User login
Update on Pediatric Atopic Dermatitis
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that occurs most frequently in children but also affects many adolescents and adults. There has been a tremendous evolution of knowledge in AD, with insights into pathogenesis, epidemiology, impact of disease, and new therapies. A variety of studies examine the epidemiology of AD and associated comorbidities. The broad developments in disease state research are reflected in new publication numbers of AD citations on PubMed. A PubMed search of articles indexed for MEDLINE at the end of 2010 using the term atopic dermatitis would have shown 965 citations during the preceding 1-year period. In the 1-year period of June 2019 to June 2020, there were more than 2000 articles. The large body of research includes work of great significance in pediatric AD, and in this article we review recent findings that are important in understanding the progress being made in the field.
Epidemiology and Comorbidities
The epidemiology of AD has evolved over the last few decades, with emerging trends and novel insights into the burden of disease.1 In a recent cross-sectional study on the epidemiology of AD in children aged 6 to 11 years, the 1-year diagnosed AD prevalence estimates worldwide included the following: United States, 10.0%; Canada, 13.3%; the EU5 Countries, 15.5%; Japan, 10.3%; and all countries studied, 12.2%.2 Another recent paper that analyzed data from the Fragile Families and Child Wellbeing Study showed that the prevalence and persistence of AD in urban US children was 15.0%.3Although pediatric AD may spontaneously remit over time, disease continuing into adolescence and adulthood is common. Paternoster et al4 studied the longitudinal course of AD in children from 2 birth cohort prospective studies, showing distinct AD phenotypes having differing course trajectories over time. Disease subsets included patients with early-onset-persistent and early-onset-late-resolving disease.4 Whether phenotyping or subgroup analysis can be used to predict disease course or risk for development of comorbidities is unknown, but it is interesting to consider how such work could influence tailoring of specific therapies to early disease presentation.
Atopic dermatitis poses a serious public health burden owing to its high prevalence, considerable morbidity and disability, increased health care utilization, and cost of care.1 Recent studies have found notably higher rates of multiple medical and mental health comorbidities in both children and adults with AD, including infections, atopic comorbidities (eg, allergic rhinitis, asthma, food allergies), eye diseases (eg, keratitis, conjunctivitis, keratoconus), and possible cardiovascular diseases and autoimmune disorders.1,5-9 Allergic comorbidities are quite common in pediatric AD patients.10 In a recent study examining the efficacy and safety of dupilumab monotherapy in 251 adolescents with moderate to severe inadequately controlled AD, most had comorbid type 2 diseases including asthma (53.6%), food allergies (60.8%), and allergic rhinitis (65.6%).11
Quality of Life/Life Impact of AD
Pediatric AD has a major impact on the quality of life of patients and their families.12 The well-being and development of children are strongly influenced by the physical and psychosocial health of parents/guardians. Two studies by Ramirez and colleagues13,14 published in 2019 examined sleep disturbances and exhaustion in mothers of children with AD. Data for the studies came from the Avon Longitudinal Study of Parents and Children. Children with active AD reported worse sleep quality than those without AD, with nearly 50% higher odds of sleep-quality disturbances. Analysis of the cohort data from 11,649 mother-child pairs who were followed up with a time-varying measure of child AD activity and severity as well as self-reported maternal sleep measures repeated at multiple time points for children aged 6 months to 11 years showed that mothers of children with AD reported difficulty falling asleep, subjectively insufficient sleep, and daytime exhaustion throughout the first 11 years of childhood.13,14 These data suggest that sleep disturbance may be a family affair.
A cross-sectional, real-world study on the burden of AD in children aged 6 to 11 years assessed by self-report demonstrated a substantial and multidimensional impact of AD, including itch, sleep disturbance, skin pain, and health-related quality-of-life impact, as well as comorbidities and school productivity losses. The burden associated with AD was remarkable and increased with disease severity.15
Drucker et al16 completed a comprehensive literature review on the burden of AD, summarized as a report for the National Eczema Association. Quality-of-life impact on pediatric patients included high rates of emotional distress; social isolation; depression; limitations in activities due to lesions with fear of triggers; and behavioral problems such as irritability, crying, and sleep disturbance resulting in difficulty performing at school.16 The psychological impact on children as well as emotional and behavioral difficulties may impact the ability for parents/guardians to implement treatment plans.17
There is a striking association between mental health disorders and AD in the US pediatric population, with a clear dose-dependent relationship that has been observed between the prevalence of a mental health disorder and the reported severity of the skin disease. Data suggest children with AD may be at increased risk for developing mental health disorders. The National Survey of Children’s Health found statistically significant increases in the likelihood of attention deficit hyperactivity disorder (odds ratio [OR], 1.87), depression (OR, 1.81), anxiety (OR, 1.77), conduct disorder (OR, 1.87), and autism (OR, 3.04).6
Evolving Practices and Therapies
Bathing Practices
There has long been much controversy regarding best bathing habits for patients with AD. In a 2009 study, cutaneous hydration was quantified after various bathing and moisturizing regimens.18 The study showed clear benefits of emollient application on skin hydration, either after bathing or without bathing. Bathing followed by emollient applications did not decrease skin hydration in contrast to bathing without emollient application.18
There are limited studies evaluating bathing frequency in pediatric patients, and many families receive conflicting information regarding best practice. In one study that surveyed 354 parents, more than 75% of parents/guardians who had seen multiple providers for their child’s AD reported a substantial amount of confusion and frustration from conflicting advice on bathing frequency.19 Cardona et al20 undertook a randomized clinical trial of frequent bathing and moisturizing vs less-frequent bathing and moisturizing in pediatric patients with AD aged 6 months to 11 years. Patients were divided into 2 groups: 1 being bathed twice daily with immediate moisturizer application and the other being bathed twice weekly followed by moisturization, then a switch to the other method. Patients used standardized topical corticosteroids (TCSs) in both groups. There were significant improvements in scoring AD and other objective measures during the frequent bathing time period vs infrequent bathing; in the group that bathed more frequently, SCORAD (SCORing Atopic Dermatitis) decreased by 21.2 compared with the group that bathed less frequently (95% confidence interval, 14.9-27.6; P<.0001). These findings suggest that more-frequent bathing with immediate moisturization is superior as an acute treatment intervention for improving AD disease severity in comparison to less-frequent bathing with immediate moisturization.20
Expanding Treatment Options
Topical Phosphodiesterase Inhibitors
There are several new and evolving topical therapies in AD. Crisaborole ointment 2% is a steroid-free phosphodiesterase inhibitor approved in 2016 by the US Food and Drug Administration (FDA) for mild to moderate AD in patients aged 2 years and older. A recent multicenter, open-label, single-arm study in 137 infants (CrisADe CARE 1) evaluated the pharmacokinetics and efficacy of crisaborole ointment 2% applied twice daily for 4 weeks in pediatric patients aged 3 months to less than 24 months of age with mild to moderate AD.21 The study had 2 cohorts: one with a minimum of 5% body surface area involvement and another (the pharmacokinetic cohort) with a minimum of 35% body surface area involvement. Both cohorts demonstrated similar efficacy data. From baseline to day 29, the mean percentage change in eczema area and severity index (EASI) score was −57.5%, and an investigator global assessment (IGA) score of clear or almost clear with at least a 2-grade improvement was achieved in 30.2% of patients. Crisaborole systemic exposures in infants were comparable with those in patients aged 2 years or older. Patients tolerated crisaborole well, with a 4% rate of burning, which was similar to other studies in children and adults but perhaps lower than seen in clinical practice. Pharmacokinetic studies did not show any remarkable noticeable concern with accumulation of propylene glycol absorption.21
Based on the CrisADe CARE 1 study data, in March 2020 the FDA extended the indication of crisaborole ointment 2% from a prior lower age limit of 24 months to approval for use in treating mild to moderate AD in children as young as 3 months, making it the first nonsteroidal topical anti-inflammatory medication to be approved in children younger than 2 years in the United States.
