User login
Prospective Randomized Evaluation of Preoperative Angiotensin-Converting Enzyme Inhibition (PREOP-ACEI)
Over 7 million surgeries are performed in United States hospitals each year. Among these surgeries, approximately 85% are noncardiac, nonvascular (NCNV) procedures.1,2 Although the preoperative use of an angiotensin-converting enzyme inhibitor (ACEI) can be expected in as many as 13% of these surgeries,3 the optimal preoperative ACEI management strategy for patients undergoing NCNV surgeries is poorly understood.
High-quality evidence suggests that renin–angiotensin–aldosterone system (RAAS) inhibitors are associated with intraoperative hypotension among patients undergoing cardiac or vascular surgeries.4-6 Intraoperative hypotension increases the risk of 30-day mortality,7 and the duration of intraoperative hypotension increases the risk of end organ damage.8,9 This body of evidence suggests that withholding ACEIs prior to cardiac and vascular surgeries is safer than continuing ACEIs without interruption.
The evidence concerning perioperative management of ACEIs is inconclusive for patients undergoing NCNV procedures. Some studies comparing patients taking or not taking a RAAS inhibitor preoperatively describe negligible differences in the frequency of intraoperative hypotensive episodes or complications.3,10 Others have found an increased risk of intraoperative hypotension and associated postoperative adverse events in patients continuing RAAS inhibitors preoperatively.11,12 Current guideline discrepancies reflect the uncertainty of the evidence. The guidelines set by the American College of Cardiology and American Heart Association (ACC/AHA) suggest the uninterrupted perioperative continuation of RAAS inhibitors.13 The guidelines provided by the European Society of Cardiology and European Society of Anaesthesiology also suggest the continuation of RAAS inhibitors throughout the perioperative period for patients with systolic heart failure but recommend transient discontinuation for patients with hypertension.14
This randomized study aimed to compare the effect of two practical strategies for preoperative ACEI management on the perioperative blood pressure of patients undergoing NCNV surgery. The two strategies studied were the omission of the final preoperative ACEI dose and the uninterrupted continuation of ACEI therapy. We hypothesized that patients randomized to ACEI omission would experience intraoperative hypotensive episodes less frequently than those randomized to ACEI continuation.
METHODS
Study Design and Setting
We performed a prospective randomized controlled trial (ClinicalTrials.gov: NCT01669434). The study was carried out in a preoperative evaluation clinic and its affiliated 489-bed academic medical center. Anesthesiologists and internal medicine physicians work collaboratively in the clinic to assess more than 5,000 patients annually (one-third of the institution’s elective surgeries). Patients were randomized 1:1 in block sizes of 5 and 10 and stratified by age < 65 and ≥ 65 years to the omission or continuation of the final preoperative ACEI dose (whether that dose was scheduled for the morning of surgery or the night prior). Preoperative clinicians enrolled patients and subsequently assigned them to intervention groups on the basis of a sequentially numbered list. Patients and healthcare providers were not blinded to allocation status. Intraoperative and postoperative management was provided in accordance with usual care as decided by treatment team.
Participants
Patients who presented to the preoperative evaluation clinic between May 2015 and November 2016 and who had been taking an ACEI for at least 6 weeks were eligible for inclusion. Patients taking angiotensin receptor blockers were excluded. Enrollment was limited to patients planning NCNV surgery. Patients planning intrathoracic, major vascular, organ transplant, and oncologic surgery were excluded. Patients undergoing outpatient procedures not requiring an overnight stay in the hospital were also excluded. Patients with preoperative clinic systolic blood pressure (SBP) <90 or ≥160 or diastolic blood pressure (DBP) <60 or ≥ 95 were excluded. Patients with moderate to severe or clinically decompensated heart failure (left ventricular ejection fraction < 40% or New York Heart Association class III or IV) and those with end-stage renal disease requiring dialysis were also excluded. Patients presenting more than once during the accrual period were eligible for the initial surgery only. All participating patients provided written informed consent. This project was approved by the University of Nebraska Medical Center Institutional Review Board.
Data Collection
Baseline characteristics were recorded by study personnel at the time of enrollment. We measured serum creatinine level at the preoperative visit and on postoperative day 1. An automated anesthesia information management system was used to measure intraoperative blood pressures every three minutes. Postoperative blood pressures through discharge were measured by hospital staff per usual care. During postoperative hospitalization, we queried patients about preoperative adherence to allocation. The digital abstraction of data from the electronic medical record was supplemented by chart review when necessary.
Outcomes
The primary outcome was intraoperative hypotension defined as any SBP < 80 mm Hg occurring from the administration of the first induction agent through transfer to the postanesthesia care unit (PACU). We also examined hypotension during anesthesia induction, which we defined as the 20-minute period following the administration of the first anesthesia induction agent. Episodes of SBP < 80 were defined as being associated with vasopressor administration when any vasopressor was administered during or within 10 min of the episode.
Secondary analyses included postoperative acute kidney injury (AKI), postoperative hypotensive and hypertensive episodes, cardiac events, and mortality. When comparing postoperative day 1 creatinine levels to preoperative creatinine levels, we used the Acute Kidney Injury Network definition of AKI as an increase in creatinine of 0.3 mg/dl or 50%.15 Postoperative hypotension was defined as any SBP < 90 mm Hg and postoperative hypertension as any SBP > 180 mm Hg occurring after arrival in the PACU. Major adverse cardiac events (MACE) were defined as a composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia. Discharge from the hospital served as the study endpoint for each patient.
Analysis
Fisher’s exact test was used to compare categorical outcomes between groups. The independent sample t-test or Wilcoxon rank–sum test, as appropriate, was used to compare continuous measures. We selected Fisher’s exact test over χ2-test to produce conservative estimates. Patients were maintained in their allocated group as randomized for analytical purposes regardless of adherence to allocation. We performed all analyses using SAS version 9.4 for Windows (SAS institute, Cary, North Carolina).
We estimated that a sample size of 300 patients would achieve 80% power to detect a difference of 0.17 between the group proportions of 0.33 and 0.50 at a significance level (ɑ) of 0.05 by using a two-sided z-test with continuity correction, assuming 15% loss to follow-up. This estimate allowed for 1 interim analysis using the O’Brien-Fleming spending function truncated at three standard deviations to determine the test boundaries. The monitoring boundary P values associated with the interim analysis were .003, and the threshold P value for the final analysis was .049.
RESULTS
Study Flow
A total of 453 patients were screened for eligibility. Among these patients, 162 were excluded, and the remaining 291 patients were randomized (Figure 1). Surgery was cancelled in six patients allocated to omission and in four patients allocated to continuation arms, respectively. Moreover, three patients in the omission arm were excluded from the analysis following randomization. Specifically, one was excluded because of early discharge without overnight stay, one was excluded because of withdrawal of consent, and one was excluded because of missing primary outcome data. In addition, three cases in the continuation arm were excluded following randomization because of the preoperative (permanent) discontinuation of ACEI therapy in two cases and discharge without an overnight stay in one case. Finally, 275 patients were included in the analysis: 137 in the ACEI omission group and 138 in the ACEI continuation group. Adherence to allocation was 88% and 92% in the omission and continuation groups, respectively.
Baseline Characteristics
The demographic data of patients allocated to ACEI omission and those allocated to ACEI continuation were similar (Table 1). A large majority of patients in both groups took the ACEI lisinopril. Overall, 187 of 275 (68%) patients were taking at least 1 antihypertensive agent, most commonly a diuretic, in addition to an ACEI. SBP measured during the preoperative clinic visit averaged 136.5 mm Hg and did not differ significantly between groups (P = .84).
Surgical Variables
General anesthesia was the most commonly utilized technique, although spinal and regional anesthesia were also represented (Table 1). The majority of cases in both groups were planning for orthopedic and spinal surgery. The method of anesthesia or type of surgery between patients allocated to ACEI omission and those allocated to continuation did not differ (P = .61 and P = .45 respectively).
Episodes of Intraoperative Hypotension
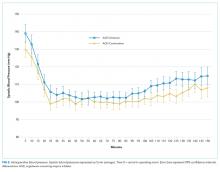
Intraoperative SBPs are displayed in Figure 2, and hemodynamic outcomes are summarized in Table 2. Episodes of SBP < 80 mm Hg during anesthesia induction were numerically less frequent in the omission group than in the continuation group; the difference between groups, however, was not statistically significant (24 of 137 [18%] vs 38 of 138 [28%], RR: 0.64, 95% CI: 0.40 to 1.00, P = .06).
Duration of Intraoperative Hypotension
The median cumulative duration of intraoperative SBP < 80 was two minutes (range 0-41) in patients allocated to the ACEI omission group compared with seven minutes (range 0-214) in those allocated to the continuation group (P < .01). The median cumulative duration of mean arterial pressure < 55 mm Hg was also shorter in the omission group (median 0 min [range 0-39] vs 3 min [range 0-122], P < .01) than in the continuation group. The duration of surgery did not differ between groups (median 141 min [range 77-554] vs 142 min [range 57-665], P = .97).
Postoperative Outcomes
RAAS inhibitor therapy was resumed within 48 h after surgery in 122 of 137 (89%) patients allocated to the omission group and in 128 of 138 (93%) patients allocated to the continuation group (RR: 0.96, 95% CI: 0.89-1.03, P = .30).
Patients allocated to the omission group were significantly less likely to experience postoperative hypotension (15 of 137 [11%] vs 31 of 138 [22%], RR: 0.49, 95% CI: 0.28 to 0.86, P = .02) and significantly more likely to experience severe postoperative hypertension (33 of 137 [24%] vs 17 of 138 [12%], RR: 1.95, 95% CI: 1.14 to 3.34, P = .01) than those allocated to the continuation group. The occurrences of postoperative AKI (RR: 0.60, 95% CI: 0.23 to 1.60, P = .44) or MACE (RR: 4.03, 95% CI: 0.46 to 35.59, P = .21) in the omission group did not differ from the continuation group. The two groups exhibited similar PACU recovery time (mean 97.2 min) and overall hospital length of stay (mean 3.0 days) (P = .49 and P = .56 ). No episodes of inpatient mortality in either group were observed.
DISCUSSION
The omission of the final preoperative ACEI dose was associated with a significant reduction in the risk of intraoperative hypotension in patients undergoing NCNV surgery. This result confirmed our hypothesis. Coupled with the knowledge that intraoperative hypotension is associated with an increased risk of complications and mortality,7-9,16 this study favors the omission of the final preoperative ACEI dose prior to NCNV surgeries.
Our findings are in agreement with those of previous randomized studies that explored this question4,5 and help extend results from cardiac and vascular surgeries to NCNV surgeries. Previous studies on the use of RAAS inhibitors in NCNV surgeries did not employ randomization and yielded mixed results.3,10-12,17 A large single-institution study (n = 18,056) noted no difference in intraoperative blood pressure between patients taking ACEIs and a matched group of non-ACEI users.3 More recently, a subgroup analysis of the international VISION study showed that omitting RAAS inhibitors on the day of surgery reduced the risk of intraoperative hypotension.11 In that analysis, however, only a small amount of the variability in preoperative RAAS inhibitor management was explainable by modeling known factors, thus allowing for the possibility of unmeasured confounding. Our study, which minimized confounding through randomization, is the first to prospectively compare protocols for patients undergoing NCNV surgery. In contrast to previous studies, the present study was able to report the lack of difference in postoperative RAAS inhibitor administration between study groups. Postoperative RAAS inhibitor management affects complications and mortality.18,19
Our present finding that preoperative ACEI management affects postoperative hypotensive and hypertensive events conflicts with some previous findings.11,20 However, recent evidence has revealed that postoperative hypotensive episodes are associated with vascular events and mortality.11,21 In the context of that evidence, our study lends further support to the omission of the final preoperative ACEI dose. However, we did not detect any decrease in AKI, MACE, or mortality in the ACEI omission group.
This study should be considered in light of its limitations. The pragmatic nature of the study allowed for certain potential biases. Although adherence to allocation was high, the specific ACEI agent taken and the exact timing of the final dose in relation to surgery were not controlled. Anesthetic and postoperative management decisions were made by the treatment team and may have systematically varied given that the treatment team was not blinded to allocation. Furthermore, all outcome data were collected as part of routine care and may not have captured events with great fidelity. Generalizability is limited by the execution of the study at a single academic institution, the preponderance of orthopedic and spine surgeries, and by the negligible representation of ethnicities other than Caucasian. Additionally, recruitment from the preoperative evaluation clinic likely resulted in a patient group with greater comorbidity than the overall population of patients undergoing NCNV surgery. This study was powered for intraoperative hypotension and not postoperative outcomes. Our primary outcome, intraoperative hypotension, is an intermediate measure but one that has well-established associations with adverse outcomes, including mortality. One study showed that sustaining an intraoperative SBP below 70 mm Hg for longer than 5 min increased the risk of mortality from less than 1% to nearly 6%.16 A large study detected an increase in mortality associated with SBP sustained below 80 mm Hg for 10 min or longer.7 Intraoperative hypotension has also been associated with postoperative AKI and myocardial injury.8,9,12
Many of the limitations of the current study could be addressed by a large randomized controlled trial of ACEI management prior to NCNV surgeries that examines clinically important endpoints beyond intraoperative hypotension. Several specific aspects of perioperative RAAS inhibitor management also deserve further investigation. Our findings may not be generalizable to patients taking ARBs or to patients with congestive heart failure. The preoperative management of ARBs and the preoperative management of RAAS inhibitors in those with congestive heart failure are important areas of focus for future research. Lastly, our finding that preoperative ACEI management decisions can affect postoperative hypotensive and hypertensive events should be substantiated by future research, and any negative consequences of those events should be further explored.
