User login
Essential Oils Debunked: Separating Fact From Myth
What is an essential oil?
An essential oil (EO) is defined by the International Organization for Standardization as a ‘‘product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes.’’1 Steam distillation is the primary method used for the production of commercial EOs,2 and believe it or not, most EOs contain 100 to 250 individual chemical components.3
The term essential oil often is incorrectly used for a variety of products obtained from plant material by methods other than distillation or cold-pressing, such as extraction. Products that are obtained via the extraction method include absolutes found in fine fragrances; hydrolates such as rose water; concretes such as jasmine or violet leaves; and vegetable oils including olive oil, coconut oil, and sesame oil.2 These products are not true EOs.
Where do EOs come from?
Essential oils are produced in many countries around the world.4 Individual oils may be obtained from species of different plants, from different parts of the same plant, or from various cultivars (plants selectively bred to obtain desirable levels of chemical constituents such as monoterpenes or sesquiterpenes and biochemical properties such as antibacterial or antioxidant activities).3,5 It is estimated that EOs can be obtained from approximately 30,000 plant species, but only 150 EOs are produced commercially.2,6
Why are people using EOs? What is their claim to fame?
Essential oils are employed by the flavor, food (eg, soft drinks, milk, candies, chocolate, meats, sausages, alcoholic beverages, spices, herbs, tea, preservatives, animal foods), fragrance, cosmetic, tobacco, and pharmaceutical industries. They also are used in household products (eg, detergents, fabric softeners, air fresheners, candles, incense) and for medicinal purposes (eg, folk and traditional medicine, phytotherapy, balneotherapy, aromatherapy).2 The oils usually are applied to the skin but also can be administered orally, inhaled, diffused through the air, or used by other means.4 One 2019 survey of Minnesota State Fair attendees (N=282) found the most common reasons for using EOs were a desire for alternative treatments (53.4%), the opinion that EOs are safer than traditional therapies (47.6%), and/or failure of standard medical treatments (10.7%). The survey results also indicated that 46.7% of EO users utilized EOs to treat medical conditions or symptoms.7 Of note, review of the website of an international company that produces EOs confirmed that EOs are marketed not only for adults but also for children to help them concentrate,8 sleep,9 improve the appearance of their skin,10 soothe upset stomachs,11 and decrease sniffles due to colds.12
Why are people selling EOs to family and friends? They must be making major bucks!
In general, the cost of EOs depends on the complexity of cultivated plant species; the mode of harvesting, which is sometimes done by hand; and the yield of oil. Prices range from $4.50 to an incredible $150,000 per kilogram.2 On average, one bottle containing 5 to 15 mL of an EO or oil blend can cost anywhere from $7 to $251.13 In the United States, the consumer EO market is partially composed of multilevel/network marketing companies in which direct consumer sales occur via a hierarchy of individual distributors. Goodier et al7 found that 36.4% of participants who obtained EOs from family and friends purchased them through multilevel/network marketing companies. In 2018, individual distributors of an international EO-producing company made on average anywhere from $4 to as much as $1.54 million annually by selling the company’s EO products and enrolling additional members/individual distributors to purchase or sell the company’s EO products.14
Sometimes EOs are described as natural and pure, but are they really?
Just because a product is labeled as “pure” or “natural” does not ensure that it is a good-quality EO. Organically produced (ie, grown without the use of herbicides or pesticides) plant material can include up to 30% of extraneous herbs and weeds, which can change the composition of the oil.2
Lesser-quality EOs are the result of adulteration, contamination, inadequate oil production, or aging.2 Adulteration (eg, cutting, stretching, bouquetting) occurs when foreign substances are introduced into pure EOs for the benefit of a higher profit; to ensure a sufficient supply of oils; or to meet demands for cheaper oils by “stretching” a more expensive, pure oil by combining with a cheaper, less pure oil. Inadequate oil production leading to lower-quality oils can occur when a biomass is incorrectly distilled, either from too much steam or temperatures that are too high or due to lack of adequate cooling units. Aging occurs when the oils are not stored properly, resulting in a change in the chemical composition due to esterification, reduction, and oxidization of chemicals, which leads to the formation of peroxides and hydroperoxides that can be contact allergens.15
Can patients develop contact allergies to EOs?
The short answer is yes! Contact allergy to almost 80 EOs has been reported,15 including tea tree oil,16,17 ylang-ylang oil,17,18 lavender oil, peppermint oil,18 jasmine absolute,17 geranium oil, rose oil,18 turpentine oil,19,20 and sandalwood oil.18 The recent increased prevalence of allergic reactions to EOs likely is due to increased consumer use as well as increased detection from availability of commercial patch-test preparations.
Essential oils have many common ingredients. De Groot and Schmidt3 documented that 14 of 23 chemicals present in more than 80% of EOs have been reported to cause contact allergy. Interestingly, allergic patients often react to more than one EO, which may be explained by the many shared chemical components in EOs.
Essential oils are “natural” so they must be safe?
In general, most safety profiles are good, but rare toxic reactions from EOs have been observed.4 A recent Australian study reviewed EO exposure calls to the New South Wales Poisons Information Centre.21 The majority of EO poisonings were accidental or the result of therapeutic error such as mistaking EOs for liquid pharmaceuticals. Additionally, this study found that from July 2014 to June 2018, there was a 5% increase in the number of calls per year. More than half of EO poisoning calls involved children, with toddlers being the most frequent cases, suggesting the need for child-resistant top closures. The most frequently involved EOs in poisonings were eucalyptus (46.4% [n=2049]), tea tree (17% [n=749]), lavender (6.1% [n=271]), clove (4.1% [n=179]), and peppermint (3.5% [n=154]).21 Essential oils do not come without potential pitfalls.
What is the clinical presentation and workup?
The workup of EO allergic contact dermatitis begins with obtaining a history to evaluate for use of EO diffusers, perfumes, hygiene products, cosmetics, massage oils, toothpastes, and/or pharmaceutical products. Exploration of potential exposures through occupation, environment, and hobbies also is indicated. Clinical presentation is dependent on the mechanism of exposure. Contact allergy may result from direct application of an allergen to the skin or mucous membranes, contact with a contaminated environmental item (eg, lavender oil on a pillow), contact with EOs used by partners or coworkers (consort dermatitis), airborne exposure (EO diffusers), or systemic exposure (flavorings). Airborne dermatitis from EO diffusers may involve the exposed areas of the face, neck, forearms, arms, behind the earlobes, bilateral eyelids, nasolabial folds, and under the chin. History and clinical presentation can raise suspicion for allergic contact dermatitis, and patch testing is necessary to confirm the diagnosis.
How do we patch test for EO contact allergy?
There are many EOs commercially available for patch testing, and they typically are tested at 2% to 5% concentrations in petrolatum.15 A North American and European study of 62,354 patch-tested patients found that 7.4% of EO-positive individuals did not react to fragrance allergens in a standard screening series including fragrance mix I, fragrance mix II, and balsam of Peru, highlighting the importance of patch testing with specific EOs.22 Currently, only 3 EOs—tea tree oil, peppermint oil, and ylang-ylang oil—are included in the 2019-2020 North American Contact Dermatitis Group screening series, making supplemental testing for other EOs important if contact allergy is suspected; however, testing the patient’s own products is imperative, as there is strong variability in the composition of EOs. Additionally, aged oils may have been exposed to light, oxygen, or varying temperatures, which could result in the formation of additional allergenic chemicals not present in commercially available preparations.15 In addition to commercially available allergens, we test patient-provided EOs either as is in semi-open fashion (ie, EOs are applied to patient’s back with a cotton swab, allowed to dry, covered with adhesive tape, and read at the same interval as other patch tests23) or occasionally dilute them to 1% or 10% (in olive oil or mineral oil).
How should I manage a positive patch-test reaction to EOs?
Patients should avoid relevant EO allergens in their products and environment, which can be easily achieved with the use of the American Contact Dermatitis Society’s Contact Allergen Management Program or similar databases.
