User login
Mass Confusion
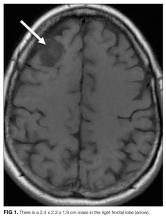
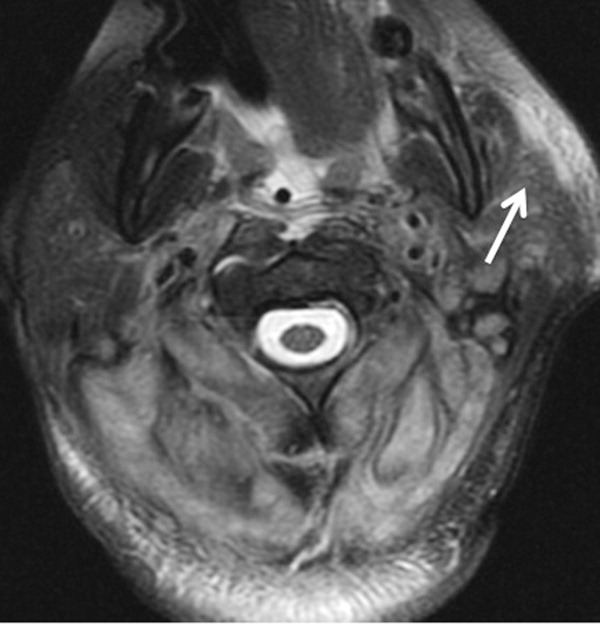
A 57-year-old woman presented to the emergency department of a community hospital with a 2-week history of dizziness, blurred vision, and poor coordination following a flu-like illness. Symptoms were initially attributed to complications from a presumed viral illness, but when they persisted for 2 weeks, she underwent magnetic resonance imaging (MRI) of the brain, which was reported as showing a 2.4 x 2.3 x 1.9 cm right frontal lobe mass with mild mass effect and contrast enhancement (Figure 1). She was discharged home at her request with plans for outpatient follow-up.
Brain masses are usually neoplastic, infectious, or less commonly, inflammatory. The isolated lesion in the right frontal lobe is unlikely to explain her symptoms, which are more suggestive of multifocal disease or elevated intracranial pressure. Although the frontal eye fields could be affected by the mass, such lesions usually cause tonic eye deviation, not blurry vision; furthermore, coordination, which is impaired here, is not governed by the frontal lobe.
Two weeks later, she returned to the same emergency department with worsening symptoms and new bilateral upper extremity dystonia, confusion, and visual hallucinations. Cerebrospinal fluid (CSF) analysis revealed clear, nonxanthochromic fluid with 4 nucleated cells (a differential was not performed), 113 red blood cells, glucose of 80 mg/dL (normal range, 50-80 mg/dL), and protein of 52 mg/dL (normal range, 15-45 mg/dL).
Confusion is generally caused by a metabolic, infectious, structural, or toxic etiology. Standard CSF test results are usually normal with most toxic or metabolic encephalopathies. The absence of significant CSF inflammation argues against infectious encephalitis; paraneoplastic and autoimmune encephalitis, however, are still possible. The CSF red blood cells were likely due to a mildly traumatic tap, but also may have arisen from the frontal lobe mass or a more diffuse invasive process, although the lack of xanthochromia argues against this. Delirium and red blood cells in the CSF should trigger consideration of herpes simplex virus (HSV) encephalitis, although the time course is a bit too protracted and the reported MRI findings do not suggest typical medial temporal lobe involvement.
The disparate neurologic findings suggest a multifocal process, perhaps embolic (eg, endocarditis), ischemic (eg, intravascular lymphoma), infiltrative (eg, malignancy, neurosarcoidosis), or demyelinating (eg, postinfectious acute disseminated encephalomyelitis, multiple sclerosis). However, most of these would have been detected on the initial MRI. Upper extremity dystonia would likely localize to the basal ganglia, whereas confusion and visual hallucinations are more global. The combination of a movement disorder and visual hallucinations is seen in Lewy body dementia, but this tempo is not typical.
Although the CSF does not have pleocytosis, her original symptoms were flu-like; therefore, CSF testing for viruses (eg, enterovirus) is reasonable. Bacterial, mycobacteria, and fungal studies are apt to be unrevealing, but CSF cytology, IgG index, and oligoclonal bands may be useful. Should the encephalopathy progress further and the general medical evaluation prove to be normal, then tests for autoimmune disorders (eg, antinuclear antibodies, NMDAR, paraneoplastic disorders) and rare causes of rapidly progressive dementias (eg, prion diseases) should be sent.
Additional CSF studies including HSV polymerase chain reaction (PCR), West Nile PCR, Lyme antibody, paraneoplastic antibodies, and cytology were sent. Intravenous acyclovir was administered. The above studies, as well as Gram stain, acid-fast bacillus stain, fungal stain, and cultures, were negative. She was started on levetiracetam for seizure prevention due to the mass lesion. An electroencephalogram (EEG) was reported as showing diffuse background slowing with superimposed semiperiodic sharp waves with a right hemispheric emphasis. Intravenous immunoglobulin (IVIG) 0.4 mg/kg/day over 5 days was administered with no improvement. The patient was transferred to an academic medical center for further evaluation.
The EEG reflects encephalopathy without pointing to a specific diagnosis. Prophylactic antiepileptic medications are not indicated for CNS mass lesions without clinical or electrophysiologic seizure activity. IVIG is often administered when an autoimmune encephalitis is suspected, but the lack of response does not rule out an autoimmune condition.
Her medical history included bilateral cataract extraction, right leg fracture, tonsillectomy, and total abdominal hysterectomy. She had a 25-year smoking history and a family history of lung cancer. She had no history of drug or alcohol use. On examination, her temperature was 37.9°C, blood pressure of 144/98 mm Hg, respiratory rate of 18 breaths per minute, a heart rate of 121 beats per minute, and oxygen saturation of 97% on ambient air. Her eyes were open but she was nonverbal. Her chest was clear to auscultation. Heart sounds were distinct and rhythm was regular. Abdomen was soft and nontender with no organomegaly. Skin examination revealed no rash. Her pupils were equal, round, and reactive to light. She did not follow verbal or gestural commands and intermittently tracked with her eyes, but not consistently enough to characterize extraocular movements. Her face was symmetric. She had a normal gag and blink reflex and an increased jaw jerk reflex. Her arms were flexed with increased tone. She had a positive palmo-mental reflex. She had spontaneous movement of all extremities. She had symmetric, 3+ reflexes of the patella and Achilles tendon with a bilateral Babinski’s sign. Sensation was intact only to withdrawal from noxious stimuli.
The physical exam does not localize to a specific brain region, but suggests a diffuse brain process. There are multiple signs of upper motor neuron involvement, including increased tone, hyperreflexia, and Babinski (plantar flexion) reflexes. A palmo-mental reflex signifies pathology in the cerebrum. Although cranial nerve testing is limited, there are no features of cranial neuropathy; similarly, no pyramidal weakness or sensory deficit has been demonstrated on limited testing. The differential diagnosis of her rapidly progressive encephalopathy includes autoimmune or paraneoplastic encephalitis, diffuse infiltrative malignancy, metabolic diseases (eg, porphyria, heavy metal intoxication), and prion disease.
Her family history of lung cancer and her smoking increases the possibility of paraneoplastic encephalitis, which often has subacute behavioral changes that precede complete neurologic impairment. Inflammatory or hemorrhagic CSF is seen with Balamuthia amoebic infection, which causes a granulomatous encephalitis and is characteristically associated with a mass lesion. Toxoplasmosis causes encephalitis that can be profound, but patients are usually immunocompromised and there are typically multiple lesions.
Laboratory results showed a normal white blood cell count and differential, basic metabolic profile and liver function tests, and C-reactive protein. Human immunodeficiency virus antibody testing was negative. Chest radiography and computed tomography of chest, abdomen, and pelvis were normal. A repeat MRI of the brain with contrast was reported as showing a 2.4 x 2.3 x 1.9 cm heterogeneously enhancing mass in the right frontal lobe with an enhancing dural tail and underlying hyperostosis consistent with a meningioma, and blooming within the mass consistent with prior hemorrhage. No mass effect was present.
The meningioma was resected 3 days after admission but her symptoms did not improve. Routine postoperative MRI was reported to show expected postsurgical changes but no infarct. Brain biopsy at the time of the operation was reported as meningioma and mild gliosis without encephalitis.
The reported MRI findings showing unchanged size and overall appearance of the mass, its connection to the dura and skull, and the pathology results all suggest that the mass is a meningioma. There is no evidence of disease outside of the CNS. Some cancers that provoke a paraneoplastic response can be quite small yet may incite an immune encephalitis; anti-NMDAR-mediated encephalitis can occur with malignancy (often ovarian), although it also arises in the absence of any tumor. Any inclination to definitively exclude conditions not seen on the brain biopsy must be tempered by the limited sensitivity of brain histology examination. Still, what was not seen warrants mention: vascular inflammation suggestive of CNS vasculitis, granulomas that might point to neurosarcoidosis, malignant cells of an infiltrating lymphoma or glioma, or inflammatory cells suggestive of encephalitis. Prion encephalopathy remains possible.
The patient remained unresponsive. A repeat EEG showed bilateral generalized periodic epileptiform discharges with accompanying twitching of the head, face, and left arm, which were suppressed with intravenous propofol and levetiracetam. Three weeks following meningioma resection, a new MRI was read as showing new abnormal signal in the right basal ganglia, abnormality of the cortex on the diffusion weighted images, and progressive generalized volume loss.
Among the aforementioned diagnoses, focal or diffuse periodic epileptiform discharges at 1-2 hertz are most characteristic of prion disease. Striatal and cortical transverse relaxation time (T2)-weighted and diffusion-weighted imaging (DWI) hyperintensities with corresponding restricted diffusion is characteristic of Creutzfeldt-Jakob disease (CJD), although metabolic disorders, seizures, and encephalitis can very rarely show similar MRI findings. The clinical course, the MRI and EEG findings, and nondiagnostic biopsy results, which were initially not assessed for prion disease, collectively point to prion disease. Detection of abnormal prion protein in the brain tissue by immunohistochemistry or molecular methods would confirm the diagnosis.
Review of the original right frontal cortex biopsy specimen at the National Prion Disease Pathology Surveillance Center, including immunostaining with 3F4, a monoclonal antibody to the prion protein, revealed granular deposits typical of prion disease. This finding established a diagnosis of prion disease, likely sporadic CJD. The patient was transitioned to palliative care and died shortly thereafter.
Brain autopsy showed regions with transcortical vacuolation (spongiform change), other cortical regions with varying degrees of vacuolation, abundant reactive astrocytes, paucity of neurons, and dark shrunken neurons. Vacuolation and gliosis were observed in the striatum and were most pronounced in the thalamus. There was no evidence of an inflammatory infiltrate or a neoplastic process. These findings with the positive 3F4 immunohistochemistry and positive Western blot from brain autopsy, as well as the absence of a mutation in the prion protein gene, were diagnostic for CJD.
An investigation was initiated to track the nondisposable surgical instruments used in the meningioma resection that may have been subsequently used in other patients. It was determined that 52 neurosurgical patients may have been exposed to prion-contaminated instruments. The instruments were subsequently processed specifically for prion decontamination. After 7 years, no cases of CJD have been diagnosed in the potentially exposed patients.
DISCUSSION
CJD is a rare neurodegenerative condition1 classified as one of the transmissible spongiform encephalopathies, so called because of the characteristic spongiform pattern (vacuolation) seen on histology, as well as the presence of neuronal loss, reactive gliosis in the gray matter, and the accumulation of the abnormal isoform of the cellular prion protein.2 It affects about one person in every one million people per year worldwide; in the United States there are about 300 cases per year. The most common form of human prion disease, sporadic CJD, is relentlessly progressive and invariably fatal, and in most cases, death occurs less than 5 months from onset.3 There is no cure, although temporizing treatments for symptoms can be helpful.
Sporadic CJD, which accounts for approximately 85% of all cases of prion disease in humans, typically manifests with rapidly progressive dementia and myoclonus after a prolonged incubation period in persons between 55 and 75 years of age. Genetic forms account for approximately 15% and acquired forms less than 1% of human prion diseases.1 Prion diseases have a broad spectrum of clinical manifestations, including dementia, ataxia, parkinsonism, myoclonus, insomnia, paresthesias, and abnormal or changed behavior.4 Given the protean clinical manifestations of prion diseases and rarity, the diagnosis is challenging to make antemortem. One recent study showed that most patients receive about 4 misdiagnoses and are often two-thirds of the way through their disease course before the correct diagnosis of sporadic CJD is made.5
Testing for protein markers of rapid neuronal injury8 in the CSF including 14-3-3, total tau, and neuron-specific enolase can increase suspicion for CJD, although there is a 10%-50% false positive rate with these markers.9 In this case, those tests were not performed; positive results would have been even more nonspecific in the setting of an enhancing brain mass and recent brain surgery.
Although not available at the time this patient was evaluated, the real-time quaking-induced conversion (RT-QuIC) test performed in CSF is diagnostically helpful, and, if positive, supportive of the MRI findings. The sensitivity and specificity of this test have been reported to be between 87%-91% and 98%-100%, respectively, albeit with limited data.10 Applying RT-QuIC to nasal mucosal brushings might lead to even higher sensitivity and specificity.11Seeking a premortem diagnosis for a rare disease with no known cure may seem superfluous, but it has important implications for establishing prognosis, limiting subsequent diagnostic and therapeutic measures, and safeguarding of other patients and operating room personnel. Iatrogenic CJD has occurred following invasive procedures involving neurosurgical instrumentation.12 CJD has been transmitted from grafts of dura mater, transplanted corneas, implantation of inadequately sterilized electrodes in the brain, and in the early 1980s, injections of contaminated pituitary hormones (particularly growth hormone) derived from human pituitary glands taken from cadavers. Since CJD was first described in the 1920s, less than 1% of human prion cases have been acquired iatrogenically.13In patients with rapidly progressive cognitive decline who warrant brain biopsy or surgery, the probability of prion diseases should be assessed based on clinical information and the results of MRI, EEG, and CSF testing. If prion disease is plausible, World Health Organization14 precautions should be employed for neuroinvasive procedures to reduce transmission risk. Disposable equipment should be used when possible, and nondisposable neurosurgical instruments should be quarantined until a nonprion disease diagnosis is identified, or should be regarded as contaminated and reprocessed using the aforementioned protocol.
This case highlights the challenges of seeking the correct diagnosis and its consequences, especially from an infection control perspective. The initial imaging finding of a mass lesion (a meningioma—which is a common incidental finding in older adults15) was a red herring that initially obscured the correct diagnosis. The patient’s progressive cognitive decline, EEG results, and evolving MRI findings, however, prompted further scrutiny of the brain biopsy specimen that eventually steered the clinicians away from mass confusion to diagnostic certainty.
TEACHING POINTS
- Rapidly progressive dementias (RPD) are characterized by cognitive decline over weeks to months. The RPD differential diagnosis includes fulminant forms of common neurodegenerative disorders (eg, Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia spectrum), autoimmune encephalidites, CNS cancers, and prion disease.
- Sporadic CJD is the most common human prion disease. It is a rare neurodegenerative condition with onset usually between the ages of 50 and 70 years, and most commonly manifests with rapidly progressive dementia, ataxia, and myoclonus.
- Because of its protean manifestations, the diagnosis of CJD is difficult to make antemortem, and diagnosis is often delayed. Specialist evaluation of brain MRI DWI sequences and new CSF diagnostic tests may allow for earlier diagnosis, which has management and infection control implications.
Disclosure
Dr. Dhaliwal reports receiving honoraria from ISMIE Mutual Insurance Company and Physicians’ Reciprocal Insurers. Dr Geschwind’s institution has received R01 grant funding from NIH/NIA; and Alliance Biosecure and the Michael J Homer Family Fund as paid money to his institution, Dr Geschwind has received consulting fees or honoraria from Best Doctors, Kendall Brill & Kelly, CJD Foundation, and Tau Consortium; Dr Geschwind is a consultant for Gerson Lehrman Group, Biohaven Pharmaceuticals, and Advance Medical, outside the submitted work; has grants/grantspending with Quest, Cure PSP, and Tau Consortium, and received payment for lectures from Multiple Grand Rounds Lectures, outside the submitted work. Dr Saint is on a medical advisory board of Doximity, has received honorarium for being a member of the medical advisory board; he is also on the scientifice advisory board of Jvion. Dr Safdar’s institution has received a grant from the VA Patient Safety Center.
1. Brown P, Gibbs CJ, Jr., Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513-529. PubMed
2. Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol. 1996;53:913-920. PubMed
3. Johnson RT, Gibbs CJ, Jr. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994-2004. PubMed
4. Will RG, Alpers MP, Dormont D, Schonberger LB. Infectious and sporadic prion diseases. In: Prusiner SB, ed. Prion biology and diseases. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999:465-507. \
5. Paterson RW, Torres-Chae CC, Kuo AL, et al. Differential diagnosis of Jakob-Creutzfeldt disease. Arch Neurol. 2012;69:1578-1582. PubMed
6. Tschampa HJ, Kallenberg K, Kretzschmar HA, et al. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2007;28:1114-1118. PubMed
7. Carswell C, Thompson A, Lukic A, et al. MRI findings are often missed in the diagnosis of Creutzfeldt-Jakob disease. BMC Neurol. 2012;12:153. PubMed
8. Geschwind MD, Martindale J, Miller D, et al. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol. 2003;60:813-816. PubMed
9. Burkhard PR, Sanchez JC, Landis T, Hochstrasser DF. CSF detection of the 14-3-3 protein in unselected patients with dementia. Neurology. 2001;56:1528-1533. PubMed
10. Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6:pii: e02451-14 PubMed
11. Orrú CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014;371:519-529. PubMed
12. Brown P, Preece M, Brandel JP, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology. 2000;55:1075-1081. PubMed
13. Brown P, Brandel JP, Sato T, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18:901-907. PubMed
14. WHO infection control guidelines for transmissible spongiform encephalopathies. Report of a WHO consultation, Geneva, Switzerland, 23-26 March 1999. http://www.who.int/csr/resources/publications/bse/whocdscsraph2003.pdf. Accessed on July 10, 2017.
15. Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197-205. PubMed
A 57-year-old woman presented to the emergency department of a community hospital with a 2-week history of dizziness, blurred vision, and poor coordination following a flu-like illness. Symptoms were initially attributed to complications from a presumed viral illness, but when they persisted for 2 weeks, she underwent magnetic resonance imaging (MRI) of the brain, which was reported as showing a 2.4 x 2.3 x 1.9 cm right frontal lobe mass with mild mass effect and contrast enhancement (Figure 1). She was discharged home at her request with plans for outpatient follow-up.
Brain masses are usually neoplastic, infectious, or less commonly, inflammatory. The isolated lesion in the right frontal lobe is unlikely to explain her symptoms, which are more suggestive of multifocal disease or elevated intracranial pressure. Although the frontal eye fields could be affected by the mass, such lesions usually cause tonic eye deviation, not blurry vision; furthermore, coordination, which is impaired here, is not governed by the frontal lobe.
Two weeks later, she returned to the same emergency department with worsening symptoms and new bilateral upper extremity dystonia, confusion, and visual hallucinations. Cerebrospinal fluid (CSF) analysis revealed clear, nonxanthochromic fluid with 4 nucleated cells (a differential was not performed), 113 red blood cells, glucose of 80 mg/dL (normal range, 50-80 mg/dL), and protein of 52 mg/dL (normal range, 15-45 mg/dL).
Confusion is generally caused by a metabolic, infectious, structural, or toxic etiology. Standard CSF test results are usually normal with most toxic or metabolic encephalopathies. The absence of significant CSF inflammation argues against infectious encephalitis; paraneoplastic and autoimmune encephalitis, however, are still possible. The CSF red blood cells were likely due to a mildly traumatic tap, but also may have arisen from the frontal lobe mass or a more diffuse invasive process, although the lack of xanthochromia argues against this. Delirium and red blood cells in the CSF should trigger consideration of herpes simplex virus (HSV) encephalitis, although the time course is a bit too protracted and the reported MRI findings do not suggest typical medial temporal lobe involvement.
The disparate neurologic findings suggest a multifocal process, perhaps embolic (eg, endocarditis), ischemic (eg, intravascular lymphoma), infiltrative (eg, malignancy, neurosarcoidosis), or demyelinating (eg, postinfectious acute disseminated encephalomyelitis, multiple sclerosis). However, most of these would have been detected on the initial MRI. Upper extremity dystonia would likely localize to the basal ganglia, whereas confusion and visual hallucinations are more global. The combination of a movement disorder and visual hallucinations is seen in Lewy body dementia, but this tempo is not typical.
Although the CSF does not have pleocytosis, her original symptoms were flu-like; therefore, CSF testing for viruses (eg, enterovirus) is reasonable. Bacterial, mycobacteria, and fungal studies are apt to be unrevealing, but CSF cytology, IgG index, and oligoclonal bands may be useful. Should the encephalopathy progress further and the general medical evaluation prove to be normal, then tests for autoimmune disorders (eg, antinuclear antibodies, NMDAR, paraneoplastic disorders) and rare causes of rapidly progressive dementias (eg, prion diseases) should be sent.
Additional CSF studies including HSV polymerase chain reaction (PCR), West Nile PCR, Lyme antibody, paraneoplastic antibodies, and cytology were sent. Intravenous acyclovir was administered. The above studies, as well as Gram stain, acid-fast bacillus stain, fungal stain, and cultures, were negative. She was started on levetiracetam for seizure prevention due to the mass lesion. An electroencephalogram (EEG) was reported as showing diffuse background slowing with superimposed semiperiodic sharp waves with a right hemispheric emphasis. Intravenous immunoglobulin (IVIG) 0.4 mg/kg/day over 5 days was administered with no improvement. The patient was transferred to an academic medical center for further evaluation.
The EEG reflects encephalopathy without pointing to a specific diagnosis. Prophylactic antiepileptic medications are not indicated for CNS mass lesions without clinical or electrophysiologic seizure activity. IVIG is often administered when an autoimmune encephalitis is suspected, but the lack of response does not rule out an autoimmune condition.
Her medical history included bilateral cataract extraction, right leg fracture, tonsillectomy, and total abdominal hysterectomy. She had a 25-year smoking history and a family history of lung cancer. She had no history of drug or alcohol use. On examination, her temperature was 37.9°C, blood pressure of 144/98 mm Hg, respiratory rate of 18 breaths per minute, a heart rate of 121 beats per minute, and oxygen saturation of 97% on ambient air. Her eyes were open but she was nonverbal. Her chest was clear to auscultation. Heart sounds were distinct and rhythm was regular. Abdomen was soft and nontender with no organomegaly. Skin examination revealed no rash. Her pupils were equal, round, and reactive to light. She did not follow verbal or gestural commands and intermittently tracked with her eyes, but not consistently enough to characterize extraocular movements. Her face was symmetric. She had a normal gag and blink reflex and an increased jaw jerk reflex. Her arms were flexed with increased tone. She had a positive palmo-mental reflex. She had spontaneous movement of all extremities. She had symmetric, 3+ reflexes of the patella and Achilles tendon with a bilateral Babinski’s sign. Sensation was intact only to withdrawal from noxious stimuli.
The physical exam does not localize to a specific brain region, but suggests a diffuse brain process. There are multiple signs of upper motor neuron involvement, including increased tone, hyperreflexia, and Babinski (plantar flexion) reflexes. A palmo-mental reflex signifies pathology in the cerebrum. Although cranial nerve testing is limited, there are no features of cranial neuropathy; similarly, no pyramidal weakness or sensory deficit has been demonstrated on limited testing. The differential diagnosis of her rapidly progressive encephalopathy includes autoimmune or paraneoplastic encephalitis, diffuse infiltrative malignancy, metabolic diseases (eg, porphyria, heavy metal intoxication), and prion disease.
Her family history of lung cancer and her smoking increases the possibility of paraneoplastic encephalitis, which often has subacute behavioral changes that precede complete neurologic impairment. Inflammatory or hemorrhagic CSF is seen with Balamuthia amoebic infection, which causes a granulomatous encephalitis and is characteristically associated with a mass lesion. Toxoplasmosis causes encephalitis that can be profound, but patients are usually immunocompromised and there are typically multiple lesions.
Laboratory results showed a normal white blood cell count and differential, basic metabolic profile and liver function tests, and C-reactive protein. Human immunodeficiency virus antibody testing was negative. Chest radiography and computed tomography of chest, abdomen, and pelvis were normal. A repeat MRI of the brain with contrast was reported as showing a 2.4 x 2.3 x 1.9 cm heterogeneously enhancing mass in the right frontal lobe with an enhancing dural tail and underlying hyperostosis consistent with a meningioma, and blooming within the mass consistent with prior hemorrhage. No mass effect was present.
The meningioma was resected 3 days after admission but her symptoms did not improve. Routine postoperative MRI was reported to show expected postsurgical changes but no infarct. Brain biopsy at the time of the operation was reported as meningioma and mild gliosis without encephalitis.
The reported MRI findings showing unchanged size and overall appearance of the mass, its connection to the dura and skull, and the pathology results all suggest that the mass is a meningioma. There is no evidence of disease outside of the CNS. Some cancers that provoke a paraneoplastic response can be quite small yet may incite an immune encephalitis; anti-NMDAR-mediated encephalitis can occur with malignancy (often ovarian), although it also arises in the absence of any tumor. Any inclination to definitively exclude conditions not seen on the brain biopsy must be tempered by the limited sensitivity of brain histology examination. Still, what was not seen warrants mention: vascular inflammation suggestive of CNS vasculitis, granulomas that might point to neurosarcoidosis, malignant cells of an infiltrating lymphoma or glioma, or inflammatory cells suggestive of encephalitis. Prion encephalopathy remains possible.
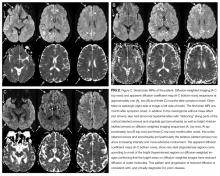
The patient remained unresponsive. A repeat EEG showed bilateral generalized periodic epileptiform discharges with accompanying twitching of the head, face, and left arm, which were suppressed with intravenous propofol and levetiracetam. Three weeks following meningioma resection, a new MRI was read as showing new abnormal signal in the right basal ganglia, abnormality of the cortex on the diffusion weighted images, and progressive generalized volume loss.
Among the aforementioned diagnoses, focal or diffuse periodic epileptiform discharges at 1-2 hertz are most characteristic of prion disease. Striatal and cortical transverse relaxation time (T2)-weighted and diffusion-weighted imaging (DWI) hyperintensities with corresponding restricted diffusion is characteristic of Creutzfeldt-Jakob disease (CJD), although metabolic disorders, seizures, and encephalitis can very rarely show similar MRI findings. The clinical course, the MRI and EEG findings, and nondiagnostic biopsy results, which were initially not assessed for prion disease, collectively point to prion disease. Detection of abnormal prion protein in the brain tissue by immunohistochemistry or molecular methods would confirm the diagnosis.
Review of the original right frontal cortex biopsy specimen at the National Prion Disease Pathology Surveillance Center, including immunostaining with 3F4, a monoclonal antibody to the prion protein, revealed granular deposits typical of prion disease. This finding established a diagnosis of prion disease, likely sporadic CJD. The patient was transitioned to palliative care and died shortly thereafter.
Brain autopsy showed regions with transcortical vacuolation (spongiform change), other cortical regions with varying degrees of vacuolation, abundant reactive astrocytes, paucity of neurons, and dark shrunken neurons. Vacuolation and gliosis were observed in the striatum and were most pronounced in the thalamus. There was no evidence of an inflammatory infiltrate or a neoplastic process. These findings with the positive 3F4 immunohistochemistry and positive Western blot from brain autopsy, as well as the absence of a mutation in the prion protein gene, were diagnostic for CJD.
An investigation was initiated to track the nondisposable surgical instruments used in the meningioma resection that may have been subsequently used in other patients. It was determined that 52 neurosurgical patients may have been exposed to prion-contaminated instruments. The instruments were subsequently processed specifically for prion decontamination. After 7 years, no cases of CJD have been diagnosed in the potentially exposed patients.
DISCUSSION
CJD is a rare neurodegenerative condition1 classified as one of the transmissible spongiform encephalopathies, so called because of the characteristic spongiform pattern (vacuolation) seen on histology, as well as the presence of neuronal loss, reactive gliosis in the gray matter, and the accumulation of the abnormal isoform of the cellular prion protein.2 It affects about one person in every one million people per year worldwide; in the United States there are about 300 cases per year. The most common form of human prion disease, sporadic CJD, is relentlessly progressive and invariably fatal, and in most cases, death occurs less than 5 months from onset.3 There is no cure, although temporizing treatments for symptoms can be helpful.
Sporadic CJD, which accounts for approximately 85% of all cases of prion disease in humans, typically manifests with rapidly progressive dementia and myoclonus after a prolonged incubation period in persons between 55 and 75 years of age. Genetic forms account for approximately 15% and acquired forms less than 1% of human prion diseases.1 Prion diseases have a broad spectrum of clinical manifestations, including dementia, ataxia, parkinsonism, myoclonus, insomnia, paresthesias, and abnormal or changed behavior.4 Given the protean clinical manifestations of prion diseases and rarity, the diagnosis is challenging to make antemortem. One recent study showed that most patients receive about 4 misdiagnoses and are often two-thirds of the way through their disease course before the correct diagnosis of sporadic CJD is made.5
Testing for protein markers of rapid neuronal injury8 in the CSF including 14-3-3, total tau, and neuron-specific enolase can increase suspicion for CJD, although there is a 10%-50% false positive rate with these markers.9 In this case, those tests were not performed; positive results would have been even more nonspecific in the setting of an enhancing brain mass and recent brain surgery.
Although not available at the time this patient was evaluated, the real-time quaking-induced conversion (RT-QuIC) test performed in CSF is diagnostically helpful, and, if positive, supportive of the MRI findings. The sensitivity and specificity of this test have been reported to be between 87%-91% and 98%-100%, respectively, albeit with limited data.10 Applying RT-QuIC to nasal mucosal brushings might lead to even higher sensitivity and specificity.11Seeking a premortem diagnosis for a rare disease with no known cure may seem superfluous, but it has important implications for establishing prognosis, limiting subsequent diagnostic and therapeutic measures, and safeguarding of other patients and operating room personnel. Iatrogenic CJD has occurred following invasive procedures involving neurosurgical instrumentation.12 CJD has been transmitted from grafts of dura mater, transplanted corneas, implantation of inadequately sterilized electrodes in the brain, and in the early 1980s, injections of contaminated pituitary hormones (particularly growth hormone) derived from human pituitary glands taken from cadavers. Since CJD was first described in the 1920s, less than 1% of human prion cases have been acquired iatrogenically.13In patients with rapidly progressive cognitive decline who warrant brain biopsy or surgery, the probability of prion diseases should be assessed based on clinical information and the results of MRI, EEG, and CSF testing. If prion disease is plausible, World Health Organization14 precautions should be employed for neuroinvasive procedures to reduce transmission risk. Disposable equipment should be used when possible, and nondisposable neurosurgical instruments should be quarantined until a nonprion disease diagnosis is identified, or should be regarded as contaminated and reprocessed using the aforementioned protocol.
This case highlights the challenges of seeking the correct diagnosis and its consequences, especially from an infection control perspective. The initial imaging finding of a mass lesion (a meningioma—which is a common incidental finding in older adults15) was a red herring that initially obscured the correct diagnosis. The patient’s progressive cognitive decline, EEG results, and evolving MRI findings, however, prompted further scrutiny of the brain biopsy specimen that eventually steered the clinicians away from mass confusion to diagnostic certainty.
TEACHING POINTS
- Rapidly progressive dementias (RPD) are characterized by cognitive decline over weeks to months. The RPD differential diagnosis includes fulminant forms of common neurodegenerative disorders (eg, Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia spectrum), autoimmune encephalidites, CNS cancers, and prion disease.
- Sporadic CJD is the most common human prion disease. It is a rare neurodegenerative condition with onset usually between the ages of 50 and 70 years, and most commonly manifests with rapidly progressive dementia, ataxia, and myoclonus.
- Because of its protean manifestations, the diagnosis of CJD is difficult to make antemortem, and diagnosis is often delayed. Specialist evaluation of brain MRI DWI sequences and new CSF diagnostic tests may allow for earlier diagnosis, which has management and infection control implications.
Disclosure
Dr. Dhaliwal reports receiving honoraria from ISMIE Mutual Insurance Company and Physicians’ Reciprocal Insurers. Dr Geschwind’s institution has received R01 grant funding from NIH/NIA; and Alliance Biosecure and the Michael J Homer Family Fund as paid money to his institution, Dr Geschwind has received consulting fees or honoraria from Best Doctors, Kendall Brill & Kelly, CJD Foundation, and Tau Consortium; Dr Geschwind is a consultant for Gerson Lehrman Group, Biohaven Pharmaceuticals, and Advance Medical, outside the submitted work; has grants/grantspending with Quest, Cure PSP, and Tau Consortium, and received payment for lectures from Multiple Grand Rounds Lectures, outside the submitted work. Dr Saint is on a medical advisory board of Doximity, has received honorarium for being a member of the medical advisory board; he is also on the scientifice advisory board of Jvion. Dr Safdar’s institution has received a grant from the VA Patient Safety Center.
A 57-year-old woman presented to the emergency department of a community hospital with a 2-week history of dizziness, blurred vision, and poor coordination following a flu-like illness. Symptoms were initially attributed to complications from a presumed viral illness, but when they persisted for 2 weeks, she underwent magnetic resonance imaging (MRI) of the brain, which was reported as showing a 2.4 x 2.3 x 1.9 cm right frontal lobe mass with mild mass effect and contrast enhancement (Figure 1). She was discharged home at her request with plans for outpatient follow-up.
Brain masses are usually neoplastic, infectious, or less commonly, inflammatory. The isolated lesion in the right frontal lobe is unlikely to explain her symptoms, which are more suggestive of multifocal disease or elevated intracranial pressure. Although the frontal eye fields could be affected by the mass, such lesions usually cause tonic eye deviation, not blurry vision; furthermore, coordination, which is impaired here, is not governed by the frontal lobe.
Two weeks later, she returned to the same emergency department with worsening symptoms and new bilateral upper extremity dystonia, confusion, and visual hallucinations. Cerebrospinal fluid (CSF) analysis revealed clear, nonxanthochromic fluid with 4 nucleated cells (a differential was not performed), 113 red blood cells, glucose of 80 mg/dL (normal range, 50-80 mg/dL), and protein of 52 mg/dL (normal range, 15-45 mg/dL).
Confusion is generally caused by a metabolic, infectious, structural, or toxic etiology. Standard CSF test results are usually normal with most toxic or metabolic encephalopathies. The absence of significant CSF inflammation argues against infectious encephalitis; paraneoplastic and autoimmune encephalitis, however, are still possible. The CSF red blood cells were likely due to a mildly traumatic tap, but also may have arisen from the frontal lobe mass or a more diffuse invasive process, although the lack of xanthochromia argues against this. Delirium and red blood cells in the CSF should trigger consideration of herpes simplex virus (HSV) encephalitis, although the time course is a bit too protracted and the reported MRI findings do not suggest typical medial temporal lobe involvement.
The disparate neurologic findings suggest a multifocal process, perhaps embolic (eg, endocarditis), ischemic (eg, intravascular lymphoma), infiltrative (eg, malignancy, neurosarcoidosis), or demyelinating (eg, postinfectious acute disseminated encephalomyelitis, multiple sclerosis). However, most of these would have been detected on the initial MRI. Upper extremity dystonia would likely localize to the basal ganglia, whereas confusion and visual hallucinations are more global. The combination of a movement disorder and visual hallucinations is seen in Lewy body dementia, but this tempo is not typical.
Although the CSF does not have pleocytosis, her original symptoms were flu-like; therefore, CSF testing for viruses (eg, enterovirus) is reasonable. Bacterial, mycobacteria, and fungal studies are apt to be unrevealing, but CSF cytology, IgG index, and oligoclonal bands may be useful. Should the encephalopathy progress further and the general medical evaluation prove to be normal, then tests for autoimmune disorders (eg, antinuclear antibodies, NMDAR, paraneoplastic disorders) and rare causes of rapidly progressive dementias (eg, prion diseases) should be sent.
Additional CSF studies including HSV polymerase chain reaction (PCR), West Nile PCR, Lyme antibody, paraneoplastic antibodies, and cytology were sent. Intravenous acyclovir was administered. The above studies, as well as Gram stain, acid-fast bacillus stain, fungal stain, and cultures, were negative. She was started on levetiracetam for seizure prevention due to the mass lesion. An electroencephalogram (EEG) was reported as showing diffuse background slowing with superimposed semiperiodic sharp waves with a right hemispheric emphasis. Intravenous immunoglobulin (IVIG) 0.4 mg/kg/day over 5 days was administered with no improvement. The patient was transferred to an academic medical center for further evaluation.
The EEG reflects encephalopathy without pointing to a specific diagnosis. Prophylactic antiepileptic medications are not indicated for CNS mass lesions without clinical or electrophysiologic seizure activity. IVIG is often administered when an autoimmune encephalitis is suspected, but the lack of response does not rule out an autoimmune condition.
Her medical history included bilateral cataract extraction, right leg fracture, tonsillectomy, and total abdominal hysterectomy. She had a 25-year smoking history and a family history of lung cancer. She had no history of drug or alcohol use. On examination, her temperature was 37.9°C, blood pressure of 144/98 mm Hg, respiratory rate of 18 breaths per minute, a heart rate of 121 beats per minute, and oxygen saturation of 97% on ambient air. Her eyes were open but she was nonverbal. Her chest was clear to auscultation. Heart sounds were distinct and rhythm was regular. Abdomen was soft and nontender with no organomegaly. Skin examination revealed no rash. Her pupils were equal, round, and reactive to light. She did not follow verbal or gestural commands and intermittently tracked with her eyes, but not consistently enough to characterize extraocular movements. Her face was symmetric. She had a normal gag and blink reflex and an increased jaw jerk reflex. Her arms were flexed with increased tone. She had a positive palmo-mental reflex. She had spontaneous movement of all extremities. She had symmetric, 3+ reflexes of the patella and Achilles tendon with a bilateral Babinski’s sign. Sensation was intact only to withdrawal from noxious stimuli.
The physical exam does not localize to a specific brain region, but suggests a diffuse brain process. There are multiple signs of upper motor neuron involvement, including increased tone, hyperreflexia, and Babinski (plantar flexion) reflexes. A palmo-mental reflex signifies pathology in the cerebrum. Although cranial nerve testing is limited, there are no features of cranial neuropathy; similarly, no pyramidal weakness or sensory deficit has been demonstrated on limited testing. The differential diagnosis of her rapidly progressive encephalopathy includes autoimmune or paraneoplastic encephalitis, diffuse infiltrative malignancy, metabolic diseases (eg, porphyria, heavy metal intoxication), and prion disease.
Her family history of lung cancer and her smoking increases the possibility of paraneoplastic encephalitis, which often has subacute behavioral changes that precede complete neurologic impairment. Inflammatory or hemorrhagic CSF is seen with Balamuthia amoebic infection, which causes a granulomatous encephalitis and is characteristically associated with a mass lesion. Toxoplasmosis causes encephalitis that can be profound, but patients are usually immunocompromised and there are typically multiple lesions.
Laboratory results showed a normal white blood cell count and differential, basic metabolic profile and liver function tests, and C-reactive protein. Human immunodeficiency virus antibody testing was negative. Chest radiography and computed tomography of chest, abdomen, and pelvis were normal. A repeat MRI of the brain with contrast was reported as showing a 2.4 x 2.3 x 1.9 cm heterogeneously enhancing mass in the right frontal lobe with an enhancing dural tail and underlying hyperostosis consistent with a meningioma, and blooming within the mass consistent with prior hemorrhage. No mass effect was present.
The meningioma was resected 3 days after admission but her symptoms did not improve. Routine postoperative MRI was reported to show expected postsurgical changes but no infarct. Brain biopsy at the time of the operation was reported as meningioma and mild gliosis without encephalitis.
The reported MRI findings showing unchanged size and overall appearance of the mass, its connection to the dura and skull, and the pathology results all suggest that the mass is a meningioma. There is no evidence of disease outside of the CNS. Some cancers that provoke a paraneoplastic response can be quite small yet may incite an immune encephalitis; anti-NMDAR-mediated encephalitis can occur with malignancy (often ovarian), although it also arises in the absence of any tumor. Any inclination to definitively exclude conditions not seen on the brain biopsy must be tempered by the limited sensitivity of brain histology examination. Still, what was not seen warrants mention: vascular inflammation suggestive of CNS vasculitis, granulomas that might point to neurosarcoidosis, malignant cells of an infiltrating lymphoma or glioma, or inflammatory cells suggestive of encephalitis. Prion encephalopathy remains possible.
The patient remained unresponsive. A repeat EEG showed bilateral generalized periodic epileptiform discharges with accompanying twitching of the head, face, and left arm, which were suppressed with intravenous propofol and levetiracetam. Three weeks following meningioma resection, a new MRI was read as showing new abnormal signal in the right basal ganglia, abnormality of the cortex on the diffusion weighted images, and progressive generalized volume loss.
Among the aforementioned diagnoses, focal or diffuse periodic epileptiform discharges at 1-2 hertz are most characteristic of prion disease. Striatal and cortical transverse relaxation time (T2)-weighted and diffusion-weighted imaging (DWI) hyperintensities with corresponding restricted diffusion is characteristic of Creutzfeldt-Jakob disease (CJD), although metabolic disorders, seizures, and encephalitis can very rarely show similar MRI findings. The clinical course, the MRI and EEG findings, and nondiagnostic biopsy results, which were initially not assessed for prion disease, collectively point to prion disease. Detection of abnormal prion protein in the brain tissue by immunohistochemistry or molecular methods would confirm the diagnosis.
Review of the original right frontal cortex biopsy specimen at the National Prion Disease Pathology Surveillance Center, including immunostaining with 3F4, a monoclonal antibody to the prion protein, revealed granular deposits typical of prion disease. This finding established a diagnosis of prion disease, likely sporadic CJD. The patient was transitioned to palliative care and died shortly thereafter.
Brain autopsy showed regions with transcortical vacuolation (spongiform change), other cortical regions with varying degrees of vacuolation, abundant reactive astrocytes, paucity of neurons, and dark shrunken neurons. Vacuolation and gliosis were observed in the striatum and were most pronounced in the thalamus. There was no evidence of an inflammatory infiltrate or a neoplastic process. These findings with the positive 3F4 immunohistochemistry and positive Western blot from brain autopsy, as well as the absence of a mutation in the prion protein gene, were diagnostic for CJD.
An investigation was initiated to track the nondisposable surgical instruments used in the meningioma resection that may have been subsequently used in other patients. It was determined that 52 neurosurgical patients may have been exposed to prion-contaminated instruments. The instruments were subsequently processed specifically for prion decontamination. After 7 years, no cases of CJD have been diagnosed in the potentially exposed patients.
DISCUSSION
CJD is a rare neurodegenerative condition1 classified as one of the transmissible spongiform encephalopathies, so called because of the characteristic spongiform pattern (vacuolation) seen on histology, as well as the presence of neuronal loss, reactive gliosis in the gray matter, and the accumulation of the abnormal isoform of the cellular prion protein.2 It affects about one person in every one million people per year worldwide; in the United States there are about 300 cases per year. The most common form of human prion disease, sporadic CJD, is relentlessly progressive and invariably fatal, and in most cases, death occurs less than 5 months from onset.3 There is no cure, although temporizing treatments for symptoms can be helpful.
Sporadic CJD, which accounts for approximately 85% of all cases of prion disease in humans, typically manifests with rapidly progressive dementia and myoclonus after a prolonged incubation period in persons between 55 and 75 years of age. Genetic forms account for approximately 15% and acquired forms less than 1% of human prion diseases.1 Prion diseases have a broad spectrum of clinical manifestations, including dementia, ataxia, parkinsonism, myoclonus, insomnia, paresthesias, and abnormal or changed behavior.4 Given the protean clinical manifestations of prion diseases and rarity, the diagnosis is challenging to make antemortem. One recent study showed that most patients receive about 4 misdiagnoses and are often two-thirds of the way through their disease course before the correct diagnosis of sporadic CJD is made.5
Testing for protein markers of rapid neuronal injury8 in the CSF including 14-3-3, total tau, and neuron-specific enolase can increase suspicion for CJD, although there is a 10%-50% false positive rate with these markers.9 In this case, those tests were not performed; positive results would have been even more nonspecific in the setting of an enhancing brain mass and recent brain surgery.
Although not available at the time this patient was evaluated, the real-time quaking-induced conversion (RT-QuIC) test performed in CSF is diagnostically helpful, and, if positive, supportive of the MRI findings. The sensitivity and specificity of this test have been reported to be between 87%-91% and 98%-100%, respectively, albeit with limited data.10 Applying RT-QuIC to nasal mucosal brushings might lead to even higher sensitivity and specificity.11Seeking a premortem diagnosis for a rare disease with no known cure may seem superfluous, but it has important implications for establishing prognosis, limiting subsequent diagnostic and therapeutic measures, and safeguarding of other patients and operating room personnel. Iatrogenic CJD has occurred following invasive procedures involving neurosurgical instrumentation.12 CJD has been transmitted from grafts of dura mater, transplanted corneas, implantation of inadequately sterilized electrodes in the brain, and in the early 1980s, injections of contaminated pituitary hormones (particularly growth hormone) derived from human pituitary glands taken from cadavers. Since CJD was first described in the 1920s, less than 1% of human prion cases have been acquired iatrogenically.13In patients with rapidly progressive cognitive decline who warrant brain biopsy or surgery, the probability of prion diseases should be assessed based on clinical information and the results of MRI, EEG, and CSF testing. If prion disease is plausible, World Health Organization14 precautions should be employed for neuroinvasive procedures to reduce transmission risk. Disposable equipment should be used when possible, and nondisposable neurosurgical instruments should be quarantined until a nonprion disease diagnosis is identified, or should be regarded as contaminated and reprocessed using the aforementioned protocol.
This case highlights the challenges of seeking the correct diagnosis and its consequences, especially from an infection control perspective. The initial imaging finding of a mass lesion (a meningioma—which is a common incidental finding in older adults15) was a red herring that initially obscured the correct diagnosis. The patient’s progressive cognitive decline, EEG results, and evolving MRI findings, however, prompted further scrutiny of the brain biopsy specimen that eventually steered the clinicians away from mass confusion to diagnostic certainty.
TEACHING POINTS
- Rapidly progressive dementias (RPD) are characterized by cognitive decline over weeks to months. The RPD differential diagnosis includes fulminant forms of common neurodegenerative disorders (eg, Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia spectrum), autoimmune encephalidites, CNS cancers, and prion disease.
- Sporadic CJD is the most common human prion disease. It is a rare neurodegenerative condition with onset usually between the ages of 50 and 70 years, and most commonly manifests with rapidly progressive dementia, ataxia, and myoclonus.
- Because of its protean manifestations, the diagnosis of CJD is difficult to make antemortem, and diagnosis is often delayed. Specialist evaluation of brain MRI DWI sequences and new CSF diagnostic tests may allow for earlier diagnosis, which has management and infection control implications.
Disclosure
Dr. Dhaliwal reports receiving honoraria from ISMIE Mutual Insurance Company and Physicians’ Reciprocal Insurers. Dr Geschwind’s institution has received R01 grant funding from NIH/NIA; and Alliance Biosecure and the Michael J Homer Family Fund as paid money to his institution, Dr Geschwind has received consulting fees or honoraria from Best Doctors, Kendall Brill & Kelly, CJD Foundation, and Tau Consortium; Dr Geschwind is a consultant for Gerson Lehrman Group, Biohaven Pharmaceuticals, and Advance Medical, outside the submitted work; has grants/grantspending with Quest, Cure PSP, and Tau Consortium, and received payment for lectures from Multiple Grand Rounds Lectures, outside the submitted work. Dr Saint is on a medical advisory board of Doximity, has received honorarium for being a member of the medical advisory board; he is also on the scientifice advisory board of Jvion. Dr Safdar’s institution has received a grant from the VA Patient Safety Center.
1. Brown P, Gibbs CJ, Jr., Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513-529. PubMed
2. Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol. 1996;53:913-920. PubMed
3. Johnson RT, Gibbs CJ, Jr. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994-2004. PubMed
4. Will RG, Alpers MP, Dormont D, Schonberger LB. Infectious and sporadic prion diseases. In: Prusiner SB, ed. Prion biology and diseases. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999:465-507. \
5. Paterson RW, Torres-Chae CC, Kuo AL, et al. Differential diagnosis of Jakob-Creutzfeldt disease. Arch Neurol. 2012;69:1578-1582. PubMed
6. Tschampa HJ, Kallenberg K, Kretzschmar HA, et al. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2007;28:1114-1118. PubMed
7. Carswell C, Thompson A, Lukic A, et al. MRI findings are often missed in the diagnosis of Creutzfeldt-Jakob disease. BMC Neurol. 2012;12:153. PubMed
8. Geschwind MD, Martindale J, Miller D, et al. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol. 2003;60:813-816. PubMed
9. Burkhard PR, Sanchez JC, Landis T, Hochstrasser DF. CSF detection of the 14-3-3 protein in unselected patients with dementia. Neurology. 2001;56:1528-1533. PubMed
10. Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6:pii: e02451-14 PubMed
11. Orrú CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014;371:519-529. PubMed
12. Brown P, Preece M, Brandel JP, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology. 2000;55:1075-1081. PubMed
13. Brown P, Brandel JP, Sato T, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18:901-907. PubMed
14. WHO infection control guidelines for transmissible spongiform encephalopathies. Report of a WHO consultation, Geneva, Switzerland, 23-26 March 1999. http://www.who.int/csr/resources/publications/bse/whocdscsraph2003.pdf. Accessed on July 10, 2017.
15. Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197-205. PubMed
1. Brown P, Gibbs CJ, Jr., Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513-529. PubMed
2. Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol. 1996;53:913-920. PubMed
3. Johnson RT, Gibbs CJ, Jr. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994-2004. PubMed
4. Will RG, Alpers MP, Dormont D, Schonberger LB. Infectious and sporadic prion diseases. In: Prusiner SB, ed. Prion biology and diseases. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999:465-507. \
5. Paterson RW, Torres-Chae CC, Kuo AL, et al. Differential diagnosis of Jakob-Creutzfeldt disease. Arch Neurol. 2012;69:1578-1582. PubMed
6. Tschampa HJ, Kallenberg K, Kretzschmar HA, et al. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2007;28:1114-1118. PubMed
7. Carswell C, Thompson A, Lukic A, et al. MRI findings are often missed in the diagnosis of Creutzfeldt-Jakob disease. BMC Neurol. 2012;12:153. PubMed
8. Geschwind MD, Martindale J, Miller D, et al. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol. 2003;60:813-816. PubMed
9. Burkhard PR, Sanchez JC, Landis T, Hochstrasser DF. CSF detection of the 14-3-3 protein in unselected patients with dementia. Neurology. 2001;56:1528-1533. PubMed
10. Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6:pii: e02451-14 PubMed
11. Orrú CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014;371:519-529. PubMed
12. Brown P, Preece M, Brandel JP, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology. 2000;55:1075-1081. PubMed
13. Brown P, Brandel JP, Sato T, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18:901-907. PubMed
14. WHO infection control guidelines for transmissible spongiform encephalopathies. Report of a WHO consultation, Geneva, Switzerland, 23-26 March 1999. http://www.who.int/csr/resources/publications/bse/whocdscsraph2003.pdf. Accessed on July 10, 2017.
15. Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197-205. PubMed
© 2017 Society of Hospital Medicine
Idle intravenous catheters are associated with preventable complications
Intravenous catheters (ICs) are common and necessary for inpatient care. However, peripheral and especially central venous catheters (CVCs) are associated with increased risk for local and systemic complications, including bloodstream infections and endocarditis.
Prevention of these complications is important and should be a major focus of infection control and patient safety practices. There are three main points of focus on infection prevention with regard to ICs – proper insertion techniques, proper care of the catheter, and prompt removal when it is no longer necessary.
We focused our review, published in the American Journal of Infection Control (2016 Oct. doi: 10.1016/j.ajic.2016.03.073), on the final point – determining the prevalence, risk factors, and outcomes related to idle intravenous catheters. To accomplish this, we conducted an integrative review of published studies related to idle catheters, excluding reviews, abstracts, and commentaries. Thirteen studies met the inclusion criteria and four of these focused on CVCs.
Generally, an idle catheter is one that remains in place even though it is not being used for patient care. However, the definition of an “idle” catheter varied amongst the reviewed studies, as did the unit of measure, especially for peripheral catheters. Central venous catheter-focused studies were more consistent in using “idle catheter days” and “catheter days.”
Studies of peripheral catheters revealed that 16%-50% of patients had an idle catheter of some type. For the studies focused on CVCs, the percentage of patients with idle catheters ranged from 2.7% in one intensive care unit to 26.2% in a different study. Interestingly, in the study with 2.7% idle CVCs in the ICU, there was a higher percentage of idle CVCs outside of the ICU in the same hospital.
The major reasons for leaving catheters in place in studies where reasons were noted were convenience, future intention to use intravenous medication, and inappropriate use of intravenous medications when oral could be used.
Although data are scarce, complications in the reviewed studies were relatively common with idle peripheral catheters, where 9%-12% suffered thrombophlebitis. Obviously, the risk for catheter-related bloodstream infection increases as the number of catheter days increases – this is especially important with regard to idle CVCs.
Decreasing the prevalence of idle catheters is likely to decrease the risk for infection and improve patient safety. Based on our review of the data, a standardized definition of an “idle catheter” is needed. At the very least, a standard definition should be developed at each institution. This would allow an individual hospital the ability to identify and track the presence of these lines, and implement targeted interventions to decrease the proportion of idle lines. Ideally, a common definition would be created and validated so that data and interventions could be comparable across institutions and guidelines could be developed.
The goal of targeted interventions should be zero idle lines. Prevention of idle peripheral catheters should also be pursued, but because CVC-related complications are often more serious, these lines are often the focus of efforts. Use of peripherally inserted central catheters (PICCs) has increased and while these catheters in some settings may have decreased complication risk, compared with femoral/internal jugular/subclavian CVCs, prevention of idle catheter days is paramount for these catheters as well.
Many ICUs, including at our own institution, have instituted programs to closely monitor for ongoing need for CVCs. This increased focus on the CVC likely explains the lower rates of idle catheters in ICUs noted in the reviewed studies. This close surveillance can be done outside of the ICU as well, and could include peripheral catheters.
At our own institution, the need for catheters is reviewed on some units as part of formalized patient safety rounds. Another potential group of interventions could focus on electronic medical record (EMR)-based changes such as limits on the duration of the order, requirement for renewal of the order, or on-screen reminders of the presence of a catheter. This sort of intervention could possibly be expanded as EMR use becomes more common and robust. For instance, if intravenous medications have not been ordered or given in a certain amount of time, an alert might be triggered. Another EMR-based mechanism could be to require an indication for ongoing catheter use.
Education about the potential adverse outcomes of idle catheters is important. Promoting a team-based approach to interventions, where all involved team members can discuss patient safety issues on equal ground is paramount to successfully decreasing idle catheters and improving patient care and safety in general. As with other hospital-wide initiatives, engagement of hospital administration is important to decrease barriers to implementation.
Intravenous catheter use will remain an integral part of patient care, but efforts should be made to create standardization around the definition of an idle catheter, standardize units of measure, and institute programs to prevent idle catheters.
Daniel Shirley, MD, MS, is assistant professor in the division of infectious disease at the University of Wisconsin–Madison School of Medicine and Public Health and the William S. Middleton Memorial Veterans Hospital. Nasia Safdar, MD, PhD, is associate professor in the division of infectious disease at the University of Wisconsin–Madison School of Medicine and Public Health and the William S. Middleton Memorial Veterans Hospital.
Intravenous catheters (ICs) are common and necessary for inpatient care. However, peripheral and especially central venous catheters (CVCs) are associated with increased risk for local and systemic complications, including bloodstream infections and endocarditis.
Prevention of these complications is important and should be a major focus of infection control and patient safety practices. There are three main points of focus on infection prevention with regard to ICs – proper insertion techniques, proper care of the catheter, and prompt removal when it is no longer necessary.
We focused our review, published in the American Journal of Infection Control (2016 Oct. doi: 10.1016/j.ajic.2016.03.073), on the final point – determining the prevalence, risk factors, and outcomes related to idle intravenous catheters. To accomplish this, we conducted an integrative review of published studies related to idle catheters, excluding reviews, abstracts, and commentaries. Thirteen studies met the inclusion criteria and four of these focused on CVCs.
Generally, an idle catheter is one that remains in place even though it is not being used for patient care. However, the definition of an “idle” catheter varied amongst the reviewed studies, as did the unit of measure, especially for peripheral catheters. Central venous catheter-focused studies were more consistent in using “idle catheter days” and “catheter days.”
Studies of peripheral catheters revealed that 16%-50% of patients had an idle catheter of some type. For the studies focused on CVCs, the percentage of patients with idle catheters ranged from 2.7% in one intensive care unit to 26.2% in a different study. Interestingly, in the study with 2.7% idle CVCs in the ICU, there was a higher percentage of idle CVCs outside of the ICU in the same hospital.
The major reasons for leaving catheters in place in studies where reasons were noted were convenience, future intention to use intravenous medication, and inappropriate use of intravenous medications when oral could be used.
Although data are scarce, complications in the reviewed studies were relatively common with idle peripheral catheters, where 9%-12% suffered thrombophlebitis. Obviously, the risk for catheter-related bloodstream infection increases as the number of catheter days increases – this is especially important with regard to idle CVCs.
Decreasing the prevalence of idle catheters is likely to decrease the risk for infection and improve patient safety. Based on our review of the data, a standardized definition of an “idle catheter” is needed. At the very least, a standard definition should be developed at each institution. This would allow an individual hospital the ability to identify and track the presence of these lines, and implement targeted interventions to decrease the proportion of idle lines. Ideally, a common definition would be created and validated so that data and interventions could be comparable across institutions and guidelines could be developed.
The goal of targeted interventions should be zero idle lines. Prevention of idle peripheral catheters should also be pursued, but because CVC-related complications are often more serious, these lines are often the focus of efforts. Use of peripherally inserted central catheters (PICCs) has increased and while these catheters in some settings may have decreased complication risk, compared with femoral/internal jugular/subclavian CVCs, prevention of idle catheter days is paramount for these catheters as well.
Many ICUs, including at our own institution, have instituted programs to closely monitor for ongoing need for CVCs. This increased focus on the CVC likely explains the lower rates of idle catheters in ICUs noted in the reviewed studies. This close surveillance can be done outside of the ICU as well, and could include peripheral catheters.
At our own institution, the need for catheters is reviewed on some units as part of formalized patient safety rounds. Another potential group of interventions could focus on electronic medical record (EMR)-based changes such as limits on the duration of the order, requirement for renewal of the order, or on-screen reminders of the presence of a catheter. This sort of intervention could possibly be expanded as EMR use becomes more common and robust. For instance, if intravenous medications have not been ordered or given in a certain amount of time, an alert might be triggered. Another EMR-based mechanism could be to require an indication for ongoing catheter use.
Education about the potential adverse outcomes of idle catheters is important. Promoting a team-based approach to interventions, where all involved team members can discuss patient safety issues on equal ground is paramount to successfully decreasing idle catheters and improving patient care and safety in general. As with other hospital-wide initiatives, engagement of hospital administration is important to decrease barriers to implementation.
Intravenous catheter use will remain an integral part of patient care, but efforts should be made to create standardization around the definition of an idle catheter, standardize units of measure, and institute programs to prevent idle catheters.
Daniel Shirley, MD, MS, is assistant professor in the division of infectious disease at the University of Wisconsin–Madison School of Medicine and Public Health and the William S. Middleton Memorial Veterans Hospital. Nasia Safdar, MD, PhD, is associate professor in the division of infectious disease at the University of Wisconsin–Madison School of Medicine and Public Health and the William S. Middleton Memorial Veterans Hospital.
Intravenous catheters (ICs) are common and necessary for inpatient care. However, peripheral and especially central venous catheters (CVCs) are associated with increased risk for local and systemic complications, including bloodstream infections and endocarditis.
Prevention of these complications is important and should be a major focus of infection control and patient safety practices. There are three main points of focus on infection prevention with regard to ICs – proper insertion techniques, proper care of the catheter, and prompt removal when it is no longer necessary.
We focused our review, published in the American Journal of Infection Control (2016 Oct. doi: 10.1016/j.ajic.2016.03.073), on the final point – determining the prevalence, risk factors, and outcomes related to idle intravenous catheters. To accomplish this, we conducted an integrative review of published studies related to idle catheters, excluding reviews, abstracts, and commentaries. Thirteen studies met the inclusion criteria and four of these focused on CVCs.
Generally, an idle catheter is one that remains in place even though it is not being used for patient care. However, the definition of an “idle” catheter varied amongst the reviewed studies, as did the unit of measure, especially for peripheral catheters. Central venous catheter-focused studies were more consistent in using “idle catheter days” and “catheter days.”
Studies of peripheral catheters revealed that 16%-50% of patients had an idle catheter of some type. For the studies focused on CVCs, the percentage of patients with idle catheters ranged from 2.7% in one intensive care unit to 26.2% in a different study. Interestingly, in the study with 2.7% idle CVCs in the ICU, there was a higher percentage of idle CVCs outside of the ICU in the same hospital.
The major reasons for leaving catheters in place in studies where reasons were noted were convenience, future intention to use intravenous medication, and inappropriate use of intravenous medications when oral could be used.
Although data are scarce, complications in the reviewed studies were relatively common with idle peripheral catheters, where 9%-12% suffered thrombophlebitis. Obviously, the risk for catheter-related bloodstream infection increases as the number of catheter days increases – this is especially important with regard to idle CVCs.
Decreasing the prevalence of idle catheters is likely to decrease the risk for infection and improve patient safety. Based on our review of the data, a standardized definition of an “idle catheter” is needed. At the very least, a standard definition should be developed at each institution. This would allow an individual hospital the ability to identify and track the presence of these lines, and implement targeted interventions to decrease the proportion of idle lines. Ideally, a common definition would be created and validated so that data and interventions could be comparable across institutions and guidelines could be developed.
The goal of targeted interventions should be zero idle lines. Prevention of idle peripheral catheters should also be pursued, but because CVC-related complications are often more serious, these lines are often the focus of efforts. Use of peripherally inserted central catheters (PICCs) has increased and while these catheters in some settings may have decreased complication risk, compared with femoral/internal jugular/subclavian CVCs, prevention of idle catheter days is paramount for these catheters as well.
Many ICUs, including at our own institution, have instituted programs to closely monitor for ongoing need for CVCs. This increased focus on the CVC likely explains the lower rates of idle catheters in ICUs noted in the reviewed studies. This close surveillance can be done outside of the ICU as well, and could include peripheral catheters.
At our own institution, the need for catheters is reviewed on some units as part of formalized patient safety rounds. Another potential group of interventions could focus on electronic medical record (EMR)-based changes such as limits on the duration of the order, requirement for renewal of the order, or on-screen reminders of the presence of a catheter. This sort of intervention could possibly be expanded as EMR use becomes more common and robust. For instance, if intravenous medications have not been ordered or given in a certain amount of time, an alert might be triggered. Another EMR-based mechanism could be to require an indication for ongoing catheter use.
Education about the potential adverse outcomes of idle catheters is important. Promoting a team-based approach to interventions, where all involved team members can discuss patient safety issues on equal ground is paramount to successfully decreasing idle catheters and improving patient care and safety in general. As with other hospital-wide initiatives, engagement of hospital administration is important to decrease barriers to implementation.
Intravenous catheter use will remain an integral part of patient care, but efforts should be made to create standardization around the definition of an idle catheter, standardize units of measure, and institute programs to prevent idle catheters.
Daniel Shirley, MD, MS, is assistant professor in the division of infectious disease at the University of Wisconsin–Madison School of Medicine and Public Health and the William S. Middleton Memorial Veterans Hospital. Nasia Safdar, MD, PhD, is associate professor in the division of infectious disease at the University of Wisconsin–Madison School of Medicine and Public Health and the William S. Middleton Memorial Veterans Hospital.
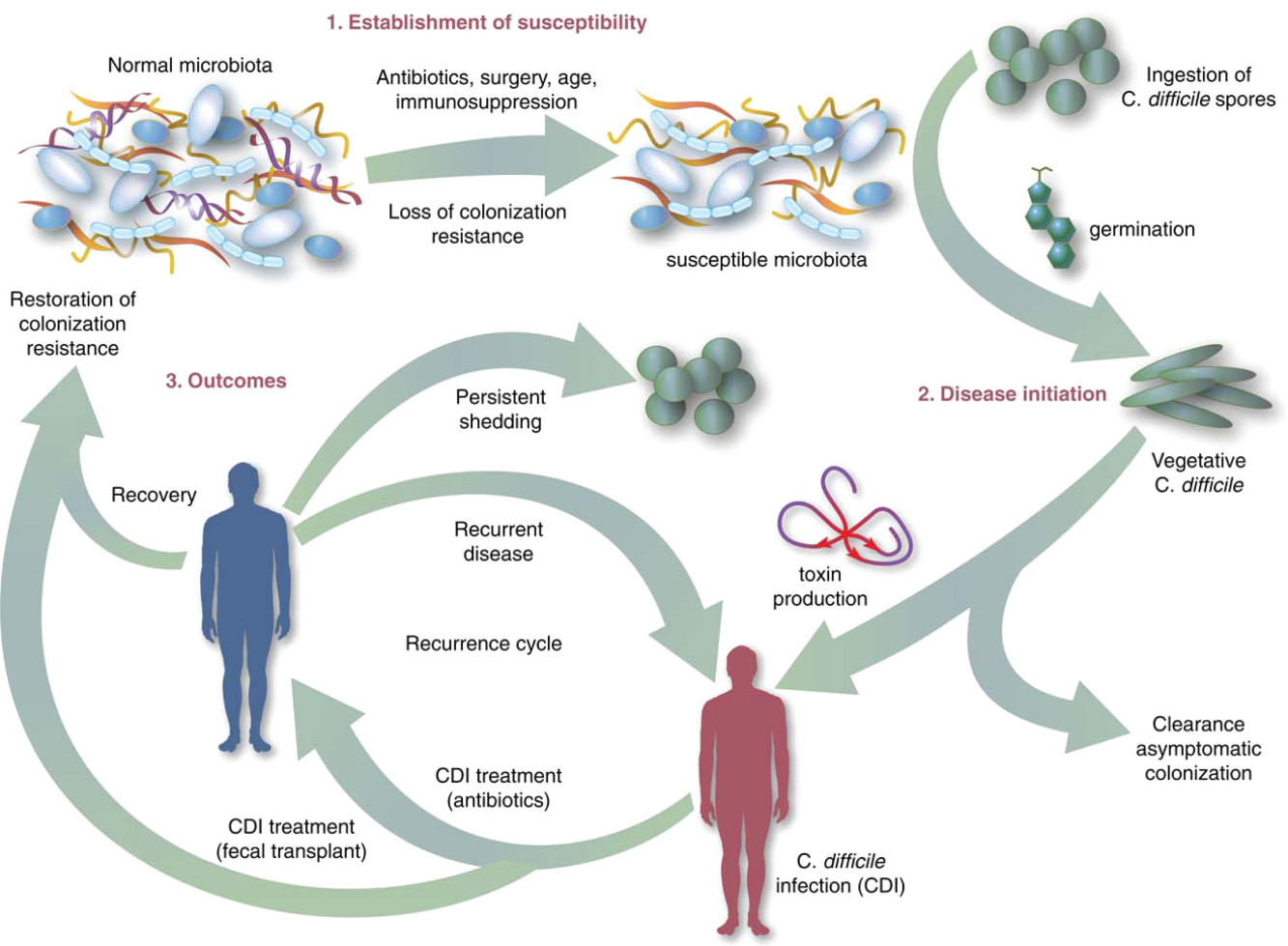
Fecal Microbiota Transplant for CDI
Symptomatic Clostridium difficile infection (CDI) results when C difficile, a gram‐positive bacillus that is an obligate‐anaerobe, produces cytotoxins TcdA and TcdB, causing epithelial and mucosal injury in the gastrointestinal tract.[1] Though it was first identified in 1978 as the causative agent of pseudomembranous colitis, and several effective treatments have subsequently been discovered,[2] nearly 3 decades later C difficile remains a major nosocomial pathogen. C difficile is the most frequent infectious cause of healthcare‐associated diarrhea and causes toxin mediated infection. The incidence of CDI in the United States has increased dramatically, especially in hospitals and nursing homes where there are now nearly 500,000 new cases and 30,000 deaths per year.[3, 4, 5, 6] This increased burden of disease is due both to the emergence of several strains that have led to a worldwide epidemic[7] and to a predilection for CDI in older adults, who constitute a growing proportion of hospitalized patients.[8] Ninety‐two percent of CDI‐related deaths occur in adults >65 years old,[9] and the risk of recurrent CDI is 2‐fold higher with each decade of life.[10] It is estimated that CDI is responsible for $1.5 billion in excess healthcare costs each year in the United States,[11] and that much of the additional cost and morbidity of CDI is due to recurrence, with around 83,000 cases per year.[6]
The human gut microbiota, which is a diverse ecosystem consisting of thousands of bacterial species,[12] protects against invasive pathogens such as C difficile.[13, 14] The pathogenesis of CDI requires disruption of the gut microbiota before onset of symptomatic disease,[15] and exposure to antibiotics is the most common precipitant (Figure 1).[16] Following exposure, the manifestations can vary from asymptomatic colonization, to a self‐limited diarrheal illness, to a fulminant, life‐threatening colitis.[1] Even among those who recover, recurrent disease is common.[10] A first recurrence will occur in 15% to 20% of successfully treated patients, a second recurrence will occur in 45% of those patients, and up to 5% of all patients enter a prolonged cycle of CDI with multiple recurrences.[17, 18, 19]
THE NEED FOR BETTER TREATMENT MODALITIES: RATIONALE
Conventional treatments (Table 1) utilize antibiotics with activity against C difficile,[20, 21] but these antibiotics have activity against other gut bacteria, limiting the ability of the microbiota to fully recover following CDI and predisposing patients to recurrence.[22] Traditional treatments for CDI result in a high incidence of recurrence (35%), with up to 65% of these patients who are again treated with conventional approaches developing a chronic pattern of recurrent CDI.[23] Though other factors may also explain why patients have recurrence (such as low serum antibody response to C difficile toxins,[24] use of medications such as proton pump inhibitors,[10] and the specific strain of C difficile causing infection[10, 21], restoration of the gut microbiome through fecal microbiota transplantation (FMT) is the treatment strategy that has garnered the most attention and has gained acceptance among practitioners in the treatment of recurrent CDI when conventional treatments have failed.[25] A review of the practices and evidence for use of FMT in the treatment of CDI in hospitalized patients is presented here, with recommendations shown in Table 2.
| Type of CDI | Associated Signs/Symptoms | Usual Treatment(s)[20] |
|---|---|---|
| ||
| Primary CDI, nonsevere | Diarrhea without signs of systemic infection, WBC <15,000 cells/mL, and serum creatinine <1.5 times the premorbid level | Metronidazole 500mg by mouth 3 times daily for 1014 days OR vancomycin 125mg by mouth 4 times daily for 1014 days OR fidaxomicin 200mg by mouth twice daily for 10 daysa |
| Primary CDI, severe | Signs of systemic infection and/or WBC15,000 cells/mL, or serum creatinine 1.5 times the premorbid level | vancomycin 125mg by mouth 4 times daily for 1014 days OR fidaxomicin 200mg by mouth twice daily for 10 daysa |
| Primary CDI, complicated | Signs of systemic infection including hypotension, ileus, or megacolon | vancomycin 500mg by mouth 4 times daily AND vancomycin 500mg by rectum 4 times daily AND intravenous metronidazole 500mg 3 times daily |
| Recurrent CDI | Return of symptoms with positive Clostridium difficile testing within 8 weeks of onset, but after initial symptoms resolved with treatment | First recurrence: same as initial treatment, based on severity. Second recurrence: Start treatment based on severity, followed by a vancomycin pulsed and/or tapered regimen over 6 or more weeks |
| Type of CDI | Recommendation on Use of FMT |
|---|---|
| |
| Primary CDI, nonsevere | Insufficient data on safety/efficacy to make a recommendation; effective conventional treatments exist |
| Primary CDI, severe | Not recommended due to insufficient data on safety/efficacy with documented adverse events |
| Primary CDI, complicated | Not recommended due to insufficient data on safety/efficacy with documented adverse events |
| Recurrent CDI (usually second recurrence) | Recommended based on data from case reports, systematic reviews, and 2 randomized, controlled clinical trials demonstrating safety and efficacy |
OVERVIEW OF FMT
FMT is not new to modern times, as there are reports of its use in ancient China for various purposes.[26] It was first described as a treatment for pseudomembranous colitis in the 1950s,[27] and in the past several years the use of FMT for CDI has increasingly gained acceptance as a safe and effective treatment. The optimal protocol for FMT is unknown; there are numerous published methods of stool preparation, infusion, and recipient and donor preparation. Diluents include tap water, normal saline, or even yogurt.[23, 28, 29] Sites of instillation of the stool include the stomach, small intestine, and large intestine.[23, 29, 30] Methods of recipient preparation for the infusion include cessation of antibiotic therapy for 24 to 48 hours prior to FMT, a bowel preparation or lavage, and use of antimotility agents, such as loperamide, to aid in retention of transplanted stool.[28] Donors may include friends or family members of the patients or 1 or more universal donors for an entire center. In both cases, screening for blood‐borne and fecal pathogens is performed before one can donate stool, though the tests performed vary between centers. FMT has been performed in both inpatient and outpatient settings, and a published study that instructed patients on self‐administration of fecal enema at home also demonstrated success.[30]
Although there are numerous variables to consider in designing a protocol, as discussed further below, it is encouraging that FMT appears to be highly effective regardless of the specific details of the protocol.[28] If the first procedure fails, evidence suggests a second or third treatment can be quite effective.[28] In a recent advance, successful FMT via administration of frozen stool oral capsules has been demonstrated,[31] which potentially removes many system‐ and patient‐level barriers to receipt of this treatment.
CLINICAL EVIDENCE FOR EFFICACY OF FMT IN TREATMENT OF CDI
Recurrent CDI
The clinical evidence for FMT is most robust for recurrent CDI, consisting of case reports or case series, recently aggregated by 2 large systematic reviews, as well as several clinical trials.[23, 29] Gough et al. published the larger of the 2 reviews with data from 317 patients treated via FMT for recurrent CDI,[23] including FMT via retention enema (35%), colonoscopic infusion (42%), and gastric infusion (23%). Though the authors noted differences in resolution proportions among routes of infusion, types of donors, and types of infusates, it is not possible to draw definite conclusions form these data given their anecdotal nature. Regardless of the specific protocol's details, 92% of patients in the review had resolution of recurrent CDI overall after 1 or more treatments, with 89% improving after only 1 treatment. Another systematic review of FMT, both for CDI and non‐CDI indications, reinforced its efficacy in CDI and overall benign safety profile.[32] Other individual case series and reports of FMT for CDI not included in these reviews have been published; they too demonstrate an excellent resolution rate.[33, 34, 35, 36, 37, 38] As with any case reports/series, generalizing from these data to arrive at conclusions about the safety and efficacy of FMT for CDI is limited by potential confounding and publication bias; thus, there emerged a need for high‐quality prospective trials.
The first randomized, controlled clinical trial (RCT) of FMT for recurrent CDI was reported in 2013.[39] Three treatment groups were compared: vancomycin for 5 days followed by FMT (n=16), vancomycin alone for 14 days (n=13), or vancomycin for 14 days with bowel lavage (n=13). Despite a strict definition of cure (absence of diarrhea or persistent diarrhea from another cause with 3 consecutive negative stool tests for C difficile toxin), the study was stopped early after an interim analysis due to resolution of CDI in 94% of patients in the FMT arm (81% after just 1 infusion) versus 23% to 31% in the others. Off‐protocol FMT was offered to the patients in the other 2 groups and 83% of them were also cured.
Youngster et al. conducted a pilot RCT with 10 patients in each group, where patients were randomized to receive FMT via either colonoscopy or nasogastric tube from a frozen fecal suspension, and no difference in efficacy was seen between administration routes, with an overall cure rate of 90%.[40] Subsequently, Youngster et al. conducted an open‐label noncomparative study with frozen fecal capsules for FMT in 20 patients with recurrent CDI.[31] Resolution occurred in 14 (70%) patients after a single treatment, and 4 of the 6 nonresponders had resolution upon retreatment for an overall efficacy of 90%.
Finally, Cammarota et al. conducted an open‐label RCT on FMT for recurrent CDI,[41] comparing FMT to a standard course of vancomycin for 10 days, followed by pulsed dosing every 2 to 3 days for 3 weeks. The study was stopped after a 1‐year interim analysis as 18 of 20 patients (90%) treated by FMT exhibited resolution of CDI‐associated diarrhea compared to only 5 of 19 patients (26%) in the vancomycin‐treated group (P<0.001).
Primary and Severe CDI
There are few data on the use of FMT for primary, nonrecurrent CDI aside from a few case reports, which are included in the data presented above. A mathematical model of CDI in an intensive care unit assessed the role of FMT on primary CDI,[42] and predicted a decreased median incidence of recurrent CDI in patients treated with FMT for primary CDI. In addition to the general limitations inherent in any mathematical model, the study had specific assumptions for model parameters that limited generalizability, such as lack of incorporation of known risk factors for CDI and assumed immediate, persistent disruption of the microbiota after any antimicrobial exposure until FMT occurred.[43]
Lagier et al.[44] conducted a nonrandomized, open‐label, before and after prospective study comparing mortality between 2 intervention periods: conventional antibiotic treatment for CDI versus early FMT via nasogastric infusion. This shift happened due to clinical need, as their hospital in Marseille developed a ribotype 027 outbreak with a dramatic global mortality rate (50.8%). Mortality in the FMT group was significantly less (64.4% vs 18.8%, P<0.01). This was an older cohort (mean age 84 years), suggesting that in an epidemic setting with a high mortality rate, early FMT may be beneficial, but one cannot extrapolate these data to support a position of early FMT for primary CDI in a nonepidemic setting.
Similarly, the evidence for use of FMT in severe CDI (defined in Table 1) consists of published case reports, which suggest efficacy.[45, 46, 47, 48] Similarly, the study by Lagier et al.[44] does not provide data on severity classification, but had a high mortality rate and found a benefit of FMT versus conventional therapy, suggesting that at least some patients presented with severe CDI and benefited. However, 1 documented death (discussed further below) following FMT for severe CDI highlights the need for caution before this treatment is used in that setting.[49]
Patient and Provider Perceptions Regarding Acceptability of FMT as a Treatment Option for CDI
A commonly cited reason for a limited role of FMT is the aesthetics of the treatment. However, few studies exist on the perceptions of patients and providers regarding FMT. Zipursky et al. surveyed 192 outpatients on their attitudes toward FMT using hypothetical case scenarios.[50] Only 1 patient had a history of CDI. The results were largely positive, with 81% of respondents agreeing to FMT for CDI. However, the need to handle stool and the nasogastric route of administration were identified as the most unappealing aspects of FMT. More respondents (90%, P=0.002) agreed to FMT when offered as a pill.
The same group of investigators undertook an electronic survey to examine physician attitudes toward FMT,[51] and found that 83 of 135 physicians (65%) in their sample had not offered or referred a patient for FMT. Frequent reasons for this included institutional barriers, concern that patients would find it too unappealing, and uncertainty regarding indications for FMT. Only 8% of physicians believed that patients would choose FMT if given the option. As the role of FMT in CDI continues to grow, it is likely that patient and provider perceptions and attitudes regarding this treatment will evolve to better align.
SAFETY OF FMT
Short‐term Complications
Serious adverse effects directly attributable to FMT in patients with normal immune function are uncommon. Symptoms of an irritable bowel (constipation, diarrhea, cramping, bloating) shortly after FMT are observed and usually last less than 48 hours.[23] A recent case series of immunocompromised patients (excluding those with inflammatory bowel disease [IBD]) treated for CDI with FMT did not find many adverse events in this group.[35] However, patients with IBD may have a different risk profile; the same case series noted adverse events occurred in 14% of IBD patients, who experienced disease flare requiring hospitalization in some cases.[35] No cases of septicemia or other infections were observed in this series. An increased risk of IBD flare, fever, and elevation in inflammatory markers following FMT has also been observed in other studies.[52, 53, 54] However, the interaction between IBD and the microbiome is complex, and a recent RCT for patients with ulcerative colitis (without CDI) treated via FMT did not show any significant adverse events.[55] FMT side effects may vary by the administration method and may be related to complications of the method itself rather than FMT (for example, misplacement of a nasogastric tube, perforation risk with colonoscopy).
Deaths following FMT are rare and often are not directly attributed to FMT. One reported death occurred as a result of aspiration pneumonia during sedation for colonoscopy for FMT.[35] In another case, a patient with severe CDI was treated with FMT, did not achieve cure, and developed toxic megacolon and shock, dying shortly after. The authors speculate that withdrawal of antibiotics with activity against CDI following FMT contributed to the outcome, rather than FMT itself.[49] FMT is largely untested in patients with severe CDI,[45, 46, 47, 48] and this fatal case of toxic megacolon warrants caution.
Long‐term Complications
The long‐term safety of FMT is unknown. There is an incomplete understanding of the interaction between the gut microbiome and the host, but this is a complex system, and associations with disease processes have been demonstrated. The gut microbiome may be associated with colon cancer, diabetes, obesity, and atopic disorders.[56] The role of FMT in contributing to these conditions is unknown. It is also not known whether targeted screening/selection of stool for infusion can mitigate these potential risks.
In the only study to capture long‐term outcomes after FMT, 77 patients were followed for 3 to 68 months (mean 17 months).[57] New conditions such as ovarian cancer, myocardial infarction, autoimmune disease, and stroke were observed. Although it is not possible to establish causality from this study or infer an increased risk of these conditions from FMT, the results underscore the need for long‐term follow‐up after FMT.
Regulatory Status
The increased use of FMT for CDI and interest in non‐CDI indications led the US Food and Drug Administration (FDA) in 2013 to publish an initial guidance statement regulating stool as a biologic agent.[58] However, subsequently, the United States Department of Health and Human Services' FDA issued guidance stating that it would exercise enforcement discretion for physicians administering FMT to treat patients with C difficile infections; thus, an investigational new drug approval is not required, but appropriate informed consent from the patient indicating that FMT is an investigational therapy is needed. Revision to this guidance is in progress.[59]
Future Directions
Expansion of the indications for FMT and use of synthetic and/or frozen stool are directions currently under active exploration. There are a number of clinical trials studying FMT for CDI underway that are not yet completed,[60, 61, 62, 63, 64, 65] and these may shed light on the safety and efficacy of FMT for primary CDI, severe CDI, and FMT as a preemptive therapy for high‐risk patients on antibiotics. Frozen stool preparations, often from a known set of prescreened donors and recently in capsule form, have been used for FMT and are gaining popularity.[31, 33] A synthetic intestinal microbiota suspension for use in FMT is currently being tested.[62] There also exists a nonprofit organization, OpenBiome (
CONCLUSIONS
Based on several prospective trials and observational data, FMT appears to be a safe and effective treatment for recurrent CDI that is superior to conventional approaches. Despite recent pivotal advances in the field of FMT, there remain many unanswered questions, and further research is needed to examine the optimal parameters, indications, and outcomes with FMT.
Disclosures
K.R. is supported by grants from the Claude D. Pepper Older Americans Independence Center (grant number AG‐024824) and the Michigan Institute for Clinical and Health Research (grant number 2UL1TR000433). N.S. is supported by a VA MERIT award. The contents of this article do not necessarily represent the views of the Department of Veterans Affairs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors report no conflicts of interest.
- , , . Emergence of Clostridium difficile‐associated disease in North America and Europe. Clin Microbiol Infect. 2006;12:2–18.
- , , , , . Antibiotic‐associated pseudomembranous colitis due to toxin‐producing clostridia. N Engl J Med. 1978;298(10):531–534.
- , , ,, et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30(6):526–533.
- , , , , . Attributable burden of hospital‐onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol. 2013;34(6):588–596.
- Centers for Disease Control and Prevention. Vital Signs. Making health care safer. Stopping C. difficile infections. Available at: http://www.cdc.gov/VitalSigns/Hai/StoppingCdifficile. Accessed January 15, 2015.
- , , , et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–834.
- , , , et al. Emergence and global spread of epidemic healthcare‐associated Clostridium difficile. Nat Genet. 2013;45(1):109–113.
- , , , et al. Effect of age on treatment outcomes in Clostridium difficile infection. J Am Geriatr Soc. 2013;61(2):222–230.
- , , . Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(suppl 2):S65–S70.
- , , , . Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One. 2014;9(6):e98400.
- , , , et al. Health care‐associated infections: a meta‐analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039–2046.
- , , , et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227.
- , , . Colonization resistance of the digestive tract in conventional and antibiotic‐treated mice. Epidemiol Infect. 1971;69(03):405–411.
- , . Colonization resistance. Antimicrob Agents Chemother. 1994;38(3):409.
- , . Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterol. 2014;146(6):1547–1553.
- , , , et al. Antibiotic‐induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114.
- . Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe. 2009;15(6):285–289.
- , . Treatment of recurrent Clostridium difficile diarrhea. Gastroenterol Hepatol. 2006;2(3):203–208.
- , , , , , . Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38(6):475–483.
- , , , et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–455.
- , , , et al. Fidaxomicin Versus Vancomycin for Clostridium difficile Infection: meta‐analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(suppl 2):S93–S103.
- , , , et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile‐associated diarrhea. J Infect Dis. 2008;197(3):435–438.
- , , . Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53(10):994–1002.
- , , , . Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251):189–193.
- , , , , . Treatment approaches including fecal microbiota transplantation for recurrent Clostridium difficile infection (RCDI) among infectious disease physicians. Anaerobe. 2013;24:20–24.
- , , , , . Should we standardize the 1,700‐year‐old fecal microbiota transplantation? Am J Gastroenterol. 2012;107(11):1755.
- , , , . Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–859.
- , , , et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–1049.
- , , , . Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta‐analysis. Am J Gastroenterol. 2013;108(4):500–508.
- , , . Success of self‐administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8(5):471–473.
- , , , , , . Oral, Capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772–1778.
- , , , et al. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39(10):1003–1032.
- , , , . Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile Infection. Am J Gastroenterol. 2012;107(5):761–767.
- , , , . Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med. 2012;172(2):191–193.
- , , , et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–1071.
- , , , et al. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2014;12(9):1572–1576.
- , , , . Fecal microbiota transplantation for refractory Clostridium difficile colitis in solid organ transplant recipients. Am J Transplant. 2014;14(2):477–480.
- , , , , . Faecal microbiota transplantation and bacteriotherapy for recurrent Clostridium difficile infection: a retrospective evaluation of 31 patients. Scand J Infect Dis. 2014;46(2):89–97.
- , , , et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415.
- , , , et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open‐label, controlled pilot study. Clin Infect Dis. 2014;58(11):1515–1522.
- , , , et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41(9):835–843.
- , , , , . A mathematical model to evaluate the routine use of fecal microbiota transplantation to prevent incident and recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;35(1):18–27.
- , , . Commentary: fecal microbiota therapy: ready for prime time? Infect Control Hosp Epidemiol. 2014;35(1):28–30.
- , , , et al. Dramatic reduction in Clostridium difficile ribotype 027‐associated mortality with early fecal transplantation by the nasogastric route: a preliminary report. Eur J Clin Microbiol Infect Dis. 2015;34(8):1597–1601.
- , , , , , . Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem cell transplant patient. Transplant Infect Dis. 2012;14(6):E161–E165.
- , , , , , . Faecal microbiota transplantation for severe Clostridium difficile infection in the intensive care unit. Eur J Gastroenterol Hepatol. 2013;25(2):255–257.
- , , , . Successful colonoscopic fecal transplant for severe acute Clostridium difficile pseudomembranous colitis. Rev Gastroenterol Mex. 2011;77(1):40–42.
- , , . Successful treatment of fulminant Clostridium difficile infection with fecal bacteriotherapy. Ann Intern Med. 2008;148(8):632–633.
- , , , . Tempered enthusiasm for fecal transplant. Clin Infect Dis. 2014;59(2):319.
- , , , , . Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis. 2012;55(12):1652–1658.
- , , , , . Physician attitudes toward the use of fecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection. Can J Gastroenterol Hepatol. 2014;28(6):319–324.
- , , . Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11(8):1036–1038.
- , , , et al. Temporal Bacterial Community Dynamics Vary Among Ulcerative Colitis Patients After Fecal Microbiota Transplantation. Am J Gastroenterol. 2013;108(10):1620–1630.
- , , , et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19(10):2155–2165.
- , , , et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterol. 2015;149(1):110–118.e4.
- , , , . Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904.
- , , , et al. Long‐term follow‐up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–1087.
- US Food and Drug Administration. Guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. Available at: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm361379.htm. Accessed July 1, 2014.
- US Food and Drug Administration. Draft guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. Available at: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm387023.htm. Accessed July 1, 2014.
- University Health Network Toronto. Oral vancomycin followed by fecal transplant versus tapering oral vancomycin. Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01226992. Available at: http://clinicaltrials.gov/ct2/show/NCT01226992. Accessed July 1, 2014.
- Tel‐Aviv Sourasky Medical Center. Transplantation of fecal microbiota for Clostridium difficile infection. Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01958463. Available at: http://clinicaltrials.gov/ct2/show/NCT01958463. Accessed July 1, 2014.
- Rebiotix Inc. Microbiota restoration therapy for recurrent Clostridium difficile‐associated diarrhea (PUNCH CD). Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01925417. Available at: http://clinicaltrials.gov/ct2/show/NCT01925417. Accessed July 1, 2014.
- Hadassah Medical Organization. Efficacy and safety of fecal microbiota transplantation for severe Clostridium difficile‐associated colitis. Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01959048. Available at: http://clinicaltrials.gov/ct2/show/NCT01959048. Accessed July 1, 2014.
- University Hospital Tuebingen. Fecal microbiota transplantation in recurrent or refractory Clostridium difficile colitis (TOCSIN). Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01942447. Available at: http://clinicaltrials.gov/ct2/show/NCT01942447. Accessed July 1, 2014.
- Duke University. Stool transplants to treat refractory Clostridium difficile colitis. Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT02127398. Available at: http://clinicaltrials.gov/ct2/show/NCT02127398. Accessed July 1, 2014.
Symptomatic Clostridium difficile infection (CDI) results when C difficile, a gram‐positive bacillus that is an obligate‐anaerobe, produces cytotoxins TcdA and TcdB, causing epithelial and mucosal injury in the gastrointestinal tract.[1] Though it was first identified in 1978 as the causative agent of pseudomembranous colitis, and several effective treatments have subsequently been discovered,[2] nearly 3 decades later C difficile remains a major nosocomial pathogen. C difficile is the most frequent infectious cause of healthcare‐associated diarrhea and causes toxin mediated infection. The incidence of CDI in the United States has increased dramatically, especially in hospitals and nursing homes where there are now nearly 500,000 new cases and 30,000 deaths per year.[3, 4, 5, 6] This increased burden of disease is due both to the emergence of several strains that have led to a worldwide epidemic[7] and to a predilection for CDI in older adults, who constitute a growing proportion of hospitalized patients.[8] Ninety‐two percent of CDI‐related deaths occur in adults >65 years old,[9] and the risk of recurrent CDI is 2‐fold higher with each decade of life.[10] It is estimated that CDI is responsible for $1.5 billion in excess healthcare costs each year in the United States,[11] and that much of the additional cost and morbidity of CDI is due to recurrence, with around 83,000 cases per year.[6]
The human gut microbiota, which is a diverse ecosystem consisting of thousands of bacterial species,[12] protects against invasive pathogens such as C difficile.[13, 14] The pathogenesis of CDI requires disruption of the gut microbiota before onset of symptomatic disease,[15] and exposure to antibiotics is the most common precipitant (Figure 1).[16] Following exposure, the manifestations can vary from asymptomatic colonization, to a self‐limited diarrheal illness, to a fulminant, life‐threatening colitis.[1] Even among those who recover, recurrent disease is common.[10] A first recurrence will occur in 15% to 20% of successfully treated patients, a second recurrence will occur in 45% of those patients, and up to 5% of all patients enter a prolonged cycle of CDI with multiple recurrences.[17, 18, 19]

