User login
Improving Patient Satisfaction
INTRODUCTION
Patient experience and satisfaction is intrinsically valued, as strong physician‐patient communication, empathy, and patient comfort require little justification. However, studies have also shown that patient satisfaction is associated with better health outcomes and greater compliance.[1, 2, 3] A systematic review of studies linking patient satisfaction to outcomes found that patient experience is positively associated with patient safety, clinical effectiveness, health outcomes, adherence, and lower resource utilization.[4] Of 378 associations studied between patient experience and health outcomes, there were 312 positive associations.[4] However, not all studies have shown a positive association between patient satisfaction and outcomes.
Nevertheless, hospitals now have to strive to improve patient satisfaction, as Centers for Medicare & Medicaid Services (CMS) has introduced Hospital Value‐Based Purchasing. CMS started to withhold Medicare Severity Diagnosis‐Related Groups payments, starting at 1.0% in 2013, 1.25% in 2014, and increasing to 2.0% in 2017. This money is redistributed based on performance on core quality measures, including patient satisfaction measured through the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.[5]
Various studies have evaluated interventions to improve patient satisfaction, but to our knowledge, no study published in a peer‐reviewed research journal has shown a significant improvement in HCAHPS scores.[6, 7, 8, 9, 10, 11, 12] Levinson et al. argue that physician communication skills should be taught during residency, and that individualized feedback is an effective way to allow physicians to track their progress over time and compared to their peers.[13] We thus aimed to evaluate an intervention to improve patient satisfaction designed by the Patient Affairs Department for Ronald Reagan University of California, Los Angeles (UCLA) Medical Center (RRUCLAMC) and the UCLA Department of Medicine.
METHODOLOGY
Design Overview
The intervention for the IM residents consisted of education on improving physician‐patient communication provided at a conference, frequent individualized patient feedback, and an incentive program in addition to existing patient satisfaction training. The results of the intervention were measured by comparing the postintervention HCAHPS scores in the Department of Medicine versus the rest of the hospital and the national averages.
Setting and Participants
The study setting was RRUCLAMC, a large university‐affiliated academic center. The internal medicine (IM) residents and patients in the Department of Medicine were in the intervention cohort. The residents in all other departments that were involved with direct adult patient care and their patients were the control cohort. Our intervention targeted resident physicians because they were most involved in the majority of direct patient care at RRUCLAMC. Residents are in house 24 hours a day, are the first line of contact for nurses and patients, and provide the most continuity, as attendings often rotate every 1 to 2 weeks, but residents are on service for at least 2 to 4 weeks for each rotation. IM residents are on all inpatient general medicine, critical care, and cardiology services at RRUCLAMC. RRUMCLA does not have a nonteaching service for adult IM patients.
Interventions
Since 2006, there has been a program at RRUCLAMC called Assessing Residents' CICARE (ARC). CICARE is an acronym that represents UCLA's patient communication model and training elements (Connect with patients, Introduce yourself and role, Communicate, Ask and anticipate, Respond, Exit courteously). The ARC program consists of trained undergraduate student volunteers surveying hospitalized patients with an optional and anonymous survey regarding specific resident physician's communication skills (see Supporting Information, Appendix A, in the online version of this article). Patients were randomly selected for the ARC and HCAHPS survey, but they were selected separately for each survey. There may have been some overlap between patients selected for ARC and HCAHPS surveys. Residents received feedback from 7 to 10 patients a year on average.
The volunteers show the patients a picture of individual resident physicians assigned to their care to confirm the resident's identity. The volunteer then asks 18 multiple‐choice questions about their physician‐patient communication skills. The patients are also asked to provide general comments regarding the resident physician.[14] The patients were interviewed in private hospital rooms by ARC volunteers. No information linking the patient to the survey is recorded. Survey data are entered into a database, and individual residents are assigned a code that links them to their patient feedback. These survey results and comments are sent to the program directors of the residency programs weekly. However, a review of the practice revealed that results were only reviewed semiannually by the residents with their program director.
Starting December 2011, the results of the ARC survey were directly e‐mailed to the interns and residents in the Department of Medicine in real time while they were on general medicine wards and the cardiology inpatient service at RRUCLAMC. Residents in other departments at RRUCLAMC continued to review the patient feedback with program directors at most biannually. This continued until June 2012 and had to be stopped during July 2012 because many of the CICARE volunteers were away on summer break.
Starting January 2012, IM residents who stood out in the ARC survey received a Commendation of Excellence. Each month, 3 residents were selected for this award based on their patient comments and if they had over 90% overall satisfaction on the survey questions. These residents received department‐wide recognition via e‐mail and a movie package (2 movie tickets, popcorn, and a drink) as a reward.
In January 2012, a 1‐hour lunchtime conference was held for IM residents to discuss best practices in physician‐patient communication, upcoming changes with Hospital Value‐Based Purchasing, and strengths and weaknesses of the Department of Medicine in patient communication. About 50% of the IM residents included in the study arm were not able to attend the education session and so no universal training was provided.
Outcomes
We analyzed the before and after intervention impact on the HCAHPS results. HCAHPS is a standardized national survey measuring patient perspectives after they are discharged from hospitals across the nation. The survey addresses communication with doctors and nurses, responsiveness of hospital staff, pain management, communication about medicines, discharge information, cleanliness of the hospital environment, and quietness of the hospital environment. The survey also includes demographic questions.[15]
Our analysis focused on the following specific questions: Would you recommend this hospital to your friends and family? During this hospital stay, how often did doctors: (1) treat you with courtesy and respect, (2) listen carefully to you, and (3) explain things in a way you could understand? Responders who did not answer all of the above questions were excluded.
Our outcomes focused on the change from January to June 2011 to January to June 2012, during which time the intervention was ongoing. We did not include data past July 2012 in the primary outcome, because the intervention did not continue due to volunteers being away for summer break. In addition, July also marks the time when the third‐year IM residents graduate and the new interns start. Thus, one‐third of the residents in the IM department had never been exposed to the intervention after June of 2012.
Statistical Analysis
We used a difference‐in‐differences regression analysis (DDRA) for these outcomes and controlled for other covariates in the patient populations to predict adjusted probabilities for each of the outcomes studied. The key predictors in the models were indicator variables for year (2011, 2012) and service (IM, all others) and an interaction between year and service. We controlled for perceived patient health, admission through emergency room (ER), age, race, patient education level, intensive care unit (ICU) stay, length of stay, and gender.[16] We calculated adjusted probabilities for each level of the interaction between service and year, holding all controls at their means. The 95% confidence intervals for these predictions were generated using the delta method.
We compared the changes in HCAHPS results for the RRUCLAMC Department of Medicine patients with all other RRUCLAMC department patients and to the national averages. We only had access to national average point estimates and not individual responses from the national sample and so were unable to do statistical analysis involving the national cohort. The prespecified significant P value was 0.05. Stata 13 (StataCorp, College Station, TX) was used for statistical analysis. The study received institutional review board exempt status.
RESULTS
Sample Size and Excluded Cases
There were initially 3637 HCAHPS patient cases. We dropped all HCAHPS cases that were missing values for outcome or demographic/explanatory variables. We dropped 226 cases due to 1 or more missing outcome variables, and we dropped 322 cases due to 1 or more missing demographic/explanatory variables. This resulted in 548 total dropped cases and a final sample size of 3089 (see Supporting Information, Appendix B, in the online version of this article). Of the 548 dropped cases, 228 cases were in the IM cohort and 320 cases from the rest of the hospital. There were 993 patients in the UCLA IM cohort and 2096 patients in the control cohort from all other UCLA adult departments. Patients excluded due to missing data were similar to the patients included in the final analysis except for 2 differences. Patients excluded were older (63 years vs 58 years, P<0.01) and more likely to have been admitted from the ER (57.4% vs 39.6%, P<0.01) than the patients we had included.
Patient Characteristics
The patient population demographics from all patients discharged from RRUCLAMC who completed HCAHPS surveys January to June 2011 and 2012 are displayed in Table 1. In both 2011 and 2012, the patients in the IM cohort were significantly older, more likely to be male, had lower perceived health, and more likely to be admitted through the emergency room than the HCAHPS patients in all other UCLA adult departments. In 2011, the IM cohort had a lower percentage of patients than the non‐IM cohort that required an ICU stay (8.0% vs 20.5%, P<0.01), but there was no statistically significant difference in 2012 (20.6% vs 20.8%, P=0.9). Other than differences in ICU stay, the demographic characteristics from 2011 to 2012 did not change in the intervention and control cohorts. The response rate for UCLA on HCAHPS during the study period was 29%, consistent with national results.[17, 18]
| 2011 | 2012 | |||||
|---|---|---|---|---|---|---|
| UCLA Internal Medicine | All Other UCLA Adult Departments | P | UCLA Internal Medicine | All Other UCLA Adult Departments | P | |
| ||||||
| Total no. | 465 | 865 | 528 | 1,231 | ||
| Age, y | 62.8 | 55.3 | <0.01 | 65.1 | 54.9 | <0.01 |
| Length of stay, d | 5.7 | 5.7 | 0.94 | 5.8 | 4.9 | 0.19 |
| Gender, male | 56.6 | 44.1 | <0.01 | 55.3 | 41.4 | <0.01 |
| Education (4 years of college or greater) | 47.3 | 49.3 | 0.5 | 47.3 | 51.3 | 0.13 |
| Patient‐perceived overall health (responding very good or excellent) | 30.5 | 55.0 | <0.01 | 27.5 | 58.2 | <0.01 |
| Admission through emergency room, yes | 75.5 | 23.8 | <0.01 | 72.4 | 23.1 | <0.01 |
| Intensive care unit, yes | 8.0 | 20.5 | <0.01 | 20.6 | 20.8 | 0.9 |
| Ethnicity (non‐Hispanic white) | 63.2 | 61.4 | 0.6 | 62.5 | 60.9 | 0.5 |
Difference‐in‐Differences Regression Analysis
The adjusted results of the DDRA for the physician‐related HCAHPS questions are presented in Table 2. The adjusted results for the percentage of patients responding positively to all 3 physician‐related HCAHPS questions in the DDRA increased by 8.1% in the IM cohort (from 65.7% to 73.8%) and by 1.5% in the control cohort (from 64.4% to 65.9%) (P=0.04). The adjusted results for the percentage of patients responding always to How often did doctors treat you with courtesy and respect? in the DDRA increased by 5.1% (from 83.8% to 88.9%) in the IM cohort and by 1.0% (from 83.3% to 84.3%) in the control cohort (P=0.09). The adjusted results for the percentage of patients responding always to Does your doctor listen carefully to you? in the DDRA increased by 6.0% in the IM department (75.6% to 81.6%) and by 1.2% (75.2% to 76.4%) in the control (P=0.1). The adjusted results for the percentage of patients responding always to Does your doctor explain things in a way you could understand? in the DDRA increased by 7.8% in the IM department (from 72.1% to 79.9%) and by 1.0% in the control cohort (from 72.2% to 73.2%) (P=0.03). There was no more than 3.1% absolute increase in any of the 4 questions in the national average. There was also a significant improvement in percentage of patients who would definitely recommend this hospital to their friends and family. The adjusted results in the DDRA for the percentage of patients responding that they would definitely recommend this hospital increased by 7.1% in the IM cohort (from 82.7% to 89.8%) and 1.5% in the control group (from 84.1% to 85.6%) (P=0.02).
| UCLA IM | All Other UCLA Adult Departments | National Average | |
|---|---|---|---|
| |||
| % Patients responding that their doctors always treated them with courtesy and respect | |||
| January to June 2011, preintervention (95% CI) | 83.8 (80.587.1) | 83.3 (80.785.9) | 82.4 |
| January to June 2012, postintervention | 88.9 (86.391.4) | 84.3 (82.186.5) | 85.5 |
| Change from 2011 to 2012, January to June | 5.1 | 1.0 | 3.1 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 4.1 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.09 | ||
| % Patients responding that their doctors always listened carefully | |||
| January to June 2011, preintervention (95% CI) | 75.6 (71.779.5) | 75.2 (72.278.1) | 76.4 |
| January to June 2012, postintervention (95% CI) | 81.6 (78.484.8) | 76.4 (73.978.9) | 73.7 |
| Change from 2011 to 2012, January to June | 6.0 | 1.2 | 2.7 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 4.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.1 | ||
| % Patients responding that their doctors always explained things in a way they could understand | |||
| January to June 2011, preintervention (95% CI) | 72.1 (6876.1) | 72.2 (69.275.4) | 70.1 |
| January to June 2012, postintervention | 79.9 (76.683.1) | 73.2 (70.675.8) | 72.2 |
| Change from 2011 to 2012, January to June | 7.8 | 1.0 | 2.1 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 6.8 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.03 | ||
| % Patients responding "always" for all 3 physician‐related HCAHPS questions | |||
| January to June 2011, preintervention (95% CI) | 65.7 (61.370.1) | 64.4 (61.267.7) | 80.1 |
| January to June 2012, postintervention | 73.8 (70.177.5) | 65.9 (63.168.6) | 87.8 |
| Change from 2011 to 2012, January to June | 8.1 | 1.5 | 7.7 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 6.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.04 | ||
| % Patients who would definitely recommend this hospital to their friends and family | |||
| January to June 2011, preintervention (95% CI) | 82.7 (79.386.1) | 84.1 (81.586.6) | 68.8 |
| January to June 2012, postintervention | 89.8 (87.392.3) | 85.6 (83.587.7) | 71.2 |
| Change from 2011 to 2012, January to June | 7.1 | 1.5 | 2.4 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 5.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.02 | ||
DISCUSSION
Our intervention, which included real‐time feedback to physicians on results of the patient survey, monthly recognition of physicians who stood out on this survey, and an educational conference, was associated with a clear improvement in patient satisfaction with physician‐patient communication and overall recommendation of the hospital. These results are significant because they demonstrate a cost‐effective intervention that can be applied to academic hospitals across the country with the use of nonmedically trained volunteers, such as the undergraduate volunteers involved in our program. The limited costs associated with the intervention were the time in managing the volunteers and movie package award ($20). To our knowledge, it is the first study published in a peer‐reviewed research journal that has demonstrated an intervention associated with significant improvements in HCAHPS scores, the standard by which CMS reimbursement will be affected.
The improvements associated with this intervention could be very valuable to hospitals and patient care. The positive correlation of higher patient satisfaction with improved outcomes suggests this intervention may have additional benefits.[4] Last, these improvements in patient satisfaction in the HCAHPS scores could minimize losses to hospital revenue, as hospitals with low patient‐satisfaction scores will be penalized.
There was a statistically significant improvement in adjusted scores for the question Did your physicians explain things understandably? with patients responding always to all 3 physician‐related HCAHPS questions and Would you recommend this hospital to friends and family. The results for the 2 other physician‐related questions (Did your doctor explain things understandably? and Did your doctor listen carefully?) did show a trend toward significance, with p values of <0.1, and a larger study may have been better powered to detect a statistically significant difference. The improvement in response to the adjusted scores for the question Did your physicians explain things understandably? was the primary driver in the improvement in the adjusted percentage of patients who responded always to all 3 physician‐related HCAHPS questions. This was likely because the IM cohort had the lowest score on this question, and so the feedback to the residents may have helped to address this area of weakness. The UCLA IM HCAHPS scores prior to 2012 have always been lower than other programs at UCLA. As a result, we do not believe the change was due to a regression to the mean.
We believe that the intervention had a positive effect on patient satisfaction for several reasons. The regular e‐mails with the results of the survey may have served as a reminder to residents that patient satisfaction was being monitored and linked to them. The immediate and individualized feedback also may have facilitated adjustments of clinical practice in real time. The residents were able to compare their own scores and comments to the anonymous results of their peers. The monthly department‐wide recognition for residents who excelled in patient communication may have created an incentive and competition among the residents. It is possible that there may be an element of the Hawthorne effect that explained the improvement in HCAHPS scores. However, all of the residents in the departments studied were already being measured through the ARC survey. The primary change was more frequent reporting of ARC survey results, and so we believe that perception of measurement alone was less likely driving the results. The findings from this study are similar to those from provider‐specific report cards, which have shown that outcomes can be improved by forcing greater accountability and competition among physicians.[19]
Brown et al. demonstrated that 2, 4‐hour physician communication workshops in their study had no impact on patient satisfaction, and so we believe that our 1‐hour workshop with only 50% attendance had minimal impact on the improved patient satisfaction scores in our study.[20] Our intervention also coincided with the implementation of the Accreditation Council for Graduate Medical Education (ACGME) work‐hour restrictions implemented in July 2011. These restrictions limited residents to 80 hours per week, intern duty periods were restricted to 16 hours and residents to 28 hours, and interns and residents required 8 to 10 hours free of duty between scheduled duty periods.[21] One of the biggest impacts of ACGME work‐hour restrictions was that interns were doing more day and night shifts rather than 28‐hour calls. However, these work‐hour restrictions were the same for all specialties and so were unlikely to explain the improved patient satisfaction associated with our intervention.
Our study has limitations. The study was a nonrandomized pre‐post study. We attempted to control for the differences in the cohorts with a multivariable regression analysis, but there may be unmeasured differences that we were unable to control for. Due to deidentification of the data, we could only control for patient health based on patient perceived health. In addition, the percentage of patients requiring ICU care in the IM cohort was higher in 2012 than in 2011. We did not identify differences in outcomes from analyses stratified by ICU or non‐ICU patients. In addition, patients who were excluded because of missing outcomes were more likely to be older and admitted through the ER. Further investigation would be needed to see if the findings of this study could be extended to other clinical situations.
In conclusion, our study found an intervention program that was associated with a significant improvement in patient satisfaction in the intervention cohort, even after adjusting for differences in the patient population, whereas there was no change in the control group. This intervention can serve as a model for academic hospitals to improve patient satisfaction, avoid revenue loss in the era of Hospital Value‐Based Purchasing, and to train the next generation of physicians on providing patient‐centered care.
Disclosure
This work was supported by the Beryl Institute and UCLA QI Initiative.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 Days. Am J Manag Care. 2011;17:41–48.
- , , , . Patients' Perception of Hospital Care in the United States. N Engl J Med. 2008;359:1921–1931.
- , , , et al. Patient satisfaction and its relationship with clinical quality and inpatient mortality in acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2010;3:188–195.
- , , . A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1).
- Centers for Medicare 70:729–732.
- , , , . Emergency department patient satisfaction: customer service training improves patient satisfaction and ratings of physician and nurse skill. J Healthc Manag. 1998;43:427–440; discussion 441–442.
- , , . Emergency department information: does it effect patients' perception and satisfaction about the care given in an emergency department? Eur J Emerg Med 1999;6:245–248.
- . Can communication skills workshops for emergency department doctors improve patient satisfaction? J Accid Emerg Med. 2000;17:251–253.
- , , , . Effects of a physician communication intervention on patient care outcomes. J Gen Intern Med. 1996;11:147–155.
- , , , , . Health‐related quality‐of‐life assessments and patient‐physician communication: a randomized controlled trial. JAMA. 2002;288:3027–3034.
- , , , . Modification of residents' behavior by preceptor feedback of patient satisfaction. J Gen Intern Med. 1986;1:394–398.
- , , . Developing physician communication skills for patient‐centered care. Health Aff (Millwood) 2010;29:1310–1318.
- ARC Medical Program @ UCLA. Available at: http://Arcmedicalprogram.Wordpress.com. Accessed July 1, 2013.
- Hospital Consumer Assessment of Healthcare Providers 12:151–162.
- Summary of HCAHPS survey results January 2010 to December 2010 discharges. Available at: http://Www.Hcahpsonline.Org/Files/Hcahps survey results table %28report_Hei_October_2011_States%29.Pdf. Accessed October 18, 2013.
- , , , et al. A randomized experiment investigating the suitability of speech‐enabled IVR and web modes for publicly reported surveys of patients' experience of hospital care. Med Care Res Rev. 2013;70:165–184.
- . Provider‐specific report cards: a tool for health sector accountability in developing countries. Health Policy Plan. 2006;21:101–109.
- , , , . Effect of clinician communication skills training on patient satisfaction: a randomized, controlled trial. Ann Intern Med. 1999;131:822–829.
- Frequently asked questions: ACGME common duty hour requirements. Available at: http://www.Acgme.Org/Acgmeweb/Portals/0/Pdfs/Dh‐Faqs2011.Pdf. Accessed January 3, 2015.
INTRODUCTION
Patient experience and satisfaction is intrinsically valued, as strong physician‐patient communication, empathy, and patient comfort require little justification. However, studies have also shown that patient satisfaction is associated with better health outcomes and greater compliance.[1, 2, 3] A systematic review of studies linking patient satisfaction to outcomes found that patient experience is positively associated with patient safety, clinical effectiveness, health outcomes, adherence, and lower resource utilization.[4] Of 378 associations studied between patient experience and health outcomes, there were 312 positive associations.[4] However, not all studies have shown a positive association between patient satisfaction and outcomes.
Nevertheless, hospitals now have to strive to improve patient satisfaction, as Centers for Medicare & Medicaid Services (CMS) has introduced Hospital Value‐Based Purchasing. CMS started to withhold Medicare Severity Diagnosis‐Related Groups payments, starting at 1.0% in 2013, 1.25% in 2014, and increasing to 2.0% in 2017. This money is redistributed based on performance on core quality measures, including patient satisfaction measured through the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.[5]
Various studies have evaluated interventions to improve patient satisfaction, but to our knowledge, no study published in a peer‐reviewed research journal has shown a significant improvement in HCAHPS scores.[6, 7, 8, 9, 10, 11, 12] Levinson et al. argue that physician communication skills should be taught during residency, and that individualized feedback is an effective way to allow physicians to track their progress over time and compared to their peers.[13] We thus aimed to evaluate an intervention to improve patient satisfaction designed by the Patient Affairs Department for Ronald Reagan University of California, Los Angeles (UCLA) Medical Center (RRUCLAMC) and the UCLA Department of Medicine.
METHODOLOGY
Design Overview
The intervention for the IM residents consisted of education on improving physician‐patient communication provided at a conference, frequent individualized patient feedback, and an incentive program in addition to existing patient satisfaction training. The results of the intervention were measured by comparing the postintervention HCAHPS scores in the Department of Medicine versus the rest of the hospital and the national averages.
Setting and Participants
The study setting was RRUCLAMC, a large university‐affiliated academic center. The internal medicine (IM) residents and patients in the Department of Medicine were in the intervention cohort. The residents in all other departments that were involved with direct adult patient care and their patients were the control cohort. Our intervention targeted resident physicians because they were most involved in the majority of direct patient care at RRUCLAMC. Residents are in house 24 hours a day, are the first line of contact for nurses and patients, and provide the most continuity, as attendings often rotate every 1 to 2 weeks, but residents are on service for at least 2 to 4 weeks for each rotation. IM residents are on all inpatient general medicine, critical care, and cardiology services at RRUCLAMC. RRUMCLA does not have a nonteaching service for adult IM patients.
Interventions
Since 2006, there has been a program at RRUCLAMC called Assessing Residents' CICARE (ARC). CICARE is an acronym that represents UCLA's patient communication model and training elements (Connect with patients, Introduce yourself and role, Communicate, Ask and anticipate, Respond, Exit courteously). The ARC program consists of trained undergraduate student volunteers surveying hospitalized patients with an optional and anonymous survey regarding specific resident physician's communication skills (see Supporting Information, Appendix A, in the online version of this article). Patients were randomly selected for the ARC and HCAHPS survey, but they were selected separately for each survey. There may have been some overlap between patients selected for ARC and HCAHPS surveys. Residents received feedback from 7 to 10 patients a year on average.
The volunteers show the patients a picture of individual resident physicians assigned to their care to confirm the resident's identity. The volunteer then asks 18 multiple‐choice questions about their physician‐patient communication skills. The patients are also asked to provide general comments regarding the resident physician.[14] The patients were interviewed in private hospital rooms by ARC volunteers. No information linking the patient to the survey is recorded. Survey data are entered into a database, and individual residents are assigned a code that links them to their patient feedback. These survey results and comments are sent to the program directors of the residency programs weekly. However, a review of the practice revealed that results were only reviewed semiannually by the residents with their program director.
Starting December 2011, the results of the ARC survey were directly e‐mailed to the interns and residents in the Department of Medicine in real time while they were on general medicine wards and the cardiology inpatient service at RRUCLAMC. Residents in other departments at RRUCLAMC continued to review the patient feedback with program directors at most biannually. This continued until June 2012 and had to be stopped during July 2012 because many of the CICARE volunteers were away on summer break.
Starting January 2012, IM residents who stood out in the ARC survey received a Commendation of Excellence. Each month, 3 residents were selected for this award based on their patient comments and if they had over 90% overall satisfaction on the survey questions. These residents received department‐wide recognition via e‐mail and a movie package (2 movie tickets, popcorn, and a drink) as a reward.
In January 2012, a 1‐hour lunchtime conference was held for IM residents to discuss best practices in physician‐patient communication, upcoming changes with Hospital Value‐Based Purchasing, and strengths and weaknesses of the Department of Medicine in patient communication. About 50% of the IM residents included in the study arm were not able to attend the education session and so no universal training was provided.
Outcomes
We analyzed the before and after intervention impact on the HCAHPS results. HCAHPS is a standardized national survey measuring patient perspectives after they are discharged from hospitals across the nation. The survey addresses communication with doctors and nurses, responsiveness of hospital staff, pain management, communication about medicines, discharge information, cleanliness of the hospital environment, and quietness of the hospital environment. The survey also includes demographic questions.[15]
Our analysis focused on the following specific questions: Would you recommend this hospital to your friends and family? During this hospital stay, how often did doctors: (1) treat you with courtesy and respect, (2) listen carefully to you, and (3) explain things in a way you could understand? Responders who did not answer all of the above questions were excluded.
Our outcomes focused on the change from January to June 2011 to January to June 2012, during which time the intervention was ongoing. We did not include data past July 2012 in the primary outcome, because the intervention did not continue due to volunteers being away for summer break. In addition, July also marks the time when the third‐year IM residents graduate and the new interns start. Thus, one‐third of the residents in the IM department had never been exposed to the intervention after June of 2012.