Nonetheless, our study is the largest randomized study of preoperative RAAS inhibition published to date. More than twice as many patients were randomized in this study than in all previous randomized studies combined.4-6 To the best of our knowledge, this is also the first randomized study evaluating NCNV surgeries. Finally, our use of a practical ACEI omission protocol based on known pharmacokinetics allows for direct application to clinical practice.
CONCLUSION
Hypertension is among the most common chronic conditions encountered in patients planning surgery, and ACEIs are among the most frequently prescribed antihypertensive medications. This study showed that ACEI continuation is associated with an increased frequency and cumulative duration of intraoperative hypotension. These findings, while at odds with current ACC/AHA guidelines, align with the findings of a meta-analysis on this subject and with recent literature.3,11-13,22
Acknowledgments
The authors wish to thank Miranda M Fricke, MS, PA-C; Tiffany K Hillyard, APRN-FNP; and Barbara Sink, MPAS, PA-C who assisted in the design and conduct of patient enrollment and randomization procedures.
Disclosures
The authors have no relevant financial conflicts of interest to report.
Funding
This study was subsidized by a grant from the University of Nebraska Medical Center Research Support Fund. The funding source had no role in the design, conduct, analysis, or reporting of the study.
1. Steiner CA KZ, Moore BJ, Imshaug MC, Pickens G. Surgeries in hospital-based ambulatory surgery and hospital inpatient settings, 2014. Statistical Brief 2017; 1-18. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb223-Ambulatory-Inpatient-Surgeries-2014.pdf. Accessed August 30, 2017. PubMed
2. Rate of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. National Hospital Discharge Survey 2010; https://www.cdc.gov/nchs/nhds/nhds_tables.htm. Accessed August 30, 2017.
3. Turan A, You J, Shiba A, Kurz A, Saager L, Sessler DI. Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114(3):552-560. doi: 10.1213/ANE.0b013e318241f6af. PubMed
4. Coriat P, Richer C, Douraki T, et al. Influence of chronic angiotensin-converting enzyme inhibition on anesthetic induction. Anesthesiology. 1994;81:299-307. PubMed
5. Pigott DW, Nagle C, Allman K, S. W, D. ER. Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements. Br J Anaesth. 1999;83:715-720. doi: 10.1093/bja/83.5.715 PubMed
6. Bertrand M, Godet G, Meersschaert K, Brun L, Salcedo E, Coriat P. Should the angiotensin II antagonists be discontinued before surgery? Anesth Analg. 2001;92:26-30. PubMed
7. Mascha EJ, Yang D, Weiss S, Sessler DI. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015;123(1):79-91. doi: 10.1097/ALN.0000000000000686. PubMed
8. Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507-515. doi: 10.1097/ALN.0b013e3182a10e26. PubMed
9. Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47-65. doi: 10.1097/ALN.0000000000001432. PubMed
10. Comfere T, Sprung J, Kumar MM, et al. Angiotensin system inhibitors in a general surgical population. Anesth Analg. 2005;100(3):636-644. doi: 10.1213/01.ANE.0000146521.68059.A1. PubMed
11. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the vascular events in noncardiac surgery patIents cohort evaluation prospective cohort. Anesthesiology. 2017;126(1):16-27. doi: 10.1097/ALN.0000000000001404. PubMed
12. Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med. 2014;9(5):283-288. doi: 10.1002/jhm.2155. PubMed
13. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-137. doi: 10.1016/j.jacc.2014.07.944. PubMed
14. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. doi: 10.1093/eurheartj/ehu282 PubMed
15. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713 PubMed
16. Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123(2):307-319. doi: 10.1097/ALN.0000000000000756. PubMed
17. Kheterpal S, Khodaparast O, Shanks A, O’Reilly M, Tremper KK. Chronic angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy combined with diuretic therapy is associated with increased episodes of hypotension in noncardiac surgery. J Cardiothorac Vasc Anesth. 2008;22(2):180-186. 10.1053/j.jvca.2007.12.020. PubMed
18. Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the veterans affairs healthcare system. Anesthesiology. 2015;123(2):288-306. 10.1097/ALN.0000000000000739. PubMed
19. Drenger B, Fontes ML, Miao Y, et al. Patterns of use of perioperative angiotensin-converting enzyme inhibitors in coronary artery bypass graft surgery with cardiopulmonary bypass: effects on in-hospital morbidity and mortality. Circulation. 2012;126(3):261-269. doi: 10.1161/CIRCULATIONAHA.111.059527. PubMed
20. Twersky RS, Goel V, Narayan P, Weedon J. The risk of hypertension after preoperative discontinuation of angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists in ambulatory and same-day admission patients. Anesth Analg. 2014;118(5):938-944. doi: 10.1213/ANE.0000000000000076. PubMed
21. Tan TW, Eslami MH, Kalish JA, et al. The need for treatment of hemodynamic instability following carotid endarterectomy is associated with increased perioperative and 1-year morbidity and mortality. J Vasc Surg. 2014;59(1):16-24 e11-12. https://doi.org/10.1053/j.jvca.2014.12.002 PubMed
22. Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319-325. doi: 10.1002/jhm.323. PubMed
Over 7 million surgeries are performed in United States hospitals each year. Among these surgeries, approximately 85% are noncardiac, nonvascular (NCNV) procedures.1,2 Although the preoperative use of an angiotensin-converting enzyme inhibitor (ACEI) can be expected in as many as 13% of these surgeries,3 the optimal preoperative ACEI management strategy for patients undergoing NCNV surgeries is poorly understood.
High-quality evidence suggests that renin–angiotensin–aldosterone system (RAAS) inhibitors are associated with intraoperative hypotension among patients undergoing cardiac or vascular surgeries.4-6 Intraoperative hypotension increases the risk of 30-day mortality,7 and the duration of intraoperative hypotension increases the risk of end organ damage.8,9 This body of evidence suggests that withholding ACEIs prior to cardiac and vascular surgeries is safer than continuing ACEIs without interruption.
The evidence concerning perioperative management of ACEIs is inconclusive for patients undergoing NCNV procedures. Some studies comparing patients taking or not taking a RAAS inhibitor preoperatively describe negligible differences in the frequency of intraoperative hypotensive episodes or complications.3,10 Others have found an increased risk of intraoperative hypotension and associated postoperative adverse events in patients continuing RAAS inhibitors preoperatively.11,12 Current guideline discrepancies reflect the uncertainty of the evidence. The guidelines set by the American College of Cardiology and American Heart Association (ACC/AHA) suggest the uninterrupted perioperative continuation of RAAS inhibitors.13 The guidelines provided by the European Society of Cardiology and European Society of Anaesthesiology also suggest the continuation of RAAS inhibitors throughout the perioperative period for patients with systolic heart failure but recommend transient discontinuation for patients with hypertension.14
This randomized study aimed to compare the effect of two practical strategies for preoperative ACEI management on the perioperative blood pressure of patients undergoing NCNV surgery. The two strategies studied were the omission of the final preoperative ACEI dose and the uninterrupted continuation of ACEI therapy. We hypothesized that patients randomized to ACEI omission would experience intraoperative hypotensive episodes less frequently than those randomized to ACEI continuation.
METHODS
Study Design and Setting
We performed a prospective randomized controlled trial (ClinicalTrials.gov: NCT01669434). The study was carried out in a preoperative evaluation clinic and its affiliated 489-bed academic medical center. Anesthesiologists and internal medicine physicians work collaboratively in the clinic to assess more than 5,000 patients annually (one-third of the institution’s elective surgeries). Patients were randomized 1:1 in block sizes of 5 and 10 and stratified by age < 65 and ≥ 65 years to the omission or continuation of the final preoperative ACEI dose (whether that dose was scheduled for the morning of surgery or the night prior). Preoperative clinicians enrolled patients and subsequently assigned them to intervention groups on the basis of a sequentially numbered list. Patients and healthcare providers were not blinded to allocation status. Intraoperative and postoperative management was provided in accordance with usual care as decided by treatment team.
Participants
Patients who presented to the preoperative evaluation clinic between May 2015 and November 2016 and who had been taking an ACEI for at least 6 weeks were eligible for inclusion. Patients taking angiotensin receptor blockers were excluded. Enrollment was limited to patients planning NCNV surgery. Patients planning intrathoracic, major vascular, organ transplant, and oncologic surgery were excluded. Patients undergoing outpatient procedures not requiring an overnight stay in the hospital were also excluded. Patients with preoperative clinic systolic blood pressure (SBP) <90 or ≥160 or diastolic blood pressure (DBP) <60 or ≥ 95 were excluded. Patients with moderate to severe or clinically decompensated heart failure (left ventricular ejection fraction < 40% or New York Heart Association class III or IV) and those with end-stage renal disease requiring dialysis were also excluded. Patients presenting more than once during the accrual period were eligible for the initial surgery only. All participating patients provided written informed consent. This project was approved by the University of Nebraska Medical Center Institutional Review Board.
Data Collection
Baseline characteristics were recorded by study personnel at the time of enrollment. We measured serum creatinine level at the preoperative visit and on postoperative day 1. An automated anesthesia information management system was used to measure intraoperative blood pressures every three minutes. Postoperative blood pressures through discharge were measured by hospital staff per usual care. During postoperative hospitalization, we queried patients about preoperative adherence to allocation. The digital abstraction of data from the electronic medical record was supplemented by chart review when necessary.
Outcomes
The primary outcome was intraoperative hypotension defined as any SBP < 80 mm Hg occurring from the administration of the first induction agent through transfer to the postanesthesia care unit (PACU). We also examined hypotension during anesthesia induction, which we defined as the 20-minute period following the administration of the first anesthesia induction agent. Episodes of SBP < 80 were defined as being associated with vasopressor administration when any vasopressor was administered during or within 10 min of the episode.
Secondary analyses included postoperative acute kidney injury (AKI), postoperative hypotensive and hypertensive episodes, cardiac events, and mortality. When comparing postoperative day 1 creatinine levels to preoperative creatinine levels, we used the Acute Kidney Injury Network definition of AKI as an increase in creatinine of 0.3 mg/dl or 50%.15 Postoperative hypotension was defined as any SBP < 90 mm Hg and postoperative hypertension as any SBP > 180 mm Hg occurring after arrival in the PACU. Major adverse cardiac events (MACE) were defined as a composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia. Discharge from the hospital served as the study endpoint for each patient.
Analysis
Fisher’s exact test was used to compare categorical outcomes between groups. The independent sample t-test or Wilcoxon rank–sum test, as appropriate, was used to compare continuous measures. We selected Fisher’s exact test over χ2-test to produce conservative estimates. Patients were maintained in their allocated group as randomized for analytical purposes regardless of adherence to allocation. We performed all analyses using SAS version 9.4 for Windows (SAS institute, Cary, North Carolina).
We estimated that a sample size of 300 patients would achieve 80% power to detect a difference of 0.17 between the group proportions of 0.33 and 0.50 at a significance level (ɑ) of 0.05 by using a two-sided z-test with continuity correction, assuming 15% loss to follow-up. This estimate allowed for 1 interim analysis using the O’Brien-Fleming spending function truncated at three standard deviations to determine the test boundaries. The monitoring boundary P values associated with the interim analysis were .003, and the threshold P value for the final analysis was .049.
RESULTS
Study Flow
A total of 453 patients were screened for eligibility. Among these patients, 162 were excluded, and the remaining 291 patients were randomized (Figure 1). Surgery was cancelled in six patients allocated to omission and in four patients allocated to continuation arms, respectively. Moreover, three patients in the omission arm were excluded from the analysis following randomization. Specifically, one was excluded because of early discharge without overnight stay, one was excluded because of withdrawal of consent, and one was excluded because of missing primary outcome data. In addition, three cases in the continuation arm were excluded following randomization because of the preoperative (permanent) discontinuation of ACEI therapy in two cases and discharge without an overnight stay in one case. Finally, 275 patients were included in the analysis: 137 in the ACEI omission group and 138 in the ACEI continuation group. Adherence to allocation was 88% and 92% in the omission and continuation groups, respectively.
Baseline Characteristics
The demographic data of patients allocated to ACEI omission and those allocated to ACEI continuation were similar (Table 1). A large majority of patients in both groups took the ACEI lisinopril. Overall, 187 of 275 (68%) patients were taking at least 1 antihypertensive agent, most commonly a diuretic, in addition to an ACEI. SBP measured during the preoperative clinic visit averaged 136.5 mm Hg and did not differ significantly between groups (P = .84).
Surgical Variables
General anesthesia was the most commonly utilized technique, although spinal and regional anesthesia were also represented (Table 1). The majority of cases in both groups were planning for orthopedic and spinal surgery. The method of anesthesia or type of surgery between patients allocated to ACEI omission and those allocated to continuation did not differ (P = .61 and P = .45 respectively).