Final Interpretation
We are ubiquitously exposed to EOs every day—through the products we use at home, at work, and in our environment. Essential oils make their place in the world by providing sweet-smelling aromas in addition to their alleged therapeutic properties; however, beware, EOs may be the culprit of your next patient’s allergic contact dermatitis.
- International Organization for Standardization. ISO 9235:2013. aromatic natural raw materials—vocabulary. https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. Accessed March 24, 2020.
- De Groot AC, Schmidt E. Essential oils: part II: general aspects. Dermatitis. 2016;27:43-49.
De Groot AC, Schmidt E. Essential oils: part III: chemical composition. Dermatitis. 2016;27:161-169. - De Groot AC, Schmidt E. Essential oils: part I: introduction. Dermatitis. 2016;27:39-42.
- Insawang S, Pripdeevech P, Tanapichatsakul C, et al. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem Biodivers. 2019;16:e1900371.
- Lawrence BM. A preliminary report on the world production of some selected essential oils and countries. Perfum Flavor. 2009;34:38-44.
- Goodier MC, Zhang AJ, Nikle AB, et al. Use of essential oils: a general population survey. Contact Dermatitis. 2019;80:391-393.
- KidScents GeneYus. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-geneyus. Accessed March 25, 2020.
- KidScents SleepyIze. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sleepyize-5ml. Accessed March 25, 2020.
- KidScents® Lotion. Young Living Essential Oils website. www.youngliving.com/en_US/products/kidscents-lotion. Accessed March 25, 2020.
- KidScents TummyGize. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-tummygize-5ml. Accessed March 25, 2020.
- KidScents SniffleEase. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sniffleease. Accessed March 25, 2020.
- 2019 Product Guide. Young Living Essential Oils website. https://issuu.com/youngliving/docs/yl_productguide. Accessed March 25, 2020.
- 2018 Income Disclosure Statement. Young Living Essential Oils website. https://www.youngliving.com/en_US/opportunity/income-disclosure. Accessed March 25, 2020.
- De Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. a multicenter study of the German Contact Dermatitis Group. J Dtsch Dermatol Ges. 2003;1:629-634.
- Larsen W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (part II). Contact Dermatitis. 2001;44:344-346.
- Bleasel N, Tate B, Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australas J Dermatol. 2002;43:211-213.
- Noiles K, Pratt M. Contact dermatitis to Vicks VapoRub. Dermatitis. 2010;21:167-169.
- Barchino-Ortiz L, Cabeza-Martinez R, Leis-Dosil VM, et al. Allergic contact hobby dermatitis from turpentine. Allergol Immunopathol (Madr). 2008;36:117-119.
- Lee KA, Harnett JE, Cairns R. Essential oil exposures in Australia: analysis of cases reported to the NSW Poisons Information Centre. Med J Aust. 2020;212:132-133.
- Warshaw EM, Zug KA, Belsito DV, et al. Positive patch test reactions to essential oils in consecutive patients: results from North America and central Europe. Dermatitis. 2017;28:246-252.
- Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88:879-888.
What is an essential oil?
An essential oil (EO) is defined by the International Organization for Standardization as a ‘‘product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes.’’1 Steam distillation is the primary method used for the production of commercial EOs,2 and believe it or not, most EOs contain 100 to 250 individual chemical components.3
The term essential oil often is incorrectly used for a variety of products obtained from plant material by methods other than distillation or cold-pressing, such as extraction. Products that are obtained via the extraction method include absolutes found in fine fragrances; hydrolates such as rose water; concretes such as jasmine or violet leaves; and vegetable oils including olive oil, coconut oil, and sesame oil.2 These products are not true EOs.
Where do EOs come from?
Essential oils are produced in many countries around the world.4 Individual oils may be obtained from species of different plants, from different parts of the same plant, or from various cultivars (plants selectively bred to obtain desirable levels of chemical constituents such as monoterpenes or sesquiterpenes and biochemical properties such as antibacterial or antioxidant activities).3,5 It is estimated that EOs can be obtained from approximately 30,000 plant species, but only 150 EOs are produced commercially.2,6
Why are people using EOs? What is their claim to fame?
Essential oils are employed by the flavor, food (eg, soft drinks, milk, candies, chocolate, meats, sausages, alcoholic beverages, spices, herbs, tea, preservatives, animal foods), fragrance, cosmetic, tobacco, and pharmaceutical industries. They also are used in household products (eg, detergents, fabric softeners, air fresheners, candles, incense) and for medicinal purposes (eg, folk and traditional medicine, phytotherapy, balneotherapy, aromatherapy).2 The oils usually are applied to the skin but also can be administered orally, inhaled, diffused through the air, or used by other means.4 One 2019 survey of Minnesota State Fair attendees (N=282) found the most common reasons for using EOs were a desire for alternative treatments (53.4%), the opinion that EOs are safer than traditional therapies (47.6%), and/or failure of standard medical treatments (10.7%). The survey results also indicated that 46.7% of EO users utilized EOs to treat medical conditions or symptoms.7 Of note, review of the website of an international company that produces EOs confirmed that EOs are marketed not only for adults but also for children to help them concentrate,8 sleep,9 improve the appearance of their skin,10 soothe upset stomachs,11 and decrease sniffles due to colds.12
Why are people selling EOs to family and friends? They must be making major bucks!
In general, the cost of EOs depends on the complexity of cultivated plant species; the mode of harvesting, which is sometimes done by hand; and the yield of oil. Prices range from $4.50 to an incredible $150,000 per kilogram.2 On average, one bottle containing 5 to 15 mL of an EO or oil blend can cost anywhere from $7 to $251.13 In the United States, the consumer EO market is partially composed of multilevel/network marketing companies in which direct consumer sales occur via a hierarchy of individual distributors. Goodier et al7 found that 36.4% of participants who obtained EOs from family and friends purchased them through multilevel/network marketing companies. In 2018, individual distributors of an international EO-producing company made on average anywhere from $4 to as much as $1.54 million annually by selling the company’s EO products and enrolling additional members/individual distributors to purchase or sell the company’s EO products.14
Sometimes EOs are described as natural and pure, but are they really?
Just because a product is labeled as “pure” or “natural” does not ensure that it is a good-quality EO. Organically produced (ie, grown without the use of herbicides or pesticides) plant material can include up to 30% of extraneous herbs and weeds, which can change the composition of the oil.2
Lesser-quality EOs are the result of adulteration, contamination, inadequate oil production, or aging.2 Adulteration (eg, cutting, stretching, bouquetting) occurs when foreign substances are introduced into pure EOs for the benefit of a higher profit; to ensure a sufficient supply of oils; or to meet demands for cheaper oils by “stretching” a more expensive, pure oil by combining with a cheaper, less pure oil. Inadequate oil production leading to lower-quality oils can occur when a biomass is incorrectly distilled, either from too much steam or temperatures that are too high or due to lack of adequate cooling units. Aging occurs when the oils are not stored properly, resulting in a change in the chemical composition due to esterification, reduction, and oxidization of chemicals, which leads to the formation of peroxides and hydroperoxides that can be contact allergens.15
Can patients develop contact allergies to EOs?
The short answer is yes! Contact allergy to almost 80 EOs has been reported,15 including tea tree oil,16,17 ylang-ylang oil,17,18 lavender oil, peppermint oil,18 jasmine absolute,17 geranium oil, rose oil,18 turpentine oil,19,20 and sandalwood oil.18 The recent increased prevalence of allergic reactions to EOs likely is due to increased consumer use as well as increased detection from availability of commercial patch-test preparations.
Essential oils have many common ingredients. De Groot and Schmidt3 documented that 14 of 23 chemicals present in more than 80% of EOs have been reported to cause contact allergy. Interestingly, allergic patients often react to more than one EO, which may be explained by the many shared chemical components in EOs.
Essential oils are “natural” so they must be safe?
