User login
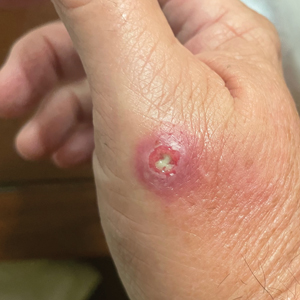
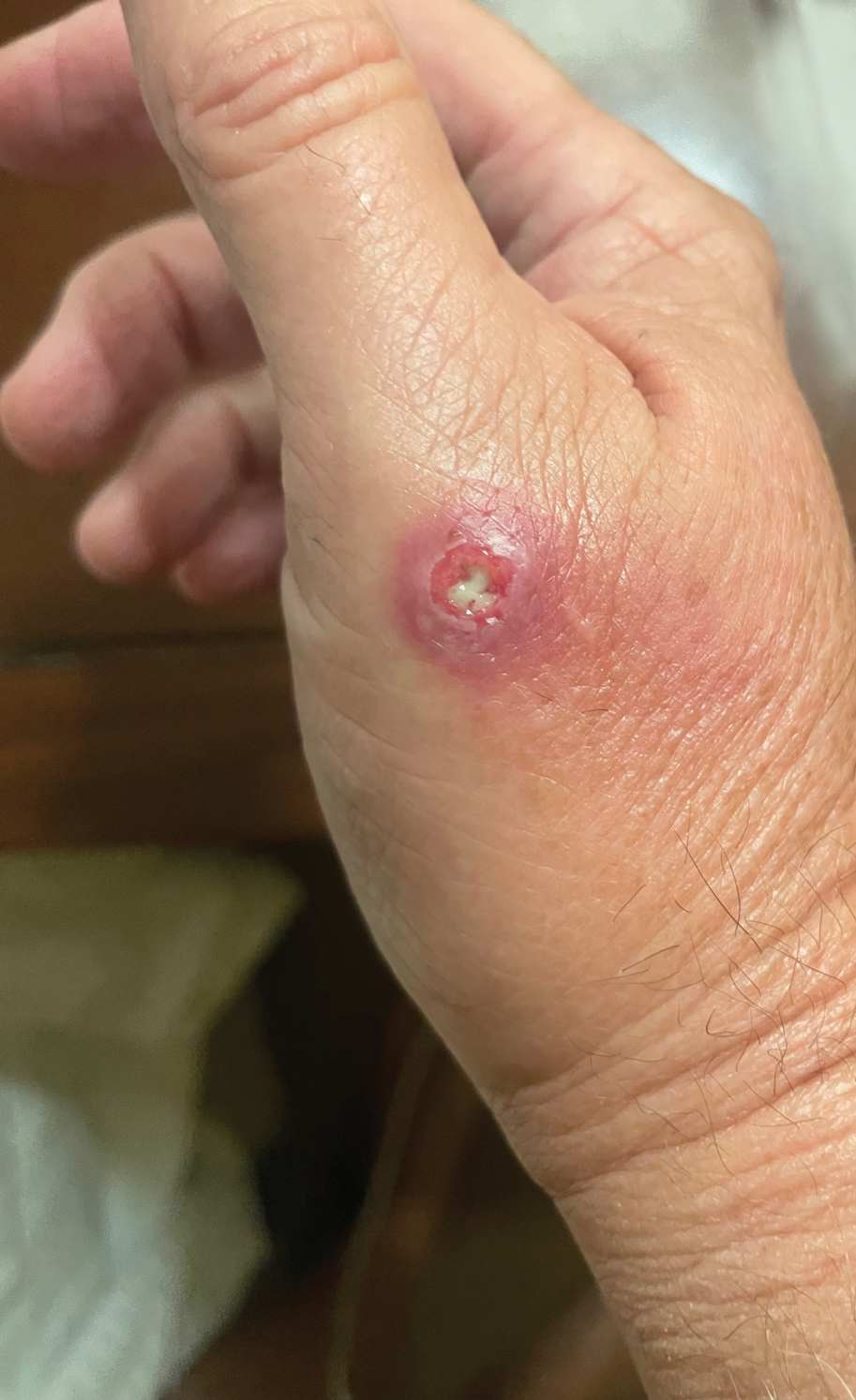
Draining Nodule of the Hand
The Diagnosis: Cutaneous Nocardiosis
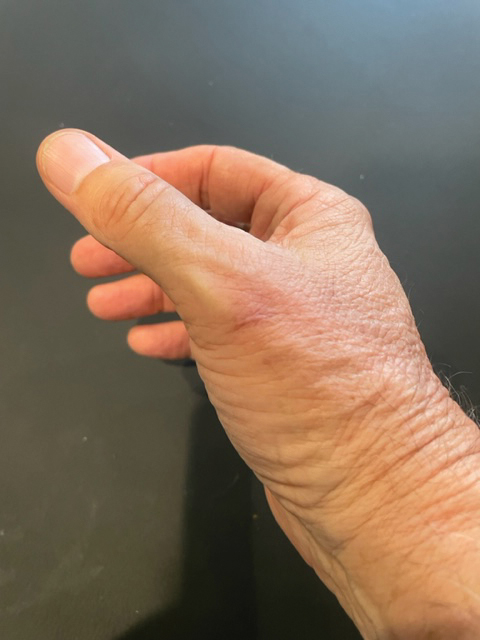
The wound culture was positive for Nocardia farcinica. The patient received a 5-day course of intravenous sulfamethoxazole-trimethoprim in the hospital and was transitioned to oral sulfamethoxazoletrimethoprim (800 mg/160 mg taken as 1 tablet twice daily) for 6 months. Complete resolution of the infection was noted at 6-month follow-up (Figure).
