User login
Disparities in Melanoma Demographics, Tumor Stage, and Metastases in Hispanic and Latino Patients: A Retrospective Study
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.
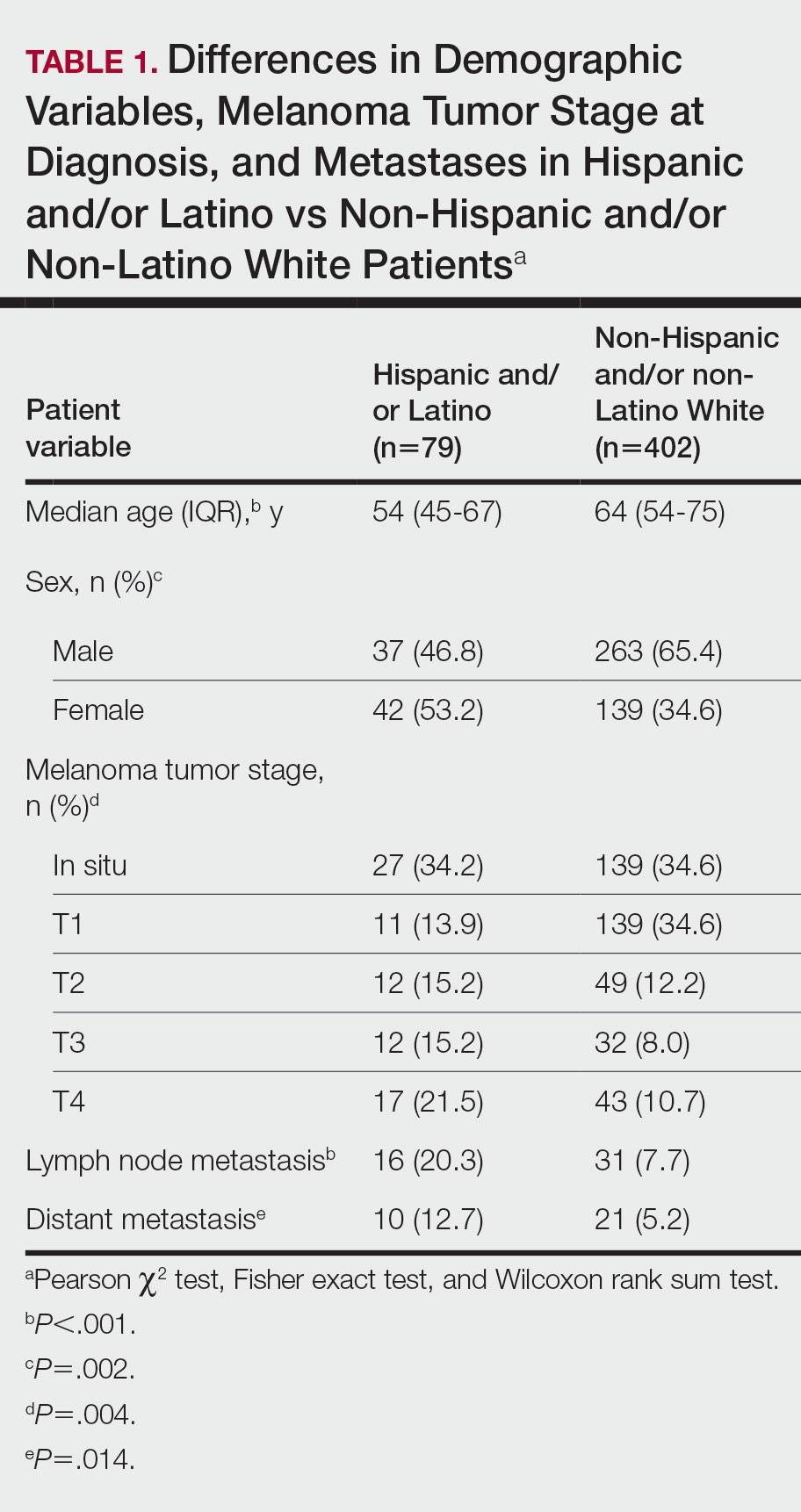
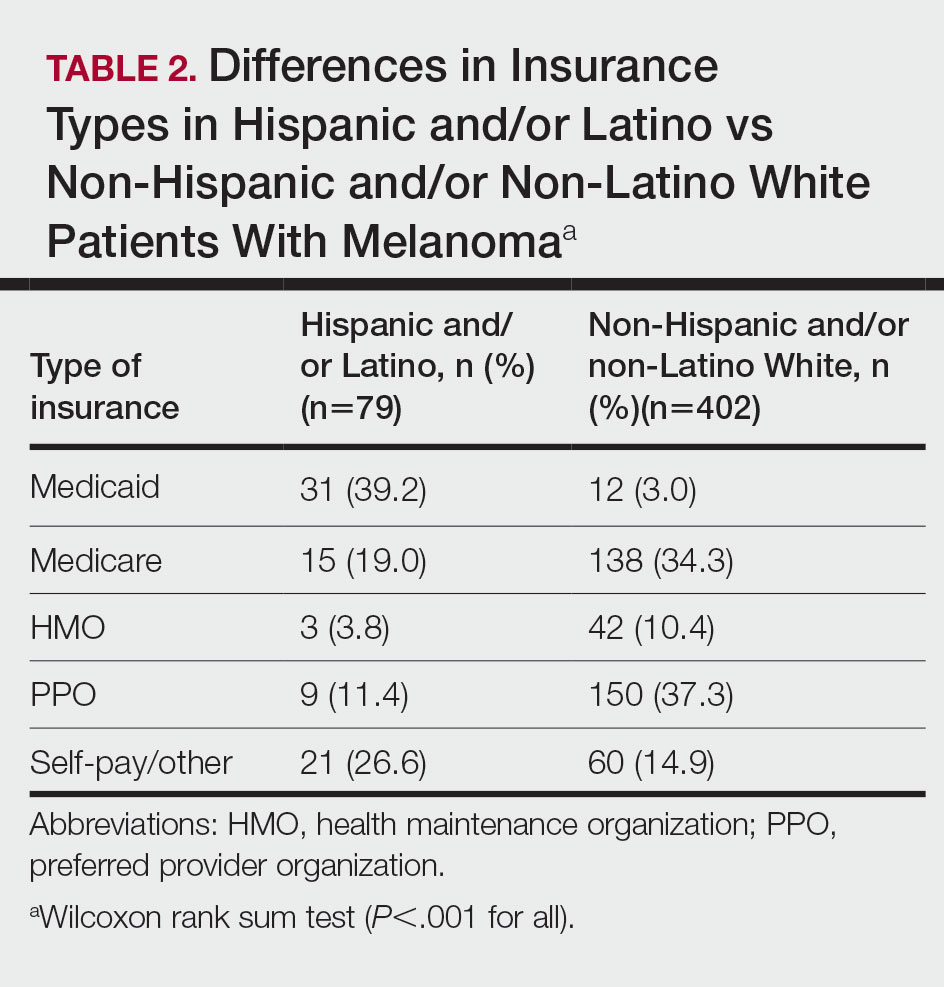
The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).
This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.

The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).

This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.

The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).

This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
Practice Points
- Hispanic and/or Latino patients often present with more advanced-stage melanomas and have decreased survival rates compared with non-Hispanic and/or non-Latino White patients.
- More education and awareness on the risk for melanoma as well as sun-protective behaviors in the Hispanic and/or Latino population is needed among both health care providers and patients to prevent diagnosis of melanoma in later stages and improve outcomes.
Field Cancerization in Dermatology: Updates on Treatment Considerations and Emerging Therapies
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
Combination Therapy With Fillers for Facial Rejuvenation
Dr. Hu discusses fillers and laser or light therapies used in combination for facial rejuvenation. For more information, read Dr. Hu's article in the April 2012 issue, "Pearls on Fillers and Combination Cosmetic Therapy."
Dr. Hu discusses fillers and laser or light therapies used in combination for facial rejuvenation. For more information, read Dr. Hu's article in the April 2012 issue, "Pearls on Fillers and Combination Cosmetic Therapy."
Dr. Hu discusses fillers and laser or light therapies used in combination for facial rejuvenation. For more information, read Dr. Hu's article in the April 2012 issue, "Pearls on Fillers and Combination Cosmetic Therapy."

