User login
Contact Allergy to Topical Medicaments, Part 2: Steroids, Immunomodulators, and Anesthetics, Oh My!
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
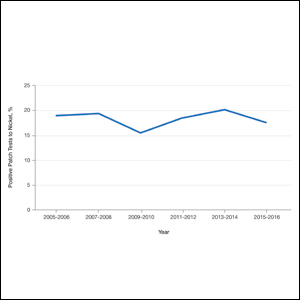
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
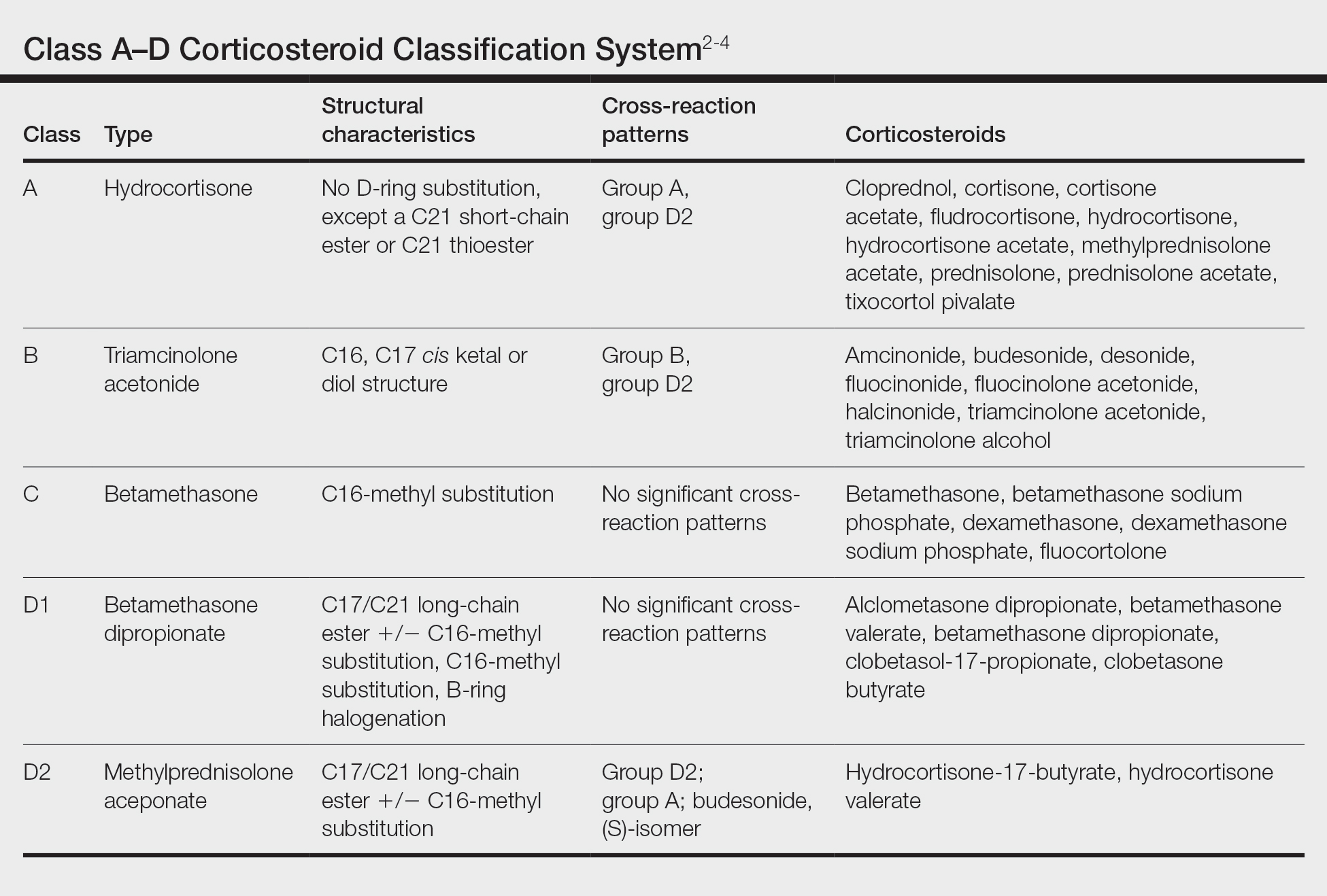
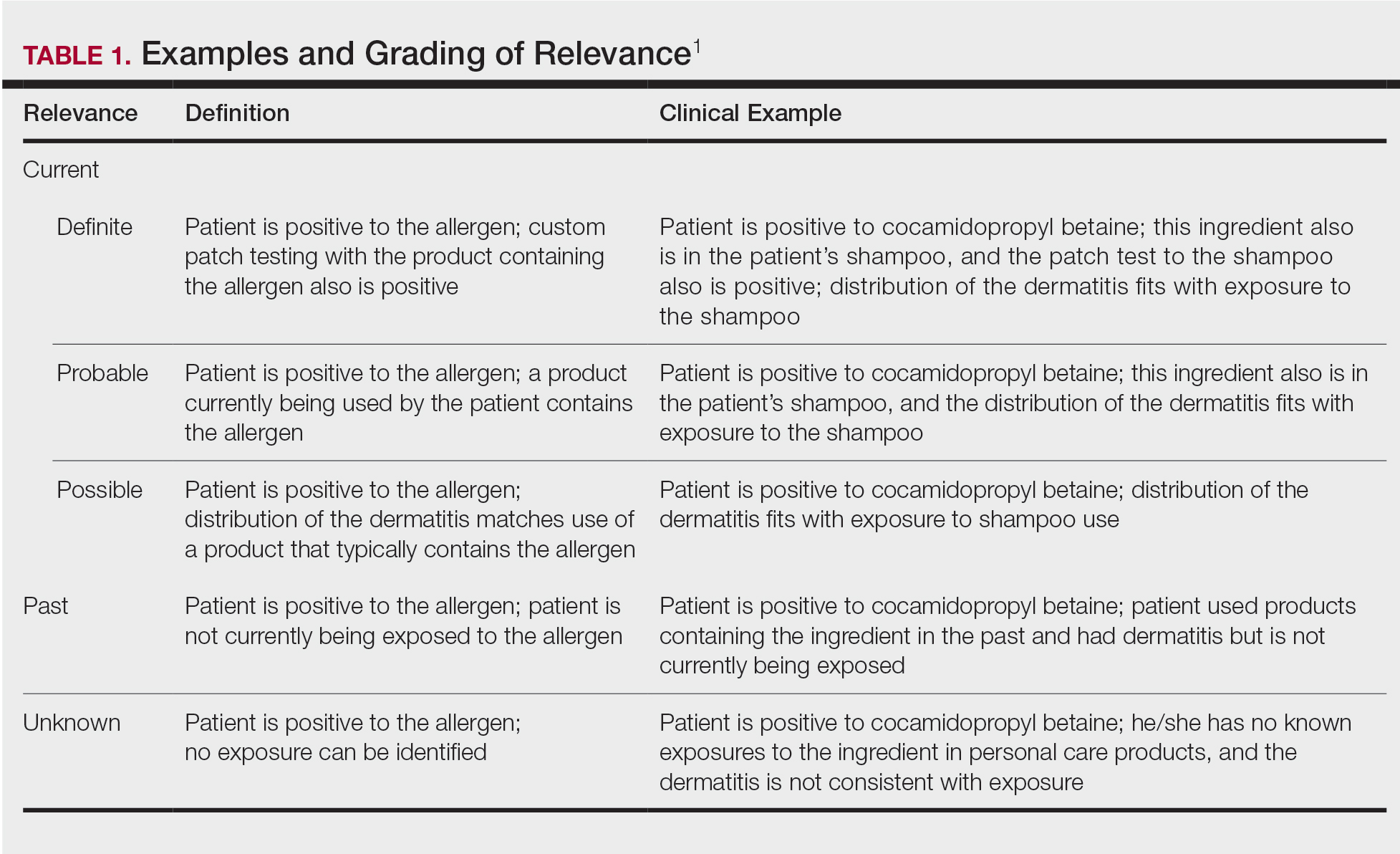
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5
Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
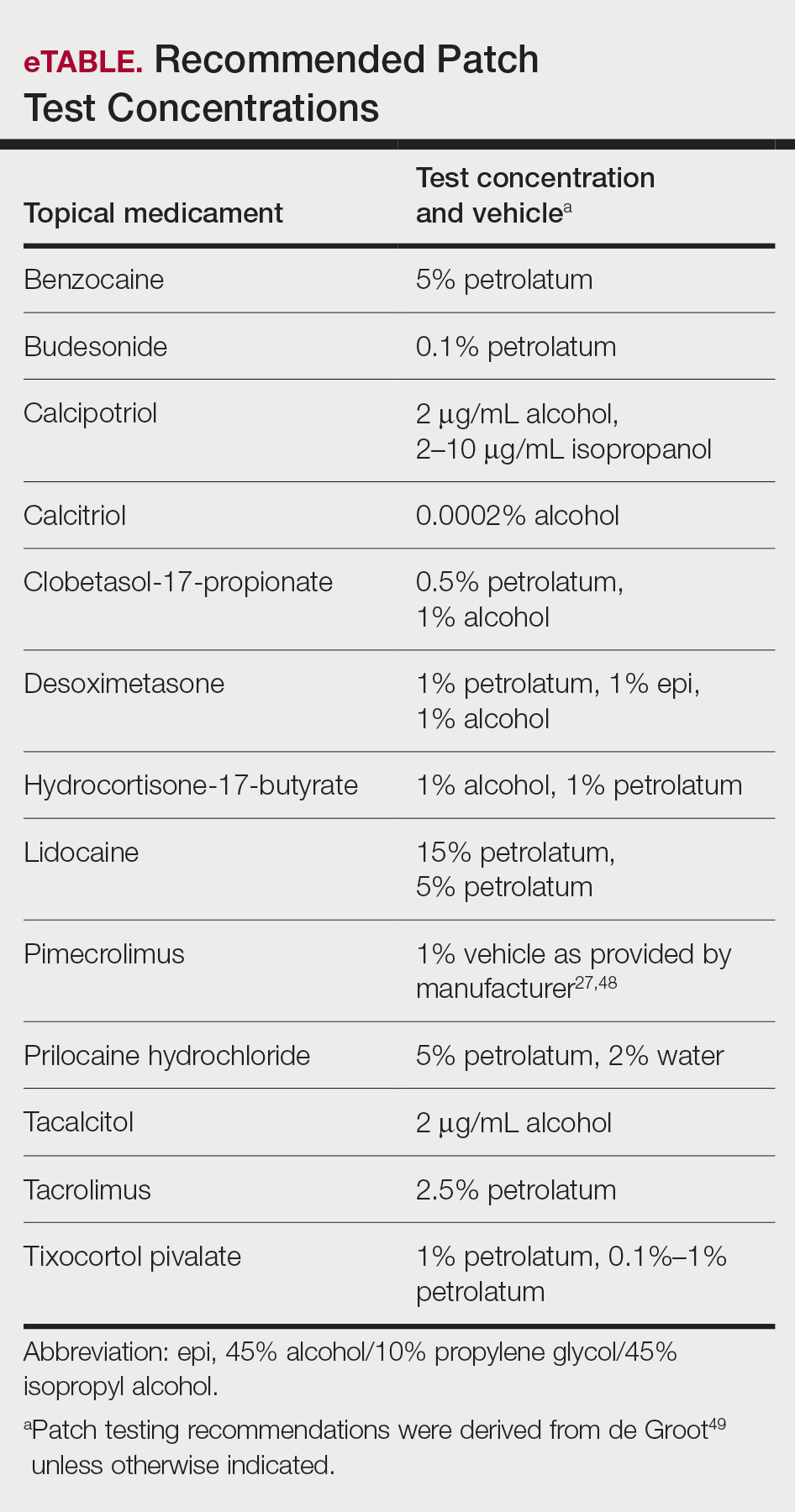
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.
Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5
Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.
Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5
Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.
Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
Practice Points
- Allergic contact dermatitis (ACD) should be suspected in patients with persistent or worsening dermatitis after use of topical medications.
- Cross-reactions commonly occur between structurally similar compounds and occasionally between molecules from different drug classes.
- Some cases of topical medicament ACD remain elusive after patch testing, particularly drugs with potent immunomodulating effects.
Contact Allergy to Topical Medicaments, Part 1: A Double-edged Sword
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
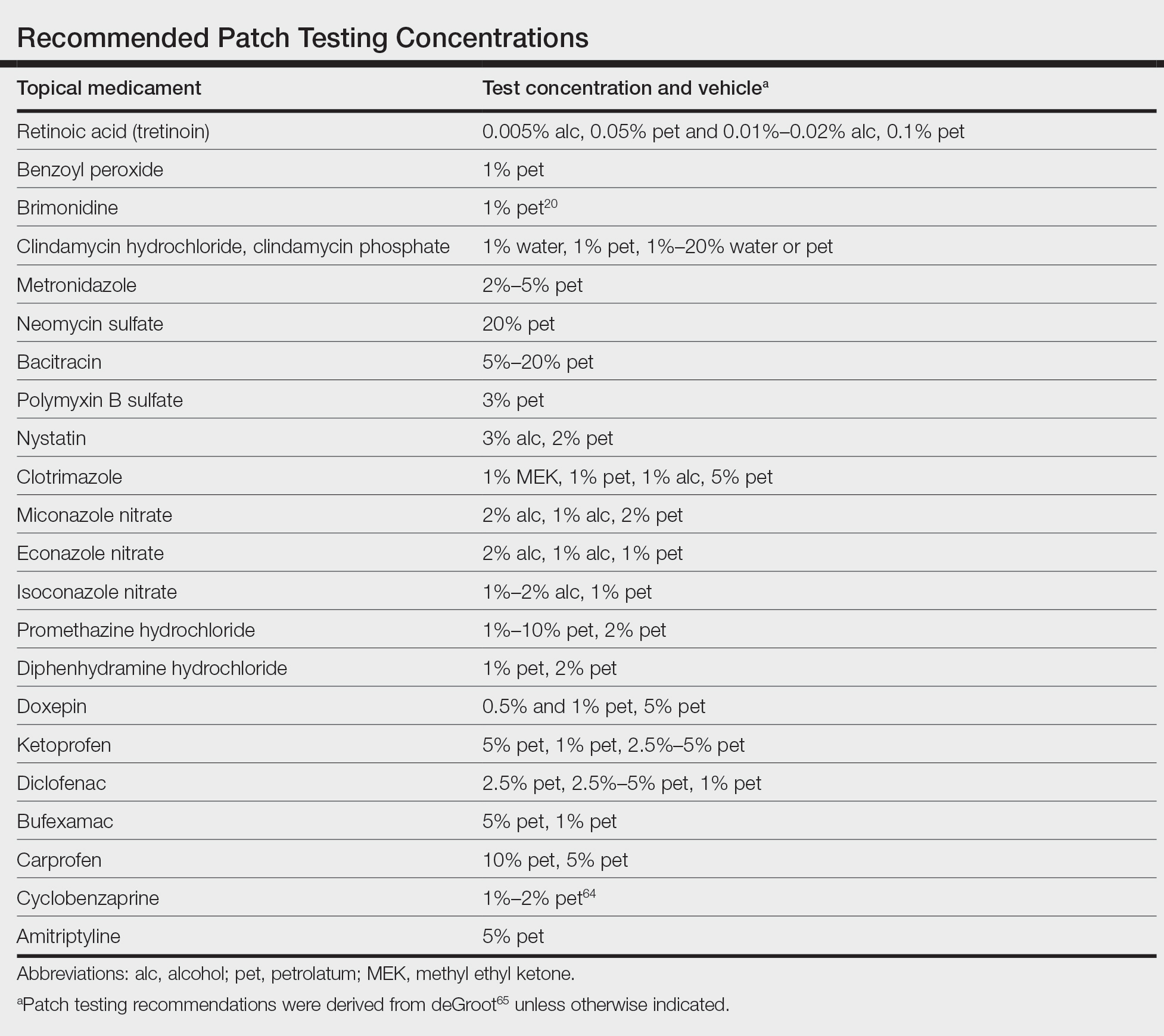
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.
Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.
Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.
Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
Practice Points
- Allergic contact dermatitis should be suspected in patients with persistent or worsening dermatitis after use of topical medications.
- Prior sensitization is not always apparent, and cross-reactions may occur between structurally similar compounds.
- Although most medicaments can be patch tested as is, patch testing to the individual components may be necessary to identify the causative allergen.
Plant Dermatitis: More Than Just Poison Ivy
Plants can contribute to a variety of dermatoses. The Toxicodendron genus, which includes poison ivy, poison oak, and poison sumac, is a well-known and common cause of allergic contact dermatitis (ACD), but many other plants can cause direct or airborne contact dermatitis, especially in gardeners, florists, and farmers. This article provides an overview of different plant-related dermatoses and culprit plants as well as how these dermatoses should be diagnosed and treated.
Epidemiology
Plant dermatoses affect more than 50 million individuals each year.1,2 In the United States, the Toxicodendron genus causes ACD in more than 70% of exposed individuals, leading to medical visits.3 An urgent care visit for a plant-related dermatitis is estimated to cost $168, while an emergency department visit can cost 3 times as much.4 Although less common, Compositae plants are another important culprit of plant dermatitis, particularly in gardeners, florists, and farmers. Data from the 2017-2018 North American Contact Dermatitis Group screening series (N=4947) showed sesquiterpene lactones and Compositae to be positive in 0.5% of patch-tested patients.5
Plant Dermatitis Classifications
Plant dermatitis can be classified into 5 main categories: ACD, mechanical irritant contact dermatitis, chemical irritant contact dermatitis, light-mediated dermatitis, and pseudophytodermatitis.6
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The common molecular allergens in plants include phenols, α-methylene-γ-butyrolactones, quinones, terpenes, disulfides, isothiocyanates, and polyacetylenic derivatives.6
Plant contact dermatitis due to mechanical and chemical irritants is precipitated by multiple mechanisms, including disruption of the epidermal barrier and subsequent cytokine release from keratinocytes.7 Nonimmunologic contact urticaria from plants is thought to be a type of irritant reaction precipitated by mechanical or chemical trauma.8
Light-mediated dermatitis includes phytophotodermatitis and photoallergic contact dermatitis. Phytophotodermatitis is a phototoxic reaction triggered by exposure to both plant-derived furanocoumarin and UVA light.9 By contrast, photoallergic contact dermatitis is a delayed hypersensitivity reaction from prior sensitization to a light-activated antigen.10
Pseudophytodermatitis, as its name implies, is not truly mediated by an allergen or irritant intrinsic to the plant but rather by dyes, waxes, insecticides, or arthropods that inhabit the plant or are secondarily applied.6
Common Plant Allergens
Anacardiaceae Family
Most of the allergenic plants within the Anacardiaceae family belong to the Toxicodendron genus, which encompasses poison ivy (Toxicodendron radicans), poison oak (Toxicodendron pubescens,Toxicodendron quercifolium, Toxicodendron diversiloum), and poison sumac (Toxicodendron vernix). Poison ivy is the celebrity of the Anacardiaceae family and contributes to most cases of plant-related ACD. It is found in every state in the continental United States. Poison oak is another common culprit found in the western and southeastern United States.11 Plants within the Anacardiaceae family contain an oleoresin called urushiol, which is the primary sensitizing substance. Although poison ivy and poison oak grow well in full sun to partial shade, poison sumac typically is found in damp swampy areas east of the Rocky Mountains. Most cases of ACD related to Anacardiaceae species are due to direct contact with urushiol from a Toxicodendron plant, but burning of brush containing Toxicodendron can cause airborne exposure when urushiol oil is carried by smoke particles.12 Sensitization to Toxicodendron can cause ACD to other Anacardiaceae species such as the Japanese lacquer tree (Toxicodendron vernicifluum), mango tree (Mangifera indica), cashew tree (Anacardium occidentale), and Indian marking nut tree (Semecarpus anacardium).6 Cross-reactions to components of the ginkgo tree (Ginkgo biloba) also are possible.
Toxicodendron plants can be more easily identified and avoided with knowledge of their characteristic leaf patterns. The most dependable way to identify poison ivy and poison oak species is to look for plants with 3 leaves, giving rise to the common saying, “Leaves of three, leave them be.” Poison sumac plants have groups of 7 to 13 leaves arranged as pairs along a central rib. Another helpful finding is a black deposit that Toxicodendron species leave behind following trauma to the leaves. Urushiol oxidizes when exposed to air and turns into a black deposit that can be seen on damaged leaves themselves or can be demonstrated in a black spot test to verify if a plant is a Toxicodendron species. The test is performed by gathering (carefully, without direct contact) a few leaves in a paper towel and crushing them to release sap. Within minutes, the sap will turn black if the plant is indeed a Toxicodendron species.13Pruritic, edematous, erythematous papules, plaques, and eventual vesicles in a linear distribution are suspicious for Toxicodendron exposure. Although your pet will not develop Toxicodendron ACD, oleoresin-contaminated pets can transfer the oils to their owners after coming into contact with these plants. Toxicodendron dermatitis also can be acquired from oleoresin-contaminated fomites such as clothing and shoes worn in the garden or when hiking. Toxicodendron dermatitis can appear at different sites on the body at different times depending on the amount of oleoresin exposure as well as epidermal thickness. For example, the oleoresin can be transferred from the hands to body areas with a thinner stratum corneum (eg, genitalia) and cause subsequent dermatitis.1
Compositae Family
The Compositae family (also known as Asteraceae) is a large plant family with more than 20,000 species, including numerous weeds, wildflowers, and vegetables. The flowers, leaves, stems, and pollens of the Compositae family are coated by cyclic esters called sesquiterpene lactones. Mitchell and Dupuis14 showed that sesquiterpene lactones are the allergens responsible for ACD to various Compositae plants, including ragweed (Ambrosia), sneezeweed (Helenium), and chrysanthemums (Chrysanthemum). Common Compositae vegetables such as lettuce (Lactuca sativa) have been reported to cause ACD in chefs, grocery store produce handlers, gardeners, and even owners of lettuce-eating pet guinea pigs and turtles.15 Similarly, artichokes (Cynara scolymus) can cause ACD in gardeners.16 Exposure to Compositae species also has been implicated in photoallergic reactions, and studies have demonstrated that some patients with chronic actinic dermatitis also have positive patch test reactions to Compositae species and/or sesquiterpene lactones.17,18
In addition to direct contact with Compositae plants, airborne exposure to sesquiterpene lactones can cause ACD.14 The pattern of airborne contact dermatitis typically involves exposed areas such as the eyelids, central face, and/or neck. The beak sign also can be a clue to airborne contact dermatitis, which involves dermatitis of the face that spares the nasal tip and/or nasal ridge. It is thought that the beak sign may result from increased sebaceous gland concentration on the nose, which prevents penetration of allergens and irritants.19 Unlike photoallergic contact dermatitis, which also can involve the face, airborne ACD frequently involves photoprotected areas such as the submandibular chin and the upper lip. Davies and Kersey20 reported the case of a groundsman who was cutting grass with dandelions (Taraxacum officinale) and was found to have associated airborne ACD of the face, neck, and forearms due to Compositae allergy. In a different setting, the aromas of chamomile (Matricaria chamomilla) have been reported to cause airborne ACD in a tea drinker.21 Paulsen22 found that ingestion of chamomile tea can induce systemic ACD in sensitized individuals.
Alstroemeriaceae, Liliaceae, and Primulaceae
Florists are exposed to many plant species and have a high prevalence of ACD. Thiboutot et al23 found that 15 of 57 (26%) floral workers experienced hand dermatitis that cleared with time away from work. The Peruvian lily (Alstroemeria, Alstroemeriaceae family), which contains tuliposide A, was found to be the leading cause of sensitization.23 Tulips (Tulipa, Liliaceae family), as the flower name suggests, also contain tuliposide A, which along with mechanical irritation from the course tecta fibers on the bulbs lead to a dermatitis known as tulip fingers.24,25 Poison primrose (Primula obconica, Primulaceae family), cultivated for its highly colorful flowers, contains the contact allergen primin.6 A common clinical presentation of ACD for any of these culprit flowers is localized dermatitis of the thumb and index finger in a florist or gardener.
Plants That Cause Irritant Reactions
Cactuses
Although the long spines of the Cactaceae family of cactuses is a warning for passersby, it is the small and nearly invisible barbed hairs (glochids) that inflict a more dramatic cutaneous reaction. The prickly pear cactus (Opuntia species) is a good example of such a plant, as its glochids cause mechanical irritation but also can become embedded in the skin and result in subcutaneous granulomas known as sabra dermatitis.26
Stinging Nettle
The dermatologic term urticaria owes its namesake to the stinging nettle plant, which comes from the family Urticaceae. The stinging nettle has small hairs on its leaves, referred to as stinging trichomes, which have needlelike tips that pierce the skin and inject a mix of histamine, formic acid, and acetylcholine, causing a pruritic dermatitis that may last up to 12 hours.27 The plant is found worldwide and is a common weed in North America.
Phytophotodermatitis
Lemons and limes (Rutaceae family) are common culprits of phytophotodermatitis, often causing what is known as a margarita burn after outdoor consumption or preparation of this tasty citrus beverage.28 An accidental spray of lime juice on the skin while adding it to a beer, guacamole, salsa, or any other food or beverage also can cause phytophotodermatitis.29-31 Although the juice of lemons and limes contains psoralens, the rind can contain a 6- to 186-fold increased concentration.32 Psoralen is the photoactive agent in Rutaceae plants that intercalates in double-stranded DNA and promotes intrastrand cross-links when exposed to UVA light, which ultimately leads to dermatitis.9 Phytophotodermatitis commonly causes erythema, edema, and painful bullae on sun-exposed areas and classically heals with hyperpigmentation.
Pseudophytodermatitis can occur in grain farmers and harvesters who handle wheat and/or barley and incidentally come in contact with insects and chemicals on the plant material. Pseudophytodermatitis from mites in the wheat and/or barley plant can occur at harvest time when contact with the plant material is high. Insects such as the North American itch mite (Pediculoides ventricosus) can cause petechiae, wheals, and pustules. In addition, insecticides such as malathion and arsenical sprays that are applied to plant leaves can cause pseudophytodermatitis, which may be initially diagnosed as dermatitis to the plant itself.6
Patch Testing to Plants
When a patient presents with recurrent or persistent dermatitis and a plant contact allergen is suspected, patch testing is indicated. Most comprehensive patch test series contain various plant allergens, such as sesquiterpene lactones, Compositae mix, and limonene hydroperoxides, and patch testing to a specialized plant series may be necessary. Poison ivy/oak/sumac allergens typically are not included in patch test series because of the high prevalence of allergic reactions to these chemicals and the likelihood of sensitization when patch testing with urushiol. Compositae contact sensitization can be difficult to diagnose because neither sesquiterpene lactone mix 0.1% nor parthenolide 0.1% are sensitive enough to pick up all Compositae allergies.33,34 Paulsen and Andersen34 proposed that if Compositae sensitization is suspected, testing should include sesquiterpene lactone, parthenolide, and Compositae mix II 2.5%, as well as other potential Compositae allergens based on the patient’s history.34
Because plants can have geographic variability and contain potentially unknown allergens,35 testing to plant components may increase the diagnostic yield of patch testing. Dividing the plant into component parts (ie, stem, bulb, leaf, flower) is helpful, as different components have different allergen concentrations. It is important to consult expert resources before proceeding with plant component patch testing because irritant reactions are frequent and may confound the testing.36
Prevention and Treatment
For all plant dermatoses, the mainstay of prevention is to avoid contact with the offending plant material. Gloves can be an important protective tool for plant dermatitis prevention; the correct material depends on the plant species being handled. Rubber gloves should not be worn to protect against Toxicodendron plants since the catechols in urushiol are soluble in rubber; vinyl gloves should be worn instead.6 Marks37 found that tuliposide A, the allergen in the Peruvian lily (Alstroemeria), penetrates both vinyl and latex gloves; it does not penetrate nitrile gloves. If exposed, the risk of dermatitis can be decreased if the allergen is washed away with soap and water as soon as possible. Some allergens such as Toxicodendron are absorbed quickly and need to be washed off within 10 minutes of exposure.6 Importantly, exposed gardening gloves may continue to perpetuate ACD if the allergen is not also washed off the gloves themselves.
For light-mediated dermatoses, sun avoidance or use of an effective sunscreen can reduce symptoms in an individual who has already been exposed.10 UVA light activates psoralen-mediated dermatitis but not until 30 to 120 minutes after absorption into the skin.38
Barrier creams are thought to be protective against plant ACD through a variety of mechanisms. The cream itself is meant to reduce skin contact to an allergen or irritant. Additionally, barrier creams contain active ingredients such as silicone, hydrocarbons, and aluminum chlorohydrate, which are thought to trap or transform offending agents before contacting the skin. When contact with a Toxicodendron species is anticipated, Marks et al39 found that dermatitis was absent or significantly reduced when 144 patients were pretreated with quaternium-18 bentonite lotion 5% (P<.0001).
Although allergen avoidance and use of gloves and barrier creams are the mainstays of preventing plant dermatoses, treatment often is required to control postexposure symptoms. For all plant dermatoses, topical corticosteroids can be used to reduce inflammation and pruritus. In some cases, systemic steroids may be necessary. To prevent rebound of dermatitis, patients often require a 3-week or longer course of oral steroids to quell the reaction, particularly if the dermatitis is vigorous or an id reaction is present.40 Antihistamines and cold compresses also can provide symptomatic relief.
Final Interpretation
Plants can cause a variety of dermatoses. Although Toxicodendron plants are the most frequent cause of ACD, it is important to keep in mind that florists, gardeners, and farmers are exposed to a large variety of allergens, irritants, and phototoxic agents that cause dermatoses as well. Confirmation of plant-induced ACD involves patch testing against suspected species. Prevention involves use of appropriate barriers and avoidance of implicated plants. Treatment includes topical steroids, antihistamines, and prednisone.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128.
- Pariser D, Ceilley R, Lefkovits A, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Wolff K, Johnson R. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. McGraw Hill Education; 2009.
- Zomorodi N, Butt M, Maczuga S, et al. Cost and diagnostic characteristics of Toxicodendron dermatitis in the USA: a retrospective cross-sectional analysis. Br J Dermatol. 2020;183:772-773.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123.
- Fowler JF, Zirwas MJ. Fisher’s Contact Dermatitis. 7th ed. Contact Dermatitis Institute; 2019.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Wakelin SH. Contact urticaria. Clin Exp Dermatol. 2001;26:132-136.
- Ellis CR, Elston DM. Psoralen-induced phytophotodermatitis. Dermatitis. 2021;32:140-143.
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- National Institute for Occupational Safety and Health. Poisonous plants. Centers for Disease Control and Prevention website. Updated June 1, 2018. Accessed August 10, 2021. https://www.cdc.gov/niosh/topics/plants/geographic.html
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mitchell J, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150.
- Paulsen E, Andersen KE. Lettuce contact allergy. Contact Dermatitis. 2016;74:67-75.
- Samaran Q, Clark E, Dereure O, et al. Airborne allergic contact dermatitis caused by artichoke. Contact Dermatitis. 2020;82:395-397.
- Du H, Ross JS, Norris PG, et al. Contact and photocontact sensitization in chronic actinic dermatitis: sesquiterpene lactone mix is an important allergen. Br J Dermatol. 1995;132:543-547.
- Wrangsjo K, Marie Ros A, Walhberg JE. Contact allergy to Compositae plants in patients with summer-exacerbated dermatitis. Contact Dermatitis. 1990;22:148-154.
- Staser K, Ezra N, Sheehan MP, et al. The beak sign: a clinical clue to airborne contact dermatitis. Dermatitis. 2014;25:97-98.
- Davies M, Kersey J. Contact allergy to yarrow and dandelion. Contact Dermatitis. 1986;14:256-257.
- Anzai A, Vázquez Herrera NE, Tosti A. Airborne allergic contact dermatitis caused by chamomile tea. Contact Dermatitis. 2015;72:254-255.
- Paulsen E. Systemic allergic dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2017;76:1-10.
- Thiboutot DM, Hamory BH, Marks JG. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58.
- Hjorth N, Wilkinson DS. Contact dermatitis IV. tulip fingers, hyacinth itch and lily rash. Br J Dermatol. 1968;80:696-698.
- Guin JD, Franks H. Fingertip dermatitis in a retail florist. Cutis. 2001;67:328-330.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Cummings AJ, Olsen M. Mechanism of action of stinging nettles. Wilderness Environ Med. 2011;22:136-139.
- Maniam G, Light KML, Wilson J. Margarita burn: recognition and treatment of phytophotodermatitis. J Am Board Fam Med. 2021;34:398-401.
- Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
- Kung AC, Stephens MB, Darling T. Phytophotodermatitis: bulla formation and hyperpigmentation during spring break. Mil Med. 2009;174:657-661.
- Smith LG. Phytophotodermatitis. Images Emerg Med. 2017;1:146-147.
- Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
- Green C, Ferguson J. Sesquiterpene lactone mix is not an adequate screen for Compositae allergy. Contact Dermatitis. 1994;31:151-153.
- Paulsen E, Andersen KE. Screening for Compositae contact sensitization with sesquiterpene lactones and Compositae mix 2.5% pet. Contact Dermatitis. 2019;81:368-373.
- Paulsen E, Andersen KE. Patch testing with constituents of Compositae mixes. Contact Dermatitis. 2012;66:241-246.
