User login
Consensus Statement Supporting the Presence of Onsite Radiation Oncology Departments at VHA Medical Centers
Radiation therapy, along with surgery and systemic therapy, is a primary therapeutic modality for cancer management. At least half of cancer patients receive radiation as part of their treatment regimen.1 Multiple studies demonstrate that radiotherapy is underutilized worldwide.2 One reason for underutilization of radiotherapy globally is poor access to this treatment modality. Factors that contribute to poor access include long wait times for consultation, delays in treatment initiation, distance to a treatment facility, and poor coordination of care.
Taskforce Findings
The presence of onsite radiation oncology and its impact on utilization of radiotherapy is poorly studied. The Veterans Health Administration (VHA) Palliative Radiotherapy Taskforce recently conducted a survey to determine the barriers to referral and timeliness of treatment for palliative radiotherapy within the VHA.3 Key findings of this study comparing centers with onsite radiation departments with centers without onsite radiation departments include:
a. Radiation consults are more likely to be completed within 1 week of consult request at centers with onsite radiation therapy (68% vs 31%, respectively; P = .01).
b. Centers with onsite radiation therapy more frequently deliver emergent treatment within 24 hours for patients with spinal cord compression, an emergency condition in which prompt radiation can prevent or minimize long-term neurologic disability (94% vs 70%, respectively; P = .01).
c. Referring practitioners with onsite radiation departments are less likely to report difficulty contacting a radiation oncologist as a barrier to referral for palliative radiotherapy (0% vs 20%, respectively; P = .006).
d. Referring practitioners with onsite radiotherapy report patient travel as a barrier to referral for palliative radiotherapy less frequently (28% vs 71%, respectively; P < .001).
e. Practitioners with onsite radiation oncology departments are more likely to have multidisciplinary tumor boards (31% vs 3%, respectively; P = .01) and are more likely to be influenced by radiation oncology recommendations at tumor boards (69% vs 44%, respectively; P = .02).
Based on the findings of this study, the VHA Palliative Radiotherapy Taskforce has prepared this consensus statement regarding the importance of onsite radiation oncology departments at VHA medical centers. More information regarding our 5 key findings and their implications for patient care are as follows:
Timeliness of Radiation Oncology Consultation
Delays in radiation oncology consultation, which can also delay treatment initiation, are associated with poor satisfaction among both patients and referring clinicians.4 Wait times have been identified as a barrier to utilization of radiotherapy by both patients and clinicians.5,6 Furthermore, delays in initiation of definitive therapy have been associated with worse outcomes, including worse overall survival.7,8 Our survey study demonstrates that consults for palliative radiotherapy are occurring in a more timely manner at centers with onsite radiation departments. Radiation oncology consults are more frequently completed within 1 week at centers with onsite radiation oncology departments compared with centers without onsite radiation oncology departments (68% vs 31%, P = .01). This trend would likely be seen for nonpalliative, definitive cases as well. The presence of radiation oncology departments onsite at VHA medical centers is an important component of timely care for veterans to optimize outcomes of cancer treatment.
Timely Delivery of Radiotherapy for Oncologic Emergencies
There are a few scenarios in which emergent radiation treatment, within 24 hours, is indicated. These include malignant spinal cord compression, uncal herniation from brain metastasis, superior vena cava syndrome, and tumor hemorrhage.9 Studies on management of metastatic spinal cord compression demonstrate that delays in treatment are associated with reduced ambulation10 as well as loss of sphincter function and incontinence.11
Our study demonstrates that VHA medical centers with onsite radiotherapy more frequently deliver radiotherapy within 24 hours for patients with metastatic spinal cord compression. This timely delivery of treatment is critical to optimizing functional status and quality of life in patients requiring treatment for oncologic emergencies. Revisiting treatment pathways for such situations at regular intervals is crucial given that residents and staff may rotate and be unfamiliar with emergency protocols.
Communication With Radiation Oncologists
Several studies have demonstrated that the inability to contact a radiation oncologist and poor communication result in decreased referrals for palliative radiotherapy.12,13 Our study demonstrates that onsite radiation oncology is associated with improved ability to contact a radiation oncologist. About 20% of clinicians at facilities without onsite radiation oncology reported difficulty contacting a radiation oncologist, compared with 0% at facilities with onsite radiation departments (P = .006).
It is possible that increased radiation oncology presence at VHA medical centers, through attenuation of barriers related to contacting a radiation oncologist and improved communication, would lead to increased use of radiotherapy. Increased communication between referring clinicians and radiation oncologists also can help with education of those clinicians making the referral. Since knowledge gaps have been identified in multiple studies as a barrier to referral for radiotherapy, such communication and increased education on the role of radiotherapy could increase use.12-14
Patient Travel
Patient ability to travel was the most commonly reported barrier (81%) to referral for palliative radiotherapy in our study. Travel time and transportation difficulties have been established in multiple studies as barriers to radiotherapy for both definitive and palliative management.15-18 Travel for radiotherapy was much less frequently reported as a barrier among respondents with onsite radiation oncology departments compared with those without onsite radiation departments (28% vs 71%, respectively; P < .001).
It is therefore possible that expansion of VHA radiation oncology services, allowing for provision of onsite radiotherapy at more VHA facilities, would reduce travel burden. Increasing travel accommodations for patients and provision of patient lodging on hospital campuses, which is already offered at some VHA medical centers (ie, Fisher House Foundation), could also help attenuate this barrier.
Multidisciplinary Tumor Boards
Our study demonstrates that centers with onsite radiation departments more frequently hold multidisciplinary tumor boards compared with centers without radiation departments (31% vs 3%, respectively; P = .01). Multidisciplinary tumor boards allow subspecialties to meet regularly to communicate about patient care and can help mitigate barriers related to communication and education of the referring health care practitioners.
As cases are discussed in multidisciplinary tumor boards, health care practitioners have the opportunity to make recommendations and provide education on potential benefits and/or downsides of treatments offered by their respective specialties. Several studies have demonstrated that cases discussed at multidisciplinary tumor boards are more likely to be referred for radiation therapy.19-21 Furthermore, multidisciplinary tumor boards have been associated with improved treatment outcomes.22
Conclusions
In this consensus statement the VHA Palliative Radiotherapy Taskforce recommends the optimization of use of radiotherapy within the VHA. Radiation oncology services should be maintained where present in the VHA, with consideration for expansion of services to additional facilities. Telehealth should be used to expedite consults and treatment. Hypofractionation should be used, when appropriate, to ease travel burden. Options for transportation services and onsite housing, or hospitalization, should be understood by practitioners and offered to patients to mitigate barriers related to travel.
1. Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024
2. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045(15)00222-3
3. Gutt R, Malhotra S, Hagan MP, et al. Palliative radiotherapy within the Veterans Health Administration: barriers to referral and timeliness of treatment. JCO Oncol Pract. 2021;17(12):e1913-e1922. doi:10.1200/OP.20.00981
4. Agazaryan N, Chow P, Lamb J, et al. The timeliness initiative: continuous process improvement for prompt initiation of radiation therapy treatment. Adv Radiat Oncol. 2020;5(5):1014-1021. Published 2020 Mar 10. doi:10.1016/j.adro.2020.01.007
5. Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. Published 2012 Oct 12. doi:10.1186/1748-717X-7-167
6. Hanna TP, Richardson H, Peng Y, Kong W, Zhang-Salomons J, Mackillop WJ. A population-based study of factors affecting the use of radiotherapy for endometrial cancer. Clin Oncol (R Coll Radiol). 2012;24(8):e113-e124. doi:10.1016/j.clon.2012.01.007
7. Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi:10.1002/cncr.31533
8. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. Published 2020 Dec 1. doi:10.1001/jamanetworkopen.2020.30072
9. Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol. 2009;16(4):55-60. doi:10.3747/co.v16i4.352
10. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976). 2016;41 (Suppl 20):S224-S230. doi:10.1097/BRS.0000000000001827
11. Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21. doi:10.1136/bmj.317.7150.18
12. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101-106. doi:10.1016/j.radonc.2005.11.008
13. McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137. doi:10.1097/PPO.0b013e31804675d4
14. Chierchini S, Ingrosso G, Saldi S, Stracci F, Aristei C. Physician and patient barriers to radiotherapy service access: treatment referral implications. Cancer Manag Res. 2019;11:8829-8833. Published 2019 Oct 7. doi:10.2147/CMAR.S168941
15. Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Travel, treatment choice, and survival among breast cancer patients: a population-based analysis. Womens Health Rep (New Rochelle). 2021;2(1):1-10. Published 2021 Jan 11. doi:10.1089/whr.2020.0094
16. Yang DD, Muralidhar V, Mahal BA, et al. Travel distance as a barrier to receipt of adjuvant radiation therapy after radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953-959. doi:10.1097/COC.0000000000000410
17. Sundaresan P, King M, Stockler M, Costa D, Milross C. Barriers to radiotherapy utilization: Consumer perceptions of issues influencing radiotherapy-related decisions. Asia Pac J Clin Oncol. 2017;13(5):e489-e496. doi:10.1111/ajco.12579
18. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi:10.1634/theoncologist.2015-0110
19. Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838-841. doi:10.1111/j.1445-5994.2009.02019.x
20. Brännström F, Bjerregaard JK, Winbladh A, et al. Multidisciplinary team conferences promote treatment according to guidelines in rectal cancer. Acta Oncol. 2015;54(4):447-453. doi:10.3109/0284186X.2014.952387
21. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. doi:10.1016/j.ctrv.2015.11.007
22. Freytag M, Herrlinger U, Hauser S, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer. 2020;20(1):355. Published 2020 Apr 28. doi:10.1186/s12885-020-06809-1
Radiation therapy, along with surgery and systemic therapy, is a primary therapeutic modality for cancer management. At least half of cancer patients receive radiation as part of their treatment regimen.1 Multiple studies demonstrate that radiotherapy is underutilized worldwide.2 One reason for underutilization of radiotherapy globally is poor access to this treatment modality. Factors that contribute to poor access include long wait times for consultation, delays in treatment initiation, distance to a treatment facility, and poor coordination of care.