In general, most safety profiles are good, but rare toxic reactions from EOs have been observed.4 A recent Australian study reviewed EO exposure calls to the New South Wales Poisons Information Centre.21 The majority of EO poisonings were accidental or the result of therapeutic error such as mistaking EOs for liquid pharmaceuticals. Additionally, this study found that from July 2014 to June 2018, there was a 5% increase in the number of calls per year. More than half of EO poisoning calls involved children, with toddlers being the most frequent cases, suggesting the need for child-resistant top closures. The most frequently involved EOs in poisonings were eucalyptus (46.4% [n=2049]), tea tree (17% [n=749]), lavender (6.1% [n=271]), clove (4.1% [n=179]), and peppermint (3.5% [n=154]).21 Essential oils do not come without potential pitfalls.
What is the clinical presentation and workup?
The workup of EO allergic contact dermatitis begins with obtaining a history to evaluate for use of EO diffusers, perfumes, hygiene products, cosmetics, massage oils, toothpastes, and/or pharmaceutical products. Exploration of potential exposures through occupation, environment, and hobbies also is indicated. Clinical presentation is dependent on the mechanism of exposure. Contact allergy may result from direct application of an allergen to the skin or mucous membranes, contact with a contaminated environmental item (eg, lavender oil on a pillow), contact with EOs used by partners or coworkers (consort dermatitis), airborne exposure (EO diffusers), or systemic exposure (flavorings). Airborne dermatitis from EO diffusers may involve the exposed areas of the face, neck, forearms, arms, behind the earlobes, bilateral eyelids, nasolabial folds, and under the chin. History and clinical presentation can raise suspicion for allergic contact dermatitis, and patch testing is necessary to confirm the diagnosis.
How do we patch test for EO contact allergy?
There are many EOs commercially available for patch testing, and they typically are tested at 2% to 5% concentrations in petrolatum.15 A North American and European study of 62,354 patch-tested patients found that 7.4% of EO-positive individuals did not react to fragrance allergens in a standard screening series including fragrance mix I, fragrance mix II, and balsam of Peru, highlighting the importance of patch testing with specific EOs.22 Currently, only 3 EOs—tea tree oil, peppermint oil, and ylang-ylang oil—are included in the 2019-2020 North American Contact Dermatitis Group screening series, making supplemental testing for other EOs important if contact allergy is suspected; however, testing the patient’s own products is imperative, as there is strong variability in the composition of EOs. Additionally, aged oils may have been exposed to light, oxygen, or varying temperatures, which could result in the formation of additional allergenic chemicals not present in commercially available preparations.15 In addition to commercially available allergens, we test patient-provided EOs either as is in semi-open fashion (ie, EOs are applied to patient’s back with a cotton swab, allowed to dry, covered with adhesive tape, and read at the same interval as other patch tests23) or occasionally dilute them to 1% or 10% (in olive oil or mineral oil).
How should I manage a positive patch-test reaction to EOs?
Patients should avoid relevant EO allergens in their products and environment, which can be easily achieved with the use of the American Contact Dermatitis Society’s Contact Allergen Management Program or similar databases.
Final Interpretation
We are ubiquitously exposed to EOs every day—through the products we use at home, at work, and in our environment. Essential oils make their place in the world by providing sweet-smelling aromas in addition to their alleged therapeutic properties; however, beware, EOs may be the culprit of your next patient’s allergic contact dermatitis.
What is an essential oil?
An essential oil (EO) is defined by the International Organization for Standardization as a ‘‘product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes.’’1 Steam distillation is the primary method used for the production of commercial EOs,2 and believe it or not, most EOs contain 100 to 250 individual chemical components.3
The term essential oil often is incorrectly used for a variety of products obtained from plant material by methods other than distillation or cold-pressing, such as extraction. Products that are obtained via the extraction method include absolutes found in fine fragrances; hydrolates such as rose water; concretes such as jasmine or violet leaves; and vegetable oils including olive oil, coconut oil, and sesame oil.2 These products are not true EOs.
Where do EOs come from?
Essential oils are produced in many countries around the world.4 Individual oils may be obtained from species of different plants, from different parts of the same plant, or from various cultivars (plants selectively bred to obtain desirable levels of chemical constituents such as monoterpenes or sesquiterpenes and biochemical properties such as antibacterial or antioxidant activities).3,5 It is estimated that EOs can be obtained from approximately 30,000 plant species, but only 150 EOs are produced commercially.2,6
Why are people using EOs? What is their claim to fame?
Essential oils are employed by the flavor, food (eg, soft drinks, milk, candies, chocolate, meats, sausages, alcoholic beverages, spices, herbs, tea, preservatives, animal foods), fragrance, cosmetic, tobacco, and pharmaceutical industries. They also are used in household products (eg, detergents, fabric softeners, air fresheners, candles, incense) and for medicinal purposes (eg, folk and traditional medicine, phytotherapy, balneotherapy, aromatherapy).2 The oils usually are applied to the skin but also can be administered orally, inhaled, diffused through the air, or used by other means.4 One 2019 survey of Minnesota State Fair attendees (N=282) found the most common reasons for using EOs were a desire for alternative treatments (53.4%), the opinion that EOs are safer than traditional therapies (47.6%), and/or failure of standard medical treatments (10.7%). The survey results also indicated that 46.7% of EO users utilized EOs to treat medical conditions or symptoms.7 Of note, review of the website of an international company that produces EOs confirmed that EOs are marketed not only for adults but also for children to help them concentrate,8 sleep,9 improve the appearance of their skin,10 soothe upset stomachs,11 and decrease sniffles due to colds.12
Why are people selling EOs to family and friends? They must be making major bucks!
In general, the cost of EOs depends on the complexity of cultivated plant species; the mode of harvesting, which is sometimes done by hand; and the yield of oil. Prices range from $4.50 to an incredible $150,000 per kilogram.2 On average, one bottle containing 5 to 15 mL of an EO or oil blend can cost anywhere from $7 to $251.13 In the United States, the consumer EO market is partially composed of multilevel/network marketing companies in which direct consumer sales occur via a hierarchy of individual distributors. Goodier et al7 found that 36.4% of participants who obtained EOs from family and friends purchased them through multilevel/network marketing companies. In 2018, individual distributors of an international EO-producing company made on average anywhere from $4 to as much as $1.54 million annually by selling the company’s EO products and enrolling additional members/individual distributors to purchase or sell the company’s EO products.14
Sometimes EOs are described as natural and pure, but are they really?
Just because a product is labeled as “pure” or “natural” does not ensure that it is a good-quality EO. Organically produced (ie, grown without the use of herbicides or pesticides) plant material can include up to 30% of extraneous herbs and weeds, which can change the composition of the oil.2
Lesser-quality EOs are the result of adulteration, contamination, inadequate oil production, or aging.2 Adulteration (eg, cutting, stretching, bouquetting) occurs when foreign substances are introduced into pure EOs for the benefit of a higher profit; to ensure a sufficient supply of oils; or to meet demands for cheaper oils by “stretching” a more expensive, pure oil by combining with a cheaper, less pure oil. Inadequate oil production leading to lower-quality oils can occur when a biomass is incorrectly distilled, either from too much steam or temperatures that are too high or due to lack of adequate cooling units. Aging occurs when the oils are not stored properly, resulting in a change in the chemical composition due to esterification, reduction, and oxidization of chemicals, which leads to the formation of peroxides and hydroperoxides that can be contact allergens.15
Can patients develop contact allergies to EOs?
The short answer is yes! Contact allergy to almost 80 EOs has been reported,15 including tea tree oil,16,17 ylang-ylang oil,17,18 lavender oil, peppermint oil,18 jasmine absolute,17 geranium oil, rose oil,18 turpentine oil,19,20 and sandalwood oil.18 The recent increased prevalence of allergic reactions to EOs likely is due to increased consumer use as well as increased detection from availability of commercial patch-test preparations.
Essential oils have many common ingredients. De Groot and Schmidt3 documented that 14 of 23 chemicals present in more than 80% of EOs have been reported to cause contact allergy. Interestingly, allergic patients often react to more than one EO, which may be explained by the many shared chemical components in EOs.
Essential oils are “natural” so they must be safe?
