User login
Assessment of the Efficacy of Tranexamic Acid Solution 5% in the Treatment of Melasma in Patients of South Asian Descent
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
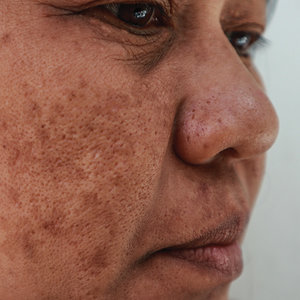
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

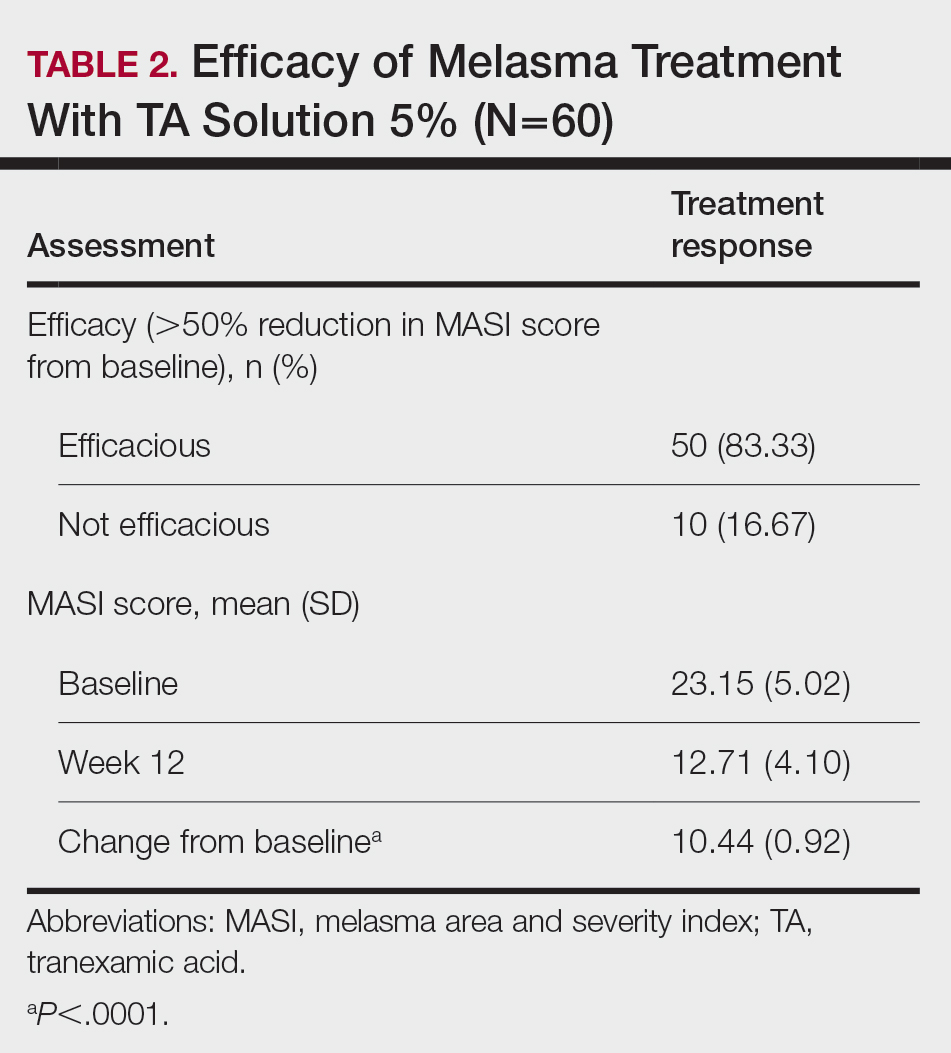
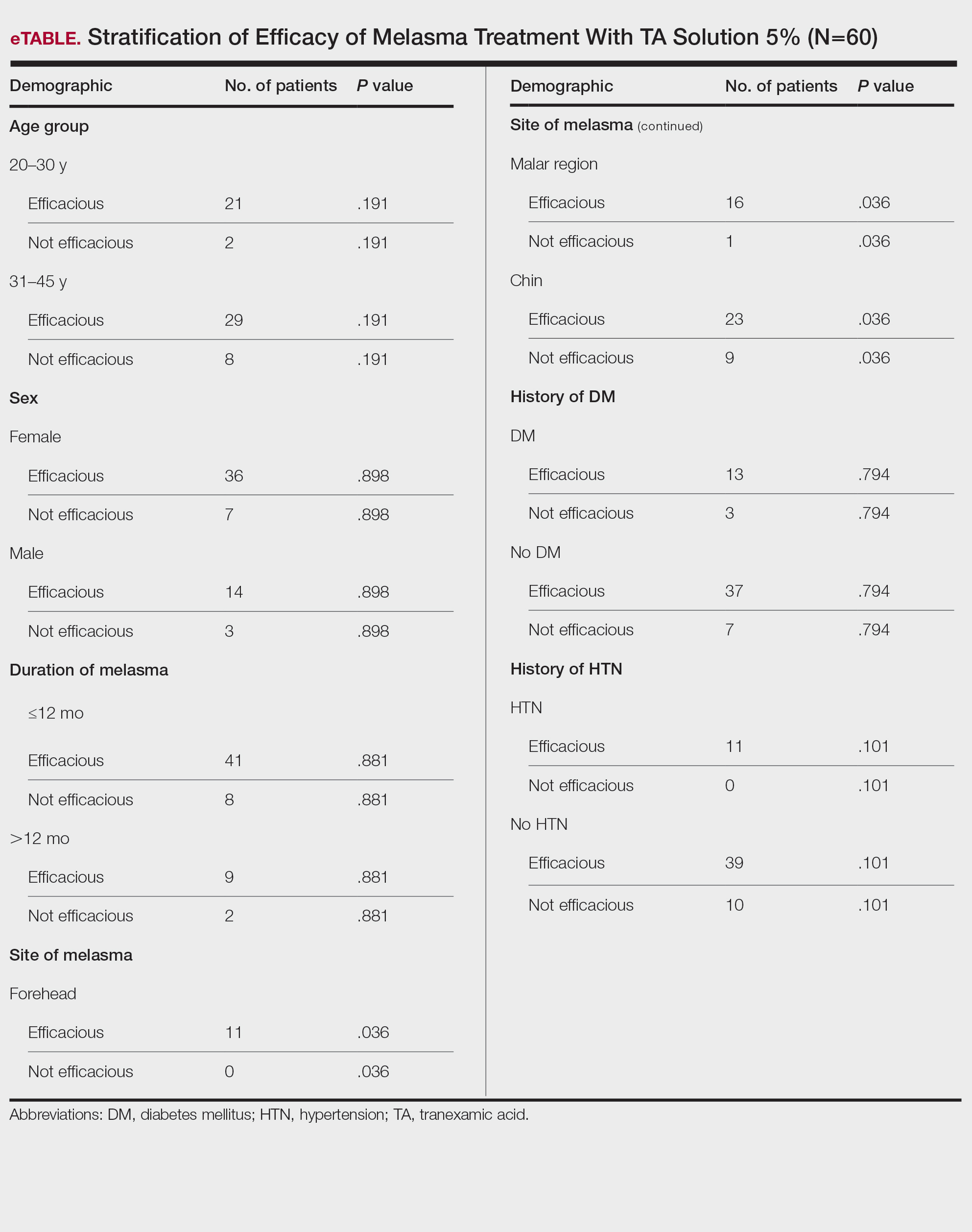
Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).
Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19
Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).

Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19

Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).

Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19

Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
PRATICE POINTS
- Tranexamic acid (TA) solution 5% is an efficacious treatment for skin of color patients with melasma.
- Topical TA is a treatment alternative for patients who may not be able to tolerate oral TA.
- Our study revealed the greatest efficacy for TA solution 5% was seen on the forehead and malar region, with less efficacy on the chin.
Imaging Tools for Noninvasive Hair Assessment
New imaging tools along with adaptations to existing technologies have been emerging in recent years, with the potential to improve hair diagnostics and treatment monitoring. We provide an overview of 4 noninvasive hair imaging technologies: global photography, trichoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT). For each instrument, we discuss current and future applications in clinical practice and research along with advantages and disadvantages.