THE NEED FOR BETTER TREATMENT MODALITIES: RATIONALE
Conventional treatments (Table 1) utilize antibiotics with activity against C difficile,[20, 21] but these antibiotics have activity against other gut bacteria, limiting the ability of the microbiota to fully recover following CDI and predisposing patients to recurrence.[22] Traditional treatments for CDI result in a high incidence of recurrence (35%), with up to 65% of these patients who are again treated with conventional approaches developing a chronic pattern of recurrent CDI.[23] Though other factors may also explain why patients have recurrence (such as low serum antibody response to C difficile toxins,[24] use of medications such as proton pump inhibitors,[10] and the specific strain of C difficile causing infection[10, 21], restoration of the gut microbiome through fecal microbiota transplantation (FMT) is the treatment strategy that has garnered the most attention and has gained acceptance among practitioners in the treatment of recurrent CDI when conventional treatments have failed.[25] A review of the practices and evidence for use of FMT in the treatment of CDI in hospitalized patients is presented here, with recommendations shown in Table 2.
| Type of CDI | Associated Signs/Symptoms | Usual Treatment(s)[20] |
|---|---|---|
| ||
| Primary CDI, nonsevere | Diarrhea without signs of systemic infection, WBC <15,000 cells/mL, and serum creatinine <1.5 times the premorbid level | Metronidazole 500mg by mouth 3 times daily for 1014 days OR vancomycin 125mg by mouth 4 times daily for 1014 days OR fidaxomicin 200mg by mouth twice daily for 10 daysa |
| Primary CDI, severe | Signs of systemic infection and/or WBC15,000 cells/mL, or serum creatinine 1.5 times the premorbid level | vancomycin 125mg by mouth 4 times daily for 1014 days OR fidaxomicin 200mg by mouth twice daily for 10 daysa |
| Primary CDI, complicated | Signs of systemic infection including hypotension, ileus, or megacolon | vancomycin 500mg by mouth 4 times daily AND vancomycin 500mg by rectum 4 times daily AND intravenous metronidazole 500mg 3 times daily |
| Recurrent CDI | Return of symptoms with positive Clostridium difficile testing within 8 weeks of onset, but after initial symptoms resolved with treatment | First recurrence: same as initial treatment, based on severity. Second recurrence: Start treatment based on severity, followed by a vancomycin pulsed and/or tapered regimen over 6 or more weeks |
| Type of CDI | Recommendation on Use of FMT |
|---|---|
| |
| Primary CDI, nonsevere | Insufficient data on safety/efficacy to make a recommendation; effective conventional treatments exist |
| Primary CDI, severe | Not recommended due to insufficient data on safety/efficacy with documented adverse events |
| Primary CDI, complicated | Not recommended due to insufficient data on safety/efficacy with documented adverse events |
| Recurrent CDI (usually second recurrence) | Recommended based on data from case reports, systematic reviews, and 2 randomized, controlled clinical trials demonstrating safety and efficacy |
OVERVIEW OF FMT
FMT is not new to modern times, as there are reports of its use in ancient China for various purposes.[26] It was first described as a treatment for pseudomembranous colitis in the 1950s,[27] and in the past several years the use of FMT for CDI has increasingly gained acceptance as a safe and effective treatment. The optimal protocol for FMT is unknown; there are numerous published methods of stool preparation, infusion, and recipient and donor preparation. Diluents include tap water, normal saline, or even yogurt.[23, 28, 29] Sites of instillation of the stool include the stomach, small intestine, and large intestine.[23, 29, 30] Methods of recipient preparation for the infusion include cessation of antibiotic therapy for 24 to 48 hours prior to FMT, a bowel preparation or lavage, and use of antimotility agents, such as loperamide, to aid in retention of transplanted stool.[28] Donors may include friends or family members of the patients or 1 or more universal donors for an entire center. In both cases, screening for blood‐borne and fecal pathogens is performed before one can donate stool, though the tests performed vary between centers. FMT has been performed in both inpatient and outpatient settings, and a published study that instructed patients on self‐administration of fecal enema at home also demonstrated success.[30]
Although there are numerous variables to consider in designing a protocol, as discussed further below, it is encouraging that FMT appears to be highly effective regardless of the specific details of the protocol.[28] If the first procedure fails, evidence suggests a second or third treatment can be quite effective.[28] In a recent advance, successful FMT via administration of frozen stool oral capsules has been demonstrated,[31] which potentially removes many system‐ and patient‐level barriers to receipt of this treatment.
CLINICAL EVIDENCE FOR EFFICACY OF FMT IN TREATMENT OF CDI
Recurrent CDI
The clinical evidence for FMT is most robust for recurrent CDI, consisting of case reports or case series, recently aggregated by 2 large systematic reviews, as well as several clinical trials.[23, 29] Gough et al. published the larger of the 2 reviews with data from 317 patients treated via FMT for recurrent CDI,[23] including FMT via retention enema (35%), colonoscopic infusion (42%), and gastric infusion (23%). Though the authors noted differences in resolution proportions among routes of infusion, types of donors, and types of infusates, it is not possible to draw definite conclusions form these data given their anecdotal nature. Regardless of the specific protocol's details, 92% of patients in the review had resolution of recurrent CDI overall after 1 or more treatments, with 89% improving after only 1 treatment. Another systematic review of FMT, both for CDI and non‐CDI indications, reinforced its efficacy in CDI and overall benign safety profile.[32] Other individual case series and reports of FMT for CDI not included in these reviews have been published; they too demonstrate an excellent resolution rate.[33, 34, 35, 36, 37, 38] As with any case reports/series, generalizing from these data to arrive at conclusions about the safety and efficacy of FMT for CDI is limited by potential confounding and publication bias; thus, there emerged a need for high‐quality prospective trials.
The first randomized, controlled clinical trial (RCT) of FMT for recurrent CDI was reported in 2013.[39] Three treatment groups were compared: vancomycin for 5 days followed by FMT (n=16), vancomycin alone for 14 days (n=13), or vancomycin for 14 days with bowel lavage (n=13). Despite a strict definition of cure (absence of diarrhea or persistent diarrhea from another cause with 3 consecutive negative stool tests for C difficile toxin), the study was stopped early after an interim analysis due to resolution of CDI in 94% of patients in the FMT arm (81% after just 1 infusion) versus 23% to 31% in the others. Off‐protocol FMT was offered to the patients in the other 2 groups and 83% of them were also cured.
Youngster et al. conducted a pilot RCT with 10 patients in each group, where patients were randomized to receive FMT via either colonoscopy or nasogastric tube from a frozen fecal suspension, and no difference in efficacy was seen between administration routes, with an overall cure rate of 90%.[40] Subsequently, Youngster et al. conducted an open‐label noncomparative study with frozen fecal capsules for FMT in 20 patients with recurrent CDI.[31] Resolution occurred in 14 (70%) patients after a single treatment, and 4 of the 6 nonresponders had resolution upon retreatment for an overall efficacy of 90%.
Finally, Cammarota et al. conducted an open‐label RCT on FMT for recurrent CDI,[41] comparing FMT to a standard course of vancomycin for 10 days, followed by pulsed dosing every 2 to 3 days for 3 weeks. The study was stopped after a 1‐year interim analysis as 18 of 20 patients (90%) treated by FMT exhibited resolution of CDI‐associated diarrhea compared to only 5 of 19 patients (26%) in the vancomycin‐treated group (P<0.001).
Primary and Severe CDI
There are few data on the use of FMT for primary, nonrecurrent CDI aside from a few case reports, which are included in the data presented above. A mathematical model of CDI in an intensive care unit assessed the role of FMT on primary CDI,[42] and predicted a decreased median incidence of recurrent CDI in patients treated with FMT for primary CDI. In addition to the general limitations inherent in any mathematical model, the study had specific assumptions for model parameters that limited generalizability, such as lack of incorporation of known risk factors for CDI and assumed immediate, persistent disruption of the microbiota after any antimicrobial exposure until FMT occurred.[43]
Lagier et al.[44] conducted a nonrandomized, open‐label, before and after prospective study comparing mortality between 2 intervention periods: conventional antibiotic treatment for CDI versus early FMT via nasogastric infusion. This shift happened due to clinical need, as their hospital in Marseille developed a ribotype 027 outbreak with a dramatic global mortality rate (50.8%). Mortality in the FMT group was significantly less (64.4% vs 18.8%, P<0.01). This was an older cohort (mean age 84 years), suggesting that in an epidemic setting with a high mortality rate, early FMT may be beneficial, but one cannot extrapolate these data to support a position of early FMT for primary CDI in a nonepidemic setting.
Similarly, the evidence for use of FMT in severe CDI (defined in Table 1) consists of published case reports, which suggest efficacy.[45, 46, 47, 48] Similarly, the study by Lagier et al.[44] does not provide data on severity classification, but had a high mortality rate and found a benefit of FMT versus conventional therapy, suggesting that at least some patients presented with severe CDI and benefited. However, 1 documented death (discussed further below) following FMT for severe CDI highlights the need for caution before this treatment is used in that setting.[49]
Patient and Provider Perceptions Regarding Acceptability of FMT as a Treatment Option for CDI
A commonly cited reason for a limited role of FMT is the aesthetics of the treatment. However, few studies exist on the perceptions of patients and providers regarding FMT. Zipursky et al. surveyed 192 outpatients on their attitudes toward FMT using hypothetical case scenarios.[50] Only 1 patient had a history of CDI. The results were largely positive, with 81% of respondents agreeing to FMT for CDI. However, the need to handle stool and the nasogastric route of administration were identified as the most unappealing aspects of FMT. More respondents (90%, P=0.002) agreed to FMT when offered as a pill.
The same group of investigators undertook an electronic survey to examine physician attitudes toward FMT,[51] and found that 83 of 135 physicians (65%) in their sample had not offered or referred a patient for FMT. Frequent reasons for this included institutional barriers, concern that patients would find it too unappealing, and uncertainty regarding indications for FMT. Only 8% of physicians believed that patients would choose FMT if given the option. As the role of FMT in CDI continues to grow, it is likely that patient and provider perceptions and attitudes regarding this treatment will evolve to better align.
SAFETY OF FMT
Short‐term Complications
Serious adverse effects directly attributable to FMT in patients with normal immune function are uncommon. Symptoms of an irritable bowel (constipation, diarrhea, cramping, bloating) shortly after FMT are observed and usually last less than 48 hours.[23] A recent case series of immunocompromised patients (excluding those with inflammatory bowel disease [IBD]) treated for CDI with FMT did not find many adverse events in this group.[35] However, patients with IBD may have a different risk profile; the same case series noted adverse events occurred in 14% of IBD patients, who experienced disease flare requiring hospitalization in some cases.[35] No cases of septicemia or other infections were observed in this series. An increased risk of IBD flare, fever, and elevation in inflammatory markers following FMT has also been observed in other studies.[52, 53, 54] However, the interaction between IBD and the microbiome is complex, and a recent RCT for patients with ulcerative colitis (without CDI) treated via FMT did not show any significant adverse events.[55] FMT side effects may vary by the administration method and may be related to complications of the method itself rather than FMT (for example, misplacement of a nasogastric tube, perforation risk with colonoscopy).
Deaths following FMT are rare and often are not directly attributed to FMT. One reported death occurred as a result of aspiration pneumonia during sedation for colonoscopy for FMT.[35] In another case, a patient with severe CDI was treated with FMT, did not achieve cure, and developed toxic megacolon and shock, dying shortly after. The authors speculate that withdrawal of antibiotics with activity against CDI following FMT contributed to the outcome, rather than FMT itself.[49] FMT is largely untested in patients with severe CDI,[45, 46, 47, 48] and this fatal case of toxic megacolon warrants caution.
Long‐term Complications
The long‐term safety of FMT is unknown. There is an incomplete understanding of the interaction between the gut microbiome and the host, but this is a complex system, and associations with disease processes have been demonstrated. The gut microbiome may be associated with colon cancer, diabetes, obesity, and atopic disorders.[56] The role of FMT in contributing to these conditions is unknown. It is also not known whether targeted screening/selection of stool for infusion can mitigate these potential risks.
In the only study to capture long‐term outcomes after FMT, 77 patients were followed for 3 to 68 months (mean 17 months).[57] New conditions such as ovarian cancer, myocardial infarction, autoimmune disease, and stroke were observed. Although it is not possible to establish causality from this study or infer an increased risk of these conditions from FMT, the results underscore the need for long‐term follow‐up after FMT.
Regulatory Status
The increased use of FMT for CDI and interest in non‐CDI indications led the US Food and Drug Administration (FDA) in 2013 to publish an initial guidance statement regulating stool as a biologic agent.[58] However, subsequently, the United States Department of Health and Human Services' FDA issued guidance stating that it would exercise enforcement discretion for physicians administering FMT to treat patients with C difficile infections; thus, an investigational new drug approval is not required, but appropriate informed consent from the patient indicating that FMT is an investigational therapy is needed. Revision to this guidance is in progress.[59]
Future Directions
Expansion of the indications for FMT and use of synthetic and/or frozen stool are directions currently under active exploration. There are a number of clinical trials studying FMT for CDI underway that are not yet completed,[60, 61, 62, 63, 64, 65] and these may shed light on the safety and efficacy of FMT for primary CDI, severe CDI, and FMT as a preemptive therapy for high‐risk patients on antibiotics. Frozen stool preparations, often from a known set of prescreened donors and recently in capsule form, have been used for FMT and are gaining popularity.[31, 33] A synthetic intestinal microbiota suspension for use in FMT is currently being tested.[62] There also exists a nonprofit organization, OpenBiome (
CONCLUSIONS
Based on several prospective trials and observational data, FMT appears to be a safe and effective treatment for recurrent CDI that is superior to conventional approaches. Despite recent pivotal advances in the field of FMT, there remain many unanswered questions, and further research is needed to examine the optimal parameters, indications, and outcomes with FMT.
Disclosures
K.R. is supported by grants from the Claude D. Pepper Older Americans Independence Center (grant number AG‐024824) and the Michigan Institute for Clinical and Health Research (grant number 2UL1TR000433). N.S. is supported by a VA MERIT award. The contents of this article do not necessarily represent the views of the Department of Veterans Affairs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors report no conflicts of interest.
Symptomatic Clostridium difficile infection (CDI) results when C difficile, a gram‐positive bacillus that is an obligate‐anaerobe, produces cytotoxins TcdA and TcdB, causing epithelial and mucosal injury in the gastrointestinal tract.[1] Though it was first identified in 1978 as the causative agent of pseudomembranous colitis, and several effective treatments have subsequently been discovered,[2] nearly 3 decades later C difficile remains a major nosocomial pathogen. C difficile is the most frequent infectious cause of healthcare‐associated diarrhea and causes toxin mediated infection. The incidence of CDI in the United States has increased dramatically, especially in hospitals and nursing homes where there are now nearly 500,000 new cases and 30,000 deaths per year.[3, 4, 5, 6] This increased burden of disease is due both to the emergence of several strains that have led to a worldwide epidemic[7] and to a predilection for CDI in older adults, who constitute a growing proportion of hospitalized patients.[8] Ninety‐two percent of CDI‐related deaths occur in adults >65 years old,[9] and the risk of recurrent CDI is 2‐fold higher with each decade of life.[10] It is estimated that CDI is responsible for $1.5 billion in excess healthcare costs each year in the United States,[11] and that much of the additional cost and morbidity of CDI is due to recurrence, with around 83,000 cases per year.[6]
The human gut microbiota, which is a diverse ecosystem consisting of thousands of bacterial species,[12] protects against invasive pathogens such as C difficile.[13, 14] The pathogenesis of CDI requires disruption of the gut microbiota before onset of symptomatic disease,[15] and exposure to antibiotics is the most common precipitant (Figure 1).[16] Following exposure, the manifestations can vary from asymptomatic colonization, to a self‐limited diarrheal illness, to a fulminant, life‐threatening colitis.[1] Even among those who recover, recurrent disease is common.[10] A first recurrence will occur in 15% to 20% of successfully treated patients, a second recurrence will occur in 45% of those patients, and up to 5% of all patients enter a prolonged cycle of CDI with multiple recurrences.[17, 18, 19]