In general, most safety profiles are good, but rare toxic reactions from EOs have been observed.4 A recent Australian study reviewed EO exposure calls to the New South Wales Poisons Information Centre.21 The majority of EO poisonings were accidental or the result of therapeutic error such as mistaking EOs for liquid pharmaceuticals. Additionally, this study found that from July 2014 to June 2018, there was a 5% increase in the number of calls per year. More than half of EO poisoning calls involved children, with toddlers being the most frequent cases, suggesting the need for child-resistant top closures. The most frequently involved EOs in poisonings were eucalyptus (46.4% [n=2049]), tea tree (17% [n=749]), lavender (6.1% [n=271]), clove (4.1% [n=179]), and peppermint (3.5% [n=154]).21 Essential oils do not come without potential pitfalls.
What is the clinical presentation and workup?
The workup of EO allergic contact dermatitis begins with obtaining a history to evaluate for use of EO diffusers, perfumes, hygiene products, cosmetics, massage oils, toothpastes, and/or pharmaceutical products. Exploration of potential exposures through occupation, environment, and hobbies also is indicated. Clinical presentation is dependent on the mechanism of exposure. Contact allergy may result from direct application of an allergen to the skin or mucous membranes, contact with a contaminated environmental item (eg, lavender oil on a pillow), contact with EOs used by partners or coworkers (consort dermatitis), airborne exposure (EO diffusers), or systemic exposure (flavorings). Airborne dermatitis from EO diffusers may involve the exposed areas of the face, neck, forearms, arms, behind the earlobes, bilateral eyelids, nasolabial folds, and under the chin. History and clinical presentation can raise suspicion for allergic contact dermatitis, and patch testing is necessary to confirm the diagnosis.
How do we patch test for EO contact allergy?
There are many EOs commercially available for patch testing, and they typically are tested at 2% to 5% concentrations in petrolatum.15 A North American and European study of 62,354 patch-tested patients found that 7.4% of EO-positive individuals did not react to fragrance allergens in a standard screening series including fragrance mix I, fragrance mix II, and balsam of Peru, highlighting the importance of patch testing with specific EOs.22 Currently, only 3 EOs—tea tree oil, peppermint oil, and ylang-ylang oil—are included in the 2019-2020 North American Contact Dermatitis Group screening series, making supplemental testing for other EOs important if contact allergy is suspected; however, testing the patient’s own products is imperative, as there is strong variability in the composition of EOs. Additionally, aged oils may have been exposed to light, oxygen, or varying temperatures, which could result in the formation of additional allergenic chemicals not present in commercially available preparations.15 In addition to commercially available allergens, we test patient-provided EOs either as is in semi-open fashion (ie, EOs are applied to patient’s back with a cotton swab, allowed to dry, covered with adhesive tape, and read at the same interval as other patch tests23) or occasionally dilute them to 1% or 10% (in olive oil or mineral oil).
How should I manage a positive patch-test reaction to EOs?
Patients should avoid relevant EO allergens in their products and environment, which can be easily achieved with the use of the American Contact Dermatitis Society’s Contact Allergen Management Program or similar databases.
Final Interpretation
We are ubiquitously exposed to EOs every day—through the products we use at home, at work, and in our environment. Essential oils make their place in the world by providing sweet-smelling aromas in addition to their alleged therapeutic properties; however, beware, EOs may be the culprit of your next patient’s allergic contact dermatitis.
- International Organization for Standardization. ISO 9235:2013. aromatic natural raw materials—vocabulary. https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. Accessed March 24, 2020.
- De Groot AC, Schmidt E. Essential oils: part II: general aspects. Dermatitis. 2016;27:43-49.
De Groot AC, Schmidt E. Essential oils: part III: chemical composition. Dermatitis. 2016;27:161-169. - De Groot AC, Schmidt E. Essential oils: part I: introduction. Dermatitis. 2016;27:39-42.
- Insawang S, Pripdeevech P, Tanapichatsakul C, et al. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem Biodivers. 2019;16:e1900371.
- Lawrence BM. A preliminary report on the world production of some selected essential oils and countries. Perfum Flavor. 2009;34:38-44.
- Goodier MC, Zhang AJ, Nikle AB, et al. Use of essential oils: a general population survey. Contact Dermatitis. 2019;80:391-393.
- KidScents GeneYus. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-geneyus. Accessed March 25, 2020.
- KidScents SleepyIze. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sleepyize-5ml. Accessed March 25, 2020.
- KidScents® Lotion. Young Living Essential Oils website. www.youngliving.com/en_US/products/kidscents-lotion. Accessed March 25, 2020.
- KidScents TummyGize. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-tummygize-5ml. Accessed March 25, 2020.
- KidScents SniffleEase. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sniffleease. Accessed March 25, 2020.
- 2019 Product Guide. Young Living Essential Oils website. https://issuu.com/youngliving/docs/yl_productguide. Accessed March 25, 2020.
- 2018 Income Disclosure Statement. Young Living Essential Oils website. https://www.youngliving.com/en_US/opportunity/income-disclosure. Accessed March 25, 2020.
- De Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. a multicenter study of the German Contact Dermatitis Group. J Dtsch Dermatol Ges. 2003;1:629-634.
- Larsen W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (part II). Contact Dermatitis. 2001;44:344-346.
- Bleasel N, Tate B, Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australas J Dermatol. 2002;43:211-213.
- Noiles K, Pratt M. Contact dermatitis to Vicks VapoRub. Dermatitis. 2010;21:167-169.
- Barchino-Ortiz L, Cabeza-Martinez R, Leis-Dosil VM, et al. Allergic contact hobby dermatitis from turpentine. Allergol Immunopathol (Madr). 2008;36:117-119.
- Lee KA, Harnett JE, Cairns R. Essential oil exposures in Australia: analysis of cases reported to the NSW Poisons Information Centre. Med J Aust. 2020;212:132-133.
- Warshaw EM, Zug KA, Belsito DV, et al. Positive patch test reactions to essential oils in consecutive patients: results from North America and central Europe. Dermatitis. 2017;28:246-252.
- Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88:879-888.
- International Organization for Standardization. ISO 9235:2013. aromatic natural raw materials—vocabulary. https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. Accessed March 24, 2020.
- De Groot AC, Schmidt E. Essential oils: part II: general aspects. Dermatitis. 2016;27:43-49.
De Groot AC, Schmidt E. Essential oils: part III: chemical composition. Dermatitis. 2016;27:161-169. - De Groot AC, Schmidt E. Essential oils: part I: introduction. Dermatitis. 2016;27:39-42.
- Insawang S, Pripdeevech P, Tanapichatsakul C, et al. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem Biodivers. 2019;16:e1900371.
- Lawrence BM. A preliminary report on the world production of some selected essential oils and countries. Perfum Flavor. 2009;34:38-44.
- Goodier MC, Zhang AJ, Nikle AB, et al. Use of essential oils: a general population survey. Contact Dermatitis. 2019;80:391-393.
- KidScents GeneYus. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-geneyus. Accessed March 25, 2020.
- KidScents SleepyIze. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sleepyize-5ml. Accessed March 25, 2020.
- KidScents® Lotion. Young Living Essential Oils website. www.youngliving.com/en_US/products/kidscents-lotion. Accessed March 25, 2020.
- KidScents TummyGize. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-tummygize-5ml. Accessed March 25, 2020.
- KidScents SniffleEase. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sniffleease. Accessed March 25, 2020.
- 2019 Product Guide. Young Living Essential Oils website. https://issuu.com/youngliving/docs/yl_productguide. Accessed March 25, 2020.
- 2018 Income Disclosure Statement. Young Living Essential Oils website. https://www.youngliving.com/en_US/opportunity/income-disclosure. Accessed March 25, 2020.
- De Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. a multicenter study of the German Contact Dermatitis Group. J Dtsch Dermatol Ges. 2003;1:629-634.
- Larsen W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (part II). Contact Dermatitis. 2001;44:344-346.
- Bleasel N, Tate B, Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australas J Dermatol. 2002;43:211-213.
- Noiles K, Pratt M. Contact dermatitis to Vicks VapoRub. Dermatitis. 2010;21:167-169.