Nocardia is a gram-positive, aerobic bacterium that typically is found in soil, water, and decaying organic matter.1 There are more than 50 species; N farcinica, Nocardia nova, and Nocardia asteroides are the leading causes of infection in humans and animals. Nocardia asteroides is the most common cause of infection in humans.1,2 Nocardiosis is an uncommon opportunistic infection that usually targets the skin, lungs, and central nervous system.3 Although it mainly affects individuals who are immunocompromised, up to 30% of infections can be seen in immunocompetent hosts who can contract cutaneous nocardiosis after experiencing traumatic injury to the skin.1
Nocardiosis is difficult to diagnose due to its diverse clinical presentations. For example, cutaneous nocardiosis can manifest similar to mycetoma, sporotrichosis, spider bites, nontuberculous mycobacteria such as Mycobacterium marinum, or methicillin-resistant Staphylococcus aureus infections, thus making cutaneous nocardiosis one of the great imitators.1 A culture is required for definitive diagnosis, as Nocardia grows well on nonselective media such as blood or Löwenstein-Jensen agar. It grows as waxy, pigmented, cerebriform colonies 3 to 5 days following incubation.3 The bacterium can be difficult to culture, and it is important to notify the microbiology laboratory if there is a high index of clinical suspicion for infection.
A history of exposure to gardening or handling animals can increase the risk for an individual contracting Nocardia.3 Although nocardiosis can be found across the world, it is native to tropical and subtropical climates such as those found in India, Africa, Latin America, and Southeast Asia.1 Infections mostly are observed in individuals aged 20 to 40 years and tend to affect men more than women. Lesions typically are seen on the lower extremities, but localized infections also can be found on the torso, neck, and upper extremities.1
Cutaneous nocardiosis is a granulomatous infection encompassing both cutaneous and subcutaneous tissue, which ultimately can lead to injury of bone and viscera.1 Primary cutaneous nocardiosis can manifest as tumors or nodules that have a sporotrichoid pattern, in which they ascend along the lymphatics. Histopathology of infected tissue frequently shows a subcutaneous dermal infiltrate of neutrophils accompanied with abscess formation, and everlasting lesions may show signs of chronic inflammation and nonspecific granulomas.3
Treatment of nocardiosis should be guided by in vitro susceptibility tests. Sulfamethoxazole-trimethoprim 800 mg/160 mg taken as 1 tablet twice daily is the first-line option. The treatment duration is contingent on the extent, severity, and complications of infection but typically is 3 to 6 months.1
- Yu Q, Song J, Liu Y, et al. Progressive primary cutaneous nocardiosis in an immunocompetent patient. Cutis. 2023;111:E22-E25.
- Gaines RJ, Randall CJ, Ruland RT. Lymphocutaneous nocardiosis from commercially treated lumber: a case report. Cutis. 2006;78:249-251.
- Riswold KJ, Tjarks BJ, Kerkvliet AM. Cutaneous nocardiosis in an immunocompromised patient. Cutis. 2019;104:226-229.
The Diagnosis: Cutaneous Nocardiosis
The wound culture was positive for Nocardia farcinica. The patient received a 5-day course of intravenous sulfamethoxazole-trimethoprim in the hospital and was transitioned to oral sulfamethoxazoletrimethoprim (800 mg/160 mg taken as 1 tablet twice daily) for 6 months. Complete resolution of the infection was noted at 6-month follow-up (Figure).
