User login
Metformin associated with acidosis only in patients with eGFR 30 mL/min per 1.73 m 2
Clinical question: Does metformin increase the risk of lactic acidosis in chronic kidney disease (CKD)?
Background: Metformin is first-line therapy for type 2 diabetes mellitus (DM) because of its low cost, safety, and potential cardiovascular benefit, but fear of lactic acidosis has limited its use in CKD. The risk of acidosis in CKD patients with varying levels of renal function has not been clearly defined.
Study design: Retrospective community-based cohort study.
Setting: Geisinger Health System in Pennsylvania.
Synopsis: A total of 75,413 patients were identified with diagnostic codes or medication prescriptions indicating DM. Forty-five percent of patients were taking metformin at enrollment, increasing by 18% over the 5.7 years of median follow-up. The primary outcome was inpatient acidosis, defined by an ICD-9-CM code capturing multiple forms of acidosis but excluding diabetic ketoacidosis.
When metformin users and nonusers were compared, risk of acidosis was similar for the entire cohort and for subgroups of patients with an estimated glomerular filtration rate (eGFR) greater than 90, 60-89, 45-59, and 30-44. Conversely, metformin use was associated with a higher risk of acidosis in patients with eGFR less than 30 (adjusted hazard ratio, 2.07; 95% confidence interval, 1.33-3.22). Metformin not increasing the risk of acidosis at eGFR greater than 30 also was noted in an additional analysis using sulfonylurea medications as an active comparator and was replicated in a separate database with 82,000 patients from 350 private health systems. As with all observational studies, this study is limited by the potential for residual confounding.
Bottom line: Metformin appears to be safe in CKD patients with eGFR above 30 mL/min per 1.73 m2.
Citation: Lazarus B et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: A community- based cohort study. JAMA Int Med. 2018;178(7):903-10.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Clinical question: Does metformin increase the risk of lactic acidosis in chronic kidney disease (CKD)?
Background: Metformin is first-line therapy for type 2 diabetes mellitus (DM) because of its low cost, safety, and potential cardiovascular benefit, but fear of lactic acidosis has limited its use in CKD. The risk of acidosis in CKD patients with varying levels of renal function has not been clearly defined.
Study design: Retrospective community-based cohort study.
Setting: Geisinger Health System in Pennsylvania.
Synopsis: A total of 75,413 patients were identified with diagnostic codes or medication prescriptions indicating DM. Forty-five percent of patients were taking metformin at enrollment, increasing by 18% over the 5.7 years of median follow-up. The primary outcome was inpatient acidosis, defined by an ICD-9-CM code capturing multiple forms of acidosis but excluding diabetic ketoacidosis.
When metformin users and nonusers were compared, risk of acidosis was similar for the entire cohort and for subgroups of patients with an estimated glomerular filtration rate (eGFR) greater than 90, 60-89, 45-59, and 30-44. Conversely, metformin use was associated with a higher risk of acidosis in patients with eGFR less than 30 (adjusted hazard ratio, 2.07; 95% confidence interval, 1.33-3.22). Metformin not increasing the risk of acidosis at eGFR greater than 30 also was noted in an additional analysis using sulfonylurea medications as an active comparator and was replicated in a separate database with 82,000 patients from 350 private health systems. As with all observational studies, this study is limited by the potential for residual confounding.
Bottom line: Metformin appears to be safe in CKD patients with eGFR above 30 mL/min per 1.73 m2.
Citation: Lazarus B et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: A community- based cohort study. JAMA Int Med. 2018;178(7):903-10.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Clinical question: Does metformin increase the risk of lactic acidosis in chronic kidney disease (CKD)?
Background: Metformin is first-line therapy for type 2 diabetes mellitus (DM) because of its low cost, safety, and potential cardiovascular benefit, but fear of lactic acidosis has limited its use in CKD. The risk of acidosis in CKD patients with varying levels of renal function has not been clearly defined.
Study design: Retrospective community-based cohort study.
Setting: Geisinger Health System in Pennsylvania.
Synopsis: A total of 75,413 patients were identified with diagnostic codes or medication prescriptions indicating DM. Forty-five percent of patients were taking metformin at enrollment, increasing by 18% over the 5.7 years of median follow-up. The primary outcome was inpatient acidosis, defined by an ICD-9-CM code capturing multiple forms of acidosis but excluding diabetic ketoacidosis.
When metformin users and nonusers were compared, risk of acidosis was similar for the entire cohort and for subgroups of patients with an estimated glomerular filtration rate (eGFR) greater than 90, 60-89, 45-59, and 30-44. Conversely, metformin use was associated with a higher risk of acidosis in patients with eGFR less than 30 (adjusted hazard ratio, 2.07; 95% confidence interval, 1.33-3.22). Metformin not increasing the risk of acidosis at eGFR greater than 30 also was noted in an additional analysis using sulfonylurea medications as an active comparator and was replicated in a separate database with 82,000 patients from 350 private health systems. As with all observational studies, this study is limited by the potential for residual confounding.
Bottom line: Metformin appears to be safe in CKD patients with eGFR above 30 mL/min per 1.73 m2.
Citation: Lazarus B et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: A community- based cohort study. JAMA Int Med. 2018;178(7):903-10.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Prioritize oral route for inpatient opioids with subcutaneous route as alternative
Clinical question: Can adoption of a local opioid standard of practice for hospitalized patients reduce intravenous and overall opioid exposure while providing effective pain control?
Background: Inpatient use of intravenous opioids may be excessive, considering that oral opioids may provide more consistent pain control with less risk of adverse effects. If oral treatment is not possible, subcutaneous administration of opioids is an effective and possibly less addictive alternative to the intravenous route.
Study design: Historical control pilot study.
Setting: Single adult general medicine unit in an urban academic medical center.
Synopsis: A 6-month historical period with 287 patients was compared with a 3-month intervention period with 127 patients. The intervention consisted of a clinical practice standard that was presented to medical and nursing staff via didactic sessions and email. The standard recommended the oral route for opioids in patients tolerating oral intake and endorsed subcutaneous over intravenous administration.
Intravenous doses decreased by 84% (0.06 vs. 0.39 doses/patient-day; P less than .001), the daily rate of patients receiving any parenteral opioid decreased by 57% (6% vs. 14%; P less than .001), and the mean daily overall morphine-milligram equivalents decreased by 31% (6.30 vs. 9.11). Pain scores were unchanged for hospital days 1 through 3 but were significantly improved on day 4 (P = .004) and day 5 (P = .009).
Limitations of this study include the small number of patients on one unit, in one institution, with one clinician group. Attractive features of the intervention include its scalability and potential for augmentation via additional processes such as EHR changes, prescribing restrictions, and pharmacy monitoring.
Bottom line: A standard of practice intervention with peer-to-peer education was associated with decreased intravenous opioid exposure, decreased total opioid exposure, and effective pain control.
Citation: Ackerman AL et al. Association of an opioid standard of practice intervention with intravenous opioid exposure in hospitalized patients. JAMA Int Med. 2018;178(6):759-63.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Clinical question: Can adoption of a local opioid standard of practice for hospitalized patients reduce intravenous and overall opioid exposure while providing effective pain control?
Background: Inpatient use of intravenous opioids may be excessive, considering that oral opioids may provide more consistent pain control with less risk of adverse effects. If oral treatment is not possible, subcutaneous administration of opioids is an effective and possibly less addictive alternative to the intravenous route.
Study design: Historical control pilot study.
Setting: Single adult general medicine unit in an urban academic medical center.
Synopsis: A 6-month historical period with 287 patients was compared with a 3-month intervention period with 127 patients. The intervention consisted of a clinical practice standard that was presented to medical and nursing staff via didactic sessions and email. The standard recommended the oral route for opioids in patients tolerating oral intake and endorsed subcutaneous over intravenous administration.
Intravenous doses decreased by 84% (0.06 vs. 0.39 doses/patient-day; P less than .001), the daily rate of patients receiving any parenteral opioid decreased by 57% (6% vs. 14%; P less than .001), and the mean daily overall morphine-milligram equivalents decreased by 31% (6.30 vs. 9.11). Pain scores were unchanged for hospital days 1 through 3 but were significantly improved on day 4 (P = .004) and day 5 (P = .009).
Limitations of this study include the small number of patients on one unit, in one institution, with one clinician group. Attractive features of the intervention include its scalability and potential for augmentation via additional processes such as EHR changes, prescribing restrictions, and pharmacy monitoring.
Bottom line: A standard of practice intervention with peer-to-peer education was associated with decreased intravenous opioid exposure, decreased total opioid exposure, and effective pain control.
Citation: Ackerman AL et al. Association of an opioid standard of practice intervention with intravenous opioid exposure in hospitalized patients. JAMA Int Med. 2018;178(6):759-63.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Clinical question: Can adoption of a local opioid standard of practice for hospitalized patients reduce intravenous and overall opioid exposure while providing effective pain control?
Background: Inpatient use of intravenous opioids may be excessive, considering that oral opioids may provide more consistent pain control with less risk of adverse effects. If oral treatment is not possible, subcutaneous administration of opioids is an effective and possibly less addictive alternative to the intravenous route.
Study design: Historical control pilot study.
Setting: Single adult general medicine unit in an urban academic medical center.
Synopsis: A 6-month historical period with 287 patients was compared with a 3-month intervention period with 127 patients. The intervention consisted of a clinical practice standard that was presented to medical and nursing staff via didactic sessions and email. The standard recommended the oral route for opioids in patients tolerating oral intake and endorsed subcutaneous over intravenous administration.
Intravenous doses decreased by 84% (0.06 vs. 0.39 doses/patient-day; P less than .001), the daily rate of patients receiving any parenteral opioid decreased by 57% (6% vs. 14%; P less than .001), and the mean daily overall morphine-milligram equivalents decreased by 31% (6.30 vs. 9.11). Pain scores were unchanged for hospital days 1 through 3 but were significantly improved on day 4 (P = .004) and day 5 (P = .009).
Limitations of this study include the small number of patients on one unit, in one institution, with one clinician group. Attractive features of the intervention include its scalability and potential for augmentation via additional processes such as EHR changes, prescribing restrictions, and pharmacy monitoring.
Bottom line: A standard of practice intervention with peer-to-peer education was associated with decreased intravenous opioid exposure, decreased total opioid exposure, and effective pain control.
Citation: Ackerman AL et al. Association of an opioid standard of practice intervention with intravenous opioid exposure in hospitalized patients. JAMA Int Med. 2018;178(6):759-63.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Decrease in Inpatient Telemetry Utilization Through a System-Wide Electronic Health Record Change and a Multifaceted Hospitalist Intervention
Wasteful care may account for between 21% and 34% of the United States’ $3.2 trillion in annual healthcare expenditures, making it a prime target for cost-saving initiatives.1,2 Telemetry is a target for value improvement strategies because telemetry is overutilized, rarely leads to a change in management, and has associated guidelines on appropriate use.3-10 Telemetry use has been a focus of the Joint Commission’s National Patient Safety Goals since 2014, and it is also a focus of the Society of Hospital Medicine’s Choosing Wisely® campaign.11-13
Previous initiatives have evaluated how changes to telemetry orders or education and feedback affect telemetry use. Few studies have compared a system-wide electronic health record (EHR) approach to a multifaceted intervention. In seeking to address this gap, we adapted published guidelines from the American Heart Association (AHA) and incorporated them into our EHR ordering process.3 Simultaneously, we implemented a multifaceted quality improvement initiative and compared this combined program’s effectiveness to that of the EHR approach alone.
METHODS
Study Design, Setting, and Population
We performed a 2-group observational pre- to postintervention study at University of Utah Health. Hospital encounters of patients 18 years and older who had at least 1 inpatient acute care, nonintensive care unit (ICU) room charge and an admission date between January 1, 2014, and July 31, 2016, were included. Patient encounters with missing encounter-level covariates, such as case mix index (CMI) or attending provider identification, were excluded. The Institutional Review Board classified this project as quality improvement and did not require review and oversight.
Intervention
On July 6, 2015, our Epic (Epic Systems Corporation, Madison, WI) EHR telemetry order was modified to discourage unnecessary telemetry monitoring. The new order required providers ordering telemetry to choose a clinical indication and select a duration for monitoring, after which the order would expire and require physician renewal or discontinuation. These were the only changes that occurred for nonhospitalist providers. The nonhospitalist group included all admitting providers who were not hospitalists. This group included neurology (6.98%); cardiology (8.13%); other medical specialties such as pulmonology, hematology, and oncology (21.30%); cardiothoracic surgery (3.72%); orthopedic surgery (14.84%); general surgery (11.11%); neurosurgery (11.07%); and other surgical specialties, including urology, transplant, vascular surgery, and plastics (16.68%).
Between January 2015 and June 2015, we implemented a multicomponent program among our hospitalist service. The hospitalist service is composed of 4 teams with internal medicine residents and 2 teams with advanced practice providers, all staffed by academic hospitalists. Our program was composed of 5 elements, all of which were made before the hospital-wide changes to electronic telemetry orders and maintained throughout the study period, as follows: (1) a single provider education session reviewing available evidence (eg, AHA guidelines, Choosing Wisely® campaign), (2) removal of the telemetry order from hospitalist admission order set on March 23, 2015, (3) inclusion of telemetry discussion in the hospitalist group’s daily “Rounding Checklist,”14 (4) monthly feedback provided as part of hospitalist group meetings, and (5) a financial incentive, awarded to the division (no individual provider payment) if performance targets were met. See supplementary Appendix (“Implementation Manual”) for further details.
Data Source
We obtained data on patient age, gender, Medicare Severity-Diagnosis Related Group, Charlson comorbidity index (CCI), CMI, admitting unit, attending physician, admission and discharge dates, length of stay (LOS), 30-day readmission, bed charge (telemetry or nontelemetry), ICU stay, and inpatient mortality from the enterprise data warehouse. Telemetry days were determined through room billing charges, which are assigned based on the presence or absence of an active telemetry order at midnight. Code events came from a log kept by the hospital telephone operator, who is responsible for sending out all calls to the code team. Code event data were available starting July 19, 2014.
Measures
Our primary outcome was the percentage of hospital days that had telemetry charges for individual patients. All billed telemetry days on acute care floors were included regardless of admission status (inpatient vs observation), service, indication, or ordering provider. Secondary outcomes were inpatient mortality, escalation of care, code event rates, and appropriate telemetry utilization rates. Escalation of care was defined as transfer to an ICU after initially being admitted to an acute care floor. The code event rate was defined as the ratio of the number of code team activations to the number of patient days. Appropriate telemetry utilization rates were determined via chart review, as detailed below.
In order to evaluate changes in appropriateness of telemetry monitoring, 4 of the authors who are internal medicine physicians (KE, CC, JC, DG) performed chart reviews of 25 randomly selected patients in each group (hospitalist and nonhospitalist) before and after the intervention who received at least 1 day of telemetry monitoring. Each reviewer was provided a key based on AHA guidelines for monitoring indications and associated maximum allowable durations.3 Chart reviews were performed to determine the indication (if any) for monitoring, as well as the number of days that were indicated. The number of indicated days was compared to the number of telemetry days the patient received to determine the overall proportion of days that were indicated (“Telemetry appropriateness per visit”). Three reviewers (KE, AR, CC) also evaluated 100 patients on the hospitalist service after the intervention who did not receive any telemetry monitoring to evaluate whether patients with indications for telemetry monitoring were not receiving it after the intervention. For patients who had a possible indication, the indication was classified as Class I (“Cardiac monitoring is indicated in most, if not all, patients in this group”) or Class II (“Cardiac monitoring may be of benefit in some patients but is not considered essential for all patients”).3
Adjustment Variables
To account for differences in patient characteristics between hospitalist and nonhospitalist groups, we included age, gender, CMI, and CCI in statistical models. CCI was calculated according to the algorithm specified by Quan et al.15 using all patient diagnoses from previous visits and the index visit identified from the facility billing system.
Statistical Analysis
We computed descriptive statistics for study outcomes and visit characteristics for hospitalist and nonhospitalist visits for pre- and postintervention periods. Descriptive statistics were expressed as n (%) for categorical patient characteristics and outcome variables. For continuous patient characteristics, we expressed the variability of individual observations as the mean ± the standard deviation. For continuous outcomes, we expressed the precision of the mean estimates using standard error. Telemetry utilization per visit was weighted by the number of total acute care days per visit. Telemetry appropriateness per visit was weighted by the number of telemetry days per visit. Patients who did not receive any telemetry monitoring were included in the analysis and noted to have 0 telemetry days. All patients had at least 1 acute care day. Categorical variables were compared using χ2 tests, and continuous variables were compared using t tests. Code event rates were compared using the binomial probability mid-p exact test for person-time data.16
We fitted generalized linear regression models using generalized estimating equations to evaluate the relative change in outcomes of interest in the postintervention period compared with the preintervention period after adjusting for study covariates. The models included study group (hospitalist and nonhospitalist), time period (pre- and postintervention), an interaction term between study group and time period, and study covariates (age, gender, CMI, and CCI). The models were defined using a binomial distributional assumption and logit link function for mortality, escalation of care, and whether patients had at least 1 telemetry day. A gamma distributional assumption and log link function were used for LOS, telemetry acute care days per visit, and total acute care days per visit. A negative binomial distributional assumption and log link function were used for telemetry utilization and telemetry appropriateness. We used the log of the acute care days as an offset for telemetry utilization and the log of the telemetry days per visit as an offset for telemetry appropriateness. An exchangeable working correlation matrix was used to account for physician-level clustering for all outcomes. Intervention effects, representing the difference in odds for categorical variables and in amount for continuous variables, were calculated as exponentiation of the beta parameters for the covariate minus 1.
P values <.05 were considered significant. We used SAS version 9.4 statistical software (SAS Institute Inc., Cary, NC) for data analysis.
RESULTS
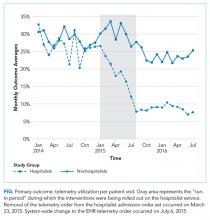
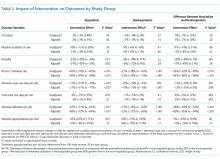
The percent of patients who had any telemetry charges decreased from 36.2% to 15.9% (P < .001) in the hospitalist group and from 31.8% to 28.0% in the nonhospitalist group (P < .001; Table 1). Rates of code events did not change over time (P = .9).
In the randomly selected sample of patients pre- and postintervention who received telemetry monitoring, there was an increase in telemetry appropriateness on the hospitalist service (46% to 72%, P = .025; Table 1). In the nonhospitalist group, appropriate telemetry utilization did not change significantly. Of the 100 randomly selected patients in the hospitalist group after the intervention who did not receive telemetry, no patient had an AHA Class I indication, and only 4 patients had a Class II indication.3,17
DISCUSSION
In this study, implementing a change in the EHR telemetry order produced reductions in telemetry days. However, when combined with a multicomponent program including education, audit and feedback, financial incentives, and changes to remove telemetry orders from admission orders sets, an even more marked improvement was seen. Neither intervention reduced LOS, increased code event rates, or increased rates of escalation of care.