- Frosch PJ, Geier J, Uter W, et al. Patch testing with the patients’ own products. Contact Dermatitis. 2011:929-941.
- Marks JG. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Moreau JF, English JC, Gehris RP. Phytophotodermatitis. J Pediatr Adolesc Gynecol. 2014;27:93-94.
- Marks JG, Fowler JF, Sherertz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216.
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (rhus)? J Fam Pract. 2006;55:166-167.
Plants can contribute to a variety of dermatoses. The Toxicodendron genus, which includes poison ivy, poison oak, and poison sumac, is a well-known and common cause of allergic contact dermatitis (ACD), but many other plants can cause direct or airborne contact dermatitis, especially in gardeners, florists, and farmers. This article provides an overview of different plant-related dermatoses and culprit plants as well as how these dermatoses should be diagnosed and treated.
Epidemiology
Plant dermatoses affect more than 50 million individuals each year.1,2 In the United States, the Toxicodendron genus causes ACD in more than 70% of exposed individuals, leading to medical visits.3 An urgent care visit for a plant-related dermatitis is estimated to cost $168, while an emergency department visit can cost 3 times as much.4 Although less common, Compositae plants are another important culprit of plant dermatitis, particularly in gardeners, florists, and farmers. Data from the 2017-2018 North American Contact Dermatitis Group screening series (N=4947) showed sesquiterpene lactones and Compositae to be positive in 0.5% of patch-tested patients.5
Plant Dermatitis Classifications
Plant dermatitis can be classified into 5 main categories: ACD, mechanical irritant contact dermatitis, chemical irritant contact dermatitis, light-mediated dermatitis, and pseudophytodermatitis.6
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The common molecular allergens in plants include phenols, α-methylene-γ-butyrolactones, quinones, terpenes, disulfides, isothiocyanates, and polyacetylenic derivatives.6
Plant contact dermatitis due to mechanical and chemical irritants is precipitated by multiple mechanisms, including disruption of the epidermal barrier and subsequent cytokine release from keratinocytes.7 Nonimmunologic contact urticaria from plants is thought to be a type of irritant reaction precipitated by mechanical or chemical trauma.8
Light-mediated dermatitis includes phytophotodermatitis and photoallergic contact dermatitis. Phytophotodermatitis is a phototoxic reaction triggered by exposure to both plant-derived furanocoumarin and UVA light.9 By contrast, photoallergic contact dermatitis is a delayed hypersensitivity reaction from prior sensitization to a light-activated antigen.10
Pseudophytodermatitis, as its name implies, is not truly mediated by an allergen or irritant intrinsic to the plant but rather by dyes, waxes, insecticides, or arthropods that inhabit the plant or are secondarily applied.6
Common Plant Allergens
Anacardiaceae Family
Most of the allergenic plants within the Anacardiaceae family belong to the Toxicodendron genus, which encompasses poison ivy (Toxicodendron radicans), poison oak (Toxicodendron pubescens,Toxicodendron quercifolium, Toxicodendron diversiloum), and poison sumac (Toxicodendron vernix). Poison ivy is the celebrity of the Anacardiaceae family and contributes to most cases of plant-related ACD. It is found in every state in the continental United States. Poison oak is another common culprit found in the western and southeastern United States.11 Plants within the Anacardiaceae family contain an oleoresin called urushiol, which is the primary sensitizing substance. Although poison ivy and poison oak grow well in full sun to partial shade, poison sumac typically is found in damp swampy areas east of the Rocky Mountains. Most cases of ACD related to Anacardiaceae species are due to direct contact with urushiol from a Toxicodendron plant, but burning of brush containing Toxicodendron can cause airborne exposure when urushiol oil is carried by smoke particles.12 Sensitization to Toxicodendron can cause ACD to other Anacardiaceae species such as the Japanese lacquer tree (Toxicodendron vernicifluum), mango tree (Mangifera indica), cashew tree (Anacardium occidentale), and Indian marking nut tree (Semecarpus anacardium).6 Cross-reactions to components of the ginkgo tree (Ginkgo biloba) also are possible.
Toxicodendron plants can be more easily identified and avoided with knowledge of their characteristic leaf patterns. The most dependable way to identify poison ivy and poison oak species is to look for plants with 3 leaves, giving rise to the common saying, “Leaves of three, leave them be.” Poison sumac plants have groups of 7 to 13 leaves arranged as pairs along a central rib. Another helpful finding is a black deposit that Toxicodendron species leave behind following trauma to the leaves. Urushiol oxidizes when exposed to air and turns into a black deposit that can be seen on damaged leaves themselves or can be demonstrated in a black spot test to verify if a plant is a Toxicodendron species. The test is performed by gathering (carefully, without direct contact) a few leaves in a paper towel and crushing them to release sap. Within minutes, the sap will turn black if the plant is indeed a Toxicodendron species.13Pruritic, edematous, erythematous papules, plaques, and eventual vesicles in a linear distribution are suspicious for Toxicodendron exposure. Although your pet will not develop Toxicodendron ACD, oleoresin-contaminated pets can transfer the oils to their owners after coming into contact with these plants. Toxicodendron dermatitis also can be acquired from oleoresin-contaminated fomites such as clothing and shoes worn in the garden or when hiking. Toxicodendron dermatitis can appear at different sites on the body at different times depending on the amount of oleoresin exposure as well as epidermal thickness. For example, the oleoresin can be transferred from the hands to body areas with a thinner stratum corneum (eg, genitalia) and cause subsequent dermatitis.1
Compositae Family
The Compositae family (also known as Asteraceae) is a large plant family with more than 20,000 species, including numerous weeds, wildflowers, and vegetables. The flowers, leaves, stems, and pollens of the Compositae family are coated by cyclic esters called sesquiterpene lactones. Mitchell and Dupuis14 showed that sesquiterpene lactones are the allergens responsible for ACD to various Compositae plants, including ragweed (Ambrosia), sneezeweed (Helenium), and chrysanthemums (Chrysanthemum). Common Compositae vegetables such as lettuce (Lactuca sativa) have been reported to cause ACD in chefs, grocery store produce handlers, gardeners, and even owners of lettuce-eating pet guinea pigs and turtles.15 Similarly, artichokes (Cynara scolymus) can cause ACD in gardeners.16 Exposure to Compositae species also has been implicated in photoallergic reactions, and studies have demonstrated that some patients with chronic actinic dermatitis also have positive patch test reactions to Compositae species and/or sesquiterpene lactones.17,18
In addition to direct contact with Compositae plants, airborne exposure to sesquiterpene lactones can cause ACD.14 The pattern of airborne contact dermatitis typically involves exposed areas such as the eyelids, central face, and/or neck. The beak sign also can be a clue to airborne contact dermatitis, which involves dermatitis of the face that spares the nasal tip and/or nasal ridge. It is thought that the beak sign may result from increased sebaceous gland concentration on the nose, which prevents penetration of allergens and irritants.19 Unlike photoallergic contact dermatitis, which also can involve the face, airborne ACD frequently involves photoprotected areas such as the submandibular chin and the upper lip. Davies and Kersey20 reported the case of a groundsman who was cutting grass with dandelions (Taraxacum officinale) and was found to have associated airborne ACD of the face, neck, and forearms due to Compositae allergy. In a different setting, the aromas of chamomile (Matricaria chamomilla) have been reported to cause airborne ACD in a tea drinker.21 Paulsen22 found that ingestion of chamomile tea can induce systemic ACD in sensitized individuals.
Alstroemeriaceae, Liliaceae, and Primulaceae
Florists are exposed to many plant species and have a high prevalence of ACD. Thiboutot et al23 found that 15 of 57 (26%) floral workers experienced hand dermatitis that cleared with time away from work. The Peruvian lily (Alstroemeria, Alstroemeriaceae family), which contains tuliposide A, was found to be the leading cause of sensitization.23 Tulips (Tulipa, Liliaceae family), as the flower name suggests, also contain tuliposide A, which along with mechanical irritation from the course tecta fibers on the bulbs lead to a dermatitis known as tulip fingers.24,25 Poison primrose (Primula obconica, Primulaceae family), cultivated for its highly colorful flowers, contains the contact allergen primin.6 A common clinical presentation of ACD for any of these culprit flowers is localized dermatitis of the thumb and index finger in a florist or gardener.
Plants That Cause Irritant Reactions
Cactuses
Although the long spines of the Cactaceae family of cactuses is a warning for passersby, it is the small and nearly invisible barbed hairs (glochids) that inflict a more dramatic cutaneous reaction. The prickly pear cactus (Opuntia species) is a good example of such a plant, as its glochids cause mechanical irritation but also can become embedded in the skin and result in subcutaneous granulomas known as sabra dermatitis.26
Stinging Nettle
The dermatologic term urticaria owes its namesake to the stinging nettle plant, which comes from the family Urticaceae. The stinging nettle has small hairs on its leaves, referred to as stinging trichomes, which have needlelike tips that pierce the skin and inject a mix of histamine, formic acid, and acetylcholine, causing a pruritic dermatitis that may last up to 12 hours.27 The plant is found worldwide and is a common weed in North America.
Phytophotodermatitis
Lemons and limes (Rutaceae family) are common culprits of phytophotodermatitis, often causing what is known as a margarita burn after outdoor consumption or preparation of this tasty citrus beverage.28 An accidental spray of lime juice on the skin while adding it to a beer, guacamole, salsa, or any other food or beverage also can cause phytophotodermatitis.29-31 Although the juice of lemons and limes contains psoralens, the rind can contain a 6- to 186-fold increased concentration.32 Psoralen is the photoactive agent in Rutaceae plants that intercalates in double-stranded DNA and promotes intrastrand cross-links when exposed to UVA light, which ultimately leads to dermatitis.9 Phytophotodermatitis commonly causes erythema, edema, and painful bullae on sun-exposed areas and classically heals with hyperpigmentation.
Pseudophytodermatitis can occur in grain farmers and harvesters who handle wheat and/or barley and incidentally come in contact with insects and chemicals on the plant material. Pseudophytodermatitis from mites in the wheat and/or barley plant can occur at harvest time when contact with the plant material is high. Insects such as the North American itch mite (Pediculoides ventricosus) can cause petechiae, wheals, and pustules. In addition, insecticides such as malathion and arsenical sprays that are applied to plant leaves can cause pseudophytodermatitis, which may be initially diagnosed as dermatitis to the plant itself.6
Patch Testing to Plants
When a patient presents with recurrent or persistent dermatitis and a plant contact allergen is suspected, patch testing is indicated. Most comprehensive patch test series contain various plant allergens, such as sesquiterpene lactones, Compositae mix, and limonene hydroperoxides, and patch testing to a specialized plant series may be necessary. Poison ivy/oak/sumac allergens typically are not included in patch test series because of the high prevalence of allergic reactions to these chemicals and the likelihood of sensitization when patch testing with urushiol. Compositae contact sensitization can be difficult to diagnose because neither sesquiterpene lactone mix 0.1% nor parthenolide 0.1% are sensitive enough to pick up all Compositae allergies.33,34 Paulsen and Andersen34 proposed that if Compositae sensitization is suspected, testing should include sesquiterpene lactone, parthenolide, and Compositae mix II 2.5%, as well as other potential Compositae allergens based on the patient’s history.34
Because plants can have geographic variability and contain potentially unknown allergens,35 testing to plant components may increase the diagnostic yield of patch testing. Dividing the plant into component parts (ie, stem, bulb, leaf, flower) is helpful, as different components have different allergen concentrations. It is important to consult expert resources before proceeding with plant component patch testing because irritant reactions are frequent and may confound the testing.36
Prevention and Treatment
For all plant dermatoses, the mainstay of prevention is to avoid contact with the offending plant material. Gloves can be an important protective tool for plant dermatitis prevention; the correct material depends on the plant species being handled. Rubber gloves should not be worn to protect against Toxicodendron plants since the catechols in urushiol are soluble in rubber; vinyl gloves should be worn instead.6 Marks37 found that tuliposide A, the allergen in the Peruvian lily (Alstroemeria), penetrates both vinyl and latex gloves; it does not penetrate nitrile gloves. If exposed, the risk of dermatitis can be decreased if the allergen is washed away with soap and water as soon as possible. Some allergens such as Toxicodendron are absorbed quickly and need to be washed off within 10 minutes of exposure.6 Importantly, exposed gardening gloves may continue to perpetuate ACD if the allergen is not also washed off the gloves themselves.
For light-mediated dermatoses, sun avoidance or use of an effective sunscreen can reduce symptoms in an individual who has already been exposed.10 UVA light activates psoralen-mediated dermatitis but not until 30 to 120 minutes after absorption into the skin.38
Barrier creams are thought to be protective against plant ACD through a variety of mechanisms. The cream itself is meant to reduce skin contact to an allergen or irritant. Additionally, barrier creams contain active ingredients such as silicone, hydrocarbons, and aluminum chlorohydrate, which are thought to trap or transform offending agents before contacting the skin. When contact with a Toxicodendron species is anticipated, Marks et al39 found that dermatitis was absent or significantly reduced when 144 patients were pretreated with quaternium-18 bentonite lotion 5% (P<.0001).
Although allergen avoidance and use of gloves and barrier creams are the mainstays of preventing plant dermatoses, treatment often is required to control postexposure symptoms. For all plant dermatoses, topical corticosteroids can be used to reduce inflammation and pruritus. In some cases, systemic steroids may be necessary. To prevent rebound of dermatitis, patients often require a 3-week or longer course of oral steroids to quell the reaction, particularly if the dermatitis is vigorous or an id reaction is present.40 Antihistamines and cold compresses also can provide symptomatic relief.
Final Interpretation
Plants can cause a variety of dermatoses. Although Toxicodendron plants are the most frequent cause of ACD, it is important to keep in mind that florists, gardeners, and farmers are exposed to a large variety of allergens, irritants, and phototoxic agents that cause dermatoses as well. Confirmation of plant-induced ACD involves patch testing against suspected species. Prevention involves use of appropriate barriers and avoidance of implicated plants. Treatment includes topical steroids, antihistamines, and prednisone.
Plants can contribute to a variety of dermatoses. The Toxicodendron genus, which includes poison ivy, poison oak, and poison sumac, is a well-known and common cause of allergic contact dermatitis (ACD), but many other plants can cause direct or airborne contact dermatitis, especially in gardeners, florists, and farmers. This article provides an overview of different plant-related dermatoses and culprit plants as well as how these dermatoses should be diagnosed and treated.
Epidemiology
Plant dermatoses affect more than 50 million individuals each year.1,2 In the United States, the Toxicodendron genus causes ACD in more than 70% of exposed individuals, leading to medical visits.3 An urgent care visit for a plant-related dermatitis is estimated to cost $168, while an emergency department visit can cost 3 times as much.4 Although less common, Compositae plants are another important culprit of plant dermatitis, particularly in gardeners, florists, and farmers. Data from the 2017-2018 North American Contact Dermatitis Group screening series (N=4947) showed sesquiterpene lactones and Compositae to be positive in 0.5% of patch-tested patients.5
Plant Dermatitis Classifications
Plant dermatitis can be classified into 5 main categories: ACD, mechanical irritant contact dermatitis, chemical irritant contact dermatitis, light-mediated dermatitis, and pseudophytodermatitis.6
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The common molecular allergens in plants include phenols, α-methylene-γ-butyrolactones, quinones, terpenes, disulfides, isothiocyanates, and polyacetylenic derivatives.6
Plant contact dermatitis due to mechanical and chemical irritants is precipitated by multiple mechanisms, including disruption of the epidermal barrier and subsequent cytokine release from keratinocytes.7 Nonimmunologic contact urticaria from plants is thought to be a type of irritant reaction precipitated by mechanical or chemical trauma.8
Light-mediated dermatitis includes phytophotodermatitis and photoallergic contact dermatitis. Phytophotodermatitis is a phototoxic reaction triggered by exposure to both plant-derived furanocoumarin and UVA light.9 By contrast, photoallergic contact dermatitis is a delayed hypersensitivity reaction from prior sensitization to a light-activated antigen.10
Pseudophytodermatitis, as its name implies, is not truly mediated by an allergen or irritant intrinsic to the plant but rather by dyes, waxes, insecticides, or arthropods that inhabit the plant or are secondarily applied.6
Common Plant Allergens
Anacardiaceae Family
Most of the allergenic plants within the Anacardiaceae family belong to the Toxicodendron genus, which encompasses poison ivy (Toxicodendron radicans), poison oak (Toxicodendron pubescens,Toxicodendron quercifolium, Toxicodendron diversiloum), and poison sumac (Toxicodendron vernix). Poison ivy is the celebrity of the Anacardiaceae family and contributes to most cases of plant-related ACD. It is found in every state in the continental United States. Poison oak is another common culprit found in the western and southeastern United States.11 Plants within the Anacardiaceae family contain an oleoresin called urushiol, which is the primary sensitizing substance. Although poison ivy and poison oak grow well in full sun to partial shade, poison sumac typically is found in damp swampy areas east of the Rocky Mountains. Most cases of ACD related to Anacardiaceae species are due to direct contact with urushiol from a Toxicodendron plant, but burning of brush containing Toxicodendron can cause airborne exposure when urushiol oil is carried by smoke particles.12 Sensitization to Toxicodendron can cause ACD to other Anacardiaceae species such as the Japanese lacquer tree (Toxicodendron vernicifluum), mango tree (Mangifera indica), cashew tree (Anacardium occidentale), and Indian marking nut tree (Semecarpus anacardium).6 Cross-reactions to components of the ginkgo tree (Ginkgo biloba) also are possible.
Toxicodendron plants can be more easily identified and avoided with knowledge of their characteristic leaf patterns. The most dependable way to identify poison ivy and poison oak species is to look for plants with 3 leaves, giving rise to the common saying, “Leaves of three, leave them be.” Poison sumac plants have groups of 7 to 13 leaves arranged as pairs along a central rib. Another helpful finding is a black deposit that Toxicodendron species leave behind following trauma to the leaves. Urushiol oxidizes when exposed to air and turns into a black deposit that can be seen on damaged leaves themselves or can be demonstrated in a black spot test to verify if a plant is a Toxicodendron species. The test is performed by gathering (carefully, without direct contact) a few leaves in a paper towel and crushing them to release sap. Within minutes, the sap will turn black if the plant is indeed a Toxicodendron species.13Pruritic, edematous, erythematous papules, plaques, and eventual vesicles in a linear distribution are suspicious for Toxicodendron exposure. Although your pet will not develop Toxicodendron ACD, oleoresin-contaminated pets can transfer the oils to their owners after coming into contact with these plants. Toxicodendron dermatitis also can be acquired from oleoresin-contaminated fomites such as clothing and shoes worn in the garden or when hiking. Toxicodendron dermatitis can appear at different sites on the body at different times depending on the amount of oleoresin exposure as well as epidermal thickness. For example, the oleoresin can be transferred from the hands to body areas with a thinner stratum corneum (eg, genitalia) and cause subsequent dermatitis.1
Compositae Family
The Compositae family (also known as Asteraceae) is a large plant family with more than 20,000 species, including numerous weeds, wildflowers, and vegetables. The flowers, leaves, stems, and pollens of the Compositae family are coated by cyclic esters called sesquiterpene lactones. Mitchell and Dupuis14 showed that sesquiterpene lactones are the allergens responsible for ACD to various Compositae plants, including ragweed (Ambrosia), sneezeweed (Helenium), and chrysanthemums (Chrysanthemum). Common Compositae vegetables such as lettuce (Lactuca sativa) have been reported to cause ACD in chefs, grocery store produce handlers, gardeners, and even owners of lettuce-eating pet guinea pigs and turtles.15 Similarly, artichokes (Cynara scolymus) can cause ACD in gardeners.16 Exposure to Compositae species also has been implicated in photoallergic reactions, and studies have demonstrated that some patients with chronic actinic dermatitis also have positive patch test reactions to Compositae species and/or sesquiterpene lactones.17,18
In addition to direct contact with Compositae plants, airborne exposure to sesquiterpene lactones can cause ACD.14 The pattern of airborne contact dermatitis typically involves exposed areas such as the eyelids, central face, and/or neck. The beak sign also can be a clue to airborne contact dermatitis, which involves dermatitis of the face that spares the nasal tip and/or nasal ridge. It is thought that the beak sign may result from increased sebaceous gland concentration on the nose, which prevents penetration of allergens and irritants.19 Unlike photoallergic contact dermatitis, which also can involve the face, airborne ACD frequently involves photoprotected areas such as the submandibular chin and the upper lip. Davies and Kersey20 reported the case of a groundsman who was cutting grass with dandelions (Taraxacum officinale) and was found to have associated airborne ACD of the face, neck, and forearms due to Compositae allergy. In a different setting, the aromas of chamomile (Matricaria chamomilla) have been reported to cause airborne ACD in a tea drinker.21 Paulsen22 found that ingestion of chamomile tea can induce systemic ACD in sensitized individuals.
Alstroemeriaceae, Liliaceae, and Primulaceae
Florists are exposed to many plant species and have a high prevalence of ACD. Thiboutot et al23 found that 15 of 57 (26%) floral workers experienced hand dermatitis that cleared with time away from work. The Peruvian lily (Alstroemeria, Alstroemeriaceae family), which contains tuliposide A, was found to be the leading cause of sensitization.23 Tulips (Tulipa, Liliaceae family), as the flower name suggests, also contain tuliposide A, which along with mechanical irritation from the course tecta fibers on the bulbs lead to a dermatitis known as tulip fingers.24,25 Poison primrose (Primula obconica, Primulaceae family), cultivated for its highly colorful flowers, contains the contact allergen primin.6 A common clinical presentation of ACD for any of these culprit flowers is localized dermatitis of the thumb and index finger in a florist or gardener.
Plants That Cause Irritant Reactions
Cactuses
Although the long spines of the Cactaceae family of cactuses is a warning for passersby, it is the small and nearly invisible barbed hairs (glochids) that inflict a more dramatic cutaneous reaction. The prickly pear cactus (Opuntia species) is a good example of such a plant, as its glochids cause mechanical irritation but also can become embedded in the skin and result in subcutaneous granulomas known as sabra dermatitis.26
Stinging Nettle
The dermatologic term urticaria owes its namesake to the stinging nettle plant, which comes from the family Urticaceae. The stinging nettle has small hairs on its leaves, referred to as stinging trichomes, which have needlelike tips that pierce the skin and inject a mix of histamine, formic acid, and acetylcholine, causing a pruritic dermatitis that may last up to 12 hours.27 The plant is found worldwide and is a common weed in North America.
Phytophotodermatitis
Lemons and limes (Rutaceae family) are common culprits of phytophotodermatitis, often causing what is known as a margarita burn after outdoor consumption or preparation of this tasty citrus beverage.28 An accidental spray of lime juice on the skin while adding it to a beer, guacamole, salsa, or any other food or beverage also can cause phytophotodermatitis.29-31 Although the juice of lemons and limes contains psoralens, the rind can contain a 6- to 186-fold increased concentration.32 Psoralen is the photoactive agent in Rutaceae plants that intercalates in double-stranded DNA and promotes intrastrand cross-links when exposed to UVA light, which ultimately leads to dermatitis.9 Phytophotodermatitis commonly causes erythema, edema, and painful bullae on sun-exposed areas and classically heals with hyperpigmentation.
Pseudophytodermatitis can occur in grain farmers and harvesters who handle wheat and/or barley and incidentally come in contact with insects and chemicals on the plant material. Pseudophytodermatitis from mites in the wheat and/or barley plant can occur at harvest time when contact with the plant material is high. Insects such as the North American itch mite (Pediculoides ventricosus) can cause petechiae, wheals, and pustules. In addition, insecticides such as malathion and arsenical sprays that are applied to plant leaves can cause pseudophytodermatitis, which may be initially diagnosed as dermatitis to the plant itself.6
Patch Testing to Plants
When a patient presents with recurrent or persistent dermatitis and a plant contact allergen is suspected, patch testing is indicated. Most comprehensive patch test series contain various plant allergens, such as sesquiterpene lactones, Compositae mix, and limonene hydroperoxides, and patch testing to a specialized plant series may be necessary. Poison ivy/oak/sumac allergens typically are not included in patch test series because of the high prevalence of allergic reactions to these chemicals and the likelihood of sensitization when patch testing with urushiol. Compositae contact sensitization can be difficult to diagnose because neither sesquiterpene lactone mix 0.1% nor parthenolide 0.1% are sensitive enough to pick up all Compositae allergies.33,34 Paulsen and Andersen34 proposed that if Compositae sensitization is suspected, testing should include sesquiterpene lactone, parthenolide, and Compositae mix II 2.5%, as well as other potential Compositae allergens based on the patient’s history.34
Because plants can have geographic variability and contain potentially unknown allergens,35 testing to plant components may increase the diagnostic yield of patch testing. Dividing the plant into component parts (ie, stem, bulb, leaf, flower) is helpful, as different components have different allergen concentrations. It is important to consult expert resources before proceeding with plant component patch testing because irritant reactions are frequent and may confound the testing.36
Prevention and Treatment
For all plant dermatoses, the mainstay of prevention is to avoid contact with the offending plant material. Gloves can be an important protective tool for plant dermatitis prevention; the correct material depends on the plant species being handled. Rubber gloves should not be worn to protect against Toxicodendron plants since the catechols in urushiol are soluble in rubber; vinyl gloves should be worn instead.6 Marks37 found that tuliposide A, the allergen in the Peruvian lily (Alstroemeria), penetrates both vinyl and latex gloves; it does not penetrate nitrile gloves. If exposed, the risk of dermatitis can be decreased if the allergen is washed away with soap and water as soon as possible. Some allergens such as Toxicodendron are absorbed quickly and need to be washed off within 10 minutes of exposure.6 Importantly, exposed gardening gloves may continue to perpetuate ACD if the allergen is not also washed off the gloves themselves.
For light-mediated dermatoses, sun avoidance or use of an effective sunscreen can reduce symptoms in an individual who has already been exposed.10 UVA light activates psoralen-mediated dermatitis but not until 30 to 120 minutes after absorption into the skin.38
Barrier creams are thought to be protective against plant ACD through a variety of mechanisms. The cream itself is meant to reduce skin contact to an allergen or irritant. Additionally, barrier creams contain active ingredients such as silicone, hydrocarbons, and aluminum chlorohydrate, which are thought to trap or transform offending agents before contacting the skin. When contact with a Toxicodendron species is anticipated, Marks et al39 found that dermatitis was absent or significantly reduced when 144 patients were pretreated with quaternium-18 bentonite lotion 5% (P<.0001).
Although allergen avoidance and use of gloves and barrier creams are the mainstays of preventing plant dermatoses, treatment often is required to control postexposure symptoms. For all plant dermatoses, topical corticosteroids can be used to reduce inflammation and pruritus. In some cases, systemic steroids may be necessary. To prevent rebound of dermatitis, patients often require a 3-week or longer course of oral steroids to quell the reaction, particularly if the dermatitis is vigorous or an id reaction is present.40 Antihistamines and cold compresses also can provide symptomatic relief.
Final Interpretation
Plants can cause a variety of dermatoses. Although Toxicodendron plants are the most frequent cause of ACD, it is important to keep in mind that florists, gardeners, and farmers are exposed to a large variety of allergens, irritants, and phototoxic agents that cause dermatoses as well. Confirmation of plant-induced ACD involves patch testing against suspected species. Prevention involves use of appropriate barriers and avoidance of implicated plants. Treatment includes topical steroids, antihistamines, and prednisone.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128.
- Pariser D, Ceilley R, Lefkovits A, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Wolff K, Johnson R. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. McGraw Hill Education; 2009.
- Zomorodi N, Butt M, Maczuga S, et al. Cost and diagnostic characteristics of Toxicodendron dermatitis in the USA: a retrospective cross-sectional analysis. Br J Dermatol. 2020;183:772-773.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123.
- Fowler JF, Zirwas MJ. Fisher’s Contact Dermatitis. 7th ed. Contact Dermatitis Institute; 2019.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Wakelin SH. Contact urticaria. Clin Exp Dermatol. 2001;26:132-136.
- Ellis CR, Elston DM. Psoralen-induced phytophotodermatitis. Dermatitis. 2021;32:140-143.
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- National Institute for Occupational Safety and Health. Poisonous plants. Centers for Disease Control and Prevention website. Updated June 1, 2018. Accessed August 10, 2021. https://www.cdc.gov/niosh/topics/plants/geographic.html
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mitchell J, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150.
- Paulsen E, Andersen KE. Lettuce contact allergy. Contact Dermatitis. 2016;74:67-75.
- Samaran Q, Clark E, Dereure O, et al. Airborne allergic contact dermatitis caused by artichoke. Contact Dermatitis. 2020;82:395-397.
- Du H, Ross JS, Norris PG, et al. Contact and photocontact sensitization in chronic actinic dermatitis: sesquiterpene lactone mix is an important allergen. Br J Dermatol. 1995;132:543-547.
- Wrangsjo K, Marie Ros A, Walhberg JE. Contact allergy to Compositae plants in patients with summer-exacerbated dermatitis. Contact Dermatitis. 1990;22:148-154.
- Staser K, Ezra N, Sheehan MP, et al. The beak sign: a clinical clue to airborne contact dermatitis. Dermatitis. 2014;25:97-98.
- Davies M, Kersey J. Contact allergy to yarrow and dandelion. Contact Dermatitis. 1986;14:256-257.
- Anzai A, Vázquez Herrera NE, Tosti A. Airborne allergic contact dermatitis caused by chamomile tea. Contact Dermatitis. 2015;72:254-255.
- Paulsen E. Systemic allergic dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2017;76:1-10.
- Thiboutot DM, Hamory BH, Marks JG. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58.
- Hjorth N, Wilkinson DS. Contact dermatitis IV. tulip fingers, hyacinth itch and lily rash. Br J Dermatol. 1968;80:696-698.
- Guin JD, Franks H. Fingertip dermatitis in a retail florist. Cutis. 2001;67:328-330.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Cummings AJ, Olsen M. Mechanism of action of stinging nettles. Wilderness Environ Med. 2011;22:136-139.
- Maniam G, Light KML, Wilson J. Margarita burn: recognition and treatment of phytophotodermatitis. J Am Board Fam Med. 2021;34:398-401.
- Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
- Kung AC, Stephens MB, Darling T. Phytophotodermatitis: bulla formation and hyperpigmentation during spring break. Mil Med. 2009;174:657-661.
- Smith LG. Phytophotodermatitis. Images Emerg Med. 2017;1:146-147.
- Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
- Green C, Ferguson J. Sesquiterpene lactone mix is not an adequate screen for Compositae allergy. Contact Dermatitis. 1994;31:151-153.
- Paulsen E, Andersen KE. Screening for Compositae contact sensitization with sesquiterpene lactones and Compositae mix 2.5% pet. Contact Dermatitis. 2019;81:368-373.
- Paulsen E, Andersen KE. Patch testing with constituents of Compositae mixes. Contact Dermatitis. 2012;66:241-246.
- Frosch PJ, Geier J, Uter W, et al. Patch testing with the patients’ own products. Contact Dermatitis. 2011:929-941.
- Marks JG. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Moreau JF, English JC, Gehris RP. Phytophotodermatitis. J Pediatr Adolesc Gynecol. 2014;27:93-94.
- Marks JG, Fowler JF, Sherertz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216.
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (rhus)? J Fam Pract. 2006;55:166-167.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128.
- Pariser D, Ceilley R, Lefkovits A, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Wolff K, Johnson R. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. McGraw Hill Education; 2009.
- Zomorodi N, Butt M, Maczuga S, et al. Cost and diagnostic characteristics of Toxicodendron dermatitis in the USA: a retrospective cross-sectional analysis. Br J Dermatol. 2020;183:772-773.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123.
- Fowler JF, Zirwas MJ. Fisher’s Contact Dermatitis. 7th ed. Contact Dermatitis Institute; 2019.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Wakelin SH. Contact urticaria. Clin Exp Dermatol. 2001;26:132-136.
- Ellis CR, Elston DM. Psoralen-induced phytophotodermatitis. Dermatitis. 2021;32:140-143.
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- National Institute for Occupational Safety and Health. Poisonous plants. Centers for Disease Control and Prevention website. Updated June 1, 2018. Accessed August 10, 2021. https://www.cdc.gov/niosh/topics/plants/geographic.html
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mitchell J, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150.
- Paulsen E, Andersen KE. Lettuce contact allergy. Contact Dermatitis. 2016;74:67-75.
- Samaran Q, Clark E, Dereure O, et al. Airborne allergic contact dermatitis caused by artichoke. Contact Dermatitis. 2020;82:395-397.
- Du H, Ross JS, Norris PG, et al. Contact and photocontact sensitization in chronic actinic dermatitis: sesquiterpene lactone mix is an important allergen. Br J Dermatol. 1995;132:543-547.
- Wrangsjo K, Marie Ros A, Walhberg JE. Contact allergy to Compositae plants in patients with summer-exacerbated dermatitis. Contact Dermatitis. 1990;22:148-154.
- Staser K, Ezra N, Sheehan MP, et al. The beak sign: a clinical clue to airborne contact dermatitis. Dermatitis. 2014;25:97-98.
- Davies M, Kersey J. Contact allergy to yarrow and dandelion. Contact Dermatitis. 1986;14:256-257.
- Anzai A, Vázquez Herrera NE, Tosti A. Airborne allergic contact dermatitis caused by chamomile tea. Contact Dermatitis. 2015;72:254-255.