Nocardia is a gram-positive, aerobic bacterium that typically is found in soil, water, and decaying organic matter.1 There are more than 50 species; N farcinica, Nocardia nova, and Nocardia asteroides are the leading causes of infection in humans and animals. Nocardia asteroides is the most common cause of infection in humans.1,2 Nocardiosis is an uncommon opportunistic infection that usually targets the skin, lungs, and central nervous system.3 Although it mainly affects individuals who are immunocompromised, up to 30% of infections can be seen in immunocompetent hosts who can contract cutaneous nocardiosis after experiencing traumatic injury to the skin.1
Nocardiosis is difficult to diagnose due to its diverse clinical presentations. For example, cutaneous nocardiosis can manifest similar to mycetoma, sporotrichosis, spider bites, nontuberculous mycobacteria such as Mycobacterium marinum, or methicillin-resistant Staphylococcus aureus infections, thus making cutaneous nocardiosis one of the great imitators.1 A culture is required for definitive diagnosis, as Nocardia grows well on nonselective media such as blood or Löwenstein-Jensen agar. It grows as waxy, pigmented, cerebriform colonies 3 to 5 days following incubation.3 The bacterium can be difficult to culture, and it is important to notify the microbiology laboratory if there is a high index of clinical suspicion for infection.
A history of exposure to gardening or handling animals can increase the risk for an individual contracting Nocardia.3 Although nocardiosis can be found across the world, it is native to tropical and subtropical climates such as those found in India, Africa, Latin America, and Southeast Asia.1 Infections mostly are observed in individuals aged 20 to 40 years and tend to affect men more than women. Lesions typically are seen on the lower extremities, but localized infections also can be found on the torso, neck, and upper extremities.1

Cutaneous nocardiosis is a granulomatous infection encompassing both cutaneous and subcutaneous tissue, which ultimately can lead to injury of bone and viscera.1 Primary cutaneous nocardiosis can manifest as tumors or nodules that have a sporotrichoid pattern, in which they ascend along the lymphatics. Histopathology of infected tissue frequently shows a subcutaneous dermal infiltrate of neutrophils accompanied with abscess formation, and everlasting lesions may show signs of chronic inflammation and nonspecific granulomas.3
Treatment of nocardiosis should be guided by in vitro susceptibility tests. Sulfamethoxazole-trimethoprim 800 mg/160 mg taken as 1 tablet twice daily is the first-line option. The treatment duration is contingent on the extent, severity, and complications of infection but typically is 3 to 6 months.1
The Diagnosis: Cutaneous Nocardiosis
The wound culture was positive for Nocardia farcinica. The patient received a 5-day course of intravenous sulfamethoxazole-trimethoprim in the hospital and was transitioned to oral sulfamethoxazoletrimethoprim (800 mg/160 mg taken as 1 tablet twice daily) for 6 months. Complete resolution of the infection was noted at 6-month follow-up (Figure).
Nocardia is a gram-positive, aerobic bacterium that typically is found in soil, water, and decaying organic matter.1 There are more than 50 species; N farcinica, Nocardia nova, and Nocardia asteroides are the leading causes of infection in humans and animals. Nocardia asteroides is the most common cause of infection in humans.1,2 Nocardiosis is an uncommon opportunistic infection that usually targets the skin, lungs, and central nervous system.3 Although it mainly affects individuals who are immunocompromised, up to 30% of infections can be seen in immunocompetent hosts who can contract cutaneous nocardiosis after experiencing traumatic injury to the skin.1
Nocardiosis is difficult to diagnose due to its diverse clinical presentations. For example, cutaneous nocardiosis can manifest similar to mycetoma, sporotrichosis, spider bites, nontuberculous mycobacteria such as Mycobacterium marinum, or methicillin-resistant Staphylococcus aureus infections, thus making cutaneous nocardiosis one of the great imitators.1 A culture is required for definitive diagnosis, as Nocardia grows well on nonselective media such as blood or Löwenstein-Jensen agar. It grows as waxy, pigmented, cerebriform colonies 3 to 5 days following incubation.3 The bacterium can be difficult to culture, and it is important to notify the microbiology laboratory if there is a high index of clinical suspicion for infection.