Prior studies have evaluated interventions to reduce unnecessary telemetry monitoring with varying degrees of success. The most successful EHR intervention to date, from Dressler et al.,18 achieved a 70% reduction in overall telemetry use by integrating the AHA guidelines into their EHR and incorporating nursing discontinuation guidelines to ensure that telemetry discontinuation was both safe and timely. Other studies using stewardship approaches and standardized protocols have been less successful.19,20 One study utilizing a multidisciplinary approach but not including an EHR component showed modest improvements in telemetry.21
Although we are unable to differentiate the exact effect of each component of the intervention, we did note an immediate decrease in telemetry orders after removing the telemetry order from our admission order set, a trend that was magnified after the addition of broader EHR changes (Figure 1). Important additional contributors to our success seem to have been the standardization of rounds to include daily discussion of telemetry and the provision of routine feedback. We cannot discern whether other components of our program (such as the financial incentives) contributed more or less to our program, though the sum of these interventions produced an overall program that required substantial buy in and sustained focus from the hospitalist group. The importance of the hospitalist program is highlighted by the relatively large differences in improvement compared with the nonhospitalist group.
Our study has several limitations. First, the study was conducted at a single center, which may limit its generalizability. Second, the intervention was multifaceted, diminishing our ability to discern which aspects beyond the system-wide change in the telemetry order were most responsible for the observed effect among hospitalists. Third, we are unable to fully account for baseline differences in telemetry utilization between hospitalist and nonhospitalist groups. It is likely that different services utilize telemetry monitoring in different ways, and the hospitalist group may have been more aware of the existing guidelines for monitoring prior to the intervention. Furthermore, we had a limited sample size for the chart audits, which reduced the available statistical power for determining changes in the appropriateness of telemetry utilization. Additionally, because internal medicine residents rotate through various services, it is possible that the education they received on their hospitalist rotation as part of our intervention had a spillover effect in the nonhospitalist group. However, any effect should have decreased the difference between the groups. Lastly, although our postintervention time period was 1 year, we do not have data beyond that to monitor for sustainability of the results.
CONCLUSION
In this single-site study, combining EHR orders prompting physicians to choose a clinical indication and duration for monitoring with a broader program—including upstream changes in ordering as well as education, audit, and feedback—produced reductions in telemetry usage. Whether this reduction improves the appropriateness of telemetry utilization or reduces other effects of telemetry (eg, alert fatigue, calls for benign arrhythmias) cannot be discerned from our study. However, our results support the idea that multipronged approaches to telemetry use are most likely to produce improvements.
Acknowledgments
The authors thank Dr. Frank Thomas for his assistance with process engineering and Mr. Andrew Wood for his routine provision of data. The statistical analysis was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067-05 (formerly 8UL1TR000105 and UL1RR025764).
Disclosure
The authors have no conflicts of interest to report.
1. National Health Expenditure Fact Sheet. 2015; https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html. Accessed June 27, 2017.
2. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516. PubMed
3. Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721-2746. PubMed
4. Sandau KE, Funk M, Auerbach A, et al. Update to Practice Standards for Electrocardiographic Monitoring in Hospital Settings: A Scientific Statement From the American Heart Association. Circulation. 2017;136(19):e273-e344. PubMed
5. Mohammad R, Shah S, Donath E, et al. Non-critical care telemetry and in-hospital cardiac arrest outcomes. J Electrocardiol. 2015;48(3):426-429. PubMed
6. Dhillon SK, Rachko M, Hanon S, Schweitzer P, Bergmann SR. Telemetry monitoring guidelines for efficient and safe delivery of cardiac rhythm monitoring to noncritical hospital inpatients. Crit Pathw Cardiol. 2009;8(3):125-126. PubMed
7. Estrada CA, Rosman HS, Prasad NK, et al. Evaluation of guidelines for the use of telemetry in the non-intensive-care setting. J Gen Intern Med. 2000;15(1):51-55. PubMed
8. Estrada CA, Prasad NK, Rosman HS, Young MJ. Outcomes of patients hospitalized to a telemetry unit. Am J Cardiol. 1994;74(4):357-362. PubMed
9. Atzema C, Schull MJ, Borgundvaag B, Slaughter GR, Lee CK. ALARMED: adverse events in low-risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med. 2006;24(1):62-67. PubMed
10. Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med. 2000;7(6):647-652. PubMed
11. The Joint Commission 2017 National Patient Safety Goals https://www.jointcommission.org/hap_2017_npsgs/. Accessed on February 15, 2017.
12. Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission announces 2014 National Patient Safety Goal. Jt Comm Perspect. 2013;33(7):1, 3-4. PubMed
13. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
14. Yarbrough PM, Kukhareva PV, Horton D, Edholm K, Kawamoto K. Multifaceted intervention including education, rounding checklist implementation, cost feedback, and financial incentives reduces inpatient laboratory costs. J Hosp Med. 2016;11(5):348-354. PubMed
15. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682. PubMed
16. Greenland S, Rothman KJ. Introduction to categorical statistics In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology. Vol 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2008: 238-257.
17. Henriques-Forsythe MN, Ivonye CC, Jamched U, Kamuguisha LK, Olejeme KA, Onwuanyi AE. Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med. 2009;76(6):368-372. PubMed
18. Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. PubMed
19. Boggan JC, Navar-Boggan AM, Patel V, Schulteis RD, Simel DL. Reductions in telemetry order duration do not reduce telemetry utilization. J Hosp Med. 2014;9(12):795-796. PubMed
20. Cantillon DJ, Loy M, Burkle A, et al. Association Between Off-site Central Monitoring Using Standardized Cardiac Telemetry and Clinical Outcomes Among Non-Critically Ill Patients. JAMA. 2016;316(5):519-524. PubMed
21. Svec D, Ahuja N, Evans KH, et al. Hospitalist intervention for appropriate use of telemetry reduces length of stay and cost. J Hosp Med. 2015;10(9):627-632. PubMed
Wasteful care may account for between 21% and 34% of the United States’ $3.2 trillion in annual healthcare expenditures, making it a prime target for cost-saving initiatives.1,2 Telemetry is a target for value improvement strategies because telemetry is overutilized, rarely leads to a change in management, and has associated guidelines on appropriate use.3-10 Telemetry use has been a focus of the Joint Commission’s National Patient Safety Goals since 2014, and it is also a focus of the Society of Hospital Medicine’s Choosing Wisely® campaign.11-13
Previous initiatives have evaluated how changes to telemetry orders or education and feedback affect telemetry use. Few studies have compared a system-wide electronic health record (EHR) approach to a multifaceted intervention. In seeking to address this gap, we adapted published guidelines from the American Heart Association (AHA) and incorporated them into our EHR ordering process.3 Simultaneously, we implemented a multifaceted quality improvement initiative and compared this combined program’s effectiveness to that of the EHR approach alone.
METHODS
Study Design, Setting, and Population
We performed a 2-group observational pre- to postintervention study at University of Utah Health. Hospital encounters of patients 18 years and older who had at least 1 inpatient acute care, nonintensive care unit (ICU) room charge and an admission date between January 1, 2014, and July 31, 2016, were included. Patient encounters with missing encounter-level covariates, such as case mix index (CMI) or attending provider identification, were excluded. The Institutional Review Board classified this project as quality improvement and did not require review and oversight.
Intervention
On July 6, 2015, our Epic (Epic Systems Corporation, Madison, WI) EHR telemetry order was modified to discourage unnecessary telemetry monitoring. The new order required providers ordering telemetry to choose a clinical indication and select a duration for monitoring, after which the order would expire and require physician renewal or discontinuation. These were the only changes that occurred for nonhospitalist providers. The nonhospitalist group included all admitting providers who were not hospitalists. This group included neurology (6.98%); cardiology (8.13%); other medical specialties such as pulmonology, hematology, and oncology (21.30%); cardiothoracic surgery (3.72%); orthopedic surgery (14.84%); general surgery (11.11%); neurosurgery (11.07%); and other surgical specialties, including urology, transplant, vascular surgery, and plastics (16.68%).
Between January 2015 and June 2015, we implemented a multicomponent program among our hospitalist service. The hospitalist service is composed of 4 teams with internal medicine residents and 2 teams with advanced practice providers, all staffed by academic hospitalists. Our program was composed of 5 elements, all of which were made before the hospital-wide changes to electronic telemetry orders and maintained throughout the study period, as follows: (1) a single provider education session reviewing available evidence (eg, AHA guidelines, Choosing Wisely® campaign), (2) removal of the telemetry order from hospitalist admission order set on March 23, 2015, (3) inclusion of telemetry discussion in the hospitalist group’s daily “Rounding Checklist,”14 (4) monthly feedback provided as part of hospitalist group meetings, and (5) a financial incentive, awarded to the division (no individual provider payment) if performance targets were met. See supplementary Appendix (“Implementation Manual”) for further details.
Data Source
We obtained data on patient age, gender, Medicare Severity-Diagnosis Related Group, Charlson comorbidity index (CCI), CMI, admitting unit, attending physician, admission and discharge dates, length of stay (LOS), 30-day readmission, bed charge (telemetry or nontelemetry), ICU stay, and inpatient mortality from the enterprise data warehouse. Telemetry days were determined through room billing charges, which are assigned based on the presence or absence of an active telemetry order at midnight. Code events came from a log kept by the hospital telephone operator, who is responsible for sending out all calls to the code team. Code event data were available starting July 19, 2014.
Measures
Our primary outcome was the percentage of hospital days that had telemetry charges for individual patients. All billed telemetry days on acute care floors were included regardless of admission status (inpatient vs observation), service, indication, or ordering provider. Secondary outcomes were inpatient mortality, escalation of care, code event rates, and appropriate telemetry utilization rates. Escalation of care was defined as transfer to an ICU after initially being admitted to an acute care floor. The code event rate was defined as the ratio of the number of code team activations to the number of patient days. Appropriate telemetry utilization rates were determined via chart review, as detailed below.
In order to evaluate changes in appropriateness of telemetry monitoring, 4 of the authors who are internal medicine physicians (KE, CC, JC, DG) performed chart reviews of 25 randomly selected patients in each group (hospitalist and nonhospitalist) before and after the intervention who received at least 1 day of telemetry monitoring. Each reviewer was provided a key based on AHA guidelines for monitoring indications and associated maximum allowable durations.3 Chart reviews were performed to determine the indication (if any) for monitoring, as well as the number of days that were indicated. The number of indicated days was compared to the number of telemetry days the patient received to determine the overall proportion of days that were indicated (“Telemetry appropriateness per visit”). Three reviewers (KE, AR, CC) also evaluated 100 patients on the hospitalist service after the intervention who did not receive any telemetry monitoring to evaluate whether patients with indications for telemetry monitoring were not receiving it after the intervention. For patients who had a possible indication, the indication was classified as Class I (“Cardiac monitoring is indicated in most, if not all, patients in this group”) or Class II (“Cardiac monitoring may be of benefit in some patients but is not considered essential for all patients”).3
Adjustment Variables
To account for differences in patient characteristics between hospitalist and nonhospitalist groups, we included age, gender, CMI, and CCI in statistical models. CCI was calculated according to the algorithm specified by Quan et al.15 using all patient diagnoses from previous visits and the index visit identified from the facility billing system.
Statistical Analysis
We computed descriptive statistics for study outcomes and visit characteristics for hospitalist and nonhospitalist visits for pre- and postintervention periods. Descriptive statistics were expressed as n (%) for categorical patient characteristics and outcome variables. For continuous patient characteristics, we expressed the variability of individual observations as the mean ± the standard deviation. For continuous outcomes, we expressed the precision of the mean estimates using standard error. Telemetry utilization per visit was weighted by the number of total acute care days per visit. Telemetry appropriateness per visit was weighted by the number of telemetry days per visit. Patients who did not receive any telemetry monitoring were included in the analysis and noted to have 0 telemetry days. All patients had at least 1 acute care day. Categorical variables were compared using χ2 tests, and continuous variables were compared using t tests. Code event rates were compared using the binomial probability mid-p exact test for person-time data.16
We fitted generalized linear regression models using generalized estimating equations to evaluate the relative change in outcomes of interest in the postintervention period compared with the preintervention period after adjusting for study covariates. The models included study group (hospitalist and nonhospitalist), time period (pre- and postintervention), an interaction term between study group and time period, and study covariates (age, gender, CMI, and CCI). The models were defined using a binomial distributional assumption and logit link function for mortality, escalation of care, and whether patients had at least 1 telemetry day. A gamma distributional assumption and log link function were used for LOS, telemetry acute care days per visit, and total acute care days per visit. A negative binomial distributional assumption and log link function were used for telemetry utilization and telemetry appropriateness. We used the log of the acute care days as an offset for telemetry utilization and the log of the telemetry days per visit as an offset for telemetry appropriateness. An exchangeable working correlation matrix was used to account for physician-level clustering for all outcomes. Intervention effects, representing the difference in odds for categorical variables and in amount for continuous variables, were calculated as exponentiation of the beta parameters for the covariate minus 1.
P values <.05 were considered significant. We used SAS version 9.4 statistical software (SAS Institute Inc., Cary, NC) for data analysis.
RESULTS
The percent of patients who had any telemetry charges decreased from 36.2% to 15.9% (P < .001) in the hospitalist group and from 31.8% to 28.0% in the nonhospitalist group (P < .001; Table 1). Rates of code events did not change over time (P = .9).
In the randomly selected sample of patients pre- and postintervention who received telemetry monitoring, there was an increase in telemetry appropriateness on the hospitalist service (46% to 72%, P = .025; Table 1). In the nonhospitalist group, appropriate telemetry utilization did not change significantly. Of the 100 randomly selected patients in the hospitalist group after the intervention who did not receive telemetry, no patient had an AHA Class I indication, and only 4 patients had a Class II indication.3,17
DISCUSSION
In this study, implementing a change in the EHR telemetry order produced reductions in telemetry days. However, when combined with a multicomponent program including education, audit and feedback, financial incentives, and changes to remove telemetry orders from admission orders sets, an even more marked improvement was seen. Neither intervention reduced LOS, increased code event rates, or increased rates of escalation of care.
Prior studies have evaluated interventions to reduce unnecessary telemetry monitoring with varying degrees of success. The most successful EHR intervention to date, from Dressler et al.,18 achieved a 70% reduction in overall telemetry use by integrating the AHA guidelines into their EHR and incorporating nursing discontinuation guidelines to ensure that telemetry discontinuation was both safe and timely. Other studies using stewardship approaches and standardized protocols have been less successful.19,20 One study utilizing a multidisciplinary approach but not including an EHR component showed modest improvements in telemetry.21
Although we are unable to differentiate the exact effect of each component of the intervention, we did note an immediate decrease in telemetry orders after removing the telemetry order from our admission order set, a trend that was magnified after the addition of broader EHR changes (Figure 1). Important additional contributors to our success seem to have been the standardization of rounds to include daily discussion of telemetry and the provision of routine feedback. We cannot discern whether other components of our program (such as the financial incentives) contributed more or less to our program, though the sum of these interventions produced an overall program that required substantial buy in and sustained focus from the hospitalist group. The importance of the hospitalist program is highlighted by the relatively large differences in improvement compared with the nonhospitalist group.
Our study has several limitations. First, the study was conducted at a single center, which may limit its generalizability. Second, the intervention was multifaceted, diminishing our ability to discern which aspects beyond the system-wide change in the telemetry order were most responsible for the observed effect among hospitalists. Third, we are unable to fully account for baseline differences in telemetry utilization between hospitalist and nonhospitalist groups. It is likely that different services utilize telemetry monitoring in different ways, and the hospitalist group may have been more aware of the existing guidelines for monitoring prior to the intervention. Furthermore, we had a limited sample size for the chart audits, which reduced the available statistical power for determining changes in the appropriateness of telemetry utilization. Additionally, because internal medicine residents rotate through various services, it is possible that the education they received on their hospitalist rotation as part of our intervention had a spillover effect in the nonhospitalist group. However, any effect should have decreased the difference between the groups. Lastly, although our postintervention time period was 1 year, we do not have data beyond that to monitor for sustainability of the results.
CONCLUSION
In this single-site study, combining EHR orders prompting physicians to choose a clinical indication and duration for monitoring with a broader program—including upstream changes in ordering as well as education, audit, and feedback—produced reductions in telemetry usage. Whether this reduction improves the appropriateness of telemetry utilization or reduces other effects of telemetry (eg, alert fatigue, calls for benign arrhythmias) cannot be discerned from our study. However, our results support the idea that multipronged approaches to telemetry use are most likely to produce improvements.
Acknowledgments
The authors thank Dr. Frank Thomas for his assistance with process engineering and Mr. Andrew Wood for his routine provision of data. The statistical analysis was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067-05 (formerly 8UL1TR000105 and UL1RR025764).
Disclosure
The authors have no conflicts of interest to report.
Wasteful care may account for between 21% and 34% of the United States’ $3.2 trillion in annual healthcare expenditures, making it a prime target for cost-saving initiatives.1,2 Telemetry is a target for value improvement strategies because telemetry is overutilized, rarely leads to a change in management, and has associated guidelines on appropriate use.3-10 Telemetry use has been a focus of the Joint Commission’s National Patient Safety Goals since 2014, and it is also a focus of the Society of Hospital Medicine’s Choosing Wisely® campaign.11-13
Previous initiatives have evaluated how changes to telemetry orders or education and feedback affect telemetry use. Few studies have compared a system-wide electronic health record (EHR) approach to a multifaceted intervention. In seeking to address this gap, we adapted published guidelines from the American Heart Association (AHA) and incorporated them into our EHR ordering process.3 Simultaneously, we implemented a multifaceted quality improvement initiative and compared this combined program’s effectiveness to that of the EHR approach alone.
METHODS
Study Design, Setting, and Population
We performed a 2-group observational pre- to postintervention study at University of Utah Health. Hospital encounters of patients 18 years and older who had at least 1 inpatient acute care, nonintensive care unit (ICU) room charge and an admission date between January 1, 2014, and July 31, 2016, were included. Patient encounters with missing encounter-level covariates, such as case mix index (CMI) or attending provider identification, were excluded. The Institutional Review Board classified this project as quality improvement and did not require review and oversight.
Intervention
On July 6, 2015, our Epic (Epic Systems Corporation, Madison, WI) EHR telemetry order was modified to discourage unnecessary telemetry monitoring. The new order required providers ordering telemetry to choose a clinical indication and select a duration for monitoring, after which the order would expire and require physician renewal or discontinuation. These were the only changes that occurred for nonhospitalist providers. The nonhospitalist group included all admitting providers who were not hospitalists. This group included neurology (6.98%); cardiology (8.13%); other medical specialties such as pulmonology, hematology, and oncology (21.30%); cardiothoracic surgery (3.72%); orthopedic surgery (14.84%); general surgery (11.11%); neurosurgery (11.07%); and other surgical specialties, including urology, transplant, vascular surgery, and plastics (16.68%).
Between January 2015 and June 2015, we implemented a multicomponent program among our hospitalist service. The hospitalist service is composed of 4 teams with internal medicine residents and 2 teams with advanced practice providers, all staffed by academic hospitalists. Our program was composed of 5 elements, all of which were made before the hospital-wide changes to electronic telemetry orders and maintained throughout the study period, as follows: (1) a single provider education session reviewing available evidence (eg, AHA guidelines, Choosing Wisely® campaign), (2) removal of the telemetry order from hospitalist admission order set on March 23, 2015, (3) inclusion of telemetry discussion in the hospitalist group’s daily “Rounding Checklist,”14 (4) monthly feedback provided as part of hospitalist group meetings, and (5) a financial incentive, awarded to the division (no individual provider payment) if performance targets were met. See supplementary Appendix (“Implementation Manual”) for further details.