THE NEED FOR BETTER TREATMENT MODALITIES: RATIONALE
Conventional treatments (Table 1) utilize antibiotics with activity against C difficile,[20, 21] but these antibiotics have activity against other gut bacteria, limiting the ability of the microbiota to fully recover following CDI and predisposing patients to recurrence.[22] Traditional treatments for CDI result in a high incidence of recurrence (35%), with up to 65% of these patients who are again treated with conventional approaches developing a chronic pattern of recurrent CDI.[23] Though other factors may also explain why patients have recurrence (such as low serum antibody response to C difficile toxins,[24] use of medications such as proton pump inhibitors,[10] and the specific strain of C difficile causing infection[10, 21], restoration of the gut microbiome through fecal microbiota transplantation (FMT) is the treatment strategy that has garnered the most attention and has gained acceptance among practitioners in the treatment of recurrent CDI when conventional treatments have failed.[25] A review of the practices and evidence for use of FMT in the treatment of CDI in hospitalized patients is presented here, with recommendations shown in Table 2.
| Type of CDI | Associated Signs/Symptoms | Usual Treatment(s)[20] |
|---|---|---|
| ||
| Primary CDI, nonsevere | Diarrhea without signs of systemic infection, WBC <15,000 cells/mL, and serum creatinine <1.5 times the premorbid level | Metronidazole 500mg by mouth 3 times daily for 1014 days OR vancomycin 125mg by mouth 4 times daily for 1014 days OR fidaxomicin 200mg by mouth twice daily for 10 daysa |
| Primary CDI, severe | Signs of systemic infection and/or WBC15,000 cells/mL, or serum creatinine 1.5 times the premorbid level | vancomycin 125mg by mouth 4 times daily for 1014 days OR fidaxomicin 200mg by mouth twice daily for 10 daysa |
| Primary CDI, complicated | Signs of systemic infection including hypotension, ileus, or megacolon | vancomycin 500mg by mouth 4 times daily AND vancomycin 500mg by rectum 4 times daily AND intravenous metronidazole 500mg 3 times daily |
| Recurrent CDI | Return of symptoms with positive Clostridium difficile testing within 8 weeks of onset, but after initial symptoms resolved with treatment | First recurrence: same as initial treatment, based on severity. Second recurrence: Start treatment based on severity, followed by a vancomycin pulsed and/or tapered regimen over 6 or more weeks |
| Type of CDI | Recommendation on Use of FMT |
|---|---|
| |
| Primary CDI, nonsevere | Insufficient data on safety/efficacy to make a recommendation; effective conventional treatments exist |
| Primary CDI, severe | Not recommended due to insufficient data on safety/efficacy with documented adverse events |
| Primary CDI, complicated | Not recommended due to insufficient data on safety/efficacy with documented adverse events |
| Recurrent CDI (usually second recurrence) | Recommended based on data from case reports, systematic reviews, and 2 randomized, controlled clinical trials demonstrating safety and efficacy |
OVERVIEW OF FMT
FMT is not new to modern times, as there are reports of its use in ancient China for various purposes.[26] It was first described as a treatment for pseudomembranous colitis in the 1950s,[27] and in the past several years the use of FMT for CDI has increasingly gained acceptance as a safe and effective treatment. The optimal protocol for FMT is unknown; there are numerous published methods of stool preparation, infusion, and recipient and donor preparation. Diluents include tap water, normal saline, or even yogurt.[23, 28, 29] Sites of instillation of the stool include the stomach, small intestine, and large intestine.[23, 29, 30] Methods of recipient preparation for the infusion include cessation of antibiotic therapy for 24 to 48 hours prior to FMT, a bowel preparation or lavage, and use of antimotility agents, such as loperamide, to aid in retention of transplanted stool.[28] Donors may include friends or family members of the patients or 1 or more universal donors for an entire center. In both cases, screening for blood‐borne and fecal pathogens is performed before one can donate stool, though the tests performed vary between centers. FMT has been performed in both inpatient and outpatient settings, and a published study that instructed patients on self‐administration of fecal enema at home also demonstrated success.[30]
Although there are numerous variables to consider in designing a protocol, as discussed further below, it is encouraging that FMT appears to be highly effective regardless of the specific details of the protocol.[28] If the first procedure fails, evidence suggests a second or third treatment can be quite effective.[28] In a recent advance, successful FMT via administration of frozen stool oral capsules has been demonstrated,[31] which potentially removes many system‐ and patient‐level barriers to receipt of this treatment.
CLINICAL EVIDENCE FOR EFFICACY OF FMT IN TREATMENT OF CDI
Recurrent CDI
The clinical evidence for FMT is most robust for recurrent CDI, consisting of case reports or case series, recently aggregated by 2 large systematic reviews, as well as several clinical trials.[23, 29] Gough et al. published the larger of the 2 reviews with data from 317 patients treated via FMT for recurrent CDI,[23] including FMT via retention enema (35%), colonoscopic infusion (42%), and gastric infusion (23%). Though the authors noted differences in resolution proportions among routes of infusion, types of donors, and types of infusates, it is not possible to draw definite conclusions form these data given their anecdotal nature. Regardless of the specific protocol's details, 92% of patients in the review had resolution of recurrent CDI overall after 1 or more treatments, with 89% improving after only 1 treatment. Another systematic review of FMT, both for CDI and non‐CDI indications, reinforced its efficacy in CDI and overall benign safety profile.[32] Other individual case series and reports of FMT for CDI not included in these reviews have been published; they too demonstrate an excellent resolution rate.[33, 34, 35, 36, 37, 38] As with any case reports/series, generalizing from these data to arrive at conclusions about the safety and efficacy of FMT for CDI is limited by potential confounding and publication bias; thus, there emerged a need for high‐quality prospective trials.
The first randomized, controlled clinical trial (RCT) of FMT for recurrent CDI was reported in 2013.[39] Three treatment groups were compared: vancomycin for 5 days followed by FMT (n=16), vancomycin alone for 14 days (n=13), or vancomycin for 14 days with bowel lavage (n=13). Despite a strict definition of cure (absence of diarrhea or persistent diarrhea from another cause with 3 consecutive negative stool tests for C difficile toxin), the study was stopped early after an interim analysis due to resolution of CDI in 94% of patients in the FMT arm (81% after just 1 infusion) versus 23% to 31% in the others. Off‐protocol FMT was offered to the patients in the other 2 groups and 83% of them were also cured.
Youngster et al. conducted a pilot RCT with 10 patients in each group, where patients were randomized to receive FMT via either colonoscopy or nasogastric tube from a frozen fecal suspension, and no difference in efficacy was seen between administration routes, with an overall cure rate of 90%.[40] Subsequently, Youngster et al. conducted an open‐label noncomparative study with frozen fecal capsules for FMT in 20 patients with recurrent CDI.[31] Resolution occurred in 14 (70%) patients after a single treatment, and 4 of the 6 nonresponders had resolution upon retreatment for an overall efficacy of 90%.
Finally, Cammarota et al. conducted an open‐label RCT on FMT for recurrent CDI,[41] comparing FMT to a standard course of vancomycin for 10 days, followed by pulsed dosing every 2 to 3 days for 3 weeks. The study was stopped after a 1‐year interim analysis as 18 of 20 patients (90%) treated by FMT exhibited resolution of CDI‐associated diarrhea compared to only 5 of 19 patients (26%) in the vancomycin‐treated group (P<0.001).
Primary and Severe CDI
There are few data on the use of FMT for primary, nonrecurrent CDI aside from a few case reports, which are included in the data presented above. A mathematical model of CDI in an intensive care unit assessed the role of FMT on primary CDI,[42] and predicted a decreased median incidence of recurrent CDI in patients treated with FMT for primary CDI. In addition to the general limitations inherent in any mathematical model, the study had specific assumptions for model parameters that limited generalizability, such as lack of incorporation of known risk factors for CDI and assumed immediate, persistent disruption of the microbiota after any antimicrobial exposure until FMT occurred.[43]
Lagier et al.[44] conducted a nonrandomized, open‐label, before and after prospective study comparing mortality between 2 intervention periods: conventional antibiotic treatment for CDI versus early FMT via nasogastric infusion. This shift happened due to clinical need, as their hospital in Marseille developed a ribotype 027 outbreak with a dramatic global mortality rate (50.8%). Mortality in the FMT group was significantly less (64.4% vs 18.8%, P<0.01). This was an older cohort (mean age 84 years), suggesting that in an epidemic setting with a high mortality rate, early FMT may be beneficial, but one cannot extrapolate these data to support a position of early FMT for primary CDI in a nonepidemic setting.
Similarly, the evidence for use of FMT in severe CDI (defined in Table 1) consists of published case reports, which suggest efficacy.[45, 46, 47, 48] Similarly, the study by Lagier et al.[44] does not provide data on severity classification, but had a high mortality rate and found a benefit of FMT versus conventional therapy, suggesting that at least some patients presented with severe CDI and benefited. However, 1 documented death (discussed further below) following FMT for severe CDI highlights the need for caution before this treatment is used in that setting.[49]
Patient and Provider Perceptions Regarding Acceptability of FMT as a Treatment Option for CDI
A commonly cited reason for a limited role of FMT is the aesthetics of the treatment. However, few studies exist on the perceptions of patients and providers regarding FMT. Zipursky et al. surveyed 192 outpatients on their attitudes toward FMT using hypothetical case scenarios.[50] Only 1 patient had a history of CDI. The results were largely positive, with 81% of respondents agreeing to FMT for CDI. However, the need to handle stool and the nasogastric route of administration were identified as the most unappealing aspects of FMT. More respondents (90%, P=0.002) agreed to FMT when offered as a pill.
The same group of investigators undertook an electronic survey to examine physician attitudes toward FMT,[51] and found that 83 of 135 physicians (65%) in their sample had not offered or referred a patient for FMT. Frequent reasons for this included institutional barriers, concern that patients would find it too unappealing, and uncertainty regarding indications for FMT. Only 8% of physicians believed that patients would choose FMT if given the option. As the role of FMT in CDI continues to grow, it is likely that patient and provider perceptions and attitudes regarding this treatment will evolve to better align.
SAFETY OF FMT
Short‐term Complications
Serious adverse effects directly attributable to FMT in patients with normal immune function are uncommon. Symptoms of an irritable bowel (constipation, diarrhea, cramping, bloating) shortly after FMT are observed and usually last less than 48 hours.[23] A recent case series of immunocompromised patients (excluding those with inflammatory bowel disease [IBD]) treated for CDI with FMT did not find many adverse events in this group.[35] However, patients with IBD may have a different risk profile; the same case series noted adverse events occurred in 14% of IBD patients, who experienced disease flare requiring hospitalization in some cases.[35] No cases of septicemia or other infections were observed in this series. An increased risk of IBD flare, fever, and elevation in inflammatory markers following FMT has also been observed in other studies.[52, 53, 54] However, the interaction between IBD and the microbiome is complex, and a recent RCT for patients with ulcerative colitis (without CDI) treated via FMT did not show any significant adverse events.[55] FMT side effects may vary by the administration method and may be related to complications of the method itself rather than FMT (for example, misplacement of a nasogastric tube, perforation risk with colonoscopy).
Deaths following FMT are rare and often are not directly attributed to FMT. One reported death occurred as a result of aspiration pneumonia during sedation for colonoscopy for FMT.[35] In another case, a patient with severe CDI was treated with FMT, did not achieve cure, and developed toxic megacolon and shock, dying shortly after. The authors speculate that withdrawal of antibiotics with activity against CDI following FMT contributed to the outcome, rather than FMT itself.[49] FMT is largely untested in patients with severe CDI,[45, 46, 47, 48] and this fatal case of toxic megacolon warrants caution.
Long‐term Complications
The long‐term safety of FMT is unknown. There is an incomplete understanding of the interaction between the gut microbiome and the host, but this is a complex system, and associations with disease processes have been demonstrated. The gut microbiome may be associated with colon cancer, diabetes, obesity, and atopic disorders.[56] The role of FMT in contributing to these conditions is unknown. It is also not known whether targeted screening/selection of stool for infusion can mitigate these potential risks.
In the only study to capture long‐term outcomes after FMT, 77 patients were followed for 3 to 68 months (mean 17 months).[57] New conditions such as ovarian cancer, myocardial infarction, autoimmune disease, and stroke were observed. Although it is not possible to establish causality from this study or infer an increased risk of these conditions from FMT, the results underscore the need for long‐term follow‐up after FMT.
Regulatory Status
The increased use of FMT for CDI and interest in non‐CDI indications led the US Food and Drug Administration (FDA) in 2013 to publish an initial guidance statement regulating stool as a biologic agent.[58] However, subsequently, the United States Department of Health and Human Services' FDA issued guidance stating that it would exercise enforcement discretion for physicians administering FMT to treat patients with C difficile infections; thus, an investigational new drug approval is not required, but appropriate informed consent from the patient indicating that FMT is an investigational therapy is needed. Revision to this guidance is in progress.[59]
Future Directions
Expansion of the indications for FMT and use of synthetic and/or frozen stool are directions currently under active exploration. There are a number of clinical trials studying FMT for CDI underway that are not yet completed,[60, 61, 62, 63, 64, 65] and these may shed light on the safety and efficacy of FMT for primary CDI, severe CDI, and FMT as a preemptive therapy for high‐risk patients on antibiotics. Frozen stool preparations, often from a known set of prescreened donors and recently in capsule form, have been used for FMT and are gaining popularity.[31, 33] A synthetic intestinal microbiota suspension for use in FMT is currently being tested.[62] There also exists a nonprofit organization, OpenBiome (
CONCLUSIONS
Based on several prospective trials and observational data, FMT appears to be a safe and effective treatment for recurrent CDI that is superior to conventional approaches. Despite recent pivotal advances in the field of FMT, there remain many unanswered questions, and further research is needed to examine the optimal parameters, indications, and outcomes with FMT.
Disclosures
K.R. is supported by grants from the Claude D. Pepper Older Americans Independence Center (grant number AG‐024824) and the Michigan Institute for Clinical and Health Research (grant number 2UL1TR000433). N.S. is supported by a VA MERIT award. The contents of this article do not necessarily represent the views of the Department of Veterans Affairs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors report no conflicts of interest.
- , , . Emergence of Clostridium difficile‐associated disease in North America and Europe. Clin Microbiol Infect. 2006;12:2–18.
- , , , , . Antibiotic‐associated pseudomembranous colitis due to toxin‐producing clostridia. N Engl J Med. 1978;298(10):531–534.
- , , ,, et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30(6):526–533.
- , , , , . Attributable burden of hospital‐onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol. 2013;34(6):588–596.
- Centers for Disease Control and Prevention. Vital Signs. Making health care safer. Stopping C. difficile infections. Available at: http://www.cdc.gov/VitalSigns/Hai/StoppingCdifficile. Accessed January 15, 2015.
- , , , et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–834.
- , , , et al. Emergence and global spread of epidemic healthcare‐associated Clostridium difficile. Nat Genet. 2013;45(1):109–113.
- , , , et al. Effect of age on treatment outcomes in Clostridium difficile infection. J Am Geriatr Soc. 2013;61(2):222–230.
- , , . Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(suppl 2):S65–S70.
- , , , . Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One. 2014;9(6):e98400.
- , , , et al. Health care‐associated infections: a meta‐analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039–2046.
- , , , et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227.
- , , . Colonization resistance of the digestive tract in conventional and antibiotic‐treated mice. Epidemiol Infect. 1971;69(03):405–411.
- , . Colonization resistance. Antimicrob Agents Chemother. 1994;38(3):409.
- , . Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterol. 2014;146(6):1547–1553.
- , , , et al. Antibiotic‐induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114.
- . Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe. 2009;15(6):285–289.
- , . Treatment of recurrent Clostridium difficile diarrhea. Gastroenterol Hepatol. 2006;2(3):203–208.
- , , , , , . Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38(6):475–483.
- , , , et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–455.
- , , , et al. Fidaxomicin Versus Vancomycin for Clostridium difficile Infection: meta‐analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(suppl 2):S93–S103.
- , , , et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile‐associated diarrhea. J Infect Dis. 2008;197(3):435–438.
- , , . Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53(10):994–1002.
- , , , . Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251):189–193.
- , , , , . Treatment approaches including fecal microbiota transplantation for recurrent Clostridium difficile infection (RCDI) among infectious disease physicians. Anaerobe. 2013;24:20–24.
- , , , , . Should we standardize the 1,700‐year‐old fecal microbiota transplantation? Am J Gastroenterol. 2012;107(11):1755.
- , , , . Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–859.
- , , , et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–1049.
- , , , . Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta‐analysis. Am J Gastroenterol. 2013;108(4):500–508.
- , , . Success of self‐administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8(5):471–473.
- , , , , , . Oral, Capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772–1778.
- , , , et al. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39(10):1003–1032.
- , , , . Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile Infection. Am J Gastroenterol. 2012;107(5):761–767.
- , , , . Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med. 2012;172(2):191–193.
- , , , et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–1071.
- , , , et al. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2014;12(9):1572–1576.
- , , , . Fecal microbiota transplantation for refractory Clostridium difficile colitis in solid organ transplant recipients. Am J Transplant. 2014;14(2):477–480.
- , , , , . Faecal microbiota transplantation and bacteriotherapy for recurrent Clostridium difficile infection: a retrospective evaluation of 31 patients. Scand J Infect Dis. 2014;46(2):89–97.
- , , , et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415.
- , , , et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open‐label, controlled pilot study. Clin Infect Dis. 2014;58(11):1515–1522.
- , , , et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41(9):835–843.
- , , , , . A mathematical model to evaluate the routine use of fecal microbiota transplantation to prevent incident and recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;35(1):18–27.
- , , . Commentary: fecal microbiota therapy: ready for prime time? Infect Control Hosp Epidemiol. 2014;35(1):28–30.
- , , , et al. Dramatic reduction in Clostridium difficile ribotype 027‐associated mortality with early fecal transplantation by the nasogastric route: a preliminary report. Eur J Clin Microbiol Infect Dis. 2015;34(8):1597–1601.
- , , , , , . Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem cell transplant patient. Transplant Infect Dis. 2012;14(6):E161–E165.
- , , , , , . Faecal microbiota transplantation for severe Clostridium difficile infection in the intensive care unit. Eur J Gastroenterol Hepatol. 2013;25(2):255–257.
- , , , . Successful colonoscopic fecal transplant for severe acute Clostridium difficile pseudomembranous colitis. Rev Gastroenterol Mex. 2011;77(1):40–42.
- , , . Successful treatment of fulminant Clostridium difficile infection with fecal bacteriotherapy. Ann Intern Med. 2008;148(8):632–633.
- , , , . Tempered enthusiasm for fecal transplant. Clin Infect Dis. 2014;59(2):319.
- , , , , . Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis. 2012;55(12):1652–1658.
- , , , , . Physician attitudes toward the use of fecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection. Can J Gastroenterol Hepatol. 2014;28(6):319–324.
- , , . Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11(8):1036–1038.
- , , , et al. Temporal Bacterial Community Dynamics Vary Among Ulcerative Colitis Patients After Fecal Microbiota Transplantation. Am J Gastroenterol. 2013;108(10):1620–1630.
- , , , et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19(10):2155–2165.
- , , , et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterol. 2015;149(1):110–118.e4.
- , , , . Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904.
- , , , et al. Long‐term follow‐up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–1087.
- US Food and Drug Administration. Guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. Available at: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm361379.htm. Accessed July 1, 2014.
- US Food and Drug Administration. Draft guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. Available at: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm387023.htm. Accessed July 1, 2014.
- University Health Network Toronto. Oral vancomycin followed by fecal transplant versus tapering oral vancomycin. Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01226992. Available at: http://clinicaltrials.gov/ct2/show/NCT01226992. Accessed July 1, 2014.
- Tel‐Aviv Sourasky Medical Center. Transplantation of fecal microbiota for Clostridium difficile infection. Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01958463. Available at: http://clinicaltrials.gov/ct2/show/NCT01958463. Accessed July 1, 2014.
- Rebiotix Inc. Microbiota restoration therapy for recurrent Clostridium difficile‐associated diarrhea (PUNCH CD). Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01925417. Available at: http://clinicaltrials.gov/ct2/show/NCT01925417. Accessed July 1, 2014.
- Hadassah Medical Organization. Efficacy and safety of fecal microbiota transplantation for severe Clostridium difficile‐associated colitis. Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01959048. Available at: http://clinicaltrials.gov/ct2/show/NCT01959048. Accessed July 1, 2014.
- University Hospital Tuebingen. Fecal microbiota transplantation in recurrent or refractory Clostridium difficile colitis (TOCSIN). Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01942447. Available at: http://clinicaltrials.gov/ct2/show/NCT01942447. Accessed July 1, 2014.
- Duke University. Stool transplants to treat refractory Clostridium difficile colitis. Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT02127398. Available at: http://clinicaltrials.gov/ct2/show/NCT02127398. Accessed July 1, 2014.
- , , . Emergence of Clostridium difficile‐associated disease in North America and Europe. Clin Microbiol Infect. 2006;12:2–18.
- , , , , . Antibiotic‐associated pseudomembranous colitis due to toxin‐producing clostridia. N Engl J Med. 1978;298(10):531–534.
- , , ,, et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30(6):526–533.
- , , , , . Attributable burden of hospital‐onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol. 2013;34(6):588–596.
- Centers for Disease Control and Prevention. Vital Signs. Making health care safer. Stopping C. difficile infections. Available at: http://www.cdc.gov/VitalSigns/Hai/StoppingCdifficile. Accessed January 15, 2015.
- , , , et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–834.
- , , , et al. Emergence and global spread of epidemic healthcare‐associated Clostridium difficile. Nat Genet. 2013;45(1):109–113.
- , , , et al. Effect of age on treatment outcomes in Clostridium difficile infection. J Am Geriatr Soc. 2013;61(2):222–230.
- , , . Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(suppl 2):S65–S70.
- , , , . Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One. 2014;9(6):e98400.
- , , , et al. Health care‐associated infections: a meta‐analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039–2046.
- , , , et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227.
- , , . Colonization resistance of the digestive tract in conventional and antibiotic‐treated mice. Epidemiol Infect. 1971;69(03):405–411.
- , . Colonization resistance. Antimicrob Agents Chemother. 1994;38(3):409.
- , . Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterol. 2014;146(6):1547–1553.
- , , , et al. Antibiotic‐induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114.
- . Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe. 2009;15(6):285–289.
- , . Treatment of recurrent Clostridium difficile diarrhea. Gastroenterol Hepatol. 2006;2(3):203–208.
- , , , , , . Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38(6):475–483.
- , , , et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–455.
- , , , et al. Fidaxomicin Versus Vancomycin for Clostridium difficile Infection: meta‐analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(suppl 2):S93–S103.
- , , , et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile‐associated diarrhea. J Infect Dis. 2008;197(3):435–438.
- , , . Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53(10):994–1002.
- , , , . Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251):189–193.
- , , , , . Treatment approaches including fecal microbiota transplantation for recurrent Clostridium difficile infection (RCDI) among infectious disease physicians. Anaerobe. 2013;24:20–24.
- , , , , . Should we standardize the 1,700‐year‐old fecal microbiota transplantation? Am J Gastroenterol. 2012;107(11):1755.
- , , , . Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–859.
- , , , et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–1049.
- , , , . Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta‐analysis. Am J Gastroenterol. 2013;108(4):500–508.
- , , . Success of self‐administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8(5):471–473.
- , , , , , . Oral, Capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772–1778.
- , , , et al. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39(10):1003–1032.
- , , , . Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile Infection. Am J Gastroenterol. 2012;107(5):761–767.
- , , , . Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med. 2012;172(2):191–193.
- , , , et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–1071.
- , , , et al. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2014;12(9):1572–1576.
- , , , . Fecal microbiota transplantation for refractory Clostridium difficile colitis in solid organ transplant recipients. Am J Transplant. 2014;14(2):477–480.
- , , , , . Faecal microbiota transplantation and bacteriotherapy for recurrent Clostridium difficile infection: a retrospective evaluation of 31 patients. Scand J Infect Dis. 2014;46(2):89–97.
- , , , et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415.
- , , , et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open‐label, controlled pilot study. Clin Infect Dis. 2014;58(11):1515–1522.
- , , , et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41(9):835–843.
- , , , , . A mathematical model to evaluate the routine use of fecal microbiota transplantation to prevent incident and recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;35(1):18–27.
- , , . Commentary: fecal microbiota therapy: ready for prime time? Infect Control Hosp Epidemiol. 2014;35(1):28–30.
- , , , et al. Dramatic reduction in Clostridium difficile ribotype 027‐associated mortality with early fecal transplantation by the nasogastric route: a preliminary report. Eur J Clin Microbiol Infect Dis. 2015;34(8):1597–1601.
- , , , , , . Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem cell transplant patient. Transplant Infect Dis. 2012;14(6):E161–E165.
- , , , , , . Faecal microbiota transplantation for severe Clostridium difficile infection in the intensive care unit. Eur J Gastroenterol Hepatol. 2013;25(2):255–257.
- , , , . Successful colonoscopic fecal transplant for severe acute Clostridium difficile pseudomembranous colitis. Rev Gastroenterol Mex. 2011;77(1):40–42.
- , , . Successful treatment of fulminant Clostridium difficile infection with fecal bacteriotherapy. Ann Intern Med. 2008;148(8):632–633.
- , , , . Tempered enthusiasm for fecal transplant. Clin Infect Dis. 2014;59(2):319.
- , , , , . Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis. 2012;55(12):1652–1658.
- , , , , . Physician attitudes toward the use of fecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection. Can J Gastroenterol Hepatol. 2014;28(6):319–324.
- , , . Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11(8):1036–1038.
- , , , et al. Temporal Bacterial Community Dynamics Vary Among Ulcerative Colitis Patients After Fecal Microbiota Transplantation. Am J Gastroenterol. 2013;108(10):1620–1630.
- , , , et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19(10):2155–2165.
- , , , et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterol. 2015;149(1):110–118.e4.
- , , , . Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904.
- , , , et al. Long‐term follow‐up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–1087.
- US Food and Drug Administration. Guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. Available at: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm361379.htm. Accessed July 1, 2014.
- US Food and Drug Administration. Draft guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. Available at: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm387023.htm. Accessed July 1, 2014.
- University Health Network Toronto. Oral vancomycin followed by fecal transplant versus tapering oral vancomycin. Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01226992. Available at: http://clinicaltrials.gov/ct2/show/NCT01226992. Accessed July 1, 2014.
- Tel‐Aviv Sourasky Medical Center. Transplantation of fecal microbiota for Clostridium difficile infection. Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01958463. Available at: http://clinicaltrials.gov/ct2/show/NCT01958463. Accessed July 1, 2014.
- Rebiotix Inc. Microbiota restoration therapy for recurrent Clostridium difficile‐associated diarrhea (PUNCH CD). Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01925417. Available at: http://clinicaltrials.gov/ct2/show/NCT01925417. Accessed July 1, 2014.
- Hadassah Medical Organization. Efficacy and safety of fecal microbiota transplantation for severe Clostridium difficile‐associated colitis. Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01959048. Available at: http://clinicaltrials.gov/ct2/show/NCT01959048. Accessed July 1, 2014.
- University Hospital Tuebingen. Fecal microbiota transplantation in recurrent or refractory Clostridium difficile colitis (TOCSIN). Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT01942447. Available at: http://clinicaltrials.gov/ct2/show/NCT01942447. Accessed July 1, 2014.
- Duke University. Stool transplants to treat refractory Clostridium difficile colitis. Bethesda, MD: National Library of Medicine; 2000. NLM identifier: NCT02127398. Available at: http://clinicaltrials.gov/ct2/show/NCT02127398. Accessed July 1, 2014.
Bedside Swallow Examination Review
Dysphagia is a serious medical condition that can lead to aspiration pneumonia, malnutrition, and dehydration.[1] Dysphagia is the result of a variety of medical etiologies, including stroke, traumatic brain injury, progressive neurologic conditions, head and neck cancers, and general deconditioning. Prevalence estimates for dysphagia vary depending upon the etiology and patient age, but estimates as high as 38% for lifetime prevalence have been reported in those over age 65 years.[2]
To avoid adverse health outcomes, early detection of dysphagia is essential. In hospitalized patients, early detection has been associated with reduced risk of pneumonia, decreased length of hospital stay, and improved cost‐effectiveness resulting from a reduction in hospital days due to fewer cases of aspiration pneumonia.[3, 4, 5] Stroke guidelines in the United States recommend screening for dysphagia for all patients admitted with stroke.[6] Consequently, the majority of screening procedures have been designed for and tested in this population.[7, 8, 9, 10]
The videofluoroscopic swallow study (VFSS) is a commonly accepted, reference standard, instrumental evaluation technique for dysphagia, as it provides the most comprehensive information regarding anatomic and physiologic function for swallowing diagnosis and treatment. Flexible endoscopic evaluation of swallowing (FEES) is also available, as are several less commonly used techniques (scintigraphy, manometry, and ultrasound). Due to availability, patient compliance, and expertise needed, it is not possible to perform instrumental examination on every patient with suspected dysphagia. Therefore, a number of minimally invasive bedside screening procedures for dysphagia have been developed.
The value of any diagnostic screening test centers on performance characteristics, which under ideal circumstances include a positive result for all those who have dysphagia (sensitivity) and negative result for all those who do not have dysphagia (specificity). Such an ideal screening procedure would reduce unnecessary referrals and testing, thus resulting in cost savings, more effective utilization of speech‐language pathology consultation services, and less unnecessary radiation exposure. In addition, an effective screen would detect all those at risk for aspiration pneumonia in need of intervention. However, most available bedside screening tools are lacking in some or all of these desirable attributes.[11, 12] We undertook a systematic review and meta‐analysis of bedside procedures to screen for dysphagia.
METHODS
Data Sources and Searches
We conducted a comprehensive search of 7 databases, including MEDLINE, Embase, and Scopus, from each database's earliest inception through June 9, 2014 for English‐language articles and abstracts. The search strategy was designed and conducted by an experienced librarian with input from 1 researcher (J.C.O.). Controlled vocabulary supplemented with keywords was used to search for comparative studies of bedside screening tests for predicting dysphagia (see Supporting Information, Appendix 1, in the online version of this article for the full strategy).
All abstracts were screened, and potentially relevant articles were identified for full‐text review. Those references were manually inspected to identify all relevant studies.
Study Selection
A study was eligible for inclusion if it tested a diagnostic swallow study of any variety against an acceptable reference standard (VFSS or flexible endoscopic evaluation of swallowing with sensory testing [FEEST]).
Data Extraction and Quality Assessment
The primary outcome of the study was aspiration, as predicted by a bedside exam, compared to gold‐standard visualization of aspirated material entering below the vocal cords. From each study, data were abstracted based on the type of diagnostic method and reference standard study population and inclusion/exclusion characteristics, design, and prediction of aspiration. Prediction of aspiration was compared against the reference standard to yield true positives, true negatives, false positives, and false negatives. Additional potential confounding variables were abstracted using a standard form based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis[13] (see Supporting Information, Appendix 2, in the online version of this article for the full abstraction template).
Data Synthesis and Analysis
Sensitivity and specificity for each test that identified the presence of dysphagia was calculated for each study. These were used to generate positive and negative likelihood ratios (LRs), which were plotted on a likelihood matrix, a graphic depiction of the logarithm of the +LR on the ordinate versus the logarithm of the LR on the abscissa, dividing the graphic into quadrants such that the right upper quadrant is tests that can be used for confirmation, right lower quadrant neither confirmation nor exclusion, left lower quadrant exclusion only, and left upper quadrant an ideal test with both exclusionary and confirmatory properties.[14] A good screening test would thus be on the left half of the graphic to effectively rule out dysphagia, and the ideal test with both good sensitivity and specificity would be found in the left upper quadrant. Graphics were constructed using the Stata MIDAS package (Stata Corp., College Station, TX).[15]
RESULTS
We identified 891 distinct articles. Of these, 749 were excluded based on abstract review. After reviewing the remaining 142 full‐text articles, 48 articles were determined to meet inclusion criteria, which included 10,437 observations across 7414 patients (Figure 1). We initially intended to conduct a meta‐analysis on each type, but heterogeneity in design and statistical heterogeneity in aggregate measures precluded pooling of results.
Characteristics of Included Studies
Of the 48 included studies, the majority (n=42) were prospective observational studies,[7, 8, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53] whereas 2 were randomized trials,[9, 54] 2 studies were double‐blind observational,[9, 16] 1 was a case‐control design,[55] and 1 was a retrospective case series.[56] The majority of studies were exclusively inpatient,[7, 8, 9, 14, 17, 18, 19, 21, 22, 24, 25, 26, 31, 32, 33, 35, 36, 38, 41, 43, 44, 45, 46, 47, 49, 51, 52, 53, 55, 57] with 5 in mixed in and outpatient populations,[20, 27, 40, 55, 58] 2 in outpatient populations,[23, 41] and the remainder not reporting the setting from which they drew their study populations.
The indications for swallow evaluations fit broadly into 4 categories: stroke,[7, 8, 9, 14, 21, 22, 24, 25, 26, 31, 33, 34, 35, 38, 40, 41, 42, 43, 45, 48, 52, 56, 58] other neurologic disorders,[17, 18, 23, 28, 39, 47] all causes,[16, 20, 27, 29, 30, 36, 37, 44, 46, 49, 51, 52, 53, 54, 58] and postsurgical.[19, 32, 34] Most used VFSS as a reference standard,[7, 8, 9, 14, 16, 17, 18, 19, 21, 22, 23, 25, 26, 27, 28, 29, 30, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 50, 51, 52, 53, 54, 56, 57, 58] with 8 using FEEST,[20, 24, 31, 32, 33, 35, 49, 55] and 1 accepting either videofluoroscopic evaluation of swallow or FEEST.[48]
Studies were placed into 1 or more of the following 4 categories: subjective bedside examination,[8, 9, 18, 19, 31, 34, 48] questionnaire‐based tools,[17, 23, 46, 53] protocolized multi‐item evaluations,[20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] and single‐item exam maneuvers, symptoms, or signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 56, 58, 59] The characteristics of all studies are detailed in Table 1.
| Study | Location | Design | Mean Age (SD) | Reason(s) for Dysphagia | Indx Test | Description | Reference Standard | Sample Size, No. of Patients | Sample Size, No. of Observations |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Splaingard et al., 198844 | Milwaukee, WI, USA | Prospective observational study | NR | Multiple | Clinical bedside swallow exam | Combination of scored comprehensive physical exam, history, and observed swallow. | VFSS | 107 | 107 |
| DePippo et al., 199243 | White Plains, NY, USA | Prospective observational study | 71 (10) | Stroke | WST | Observation of swallow. | VFSS | 44 | |
| Horner et al., 199356 | Durham, NC, USA | Retrospective case series | 64* | Stroke | Clinical bedside swallow evaluation | VFSS | 38 | 114 | |
| Kidd et al., 199342 | Belfast, UK | Prospective observational study | 72 (10) | Stroke | Bedside 50‐mL swallow evaluation | Patient swallows 50 mL of water in 5‐mL aliquots, with therapist assessing for choking, coughing, or change in vocal quality after each swallow. | VFSS | 60 | 240 |
| Collins and Bakheit, 199741 | Southampton, UK | Prospective observational study | 65* | Stroke | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 54 | 54 |
| Daniels et al., 199740 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside examination | 6 individual bedside assessments (dysphonia, dysphagia, cough before/after swallow, gag reflex and voice change) examined as predictors for aspiration risk. | VFSS | 59 | 354 |
| Mari et al., 199739 | Ancona, Italy | Prospective observational study | 60 (16) | Mixed neurologic diseases | Combined history and exam | Assessed symptoms of dysphagia, cough, and 3‐oz water swallow. | VFSS | 93 | 372 |
| Daniels et al., 19987 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 55 | 330 |
| Smithard et al., 19988 | Ashford, UK | Prospective observational study | 79* | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 83 | 249 |
| Addington et al., 199938 | Kansas City, MO, USA | Prospective observational study | 80* | Stroke | NR | Reflex cough. | VFSS | 40 | 40 |
| Logemann et al., 199937 | Evanston, IL, USA | Prospective observational study | 65 | Multiple | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 200 | 1400 |
| Smith et al., 20009 | Manchester, UK | Double blind observational | 69 | Stroke | Clinical bedside swallow evaluation, pulse oximetry evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. Also evaluated if patient desaturates by at least 2% during evaluation. | VFSS | 53 | 53 |
| Warms et al., 200036 | Melbourne, Australia | Prospective observational study | 67 | Multiple | Wet voice | Voice was recorded and analyzed with Sony digital audio tape during videofluoroscopy. | VFSS | 23 | 708 |
| Lim et al., 200135 | Singapore, Singapore | Prospective observational study | NR | Stroke | Water swallow test, desaturation during swallow | 50‐mL swallow done in 5‐mL aliquots with assessment of phonation/choking afterward; desaturation >2% during swallow, | FEEST | 50 | 100 |
| McCullough et al., 200134 | Nashville, TN, USA | Prospective observational study | 60 (10) | Stroke | Clinical bedside swallow evaluation | 15‐item physical exam with observed swallow. | VFSS | 2040 | 60 |
| Rosen et al., 2001[74] | Newark, NJ, USA | Prospective observational study | 60 | Head and Neck cancer | Wet voice | Observation of swallow. | VFSS | 26 | 26 |
| Leder and Espinosa, 200233 | New Haven, CT, USA | Prospective observational study | 70* | Stroke | Clinical exam | Checklist evaluation of cough and voice change after swallow, volitional cough, dysphonia, dysarthria, and abnormal gag. | FEEST | 49 | 49 |
| Belafsky et al., 200332 | San Francisco, CA, USA | Prospective observational study | 65 (11) | Post‐tracheostomy patients | Modified Evans Blue Dye Test | 3 boluses of dye‐impregnated ice are given to patient. Tracheal secretions are suctioned, and evaluated for the presence of dye. | FEES | 30 | 30 |
| Chong et al., 200331 | Jalan Tan Tock Seng, Singapore | Prospective observational study | 75 (7) | Stroke | Water swallow test, desaturation during, clinical exam | Subjective exam, drinking 50 mL of water in 10‐mL aliquots, and evaluating for desaturation >2% during FEES. | FEEST | 50 | 150 |
| Tohara et al., 200330 | Tokyo, Japan | Prospective observational study | 63 (17) | Multiple | Food/water swallow tests, and a combination of the 2 | Protocolized observation of sequential food and water swallows with scored outcomes. | VFSS | 63 | 63 |
| Rosenbek et al., 200414 | Gainesville, FL, USA | Prospective observational study | 68* | Stroke | Clinical bedside swallow evaluation | Describes 5 parameters of voice quality and 15 physical examination maneuvers used. | VFSS | 60 | 1200 |
| Ryu et al., 200429 | Seoul, South Korea | Prospective observational study | 64 (14) | Multiple | Voice analysis parameters | Analysis of the/a/vowel sound with Visi‐Pitch II 3300. | VFSS | 93 | 372 |
| Shaw et al., 200428 | Sheffield, UK | Prospective observational study | 71 | Neurologic disease | Bronchial auscultation | Auscultation over the right main bronchus during trial feeding to listen for sounds of aspiration. | VFSS | 105 | 105 |
| Wu et al., 200427 | Taipei, Taiwan | Prospective observational study | 72 (11) | Multiple | 100‐mL swallow test | Patient lifts a glass of 100 mL of water and drinks as quickly as possible, and is assessed for signs of choking, coughing, or wet voice, and is timed for speed of drinking. | VFSS | 54 | 54 |
| Nishiwaki et al., 200526 | Shizuoaka, Japan | Prospective observational study | 70* | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 31 | 248 |
| Wang et al., 200554 | Taipei, Taiwan | Prospective double‐blind study | 41* | Multiple | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 60 | 60 |
| Ramsey et al., 200625 | Kent, UK | Prospective observational study | 71 (10) | Stroke | BSA | Assessment of lip seal, tongue movement, voice quality, cough, and observed 5‐mL swallow. | VFSS | 54 | 54 |
| Trapl et al., 200724 | Krems, Austria | Prospective observational study | 76 (2) | Stroke | Gugging Swallow Screen | Progressive observed swallow trials with saliva, then with 350 mL liquid, then dry bread. | FEEST | 49 | 49 |
| Suiter and Leder, 200849 | Several centers across the USA | Prospective observational study | 68.3 | Multiple | 3‐oz water swallow test | Observation of swallow. | FEEST | 3000 | 3000 |
| Wagasugi et al., 200850 | Tokyo, Japan | Prospective observational study | NR | Multiple | Cough test | Acoustic analysis of cough. | VFSS | 204 | 204 |
| Baylow et al., 200945 | New York, NY, USA | Prospective observational study | NR | Stroke | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 15 | 30 |
| Cox et al., 200923 | Leiden, the Netherlands | Prospective observational study | 68 (8) | Inclusion body myositis | Dysphagia questionnaire | Questionnaire assessing symptoms of dysphagia. | VFSS | 57 | 57 |
| Kagaya et al., 201051 | Tokyo, Japan | Prospective observational study | NR | Multiple | Simple Swallow Provocation Test | Injection of 1‐2 mL of water through nasal tube directed at the suprapharynx. | VFSS | 46 | 46 |
| Martino et al., 200957 | Toronto, Canada | Randomized trial | 69 (14) | Stroke | Toronto Bedside Swallow Screening Test | 4‐item physical assessment including Kidd water swallow test, pharyngeal sensation, tongue movement, and dysphonia (before and after water swallow). | VFSS | 59 | 59 |
| Santamato et al., 200955 | Bari, Italy | Case control | NR | Multiple | Acoustic analysis, postswallow apnea | Acoustic analysis of cough. | VFSS | 15 | 15 |
| Smith Hammond et al., 200948 | Durham, NC, USA | Prospective observational study | 67.7 (1.2) | Multiple | Cough, expiratory phase peak flow | Acoustic analysis of cough. | VFSS or FEES | 96 | 288 |
| Leigh et al., 201022 | Seoul, South Korea | Prospective observational study | NR | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 167 | 167 |
| Pitts et al., 201047 | Gainesville, FL, USA | Prospective observational study | NR | Parkinson | Cough compression phase duration | Acoustic analysis of cough. | VFSS | 58 | 232 |
| Cohen and Manor, 201146 | Tel Aviv, Israel | Prospective observational Study | NR | Multiple | Swallow Disturbance Questionnaire | 15‐item questionnaire. | FEES | 100 | 100 |
| Edmiaston et al., 201121 | St. Louis, MO, USA | Prospective observational study | 63* | Stroke | SWALLOW‐3D Acute Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Mandysova et al., 201120 | Pardubice, Czech Republic | Prospective observational study | 69 (13) | Multiple | Brief Bedside Dysphagia Screening Test | 8‐item physician exam including ability to clench teeth; symmetry/strength of tongue, facial, and shoulder muscles; dysarthria; and choking, coughing, or dripping of food after taking thick liquid. | FEES | 87 | 87 |
| Steele et al., 201158 | Toronto, Canada | Double blind observational | 67 | Stroke | 4‐item bedside exam | Tongue lateralization, cough, throat clear, and voice quality. | VFSS | 400 | 40 |
| Yamamoto et al., 201117 | Kodaira, Japan | Prospective observational study | 67 (9) | Parkinson's Disease | Swallowing Disturbance Questionnaire | 15‐item questionnaire. | VFSS | 61 | 61 |
| Bhama et al., 201219 | Pittsburgh, PA, USA | Prospective observational study | 57 (14) | Post‐lung transplant | Clinical bedside swallow evaluation | Not described. | VFSS | 128 | 128 |
| Shem et al., 201218 | San Jose, CA, USA | Prospective observational study | 42 (17) | Spinal cord injuries resulting in tetraplegia | Clinical bedside swallow evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. | VFSS | 26 | 26 |
| Steele et al., 201316 | Toronto, Canada | Prospective observational study | 67 (14) | Multiple | Dual‐axis accelerometry | Computed accelerometry of swallow. | VFSS | 37 | 37 |
| Edmiaston et al., 201452 | St. Louis, MO, USA | Prospective observational study | 63 (15) | Stroke | Barnes Jewish Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Rofes et al., 201453 | Barcelona, Spain | Prospective observational study | 74 (12) | Mixed | EAT‐10 questionnaire and variable viscosity swallow test | Symptom‐based questionnaire (EAT‐10) and repeated observations and measurements of swallow with different thickness liquids. | VFS | 134 | 134 |
Subjective Clinical Exam
Seven studies reported the sensitivity and specificity of subjective assessments of nurses and speech‐language pathologists in observing swallowing and predicting aspiration.[8, 9, 18, 19, 31, 34, 48] The overall distribution of studies is summarized in the likelihood matrix in Figure 2. Two studies, Chong et al.[31] and Shem et al.,[18] were on the left side of the matrix, indicating a sensitive rule‐out test. However, both were small studies, and only Chong et al. reported reasonable sensitivity with incorporation bias from knowledge of a desaturation study outcome. Overall, subjective exams did not appear reliable in ruling out dysphagia.
Questionnaire‐Based Tools
Only 4 studies used questionnaire‐based tools filled out by the patient, asking about subjective assessment of dysphagia symptoms and frequency.[17, 23, 46, 53] Yamamoto et al. reported results of using the swallow dysphagia questionnaire in patients with Parkinson's disease.[17] Rofes et al. looked at the Eating Assessment Tool (EAT‐10) questionnaire among all referred patients and a small population of healthy volunteers.[53] Each was administered the questionnaire before undergoing a videofluoroscopic study. Overall, sensitivity and specificity were 77.8% and 84.6%, respectively. Cox et al. studied a different questionnaire in a group of patients with inclusion body myositis, finding 70% sensitivity and 44% specificity.[23] Cohen and Manor examined the swallow dysphagia questionnaire across several different causes of dysphagia, finding at optimum, the test is 78% specific and 73% sensitive.[46] Rofes et al. had an 86% sensitivity and 68% specificity for the EAT‐10 tool.[53]
Multi‐Item Exam Protocols
Sixteen studies reported multistep protocols for determining a patient's risk for aspiration.[9, 20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] Each involved a combination of physical exam maneuvers and history elements, detailed in Table 1. This is shown in the likelihood matrix in Figure 3. Only 2 of these studies were in the left lower quadrant, Edmiaston et al. 201121 and 2014.[52] Both studies were restricted to stroke populations, but found reasonable sensitivity and specificity in identifying dysphagia.
Individual Exam Maneuvers
Thirty studies reported the diagnostic performance of individual exam maneuvers and signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 54, 56, 58] Each is depicted in Figure 4 as a likelihood matrix demonstrating the +LR and LR for individual maneuvers as seen in the figure; most fall into the right lower quadrant, where they are not diagnostically useful tests. Studies in the left lower quadrant demonstrating the ability to exclude aspiration desirable in a screening test were dysphonia in McCullough et al.,[34] dual‐axis accelerometry in Steele et al.,[16] and the water swallow test in DePippo et al.[43] and Suiter and Leder.[49]
McCullough et al. found dysphonia to be the most discriminatory sign or symptom assessed, with an area under the curve (AUC) of 0.818. Dysphonia was judged by a sustained/a/and had 100% sensitivity but only 27% specificity. Wet voice within the same study was slightly less informative, with an AUC of 0.77 (sensitivity 50% and specificity 84%).[34]
Kidd et al. verified the diagnosis of stroke, and then assessed several neurologic parameters, including speech, muscle strength, and sensation. Pharyngeal sensation was assessed by touching each side of the pharyngeal wall and asking patients if they felt sensation that differed from each side. Patient report of abnormal sensation during this maneuver was 80% sensitive and 86% specific as a predictor of aspiration on VFSS.[42]
Steele et al. described the technique of dual axis accelerometry, where an accelerometer was placed at the midline of the neck over the cricoid cartilage during VFSS. The movement of the cricoid cartilage was captured for analysis in a computer algorithm to identify abnormal pharyngeal swallow behavior. Sensitivity was 100%, and specificity was 54%. Although the study was small (n=40), this novel method demonstrated good discrimination.[58]
DePippo et al. evaluated a 3‐oz water swallow in stroke patients. This protocol called for patients to drink the bolus of water without interruption, and be observed for 1 minute after for cough or wet‐hoarse voice. Presence of either sign was considered abnormal. Overall, sensitivity was 94% and specificity 30% looking for the presence of either sign.[43] Suiter and Leder used a similar protocol, with sensitivity of 97% and specificity of 49%.[49]
DISCUSSION
Our results show that most bedside swallow examinations lack the sensitivity to be used as a screening test for dysphagia across all patient populations examined. This is unfortunate as the ability to determine which patients require formal speech language pathology consultation or imaging as part of their diagnostic evaluation early in the hospital stay would lead to improved allocation of resources, cost reductions, and earlier implementation of effective therapy approaches. Furthermore, although radiation doses received during VFSS are not high when compared with other radiologic exams like computed tomography scans,[60] increasing awareness about the long‐term malignancy risks associated with medical imaging makes it desirable to reduce any test involving ionizing radiation.
There were several categories of screening procedures identified during this review process. Those classified as subjective bedside exams and protocolized multi‐item evaluations were found to have high heterogeneity in their sensitivity and specificity, though a few exam protocols did have a reasonable sensitivity and specificity.[21, 31, 52] The following individual exam maneuvers were found to demonstrate high sensitivity and an ability to exclude aspiration: a test for dysphonia through production of a sustained/a/34 and use of dual‐axis accelerometry.[16] Two other tests, the 3‐oz water swallow test[43] and testing of abnormal pharyngeal sensation,[42] were each found effective in a single study, with conflicting results from other studies.
Our results extend the findings from previous systematic reviews on this subject, most of which focused only on stroke patients.[5, 12, 61, 62] Martino and colleagues[5] conducted a review focused on screening for adults poststroke. From 13 identified articles, it was concluded that evidence to support inclusion or exclusion of screening was poor. Daniels et al. conducted a systematic review of swallowing screening tools specific to patients with acute or chronic stroke.[12] Based on 16 articles, the authors concluded that a combination of swallowing and nonswallowing features may be necessary for development of a valid screening tool. The generalizability of these reviews is limited given that all were conducted in patients poststroke, and therefore results and recommendations may not be generalizable to other patients.
Wilkinson et al.[62] conducted a recent systematic review that focused on screening techniques for inpatients 65 years or older that excluded patients with stroke or Parkinson's disease. The purpose of that review was to examine sensitivity and specificity of bedside screening tests as well as ability to accurately predict pneumonia. The authors concluded that existing evidence is not sufficient to recommend the use of bedside tests in a general older population.[62]
Specific screening tools identified by Martino and colleagues[5] to have good predictive value in detecting aspiration as a diagnostic marker of dysphagia were an abnormal test of pharyngeal sensation[42] and the 50‐mL water swallow test. Daniels et al. identified a water swallow test as an important component of a screen.[7] These results were consistent with those of this review in that the abnormal test of pharyngeal sensation[42] was identified for high levels of sensitivity. However, the 3‐oz water swallow test,[43, 49] rather than the 50‐mL water swallow test,[42] was identified in this review as the version of the water swallow test with the best predictive value in ruling out aspiration. Results of our review identified 2 additional individual items, dual‐axis accelerometry[16] and dysphonia,[34] that may be important to include in a comprehensive screening tool. In the absence of better tools, the 3 oz swallow test, properly executed, seems to be the best currently available tool validated in more than 1 study.
Several studies in the current review included an assessment of oral tongue movement that is not described thoroughly and varies between studies. Tongue movement as an individual item on a screening protocol was not found to yield high sensitivity or specificity. However, tongue movement or range of motion is only 1 aspect of oral tongue function; pressures produced by the tongue reflecting strength also may be important and warrant evaluation. Multiple studies have shown patients with dysphagia resulting from a variety of etiologies to produce lower than normal maximum isometric lingual pressures,[63, 64, 65, 66, 67, 68] or pressures produced when the tongue is pushed as hard as possible against the hard palate. Tongue strengthening protocols that result in higher maximum isometric lingual pressures have been shown to carry over to positive changes in swallow function.[69, 70, 71, 72, 73] Inclusion of tongue pressure measurement in a comprehensive screening tool may help to improve predictive capabilities.
We believe our results have implications for practicing clinicians, and serve as a call to action for development of an easy‐to‐perform, accurate tool for dysphagia screening. Future prospective studies should focus on practical tools that can be deployed at the bedside, and correlate the results with not only gold‐standard VFSS and FEES, but with clinical outcomes such as pneumonia and aspiration events leading to prolonged length of stay.
There were several limitations to this review. High levels of heterogeneity were reported in the screening tests present in the literature, precluding meaningful meta‐analysis. In addition, the majority of studies included were in poststroke adults, which limits the generalizability of results.
In conclusion, no screening protocol has been shown to provide adequate predictive value for presence of aspiration. Several individual exam maneuvers demonstrate high sensitivity; however, the most effective combination of screening protocol components is unknown. There is a need for future research focused on the development of a comprehensive screening tool that can be applied across patient populations for accurate detection of dysphagia as well as prediction of other adverse health outcomes, including pneumonia.
Acknowledgements
The authors thank Drs. Byun‐Mo Oh and Catrionia Steele for providing additional information in response to requests for unpublished information.
Disclosures: Nasia Safdar MD, is supported by a National Institutes of Health R03 GEMSSTAR award and a VA MERIT award. The authors report no conflicts of interest.
- , , , et al. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser. 2012;72:57–66.
- , , , Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–865.
- , , Formal dysphagia screening protocols prevent pneumonia. Stroke. 2006;37(3):765.
- , , Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995;76(12):1130–1133.
- , , Screening for oropharyngeal dysphagia in stroke: insufficient evidence for guidelines. Dysphagia. 2000;15(1):19–30.
- , , , et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(3):870–947.
- , , , , , Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79(1):14–19.
- , , , et al. Can bedside assessment reliably exclude aspiration following acute stroke? Age Ageing. 1998;27(2):99–106.
- , , , The combination of bedside swallowing assessment and oxygen saturation monitoring of swallowing in acute stroke: a safe and humane screening tool. Age Ageing. 2000;29(6):495–499.
- , , , Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010;19(4):357–364.
- , Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001;16(1):7–18.
- , , Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke. 2012;43(3):892–897.
- , , , , Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–269, W64.
- , , Is the information about a test important? Applying the methods of evidence‐based medicine to the clinical examination of swallowing. J Commun Disord. 2004;37(5):437–450.
- MIDAS: Stata module for meta‐analytical integration of diagnostic test accuracy studies. Statistical Software Components. S456880, Boston College Department of Economics, 2009.
- , , Noninvasive detection of thin‐liquid aspiration using dual‐axis swallowing accelerometry. Dysphagia. 2013;28(1):105–112.
- , , , , Validation of the Japanese translation of the Swallowing Disturbance Questionnaire in Parkinson's disease patients. Qual Life Res. 2012;21(7):1299–1303.
- , , , , , Diagnostic accuracy of bedside swallow evaluation versus videofluoroscopy to assess dysphagia in individuals with tetraplegia. PM R. 2012;4(4):283–289.
- , et al. 723 Aspiration after Lung Transplantation: Incidence, Risk Factors, and Accuracy of the Bedside Swallow Evaluation. The J Heart Lung Transplant. 2012;31(4 suppl 1):S247–S248.
- , , , Cerny M. Development of the brief bedside dysphagia screening test in the Czech Republic. Nurs Health Sci. 2011;13(4):388–395.
- , , SWALLOW‐3D, a simple 2‐minute bedside screening test, detects dysphagia in acute stroke patients with high sensitivity when validated against video‐fluoroscopy. Stroke. 2011;42(3):e352.
- , , , , Bedside screening and subacute reassessment of post‐stroke dysphagia: a prospective study. Int J Stroke. 2010;5:200.
- , , , , , Detecting dysphagia in inclusion body myositis. J Neurol. 2009;256(12):2009–2013.
- , , , et al. Dysphagia bedside screening for acute‐stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38(11):2948–2952.
- , , Can pulse oximetry or a bedside swallowing assessment be used to detect aspiration after stroke? Stroke. 2006;37(12):2984–2988.
- , , , , , Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variables. J Rehabil Med. 2005;37(4):247–251.
- , , , Evaluating swallowing dysfunction using a 100‐ml water swallowing test. Dysphagia. 2004;19(1):43–47.
- , , , et al. Bronchial auscultation: an effective adjunct to speech and language therapy bedside assessment when detecting dysphagia and aspiration? Dysphagia. 2004;19(4):211–218.
- , , Prediction of laryngeal aspiration using voice analysis. Am J Phys Med Rehabil. 2004;83(10):753–757.
- , , , , Three tests for predicting aspiration without videofluorography. Dysphagia. 2003;18(2):126–134.
- , , , , Bedside clinical methods useful as screening test for aspiration in elderly patients with recent and previous strokes. Ann Acad Med Singapore. 2003;32(6):790–794.
- , , , The accuracy of the modified Evan's blue dye test in predicting aspiration. Laryngoscope. 2003;113(11):1969–1972.
- , Aspiration risk after acute stroke: comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17(3):214–218.
- , , Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord. 2001;34(1‐2):55–72.
- , , , et al. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16(1):1–6.
- , “Wet Voice” as a predictor of penetration and aspiration in oropharyngeal dysphagia. Dysphagia. 2000;15(2):84–88.
- , , A screening procedure for oropharyngeal dysphagia. Dysphagia. 1999;14(1):44–51.
- , , , Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil. 1999;80(2):150–154.
- , , , , , Predictive value of clinical indices in detecting aspiration in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 1997;63(4):456–460.
- , , , Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol. 1997;6(4):17–24.
- , Does pulse oximetry reliably detect aspiration in dysphagic stroke patients? Stroke. 1997;28(9):1773–1775.
- , , , Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med. 1993;86(12):825–829.
- , , Validation of the 3‐oz water swallow test for aspiration following stroke. Arch Neurol. 1992;49(12):1259–1261.
- , , , Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil. 1988;69(8):637–640.
- , , , Accuracy of clinical judgment of the chin‐down posture for dysphagia during the clinical/bedside assessment as corroborated by videofluoroscopy in adults with acute stroke. Dysphagia. 2009;24(4):423–433.
- , Swallowing disturbance questionnaire for detecting dysphagia. Laryngoscope. 2011;121(7):1383–1387.
- , , , , , . Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–1431.
- , , , et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777.
- , Clinical utility of the 3‐ounce water swallow test. Dysphagia. 2008;23(3):244–250.
- , , , et al. Screening test for silent aspiration at the bedside. Dysphagia. 2008;23(4):364–370.
- , , , , , Simple swallowing provocation test has limited applicability as a screening tool for detecting aspiration, silent aspiration, or penetration. Dysphagia. 2010;25(1):6–10.
- , , , A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. J Stroke Cerebrovasc Dis. 2014;23 (4):712–716.
- , , , Sensitivity and specificity of the Eating Assessment Tool and the Volume‐Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–1265.
- , , , Pulse oximetry does not reliably detect aspiration on videofluoroscopic swallowing study. Arch Phys Med Rehabil. 2005;86(4):730–734.
- , , , et al. Acoustic analysis of swallowing sounds: a new technique for assessing dysphagia. J Rehabil Med. 2009;41(8):639–645.
- , , Aspiration in bilateral stroke patients: a validation study. Neurology. 1993;43(2):430–433.
- , , , et al. The Toronto Bedside Swallowing Screening Test (TOR‐BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40(2):555–561.
- , , , et al. Exploration of the utility of a brief swallow screening protocol with comparison to concurrent videofluoroscopy. Can J Speech Lang Pathol Audiol. 2011;35(3):228–242.
- , , , et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–1976.
- , , , et al. Radiation exposure time during MBSS: influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia. 2013;28(1):77–85.
- Detection of eating difficulties after stroke: a systematic review. Int Nurs Rev. 2006;53(2):143–149.
- , , Aspiration in older patients without stroke: A systematic review of bedside diagnostic tests and predictors of pneumonia. Eur Geriatr Med. 2012;3(3):145–152.
- , , A tongue force measurement system for the assessment of oral‐phase swallowing disorders. Arch Phys Med Rehabil. 1991;72(1):38–42.
- , , Strength, Endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res. 2000;43(1):256–267.
- , , , et al. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007;29(7):632–637.
- , , , , Tongue pressure against hard palate during swallowing in post‐stroke patients. Gerodontology. 2005;22(4):227–233.
- , Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–156.
- , , , et al. Tongue strength as a predictor of functional outcomes and quality of life after tongue cancer surgery. Ann Otol Rhinol Laryngol. 2013;122(6):386–397.
- , , , Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55(4):199–205.
- , , , , , The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. S2005;53(9):1483–1489.
- , , , et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–158.
- , , , , , Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118(1):39–43.
- , , , Effects of directional exercise on lingual strength. J Speech Lang Hear Res. 2009;52(4):1034–1047.
- , , et al. Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Archives of Otolaryngology – Head 127(8):975–979.
Dysphagia is a serious medical condition that can lead to aspiration pneumonia, malnutrition, and dehydration.[1] Dysphagia is the result of a variety of medical etiologies, including stroke, traumatic brain injury, progressive neurologic conditions, head and neck cancers, and general deconditioning. Prevalence estimates for dysphagia vary depending upon the etiology and patient age, but estimates as high as 38% for lifetime prevalence have been reported in those over age 65 years.[2]
To avoid adverse health outcomes, early detection of dysphagia is essential. In hospitalized patients, early detection has been associated with reduced risk of pneumonia, decreased length of hospital stay, and improved cost‐effectiveness resulting from a reduction in hospital days due to fewer cases of aspiration pneumonia.[3, 4, 5] Stroke guidelines in the United States recommend screening for dysphagia for all patients admitted with stroke.[6] Consequently, the majority of screening procedures have been designed for and tested in this population.[7, 8, 9, 10]
The videofluoroscopic swallow study (VFSS) is a commonly accepted, reference standard, instrumental evaluation technique for dysphagia, as it provides the most comprehensive information regarding anatomic and physiologic function for swallowing diagnosis and treatment. Flexible endoscopic evaluation of swallowing (FEES) is also available, as are several less commonly used techniques (scintigraphy, manometry, and ultrasound). Due to availability, patient compliance, and expertise needed, it is not possible to perform instrumental examination on every patient with suspected dysphagia. Therefore, a number of minimally invasive bedside screening procedures for dysphagia have been developed.
The value of any diagnostic screening test centers on performance characteristics, which under ideal circumstances include a positive result for all those who have dysphagia (sensitivity) and negative result for all those who do not have dysphagia (specificity). Such an ideal screening procedure would reduce unnecessary referrals and testing, thus resulting in cost savings, more effective utilization of speech‐language pathology consultation services, and less unnecessary radiation exposure. In addition, an effective screen would detect all those at risk for aspiration pneumonia in need of intervention. However, most available bedside screening tools are lacking in some or all of these desirable attributes.[11, 12] We undertook a systematic review and meta‐analysis of bedside procedures to screen for dysphagia.
METHODS
Data Sources and Searches
We conducted a comprehensive search of 7 databases, including MEDLINE, Embase, and Scopus, from each database's earliest inception through June 9, 2014 for English‐language articles and abstracts. The search strategy was designed and conducted by an experienced librarian with input from 1 researcher (J.C.O.). Controlled vocabulary supplemented with keywords was used to search for comparative studies of bedside screening tests for predicting dysphagia (see Supporting Information, Appendix 1, in the online version of this article for the full strategy).
All abstracts were screened, and potentially relevant articles were identified for full‐text review. Those references were manually inspected to identify all relevant studies.
Study Selection
A study was eligible for inclusion if it tested a diagnostic swallow study of any variety against an acceptable reference standard (VFSS or flexible endoscopic evaluation of swallowing with sensory testing [FEEST]).
Data Extraction and Quality Assessment
The primary outcome of the study was aspiration, as predicted by a bedside exam, compared to gold‐standard visualization of aspirated material entering below the vocal cords. From each study, data were abstracted based on the type of diagnostic method and reference standard study population and inclusion/exclusion characteristics, design, and prediction of aspiration. Prediction of aspiration was compared against the reference standard to yield true positives, true negatives, false positives, and false negatives. Additional potential confounding variables were abstracted using a standard form based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis[13] (see Supporting Information, Appendix 2, in the online version of this article for the full abstraction template).
Data Synthesis and Analysis
Sensitivity and specificity for each test that identified the presence of dysphagia was calculated for each study. These were used to generate positive and negative likelihood ratios (LRs), which were plotted on a likelihood matrix, a graphic depiction of the logarithm of the +LR on the ordinate versus the logarithm of the LR on the abscissa, dividing the graphic into quadrants such that the right upper quadrant is tests that can be used for confirmation, right lower quadrant neither confirmation nor exclusion, left lower quadrant exclusion only, and left upper quadrant an ideal test with both exclusionary and confirmatory properties.[14] A good screening test would thus be on the left half of the graphic to effectively rule out dysphagia, and the ideal test with both good sensitivity and specificity would be found in the left upper quadrant. Graphics were constructed using the Stata MIDAS package (Stata Corp., College Station, TX).[15]
RESULTS
We identified 891 distinct articles. Of these, 749 were excluded based on abstract review. After reviewing the remaining 142 full‐text articles, 48 articles were determined to meet inclusion criteria, which included 10,437 observations across 7414 patients (Figure 1). We initially intended to conduct a meta‐analysis on each type, but heterogeneity in design and statistical heterogeneity in aggregate measures precluded pooling of results.