Episodes of Intraoperative Hypotension
Intraoperative SBPs are displayed in Figure 2, and hemodynamic outcomes are summarized in Table 2. Episodes of SBP < 80 mm Hg during anesthesia induction were numerically less frequent in the omission group than in the continuation group; the difference between groups, however, was not statistically significant (24 of 137 [18%] vs 38 of 138 [28%], RR: 0.64, 95% CI: 0.40 to 1.00, P = .06).
Duration of Intraoperative Hypotension
The median cumulative duration of intraoperative SBP < 80 was two minutes (range 0-41) in patients allocated to the ACEI omission group compared with seven minutes (range 0-214) in those allocated to the continuation group (P < .01). The median cumulative duration of mean arterial pressure < 55 mm Hg was also shorter in the omission group (median 0 min [range 0-39] vs 3 min [range 0-122], P < .01) than in the continuation group. The duration of surgery did not differ between groups (median 141 min [range 77-554] vs 142 min [range 57-665], P = .97).
Postoperative Outcomes
RAAS inhibitor therapy was resumed within 48 h after surgery in 122 of 137 (89%) patients allocated to the omission group and in 128 of 138 (93%) patients allocated to the continuation group (RR: 0.96, 95% CI: 0.89-1.03, P = .30).
Patients allocated to the omission group were significantly less likely to experience postoperative hypotension (15 of 137 [11%] vs 31 of 138 [22%], RR: 0.49, 95% CI: 0.28 to 0.86, P = .02) and significantly more likely to experience severe postoperative hypertension (33 of 137 [24%] vs 17 of 138 [12%], RR: 1.95, 95% CI: 1.14 to 3.34, P = .01) than those allocated to the continuation group. The occurrences of postoperative AKI (RR: 0.60, 95% CI: 0.23 to 1.60, P = .44) or MACE (RR: 4.03, 95% CI: 0.46 to 35.59, P = .21) in the omission group did not differ from the continuation group. The two groups exhibited similar PACU recovery time (mean 97.2 min) and overall hospital length of stay (mean 3.0 days) (P = .49 and P = .56 ). No episodes of inpatient mortality in either group were observed.
DISCUSSION
The omission of the final preoperative ACEI dose was associated with a significant reduction in the risk of intraoperative hypotension in patients undergoing NCNV surgery. This result confirmed our hypothesis. Coupled with the knowledge that intraoperative hypotension is associated with an increased risk of complications and mortality,7-9,16 this study favors the omission of the final preoperative ACEI dose prior to NCNV surgeries.
Our findings are in agreement with those of previous randomized studies that explored this question4,5 and help extend results from cardiac and vascular surgeries to NCNV surgeries. Previous studies on the use of RAAS inhibitors in NCNV surgeries did not employ randomization and yielded mixed results.3,10-12,17 A large single-institution study (n = 18,056) noted no difference in intraoperative blood pressure between patients taking ACEIs and a matched group of non-ACEI users.3 More recently, a subgroup analysis of the international VISION study showed that omitting RAAS inhibitors on the day of surgery reduced the risk of intraoperative hypotension.11 In that analysis, however, only a small amount of the variability in preoperative RAAS inhibitor management was explainable by modeling known factors, thus allowing for the possibility of unmeasured confounding. Our study, which minimized confounding through randomization, is the first to prospectively compare protocols for patients undergoing NCNV surgery. In contrast to previous studies, the present study was able to report the lack of difference in postoperative RAAS inhibitor administration between study groups. Postoperative RAAS inhibitor management affects complications and mortality.18,19
Our present finding that preoperative ACEI management affects postoperative hypotensive and hypertensive events conflicts with some previous findings.11,20 However, recent evidence has revealed that postoperative hypotensive episodes are associated with vascular events and mortality.11,21 In the context of that evidence, our study lends further support to the omission of the final preoperative ACEI dose. However, we did not detect any decrease in AKI, MACE, or mortality in the ACEI omission group.
This study should be considered in light of its limitations. The pragmatic nature of the study allowed for certain potential biases. Although adherence to allocation was high, the specific ACEI agent taken and the exact timing of the final dose in relation to surgery were not controlled. Anesthetic and postoperative management decisions were made by the treatment team and may have systematically varied given that the treatment team was not blinded to allocation. Furthermore, all outcome data were collected as part of routine care and may not have captured events with great fidelity. Generalizability is limited by the execution of the study at a single academic institution, the preponderance of orthopedic and spine surgeries, and by the negligible representation of ethnicities other than Caucasian. Additionally, recruitment from the preoperative evaluation clinic likely resulted in a patient group with greater comorbidity than the overall population of patients undergoing NCNV surgery. This study was powered for intraoperative hypotension and not postoperative outcomes. Our primary outcome, intraoperative hypotension, is an intermediate measure but one that has well-established associations with adverse outcomes, including mortality. One study showed that sustaining an intraoperative SBP below 70 mm Hg for longer than 5 min increased the risk of mortality from less than 1% to nearly 6%.16 A large study detected an increase in mortality associated with SBP sustained below 80 mm Hg for 10 min or longer.7 Intraoperative hypotension has also been associated with postoperative AKI and myocardial injury.8,9,12
Many of the limitations of the current study could be addressed by a large randomized controlled trial of ACEI management prior to NCNV surgeries that examines clinically important endpoints beyond intraoperative hypotension. Several specific aspects of perioperative RAAS inhibitor management also deserve further investigation. Our findings may not be generalizable to patients taking ARBs or to patients with congestive heart failure. The preoperative management of ARBs and the preoperative management of RAAS inhibitors in those with congestive heart failure are important areas of focus for future research. Lastly, our finding that preoperative ACEI management decisions can affect postoperative hypotensive and hypertensive events should be substantiated by future research, and any negative consequences of those events should be further explored.
Nonetheless, our study is the largest randomized study of preoperative RAAS inhibition published to date. More than twice as many patients were randomized in this study than in all previous randomized studies combined.4-6 To the best of our knowledge, this is also the first randomized study evaluating NCNV surgeries. Finally, our use of a practical ACEI omission protocol based on known pharmacokinetics allows for direct application to clinical practice.
CONCLUSION
Hypertension is among the most common chronic conditions encountered in patients planning surgery, and ACEIs are among the most frequently prescribed antihypertensive medications. This study showed that ACEI continuation is associated with an increased frequency and cumulative duration of intraoperative hypotension. These findings, while at odds with current ACC/AHA guidelines, align with the findings of a meta-analysis on this subject and with recent literature.3,11-13,22
Acknowledgments
The authors wish to thank Miranda M Fricke, MS, PA-C; Tiffany K Hillyard, APRN-FNP; and Barbara Sink, MPAS, PA-C who assisted in the design and conduct of patient enrollment and randomization procedures.
Disclosures
The authors have no relevant financial conflicts of interest to report.
Funding
This study was subsidized by a grant from the University of Nebraska Medical Center Research Support Fund. The funding source had no role in the design, conduct, analysis, or reporting of the study.
Over 7 million surgeries are performed in United States hospitals each year. Among these surgeries, approximately 85% are noncardiac, nonvascular (NCNV) procedures.1,2 Although the preoperative use of an angiotensin-converting enzyme inhibitor (ACEI) can be expected in as many as 13% of these surgeries,3 the optimal preoperative ACEI management strategy for patients undergoing NCNV surgeries is poorly understood.
High-quality evidence suggests that renin–angiotensin–aldosterone system (RAAS) inhibitors are associated with intraoperative hypotension among patients undergoing cardiac or vascular surgeries.4-6 Intraoperative hypotension increases the risk of 30-day mortality,7 and the duration of intraoperative hypotension increases the risk of end organ damage.8,9 This body of evidence suggests that withholding ACEIs prior to cardiac and vascular surgeries is safer than continuing ACEIs without interruption.
The evidence concerning perioperative management of ACEIs is inconclusive for patients undergoing NCNV procedures. Some studies comparing patients taking or not taking a RAAS inhibitor preoperatively describe negligible differences in the frequency of intraoperative hypotensive episodes or complications.3,10 Others have found an increased risk of intraoperative hypotension and associated postoperative adverse events in patients continuing RAAS inhibitors preoperatively.11,12 Current guideline discrepancies reflect the uncertainty of the evidence. The guidelines set by the American College of Cardiology and American Heart Association (ACC/AHA) suggest the uninterrupted perioperative continuation of RAAS inhibitors.13 The guidelines provided by the European Society of Cardiology and European Society of Anaesthesiology also suggest the continuation of RAAS inhibitors throughout the perioperative period for patients with systolic heart failure but recommend transient discontinuation for patients with hypertension.14
This randomized study aimed to compare the effect of two practical strategies for preoperative ACEI management on the perioperative blood pressure of patients undergoing NCNV surgery. The two strategies studied were the omission of the final preoperative ACEI dose and the uninterrupted continuation of ACEI therapy. We hypothesized that patients randomized to ACEI omission would experience intraoperative hypotensive episodes less frequently than those randomized to ACEI continuation.
METHODS
Study Design and Setting
We performed a prospective randomized controlled trial (ClinicalTrials.gov: NCT01669434). The study was carried out in a preoperative evaluation clinic and its affiliated 489-bed academic medical center. Anesthesiologists and internal medicine physicians work collaboratively in the clinic to assess more than 5,000 patients annually (one-third of the institution’s elective surgeries). Patients were randomized 1:1 in block sizes of 5 and 10 and stratified by age < 65 and ≥ 65 years to the omission or continuation of the final preoperative ACEI dose (whether that dose was scheduled for the morning of surgery or the night prior). Preoperative clinicians enrolled patients and subsequently assigned them to intervention groups on the basis of a sequentially numbered list. Patients and healthcare providers were not blinded to allocation status. Intraoperative and postoperative management was provided in accordance with usual care as decided by treatment team.
Participants
Patients who presented to the preoperative evaluation clinic between May 2015 and November 2016 and who had been taking an ACEI for at least 6 weeks were eligible for inclusion. Patients taking angiotensin receptor blockers were excluded. Enrollment was limited to patients planning NCNV surgery. Patients planning intrathoracic, major vascular, organ transplant, and oncologic surgery were excluded. Patients undergoing outpatient procedures not requiring an overnight stay in the hospital were also excluded. Patients with preoperative clinic systolic blood pressure (SBP) <90 or ≥160 or diastolic blood pressure (DBP) <60 or ≥ 95 were excluded. Patients with moderate to severe or clinically decompensated heart failure (left ventricular ejection fraction < 40% or New York Heart Association class III or IV) and those with end-stage renal disease requiring dialysis were also excluded. Patients presenting more than once during the accrual period were eligible for the initial surgery only. All participating patients provided written informed consent. This project was approved by the University of Nebraska Medical Center Institutional Review Board.
Data Collection
Baseline characteristics were recorded by study personnel at the time of enrollment. We measured serum creatinine level at the preoperative visit and on postoperative day 1. An automated anesthesia information management system was used to measure intraoperative blood pressures every three minutes. Postoperative blood pressures through discharge were measured by hospital staff per usual care. During postoperative hospitalization, we queried patients about preoperative adherence to allocation. The digital abstraction of data from the electronic medical record was supplemented by chart review when necessary.
Outcomes
The primary outcome was intraoperative hypotension defined as any SBP < 80 mm Hg occurring from the administration of the first induction agent through transfer to the postanesthesia care unit (PACU). We also examined hypotension during anesthesia induction, which we defined as the 20-minute period following the administration of the first anesthesia induction agent. Episodes of SBP < 80 were defined as being associated with vasopressor administration when any vasopressor was administered during or within 10 min of the episode.
Secondary analyses included postoperative acute kidney injury (AKI), postoperative hypotensive and hypertensive episodes, cardiac events, and mortality. When comparing postoperative day 1 creatinine levels to preoperative creatinine levels, we used the Acute Kidney Injury Network definition of AKI as an increase in creatinine of 0.3 mg/dl or 50%.15 Postoperative hypotension was defined as any SBP < 90 mm Hg and postoperative hypertension as any SBP > 180 mm Hg occurring after arrival in the PACU. Major adverse cardiac events (MACE) were defined as a composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia. Discharge from the hospital served as the study endpoint for each patient.
Analysis
Fisher’s exact test was used to compare categorical outcomes between groups. The independent sample t-test or Wilcoxon rank–sum test, as appropriate, was used to compare continuous measures. We selected Fisher’s exact test over χ2-test to produce conservative estimates. Patients were maintained in their allocated group as randomized for analytical purposes regardless of adherence to allocation. We performed all analyses using SAS version 9.4 for Windows (SAS institute, Cary, North Carolina).
We estimated that a sample size of 300 patients would achieve 80% power to detect a difference of 0.17 between the group proportions of 0.33 and 0.50 at a significance level (ɑ) of 0.05 by using a two-sided z-test with continuity correction, assuming 15% loss to follow-up. This estimate allowed for 1 interim analysis using the O’Brien-Fleming spending function truncated at three standard deviations to determine the test boundaries. The monitoring boundary P values associated with the interim analysis were .003, and the threshold P value for the final analysis was .049.
