User login
Rules of Engagement
Peripheral arterial disease (PAD) is defined by the presence of stenosis or occlusion in peripheral arterial beds.1, 2 Based on large population‐based screening surveys, the prevalence of this disease ranges between 5.5% and 26.7% and is dependent on age, atherothrombotic risk factors, and the coexistence of other atherothrombotic diseases.35 Symptoms of PAD include mild to intermittent claudication, ischemic rest pain, and tissue loss.2 Disease severity is classified according to either Fontaine's stages or Rutherford categories. These categorization schema have value in improving communication between physicians, which is important in ensuring continuity of care between the inpatient and outpatient settings (Table 1).2
| Stage | Fontaine | Rutherford | ||
|---|---|---|---|---|
| Clinical | Grade | Category | Clinical | |
| ||||
| I | Asymptomatic | 0 | 0 | Asymptomatic |
| IIa | Mild claudication | I | 1 | Mild claudication |
| IIb | Moderate‐severe claudication | I | 2 | Moderate claudication |
| III | Ischemic rest pain | I | 3 | Severe claudication |
| IV | Ulceration or gangrene | II | 4 | Ischemic rest pain |
| III | 5 | Minor tissue loss | ||
| IV | 6 | Ulceration or gangrene | ||
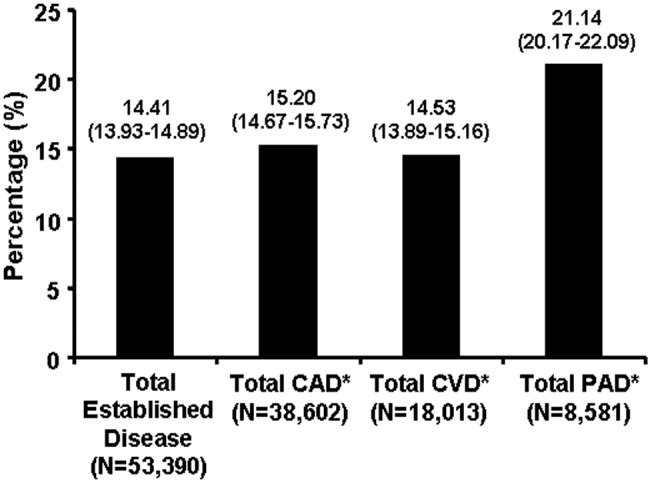
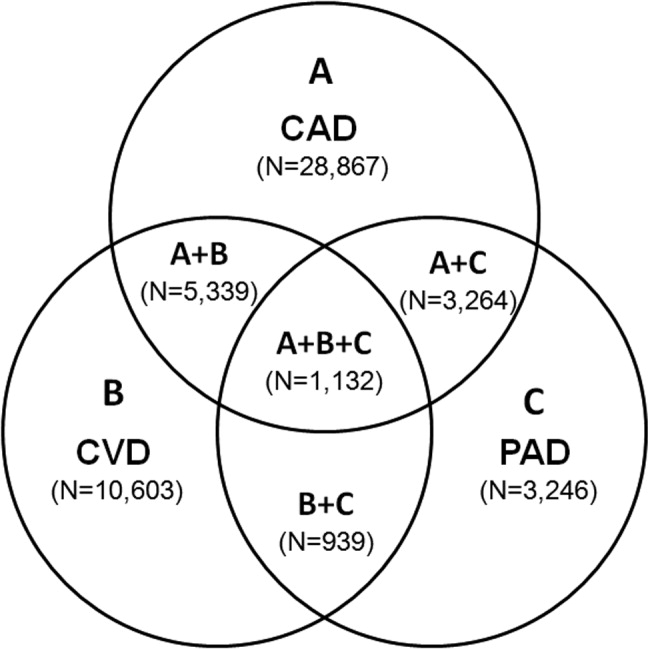
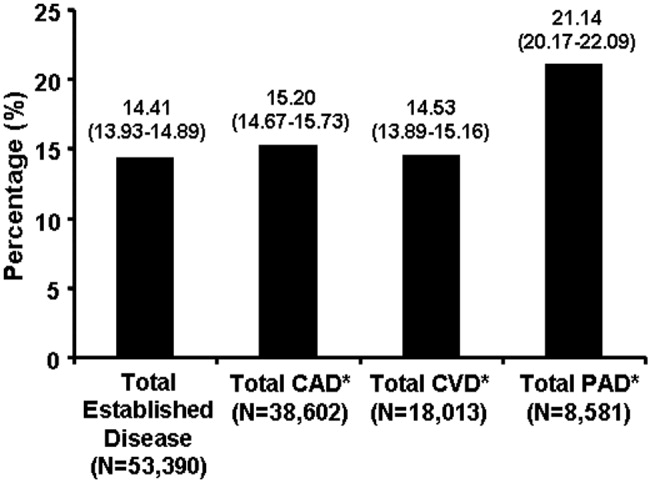
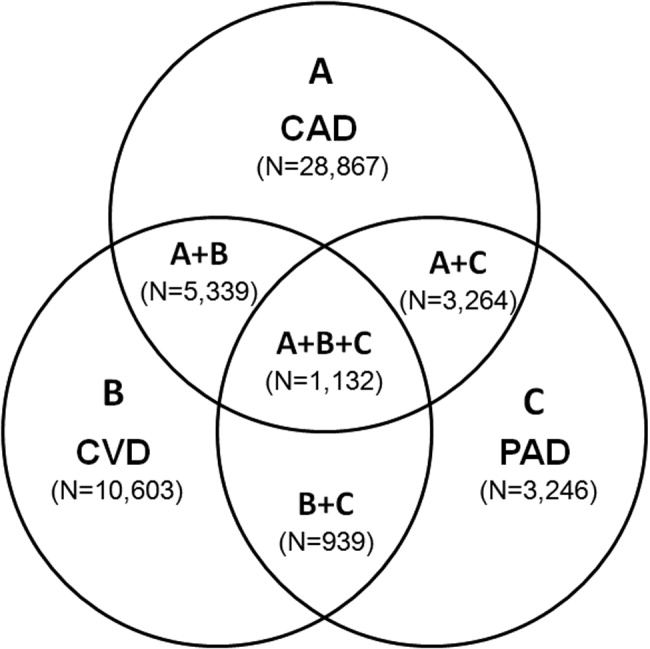
Patients with PAD are at increased risk of dying from or experiencing a cardiovascular event.68 Among patients diagnosed with PAD, coronary artery disease (CAD), or cerebrovascular disease (CVD), those with PAD have the highest 1‐year rate of cardiovascular death, MI, stroke, or vascular‐related hospitalization (Fig. 1).8 This risk is attributable in part to the high rate of association of PAD with other atherothrombotic diseases. The Reduction of Atherothrombosis for Continued Health (REACH) Registry found that approximately 60% of participants with documented PAD have polyvascular disease, defined by the coexistence of CAD and/or CVD. In comparison, 25% of participants with CAD and 40% of participants with CVD have polyvascular disease.8 Thus, PAD can be considered a powerful indicator of systemic atherothrombotic disease and a predictor of cardiovascular and cerebrovascular morbidity and mortality.1
Unfortunately, asymptomatic PAD is more common than its symptomatic counterpart.3 In addition, symptomatic patients often fail to notify their physicians about PAD‐associated symptoms because they attribute them to aging.3 As a result, this disease is underdiagnosed and undertreated.1 Accordingly, several medical associations and physician task forces have called for an increase in screening for PAD in at‐risk populations that include: patients older than 70, patients older than 50 who have concomitant atherothrombotic risk factors, and patients with atherothrombotic disease of single or multiple vascular beds.1, 9 In many cases hospitalists encounter patients at high‐risk for PAD whose DRG for admission might be unrelated to this disease. Nonetheless, hospitalists have the opportunity to improve patient outcomes by being capable of screening for undiagnosed PAD and initiating appropriate interventions to reduce the risk of life‐threatening cardiovascular events.