Taskforce Findings
The presence of onsite radiation oncology and its impact on utilization of radiotherapy is poorly studied. The Veterans Health Administration (VHA) Palliative Radiotherapy Taskforce recently conducted a survey to determine the barriers to referral and timeliness of treatment for palliative radiotherapy within the VHA.3 Key findings of this study comparing centers with onsite radiation departments with centers without onsite radiation departments include:
a. Radiation consults are more likely to be completed within 1 week of consult request at centers with onsite radiation therapy (68% vs 31%, respectively; P = .01).
b. Centers with onsite radiation therapy more frequently deliver emergent treatment within 24 hours for patients with spinal cord compression, an emergency condition in which prompt radiation can prevent or minimize long-term neurologic disability (94% vs 70%, respectively; P = .01).
c. Referring practitioners with onsite radiation departments are less likely to report difficulty contacting a radiation oncologist as a barrier to referral for palliative radiotherapy (0% vs 20%, respectively; P = .006).
d. Referring practitioners with onsite radiotherapy report patient travel as a barrier to referral for palliative radiotherapy less frequently (28% vs 71%, respectively; P < .001).
e. Practitioners with onsite radiation oncology departments are more likely to have multidisciplinary tumor boards (31% vs 3%, respectively; P = .01) and are more likely to be influenced by radiation oncology recommendations at tumor boards (69% vs 44%, respectively; P = .02).
Based on the findings of this study, the VHA Palliative Radiotherapy Taskforce has prepared this consensus statement regarding the importance of onsite radiation oncology departments at VHA medical centers. More information regarding our 5 key findings and their implications for patient care are as follows:
Timeliness of Radiation Oncology Consultation
Delays in radiation oncology consultation, which can also delay treatment initiation, are associated with poor satisfaction among both patients and referring clinicians.4 Wait times have been identified as a barrier to utilization of radiotherapy by both patients and clinicians.5,6 Furthermore, delays in initiation of definitive therapy have been associated with worse outcomes, including worse overall survival.7,8 Our survey study demonstrates that consults for palliative radiotherapy are occurring in a more timely manner at centers with onsite radiation departments. Radiation oncology consults are more frequently completed within 1 week at centers with onsite radiation oncology departments compared with centers without onsite radiation oncology departments (68% vs 31%, P = .01). This trend would likely be seen for nonpalliative, definitive cases as well. The presence of radiation oncology departments onsite at VHA medical centers is an important component of timely care for veterans to optimize outcomes of cancer treatment.
Timely Delivery of Radiotherapy for Oncologic Emergencies
There are a few scenarios in which emergent radiation treatment, within 24 hours, is indicated. These include malignant spinal cord compression, uncal herniation from brain metastasis, superior vena cava syndrome, and tumor hemorrhage.9 Studies on management of metastatic spinal cord compression demonstrate that delays in treatment are associated with reduced ambulation10 as well as loss of sphincter function and incontinence.11
Our study demonstrates that VHA medical centers with onsite radiotherapy more frequently deliver radiotherapy within 24 hours for patients with metastatic spinal cord compression. This timely delivery of treatment is critical to optimizing functional status and quality of life in patients requiring treatment for oncologic emergencies. Revisiting treatment pathways for such situations at regular intervals is crucial given that residents and staff may rotate and be unfamiliar with emergency protocols.
Communication With Radiation Oncologists
Several studies have demonstrated that the inability to contact a radiation oncologist and poor communication result in decreased referrals for palliative radiotherapy.12,13 Our study demonstrates that onsite radiation oncology is associated with improved ability to contact a radiation oncologist. About 20% of clinicians at facilities without onsite radiation oncology reported difficulty contacting a radiation oncologist, compared with 0% at facilities with onsite radiation departments (P = .006).
It is possible that increased radiation oncology presence at VHA medical centers, through attenuation of barriers related to contacting a radiation oncologist and improved communication, would lead to increased use of radiotherapy. Increased communication between referring clinicians and radiation oncologists also can help with education of those clinicians making the referral. Since knowledge gaps have been identified in multiple studies as a barrier to referral for radiotherapy, such communication and increased education on the role of radiotherapy could increase use.12-14
Patient Travel
Patient ability to travel was the most commonly reported barrier (81%) to referral for palliative radiotherapy in our study. Travel time and transportation difficulties have been established in multiple studies as barriers to radiotherapy for both definitive and palliative management.15-18 Travel for radiotherapy was much less frequently reported as a barrier among respondents with onsite radiation oncology departments compared with those without onsite radiation departments (28% vs 71%, respectively; P < .001).
It is therefore possible that expansion of VHA radiation oncology services, allowing for provision of onsite radiotherapy at more VHA facilities, would reduce travel burden. Increasing travel accommodations for patients and provision of patient lodging on hospital campuses, which is already offered at some VHA medical centers (ie, Fisher House Foundation), could also help attenuate this barrier.
Multidisciplinary Tumor Boards
Our study demonstrates that centers with onsite radiation departments more frequently hold multidisciplinary tumor boards compared with centers without radiation departments (31% vs 3%, respectively; P = .01). Multidisciplinary tumor boards allow subspecialties to meet regularly to communicate about patient care and can help mitigate barriers related to communication and education of the referring health care practitioners.
As cases are discussed in multidisciplinary tumor boards, health care practitioners have the opportunity to make recommendations and provide education on potential benefits and/or downsides of treatments offered by their respective specialties. Several studies have demonstrated that cases discussed at multidisciplinary tumor boards are more likely to be referred for radiation therapy.19-21 Furthermore, multidisciplinary tumor boards have been associated with improved treatment outcomes.22
Conclusions
In this consensus statement the VHA Palliative Radiotherapy Taskforce recommends the optimization of use of radiotherapy within the VHA. Radiation oncology services should be maintained where present in the VHA, with consideration for expansion of services to additional facilities. Telehealth should be used to expedite consults and treatment. Hypofractionation should be used, when appropriate, to ease travel burden. Options for transportation services and onsite housing, or hospitalization, should be understood by practitioners and offered to patients to mitigate barriers related to travel.
Radiation therapy, along with surgery and systemic therapy, is a primary therapeutic modality for cancer management. At least half of cancer patients receive radiation as part of their treatment regimen.1 Multiple studies demonstrate that radiotherapy is underutilized worldwide.2 One reason for underutilization of radiotherapy globally is poor access to this treatment modality. Factors that contribute to poor access include long wait times for consultation, delays in treatment initiation, distance to a treatment facility, and poor coordination of care.
Taskforce Findings
The presence of onsite radiation oncology and its impact on utilization of radiotherapy is poorly studied. The Veterans Health Administration (VHA) Palliative Radiotherapy Taskforce recently conducted a survey to determine the barriers to referral and timeliness of treatment for palliative radiotherapy within the VHA.3 Key findings of this study comparing centers with onsite radiation departments with centers without onsite radiation departments include:
a. Radiation consults are more likely to be completed within 1 week of consult request at centers with onsite radiation therapy (68% vs 31%, respectively; P = .01).
b. Centers with onsite radiation therapy more frequently deliver emergent treatment within 24 hours for patients with spinal cord compression, an emergency condition in which prompt radiation can prevent or minimize long-term neurologic disability (94% vs 70%, respectively; P = .01).
c. Referring practitioners with onsite radiation departments are less likely to report difficulty contacting a radiation oncologist as a barrier to referral for palliative radiotherapy (0% vs 20%, respectively; P = .006).
d. Referring practitioners with onsite radiotherapy report patient travel as a barrier to referral for palliative radiotherapy less frequently (28% vs 71%, respectively; P < .001).
e. Practitioners with onsite radiation oncology departments are more likely to have multidisciplinary tumor boards (31% vs 3%, respectively; P = .01) and are more likely to be influenced by radiation oncology recommendations at tumor boards (69% vs 44%, respectively; P = .02).
Based on the findings of this study, the VHA Palliative Radiotherapy Taskforce has prepared this consensus statement regarding the importance of onsite radiation oncology departments at VHA medical centers. More information regarding our 5 key findings and their implications for patient care are as follows:
Timeliness of Radiation Oncology Consultation
Delays in radiation oncology consultation, which can also delay treatment initiation, are associated with poor satisfaction among both patients and referring clinicians.4 Wait times have been identified as a barrier to utilization of radiotherapy by both patients and clinicians.5,6 Furthermore, delays in initiation of definitive therapy have been associated with worse outcomes, including worse overall survival.7,8 Our survey study demonstrates that consults for palliative radiotherapy are occurring in a more timely manner at centers with onsite radiation departments. Radiation oncology consults are more frequently completed within 1 week at centers with onsite radiation oncology departments compared with centers without onsite radiation oncology departments (68% vs 31%, P = .01). This trend would likely be seen for nonpalliative, definitive cases as well. The presence of radiation oncology departments onsite at VHA medical centers is an important component of timely care for veterans to optimize outcomes of cancer treatment.
Timely Delivery of Radiotherapy for Oncologic Emergencies
There are a few scenarios in which emergent radiation treatment, within 24 hours, is indicated. These include malignant spinal cord compression, uncal herniation from brain metastasis, superior vena cava syndrome, and tumor hemorrhage.9 Studies on management of metastatic spinal cord compression demonstrate that delays in treatment are associated with reduced ambulation10 as well as loss of sphincter function and incontinence.11
Our study demonstrates that VHA medical centers with onsite radiotherapy more frequently deliver radiotherapy within 24 hours for patients with metastatic spinal cord compression. This timely delivery of treatment is critical to optimizing functional status and quality of life in patients requiring treatment for oncologic emergencies. Revisiting treatment pathways for such situations at regular intervals is crucial given that residents and staff may rotate and be unfamiliar with emergency protocols.
Communication With Radiation Oncologists
Several studies have demonstrated that the inability to contact a radiation oncologist and poor communication result in decreased referrals for palliative radiotherapy.12,13 Our study demonstrates that onsite radiation oncology is associated with improved ability to contact a radiation oncologist. About 20% of clinicians at facilities without onsite radiation oncology reported difficulty contacting a radiation oncologist, compared with 0% at facilities with onsite radiation departments (P = .006).
It is possible that increased radiation oncology presence at VHA medical centers, through attenuation of barriers related to contacting a radiation oncologist and improved communication, would lead to increased use of radiotherapy. Increased communication between referring clinicians and radiation oncologists also can help with education of those clinicians making the referral. Since knowledge gaps have been identified in multiple studies as a barrier to referral for radiotherapy, such communication and increased education on the role of radiotherapy could increase use.12-14
Patient Travel
Patient ability to travel was the most commonly reported barrier (81%) to referral for palliative radiotherapy in our study. Travel time and transportation difficulties have been established in multiple studies as barriers to radiotherapy for both definitive and palliative management.15-18 Travel for radiotherapy was much less frequently reported as a barrier among respondents with onsite radiation oncology departments compared with those without onsite radiation departments (28% vs 71%, respectively; P < .001).