Characteristics of Included Studies
Of the 48 included studies, the majority (n=42) were prospective observational studies,[7, 8, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53] whereas 2 were randomized trials,[9, 54] 2 studies were double‐blind observational,[9, 16] 1 was a case‐control design,[55] and 1 was a retrospective case series.[56] The majority of studies were exclusively inpatient,[7, 8, 9, 14, 17, 18, 19, 21, 22, 24, 25, 26, 31, 32, 33, 35, 36, 38, 41, 43, 44, 45, 46, 47, 49, 51, 52, 53, 55, 57] with 5 in mixed in and outpatient populations,[20, 27, 40, 55, 58] 2 in outpatient populations,[23, 41] and the remainder not reporting the setting from which they drew their study populations.
The indications for swallow evaluations fit broadly into 4 categories: stroke,[7, 8, 9, 14, 21, 22, 24, 25, 26, 31, 33, 34, 35, 38, 40, 41, 42, 43, 45, 48, 52, 56, 58] other neurologic disorders,[17, 18, 23, 28, 39, 47] all causes,[16, 20, 27, 29, 30, 36, 37, 44, 46, 49, 51, 52, 53, 54, 58] and postsurgical.[19, 32, 34] Most used VFSS as a reference standard,[7, 8, 9, 14, 16, 17, 18, 19, 21, 22, 23, 25, 26, 27, 28, 29, 30, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 50, 51, 52, 53, 54, 56, 57, 58] with 8 using FEEST,[20, 24, 31, 32, 33, 35, 49, 55] and 1 accepting either videofluoroscopic evaluation of swallow or FEEST.[48]
Studies were placed into 1 or more of the following 4 categories: subjective bedside examination,[8, 9, 18, 19, 31, 34, 48] questionnaire‐based tools,[17, 23, 46, 53] protocolized multi‐item evaluations,[20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] and single‐item exam maneuvers, symptoms, or signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 56, 58, 59] The characteristics of all studies are detailed in Table 1.
| Study | Location | Design | Mean Age (SD) | Reason(s) for Dysphagia | Indx Test | Description | Reference Standard | Sample Size, No. of Patients | Sample Size, No. of Observations |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Splaingard et al., 198844 | Milwaukee, WI, USA | Prospective observational study | NR | Multiple | Clinical bedside swallow exam | Combination of scored comprehensive physical exam, history, and observed swallow. | VFSS | 107 | 107 |
| DePippo et al., 199243 | White Plains, NY, USA | Prospective observational study | 71 (10) | Stroke | WST | Observation of swallow. | VFSS | 44 | |
| Horner et al., 199356 | Durham, NC, USA | Retrospective case series | 64* | Stroke | Clinical bedside swallow evaluation | VFSS | 38 | 114 | |
| Kidd et al., 199342 | Belfast, UK | Prospective observational study | 72 (10) | Stroke | Bedside 50‐mL swallow evaluation | Patient swallows 50 mL of water in 5‐mL aliquots, with therapist assessing for choking, coughing, or change in vocal quality after each swallow. | VFSS | 60 | 240 |
| Collins and Bakheit, 199741 | Southampton, UK | Prospective observational study | 65* | Stroke | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 54 | 54 |
| Daniels et al., 199740 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside examination | 6 individual bedside assessments (dysphonia, dysphagia, cough before/after swallow, gag reflex and voice change) examined as predictors for aspiration risk. | VFSS | 59 | 354 |
| Mari et al., 199739 | Ancona, Italy | Prospective observational study | 60 (16) | Mixed neurologic diseases | Combined history and exam | Assessed symptoms of dysphagia, cough, and 3‐oz water swallow. | VFSS | 93 | 372 |
| Daniels et al., 19987 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 55 | 330 |
| Smithard et al., 19988 | Ashford, UK | Prospective observational study | 79* | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 83 | 249 |
| Addington et al., 199938 | Kansas City, MO, USA | Prospective observational study | 80* | Stroke | NR | Reflex cough. | VFSS | 40 | 40 |
| Logemann et al., 199937 | Evanston, IL, USA | Prospective observational study | 65 | Multiple | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 200 | 1400 |
| Smith et al., 20009 | Manchester, UK | Double blind observational | 69 | Stroke | Clinical bedside swallow evaluation, pulse oximetry evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. Also evaluated if patient desaturates by at least 2% during evaluation. | VFSS | 53 | 53 |
| Warms et al., 200036 | Melbourne, Australia | Prospective observational study | 67 | Multiple | Wet voice | Voice was recorded and analyzed with Sony digital audio tape during videofluoroscopy. | VFSS | 23 | 708 |
| Lim et al., 200135 | Singapore, Singapore | Prospective observational study | NR | Stroke | Water swallow test, desaturation during swallow | 50‐mL swallow done in 5‐mL aliquots with assessment of phonation/choking afterward; desaturation >2% during swallow, | FEEST | 50 | 100 |
| McCullough et al., 200134 | Nashville, TN, USA | Prospective observational study | 60 (10) | Stroke | Clinical bedside swallow evaluation | 15‐item physical exam with observed swallow. | VFSS | 2040 | 60 |
| Rosen et al., 2001[74] | Newark, NJ, USA | Prospective observational study | 60 | Head and Neck cancer | Wet voice | Observation of swallow. | VFSS | 26 | 26 |
| Leder and Espinosa, 200233 | New Haven, CT, USA | Prospective observational study | 70* | Stroke | Clinical exam | Checklist evaluation of cough and voice change after swallow, volitional cough, dysphonia, dysarthria, and abnormal gag. | FEEST | 49 | 49 |
| Belafsky et al., 200332 | San Francisco, CA, USA | Prospective observational study | 65 (11) | Post‐tracheostomy patients | Modified Evans Blue Dye Test | 3 boluses of dye‐impregnated ice are given to patient. Tracheal secretions are suctioned, and evaluated for the presence of dye. | FEES | 30 | 30 |
| Chong et al., 200331 | Jalan Tan Tock Seng, Singapore | Prospective observational study | 75 (7) | Stroke | Water swallow test, desaturation during, clinical exam | Subjective exam, drinking 50 mL of water in 10‐mL aliquots, and evaluating for desaturation >2% during FEES. | FEEST | 50 | 150 |
| Tohara et al., 200330 | Tokyo, Japan | Prospective observational study | 63 (17) | Multiple | Food/water swallow tests, and a combination of the 2 | Protocolized observation of sequential food and water swallows with scored outcomes. | VFSS | 63 | 63 |
| Rosenbek et al., 200414 | Gainesville, FL, USA | Prospective observational study | 68* | Stroke | Clinical bedside swallow evaluation | Describes 5 parameters of voice quality and 15 physical examination maneuvers used. | VFSS | 60 | 1200 |
| Ryu et al., 200429 | Seoul, South Korea | Prospective observational study | 64 (14) | Multiple | Voice analysis parameters | Analysis of the/a/vowel sound with Visi‐Pitch II 3300. | VFSS | 93 | 372 |
| Shaw et al., 200428 | Sheffield, UK | Prospective observational study | 71 | Neurologic disease | Bronchial auscultation | Auscultation over the right main bronchus during trial feeding to listen for sounds of aspiration. | VFSS | 105 | 105 |
| Wu et al., 200427 | Taipei, Taiwan | Prospective observational study | 72 (11) | Multiple | 100‐mL swallow test | Patient lifts a glass of 100 mL of water and drinks as quickly as possible, and is assessed for signs of choking, coughing, or wet voice, and is timed for speed of drinking. | VFSS | 54 | 54 |
| Nishiwaki et al., 200526 | Shizuoaka, Japan | Prospective observational study | 70* | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 31 | 248 |
| Wang et al., 200554 | Taipei, Taiwan | Prospective double‐blind study | 41* | Multiple | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 60 | 60 |
| Ramsey et al., 200625 | Kent, UK | Prospective observational study | 71 (10) | Stroke | BSA | Assessment of lip seal, tongue movement, voice quality, cough, and observed 5‐mL swallow. | VFSS | 54 | 54 |
| Trapl et al., 200724 | Krems, Austria | Prospective observational study | 76 (2) | Stroke | Gugging Swallow Screen | Progressive observed swallow trials with saliva, then with 350 mL liquid, then dry bread. | FEEST | 49 | 49 |
| Suiter and Leder, 200849 | Several centers across the USA | Prospective observational study | 68.3 | Multiple | 3‐oz water swallow test | Observation of swallow. | FEEST | 3000 | 3000 |
| Wagasugi et al., 200850 | Tokyo, Japan | Prospective observational study | NR | Multiple | Cough test | Acoustic analysis of cough. | VFSS | 204 | 204 |
| Baylow et al., 200945 | New York, NY, USA | Prospective observational study | NR | Stroke | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 15 | 30 |
| Cox et al., 200923 | Leiden, the Netherlands | Prospective observational study | 68 (8) | Inclusion body myositis | Dysphagia questionnaire | Questionnaire assessing symptoms of dysphagia. | VFSS | 57 | 57 |
| Kagaya et al., 201051 | Tokyo, Japan | Prospective observational study | NR | Multiple | Simple Swallow Provocation Test | Injection of 1‐2 mL of water through nasal tube directed at the suprapharynx. | VFSS | 46 | 46 |
| Martino et al., 200957 | Toronto, Canada | Randomized trial | 69 (14) | Stroke | Toronto Bedside Swallow Screening Test | 4‐item physical assessment including Kidd water swallow test, pharyngeal sensation, tongue movement, and dysphonia (before and after water swallow). | VFSS | 59 | 59 |
| Santamato et al., 200955 | Bari, Italy | Case control | NR | Multiple | Acoustic analysis, postswallow apnea | Acoustic analysis of cough. | VFSS | 15 | 15 |
| Smith Hammond et al., 200948 | Durham, NC, USA | Prospective observational study | 67.7 (1.2) | Multiple | Cough, expiratory phase peak flow | Acoustic analysis of cough. | VFSS or FEES | 96 | 288 |
| Leigh et al., 201022 | Seoul, South Korea | Prospective observational study | NR | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 167 | 167 |
| Pitts et al., 201047 | Gainesville, FL, USA | Prospective observational study | NR | Parkinson | Cough compression phase duration | Acoustic analysis of cough. | VFSS | 58 | 232 |
| Cohen and Manor, 201146 | Tel Aviv, Israel | Prospective observational Study | NR | Multiple | Swallow Disturbance Questionnaire | 15‐item questionnaire. | FEES | 100 | 100 |
| Edmiaston et al., 201121 | St. Louis, MO, USA | Prospective observational study | 63* | Stroke | SWALLOW‐3D Acute Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Mandysova et al., 201120 | Pardubice, Czech Republic | Prospective observational study | 69 (13) | Multiple | Brief Bedside Dysphagia Screening Test | 8‐item physician exam including ability to clench teeth; symmetry/strength of tongue, facial, and shoulder muscles; dysarthria; and choking, coughing, or dripping of food after taking thick liquid. | FEES | 87 | 87 |
| Steele et al., 201158 | Toronto, Canada | Double blind observational | 67 | Stroke | 4‐item bedside exam | Tongue lateralization, cough, throat clear, and voice quality. | VFSS | 400 | 40 |
| Yamamoto et al., 201117 | Kodaira, Japan | Prospective observational study | 67 (9) | Parkinson's Disease | Swallowing Disturbance Questionnaire | 15‐item questionnaire. | VFSS | 61 | 61 |
| Bhama et al., 201219 | Pittsburgh, PA, USA | Prospective observational study | 57 (14) | Post‐lung transplant | Clinical bedside swallow evaluation | Not described. | VFSS | 128 | 128 |
| Shem et al., 201218 | San Jose, CA, USA | Prospective observational study | 42 (17) | Spinal cord injuries resulting in tetraplegia | Clinical bedside swallow evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. | VFSS | 26 | 26 |
| Steele et al., 201316 | Toronto, Canada | Prospective observational study | 67 (14) | Multiple | Dual‐axis accelerometry | Computed accelerometry of swallow. | VFSS | 37 | 37 |
| Edmiaston et al., 201452 | St. Louis, MO, USA | Prospective observational study | 63 (15) | Stroke | Barnes Jewish Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Rofes et al., 201453 | Barcelona, Spain | Prospective observational study | 74 (12) | Mixed | EAT‐10 questionnaire and variable viscosity swallow test | Symptom‐based questionnaire (EAT‐10) and repeated observations and measurements of swallow with different thickness liquids. | VFS | 134 | 134 |
Subjective Clinical Exam
Seven studies reported the sensitivity and specificity of subjective assessments of nurses and speech‐language pathologists in observing swallowing and predicting aspiration.[8, 9, 18, 19, 31, 34, 48] The overall distribution of studies is summarized in the likelihood matrix in Figure 2. Two studies, Chong et al.[31] and Shem et al.,[18] were on the left side of the matrix, indicating a sensitive rule‐out test. However, both were small studies, and only Chong et al. reported reasonable sensitivity with incorporation bias from knowledge of a desaturation study outcome. Overall, subjective exams did not appear reliable in ruling out dysphagia.

Questionnaire‐Based Tools
Only 4 studies used questionnaire‐based tools filled out by the patient, asking about subjective assessment of dysphagia symptoms and frequency.[17, 23, 46, 53] Yamamoto et al. reported results of using the swallow dysphagia questionnaire in patients with Parkinson's disease.[17] Rofes et al. looked at the Eating Assessment Tool (EAT‐10) questionnaire among all referred patients and a small population of healthy volunteers.[53] Each was administered the questionnaire before undergoing a videofluoroscopic study. Overall, sensitivity and specificity were 77.8% and 84.6%, respectively. Cox et al. studied a different questionnaire in a group of patients with inclusion body myositis, finding 70% sensitivity and 44% specificity.[23] Cohen and Manor examined the swallow dysphagia questionnaire across several different causes of dysphagia, finding at optimum, the test is 78% specific and 73% sensitive.[46] Rofes et al. had an 86% sensitivity and 68% specificity for the EAT‐10 tool.[53]
Multi‐Item Exam Protocols
Sixteen studies reported multistep protocols for determining a patient's risk for aspiration.[9, 20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] Each involved a combination of physical exam maneuvers and history elements, detailed in Table 1. This is shown in the likelihood matrix in Figure 3. Only 2 of these studies were in the left lower quadrant, Edmiaston et al. 201121 and 2014.[52] Both studies were restricted to stroke populations, but found reasonable sensitivity and specificity in identifying dysphagia.

Individual Exam Maneuvers
Thirty studies reported the diagnostic performance of individual exam maneuvers and signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 54, 56, 58] Each is depicted in Figure 4 as a likelihood matrix demonstrating the +LR and LR for individual maneuvers as seen in the figure; most fall into the right lower quadrant, where they are not diagnostically useful tests. Studies in the left lower quadrant demonstrating the ability to exclude aspiration desirable in a screening test were dysphonia in McCullough et al.,[34] dual‐axis accelerometry in Steele et al.,[16] and the water swallow test in DePippo et al.[43] and Suiter and Leder.[49]

McCullough et al. found dysphonia to be the most discriminatory sign or symptom assessed, with an area under the curve (AUC) of 0.818. Dysphonia was judged by a sustained/a/and had 100% sensitivity but only 27% specificity. Wet voice within the same study was slightly less informative, with an AUC of 0.77 (sensitivity 50% and specificity 84%).[34]
Kidd et al. verified the diagnosis of stroke, and then assessed several neurologic parameters, including speech, muscle strength, and sensation. Pharyngeal sensation was assessed by touching each side of the pharyngeal wall and asking patients if they felt sensation that differed from each side. Patient report of abnormal sensation during this maneuver was 80% sensitive and 86% specific as a predictor of aspiration on VFSS.[42]
Steele et al. described the technique of dual axis accelerometry, where an accelerometer was placed at the midline of the neck over the cricoid cartilage during VFSS. The movement of the cricoid cartilage was captured for analysis in a computer algorithm to identify abnormal pharyngeal swallow behavior. Sensitivity was 100%, and specificity was 54%. Although the study was small (n=40), this novel method demonstrated good discrimination.[58]
DePippo et al. evaluated a 3‐oz water swallow in stroke patients. This protocol called for patients to drink the bolus of water without interruption, and be observed for 1 minute after for cough or wet‐hoarse voice. Presence of either sign was considered abnormal. Overall, sensitivity was 94% and specificity 30% looking for the presence of either sign.[43] Suiter and Leder used a similar protocol, with sensitivity of 97% and specificity of 49%.[49]
DISCUSSION
Our results show that most bedside swallow examinations lack the sensitivity to be used as a screening test for dysphagia across all patient populations examined. This is unfortunate as the ability to determine which patients require formal speech language pathology consultation or imaging as part of their diagnostic evaluation early in the hospital stay would lead to improved allocation of resources, cost reductions, and earlier implementation of effective therapy approaches. Furthermore, although radiation doses received during VFSS are not high when compared with other radiologic exams like computed tomography scans,[60] increasing awareness about the long‐term malignancy risks associated with medical imaging makes it desirable to reduce any test involving ionizing radiation.
There were several categories of screening procedures identified during this review process. Those classified as subjective bedside exams and protocolized multi‐item evaluations were found to have high heterogeneity in their sensitivity and specificity, though a few exam protocols did have a reasonable sensitivity and specificity.[21, 31, 52] The following individual exam maneuvers were found to demonstrate high sensitivity and an ability to exclude aspiration: a test for dysphonia through production of a sustained/a/34 and use of dual‐axis accelerometry.[16] Two other tests, the 3‐oz water swallow test[43] and testing of abnormal pharyngeal sensation,[42] were each found effective in a single study, with conflicting results from other studies.
Our results extend the findings from previous systematic reviews on this subject, most of which focused only on stroke patients.[5, 12, 61, 62] Martino and colleagues[5] conducted a review focused on screening for adults poststroke. From 13 identified articles, it was concluded that evidence to support inclusion or exclusion of screening was poor. Daniels et al. conducted a systematic review of swallowing screening tools specific to patients with acute or chronic stroke.[12] Based on 16 articles, the authors concluded that a combination of swallowing and nonswallowing features may be necessary for development of a valid screening tool. The generalizability of these reviews is limited given that all were conducted in patients poststroke, and therefore results and recommendations may not be generalizable to other patients.
Wilkinson et al.[62] conducted a recent systematic review that focused on screening techniques for inpatients 65 years or older that excluded patients with stroke or Parkinson's disease. The purpose of that review was to examine sensitivity and specificity of bedside screening tests as well as ability to accurately predict pneumonia. The authors concluded that existing evidence is not sufficient to recommend the use of bedside tests in a general older population.[62]
Specific screening tools identified by Martino and colleagues[5] to have good predictive value in detecting aspiration as a diagnostic marker of dysphagia were an abnormal test of pharyngeal sensation[42] and the 50‐mL water swallow test. Daniels et al. identified a water swallow test as an important component of a screen.[7] These results were consistent with those of this review in that the abnormal test of pharyngeal sensation[42] was identified for high levels of sensitivity. However, the 3‐oz water swallow test,[43, 49] rather than the 50‐mL water swallow test,[42] was identified in this review as the version of the water swallow test with the best predictive value in ruling out aspiration. Results of our review identified 2 additional individual items, dual‐axis accelerometry[16] and dysphonia,[34] that may be important to include in a comprehensive screening tool. In the absence of better tools, the 3 oz swallow test, properly executed, seems to be the best currently available tool validated in more than 1 study.
Several studies in the current review included an assessment of oral tongue movement that is not described thoroughly and varies between studies. Tongue movement as an individual item on a screening protocol was not found to yield high sensitivity or specificity. However, tongue movement or range of motion is only 1 aspect of oral tongue function; pressures produced by the tongue reflecting strength also may be important and warrant evaluation. Multiple studies have shown patients with dysphagia resulting from a variety of etiologies to produce lower than normal maximum isometric lingual pressures,[63, 64, 65, 66, 67, 68] or pressures produced when the tongue is pushed as hard as possible against the hard palate. Tongue strengthening protocols that result in higher maximum isometric lingual pressures have been shown to carry over to positive changes in swallow function.[69, 70, 71, 72, 73] Inclusion of tongue pressure measurement in a comprehensive screening tool may help to improve predictive capabilities.
We believe our results have implications for practicing clinicians, and serve as a call to action for development of an easy‐to‐perform, accurate tool for dysphagia screening. Future prospective studies should focus on practical tools that can be deployed at the bedside, and correlate the results with not only gold‐standard VFSS and FEES, but with clinical outcomes such as pneumonia and aspiration events leading to prolonged length of stay.
There were several limitations to this review. High levels of heterogeneity were reported in the screening tests present in the literature, precluding meaningful meta‐analysis. In addition, the majority of studies included were in poststroke adults, which limits the generalizability of results.
In conclusion, no screening protocol has been shown to provide adequate predictive value for presence of aspiration. Several individual exam maneuvers demonstrate high sensitivity; however, the most effective combination of screening protocol components is unknown. There is a need for future research focused on the development of a comprehensive screening tool that can be applied across patient populations for accurate detection of dysphagia as well as prediction of other adverse health outcomes, including pneumonia.
Acknowledgements
The authors thank Drs. Byun‐Mo Oh and Catrionia Steele for providing additional information in response to requests for unpublished information.
Disclosures: Nasia Safdar MD, is supported by a National Institutes of Health R03 GEMSSTAR award and a VA MERIT award. The authors report no conflicts of interest.
Dysphagia is a serious medical condition that can lead to aspiration pneumonia, malnutrition, and dehydration.[1] Dysphagia is the result of a variety of medical etiologies, including stroke, traumatic brain injury, progressive neurologic conditions, head and neck cancers, and general deconditioning. Prevalence estimates for dysphagia vary depending upon the etiology and patient age, but estimates as high as 38% for lifetime prevalence have been reported in those over age 65 years.[2]
To avoid adverse health outcomes, early detection of dysphagia is essential. In hospitalized patients, early detection has been associated with reduced risk of pneumonia, decreased length of hospital stay, and improved cost‐effectiveness resulting from a reduction in hospital days due to fewer cases of aspiration pneumonia.[3, 4, 5] Stroke guidelines in the United States recommend screening for dysphagia for all patients admitted with stroke.[6] Consequently, the majority of screening procedures have been designed for and tested in this population.[7, 8, 9, 10]
The videofluoroscopic swallow study (VFSS) is a commonly accepted, reference standard, instrumental evaluation technique for dysphagia, as it provides the most comprehensive information regarding anatomic and physiologic function for swallowing diagnosis and treatment. Flexible endoscopic evaluation of swallowing (FEES) is also available, as are several less commonly used techniques (scintigraphy, manometry, and ultrasound). Due to availability, patient compliance, and expertise needed, it is not possible to perform instrumental examination on every patient with suspected dysphagia. Therefore, a number of minimally invasive bedside screening procedures for dysphagia have been developed.
The value of any diagnostic screening test centers on performance characteristics, which under ideal circumstances include a positive result for all those who have dysphagia (sensitivity) and negative result for all those who do not have dysphagia (specificity). Such an ideal screening procedure would reduce unnecessary referrals and testing, thus resulting in cost savings, more effective utilization of speech‐language pathology consultation services, and less unnecessary radiation exposure. In addition, an effective screen would detect all those at risk for aspiration pneumonia in need of intervention. However, most available bedside screening tools are lacking in some or all of these desirable attributes.[11, 12] We undertook a systematic review and meta‐analysis of bedside procedures to screen for dysphagia.
METHODS
Data Sources and Searches
We conducted a comprehensive search of 7 databases, including MEDLINE, Embase, and Scopus, from each database's earliest inception through June 9, 2014 for English‐language articles and abstracts. The search strategy was designed and conducted by an experienced librarian with input from 1 researcher (J.C.O.). Controlled vocabulary supplemented with keywords was used to search for comparative studies of bedside screening tests for predicting dysphagia (see Supporting Information, Appendix 1, in the online version of this article for the full strategy).
All abstracts were screened, and potentially relevant articles were identified for full‐text review. Those references were manually inspected to identify all relevant studies.
Study Selection
A study was eligible for inclusion if it tested a diagnostic swallow study of any variety against an acceptable reference standard (VFSS or flexible endoscopic evaluation of swallowing with sensory testing [FEEST]).
Data Extraction and Quality Assessment
The primary outcome of the study was aspiration, as predicted by a bedside exam, compared to gold‐standard visualization of aspirated material entering below the vocal cords. From each study, data were abstracted based on the type of diagnostic method and reference standard study population and inclusion/exclusion characteristics, design, and prediction of aspiration. Prediction of aspiration was compared against the reference standard to yield true positives, true negatives, false positives, and false negatives. Additional potential confounding variables were abstracted using a standard form based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis[13] (see Supporting Information, Appendix 2, in the online version of this article for the full abstraction template).
Data Synthesis and Analysis
Sensitivity and specificity for each test that identified the presence of dysphagia was calculated for each study. These were used to generate positive and negative likelihood ratios (LRs), which were plotted on a likelihood matrix, a graphic depiction of the logarithm of the +LR on the ordinate versus the logarithm of the LR on the abscissa, dividing the graphic into quadrants such that the right upper quadrant is tests that can be used for confirmation, right lower quadrant neither confirmation nor exclusion, left lower quadrant exclusion only, and left upper quadrant an ideal test with both exclusionary and confirmatory properties.[14] A good screening test would thus be on the left half of the graphic to effectively rule out dysphagia, and the ideal test with both good sensitivity and specificity would be found in the left upper quadrant. Graphics were constructed using the Stata MIDAS package (Stata Corp., College Station, TX).[15]
RESULTS
We identified 891 distinct articles. Of these, 749 were excluded based on abstract review. After reviewing the remaining 142 full‐text articles, 48 articles were determined to meet inclusion criteria, which included 10,437 observations across 7414 patients (Figure 1). We initially intended to conduct a meta‐analysis on each type, but heterogeneity in design and statistical heterogeneity in aggregate measures precluded pooling of results.

Characteristics of Included Studies
Of the 48 included studies, the majority (n=42) were prospective observational studies,[7, 8, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53] whereas 2 were randomized trials,[9, 54] 2 studies were double‐blind observational,[9, 16] 1 was a case‐control design,[55] and 1 was a retrospective case series.[56] The majority of studies were exclusively inpatient,[7, 8, 9, 14, 17, 18, 19, 21, 22, 24, 25, 26, 31, 32, 33, 35, 36, 38, 41, 43, 44, 45, 46, 47, 49, 51, 52, 53, 55, 57] with 5 in mixed in and outpatient populations,[20, 27, 40, 55, 58] 2 in outpatient populations,[23, 41] and the remainder not reporting the setting from which they drew their study populations.
The indications for swallow evaluations fit broadly into 4 categories: stroke,[7, 8, 9, 14, 21, 22, 24, 25, 26, 31, 33, 34, 35, 38, 40, 41, 42, 43, 45, 48, 52, 56, 58] other neurologic disorders,[17, 18, 23, 28, 39, 47] all causes,[16, 20, 27, 29, 30, 36, 37, 44, 46, 49, 51, 52, 53, 54, 58] and postsurgical.[19, 32, 34] Most used VFSS as a reference standard,[7, 8, 9, 14, 16, 17, 18, 19, 21, 22, 23, 25, 26, 27, 28, 29, 30, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 50, 51, 52, 53, 54, 56, 57, 58] with 8 using FEEST,[20, 24, 31, 32, 33, 35, 49, 55] and 1 accepting either videofluoroscopic evaluation of swallow or FEEST.[48]
Studies were placed into 1 or more of the following 4 categories: subjective bedside examination,[8, 9, 18, 19, 31, 34, 48] questionnaire‐based tools,[17, 23, 46, 53] protocolized multi‐item evaluations,[20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] and single‐item exam maneuvers, symptoms, or signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 56, 58, 59] The characteristics of all studies are detailed in Table 1.
| Study | Location | Design | Mean Age (SD) | Reason(s) for Dysphagia | Indx Test | Description | Reference Standard | Sample Size, No. of Patients | Sample Size, No. of Observations |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Splaingard et al., 198844 | Milwaukee, WI, USA | Prospective observational study | NR | Multiple | Clinical bedside swallow exam | Combination of scored comprehensive physical exam, history, and observed swallow. | VFSS | 107 | 107 |
| DePippo et al., 199243 | White Plains, NY, USA | Prospective observational study | 71 (10) | Stroke | WST | Observation of swallow. | VFSS | 44 | |
| Horner et al., 199356 | Durham, NC, USA | Retrospective case series | 64* | Stroke | Clinical bedside swallow evaluation | VFSS | 38 | 114 | |
| Kidd et al., 199342 | Belfast, UK | Prospective observational study | 72 (10) | Stroke | Bedside 50‐mL swallow evaluation | Patient swallows 50 mL of water in 5‐mL aliquots, with therapist assessing for choking, coughing, or change in vocal quality after each swallow. | VFSS | 60 | 240 |
| Collins and Bakheit, 199741 | Southampton, UK | Prospective observational study | 65* | Stroke | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 54 | 54 |
| Daniels et al., 199740 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside examination | 6 individual bedside assessments (dysphonia, dysphagia, cough before/after swallow, gag reflex and voice change) examined as predictors for aspiration risk. | VFSS | 59 | 354 |
| Mari et al., 199739 | Ancona, Italy | Prospective observational study | 60 (16) | Mixed neurologic diseases | Combined history and exam | Assessed symptoms of dysphagia, cough, and 3‐oz water swallow. | VFSS | 93 | 372 |
| Daniels et al., 19987 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 55 | 330 |
| Smithard et al., 19988 | Ashford, UK | Prospective observational study | 79* | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 83 | 249 |
| Addington et al., 199938 | Kansas City, MO, USA | Prospective observational study | 80* | Stroke | NR | Reflex cough. | VFSS | 40 | 40 |
| Logemann et al., 199937 | Evanston, IL, USA | Prospective observational study | 65 | Multiple | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 200 | 1400 |
| Smith et al., 20009 | Manchester, UK | Double blind observational | 69 | Stroke | Clinical bedside swallow evaluation, pulse oximetry evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. Also evaluated if patient desaturates by at least 2% during evaluation. | VFSS | 53 | 53 |
| Warms et al., 200036 | Melbourne, Australia | Prospective observational study | 67 | Multiple | Wet voice | Voice was recorded and analyzed with Sony digital audio tape during videofluoroscopy. | VFSS | 23 | 708 |
| Lim et al., 200135 | Singapore, Singapore | Prospective observational study | NR | Stroke | Water swallow test, desaturation during swallow | 50‐mL swallow done in 5‐mL aliquots with assessment of phonation/choking afterward; desaturation >2% during swallow, | FEEST | 50 | 100 |
| McCullough et al., 200134 | Nashville, TN, USA | Prospective observational study | 60 (10) | Stroke | Clinical bedside swallow evaluation | 15‐item physical exam with observed swallow. | VFSS | 2040 | 60 |
| Rosen et al., 2001[74] | Newark, NJ, USA | Prospective observational study | 60 | Head and Neck cancer | Wet voice | Observation of swallow. | VFSS | 26 | 26 |
| Leder and Espinosa, 200233 | New Haven, CT, USA | Prospective observational study | 70* | Stroke | Clinical exam | Checklist evaluation of cough and voice change after swallow, volitional cough, dysphonia, dysarthria, and abnormal gag. | FEEST | 49 | 49 |
| Belafsky et al., 200332 | San Francisco, CA, USA | Prospective observational study | 65 (11) | Post‐tracheostomy patients | Modified Evans Blue Dye Test | 3 boluses of dye‐impregnated ice are given to patient. Tracheal secretions are suctioned, and evaluated for the presence of dye. | FEES | 30 | 30 |
| Chong et al., 200331 | Jalan Tan Tock Seng, Singapore | Prospective observational study | 75 (7) | Stroke | Water swallow test, desaturation during, clinical exam | Subjective exam, drinking 50 mL of water in 10‐mL aliquots, and evaluating for desaturation >2% during FEES. | FEEST | 50 | 150 |
| Tohara et al., 200330 | Tokyo, Japan | Prospective observational study | 63 (17) | Multiple | Food/water swallow tests, and a combination of the 2 | Protocolized observation of sequential food and water swallows with scored outcomes. | VFSS | 63 | 63 |
| Rosenbek et al., 200414 | Gainesville, FL, USA | Prospective observational study | 68* | Stroke | Clinical bedside swallow evaluation | Describes 5 parameters of voice quality and 15 physical examination maneuvers used. | VFSS | 60 | 1200 |
| Ryu et al., 200429 | Seoul, South Korea | Prospective observational study | 64 (14) | Multiple | Voice analysis parameters | Analysis of the/a/vowel sound with Visi‐Pitch II 3300. | VFSS | 93 | 372 |
| Shaw et al., 200428 | Sheffield, UK | Prospective observational study | 71 | Neurologic disease | Bronchial auscultation | Auscultation over the right main bronchus during trial feeding to listen for sounds of aspiration. | VFSS | 105 | 105 |
| Wu et al., 200427 | Taipei, Taiwan | Prospective observational study | 72 (11) | Multiple | 100‐mL swallow test | Patient lifts a glass of 100 mL of water and drinks as quickly as possible, and is assessed for signs of choking, coughing, or wet voice, and is timed for speed of drinking. | VFSS | 54 | 54 |
| Nishiwaki et al., 200526 | Shizuoaka, Japan | Prospective observational study | 70* | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 31 | 248 |
| Wang et al., 200554 | Taipei, Taiwan | Prospective double‐blind study | 41* | Multiple | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 60 | 60 |
| Ramsey et al., 200625 | Kent, UK | Prospective observational study | 71 (10) | Stroke | BSA | Assessment of lip seal, tongue movement, voice quality, cough, and observed 5‐mL swallow. | VFSS | 54 | 54 |
| Trapl et al., 200724 | Krems, Austria | Prospective observational study | 76 (2) | Stroke | Gugging Swallow Screen | Progressive observed swallow trials with saliva, then with 350 mL liquid, then dry bread. | FEEST | 49 | 49 |
| Suiter and Leder, 200849 | Several centers across the USA | Prospective observational study | 68.3 | Multiple | 3‐oz water swallow test | Observation of swallow. | FEEST | 3000 | 3000 |
| Wagasugi et al., 200850 | Tokyo, Japan | Prospective observational study | NR | Multiple | Cough test | Acoustic analysis of cough. | VFSS | 204 | 204 |
| Baylow et al., 200945 | New York, NY, USA | Prospective observational study | NR | Stroke | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 15 | 30 |
| Cox et al., 200923 | Leiden, the Netherlands | Prospective observational study | 68 (8) | Inclusion body myositis | Dysphagia questionnaire | Questionnaire assessing symptoms of dysphagia. | VFSS | 57 | 57 |
| Kagaya et al., 201051 | Tokyo, Japan | Prospective observational study | NR | Multiple | Simple Swallow Provocation Test | Injection of 1‐2 mL of water through nasal tube directed at the suprapharynx. | VFSS | 46 | 46 |
| Martino et al., 200957 | Toronto, Canada | Randomized trial | 69 (14) | Stroke | Toronto Bedside Swallow Screening Test | 4‐item physical assessment including Kidd water swallow test, pharyngeal sensation, tongue movement, and dysphonia (before and after water swallow). | VFSS | 59 | 59 |
| Santamato et al., 200955 | Bari, Italy | Case control | NR | Multiple | Acoustic analysis, postswallow apnea | Acoustic analysis of cough. | VFSS | 15 | 15 |
| Smith Hammond et al., 200948 | Durham, NC, USA | Prospective observational study | 67.7 (1.2) | Multiple | Cough, expiratory phase peak flow | Acoustic analysis of cough. | VFSS or FEES | 96 | 288 |
| Leigh et al., 201022 | Seoul, South Korea | Prospective observational study | NR | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 167 | 167 |
| Pitts et al., 201047 | Gainesville, FL, USA | Prospective observational study | NR | Parkinson | Cough compression phase duration | Acoustic analysis of cough. | VFSS | 58 | 232 |
| Cohen and Manor, 201146 | Tel Aviv, Israel | Prospective observational Study | NR | Multiple | Swallow Disturbance Questionnaire | 15‐item questionnaire. | FEES | 100 | 100 |
| Edmiaston et al., 201121 | St. Louis, MO, USA | Prospective observational study | 63* | Stroke | SWALLOW‐3D Acute Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Mandysova et al., 201120 | Pardubice, Czech Republic | Prospective observational study | 69 (13) | Multiple | Brief Bedside Dysphagia Screening Test | 8‐item physician exam including ability to clench teeth; symmetry/strength of tongue, facial, and shoulder muscles; dysarthria; and choking, coughing, or dripping of food after taking thick liquid. | FEES | 87 | 87 |
| Steele et al., 201158 | Toronto, Canada | Double blind observational | 67 | Stroke | 4‐item bedside exam | Tongue lateralization, cough, throat clear, and voice quality. | VFSS | 400 | 40 |
| Yamamoto et al., 201117 | Kodaira, Japan | Prospective observational study | 67 (9) | Parkinson's Disease | Swallowing Disturbance Questionnaire | 15‐item questionnaire. | VFSS | 61 | 61 |
| Bhama et al., 201219 | Pittsburgh, PA, USA | Prospective observational study | 57 (14) | Post‐lung transplant | Clinical bedside swallow evaluation | Not described. | VFSS | 128 | 128 |
| Shem et al., 201218 | San Jose, CA, USA | Prospective observational study | 42 (17) | Spinal cord injuries resulting in tetraplegia | Clinical bedside swallow evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. | VFSS | 26 | 26 |
| Steele et al., 201316 | Toronto, Canada | Prospective observational study | 67 (14) | Multiple | Dual‐axis accelerometry | Computed accelerometry of swallow. | VFSS | 37 | 37 |
| Edmiaston et al., 201452 | St. Louis, MO, USA | Prospective observational study | 63 (15) | Stroke | Barnes Jewish Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Rofes et al., 201453 | Barcelona, Spain | Prospective observational study | 74 (12) | Mixed | EAT‐10 questionnaire and variable viscosity swallow test | Symptom‐based questionnaire (EAT‐10) and repeated observations and measurements of swallow with different thickness liquids. | VFS | 134 | 134 |
Subjective Clinical Exam
Seven studies reported the sensitivity and specificity of subjective assessments of nurses and speech‐language pathologists in observing swallowing and predicting aspiration.[8, 9, 18, 19, 31, 34, 48] The overall distribution of studies is summarized in the likelihood matrix in Figure 2. Two studies, Chong et al.[31] and Shem et al.,[18] were on the left side of the matrix, indicating a sensitive rule‐out test. However, both were small studies, and only Chong et al. reported reasonable sensitivity with incorporation bias from knowledge of a desaturation study outcome. Overall, subjective exams did not appear reliable in ruling out dysphagia.

Questionnaire‐Based Tools
Only 4 studies used questionnaire‐based tools filled out by the patient, asking about subjective assessment of dysphagia symptoms and frequency.[17, 23, 46, 53] Yamamoto et al. reported results of using the swallow dysphagia questionnaire in patients with Parkinson's disease.[17] Rofes et al. looked at the Eating Assessment Tool (EAT‐10) questionnaire among all referred patients and a small population of healthy volunteers.[53] Each was administered the questionnaire before undergoing a videofluoroscopic study. Overall, sensitivity and specificity were 77.8% and 84.6%, respectively. Cox et al. studied a different questionnaire in a group of patients with inclusion body myositis, finding 70% sensitivity and 44% specificity.[23] Cohen and Manor examined the swallow dysphagia questionnaire across several different causes of dysphagia, finding at optimum, the test is 78% specific and 73% sensitive.[46] Rofes et al. had an 86% sensitivity and 68% specificity for the EAT‐10 tool.[53]
Multi‐Item Exam Protocols
Sixteen studies reported multistep protocols for determining a patient's risk for aspiration.[9, 20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] Each involved a combination of physical exam maneuvers and history elements, detailed in Table 1. This is shown in the likelihood matrix in Figure 3. Only 2 of these studies were in the left lower quadrant, Edmiaston et al. 201121 and 2014.[52] Both studies were restricted to stroke populations, but found reasonable sensitivity and specificity in identifying dysphagia.