Evolving Topical Therapies
Topical Janus Kinase Inhibitors
Ruxolitinib is a potent inhibitor of Janus kinase 1 (JAK-1) and Janus kinase 2 (JAK-2) and has been developed in topical formulations. In recent phase 3 clinical trials of patients with AD aged 12 years and older with mild to moderate disease (TRuE-AD1 and TRuE-AD2), more than half of the patients treated with either ruxolitinib cream in a 0.75% or 1.5% concentration reached EASI-75 after 8 weeks of treatment.22 Additionally, more patients treated with topical ruxolitinib reached an IGA score of clear to almost clear than patients treated with vehicle at the end of treatment. Thus far, it appears to be very well tolerated, significantly decreases EASI score (P<.0001), and improves overall pruritus.22
Delgocitinib is a topical pan-JAK inhibitor that blocks several cytokine-signaling cascade pathways. It was first developed and approved in Japan in an ointment formulation for use in patients with AD aged 16 years and older.23 The efficacy and safety profile of delgocitinib is currently being evaluated in pediatric patients with AD in Japan. In a recent phase 2 clinical study of 103 Japanese patients aged 2 to 15 years with moderate to severe AD, patients were randomized to receive either delgocitinib ointment in 0.25% or 0.5% concentrations or vehicle ointment twice daily for 4 weeks. The proportion of patients with a modified EASI-75 score was 38.2% (13/34) in the 0.25% group and 50.0% (17/34) in the 0.5% group vs 8.6% (3/35) in the placebo group. More patients treated with delgocitinib ointment received an IGA score of clear or almost clear than patients treated with vehicle at the end of treatment. Overall, both delgocitinib groups demonstrated superior improvement in clinical symptoms and signs without notable side effects.24
Tapinarof
Tapinarof is a topical therapeutic aryl hydrocarbon receptor agonist. In a recent phase 2 randomized study of 2 concentrations and 2 frequencies of tapinarof cream vs vehicle in 247 randomized patients aged 12 to 65 years with moderate to severe disease, tapinarof demonstrated greater success with both concentrations than vehicle at all visits beyond week 2.25 Additionally, in patients treated with tapinarof cream 1%, nearly 50% reached an IGA score of clear to almost clear with at least a 2-grade improvement. More than 50% of patients achieved EASI-75 improvement at 12 weeks of treatment with tapinarof cream 1% used daily. These findings suggest that tapinarof may be an efficacious and well-tolerated treatment for both adolescents and adults with AD; however, large confirmation trials are needed to further investigate.25
Systemic Treatments
Oral JAK Inhibitors
Some of the most exciting novel therapies include several oral JAK inhibitors that target different combinations of kinases and have been shown to decrease AD severity and symptoms. Some of these agents have indications in other disease states, such as baricitinib and upadacitinib, which are both FDA approved for the treatment of rheumatoid arthritis, whereas others, such as abrocitinib, have been studied specifically for AD.
Although some agents have only been studied in adults to date, others have included adolescents in their core studies, such as abrocitinib, which received Breakthrough Therapy designation from the FDA for the treatment of patients with moderate to severe AD in February 2018. In recent phase 3 trials of patients aged 12 years and older with moderate to severe AD (JADE MONO-1 and JADE MONO-2), both doses of abrocitinib improved the IGA and EASI-75 outcomes compared with placebo.26 Additional studies will be conducted to further investigate the relative efficacy and safety in patients younger than 18 years.
Biologics
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling without suppressing the immune system. It is approved for use in patients aged 12 years and older with moderate to severe asthma and in adults with chronic rhinosinusitis with nasal polyposis. It is the first biologic to show positive results in the moderate to severe pediatric AD population. There are now extended data available exhibiting sustained benefit in adolescent patients who were continued on dupilumab therapy, evidenced by further improvement in EASI scores at the 1-year mark.27
Recently, dupilumab received approval for use in patients aged 6 to 11 years, making it the first biologic for AD to be approved for use in patients younger than 12 years. The expedited FDA approval was based on the phase 3 results in which the efficacy and safety of dupilumab combined with TCSs were compared to TCSs alone (N=367).28 In this trial, more than twice as many children achieved clear or almost clear skin and more than 4 times as many achieved itch reduction with dupilumab plus TCSs than with TCSs alone. Three-quarters of patients receiving dupilumab at the subsequently approved dosing achieved at least a 75% improvement in overall disease.28 An additional study is being conducted that includes pediatric patients aged 6 months to younger than 6 years (ClinicalTrials.gov Identifier NCT03346434).
Future Directions in Pediatric AD
Our review summarizes only some of the agents under clinical investigation for use in pediatric AD. Early treatment to establish excellent long-term disease control with aggressive topical regimens or with systemic agents may alter the course of AD and influence the development of comorbidities, though this has not yet been shown in clinical studies. The long-term impact of early treatment, along with many other intriguing issues, will be studied more in the near future.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paper presented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1.
- Paternoster L, Savenije OEM, Heron J, et al. IJ Allergy Clin Immunol. 2018;141:964-971.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Yaghmaie P, Koudelka CW, Simpson Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
- Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468.
- al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.
- Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074-1081.
- Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821-838.
- Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis.
- Quality of life in families with children with atopic dermatitis. Pediatr Dermatol. 2016;33:28-32.
- Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556-563.
- Association of atopic dermatitis with sleep quality in children.
- Weidinger S, Simpson EL, Eckert L, et al. The patient-reported disease burden in pediatric patients with atopic dermatitis: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paperpresented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag. 2018;11:289-298.
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Kempe E, Jain N, Cardona I. Bathing frequency recommendations for pediatric atopic dermatitis: are we adding to parental frustration? Ann Allergy Asthma Immunol. 2013;111:298‐299.
- Cardona ID, Kempe EE, Lary C, et al. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8:1014‐1021.
- Gower , Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275-284.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment atopic dermatitis: results from two phase 3, randomized, double-blind studies. Presented at: 2nd Annual Revolutionizing Atopic Dermatitis Conference; April 5, 2020; Chicago, IL.
- Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609‐615.
- Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575‐1583.
- Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89‐98.e3.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85‐96.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published online June 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.054.
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that occurs most frequently in children but also affects many adolescents and adults. There has been a tremendous evolution of knowledge in AD, with insights into pathogenesis, epidemiology, impact of disease, and new therapies. A variety of studies examine the epidemiology of AD and associated comorbidities. The broad developments in disease state research are reflected in new publication numbers of AD citations on PubMed. A PubMed search of articles indexed for MEDLINE at the end of 2010 using the term atopic dermatitis would have shown 965 citations during the preceding 1-year period. In the 1-year period of June 2019 to June 2020, there were more than 2000 articles. The large body of research includes work of great significance in pediatric AD, and in this article we review recent findings that are important in understanding the progress being made in the field.
Epidemiology and Comorbidities
The epidemiology of AD has evolved over the last few decades, with emerging trends and novel insights into the burden of disease.1 In a recent cross-sectional study on the epidemiology of AD in children aged 6 to 11 years, the 1-year diagnosed AD prevalence estimates worldwide included the following: United States, 10.0%; Canada, 13.3%; the EU5 Countries, 15.5%; Japan, 10.3%; and all countries studied, 12.2%.2 Another recent paper that analyzed data from the Fragile Families and Child Wellbeing Study showed that the prevalence and persistence of AD in urban US children was 15.0%.3Although pediatric AD may spontaneously remit over time, disease continuing into adolescence and adulthood is common. Paternoster et al4 studied the longitudinal course of AD in children from 2 birth cohort prospective studies, showing distinct AD phenotypes having differing course trajectories over time. Disease subsets included patients with early-onset-persistent and early-onset-late-resolving disease.4 Whether phenotyping or subgroup analysis can be used to predict disease course or risk for development of comorbidities is unknown, but it is interesting to consider how such work could influence tailoring of specific therapies to early disease presentation.