It is therefore possible that expansion of VHA radiation oncology services, allowing for provision of onsite radiotherapy at more VHA facilities, would reduce travel burden. Increasing travel accommodations for patients and provision of patient lodging on hospital campuses, which is already offered at some VHA medical centers (ie, Fisher House Foundation), could also help attenuate this barrier.
Multidisciplinary Tumor Boards
Our study demonstrates that centers with onsite radiation departments more frequently hold multidisciplinary tumor boards compared with centers without radiation departments (31% vs 3%, respectively; P = .01). Multidisciplinary tumor boards allow subspecialties to meet regularly to communicate about patient care and can help mitigate barriers related to communication and education of the referring health care practitioners.
As cases are discussed in multidisciplinary tumor boards, health care practitioners have the opportunity to make recommendations and provide education on potential benefits and/or downsides of treatments offered by their respective specialties. Several studies have demonstrated that cases discussed at multidisciplinary tumor boards are more likely to be referred for radiation therapy.19-21 Furthermore, multidisciplinary tumor boards have been associated with improved treatment outcomes.22
Conclusions
In this consensus statement the VHA Palliative Radiotherapy Taskforce recommends the optimization of use of radiotherapy within the VHA. Radiation oncology services should be maintained where present in the VHA, with consideration for expansion of services to additional facilities. Telehealth should be used to expedite consults and treatment. Hypofractionation should be used, when appropriate, to ease travel burden. Options for transportation services and onsite housing, or hospitalization, should be understood by practitioners and offered to patients to mitigate barriers related to travel.
1. Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024
2. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045(15)00222-3
3. Gutt R, Malhotra S, Hagan MP, et al. Palliative radiotherapy within the Veterans Health Administration: barriers to referral and timeliness of treatment. JCO Oncol Pract. 2021;17(12):e1913-e1922. doi:10.1200/OP.20.00981
4. Agazaryan N, Chow P, Lamb J, et al. The timeliness initiative: continuous process improvement for prompt initiation of radiation therapy treatment. Adv Radiat Oncol. 2020;5(5):1014-1021. Published 2020 Mar 10. doi:10.1016/j.adro.2020.01.007
5. Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. Published 2012 Oct 12. doi:10.1186/1748-717X-7-167
6. Hanna TP, Richardson H, Peng Y, Kong W, Zhang-Salomons J, Mackillop WJ. A population-based study of factors affecting the use of radiotherapy for endometrial cancer. Clin Oncol (R Coll Radiol). 2012;24(8):e113-e124. doi:10.1016/j.clon.2012.01.007
7. Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi:10.1002/cncr.31533
8. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. Published 2020 Dec 1. doi:10.1001/jamanetworkopen.2020.30072
9. Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol. 2009;16(4):55-60. doi:10.3747/co.v16i4.352
10. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976). 2016;41 (Suppl 20):S224-S230. doi:10.1097/BRS.0000000000001827
11. Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21. doi:10.1136/bmj.317.7150.18
12. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101-106. doi:10.1016/j.radonc.2005.11.008
13. McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137. doi:10.1097/PPO.0b013e31804675d4
14. Chierchini S, Ingrosso G, Saldi S, Stracci F, Aristei C. Physician and patient barriers to radiotherapy service access: treatment referral implications. Cancer Manag Res. 2019;11:8829-8833. Published 2019 Oct 7. doi:10.2147/CMAR.S168941
15. Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Travel, treatment choice, and survival among breast cancer patients: a population-based analysis. Womens Health Rep (New Rochelle). 2021;2(1):1-10. Published 2021 Jan 11. doi:10.1089/whr.2020.0094
16. Yang DD, Muralidhar V, Mahal BA, et al. Travel distance as a barrier to receipt of adjuvant radiation therapy after radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953-959. doi:10.1097/COC.0000000000000410
17. Sundaresan P, King M, Stockler M, Costa D, Milross C. Barriers to radiotherapy utilization: Consumer perceptions of issues influencing radiotherapy-related decisions. Asia Pac J Clin Oncol. 2017;13(5):e489-e496. doi:10.1111/ajco.12579
18. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi:10.1634/theoncologist.2015-0110
19. Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838-841. doi:10.1111/j.1445-5994.2009.02019.x
20. Brännström F, Bjerregaard JK, Winbladh A, et al. Multidisciplinary team conferences promote treatment according to guidelines in rectal cancer. Acta Oncol. 2015;54(4):447-453. doi:10.3109/0284186X.2014.952387
21. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. doi:10.1016/j.ctrv.2015.11.007
22. Freytag M, Herrlinger U, Hauser S, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer. 2020;20(1):355. Published 2020 Apr 28. doi:10.1186/s12885-020-06809-1
1. Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024
2. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045(15)00222-3
3. Gutt R, Malhotra S, Hagan MP, et al. Palliative radiotherapy within the Veterans Health Administration: barriers to referral and timeliness of treatment. JCO Oncol Pract. 2021;17(12):e1913-e1922. doi:10.1200/OP.20.00981
4. Agazaryan N, Chow P, Lamb J, et al. The timeliness initiative: continuous process improvement for prompt initiation of radiation therapy treatment. Adv Radiat Oncol. 2020;5(5):1014-1021. Published 2020 Mar 10. doi:10.1016/j.adro.2020.01.007
5. Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. Published 2012 Oct 12. doi:10.1186/1748-717X-7-167
6. Hanna TP, Richardson H, Peng Y, Kong W, Zhang-Salomons J, Mackillop WJ. A population-based study of factors affecting the use of radiotherapy for endometrial cancer. Clin Oncol (R Coll Radiol). 2012;24(8):e113-e124. doi:10.1016/j.clon.2012.01.007
7. Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi:10.1002/cncr.31533
8. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. Published 2020 Dec 1. doi:10.1001/jamanetworkopen.2020.30072
9. Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol. 2009;16(4):55-60. doi:10.3747/co.v16i4.352
10. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976). 2016;41 (Suppl 20):S224-S230. doi:10.1097/BRS.0000000000001827
11. Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21. doi:10.1136/bmj.317.7150.18
12. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101-106. doi:10.1016/j.radonc.2005.11.008
13. McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137. doi:10.1097/PPO.0b013e31804675d4
14. Chierchini S, Ingrosso G, Saldi S, Stracci F, Aristei C. Physician and patient barriers to radiotherapy service access: treatment referral implications. Cancer Manag Res. 2019;11:8829-8833. Published 2019 Oct 7. doi:10.2147/CMAR.S168941
15. Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Travel, treatment choice, and survival among breast cancer patients: a population-based analysis. Womens Health Rep (New Rochelle). 2021;2(1):1-10. Published 2021 Jan 11. doi:10.1089/whr.2020.0094
16. Yang DD, Muralidhar V, Mahal BA, et al. Travel distance as a barrier to receipt of adjuvant radiation therapy after radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953-959. doi:10.1097/COC.0000000000000410
17. Sundaresan P, King M, Stockler M, Costa D, Milross C. Barriers to radiotherapy utilization: Consumer perceptions of issues influencing radiotherapy-related decisions. Asia Pac J Clin Oncol. 2017;13(5):e489-e496. doi:10.1111/ajco.12579
18. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi:10.1634/theoncologist.2015-0110
19. Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838-841. doi:10.1111/j.1445-5994.2009.02019.x
20. Brännström F, Bjerregaard JK, Winbladh A, et al. Multidisciplinary team conferences promote treatment according to guidelines in rectal cancer. Acta Oncol. 2015;54(4):447-453. doi:10.3109/0284186X.2014.952387
21. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. doi:10.1016/j.ctrv.2015.11.007
22. Freytag M, Herrlinger U, Hauser S, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer. 2020;20(1):355. Published 2020 Apr 28. doi:10.1186/s12885-020-06809-1
Skull Base Regeneration During Treatment With Chemoradiation for Nasopharyngeal Carcinoma: A Case Report
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
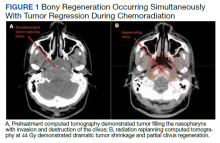
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
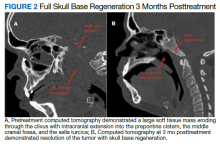
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
Radiotherapeutic Care of Patients With Stage IV Lung Cancer with Thoracic Symptoms in the Veterans Health Administration (FULL)
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
Palliative Radiotherapy for the Management of Metastatic Cancer
In recent years, there has been increasing interest in palliative care for patients with cancer at the end of life. Up to 23% of patients have metastatic disease at presentation, and symptoms from metastatic lesions can cause significant anxiety and impair patients’ quality of life (QOL).1
Palliative radiotherapy (RT) plays a valuable role in the management of metastatic disease to relieve tumor-related symptoms. Although palliative RT does not provide a chance for a cure, it improves QOL and may prolong survival time.2-4 An estimated 20% to 50% of radiation courses are prescribed with palliative intent, because RT is highly effective in providing symptom relief, and the toxicity associated with palliative doses is typically mild.5,6 Palliative RT can be used to manage bone and brain metastases, prevent or treat spinal cord compression, and manage numerous tumor-related symptoms, such as pain and bleeding in patients with terminal cancer.
Palliative RT for bone and brain metastases is supported by high-quality evidence and is considered one of the most effective and cost-effective options available.7,8 This article aims to review the role of RT in treating 3 conditions commonly encountered in patients with metastatic disease—bone metastases, spinal cord compression, and brain metastases—and to emphasize the importance of timely integration of RT for optimal results.
