User login
ACUTE Center for Eating Disorders
Anorexia nervosa occurs in 0.9% of women and 0.3% of men in the United States1 and is associated with a prolonged course,2 extensive medical complications that can affect almost every organ system,3, 4 and a 5% mean crude mortality rate9.6 times expected for age‐matched women in the United States.2, 5 Those with anorexia nervosa die as a complication of their illness more frequently than any other mental illness.3 Anorexia nervosa is commonly diagnosed during the adolescent years,2 with almost 25% going on to develop chronic anorexia nervosa.2, 6 Consequently, many patients with severe anorexia nervosa will receive treatment by adult medicine practitioners.
Patients with anorexia nervosa frequently require hospitalization. Published guidelines suggest that those who are 70% or less than ideal body weight, bradycardic, hypotensive, or those with severe electrolyte disturbances warrant admission for medical stabilization.79 Once admitted, however, there are no published guidelines for best practices to medically stabilize patients.7, 10 Although most experts advocate a multidisciplinary approach with weight restoration and medical stability as the goals of hospital admission,8, 9 controversy exists in the literature about how best to achieve these goals.7, 10
It is known, however, that for patients with complicated medical illnesses, such as human immunodeficiency virus (HIV) and sepsis, higher volumes of patient caseloads treated by physicians with disease‐specific expertise has been found to lead to improved outcomes in patients.11, 12 The adult patient with severe anorexia nervosa who requires inpatient medical stabilization may also benefit from a multidisciplinary trained staff familiar with the medical management of anorexia nervosa. Accordingly, we have developed the Acute Comprehensive Urgent Treatment for Eating Disorders (ACUTE) Center.
PROGRAM DESCRIPTION
The ACUTE Center at Denver Health is a 5‐bed unit dedicated to the medical stabilization of patients with severe malnutrition due to anorexia nervosa or severe electrolyte disorders due to bulimia nervosa. ACUTE accepts patients 17 years and older with medical complications related to chronic malnutrition and refeeding.
ACUTE uses a multidisciplinary approach to patient care. The physician team is composed of a hospital medicine attending physician, consultative expertise by an internal medicine specialist in the management of the medical complications of eating disorders, and a psychiatrist specializing in eating disorders. There is a dedicated team of nurses, two dieticians, physical therapists, certified nursing assistants, speech therapists, a psychotherapist, and a chaplain.
ACUTE patients are on continuous telemetry monitoring for the duration of their hospitalization to monitor for arrhythmias as well as signs of covert exercise. As part of the initial intake, a full set of vital signs is obtained, including height and weight. Patients are weighed daily with their back to the scale. There is no discussion of weight fluctuations. Patients may walk at a slow pace around the unit. No exercise is allowed.
Each patient at the ACUTE Center has an individualized meal plan and are started on an oral caloric intake 200 kcal below their basal energy expenditure (BEE). Indirect calorimetry is performed on the first hospital day. Each patient meets on a daily basis with the registered dietician to choose meals that meet their caloric goals.
All patients have a sitter continuously for their first week, and thereafter sitter time may be reduced to supervision surrounding each meal. Patients who fail to finish their prescribed meal are required to drink a liquid supplement to meet caloric goals. Calories are increased weekly until the patient's weight shows a clear pattern of weight increase. 0
Patients are discharged from the ACUTE Center when they have achieved several basic goals: They are consuming greater than 2000 kcal per day, they are consistently gaining 23 pounds per week, their laboratory values have stabilized without electrolyte supplementation, and they are strong enough for an inpatient eating disorder program.
METHODS
Patients admitted to the ACUTE Center between October 2008 and December 2010 for medical stabilization and monitored refeeding were included. Patients with a diagnosis of bulimia nervosa were excluded. Demographic data and laboratory results were obtained electronically from our data repository, whereas weight, height, and other clinical characteristics were obtained by manual chart abstraction. The statistical analysis was conducted in SAS Enterprise Guide v4.1 (SAS Institute, Cary, NC).
RESULTS
In its first 27 months, the ACUTE Center had 76 total admissions, comprising 59 patients. Of the 76 admissions, the 62 admissions for medical stabilization and monitored refeeding of 54 patients with anorexia nervosa were included. Forty‐eight of the 54 (89%) included patients were female. Six patients were hospitalized twice, and 1 patient 3 times. There were 3 transfers to the intensive care unit, and no inpatient mortality. Of the 62 admissions, 11 (18%) discharges were to home, and 51 (82%) were to inpatient psychiatric eating disorder units.
The mean age at admission was 27 years (range 1765 years). The mean percent of ideal body weight (IBW) on admission was 62.2% 10.2%. The mean body mass index (BMI) was 12.9 2.0 kg/m2 on admission, and 13.1 1.9 kg/m2 upon discharge. The median length of stay was 16 days (interquartile range [IQR] 929 days). Median calculated BEE (1119 [10671184 IQR]) was higher than measured BEE by indirect calorimetry (792 [6341094]), (Table 1).
| Median (Interquartile Range)* | Range | |
|---|---|---|
| ||
| Age, yr | 27 (2135) | 1765 |
| Female | 56 | 90% |
| Length of hospitalization, days | 16 (929) | 570 |
| Calculated BEE | 1119 (10671184) | 9061491 |
| Measured BEE | 792 (6341094) | 5001742 |
| DEXA Z‐score | 2.2 1.1 | 4.40.7 |
| Height, in | 65 (6167) | 5774 |
| Weight on admission, lb | 76.1 14.4 | 50.8110.0 |
| % Ideal body weight on admission | 62.2 10.2 | 42.4101.0 |
| % Ideal body weight on discharge | 63.2 9.1 | 42.3 82.7 |
| BMI on admission | 12.9 2.0 | 8.719.7 |
| BMI nadir | 12.4 1.9 | 8.415.7 |
| BMI on discharge | 13.1 1.9 | 8.717.0 |
The majority of admission laboratory values, including serum albumin, blood urea nitrogen (BUN), creatinine, potassium, magnesium, and phosphate levels, were within normal limits. Fifty‐six percent were hyponatremic at admission, with a mean serum sodium level of 133 6 mmol/L (Table 2).
| Median (Interquartile Range)* | Range | |
|---|---|---|
| ||
| Sodium (135143 mmol/L) | 133 6 | 117145 |
| Potassium (3.65.1 mmol/L) | 3.8 (3.0 4.0) | 1.85.5 |
| Carbon dioxide (1827 mmol/L) | 28 (2531) | 1845 |
| Glucose (60199 mg/dL) | 85 (76105) | 41166 |
| BUN (622 mg/dL) | 16 (923) | 344 |
| Creatinine (0.61.2 mg/dL) | 0.7 (0.61.0) | 0.31.6 |
| Calcium (8.110.5 mg/dL) | 8.9 0.6 | 7.610.1 |
| Phosphorus (2.74.8 mg/dL) | 3.2 (2.83.7) | 2.15.7 |
| Magnesium (1.32.1 mEq/L) | 1.8 0.3 | 1.22.5 |
| AST (1040 U/L) | 38 (2391) | 122402 |
| ALT (745 U/L) | 45 (2498) | 152436 |
| Total bilirubin (0.01.2 mg/dL) | 0.5 (0.30.7) | 0.12.2 |
| Pre‐albumin (2052 mg/dL) | 21 7 | 842 |
| Albumin (3.05.3 g/dL) | 3.7 0.7 | 1.64.8 |
| WBC (4.510.0 k/L) | 4.0 (3.25.7) | 1.120.3 |
| Neutrophils (%) (48.069.0%) | 55.5 13.1 | 17.082.0 |
| Lymphocytes (%) (21.043.0%) | 34.9 13.0 | 10.864.0 |
| Platelet count (150450 k/L) | 266 (193371) | 40819 |
| Hematocrit (37.047.0%) | 36.1 5.4 | 19.145.7 |
| MCV (80100 fL) | 91 7 | 73105 |
| TSH (0.346.00 IU/mL) | 1.52 (0.962.84) | 0.1864.1 |
| INR (0.821.17) | 1.09 (1.001.22) | 0.812.05 |
| 1,25 Hydroxy vitamin D (3080 ng/mL) | 41 (3058) | 8171 |
DISCUSSION
Hospital Medicine is currently the fastest growing area of specialization in medicine.13 Palliative care, inpatient geriatrics, short stay units, and bedside procedures have evolved into hospitalist‐led services.1418 The management of the medical complications of severe eating disorders is another potential niche for hospitalists.
The ACUTE Center at Denver Health represents a center in which highly specialized, multidisciplinary care is provided for a rare and extremely ill population of patients. Prior to entering the ACUTE Center, the patients described in our program had each experienced prolonged and unsuccessful stays for medical stabilization in acute care hospitals across the country, after being denied treatment in eating disorder programs due to medical instability.
Patients transferred to ACUTE often received medical care reflecting a lack of specific expertise, training, and exposure. The most common management discrepancy we noted was over‐aggressive provision of intravenous fluids. Consequently, we often diurese 1020 pounds of edema weight, gained during a prior medical hospitalization, before beginning the process of weight restoration. This edema weight artificially increases admission weight and results in less than expected weight gain from admission to discharge.
Even without substantial weight gain, medical stabilization is evidenced by consistent caloric oral intake, and fluid and electrolyte stabilization after initial refeeding. Accordingly, patients who have been treated at the ACUTE Center often become eligible for admission to eating disorder programs at body weights below the typical 70% of ideal body weight that most programs use as a threshold for admission.