Global Photography
Global photography allows for the analysis of hair growth, volume, distribution, and density through serial standardized photographs.1 Global photography was first introduced for hair growth studies in 1987 and soon after was used for hair and scalp assessments in finasteride clinical trials.2
Hair Assessment—Washed, dried, and combed hair, without hair product, are required for accurate imaging; wet conditions increase reflection and promote hair clumping, thus revealing more scalp and depicting the patient as having less hair.1 Headshots are taken from short distances and use stereotactic positioning devices to create 4 global views: vertex, midline, frontal, and temporal.3 Stereotactic positioning involves fixing the patient’s chin and forehead as well as mounting the camera and flash device to ensure proper magnification. These adjustments ensure lighting remains consistent throughout consecutive study visits.4 Various grading scales are available for use in hair growth clinical studies to increase objectivity in the analysis of serial global photographs. A blinded evaluator should assess the before and after photographs to limit experimenter bias. Global photography often is combined with quantitative software analysis for improved detection of hair changes.1
Advancements—Growing interest in improving global photography has resulted in various application-based, artificial intelligence (AI)–mediated tools to simplify photograph collection and analysis. For instance, new hair analysis software utilizes AI algorithms to account for facial features in determining the optimal angle for capturing global photographs (Figure 1), which simplifies the generation of global photography images through smartphone applications and obviates the need for additional stereotactic positioning equipment.5,6

Limitations—Clinicians should be aware of global photography’s requirements for consistency in lighting, camera settings, film, and image processing, which can limit the accuracy of hair assessment over time if not replicated correctly.7,8 Emerging global photography software has helped to overcome some of these limitations.
Global photography is less precise when a patient’s hair loss is less than 50%, as it is difficult to discern subtle hair changes. Thus, global photography provides limited utility in assessing minimal to moderate hair loss.9 Currently, global photography largely functions as an adjunct tool for other hair analysis methods rather than as a stand-alone tool.
Trichoscopy
Trichoscopy (also known as dermoscopy of the hair and scalp) may be performed with a manual dermoscope (with 10× magnification) or a digital videodermatoscope (up to 1000× magnification).10-12 Unlike global photography, trichoscopy provides a detailed structural analysis of hair shafts, follicular openings, and perifollicular and interfollicular areas.13 Kinoshita-Ise and Sachdeva13 provided an in-depth, updated review of trichoscopy terminology with their definitions and associated conditions (with prevalence), which should be referenced when performing trichoscopic examination.
Hair Assessment—Trichoscopic assessment begins with inspection of follicular openings (also referred to as “dots”), which vary in color depending on the material filling them—degrading keratinocytes, keratin, sebaceous debris, melanin, or fractured hairs.13 The structure of hair shafts also is examined, showing broken hairs, short vellus hairs, and comma hairs, among others. Perifollicular areas are examined for scale, erythema, blue-gray dots, and whitish halos. Interfollicular areas are examined for pigment pattern as well as vascularization, which often presents in a looping configuration under dermoscopy. A combination of dot colorization, hair shaft structure, and perifollicular and interfollicular findings inform diagnostic algorithms of hair and scalp conditions. For example, central centrifugal cicatricial alopecia, the most common alopecia seen in Black women, has been associated with a combination of honeycomb pigment pattern, perifollicular whitish halo, pinpoint white dots, white patches, and perifollicular erythema.13
Advantages—Perhaps the most useful feature of trichoscopy is its ability to translate visualized features into simple diagnostic algorithms. For instance, if the clinician has diagnosed the patient with noncicatricial alopecia, they would next focus on dot colors. With black dots, the next step would be to determine whether the hairs are tapered or coiled, and so on. This systematic approach enables the clinician to narrow possible diagnoses.2 An additional advantage of trichoscopy is that it examines large surface areas noninvasively as compared to hair-pull tests and scalp biopsy.14,15 Trichoscopy allows temporal comparisons of the same area for disease and treatment monitoring with more diagnostic detail than global photography.16 Trichoscopy also is useful in selecting biopsy locations by discerning and avoiding areas of scar tissue.17
Limitations—Diagnosis via the trichoscopy algorithm is limiting because it is not comprehensive of all hair and scalp disease.18 Additionally, many pathologies exhibit overlapping follicular and interfollicular patterning. For example, almost all subtypes of scarring alopecia present with hair loss and scarred follicles once they have progressed to advanced stages. Further studies should identify more specific patterns of hair and scalp pathologies, which could then be incorporated into a diagnostic algorithm.13
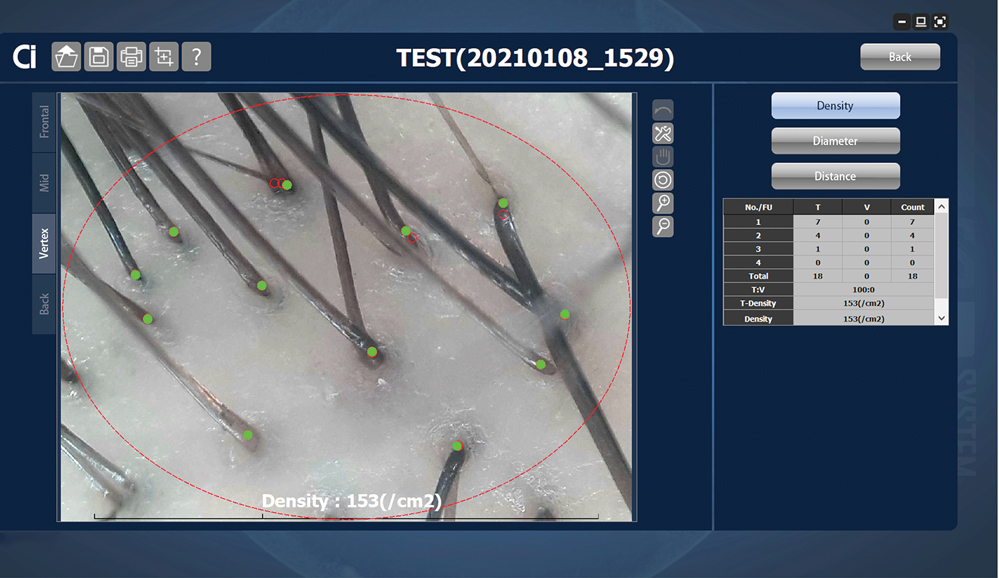
Advancements—The advent of hair analysis software has expanded the role of videodermoscopy by rapidly quantifying hair growth parameters such as hair count, follicular density, and follicular diameter, as well as interfollicular distances (Figure 2).14,17 Vellus and terminal hairs are differentiated according to their thickness and length.17 Moreover, the software can analyze the same area of the scalp over time by either virtual tattoos, semipermanent markings, or precise location measurements, increasing intra- and interclass correlation. The rate of hair growth, hair shedding, and parameters of anagen and telogen hairs can be studied by a method termed phototrichogram whereby a transitional area of hair loss and normal hair growth is identified and trimmed to less than 1 mm from the skin surface.19 A baseline photograph is taken using videodermoscopy. After approximately 3 days, the identical region is photographed and compared with the initial image to observe changes in the hair. Software programs can distinguish the growing hair as anagen and nongrowing hair as telogen, calculating the anagen-to-telogen ratio as well as hair growth rate, which are essential measurements in hair research and clinical studies. Software programs have replaced laborious and time-consuming manual hair counts and have rapidly grown in popularity in evaluating patterned hair loss.
Reflectance Confocal Microscopy
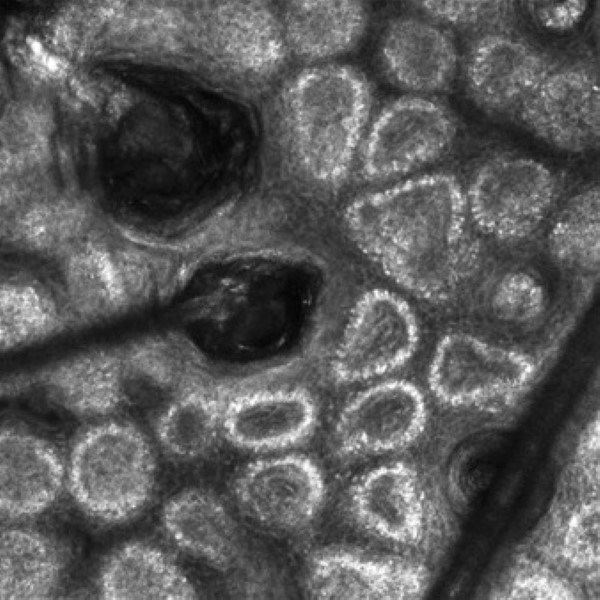
Reflectance confocal microscopy is a noninvasive imaging tool that visualizes skin and its appendages at near-histologic resolution (lateral resolution of 0.5–1 μm). It produces grayscale horizontal images that can be taken at levels ranging from the stratum corneum to the superficial papillary dermis, corresponding to a depth of approximately 100 to 150 µm. Thus, a hair follicle can be imaged starting from the follicular ostia down to the reachable papillary dermis (Figure 3).20 Image contrast is provided by differences in the size and refractive indices of cellular organelles.21,22 There are 2 commercially available RCM devices: VivaScope 1500 and VivaScope 3000 (Caliber Imaging & Diagnostics, Inc).