Data Source
We obtained data on patient age, gender, Medicare Severity-Diagnosis Related Group, Charlson comorbidity index (CCI), CMI, admitting unit, attending physician, admission and discharge dates, length of stay (LOS), 30-day readmission, bed charge (telemetry or nontelemetry), ICU stay, and inpatient mortality from the enterprise data warehouse. Telemetry days were determined through room billing charges, which are assigned based on the presence or absence of an active telemetry order at midnight. Code events came from a log kept by the hospital telephone operator, who is responsible for sending out all calls to the code team. Code event data were available starting July 19, 2014.
Measures
Our primary outcome was the percentage of hospital days that had telemetry charges for individual patients. All billed telemetry days on acute care floors were included regardless of admission status (inpatient vs observation), service, indication, or ordering provider. Secondary outcomes were inpatient mortality, escalation of care, code event rates, and appropriate telemetry utilization rates. Escalation of care was defined as transfer to an ICU after initially being admitted to an acute care floor. The code event rate was defined as the ratio of the number of code team activations to the number of patient days. Appropriate telemetry utilization rates were determined via chart review, as detailed below.
In order to evaluate changes in appropriateness of telemetry monitoring, 4 of the authors who are internal medicine physicians (KE, CC, JC, DG) performed chart reviews of 25 randomly selected patients in each group (hospitalist and nonhospitalist) before and after the intervention who received at least 1 day of telemetry monitoring. Each reviewer was provided a key based on AHA guidelines for monitoring indications and associated maximum allowable durations.3 Chart reviews were performed to determine the indication (if any) for monitoring, as well as the number of days that were indicated. The number of indicated days was compared to the number of telemetry days the patient received to determine the overall proportion of days that were indicated (“Telemetry appropriateness per visit”). Three reviewers (KE, AR, CC) also evaluated 100 patients on the hospitalist service after the intervention who did not receive any telemetry monitoring to evaluate whether patients with indications for telemetry monitoring were not receiving it after the intervention. For patients who had a possible indication, the indication was classified as Class I (“Cardiac monitoring is indicated in most, if not all, patients in this group”) or Class II (“Cardiac monitoring may be of benefit in some patients but is not considered essential for all patients”).3
Adjustment Variables
To account for differences in patient characteristics between hospitalist and nonhospitalist groups, we included age, gender, CMI, and CCI in statistical models. CCI was calculated according to the algorithm specified by Quan et al.15 using all patient diagnoses from previous visits and the index visit identified from the facility billing system.
Statistical Analysis
We computed descriptive statistics for study outcomes and visit characteristics for hospitalist and nonhospitalist visits for pre- and postintervention periods. Descriptive statistics were expressed as n (%) for categorical patient characteristics and outcome variables. For continuous patient characteristics, we expressed the variability of individual observations as the mean ± the standard deviation. For continuous outcomes, we expressed the precision of the mean estimates using standard error. Telemetry utilization per visit was weighted by the number of total acute care days per visit. Telemetry appropriateness per visit was weighted by the number of telemetry days per visit. Patients who did not receive any telemetry monitoring were included in the analysis and noted to have 0 telemetry days. All patients had at least 1 acute care day. Categorical variables were compared using χ2 tests, and continuous variables were compared using t tests. Code event rates were compared using the binomial probability mid-p exact test for person-time data.16
We fitted generalized linear regression models using generalized estimating equations to evaluate the relative change in outcomes of interest in the postintervention period compared with the preintervention period after adjusting for study covariates. The models included study group (hospitalist and nonhospitalist), time period (pre- and postintervention), an interaction term between study group and time period, and study covariates (age, gender, CMI, and CCI). The models were defined using a binomial distributional assumption and logit link function for mortality, escalation of care, and whether patients had at least 1 telemetry day. A gamma distributional assumption and log link function were used for LOS, telemetry acute care days per visit, and total acute care days per visit. A negative binomial distributional assumption and log link function were used for telemetry utilization and telemetry appropriateness. We used the log of the acute care days as an offset for telemetry utilization and the log of the telemetry days per visit as an offset for telemetry appropriateness. An exchangeable working correlation matrix was used to account for physician-level clustering for all outcomes. Intervention effects, representing the difference in odds for categorical variables and in amount for continuous variables, were calculated as exponentiation of the beta parameters for the covariate minus 1.
P values <.05 were considered significant. We used SAS version 9.4 statistical software (SAS Institute Inc., Cary, NC) for data analysis.
RESULTS
The percent of patients who had any telemetry charges decreased from 36.2% to 15.9% (P < .001) in the hospitalist group and from 31.8% to 28.0% in the nonhospitalist group (P < .001; Table 1). Rates of code events did not change over time (P = .9).
In the randomly selected sample of patients pre- and postintervention who received telemetry monitoring, there was an increase in telemetry appropriateness on the hospitalist service (46% to 72%, P = .025; Table 1). In the nonhospitalist group, appropriate telemetry utilization did not change significantly. Of the 100 randomly selected patients in the hospitalist group after the intervention who did not receive telemetry, no patient had an AHA Class I indication, and only 4 patients had a Class II indication.3,17
DISCUSSION
In this study, implementing a change in the EHR telemetry order produced reductions in telemetry days. However, when combined with a multicomponent program including education, audit and feedback, financial incentives, and changes to remove telemetry orders from admission orders sets, an even more marked improvement was seen. Neither intervention reduced LOS, increased code event rates, or increased rates of escalation of care.
Prior studies have evaluated interventions to reduce unnecessary telemetry monitoring with varying degrees of success. The most successful EHR intervention to date, from Dressler et al.,18 achieved a 70% reduction in overall telemetry use by integrating the AHA guidelines into their EHR and incorporating nursing discontinuation guidelines to ensure that telemetry discontinuation was both safe and timely. Other studies using stewardship approaches and standardized protocols have been less successful.19,20 One study utilizing a multidisciplinary approach but not including an EHR component showed modest improvements in telemetry.21
Although we are unable to differentiate the exact effect of each component of the intervention, we did note an immediate decrease in telemetry orders after removing the telemetry order from our admission order set, a trend that was magnified after the addition of broader EHR changes (Figure 1). Important additional contributors to our success seem to have been the standardization of rounds to include daily discussion of telemetry and the provision of routine feedback. We cannot discern whether other components of our program (such as the financial incentives) contributed more or less to our program, though the sum of these interventions produced an overall program that required substantial buy in and sustained focus from the hospitalist group. The importance of the hospitalist program is highlighted by the relatively large differences in improvement compared with the nonhospitalist group.
Our study has several limitations. First, the study was conducted at a single center, which may limit its generalizability. Second, the intervention was multifaceted, diminishing our ability to discern which aspects beyond the system-wide change in the telemetry order were most responsible for the observed effect among hospitalists. Third, we are unable to fully account for baseline differences in telemetry utilization between hospitalist and nonhospitalist groups. It is likely that different services utilize telemetry monitoring in different ways, and the hospitalist group may have been more aware of the existing guidelines for monitoring prior to the intervention. Furthermore, we had a limited sample size for the chart audits, which reduced the available statistical power for determining changes in the appropriateness of telemetry utilization. Additionally, because internal medicine residents rotate through various services, it is possible that the education they received on their hospitalist rotation as part of our intervention had a spillover effect in the nonhospitalist group. However, any effect should have decreased the difference between the groups. Lastly, although our postintervention time period was 1 year, we do not have data beyond that to monitor for sustainability of the results.
CONCLUSION
In this single-site study, combining EHR orders prompting physicians to choose a clinical indication and duration for monitoring with a broader program—including upstream changes in ordering as well as education, audit, and feedback—produced reductions in telemetry usage. Whether this reduction improves the appropriateness of telemetry utilization or reduces other effects of telemetry (eg, alert fatigue, calls for benign arrhythmias) cannot be discerned from our study. However, our results support the idea that multipronged approaches to telemetry use are most likely to produce improvements.
Acknowledgments
The authors thank Dr. Frank Thomas for his assistance with process engineering and Mr. Andrew Wood for his routine provision of data. The statistical analysis was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067-05 (formerly 8UL1TR000105 and UL1RR025764).
Disclosure
The authors have no conflicts of interest to report.
1. National Health Expenditure Fact Sheet. 2015; https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html. Accessed June 27, 2017.
2. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516. PubMed
3. Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721-2746. PubMed
4. Sandau KE, Funk M, Auerbach A, et al. Update to Practice Standards for Electrocardiographic Monitoring in Hospital Settings: A Scientific Statement From the American Heart Association. Circulation. 2017;136(19):e273-e344. PubMed
5. Mohammad R, Shah S, Donath E, et al. Non-critical care telemetry and in-hospital cardiac arrest outcomes. J Electrocardiol. 2015;48(3):426-429. PubMed
6. Dhillon SK, Rachko M, Hanon S, Schweitzer P, Bergmann SR. Telemetry monitoring guidelines for efficient and safe delivery of cardiac rhythm monitoring to noncritical hospital inpatients. Crit Pathw Cardiol. 2009;8(3):125-126. PubMed
7. Estrada CA, Rosman HS, Prasad NK, et al. Evaluation of guidelines for the use of telemetry in the non-intensive-care setting. J Gen Intern Med. 2000;15(1):51-55. PubMed
8. Estrada CA, Prasad NK, Rosman HS, Young MJ. Outcomes of patients hospitalized to a telemetry unit. Am J Cardiol. 1994;74(4):357-362. PubMed
9. Atzema C, Schull MJ, Borgundvaag B, Slaughter GR, Lee CK. ALARMED: adverse events in low-risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med. 2006;24(1):62-67. PubMed
10. Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med. 2000;7(6):647-652. PubMed
11. The Joint Commission 2017 National Patient Safety Goals https://www.jointcommission.org/hap_2017_npsgs/. Accessed on February 15, 2017.
12. Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission announces 2014 National Patient Safety Goal. Jt Comm Perspect. 2013;33(7):1, 3-4. PubMed
13. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
14. Yarbrough PM, Kukhareva PV, Horton D, Edholm K, Kawamoto K. Multifaceted intervention including education, rounding checklist implementation, cost feedback, and financial incentives reduces inpatient laboratory costs. J Hosp Med. 2016;11(5):348-354. PubMed
15. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682. PubMed
16. Greenland S, Rothman KJ. Introduction to categorical statistics In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology. Vol 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2008: 238-257.
17. Henriques-Forsythe MN, Ivonye CC, Jamched U, Kamuguisha LK, Olejeme KA, Onwuanyi AE. Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med. 2009;76(6):368-372. PubMed
18. Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. PubMed
19. Boggan JC, Navar-Boggan AM, Patel V, Schulteis RD, Simel DL. Reductions in telemetry order duration do not reduce telemetry utilization. J Hosp Med. 2014;9(12):795-796. PubMed
20. Cantillon DJ, Loy M, Burkle A, et al. Association Between Off-site Central Monitoring Using Standardized Cardiac Telemetry and Clinical Outcomes Among Non-Critically Ill Patients. JAMA. 2016;316(5):519-524. PubMed
21. Svec D, Ahuja N, Evans KH, et al. Hospitalist intervention for appropriate use of telemetry reduces length of stay and cost. J Hosp Med. 2015;10(9):627-632. PubMed
1. National Health Expenditure Fact Sheet. 2015; https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html. Accessed June 27, 2017.
2. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516. PubMed
3. Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721-2746. PubMed
4. Sandau KE, Funk M, Auerbach A, et al. Update to Practice Standards for Electrocardiographic Monitoring in Hospital Settings: A Scientific Statement From the American Heart Association. Circulation. 2017;136(19):e273-e344. PubMed
5. Mohammad R, Shah S, Donath E, et al. Non-critical care telemetry and in-hospital cardiac arrest outcomes. J Electrocardiol. 2015;48(3):426-429. PubMed
6. Dhillon SK, Rachko M, Hanon S, Schweitzer P, Bergmann SR. Telemetry monitoring guidelines for efficient and safe delivery of cardiac rhythm monitoring to noncritical hospital inpatients. Crit Pathw Cardiol. 2009;8(3):125-126. PubMed
7. Estrada CA, Rosman HS, Prasad NK, et al. Evaluation of guidelines for the use of telemetry in the non-intensive-care setting. J Gen Intern Med. 2000;15(1):51-55. PubMed
8. Estrada CA, Prasad NK, Rosman HS, Young MJ. Outcomes of patients hospitalized to a telemetry unit. Am J Cardiol. 1994;74(4):357-362. PubMed
9. Atzema C, Schull MJ, Borgundvaag B, Slaughter GR, Lee CK. ALARMED: adverse events in low-risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med. 2006;24(1):62-67. PubMed
10. Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med. 2000;7(6):647-652. PubMed
11. The Joint Commission 2017 National Patient Safety Goals https://www.jointcommission.org/hap_2017_npsgs/. Accessed on February 15, 2017.
12. Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission announces 2014 National Patient Safety Goal. Jt Comm Perspect. 2013;33(7):1, 3-4. PubMed
13. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
14. Yarbrough PM, Kukhareva PV, Horton D, Edholm K, Kawamoto K. Multifaceted intervention including education, rounding checklist implementation, cost feedback, and financial incentives reduces inpatient laboratory costs. J Hosp Med. 2016;11(5):348-354. PubMed
15. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682. PubMed
16. Greenland S, Rothman KJ. Introduction to categorical statistics In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology. Vol 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2008: 238-257.
17. Henriques-Forsythe MN, Ivonye CC, Jamched U, Kamuguisha LK, Olejeme KA, Onwuanyi AE. Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med. 2009;76(6):368-372. PubMed
18. Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. PubMed
19. Boggan JC, Navar-Boggan AM, Patel V, Schulteis RD, Simel DL. Reductions in telemetry order duration do not reduce telemetry utilization. J Hosp Med. 2014;9(12):795-796. PubMed
20. Cantillon DJ, Loy M, Burkle A, et al. Association Between Off-site Central Monitoring Using Standardized Cardiac Telemetry and Clinical Outcomes Among Non-Critically Ill Patients. JAMA. 2016;316(5):519-524. PubMed
21. Svec D, Ahuja N, Evans KH, et al. Hospitalist intervention for appropriate use of telemetry reduces length of stay and cost. J Hosp Med. 2015;10(9):627-632. PubMed
© 2018 Society of Hospital Medicine
Continuing Medical Education Program in
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
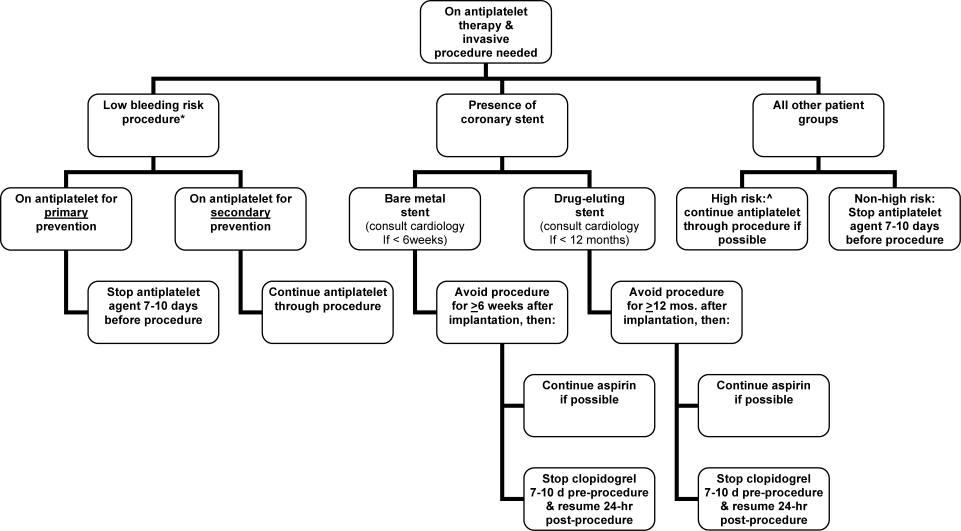
Periprocedural Antithrombotic Management
The management of patients on long‐term antithrombotic therapy (vitamin K antagonists [VKA] or antiplatelet agents) who may require temporary disruption for an invasive procedure is challenging. Management is controversial due to methodologically limited prospective data and varied consensus opinions. Yet periprocedural anticoagulation management is a commonly encountered clinical problem. It is estimated that there are 2.5 million patients on long‐term VKA therapy in North America1 and 41% of the U.S. population over age 40 years is on antiplatelet therapy.2 Further, the need for temporary disruption of these therapies for an invasive procedure is frequent. As an example, in 1 European study, approximately 15% of patients on long‐term VKA required a major surgical procedure in 4 years of follow‐up.3 The role of the hospitalist physician in managing these patients is increasing as hospitalists care for an increasing number of surgical patients and provide periprocedural consultation both in and out of the hospital. Therefore, it is imperative for the hospitalist physician to be proficient in making thoughtful and individualized recommendations on the appropriate management of periprocedural anticoagulants, drawing from the available literature and evidence‐based practice guidelines. Importantly, the Society of Hospital Medicine has cited perioperative management as an important core competency.4
The hospitalist physician is likely to encounter numerous periprocedural scenarios, including the management of antiplatelet agents, identifying low bleeding risk procedures wherein interruption of anticoagulants is unnecessary, and recognizing patients with a low short‐term thromboembolic risk where anticoagulants can be disrupted without the need for heparin or low molecular weight heparin (LMWH) in the periprocedural period (defined as bridging therapy). Further, all other clinical scenarios require both a careful individualized assessment of the patient's risk of periprocedural bleeding and thromboembolism and a thoughtful discussion with all involved parties. This discussion may involve the person performing the procedure, the anesthesiologist, and the patient. The purpose of this work is to explore these relevant areas through a review of the literature with a particular focus on the recently published 2008 American College of Chest Physicians (ACCP) evidence‐based clinical practice guidelines.
We reviewed medical literature from 1990 through May 2008 with the following key words: bridging, anticoagulation, perioperative, antiplatelet, heparin, and low molecular weight heparin. Individual studies were then independently reviewed by the authors. Studies that were felt relevant to a hospitalist physician were retrieved and reviewed. If there was uncertainty regarding applicability to a hospitalist setting, a second author's opinion was rendered. Additionally, we reviewed 1 author's personal reference list of articles relating to periprocedural anticoagulation that has been compiled over the past 10 years. This list and the reference lists of retrieved articles were also reviewed. Data were summarized to answer 4 clinically relevant questions:
-
What is the optimal management of antiplatelet therapy in the periprocedural period?
-
Are there very low‐bleeding risk procedures that do not require interruption of oral anticoagulation?
-
Are there low thromboembolic risk populations who do not require periprocedural bridging?
-
How do you manage patients who must discontinue anticoagulants but are at an increased thrombotic risk?
Clinical Question #1: What Is the Optimal Management of Antiplatelet Therapy in the Periprocedural Period?