RESULTS
Study Flow
A total of 453 patients were screened for eligibility. Among these patients, 162 were excluded, and the remaining 291 patients were randomized (Figure 1). Surgery was cancelled in six patients allocated to omission and in four patients allocated to continuation arms, respectively. Moreover, three patients in the omission arm were excluded from the analysis following randomization. Specifically, one was excluded because of early discharge without overnight stay, one was excluded because of withdrawal of consent, and one was excluded because of missing primary outcome data. In addition, three cases in the continuation arm were excluded following randomization because of the preoperative (permanent) discontinuation of ACEI therapy in two cases and discharge without an overnight stay in one case. Finally, 275 patients were included in the analysis: 137 in the ACEI omission group and 138 in the ACEI continuation group. Adherence to allocation was 88% and 92% in the omission and continuation groups, respectively.
Baseline Characteristics
The demographic data of patients allocated to ACEI omission and those allocated to ACEI continuation were similar (Table 1). A large majority of patients in both groups took the ACEI lisinopril. Overall, 187 of 275 (68%) patients were taking at least 1 antihypertensive agent, most commonly a diuretic, in addition to an ACEI. SBP measured during the preoperative clinic visit averaged 136.5 mm Hg and did not differ significantly between groups (P = .84).
Surgical Variables
General anesthesia was the most commonly utilized technique, although spinal and regional anesthesia were also represented (Table 1). The majority of cases in both groups were planning for orthopedic and spinal surgery. The method of anesthesia or type of surgery between patients allocated to ACEI omission and those allocated to continuation did not differ (P = .61 and P = .45 respectively).
Episodes of Intraoperative Hypotension
Intraoperative SBPs are displayed in Figure 2, and hemodynamic outcomes are summarized in Table 2. Episodes of SBP < 80 mm Hg during anesthesia induction were numerically less frequent in the omission group than in the continuation group; the difference between groups, however, was not statistically significant (24 of 137 [18%] vs 38 of 138 [28%], RR: 0.64, 95% CI: 0.40 to 1.00, P = .06).
Duration of Intraoperative Hypotension
The median cumulative duration of intraoperative SBP < 80 was two minutes (range 0-41) in patients allocated to the ACEI omission group compared with seven minutes (range 0-214) in those allocated to the continuation group (P < .01). The median cumulative duration of mean arterial pressure < 55 mm Hg was also shorter in the omission group (median 0 min [range 0-39] vs 3 min [range 0-122], P < .01) than in the continuation group. The duration of surgery did not differ between groups (median 141 min [range 77-554] vs 142 min [range 57-665], P = .97).
Postoperative Outcomes
RAAS inhibitor therapy was resumed within 48 h after surgery in 122 of 137 (89%) patients allocated to the omission group and in 128 of 138 (93%) patients allocated to the continuation group (RR: 0.96, 95% CI: 0.89-1.03, P = .30).
Patients allocated to the omission group were significantly less likely to experience postoperative hypotension (15 of 137 [11%] vs 31 of 138 [22%], RR: 0.49, 95% CI: 0.28 to 0.86, P = .02) and significantly more likely to experience severe postoperative hypertension (33 of 137 [24%] vs 17 of 138 [12%], RR: 1.95, 95% CI: 1.14 to 3.34, P = .01) than those allocated to the continuation group. The occurrences of postoperative AKI (RR: 0.60, 95% CI: 0.23 to 1.60, P = .44) or MACE (RR: 4.03, 95% CI: 0.46 to 35.59, P = .21) in the omission group did not differ from the continuation group. The two groups exhibited similar PACU recovery time (mean 97.2 min) and overall hospital length of stay (mean 3.0 days) (P = .49 and P = .56 ). No episodes of inpatient mortality in either group were observed.
DISCUSSION
The omission of the final preoperative ACEI dose was associated with a significant reduction in the risk of intraoperative hypotension in patients undergoing NCNV surgery. This result confirmed our hypothesis. Coupled with the knowledge that intraoperative hypotension is associated with an increased risk of complications and mortality,7-9,16 this study favors the omission of the final preoperative ACEI dose prior to NCNV surgeries.
Our findings are in agreement with those of previous randomized studies that explored this question4,5 and help extend results from cardiac and vascular surgeries to NCNV surgeries. Previous studies on the use of RAAS inhibitors in NCNV surgeries did not employ randomization and yielded mixed results.3,10-12,17 A large single-institution study (n = 18,056) noted no difference in intraoperative blood pressure between patients taking ACEIs and a matched group of non-ACEI users.3 More recently, a subgroup analysis of the international VISION study showed that omitting RAAS inhibitors on the day of surgery reduced the risk of intraoperative hypotension.11 In that analysis, however, only a small amount of the variability in preoperative RAAS inhibitor management was explainable by modeling known factors, thus allowing for the possibility of unmeasured confounding. Our study, which minimized confounding through randomization, is the first to prospectively compare protocols for patients undergoing NCNV surgery. In contrast to previous studies, the present study was able to report the lack of difference in postoperative RAAS inhibitor administration between study groups. Postoperative RAAS inhibitor management affects complications and mortality.18,19
Our present finding that preoperative ACEI management affects postoperative hypotensive and hypertensive events conflicts with some previous findings.11,20 However, recent evidence has revealed that postoperative hypotensive episodes are associated with vascular events and mortality.11,21 In the context of that evidence, our study lends further support to the omission of the final preoperative ACEI dose. However, we did not detect any decrease in AKI, MACE, or mortality in the ACEI omission group.
This study should be considered in light of its limitations. The pragmatic nature of the study allowed for certain potential biases. Although adherence to allocation was high, the specific ACEI agent taken and the exact timing of the final dose in relation to surgery were not controlled. Anesthetic and postoperative management decisions were made by the treatment team and may have systematically varied given that the treatment team was not blinded to allocation. Furthermore, all outcome data were collected as part of routine care and may not have captured events with great fidelity. Generalizability is limited by the execution of the study at a single academic institution, the preponderance of orthopedic and spine surgeries, and by the negligible representation of ethnicities other than Caucasian. Additionally, recruitment from the preoperative evaluation clinic likely resulted in a patient group with greater comorbidity than the overall population of patients undergoing NCNV surgery. This study was powered for intraoperative hypotension and not postoperative outcomes. Our primary outcome, intraoperative hypotension, is an intermediate measure but one that has well-established associations with adverse outcomes, including mortality. One study showed that sustaining an intraoperative SBP below 70 mm Hg for longer than 5 min increased the risk of mortality from less than 1% to nearly 6%.16 A large study detected an increase in mortality associated with SBP sustained below 80 mm Hg for 10 min or longer.7 Intraoperative hypotension has also been associated with postoperative AKI and myocardial injury.8,9,12
Many of the limitations of the current study could be addressed by a large randomized controlled trial of ACEI management prior to NCNV surgeries that examines clinically important endpoints beyond intraoperative hypotension. Several specific aspects of perioperative RAAS inhibitor management also deserve further investigation. Our findings may not be generalizable to patients taking ARBs or to patients with congestive heart failure. The preoperative management of ARBs and the preoperative management of RAAS inhibitors in those with congestive heart failure are important areas of focus for future research. Lastly, our finding that preoperative ACEI management decisions can affect postoperative hypotensive and hypertensive events should be substantiated by future research, and any negative consequences of those events should be further explored.
Nonetheless, our study is the largest randomized study of preoperative RAAS inhibition published to date. More than twice as many patients were randomized in this study than in all previous randomized studies combined.4-6 To the best of our knowledge, this is also the first randomized study evaluating NCNV surgeries. Finally, our use of a practical ACEI omission protocol based on known pharmacokinetics allows for direct application to clinical practice.
CONCLUSION
Hypertension is among the most common chronic conditions encountered in patients planning surgery, and ACEIs are among the most frequently prescribed antihypertensive medications. This study showed that ACEI continuation is associated with an increased frequency and cumulative duration of intraoperative hypotension. These findings, while at odds with current ACC/AHA guidelines, align with the findings of a meta-analysis on this subject and with recent literature.3,11-13,22
Acknowledgments
The authors wish to thank Miranda M Fricke, MS, PA-C; Tiffany K Hillyard, APRN-FNP; and Barbara Sink, MPAS, PA-C who assisted in the design and conduct of patient enrollment and randomization procedures.
Disclosures
The authors have no relevant financial conflicts of interest to report.
Funding
This study was subsidized by a grant from the University of Nebraska Medical Center Research Support Fund. The funding source had no role in the design, conduct, analysis, or reporting of the study.
1. Steiner CA KZ, Moore BJ, Imshaug MC, Pickens G. Surgeries in hospital-based ambulatory surgery and hospital inpatient settings, 2014. Statistical Brief 2017; 1-18. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb223-Ambulatory-Inpatient-Surgeries-2014.pdf. Accessed August 30, 2017. PubMed
2. Rate of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. National Hospital Discharge Survey 2010; https://www.cdc.gov/nchs/nhds/nhds_tables.htm. Accessed August 30, 2017.
3. Turan A, You J, Shiba A, Kurz A, Saager L, Sessler DI. Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114(3):552-560. doi: 10.1213/ANE.0b013e318241f6af. PubMed
4. Coriat P, Richer C, Douraki T, et al. Influence of chronic angiotensin-converting enzyme inhibition on anesthetic induction. Anesthesiology. 1994;81:299-307. PubMed
5. Pigott DW, Nagle C, Allman K, S. W, D. ER. Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements. Br J Anaesth. 1999;83:715-720. doi: 10.1093/bja/83.5.715 PubMed
6. Bertrand M, Godet G, Meersschaert K, Brun L, Salcedo E, Coriat P. Should the angiotensin II antagonists be discontinued before surgery? Anesth Analg. 2001;92:26-30. PubMed
7. Mascha EJ, Yang D, Weiss S, Sessler DI. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015;123(1):79-91. doi: 10.1097/ALN.0000000000000686. PubMed
8. Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507-515. doi: 10.1097/ALN.0b013e3182a10e26. PubMed
9. Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47-65. doi: 10.1097/ALN.0000000000001432. PubMed
10. Comfere T, Sprung J, Kumar MM, et al. Angiotensin system inhibitors in a general surgical population. Anesth Analg. 2005;100(3):636-644. doi: 10.1213/01.ANE.0000146521.68059.A1. PubMed
11. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the vascular events in noncardiac surgery patIents cohort evaluation prospective cohort. Anesthesiology. 2017;126(1):16-27. doi: 10.1097/ALN.0000000000001404. PubMed
12. Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med. 2014;9(5):283-288. doi: 10.1002/jhm.2155. PubMed
13. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-137. doi: 10.1016/j.jacc.2014.07.944. PubMed
14. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. doi: 10.1093/eurheartj/ehu282 PubMed
15. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713 PubMed
16. Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123(2):307-319. doi: 10.1097/ALN.0000000000000756. PubMed
17. Kheterpal S, Khodaparast O, Shanks A, O’Reilly M, Tremper KK. Chronic angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy combined with diuretic therapy is associated with increased episodes of hypotension in noncardiac surgery. J Cardiothorac Vasc Anesth. 2008;22(2):180-186. 10.1053/j.jvca.2007.12.020. PubMed
18. Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the veterans affairs healthcare system. Anesthesiology. 2015;123(2):288-306. 10.1097/ALN.0000000000000739. PubMed
19. Drenger B, Fontes ML, Miao Y, et al. Patterns of use of perioperative angiotensin-converting enzyme inhibitors in coronary artery bypass graft surgery with cardiopulmonary bypass: effects on in-hospital morbidity and mortality. Circulation. 2012;126(3):261-269. doi: 10.1161/CIRCULATIONAHA.111.059527. PubMed
20. Twersky RS, Goel V, Narayan P, Weedon J. The risk of hypertension after preoperative discontinuation of angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists in ambulatory and same-day admission patients. Anesth Analg. 2014;118(5):938-944. doi: 10.1213/ANE.0000000000000076. PubMed
21. Tan TW, Eslami MH, Kalish JA, et al. The need for treatment of hemodynamic instability following carotid endarterectomy is associated with increased perioperative and 1-year morbidity and mortality. J Vasc Surg. 2014;59(1):16-24 e11-12. https://doi.org/10.1053/j.jvca.2014.12.002 PubMed
22. Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319-325. doi: 10.1002/jhm.323. PubMed
1. Steiner CA KZ, Moore BJ, Imshaug MC, Pickens G. Surgeries in hospital-based ambulatory surgery and hospital inpatient settings, 2014. Statistical Brief 2017; 1-18. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb223-Ambulatory-Inpatient-Surgeries-2014.pdf. Accessed August 30, 2017. PubMed
2. Rate of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. National Hospital Discharge Survey 2010; https://www.cdc.gov/nchs/nhds/nhds_tables.htm. Accessed August 30, 2017.