DIAGNOSIS
Peripheral arterial disease can be diagnosed by either noninvasive or invasive methods. The ankle‐to‐brachial index (ABI) is an accurate, practical, inexpensive, and noninvasive method for detecting PAD.1 The sensitivity of ABI in detecting PAD is 95% with 99% specificity,10 which makes the method superior to other indicators (eg, absence of a pedal pulse, presence of a femoral arterial bruit, slow venous filling, or cold/abnormally colored skin) assessed during a physical examination.11 Under normal conditions, the systolic pressure at the ankle should be equal to or greater than that recorded from the upper arm. As PAD narrows arteries, the systolic pressure decreases at sites distal to the area of arterial narrowing. A resting ABI is quantified by taking 2 readings each of ankle and brachial blood pressures with a handheld Doppler device while the patient is supine and dividing the highest ankle systolic pressure by the highest brachial pressure.12
An ABI between 0.9 and 1.30 is considered normal. Ratios between 0.7 and 0.89 indicate mild PAD, 0.4 and 0.69 moderate PAD, and an ABI < 0.4 severe PAD when patients are more likely to have ischemic pain when at rest. An ABI > 1.3 usually indicates the presence of noncompressible vessels, which can be common in the elderly and patients with diabetes mellitus who have calcification of the distal arteries.1, 2 The ABI is also inversely related to the number of atherosclerotic risk factors and the risk of adverse cardiovascular events and death.6, 1316 To identify individuals with suspected or asymptomatic lower‐extremity PAD, ABI has a class I recommendation from the American College of Cardiology and American Heart Association (ACC/AHA) for patients who present with leg symptoms, who are 70 years and older, or who are 50 years and older with a history of smoking or diabetes.2 This enables physicians to make therapeutic interventions to reduce the risk of adverse vascular events in these patient cohorts.
Additional detection methods for PAD include measuring the ABI before and after exercise on a treadmill, if the patient is ambulatory, or exercise by performing 50 repetitions of raising the heels maximally off the floor, if the patient is not ambulatory. These tests determine the extent of claudication.2 Duplex ultrasound is used to establish the location and severity of stenosis and to follow PAD progression.2
Invasive evaluations for PAD are used primarily to confirm an initial diagnosis of PAD and assess its severity. These methods include a conventional angiogram, which is the most readily available and widely used technique for defining arterial stenosis. Magnetic resonance (MR) angiography with gadolinium and computed tomographic (CT) angiography are used to determine the location and degree of stenosis. Both MR and CT angiography have advantages and disadvantages but are considered interchangeable with one another in patients with contraindications to either method (Table 2).2
| Diagostic method | Benefits | Limitations |
|---|---|---|
| ||
| Magnetic resonance angiography (MRA) | Useful to assess PAD anatomy and presence of significant stenosis | Tends to overestimate degree of stenosis |
| Useful to select patients who are candidates for endovascular of surgical revascularization | May be inaccurate in arteries treated with metal stents | |
| Cannot be used in patients with contraindication to magnetic resonance technique | ||
| Computed tomographic angiography (CTA) | Useful to assess PAD anatomy and presence of significant stenosis | Single‐detector CT lacks accuracy to detect stenoses |
| Useful to select patients who are candidates for endovascular or surgical revascularization | Spatial Resolution lower than digital subtraction angiography | |
| Helpful to provide associated soft‐tissue diagnostic information that may be associated with PAD | Venous opacification can obscure arterial filling | |
| Patients with contraindications to MRA | Asymmetric opacification of legs may obscure arterial phase in some vessels | |
| Metal clips, stents, and prostheses do not cause significant CTA artifacts | Accuracy and effectiveness not as well determined as MRA | |
| Scan times are significantly faster | Treatment plans based on CTA have not been compared to those of catheter angiography | |
| Requires contrast and radiation | ||
| Use may be limited in individuals with renal dysfunction | ||
ANTIPLATELET THERAPY FOR REDUCTION OF VASCULAR EVENTS
Hospitalists utilize a wide array of therapies to treat and manage PAD. Acute complications of PAD may require interventions to prevent tissue loss or infection, revascularization procedures, or surgical amputation. Treatment of mild to moderate PAD focuses on atherothrombotic risk factor management, exercise therapy to improve limb function, and interventions to reduce the risk of adverse vascular events.2, 9 The remainder of this report focuses on the role of antiplatelet therapy (eg, aspirin and thienopyridines) in reducing the risk of vascular events in patients with PAD.
The Antiplatelet Trialists' Collaboration performed an overview analysis of randomized trials conducted prior to 1990 in order to determine the association of prolonged antiplatelet therapy with the occurrence of major vascular events. As a whole, therapies thought to act through inhibition of platelet aggregation, adhesion, or both reduced the incidence of vascular events by 33% in patients with PAD and those at high risk, and by 25% in all patient groups. Antiplatelet agents were also well tolerated; the absolute risk of fatal or nonmajor hemorrhage was low.17
A similar meta‐analysis was conducted of antiplatelet therapies in high‐risk patients with atherothrombosis by the Antithrombotic Trialists' Collaboration. Antiplatelet therapies taken together reduced the odds of patients experiencing vascular events by 22% (SE = 2%) across all trials and 23% (SE = 8%) in patients with PAD.18 Similar to the Antiplatelet Trialists' Collaboration study, the absolute risk of major and minor bleeding was low compared to the benefits of antiplatelet therapy.18 The results of these studies provide supporting evidence for the ACC/AHA class I recommendation for the use of antiplatelet therapy to reduce the risk of MI, stroke, or vascular death in patients with PAD.