VivaScope 1500, a wide-probe microscope, requires the attachment of a plastic window to the desired imaging area. The plastic window is lined with medical adhesive tape to prevent movement during imaging. The adhesive tape can pull on hair upon removal, which is not ideal for patients with existing hair loss. Additionally, the image quality of VivaSope 1500 is best in flat areas and areas where hair is shaved.20,23,24 Despite these disadvantages, VivaScope 1500 has successfully shown utility in research studies, which suggests that these obstacles can be overcome by experienced users. The handheld VivaScope 3000 is ergonomically designed and suitable for curved surfaces such as the scalp, with the advantage of not requiring any adhesive. However, the images acquired from the VivaScope 3000 cover a smaller surface area.
Structures Visualized—Structures distinguished with RCM include keratinocytes, melanocytes, inflammatory cells, hair follicles, hair shafts, adnexal infundibular epithelium, blood vessels, fibroblasts, and collagen.23 Real-time visualization of blood flow also can be seen.
Applications of RCM—Reflectance confocal microscopy has been used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, chemotherapy-induced alopecia (CIA), alopecia areata, and androgenetic alopecia. Diagnostic RCM criteria for such alopecias have been developed based on their correspondence to histopathology. An RCM study of classic lichen planopilaris and frontal fibrosing alopecia identified features of epidermal disarray, infundibular hyperkeratosis, inflammatory cells, pigment incontinence, perifollicular fibrosis, bandlike scarring, melanophages in the dermis, dilated blood vessels, basal layer vacuolar degeneration, and necrotic keratinocytes.25 Pigment incontinence in the superficial epidermis, perifollicular lichenoid inflammation, and hyperkeratosis were characteristic RCM features of early-stage lichen planopilaris, while perifollicular fibrosis and dilated blood vessels were characteristic RCM features of late-stage disease. The ability of RCM features to distinguish different stages of lichen planopilaris shows its potential in treating early disease and preventing irreversible hair loss.
Differentiating between scarring and nonscarring alopecia also is possible through RCM. The presence of periadnexal, epidermal, and dermal inflammatory cells, in addition to periadnexal sclerosis, are defining RCM features of scarring alopecia.26 These features are absent in nonscarring alopecias. Reflectance confocal microscopy additionally has been shown to be useful in the treatment monitoring of lichen planopilaris and discoid lupus erythematosus.20 Independent reviewers, blinded to the patients’ identities, were able to characterize and follow features of these scarring alopecias by RCM. The assessed RCM features were comparable to those observed by histopathologic evaluation: epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intra-adnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells and melanophages, and dermal sclerosis. A reduction in inflammatory cells across multiple skin layers and at the level of the adnexal epithelium correlated with clinical response to treatment. Reflectance confocal microscopy also was able to detect recurrence of inflammation in cases where treatment had been interrupted before clinical signs of disease recurrence were evident. The authors thus concluded that RCM’s sensitivity can guide timing of treatment and avoid delays in starting or restarting treatment.20
Reflectance confocal microscopy also has served as a learning tool for new subclinical understandings of alopecia. In a study of CIA, the disease was found to be a dynamic process that could be categorized into 4 distinct phases distinguishable by combined confocal and dermoscopic features. This study also identified a new feature observable on RCM images—a CIA dot—defined as a dilated follicular infundibulum containing mashed, malted, nonhomogeneous material and normal or fragmented hair. This dot is thought to represent the initial microscopic sign of direct toxicity of chemotherapy on the hair follicle. Chemotherapy-induced alopecia dots persist throughout chemotherapy and subsequently disappear after chemotherapy ends.27
Limitations and Advantages—Currently, subtypes of cicatricial alopecias cannot be characterized on RCM because inflammatory cell types are not distinguished from each other (eg, eosinophils vs neutrophils). Another limitation of RCM is the loss of resolution below the superficial papillary dermis (a depth of approximately 150 µm); thus, deeper structures, such as the hair bulb, cannot be visualized.
Unlike global photography and trichoscopy, which are low-cost methods, RCM is much more costly, ranging upwards of several thousand dollars, and it may require additional technical support fees, making it less accessible for clinical practice. However, RCM imaging continues to be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.26 If a biopsy is required, RCM can aid in the selection of a biopsy site, as areas with active inflammation are more informative than atrophic and fibrosed areas.23 The role of RCM in trichoscopy can be expanded by designing a more cost-effective and ergonomically suited scope for hair and scalp assessment.
Optical Coherence Tomography
Optical coherence tomography is a noninvasive handheld device that emits low-power infrared light to visualize the skin and adnexal structures. Optical coherence tomography relies on the principle of interferometry to detect phase differences in optical backscattering at varying tissue depths.28,29 It allows visualization up to 2 mm, which is 2 to 5 times deeper than RCM.36 Unlike RCM, which has cellular resolution, OCT has an axial resolution of 3 to 15 μm, which allows only for the detection of structural boundaries.30 There are various OCT modalities that differ in lateral and axial resolutions and maximum depth. Commercial software is available that measures changes in vascular density by depth, epidermal thickness, skin surface texture, and optical attenuation—the latter being an indirect measurement of collagen density and skin hydration.
Structures Visualized—Hair follicles can be well distinguished on OCT images, and as such, OCT is recognized as a diagnostic tool in trichology (Figure 4).31 Follicular openings, interfollicular collagen, and outlines of the hair shafts are visible; however, detailed components of the follicular unit cannot be visualized by OCT. Keratin hyperrefractivity identifies the hair shaft. Additionally, the hair matrix is denoted by a slightly granular texture in the dermis. Dynamic OCT produces colorized images that visualize blood flow within vessels.
Applications of OCT—Optical coherence tomography is utilized in investigative trichology because it provides highly reproducible measurements of hair shaft diameters, cross-sectional surface areas, and form factor, which is a surrogate parameter for hair shape. The cross-section of hair shafts provides insight into local metabolism and perifollicular inflammation. Cross-sections of hair shafts in areas of alopecia areata were found to be smaller than cross-sections in the unaffected scalp within the same individual.32 Follicular density can be manually quantified on OCT images, but there also is promise for automated quantification. A recent study by Urban et al33 described training a convolutional neural network to automatically count hair as well as hair-bearing and non–hair-bearing follicles in OCT scans. These investigators also were able to color-code hair according to height, resulting in the creation of a “height” map.
Optical coherence tomography has furthered our understanding of the pathophysiology of cicatricial and nonscarring alopecias. Vazquez-Herrera et al34 assessed the inflammatory and cicatricial stages of frontal fibrosing alopecia by OCT imaging. Inflammatory hairlines, which are seen in the early stages of frontal fibrosing alopecia, exhibited a thickened dermis, irregular distribution of collagen, and increased vascularity in both the superficial and deep dermal layers compared to cicatricial and healthy scalp. Conversely, late-stage cicatricial areas exhibited a thin dermis and collagen that appeared in a hyperreflective, concentric, onion-shaped pattern around remnant follicular openings. Vascular flow was reduced in the superficial dermis of a cicatricial scalp but increased in the deep dermal layers compared with a healthy scalp. The attenuation coefficients of these disease stages also were assessed. The attenuation coefficient of the inflammatory hairline was higher compared with normal skin, likely as a reflection of inflammatory infiltrate and edema, whereas the attenuation coefficient of cicatricial scalp was lower compared with normal skin, likely reflecting the reduced water content of atrophic skin.34 This differentiation of early- and late-stage cicatricial alopecias has implications for early treatment and improved prognosis. Additionally, there is potential for OCT to assist in the differentiation of alopecia subtypes, as it can measure the epidermal thickness and follicular density and was previously used to compare scarring and nonscarring alopecia.35
Advantages and Limitations—Similar to RCM, OCT may be cost prohibitive for some clinicians. In addition, OCT cannot visualize the follicular unit in cellular detail. However, the extent of OCT’s capabilities may not be fully realized. Dynamic OCT is a new angiographic type of OCT that shows potential in monitoring early subclinical responses to novel alopecia therapies, such as platelet-rich plasminogen, which is hypothesized to stimulate hair growth through angiogenesis. Additionally, OCT may improve outcomes of hair transplantation procedures by allowing for visualization of the subcutaneous angle of hair follicles. Blind extraction of hair follicles in follicular unit extraction procedures can result in inadvertent transection and damage to the hair follicle; OCT could help identify good candidates for follicular unit extraction, such as patients with hair follicles in parallel arrangement, who are predicted to have better results.36
Conclusion
The field of trichology will continue to evolve with the emergence of noninvasive imaging technologies that diagnose hair disease in early stages and enable treatment monitoring with quantification of hair parameters. As discussed in this review, global photography, trichoscopy, RCM, and OCT have furthered our understanding of alopecia pathophysiology and provided objective methods of treatment evaluation. The capabilities of these tools will continue to expand with advancements in add-on software and AI algorithms.
- Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-721.
- Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Peytavi U, Hillmann K, Guarrera M, eds. Hair Growth and Disorders. 4th ed. Springer; 2008:140-144.
- Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10-18.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578-579.
- Capily Institute. Artificial intelligence (A.I.) powered hair growth tracking. Accessed July 31, 2023. https://tss-aesthetics.com/capily-hair-tracking-syst
- Dinh Q, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189-199.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4:259-264.
- Alessandrini A, Bruni F, Piraccini BM, et al. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629-640.
- Ashique K, Kaliyadan F. Clinical photography for trichology practice: tips and tricks. Int J Trichology. 2011;3:7-13.
- Rudnicka L, Olszewska M, Rakowska A, et al. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-654.
- Kinoshita-Ise M, Sachdeva M. Update on trichoscopy: integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J Dermatol. 2022;49:4-18. doi:10.1111/1346-8138.16233
- Van Neste D, Trüeb RM. Critical study of hair growth analysis with computer-assisted methods. J Eur Acad Dermatol Venereol. 2006;20:578-583.
- Romero J, Grimalt R. Trichoscopy: essentials for the dermatologist. World J Dermatol. 2015;4:63-68.
- Trichoscopy: a new frontier for the diagnosis of hair diseases. Exp Rev Dermatol. 2012;7:429-437.
- Lee B, Chan J, Monselise A, et al. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66:166-167.
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2010;38:71-75.
- Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242-244.
- Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165:534-540.
- Kuck M, Schanzer S, Ulrich M, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Opt. 2012;17:101504.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4:1014-1023.
- Agozzino M, Ardigò M. Scalp confocal microscopy. In: Humbert P, Maibach H, Fanian F, et al, eds. Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. 2nd ed. Springer International Publishing; 2016:311-326.
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008;2:55-59.
- Kurzeja M, Czuwara J, Walecka I, et al. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27:266-271.
- Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-318.
- Franceschini C, Garelli V, Persechino F, et al. Dermoscopy and confocal microscopy for different chemotherapy-induced alopecia (CIA) phases characterization: preliminary study. Skin Res Technol. 2020;26:269-276.
- Martinez-Velasco MA, Perper M, Maddy AJ, et al. In vitro determination of Mexican Mestizo hair shaft diameter using optical coherence tomography. Skin Res Technol. 2018;24;274-277.
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019;104:108-113.
- Wan B, Ganier C, Du-Harpur X, et al. Applications and future directions for optical coherence tomography in dermatology. Br J Dermatol. 2021;184:1014-1022.
- Blume-Peytavi U, Vieten J, Knuttel A et al. Optical coherent tomography (OCT): a new method for online-measurement of hair shaft thickness. J Dtsch Dermatol Ges. 2004;2:546.
- Garcia Bartels N, Jahnke I, Patzelt A, et al. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol. 2011;17:201-205.
- Urban G, Feil N, Csuka E, et al. Combining deep learning with optical coherence tomography imaging to determine scalp hair and follicle counts. Lasers Surg Med. 2021;53:171-178.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, et al. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32:318-322.
- Ekelem C, Feil N, Csuka E, et al. Optical coherence tomography in the evaluation of the scalp and hair: common features and clinical utility. Lasers Surg Med. 2021;53:129-140.
- Schicho K, Seemann R, Binder M, et al. Optical coherence tomography for planning of follicular unit extraction. Dermatol Surg. 2015;41:358-363.
New imaging tools along with adaptations to existing technologies have been emerging in recent years, with the potential to improve hair diagnostics and treatment monitoring. We provide an overview of 4 noninvasive hair imaging technologies: global photography, trichoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT). For each instrument, we discuss current and future applications in clinical practice and research along with advantages and disadvantages.
Global Photography
Global photography allows for the analysis of hair growth, volume, distribution, and density through serial standardized photographs.1 Global photography was first introduced for hair growth studies in 1987 and soon after was used for hair and scalp assessments in finasteride clinical trials.2
Hair Assessment—Washed, dried, and combed hair, without hair product, are required for accurate imaging; wet conditions increase reflection and promote hair clumping, thus revealing more scalp and depicting the patient as having less hair.1 Headshots are taken from short distances and use stereotactic positioning devices to create 4 global views: vertex, midline, frontal, and temporal.3 Stereotactic positioning involves fixing the patient’s chin and forehead as well as mounting the camera and flash device to ensure proper magnification. These adjustments ensure lighting remains consistent throughout consecutive study visits.4 Various grading scales are available for use in hair growth clinical studies to increase objectivity in the analysis of serial global photographs. A blinded evaluator should assess the before and after photographs to limit experimenter bias. Global photography often is combined with quantitative software analysis for improved detection of hair changes.1
Advancements—Growing interest in improving global photography has resulted in various application-based, artificial intelligence (AI)–mediated tools to simplify photograph collection and analysis. For instance, new hair analysis software utilizes AI algorithms to account for facial features in determining the optimal angle for capturing global photographs (Figure 1), which simplifies the generation of global photography images through smartphone applications and obviates the need for additional stereotactic positioning equipment.5,6

Limitations—Clinicians should be aware of global photography’s requirements for consistency in lighting, camera settings, film, and image processing, which can limit the accuracy of hair assessment over time if not replicated correctly.7,8 Emerging global photography software has helped to overcome some of these limitations.
Global photography is less precise when a patient’s hair loss is less than 50%, as it is difficult to discern subtle hair changes. Thus, global photography provides limited utility in assessing minimal to moderate hair loss.9 Currently, global photography largely functions as an adjunct tool for other hair analysis methods rather than as a stand-alone tool.
Trichoscopy
Trichoscopy (also known as dermoscopy of the hair and scalp) may be performed with a manual dermoscope (with 10× magnification) or a digital videodermatoscope (up to 1000× magnification).10-12 Unlike global photography, trichoscopy provides a detailed structural analysis of hair shafts, follicular openings, and perifollicular and interfollicular areas.13 Kinoshita-Ise and Sachdeva13 provided an in-depth, updated review of trichoscopy terminology with their definitions and associated conditions (with prevalence), which should be referenced when performing trichoscopic examination.