Individual Exam Maneuvers
Thirty studies reported the diagnostic performance of individual exam maneuvers and signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 54, 56, 58] Each is depicted in Figure 4 as a likelihood matrix demonstrating the +LR and LR for individual maneuvers as seen in the figure; most fall into the right lower quadrant, where they are not diagnostically useful tests. Studies in the left lower quadrant demonstrating the ability to exclude aspiration desirable in a screening test were dysphonia in McCullough et al.,[34] dual‐axis accelerometry in Steele et al.,[16] and the water swallow test in DePippo et al.[43] and Suiter and Leder.[49]

McCullough et al. found dysphonia to be the most discriminatory sign or symptom assessed, with an area under the curve (AUC) of 0.818. Dysphonia was judged by a sustained/a/and had 100% sensitivity but only 27% specificity. Wet voice within the same study was slightly less informative, with an AUC of 0.77 (sensitivity 50% and specificity 84%).[34]
Kidd et al. verified the diagnosis of stroke, and then assessed several neurologic parameters, including speech, muscle strength, and sensation. Pharyngeal sensation was assessed by touching each side of the pharyngeal wall and asking patients if they felt sensation that differed from each side. Patient report of abnormal sensation during this maneuver was 80% sensitive and 86% specific as a predictor of aspiration on VFSS.[42]
Steele et al. described the technique of dual axis accelerometry, where an accelerometer was placed at the midline of the neck over the cricoid cartilage during VFSS. The movement of the cricoid cartilage was captured for analysis in a computer algorithm to identify abnormal pharyngeal swallow behavior. Sensitivity was 100%, and specificity was 54%. Although the study was small (n=40), this novel method demonstrated good discrimination.[58]
DePippo et al. evaluated a 3‐oz water swallow in stroke patients. This protocol called for patients to drink the bolus of water without interruption, and be observed for 1 minute after for cough or wet‐hoarse voice. Presence of either sign was considered abnormal. Overall, sensitivity was 94% and specificity 30% looking for the presence of either sign.[43] Suiter and Leder used a similar protocol, with sensitivity of 97% and specificity of 49%.[49]
DISCUSSION
Our results show that most bedside swallow examinations lack the sensitivity to be used as a screening test for dysphagia across all patient populations examined. This is unfortunate as the ability to determine which patients require formal speech language pathology consultation or imaging as part of their diagnostic evaluation early in the hospital stay would lead to improved allocation of resources, cost reductions, and earlier implementation of effective therapy approaches. Furthermore, although radiation doses received during VFSS are not high when compared with other radiologic exams like computed tomography scans,[60] increasing awareness about the long‐term malignancy risks associated with medical imaging makes it desirable to reduce any test involving ionizing radiation.
There were several categories of screening procedures identified during this review process. Those classified as subjective bedside exams and protocolized multi‐item evaluations were found to have high heterogeneity in their sensitivity and specificity, though a few exam protocols did have a reasonable sensitivity and specificity.[21, 31, 52] The following individual exam maneuvers were found to demonstrate high sensitivity and an ability to exclude aspiration: a test for dysphonia through production of a sustained/a/34 and use of dual‐axis accelerometry.[16] Two other tests, the 3‐oz water swallow test[43] and testing of abnormal pharyngeal sensation,[42] were each found effective in a single study, with conflicting results from other studies.
Our results extend the findings from previous systematic reviews on this subject, most of which focused only on stroke patients.[5, 12, 61, 62] Martino and colleagues[5] conducted a review focused on screening for adults poststroke. From 13 identified articles, it was concluded that evidence to support inclusion or exclusion of screening was poor. Daniels et al. conducted a systematic review of swallowing screening tools specific to patients with acute or chronic stroke.[12] Based on 16 articles, the authors concluded that a combination of swallowing and nonswallowing features may be necessary for development of a valid screening tool. The generalizability of these reviews is limited given that all were conducted in patients poststroke, and therefore results and recommendations may not be generalizable to other patients.
Wilkinson et al.[62] conducted a recent systematic review that focused on screening techniques for inpatients 65 years or older that excluded patients with stroke or Parkinson's disease. The purpose of that review was to examine sensitivity and specificity of bedside screening tests as well as ability to accurately predict pneumonia. The authors concluded that existing evidence is not sufficient to recommend the use of bedside tests in a general older population.[62]
Specific screening tools identified by Martino and colleagues[5] to have good predictive value in detecting aspiration as a diagnostic marker of dysphagia were an abnormal test of pharyngeal sensation[42] and the 50‐mL water swallow test. Daniels et al. identified a water swallow test as an important component of a screen.[7] These results were consistent with those of this review in that the abnormal test of pharyngeal sensation[42] was identified for high levels of sensitivity. However, the 3‐oz water swallow test,[43, 49] rather than the 50‐mL water swallow test,[42] was identified in this review as the version of the water swallow test with the best predictive value in ruling out aspiration. Results of our review identified 2 additional individual items, dual‐axis accelerometry[16] and dysphonia,[34] that may be important to include in a comprehensive screening tool. In the absence of better tools, the 3 oz swallow test, properly executed, seems to be the best currently available tool validated in more than 1 study.
Several studies in the current review included an assessment of oral tongue movement that is not described thoroughly and varies between studies. Tongue movement as an individual item on a screening protocol was not found to yield high sensitivity or specificity. However, tongue movement or range of motion is only 1 aspect of oral tongue function; pressures produced by the tongue reflecting strength also may be important and warrant evaluation. Multiple studies have shown patients with dysphagia resulting from a variety of etiologies to produce lower than normal maximum isometric lingual pressures,[63, 64, 65, 66, 67, 68] or pressures produced when the tongue is pushed as hard as possible against the hard palate. Tongue strengthening protocols that result in higher maximum isometric lingual pressures have been shown to carry over to positive changes in swallow function.[69, 70, 71, 72, 73] Inclusion of tongue pressure measurement in a comprehensive screening tool may help to improve predictive capabilities.
We believe our results have implications for practicing clinicians, and serve as a call to action for development of an easy‐to‐perform, accurate tool for dysphagia screening. Future prospective studies should focus on practical tools that can be deployed at the bedside, and correlate the results with not only gold‐standard VFSS and FEES, but with clinical outcomes such as pneumonia and aspiration events leading to prolonged length of stay.
There were several limitations to this review. High levels of heterogeneity were reported in the screening tests present in the literature, precluding meaningful meta‐analysis. In addition, the majority of studies included were in poststroke adults, which limits the generalizability of results.
In conclusion, no screening protocol has been shown to provide adequate predictive value for presence of aspiration. Several individual exam maneuvers demonstrate high sensitivity; however, the most effective combination of screening protocol components is unknown. There is a need for future research focused on the development of a comprehensive screening tool that can be applied across patient populations for accurate detection of dysphagia as well as prediction of other adverse health outcomes, including pneumonia.
Acknowledgements
The authors thank Drs. Byun‐Mo Oh and Catrionia Steele for providing additional information in response to requests for unpublished information.
Disclosures: Nasia Safdar MD, is supported by a National Institutes of Health R03 GEMSSTAR award and a VA MERIT award. The authors report no conflicts of interest.
- , , , et al. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser. 2012;72:57–66.
- , , , Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–865.
- , , Formal dysphagia screening protocols prevent pneumonia. Stroke. 2006;37(3):765.
- , , Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995;76(12):1130–1133.
- , , Screening for oropharyngeal dysphagia in stroke: insufficient evidence for guidelines. Dysphagia. 2000;15(1):19–30.
- , , , et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(3):870–947.
- , , , , , Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79(1):14–19.
- , , , et al. Can bedside assessment reliably exclude aspiration following acute stroke? Age Ageing. 1998;27(2):99–106.
- , , , The combination of bedside swallowing assessment and oxygen saturation monitoring of swallowing in acute stroke: a safe and humane screening tool. Age Ageing. 2000;29(6):495–499.
- , , , Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010;19(4):357–364.
- , Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001;16(1):7–18.
- , , Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke. 2012;43(3):892–897.
- , , , , Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–269, W64.
- , , Is the information about a test important? Applying the methods of evidence‐based medicine to the clinical examination of swallowing. J Commun Disord. 2004;37(5):437–450.
- MIDAS: Stata module for meta‐analytical integration of diagnostic test accuracy studies. Statistical Software Components. S456880, Boston College Department of Economics, 2009.
- , , Noninvasive detection of thin‐liquid aspiration using dual‐axis swallowing accelerometry. Dysphagia. 2013;28(1):105–112.
- , , , , Validation of the Japanese translation of the Swallowing Disturbance Questionnaire in Parkinson's disease patients. Qual Life Res. 2012;21(7):1299–1303.
- , , , , , Diagnostic accuracy of bedside swallow evaluation versus videofluoroscopy to assess dysphagia in individuals with tetraplegia. PM R. 2012;4(4):283–289.
- , et al. 723 Aspiration after Lung Transplantation: Incidence, Risk Factors, and Accuracy of the Bedside Swallow Evaluation. The J Heart Lung Transplant. 2012;31(4 suppl 1):S247–S248.
- , , , Cerny M. Development of the brief bedside dysphagia screening test in the Czech Republic. Nurs Health Sci. 2011;13(4):388–395.
- , , SWALLOW‐3D, a simple 2‐minute bedside screening test, detects dysphagia in acute stroke patients with high sensitivity when validated against video‐fluoroscopy. Stroke. 2011;42(3):e352.
- , , , , Bedside screening and subacute reassessment of post‐stroke dysphagia: a prospective study. Int J Stroke. 2010;5:200.
- , , , , , Detecting dysphagia in inclusion body myositis. J Neurol. 2009;256(12):2009–2013.
- , , , et al. Dysphagia bedside screening for acute‐stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38(11):2948–2952.
- , , Can pulse oximetry or a bedside swallowing assessment be used to detect aspiration after stroke? Stroke. 2006;37(12):2984–2988.
- , , , , , Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variables. J Rehabil Med. 2005;37(4):247–251.
- , , , Evaluating swallowing dysfunction using a 100‐ml water swallowing test. Dysphagia. 2004;19(1):43–47.
- , , , et al. Bronchial auscultation: an effective adjunct to speech and language therapy bedside assessment when detecting dysphagia and aspiration? Dysphagia. 2004;19(4):211–218.
- , , Prediction of laryngeal aspiration using voice analysis. Am J Phys Med Rehabil. 2004;83(10):753–757.
- , , , , Three tests for predicting aspiration without videofluorography. Dysphagia. 2003;18(2):126–134.
- , , , , Bedside clinical methods useful as screening test for aspiration in elderly patients with recent and previous strokes. Ann Acad Med Singapore. 2003;32(6):790–794.
- , , , The accuracy of the modified Evan's blue dye test in predicting aspiration. Laryngoscope. 2003;113(11):1969–1972.
- , Aspiration risk after acute stroke: comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17(3):214–218.
- , , Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord. 2001;34(1‐2):55–72.
- , , , et al. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16(1):1–6.
- , “Wet Voice” as a predictor of penetration and aspiration in oropharyngeal dysphagia. Dysphagia. 2000;15(2):84–88.
- , , A screening procedure for oropharyngeal dysphagia. Dysphagia. 1999;14(1):44–51.
- , , , Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil. 1999;80(2):150–154.
- , , , , , Predictive value of clinical indices in detecting aspiration in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 1997;63(4):456–460.
- , , , Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol. 1997;6(4):17–24.
- , Does pulse oximetry reliably detect aspiration in dysphagic stroke patients? Stroke. 1997;28(9):1773–1775.
- , , , Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med. 1993;86(12):825–829.
- , , Validation of the 3‐oz water swallow test for aspiration following stroke. Arch Neurol. 1992;49(12):1259–1261.
- , , , Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil. 1988;69(8):637–640.
- , , , Accuracy of clinical judgment of the chin‐down posture for dysphagia during the clinical/bedside assessment as corroborated by videofluoroscopy in adults with acute stroke. Dysphagia. 2009;24(4):423–433.
- , Swallowing disturbance questionnaire for detecting dysphagia. Laryngoscope. 2011;121(7):1383–1387.
- , , , , , . Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–1431.
- , , , et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777.
- , Clinical utility of the 3‐ounce water swallow test. Dysphagia. 2008;23(3):244–250.
- , , , et al. Screening test for silent aspiration at the bedside. Dysphagia. 2008;23(4):364–370.
- , , , , , Simple swallowing provocation test has limited applicability as a screening tool for detecting aspiration, silent aspiration, or penetration. Dysphagia. 2010;25(1):6–10.
- , , , A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. J Stroke Cerebrovasc Dis. 2014;23 (4):712–716.
- , , , Sensitivity and specificity of the Eating Assessment Tool and the Volume‐Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–1265.
- , , , Pulse oximetry does not reliably detect aspiration on videofluoroscopic swallowing study. Arch Phys Med Rehabil. 2005;86(4):730–734.
- , , , et al. Acoustic analysis of swallowing sounds: a new technique for assessing dysphagia. J Rehabil Med. 2009;41(8):639–645.
- , , Aspiration in bilateral stroke patients: a validation study. Neurology. 1993;43(2):430–433.
- , , , et al. The Toronto Bedside Swallowing Screening Test (TOR‐BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40(2):555–561.
- , , , et al. Exploration of the utility of a brief swallow screening protocol with comparison to concurrent videofluoroscopy. Can J Speech Lang Pathol Audiol. 2011;35(3):228–242.
- , , , et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–1976.
- , , , et al. Radiation exposure time during MBSS: influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia. 2013;28(1):77–85.
- Detection of eating difficulties after stroke: a systematic review. Int Nurs Rev. 2006;53(2):143–149.
- , , Aspiration in older patients without stroke: A systematic review of bedside diagnostic tests and predictors of pneumonia. Eur Geriatr Med. 2012;3(3):145–152.
- , , A tongue force measurement system for the assessment of oral‐phase swallowing disorders. Arch Phys Med Rehabil. 1991;72(1):38–42.
- , , Strength, Endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res. 2000;43(1):256–267.
- , , , et al. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007;29(7):632–637.
- , , , , Tongue pressure against hard palate during swallowing in post‐stroke patients. Gerodontology. 2005;22(4):227–233.
- , Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–156.
- , , , et al. Tongue strength as a predictor of functional outcomes and quality of life after tongue cancer surgery. Ann Otol Rhinol Laryngol. 2013;122(6):386–397.
- , , , Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55(4):199–205.
- , , , , , The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. S2005;53(9):1483–1489.
- , , , et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–158.
- , , , , , Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118(1):39–43.
- , , , Effects of directional exercise on lingual strength. J Speech Lang Hear Res. 2009;52(4):1034–1047.
- , , et al. Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Archives of Otolaryngology – Head 127(8):975–979.
- , , , et al. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser. 2012;72:57–66.
- , , , Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–865.
- , , Formal dysphagia screening protocols prevent pneumonia. Stroke. 2006;37(3):765.
- , , Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995;76(12):1130–1133.
- , , Screening for oropharyngeal dysphagia in stroke: insufficient evidence for guidelines. Dysphagia. 2000;15(1):19–30.
- , , , et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(3):870–947.
- , , , , , Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79(1):14–19.
- , , , et al. Can bedside assessment reliably exclude aspiration following acute stroke? Age Ageing. 1998;27(2):99–106.
- , , , The combination of bedside swallowing assessment and oxygen saturation monitoring of swallowing in acute stroke: a safe and humane screening tool. Age Ageing. 2000;29(6):495–499.
- , , , Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010;19(4):357–364.
- , Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001;16(1):7–18.
- , , Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke. 2012;43(3):892–897.
- , , , , Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–269, W64.
- , , Is the information about a test important? Applying the methods of evidence‐based medicine to the clinical examination of swallowing. J Commun Disord. 2004;37(5):437–450.
- MIDAS: Stata module for meta‐analytical integration of diagnostic test accuracy studies. Statistical Software Components. S456880, Boston College Department of Economics, 2009.
- , , Noninvasive detection of thin‐liquid aspiration using dual‐axis swallowing accelerometry. Dysphagia. 2013;28(1):105–112.
- , , , , Validation of the Japanese translation of the Swallowing Disturbance Questionnaire in Parkinson's disease patients. Qual Life Res. 2012;21(7):1299–1303.
- , , , , , Diagnostic accuracy of bedside swallow evaluation versus videofluoroscopy to assess dysphagia in individuals with tetraplegia. PM R. 2012;4(4):283–289.
- , et al. 723 Aspiration after Lung Transplantation: Incidence, Risk Factors, and Accuracy of the Bedside Swallow Evaluation. The J Heart Lung Transplant. 2012;31(4 suppl 1):S247–S248.
- , , , Cerny M. Development of the brief bedside dysphagia screening test in the Czech Republic. Nurs Health Sci. 2011;13(4):388–395.
- , , SWALLOW‐3D, a simple 2‐minute bedside screening test, detects dysphagia in acute stroke patients with high sensitivity when validated against video‐fluoroscopy. Stroke. 2011;42(3):e352.
- , , , , Bedside screening and subacute reassessment of post‐stroke dysphagia: a prospective study. Int J Stroke. 2010;5:200.
- , , , , , Detecting dysphagia in inclusion body myositis. J Neurol. 2009;256(12):2009–2013.
- , , , et al. Dysphagia bedside screening for acute‐stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38(11):2948–2952.
- , , Can pulse oximetry or a bedside swallowing assessment be used to detect aspiration after stroke? Stroke. 2006;37(12):2984–2988.
- , , , , , Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variables. J Rehabil Med. 2005;37(4):247–251.
- , , , Evaluating swallowing dysfunction using a 100‐ml water swallowing test. Dysphagia. 2004;19(1):43–47.
- , , , et al. Bronchial auscultation: an effective adjunct to speech and language therapy bedside assessment when detecting dysphagia and aspiration? Dysphagia. 2004;19(4):211–218.
- , , Prediction of laryngeal aspiration using voice analysis. Am J Phys Med Rehabil. 2004;83(10):753–757.
- , , , , Three tests for predicting aspiration without videofluorography. Dysphagia. 2003;18(2):126–134.
- , , , , Bedside clinical methods useful as screening test for aspiration in elderly patients with recent and previous strokes. Ann Acad Med Singapore. 2003;32(6):790–794.
- , , , The accuracy of the modified Evan's blue dye test in predicting aspiration. Laryngoscope. 2003;113(11):1969–1972.
- , Aspiration risk after acute stroke: comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17(3):214–218.
- , , Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord. 2001;34(1‐2):55–72.
- , , , et al. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16(1):1–6.
- , “Wet Voice” as a predictor of penetration and aspiration in oropharyngeal dysphagia. Dysphagia. 2000;15(2):84–88.
- , , A screening procedure for oropharyngeal dysphagia. Dysphagia. 1999;14(1):44–51.
- , , , Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil. 1999;80(2):150–154.
- , , , , , Predictive value of clinical indices in detecting aspiration in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 1997;63(4):456–460.
- , , , Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol. 1997;6(4):17–24.
- , Does pulse oximetry reliably detect aspiration in dysphagic stroke patients? Stroke. 1997;28(9):1773–1775.
- , , , Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med. 1993;86(12):825–829.
- , , Validation of the 3‐oz water swallow test for aspiration following stroke. Arch Neurol. 1992;49(12):1259–1261.
- , , , Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil. 1988;69(8):637–640.
- , , , Accuracy of clinical judgment of the chin‐down posture for dysphagia during the clinical/bedside assessment as corroborated by videofluoroscopy in adults with acute stroke. Dysphagia. 2009;24(4):423–433.
- , Swallowing disturbance questionnaire for detecting dysphagia. Laryngoscope. 2011;121(7):1383–1387.
- , , , , , . Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–1431.
- , , , et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777.
- , Clinical utility of the 3‐ounce water swallow test. Dysphagia. 2008;23(3):244–250.
- , , , et al. Screening test for silent aspiration at the bedside. Dysphagia. 2008;23(4):364–370.
- , , , , , Simple swallowing provocation test has limited applicability as a screening tool for detecting aspiration, silent aspiration, or penetration. Dysphagia. 2010;25(1):6–10.
- , , , A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. J Stroke Cerebrovasc Dis. 2014;23 (4):712–716.
- , , , Sensitivity and specificity of the Eating Assessment Tool and the Volume‐Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–1265.
- , , , Pulse oximetry does not reliably detect aspiration on videofluoroscopic swallowing study. Arch Phys Med Rehabil. 2005;86(4):730–734.
- , , , et al. Acoustic analysis of swallowing sounds: a new technique for assessing dysphagia. J Rehabil Med. 2009;41(8):639–645.
- , , Aspiration in bilateral stroke patients: a validation study. Neurology. 1993;43(2):430–433.
- , , , et al. The Toronto Bedside Swallowing Screening Test (TOR‐BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40(2):555–561.
- , , , et al. Exploration of the utility of a brief swallow screening protocol with comparison to concurrent videofluoroscopy. Can J Speech Lang Pathol Audiol. 2011;35(3):228–242.
- , , , et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–1976.
- , , , et al. Radiation exposure time during MBSS: influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia. 2013;28(1):77–85.
- Detection of eating difficulties after stroke: a systematic review. Int Nurs Rev. 2006;53(2):143–149.
- , , Aspiration in older patients without stroke: A systematic review of bedside diagnostic tests and predictors of pneumonia. Eur Geriatr Med. 2012;3(3):145–152.
- , , A tongue force measurement system for the assessment of oral‐phase swallowing disorders. Arch Phys Med Rehabil. 1991;72(1):38–42.
- , , Strength, Endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res. 2000;43(1):256–267.
- , , , et al. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007;29(7):632–637.
- , , , , Tongue pressure against hard palate during swallowing in post‐stroke patients. Gerodontology. 2005;22(4):227–233.
- , Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–156.
- , , , et al. Tongue strength as a predictor of functional outcomes and quality of life after tongue cancer surgery. Ann Otol Rhinol Laryngol. 2013;122(6):386–397.
- , , , Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55(4):199–205.
- , , , , , The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. S2005;53(9):1483–1489.
- , , , et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–158.
- , , , , , Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118(1):39–43.
- , , , Effects of directional exercise on lingual strength. J Speech Lang Hear Res. 2009;52(4):1034–1047.
- , , et al. Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Archives of Otolaryngology – Head 127(8):975–979.
Reducing Transmission of Methicillin-Resistant <em>Staphylococcus aureus</em> and Vancomycin-Resistant <em>Enterococcus</em> in the ICU—An Update on Prevention and Infection Control Practices
From the Department of Medicine, Infectious Disease Practice and Innovations, The Medical City, Pasig City, Philippines (Dr. Abad), the Division of Emergency Medicine, University of Wisconsin Medical School, Madison, WI (Dr. Pulia), University of Wisconsin Hospital and Clinics, Madison, WI (Ms. Krupp), and the Willam S. Middleton Memorial Veterans Affairs Hospital, Madison, WI (Dr. Safdar).
Patients in intensive care units (ICUs) are at greatly increased risk of developing health care-associated infections (HAIs) [1]. More than 70% of the bacteria that cause HAIs are resistant to at least one of the antimicrobials commonly used to treat these infections [2]. Two such pathogens, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) are responsible for a considerable proportion of ICU infections that are associated with increased morbidity, mortality, and costs [3–5]. In this review, we discuss the epidemiology of colonization and infection by MRSA and VRE and provide an update on practices for prevention of transmission and infection by MRSA and VRE in the ICU.
EPIDEMIOLOGY AND MECHANISMS OF RESISTANCE
MRSA is the major cause of HAIs worldwide [6]. Among ICUs in the United States, the proportion of methicillin resistance among S. aureus isolates increased from 35.9% in 1992 to 64.4% in 2003 [4]. Approximately 8% of patients are colonized with MRSA upon admission, and an average of 5% will acquire MRSA colonization in the ICU [7,8]. A comparison study of academic tertiary care facilities found medical ICUs had higher MRSA admission prevalence rates than surgical ICUs, whereas surgical ICUs had a higher incidence rate [7]. Enteroccoccus is the third most common pathogen associated with HAIs, with 33% resistant to vancomycin [9]. VRE infection is associated with increased ICU cost and increased length of stay [10]. Incidence of ICU-acquired VRE varies among regions and countries. For example, in the United States, Warren et al [11] reported a VRE incidence of 27 cases per 1000 patient ICU days, whereas Kohlenberg et al [12] reported a mean incidence of 0.29 cases per 1000 patient ICU days in Germany.
Understanding the mechanisms that allow development of resistant strains of S. aureus and Enterococcus species is important to devise preventive strategies. Methicillin resistance in MRSA is determined by the staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element that carries the mecA gene. The mecA gene codes for an additional penicillin-binding protein (PBP) that has a reduced affinity towards methicillin (PBP2a/PBP2'). This results in a reduced ability to bind to the bacterial cell wall and inhibit synthesis [13,14]. Study of molecular epidemiology has identified MRSA as originating from 8 major variants of the mecA gene [15]. The majority of MRSA infections are caused by strains belonging to a few internationally disseminated clones [14]. The first identified strains were associated with infections in hospitalized patients (hospital-associated MRSA), but community-associated MRSA strains have since emerged and have become established globally, including in health care institutions [16].
Community-acquired MRSA can cause severe infections in health hosts [17]; possible explanations include increased CA-MRSA virulence due to the acquisition of mobile genetic elements, namely those containing Panton-Valentine leukocidin (PVL) [18] or increased expression of core genome-encoded virulence genes, such as phenol-soluble modulin (PSM) cytolysins, α-toxin, and other virulence determinants [19].
Enterococcus is intrinsically resistant to several antimicrobial drugs, with resistance to vancomycin encoded by several clusters of genes known as vancomycin resistance gene clusters (eg, vanA, vanB). The gene clusters generate resistance through multiple pathways which encode enzymes to determine the structure of peptidoglycan precursors [20,21]. Genetically diverse, hospital-associated VRE outbreaks have been associated with single clones, multiple clones, and changing molecular epidemiology over time [21]. While up to 25% of the VRE genome includes acquired elements, the majority of hospital-associated infections are traced to a few clonal complexes, which differ from community-associated VRE strains [22].
The evolution of these efficient mechanisms that promote drug resistance has made it extremely challenging to eradicate organisms such as MRSA and VRE. However, advances in recent years have furthered our understanding of the epidemiology, pathogenesis, and methods of prevention and containment.
RISK FACTORS FOR COLONIZATION AND INFECTION
MRSA
The risk factors underlying MRSA colonization and infection in the ICU setting can be categorized as either patient/host or environmental factors. A wide range of patient-level factors is associated with MRSA colonization upon admission. General principles regarding the transmission of MRSA in the community include close contact with colonized or infected individuals, breaks in the skin, crowded living conditions and poor hygiene. These factors, alone or in combination, are thought to underlie observed outbreaks among sports teams, military personnel, correction facilities, American Indian communities, and daycare centers [23–34].
Two recently published systematic reviews have summarized important patient-level factors associated with MRSA colonization at the time of hospital admission. Forster et al [35] examined 27 studies and identified previous admission to hospital, transfer from nursing home or long-term care facility, and previous antibiotic use as the top 3 factors associated with MRSA colonization. A similar review conducted by McKinnell and colleagues [36] found that prior hospitalization, nursing home contact, recent antibiotic use, and exposure to health care-associated pathogens (MRSA carriage, VRE carriage, or Clostridium difficile infection) were found to portend the highest risk. Specific comorbid conditions also conveyed an increased risk, including congestive heart failure, chronic wounds/bedsores, diabetes mellitus, pulmonary disease, immunosuppression, urinary catheter, and renal failure/dialysis. It is clear that health care contacts, especially recent hospitalization, residence in a long-term care facility, and antibiotic use, are significant risk factors for MRSA colonization [37–39].
 In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
Regardless of whether MRSA colonization precedes admission or occurs due to nosocomial spread, it is associated with increased risk of developing a HAI [46–49]. In 2 large prospective observational cohort studies, the hazard ratios of MRSA colonization developing into S. aureus infections during the ICU stay were 3.84 and 4.70, respectively [50,51]. High levels of concordance between MRSA colonization strains and HAI strains have also been reported [52]. Nasal colonization with S. aureus has also been identified as an independent risk factor for developing ventilator-associated pneumonia (VAP) and bacteremia [53,54]. A case series of ICU patients with S. aureus nasal colonization who developed lower respiratory tract infections demonstrated genetically identical nasal and bronchial strains in 15/16 cases [55]. This finding strongly suggests that nasopharyngeal colonization with S. aureus contaminates oral secretions that are aspirated by critically ill patients, resulting in subsequent pneumonia. In a long-term outcomes study among a matched cohort of veterans, MRSA colonization was associated with an increased risk of infection-related readmission and mortality [56]. These findings reflect the critically important nature of measures designed to curb nosocomial transmission and acquisition of MRSA, especially among the vulnerable ICU population.
VRE
As with MRSA, risk factors associated with VRE colonization include both patient-level and ICU-level (or environmental) factors [57]. Examples of patient-level factors include previous antimicrobial exposure [58–62], underlying medical illnesses such as chronic renal failure requiring hemodialysis [11,63], length of hospital or ICU stay [11,59,64,65], and recent exposure to health care facilities. ICU-level factors of relevance are the prevalence of VRE in the unit, with high levels of endemicity leading to higher risk of colonization and transmission.
Antibiotic use is a major risk factor for VRE acquisition, although the type and class of antibiotic varies considerably across studies; the most frequently identified antibiotics are broad-spectrum cephalosporins, vancomycin, and anti-anaerobic agents [58,62,64]. Patients with chronic liver disease and post-transplantation are at exceedingly high risk for VRE acquisition [59]. In a recent study by Pan [66], for example, the authors found that the incidence of newly acquired VRE was 21.9 per 1000 patient-days in an ICU setting. On multivariate analysis, the authors found that, similar to other reports [11,59,67], length of stay in the ICU was associated with increased risk of VRE acquisition, with each additional day of stay increasing risk of VRE by 1.03 times. Warren et al undertook a prospective cohort study involving 519 patients admitted to the ICU for more than 48 hours [11]. Seventy-four (21%) of 352 patients were subsequently colonized with VRE. The median time to development of a positive VRE culture after ICU admission was 6 days. Increased mean APACHE II score on ICU admission (P = 0.002), sucralfate use (P = 0.003), vasopressor use (P = 0.01), tracheostomy in the ICU (P = 0.02), and C. difficile diarrhea (P = 0.002) appeared to be associated with VRE acquisition.
 It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
REDUCING TRANSMISSION—MRSA AND VRE PREVENTION STRATEGIES
 Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Antimicrobial Stewardship
Antibiotic use is a major driver of antibiotic resistance. A meta-analysis by de Bruin and Riley [71] studied the effect of vancomycin usage on VRE colonization and infection. A total of 12 articles describing 13 studies were included; none were randomized controlled trials. All studies were quasi-experimental and lacked control groups. Among all studies, less than half (46%) implemented vancomycin reduction measures as the sole type of intervention [72–76]. The remaining studies implemented other infection control modalities and or restricted the use of other antimicrobials [77–83]. Although all studies that implemented vancomycin restriction alone as a single strategy showed a decline in vancomycin usage, only 2 of these [74,75] showed a relative risk reduction in VRE acquisition post-intervention. Also, studies that restricted vancomycin alone revealed a trend towards lower efficacy in reducing VRE colonization and infection (33%) when compared with those that used additional measures (71%). While judicious antibiotic use should always be practiced, the evidence for vancomycin restriction as a sole intervention to control VRE is scant. It may be that other antibiotics are as big or bigger drivers of resistance in enterococci than vancomycin. For example, a growing body of literature supports antibiotic restriction, especially fluoroquinolones, for reducing MRSA. In several time-series quasi-experimental studies, restriction of fluorquinolones was associated with reduced trends in MRSA infections in the acute care setting, and consideration should be given to monitor and optimize fluoroquinolone use in the ICU setting [84,85].
Antimicrobial stewardship programs are fundamental to optimizing antibiotic use in the ICU and the authors strongly recommend that all ICUs should have such a program in place.
Educational Interventions
Infection control and multidrug-resistant organism (MDRO)–specific education programs for health care workers is a core principle of the CDC’s prevention guidelines. The HICPAC VRE guideline also explicitly states “continuing education programs for hospital staff (including attending and consulting physicians, medical residents, and students; pharmacy, nursing, and laboratory personnel; and other direct patient-care providers) should include information concerning the epidemiology of VRE and the potential impact of this pathogen on the cost and outcome of patient care [70].” A systematic review published in 2008 [86] that included 26 studies showed that such interventions to prevent HCAIs are usually successful; in this review, 20 of 26 studies showed a statistically significant decrease in infection rates, with risk ratios ranging from 0 to 1.6. Education was usually part of a broader array of infection control interventions. While clearly essential, education alone is unlikely to have a sustained impact on reducing MRSA and VRE infections.
Infection Control Measures
Major infection control interventions include hand hygiene, the use of personal protective equipment (PPE), and cohorting. These measures can be grouped into “horizontal” (or global) vs. “vertical” (or targeted) strategies. Although not mutually exclusive, horizontal approaches are designed to have an impact on multiple pathogens (pathogen nonspecific), whereas vertical approaches are designed to reduce the impact of specific pathogens (such as VRE). For the purposes of this review, we will discuss both strategies for containment of MRSA and VRE. Horizontal strategies include hand hygiene, universal gloving and/or gowning, environmental cleaning, and daily bathing with chlorhexidine. Vertical strategies include screening for either MRSA or VRE followed by placement in contact precautions and decolonization with mupirocin.
Hand Hygiene
Hand washing is fundamental to reducing transmission of MDROs in health care institutions; however, optimal compliance is hard to achieve and sustain. Barriers to adherence may include unavailability of sinks or hand hygiene materials (eg, alcohol-based gels, gloves) time constraints, forgetfulness, or lack of knowledge [87–95]. Several monitoring strategies have been evaluated to increase compliance with hand hygiene. Most involve direct observation followed by performance assessment and feedback.
Trials examining the impact of improvements in hand hygiene compliance on HAIs in the ICU setting have largely found benefit, although not all studies showed a decline in HAI. In a prospective crossover trial, Rupp et al [96] found dramatic improvements in compliance with hand gel availability, but this did not translate to decreased nosocomial MRSA infections. Venkatesh et al [97] carried out a before-and-after interventional prospective study in a hematology unit in a tertiary level hospital to evaluate the use of an electronic method of surveillance to determine compliance with hand hygiene. The authors also used rates of horizontal transmission of VRE as a secondary end-point. Results of the study showed that hand hygiene compliance improved from 36.3% at baseline to 70.1%. This represented an OR of 4.1 (95% confidence interval, 3.7–4.5), which the authors attributed to the use of automated alerts. VRE transmission rates before and during intervention were not statistically different, but the rates of infection were lower at 1.0 per month in comparison with 4.7 infections per month in the preceding 6 months (P = 0.096).
While improved hand hygiene may result in significant reductions in HAIs [40], research indicates hand hygiene alone influences about 40% of infections in the ICU setting [98]. As such, hand hygiene should be viewed as a necessary component of a comprehensive infection control program [99]. Despite the success of hand hygiene in reducing HAIs in the ICU, effective strategies to improve compliance remain elusive even under study conditions and further research is needed in this area [100].
Personal Protective Equipment
Tenorio et al [101] conducted a study to assess the effectiveness of gloving in the prevention of hand carriage of VRE by health care workers. The study showed that among 50 health care workers who had contact with patients colonized with VRE, 6 carried a similar patient strain even prior to known contact, and 17 of 44 (69%) had a patient-related VRE strain on their gloves after contact. This suggests a relatively high rate of colonization after usual patient-care contact. Factors associated with acquisition of VRE on gloves included duration of contact, contact with a patient’s body fluids, presence of diarrhea in a patient, mean VRE colony counts on a patient’s skin, and number of body sites colonized with VRE. Although gloves reduced the risk of VRE acquisition of VRE by 71% (ie, 12/17 did not have VRE on their hands after de-gloving) the protection afforded by gloves was incomplete. As such, hand hygiene after glove removal is recommended.
Slaughter et al [102] compared the use of personal protective equipment in the acquisition of VRE. During this study, 93 patients in glove-and-gown rooms and 88 patients in glove-only rooms had similar rates of VRE at baseline entry into the ICU and after the intervention. Mean times to colonization among the patients who became colonized were 8.0 days in the glove-and-gown group and 7.1 days in the glove-only group. None of these comparisons were statistically significant and the authors concluded that the universal use of gown and gloves was no better than the use of gloves alone in preventing VRE colonization.
A recent cluster randomized trial compared the effect of universal PPE (ie, gowning and gloving) with usual care for reducing acquisition of MRSA or VRE as a composite outcome [103]. The study did not find that universal gowning and gloving reduced VRE or MRSA acquisition but found a 40% decline in MRSA acquisition in the intervention ICUs compared with baseline rates of MRSA. No major adverse effects of universal gowning and gloving were noted in this study. A thoughtful editorial commenting on this article proposes that several aspects of the study deserve consideration, including the possibility of false-negative screening tests for VRE, which may have partially accounted for the negative primary outcome [69].
Based on these studies, it appears that the use of barrier precautions may be of value more for MRSA than VRE but further studies are needed to examine its impact on other types of pathogens, including new and emerging MDROs. Until further evidence becomes available, routine gowning and gloving may be of value in units with a high prevalence of MRSA.
Environmental Cleaning
Accumulating data suggests that the environment may play a major role in transmission of pathogens. MRSA has the ability to survive for days to weeks on inanimate objects [104–107]. Environmental contamination results in contamination of staff clothing and gloves [107,108] and is highly correlated with colonization strains among inpatients [109]. Although some studies of enhanced cleaning techniques and increased environmental services staff time have demonstrated reductions in MRSA outbreaks [110–112], the results are not universally favorable [113,114] and further studies are needed to examine the impact of environmental cleaning on rates of MRSA colonization or infection.
Several studies have implicated contaminated equipment as vectors for transmission of VRE during outbreaks [115–117], but the direction of fomite transfer from patient to environment has been difficult to ascertain. VRE have been found frequently on a variety of inanimate objects and surfaces in different health care environments [118–123], including gloved or ungloved hands of health care workers [101,124,125]. Hayden et al [126] determined the effect of improved environmental cleaning on VRE acquisition rates. This study was a pre-and-post intervention study carried out in a 21-bed medical intensive care unit (MICU) in a tertiary hospital over several phases. The intervention included the creation of a unique and improved cleaning program, as well as in-services to housekeeper services, education of the MICU staff, and a hand hygiene campaign. The results of the study showed decreased acquisition of VRE from 33.47 cases per 1000 patient days at risk in period 1 to 10.40 cases per 1000 patient-days at risk by period 4 of the study. Increased environmental cleaning was also associated with reduced growth of VRE from environmental cultures. At baseline, weekly contamination rates were 0.15 and 0.1 for samples obtained before and after cleaning, respectively. Culture positivity decreased to 0.07 and 0.04 for before and after cleaning in period 2 and then remained at low levels during the remainder of the study. It is important to note that the method for disinfecting used in this study was the “bucket method” as promoted by Byers [127]. This study provides further support for the importance of an environmental reservoir and of environmental decontamination to prevent endemic cross-transmission of VRE [126].
Goodman et al [128] used similar interventions but added a feedback tool using a black-light monitoring system (ie, use of an invisible, nontoxic marker to delineate areas that are adequately or inadequately cleaned) to reduce the likelihood of isolating either MRSA or VRE from an ICU environment. This study also showed favorable results, and notably, the use of the black-light monitoring system identified specific areas that were typically inadequately disinfected. Results showed that flat, horizontal surfaces (eg, countertops, bedside tray tables, and hamper tops) were adequately cleaned more often than small, vertical surfaces (eg, doorknobs, toilet handles, light switches, and electronics).
Part of the controversy surrounding the impact of environmental cleaning is the difficulty in determining its individual value as part of an overall infection control bundle [129]. A proposed area of demonstrable impact for environmental cleaning are frequently touched sites which are more likely to be contaminated with pathogens. Focusing on these “hot-bed” areas of the care environment may offer a useful adjunct to other infection control measures [129].
Active Surveillance
Active surveillance refers to periodic screening for asymp-tomatic carriers followed by placement of colonized patients in contact isolation. This practice is highly variable across institutions, as the evidence supporting this practice is conflicting and there are concerns about the cost of implementing this approach without solid evidence [70,130,131]. Despite lack of randomized controlled trials to guide this practice for MRSA prevention, many hospitals utilize MRSA surveillance and it is mandated by law in 9 states [132,133].
A prospective, interventional cohort study of universal MRSA screening on admission to surgical wards failed to reduce nosocomial MRSA infections [134]. Most recently, a pragmatic, cluster-randomized ICU trial reported that universal decolonization with chlorhexidine wipes and mupirocin use was more effective than screening and isolation in reducing rates of MRSA clinical isolates [65]. However, concerns regarding the risk of mupirocin resistance have been expressed [135,136]. The only randomized trial that compared active surveillance for MRSA and VRE followed by contact precautions to usual care did not find a benefit to active surveillance.
Huskins et al [137], in a large, cluster-randomized trial of 19 ICUs from different hospitals, determined the utility of using a culture-based active surveillance and contact isolation, compared with usual care (contact isolation for patients colonized with MRSA or VRE) as identified by existing hospital protocols, to reduce the incidence of colonization or infection with MRSA or VRE. In this trial, which spanned 6 months and involved 3488 participants, the authors found no significant difference between the intervention and control ICUs in terms of MRSA and VRE colonization or infection rates.
Conflicting with these findings is an observational study comparing MRSA infection rates before and after institution of a universal screening protocol, which demonstrated a 69.6% (CI, –89.2% to –19.6%]; P = 0.03) reduction in hospital wide MRSA prevalence density with screening [138]. The “MRSA bundle” implemented in 2007 at VA hospitals nationwide, which included universal screening, produced a 62% (P < 0.001) reduction in MRSA ICU infections; the relative contribution of the various bundle components is uncertain [139,140].
A proposed cost-saving alternative to universal screening is selective screening based on risk factor assessment [141]. The effectiveness of this type of program depends on creating a clinical decision-making tool capable of accurately identifying high-risk individuals while also accounting for the different risk factor profiles between HA-MRSA and CA-MRSA [142]. It has been proposed that targeted screening protocols may be more cost-effective in settings with < 5% prevalence of MRSA colonization on admission [143].
Many studies [61,144–149] have shown that active surveillance against VRE is cost-effective. For example, Calfee et al [144] showed that an established active surveillance program results in control of endemic VRE in high-risk patients. The infection control program was established in response to a hospital-wide VRE outbreak, and was sustained after the outbreak was controlled. The study by Calfee et al spanned 5 years and was performed at a tertiary-level university hospital, where cultures from perirectal areas were used to identify high-risk patients who were asymptomatically colonized with VRE. During the latter 2 years, 768 new cases of VRE colonization were detected among 69,672 admissions (1.1% of admissions), of which 730 (95.1%) were identified by active surveillance methods. This implies that routine clinical cultures would probably have missed the majority of colonized patients. During this period, the incidence of VRE infection was likewise extremely low at 0.12/1000 patient days (ie, 90 nosocomial VRE infections were identified in 83 patients during 743,956 days of patient care). Sixty-nine of the 83 patients (83%) who developed nosocomial VRE infections were found to be colonized with VRE by surveillance culture before the onset of infection.
Patient Decolonization
Chlorhexidine gluconate has been used in several settings to control outbreaks and infections related to MRSA and VRE due to its broad-spectrum activity against these pathogens. Chlorhexidine-based solutions reduce the density of skin colonization with pathogens such as MRSA and VRE (skin asepsis), thus lowering the risk for horizontal transmission between health care workers and patients.
Decolonization with chlorhexidine as an MRSA infection reduction technique has demonstrated benefit in the ICU setting [150]. The previously mentioned large, cluster-randomized ICU trial by Huang and colleagues found universal decolonization with twice-daily intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the entire ICU stay was superior to targeted decolonization of known MRSA carriers in preventing overall MRSA isolates. However, universal decolonization failed to show a reduction in MRSA bacteremia [151], and concerns about mupirocin resistance may limit the applicability of this approach.
There are now several studies [152–154] that show decreased acquisition of VRE with use of daily chlorhexidine bathing. In a study including 1787 ICU patients, Vernon et al found [154] that the reducing microbial density of VRE on patient’s skin by using chlorhexidine led to decreased transmission. In another study by Climo et al [153] that involved 6 ICUs at 4 academic centers and measured the incidence of MRSA and VRE colonization and blood stream infections (BSI) during a period of bathing with routine soap for 6 months compared with a 6-month period where all admitted patients received daily bathing with a chlorhexidine solution, results found decreased acquisition of VRE by 50% (4.35 vs. 2.19 cases/1000 patient days, P < 0.008) following the introduction of daily chlorhexidine bathing. Furthermore, compared with 16 of 270 patients colonized with VRE who subsequently developed VRE bacteremia at baseline, only 4 of 226 VRE-colonized patients bathed with chlorhexidine in the intervention period developed a BSI, translating into a relative risk reduction of 3.35 (95% CI, 1.13–9.87; P < 0.035). Patients colonized with VRE were 3 times less likely to develop VRE bacteremia when bathed with chlorhexidine compared with regular bathing. Despite the success of this protocol for VRE, when analyzed by individual organism no significant reductions in MRSA acquisition or BSI were reported. This finding is similarly corroborated by a trial conducted in the pediatric ICU setting which found an overall reduction in bacteremia with daily chlorhexidine washes but no significant decrease in cases due to S. aureus [155].
The results of these studies suggest that daily bathing with chlorhexidine should be part of routine practice in health care, especially in ICUs where endemic MRSA or VRE rates are high. Whether there is benefit in other settings needs to be studied.
In addition to chlorhexidine washes, other decolonization techniques have been proposed to reduce colonization and the spread of HAIs in the ICU setting. A randomized controlled trial of daily 5% tea tree oil body washes for the prevention of MRSA colonization failed to significantly reduce rates compared to standard soap body washes [156]. Another proposed decolonization intervention that has not been widely adopted in the United States due to concerns related to development of resistant organisms is selective digestive decontamination (SDD) or selective oropharyngeal decontamination (SOD) with antimicrobial agents [157,158]. In terms of clinical benefit, SDD/SOD have been found to decrease MDRO infection rate [159] and mortality [160].
Cohorting
There is insufficient evidence to conclude that cohorting isolated patients is of benefit for routine use in the endemic ICU setting. A few studies, mainly in the outbreak setting, have examined this approach and the results are conflicting [161,162]. Pending further studies in this area, it is reasonable to cohort patients colonized with the same microorganisms, especially if patients cannot be placed in single rooms.
CONCLUSION
The emergence of MRSA and VRE has led to a resurgence of interest and emphasis on infection control practices and prevention. CDC guidelines to help health care practitioners manage these MDROs in the hospital and ICU-setting exist; however, many questions remain regarding best practice.
Prevention of MRSA and VRE needs to be a 2-pronged approach—antimicrobial stewardship [163] and infection control. A robust antimicrobial stewardship program to optimize and minimize inappropriate antibiotic use is necessary in every institution. From the infection prevention standpoint, it is unclear if systematic identification of MRSA and VRE colonization followed by contact precautions is useful in reducing transmission. It is clear that a strong institutional climate of promoting patient safety and a culture of infection prevention will help in reducing MRSA and VRE facility-wide. It also appears that universal gowning and gloving may be useful for reducing MRSA, but not VRE, transmission. While universal decolonization with mupirocin is efficacious in reducing MRSA, this strategy is not recommended because of promoting mupirocin resistance. However, the use of daily bathing with chlorhexidine represents a relatively low-cost, high-yield intervention that should be adopted. Pending further data, patients known to be colonized or infected with MRSA should be placed in contact precuations as is current practice in most institutions. Finally, in this era of MDROs, hand hygiene remains our best defense against the spread of pathogens in the health care environment.
Note: This article does not represent the views of the Department of Veterans Affairs.
Corresponding author: Nasia Safdar, MD, Willam S. Middleton Memorial Veterans Affairs Hospital, 2500 Overlook Terrace, Madison, WI 53705, ns2@medicine.wisc.edu.
Funding/support: This work is funded by a MERIT award from the Department of Veterans Affairs to Nasia Safdar.
Financial disclosures: None.
REFERENCES
1. Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA 2009;301:727–36.
2. LeDell K, Muto CA, Jarvis WR, Farr BM. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol 2003;24:639–41.
3. Giske CG, Monnet DL, Cars O, Carmeli Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 2008;52:813–21.
4. Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Pub Health Rep 2007;122:160–6.
5. Schwaber MJ, Carmeli Y. The effect of antimicrobial resistance on patient outcomes: importance of proper evaluation of appropriate therapy. Crit Care 2009;13:106.
6. Grundmann H, Hori S, Winter B, et al. Risk factors for the transmission of methicillin-resistant Staphylococcus aureus in an adult intensive care unit: fitting a model to the data. J Inf Dis 2002;185:481–8.
7. Huang SS, Rifas-Shiman SL, Warren DK, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis 2007;195:330–8.
8. Muder RR, Cunningham C, McCray E, et al. Implementation of an industrial systems-engineering approach to reduce the incidence of methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2008;29:702–8.
9. Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008;29:996–1011.
10. Pelz RK, Lipsett PA, Swoboda SM, et al. Vancomycin-sensitive and vancomycin-resistant enterococcal infections in the ICU: attributable costs and outcomes. Intensive Care Med 2002;28:692–7.
11. Warren DK, Kollef MH, Seiler SM, et al. The epidemiology of vancomycin-resistant Enterococcus colonization in a medical intensive care unit. Infect Control Hosp Epidemiol 2003;24:257–63.
12. Kohlenberg A, Schwab F, Meyer E, et al. Regional trends in multidrug-resistant infections in German intensive care units: a real-time model for epidemiological monitoring and analysis. J Hosp Infect 2009;73:239–45.
13. Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol 2008;8:747–63.
14. Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008;46 Suppl 5:S350–9.
15. Zhang K, McClure JA, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009;53:531–40.
16. Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol 2013;5:205–17.
17. Otto M. MRSA virulence and spread. Cell Microbiol 2012;14:1513–21.
18. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978–84.
19. Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 2009;106:5883–8.
20. Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis 2006;42 Suppl 1:S25–34.
21. Gold HS. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis 2001;33:210–9.
22. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012;10:266–78.
23. Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis 1998;178:577–80.
24. Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 2004;113:e347–52.
25. Groom AV, Wolsey DH, Naimi TS, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 2001;286:1201–5.
26. Hewlett AL, Falk PS, Hughes KS, Mayhall CG. Epidemiology of methicillin-resistant Staphylococcus aureus in a university medical center day care facility. Infect Control Hosp Epidemiol 2009;30:985–92.
27. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005;352:468–75.
28. Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA 2012;308:50–9.
29. Lindenmayer JM, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med 1998;158:895–9.
30. Malcolm B. The rise of methicillin-resistant staphylococcus aureus in U.S. correctional populations. J Correct Health Care 2011;17:254–65.
31. Nerby JM, Gorwitz R, Lesher L, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2011;30:927–32.
32. Stemper ME, Shukla SK, Reed KD. Emergence and spread of community-associated methicillin-resistant Staphylococcus aureus in rural Wisconsin, 1989 to 1999. J Clin Microbiol 2004;42:5673–80.
33. Turabelidze G, Lin M, Wolkoff B, et al. Personal hygiene and methicillin-resistant Staphylococcus aureus infection. Emerg Infect Dis 2006;12:422–7.
34. Ellis MW, Hospenthal DR, Dooley DP, et al. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004;39:971–9.
35. Forster AJ, Oake N, Roth V, et al. Patient-level factors associated with methicillin-resistant Staphylococcus aureus carriage at hospital admission: a systematic review. Am J Infect Control 2013;41:214–20.
36. McKinnell JA, Miller LG, Eells SJ, et al. A systematic literature review and meta-analysis of factors associated with methicillin-resistant staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Contol Hosp Epidemiol 2013;34:1077–86.
37. Furuno JP, McGregor JC, Harris AD, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med 2006;166:580–5.
38. Jernigan JA, Pullen AL, Flowers L, et al. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol 2003;24:409–14.
39. Horner C, Parnell P, Hall D, Kearns A, Heritage J, Wilcox M. Meticillin-resistant Staphylococcus aureus in elderly residents of care homes: colonization rates and molecular epidemiology. J Hosp Infect 2013;83:212–8.
40. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 2009;73:305–15.
41. Boyce JM. Methicillin-resistant Staphylococcus aureus. Detection, epidemiology, and control measures. Infect Dis Clin North Am 1989;3:901–13.
42. Jernigan JA. Methicillin-resistant Staphylococcus aureus colonization among health care personnel in the emergency department: what does it tell us? Ann Emerg Med 2008;52:534–6.
43. Carnicer-Pont D, Bailey KA, Mason BW, Walker AM, Evans MR, Salmon RL. Risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus bacteraemia: a case-control study. Epidemiol Infect 2006;134:1167–73.
44. Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 2002;49:999–1005.
45. Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med 1982;97:309–17.
46. Davis KA, Stewart JJ, Crouch HK,et al. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 2004;39:776–82.
47. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997;10:505–20.
48. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med 2008;121:310–5.
49. Wertheim HFL, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703–5.
50. Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol 2001;22:687–92.
51. Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol;31:584–91.
52. von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001;344:11–6.
53. Pujol M, Pea C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am J Med 1996;100:509–16.
54. Rocha LA, Marques Ribas R, da Costa Darini AL, Gontijo Filho PP. Relationship between nasal colonization and ventilator-associated pneumonia and the role of the environment in transmission of Staphylococcus aureus in intensive care units. Am J Infect Control 2013;41:236–40.
55. Corne P, Marchandin Hln, Jonquet O, Campos J, Bauls A-L. Molecular evidence that nasal carriage of Staphylococcus aureus plays a role in respiratory tract infections of critically ill patients. J Clin Microbiol 2005;43:3491–3.
56. Quezada Joaquin NM, Diekema DJ, Perencevich EN, et al. Long-term risk for readmission, methicillin-resistant Staphylococcus aureus (MRSA) infection, and death among MRSA-colonized veterans. Antimicrob Agents Chemother 2013;57:1169–72.
57. Lin MY, Hayden MK. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus: recognition and prevention in intensive care units. Crit Care Med 2010;38:S335–44.
58. Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis 2002;8:802–7.
59. Ostrowsky BE, Venkataraman L, D’Agata EM, et al. Vancomycin-resistant enterococci in intensive care units: high frequency of stool carriage during a non-outbreak period. Arch Intern Med 1999;159:1467–72.
60. Bonten MJ, Hayden MK, Nathan C, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet 1996;348:1615–9.
61. Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med 2001;344:1427–33.
62. Padiglione AA, Wolfe R, Grabsch EA, et al. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob Agents Chemother 2003;47:2492–8.
63. Batistao DW, Gontijo-Filho PP, Conceicao N, et al. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz 2012;107:57–63.
64. Furtado GH, Martins ST, Coutinho AP, et al. Prevalence and factors associated with rectal vancomycin-resistant enterococci colonization in two intensive care units in Sao Paulo, Brazil. Braz J Infect Dis 2005;9:64–9.
65. Huang SS, Datta R, Rifas-Shiman S, et al. Colonization with antibiotic-susceptible strains protects against methicillin-resistant Staphylococcus aureus but not vancomycin-resistant enterococci acquisition: a nested case-control study. Crit Care 2011;15:R210.
66. Pan SC, Wang JT, Chen YC, et al. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS One 2012;7:e47297.
67. Se YB, Chun HJ, Yi HJ, et al. Incidence and risk factors of infection caused by vancomycin-resistant enterococcus colonization in neurosurgical intensive care unit patients. J Korean Neurosurg Soc 2009;46:123–9.
68. Healthcare Infection Control Practices Advisory Committee (HICPAC). Management of multidrug-resistant organisms in healthcare settings, 2006. Accessed 11 Oct 2013 at www.cdc.gov/hicpac/mdro/mdro_toc.html.
69. Malani PN. Preventing infections in the ICU: one size does not fit all. JAMA 2013;310:1567–8.
70. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 1995;44:1–13.
71. de Bruin MA, Riley LW. Does vancomycin prescribing intervention affect vancomycin-resistant enterococcus infection and colonization in hospitals? A systematic review. BMC Infect Dis 2007;7:24.
72. Adachi W, Bolding F, Armstrong R. Experience with vancomycin education and order sheet to limit vancomycin use. Hosp Pharm 1997:1370–3.
73. Fridkin SK, Lawton R, Edwards JR, et al. Monitoring antimicrobial use and resistance: comparison with a national benchmark on reducing vancomycin use and vancomycin-resistant enterococci. Emerg Infect Dis 2002;8:702–7.
74. Guglielmo BJ, Dudas V, Maewal I, et al. Impact of a series of interventions in vancomycin prescribing on use and prevalence of vancomycin-resistant enterococci. Jt Comm J Qual Patient Saf 2005;31:469–75.
75. Lautenbach E, LaRosa LA, Marr AM, et al. Changes in the prevalence of vancomycin-resistant enterococci in response to antimicrobial formulary interventions: impact of progressive restrictions on use of vancomycin and third-generation cephalosporins. Clin Infect Dis 2003;36:440–6.
76. Morgan AS, Brennan PJ, Fishman NO. Impact of a vancomycin restriction policy on use and cost of vancomycin and incidence of vancomycin-resistant Enterococcus. Ann Pharmacother 1997;31:970–3.
77. Anglim AM, Klym B, Byers KE, et al. Effect of a vancomycin restriction policy on ordering practices during an outbreak of vancomycin-resistant Enterococcus faecium. Arch Intern Med 1997;157:1132–6.
78. Montecalvo MA, Jarvis WR, Uman J, et al. Infection-control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med 1999;131:269–72.
79. Morris JG Jr, Shay DK, Hebden JN, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med 1995;123:250–9.
80. Quale J, Landman D, Saurina G, et al. Manipulation of a hospital antimicrobial formulary to control an outbreak of vancomycin-resistant enterococci. Clin Infect Dis 1996;23:1020-5.
81. Rubin LG, Tucci V, Cercenado E, et al. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect Control Hosp Epidemiol 1992;13:700–5.
82. Lai KK, Kelley AL, Melvin ZS, et al. Failure to eradicate vancomycin-resistant enterococci in a university hospital and the cost of barrier precautions. Infect Control Hosp Epidemiol 1998;19:647–52.
83. Shaikh ZH, Osting CA, Hanna HA, et al. Effectiveness of a multifaceted infection control policy in reducing vancomycin usage and vancomycin-resistant enterococci at a tertiary care cancer centre. J Hosp Infect 2002;51:52–8.
84. Lafaurie M, Porcher R, Donay JL, et al. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother 2012;67:1010–5.
85. Parienti JJ, Cattoir V, Thibon P, et al. Hospital-wide modification of fluoroquinolone policy and meticillin-resistant Staphylococcus aureus rates: a 10-year interrupted time-series analysis. J Hosp Infect 2011;78:118–22.
86. Safdar N, Abad C. Educational interventions for prevention of healthcare-associated infection: a systematic review. Crit Care Med 2008;36:933–40.
87. Boyce JM. It is time for action: improving hand hygiene in hospitals. Ann Intern Med 1999;130:153–5.
88. Jackson M, Chiarello LA, Gaynes RP, Gerberding JL. Nurse staffing and healthcare-associated infections: proceedings from a working group meeting. J Nurs Adm 2002;32:314–22.
89. Kuzu N, Ozer F, Aydemir S, et al. Compliance with hand hygiene and glove use in a university-affiliated hospital. Infect Control Hosp Epidemiol 2005;26:312–5.
90. Larson E, Killien M. Factors influencing handwashing behavior of patient care personnel. Am J Infect Control 1982;10:93–9.
91. Larson E, Kretzer EK. Compliance with handwashing and barrier precautions. J Hosp Infect 1995;30 Suppl:88–106.
92. Naikoba S, Hayward A. The effectiveness of interventions aimed at increasing handwashing in healthcare workers - a systematic review. J Hosp Infect 2001;47:173–80.
93. Pittet D, Simon A, Hugonnet S, et al. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med 2004;141:1–8.
94. Trick WE, Vernon MO, Welbel SF, et al. Multicenter intervention program to increase adherence to hand hygiene recommendations and glove use and to reduce the incidence of antimicrobial resistance. Infect Control Hosp Epidemiol 2007;28:42–9.
95. Wisniewski MF, Kim S, Trick WE, et al. Effect of education on hand hygiene beliefs and practices: a 5-year program. Infect Control Hosp Epidemiol 2007;28:88–91.
96. Rupp ME, Fitzgerald T, Puumala S, et al. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect Control Hosp Epidemiol 2008;29:8–15.
97. Venkatesh AK, Lankford MG, Rooney DM, et al. Use of electronic alerts to enhance hand hygiene compliance and decrease transmission of vancomycin-resistant Enterococcus in a hematology unit. Am J Infect Control 2008;36:199–205.
98. Silvestri L, Petros AJ, Sarginson RE, et al. Handwashing in the intensive care unit: a big measure with modest effects. J Hosp Infect 2005;59:172–9.
99. Akyol A, Ulusoy H, Ozen I. Handwashing: a simple, economical and effective method for preventing nosocomial infections in intensive care units. J Hosp Infect 2006;62:395–405.
100. Simmons B, Bryant J, Neiman K, et al. The role of handwashing in prevention of endemic intensive care unit infections. Infect Control Hosp Epidemiol 1990;11:589–94.
101. Tenorio AR, Badri SM, Sahgal NB, et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin Infect Dis 2001;32:826–9.
102. Slaughter S, Hayden MK, Nathan C, et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med 1996;125:448–56.
103. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013;310:1571–80.
104. Dietze B, Rath A, Wendt C, Martiny H. Survival of MRSA on sterile goods packaging. J Hosp Infect 2001;49:255–61.
105. Hardy KJ, Oppenheim BA, Gossain S, et al. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect Control Hosp Epidemiol 2006;27:127–32.
106. Jawad A, Heritage J, Snelling AM, et al. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol 1996;34:2881–7.
107. Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol 2007;28:1142–7.
108. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997;18:622–7.
109. Sexton T, Clarke P, O’Neill E, et al. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect 2006;62:187–94.
110. Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet infect dis 2008;8:101–13.
111. Dancer SJ, White LF, Lamb J, et al. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC med 2009;7.
112. Rampling A, Wiseman S, Davis L, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect 2001;49:109–16.
113. Wilson APR, Smyth D, Moore G, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med 2011;39:651–8.
114. Hess AS, Shardell M, Johnson JK, et al. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria. Infection Control Hosp Epidemiol 2013;34:487–93.
115. Falk PS, Winnike J, Woodmansee C, et al. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol 2000;21:575–82.
116. Livornese LL Jr, Dias S, Samel C, et al. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med 1992;117:112–6.
117. Porwancher R, Sheth A, Remphrey S, et al. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium: possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemiol 1997;18:771–3.
118. Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000;343:1925–32.
119. Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol 2000;38:724–6.
120. Noskin GA, Bednarz P, Suriano T, et al. Persistent contamination of fabric-covered furniture by vancomycin-resistant enterococci: implications for upholstery selection in hospitals. Am J Infect Control 2000;28:311–3.
121. Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol 1995;16:577–81.
122. Smith TL, Iwen PC, Olson SB, Rupp ME. Environmental contamination with vancomycin-resistant enterococci in an outpatient setting. Infect Control Hosp Epidemiol 1998;19:515–8.
123. Wendt C, Wiesenthal B, Dietz E, Ruden H. Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. J Clin Microbiol 1998;36:3734–6.
124. Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol 2004;25:164–7.
125. Ray AJ, Hoyen CK, Taub TF, et al. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA 2002;287:1400–1.
126. Hayden MK, Bonten MJ, Blom DW, et al. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 2006;42:1552–60.
127. Byers KE, Durbin LJ, Simonton BM, et al. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol 1998;19:261–4.
128. Goodman ER, Platt R, Bass R, et al. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol 2008;29:593–9.
129. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009;73:378–85.
130. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA) infections. Accessed 11 Oct 2013 at www.cdc.gov/mrsa/index.html.
131. Edmond MB, Wenzel RP. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1471.
132. Lai KK, Fontecchio S, Melvin Z, Baker SP. Impact of alcohol-based, waterless hand antiseptic on the incidence of infection and colonization with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Infect Control Hosp Epidemiol 2006;27:1018–24.
133. Ostrowsky B, Steinberg JT, Farr B, et al. Reality check: should we try to detect and isolate vancomycin-resistant enterococci patients? Infect Control Hosp Epidemiol 2001;22:116–9.
134. Harbarth S, Sax H, Uckay I, et al. A predictive model for identifying surgical patients at risk of methicillin-resistant Staphylococcus aureus carriage on admission. J Am Coll Surg 2008;207:683–9.
135. Jarvis WR. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1469.
136. Krause R, Honigl M, Zollner-Schwetz I. Targeted decolonization to prevent ICU infections. N Engl J Med;369:1469–70.
137. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med;364:1407–18.
138. Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008;148:409–18.
139. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30.
140. Gurieva T, Bootsma MCJ, Bonten MJM. Successful Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections revisited. Clin Infect Dis 2012:54:1618–20.
141. Gavalda L, Masuet C, Beltran J, et al. Comparative cost of selective screening to prevent transmission of methicillin-resistant Staphylococcus aureus (MRSA), compared with the attributable costs of MRSA infection. Infection control and hospital epidemiology 2006;27:1264–6.
142. Otter JA, Herdman MT, Williams B, et al. Low prevalence of methicillin-resistant Staphylococcus aureus carriage at hospital admission: implications for risk-factor-based vs universal screening. J Hosp Infect 2013;83:114–21.
143. Harbarth S, Hawkey PM, Tenover F, et al. Update on screening and clinical diagnosis of methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 2011;37:110–7.
144. Calfee DP, Giannetta ET, Durbin LJ, et al. Control of endemic vancomycin-resistant Enterococcus among inpatients at a university hospital. Clin Infect Dis 2003;37:326–32.
145. Hendrix CW, Hammond JM, Swoboda SM, et al. Surveillance strategies and impact of vancomycin-resistant enterococcal colonization and infection in critically ill patients. Ann Surg 2001;233:259–65.
146. Muto CA, Giannetta ET, Durbin LJ, et al. Cost-effectiveness of perirectal surveillance cultures for controlling vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol 2002;23:429–35.
147. Price CS, Paule S, Noskin GA, Peterson LR. Active surveillance reduces the incidence of vancomycin-resistant enterococcal bacteremia. Clin Infect Dis 2003;37:921–8.
148. Shadel BN, Puzniak LA, Gillespie KN, et al. Surveillance for vancomycin-resistant enterococci: type, rates, costs, and implications. Infect Control Hosp Epidemiol 2006;27:1068–75.
149. Siddiqui AH, Harris AD, Hebden J, et al. The effect of active surveillance for vancomycin-resistant enterococci in high-risk units on vancomycin-resistant enterococci incidence hospital-wide. Am J Infect Control 2002;30:40–3.
150. Sandri AM, Dalarosa MG, Ruschel de Alcantara L, et al. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol 2006;27:185–7.
151. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255–65.
152. Bleasdale SC, Trick WE, Gonzalez IM, et al. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–9.
153. Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009;37:1858–65.
154. Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166:306–12.
155. Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 2013;381:1099–106.
156. Blackwood B, Thompson G, McMullan R, et al. Tea tree oil (5%) body wash versus standard care (Johnson’s Baby Softwash) to prevent colonization with methicillin-resistant Staphylococcus aureus in critically ill adults: a randomized controlled trial. J Antimicrob Chemother 2013;68:1193–9.
157. Daneman N, Sarwar S, Fowler RA, et al. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:328–41.
158. Verwaest C, Verhaegen J, Ferdinande P, et al. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med 1997;25:63–71.
159. de Smet AMGA, Kluytmans JAJW, Blok HEM, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis 2011;11:372–80.
160. de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003;362:1011–6.
161. Cepeda JA, Whitehouse T, Cooper B, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet 2005;365:295–304.
162. Dhaliwal J, McGeer A. Does isolation prevent the spread of methicillin-resistant Staphylococcus aureus? CMAJ 2005;172:875.
163. Kollef MH, Micek ST. Antimicrobial stewardship programs: mandatory for all ICUs. Crit Care 2012;16:179.
164. McKinnell JA, Huang SS, Eells SJ, et al. Quantifying the impact of extranasal testing of body sites for methicillin-resistant Staphylococcus aureus colonization at the time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 2013;34:161–70.
165. Denkinger CM, Grant AD, Denkinger M, et al. Increased multi-drug resistance among the elderly on admission to the hospital—a 12-year surveillance study. Arch Gerontol Geriatr 2013;56:227–30.
166. Boisseau D, Alfandari S, Gauzit R, et al. Staphylococcus aureus nasal carriage during the infectious diseases national congress in France. Med Mal Infect 2012;42:435–9.
167. Fritz SA, Hogan PG, Hayek G, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med 2012;166:551–7.
168. Rafee Y, Abdel-Haq N, Asmar B, et al. Increased prevalence of methicillin-resistant Staphylococcus aureus nasal colonization in household contacts of children with community acquired disease. BMC Infect Dis 2012;12:45.
169. Schechter-Perkins EM, Mitchell PM, Murray KA, et al. Prevalence and predictors of nasal and extranasal staphylococcal colonization in patients presenting to the emergency department. Ann Emerg Med 2011;57:492–9.
170. Bisaga A, Paquette K, Sabatini L, Lovell E. A prevalence study of methicillin-resistant staphylococcus aureus colonization in emergency department health care workers. Ann Emerg Med 2008;52:525–8.
171. Suffoletto B, Cannon E, Ilkhanipour K, Yealy D. Prevalence of Staphylococcus aureus nasal colonization in emergency department personnel. Ann Emerg Med 2008;52:529–33.
172. Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg 2004;139:947-51; discussion 51–3.
173. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis 2003;36:131–9.
From the Department of Medicine, Infectious Disease Practice and Innovations, The Medical City, Pasig City, Philippines (Dr. Abad), the Division of Emergency Medicine, University of Wisconsin Medical School, Madison, WI (Dr. Pulia), University of Wisconsin Hospital and Clinics, Madison, WI (Ms. Krupp), and the Willam S. Middleton Memorial Veterans Affairs Hospital, Madison, WI (Dr. Safdar).
Patients in intensive care units (ICUs) are at greatly increased risk of developing health care-associated infections (HAIs) [1]. More than 70% of the bacteria that cause HAIs are resistant to at least one of the antimicrobials commonly used to treat these infections [2]. Two such pathogens, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) are responsible for a considerable proportion of ICU infections that are associated with increased morbidity, mortality, and costs [3–5]. In this review, we discuss the epidemiology of colonization and infection by MRSA and VRE and provide an update on practices for prevention of transmission and infection by MRSA and VRE in the ICU.
EPIDEMIOLOGY AND MECHANISMS OF RESISTANCE
MRSA is the major cause of HAIs worldwide [6]. Among ICUs in the United States, the proportion of methicillin resistance among S. aureus isolates increased from 35.9% in 1992 to 64.4% in 2003 [4]. Approximately 8% of patients are colonized with MRSA upon admission, and an average of 5% will acquire MRSA colonization in the ICU [7,8]. A comparison study of academic tertiary care facilities found medical ICUs had higher MRSA admission prevalence rates than surgical ICUs, whereas surgical ICUs had a higher incidence rate [7]. Enteroccoccus is the third most common pathogen associated with HAIs, with 33% resistant to vancomycin [9]. VRE infection is associated with increased ICU cost and increased length of stay [10]. Incidence of ICU-acquired VRE varies among regions and countries. For example, in the United States, Warren et al [11] reported a VRE incidence of 27 cases per 1000 patient ICU days, whereas Kohlenberg et al [12] reported a mean incidence of 0.29 cases per 1000 patient ICU days in Germany.
Understanding the mechanisms that allow development of resistant strains of S. aureus and Enterococcus species is important to devise preventive strategies. Methicillin resistance in MRSA is determined by the staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element that carries the mecA gene. The mecA gene codes for an additional penicillin-binding protein (PBP) that has a reduced affinity towards methicillin (PBP2a/PBP2'). This results in a reduced ability to bind to the bacterial cell wall and inhibit synthesis [13,14]. Study of molecular epidemiology has identified MRSA as originating from 8 major variants of the mecA gene [15]. The majority of MRSA infections are caused by strains belonging to a few internationally disseminated clones [14]. The first identified strains were associated with infections in hospitalized patients (hospital-associated MRSA), but community-associated MRSA strains have since emerged and have become established globally, including in health care institutions [16].
Community-acquired MRSA can cause severe infections in health hosts [17]; possible explanations include increased CA-MRSA virulence due to the acquisition of mobile genetic elements, namely those containing Panton-Valentine leukocidin (PVL) [18] or increased expression of core genome-encoded virulence genes, such as phenol-soluble modulin (PSM) cytolysins, α-toxin, and other virulence determinants [19].
Enterococcus is intrinsically resistant to several antimicrobial drugs, with resistance to vancomycin encoded by several clusters of genes known as vancomycin resistance gene clusters (eg, vanA, vanB). The gene clusters generate resistance through multiple pathways which encode enzymes to determine the structure of peptidoglycan precursors [20,21]. Genetically diverse, hospital-associated VRE outbreaks have been associated with single clones, multiple clones, and changing molecular epidemiology over time [21]. While up to 25% of the VRE genome includes acquired elements, the majority of hospital-associated infections are traced to a few clonal complexes, which differ from community-associated VRE strains [22].
The evolution of these efficient mechanisms that promote drug resistance has made it extremely challenging to eradicate organisms such as MRSA and VRE. However, advances in recent years have furthered our understanding of the epidemiology, pathogenesis, and methods of prevention and containment.
RISK FACTORS FOR COLONIZATION AND INFECTION
MRSA
The risk factors underlying MRSA colonization and infection in the ICU setting can be categorized as either patient/host or environmental factors. A wide range of patient-level factors is associated with MRSA colonization upon admission. General principles regarding the transmission of MRSA in the community include close contact with colonized or infected individuals, breaks in the skin, crowded living conditions and poor hygiene. These factors, alone or in combination, are thought to underlie observed outbreaks among sports teams, military personnel, correction facilities, American Indian communities, and daycare centers [23–34].
Two recently published systematic reviews have summarized important patient-level factors associated with MRSA colonization at the time of hospital admission. Forster et al [35] examined 27 studies and identified previous admission to hospital, transfer from nursing home or long-term care facility, and previous antibiotic use as the top 3 factors associated with MRSA colonization. A similar review conducted by McKinnell and colleagues [36] found that prior hospitalization, nursing home contact, recent antibiotic use, and exposure to health care-associated pathogens (MRSA carriage, VRE carriage, or Clostridium difficile infection) were found to portend the highest risk. Specific comorbid conditions also conveyed an increased risk, including congestive heart failure, chronic wounds/bedsores, diabetes mellitus, pulmonary disease, immunosuppression, urinary catheter, and renal failure/dialysis. It is clear that health care contacts, especially recent hospitalization, residence in a long-term care facility, and antibiotic use, are significant risk factors for MRSA colonization [37–39].
 In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
Regardless of whether MRSA colonization precedes admission or occurs due to nosocomial spread, it is associated with increased risk of developing a HAI [46–49]. In 2 large prospective observational cohort studies, the hazard ratios of MRSA colonization developing into S. aureus infections during the ICU stay were 3.84 and 4.70, respectively [50,51]. High levels of concordance between MRSA colonization strains and HAI strains have also been reported [52]. Nasal colonization with S. aureus has also been identified as an independent risk factor for developing ventilator-associated pneumonia (VAP) and bacteremia [53,54]. A case series of ICU patients with S. aureus nasal colonization who developed lower respiratory tract infections demonstrated genetically identical nasal and bronchial strains in 15/16 cases [55]. This finding strongly suggests that nasopharyngeal colonization with S. aureus contaminates oral secretions that are aspirated by critically ill patients, resulting in subsequent pneumonia. In a long-term outcomes study among a matched cohort of veterans, MRSA colonization was associated with an increased risk of infection-related readmission and mortality [56]. These findings reflect the critically important nature of measures designed to curb nosocomial transmission and acquisition of MRSA, especially among the vulnerable ICU population.
VRE
As with MRSA, risk factors associated with VRE colonization include both patient-level and ICU-level (or environmental) factors [57]. Examples of patient-level factors include previous antimicrobial exposure [58–62], underlying medical illnesses such as chronic renal failure requiring hemodialysis [11,63], length of hospital or ICU stay [11,59,64,65], and recent exposure to health care facilities. ICU-level factors of relevance are the prevalence of VRE in the unit, with high levels of endemicity leading to higher risk of colonization and transmission.
Antibiotic use is a major risk factor for VRE acquisition, although the type and class of antibiotic varies considerably across studies; the most frequently identified antibiotics are broad-spectrum cephalosporins, vancomycin, and anti-anaerobic agents [58,62,64]. Patients with chronic liver disease and post-transplantation are at exceedingly high risk for VRE acquisition [59]. In a recent study by Pan [66], for example, the authors found that the incidence of newly acquired VRE was 21.9 per 1000 patient-days in an ICU setting. On multivariate analysis, the authors found that, similar to other reports [11,59,67], length of stay in the ICU was associated with increased risk of VRE acquisition, with each additional day of stay increasing risk of VRE by 1.03 times. Warren et al undertook a prospective cohort study involving 519 patients admitted to the ICU for more than 48 hours [11]. Seventy-four (21%) of 352 patients were subsequently colonized with VRE. The median time to development of a positive VRE culture after ICU admission was 6 days. Increased mean APACHE II score on ICU admission (P = 0.002), sucralfate use (P = 0.003), vasopressor use (P = 0.01), tracheostomy in the ICU (P = 0.02), and C. difficile diarrhea (P = 0.002) appeared to be associated with VRE acquisition.
 It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
REDUCING TRANSMISSION—MRSA AND VRE PREVENTION STRATEGIES
 Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Antimicrobial Stewardship
Antibiotic use is a major driver of antibiotic resistance. A meta-analysis by de Bruin and Riley [71] studied the effect of vancomycin usage on VRE colonization and infection. A total of 12 articles describing 13 studies were included; none were randomized controlled trials. All studies were quasi-experimental and lacked control groups. Among all studies, less than half (46%) implemented vancomycin reduction measures as the sole type of intervention [72–76]. The remaining studies implemented other infection control modalities and or restricted the use of other antimicrobials [77–83]. Although all studies that implemented vancomycin restriction alone as a single strategy showed a decline in vancomycin usage, only 2 of these [74,75] showed a relative risk reduction in VRE acquisition post-intervention. Also, studies that restricted vancomycin alone revealed a trend towards lower efficacy in reducing VRE colonization and infection (33%) when compared with those that used additional measures (71%). While judicious antibiotic use should always be practiced, the evidence for vancomycin restriction as a sole intervention to control VRE is scant. It may be that other antibiotics are as big or bigger drivers of resistance in enterococci than vancomycin. For example, a growing body of literature supports antibiotic restriction, especially fluoroquinolones, for reducing MRSA. In several time-series quasi-experimental studies, restriction of fluorquinolones was associated with reduced trends in MRSA infections in the acute care setting, and consideration should be given to monitor and optimize fluoroquinolone use in the ICU setting [84,85].
Antimicrobial stewardship programs are fundamental to optimizing antibiotic use in the ICU and the authors strongly recommend that all ICUs should have such a program in place.
Educational Interventions
Infection control and multidrug-resistant organism (MDRO)–specific education programs for health care workers is a core principle of the CDC’s prevention guidelines. The HICPAC VRE guideline also explicitly states “continuing education programs for hospital staff (including attending and consulting physicians, medical residents, and students; pharmacy, nursing, and laboratory personnel; and other direct patient-care providers) should include information concerning the epidemiology of VRE and the potential impact of this pathogen on the cost and outcome of patient care [70].” A systematic review published in 2008 [86] that included 26 studies showed that such interventions to prevent HCAIs are usually successful; in this review, 20 of 26 studies showed a statistically significant decrease in infection rates, with risk ratios ranging from 0 to 1.6. Education was usually part of a broader array of infection control interventions. While clearly essential, education alone is unlikely to have a sustained impact on reducing MRSA and VRE infections.
Infection Control Measures
Major infection control interventions include hand hygiene, the use of personal protective equipment (PPE), and cohorting. These measures can be grouped into “horizontal” (or global) vs. “vertical” (or targeted) strategies. Although not mutually exclusive, horizontal approaches are designed to have an impact on multiple pathogens (pathogen nonspecific), whereas vertical approaches are designed to reduce the impact of specific pathogens (such as VRE). For the purposes of this review, we will discuss both strategies for containment of MRSA and VRE. Horizontal strategies include hand hygiene, universal gloving and/or gowning, environmental cleaning, and daily bathing with chlorhexidine. Vertical strategies include screening for either MRSA or VRE followed by placement in contact precautions and decolonization with mupirocin.
Hand Hygiene
Hand washing is fundamental to reducing transmission of MDROs in health care institutions; however, optimal compliance is hard to achieve and sustain. Barriers to adherence may include unavailability of sinks or hand hygiene materials (eg, alcohol-based gels, gloves) time constraints, forgetfulness, or lack of knowledge [87–95]. Several monitoring strategies have been evaluated to increase compliance with hand hygiene. Most involve direct observation followed by performance assessment and feedback.
Trials examining the impact of improvements in hand hygiene compliance on HAIs in the ICU setting have largely found benefit, although not all studies showed a decline in HAI. In a prospective crossover trial, Rupp et al [96] found dramatic improvements in compliance with hand gel availability, but this did not translate to decreased nosocomial MRSA infections. Venkatesh et al [97] carried out a before-and-after interventional prospective study in a hematology unit in a tertiary level hospital to evaluate the use of an electronic method of surveillance to determine compliance with hand hygiene. The authors also used rates of horizontal transmission of VRE as a secondary end-point. Results of the study showed that hand hygiene compliance improved from 36.3% at baseline to 70.1%. This represented an OR of 4.1 (95% confidence interval, 3.7–4.5), which the authors attributed to the use of automated alerts. VRE transmission rates before and during intervention were not statistically different, but the rates of infection were lower at 1.0 per month in comparison with 4.7 infections per month in the preceding 6 months (P = 0.096).
While improved hand hygiene may result in significant reductions in HAIs [40], research indicates hand hygiene alone influences about 40% of infections in the ICU setting [98]. As such, hand hygiene should be viewed as a necessary component of a comprehensive infection control program [99]. Despite the success of hand hygiene in reducing HAIs in the ICU, effective strategies to improve compliance remain elusive even under study conditions and further research is needed in this area [100].
Personal Protective Equipment
Tenorio et al [101] conducted a study to assess the effectiveness of gloving in the prevention of hand carriage of VRE by health care workers. The study showed that among 50 health care workers who had contact with patients colonized with VRE, 6 carried a similar patient strain even prior to known contact, and 17 of 44 (69%) had a patient-related VRE strain on their gloves after contact. This suggests a relatively high rate of colonization after usual patient-care contact. Factors associated with acquisition of VRE on gloves included duration of contact, contact with a patient’s body fluids, presence of diarrhea in a patient, mean VRE colony counts on a patient’s skin, and number of body sites colonized with VRE. Although gloves reduced the risk of VRE acquisition of VRE by 71% (ie, 12/17 did not have VRE on their hands after de-gloving) the protection afforded by gloves was incomplete. As such, hand hygiene after glove removal is recommended.
Slaughter et al [102] compared the use of personal protective equipment in the acquisition of VRE. During this study, 93 patients in glove-and-gown rooms and 88 patients in glove-only rooms had similar rates of VRE at baseline entry into the ICU and after the intervention. Mean times to colonization among the patients who became colonized were 8.0 days in the glove-and-gown group and 7.1 days in the glove-only group. None of these comparisons were statistically significant and the authors concluded that the universal use of gown and gloves was no better than the use of gloves alone in preventing VRE colonization.
A recent cluster randomized trial compared the effect of universal PPE (ie, gowning and gloving) with usual care for reducing acquisition of MRSA or VRE as a composite outcome [103]. The study did not find that universal gowning and gloving reduced VRE or MRSA acquisition but found a 40% decline in MRSA acquisition in the intervention ICUs compared with baseline rates of MRSA. No major adverse effects of universal gowning and gloving were noted in this study. A thoughtful editorial commenting on this article proposes that several aspects of the study deserve consideration, including the possibility of false-negative screening tests for VRE, which may have partially accounted for the negative primary outcome [69].
Based on these studies, it appears that the use of barrier precautions may be of value more for MRSA than VRE but further studies are needed to examine its impact on other types of pathogens, including new and emerging MDROs. Until further evidence becomes available, routine gowning and gloving may be of value in units with a high prevalence of MRSA.