From a clinical research perspective, centers such as ACUTE allow for opportunities to better understand and investigate the nuances of patient care in the setting of severe malnutrition. From our cohort of patients to date, we have noted unique issues in albumin levels,19 coagulopathy,20 and liver function,21 among others. As an example, the cohort of patients with anorexia nervosa described here had profoundly low body weight, but relatively normal admission labs. Even the serum albumin, a parameter often used to reflect nutrition in an adult internal medicine setting, is usually normal, reflecting, in an otherwise generally healthy young population, the absence of a malignant, inflammatory, or infectious etiology of weight loss.19
Hospitalists also advocate for their patients by helping to maximize the benefits of their health care coverage. Many health care plans place limits on inpatient psychiatric care benefits. Patients who are severely malnourished from their eating disorder may waste valuable psychiatric care benefits undergoing medical stabilization in psychiatric units while physically unable to undergo psychotherapy. This has become increasingly important as health insurance plans continue to decrease coverage for residential care of patients with anorexia.22
In contrast, the medical benefits of most health plans are more robust. Accordingly, from the patient perspective, medical stabilization in an acute medical unit before admission to a psychiatry unit maximizes their ability to participate in the intensive psychiatric therapy which is still needed after medical stabilization. A recent study from a residential eating disorder program confirmed that a higher discharge BMI was the single best predictor of full recovery from anorexia nervosa.23
In the future, we believe that a continuing concentration of care and experience may also lend itself to the development of protocols and management guidelines which may benefit patients beyond our own unit. Severely malnourished patients with anorexia nervosa, or bulimic patients with complicated electrolyte disorders, are likely to benefit both medically and financially from centers of excellence. Inpatient or residential psychiatric eating disorder programs may act in synergy with medical eating disorders units, like ACUTE, to most efficiently care for the severely malnourished patient. Hospitalists, with the proper training and experience, are uniquely positioned to develop such centers of excellence.
- ,,,.The prevalence and correlates of eating disorders in the national comorbidity survey replication.Biol Psychiatry.2007;61:348–358.
- .The outcome of anorexia nervosa in the 20th century.Am J Psychiatry.2002;159:1284–1293.
- ,.Anorexia nervosa medical issues.J Womens Health.2003;12:331–340.
- .Diagnosis and care of patients with anorexia nervosa in primary care settings.Ann Intern Med.2001;134:1048–1059.
- ,,, et al.Mortality in eating disorders: a descriptive study.Int J Eat Disord.2000;28:20–26.
- ,,,,.Long‐term prognosis in anorexia nervosa: lessons from a 21‐year follow‐up study.Lancet.2000;355:721–722.
- ,,,,.Variations in admissions practices for adolescents with anorexia nervosa: a North American sample.J Adolesc Health.2008;43:425–431.
- American Psychiatric Association.Treatment of patients with eating disorders, third edition.Am J Psychiatry.2006;163(suppl 7):4–54.
- American Dietetic Association.Position of the American Dietetic Association: nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders (ADA reports).J Am Diet Assoc.2006;106:2073–2082.
- ,.Clinical practice guidelines for treating restrictive eating disorder patients during medical hospitalization.Curr Opin Pediatr.2008;20:390–397.
- .Practice makes perfect: a volume‐outcome study of hospital patients with HIV disease.J Acquir Immune Defic Syndr.2008;47:226–233.
- ,,,.Association between physician caseload and patient outcome for sepsis treatment.Infect Control Hosp Epidemiol.2009;30:556–562.
- .Reflections: the hospitalist movement ten years later.J Hosp Med.2006;1:248–252.
- What will board certification be‐and mean‐for hospitalists?.Palliative care in hospitals.J Hosp Med.2006;1:21–28.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1:5–6.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial.J Hosp Med.2007;2:143–149.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- ,,, et al.A hospitalist run short stay unit: features that predict length of stay and eventual admission to traditional inpatient services.J Hosp Med.2009;4:276–284.
- ,,.Serum albumin levels may not correlate with weight status in severe anorexia nervosa.Eat Disord.2009;17:322–326.
- ,,,,.The use of thrombelastography to determine coagulation status in severe anorexia nervosa: a case series.Int J Eat Disord.2010;43(4):382–385.
- ,,,.Liver function test abnormalities in anorexia nervosa—cause or effect.Int J Eat Disord.2010;43(4):378–381.
- .Eating disorders: a new front in insurance fight.New York Times. October 13, 2011. Available at: http://www.nytimes.com/2011/10/14/business/ruling‐offers‐hope‐to‐eating‐disorder‐sufferers. html?ref=business.
- ,.Long‐term outcome of residential treatment for anorexia nervosa and bulimia nervosa.Eat Disord.2011;19:132–144.
Anorexia nervosa occurs in 0.9% of women and 0.3% of men in the United States1 and is associated with a prolonged course,2 extensive medical complications that can affect almost every organ system,3, 4 and a 5% mean crude mortality rate9.6 times expected for age‐matched women in the United States.2, 5 Those with anorexia nervosa die as a complication of their illness more frequently than any other mental illness.3 Anorexia nervosa is commonly diagnosed during the adolescent years,2 with almost 25% going on to develop chronic anorexia nervosa.2, 6 Consequently, many patients with severe anorexia nervosa will receive treatment by adult medicine practitioners.
Patients with anorexia nervosa frequently require hospitalization. Published guidelines suggest that those who are 70% or less than ideal body weight, bradycardic, hypotensive, or those with severe electrolyte disturbances warrant admission for medical stabilization.79 Once admitted, however, there are no published guidelines for best practices to medically stabilize patients.7, 10 Although most experts advocate a multidisciplinary approach with weight restoration and medical stability as the goals of hospital admission,8, 9 controversy exists in the literature about how best to achieve these goals.7, 10
It is known, however, that for patients with complicated medical illnesses, such as human immunodeficiency virus (HIV) and sepsis, higher volumes of patient caseloads treated by physicians with disease‐specific expertise has been found to lead to improved outcomes in patients.11, 12 The adult patient with severe anorexia nervosa who requires inpatient medical stabilization may also benefit from a multidisciplinary trained staff familiar with the medical management of anorexia nervosa. Accordingly, we have developed the Acute Comprehensive Urgent Treatment for Eating Disorders (ACUTE) Center.
PROGRAM DESCRIPTION
The ACUTE Center at Denver Health is a 5‐bed unit dedicated to the medical stabilization of patients with severe malnutrition due to anorexia nervosa or severe electrolyte disorders due to bulimia nervosa. ACUTE accepts patients 17 years and older with medical complications related to chronic malnutrition and refeeding.
ACUTE uses a multidisciplinary approach to patient care. The physician team is composed of a hospital medicine attending physician, consultative expertise by an internal medicine specialist in the management of the medical complications of eating disorders, and a psychiatrist specializing in eating disorders. There is a dedicated team of nurses, two dieticians, physical therapists, certified nursing assistants, speech therapists, a psychotherapist, and a chaplain.
ACUTE patients are on continuous telemetry monitoring for the duration of their hospitalization to monitor for arrhythmias as well as signs of covert exercise. As part of the initial intake, a full set of vital signs is obtained, including height and weight. Patients are weighed daily with their back to the scale. There is no discussion of weight fluctuations. Patients may walk at a slow pace around the unit. No exercise is allowed.
Each patient at the ACUTE Center has an individualized meal plan and are started on an oral caloric intake 200 kcal below their basal energy expenditure (BEE). Indirect calorimetry is performed on the first hospital day. Each patient meets on a daily basis with the registered dietician to choose meals that meet their caloric goals.
All patients have a sitter continuously for their first week, and thereafter sitter time may be reduced to supervision surrounding each meal. Patients who fail to finish their prescribed meal are required to drink a liquid supplement to meet caloric goals. Calories are increased weekly until the patient's weight shows a clear pattern of weight increase. 0

Patients are discharged from the ACUTE Center when they have achieved several basic goals: They are consuming greater than 2000 kcal per day, they are consistently gaining 23 pounds per week, their laboratory values have stabilized without electrolyte supplementation, and they are strong enough for an inpatient eating disorder program.
METHODS
Patients admitted to the ACUTE Center between October 2008 and December 2010 for medical stabilization and monitored refeeding were included. Patients with a diagnosis of bulimia nervosa were excluded. Demographic data and laboratory results were obtained electronically from our data repository, whereas weight, height, and other clinical characteristics were obtained by manual chart abstraction. The statistical analysis was conducted in SAS Enterprise Guide v4.1 (SAS Institute, Cary, NC).
RESULTS
In its first 27 months, the ACUTE Center had 76 total admissions, comprising 59 patients. Of the 76 admissions, the 62 admissions for medical stabilization and monitored refeeding of 54 patients with anorexia nervosa were included. Forty‐eight of the 54 (89%) included patients were female. Six patients were hospitalized twice, and 1 patient 3 times. There were 3 transfers to the intensive care unit, and no inpatient mortality. Of the 62 admissions, 11 (18%) discharges were to home, and 51 (82%) were to inpatient psychiatric eating disorder units.