Hair Assessment—Trichoscopic assessment begins with inspection of follicular openings (also referred to as “dots”), which vary in color depending on the material filling them—degrading keratinocytes, keratin, sebaceous debris, melanin, or fractured hairs.13 The structure of hair shafts also is examined, showing broken hairs, short vellus hairs, and comma hairs, among others. Perifollicular areas are examined for scale, erythema, blue-gray dots, and whitish halos. Interfollicular areas are examined for pigment pattern as well as vascularization, which often presents in a looping configuration under dermoscopy. A combination of dot colorization, hair shaft structure, and perifollicular and interfollicular findings inform diagnostic algorithms of hair and scalp conditions. For example, central centrifugal cicatricial alopecia, the most common alopecia seen in Black women, has been associated with a combination of honeycomb pigment pattern, perifollicular whitish halo, pinpoint white dots, white patches, and perifollicular erythema.13
Advantages—Perhaps the most useful feature of trichoscopy is its ability to translate visualized features into simple diagnostic algorithms. For instance, if the clinician has diagnosed the patient with noncicatricial alopecia, they would next focus on dot colors. With black dots, the next step would be to determine whether the hairs are tapered or coiled, and so on. This systematic approach enables the clinician to narrow possible diagnoses.2 An additional advantage of trichoscopy is that it examines large surface areas noninvasively as compared to hair-pull tests and scalp biopsy.14,15 Trichoscopy allows temporal comparisons of the same area for disease and treatment monitoring with more diagnostic detail than global photography.16 Trichoscopy also is useful in selecting biopsy locations by discerning and avoiding areas of scar tissue.17
Limitations—Diagnosis via the trichoscopy algorithm is limiting because it is not comprehensive of all hair and scalp disease.18 Additionally, many pathologies exhibit overlapping follicular and interfollicular patterning. For example, almost all subtypes of scarring alopecia present with hair loss and scarred follicles once they have progressed to advanced stages. Further studies should identify more specific patterns of hair and scalp pathologies, which could then be incorporated into a diagnostic algorithm.13
Advancements—The advent of hair analysis software has expanded the role of videodermoscopy by rapidly quantifying hair growth parameters such as hair count, follicular density, and follicular diameter, as well as interfollicular distances (Figure 2).14,17 Vellus and terminal hairs are differentiated according to their thickness and length.17 Moreover, the software can analyze the same area of the scalp over time by either virtual tattoos, semipermanent markings, or precise location measurements, increasing intra- and interclass correlation. The rate of hair growth, hair shedding, and parameters of anagen and telogen hairs can be studied by a method termed phototrichogram whereby a transitional area of hair loss and normal hair growth is identified and trimmed to less than 1 mm from the skin surface.19 A baseline photograph is taken using videodermoscopy. After approximately 3 days, the identical region is photographed and compared with the initial image to observe changes in the hair. Software programs can distinguish the growing hair as anagen and nongrowing hair as telogen, calculating the anagen-to-telogen ratio as well as hair growth rate, which are essential measurements in hair research and clinical studies. Software programs have replaced laborious and time-consuming manual hair counts and have rapidly grown in popularity in evaluating patterned hair loss.

Reflectance Confocal Microscopy
Reflectance confocal microscopy is a noninvasive imaging tool that visualizes skin and its appendages at near-histologic resolution (lateral resolution of 0.5–1 μm). It produces grayscale horizontal images that can be taken at levels ranging from the stratum corneum to the superficial papillary dermis, corresponding to a depth of approximately 100 to 150 µm. Thus, a hair follicle can be imaged starting from the follicular ostia down to the reachable papillary dermis (Figure 3).20 Image contrast is provided by differences in the size and refractive indices of cellular organelles.21,22 There are 2 commercially available RCM devices: VivaScope 1500 and VivaScope 3000 (Caliber Imaging & Diagnostics, Inc).

VivaScope 1500, a wide-probe microscope, requires the attachment of a plastic window to the desired imaging area. The plastic window is lined with medical adhesive tape to prevent movement during imaging. The adhesive tape can pull on hair upon removal, which is not ideal for patients with existing hair loss. Additionally, the image quality of VivaSope 1500 is best in flat areas and areas where hair is shaved.20,23,24 Despite these disadvantages, VivaScope 1500 has successfully shown utility in research studies, which suggests that these obstacles can be overcome by experienced users. The handheld VivaScope 3000 is ergonomically designed and suitable for curved surfaces such as the scalp, with the advantage of not requiring any adhesive. However, the images acquired from the VivaScope 3000 cover a smaller surface area.
Structures Visualized—Structures distinguished with RCM include keratinocytes, melanocytes, inflammatory cells, hair follicles, hair shafts, adnexal infundibular epithelium, blood vessels, fibroblasts, and collagen.23 Real-time visualization of blood flow also can be seen.
Applications of RCM—Reflectance confocal microscopy has been used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, chemotherapy-induced alopecia (CIA), alopecia areata, and androgenetic alopecia. Diagnostic RCM criteria for such alopecias have been developed based on their correspondence to histopathology. An RCM study of classic lichen planopilaris and frontal fibrosing alopecia identified features of epidermal disarray, infundibular hyperkeratosis, inflammatory cells, pigment incontinence, perifollicular fibrosis, bandlike scarring, melanophages in the dermis, dilated blood vessels, basal layer vacuolar degeneration, and necrotic keratinocytes.25 Pigment incontinence in the superficial epidermis, perifollicular lichenoid inflammation, and hyperkeratosis were characteristic RCM features of early-stage lichen planopilaris, while perifollicular fibrosis and dilated blood vessels were characteristic RCM features of late-stage disease. The ability of RCM features to distinguish different stages of lichen planopilaris shows its potential in treating early disease and preventing irreversible hair loss.
Differentiating between scarring and nonscarring alopecia also is possible through RCM. The presence of periadnexal, epidermal, and dermal inflammatory cells, in addition to periadnexal sclerosis, are defining RCM features of scarring alopecia.26 These features are absent in nonscarring alopecias. Reflectance confocal microscopy additionally has been shown to be useful in the treatment monitoring of lichen planopilaris and discoid lupus erythematosus.20 Independent reviewers, blinded to the patients’ identities, were able to characterize and follow features of these scarring alopecias by RCM. The assessed RCM features were comparable to those observed by histopathologic evaluation: epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intra-adnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells and melanophages, and dermal sclerosis. A reduction in inflammatory cells across multiple skin layers and at the level of the adnexal epithelium correlated with clinical response to treatment. Reflectance confocal microscopy also was able to detect recurrence of inflammation in cases where treatment had been interrupted before clinical signs of disease recurrence were evident. The authors thus concluded that RCM’s sensitivity can guide timing of treatment and avoid delays in starting or restarting treatment.20
Reflectance confocal microscopy also has served as a learning tool for new subclinical understandings of alopecia. In a study of CIA, the disease was found to be a dynamic process that could be categorized into 4 distinct phases distinguishable by combined confocal and dermoscopic features. This study also identified a new feature observable on RCM images—a CIA dot—defined as a dilated follicular infundibulum containing mashed, malted, nonhomogeneous material and normal or fragmented hair. This dot is thought to represent the initial microscopic sign of direct toxicity of chemotherapy on the hair follicle. Chemotherapy-induced alopecia dots persist throughout chemotherapy and subsequently disappear after chemotherapy ends.27
Limitations and Advantages—Currently, subtypes of cicatricial alopecias cannot be characterized on RCM because inflammatory cell types are not distinguished from each other (eg, eosinophils vs neutrophils). Another limitation of RCM is the loss of resolution below the superficial papillary dermis (a depth of approximately 150 µm); thus, deeper structures, such as the hair bulb, cannot be visualized.
Unlike global photography and trichoscopy, which are low-cost methods, RCM is much more costly, ranging upwards of several thousand dollars, and it may require additional technical support fees, making it less accessible for clinical practice. However, RCM imaging continues to be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.26 If a biopsy is required, RCM can aid in the selection of a biopsy site, as areas with active inflammation are more informative than atrophic and fibrosed areas.23 The role of RCM in trichoscopy can be expanded by designing a more cost-effective and ergonomically suited scope for hair and scalp assessment.
Optical Coherence Tomography
Optical coherence tomography is a noninvasive handheld device that emits low-power infrared light to visualize the skin and adnexal structures. Optical coherence tomography relies on the principle of interferometry to detect phase differences in optical backscattering at varying tissue depths.28,29 It allows visualization up to 2 mm, which is 2 to 5 times deeper than RCM.36 Unlike RCM, which has cellular resolution, OCT has an axial resolution of 3 to 15 μm, which allows only for the detection of structural boundaries.30 There are various OCT modalities that differ in lateral and axial resolutions and maximum depth. Commercial software is available that measures changes in vascular density by depth, epidermal thickness, skin surface texture, and optical attenuation—the latter being an indirect measurement of collagen density and skin hydration.
Structures Visualized—Hair follicles can be well distinguished on OCT images, and as such, OCT is recognized as a diagnostic tool in trichology (Figure 4).31 Follicular openings, interfollicular collagen, and outlines of the hair shafts are visible; however, detailed components of the follicular unit cannot be visualized by OCT. Keratin hyperrefractivity identifies the hair shaft. Additionally, the hair matrix is denoted by a slightly granular texture in the dermis. Dynamic OCT produces colorized images that visualize blood flow within vessels.

Applications of OCT—Optical coherence tomography is utilized in investigative trichology because it provides highly reproducible measurements of hair shaft diameters, cross-sectional surface areas, and form factor, which is a surrogate parameter for hair shape. The cross-section of hair shafts provides insight into local metabolism and perifollicular inflammation. Cross-sections of hair shafts in areas of alopecia areata were found to be smaller than cross-sections in the unaffected scalp within the same individual.32 Follicular density can be manually quantified on OCT images, but there also is promise for automated quantification. A recent study by Urban et al33 described training a convolutional neural network to automatically count hair as well as hair-bearing and non–hair-bearing follicles in OCT scans. These investigators also were able to color-code hair according to height, resulting in the creation of a “height” map.