The Antithrombotic Trialists' Collaboration also examined the risk reduction associated with a specific antiplatelet agent, aspirin. All doses of aspirin (75‐150, 160‐325, and 500‐1500 mg/day) reduced the odds by 23% (SE = 2%); high doses were no more effective than medium or low doses.18 Although the effects of aspirin was not analyzed in a subgroup analysis of patients with PAD, this study and others support the ACC/AHA class I recommendations for the use of aspirin to reduce the risk of MI, stroke, or vascular death in patients with PAD.2, 1921
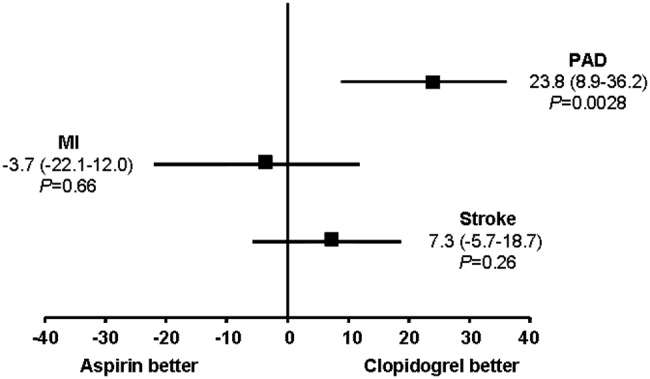
The CAPRIE trial compared the efficacy of another antiplatelet agent, clopidogrel, against aspirin in patients with PAD.22 Patients with a history of recent ischemic stroke, MI, or symptomatic PAD were randomized to receive either clopidogrel (75 mg/day) or aspirin (325 mg/day) for 1‐3 years (mean follow‐up time, 1.91 years). Study outcomes were the incidence of nonfatal MI, ischemic stroke, hemorrhagic stroke, leg amputation, and vascular deaths. The absolute risk reduction for all patients was 8.7% (95% confidence interval [CI], 0.3%‐16.5%) in favor of clopidogrel over aspirin. Moreover, subgroup analysis in patients with PAD revealed that clopidogrel reduced the risk of a vascular event by 23.8% (95% CI, 8.9%‐36.2%; P = 0.0028) compared with aspirin (Fig. 2). Clopidogrel and aspirin had similar safety profiles, but other studies have revealed bleeding incidence is numerically greater in patients treated with clopidogrel.2224 Although the CAPRIE trial is the only study to date to compare the efficacy of clopidogrel over aspirin in reducing vascular event in patients with PAD, its outcomes underlie the class I ACC/AHA recommendation for clopidogrel (75 mg/day) as an effective alternative to aspirin to reduce the risk of MI, stroke, or death in patients with PAD.2
CONCLUSIONS
Despite the availability of accurate, practical, and inexpensive diagnostic testing, PAD remains underdiagnosed and undertreated. Early detection of PAD and subsequent intervention by hospitalists are important because peripheral arterial disease is strongly associated with an increased risk of mortality and morbidity from adverse vascular events. The ACC/AHA recommends screening for asymptomatic patients at risk for this disease so that therapies that reduce the risk of an MI, stroke, or vascular death can be administered immediately. Antiplatelet agents reduce the risk of adverse vascular events in patients with PAD. The use of aspirin or clopidogrel is recommended in this cohort of patients. However, further study is necessary to determine the efficacy and safety of combination therapy with aspirin and clopidogrel in patients with PAD. It is also important to note that coordination of care between hospitalists and cardiologists is critical in the management of patients with this disease. However, the appropriate handoff of patients between these 2 groups of physicians depends on the local expertise and support structure of these health care professionals. Thus, an interdisciplinary approach utilizing guideline‐based patient care will allow hospitalists to refer patients accordingly, ensuring optimal outcomes in patients with PAD.
- ,,, et al.Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action.Arch Intern Med.2003;163:884–892.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:e463–e654.
- ,,,,,.Peripheral arterial disease in the elderly: the Rotterdam Study.Arterioscler Thromb Vasc Biol.1998;18:185–192.
- ,,, et al.Peripheral arterial disease detection, awareness, and treatment in primary care.JAMA.2001;286:1317–1324.
- ,.Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999‐2000.Circulation.2004;110:738–743.
- ,,, et al.Mortality over a period of 10 years in patients with peripheral arterial disease.N Engl J Med.1992;326:381–386.
- ,.Vascular event rates in patients with atherosclerotic cerebrovascular disease.Arch Neurol.1992;49:857–863.
- ,,, et al.;REACH Registry Investigators. One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review.Circulation.1996;94:3026–3049.
- ,.Management of peripheral arterial disease (PAD): TASC Working Group. TransAtlantic Inter‐Society Consensus (TASC).J Vasc Surg.2000:31(1Pt 2):S1–S296.
- ,.Physical examination and chronic lower‐extremity ischemia.Arch Intern Med.1998;158:1357–1364.
- .Medical treatment of peripheral artery disease and claudication.N Engl J Med.2001;344:1608–1621.
- ,,, et al.Ankle‐arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group.Circulation.1993;88:837–845.
- ,,,.Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index.JAMA.1993;270:487–489.
- ,,, et al.Ankle‐arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group.Arterioscler Thromb Vasc Biol.1999;19:538–545.
- ,,,,,;Framingham Study. The ankle‐brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study.Arch Intern Med.2003;163:1939–1942.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy—1: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- The Medical Research Council's General Practice Research Framework.Thrombosis prevention trial: randomised trial of low‐intensity oral anticoagulation with warfarin and low‐dose aspirin in the primary prevention of ischemic heart disease in men at increased risk.Lancet.1998;351:233–241.
- ,,, for theHOT Study Group.Effects of intensive blood pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial.Lancet1998;280:1930–1935.
- Collaborative Group of the Primary Prevention Project (PPP).Low‐dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice.Lancet.2001;357:89–95.
- CAPRIE Steering Committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,; for theCHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,; on behalf of theMATCH investigators.Aspirin and clopidogrel compared with clopidogrel alone after ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
Peripheral arterial disease (PAD) is defined by the presence of stenosis or occlusion in peripheral arterial beds.1, 2 Based on large population‐based screening surveys, the prevalence of this disease ranges between 5.5% and 26.7% and is dependent on age, atherothrombotic risk factors, and the coexistence of other atherothrombotic diseases.35 Symptoms of PAD include mild to intermittent claudication, ischemic rest pain, and tissue loss.2 Disease severity is classified according to either Fontaine's stages or Rutherford categories. These categorization schema have value in improving communication between physicians, which is important in ensuring continuity of care between the inpatient and outpatient settings (Table 1).2
| Stage | Fontaine | Rutherford | ||
|---|---|---|---|---|
| Clinical | Grade | Category | Clinical | |
| ||||
| I | Asymptomatic | 0 | 0 | Asymptomatic |
| IIa | Mild claudication | I | 1 | Mild claudication |
| IIb | Moderate‐severe claudication | I | 2 | Moderate claudication |
| III | Ischemic rest pain | I | 3 | Severe claudication |
| IV | Ulceration or gangrene | II | 4 | Ischemic rest pain |
| III | 5 | Minor tissue loss | ||
| IV | 6 | Ulceration or gangrene | ||
Patients with PAD are at increased risk of dying from or experiencing a cardiovascular event.68 Among patients diagnosed with PAD, coronary artery disease (CAD), or cerebrovascular disease (CVD), those with PAD have the highest 1‐year rate of cardiovascular death, MI, stroke, or vascular‐related hospitalization (Fig. 1).8 This risk is attributable in part to the high rate of association of PAD with other atherothrombotic diseases. The Reduction of Atherothrombosis for Continued Health (REACH) Registry found that approximately 60% of participants with documented PAD have polyvascular disease, defined by the coexistence of CAD and/or CVD. In comparison, 25% of participants with CAD and 40% of participants with CVD have polyvascular disease.8 Thus, PAD can be considered a powerful indicator of systemic atherothrombotic disease and a predictor of cardiovascular and cerebrovascular morbidity and mortality.1

Unfortunately, asymptomatic PAD is more common than its symptomatic counterpart.3 In addition, symptomatic patients often fail to notify their physicians about PAD‐associated symptoms because they attribute them to aging.3 As a result, this disease is underdiagnosed and undertreated.1 Accordingly, several medical associations and physician task forces have called for an increase in screening for PAD in at‐risk populations that include: patients older than 70, patients older than 50 who have concomitant atherothrombotic risk factors, and patients with atherothrombotic disease of single or multiple vascular beds.1, 9 In many cases hospitalists encounter patients at high‐risk for PAD whose DRG for admission might be unrelated to this disease. Nonetheless, hospitalists have the opportunity to improve patient outcomes by being capable of screening for undiagnosed PAD and initiating appropriate interventions to reduce the risk of life‐threatening cardiovascular events.