The mean age at admission was 27 years (range 1765 years). The mean percent of ideal body weight (IBW) on admission was 62.2% 10.2%. The mean body mass index (BMI) was 12.9 2.0 kg/m2 on admission, and 13.1 1.9 kg/m2 upon discharge. The median length of stay was 16 days (interquartile range [IQR] 929 days). Median calculated BEE (1119 [10671184 IQR]) was higher than measured BEE by indirect calorimetry (792 [6341094]), (Table 1).
| Median (Interquartile Range)* | Range | |
|---|---|---|
| ||
| Age, yr | 27 (2135) | 1765 |
| Female | 56 | 90% |
| Length of hospitalization, days | 16 (929) | 570 |
| Calculated BEE | 1119 (10671184) | 9061491 |
| Measured BEE | 792 (6341094) | 5001742 |
| DEXA Z‐score | 2.2 1.1 | 4.40.7 |
| Height, in | 65 (6167) | 5774 |
| Weight on admission, lb | 76.1 14.4 | 50.8110.0 |
| % Ideal body weight on admission | 62.2 10.2 | 42.4101.0 |
| % Ideal body weight on discharge | 63.2 9.1 | 42.3 82.7 |
| BMI on admission | 12.9 2.0 | 8.719.7 |
| BMI nadir | 12.4 1.9 | 8.415.7 |
| BMI on discharge | 13.1 1.9 | 8.717.0 |
The majority of admission laboratory values, including serum albumin, blood urea nitrogen (BUN), creatinine, potassium, magnesium, and phosphate levels, were within normal limits. Fifty‐six percent were hyponatremic at admission, with a mean serum sodium level of 133 6 mmol/L (Table 2).
| Median (Interquartile Range)* | Range | |
|---|---|---|
| ||
| Sodium (135143 mmol/L) | 133 6 | 117145 |
| Potassium (3.65.1 mmol/L) | 3.8 (3.0 4.0) | 1.85.5 |
| Carbon dioxide (1827 mmol/L) | 28 (2531) | 1845 |
| Glucose (60199 mg/dL) | 85 (76105) | 41166 |
| BUN (622 mg/dL) | 16 (923) | 344 |
| Creatinine (0.61.2 mg/dL) | 0.7 (0.61.0) | 0.31.6 |
| Calcium (8.110.5 mg/dL) | 8.9 0.6 | 7.610.1 |
| Phosphorus (2.74.8 mg/dL) | 3.2 (2.83.7) | 2.15.7 |
| Magnesium (1.32.1 mEq/L) | 1.8 0.3 | 1.22.5 |
| AST (1040 U/L) | 38 (2391) | 122402 |
| ALT (745 U/L) | 45 (2498) | 152436 |
| Total bilirubin (0.01.2 mg/dL) | 0.5 (0.30.7) | 0.12.2 |
| Pre‐albumin (2052 mg/dL) | 21 7 | 842 |
| Albumin (3.05.3 g/dL) | 3.7 0.7 | 1.64.8 |
| WBC (4.510.0 k/L) | 4.0 (3.25.7) | 1.120.3 |
| Neutrophils (%) (48.069.0%) | 55.5 13.1 | 17.082.0 |
| Lymphocytes (%) (21.043.0%) | 34.9 13.0 | 10.864.0 |
| Platelet count (150450 k/L) | 266 (193371) | 40819 |
| Hematocrit (37.047.0%) | 36.1 5.4 | 19.145.7 |
| MCV (80100 fL) | 91 7 | 73105 |
| TSH (0.346.00 IU/mL) | 1.52 (0.962.84) | 0.1864.1 |
| INR (0.821.17) | 1.09 (1.001.22) | 0.812.05 |
| 1,25 Hydroxy vitamin D (3080 ng/mL) | 41 (3058) | 8171 |
DISCUSSION
Hospital Medicine is currently the fastest growing area of specialization in medicine.13 Palliative care, inpatient geriatrics, short stay units, and bedside procedures have evolved into hospitalist‐led services.1418 The management of the medical complications of severe eating disorders is another potential niche for hospitalists.
The ACUTE Center at Denver Health represents a center in which highly specialized, multidisciplinary care is provided for a rare and extremely ill population of patients. Prior to entering the ACUTE Center, the patients described in our program had each experienced prolonged and unsuccessful stays for medical stabilization in acute care hospitals across the country, after being denied treatment in eating disorder programs due to medical instability.
Patients transferred to ACUTE often received medical care reflecting a lack of specific expertise, training, and exposure. The most common management discrepancy we noted was over‐aggressive provision of intravenous fluids. Consequently, we often diurese 1020 pounds of edema weight, gained during a prior medical hospitalization, before beginning the process of weight restoration. This edema weight artificially increases admission weight and results in less than expected weight gain from admission to discharge.
Even without substantial weight gain, medical stabilization is evidenced by consistent caloric oral intake, and fluid and electrolyte stabilization after initial refeeding. Accordingly, patients who have been treated at the ACUTE Center often become eligible for admission to eating disorder programs at body weights below the typical 70% of ideal body weight that most programs use as a threshold for admission.
From a clinical research perspective, centers such as ACUTE allow for opportunities to better understand and investigate the nuances of patient care in the setting of severe malnutrition. From our cohort of patients to date, we have noted unique issues in albumin levels,19 coagulopathy,20 and liver function,21 among others. As an example, the cohort of patients with anorexia nervosa described here had profoundly low body weight, but relatively normal admission labs. Even the serum albumin, a parameter often used to reflect nutrition in an adult internal medicine setting, is usually normal, reflecting, in an otherwise generally healthy young population, the absence of a malignant, inflammatory, or infectious etiology of weight loss.19
Hospitalists also advocate for their patients by helping to maximize the benefits of their health care coverage. Many health care plans place limits on inpatient psychiatric care benefits. Patients who are severely malnourished from their eating disorder may waste valuable psychiatric care benefits undergoing medical stabilization in psychiatric units while physically unable to undergo psychotherapy. This has become increasingly important as health insurance plans continue to decrease coverage for residential care of patients with anorexia.22
In contrast, the medical benefits of most health plans are more robust. Accordingly, from the patient perspective, medical stabilization in an acute medical unit before admission to a psychiatry unit maximizes their ability to participate in the intensive psychiatric therapy which is still needed after medical stabilization. A recent study from a residential eating disorder program confirmed that a higher discharge BMI was the single best predictor of full recovery from anorexia nervosa.23
In the future, we believe that a continuing concentration of care and experience may also lend itself to the development of protocols and management guidelines which may benefit patients beyond our own unit. Severely malnourished patients with anorexia nervosa, or bulimic patients with complicated electrolyte disorders, are likely to benefit both medically and financially from centers of excellence. Inpatient or residential psychiatric eating disorder programs may act in synergy with medical eating disorders units, like ACUTE, to most efficiently care for the severely malnourished patient. Hospitalists, with the proper training and experience, are uniquely positioned to develop such centers of excellence.
Anorexia nervosa occurs in 0.9% of women and 0.3% of men in the United States1 and is associated with a prolonged course,2 extensive medical complications that can affect almost every organ system,3, 4 and a 5% mean crude mortality rate9.6 times expected for age‐matched women in the United States.2, 5 Those with anorexia nervosa die as a complication of their illness more frequently than any other mental illness.3 Anorexia nervosa is commonly diagnosed during the adolescent years,2 with almost 25% going on to develop chronic anorexia nervosa.2, 6 Consequently, many patients with severe anorexia nervosa will receive treatment by adult medicine practitioners.
Patients with anorexia nervosa frequently require hospitalization. Published guidelines suggest that those who are 70% or less than ideal body weight, bradycardic, hypotensive, or those with severe electrolyte disturbances warrant admission for medical stabilization.79 Once admitted, however, there are no published guidelines for best practices to medically stabilize patients.7, 10 Although most experts advocate a multidisciplinary approach with weight restoration and medical stability as the goals of hospital admission,8, 9 controversy exists in the literature about how best to achieve these goals.7, 10
It is known, however, that for patients with complicated medical illnesses, such as human immunodeficiency virus (HIV) and sepsis, higher volumes of patient caseloads treated by physicians with disease‐specific expertise has been found to lead to improved outcomes in patients.11, 12 The adult patient with severe anorexia nervosa who requires inpatient medical stabilization may also benefit from a multidisciplinary trained staff familiar with the medical management of anorexia nervosa. Accordingly, we have developed the Acute Comprehensive Urgent Treatment for Eating Disorders (ACUTE) Center.
PROGRAM DESCRIPTION
The ACUTE Center at Denver Health is a 5‐bed unit dedicated to the medical stabilization of patients with severe malnutrition due to anorexia nervosa or severe electrolyte disorders due to bulimia nervosa. ACUTE accepts patients 17 years and older with medical complications related to chronic malnutrition and refeeding.
ACUTE uses a multidisciplinary approach to patient care. The physician team is composed of a hospital medicine attending physician, consultative expertise by an internal medicine specialist in the management of the medical complications of eating disorders, and a psychiatrist specializing in eating disorders. There is a dedicated team of nurses, two dieticians, physical therapists, certified nursing assistants, speech therapists, a psychotherapist, and a chaplain.
ACUTE patients are on continuous telemetry monitoring for the duration of their hospitalization to monitor for arrhythmias as well as signs of covert exercise. As part of the initial intake, a full set of vital signs is obtained, including height and weight. Patients are weighed daily with their back to the scale. There is no discussion of weight fluctuations. Patients may walk at a slow pace around the unit. No exercise is allowed.
Each patient at the ACUTE Center has an individualized meal plan and are started on an oral caloric intake 200 kcal below their basal energy expenditure (BEE). Indirect calorimetry is performed on the first hospital day. Each patient meets on a daily basis with the registered dietician to choose meals that meet their caloric goals.
All patients have a sitter continuously for their first week, and thereafter sitter time may be reduced to supervision surrounding each meal. Patients who fail to finish their prescribed meal are required to drink a liquid supplement to meet caloric goals. Calories are increased weekly until the patient's weight shows a clear pattern of weight increase. 0

Patients are discharged from the ACUTE Center when they have achieved several basic goals: They are consuming greater than 2000 kcal per day, they are consistently gaining 23 pounds per week, their laboratory values have stabilized without electrolyte supplementation, and they are strong enough for an inpatient eating disorder program.
METHODS
Patients admitted to the ACUTE Center between October 2008 and December 2010 for medical stabilization and monitored refeeding were included. Patients with a diagnosis of bulimia nervosa were excluded. Demographic data and laboratory results were obtained electronically from our data repository, whereas weight, height, and other clinical characteristics were obtained by manual chart abstraction. The statistical analysis was conducted in SAS Enterprise Guide v4.1 (SAS Institute, Cary, NC).
RESULTS
In its first 27 months, the ACUTE Center had 76 total admissions, comprising 59 patients. Of the 76 admissions, the 62 admissions for medical stabilization and monitored refeeding of 54 patients with anorexia nervosa were included. Forty‐eight of the 54 (89%) included patients were female. Six patients were hospitalized twice, and 1 patient 3 times. There were 3 transfers to the intensive care unit, and no inpatient mortality. Of the 62 admissions, 11 (18%) discharges were to home, and 51 (82%) were to inpatient psychiatric eating disorder units.