- Barchino-Ortiz L, Cabeza-Martinez R, Leis-Dosil VM, et al. Allergic contact hobby dermatitis from turpentine. Allergol Immunopathol (Madr). 2008;36:117-119.
- Lee KA, Harnett JE, Cairns R. Essential oil exposures in Australia: analysis of cases reported to the NSW Poisons Information Centre. Med J Aust. 2020;212:132-133.
- Warshaw EM, Zug KA, Belsito DV, et al. Positive patch test reactions to essential oils in consecutive patients: results from North America and central Europe. Dermatitis. 2017;28:246-252.
- Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88:879-888.
Practice Points
- Essential oils (EOs) are present in many consumer products, including foods, cosmetics, pharmaceuticals, and household products; patients can develop contact allergy to EOs.
- Common EO allergens include tea tree oil, ylang-ylang oil, lavender oil, peppermint oil, jasmine absolute, geranium oil, rose oil, turpentine oil, and sandalwood oil.
- In general, EOs have good safety profiles, but caution must be taken when storing them.
- When patch testing for potential EO contact allergy, supplemental testing with both commercially available EOs as well as a patient’s own products is necessary given there is strong variability in the composition of EO products.
Recurrence of Linear Basal Cell Carcinoma
Case Report
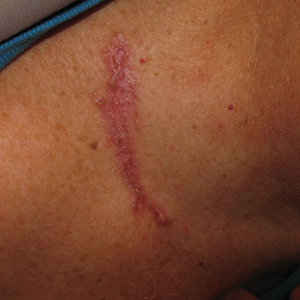
A 63-year-old man was evaluated in the Mohs clinic for a lesion on the right supraclavicular neck, which he described as a linear asymptomatic “birthmark” that had been present since childhood and stable for many years. It began to enlarge approximately 5 years prior, became increasingly red, and had occasional crusting. The lesion also gradually became more irritated with repeated mild trauma when he carried a backpack while hiking. On physical examination, a 10×2-cm, linear, pink plaque with an irregular border, translucent rolled edges, and central smooth atrophic skin was seen on the right supraclavicular neck (Figure). There was no visible epidermal nevus or nevus sebaceous in the area. A shave biopsy of the lesion confirmed the pathologic diagnosis of basal cell carcinoma, nodular type, along with the morphologic diagnosis of linear basal cell carcinoma (LBCC). The tumor was completely removed with standard excision using 5-mm margins.
Approximately 10 months after the original excision, the patient developed an irritated erosion that occasionally bled when his backpack rubbed against it. He returned to the clinic after the erosion failed to heal. Physical examination revealed a 1.4×0.7-cm, eroded, pink papule with large telangiectases at the superior pole of the excision scar. A shave biopsy confirmed the diagnosis of a recurrent infiltrative basal cell carcinoma. The tumor was then completely excised using Mohs micrographic surgery.
Comment
Linear basal cell carcinoma, first described by Lewis1 in 1985, is a rare morphologic variant of basal cell carcinoma. In 2011, Al-Niaimi and Lyon2 performed a comprehensive literature search on LBCC (1985-2008) and found only 39 cases (including 2 of their own) had been published since the pioneer case in 1985. It was determined that the most common sites affected were the periorbital area and neck (n=13 each [67%]), and the majority were histologically nodular (n=27 [69%]). Mohs micrographic surgery was the most common treatment method (n=23 [59%]), followed by primary excision (n=17 [44%]). A history of trauma, radiotherapy, or prior operation in association with the site of the LBCC was discovered in only 7 cases (18%).2 Although Peschen et al3 proposed that trauma—both physical and surgical—and radiotherapy may play a role in the development of LBCCs, the low incidence reported suggests that other factors may be involved. To determine if genetic factors were contributing to the development of LBCCs, Yamaguchi et al4 investigated the expression of p27 and PCTAIRE1, both known to contribute to tumorigenesis when mutated, as well as somatic gene mutations using deep sequencing in a case of LBCC; they found no associated genetic mutation.
Reported Cases of LBCC
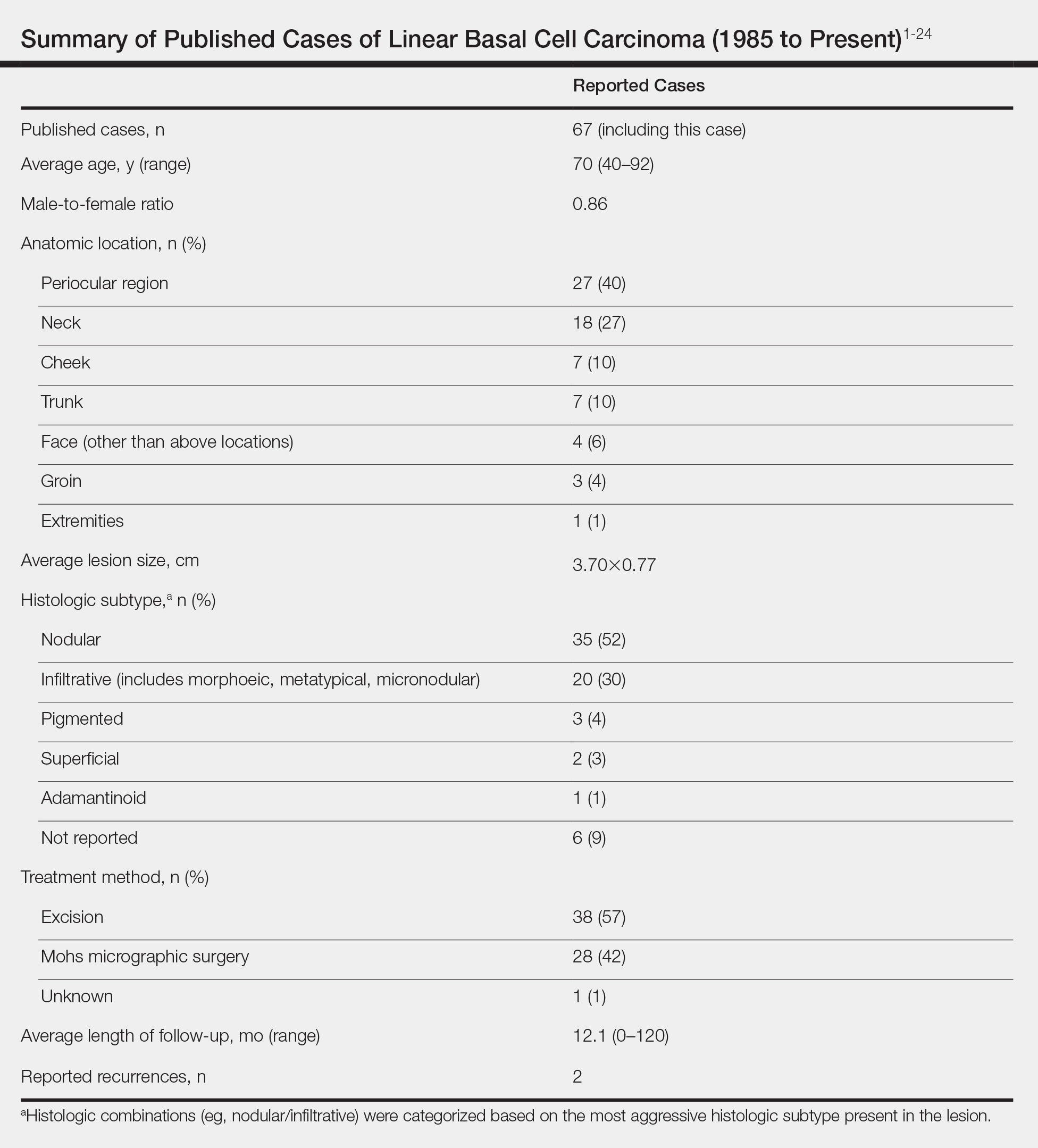
According to a PubMed search of articles indexed for MEDLINE using the terms linear and basal cell carcinoma, 67 cases (including the current case) of LBCC have been published since 1985. The patient demographics, anatomic location, histologic subtype, treatment methods, and frequency of recurrence for all reported cases of LBCC are summarized in the Table.1-24 There were 36 women and 31 men, with an average age of 70 years (range, 40–92 years). The most commonly affected sites were the periocular region (n=27) and neck (n=18). Histologically, most LBCCs were nodular (n=35), with the next most common histologic subtype being infiltrative (n=20), which included the morphoeic, metatypical, and micronodular subtypes under the overarching infiltrative subtype. The most frequently chosen treatment option was primary excision (n=38 [57%]), followed by Mohs micrographic surgery (n=28 [42%]). Risk factors previously identified by Al-Niaimi and Lyon,2 including trauma, radiotherapy, or prior operation, were reported in 12 of 67 cases. Recurrence was reported in only 2 of 67 cases, 1 being the current case; however, an accurate recurrence rate could not be calculated due to lack of follow-up or short length of follow-up in most of the reported cases.