Bone Metastases
About 80% of patients with metastatic solid tumors develop bone metastases, and about 350,000 deaths are linked to bone metastases in the U.S. each year.9 Osseous
metastases can lead to pain, fracture, hypercalcemia, and spinal cord compression. The primary modality for treatment of pain and prevention of morbidity from bone metastases is external beam RT.10
The likelihood of bone pain relief with palliative RT is 60% to 80%, and 30% to 40% of patients achieving complete pain relief. Randomized studies have shown multiple-dose and fractionation regimens provided effective symptom relief for bone metastases. Most commonly used regimens include a single fraction of 8 gray (Gy) delivered in 1 treatment, 20 Gy in 5 fractions delivered daily over 1 week, and 30 Gy in 10 fractions delivered over 2 weeks. Treatment with a single fraction improves access to treatment and patient convenience, whereas more prolonged courses have been associated with lower rates of retreatment.11,12 Regarding the higher rate of retreatment with single-fraction RT, no clear evidence exists that this is due to a less durable pain response or lower level of pain relief.13
There has been recent interest in using predictive models to estimate life expectancy to avoid long courses of RT at the end of life.14,15 Shorter treatment courses of 8 Gyonce or 20 Gy in 5 fractions are particularly valuable for patients with a life expectancy < 3 months to avoid long courses of treatment, and thereby improve QOL as patients transition into hospice. A recent survey demonstrated that 93% of radiation oncologists within the VHA are willing to prescribe short courses of RT consisting of ≤ 6 fractions, and 76% have experience with single-fraction RT.16 These findings are in contradiction to the findings in the non-VA radiation oncology community, in which < 10% of patients with uncomplicated bone metastases are treated with a single fraction.17,18
In addition to providing pain relief, RT is used in the treatment of impending fractures either, adjuvant after surgical stabilization or alone for lower risk lesions.19 Factors that impact fracture risk include location of the metastasis (weight-bearing bones, such as femurs, which are at particularly high risk), length of bone involved, and extent of cortical involvement. Mirels’ scoring system was developed to predict fracture risk in patients with bone metastasis, based on 4 criteria: the
extent of cortical involvement, the location of the metastasis, the osteolytic vs osteoblastic appearance of the lesion, and the degree of pain.20 Surgical fixation can be considered, based on the total score and corresponding fracture risk. When appropriate, surgical stabilization should be considered by an orthopedic surgeon prior to initiating RT.
Postoperative RT after surgical stabilization has been associated with a reduced rate of secondary surgical procedures as well as with improved functional status.21 Radiotherapy promotes remineralization and bone healing and prevents the loss of surgical fixation by treating any residual tumor. A retrospective review of 60 patients with metastatic disease in weight-bearing bones with pathologic fracture or impending pathologic fracture demonstrated that surgery followed by RT was associated with improved functional status as well as with improved overall survival (OS).22,23 For patients in whom surgery is not indicated, the consulting radiation oncologist should consider factors such as the location of the metastasis in weight-bearing vs nonweight bearing bones, the size and extent of the metastasis, and associated symptoms when making a treatment recommendation. In patients at fracture risk from bone metastases, bisphosphonates should also be considered as part of the treatment regimen.24
Spinal Cord Compression
About 5% to 10% of patients diagnosed with cancer will develop spinal cord compression during the course of their disease.25 Spinal cord compression is considered a medical emergency that can result in significant pain and neurologic symptoms, including weakness, paralysis, parasthesias, and incontinence. Early treatment of spinal cord compression can prevent onset or progression of these symptoms; furthermore, early treatment prior to loss of ambulation is associated with improved long-term ambulatory function.26,27
Treatment decisions for spinal metastases with an associated concern for cord compression should be made after a consultation with both a neurosurgeon and a radiation oncologist. Early initiation of steroids is recommended to aid in tumor shrinkage for potential symptom relief.28 A standard way to administer dexamethasone is with a 10-mg loading dose followed by 16 mg per day, divided into 4 doses of 4 mg. Higher steroid doses showed no benefit in a prospective randomized trial comparing 96 mg with 16 mg of dexamethasone daily.29
Surgical decompression should be considered initial management of spinal cord compression. For patients treated surgically, local RT is indicated postoperatively as well. Randomized data show that surgery followed by RT provides better ambulatory function than does RT alone in patients with paralysis of < 2 days’ duration.30 Some patients with metastatic disease are not good candidates for surgery due to comorbidities, poor performance status, life expectancy < 3 months, or multilevel spinal involvement.
In patients who are not operative candidates, radiation alone is an appropriate alternative. However, several factors need consideration in deciding whether to manage cord compression with surgery followed by RT vs RT alone. These factors include life expectancy, tumor type (myeloma and lymphoma are more radiosensitive), interval since tumor diagnosis, and the presence of visceral metastases.31 Factors favoring surgical decompression plus postoperative RT over RT alone include spinal instability, KPS (Karnofsky Performance Status) > 70, radio-resistant tumor histology, minimal metastatic disease, and projected survival > 3 months.10
For patients managed with RT alone, early diagnosis and treatment is associated with improved outcomes. A prospective study of patients treated with RT without surgery for spinal cord compression demonstrated that 82% of patients experienced back pain relief, 76% achieved improvement in or preservation of ambulation, and 44% of patients with sphincter dysfunction experienced improvement with treatment.32 Patients with certain tumor histologies, such as myeloma, breast cancer, and prostate cancer, had better responses to RT.32
In the setting of spinal cord compression, longer courses of RT may provide better local control than do shorter courses.33 Therefore, longer courses of RT, such as 30 Gy in 10 fractions delivered over 2 weeks, are often preferred in cases of spinal cord compression treated with definitive RT as well as after surgical decompression. However, overall life expectancy is an important factor considered by the treating radiation oncologist when selecting a short course vs a longer course of RT.
In the instance of painful vertebral body metastases without spinal cord compression, a new subset analysis of the Radiation Therapy Oncology Group (RTOG) 9714 randomized trial indicated that single fraction RT (8 Gy) is just as effective as multiple fractions (30 Gy in 10 fractions), with this study demonstrating comparable rates of pain relief and narcotic use in both groups 3 months after RT.34 Advantages to the single-fraction plan compared with those of multiple fractions include mitigation of logistic concerns for patients and family at the end of life and less acute adverse effects.
Brain Metastases
An estimated 20% to 40% of patients with cancer develop brain metastases.35 The incidence of brain metastases has been rising most likely due to improved detection rates with magnetic resonance imaging (MRI) and improved cancer survival, because treatment regimens have improved with targeted chemotherapy and radiation techniques. Currently, the annual incidence of brain metastases is 170,000 to 200,000 in the U.S.36 Prognosis for these patients is poor, with median survival of 1 month without treatment and about 4 months with whole brain RT (WBRT) (Table).25,37-39
The goal of management for patients with brain metastases is to prevent or treat neurologic symptoms and to prolong survival. Treatment options include corticosteroids, WBRT, surgery, and stereotactic radiosurgery (SRS). Recommendations for treatment should involve both a radiation oncologist and neurosurgeon to determine the best treatment for an individual based on patient age, performance status, extent of systemic disease, and number of brain metastases. These prognostic factors that may predict life expectancy and impact treatment recommendations.40
Factors that have been correlated with improved survival include younger age, better performance status, fewer brain metastases, and lower burden of systemic disease.41,42 Prognostic assessment tools such as the Graded Prognostic Assessment and RTOG-Recursive Partitioning Analysis can be used to predict life expectancy in patients with brain metastases.41,43 However, routine use of these tools is lagging, as evidenced by a recent survey of VHA radiation oncologists. Use of these tools in the clinic will enhance the quality of end of life care and decision making.
Corticosteroids have classically been used in the treatment of brain metastases either alone for supportive care or in combination with RT. Steroids are recommended to provide symptom relief in patients with symptoms related to cerebral edema or mass effect.44 Steroids have been shown to mitigate edema and improve neurologic deficits in about two-thirds of patients with brain metastases.36,45 The effect of corticosteroids is thought to be mediated through inhibition of prostaglandin synthesis, reduction in vascular permeability, and anti-inflammatory properties.46 A common corticosteroid regimen is a 10-mg loading dose of dexamethasone, followed by 16 mg daily in divided doses. For patients without neurologic deficits or cerebral edema, it is reasonable to defer corticosteroid use only when patients are symptomatic.
In general, WBRT is considered an appropriate treatment option for patients with multiple brain metastases based on data suggesting an improvement in OS compared with the use of corticosteroids alone.47 Whole brain radiation has been shown to result in the improvement of baseline neurologic deficits or the prevention of further symptom progression.48 The partial or complete metastasis response rates are on the order of 60%.38 Tumor regression after WBRT has been associated with preservation of neurocognitive function as well as prolonged survival.49
For good prognosis patients with a single brain metastasis and good performance status, the use of surgery or radiosurgery added to WBRT has been associated with improved OS (Table). The RTOG 9508 randomized trial of WBRT with or without SRS demonstrated a survival advantage with SRS, with median survival times of 6.5 months with WBRT + SRS vs 4.9 months with WBRT alone.50 Similarly, a randomized trial evaluating WBRT alone compared with surgery followed by WBRT in patients
with good prognosis demonstrated significantly improved OS in the surgery group (median 40 weeks vs 15 weeks).51 In general, WBRT or postoperative RT to the tumor bed is still indicated after surgical resection, based on randomized data showing a reduction in tumor bed recurrence with postoperative RT.52
For patients with only 1 to 3 brain metastases and a favorable prognosis, surgery and SRS can be considered treatment options, oftentimes with WBRT. The EORTC randomized trial of patients with 1 to 3 brain metastases was designed to determine the benefit of WBRT after treatment with surgery or SRS. In this study, 119 patients underwent SRS and 160 patients underwent surgical resection.53 Both groups of patients were randomized to observation vs adjuvant WBRT. This study demonstrated reduced rates of intracranial relapse with WBRT, however, without any change in OS. Although there is concern that WBRT may impair cognitive function with no clear survival benefit after surgery or SRS, WBRT does reduce recurrence rates in the brain and the need for further treatment.54 Therefore, decisions regarding WBRT in such a setting should be made only after a detailed discussion with a radiation oncologist regarding risks vs benefits of treatment as part of the informed decision-making process.
Conclusions
Palliative RT plays an important role in the management of metastatic cancer to provide symptom relief and is a cost-effective treatment option for bone and brain metastases. Life expectancy and tumor characteristics should be considered when making treatment recommendations to ensure selection of regimens that complement patients’ unique situations. Timely referrals for treatment are important to optimize treatment results.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Porter A and David M. Palliative care for bone, spinal cord, brain, and liver metastases. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. 2nd ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2007:437-451.