The mean age at admission was 27 years (range 1765 years). The mean percent of ideal body weight (IBW) on admission was 62.2% 10.2%. The mean body mass index (BMI) was 12.9 2.0 kg/m2 on admission, and 13.1 1.9 kg/m2 upon discharge. The median length of stay was 16 days (interquartile range [IQR] 929 days). Median calculated BEE (1119 [10671184 IQR]) was higher than measured BEE by indirect calorimetry (792 [6341094]), (Table 1).
| Median (Interquartile Range)* | Range | |
|---|---|---|
| ||
| Age, yr | 27 (2135) | 1765 |
| Female | 56 | 90% |
| Length of hospitalization, days | 16 (929) | 570 |
| Calculated BEE | 1119 (10671184) | 9061491 |
| Measured BEE | 792 (6341094) | 5001742 |
| DEXA Z‐score | 2.2 1.1 | 4.40.7 |
| Height, in | 65 (6167) | 5774 |
| Weight on admission, lb | 76.1 14.4 | 50.8110.0 |
| % Ideal body weight on admission | 62.2 10.2 | 42.4101.0 |
| % Ideal body weight on discharge | 63.2 9.1 | 42.3 82.7 |
| BMI on admission | 12.9 2.0 | 8.719.7 |
| BMI nadir | 12.4 1.9 | 8.415.7 |
| BMI on discharge | 13.1 1.9 | 8.717.0 |
The majority of admission laboratory values, including serum albumin, blood urea nitrogen (BUN), creatinine, potassium, magnesium, and phosphate levels, were within normal limits. Fifty‐six percent were hyponatremic at admission, with a mean serum sodium level of 133 6 mmol/L (Table 2).
| Median (Interquartile Range)* | Range | |
|---|---|---|
| ||
| Sodium (135143 mmol/L) | 133 6 | 117145 |
| Potassium (3.65.1 mmol/L) | 3.8 (3.0 4.0) | 1.85.5 |
| Carbon dioxide (1827 mmol/L) | 28 (2531) | 1845 |
| Glucose (60199 mg/dL) | 85 (76105) | 41166 |
| BUN (622 mg/dL) | 16 (923) | 344 |
| Creatinine (0.61.2 mg/dL) | 0.7 (0.61.0) | 0.31.6 |
| Calcium (8.110.5 mg/dL) | 8.9 0.6 | 7.610.1 |
| Phosphorus (2.74.8 mg/dL) | 3.2 (2.83.7) | 2.15.7 |
| Magnesium (1.32.1 mEq/L) | 1.8 0.3 | 1.22.5 |
| AST (1040 U/L) | 38 (2391) | 122402 |
| ALT (745 U/L) | 45 (2498) | 152436 |
| Total bilirubin (0.01.2 mg/dL) | 0.5 (0.30.7) | 0.12.2 |
| Pre‐albumin (2052 mg/dL) | 21 7 | 842 |
| Albumin (3.05.3 g/dL) | 3.7 0.7 | 1.64.8 |
| WBC (4.510.0 k/L) | 4.0 (3.25.7) | 1.120.3 |
| Neutrophils (%) (48.069.0%) | 55.5 13.1 | 17.082.0 |
| Lymphocytes (%) (21.043.0%) | 34.9 13.0 | 10.864.0 |
| Platelet count (150450 k/L) | 266 (193371) | 40819 |
| Hematocrit (37.047.0%) | 36.1 5.4 | 19.145.7 |
| MCV (80100 fL) | 91 7 | 73105 |
| TSH (0.346.00 IU/mL) | 1.52 (0.962.84) | 0.1864.1 |
| INR (0.821.17) | 1.09 (1.001.22) | 0.812.05 |
| 1,25 Hydroxy vitamin D (3080 ng/mL) | 41 (3058) | 8171 |
DISCUSSION
Hospital Medicine is currently the fastest growing area of specialization in medicine.13 Palliative care, inpatient geriatrics, short stay units, and bedside procedures have evolved into hospitalist‐led services.1418 The management of the medical complications of severe eating disorders is another potential niche for hospitalists.
The ACUTE Center at Denver Health represents a center in which highly specialized, multidisciplinary care is provided for a rare and extremely ill population of patients. Prior to entering the ACUTE Center, the patients described in our program had each experienced prolonged and unsuccessful stays for medical stabilization in acute care hospitals across the country, after being denied treatment in eating disorder programs due to medical instability.
Patients transferred to ACUTE often received medical care reflecting a lack of specific expertise, training, and exposure. The most common management discrepancy we noted was over‐aggressive provision of intravenous fluids. Consequently, we often diurese 1020 pounds of edema weight, gained during a prior medical hospitalization, before beginning the process of weight restoration. This edema weight artificially increases admission weight and results in less than expected weight gain from admission to discharge.
Even without substantial weight gain, medical stabilization is evidenced by consistent caloric oral intake, and fluid and electrolyte stabilization after initial refeeding. Accordingly, patients who have been treated at the ACUTE Center often become eligible for admission to eating disorder programs at body weights below the typical 70% of ideal body weight that most programs use as a threshold for admission.
From a clinical research perspective, centers such as ACUTE allow for opportunities to better understand and investigate the nuances of patient care in the setting of severe malnutrition. From our cohort of patients to date, we have noted unique issues in albumin levels,19 coagulopathy,20 and liver function,21 among others. As an example, the cohort of patients with anorexia nervosa described here had profoundly low body weight, but relatively normal admission labs. Even the serum albumin, a parameter often used to reflect nutrition in an adult internal medicine setting, is usually normal, reflecting, in an otherwise generally healthy young population, the absence of a malignant, inflammatory, or infectious etiology of weight loss.19
Hospitalists also advocate for their patients by helping to maximize the benefits of their health care coverage. Many health care plans place limits on inpatient psychiatric care benefits. Patients who are severely malnourished from their eating disorder may waste valuable psychiatric care benefits undergoing medical stabilization in psychiatric units while physically unable to undergo psychotherapy. This has become increasingly important as health insurance plans continue to decrease coverage for residential care of patients with anorexia.22
In contrast, the medical benefits of most health plans are more robust. Accordingly, from the patient perspective, medical stabilization in an acute medical unit before admission to a psychiatry unit maximizes their ability to participate in the intensive psychiatric therapy which is still needed after medical stabilization. A recent study from a residential eating disorder program confirmed that a higher discharge BMI was the single best predictor of full recovery from anorexia nervosa.23
In the future, we believe that a continuing concentration of care and experience may also lend itself to the development of protocols and management guidelines which may benefit patients beyond our own unit. Severely malnourished patients with anorexia nervosa, or bulimic patients with complicated electrolyte disorders, are likely to benefit both medically and financially from centers of excellence. Inpatient or residential psychiatric eating disorder programs may act in synergy with medical eating disorders units, like ACUTE, to most efficiently care for the severely malnourished patient. Hospitalists, with the proper training and experience, are uniquely positioned to develop such centers of excellence.
- ,,,.The prevalence and correlates of eating disorders in the national comorbidity survey replication.Biol Psychiatry.2007;61:348–358.
- .The outcome of anorexia nervosa in the 20th century.Am J Psychiatry.2002;159:1284–1293.
- ,.Anorexia nervosa medical issues.J Womens Health.2003;12:331–340.
- .Diagnosis and care of patients with anorexia nervosa in primary care settings.Ann Intern Med.2001;134:1048–1059.
- ,,, et al.Mortality in eating disorders: a descriptive study.Int J Eat Disord.2000;28:20–26.
- ,,,,.Long‐term prognosis in anorexia nervosa: lessons from a 21‐year follow‐up study.Lancet.2000;355:721–722.
- ,,,,.Variations in admissions practices for adolescents with anorexia nervosa: a North American sample.J Adolesc Health.2008;43:425–431.
- American Psychiatric Association.Treatment of patients with eating disorders, third edition.Am J Psychiatry.2006;163(suppl 7):4–54.
- American Dietetic Association.Position of the American Dietetic Association: nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders (ADA reports).J Am Diet Assoc.2006;106:2073–2082.
- ,.Clinical practice guidelines for treating restrictive eating disorder patients during medical hospitalization.Curr Opin Pediatr.2008;20:390–397.
- .Practice makes perfect: a volume‐outcome study of hospital patients with HIV disease.J Acquir Immune Defic Syndr.2008;47:226–233.
- ,,,.Association between physician caseload and patient outcome for sepsis treatment.Infect Control Hosp Epidemiol.2009;30:556–562.
- .Reflections: the hospitalist movement ten years later.J Hosp Med.2006;1:248–252.
- What will board certification be‐and mean‐for hospitalists?.Palliative care in hospitals.J Hosp Med.2006;1:21–28.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1:5–6.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial.J Hosp Med.2007;2:143–149.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- ,,, et al.A hospitalist run short stay unit: features that predict length of stay and eventual admission to traditional inpatient services.J Hosp Med.2009;4:276–284.
- ,,.Serum albumin levels may not correlate with weight status in severe anorexia nervosa.Eat Disord.2009;17:322–326.
- ,,,,.The use of thrombelastography to determine coagulation status in severe anorexia nervosa: a case series.Int J Eat Disord.2010;43(4):382–385.
- ,,,.Liver function test abnormalities in anorexia nervosa—cause or effect.Int J Eat Disord.2010;43(4):378–381.
- .Eating disorders: a new front in insurance fight.New York Times. October 13, 2011. Available at: http://www.nytimes.com/2011/10/14/business/ruling‐offers‐hope‐to‐eating‐disorder‐sufferers. html?ref=business.
- ,.Long‐term outcome of residential treatment for anorexia nervosa and bulimia nervosa.Eat Disord.2011;19:132–144.