Optical coherence tomography has furthered our understanding of the pathophysiology of cicatricial and nonscarring alopecias. Vazquez-Herrera et al34 assessed the inflammatory and cicatricial stages of frontal fibrosing alopecia by OCT imaging. Inflammatory hairlines, which are seen in the early stages of frontal fibrosing alopecia, exhibited a thickened dermis, irregular distribution of collagen, and increased vascularity in both the superficial and deep dermal layers compared to cicatricial and healthy scalp. Conversely, late-stage cicatricial areas exhibited a thin dermis and collagen that appeared in a hyperreflective, concentric, onion-shaped pattern around remnant follicular openings. Vascular flow was reduced in the superficial dermis of a cicatricial scalp but increased in the deep dermal layers compared with a healthy scalp. The attenuation coefficients of these disease stages also were assessed. The attenuation coefficient of the inflammatory hairline was higher compared with normal skin, likely as a reflection of inflammatory infiltrate and edema, whereas the attenuation coefficient of cicatricial scalp was lower compared with normal skin, likely reflecting the reduced water content of atrophic skin.34 This differentiation of early- and late-stage cicatricial alopecias has implications for early treatment and improved prognosis. Additionally, there is potential for OCT to assist in the differentiation of alopecia subtypes, as it can measure the epidermal thickness and follicular density and was previously used to compare scarring and nonscarring alopecia.35
Advantages and Limitations—Similar to RCM, OCT may be cost prohibitive for some clinicians. In addition, OCT cannot visualize the follicular unit in cellular detail. However, the extent of OCT’s capabilities may not be fully realized. Dynamic OCT is a new angiographic type of OCT that shows potential in monitoring early subclinical responses to novel alopecia therapies, such as platelet-rich plasminogen, which is hypothesized to stimulate hair growth through angiogenesis. Additionally, OCT may improve outcomes of hair transplantation procedures by allowing for visualization of the subcutaneous angle of hair follicles. Blind extraction of hair follicles in follicular unit extraction procedures can result in inadvertent transection and damage to the hair follicle; OCT could help identify good candidates for follicular unit extraction, such as patients with hair follicles in parallel arrangement, who are predicted to have better results.36
Conclusion
The field of trichology will continue to evolve with the emergence of noninvasive imaging technologies that diagnose hair disease in early stages and enable treatment monitoring with quantification of hair parameters. As discussed in this review, global photography, trichoscopy, RCM, and OCT have furthered our understanding of alopecia pathophysiology and provided objective methods of treatment evaluation. The capabilities of these tools will continue to expand with advancements in add-on software and AI algorithms.
New imaging tools along with adaptations to existing technologies have been emerging in recent years, with the potential to improve hair diagnostics and treatment monitoring. We provide an overview of 4 noninvasive hair imaging technologies: global photography, trichoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT). For each instrument, we discuss current and future applications in clinical practice and research along with advantages and disadvantages.
Global Photography
Global photography allows for the analysis of hair growth, volume, distribution, and density through serial standardized photographs.1 Global photography was first introduced for hair growth studies in 1987 and soon after was used for hair and scalp assessments in finasteride clinical trials.2
Hair Assessment—Washed, dried, and combed hair, without hair product, are required for accurate imaging; wet conditions increase reflection and promote hair clumping, thus revealing more scalp and depicting the patient as having less hair.1 Headshots are taken from short distances and use stereotactic positioning devices to create 4 global views: vertex, midline, frontal, and temporal.3 Stereotactic positioning involves fixing the patient’s chin and forehead as well as mounting the camera and flash device to ensure proper magnification. These adjustments ensure lighting remains consistent throughout consecutive study visits.4 Various grading scales are available for use in hair growth clinical studies to increase objectivity in the analysis of serial global photographs. A blinded evaluator should assess the before and after photographs to limit experimenter bias. Global photography often is combined with quantitative software analysis for improved detection of hair changes.1
Advancements—Growing interest in improving global photography has resulted in various application-based, artificial intelligence (AI)–mediated tools to simplify photograph collection and analysis. For instance, new hair analysis software utilizes AI algorithms to account for facial features in determining the optimal angle for capturing global photographs (Figure 1), which simplifies the generation of global photography images through smartphone applications and obviates the need for additional stereotactic positioning equipment.5,6

Limitations—Clinicians should be aware of global photography’s requirements for consistency in lighting, camera settings, film, and image processing, which can limit the accuracy of hair assessment over time if not replicated correctly.7,8 Emerging global photography software has helped to overcome some of these limitations.
Global photography is less precise when a patient’s hair loss is less than 50%, as it is difficult to discern subtle hair changes. Thus, global photography provides limited utility in assessing minimal to moderate hair loss.9 Currently, global photography largely functions as an adjunct tool for other hair analysis methods rather than as a stand-alone tool.
Trichoscopy
Trichoscopy (also known as dermoscopy of the hair and scalp) may be performed with a manual dermoscope (with 10× magnification) or a digital videodermatoscope (up to 1000× magnification).10-12 Unlike global photography, trichoscopy provides a detailed structural analysis of hair shafts, follicular openings, and perifollicular and interfollicular areas.13 Kinoshita-Ise and Sachdeva13 provided an in-depth, updated review of trichoscopy terminology with their definitions and associated conditions (with prevalence), which should be referenced when performing trichoscopic examination.
Hair Assessment—Trichoscopic assessment begins with inspection of follicular openings (also referred to as “dots”), which vary in color depending on the material filling them—degrading keratinocytes, keratin, sebaceous debris, melanin, or fractured hairs.13 The structure of hair shafts also is examined, showing broken hairs, short vellus hairs, and comma hairs, among others. Perifollicular areas are examined for scale, erythema, blue-gray dots, and whitish halos. Interfollicular areas are examined for pigment pattern as well as vascularization, which often presents in a looping configuration under dermoscopy. A combination of dot colorization, hair shaft structure, and perifollicular and interfollicular findings inform diagnostic algorithms of hair and scalp conditions. For example, central centrifugal cicatricial alopecia, the most common alopecia seen in Black women, has been associated with a combination of honeycomb pigment pattern, perifollicular whitish halo, pinpoint white dots, white patches, and perifollicular erythema.13
Advantages—Perhaps the most useful feature of trichoscopy is its ability to translate visualized features into simple diagnostic algorithms. For instance, if the clinician has diagnosed the patient with noncicatricial alopecia, they would next focus on dot colors. With black dots, the next step would be to determine whether the hairs are tapered or coiled, and so on. This systematic approach enables the clinician to narrow possible diagnoses.2 An additional advantage of trichoscopy is that it examines large surface areas noninvasively as compared to hair-pull tests and scalp biopsy.14,15 Trichoscopy allows temporal comparisons of the same area for disease and treatment monitoring with more diagnostic detail than global photography.16 Trichoscopy also is useful in selecting biopsy locations by discerning and avoiding areas of scar tissue.17
Limitations—Diagnosis via the trichoscopy algorithm is limiting because it is not comprehensive of all hair and scalp disease.18 Additionally, many pathologies exhibit overlapping follicular and interfollicular patterning. For example, almost all subtypes of scarring alopecia present with hair loss and scarred follicles once they have progressed to advanced stages. Further studies should identify more specific patterns of hair and scalp pathologies, which could then be incorporated into a diagnostic algorithm.13
Advancements—The advent of hair analysis software has expanded the role of videodermoscopy by rapidly quantifying hair growth parameters such as hair count, follicular density, and follicular diameter, as well as interfollicular distances (Figure 2).14,17 Vellus and terminal hairs are differentiated according to their thickness and length.17 Moreover, the software can analyze the same area of the scalp over time by either virtual tattoos, semipermanent markings, or precise location measurements, increasing intra- and interclass correlation. The rate of hair growth, hair shedding, and parameters of anagen and telogen hairs can be studied by a method termed phototrichogram whereby a transitional area of hair loss and normal hair growth is identified and trimmed to less than 1 mm from the skin surface.19 A baseline photograph is taken using videodermoscopy. After approximately 3 days, the identical region is photographed and compared with the initial image to observe changes in the hair. Software programs can distinguish the growing hair as anagen and nongrowing hair as telogen, calculating the anagen-to-telogen ratio as well as hair growth rate, which are essential measurements in hair research and clinical studies. Software programs have replaced laborious and time-consuming manual hair counts and have rapidly grown in popularity in evaluating patterned hair loss.