Environmental Cleaning
Accumulating data suggests that the environment may play a major role in transmission of pathogens. MRSA has the ability to survive for days to weeks on inanimate objects [104–107]. Environmental contamination results in contamination of staff clothing and gloves [107,108] and is highly correlated with colonization strains among inpatients [109]. Although some studies of enhanced cleaning techniques and increased environmental services staff time have demonstrated reductions in MRSA outbreaks [110–112], the results are not universally favorable [113,114] and further studies are needed to examine the impact of environmental cleaning on rates of MRSA colonization or infection.
Several studies have implicated contaminated equipment as vectors for transmission of VRE during outbreaks [115–117], but the direction of fomite transfer from patient to environment has been difficult to ascertain. VRE have been found frequently on a variety of inanimate objects and surfaces in different health care environments [118–123], including gloved or ungloved hands of health care workers [101,124,125]. Hayden et al [126] determined the effect of improved environmental cleaning on VRE acquisition rates. This study was a pre-and-post intervention study carried out in a 21-bed medical intensive care unit (MICU) in a tertiary hospital over several phases. The intervention included the creation of a unique and improved cleaning program, as well as in-services to housekeeper services, education of the MICU staff, and a hand hygiene campaign. The results of the study showed decreased acquisition of VRE from 33.47 cases per 1000 patient days at risk in period 1 to 10.40 cases per 1000 patient-days at risk by period 4 of the study. Increased environmental cleaning was also associated with reduced growth of VRE from environmental cultures. At baseline, weekly contamination rates were 0.15 and 0.1 for samples obtained before and after cleaning, respectively. Culture positivity decreased to 0.07 and 0.04 for before and after cleaning in period 2 and then remained at low levels during the remainder of the study. It is important to note that the method for disinfecting used in this study was the “bucket method” as promoted by Byers [127]. This study provides further support for the importance of an environmental reservoir and of environmental decontamination to prevent endemic cross-transmission of VRE [126].
Goodman et al [128] used similar interventions but added a feedback tool using a black-light monitoring system (ie, use of an invisible, nontoxic marker to delineate areas that are adequately or inadequately cleaned) to reduce the likelihood of isolating either MRSA or VRE from an ICU environment. This study also showed favorable results, and notably, the use of the black-light monitoring system identified specific areas that were typically inadequately disinfected. Results showed that flat, horizontal surfaces (eg, countertops, bedside tray tables, and hamper tops) were adequately cleaned more often than small, vertical surfaces (eg, doorknobs, toilet handles, light switches, and electronics).
Part of the controversy surrounding the impact of environmental cleaning is the difficulty in determining its individual value as part of an overall infection control bundle [129]. A proposed area of demonstrable impact for environmental cleaning are frequently touched sites which are more likely to be contaminated with pathogens. Focusing on these “hot-bed” areas of the care environment may offer a useful adjunct to other infection control measures [129].
Active Surveillance
Active surveillance refers to periodic screening for asymp-tomatic carriers followed by placement of colonized patients in contact isolation. This practice is highly variable across institutions, as the evidence supporting this practice is conflicting and there are concerns about the cost of implementing this approach without solid evidence [70,130,131]. Despite lack of randomized controlled trials to guide this practice for MRSA prevention, many hospitals utilize MRSA surveillance and it is mandated by law in 9 states [132,133].
A prospective, interventional cohort study of universal MRSA screening on admission to surgical wards failed to reduce nosocomial MRSA infections [134]. Most recently, a pragmatic, cluster-randomized ICU trial reported that universal decolonization with chlorhexidine wipes and mupirocin use was more effective than screening and isolation in reducing rates of MRSA clinical isolates [65]. However, concerns regarding the risk of mupirocin resistance have been expressed [135,136]. The only randomized trial that compared active surveillance for MRSA and VRE followed by contact precautions to usual care did not find a benefit to active surveillance.
Huskins et al [137], in a large, cluster-randomized trial of 19 ICUs from different hospitals, determined the utility of using a culture-based active surveillance and contact isolation, compared with usual care (contact isolation for patients colonized with MRSA or VRE) as identified by existing hospital protocols, to reduce the incidence of colonization or infection with MRSA or VRE. In this trial, which spanned 6 months and involved 3488 participants, the authors found no significant difference between the intervention and control ICUs in terms of MRSA and VRE colonization or infection rates.
Conflicting with these findings is an observational study comparing MRSA infection rates before and after institution of a universal screening protocol, which demonstrated a 69.6% (CI, –89.2% to –19.6%]; P = 0.03) reduction in hospital wide MRSA prevalence density with screening [138]. The “MRSA bundle” implemented in 2007 at VA hospitals nationwide, which included universal screening, produced a 62% (P < 0.001) reduction in MRSA ICU infections; the relative contribution of the various bundle components is uncertain [139,140].
A proposed cost-saving alternative to universal screening is selective screening based on risk factor assessment [141]. The effectiveness of this type of program depends on creating a clinical decision-making tool capable of accurately identifying high-risk individuals while also accounting for the different risk factor profiles between HA-MRSA and CA-MRSA [142]. It has been proposed that targeted screening protocols may be more cost-effective in settings with < 5% prevalence of MRSA colonization on admission [143].
Many studies [61,144–149] have shown that active surveillance against VRE is cost-effective. For example, Calfee et al [144] showed that an established active surveillance program results in control of endemic VRE in high-risk patients. The infection control program was established in response to a hospital-wide VRE outbreak, and was sustained after the outbreak was controlled. The study by Calfee et al spanned 5 years and was performed at a tertiary-level university hospital, where cultures from perirectal areas were used to identify high-risk patients who were asymptomatically colonized with VRE. During the latter 2 years, 768 new cases of VRE colonization were detected among 69,672 admissions (1.1% of admissions), of which 730 (95.1%) were identified by active surveillance methods. This implies that routine clinical cultures would probably have missed the majority of colonized patients. During this period, the incidence of VRE infection was likewise extremely low at 0.12/1000 patient days (ie, 90 nosocomial VRE infections were identified in 83 patients during 743,956 days of patient care). Sixty-nine of the 83 patients (83%) who developed nosocomial VRE infections were found to be colonized with VRE by surveillance culture before the onset of infection.
Patient Decolonization
Chlorhexidine gluconate has been used in several settings to control outbreaks and infections related to MRSA and VRE due to its broad-spectrum activity against these pathogens. Chlorhexidine-based solutions reduce the density of skin colonization with pathogens such as MRSA and VRE (skin asepsis), thus lowering the risk for horizontal transmission between health care workers and patients.
Decolonization with chlorhexidine as an MRSA infection reduction technique has demonstrated benefit in the ICU setting [150]. The previously mentioned large, cluster-randomized ICU trial by Huang and colleagues found universal decolonization with twice-daily intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the entire ICU stay was superior to targeted decolonization of known MRSA carriers in preventing overall MRSA isolates. However, universal decolonization failed to show a reduction in MRSA bacteremia [151], and concerns about mupirocin resistance may limit the applicability of this approach.
There are now several studies [152–154] that show decreased acquisition of VRE with use of daily chlorhexidine bathing. In a study including 1787 ICU patients, Vernon et al found [154] that the reducing microbial density of VRE on patient’s skin by using chlorhexidine led to decreased transmission. In another study by Climo et al [153] that involved 6 ICUs at 4 academic centers and measured the incidence of MRSA and VRE colonization and blood stream infections (BSI) during a period of bathing with routine soap for 6 months compared with a 6-month period where all admitted patients received daily bathing with a chlorhexidine solution, results found decreased acquisition of VRE by 50% (4.35 vs. 2.19 cases/1000 patient days, P < 0.008) following the introduction of daily chlorhexidine bathing. Furthermore, compared with 16 of 270 patients colonized with VRE who subsequently developed VRE bacteremia at baseline, only 4 of 226 VRE-colonized patients bathed with chlorhexidine in the intervention period developed a BSI, translating into a relative risk reduction of 3.35 (95% CI, 1.13–9.87; P < 0.035). Patients colonized with VRE were 3 times less likely to develop VRE bacteremia when bathed with chlorhexidine compared with regular bathing. Despite the success of this protocol for VRE, when analyzed by individual organism no significant reductions in MRSA acquisition or BSI were reported. This finding is similarly corroborated by a trial conducted in the pediatric ICU setting which found an overall reduction in bacteremia with daily chlorhexidine washes but no significant decrease in cases due to S. aureus [155].
The results of these studies suggest that daily bathing with chlorhexidine should be part of routine practice in health care, especially in ICUs where endemic MRSA or VRE rates are high. Whether there is benefit in other settings needs to be studied.
In addition to chlorhexidine washes, other decolonization techniques have been proposed to reduce colonization and the spread of HAIs in the ICU setting. A randomized controlled trial of daily 5% tea tree oil body washes for the prevention of MRSA colonization failed to significantly reduce rates compared to standard soap body washes [156]. Another proposed decolonization intervention that has not been widely adopted in the United States due to concerns related to development of resistant organisms is selective digestive decontamination (SDD) or selective oropharyngeal decontamination (SOD) with antimicrobial agents [157,158]. In terms of clinical benefit, SDD/SOD have been found to decrease MDRO infection rate [159] and mortality [160].
Cohorting
There is insufficient evidence to conclude that cohorting isolated patients is of benefit for routine use in the endemic ICU setting. A few studies, mainly in the outbreak setting, have examined this approach and the results are conflicting [161,162]. Pending further studies in this area, it is reasonable to cohort patients colonized with the same microorganisms, especially if patients cannot be placed in single rooms.
CONCLUSION
The emergence of MRSA and VRE has led to a resurgence of interest and emphasis on infection control practices and prevention. CDC guidelines to help health care practitioners manage these MDROs in the hospital and ICU-setting exist; however, many questions remain regarding best practice.
Prevention of MRSA and VRE needs to be a 2-pronged approach—antimicrobial stewardship [163] and infection control. A robust antimicrobial stewardship program to optimize and minimize inappropriate antibiotic use is necessary in every institution. From the infection prevention standpoint, it is unclear if systematic identification of MRSA and VRE colonization followed by contact precautions is useful in reducing transmission. It is clear that a strong institutional climate of promoting patient safety and a culture of infection prevention will help in reducing MRSA and VRE facility-wide. It also appears that universal gowning and gloving may be useful for reducing MRSA, but not VRE, transmission. While universal decolonization with mupirocin is efficacious in reducing MRSA, this strategy is not recommended because of promoting mupirocin resistance. However, the use of daily bathing with chlorhexidine represents a relatively low-cost, high-yield intervention that should be adopted. Pending further data, patients known to be colonized or infected with MRSA should be placed in contact precuations as is current practice in most institutions. Finally, in this era of MDROs, hand hygiene remains our best defense against the spread of pathogens in the health care environment.
Note: This article does not represent the views of the Department of Veterans Affairs.
Corresponding author: Nasia Safdar, MD, Willam S. Middleton Memorial Veterans Affairs Hospital, 2500 Overlook Terrace, Madison, WI 53705, ns2@medicine.wisc.edu.
Funding/support: This work is funded by a MERIT award from the Department of Veterans Affairs to Nasia Safdar.
Financial disclosures: None.
REFERENCES
1. Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA 2009;301:727–36.
2. LeDell K, Muto CA, Jarvis WR, Farr BM. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol 2003;24:639–41.
3. Giske CG, Monnet DL, Cars O, Carmeli Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 2008;52:813–21.
4. Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Pub Health Rep 2007;122:160–6.
5. Schwaber MJ, Carmeli Y. The effect of antimicrobial resistance on patient outcomes: importance of proper evaluation of appropriate therapy. Crit Care 2009;13:106.
6. Grundmann H, Hori S, Winter B, et al. Risk factors for the transmission of methicillin-resistant Staphylococcus aureus in an adult intensive care unit: fitting a model to the data. J Inf Dis 2002;185:481–8.
7. Huang SS, Rifas-Shiman SL, Warren DK, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis 2007;195:330–8.
8. Muder RR, Cunningham C, McCray E, et al. Implementation of an industrial systems-engineering approach to reduce the incidence of methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2008;29:702–8.
9. Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008;29:996–1011.
10. Pelz RK, Lipsett PA, Swoboda SM, et al. Vancomycin-sensitive and vancomycin-resistant enterococcal infections in the ICU: attributable costs and outcomes. Intensive Care Med 2002;28:692–7.
11. Warren DK, Kollef MH, Seiler SM, et al. The epidemiology of vancomycin-resistant Enterococcus colonization in a medical intensive care unit. Infect Control Hosp Epidemiol 2003;24:257–63.
12. Kohlenberg A, Schwab F, Meyer E, et al. Regional trends in multidrug-resistant infections in German intensive care units: a real-time model for epidemiological monitoring and analysis. J Hosp Infect 2009;73:239–45.
13. Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol 2008;8:747–63.
14. Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008;46 Suppl 5:S350–9.
15. Zhang K, McClure JA, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009;53:531–40.
16. Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol 2013;5:205–17.
17. Otto M. MRSA virulence and spread. Cell Microbiol 2012;14:1513–21.
18. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978–84.
19. Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 2009;106:5883–8.
20. Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis 2006;42 Suppl 1:S25–34.
21. Gold HS. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis 2001;33:210–9.
22. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012;10:266–78.
23. Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis 1998;178:577–80.
24. Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 2004;113:e347–52.
25. Groom AV, Wolsey DH, Naimi TS, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 2001;286:1201–5.
26. Hewlett AL, Falk PS, Hughes KS, Mayhall CG. Epidemiology of methicillin-resistant Staphylococcus aureus in a university medical center day care facility. Infect Control Hosp Epidemiol 2009;30:985–92.
27. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005;352:468–75.
28. Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA 2012;308:50–9.
29. Lindenmayer JM, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med 1998;158:895–9.
30. Malcolm B. The rise of methicillin-resistant staphylococcus aureus in U.S. correctional populations. J Correct Health Care 2011;17:254–65.
31. Nerby JM, Gorwitz R, Lesher L, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2011;30:927–32.
32. Stemper ME, Shukla SK, Reed KD. Emergence and spread of community-associated methicillin-resistant Staphylococcus aureus in rural Wisconsin, 1989 to 1999. J Clin Microbiol 2004;42:5673–80.
33. Turabelidze G, Lin M, Wolkoff B, et al. Personal hygiene and methicillin-resistant Staphylococcus aureus infection. Emerg Infect Dis 2006;12:422–7.
34. Ellis MW, Hospenthal DR, Dooley DP, et al. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004;39:971–9.
35. Forster AJ, Oake N, Roth V, et al. Patient-level factors associated with methicillin-resistant Staphylococcus aureus carriage at hospital admission: a systematic review. Am J Infect Control 2013;41:214–20.
36. McKinnell JA, Miller LG, Eells SJ, et al. A systematic literature review and meta-analysis of factors associated with methicillin-resistant staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Contol Hosp Epidemiol 2013;34:1077–86.
37. Furuno JP, McGregor JC, Harris AD, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med 2006;166:580–5.
38. Jernigan JA, Pullen AL, Flowers L, et al. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol 2003;24:409–14.
39. Horner C, Parnell P, Hall D, Kearns A, Heritage J, Wilcox M. Meticillin-resistant Staphylococcus aureus in elderly residents of care homes: colonization rates and molecular epidemiology. J Hosp Infect 2013;83:212–8.
40. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 2009;73:305–15.
41. Boyce JM. Methicillin-resistant Staphylococcus aureus. Detection, epidemiology, and control measures. Infect Dis Clin North Am 1989;3:901–13.
42. Jernigan JA. Methicillin-resistant Staphylococcus aureus colonization among health care personnel in the emergency department: what does it tell us? Ann Emerg Med 2008;52:534–6.
43. Carnicer-Pont D, Bailey KA, Mason BW, Walker AM, Evans MR, Salmon RL. Risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus bacteraemia: a case-control study. Epidemiol Infect 2006;134:1167–73.
44. Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 2002;49:999–1005.
45. Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med 1982;97:309–17.
46. Davis KA, Stewart JJ, Crouch HK,et al. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 2004;39:776–82.
47. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997;10:505–20.
48. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med 2008;121:310–5.
49. Wertheim HFL, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703–5.
50. Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol 2001;22:687–92.
51. Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol;31:584–91.
52. von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001;344:11–6.
53. Pujol M, Pea C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am J Med 1996;100:509–16.
54. Rocha LA, Marques Ribas R, da Costa Darini AL, Gontijo Filho PP. Relationship between nasal colonization and ventilator-associated pneumonia and the role of the environment in transmission of Staphylococcus aureus in intensive care units. Am J Infect Control 2013;41:236–40.
55. Corne P, Marchandin Hln, Jonquet O, Campos J, Bauls A-L. Molecular evidence that nasal carriage of Staphylococcus aureus plays a role in respiratory tract infections of critically ill patients. J Clin Microbiol 2005;43:3491–3.
56. Quezada Joaquin NM, Diekema DJ, Perencevich EN, et al. Long-term risk for readmission, methicillin-resistant Staphylococcus aureus (MRSA) infection, and death among MRSA-colonized veterans. Antimicrob Agents Chemother 2013;57:1169–72.
57. Lin MY, Hayden MK. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus: recognition and prevention in intensive care units. Crit Care Med 2010;38:S335–44.
58. Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis 2002;8:802–7.
59. Ostrowsky BE, Venkataraman L, D’Agata EM, et al. Vancomycin-resistant enterococci in intensive care units: high frequency of stool carriage during a non-outbreak period. Arch Intern Med 1999;159:1467–72.
60. Bonten MJ, Hayden MK, Nathan C, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet 1996;348:1615–9.
61. Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med 2001;344:1427–33.
62. Padiglione AA, Wolfe R, Grabsch EA, et al. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob Agents Chemother 2003;47:2492–8.
63. Batistao DW, Gontijo-Filho PP, Conceicao N, et al. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz 2012;107:57–63.
64. Furtado GH, Martins ST, Coutinho AP, et al. Prevalence and factors associated with rectal vancomycin-resistant enterococci colonization in two intensive care units in Sao Paulo, Brazil. Braz J Infect Dis 2005;9:64–9.
65. Huang SS, Datta R, Rifas-Shiman S, et al. Colonization with antibiotic-susceptible strains protects against methicillin-resistant Staphylococcus aureus but not vancomycin-resistant enterococci acquisition: a nested case-control study. Crit Care 2011;15:R210.
66. Pan SC, Wang JT, Chen YC, et al. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS One 2012;7:e47297.
67. Se YB, Chun HJ, Yi HJ, et al. Incidence and risk factors of infection caused by vancomycin-resistant enterococcus colonization in neurosurgical intensive care unit patients. J Korean Neurosurg Soc 2009;46:123–9.
68. Healthcare Infection Control Practices Advisory Committee (HICPAC). Management of multidrug-resistant organisms in healthcare settings, 2006. Accessed 11 Oct 2013 at www.cdc.gov/hicpac/mdro/mdro_toc.html.
69. Malani PN. Preventing infections in the ICU: one size does not fit all. JAMA 2013;310:1567–8.
70. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 1995;44:1–13.
71. de Bruin MA, Riley LW. Does vancomycin prescribing intervention affect vancomycin-resistant enterococcus infection and colonization in hospitals? A systematic review. BMC Infect Dis 2007;7:24.
72. Adachi W, Bolding F, Armstrong R. Experience with vancomycin education and order sheet to limit vancomycin use. Hosp Pharm 1997:1370–3.
73. Fridkin SK, Lawton R, Edwards JR, et al. Monitoring antimicrobial use and resistance: comparison with a national benchmark on reducing vancomycin use and vancomycin-resistant enterococci. Emerg Infect Dis 2002;8:702–7.
74. Guglielmo BJ, Dudas V, Maewal I, et al. Impact of a series of interventions in vancomycin prescribing on use and prevalence of vancomycin-resistant enterococci. Jt Comm J Qual Patient Saf 2005;31:469–75.
75. Lautenbach E, LaRosa LA, Marr AM, et al. Changes in the prevalence of vancomycin-resistant enterococci in response to antimicrobial formulary interventions: impact of progressive restrictions on use of vancomycin and third-generation cephalosporins. Clin Infect Dis 2003;36:440–6.
76. Morgan AS, Brennan PJ, Fishman NO. Impact of a vancomycin restriction policy on use and cost of vancomycin and incidence of vancomycin-resistant Enterococcus. Ann Pharmacother 1997;31:970–3.
77. Anglim AM, Klym B, Byers KE, et al. Effect of a vancomycin restriction policy on ordering practices during an outbreak of vancomycin-resistant Enterococcus faecium. Arch Intern Med 1997;157:1132–6.
78. Montecalvo MA, Jarvis WR, Uman J, et al. Infection-control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med 1999;131:269–72.
79. Morris JG Jr, Shay DK, Hebden JN, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med 1995;123:250–9.
80. Quale J, Landman D, Saurina G, et al. Manipulation of a hospital antimicrobial formulary to control an outbreak of vancomycin-resistant enterococci. Clin Infect Dis 1996;23:1020-5.
81. Rubin LG, Tucci V, Cercenado E, et al. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect Control Hosp Epidemiol 1992;13:700–5.
82. Lai KK, Kelley AL, Melvin ZS, et al. Failure to eradicate vancomycin-resistant enterococci in a university hospital and the cost of barrier precautions. Infect Control Hosp Epidemiol 1998;19:647–52.
83. Shaikh ZH, Osting CA, Hanna HA, et al. Effectiveness of a multifaceted infection control policy in reducing vancomycin usage and vancomycin-resistant enterococci at a tertiary care cancer centre. J Hosp Infect 2002;51:52–8.
84. Lafaurie M, Porcher R, Donay JL, et al. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother 2012;67:1010–5.
85. Parienti JJ, Cattoir V, Thibon P, et al. Hospital-wide modification of fluoroquinolone policy and meticillin-resistant Staphylococcus aureus rates: a 10-year interrupted time-series analysis. J Hosp Infect 2011;78:118–22.
86. Safdar N, Abad C. Educational interventions for prevention of healthcare-associated infection: a systematic review. Crit Care Med 2008;36:933–40.
87. Boyce JM. It is time for action: improving hand hygiene in hospitals. Ann Intern Med 1999;130:153–5.
88. Jackson M, Chiarello LA, Gaynes RP, Gerberding JL. Nurse staffing and healthcare-associated infections: proceedings from a working group meeting. J Nurs Adm 2002;32:314–22.
89. Kuzu N, Ozer F, Aydemir S, et al. Compliance with hand hygiene and glove use in a university-affiliated hospital. Infect Control Hosp Epidemiol 2005;26:312–5.
90. Larson E, Killien M. Factors influencing handwashing behavior of patient care personnel. Am J Infect Control 1982;10:93–9.
91. Larson E, Kretzer EK. Compliance with handwashing and barrier precautions. J Hosp Infect 1995;30 Suppl:88–106.
92. Naikoba S, Hayward A. The effectiveness of interventions aimed at increasing handwashing in healthcare workers - a systematic review. J Hosp Infect 2001;47:173–80.
93. Pittet D, Simon A, Hugonnet S, et al. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med 2004;141:1–8.
94. Trick WE, Vernon MO, Welbel SF, et al. Multicenter intervention program to increase adherence to hand hygiene recommendations and glove use and to reduce the incidence of antimicrobial resistance. Infect Control Hosp Epidemiol 2007;28:42–9.
95. Wisniewski MF, Kim S, Trick WE, et al. Effect of education on hand hygiene beliefs and practices: a 5-year program. Infect Control Hosp Epidemiol 2007;28:88–91.
96. Rupp ME, Fitzgerald T, Puumala S, et al. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect Control Hosp Epidemiol 2008;29:8–15.
97. Venkatesh AK, Lankford MG, Rooney DM, et al. Use of electronic alerts to enhance hand hygiene compliance and decrease transmission of vancomycin-resistant Enterococcus in a hematology unit. Am J Infect Control 2008;36:199–205.
98. Silvestri L, Petros AJ, Sarginson RE, et al. Handwashing in the intensive care unit: a big measure with modest effects. J Hosp Infect 2005;59:172–9.
99. Akyol A, Ulusoy H, Ozen I. Handwashing: a simple, economical and effective method for preventing nosocomial infections in intensive care units. J Hosp Infect 2006;62:395–405.
100. Simmons B, Bryant J, Neiman K, et al. The role of handwashing in prevention of endemic intensive care unit infections. Infect Control Hosp Epidemiol 1990;11:589–94.
101. Tenorio AR, Badri SM, Sahgal NB, et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin Infect Dis 2001;32:826–9.
102. Slaughter S, Hayden MK, Nathan C, et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med 1996;125:448–56.
103. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013;310:1571–80.
104. Dietze B, Rath A, Wendt C, Martiny H. Survival of MRSA on sterile goods packaging. J Hosp Infect 2001;49:255–61.
105. Hardy KJ, Oppenheim BA, Gossain S, et al. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect Control Hosp Epidemiol 2006;27:127–32.
106. Jawad A, Heritage J, Snelling AM, et al. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol 1996;34:2881–7.
107. Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol 2007;28:1142–7.
108. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997;18:622–7.
109. Sexton T, Clarke P, O’Neill E, et al. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect 2006;62:187–94.
110. Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet infect dis 2008;8:101–13.
111. Dancer SJ, White LF, Lamb J, et al. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC med 2009;7.
112. Rampling A, Wiseman S, Davis L, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect 2001;49:109–16.
113. Wilson APR, Smyth D, Moore G, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med 2011;39:651–8.
114. Hess AS, Shardell M, Johnson JK, et al. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria. Infection Control Hosp Epidemiol 2013;34:487–93.
115. Falk PS, Winnike J, Woodmansee C, et al. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol 2000;21:575–82.
116. Livornese LL Jr, Dias S, Samel C, et al. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med 1992;117:112–6.
117. Porwancher R, Sheth A, Remphrey S, et al. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium: possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemiol 1997;18:771–3.
118. Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000;343:1925–32.
119. Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol 2000;38:724–6.
120. Noskin GA, Bednarz P, Suriano T, et al. Persistent contamination of fabric-covered furniture by vancomycin-resistant enterococci: implications for upholstery selection in hospitals. Am J Infect Control 2000;28:311–3.
121. Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol 1995;16:577–81.
122. Smith TL, Iwen PC, Olson SB, Rupp ME. Environmental contamination with vancomycin-resistant enterococci in an outpatient setting. Infect Control Hosp Epidemiol 1998;19:515–8.
123. Wendt C, Wiesenthal B, Dietz E, Ruden H. Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. J Clin Microbiol 1998;36:3734–6.
124. Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol 2004;25:164–7.
125. Ray AJ, Hoyen CK, Taub TF, et al. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA 2002;287:1400–1.
126. Hayden MK, Bonten MJ, Blom DW, et al. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 2006;42:1552–60.
127. Byers KE, Durbin LJ, Simonton BM, et al. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol 1998;19:261–4.
128. Goodman ER, Platt R, Bass R, et al. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol 2008;29:593–9.
129. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009;73:378–85.
130. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA) infections. Accessed 11 Oct 2013 at www.cdc.gov/mrsa/index.html.
131. Edmond MB, Wenzel RP. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1471.
132. Lai KK, Fontecchio S, Melvin Z, Baker SP. Impact of alcohol-based, waterless hand antiseptic on the incidence of infection and colonization with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Infect Control Hosp Epidemiol 2006;27:1018–24.
133. Ostrowsky B, Steinberg JT, Farr B, et al. Reality check: should we try to detect and isolate vancomycin-resistant enterococci patients? Infect Control Hosp Epidemiol 2001;22:116–9.
134. Harbarth S, Sax H, Uckay I, et al. A predictive model for identifying surgical patients at risk of methicillin-resistant Staphylococcus aureus carriage on admission. J Am Coll Surg 2008;207:683–9.
135. Jarvis WR. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1469.
136. Krause R, Honigl M, Zollner-Schwetz I. Targeted decolonization to prevent ICU infections. N Engl J Med;369:1469–70.
137. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med;364:1407–18.
138. Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008;148:409–18.
139. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30.
140. Gurieva T, Bootsma MCJ, Bonten MJM. Successful Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections revisited. Clin Infect Dis 2012:54:1618–20.
141. Gavalda L, Masuet C, Beltran J, et al. Comparative cost of selective screening to prevent transmission of methicillin-resistant Staphylococcus aureus (MRSA), compared with the attributable costs of MRSA infection. Infection control and hospital epidemiology 2006;27:1264–6.
142. Otter JA, Herdman MT, Williams B, et al. Low prevalence of methicillin-resistant Staphylococcus aureus carriage at hospital admission: implications for risk-factor-based vs universal screening. J Hosp Infect 2013;83:114–21.
143. Harbarth S, Hawkey PM, Tenover F, et al. Update on screening and clinical diagnosis of methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 2011;37:110–7.
144. Calfee DP, Giannetta ET, Durbin LJ, et al. Control of endemic vancomycin-resistant Enterococcus among inpatients at a university hospital. Clin Infect Dis 2003;37:326–32.
145. Hendrix CW, Hammond JM, Swoboda SM, et al. Surveillance strategies and impact of vancomycin-resistant enterococcal colonization and infection in critically ill patients. Ann Surg 2001;233:259–65.
146. Muto CA, Giannetta ET, Durbin LJ, et al. Cost-effectiveness of perirectal surveillance cultures for controlling vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol 2002;23:429–35.
147. Price CS, Paule S, Noskin GA, Peterson LR. Active surveillance reduces the incidence of vancomycin-resistant enterococcal bacteremia. Clin Infect Dis 2003;37:921–8.
148. Shadel BN, Puzniak LA, Gillespie KN, et al. Surveillance for vancomycin-resistant enterococci: type, rates, costs, and implications. Infect Control Hosp Epidemiol 2006;27:1068–75.
149. Siddiqui AH, Harris AD, Hebden J, et al. The effect of active surveillance for vancomycin-resistant enterococci in high-risk units on vancomycin-resistant enterococci incidence hospital-wide. Am J Infect Control 2002;30:40–3.
150. Sandri AM, Dalarosa MG, Ruschel de Alcantara L, et al. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol 2006;27:185–7.
151. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255–65.
152. Bleasdale SC, Trick WE, Gonzalez IM, et al. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–9.
153. Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009;37:1858–65.
154. Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166:306–12.
155. Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 2013;381:1099–106.
156. Blackwood B, Thompson G, McMullan R, et al. Tea tree oil (5%) body wash versus standard care (Johnson’s Baby Softwash) to prevent colonization with methicillin-resistant Staphylococcus aureus in critically ill adults: a randomized controlled trial. J Antimicrob Chemother 2013;68:1193–9.
157. Daneman N, Sarwar S, Fowler RA, et al. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:328–41.
158. Verwaest C, Verhaegen J, Ferdinande P, et al. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med 1997;25:63–71.
159. de Smet AMGA, Kluytmans JAJW, Blok HEM, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis 2011;11:372–80.
160. de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003;362:1011–6.
161. Cepeda JA, Whitehouse T, Cooper B, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet 2005;365:295–304.
162. Dhaliwal J, McGeer A. Does isolation prevent the spread of methicillin-resistant Staphylococcus aureus? CMAJ 2005;172:875.
163. Kollef MH, Micek ST. Antimicrobial stewardship programs: mandatory for all ICUs. Crit Care 2012;16:179.
164. McKinnell JA, Huang SS, Eells SJ, et al. Quantifying the impact of extranasal testing of body sites for methicillin-resistant Staphylococcus aureus colonization at the time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 2013;34:161–70.
165. Denkinger CM, Grant AD, Denkinger M, et al. Increased multi-drug resistance among the elderly on admission to the hospital—a 12-year surveillance study. Arch Gerontol Geriatr 2013;56:227–30.
166. Boisseau D, Alfandari S, Gauzit R, et al. Staphylococcus aureus nasal carriage during the infectious diseases national congress in France. Med Mal Infect 2012;42:435–9.
167. Fritz SA, Hogan PG, Hayek G, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med 2012;166:551–7.
168. Rafee Y, Abdel-Haq N, Asmar B, et al. Increased prevalence of methicillin-resistant Staphylococcus aureus nasal colonization in household contacts of children with community acquired disease. BMC Infect Dis 2012;12:45.
169. Schechter-Perkins EM, Mitchell PM, Murray KA, et al. Prevalence and predictors of nasal and extranasal staphylococcal colonization in patients presenting to the emergency department. Ann Emerg Med 2011;57:492–9.
170. Bisaga A, Paquette K, Sabatini L, Lovell E. A prevalence study of methicillin-resistant staphylococcus aureus colonization in emergency department health care workers. Ann Emerg Med 2008;52:525–8.
171. Suffoletto B, Cannon E, Ilkhanipour K, Yealy D. Prevalence of Staphylococcus aureus nasal colonization in emergency department personnel. Ann Emerg Med 2008;52:529–33.
172. Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg 2004;139:947-51; discussion 51–3.
173. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis 2003;36:131–9.
From the Department of Medicine, Infectious Disease Practice and Innovations, The Medical City, Pasig City, Philippines (Dr. Abad), the Division of Emergency Medicine, University of Wisconsin Medical School, Madison, WI (Dr. Pulia), University of Wisconsin Hospital and Clinics, Madison, WI (Ms. Krupp), and the Willam S. Middleton Memorial Veterans Affairs Hospital, Madison, WI (Dr. Safdar).
Patients in intensive care units (ICUs) are at greatly increased risk of developing health care-associated infections (HAIs) [1]. More than 70% of the bacteria that cause HAIs are resistant to at least one of the antimicrobials commonly used to treat these infections [2]. Two such pathogens, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) are responsible for a considerable proportion of ICU infections that are associated with increased morbidity, mortality, and costs [3–5]. In this review, we discuss the epidemiology of colonization and infection by MRSA and VRE and provide an update on practices for prevention of transmission and infection by MRSA and VRE in the ICU.
EPIDEMIOLOGY AND MECHANISMS OF RESISTANCE
MRSA is the major cause of HAIs worldwide [6]. Among ICUs in the United States, the proportion of methicillin resistance among S. aureus isolates increased from 35.9% in 1992 to 64.4% in 2003 [4]. Approximately 8% of patients are colonized with MRSA upon admission, and an average of 5% will acquire MRSA colonization in the ICU [7,8]. A comparison study of academic tertiary care facilities found medical ICUs had higher MRSA admission prevalence rates than surgical ICUs, whereas surgical ICUs had a higher incidence rate [7]. Enteroccoccus is the third most common pathogen associated with HAIs, with 33% resistant to vancomycin [9]. VRE infection is associated with increased ICU cost and increased length of stay [10]. Incidence of ICU-acquired VRE varies among regions and countries. For example, in the United States, Warren et al [11] reported a VRE incidence of 27 cases per 1000 patient ICU days, whereas Kohlenberg et al [12] reported a mean incidence of 0.29 cases per 1000 patient ICU days in Germany.
Understanding the mechanisms that allow development of resistant strains of S. aureus and Enterococcus species is important to devise preventive strategies. Methicillin resistance in MRSA is determined by the staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element that carries the mecA gene. The mecA gene codes for an additional penicillin-binding protein (PBP) that has a reduced affinity towards methicillin (PBP2a/PBP2'). This results in a reduced ability to bind to the bacterial cell wall and inhibit synthesis [13,14]. Study of molecular epidemiology has identified MRSA as originating from 8 major variants of the mecA gene [15]. The majority of MRSA infections are caused by strains belonging to a few internationally disseminated clones [14]. The first identified strains were associated with infections in hospitalized patients (hospital-associated MRSA), but community-associated MRSA strains have since emerged and have become established globally, including in health care institutions [16].
Community-acquired MRSA can cause severe infections in health hosts [17]; possible explanations include increased CA-MRSA virulence due to the acquisition of mobile genetic elements, namely those containing Panton-Valentine leukocidin (PVL) [18] or increased expression of core genome-encoded virulence genes, such as phenol-soluble modulin (PSM) cytolysins, α-toxin, and other virulence determinants [19].
Enterococcus is intrinsically resistant to several antimicrobial drugs, with resistance to vancomycin encoded by several clusters of genes known as vancomycin resistance gene clusters (eg, vanA, vanB). The gene clusters generate resistance through multiple pathways which encode enzymes to determine the structure of peptidoglycan precursors [20,21]. Genetically diverse, hospital-associated VRE outbreaks have been associated with single clones, multiple clones, and changing molecular epidemiology over time [21]. While up to 25% of the VRE genome includes acquired elements, the majority of hospital-associated infections are traced to a few clonal complexes, which differ from community-associated VRE strains [22].
The evolution of these efficient mechanisms that promote drug resistance has made it extremely challenging to eradicate organisms such as MRSA and VRE. However, advances in recent years have furthered our understanding of the epidemiology, pathogenesis, and methods of prevention and containment.
RISK FACTORS FOR COLONIZATION AND INFECTION
MRSA
The risk factors underlying MRSA colonization and infection in the ICU setting can be categorized as either patient/host or environmental factors. A wide range of patient-level factors is associated with MRSA colonization upon admission. General principles regarding the transmission of MRSA in the community include close contact with colonized or infected individuals, breaks in the skin, crowded living conditions and poor hygiene. These factors, alone or in combination, are thought to underlie observed outbreaks among sports teams, military personnel, correction facilities, American Indian communities, and daycare centers [23–34].
Two recently published systematic reviews have summarized important patient-level factors associated with MRSA colonization at the time of hospital admission. Forster et al [35] examined 27 studies and identified previous admission to hospital, transfer from nursing home or long-term care facility, and previous antibiotic use as the top 3 factors associated with MRSA colonization. A similar review conducted by McKinnell and colleagues [36] found that prior hospitalization, nursing home contact, recent antibiotic use, and exposure to health care-associated pathogens (MRSA carriage, VRE carriage, or Clostridium difficile infection) were found to portend the highest risk. Specific comorbid conditions also conveyed an increased risk, including congestive heart failure, chronic wounds/bedsores, diabetes mellitus, pulmonary disease, immunosuppression, urinary catheter, and renal failure/dialysis. It is clear that health care contacts, especially recent hospitalization, residence in a long-term care facility, and antibiotic use, are significant risk factors for MRSA colonization [37–39].
 In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
Regardless of whether MRSA colonization precedes admission or occurs due to nosocomial spread, it is associated with increased risk of developing a HAI [46–49]. In 2 large prospective observational cohort studies, the hazard ratios of MRSA colonization developing into S. aureus infections during the ICU stay were 3.84 and 4.70, respectively [50,51]. High levels of concordance between MRSA colonization strains and HAI strains have also been reported [52]. Nasal colonization with S. aureus has also been identified as an independent risk factor for developing ventilator-associated pneumonia (VAP) and bacteremia [53,54]. A case series of ICU patients with S. aureus nasal colonization who developed lower respiratory tract infections demonstrated genetically identical nasal and bronchial strains in 15/16 cases [55]. This finding strongly suggests that nasopharyngeal colonization with S. aureus contaminates oral secretions that are aspirated by critically ill patients, resulting in subsequent pneumonia. In a long-term outcomes study among a matched cohort of veterans, MRSA colonization was associated with an increased risk of infection-related readmission and mortality [56]. These findings reflect the critically important nature of measures designed to curb nosocomial transmission and acquisition of MRSA, especially among the vulnerable ICU population.
VRE
As with MRSA, risk factors associated with VRE colonization include both patient-level and ICU-level (or environmental) factors [57]. Examples of patient-level factors include previous antimicrobial exposure [58–62], underlying medical illnesses such as chronic renal failure requiring hemodialysis [11,63], length of hospital or ICU stay [11,59,64,65], and recent exposure to health care facilities. ICU-level factors of relevance are the prevalence of VRE in the unit, with high levels of endemicity leading to higher risk of colonization and transmission.
Antibiotic use is a major risk factor for VRE acquisition, although the type and class of antibiotic varies considerably across studies; the most frequently identified antibiotics are broad-spectrum cephalosporins, vancomycin, and anti-anaerobic agents [58,62,64]. Patients with chronic liver disease and post-transplantation are at exceedingly high risk for VRE acquisition [59]. In a recent study by Pan [66], for example, the authors found that the incidence of newly acquired VRE was 21.9 per 1000 patient-days in an ICU setting. On multivariate analysis, the authors found that, similar to other reports [11,59,67], length of stay in the ICU was associated with increased risk of VRE acquisition, with each additional day of stay increasing risk of VRE by 1.03 times. Warren et al undertook a prospective cohort study involving 519 patients admitted to the ICU for more than 48 hours [11]. Seventy-four (21%) of 352 patients were subsequently colonized with VRE. The median time to development of a positive VRE culture after ICU admission was 6 days. Increased mean APACHE II score on ICU admission (P = 0.002), sucralfate use (P = 0.003), vasopressor use (P = 0.01), tracheostomy in the ICU (P = 0.02), and C. difficile diarrhea (P = 0.002) appeared to be associated with VRE acquisition.
 It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
REDUCING TRANSMISSION—MRSA AND VRE PREVENTION STRATEGIES
 Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Antimicrobial Stewardship
Antibiotic use is a major driver of antibiotic resistance. A meta-analysis by de Bruin and Riley [71] studied the effect of vancomycin usage on VRE colonization and infection. A total of 12 articles describing 13 studies were included; none were randomized controlled trials. All studies were quasi-experimental and lacked control groups. Among all studies, less than half (46%) implemented vancomycin reduction measures as the sole type of intervention [72–76]. The remaining studies implemented other infection control modalities and or restricted the use of other antimicrobials [77–83]. Although all studies that implemented vancomycin restriction alone as a single strategy showed a decline in vancomycin usage, only 2 of these [74,75] showed a relative risk reduction in VRE acquisition post-intervention. Also, studies that restricted vancomycin alone revealed a trend towards lower efficacy in reducing VRE colonization and infection (33%) when compared with those that used additional measures (71%). While judicious antibiotic use should always be practiced, the evidence for vancomycin restriction as a sole intervention to control VRE is scant. It may be that other antibiotics are as big or bigger drivers of resistance in enterococci than vancomycin. For example, a growing body of literature supports antibiotic restriction, especially fluoroquinolones, for reducing MRSA. In several time-series quasi-experimental studies, restriction of fluorquinolones was associated with reduced trends in MRSA infections in the acute care setting, and consideration should be given to monitor and optimize fluoroquinolone use in the ICU setting [84,85].
Antimicrobial stewardship programs are fundamental to optimizing antibiotic use in the ICU and the authors strongly recommend that all ICUs should have such a program in place.
Educational Interventions
Infection control and multidrug-resistant organism (MDRO)–specific education programs for health care workers is a core principle of the CDC’s prevention guidelines. The HICPAC VRE guideline also explicitly states “continuing education programs for hospital staff (including attending and consulting physicians, medical residents, and students; pharmacy, nursing, and laboratory personnel; and other direct patient-care providers) should include information concerning the epidemiology of VRE and the potential impact of this pathogen on the cost and outcome of patient care [70].” A systematic review published in 2008 [86] that included 26 studies showed that such interventions to prevent HCAIs are usually successful; in this review, 20 of 26 studies showed a statistically significant decrease in infection rates, with risk ratios ranging from 0 to 1.6. Education was usually part of a broader array of infection control interventions. While clearly essential, education alone is unlikely to have a sustained impact on reducing MRSA and VRE infections.
Infection Control Measures
Major infection control interventions include hand hygiene, the use of personal protective equipment (PPE), and cohorting. These measures can be grouped into “horizontal” (or global) vs. “vertical” (or targeted) strategies. Although not mutually exclusive, horizontal approaches are designed to have an impact on multiple pathogens (pathogen nonspecific), whereas vertical approaches are designed to reduce the impact of specific pathogens (such as VRE). For the purposes of this review, we will discuss both strategies for containment of MRSA and VRE. Horizontal strategies include hand hygiene, universal gloving and/or gowning, environmental cleaning, and daily bathing with chlorhexidine. Vertical strategies include screening for either MRSA or VRE followed by placement in contact precautions and decolonization with mupirocin.
Hand Hygiene
Hand washing is fundamental to reducing transmission of MDROs in health care institutions; however, optimal compliance is hard to achieve and sustain. Barriers to adherence may include unavailability of sinks or hand hygiene materials (eg, alcohol-based gels, gloves) time constraints, forgetfulness, or lack of knowledge [87–95]. Several monitoring strategies have been evaluated to increase compliance with hand hygiene. Most involve direct observation followed by performance assessment and feedback.
Trials examining the impact of improvements in hand hygiene compliance on HAIs in the ICU setting have largely found benefit, although not all studies showed a decline in HAI. In a prospective crossover trial, Rupp et al [96] found dramatic improvements in compliance with hand gel availability, but this did not translate to decreased nosocomial MRSA infections. Venkatesh et al [97] carried out a before-and-after interventional prospective study in a hematology unit in a tertiary level hospital to evaluate the use of an electronic method of surveillance to determine compliance with hand hygiene. The authors also used rates of horizontal transmission of VRE as a secondary end-point. Results of the study showed that hand hygiene compliance improved from 36.3% at baseline to 70.1%. This represented an OR of 4.1 (95% confidence interval, 3.7–4.5), which the authors attributed to the use of automated alerts. VRE transmission rates before and during intervention were not statistically different, but the rates of infection were lower at 1.0 per month in comparison with 4.7 infections per month in the preceding 6 months (P = 0.096).
While improved hand hygiene may result in significant reductions in HAIs [40], research indicates hand hygiene alone influences about 40% of infections in the ICU setting [98]. As such, hand hygiene should be viewed as a necessary component of a comprehensive infection control program [99]. Despite the success of hand hygiene in reducing HAIs in the ICU, effective strategies to improve compliance remain elusive even under study conditions and further research is needed in this area [100].
Personal Protective Equipment
Tenorio et al [101] conducted a study to assess the effectiveness of gloving in the prevention of hand carriage of VRE by health care workers. The study showed that among 50 health care workers who had contact with patients colonized with VRE, 6 carried a similar patient strain even prior to known contact, and 17 of 44 (69%) had a patient-related VRE strain on their gloves after contact. This suggests a relatively high rate of colonization after usual patient-care contact. Factors associated with acquisition of VRE on gloves included duration of contact, contact with a patient’s body fluids, presence of diarrhea in a patient, mean VRE colony counts on a patient’s skin, and number of body sites colonized with VRE. Although gloves reduced the risk of VRE acquisition of VRE by 71% (ie, 12/17 did not have VRE on their hands after de-gloving) the protection afforded by gloves was incomplete. As such, hand hygiene after glove removal is recommended.
Slaughter et al [102] compared the use of personal protective equipment in the acquisition of VRE. During this study, 93 patients in glove-and-gown rooms and 88 patients in glove-only rooms had similar rates of VRE at baseline entry into the ICU and after the intervention. Mean times to colonization among the patients who became colonized were 8.0 days in the glove-and-gown group and 7.1 days in the glove-only group. None of these comparisons were statistically significant and the authors concluded that the universal use of gown and gloves was no better than the use of gloves alone in preventing VRE colonization.
A recent cluster randomized trial compared the effect of universal PPE (ie, gowning and gloving) with usual care for reducing acquisition of MRSA or VRE as a composite outcome [103]. The study did not find that universal gowning and gloving reduced VRE or MRSA acquisition but found a 40% decline in MRSA acquisition in the intervention ICUs compared with baseline rates of MRSA. No major adverse effects of universal gowning and gloving were noted in this study. A thoughtful editorial commenting on this article proposes that several aspects of the study deserve consideration, including the possibility of false-negative screening tests for VRE, which may have partially accounted for the negative primary outcome [69].
Based on these studies, it appears that the use of barrier precautions may be of value more for MRSA than VRE but further studies are needed to examine its impact on other types of pathogens, including new and emerging MDROs. Until further evidence becomes available, routine gowning and gloving may be of value in units with a high prevalence of MRSA.
Environmental Cleaning
Accumulating data suggests that the environment may play a major role in transmission of pathogens. MRSA has the ability to survive for days to weeks on inanimate objects [104–107]. Environmental contamination results in contamination of staff clothing and gloves [107,108] and is highly correlated with colonization strains among inpatients [109]. Although some studies of enhanced cleaning techniques and increased environmental services staff time have demonstrated reductions in MRSA outbreaks [110–112], the results are not universally favorable [113,114] and further studies are needed to examine the impact of environmental cleaning on rates of MRSA colonization or infection.
Several studies have implicated contaminated equipment as vectors for transmission of VRE during outbreaks [115–117], but the direction of fomite transfer from patient to environment has been difficult to ascertain. VRE have been found frequently on a variety of inanimate objects and surfaces in different health care environments [118–123], including gloved or ungloved hands of health care workers [101,124,125]. Hayden et al [126] determined the effect of improved environmental cleaning on VRE acquisition rates. This study was a pre-and-post intervention study carried out in a 21-bed medical intensive care unit (MICU) in a tertiary hospital over several phases. The intervention included the creation of a unique and improved cleaning program, as well as in-services to housekeeper services, education of the MICU staff, and a hand hygiene campaign. The results of the study showed decreased acquisition of VRE from 33.47 cases per 1000 patient days at risk in period 1 to 10.40 cases per 1000 patient-days at risk by period 4 of the study. Increased environmental cleaning was also associated with reduced growth of VRE from environmental cultures. At baseline, weekly contamination rates were 0.15 and 0.1 for samples obtained before and after cleaning, respectively. Culture positivity decreased to 0.07 and 0.04 for before and after cleaning in period 2 and then remained at low levels during the remainder of the study. It is important to note that the method for disinfecting used in this study was the “bucket method” as promoted by Byers [127]. This study provides further support for the importance of an environmental reservoir and of environmental decontamination to prevent endemic cross-transmission of VRE [126].
Goodman et al [128] used similar interventions but added a feedback tool using a black-light monitoring system (ie, use of an invisible, nontoxic marker to delineate areas that are adequately or inadequately cleaned) to reduce the likelihood of isolating either MRSA or VRE from an ICU environment. This study also showed favorable results, and notably, the use of the black-light monitoring system identified specific areas that were typically inadequately disinfected. Results showed that flat, horizontal surfaces (eg, countertops, bedside tray tables, and hamper tops) were adequately cleaned more often than small, vertical surfaces (eg, doorknobs, toilet handles, light switches, and electronics).
Part of the controversy surrounding the impact of environmental cleaning is the difficulty in determining its individual value as part of an overall infection control bundle [129]. A proposed area of demonstrable impact for environmental cleaning are frequently touched sites which are more likely to be contaminated with pathogens. Focusing on these “hot-bed” areas of the care environment may offer a useful adjunct to other infection control measures [129].
Active Surveillance
Active surveillance refers to periodic screening for asymp-tomatic carriers followed by placement of colonized patients in contact isolation. This practice is highly variable across institutions, as the evidence supporting this practice is conflicting and there are concerns about the cost of implementing this approach without solid evidence [70,130,131]. Despite lack of randomized controlled trials to guide this practice for MRSA prevention, many hospitals utilize MRSA surveillance and it is mandated by law in 9 states [132,133].
A prospective, interventional cohort study of universal MRSA screening on admission to surgical wards failed to reduce nosocomial MRSA infections [134]. Most recently, a pragmatic, cluster-randomized ICU trial reported that universal decolonization with chlorhexidine wipes and mupirocin use was more effective than screening and isolation in reducing rates of MRSA clinical isolates [65]. However, concerns regarding the risk of mupirocin resistance have been expressed [135,136]. The only randomized trial that compared active surveillance for MRSA and VRE followed by contact precautions to usual care did not find a benefit to active surveillance.
Huskins et al [137], in a large, cluster-randomized trial of 19 ICUs from different hospitals, determined the utility of using a culture-based active surveillance and contact isolation, compared with usual care (contact isolation for patients colonized with MRSA or VRE) as identified by existing hospital protocols, to reduce the incidence of colonization or infection with MRSA or VRE. In this trial, which spanned 6 months and involved 3488 participants, the authors found no significant difference between the intervention and control ICUs in terms of MRSA and VRE colonization or infection rates.
Conflicting with these findings is an observational study comparing MRSA infection rates before and after institution of a universal screening protocol, which demonstrated a 69.6% (CI, –89.2% to –19.6%]; P = 0.03) reduction in hospital wide MRSA prevalence density with screening [138]. The “MRSA bundle” implemented in 2007 at VA hospitals nationwide, which included universal screening, produced a 62% (P < 0.001) reduction in MRSA ICU infections; the relative contribution of the various bundle components is uncertain [139,140].
A proposed cost-saving alternative to universal screening is selective screening based on risk factor assessment [141]. The effectiveness of this type of program depends on creating a clinical decision-making tool capable of accurately identifying high-risk individuals while also accounting for the different risk factor profiles between HA-MRSA and CA-MRSA [142]. It has been proposed that targeted screening protocols may be more cost-effective in settings with < 5% prevalence of MRSA colonization on admission [143].
Many studies [61,144–149] have shown that active surveillance against VRE is cost-effective. For example, Calfee et al [144] showed that an established active surveillance program results in control of endemic VRE in high-risk patients. The infection control program was established in response to a hospital-wide VRE outbreak, and was sustained after the outbreak was controlled. The study by Calfee et al spanned 5 years and was performed at a tertiary-level university hospital, where cultures from perirectal areas were used to identify high-risk patients who were asymptomatically colonized with VRE. During the latter 2 years, 768 new cases of VRE colonization were detected among 69,672 admissions (1.1% of admissions), of which 730 (95.1%) were identified by active surveillance methods. This implies that routine clinical cultures would probably have missed the majority of colonized patients. During this period, the incidence of VRE infection was likewise extremely low at 0.12/1000 patient days (ie, 90 nosocomial VRE infections were identified in 83 patients during 743,956 days of patient care). Sixty-nine of the 83 patients (83%) who developed nosocomial VRE infections were found to be colonized with VRE by surveillance culture before the onset of infection.
Patient Decolonization
Chlorhexidine gluconate has been used in several settings to control outbreaks and infections related to MRSA and VRE due to its broad-spectrum activity against these pathogens. Chlorhexidine-based solutions reduce the density of skin colonization with pathogens such as MRSA and VRE (skin asepsis), thus lowering the risk for horizontal transmission between health care workers and patients.
Decolonization with chlorhexidine as an MRSA infection reduction technique has demonstrated benefit in the ICU setting [150]. The previously mentioned large, cluster-randomized ICU trial by Huang and colleagues found universal decolonization with twice-daily intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the entire ICU stay was superior to targeted decolonization of known MRSA carriers in preventing overall MRSA isolates. However, universal decolonization failed to show a reduction in MRSA bacteremia [151], and concerns about mupirocin resistance may limit the applicability of this approach.
There are now several studies [152–154] that show decreased acquisition of VRE with use of daily chlorhexidine bathing. In a study including 1787 ICU patients, Vernon et al found [154] that the reducing microbial density of VRE on patient’s skin by using chlorhexidine led to decreased transmission. In another study by Climo et al [153] that involved 6 ICUs at 4 academic centers and measured the incidence of MRSA and VRE colonization and blood stream infections (BSI) during a period of bathing with routine soap for 6 months compared with a 6-month period where all admitted patients received daily bathing with a chlorhexidine solution, results found decreased acquisition of VRE by 50% (4.35 vs. 2.19 cases/1000 patient days, P < 0.008) following the introduction of daily chlorhexidine bathing. Furthermore, compared with 16 of 270 patients colonized with VRE who subsequently developed VRE bacteremia at baseline, only 4 of 226 VRE-colonized patients bathed with chlorhexidine in the intervention period developed a BSI, translating into a relative risk reduction of 3.35 (95% CI, 1.13–9.87; P < 0.035). Patients colonized with VRE were 3 times less likely to develop VRE bacteremia when bathed with chlorhexidine compared with regular bathing. Despite the success of this protocol for VRE, when analyzed by individual organism no significant reductions in MRSA acquisition or BSI were reported. This finding is similarly corroborated by a trial conducted in the pediatric ICU setting which found an overall reduction in bacteremia with daily chlorhexidine washes but no significant decrease in cases due to S. aureus [155].
The results of these studies suggest that daily bathing with chlorhexidine should be part of routine practice in health care, especially in ICUs where endemic MRSA or VRE rates are high. Whether there is benefit in other settings needs to be studied.
In addition to chlorhexidine washes, other decolonization techniques have been proposed to reduce colonization and the spread of HAIs in the ICU setting. A randomized controlled trial of daily 5% tea tree oil body washes for the prevention of MRSA colonization failed to significantly reduce rates compared to standard soap body washes [156]. Another proposed decolonization intervention that has not been widely adopted in the United States due to concerns related to development of resistant organisms is selective digestive decontamination (SDD) or selective oropharyngeal decontamination (SOD) with antimicrobial agents [157,158]. In terms of clinical benefit, SDD/SOD have been found to decrease MDRO infection rate [159] and mortality [160].
Cohorting
There is insufficient evidence to conclude that cohorting isolated patients is of benefit for routine use in the endemic ICU setting. A few studies, mainly in the outbreak setting, have examined this approach and the results are conflicting [161,162]. Pending further studies in this area, it is reasonable to cohort patients colonized with the same microorganisms, especially if patients cannot be placed in single rooms.
CONCLUSION
The emergence of MRSA and VRE has led to a resurgence of interest and emphasis on infection control practices and prevention. CDC guidelines to help health care practitioners manage these MDROs in the hospital and ICU-setting exist; however, many questions remain regarding best practice.
Prevention of MRSA and VRE needs to be a 2-pronged approach—antimicrobial stewardship [163] and infection control. A robust antimicrobial stewardship program to optimize and minimize inappropriate antibiotic use is necessary in every institution. From the infection prevention standpoint, it is unclear if systematic identification of MRSA and VRE colonization followed by contact precautions is useful in reducing transmission. It is clear that a strong institutional climate of promoting patient safety and a culture of infection prevention will help in reducing MRSA and VRE facility-wide. It also appears that universal gowning and gloving may be useful for reducing MRSA, but not VRE, transmission. While universal decolonization with mupirocin is efficacious in reducing MRSA, this strategy is not recommended because of promoting mupirocin resistance. However, the use of daily bathing with chlorhexidine represents a relatively low-cost, high-yield intervention that should be adopted. Pending further data, patients known to be colonized or infected with MRSA should be placed in contact precuations as is current practice in most institutions. Finally, in this era of MDROs, hand hygiene remains our best defense against the spread of pathogens in the health care environment.
Note: This article does not represent the views of the Department of Veterans Affairs.
Corresponding author: Nasia Safdar, MD, Willam S. Middleton Memorial Veterans Affairs Hospital, 2500 Overlook Terrace, Madison, WI 53705, ns2@medicine.wisc.edu.
Funding/support: This work is funded by a MERIT award from the Department of Veterans Affairs to Nasia Safdar.
Financial disclosures: None.
REFERENCES
1. Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA 2009;301:727–36.
2. LeDell K, Muto CA, Jarvis WR, Farr BM. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol 2003;24:639–41.
3. Giske CG, Monnet DL, Cars O, Carmeli Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 2008;52:813–21.
4. Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Pub Health Rep 2007;122:160–6.
5. Schwaber MJ, Carmeli Y. The effect of antimicrobial resistance on patient outcomes: importance of proper evaluation of appropriate therapy. Crit Care 2009;13:106.
6. Grundmann H, Hori S, Winter B, et al. Risk factors for the transmission of methicillin-resistant Staphylococcus aureus in an adult intensive care unit: fitting a model to the data. J Inf Dis 2002;185:481–8.
7. Huang SS, Rifas-Shiman SL, Warren DK, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis 2007;195:330–8.
8. Muder RR, Cunningham C, McCray E, et al. Implementation of an industrial systems-engineering approach to reduce the incidence of methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2008;29:702–8.
9. Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008;29:996–1011.
10. Pelz RK, Lipsett PA, Swoboda SM, et al. Vancomycin-sensitive and vancomycin-resistant enterococcal infections in the ICU: attributable costs and outcomes. Intensive Care Med 2002;28:692–7.
11. Warren DK, Kollef MH, Seiler SM, et al. The epidemiology of vancomycin-resistant Enterococcus colonization in a medical intensive care unit. Infect Control Hosp Epidemiol 2003;24:257–63.
12. Kohlenberg A, Schwab F, Meyer E, et al. Regional trends in multidrug-resistant infections in German intensive care units: a real-time model for epidemiological monitoring and analysis. J Hosp Infect 2009;73:239–45.
13. Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol 2008;8:747–63.
14. Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008;46 Suppl 5:S350–9.
15. Zhang K, McClure JA, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009;53:531–40.
16. Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol 2013;5:205–17.
17. Otto M. MRSA virulence and spread. Cell Microbiol 2012;14:1513–21.
18. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978–84.
19. Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 2009;106:5883–8.
20. Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis 2006;42 Suppl 1:S25–34.
21. Gold HS. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis 2001;33:210–9.
22. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012;10:266–78.
23. Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis 1998;178:577–80.
24. Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 2004;113:e347–52.
25. Groom AV, Wolsey DH, Naimi TS, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 2001;286:1201–5.
26. Hewlett AL, Falk PS, Hughes KS, Mayhall CG. Epidemiology of methicillin-resistant Staphylococcus aureus in a university medical center day care facility. Infect Control Hosp Epidemiol 2009;30:985–92.
27. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005;352:468–75.
28. Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA 2012;308:50–9.
29. Lindenmayer JM, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med 1998;158:895–9.
30. Malcolm B. The rise of methicillin-resistant staphylococcus aureus in U.S. correctional populations. J Correct Health Care 2011;17:254–65.
31. Nerby JM, Gorwitz R, Lesher L, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2011;30:927–32.
32. Stemper ME, Shukla SK, Reed KD. Emergence and spread of community-associated methicillin-resistant Staphylococcus aureus in rural Wisconsin, 1989 to 1999. J Clin Microbiol 2004;42:5673–80.
33. Turabelidze G, Lin M, Wolkoff B, et al. Personal hygiene and methicillin-resistant Staphylococcus aureus infection. Emerg Infect Dis 2006;12:422–7.
34. Ellis MW, Hospenthal DR, Dooley DP, et al. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004;39:971–9.
35. Forster AJ, Oake N, Roth V, et al. Patient-level factors associated with methicillin-resistant Staphylococcus aureus carriage at hospital admission: a systematic review. Am J Infect Control 2013;41:214–20.
36. McKinnell JA, Miller LG, Eells SJ, et al. A systematic literature review and meta-analysis of factors associated with methicillin-resistant staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Contol Hosp Epidemiol 2013;34:1077–86.
37. Furuno JP, McGregor JC, Harris AD, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med 2006;166:580–5.
38. Jernigan JA, Pullen AL, Flowers L, et al. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol 2003;24:409–14.
39. Horner C, Parnell P, Hall D, Kearns A, Heritage J, Wilcox M. Meticillin-resistant Staphylococcus aureus in elderly residents of care homes: colonization rates and molecular epidemiology. J Hosp Infect 2013;83:212–8.
40. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 2009;73:305–15.
41. Boyce JM. Methicillin-resistant Staphylococcus aureus. Detection, epidemiology, and control measures. Infect Dis Clin North Am 1989;3:901–13.
42. Jernigan JA. Methicillin-resistant Staphylococcus aureus colonization among health care personnel in the emergency department: what does it tell us? Ann Emerg Med 2008;52:534–6.
43. Carnicer-Pont D, Bailey KA, Mason BW, Walker AM, Evans MR, Salmon RL. Risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus bacteraemia: a case-control study. Epidemiol Infect 2006;134:1167–73.
44. Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 2002;49:999–1005.
45. Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med 1982;97:309–17.
46. Davis KA, Stewart JJ, Crouch HK,et al. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 2004;39:776–82.
47. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997;10:505–20.
48. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med 2008;121:310–5.
49. Wertheim HFL, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703–5.
50. Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol 2001;22:687–92.
51. Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol;31:584–91.
52. von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001;344:11–6.
53. Pujol M, Pea C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am J Med 1996;100:509–16.
54. Rocha LA, Marques Ribas R, da Costa Darini AL, Gontijo Filho PP. Relationship between nasal colonization and ventilator-associated pneumonia and the role of the environment in transmission of Staphylococcus aureus in intensive care units. Am J Infect Control 2013;41:236–40.
55. Corne P, Marchandin Hln, Jonquet O, Campos J, Bauls A-L. Molecular evidence that nasal carriage of Staphylococcus aureus plays a role in respiratory tract infections of critically ill patients. J Clin Microbiol 2005;43:3491–3.
56. Quezada Joaquin NM, Diekema DJ, Perencevich EN, et al. Long-term risk for readmission, methicillin-resistant Staphylococcus aureus (MRSA) infection, and death among MRSA-colonized veterans. Antimicrob Agents Chemother 2013;57:1169–72.
57. Lin MY, Hayden MK. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus: recognition and prevention in intensive care units. Crit Care Med 2010;38:S335–44.
58. Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis 2002;8:802–7.
59. Ostrowsky BE, Venkataraman L, D’Agata EM, et al. Vancomycin-resistant enterococci in intensive care units: high frequency of stool carriage during a non-outbreak period. Arch Intern Med 1999;159:1467–72.
60. Bonten MJ, Hayden MK, Nathan C, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet 1996;348:1615–9.
61. Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med 2001;344:1427–33.
62. Padiglione AA, Wolfe R, Grabsch EA, et al. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob Agents Chemother 2003;47:2492–8.
63. Batistao DW, Gontijo-Filho PP, Conceicao N, et al. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz 2012;107:57–63.
64. Furtado GH, Martins ST, Coutinho AP, et al. Prevalence and factors associated with rectal vancomycin-resistant enterococci colonization in two intensive care units in Sao Paulo, Brazil. Braz J Infect Dis 2005;9:64–9.
65. Huang SS, Datta R, Rifas-Shiman S, et al. Colonization with antibiotic-susceptible strains protects against methicillin-resistant Staphylococcus aureus but not vancomycin-resistant enterococci acquisition: a nested case-control study. Crit Care 2011;15:R210.
66. Pan SC, Wang JT, Chen YC, et al. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS One 2012;7:e47297.
67. Se YB, Chun HJ, Yi HJ, et al. Incidence and risk factors of infection caused by vancomycin-resistant enterococcus colonization in neurosurgical intensive care unit patients. J Korean Neurosurg Soc 2009;46:123–9.
68. Healthcare Infection Control Practices Advisory Committee (HICPAC). Management of multidrug-resistant organisms in healthcare settings, 2006. Accessed 11 Oct 2013 at www.cdc.gov/hicpac/mdro/mdro_toc.html.
69. Malani PN. Preventing infections in the ICU: one size does not fit all. JAMA 2013;310:1567–8.
70. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 1995;44:1–13.
71. de Bruin MA, Riley LW. Does vancomycin prescribing intervention affect vancomycin-resistant enterococcus infection and colonization in hospitals? A systematic review. BMC Infect Dis 2007;7:24.
72. Adachi W, Bolding F, Armstrong R. Experience with vancomycin education and order sheet to limit vancomycin use. Hosp Pharm 1997:1370–3.
73. Fridkin SK, Lawton R, Edwards JR, et al. Monitoring antimicrobial use and resistance: comparison with a national benchmark on reducing vancomycin use and vancomycin-resistant enterococci. Emerg Infect Dis 2002;8:702–7.
74. Guglielmo BJ, Dudas V, Maewal I, et al. Impact of a series of interventions in vancomycin prescribing on use and prevalence of vancomycin-resistant enterococci. Jt Comm J Qual Patient Saf 2005;31:469–75.
75. Lautenbach E, LaRosa LA, Marr AM, et al. Changes in the prevalence of vancomycin-resistant enterococci in response to antimicrobial formulary interventions: impact of progressive restrictions on use of vancomycin and third-generation cephalosporins. Clin Infect Dis 2003;36:440–6.
76. Morgan AS, Brennan PJ, Fishman NO. Impact of a vancomycin restriction policy on use and cost of vancomycin and incidence of vancomycin-resistant Enterococcus. Ann Pharmacother 1997;31:970–3.
77. Anglim AM, Klym B, Byers KE, et al. Effect of a vancomycin restriction policy on ordering practices during an outbreak of vancomycin-resistant Enterococcus faecium. Arch Intern Med 1997;157:1132–6.
78. Montecalvo MA, Jarvis WR, Uman J, et al. Infection-control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med 1999;131:269–72.
79. Morris JG Jr, Shay DK, Hebden JN, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med 1995;123:250–9.
80. Quale J, Landman D, Saurina G, et al. Manipulation of a hospital antimicrobial formulary to control an outbreak of vancomycin-resistant enterococci. Clin Infect Dis 1996;23:1020-5.
81. Rubin LG, Tucci V, Cercenado E, et al. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect Control Hosp Epidemiol 1992;13:700–5.
82. Lai KK, Kelley AL, Melvin ZS, et al. Failure to eradicate vancomycin-resistant enterococci in a university hospital and the cost of barrier precautions. Infect Control Hosp Epidemiol 1998;19:647–52.
83. Shaikh ZH, Osting CA, Hanna HA, et al. Effectiveness of a multifaceted infection control policy in reducing vancomycin usage and vancomycin-resistant enterococci at a tertiary care cancer centre. J Hosp Infect 2002;51:52–8.
84. Lafaurie M, Porcher R, Donay JL, et al. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother 2012;67:1010–5.
85. Parienti JJ, Cattoir V, Thibon P, et al. Hospital-wide modification of fluoroquinolone policy and meticillin-resistant Staphylococcus aureus rates: a 10-year interrupted time-series analysis. J Hosp Infect 2011;78:118–22.
86. Safdar N, Abad C. Educational interventions for prevention of healthcare-associated infection: a systematic review. Crit Care Med 2008;36:933–40.
87. Boyce JM. It is time for action: improving hand hygiene in hospitals. Ann Intern Med 1999;130:153–5.
88. Jackson M, Chiarello LA, Gaynes RP, Gerberding JL. Nurse staffing and healthcare-associated infections: proceedings from a working group meeting. J Nurs Adm 2002;32:314–22.
89. Kuzu N, Ozer F, Aydemir S, et al. Compliance with hand hygiene and glove use in a university-affiliated hospital. Infect Control Hosp Epidemiol 2005;26:312–5.
90. Larson E, Killien M. Factors influencing handwashing behavior of patient care personnel. Am J Infect Control 1982;10:93–9.
91. Larson E, Kretzer EK. Compliance with handwashing and barrier precautions. J Hosp Infect 1995;30 Suppl:88–106.
92. Naikoba S, Hayward A. The effectiveness of interventions aimed at increasing handwashing in healthcare workers - a systematic review. J Hosp Infect 2001;47:173–80.
93. Pittet D, Simon A, Hugonnet S, et al. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med 2004;141:1–8.
94. Trick WE, Vernon MO, Welbel SF, et al. Multicenter intervention program to increase adherence to hand hygiene recommendations and glove use and to reduce the incidence of antimicrobial resistance. Infect Control Hosp Epidemiol 2007;28:42–9.
95. Wisniewski MF, Kim S, Trick WE, et al. Effect of education on hand hygiene beliefs and practices: a 5-year program. Infect Control Hosp Epidemiol 2007;28:88–91.
96. Rupp ME, Fitzgerald T, Puumala S, et al. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect Control Hosp Epidemiol 2008;29:8–15.
97. Venkatesh AK, Lankford MG, Rooney DM, et al. Use of electronic alerts to enhance hand hygiene compliance and decrease transmission of vancomycin-resistant Enterococcus in a hematology unit. Am J Infect Control 2008;36:199–205.
98. Silvestri L, Petros AJ, Sarginson RE, et al. Handwashing in the intensive care unit: a big measure with modest effects. J Hosp Infect 2005;59:172–9.
99. Akyol A, Ulusoy H, Ozen I. Handwashing: a simple, economical and effective method for preventing nosocomial infections in intensive care units. J Hosp Infect 2006;62:395–405.
100. Simmons B, Bryant J, Neiman K, et al. The role of handwashing in prevention of endemic intensive care unit infections. Infect Control Hosp Epidemiol 1990;11:589–94.
101. Tenorio AR, Badri SM, Sahgal NB, et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin Infect Dis 2001;32:826–9.
102. Slaughter S, Hayden MK, Nathan C, et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med 1996;125:448–56.
103. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013;310:1571–80.
104. Dietze B, Rath A, Wendt C, Martiny H. Survival of MRSA on sterile goods packaging. J Hosp Infect 2001;49:255–61.
105. Hardy KJ, Oppenheim BA, Gossain S, et al. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect Control Hosp Epidemiol 2006;27:127–32.
106. Jawad A, Heritage J, Snelling AM, et al. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol 1996;34:2881–7.
107. Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol 2007;28:1142–7.
108. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997;18:622–7.
109. Sexton T, Clarke P, O’Neill E, et al. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect 2006;62:187–94.
110. Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet infect dis 2008;8:101–13.
111. Dancer SJ, White LF, Lamb J, et al. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC med 2009;7.
112. Rampling A, Wiseman S, Davis L, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect 2001;49:109–16.
113. Wilson APR, Smyth D, Moore G, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med 2011;39:651–8.
114. Hess AS, Shardell M, Johnson JK, et al. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria. Infection Control Hosp Epidemiol 2013;34:487–93.
115. Falk PS, Winnike J, Woodmansee C, et al. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol 2000;21:575–82.
116. Livornese LL Jr, Dias S, Samel C, et al. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med 1992;117:112–6.
117. Porwancher R, Sheth A, Remphrey S, et al. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium: possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemiol 1997;18:771–3.
118. Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000;343:1925–32.
119. Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol 2000;38:724–6.
120. Noskin GA, Bednarz P, Suriano T, et al. Persistent contamination of fabric-covered furniture by vancomycin-resistant enterococci: implications for upholstery selection in hospitals. Am J Infect Control 2000;28:311–3.
121. Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol 1995;16:577–81.
122. Smith TL, Iwen PC, Olson SB, Rupp ME. Environmental contamination with vancomycin-resistant enterococci in an outpatient setting. Infect Control Hosp Epidemiol 1998;19:515–8.
123. Wendt C, Wiesenthal B, Dietz E, Ruden H. Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. J Clin Microbiol 1998;36:3734–6.
124. Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol 2004;25:164–7.
125. Ray AJ, Hoyen CK, Taub TF, et al. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA 2002;287:1400–1.
126. Hayden MK, Bonten MJ, Blom DW, et al. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 2006;42:1552–60.
127. Byers KE, Durbin LJ, Simonton BM, et al. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol 1998;19:261–4.
128. Goodman ER, Platt R, Bass R, et al. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol 2008;29:593–9.
129. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009;73:378–85.
130. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA) infections. Accessed 11 Oct 2013 at www.cdc.gov/mrsa/index.html.
131. Edmond MB, Wenzel RP. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1471.
132. Lai KK, Fontecchio S, Melvin Z, Baker SP. Impact of alcohol-based, waterless hand antiseptic on the incidence of infection and colonization with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Infect Control Hosp Epidemiol 2006;27:1018–24.
133. Ostrowsky B, Steinberg JT, Farr B, et al. Reality check: should we try to detect and isolate vancomycin-resistant enterococci patients? Infect Control Hosp Epidemiol 2001;22:116–9.
134. Harbarth S, Sax H, Uckay I, et al. A predictive model for identifying surgical patients at risk of methicillin-resistant Staphylococcus aureus carriage on admission. J Am Coll Surg 2008;207:683–9.
135. Jarvis WR. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1469.
136. Krause R, Honigl M, Zollner-Schwetz I. Targeted decolonization to prevent ICU infections. N Engl J Med;369:1469–70.
137. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med;364:1407–18.
138. Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008;148:409–18.
139. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30.
140. Gurieva T, Bootsma MCJ, Bonten MJM. Successful Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections revisited. Clin Infect Dis 2012:54:1618–20.
141. Gavalda L, Masuet C, Beltran J, et al. Comparative cost of selective screening to prevent transmission of methicillin-resistant Staphylococcus aureus (MRSA), compared with the attributable costs of MRSA infection. Infection control and hospital epidemiology 2006;27:1264–6.
142. Otter JA, Herdman MT, Williams B, et al. Low prevalence of methicillin-resistant Staphylococcus aureus carriage at hospital admission: implications for risk-factor-based vs universal screening. J Hosp Infect 2013;83:114–21.
143. Harbarth S, Hawkey PM, Tenover F, et al. Update on screening and clinical diagnosis of methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 2011;37:110–7.
144. Calfee DP, Giannetta ET, Durbin LJ, et al. Control of endemic vancomycin-resistant Enterococcus among inpatients at a university hospital. Clin Infect Dis 2003;37:326–32.
145. Hendrix CW, Hammond JM, Swoboda SM, et al. Surveillance strategies and impact of vancomycin-resistant enterococcal colonization and infection in critically ill patients. Ann Surg 2001;233:259–65.
146. Muto CA, Giannetta ET, Durbin LJ, et al. Cost-effectiveness of perirectal surveillance cultures for controlling vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol 2002;23:429–35.
147. Price CS, Paule S, Noskin GA, Peterson LR. Active surveillance reduces the incidence of vancomycin-resistant enterococcal bacteremia. Clin Infect Dis 2003;37:921–8.
148. Shadel BN, Puzniak LA, Gillespie KN, et al. Surveillance for vancomycin-resistant enterococci: type, rates, costs, and implications. Infect Control Hosp Epidemiol 2006;27:1068–75.
149. Siddiqui AH, Harris AD, Hebden J, et al. The effect of active surveillance for vancomycin-resistant enterococci in high-risk units on vancomycin-resistant enterococci incidence hospital-wide. Am J Infect Control 2002;30:40–3.
150. Sandri AM, Dalarosa MG, Ruschel de Alcantara L, et al. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol 2006;27:185–7.
151. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255–65.
152. Bleasdale SC, Trick WE, Gonzalez IM, et al. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–9.
153. Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009;37:1858–65.
154. Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166:306–12.
155. Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 2013;381:1099–106.
156. Blackwood B, Thompson G, McMullan R, et al. Tea tree oil (5%) body wash versus standard care (Johnson’s Baby Softwash) to prevent colonization with methicillin-resistant Staphylococcus aureus in critically ill adults: a randomized controlled trial. J Antimicrob Chemother 2013;68:1193–9.
157. Daneman N, Sarwar S, Fowler RA, et al. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:328–41.
158. Verwaest C, Verhaegen J, Ferdinande P, et al. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med 1997;25:63–71.
159. de Smet AMGA, Kluytmans JAJW, Blok HEM, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis 2011;11:372–80.
160. de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003;362:1011–6.
161. Cepeda JA, Whitehouse T, Cooper B, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet 2005;365:295–304.
162. Dhaliwal J, McGeer A. Does isolation prevent the spread of methicillin-resistant Staphylococcus aureus? CMAJ 2005;172:875.
163. Kollef MH, Micek ST. Antimicrobial stewardship programs: mandatory for all ICUs. Crit Care 2012;16:179.
164. McKinnell JA, Huang SS, Eells SJ, et al. Quantifying the impact of extranasal testing of body sites for methicillin-resistant Staphylococcus aureus colonization at the time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 2013;34:161–70.
165. Denkinger CM, Grant AD, Denkinger M, et al. Increased multi-drug resistance among the elderly on admission to the hospital—a 12-year surveillance study. Arch Gerontol Geriatr 2013;56:227–30.
166. Boisseau D, Alfandari S, Gauzit R, et al. Staphylococcus aureus nasal carriage during the infectious diseases national congress in France. Med Mal Infect 2012;42:435–9.
167. Fritz SA, Hogan PG, Hayek G, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med 2012;166:551–7.
168. Rafee Y, Abdel-Haq N, Asmar B, et al. Increased prevalence of methicillin-resistant Staphylococcus aureus nasal colonization in household contacts of children with community acquired disease. BMC Infect Dis 2012;12:45.
169. Schechter-Perkins EM, Mitchell PM, Murray KA, et al. Prevalence and predictors of nasal and extranasal staphylococcal colonization in patients presenting to the emergency department. Ann Emerg Med 2011;57:492–9.
170. Bisaga A, Paquette K, Sabatini L, Lovell E. A prevalence study of methicillin-resistant staphylococcus aureus colonization in emergency department health care workers. Ann Emerg Med 2008;52:525–8.
171. Suffoletto B, Cannon E, Ilkhanipour K, Yealy D. Prevalence of Staphylococcus aureus nasal colonization in emergency department personnel. Ann Emerg Med 2008;52:529–33.
172. Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg 2004;139:947-51; discussion 51–3.
173. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis 2003;36:131–9.
Different Strokes for Different Folks
A 35‐year‐old woman presented to her primary care physician complaining of left post‐auricular pain, swelling, and redness. She described the pain as 8 out of 10, constant, sharp, and nonradiating. She denied fever or chills. A presumptive diagnosis of cellulitis led to a prescription for oral trimethoprim‐sulfamethoxazole. Left facial swelling worsened despite 4 days of antibiotics, so she came to the emergency department.
Noninfectious causes of this woman's symptoms include trauma, or an inflammatory condition such as polychondritis. Key infectious considerations are mastoiditis or a mastoid abscess. Herpes zoster with involvement of the pinna and auditory canal may also present with pain and redness. In the absence of findings suggestive of an infection arising from the auditory canal, cellulitis is a reasonable consideration. With the growing incidence of community‐acquired methicillin‐resistant Staphylococcus aureus infections, an agent effective against this pathogen such as trimethoprim‐sulfamethoxazole may be used, usually in combination with an antibiotic that provides more reliable coverage for group A streptococcus.
Her past medical history included poorly controlled type II diabetes mellitus and asthma. She reported no previous surgical history. Her current medications were insulin, albuterol inhaler, and trimethoprim‐sulfamethoxazole, although she had a history of noncompliance with her insulin. She was married with 1 child and was unemployed. She smoked 1 pack of cigarettes daily, drank up to 6 beers daily, and denied use of illicit drugs.
Her history of diabetes increases her risk of malignant otitis externa. Both diabetes and excess alcohol consumption are risk factors for herpes zoster. Smoking has been shown to increase the risk of otitis media and carriage by S. pneumoniae, a common pathogen in ear infections.
She was ill‐appearing and in moderate respiratory distress. Her temperature was 39C, blood pressure 149/93 mmHg, pulse 95 beats per minute, respiratory rate of 26 times per minute, with an oxygen saturation of 96% while breathing ambient air. She had swelling of the left side of the face extending to the left forehead and lateral neck. Examination of the external ear and auditory canal were unremarkable. The swelling had no associated erythema, tenderness, or lymphadenopathy. She had no oropharyngeal or nasal ulcers present. Her pupils were equal, round, and reactive to light and accommodation with normal sclera. Her trachea was midline; thyroid exam was normal. The heart sounds included normal S1 and S2 without murmurs, rubs, or gallops. Her lung exam was remarkable for inspiratory stridor. The abdominal examination revealed no distention, tenderness, organomegaly, or masses. Cranial nerve testing revealed a left‐sided central seventh nerve palsy along with decreased visual acuity of the left eye. Strength, sensation, and deep tendon reflexes were normal.
While there are many causes of facial nerve palsy, distinguishing between a peripheral palsy (which causes paralysis of the entire ipsilateral side of the face) and a central palsy (which spares the musculature of the forehead) is important. The most common type of peripheral facial nerve palsy is Bell's palsy. Infections such as meningitis or tumors of the central nervous system can cause central facial nerve or other cranial nerve palsy. Important infections to consider in this case would be viral such as herpes zoster or simplex, or atypical bacteria such as Mycoplasma and Rickettsia, which may explain the neurologic but not all of the other clinical findings in this case. It is also critical to determine whether she has an isolated seventh cranial nerve palsy or if other cranial nerves are involved such as may occur with basilar meningitis, which has a myriad of infectious and noninfectious causes. The decreased visual acuity may be a result of corneal dryness and abrasions from inability to close the eye but may also represent optic nerve problems, so detailed ophthalmologic exam is essential. Her ill appearance coupled with facial and neck swelling leads me to at least consider Lemierre's syndrome with central nervous system involvement. Finally, facial swelling and the inspiratory stridor may represent angioedema, although one‐sided involvement of the face would be unusual.
The results of initial laboratory testing were as follows: sodium, 138 mmol/L; potassium, 3.4 mmol/L; chloride, 109 mmol/L; bicarbonate, 14 mmol/L; blood urea nitrogen level, 19 mg/dL; creatinine, 1.1 mg/dL; white cell count, 23,510/mm3; differential, 90% neutrophils, 1% bands, 7% lymphocytes, 2% monocytes; hemoglobin level, 12.5 g/dL; platelet count, 566,000/mm3; hemoglobin A1c, 11%; albumin, 1.6 g/dL; total protein, 6.2 g/dL; total bilirubin, 0.8 mg/dL; alkaline phosphatase, 103 U/L; alanine aminotransferase level, 14 U/L; international normalized ratio of 1.2; partial thromboplastin time, 29 seconds (normal value, 2434 seconds); erythrocyte sedimentation rate, 121 mm/hr; creatine kinase, 561 U/L (normal value 25190). Arterial blood gas measurements with the patient breathing 50% oxygen revealed a pH of 7.34, a partial pressure of carbon dioxide of 28 mmHg, and a partial pressure of oxygen of 228 mmHg.
I am concerned that this patient has sepsis, likely due to an infectious trigger. With her clinical presentation localized to the head and neck, her history of diabetes, and the accelerated sedimentation rate, malignant otitis externa would explain many of her findings. Empiric anti‐infective therapy directed toward Pseudomonas aeruginosa should be initiated, and imaging of the head and ear should be undertaken.
The patient required intubation due to increased respiratory distress and stridor. Her physicians used intravenous vancomycin, clindamycin, and piperacillin/tazobactam to treat presumed cellulitis. Her abnormal neurologic exam led to magnetic resonance (MR) imaging and MR angiography of her neck and brain, which showed evidence of multiple regions of ischemia in the left occipital and inferior parietal distributions, as well as bilateral cerebellar distributions and enhancement of the parotid gland and mastoid air cells (Figure 1). A cerebral angiogram revealed irregularity and caliber reduction in multiple cervical and intracranial arteries, associated with intraluminal thrombi within the left intracranial vertebral artery, consistent with either vasculitis or infectious angioinvasion (Figure 2).
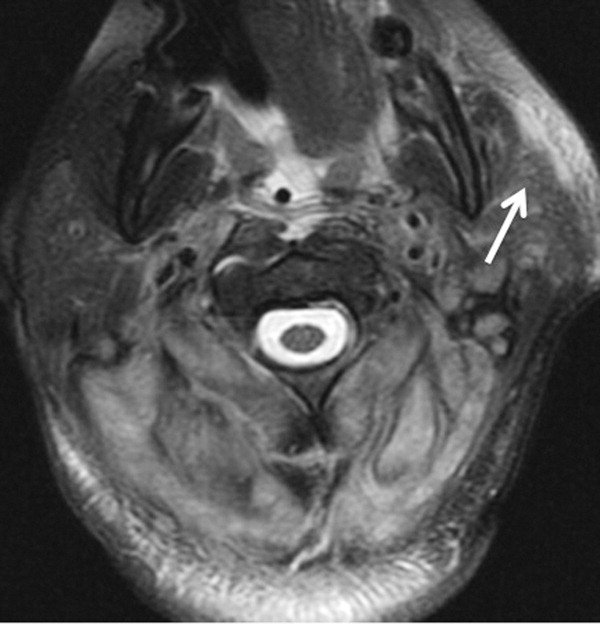