2. Yamaguchi S, Ohguri T, Matsuki Y, et al. Palliative radiotherapy in patients with a poor performance status: the palliative effect is correlated with prolongation of survival time. Radiat Oncol. 2013;8:166.
3. Mac Manus MP, Matthews JP, Wada M, Wirth A, Worotniuk V, Ball DL. Unexpected long-term survival after low-dose palliative radiotherapy for non-small cell lung cancer. Cancer. 2006;106(5):1110-1116.
4. Rastogi M, Revannasiddaiah S, Gupta MK, Seam RK, Thakur P, Gupta M. When palliative treatment achieves more than palliation: instances of long-term survival after palliative radiotherapy. Indian J Palliat Care. 2012;18(2):117-121.
5. Nieder C, Pawinski A, Haukland E, Dokmo R, Phillipi I, Dalhaug A. Estimating need for palliative external beam radiotherapy in adult cancer patients. Int J Radiat Oncol Biol Phys. 2010;76(1):207-211.
6. Hoegler D. Radiotherapy for palliation of symptoms in incurable cancer. Curr Probl Cancer. 1997;21(3):129-183.
7. Expósito J, Jaén J, Alonso E, Tovar I. Use of palliative radiotherapy in brain and bone metastases (VARA II study). Radiat Oncol. 2012;7:131.
8. Konski A. Radiotherapy is a cost-effective palliative treatment for patients with bone metastasis from prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60(5):1373-1378.
9. Popovic M, den Hartogh M, Zhang L, et al. Review of international patterns of practice for the treatment of painful bone metastases with palliative radiotherapy from 1993 to 2013. Radiother Oncol. 2014;111(1):11-17.
10. Lutz S, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79(4):965-976.
11. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systemic review. J Clin Oncol. 2007;25(11):1423-1436.
12. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy—a systemic review of the randomized trials. Cochrane Database Syst Rev. 2004;(2):CD004721.
13. Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101-109.
14. Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectance in patients with metastatic cancer receiving palliative radiotherapy: the TEACHH model. Cancer. 2014;120(1):134-141.
15. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buccholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
16. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
17. Ellsworth SG, Alcorn SR, Hales RK, McNutt TR, DeWeese TL, Smith TJ. Patterns of care among patients receiving radiation therapy for bone metastases at a large academic institution. Int J Radiat Oncol Biol Phys. 2014;89(5):1100-1105.
18. Bradley NM, Husted J, Sey MS, et al. Review of patterns of practice and patients’ preferences in the treatment of bone metastases with palliative radiotherapy. Support Care Cancer. 2007;15(4):373-385.
19. Haidukewych GJ. Metastatic disease around the hip: maintaining quality of life. J Bone Joint Surg Br. 2012;94(11 suppl A):22-25.
20. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;(249):256-264.
21. Jacofsky DJ, Haidukewych GJ. Management of pathologic fractures of the proximal femur: state of the art. J Orthop Trauma. 2004;18(7):459-469.
22. Townsend PW, Rosenthal HG, Smalley SR, Cozad SC, Hassanein RE. Impact of postoperative radiation therapy and other perioperative factors on outcome after orthopedic stabilization of impending or pathologic fractures due to metastatic disease. J Clin Oncol. 1994;12(11):2345-2350.
23. Townsend PW, Smalley SR, Cozad SC, Rosenthal HG, Hassanein RE. Role of postoperative radiation therapy after stabilization of fractures caused by metastatic disease. Int J Radiat Oncol Biol Phys. 1995;31(1):43-49.
24. Farooki A. NCCN bone health task force: key recommendations. J Natl Compr Canc Netw. 2014;12(5 suppl):813-816.
25. Sejpal SV, Bhate A, Small W. Palliative radiation therapy in the management of brain metastases, spinal cord compression, and bone metastases. Semin Intervent Radiol. 2007;24(4):362-374.
26. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “all I care about is walking and living my life.” JAMA. 2008;299(8):937-946.
27. Kim RY, Spencer SA, Meredith RF, et al. Extradural spinal cord compression: analysis of factors determining functional prognosis—prospective study. Radiology. 1990;176(1):279-282.
28. Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21(2):140-143.
29. Graham PH, Capp A, Delaney G, et al. A pilot randomized comparison of dexamethasone 96 mg vs 16 mg per day for malignant spinal-cord compression treated by radiotherapy: TROG 01.05 Superdex study. Clin Oncol (R Coll Radiol). 2006;18(1):70-76.
30. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
31. Rades D, Huttenlocher S, Bajrovic A, et al. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int J Radiat Oncol Biol Phys. 2011;81(5):e861-e868.
32. Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32(4):959-967.
33. Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393.
34. Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer. 2013;119(4):888-896.
35. Wong J, Hird A, Kirou-Mauro, Napolskikh J, Chow E. Quality of life in brain metastases radiation trials: a literature review. Curr Oncol. 2008;15(5):25-45.
36. Nichols EM, Patchell RA, Regine WF, Kwok Y. Palliation of brain and spinal cord metastases. In: Halperin EC, Brady LW, Perez CA, Wazer DE, eds. Perez and Brady’s Principles and Practice of Radiation Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013:1974.
37. Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48(2):384-394.
38. Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24(8):1295-1304.
39. Sundström JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med. 1998;30(3):296-299.
40. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
41. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
42. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG databases. Int J Radiat Oncol Biol Phys. 2008;70(2):510-514.
43. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419-425.
44. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
45. Ruderman NB, Hall TC. Use of glucocorticoids in the palliative treatment of metastatic brain tumors. Cancer. 1965;18:298-306.
46. Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21(2):140-143.
47. Horton J, Baxter DH, Olson KB. The management of metastases to the brain by irradiation and corticosteroids. Am J Roentgenol Radium Ther Nucl Med. 1971;111(2)334-336.
48. Wong J, Hird A, Zhang L, et al. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(4):1125-1131.
49. Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260-1266.
50. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
51. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494-500.
52. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
53. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141.
54. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
In recent years, there has been increasing interest in palliative care for patients with cancer at the end of life. Up to 23% of patients have metastatic disease at presentation, and symptoms from metastatic lesions can cause significant anxiety and impair patients’ quality of life (QOL).1
Palliative radiotherapy (RT) plays a valuable role in the management of metastatic disease to relieve tumor-related symptoms. Although palliative RT does not provide a chance for a cure, it improves QOL and may prolong survival time.2-4 An estimated 20% to 50% of radiation courses are prescribed with palliative intent, because RT is highly effective in providing symptom relief, and the toxicity associated with palliative doses is typically mild.5,6 Palliative RT can be used to manage bone and brain metastases, prevent or treat spinal cord compression, and manage numerous tumor-related symptoms, such as pain and bleeding in patients with terminal cancer.
Palliative RT for bone and brain metastases is supported by high-quality evidence and is considered one of the most effective and cost-effective options available.7,8 This article aims to review the role of RT in treating 3 conditions commonly encountered in patients with metastatic disease—bone metastases, spinal cord compression, and brain metastases—and to emphasize the importance of timely integration of RT for optimal results.
Bone Metastases
About 80% of patients with metastatic solid tumors develop bone metastases, and about 350,000 deaths are linked to bone metastases in the U.S. each year.9 Osseous
metastases can lead to pain, fracture, hypercalcemia, and spinal cord compression. The primary modality for treatment of pain and prevention of morbidity from bone metastases is external beam RT.10
The likelihood of bone pain relief with palliative RT is 60% to 80%, and 30% to 40% of patients achieving complete pain relief. Randomized studies have shown multiple-dose and fractionation regimens provided effective symptom relief for bone metastases. Most commonly used regimens include a single fraction of 8 gray (Gy) delivered in 1 treatment, 20 Gy in 5 fractions delivered daily over 1 week, and 30 Gy in 10 fractions delivered over 2 weeks. Treatment with a single fraction improves access to treatment and patient convenience, whereas more prolonged courses have been associated with lower rates of retreatment.11,12 Regarding the higher rate of retreatment with single-fraction RT, no clear evidence exists that this is due to a less durable pain response or lower level of pain relief.13
There has been recent interest in using predictive models to estimate life expectancy to avoid long courses of RT at the end of life.14,15 Shorter treatment courses of 8 Gyonce or 20 Gy in 5 fractions are particularly valuable for patients with a life expectancy < 3 months to avoid long courses of treatment, and thereby improve QOL as patients transition into hospice. A recent survey demonstrated that 93% of radiation oncologists within the VHA are willing to prescribe short courses of RT consisting of ≤ 6 fractions, and 76% have experience with single-fraction RT.16 These findings are in contradiction to the findings in the non-VA radiation oncology community, in which < 10% of patients with uncomplicated bone metastases are treated with a single fraction.17,18
In addition to providing pain relief, RT is used in the treatment of impending fractures either, adjuvant after surgical stabilization or alone for lower risk lesions.19 Factors that impact fracture risk include location of the metastasis (weight-bearing bones, such as femurs, which are at particularly high risk), length of bone involved, and extent of cortical involvement. Mirels’ scoring system was developed to predict fracture risk in patients with bone metastasis, based on 4 criteria: the
extent of cortical involvement, the location of the metastasis, the osteolytic vs osteoblastic appearance of the lesion, and the degree of pain.20 Surgical fixation can be considered, based on the total score and corresponding fracture risk. When appropriate, surgical stabilization should be considered by an orthopedic surgeon prior to initiating RT.
Postoperative RT after surgical stabilization has been associated with a reduced rate of secondary surgical procedures as well as with improved functional status.21 Radiotherapy promotes remineralization and bone healing and prevents the loss of surgical fixation by treating any residual tumor. A retrospective review of 60 patients with metastatic disease in weight-bearing bones with pathologic fracture or impending pathologic fracture demonstrated that surgery followed by RT was associated with improved functional status as well as with improved overall survival (OS).22,23 For patients in whom surgery is not indicated, the consulting radiation oncologist should consider factors such as the location of the metastasis in weight-bearing vs nonweight bearing bones, the size and extent of the metastasis, and associated symptoms when making a treatment recommendation. In patients at fracture risk from bone metastases, bisphosphonates should also be considered as part of the treatment regimen.24
Spinal Cord Compression
About 5% to 10% of patients diagnosed with cancer will develop spinal cord compression during the course of their disease.25 Spinal cord compression is considered a medical emergency that can result in significant pain and neurologic symptoms, including weakness, paralysis, parasthesias, and incontinence. Early treatment of spinal cord compression can prevent onset or progression of these symptoms; furthermore, early treatment prior to loss of ambulation is associated with improved long-term ambulatory function.26,27
Treatment decisions for spinal metastases with an associated concern for cord compression should be made after a consultation with both a neurosurgeon and a radiation oncologist. Early initiation of steroids is recommended to aid in tumor shrinkage for potential symptom relief.28 A standard way to administer dexamethasone is with a 10-mg loading dose followed by 16 mg per day, divided into 4 doses of 4 mg. Higher steroid doses showed no benefit in a prospective randomized trial comparing 96 mg with 16 mg of dexamethasone daily.29
Surgical decompression should be considered initial management of spinal cord compression. For patients treated surgically, local RT is indicated postoperatively as well. Randomized data show that surgery followed by RT provides better ambulatory function than does RT alone in patients with paralysis of < 2 days’ duration.30 Some patients with metastatic disease are not good candidates for surgery due to comorbidities, poor performance status, life expectancy < 3 months, or multilevel spinal involvement.