DIAGNOSIS
Peripheral arterial disease can be diagnosed by either noninvasive or invasive methods. The ankle‐to‐brachial index (ABI) is an accurate, practical, inexpensive, and noninvasive method for detecting PAD.1 The sensitivity of ABI in detecting PAD is 95% with 99% specificity,10 which makes the method superior to other indicators (eg, absence of a pedal pulse, presence of a femoral arterial bruit, slow venous filling, or cold/abnormally colored skin) assessed during a physical examination.11 Under normal conditions, the systolic pressure at the ankle should be equal to or greater than that recorded from the upper arm. As PAD narrows arteries, the systolic pressure decreases at sites distal to the area of arterial narrowing. A resting ABI is quantified by taking 2 readings each of ankle and brachial blood pressures with a handheld Doppler device while the patient is supine and dividing the highest ankle systolic pressure by the highest brachial pressure.12
An ABI between 0.9 and 1.30 is considered normal. Ratios between 0.7 and 0.89 indicate mild PAD, 0.4 and 0.69 moderate PAD, and an ABI < 0.4 severe PAD when patients are more likely to have ischemic pain when at rest. An ABI > 1.3 usually indicates the presence of noncompressible vessels, which can be common in the elderly and patients with diabetes mellitus who have calcification of the distal arteries.1, 2 The ABI is also inversely related to the number of atherosclerotic risk factors and the risk of adverse cardiovascular events and death.6, 1316 To identify individuals with suspected or asymptomatic lower‐extremity PAD, ABI has a class I recommendation from the American College of Cardiology and American Heart Association (ACC/AHA) for patients who present with leg symptoms, who are 70 years and older, or who are 50 years and older with a history of smoking or diabetes.2 This enables physicians to make therapeutic interventions to reduce the risk of adverse vascular events in these patient cohorts.
Additional detection methods for PAD include measuring the ABI before and after exercise on a treadmill, if the patient is ambulatory, or exercise by performing 50 repetitions of raising the heels maximally off the floor, if the patient is not ambulatory. These tests determine the extent of claudication.2 Duplex ultrasound is used to establish the location and severity of stenosis and to follow PAD progression.2
Invasive evaluations for PAD are used primarily to confirm an initial diagnosis of PAD and assess its severity. These methods include a conventional angiogram, which is the most readily available and widely used technique for defining arterial stenosis. Magnetic resonance (MR) angiography with gadolinium and computed tomographic (CT) angiography are used to determine the location and degree of stenosis. Both MR and CT angiography have advantages and disadvantages but are considered interchangeable with one another in patients with contraindications to either method (Table 2).2
| Diagostic method | Benefits | Limitations |
|---|---|---|
| ||
| Magnetic resonance angiography (MRA) | Useful to assess PAD anatomy and presence of significant stenosis | Tends to overestimate degree of stenosis |
| Useful to select patients who are candidates for endovascular of surgical revascularization | May be inaccurate in arteries treated with metal stents | |
| Cannot be used in patients with contraindication to magnetic resonance technique | ||
| Computed tomographic angiography (CTA) | Useful to assess PAD anatomy and presence of significant stenosis | Single‐detector CT lacks accuracy to detect stenoses |
| Useful to select patients who are candidates for endovascular or surgical revascularization | Spatial Resolution lower than digital subtraction angiography | |
| Helpful to provide associated soft‐tissue diagnostic information that may be associated with PAD | Venous opacification can obscure arterial filling | |
| Patients with contraindications to MRA | Asymmetric opacification of legs may obscure arterial phase in some vessels | |
| Metal clips, stents, and prostheses do not cause significant CTA artifacts | Accuracy and effectiveness not as well determined as MRA | |
| Scan times are significantly faster | Treatment plans based on CTA have not been compared to those of catheter angiography | |
| Requires contrast and radiation | ||
| Use may be limited in individuals with renal dysfunction | ||
ANTIPLATELET THERAPY FOR REDUCTION OF VASCULAR EVENTS
Hospitalists utilize a wide array of therapies to treat and manage PAD. Acute complications of PAD may require interventions to prevent tissue loss or infection, revascularization procedures, or surgical amputation. Treatment of mild to moderate PAD focuses on atherothrombotic risk factor management, exercise therapy to improve limb function, and interventions to reduce the risk of adverse vascular events.2, 9 The remainder of this report focuses on the role of antiplatelet therapy (eg, aspirin and thienopyridines) in reducing the risk of vascular events in patients with PAD.
The Antiplatelet Trialists' Collaboration performed an overview analysis of randomized trials conducted prior to 1990 in order to determine the association of prolonged antiplatelet therapy with the occurrence of major vascular events. As a whole, therapies thought to act through inhibition of platelet aggregation, adhesion, or both reduced the incidence of vascular events by 33% in patients with PAD and those at high risk, and by 25% in all patient groups. Antiplatelet agents were also well tolerated; the absolute risk of fatal or nonmajor hemorrhage was low.17
A similar meta‐analysis was conducted of antiplatelet therapies in high‐risk patients with atherothrombosis by the Antithrombotic Trialists' Collaboration. Antiplatelet therapies taken together reduced the odds of patients experiencing vascular events by 22% (SE = 2%) across all trials and 23% (SE = 8%) in patients with PAD.18 Similar to the Antiplatelet Trialists' Collaboration study, the absolute risk of major and minor bleeding was low compared to the benefits of antiplatelet therapy.18 The results of these studies provide supporting evidence for the ACC/AHA class I recommendation for the use of antiplatelet therapy to reduce the risk of MI, stroke, or vascular death in patients with PAD.
The Antithrombotic Trialists' Collaboration also examined the risk reduction associated with a specific antiplatelet agent, aspirin. All doses of aspirin (75‐150, 160‐325, and 500‐1500 mg/day) reduced the odds by 23% (SE = 2%); high doses were no more effective than medium or low doses.18 Although the effects of aspirin was not analyzed in a subgroup analysis of patients with PAD, this study and others support the ACC/AHA class I recommendations for the use of aspirin to reduce the risk of MI, stroke, or vascular death in patients with PAD.2, 1921
The CAPRIE trial compared the efficacy of another antiplatelet agent, clopidogrel, against aspirin in patients with PAD.22 Patients with a history of recent ischemic stroke, MI, or symptomatic PAD were randomized to receive either clopidogrel (75 mg/day) or aspirin (325 mg/day) for 1‐3 years (mean follow‐up time, 1.91 years). Study outcomes were the incidence of nonfatal MI, ischemic stroke, hemorrhagic stroke, leg amputation, and vascular deaths. The absolute risk reduction for all patients was 8.7% (95% confidence interval [CI], 0.3%‐16.5%) in favor of clopidogrel over aspirin. Moreover, subgroup analysis in patients with PAD revealed that clopidogrel reduced the risk of a vascular event by 23.8% (95% CI, 8.9%‐36.2%; P = 0.0028) compared with aspirin (Fig. 2). Clopidogrel and aspirin had similar safety profiles, but other studies have revealed bleeding incidence is numerically greater in patients treated with clopidogrel.2224 Although the CAPRIE trial is the only study to date to compare the efficacy of clopidogrel over aspirin in reducing vascular event in patients with PAD, its outcomes underlie the class I ACC/AHA recommendation for clopidogrel (75 mg/day) as an effective alternative to aspirin to reduce the risk of MI, stroke, or death in patients with PAD.2

CONCLUSIONS
Despite the availability of accurate, practical, and inexpensive diagnostic testing, PAD remains underdiagnosed and undertreated. Early detection of PAD and subsequent intervention by hospitalists are important because peripheral arterial disease is strongly associated with an increased risk of mortality and morbidity from adverse vascular events. The ACC/AHA recommends screening for asymptomatic patients at risk for this disease so that therapies that reduce the risk of an MI, stroke, or vascular death can be administered immediately. Antiplatelet agents reduce the risk of adverse vascular events in patients with PAD. The use of aspirin or clopidogrel is recommended in this cohort of patients. However, further study is necessary to determine the efficacy and safety of combination therapy with aspirin and clopidogrel in patients with PAD. It is also important to note that coordination of care between hospitalists and cardiologists is critical in the management of patients with this disease. However, the appropriate handoff of patients between these 2 groups of physicians depends on the local expertise and support structure of these health care professionals. Thus, an interdisciplinary approach utilizing guideline‐based patient care will allow hospitalists to refer patients accordingly, ensuring optimal outcomes in patients with PAD.