Atopic dermatitis poses a serious public health burden owing to its high prevalence, considerable morbidity and disability, increased health care utilization, and cost of care.1 Recent studies have found notably higher rates of multiple medical and mental health comorbidities in both children and adults with AD, including infections, atopic comorbidities (eg, allergic rhinitis, asthma, food allergies), eye diseases (eg, keratitis, conjunctivitis, keratoconus), and possible cardiovascular diseases and autoimmune disorders.1,5-9 Allergic comorbidities are quite common in pediatric AD patients.10 In a recent study examining the efficacy and safety of dupilumab monotherapy in 251 adolescents with moderate to severe inadequately controlled AD, most had comorbid type 2 diseases including asthma (53.6%), food allergies (60.8%), and allergic rhinitis (65.6%).11
Quality of Life/Life Impact of AD
Pediatric AD has a major impact on the quality of life of patients and their families.12 The well-being and development of children are strongly influenced by the physical and psychosocial health of parents/guardians. Two studies by Ramirez and colleagues13,14 published in 2019 examined sleep disturbances and exhaustion in mothers of children with AD. Data for the studies came from the Avon Longitudinal Study of Parents and Children. Children with active AD reported worse sleep quality than those without AD, with nearly 50% higher odds of sleep-quality disturbances. Analysis of the cohort data from 11,649 mother-child pairs who were followed up with a time-varying measure of child AD activity and severity as well as self-reported maternal sleep measures repeated at multiple time points for children aged 6 months to 11 years showed that mothers of children with AD reported difficulty falling asleep, subjectively insufficient sleep, and daytime exhaustion throughout the first 11 years of childhood.13,14 These data suggest that sleep disturbance may be a family affair.
A cross-sectional, real-world study on the burden of AD in children aged 6 to 11 years assessed by self-report demonstrated a substantial and multidimensional impact of AD, including itch, sleep disturbance, skin pain, and health-related quality-of-life impact, as well as comorbidities and school productivity losses. The burden associated with AD was remarkable and increased with disease severity.15
Drucker et al16 completed a comprehensive literature review on the burden of AD, summarized as a report for the National Eczema Association. Quality-of-life impact on pediatric patients included high rates of emotional distress; social isolation; depression; limitations in activities due to lesions with fear of triggers; and behavioral problems such as irritability, crying, and sleep disturbance resulting in difficulty performing at school.16 The psychological impact on children as well as emotional and behavioral difficulties may impact the ability for parents/guardians to implement treatment plans.17
There is a striking association between mental health disorders and AD in the US pediatric population, with a clear dose-dependent relationship that has been observed between the prevalence of a mental health disorder and the reported severity of the skin disease. Data suggest children with AD may be at increased risk for developing mental health disorders. The National Survey of Children’s Health found statistically significant increases in the likelihood of attention deficit hyperactivity disorder (odds ratio [OR], 1.87), depression (OR, 1.81), anxiety (OR, 1.77), conduct disorder (OR, 1.87), and autism (OR, 3.04).6
Evolving Practices and Therapies
Bathing Practices
There has long been much controversy regarding best bathing habits for patients with AD. In a 2009 study, cutaneous hydration was quantified after various bathing and moisturizing regimens.18 The study showed clear benefits of emollient application on skin hydration, either after bathing or without bathing. Bathing followed by emollient applications did not decrease skin hydration in contrast to bathing without emollient application.18
There are limited studies evaluating bathing frequency in pediatric patients, and many families receive conflicting information regarding best practice. In one study that surveyed 354 parents, more than 75% of parents/guardians who had seen multiple providers for their child’s AD reported a substantial amount of confusion and frustration from conflicting advice on bathing frequency.19 Cardona et al20 undertook a randomized clinical trial of frequent bathing and moisturizing vs less-frequent bathing and moisturizing in pediatric patients with AD aged 6 months to 11 years. Patients were divided into 2 groups: 1 being bathed twice daily with immediate moisturizer application and the other being bathed twice weekly followed by moisturization, then a switch to the other method. Patients used standardized topical corticosteroids (TCSs) in both groups. There were significant improvements in scoring AD and other objective measures during the frequent bathing time period vs infrequent bathing; in the group that bathed more frequently, SCORAD (SCORing Atopic Dermatitis) decreased by 21.2 compared with the group that bathed less frequently (95% confidence interval, 14.9-27.6; P<.0001). These findings suggest that more-frequent bathing with immediate moisturization is superior as an acute treatment intervention for improving AD disease severity in comparison to less-frequent bathing with immediate moisturization.20
Expanding Treatment Options
Topical Phosphodiesterase Inhibitors
There are several new and evolving topical therapies in AD. Crisaborole ointment 2% is a steroid-free phosphodiesterase inhibitor approved in 2016 by the US Food and Drug Administration (FDA) for mild to moderate AD in patients aged 2 years and older. A recent multicenter, open-label, single-arm study in 137 infants (CrisADe CARE 1) evaluated the pharmacokinetics and efficacy of crisaborole ointment 2% applied twice daily for 4 weeks in pediatric patients aged 3 months to less than 24 months of age with mild to moderate AD.21 The study had 2 cohorts: one with a minimum of 5% body surface area involvement and another (the pharmacokinetic cohort) with a minimum of 35% body surface area involvement. Both cohorts demonstrated similar efficacy data. From baseline to day 29, the mean percentage change in eczema area and severity index (EASI) score was −57.5%, and an investigator global assessment (IGA) score of clear or almost clear with at least a 2-grade improvement was achieved in 30.2% of patients. Crisaborole systemic exposures in infants were comparable with those in patients aged 2 years or older. Patients tolerated crisaborole well, with a 4% rate of burning, which was similar to other studies in children and adults but perhaps lower than seen in clinical practice. Pharmacokinetic studies did not show any remarkable noticeable concern with accumulation of propylene glycol absorption.21
Based on the CrisADe CARE 1 study data, in March 2020 the FDA extended the indication of crisaborole ointment 2% from a prior lower age limit of 24 months to approval for use in treating mild to moderate AD in children as young as 3 months, making it the first nonsteroidal topical anti-inflammatory medication to be approved in children younger than 2 years in the United States.
Evolving Topical Therapies
Topical Janus Kinase Inhibitors
Ruxolitinib is a potent inhibitor of Janus kinase 1 (JAK-1) and Janus kinase 2 (JAK-2) and has been developed in topical formulations. In recent phase 3 clinical trials of patients with AD aged 12 years and older with mild to moderate disease (TRuE-AD1 and TRuE-AD2), more than half of the patients treated with either ruxolitinib cream in a 0.75% or 1.5% concentration reached EASI-75 after 8 weeks of treatment.22 Additionally, more patients treated with topical ruxolitinib reached an IGA score of clear to almost clear than patients treated with vehicle at the end of treatment. Thus far, it appears to be very well tolerated, significantly decreases EASI score (P<.0001), and improves overall pruritus.22
Delgocitinib is a topical pan-JAK inhibitor that blocks several cytokine-signaling cascade pathways. It was first developed and approved in Japan in an ointment formulation for use in patients with AD aged 16 years and older.23 The efficacy and safety profile of delgocitinib is currently being evaluated in pediatric patients with AD in Japan. In a recent phase 2 clinical study of 103 Japanese patients aged 2 to 15 years with moderate to severe AD, patients were randomized to receive either delgocitinib ointment in 0.25% or 0.5% concentrations or vehicle ointment twice daily for 4 weeks. The proportion of patients with a modified EASI-75 score was 38.2% (13/34) in the 0.25% group and 50.0% (17/34) in the 0.5% group vs 8.6% (3/35) in the placebo group. More patients treated with delgocitinib ointment received an IGA score of clear or almost clear than patients treated with vehicle at the end of treatment. Overall, both delgocitinib groups demonstrated superior improvement in clinical symptoms and signs without notable side effects.24
Tapinarof
Tapinarof is a topical therapeutic aryl hydrocarbon receptor agonist. In a recent phase 2 randomized study of 2 concentrations and 2 frequencies of tapinarof cream vs vehicle in 247 randomized patients aged 12 to 65 years with moderate to severe disease, tapinarof demonstrated greater success with both concentrations than vehicle at all visits beyond week 2.25 Additionally, in patients treated with tapinarof cream 1%, nearly 50% reached an IGA score of clear to almost clear with at least a 2-grade improvement. More than 50% of patients achieved EASI-75 improvement at 12 weeks of treatment with tapinarof cream 1% used daily. These findings suggest that tapinarof may be an efficacious and well-tolerated treatment for both adolescents and adults with AD; however, large confirmation trials are needed to further investigate.25
Systemic Treatments
Oral JAK Inhibitors
Some of the most exciting novel therapies include several oral JAK inhibitors that target different combinations of kinases and have been shown to decrease AD severity and symptoms. Some of these agents have indications in other disease states, such as baricitinib and upadacitinib, which are both FDA approved for the treatment of rheumatoid arthritis, whereas others, such as abrocitinib, have been studied specifically for AD.