In patients who are not operative candidates, radiation alone is an appropriate alternative. However, several factors need consideration in deciding whether to manage cord compression with surgery followed by RT vs RT alone. These factors include life expectancy, tumor type (myeloma and lymphoma are more radiosensitive), interval since tumor diagnosis, and the presence of visceral metastases.31 Factors favoring surgical decompression plus postoperative RT over RT alone include spinal instability, KPS (Karnofsky Performance Status) > 70, radio-resistant tumor histology, minimal metastatic disease, and projected survival > 3 months.10
For patients managed with RT alone, early diagnosis and treatment is associated with improved outcomes. A prospective study of patients treated with RT without surgery for spinal cord compression demonstrated that 82% of patients experienced back pain relief, 76% achieved improvement in or preservation of ambulation, and 44% of patients with sphincter dysfunction experienced improvement with treatment.32 Patients with certain tumor histologies, such as myeloma, breast cancer, and prostate cancer, had better responses to RT.32
In the setting of spinal cord compression, longer courses of RT may provide better local control than do shorter courses.33 Therefore, longer courses of RT, such as 30 Gy in 10 fractions delivered over 2 weeks, are often preferred in cases of spinal cord compression treated with definitive RT as well as after surgical decompression. However, overall life expectancy is an important factor considered by the treating radiation oncologist when selecting a short course vs a longer course of RT.
In the instance of painful vertebral body metastases without spinal cord compression, a new subset analysis of the Radiation Therapy Oncology Group (RTOG) 9714 randomized trial indicated that single fraction RT (8 Gy) is just as effective as multiple fractions (30 Gy in 10 fractions), with this study demonstrating comparable rates of pain relief and narcotic use in both groups 3 months after RT.34 Advantages to the single-fraction plan compared with those of multiple fractions include mitigation of logistic concerns for patients and family at the end of life and less acute adverse effects.
Brain Metastases
An estimated 20% to 40% of patients with cancer develop brain metastases.35 The incidence of brain metastases has been rising most likely due to improved detection rates with magnetic resonance imaging (MRI) and improved cancer survival, because treatment regimens have improved with targeted chemotherapy and radiation techniques. Currently, the annual incidence of brain metastases is 170,000 to 200,000 in the U.S.36 Prognosis for these patients is poor, with median survival of 1 month without treatment and about 4 months with whole brain RT (WBRT) (Table).25,37-39
The goal of management for patients with brain metastases is to prevent or treat neurologic symptoms and to prolong survival. Treatment options include corticosteroids, WBRT, surgery, and stereotactic radiosurgery (SRS). Recommendations for treatment should involve both a radiation oncologist and neurosurgeon to determine the best treatment for an individual based on patient age, performance status, extent of systemic disease, and number of brain metastases. These prognostic factors that may predict life expectancy and impact treatment recommendations.40
Factors that have been correlated with improved survival include younger age, better performance status, fewer brain metastases, and lower burden of systemic disease.41,42 Prognostic assessment tools such as the Graded Prognostic Assessment and RTOG-Recursive Partitioning Analysis can be used to predict life expectancy in patients with brain metastases.41,43 However, routine use of these tools is lagging, as evidenced by a recent survey of VHA radiation oncologists. Use of these tools in the clinic will enhance the quality of end of life care and decision making.
Corticosteroids have classically been used in the treatment of brain metastases either alone for supportive care or in combination with RT. Steroids are recommended to provide symptom relief in patients with symptoms related to cerebral edema or mass effect.44 Steroids have been shown to mitigate edema and improve neurologic deficits in about two-thirds of patients with brain metastases.36,45 The effect of corticosteroids is thought to be mediated through inhibition of prostaglandin synthesis, reduction in vascular permeability, and anti-inflammatory properties.46 A common corticosteroid regimen is a 10-mg loading dose of dexamethasone, followed by 16 mg daily in divided doses. For patients without neurologic deficits or cerebral edema, it is reasonable to defer corticosteroid use only when patients are symptomatic.
In general, WBRT is considered an appropriate treatment option for patients with multiple brain metastases based on data suggesting an improvement in OS compared with the use of corticosteroids alone.47 Whole brain radiation has been shown to result in the improvement of baseline neurologic deficits or the prevention of further symptom progression.48 The partial or complete metastasis response rates are on the order of 60%.38 Tumor regression after WBRT has been associated with preservation of neurocognitive function as well as prolonged survival.49
For good prognosis patients with a single brain metastasis and good performance status, the use of surgery or radiosurgery added to WBRT has been associated with improved OS (Table). The RTOG 9508 randomized trial of WBRT with or without SRS demonstrated a survival advantage with SRS, with median survival times of 6.5 months with WBRT + SRS vs 4.9 months with WBRT alone.50 Similarly, a randomized trial evaluating WBRT alone compared with surgery followed by WBRT in patients
with good prognosis demonstrated significantly improved OS in the surgery group (median 40 weeks vs 15 weeks).51 In general, WBRT or postoperative RT to the tumor bed is still indicated after surgical resection, based on randomized data showing a reduction in tumor bed recurrence with postoperative RT.52
For patients with only 1 to 3 brain metastases and a favorable prognosis, surgery and SRS can be considered treatment options, oftentimes with WBRT. The EORTC randomized trial of patients with 1 to 3 brain metastases was designed to determine the benefit of WBRT after treatment with surgery or SRS. In this study, 119 patients underwent SRS and 160 patients underwent surgical resection.53 Both groups of patients were randomized to observation vs adjuvant WBRT. This study demonstrated reduced rates of intracranial relapse with WBRT, however, without any change in OS. Although there is concern that WBRT may impair cognitive function with no clear survival benefit after surgery or SRS, WBRT does reduce recurrence rates in the brain and the need for further treatment.54 Therefore, decisions regarding WBRT in such a setting should be made only after a detailed discussion with a radiation oncologist regarding risks vs benefits of treatment as part of the informed decision-making process.
Conclusions
Palliative RT plays an important role in the management of metastatic cancer to provide symptom relief and is a cost-effective treatment option for bone and brain metastases. Life expectancy and tumor characteristics should be considered when making treatment recommendations to ensure selection of regimens that complement patients’ unique situations. Timely referrals for treatment are important to optimize treatment results.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
In recent years, there has been increasing interest in palliative care for patients with cancer at the end of life. Up to 23% of patients have metastatic disease at presentation, and symptoms from metastatic lesions can cause significant anxiety and impair patients’ quality of life (QOL).1
Palliative radiotherapy (RT) plays a valuable role in the management of metastatic disease to relieve tumor-related symptoms. Although palliative RT does not provide a chance for a cure, it improves QOL and may prolong survival time.2-4 An estimated 20% to 50% of radiation courses are prescribed with palliative intent, because RT is highly effective in providing symptom relief, and the toxicity associated with palliative doses is typically mild.5,6 Palliative RT can be used to manage bone and brain metastases, prevent or treat spinal cord compression, and manage numerous tumor-related symptoms, such as pain and bleeding in patients with terminal cancer.
Palliative RT for bone and brain metastases is supported by high-quality evidence and is considered one of the most effective and cost-effective options available.7,8 This article aims to review the role of RT in treating 3 conditions commonly encountered in patients with metastatic disease—bone metastases, spinal cord compression, and brain metastases—and to emphasize the importance of timely integration of RT for optimal results.
Bone Metastases
About 80% of patients with metastatic solid tumors develop bone metastases, and about 350,000 deaths are linked to bone metastases in the U.S. each year.9 Osseous
metastases can lead to pain, fracture, hypercalcemia, and spinal cord compression. The primary modality for treatment of pain and prevention of morbidity from bone metastases is external beam RT.10
The likelihood of bone pain relief with palliative RT is 60% to 80%, and 30% to 40% of patients achieving complete pain relief. Randomized studies have shown multiple-dose and fractionation regimens provided effective symptom relief for bone metastases. Most commonly used regimens include a single fraction of 8 gray (Gy) delivered in 1 treatment, 20 Gy in 5 fractions delivered daily over 1 week, and 30 Gy in 10 fractions delivered over 2 weeks. Treatment with a single fraction improves access to treatment and patient convenience, whereas more prolonged courses have been associated with lower rates of retreatment.11,12 Regarding the higher rate of retreatment with single-fraction RT, no clear evidence exists that this is due to a less durable pain response or lower level of pain relief.13
There has been recent interest in using predictive models to estimate life expectancy to avoid long courses of RT at the end of life.14,15 Shorter treatment courses of 8 Gyonce or 20 Gy in 5 fractions are particularly valuable for patients with a life expectancy < 3 months to avoid long courses of treatment, and thereby improve QOL as patients transition into hospice. A recent survey demonstrated that 93% of radiation oncologists within the VHA are willing to prescribe short courses of RT consisting of ≤ 6 fractions, and 76% have experience with single-fraction RT.16 These findings are in contradiction to the findings in the non-VA radiation oncology community, in which < 10% of patients with uncomplicated bone metastases are treated with a single fraction.17,18
In addition to providing pain relief, RT is used in the treatment of impending fractures either, adjuvant after surgical stabilization or alone for lower risk lesions.19 Factors that impact fracture risk include location of the metastasis (weight-bearing bones, such as femurs, which are at particularly high risk), length of bone involved, and extent of cortical involvement. Mirels’ scoring system was developed to predict fracture risk in patients with bone metastasis, based on 4 criteria: the
extent of cortical involvement, the location of the metastasis, the osteolytic vs osteoblastic appearance of the lesion, and the degree of pain.20 Surgical fixation can be considered, based on the total score and corresponding fracture risk. When appropriate, surgical stabilization should be considered by an orthopedic surgeon prior to initiating RT.
