ron Keren, and Raj Srivastava are all Executive Council members of the Pediatric Research in Inpatient Settings (PRIS) Network. PRIS, Sanjay Mahant, Ron Keren, and Raj Srivastava are all supported by grants from the Children's Hospital Association. Sanjay Mahant is also supported by research grants from the Canadian Institute of Health Research and Physician Services Incorporated. Ron Keren and Raj Srivastava also serve as medical legal consultants. The remaining authors have no financial relationships relevant to this article to disclose.
- , . Development of the Pediatric Research in Inpatient Settings (PRIS) Network: lessons learned. J Hosp Med. 2012;7(8):661–664.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1–10.
- , . How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185–1191.
- . Making it easy to do it right. N Engl J Med. 2001;345(13):991–993.
- , , , , . Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318(7182):527–530.
- , , , et al. The Joint Commission Children's Asthma Care quality measures and asthma readmissions. Pediatrics. 2012;130(3):482–491.
- , , , et al. Toward transparent clinical policies. Pediatrics. 2008;121(3):643–646.
- Field MJ, Lohr KN, eds.; Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
- , , , , . Development and application of a generic methodology to assess the quality of clinical guidelines. Int J Qual Health Care. 1999;11(1):21–28.
- , , , , . A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int J Qual Health Care. 2005;17(3):235–242.
- , , . How to decide whether a clinical practice guideline is trustworthy. JAMA. 2013;309(2):139–140.
- Field MJ, Lohr KN, eds.; Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Guidelines for Clinical Practice: From Development to Use. Washington, DC: National Academies Press; 1992.
- The AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12:18–23.
- . AGREE II‐improving the quality of clinical care. Lancet. 2010;376(9747):1128–1129.
- , , , . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055.
- , , , et al. Clinical practice guideline: polysomnography for sleep‐disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1 suppl):S1–S15.
- , , , et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–E842.
- , . The Delphi method as a research tool: an example, design considerations and applications. Inform Manag. 2004;42(1):15–29.
- , . The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174.
- National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma‐full report 2007. Pages 1–440. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. Accessed on 24 August 2012.
- , , , et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1–S30.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- Health supervision for children with sickle cell disease. Pediatrics. 2002;109(3):526–535.
- National Heart, Lung, and Blood Institute, National Institutes of Health. The management of sickle cell disease. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Available at: http://www.nhlbi.nih.gov/health/prof/blood/sickle/sc_mngt.pdf. Revised June 2002. Accessed on 13 October 2012.
- American Academy on Pediatric Dentistry Clinical Affairs Committee–Pulp Therapy Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;30:170–174.
- , , , et al.; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23.
- American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012;130(3):e756–e785.
- , , , et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498–547.
- , , , et al. The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76.
- American Academy of Family Physicians; American Academy of Otolaryngology‐Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Clinical Practice Guidelines: Otitis media with effusion. Pediatrics. 2004 May;113(5):1412–29.
- , , . Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer‐reviewed medical literature. JAMA. 1999;281(20):1900–1905.
- , , , . Quality of reporting and evidence in American Academy of Pediatrics guidelines. Pediatrics. 2013;131(4):732–738.
- , , . Quality of evidence‐based pediatric guidelines. Pediatrics. 2005;115(5):1378–1391.
- , , . The Yale Guideline Recommendation Corpus: a representative sample of the knowledge content of guidelines. Int J Med Inform. 2009;78(5):354–363.
- , , . How often is strength of recommendation indicated in guidelines? Analysis of the Yale Guideline Recommendation Corpus. AMIA Annu Symp Proc. 2008:984.
- , , . Clinical practice guideline development manual, third edition: a quality‐driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2013;148(1 suppl):S1–S55.
- , , , , , . Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ. 1998;317(7162):858–861.
- , , , et al. Toward evidence‐based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966–1998. J Gen Intern Med. 2006;21(suppl 2):S14–S20.
- , , , . Effectiveness of antimicrobial guidelines for community‐acquired pneumonia in children. Pediatrics. 2012;129(5):e1326–e1333.
- , . Development of the Pediatric Research in Inpatient Settings (PRIS) Network: lessons learned. J Hosp Med. 2012;7(8):661–664.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1–10.
- , . How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185–1191.
- . Making it easy to do it right. N Engl J Med. 2001;345(13):991–993.
- , , , , . Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318(7182):527–530.
- , , , et al. The Joint Commission Children's Asthma Care quality measures and asthma readmissions. Pediatrics. 2012;130(3):482–491.
- , , , et al. Toward transparent clinical policies. Pediatrics. 2008;121(3):643–646.
- Field MJ, Lohr KN, eds.; Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
- , , , , . Development and application of a generic methodology to assess the quality of clinical guidelines. Int J Qual Health Care. 1999;11(1):21–28.
- , , , , . A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int J Qual Health Care. 2005;17(3):235–242.
- , , . How to decide whether a clinical practice guideline is trustworthy. JAMA. 2013;309(2):139–140.
- Field MJ, Lohr KN, eds.; Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Guidelines for Clinical Practice: From Development to Use. Washington, DC: National Academies Press; 1992.
- The AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12:18–23.
- . AGREE II‐improving the quality of clinical care. Lancet. 2010;376(9747):1128–1129.
- , , , . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055.
- , , , et al. Clinical practice guideline: polysomnography for sleep‐disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1 suppl):S1–S15.
- , , , et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–E842.
- , . The Delphi method as a research tool: an example, design considerations and applications. Inform Manag. 2004;42(1):15–29.
- , . The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174.
- National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma‐full report 2007. Pages 1–440. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. Accessed on 24 August 2012.
- , , , et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1–S30.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- Health supervision for children with sickle cell disease. Pediatrics. 2002;109(3):526–535.
- National Heart, Lung, and Blood Institute, National Institutes of Health. The management of sickle cell disease. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Available at: http://www.nhlbi.nih.gov/health/prof/blood/sickle/sc_mngt.pdf. Revised June 2002. Accessed on 13 October 2012.
- American Academy on Pediatric Dentistry Clinical Affairs Committee–Pulp Therapy Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;30:170–174.
- , , , et al.; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23.
- American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012;130(3):e756–e785.
- , , , et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498–547.
- , , , et al. The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76.
- American Academy of Family Physicians; American Academy of Otolaryngology‐Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Clinical Practice Guidelines: Otitis media with effusion. Pediatrics. 2004 May;113(5):1412–29.
- , , . Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer‐reviewed medical literature. JAMA. 1999;281(20):1900–1905.
- , , , . Quality of reporting and evidence in American Academy of Pediatrics guidelines. Pediatrics. 2013;131(4):732–738.
- , , . Quality of evidence‐based pediatric guidelines. Pediatrics. 2005;115(5):1378–1391.
- , , . The Yale Guideline Recommendation Corpus: a representative sample of the knowledge content of guidelines. Int J Med Inform. 2009;78(5):354–363.
- , , . How often is strength of recommendation indicated in guidelines? Analysis of the Yale Guideline Recommendation Corpus. AMIA Annu Symp Proc. 2008:984.
- , , . Clinical practice guideline development manual, third edition: a quality‐driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2013;148(1 suppl):S1–S55.
- , , , , , . Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ. 1998;317(7162):858–861.
- , , , et al. Toward evidence‐based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966–1998. J Gen Intern Med. 2006;21(suppl 2):S14–S20.
- , , , . Effectiveness of antimicrobial guidelines for community‐acquired pneumonia in children. Pediatrics. 2012;129(5):e1326–e1333.
© 2014 Society of Hospital Medicine