Although some agents have only been studied in adults to date, others have included adolescents in their core studies, such as abrocitinib, which received Breakthrough Therapy designation from the FDA for the treatment of patients with moderate to severe AD in February 2018. In recent phase 3 trials of patients aged 12 years and older with moderate to severe AD (JADE MONO-1 and JADE MONO-2), both doses of abrocitinib improved the IGA and EASI-75 outcomes compared with placebo.26 Additional studies will be conducted to further investigate the relative efficacy and safety in patients younger than 18 years.
Biologics
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling without suppressing the immune system. It is approved for use in patients aged 12 years and older with moderate to severe asthma and in adults with chronic rhinosinusitis with nasal polyposis. It is the first biologic to show positive results in the moderate to severe pediatric AD population. There are now extended data available exhibiting sustained benefit in adolescent patients who were continued on dupilumab therapy, evidenced by further improvement in EASI scores at the 1-year mark.27
Recently, dupilumab received approval for use in patients aged 6 to 11 years, making it the first biologic for AD to be approved for use in patients younger than 12 years. The expedited FDA approval was based on the phase 3 results in which the efficacy and safety of dupilumab combined with TCSs were compared to TCSs alone (N=367).28 In this trial, more than twice as many children achieved clear or almost clear skin and more than 4 times as many achieved itch reduction with dupilumab plus TCSs than with TCSs alone. Three-quarters of patients receiving dupilumab at the subsequently approved dosing achieved at least a 75% improvement in overall disease.28 An additional study is being conducted that includes pediatric patients aged 6 months to younger than 6 years (ClinicalTrials.gov Identifier NCT03346434).
Future Directions in Pediatric AD
Our review summarizes only some of the agents under clinical investigation for use in pediatric AD. Early treatment to establish excellent long-term disease control with aggressive topical regimens or with systemic agents may alter the course of AD and influence the development of comorbidities, though this has not yet been shown in clinical studies. The long-term impact of early treatment, along with many other intriguing issues, will be studied more in the near future.
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that occurs most frequently in children but also affects many adolescents and adults. There has been a tremendous evolution of knowledge in AD, with insights into pathogenesis, epidemiology, impact of disease, and new therapies. A variety of studies examine the epidemiology of AD and associated comorbidities. The broad developments in disease state research are reflected in new publication numbers of AD citations on PubMed. A PubMed search of articles indexed for MEDLINE at the end of 2010 using the term atopic dermatitis would have shown 965 citations during the preceding 1-year period. In the 1-year period of June 2019 to June 2020, there were more than 2000 articles. The large body of research includes work of great significance in pediatric AD, and in this article we review recent findings that are important in understanding the progress being made in the field.
Epidemiology and Comorbidities
The epidemiology of AD has evolved over the last few decades, with emerging trends and novel insights into the burden of disease.1 In a recent cross-sectional study on the epidemiology of AD in children aged 6 to 11 years, the 1-year diagnosed AD prevalence estimates worldwide included the following: United States, 10.0%; Canada, 13.3%; the EU5 Countries, 15.5%; Japan, 10.3%; and all countries studied, 12.2%.2 Another recent paper that analyzed data from the Fragile Families and Child Wellbeing Study showed that the prevalence and persistence of AD in urban US children was 15.0%.3Although pediatric AD may spontaneously remit over time, disease continuing into adolescence and adulthood is common. Paternoster et al4 studied the longitudinal course of AD in children from 2 birth cohort prospective studies, showing distinct AD phenotypes having differing course trajectories over time. Disease subsets included patients with early-onset-persistent and early-onset-late-resolving disease.4 Whether phenotyping or subgroup analysis can be used to predict disease course or risk for development of comorbidities is unknown, but it is interesting to consider how such work could influence tailoring of specific therapies to early disease presentation.
Atopic dermatitis poses a serious public health burden owing to its high prevalence, considerable morbidity and disability, increased health care utilization, and cost of care.1 Recent studies have found notably higher rates of multiple medical and mental health comorbidities in both children and adults with AD, including infections, atopic comorbidities (eg, allergic rhinitis, asthma, food allergies), eye diseases (eg, keratitis, conjunctivitis, keratoconus), and possible cardiovascular diseases and autoimmune disorders.1,5-9 Allergic comorbidities are quite common in pediatric AD patients.10 In a recent study examining the efficacy and safety of dupilumab monotherapy in 251 adolescents with moderate to severe inadequately controlled AD, most had comorbid type 2 diseases including asthma (53.6%), food allergies (60.8%), and allergic rhinitis (65.6%).11
Quality of Life/Life Impact of AD
Pediatric AD has a major impact on the quality of life of patients and their families.12 The well-being and development of children are strongly influenced by the physical and psychosocial health of parents/guardians. Two studies by Ramirez and colleagues13,14 published in 2019 examined sleep disturbances and exhaustion in mothers of children with AD. Data for the studies came from the Avon Longitudinal Study of Parents and Children. Children with active AD reported worse sleep quality than those without AD, with nearly 50% higher odds of sleep-quality disturbances. Analysis of the cohort data from 11,649 mother-child pairs who were followed up with a time-varying measure of child AD activity and severity as well as self-reported maternal sleep measures repeated at multiple time points for children aged 6 months to 11 years showed that mothers of children with AD reported difficulty falling asleep, subjectively insufficient sleep, and daytime exhaustion throughout the first 11 years of childhood.13,14 These data suggest that sleep disturbance may be a family affair.
A cross-sectional, real-world study on the burden of AD in children aged 6 to 11 years assessed by self-report demonstrated a substantial and multidimensional impact of AD, including itch, sleep disturbance, skin pain, and health-related quality-of-life impact, as well as comorbidities and school productivity losses. The burden associated with AD was remarkable and increased with disease severity.15
Drucker et al16 completed a comprehensive literature review on the burden of AD, summarized as a report for the National Eczema Association. Quality-of-life impact on pediatric patients included high rates of emotional distress; social isolation; depression; limitations in activities due to lesions with fear of triggers; and behavioral problems such as irritability, crying, and sleep disturbance resulting in difficulty performing at school.16 The psychological impact on children as well as emotional and behavioral difficulties may impact the ability for parents/guardians to implement treatment plans.17
There is a striking association between mental health disorders and AD in the US pediatric population, with a clear dose-dependent relationship that has been observed between the prevalence of a mental health disorder and the reported severity of the skin disease. Data suggest children with AD may be at increased risk for developing mental health disorders. The National Survey of Children’s Health found statistically significant increases in the likelihood of attention deficit hyperactivity disorder (odds ratio [OR], 1.87), depression (OR, 1.81), anxiety (OR, 1.77), conduct disorder (OR, 1.87), and autism (OR, 3.04).6
Evolving Practices and Therapies
Bathing Practices
There has long been much controversy regarding best bathing habits for patients with AD. In a 2009 study, cutaneous hydration was quantified after various bathing and moisturizing regimens.18 The study showed clear benefits of emollient application on skin hydration, either after bathing or without bathing. Bathing followed by emollient applications did not decrease skin hydration in contrast to bathing without emollient application.18
There are limited studies evaluating bathing frequency in pediatric patients, and many families receive conflicting information regarding best practice. In one study that surveyed 354 parents, more than 75% of parents/guardians who had seen multiple providers for their child’s AD reported a substantial amount of confusion and frustration from conflicting advice on bathing frequency.19 Cardona et al20 undertook a randomized clinical trial of frequent bathing and moisturizing vs less-frequent bathing and moisturizing in pediatric patients with AD aged 6 months to 11 years. Patients were divided into 2 groups: 1 being bathed twice daily with immediate moisturizer application and the other being bathed twice weekly followed by moisturization, then a switch to the other method. Patients used standardized topical corticosteroids (TCSs) in both groups. There were significant improvements in scoring AD and other objective measures during the frequent bathing time period vs infrequent bathing; in the group that bathed more frequently, SCORAD (SCORing Atopic Dermatitis) decreased by 21.2 compared with the group that bathed less frequently (95% confidence interval, 14.9-27.6; P<.0001). These findings suggest that more-frequent bathing with immediate moisturization is superior as an acute treatment intervention for improving AD disease severity in comparison to less-frequent bathing with immediate moisturization.20
Expanding Treatment Options
Topical Phosphodiesterase Inhibitors
There are several new and evolving topical therapies in AD. Crisaborole ointment 2% is a steroid-free phosphodiesterase inhibitor approved in 2016 by the US Food and Drug Administration (FDA) for mild to moderate AD in patients aged 2 years and older. A recent multicenter, open-label, single-arm study in 137 infants (CrisADe CARE 1) evaluated the pharmacokinetics and efficacy of crisaborole ointment 2% applied twice daily for 4 weeks in pediatric patients aged 3 months to less than 24 months of age with mild to moderate AD.21 The study had 2 cohorts: one with a minimum of 5% body surface area involvement and another (the pharmacokinetic cohort) with a minimum of 35% body surface area involvement. Both cohorts demonstrated similar efficacy data. From baseline to day 29, the mean percentage change in eczema area and severity index (EASI) score was −57.5%, and an investigator global assessment (IGA) score of clear or almost clear with at least a 2-grade improvement was achieved in 30.2% of patients. Crisaborole systemic exposures in infants were comparable with those in patients aged 2 years or older. Patients tolerated crisaborole well, with a 4% rate of burning, which was similar to other studies in children and adults but perhaps lower than seen in clinical practice. Pharmacokinetic studies did not show any remarkable noticeable concern with accumulation of propylene glycol absorption.21
Based on the CrisADe CARE 1 study data, in March 2020 the FDA extended the indication of crisaborole ointment 2% from a prior lower age limit of 24 months to approval for use in treating mild to moderate AD in children as young as 3 months, making it the first nonsteroidal topical anti-inflammatory medication to be approved in children younger than 2 years in the United States.