Statistical Analysis
We used a difference‐in‐differences regression analysis (DDRA) for these outcomes and controlled for other covariates in the patient populations to predict adjusted probabilities for each of the outcomes studied. The key predictors in the models were indicator variables for year (2011, 2012) and service (IM, all others) and an interaction between year and service. We controlled for perceived patient health, admission through emergency room (ER), age, race, patient education level, intensive care unit (ICU) stay, length of stay, and gender.[16] We calculated adjusted probabilities for each level of the interaction between service and year, holding all controls at their means. The 95% confidence intervals for these predictions were generated using the delta method.
We compared the changes in HCAHPS results for the RRUCLAMC Department of Medicine patients with all other RRUCLAMC department patients and to the national averages. We only had access to national average point estimates and not individual responses from the national sample and so were unable to do statistical analysis involving the national cohort. The prespecified significant P value was 0.05. Stata 13 (StataCorp, College Station, TX) was used for statistical analysis. The study received institutional review board exempt status.
RESULTS
Sample Size and Excluded Cases
There were initially 3637 HCAHPS patient cases. We dropped all HCAHPS cases that were missing values for outcome or demographic/explanatory variables. We dropped 226 cases due to 1 or more missing outcome variables, and we dropped 322 cases due to 1 or more missing demographic/explanatory variables. This resulted in 548 total dropped cases and a final sample size of 3089 (see Supporting Information, Appendix B, in the online version of this article). Of the 548 dropped cases, 228 cases were in the IM cohort and 320 cases from the rest of the hospital. There were 993 patients in the UCLA IM cohort and 2096 patients in the control cohort from all other UCLA adult departments. Patients excluded due to missing data were similar to the patients included in the final analysis except for 2 differences. Patients excluded were older (63 years vs 58 years, P<0.01) and more likely to have been admitted from the ER (57.4% vs 39.6%, P<0.01) than the patients we had included.
Patient Characteristics
The patient population demographics from all patients discharged from RRUCLAMC who completed HCAHPS surveys January to June 2011 and 2012 are displayed in Table 1. In both 2011 and 2012, the patients in the IM cohort were significantly older, more likely to be male, had lower perceived health, and more likely to be admitted through the emergency room than the HCAHPS patients in all other UCLA adult departments. In 2011, the IM cohort had a lower percentage of patients than the non‐IM cohort that required an ICU stay (8.0% vs 20.5%, P<0.01), but there was no statistically significant difference in 2012 (20.6% vs 20.8%, P=0.9). Other than differences in ICU stay, the demographic characteristics from 2011 to 2012 did not change in the intervention and control cohorts. The response rate for UCLA on HCAHPS during the study period was 29%, consistent with national results.[17, 18]
| 2011 | 2012 | |||||
|---|---|---|---|---|---|---|
| UCLA Internal Medicine | All Other UCLA Adult Departments | P | UCLA Internal Medicine | All Other UCLA Adult Departments | P | |
| ||||||
| Total no. | 465 | 865 | 528 | 1,231 | ||
| Age, y | 62.8 | 55.3 | <0.01 | 65.1 | 54.9 | <0.01 |
| Length of stay, d | 5.7 | 5.7 | 0.94 | 5.8 | 4.9 | 0.19 |
| Gender, male | 56.6 | 44.1 | <0.01 | 55.3 | 41.4 | <0.01 |
| Education (4 years of college or greater) | 47.3 | 49.3 | 0.5 | 47.3 | 51.3 | 0.13 |
| Patient‐perceived overall health (responding very good or excellent) | 30.5 | 55.0 | <0.01 | 27.5 | 58.2 | <0.01 |
| Admission through emergency room, yes | 75.5 | 23.8 | <0.01 | 72.4 | 23.1 | <0.01 |
| Intensive care unit, yes | 8.0 | 20.5 | <0.01 | 20.6 | 20.8 | 0.9 |
| Ethnicity (non‐Hispanic white) | 63.2 | 61.4 | 0.6 | 62.5 | 60.9 | 0.5 |
Difference‐in‐Differences Regression Analysis
The adjusted results of the DDRA for the physician‐related HCAHPS questions are presented in Table 2. The adjusted results for the percentage of patients responding positively to all 3 physician‐related HCAHPS questions in the DDRA increased by 8.1% in the IM cohort (from 65.7% to 73.8%) and by 1.5% in the control cohort (from 64.4% to 65.9%) (P=0.04). The adjusted results for the percentage of patients responding always to How often did doctors treat you with courtesy and respect? in the DDRA increased by 5.1% (from 83.8% to 88.9%) in the IM cohort and by 1.0% (from 83.3% to 84.3%) in the control cohort (P=0.09). The adjusted results for the percentage of patients responding always to Does your doctor listen carefully to you? in the DDRA increased by 6.0% in the IM department (75.6% to 81.6%) and by 1.2% (75.2% to 76.4%) in the control (P=0.1). The adjusted results for the percentage of patients responding always to Does your doctor explain things in a way you could understand? in the DDRA increased by 7.8% in the IM department (from 72.1% to 79.9%) and by 1.0% in the control cohort (from 72.2% to 73.2%) (P=0.03). There was no more than 3.1% absolute increase in any of the 4 questions in the national average. There was also a significant improvement in percentage of patients who would definitely recommend this hospital to their friends and family. The adjusted results in the DDRA for the percentage of patients responding that they would definitely recommend this hospital increased by 7.1% in the IM cohort (from 82.7% to 89.8%) and 1.5% in the control group (from 84.1% to 85.6%) (P=0.02).
| UCLA IM | All Other UCLA Adult Departments | National Average | |
|---|---|---|---|
| |||
| % Patients responding that their doctors always treated them with courtesy and respect | |||
| January to June 2011, preintervention (95% CI) | 83.8 (80.587.1) | 83.3 (80.785.9) | 82.4 |
| January to June 2012, postintervention | 88.9 (86.391.4) | 84.3 (82.186.5) | 85.5 |
| Change from 2011 to 2012, January to June | 5.1 | 1.0 | 3.1 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 4.1 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.09 | ||
| % Patients responding that their doctors always listened carefully | |||
| January to June 2011, preintervention (95% CI) | 75.6 (71.779.5) | 75.2 (72.278.1) | 76.4 |
| January to June 2012, postintervention (95% CI) | 81.6 (78.484.8) | 76.4 (73.978.9) | 73.7 |
| Change from 2011 to 2012, January to June | 6.0 | 1.2 | 2.7 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 4.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.1 | ||
| % Patients responding that their doctors always explained things in a way they could understand | |||
| January to June 2011, preintervention (95% CI) | 72.1 (6876.1) | 72.2 (69.275.4) | 70.1 |
| January to June 2012, postintervention | 79.9 (76.683.1) | 73.2 (70.675.8) | 72.2 |
| Change from 2011 to 2012, January to June | 7.8 | 1.0 | 2.1 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 6.8 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.03 | ||
| % Patients responding "always" for all 3 physician‐related HCAHPS questions | |||
| January to June 2011, preintervention (95% CI) | 65.7 (61.370.1) | 64.4 (61.267.7) | 80.1 |
| January to June 2012, postintervention | 73.8 (70.177.5) | 65.9 (63.168.6) | 87.8 |
| Change from 2011 to 2012, January to June | 8.1 | 1.5 | 7.7 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 6.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.04 | ||
| % Patients who would definitely recommend this hospital to their friends and family | |||
| January to June 2011, preintervention (95% CI) | 82.7 (79.386.1) | 84.1 (81.586.6) | 68.8 |
| January to June 2012, postintervention | 89.8 (87.392.3) | 85.6 (83.587.7) | 71.2 |
| Change from 2011 to 2012, January to June | 7.1 | 1.5 | 2.4 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 5.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.02 | ||
DISCUSSION
Our intervention, which included real‐time feedback to physicians on results of the patient survey, monthly recognition of physicians who stood out on this survey, and an educational conference, was associated with a clear improvement in patient satisfaction with physician‐patient communication and overall recommendation of the hospital. These results are significant because they demonstrate a cost‐effective intervention that can be applied to academic hospitals across the country with the use of nonmedically trained volunteers, such as the undergraduate volunteers involved in our program. The limited costs associated with the intervention were the time in managing the volunteers and movie package award ($20). To our knowledge, it is the first study published in a peer‐reviewed research journal that has demonstrated an intervention associated with significant improvements in HCAHPS scores, the standard by which CMS reimbursement will be affected.
The improvements associated with this intervention could be very valuable to hospitals and patient care. The positive correlation of higher patient satisfaction with improved outcomes suggests this intervention may have additional benefits.[4] Last, these improvements in patient satisfaction in the HCAHPS scores could minimize losses to hospital revenue, as hospitals with low patient‐satisfaction scores will be penalized.
There was a statistically significant improvement in adjusted scores for the question Did your physicians explain things understandably? with patients responding always to all 3 physician‐related HCAHPS questions and Would you recommend this hospital to friends and family. The results for the 2 other physician‐related questions (Did your doctor explain things understandably? and Did your doctor listen carefully?) did show a trend toward significance, with p values of <0.1, and a larger study may have been better powered to detect a statistically significant difference. The improvement in response to the adjusted scores for the question Did your physicians explain things understandably? was the primary driver in the improvement in the adjusted percentage of patients who responded always to all 3 physician‐related HCAHPS questions. This was likely because the IM cohort had the lowest score on this question, and so the feedback to the residents may have helped to address this area of weakness. The UCLA IM HCAHPS scores prior to 2012 have always been lower than other programs at UCLA. As a result, we do not believe the change was due to a regression to the mean.
We believe that the intervention had a positive effect on patient satisfaction for several reasons. The regular e‐mails with the results of the survey may have served as a reminder to residents that patient satisfaction was being monitored and linked to them. The immediate and individualized feedback also may have facilitated adjustments of clinical practice in real time. The residents were able to compare their own scores and comments to the anonymous results of their peers. The monthly department‐wide recognition for residents who excelled in patient communication may have created an incentive and competition among the residents. It is possible that there may be an element of the Hawthorne effect that explained the improvement in HCAHPS scores. However, all of the residents in the departments studied were already being measured through the ARC survey. The primary change was more frequent reporting of ARC survey results, and so we believe that perception of measurement alone was less likely driving the results. The findings from this study are similar to those from provider‐specific report cards, which have shown that outcomes can be improved by forcing greater accountability and competition among physicians.[19]
Brown et al. demonstrated that 2, 4‐hour physician communication workshops in their study had no impact on patient satisfaction, and so we believe that our 1‐hour workshop with only 50% attendance had minimal impact on the improved patient satisfaction scores in our study.[20] Our intervention also coincided with the implementation of the Accreditation Council for Graduate Medical Education (ACGME) work‐hour restrictions implemented in July 2011. These restrictions limited residents to 80 hours per week, intern duty periods were restricted to 16 hours and residents to 28 hours, and interns and residents required 8 to 10 hours free of duty between scheduled duty periods.[21] One of the biggest impacts of ACGME work‐hour restrictions was that interns were doing more day and night shifts rather than 28‐hour calls. However, these work‐hour restrictions were the same for all specialties and so were unlikely to explain the improved patient satisfaction associated with our intervention.
Our study has limitations. The study was a nonrandomized pre‐post study. We attempted to control for the differences in the cohorts with a multivariable regression analysis, but there may be unmeasured differences that we were unable to control for. Due to deidentification of the data, we could only control for patient health based on patient perceived health. In addition, the percentage of patients requiring ICU care in the IM cohort was higher in 2012 than in 2011. We did not identify differences in outcomes from analyses stratified by ICU or non‐ICU patients. In addition, patients who were excluded because of missing outcomes were more likely to be older and admitted through the ER. Further investigation would be needed to see if the findings of this study could be extended to other clinical situations.
In conclusion, our study found an intervention program that was associated with a significant improvement in patient satisfaction in the intervention cohort, even after adjusting for differences in the patient population, whereas there was no change in the control group. This intervention can serve as a model for academic hospitals to improve patient satisfaction, avoid revenue loss in the era of Hospital Value‐Based Purchasing, and to train the next generation of physicians on providing patient‐centered care.
Disclosure
This work was supported by the Beryl Institute and UCLA QI Initiative.
INTRODUCTION
Patient experience and satisfaction is intrinsically valued, as strong physician‐patient communication, empathy, and patient comfort require little justification. However, studies have also shown that patient satisfaction is associated with better health outcomes and greater compliance.[1, 2, 3] A systematic review of studies linking patient satisfaction to outcomes found that patient experience is positively associated with patient safety, clinical effectiveness, health outcomes, adherence, and lower resource utilization.[4] Of 378 associations studied between patient experience and health outcomes, there were 312 positive associations.[4] However, not all studies have shown a positive association between patient satisfaction and outcomes.
Nevertheless, hospitals now have to strive to improve patient satisfaction, as Centers for Medicare & Medicaid Services (CMS) has introduced Hospital Value‐Based Purchasing. CMS started to withhold Medicare Severity Diagnosis‐Related Groups payments, starting at 1.0% in 2013, 1.25% in 2014, and increasing to 2.0% in 2017. This money is redistributed based on performance on core quality measures, including patient satisfaction measured through the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey.[5]
Various studies have evaluated interventions to improve patient satisfaction, but to our knowledge, no study published in a peer‐reviewed research journal has shown a significant improvement in HCAHPS scores.[6, 7, 8, 9, 10, 11, 12] Levinson et al. argue that physician communication skills should be taught during residency, and that individualized feedback is an effective way to allow physicians to track their progress over time and compared to their peers.[13] We thus aimed to evaluate an intervention to improve patient satisfaction designed by the Patient Affairs Department for Ronald Reagan University of California, Los Angeles (UCLA) Medical Center (RRUCLAMC) and the UCLA Department of Medicine.
METHODOLOGY
Design Overview
The intervention for the IM residents consisted of education on improving physician‐patient communication provided at a conference, frequent individualized patient feedback, and an incentive program in addition to existing patient satisfaction training. The results of the intervention were measured by comparing the postintervention HCAHPS scores in the Department of Medicine versus the rest of the hospital and the national averages.
Setting and Participants
The study setting was RRUCLAMC, a large university‐affiliated academic center. The internal medicine (IM) residents and patients in the Department of Medicine were in the intervention cohort. The residents in all other departments that were involved with direct adult patient care and their patients were the control cohort. Our intervention targeted resident physicians because they were most involved in the majority of direct patient care at RRUCLAMC. Residents are in house 24 hours a day, are the first line of contact for nurses and patients, and provide the most continuity, as attendings often rotate every 1 to 2 weeks, but residents are on service for at least 2 to 4 weeks for each rotation. IM residents are on all inpatient general medicine, critical care, and cardiology services at RRUCLAMC. RRUMCLA does not have a nonteaching service for adult IM patients.
Interventions
Since 2006, there has been a program at RRUCLAMC called Assessing Residents' CICARE (ARC). CICARE is an acronym that represents UCLA's patient communication model and training elements (Connect with patients, Introduce yourself and role, Communicate, Ask and anticipate, Respond, Exit courteously). The ARC program consists of trained undergraduate student volunteers surveying hospitalized patients with an optional and anonymous survey regarding specific resident physician's communication skills (see Supporting Information, Appendix A, in the online version of this article). Patients were randomly selected for the ARC and HCAHPS survey, but they were selected separately for each survey. There may have been some overlap between patients selected for ARC and HCAHPS surveys. Residents received feedback from 7 to 10 patients a year on average.
The volunteers show the patients a picture of individual resident physicians assigned to their care to confirm the resident's identity. The volunteer then asks 18 multiple‐choice questions about their physician‐patient communication skills. The patients are also asked to provide general comments regarding the resident physician.[14] The patients were interviewed in private hospital rooms by ARC volunteers. No information linking the patient to the survey is recorded. Survey data are entered into a database, and individual residents are assigned a code that links them to their patient feedback. These survey results and comments are sent to the program directors of the residency programs weekly. However, a review of the practice revealed that results were only reviewed semiannually by the residents with their program director.
Starting December 2011, the results of the ARC survey were directly e‐mailed to the interns and residents in the Department of Medicine in real time while they were on general medicine wards and the cardiology inpatient service at RRUCLAMC. Residents in other departments at RRUCLAMC continued to review the patient feedback with program directors at most biannually. This continued until June 2012 and had to be stopped during July 2012 because many of the CICARE volunteers were away on summer break.
Starting January 2012, IM residents who stood out in the ARC survey received a Commendation of Excellence. Each month, 3 residents were selected for this award based on their patient comments and if they had over 90% overall satisfaction on the survey questions. These residents received department‐wide recognition via e‐mail and a movie package (2 movie tickets, popcorn, and a drink) as a reward.
In January 2012, a 1‐hour lunchtime conference was held for IM residents to discuss best practices in physician‐patient communication, upcoming changes with Hospital Value‐Based Purchasing, and strengths and weaknesses of the Department of Medicine in patient communication. About 50% of the IM residents included in the study arm were not able to attend the education session and so no universal training was provided.
Outcomes
We analyzed the before and after intervention impact on the HCAHPS results. HCAHPS is a standardized national survey measuring patient perspectives after they are discharged from hospitals across the nation. The survey addresses communication with doctors and nurses, responsiveness of hospital staff, pain management, communication about medicines, discharge information, cleanliness of the hospital environment, and quietness of the hospital environment. The survey also includes demographic questions.[15]
Our analysis focused on the following specific questions: Would you recommend this hospital to your friends and family? During this hospital stay, how often did doctors: (1) treat you with courtesy and respect, (2) listen carefully to you, and (3) explain things in a way you could understand? Responders who did not answer all of the above questions were excluded.
Our outcomes focused on the change from January to June 2011 to January to June 2012, during which time the intervention was ongoing. We did not include data past July 2012 in the primary outcome, because the intervention did not continue due to volunteers being away for summer break. In addition, July also marks the time when the third‐year IM residents graduate and the new interns start. Thus, one‐third of the residents in the IM department had never been exposed to the intervention after June of 2012.
Statistical Analysis
We used a difference‐in‐differences regression analysis (DDRA) for these outcomes and controlled for other covariates in the patient populations to predict adjusted probabilities for each of the outcomes studied. The key predictors in the models were indicator variables for year (2011, 2012) and service (IM, all others) and an interaction between year and service. We controlled for perceived patient health, admission through emergency room (ER), age, race, patient education level, intensive care unit (ICU) stay, length of stay, and gender.[16] We calculated adjusted probabilities for each level of the interaction between service and year, holding all controls at their means. The 95% confidence intervals for these predictions were generated using the delta method.
We compared the changes in HCAHPS results for the RRUCLAMC Department of Medicine patients with all other RRUCLAMC department patients and to the national averages. We only had access to national average point estimates and not individual responses from the national sample and so were unable to do statistical analysis involving the national cohort. The prespecified significant P value was 0.05. Stata 13 (StataCorp, College Station, TX) was used for statistical analysis. The study received institutional review board exempt status.
RESULTS
Sample Size and Excluded Cases
There were initially 3637 HCAHPS patient cases. We dropped all HCAHPS cases that were missing values for outcome or demographic/explanatory variables. We dropped 226 cases due to 1 or more missing outcome variables, and we dropped 322 cases due to 1 or more missing demographic/explanatory variables. This resulted in 548 total dropped cases and a final sample size of 3089 (see Supporting Information, Appendix B, in the online version of this article). Of the 548 dropped cases, 228 cases were in the IM cohort and 320 cases from the rest of the hospital. There were 993 patients in the UCLA IM cohort and 2096 patients in the control cohort from all other UCLA adult departments. Patients excluded due to missing data were similar to the patients included in the final analysis except for 2 differences. Patients excluded were older (63 years vs 58 years, P<0.01) and more likely to have been admitted from the ER (57.4% vs 39.6%, P<0.01) than the patients we had included.
Patient Characteristics
The patient population demographics from all patients discharged from RRUCLAMC who completed HCAHPS surveys January to June 2011 and 2012 are displayed in Table 1. In both 2011 and 2012, the patients in the IM cohort were significantly older, more likely to be male, had lower perceived health, and more likely to be admitted through the emergency room than the HCAHPS patients in all other UCLA adult departments. In 2011, the IM cohort had a lower percentage of patients than the non‐IM cohort that required an ICU stay (8.0% vs 20.5%, P<0.01), but there was no statistically significant difference in 2012 (20.6% vs 20.8%, P=0.9). Other than differences in ICU stay, the demographic characteristics from 2011 to 2012 did not change in the intervention and control cohorts. The response rate for UCLA on HCAHPS during the study period was 29%, consistent with national results.[17, 18]
| 2011 | 2012 | |||||
|---|---|---|---|---|---|---|
| UCLA Internal Medicine | All Other UCLA Adult Departments | P | UCLA Internal Medicine | All Other UCLA Adult Departments | P | |
| ||||||
| Total no. | 465 | 865 | 528 | 1,231 | ||
| Age, y | 62.8 | 55.3 | <0.01 | 65.1 | 54.9 | <0.01 |
| Length of stay, d | 5.7 | 5.7 | 0.94 | 5.8 | 4.9 | 0.19 |
| Gender, male | 56.6 | 44.1 | <0.01 | 55.3 | 41.4 | <0.01 |
| Education (4 years of college or greater) | 47.3 | 49.3 | 0.5 | 47.3 | 51.3 | 0.13 |
| Patient‐perceived overall health (responding very good or excellent) | 30.5 | 55.0 | <0.01 | 27.5 | 58.2 | <0.01 |
| Admission through emergency room, yes | 75.5 | 23.8 | <0.01 | 72.4 | 23.1 | <0.01 |
| Intensive care unit, yes | 8.0 | 20.5 | <0.01 | 20.6 | 20.8 | 0.9 |
| Ethnicity (non‐Hispanic white) | 63.2 | 61.4 | 0.6 | 62.5 | 60.9 | 0.5 |
Difference‐in‐Differences Regression Analysis
The adjusted results of the DDRA for the physician‐related HCAHPS questions are presented in Table 2. The adjusted results for the percentage of patients responding positively to all 3 physician‐related HCAHPS questions in the DDRA increased by 8.1% in the IM cohort (from 65.7% to 73.8%) and by 1.5% in the control cohort (from 64.4% to 65.9%) (P=0.04). The adjusted results for the percentage of patients responding always to How often did doctors treat you with courtesy and respect? in the DDRA increased by 5.1% (from 83.8% to 88.9%) in the IM cohort and by 1.0% (from 83.3% to 84.3%) in the control cohort (P=0.09). The adjusted results for the percentage of patients responding always to Does your doctor listen carefully to you? in the DDRA increased by 6.0% in the IM department (75.6% to 81.6%) and by 1.2% (75.2% to 76.4%) in the control (P=0.1). The adjusted results for the percentage of patients responding always to Does your doctor explain things in a way you could understand? in the DDRA increased by 7.8% in the IM department (from 72.1% to 79.9%) and by 1.0% in the control cohort (from 72.2% to 73.2%) (P=0.03). There was no more than 3.1% absolute increase in any of the 4 questions in the national average. There was also a significant improvement in percentage of patients who would definitely recommend this hospital to their friends and family. The adjusted results in the DDRA for the percentage of patients responding that they would definitely recommend this hospital increased by 7.1% in the IM cohort (from 82.7% to 89.8%) and 1.5% in the control group (from 84.1% to 85.6%) (P=0.02).
| UCLA IM | All Other UCLA Adult Departments | National Average | |
|---|---|---|---|
| |||
| % Patients responding that their doctors always treated them with courtesy and respect | |||
| January to June 2011, preintervention (95% CI) | 83.8 (80.587.1) | 83.3 (80.785.9) | 82.4 |
| January to June 2012, postintervention | 88.9 (86.391.4) | 84.3 (82.186.5) | 85.5 |
| Change from 2011 to 2012, January to June | 5.1 | 1.0 | 3.1 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 4.1 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.09 | ||
| % Patients responding that their doctors always listened carefully | |||
| January to June 2011, preintervention (95% CI) | 75.6 (71.779.5) | 75.2 (72.278.1) | 76.4 |
| January to June 2012, postintervention (95% CI) | 81.6 (78.484.8) | 76.4 (73.978.9) | 73.7 |
| Change from 2011 to 2012, January to June | 6.0 | 1.2 | 2.7 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 4.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.1 | ||
| % Patients responding that their doctors always explained things in a way they could understand | |||
| January to June 2011, preintervention (95% CI) | 72.1 (6876.1) | 72.2 (69.275.4) | 70.1 |
| January to June 2012, postintervention | 79.9 (76.683.1) | 73.2 (70.675.8) | 72.2 |
| Change from 2011 to 2012, January to June | 7.8 | 1.0 | 2.1 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 6.8 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.03 | ||
| % Patients responding "always" for all 3 physician‐related HCAHPS questions | |||
| January to June 2011, preintervention (95% CI) | 65.7 (61.370.1) | 64.4 (61.267.7) | 80.1 |
| January to June 2012, postintervention | 73.8 (70.177.5) | 65.9 (63.168.6) | 87.8 |
| Change from 2011 to 2012, January to June | 8.1 | 1.5 | 7.7 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 6.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.04 | ||
| % Patients who would definitely recommend this hospital to their friends and family | |||
| January to June 2011, preintervention (95% CI) | 82.7 (79.386.1) | 84.1 (81.586.6) | 68.8 |
| January to June 2012, postintervention | 89.8 (87.392.3) | 85.6 (83.587.7) | 71.2 |
| Change from 2011 to 2012, January to June | 7.1 | 1.5 | 2.4 |
| Change in UCLA IM minus change in all other UCLA adult departments, difference in differences | 5.6 | ||
| P value of difference in differences between IM and the rest of the hospital | 0.02 | ||
DISCUSSION
Our intervention, which included real‐time feedback to physicians on results of the patient survey, monthly recognition of physicians who stood out on this survey, and an educational conference, was associated with a clear improvement in patient satisfaction with physician‐patient communication and overall recommendation of the hospital. These results are significant because they demonstrate a cost‐effective intervention that can be applied to academic hospitals across the country with the use of nonmedically trained volunteers, such as the undergraduate volunteers involved in our program. The limited costs associated with the intervention were the time in managing the volunteers and movie package award ($20). To our knowledge, it is the first study published in a peer‐reviewed research journal that has demonstrated an intervention associated with significant improvements in HCAHPS scores, the standard by which CMS reimbursement will be affected.