3. Turan A, You J, Shiba A, Kurz A, Saager L, Sessler DI. Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114(3):552-560. doi: 10.1213/ANE.0b013e318241f6af. PubMed
4. Coriat P, Richer C, Douraki T, et al. Influence of chronic angiotensin-converting enzyme inhibition on anesthetic induction. Anesthesiology. 1994;81:299-307. PubMed
5. Pigott DW, Nagle C, Allman K, S. W, D. ER. Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements. Br J Anaesth. 1999;83:715-720. doi: 10.1093/bja/83.5.715 PubMed
6. Bertrand M, Godet G, Meersschaert K, Brun L, Salcedo E, Coriat P. Should the angiotensin II antagonists be discontinued before surgery? Anesth Analg. 2001;92:26-30. PubMed
7. Mascha EJ, Yang D, Weiss S, Sessler DI. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015;123(1):79-91. doi: 10.1097/ALN.0000000000000686. PubMed
8. Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507-515. doi: 10.1097/ALN.0b013e3182a10e26. PubMed
9. Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47-65. doi: 10.1097/ALN.0000000000001432. PubMed
10. Comfere T, Sprung J, Kumar MM, et al. Angiotensin system inhibitors in a general surgical population. Anesth Analg. 2005;100(3):636-644. doi: 10.1213/01.ANE.0000146521.68059.A1. PubMed
11. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the vascular events in noncardiac surgery patIents cohort evaluation prospective cohort. Anesthesiology. 2017;126(1):16-27. doi: 10.1097/ALN.0000000000001404. PubMed
12. Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med. 2014;9(5):283-288. doi: 10.1002/jhm.2155. PubMed
13. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-137. doi: 10.1016/j.jacc.2014.07.944. PubMed
14. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. doi: 10.1093/eurheartj/ehu282 PubMed
15. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713 PubMed
16. Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123(2):307-319. doi: 10.1097/ALN.0000000000000756. PubMed
17. Kheterpal S, Khodaparast O, Shanks A, O’Reilly M, Tremper KK. Chronic angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy combined with diuretic therapy is associated with increased episodes of hypotension in noncardiac surgery. J Cardiothorac Vasc Anesth. 2008;22(2):180-186. 10.1053/j.jvca.2007.12.020. PubMed
18. Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the veterans affairs healthcare system. Anesthesiology. 2015;123(2):288-306. 10.1097/ALN.0000000000000739. PubMed
19. Drenger B, Fontes ML, Miao Y, et al. Patterns of use of perioperative angiotensin-converting enzyme inhibitors in coronary artery bypass graft surgery with cardiopulmonary bypass: effects on in-hospital morbidity and mortality. Circulation. 2012;126(3):261-269. doi: 10.1161/CIRCULATIONAHA.111.059527. PubMed
20. Twersky RS, Goel V, Narayan P, Weedon J. The risk of hypertension after preoperative discontinuation of angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists in ambulatory and same-day admission patients. Anesth Analg. 2014;118(5):938-944. doi: 10.1213/ANE.0000000000000076. PubMed
21. Tan TW, Eslami MH, Kalish JA, et al. The need for treatment of hemodynamic instability following carotid endarterectomy is associated with increased perioperative and 1-year morbidity and mortality. J Vasc Surg. 2014;59(1):16-24 e11-12. https://doi.org/10.1053/j.jvca.2014.12.002 PubMed
22. Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319-325. doi: 10.1002/jhm.323. PubMed
© 2018 Society of Hospital Medicine
Update in Hospital Medicine: Practical Lessons from the Literature
ESSENTIAL PUBLICATIONS
Prevalence of Pulmonary Embolism among Patients Hospitalized for Syncope. Prandoni P et al. New England Journal of Medicine, 2016;375(16):1524-31.1
Background
Pulmonary embolism (PE), a potentially fatal disease, is rarely considered as a likely cause of syncope. To determine the prevalence of PE among patients presenting with their first episode of syncope, the authors performed a systematic workup for pulmonary embolism in adult patients admitted for syncope at 11 hospitals in Italy.
Findings
Of the 2584 patients who presented to the emergency department (ED) with syncope during the study, 560 patients were admitted and met the inclusion criteria. A modified Wells Score was applied, and a D-dimer was measured on every hospitalized patient. Those with a high pretest probability, a Wells Score of 4.0 or higher, or a positive D-dimer underwent further testing for pulmonary embolism by a CT scan, a ventilation perfusion scan, or an autopsy. Ninety-seven of the 560 patients admitted to the hospital for syncope were found to have a PE (17%). One in
Cautions
Nearly 72% of the patients with common explanations for syncope, such as vasovagal, drug-induced, or volume depletion, were discharged from the ED and not included in the study. The authors focused on the prevalence of PE. The causation between PE and syncope is not clear in each of the patients. Of the patients’ diagnosis by a CT, only 67% of the PEs were found to be in a main pulmonary artery or lobar artery. The other 33% were segmental or subsegmental. Of those diagnosed by a ventilation perfusion scan, 50% of the patients had 25% or more of the area of both lungs involved. The other 50% involved less than 25% of the area of both lungs. Also, it is important to note that 75% of the patients admitted to the hospital in this study were 70 years of age or older.
Implications
After common diagnoses are ruled out, it is important to consider pulmonary embolism in patients hospitalized with syncope. Providers should calculate a Wells Score and measure a D-dimer to guide the decision making.
Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. Russo RJ et al. New England Journal of Medicine, 2017;376(8):755-64.2
Background
Magnetic resonance imaging (MRI) in patients with implantable cardiac devices is considered a safety risk due to the potential of cardiac lead heating and subsequent myocardial injury or alterations of the pacing properties. Although manufacturers have developed “MRI-conditional” devices designed to reduce these risks, still 2 million people in the United States and 6 million people worldwide have “non–MRI-conditional” devices. The authors evaluated the event rates in patients with “non-MRI-conditional” devices undergoing an MRI.
Findings
The authors prospectively followed up 1500 adults with cardiac devices placed since 2001 who received nonthoracic MRIs according to a specific protocol available in the supplemental materials published with this article in the New England Journal of Medicine. Of the 1000 patients with pacemakers only, they observed 5 atrial arrhythmias and 6 electrical resets. Of the 500 patients with implantable cardioverter defibrillators (ICDs), they observed 1 atrial arrhythmia and 1 generator failure (although this case had deviated from the protocol). All of the atrial arrhythmias were self-terminating. No deaths, lead failure requiring an immediate replacement, a loss of capture, or ventricular arrhythmias were observed.
Cautions
Patients who were pacing dependent were excluded. No devices implanted before 2001 were included in the study, and the MRIs performed were only 1.5 Tesla (a lower field strength than the also available 3 Tesla MRIs).
Implications
It is safe to proceed with 1.5 Tesla nonthoracic MRIs in patients, following the protocol outlined in this article, with non–MRI conditional cardiac devices implanted since 2001.
Culture If Spikes? Indications and Yield of Blood Cultures in Hospitalized Medical Patients. Linsenmeyer K et al. Journal of Hospital Medicine, 2016;11(5):336-40.3
Background
Blood cultures are frequently drawn for the evaluation of an inpatient fever. This “culture if spikes” approach may lead to unnecessary testing and false positive results. In this study, the authors evaluated rates of true positive and false positive blood cultures in the setting of an inpatient fever.
Findings
The patients hospitalized on the general medicine or cardiology floors at a Veterans Affairs teaching hospital were prospectively followed over 7 months. A total of 576 blood cultures were ordered among 323 unique patients. The patients were older (average age of 70 years) and predominantly male (94%). The true-positive rate for cultures, determined by a consensus among the microbiology and infectious disease departments based on a review of clinical and laboratory data, was 3.6% compared with a false-positive rate of 2.3%. The clinical characteristics associated with a higher likelihood of a true positive included: the indication for a culture as a follow-up from a previous culture (likelihood ratio [LR] 3.4), a working diagnosis of bacteremia or endocarditis (LR 3.7), and the constellation of fever and leukocytosis in a patient who has not been on antibiotics (LR 5.6).
Cautions
This study was performed at a single center with patients in the medicine and cardiology services, and thus, the data is representative of clinical practice patterns specific to that site.
Implications
Reflexive ordering of blood cultures for inpatient fever is of a low yield with a false-positive rate that approximates the true positive rate. A large number of patients are tested unnecessarily, and for those with positive tests, physicians are as likely to be misled as they are certain to truly identify a pathogen. The positive predictive value of blood cultures is improved when drawn on patients who are not on antibiotics and when the patient has a specific diagnosis, such as pneumonia, previous bacteremia, or suspected endocarditis.
Incidence of and Risk Factors for Chronic Opioid Use among Opioid-Naive Patients in the Postoperative Period. Sun EC et al. JAMA Internal Medicine, 2016;176(9):1286-93.4
Background
Each day in the United States, 650,000 opioid prescriptions are filled, and 78 people suffer an opiate-related death. Opioids are frequently prescribed for inpatient management of postoperative pain. In this study, authors compared the development of chronic opioid use between patients who had undergone surgery and those who had not.
Findings
This was a retrospective analysis of a nationwide insurance claims database. A total of 641,941 opioid-naive patients underwent 1 of 11 designated surgeries in the study period and were compared with 18,011,137 opioid-naive patients who did not undergo surgery. Chronic opioid use was defined as the filling of 10 or more prescriptions or receiving more than a 120-day supply between 90 and 365 days postoperatively (or following the assigned faux surgical date in those not having surgery). This was observed in a small proportion of the surgical patients (less than 0.5%). However, several procedures were associated with the increased odds of postoperative chronic opioid use, including a simple mastectomy (Odds ratio [OR] 2.65), a cesarean delivery (OR 1.28), an open appendectomy (OR 1.69), an open and laparoscopic cholecystectomy (ORs 3.60 and 1.62, respectively), and a total hip and total knee arthroplasty (ORs 2.52 and 5.10, respectively). Also, male sex, age greater than 50 years, preoperative benzodiazepines or antidepressants, and a history of drug abuse were associated with increased odds.
Cautions
This study was limited by the claims-based data and that the nonsurgical population was inherently different from the surgical population in ways that could lead to confounding.
Implications
In perioperative care, there is a need to focus on multimodal approaches to pain and to implement opioid reducing and sparing strategies that might include options such as acetaminophen, NSAIDs, neuropathic pain medications, and Lidocaine patches. Moreover, at discharge, careful consideration should be given to the quantity and duration of the postoperative opioids.
Rapid Rule-out of Acute Myocardial Infarction with a Single High-Sensitivity Cardiac Troponin T Measurement below the Limit of Detection: A Collaborative Meta-Analysis. Pickering JW et al. Annals of Internal Medicine, 2017;166:715-24.5
Background
High-sensitivity cardiac troponin testing (hs-cTnT) is now available in the United States. Studies have found that these can play a significant role in a rapid rule-out of acute myocardial infarction (AMI).
Findings
In this meta-analysis, the authors identified 11 studies with 9241 participants that prospectively evaluated patients presenting to the emergency department (ED) with chest pain, underwent an ECG, and had hs-cTnT drawn. A total of 30% of the patients were classified as low risk with negative hs-cTnT and negative ECG (defined as no ST changes or T-wave inversions indicative of ischemia). Among the low risk patients, only 14 of the 2825 (0.5%) had AMI according to the Global Task Forces definition.6 Seven of these were in patients with hs-cTnT drawn within 3 hours of a chest pain onset. The pooled negative predictive value was 99.0% (CI 93.8%–99.8%).
Cautions
The heterogeneity between the studies in this meta-analysis, especially in the exclusion criteria, warrants careful consideration when being implemented in new settings. A more sensitive test will result in more positive troponins due to different limits of detection. Thus, medical teams and institutions need to plan accordingly. Caution should be taken for any patient presenting within 3 hours of a chest pain onset.
Implications
Rapid rule-out protocols—which include clinical evaluation, a negative ECG, and a negative high-sensitivity cardiac troponin—identify a large proportion of low-risk patients who are unlikely to have a true AMI.
Prevalence and Localization of Pulmonary Embolism in Unexplained Acute Exacerbations of COPD: A Systematic Review and Meta-analysis. Aleva FE et al. Chest, 2017;151(3):544-54.7
Background
Acute exacerbations of chronic obstructive pulmonary disease (AE-COPD) are frequent. In up to 30%, no clear trigger is found. Previous studies suggested that 1 in 4 of these patients may have a pulmonary embolus (PE).7 This study reviewed the literature and meta-data to describe the prevalence, the embolism location, and the clinical predictors of PE among patients with unexplained AE-COPD.
Findings
A systematic review of the literature and meta-analysis identified 7 studies with 880 patients. In the pooled analysis, 16% had PE (range: 3%–29%). Of the 120 patients with PE, two-thirds were in lobar or larger arteries and one-third in segmental or smaller. Pleuritic chest pain and signs of cardiac compromise (hypotension, syncope, and right-sided heart failure) were associated with PE.
Cautions
This study was heterogeneous leading to a broad confidence interval for prevalence ranging from 8%–25%. Given the frequency of AE-COPD with no identified trigger, physicians need to attend to risks of repeat radiation exposure when considering an evaluation for PE.
Implications
One in 6 patients with unexplained AE-COPD was found to have PE; the odds were greater in those with pleuritic chest pain or signs of cardiac compromise. In patients with AE-COPD with an unclear trigger, the providers should consider an evaluation for PE by using a clinical prediction rule and/or a D-dimer.
Sitting at Patients’ Bedsides May Improve Patients’ Perceptions of Physician Communication Skills. Merel SE et al. Journal of Hospital Medicine, 2016;11(12):865-8.9
Background
Sitting at a patient’s bedside in the inpatient setting is considered a best practice, yet it has not been widely adopted. The authors conducted a cluster-randomized trial of physicians on a single 28-bed hospitalist only run unit where physicians were assigned to sitting or standing for the first 3 days of a 7-day workweek assignment. New admissions or transfers to the unit were considered eligible for the study.