- ,,,.The prevalence and correlates of eating disorders in the national comorbidity survey replication.Biol Psychiatry.2007;61:348–358.
- .The outcome of anorexia nervosa in the 20th century.Am J Psychiatry.2002;159:1284–1293.
- ,.Anorexia nervosa medical issues.J Womens Health.2003;12:331–340.
- .Diagnosis and care of patients with anorexia nervosa in primary care settings.Ann Intern Med.2001;134:1048–1059.
- ,,, et al.Mortality in eating disorders: a descriptive study.Int J Eat Disord.2000;28:20–26.
- ,,,,.Long‐term prognosis in anorexia nervosa: lessons from a 21‐year follow‐up study.Lancet.2000;355:721–722.
- ,,,,.Variations in admissions practices for adolescents with anorexia nervosa: a North American sample.J Adolesc Health.2008;43:425–431.
- American Psychiatric Association.Treatment of patients with eating disorders, third edition.Am J Psychiatry.2006;163(suppl 7):4–54.
- American Dietetic Association.Position of the American Dietetic Association: nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders (ADA reports).J Am Diet Assoc.2006;106:2073–2082.
- ,.Clinical practice guidelines for treating restrictive eating disorder patients during medical hospitalization.Curr Opin Pediatr.2008;20:390–397.
- .Practice makes perfect: a volume‐outcome study of hospital patients with HIV disease.J Acquir Immune Defic Syndr.2008;47:226–233.
- ,,,.Association between physician caseload and patient outcome for sepsis treatment.Infect Control Hosp Epidemiol.2009;30:556–562.
- .Reflections: the hospitalist movement ten years later.J Hosp Med.2006;1:248–252.
- What will board certification be‐and mean‐for hospitalists?.Palliative care in hospitals.J Hosp Med.2006;1:21–28.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1:5–6.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial.J Hosp Med.2007;2:143–149.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- ,,, et al.A hospitalist run short stay unit: features that predict length of stay and eventual admission to traditional inpatient services.J Hosp Med.2009;4:276–284.
- ,,.Serum albumin levels may not correlate with weight status in severe anorexia nervosa.Eat Disord.2009;17:322–326.
- ,,,,.The use of thrombelastography to determine coagulation status in severe anorexia nervosa: a case series.Int J Eat Disord.2010;43(4):382–385.
- ,,,.Liver function test abnormalities in anorexia nervosa—cause or effect.Int J Eat Disord.2010;43(4):378–381.
- .Eating disorders: a new front in insurance fight.New York Times. October 13, 2011. Available at: http://www.nytimes.com/2011/10/14/business/ruling‐offers‐hope‐to‐eating‐disorder‐sufferers. html?ref=business.
- ,.Long‐term outcome of residential treatment for anorexia nervosa and bulimia nervosa.Eat Disord.2011;19:132–144.
In response to: A quality conundrum: Well done but not enough—Quality improvement conundrums: Looking back before moving forward
If clinician‐quality improvers are to gain traction as academicians,1 their first objective should be to bring quality improvement (QI) sandly into the world of scientific method. We believe that Dr. Chakraborti's 2 pointsthat the reasons for afferent limb failure need to be more closely investigated, and that lessons learned from 1 hospital's rapid response system (RRS) may not generalize to other hospitalsreflect the immaturity of QI as a science. In clinical science, 3 well‐defined testing phases bring 1 homogeneous, rigorously tested product to market that is monitored in a fourth phase. While Dr. Chakraborti urges us to examine our afferent limb failures more closely, the monitoring and reporting strategies used in the Josie King Patient Safety Program2 resonate with the postmarketing surveillance of Phase IV trials.
Although necessary and valid, we believe that the majority of the QI conundrum of RRS lies in the lack of premarket, stepwise testing of QI products. QI initiatives are often promulgated before an appropriate evidence base has been established. This lack of scientific rigor has resulted in RRS with calling criteria that have poor operating characteristics,3 undetermined methods for achieving afferent success,4 and efferent response arms of varying sizes and compositions.5 Consequently, a heterogeneous group of RRS have produced equivocal outcomes6 and diminished the applicability of lessons learned across institutions.
Indeed, while it is important to ask, What do we do now?, it may be more informative to answer the question, How did we get here?
- ,.Clinicians in quality improvement. A new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- Josie King Foundation. Josie King Patient Safety Program. Available at: http://www.josieking.org/page.cfm?pageID=27. Accessed September2009.
- ,,,.Rapid response: a quality improvement conundrum.J Hosp Med.2009;4(4):255–257.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,.Dress for the occasion.Jt Comm J Qual Patient Saf.2009;35(6):295.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
If clinician‐quality improvers are to gain traction as academicians,1 their first objective should be to bring quality improvement (QI) sandly into the world of scientific method. We believe that Dr. Chakraborti's 2 pointsthat the reasons for afferent limb failure need to be more closely investigated, and that lessons learned from 1 hospital's rapid response system (RRS) may not generalize to other hospitalsreflect the immaturity of QI as a science. In clinical science, 3 well‐defined testing phases bring 1 homogeneous, rigorously tested product to market that is monitored in a fourth phase. While Dr. Chakraborti urges us to examine our afferent limb failures more closely, the monitoring and reporting strategies used in the Josie King Patient Safety Program2 resonate with the postmarketing surveillance of Phase IV trials.
Although necessary and valid, we believe that the majority of the QI conundrum of RRS lies in the lack of premarket, stepwise testing of QI products. QI initiatives are often promulgated before an appropriate evidence base has been established. This lack of scientific rigor has resulted in RRS with calling criteria that have poor operating characteristics,3 undetermined methods for achieving afferent success,4 and efferent response arms of varying sizes and compositions.5 Consequently, a heterogeneous group of RRS have produced equivocal outcomes6 and diminished the applicability of lessons learned across institutions.
Indeed, while it is important to ask, What do we do now?, it may be more informative to answer the question, How did we get here?
If clinician‐quality improvers are to gain traction as academicians,1 their first objective should be to bring quality improvement (QI) sandly into the world of scientific method. We believe that Dr. Chakraborti's 2 pointsthat the reasons for afferent limb failure need to be more closely investigated, and that lessons learned from 1 hospital's rapid response system (RRS) may not generalize to other hospitalsreflect the immaturity of QI as a science. In clinical science, 3 well‐defined testing phases bring 1 homogeneous, rigorously tested product to market that is monitored in a fourth phase. While Dr. Chakraborti urges us to examine our afferent limb failures more closely, the monitoring and reporting strategies used in the Josie King Patient Safety Program2 resonate with the postmarketing surveillance of Phase IV trials.
Although necessary and valid, we believe that the majority of the QI conundrum of RRS lies in the lack of premarket, stepwise testing of QI products. QI initiatives are often promulgated before an appropriate evidence base has been established. This lack of scientific rigor has resulted in RRS with calling criteria that have poor operating characteristics,3 undetermined methods for achieving afferent success,4 and efferent response arms of varying sizes and compositions.5 Consequently, a heterogeneous group of RRS have produced equivocal outcomes6 and diminished the applicability of lessons learned across institutions.
Indeed, while it is important to ask, What do we do now?, it may be more informative to answer the question, How did we get here?
- ,.Clinicians in quality improvement. A new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- Josie King Foundation. Josie King Patient Safety Program. Available at: http://www.josieking.org/page.cfm?pageID=27. Accessed September2009.
- ,,,.Rapid response: a quality improvement conundrum.J Hosp Med.2009;4(4):255–257.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,.Dress for the occasion.Jt Comm J Qual Patient Saf.2009;35(6):295.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
- ,.Clinicians in quality improvement. A new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- Josie King Foundation. Josie King Patient Safety Program. Available at: http://www.josieking.org/page.cfm?pageID=27. Accessed September2009.