Evolving Topical Therapies
Topical Janus Kinase Inhibitors
Ruxolitinib is a potent inhibitor of Janus kinase 1 (JAK-1) and Janus kinase 2 (JAK-2) and has been developed in topical formulations. In recent phase 3 clinical trials of patients with AD aged 12 years and older with mild to moderate disease (TRuE-AD1 and TRuE-AD2), more than half of the patients treated with either ruxolitinib cream in a 0.75% or 1.5% concentration reached EASI-75 after 8 weeks of treatment.22 Additionally, more patients treated with topical ruxolitinib reached an IGA score of clear to almost clear than patients treated with vehicle at the end of treatment. Thus far, it appears to be very well tolerated, significantly decreases EASI score (P<.0001), and improves overall pruritus.22
Delgocitinib is a topical pan-JAK inhibitor that blocks several cytokine-signaling cascade pathways. It was first developed and approved in Japan in an ointment formulation for use in patients with AD aged 16 years and older.23 The efficacy and safety profile of delgocitinib is currently being evaluated in pediatric patients with AD in Japan. In a recent phase 2 clinical study of 103 Japanese patients aged 2 to 15 years with moderate to severe AD, patients were randomized to receive either delgocitinib ointment in 0.25% or 0.5% concentrations or vehicle ointment twice daily for 4 weeks. The proportion of patients with a modified EASI-75 score was 38.2% (13/34) in the 0.25% group and 50.0% (17/34) in the 0.5% group vs 8.6% (3/35) in the placebo group. More patients treated with delgocitinib ointment received an IGA score of clear or almost clear than patients treated with vehicle at the end of treatment. Overall, both delgocitinib groups demonstrated superior improvement in clinical symptoms and signs without notable side effects.24
Tapinarof
Tapinarof is a topical therapeutic aryl hydrocarbon receptor agonist. In a recent phase 2 randomized study of 2 concentrations and 2 frequencies of tapinarof cream vs vehicle in 247 randomized patients aged 12 to 65 years with moderate to severe disease, tapinarof demonstrated greater success with both concentrations than vehicle at all visits beyond week 2.25 Additionally, in patients treated with tapinarof cream 1%, nearly 50% reached an IGA score of clear to almost clear with at least a 2-grade improvement. More than 50% of patients achieved EASI-75 improvement at 12 weeks of treatment with tapinarof cream 1% used daily. These findings suggest that tapinarof may be an efficacious and well-tolerated treatment for both adolescents and adults with AD; however, large confirmation trials are needed to further investigate.25
Systemic Treatments
Oral JAK Inhibitors
Some of the most exciting novel therapies include several oral JAK inhibitors that target different combinations of kinases and have been shown to decrease AD severity and symptoms. Some of these agents have indications in other disease states, such as baricitinib and upadacitinib, which are both FDA approved for the treatment of rheumatoid arthritis, whereas others, such as abrocitinib, have been studied specifically for AD.
Although some agents have only been studied in adults to date, others have included adolescents in their core studies, such as abrocitinib, which received Breakthrough Therapy designation from the FDA for the treatment of patients with moderate to severe AD in February 2018. In recent phase 3 trials of patients aged 12 years and older with moderate to severe AD (JADE MONO-1 and JADE MONO-2), both doses of abrocitinib improved the IGA and EASI-75 outcomes compared with placebo.26 Additional studies will be conducted to further investigate the relative efficacy and safety in patients younger than 18 years.
Biologics
Dupilumab is a fully human monoclonal antibody that inhibits IL-4 and IL-13 signaling without suppressing the immune system. It is approved for use in patients aged 12 years and older with moderate to severe asthma and in adults with chronic rhinosinusitis with nasal polyposis. It is the first biologic to show positive results in the moderate to severe pediatric AD population. There are now extended data available exhibiting sustained benefit in adolescent patients who were continued on dupilumab therapy, evidenced by further improvement in EASI scores at the 1-year mark.27
Recently, dupilumab received approval for use in patients aged 6 to 11 years, making it the first biologic for AD to be approved for use in patients younger than 12 years. The expedited FDA approval was based on the phase 3 results in which the efficacy and safety of dupilumab combined with TCSs were compared to TCSs alone (N=367).28 In this trial, more than twice as many children achieved clear or almost clear skin and more than 4 times as many achieved itch reduction with dupilumab plus TCSs than with TCSs alone. Three-quarters of patients receiving dupilumab at the subsequently approved dosing achieved at least a 75% improvement in overall disease.28 An additional study is being conducted that includes pediatric patients aged 6 months to younger than 6 years (ClinicalTrials.gov Identifier NCT03346434).
Future Directions in Pediatric AD
Our review summarizes only some of the agents under clinical investigation for use in pediatric AD. Early treatment to establish excellent long-term disease control with aggressive topical regimens or with systemic agents may alter the course of AD and influence the development of comorbidities, though this has not yet been shown in clinical studies. The long-term impact of early treatment, along with many other intriguing issues, will be studied more in the near future.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paper presented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1.
- Paternoster L, Savenije OEM, Heron J, et al. IJ Allergy Clin Immunol. 2018;141:964-971.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Yaghmaie P, Koudelka CW, Simpson Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
- Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468.
- al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.
- Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074-1081.
- Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821-838.
- Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis.
- Quality of life in families with children with atopic dermatitis. Pediatr Dermatol. 2016;33:28-32.
- Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556-563.
- Association of atopic dermatitis with sleep quality in children.
- Weidinger S, Simpson EL, Eckert L, et al. The patient-reported disease burden in pediatric patients with atopic dermatitis: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paperpresented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag. 2018;11:289-298.
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Kempe E, Jain N, Cardona I. Bathing frequency recommendations for pediatric atopic dermatitis: are we adding to parental frustration? Ann Allergy Asthma Immunol. 2013;111:298‐299.
- Cardona ID, Kempe EE, Lary C, et al. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8:1014‐1021.
- Gower , Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275-284.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment atopic dermatitis: results from two phase 3, randomized, double-blind studies. Presented at: 2nd Annual Revolutionizing Atopic Dermatitis Conference; April 5, 2020; Chicago, IL.
- Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609‐615.
- Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575‐1583.
- Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89‐98.e3.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85‐96.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published online June 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.054.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paper presented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1.
- Paternoster L, Savenije OEM, Heron J, et al. IJ Allergy Clin Immunol. 2018;141:964-971.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Yaghmaie P, Koudelka CW, Simpson Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428-433.
- Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468.
- al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.
- Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074-1081.
- Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821-838.
- Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis.
- Quality of life in families with children with atopic dermatitis. Pediatr Dermatol. 2016;33:28-32.
- Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556-563.
- Association of atopic dermatitis with sleep quality in children.
- Weidinger S, Simpson EL, Eckert L, et al. The patient-reported disease burden in pediatric patients with atopic dermatitis: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Paperpresented at: American Academy of Dermatology Annual Meeting; March 20-24, 2020; Denver, CO.