The optimal management of oral antiplatelet therapy in the periprocedural period is not well studied. Most reviews, expert recommendations, and consensus statements either do not comment on periprocedural antiplatelet management or recommend the routine discontinuation of therapy at least 7 days prior to surgery.3, 5, 6 However, as the 2008 ACCP guidelines highlight, the recommendation to routinely discontinue antiplatelet therapy 7 days prior to the procedure is an oversimplification.1 In the era of both bare metal cardiac stents and drug‐eluting stents, the optimal management of these patients requires that 2 primary questions be asked: (1) Is this a low‐bleeding risk procedure whereby antiplatelet therapy can be continued? (2) Does the patient have a coronary stent whereby the continuation of antiplatelet therapy or delay of the intervention is necessary?
In the context of ongoing aspirin therapy, certain procedures have a low risk of significant hemorrhagic complications. These low bleeding risk procedures include cataract surgery, cutaneous surgery, oral surgery, and endoscopic procedures, including those with mucosal biopsies.710 Patients undergoing these procedures may safely continue low dose aspirin therapy, especially if they have a high‐risk indication for their aspirin such as recent myocardial infarction, stroke, or the presence of a coronary stent.5, 710 Whether these procedures can be safely performed in the setting of a thienopyridine or combination antiplatelet therapy is uncertain.
In the past several decades, the management of obstructive coronary artery disease has undergone a major evolution. Placement of coronary stents has become commonplace, and there are now several million patients with drug‐eluting stents.11 The major complication of these devices is stent thrombosis, which results in death or myocardial infarction in up to 64% of patients.12 Fortunately, dual antiplatelet therapy (aspirin and a thienopyridine such as clopidogrel) markedly reduces this risk.13 Current guidelines recommend using combination antiplatelet therapy for at least 4 to 6 weeks and ideally up to 12 months after placement of a bare metal stent and at least 12 months after placement of either a sirolimus‐ or paclitaxel‐eluting stent.1, 14 During this period of dual antiplatelet therapy, the premature discontinuation of the thienopyridine may be catastrophic. To guide clinicians in managing these patients in the periprocedural period, recent consensus guidelines recommend the following:1, 12
-
In patients who are expected to need an invasive surgical procedure in the next 12 months, consideration should be given to avoiding drug‐eluting stents.
-
Elective procedures which have an increased risk of bleeding should be deferred for at least 6 weeks after bare metal stent implantation and 12 months after drug‐eluting stent implantation.
-
For patients undergoing a surgical procedure within 6 weeks of bare metal stent implantation and 12 months of drug‐eluting stent implantation, continuation of aspirin and clopidogrel is recommended. If bleeding risk prohibits this, then a cardiologist should be consulted.
-
In patients with a drug‐eluting stent who need to undergo a procedure whereby the thienopyridine needs to be discontinued, aspirin should be continued if at all possible, and the thienopyridine should be resumed as soon as possible after the procedure. It may be reasonable to consider a loading dose of clopidogrel, up to 600 mg, in this setting, although prospective supportive data is lacking.1
It is important to recognize that delayed stent thrombosis is now reported well beyond 1 year after drug‐eluting stent implantation, and that there may not be a diminution in risk after the initial 12 months.1517 Until additional data is available, it seems prudent, if possible, to at least continue aspirin in the periprocedural period in these patients. If bleeding concerns obviate this, then antiplatelet therapy should be discontinued and resumed as soon as possible.
For patients on chronic antiplatelet therapy who do not have a cardiac stent and who are not undergoing a low‐bleeding‐risk procedure, the risks and benefits of the continuation or discontinuation of antiplatelet therapy in the periprocedural period are uncertain as absolute risks in the periprocedural period have not been well studied. Relative risks/benefits, however, can be estimated from prior studies. Aspirin leads to an approximate 25% relative risk reduction in cardiac or thrombotic event rates compared to placebo.14, 18 Although important, the absolute benefit of 1 week of therapy (vs. no therapy during the periprocedural period) is estimated to be small. The small absolute benefit of continued aspirin therapy may be offset by an increase in significant bleeding events. Although, not well studied, continued aspirin increases significant bleeding by 50% with absolute event rates varying by type of procedure.8 In some procedures, such as intracranial surgery or transurethral prostatectomy, this bleeding risk is prohibitive. For others, the risk may be modest and the decision to continue vs. discontinue aspirin therapy may be at the discretion of the person performing the procedure. In general, for most patients who do not have a coronary stent and have not had a recent (past 3 months) myocardial infarction or stroke, discontinuation of antiplatelet therapy 7 to 10 days prior to the procedure seems prudent. The primary exceptions are patients who are undergoing percutaneous coronary intervention or coronary artery bypass grafting. For these procedures continuing aspirin is recommended.1 Figure 1 outlines a proposed management strategy based upon available evidence and guidelines.
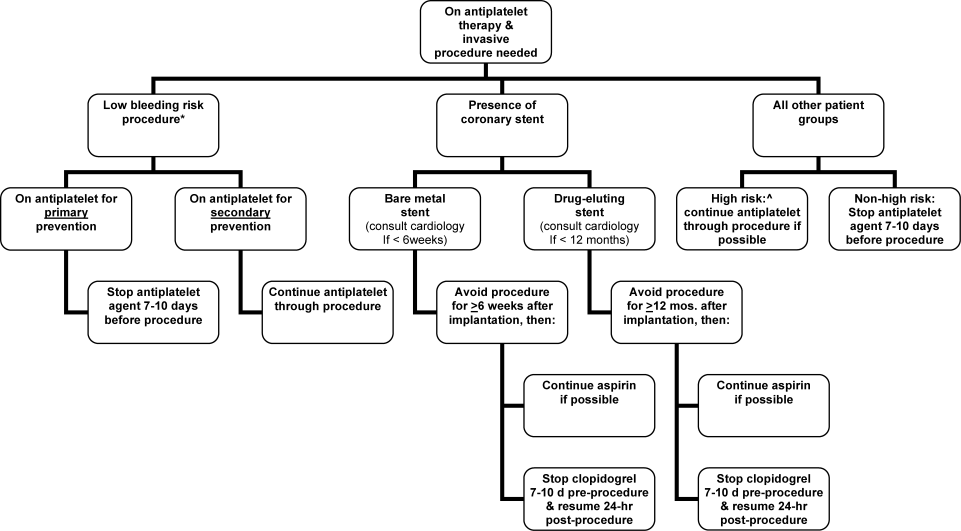
Clinical Question #2: Are There Very‐Low‐Bleeding‐Risk Procedures That Do Not Require Interruption of Oral Anticoagulation?
Some procedures are associated with a low‐enough risk of bleeding that it is safe to proceed without interrupting VKA anticoagulation. This approach spares the risk and cost that occur with the holding of oral anticoagulants and institution of bridging therapy. When considering this strategy, it is important that the specialist performing the procedure is included in the discussion. Dental, dermatologic, and cataract procedures are common outpatient procedures that are associated with low bleeding risk. The relative safety of these procedures in patients who are anticoagulated is discussed thoroughly in the ACCP guidelines.1 Other low‐bleeding‐risk procedures for which a hospitalist may be consulted include certain endoscopic procedures, paracentesis, central venous catheter placement, and arthrocentesis.
The American Society for Gastrointestinal Endoscopy has published guidelines recommending that anticoagulation can be safely continued in patients undergoing the following endoscopic procedures with a low bleeding risk: esophagogastroduodenoscopy (EGD), flexible sigmoidoscopy, and colonoscopy, all with or without mucosal biopsy; enteroscopy, biliary/pancreatic stent placement, endoscopic ultrasound without biopsy, and endoscopic retrograde cholangiopancreatography (ERCP) without sphincterotomy.19 Conversely, high‐risk procedures for which interruption of anticoagulation is recommended include polypectomy, biliary sphincterotomy, variceal treatment, percutaneous endoscopic gastrostomy (PEG) placement, dilation of strictures, and endoscopic ultrasound‐guided fine‐needle aspiration.
Limited data suggest that paracentesis, central venous catheter placement, and arthrocentesis may be safe to perform in the setting of anticoagulation. For patients undergoing paracentesis there is little evidence in anticoagulated patients; however, it is probably safe to continue anticoagulation as studies have demonstrated the safety of this procedure in patients with significant thrombocytopenia and coagulopathy.20, 21 Limited data also supports that central venous catheter placement may be safely performed in the setting of abnormal coagulation tests, although some recommend avoiding the subclavian site due to the risk of hemothorax and the inability to apply adequate compression.2226 With regard to arthrocentesis, multiple authors have endorsed the idea that joint and soft‐tissue aspirations and injections present a low risk of serious bleeding even with anticoagulation.2729 This is supported by limited data.30, 31
Other procedures such as lumbar puncture, thoracentesis, and cardiac catheterization are somewhat more controversial in the anticoagulated patient. Anticoagulation should generally be interrupted for lumbar puncture,29, 32 as 1 study involving patients who were started on heparin immediately after the procedure had a 2% incidence of spinal hematoma and 6.7% major complication rate.33 With regard to thoracentesis, evidence is very limited, but experts generally accept that it may be safely performed in patients with mild coagulopathy.34, 35 One frequently‐cited study found no bleeding complications in 57 patients with mild elevation in prothrombin time, which correlated to an International Normalized Ratio of approximately 2.2 or less.36 A recent report also revealed no serious bleeding complications in 33 thoracenteses performed on patients receiving full anticoagulation with warfarin, heparin, and/or low molecular weight heparin.37
Therapeutic anticoagulation has traditionally been felt to be a relative contraindication to cardiac catheterization.38, 39 In spite of this, several observational studies have suggested it may be safely performed using a standard approach,40 using vascular closure devices,41 or using a radial artery approach instead of the more commonly used femoral site.4244 The small size of these observational reports, the diagnostic rather than therapeutic nature of most cases, the limited use of other antithrombotic and antiplatelet medications, and the experience required to use the transradial approach are all major limitations preventing widespread acceptance of cardiac catheterization in therapeutically anticoagulated patients.
In summary, there are numerous procedures that may be safely pursued in the setting of therapeutic anticoagulation. However, for most of these procedures the data is somewhat limited. As such, it is paramount for the hospitalist physician to recognize these clinical scenarios and to discuss management options with the patient and the person performing the procedure, if applicable.
Clinical Question #3: Are There LowThromboembolic‐Risk Populations Who Do Not Require Periprocedural Bridging?
Although it has previously been noted that there is a wide variation of opinion on when and how to perform periprocedural bridging, it is generally agreed that in the following conditions the risk of thrombosis is low enough that bridging with full dose heparin or LMWH is not necessary:1, 5, 4549
-
Atrial fibrillation without previous stroke or transient ischemic attack (TIA) and no more than 2 additional thrombotic risk factors on the CHADS2 scoring system (Table 1).
-
A single venous thromboembolic event that occurred greater than 12 months ago with no ongoing risk factors such as active malignancy, high risk thrombophilia, or the antiphospholipid antibody syndrome.
-
Bileaflet aortic valve without the presence of additional risk factors (ie, patients <75 years of age with the absence of atrial fibrillation, prior stroke or transient ischemic attack, hypertension, diabetes, or congestive heart failure).
| CHADS2 Score* | Annual Risk of Stroke (%) |
|---|---|
| |
| 0 | 1.9 |
| 1 | 2.8 |
| 2 | 4.0 |
| 3 | 5.9 |
| 4 | 8.5 |
| 5 | 12.5 |
| 6 | 18.2 |
Clinical Question #4: How Do You Manage Patients Who Must Discontinue Anticoagulants But Are at an Increased Thrombotic Risk?
When anticoagulation must be held and the patient does not have a very low thromboembolic risk, a decision of whether or not to use bridging anticoagulation must be made. The current ACCP guideline gives grade 1C and 2C recommendations (evidence from observational studies, case series, or controlled trials with serious flaws) regarding for whom and how to implement bridging.1 The grade C designation is due to a lack of high‐quality randomized clinical trials. As such, the clinician must carefully consider an individual patient's estimated thromboembolic risk, procedurally‐related bleeding risk, patient‐related bleeding risk factors, and the patient's values regarding concerns of thromboembolism or bleeding. In these situations it is also imperative that the person performing the procedure is involved in the risk‐to‐benefit discussion.
When evaluating an individual patient's risk of thromboembolism, clinicians sometimes estimate the perioperative risk by prorating the annual incidence of thromboembolic complications to the few days that anticoagulation is withheld.67 Making this extrapolation discounts the effect of a potential increase in thromboembolic risk induced by surgery. As an example, an average patient with atrial fibrillation who has a 5% predicted annual stroke rate would be estimated to have a stroke risk of 0.05% if they are not anticoagulated for 4 days. However, studies have shown that the actual rate of perioperative thromboembolism is approximately 1%.1 With these limitations and uncertainties in mind, and until there is better prospective outcomes data, we must consider relative risks in the context of absolute event rate estimates when deciding a perioperative anticoagulant management plan. The estimated annual incidence of thrombosis without anticoagulation for various indications and the current guideline recommendations are presented in Table 2.
| Practice Guideline Preferred Management Recommendations | ||||
|---|---|---|---|---|
| Indication for chronic anticoagulation | Estimated Annual Thrombotic Risk Without Anticoagulation | ACCP*1 | ACC/AHA45, 46 | British Haematologic Society70 |
| ||||
| Dual prosthetic or older‐generation valve | >10% | Bridge | Bridge | Bridge |
| VTE within 3 months or severe thrombophilias | Bridge | N/A | Bridge | |
| Pregnancy with prosthetic valve | Bridge | Bridge | N/A | |
| Bileaflet valve in the mitral position | Bridge | Bridge | Prophylaxis | |
| Valve with acute embolism <6 months | Bridge | N/A | Bridge | |
| A‐fib valvular or CHADS2 score 5‐6 | Bridge | Consider bridging | N/A | |
| Recurrent venous thromboembolism | 4‐10% | Bridge | N/A | N/A |
| VTE within 3‐12 months or active cancer | Bridge | N/A | Prophylaxis | |
| Bileaflet aortic valve with additional risk factors | Bridge | Bridge | Prophylaxis | |
| A‐fib CHADS2 score 3‐4 | Bridge | Consider bridging | N/A | |
| Bileaflet aortic valve without additional risk factors | <4% | Prophylaxis or no bridging | No bridging | Prophylaxis |
| VTE >12 months | Prophylaxis or no bridging | N/A | Prophylaxis | |
| A‐fib CHADS2 score 0‐2 and no previous CVA/TIA | Prophylaxis or no bridging | No bridging | N/A | |
In addition to thromboembolic risk, we must also consider the bleeding risk associated with the procedure/surgery. Importantly, therapeutic heparin started early in the postoperative period is associated with major bleeding event rates as high as 10% to 20%.1, 50 Once a major bleeding event occurs, this will often lead to an extended interruption of anticoagulant therapy, placing the patient at a more prolonged risk of an associated thromboembolic event. For this reason, the resumption of full‐dose anticoagulation with LMWH/heparin should be delayed for at least 48 hours in most patients undergoing a surgery or procedure associated with an increased risk of bleeding. Examples of these higher‐bleeding‐risk procedures include major thoracic surgery, intracranial or spinal surgery, major vascular surgery, major orthopedic surgery, urologic surgery involving the bladder or prostrate, major oncologic surgery, reconstructive plastic surgery, colonoscopy with associated polypectomy, renal or prostate biopsies, and placement of a cardiac pacemaker/defibrillator.1, 5157
Taken together, these uncertainties surrounding thromboembolic and bleeding risk estimates imply that there are multiple options for periprocedural management. Several studies, many of which included patients with mechanical heart valves, have shown similar safety and efficacy between LMWH and intravenous (IV) unfractionated heparin.5864 Table 3 summarizes these studies. The ACCP recommends bridging with LMWH over IV unfractionated heparin due to equal efficacy and cost savings with LMWH.1 When bridging is used, careful attention must be given to the timing and dose of anticoagulation in both the preoperative and postoperative periods. Table 4 lists dosing of commonly used LMWHs in North America. When using LMWHs in the preprocedural setting it is important to note that unacceptably high levels of anticoagulation remain present when a patient is given a full once‐daily LMWH dose the morning prior to the procedure or when a full‐dose, twice‐daily LMWH dose is given the evening prior to the procedure.65, 66 For this reason, the ACCP recommends administering the last preoperative dose 24 hours before surgery and if full‐dose once‐daily LMWH is used, the dose should be decreased by one‐half on the day before the surgery in order to ensure that no residual anticoagulant effect remains at the time of surgery.
| AuthorReference/Study Type | Number of Patients | Patient Population | Type of Procedure | Bridging Strategy | Major Bleeds | Minor Bleeds | TE Rate |
|---|---|---|---|---|---|---|---|
| |||||||
| Turpie and Douketis63/single arm cohort | 174 | 66% aortic valve; 34% mitral or dual prosthetic valve | Not specified | Enoxaparin 1 mg/kg twice daily | 2.3% | Not specified | None |
| Kovacs et al.61/single arm cohort | 224 | Prosthetic heart valves or a‐fib plus 1 major risk factor | 67 surgical; 157 nonsurgical | Preoperative bridging with dalteparin 200 IU/kg daily; dose reduced to 100 IU/kg on preoperative day 1; restarted at 100 IU/kg on POD 1; dose reduced to 5000 IU daily if high risk for bleeding | 6.7%; 8/15 occurred intraoperatively or <6 hours postoperatively; 2/15 occurred after 4 weeks | Not specified | 3.6%; 6/8 episodes occurred after warfarin held secondary to bleeding; 2/8 thrombotic episodes judged to be due to cardioembolism |
| Douketis et al.59/prospective registry | 650 | A‐fib 58%; mechanical heart valve 33% | 251 surgical; 399 nonsurgical | Dalteparin 100 IU/kg twice daily; held after high bleeding risk procedure and patients with poor hemostasis | 0.92% | 5.9% | 0.6% |
| Spyropolous et al.62/prospective registry; 14 centers in United States and Canada | 901 | UFH: 40% mechanical valves, 33% a‐fib; LMWH: 24% mechanical valve, 40% a‐fib | 394 surgical; 507 nonsurgical | LMWH mostly given twice daily 80%; UFH 20% | 5.5% UFH; 3.3% LMWH | 9.1% UFH; 12.0% LMWH | 2.4% UFH; 0.9% LMWH |
| Dunn et al.66/prospective cohort | 260 | A‐fib 68% or prior DVT 37% (excluding prosthetic heart valves) | 105 surgical; 145 nonsurgical | Enoxaparin 1.5 mg/kg daily | 3.5% overall; minor surgery/procedures 0.9%; major surgery 28% | 42% | 1.9%; 1/5 events occurred after bleeding led to withdrawal of AC |
| Omran et al.77/prospective registry | 779 | Various indications | Major and minor procedures | All patients bridged with enoxaparin; moderate TE risk 1 mg/kg daily; high TE risk 1 mg/kg twice daily | 0.5%; all in high‐risk group | 5.9% | 0 |
| Garcia et al.71/prospective, observational cohort of 101 sites in United States | 1024 patients with 1293 interruptions of AC | A‐fib 53%; VTE 14%; prosthetic valve 13% | Outpatient procedures only | At discretion of provider. Bridging performed in 8.3% of interruptions; 3% a‐fib, 10% VTE, and 29% mechanical valves | 0.6%; 4/6 patients with major bleed received bridging | 1.7%;10/17 patients with minor bleed received bridging | 0.7%; no events in patients who were bridged |
| Wysokinski et al.64/prospective cohort | 345 consecutive patients undergoing 386 procedures | 100% nonvalvular a‐fib | Major and minor surgeries/procedures | Individualized in AC clinic; 52% of patients bridged | 2.7%; no difference whether patient received bridging or not | 3.0%; 10/11 occurred in bridged patients | 1.1%; no difference in bridged vs. nonbridged patients |
| Low Molecular Weight Heparin | Subcutaneous Dose |
|---|---|
| |
| Dalteparin | |
| Low dose (prophylaxis dose) | 5,000 IU once daily |
| Full dose | 100 IU/kg twice daily or 200 IU/kg once daily |
| Enoxaparin | |
| Low dose (prophylaxis dose) | 30 mg twice daily or 40mg daily |
| Full dose | 1 mg/kg twice daily or 1.5 mg/kg once daily |
| Tinzaparin (full dose) | 175 IU/kg once daily |
In the postprocedural setting, timing and dose of anticoagulant is important, as major bleeding with the use of therapeutic anticoagulation can occur in up to 10% to 20% of cases. When restarting anticoagulation after the procedure, it is important to evaluate intraoperative hemostasis and to consider patient‐related factors that may further increase bleeding risk. These include advanced age, concomitant antiplatelet or nonsteroidal antiinflammatory medications, renal insufficiency, placement of spinal/epidural catheter, worsening liver disease, or the presence of other comorbid illnesses such as cancer.30, 67, 68 The ACCP recommends withholding full‐dose anticoagulation for at least 48 to 72 hours in patients who are felt to be at a high risk for postoperative bleeding.1 Figure 2 is a proposed management approach to the use of bridging anticoagulants that is consistent with the 2008 ACCP recommendations.