- ,,,.Rapid response: a quality improvement conundrum.J Hosp Med.2009;4(4):255–257.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,.Dress for the occasion.Jt Comm J Qual Patient Saf.2009;35(6):295.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
Rapid Response: A QI Conundrum
Many in‐hospital cardiac arrests and other adverse events are heralded by warning signs that are evident in the preceding 6 to 8 hours.1 By promptly intervening before further deterioration occurs, rapid response teams (RRTs) are designed to decrease unexpected intensive care unit (ICU) transfers, cardiac arrests, and inpatient mortality. While implementing RRTs is 1 of the 6 initiatives recommended by the Institute for Healthcare Improvement,2 data supporting their effectiveness is equivocal.3, 4
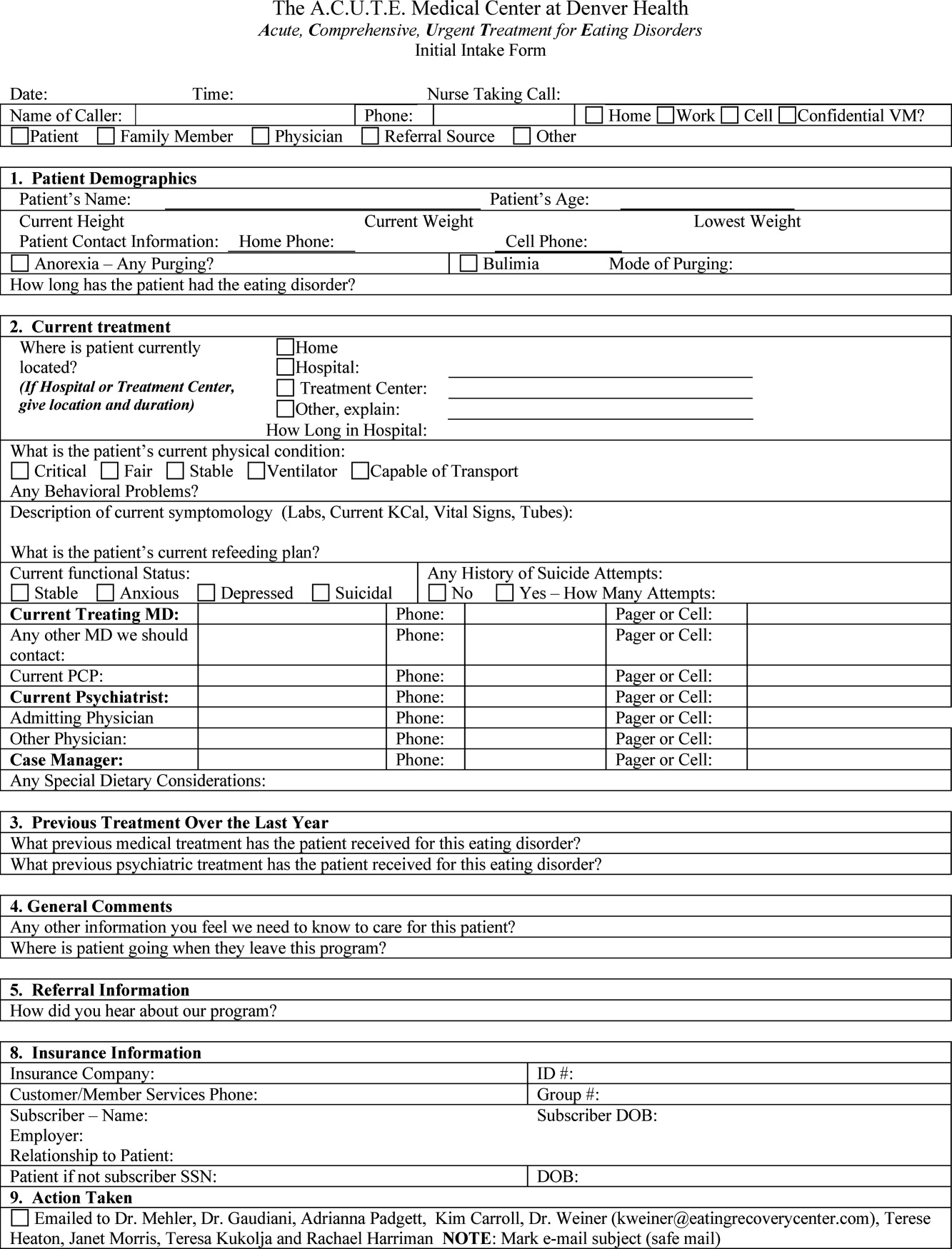
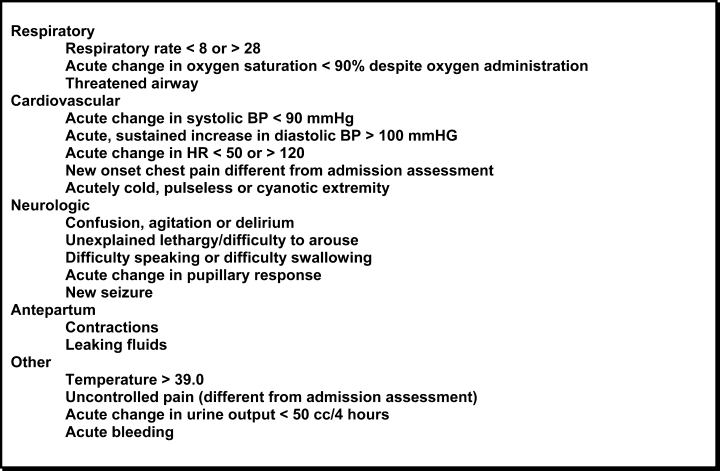
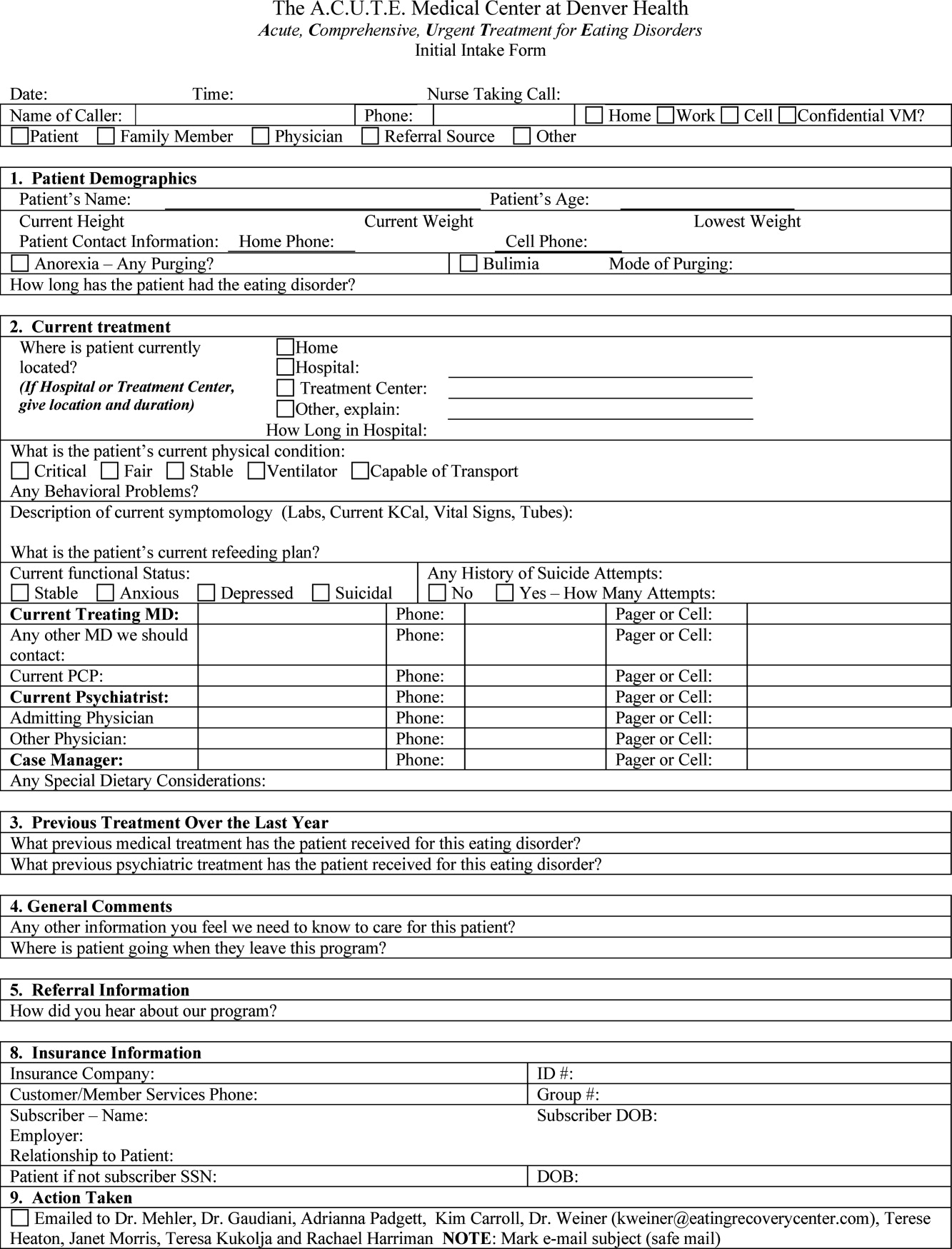
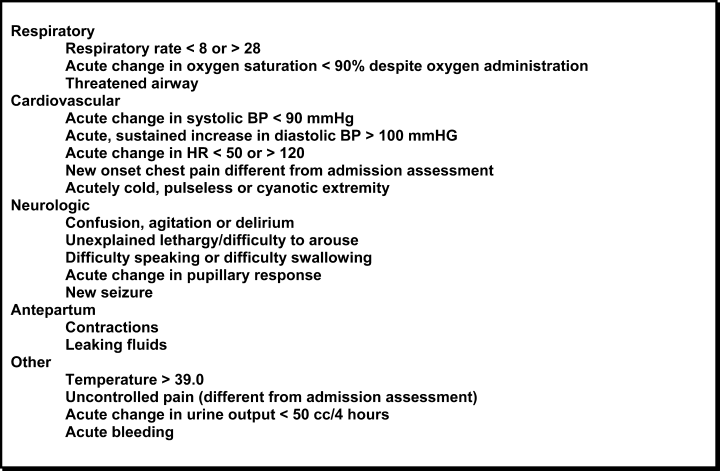
In October 2006, at Denver Health Medical Center, an academic, safety net hospital, we initiated a rapid response systemclinical triggers program (RRS‐CTP).5 In our RRS‐CTP, an abrupt change in patient status (Figure 1) triggers a mandatory call by the patient's nurse to the primary team, which is then required to perform an immediate bedside evaluation. By incorporating the primary team into the RRT‐CTP, we sought to preserve as much continuity of care as possible. Also, since the same house staff compose our cardiopulmonary arrest or cor team, and staff the ICUs and non‐ICU hospital wards, we did not feel that creating a separate RRT was an efficient use of resources. Our nurses have undergone extensive education about the necessity of a prompt bedside evaluation and have been instructed and empowered to escalate concerns to senior physicians if needed. We present a case that illustrates challenges to both implementing an RRS and measuring its potential benefits.
Case
A 59‐year‐old woman with a history of bipolar mood disorder was admitted for altered mental status. At presentation, she had signs of acute mania with normal vital signs. After initial laboratory workup, her altered mental status was felt to be multifactorial due to urinary tract infection, hypernatremia (attributed to lithium‐induced nephrogenic diabetes insipidus), and acute mania (attributed to medication discontinuation). Because she was slow to recover from the acute mania, her hospital stay was prolonged. From admission, the patient was treated with heparin 5000 units subcutaneously twice daily for venous thromboembolism prophylaxis.