- The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Mitchell AE. Bidirectional relationships between psychological health and dermatological conditions in children. Psychol Res Behav Manag. 2018;11:289-298.
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Kempe E, Jain N, Cardona I. Bathing frequency recommendations for pediatric atopic dermatitis: are we adding to parental frustration? Ann Allergy Asthma Immunol. 2013;111:298‐299.
- Cardona ID, Kempe EE, Lary C, et al. Frequent versus infrequent bathing in pediatric atopic dermatitis: a randomized clinical trial. J Allergy Clin Immunol Pract. 2020;8:1014‐1021.
- Gower , Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to <24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21:275-284.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment atopic dermatitis: results from two phase 3, randomized, double-blind studies. Presented at: 2nd Annual Revolutionizing Atopic Dermatitis Conference; April 5, 2020; Chicago, IL.
- Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609‐615.
- Nakagawa H, Nemoto O, Igarashi A, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:1575‐1583.
- Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89‐98.e3.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85‐96.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published online June 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.054.
Practice Points
- There has been tremendous growth in our understanding of atopic dermatitis, with further insight into epidemiology, the impact on quality of life of affected individuals and their families, best bathing practices, and expanding treatment options.
- There are several novel topical and systemic agents recently approved and in late-stage clinical development programs that are evolving therapeutic approaches to pediatric disease.
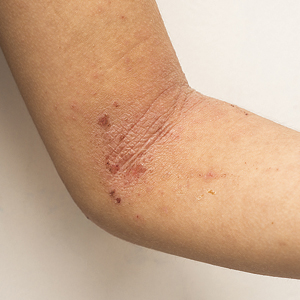
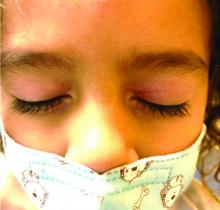
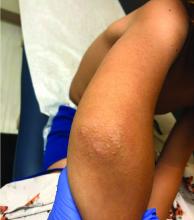
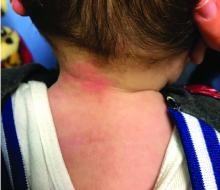
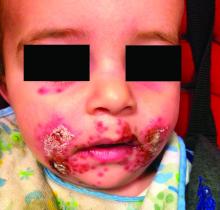
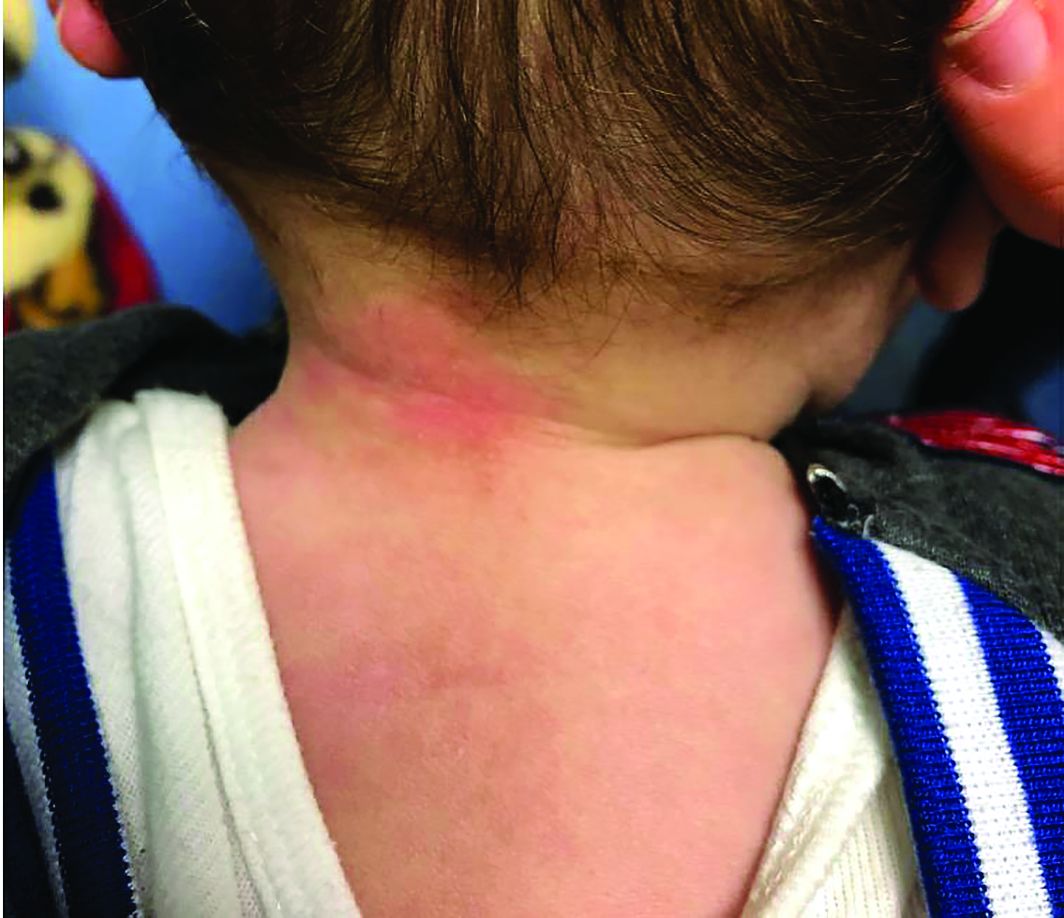
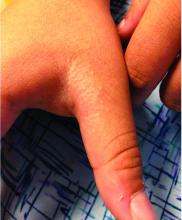
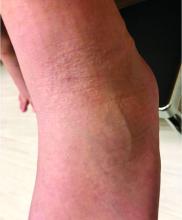
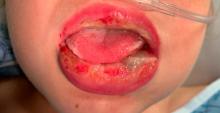
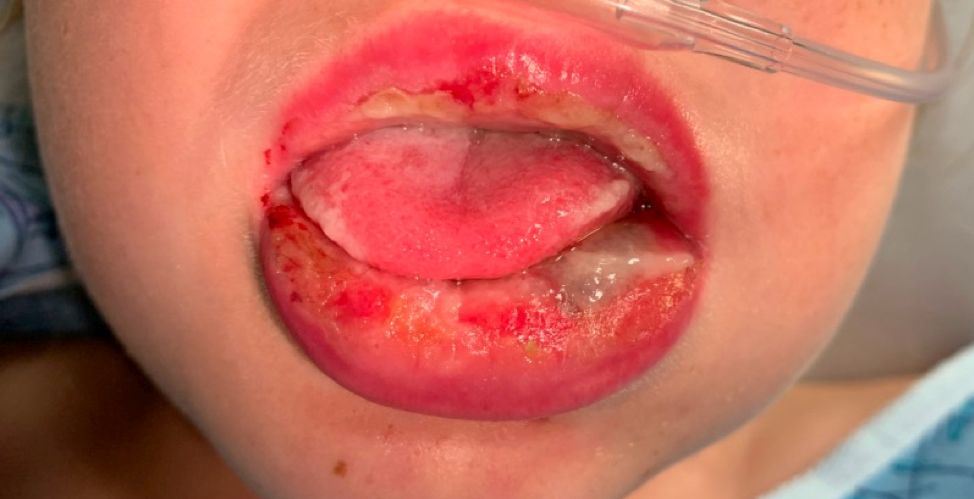
Four-year-old boy presents with itchy rash on face, extremities
Contact dermatitis is an eczematous, pruritic eruption caused by direct contact with a substance and an irritant or allergic reaction. While it may not be contagious or life-threatening, contact dermatitis may be tremendously uncomfortable and impactful. Contact dermatitis may occur from exposure to chemicals in soaps, shampoos, cosmetics, metals, plants and topical products, and medications. The hallmark of contact dermatitis is localized eczematous reactions on the portion of the body that has been directly exposed to the reaction-causing substance. – often with oozing and crusting.