Reflectance Confocal Microscopy
Reflectance confocal microscopy is a noninvasive imaging tool that visualizes skin and its appendages at near-histologic resolution (lateral resolution of 0.5–1 μm). It produces grayscale horizontal images that can be taken at levels ranging from the stratum corneum to the superficial papillary dermis, corresponding to a depth of approximately 100 to 150 µm. Thus, a hair follicle can be imaged starting from the follicular ostia down to the reachable papillary dermis (Figure 3).20 Image contrast is provided by differences in the size and refractive indices of cellular organelles.21,22 There are 2 commercially available RCM devices: VivaScope 1500 and VivaScope 3000 (Caliber Imaging & Diagnostics, Inc).

VivaScope 1500, a wide-probe microscope, requires the attachment of a plastic window to the desired imaging area. The plastic window is lined with medical adhesive tape to prevent movement during imaging. The adhesive tape can pull on hair upon removal, which is not ideal for patients with existing hair loss. Additionally, the image quality of VivaSope 1500 is best in flat areas and areas where hair is shaved.20,23,24 Despite these disadvantages, VivaScope 1500 has successfully shown utility in research studies, which suggests that these obstacles can be overcome by experienced users. The handheld VivaScope 3000 is ergonomically designed and suitable for curved surfaces such as the scalp, with the advantage of not requiring any adhesive. However, the images acquired from the VivaScope 3000 cover a smaller surface area.
Structures Visualized—Structures distinguished with RCM include keratinocytes, melanocytes, inflammatory cells, hair follicles, hair shafts, adnexal infundibular epithelium, blood vessels, fibroblasts, and collagen.23 Real-time visualization of blood flow also can be seen.
Applications of RCM—Reflectance confocal microscopy has been used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, chemotherapy-induced alopecia (CIA), alopecia areata, and androgenetic alopecia. Diagnostic RCM criteria for such alopecias have been developed based on their correspondence to histopathology. An RCM study of classic lichen planopilaris and frontal fibrosing alopecia identified features of epidermal disarray, infundibular hyperkeratosis, inflammatory cells, pigment incontinence, perifollicular fibrosis, bandlike scarring, melanophages in the dermis, dilated blood vessels, basal layer vacuolar degeneration, and necrotic keratinocytes.25 Pigment incontinence in the superficial epidermis, perifollicular lichenoid inflammation, and hyperkeratosis were characteristic RCM features of early-stage lichen planopilaris, while perifollicular fibrosis and dilated blood vessels were characteristic RCM features of late-stage disease. The ability of RCM features to distinguish different stages of lichen planopilaris shows its potential in treating early disease and preventing irreversible hair loss.
Differentiating between scarring and nonscarring alopecia also is possible through RCM. The presence of periadnexal, epidermal, and dermal inflammatory cells, in addition to periadnexal sclerosis, are defining RCM features of scarring alopecia.26 These features are absent in nonscarring alopecias. Reflectance confocal microscopy additionally has been shown to be useful in the treatment monitoring of lichen planopilaris and discoid lupus erythematosus.20 Independent reviewers, blinded to the patients’ identities, were able to characterize and follow features of these scarring alopecias by RCM. The assessed RCM features were comparable to those observed by histopathologic evaluation: epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intra-adnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells and melanophages, and dermal sclerosis. A reduction in inflammatory cells across multiple skin layers and at the level of the adnexal epithelium correlated with clinical response to treatment. Reflectance confocal microscopy also was able to detect recurrence of inflammation in cases where treatment had been interrupted before clinical signs of disease recurrence were evident. The authors thus concluded that RCM’s sensitivity can guide timing of treatment and avoid delays in starting or restarting treatment.20
Reflectance confocal microscopy also has served as a learning tool for new subclinical understandings of alopecia. In a study of CIA, the disease was found to be a dynamic process that could be categorized into 4 distinct phases distinguishable by combined confocal and dermoscopic features. This study also identified a new feature observable on RCM images—a CIA dot—defined as a dilated follicular infundibulum containing mashed, malted, nonhomogeneous material and normal or fragmented hair. This dot is thought to represent the initial microscopic sign of direct toxicity of chemotherapy on the hair follicle. Chemotherapy-induced alopecia dots persist throughout chemotherapy and subsequently disappear after chemotherapy ends.27
Limitations and Advantages—Currently, subtypes of cicatricial alopecias cannot be characterized on RCM because inflammatory cell types are not distinguished from each other (eg, eosinophils vs neutrophils). Another limitation of RCM is the loss of resolution below the superficial papillary dermis (a depth of approximately 150 µm); thus, deeper structures, such as the hair bulb, cannot be visualized.
Unlike global photography and trichoscopy, which are low-cost methods, RCM is much more costly, ranging upwards of several thousand dollars, and it may require additional technical support fees, making it less accessible for clinical practice. However, RCM imaging continues to be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.26 If a biopsy is required, RCM can aid in the selection of a biopsy site, as areas with active inflammation are more informative than atrophic and fibrosed areas.23 The role of RCM in trichoscopy can be expanded by designing a more cost-effective and ergonomically suited scope for hair and scalp assessment.
Optical Coherence Tomography
Optical coherence tomography is a noninvasive handheld device that emits low-power infrared light to visualize the skin and adnexal structures. Optical coherence tomography relies on the principle of interferometry to detect phase differences in optical backscattering at varying tissue depths.28,29 It allows visualization up to 2 mm, which is 2 to 5 times deeper than RCM.36 Unlike RCM, which has cellular resolution, OCT has an axial resolution of 3 to 15 μm, which allows only for the detection of structural boundaries.30 There are various OCT modalities that differ in lateral and axial resolutions and maximum depth. Commercial software is available that measures changes in vascular density by depth, epidermal thickness, skin surface texture, and optical attenuation—the latter being an indirect measurement of collagen density and skin hydration.
Structures Visualized—Hair follicles can be well distinguished on OCT images, and as such, OCT is recognized as a diagnostic tool in trichology (Figure 4).31 Follicular openings, interfollicular collagen, and outlines of the hair shafts are visible; however, detailed components of the follicular unit cannot be visualized by OCT. Keratin hyperrefractivity identifies the hair shaft. Additionally, the hair matrix is denoted by a slightly granular texture in the dermis. Dynamic OCT produces colorized images that visualize blood flow within vessels.

Applications of OCT—Optical coherence tomography is utilized in investigative trichology because it provides highly reproducible measurements of hair shaft diameters, cross-sectional surface areas, and form factor, which is a surrogate parameter for hair shape. The cross-section of hair shafts provides insight into local metabolism and perifollicular inflammation. Cross-sections of hair shafts in areas of alopecia areata were found to be smaller than cross-sections in the unaffected scalp within the same individual.32 Follicular density can be manually quantified on OCT images, but there also is promise for automated quantification. A recent study by Urban et al33 described training a convolutional neural network to automatically count hair as well as hair-bearing and non–hair-bearing follicles in OCT scans. These investigators also were able to color-code hair according to height, resulting in the creation of a “height” map.
Optical coherence tomography has furthered our understanding of the pathophysiology of cicatricial and nonscarring alopecias. Vazquez-Herrera et al34 assessed the inflammatory and cicatricial stages of frontal fibrosing alopecia by OCT imaging. Inflammatory hairlines, which are seen in the early stages of frontal fibrosing alopecia, exhibited a thickened dermis, irregular distribution of collagen, and increased vascularity in both the superficial and deep dermal layers compared to cicatricial and healthy scalp. Conversely, late-stage cicatricial areas exhibited a thin dermis and collagen that appeared in a hyperreflective, concentric, onion-shaped pattern around remnant follicular openings. Vascular flow was reduced in the superficial dermis of a cicatricial scalp but increased in the deep dermal layers compared with a healthy scalp. The attenuation coefficients of these disease stages also were assessed. The attenuation coefficient of the inflammatory hairline was higher compared with normal skin, likely as a reflection of inflammatory infiltrate and edema, whereas the attenuation coefficient of cicatricial scalp was lower compared with normal skin, likely reflecting the reduced water content of atrophic skin.34 This differentiation of early- and late-stage cicatricial alopecias has implications for early treatment and improved prognosis. Additionally, there is potential for OCT to assist in the differentiation of alopecia subtypes, as it can measure the epidermal thickness and follicular density and was previously used to compare scarring and nonscarring alopecia.35
Advantages and Limitations—Similar to RCM, OCT may be cost prohibitive for some clinicians. In addition, OCT cannot visualize the follicular unit in cellular detail. However, the extent of OCT’s capabilities may not be fully realized. Dynamic OCT is a new angiographic type of OCT that shows potential in monitoring early subclinical responses to novel alopecia therapies, such as platelet-rich plasminogen, which is hypothesized to stimulate hair growth through angiogenesis. Additionally, OCT may improve outcomes of hair transplantation procedures by allowing for visualization of the subcutaneous angle of hair follicles. Blind extraction of hair follicles in follicular unit extraction procedures can result in inadvertent transection and damage to the hair follicle; OCT could help identify good candidates for follicular unit extraction, such as patients with hair follicles in parallel arrangement, who are predicted to have better results.36
Conclusion
The field of trichology will continue to evolve with the emergence of noninvasive imaging technologies that diagnose hair disease in early stages and enable treatment monitoring with quantification of hair parameters. As discussed in this review, global photography, trichoscopy, RCM, and OCT have furthered our understanding of alopecia pathophysiology and provided objective methods of treatment evaluation. The capabilities of these tools will continue to expand with advancements in add-on software and AI algorithms.
- Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-721.
- Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Peytavi U, Hillmann K, Guarrera M, eds. Hair Growth and Disorders. 4th ed. Springer; 2008:140-144.
- Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10-18.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578-579.
- Capily Institute. Artificial intelligence (A.I.) powered hair growth tracking. Accessed July 31, 2023. https://tss-aesthetics.com/capily-hair-tracking-syst
- Dinh Q, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189-199.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4:259-264.
- Alessandrini A, Bruni F, Piraccini BM, et al. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629-640.
- Ashique K, Kaliyadan F. Clinical photography for trichology practice: tips and tricks. Int J Trichology. 2011;3:7-13.
- Rudnicka L, Olszewska M, Rakowska A, et al. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-654.
- Kinoshita-Ise M, Sachdeva M. Update on trichoscopy: integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J Dermatol. 2022;49:4-18. doi:10.1111/1346-8138.16233
- Van Neste D, Trüeb RM. Critical study of hair growth analysis with computer-assisted methods. J Eur Acad Dermatol Venereol. 2006;20:578-583.
- Romero J, Grimalt R. Trichoscopy: essentials for the dermatologist. World J Dermatol. 2015;4:63-68.
- Trichoscopy: a new frontier for the diagnosis of hair diseases. Exp Rev Dermatol. 2012;7:429-437.
- Lee B, Chan J, Monselise A, et al. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66:166-167.
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2010;38:71-75.
- Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242-244.
- Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165:534-540.
- Kuck M, Schanzer S, Ulrich M, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Opt. 2012;17:101504.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4:1014-1023.
- Agozzino M, Ardigò M. Scalp confocal microscopy. In: Humbert P, Maibach H, Fanian F, et al, eds. Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. 2nd ed. Springer International Publishing; 2016:311-326.
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008;2:55-59.
- Kurzeja M, Czuwara J, Walecka I, et al. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27:266-271.
- Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-318.
- Franceschini C, Garelli V, Persechino F, et al. Dermoscopy and confocal microscopy for different chemotherapy-induced alopecia (CIA) phases characterization: preliminary study. Skin Res Technol. 2020;26:269-276.
- Martinez-Velasco MA, Perper M, Maddy AJ, et al. In vitro determination of Mexican Mestizo hair shaft diameter using optical coherence tomography. Skin Res Technol. 2018;24;274-277.
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019;104:108-113.
- Wan B, Ganier C, Du-Harpur X, et al. Applications and future directions for optical coherence tomography in dermatology. Br J Dermatol. 2021;184:1014-1022.
- Blume-Peytavi U, Vieten J, Knuttel A et al. Optical coherent tomography (OCT): a new method for online-measurement of hair shaft thickness. J Dtsch Dermatol Ges. 2004;2:546.
- Garcia Bartels N, Jahnke I, Patzelt A, et al. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol. 2011;17:201-205.
- Urban G, Feil N, Csuka E, et al. Combining deep learning with optical coherence tomography imaging to determine scalp hair and follicle counts. Lasers Surg Med. 2021;53:171-178.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, et al. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32:318-322.
- Ekelem C, Feil N, Csuka E, et al. Optical coherence tomography in the evaluation of the scalp and hair: common features and clinical utility. Lasers Surg Med. 2021;53:129-140.
- Schicho K, Seemann R, Binder M, et al. Optical coherence tomography for planning of follicular unit extraction. Dermatol Surg. 2015;41:358-363.
- Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-721.
- Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Peytavi U, Hillmann K, Guarrera M, eds. Hair Growth and Disorders. 4th ed. Springer; 2008:140-144.
- Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10-18.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578-579.
- Capily Institute. Artificial intelligence (A.I.) powered hair growth tracking. Accessed July 31, 2023. https://tss-aesthetics.com/capily-hair-tracking-syst
- Dinh Q, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189-199.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4:259-264.
- Alessandrini A, Bruni F, Piraccini BM, et al. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629-640.
- Ashique K, Kaliyadan F. Clinical photography for trichology practice: tips and tricks. Int J Trichology. 2011;3:7-13.
- Rudnicka L, Olszewska M, Rakowska A, et al. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-654.
- Kinoshita-Ise M, Sachdeva M. Update on trichoscopy: integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J Dermatol. 2022;49:4-18. doi:10.1111/1346-8138.16233
- Van Neste D, Trüeb RM. Critical study of hair growth analysis with computer-assisted methods. J Eur Acad Dermatol Venereol. 2006;20:578-583.
- Romero J, Grimalt R. Trichoscopy: essentials for the dermatologist. World J Dermatol. 2015;4:63-68.
- Trichoscopy: a new frontier for the diagnosis of hair diseases. Exp Rev Dermatol. 2012;7:429-437.
- Lee B, Chan J, Monselise A, et al. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66:166-167.
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2010;38:71-75.
- Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242-244.
- Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165:534-540.
- Kuck M, Schanzer S, Ulrich M, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Opt. 2012;17:101504.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4:1014-1023.
- Agozzino M, Ardigò M. Scalp confocal microscopy. In: Humbert P, Maibach H, Fanian F, et al, eds. Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. 2nd ed. Springer International Publishing; 2016:311-326.
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008;2:55-59.
- Kurzeja M, Czuwara J, Walecka I, et al. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27:266-271.
- Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-318.
- Franceschini C, Garelli V, Persechino F, et al. Dermoscopy and confocal microscopy for different chemotherapy-induced alopecia (CIA) phases characterization: preliminary study. Skin Res Technol. 2020;26:269-276.
- Martinez-Velasco MA, Perper M, Maddy AJ, et al. In vitro determination of Mexican Mestizo hair shaft diameter using optical coherence tomography. Skin Res Technol. 2018;24;274-277.
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019;104:108-113.
- Wan B, Ganier C, Du-Harpur X, et al. Applications and future directions for optical coherence tomography in dermatology. Br J Dermatol. 2021;184:1014-1022.
- Blume-Peytavi U, Vieten J, Knuttel A et al. Optical coherent tomography (OCT): a new method for online-measurement of hair shaft thickness. J Dtsch Dermatol Ges. 2004;2:546.
- Garcia Bartels N, Jahnke I, Patzelt A, et al. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol. 2011;17:201-205.
- Urban G, Feil N, Csuka E, et al. Combining deep learning with optical coherence tomography imaging to determine scalp hair and follicle counts. Lasers Surg Med. 2021;53:171-178.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, et al. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32:318-322.
- Ekelem C, Feil N, Csuka E, et al. Optical coherence tomography in the evaluation of the scalp and hair: common features and clinical utility. Lasers Surg Med. 2021;53:129-140.
- Schicho K, Seemann R, Binder M, et al. Optical coherence tomography for planning of follicular unit extraction. Dermatol Surg. 2015;41:358-363.
Practice Points
- Reflectance confocal microscopy (RCM) imaging can be taken at levels from the stratum corneum to the papillary dermis and can be used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, alopecia areata, and androgenetic alopecia.
- Because of its ability to distinguish different stages of disease, RCM can be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.
- Optical coherence tomography has the potential to monitor early subclinical responses to alopecia therapies while also improving hair transplantation outcomes by allowing for visualization of the subcutaneous angle of hair follicles.
- Software development paired with trichoscopy has the ability to quantify hair growth parameters such as hair count, density, and diameter.