The improvements associated with this intervention could be very valuable to hospitals and patient care. The positive correlation of higher patient satisfaction with improved outcomes suggests this intervention may have additional benefits.[4] Last, these improvements in patient satisfaction in the HCAHPS scores could minimize losses to hospital revenue, as hospitals with low patient‐satisfaction scores will be penalized.
There was a statistically significant improvement in adjusted scores for the question Did your physicians explain things understandably? with patients responding always to all 3 physician‐related HCAHPS questions and Would you recommend this hospital to friends and family. The results for the 2 other physician‐related questions (Did your doctor explain things understandably? and Did your doctor listen carefully?) did show a trend toward significance, with p values of <0.1, and a larger study may have been better powered to detect a statistically significant difference. The improvement in response to the adjusted scores for the question Did your physicians explain things understandably? was the primary driver in the improvement in the adjusted percentage of patients who responded always to all 3 physician‐related HCAHPS questions. This was likely because the IM cohort had the lowest score on this question, and so the feedback to the residents may have helped to address this area of weakness. The UCLA IM HCAHPS scores prior to 2012 have always been lower than other programs at UCLA. As a result, we do not believe the change was due to a regression to the mean.
We believe that the intervention had a positive effect on patient satisfaction for several reasons. The regular e‐mails with the results of the survey may have served as a reminder to residents that patient satisfaction was being monitored and linked to them. The immediate and individualized feedback also may have facilitated adjustments of clinical practice in real time. The residents were able to compare their own scores and comments to the anonymous results of their peers. The monthly department‐wide recognition for residents who excelled in patient communication may have created an incentive and competition among the residents. It is possible that there may be an element of the Hawthorne effect that explained the improvement in HCAHPS scores. However, all of the residents in the departments studied were already being measured through the ARC survey. The primary change was more frequent reporting of ARC survey results, and so we believe that perception of measurement alone was less likely driving the results. The findings from this study are similar to those from provider‐specific report cards, which have shown that outcomes can be improved by forcing greater accountability and competition among physicians.[19]
Brown et al. demonstrated that 2, 4‐hour physician communication workshops in their study had no impact on patient satisfaction, and so we believe that our 1‐hour workshop with only 50% attendance had minimal impact on the improved patient satisfaction scores in our study.[20] Our intervention also coincided with the implementation of the Accreditation Council for Graduate Medical Education (ACGME) work‐hour restrictions implemented in July 2011. These restrictions limited residents to 80 hours per week, intern duty periods were restricted to 16 hours and residents to 28 hours, and interns and residents required 8 to 10 hours free of duty between scheduled duty periods.[21] One of the biggest impacts of ACGME work‐hour restrictions was that interns were doing more day and night shifts rather than 28‐hour calls. However, these work‐hour restrictions were the same for all specialties and so were unlikely to explain the improved patient satisfaction associated with our intervention.
Our study has limitations. The study was a nonrandomized pre‐post study. We attempted to control for the differences in the cohorts with a multivariable regression analysis, but there may be unmeasured differences that we were unable to control for. Due to deidentification of the data, we could only control for patient health based on patient perceived health. In addition, the percentage of patients requiring ICU care in the IM cohort was higher in 2012 than in 2011. We did not identify differences in outcomes from analyses stratified by ICU or non‐ICU patients. In addition, patients who were excluded because of missing outcomes were more likely to be older and admitted through the ER. Further investigation would be needed to see if the findings of this study could be extended to other clinical situations.
In conclusion, our study found an intervention program that was associated with a significant improvement in patient satisfaction in the intervention cohort, even after adjusting for differences in the patient population, whereas there was no change in the control group. This intervention can serve as a model for academic hospitals to improve patient satisfaction, avoid revenue loss in the era of Hospital Value‐Based Purchasing, and to train the next generation of physicians on providing patient‐centered care.
Disclosure
This work was supported by the Beryl Institute and UCLA QI Initiative.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 Days. Am J Manag Care. 2011;17:41–48.
- , , , . Patients' Perception of Hospital Care in the United States. N Engl J Med. 2008;359:1921–1931.
- , , , et al. Patient satisfaction and its relationship with clinical quality and inpatient mortality in acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2010;3:188–195.
- , , . A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1).
- Centers for Medicare 70:729–732.
- , , , . Emergency department patient satisfaction: customer service training improves patient satisfaction and ratings of physician and nurse skill. J Healthc Manag. 1998;43:427–440; discussion 441–442.
- , , . Emergency department information: does it effect patients' perception and satisfaction about the care given in an emergency department? Eur J Emerg Med 1999;6:245–248.
- . Can communication skills workshops for emergency department doctors improve patient satisfaction? J Accid Emerg Med. 2000;17:251–253.
- , , , . Effects of a physician communication intervention on patient care outcomes. J Gen Intern Med. 1996;11:147–155.
- , , , , . Health‐related quality‐of‐life assessments and patient‐physician communication: a randomized controlled trial. JAMA. 2002;288:3027–3034.
- , , , . Modification of residents' behavior by preceptor feedback of patient satisfaction. J Gen Intern Med. 1986;1:394–398.
- , , . Developing physician communication skills for patient‐centered care. Health Aff (Millwood) 2010;29:1310–1318.
- ARC Medical Program @ UCLA. Available at: http://Arcmedicalprogram.Wordpress.com. Accessed July 1, 2013.
- Hospital Consumer Assessment of Healthcare Providers 12:151–162.
- Summary of HCAHPS survey results January 2010 to December 2010 discharges. Available at: http://Www.Hcahpsonline.Org/Files/Hcahps survey results table %28report_Hei_October_2011_States%29.Pdf. Accessed October 18, 2013.
- , , , et al. A randomized experiment investigating the suitability of speech‐enabled IVR and web modes for publicly reported surveys of patients' experience of hospital care. Med Care Res Rev. 2013;70:165–184.
- . Provider‐specific report cards: a tool for health sector accountability in developing countries. Health Policy Plan. 2006;21:101–109.
- , , , . Effect of clinician communication skills training on patient satisfaction: a randomized, controlled trial. Ann Intern Med. 1999;131:822–829.
- Frequently asked questions: ACGME common duty hour requirements. Available at: http://www.Acgme.Org/Acgmeweb/Portals/0/Pdfs/Dh‐Faqs2011.Pdf. Accessed January 3, 2015.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 Days. Am J Manag Care. 2011;17:41–48.
- , , , . Patients' Perception of Hospital Care in the United States. N Engl J Med. 2008;359:1921–1931.
- , , , et al. Patient satisfaction and its relationship with clinical quality and inpatient mortality in acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2010;3:188–195.
- , , . A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1).
- Centers for Medicare 70:729–732.
- , , , . Emergency department patient satisfaction: customer service training improves patient satisfaction and ratings of physician and nurse skill. J Healthc Manag. 1998;43:427–440; discussion 441–442.
- , , . Emergency department information: does it effect patients' perception and satisfaction about the care given in an emergency department? Eur J Emerg Med 1999;6:245–248.
- . Can communication skills workshops for emergency department doctors improve patient satisfaction? J Accid Emerg Med. 2000;17:251–253.
- , , , . Effects of a physician communication intervention on patient care outcomes. J Gen Intern Med. 1996;11:147–155.
- , , , , . Health‐related quality‐of‐life assessments and patient‐physician communication: a randomized controlled trial. JAMA. 2002;288:3027–3034.
- , , , . Modification of residents' behavior by preceptor feedback of patient satisfaction. J Gen Intern Med. 1986;1:394–398.
- , , . Developing physician communication skills for patient‐centered care. Health Aff (Millwood) 2010;29:1310–1318.
- ARC Medical Program @ UCLA. Available at: http://Arcmedicalprogram.Wordpress.com. Accessed July 1, 2013.
- Hospital Consumer Assessment of Healthcare Providers 12:151–162.
- Summary of HCAHPS survey results January 2010 to December 2010 discharges. Available at: http://Www.Hcahpsonline.Org/Files/Hcahps survey results table %28report_Hei_October_2011_States%29.Pdf. Accessed October 18, 2013.
- , , , et al. A randomized experiment investigating the suitability of speech‐enabled IVR and web modes for publicly reported surveys of patients' experience of hospital care. Med Care Res Rev. 2013;70:165–184.
- . Provider‐specific report cards: a tool for health sector accountability in developing countries. Health Policy Plan. 2006;21:101–109.
- , , , . Effect of clinician communication skills training on patient satisfaction: a randomized, controlled trial. Ann Intern Med. 1999;131:822–829.
- Frequently asked questions: ACGME common duty hour requirements. Available at: http://www.Acgme.Org/Acgmeweb/Portals/0/Pdfs/Dh‐Faqs2011.Pdf. Accessed January 3, 2015.
© 2015 Society of Hospital Medicine
Diagnosis by Treatment
A 46‐year‐old Mexican woman with acquired immune deficiency syndrome (AIDS), admitted for 6 months of diarrhea and failure to thrive, developed acute shortness of breath following colonoscopy. She reported dyspnea in the recumbent position, associated with a nonproductive cough, which improved with elevation of the head of the bed. She denied chest pain, palpitations, lightheadedness, hemoptysis, abdominal pain, nausea, and fever.
The approach to acute shortness of breath in hospitalized patients should include evaluation for life‐threatening cardiopulmonary processes. The patient should be assessed for cardiopulmonary process, including myocardial infarction, pulmonary embolism, aortic dissection, congestive heart failure, unstable arrhythmias, cardiac tamponade, and pneumothorax. The presence of orthopnea does suggest pulmonary congestion and a cardiac process. Given the timing of her symptoms, there is also concern for complications related to the colonoscopy, including aspiration pneumonitis, bronchospasm due to ethylene glycol, and methemoglobinemia from benzocaine used during the procedure.
The patient had been admitted the prior day for 6 months of diarrhea, weight loss, and failure to thrive. On admission, she was afebrile with a blood pressure of 110/50 mmHg and a pulse of 110 beats per minute; electrocardiogram (EKG) at the time revealed normal sinus rhythm. Her oxygen saturation was 100% on ambient air, and she had no complaints of cough, fevers, or dyspnea.
On admission, a peripherally inserted central catheter (PICC) was placed and total parenteral nutrition (TPN) was initiated. A gastroenterology consult was obtained, and colonoscopy was recommended to evaluate the cause of her chronic diarrhea. Overnight, the patient was started on polyethylene glycol electrolyte solution, with nothing else by mouth, and initiation of maintenance intravenous normal saline at 50 ml/hr in addition to her TPN. The patient expressed difficulty completing the colonoscopy preparation, but her preparation was acceptable to proceed with the procedure. She denied fever, chills, abdominal pain, and respiratory symptoms. She was taken down to endoscopy where she underwent conscious sedation, followed by an uneventful colonoscopy with mucosal biopsies. She subsequently was transported back to her hospital room in the supine position and almost immediately began to complain of mild shortness of breath. No aspiration event was witnessed following her procedure and transport.
Considering the patient's chronic diarrhea, there may be a unifying cause of both the gastrointestinal (GI) and pulmonary symptoms. Possibilities include infectious causes (Toxoplasma gondii and Trypanosoma cruzi), infiltrative diseases (amyloidosis), and metabolic processes (hyperthyroidism). More specifically, T. cruzi can cause dilated cardiomyopathy, with subsequent congestive heart failure and associated pulmonary symptoms; furthermore, it can lead to a dilated colon with abnormal bowel movements. Opportunistic infections, including Microsporidia, Cryptosporidium, Mycobacterium avium complex (MAC), and cytomegalovirus (CMV) should be considered. MAC and CMV can present with non‐bloody diarrhea and evolve into respiratory illnesses. Lastly, human immunodeficiency virus (HIV) is known to involve multiple organ systems, including the heart and gastrointestinal tract. History of prior cardiac or pulmonary disease, CD4 count and viral load, use of antiretroviral and prophylactic medications, and recent travel should be obtained.
Thirteen years previously, the patient was diagnosed with HIV, and subsequently developed AIDS with thrush and uncomplicated CMV viremia. At that time, highly active antiretroviral therapy (HAART)was initiated, but she was intolerant of her medications and received therapy intermittently. Her past medical history included multiple fractures secondary to osteoporosis. She denied any history of respiratory or cardiac symptoms. The patient was born in rural Mexico and immigrated to the United States 20 years prior. Her last visit to Mexico occurred 2 months prior to admission, and 4 months following the development of chronic diarrhea. Previously, she worked as a housekeeper and was not aware of any toxic exposures during cleaning. She denied a history of alcohol or recreational drug use. Despite her generalized weakness, her baseline functional status included performing all activities of daily living without symptoms.
Over the prior 6 months, the patient had developed diffuse watery diarrhea, associated with a 20‐pound weight loss. Stool evaluation, 1 week prior, was negative for Clostridium difficile, Microsporidia, Isospora, Cryptosporidium, Escherichia coli, Campylobacter, and ova and parasites. Her CD4 count was 8 cells per cubic millimeter.
The low CD4 count predisposes the patient to all opportunistic infections. Considering the history of CMV viremia, there is likelihood of reactivation with viremia and colitis, leading to chronic diarrhea and pneumonia. Disseminated MAC infection is also a consideration and would account for wasting, diarrhea, and dyspnea. However, it is important to note that the acute onset of dyspnea is atypical for CMV and MAC infections.
On physical exam, she was a thin woman with temporal wasting, in mild respiratory distress. Her temperature was 37.3C, blood pressure 133/55 mmHg, heart rate 140 beats/min, respiratory rate 22 breaths/min, and oxygen saturation 89% on room air. Her oropharynx was clear and without acral cyanosis. Use of accessory muscles for breathing was noted. The trachea was midline and no lymphadenopathy or thyromegaly were present. Her jugular venous pulse was normal. Cardiac exam revealed tachycardia with a new S4 gallop. A prominent apical impulse was noted. No murmurs or rubs were appreciated. There was no pulsus paradoxus. Her radial, femoral, and dorsalis pedis pulses were 2+ without delay. Her lung exam revealed inspiratory crackles involving the lower one‐third of both lungs. The lower extremities revealed 2+ pitting edema to the knees. The rest of her exam, including her neurologic evaluation, was unremarkable.
These clinical findings are consistent with left‐sided heart failure, concerning for ischemic injury or structural disorders of the heart. It is possible that the patient has had progressive heart failure, which is now unmasked by the volume received with TPN and endoscopy. If the heart failure has been longstanding, one has to consider potential non‐ischemic causes of cardiomyopathy, including infectious etiologies such as HIV, Epstein‐Barr virus (EBV), coxsackie virus, CMV, Toxoplasma gondii, and Trypanosoma cruzi; alcohol‐associated; and pericardial disease with Mycobacterium tuberculosis (MTB). Toxoplasma should be evaluated if the patient has exposure to cats. Considering her country of origin and travel history, risk factors for trypanosomiasis and MTB should be assessed.
At this point, the patient's respiratory failure should be aggressively addressed. Supplemental oxygen should be administered. She should be evaluated for acute coronary syndrome with an EKG and serial cardiac enzymes. A chest x‐ray should be obtained to grossly evaluate for pulmonary, pericardial, and aortic illnesses. Brain natriuretic peptide (BNP) levels should also be sent. Considering the evidence of volume overload and her HIV status, liver function tests, serum electrolytes, and urinalysis should be sent to exclude liver and renal involvement.
The patient was placed on 2 liters of oxygen by nasal cannula with resolution of her symptoms and improvement in her oxygen saturation to 95%. An EKG demonstrated sinus tachycardia, without evidence of ischemia. Metabolic panel revealed sodium 134 mmol/L; potassium 4.3 mmol/L; chloride 105 mmol/L; bicarbonate 16 mmol/L; creatinine 0.6 mg/dL, and liver function tests were within normal limits. Her troponin level was within the normal range for a negative value, and BNP was 823 pg/ml (normal <100). The complete blood count demonstrated leukopenia and anemia (hemoglobin 9.8 g/dL), which were unchanged from admission. Urinalysis was negative. A portable chest x‐ray demonstrated vascular congestion and mild pulmonary edema, without evidence of pneumothorax or pleural effusion.
The significantly elevated BNP and pulmonary vascular congestion seen on chest x‐ray confirm the clinical diagnosis of heart failure. However, the negative troponin and unremarkable EKG suggest a non‐ischemic cause for her symptoms. An echocardiogram should be obtained with specific emphasis on the presence of valve regurgitation, pericardial effusion, and ventricular/atrial thickening consistent with infiltrative disorders. Thyroid stimulating hormone (TSH) and serologies for infectious agents, including, T. cruzi, HIV, CMV, and Toxoplasmosis gondii, should also be sent. The patient should receive intravenous loop diuretics to improve her cardiac dynamics and pulmonary edema.
Intravenous furosemide was administered. Her symptoms improved and oxygen saturation on room air was 92%. An echocardiogram revealed global hypokinesis with left ventricular ejection fraction (LVEF) of 35% to 40%. There was no evidence of an underlying valvular or infiltrative process. TSH was normal. T. cruzi antibodies were sent.
The echocardiogram did not reveal an underlying structural heart abnormality. Infiltrative cardiomyopathies do not typically demonstrate global hypokinesis on echocardiogram, particularly without evidence of ventricular wall thickening or increased echogenicity, that can be seen in amyloid and sarcoid cardiomyopathies. Therefore, infiltrative cardiomyopathy is unlikely to be a cause of this patient's heart failure. The rapid improvement of her symptoms with furosemide decreases the likelihood of infectious causes for her acute decompensation. In reviewing the patient's history, she had developed severe chronic diarrhea associated with poor oral intake and a 20‐pound weight loss prior to hospitalization. These symptoms, along with a history of osteoporosis at an early age without traditional risk factors, indicate a state of severe malnutrition, placing her at risk for thiamine deficiency. Checking the thiamine level would be appropriate.
Considering the patient's long history of malnutrition and negative infectious and ischemic evaluation, she was empirically treated for wet beriberi with thiamine supplementation through her TPN. A serum thiamine B1 was obtained prior to supplementation. A vitamin D 25OH level was also sent, which was 15 ng/mL (normal >30 ng/mL), further suggesting malnutrition.
The patient continued to improve and furosemide was discontinued. Her initial serum thiamine level was 49 nmol/L (reference range: 70‐180 nmol/L). A repeat echocardiogram 5 days later revealed resolution of her systolic dysfunction and regional wall motion abnormality. The LVEF improved to 60%. Her colonoscopy biopsies revealed evidence of HIV enteropathy and CMV inclusion bodies. Her CMV viral load was 1223 genomes/mL. The T. cruzi antibodies were negative. She was restarted on HAART and ganciclovir. She continued to have diarrhea and was discharged home with TPN. Her serum thiamine level at discharge was 123 nmol/L.
Heart failure due to thiamine deficiency, or wet beriberi, was diagnosed considering the rapid clinical improvement in cardiac function after initiating thiamine therapy. While HIV cardiomyopathy could have contributed to heart failure in this patient, it is unlikely to improve so significantly over such a brief period of time.
DISCUSSION
Beriberi is a disease caused by severe thiamine deficiency. In fact, thiamine, also known as vitamin B1, was first named the anti‐beriberi factor in 1926. However, the earliest descriptions of beriberi can be found in Chinese medical texts dating back to 2697 BC.1 Beriberi is most commonly seen in Asia, where the diet is high in polished rice and the thiamine‐containing rice germs and husks have been removed. In the United States, thiamine‐enriched bread has virtually abolished the disease, except in severely malnourished populations such as alcoholics, those on fad diets, and patients with chronic diarrhea. Beriberi may also occur in patients with altered intestinal absorption such as post‐bariatric surgery patients.2 In 1985, the first case of beriberi as a complication of TPN without vitamin supplementation was reported.3 Subsequent cases of Wernicke's encephalopathy and beriberi have been noted in patients with gastrointestinal diseases and malabsorption on chronic TPN. More recently, thiamine deficiency has also been recognized in patients on long‐term diuretic therapy, as diuretics increase urinary excretion of this water‐soluble vitamin.4, 5 Since there is limited tissue storage of thiamine and its biologic half‐life is 10 to 20 days, high‐risk patients can develop thiamine deficiency within 4 weeks of initiation of diuretic therapy.6
Beriberi is classically divided into 2 types: wet, characterized by congestive heart failure, and dry, manifested as a symmetric peripheral neuropathy with both sensory and motor impairments.7 These 2 types of beriberi can coexist in the same patient; however, it is unclear why both types occur in some patients and not in others. Wet beriberi, also known as beriberi cardiomyopathy, typically presents as high‐output heart failure secondary to vasodilation, with a compensatory increase in blood volume and tachycardia.8 This state eventually leads to myocardial injury with systolic dysfunction and development of a low‐output state.8 Patients experience hypotension, lactic acidosis, and eventually fulminant vascular collapse. Although minor EKG changes such as sinus tachycardia, low‐voltage ventricular complexes, QT prolongation, and biphasic or inverted T waves are not uncommon in beriberi cardiomyopathy, major EKG changes, such as ST segment elevations and tall or deeply inverted T waves, are rare. Similarly, troponin elevation in beriberi cardiomyopathy is uncommon, but has been described.6
The pathogenesis of heart failure in beriberi is multifactorial. Thiamine is required for glucose to enter the Krebs cycle for aerobic metabolism, serving as a catalyst in the conversion of pyruvate to acetyl‐CoA. Without thiamine, anaerobic metabolism occurs, leading to the development of lactic acidosis and cellular malfunction. In fact, severe metabolic acidosis with serum pH values as low as 6.70 have been reported in cases of fulminant beriberi (although it is unclear if the lactic acidosis is mostly from anaerobic metabolism or from the low‐output state ultimately caused by thiamine deficiency).3
Laboratory diagnosis of thiamine deficiency, based on measurements of thiamine stores and metabolites, is often fraught with error and therefore unreliable. Serum pyruvate and lactate levels are commonly measured, and while elevated levels may be sensitive for thiamine deficiency, they are nonspecific. Measurement of whole blood thiamine is easy and the test is widely available; however, a low blood thiamine concentration is not always a sensitive indicator of deficiency since less than 1% of total body thiamine is found in whole blood.9 Additionally, this value may also be artificially elevated by thiamine intake immediately preceding the measurement. Urinary thiamine excretion has been proposed as a more accurate measurement, but this laboratory test is also problematic since urinary thiamine excretion reflects dietary intake more than total body stores.9 Erythrocyte transketolase activity (ETKA) is a functional enzyme test in which transketolase uses thiamine pyrophosphate as a catalyzer. This may be a more reliable measurement since red blood cells are among the first cells to be affected by thiamine depletion.9 Although a low ETKA level often indicates thiamine deficiency, this test is influenced by the hemoglobin concentration, and it is not widely available. Thus, the diagnosis of wet beriberi is usually made on the basis of rapid response to thiamine replacement.
Similar to the patient discussed, the clinical improvement in wet beriberi occurs within hours of treatment. There is an initial elevation in blood pressure and resolution of acidosis, followed by decrease in heart rate and normalization of cardiac output. Overall cardiac function improves within 24 to 48 hours after treatment, and return to a normal hemodynamic condition often occurs within 2 weeks of the start of treatment.10
There are no well‐established guidelines for the treatment of patients with beriberi, but general recommendations are an initial loading dose of intravenous thiamine 100 to 500 mg followed by 25 to 100 mg orally for 7 to 14 days.1 Thereafter, the daily thiamine requirement can be calculated based upon total caloric intake. The current recommendations in the United States are 0.5 mg of thiamine per 1000 kcal.1 However, one must consider whether a patient has impaired intestinal absorption or increased urinary losses when determining an appropriate maintenance dose.
Chronic malnutrition can lead to significant morbidity and mortality. Prior to admission, this patient already exhibited signs of severe malnutrition with a history of multiple pathologic fractures and diagnosis of osteoporosis. Considering her age and lack of risk factors for bone disease, osteoporosis suggests vitamin D deficiency. In this patient with chronic diarrhea caused by CMV, it is unlikely that a selective absorptive deficiency would occur. When the common causes of acute heart failure following volume challenge were excluded, the diagnosis of thiamine deficiency became more likely. Fortunately, an empiric trial of intravenous thiamine resulted in diagnosis by treatment and improvement of her cardiac function.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
KEY TEACHING POINTS
-
Hospitalists should consider vitamin B1 deficiency in patients with chronic illness and malnutrition.
-
Diagnosis of wet beriberi based on laboratory values can be challenging and, therefore, high clinical suspicion should prompt immediate treatment with thiamine.
-
Congestive heart failure due to thiamine deficiency can be reversed with thiamine replacement.
- ,,, et al.Thiamin. Modern Nutrition in Health and Disease.9th ed.1999:381–389.
- ,,, et al.One‐year outcomes of Roux‐en‐Y gastric bypass for morbidly obese adolescents: a multicenter study from the Pediatric Bariatric Study Group.J Pediatr Surg.2006;41:137–143.
- ,,.Severe acute metabolic acidosis (acute beriberi): an avoidable complication of total parenteral nutrition.J Parenter Enteral Nutr.1985;9(2):216–219.
- ,,, et al.Urinary thiamine excretion in the rat: effects of furosemide, other diuretics, and volume load.J Lab Clin Med.1999;134:232–237.
- ,,, et al.Urinary loss of thiamine is increased by low doses of furosemide in healthy volunteers.J Lab ClinMed.1999;134:238–243.
- .Increased troponin I in “wet” beriberi.J Clin Pathol.2006;59(5):555.