CONCLUSION
The evaluation and management of patients on long‐term antiplatelet or VKA therapy who require an invasive procedure or surgery is a common, complicated, and controversial area. Importantly, it is an area in which the hospitalist physician must be adept. Although there remain many unanswered clinical questions, an evolving literature base and recent practice guidelines can help guide management decisions.
- ,,, et al.The Perioperative Management of Antithrombotic Therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6_suppl):299S–339S.
- ,,,,.Aspirin use among adults aged 40 and older in the United States: results of a national survey.Am J Prev Med.2007;32(5):403–407.
- ,.Oral anticoagulation in surgical procedures: risks and recommendations.Br J Haematol.2003;123(4):676–682.
- Perioperative medicine.J Hosp Med.2006;1(supp 1):30–31.
- .Perioperative anticoagulation management in patients who are receiving oral anticoagulant therapy: a practical guide for clinicians.Thromb Res.2002;108(1):3–13.
- ,,,.Periprocedural bridging therapy in patients receiving chronic oral anticoagulation therapy.Curr Med Res Opin.2006;22(6):1109–1122.
- ,,,,.Effect of aspirin intake on bleeding during cataract surgery.J Cataract Refract Surg.1998;24(9):1243–1246.
- ,,,.Low‐dose aspirin for secondary cardiovascular prevention–cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation—review and meta‐analysis.J Intern Med.2005;257(5):399–414.
- ,,.Aspirin does not increase bleeding complications after transbronchial biopsy.Chest.2002;122(4):1461–1464.
- ,.The management of patients on anticoagulants prior to cutaneous surgery: case report of a thromboembolic complication, review of the literature, and evidence‐based recommendations.Plast Reconstr Surg.2006;118(5):110e–117e.
- .Unanswered questions—drug‐eluting stents and the risk of late thrombosis.N Engl J Med.2007;356(10):981–984.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115(6):813–818.
- ,,, et al.The Primary and Secondary Prevention of Coronary Artery Disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6_suppl):776S–814S.
- ,,, et al.2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee.Circulation.2008;117(2):261–295.
- ,,,,,.Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials.Am J Med.2006;119(12):1056–1061.
- ,,, et al.Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study.Lancet.2007;369(9562):667–678.
- ,,, et al.Clopidogrel use and long‐term clinical outcomes after drug‐eluting stent implantation.JAMA.2007;297(2):159–168.
- Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.Antiplatelet Trialists' Collaboration.Br Med J (Clin Res Ed).1994;308(6921):81–106.
- ,,, et al.Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures.Gastrointest Endosc.2002;55(7):775–779.
- ,.Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease.Aliment Pharmacol Ther.2005;21(5):525–529.
- ,,, et al.Performance standards for therapeutic abdominal paracentesis.Hepatology.2004;40(2):484–488.
- ,.Percutaneous cannulation of the internal jugular vein in patients with coagulopathies: an experience based on 1,000 attempts.Anesthesiology.1982;56(4):321–323.
- ,,,,,.Central venous catheterization in patients with coagulopathy.Arch Surg.1992;127(3):273–275.
- ,,.Central venous catheter placement in patients with disorders of hemostasis.Chest.1996;110(1):185–188.
- ,,.US‐guided placement of central vein catheters in patients with disorders of hemostasis.Eur J Radiol.2008;65(2):253–256.
- ,.Central venous cannulation in patients with liver disease and coagulopathy—a prospective audit.Intensive Care Med.1999;25(5):481–485.
- ,.Perioperative management of patients receiving oral anticoagulants: a systematic review.Arch Intern Med.2003;163(8):901–908.
- ,,, et al.Kelley's Textbook of Rheumatology.7th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,.Clinical Procedures in Emergency Medicine.4th ed.Philadelphia, PA:W.B. Saunders;2004.
- ,.A prospective study of the safety of joint and soft tissue aspirations and injections in patients taking warfarin sodium.Arthritis Rheum.1998;41(4):736–739.
- ,,, et al.[Frequency of the bleeding risk in patients receiving warfarin submitted to arthrocentesis of the knee].Reumatismo.2003;55(3):159–163.
- .Textbook of Critical Care.5th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,.Complications of lumbar puncture followed by anticoagulation.Stroke.1981;12(6):879–881.
- ,,,.Mason, Murray and Nadel's Textbook of Respiratory Medicine.4th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,,.Videos in clinical medicine. Thoracentesis.N Engl J Med.2006;355(15):e16.
- ,.Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities.Transfusion.1991;31(2):164–171.
- .Risk of bleeding during thoracentesis in anticoagulated patients.Chest.2006;130(4):141S–d‐2.
- ,,, et al.Guidelines for prevention of thromboembolic events in valvular heart disease. Study Group of the Working Group on Valvular Heart Disease of the European Society of Cardiology.Eur Heart J.1995;16(10):1320–1330.
- ,.Coronary angiography and intravascular ultrasonography. In: Braunwald E, Zipes DP, Libby P, eds.Heart Disease: A Textbook of Cardiovascular Medicine.6th ed.Philadelphia, PA:WB Saunders;2001:387–421.
- ,,, et al.Effectiveness of manual pressure hemostasis following transfemoral coronary angiography in patients on therapeutic warfarin anticoagulation.Am J Cardiol.2006;97(4):485–488.
- ,,,,,.Elective coronary angiography and percutaneous coronary intervention during uninterrupted warfarin therapy.Catheter Cardiovasc Interv.2003;60(2):180–184.
- ,,,.Coronary angiography in the fully anticoagulated patient: the transradial route is successful and safe.Catheter Cardiovasc Interv.2003;58(1):8–10.
- ,,,,.Percutaneous left and right heart catheterization in fully anticoagulated patients utilizing the radial artery and forearm vein: a two‐center experience.J Interv Cardiol.2006;19(3):258–263.
- ,,, et al.[Safety of diagnostic transradial catheterization in patients undergoing long‐term anticoagulation with coumarin derivatives].Rev Esp Cardiol.2007;60(9):988–991. [Spanish]
- ,,,,,,,,,,,,,2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Valvular Heart Disease).J Am Coll Cardiol.2008;52:e1–142.
- ,,, et al.ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in Collaboration With the North American Society of Pacing and Electrophysiology.J Am Coll Cardiol.2001;38(4):1231–1266.
- .Periprocedural thromboprophylaxis in patients receiving chronic anticoagulation therapy.Am Heart J.2004;147(1):3–15.
- ,.Management of anticoagulation before and after elective surgery.N Engl J Med.1997;336(21):1506–1511.
- ,,,,,.Modern management of prosthetic valve anticoagulation.Mayo Clin Proc.1998;73(7):665–680.
- ,.Anticoagulant‐related bleeding: clinical epidemiology, prediction, and prevention.Am J Med.1993;95(3):315–328.
- ,,,,,.Reconstruction of musculoskeletal defects following oncologic resection in 76 patients.Ann Plast Surg.2006;57(2):190–194.
- ,,,,.Biopsy of the prostate guided by transrectal ultrasound: relation between warfarin use and incidence of bleeding complications.Clin Radiol.2005;60(4):459–463; discussion 457‐458.
- ,.Anticoagulation in neurosurgical patients.Neurosurgery.1999;45(4):838–847; discussion 847‐848.
- ,,,,.Post‐operative blood loss after transurethral prostatectomy is dependent on in situ fibrinolysis.Br J Urol.1997;80(6):889–893.
- ,,.Complications of heparin therapy after total joint arthroplasty.J Bone Joint Surg.1989;71(8):1130–1134.
- ,,,,,.Postpolypectomy lower GI bleeding: descriptive analysis.Gastrointest Endosc.2000;51(6):690–696.
- ,,, et al.Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy.Chest.2004;126(4):1177–1186.
- ,,.[Perioperative bridging with enoxaparin. results of the prospective BRAVE registry with 779 patients].Med Klin (Munich).2007;102(10):809–815. [German]
- ,,.Low molecular weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen.Arch Intern Med.2004;164(12):1319–1326.
- ,,.Bridging therapy in patients on long‐term oral anticoagulants who require surgery: the Prospective Peri‐operative Enoxaparin Cohort Trial (PROSPECT).J Thromb Haemost.2007;5(11):2211–2218.
- ,,, et al.Single‐arm study of bridging therapy with low molecular weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin.Circulation.2004;110(12):1658–1663.
- ,,,.Costs and clinical outcomes associated with low molecular weight heparin vs unfractionated heparin for perioperative bridging in patients receiving long‐term oral anticoagulant therapy.Chest.2004;125(5):1642–1650.
- ,.Enoxaparin is effective and safe as bridging anticoagulation in patients with a mechanical prosthetic heart valve who require temporary interruption of warfarin because of surgery or an invasive procedure. [Abstract #703]. ASH Annual Meeting Abstracts.Blood.2004;104:202A.
- ,,, et al.Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation.Mayo Clin Proc.2008;83(6):639–645.
- ,,,.Bridging anticoagulation with low molecular weight heparin after interruption of warfarin therapy is associated with a residual anticoagulant effect prior to surgery.Thromb Haemost.2005;94(3):528–531.
- ,,, et al.Brief communication: preoperative anticoagulant activity after bridging low molecular weight heparin for temporary interruption of warfarin.Ann Intern Med.2007;146(3):184–187.
- National Institute for Clinical Excellence. Atrial fibrillation: national clinical guideline for management in primary and secondary care.2006. Available at: http://www.nice.org.uk/nicemedia/pdf/cg036fullguideline. pdf. Accessed May 2009.
- ,,, et al.Clinical outcomes with unfractionated heparin or low molecular weight heparin as bridging therapy in patients on long‐term oral anticoagulants: the REGIMEN registry.J Thromb Haemost.2006;4(6):1246–1252.
- ,,,,,.Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation.JAMA.2001;285(22):2864–2870.
- ,,.Guidelines on oral anticoagulation (warfarin). 3rd ed. 2005 update.Br J Haematol.2006;132(3):277–285.
- ,,, et al.Risk of thromboembolism with short‐term interruption of warfarin therapy.Arch Intern Med.2008;168(1):63–69.
The management of patients on long‐term antithrombotic therapy (vitamin K antagonists [VKA] or antiplatelet agents) who may require temporary disruption for an invasive procedure is challenging. Management is controversial due to methodologically limited prospective data and varied consensus opinions. Yet periprocedural anticoagulation management is a commonly encountered clinical problem. It is estimated that there are 2.5 million patients on long‐term VKA therapy in North America1 and 41% of the U.S. population over age 40 years is on antiplatelet therapy.2 Further, the need for temporary disruption of these therapies for an invasive procedure is frequent. As an example, in 1 European study, approximately 15% of patients on long‐term VKA required a major surgical procedure in 4 years of follow‐up.3 The role of the hospitalist physician in managing these patients is increasing as hospitalists care for an increasing number of surgical patients and provide periprocedural consultation both in and out of the hospital. Therefore, it is imperative for the hospitalist physician to be proficient in making thoughtful and individualized recommendations on the appropriate management of periprocedural anticoagulants, drawing from the available literature and evidence‐based practice guidelines. Importantly, the Society of Hospital Medicine has cited perioperative management as an important core competency.4
The hospitalist physician is likely to encounter numerous periprocedural scenarios, including the management of antiplatelet agents, identifying low bleeding risk procedures wherein interruption of anticoagulants is unnecessary, and recognizing patients with a low short‐term thromboembolic risk where anticoagulants can be disrupted without the need for heparin or low molecular weight heparin (LMWH) in the periprocedural period (defined as bridging therapy). Further, all other clinical scenarios require both a careful individualized assessment of the patient's risk of periprocedural bleeding and thromboembolism and a thoughtful discussion with all involved parties. This discussion may involve the person performing the procedure, the anesthesiologist, and the patient. The purpose of this work is to explore these relevant areas through a review of the literature with a particular focus on the recently published 2008 American College of Chest Physicians (ACCP) evidence‐based clinical practice guidelines.
We reviewed medical literature from 1990 through May 2008 with the following key words: bridging, anticoagulation, perioperative, antiplatelet, heparin, and low molecular weight heparin. Individual studies were then independently reviewed by the authors. Studies that were felt relevant to a hospitalist physician were retrieved and reviewed. If there was uncertainty regarding applicability to a hospitalist setting, a second author's opinion was rendered. Additionally, we reviewed 1 author's personal reference list of articles relating to periprocedural anticoagulation that has been compiled over the past 10 years. This list and the reference lists of retrieved articles were also reviewed. Data were summarized to answer 4 clinically relevant questions:
-
What is the optimal management of antiplatelet therapy in the periprocedural period?
-
Are there very low‐bleeding risk procedures that do not require interruption of oral anticoagulation?
-
Are there low thromboembolic risk populations who do not require periprocedural bridging?
-
How do you manage patients who must discontinue anticoagulants but are at an increased thrombotic risk?
Clinical Question #1: What Is the Optimal Management of Antiplatelet Therapy in the Periprocedural Period?
The optimal management of oral antiplatelet therapy in the periprocedural period is not well studied. Most reviews, expert recommendations, and consensus statements either do not comment on periprocedural antiplatelet management or recommend the routine discontinuation of therapy at least 7 days prior to surgery.3, 5, 6 However, as the 2008 ACCP guidelines highlight, the recommendation to routinely discontinue antiplatelet therapy 7 days prior to the procedure is an oversimplification.1 In the era of both bare metal cardiac stents and drug‐eluting stents, the optimal management of these patients requires that 2 primary questions be asked: (1) Is this a low‐bleeding risk procedure whereby antiplatelet therapy can be continued? (2) Does the patient have a coronary stent whereby the continuation of antiplatelet therapy or delay of the intervention is necessary?
In the context of ongoing aspirin therapy, certain procedures have a low risk of significant hemorrhagic complications. These low bleeding risk procedures include cataract surgery, cutaneous surgery, oral surgery, and endoscopic procedures, including those with mucosal biopsies.710 Patients undergoing these procedures may safely continue low dose aspirin therapy, especially if they have a high‐risk indication for their aspirin such as recent myocardial infarction, stroke, or the presence of a coronary stent.5, 710 Whether these procedures can be safely performed in the setting of a thienopyridine or combination antiplatelet therapy is uncertain.
In the past several decades, the management of obstructive coronary artery disease has undergone a major evolution. Placement of coronary stents has become commonplace, and there are now several million patients with drug‐eluting stents.11 The major complication of these devices is stent thrombosis, which results in death or myocardial infarction in up to 64% of patients.12 Fortunately, dual antiplatelet therapy (aspirin and a thienopyridine such as clopidogrel) markedly reduces this risk.13 Current guidelines recommend using combination antiplatelet therapy for at least 4 to 6 weeks and ideally up to 12 months after placement of a bare metal stent and at least 12 months after placement of either a sirolimus‐ or paclitaxel‐eluting stent.1, 14 During this period of dual antiplatelet therapy, the premature discontinuation of the thienopyridine may be catastrophic. To guide clinicians in managing these patients in the periprocedural period, recent consensus guidelines recommend the following:1, 12
-
In patients who are expected to need an invasive surgical procedure in the next 12 months, consideration should be given to avoiding drug‐eluting stents.
-
Elective procedures which have an increased risk of bleeding should be deferred for at least 6 weeks after bare metal stent implantation and 12 months after drug‐eluting stent implantation.
-
For patients undergoing a surgical procedure within 6 weeks of bare metal stent implantation and 12 months of drug‐eluting stent implantation, continuation of aspirin and clopidogrel is recommended. If bleeding risk prohibits this, then a cardiologist should be consulted.
-
In patients with a drug‐eluting stent who need to undergo a procedure whereby the thienopyridine needs to be discontinued, aspirin should be continued if at all possible, and the thienopyridine should be resumed as soon as possible after the procedure. It may be reasonable to consider a loading dose of clopidogrel, up to 600 mg, in this setting, although prospective supportive data is lacking.1
It is important to recognize that delayed stent thrombosis is now reported well beyond 1 year after drug‐eluting stent implantation, and that there may not be a diminution in risk after the initial 12 months.1517 Until additional data is available, it seems prudent, if possible, to at least continue aspirin in the periprocedural period in these patients. If bleeding concerns obviate this, then antiplatelet therapy should be discontinued and resumed as soon as possible.
For patients on chronic antiplatelet therapy who do not have a cardiac stent and who are not undergoing a low‐bleeding‐risk procedure, the risks and benefits of the continuation or discontinuation of antiplatelet therapy in the periprocedural period are uncertain as absolute risks in the periprocedural period have not been well studied. Relative risks/benefits, however, can be estimated from prior studies. Aspirin leads to an approximate 25% relative risk reduction in cardiac or thrombotic event rates compared to placebo.14, 18 Although important, the absolute benefit of 1 week of therapy (vs. no therapy during the periprocedural period) is estimated to be small. The small absolute benefit of continued aspirin therapy may be offset by an increase in significant bleeding events. Although, not well studied, continued aspirin increases significant bleeding by 50% with absolute event rates varying by type of procedure.8 In some procedures, such as intracranial surgery or transurethral prostatectomy, this bleeding risk is prohibitive. For others, the risk may be modest and the decision to continue vs. discontinue aspirin therapy may be at the discretion of the person performing the procedure. In general, for most patients who do not have a coronary stent and have not had a recent (past 3 months) myocardial infarction or stroke, discontinuation of antiplatelet therapy 7 to 10 days prior to the procedure seems prudent. The primary exceptions are patients who are undergoing percutaneous coronary intervention or coronary artery bypass grafting. For these procedures continuing aspirin is recommended.1 Figure 1 outlines a proposed management strategy based upon available evidence and guidelines.