Findings
Sixteen hospitalists saw on an average 13 patients daily during the study (a total of 159 patients were included in the analysis after 52 patients were excluded or declined to participate). The hospitalists were 69% female, and 81% had been in practice 3 years or less. The average time spent in the patient’s room was 12:00 minutes while seated and 12:10 minutes while standing. There was no difference in the patients’ perception of the amount of time spent—the patients overestimated this by 4 minutes in both groups. Sitting was associated with higher ratings for “listening carefully” and “explaining things in a way that was easy to understand.” There was no difference in ratings on the physicians interrupting the patient when talking or in treating patients with courtesy and respect.
Cautions
The study had a small sample size, was limited to English-speaking patients, and was a single-site study. It involved only attending-level physicians and did not involve nonphysician team members. The physicians were not blinded and were aware that the interactions were monitored, perhaps creating a Hawthorne effect. The analysis did not control for other factors such as the severity of the illness, the number of consultants used, or the degree of health literacy.
Implications
This study supports an important best practice highlighted in etiquette-based medicine 10: sitting at the bedside provided a benefit in the patient’s perception of communication by physicians without a negative effect on the physician’s workflow.
The Duration of Antibiotic Treatment in Community-Acquired Pneumonia: A Multi-Center Randomized Clinical Trial. Uranga A et al. JAMA Intern Medicine, 2016;176(9):1257-65.11
Background
The optimal duration of treatment for community-acquired pneumonia (CAP) is unclear; a growing body of evidence suggests shorter and longer durations may be equivalent.
Findings
At 4 hospitals in Spain, 312 adults with a mean age of 65 years and a diagnosis of CAP (non-ICU) were randomized to a short (5 days) versus a long (provider discretion) course of antibiotics. In the short-course group, the antibiotics were stopped after 5 days if the body temperature had been 37.8o C or less for 48 hours, and no more than 1 sign of clinical instability was present (SBP < 90 mmHg, HR >100/min, RR > 24/min, O2Sat < 90%). The median number of antibiotic days was 5 for the short-course group and 10 for the long-course group (P < .01). There was no difference in the resolution of pneumonia symptoms at 10 days or 30 days or in 30-day mortality. There were no differences in in-hospital side effects. However, 30-day readmissions were higher in the long-course group compared with the short-course group (6.6% vs 1.4%; P = .02). The results were similar across all of the Pneumonia Severity Index (PSI) classes.
Cautions
Most of the patients were not severely ill (~60% PSI I-III), the level of comorbid disease was low, and nearly 80% of the patients received fluoroquinolone. There was a significant cross over with 30% of patients assigned to the short-course group receiving antibiotics for more than 5 days.
Implications
Inpatient providers should aim to treat patients with community-acquired pneumonia (regardless of the severity of the illness) for 5 days. At day 5, if the patient is afebrile and has no signs of clinical instability, clinicians should be comfortable stopping antibiotics.
Is the Era of Intravenous Proton Pump Inhibitors Coming to an End in Patients with Bleeding Peptic Ulcers? A Meta-Analysis of the Published Literature. Jian Z et al. British Journal of Clinical Pharmacology, 2016;82(3):880-9.12
Background
Guidelines recommend intravenous proton pump inhibitors (PPI) after an endoscopy for patients with a bleeding peptic ulcer. Yet, acid suppression with oral PPI is deemed equivalent to the intravenous route.
Findings
This systematic review and meta-analysis identified 7 randomized controlled trials involving 859 patients. After an endoscopy, the patients were randomized to receive either oral or intravenous PPI. Most of the patients had “high-risk” peptic ulcers (active bleeding, a visible vessel, an adherent clot). The PPI dose and frequency varied between the studies. Re-bleeding rates were no different between the oral and intravenous route at 72 hours (2.4% vs 5.1%; P = .26), 7 days (5.6% vs 6.8%; P =.68), or 30 days (7.9% vs 8.8%; P = .62). There was also no difference in 30-day mortality (2.1% vs 2.4%; P = .88), and the length of stay was the same in both groups. Side effects were not reported.
Cautions
This systematic review and meta-analysis included multiple heterogeneous small studies of moderate quality. A large number of patients were excluded, increasing the risk of a selection bias.
Implications
There is no clear indication for intravenous PPI in the treatment of bleeding peptic ulcers following an endoscopy. Converting to oral PPI is equivalent to intravenous and is a safe, effective, and cost-saving option for patients with bleeding peptic ulcers.
1. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016; 375(16):1524-1531. PubMed
2. Russo RJ, Costa HS, Silva PD, et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med. 2017;376(8):755-764. PubMed
3. Linsenmeyer K, Gupta K, Strymish JM, Dhanani M, Brecher SM, Breu AC. Culture if spikes? Indications and yield of blood cultures in hospitalized medical patients. J Hosp Med. 2016;11(5):336-340. PubMed
4. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293. PubMed
5. Pickering JW, Than MP, Cullen L, et al. Rapid rule-out of acute myocardial infarction with a single high-sensitivity cardiac troponin T measurement below the limit of detection: A collaborative meta-analysis. Ann Intern Med. 2017;166(10):715-724. PubMed
6. Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634-2653. PubMed
7. Aleva FE, Voets LWLM, Simons SO, de Mast Q, van der Ven AJAM, Heijdra YF. Prevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD: A systematic review and meta-analysis. Chest. 2017; 151(3):544-554. PubMed
8. Rizkallah J, Man SFP, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: A systematic review and meta-analysis. Chest. 2009;135(3):786-793. PubMed
9. Merel SE, McKinney CM, Ufkes P, Kwan AC, White AA. Sitting at patients’ bedsides may improve patients’ perceptions of physician communication skills. J Hosp Med. 2016;11(12):865-868. PubMed
10. Kahn MW. Etiquette-based medicine. N Engl J Med. 2008;358(19):1988-1989. PubMed
11. Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: A multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-1265. PubMed
12. Jian Z, Li H, Race NS, Ma T, Jin H, Yin Z. Is the era of intravenous proton pump inhibitors coming to an end in patients with bleeding peptic ulcers? Meta-analysis of the published literature. Br J Clin Pharmacol. 2016;82(3):880-889. PubMed
ESSENTIAL PUBLICATIONS
Prevalence of Pulmonary Embolism among Patients Hospitalized for Syncope. Prandoni P et al. New England Journal of Medicine, 2016;375(16):1524-31.1
Background
Pulmonary embolism (PE), a potentially fatal disease, is rarely considered as a likely cause of syncope. To determine the prevalence of PE among patients presenting with their first episode of syncope, the authors performed a systematic workup for pulmonary embolism in adult patients admitted for syncope at 11 hospitals in Italy.
Findings
Of the 2584 patients who presented to the emergency department (ED) with syncope during the study, 560 patients were admitted and met the inclusion criteria. A modified Wells Score was applied, and a D-dimer was measured on every hospitalized patient. Those with a high pretest probability, a Wells Score of 4.0 or higher, or a positive D-dimer underwent further testing for pulmonary embolism by a CT scan, a ventilation perfusion scan, or an autopsy. Ninety-seven of the 560 patients admitted to the hospital for syncope were found to have a PE (17%). One in
Cautions
Nearly 72% of the patients with common explanations for syncope, such as vasovagal, drug-induced, or volume depletion, were discharged from the ED and not included in the study. The authors focused on the prevalence of PE. The causation between PE and syncope is not clear in each of the patients. Of the patients’ diagnosis by a CT, only 67% of the PEs were found to be in a main pulmonary artery or lobar artery. The other 33% were segmental or subsegmental. Of those diagnosed by a ventilation perfusion scan, 50% of the patients had 25% or more of the area of both lungs involved. The other 50% involved less than 25% of the area of both lungs. Also, it is important to note that 75% of the patients admitted to the hospital in this study were 70 years of age or older.
Implications
After common diagnoses are ruled out, it is important to consider pulmonary embolism in patients hospitalized with syncope. Providers should calculate a Wells Score and measure a D-dimer to guide the decision making.
Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. Russo RJ et al. New England Journal of Medicine, 2017;376(8):755-64.2
Background
Magnetic resonance imaging (MRI) in patients with implantable cardiac devices is considered a safety risk due to the potential of cardiac lead heating and subsequent myocardial injury or alterations of the pacing properties. Although manufacturers have developed “MRI-conditional” devices designed to reduce these risks, still 2 million people in the United States and 6 million people worldwide have “non–MRI-conditional” devices. The authors evaluated the event rates in patients with “non-MRI-conditional” devices undergoing an MRI.
Findings
The authors prospectively followed up 1500 adults with cardiac devices placed since 2001 who received nonthoracic MRIs according to a specific protocol available in the supplemental materials published with this article in the New England Journal of Medicine. Of the 1000 patients with pacemakers only, they observed 5 atrial arrhythmias and 6 electrical resets. Of the 500 patients with implantable cardioverter defibrillators (ICDs), they observed 1 atrial arrhythmia and 1 generator failure (although this case had deviated from the protocol). All of the atrial arrhythmias were self-terminating. No deaths, lead failure requiring an immediate replacement, a loss of capture, or ventricular arrhythmias were observed.
Cautions
Patients who were pacing dependent were excluded. No devices implanted before 2001 were included in the study, and the MRIs performed were only 1.5 Tesla (a lower field strength than the also available 3 Tesla MRIs).
Implications
It is safe to proceed with 1.5 Tesla nonthoracic MRIs in patients, following the protocol outlined in this article, with non–MRI conditional cardiac devices implanted since 2001.
Culture If Spikes? Indications and Yield of Blood Cultures in Hospitalized Medical Patients. Linsenmeyer K et al. Journal of Hospital Medicine, 2016;11(5):336-40.3
Background
Blood cultures are frequently drawn for the evaluation of an inpatient fever. This “culture if spikes” approach may lead to unnecessary testing and false positive results. In this study, the authors evaluated rates of true positive and false positive blood cultures in the setting of an inpatient fever.
Findings
The patients hospitalized on the general medicine or cardiology floors at a Veterans Affairs teaching hospital were prospectively followed over 7 months. A total of 576 blood cultures were ordered among 323 unique patients. The patients were older (average age of 70 years) and predominantly male (94%). The true-positive rate for cultures, determined by a consensus among the microbiology and infectious disease departments based on a review of clinical and laboratory data, was 3.6% compared with a false-positive rate of 2.3%. The clinical characteristics associated with a higher likelihood of a true positive included: the indication for a culture as a follow-up from a previous culture (likelihood ratio [LR] 3.4), a working diagnosis of bacteremia or endocarditis (LR 3.7), and the constellation of fever and leukocytosis in a patient who has not been on antibiotics (LR 5.6).
Cautions
This study was performed at a single center with patients in the medicine and cardiology services, and thus, the data is representative of clinical practice patterns specific to that site.
Implications
Reflexive ordering of blood cultures for inpatient fever is of a low yield with a false-positive rate that approximates the true positive rate. A large number of patients are tested unnecessarily, and for those with positive tests, physicians are as likely to be misled as they are certain to truly identify a pathogen. The positive predictive value of blood cultures is improved when drawn on patients who are not on antibiotics and when the patient has a specific diagnosis, such as pneumonia, previous bacteremia, or suspected endocarditis.
Incidence of and Risk Factors for Chronic Opioid Use among Opioid-Naive Patients in the Postoperative Period. Sun EC et al. JAMA Internal Medicine, 2016;176(9):1286-93.4
Background
Each day in the United States, 650,000 opioid prescriptions are filled, and 78 people suffer an opiate-related death. Opioids are frequently prescribed for inpatient management of postoperative pain. In this study, authors compared the development of chronic opioid use between patients who had undergone surgery and those who had not.
Findings
This was a retrospective analysis of a nationwide insurance claims database. A total of 641,941 opioid-naive patients underwent 1 of 11 designated surgeries in the study period and were compared with 18,011,137 opioid-naive patients who did not undergo surgery. Chronic opioid use was defined as the filling of 10 or more prescriptions or receiving more than a 120-day supply between 90 and 365 days postoperatively (or following the assigned faux surgical date in those not having surgery). This was observed in a small proportion of the surgical patients (less than 0.5%). However, several procedures were associated with the increased odds of postoperative chronic opioid use, including a simple mastectomy (Odds ratio [OR] 2.65), a cesarean delivery (OR 1.28), an open appendectomy (OR 1.69), an open and laparoscopic cholecystectomy (ORs 3.60 and 1.62, respectively), and a total hip and total knee arthroplasty (ORs 2.52 and 5.10, respectively). Also, male sex, age greater than 50 years, preoperative benzodiazepines or antidepressants, and a history of drug abuse were associated with increased odds.
Cautions
This study was limited by the claims-based data and that the nonsurgical population was inherently different from the surgical population in ways that could lead to confounding.
Implications
In perioperative care, there is a need to focus on multimodal approaches to pain and to implement opioid reducing and sparing strategies that might include options such as acetaminophen, NSAIDs, neuropathic pain medications, and Lidocaine patches. Moreover, at discharge, careful consideration should be given to the quantity and duration of the postoperative opioids.