A history of exposure to gardening or handling animals can increase the risk for an individual contracting Nocardia.3 Although nocardiosis can be found across the world, it is native to tropical and subtropical climates such as those found in India, Africa, Latin America, and Southeast Asia.1 Infections mostly are observed in individuals aged 20 to 40 years and tend to affect men more than women. Lesions typically are seen on the lower extremities, but localized infections also can be found on the torso, neck, and upper extremities.1

Cutaneous nocardiosis is a granulomatous infection encompassing both cutaneous and subcutaneous tissue, which ultimately can lead to injury of bone and viscera.1 Primary cutaneous nocardiosis can manifest as tumors or nodules that have a sporotrichoid pattern, in which they ascend along the lymphatics. Histopathology of infected tissue frequently shows a subcutaneous dermal infiltrate of neutrophils accompanied with abscess formation, and everlasting lesions may show signs of chronic inflammation and nonspecific granulomas.3
Treatment of nocardiosis should be guided by in vitro susceptibility tests. Sulfamethoxazole-trimethoprim 800 mg/160 mg taken as 1 tablet twice daily is the first-line option. The treatment duration is contingent on the extent, severity, and complications of infection but typically is 3 to 6 months.1
- Yu Q, Song J, Liu Y, et al. Progressive primary cutaneous nocardiosis in an immunocompetent patient. Cutis. 2023;111:E22-E25.
- Gaines RJ, Randall CJ, Ruland RT. Lymphocutaneous nocardiosis from commercially treated lumber: a case report. Cutis. 2006;78:249-251.
- Riswold KJ, Tjarks BJ, Kerkvliet AM. Cutaneous nocardiosis in an immunocompromised patient. Cutis. 2019;104:226-229.
- Yu Q, Song J, Liu Y, et al. Progressive primary cutaneous nocardiosis in an immunocompetent patient. Cutis. 2023;111:E22-E25.
- Gaines RJ, Randall CJ, Ruland RT. Lymphocutaneous nocardiosis from commercially treated lumber: a case report. Cutis. 2006;78:249-251.
- Riswold KJ, Tjarks BJ, Kerkvliet AM. Cutaneous nocardiosis in an immunocompromised patient. Cutis. 2019;104:226-229.
A 67-year-old man presented to the emergency department with a draining nodule on the right hand of 4 days’ duration. He reported that the swelling and redness started 1 hour after handling a succulent plant. The following day, the nodule increased in size and exudated yellow pus. He presented with swelling of the thumb and hand, which resulted in a decreased range of motion. He had a history of prediabetes and denied any recent travel, allergies, or animal exposures. Physical examination revealed a draining nodule on the dorsal aspect of the right hand that measured approximately 15×15 mm with surrounding erythema and tenderness. There also was progression of ascending erythema up to the axilla. The patient was admitted to the hospital.
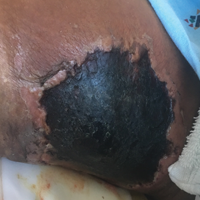
Necrotic Ulcer on the Thigh
The Diagnosis: Disseminated Cryptococcosis
Histopathologic examination of a 3-mm punch biopsy showed a diffuse dermal neutrophilic infiltrate with necrosis and subcutaneous tissue with round yeast surrounded by a prominent halo staining bright red with mucicarmine, representing a thick mucinous capsule (Figure). Grocott-Gomori methenamine-silver and periodic acid-Schiff stains also demonstrated fungal spores morphologically. Cerebrospinal fluid culture grew Cryptococcus neoformans, and cryptococcal antigen titers were positive in both serum and cerebrospinal fluid samples (>1:4096). The patient had autolytic debridement of the ulcer after completing a 4-week induction course of intravenous liposomal amphotericin B with oral flucytosine. He was transitioned to oral fluconazole for the consolidation phase of treatment.