- ,,, et al.Shoshin syndrome: two case reports representing opposite ends of the same disease spectrum.Acta Cardiol.1998;53:195.
- ,.The challenge of cardiomyopathy.J Am Coll Cardiol.1989;13:1219–1239.
- .Loop diuretic therapy, thiamine balance, and heart failure.Congest Heart Fail.2007;13(4):244–247.
- ,,.Cardiovascular beriberi.Am J Cardiol.1972;30:418–422.
A 46‐year‐old Mexican woman with acquired immune deficiency syndrome (AIDS), admitted for 6 months of diarrhea and failure to thrive, developed acute shortness of breath following colonoscopy. She reported dyspnea in the recumbent position, associated with a nonproductive cough, which improved with elevation of the head of the bed. She denied chest pain, palpitations, lightheadedness, hemoptysis, abdominal pain, nausea, and fever.
The approach to acute shortness of breath in hospitalized patients should include evaluation for life‐threatening cardiopulmonary processes. The patient should be assessed for cardiopulmonary process, including myocardial infarction, pulmonary embolism, aortic dissection, congestive heart failure, unstable arrhythmias, cardiac tamponade, and pneumothorax. The presence of orthopnea does suggest pulmonary congestion and a cardiac process. Given the timing of her symptoms, there is also concern for complications related to the colonoscopy, including aspiration pneumonitis, bronchospasm due to ethylene glycol, and methemoglobinemia from benzocaine used during the procedure.
The patient had been admitted the prior day for 6 months of diarrhea, weight loss, and failure to thrive. On admission, she was afebrile with a blood pressure of 110/50 mmHg and a pulse of 110 beats per minute; electrocardiogram (EKG) at the time revealed normal sinus rhythm. Her oxygen saturation was 100% on ambient air, and she had no complaints of cough, fevers, or dyspnea.
On admission, a peripherally inserted central catheter (PICC) was placed and total parenteral nutrition (TPN) was initiated. A gastroenterology consult was obtained, and colonoscopy was recommended to evaluate the cause of her chronic diarrhea. Overnight, the patient was started on polyethylene glycol electrolyte solution, with nothing else by mouth, and initiation of maintenance intravenous normal saline at 50 ml/hr in addition to her TPN. The patient expressed difficulty completing the colonoscopy preparation, but her preparation was acceptable to proceed with the procedure. She denied fever, chills, abdominal pain, and respiratory symptoms. She was taken down to endoscopy where she underwent conscious sedation, followed by an uneventful colonoscopy with mucosal biopsies. She subsequently was transported back to her hospital room in the supine position and almost immediately began to complain of mild shortness of breath. No aspiration event was witnessed following her procedure and transport.
Considering the patient's chronic diarrhea, there may be a unifying cause of both the gastrointestinal (GI) and pulmonary symptoms. Possibilities include infectious causes (Toxoplasma gondii and Trypanosoma cruzi), infiltrative diseases (amyloidosis), and metabolic processes (hyperthyroidism). More specifically, T. cruzi can cause dilated cardiomyopathy, with subsequent congestive heart failure and associated pulmonary symptoms; furthermore, it can lead to a dilated colon with abnormal bowel movements. Opportunistic infections, including Microsporidia, Cryptosporidium, Mycobacterium avium complex (MAC), and cytomegalovirus (CMV) should be considered. MAC and CMV can present with non‐bloody diarrhea and evolve into respiratory illnesses. Lastly, human immunodeficiency virus (HIV) is known to involve multiple organ systems, including the heart and gastrointestinal tract. History of prior cardiac or pulmonary disease, CD4 count and viral load, use of antiretroviral and prophylactic medications, and recent travel should be obtained.
Thirteen years previously, the patient was diagnosed with HIV, and subsequently developed AIDS with thrush and uncomplicated CMV viremia. At that time, highly active antiretroviral therapy (HAART)was initiated, but she was intolerant of her medications and received therapy intermittently. Her past medical history included multiple fractures secondary to osteoporosis. She denied any history of respiratory or cardiac symptoms. The patient was born in rural Mexico and immigrated to the United States 20 years prior. Her last visit to Mexico occurred 2 months prior to admission, and 4 months following the development of chronic diarrhea. Previously, she worked as a housekeeper and was not aware of any toxic exposures during cleaning. She denied a history of alcohol or recreational drug use. Despite her generalized weakness, her baseline functional status included performing all activities of daily living without symptoms.
Over the prior 6 months, the patient had developed diffuse watery diarrhea, associated with a 20‐pound weight loss. Stool evaluation, 1 week prior, was negative for Clostridium difficile, Microsporidia, Isospora, Cryptosporidium, Escherichia coli, Campylobacter, and ova and parasites. Her CD4 count was 8 cells per cubic millimeter.
The low CD4 count predisposes the patient to all opportunistic infections. Considering the history of CMV viremia, there is likelihood of reactivation with viremia and colitis, leading to chronic diarrhea and pneumonia. Disseminated MAC infection is also a consideration and would account for wasting, diarrhea, and dyspnea. However, it is important to note that the acute onset of dyspnea is atypical for CMV and MAC infections.
On physical exam, she was a thin woman with temporal wasting, in mild respiratory distress. Her temperature was 37.3C, blood pressure 133/55 mmHg, heart rate 140 beats/min, respiratory rate 22 breaths/min, and oxygen saturation 89% on room air. Her oropharynx was clear and without acral cyanosis. Use of accessory muscles for breathing was noted. The trachea was midline and no lymphadenopathy or thyromegaly were present. Her jugular venous pulse was normal. Cardiac exam revealed tachycardia with a new S4 gallop. A prominent apical impulse was noted. No murmurs or rubs were appreciated. There was no pulsus paradoxus. Her radial, femoral, and dorsalis pedis pulses were 2+ without delay. Her lung exam revealed inspiratory crackles involving the lower one‐third of both lungs. The lower extremities revealed 2+ pitting edema to the knees. The rest of her exam, including her neurologic evaluation, was unremarkable.
These clinical findings are consistent with left‐sided heart failure, concerning for ischemic injury or structural disorders of the heart. It is possible that the patient has had progressive heart failure, which is now unmasked by the volume received with TPN and endoscopy. If the heart failure has been longstanding, one has to consider potential non‐ischemic causes of cardiomyopathy, including infectious etiologies such as HIV, Epstein‐Barr virus (EBV), coxsackie virus, CMV, Toxoplasma gondii, and Trypanosoma cruzi; alcohol‐associated; and pericardial disease with Mycobacterium tuberculosis (MTB). Toxoplasma should be evaluated if the patient has exposure to cats. Considering her country of origin and travel history, risk factors for trypanosomiasis and MTB should be assessed.
At this point, the patient's respiratory failure should be aggressively addressed. Supplemental oxygen should be administered. She should be evaluated for acute coronary syndrome with an EKG and serial cardiac enzymes. A chest x‐ray should be obtained to grossly evaluate for pulmonary, pericardial, and aortic illnesses. Brain natriuretic peptide (BNP) levels should also be sent. Considering the evidence of volume overload and her HIV status, liver function tests, serum electrolytes, and urinalysis should be sent to exclude liver and renal involvement.
The patient was placed on 2 liters of oxygen by nasal cannula with resolution of her symptoms and improvement in her oxygen saturation to 95%. An EKG demonstrated sinus tachycardia, without evidence of ischemia. Metabolic panel revealed sodium 134 mmol/L; potassium 4.3 mmol/L; chloride 105 mmol/L; bicarbonate 16 mmol/L; creatinine 0.6 mg/dL, and liver function tests were within normal limits. Her troponin level was within the normal range for a negative value, and BNP was 823 pg/ml (normal <100). The complete blood count demonstrated leukopenia and anemia (hemoglobin 9.8 g/dL), which were unchanged from admission. Urinalysis was negative. A portable chest x‐ray demonstrated vascular congestion and mild pulmonary edema, without evidence of pneumothorax or pleural effusion.
The significantly elevated BNP and pulmonary vascular congestion seen on chest x‐ray confirm the clinical diagnosis of heart failure. However, the negative troponin and unremarkable EKG suggest a non‐ischemic cause for her symptoms. An echocardiogram should be obtained with specific emphasis on the presence of valve regurgitation, pericardial effusion, and ventricular/atrial thickening consistent with infiltrative disorders. Thyroid stimulating hormone (TSH) and serologies for infectious agents, including, T. cruzi, HIV, CMV, and Toxoplasmosis gondii, should also be sent. The patient should receive intravenous loop diuretics to improve her cardiac dynamics and pulmonary edema.
Intravenous furosemide was administered. Her symptoms improved and oxygen saturation on room air was 92%. An echocardiogram revealed global hypokinesis with left ventricular ejection fraction (LVEF) of 35% to 40%. There was no evidence of an underlying valvular or infiltrative process. TSH was normal. T. cruzi antibodies were sent.
The echocardiogram did not reveal an underlying structural heart abnormality. Infiltrative cardiomyopathies do not typically demonstrate global hypokinesis on echocardiogram, particularly without evidence of ventricular wall thickening or increased echogenicity, that can be seen in amyloid and sarcoid cardiomyopathies. Therefore, infiltrative cardiomyopathy is unlikely to be a cause of this patient's heart failure. The rapid improvement of her symptoms with furosemide decreases the likelihood of infectious causes for her acute decompensation. In reviewing the patient's history, she had developed severe chronic diarrhea associated with poor oral intake and a 20‐pound weight loss prior to hospitalization. These symptoms, along with a history of osteoporosis at an early age without traditional risk factors, indicate a state of severe malnutrition, placing her at risk for thiamine deficiency. Checking the thiamine level would be appropriate.
Considering the patient's long history of malnutrition and negative infectious and ischemic evaluation, she was empirically treated for wet beriberi with thiamine supplementation through her TPN. A serum thiamine B1 was obtained prior to supplementation. A vitamin D 25OH level was also sent, which was 15 ng/mL (normal >30 ng/mL), further suggesting malnutrition.
The patient continued to improve and furosemide was discontinued. Her initial serum thiamine level was 49 nmol/L (reference range: 70‐180 nmol/L). A repeat echocardiogram 5 days later revealed resolution of her systolic dysfunction and regional wall motion abnormality. The LVEF improved to 60%. Her colonoscopy biopsies revealed evidence of HIV enteropathy and CMV inclusion bodies. Her CMV viral load was 1223 genomes/mL. The T. cruzi antibodies were negative. She was restarted on HAART and ganciclovir. She continued to have diarrhea and was discharged home with TPN. Her serum thiamine level at discharge was 123 nmol/L.
Heart failure due to thiamine deficiency, or wet beriberi, was diagnosed considering the rapid clinical improvement in cardiac function after initiating thiamine therapy. While HIV cardiomyopathy could have contributed to heart failure in this patient, it is unlikely to improve so significantly over such a brief period of time.
DISCUSSION
Beriberi is a disease caused by severe thiamine deficiency. In fact, thiamine, also known as vitamin B1, was first named the anti‐beriberi factor in 1926. However, the earliest descriptions of beriberi can be found in Chinese medical texts dating back to 2697 BC.1 Beriberi is most commonly seen in Asia, where the diet is high in polished rice and the thiamine‐containing rice germs and husks have been removed. In the United States, thiamine‐enriched bread has virtually abolished the disease, except in severely malnourished populations such as alcoholics, those on fad diets, and patients with chronic diarrhea. Beriberi may also occur in patients with altered intestinal absorption such as post‐bariatric surgery patients.2 In 1985, the first case of beriberi as a complication of TPN without vitamin supplementation was reported.3 Subsequent cases of Wernicke's encephalopathy and beriberi have been noted in patients with gastrointestinal diseases and malabsorption on chronic TPN. More recently, thiamine deficiency has also been recognized in patients on long‐term diuretic therapy, as diuretics increase urinary excretion of this water‐soluble vitamin.4, 5 Since there is limited tissue storage of thiamine and its biologic half‐life is 10 to 20 days, high‐risk patients can develop thiamine deficiency within 4 weeks of initiation of diuretic therapy.6
Beriberi is classically divided into 2 types: wet, characterized by congestive heart failure, and dry, manifested as a symmetric peripheral neuropathy with both sensory and motor impairments.7 These 2 types of beriberi can coexist in the same patient; however, it is unclear why both types occur in some patients and not in others. Wet beriberi, also known as beriberi cardiomyopathy, typically presents as high‐output heart failure secondary to vasodilation, with a compensatory increase in blood volume and tachycardia.8 This state eventually leads to myocardial injury with systolic dysfunction and development of a low‐output state.8 Patients experience hypotension, lactic acidosis, and eventually fulminant vascular collapse. Although minor EKG changes such as sinus tachycardia, low‐voltage ventricular complexes, QT prolongation, and biphasic or inverted T waves are not uncommon in beriberi cardiomyopathy, major EKG changes, such as ST segment elevations and tall or deeply inverted T waves, are rare. Similarly, troponin elevation in beriberi cardiomyopathy is uncommon, but has been described.6
The pathogenesis of heart failure in beriberi is multifactorial. Thiamine is required for glucose to enter the Krebs cycle for aerobic metabolism, serving as a catalyst in the conversion of pyruvate to acetyl‐CoA. Without thiamine, anaerobic metabolism occurs, leading to the development of lactic acidosis and cellular malfunction. In fact, severe metabolic acidosis with serum pH values as low as 6.70 have been reported in cases of fulminant beriberi (although it is unclear if the lactic acidosis is mostly from anaerobic metabolism or from the low‐output state ultimately caused by thiamine deficiency).3
Laboratory diagnosis of thiamine deficiency, based on measurements of thiamine stores and metabolites, is often fraught with error and therefore unreliable. Serum pyruvate and lactate levels are commonly measured, and while elevated levels may be sensitive for thiamine deficiency, they are nonspecific. Measurement of whole blood thiamine is easy and the test is widely available; however, a low blood thiamine concentration is not always a sensitive indicator of deficiency since less than 1% of total body thiamine is found in whole blood.9 Additionally, this value may also be artificially elevated by thiamine intake immediately preceding the measurement. Urinary thiamine excretion has been proposed as a more accurate measurement, but this laboratory test is also problematic since urinary thiamine excretion reflects dietary intake more than total body stores.9 Erythrocyte transketolase activity (ETKA) is a functional enzyme test in which transketolase uses thiamine pyrophosphate as a catalyzer. This may be a more reliable measurement since red blood cells are among the first cells to be affected by thiamine depletion.9 Although a low ETKA level often indicates thiamine deficiency, this test is influenced by the hemoglobin concentration, and it is not widely available. Thus, the diagnosis of wet beriberi is usually made on the basis of rapid response to thiamine replacement.
Similar to the patient discussed, the clinical improvement in wet beriberi occurs within hours of treatment. There is an initial elevation in blood pressure and resolution of acidosis, followed by decrease in heart rate and normalization of cardiac output. Overall cardiac function improves within 24 to 48 hours after treatment, and return to a normal hemodynamic condition often occurs within 2 weeks of the start of treatment.10
There are no well‐established guidelines for the treatment of patients with beriberi, but general recommendations are an initial loading dose of intravenous thiamine 100 to 500 mg followed by 25 to 100 mg orally for 7 to 14 days.1 Thereafter, the daily thiamine requirement can be calculated based upon total caloric intake. The current recommendations in the United States are 0.5 mg of thiamine per 1000 kcal.1 However, one must consider whether a patient has impaired intestinal absorption or increased urinary losses when determining an appropriate maintenance dose.
Chronic malnutrition can lead to significant morbidity and mortality. Prior to admission, this patient already exhibited signs of severe malnutrition with a history of multiple pathologic fractures and diagnosis of osteoporosis. Considering her age and lack of risk factors for bone disease, osteoporosis suggests vitamin D deficiency. In this patient with chronic diarrhea caused by CMV, it is unlikely that a selective absorptive deficiency would occur. When the common causes of acute heart failure following volume challenge were excluded, the diagnosis of thiamine deficiency became more likely. Fortunately, an empiric trial of intravenous thiamine resulted in diagnosis by treatment and improvement of her cardiac function.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
KEY TEACHING POINTS
-
Hospitalists should consider vitamin B1 deficiency in patients with chronic illness and malnutrition.
-
Diagnosis of wet beriberi based on laboratory values can be challenging and, therefore, high clinical suspicion should prompt immediate treatment with thiamine.
-
Congestive heart failure due to thiamine deficiency can be reversed with thiamine replacement.
A 46‐year‐old Mexican woman with acquired immune deficiency syndrome (AIDS), admitted for 6 months of diarrhea and failure to thrive, developed acute shortness of breath following colonoscopy. She reported dyspnea in the recumbent position, associated with a nonproductive cough, which improved with elevation of the head of the bed. She denied chest pain, palpitations, lightheadedness, hemoptysis, abdominal pain, nausea, and fever.
The approach to acute shortness of breath in hospitalized patients should include evaluation for life‐threatening cardiopulmonary processes. The patient should be assessed for cardiopulmonary process, including myocardial infarction, pulmonary embolism, aortic dissection, congestive heart failure, unstable arrhythmias, cardiac tamponade, and pneumothorax. The presence of orthopnea does suggest pulmonary congestion and a cardiac process. Given the timing of her symptoms, there is also concern for complications related to the colonoscopy, including aspiration pneumonitis, bronchospasm due to ethylene glycol, and methemoglobinemia from benzocaine used during the procedure.
The patient had been admitted the prior day for 6 months of diarrhea, weight loss, and failure to thrive. On admission, she was afebrile with a blood pressure of 110/50 mmHg and a pulse of 110 beats per minute; electrocardiogram (EKG) at the time revealed normal sinus rhythm. Her oxygen saturation was 100% on ambient air, and she had no complaints of cough, fevers, or dyspnea.
On admission, a peripherally inserted central catheter (PICC) was placed and total parenteral nutrition (TPN) was initiated. A gastroenterology consult was obtained, and colonoscopy was recommended to evaluate the cause of her chronic diarrhea. Overnight, the patient was started on polyethylene glycol electrolyte solution, with nothing else by mouth, and initiation of maintenance intravenous normal saline at 50 ml/hr in addition to her TPN. The patient expressed difficulty completing the colonoscopy preparation, but her preparation was acceptable to proceed with the procedure. She denied fever, chills, abdominal pain, and respiratory symptoms. She was taken down to endoscopy where she underwent conscious sedation, followed by an uneventful colonoscopy with mucosal biopsies. She subsequently was transported back to her hospital room in the supine position and almost immediately began to complain of mild shortness of breath. No aspiration event was witnessed following her procedure and transport.
Considering the patient's chronic diarrhea, there may be a unifying cause of both the gastrointestinal (GI) and pulmonary symptoms. Possibilities include infectious causes (Toxoplasma gondii and Trypanosoma cruzi), infiltrative diseases (amyloidosis), and metabolic processes (hyperthyroidism). More specifically, T. cruzi can cause dilated cardiomyopathy, with subsequent congestive heart failure and associated pulmonary symptoms; furthermore, it can lead to a dilated colon with abnormal bowel movements. Opportunistic infections, including Microsporidia, Cryptosporidium, Mycobacterium avium complex (MAC), and cytomegalovirus (CMV) should be considered. MAC and CMV can present with non‐bloody diarrhea and evolve into respiratory illnesses. Lastly, human immunodeficiency virus (HIV) is known to involve multiple organ systems, including the heart and gastrointestinal tract. History of prior cardiac or pulmonary disease, CD4 count and viral load, use of antiretroviral and prophylactic medications, and recent travel should be obtained.
Thirteen years previously, the patient was diagnosed with HIV, and subsequently developed AIDS with thrush and uncomplicated CMV viremia. At that time, highly active antiretroviral therapy (HAART)was initiated, but she was intolerant of her medications and received therapy intermittently. Her past medical history included multiple fractures secondary to osteoporosis. She denied any history of respiratory or cardiac symptoms. The patient was born in rural Mexico and immigrated to the United States 20 years prior. Her last visit to Mexico occurred 2 months prior to admission, and 4 months following the development of chronic diarrhea. Previously, she worked as a housekeeper and was not aware of any toxic exposures during cleaning. She denied a history of alcohol or recreational drug use. Despite her generalized weakness, her baseline functional status included performing all activities of daily living without symptoms.
Over the prior 6 months, the patient had developed diffuse watery diarrhea, associated with a 20‐pound weight loss. Stool evaluation, 1 week prior, was negative for Clostridium difficile, Microsporidia, Isospora, Cryptosporidium, Escherichia coli, Campylobacter, and ova and parasites. Her CD4 count was 8 cells per cubic millimeter.
The low CD4 count predisposes the patient to all opportunistic infections. Considering the history of CMV viremia, there is likelihood of reactivation with viremia and colitis, leading to chronic diarrhea and pneumonia. Disseminated MAC infection is also a consideration and would account for wasting, diarrhea, and dyspnea. However, it is important to note that the acute onset of dyspnea is atypical for CMV and MAC infections.
On physical exam, she was a thin woman with temporal wasting, in mild respiratory distress. Her temperature was 37.3C, blood pressure 133/55 mmHg, heart rate 140 beats/min, respiratory rate 22 breaths/min, and oxygen saturation 89% on room air. Her oropharynx was clear and without acral cyanosis. Use of accessory muscles for breathing was noted. The trachea was midline and no lymphadenopathy or thyromegaly were present. Her jugular venous pulse was normal. Cardiac exam revealed tachycardia with a new S4 gallop. A prominent apical impulse was noted. No murmurs or rubs were appreciated. There was no pulsus paradoxus. Her radial, femoral, and dorsalis pedis pulses were 2+ without delay. Her lung exam revealed inspiratory crackles involving the lower one‐third of both lungs. The lower extremities revealed 2+ pitting edema to the knees. The rest of her exam, including her neurologic evaluation, was unremarkable.
These clinical findings are consistent with left‐sided heart failure, concerning for ischemic injury or structural disorders of the heart. It is possible that the patient has had progressive heart failure, which is now unmasked by the volume received with TPN and endoscopy. If the heart failure has been longstanding, one has to consider potential non‐ischemic causes of cardiomyopathy, including infectious etiologies such as HIV, Epstein‐Barr virus (EBV), coxsackie virus, CMV, Toxoplasma gondii, and Trypanosoma cruzi; alcohol‐associated; and pericardial disease with Mycobacterium tuberculosis (MTB). Toxoplasma should be evaluated if the patient has exposure to cats. Considering her country of origin and travel history, risk factors for trypanosomiasis and MTB should be assessed.
At this point, the patient's respiratory failure should be aggressively addressed. Supplemental oxygen should be administered. She should be evaluated for acute coronary syndrome with an EKG and serial cardiac enzymes. A chest x‐ray should be obtained to grossly evaluate for pulmonary, pericardial, and aortic illnesses. Brain natriuretic peptide (BNP) levels should also be sent. Considering the evidence of volume overload and her HIV status, liver function tests, serum electrolytes, and urinalysis should be sent to exclude liver and renal involvement.
The patient was placed on 2 liters of oxygen by nasal cannula with resolution of her symptoms and improvement in her oxygen saturation to 95%. An EKG demonstrated sinus tachycardia, without evidence of ischemia. Metabolic panel revealed sodium 134 mmol/L; potassium 4.3 mmol/L; chloride 105 mmol/L; bicarbonate 16 mmol/L; creatinine 0.6 mg/dL, and liver function tests were within normal limits. Her troponin level was within the normal range for a negative value, and BNP was 823 pg/ml (normal <100). The complete blood count demonstrated leukopenia and anemia (hemoglobin 9.8 g/dL), which were unchanged from admission. Urinalysis was negative. A portable chest x‐ray demonstrated vascular congestion and mild pulmonary edema, without evidence of pneumothorax or pleural effusion.
The significantly elevated BNP and pulmonary vascular congestion seen on chest x‐ray confirm the clinical diagnosis of heart failure. However, the negative troponin and unremarkable EKG suggest a non‐ischemic cause for her symptoms. An echocardiogram should be obtained with specific emphasis on the presence of valve regurgitation, pericardial effusion, and ventricular/atrial thickening consistent with infiltrative disorders. Thyroid stimulating hormone (TSH) and serologies for infectious agents, including, T. cruzi, HIV, CMV, and Toxoplasmosis gondii, should also be sent. The patient should receive intravenous loop diuretics to improve her cardiac dynamics and pulmonary edema.
Intravenous furosemide was administered. Her symptoms improved and oxygen saturation on room air was 92%. An echocardiogram revealed global hypokinesis with left ventricular ejection fraction (LVEF) of 35% to 40%. There was no evidence of an underlying valvular or infiltrative process. TSH was normal. T. cruzi antibodies were sent.
The echocardiogram did not reveal an underlying structural heart abnormality. Infiltrative cardiomyopathies do not typically demonstrate global hypokinesis on echocardiogram, particularly without evidence of ventricular wall thickening or increased echogenicity, that can be seen in amyloid and sarcoid cardiomyopathies. Therefore, infiltrative cardiomyopathy is unlikely to be a cause of this patient's heart failure. The rapid improvement of her symptoms with furosemide decreases the likelihood of infectious causes for her acute decompensation. In reviewing the patient's history, she had developed severe chronic diarrhea associated with poor oral intake and a 20‐pound weight loss prior to hospitalization. These symptoms, along with a history of osteoporosis at an early age without traditional risk factors, indicate a state of severe malnutrition, placing her at risk for thiamine deficiency. Checking the thiamine level would be appropriate.
Considering the patient's long history of malnutrition and negative infectious and ischemic evaluation, she was empirically treated for wet beriberi with thiamine supplementation through her TPN. A serum thiamine B1 was obtained prior to supplementation. A vitamin D 25OH level was also sent, which was 15 ng/mL (normal >30 ng/mL), further suggesting malnutrition.
The patient continued to improve and furosemide was discontinued. Her initial serum thiamine level was 49 nmol/L (reference range: 70‐180 nmol/L). A repeat echocardiogram 5 days later revealed resolution of her systolic dysfunction and regional wall motion abnormality. The LVEF improved to 60%. Her colonoscopy biopsies revealed evidence of HIV enteropathy and CMV inclusion bodies. Her CMV viral load was 1223 genomes/mL. The T. cruzi antibodies were negative. She was restarted on HAART and ganciclovir. She continued to have diarrhea and was discharged home with TPN. Her serum thiamine level at discharge was 123 nmol/L.