Presentation and Treatment
Currently, there are no set criteria for the diagnosis of LBCC, but it has been shown to follow a characteristic morphologic pattern, favoring extension in one direction leading to a length-to-width ratio that typically is at least 3 to 1.5 With most lesions presenting in the periocular region along relaxed skin tension lines, it has been speculated that these tumors expand along wrinkles.2 Pierard and Lapiere25 proposed that the preferential parallel orientation and a straightening of thin collagen bundles and elastic fibers within the reticular dermis combined with relaxed skin tension lines and muscle contraction perpendicular to these stromal parts may influence the growth of tumors preferentially in one direction, contributing to linearity of the lesion. In addition, the clinical appearance is not a reliable indicator of subclinical extension.2 Therefore, Lim et al6 recommended Mohs micrographic surgery as the best initial treatment of LBCCs.
Conclusion
Linear basal cell carcinoma should be considered a distinct morphologic variant of basal cell carcinoma. Although likely underreported, this variant is uncommon. It presents most often in the periocular and neck regions. The most common histologic subtypes are nodular and infiltrative. Because of the likelihood of subclinical spread, LBCC should be regarded as a high-risk subtype. As such, Mohs micrographic surgery or excision with complete circumferential peripheral and deep margin assessment is recommended as first-line treatment of LBCC.6
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1985;24:124-125.
- Al-Niaimi F, Lyon CC. Linear basal cell carcinoma: a distinct condition? Clin Exp Dermatol. 2011;36:231-234.
- Peschen M, Lo JS, Snow SN, et al. Linear basal cell carcinoma. Cutis. 1993;51:287-289.
- Yamaguchi Y, Yanagi T, Imafuku K, et al. A case of linear basal cell carcinoma: evaluation of proliferative activity by immunohistochemical staining of PCTAIRE1 and p27. J Eur Acad Dermatol Venereol. 2017;31:E359-E362.
- Mavirakis I, Malhotra R, Selva D, et al. Linear basal cell carcinoma: a distinct clinical entity. J Plast Reconstr Aesthet Surg. 2006;59:419-423.
- Lim KK, Randle HW, Roenigk RK, et al. Linear basal cell carcinoma: report of seventeen cases and review of the presentation and treatment. Dermatol Surg. 1999;25:63-67.
- Pardavila R, Rosón E, De la torre C, et al. Linear basal cell carcinoma. report of two cases [in Spanish]. Actas Dermosifiliogr. 2007;98:291.
- Shinsuke K, Hirohiko K, Yasuhiro T, et al. Linear basal cell carcinoma in an Asian patient. Open Ophthalmol J. 2007;1:20-22.
- Ning C, Chao S. Linear basal cell carcinoma of the scrotum. Dermatol Sinica. 2002;20:57-62.
- Chopra KF, Cohen PR. Linear basal cell carcinomas: report of multiple sequential tumors localized to a radiotherapy port and review of the literature. Tex Med. 1997;93:57-59.
- da Silva MO, Dadalt P, Santos OL, et al. Linear basal cell carcinoma. Int J Dermatol. 1995;34:488.
- Warthan TL, Lewis JE. Giant linear basal cell epithelioma. Int J Dermatol. 1994;33:284.
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1989;28:682-684.
- Alcántara-Reifs CM, Salido-Vallejo R, González-Menchen A, et al. Linear basal cell carcinoma: report of three cases with dermoscopic findings. Indian J Dermatol Venereol Leprol. 2016;82:708-711.
- Lee MS, Cho E, Lee JH, et al. Linearly curved, blackish macule on the wrist. Cutis. 2016;97:384, 406-407.
- Bajaj S, Sharma PK, Kar HK. Linear adamantinoid basal cell carcinoma in the axilla. Dermatol Online J. 2015;21. pii:13030/qt8k0713nb.
- Iga N, Sakurai K, Fujii H, et al. Linear basal cell carcinoma at the external genitalia. J Dermatol. 2014;41:275-276.
- Ichinokawa Y, Ohtuki A, Hattori M, et al. Linear basal cell carcinoma: a case report. Case Rep Dermatol. 2011;3:142-146.
- Becher GL, Affleck A, Fleming C, et al. Linear basal cell carcinoma occurs most commonly on the lower eyelid. Clin Exp Dermatol. 2011;36:311-312.
- Jellouli A, Triki S, Zghal M, et al. Linear basal cell carcinoma. Actas Dermosifiliogr. 2010;101:648-650.
- Takiyoshi N, Nakano H, Kaneko T, et al. A linear basal cell carcinoma undergoing spontaneous regression. Clin Exp Dermatol. 2009;34:E411-E413.
- Yoleri L, Ozden S, Kandiloglu A. A 46-year-old male with an ulcerated linear lesion on his neck. Ann Saudi Med. 2008;28:57-58.
- Palleschi GM, Corradini D, Bruscino N, et al. Linear basal cell carcinoma: clinical significance and better surgical approach. G Ital Dermatol Venereol. 2016;151:119-121.
- Rodriguez-Garijo N, Redondo P. Linear basal cell carcinoma of the lower eyelid: reconstruction with a musculocutaneous transposition flap. JAAD Case Rep. 2018;4:633-635.
- Pierard GE, Lapiere CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer’s lines. Am J Dermatopathol. 1987;9:219-224.
Case Report
A 63-year-old man was evaluated in the Mohs clinic for a lesion on the right supraclavicular neck, which he described as a linear asymptomatic “birthmark” that had been present since childhood and stable for many years. It began to enlarge approximately 5 years prior, became increasingly red, and had occasional crusting. The lesion also gradually became more irritated with repeated mild trauma when he carried a backpack while hiking. On physical examination, a 10×2-cm, linear, pink plaque with an irregular border, translucent rolled edges, and central smooth atrophic skin was seen on the right supraclavicular neck (Figure). There was no visible epidermal nevus or nevus sebaceous in the area. A shave biopsy of the lesion confirmed the pathologic diagnosis of basal cell carcinoma, nodular type, along with the morphologic diagnosis of linear basal cell carcinoma (LBCC). The tumor was completely removed with standard excision using 5-mm margins.
Approximately 10 months after the original excision, the patient developed an irritated erosion that occasionally bled when his backpack rubbed against it. He returned to the clinic after the erosion failed to heal. Physical examination revealed a 1.4×0.7-cm, eroded, pink papule with large telangiectases at the superior pole of the excision scar. A shave biopsy confirmed the diagnosis of a recurrent infiltrative basal cell carcinoma. The tumor was then completely excised using Mohs micrographic surgery.
Comment
Linear basal cell carcinoma, first described by Lewis1 in 1985, is a rare morphologic variant of basal cell carcinoma. In 2011, Al-Niaimi and Lyon2 performed a comprehensive literature search on LBCC (1985-2008) and found only 39 cases (including 2 of their own) had been published since the pioneer case in 1985. It was determined that the most common sites affected were the periorbital area and neck (n=13 each [67%]), and the majority were histologically nodular (n=27 [69%]). Mohs micrographic surgery was the most common treatment method (n=23 [59%]), followed by primary excision (n=17 [44%]). A history of trauma, radiotherapy, or prior operation in association with the site of the LBCC was discovered in only 7 cases (18%).2 Although Peschen et al3 proposed that trauma—both physical and surgical—and radiotherapy may play a role in the development of LBCCs, the low incidence reported suggests that other factors may be involved. To determine if genetic factors were contributing to the development of LBCCs, Yamaguchi et al4 investigated the expression of p27 and PCTAIRE1, both known to contribute to tumorigenesis when mutated, as well as somatic gene mutations using deep sequencing in a case of LBCC; they found no associated genetic mutation.