- Paulsen E. Systemic allergic dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2017;76:1-10.
- Thiboutot DM, Hamory BH, Marks JG. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58.
- Hjorth N, Wilkinson DS. Contact dermatitis IV. tulip fingers, hyacinth itch and lily rash. Br J Dermatol. 1968;80:696-698.
- Guin JD, Franks H. Fingertip dermatitis in a retail florist. Cutis. 2001;67:328-330.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Cummings AJ, Olsen M. Mechanism of action of stinging nettles. Wilderness Environ Med. 2011;22:136-139.
- Maniam G, Light KML, Wilson J. Margarita burn: recognition and treatment of phytophotodermatitis. J Am Board Fam Med. 2021;34:398-401.
- Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
- Kung AC, Stephens MB, Darling T. Phytophotodermatitis: bulla formation and hyperpigmentation during spring break. Mil Med. 2009;174:657-661.
- Smith LG. Phytophotodermatitis. Images Emerg Med. 2017;1:146-147.
- Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
- Green C, Ferguson J. Sesquiterpene lactone mix is not an adequate screen for Compositae allergy. Contact Dermatitis. 1994;31:151-153.
- Paulsen E, Andersen KE. Screening for Compositae contact sensitization with sesquiterpene lactones and Compositae mix 2.5% pet. Contact Dermatitis. 2019;81:368-373.
- Paulsen E, Andersen KE. Patch testing with constituents of Compositae mixes. Contact Dermatitis. 2012;66:241-246.
- Frosch PJ, Geier J, Uter W, et al. Patch testing with the patients’ own products. Contact Dermatitis. 2011:929-941.
- Marks JG. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Moreau JF, English JC, Gehris RP. Phytophotodermatitis. J Pediatr Adolesc Gynecol. 2014;27:93-94.
- Marks JG, Fowler JF, Sherertz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216.
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (rhus)? J Fam Pract. 2006;55:166-167.
Practice Points
- Gardeners, florists, farmers, and outdoor enthusiasts are at risk for various plant dermatoses, which can be classified into 5 main categories: allergic contact dermatitis (ACD), mechanical irritant contact dermatitis, chemical irritant contact dermatitis, light-mediated dermatitis, and pseudophytodermatitis.
- Poison ivy, from the Toxicodendron genus, is the leading cause of plant ACD; however, a myriad of other plants also can cause dermatoses.
- Patch testing can be used to identify the source of immune-mediated type IV delayed hypersensitivity reactions to various plant species in individuals with recurrent or persistent dermatitis.
- Treatment options for all plant dermatoses can include topical steroids, antihistamines, and oral prednisone. Prevention involves avoidance or use of an effective barrier.
Update on Contact Dermatitis and Patch Testing in Patients With Skin of Color
The world is an increasingly diverse place, which has particular relevance for the dermatologist. Skin color plays a significant role in diagnostic approach, as there are important differences in how cutaneous disease presents in patients with skin of color (SOC). Therefore, education about these differences is imperative. In this review, we focus on allergic contact dermatitis (ACD) and patch testing in patients with SOC. We discuss allergens common to this demographic and challenges encountered in patch testing patients with SOC. We also identify key health care disparities in the evaluation and management of ACD in this population.
Has contact allergy in SOC populations been studied in North America?
Over the last 2 decades, there have been only a handful of North American studies that address contact allergy in SOC populations. Patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic from 1988 to 1991 showed that overall allergy frequency was relatively similar (43.0% vs 43.6%). There were notable differences in allergen sensitization. Paraphenylenediamine (PPD), which is used in hair dye, had more positive patch test reactions in Black patients (10.6% vs 4.5%), and both PPD (21.2% vs 4.2%) and imidazolidinyl urea, a formaldehyde-releasing preservative (9.1% vs 2.6%), were more frequently allergenic in Black men compared to White men.1 Patch test results from the North American Contact Dermatitis Group from 1992 to 1998 described similar results, with minimal variation in the prevalence of ACD among 1014 Black and 8610 White patients (47%–49% vs 46%–49%).2 Positive patch test reactions to PPD were higher in Black patients for 2 of 3 test cycles (13.5% vs 5.8% [1994-1996] and 10.3% vs 5.3% [1996-1998]). Positive patch test reactions were higher in White patients for dimethylol dimethyl hydantoin, a formaldehyde-releasing preservative, also for 2 of 3 test cycles (1.8% vs 0% [1992-1994] and 2.8% vs 0.3% [1994-1996]). Finally, positive patch test reactions to thioureas (rubber accelerators) had a mixed picture: 2 test cycles were higher in Black patients (1.9% vs 1.0% [1992-1994] and 1.3% vs 0.7% [1994-1996]), but the third cycle (1996-1998) was lower (0.7% vs 1.4%). Positive patch test reactions to the metal cobalt chloride were higher in Black patients in just 1 test cycle (9.2% vs 6.6% [1992-1994]). The authors suggested that the use of darker hair dyes in the Black community may lead to more sensitization to PPD. They also theorized that this population’s more frequent use of ointment-based skin care products may make them less susceptible to sensitization to preservatives such as formaldehyde, which more commonly are found in water-based products such as creams. They concluded that differences in sensitization patterns likely were driven by cultural practices affecting exposures.2
In 2016, the North American Contact Dermatitis Group reported patch test results in 434 Black and 6634 White patients (1998-2006).3 Again, ACD prevalence was about the same in both groups (45.9% vs 43.6%). However, they reported several allergens with different reaction patterns. Black patients had higher risk ratios (RRs) for 3 rubber accelerators: mercaptobenzothiazole (RR, 2.10), mercapto mix (RR, 2.27), and thiuram mix (RR, 1.44). They also reacted to PPD (RR, 1.56) and the antibiotic bacitracin (RR, 1.34) at higher frequencies than White patients, who more frequently reacted to formaldehyde (RR, 0.58); the formaldehyde-releasing preservatives quaternium-15 (RR, 0.63) and diazolidinyl urea (in petrolatum: RR, 0.44; aqueous: RR, 0.47); the clothing finish ethylene urea melamine formalin resin (RR, 0.45); and the fragrances fragrance mix 1 (RR, 0.65) and balsam of Peru (RR, 0.55).3
Patch testing of 139 African American or Black patients at the Cleveland Clinic (2003-2012) revealed that this population most commonly had positive reactions to nickel (27.5%), fragrance mix (18.1%), bacitracin (13.0%), balsam of Peru (12.3%), and PPD (10.9%). The authors highlighted unique features of physical examination in patients with darker skin types, including lichenification and/or hyperpigmentation in those with ACD and the potential for lack of erythema and/or a papular reaction with patch test readings.4 Recently, data was presented at the American Contact Dermatitis Society Annual Meeting (March 2021) on patterns of ACD in Black and White patch tested patients in Philadelphia (2009-2019).5 Using the North American 80 comprehensive series, the researchers documented statistically significant differences in allergen sensitivity between the 2 groups. Black patients reacted to disperse blue dye (P=.019) and textile dye mix (P=.001) at higher frequencies. There was a nonsignificant trend of more frequent positive reactions to PPD in Black patients (11% vs 6%).5
Notably, all of these studies examined only 1 or 2 racial groups with a focus on Black patients. Some authors commented that this was due to low numbers of Hispanic, Asian and Pacific Islander, and Native American patients in tested populations.2,3,5 With approximately 13% of the US population self-identifying as Black,6 these patients and other minority races typically are underrepresented in large patch test studies. More data on patch test results for these groups is necessary for a complete understanding of patch testing in patients with SOC.
What are the challenges in patch testing SOC populations?
Patch testing in patients with SOC requires additional skills and experience. Darker skin does not reveal erythema as strikingly as lighter skin, making it more difficult to appreciate subtle color changes. Moreover, multiple studies have shown that ACD can have different presentations in Black patients.4,7,8 Lichenification and hyperpigmentation may be early signs of ACD in comparison to bright erythema and vesicles that can be seen in lighter skin types. It also has been reported that scalp ACD can be mistaken for seborrheic dermatitis due to lack of erythema.7 Without a high degree of clinical suspicion, a diagnosis of ACD can be missed in this patient population.
Patch test interpretation also can be challenging in patients with SOC. An early papular or follicular eruption with minimal erythema can signal a positive reaction.4,7 Because of these potentially subtle changes, patch testers should exercise care and attention when reading results for SOC populations. We recommend ample side lighting, palpation for adequate identification of positive reactions, and double-checking for positives that may have been overlooked on the initial review of findings.4,7
What health care disparities impact the evaluation and management of ACD?
There are many factors at play in this dialogue. The challenges we identified in diagnosing ACD in darker skin types are important to consider. Lack of familiarity with these unique features can lead to a delay in diagnosis and ultimately a delay in referral for patch testing. This is where dermatology training can help fill in the gap, but are the majority of programs equipped to do so? Inadequate education and exposure to patients with SOC is an issue for many dermatology residency programs. Surveys of residents and program directors in geographically less diverse regions may not receive adequate education or exposure to patients with SOC.9 Further, there is a lack of representation of SOC images for general dermatologic conditions in textbooks,10,11 which has a profound impact on the dermatologist’s ability to recognize common diseases in darker skin types. A 2019 survey of more than 5000 images from 2 dermatology textbooks showed SOC images comprised 22% to 32% of the total images.11 However, SOC images are overrepresented in textbooks for sexually transmitted infections, constituting 47% to 58% of the images; they made up 28% of images for nonvenereal infections.11 Why is that? In this article, we have shown the prevalence of ACD to be nearly equivalent in Black and White patients, yet a perusal of ACD images in dermatology textbooks will tell a different story. This trend deserves our attention; perhaps it is highlighting patterns of systemic racism seen in medicine. If our primary teaching materials are perpetuating stereotypes, we must consider the impact this can have on our personal implicit biases and the health care disparities that can ensue.
Additional factors impact time to diagnosis of ACD and referral for patch testing. A retrospective study examining distance to a North Carolina patch test referral clinic showed that patients living further from the clinic experienced a longer duration of dermatitis prior to patch test consultation and tended to live in areas with a higher county poverty rate.12 Specifically, a 17.9% increase (P<.001) in the median duration of dermatitis was observed for every 50-mile increase in distance to the patch test clinic. County poverty rate was measured by the percentage of residents living below the poverty threshold; for every 5% increase in county poverty rate, a 16.3% increase (P<.032) in duration of dermatitis was found.12
These data highlight a relationship with which many dermatologists are familiar and underscore a need for dermatologists to practice in areas that are more geographically accessible. The recently increased utilization of telehealth modalities can potentially help to bridge this gap by decreasing delays in diagnosis and providing more affordable options for evaluation by a dermatologist for patients with socioeconomic obstacles.
Final Interpretation
The prevalence of ACD among Black and White patients is similar; however, there are important differences in patch test reaction frequencies that may be related to the diverse exposure patterns for each group. Additionally, patients with SOC may have unique clinical presentations of ACD, such as lichenification and hyperpigmentation. Darker skin types also may require specialized techniques for accurate patch test readings. It is imperative that dermatologists are trained to recognize all of these features. Health care disparities come in many forms and, in this setting, can result in delayed referral for patch testing. Additional studies are needed to further examine these health care disparities and identify potential solutions.
- Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermat. 2001;12:77-82.
- Deleo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl understanding):S107-S112.
- Deleo VA, Alexis A, Warshaw EM, et al. The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292.
- Yu SH, Khanna U, Taylor JS, et al. Patch testing in the African American population: a 10-year experience. Dermatitis. 2019;30:277-278.
- Garg VS, Zhan, T, Brod B, et al. Patterns of allergic contact dermatitis in African Americans and Caucasians in a major metropolitan area over a ten-year period. Presented at: 32nd American Contact Dermatitis Society Annual Meeting (virtual); March 17-18, 2021.
- United States Census Bureau. QuickFacts—United States. Accessed June 11, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- Stallings A, Sood A. Hair-care practices in African American women: potential for allergic contact dermatitis. Semin Cutan Med Surg. 2016;35:207-210.
- Otrofanowei E, Ayanlowo OO, Akinkugbe A, et al. Clinico-etiologic profile of hand dermatitis and patch response of patients at a tertiary hospital in Lagos, Nigeria: results of a prospective observational study. Int J Dermatol. 2018;57:149-155.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Lester JC, Taylor SC, Chren MM. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264.
The world is an increasingly diverse place, which has particular relevance for the dermatologist. Skin color plays a significant role in diagnostic approach, as there are important differences in how cutaneous disease presents in patients with skin of color (SOC). Therefore, education about these differences is imperative. In this review, we focus on allergic contact dermatitis (ACD) and patch testing in patients with SOC. We discuss allergens common to this demographic and challenges encountered in patch testing patients with SOC. We also identify key health care disparities in the evaluation and management of ACD in this population.
Has contact allergy in SOC populations been studied in North America?
Over the last 2 decades, there have been only a handful of North American studies that address contact allergy in SOC populations. Patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic from 1988 to 1991 showed that overall allergy frequency was relatively similar (43.0% vs 43.6%). There were notable differences in allergen sensitization. Paraphenylenediamine (PPD), which is used in hair dye, had more positive patch test reactions in Black patients (10.6% vs 4.5%), and both PPD (21.2% vs 4.2%) and imidazolidinyl urea, a formaldehyde-releasing preservative (9.1% vs 2.6%), were more frequently allergenic in Black men compared to White men.1 Patch test results from the North American Contact Dermatitis Group from 1992 to 1998 described similar results, with minimal variation in the prevalence of ACD among 1014 Black and 8610 White patients (47%–49% vs 46%–49%).2 Positive patch test reactions to PPD were higher in Black patients for 2 of 3 test cycles (13.5% vs 5.8% [1994-1996] and 10.3% vs 5.3% [1996-1998]). Positive patch test reactions were higher in White patients for dimethylol dimethyl hydantoin, a formaldehyde-releasing preservative, also for 2 of 3 test cycles (1.8% vs 0% [1992-1994] and 2.8% vs 0.3% [1994-1996]). Finally, positive patch test reactions to thioureas (rubber accelerators) had a mixed picture: 2 test cycles were higher in Black patients (1.9% vs 1.0% [1992-1994] and 1.3% vs 0.7% [1994-1996]), but the third cycle (1996-1998) was lower (0.7% vs 1.4%). Positive patch test reactions to the metal cobalt chloride were higher in Black patients in just 1 test cycle (9.2% vs 6.6% [1992-1994]). The authors suggested that the use of darker hair dyes in the Black community may lead to more sensitization to PPD. They also theorized that this population’s more frequent use of ointment-based skin care products may make them less susceptible to sensitization to preservatives such as formaldehyde, which more commonly are found in water-based products such as creams. They concluded that differences in sensitization patterns likely were driven by cultural practices affecting exposures.2
In 2016, the North American Contact Dermatitis Group reported patch test results in 434 Black and 6634 White patients (1998-2006).3 Again, ACD prevalence was about the same in both groups (45.9% vs 43.6%). However, they reported several allergens with different reaction patterns. Black patients had higher risk ratios (RRs) for 3 rubber accelerators: mercaptobenzothiazole (RR, 2.10), mercapto mix (RR, 2.27), and thiuram mix (RR, 1.44). They also reacted to PPD (RR, 1.56) and the antibiotic bacitracin (RR, 1.34) at higher frequencies than White patients, who more frequently reacted to formaldehyde (RR, 0.58); the formaldehyde-releasing preservatives quaternium-15 (RR, 0.63) and diazolidinyl urea (in petrolatum: RR, 0.44; aqueous: RR, 0.47); the clothing finish ethylene urea melamine formalin resin (RR, 0.45); and the fragrances fragrance mix 1 (RR, 0.65) and balsam of Peru (RR, 0.55).3
Patch testing of 139 African American or Black patients at the Cleveland Clinic (2003-2012) revealed that this population most commonly had positive reactions to nickel (27.5%), fragrance mix (18.1%), bacitracin (13.0%), balsam of Peru (12.3%), and PPD (10.9%). The authors highlighted unique features of physical examination in patients with darker skin types, including lichenification and/or hyperpigmentation in those with ACD and the potential for lack of erythema and/or a papular reaction with patch test readings.4 Recently, data was presented at the American Contact Dermatitis Society Annual Meeting (March 2021) on patterns of ACD in Black and White patch tested patients in Philadelphia (2009-2019).5 Using the North American 80 comprehensive series, the researchers documented statistically significant differences in allergen sensitivity between the 2 groups. Black patients reacted to disperse blue dye (P=.019) and textile dye mix (P=.001) at higher frequencies. There was a nonsignificant trend of more frequent positive reactions to PPD in Black patients (11% vs 6%).5
Notably, all of these studies examined only 1 or 2 racial groups with a focus on Black patients. Some authors commented that this was due to low numbers of Hispanic, Asian and Pacific Islander, and Native American patients in tested populations.2,3,5 With approximately 13% of the US population self-identifying as Black,6 these patients and other minority races typically are underrepresented in large patch test studies. More data on patch test results for these groups is necessary for a complete understanding of patch testing in patients with SOC.
What are the challenges in patch testing SOC populations?
Patch testing in patients with SOC requires additional skills and experience. Darker skin does not reveal erythema as strikingly as lighter skin, making it more difficult to appreciate subtle color changes. Moreover, multiple studies have shown that ACD can have different presentations in Black patients.4,7,8 Lichenification and hyperpigmentation may be early signs of ACD in comparison to bright erythema and vesicles that can be seen in lighter skin types. It also has been reported that scalp ACD can be mistaken for seborrheic dermatitis due to lack of erythema.7 Without a high degree of clinical suspicion, a diagnosis of ACD can be missed in this patient population.
Patch test interpretation also can be challenging in patients with SOC. An early papular or follicular eruption with minimal erythema can signal a positive reaction.4,7 Because of these potentially subtle changes, patch testers should exercise care and attention when reading results for SOC populations. We recommend ample side lighting, palpation for adequate identification of positive reactions, and double-checking for positives that may have been overlooked on the initial review of findings.4,7
What health care disparities impact the evaluation and management of ACD?
There are many factors at play in this dialogue. The challenges we identified in diagnosing ACD in darker skin types are important to consider. Lack of familiarity with these unique features can lead to a delay in diagnosis and ultimately a delay in referral for patch testing. This is where dermatology training can help fill in the gap, but are the majority of programs equipped to do so? Inadequate education and exposure to patients with SOC is an issue for many dermatology residency programs. Surveys of residents and program directors in geographically less diverse regions may not receive adequate education or exposure to patients with SOC.9 Further, there is a lack of representation of SOC images for general dermatologic conditions in textbooks,10,11 which has a profound impact on the dermatologist’s ability to recognize common diseases in darker skin types. A 2019 survey of more than 5000 images from 2 dermatology textbooks showed SOC images comprised 22% to 32% of the total images.11 However, SOC images are overrepresented in textbooks for sexually transmitted infections, constituting 47% to 58% of the images; they made up 28% of images for nonvenereal infections.11 Why is that? In this article, we have shown the prevalence of ACD to be nearly equivalent in Black and White patients, yet a perusal of ACD images in dermatology textbooks will tell a different story. This trend deserves our attention; perhaps it is highlighting patterns of systemic racism seen in medicine. If our primary teaching materials are perpetuating stereotypes, we must consider the impact this can have on our personal implicit biases and the health care disparities that can ensue.
Additional factors impact time to diagnosis of ACD and referral for patch testing. A retrospective study examining distance to a North Carolina patch test referral clinic showed that patients living further from the clinic experienced a longer duration of dermatitis prior to patch test consultation and tended to live in areas with a higher county poverty rate.12 Specifically, a 17.9% increase (P<.001) in the median duration of dermatitis was observed for every 50-mile increase in distance to the patch test clinic. County poverty rate was measured by the percentage of residents living below the poverty threshold; for every 5% increase in county poverty rate, a 16.3% increase (P<.032) in duration of dermatitis was found.12
These data highlight a relationship with which many dermatologists are familiar and underscore a need for dermatologists to practice in areas that are more geographically accessible. The recently increased utilization of telehealth modalities can potentially help to bridge this gap by decreasing delays in diagnosis and providing more affordable options for evaluation by a dermatologist for patients with socioeconomic obstacles.
Final Interpretation
The prevalence of ACD among Black and White patients is similar; however, there are important differences in patch test reaction frequencies that may be related to the diverse exposure patterns for each group. Additionally, patients with SOC may have unique clinical presentations of ACD, such as lichenification and hyperpigmentation. Darker skin types also may require specialized techniques for accurate patch test readings. It is imperative that dermatologists are trained to recognize all of these features. Health care disparities come in many forms and, in this setting, can result in delayed referral for patch testing. Additional studies are needed to further examine these health care disparities and identify potential solutions.
The world is an increasingly diverse place, which has particular relevance for the dermatologist. Skin color plays a significant role in diagnostic approach, as there are important differences in how cutaneous disease presents in patients with skin of color (SOC). Therefore, education about these differences is imperative. In this review, we focus on allergic contact dermatitis (ACD) and patch testing in patients with SOC. We discuss allergens common to this demographic and challenges encountered in patch testing patients with SOC. We also identify key health care disparities in the evaluation and management of ACD in this population.
Has contact allergy in SOC populations been studied in North America?
Over the last 2 decades, there have been only a handful of North American studies that address contact allergy in SOC populations. Patch test results from 114 Black patients and 877 White patients at the Cleveland Clinic from 1988 to 1991 showed that overall allergy frequency was relatively similar (43.0% vs 43.6%). There were notable differences in allergen sensitization. Paraphenylenediamine (PPD), which is used in hair dye, had more positive patch test reactions in Black patients (10.6% vs 4.5%), and both PPD (21.2% vs 4.2%) and imidazolidinyl urea, a formaldehyde-releasing preservative (9.1% vs 2.6%), were more frequently allergenic in Black men compared to White men.1 Patch test results from the North American Contact Dermatitis Group from 1992 to 1998 described similar results, with minimal variation in the prevalence of ACD among 1014 Black and 8610 White patients (47%–49% vs 46%–49%).2 Positive patch test reactions to PPD were higher in Black patients for 2 of 3 test cycles (13.5% vs 5.8% [1994-1996] and 10.3% vs 5.3% [1996-1998]). Positive patch test reactions were higher in White patients for dimethylol dimethyl hydantoin, a formaldehyde-releasing preservative, also for 2 of 3 test cycles (1.8% vs 0% [1992-1994] and 2.8% vs 0.3% [1994-1996]). Finally, positive patch test reactions to thioureas (rubber accelerators) had a mixed picture: 2 test cycles were higher in Black patients (1.9% vs 1.0% [1992-1994] and 1.3% vs 0.7% [1994-1996]), but the third cycle (1996-1998) was lower (0.7% vs 1.4%). Positive patch test reactions to the metal cobalt chloride were higher in Black patients in just 1 test cycle (9.2% vs 6.6% [1992-1994]). The authors suggested that the use of darker hair dyes in the Black community may lead to more sensitization to PPD. They also theorized that this population’s more frequent use of ointment-based skin care products may make them less susceptible to sensitization to preservatives such as formaldehyde, which more commonly are found in water-based products such as creams. They concluded that differences in sensitization patterns likely were driven by cultural practices affecting exposures.2
In 2016, the North American Contact Dermatitis Group reported patch test results in 434 Black and 6634 White patients (1998-2006).3 Again, ACD prevalence was about the same in both groups (45.9% vs 43.6%). However, they reported several allergens with different reaction patterns. Black patients had higher risk ratios (RRs) for 3 rubber accelerators: mercaptobenzothiazole (RR, 2.10), mercapto mix (RR, 2.27), and thiuram mix (RR, 1.44). They also reacted to PPD (RR, 1.56) and the antibiotic bacitracin (RR, 1.34) at higher frequencies than White patients, who more frequently reacted to formaldehyde (RR, 0.58); the formaldehyde-releasing preservatives quaternium-15 (RR, 0.63) and diazolidinyl urea (in petrolatum: RR, 0.44; aqueous: RR, 0.47); the clothing finish ethylene urea melamine formalin resin (RR, 0.45); and the fragrances fragrance mix 1 (RR, 0.65) and balsam of Peru (RR, 0.55).3
Patch testing of 139 African American or Black patients at the Cleveland Clinic (2003-2012) revealed that this population most commonly had positive reactions to nickel (27.5%), fragrance mix (18.1%), bacitracin (13.0%), balsam of Peru (12.3%), and PPD (10.9%). The authors highlighted unique features of physical examination in patients with darker skin types, including lichenification and/or hyperpigmentation in those with ACD and the potential for lack of erythema and/or a papular reaction with patch test readings.4 Recently, data was presented at the American Contact Dermatitis Society Annual Meeting (March 2021) on patterns of ACD in Black and White patch tested patients in Philadelphia (2009-2019).5 Using the North American 80 comprehensive series, the researchers documented statistically significant differences in allergen sensitivity between the 2 groups. Black patients reacted to disperse blue dye (P=.019) and textile dye mix (P=.001) at higher frequencies. There was a nonsignificant trend of more frequent positive reactions to PPD in Black patients (11% vs 6%).5
Notably, all of these studies examined only 1 or 2 racial groups with a focus on Black patients. Some authors commented that this was due to low numbers of Hispanic, Asian and Pacific Islander, and Native American patients in tested populations.2,3,5 With approximately 13% of the US population self-identifying as Black,6 these patients and other minority races typically are underrepresented in large patch test studies. More data on patch test results for these groups is necessary for a complete understanding of patch testing in patients with SOC.
What are the challenges in patch testing SOC populations?
Patch testing in patients with SOC requires additional skills and experience. Darker skin does not reveal erythema as strikingly as lighter skin, making it more difficult to appreciate subtle color changes. Moreover, multiple studies have shown that ACD can have different presentations in Black patients.4,7,8 Lichenification and hyperpigmentation may be early signs of ACD in comparison to bright erythema and vesicles that can be seen in lighter skin types. It also has been reported that scalp ACD can be mistaken for seborrheic dermatitis due to lack of erythema.7 Without a high degree of clinical suspicion, a diagnosis of ACD can be missed in this patient population.
Patch test interpretation also can be challenging in patients with SOC. An early papular or follicular eruption with minimal erythema can signal a positive reaction.4,7 Because of these potentially subtle changes, patch testers should exercise care and attention when reading results for SOC populations. We recommend ample side lighting, palpation for adequate identification of positive reactions, and double-checking for positives that may have been overlooked on the initial review of findings.4,7
What health care disparities impact the evaluation and management of ACD?
There are many factors at play in this dialogue. The challenges we identified in diagnosing ACD in darker skin types are important to consider. Lack of familiarity with these unique features can lead to a delay in diagnosis and ultimately a delay in referral for patch testing. This is where dermatology training can help fill in the gap, but are the majority of programs equipped to do so? Inadequate education and exposure to patients with SOC is an issue for many dermatology residency programs. Surveys of residents and program directors in geographically less diverse regions may not receive adequate education or exposure to patients with SOC.9 Further, there is a lack of representation of SOC images for general dermatologic conditions in textbooks,10,11 which has a profound impact on the dermatologist’s ability to recognize common diseases in darker skin types. A 2019 survey of more than 5000 images from 2 dermatology textbooks showed SOC images comprised 22% to 32% of the total images.11 However, SOC images are overrepresented in textbooks for sexually transmitted infections, constituting 47% to 58% of the images; they made up 28% of images for nonvenereal infections.11 Why is that? In this article, we have shown the prevalence of ACD to be nearly equivalent in Black and White patients, yet a perusal of ACD images in dermatology textbooks will tell a different story. This trend deserves our attention; perhaps it is highlighting patterns of systemic racism seen in medicine. If our primary teaching materials are perpetuating stereotypes, we must consider the impact this can have on our personal implicit biases and the health care disparities that can ensue.
Additional factors impact time to diagnosis of ACD and referral for patch testing. A retrospective study examining distance to a North Carolina patch test referral clinic showed that patients living further from the clinic experienced a longer duration of dermatitis prior to patch test consultation and tended to live in areas with a higher county poverty rate.12 Specifically, a 17.9% increase (P<.001) in the median duration of dermatitis was observed for every 50-mile increase in distance to the patch test clinic. County poverty rate was measured by the percentage of residents living below the poverty threshold; for every 5% increase in county poverty rate, a 16.3% increase (P<.032) in duration of dermatitis was found.12
These data highlight a relationship with which many dermatologists are familiar and underscore a need for dermatologists to practice in areas that are more geographically accessible. The recently increased utilization of telehealth modalities can potentially help to bridge this gap by decreasing delays in diagnosis and providing more affordable options for evaluation by a dermatologist for patients with socioeconomic obstacles.
Final Interpretation
The prevalence of ACD among Black and White patients is similar; however, there are important differences in patch test reaction frequencies that may be related to the diverse exposure patterns for each group. Additionally, patients with SOC may have unique clinical presentations of ACD, such as lichenification and hyperpigmentation. Darker skin types also may require specialized techniques for accurate patch test readings. It is imperative that dermatologists are trained to recognize all of these features. Health care disparities come in many forms and, in this setting, can result in delayed referral for patch testing. Additional studies are needed to further examine these health care disparities and identify potential solutions.
- Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermat. 2001;12:77-82.
- Deleo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl understanding):S107-S112.
- Deleo VA, Alexis A, Warshaw EM, et al. The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292.
- Yu SH, Khanna U, Taylor JS, et al. Patch testing in the African American population: a 10-year experience. Dermatitis. 2019;30:277-278.
- Garg VS, Zhan, T, Brod B, et al. Patterns of allergic contact dermatitis in African Americans and Caucasians in a major metropolitan area over a ten-year period. Presented at: 32nd American Contact Dermatitis Society Annual Meeting (virtual); March 17-18, 2021.
- United States Census Bureau. QuickFacts—United States. Accessed June 11, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- Stallings A, Sood A. Hair-care practices in African American women: potential for allergic contact dermatitis. Semin Cutan Med Surg. 2016;35:207-210.
- Otrofanowei E, Ayanlowo OO, Akinkugbe A, et al. Clinico-etiologic profile of hand dermatitis and patch response of patients at a tertiary hospital in Lagos, Nigeria: results of a prospective observational study. Int J Dermatol. 2018;57:149-155.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Lester JC, Taylor SC, Chren MM. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264.
- Dickel H, Taylor JS, Evey P, et al. Comparison of patch test results with a standard series among white and black racial groups. Am J Contact Dermat. 2001;12:77-82.
- Deleo VA, Taylor SC, Belsito DV, et al. The effect of race and ethnicity on patch test results. J Am Acad Dermatol. 2002;46(2 suppl understanding):S107-S112.
- Deleo VA, Alexis A, Warshaw EM, et al. The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-292.
- Yu SH, Khanna U, Taylor JS, et al. Patch testing in the African American population: a 10-year experience. Dermatitis. 2019;30:277-278.
- Garg VS, Zhan, T, Brod B, et al. Patterns of allergic contact dermatitis in African Americans and Caucasians in a major metropolitan area over a ten-year period. Presented at: 32nd American Contact Dermatitis Society Annual Meeting (virtual); March 17-18, 2021.
- United States Census Bureau. QuickFacts—United States. Accessed June 11, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- Stallings A, Sood A. Hair-care practices in African American women: potential for allergic contact dermatitis. Semin Cutan Med Surg. 2016;35:207-210.
- Otrofanowei E, Ayanlowo OO, Akinkugbe A, et al. Clinico-etiologic profile of hand dermatitis and patch response of patients at a tertiary hospital in Lagos, Nigeria: results of a prospective observational study. Int J Dermatol. 2018;57:149-155.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Lester JC, Taylor SC, Chren MM. Under-representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Rodriguez-Homs LG, Liu B, Green CL, et al. Duration of dermatitis before patch test appointment is associated with distance to clinic and county poverty rate. Dermatitis. 2020;31:259-264.
Practice Points
- Similar rates of allergic contact dermatitis (ACD) exist between Black and White patients, with some differences in allergen profiles.
- Patch testing in patients with skin of color (SOC) may require side lighting and palpation, as erythema may be absent or minimal.
- Dermatologic training in evaluation and management of patients with SOC and ACD is vital.
- Distance to clinic and county poverty rate may adversely affect timely referral to a contact dermatitis specialist.
Acetophenone Azine: The 2021 American Contact Dermatitis Society Allergen of the Year
It’s time for the American Contact Dermatitis Society (ACDS) Allergen of the Year! For 2021, the esteemed award goes to acetophenone azine (AA). If you have never heard of this chemical, you are not alone. Acetophenone azine has been identified in foam materials made of the copolymer ethyl-vinyl acetate (EVA). Contact allergy to AA initially was reported in 2016.1 There are only a few European and Canadian case reports and one case series of AA contact allergy in the literature, all of which are associated with foam shin pads or shin guards, shoe insoles, and/or flip-flops.2-6 Acetophenone azine is an important emerging allergen, and in this column, we will introduce you to AA and the sneaky places it can lurk and cause allergic contact dermatitis (ACD). We also highlight diagnosis, management, and patch testing for AA contact allergy.