Irritant contact dermatitis is the most common type, which occurs when a substance damages the skin’s outer protective layer and does not require prior exposure or sensitization. Allergic contact dermatitis (ACD) can develop after exposure and sensitization, with an external allergen triggering an acute inflammatory response.1 Common causes of ACD include nickel, cobalt, gold, chromium, poison ivy/oak/sumac, cosmetics/personal care products that contain formaldehyde, fragrances, topical medications (anesthetics, antibiotics, corticosteroids), baby wipes, sunscreens, latex materials, protective equipment, soap/cleansers, resins, and acrylics. Among children, nickel sulfate, ammonium persulfate, gold sodium thiosulfate, thimerosal, and toluene-2,5-diamine are the most common sensitizers. Rarely, ACD can be triggered by something that enters the body through foods, flavorings, medicine, or medical or dental procedures (systemic contact dermatitis).
An Id reaction, or autoeczematization, is a generalized acute cutaneous reaction to a variety of stimuli, including infectious and inflammatory skin conditions such as contact dermatitis, stasis dermatitis, or other eczematous dermatitis.3 Id reactions usually are preceded by a preexisting dermatitis. Lesions are, by definition, at a site distant from the primary infection or dermatitis. They often are distributed symmetrically. Papular or papular-vesicular lesions of the extremities and or trunk are common in children.
Our patient had evidence of a localized periocular contact dermatitis reaction that preceded the symmetric papular, eczematous eruption consistent with an id reaction. Our patient was prescribed hydrocortisone 2.5 % ointment for the eyes and triamcinolone 0.1% ointment for the rash on the body, which resulted in significant improvement.
Rosacea is a chronic and relapsing inflammatory skin disorder that primarily involves the central face. Common clinical features include facial erythema, telangiectasias, and inflammatory papules or pustules. Ocular involvement may occur in the presence or absence of cutaneous manifestations. Patients may report the presence of ocular foreign body sensation, burning, photophobia, blurred vision, redness, and tearing. Ocular disease is usually bilateral and is not proportional to the severity of the skin disease.4 Common skin findings are blepharitis, lid margin telangiectasia, tear abnormalities, meibomian gland inflammation, frequent chalazion, bilateral hordeolum, conjunctivitis, and, rarely, corneal ulcers and vascularization. Our patient initially did have bilateral hordeolum in what may seem to be ocular rosacea. However, given the use of a recent topical antibiotic with subsequent eczematous rash of the eyelids and then resulting distant rash on the body 1week later made the rash likely allergic contact dermatitis with id reaction.
Seborrheic dermatitis is a chronic, relapsing, and usually mild form of dermatitis that occurs in infants and in adults. The severity may vary from minimal, asymptomatic scaliness of the scalp (dandruff) to more widespread involvement. It is usually characterized by well-demarcated, erythematous plaques with greasy-looking, yellowish scales distributed on areas rich in sebaceous glands, such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas.
Psoriasis typically affects the outside of the elbows, knees, or scalp, although it can appear on any location. It tends to go through cycles, flaring for a few weeks or months, then subsiding for a while or going into remission. Ocular involvement is a well known manifestation of psoriasis.5 Psoriatic lesions of the eyelid are rare, even in the erythrodermic variant of the disease. Occasionally, pustular psoriasis may involve the eyelids, with typical psoriatic lesions visible on the skin and lid margin. The reason for the relative sparing of the eyelid skin in patients with psoriasis is unknown. Other manifestations include meibomian gland dysfunction, decreased tear film break-up time, a nonspecific conjunctivitis, and corneal disease secondary to lid disease such as trichiasis.
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis, papular acrodermatitis of childhood, and infantile papular acrodermatitis, is a self-limited skin disorder that most often occurs in young children. Viral infections are common GCS precipitating factors . GCS typically manifests as a symmetric, papular eruption, often with larger (3- to 10-mm) flat topped papulovesicles. Classic sites of involvement include the cheeks, buttocks, and extensor surfaces of the forearms and legs. GCS may be pruritic or asymptomatic, and papules typically resolve spontaneously within 2 months. Occasionally, GCS persists for longer periods. The eyelid lesions and localized pattern, with the absence of larger symmetric papules of the buttocks and legs, was not consistent with papular acrodermatitis of childhood.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. They had no conflicts of interest to disclose. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol 2016 Jun; 74(6):1043-54.
2. Pediatr Dermatol 2016 Jul; 33(4):399-404.
3. Evans M & Bronson D. (2019) Id Reaction (Autoeczematization). Retrieved from emedicine.medscape.com/article/1049760-overview.
4. Curr Opin Ophthalmol. 2004 Dec;15(6):499-502.
5. Clin Dermatol. Mar-Apr 2016;34(2):146-50.
Contact dermatitis is an eczematous, pruritic eruption caused by direct contact with a substance and an irritant or allergic reaction. While it may not be contagious or life-threatening, contact dermatitis may be tremendously uncomfortable and impactful. Contact dermatitis may occur from exposure to chemicals in soaps, shampoos, cosmetics, metals, plants and topical products, and medications. The hallmark of contact dermatitis is localized eczematous reactions on the portion of the body that has been directly exposed to the reaction-causing substance. – often with oozing and crusting.
Irritant contact dermatitis is the most common type, which occurs when a substance damages the skin’s outer protective layer and does not require prior exposure or sensitization. Allergic contact dermatitis (ACD) can develop after exposure and sensitization, with an external allergen triggering an acute inflammatory response.1 Common causes of ACD include nickel, cobalt, gold, chromium, poison ivy/oak/sumac, cosmetics/personal care products that contain formaldehyde, fragrances, topical medications (anesthetics, antibiotics, corticosteroids), baby wipes, sunscreens, latex materials, protective equipment, soap/cleansers, resins, and acrylics. Among children, nickel sulfate, ammonium persulfate, gold sodium thiosulfate, thimerosal, and toluene-2,5-diamine are the most common sensitizers. Rarely, ACD can be triggered by something that enters the body through foods, flavorings, medicine, or medical or dental procedures (systemic contact dermatitis).
An Id reaction, or autoeczematization, is a generalized acute cutaneous reaction to a variety of stimuli, including infectious and inflammatory skin conditions such as contact dermatitis, stasis dermatitis, or other eczematous dermatitis.3 Id reactions usually are preceded by a preexisting dermatitis. Lesions are, by definition, at a site distant from the primary infection or dermatitis. They often are distributed symmetrically. Papular or papular-vesicular lesions of the extremities and or trunk are common in children.
Our patient had evidence of a localized periocular contact dermatitis reaction that preceded the symmetric papular, eczematous eruption consistent with an id reaction. Our patient was prescribed hydrocortisone 2.5 % ointment for the eyes and triamcinolone 0.1% ointment for the rash on the body, which resulted in significant improvement.
Rosacea is a chronic and relapsing inflammatory skin disorder that primarily involves the central face. Common clinical features include facial erythema, telangiectasias, and inflammatory papules or pustules. Ocular involvement may occur in the presence or absence of cutaneous manifestations. Patients may report the presence of ocular foreign body sensation, burning, photophobia, blurred vision, redness, and tearing. Ocular disease is usually bilateral and is not proportional to the severity of the skin disease.4 Common skin findings are blepharitis, lid margin telangiectasia, tear abnormalities, meibomian gland inflammation, frequent chalazion, bilateral hordeolum, conjunctivitis, and, rarely, corneal ulcers and vascularization. Our patient initially did have bilateral hordeolum in what may seem to be ocular rosacea. However, given the use of a recent topical antibiotic with subsequent eczematous rash of the eyelids and then resulting distant rash on the body 1week later made the rash likely allergic contact dermatitis with id reaction.
Seborrheic dermatitis is a chronic, relapsing, and usually mild form of dermatitis that occurs in infants and in adults. The severity may vary from minimal, asymptomatic scaliness of the scalp (dandruff) to more widespread involvement. It is usually characterized by well-demarcated, erythematous plaques with greasy-looking, yellowish scales distributed on areas rich in sebaceous glands, such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas.
Psoriasis typically affects the outside of the elbows, knees, or scalp, although it can appear on any location. It tends to go through cycles, flaring for a few weeks or months, then subsiding for a while or going into remission. Ocular involvement is a well known manifestation of psoriasis.5 Psoriatic lesions of the eyelid are rare, even in the erythrodermic variant of the disease. Occasionally, pustular psoriasis may involve the eyelids, with typical psoriatic lesions visible on the skin and lid margin. The reason for the relative sparing of the eyelid skin in patients with psoriasis is unknown. Other manifestations include meibomian gland dysfunction, decreased tear film break-up time, a nonspecific conjunctivitis, and corneal disease secondary to lid disease such as trichiasis.