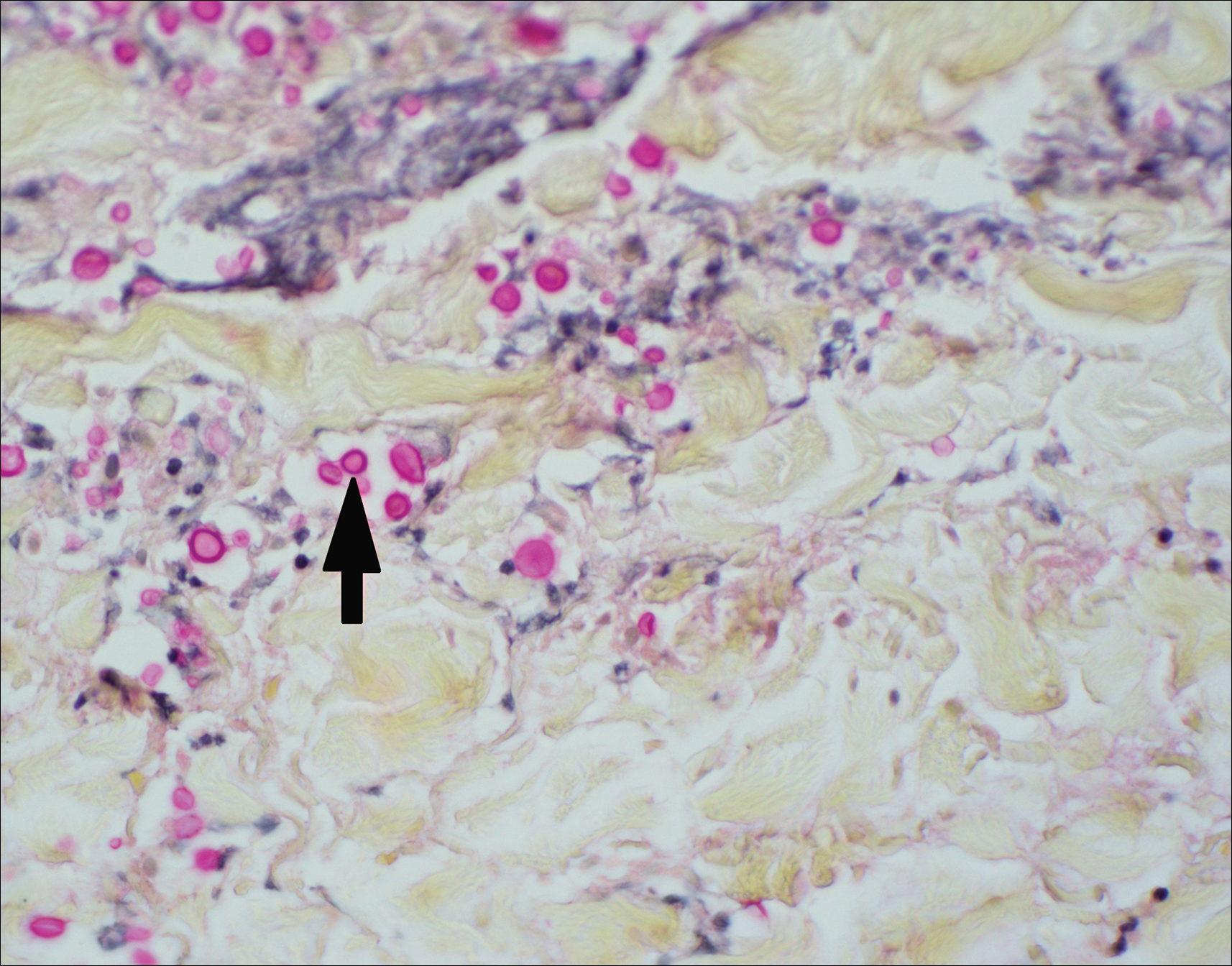
Cryptococcus is an opportunistic basidiomycetous yeast with worldwide distribution and 2 primary pathogenic species in humans: C neoformans and Cryptococcus gattii. It is associated with bird feces, composted food, and decayed wood.1,2 A predilection toward an immunosuppressed host is recognized in 70% to 90% of the infections caused by C neoformans; however, C gattii commonly affects individuals with apparently intact immune systems.1,3 Risk factors for infection include advanced human immunodeficiency virus infection, solid organ transplantation, chronic liver disease, autoimmune disease, hematological malignancy, and underlying genetic susceptibility.1,2
Initial exposure is through the respiratory tract with formation of latent reservoirs in the pulmonary lymph nodes with subsequent reactivation that can result in hematogenous dissemination.1,2 Cutaneous involvement was described in 108 patients (5%) in a large review of 1974 cases in France.4 Among those with cutaneous involvement, disseminated disease was diagnosed in 80 cases (74%), and 28 cases (26%) were considered primary cutaneous cryptococcosis. Primary cutaneous cryptococcosis typically presents as a single lesion, predominantly on the hand, with whitlow and more rarely with extensive cellulitis or necrotizing fasciitis.4 In disseminated cutaneous disease, there is no pathognomonic single lesion; however, it is commonly associated with multiple cutaneous lesions predominantly involving the head and neck. Plaques, abscesses, nodules, and pustular or umbilicated papules have been reported.1,5 There are few case reports that describe a single isolated necrotic ulcer with disseminated disease similar to our presented case, and more typically the necrotic ulcer is seen in transplanted patients.6 The differential diagnosis of a necrotic thigh ulcer includes pseudomonal ecthyma gangrenosum, cutaneous anthrax and aspergillosis, fusariosis, and a bite from the brown recluse spider.7 Our patient had an increased susceptibility to infection from his ongoing chemotherapy, a risk previously described in oncology patients with cell-mediated immunosuppression.8
Management for disseminated cryptococcosis is a 3-phase therapy including induction with intravenous amphotericin B and oral flucytosine for a minimum of 2 weeks, with consolidation and maintenance phases both with oral fluconazole for a length depending on underlying immunosuppression.9
- Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev. 2014;27:980-1024.