Reported Cases of LBCC
According to a PubMed search of articles indexed for MEDLINE using the terms linear and basal cell carcinoma, 67 cases (including the current case) of LBCC have been published since 1985. The patient demographics, anatomic location, histologic subtype, treatment methods, and frequency of recurrence for all reported cases of LBCC are summarized in the Table.1-24 There were 36 women and 31 men, with an average age of 70 years (range, 40–92 years). The most commonly affected sites were the periocular region (n=27) and neck (n=18). Histologically, most LBCCs were nodular (n=35), with the next most common histologic subtype being infiltrative (n=20), which included the morphoeic, metatypical, and micronodular subtypes under the overarching infiltrative subtype. The most frequently chosen treatment option was primary excision (n=38 [57%]), followed by Mohs micrographic surgery (n=28 [42%]). Risk factors previously identified by Al-Niaimi and Lyon,2 including trauma, radiotherapy, or prior operation, were reported in 12 of 67 cases. Recurrence was reported in only 2 of 67 cases, 1 being the current case; however, an accurate recurrence rate could not be calculated due to lack of follow-up or short length of follow-up in most of the reported cases.
Presentation and Treatment
Currently, there are no set criteria for the diagnosis of LBCC, but it has been shown to follow a characteristic morphologic pattern, favoring extension in one direction leading to a length-to-width ratio that typically is at least 3 to 1.5 With most lesions presenting in the periocular region along relaxed skin tension lines, it has been speculated that these tumors expand along wrinkles.2 Pierard and Lapiere25 proposed that the preferential parallel orientation and a straightening of thin collagen bundles and elastic fibers within the reticular dermis combined with relaxed skin tension lines and muscle contraction perpendicular to these stromal parts may influence the growth of tumors preferentially in one direction, contributing to linearity of the lesion. In addition, the clinical appearance is not a reliable indicator of subclinical extension.2 Therefore, Lim et al6 recommended Mohs micrographic surgery as the best initial treatment of LBCCs.
Conclusion
Linear basal cell carcinoma should be considered a distinct morphologic variant of basal cell carcinoma. Although likely underreported, this variant is uncommon. It presents most often in the periocular and neck regions. The most common histologic subtypes are nodular and infiltrative. Because of the likelihood of subclinical spread, LBCC should be regarded as a high-risk subtype. As such, Mohs micrographic surgery or excision with complete circumferential peripheral and deep margin assessment is recommended as first-line treatment of LBCC.6
Case Report
A 63-year-old man was evaluated in the Mohs clinic for a lesion on the right supraclavicular neck, which he described as a linear asymptomatic “birthmark” that had been present since childhood and stable for many years. It began to enlarge approximately 5 years prior, became increasingly red, and had occasional crusting. The lesion also gradually became more irritated with repeated mild trauma when he carried a backpack while hiking. On physical examination, a 10×2-cm, linear, pink plaque with an irregular border, translucent rolled edges, and central smooth atrophic skin was seen on the right supraclavicular neck (Figure). There was no visible epidermal nevus or nevus sebaceous in the area. A shave biopsy of the lesion confirmed the pathologic diagnosis of basal cell carcinoma, nodular type, along with the morphologic diagnosis of linear basal cell carcinoma (LBCC). The tumor was completely removed with standard excision using 5-mm margins.
Approximately 10 months after the original excision, the patient developed an irritated erosion that occasionally bled when his backpack rubbed against it. He returned to the clinic after the erosion failed to heal. Physical examination revealed a 1.4×0.7-cm, eroded, pink papule with large telangiectases at the superior pole of the excision scar. A shave biopsy confirmed the diagnosis of a recurrent infiltrative basal cell carcinoma. The tumor was then completely excised using Mohs micrographic surgery.
Comment
Linear basal cell carcinoma, first described by Lewis1 in 1985, is a rare morphologic variant of basal cell carcinoma. In 2011, Al-Niaimi and Lyon2 performed a comprehensive literature search on LBCC (1985-2008) and found only 39 cases (including 2 of their own) had been published since the pioneer case in 1985. It was determined that the most common sites affected were the periorbital area and neck (n=13 each [67%]), and the majority were histologically nodular (n=27 [69%]). Mohs micrographic surgery was the most common treatment method (n=23 [59%]), followed by primary excision (n=17 [44%]). A history of trauma, radiotherapy, or prior operation in association with the site of the LBCC was discovered in only 7 cases (18%).2 Although Peschen et al3 proposed that trauma—both physical and surgical—and radiotherapy may play a role in the development of LBCCs, the low incidence reported suggests that other factors may be involved. To determine if genetic factors were contributing to the development of LBCCs, Yamaguchi et al4 investigated the expression of p27 and PCTAIRE1, both known to contribute to tumorigenesis when mutated, as well as somatic gene mutations using deep sequencing in a case of LBCC; they found no associated genetic mutation.
Reported Cases of LBCC
According to a PubMed search of articles indexed for MEDLINE using the terms linear and basal cell carcinoma, 67 cases (including the current case) of LBCC have been published since 1985. The patient demographics, anatomic location, histologic subtype, treatment methods, and frequency of recurrence for all reported cases of LBCC are summarized in the Table.1-24 There were 36 women and 31 men, with an average age of 70 years (range, 40–92 years). The most commonly affected sites were the periocular region (n=27) and neck (n=18). Histologically, most LBCCs were nodular (n=35), with the next most common histologic subtype being infiltrative (n=20), which included the morphoeic, metatypical, and micronodular subtypes under the overarching infiltrative subtype. The most frequently chosen treatment option was primary excision (n=38 [57%]), followed by Mohs micrographic surgery (n=28 [42%]). Risk factors previously identified by Al-Niaimi and Lyon,2 including trauma, radiotherapy, or prior operation, were reported in 12 of 67 cases. Recurrence was reported in only 2 of 67 cases, 1 being the current case; however, an accurate recurrence rate could not be calculated due to lack of follow-up or short length of follow-up in most of the reported cases.
Presentation and Treatment
Currently, there are no set criteria for the diagnosis of LBCC, but it has been shown to follow a characteristic morphologic pattern, favoring extension in one direction leading to a length-to-width ratio that typically is at least 3 to 1.5 With most lesions presenting in the periocular region along relaxed skin tension lines, it has been speculated that these tumors expand along wrinkles.2 Pierard and Lapiere25 proposed that the preferential parallel orientation and a straightening of thin collagen bundles and elastic fibers within the reticular dermis combined with relaxed skin tension lines and muscle contraction perpendicular to these stromal parts may influence the growth of tumors preferentially in one direction, contributing to linearity of the lesion. In addition, the clinical appearance is not a reliable indicator of subclinical extension.2 Therefore, Lim et al6 recommended Mohs micrographic surgery as the best initial treatment of LBCCs.
Conclusion
Linear basal cell carcinoma should be considered a distinct morphologic variant of basal cell carcinoma. Although likely underreported, this variant is uncommon. It presents most often in the periocular and neck regions. The most common histologic subtypes are nodular and infiltrative. Because of the likelihood of subclinical spread, LBCC should be regarded as a high-risk subtype. As such, Mohs micrographic surgery or excision with complete circumferential peripheral and deep margin assessment is recommended as first-line treatment of LBCC.6
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1985;24:124-125.
- Al-Niaimi F, Lyon CC. Linear basal cell carcinoma: a distinct condition? Clin Exp Dermatol. 2011;36:231-234.
- Peschen M, Lo JS, Snow SN, et al. Linear basal cell carcinoma. Cutis. 1993;51:287-289.