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis, papular acrodermatitis of childhood, and infantile papular acrodermatitis, is a self-limited skin disorder that most often occurs in young children. Viral infections are common GCS precipitating factors . GCS typically manifests as a symmetric, papular eruption, often with larger (3- to 10-mm) flat topped papulovesicles. Classic sites of involvement include the cheeks, buttocks, and extensor surfaces of the forearms and legs. GCS may be pruritic or asymptomatic, and papules typically resolve spontaneously within 2 months. Occasionally, GCS persists for longer periods. The eyelid lesions and localized pattern, with the absence of larger symmetric papules of the buttocks and legs, was not consistent with papular acrodermatitis of childhood.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. They had no conflicts of interest to disclose. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol 2016 Jun; 74(6):1043-54.
2. Pediatr Dermatol 2016 Jul; 33(4):399-404.
3. Evans M & Bronson D. (2019) Id Reaction (Autoeczematization). Retrieved from emedicine.medscape.com/article/1049760-overview.
4. Curr Opin Ophthalmol. 2004 Dec;15(6):499-502.
5. Clin Dermatol. Mar-Apr 2016;34(2):146-50.
Contact dermatitis is an eczematous, pruritic eruption caused by direct contact with a substance and an irritant or allergic reaction. While it may not be contagious or life-threatening, contact dermatitis may be tremendously uncomfortable and impactful. Contact dermatitis may occur from exposure to chemicals in soaps, shampoos, cosmetics, metals, plants and topical products, and medications. The hallmark of contact dermatitis is localized eczematous reactions on the portion of the body that has been directly exposed to the reaction-causing substance. – often with oozing and crusting.
Irritant contact dermatitis is the most common type, which occurs when a substance damages the skin’s outer protective layer and does not require prior exposure or sensitization. Allergic contact dermatitis (ACD) can develop after exposure and sensitization, with an external allergen triggering an acute inflammatory response.1 Common causes of ACD include nickel, cobalt, gold, chromium, poison ivy/oak/sumac, cosmetics/personal care products that contain formaldehyde, fragrances, topical medications (anesthetics, antibiotics, corticosteroids), baby wipes, sunscreens, latex materials, protective equipment, soap/cleansers, resins, and acrylics. Among children, nickel sulfate, ammonium persulfate, gold sodium thiosulfate, thimerosal, and toluene-2,5-diamine are the most common sensitizers. Rarely, ACD can be triggered by something that enters the body through foods, flavorings, medicine, or medical or dental procedures (systemic contact dermatitis).
An Id reaction, or autoeczematization, is a generalized acute cutaneous reaction to a variety of stimuli, including infectious and inflammatory skin conditions such as contact dermatitis, stasis dermatitis, or other eczematous dermatitis.3 Id reactions usually are preceded by a preexisting dermatitis. Lesions are, by definition, at a site distant from the primary infection or dermatitis. They often are distributed symmetrically. Papular or papular-vesicular lesions of the extremities and or trunk are common in children.
Our patient had evidence of a localized periocular contact dermatitis reaction that preceded the symmetric papular, eczematous eruption consistent with an id reaction. Our patient was prescribed hydrocortisone 2.5 % ointment for the eyes and triamcinolone 0.1% ointment for the rash on the body, which resulted in significant improvement.
Rosacea is a chronic and relapsing inflammatory skin disorder that primarily involves the central face. Common clinical features include facial erythema, telangiectasias, and inflammatory papules or pustules. Ocular involvement may occur in the presence or absence of cutaneous manifestations. Patients may report the presence of ocular foreign body sensation, burning, photophobia, blurred vision, redness, and tearing. Ocular disease is usually bilateral and is not proportional to the severity of the skin disease.4 Common skin findings are blepharitis, lid margin telangiectasia, tear abnormalities, meibomian gland inflammation, frequent chalazion, bilateral hordeolum, conjunctivitis, and, rarely, corneal ulcers and vascularization. Our patient initially did have bilateral hordeolum in what may seem to be ocular rosacea. However, given the use of a recent topical antibiotic with subsequent eczematous rash of the eyelids and then resulting distant rash on the body 1week later made the rash likely allergic contact dermatitis with id reaction.
Seborrheic dermatitis is a chronic, relapsing, and usually mild form of dermatitis that occurs in infants and in adults. The severity may vary from minimal, asymptomatic scaliness of the scalp (dandruff) to more widespread involvement. It is usually characterized by well-demarcated, erythematous plaques with greasy-looking, yellowish scales distributed on areas rich in sebaceous glands, such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas.
Psoriasis typically affects the outside of the elbows, knees, or scalp, although it can appear on any location. It tends to go through cycles, flaring for a few weeks or months, then subsiding for a while or going into remission. Ocular involvement is a well known manifestation of psoriasis.5 Psoriatic lesions of the eyelid are rare, even in the erythrodermic variant of the disease. Occasionally, pustular psoriasis may involve the eyelids, with typical psoriatic lesions visible on the skin and lid margin. The reason for the relative sparing of the eyelid skin in patients with psoriasis is unknown. Other manifestations include meibomian gland dysfunction, decreased tear film break-up time, a nonspecific conjunctivitis, and corneal disease secondary to lid disease such as trichiasis.
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis, papular acrodermatitis of childhood, and infantile papular acrodermatitis, is a self-limited skin disorder that most often occurs in young children. Viral infections are common GCS precipitating factors . GCS typically manifests as a symmetric, papular eruption, often with larger (3- to 10-mm) flat topped papulovesicles. Classic sites of involvement include the cheeks, buttocks, and extensor surfaces of the forearms and legs. GCS may be pruritic or asymptomatic, and papules typically resolve spontaneously within 2 months. Occasionally, GCS persists for longer periods. The eyelid lesions and localized pattern, with the absence of larger symmetric papules of the buttocks and legs, was not consistent with papular acrodermatitis of childhood.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. They had no conflicts of interest to disclose. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol 2016 Jun; 74(6):1043-54.
2. Pediatr Dermatol 2016 Jul; 33(4):399-404.
3. Evans M & Bronson D. (2019) Id Reaction (Autoeczematization). Retrieved from emedicine.medscape.com/article/1049760-overview.
4. Curr Opin Ophthalmol. 2004 Dec;15(6):499-502.
5. Clin Dermatol. Mar-Apr 2016;34(2):146-50.
A 4-year-old healthy male with no significant prior medical history presents for evaluation of "itchy bumps" on the face and extremities of 2 weeks' duration.
The child was well until around 2 and a half weeks ago when he presented for evaluation of two lesions on the lower eyelids, diagnosed as hordeolum (a stye). He was prescribed ofloxacin ophthalmic solution.
One week later he developed bilateral itchy red eyes with red, thickened areas on the upper lids, followed several days later by pruritic papules on the ears, wrists, elbows, knees, and ankles. His mother used Vaseline for the eyelids for 1 week with no improvement. Physical exam at the dermatologist's office showed mild erythema, induration, and lichenification of the upper eyelids, and bilateral periocular eczematous patches with overlying scale. Subtle papules were evident on the elbows and feet.
A 7-month-old male presents with perioral rash and fever
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at pdnews@mdedge.com.
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at pdnews@mdedge.com.
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at pdnews@mdedge.com.
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Rash on hands and feet
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at pdnews@mdedge.com.
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
A 9-year-old healthy Kuwaiti male with no significant past medical history presents with a rash on his hands and feet that has been present for 3 years.
His mother reports that he has been seen by dermatologists in various countries and was last seen by a dermatologist in Kuwait 3 years ago. At that time, he was told that it was dryness and advised to not shower daily. Since then he has been taking showers three times weekly and using Cetaphil once weekly without improvement. He was seen by his pediatrician 6 months ago, diagnosed with xerosis, and was given hydrocortisone 2.5% to use twice daily, again without any improvement.
The rash is not itchy, and he has no oral lesions or nail involvement. Exam revealed lichenoid papules on bilateral dorsal hands and feet, bilateral upper arms, bilateral axilla, lower abdomen, and left upper chest.
Pneumonia with tender, dry, crusted lips
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at pdnews@mdedge.com.
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at pdnews@mdedge.com.
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at pdnews@mdedge.com.
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.