Heart failure due to thiamine deficiency, or wet beriberi, was diagnosed considering the rapid clinical improvement in cardiac function after initiating thiamine therapy. While HIV cardiomyopathy could have contributed to heart failure in this patient, it is unlikely to improve so significantly over such a brief period of time.
DISCUSSION
Beriberi is a disease caused by severe thiamine deficiency. In fact, thiamine, also known as vitamin B1, was first named the anti‐beriberi factor in 1926. However, the earliest descriptions of beriberi can be found in Chinese medical texts dating back to 2697 BC.1 Beriberi is most commonly seen in Asia, where the diet is high in polished rice and the thiamine‐containing rice germs and husks have been removed. In the United States, thiamine‐enriched bread has virtually abolished the disease, except in severely malnourished populations such as alcoholics, those on fad diets, and patients with chronic diarrhea. Beriberi may also occur in patients with altered intestinal absorption such as post‐bariatric surgery patients.2 In 1985, the first case of beriberi as a complication of TPN without vitamin supplementation was reported.3 Subsequent cases of Wernicke's encephalopathy and beriberi have been noted in patients with gastrointestinal diseases and malabsorption on chronic TPN. More recently, thiamine deficiency has also been recognized in patients on long‐term diuretic therapy, as diuretics increase urinary excretion of this water‐soluble vitamin.4, 5 Since there is limited tissue storage of thiamine and its biologic half‐life is 10 to 20 days, high‐risk patients can develop thiamine deficiency within 4 weeks of initiation of diuretic therapy.6
Beriberi is classically divided into 2 types: wet, characterized by congestive heart failure, and dry, manifested as a symmetric peripheral neuropathy with both sensory and motor impairments.7 These 2 types of beriberi can coexist in the same patient; however, it is unclear why both types occur in some patients and not in others. Wet beriberi, also known as beriberi cardiomyopathy, typically presents as high‐output heart failure secondary to vasodilation, with a compensatory increase in blood volume and tachycardia.8 This state eventually leads to myocardial injury with systolic dysfunction and development of a low‐output state.8 Patients experience hypotension, lactic acidosis, and eventually fulminant vascular collapse. Although minor EKG changes such as sinus tachycardia, low‐voltage ventricular complexes, QT prolongation, and biphasic or inverted T waves are not uncommon in beriberi cardiomyopathy, major EKG changes, such as ST segment elevations and tall or deeply inverted T waves, are rare. Similarly, troponin elevation in beriberi cardiomyopathy is uncommon, but has been described.6
The pathogenesis of heart failure in beriberi is multifactorial. Thiamine is required for glucose to enter the Krebs cycle for aerobic metabolism, serving as a catalyst in the conversion of pyruvate to acetyl‐CoA. Without thiamine, anaerobic metabolism occurs, leading to the development of lactic acidosis and cellular malfunction. In fact, severe metabolic acidosis with serum pH values as low as 6.70 have been reported in cases of fulminant beriberi (although it is unclear if the lactic acidosis is mostly from anaerobic metabolism or from the low‐output state ultimately caused by thiamine deficiency).3
Laboratory diagnosis of thiamine deficiency, based on measurements of thiamine stores and metabolites, is often fraught with error and therefore unreliable. Serum pyruvate and lactate levels are commonly measured, and while elevated levels may be sensitive for thiamine deficiency, they are nonspecific. Measurement of whole blood thiamine is easy and the test is widely available; however, a low blood thiamine concentration is not always a sensitive indicator of deficiency since less than 1% of total body thiamine is found in whole blood.9 Additionally, this value may also be artificially elevated by thiamine intake immediately preceding the measurement. Urinary thiamine excretion has been proposed as a more accurate measurement, but this laboratory test is also problematic since urinary thiamine excretion reflects dietary intake more than total body stores.9 Erythrocyte transketolase activity (ETKA) is a functional enzyme test in which transketolase uses thiamine pyrophosphate as a catalyzer. This may be a more reliable measurement since red blood cells are among the first cells to be affected by thiamine depletion.9 Although a low ETKA level often indicates thiamine deficiency, this test is influenced by the hemoglobin concentration, and it is not widely available. Thus, the diagnosis of wet beriberi is usually made on the basis of rapid response to thiamine replacement.
Similar to the patient discussed, the clinical improvement in wet beriberi occurs within hours of treatment. There is an initial elevation in blood pressure and resolution of acidosis, followed by decrease in heart rate and normalization of cardiac output. Overall cardiac function improves within 24 to 48 hours after treatment, and return to a normal hemodynamic condition often occurs within 2 weeks of the start of treatment.10
There are no well‐established guidelines for the treatment of patients with beriberi, but general recommendations are an initial loading dose of intravenous thiamine 100 to 500 mg followed by 25 to 100 mg orally for 7 to 14 days.1 Thereafter, the daily thiamine requirement can be calculated based upon total caloric intake. The current recommendations in the United States are 0.5 mg of thiamine per 1000 kcal.1 However, one must consider whether a patient has impaired intestinal absorption or increased urinary losses when determining an appropriate maintenance dose.
Chronic malnutrition can lead to significant morbidity and mortality. Prior to admission, this patient already exhibited signs of severe malnutrition with a history of multiple pathologic fractures and diagnosis of osteoporosis. Considering her age and lack of risk factors for bone disease, osteoporosis suggests vitamin D deficiency. In this patient with chronic diarrhea caused by CMV, it is unlikely that a selective absorptive deficiency would occur. When the common causes of acute heart failure following volume challenge were excluded, the diagnosis of thiamine deficiency became more likely. Fortunately, an empiric trial of intravenous thiamine resulted in diagnosis by treatment and improvement of her cardiac function.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
KEY TEACHING POINTS
-
Hospitalists should consider vitamin B1 deficiency in patients with chronic illness and malnutrition.
-
Diagnosis of wet beriberi based on laboratory values can be challenging and, therefore, high clinical suspicion should prompt immediate treatment with thiamine.
-
Congestive heart failure due to thiamine deficiency can be reversed with thiamine replacement.
- ,,, et al.Thiamin. Modern Nutrition in Health and Disease.9th ed.1999:381–389.
- ,,, et al.One‐year outcomes of Roux‐en‐Y gastric bypass for morbidly obese adolescents: a multicenter study from the Pediatric Bariatric Study Group.J Pediatr Surg.2006;41:137–143.
- ,,.Severe acute metabolic acidosis (acute beriberi): an avoidable complication of total parenteral nutrition.J Parenter Enteral Nutr.1985;9(2):216–219.
- ,,, et al.Urinary thiamine excretion in the rat: effects of furosemide, other diuretics, and volume load.J Lab Clin Med.1999;134:232–237.
- ,,, et al.Urinary loss of thiamine is increased by low doses of furosemide in healthy volunteers.J Lab ClinMed.1999;134:238–243.
- .Increased troponin I in “wet” beriberi.J Clin Pathol.2006;59(5):555.
- ,,, et al.Shoshin syndrome: two case reports representing opposite ends of the same disease spectrum.Acta Cardiol.1998;53:195.
- ,.The challenge of cardiomyopathy.J Am Coll Cardiol.1989;13:1219–1239.
- .Loop diuretic therapy, thiamine balance, and heart failure.Congest Heart Fail.2007;13(4):244–247.
- ,,.Cardiovascular beriberi.Am J Cardiol.1972;30:418–422.
- ,,, et al.Thiamin. Modern Nutrition in Health and Disease.9th ed.1999:381–389.
- ,,, et al.One‐year outcomes of Roux‐en‐Y gastric bypass for morbidly obese adolescents: a multicenter study from the Pediatric Bariatric Study Group.J Pediatr Surg.2006;41:137–143.
- ,,.Severe acute metabolic acidosis (acute beriberi): an avoidable complication of total parenteral nutrition.J Parenter Enteral Nutr.1985;9(2):216–219.
- ,,, et al.Urinary thiamine excretion in the rat: effects of furosemide, other diuretics, and volume load.J Lab Clin Med.1999;134:232–237.
- ,,, et al.Urinary loss of thiamine is increased by low doses of furosemide in healthy volunteers.J Lab ClinMed.1999;134:238–243.
- .Increased troponin I in “wet” beriberi.J Clin Pathol.2006;59(5):555.
- ,,, et al.Shoshin syndrome: two case reports representing opposite ends of the same disease spectrum.Acta Cardiol.1998;53:195.
- ,.The challenge of cardiomyopathy.J Am Coll Cardiol.1989;13:1219–1239.
- .Loop diuretic therapy, thiamine balance, and heart failure.Congest Heart Fail.2007;13(4):244–247.
- ,,.Cardiovascular beriberi.Am J Cardiol.1972;30:418–422.
Satisfaction Challenge
In 1973, a survey was conducted to evaluate physician satisfaction. Less than 15% of physicians reported any doubt that they had made the right career choice, with 3.7% stating that they were “not happy.”1 Twenty years later, surveys revealed a different story: Forty percent of physicians stated that they would not choose the medical profession if they had to choose a career again.2
Dissatisfaction in medicine has been reported in diverse age groups, different areas of the country, and various medical specialties.3 When dissatisfied, physicians often leave their jobs and, consequently, the patient-physician relationship is disrupted. This turnover is quite costly to the healthcare system. In primary care, the cost of replacing a physician is estimated at $250,000.4
Here are some of the factors that contribute to burnout, as well as solutions for ensuring job satisfaction.
Challenges Ahead
Burnout is an interesting phenomenon in the medical profession. Unlike many other professionals, physicians often experience extreme fatigue and emotional exhaustion at an early stage in their careers—during medical school and residency. By midcareer, the momentum is maintained as colleagues recognize their hard work, and they continue to place service to others before themselves. Physicians who encounter burnout often experience emotional exhaustion, impaired job performance, relationship difficulties, and poor health, including irritability, sleep disturbances, headaches, depression, and drug addictions.
Increased rates of burnout have been linked to several internal and external factors. Internal factors—management style in a workplace, multiple demands at work, social support from colleagues, lack of control over the work environment—have been illustrated to correlate with higher rates of burnout. The ever-increasing demand on physicians’ time leads to higher rates of dissatisfaction. There are an exponentially increasing number of medications, tests, and procedures to discuss with patients and families. This is complicated by the rise of e-mail and the Internet, as some patients expect immediate responses to their concerns.
Some studies have shown that personality factors can lead to burnout. Compulsiveness, a trait often seen in physicians, is an adaptive behavior for the demands of medical education and practice. However, it can lead to chronic feelings of inadequacy, an exaggerated sense of responsibility, and difficulty setting limits. Furthermore, physicians often are conditioned in the psychology of postponement. It takes root in the early years of medical education and leads to habitually delaying various sources of renewal, such as vacations and relationships.
External factors include payment reductions, managing various insurers, and increasing malpractice cases.1,2,5 Evaluating the changing landscape of managed-care organizations reveals that while a small fraction of physicians are employed by them, more than 90% contract with them. Commonly cited reasons for dissatisfaction with managed care include “trafficking” of patients in and out of care, administrative paperwork, limitations on referring patients to specialists, financial incentives to curb medical workups, and pressure to evaluate increasing numbers of patients.6
Malpractice cases have increased in the past 30 years. The American Medical Association (AMA) has identified 18 states where providers are finding it challenging to purchase affordable insurance.7 An additional 26 states have been placed on “orange alert,” indicating a worsening situation in availability and affordability of insurance. Physicians who are not personally burdened by malpractice suits feel its repercussions. They practice “defensive medicine” by ordering increasing numbers of tests and procedures to avoid potential litigation. Physicians involved in lawsuits, regardless of the outcome, describe feeling shame, self-doubt, and disillusionment with medical practice.
What Makes You Happy?
In the December 2006 issue of The Hospitalist (see “Are You Satisfied?” p. 4), Mary Jo Gorman, MD, MBA, FHM, then president of SHM, pointed out five factors that contribute to physician satisfaction:
- Stimulation and challenge at work. It’s critical to have a job that requires technically difficult tasks, procedures, or intellectual challenges. The ability to interact and collaborate with other physicians further adds depth and richness to hospitalists’ clinical practice. However, it’s important to realize that overstimulation can lead to discomfort and unhappiness.
- Feeling appreciated. Recog-nition for your performance leads to feeling valued at work and has a strong correlation with overall job satisfaction. It keeps hospitalists interested and motivated. However, recognition should be personalized; otherwise, it can have a detrimental effect.
- Control over work. Auto-nomy and control over work is important to ensuring job satisfaction. This includes actively participating in the design of your work schedule and other work-related matters. When decisions are imposed on physicians, it creates tension and stress.
- Work environment. This includes the type of work, support, and opportunities for growth and development, as well as interactions with colleagues and staff.
- Income. Compensation is often fourth or fifth on the list of priorities for physicians. While all of us seek fair compensation for our work, it often is not the main reason we choose an employment.
Solutions
Burnout prevention is the responsibility of all healthcare professionals. It’s critical to promote well-being on all levels: physical, emotional, psychological, and spiritual. The following recommendations are based on various interventions established nationally to address physician burnout:
Establish realistic goals. Identify realistic goals for your professional and personal life, and actively work on balancing the two. Emphasize these goals throughout your professional career, avoiding the natural tendency for postponement.
Improve your work environment. Involve physicians in the design and management of the practice; build flexible schedules that allow coverage during important life events (i.e., graduations, births, weddings); minimize paperwork and improve efficiency; and establish a committee for open discussion of physician wellness issues.
Take care of yourself. Mentorship programs support junior members in their career development and help them balance the challenges of their personal and professional lives. Mentors can detect dissatisfaction and help physicians re-evaluate their interests and career paths. Require physicians to have their own primary-care physician (PCP) to ensure their physical and mental well-being. Offer memberships to fitness centers.
Provide opportunities to grow. Seek opportunities for medical education; address personal goals and aspirations, such as hobbies and interests; and establish sabbatical programs to gain perspective and broaden your horizons.
Fortunately, medicine has an enthusiastic applicant pool. There is hope that highly motivated and qualified students will continue to apply and enter the medical profession. However, there is concern that the dissatisfaction in medicine might influence the caliber of applicants who apply.
Medical education and training needs to address the challenges of practicing medicine. Students should be taught about the challenges of delivering high-quality care, risk management, cost containment, and utilization review. During the clinical years in medical school and residency, trainees need to experience the fast pace of medicine, the realities of payment dilemmas, and increased paperwork. It ultimately is the responsibility of educators in the medical profession to encourage students and residents to establish more accurate expectations of the practice of medicine. TH
Dr. Afsarmanesh is director of hospital medicine quality initiatives at Ronald Reagan UCLA Medical Center in Los Angeles.
References
- Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
- Hadley J, Cantor JC, Willke RJ, Feder J, Cohen AB. Young physicians most and least likely to have second thoughts about a career in medicine. Acad Med. 1992;67:180-190.
- Harvey LK, Shubat SC. AMA Public Opinion on Healthcare Issues. Chicago: American Medical Association Press; 1988.
- Leigh JP, Tancredi DJ, Kravitz RL. Physician career satisfaction within specialties. BMC Health Serv Res. 2009;9:166.
- Buchbinder SB, Wilson M, Melick CF, Powe NR. Estimates of costs of primary care physician turnover. Am J Manag Care. 1999;5(11):1431-1438.
- Reams HR, Dunstone DC. Professional satisfaction of physicians. Arch Intern Med. 1989;149:1951-1956.
- McMurray JE, Williams E, Schwartz MD, et al. Developing a model using qualitative data. J Gen Intern Med. 1997;12(11):711–714.
In 1973, a survey was conducted to evaluate physician satisfaction. Less than 15% of physicians reported any doubt that they had made the right career choice, with 3.7% stating that they were “not happy.”1 Twenty years later, surveys revealed a different story: Forty percent of physicians stated that they would not choose the medical profession if they had to choose a career again.2
Dissatisfaction in medicine has been reported in diverse age groups, different areas of the country, and various medical specialties.3 When dissatisfied, physicians often leave their jobs and, consequently, the patient-physician relationship is disrupted. This turnover is quite costly to the healthcare system. In primary care, the cost of replacing a physician is estimated at $250,000.4
Here are some of the factors that contribute to burnout, as well as solutions for ensuring job satisfaction.
Challenges Ahead
Burnout is an interesting phenomenon in the medical profession. Unlike many other professionals, physicians often experience extreme fatigue and emotional exhaustion at an early stage in their careers—during medical school and residency. By midcareer, the momentum is maintained as colleagues recognize their hard work, and they continue to place service to others before themselves. Physicians who encounter burnout often experience emotional exhaustion, impaired job performance, relationship difficulties, and poor health, including irritability, sleep disturbances, headaches, depression, and drug addictions.
Increased rates of burnout have been linked to several internal and external factors. Internal factors—management style in a workplace, multiple demands at work, social support from colleagues, lack of control over the work environment—have been illustrated to correlate with higher rates of burnout. The ever-increasing demand on physicians’ time leads to higher rates of dissatisfaction. There are an exponentially increasing number of medications, tests, and procedures to discuss with patients and families. This is complicated by the rise of e-mail and the Internet, as some patients expect immediate responses to their concerns.
Some studies have shown that personality factors can lead to burnout. Compulsiveness, a trait often seen in physicians, is an adaptive behavior for the demands of medical education and practice. However, it can lead to chronic feelings of inadequacy, an exaggerated sense of responsibility, and difficulty setting limits. Furthermore, physicians often are conditioned in the psychology of postponement. It takes root in the early years of medical education and leads to habitually delaying various sources of renewal, such as vacations and relationships.
External factors include payment reductions, managing various insurers, and increasing malpractice cases.1,2,5 Evaluating the changing landscape of managed-care organizations reveals that while a small fraction of physicians are employed by them, more than 90% contract with them. Commonly cited reasons for dissatisfaction with managed care include “trafficking” of patients in and out of care, administrative paperwork, limitations on referring patients to specialists, financial incentives to curb medical workups, and pressure to evaluate increasing numbers of patients.6
Malpractice cases have increased in the past 30 years. The American Medical Association (AMA) has identified 18 states where providers are finding it challenging to purchase affordable insurance.7 An additional 26 states have been placed on “orange alert,” indicating a worsening situation in availability and affordability of insurance. Physicians who are not personally burdened by malpractice suits feel its repercussions. They practice “defensive medicine” by ordering increasing numbers of tests and procedures to avoid potential litigation. Physicians involved in lawsuits, regardless of the outcome, describe feeling shame, self-doubt, and disillusionment with medical practice.
What Makes You Happy?
In the December 2006 issue of The Hospitalist (see “Are You Satisfied?” p. 4), Mary Jo Gorman, MD, MBA, FHM, then president of SHM, pointed out five factors that contribute to physician satisfaction:
- Stimulation and challenge at work. It’s critical to have a job that requires technically difficult tasks, procedures, or intellectual challenges. The ability to interact and collaborate with other physicians further adds depth and richness to hospitalists’ clinical practice. However, it’s important to realize that overstimulation can lead to discomfort and unhappiness.
- Feeling appreciated. Recog-nition for your performance leads to feeling valued at work and has a strong correlation with overall job satisfaction. It keeps hospitalists interested and motivated. However, recognition should be personalized; otherwise, it can have a detrimental effect.
- Control over work. Auto-nomy and control over work is important to ensuring job satisfaction. This includes actively participating in the design of your work schedule and other work-related matters. When decisions are imposed on physicians, it creates tension and stress.
- Work environment. This includes the type of work, support, and opportunities for growth and development, as well as interactions with colleagues and staff.
- Income. Compensation is often fourth or fifth on the list of priorities for physicians. While all of us seek fair compensation for our work, it often is not the main reason we choose an employment.
Solutions
Burnout prevention is the responsibility of all healthcare professionals. It’s critical to promote well-being on all levels: physical, emotional, psychological, and spiritual. The following recommendations are based on various interventions established nationally to address physician burnout:
Establish realistic goals. Identify realistic goals for your professional and personal life, and actively work on balancing the two. Emphasize these goals throughout your professional career, avoiding the natural tendency for postponement.
Improve your work environment. Involve physicians in the design and management of the practice; build flexible schedules that allow coverage during important life events (i.e., graduations, births, weddings); minimize paperwork and improve efficiency; and establish a committee for open discussion of physician wellness issues.
Take care of yourself. Mentorship programs support junior members in their career development and help them balance the challenges of their personal and professional lives. Mentors can detect dissatisfaction and help physicians re-evaluate their interests and career paths. Require physicians to have their own primary-care physician (PCP) to ensure their physical and mental well-being. Offer memberships to fitness centers.
Provide opportunities to grow. Seek opportunities for medical education; address personal goals and aspirations, such as hobbies and interests; and establish sabbatical programs to gain perspective and broaden your horizons.
Fortunately, medicine has an enthusiastic applicant pool. There is hope that highly motivated and qualified students will continue to apply and enter the medical profession. However, there is concern that the dissatisfaction in medicine might influence the caliber of applicants who apply.
Medical education and training needs to address the challenges of practicing medicine. Students should be taught about the challenges of delivering high-quality care, risk management, cost containment, and utilization review. During the clinical years in medical school and residency, trainees need to experience the fast pace of medicine, the realities of payment dilemmas, and increased paperwork. It ultimately is the responsibility of educators in the medical profession to encourage students and residents to establish more accurate expectations of the practice of medicine. TH
Dr. Afsarmanesh is director of hospital medicine quality initiatives at Ronald Reagan UCLA Medical Center in Los Angeles.
References
- Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
- Hadley J, Cantor JC, Willke RJ, Feder J, Cohen AB. Young physicians most and least likely to have second thoughts about a career in medicine. Acad Med. 1992;67:180-190.
- Harvey LK, Shubat SC. AMA Public Opinion on Healthcare Issues. Chicago: American Medical Association Press; 1988.
- Leigh JP, Tancredi DJ, Kravitz RL. Physician career satisfaction within specialties. BMC Health Serv Res. 2009;9:166.
- Buchbinder SB, Wilson M, Melick CF, Powe NR. Estimates of costs of primary care physician turnover. Am J Manag Care. 1999;5(11):1431-1438.
- Reams HR, Dunstone DC. Professional satisfaction of physicians. Arch Intern Med. 1989;149:1951-1956.
- McMurray JE, Williams E, Schwartz MD, et al. Developing a model using qualitative data. J Gen Intern Med. 1997;12(11):711–714.
In 1973, a survey was conducted to evaluate physician satisfaction. Less than 15% of physicians reported any doubt that they had made the right career choice, with 3.7% stating that they were “not happy.”1 Twenty years later, surveys revealed a different story: Forty percent of physicians stated that they would not choose the medical profession if they had to choose a career again.2
Dissatisfaction in medicine has been reported in diverse age groups, different areas of the country, and various medical specialties.3 When dissatisfied, physicians often leave their jobs and, consequently, the patient-physician relationship is disrupted. This turnover is quite costly to the healthcare system. In primary care, the cost of replacing a physician is estimated at $250,000.4
Here are some of the factors that contribute to burnout, as well as solutions for ensuring job satisfaction.
Challenges Ahead
Burnout is an interesting phenomenon in the medical profession. Unlike many other professionals, physicians often experience extreme fatigue and emotional exhaustion at an early stage in their careers—during medical school and residency. By midcareer, the momentum is maintained as colleagues recognize their hard work, and they continue to place service to others before themselves. Physicians who encounter burnout often experience emotional exhaustion, impaired job performance, relationship difficulties, and poor health, including irritability, sleep disturbances, headaches, depression, and drug addictions.
Increased rates of burnout have been linked to several internal and external factors. Internal factors—management style in a workplace, multiple demands at work, social support from colleagues, lack of control over the work environment—have been illustrated to correlate with higher rates of burnout. The ever-increasing demand on physicians’ time leads to higher rates of dissatisfaction. There are an exponentially increasing number of medications, tests, and procedures to discuss with patients and families. This is complicated by the rise of e-mail and the Internet, as some patients expect immediate responses to their concerns.
Some studies have shown that personality factors can lead to burnout. Compulsiveness, a trait often seen in physicians, is an adaptive behavior for the demands of medical education and practice. However, it can lead to chronic feelings of inadequacy, an exaggerated sense of responsibility, and difficulty setting limits. Furthermore, physicians often are conditioned in the psychology of postponement. It takes root in the early years of medical education and leads to habitually delaying various sources of renewal, such as vacations and relationships.
External factors include payment reductions, managing various insurers, and increasing malpractice cases.1,2,5 Evaluating the changing landscape of managed-care organizations reveals that while a small fraction of physicians are employed by them, more than 90% contract with them. Commonly cited reasons for dissatisfaction with managed care include “trafficking” of patients in and out of care, administrative paperwork, limitations on referring patients to specialists, financial incentives to curb medical workups, and pressure to evaluate increasing numbers of patients.6
Malpractice cases have increased in the past 30 years. The American Medical Association (AMA) has identified 18 states where providers are finding it challenging to purchase affordable insurance.7 An additional 26 states have been placed on “orange alert,” indicating a worsening situation in availability and affordability of insurance. Physicians who are not personally burdened by malpractice suits feel its repercussions. They practice “defensive medicine” by ordering increasing numbers of tests and procedures to avoid potential litigation. Physicians involved in lawsuits, regardless of the outcome, describe feeling shame, self-doubt, and disillusionment with medical practice.
What Makes You Happy?
In the December 2006 issue of The Hospitalist (see “Are You Satisfied?” p. 4), Mary Jo Gorman, MD, MBA, FHM, then president of SHM, pointed out five factors that contribute to physician satisfaction:
- Stimulation and challenge at work. It’s critical to have a job that requires technically difficult tasks, procedures, or intellectual challenges. The ability to interact and collaborate with other physicians further adds depth and richness to hospitalists’ clinical practice. However, it’s important to realize that overstimulation can lead to discomfort and unhappiness.