- Williamson PR, Jarvis JN, Panackal AA, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis, and therapy [published online November 25, 2016]. Nat Rev Neurol. 2017;13:13-24.
- Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21:28-34.
- Neuville S, Dromer F, Morin O, et al; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity [published online January 17, 2003]. Clin Infect Dis. 2003;36:337-347.
- Murakawa GJ, Kerschmann R, Berger T. Cutaneous cryptococcus infection and AIDS: report of 12 cases and review of the literature. JAMA Dermatol. 1996;132:545-548.
- Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
- Grossman ME, Fox LP, Kovarik C, et al. Cutaneous Manifestations of Infection in the Immunocompromised Host. Baltimore, MD: Williams & Wilkins; 2012.
- Korfel A, Menssen HD, Schwartz S, et al. Cryptococcosis in Hodgkin's disease: description of two cases and review of the literature. Ann Hematol. 1998;76:283-286.
- Perfect JR, Dismukes WE, Dromer F. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291-322.
The Diagnosis: Disseminated Cryptococcosis
Histopathologic examination of a 3-mm punch biopsy showed a diffuse dermal neutrophilic infiltrate with necrosis and subcutaneous tissue with round yeast surrounded by a prominent halo staining bright red with mucicarmine, representing a thick mucinous capsule (Figure). Grocott-Gomori methenamine-silver and periodic acid-Schiff stains also demonstrated fungal spores morphologically. Cerebrospinal fluid culture grew Cryptococcus neoformans, and cryptococcal antigen titers were positive in both serum and cerebrospinal fluid samples (>1:4096). The patient had autolytic debridement of the ulcer after completing a 4-week induction course of intravenous liposomal amphotericin B with oral flucytosine. He was transitioned to oral fluconazole for the consolidation phase of treatment.

Cryptococcus is an opportunistic basidiomycetous yeast with worldwide distribution and 2 primary pathogenic species in humans: C neoformans and Cryptococcus gattii. It is associated with bird feces, composted food, and decayed wood.1,2 A predilection toward an immunosuppressed host is recognized in 70% to 90% of the infections caused by C neoformans; however, C gattii commonly affects individuals with apparently intact immune systems.1,3 Risk factors for infection include advanced human immunodeficiency virus infection, solid organ transplantation, chronic liver disease, autoimmune disease, hematological malignancy, and underlying genetic susceptibility.1,2
Initial exposure is through the respiratory tract with formation of latent reservoirs in the pulmonary lymph nodes with subsequent reactivation that can result in hematogenous dissemination.1,2 Cutaneous involvement was described in 108 patients (5%) in a large review of 1974 cases in France.4 Among those with cutaneous involvement, disseminated disease was diagnosed in 80 cases (74%), and 28 cases (26%) were considered primary cutaneous cryptococcosis. Primary cutaneous cryptococcosis typically presents as a single lesion, predominantly on the hand, with whitlow and more rarely with extensive cellulitis or necrotizing fasciitis.4 In disseminated cutaneous disease, there is no pathognomonic single lesion; however, it is commonly associated with multiple cutaneous lesions predominantly involving the head and neck. Plaques, abscesses, nodules, and pustular or umbilicated papules have been reported.1,5 There are few case reports that describe a single isolated necrotic ulcer with disseminated disease similar to our presented case, and more typically the necrotic ulcer is seen in transplanted patients.6 The differential diagnosis of a necrotic thigh ulcer includes pseudomonal ecthyma gangrenosum, cutaneous anthrax and aspergillosis, fusariosis, and a bite from the brown recluse spider.7 Our patient had an increased susceptibility to infection from his ongoing chemotherapy, a risk previously described in oncology patients with cell-mediated immunosuppression.8
Management for disseminated cryptococcosis is a 3-phase therapy including induction with intravenous amphotericin B and oral flucytosine for a minimum of 2 weeks, with consolidation and maintenance phases both with oral fluconazole for a length depending on underlying immunosuppression.9
The Diagnosis: Disseminated Cryptococcosis
Histopathologic examination of a 3-mm punch biopsy showed a diffuse dermal neutrophilic infiltrate with necrosis and subcutaneous tissue with round yeast surrounded by a prominent halo staining bright red with mucicarmine, representing a thick mucinous capsule (Figure). Grocott-Gomori methenamine-silver and periodic acid-Schiff stains also demonstrated fungal spores morphologically. Cerebrospinal fluid culture grew Cryptococcus neoformans, and cryptococcal antigen titers were positive in both serum and cerebrospinal fluid samples (>1:4096). The patient had autolytic debridement of the ulcer after completing a 4-week induction course of intravenous liposomal amphotericin B with oral flucytosine. He was transitioned to oral fluconazole for the consolidation phase of treatment.