Peripheral arterial disease (PAD) is defined by the presence of stenosis or occlusion in peripheral arterial beds.1, 2 Based on large population‐based screening surveys, the prevalence of this disease ranges between 5.5% and 26.7% and is dependent on age, atherothrombotic risk factors, and the coexistence of other atherothrombotic diseases.35 Symptoms of PAD include mild to intermittent claudication, ischemic rest pain, and tissue loss.2 Disease severity is classified according to either Fontaine's stages or Rutherford categories. These categorization schema have value in improving communication between physicians, which is important in ensuring continuity of care between the inpatient and outpatient settings (Table 1).2
| Stage | Fontaine | Rutherford | ||
|---|---|---|---|---|
| Clinical | Grade | Category | Clinical | |
| ||||
| I | Asymptomatic | 0 | 0 | Asymptomatic |
| IIa | Mild claudication | I | 1 | Mild claudication |
| IIb | Moderate‐severe claudication | I | 2 | Moderate claudication |
| III | Ischemic rest pain | I | 3 | Severe claudication |
| IV | Ulceration or gangrene | II | 4 | Ischemic rest pain |
| III | 5 | Minor tissue loss | ||
| IV | 6 | Ulceration or gangrene | ||
Patients with PAD are at increased risk of dying from or experiencing a cardiovascular event.68 Among patients diagnosed with PAD, coronary artery disease (CAD), or cerebrovascular disease (CVD), those with PAD have the highest 1‐year rate of cardiovascular death, MI, stroke, or vascular‐related hospitalization (Fig. 1).8 This risk is attributable in part to the high rate of association of PAD with other atherothrombotic diseases. The Reduction of Atherothrombosis for Continued Health (REACH) Registry found that approximately 60% of participants with documented PAD have polyvascular disease, defined by the coexistence of CAD and/or CVD. In comparison, 25% of participants with CAD and 40% of participants with CVD have polyvascular disease.8 Thus, PAD can be considered a powerful indicator of systemic atherothrombotic disease and a predictor of cardiovascular and cerebrovascular morbidity and mortality.1

Unfortunately, asymptomatic PAD is more common than its symptomatic counterpart.3 In addition, symptomatic patients often fail to notify their physicians about PAD‐associated symptoms because they attribute them to aging.3 As a result, this disease is underdiagnosed and undertreated.1 Accordingly, several medical associations and physician task forces have called for an increase in screening for PAD in at‐risk populations that include: patients older than 70, patients older than 50 who have concomitant atherothrombotic risk factors, and patients with atherothrombotic disease of single or multiple vascular beds.1, 9 In many cases hospitalists encounter patients at high‐risk for PAD whose DRG for admission might be unrelated to this disease. Nonetheless, hospitalists have the opportunity to improve patient outcomes by being capable of screening for undiagnosed PAD and initiating appropriate interventions to reduce the risk of life‐threatening cardiovascular events.
DIAGNOSIS
Peripheral arterial disease can be diagnosed by either noninvasive or invasive methods. The ankle‐to‐brachial index (ABI) is an accurate, practical, inexpensive, and noninvasive method for detecting PAD.1 The sensitivity of ABI in detecting PAD is 95% with 99% specificity,10 which makes the method superior to other indicators (eg, absence of a pedal pulse, presence of a femoral arterial bruit, slow venous filling, or cold/abnormally colored skin) assessed during a physical examination.11 Under normal conditions, the systolic pressure at the ankle should be equal to or greater than that recorded from the upper arm. As PAD narrows arteries, the systolic pressure decreases at sites distal to the area of arterial narrowing. A resting ABI is quantified by taking 2 readings each of ankle and brachial blood pressures with a handheld Doppler device while the patient is supine and dividing the highest ankle systolic pressure by the highest brachial pressure.12
An ABI between 0.9 and 1.30 is considered normal. Ratios between 0.7 and 0.89 indicate mild PAD, 0.4 and 0.69 moderate PAD, and an ABI < 0.4 severe PAD when patients are more likely to have ischemic pain when at rest. An ABI > 1.3 usually indicates the presence of noncompressible vessels, which can be common in the elderly and patients with diabetes mellitus who have calcification of the distal arteries.1, 2 The ABI is also inversely related to the number of atherosclerotic risk factors and the risk of adverse cardiovascular events and death.6, 1316 To identify individuals with suspected or asymptomatic lower‐extremity PAD, ABI has a class I recommendation from the American College of Cardiology and American Heart Association (ACC/AHA) for patients who present with leg symptoms, who are 70 years and older, or who are 50 years and older with a history of smoking or diabetes.2 This enables physicians to make therapeutic interventions to reduce the risk of adverse vascular events in these patient cohorts.
Additional detection methods for PAD include measuring the ABI before and after exercise on a treadmill, if the patient is ambulatory, or exercise by performing 50 repetitions of raising the heels maximally off the floor, if the patient is not ambulatory. These tests determine the extent of claudication.2 Duplex ultrasound is used to establish the location and severity of stenosis and to follow PAD progression.2
Invasive evaluations for PAD are used primarily to confirm an initial diagnosis of PAD and assess its severity. These methods include a conventional angiogram, which is the most readily available and widely used technique for defining arterial stenosis. Magnetic resonance (MR) angiography with gadolinium and computed tomographic (CT) angiography are used to determine the location and degree of stenosis. Both MR and CT angiography have advantages and disadvantages but are considered interchangeable with one another in patients with contraindications to either method (Table 2).2
| Diagostic method | Benefits | Limitations |
|---|---|---|
| ||
| Magnetic resonance angiography (MRA) | Useful to assess PAD anatomy and presence of significant stenosis | Tends to overestimate degree of stenosis |
| Useful to select patients who are candidates for endovascular of surgical revascularization | May be inaccurate in arteries treated with metal stents | |
| Cannot be used in patients with contraindication to magnetic resonance technique | ||
| Computed tomographic angiography (CTA) | Useful to assess PAD anatomy and presence of significant stenosis | Single‐detector CT lacks accuracy to detect stenoses |
| Useful to select patients who are candidates for endovascular or surgical revascularization | Spatial Resolution lower than digital subtraction angiography | |
| Helpful to provide associated soft‐tissue diagnostic information that may be associated with PAD | Venous opacification can obscure arterial filling | |
| Patients with contraindications to MRA | Asymmetric opacification of legs may obscure arterial phase in some vessels | |
| Metal clips, stents, and prostheses do not cause significant CTA artifacts | Accuracy and effectiveness not as well determined as MRA | |
| Scan times are significantly faster | Treatment plans based on CTA have not been compared to those of catheter angiography | |
| Requires contrast and radiation | ||
| Use may be limited in individuals with renal dysfunction | ||
ANTIPLATELET THERAPY FOR REDUCTION OF VASCULAR EVENTS
Hospitalists utilize a wide array of therapies to treat and manage PAD. Acute complications of PAD may require interventions to prevent tissue loss or infection, revascularization procedures, or surgical amputation. Treatment of mild to moderate PAD focuses on atherothrombotic risk factor management, exercise therapy to improve limb function, and interventions to reduce the risk of adverse vascular events.2, 9 The remainder of this report focuses on the role of antiplatelet therapy (eg, aspirin and thienopyridines) in reducing the risk of vascular events in patients with PAD.