- Feeling appreciated. Recog-nition for your performance leads to feeling valued at work and has a strong correlation with overall job satisfaction. It keeps hospitalists interested and motivated. However, recognition should be personalized; otherwise, it can have a detrimental effect.
- Control over work. Auto-nomy and control over work is important to ensuring job satisfaction. This includes actively participating in the design of your work schedule and other work-related matters. When decisions are imposed on physicians, it creates tension and stress.
- Work environment. This includes the type of work, support, and opportunities for growth and development, as well as interactions with colleagues and staff.
- Income. Compensation is often fourth or fifth on the list of priorities for physicians. While all of us seek fair compensation for our work, it often is not the main reason we choose an employment.
Solutions
Burnout prevention is the responsibility of all healthcare professionals. It’s critical to promote well-being on all levels: physical, emotional, psychological, and spiritual. The following recommendations are based on various interventions established nationally to address physician burnout:
Establish realistic goals. Identify realistic goals for your professional and personal life, and actively work on balancing the two. Emphasize these goals throughout your professional career, avoiding the natural tendency for postponement.
Improve your work environment. Involve physicians in the design and management of the practice; build flexible schedules that allow coverage during important life events (i.e., graduations, births, weddings); minimize paperwork and improve efficiency; and establish a committee for open discussion of physician wellness issues.
Take care of yourself. Mentorship programs support junior members in their career development and help them balance the challenges of their personal and professional lives. Mentors can detect dissatisfaction and help physicians re-evaluate their interests and career paths. Require physicians to have their own primary-care physician (PCP) to ensure their physical and mental well-being. Offer memberships to fitness centers.
Provide opportunities to grow. Seek opportunities for medical education; address personal goals and aspirations, such as hobbies and interests; and establish sabbatical programs to gain perspective and broaden your horizons.
Fortunately, medicine has an enthusiastic applicant pool. There is hope that highly motivated and qualified students will continue to apply and enter the medical profession. However, there is concern that the dissatisfaction in medicine might influence the caliber of applicants who apply.
Medical education and training needs to address the challenges of practicing medicine. Students should be taught about the challenges of delivering high-quality care, risk management, cost containment, and utilization review. During the clinical years in medical school and residency, trainees need to experience the fast pace of medicine, the realities of payment dilemmas, and increased paperwork. It ultimately is the responsibility of educators in the medical profession to encourage students and residents to establish more accurate expectations of the practice of medicine. TH
Dr. Afsarmanesh is director of hospital medicine quality initiatives at Ronald Reagan UCLA Medical Center in Los Angeles.
References
- Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
- Hadley J, Cantor JC, Willke RJ, Feder J, Cohen AB. Young physicians most and least likely to have second thoughts about a career in medicine. Acad Med. 1992;67:180-190.
- Harvey LK, Shubat SC. AMA Public Opinion on Healthcare Issues. Chicago: American Medical Association Press; 1988.
- Leigh JP, Tancredi DJ, Kravitz RL. Physician career satisfaction within specialties. BMC Health Serv Res. 2009;9:166.
- Buchbinder SB, Wilson M, Melick CF, Powe NR. Estimates of costs of primary care physician turnover. Am J Manag Care. 1999;5(11):1431-1438.
- Reams HR, Dunstone DC. Professional satisfaction of physicians. Arch Intern Med. 1989;149:1951-1956.
- McMurray JE, Williams E, Schwartz MD, et al. Developing a model using qualitative data. J Gen Intern Med. 1997;12(11):711–714.
Aching for a Diagnosis
A 23‐year‐old Caucasian man presented to an outpatient clinic with a sore throat and associated subjective fevers. His evaluation included a negative rapid streptococcus test; nevertheless, he was empirically treated with amoxicillin. The following day, he experienced increasing sore throat and presented to the emergency department (ED). He was treated with prednisone and morphine sulfate and discharged home with azithromycin.
Initial considerations in a healthy young man who presents with fever and pharyngitis should focus on common infectious etiologies. Viral illnesses are the most frequent causes of sore throat and fever. These often manifest as mononucleosis‐like illnesses and include Epstein‐Barr virus (EBV) and cytomegalovirus (CMV). In this age group, it is also critical to consider sexually transmitted diseases (STDs) such as gonorrhea, human immunodeficiency virus (HIV), herpes simplex virus, and syphilis. Consideration of streptococcal pharyngitis is important. Since the rapid streptococcal antigen test is neither sensitive nor specific, confirmation of infection should be based on clinical findings and a culture of the pharynx for group A Streptococcus. Other common etiologies of fever and pharyngitis include acute or chronic sinusitis with postnasal drainage. Due to the progressive nature of the sore throat, there should be an evaluation for difficulty swallowing, problems phonating, or neck discomfort, any of which would be concerning for a retropharyngeal abscess. Additional history should be obtained with focus on sexual history, previous STDs, recent sick contacts, and other supporting signs and symptoms of viral illnesses.
Eight days after the initial onset of symptoms, the patient developed acute low back pain. The back pain was midline, severe, and constant around the lumbar spine. There was no saddle anesthesia, bowel or bladder dysfunction, or weakness or numbness in the extremities. He also noted swelling of the left fourth metacarpophalangeal joint and an erythematous rash on his right knee and anterior tibial region of the right leg. He continued to experience subjective fevers, sore throat, and swollen neck glands. Due to the severity and discomfort of symptoms, the patient returned to the ED.
With no history of trauma, the subsequent development of acute low back pain may be related to the patient's sore throat and fever. Monoarticular arthritis with contralateral skin lesions should raise suspicion for a systemic process, particularly infection or a rheumatologic syndrome. Infectious etiologies would include rheumatic fever, endocarditis with septic emboli, and osteomyelitis. Rheumatologic causes, such as ankylosing spondylitis and juvenile rheumatoid arthritis (RA), are also possibilities. The infectious evaluation should include an assessment of a history of intravenous drug use (IVDU) and underlying valvular disorders, which will increase the risk for endocarditis and therefore septic emboli. Acute HIV infection can be seen as early as 1 to 2 weeks postexposure and should be considered as well. Appropriate testing would include both conventional HIV antibody tests and HIV viral load assay. Lastly, in considering the patient's symptoms, obtaining his travel history to identify risk for Lyme disease would also be appropriate.
The patient did not report any further positive findings on review of systems. He did not have any significant past medical history and did not take any chronic medications. He had no sick contacts. He rarely drank alcohol and denied IVDU and sexual activity over the past year. He was previously involved in monogamous relationships with women. His last HIV test, 1 year prior, was negative. He did not have any history of STDs. He was a graduate student in computer science and lived in southern California. He had recently traveled to central California and France for 2 weeks, staying in larger cities. He had not been hiking during that time. His family history was significant for hypertension.
The travel history is provocative for 3 diseases of the reticuloendothelial system with possible systemic manifestations. First, toxoplasmosis, which is endemic in France where rare or raw beef and lamb are frequently consumed. It may present as a mononucleosis‐like illness and rarely as atypical pneumonia. Second, tuberculosis, which is also endemic in France, especially in major cities. Although most commonly a self‐limited respiratory disease, it may disseminate with systemic symptoms. Third, primary coccidioidomycosis, which is prevalent in the central valleys of California. The climate and wind patterns lead to aerosolization of the spores and make this a common respiratory pathogen.
The physical exam should include a detailed evaluation of the eyes for uveitis and iritis, seen in some rheumatologic disorders. A pharyngeal exam with assessment for exudate can support streptococcal pharyngitis or diphtheria. Evaluation for lymphadenopathy, while nonspecific, would be important for streptococcal pharyngitis, rheumatic fever, and juvenile RA. Further characterizing the rash is essential in distinguishing viral exanthems from the fleeting salmon‐colored maculopapular rash of juvenile RA. Assessment for peripheral stigmata of endocarditis should be done. A thorough joint exam should evaluate evidence of inflammatory or infectious joint disease.
On physical exam, he was a thin man who appeared anxious but in no acute distress. His temperature was 36.7C, blood pressure 111/68 mm Hg, heart rate 83 beats/minute, respiratory rate 16 breaths/minute, and oxygen saturation 99% on room air. Erythema was noted in the posterior oropharynx with no tonsillar exudate. There were several subcentimeter, nontender, and mobile lymph nodes in the anterior cervical chain bilaterally. The cardiovascular exam revealed normal sinus rhythm with a 2/6 systolic murmur at the apex, without radiation. His lungs were clear to auscultation. Skin exam revealed 2 blanching erythematous, indurated, and tender lesions on the right pretibial region, 2‐cm and 4‐cm in diameter. Two other similar, but smaller, lesions were noted on the left upper extremity and left ankle. His lumbar spine was slightly tender to touch. A complete joint exam was normal, including the left fourth metacarpophalangeal joint. Neurological exam, including bilateral strength, sensation, reflexes, and gait, was unremarkable.
Younger patients are subject to social‐acceptance bias and can deny sexual activity on initial inquiry. An objective evaluation for STDs with serologic workup should still be pursued. The cervical lymphadenopathy and tonsillar erythema continue to suggest a viral illness. While the systolic murmur may be physiologic, subjective fevers, disseminated cutaneous lesions, and arthritis warrant evaluation for bacterial endocarditis with blood cultures and an echocardiogram.
On exam, there is no evidence of true joint involvement and this decreases the likelihood of rheumatologic conditions, such as ankylosing spondylitis and juvenile RA. However, the skin lesions are suspicious for erythema nodosum (EN), which should prompt a biopsy and an evaluation for infectious etiologies. Serologies should include evaluation of Chlamydia, Mycoplasma, Coccidioides, and Histoplasma. I would also examine the feet carefully for potential transcutaneous inoculation by microorganisms that can produce a rash similar to EN. For instance, penetrating skin trauma can lead to pseudomonal infection. Brucella (from ingesting unpasteurized milk or milk products), Bartonella (from the scratches of feline animals), and Francisella tularensis (from rabbit exposure) can also produce skin lesions that mimic EN. These are best distinguished through a detailed history, concomitant serologic workup, and biopsy. Other noninfectious etiologies of EN can include inflammatory bowel disease, Behcet's, and sarcoidosis; however, the patient does not currently report any symptoms supporting these diagnoses. In addition to the above evaluation, complete blood count with differential, liver function tests, creatinine, and urinalysis should be obtained.
The patient's white blood cell (WBC) count was 12,100/L with 73% neutrophils, 14% lymphocytes, and 12% monocytes. Hemoglobin was 11.8 g/dL and platelet count 292,000/L. Chemistry panel and liver function tests were unremarkable. Erythrocyte sedimentation rate (ESR) was 71 mm/hour (range, 010). Urinalysis was negative for protein and red blood cells. Chest x‐ray did not illustrate any abnormalities. Computed tomography (CT) of the lumbar spine revealed a small posterior disc bulge at L4‐5 and L5‐S1.
The moderate leukocytosis with neutrophilic predominance and monocytosis raises concern for a systemic inflammatory process; the elevated ESR further supports this. Monocytosis can be seen in a number of infectious, autoimmune, and malignant conditions. Tuberculosis, brucellosis, bacterial endocarditis, syphilis, infectious mononucleosis, and viral illnesses are among the infections typically characterized by monocytosis. Autoimmune illnesses, such as systemic lupus erythematosus and RA can also have similar presentations. The patient does not have any features of an underlying malignancy, such as weight loss or night sweats; however, if the autoimmune and infectious evaluations are negative, Hodgkin's disease and certain leukemias should be considered. There is no evidence of osteomyelitis on the spine CT, which decreases the possibility of (but does not exclude) infectious or rheumatologic conditions of the spine. I would suggest a comprehensive laboratory evaluation for the discussed infectious and rheumatologic disorders.
The patient's back pain was controlled with antiinflammatory medications overnight. Due to the patient's stable condition and lack of a diagnosis, empiric antibiotics were not initiated. An extensive workup was sent, including antistreptolysin O, polymerase chain reaction for Chlamydia, Neisseria gonorrhoeae, EBV, and parvovirus B19 DNA, serologies for Coccidioides immunoglobulin G (IgG) and IgM, urinary antigen for Histoplasma, HIV enzyme‐linked immunosorbent assay (ELISA) and Western blot, serum angiotensin‐converting enzyme level, C‐reactive protein, rheumatoid factor, antinuclear antibody, and antidouble‐stranded DNA antibodies.
Without a clear diagnosis, I would recommend against treatment with empiric antibiotics. At this point, I agree with waiting for the results of the pending workup.
On hospital day 1, the patient developed severe acute left ankle pain. On examination, the joint was exquisitely tender with decreased range of motion. Arthrocentesis was promptly performed. The synovial fluid WBC count was 1370/L with a differential of 82% neutrophils and 18% monocytes. No crystals were identified and the bacterial Gram stain was negative. He was treated with antiinflammatory medications. Bacterial blood cultures, obtained from the day of admission, were negative.
The arthrocentesis reveals a polymorphonuclear‐predominant fluid; however, the WBC count in the fluid is only mildly elevated. While the elevated monocyte count could again be consistent with viral arthropathies or juvenile RA, there is currently no systemic evidence of either illness. It is important to await the results of the final cultures, but the low WBC count and negative Gram stain decrease the probability of a septic joint. Empiric antibiotics to cover Gram‐positive organisms and gonococci would not be unreasonable, pending joint fluid culture results. The monocytosis could also be consistent with a fungal arthritis.
On hospital day 2, the results of the rheumatologic and infectious evaluation were negative with the exception of C‐reactive protein, which was 11.8 mg/dL (normal, <0.8), antinuclear antibody titer of 1:160 (normal, <1:40), Coccidioides IgM enzyme immunoassay (EIA) 0.710 (negative, <0.150), and Coccidioides tube‐precipitin (TP) immunodiffusion (ID) antibody‐positive. Coccidioides IgG EIA was negative.
The serologic tests are consistent with primary coccidioidomycosis. This is often a challenging diagnosis due to the nonspecific signs and symptoms, such as cough, fever, myalgias, and fatigue. Since screening EIAs are sensitive but not specific, concern for coccidioidomycosis or abnormal EIA results should prompt confirmatory testing with complement fixation titers (CF) and TP ID. Treatment with fluconazole should be initiated. Since the patient does not have central nervous system (CNS) symptoms, I would not recommend lumbar puncture at this point. However, a bone scan should be done for assessment of the back pain.
The patient was diagnosed with primary coccidioidomycosis infection with immune‐complexmediated arthritis and EN. A bone scan was negative. The patient was treated with fluconazole and discharged with 3 months of therapy. At follow‐up clinic visits after completion of therapy, his symptoms had resolved and his titers had normalized.
Discussion
The diagnosis of coccidioidomycosis is often challenging due to its protean manifestations. Four clinical syndromes are commonly seen: (1) acute pneumonia, (2) chronic progressive pneumonia, (3) pulmonary cavities and nodules, and (4) extrapulmonary disease involving the skin, lymph nodes, bones, joints, and meninges. The most common clinical manifestation, acute pneumonia, may be indistinguishable from other causes of community‐acquired pneumonia (CAP). In a study of CAP in Arizona, 29% of cases were positive for coccidioidal infection through serologic evaluation.1 Features suggestive of coccidioidal infection include fatigue, severe headache, and pleuritic chest pain. Adenopathy in the hilar or paratracheal regions can be seen in 25% of infections.2 Chronic progressive pneumonia refers to infections in which symptoms, including cough, hemoptysis, and weight loss, persist for longer than 3 months. Pulmonary nodules and cavities are residual manifestations of primary pulmonary infection and occur in 2% to 8% of cases. Extrapulmonary disease develops in less than 5% of immunocompetent patients with primary pulmonary infection, with higher prevalence in patients of African American and Filipino decent. Immunocompromised patients are at increased risk for extrapulmonary infection. The most serious site of extrapulmonary disease is the meninges. Coccidioidal meningitis carries nearly 100% mortality rate if left untreated. The presentation is variable with up to 75% of cases reporting headache. While coccidioidal pneumonia also frequently presents with headache, symptoms including altered mental status, focal neurological deficits, and persistent or progressive headache are more suggestive of meningeal disease.3
Patients with any presentation of coccidioidomycosis can display immune‐mediated manifestations such as EN, arthralgias (desert rheumatism), and in some cases mild conjunctivitis.4 It is hypothesized that these findings occur due to a hypersensitivity reaction to coccidioidomycosis.4 EN is an inflammatory process of the subcutaneous fat, which presents as tender and erythematous nodules typically on the lower extremities. EN is not a disease entity or site of metastatic infection, but a response to underlying illness. Its recognition should trigger a search for the primary etiology, as guided by the patient's history and clinical presentation. The differential diagnosis for EN is broad and includes rheumatologic, infectious, medication‐related, inflammatory, and idiopathic processes (Table 1). Coccidioidomycosis should be strongly considered based on geographical location, with the vast majority of cases seen in southern California, Arizona, Nevada, New Mexico, and Texas. While the pathophysiology of EN has not been completely elucidated, the lesions may reflect a vigorous immune response conferring a protective advantage. Interestingly, a study of pregnant women with coccidioidomycosis revealed a decreased incidence of disseminated disease in patients with EN.5, 6
| Rheumatologic/autoimmune |
| Systemic lupus erythematosus |
| Wegener's granulomatosis |
| Sarcoidosis |
| Infectious |
| Streptococcus pyogenes causing pharyngitis (most common) |
| Borrelia burgdorferi |
| Mycoplasma pneumoniae |
| Bartonella henselae |
| Shigella |
| Campylobacter jejuni |
| Salmonella |
| Yersinia enterocolitica |
| Chlamydia |
| Brucella |
| Escherichia coli |
| Treponema pallidum |
| Mycobacterium leprae |
| Neisseria gonorrhoeae |
| Mycobacterium tuberculosis |
| Human immunodeficiency virus |
| Epstein‐Barr virus |
| Cytomegalovirus |
| Influenza |
| Varicella Zoster virus |
| Coccidioides immitis |
| Histoplasma capsulatum |
| Blastomyces dermatitidis |
| Dermatophytic fungal infections (rare) |
| Gastrointestinal |
| Ulcerative colitis |
| Crohn's disease |
| Celiac disease |
| Behcet's disease |
| Medications |
| Oral contraceptives |
| Proton pump inhibitors |
| Sulfonamides |
| Leukotriene modifiers (montelukast) |
| Hepatitis B vaccine |
| Isoretinoin |
| Miscellaneous |
| Hodgkins lymphoma |
| Sweet's syndrome |
Coccidioidomycosis is also associated with immune‐mediated arthralgias and arthritis. These manifestations occur in up to one‐third of patients with concomitant EN. Arthritis may be monoarticular or polyarticular, often affecting large joints such as the knees or ankles. It is important to note that septic arthritis can also occur and should be differentiated from rheumatism by joint aspiration.
The diagnosis of coccidioidomycosis can be made by serologic testing, direct isolation of the organism on culture, or visualization on tissue biopsy. Of these methods, serologic testing is most commonly utilized. The 2007 Infectious Disease Society of America (IDSA) and American Thoracic Society guidelines recommend diagnostic testing in hospitalized patients with CAP who reside in or have recently traveled (within 2 weeks) to endemic areas.7 There are multiple approaches to serologic diagnosis based on identification of IgM or IgG antibodies to various coccidioidal antigens. During the early phase of infection, TP ID and EIA can be utilized to detect IgM antibodies. While EIA testing has 92% sensitivity, it has high rates of false‐positive results, and therefore confirmatory testing with ID is recommended. ID has variable sensitivity, but 90% of patients will test positive by 3 weeks of infection.8 During the later phase of the infection, IgG antibodies are detected either quantitatively by CF or qualitatively by ID and EIA. CF can provide information on the severity of illness and prognosis based on titer levels, as well as serving as a marker for response to treatment.2 Positive titers greater than 1:32 suggest disseminated disease. In addition, CF titer in the cerebrospinal fluid is the test of choice in diagnosis of coccidioidal meningitis. An evaluation for disseminated disease should be initiated if the patient has any risk factors or clinically concerning symptoms for bone or CNS involvement. This evaluation includes a bone scan and lumbar puncture. All patients should be assessed for immunocompromised status.
The management of coccidioidomycosis is based on the extent of infection, the severity of illness, and the immune status of the patient. In 95% of cases of uncomplicated pulmonary disease in an immunocompetent host, the symptoms will resolve without treatment with antifungal agents.9 The decision to treat uncomplicated pulmonary disease is based on severity of illness. While there is no consensus recommendation, commonly used indicators for treatment include persistent fever, age >55 years, symptoms greater than 2 months, hilar adenopathy, diffuse pulmonary infiltrates, weight loss, and inability to work.9 In patients with chronic progressive pneumonia or extrapulmonary involvement, treatment with antifungal medications should be initiated. While fluconazole remains the preferred treatment in coccidioidal pneumonia and meningitis, amphotericin B preparations should be considered for diffuse coccidioidal pneumonia and disseminated disease, including refractory meningitis.9 The use of newer azoles, particularly posaconazole, has been studied in a limited number of patients with refractory coccidioidomycosis with improvement in symptoms.10 Frequent follow‐up visits are recommended to detect progression of disease or to document resolution, with improving symptoms and decreasing titers. Duration of therapy in uncomplicated cases should be at least 3 months. Treatment of extrapulmonary disease can span years, and in the case of meningitis lifetime treatment is recommended given the high rate of relapse.
While the patient and the clinicians were aching for a diagnosis after the initial negative evaluation, recognition of the immunologic manifestations of coccidioidomycosis was essential in this case. Coccidioidomycosis should be considered in patients presenting with EN, regardless of presence of concurrent pulmonary symptoms; particularly in patients living in or with recent travel to endemic areas. Furthermore, the severity of symptoms can guide the decision and duration of treatment.
Teaching Points
-
Coccidioidomycosis has 4 main clinical presentations: (1) acute pneumonia, (2) chronic progressive pneumonia, (3) pulmonary cavities and nodules, and (4) extrapulmonary disease.
-
Independent of pulmonary symptoms, coccidioidomycosis can present with immune‐mediated manifestations, such as EN and arthritis.
-
The diagnosis of coccidioidomycosis often relies on serologic testing for early and late infection.
-
Treatment of coccidioidomycosis is based on risk factors and severity of symptoms. High‐risk and symptomatic patients can be treated with fluconazole or amphotericin B.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring the patient and the discussant.
- ,,, et al.Coccidioidomycosis as a common cause of community‐acquired pneumonia.Emerg Infect Dis.2006;12:958–962.
- ,.Coccidioidomycosis.Mayo Clin Proc.2008;83:343–349.
- ,.Coccidioidal meningitis.Clin Infect Dis.2006;42:103–107.
- ,.Coccidioidomycosis: host response and vaccine development.Clin Microbiol Rev.2004;17:804–839.
- ,,.Erythema nodosum in pregnant patients with coccidioidomycosis.Clin Infect Dis.1998;27:1201–1203.
- .Protective effects of erythema nodosum in coccidioidomycosis.Lancet.1999;353:168.
- ,,, et al.Infectious Disease Society of America/American Thoracic Society consensus guidelines on management of community acquired pneumonia in adults.Clin Infect Dis.2007;44:S27–S72
- .Laboratory aspects in the diagnosis of coccidioidomycosis.Ann N Y Acad Sci.2007;1111:301–314.
- ,,, et al.Coccidioidomycosis.Clin Infect Dis.2005;41:1217–1223.
- ,,,,.Refractory coccidioidomycosis treated with posaconazole.Clin Infect Dis.2005;40:1770–1776.
A 23‐year‐old Caucasian man presented to an outpatient clinic with a sore throat and associated subjective fevers. His evaluation included a negative rapid streptococcus test; nevertheless, he was empirically treated with amoxicillin. The following day, he experienced increasing sore throat and presented to the emergency department (ED). He was treated with prednisone and morphine sulfate and discharged home with azithromycin.
Initial considerations in a healthy young man who presents with fever and pharyngitis should focus on common infectious etiologies. Viral illnesses are the most frequent causes of sore throat and fever. These often manifest as mononucleosis‐like illnesses and include Epstein‐Barr virus (EBV) and cytomegalovirus (CMV). In this age group, it is also critical to consider sexually transmitted diseases (STDs) such as gonorrhea, human immunodeficiency virus (HIV), herpes simplex virus, and syphilis. Consideration of streptococcal pharyngitis is important. Since the rapid streptococcal antigen test is neither sensitive nor specific, confirmation of infection should be based on clinical findings and a culture of the pharynx for group A Streptococcus. Other common etiologies of fever and pharyngitis include acute or chronic sinusitis with postnasal drainage. Due to the progressive nature of the sore throat, there should be an evaluation for difficulty swallowing, problems phonating, or neck discomfort, any of which would be concerning for a retropharyngeal abscess. Additional history should be obtained with focus on sexual history, previous STDs, recent sick contacts, and other supporting signs and symptoms of viral illnesses.
Eight days after the initial onset of symptoms, the patient developed acute low back pain. The back pain was midline, severe, and constant around the lumbar spine. There was no saddle anesthesia, bowel or bladder dysfunction, or weakness or numbness in the extremities. He also noted swelling of the left fourth metacarpophalangeal joint and an erythematous rash on his right knee and anterior tibial region of the right leg. He continued to experience subjective fevers, sore throat, and swollen neck glands. Due to the severity and discomfort of symptoms, the patient returned to the ED.
With no history of trauma, the subsequent development of acute low back pain may be related to the patient's sore throat and fever. Monoarticular arthritis with contralateral skin lesions should raise suspicion for a systemic process, particularly infection or a rheumatologic syndrome. Infectious etiologies would include rheumatic fever, endocarditis with septic emboli, and osteomyelitis. Rheumatologic causes, such as ankylosing spondylitis and juvenile rheumatoid arthritis (RA), are also possibilities. The infectious evaluation should include an assessment of a history of intravenous drug use (IVDU) and underlying valvular disorders, which will increase the risk for endocarditis and therefore septic emboli. Acute HIV infection can be seen as early as 1 to 2 weeks postexposure and should be considered as well. Appropriate testing would include both conventional HIV antibody tests and HIV viral load assay. Lastly, in considering the patient's symptoms, obtaining his travel history to identify risk for Lyme disease would also be appropriate.