Postoperative RT after surgical stabilization has been associated with a reduced rate of secondary surgical procedures as well as with improved functional status.21 Radiotherapy promotes remineralization and bone healing and prevents the loss of surgical fixation by treating any residual tumor. A retrospective review of 60 patients with metastatic disease in weight-bearing bones with pathologic fracture or impending pathologic fracture demonstrated that surgery followed by RT was associated with improved functional status as well as with improved overall survival (OS).22,23 For patients in whom surgery is not indicated, the consulting radiation oncologist should consider factors such as the location of the metastasis in weight-bearing vs nonweight bearing bones, the size and extent of the metastasis, and associated symptoms when making a treatment recommendation. In patients at fracture risk from bone metastases, bisphosphonates should also be considered as part of the treatment regimen.24
Spinal Cord Compression
About 5% to 10% of patients diagnosed with cancer will develop spinal cord compression during the course of their disease.25 Spinal cord compression is considered a medical emergency that can result in significant pain and neurologic symptoms, including weakness, paralysis, parasthesias, and incontinence. Early treatment of spinal cord compression can prevent onset or progression of these symptoms; furthermore, early treatment prior to loss of ambulation is associated with improved long-term ambulatory function.26,27
Treatment decisions for spinal metastases with an associated concern for cord compression should be made after a consultation with both a neurosurgeon and a radiation oncologist. Early initiation of steroids is recommended to aid in tumor shrinkage for potential symptom relief.28 A standard way to administer dexamethasone is with a 10-mg loading dose followed by 16 mg per day, divided into 4 doses of 4 mg. Higher steroid doses showed no benefit in a prospective randomized trial comparing 96 mg with 16 mg of dexamethasone daily.29
Surgical decompression should be considered initial management of spinal cord compression. For patients treated surgically, local RT is indicated postoperatively as well. Randomized data show that surgery followed by RT provides better ambulatory function than does RT alone in patients with paralysis of < 2 days’ duration.30 Some patients with metastatic disease are not good candidates for surgery due to comorbidities, poor performance status, life expectancy < 3 months, or multilevel spinal involvement.
In patients who are not operative candidates, radiation alone is an appropriate alternative. However, several factors need consideration in deciding whether to manage cord compression with surgery followed by RT vs RT alone. These factors include life expectancy, tumor type (myeloma and lymphoma are more radiosensitive), interval since tumor diagnosis, and the presence of visceral metastases.31 Factors favoring surgical decompression plus postoperative RT over RT alone include spinal instability, KPS (Karnofsky Performance Status) > 70, radio-resistant tumor histology, minimal metastatic disease, and projected survival > 3 months.10
For patients managed with RT alone, early diagnosis and treatment is associated with improved outcomes. A prospective study of patients treated with RT without surgery for spinal cord compression demonstrated that 82% of patients experienced back pain relief, 76% achieved improvement in or preservation of ambulation, and 44% of patients with sphincter dysfunction experienced improvement with treatment.32 Patients with certain tumor histologies, such as myeloma, breast cancer, and prostate cancer, had better responses to RT.32
In the setting of spinal cord compression, longer courses of RT may provide better local control than do shorter courses.33 Therefore, longer courses of RT, such as 30 Gy in 10 fractions delivered over 2 weeks, are often preferred in cases of spinal cord compression treated with definitive RT as well as after surgical decompression. However, overall life expectancy is an important factor considered by the treating radiation oncologist when selecting a short course vs a longer course of RT.
In the instance of painful vertebral body metastases without spinal cord compression, a new subset analysis of the Radiation Therapy Oncology Group (RTOG) 9714 randomized trial indicated that single fraction RT (8 Gy) is just as effective as multiple fractions (30 Gy in 10 fractions), with this study demonstrating comparable rates of pain relief and narcotic use in both groups 3 months after RT.34 Advantages to the single-fraction plan compared with those of multiple fractions include mitigation of logistic concerns for patients and family at the end of life and less acute adverse effects.
Brain Metastases
An estimated 20% to 40% of patients with cancer develop brain metastases.35 The incidence of brain metastases has been rising most likely due to improved detection rates with magnetic resonance imaging (MRI) and improved cancer survival, because treatment regimens have improved with targeted chemotherapy and radiation techniques. Currently, the annual incidence of brain metastases is 170,000 to 200,000 in the U.S.36 Prognosis for these patients is poor, with median survival of 1 month without treatment and about 4 months with whole brain RT (WBRT) (Table).25,37-39
The goal of management for patients with brain metastases is to prevent or treat neurologic symptoms and to prolong survival. Treatment options include corticosteroids, WBRT, surgery, and stereotactic radiosurgery (SRS). Recommendations for treatment should involve both a radiation oncologist and neurosurgeon to determine the best treatment for an individual based on patient age, performance status, extent of systemic disease, and number of brain metastases. These prognostic factors that may predict life expectancy and impact treatment recommendations.40
Factors that have been correlated with improved survival include younger age, better performance status, fewer brain metastases, and lower burden of systemic disease.41,42 Prognostic assessment tools such as the Graded Prognostic Assessment and RTOG-Recursive Partitioning Analysis can be used to predict life expectancy in patients with brain metastases.41,43 However, routine use of these tools is lagging, as evidenced by a recent survey of VHA radiation oncologists. Use of these tools in the clinic will enhance the quality of end of life care and decision making.
Corticosteroids have classically been used in the treatment of brain metastases either alone for supportive care or in combination with RT. Steroids are recommended to provide symptom relief in patients with symptoms related to cerebral edema or mass effect.44 Steroids have been shown to mitigate edema and improve neurologic deficits in about two-thirds of patients with brain metastases.36,45 The effect of corticosteroids is thought to be mediated through inhibition of prostaglandin synthesis, reduction in vascular permeability, and anti-inflammatory properties.46 A common corticosteroid regimen is a 10-mg loading dose of dexamethasone, followed by 16 mg daily in divided doses. For patients without neurologic deficits or cerebral edema, it is reasonable to defer corticosteroid use only when patients are symptomatic.
In general, WBRT is considered an appropriate treatment option for patients with multiple brain metastases based on data suggesting an improvement in OS compared with the use of corticosteroids alone.47 Whole brain radiation has been shown to result in the improvement of baseline neurologic deficits or the prevention of further symptom progression.48 The partial or complete metastasis response rates are on the order of 60%.38 Tumor regression after WBRT has been associated with preservation of neurocognitive function as well as prolonged survival.49
For good prognosis patients with a single brain metastasis and good performance status, the use of surgery or radiosurgery added to WBRT has been associated with improved OS (Table). The RTOG 9508 randomized trial of WBRT with or without SRS demonstrated a survival advantage with SRS, with median survival times of 6.5 months with WBRT + SRS vs 4.9 months with WBRT alone.50 Similarly, a randomized trial evaluating WBRT alone compared with surgery followed by WBRT in patients
with good prognosis demonstrated significantly improved OS in the surgery group (median 40 weeks vs 15 weeks).51 In general, WBRT or postoperative RT to the tumor bed is still indicated after surgical resection, based on randomized data showing a reduction in tumor bed recurrence with postoperative RT.52
For patients with only 1 to 3 brain metastases and a favorable prognosis, surgery and SRS can be considered treatment options, oftentimes with WBRT. The EORTC randomized trial of patients with 1 to 3 brain metastases was designed to determine the benefit of WBRT after treatment with surgery or SRS. In this study, 119 patients underwent SRS and 160 patients underwent surgical resection.53 Both groups of patients were randomized to observation vs adjuvant WBRT. This study demonstrated reduced rates of intracranial relapse with WBRT, however, without any change in OS. Although there is concern that WBRT may impair cognitive function with no clear survival benefit after surgery or SRS, WBRT does reduce recurrence rates in the brain and the need for further treatment.54 Therefore, decisions regarding WBRT in such a setting should be made only after a detailed discussion with a radiation oncologist regarding risks vs benefits of treatment as part of the informed decision-making process.
Conclusions
Palliative RT plays an important role in the management of metastatic cancer to provide symptom relief and is a cost-effective treatment option for bone and brain metastases. Life expectancy and tumor characteristics should be considered when making treatment recommendations to ensure selection of regimens that complement patients’ unique situations. Timely referrals for treatment are important to optimize treatment results.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Porter A and David M. Palliative care for bone, spinal cord, brain, and liver metastases. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. 2nd ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2007:437-451.
2. Yamaguchi S, Ohguri T, Matsuki Y, et al. Palliative radiotherapy in patients with a poor performance status: the palliative effect is correlated with prolongation of survival time. Radiat Oncol. 2013;8:166.
3. Mac Manus MP, Matthews JP, Wada M, Wirth A, Worotniuk V, Ball DL. Unexpected long-term survival after low-dose palliative radiotherapy for non-small cell lung cancer. Cancer. 2006;106(5):1110-1116.
4. Rastogi M, Revannasiddaiah S, Gupta MK, Seam RK, Thakur P, Gupta M. When palliative treatment achieves more than palliation: instances of long-term survival after palliative radiotherapy. Indian J Palliat Care. 2012;18(2):117-121.
5. Nieder C, Pawinski A, Haukland E, Dokmo R, Phillipi I, Dalhaug A. Estimating need for palliative external beam radiotherapy in adult cancer patients. Int J Radiat Oncol Biol Phys. 2010;76(1):207-211.
6. Hoegler D. Radiotherapy for palliation of symptoms in incurable cancer. Curr Probl Cancer. 1997;21(3):129-183.
7. Expósito J, Jaén J, Alonso E, Tovar I. Use of palliative radiotherapy in brain and bone metastases (VARA II study). Radiat Oncol. 2012;7:131.
8. Konski A. Radiotherapy is a cost-effective palliative treatment for patients with bone metastasis from prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60(5):1373-1378.
9. Popovic M, den Hartogh M, Zhang L, et al. Review of international patterns of practice for the treatment of painful bone metastases with palliative radiotherapy from 1993 to 2013. Radiother Oncol. 2014;111(1):11-17.