Clinical Question #2: Are There Very‐Low‐Bleeding‐Risk Procedures That Do Not Require Interruption of Oral Anticoagulation?
Some procedures are associated with a low‐enough risk of bleeding that it is safe to proceed without interrupting VKA anticoagulation. This approach spares the risk and cost that occur with the holding of oral anticoagulants and institution of bridging therapy. When considering this strategy, it is important that the specialist performing the procedure is included in the discussion. Dental, dermatologic, and cataract procedures are common outpatient procedures that are associated with low bleeding risk. The relative safety of these procedures in patients who are anticoagulated is discussed thoroughly in the ACCP guidelines.1 Other low‐bleeding‐risk procedures for which a hospitalist may be consulted include certain endoscopic procedures, paracentesis, central venous catheter placement, and arthrocentesis.
The American Society for Gastrointestinal Endoscopy has published guidelines recommending that anticoagulation can be safely continued in patients undergoing the following endoscopic procedures with a low bleeding risk: esophagogastroduodenoscopy (EGD), flexible sigmoidoscopy, and colonoscopy, all with or without mucosal biopsy; enteroscopy, biliary/pancreatic stent placement, endoscopic ultrasound without biopsy, and endoscopic retrograde cholangiopancreatography (ERCP) without sphincterotomy.19 Conversely, high‐risk procedures for which interruption of anticoagulation is recommended include polypectomy, biliary sphincterotomy, variceal treatment, percutaneous endoscopic gastrostomy (PEG) placement, dilation of strictures, and endoscopic ultrasound‐guided fine‐needle aspiration.
Limited data suggest that paracentesis, central venous catheter placement, and arthrocentesis may be safe to perform in the setting of anticoagulation. For patients undergoing paracentesis there is little evidence in anticoagulated patients; however, it is probably safe to continue anticoagulation as studies have demonstrated the safety of this procedure in patients with significant thrombocytopenia and coagulopathy.20, 21 Limited data also supports that central venous catheter placement may be safely performed in the setting of abnormal coagulation tests, although some recommend avoiding the subclavian site due to the risk of hemothorax and the inability to apply adequate compression.2226 With regard to arthrocentesis, multiple authors have endorsed the idea that joint and soft‐tissue aspirations and injections present a low risk of serious bleeding even with anticoagulation.2729 This is supported by limited data.30, 31
Other procedures such as lumbar puncture, thoracentesis, and cardiac catheterization are somewhat more controversial in the anticoagulated patient. Anticoagulation should generally be interrupted for lumbar puncture,29, 32 as 1 study involving patients who were started on heparin immediately after the procedure had a 2% incidence of spinal hematoma and 6.7% major complication rate.33 With regard to thoracentesis, evidence is very limited, but experts generally accept that it may be safely performed in patients with mild coagulopathy.34, 35 One frequently‐cited study found no bleeding complications in 57 patients with mild elevation in prothrombin time, which correlated to an International Normalized Ratio of approximately 2.2 or less.36 A recent report also revealed no serious bleeding complications in 33 thoracenteses performed on patients receiving full anticoagulation with warfarin, heparin, and/or low molecular weight heparin.37
Therapeutic anticoagulation has traditionally been felt to be a relative contraindication to cardiac catheterization.38, 39 In spite of this, several observational studies have suggested it may be safely performed using a standard approach,40 using vascular closure devices,41 or using a radial artery approach instead of the more commonly used femoral site.4244 The small size of these observational reports, the diagnostic rather than therapeutic nature of most cases, the limited use of other antithrombotic and antiplatelet medications, and the experience required to use the transradial approach are all major limitations preventing widespread acceptance of cardiac catheterization in therapeutically anticoagulated patients.
In summary, there are numerous procedures that may be safely pursued in the setting of therapeutic anticoagulation. However, for most of these procedures the data is somewhat limited. As such, it is paramount for the hospitalist physician to recognize these clinical scenarios and to discuss management options with the patient and the person performing the procedure, if applicable.
Clinical Question #3: Are There LowThromboembolic‐Risk Populations Who Do Not Require Periprocedural Bridging?
Although it has previously been noted that there is a wide variation of opinion on when and how to perform periprocedural bridging, it is generally agreed that in the following conditions the risk of thrombosis is low enough that bridging with full dose heparin or LMWH is not necessary:1, 5, 4549
-
Atrial fibrillation without previous stroke or transient ischemic attack (TIA) and no more than 2 additional thrombotic risk factors on the CHADS2 scoring system (Table 1).
-
A single venous thromboembolic event that occurred greater than 12 months ago with no ongoing risk factors such as active malignancy, high risk thrombophilia, or the antiphospholipid antibody syndrome.
-
Bileaflet aortic valve without the presence of additional risk factors (ie, patients <75 years of age with the absence of atrial fibrillation, prior stroke or transient ischemic attack, hypertension, diabetes, or congestive heart failure).
| CHADS2 Score* | Annual Risk of Stroke (%) |
|---|---|
| |
| 0 | 1.9 |
| 1 | 2.8 |
| 2 | 4.0 |
| 3 | 5.9 |
| 4 | 8.5 |
| 5 | 12.5 |
| 6 | 18.2 |
Clinical Question #4: How Do You Manage Patients Who Must Discontinue Anticoagulants But Are at an Increased Thrombotic Risk?
When anticoagulation must be held and the patient does not have a very low thromboembolic risk, a decision of whether or not to use bridging anticoagulation must be made. The current ACCP guideline gives grade 1C and 2C recommendations (evidence from observational studies, case series, or controlled trials with serious flaws) regarding for whom and how to implement bridging.1 The grade C designation is due to a lack of high‐quality randomized clinical trials. As such, the clinician must carefully consider an individual patient's estimated thromboembolic risk, procedurally‐related bleeding risk, patient‐related bleeding risk factors, and the patient's values regarding concerns of thromboembolism or bleeding. In these situations it is also imperative that the person performing the procedure is involved in the risk‐to‐benefit discussion.
When evaluating an individual patient's risk of thromboembolism, clinicians sometimes estimate the perioperative risk by prorating the annual incidence of thromboembolic complications to the few days that anticoagulation is withheld.67 Making this extrapolation discounts the effect of a potential increase in thromboembolic risk induced by surgery. As an example, an average patient with atrial fibrillation who has a 5% predicted annual stroke rate would be estimated to have a stroke risk of 0.05% if they are not anticoagulated for 4 days. However, studies have shown that the actual rate of perioperative thromboembolism is approximately 1%.1 With these limitations and uncertainties in mind, and until there is better prospective outcomes data, we must consider relative risks in the context of absolute event rate estimates when deciding a perioperative anticoagulant management plan. The estimated annual incidence of thrombosis without anticoagulation for various indications and the current guideline recommendations are presented in Table 2.
| Practice Guideline Preferred Management Recommendations | ||||
|---|---|---|---|---|
| Indication for chronic anticoagulation | Estimated Annual Thrombotic Risk Without Anticoagulation | ACCP*1 | ACC/AHA45, 46 | British Haematologic Society70 |
| ||||
| Dual prosthetic or older‐generation valve | >10% | Bridge | Bridge | Bridge |
| VTE within 3 months or severe thrombophilias | Bridge | N/A | Bridge | |
| Pregnancy with prosthetic valve | Bridge | Bridge | N/A | |
| Bileaflet valve in the mitral position | Bridge | Bridge | Prophylaxis | |
| Valve with acute embolism <6 months | Bridge | N/A | Bridge | |
| A‐fib valvular or CHADS2 score 5‐6 | Bridge | Consider bridging | N/A | |
| Recurrent venous thromboembolism | 4‐10% | Bridge | N/A | N/A |
| VTE within 3‐12 months or active cancer | Bridge | N/A | Prophylaxis | |
| Bileaflet aortic valve with additional risk factors | Bridge | Bridge | Prophylaxis | |
| A‐fib CHADS2 score 3‐4 | Bridge | Consider bridging | N/A | |
| Bileaflet aortic valve without additional risk factors | <4% | Prophylaxis or no bridging | No bridging | Prophylaxis |
| VTE >12 months | Prophylaxis or no bridging | N/A | Prophylaxis | |
| A‐fib CHADS2 score 0‐2 and no previous CVA/TIA | Prophylaxis or no bridging | No bridging | N/A | |
In addition to thromboembolic risk, we must also consider the bleeding risk associated with the procedure/surgery. Importantly, therapeutic heparin started early in the postoperative period is associated with major bleeding event rates as high as 10% to 20%.1, 50 Once a major bleeding event occurs, this will often lead to an extended interruption of anticoagulant therapy, placing the patient at a more prolonged risk of an associated thromboembolic event. For this reason, the resumption of full‐dose anticoagulation with LMWH/heparin should be delayed for at least 48 hours in most patients undergoing a surgery or procedure associated with an increased risk of bleeding. Examples of these higher‐bleeding‐risk procedures include major thoracic surgery, intracranial or spinal surgery, major vascular surgery, major orthopedic surgery, urologic surgery involving the bladder or prostrate, major oncologic surgery, reconstructive plastic surgery, colonoscopy with associated polypectomy, renal or prostate biopsies, and placement of a cardiac pacemaker/defibrillator.1, 5157
Taken together, these uncertainties surrounding thromboembolic and bleeding risk estimates imply that there are multiple options for periprocedural management. Several studies, many of which included patients with mechanical heart valves, have shown similar safety and efficacy between LMWH and intravenous (IV) unfractionated heparin.5864 Table 3 summarizes these studies. The ACCP recommends bridging with LMWH over IV unfractionated heparin due to equal efficacy and cost savings with LMWH.1 When bridging is used, careful attention must be given to the timing and dose of anticoagulation in both the preoperative and postoperative periods. Table 4 lists dosing of commonly used LMWHs in North America. When using LMWHs in the preprocedural setting it is important to note that unacceptably high levels of anticoagulation remain present when a patient is given a full once‐daily LMWH dose the morning prior to the procedure or when a full‐dose, twice‐daily LMWH dose is given the evening prior to the procedure.65, 66 For this reason, the ACCP recommends administering the last preoperative dose 24 hours before surgery and if full‐dose once‐daily LMWH is used, the dose should be decreased by one‐half on the day before the surgery in order to ensure that no residual anticoagulant effect remains at the time of surgery.
| AuthorReference/Study Type | Number of Patients | Patient Population | Type of Procedure | Bridging Strategy | Major Bleeds | Minor Bleeds | TE Rate |
|---|---|---|---|---|---|---|---|
| |||||||
| Turpie and Douketis63/single arm cohort | 174 | 66% aortic valve; 34% mitral or dual prosthetic valve | Not specified | Enoxaparin 1 mg/kg twice daily | 2.3% | Not specified | None |
| Kovacs et al.61/single arm cohort | 224 | Prosthetic heart valves or a‐fib plus 1 major risk factor | 67 surgical; 157 nonsurgical | Preoperative bridging with dalteparin 200 IU/kg daily; dose reduced to 100 IU/kg on preoperative day 1; restarted at 100 IU/kg on POD 1; dose reduced to 5000 IU daily if high risk for bleeding | 6.7%; 8/15 occurred intraoperatively or <6 hours postoperatively; 2/15 occurred after 4 weeks | Not specified | 3.6%; 6/8 episodes occurred after warfarin held secondary to bleeding; 2/8 thrombotic episodes judged to be due to cardioembolism |
| Douketis et al.59/prospective registry | 650 | A‐fib 58%; mechanical heart valve 33% | 251 surgical; 399 nonsurgical | Dalteparin 100 IU/kg twice daily; held after high bleeding risk procedure and patients with poor hemostasis | 0.92% | 5.9% | 0.6% |
| Spyropolous et al.62/prospective registry; 14 centers in United States and Canada | 901 | UFH: 40% mechanical valves, 33% a‐fib; LMWH: 24% mechanical valve, 40% a‐fib | 394 surgical; 507 nonsurgical | LMWH mostly given twice daily 80%; UFH 20% | 5.5% UFH; 3.3% LMWH | 9.1% UFH; 12.0% LMWH | 2.4% UFH; 0.9% LMWH |
| Dunn et al.66/prospective cohort | 260 | A‐fib 68% or prior DVT 37% (excluding prosthetic heart valves) | 105 surgical; 145 nonsurgical | Enoxaparin 1.5 mg/kg daily | 3.5% overall; minor surgery/procedures 0.9%; major surgery 28% | 42% | 1.9%; 1/5 events occurred after bleeding led to withdrawal of AC |
| Omran et al.77/prospective registry | 779 | Various indications | Major and minor procedures | All patients bridged with enoxaparin; moderate TE risk 1 mg/kg daily; high TE risk 1 mg/kg twice daily | 0.5%; all in high‐risk group | 5.9% | 0 |
| Garcia et al.71/prospective, observational cohort of 101 sites in United States | 1024 patients with 1293 interruptions of AC | A‐fib 53%; VTE 14%; prosthetic valve 13% | Outpatient procedures only | At discretion of provider. Bridging performed in 8.3% of interruptions; 3% a‐fib, 10% VTE, and 29% mechanical valves | 0.6%; 4/6 patients with major bleed received bridging | 1.7%;10/17 patients with minor bleed received bridging | 0.7%; no events in patients who were bridged |
| Wysokinski et al.64/prospective cohort | 345 consecutive patients undergoing 386 procedures | 100% nonvalvular a‐fib | Major and minor surgeries/procedures | Individualized in AC clinic; 52% of patients bridged | 2.7%; no difference whether patient received bridging or not | 3.0%; 10/11 occurred in bridged patients | 1.1%; no difference in bridged vs. nonbridged patients |
| Low Molecular Weight Heparin | Subcutaneous Dose |
|---|---|
| |
| Dalteparin | |
| Low dose (prophylaxis dose) | 5,000 IU once daily |
| Full dose | 100 IU/kg twice daily or 200 IU/kg once daily |
| Enoxaparin | |
| Low dose (prophylaxis dose) | 30 mg twice daily or 40mg daily |
| Full dose | 1 mg/kg twice daily or 1.5 mg/kg once daily |
| Tinzaparin (full dose) | 175 IU/kg once daily |
In the postprocedural setting, timing and dose of anticoagulant is important, as major bleeding with the use of therapeutic anticoagulation can occur in up to 10% to 20% of cases. When restarting anticoagulation after the procedure, it is important to evaluate intraoperative hemostasis and to consider patient‐related factors that may further increase bleeding risk. These include advanced age, concomitant antiplatelet or nonsteroidal antiinflammatory medications, renal insufficiency, placement of spinal/epidural catheter, worsening liver disease, or the presence of other comorbid illnesses such as cancer.30, 67, 68 The ACCP recommends withholding full‐dose anticoagulation for at least 48 to 72 hours in patients who are felt to be at a high risk for postoperative bleeding.1 Figure 2 is a proposed management approach to the use of bridging anticoagulants that is consistent with the 2008 ACCP recommendations.

CONCLUSION
The evaluation and management of patients on long‐term antiplatelet or VKA therapy who require an invasive procedure or surgery is a common, complicated, and controversial area. Importantly, it is an area in which the hospitalist physician must be adept. Although there remain many unanswered clinical questions, an evolving literature base and recent practice guidelines can help guide management decisions.
The management of patients on long‐term antithrombotic therapy (vitamin K antagonists [VKA] or antiplatelet agents) who may require temporary disruption for an invasive procedure is challenging. Management is controversial due to methodologically limited prospective data and varied consensus opinions. Yet periprocedural anticoagulation management is a commonly encountered clinical problem. It is estimated that there are 2.5 million patients on long‐term VKA therapy in North America1 and 41% of the U.S. population over age 40 years is on antiplatelet therapy.2 Further, the need for temporary disruption of these therapies for an invasive procedure is frequent. As an example, in 1 European study, approximately 15% of patients on long‐term VKA required a major surgical procedure in 4 years of follow‐up.3 The role of the hospitalist physician in managing these patients is increasing as hospitalists care for an increasing number of surgical patients and provide periprocedural consultation both in and out of the hospital. Therefore, it is imperative for the hospitalist physician to be proficient in making thoughtful and individualized recommendations on the appropriate management of periprocedural anticoagulants, drawing from the available literature and evidence‐based practice guidelines. Importantly, the Society of Hospital Medicine has cited perioperative management as an important core competency.4
The hospitalist physician is likely to encounter numerous periprocedural scenarios, including the management of antiplatelet agents, identifying low bleeding risk procedures wherein interruption of anticoagulants is unnecessary, and recognizing patients with a low short‐term thromboembolic risk where anticoagulants can be disrupted without the need for heparin or low molecular weight heparin (LMWH) in the periprocedural period (defined as bridging therapy). Further, all other clinical scenarios require both a careful individualized assessment of the patient's risk of periprocedural bleeding and thromboembolism and a thoughtful discussion with all involved parties. This discussion may involve the person performing the procedure, the anesthesiologist, and the patient. The purpose of this work is to explore these relevant areas through a review of the literature with a particular focus on the recently published 2008 American College of Chest Physicians (ACCP) evidence‐based clinical practice guidelines.
We reviewed medical literature from 1990 through May 2008 with the following key words: bridging, anticoagulation, perioperative, antiplatelet, heparin, and low molecular weight heparin. Individual studies were then independently reviewed by the authors. Studies that were felt relevant to a hospitalist physician were retrieved and reviewed. If there was uncertainty regarding applicability to a hospitalist setting, a second author's opinion was rendered. Additionally, we reviewed 1 author's personal reference list of articles relating to periprocedural anticoagulation that has been compiled over the past 10 years. This list and the reference lists of retrieved articles were also reviewed. Data were summarized to answer 4 clinically relevant questions:
-
What is the optimal management of antiplatelet therapy in the periprocedural period?
-
Are there very low‐bleeding risk procedures that do not require interruption of oral anticoagulation?
-
Are there low thromboembolic risk populations who do not require periprocedural bridging?
-
How do you manage patients who must discontinue anticoagulants but are at an increased thrombotic risk?
Clinical Question #1: What Is the Optimal Management of Antiplatelet Therapy in the Periprocedural Period?
The optimal management of oral antiplatelet therapy in the periprocedural period is not well studied. Most reviews, expert recommendations, and consensus statements either do not comment on periprocedural antiplatelet management or recommend the routine discontinuation of therapy at least 7 days prior to surgery.3, 5, 6 However, as the 2008 ACCP guidelines highlight, the recommendation to routinely discontinue antiplatelet therapy 7 days prior to the procedure is an oversimplification.1 In the era of both bare metal cardiac stents and drug‐eluting stents, the optimal management of these patients requires that 2 primary questions be asked: (1) Is this a low‐bleeding risk procedure whereby antiplatelet therapy can be continued? (2) Does the patient have a coronary stent whereby the continuation of antiplatelet therapy or delay of the intervention is necessary?