AA Contact Allergy in the Literature
The first case of AA contact allergy was reported in Europe in 2016 when a 13-year-old male soccer player developed severe lower leg dermatitis and later generalized dermatitis associated with wearing foam shin guards.1 Patch testing to standard and supplemental trays was negative or not relevant; however, the patient exhibited strong reactions when patch tested directly to a piece of the shin guard soaked in acetone, water, and ethanol. Additional testing with AA diluted in acetone, water, and petrolatum resulted in positive patch test reactions to acetone dilutions of 1%, 0.1%, 0.01%, and 0.001% and aqueous solutions of 1% and 0.1%. Chromatographic analyses with high-performance liquid chromatography (HPLC) of shin guard extracts confirmed the culprit allergen to be AA.1
In the following months, the same clinic saw 2 more cases of AA contact allergy.2 An 11-year-old male soccer player developed lower leg dermatitis and later generalized dermatitis from wearing shin guards. Months later, he also developed dermatitis on the soles of the feet, which was attributed to wearing flip-flops. Patch tests to pieces of the shin guards and flip-flops were positive; AA in acetone 0.1% and 0.01% also was positive. As you might expect, HPLC again confirmed the presence of AA in the shin guards and flip-flops. The third patient was a 12-year-old boy with dermatitis on the soles of both feet; later he also developed a generalized dermatitis. Patch testing to pieces of the insoles of his sneakers and AA in acetone 0.1% and 0.01% was positive. Again, HPLC was positive for the presence of AA in the insoles of his sneakers.2
Several more cases of AA contact allergy have been reported in the literature. A 29-year-old European male hockey player demonstrated contact allergy to the gray foam of his shin pads as well as localized leg dermatitis followed by generalized dermatitis (are you noticing a trend yet?), and later dermatitis on the soles of the feet with positive patch-test reactions to pieces of his shin pads and shoe insoles as well as AA 0.1% and 0.01% in acetone.3 A 6-year-old Canadian male soccer player presented with leg dermatitis and later generalized dermatitis and dermatitis on the soles of the feet with positive reactions to pieces of his shin pads and shoe insoles as well as to AA 1% and 0.1% in petrolatum.4 A 17-year-old British male (another trend, all males so far!) hockey player developed dermatitis localized to the legs and positive patch tests to the worn foam inner lining of his shin pads as well as to AA 0.1%, 0.01%, and 0.001% in acetone.5Finally, Darrigade et al6 published a case series of 6 European children with AA contact allergy associated with shin pads and shoes; all had localized leg dermatitis, and some had generalized dermatitis. Patch testing to pieces of shin pads and shoe parts as well as to AA 0.1% in petrolatum and/or acetone showed with positive reactions to the foam pieces and AA in all 6 patients.
What’s the Deal With AA?
Acetophenone azine (also known as methylphenylketazine or bis[1-phenylethylidene]hydrazine) is composed of 2 acetophenone structures and a hydrazine moiety. It has been identified in EVA foam, which can be found in sports equipment such as shin pads or shin guards, shoes, and flip-flops. Raison-Peyron et al1 confirmed the presence of AA in EVA foam but reported that they did not know the exact reason for its presence. The authors theorized that AA might be a catalyst during EVA polymerization and also noted that it has antimicrobial and antihelminthic activity.1 Several authors noted that AA could be a by-product of EVA synthesis and that sports equipment manufacturers might not be aware of its presence in EVA.2,4-6 Some noted that AA concentration was higher in shin guards than in shoe insoles; they thought this explained why patients reacted first to their shin guards and were perhaps even initially sensitized to the shin guards, as well as why shoe insole contact allergy commonly was reported later or only after allergy to shin guards had already developed.4,6
Differential Diagnosis of Shin Pad or Shin Guard Dermatitis
We would be remiss if we did not mention the appropriate differential diagnosis when shin pad or shin guard dermatitis is identified. In fact, in most cases, shin guard dermatitis results from irritant contact dermatitis from friction, heat, and/or perspiration. Acetophenone azine contact allergy is not the most likely diagnosis when your sports-savvy, shin guard–wearing patient presents with anterior lower leg dermatitis. However, when conservative therapy (eg, barrier between the shin guard and the skin, control or management of perspiration, topical corticosteroid therapy) fails, patch testing to evaluate for ACD is indicated.
Management of AA Contact Allergy
As astute readers of this column are already aware, treatment of ACD requires strict allergen avoidance. You will find that we have the same recommendations for AA contact allergy. Given that there are only a handful of cases in the literature, there are limited recommendations on practical allergen avoidance other than “don’t wear the problem shin guards, shoe insoles, or flip-flops.” However, Darrigade et al6 recommended wearing polyurethane shin guards and leather insoles as alternatives when AA contact allergy is suspected or confirmed. They also made it clear that thick socks worn between shin guards and the skin often are not good enough to avoid ACD because the relevant allergens may achieve skin contact despite the barrier.6
Patch Testing for AA Contact Allergy
Historically, ACD to shin guards or shin pads, insoles of shoes, and even flip-flops has been associated with rubber-related chemicals such as mercapto mix, thiuram mix, N-isopropyl-N’-phenyl-p-phenylenediamine, thioureas, and carbamates, as well as dyes, benzoyl peroxide, and urea formaldehyde or phenol formaldehyde resins.1 Most of these chemicals can be tested with standard screening series or supplemental series. Patients with contact allergy to AA may have negative patch testing to screening series and/or supplemental series and may have strong positive reactions to pieces of suspected foam shin pads or shin guards, shoes, and/or flip-flops. Although Koumaki et al5 recommended patch testing for AA contact allergy with AA 0.1% in acetone, Besner Morin et al4 mentioned that petrolatum may be a more desirable vehicle because it could maintain stability for a longer period of time. In fact, a 2021 article highlighting the American Contact Dermatitis Society Allergen of the Year recommends testing with either AA 0.1% in acetone or AA 0.1% in petrolatum.7 Unfortunately, AA is not commercially available for purchase at the time of publication. We are hopeful that this will change in the near future.
Final Interpretation
Acetophenone azine is an emerging allergen commonly identified in EVA foam and attributed to contact allergy to shin guards or pads, soles of shoes, and flip-flops. Most cases have been reported in Europe and Canada and have been identified in young male athletes. In addition to standard patch testing, athletes with lower leg dermatitis and/or dermatitis of the soles of the feet should undergo patch testing with AA 0.1% in acetone or petrolatum and pieces of the equipment and/or footwear.
- Raison-Peyron N, Bergendorff O, Bourrain JL, et al. Acetophenone azine: a new allergen responsible for severe contact dermatitis from shin pads. Contact Dermatitis. 2016;75:106-110.
- Raison-Peyron N, Bergendorff O, Du-Thanh A, et al. Two new cases of severe allergic contact dermatitis caused by acetophenone azine. Contact Dermatitis. 2017;76:380-381.
- De Fré C, Bergendorff O, Raison-Peyron N, et al. Acetophenone azine: a new shoe allergen causing severe foot dermatitis. Contact Dermatitis. 2017;77:416-417.
- Besner Morin C, Stanciu M, Miedzybrodzki B, et al. Allergic contact dermatitis from acetophenone azine in a Canadian child. Contact Dermatitis. 2020;83:41-42.
- Koumaki D, Bergendorff O, Bruze M, et al. Allergic contact dermatitis to shin pads in a hockey player: acetophenone is an emerging allergen. Dermatitis. 2019;30:162-163.
- Darrigade AS, Raison-Peyron N, Courouge-Dorcier D, et al. The chemical acetophenone azine: an important cause of shin and foot dermatitis in children. J Eur Acad Dermatol Venereol. 2020;34:E61-E62.
- Raison-Peyron N, Sasseville D. Acetophenone azine. Dermatitis. 2021;32:5-9.
It’s time for the American Contact Dermatitis Society (ACDS) Allergen of the Year! For 2021, the esteemed award goes to acetophenone azine (AA). If you have never heard of this chemical, you are not alone. Acetophenone azine has been identified in foam materials made of the copolymer ethyl-vinyl acetate (EVA). Contact allergy to AA initially was reported in 2016.1 There are only a few European and Canadian case reports and one case series of AA contact allergy in the literature, all of which are associated with foam shin pads or shin guards, shoe insoles, and/or flip-flops.2-6 Acetophenone azine is an important emerging allergen, and in this column, we will introduce you to AA and the sneaky places it can lurk and cause allergic contact dermatitis (ACD). We also highlight diagnosis, management, and patch testing for AA contact allergy.
AA Contact Allergy in the Literature
The first case of AA contact allergy was reported in Europe in 2016 when a 13-year-old male soccer player developed severe lower leg dermatitis and later generalized dermatitis associated with wearing foam shin guards.1 Patch testing to standard and supplemental trays was negative or not relevant; however, the patient exhibited strong reactions when patch tested directly to a piece of the shin guard soaked in acetone, water, and ethanol. Additional testing with AA diluted in acetone, water, and petrolatum resulted in positive patch test reactions to acetone dilutions of 1%, 0.1%, 0.01%, and 0.001% and aqueous solutions of 1% and 0.1%. Chromatographic analyses with high-performance liquid chromatography (HPLC) of shin guard extracts confirmed the culprit allergen to be AA.1
In the following months, the same clinic saw 2 more cases of AA contact allergy.2 An 11-year-old male soccer player developed lower leg dermatitis and later generalized dermatitis from wearing shin guards. Months later, he also developed dermatitis on the soles of the feet, which was attributed to wearing flip-flops. Patch tests to pieces of the shin guards and flip-flops were positive; AA in acetone 0.1% and 0.01% also was positive. As you might expect, HPLC again confirmed the presence of AA in the shin guards and flip-flops. The third patient was a 12-year-old boy with dermatitis on the soles of both feet; later he also developed a generalized dermatitis. Patch testing to pieces of the insoles of his sneakers and AA in acetone 0.1% and 0.01% was positive. Again, HPLC was positive for the presence of AA in the insoles of his sneakers.2
Several more cases of AA contact allergy have been reported in the literature. A 29-year-old European male hockey player demonstrated contact allergy to the gray foam of his shin pads as well as localized leg dermatitis followed by generalized dermatitis (are you noticing a trend yet?), and later dermatitis on the soles of the feet with positive patch-test reactions to pieces of his shin pads and shoe insoles as well as AA 0.1% and 0.01% in acetone.3 A 6-year-old Canadian male soccer player presented with leg dermatitis and later generalized dermatitis and dermatitis on the soles of the feet with positive reactions to pieces of his shin pads and shoe insoles as well as to AA 1% and 0.1% in petrolatum.4 A 17-year-old British male (another trend, all males so far!) hockey player developed dermatitis localized to the legs and positive patch tests to the worn foam inner lining of his shin pads as well as to AA 0.1%, 0.01%, and 0.001% in acetone.5Finally, Darrigade et al6 published a case series of 6 European children with AA contact allergy associated with shin pads and shoes; all had localized leg dermatitis, and some had generalized dermatitis. Patch testing to pieces of shin pads and shoe parts as well as to AA 0.1% in petrolatum and/or acetone showed with positive reactions to the foam pieces and AA in all 6 patients.
What’s the Deal With AA?
Acetophenone azine (also known as methylphenylketazine or bis[1-phenylethylidene]hydrazine) is composed of 2 acetophenone structures and a hydrazine moiety. It has been identified in EVA foam, which can be found in sports equipment such as shin pads or shin guards, shoes, and flip-flops. Raison-Peyron et al1 confirmed the presence of AA in EVA foam but reported that they did not know the exact reason for its presence. The authors theorized that AA might be a catalyst during EVA polymerization and also noted that it has antimicrobial and antihelminthic activity.1 Several authors noted that AA could be a by-product of EVA synthesis and that sports equipment manufacturers might not be aware of its presence in EVA.2,4-6 Some noted that AA concentration was higher in shin guards than in shoe insoles; they thought this explained why patients reacted first to their shin guards and were perhaps even initially sensitized to the shin guards, as well as why shoe insole contact allergy commonly was reported later or only after allergy to shin guards had already developed.4,6
Differential Diagnosis of Shin Pad or Shin Guard Dermatitis
We would be remiss if we did not mention the appropriate differential diagnosis when shin pad or shin guard dermatitis is identified. In fact, in most cases, shin guard dermatitis results from irritant contact dermatitis from friction, heat, and/or perspiration. Acetophenone azine contact allergy is not the most likely diagnosis when your sports-savvy, shin guard–wearing patient presents with anterior lower leg dermatitis. However, when conservative therapy (eg, barrier between the shin guard and the skin, control or management of perspiration, topical corticosteroid therapy) fails, patch testing to evaluate for ACD is indicated.
Management of AA Contact Allergy
As astute readers of this column are already aware, treatment of ACD requires strict allergen avoidance. You will find that we have the same recommendations for AA contact allergy. Given that there are only a handful of cases in the literature, there are limited recommendations on practical allergen avoidance other than “don’t wear the problem shin guards, shoe insoles, or flip-flops.” However, Darrigade et al6 recommended wearing polyurethane shin guards and leather insoles as alternatives when AA contact allergy is suspected or confirmed. They also made it clear that thick socks worn between shin guards and the skin often are not good enough to avoid ACD because the relevant allergens may achieve skin contact despite the barrier.6
Patch Testing for AA Contact Allergy
Historically, ACD to shin guards or shin pads, insoles of shoes, and even flip-flops has been associated with rubber-related chemicals such as mercapto mix, thiuram mix, N-isopropyl-N’-phenyl-p-phenylenediamine, thioureas, and carbamates, as well as dyes, benzoyl peroxide, and urea formaldehyde or phenol formaldehyde resins.1 Most of these chemicals can be tested with standard screening series or supplemental series. Patients with contact allergy to AA may have negative patch testing to screening series and/or supplemental series and may have strong positive reactions to pieces of suspected foam shin pads or shin guards, shoes, and/or flip-flops. Although Koumaki et al5 recommended patch testing for AA contact allergy with AA 0.1% in acetone, Besner Morin et al4 mentioned that petrolatum may be a more desirable vehicle because it could maintain stability for a longer period of time. In fact, a 2021 article highlighting the American Contact Dermatitis Society Allergen of the Year recommends testing with either AA 0.1% in acetone or AA 0.1% in petrolatum.7 Unfortunately, AA is not commercially available for purchase at the time of publication. We are hopeful that this will change in the near future.
Final Interpretation
Acetophenone azine is an emerging allergen commonly identified in EVA foam and attributed to contact allergy to shin guards or pads, soles of shoes, and flip-flops. Most cases have been reported in Europe and Canada and have been identified in young male athletes. In addition to standard patch testing, athletes with lower leg dermatitis and/or dermatitis of the soles of the feet should undergo patch testing with AA 0.1% in acetone or petrolatum and pieces of the equipment and/or footwear.
It’s time for the American Contact Dermatitis Society (ACDS) Allergen of the Year! For 2021, the esteemed award goes to acetophenone azine (AA). If you have never heard of this chemical, you are not alone. Acetophenone azine has been identified in foam materials made of the copolymer ethyl-vinyl acetate (EVA). Contact allergy to AA initially was reported in 2016.1 There are only a few European and Canadian case reports and one case series of AA contact allergy in the literature, all of which are associated with foam shin pads or shin guards, shoe insoles, and/or flip-flops.2-6 Acetophenone azine is an important emerging allergen, and in this column, we will introduce you to AA and the sneaky places it can lurk and cause allergic contact dermatitis (ACD). We also highlight diagnosis, management, and patch testing for AA contact allergy.
AA Contact Allergy in the Literature
The first case of AA contact allergy was reported in Europe in 2016 when a 13-year-old male soccer player developed severe lower leg dermatitis and later generalized dermatitis associated with wearing foam shin guards.1 Patch testing to standard and supplemental trays was negative or not relevant; however, the patient exhibited strong reactions when patch tested directly to a piece of the shin guard soaked in acetone, water, and ethanol. Additional testing with AA diluted in acetone, water, and petrolatum resulted in positive patch test reactions to acetone dilutions of 1%, 0.1%, 0.01%, and 0.001% and aqueous solutions of 1% and 0.1%. Chromatographic analyses with high-performance liquid chromatography (HPLC) of shin guard extracts confirmed the culprit allergen to be AA.1
In the following months, the same clinic saw 2 more cases of AA contact allergy.2 An 11-year-old male soccer player developed lower leg dermatitis and later generalized dermatitis from wearing shin guards. Months later, he also developed dermatitis on the soles of the feet, which was attributed to wearing flip-flops. Patch tests to pieces of the shin guards and flip-flops were positive; AA in acetone 0.1% and 0.01% also was positive. As you might expect, HPLC again confirmed the presence of AA in the shin guards and flip-flops. The third patient was a 12-year-old boy with dermatitis on the soles of both feet; later he also developed a generalized dermatitis. Patch testing to pieces of the insoles of his sneakers and AA in acetone 0.1% and 0.01% was positive. Again, HPLC was positive for the presence of AA in the insoles of his sneakers.2
Several more cases of AA contact allergy have been reported in the literature. A 29-year-old European male hockey player demonstrated contact allergy to the gray foam of his shin pads as well as localized leg dermatitis followed by generalized dermatitis (are you noticing a trend yet?), and later dermatitis on the soles of the feet with positive patch-test reactions to pieces of his shin pads and shoe insoles as well as AA 0.1% and 0.01% in acetone.3 A 6-year-old Canadian male soccer player presented with leg dermatitis and later generalized dermatitis and dermatitis on the soles of the feet with positive reactions to pieces of his shin pads and shoe insoles as well as to AA 1% and 0.1% in petrolatum.4 A 17-year-old British male (another trend, all males so far!) hockey player developed dermatitis localized to the legs and positive patch tests to the worn foam inner lining of his shin pads as well as to AA 0.1%, 0.01%, and 0.001% in acetone.5Finally, Darrigade et al6 published a case series of 6 European children with AA contact allergy associated with shin pads and shoes; all had localized leg dermatitis, and some had generalized dermatitis. Patch testing to pieces of shin pads and shoe parts as well as to AA 0.1% in petrolatum and/or acetone showed with positive reactions to the foam pieces and AA in all 6 patients.
What’s the Deal With AA?
Acetophenone azine (also known as methylphenylketazine or bis[1-phenylethylidene]hydrazine) is composed of 2 acetophenone structures and a hydrazine moiety. It has been identified in EVA foam, which can be found in sports equipment such as shin pads or shin guards, shoes, and flip-flops. Raison-Peyron et al1 confirmed the presence of AA in EVA foam but reported that they did not know the exact reason for its presence. The authors theorized that AA might be a catalyst during EVA polymerization and also noted that it has antimicrobial and antihelminthic activity.1 Several authors noted that AA could be a by-product of EVA synthesis and that sports equipment manufacturers might not be aware of its presence in EVA.2,4-6 Some noted that AA concentration was higher in shin guards than in shoe insoles; they thought this explained why patients reacted first to their shin guards and were perhaps even initially sensitized to the shin guards, as well as why shoe insole contact allergy commonly was reported later or only after allergy to shin guards had already developed.4,6
Differential Diagnosis of Shin Pad or Shin Guard Dermatitis
We would be remiss if we did not mention the appropriate differential diagnosis when shin pad or shin guard dermatitis is identified. In fact, in most cases, shin guard dermatitis results from irritant contact dermatitis from friction, heat, and/or perspiration. Acetophenone azine contact allergy is not the most likely diagnosis when your sports-savvy, shin guard–wearing patient presents with anterior lower leg dermatitis. However, when conservative therapy (eg, barrier between the shin guard and the skin, control or management of perspiration, topical corticosteroid therapy) fails, patch testing to evaluate for ACD is indicated.
Management of AA Contact Allergy
As astute readers of this column are already aware, treatment of ACD requires strict allergen avoidance. You will find that we have the same recommendations for AA contact allergy. Given that there are only a handful of cases in the literature, there are limited recommendations on practical allergen avoidance other than “don’t wear the problem shin guards, shoe insoles, or flip-flops.” However, Darrigade et al6 recommended wearing polyurethane shin guards and leather insoles as alternatives when AA contact allergy is suspected or confirmed. They also made it clear that thick socks worn between shin guards and the skin often are not good enough to avoid ACD because the relevant allergens may achieve skin contact despite the barrier.6
Patch Testing for AA Contact Allergy
Historically, ACD to shin guards or shin pads, insoles of shoes, and even flip-flops has been associated with rubber-related chemicals such as mercapto mix, thiuram mix, N-isopropyl-N’-phenyl-p-phenylenediamine, thioureas, and carbamates, as well as dyes, benzoyl peroxide, and urea formaldehyde or phenol formaldehyde resins.1 Most of these chemicals can be tested with standard screening series or supplemental series. Patients with contact allergy to AA may have negative patch testing to screening series and/or supplemental series and may have strong positive reactions to pieces of suspected foam shin pads or shin guards, shoes, and/or flip-flops. Although Koumaki et al5 recommended patch testing for AA contact allergy with AA 0.1% in acetone, Besner Morin et al4 mentioned that petrolatum may be a more desirable vehicle because it could maintain stability for a longer period of time. In fact, a 2021 article highlighting the American Contact Dermatitis Society Allergen of the Year recommends testing with either AA 0.1% in acetone or AA 0.1% in petrolatum.7 Unfortunately, AA is not commercially available for purchase at the time of publication. We are hopeful that this will change in the near future.
Final Interpretation
Acetophenone azine is an emerging allergen commonly identified in EVA foam and attributed to contact allergy to shin guards or pads, soles of shoes, and flip-flops. Most cases have been reported in Europe and Canada and have been identified in young male athletes. In addition to standard patch testing, athletes with lower leg dermatitis and/or dermatitis of the soles of the feet should undergo patch testing with AA 0.1% in acetone or petrolatum and pieces of the equipment and/or footwear.
- Raison-Peyron N, Bergendorff O, Bourrain JL, et al. Acetophenone azine: a new allergen responsible for severe contact dermatitis from shin pads. Contact Dermatitis. 2016;75:106-110.
- Raison-Peyron N, Bergendorff O, Du-Thanh A, et al. Two new cases of severe allergic contact dermatitis caused by acetophenone azine. Contact Dermatitis. 2017;76:380-381.
- De Fré C, Bergendorff O, Raison-Peyron N, et al. Acetophenone azine: a new shoe allergen causing severe foot dermatitis. Contact Dermatitis. 2017;77:416-417.
- Besner Morin C, Stanciu M, Miedzybrodzki B, et al. Allergic contact dermatitis from acetophenone azine in a Canadian child. Contact Dermatitis. 2020;83:41-42.
- Koumaki D, Bergendorff O, Bruze M, et al. Allergic contact dermatitis to shin pads in a hockey player: acetophenone is an emerging allergen. Dermatitis. 2019;30:162-163.
- Darrigade AS, Raison-Peyron N, Courouge-Dorcier D, et al. The chemical acetophenone azine: an important cause of shin and foot dermatitis in children. J Eur Acad Dermatol Venereol. 2020;34:E61-E62.
- Raison-Peyron N, Sasseville D. Acetophenone azine. Dermatitis. 2021;32:5-9.
- Raison-Peyron N, Bergendorff O, Bourrain JL, et al. Acetophenone azine: a new allergen responsible for severe contact dermatitis from shin pads. Contact Dermatitis. 2016;75:106-110.
- Raison-Peyron N, Bergendorff O, Du-Thanh A, et al. Two new cases of severe allergic contact dermatitis caused by acetophenone azine. Contact Dermatitis. 2017;76:380-381.
- De Fré C, Bergendorff O, Raison-Peyron N, et al. Acetophenone azine: a new shoe allergen causing severe foot dermatitis. Contact Dermatitis. 2017;77:416-417.
- Besner Morin C, Stanciu M, Miedzybrodzki B, et al. Allergic contact dermatitis from acetophenone azine in a Canadian child. Contact Dermatitis. 2020;83:41-42.
- Koumaki D, Bergendorff O, Bruze M, et al. Allergic contact dermatitis to shin pads in a hockey player: acetophenone is an emerging allergen. Dermatitis. 2019;30:162-163.
- Darrigade AS, Raison-Peyron N, Courouge-Dorcier D, et al. The chemical acetophenone azine: an important cause of shin and foot dermatitis in children. J Eur Acad Dermatol Venereol. 2020;34:E61-E62.
- Raison-Peyron N, Sasseville D. Acetophenone azine. Dermatitis. 2021;32:5-9.
Practice Points
- Acetophenone azine is an emerging allergen identified in ethyl-vinyl acetate foam used in shin guards, shoe soles, and flip-flops.
- Cases have been reported in young male athletes in Europe and Canada.
- Patch testing can be completed with acetophenone azine 0.1% in acetone or petrolatum.
Contact Dermatitis of the Hands: Is It Irritant or Allergic?
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
Practice Points
- For the hands, irritant contact dermatitis (ICD) is more common than allergic contact dermatitis in both occupational and nonoccupational settings. Because of overlapping clinical features, it can be difficult to differentiate between these conditions.
- Use of hand hygiene products, frequent handwashing, wet work, mechanical trauma, and occlusion can contribute to ICD of the hands.
- Common hand contact allergens include preservatives, metals, fragrances, and rubber accelerators.
- Patch testing often is necessary for diagnosis of hand dermatitis, and both screening and supplemental allergen series may be required.
Contact Allergy to Nickel: Still #1 After All These Years
Nickel is unrivaled as the most common cause of contact allergy worldwide.1 Nickel is commonly used as a hardening agent in metal products, and complete avoidance is challenging due to numerous potential exposures (eg, direct contact, airborne, dietary, medical implantation). Allergic contact dermatitis to nickel (Ni-ACD) can lead to decreased quality of life, inability to work, and considerable health care expenses.1 Here, we review the epidemiology of nickel allergy, regulation of nickel in the United States and Europe, common clinical presentations, and pearls on avoidance.
Epidemiology
Nickel continues to be the most common cause of contact allergy worldwide. Data from the 2015-2016 North American Contact Dermatitis Group patch test cycle (N=5597) showed nickel sulfate to be positive in 17.5% of patients patch tested to nickel.2 The prevalence of nickel allergy has been relatively stable in North America since 2005 (Figure 1). Although Ni-ACD historically was identified as an occupational disease of the hands in male nickel platers, the epidemiology of nickel allergy has shifted.1 Today, most cases are nonoccupational and affect women more often than men,3 in part due to improved industrial hygiene, pervasive incorporation of nickel in consumer items, and differences in cultural practices such as piercings.1,3 Piercings in particular have been implicated as important sources of nickel exposure, as this practice disrupts normal skin barrier function and is a potentially sensitizing event. Multiple studies including a large-scale epidemiologic analysis from 2017 have found piercings to be associated with an increased frequency of Ni-ACD (24.4% with piercing vs 9.6% without piercing). Interestingly, the degree of nickel sensitivity also was found to increase with the number of piercings (14.3% with 1 piercing vs 34.0% with ≥5 piercings).4
Regulation
Nickel content has been regulated in parts of the European Union (EU) since the 1990s, but regulation in the United States is lacking. In an attempt to reduce the prevalence of nickel allergy, the EU limits the level of nickel release from consumer items intended to be in direct and prolonged contact with the skin. These limits were first introduced in Denmark in 1990, followed closely by the EU Nickel Directive in 1994, which has resulted in consistent patterns of decreasing prevalence of Ni-ACD in multiple European countries.5 Notably, a Danish study comparing the prevalence of sensitization between girls with ears pierced before vs after implementation of nickel regulation found a decrease in prevalence from 17.1% to 3.9%.6 Additionally, this initiative has greatly reduced the economic burden of nickel dermatitis. It is estimated that Denmark alone has saved US $2 billion over a 20-year period in both direct and indirect health care costs.7
However, a policy is only effective if it is enforced, and it has been reported in the EU that 8% to 32% of tested jewelry exceeds the limit placed on nickel release, with imported jewelry being especially problematic.5 Also of interest, the 1 and 2 euro coins are known to release more nickel than pure nickel itself, releasing 240 to 320 times more than is allowed under the EU Nickel Directive (Figure 2).8 Although coins are not explicitly mentioned as items having prolonged contact with the skin, they can and do exacerbate allergic contact dermatitis of the hands, especially in occupational groups such as cashiers.9 Unsurprisingly, during the discussions to determine the composition of coins prior to the mass adoption of the euro in the EU in 2002, dermatologists and nickel industry experts remained divided in their recommendations.10 However, the EU regulation is considered a public health success overall, and the trends of Ni-ACD and economic burden are opposite of the United States, where legislation has yet to be adopted.
Patch Testing to Nickel
In North America, the 2 available patch test systems are the chamber method and the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice). In the T.R.U.E. test, nickel sulfate is used to formulate the patch at 200 µg/cm2 using hydroxypropyl cellulose as the gel vehicle. In the chamber method, nickel sulfate is used on either an aluminum or plastic chamber, most commonly at concentrations of 2.5% or 5% in petrolatum. Nickel sulfate 2.5% is most frequently used in US-based patch test clinics. A 2018 study (N=205) comparing the sensitivities of the 2.5% and 5% concentrations of nickel found 5% to be more sensitive; 31% of the cohort tested positive at 5% but only 20% at 2.5%, suggesting the 5% formulation is superior at detecting nickel allergy.11
Similar to other metals, nickel may react later than other allergens. A 2019 analysis of the prevalence of new patch test reactions on day 7 showed that 17% of 607 patients were negative on day 3 but were positive on day 7, further emphasizing the importance of a properly timed delayed reading.12
Clinical Presentation
Localized
The classic presentation of Ni-ACD is a scaly erythematous dermatitis in a typical distribution (eg, earlobes [earrings], wrists [watch], periumbilical [belt]). These scenarios usually can be diagnosed by the astute clinician without patch testing; however, the source of exposure may be less obvious if the nickel-releasing item has intermittent contact with the skin (eg, coins in the pocket, furniture hardware, personal grooming devices).13 Other reported exposures include facial dermatitis from mobile phones, dermatitis of the ulnar hands from laptop use, and hand dermatitis from gaming controllers,14-16 perhaps another reason for some to unplug.
Systemic
Sensitized individuals also may present with systemic contact dermatitis after airborne, oral, mucosal, or intravenous exposure. Presentations vary but have been reported to manifest as flare-up reactions in previously affected areas, pompholyx, diffuse dermatitis, flexural dermatitis, and baboon syndrome.17 Although it is unknown if airborne exposure alone is sufficient for sensitization, cases have been reported in occupational settings.18 One report described a man presenting with widespread dermatitis involving the extremities, chest, and genital area after his first day working at an electroplating plant.19
Systemic contact dermatitis from foods high in nickel (eg, chocolate, sunflower seeds, whole-grain flour, dried beans) and occasionally nonfood items (eg, coins) also has occurred. The so-called Easter egg hunt dermatitis has been described in children with Ni-ACD after candy ingestion.20 Another case described an 8-year-old girl and budding illusionist with severe diffuse dermatitis; a thorough history revealed the dermatitis began after she ingested a coin while performing a magic trick.21
Cases of nickel systemic contact dermatitis have been reported following medical device implantation, including reactions to cardiac devices, orthopedic implants, neurosurgery materials, and others.22 In addition, both intraoral and extraoral manifestations following application of orthodontic materials and dental implants have been reported.23,24 Although nickel-containing medical devices generally are well tolerated even in nickel-sensitive individuals, the development of systemic Ni-ACD has at times required device or hardware removal.22,23
After the Patch Test: Avoidance of Nickel
Counseling patients on nickel avoidance is critical to clinical improvement. Common nickel-containing items include jewelry, metal on clothing (eg, zippers, clasps, grommets), belt buckles, watches, glasses, furniture, coins, and keys. Numerous personal care products may also contain nickel, including nail clippers, eyelash curlers, tweezers, mascara tubes, and razors.25,26 Patients should be made aware that nickel-free alternatives are available for the majority of these products. Internet-based tips such as painting nail polish on products or iron-on patches tend to be of limited use in our experience. Patients may consider purchasing a nickel spot test to detect nickel in their environment; the dimethylglyoxime nickel spot test is inexpensive, rapid, and easy-to-use. To use the test, a small amount of the chemical is rubbed on the metal item using a cotton swab; a pink color indicates nickel release. Patients can be reassured that dimethylglyoxime does not harm jewelry.
Some general advice for patients regarding jewelry, the most common source of nickel exposure, is to only wear jewelry that is made from metals such as surgical-grade stainless steel, pure sterling silver, or platinum. If yellow gold is the preferred metal, it is prudent to be aware that lower karat items could potentially contain nickel. White gold should be avoided, as it often contains nickel to contribute to its color. Finally, gold-plated jewelry should be avoided, as the plating can wear off and expose a possibly nickel-containing base.
A low-nickel diet may be of benefit in select patients. A meta-analysis assessing systemic contact dermatitis from nickel ingestion found that 1% of nickel-sensitive individuals may be expected to react to nickel found in a normal diet.27 However, as with any diet, adherence can be difficult. Thankfully, Mislankar and Zirwas28 have developed a simple point-based system to help increase compliance. Additionally, a free mobile application is now available; Nickel Navigator can be used to track daily nickel intake and may be especially convenient for our more tech-savvy patients. In conjunction with a low-nickel diet, some authors also recommend eating meals high in vitamin C or supplementation with vitamin C, as co-ingestion has been shown to reduce nickel absorption.29
Final Interpretation
Nickel allergy remains common, found in up to 17.5% of patch tested patients. Despite regulation in the EU, nickel continues to have high prevalence of positive patch test reactions around the world. Nickel is not only found in jewelry and belt buckles but also in personal care products, electronics, and food. Allergen avoidance is key and requires knowledge of common items containing nickel and a low nickel diet for select patients.
- Ahlström MG, Thyssen JP, Wennervaldt M, et al. Nickel allergy and allergic contact dermatitis: a clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis. 2019;81:227-241.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Thyssen JP, Menné T. Metal allergy—a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23:309-318.