On hospital day 7, at 21:32, the patient was noted to have asymptomatic tachycardia at 149 beats per minute and a new oxygen requirement of 3 L/minute. The cross‐cover team was called; however, although criteria were met, the RRS‐CTP was not activated and a bedside evaluation was not performed. A chest X‐ray was found to be normal and, with the exception of the oxygen requirement, her vital signs normalized by 23:45. No further diagnostic testing was performed at the time.
The next morning, at 11:58, the patient was found to have a blood pressure of 60/40 mmHg and heart rate of 42 beats per minute. The RRS‐CTP was activated. The primary team arrived at the bedside at 12:00 and found the patient to be alert, oriented, and without complaints. Her respiratory rate was 30/minute, and her oxygen saturation was 86% on 3 L/minute. An arterial blood gas analysis demonstrated acute respiratory alkalosis with hypoxemia and an electrocardiogram showed sinus tachycardia with a new S1Q3T3 pattern. A computed tomography angiogram revealed a large, nearly occlusive pulmonary embolus (PE) filling an enlarged right pulmonary artery, as well as thrombus within the left main pulmonary artery. She was transferred to the medical ICU and alteplase was administered. The patient survived and was discharged in good clinical condition.
Discussion
Despite the strong theoretical benefit of the RRT concept, a recent review by Ranji et al.4 concluded that RRTs had not yet been shown to improve patient outcomes. In contrast to dedicated RRTs, this case illustrates a different type of RRS that was designed to address abrupt changes in patient status, while maintaining continuity of care and efficiently utilizing resources.
If one considers an RRS to have both afferent (criteria recognition) and efferent (RRT or primary team response) limbs, the afferent limb must be consistently activated in order to obtain the efferent limb's response.6 The greatest opportunities to improve RRSs are thought to lie in the afferent limb.3 Our RRS‐CTP was not triggered in 1 of 2 instances in which criteria for mandatory initiation of the system were met. This is consistent with the findings of the Medical Early Response Intervention and Therapy (MERIT) trial, in which RRTs were called in only 41% of the patients meeting criteria and subsequently having adverse events,7 and with the ongoing monitoring of the use of the system at our hospital. Had the cross‐covering team seen the patient at the bedside initially, the PE might have been diagnosed while the patient was hemodynamically stable, giving the patient nearly a 3‐fold lower relative mortality.8 When the RRS‐CTP was activated, a prompt bedside evaluation occurred, allowing for lytic therapy to be administered before cardiopulmonary arrest (attendant mortality of 90%).9
While rapid response criteria were originally based upon published sensitivity analyses, more recent studies suggest that these criteria lack diagnostic accuracy. As demonstrated by Cretikos et al,10 to reach a sensitivity of 70%, the corresponding specificity would be only 86%. Given that the prevalence of adverse events in the MERIT trial was only 0.6%, the resulting positive predictive value (PPV) of rapid response call criteria is 3%. Accordingly, 33 calls would be needed to prevent 1 unplanned ICU transfer, cardiac arrest, or death. Nurses' attempts to minimize false‐positive calls may help explain the low call rates for patients meeting RRT criteria. The 2 avenues to increase the PPV of criteria are:
-
Increase the prevalence of disease in the population screened by risk factor stratification.
-
Increase the specificity of the call criteria, which has been limited by the associated decrease in sensitivity.10
Regarding the efferent response limb of RRS, our case demonstrates that the primary team (rather than a separate group of caregivers), when alerted appropriately, can effectively respond to critical changes in patient status. Accordingly, our data show that since the inception of the program, cardiopulmonary arrests have decreased from a mean of 4.1 per month to 2.3 per month (P = 0.03).
Many clinical trials of RRTs would not capture the success demonstrated in this case. For example, due to the low prevalence of events, the MERIT trial used a composite endpoint that included unplanned ICU transfers, cardiac arrests, and mortality. Because our patient still required an unplanned ICU transfer after being evaluated by the responding team, she would have been counted as a system failure.
Conclusion
While local needs should inform the type of RRS implemented, this case illustrates one of the major obstacles ubiquitous to RRS implementation: failure of system activation. With appropriate activation, an RRS‐CTP can meet RRS goals while maintaining continuity of care and maximizing existing resources. This case also illustrates the difficulty of achieving a statistically relevant outcome, while demonstrating the potential benefits of evolving RRSs.
- ,,,,.Rapid response teams—do they make a difference.Dimens Crit Care Nurs.2007;26(6):253–260.
- Institute for Healthcare Improvement. 5 Million Lives Campaign. Available at: http://www.ihi.org/IHI/Programs/Campaign/Campaign.htm?TabId=1IHI. Accessed February2009.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,,.Effects of rapid response systems on clinical outcomes: review and meta‐analyses.J Hosp Med.2007;2:422–432.
- ,,.Clinical triggers and rapid response escalation criteria.Patient Saf Qual Healthc.2007;4(2):12–13. Available at: http://www.psqh.com/archives.html. Accessed February 2009.
- ,,, et al.Use of medical emergency team responses to reduce hospital cardiopulmonary arrest.Qual Saf Health Care.2004;13:251–254.
- MERIT Study Investigators.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,.Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER).Lancet.1999;353(9162):1386–1389.
- ,,,.Early predictors of mortality for hospitalized patients suffering cardiopulmonary arrest.Chest.1990;97(2):413–419.
- ,,,,,.The objective medical emergency team activation criteria: a case–control study.Resuscitation.2007;73:62–72.
Many in‐hospital cardiac arrests and other adverse events are heralded by warning signs that are evident in the preceding 6 to 8 hours.1 By promptly intervening before further deterioration occurs, rapid response teams (RRTs) are designed to decrease unexpected intensive care unit (ICU) transfers, cardiac arrests, and inpatient mortality. While implementing RRTs is 1 of the 6 initiatives recommended by the Institute for Healthcare Improvement,2 data supporting their effectiveness is equivocal.3, 4
In October 2006, at Denver Health Medical Center, an academic, safety net hospital, we initiated a rapid response systemclinical triggers program (RRS‐CTP).5 In our RRS‐CTP, an abrupt change in patient status (Figure 1) triggers a mandatory call by the patient's nurse to the primary team, which is then required to perform an immediate bedside evaluation. By incorporating the primary team into the RRT‐CTP, we sought to preserve as much continuity of care as possible. Also, since the same house staff compose our cardiopulmonary arrest or cor team, and staff the ICUs and non‐ICU hospital wards, we did not feel that creating a separate RRT was an efficient use of resources. Our nurses have undergone extensive education about the necessity of a prompt bedside evaluation and have been instructed and empowered to escalate concerns to senior physicians if needed. We present a case that illustrates challenges to both implementing an RRS and measuring its potential benefits.

Case
A 59‐year‐old woman with a history of bipolar mood disorder was admitted for altered mental status. At presentation, she had signs of acute mania with normal vital signs. After initial laboratory workup, her altered mental status was felt to be multifactorial due to urinary tract infection, hypernatremia (attributed to lithium‐induced nephrogenic diabetes insipidus), and acute mania (attributed to medication discontinuation). Because she was slow to recover from the acute mania, her hospital stay was prolonged. From admission, the patient was treated with heparin 5000 units subcutaneously twice daily for venous thromboembolism prophylaxis.
On hospital day 7, at 21:32, the patient was noted to have asymptomatic tachycardia at 149 beats per minute and a new oxygen requirement of 3 L/minute. The cross‐cover team was called; however, although criteria were met, the RRS‐CTP was not activated and a bedside evaluation was not performed. A chest X‐ray was found to be normal and, with the exception of the oxygen requirement, her vital signs normalized by 23:45. No further diagnostic testing was performed at the time.
The next morning, at 11:58, the patient was found to have a blood pressure of 60/40 mmHg and heart rate of 42 beats per minute. The RRS‐CTP was activated. The primary team arrived at the bedside at 12:00 and found the patient to be alert, oriented, and without complaints. Her respiratory rate was 30/minute, and her oxygen saturation was 86% on 3 L/minute. An arterial blood gas analysis demonstrated acute respiratory alkalosis with hypoxemia and an electrocardiogram showed sinus tachycardia with a new S1Q3T3 pattern. A computed tomography angiogram revealed a large, nearly occlusive pulmonary embolus (PE) filling an enlarged right pulmonary artery, as well as thrombus within the left main pulmonary artery. She was transferred to the medical ICU and alteplase was administered. The patient survived and was discharged in good clinical condition.
Discussion
Despite the strong theoretical benefit of the RRT concept, a recent review by Ranji et al.4 concluded that RRTs had not yet been shown to improve patient outcomes. In contrast to dedicated RRTs, this case illustrates a different type of RRS that was designed to address abrupt changes in patient status, while maintaining continuity of care and efficiently utilizing resources.
If one considers an RRS to have both afferent (criteria recognition) and efferent (RRT or primary team response) limbs, the afferent limb must be consistently activated in order to obtain the efferent limb's response.6 The greatest opportunities to improve RRSs are thought to lie in the afferent limb.3 Our RRS‐CTP was not triggered in 1 of 2 instances in which criteria for mandatory initiation of the system were met. This is consistent with the findings of the Medical Early Response Intervention and Therapy (MERIT) trial, in which RRTs were called in only 41% of the patients meeting criteria and subsequently having adverse events,7 and with the ongoing monitoring of the use of the system at our hospital. Had the cross‐covering team seen the patient at the bedside initially, the PE might have been diagnosed while the patient was hemodynamically stable, giving the patient nearly a 3‐fold lower relative mortality.8 When the RRS‐CTP was activated, a prompt bedside evaluation occurred, allowing for lytic therapy to be administered before cardiopulmonary arrest (attendant mortality of 90%).9
While rapid response criteria were originally based upon published sensitivity analyses, more recent studies suggest that these criteria lack diagnostic accuracy. As demonstrated by Cretikos et al,10 to reach a sensitivity of 70%, the corresponding specificity would be only 86%. Given that the prevalence of adverse events in the MERIT trial was only 0.6%, the resulting positive predictive value (PPV) of rapid response call criteria is 3%. Accordingly, 33 calls would be needed to prevent 1 unplanned ICU transfer, cardiac arrest, or death. Nurses' attempts to minimize false‐positive calls may help explain the low call rates for patients meeting RRT criteria. The 2 avenues to increase the PPV of criteria are:
-
Increase the prevalence of disease in the population screened by risk factor stratification.