The Antiplatelet Trialists' Collaboration performed an overview analysis of randomized trials conducted prior to 1990 in order to determine the association of prolonged antiplatelet therapy with the occurrence of major vascular events. As a whole, therapies thought to act through inhibition of platelet aggregation, adhesion, or both reduced the incidence of vascular events by 33% in patients with PAD and those at high risk, and by 25% in all patient groups. Antiplatelet agents were also well tolerated; the absolute risk of fatal or nonmajor hemorrhage was low.17
A similar meta‐analysis was conducted of antiplatelet therapies in high‐risk patients with atherothrombosis by the Antithrombotic Trialists' Collaboration. Antiplatelet therapies taken together reduced the odds of patients experiencing vascular events by 22% (SE = 2%) across all trials and 23% (SE = 8%) in patients with PAD.18 Similar to the Antiplatelet Trialists' Collaboration study, the absolute risk of major and minor bleeding was low compared to the benefits of antiplatelet therapy.18 The results of these studies provide supporting evidence for the ACC/AHA class I recommendation for the use of antiplatelet therapy to reduce the risk of MI, stroke, or vascular death in patients with PAD.
The Antithrombotic Trialists' Collaboration also examined the risk reduction associated with a specific antiplatelet agent, aspirin. All doses of aspirin (75‐150, 160‐325, and 500‐1500 mg/day) reduced the odds by 23% (SE = 2%); high doses were no more effective than medium or low doses.18 Although the effects of aspirin was not analyzed in a subgroup analysis of patients with PAD, this study and others support the ACC/AHA class I recommendations for the use of aspirin to reduce the risk of MI, stroke, or vascular death in patients with PAD.2, 1921
The CAPRIE trial compared the efficacy of another antiplatelet agent, clopidogrel, against aspirin in patients with PAD.22 Patients with a history of recent ischemic stroke, MI, or symptomatic PAD were randomized to receive either clopidogrel (75 mg/day) or aspirin (325 mg/day) for 1‐3 years (mean follow‐up time, 1.91 years). Study outcomes were the incidence of nonfatal MI, ischemic stroke, hemorrhagic stroke, leg amputation, and vascular deaths. The absolute risk reduction for all patients was 8.7% (95% confidence interval [CI], 0.3%‐16.5%) in favor of clopidogrel over aspirin. Moreover, subgroup analysis in patients with PAD revealed that clopidogrel reduced the risk of a vascular event by 23.8% (95% CI, 8.9%‐36.2%; P = 0.0028) compared with aspirin (Fig. 2). Clopidogrel and aspirin had similar safety profiles, but other studies have revealed bleeding incidence is numerically greater in patients treated with clopidogrel.2224 Although the CAPRIE trial is the only study to date to compare the efficacy of clopidogrel over aspirin in reducing vascular event in patients with PAD, its outcomes underlie the class I ACC/AHA recommendation for clopidogrel (75 mg/day) as an effective alternative to aspirin to reduce the risk of MI, stroke, or death in patients with PAD.2

CONCLUSIONS
Despite the availability of accurate, practical, and inexpensive diagnostic testing, PAD remains underdiagnosed and undertreated. Early detection of PAD and subsequent intervention by hospitalists are important because peripheral arterial disease is strongly associated with an increased risk of mortality and morbidity from adverse vascular events. The ACC/AHA recommends screening for asymptomatic patients at risk for this disease so that therapies that reduce the risk of an MI, stroke, or vascular death can be administered immediately. Antiplatelet agents reduce the risk of adverse vascular events in patients with PAD. The use of aspirin or clopidogrel is recommended in this cohort of patients. However, further study is necessary to determine the efficacy and safety of combination therapy with aspirin and clopidogrel in patients with PAD. It is also important to note that coordination of care between hospitalists and cardiologists is critical in the management of patients with this disease. However, the appropriate handoff of patients between these 2 groups of physicians depends on the local expertise and support structure of these health care professionals. Thus, an interdisciplinary approach utilizing guideline‐based patient care will allow hospitalists to refer patients accordingly, ensuring optimal outcomes in patients with PAD.
- ,,, et al.Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action.Arch Intern Med.2003;163:884–892.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:e463–e654.
- ,,,,,.Peripheral arterial disease in the elderly: the Rotterdam Study.Arterioscler Thromb Vasc Biol.1998;18:185–192.
- ,,, et al.Peripheral arterial disease detection, awareness, and treatment in primary care.JAMA.2001;286:1317–1324.
- ,.Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999‐2000.Circulation.2004;110:738–743.
- ,,, et al.Mortality over a period of 10 years in patients with peripheral arterial disease.N Engl J Med.1992;326:381–386.
- ,.Vascular event rates in patients with atherosclerotic cerebrovascular disease.Arch Neurol.1992;49:857–863.
- ,,, et al.;REACH Registry Investigators. One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review.Circulation.1996;94:3026–3049.
- ,.Management of peripheral arterial disease (PAD): TASC Working Group. TransAtlantic Inter‐Society Consensus (TASC).J Vasc Surg.2000:31(1Pt 2):S1–S296.
- ,.Physical examination and chronic lower‐extremity ischemia.Arch Intern Med.1998;158:1357–1364.
- .Medical treatment of peripheral artery disease and claudication.N Engl J Med.2001;344:1608–1621.
- ,,, et al.Ankle‐arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group.Circulation.1993;88:837–845.
- ,,,.Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index.JAMA.1993;270:487–489.
- ,,, et al.Ankle‐arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group.Arterioscler Thromb Vasc Biol.1999;19:538–545.
- ,,,,,;Framingham Study. The ankle‐brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study.Arch Intern Med.2003;163:1939–1942.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy—1: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- The Medical Research Council's General Practice Research Framework.Thrombosis prevention trial: randomised trial of low‐intensity oral anticoagulation with warfarin and low‐dose aspirin in the primary prevention of ischemic heart disease in men at increased risk.Lancet.1998;351:233–241.
- ,,, for theHOT Study Group.Effects of intensive blood pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial.Lancet1998;280:1930–1935.