- Warshaw EM, Aschenbeck KA, DeKoven JG, et al. Piercing and metal sensitivity: extended analysis of the North American Contact Dermatitis Group data, 2007-2014. Dermatitis. 2017;28:333-341.
- Ahlström MG, Thyssen JP, Menné T, et al. Prevalence of nickel allergy in Europe following the EU Nickel Directive—a review. Contact Dermatitis. 2017;77:193-200.
- Jensen CS, Lisby S, Baadsgaard O, et al. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br J Dermatol. 2002;146:636-642.
- Serup-Hansen N, Gudum A, Sørensen MM. Valuation of Chemical Related Health Impacts. Danish Environmental Protection Agency. Published 2004. Accessed December 14, 2020. https://www2.mst.dk/udgiv/publications/2004/87-7614-295-7/pdf/87-7614-296-5.pdf
- Nestle FO, Speidel H, Speidel MO. Metallurgy: high nickel release from 1- and 2-euro coins. Nature. 2002;419:132.
- Kanerva L, Estlander T, Jolanki R. Bank clerk’s occupational allergic nickel and cobalt contact dermatitis from coins. Contact Dermatitis. 1998;38:217-218.
- Aberer W. Platitudes in allergy—based on the example of the euro. Contact Dermatitis. 2001;45:254-255.
- Goldminz AM, Scheinman PL. Comparison of nickel sulfate 2.5% and nickel sulfate 5% for detecting nickel contact allergy. Dermatitis. 2018;29:321-323.
- van Amerongen CCA, Ofenloch R, Dittmar D, et al. New positive patch test reactions on day 7—the additional value of the day 7 patch test reading. Contact Dermatitis. 2019;81:280-287.
- Silverberg NB, Pelletier JL, Jacob SE, et al; Section of Dermatology, Section on Allergy and Immunology. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628.
- Aquino M, Mucci T, Chong M, et al. Mobile phones: potential sources of nickel and cobalt exposure for metal allergic patients. Pediatr Allergy Immunol Pulmonol. 2013;26:181-186.
- Jensen P, Jellesen MS, Møller P, et al. Nickel allergy and dermatitis following use of a laptop computer. J Am Acad Dermatol. 2012;67:E170-E171.
- Jacob SE. Xbox—a source of nickel exposure in children. Pediatr Dermatol. 2014;31:115-116.
- Menné T, Veien NK. Systemic contact dermatitis. In: Rycroft RJG, Menné T, Frosch PJ, et al, eds. Textbook of Contact Dermatitis. Springer; 2001:355-366.
- Mann E, Ranft U, Eberwein G, et al. Does airborne nickel exposure induce nickel sensitization? Contact Dermatitis. 2010;62:355-362.
- Candura SM, Locatelli C, Butera R, et al. Widespread nickel dermatitis from inhalation. Contact Dermatitis. 2001;45:174-175.
- Jacob SE, Hamann D, Goldenberg A, et al. Easter egg hunt dermatitis: systemic allergic contact dermatitis associated with chocolate ingestion. Pediatr Dermatol. 2015;32:231-233.
- Mahdi G, Israel DM, Hassall E. Nickel dermatitis and associated gastritis after coin ingestion. J Pediatr Gastroenterol Nutr. 1996;23:74-76.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schultz JC, Connelly E, Glesne L, et al. Cutaneous and oral eruption from oral exposure to nickel in dental braces. Dermatitis. 2004;15:154-157.
- Pigatto PD, Brambilla L, Ferrucci S, et al. Systemic allergic contact dermatitis associated with allergy to intraoral metals. Dermatol Online J. 2014;20:13030/qt74632201.
- Brandrup F. Nickel eyelid dermatitis from an eyelash curler. Contact Dermatitis. 1991;25:77.
- Walsh G, Wilkinson SM. Materials and allergens within spectacle frames: a review. Contact Dermatitis. 2006;55:130-139.
- Bergman D, Goldenberg A, Rundle C, et al. Low nickel diet: a patient-centered review [published May 24, 2016]. J Clin Exp Dermatol Res. doi:10.4172/2155-9554.1000355
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Zirwas MJ, Molenda MA. Dietary nickel as a cause of systemic contact dermatitis. J Clin Aesthet Dermatol. 2009;2:39-43.
Nickel is unrivaled as the most common cause of contact allergy worldwide.1 Nickel is commonly used as a hardening agent in metal products, and complete avoidance is challenging due to numerous potential exposures (eg, direct contact, airborne, dietary, medical implantation). Allergic contact dermatitis to nickel (Ni-ACD) can lead to decreased quality of life, inability to work, and considerable health care expenses.1 Here, we review the epidemiology of nickel allergy, regulation of nickel in the United States and Europe, common clinical presentations, and pearls on avoidance.
Epidemiology
Nickel continues to be the most common cause of contact allergy worldwide. Data from the 2015-2016 North American Contact Dermatitis Group patch test cycle (N=5597) showed nickel sulfate to be positive in 17.5% of patients patch tested to nickel.2 The prevalence of nickel allergy has been relatively stable in North America since 2005 (Figure 1). Although Ni-ACD historically was identified as an occupational disease of the hands in male nickel platers, the epidemiology of nickel allergy has shifted.1 Today, most cases are nonoccupational and affect women more often than men,3 in part due to improved industrial hygiene, pervasive incorporation of nickel in consumer items, and differences in cultural practices such as piercings.1,3 Piercings in particular have been implicated as important sources of nickel exposure, as this practice disrupts normal skin barrier function and is a potentially sensitizing event. Multiple studies including a large-scale epidemiologic analysis from 2017 have found piercings to be associated with an increased frequency of Ni-ACD (24.4% with piercing vs 9.6% without piercing). Interestingly, the degree of nickel sensitivity also was found to increase with the number of piercings (14.3% with 1 piercing vs 34.0% with ≥5 piercings).4
Regulation
Nickel content has been regulated in parts of the European Union (EU) since the 1990s, but regulation in the United States is lacking. In an attempt to reduce the prevalence of nickel allergy, the EU limits the level of nickel release from consumer items intended to be in direct and prolonged contact with the skin. These limits were first introduced in Denmark in 1990, followed closely by the EU Nickel Directive in 1994, which has resulted in consistent patterns of decreasing prevalence of Ni-ACD in multiple European countries.5 Notably, a Danish study comparing the prevalence of sensitization between girls with ears pierced before vs after implementation of nickel regulation found a decrease in prevalence from 17.1% to 3.9%.6 Additionally, this initiative has greatly reduced the economic burden of nickel dermatitis. It is estimated that Denmark alone has saved US $2 billion over a 20-year period in both direct and indirect health care costs.7
However, a policy is only effective if it is enforced, and it has been reported in the EU that 8% to 32% of tested jewelry exceeds the limit placed on nickel release, with imported jewelry being especially problematic.5 Also of interest, the 1 and 2 euro coins are known to release more nickel than pure nickel itself, releasing 240 to 320 times more than is allowed under the EU Nickel Directive (Figure 2).8 Although coins are not explicitly mentioned as items having prolonged contact with the skin, they can and do exacerbate allergic contact dermatitis of the hands, especially in occupational groups such as cashiers.9 Unsurprisingly, during the discussions to determine the composition of coins prior to the mass adoption of the euro in the EU in 2002, dermatologists and nickel industry experts remained divided in their recommendations.10 However, the EU regulation is considered a public health success overall, and the trends of Ni-ACD and economic burden are opposite of the United States, where legislation has yet to be adopted.
Patch Testing to Nickel
In North America, the 2 available patch test systems are the chamber method and the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice). In the T.R.U.E. test, nickel sulfate is used to formulate the patch at 200 µg/cm2 using hydroxypropyl cellulose as the gel vehicle. In the chamber method, nickel sulfate is used on either an aluminum or plastic chamber, most commonly at concentrations of 2.5% or 5% in petrolatum. Nickel sulfate 2.5% is most frequently used in US-based patch test clinics. A 2018 study (N=205) comparing the sensitivities of the 2.5% and 5% concentrations of nickel found 5% to be more sensitive; 31% of the cohort tested positive at 5% but only 20% at 2.5%, suggesting the 5% formulation is superior at detecting nickel allergy.11
Similar to other metals, nickel may react later than other allergens. A 2019 analysis of the prevalence of new patch test reactions on day 7 showed that 17% of 607 patients were negative on day 3 but were positive on day 7, further emphasizing the importance of a properly timed delayed reading.12
Clinical Presentation
Localized
The classic presentation of Ni-ACD is a scaly erythematous dermatitis in a typical distribution (eg, earlobes [earrings], wrists [watch], periumbilical [belt]). These scenarios usually can be diagnosed by the astute clinician without patch testing; however, the source of exposure may be less obvious if the nickel-releasing item has intermittent contact with the skin (eg, coins in the pocket, furniture hardware, personal grooming devices).13 Other reported exposures include facial dermatitis from mobile phones, dermatitis of the ulnar hands from laptop use, and hand dermatitis from gaming controllers,14-16 perhaps another reason for some to unplug.
Systemic
Sensitized individuals also may present with systemic contact dermatitis after airborne, oral, mucosal, or intravenous exposure. Presentations vary but have been reported to manifest as flare-up reactions in previously affected areas, pompholyx, diffuse dermatitis, flexural dermatitis, and baboon syndrome.17 Although it is unknown if airborne exposure alone is sufficient for sensitization, cases have been reported in occupational settings.18 One report described a man presenting with widespread dermatitis involving the extremities, chest, and genital area after his first day working at an electroplating plant.19
Systemic contact dermatitis from foods high in nickel (eg, chocolate, sunflower seeds, whole-grain flour, dried beans) and occasionally nonfood items (eg, coins) also has occurred. The so-called Easter egg hunt dermatitis has been described in children with Ni-ACD after candy ingestion.20 Another case described an 8-year-old girl and budding illusionist with severe diffuse dermatitis; a thorough history revealed the dermatitis began after she ingested a coin while performing a magic trick.21
Cases of nickel systemic contact dermatitis have been reported following medical device implantation, including reactions to cardiac devices, orthopedic implants, neurosurgery materials, and others.22 In addition, both intraoral and extraoral manifestations following application of orthodontic materials and dental implants have been reported.23,24 Although nickel-containing medical devices generally are well tolerated even in nickel-sensitive individuals, the development of systemic Ni-ACD has at times required device or hardware removal.22,23
After the Patch Test: Avoidance of Nickel
Counseling patients on nickel avoidance is critical to clinical improvement. Common nickel-containing items include jewelry, metal on clothing (eg, zippers, clasps, grommets), belt buckles, watches, glasses, furniture, coins, and keys. Numerous personal care products may also contain nickel, including nail clippers, eyelash curlers, tweezers, mascara tubes, and razors.25,26 Patients should be made aware that nickel-free alternatives are available for the majority of these products. Internet-based tips such as painting nail polish on products or iron-on patches tend to be of limited use in our experience. Patients may consider purchasing a nickel spot test to detect nickel in their environment; the dimethylglyoxime nickel spot test is inexpensive, rapid, and easy-to-use. To use the test, a small amount of the chemical is rubbed on the metal item using a cotton swab; a pink color indicates nickel release. Patients can be reassured that dimethylglyoxime does not harm jewelry.
Some general advice for patients regarding jewelry, the most common source of nickel exposure, is to only wear jewelry that is made from metals such as surgical-grade stainless steel, pure sterling silver, or platinum. If yellow gold is the preferred metal, it is prudent to be aware that lower karat items could potentially contain nickel. White gold should be avoided, as it often contains nickel to contribute to its color. Finally, gold-plated jewelry should be avoided, as the plating can wear off and expose a possibly nickel-containing base.
A low-nickel diet may be of benefit in select patients. A meta-analysis assessing systemic contact dermatitis from nickel ingestion found that 1% of nickel-sensitive individuals may be expected to react to nickel found in a normal diet.27 However, as with any diet, adherence can be difficult. Thankfully, Mislankar and Zirwas28 have developed a simple point-based system to help increase compliance. Additionally, a free mobile application is now available; Nickel Navigator can be used to track daily nickel intake and may be especially convenient for our more tech-savvy patients. In conjunction with a low-nickel diet, some authors also recommend eating meals high in vitamin C or supplementation with vitamin C, as co-ingestion has been shown to reduce nickel absorption.29
Final Interpretation
Nickel allergy remains common, found in up to 17.5% of patch tested patients. Despite regulation in the EU, nickel continues to have high prevalence of positive patch test reactions around the world. Nickel is not only found in jewelry and belt buckles but also in personal care products, electronics, and food. Allergen avoidance is key and requires knowledge of common items containing nickel and a low nickel diet for select patients.
Nickel is unrivaled as the most common cause of contact allergy worldwide.1 Nickel is commonly used as a hardening agent in metal products, and complete avoidance is challenging due to numerous potential exposures (eg, direct contact, airborne, dietary, medical implantation). Allergic contact dermatitis to nickel (Ni-ACD) can lead to decreased quality of life, inability to work, and considerable health care expenses.1 Here, we review the epidemiology of nickel allergy, regulation of nickel in the United States and Europe, common clinical presentations, and pearls on avoidance.
Epidemiology
Nickel continues to be the most common cause of contact allergy worldwide. Data from the 2015-2016 North American Contact Dermatitis Group patch test cycle (N=5597) showed nickel sulfate to be positive in 17.5% of patients patch tested to nickel.2 The prevalence of nickel allergy has been relatively stable in North America since 2005 (Figure 1). Although Ni-ACD historically was identified as an occupational disease of the hands in male nickel platers, the epidemiology of nickel allergy has shifted.1 Today, most cases are nonoccupational and affect women more often than men,3 in part due to improved industrial hygiene, pervasive incorporation of nickel in consumer items, and differences in cultural practices such as piercings.1,3 Piercings in particular have been implicated as important sources of nickel exposure, as this practice disrupts normal skin barrier function and is a potentially sensitizing event. Multiple studies including a large-scale epidemiologic analysis from 2017 have found piercings to be associated with an increased frequency of Ni-ACD (24.4% with piercing vs 9.6% without piercing). Interestingly, the degree of nickel sensitivity also was found to increase with the number of piercings (14.3% with 1 piercing vs 34.0% with ≥5 piercings).4
Regulation
Nickel content has been regulated in parts of the European Union (EU) since the 1990s, but regulation in the United States is lacking. In an attempt to reduce the prevalence of nickel allergy, the EU limits the level of nickel release from consumer items intended to be in direct and prolonged contact with the skin. These limits were first introduced in Denmark in 1990, followed closely by the EU Nickel Directive in 1994, which has resulted in consistent patterns of decreasing prevalence of Ni-ACD in multiple European countries.5 Notably, a Danish study comparing the prevalence of sensitization between girls with ears pierced before vs after implementation of nickel regulation found a decrease in prevalence from 17.1% to 3.9%.6 Additionally, this initiative has greatly reduced the economic burden of nickel dermatitis. It is estimated that Denmark alone has saved US $2 billion over a 20-year period in both direct and indirect health care costs.7
However, a policy is only effective if it is enforced, and it has been reported in the EU that 8% to 32% of tested jewelry exceeds the limit placed on nickel release, with imported jewelry being especially problematic.5 Also of interest, the 1 and 2 euro coins are known to release more nickel than pure nickel itself, releasing 240 to 320 times more than is allowed under the EU Nickel Directive (Figure 2).8 Although coins are not explicitly mentioned as items having prolonged contact with the skin, they can and do exacerbate allergic contact dermatitis of the hands, especially in occupational groups such as cashiers.9 Unsurprisingly, during the discussions to determine the composition of coins prior to the mass adoption of the euro in the EU in 2002, dermatologists and nickel industry experts remained divided in their recommendations.10 However, the EU regulation is considered a public health success overall, and the trends of Ni-ACD and economic burden are opposite of the United States, where legislation has yet to be adopted.
Patch Testing to Nickel
In North America, the 2 available patch test systems are the chamber method and the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice). In the T.R.U.E. test, nickel sulfate is used to formulate the patch at 200 µg/cm2 using hydroxypropyl cellulose as the gel vehicle. In the chamber method, nickel sulfate is used on either an aluminum or plastic chamber, most commonly at concentrations of 2.5% or 5% in petrolatum. Nickel sulfate 2.5% is most frequently used in US-based patch test clinics. A 2018 study (N=205) comparing the sensitivities of the 2.5% and 5% concentrations of nickel found 5% to be more sensitive; 31% of the cohort tested positive at 5% but only 20% at 2.5%, suggesting the 5% formulation is superior at detecting nickel allergy.11
Similar to other metals, nickel may react later than other allergens. A 2019 analysis of the prevalence of new patch test reactions on day 7 showed that 17% of 607 patients were negative on day 3 but were positive on day 7, further emphasizing the importance of a properly timed delayed reading.12
Clinical Presentation
Localized
The classic presentation of Ni-ACD is a scaly erythematous dermatitis in a typical distribution (eg, earlobes [earrings], wrists [watch], periumbilical [belt]). These scenarios usually can be diagnosed by the astute clinician without patch testing; however, the source of exposure may be less obvious if the nickel-releasing item has intermittent contact with the skin (eg, coins in the pocket, furniture hardware, personal grooming devices).13 Other reported exposures include facial dermatitis from mobile phones, dermatitis of the ulnar hands from laptop use, and hand dermatitis from gaming controllers,14-16 perhaps another reason for some to unplug.
Systemic
Sensitized individuals also may present with systemic contact dermatitis after airborne, oral, mucosal, or intravenous exposure. Presentations vary but have been reported to manifest as flare-up reactions in previously affected areas, pompholyx, diffuse dermatitis, flexural dermatitis, and baboon syndrome.17 Although it is unknown if airborne exposure alone is sufficient for sensitization, cases have been reported in occupational settings.18 One report described a man presenting with widespread dermatitis involving the extremities, chest, and genital area after his first day working at an electroplating plant.19
Systemic contact dermatitis from foods high in nickel (eg, chocolate, sunflower seeds, whole-grain flour, dried beans) and occasionally nonfood items (eg, coins) also has occurred. The so-called Easter egg hunt dermatitis has been described in children with Ni-ACD after candy ingestion.20 Another case described an 8-year-old girl and budding illusionist with severe diffuse dermatitis; a thorough history revealed the dermatitis began after she ingested a coin while performing a magic trick.21
Cases of nickel systemic contact dermatitis have been reported following medical device implantation, including reactions to cardiac devices, orthopedic implants, neurosurgery materials, and others.22 In addition, both intraoral and extraoral manifestations following application of orthodontic materials and dental implants have been reported.23,24 Although nickel-containing medical devices generally are well tolerated even in nickel-sensitive individuals, the development of systemic Ni-ACD has at times required device or hardware removal.22,23
After the Patch Test: Avoidance of Nickel
Counseling patients on nickel avoidance is critical to clinical improvement. Common nickel-containing items include jewelry, metal on clothing (eg, zippers, clasps, grommets), belt buckles, watches, glasses, furniture, coins, and keys. Numerous personal care products may also contain nickel, including nail clippers, eyelash curlers, tweezers, mascara tubes, and razors.25,26 Patients should be made aware that nickel-free alternatives are available for the majority of these products. Internet-based tips such as painting nail polish on products or iron-on patches tend to be of limited use in our experience. Patients may consider purchasing a nickel spot test to detect nickel in their environment; the dimethylglyoxime nickel spot test is inexpensive, rapid, and easy-to-use. To use the test, a small amount of the chemical is rubbed on the metal item using a cotton swab; a pink color indicates nickel release. Patients can be reassured that dimethylglyoxime does not harm jewelry.
Some general advice for patients regarding jewelry, the most common source of nickel exposure, is to only wear jewelry that is made from metals such as surgical-grade stainless steel, pure sterling silver, or platinum. If yellow gold is the preferred metal, it is prudent to be aware that lower karat items could potentially contain nickel. White gold should be avoided, as it often contains nickel to contribute to its color. Finally, gold-plated jewelry should be avoided, as the plating can wear off and expose a possibly nickel-containing base.
A low-nickel diet may be of benefit in select patients. A meta-analysis assessing systemic contact dermatitis from nickel ingestion found that 1% of nickel-sensitive individuals may be expected to react to nickel found in a normal diet.27 However, as with any diet, adherence can be difficult. Thankfully, Mislankar and Zirwas28 have developed a simple point-based system to help increase compliance. Additionally, a free mobile application is now available; Nickel Navigator can be used to track daily nickel intake and may be especially convenient for our more tech-savvy patients. In conjunction with a low-nickel diet, some authors also recommend eating meals high in vitamin C or supplementation with vitamin C, as co-ingestion has been shown to reduce nickel absorption.29
Final Interpretation
Nickel allergy remains common, found in up to 17.5% of patch tested patients. Despite regulation in the EU, nickel continues to have high prevalence of positive patch test reactions around the world. Nickel is not only found in jewelry and belt buckles but also in personal care products, electronics, and food. Allergen avoidance is key and requires knowledge of common items containing nickel and a low nickel diet for select patients.
- Ahlström MG, Thyssen JP, Wennervaldt M, et al. Nickel allergy and allergic contact dermatitis: a clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis. 2019;81:227-241.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Thyssen JP, Menné T. Metal allergy—a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23:309-318.
- Warshaw EM, Aschenbeck KA, DeKoven JG, et al. Piercing and metal sensitivity: extended analysis of the North American Contact Dermatitis Group data, 2007-2014. Dermatitis. 2017;28:333-341.
- Ahlström MG, Thyssen JP, Menné T, et al. Prevalence of nickel allergy in Europe following the EU Nickel Directive—a review. Contact Dermatitis. 2017;77:193-200.
- Jensen CS, Lisby S, Baadsgaard O, et al. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br J Dermatol. 2002;146:636-642.
- Serup-Hansen N, Gudum A, Sørensen MM. Valuation of Chemical Related Health Impacts. Danish Environmental Protection Agency. Published 2004. Accessed December 14, 2020. https://www2.mst.dk/udgiv/publications/2004/87-7614-295-7/pdf/87-7614-296-5.pdf
- Nestle FO, Speidel H, Speidel MO. Metallurgy: high nickel release from 1- and 2-euro coins. Nature. 2002;419:132.
- Kanerva L, Estlander T, Jolanki R. Bank clerk’s occupational allergic nickel and cobalt contact dermatitis from coins. Contact Dermatitis. 1998;38:217-218.
- Aberer W. Platitudes in allergy—based on the example of the euro. Contact Dermatitis. 2001;45:254-255.
- Goldminz AM, Scheinman PL. Comparison of nickel sulfate 2.5% and nickel sulfate 5% for detecting nickel contact allergy. Dermatitis. 2018;29:321-323.
- van Amerongen CCA, Ofenloch R, Dittmar D, et al. New positive patch test reactions on day 7—the additional value of the day 7 patch test reading. Contact Dermatitis. 2019;81:280-287.
- Silverberg NB, Pelletier JL, Jacob SE, et al; Section of Dermatology, Section on Allergy and Immunology. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628.
- Aquino M, Mucci T, Chong M, et al. Mobile phones: potential sources of nickel and cobalt exposure for metal allergic patients. Pediatr Allergy Immunol Pulmonol. 2013;26:181-186.
- Jensen P, Jellesen MS, Møller P, et al. Nickel allergy and dermatitis following use of a laptop computer. J Am Acad Dermatol. 2012;67:E170-E171.
- Jacob SE. Xbox—a source of nickel exposure in children. Pediatr Dermatol. 2014;31:115-116.
- Menné T, Veien NK. Systemic contact dermatitis. In: Rycroft RJG, Menné T, Frosch PJ, et al, eds. Textbook of Contact Dermatitis. Springer; 2001:355-366.
- Mann E, Ranft U, Eberwein G, et al. Does airborne nickel exposure induce nickel sensitization? Contact Dermatitis. 2010;62:355-362.
- Candura SM, Locatelli C, Butera R, et al. Widespread nickel dermatitis from inhalation. Contact Dermatitis. 2001;45:174-175.
- Jacob SE, Hamann D, Goldenberg A, et al. Easter egg hunt dermatitis: systemic allergic contact dermatitis associated with chocolate ingestion. Pediatr Dermatol. 2015;32:231-233.
- Mahdi G, Israel DM, Hassall E. Nickel dermatitis and associated gastritis after coin ingestion. J Pediatr Gastroenterol Nutr. 1996;23:74-76.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schultz JC, Connelly E, Glesne L, et al. Cutaneous and oral eruption from oral exposure to nickel in dental braces. Dermatitis. 2004;15:154-157.
- Pigatto PD, Brambilla L, Ferrucci S, et al. Systemic allergic contact dermatitis associated with allergy to intraoral metals. Dermatol Online J. 2014;20:13030/qt74632201.
- Brandrup F. Nickel eyelid dermatitis from an eyelash curler. Contact Dermatitis. 1991;25:77.
- Walsh G, Wilkinson SM. Materials and allergens within spectacle frames: a review. Contact Dermatitis. 2006;55:130-139.
- Bergman D, Goldenberg A, Rundle C, et al. Low nickel diet: a patient-centered review [published May 24, 2016]. J Clin Exp Dermatol Res. doi:10.4172/2155-9554.1000355
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Zirwas MJ, Molenda MA. Dietary nickel as a cause of systemic contact dermatitis. J Clin Aesthet Dermatol. 2009;2:39-43.
- Ahlström MG, Thyssen JP, Wennervaldt M, et al. Nickel allergy and allergic contact dermatitis: a clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis. 2019;81:227-241.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Thyssen JP, Menné T. Metal allergy—a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23:309-318.
- Warshaw EM, Aschenbeck KA, DeKoven JG, et al. Piercing and metal sensitivity: extended analysis of the North American Contact Dermatitis Group data, 2007-2014. Dermatitis. 2017;28:333-341.
- Ahlström MG, Thyssen JP, Menné T, et al. Prevalence of nickel allergy in Europe following the EU Nickel Directive—a review. Contact Dermatitis. 2017;77:193-200.
- Jensen CS, Lisby S, Baadsgaard O, et al. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br J Dermatol. 2002;146:636-642.
- Serup-Hansen N, Gudum A, Sørensen MM. Valuation of Chemical Related Health Impacts. Danish Environmental Protection Agency. Published 2004. Accessed December 14, 2020. https://www2.mst.dk/udgiv/publications/2004/87-7614-295-7/pdf/87-7614-296-5.pdf
- Nestle FO, Speidel H, Speidel MO. Metallurgy: high nickel release from 1- and 2-euro coins. Nature. 2002;419:132.
- Kanerva L, Estlander T, Jolanki R. Bank clerk’s occupational allergic nickel and cobalt contact dermatitis from coins. Contact Dermatitis. 1998;38:217-218.
- Aberer W. Platitudes in allergy—based on the example of the euro. Contact Dermatitis. 2001;45:254-255.
- Goldminz AM, Scheinman PL. Comparison of nickel sulfate 2.5% and nickel sulfate 5% for detecting nickel contact allergy. Dermatitis. 2018;29:321-323.
- van Amerongen CCA, Ofenloch R, Dittmar D, et al. New positive patch test reactions on day 7—the additional value of the day 7 patch test reading. Contact Dermatitis. 2019;81:280-287.
- Silverberg NB, Pelletier JL, Jacob SE, et al; Section of Dermatology, Section on Allergy and Immunology. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628.
- Aquino M, Mucci T, Chong M, et al. Mobile phones: potential sources of nickel and cobalt exposure for metal allergic patients. Pediatr Allergy Immunol Pulmonol. 2013;26:181-186.
- Jensen P, Jellesen MS, Møller P, et al. Nickel allergy and dermatitis following use of a laptop computer. J Am Acad Dermatol. 2012;67:E170-E171.
- Jacob SE. Xbox—a source of nickel exposure in children. Pediatr Dermatol. 2014;31:115-116.
- Menné T, Veien NK. Systemic contact dermatitis. In: Rycroft RJG, Menné T, Frosch PJ, et al, eds. Textbook of Contact Dermatitis. Springer; 2001:355-366.
- Mann E, Ranft U, Eberwein G, et al. Does airborne nickel exposure induce nickel sensitization? Contact Dermatitis. 2010;62:355-362.
- Candura SM, Locatelli C, Butera R, et al. Widespread nickel dermatitis from inhalation. Contact Dermatitis. 2001;45:174-175.
- Jacob SE, Hamann D, Goldenberg A, et al. Easter egg hunt dermatitis: systemic allergic contact dermatitis associated with chocolate ingestion. Pediatr Dermatol. 2015;32:231-233.
- Mahdi G, Israel DM, Hassall E. Nickel dermatitis and associated gastritis after coin ingestion. J Pediatr Gastroenterol Nutr. 1996;23:74-76.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schultz JC, Connelly E, Glesne L, et al. Cutaneous and oral eruption from oral exposure to nickel in dental braces. Dermatitis. 2004;15:154-157.
- Pigatto PD, Brambilla L, Ferrucci S, et al. Systemic allergic contact dermatitis associated with allergy to intraoral metals. Dermatol Online J. 2014;20:13030/qt74632201.
- Brandrup F. Nickel eyelid dermatitis from an eyelash curler. Contact Dermatitis. 1991;25:77.
- Walsh G, Wilkinson SM. Materials and allergens within spectacle frames: a review. Contact Dermatitis. 2006;55:130-139.
- Bergman D, Goldenberg A, Rundle C, et al. Low nickel diet: a patient-centered review [published May 24, 2016]. J Clin Exp Dermatol Res. doi:10.4172/2155-9554.1000355
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Zirwas MJ, Molenda MA. Dietary nickel as a cause of systemic contact dermatitis. J Clin Aesthet Dermatol. 2009;2:39-43.
Practice Points
- Nickel is the most common cause of contact allergy worldwide. It is ubiquitous in our daily environment, making avoidance challenging.
- Nickel allergic contact dermatitis typically presents in a localized distribution but also can present as systemic contact dermatitis.
- Nickel regulation has been adopted in Europe, but similar legislation does not exist in the United States.
Patch Testing 101, Part 2: After the Patch Test
The first part of this 2-part series addressed the basics of patch testing, including patch test systems, allergens, and patch test readings. In the second part of this series, we examine the incredibly important and absolutely vital steps that come after the patch test: determining relevance, patient counseling, and identifying allergen-free products for patient use. Let’s dive in!
Determining Relevance
The purpose of determining relevance is to assess whether the positive patch test explains the patient’s dermatitis. It is important to consider all of the patient’s exposures, including at home, at work, and during recreational activities. Several relevance grading scales exist. The North American Contact Dermatitis Group grades relevance as current, past, or unknown. Current relevance is further divided into definite, probable, and possible.1 Table 1 includes explanations and clinical examples of each relevance type.
True relevance is only known weeks or months after patch testing is complete. If the patient avoids allergens and is subsequently free of dermatitis, the allergens identified through patch testing were relevant. However, if the patient avoids allergens and sees no improvement in dermatitis, the allergens were not relevant. Gipson et al2 analyzed relevance as documented by the physician at final patch test reading vs patient opinion of relevance 30 days to 3 years after the final reading and found that there was variable agreement between the 2 groups; percentage agreement for formaldehyde-releasing preservatives was 88%, neomycin was 78%, nickel was 71%, fragrances was 65%, and gold was 56%. These differences underscore the need for ongoing research on patch test methods, determination of relevance, and standards for patient follow-up.2
Patient Counseling
Patient counseling is one of the most important and complex parts of patch testing. We have consulted with patients who had already completed patch testing with other providers but did not receive comprehensive allergen counseling and therefore did not improve. It is up to you to explain positive allergens to your patients in a way that they understand, can retain long-term, and can use to their advantage to keep their skin free of dermatitis, which is an incredibly difficult feat to accomplish. The resources that we describe next are the very basic requirements for proficient patch testing.
There are several tools that can be utilized to develop patch test counseling skills (Table 2). Membership with the American Contact Dermatitis Society (ACDS) includes opportunities for virtual and in-person (post–coronavirus disease 2019) lectures and conferences, videos, patch test support information, and patient resources. The European Society of Contact Dermatitis is similar, with a focus on European-based patch testers. Both societies are affiliated with academic journals—Dermatitis and Contact Dermatitis, respectively—which are phenomenal educational resources. Dermatitis Academy (https://www.dermatitisacademy.com) and Contact Dermatitis Institute (https://www.contactdermatitisinstitute.com) are websites that are privately designed and managed by US-based patch test experts.
Allergen Information Handouts
Allergen information should be presented in both verbal and written formats as well as in the patient’s preferred language and education level. Patch test counseling is detailed and complex. Patients rarely remember everything that is discussed; written information allows them to review again when necessary. Allergen information sheets typically include the name of the allergen, alternative names, types of products that might contain the allergen, and other pertinent facts. They also can be helpful for the physician who does not patch test full time; in this case, they can be used as a quick reference to guide patient counseling. It is helpful to highlight or underline important points and make notes when relevant. Importantly, reviewing information sheets with the patient allows time for questions.
Allergen information sheets are provided by manufacturers of patch test materials, including SmartPractice (allergEAZE, T.R.U.E. Test) and Chemotechnique (Dormer)(Table 2). The ACDS also provides a selection of allergen information sheets for members to share with their patients. The ACDS allergen handouts are designed for patient use, are vetted by practicing patch test dermatologists, and contain up-to-date information for patients. We recommend that you choose the handout(s) that are most appropriate for your patient; this decision can be made based on patient education or reading level, the region of the world where you are patch testing or where the patient lives, the patient’s primary language, and the specific allergen. Information on rare or new allergens may not be available on every website resource.