In the context of ongoing aspirin therapy, certain procedures have a low risk of significant hemorrhagic complications. These low bleeding risk procedures include cataract surgery, cutaneous surgery, oral surgery, and endoscopic procedures, including those with mucosal biopsies.710 Patients undergoing these procedures may safely continue low dose aspirin therapy, especially if they have a high‐risk indication for their aspirin such as recent myocardial infarction, stroke, or the presence of a coronary stent.5, 710 Whether these procedures can be safely performed in the setting of a thienopyridine or combination antiplatelet therapy is uncertain.
In the past several decades, the management of obstructive coronary artery disease has undergone a major evolution. Placement of coronary stents has become commonplace, and there are now several million patients with drug‐eluting stents.11 The major complication of these devices is stent thrombosis, which results in death or myocardial infarction in up to 64% of patients.12 Fortunately, dual antiplatelet therapy (aspirin and a thienopyridine such as clopidogrel) markedly reduces this risk.13 Current guidelines recommend using combination antiplatelet therapy for at least 4 to 6 weeks and ideally up to 12 months after placement of a bare metal stent and at least 12 months after placement of either a sirolimus‐ or paclitaxel‐eluting stent.1, 14 During this period of dual antiplatelet therapy, the premature discontinuation of the thienopyridine may be catastrophic. To guide clinicians in managing these patients in the periprocedural period, recent consensus guidelines recommend the following:1, 12
-
In patients who are expected to need an invasive surgical procedure in the next 12 months, consideration should be given to avoiding drug‐eluting stents.
-
Elective procedures which have an increased risk of bleeding should be deferred for at least 6 weeks after bare metal stent implantation and 12 months after drug‐eluting stent implantation.
-
For patients undergoing a surgical procedure within 6 weeks of bare metal stent implantation and 12 months of drug‐eluting stent implantation, continuation of aspirin and clopidogrel is recommended. If bleeding risk prohibits this, then a cardiologist should be consulted.
-
In patients with a drug‐eluting stent who need to undergo a procedure whereby the thienopyridine needs to be discontinued, aspirin should be continued if at all possible, and the thienopyridine should be resumed as soon as possible after the procedure. It may be reasonable to consider a loading dose of clopidogrel, up to 600 mg, in this setting, although prospective supportive data is lacking.1
It is important to recognize that delayed stent thrombosis is now reported well beyond 1 year after drug‐eluting stent implantation, and that there may not be a diminution in risk after the initial 12 months.1517 Until additional data is available, it seems prudent, if possible, to at least continue aspirin in the periprocedural period in these patients. If bleeding concerns obviate this, then antiplatelet therapy should be discontinued and resumed as soon as possible.
For patients on chronic antiplatelet therapy who do not have a cardiac stent and who are not undergoing a low‐bleeding‐risk procedure, the risks and benefits of the continuation or discontinuation of antiplatelet therapy in the periprocedural period are uncertain as absolute risks in the periprocedural period have not been well studied. Relative risks/benefits, however, can be estimated from prior studies. Aspirin leads to an approximate 25% relative risk reduction in cardiac or thrombotic event rates compared to placebo.14, 18 Although important, the absolute benefit of 1 week of therapy (vs. no therapy during the periprocedural period) is estimated to be small. The small absolute benefit of continued aspirin therapy may be offset by an increase in significant bleeding events. Although, not well studied, continued aspirin increases significant bleeding by 50% with absolute event rates varying by type of procedure.8 In some procedures, such as intracranial surgery or transurethral prostatectomy, this bleeding risk is prohibitive. For others, the risk may be modest and the decision to continue vs. discontinue aspirin therapy may be at the discretion of the person performing the procedure. In general, for most patients who do not have a coronary stent and have not had a recent (past 3 months) myocardial infarction or stroke, discontinuation of antiplatelet therapy 7 to 10 days prior to the procedure seems prudent. The primary exceptions are patients who are undergoing percutaneous coronary intervention or coronary artery bypass grafting. For these procedures continuing aspirin is recommended.1 Figure 1 outlines a proposed management strategy based upon available evidence and guidelines.

Clinical Question #2: Are There Very‐Low‐Bleeding‐Risk Procedures That Do Not Require Interruption of Oral Anticoagulation?
Some procedures are associated with a low‐enough risk of bleeding that it is safe to proceed without interrupting VKA anticoagulation. This approach spares the risk and cost that occur with the holding of oral anticoagulants and institution of bridging therapy. When considering this strategy, it is important that the specialist performing the procedure is included in the discussion. Dental, dermatologic, and cataract procedures are common outpatient procedures that are associated with low bleeding risk. The relative safety of these procedures in patients who are anticoagulated is discussed thoroughly in the ACCP guidelines.1 Other low‐bleeding‐risk procedures for which a hospitalist may be consulted include certain endoscopic procedures, paracentesis, central venous catheter placement, and arthrocentesis.
The American Society for Gastrointestinal Endoscopy has published guidelines recommending that anticoagulation can be safely continued in patients undergoing the following endoscopic procedures with a low bleeding risk: esophagogastroduodenoscopy (EGD), flexible sigmoidoscopy, and colonoscopy, all with or without mucosal biopsy; enteroscopy, biliary/pancreatic stent placement, endoscopic ultrasound without biopsy, and endoscopic retrograde cholangiopancreatography (ERCP) without sphincterotomy.19 Conversely, high‐risk procedures for which interruption of anticoagulation is recommended include polypectomy, biliary sphincterotomy, variceal treatment, percutaneous endoscopic gastrostomy (PEG) placement, dilation of strictures, and endoscopic ultrasound‐guided fine‐needle aspiration.
Limited data suggest that paracentesis, central venous catheter placement, and arthrocentesis may be safe to perform in the setting of anticoagulation. For patients undergoing paracentesis there is little evidence in anticoagulated patients; however, it is probably safe to continue anticoagulation as studies have demonstrated the safety of this procedure in patients with significant thrombocytopenia and coagulopathy.20, 21 Limited data also supports that central venous catheter placement may be safely performed in the setting of abnormal coagulation tests, although some recommend avoiding the subclavian site due to the risk of hemothorax and the inability to apply adequate compression.2226 With regard to arthrocentesis, multiple authors have endorsed the idea that joint and soft‐tissue aspirations and injections present a low risk of serious bleeding even with anticoagulation.2729 This is supported by limited data.30, 31
Other procedures such as lumbar puncture, thoracentesis, and cardiac catheterization are somewhat more controversial in the anticoagulated patient. Anticoagulation should generally be interrupted for lumbar puncture,29, 32 as 1 study involving patients who were started on heparin immediately after the procedure had a 2% incidence of spinal hematoma and 6.7% major complication rate.33 With regard to thoracentesis, evidence is very limited, but experts generally accept that it may be safely performed in patients with mild coagulopathy.34, 35 One frequently‐cited study found no bleeding complications in 57 patients with mild elevation in prothrombin time, which correlated to an International Normalized Ratio of approximately 2.2 or less.36 A recent report also revealed no serious bleeding complications in 33 thoracenteses performed on patients receiving full anticoagulation with warfarin, heparin, and/or low molecular weight heparin.37
Therapeutic anticoagulation has traditionally been felt to be a relative contraindication to cardiac catheterization.38, 39 In spite of this, several observational studies have suggested it may be safely performed using a standard approach,40 using vascular closure devices,41 or using a radial artery approach instead of the more commonly used femoral site.4244 The small size of these observational reports, the diagnostic rather than therapeutic nature of most cases, the limited use of other antithrombotic and antiplatelet medications, and the experience required to use the transradial approach are all major limitations preventing widespread acceptance of cardiac catheterization in therapeutically anticoagulated patients.
In summary, there are numerous procedures that may be safely pursued in the setting of therapeutic anticoagulation. However, for most of these procedures the data is somewhat limited. As such, it is paramount for the hospitalist physician to recognize these clinical scenarios and to discuss management options with the patient and the person performing the procedure, if applicable.
Clinical Question #3: Are There LowThromboembolic‐Risk Populations Who Do Not Require Periprocedural Bridging?
Although it has previously been noted that there is a wide variation of opinion on when and how to perform periprocedural bridging, it is generally agreed that in the following conditions the risk of thrombosis is low enough that bridging with full dose heparin or LMWH is not necessary:1, 5, 4549
-
Atrial fibrillation without previous stroke or transient ischemic attack (TIA) and no more than 2 additional thrombotic risk factors on the CHADS2 scoring system (Table 1).
-
A single venous thromboembolic event that occurred greater than 12 months ago with no ongoing risk factors such as active malignancy, high risk thrombophilia, or the antiphospholipid antibody syndrome.
-
Bileaflet aortic valve without the presence of additional risk factors (ie, patients <75 years of age with the absence of atrial fibrillation, prior stroke or transient ischemic attack, hypertension, diabetes, or congestive heart failure).
| CHADS2 Score* | Annual Risk of Stroke (%) |
|---|---|
| |
| 0 | 1.9 |
| 1 | 2.8 |
| 2 | 4.0 |
| 3 | 5.9 |
| 4 | 8.5 |
| 5 | 12.5 |
| 6 | 18.2 |
Clinical Question #4: How Do You Manage Patients Who Must Discontinue Anticoagulants But Are at an Increased Thrombotic Risk?
When anticoagulation must be held and the patient does not have a very low thromboembolic risk, a decision of whether or not to use bridging anticoagulation must be made. The current ACCP guideline gives grade 1C and 2C recommendations (evidence from observational studies, case series, or controlled trials with serious flaws) regarding for whom and how to implement bridging.1 The grade C designation is due to a lack of high‐quality randomized clinical trials. As such, the clinician must carefully consider an individual patient's estimated thromboembolic risk, procedurally‐related bleeding risk, patient‐related bleeding risk factors, and the patient's values regarding concerns of thromboembolism or bleeding. In these situations it is also imperative that the person performing the procedure is involved in the risk‐to‐benefit discussion.
When evaluating an individual patient's risk of thromboembolism, clinicians sometimes estimate the perioperative risk by prorating the annual incidence of thromboembolic complications to the few days that anticoagulation is withheld.67 Making this extrapolation discounts the effect of a potential increase in thromboembolic risk induced by surgery. As an example, an average patient with atrial fibrillation who has a 5% predicted annual stroke rate would be estimated to have a stroke risk of 0.05% if they are not anticoagulated for 4 days. However, studies have shown that the actual rate of perioperative thromboembolism is approximately 1%.1 With these limitations and uncertainties in mind, and until there is better prospective outcomes data, we must consider relative risks in the context of absolute event rate estimates when deciding a perioperative anticoagulant management plan. The estimated annual incidence of thrombosis without anticoagulation for various indications and the current guideline recommendations are presented in Table 2.
| Practice Guideline Preferred Management Recommendations | ||||
|---|---|---|---|---|
| Indication for chronic anticoagulation | Estimated Annual Thrombotic Risk Without Anticoagulation | ACCP*1 | ACC/AHA45, 46 | British Haematologic Society70 |
| ||||
| Dual prosthetic or older‐generation valve | >10% | Bridge | Bridge | Bridge |
| VTE within 3 months or severe thrombophilias | Bridge | N/A | Bridge | |
| Pregnancy with prosthetic valve | Bridge | Bridge | N/A | |
| Bileaflet valve in the mitral position | Bridge | Bridge | Prophylaxis | |
| Valve with acute embolism <6 months | Bridge | N/A | Bridge | |
| A‐fib valvular or CHADS2 score 5‐6 | Bridge | Consider bridging | N/A | |
| Recurrent venous thromboembolism | 4‐10% | Bridge | N/A | N/A |
| VTE within 3‐12 months or active cancer | Bridge | N/A | Prophylaxis | |
| Bileaflet aortic valve with additional risk factors | Bridge | Bridge | Prophylaxis | |
| A‐fib CHADS2 score 3‐4 | Bridge | Consider bridging | N/A | |
| Bileaflet aortic valve without additional risk factors | <4% | Prophylaxis or no bridging | No bridging | Prophylaxis |
| VTE >12 months | Prophylaxis or no bridging | N/A | Prophylaxis | |
| A‐fib CHADS2 score 0‐2 and no previous CVA/TIA | Prophylaxis or no bridging | No bridging | N/A | |
In addition to thromboembolic risk, we must also consider the bleeding risk associated with the procedure/surgery. Importantly, therapeutic heparin started early in the postoperative period is associated with major bleeding event rates as high as 10% to 20%.1, 50 Once a major bleeding event occurs, this will often lead to an extended interruption of anticoagulant therapy, placing the patient at a more prolonged risk of an associated thromboembolic event. For this reason, the resumption of full‐dose anticoagulation with LMWH/heparin should be delayed for at least 48 hours in most patients undergoing a surgery or procedure associated with an increased risk of bleeding. Examples of these higher‐bleeding‐risk procedures include major thoracic surgery, intracranial or spinal surgery, major vascular surgery, major orthopedic surgery, urologic surgery involving the bladder or prostrate, major oncologic surgery, reconstructive plastic surgery, colonoscopy with associated polypectomy, renal or prostate biopsies, and placement of a cardiac pacemaker/defibrillator.1, 5157
Taken together, these uncertainties surrounding thromboembolic and bleeding risk estimates imply that there are multiple options for periprocedural management. Several studies, many of which included patients with mechanical heart valves, have shown similar safety and efficacy between LMWH and intravenous (IV) unfractionated heparin.5864 Table 3 summarizes these studies. The ACCP recommends bridging with LMWH over IV unfractionated heparin due to equal efficacy and cost savings with LMWH.1 When bridging is used, careful attention must be given to the timing and dose of anticoagulation in both the preoperative and postoperative periods. Table 4 lists dosing of commonly used LMWHs in North America. When using LMWHs in the preprocedural setting it is important to note that unacceptably high levels of anticoagulation remain present when a patient is given a full once‐daily LMWH dose the morning prior to the procedure or when a full‐dose, twice‐daily LMWH dose is given the evening prior to the procedure.65, 66 For this reason, the ACCP recommends administering the last preoperative dose 24 hours before surgery and if full‐dose once‐daily LMWH is used, the dose should be decreased by one‐half on the day before the surgery in order to ensure that no residual anticoagulant effect remains at the time of surgery.
| AuthorReference/Study Type | Number of Patients | Patient Population | Type of Procedure | Bridging Strategy | Major Bleeds | Minor Bleeds | TE Rate |
|---|---|---|---|---|---|---|---|
| |||||||
| Turpie and Douketis63/single arm cohort | 174 | 66% aortic valve; 34% mitral or dual prosthetic valve | Not specified | Enoxaparin 1 mg/kg twice daily | 2.3% | Not specified | None |
| Kovacs et al.61/single arm cohort | 224 | Prosthetic heart valves or a‐fib plus 1 major risk factor | 67 surgical; 157 nonsurgical | Preoperative bridging with dalteparin 200 IU/kg daily; dose reduced to 100 IU/kg on preoperative day 1; restarted at 100 IU/kg on POD 1; dose reduced to 5000 IU daily if high risk for bleeding | 6.7%; 8/15 occurred intraoperatively or <6 hours postoperatively; 2/15 occurred after 4 weeks | Not specified | 3.6%; 6/8 episodes occurred after warfarin held secondary to bleeding; 2/8 thrombotic episodes judged to be due to cardioembolism |
| Douketis et al.59/prospective registry | 650 | A‐fib 58%; mechanical heart valve 33% | 251 surgical; 399 nonsurgical | Dalteparin 100 IU/kg twice daily; held after high bleeding risk procedure and patients with poor hemostasis | 0.92% | 5.9% | 0.6% |
| Spyropolous et al.62/prospective registry; 14 centers in United States and Canada | 901 | UFH: 40% mechanical valves, 33% a‐fib; LMWH: 24% mechanical valve, 40% a‐fib | 394 surgical; 507 nonsurgical | LMWH mostly given twice daily 80%; UFH 20% | 5.5% UFH; 3.3% LMWH | 9.1% UFH; 12.0% LMWH | 2.4% UFH; 0.9% LMWH |
| Dunn et al.66/prospective cohort | 260 | A‐fib 68% or prior DVT 37% (excluding prosthetic heart valves) | 105 surgical; 145 nonsurgical | Enoxaparin 1.5 mg/kg daily | 3.5% overall; minor surgery/procedures 0.9%; major surgery 28% | 42% | 1.9%; 1/5 events occurred after bleeding led to withdrawal of AC |
| Omran et al.77/prospective registry | 779 | Various indications | Major and minor procedures | All patients bridged with enoxaparin; moderate TE risk 1 mg/kg daily; high TE risk 1 mg/kg twice daily | 0.5%; all in high‐risk group | 5.9% | 0 |
| Garcia et al.71/prospective, observational cohort of 101 sites in United States | 1024 patients with 1293 interruptions of AC | A‐fib 53%; VTE 14%; prosthetic valve 13% | Outpatient procedures only | At discretion of provider. Bridging performed in 8.3% of interruptions; 3% a‐fib, 10% VTE, and 29% mechanical valves | 0.6%; 4/6 patients with major bleed received bridging | 1.7%;10/17 patients with minor bleed received bridging | 0.7%; no events in patients who were bridged |
| Wysokinski et al.64/prospective cohort | 345 consecutive patients undergoing 386 procedures | 100% nonvalvular a‐fib | Major and minor surgeries/procedures | Individualized in AC clinic; 52% of patients bridged | 2.7%; no difference whether patient received bridging or not | 3.0%; 10/11 occurred in bridged patients | 1.1%; no difference in bridged vs. nonbridged patients |
| Low Molecular Weight Heparin | Subcutaneous Dose |
|---|---|
| |
| Dalteparin | |
| Low dose (prophylaxis dose) | 5,000 IU once daily |
| Full dose | 100 IU/kg twice daily or 200 IU/kg once daily |
| Enoxaparin | |
| Low dose (prophylaxis dose) | 30 mg twice daily or 40mg daily |
| Full dose | 1 mg/kg twice daily or 1.5 mg/kg once daily |
| Tinzaparin (full dose) | 175 IU/kg once daily |
In the postprocedural setting, timing and dose of anticoagulant is important, as major bleeding with the use of therapeutic anticoagulation can occur in up to 10% to 20% of cases. When restarting anticoagulation after the procedure, it is important to evaluate intraoperative hemostasis and to consider patient‐related factors that may further increase bleeding risk. These include advanced age, concomitant antiplatelet or nonsteroidal antiinflammatory medications, renal insufficiency, placement of spinal/epidural catheter, worsening liver disease, or the presence of other comorbid illnesses such as cancer.30, 67, 68 The ACCP recommends withholding full‐dose anticoagulation for at least 48 to 72 hours in patients who are felt to be at a high risk for postoperative bleeding.1 Figure 2 is a proposed management approach to the use of bridging anticoagulants that is consistent with the 2008 ACCP recommendations.

CONCLUSION
The evaluation and management of patients on long‐term antiplatelet or VKA therapy who require an invasive procedure or surgery is a common, complicated, and controversial area. Importantly, it is an area in which the hospitalist physician must be adept. Although there remain many unanswered clinical questions, an evolving literature base and recent practice guidelines can help guide management decisions.
- ,,, et al.The Perioperative Management of Antithrombotic Therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6_suppl):299S–339S.