Rapid Rule-out of Acute Myocardial Infarction with a Single High-Sensitivity Cardiac Troponin T Measurement below the Limit of Detection: A Collaborative Meta-Analysis. Pickering JW et al. Annals of Internal Medicine, 2017;166:715-24.5
Background
High-sensitivity cardiac troponin testing (hs-cTnT) is now available in the United States. Studies have found that these can play a significant role in a rapid rule-out of acute myocardial infarction (AMI).
Findings
In this meta-analysis, the authors identified 11 studies with 9241 participants that prospectively evaluated patients presenting to the emergency department (ED) with chest pain, underwent an ECG, and had hs-cTnT drawn. A total of 30% of the patients were classified as low risk with negative hs-cTnT and negative ECG (defined as no ST changes or T-wave inversions indicative of ischemia). Among the low risk patients, only 14 of the 2825 (0.5%) had AMI according to the Global Task Forces definition.6 Seven of these were in patients with hs-cTnT drawn within 3 hours of a chest pain onset. The pooled negative predictive value was 99.0% (CI 93.8%–99.8%).
Cautions
The heterogeneity between the studies in this meta-analysis, especially in the exclusion criteria, warrants careful consideration when being implemented in new settings. A more sensitive test will result in more positive troponins due to different limits of detection. Thus, medical teams and institutions need to plan accordingly. Caution should be taken for any patient presenting within 3 hours of a chest pain onset.
Implications
Rapid rule-out protocols—which include clinical evaluation, a negative ECG, and a negative high-sensitivity cardiac troponin—identify a large proportion of low-risk patients who are unlikely to have a true AMI.
Prevalence and Localization of Pulmonary Embolism in Unexplained Acute Exacerbations of COPD: A Systematic Review and Meta-analysis. Aleva FE et al. Chest, 2017;151(3):544-54.7
Background
Acute exacerbations of chronic obstructive pulmonary disease (AE-COPD) are frequent. In up to 30%, no clear trigger is found. Previous studies suggested that 1 in 4 of these patients may have a pulmonary embolus (PE).7 This study reviewed the literature and meta-data to describe the prevalence, the embolism location, and the clinical predictors of PE among patients with unexplained AE-COPD.
Findings
A systematic review of the literature and meta-analysis identified 7 studies with 880 patients. In the pooled analysis, 16% had PE (range: 3%–29%). Of the 120 patients with PE, two-thirds were in lobar or larger arteries and one-third in segmental or smaller. Pleuritic chest pain and signs of cardiac compromise (hypotension, syncope, and right-sided heart failure) were associated with PE.
Cautions
This study was heterogeneous leading to a broad confidence interval for prevalence ranging from 8%–25%. Given the frequency of AE-COPD with no identified trigger, physicians need to attend to risks of repeat radiation exposure when considering an evaluation for PE.
Implications
One in 6 patients with unexplained AE-COPD was found to have PE; the odds were greater in those with pleuritic chest pain or signs of cardiac compromise. In patients with AE-COPD with an unclear trigger, the providers should consider an evaluation for PE by using a clinical prediction rule and/or a D-dimer.
Sitting at Patients’ Bedsides May Improve Patients’ Perceptions of Physician Communication Skills. Merel SE et al. Journal of Hospital Medicine, 2016;11(12):865-8.9
Background
Sitting at a patient’s bedside in the inpatient setting is considered a best practice, yet it has not been widely adopted. The authors conducted a cluster-randomized trial of physicians on a single 28-bed hospitalist only run unit where physicians were assigned to sitting or standing for the first 3 days of a 7-day workweek assignment. New admissions or transfers to the unit were considered eligible for the study.
Findings
Sixteen hospitalists saw on an average 13 patients daily during the study (a total of 159 patients were included in the analysis after 52 patients were excluded or declined to participate). The hospitalists were 69% female, and 81% had been in practice 3 years or less. The average time spent in the patient’s room was 12:00 minutes while seated and 12:10 minutes while standing. There was no difference in the patients’ perception of the amount of time spent—the patients overestimated this by 4 minutes in both groups. Sitting was associated with higher ratings for “listening carefully” and “explaining things in a way that was easy to understand.” There was no difference in ratings on the physicians interrupting the patient when talking or in treating patients with courtesy and respect.
Cautions
The study had a small sample size, was limited to English-speaking patients, and was a single-site study. It involved only attending-level physicians and did not involve nonphysician team members. The physicians were not blinded and were aware that the interactions were monitored, perhaps creating a Hawthorne effect. The analysis did not control for other factors such as the severity of the illness, the number of consultants used, or the degree of health literacy.
Implications
This study supports an important best practice highlighted in etiquette-based medicine 10: sitting at the bedside provided a benefit in the patient’s perception of communication by physicians without a negative effect on the physician’s workflow.
The Duration of Antibiotic Treatment in Community-Acquired Pneumonia: A Multi-Center Randomized Clinical Trial. Uranga A et al. JAMA Intern Medicine, 2016;176(9):1257-65.11
Background
The optimal duration of treatment for community-acquired pneumonia (CAP) is unclear; a growing body of evidence suggests shorter and longer durations may be equivalent.
Findings
At 4 hospitals in Spain, 312 adults with a mean age of 65 years and a diagnosis of CAP (non-ICU) were randomized to a short (5 days) versus a long (provider discretion) course of antibiotics. In the short-course group, the antibiotics were stopped after 5 days if the body temperature had been 37.8o C or less for 48 hours, and no more than 1 sign of clinical instability was present (SBP < 90 mmHg, HR >100/min, RR > 24/min, O2Sat < 90%). The median number of antibiotic days was 5 for the short-course group and 10 for the long-course group (P < .01). There was no difference in the resolution of pneumonia symptoms at 10 days or 30 days or in 30-day mortality. There were no differences in in-hospital side effects. However, 30-day readmissions were higher in the long-course group compared with the short-course group (6.6% vs 1.4%; P = .02). The results were similar across all of the Pneumonia Severity Index (PSI) classes.
Cautions
Most of the patients were not severely ill (~60% PSI I-III), the level of comorbid disease was low, and nearly 80% of the patients received fluoroquinolone. There was a significant cross over with 30% of patients assigned to the short-course group receiving antibiotics for more than 5 days.
Implications
Inpatient providers should aim to treat patients with community-acquired pneumonia (regardless of the severity of the illness) for 5 days. At day 5, if the patient is afebrile and has no signs of clinical instability, clinicians should be comfortable stopping antibiotics.
Is the Era of Intravenous Proton Pump Inhibitors Coming to an End in Patients with Bleeding Peptic Ulcers? A Meta-Analysis of the Published Literature. Jian Z et al. British Journal of Clinical Pharmacology, 2016;82(3):880-9.12
Background
Guidelines recommend intravenous proton pump inhibitors (PPI) after an endoscopy for patients with a bleeding peptic ulcer. Yet, acid suppression with oral PPI is deemed equivalent to the intravenous route.
Findings
This systematic review and meta-analysis identified 7 randomized controlled trials involving 859 patients. After an endoscopy, the patients were randomized to receive either oral or intravenous PPI. Most of the patients had “high-risk” peptic ulcers (active bleeding, a visible vessel, an adherent clot). The PPI dose and frequency varied between the studies. Re-bleeding rates were no different between the oral and intravenous route at 72 hours (2.4% vs 5.1%; P = .26), 7 days (5.6% vs 6.8%; P =.68), or 30 days (7.9% vs 8.8%; P = .62). There was also no difference in 30-day mortality (2.1% vs 2.4%; P = .88), and the length of stay was the same in both groups. Side effects were not reported.
Cautions
This systematic review and meta-analysis included multiple heterogeneous small studies of moderate quality. A large number of patients were excluded, increasing the risk of a selection bias.
Implications
There is no clear indication for intravenous PPI in the treatment of bleeding peptic ulcers following an endoscopy. Converting to oral PPI is equivalent to intravenous and is a safe, effective, and cost-saving option for patients with bleeding peptic ulcers.
ESSENTIAL PUBLICATIONS
Prevalence of Pulmonary Embolism among Patients Hospitalized for Syncope. Prandoni P et al. New England Journal of Medicine, 2016;375(16):1524-31.1
Background
Pulmonary embolism (PE), a potentially fatal disease, is rarely considered as a likely cause of syncope. To determine the prevalence of PE among patients presenting with their first episode of syncope, the authors performed a systematic workup for pulmonary embolism in adult patients admitted for syncope at 11 hospitals in Italy.
Findings
Of the 2584 patients who presented to the emergency department (ED) with syncope during the study, 560 patients were admitted and met the inclusion criteria. A modified Wells Score was applied, and a D-dimer was measured on every hospitalized patient. Those with a high pretest probability, a Wells Score of 4.0 or higher, or a positive D-dimer underwent further testing for pulmonary embolism by a CT scan, a ventilation perfusion scan, or an autopsy. Ninety-seven of the 560 patients admitted to the hospital for syncope were found to have a PE (17%). One in
Cautions
Nearly 72% of the patients with common explanations for syncope, such as vasovagal, drug-induced, or volume depletion, were discharged from the ED and not included in the study. The authors focused on the prevalence of PE. The causation between PE and syncope is not clear in each of the patients. Of the patients’ diagnosis by a CT, only 67% of the PEs were found to be in a main pulmonary artery or lobar artery. The other 33% were segmental or subsegmental. Of those diagnosed by a ventilation perfusion scan, 50% of the patients had 25% or more of the area of both lungs involved. The other 50% involved less than 25% of the area of both lungs. Also, it is important to note that 75% of the patients admitted to the hospital in this study were 70 years of age or older.
Implications
After common diagnoses are ruled out, it is important to consider pulmonary embolism in patients hospitalized with syncope. Providers should calculate a Wells Score and measure a D-dimer to guide the decision making.
Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. Russo RJ et al. New England Journal of Medicine, 2017;376(8):755-64.2
Background
Magnetic resonance imaging (MRI) in patients with implantable cardiac devices is considered a safety risk due to the potential of cardiac lead heating and subsequent myocardial injury or alterations of the pacing properties. Although manufacturers have developed “MRI-conditional” devices designed to reduce these risks, still 2 million people in the United States and 6 million people worldwide have “non–MRI-conditional” devices. The authors evaluated the event rates in patients with “non-MRI-conditional” devices undergoing an MRI.
Findings
The authors prospectively followed up 1500 adults with cardiac devices placed since 2001 who received nonthoracic MRIs according to a specific protocol available in the supplemental materials published with this article in the New England Journal of Medicine. Of the 1000 patients with pacemakers only, they observed 5 atrial arrhythmias and 6 electrical resets. Of the 500 patients with implantable cardioverter defibrillators (ICDs), they observed 1 atrial arrhythmia and 1 generator failure (although this case had deviated from the protocol). All of the atrial arrhythmias were self-terminating. No deaths, lead failure requiring an immediate replacement, a loss of capture, or ventricular arrhythmias were observed.
Cautions
Patients who were pacing dependent were excluded. No devices implanted before 2001 were included in the study, and the MRIs performed were only 1.5 Tesla (a lower field strength than the also available 3 Tesla MRIs).
Implications
It is safe to proceed with 1.5 Tesla nonthoracic MRIs in patients, following the protocol outlined in this article, with non–MRI conditional cardiac devices implanted since 2001.
Culture If Spikes? Indications and Yield of Blood Cultures in Hospitalized Medical Patients. Linsenmeyer K et al. Journal of Hospital Medicine, 2016;11(5):336-40.3
Background
Blood cultures are frequently drawn for the evaluation of an inpatient fever. This “culture if spikes” approach may lead to unnecessary testing and false positive results. In this study, the authors evaluated rates of true positive and false positive blood cultures in the setting of an inpatient fever.
Findings
The patients hospitalized on the general medicine or cardiology floors at a Veterans Affairs teaching hospital were prospectively followed over 7 months. A total of 576 blood cultures were ordered among 323 unique patients. The patients were older (average age of 70 years) and predominantly male (94%). The true-positive rate for cultures, determined by a consensus among the microbiology and infectious disease departments based on a review of clinical and laboratory data, was 3.6% compared with a false-positive rate of 2.3%. The clinical characteristics associated with a higher likelihood of a true positive included: the indication for a culture as a follow-up from a previous culture (likelihood ratio [LR] 3.4), a working diagnosis of bacteremia or endocarditis (LR 3.7), and the constellation of fever and leukocytosis in a patient who has not been on antibiotics (LR 5.6).
Cautions
This study was performed at a single center with patients in the medicine and cardiology services, and thus, the data is representative of clinical practice patterns specific to that site.
Implications
Reflexive ordering of blood cultures for inpatient fever is of a low yield with a false-positive rate that approximates the true positive rate. A large number of patients are tested unnecessarily, and for those with positive tests, physicians are as likely to be misled as they are certain to truly identify a pathogen. The positive predictive value of blood cultures is improved when drawn on patients who are not on antibiotics and when the patient has a specific diagnosis, such as pneumonia, previous bacteremia, or suspected endocarditis.