Identification of Allergen-Free Products
We ask patients to bring their personal care products to their patch test reading visit, and once positive allergens are known, we search for the presence of that allergen in their products. It is helpful for patients if products that are “safe” and “not safe” are sorted for them. We frequently emphasize that just one exposure to an allergen in a personal care product can be the source of the dermatitis. If a product label does not include ingredients, they often can be identified with a quick web search (use your favorite search engine or see Table 2 for websites); however, caution is advised, as lists found online may not match those found on in-store products.3 Reviewing the patient’s own products in the clinic is preferred over searching for ingredient lists online. If the product’s ingredients cannot be found (eg, ingredients that are found on external packaging), the patient has several choices: do not use, complete repeat open application testing if it is a leave-on product, or check to see if it is on a product database safe list.
We explain to patients that once they have confirmed that they are using only “safe” allergen-free products, it can take up to 6 to 8 weeks for dermatitis to improve, and at that point, the skin may only be about 75% to 80% clear. A clear description of what to expect and when is needed for a strong patient-physician partnership. For example, if the patient expects to be clear in 2 days but is not and stops avoiding their allergens because they think the process has failed, their dermatitis will not improve.
Product Databases
Because allergens sometimes have multiple different chemical names and cross-reactivity is abundant, avoidance of both the allergen and cross-reactors can be daunting for many patients (and dermatologists!). The use of a product database to aid in product selection is an invaluable resource. Product databases help patients avoid not only their allergens but also common cross-reactors by relying on complex cross-reactor programming. The ACDS owns and maintains the Contact Allergy Management Program (CAMP). Another resource is SkinSafe, which is powered by HER Inc and developed with the Mayo Clinic. Both CAMP and SkinSafe have mobile apps and update product lists frequently; they allow for much easier shopping and identification of safe products.
We typically use CAMP for generation of patient safe lists. We enter the patient’s allergens into the database, and a safe list is generated and shared with the patient. Next, we educate the patient on how to use the safe list. It is vital that the concept of exact product matching be explained to patients, as not all products from one brand or type of product is necessarily safe for a given individual. We also share information on how to download the CAMP app onto mobile devices and tablets.
Product safe lists are important resources for patients to be successful in avoiding allergens but are not a substitute for reading labels. Both CAMP and SkinSafe can potentially contain ingredient list errors due to companies frequently changing their product formulations.3 Although safe lists are an important part in selecting safe skin care products, they are not a substitute for label reading.
Counseling Pitfalls and Pearls
Language
Chemotechnique handouts are available in English, Swedish, French, and Spanish, and ACDS handouts are available in English and Spanish. If language interpretation is needed, inform the interpreter before the visit begins that you will be discussing patch test information and products so they can carefully interpret the details of the discussion.
Barriers to Allergen Avoidance
There are several barriers to long-term avoidance of contact allergy. In a European-based study of methylisothiazolinone (MI) contact allergy 2 to 5 years after patch testing, challenges described by patients included label reading, verifying products, difficulty obtaining ingredients of industrial products, the need to have their “safe” products always available for use, remembering allergen name, avoiding workplace allergens, finding acceptable MI-free products, and navigating the cost of MI-free products.4
Patient allergen recall is a well-documented long-term concern. In the previously mentioned European study (N=139), 11% of patients identified remembering the allergen name as a contributor to difficulty with avoidance.4 A Swedish study evaluated patient allergen recall at 1, 5, and 10 years after patch testing was completed; 96% of 252 patients remembered that they had completed patch testing, 79% (111/141) remembered that they had positive results, and only 29% (41/141) correctly recalled their allergens.5 Patients who had completed patch testing 10 years prior were less likely to correctly recall their allergens (P=.0045). Recall also was less likely if there was more than 1 allergen as well as in males.5 Korkmaz and Boyvat6 analyzed outcomes 6 months after patch testing in Turkey and found that 38 of 51 (74.5%) correctly recalled their allergens. Patients with more than 1 positive allergen were less likely to recall their allergens (P=.046), and patients with higher baseline investigator global assessment (P=.036) and dermatology life quality index (P=.041) scores were more likely to recall their allergens.6 A US-based study (N=757) noted that 34.1% of patients correctly recalled all of their allergens.7 Patients were less likely to remember if they had 3 or more positives but were more likely to remember if they were aged 50 to 59 years (compared to other age groups) or female as well as if their occupation was nursing (as compared to other occupations).
Additional barriers include hidden sources of allergens, as has been reported in the cases of undeclared MI8 and formaldehyde9 in personal care products. Although this phenomenon is thought to be the exception and not the rule, possible reasons for the presence of these undeclared allergens include their use as preservatives in raw materials,8,9 or in the case of formaldehyde, theorized release from product packaging or auto-oxidation and degradation of other chemicals present within the product.9
Readers may recall that we mentioned the option of identifying product ingredients with online search engines or databases, but it is not a perfect system. Comstock and Reeder3 reviewed and compared online ingredient lists from Amazon and several product databases to products taken off shelves at Target and Walgreens and found that 27.7% of online ingredient lists did not match the in-store labels.3 These differences likely are due to changes in product formulations, ingredient variability based on production site, outdated product on store shelves, or data entry error and may not be entirely avoidable. Regardless, patch test experts should be aware of this possibility. When in doubt, always check the product’s original packaging.
Finally, the elephant in the room: We challenge you, as dermatologists and patch test enthusiasts, to name all of the formaldehyde releasers or perhaps declare whether linalool and hydroxycitronellol are fragrances, preservatives, or surfactants. How about naming the relationship between cocamidopropyl betaine, amidoamine, and dimethylaminopropylamine? Difficult stuff, right? And we are medical specialists. It is downright impossible for many of our patients to memorize the names of these chemicals, let alone know their cross-reactors or other important chemical relationships. We mention that providing a safe list is part of patient counseling, but we bring up this knowledge gap to illustrate that patch testing without providing resources to select safe care products is almost as bad as not patch testing at all because in many cases patients may be left without the tools they need to be successful. Do not let this be your downfall!
Final Interpretation
The most challenging and nuanced part of patch testing happens after the actual patch test: assessment of relevance, allergen counseling, and identification of appropriate products for patient use. You now have the tools to successfully counsel your patients after patch testing; get to it!
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gipson KA, Carlson SW, Nedorost ST. Physician-patient agreement in the assessment of allergen relevance. Dermatitis. 2010;21:275-279.
- Comstock JR, Reeder MJ. Accuracy of product ingredient labeling: comparing drugstore products with online databases and online retailers. Dermatitis. 2020;31:106-111.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the department of dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220.
- Korkmaz P, Boyvat A. Effect of patch testing on the course of allergic contact dermatitis and prognostic factors that influence outcomes. Dermatitis. 2019;30:135-141.
- Scalf LA, Genebriera J, Davis MD, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Nikle A, Ericson M, Warshaw E. Formaldehyde release from personal care products: chromotropic acid method analysis. Dermatitis. 2019;30:67-73.
The first part of this 2-part series addressed the basics of patch testing, including patch test systems, allergens, and patch test readings. In the second part of this series, we examine the incredibly important and absolutely vital steps that come after the patch test: determining relevance, patient counseling, and identifying allergen-free products for patient use. Let’s dive in!
Determining Relevance
The purpose of determining relevance is to assess whether the positive patch test explains the patient’s dermatitis. It is important to consider all of the patient’s exposures, including at home, at work, and during recreational activities. Several relevance grading scales exist. The North American Contact Dermatitis Group grades relevance as current, past, or unknown. Current relevance is further divided into definite, probable, and possible.1 Table 1 includes explanations and clinical examples of each relevance type.
True relevance is only known weeks or months after patch testing is complete. If the patient avoids allergens and is subsequently free of dermatitis, the allergens identified through patch testing were relevant. However, if the patient avoids allergens and sees no improvement in dermatitis, the allergens were not relevant. Gipson et al2 analyzed relevance as documented by the physician at final patch test reading vs patient opinion of relevance 30 days to 3 years after the final reading and found that there was variable agreement between the 2 groups; percentage agreement for formaldehyde-releasing preservatives was 88%, neomycin was 78%, nickel was 71%, fragrances was 65%, and gold was 56%. These differences underscore the need for ongoing research on patch test methods, determination of relevance, and standards for patient follow-up.2
Patient Counseling
Patient counseling is one of the most important and complex parts of patch testing. We have consulted with patients who had already completed patch testing with other providers but did not receive comprehensive allergen counseling and therefore did not improve. It is up to you to explain positive allergens to your patients in a way that they understand, can retain long-term, and can use to their advantage to keep their skin free of dermatitis, which is an incredibly difficult feat to accomplish. The resources that we describe next are the very basic requirements for proficient patch testing.
There are several tools that can be utilized to develop patch test counseling skills (Table 2). Membership with the American Contact Dermatitis Society (ACDS) includes opportunities for virtual and in-person (post–coronavirus disease 2019) lectures and conferences, videos, patch test support information, and patient resources. The European Society of Contact Dermatitis is similar, with a focus on European-based patch testers. Both societies are affiliated with academic journals—Dermatitis and Contact Dermatitis, respectively—which are phenomenal educational resources. Dermatitis Academy (https://www.dermatitisacademy.com) and Contact Dermatitis Institute (https://www.contactdermatitisinstitute.com) are websites that are privately designed and managed by US-based patch test experts.
Allergen Information Handouts
Allergen information should be presented in both verbal and written formats as well as in the patient’s preferred language and education level. Patch test counseling is detailed and complex. Patients rarely remember everything that is discussed; written information allows them to review again when necessary. Allergen information sheets typically include the name of the allergen, alternative names, types of products that might contain the allergen, and other pertinent facts. They also can be helpful for the physician who does not patch test full time; in this case, they can be used as a quick reference to guide patient counseling. It is helpful to highlight or underline important points and make notes when relevant. Importantly, reviewing information sheets with the patient allows time for questions.
Allergen information sheets are provided by manufacturers of patch test materials, including SmartPractice (allergEAZE, T.R.U.E. Test) and Chemotechnique (Dormer)(Table 2). The ACDS also provides a selection of allergen information sheets for members to share with their patients. The ACDS allergen handouts are designed for patient use, are vetted by practicing patch test dermatologists, and contain up-to-date information for patients. We recommend that you choose the handout(s) that are most appropriate for your patient; this decision can be made based on patient education or reading level, the region of the world where you are patch testing or where the patient lives, the patient’s primary language, and the specific allergen. Information on rare or new allergens may not be available on every website resource.
Identification of Allergen-Free Products
We ask patients to bring their personal care products to their patch test reading visit, and once positive allergens are known, we search for the presence of that allergen in their products. It is helpful for patients if products that are “safe” and “not safe” are sorted for them. We frequently emphasize that just one exposure to an allergen in a personal care product can be the source of the dermatitis. If a product label does not include ingredients, they often can be identified with a quick web search (use your favorite search engine or see Table 2 for websites); however, caution is advised, as lists found online may not match those found on in-store products.3 Reviewing the patient’s own products in the clinic is preferred over searching for ingredient lists online. If the product’s ingredients cannot be found (eg, ingredients that are found on external packaging), the patient has several choices: do not use, complete repeat open application testing if it is a leave-on product, or check to see if it is on a product database safe list.
We explain to patients that once they have confirmed that they are using only “safe” allergen-free products, it can take up to 6 to 8 weeks for dermatitis to improve, and at that point, the skin may only be about 75% to 80% clear. A clear description of what to expect and when is needed for a strong patient-physician partnership. For example, if the patient expects to be clear in 2 days but is not and stops avoiding their allergens because they think the process has failed, their dermatitis will not improve.
Product Databases
Because allergens sometimes have multiple different chemical names and cross-reactivity is abundant, avoidance of both the allergen and cross-reactors can be daunting for many patients (and dermatologists!). The use of a product database to aid in product selection is an invaluable resource. Product databases help patients avoid not only their allergens but also common cross-reactors by relying on complex cross-reactor programming. The ACDS owns and maintains the Contact Allergy Management Program (CAMP). Another resource is SkinSafe, which is powered by HER Inc and developed with the Mayo Clinic. Both CAMP and SkinSafe have mobile apps and update product lists frequently; they allow for much easier shopping and identification of safe products.
We typically use CAMP for generation of patient safe lists. We enter the patient’s allergens into the database, and a safe list is generated and shared with the patient. Next, we educate the patient on how to use the safe list. It is vital that the concept of exact product matching be explained to patients, as not all products from one brand or type of product is necessarily safe for a given individual. We also share information on how to download the CAMP app onto mobile devices and tablets.
Product safe lists are important resources for patients to be successful in avoiding allergens but are not a substitute for reading labels. Both CAMP and SkinSafe can potentially contain ingredient list errors due to companies frequently changing their product formulations.3 Although safe lists are an important part in selecting safe skin care products, they are not a substitute for label reading.
Counseling Pitfalls and Pearls
Language
Chemotechnique handouts are available in English, Swedish, French, and Spanish, and ACDS handouts are available in English and Spanish. If language interpretation is needed, inform the interpreter before the visit begins that you will be discussing patch test information and products so they can carefully interpret the details of the discussion.
Barriers to Allergen Avoidance
There are several barriers to long-term avoidance of contact allergy. In a European-based study of methylisothiazolinone (MI) contact allergy 2 to 5 years after patch testing, challenges described by patients included label reading, verifying products, difficulty obtaining ingredients of industrial products, the need to have their “safe” products always available for use, remembering allergen name, avoiding workplace allergens, finding acceptable MI-free products, and navigating the cost of MI-free products.4
Patient allergen recall is a well-documented long-term concern. In the previously mentioned European study (N=139), 11% of patients identified remembering the allergen name as a contributor to difficulty with avoidance.4 A Swedish study evaluated patient allergen recall at 1, 5, and 10 years after patch testing was completed; 96% of 252 patients remembered that they had completed patch testing, 79% (111/141) remembered that they had positive results, and only 29% (41/141) correctly recalled their allergens.5 Patients who had completed patch testing 10 years prior were less likely to correctly recall their allergens (P=.0045). Recall also was less likely if there was more than 1 allergen as well as in males.5 Korkmaz and Boyvat6 analyzed outcomes 6 months after patch testing in Turkey and found that 38 of 51 (74.5%) correctly recalled their allergens. Patients with more than 1 positive allergen were less likely to recall their allergens (P=.046), and patients with higher baseline investigator global assessment (P=.036) and dermatology life quality index (P=.041) scores were more likely to recall their allergens.6 A US-based study (N=757) noted that 34.1% of patients correctly recalled all of their allergens.7 Patients were less likely to remember if they had 3 or more positives but were more likely to remember if they were aged 50 to 59 years (compared to other age groups) or female as well as if their occupation was nursing (as compared to other occupations).
Additional barriers include hidden sources of allergens, as has been reported in the cases of undeclared MI8 and formaldehyde9 in personal care products. Although this phenomenon is thought to be the exception and not the rule, possible reasons for the presence of these undeclared allergens include their use as preservatives in raw materials,8,9 or in the case of formaldehyde, theorized release from product packaging or auto-oxidation and degradation of other chemicals present within the product.9
Readers may recall that we mentioned the option of identifying product ingredients with online search engines or databases, but it is not a perfect system. Comstock and Reeder3 reviewed and compared online ingredient lists from Amazon and several product databases to products taken off shelves at Target and Walgreens and found that 27.7% of online ingredient lists did not match the in-store labels.3 These differences likely are due to changes in product formulations, ingredient variability based on production site, outdated product on store shelves, or data entry error and may not be entirely avoidable. Regardless, patch test experts should be aware of this possibility. When in doubt, always check the product’s original packaging.
Finally, the elephant in the room: We challenge you, as dermatologists and patch test enthusiasts, to name all of the formaldehyde releasers or perhaps declare whether linalool and hydroxycitronellol are fragrances, preservatives, or surfactants. How about naming the relationship between cocamidopropyl betaine, amidoamine, and dimethylaminopropylamine? Difficult stuff, right? And we are medical specialists. It is downright impossible for many of our patients to memorize the names of these chemicals, let alone know their cross-reactors or other important chemical relationships. We mention that providing a safe list is part of patient counseling, but we bring up this knowledge gap to illustrate that patch testing without providing resources to select safe care products is almost as bad as not patch testing at all because in many cases patients may be left without the tools they need to be successful. Do not let this be your downfall!
Final Interpretation
The most challenging and nuanced part of patch testing happens after the actual patch test: assessment of relevance, allergen counseling, and identification of appropriate products for patient use. You now have the tools to successfully counsel your patients after patch testing; get to it!
The first part of this 2-part series addressed the basics of patch testing, including patch test systems, allergens, and patch test readings. In the second part of this series, we examine the incredibly important and absolutely vital steps that come after the patch test: determining relevance, patient counseling, and identifying allergen-free products for patient use. Let’s dive in!
Determining Relevance
The purpose of determining relevance is to assess whether the positive patch test explains the patient’s dermatitis. It is important to consider all of the patient’s exposures, including at home, at work, and during recreational activities. Several relevance grading scales exist. The North American Contact Dermatitis Group grades relevance as current, past, or unknown. Current relevance is further divided into definite, probable, and possible.1 Table 1 includes explanations and clinical examples of each relevance type.
True relevance is only known weeks or months after patch testing is complete. If the patient avoids allergens and is subsequently free of dermatitis, the allergens identified through patch testing were relevant. However, if the patient avoids allergens and sees no improvement in dermatitis, the allergens were not relevant. Gipson et al2 analyzed relevance as documented by the physician at final patch test reading vs patient opinion of relevance 30 days to 3 years after the final reading and found that there was variable agreement between the 2 groups; percentage agreement for formaldehyde-releasing preservatives was 88%, neomycin was 78%, nickel was 71%, fragrances was 65%, and gold was 56%. These differences underscore the need for ongoing research on patch test methods, determination of relevance, and standards for patient follow-up.2
Patient Counseling
Patient counseling is one of the most important and complex parts of patch testing. We have consulted with patients who had already completed patch testing with other providers but did not receive comprehensive allergen counseling and therefore did not improve. It is up to you to explain positive allergens to your patients in a way that they understand, can retain long-term, and can use to their advantage to keep their skin free of dermatitis, which is an incredibly difficult feat to accomplish. The resources that we describe next are the very basic requirements for proficient patch testing.
There are several tools that can be utilized to develop patch test counseling skills (Table 2). Membership with the American Contact Dermatitis Society (ACDS) includes opportunities for virtual and in-person (post–coronavirus disease 2019) lectures and conferences, videos, patch test support information, and patient resources. The European Society of Contact Dermatitis is similar, with a focus on European-based patch testers. Both societies are affiliated with academic journals—Dermatitis and Contact Dermatitis, respectively—which are phenomenal educational resources. Dermatitis Academy (https://www.dermatitisacademy.com) and Contact Dermatitis Institute (https://www.contactdermatitisinstitute.com) are websites that are privately designed and managed by US-based patch test experts.
Allergen Information Handouts
Allergen information should be presented in both verbal and written formats as well as in the patient’s preferred language and education level. Patch test counseling is detailed and complex. Patients rarely remember everything that is discussed; written information allows them to review again when necessary. Allergen information sheets typically include the name of the allergen, alternative names, types of products that might contain the allergen, and other pertinent facts. They also can be helpful for the physician who does not patch test full time; in this case, they can be used as a quick reference to guide patient counseling. It is helpful to highlight or underline important points and make notes when relevant. Importantly, reviewing information sheets with the patient allows time for questions.
Allergen information sheets are provided by manufacturers of patch test materials, including SmartPractice (allergEAZE, T.R.U.E. Test) and Chemotechnique (Dormer)(Table 2). The ACDS also provides a selection of allergen information sheets for members to share with their patients. The ACDS allergen handouts are designed for patient use, are vetted by practicing patch test dermatologists, and contain up-to-date information for patients. We recommend that you choose the handout(s) that are most appropriate for your patient; this decision can be made based on patient education or reading level, the region of the world where you are patch testing or where the patient lives, the patient’s primary language, and the specific allergen. Information on rare or new allergens may not be available on every website resource.
Identification of Allergen-Free Products
We ask patients to bring their personal care products to their patch test reading visit, and once positive allergens are known, we search for the presence of that allergen in their products. It is helpful for patients if products that are “safe” and “not safe” are sorted for them. We frequently emphasize that just one exposure to an allergen in a personal care product can be the source of the dermatitis. If a product label does not include ingredients, they often can be identified with a quick web search (use your favorite search engine or see Table 2 for websites); however, caution is advised, as lists found online may not match those found on in-store products.3 Reviewing the patient’s own products in the clinic is preferred over searching for ingredient lists online. If the product’s ingredients cannot be found (eg, ingredients that are found on external packaging), the patient has several choices: do not use, complete repeat open application testing if it is a leave-on product, or check to see if it is on a product database safe list.
We explain to patients that once they have confirmed that they are using only “safe” allergen-free products, it can take up to 6 to 8 weeks for dermatitis to improve, and at that point, the skin may only be about 75% to 80% clear. A clear description of what to expect and when is needed for a strong patient-physician partnership. For example, if the patient expects to be clear in 2 days but is not and stops avoiding their allergens because they think the process has failed, their dermatitis will not improve.
Product Databases
Because allergens sometimes have multiple different chemical names and cross-reactivity is abundant, avoidance of both the allergen and cross-reactors can be daunting for many patients (and dermatologists!). The use of a product database to aid in product selection is an invaluable resource. Product databases help patients avoid not only their allergens but also common cross-reactors by relying on complex cross-reactor programming. The ACDS owns and maintains the Contact Allergy Management Program (CAMP). Another resource is SkinSafe, which is powered by HER Inc and developed with the Mayo Clinic. Both CAMP and SkinSafe have mobile apps and update product lists frequently; they allow for much easier shopping and identification of safe products.
We typically use CAMP for generation of patient safe lists. We enter the patient’s allergens into the database, and a safe list is generated and shared with the patient. Next, we educate the patient on how to use the safe list. It is vital that the concept of exact product matching be explained to patients, as not all products from one brand or type of product is necessarily safe for a given individual. We also share information on how to download the CAMP app onto mobile devices and tablets.
Product safe lists are important resources for patients to be successful in avoiding allergens but are not a substitute for reading labels. Both CAMP and SkinSafe can potentially contain ingredient list errors due to companies frequently changing their product formulations.3 Although safe lists are an important part in selecting safe skin care products, they are not a substitute for label reading.
Counseling Pitfalls and Pearls
Language
Chemotechnique handouts are available in English, Swedish, French, and Spanish, and ACDS handouts are available in English and Spanish. If language interpretation is needed, inform the interpreter before the visit begins that you will be discussing patch test information and products so they can carefully interpret the details of the discussion.
Barriers to Allergen Avoidance
There are several barriers to long-term avoidance of contact allergy. In a European-based study of methylisothiazolinone (MI) contact allergy 2 to 5 years after patch testing, challenges described by patients included label reading, verifying products, difficulty obtaining ingredients of industrial products, the need to have their “safe” products always available for use, remembering allergen name, avoiding workplace allergens, finding acceptable MI-free products, and navigating the cost of MI-free products.4
Patient allergen recall is a well-documented long-term concern. In the previously mentioned European study (N=139), 11% of patients identified remembering the allergen name as a contributor to difficulty with avoidance.4 A Swedish study evaluated patient allergen recall at 1, 5, and 10 years after patch testing was completed; 96% of 252 patients remembered that they had completed patch testing, 79% (111/141) remembered that they had positive results, and only 29% (41/141) correctly recalled their allergens.5 Patients who had completed patch testing 10 years prior were less likely to correctly recall their allergens (P=.0045). Recall also was less likely if there was more than 1 allergen as well as in males.5 Korkmaz and Boyvat6 analyzed outcomes 6 months after patch testing in Turkey and found that 38 of 51 (74.5%) correctly recalled their allergens. Patients with more than 1 positive allergen were less likely to recall their allergens (P=.046), and patients with higher baseline investigator global assessment (P=.036) and dermatology life quality index (P=.041) scores were more likely to recall their allergens.6 A US-based study (N=757) noted that 34.1% of patients correctly recalled all of their allergens.7 Patients were less likely to remember if they had 3 or more positives but were more likely to remember if they were aged 50 to 59 years (compared to other age groups) or female as well as if their occupation was nursing (as compared to other occupations).
Additional barriers include hidden sources of allergens, as has been reported in the cases of undeclared MI8 and formaldehyde9 in personal care products. Although this phenomenon is thought to be the exception and not the rule, possible reasons for the presence of these undeclared allergens include their use as preservatives in raw materials,8,9 or in the case of formaldehyde, theorized release from product packaging or auto-oxidation and degradation of other chemicals present within the product.9
Readers may recall that we mentioned the option of identifying product ingredients with online search engines or databases, but it is not a perfect system. Comstock and Reeder3 reviewed and compared online ingredient lists from Amazon and several product databases to products taken off shelves at Target and Walgreens and found that 27.7% of online ingredient lists did not match the in-store labels.3 These differences likely are due to changes in product formulations, ingredient variability based on production site, outdated product on store shelves, or data entry error and may not be entirely avoidable. Regardless, patch test experts should be aware of this possibility. When in doubt, always check the product’s original packaging.
Finally, the elephant in the room: We challenge you, as dermatologists and patch test enthusiasts, to name all of the formaldehyde releasers or perhaps declare whether linalool and hydroxycitronellol are fragrances, preservatives, or surfactants. How about naming the relationship between cocamidopropyl betaine, amidoamine, and dimethylaminopropylamine? Difficult stuff, right? And we are medical specialists. It is downright impossible for many of our patients to memorize the names of these chemicals, let alone know their cross-reactors or other important chemical relationships. We mention that providing a safe list is part of patient counseling, but we bring up this knowledge gap to illustrate that patch testing without providing resources to select safe care products is almost as bad as not patch testing at all because in many cases patients may be left without the tools they need to be successful. Do not let this be your downfall!
Final Interpretation
The most challenging and nuanced part of patch testing happens after the actual patch test: assessment of relevance, allergen counseling, and identification of appropriate products for patient use. You now have the tools to successfully counsel your patients after patch testing; get to it!
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gipson KA, Carlson SW, Nedorost ST. Physician-patient agreement in the assessment of allergen relevance. Dermatitis. 2010;21:275-279.
- Comstock JR, Reeder MJ. Accuracy of product ingredient labeling: comparing drugstore products with online databases and online retailers. Dermatitis. 2020;31:106-111.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the department of dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220.
- Korkmaz P, Boyvat A. Effect of patch testing on the course of allergic contact dermatitis and prognostic factors that influence outcomes. Dermatitis. 2019;30:135-141.
- Scalf LA, Genebriera J, Davis MD, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Nikle A, Ericson M, Warshaw E. Formaldehyde release from personal care products: chromotropic acid method analysis. Dermatitis. 2019;30:67-73.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gipson KA, Carlson SW, Nedorost ST. Physician-patient agreement in the assessment of allergen relevance. Dermatitis. 2010;21:275-279.
- Comstock JR, Reeder MJ. Accuracy of product ingredient labeling: comparing drugstore products with online databases and online retailers. Dermatitis. 2020;31:106-111.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the department of dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220.
- Korkmaz P, Boyvat A. Effect of patch testing on the course of allergic contact dermatitis and prognostic factors that influence outcomes. Dermatitis. 2019;30:135-141.
- Scalf LA, Genebriera J, Davis MD, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Nikle A, Ericson M, Warshaw E. Formaldehyde release from personal care products: chromotropic acid method analysis. Dermatitis. 2019;30:67-73.
Practice Points
- Positive patch test reactions must be interpreted in the context of the patient’s exposures, both current and past.
- Allergen information sheets and product database safe lists are invaluable tools to help patients select safe skin care products.
Patch Testing 101, Part 1: Performing the Test
Our apologies, dear reader. It seems we have gotten ahead of ourselves. While we were writing about the Allergen of the Year, systemic dermatitis, and patch testing in children, we forgot to start with the basics. Let us remedy that. This is the first of a 2-part series addressing the basics of patch testing. In this article, we examine patch test systems, allergens, patch test readings, testing while on medications, and patch testing pearls and pitfalls. Let us begin!
Patch Test Systems
There are 2 patch test systems in North America: the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice), which is approved by the US Food and Drug Administration for those 6 years and older, and the chamber method.
The T.R.U.E. test consists of 3 panels with 35 allergens and 1 negative control. The T.R.U.E. package insert1 describes surgical tape with individual polyester patches, each coated with an allergen film. Benefits of T.R.U.E. include ease of use (ie, easy storage and preparation, quick and straightforward application) and a readily identifiable set of allergens. The main drawback of T.R.U.E. is that only a limited number of allergens are tested, and as a result, it may miss the identification of some contact allergies. In an analysis of the 2015-2016 North American Contact Dermatitis Group (NACDG) patch test screening series, 25% to 40% of positive patch tests would have been missed if patch testing was performed with T.R.U.E. alone.2
Chamber method patch testing describes the process by which allergens are loaded into either metal or plastic chambers and then applied to the patient’s skin. The major benefit of the chamber method is that patches may be truly customized for the patient. The chamber method is time and labor intensive for patch preparation and application. Most comprehensive patch test clinics in North America use the chamber method, including the NACDG.
Patch test chambers largely can be divided into 2 categories: metal (aluminum) or plastic. Aluminum chambers, also known as Finn chambers, traditionally are used in patch testing. There are rare reports of hypersensitivity to aluminum chambers with associated diffuse positive patch test reactions,3,4 which may be more common in the pediatric population and likely is due to the fact that aluminum is present as an adjuvant in many childhood vaccines. As a precaution, some patch text experts recommend using plastic chambers in children younger than 16 to 18 years (M.R. and A.R.A., personal communication). Metal chambers require the additional application of diffusion discs for liquid allergens, and plastic chambers typically already contain the necessary diffusion discs. Finn chambers traditionally are applied with hypoallergenic porous surgical tape, but a waterproof tape also is available. To keep the chambers in place for the necessary 48 hours, additional tape may be applied over the patches.
Allergens
In patch test clinics, many dermatologists use a standard or screening allergen series. An appropriate standard series encompasses allergens that are most likely to be positive and relevant in the tested population. Some patch test experts recommend that allergens with a positive patch test frequency of greater than 0.5% to 1% should be included in a standard series.5 However, geographic differences in positive reactions can influence which allergens are appropriate to include. As a result, there is no universal standard series. Examples of standard or screening series include the American Contact Dermatitis Society (ACDS) allergen series,6 North American Baseline Series or North American 80 Comprehensive Series, European Baseline Series, NACDG series,2 and the Pediatric Baseline Series,7 as well as many other country- or region-specific series. There currently are 2 major commercial allergen distribution companies—Chemotechnique Diagnostics/Dormer Laboratories (series, individual allergens) and SmartPractice/allerGEAZE (series, individual allergens, T.R.U.E.).
In addition to a properly selected standard or screening series, supplemental patch testing with additional allergens can increase the diagnostic yield. Numerous supplemental series exist, including cosmetic, dental, textile, rubber, adhesive, plastics, and glue, among many others. In the NACDG 2015-2016 patch test cycle, it was found that 23% of 5597 patients reacted to an allergen that was not present on the NACDG screening series.2
In some situations, it is appropriate to patch test patient products, or nonstandard allergens. An abundance of caution, understanding of patch testing, and experience is necessary; for example, some chemicals are not recommended for testing, such as cleaning products, certain industrial chemicals, and those that may be carcinogens. We frequently consult De Groot’s Patch Testing8 for recommended allergen test concentrations and vehicles.
Patch Test Readings
The timing of the patch test reading is an important component of the test. Most North American comprehensive patch test clinics perform both first and delayed readings. After application, patches remain in place for 48 hours and then are removed, and a first reading is completed. Results are recorded as +/− (weak/doubtful), + (mild), ++ (strong), +++ (very strong), irritant, and negative.2 Many patch test specialists use side lighting to achieve the best reading and palpate to confirm the presence of induration; panel alignment devices commonly are utilized. There are some scenarios where shorter or longer application times are indicated, but this is beyond the scope of this article. A second, or delayed, reading should be completed 72 to 144 hours after initial application. We usually complete the delayed reading at 96 to 120 hours.
Certain patch test reactions may peak at different times, with fragrances often reacting earlier, and metals, topical antibiotics, and textile dyes reacting later.9 In the scenario of delayed peak reactions, third readings may be indicated.
Neglecting to complete a delayed reading is a potential pitfall and can increase the risk for both false-positive and false-negative reactions.10,11 In 1996, Uter et al10 published a large study of 9946 patients who were patch tested over a 4-year period. The authors compared patch test reactions at 48 and 72 hours and found that 34.5% of all positive reactions occurred at 72 hours; an additional 15.1% were positive at 96 hours. Importantly, one reading at 48 hours missed approximately one-third of positive patch test reactions, emphasizing the importance of delayed patch test readings.10 Furthermore, another study of 9997 consecutively patch tested patients examined reactions that were either negative or doubtful between days 3 or 4 and followed to see which of those reactions were positive at days 6 or 7. Of the negative reactions, the authors found that 4.4% were positive on days 6 or 7, and of the doubtful reactions, 9.1% were positive on days 6 or 7, meaning that up to 13.5% of positive reactions can be missed when a later reading is not performed.11
Medications During Patch Testing
Topical Medications
Topical medications generally can be continued during patch testing; however, patients should not apply topical medications to the patch test application site. Ideally, there should be no topical medication applied to the patch test application site for 1 to 2 weeks prior to patch test placement.12 Use of topical medications such as corticosteroids, calcineurin inhibitors, and theoretically even phosphodiesterase 4 inhibitors can not only result in suppression of positive patch test reactions but also can make patch adherence difficult.