- ,,,,.Aspirin use among adults aged 40 and older in the United States: results of a national survey.Am J Prev Med.2007;32(5):403–407.
- ,.Oral anticoagulation in surgical procedures: risks and recommendations.Br J Haematol.2003;123(4):676–682.
- Perioperative medicine.J Hosp Med.2006;1(supp 1):30–31.
- .Perioperative anticoagulation management in patients who are receiving oral anticoagulant therapy: a practical guide for clinicians.Thromb Res.2002;108(1):3–13.
- ,,,.Periprocedural bridging therapy in patients receiving chronic oral anticoagulation therapy.Curr Med Res Opin.2006;22(6):1109–1122.
- ,,,,.Effect of aspirin intake on bleeding during cataract surgery.J Cataract Refract Surg.1998;24(9):1243–1246.
- ,,,.Low‐dose aspirin for secondary cardiovascular prevention–cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation—review and meta‐analysis.J Intern Med.2005;257(5):399–414.
- ,,.Aspirin does not increase bleeding complications after transbronchial biopsy.Chest.2002;122(4):1461–1464.
- ,.The management of patients on anticoagulants prior to cutaneous surgery: case report of a thromboembolic complication, review of the literature, and evidence‐based recommendations.Plast Reconstr Surg.2006;118(5):110e–117e.
- .Unanswered questions—drug‐eluting stents and the risk of late thrombosis.N Engl J Med.2007;356(10):981–984.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115(6):813–818.
- ,,, et al.The Primary and Secondary Prevention of Coronary Artery Disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6_suppl):776S–814S.
- ,,, et al.2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee.Circulation.2008;117(2):261–295.
- ,,,,,.Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials.Am J Med.2006;119(12):1056–1061.
- ,,, et al.Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study.Lancet.2007;369(9562):667–678.
- ,,, et al.Clopidogrel use and long‐term clinical outcomes after drug‐eluting stent implantation.JAMA.2007;297(2):159–168.
- Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.Antiplatelet Trialists' Collaboration.Br Med J (Clin Res Ed).1994;308(6921):81–106.
- ,,, et al.Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures.Gastrointest Endosc.2002;55(7):775–779.
- ,.Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease.Aliment Pharmacol Ther.2005;21(5):525–529.
- ,,, et al.Performance standards for therapeutic abdominal paracentesis.Hepatology.2004;40(2):484–488.
- ,.Percutaneous cannulation of the internal jugular vein in patients with coagulopathies: an experience based on 1,000 attempts.Anesthesiology.1982;56(4):321–323.
- ,,,,,.Central venous catheterization in patients with coagulopathy.Arch Surg.1992;127(3):273–275.
- ,,.Central venous catheter placement in patients with disorders of hemostasis.Chest.1996;110(1):185–188.
- ,,.US‐guided placement of central vein catheters in patients with disorders of hemostasis.Eur J Radiol.2008;65(2):253–256.
- ,.Central venous cannulation in patients with liver disease and coagulopathy—a prospective audit.Intensive Care Med.1999;25(5):481–485.
- ,.Perioperative management of patients receiving oral anticoagulants: a systematic review.Arch Intern Med.2003;163(8):901–908.
- ,,, et al.Kelley's Textbook of Rheumatology.7th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,.Clinical Procedures in Emergency Medicine.4th ed.Philadelphia, PA:W.B. Saunders;2004.
- ,.A prospective study of the safety of joint and soft tissue aspirations and injections in patients taking warfarin sodium.Arthritis Rheum.1998;41(4):736–739.
- ,,, et al.[Frequency of the bleeding risk in patients receiving warfarin submitted to arthrocentesis of the knee].Reumatismo.2003;55(3):159–163.
- .Textbook of Critical Care.5th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,.Complications of lumbar puncture followed by anticoagulation.Stroke.1981;12(6):879–881.
- ,,,.Mason, Murray and Nadel's Textbook of Respiratory Medicine.4th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,,.Videos in clinical medicine. Thoracentesis.N Engl J Med.2006;355(15):e16.
- ,.Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities.Transfusion.1991;31(2):164–171.
- .Risk of bleeding during thoracentesis in anticoagulated patients.Chest.2006;130(4):141S–d‐2.
- ,,, et al.Guidelines for prevention of thromboembolic events in valvular heart disease. Study Group of the Working Group on Valvular Heart Disease of the European Society of Cardiology.Eur Heart J.1995;16(10):1320–1330.
- ,.Coronary angiography and intravascular ultrasonography. In: Braunwald E, Zipes DP, Libby P, eds.Heart Disease: A Textbook of Cardiovascular Medicine.6th ed.Philadelphia, PA:WB Saunders;2001:387–421.
- ,,, et al.Effectiveness of manual pressure hemostasis following transfemoral coronary angiography in patients on therapeutic warfarin anticoagulation.Am J Cardiol.2006;97(4):485–488.
- ,,,,,.Elective coronary angiography and percutaneous coronary intervention during uninterrupted warfarin therapy.Catheter Cardiovasc Interv.2003;60(2):180–184.
- ,,,.Coronary angiography in the fully anticoagulated patient: the transradial route is successful and safe.Catheter Cardiovasc Interv.2003;58(1):8–10.
- ,,,,.Percutaneous left and right heart catheterization in fully anticoagulated patients utilizing the radial artery and forearm vein: a two‐center experience.J Interv Cardiol.2006;19(3):258–263.
- ,,, et al.[Safety of diagnostic transradial catheterization in patients undergoing long‐term anticoagulation with coumarin derivatives].Rev Esp Cardiol.2007;60(9):988–991. [Spanish]
- ,,,,,,,,,,,,,2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Valvular Heart Disease).J Am Coll Cardiol.2008;52:e1–142.
- ,,, et al.ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in Collaboration With the North American Society of Pacing and Electrophysiology.J Am Coll Cardiol.2001;38(4):1231–1266.
- .Periprocedural thromboprophylaxis in patients receiving chronic anticoagulation therapy.Am Heart J.2004;147(1):3–15.
- ,.Management of anticoagulation before and after elective surgery.N Engl J Med.1997;336(21):1506–1511.
- ,,,,,.Modern management of prosthetic valve anticoagulation.Mayo Clin Proc.1998;73(7):665–680.
- ,.Anticoagulant‐related bleeding: clinical epidemiology, prediction, and prevention.Am J Med.1993;95(3):315–328.
- ,,,,,.Reconstruction of musculoskeletal defects following oncologic resection in 76 patients.Ann Plast Surg.2006;57(2):190–194.
- ,,,,.Biopsy of the prostate guided by transrectal ultrasound: relation between warfarin use and incidence of bleeding complications.Clin Radiol.2005;60(4):459–463; discussion 457‐458.
- ,.Anticoagulation in neurosurgical patients.Neurosurgery.1999;45(4):838–847; discussion 847‐848.
- ,,,,.Post‐operative blood loss after transurethral prostatectomy is dependent on in situ fibrinolysis.Br J Urol.1997;80(6):889–893.
- ,,.Complications of heparin therapy after total joint arthroplasty.J Bone Joint Surg.1989;71(8):1130–1134.
- ,,,,,.Postpolypectomy lower GI bleeding: descriptive analysis.Gastrointest Endosc.2000;51(6):690–696.
- ,,, et al.Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy.Chest.2004;126(4):1177–1186.
- ,,.[Perioperative bridging with enoxaparin. results of the prospective BRAVE registry with 779 patients].Med Klin (Munich).2007;102(10):809–815. [German]
- ,,.Low molecular weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen.Arch Intern Med.2004;164(12):1319–1326.
- ,,.Bridging therapy in patients on long‐term oral anticoagulants who require surgery: the Prospective Peri‐operative Enoxaparin Cohort Trial (PROSPECT).J Thromb Haemost.2007;5(11):2211–2218.
- ,,, et al.Single‐arm study of bridging therapy with low molecular weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin.Circulation.2004;110(12):1658–1663.
- ,,,.Costs and clinical outcomes associated with low molecular weight heparin vs unfractionated heparin for perioperative bridging in patients receiving long‐term oral anticoagulant therapy.Chest.2004;125(5):1642–1650.
- ,.Enoxaparin is effective and safe as bridging anticoagulation in patients with a mechanical prosthetic heart valve who require temporary interruption of warfarin because of surgery or an invasive procedure. [Abstract #703]. ASH Annual Meeting Abstracts.Blood.2004;104:202A.
- ,,, et al.Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation.Mayo Clin Proc.2008;83(6):639–645.
- ,,,.Bridging anticoagulation with low molecular weight heparin after interruption of warfarin therapy is associated with a residual anticoagulant effect prior to surgery.Thromb Haemost.2005;94(3):528–531.
- ,,, et al.Brief communication: preoperative anticoagulant activity after bridging low molecular weight heparin for temporary interruption of warfarin.Ann Intern Med.2007;146(3):184–187.
- National Institute for Clinical Excellence. Atrial fibrillation: national clinical guideline for management in primary and secondary care.2006. Available at: http://www.nice.org.uk/nicemedia/pdf/cg036fullguideline. pdf. Accessed May 2009.
- ,,, et al.Clinical outcomes with unfractionated heparin or low molecular weight heparin as bridging therapy in patients on long‐term oral anticoagulants: the REGIMEN registry.J Thromb Haemost.2006;4(6):1246–1252.
- ,,,,,.Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation.JAMA.2001;285(22):2864–2870.
- ,,.Guidelines on oral anticoagulation (warfarin). 3rd ed. 2005 update.Br J Haematol.2006;132(3):277–285.
- ,,, et al.Risk of thromboembolism with short‐term interruption of warfarin therapy.Arch Intern Med.2008;168(1):63–69.
- ,,, et al.The Perioperative Management of Antithrombotic Therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6_suppl):299S–339S.
- ,,,,.Aspirin use among adults aged 40 and older in the United States: results of a national survey.Am J Prev Med.2007;32(5):403–407.
- ,.Oral anticoagulation in surgical procedures: risks and recommendations.Br J Haematol.2003;123(4):676–682.
- Perioperative medicine.J Hosp Med.2006;1(supp 1):30–31.
- .Perioperative anticoagulation management in patients who are receiving oral anticoagulant therapy: a practical guide for clinicians.Thromb Res.2002;108(1):3–13.
- ,,,.Periprocedural bridging therapy in patients receiving chronic oral anticoagulation therapy.Curr Med Res Opin.2006;22(6):1109–1122.
- ,,,,.Effect of aspirin intake on bleeding during cataract surgery.J Cataract Refract Surg.1998;24(9):1243–1246.
- ,,,.Low‐dose aspirin for secondary cardiovascular prevention–cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation—review and meta‐analysis.J Intern Med.2005;257(5):399–414.
- ,,.Aspirin does not increase bleeding complications after transbronchial biopsy.Chest.2002;122(4):1461–1464.
- ,.The management of patients on anticoagulants prior to cutaneous surgery: case report of a thromboembolic complication, review of the literature, and evidence‐based recommendations.Plast Reconstr Surg.2006;118(5):110e–117e.
- .Unanswered questions—drug‐eluting stents and the risk of late thrombosis.N Engl J Med.2007;356(10):981–984.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115(6):813–818.
- ,,, et al.The Primary and Secondary Prevention of Coronary Artery Disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6_suppl):776S–814S.
- ,,, et al.2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee.Circulation.2008;117(2):261–295.
- ,,,,,.Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials.Am J Med.2006;119(12):1056–1061.
- ,,, et al.Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study.Lancet.2007;369(9562):667–678.
- ,,, et al.Clopidogrel use and long‐term clinical outcomes after drug‐eluting stent implantation.JAMA.2007;297(2):159–168.
- Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.Antiplatelet Trialists' Collaboration.Br Med J (Clin Res Ed).1994;308(6921):81–106.
- ,,, et al.Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures.Gastrointest Endosc.2002;55(7):775–779.
- ,.Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease.Aliment Pharmacol Ther.2005;21(5):525–529.
- ,,, et al.Performance standards for therapeutic abdominal paracentesis.Hepatology.2004;40(2):484–488.
- ,.Percutaneous cannulation of the internal jugular vein in patients with coagulopathies: an experience based on 1,000 attempts.Anesthesiology.1982;56(4):321–323.
- ,,,,,.Central venous catheterization in patients with coagulopathy.Arch Surg.1992;127(3):273–275.
- ,,.Central venous catheter placement in patients with disorders of hemostasis.Chest.1996;110(1):185–188.
- ,,.US‐guided placement of central vein catheters in patients with disorders of hemostasis.Eur J Radiol.2008;65(2):253–256.
- ,.Central venous cannulation in patients with liver disease and coagulopathy—a prospective audit.Intensive Care Med.1999;25(5):481–485.
- ,.Perioperative management of patients receiving oral anticoagulants: a systematic review.Arch Intern Med.2003;163(8):901–908.
- ,,, et al.Kelley's Textbook of Rheumatology.7th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,.Clinical Procedures in Emergency Medicine.4th ed.Philadelphia, PA:W.B. Saunders;2004.
- ,.A prospective study of the safety of joint and soft tissue aspirations and injections in patients taking warfarin sodium.Arthritis Rheum.1998;41(4):736–739.
- ,,, et al.[Frequency of the bleeding risk in patients receiving warfarin submitted to arthrocentesis of the knee].Reumatismo.2003;55(3):159–163.
- .Textbook of Critical Care.5th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,.Complications of lumbar puncture followed by anticoagulation.Stroke.1981;12(6):879–881.
- ,,,.Mason, Murray and Nadel's Textbook of Respiratory Medicine.4th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,,.Videos in clinical medicine. Thoracentesis.N Engl J Med.2006;355(15):e16.
- ,.Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities.Transfusion.1991;31(2):164–171.
- .Risk of bleeding during thoracentesis in anticoagulated patients.Chest.2006;130(4):141S–d‐2.
- ,,, et al.Guidelines for prevention of thromboembolic events in valvular heart disease. Study Group of the Working Group on Valvular Heart Disease of the European Society of Cardiology.Eur Heart J.1995;16(10):1320–1330.
- ,.Coronary angiography and intravascular ultrasonography. In: Braunwald E, Zipes DP, Libby P, eds.Heart Disease: A Textbook of Cardiovascular Medicine.6th ed.Philadelphia, PA:WB Saunders;2001:387–421.
- ,,, et al.Effectiveness of manual pressure hemostasis following transfemoral coronary angiography in patients on therapeutic warfarin anticoagulation.Am J Cardiol.2006;97(4):485–488.
- ,,,,,.Elective coronary angiography and percutaneous coronary intervention during uninterrupted warfarin therapy.Catheter Cardiovasc Interv.2003;60(2):180–184.
- ,,,.Coronary angiography in the fully anticoagulated patient: the transradial route is successful and safe.Catheter Cardiovasc Interv.2003;58(1):8–10.
- ,,,,.Percutaneous left and right heart catheterization in fully anticoagulated patients utilizing the radial artery and forearm vein: a two‐center experience.J Interv Cardiol.2006;19(3):258–263.
- ,,, et al.[Safety of diagnostic transradial catheterization in patients undergoing long‐term anticoagulation with coumarin derivatives].Rev Esp Cardiol.2007;60(9):988–991. [Spanish]
- ,,,,,,,,,,,,,2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Valvular Heart Disease).J Am Coll Cardiol.2008;52:e1–142.
- ,,, et al.ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in Collaboration With the North American Society of Pacing and Electrophysiology.J Am Coll Cardiol.2001;38(4):1231–1266.
- .Periprocedural thromboprophylaxis in patients receiving chronic anticoagulation therapy.Am Heart J.2004;147(1):3–15.
- ,.Management of anticoagulation before and after elective surgery.N Engl J Med.1997;336(21):1506–1511.
- ,,,,,.Modern management of prosthetic valve anticoagulation.Mayo Clin Proc.1998;73(7):665–680.
- ,.Anticoagulant‐related bleeding: clinical epidemiology, prediction, and prevention.Am J Med.1993;95(3):315–328.
- ,,,,,.Reconstruction of musculoskeletal defects following oncologic resection in 76 patients.Ann Plast Surg.2006;57(2):190–194.
- ,,,,.Biopsy of the prostate guided by transrectal ultrasound: relation between warfarin use and incidence of bleeding complications.Clin Radiol.2005;60(4):459–463; discussion 457‐458.
- ,.Anticoagulation in neurosurgical patients.Neurosurgery.1999;45(4):838–847; discussion 847‐848.
- ,,,,.Post‐operative blood loss after transurethral prostatectomy is dependent on in situ fibrinolysis.Br J Urol.1997;80(6):889–893.
- ,,.Complications of heparin therapy after total joint arthroplasty.J Bone Joint Surg.1989;71(8):1130–1134.
- ,,,,,.Postpolypectomy lower GI bleeding: descriptive analysis.Gastrointest Endosc.2000;51(6):690–696.
- ,,, et al.Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy.Chest.2004;126(4):1177–1186.
- ,,.[Perioperative bridging with enoxaparin. results of the prospective BRAVE registry with 779 patients].Med Klin (Munich).2007;102(10):809–815. [German]
- ,,.Low molecular weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen.Arch Intern Med.2004;164(12):1319–1326.
- ,,.Bridging therapy in patients on long‐term oral anticoagulants who require surgery: the Prospective Peri‐operative Enoxaparin Cohort Trial (PROSPECT).J Thromb Haemost.2007;5(11):2211–2218.
- ,,, et al.Single‐arm study of bridging therapy with low molecular weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin.Circulation.2004;110(12):1658–1663.
- ,,,.Costs and clinical outcomes associated with low molecular weight heparin vs unfractionated heparin for perioperative bridging in patients receiving long‐term oral anticoagulant therapy.Chest.2004;125(5):1642–1650.
- ,.Enoxaparin is effective and safe as bridging anticoagulation in patients with a mechanical prosthetic heart valve who require temporary interruption of warfarin because of surgery or an invasive procedure. [Abstract #703]. ASH Annual Meeting Abstracts.Blood.2004;104:202A.
- ,,, et al.Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation.Mayo Clin Proc.2008;83(6):639–645.
- ,,,.Bridging anticoagulation with low molecular weight heparin after interruption of warfarin therapy is associated with a residual anticoagulant effect prior to surgery.Thromb Haemost.2005;94(3):528–531.
- ,,, et al.Brief communication: preoperative anticoagulant activity after bridging low molecular weight heparin for temporary interruption of warfarin.Ann Intern Med.2007;146(3):184–187.
- National Institute for Clinical Excellence. Atrial fibrillation: national clinical guideline for management in primary and secondary care.2006. Available at: http://www.nice.org.uk/nicemedia/pdf/cg036fullguideline. pdf. Accessed May 2009.
- ,,, et al.Clinical outcomes with unfractionated heparin or low molecular weight heparin as bridging therapy in patients on long‐term oral anticoagulants: the REGIMEN registry.J Thromb Haemost.2006;4(6):1246–1252.
- ,,,,,.Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation.JAMA.2001;285(22):2864–2870.
- ,,.Guidelines on oral anticoagulation (warfarin). 3rd ed. 2005 update.Br J Haematol.2006;132(3):277–285.
- ,,, et al.Risk of thromboembolism with short‐term interruption of warfarin therapy.Arch Intern Med.2008;168(1):63–69.