The angioinvasive nature of the findings on imaging leads me to suspect fungal infection. The patient's history of diabetes mellitus and acidosis are risk factors for mucormycosis. Aspergillus and Fusarium may also be angioinvasive but would be much more likely in neutropenic or severely immunocompromised patients. S. aureus may cause septic emboli mimicking angioinvasion but should be readily detected in conventional blood cultures. At this point, I would empirically begin amphotericin B; tissue, however, is needed for definitive diagnosis and a surgical consult should be requested.
After reviewing her imaging studies, an investigation for vasculitis and hypercoagulable states including antinuclear antibody, anti‐deoxyribonucleic acid, anti‐Smith antibody, anti‐SSA antibody level, anti‐SSB level, antineutrophil cytoplasmic antibody, activated protein C resistance level, factor VIII level, human immunodeficiency virus antibody, homocysteine level, cardiolipin antibody testing, lupus anticoagulant, prothrombin 20210 mutation, and protein C level was done, and all tests were normal. Protein S level was slightly low at 64% (normal value 65%140%). Given the enlarged parotid gland and the enhancement of the left parotid bed on magnetic resonance imaging, she underwent a parotid biopsy that revealed sialadenitis.
Systemic vasculitides can result in tissue damage, mediated by the release of endogenous cellular contents from dying cells, known as damage‐associated molecular patterns, sufficient to cause systemic inflammatory response syndrome (SIRS). This patient presented with acute symptoms but has negative laboratory studies for autoantibodies. The parotid biopsy also did not reveal evidence of vasculitis. All these findings make the diagnosis of vasculitis much less likely.
She remained in the medical intensive care unit on mechanical ventilation, with minimal symptomatic improvement. On hospital day 10, the patient developed necrosis of the left external ear. A punch biopsy of the necrotic area of her left pinna was performed; the pathology report read: Sections of punch biopsy of skin show an unremarkable epidermis. There is dermal necrosis involving the stroma and adnexal structures. Intravascular thrombi within the deep dermis are seen. Within superficial dermis there are broad, elongated, nonseptated hyaline structures reminiscent of Mucor. Special stains (periodic acid‐Schiff stain and Grocott Gomori methenamine silver stain [GMS]) performed with appropriately reactive controls fail to highlight these structures (Figure 3). The infectious disease team reviewed the pathology slides with the pathologist. As there was inconclusive evidence for zygomycosis, ie, only a few hyaline structures which failed to stain with GMS stain, the consultants recommended no change in the patient's management.
The gross and microscopic evidence of necrosis and areas of intravascular thrombi are nonspecific but compatible with a fungal infection in a patient with risk factors for zygomycosis. The GMS stain is a very sensitive stain for fungal structures, so a negative stain in this case is surprising, but additional testing such as immunohistochemistry should be pursued to confirm or refute this diagnosis. While Rhizopus species can be contaminants, the laboratory finding of these organisms in specimens from patients with risk factors for zygomycosis should not be ignored.
On hospital day 12, the patient was noted to have increased facial swelling. A computed tomographic (CT) angiogram of the neck revealed necrosis of the anterior and posterior paraspinal muscles from the skull base to C34, marked swelling of the left parotid gland, and left inferior parieto‐occipital enhancing lesion. An incisional parotid biopsy was performed. Special stains were positive for broad‐based fungal hyphae consistent with mucormycosis (Figure 4).
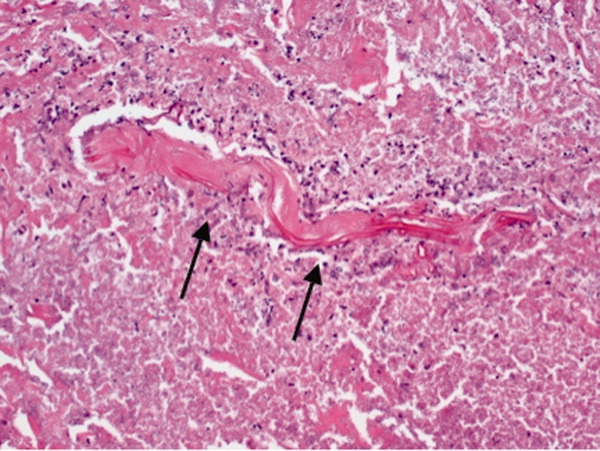
Given these findings, the patient should be started on amphotericin B immediately. Medical therapy alone generally does not suffice, and aggressive surgical debridement combined with intravenous antifungal therapy results in better outcomes. The longer the duration of symptoms and the greater the progression of disease, the less favorable the prognosis.
The patient was started on amphotericin B lipid complex and micafungin. However, after 16 days of therapy, repeat imaging of the neck showed worsening necrosis of the neck muscles. At this time, she underwent extensive debridement of face and neck, and posaconazole was added. After prolonged hospitalization, she was discharged to a rehabilitation facility on posaconazole. She resided in a nursing facility for 6 months. One year after her hospitalization, she is living at home and is able to ambulate independently, but requires feeding through a percutaneous endoscopic gastrostomy (PEG) tube because she remains dysphagic.
COMMENTARY
Infections caused by the ubiquitous fungi of the class Zygomycetes typically take 1 of 5 forms: rhinocerebral, pulmonary, gastrointestinal, disseminated, and cutaneous. The presentation varies widely, ranging from plaques, skin swelling, pustules, cellulitis, blisters, nodules, ulcerations, and ecthyma gangrenosum‐like lesions to deeper infections such as necrotizing fasciitis, osteomyelitis, and disseminated infection.1 Infections typically occur in immunocompromised hosts, including transplant recipients and patients with hematologic malignancy, but also occur in patients with diabetes mellitus, intravenous drug users, and patients on deferoxamine therapy.2 Deferoxamine and other iron‐binding therapy is thought to predispose to zygomycetes infections because of improved iron uptake of the fungal species and, thus, stimulation of growth.3 Pulmonary and rhinocerebral infections are the most common clinically encountered forms, and 44% of cutaneous infections are complicated by deep extension or dissemination.4
The articles cited above describe the more typical presentations of this rare disease. However, this patient had an unusual presentation, as parotid involvement due to zygomycosis has only been described once previously.5 Her inflammatory vasculitis and ensuing strokes from involvement of the carotid artery are recognized complications of zygomycosis, and in 1 case series of 41 patients with rhinocerebral mucormycosis, carotid involvement was seen in 31% of patients.6 After the punch biopsy of the patient's pinna showing nonseptated hyphae reminiscent of Mucor, why did her physicians delay administering amphotericin?
There are 2 likely possibilities: anchoring bias or error in medical decision‐making due to inaccurate probability estimates. Anchoring bias describes a heuristic where the initial diagnosis or gestalt biases the physician's process for assigning a final diagnosis.7, 8 This bias creates cognitive errors by limiting creativity in diagnosis. In this case, the infectious disease team carefully weighed the information obtained from the first biopsy. Given their low pretest estimate of this virtually unreported presentation of a rare disease, they decided to evaluate further without beginning antifungal therapy. Of note, there were few hyaline structures, and those structures lacked uptake of GMS. Since they considered the diagnosis yet rejected the diagnosis due to insufficient evidence, it is unlikely that anchoring bias played a role.
Was there an error in medical decision‐making? The physicians in this case faced a very common medical dilemma: whether or not to start a toxic medication empirically or wait for diagnostic confirmation prior to treatment.9 To solve this dilemma, one can apply decision analysis. Moskowitz et al described 5 phases of medical decision analysis by which a probabilistic right answer to clinical scenarios can be deduced mathematically.10 To solve this problem, probabilities must be assigned to the risk of giving a drug to a patient without the disease versus the risk of not giving a drug to a patient with the disease. For example, amphotericin deoxycholate causes acute renal failure in 30% to 47% of patients. Newer formulations of amphotericin, such as liposomal amphotericin and lipid complex, result in lower rates of nephrotoxicity (27% vs 47%). The risk of not giving amphotericin to a patient with zygomycosis is death. Even in patients treated with amphotericin, the mortality rate has been shown to be 66%, and up to 100% in those with strokes related to zygomycosis.2, 6, 11 Simply looking at these probabilities, decision analysis would favor empiric treatment.
The physicians caring for this patient did not have the luxury of retrospective speculation. After looking at all of the data, the equivocal skin biopsy and rare clinical presentation, the question to ask would change: What is the risk of giving amphotericin empirically to someone who, based on available information, has a very low probability of having zygomycosis? When phrased in this manner, there is a 47% chance of nephrotoxicity with amphotericin versus the very small probability that you have diagnosed a case of zygomycosis that has only been described once in the literature. Mathematically andmore importantlyclinically, this question becomes more difficult to answer. However, no value can be placed on the possibility of death in suspected zygomycosis, and the risk of short‐term amphotericin use is much less than that of a course of treatment. As such, empiric therapy should always be given.
Physicians are not mathematicians, and dynamic clinical scenarios are not so easily made into static math problems. Disease presentations evolve over time towards a diagnosable clinical pattern, as was the case with this patient. Two days after the aforementioned biopsy, she worsened and in less time than it would have taken to isolate zygomycosis from the first biopsy, a second biopsy revealed the typical nonseptated hyphae demarcated with the GMS stain. Even appropriate diagnostic testing, thoughtful interpretation, and avoidance of certain cognitive errors can result in incorrect diagnoses and delayed treatment. It is monitoring the progression of disease and collecting additional data that allows physicians to mold a diagnosis and create a treatment plan.
The primary treatment of zygomycosis should include amphotericin. However, there are limited data to support combination therapy with an echinocandin in severe cases, as in this patient.12 Posaconazole is not recommended for monotherapy as an initial therapy, but there is data for its use as salvage therapy in zygomycosis.13 This case highlights the difficulties that physicians face in the diagnosis and treatment of rare diseases. Cerebral infarction in a hematologic malignancy, uncontrolled diabetes, or iron chelation therapy could be the initial presentation of rhinocerebral zygomycosis. There truly are different strokes for different folks. Recognizing this and similar presentations may lead to a more rapid diagnosis and treatment of zygomycosis.
TEACHING POINTS
-
Zygomycosis has a wide range of clinical presentations ranging from skin lesions to deep tissue infections. As it is an angioinvasive organism, it can also present as cerebral infarcts and brain abscesses.
-
Zygomycosis infections should be suspected in patients with uncontrolled diabetes, hematologic or oncologic malignancies, and patients on iron chelation therapy with a potentially compatible clinical picture.
-
If zygomycosis infection is suspected, rapid histologic diagnosis should be attempted. However, as histologic diagnosis can take time, empiric therapy with amphotericin should always be administered.
-
Amphotericin remains the primary medical therapy for this disease; however, there is limited emerging evidence to suggest that echinocandins can be used in combination with amphotericin for improved treatment of severe rhinocerebral zygomyocosis. Posaconazole has a role as salvage therapy in zygomycosis, but should not be used as the sole primary treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors are indebted to Dr Glenn Roberson at the Department of Radiology, University of Alabama at Birmingham, for providing the radiographic images; to Dr Aleodor Andea at the Department of Pathology, University of Alabama at Birmingham, for providing the pathology images; and to Dr. Crysten Brinkley at the Department of Neurology at the University of Alabama at Birmingham for her assistance with this case presentation.
Disclosure: Nothing to report.
- ,,,.Mucormycosis: emerging prominence of cutaneous infections.Clin Infect Dis.1994;19:67–76.
- ,,,.Zygomycosis in the 1990s in a tertiary‐care cancer center.Clin Infect Dis.2000;30:851–856.
- ,,, et al.Mucormycosis during deferoxamine therapy is a siderophore‐mediated infection. In vitro and in vivo animal studies.J Clin Invest.1993;91:1979–1986.
- ,,, et al.Epidemiology and outcome of zygomycosis: a review of 929 reported cases.Clin Infect Dis.2005;41:634–653.
- ,,,,,.Cutaneous mucormycosis of the head and neck with parotid gland involvement: first report of a case.Ear Nose Throat J.2004;83:282–286.
- ,,,,,.A successful combined endovascular and surgical treatment of a cranial base mucormycosis with an associated internal carotid artery pseudoaneurysm.Neurosurgery.2009;65:733–740.
- ,.Judgment under uncertainty: heuristics and biases.Science.1974;185:1124–1131.
- ,,,,.Clinical problem‐solving. Anchors away.N Engl J Med.2007;356:504–509.
- ,,,.Clinical problem‐solving. Empirically incorrect.N Engl J Med.2006;354:509–514.
- ,,.Dealing with uncertainty, risks, and tradeoffs in clinical decisions. A cognitive science approach.Ann Intern Med.1988;108:435–449.
- ,,.Fatal strokes in patients with rhino‐orbito‐cerebral mucormycosis and associated vasculopathy.Scand J Infect Dis.2004;36:643–648.
- ,,, et al.Combination polyene‐caspofungin treatment of rhino‐orbital‐cerebral mucormycosis.Clin Infect Dis.2008;47:364–371.
- ,,,,.Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases.Clin Infect Dis.2006;42:e61–e65.
A 35‐year‐old woman presented to her primary care physician complaining of left post‐auricular pain, swelling, and redness. She described the pain as 8 out of 10, constant, sharp, and nonradiating. She denied fever or chills. A presumptive diagnosis of cellulitis led to a prescription for oral trimethoprim‐sulfamethoxazole. Left facial swelling worsened despite 4 days of antibiotics, so she came to the emergency department.
Noninfectious causes of this woman's symptoms include trauma, or an inflammatory condition such as polychondritis. Key infectious considerations are mastoiditis or a mastoid abscess. Herpes zoster with involvement of the pinna and auditory canal may also present with pain and redness. In the absence of findings suggestive of an infection arising from the auditory canal, cellulitis is a reasonable consideration. With the growing incidence of community‐acquired methicillin‐resistant Staphylococcus aureus infections, an agent effective against this pathogen such as trimethoprim‐sulfamethoxazole may be used, usually in combination with an antibiotic that provides more reliable coverage for group A streptococcus.
Her past medical history included poorly controlled type II diabetes mellitus and asthma. She reported no previous surgical history. Her current medications were insulin, albuterol inhaler, and trimethoprim‐sulfamethoxazole, although she had a history of noncompliance with her insulin. She was married with 1 child and was unemployed. She smoked 1 pack of cigarettes daily, drank up to 6 beers daily, and denied use of illicit drugs.
Her history of diabetes increases her risk of malignant otitis externa. Both diabetes and excess alcohol consumption are risk factors for herpes zoster. Smoking has been shown to increase the risk of otitis media and carriage by S. pneumoniae, a common pathogen in ear infections.
She was ill‐appearing and in moderate respiratory distress. Her temperature was 39C, blood pressure 149/93 mmHg, pulse 95 beats per minute, respiratory rate of 26 times per minute, with an oxygen saturation of 96% while breathing ambient air. She had swelling of the left side of the face extending to the left forehead and lateral neck. Examination of the external ear and auditory canal were unremarkable. The swelling had no associated erythema, tenderness, or lymphadenopathy. She had no oropharyngeal or nasal ulcers present. Her pupils were equal, round, and reactive to light and accommodation with normal sclera. Her trachea was midline; thyroid exam was normal. The heart sounds included normal S1 and S2 without murmurs, rubs, or gallops. Her lung exam was remarkable for inspiratory stridor. The abdominal examination revealed no distention, tenderness, organomegaly, or masses. Cranial nerve testing revealed a left‐sided central seventh nerve palsy along with decreased visual acuity of the left eye. Strength, sensation, and deep tendon reflexes were normal.
While there are many causes of facial nerve palsy, distinguishing between a peripheral palsy (which causes paralysis of the entire ipsilateral side of the face) and a central palsy (which spares the musculature of the forehead) is important. The most common type of peripheral facial nerve palsy is Bell's palsy. Infections such as meningitis or tumors of the central nervous system can cause central facial nerve or other cranial nerve palsy. Important infections to consider in this case would be viral such as herpes zoster or simplex, or atypical bacteria such as Mycoplasma and Rickettsia, which may explain the neurologic but not all of the other clinical findings in this case. It is also critical to determine whether she has an isolated seventh cranial nerve palsy or if other cranial nerves are involved such as may occur with basilar meningitis, which has a myriad of infectious and noninfectious causes. The decreased visual acuity may be a result of corneal dryness and abrasions from inability to close the eye but may also represent optic nerve problems, so detailed ophthalmologic exam is essential. Her ill appearance coupled with facial and neck swelling leads me to at least consider Lemierre's syndrome with central nervous system involvement. Finally, facial swelling and the inspiratory stridor may represent angioedema, although one‐sided involvement of the face would be unusual.
The results of initial laboratory testing were as follows: sodium, 138 mmol/L; potassium, 3.4 mmol/L; chloride, 109 mmol/L; bicarbonate, 14 mmol/L; blood urea nitrogen level, 19 mg/dL; creatinine, 1.1 mg/dL; white cell count, 23,510/mm3; differential, 90% neutrophils, 1% bands, 7% lymphocytes, 2% monocytes; hemoglobin level, 12.5 g/dL; platelet count, 566,000/mm3; hemoglobin A1c, 11%; albumin, 1.6 g/dL; total protein, 6.2 g/dL; total bilirubin, 0.8 mg/dL; alkaline phosphatase, 103 U/L; alanine aminotransferase level, 14 U/L; international normalized ratio of 1.2; partial thromboplastin time, 29 seconds (normal value, 2434 seconds); erythrocyte sedimentation rate, 121 mm/hr; creatine kinase, 561 U/L (normal value 25190). Arterial blood gas measurements with the patient breathing 50% oxygen revealed a pH of 7.34, a partial pressure of carbon dioxide of 28 mmHg, and a partial pressure of oxygen of 228 mmHg.
I am concerned that this patient has sepsis, likely due to an infectious trigger. With her clinical presentation localized to the head and neck, her history of diabetes, and the accelerated sedimentation rate, malignant otitis externa would explain many of her findings. Empiric anti‐infective therapy directed toward Pseudomonas aeruginosa should be initiated, and imaging of the head and ear should be undertaken.
The patient required intubation due to increased respiratory distress and stridor. Her physicians used intravenous vancomycin, clindamycin, and piperacillin/tazobactam to treat presumed cellulitis. Her abnormal neurologic exam led to magnetic resonance (MR) imaging and MR angiography of her neck and brain, which showed evidence of multiple regions of ischemia in the left occipital and inferior parietal distributions, as well as bilateral cerebellar distributions and enhancement of the parotid gland and mastoid air cells (Figure 1). A cerebral angiogram revealed irregularity and caliber reduction in multiple cervical and intracranial arteries, associated with intraluminal thrombi within the left intracranial vertebral artery, consistent with either vasculitis or infectious angioinvasion (Figure 2).