Phototherapy
Phototherapy can result in local cutaneous immune suppression; therefore, it is recommended that it not be applied to the patch test area either during the patch test process or for 1 to 2 weeks prior to patch test application. In addition, if heat or sweating are generated during phototherapy, they can affect the success of patch testing by poor patch adherence and/or disruption of allergen distribution.
Systemic Medications
Oral antihistamines do not affect patch testing and can be continued during the patch test process.
It is ideal to avoid systemic immunomodulatory agents during the patch test process, but they occasionally are unavoidable, either because they are necessary to manage other medical conditions or because they are needed to achieve clear enough skin to proceed with patch testing. If it is required, prednisone is not recommended to exceed 10 mg daily.12,13 If intramuscular triamcinolone acetonide has been administered, patch testing should occur at least 1 month after the most recent injection.12 Oral methotrexate can probably be continued during patch testing but should be kept at the lowest possible dose and should be held during the week of testing, if possible. Adalimumab, etanercept, infliximab, and ustekinumab can be continued, as they are unlikely to interfere with patch testing.12 There are reports of positive patch test reactions on dupilumab,14,15 and some authors have described the response as variable and potentially allergen dependent.16,17 We believe that it generally is acceptable to continue dupilumab during patch testing. Data on cyclosporine during patch testing are mixed, and caution is advised as higher doses may suppress a positive patch test. Azathioprine and mycophenolate should be avoided, if possible.12
Pearls and Pitfalls
A few tips along the way can help assure your success in patch testing.
- Proper patient counseling determines a successful test. Provide your patient with verbal and written instructions about the patch test process, patch care, and any other necessary information.
- A simple sponge bath is permissible during patch testing provided the back stays dry. One of the authors (A.R.A.) advises patients to sit in a small amount of water in a bathtub to bathe, wash only the front of the body in the shower, and wash hair in the sink.
- No sweating, swimming, heavy exercise, or heavy physical labor. If your patient is planning to run a marathon the week of patch testing, they will be sorely disappointed when you tell them no sweating or showering is allowed! Patients with an occupation that requires physical labor may require a work excuse.
- Tape does not adhere to areas of the skin with excess hair. A scissor trim or electric shave will help the patches stay occluded and in place. We use an electric razor with a disposable replaceable head. A traditional straight razor should not be used, as it can increase the risk for folliculitis, which can make patch readings quite difficult.
- Securing the patches in place with an extra layer of tape provides added security. Large sheets of transparent medical dressings work particularly well for children or if there is difficulty with tape adherence.
Avoid application of patches to areas of the skin with tattoos. In theory, tattooed skin may have a decreased immune response, and tattoo pigment can obscure results.18 However, this is sometimes unavoidable, and Fowler and McTigue18 described a case of successful patch testing on a diffusely tattooed back.
- Avoid skin lesions (eg, scars, seborrheic keratoses, dermatitis) that can affect tape application, patch adherence, or patch readings.
Final Interpretation
The first step to excellent patch testing is understanding the patch test process. Patch test systems include T.R.U.E. and the chamber method. There are several allergen screening series, and the best series for each patient is determined based on geographic region, exposures, and allergen prevalence. The timing and practice of the patch test reading is vital, and physicians should be cognizant of medications and phototherapy use during the patch test process. An understanding of common pearls and pitfalls makes the difference between a good and great patch tester.
Now that you are an expert in performing the test, watch out for part 2 of this series on patch test interpretation, relevance, education, and counseling. Happy testing!
- T.R.U.E TEST [package insert]. Phoenix, AZ: SmartPractice; 1994.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Ward JM, Walsh RK, Bellet JS, et al. Allergic contact dermatitis to aluminum-based chambers during routine patch testing. Paper presented at: American Contact Dermatitis Society Annual Meeting; March 19, 2020; Denver, CO.
- Deleuran MG, Ahlström MG, Zachariae C, et al. Patch test reactivity to aluminum chambers. Contact Dermatitis. 2019;81:318-319.
- Bruze M, Condé-Salazar L, Goossens A, et al. European Society of Contact Dermatitis. thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 update. Dermatitis. 2017;28:141-143.
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- De Groot AC. Patch Testing: Test Concentrations and Vehicles for 4900 Chemicals. 4th ed. Wapserveen, The Netherlands: Acdegroot Publishing; 2018.
- Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: an update from the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28:253-260.
- Uter WJ, Geier J, Schnuch A. Good clinical practice in patch testing: readings beyond day 2 are necessary: a confirmatory analysis. Members of the Information Network of Departments of Dermatology. Am J Contact Dermat. 1996;7:231-237.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of T.R.U.E. Tests® on D6/7. Contact Dermatitis. 2013;68:94-97.
- Lampel H, Atwater AR. Patch testing tools of the trade: use of immunosuppressants and antihistamines during patch testing. J Dermatol Nurses’ Assoc. 2016;8:209-211.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89.
- Hoot JW, Douglas JD, Falo LD. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164.
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162.
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121.
- Fowler JF, McTigue MK. Patch testing over tattoos. Am J Contact Dermat. 2002;13:19-20.
Our apologies, dear reader. It seems we have gotten ahead of ourselves. While we were writing about the Allergen of the Year, systemic dermatitis, and patch testing in children, we forgot to start with the basics. Let us remedy that. This is the first of a 2-part series addressing the basics of patch testing. In this article, we examine patch test systems, allergens, patch test readings, testing while on medications, and patch testing pearls and pitfalls. Let us begin!
Patch Test Systems
There are 2 patch test systems in North America: the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice), which is approved by the US Food and Drug Administration for those 6 years and older, and the chamber method.
The T.R.U.E. test consists of 3 panels with 35 allergens and 1 negative control. The T.R.U.E. package insert1 describes surgical tape with individual polyester patches, each coated with an allergen film. Benefits of T.R.U.E. include ease of use (ie, easy storage and preparation, quick and straightforward application) and a readily identifiable set of allergens. The main drawback of T.R.U.E. is that only a limited number of allergens are tested, and as a result, it may miss the identification of some contact allergies. In an analysis of the 2015-2016 North American Contact Dermatitis Group (NACDG) patch test screening series, 25% to 40% of positive patch tests would have been missed if patch testing was performed with T.R.U.E. alone.2
Chamber method patch testing describes the process by which allergens are loaded into either metal or plastic chambers and then applied to the patient’s skin. The major benefit of the chamber method is that patches may be truly customized for the patient. The chamber method is time and labor intensive for patch preparation and application. Most comprehensive patch test clinics in North America use the chamber method, including the NACDG.
Patch test chambers largely can be divided into 2 categories: metal (aluminum) or plastic. Aluminum chambers, also known as Finn chambers, traditionally are used in patch testing. There are rare reports of hypersensitivity to aluminum chambers with associated diffuse positive patch test reactions,3,4 which may be more common in the pediatric population and likely is due to the fact that aluminum is present as an adjuvant in many childhood vaccines. As a precaution, some patch text experts recommend using plastic chambers in children younger than 16 to 18 years (M.R. and A.R.A., personal communication). Metal chambers require the additional application of diffusion discs for liquid allergens, and plastic chambers typically already contain the necessary diffusion discs. Finn chambers traditionally are applied with hypoallergenic porous surgical tape, but a waterproof tape also is available. To keep the chambers in place for the necessary 48 hours, additional tape may be applied over the patches.
Allergens
In patch test clinics, many dermatologists use a standard or screening allergen series. An appropriate standard series encompasses allergens that are most likely to be positive and relevant in the tested population. Some patch test experts recommend that allergens with a positive patch test frequency of greater than 0.5% to 1% should be included in a standard series.5 However, geographic differences in positive reactions can influence which allergens are appropriate to include. As a result, there is no universal standard series. Examples of standard or screening series include the American Contact Dermatitis Society (ACDS) allergen series,6 North American Baseline Series or North American 80 Comprehensive Series, European Baseline Series, NACDG series,2 and the Pediatric Baseline Series,7 as well as many other country- or region-specific series. There currently are 2 major commercial allergen distribution companies—Chemotechnique Diagnostics/Dormer Laboratories (series, individual allergens) and SmartPractice/allerGEAZE (series, individual allergens, T.R.U.E.).
In addition to a properly selected standard or screening series, supplemental patch testing with additional allergens can increase the diagnostic yield. Numerous supplemental series exist, including cosmetic, dental, textile, rubber, adhesive, plastics, and glue, among many others. In the NACDG 2015-2016 patch test cycle, it was found that 23% of 5597 patients reacted to an allergen that was not present on the NACDG screening series.2
In some situations, it is appropriate to patch test patient products, or nonstandard allergens. An abundance of caution, understanding of patch testing, and experience is necessary; for example, some chemicals are not recommended for testing, such as cleaning products, certain industrial chemicals, and those that may be carcinogens. We frequently consult De Groot’s Patch Testing8 for recommended allergen test concentrations and vehicles.
Patch Test Readings
The timing of the patch test reading is an important component of the test. Most North American comprehensive patch test clinics perform both first and delayed readings. After application, patches remain in place for 48 hours and then are removed, and a first reading is completed. Results are recorded as +/− (weak/doubtful), + (mild), ++ (strong), +++ (very strong), irritant, and negative.2 Many patch test specialists use side lighting to achieve the best reading and palpate to confirm the presence of induration; panel alignment devices commonly are utilized. There are some scenarios where shorter or longer application times are indicated, but this is beyond the scope of this article. A second, or delayed, reading should be completed 72 to 144 hours after initial application. We usually complete the delayed reading at 96 to 120 hours.
Certain patch test reactions may peak at different times, with fragrances often reacting earlier, and metals, topical antibiotics, and textile dyes reacting later.9 In the scenario of delayed peak reactions, third readings may be indicated.
Neglecting to complete a delayed reading is a potential pitfall and can increase the risk for both false-positive and false-negative reactions.10,11 In 1996, Uter et al10 published a large study of 9946 patients who were patch tested over a 4-year period. The authors compared patch test reactions at 48 and 72 hours and found that 34.5% of all positive reactions occurred at 72 hours; an additional 15.1% were positive at 96 hours. Importantly, one reading at 48 hours missed approximately one-third of positive patch test reactions, emphasizing the importance of delayed patch test readings.10 Furthermore, another study of 9997 consecutively patch tested patients examined reactions that were either negative or doubtful between days 3 or 4 and followed to see which of those reactions were positive at days 6 or 7. Of the negative reactions, the authors found that 4.4% were positive on days 6 or 7, and of the doubtful reactions, 9.1% were positive on days 6 or 7, meaning that up to 13.5% of positive reactions can be missed when a later reading is not performed.11
Medications During Patch Testing
Topical Medications
Topical medications generally can be continued during patch testing; however, patients should not apply topical medications to the patch test application site. Ideally, there should be no topical medication applied to the patch test application site for 1 to 2 weeks prior to patch test placement.12 Use of topical medications such as corticosteroids, calcineurin inhibitors, and theoretically even phosphodiesterase 4 inhibitors can not only result in suppression of positive patch test reactions but also can make patch adherence difficult.
Phototherapy
Phototherapy can result in local cutaneous immune suppression; therefore, it is recommended that it not be applied to the patch test area either during the patch test process or for 1 to 2 weeks prior to patch test application. In addition, if heat or sweating are generated during phototherapy, they can affect the success of patch testing by poor patch adherence and/or disruption of allergen distribution.
Systemic Medications
Oral antihistamines do not affect patch testing and can be continued during the patch test process.
It is ideal to avoid systemic immunomodulatory agents during the patch test process, but they occasionally are unavoidable, either because they are necessary to manage other medical conditions or because they are needed to achieve clear enough skin to proceed with patch testing. If it is required, prednisone is not recommended to exceed 10 mg daily.12,13 If intramuscular triamcinolone acetonide has been administered, patch testing should occur at least 1 month after the most recent injection.12 Oral methotrexate can probably be continued during patch testing but should be kept at the lowest possible dose and should be held during the week of testing, if possible. Adalimumab, etanercept, infliximab, and ustekinumab can be continued, as they are unlikely to interfere with patch testing.12 There are reports of positive patch test reactions on dupilumab,14,15 and some authors have described the response as variable and potentially allergen dependent.16,17 We believe that it generally is acceptable to continue dupilumab during patch testing. Data on cyclosporine during patch testing are mixed, and caution is advised as higher doses may suppress a positive patch test. Azathioprine and mycophenolate should be avoided, if possible.12
Pearls and Pitfalls
A few tips along the way can help assure your success in patch testing.
- Proper patient counseling determines a successful test. Provide your patient with verbal and written instructions about the patch test process, patch care, and any other necessary information.
- A simple sponge bath is permissible during patch testing provided the back stays dry. One of the authors (A.R.A.) advises patients to sit in a small amount of water in a bathtub to bathe, wash only the front of the body in the shower, and wash hair in the sink.
- No sweating, swimming, heavy exercise, or heavy physical labor. If your patient is planning to run a marathon the week of patch testing, they will be sorely disappointed when you tell them no sweating or showering is allowed! Patients with an occupation that requires physical labor may require a work excuse.
- Tape does not adhere to areas of the skin with excess hair. A scissor trim or electric shave will help the patches stay occluded and in place. We use an electric razor with a disposable replaceable head. A traditional straight razor should not be used, as it can increase the risk for folliculitis, which can make patch readings quite difficult.
- Securing the patches in place with an extra layer of tape provides added security. Large sheets of transparent medical dressings work particularly well for children or if there is difficulty with tape adherence.
Avoid application of patches to areas of the skin with tattoos. In theory, tattooed skin may have a decreased immune response, and tattoo pigment can obscure results.18 However, this is sometimes unavoidable, and Fowler and McTigue18 described a case of successful patch testing on a diffusely tattooed back.
- Avoid skin lesions (eg, scars, seborrheic keratoses, dermatitis) that can affect tape application, patch adherence, or patch readings.
Final Interpretation
The first step to excellent patch testing is understanding the patch test process. Patch test systems include T.R.U.E. and the chamber method. There are several allergen screening series, and the best series for each patient is determined based on geographic region, exposures, and allergen prevalence. The timing and practice of the patch test reading is vital, and physicians should be cognizant of medications and phototherapy use during the patch test process. An understanding of common pearls and pitfalls makes the difference between a good and great patch tester.
Now that you are an expert in performing the test, watch out for part 2 of this series on patch test interpretation, relevance, education, and counseling. Happy testing!
Our apologies, dear reader. It seems we have gotten ahead of ourselves. While we were writing about the Allergen of the Year, systemic dermatitis, and patch testing in children, we forgot to start with the basics. Let us remedy that. This is the first of a 2-part series addressing the basics of patch testing. In this article, we examine patch test systems, allergens, patch test readings, testing while on medications, and patch testing pearls and pitfalls. Let us begin!
Patch Test Systems
There are 2 patch test systems in North America: the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice), which is approved by the US Food and Drug Administration for those 6 years and older, and the chamber method.
The T.R.U.E. test consists of 3 panels with 35 allergens and 1 negative control. The T.R.U.E. package insert1 describes surgical tape with individual polyester patches, each coated with an allergen film. Benefits of T.R.U.E. include ease of use (ie, easy storage and preparation, quick and straightforward application) and a readily identifiable set of allergens. The main drawback of T.R.U.E. is that only a limited number of allergens are tested, and as a result, it may miss the identification of some contact allergies. In an analysis of the 2015-2016 North American Contact Dermatitis Group (NACDG) patch test screening series, 25% to 40% of positive patch tests would have been missed if patch testing was performed with T.R.U.E. alone.2
Chamber method patch testing describes the process by which allergens are loaded into either metal or plastic chambers and then applied to the patient’s skin. The major benefit of the chamber method is that patches may be truly customized for the patient. The chamber method is time and labor intensive for patch preparation and application. Most comprehensive patch test clinics in North America use the chamber method, including the NACDG.
Patch test chambers largely can be divided into 2 categories: metal (aluminum) or plastic. Aluminum chambers, also known as Finn chambers, traditionally are used in patch testing. There are rare reports of hypersensitivity to aluminum chambers with associated diffuse positive patch test reactions,3,4 which may be more common in the pediatric population and likely is due to the fact that aluminum is present as an adjuvant in many childhood vaccines. As a precaution, some patch text experts recommend using plastic chambers in children younger than 16 to 18 years (M.R. and A.R.A., personal communication). Metal chambers require the additional application of diffusion discs for liquid allergens, and plastic chambers typically already contain the necessary diffusion discs. Finn chambers traditionally are applied with hypoallergenic porous surgical tape, but a waterproof tape also is available. To keep the chambers in place for the necessary 48 hours, additional tape may be applied over the patches.
Allergens
In patch test clinics, many dermatologists use a standard or screening allergen series. An appropriate standard series encompasses allergens that are most likely to be positive and relevant in the tested population. Some patch test experts recommend that allergens with a positive patch test frequency of greater than 0.5% to 1% should be included in a standard series.5 However, geographic differences in positive reactions can influence which allergens are appropriate to include. As a result, there is no universal standard series. Examples of standard or screening series include the American Contact Dermatitis Society (ACDS) allergen series,6 North American Baseline Series or North American 80 Comprehensive Series, European Baseline Series, NACDG series,2 and the Pediatric Baseline Series,7 as well as many other country- or region-specific series. There currently are 2 major commercial allergen distribution companies—Chemotechnique Diagnostics/Dormer Laboratories (series, individual allergens) and SmartPractice/allerGEAZE (series, individual allergens, T.R.U.E.).
In addition to a properly selected standard or screening series, supplemental patch testing with additional allergens can increase the diagnostic yield. Numerous supplemental series exist, including cosmetic, dental, textile, rubber, adhesive, plastics, and glue, among many others. In the NACDG 2015-2016 patch test cycle, it was found that 23% of 5597 patients reacted to an allergen that was not present on the NACDG screening series.2
In some situations, it is appropriate to patch test patient products, or nonstandard allergens. An abundance of caution, understanding of patch testing, and experience is necessary; for example, some chemicals are not recommended for testing, such as cleaning products, certain industrial chemicals, and those that may be carcinogens. We frequently consult De Groot’s Patch Testing8 for recommended allergen test concentrations and vehicles.
Patch Test Readings
The timing of the patch test reading is an important component of the test. Most North American comprehensive patch test clinics perform both first and delayed readings. After application, patches remain in place for 48 hours and then are removed, and a first reading is completed. Results are recorded as +/− (weak/doubtful), + (mild), ++ (strong), +++ (very strong), irritant, and negative.2 Many patch test specialists use side lighting to achieve the best reading and palpate to confirm the presence of induration; panel alignment devices commonly are utilized. There are some scenarios where shorter or longer application times are indicated, but this is beyond the scope of this article. A second, or delayed, reading should be completed 72 to 144 hours after initial application. We usually complete the delayed reading at 96 to 120 hours.
Certain patch test reactions may peak at different times, with fragrances often reacting earlier, and metals, topical antibiotics, and textile dyes reacting later.9 In the scenario of delayed peak reactions, third readings may be indicated.
Neglecting to complete a delayed reading is a potential pitfall and can increase the risk for both false-positive and false-negative reactions.10,11 In 1996, Uter et al10 published a large study of 9946 patients who were patch tested over a 4-year period. The authors compared patch test reactions at 48 and 72 hours and found that 34.5% of all positive reactions occurred at 72 hours; an additional 15.1% were positive at 96 hours. Importantly, one reading at 48 hours missed approximately one-third of positive patch test reactions, emphasizing the importance of delayed patch test readings.10 Furthermore, another study of 9997 consecutively patch tested patients examined reactions that were either negative or doubtful between days 3 or 4 and followed to see which of those reactions were positive at days 6 or 7. Of the negative reactions, the authors found that 4.4% were positive on days 6 or 7, and of the doubtful reactions, 9.1% were positive on days 6 or 7, meaning that up to 13.5% of positive reactions can be missed when a later reading is not performed.11
Medications During Patch Testing
Topical Medications
Topical medications generally can be continued during patch testing; however, patients should not apply topical medications to the patch test application site. Ideally, there should be no topical medication applied to the patch test application site for 1 to 2 weeks prior to patch test placement.12 Use of topical medications such as corticosteroids, calcineurin inhibitors, and theoretically even phosphodiesterase 4 inhibitors can not only result in suppression of positive patch test reactions but also can make patch adherence difficult.
Phototherapy
Phototherapy can result in local cutaneous immune suppression; therefore, it is recommended that it not be applied to the patch test area either during the patch test process or for 1 to 2 weeks prior to patch test application. In addition, if heat or sweating are generated during phototherapy, they can affect the success of patch testing by poor patch adherence and/or disruption of allergen distribution.
Systemic Medications
Oral antihistamines do not affect patch testing and can be continued during the patch test process.
It is ideal to avoid systemic immunomodulatory agents during the patch test process, but they occasionally are unavoidable, either because they are necessary to manage other medical conditions or because they are needed to achieve clear enough skin to proceed with patch testing. If it is required, prednisone is not recommended to exceed 10 mg daily.12,13 If intramuscular triamcinolone acetonide has been administered, patch testing should occur at least 1 month after the most recent injection.12 Oral methotrexate can probably be continued during patch testing but should be kept at the lowest possible dose and should be held during the week of testing, if possible. Adalimumab, etanercept, infliximab, and ustekinumab can be continued, as they are unlikely to interfere with patch testing.12 There are reports of positive patch test reactions on dupilumab,14,15 and some authors have described the response as variable and potentially allergen dependent.16,17 We believe that it generally is acceptable to continue dupilumab during patch testing. Data on cyclosporine during patch testing are mixed, and caution is advised as higher doses may suppress a positive patch test. Azathioprine and mycophenolate should be avoided, if possible.12
Pearls and Pitfalls
A few tips along the way can help assure your success in patch testing.
- Proper patient counseling determines a successful test. Provide your patient with verbal and written instructions about the patch test process, patch care, and any other necessary information.
- A simple sponge bath is permissible during patch testing provided the back stays dry. One of the authors (A.R.A.) advises patients to sit in a small amount of water in a bathtub to bathe, wash only the front of the body in the shower, and wash hair in the sink.
- No sweating, swimming, heavy exercise, or heavy physical labor. If your patient is planning to run a marathon the week of patch testing, they will be sorely disappointed when you tell them no sweating or showering is allowed! Patients with an occupation that requires physical labor may require a work excuse.
- Tape does not adhere to areas of the skin with excess hair. A scissor trim or electric shave will help the patches stay occluded and in place. We use an electric razor with a disposable replaceable head. A traditional straight razor should not be used, as it can increase the risk for folliculitis, which can make patch readings quite difficult.
- Securing the patches in place with an extra layer of tape provides added security. Large sheets of transparent medical dressings work particularly well for children or if there is difficulty with tape adherence.
Avoid application of patches to areas of the skin with tattoos. In theory, tattooed skin may have a decreased immune response, and tattoo pigment can obscure results.18 However, this is sometimes unavoidable, and Fowler and McTigue18 described a case of successful patch testing on a diffusely tattooed back.
- Avoid skin lesions (eg, scars, seborrheic keratoses, dermatitis) that can affect tape application, patch adherence, or patch readings.
Final Interpretation
The first step to excellent patch testing is understanding the patch test process. Patch test systems include T.R.U.E. and the chamber method. There are several allergen screening series, and the best series for each patient is determined based on geographic region, exposures, and allergen prevalence. The timing and practice of the patch test reading is vital, and physicians should be cognizant of medications and phototherapy use during the patch test process. An understanding of common pearls and pitfalls makes the difference between a good and great patch tester.
Now that you are an expert in performing the test, watch out for part 2 of this series on patch test interpretation, relevance, education, and counseling. Happy testing!
- T.R.U.E TEST [package insert]. Phoenix, AZ: SmartPractice; 1994.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Ward JM, Walsh RK, Bellet JS, et al. Allergic contact dermatitis to aluminum-based chambers during routine patch testing. Paper presented at: American Contact Dermatitis Society Annual Meeting; March 19, 2020; Denver, CO.
- Deleuran MG, Ahlström MG, Zachariae C, et al. Patch test reactivity to aluminum chambers. Contact Dermatitis. 2019;81:318-319.
- Bruze M, Condé-Salazar L, Goossens A, et al. European Society of Contact Dermatitis. thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 update. Dermatitis. 2017;28:141-143.
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- De Groot AC. Patch Testing: Test Concentrations and Vehicles for 4900 Chemicals. 4th ed. Wapserveen, The Netherlands: Acdegroot Publishing; 2018.
- Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: an update from the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28:253-260.
- Uter WJ, Geier J, Schnuch A. Good clinical practice in patch testing: readings beyond day 2 are necessary: a confirmatory analysis. Members of the Information Network of Departments of Dermatology. Am J Contact Dermat. 1996;7:231-237.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of T.R.U.E. Tests® on D6/7. Contact Dermatitis. 2013;68:94-97.
- Lampel H, Atwater AR. Patch testing tools of the trade: use of immunosuppressants and antihistamines during patch testing. J Dermatol Nurses’ Assoc. 2016;8:209-211.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89.
- Hoot JW, Douglas JD, Falo LD. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164.
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162.
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121.
- Fowler JF, McTigue MK. Patch testing over tattoos. Am J Contact Dermat. 2002;13:19-20.
- T.R.U.E TEST [package insert]. Phoenix, AZ: SmartPractice; 1994.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Ward JM, Walsh RK, Bellet JS, et al. Allergic contact dermatitis to aluminum-based chambers during routine patch testing. Paper presented at: American Contact Dermatitis Society Annual Meeting; March 19, 2020; Denver, CO.
- Deleuran MG, Ahlström MG, Zachariae C, et al. Patch test reactivity to aluminum chambers. Contact Dermatitis. 2019;81:318-319.
- Bruze M, Condé-Salazar L, Goossens A, et al. European Society of Contact Dermatitis. thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 update. Dermatitis. 2017;28:141-143.
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- De Groot AC. Patch Testing: Test Concentrations and Vehicles for 4900 Chemicals. 4th ed. Wapserveen, The Netherlands: Acdegroot Publishing; 2018.
- Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: an update from the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28:253-260.
- Uter WJ, Geier J, Schnuch A. Good clinical practice in patch testing: readings beyond day 2 are necessary: a confirmatory analysis. Members of the Information Network of Departments of Dermatology. Am J Contact Dermat. 1996;7:231-237.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of T.R.U.E. Tests® on D6/7. Contact Dermatitis. 2013;68:94-97.
- Lampel H, Atwater AR. Patch testing tools of the trade: use of immunosuppressants and antihistamines during patch testing. J Dermatol Nurses’ Assoc. 2016;8:209-211.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89.
- Hoot JW, Douglas JD, Falo LD. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164.
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162.
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121.
- Fowler JF, McTigue MK. Patch testing over tattoos. Am J Contact Dermat. 2002;13:19-20.
Practice Points
- There are 2 basic patch testing systems: the T.R.U.E. test and the chamber method.
- Patches should be applied to the upper back and should be removed after 48 hours. A delayed reading is necessary and should be performed 72 to 144 hours after placement.
- Certain oral and topical medications and phototherapy may interfere with patch test results.
Tattoo Hypersensitivity Reactions: Inky Business
Sometimes regrettable yet increasingly common, tattoos are an ancient art form used in modern times as a mark of artistic and cultural expression. Allergic contact dermatitis (ACD) to tattoo ink is rare, but the popularity of tattoos makes ACD an increasingly recognized occurrence. In a retrospective study of 38,543 patch-tested patients, only 29 (0.08%) had tattoo-related ACD, with the majority of patients being female and young adults. The most common contact allergy was to paraphenylenediamine (PPD), which occurred in 22 (76%) patients.1 In this article, we will walk you through the rainbow of tattoo ACD, covering hypersensitivity reactions to both temporary and permanent tattoo inks.
Temporary Tattoo Inks
Henna is the most common temporary tattoo ink. Derived from the plant Lawsonia inermis, henna is an orange dye that has been used in many parts of the world, particularly in Islamic and Hindu cultures, to dye skin, hair, and fabrics. Application of henna tattoos is common for weddings and other celebrations, and brides may wear elaborate henna patterns. To create these tattoos, henna powder is mixed with water and sometimes essential oils and is then applied to the skin for several hours. After application, the henna pigment lawsone (2-hydroxy-1,4-naphthoquinone) interacts with keratin and leaves a red-orange stain on the skin2; longer application time leads to a deeper color. Most traditional cutaneous henna designs fade in 2 to 6 weeks, but some last longer. Red henna generally is considered safe with low incidence of contact allergy. What is referred to as black henna usually is red henna mixed with PPD, a black dye, which is added to deepen the color. Paraphenylenediamine is highly sensitizing; patients can become sensitized to the PPD in the tattoo itself.2 One study confirmed the presence of PPD in black henna tattoos, with chemical analysis of common preparations revealing concentrations ranging from less than 1% to 30%.2 Patients who undergo patch testing for tattoo reactions often are strongly positive to PPD and have concomitant reactions to azo dyes, black rubber, and anesthetics. Other aromatic amines including aminophenols have been identified in black henna tattoo ink, and these chemicals also may contribute to ACD.3 Less common sources of contact allergy from temporary black henna tattoos include resorcinol,4 para-tertiary butylphenol formaldehyde resin,5 and fragrance.6
Clinically, ACD to PPD in temporary tattoos presents 1 to 3 days after application if the patient is already sensitized or 4 to 14 days if the patient is sensitized by the tattoo ink.2 Most patients notice erythema, edema, vesicles, papules, and/or bullae, but other less common reactions including generalized dermatitis, systemic symptoms, urticaria, and pustules have been described.2 Postinflammatory hypopigmentation or hyperpigmentation also can occur.
Because of the sensitizing nature of black henna tattoos, consumers are turning to natural temporary tattoos. Jagua temporary tattoos, with pigment derived from the sap of fruit from the Genipa americana tree, have been associated with ACD.7 This black dye is applied and washed off in a similar fashion to henna tattoos. Importantly, a recent analysis of jagua dye identified no PPD. In one case, a patient who developed ACD to a jagua tattoo was patch tested to components of the dye and had a positive reaction to genipin, a component of the fruit extract.7 Thus, jagua tattoos often are marketed as safe but are an emerging source of contact dermatitis to temporary tattoos.
Permanent Tattoo Inks
Permanent tattoos are created by injecting small amounts of ink into the dermis. As the name suggests, these tattoos are permanent. Tattoos are common; nearly one-third of Americans have at least 1 tattoo.1 Historically, tattoos were created using black pigment composed of amorphous carbon or black iron oxides.8,9 Metallic pigments (eg, mercury, chromium, cobalt, cadmium) were once used to add color to tattoos, but these metals are now only rarely used; in fact, a 2019 study of tattoo ink components identified 44 distinct pigments in 1416 permanent inks, with an average of 3 pigments per ink.8 Of the 44 pigments, 10 had metallic components including iron, barium, zinc, copper, molybdenum, and titanium. The remaining 34 pigments contained carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes. The authors noted that nearly one-quarter of the tattoo pigments identified in their study had been reported as contact allergens.8
Typically, reactions to permanent tattoo inks manifest as an eczematous dermatitis occurring weeks to years after tattoo application.9,10 The dermatitis usually is locally confined to the tattoo and may be limited to particular colors; occasionally, a new tattoo reaction may trigger concurrent inflammation in older tattoos. Many tattoo reactions occur as a response to red pigment but also have occurred with other tattoo ink components.9 Many researchers have speculated as to whether the reaction is related to the ink component itself or from the photochemical breakdown of the ink by exposure to UV radiation and/or laser therapy.9
Red Pigment
Red ink is the most common color reported to cause tattoo hypersensitivity reactions. Historically, red tattoo pigments include mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal,11 but today’s tattoo inks primarily are composed of other pigments, such as quinacridone and azo dyes.12 Several cases of red tattoo ink hypersensitivity reactions exist in the literature, many without completion of patch tests or without positive patch tests to relevant red pigments.11-15
Black Pigment
In general, reactions to permanent black tattoo ink are rare; however, a few case reports exist. Black pigment can be created with India ink (carbon), logwood (chrome), iron oxide, and titanium.16,17 Shellac can be used as a binding agent in tattoo ink; there is at least one report of a reaction to black tattoo ink with a positive patch test to shellac and the original black ink.18
Metals
When utilized in tattoos, metals can create a variety of colors; several have been reported to cause ACD. There has been at least one reported case of a tattoo hypersensitivity reaction to a gold tattoo, with positive patch testing for gold sodium thiosulfate.19 Green tattoo inks also have been confirmed to contain metal. One case of nickel allergy from a green tattoo has been reported, with a positive patch test for nickel sulfate and tissue confirmation of the presence of nickel with micro X-ray fluorescence and laser ablation inductively coupled plasma mass spectrometry.20 Another case series described 3 patients with pruritus and chronic dermatitis associated with green tattoos who had positive patch tests to potassium dichromate, and the green tattoo pigment flared during patch testing. Chromium oxide was presumed to be present in the green tattoo pigment, and potassium dichromate avoidance in products and food improved both the pruritus and dermatitis.21
Azo Pigments
Azo pigments frequently are used in modern tattoos due to their vibrant colors. One case of hypersensitivity to azo pigment involved an eczematous ulcerated plaque overlying yellow, red, and green ink in a recently applied tattoo. Patch testing with the inks originally used in the tattoo was negative. The authors noted that the 3 problematic ink colors all contained pigment yellow 65—an azo pigment—and attributed the reaction to this dye.22 In another azo reaction, a patient had erythema and pruritus overlying a tattoo applied 1 month prior. Patch testing was positive for aminoazobenzene, an azo pigment that was present in the orange ink of the tattoo.23
Management of Tattoo Hypersensitivity Reactions
Hypersensitivity reactions to temporary tattoos are just that—temporary. Topical steroids and time generally will allow these reactions to resolve. In the setting of vigorous reactions, patients may develop postinflammatory hypopigmentation or hyperpigmentation that may last for months. Unfortunately, bullous tattoo reactions can lead to scarring and keloid formation, requiring more aggressive therapy.