Cryptococcus is an opportunistic basidiomycetous yeast with worldwide distribution and 2 primary pathogenic species in humans: C neoformans and Cryptococcus gattii. It is associated with bird feces, composted food, and decayed wood.1,2 A predilection toward an immunosuppressed host is recognized in 70% to 90% of the infections caused by C neoformans; however, C gattii commonly affects individuals with apparently intact immune systems.1,3 Risk factors for infection include advanced human immunodeficiency virus infection, solid organ transplantation, chronic liver disease, autoimmune disease, hematological malignancy, and underlying genetic susceptibility.1,2
Initial exposure is through the respiratory tract with formation of latent reservoirs in the pulmonary lymph nodes with subsequent reactivation that can result in hematogenous dissemination.1,2 Cutaneous involvement was described in 108 patients (5%) in a large review of 1974 cases in France.4 Among those with cutaneous involvement, disseminated disease was diagnosed in 80 cases (74%), and 28 cases (26%) were considered primary cutaneous cryptococcosis. Primary cutaneous cryptococcosis typically presents as a single lesion, predominantly on the hand, with whitlow and more rarely with extensive cellulitis or necrotizing fasciitis.4 In disseminated cutaneous disease, there is no pathognomonic single lesion; however, it is commonly associated with multiple cutaneous lesions predominantly involving the head and neck. Plaques, abscesses, nodules, and pustular or umbilicated papules have been reported.1,5 There are few case reports that describe a single isolated necrotic ulcer with disseminated disease similar to our presented case, and more typically the necrotic ulcer is seen in transplanted patients.6 The differential diagnosis of a necrotic thigh ulcer includes pseudomonal ecthyma gangrenosum, cutaneous anthrax and aspergillosis, fusariosis, and a bite from the brown recluse spider.7 Our patient had an increased susceptibility to infection from his ongoing chemotherapy, a risk previously described in oncology patients with cell-mediated immunosuppression.8
Management for disseminated cryptococcosis is a 3-phase therapy including induction with intravenous amphotericin B and oral flucytosine for a minimum of 2 weeks, with consolidation and maintenance phases both with oral fluconazole for a length depending on underlying immunosuppression.9
- Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev. 2014;27:980-1024.
- Williamson PR, Jarvis JN, Panackal AA, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis, and therapy [published online November 25, 2016]. Nat Rev Neurol. 2017;13:13-24.
- Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21:28-34.
- Neuville S, Dromer F, Morin O, et al; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity [published online January 17, 2003]. Clin Infect Dis. 2003;36:337-347.
- Murakawa GJ, Kerschmann R, Berger T. Cutaneous cryptococcus infection and AIDS: report of 12 cases and review of the literature. JAMA Dermatol. 1996;132:545-548.
- Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
- Grossman ME, Fox LP, Kovarik C, et al. Cutaneous Manifestations of Infection in the Immunocompromised Host. Baltimore, MD: Williams & Wilkins; 2012.
- Korfel A, Menssen HD, Schwartz S, et al. Cryptococcosis in Hodgkin's disease: description of two cases and review of the literature. Ann Hematol. 1998;76:283-286.
- Perfect JR, Dismukes WE, Dromer F. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291-322.
- Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev. 2014;27:980-1024.
- Williamson PR, Jarvis JN, Panackal AA, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis, and therapy [published online November 25, 2016]. Nat Rev Neurol. 2017;13:13-24.
- Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21:28-34.
- Neuville S, Dromer F, Morin O, et al; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity [published online January 17, 2003]. Clin Infect Dis. 2003;36:337-347.
- Murakawa GJ, Kerschmann R, Berger T. Cutaneous cryptococcus infection and AIDS: report of 12 cases and review of the literature. JAMA Dermatol. 1996;132:545-548.
- Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
- Grossman ME, Fox LP, Kovarik C, et al. Cutaneous Manifestations of Infection in the Immunocompromised Host. Baltimore, MD: Williams & Wilkins; 2012.
- Korfel A, Menssen HD, Schwartz S, et al. Cryptococcosis in Hodgkin's disease: description of two cases and review of the literature. Ann Hematol. 1998;76:283-286.
- Perfect JR, Dismukes WE, Dromer F. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291-322.