The patient did not report any further positive findings on review of systems. He did not have any significant past medical history and did not take any chronic medications. He had no sick contacts. He rarely drank alcohol and denied IVDU and sexual activity over the past year. He was previously involved in monogamous relationships with women. His last HIV test, 1 year prior, was negative. He did not have any history of STDs. He was a graduate student in computer science and lived in southern California. He had recently traveled to central California and France for 2 weeks, staying in larger cities. He had not been hiking during that time. His family history was significant for hypertension.
The travel history is provocative for 3 diseases of the reticuloendothelial system with possible systemic manifestations. First, toxoplasmosis, which is endemic in France where rare or raw beef and lamb are frequently consumed. It may present as a mononucleosis‐like illness and rarely as atypical pneumonia. Second, tuberculosis, which is also endemic in France, especially in major cities. Although most commonly a self‐limited respiratory disease, it may disseminate with systemic symptoms. Third, primary coccidioidomycosis, which is prevalent in the central valleys of California. The climate and wind patterns lead to aerosolization of the spores and make this a common respiratory pathogen.
The physical exam should include a detailed evaluation of the eyes for uveitis and iritis, seen in some rheumatologic disorders. A pharyngeal exam with assessment for exudate can support streptococcal pharyngitis or diphtheria. Evaluation for lymphadenopathy, while nonspecific, would be important for streptococcal pharyngitis, rheumatic fever, and juvenile RA. Further characterizing the rash is essential in distinguishing viral exanthems from the fleeting salmon‐colored maculopapular rash of juvenile RA. Assessment for peripheral stigmata of endocarditis should be done. A thorough joint exam should evaluate evidence of inflammatory or infectious joint disease.
On physical exam, he was a thin man who appeared anxious but in no acute distress. His temperature was 36.7C, blood pressure 111/68 mm Hg, heart rate 83 beats/minute, respiratory rate 16 breaths/minute, and oxygen saturation 99% on room air. Erythema was noted in the posterior oropharynx with no tonsillar exudate. There were several subcentimeter, nontender, and mobile lymph nodes in the anterior cervical chain bilaterally. The cardiovascular exam revealed normal sinus rhythm with a 2/6 systolic murmur at the apex, without radiation. His lungs were clear to auscultation. Skin exam revealed 2 blanching erythematous, indurated, and tender lesions on the right pretibial region, 2‐cm and 4‐cm in diameter. Two other similar, but smaller, lesions were noted on the left upper extremity and left ankle. His lumbar spine was slightly tender to touch. A complete joint exam was normal, including the left fourth metacarpophalangeal joint. Neurological exam, including bilateral strength, sensation, reflexes, and gait, was unremarkable.
Younger patients are subject to social‐acceptance bias and can deny sexual activity on initial inquiry. An objective evaluation for STDs with serologic workup should still be pursued. The cervical lymphadenopathy and tonsillar erythema continue to suggest a viral illness. While the systolic murmur may be physiologic, subjective fevers, disseminated cutaneous lesions, and arthritis warrant evaluation for bacterial endocarditis with blood cultures and an echocardiogram.
On exam, there is no evidence of true joint involvement and this decreases the likelihood of rheumatologic conditions, such as ankylosing spondylitis and juvenile RA. However, the skin lesions are suspicious for erythema nodosum (EN), which should prompt a biopsy and an evaluation for infectious etiologies. Serologies should include evaluation of Chlamydia, Mycoplasma, Coccidioides, and Histoplasma. I would also examine the feet carefully for potential transcutaneous inoculation by microorganisms that can produce a rash similar to EN. For instance, penetrating skin trauma can lead to pseudomonal infection. Brucella (from ingesting unpasteurized milk or milk products), Bartonella (from the scratches of feline animals), and Francisella tularensis (from rabbit exposure) can also produce skin lesions that mimic EN. These are best distinguished through a detailed history, concomitant serologic workup, and biopsy. Other noninfectious etiologies of EN can include inflammatory bowel disease, Behcet's, and sarcoidosis; however, the patient does not currently report any symptoms supporting these diagnoses. In addition to the above evaluation, complete blood count with differential, liver function tests, creatinine, and urinalysis should be obtained.
The patient's white blood cell (WBC) count was 12,100/L with 73% neutrophils, 14% lymphocytes, and 12% monocytes. Hemoglobin was 11.8 g/dL and platelet count 292,000/L. Chemistry panel and liver function tests were unremarkable. Erythrocyte sedimentation rate (ESR) was 71 mm/hour (range, 010). Urinalysis was negative for protein and red blood cells. Chest x‐ray did not illustrate any abnormalities. Computed tomography (CT) of the lumbar spine revealed a small posterior disc bulge at L4‐5 and L5‐S1.
The moderate leukocytosis with neutrophilic predominance and monocytosis raises concern for a systemic inflammatory process; the elevated ESR further supports this. Monocytosis can be seen in a number of infectious, autoimmune, and malignant conditions. Tuberculosis, brucellosis, bacterial endocarditis, syphilis, infectious mononucleosis, and viral illnesses are among the infections typically characterized by monocytosis. Autoimmune illnesses, such as systemic lupus erythematosus and RA can also have similar presentations. The patient does not have any features of an underlying malignancy, such as weight loss or night sweats; however, if the autoimmune and infectious evaluations are negative, Hodgkin's disease and certain leukemias should be considered. There is no evidence of osteomyelitis on the spine CT, which decreases the possibility of (but does not exclude) infectious or rheumatologic conditions of the spine. I would suggest a comprehensive laboratory evaluation for the discussed infectious and rheumatologic disorders.
The patient's back pain was controlled with antiinflammatory medications overnight. Due to the patient's stable condition and lack of a diagnosis, empiric antibiotics were not initiated. An extensive workup was sent, including antistreptolysin O, polymerase chain reaction for Chlamydia, Neisseria gonorrhoeae, EBV, and parvovirus B19 DNA, serologies for Coccidioides immunoglobulin G (IgG) and IgM, urinary antigen for Histoplasma, HIV enzyme‐linked immunosorbent assay (ELISA) and Western blot, serum angiotensin‐converting enzyme level, C‐reactive protein, rheumatoid factor, antinuclear antibody, and antidouble‐stranded DNA antibodies.
Without a clear diagnosis, I would recommend against treatment with empiric antibiotics. At this point, I agree with waiting for the results of the pending workup.
On hospital day 1, the patient developed severe acute left ankle pain. On examination, the joint was exquisitely tender with decreased range of motion. Arthrocentesis was promptly performed. The synovial fluid WBC count was 1370/L with a differential of 82% neutrophils and 18% monocytes. No crystals were identified and the bacterial Gram stain was negative. He was treated with antiinflammatory medications. Bacterial blood cultures, obtained from the day of admission, were negative.
The arthrocentesis reveals a polymorphonuclear‐predominant fluid; however, the WBC count in the fluid is only mildly elevated. While the elevated monocyte count could again be consistent with viral arthropathies or juvenile RA, there is currently no systemic evidence of either illness. It is important to await the results of the final cultures, but the low WBC count and negative Gram stain decrease the probability of a septic joint. Empiric antibiotics to cover Gram‐positive organisms and gonococci would not be unreasonable, pending joint fluid culture results. The monocytosis could also be consistent with a fungal arthritis.
On hospital day 2, the results of the rheumatologic and infectious evaluation were negative with the exception of C‐reactive protein, which was 11.8 mg/dL (normal, <0.8), antinuclear antibody titer of 1:160 (normal, <1:40), Coccidioides IgM enzyme immunoassay (EIA) 0.710 (negative, <0.150), and Coccidioides tube‐precipitin (TP) immunodiffusion (ID) antibody‐positive. Coccidioides IgG EIA was negative.
The serologic tests are consistent with primary coccidioidomycosis. This is often a challenging diagnosis due to the nonspecific signs and symptoms, such as cough, fever, myalgias, and fatigue. Since screening EIAs are sensitive but not specific, concern for coccidioidomycosis or abnormal EIA results should prompt confirmatory testing with complement fixation titers (CF) and TP ID. Treatment with fluconazole should be initiated. Since the patient does not have central nervous system (CNS) symptoms, I would not recommend lumbar puncture at this point. However, a bone scan should be done for assessment of the back pain.
The patient was diagnosed with primary coccidioidomycosis infection with immune‐complexmediated arthritis and EN. A bone scan was negative. The patient was treated with fluconazole and discharged with 3 months of therapy. At follow‐up clinic visits after completion of therapy, his symptoms had resolved and his titers had normalized.
Discussion
The diagnosis of coccidioidomycosis is often challenging due to its protean manifestations. Four clinical syndromes are commonly seen: (1) acute pneumonia, (2) chronic progressive pneumonia, (3) pulmonary cavities and nodules, and (4) extrapulmonary disease involving the skin, lymph nodes, bones, joints, and meninges. The most common clinical manifestation, acute pneumonia, may be indistinguishable from other causes of community‐acquired pneumonia (CAP). In a study of CAP in Arizona, 29% of cases were positive for coccidioidal infection through serologic evaluation.1 Features suggestive of coccidioidal infection include fatigue, severe headache, and pleuritic chest pain. Adenopathy in the hilar or paratracheal regions can be seen in 25% of infections.2 Chronic progressive pneumonia refers to infections in which symptoms, including cough, hemoptysis, and weight loss, persist for longer than 3 months. Pulmonary nodules and cavities are residual manifestations of primary pulmonary infection and occur in 2% to 8% of cases. Extrapulmonary disease develops in less than 5% of immunocompetent patients with primary pulmonary infection, with higher prevalence in patients of African American and Filipino decent. Immunocompromised patients are at increased risk for extrapulmonary infection. The most serious site of extrapulmonary disease is the meninges. Coccidioidal meningitis carries nearly 100% mortality rate if left untreated. The presentation is variable with up to 75% of cases reporting headache. While coccidioidal pneumonia also frequently presents with headache, symptoms including altered mental status, focal neurological deficits, and persistent or progressive headache are more suggestive of meningeal disease.3
Patients with any presentation of coccidioidomycosis can display immune‐mediated manifestations such as EN, arthralgias (desert rheumatism), and in some cases mild conjunctivitis.4 It is hypothesized that these findings occur due to a hypersensitivity reaction to coccidioidomycosis.4 EN is an inflammatory process of the subcutaneous fat, which presents as tender and erythematous nodules typically on the lower extremities. EN is not a disease entity or site of metastatic infection, but a response to underlying illness. Its recognition should trigger a search for the primary etiology, as guided by the patient's history and clinical presentation. The differential diagnosis for EN is broad and includes rheumatologic, infectious, medication‐related, inflammatory, and idiopathic processes (Table 1). Coccidioidomycosis should be strongly considered based on geographical location, with the vast majority of cases seen in southern California, Arizona, Nevada, New Mexico, and Texas. While the pathophysiology of EN has not been completely elucidated, the lesions may reflect a vigorous immune response conferring a protective advantage. Interestingly, a study of pregnant women with coccidioidomycosis revealed a decreased incidence of disseminated disease in patients with EN.5, 6
| Rheumatologic/autoimmune |
| Systemic lupus erythematosus |
| Wegener's granulomatosis |
| Sarcoidosis |
| Infectious |
| Streptococcus pyogenes causing pharyngitis (most common) |
| Borrelia burgdorferi |
| Mycoplasma pneumoniae |
| Bartonella henselae |
| Shigella |
| Campylobacter jejuni |
| Salmonella |
| Yersinia enterocolitica |
| Chlamydia |
| Brucella |
| Escherichia coli |
| Treponema pallidum |
| Mycobacterium leprae |
| Neisseria gonorrhoeae |
| Mycobacterium tuberculosis |
| Human immunodeficiency virus |
| Epstein‐Barr virus |
| Cytomegalovirus |
| Influenza |
| Varicella Zoster virus |
| Coccidioides immitis |
| Histoplasma capsulatum |
| Blastomyces dermatitidis |
| Dermatophytic fungal infections (rare) |
| Gastrointestinal |
| Ulcerative colitis |
| Crohn's disease |
| Celiac disease |
| Behcet's disease |
| Medications |
| Oral contraceptives |
| Proton pump inhibitors |
| Sulfonamides |
| Leukotriene modifiers (montelukast) |
| Hepatitis B vaccine |
| Isoretinoin |
| Miscellaneous |
| Hodgkins lymphoma |
| Sweet's syndrome |
Coccidioidomycosis is also associated with immune‐mediated arthralgias and arthritis. These manifestations occur in up to one‐third of patients with concomitant EN. Arthritis may be monoarticular or polyarticular, often affecting large joints such as the knees or ankles. It is important to note that septic arthritis can also occur and should be differentiated from rheumatism by joint aspiration.
The diagnosis of coccidioidomycosis can be made by serologic testing, direct isolation of the organism on culture, or visualization on tissue biopsy. Of these methods, serologic testing is most commonly utilized. The 2007 Infectious Disease Society of America (IDSA) and American Thoracic Society guidelines recommend diagnostic testing in hospitalized patients with CAP who reside in or have recently traveled (within 2 weeks) to endemic areas.7 There are multiple approaches to serologic diagnosis based on identification of IgM or IgG antibodies to various coccidioidal antigens. During the early phase of infection, TP ID and EIA can be utilized to detect IgM antibodies. While EIA testing has 92% sensitivity, it has high rates of false‐positive results, and therefore confirmatory testing with ID is recommended. ID has variable sensitivity, but 90% of patients will test positive by 3 weeks of infection.8 During the later phase of the infection, IgG antibodies are detected either quantitatively by CF or qualitatively by ID and EIA. CF can provide information on the severity of illness and prognosis based on titer levels, as well as serving as a marker for response to treatment.2 Positive titers greater than 1:32 suggest disseminated disease. In addition, CF titer in the cerebrospinal fluid is the test of choice in diagnosis of coccidioidal meningitis. An evaluation for disseminated disease should be initiated if the patient has any risk factors or clinically concerning symptoms for bone or CNS involvement. This evaluation includes a bone scan and lumbar puncture. All patients should be assessed for immunocompromised status.
The management of coccidioidomycosis is based on the extent of infection, the severity of illness, and the immune status of the patient. In 95% of cases of uncomplicated pulmonary disease in an immunocompetent host, the symptoms will resolve without treatment with antifungal agents.9 The decision to treat uncomplicated pulmonary disease is based on severity of illness. While there is no consensus recommendation, commonly used indicators for treatment include persistent fever, age >55 years, symptoms greater than 2 months, hilar adenopathy, diffuse pulmonary infiltrates, weight loss, and inability to work.9 In patients with chronic progressive pneumonia or extrapulmonary involvement, treatment with antifungal medications should be initiated. While fluconazole remains the preferred treatment in coccidioidal pneumonia and meningitis, amphotericin B preparations should be considered for diffuse coccidioidal pneumonia and disseminated disease, including refractory meningitis.9 The use of newer azoles, particularly posaconazole, has been studied in a limited number of patients with refractory coccidioidomycosis with improvement in symptoms.10 Frequent follow‐up visits are recommended to detect progression of disease or to document resolution, with improving symptoms and decreasing titers. Duration of therapy in uncomplicated cases should be at least 3 months. Treatment of extrapulmonary disease can span years, and in the case of meningitis lifetime treatment is recommended given the high rate of relapse.
While the patient and the clinicians were aching for a diagnosis after the initial negative evaluation, recognition of the immunologic manifestations of coccidioidomycosis was essential in this case. Coccidioidomycosis should be considered in patients presenting with EN, regardless of presence of concurrent pulmonary symptoms; particularly in patients living in or with recent travel to endemic areas. Furthermore, the severity of symptoms can guide the decision and duration of treatment.
Teaching Points
-
Coccidioidomycosis has 4 main clinical presentations: (1) acute pneumonia, (2) chronic progressive pneumonia, (3) pulmonary cavities and nodules, and (4) extrapulmonary disease.
-
Independent of pulmonary symptoms, coccidioidomycosis can present with immune‐mediated manifestations, such as EN and arthritis.
-
The diagnosis of coccidioidomycosis often relies on serologic testing for early and late infection.
-
Treatment of coccidioidomycosis is based on risk factors and severity of symptoms. High‐risk and symptomatic patients can be treated with fluconazole or amphotericin B.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring the patient and the discussant.
A 23‐year‐old Caucasian man presented to an outpatient clinic with a sore throat and associated subjective fevers. His evaluation included a negative rapid streptococcus test; nevertheless, he was empirically treated with amoxicillin. The following day, he experienced increasing sore throat and presented to the emergency department (ED). He was treated with prednisone and morphine sulfate and discharged home with azithromycin.
Initial considerations in a healthy young man who presents with fever and pharyngitis should focus on common infectious etiologies. Viral illnesses are the most frequent causes of sore throat and fever. These often manifest as mononucleosis‐like illnesses and include Epstein‐Barr virus (EBV) and cytomegalovirus (CMV). In this age group, it is also critical to consider sexually transmitted diseases (STDs) such as gonorrhea, human immunodeficiency virus (HIV), herpes simplex virus, and syphilis. Consideration of streptococcal pharyngitis is important. Since the rapid streptococcal antigen test is neither sensitive nor specific, confirmation of infection should be based on clinical findings and a culture of the pharynx for group A Streptococcus. Other common etiologies of fever and pharyngitis include acute or chronic sinusitis with postnasal drainage. Due to the progressive nature of the sore throat, there should be an evaluation for difficulty swallowing, problems phonating, or neck discomfort, any of which would be concerning for a retropharyngeal abscess. Additional history should be obtained with focus on sexual history, previous STDs, recent sick contacts, and other supporting signs and symptoms of viral illnesses.
Eight days after the initial onset of symptoms, the patient developed acute low back pain. The back pain was midline, severe, and constant around the lumbar spine. There was no saddle anesthesia, bowel or bladder dysfunction, or weakness or numbness in the extremities. He also noted swelling of the left fourth metacarpophalangeal joint and an erythematous rash on his right knee and anterior tibial region of the right leg. He continued to experience subjective fevers, sore throat, and swollen neck glands. Due to the severity and discomfort of symptoms, the patient returned to the ED.
With no history of trauma, the subsequent development of acute low back pain may be related to the patient's sore throat and fever. Monoarticular arthritis with contralateral skin lesions should raise suspicion for a systemic process, particularly infection or a rheumatologic syndrome. Infectious etiologies would include rheumatic fever, endocarditis with septic emboli, and osteomyelitis. Rheumatologic causes, such as ankylosing spondylitis and juvenile rheumatoid arthritis (RA), are also possibilities. The infectious evaluation should include an assessment of a history of intravenous drug use (IVDU) and underlying valvular disorders, which will increase the risk for endocarditis and therefore septic emboli. Acute HIV infection can be seen as early as 1 to 2 weeks postexposure and should be considered as well. Appropriate testing would include both conventional HIV antibody tests and HIV viral load assay. Lastly, in considering the patient's symptoms, obtaining his travel history to identify risk for Lyme disease would also be appropriate.
The patient did not report any further positive findings on review of systems. He did not have any significant past medical history and did not take any chronic medications. He had no sick contacts. He rarely drank alcohol and denied IVDU and sexual activity over the past year. He was previously involved in monogamous relationships with women. His last HIV test, 1 year prior, was negative. He did not have any history of STDs. He was a graduate student in computer science and lived in southern California. He had recently traveled to central California and France for 2 weeks, staying in larger cities. He had not been hiking during that time. His family history was significant for hypertension.
The travel history is provocative for 3 diseases of the reticuloendothelial system with possible systemic manifestations. First, toxoplasmosis, which is endemic in France where rare or raw beef and lamb are frequently consumed. It may present as a mononucleosis‐like illness and rarely as atypical pneumonia. Second, tuberculosis, which is also endemic in France, especially in major cities. Although most commonly a self‐limited respiratory disease, it may disseminate with systemic symptoms. Third, primary coccidioidomycosis, which is prevalent in the central valleys of California. The climate and wind patterns lead to aerosolization of the spores and make this a common respiratory pathogen.
The physical exam should include a detailed evaluation of the eyes for uveitis and iritis, seen in some rheumatologic disorders. A pharyngeal exam with assessment for exudate can support streptococcal pharyngitis or diphtheria. Evaluation for lymphadenopathy, while nonspecific, would be important for streptococcal pharyngitis, rheumatic fever, and juvenile RA. Further characterizing the rash is essential in distinguishing viral exanthems from the fleeting salmon‐colored maculopapular rash of juvenile RA. Assessment for peripheral stigmata of endocarditis should be done. A thorough joint exam should evaluate evidence of inflammatory or infectious joint disease.
On physical exam, he was a thin man who appeared anxious but in no acute distress. His temperature was 36.7C, blood pressure 111/68 mm Hg, heart rate 83 beats/minute, respiratory rate 16 breaths/minute, and oxygen saturation 99% on room air. Erythema was noted in the posterior oropharynx with no tonsillar exudate. There were several subcentimeter, nontender, and mobile lymph nodes in the anterior cervical chain bilaterally. The cardiovascular exam revealed normal sinus rhythm with a 2/6 systolic murmur at the apex, without radiation. His lungs were clear to auscultation. Skin exam revealed 2 blanching erythematous, indurated, and tender lesions on the right pretibial region, 2‐cm and 4‐cm in diameter. Two other similar, but smaller, lesions were noted on the left upper extremity and left ankle. His lumbar spine was slightly tender to touch. A complete joint exam was normal, including the left fourth metacarpophalangeal joint. Neurological exam, including bilateral strength, sensation, reflexes, and gait, was unremarkable.
Younger patients are subject to social‐acceptance bias and can deny sexual activity on initial inquiry. An objective evaluation for STDs with serologic workup should still be pursued. The cervical lymphadenopathy and tonsillar erythema continue to suggest a viral illness. While the systolic murmur may be physiologic, subjective fevers, disseminated cutaneous lesions, and arthritis warrant evaluation for bacterial endocarditis with blood cultures and an echocardiogram.
On exam, there is no evidence of true joint involvement and this decreases the likelihood of rheumatologic conditions, such as ankylosing spondylitis and juvenile RA. However, the skin lesions are suspicious for erythema nodosum (EN), which should prompt a biopsy and an evaluation for infectious etiologies. Serologies should include evaluation of Chlamydia, Mycoplasma, Coccidioides, and Histoplasma. I would also examine the feet carefully for potential transcutaneous inoculation by microorganisms that can produce a rash similar to EN. For instance, penetrating skin trauma can lead to pseudomonal infection. Brucella (from ingesting unpasteurized milk or milk products), Bartonella (from the scratches of feline animals), and Francisella tularensis (from rabbit exposure) can also produce skin lesions that mimic EN. These are best distinguished through a detailed history, concomitant serologic workup, and biopsy. Other noninfectious etiologies of EN can include inflammatory bowel disease, Behcet's, and sarcoidosis; however, the patient does not currently report any symptoms supporting these diagnoses. In addition to the above evaluation, complete blood count with differential, liver function tests, creatinine, and urinalysis should be obtained.
The patient's white blood cell (WBC) count was 12,100/L with 73% neutrophils, 14% lymphocytes, and 12% monocytes. Hemoglobin was 11.8 g/dL and platelet count 292,000/L. Chemistry panel and liver function tests were unremarkable. Erythrocyte sedimentation rate (ESR) was 71 mm/hour (range, 010). Urinalysis was negative for protein and red blood cells. Chest x‐ray did not illustrate any abnormalities. Computed tomography (CT) of the lumbar spine revealed a small posterior disc bulge at L4‐5 and L5‐S1.
The moderate leukocytosis with neutrophilic predominance and monocytosis raises concern for a systemic inflammatory process; the elevated ESR further supports this. Monocytosis can be seen in a number of infectious, autoimmune, and malignant conditions. Tuberculosis, brucellosis, bacterial endocarditis, syphilis, infectious mononucleosis, and viral illnesses are among the infections typically characterized by monocytosis. Autoimmune illnesses, such as systemic lupus erythematosus and RA can also have similar presentations. The patient does not have any features of an underlying malignancy, such as weight loss or night sweats; however, if the autoimmune and infectious evaluations are negative, Hodgkin's disease and certain leukemias should be considered. There is no evidence of osteomyelitis on the spine CT, which decreases the possibility of (but does not exclude) infectious or rheumatologic conditions of the spine. I would suggest a comprehensive laboratory evaluation for the discussed infectious and rheumatologic disorders.
The patient's back pain was controlled with antiinflammatory medications overnight. Due to the patient's stable condition and lack of a diagnosis, empiric antibiotics were not initiated. An extensive workup was sent, including antistreptolysin O, polymerase chain reaction for Chlamydia, Neisseria gonorrhoeae, EBV, and parvovirus B19 DNA, serologies for Coccidioides immunoglobulin G (IgG) and IgM, urinary antigen for Histoplasma, HIV enzyme‐linked immunosorbent assay (ELISA) and Western blot, serum angiotensin‐converting enzyme level, C‐reactive protein, rheumatoid factor, antinuclear antibody, and antidouble‐stranded DNA antibodies.
Without a clear diagnosis, I would recommend against treatment with empiric antibiotics. At this point, I agree with waiting for the results of the pending workup.
On hospital day 1, the patient developed severe acute left ankle pain. On examination, the joint was exquisitely tender with decreased range of motion. Arthrocentesis was promptly performed. The synovial fluid WBC count was 1370/L with a differential of 82% neutrophils and 18% monocytes. No crystals were identified and the bacterial Gram stain was negative. He was treated with antiinflammatory medications. Bacterial blood cultures, obtained from the day of admission, were negative.
The arthrocentesis reveals a polymorphonuclear‐predominant fluid; however, the WBC count in the fluid is only mildly elevated. While the elevated monocyte count could again be consistent with viral arthropathies or juvenile RA, there is currently no systemic evidence of either illness. It is important to await the results of the final cultures, but the low WBC count and negative Gram stain decrease the probability of a septic joint. Empiric antibiotics to cover Gram‐positive organisms and gonococci would not be unreasonable, pending joint fluid culture results. The monocytosis could also be consistent with a fungal arthritis.