- Collaborative Group of the Primary Prevention Project (PPP).Low‐dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice.Lancet.2001;357:89–95.
- CAPRIE Steering Committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,; for theCHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,; on behalf of theMATCH investigators.Aspirin and clopidogrel compared with clopidogrel alone after ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,, et al.Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action.Arch Intern Med.2003;163:884–892.
- ,,, et al.ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic).Circulation.2006;113:e463–e654.
- ,,,,,.Peripheral arterial disease in the elderly: the Rotterdam Study.Arterioscler Thromb Vasc Biol.1998;18:185–192.
- ,,, et al.Peripheral arterial disease detection, awareness, and treatment in primary care.JAMA.2001;286:1317–1324.
- ,.Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999‐2000.Circulation.2004;110:738–743.
- ,,, et al.Mortality over a period of 10 years in patients with peripheral arterial disease.N Engl J Med.1992;326:381–386.
- ,.Vascular event rates in patients with atherosclerotic cerebrovascular disease.Arch Neurol.1992;49:857–863.
- ,,, et al.;REACH Registry Investigators. One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review.Circulation.1996;94:3026–3049.
- ,.Management of peripheral arterial disease (PAD): TASC Working Group. TransAtlantic Inter‐Society Consensus (TASC).J Vasc Surg.2000:31(1Pt 2):S1–S296.
- ,.Physical examination and chronic lower‐extremity ischemia.Arch Intern Med.1998;158:1357–1364.
- .Medical treatment of peripheral artery disease and claudication.N Engl J Med.2001;344:1608–1621.
- ,,, et al.Ankle‐arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group.Circulation.1993;88:837–845.
- ,,,.Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index.JAMA.1993;270:487–489.
- ,,, et al.Ankle‐arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group.Arterioscler Thromb Vasc Biol.1999;19:538–545.
- ,,,,,;Framingham Study. The ankle‐brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study.Arch Intern Med.2003;163:1939–1942.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy—1: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- The Medical Research Council's General Practice Research Framework.Thrombosis prevention trial: randomised trial of low‐intensity oral anticoagulation with warfarin and low‐dose aspirin in the primary prevention of ischemic heart disease in men at increased risk.Lancet.1998;351:233–241.
- ,,, for theHOT Study Group.Effects of intensive blood pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial.Lancet1998;280:1930–1935.
- Collaborative Group of the Primary Prevention Project (PPP).Low‐dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice.Lancet.2001;357:89–95.
- CAPRIE Steering Committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,; for theCHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,; on behalf of theMATCH investigators.Aspirin and clopidogrel compared with clopidogrel alone after ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
Editorial: Rules of Engagement
Acute atherothrombotic events associated with ischemic heart disease and stroke are the first and third most common causes of death in the United States, respectively.1 Despite an overall decrease in age‐adjusted mortality since 1970 in the United States, the worldwide prevalence of these diseases is anticipated to sharply increase by 2020.1, 2 Caring for patients with atherothrombosis is now within the purview of hospitalists to a larger extent than ever before. In recognition of the expanding role of these health care professionals and to reduce the risk of adverse cardiovascular events in the outpatient setting, the Society of Hospital Medicine held a symposium during its 10th Annual Meeting.
Rules of Engagement: The Hospitalist and Atherothrombosis took place on May 24, 2007, in Dallas, Texas. This supplement summarizes the highlights from this symposium and reviews the causes and polyvascular nature of atherothrombosis. The role of the hospitalist in managing atherothrombotic disease and evidence‐based practices for the evaluation and treatment of patients with various manifestations of atherothrombotic disease are also discussed.
ARTERIAL THROMBOSIS AND ITS POLYVASCULAR NATURE
Atherothrombosis refers to the formation of large and occlusive mural thrombi that arise from the rupture of an atherosclerotic plaque. Myocardial infarction (MI), ischemic stroke, and acute limb ischemia are the most severe manifestations of this disease.3, 4 This process begins when denuded or inflamed endothelial cells develop properties that permit platelet adhesion. At the site of endothelial dysfunction, activation of adherent platelet results in the release of inflammatory and mitogenic factors. After a series of dynamic and repetitive processes including amplified platelet activation, monocyte chemotaxis, adhesion, transmigration, and lipoprotein retention, plaque formation occurs.5 Consequently, the rupture or erosion of an atherosclerotic plaque produces a higher degree of platelet adhesion, activation, and aggregation, causing the fibrotic organization of a mural thrombus.3
The number of persons with multiple, concomitant cardiovascular disease (CAD), cerebrovascular disease (CVD), and peripheral arterial disease (PAD) accentuates the polyvascular nature of atherothrombosis (Fig. 1). The international Reduction of Atherothrombosis for Continued Health (REACH) Registry demonstrated that 1‐year incidence rates of major cardiovascular events (eg, MI, stroke, death) were high in patients with an established atherothrombotic disease and increased with the number of concomitant vascular diseases.6 These data infer that the burden on the vascular system is considered extensive on diagnosis of a single atherothrombotic disease. Thus, aggressive therapies are needed to reduce the risk of recurrent or other cardiovascular events. The management of risk factors for atherothrombosis such as hypercholesterolemia, dyslipidemia, hypertension, and diabetes mellitus fall under specific disease‐specific guidelines for patients presenting with atherothrombotic diseases.712
ANTIPLATELET THERAPIES
Antiplatelet therapies are used for the acute and long‐term treatment of patients after a thrombic event. Antiplatelet agents target the molecular mechanisms responsible for platelet activation and aggregation, such as the synthesis of thromboxane A2. On platelet activation, free arachidonic acid is converted to prostaglandin H2 (PGH2) by cyclooxygenase‐1 (COX‐1; Fig. 2). Further metabolism of PGH2 by thromboxane synthase produces thromboxane A2, which induces vasoconstriction (Fig. 2). Fortunately, the ability of platelets to produce COX‐1 is limited, and irreversible inhibition of this enzyme can impair thromboxane A2 synthesis for approximately 10 days.
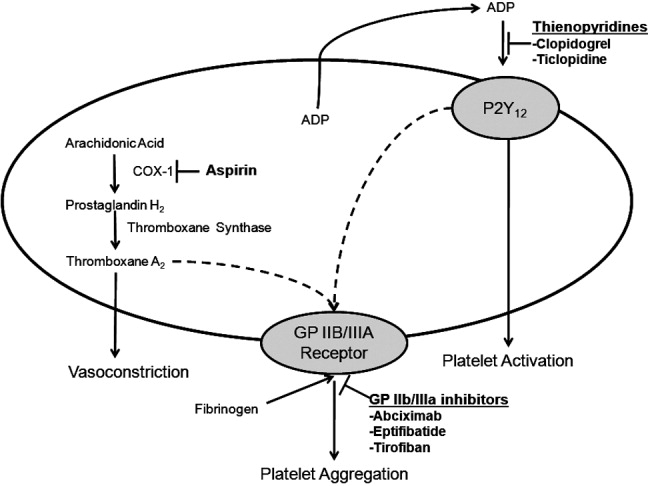
Aspirin is a potent COX‐1 inhibitor, whose effects are evident 1 hour after dosing (Fig. 2).4, 13 Aspirin effectively prevents fatal and nonfatal vascular events in healthy individuals and in patients who present with acute MI or ischemic stroke.13 Unfortunately, a proportion of patients are aspirin resistant. Recent studies have indicated that interactions with the nonsteroidal anti‐inflammatory drug (NSAID) ibuprofen may diminish the primary and secondary protective effects of aspirin and may contribute to aspirin resistance, although the origin of this remains unclear.