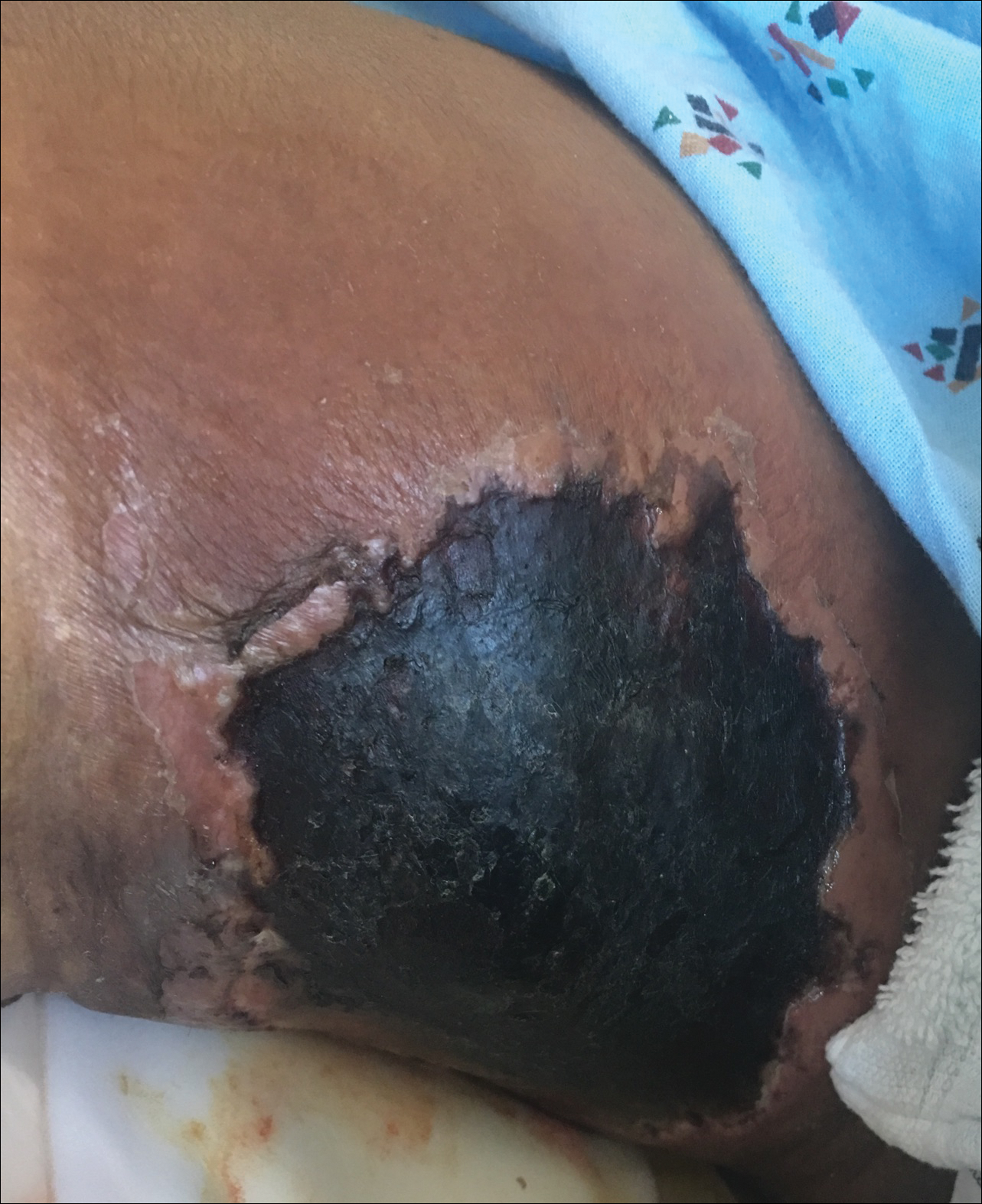
A 29-year-old man with a history of acute lymphoblastic leukemia was admitted for acute encephalopathy and a necrotic ulcer on the right thigh of 2 weeks' duration. He had received chemotherapy with pegaspargase and vincristine 6 weeks prior to admission. He reported headache with nausea and vomiting of 2 weeks' duration and had sustained a fall in the bathtub a week prior that initially resulted in a right thigh abrasion. He denied recent travel, unusual food consumption, animal exposure, exposure to sick persons, and alcohol or other drug use. On examination the patient was alert but was not oriented to person, place, or time. A 10.2 ×10-cm necrotic ulcer with surrounding mild erythema and tenderness was noted on the right inner thigh.