10. Lutz S, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79(4):965-976.
11. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systemic review. J Clin Oncol. 2007;25(11):1423-1436.
12. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy—a systemic review of the randomized trials. Cochrane Database Syst Rev. 2004;(2):CD004721.
13. Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101-109.
14. Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectance in patients with metastatic cancer receiving palliative radiotherapy: the TEACHH model. Cancer. 2014;120(1):134-141.
15. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buccholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
16. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
17. Ellsworth SG, Alcorn SR, Hales RK, McNutt TR, DeWeese TL, Smith TJ. Patterns of care among patients receiving radiation therapy for bone metastases at a large academic institution. Int J Radiat Oncol Biol Phys. 2014;89(5):1100-1105.
18. Bradley NM, Husted J, Sey MS, et al. Review of patterns of practice and patients’ preferences in the treatment of bone metastases with palliative radiotherapy. Support Care Cancer. 2007;15(4):373-385.
19. Haidukewych GJ. Metastatic disease around the hip: maintaining quality of life. J Bone Joint Surg Br. 2012;94(11 suppl A):22-25.
20. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;(249):256-264.
21. Jacofsky DJ, Haidukewych GJ. Management of pathologic fractures of the proximal femur: state of the art. J Orthop Trauma. 2004;18(7):459-469.
22. Townsend PW, Rosenthal HG, Smalley SR, Cozad SC, Hassanein RE. Impact of postoperative radiation therapy and other perioperative factors on outcome after orthopedic stabilization of impending or pathologic fractures due to metastatic disease. J Clin Oncol. 1994;12(11):2345-2350.
23. Townsend PW, Smalley SR, Cozad SC, Rosenthal HG, Hassanein RE. Role of postoperative radiation therapy after stabilization of fractures caused by metastatic disease. Int J Radiat Oncol Biol Phys. 1995;31(1):43-49.
24. Farooki A. NCCN bone health task force: key recommendations. J Natl Compr Canc Netw. 2014;12(5 suppl):813-816.
25. Sejpal SV, Bhate A, Small W. Palliative radiation therapy in the management of brain metastases, spinal cord compression, and bone metastases. Semin Intervent Radiol. 2007;24(4):362-374.
26. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “all I care about is walking and living my life.” JAMA. 2008;299(8):937-946.
27. Kim RY, Spencer SA, Meredith RF, et al. Extradural spinal cord compression: analysis of factors determining functional prognosis—prospective study. Radiology. 1990;176(1):279-282.
28. Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21(2):140-143.
29. Graham PH, Capp A, Delaney G, et al. A pilot randomized comparison of dexamethasone 96 mg vs 16 mg per day for malignant spinal-cord compression treated by radiotherapy: TROG 01.05 Superdex study. Clin Oncol (R Coll Radiol). 2006;18(1):70-76.
30. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
31. Rades D, Huttenlocher S, Bajrovic A, et al. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int J Radiat Oncol Biol Phys. 2011;81(5):e861-e868.
32. Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32(4):959-967.
33. Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393.
34. Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer. 2013;119(4):888-896.
35. Wong J, Hird A, Kirou-Mauro, Napolskikh J, Chow E. Quality of life in brain metastases radiation trials: a literature review. Curr Oncol. 2008;15(5):25-45.
36. Nichols EM, Patchell RA, Regine WF, Kwok Y. Palliation of brain and spinal cord metastases. In: Halperin EC, Brady LW, Perez CA, Wazer DE, eds. Perez and Brady’s Principles and Practice of Radiation Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013:1974.
37. Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48(2):384-394.
38. Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24(8):1295-1304.
39. Sundström JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med. 1998;30(3):296-299.
40. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
41. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
42. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG databases. Int J Radiat Oncol Biol Phys. 2008;70(2):510-514.
43. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419-425.
44. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
45. Ruderman NB, Hall TC. Use of glucocorticoids in the palliative treatment of metastatic brain tumors. Cancer. 1965;18:298-306.
46. Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21(2):140-143.
47. Horton J, Baxter DH, Olson KB. The management of metastases to the brain by irradiation and corticosteroids. Am J Roentgenol Radium Ther Nucl Med. 1971;111(2)334-336.
48. Wong J, Hird A, Zhang L, et al. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(4):1125-1131.
49. Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260-1266.
50. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
51. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494-500.
52. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
53. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141.
54. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
1. Porter A and David M. Palliative care for bone, spinal cord, brain, and liver metastases. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. 2nd ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2007:437-451.
2. Yamaguchi S, Ohguri T, Matsuki Y, et al. Palliative radiotherapy in patients with a poor performance status: the palliative effect is correlated with prolongation of survival time. Radiat Oncol. 2013;8:166.
3. Mac Manus MP, Matthews JP, Wada M, Wirth A, Worotniuk V, Ball DL. Unexpected long-term survival after low-dose palliative radiotherapy for non-small cell lung cancer. Cancer. 2006;106(5):1110-1116.
4. Rastogi M, Revannasiddaiah S, Gupta MK, Seam RK, Thakur P, Gupta M. When palliative treatment achieves more than palliation: instances of long-term survival after palliative radiotherapy. Indian J Palliat Care. 2012;18(2):117-121.
5. Nieder C, Pawinski A, Haukland E, Dokmo R, Phillipi I, Dalhaug A. Estimating need for palliative external beam radiotherapy in adult cancer patients. Int J Radiat Oncol Biol Phys. 2010;76(1):207-211.
6. Hoegler D. Radiotherapy for palliation of symptoms in incurable cancer. Curr Probl Cancer. 1997;21(3):129-183.
7. Expósito J, Jaén J, Alonso E, Tovar I. Use of palliative radiotherapy in brain and bone metastases (VARA II study). Radiat Oncol. 2012;7:131.
8. Konski A. Radiotherapy is a cost-effective palliative treatment for patients with bone metastasis from prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60(5):1373-1378.
9. Popovic M, den Hartogh M, Zhang L, et al. Review of international patterns of practice for the treatment of painful bone metastases with palliative radiotherapy from 1993 to 2013. Radiother Oncol. 2014;111(1):11-17.
10. Lutz S, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79(4):965-976.
11. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systemic review. J Clin Oncol. 2007;25(11):1423-1436.
12. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy—a systemic review of the randomized trials. Cochrane Database Syst Rev. 2004;(2):CD004721.
13. Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101-109.
14. Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectance in patients with metastatic cancer receiving palliative radiotherapy: the TEACHH model. Cancer. 2014;120(1):134-141.
15. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buccholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
16. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
17. Ellsworth SG, Alcorn SR, Hales RK, McNutt TR, DeWeese TL, Smith TJ. Patterns of care among patients receiving radiation therapy for bone metastases at a large academic institution. Int J Radiat Oncol Biol Phys. 2014;89(5):1100-1105.
18. Bradley NM, Husted J, Sey MS, et al. Review of patterns of practice and patients’ preferences in the treatment of bone metastases with palliative radiotherapy. Support Care Cancer. 2007;15(4):373-385.
19. Haidukewych GJ. Metastatic disease around the hip: maintaining quality of life. J Bone Joint Surg Br. 2012;94(11 suppl A):22-25.
20. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;(249):256-264.
21. Jacofsky DJ, Haidukewych GJ. Management of pathologic fractures of the proximal femur: state of the art. J Orthop Trauma. 2004;18(7):459-469.
22. Townsend PW, Rosenthal HG, Smalley SR, Cozad SC, Hassanein RE. Impact of postoperative radiation therapy and other perioperative factors on outcome after orthopedic stabilization of impending or pathologic fractures due to metastatic disease. J Clin Oncol. 1994;12(11):2345-2350.
23. Townsend PW, Smalley SR, Cozad SC, Rosenthal HG, Hassanein RE. Role of postoperative radiation therapy after stabilization of fractures caused by metastatic disease. Int J Radiat Oncol Biol Phys. 1995;31(1):43-49.
24. Farooki A. NCCN bone health task force: key recommendations. J Natl Compr Canc Netw. 2014;12(5 suppl):813-816.
25. Sejpal SV, Bhate A, Small W. Palliative radiation therapy in the management of brain metastases, spinal cord compression, and bone metastases. Semin Intervent Radiol. 2007;24(4):362-374.
26. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “all I care about is walking and living my life.” JAMA. 2008;299(8):937-946.
27. Kim RY, Spencer SA, Meredith RF, et al. Extradural spinal cord compression: analysis of factors determining functional prognosis—prospective study. Radiology. 1990;176(1):279-282.
28. Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21(2):140-143.
29. Graham PH, Capp A, Delaney G, et al. A pilot randomized comparison of dexamethasone 96 mg vs 16 mg per day for malignant spinal-cord compression treated by radiotherapy: TROG 01.05 Superdex study. Clin Oncol (R Coll Radiol). 2006;18(1):70-76.
30. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
31. Rades D, Huttenlocher S, Bajrovic A, et al. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int J Radiat Oncol Biol Phys. 2011;81(5):e861-e868.
32. Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32(4):959-967.
33. Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393.
34. Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer. 2013;119(4):888-896.
35. Wong J, Hird A, Kirou-Mauro, Napolskikh J, Chow E. Quality of life in brain metastases radiation trials: a literature review. Curr Oncol. 2008;15(5):25-45.
36. Nichols EM, Patchell RA, Regine WF, Kwok Y. Palliation of brain and spinal cord metastases. In: Halperin EC, Brady LW, Perez CA, Wazer DE, eds. Perez and Brady’s Principles and Practice of Radiation Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013:1974.
37. Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48(2):384-394.
38. Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24(8):1295-1304.
39. Sundström JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med. 1998;30(3):296-299.
40. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
41. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
42. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG databases. Int J Radiat Oncol Biol Phys. 2008;70(2):510-514.
43. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419-425.
44. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
45. Ruderman NB, Hall TC. Use of glucocorticoids in the palliative treatment of metastatic brain tumors. Cancer. 1965;18:298-306.
46. Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21(2):140-143.
47. Horton J, Baxter DH, Olson KB. The management of metastases to the brain by irradiation and corticosteroids. Am J Roentgenol Radium Ther Nucl Med. 1971;111(2)334-336.
48. Wong J, Hird A, Zhang L, et al. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(4):1125-1131.
49. Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260-1266.
50. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
51. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494-500.
52. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
53. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141.
54. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.