On hospital day 2, the results of the rheumatologic and infectious evaluation were negative with the exception of C‐reactive protein, which was 11.8 mg/dL (normal, <0.8), antinuclear antibody titer of 1:160 (normal, <1:40), Coccidioides IgM enzyme immunoassay (EIA) 0.710 (negative, <0.150), and Coccidioides tube‐precipitin (TP) immunodiffusion (ID) antibody‐positive. Coccidioides IgG EIA was negative.
The serologic tests are consistent with primary coccidioidomycosis. This is often a challenging diagnosis due to the nonspecific signs and symptoms, such as cough, fever, myalgias, and fatigue. Since screening EIAs are sensitive but not specific, concern for coccidioidomycosis or abnormal EIA results should prompt confirmatory testing with complement fixation titers (CF) and TP ID. Treatment with fluconazole should be initiated. Since the patient does not have central nervous system (CNS) symptoms, I would not recommend lumbar puncture at this point. However, a bone scan should be done for assessment of the back pain.
The patient was diagnosed with primary coccidioidomycosis infection with immune‐complexmediated arthritis and EN. A bone scan was negative. The patient was treated with fluconazole and discharged with 3 months of therapy. At follow‐up clinic visits after completion of therapy, his symptoms had resolved and his titers had normalized.
Discussion
The diagnosis of coccidioidomycosis is often challenging due to its protean manifestations. Four clinical syndromes are commonly seen: (1) acute pneumonia, (2) chronic progressive pneumonia, (3) pulmonary cavities and nodules, and (4) extrapulmonary disease involving the skin, lymph nodes, bones, joints, and meninges. The most common clinical manifestation, acute pneumonia, may be indistinguishable from other causes of community‐acquired pneumonia (CAP). In a study of CAP in Arizona, 29% of cases were positive for coccidioidal infection through serologic evaluation.1 Features suggestive of coccidioidal infection include fatigue, severe headache, and pleuritic chest pain. Adenopathy in the hilar or paratracheal regions can be seen in 25% of infections.2 Chronic progressive pneumonia refers to infections in which symptoms, including cough, hemoptysis, and weight loss, persist for longer than 3 months. Pulmonary nodules and cavities are residual manifestations of primary pulmonary infection and occur in 2% to 8% of cases. Extrapulmonary disease develops in less than 5% of immunocompetent patients with primary pulmonary infection, with higher prevalence in patients of African American and Filipino decent. Immunocompromised patients are at increased risk for extrapulmonary infection. The most serious site of extrapulmonary disease is the meninges. Coccidioidal meningitis carries nearly 100% mortality rate if left untreated. The presentation is variable with up to 75% of cases reporting headache. While coccidioidal pneumonia also frequently presents with headache, symptoms including altered mental status, focal neurological deficits, and persistent or progressive headache are more suggestive of meningeal disease.3
Patients with any presentation of coccidioidomycosis can display immune‐mediated manifestations such as EN, arthralgias (desert rheumatism), and in some cases mild conjunctivitis.4 It is hypothesized that these findings occur due to a hypersensitivity reaction to coccidioidomycosis.4 EN is an inflammatory process of the subcutaneous fat, which presents as tender and erythematous nodules typically on the lower extremities. EN is not a disease entity or site of metastatic infection, but a response to underlying illness. Its recognition should trigger a search for the primary etiology, as guided by the patient's history and clinical presentation. The differential diagnosis for EN is broad and includes rheumatologic, infectious, medication‐related, inflammatory, and idiopathic processes (Table 1). Coccidioidomycosis should be strongly considered based on geographical location, with the vast majority of cases seen in southern California, Arizona, Nevada, New Mexico, and Texas. While the pathophysiology of EN has not been completely elucidated, the lesions may reflect a vigorous immune response conferring a protective advantage. Interestingly, a study of pregnant women with coccidioidomycosis revealed a decreased incidence of disseminated disease in patients with EN.5, 6
| Rheumatologic/autoimmune |
| Systemic lupus erythematosus |
| Wegener's granulomatosis |
| Sarcoidosis |
| Infectious |
| Streptococcus pyogenes causing pharyngitis (most common) |
| Borrelia burgdorferi |
| Mycoplasma pneumoniae |
| Bartonella henselae |
| Shigella |
| Campylobacter jejuni |
| Salmonella |
| Yersinia enterocolitica |
| Chlamydia |
| Brucella |
| Escherichia coli |
| Treponema pallidum |
| Mycobacterium leprae |
| Neisseria gonorrhoeae |
| Mycobacterium tuberculosis |
| Human immunodeficiency virus |
| Epstein‐Barr virus |
| Cytomegalovirus |
| Influenza |
| Varicella Zoster virus |
| Coccidioides immitis |
| Histoplasma capsulatum |
| Blastomyces dermatitidis |
| Dermatophytic fungal infections (rare) |
| Gastrointestinal |
| Ulcerative colitis |
| Crohn's disease |
| Celiac disease |
| Behcet's disease |
| Medications |
| Oral contraceptives |
| Proton pump inhibitors |
| Sulfonamides |
| Leukotriene modifiers (montelukast) |
| Hepatitis B vaccine |
| Isoretinoin |
| Miscellaneous |
| Hodgkins lymphoma |
| Sweet's syndrome |
Coccidioidomycosis is also associated with immune‐mediated arthralgias and arthritis. These manifestations occur in up to one‐third of patients with concomitant EN. Arthritis may be monoarticular or polyarticular, often affecting large joints such as the knees or ankles. It is important to note that septic arthritis can also occur and should be differentiated from rheumatism by joint aspiration.
The diagnosis of coccidioidomycosis can be made by serologic testing, direct isolation of the organism on culture, or visualization on tissue biopsy. Of these methods, serologic testing is most commonly utilized. The 2007 Infectious Disease Society of America (IDSA) and American Thoracic Society guidelines recommend diagnostic testing in hospitalized patients with CAP who reside in or have recently traveled (within 2 weeks) to endemic areas.7 There are multiple approaches to serologic diagnosis based on identification of IgM or IgG antibodies to various coccidioidal antigens. During the early phase of infection, TP ID and EIA can be utilized to detect IgM antibodies. While EIA testing has 92% sensitivity, it has high rates of false‐positive results, and therefore confirmatory testing with ID is recommended. ID has variable sensitivity, but 90% of patients will test positive by 3 weeks of infection.8 During the later phase of the infection, IgG antibodies are detected either quantitatively by CF or qualitatively by ID and EIA. CF can provide information on the severity of illness and prognosis based on titer levels, as well as serving as a marker for response to treatment.2 Positive titers greater than 1:32 suggest disseminated disease. In addition, CF titer in the cerebrospinal fluid is the test of choice in diagnosis of coccidioidal meningitis. An evaluation for disseminated disease should be initiated if the patient has any risk factors or clinically concerning symptoms for bone or CNS involvement. This evaluation includes a bone scan and lumbar puncture. All patients should be assessed for immunocompromised status.
The management of coccidioidomycosis is based on the extent of infection, the severity of illness, and the immune status of the patient. In 95% of cases of uncomplicated pulmonary disease in an immunocompetent host, the symptoms will resolve without treatment with antifungal agents.9 The decision to treat uncomplicated pulmonary disease is based on severity of illness. While there is no consensus recommendation, commonly used indicators for treatment include persistent fever, age >55 years, symptoms greater than 2 months, hilar adenopathy, diffuse pulmonary infiltrates, weight loss, and inability to work.9 In patients with chronic progressive pneumonia or extrapulmonary involvement, treatment with antifungal medications should be initiated. While fluconazole remains the preferred treatment in coccidioidal pneumonia and meningitis, amphotericin B preparations should be considered for diffuse coccidioidal pneumonia and disseminated disease, including refractory meningitis.9 The use of newer azoles, particularly posaconazole, has been studied in a limited number of patients with refractory coccidioidomycosis with improvement in symptoms.10 Frequent follow‐up visits are recommended to detect progression of disease or to document resolution, with improving symptoms and decreasing titers. Duration of therapy in uncomplicated cases should be at least 3 months. Treatment of extrapulmonary disease can span years, and in the case of meningitis lifetime treatment is recommended given the high rate of relapse.
While the patient and the clinicians were aching for a diagnosis after the initial negative evaluation, recognition of the immunologic manifestations of coccidioidomycosis was essential in this case. Coccidioidomycosis should be considered in patients presenting with EN, regardless of presence of concurrent pulmonary symptoms; particularly in patients living in or with recent travel to endemic areas. Furthermore, the severity of symptoms can guide the decision and duration of treatment.
Teaching Points
-
Coccidioidomycosis has 4 main clinical presentations: (1) acute pneumonia, (2) chronic progressive pneumonia, (3) pulmonary cavities and nodules, and (4) extrapulmonary disease.
-
Independent of pulmonary symptoms, coccidioidomycosis can present with immune‐mediated manifestations, such as EN and arthritis.
-
The diagnosis of coccidioidomycosis often relies on serologic testing for early and late infection.
-
Treatment of coccidioidomycosis is based on risk factors and severity of symptoms. High‐risk and symptomatic patients can be treated with fluconazole or amphotericin B.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring the patient and the discussant.
- ,,, et al.Coccidioidomycosis as a common cause of community‐acquired pneumonia.Emerg Infect Dis.2006;12:958–962.
- ,.Coccidioidomycosis.Mayo Clin Proc.2008;83:343–349.
- ,.Coccidioidal meningitis.Clin Infect Dis.2006;42:103–107.
- ,.Coccidioidomycosis: host response and vaccine development.Clin Microbiol Rev.2004;17:804–839.
- ,,.Erythema nodosum in pregnant patients with coccidioidomycosis.Clin Infect Dis.1998;27:1201–1203.
- .Protective effects of erythema nodosum in coccidioidomycosis.Lancet.1999;353:168.
- ,,, et al.Infectious Disease Society of America/American Thoracic Society consensus guidelines on management of community acquired pneumonia in adults.Clin Infect Dis.2007;44:S27–S72
- .Laboratory aspects in the diagnosis of coccidioidomycosis.Ann N Y Acad Sci.2007;1111:301–314.
- ,,, et al.Coccidioidomycosis.Clin Infect Dis.2005;41:1217–1223.
- ,,,,.Refractory coccidioidomycosis treated with posaconazole.Clin Infect Dis.2005;40:1770–1776.
- ,,, et al.Coccidioidomycosis as a common cause of community‐acquired pneumonia.Emerg Infect Dis.2006;12:958–962.
- ,.Coccidioidomycosis.Mayo Clin Proc.2008;83:343–349.
- ,.Coccidioidal meningitis.Clin Infect Dis.2006;42:103–107.
- ,.Coccidioidomycosis: host response and vaccine development.Clin Microbiol Rev.2004;17:804–839.
- ,,.Erythema nodosum in pregnant patients with coccidioidomycosis.Clin Infect Dis.1998;27:1201–1203.
- .Protective effects of erythema nodosum in coccidioidomycosis.Lancet.1999;353:168.
- ,,, et al.Infectious Disease Society of America/American Thoracic Society consensus guidelines on management of community acquired pneumonia in adults.Clin Infect Dis.2007;44:S27–S72
- .Laboratory aspects in the diagnosis of coccidioidomycosis.Ann N Y Acad Sci.2007;1111:301–314.
- ,,, et al.Coccidioidomycosis.Clin Infect Dis.2005;41:1217–1223.
- ,,,,.Refractory coccidioidomycosis treated with posaconazole.Clin Infect Dis.2005;40:1770–1776.
Calcinosis universalis
A 38‐year‐old woman with juvenile dermatomyositis (JDM) and calcinosis universalis presented with 3 days of drainage from a lesion on her right elbow. An examination of the elbow revealed diffuse and firm subcutaneous nodules with overlying erythema. X‐rays illustrated soft‐tissue calcifications in the forearm and elbow without evidence of osteomyelitis (Figure 1). Wound cultures grew Staphylococcus aureus, and the patient was started on intravenous antibiotics for abscess treatment.
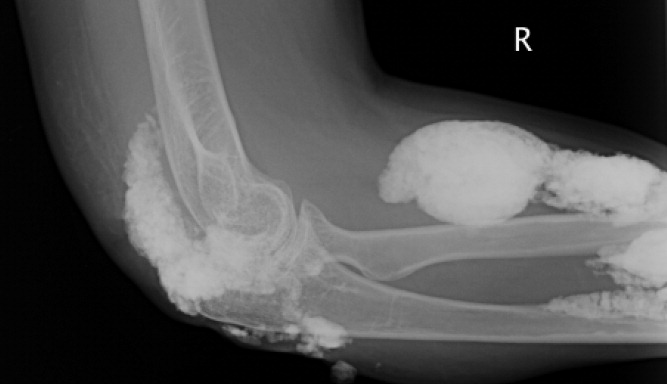
Calcinosis universalis is soft‐tissue calcification presenting as a complication of JDM. It is often detected in childhood in 30% to 70% of patients. It is hypothesized that calcinosis is due to chronic tissue inflammation, as seen in JDM, leading to muscle damage, releasing calcium, and inducing mineralization. Calcinosis universalis often presents as calcified nodules and plaques in areas of repeated trauma, such as joints, extremities, and buttocks (Figures 13). Calcification is localized in subcutaneous tissue, fascial planes, tendons, or intramuscular areas. It can cause debilitating secondary complications such as skin ulcerations expressing calcified material, superimposed infections of skin lesions, joint contractures with severe arthralgias, and muscle atrophy. Calcinosis has been correlated with severity of JDM with presence of cardiac involvement and use of more than one immunosuppression medication.1 It has also been associated with the degree of vasculopathy and delay in initiation of therapy for controlling inflammation in JDM.2
Soft‐tissue calcification can be classified into 5 categories:
-
Dystrophic calcification occurs in injured tissues with normal calcium, phosphorus, and parathyroid hormone levels, as seen in this patient. Calcified nodules or plaques occur in the extremities and buttocks. This is most often seen in JDM, scleroderma, and systemic lupus erythematosus.
-
Metastatic calcification affects normal tissues with abnormal levels of calcium and phosphorus. It is seen in large joints as well as arteries and visceral organs. It is associated with hyperparathyroidism, hypervitaminosis D, and malignancies.
-
Calciphylaxis with abnormal calcium and phosphorus metabolism causes small‐vessel calcification in patients with chronic renal failure.
-
Tumoral calcification is a familial condition with normal calcium levels but elevated phosphorus levels. Large subcutaneous calcifications are seen near high‐pressure areas and joints.
-
Idiopathic calcification is seen in healthy children and young adults with normal calcium metabolism and appears as multiple subcutaneous calcifications.2
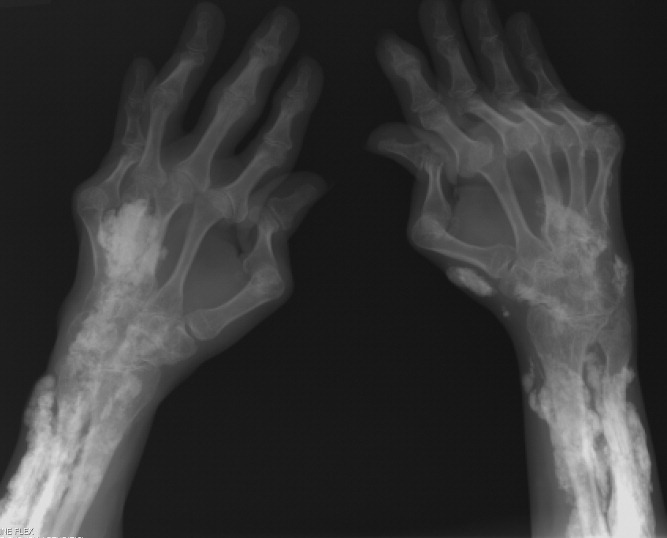
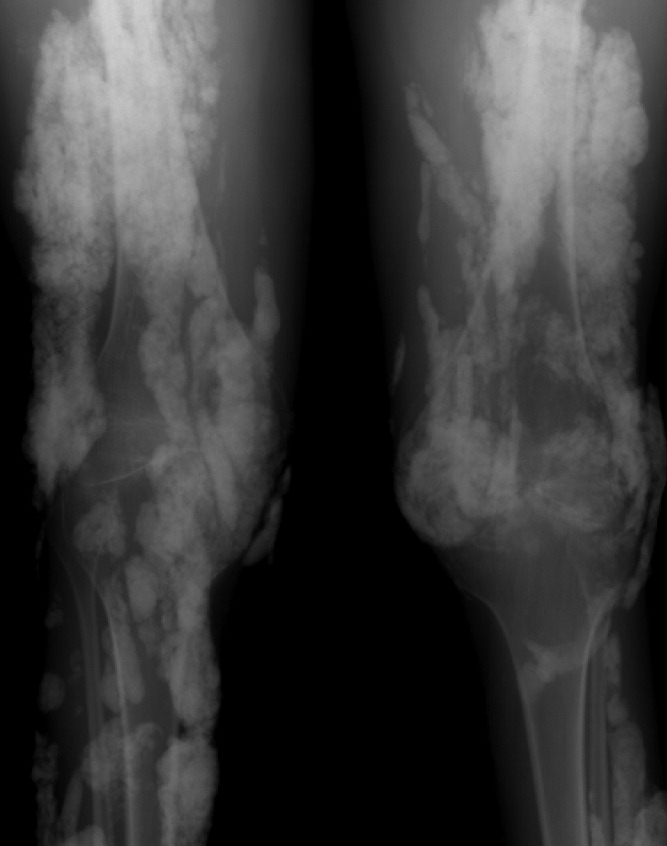
Although multiple therapeutic options have been tried for the management or prevention of calcinosis, there is currently no accepted standard of treatment. In patients with calcinosis, warfarin, probenecid, colchicine, bisphosphonates, minocycline, diltiazem, aluminum hydroxide, corticosteroids, and salicylate have been attempted with variable results. Other therapeutic options include carbon dioxide laser treatments and surgical excision of large plaques. Decreasing muscle inflammation with aggressive treatment of JDM may improve outcomes and decrease the incidence of calcification.3 Unfortunately, once calcinosis has occurred, it is highly refractory to medical therapy.
Calcinosis universalis can lead to severe functional impairment. It can be distinguished from other types of calcinosis by diffuse involvement of muscle and fascia in connective tissue disease with normal calcium and phosphorus levels. New management modalities such as cyclosporine, intravenous immunoglobulin, and tumor necrosis factor alpha inhibitors are currently being evaluated.
- ,,, et al.Risk factors associated with calcinosis of juvenile dermatomyositis.JPediatr (Rio J).2008;84(1):68–74.
- ,,,.Calcinosis in rheumatic diseases.Semin Arthritis Rheum.2005;34(6):805–812.
- ,,, et al.Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis.J Am Acad Dermatol.2002;47(4):505–511.
A 38‐year‐old woman with juvenile dermatomyositis (JDM) and calcinosis universalis presented with 3 days of drainage from a lesion on her right elbow. An examination of the elbow revealed diffuse and firm subcutaneous nodules with overlying erythema. X‐rays illustrated soft‐tissue calcifications in the forearm and elbow without evidence of osteomyelitis (Figure 1). Wound cultures grew Staphylococcus aureus, and the patient was started on intravenous antibiotics for abscess treatment.

Calcinosis universalis is soft‐tissue calcification presenting as a complication of JDM. It is often detected in childhood in 30% to 70% of patients. It is hypothesized that calcinosis is due to chronic tissue inflammation, as seen in JDM, leading to muscle damage, releasing calcium, and inducing mineralization. Calcinosis universalis often presents as calcified nodules and plaques in areas of repeated trauma, such as joints, extremities, and buttocks (Figures 13). Calcification is localized in subcutaneous tissue, fascial planes, tendons, or intramuscular areas. It can cause debilitating secondary complications such as skin ulcerations expressing calcified material, superimposed infections of skin lesions, joint contractures with severe arthralgias, and muscle atrophy. Calcinosis has been correlated with severity of JDM with presence of cardiac involvement and use of more than one immunosuppression medication.1 It has also been associated with the degree of vasculopathy and delay in initiation of therapy for controlling inflammation in JDM.2
Soft‐tissue calcification can be classified into 5 categories:
-
Dystrophic calcification occurs in injured tissues with normal calcium, phosphorus, and parathyroid hormone levels, as seen in this patient. Calcified nodules or plaques occur in the extremities and buttocks. This is most often seen in JDM, scleroderma, and systemic lupus erythematosus.
-
Metastatic calcification affects normal tissues with abnormal levels of calcium and phosphorus. It is seen in large joints as well as arteries and visceral organs. It is associated with hyperparathyroidism, hypervitaminosis D, and malignancies.
-
Calciphylaxis with abnormal calcium and phosphorus metabolism causes small‐vessel calcification in patients with chronic renal failure.
-
Tumoral calcification is a familial condition with normal calcium levels but elevated phosphorus levels. Large subcutaneous calcifications are seen near high‐pressure areas and joints.
-
Idiopathic calcification is seen in healthy children and young adults with normal calcium metabolism and appears as multiple subcutaneous calcifications.2


Although multiple therapeutic options have been tried for the management or prevention of calcinosis, there is currently no accepted standard of treatment. In patients with calcinosis, warfarin, probenecid, colchicine, bisphosphonates, minocycline, diltiazem, aluminum hydroxide, corticosteroids, and salicylate have been attempted with variable results. Other therapeutic options include carbon dioxide laser treatments and surgical excision of large plaques. Decreasing muscle inflammation with aggressive treatment of JDM may improve outcomes and decrease the incidence of calcification.3 Unfortunately, once calcinosis has occurred, it is highly refractory to medical therapy.
Calcinosis universalis can lead to severe functional impairment. It can be distinguished from other types of calcinosis by diffuse involvement of muscle and fascia in connective tissue disease with normal calcium and phosphorus levels. New management modalities such as cyclosporine, intravenous immunoglobulin, and tumor necrosis factor alpha inhibitors are currently being evaluated.
A 38‐year‐old woman with juvenile dermatomyositis (JDM) and calcinosis universalis presented with 3 days of drainage from a lesion on her right elbow. An examination of the elbow revealed diffuse and firm subcutaneous nodules with overlying erythema. X‐rays illustrated soft‐tissue calcifications in the forearm and elbow without evidence of osteomyelitis (Figure 1). Wound cultures grew Staphylococcus aureus, and the patient was started on intravenous antibiotics for abscess treatment.

Calcinosis universalis is soft‐tissue calcification presenting as a complication of JDM. It is often detected in childhood in 30% to 70% of patients. It is hypothesized that calcinosis is due to chronic tissue inflammation, as seen in JDM, leading to muscle damage, releasing calcium, and inducing mineralization. Calcinosis universalis often presents as calcified nodules and plaques in areas of repeated trauma, such as joints, extremities, and buttocks (Figures 13). Calcification is localized in subcutaneous tissue, fascial planes, tendons, or intramuscular areas. It can cause debilitating secondary complications such as skin ulcerations expressing calcified material, superimposed infections of skin lesions, joint contractures with severe arthralgias, and muscle atrophy. Calcinosis has been correlated with severity of JDM with presence of cardiac involvement and use of more than one immunosuppression medication.1 It has also been associated with the degree of vasculopathy and delay in initiation of therapy for controlling inflammation in JDM.2
Soft‐tissue calcification can be classified into 5 categories:
-
Dystrophic calcification occurs in injured tissues with normal calcium, phosphorus, and parathyroid hormone levels, as seen in this patient. Calcified nodules or plaques occur in the extremities and buttocks. This is most often seen in JDM, scleroderma, and systemic lupus erythematosus.
-
Metastatic calcification affects normal tissues with abnormal levels of calcium and phosphorus. It is seen in large joints as well as arteries and visceral organs. It is associated with hyperparathyroidism, hypervitaminosis D, and malignancies.
-
Calciphylaxis with abnormal calcium and phosphorus metabolism causes small‐vessel calcification in patients with chronic renal failure.
-
Tumoral calcification is a familial condition with normal calcium levels but elevated phosphorus levels. Large subcutaneous calcifications are seen near high‐pressure areas and joints.
-
Idiopathic calcification is seen in healthy children and young adults with normal calcium metabolism and appears as multiple subcutaneous calcifications.2


Although multiple therapeutic options have been tried for the management or prevention of calcinosis, there is currently no accepted standard of treatment. In patients with calcinosis, warfarin, probenecid, colchicine, bisphosphonates, minocycline, diltiazem, aluminum hydroxide, corticosteroids, and salicylate have been attempted with variable results. Other therapeutic options include carbon dioxide laser treatments and surgical excision of large plaques. Decreasing muscle inflammation with aggressive treatment of JDM may improve outcomes and decrease the incidence of calcification.3 Unfortunately, once calcinosis has occurred, it is highly refractory to medical therapy.
Calcinosis universalis can lead to severe functional impairment. It can be distinguished from other types of calcinosis by diffuse involvement of muscle and fascia in connective tissue disease with normal calcium and phosphorus levels. New management modalities such as cyclosporine, intravenous immunoglobulin, and tumor necrosis factor alpha inhibitors are currently being evaluated.
- ,,, et al.Risk factors associated with calcinosis of juvenile dermatomyositis.JPediatr (Rio J).2008;84(1):68–74.
- ,,,.Calcinosis in rheumatic diseases.Semin Arthritis Rheum.2005;34(6):805–812.
- ,,, et al.Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis.J Am Acad Dermatol.2002;47(4):505–511.
- ,,, et al.Risk factors associated with calcinosis of juvenile dermatomyositis.JPediatr (Rio J).2008;84(1):68–74.
- ,,,.Calcinosis in rheumatic diseases.Semin Arthritis Rheum.2005;34(6):805–812.
- ,,, et al.Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis.J Am Acad Dermatol.2002;47(4):505–511.