The angioinvasive nature of the findings on imaging leads me to suspect fungal infection. The patient's history of diabetes mellitus and acidosis are risk factors for mucormycosis. Aspergillus and Fusarium may also be angioinvasive but would be much more likely in neutropenic or severely immunocompromised patients. S. aureus may cause septic emboli mimicking angioinvasion but should be readily detected in conventional blood cultures. At this point, I would empirically begin amphotericin B; tissue, however, is needed for definitive diagnosis and a surgical consult should be requested.
After reviewing her imaging studies, an investigation for vasculitis and hypercoagulable states including antinuclear antibody, anti‐deoxyribonucleic acid, anti‐Smith antibody, anti‐SSA antibody level, anti‐SSB level, antineutrophil cytoplasmic antibody, activated protein C resistance level, factor VIII level, human immunodeficiency virus antibody, homocysteine level, cardiolipin antibody testing, lupus anticoagulant, prothrombin 20210 mutation, and protein C level was done, and all tests were normal. Protein S level was slightly low at 64% (normal value 65%140%). Given the enlarged parotid gland and the enhancement of the left parotid bed on magnetic resonance imaging, she underwent a parotid biopsy that revealed sialadenitis.
Systemic vasculitides can result in tissue damage, mediated by the release of endogenous cellular contents from dying cells, known as damage‐associated molecular patterns, sufficient to cause systemic inflammatory response syndrome (SIRS). This patient presented with acute symptoms but has negative laboratory studies for autoantibodies. The parotid biopsy also did not reveal evidence of vasculitis. All these findings make the diagnosis of vasculitis much less likely.
She remained in the medical intensive care unit on mechanical ventilation, with minimal symptomatic improvement. On hospital day 10, the patient developed necrosis of the left external ear. A punch biopsy of the necrotic area of her left pinna was performed; the pathology report read: Sections of punch biopsy of skin show an unremarkable epidermis. There is dermal necrosis involving the stroma and adnexal structures. Intravascular thrombi within the deep dermis are seen. Within superficial dermis there are broad, elongated, nonseptated hyaline structures reminiscent of Mucor. Special stains (periodic acid‐Schiff stain and Grocott Gomori methenamine silver stain [GMS]) performed with appropriately reactive controls fail to highlight these structures (Figure 3). The infectious disease team reviewed the pathology slides with the pathologist. As there was inconclusive evidence for zygomycosis, ie, only a few hyaline structures which failed to stain with GMS stain, the consultants recommended no change in the patient's management.

The gross and microscopic evidence of necrosis and areas of intravascular thrombi are nonspecific but compatible with a fungal infection in a patient with risk factors for zygomycosis. The GMS stain is a very sensitive stain for fungal structures, so a negative stain in this case is surprising, but additional testing such as immunohistochemistry should be pursued to confirm or refute this diagnosis. While Rhizopus species can be contaminants, the laboratory finding of these organisms in specimens from patients with risk factors for zygomycosis should not be ignored.
On hospital day 12, the patient was noted to have increased facial swelling. A computed tomographic (CT) angiogram of the neck revealed necrosis of the anterior and posterior paraspinal muscles from the skull base to C34, marked swelling of the left parotid gland, and left inferior parieto‐occipital enhancing lesion. An incisional parotid biopsy was performed. Special stains were positive for broad‐based fungal hyphae consistent with mucormycosis (Figure 4).

Given these findings, the patient should be started on amphotericin B immediately. Medical therapy alone generally does not suffice, and aggressive surgical debridement combined with intravenous antifungal therapy results in better outcomes. The longer the duration of symptoms and the greater the progression of disease, the less favorable the prognosis.
The patient was started on amphotericin B lipid complex and micafungin. However, after 16 days of therapy, repeat imaging of the neck showed worsening necrosis of the neck muscles. At this time, she underwent extensive debridement of face and neck, and posaconazole was added. After prolonged hospitalization, she was discharged to a rehabilitation facility on posaconazole. She resided in a nursing facility for 6 months. One year after her hospitalization, she is living at home and is able to ambulate independently, but requires feeding through a percutaneous endoscopic gastrostomy (PEG) tube because she remains dysphagic.
COMMENTARY
Infections caused by the ubiquitous fungi of the class Zygomycetes typically take 1 of 5 forms: rhinocerebral, pulmonary, gastrointestinal, disseminated, and cutaneous. The presentation varies widely, ranging from plaques, skin swelling, pustules, cellulitis, blisters, nodules, ulcerations, and ecthyma gangrenosum‐like lesions to deeper infections such as necrotizing fasciitis, osteomyelitis, and disseminated infection.1 Infections typically occur in immunocompromised hosts, including transplant recipients and patients with hematologic malignancy, but also occur in patients with diabetes mellitus, intravenous drug users, and patients on deferoxamine therapy.2 Deferoxamine and other iron‐binding therapy is thought to predispose to zygomycetes infections because of improved iron uptake of the fungal species and, thus, stimulation of growth.3 Pulmonary and rhinocerebral infections are the most common clinically encountered forms, and 44% of cutaneous infections are complicated by deep extension or dissemination.4
The articles cited above describe the more typical presentations of this rare disease. However, this patient had an unusual presentation, as parotid involvement due to zygomycosis has only been described once previously.5 Her inflammatory vasculitis and ensuing strokes from involvement of the carotid artery are recognized complications of zygomycosis, and in 1 case series of 41 patients with rhinocerebral mucormycosis, carotid involvement was seen in 31% of patients.6 After the punch biopsy of the patient's pinna showing nonseptated hyphae reminiscent of Mucor, why did her physicians delay administering amphotericin?
There are 2 likely possibilities: anchoring bias or error in medical decision‐making due to inaccurate probability estimates. Anchoring bias describes a heuristic where the initial diagnosis or gestalt biases the physician's process for assigning a final diagnosis.7, 8 This bias creates cognitive errors by limiting creativity in diagnosis. In this case, the infectious disease team carefully weighed the information obtained from the first biopsy. Given their low pretest estimate of this virtually unreported presentation of a rare disease, they decided to evaluate further without beginning antifungal therapy. Of note, there were few hyaline structures, and those structures lacked uptake of GMS. Since they considered the diagnosis yet rejected the diagnosis due to insufficient evidence, it is unlikely that anchoring bias played a role.
Was there an error in medical decision‐making? The physicians in this case faced a very common medical dilemma: whether or not to start a toxic medication empirically or wait for diagnostic confirmation prior to treatment.9 To solve this dilemma, one can apply decision analysis. Moskowitz et al described 5 phases of medical decision analysis by which a probabilistic right answer to clinical scenarios can be deduced mathematically.10 To solve this problem, probabilities must be assigned to the risk of giving a drug to a patient without the disease versus the risk of not giving a drug to a patient with the disease. For example, amphotericin deoxycholate causes acute renal failure in 30% to 47% of patients. Newer formulations of amphotericin, such as liposomal amphotericin and lipid complex, result in lower rates of nephrotoxicity (27% vs 47%). The risk of not giving amphotericin to a patient with zygomycosis is death. Even in patients treated with amphotericin, the mortality rate has been shown to be 66%, and up to 100% in those with strokes related to zygomycosis.2, 6, 11 Simply looking at these probabilities, decision analysis would favor empiric treatment.
The physicians caring for this patient did not have the luxury of retrospective speculation. After looking at all of the data, the equivocal skin biopsy and rare clinical presentation, the question to ask would change: What is the risk of giving amphotericin empirically to someone who, based on available information, has a very low probability of having zygomycosis? When phrased in this manner, there is a 47% chance of nephrotoxicity with amphotericin versus the very small probability that you have diagnosed a case of zygomycosis that has only been described once in the literature. Mathematically andmore importantlyclinically, this question becomes more difficult to answer. However, no value can be placed on the possibility of death in suspected zygomycosis, and the risk of short‐term amphotericin use is much less than that of a course of treatment. As such, empiric therapy should always be given.
Physicians are not mathematicians, and dynamic clinical scenarios are not so easily made into static math problems. Disease presentations evolve over time towards a diagnosable clinical pattern, as was the case with this patient. Two days after the aforementioned biopsy, she worsened and in less time than it would have taken to isolate zygomycosis from the first biopsy, a second biopsy revealed the typical nonseptated hyphae demarcated with the GMS stain. Even appropriate diagnostic testing, thoughtful interpretation, and avoidance of certain cognitive errors can result in incorrect diagnoses and delayed treatment. It is monitoring the progression of disease and collecting additional data that allows physicians to mold a diagnosis and create a treatment plan.
The primary treatment of zygomycosis should include amphotericin. However, there are limited data to support combination therapy with an echinocandin in severe cases, as in this patient.12 Posaconazole is not recommended for monotherapy as an initial therapy, but there is data for its use as salvage therapy in zygomycosis.13 This case highlights the difficulties that physicians face in the diagnosis and treatment of rare diseases. Cerebral infarction in a hematologic malignancy, uncontrolled diabetes, or iron chelation therapy could be the initial presentation of rhinocerebral zygomycosis. There truly are different strokes for different folks. Recognizing this and similar presentations may lead to a more rapid diagnosis and treatment of zygomycosis.
TEACHING POINTS
-
Zygomycosis has a wide range of clinical presentations ranging from skin lesions to deep tissue infections. As it is an angioinvasive organism, it can also present as cerebral infarcts and brain abscesses.
-
Zygomycosis infections should be suspected in patients with uncontrolled diabetes, hematologic or oncologic malignancies, and patients on iron chelation therapy with a potentially compatible clinical picture.
-
If zygomycosis infection is suspected, rapid histologic diagnosis should be attempted. However, as histologic diagnosis can take time, empiric therapy with amphotericin should always be administered.
-
Amphotericin remains the primary medical therapy for this disease; however, there is limited emerging evidence to suggest that echinocandins can be used in combination with amphotericin for improved treatment of severe rhinocerebral zygomyocosis. Posaconazole has a role as salvage therapy in zygomycosis, but should not be used as the sole primary treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors are indebted to Dr Glenn Roberson at the Department of Radiology, University of Alabama at Birmingham, for providing the radiographic images; to Dr Aleodor Andea at the Department of Pathology, University of Alabama at Birmingham, for providing the pathology images; and to Dr. Crysten Brinkley at the Department of Neurology at the University of Alabama at Birmingham for her assistance with this case presentation.
Disclosure: Nothing to report.
A 35‐year‐old woman presented to her primary care physician complaining of left post‐auricular pain, swelling, and redness. She described the pain as 8 out of 10, constant, sharp, and nonradiating. She denied fever or chills. A presumptive diagnosis of cellulitis led to a prescription for oral trimethoprim‐sulfamethoxazole. Left facial swelling worsened despite 4 days of antibiotics, so she came to the emergency department.
Noninfectious causes of this woman's symptoms include trauma, or an inflammatory condition such as polychondritis. Key infectious considerations are mastoiditis or a mastoid abscess. Herpes zoster with involvement of the pinna and auditory canal may also present with pain and redness. In the absence of findings suggestive of an infection arising from the auditory canal, cellulitis is a reasonable consideration. With the growing incidence of community‐acquired methicillin‐resistant Staphylococcus aureus infections, an agent effective against this pathogen such as trimethoprim‐sulfamethoxazole may be used, usually in combination with an antibiotic that provides more reliable coverage for group A streptococcus.
Her past medical history included poorly controlled type II diabetes mellitus and asthma. She reported no previous surgical history. Her current medications were insulin, albuterol inhaler, and trimethoprim‐sulfamethoxazole, although she had a history of noncompliance with her insulin. She was married with 1 child and was unemployed. She smoked 1 pack of cigarettes daily, drank up to 6 beers daily, and denied use of illicit drugs.
Her history of diabetes increases her risk of malignant otitis externa. Both diabetes and excess alcohol consumption are risk factors for herpes zoster. Smoking has been shown to increase the risk of otitis media and carriage by S. pneumoniae, a common pathogen in ear infections.
She was ill‐appearing and in moderate respiratory distress. Her temperature was 39C, blood pressure 149/93 mmHg, pulse 95 beats per minute, respiratory rate of 26 times per minute, with an oxygen saturation of 96% while breathing ambient air. She had swelling of the left side of the face extending to the left forehead and lateral neck. Examination of the external ear and auditory canal were unremarkable. The swelling had no associated erythema, tenderness, or lymphadenopathy. She had no oropharyngeal or nasal ulcers present. Her pupils were equal, round, and reactive to light and accommodation with normal sclera. Her trachea was midline; thyroid exam was normal. The heart sounds included normal S1 and S2 without murmurs, rubs, or gallops. Her lung exam was remarkable for inspiratory stridor. The abdominal examination revealed no distention, tenderness, organomegaly, or masses. Cranial nerve testing revealed a left‐sided central seventh nerve palsy along with decreased visual acuity of the left eye. Strength, sensation, and deep tendon reflexes were normal.
While there are many causes of facial nerve palsy, distinguishing between a peripheral palsy (which causes paralysis of the entire ipsilateral side of the face) and a central palsy (which spares the musculature of the forehead) is important. The most common type of peripheral facial nerve palsy is Bell's palsy. Infections such as meningitis or tumors of the central nervous system can cause central facial nerve or other cranial nerve palsy. Important infections to consider in this case would be viral such as herpes zoster or simplex, or atypical bacteria such as Mycoplasma and Rickettsia, which may explain the neurologic but not all of the other clinical findings in this case. It is also critical to determine whether she has an isolated seventh cranial nerve palsy or if other cranial nerves are involved such as may occur with basilar meningitis, which has a myriad of infectious and noninfectious causes. The decreased visual acuity may be a result of corneal dryness and abrasions from inability to close the eye but may also represent optic nerve problems, so detailed ophthalmologic exam is essential. Her ill appearance coupled with facial and neck swelling leads me to at least consider Lemierre's syndrome with central nervous system involvement. Finally, facial swelling and the inspiratory stridor may represent angioedema, although one‐sided involvement of the face would be unusual.
The results of initial laboratory testing were as follows: sodium, 138 mmol/L; potassium, 3.4 mmol/L; chloride, 109 mmol/L; bicarbonate, 14 mmol/L; blood urea nitrogen level, 19 mg/dL; creatinine, 1.1 mg/dL; white cell count, 23,510/mm3; differential, 90% neutrophils, 1% bands, 7% lymphocytes, 2% monocytes; hemoglobin level, 12.5 g/dL; platelet count, 566,000/mm3; hemoglobin A1c, 11%; albumin, 1.6 g/dL; total protein, 6.2 g/dL; total bilirubin, 0.8 mg/dL; alkaline phosphatase, 103 U/L; alanine aminotransferase level, 14 U/L; international normalized ratio of 1.2; partial thromboplastin time, 29 seconds (normal value, 2434 seconds); erythrocyte sedimentation rate, 121 mm/hr; creatine kinase, 561 U/L (normal value 25190). Arterial blood gas measurements with the patient breathing 50% oxygen revealed a pH of 7.34, a partial pressure of carbon dioxide of 28 mmHg, and a partial pressure of oxygen of 228 mmHg.
I am concerned that this patient has sepsis, likely due to an infectious trigger. With her clinical presentation localized to the head and neck, her history of diabetes, and the accelerated sedimentation rate, malignant otitis externa would explain many of her findings. Empiric anti‐infective therapy directed toward Pseudomonas aeruginosa should be initiated, and imaging of the head and ear should be undertaken.
The patient required intubation due to increased respiratory distress and stridor. Her physicians used intravenous vancomycin, clindamycin, and piperacillin/tazobactam to treat presumed cellulitis. Her abnormal neurologic exam led to magnetic resonance (MR) imaging and MR angiography of her neck and brain, which showed evidence of multiple regions of ischemia in the left occipital and inferior parietal distributions, as well as bilateral cerebellar distributions and enhancement of the parotid gland and mastoid air cells (Figure 1). A cerebral angiogram revealed irregularity and caliber reduction in multiple cervical and intracranial arteries, associated with intraluminal thrombi within the left intracranial vertebral artery, consistent with either vasculitis or infectious angioinvasion (Figure 2).


The angioinvasive nature of the findings on imaging leads me to suspect fungal infection. The patient's history of diabetes mellitus and acidosis are risk factors for mucormycosis. Aspergillus and Fusarium may also be angioinvasive but would be much more likely in neutropenic or severely immunocompromised patients. S. aureus may cause septic emboli mimicking angioinvasion but should be readily detected in conventional blood cultures. At this point, I would empirically begin amphotericin B; tissue, however, is needed for definitive diagnosis and a surgical consult should be requested.
After reviewing her imaging studies, an investigation for vasculitis and hypercoagulable states including antinuclear antibody, anti‐deoxyribonucleic acid, anti‐Smith antibody, anti‐SSA antibody level, anti‐SSB level, antineutrophil cytoplasmic antibody, activated protein C resistance level, factor VIII level, human immunodeficiency virus antibody, homocysteine level, cardiolipin antibody testing, lupus anticoagulant, prothrombin 20210 mutation, and protein C level was done, and all tests were normal. Protein S level was slightly low at 64% (normal value 65%140%). Given the enlarged parotid gland and the enhancement of the left parotid bed on magnetic resonance imaging, she underwent a parotid biopsy that revealed sialadenitis.
Systemic vasculitides can result in tissue damage, mediated by the release of endogenous cellular contents from dying cells, known as damage‐associated molecular patterns, sufficient to cause systemic inflammatory response syndrome (SIRS). This patient presented with acute symptoms but has negative laboratory studies for autoantibodies. The parotid biopsy also did not reveal evidence of vasculitis. All these findings make the diagnosis of vasculitis much less likely.
She remained in the medical intensive care unit on mechanical ventilation, with minimal symptomatic improvement. On hospital day 10, the patient developed necrosis of the left external ear. A punch biopsy of the necrotic area of her left pinna was performed; the pathology report read: Sections of punch biopsy of skin show an unremarkable epidermis. There is dermal necrosis involving the stroma and adnexal structures. Intravascular thrombi within the deep dermis are seen. Within superficial dermis there are broad, elongated, nonseptated hyaline structures reminiscent of Mucor. Special stains (periodic acid‐Schiff stain and Grocott Gomori methenamine silver stain [GMS]) performed with appropriately reactive controls fail to highlight these structures (Figure 3). The infectious disease team reviewed the pathology slides with the pathologist. As there was inconclusive evidence for zygomycosis, ie, only a few hyaline structures which failed to stain with GMS stain, the consultants recommended no change in the patient's management.

The gross and microscopic evidence of necrosis and areas of intravascular thrombi are nonspecific but compatible with a fungal infection in a patient with risk factors for zygomycosis. The GMS stain is a very sensitive stain for fungal structures, so a negative stain in this case is surprising, but additional testing such as immunohistochemistry should be pursued to confirm or refute this diagnosis. While Rhizopus species can be contaminants, the laboratory finding of these organisms in specimens from patients with risk factors for zygomycosis should not be ignored.
On hospital day 12, the patient was noted to have increased facial swelling. A computed tomographic (CT) angiogram of the neck revealed necrosis of the anterior and posterior paraspinal muscles from the skull base to C34, marked swelling of the left parotid gland, and left inferior parieto‐occipital enhancing lesion. An incisional parotid biopsy was performed. Special stains were positive for broad‐based fungal hyphae consistent with mucormycosis (Figure 4).

Given these findings, the patient should be started on amphotericin B immediately. Medical therapy alone generally does not suffice, and aggressive surgical debridement combined with intravenous antifungal therapy results in better outcomes. The longer the duration of symptoms and the greater the progression of disease, the less favorable the prognosis.
The patient was started on amphotericin B lipid complex and micafungin. However, after 16 days of therapy, repeat imaging of the neck showed worsening necrosis of the neck muscles. At this time, she underwent extensive debridement of face and neck, and posaconazole was added. After prolonged hospitalization, she was discharged to a rehabilitation facility on posaconazole. She resided in a nursing facility for 6 months. One year after her hospitalization, she is living at home and is able to ambulate independently, but requires feeding through a percutaneous endoscopic gastrostomy (PEG) tube because she remains dysphagic.
COMMENTARY
Infections caused by the ubiquitous fungi of the class Zygomycetes typically take 1 of 5 forms: rhinocerebral, pulmonary, gastrointestinal, disseminated, and cutaneous. The presentation varies widely, ranging from plaques, skin swelling, pustules, cellulitis, blisters, nodules, ulcerations, and ecthyma gangrenosum‐like lesions to deeper infections such as necrotizing fasciitis, osteomyelitis, and disseminated infection.1 Infections typically occur in immunocompromised hosts, including transplant recipients and patients with hematologic malignancy, but also occur in patients with diabetes mellitus, intravenous drug users, and patients on deferoxamine therapy.2 Deferoxamine and other iron‐binding therapy is thought to predispose to zygomycetes infections because of improved iron uptake of the fungal species and, thus, stimulation of growth.3 Pulmonary and rhinocerebral infections are the most common clinically encountered forms, and 44% of cutaneous infections are complicated by deep extension or dissemination.4
The articles cited above describe the more typical presentations of this rare disease. However, this patient had an unusual presentation, as parotid involvement due to zygomycosis has only been described once previously.5 Her inflammatory vasculitis and ensuing strokes from involvement of the carotid artery are recognized complications of zygomycosis, and in 1 case series of 41 patients with rhinocerebral mucormycosis, carotid involvement was seen in 31% of patients.6 After the punch biopsy of the patient's pinna showing nonseptated hyphae reminiscent of Mucor, why did her physicians delay administering amphotericin?
There are 2 likely possibilities: anchoring bias or error in medical decision‐making due to inaccurate probability estimates. Anchoring bias describes a heuristic where the initial diagnosis or gestalt biases the physician's process for assigning a final diagnosis.7, 8 This bias creates cognitive errors by limiting creativity in diagnosis. In this case, the infectious disease team carefully weighed the information obtained from the first biopsy. Given their low pretest estimate of this virtually unreported presentation of a rare disease, they decided to evaluate further without beginning antifungal therapy. Of note, there were few hyaline structures, and those structures lacked uptake of GMS. Since they considered the diagnosis yet rejected the diagnosis due to insufficient evidence, it is unlikely that anchoring bias played a role.
Was there an error in medical decision‐making? The physicians in this case faced a very common medical dilemma: whether or not to start a toxic medication empirically or wait for diagnostic confirmation prior to treatment.9 To solve this dilemma, one can apply decision analysis. Moskowitz et al described 5 phases of medical decision analysis by which a probabilistic right answer to clinical scenarios can be deduced mathematically.10 To solve this problem, probabilities must be assigned to the risk of giving a drug to a patient without the disease versus the risk of not giving a drug to a patient with the disease. For example, amphotericin deoxycholate causes acute renal failure in 30% to 47% of patients. Newer formulations of amphotericin, such as liposomal amphotericin and lipid complex, result in lower rates of nephrotoxicity (27% vs 47%). The risk of not giving amphotericin to a patient with zygomycosis is death. Even in patients treated with amphotericin, the mortality rate has been shown to be 66%, and up to 100% in those with strokes related to zygomycosis.2, 6, 11 Simply looking at these probabilities, decision analysis would favor empiric treatment.
The physicians caring for this patient did not have the luxury of retrospective speculation. After looking at all of the data, the equivocal skin biopsy and rare clinical presentation, the question to ask would change: What is the risk of giving amphotericin empirically to someone who, based on available information, has a very low probability of having zygomycosis? When phrased in this manner, there is a 47% chance of nephrotoxicity with amphotericin versus the very small probability that you have diagnosed a case of zygomycosis that has only been described once in the literature. Mathematically andmore importantlyclinically, this question becomes more difficult to answer. However, no value can be placed on the possibility of death in suspected zygomycosis, and the risk of short‐term amphotericin use is much less than that of a course of treatment. As such, empiric therapy should always be given.
Physicians are not mathematicians, and dynamic clinical scenarios are not so easily made into static math problems. Disease presentations evolve over time towards a diagnosable clinical pattern, as was the case with this patient. Two days after the aforementioned biopsy, she worsened and in less time than it would have taken to isolate zygomycosis from the first biopsy, a second biopsy revealed the typical nonseptated hyphae demarcated with the GMS stain. Even appropriate diagnostic testing, thoughtful interpretation, and avoidance of certain cognitive errors can result in incorrect diagnoses and delayed treatment. It is monitoring the progression of disease and collecting additional data that allows physicians to mold a diagnosis and create a treatment plan.
The primary treatment of zygomycosis should include amphotericin. However, there are limited data to support combination therapy with an echinocandin in severe cases, as in this patient.12 Posaconazole is not recommended for monotherapy as an initial therapy, but there is data for its use as salvage therapy in zygomycosis.13 This case highlights the difficulties that physicians face in the diagnosis and treatment of rare diseases. Cerebral infarction in a hematologic malignancy, uncontrolled diabetes, or iron chelation therapy could be the initial presentation of rhinocerebral zygomycosis. There truly are different strokes for different folks. Recognizing this and similar presentations may lead to a more rapid diagnosis and treatment of zygomycosis.
TEACHING POINTS
-
Zygomycosis has a wide range of clinical presentations ranging from skin lesions to deep tissue infections. As it is an angioinvasive organism, it can also present as cerebral infarcts and brain abscesses.
-
Zygomycosis infections should be suspected in patients with uncontrolled diabetes, hematologic or oncologic malignancies, and patients on iron chelation therapy with a potentially compatible clinical picture.
-
If zygomycosis infection is suspected, rapid histologic diagnosis should be attempted. However, as histologic diagnosis can take time, empiric therapy with amphotericin should always be administered.
-
Amphotericin remains the primary medical therapy for this disease; however, there is limited emerging evidence to suggest that echinocandins can be used in combination with amphotericin for improved treatment of severe rhinocerebral zygomyocosis. Posaconazole has a role as salvage therapy in zygomycosis, but should not be used as the sole primary treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors are indebted to Dr Glenn Roberson at the Department of Radiology, University of Alabama at Birmingham, for providing the radiographic images; to Dr Aleodor Andea at the Department of Pathology, University of Alabama at Birmingham, for providing the pathology images; and to Dr. Crysten Brinkley at the Department of Neurology at the University of Alabama at Birmingham for her assistance with this case presentation.
Disclosure: Nothing to report.
- ,,,.Mucormycosis: emerging prominence of cutaneous infections.Clin Infect Dis.1994;19:67–76.
- ,,,.Zygomycosis in the 1990s in a tertiary‐care cancer center.Clin Infect Dis.2000;30:851–856.
- ,,, et al.Mucormycosis during deferoxamine therapy is a siderophore‐mediated infection. In vitro and in vivo animal studies.J Clin Invest.1993;91:1979–1986.
- ,,, et al.Epidemiology and outcome of zygomycosis: a review of 929 reported cases.Clin Infect Dis.2005;41:634–653.
- ,,,,,.Cutaneous mucormycosis of the head and neck with parotid gland involvement: first report of a case.Ear Nose Throat J.2004;83:282–286.
- ,,,,,.A successful combined endovascular and surgical treatment of a cranial base mucormycosis with an associated internal carotid artery pseudoaneurysm.Neurosurgery.2009;65:733–740.
- ,.Judgment under uncertainty: heuristics and biases.Science.1974;185:1124–1131.
- ,,,,.Clinical problem‐solving. Anchors away.N Engl J Med.2007;356:504–509.
- ,,,.Clinical problem‐solving. Empirically incorrect.N Engl J Med.2006;354:509–514.
- ,,.Dealing with uncertainty, risks, and tradeoffs in clinical decisions. A cognitive science approach.Ann Intern Med.1988;108:435–449.
- ,,.Fatal strokes in patients with rhino‐orbito‐cerebral mucormycosis and associated vasculopathy.Scand J Infect Dis.2004;36:643–648.
- ,,, et al.Combination polyene‐caspofungin treatment of rhino‐orbital‐cerebral mucormycosis.Clin Infect Dis.2008;47:364–371.
- ,,,,.Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases.Clin Infect Dis.2006;42:e61–e65.
- ,,,.Mucormycosis: emerging prominence of cutaneous infections.Clin Infect Dis.1994;19:67–76.
- ,,,.Zygomycosis in the 1990s in a tertiary‐care cancer center.Clin Infect Dis.2000;30:851–856.
- ,,, et al.Mucormycosis during deferoxamine therapy is a siderophore‐mediated infection. In vitro and in vivo animal studies.J Clin Invest.1993;91:1979–1986.
- ,,, et al.Epidemiology and outcome of zygomycosis: a review of 929 reported cases.Clin Infect Dis.2005;41:634–653.
- ,,,,,.Cutaneous mucormycosis of the head and neck with parotid gland involvement: first report of a case.Ear Nose Throat J.2004;83:282–286.
- ,,,,,.A successful combined endovascular and surgical treatment of a cranial base mucormycosis with an associated internal carotid artery pseudoaneurysm.Neurosurgery.2009;65:733–740.
- ,.Judgment under uncertainty: heuristics and biases.Science.1974;185:1124–1131.
- ,,,,.Clinical problem‐solving. Anchors away.N Engl J Med.2007;356:504–509.
- ,,,.Clinical problem‐solving. Empirically incorrect.N Engl J Med.2006;354:509–514.
- ,,.Dealing with uncertainty, risks, and tradeoffs in clinical decisions. A cognitive science approach.Ann Intern Med.1988;108:435–449.
- ,,.Fatal strokes in patients with rhino‐orbito‐cerebral mucormycosis and associated vasculopathy.Scand J Infect Dis.2004;36:643–648.
- ,,, et al.Combination polyene‐caspofungin treatment of rhino‐orbital‐cerebral mucormycosis.Clin Infect Dis.2008;47:364–371.
- ,,,,.Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases.Clin Infect Dis.2006;42:e61–e65.