-
Increase the specificity of the call criteria, which has been limited by the associated decrease in sensitivity.10
Regarding the efferent response limb of RRS, our case demonstrates that the primary team (rather than a separate group of caregivers), when alerted appropriately, can effectively respond to critical changes in patient status. Accordingly, our data show that since the inception of the program, cardiopulmonary arrests have decreased from a mean of 4.1 per month to 2.3 per month (P = 0.03).
Many clinical trials of RRTs would not capture the success demonstrated in this case. For example, due to the low prevalence of events, the MERIT trial used a composite endpoint that included unplanned ICU transfers, cardiac arrests, and mortality. Because our patient still required an unplanned ICU transfer after being evaluated by the responding team, she would have been counted as a system failure.
Conclusion
While local needs should inform the type of RRS implemented, this case illustrates one of the major obstacles ubiquitous to RRS implementation: failure of system activation. With appropriate activation, an RRS‐CTP can meet RRS goals while maintaining continuity of care and maximizing existing resources. This case also illustrates the difficulty of achieving a statistically relevant outcome, while demonstrating the potential benefits of evolving RRSs.
Many in‐hospital cardiac arrests and other adverse events are heralded by warning signs that are evident in the preceding 6 to 8 hours.1 By promptly intervening before further deterioration occurs, rapid response teams (RRTs) are designed to decrease unexpected intensive care unit (ICU) transfers, cardiac arrests, and inpatient mortality. While implementing RRTs is 1 of the 6 initiatives recommended by the Institute for Healthcare Improvement,2 data supporting their effectiveness is equivocal.3, 4
In October 2006, at Denver Health Medical Center, an academic, safety net hospital, we initiated a rapid response systemclinical triggers program (RRS‐CTP).5 In our RRS‐CTP, an abrupt change in patient status (Figure 1) triggers a mandatory call by the patient's nurse to the primary team, which is then required to perform an immediate bedside evaluation. By incorporating the primary team into the RRT‐CTP, we sought to preserve as much continuity of care as possible. Also, since the same house staff compose our cardiopulmonary arrest or cor team, and staff the ICUs and non‐ICU hospital wards, we did not feel that creating a separate RRT was an efficient use of resources. Our nurses have undergone extensive education about the necessity of a prompt bedside evaluation and have been instructed and empowered to escalate concerns to senior physicians if needed. We present a case that illustrates challenges to both implementing an RRS and measuring its potential benefits.

Case
A 59‐year‐old woman with a history of bipolar mood disorder was admitted for altered mental status. At presentation, she had signs of acute mania with normal vital signs. After initial laboratory workup, her altered mental status was felt to be multifactorial due to urinary tract infection, hypernatremia (attributed to lithium‐induced nephrogenic diabetes insipidus), and acute mania (attributed to medication discontinuation). Because she was slow to recover from the acute mania, her hospital stay was prolonged. From admission, the patient was treated with heparin 5000 units subcutaneously twice daily for venous thromboembolism prophylaxis.
On hospital day 7, at 21:32, the patient was noted to have asymptomatic tachycardia at 149 beats per minute and a new oxygen requirement of 3 L/minute. The cross‐cover team was called; however, although criteria were met, the RRS‐CTP was not activated and a bedside evaluation was not performed. A chest X‐ray was found to be normal and, with the exception of the oxygen requirement, her vital signs normalized by 23:45. No further diagnostic testing was performed at the time.
The next morning, at 11:58, the patient was found to have a blood pressure of 60/40 mmHg and heart rate of 42 beats per minute. The RRS‐CTP was activated. The primary team arrived at the bedside at 12:00 and found the patient to be alert, oriented, and without complaints. Her respiratory rate was 30/minute, and her oxygen saturation was 86% on 3 L/minute. An arterial blood gas analysis demonstrated acute respiratory alkalosis with hypoxemia and an electrocardiogram showed sinus tachycardia with a new S1Q3T3 pattern. A computed tomography angiogram revealed a large, nearly occlusive pulmonary embolus (PE) filling an enlarged right pulmonary artery, as well as thrombus within the left main pulmonary artery. She was transferred to the medical ICU and alteplase was administered. The patient survived and was discharged in good clinical condition.
Discussion
Despite the strong theoretical benefit of the RRT concept, a recent review by Ranji et al.4 concluded that RRTs had not yet been shown to improve patient outcomes. In contrast to dedicated RRTs, this case illustrates a different type of RRS that was designed to address abrupt changes in patient status, while maintaining continuity of care and efficiently utilizing resources.
If one considers an RRS to have both afferent (criteria recognition) and efferent (RRT or primary team response) limbs, the afferent limb must be consistently activated in order to obtain the efferent limb's response.6 The greatest opportunities to improve RRSs are thought to lie in the afferent limb.3 Our RRS‐CTP was not triggered in 1 of 2 instances in which criteria for mandatory initiation of the system were met. This is consistent with the findings of the Medical Early Response Intervention and Therapy (MERIT) trial, in which RRTs were called in only 41% of the patients meeting criteria and subsequently having adverse events,7 and with the ongoing monitoring of the use of the system at our hospital. Had the cross‐covering team seen the patient at the bedside initially, the PE might have been diagnosed while the patient was hemodynamically stable, giving the patient nearly a 3‐fold lower relative mortality.8 When the RRS‐CTP was activated, a prompt bedside evaluation occurred, allowing for lytic therapy to be administered before cardiopulmonary arrest (attendant mortality of 90%).9
While rapid response criteria were originally based upon published sensitivity analyses, more recent studies suggest that these criteria lack diagnostic accuracy. As demonstrated by Cretikos et al,10 to reach a sensitivity of 70%, the corresponding specificity would be only 86%. Given that the prevalence of adverse events in the MERIT trial was only 0.6%, the resulting positive predictive value (PPV) of rapid response call criteria is 3%. Accordingly, 33 calls would be needed to prevent 1 unplanned ICU transfer, cardiac arrest, or death. Nurses' attempts to minimize false‐positive calls may help explain the low call rates for patients meeting RRT criteria. The 2 avenues to increase the PPV of criteria are:
-
Increase the prevalence of disease in the population screened by risk factor stratification.
-
Increase the specificity of the call criteria, which has been limited by the associated decrease in sensitivity.10
Regarding the efferent response limb of RRS, our case demonstrates that the primary team (rather than a separate group of caregivers), when alerted appropriately, can effectively respond to critical changes in patient status. Accordingly, our data show that since the inception of the program, cardiopulmonary arrests have decreased from a mean of 4.1 per month to 2.3 per month (P = 0.03).
Many clinical trials of RRTs would not capture the success demonstrated in this case. For example, due to the low prevalence of events, the MERIT trial used a composite endpoint that included unplanned ICU transfers, cardiac arrests, and mortality. Because our patient still required an unplanned ICU transfer after being evaluated by the responding team, she would have been counted as a system failure.
Conclusion
While local needs should inform the type of RRS implemented, this case illustrates one of the major obstacles ubiquitous to RRS implementation: failure of system activation. With appropriate activation, an RRS‐CTP can meet RRS goals while maintaining continuity of care and maximizing existing resources. This case also illustrates the difficulty of achieving a statistically relevant outcome, while demonstrating the potential benefits of evolving RRSs.
- ,,,,.Rapid response teams—do they make a difference.Dimens Crit Care Nurs.2007;26(6):253–260.
- Institute for Healthcare Improvement. 5 Million Lives Campaign. Available at: http://www.ihi.org/IHI/Programs/Campaign/Campaign.htm?TabId=1IHI. Accessed February2009.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,,.Effects of rapid response systems on clinical outcomes: review and meta‐analyses.J Hosp Med.2007;2:422–432.
- ,,.Clinical triggers and rapid response escalation criteria.Patient Saf Qual Healthc.2007;4(2):12–13. Available at: http://www.psqh.com/archives.html. Accessed February 2009.
- ,,, et al.Use of medical emergency team responses to reduce hospital cardiopulmonary arrest.Qual Saf Health Care.2004;13:251–254.
- MERIT Study Investigators.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,.Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER).Lancet.1999;353(9162):1386–1389.
- ,,,.Early predictors of mortality for hospitalized patients suffering cardiopulmonary arrest.Chest.1990;97(2):413–419.
- ,,,,,.The objective medical emergency team activation criteria: a case–control study.Resuscitation.2007;73:62–72.
- ,,,,.Rapid response teams—do they make a difference.Dimens Crit Care Nurs.2007;26(6):253–260.
- Institute for Healthcare Improvement. 5 Million Lives Campaign. Available at: http://www.ihi.org/IHI/Programs/Campaign/Campaign.htm?TabId=1IHI. Accessed February2009.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,,.Effects of rapid response systems on clinical outcomes: review and meta‐analyses.J Hosp Med.2007;2:422–432.
- ,,.Clinical triggers and rapid response escalation criteria.Patient Saf Qual Healthc.2007;4(2):12–13. Available at: http://www.psqh.com/archives.html. Accessed February 2009.
- ,,, et al.Use of medical emergency team responses to reduce hospital cardiopulmonary arrest.Qual Saf Health Care.2004;13:251–254.
- MERIT Study Investigators.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,.Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER).Lancet.1999;353(9162):1386–1389.
- ,,,.Early predictors of mortality for hospitalized patients suffering cardiopulmonary arrest.Chest.1990;97(2):413–419.
- ,,,,,.The objective medical emergency team activation criteria: a case–control study.Resuscitation.2007;73:62–72.