Management of reactions to permanent tattoos is more challenging. High-potency topical steroids under occlusion or intralesional corticosteroid injections may aid in treating pruritus or discomfort. For severe reactions, oral corticosteroids may be required. Patients also may consider laser tattoo removal; however, providers should be aware that there have been rare reports of systemic urticarial reactions from this procedure.24,25 Obviously limited by location and size, excision also may be offered.
Patch Testing for Tattoo Ink Contact Allergy
When patients present for evaluation and management of tattoo ACD, it is important to also consider other causes, including granulomatous tattoo reaction, pseudolymphoma, and lichenoid tattoo reaction. A biopsy can be helpful if the diagnosis is in question.
Patch testing for contact allergy to temporary tattoo inks should include PPD, fragrance, aminophenols, resorcinol, para-tertiary butylphenol formaldehyde, and essential oils. Jagua currently is not available for commercial purchase but also should be considered if the patient has the original product or in research settings. If the individual tattoo ingredients can be identified, they also should be tested. In this scenario, recall reactions may occur; testing with the tattoo paste should be avoided if the prior reaction was severe. Importantly, patients with a PPD allergy should be counseled to avoid hair dyes that contain PPD. Many patients who are sensitized to PPD have strong reactions on patch testing and are at risk for severe reactions if PPD or PPD-related compounds are encountered in hair dye.
Patch testing for ACD to permanent tattoos is complex. In most cases, patch testing is of limited utility because many of the chemicals that have been reported to cause ACD are not commercially available. Additionally, a 2014 study of 90 patients with chronic tattoo reactions found that the majority had negative patch testing to the European baseline series (66%), disperse dyes (87%), and tattoo inks (87%–92%). The investigators theorized that the allergens causing tattoo reactions are formed by haptenization of “parent” chemicals in the dermis, meaning application of chemicals present in the original tattoo ink may not identify the relevant allergen.26 If patch testing is performed, it is most ideal if individual pigment ingredients can be identified. Allergens to be considered for testing include azo dyes, aromatic amines, iron oxide, barium, zinc, copper, molybdenum, titanium, gold sodium thiosulfate, nickel sulfate, carbon, shellac, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), quinophthalone (yellow) dyes, mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal, many of which are not commercially available for purchase.
Final Interpretation
As tattoos become increasingly trendy, tattoo ACD should be recognized by the astute dermatologist. The most common allergen associated with tattoo ACD is PPD, but other potential allergens include azo dyes and newer pigments. Unlike tattoos of the past, today’s inks are unlikely to contain toxic metals. Diagnosing ACD caused by permanent tattoo inks requires a high degree of suspicion, as patch testing may be of limited utility.
- Warshaw EM, Schlarbaum JP, Taylor JS, et al. Allergic reactions to tattoos: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. J Am Acad Dermatol. 2020;82:E61-E62.
- de Groot AC. Side-effects of henna and semi-permanent ‘black henna’ tattoos: a full review. Contact Dermatitis. 2013;69:1-25.
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m-aminophenol and toluene-2,5-diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52.
- Ormerod E, Hughes TM, Stone N. Allergic contact dermatitis caused by resorcinol following a temporary black henna tattoo. Contact Dermatitis. 2017;77:187-188.
- Rodrigo-Nicolás B, de la Cuadra J, Sierra C, et al. Contact dermatitis from a temporary tattoo in a boy with contact allergy to p-tert butyl phenol formaldehyde resin. Dermatitis. 2014;25:37-38.
- Temesvári E, Podányi B, Pónyai G, et al. Fragrance sensitization caused by temporary henna tattoo. Contact Dermatitis. 2002;47:240.
- Bircher AJ, Scherer Hofmeier K, Schlegel U, et al. Genipin in temporary jagua tattoos—black dye causing severe allergic dermatitis. Dermatitis. 2019;30:375-376.
- Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379-385.
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82.
- Bjerre RD, Ulrich NH, Linneberg A, et al. Adverse reactions to tattoos in the general population of Denmark. J Am Acad Dermatol. 2018;79:770-772.
- Bhardwaj SS, Brodell RT, Taylor JS. Red tattoo reactions. Contact Dermatitis. 2003;48:236-237.
- Gaudron S, Ferrier-Le Bouëdec MC, Franck F, et al. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97-105.
- de Winter RW, van der Bent SAS, van Esch M, et al. Allergic reaction to red cosmetic lip tattoo treated with hydroxychloroquine. Dermatitis. 2019;30:82-83.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Ruiz-Villaverde R, Fernandez-Crehuet P, Aguayo-Carreras P, et al. Inflammatory reactions to red tattoo inks: three cases highlighting an emerging problem. Sultan Qaboos Univ Med J. 2018;18:E215-E218.
- Gallo R, Parodi A, Cozzani E, et al. Allergic reaction to India ink in a black tattoo. Contact Dermatitis. 1998;38:346-347.
- de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo-related hypersensitivity reactions? a case report. Contact Dermatitis. 2017;77:397-405.
- González-Villanueva I, Hispán Ocete P, Silvestre Salvador JF. Allergic contact dermatitis caused by a black tattoo ink in a patient allergic to shellac. Contact Dermatitis. 2016;75:247-248.
- Tammaro A, Tuchinda P, Persechino S, et al. Contact allergic dermatitis to gold in a tattoo: a case report. Int J Immunopathol Pharmacol. 2011;24:1111-1113.
- van der Bent SAS, Berg T, Karst U, et al. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermatitis. 2019;81:64-66.
- Jacob SE, Castanedo-Tardan MP, Blyumin ML. Inflammation in green (chromium) tattoos during patch testing. Dermatitis. 2008;19:E33-E34.
- González-Villanueva I, Álvarez-Chinchilla P, Silvestre JF. Allergic reaction to 3 tattoo inks containing pigment yellow 65. Contact Dermatitis. 2018;79:107-108.
- Tammaro A, De Marco G, D’Arino A, et al. Aminoazobenzene in tattoo: another case of allergic contact dermatitis. Int J Dermatol. 2017;56:E79-E81.
- Willardson HB, Kobayashi TT, Arnold JG, et al. Diffuse urticarial reaction associated with titanium dioxide following laser tattoo removal treatments. Photomed Laser Surg. 2017;35:176‐180.
- England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215‐217.
- Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
Sometimes regrettable yet increasingly common, tattoos are an ancient art form used in modern times as a mark of artistic and cultural expression. Allergic contact dermatitis (ACD) to tattoo ink is rare, but the popularity of tattoos makes ACD an increasingly recognized occurrence. In a retrospective study of 38,543 patch-tested patients, only 29 (0.08%) had tattoo-related ACD, with the majority of patients being female and young adults. The most common contact allergy was to paraphenylenediamine (PPD), which occurred in 22 (76%) patients.1 In this article, we will walk you through the rainbow of tattoo ACD, covering hypersensitivity reactions to both temporary and permanent tattoo inks.
Temporary Tattoo Inks
Henna is the most common temporary tattoo ink. Derived from the plant Lawsonia inermis, henna is an orange dye that has been used in many parts of the world, particularly in Islamic and Hindu cultures, to dye skin, hair, and fabrics. Application of henna tattoos is common for weddings and other celebrations, and brides may wear elaborate henna patterns. To create these tattoos, henna powder is mixed with water and sometimes essential oils and is then applied to the skin for several hours. After application, the henna pigment lawsone (2-hydroxy-1,4-naphthoquinone) interacts with keratin and leaves a red-orange stain on the skin2; longer application time leads to a deeper color. Most traditional cutaneous henna designs fade in 2 to 6 weeks, but some last longer. Red henna generally is considered safe with low incidence of contact allergy. What is referred to as black henna usually is red henna mixed with PPD, a black dye, which is added to deepen the color. Paraphenylenediamine is highly sensitizing; patients can become sensitized to the PPD in the tattoo itself.2 One study confirmed the presence of PPD in black henna tattoos, with chemical analysis of common preparations revealing concentrations ranging from less than 1% to 30%.2 Patients who undergo patch testing for tattoo reactions often are strongly positive to PPD and have concomitant reactions to azo dyes, black rubber, and anesthetics. Other aromatic amines including aminophenols have been identified in black henna tattoo ink, and these chemicals also may contribute to ACD.3 Less common sources of contact allergy from temporary black henna tattoos include resorcinol,4 para-tertiary butylphenol formaldehyde resin,5 and fragrance.6
Clinically, ACD to PPD in temporary tattoos presents 1 to 3 days after application if the patient is already sensitized or 4 to 14 days if the patient is sensitized by the tattoo ink.2 Most patients notice erythema, edema, vesicles, papules, and/or bullae, but other less common reactions including generalized dermatitis, systemic symptoms, urticaria, and pustules have been described.2 Postinflammatory hypopigmentation or hyperpigmentation also can occur.
Because of the sensitizing nature of black henna tattoos, consumers are turning to natural temporary tattoos. Jagua temporary tattoos, with pigment derived from the sap of fruit from the Genipa americana tree, have been associated with ACD.7 This black dye is applied and washed off in a similar fashion to henna tattoos. Importantly, a recent analysis of jagua dye identified no PPD. In one case, a patient who developed ACD to a jagua tattoo was patch tested to components of the dye and had a positive reaction to genipin, a component of the fruit extract.7 Thus, jagua tattoos often are marketed as safe but are an emerging source of contact dermatitis to temporary tattoos.
Permanent Tattoo Inks
Permanent tattoos are created by injecting small amounts of ink into the dermis. As the name suggests, these tattoos are permanent. Tattoos are common; nearly one-third of Americans have at least 1 tattoo.1 Historically, tattoos were created using black pigment composed of amorphous carbon or black iron oxides.8,9 Metallic pigments (eg, mercury, chromium, cobalt, cadmium) were once used to add color to tattoos, but these metals are now only rarely used; in fact, a 2019 study of tattoo ink components identified 44 distinct pigments in 1416 permanent inks, with an average of 3 pigments per ink.8 Of the 44 pigments, 10 had metallic components including iron, barium, zinc, copper, molybdenum, and titanium. The remaining 34 pigments contained carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes. The authors noted that nearly one-quarter of the tattoo pigments identified in their study had been reported as contact allergens.8
Typically, reactions to permanent tattoo inks manifest as an eczematous dermatitis occurring weeks to years after tattoo application.9,10 The dermatitis usually is locally confined to the tattoo and may be limited to particular colors; occasionally, a new tattoo reaction may trigger concurrent inflammation in older tattoos. Many tattoo reactions occur as a response to red pigment but also have occurred with other tattoo ink components.9 Many researchers have speculated as to whether the reaction is related to the ink component itself or from the photochemical breakdown of the ink by exposure to UV radiation and/or laser therapy.9
Red Pigment
Red ink is the most common color reported to cause tattoo hypersensitivity reactions. Historically, red tattoo pigments include mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal,11 but today’s tattoo inks primarily are composed of other pigments, such as quinacridone and azo dyes.12 Several cases of red tattoo ink hypersensitivity reactions exist in the literature, many without completion of patch tests or without positive patch tests to relevant red pigments.11-15
Black Pigment
In general, reactions to permanent black tattoo ink are rare; however, a few case reports exist. Black pigment can be created with India ink (carbon), logwood (chrome), iron oxide, and titanium.16,17 Shellac can be used as a binding agent in tattoo ink; there is at least one report of a reaction to black tattoo ink with a positive patch test to shellac and the original black ink.18
Metals
When utilized in tattoos, metals can create a variety of colors; several have been reported to cause ACD. There has been at least one reported case of a tattoo hypersensitivity reaction to a gold tattoo, with positive patch testing for gold sodium thiosulfate.19 Green tattoo inks also have been confirmed to contain metal. One case of nickel allergy from a green tattoo has been reported, with a positive patch test for nickel sulfate and tissue confirmation of the presence of nickel with micro X-ray fluorescence and laser ablation inductively coupled plasma mass spectrometry.20 Another case series described 3 patients with pruritus and chronic dermatitis associated with green tattoos who had positive patch tests to potassium dichromate, and the green tattoo pigment flared during patch testing. Chromium oxide was presumed to be present in the green tattoo pigment, and potassium dichromate avoidance in products and food improved both the pruritus and dermatitis.21
Azo Pigments
Azo pigments frequently are used in modern tattoos due to their vibrant colors. One case of hypersensitivity to azo pigment involved an eczematous ulcerated plaque overlying yellow, red, and green ink in a recently applied tattoo. Patch testing with the inks originally used in the tattoo was negative. The authors noted that the 3 problematic ink colors all contained pigment yellow 65—an azo pigment—and attributed the reaction to this dye.22 In another azo reaction, a patient had erythema and pruritus overlying a tattoo applied 1 month prior. Patch testing was positive for aminoazobenzene, an azo pigment that was present in the orange ink of the tattoo.23
Management of Tattoo Hypersensitivity Reactions
Hypersensitivity reactions to temporary tattoos are just that—temporary. Topical steroids and time generally will allow these reactions to resolve. In the setting of vigorous reactions, patients may develop postinflammatory hypopigmentation or hyperpigmentation that may last for months. Unfortunately, bullous tattoo reactions can lead to scarring and keloid formation, requiring more aggressive therapy.
Management of reactions to permanent tattoos is more challenging. High-potency topical steroids under occlusion or intralesional corticosteroid injections may aid in treating pruritus or discomfort. For severe reactions, oral corticosteroids may be required. Patients also may consider laser tattoo removal; however, providers should be aware that there have been rare reports of systemic urticarial reactions from this procedure.24,25 Obviously limited by location and size, excision also may be offered.
Patch Testing for Tattoo Ink Contact Allergy
When patients present for evaluation and management of tattoo ACD, it is important to also consider other causes, including granulomatous tattoo reaction, pseudolymphoma, and lichenoid tattoo reaction. A biopsy can be helpful if the diagnosis is in question.
Patch testing for contact allergy to temporary tattoo inks should include PPD, fragrance, aminophenols, resorcinol, para-tertiary butylphenol formaldehyde, and essential oils. Jagua currently is not available for commercial purchase but also should be considered if the patient has the original product or in research settings. If the individual tattoo ingredients can be identified, they also should be tested. In this scenario, recall reactions may occur; testing with the tattoo paste should be avoided if the prior reaction was severe. Importantly, patients with a PPD allergy should be counseled to avoid hair dyes that contain PPD. Many patients who are sensitized to PPD have strong reactions on patch testing and are at risk for severe reactions if PPD or PPD-related compounds are encountered in hair dye.
Patch testing for ACD to permanent tattoos is complex. In most cases, patch testing is of limited utility because many of the chemicals that have been reported to cause ACD are not commercially available. Additionally, a 2014 study of 90 patients with chronic tattoo reactions found that the majority had negative patch testing to the European baseline series (66%), disperse dyes (87%), and tattoo inks (87%–92%). The investigators theorized that the allergens causing tattoo reactions are formed by haptenization of “parent” chemicals in the dermis, meaning application of chemicals present in the original tattoo ink may not identify the relevant allergen.26 If patch testing is performed, it is most ideal if individual pigment ingredients can be identified. Allergens to be considered for testing include azo dyes, aromatic amines, iron oxide, barium, zinc, copper, molybdenum, titanium, gold sodium thiosulfate, nickel sulfate, carbon, shellac, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), quinophthalone (yellow) dyes, mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal, many of which are not commercially available for purchase.
Final Interpretation
As tattoos become increasingly trendy, tattoo ACD should be recognized by the astute dermatologist. The most common allergen associated with tattoo ACD is PPD, but other potential allergens include azo dyes and newer pigments. Unlike tattoos of the past, today’s inks are unlikely to contain toxic metals. Diagnosing ACD caused by permanent tattoo inks requires a high degree of suspicion, as patch testing may be of limited utility.
Sometimes regrettable yet increasingly common, tattoos are an ancient art form used in modern times as a mark of artistic and cultural expression. Allergic contact dermatitis (ACD) to tattoo ink is rare, but the popularity of tattoos makes ACD an increasingly recognized occurrence. In a retrospective study of 38,543 patch-tested patients, only 29 (0.08%) had tattoo-related ACD, with the majority of patients being female and young adults. The most common contact allergy was to paraphenylenediamine (PPD), which occurred in 22 (76%) patients.1 In this article, we will walk you through the rainbow of tattoo ACD, covering hypersensitivity reactions to both temporary and permanent tattoo inks.
Temporary Tattoo Inks
Henna is the most common temporary tattoo ink. Derived from the plant Lawsonia inermis, henna is an orange dye that has been used in many parts of the world, particularly in Islamic and Hindu cultures, to dye skin, hair, and fabrics. Application of henna tattoos is common for weddings and other celebrations, and brides may wear elaborate henna patterns. To create these tattoos, henna powder is mixed with water and sometimes essential oils and is then applied to the skin for several hours. After application, the henna pigment lawsone (2-hydroxy-1,4-naphthoquinone) interacts with keratin and leaves a red-orange stain on the skin2; longer application time leads to a deeper color. Most traditional cutaneous henna designs fade in 2 to 6 weeks, but some last longer. Red henna generally is considered safe with low incidence of contact allergy. What is referred to as black henna usually is red henna mixed with PPD, a black dye, which is added to deepen the color. Paraphenylenediamine is highly sensitizing; patients can become sensitized to the PPD in the tattoo itself.2 One study confirmed the presence of PPD in black henna tattoos, with chemical analysis of common preparations revealing concentrations ranging from less than 1% to 30%.2 Patients who undergo patch testing for tattoo reactions often are strongly positive to PPD and have concomitant reactions to azo dyes, black rubber, and anesthetics. Other aromatic amines including aminophenols have been identified in black henna tattoo ink, and these chemicals also may contribute to ACD.3 Less common sources of contact allergy from temporary black henna tattoos include resorcinol,4 para-tertiary butylphenol formaldehyde resin,5 and fragrance.6
Clinically, ACD to PPD in temporary tattoos presents 1 to 3 days after application if the patient is already sensitized or 4 to 14 days if the patient is sensitized by the tattoo ink.2 Most patients notice erythema, edema, vesicles, papules, and/or bullae, but other less common reactions including generalized dermatitis, systemic symptoms, urticaria, and pustules have been described.2 Postinflammatory hypopigmentation or hyperpigmentation also can occur.
Because of the sensitizing nature of black henna tattoos, consumers are turning to natural temporary tattoos. Jagua temporary tattoos, with pigment derived from the sap of fruit from the Genipa americana tree, have been associated with ACD.7 This black dye is applied and washed off in a similar fashion to henna tattoos. Importantly, a recent analysis of jagua dye identified no PPD. In one case, a patient who developed ACD to a jagua tattoo was patch tested to components of the dye and had a positive reaction to genipin, a component of the fruit extract.7 Thus, jagua tattoos often are marketed as safe but are an emerging source of contact dermatitis to temporary tattoos.
Permanent Tattoo Inks
Permanent tattoos are created by injecting small amounts of ink into the dermis. As the name suggests, these tattoos are permanent. Tattoos are common; nearly one-third of Americans have at least 1 tattoo.1 Historically, tattoos were created using black pigment composed of amorphous carbon or black iron oxides.8,9 Metallic pigments (eg, mercury, chromium, cobalt, cadmium) were once used to add color to tattoos, but these metals are now only rarely used; in fact, a 2019 study of tattoo ink components identified 44 distinct pigments in 1416 permanent inks, with an average of 3 pigments per ink.8 Of the 44 pigments, 10 had metallic components including iron, barium, zinc, copper, molybdenum, and titanium. The remaining 34 pigments contained carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes. The authors noted that nearly one-quarter of the tattoo pigments identified in their study had been reported as contact allergens.8
Typically, reactions to permanent tattoo inks manifest as an eczematous dermatitis occurring weeks to years after tattoo application.9,10 The dermatitis usually is locally confined to the tattoo and may be limited to particular colors; occasionally, a new tattoo reaction may trigger concurrent inflammation in older tattoos. Many tattoo reactions occur as a response to red pigment but also have occurred with other tattoo ink components.9 Many researchers have speculated as to whether the reaction is related to the ink component itself or from the photochemical breakdown of the ink by exposure to UV radiation and/or laser therapy.9
Red Pigment
Red ink is the most common color reported to cause tattoo hypersensitivity reactions. Historically, red tattoo pigments include mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal,11 but today’s tattoo inks primarily are composed of other pigments, such as quinacridone and azo dyes.12 Several cases of red tattoo ink hypersensitivity reactions exist in the literature, many without completion of patch tests or without positive patch tests to relevant red pigments.11-15
Black Pigment
In general, reactions to permanent black tattoo ink are rare; however, a few case reports exist. Black pigment can be created with India ink (carbon), logwood (chrome), iron oxide, and titanium.16,17 Shellac can be used as a binding agent in tattoo ink; there is at least one report of a reaction to black tattoo ink with a positive patch test to shellac and the original black ink.18
Metals
When utilized in tattoos, metals can create a variety of colors; several have been reported to cause ACD. There has been at least one reported case of a tattoo hypersensitivity reaction to a gold tattoo, with positive patch testing for gold sodium thiosulfate.19 Green tattoo inks also have been confirmed to contain metal. One case of nickel allergy from a green tattoo has been reported, with a positive patch test for nickel sulfate and tissue confirmation of the presence of nickel with micro X-ray fluorescence and laser ablation inductively coupled plasma mass spectrometry.20 Another case series described 3 patients with pruritus and chronic dermatitis associated with green tattoos who had positive patch tests to potassium dichromate, and the green tattoo pigment flared during patch testing. Chromium oxide was presumed to be present in the green tattoo pigment, and potassium dichromate avoidance in products and food improved both the pruritus and dermatitis.21
Azo Pigments
Azo pigments frequently are used in modern tattoos due to their vibrant colors. One case of hypersensitivity to azo pigment involved an eczematous ulcerated plaque overlying yellow, red, and green ink in a recently applied tattoo. Patch testing with the inks originally used in the tattoo was negative. The authors noted that the 3 problematic ink colors all contained pigment yellow 65—an azo pigment—and attributed the reaction to this dye.22 In another azo reaction, a patient had erythema and pruritus overlying a tattoo applied 1 month prior. Patch testing was positive for aminoazobenzene, an azo pigment that was present in the orange ink of the tattoo.23
Management of Tattoo Hypersensitivity Reactions
Hypersensitivity reactions to temporary tattoos are just that—temporary. Topical steroids and time generally will allow these reactions to resolve. In the setting of vigorous reactions, patients may develop postinflammatory hypopigmentation or hyperpigmentation that may last for months. Unfortunately, bullous tattoo reactions can lead to scarring and keloid formation, requiring more aggressive therapy.
Management of reactions to permanent tattoos is more challenging. High-potency topical steroids under occlusion or intralesional corticosteroid injections may aid in treating pruritus or discomfort. For severe reactions, oral corticosteroids may be required. Patients also may consider laser tattoo removal; however, providers should be aware that there have been rare reports of systemic urticarial reactions from this procedure.24,25 Obviously limited by location and size, excision also may be offered.
Patch Testing for Tattoo Ink Contact Allergy
When patients present for evaluation and management of tattoo ACD, it is important to also consider other causes, including granulomatous tattoo reaction, pseudolymphoma, and lichenoid tattoo reaction. A biopsy can be helpful if the diagnosis is in question.
Patch testing for contact allergy to temporary tattoo inks should include PPD, fragrance, aminophenols, resorcinol, para-tertiary butylphenol formaldehyde, and essential oils. Jagua currently is not available for commercial purchase but also should be considered if the patient has the original product or in research settings. If the individual tattoo ingredients can be identified, they also should be tested. In this scenario, recall reactions may occur; testing with the tattoo paste should be avoided if the prior reaction was severe. Importantly, patients with a PPD allergy should be counseled to avoid hair dyes that contain PPD. Many patients who are sensitized to PPD have strong reactions on patch testing and are at risk for severe reactions if PPD or PPD-related compounds are encountered in hair dye.
Patch testing for ACD to permanent tattoos is complex. In most cases, patch testing is of limited utility because many of the chemicals that have been reported to cause ACD are not commercially available. Additionally, a 2014 study of 90 patients with chronic tattoo reactions found that the majority had negative patch testing to the European baseline series (66%), disperse dyes (87%), and tattoo inks (87%–92%). The investigators theorized that the allergens causing tattoo reactions are formed by haptenization of “parent” chemicals in the dermis, meaning application of chemicals present in the original tattoo ink may not identify the relevant allergen.26 If patch testing is performed, it is most ideal if individual pigment ingredients can be identified. Allergens to be considered for testing include azo dyes, aromatic amines, iron oxide, barium, zinc, copper, molybdenum, titanium, gold sodium thiosulfate, nickel sulfate, carbon, shellac, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), quinophthalone (yellow) dyes, mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal, many of which are not commercially available for purchase.
Final Interpretation
As tattoos become increasingly trendy, tattoo ACD should be recognized by the astute dermatologist. The most common allergen associated with tattoo ACD is PPD, but other potential allergens include azo dyes and newer pigments. Unlike tattoos of the past, today’s inks are unlikely to contain toxic metals. Diagnosing ACD caused by permanent tattoo inks requires a high degree of suspicion, as patch testing may be of limited utility.
- Warshaw EM, Schlarbaum JP, Taylor JS, et al. Allergic reactions to tattoos: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. J Am Acad Dermatol. 2020;82:E61-E62.
- de Groot AC. Side-effects of henna and semi-permanent ‘black henna’ tattoos: a full review. Contact Dermatitis. 2013;69:1-25.
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m-aminophenol and toluene-2,5-diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52.
- Ormerod E, Hughes TM, Stone N. Allergic contact dermatitis caused by resorcinol following a temporary black henna tattoo. Contact Dermatitis. 2017;77:187-188.
- Rodrigo-Nicolás B, de la Cuadra J, Sierra C, et al. Contact dermatitis from a temporary tattoo in a boy with contact allergy to p-tert butyl phenol formaldehyde resin. Dermatitis. 2014;25:37-38.
- Temesvári E, Podányi B, Pónyai G, et al. Fragrance sensitization caused by temporary henna tattoo. Contact Dermatitis. 2002;47:240.
- Bircher AJ, Scherer Hofmeier K, Schlegel U, et al. Genipin in temporary jagua tattoos—black dye causing severe allergic dermatitis. Dermatitis. 2019;30:375-376.
- Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379-385.
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82.
- Bjerre RD, Ulrich NH, Linneberg A, et al. Adverse reactions to tattoos in the general population of Denmark. J Am Acad Dermatol. 2018;79:770-772.
- Bhardwaj SS, Brodell RT, Taylor JS. Red tattoo reactions. Contact Dermatitis. 2003;48:236-237.
- Gaudron S, Ferrier-Le Bouëdec MC, Franck F, et al. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97-105.
- de Winter RW, van der Bent SAS, van Esch M, et al. Allergic reaction to red cosmetic lip tattoo treated with hydroxychloroquine. Dermatitis. 2019;30:82-83.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Ruiz-Villaverde R, Fernandez-Crehuet P, Aguayo-Carreras P, et al. Inflammatory reactions to red tattoo inks: three cases highlighting an emerging problem. Sultan Qaboos Univ Med J. 2018;18:E215-E218.
- Gallo R, Parodi A, Cozzani E, et al. Allergic reaction to India ink in a black tattoo. Contact Dermatitis. 1998;38:346-347.
- de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo-related hypersensitivity reactions? a case report. Contact Dermatitis. 2017;77:397-405.
- González-Villanueva I, Hispán Ocete P, Silvestre Salvador JF. Allergic contact dermatitis caused by a black tattoo ink in a patient allergic to shellac. Contact Dermatitis. 2016;75:247-248.
- Tammaro A, Tuchinda P, Persechino S, et al. Contact allergic dermatitis to gold in a tattoo: a case report. Int J Immunopathol Pharmacol. 2011;24:1111-1113.
- van der Bent SAS, Berg T, Karst U, et al. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermatitis. 2019;81:64-66.
- Jacob SE, Castanedo-Tardan MP, Blyumin ML. Inflammation in green (chromium) tattoos during patch testing. Dermatitis. 2008;19:E33-E34.
- González-Villanueva I, Álvarez-Chinchilla P, Silvestre JF. Allergic reaction to 3 tattoo inks containing pigment yellow 65. Contact Dermatitis. 2018;79:107-108.
- Tammaro A, De Marco G, D’Arino A, et al. Aminoazobenzene in tattoo: another case of allergic contact dermatitis. Int J Dermatol. 2017;56:E79-E81.
- Willardson HB, Kobayashi TT, Arnold JG, et al. Diffuse urticarial reaction associated with titanium dioxide following laser tattoo removal treatments. Photomed Laser Surg. 2017;35:176‐180.
- England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215‐217.
- Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
- Warshaw EM, Schlarbaum JP, Taylor JS, et al. Allergic reactions to tattoos: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. J Am Acad Dermatol. 2020;82:E61-E62.
- de Groot AC. Side-effects of henna and semi-permanent ‘black henna’ tattoos: a full review. Contact Dermatitis. 2013;69:1-25.
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m-aminophenol and toluene-2,5-diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52.
- Ormerod E, Hughes TM, Stone N. Allergic contact dermatitis caused by resorcinol following a temporary black henna tattoo. Contact Dermatitis. 2017;77:187-188.
- Rodrigo-Nicolás B, de la Cuadra J, Sierra C, et al. Contact dermatitis from a temporary tattoo in a boy with contact allergy to p-tert butyl phenol formaldehyde resin. Dermatitis. 2014;25:37-38.
- Temesvári E, Podányi B, Pónyai G, et al. Fragrance sensitization caused by temporary henna tattoo. Contact Dermatitis. 2002;47:240.
- Bircher AJ, Scherer Hofmeier K, Schlegel U, et al. Genipin in temporary jagua tattoos—black dye causing severe allergic dermatitis. Dermatitis. 2019;30:375-376.
- Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379-385.
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82.
- Bjerre RD, Ulrich NH, Linneberg A, et al. Adverse reactions to tattoos in the general population of Denmark. J Am Acad Dermatol. 2018;79:770-772.
- Bhardwaj SS, Brodell RT, Taylor JS. Red tattoo reactions. Contact Dermatitis. 2003;48:236-237.
- Gaudron S, Ferrier-Le Bouëdec MC, Franck F, et al. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97-105.
- de Winter RW, van der Bent SAS, van Esch M, et al. Allergic reaction to red cosmetic lip tattoo treated with hydroxychloroquine. Dermatitis. 2019;30:82-83.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Ruiz-Villaverde R, Fernandez-Crehuet P, Aguayo-Carreras P, et al. Inflammatory reactions to red tattoo inks: three cases highlighting an emerging problem. Sultan Qaboos Univ Med J. 2018;18:E215-E218.
- Gallo R, Parodi A, Cozzani E, et al. Allergic reaction to India ink in a black tattoo. Contact Dermatitis. 1998;38:346-347.
- de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo-related hypersensitivity reactions? a case report. Contact Dermatitis. 2017;77:397-405.
- González-Villanueva I, Hispán Ocete P, Silvestre Salvador JF. Allergic contact dermatitis caused by a black tattoo ink in a patient allergic to shellac. Contact Dermatitis. 2016;75:247-248.
- Tammaro A, Tuchinda P, Persechino S, et al. Contact allergic dermatitis to gold in a tattoo: a case report. Int J Immunopathol Pharmacol. 2011;24:1111-1113.
- van der Bent SAS, Berg T, Karst U, et al. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermatitis. 2019;81:64-66.
- Jacob SE, Castanedo-Tardan MP, Blyumin ML. Inflammation in green (chromium) tattoos during patch testing. Dermatitis. 2008;19:E33-E34.
- González-Villanueva I, Álvarez-Chinchilla P, Silvestre JF. Allergic reaction to 3 tattoo inks containing pigment yellow 65. Contact Dermatitis. 2018;79:107-108.
- Tammaro A, De Marco G, D’Arino A, et al. Aminoazobenzene in tattoo: another case of allergic contact dermatitis. Int J Dermatol. 2017;56:E79-E81.
- Willardson HB, Kobayashi TT, Arnold JG, et al. Diffuse urticarial reaction associated with titanium dioxide following laser tattoo removal treatments. Photomed Laser Surg. 2017;35:176‐180.
- England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215‐217.
- Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
Practice Points
- Temporary tattoo pigments include red henna, black henna, and jagua.
- Black henna tattoos contain paraphenylenediamine, the most common allergen in temporary tattoos.
- Modern permanent tattoo ink components include metals, carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes.
- Patch testing for tattoo contact allergy is complex and challenging.