- Yamaguchi Y, Yanagi T, Imafuku K, et al. A case of linear basal cell carcinoma: evaluation of proliferative activity by immunohistochemical staining of PCTAIRE1 and p27. J Eur Acad Dermatol Venereol. 2017;31:E359-E362.
- Mavirakis I, Malhotra R, Selva D, et al. Linear basal cell carcinoma: a distinct clinical entity. J Plast Reconstr Aesthet Surg. 2006;59:419-423.
- Lim KK, Randle HW, Roenigk RK, et al. Linear basal cell carcinoma: report of seventeen cases and review of the presentation and treatment. Dermatol Surg. 1999;25:63-67.
- Pardavila R, Rosón E, De la torre C, et al. Linear basal cell carcinoma. report of two cases [in Spanish]. Actas Dermosifiliogr. 2007;98:291.
- Shinsuke K, Hirohiko K, Yasuhiro T, et al. Linear basal cell carcinoma in an Asian patient. Open Ophthalmol J. 2007;1:20-22.
- Ning C, Chao S. Linear basal cell carcinoma of the scrotum. Dermatol Sinica. 2002;20:57-62.
- Chopra KF, Cohen PR. Linear basal cell carcinomas: report of multiple sequential tumors localized to a radiotherapy port and review of the literature. Tex Med. 1997;93:57-59.
- da Silva MO, Dadalt P, Santos OL, et al. Linear basal cell carcinoma. Int J Dermatol. 1995;34:488.
- Warthan TL, Lewis JE. Giant linear basal cell epithelioma. Int J Dermatol. 1994;33:284.
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1989;28:682-684.
- Alcántara-Reifs CM, Salido-Vallejo R, González-Menchen A, et al. Linear basal cell carcinoma: report of three cases with dermoscopic findings. Indian J Dermatol Venereol Leprol. 2016;82:708-711.
- Lee MS, Cho E, Lee JH, et al. Linearly curved, blackish macule on the wrist. Cutis. 2016;97:384, 406-407.
- Bajaj S, Sharma PK, Kar HK. Linear adamantinoid basal cell carcinoma in the axilla. Dermatol Online J. 2015;21. pii:13030/qt8k0713nb.
- Iga N, Sakurai K, Fujii H, et al. Linear basal cell carcinoma at the external genitalia. J Dermatol. 2014;41:275-276.
- Ichinokawa Y, Ohtuki A, Hattori M, et al. Linear basal cell carcinoma: a case report. Case Rep Dermatol. 2011;3:142-146.
- Becher GL, Affleck A, Fleming C, et al. Linear basal cell carcinoma occurs most commonly on the lower eyelid. Clin Exp Dermatol. 2011;36:311-312.
- Jellouli A, Triki S, Zghal M, et al. Linear basal cell carcinoma. Actas Dermosifiliogr. 2010;101:648-650.
- Takiyoshi N, Nakano H, Kaneko T, et al. A linear basal cell carcinoma undergoing spontaneous regression. Clin Exp Dermatol. 2009;34:E411-E413.
- Yoleri L, Ozden S, Kandiloglu A. A 46-year-old male with an ulcerated linear lesion on his neck. Ann Saudi Med. 2008;28:57-58.
- Palleschi GM, Corradini D, Bruscino N, et al. Linear basal cell carcinoma: clinical significance and better surgical approach. G Ital Dermatol Venereol. 2016;151:119-121.
- Rodriguez-Garijo N, Redondo P. Linear basal cell carcinoma of the lower eyelid: reconstruction with a musculocutaneous transposition flap. JAAD Case Rep. 2018;4:633-635.
- Pierard GE, Lapiere CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer’s lines. Am J Dermatopathol. 1987;9:219-224.
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1985;24:124-125.
- Al-Niaimi F, Lyon CC. Linear basal cell carcinoma: a distinct condition? Clin Exp Dermatol. 2011;36:231-234.
- Peschen M, Lo JS, Snow SN, et al. Linear basal cell carcinoma. Cutis. 1993;51:287-289.
- Yamaguchi Y, Yanagi T, Imafuku K, et al. A case of linear basal cell carcinoma: evaluation of proliferative activity by immunohistochemical staining of PCTAIRE1 and p27. J Eur Acad Dermatol Venereol. 2017;31:E359-E362.
- Mavirakis I, Malhotra R, Selva D, et al. Linear basal cell carcinoma: a distinct clinical entity. J Plast Reconstr Aesthet Surg. 2006;59:419-423.
- Lim KK, Randle HW, Roenigk RK, et al. Linear basal cell carcinoma: report of seventeen cases and review of the presentation and treatment. Dermatol Surg. 1999;25:63-67.
- Pardavila R, Rosón E, De la torre C, et al. Linear basal cell carcinoma. report of two cases [in Spanish]. Actas Dermosifiliogr. 2007;98:291.
- Shinsuke K, Hirohiko K, Yasuhiro T, et al. Linear basal cell carcinoma in an Asian patient. Open Ophthalmol J. 2007;1:20-22.
- Ning C, Chao S. Linear basal cell carcinoma of the scrotum. Dermatol Sinica. 2002;20:57-62.
- Chopra KF, Cohen PR. Linear basal cell carcinomas: report of multiple sequential tumors localized to a radiotherapy port and review of the literature. Tex Med. 1997;93:57-59.
- da Silva MO, Dadalt P, Santos OL, et al. Linear basal cell carcinoma. Int J Dermatol. 1995;34:488.
- Warthan TL, Lewis JE. Giant linear basal cell epithelioma. Int J Dermatol. 1994;33:284.
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1989;28:682-684.
- Alcántara-Reifs CM, Salido-Vallejo R, González-Menchen A, et al. Linear basal cell carcinoma: report of three cases with dermoscopic findings. Indian J Dermatol Venereol Leprol. 2016;82:708-711.
- Lee MS, Cho E, Lee JH, et al. Linearly curved, blackish macule on the wrist. Cutis. 2016;97:384, 406-407.
- Bajaj S, Sharma PK, Kar HK. Linear adamantinoid basal cell carcinoma in the axilla. Dermatol Online J. 2015;21. pii:13030/qt8k0713nb.
- Iga N, Sakurai K, Fujii H, et al. Linear basal cell carcinoma at the external genitalia. J Dermatol. 2014;41:275-276.
- Ichinokawa Y, Ohtuki A, Hattori M, et al. Linear basal cell carcinoma: a case report. Case Rep Dermatol. 2011;3:142-146.
- Becher GL, Affleck A, Fleming C, et al. Linear basal cell carcinoma occurs most commonly on the lower eyelid. Clin Exp Dermatol. 2011;36:311-312.
- Jellouli A, Triki S, Zghal M, et al. Linear basal cell carcinoma. Actas Dermosifiliogr. 2010;101:648-650.
- Takiyoshi N, Nakano H, Kaneko T, et al. A linear basal cell carcinoma undergoing spontaneous regression. Clin Exp Dermatol. 2009;34:E411-E413.
- Yoleri L, Ozden S, Kandiloglu A. A 46-year-old male with an ulcerated linear lesion on his neck. Ann Saudi Med. 2008;28:57-58.
- Palleschi GM, Corradini D, Bruscino N, et al. Linear basal cell carcinoma: clinical significance and better surgical approach. G Ital Dermatol Venereol. 2016;151:119-121.
- Rodriguez-Garijo N, Redondo P. Linear basal cell carcinoma of the lower eyelid: reconstruction with a musculocutaneous transposition flap. JAAD Case Rep. 2018;4:633-635.
- Pierard GE, Lapiere CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer’s lines. Am J Dermatopathol. 1987;9:219-224.
Practice Points
- Linear basal cell carcinoma (LBCC) follows a characteristic morphologic pattern of a length-to-width ratio that typically is at least 3 to 1.
- Linear basal cell carcinomas most commonly present in the periocular region and on the neck along relaxed skin tension lines.
- Because of the likelihood of subclinical spread, LBCC should be regarded as a high-risk subtype of basal cell carcinoma.
- Mohs micrographic surgery or excision with complete circumferential peripheral and deep-margin assessment is recommended as first-line treatment.