Incidence of and Risk Factors for Chronic Opioid Use among Opioid-Naive Patients in the Postoperative Period. Sun EC et al. JAMA Internal Medicine, 2016;176(9):1286-93.4
Background
Each day in the United States, 650,000 opioid prescriptions are filled, and 78 people suffer an opiate-related death. Opioids are frequently prescribed for inpatient management of postoperative pain. In this study, authors compared the development of chronic opioid use between patients who had undergone surgery and those who had not.
Findings
This was a retrospective analysis of a nationwide insurance claims database. A total of 641,941 opioid-naive patients underwent 1 of 11 designated surgeries in the study period and were compared with 18,011,137 opioid-naive patients who did not undergo surgery. Chronic opioid use was defined as the filling of 10 or more prescriptions or receiving more than a 120-day supply between 90 and 365 days postoperatively (or following the assigned faux surgical date in those not having surgery). This was observed in a small proportion of the surgical patients (less than 0.5%). However, several procedures were associated with the increased odds of postoperative chronic opioid use, including a simple mastectomy (Odds ratio [OR] 2.65), a cesarean delivery (OR 1.28), an open appendectomy (OR 1.69), an open and laparoscopic cholecystectomy (ORs 3.60 and 1.62, respectively), and a total hip and total knee arthroplasty (ORs 2.52 and 5.10, respectively). Also, male sex, age greater than 50 years, preoperative benzodiazepines or antidepressants, and a history of drug abuse were associated with increased odds.
Cautions
This study was limited by the claims-based data and that the nonsurgical population was inherently different from the surgical population in ways that could lead to confounding.
Implications
In perioperative care, there is a need to focus on multimodal approaches to pain and to implement opioid reducing and sparing strategies that might include options such as acetaminophen, NSAIDs, neuropathic pain medications, and Lidocaine patches. Moreover, at discharge, careful consideration should be given to the quantity and duration of the postoperative opioids.
Rapid Rule-out of Acute Myocardial Infarction with a Single High-Sensitivity Cardiac Troponin T Measurement below the Limit of Detection: A Collaborative Meta-Analysis. Pickering JW et al. Annals of Internal Medicine, 2017;166:715-24.5
Background
High-sensitivity cardiac troponin testing (hs-cTnT) is now available in the United States. Studies have found that these can play a significant role in a rapid rule-out of acute myocardial infarction (AMI).
Findings
In this meta-analysis, the authors identified 11 studies with 9241 participants that prospectively evaluated patients presenting to the emergency department (ED) with chest pain, underwent an ECG, and had hs-cTnT drawn. A total of 30% of the patients were classified as low risk with negative hs-cTnT and negative ECG (defined as no ST changes or T-wave inversions indicative of ischemia). Among the low risk patients, only 14 of the 2825 (0.5%) had AMI according to the Global Task Forces definition.6 Seven of these were in patients with hs-cTnT drawn within 3 hours of a chest pain onset. The pooled negative predictive value was 99.0% (CI 93.8%–99.8%).
Cautions
The heterogeneity between the studies in this meta-analysis, especially in the exclusion criteria, warrants careful consideration when being implemented in new settings. A more sensitive test will result in more positive troponins due to different limits of detection. Thus, medical teams and institutions need to plan accordingly. Caution should be taken for any patient presenting within 3 hours of a chest pain onset.
Implications
Rapid rule-out protocols—which include clinical evaluation, a negative ECG, and a negative high-sensitivity cardiac troponin—identify a large proportion of low-risk patients who are unlikely to have a true AMI.
Prevalence and Localization of Pulmonary Embolism in Unexplained Acute Exacerbations of COPD: A Systematic Review and Meta-analysis. Aleva FE et al. Chest, 2017;151(3):544-54.7
Background
Acute exacerbations of chronic obstructive pulmonary disease (AE-COPD) are frequent. In up to 30%, no clear trigger is found. Previous studies suggested that 1 in 4 of these patients may have a pulmonary embolus (PE).7 This study reviewed the literature and meta-data to describe the prevalence, the embolism location, and the clinical predictors of PE among patients with unexplained AE-COPD.
Findings
A systematic review of the literature and meta-analysis identified 7 studies with 880 patients. In the pooled analysis, 16% had PE (range: 3%–29%). Of the 120 patients with PE, two-thirds were in lobar or larger arteries and one-third in segmental or smaller. Pleuritic chest pain and signs of cardiac compromise (hypotension, syncope, and right-sided heart failure) were associated with PE.
Cautions
This study was heterogeneous leading to a broad confidence interval for prevalence ranging from 8%–25%. Given the frequency of AE-COPD with no identified trigger, physicians need to attend to risks of repeat radiation exposure when considering an evaluation for PE.
Implications
One in 6 patients with unexplained AE-COPD was found to have PE; the odds were greater in those with pleuritic chest pain or signs of cardiac compromise. In patients with AE-COPD with an unclear trigger, the providers should consider an evaluation for PE by using a clinical prediction rule and/or a D-dimer.
Sitting at Patients’ Bedsides May Improve Patients’ Perceptions of Physician Communication Skills. Merel SE et al. Journal of Hospital Medicine, 2016;11(12):865-8.9
Background
Sitting at a patient’s bedside in the inpatient setting is considered a best practice, yet it has not been widely adopted. The authors conducted a cluster-randomized trial of physicians on a single 28-bed hospitalist only run unit where physicians were assigned to sitting or standing for the first 3 days of a 7-day workweek assignment. New admissions or transfers to the unit were considered eligible for the study.
Findings
Sixteen hospitalists saw on an average 13 patients daily during the study (a total of 159 patients were included in the analysis after 52 patients were excluded or declined to participate). The hospitalists were 69% female, and 81% had been in practice 3 years or less. The average time spent in the patient’s room was 12:00 minutes while seated and 12:10 minutes while standing. There was no difference in the patients’ perception of the amount of time spent—the patients overestimated this by 4 minutes in both groups. Sitting was associated with higher ratings for “listening carefully” and “explaining things in a way that was easy to understand.” There was no difference in ratings on the physicians interrupting the patient when talking or in treating patients with courtesy and respect.
Cautions
The study had a small sample size, was limited to English-speaking patients, and was a single-site study. It involved only attending-level physicians and did not involve nonphysician team members. The physicians were not blinded and were aware that the interactions were monitored, perhaps creating a Hawthorne effect. The analysis did not control for other factors such as the severity of the illness, the number of consultants used, or the degree of health literacy.
Implications
This study supports an important best practice highlighted in etiquette-based medicine 10: sitting at the bedside provided a benefit in the patient’s perception of communication by physicians without a negative effect on the physician’s workflow.
The Duration of Antibiotic Treatment in Community-Acquired Pneumonia: A Multi-Center Randomized Clinical Trial. Uranga A et al. JAMA Intern Medicine, 2016;176(9):1257-65.11
Background
The optimal duration of treatment for community-acquired pneumonia (CAP) is unclear; a growing body of evidence suggests shorter and longer durations may be equivalent.
Findings
At 4 hospitals in Spain, 312 adults with a mean age of 65 years and a diagnosis of CAP (non-ICU) were randomized to a short (5 days) versus a long (provider discretion) course of antibiotics. In the short-course group, the antibiotics were stopped after 5 days if the body temperature had been 37.8o C or less for 48 hours, and no more than 1 sign of clinical instability was present (SBP < 90 mmHg, HR >100/min, RR > 24/min, O2Sat < 90%). The median number of antibiotic days was 5 for the short-course group and 10 for the long-course group (P < .01). There was no difference in the resolution of pneumonia symptoms at 10 days or 30 days or in 30-day mortality. There were no differences in in-hospital side effects. However, 30-day readmissions were higher in the long-course group compared with the short-course group (6.6% vs 1.4%; P = .02). The results were similar across all of the Pneumonia Severity Index (PSI) classes.
Cautions
Most of the patients were not severely ill (~60% PSI I-III), the level of comorbid disease was low, and nearly 80% of the patients received fluoroquinolone. There was a significant cross over with 30% of patients assigned to the short-course group receiving antibiotics for more than 5 days.
Implications
Inpatient providers should aim to treat patients with community-acquired pneumonia (regardless of the severity of the illness) for 5 days. At day 5, if the patient is afebrile and has no signs of clinical instability, clinicians should be comfortable stopping antibiotics.
Is the Era of Intravenous Proton Pump Inhibitors Coming to an End in Patients with Bleeding Peptic Ulcers? A Meta-Analysis of the Published Literature. Jian Z et al. British Journal of Clinical Pharmacology, 2016;82(3):880-9.12
Background
Guidelines recommend intravenous proton pump inhibitors (PPI) after an endoscopy for patients with a bleeding peptic ulcer. Yet, acid suppression with oral PPI is deemed equivalent to the intravenous route.
Findings
This systematic review and meta-analysis identified 7 randomized controlled trials involving 859 patients. After an endoscopy, the patients were randomized to receive either oral or intravenous PPI. Most of the patients had “high-risk” peptic ulcers (active bleeding, a visible vessel, an adherent clot). The PPI dose and frequency varied between the studies. Re-bleeding rates were no different between the oral and intravenous route at 72 hours (2.4% vs 5.1%; P = .26), 7 days (5.6% vs 6.8%; P =.68), or 30 days (7.9% vs 8.8%; P = .62). There was also no difference in 30-day mortality (2.1% vs 2.4%; P = .88), and the length of stay was the same in both groups. Side effects were not reported.
Cautions
This systematic review and meta-analysis included multiple heterogeneous small studies of moderate quality. A large number of patients were excluded, increasing the risk of a selection bias.
Implications
There is no clear indication for intravenous PPI in the treatment of bleeding peptic ulcers following an endoscopy. Converting to oral PPI is equivalent to intravenous and is a safe, effective, and cost-saving option for patients with bleeding peptic ulcers.
1. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016; 375(16):1524-1531. PubMed
2. Russo RJ, Costa HS, Silva PD, et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med. 2017;376(8):755-764. PubMed
3. Linsenmeyer K, Gupta K, Strymish JM, Dhanani M, Brecher SM, Breu AC. Culture if spikes? Indications and yield of blood cultures in hospitalized medical patients. J Hosp Med. 2016;11(5):336-340. PubMed
4. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293. PubMed
5. Pickering JW, Than MP, Cullen L, et al. Rapid rule-out of acute myocardial infarction with a single high-sensitivity cardiac troponin T measurement below the limit of detection: A collaborative meta-analysis. Ann Intern Med. 2017;166(10):715-724. PubMed
6. Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634-2653. PubMed
7. Aleva FE, Voets LWLM, Simons SO, de Mast Q, van der Ven AJAM, Heijdra YF. Prevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD: A systematic review and meta-analysis. Chest. 2017; 151(3):544-554. PubMed
8. Rizkallah J, Man SFP, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: A systematic review and meta-analysis. Chest. 2009;135(3):786-793. PubMed
9. Merel SE, McKinney CM, Ufkes P, Kwan AC, White AA. Sitting at patients’ bedsides may improve patients’ perceptions of physician communication skills. J Hosp Med. 2016;11(12):865-868. PubMed
10. Kahn MW. Etiquette-based medicine. N Engl J Med. 2008;358(19):1988-1989. PubMed
11. Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: A multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-1265. PubMed
12. Jian Z, Li H, Race NS, Ma T, Jin H, Yin Z. Is the era of intravenous proton pump inhibitors coming to an end in patients with bleeding peptic ulcers? Meta-analysis of the published literature. Br J Clin Pharmacol. 2016;82(3):880-889. PubMed
1. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016; 375(16):1524-1531. PubMed
2. Russo RJ, Costa HS, Silva PD, et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med. 2017;376(8):755-764. PubMed
3. Linsenmeyer K, Gupta K, Strymish JM, Dhanani M, Brecher SM, Breu AC. Culture if spikes? Indications and yield of blood cultures in hospitalized medical patients. J Hosp Med. 2016;11(5):336-340. PubMed
4. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293. PubMed
5. Pickering JW, Than MP, Cullen L, et al. Rapid rule-out of acute myocardial infarction with a single high-sensitivity cardiac troponin T measurement below the limit of detection: A collaborative meta-analysis. Ann Intern Med. 2017;166(10):715-724. PubMed
6. Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634-2653. PubMed
7. Aleva FE, Voets LWLM, Simons SO, de Mast Q, van der Ven AJAM, Heijdra YF. Prevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD: A systematic review and meta-analysis. Chest. 2017; 151(3):544-554. PubMed
8. Rizkallah J, Man SFP, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: A systematic review and meta-analysis. Chest. 2009;135(3):786-793. PubMed
9. Merel SE, McKinney CM, Ufkes P, Kwan AC, White AA. Sitting at patients’ bedsides may improve patients’ perceptions of physician communication skills. J Hosp Med. 2016;11(12):865-868. PubMed
10. Kahn MW. Etiquette-based medicine. N Engl J Med. 2008;358(19):1988-1989. PubMed
11. Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: A multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-1265. PubMed
12. Jian Z, Li H, Race NS, Ma T, Jin H, Yin Z. Is the era of intravenous proton pump inhibitors coming to an end in patients with bleeding peptic ulcers? Meta-analysis of the published literature. Br J Clin Pharmacol. 2016;82(3):880-889. PubMed
© 2018 Society of Hospital Medicine
Hospital medicine and perioperative care: A framework for high-quality, high-value collaborative care
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
© 2017 Society of Hospital Medicine