The results of a post hoc subgroup analysis of 22,071 apparently healthy male physicians randomized to take aspirin or placebo for 5 years indicated that individuals who used NSAIDs for at least 60 days/year increased their risk of MI by more than 2‐fold compared with those who did not use NSAIDs.14 A second study conducted in patients following a major adverse cardiovascular event showed that the combination of aspirin plus ibuprofen increased the adjusted relative risk of cardiovascular mortality over an 8‐year period compared with aspirin alone.15 However, the effects of NSAIDS on aspirin's ability to inhibit COX‐1 are reversible and only last for the dosing interval and body clearance time of the drug.16
Adeonsine diphosphate (ADP)dependent stimulation of the P2Y12 receptor is another target for antiplatelet therapy. On its release, ADP binds to the P2Y12 receptor on platelets, resulting in activation and aggregation (Fig. 2). Ticlopidine and clopidogrel are thienopyridines that may irreversibly modify the P2Y12 receptor (Fig. 2).13 Safety concerns associated with ticlopidine use, including severe neutropenia, have limited its administration. Conversely, clopidogrel is relatively well‐tolerated and can prevent cardiovascular events in patients with CAD, ischemic stroke, and PAD. This agent is an orally administered prodrug requiring activation by hepatic cytochrome P450 enzymes.13
Aspirin and thienopyridines do not inhibit platelet aggregation induced by the binding of fibrinogen to the platelet glycoprotein (GP) IIb/IIIa receptor (Fig. 2).4, 13 However, there are 3 commonly administered GP IIb/IIIa inhibitors: abciximab, eptifibatide, and tirofiban (Fig. 2).4 Abciximab is the fab fragment of the chimeric monoclonal antibody 7E3 and irreversibly inhibits the GP IIb/IIIa receptor. By contrast, eptifibatide is a cyclic heptapeptide, tirofiban is a nonpeptide, and both agents are reversible inhibitors. These agents are administered intravenously, and boluses are reserved for the short‐term treatment of atherothrombosis in patients undergoing percutaneous coronary intervention.13
CONCLUSIONS
Atherothrombosis is a systemic disease that often affects coronary, intracranial, and peripheral arterial beds concomitantly, which increases the probability of a thrombotic event. Aggressive treatments, including acute and long‐term antiplatelet therapies, are required to reduce the risks associated with atherothrombosis. This supplement reviews the evidence‐based approaches for managing atherothrombosis. It will provide hospitalists with the knowledge needed to treat patients with PAD, stroke, and acute coronary syndrome. First, the administration of antiplatelet therapies to patients with acute coronary syndrome will be described. Then, guidelines for the management of patients with acute ischemic stroke and the use of antiplatelet therapies to reduce mortality due to primary and secondary ischemic events will be reviewed. Finally, the role of the hospitalist in the diagnosis of PAD in asymptomatic patients and in those with confirmed atherothrombosis will be discussed.
- ,,,.Trends in the leading causes of death in the United States, 1970‐2002.JAMA.2005;294:1255–1259.
- ,.The global burden of disease, 1990‐2020.Nat Med.1998:4:1241–1243.
- ,,,.The pathogenesis of coronary artery disease and the acute coronary syndromes.N Engl J Med.1992;326:242–250.
- .Antiplatelet therapy.Am J Med.1996;101:199–209.
- ,,.Platelets in inflammation and atherogenesis.J Clin Invest.2005;115:3378–3384.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.ACC/AHA 2000 guidelines for management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2000;36:970–1062.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- ,,, et al.Guidelines for the prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:557–617.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines.Circulation.2006;113:463–654.
- ,,.Inflammation and atherosclerosis.Circulation.2002;105:1135–1143.
- ,,, et al.AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update.J Am Coll Cardiol.2006;47:2130–2139.
- ,,, et al.Platelet‐active drugs: the relationships among dose, effectiveness, and side effects.Chest.2001;119:39–63.
- ,,, et al.Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal anti‐inflammatory drugs.Circulation.2003;108:1191–1195.
- ,.The effect of ibuprofen on cardioprotective effects of aspirin.Lancet.2003;361:573–574.
- ,,, et al.Cyclooxygenase inhibitors and the antiplatelet effects of aspirin.N Engl J Med.2001;345:1809–1817.
Acute atherothrombotic events associated with ischemic heart disease and stroke are the first and third most common causes of death in the United States, respectively.1 Despite an overall decrease in age‐adjusted mortality since 1970 in the United States, the worldwide prevalence of these diseases is anticipated to sharply increase by 2020.1, 2 Caring for patients with atherothrombosis is now within the purview of hospitalists to a larger extent than ever before. In recognition of the expanding role of these health care professionals and to reduce the risk of adverse cardiovascular events in the outpatient setting, the Society of Hospital Medicine held a symposium during its 10th Annual Meeting.
Rules of Engagement: The Hospitalist and Atherothrombosis took place on May 24, 2007, in Dallas, Texas. This supplement summarizes the highlights from this symposium and reviews the causes and polyvascular nature of atherothrombosis. The role of the hospitalist in managing atherothrombotic disease and evidence‐based practices for the evaluation and treatment of patients with various manifestations of atherothrombotic disease are also discussed.
ARTERIAL THROMBOSIS AND ITS POLYVASCULAR NATURE
Atherothrombosis refers to the formation of large and occlusive mural thrombi that arise from the rupture of an atherosclerotic plaque. Myocardial infarction (MI), ischemic stroke, and acute limb ischemia are the most severe manifestations of this disease.3, 4 This process begins when denuded or inflamed endothelial cells develop properties that permit platelet adhesion. At the site of endothelial dysfunction, activation of adherent platelet results in the release of inflammatory and mitogenic factors. After a series of dynamic and repetitive processes including amplified platelet activation, monocyte chemotaxis, adhesion, transmigration, and lipoprotein retention, plaque formation occurs.5 Consequently, the rupture or erosion of an atherosclerotic plaque produces a higher degree of platelet adhesion, activation, and aggregation, causing the fibrotic organization of a mural thrombus.3
The number of persons with multiple, concomitant cardiovascular disease (CAD), cerebrovascular disease (CVD), and peripheral arterial disease (PAD) accentuates the polyvascular nature of atherothrombosis (Fig. 1). The international Reduction of Atherothrombosis for Continued Health (REACH) Registry demonstrated that 1‐year incidence rates of major cardiovascular events (eg, MI, stroke, death) were high in patients with an established atherothrombotic disease and increased with the number of concomitant vascular diseases.6 These data infer that the burden on the vascular system is considered extensive on diagnosis of a single atherothrombotic disease. Thus, aggressive therapies are needed to reduce the risk of recurrent or other cardiovascular events. The management of risk factors for atherothrombosis such as hypercholesterolemia, dyslipidemia, hypertension, and diabetes mellitus fall under specific disease‐specific guidelines for patients presenting with atherothrombotic diseases.712

ANTIPLATELET THERAPIES
Antiplatelet therapies are used for the acute and long‐term treatment of patients after a thrombic event. Antiplatelet agents target the molecular mechanisms responsible for platelet activation and aggregation, such as the synthesis of thromboxane A2. On platelet activation, free arachidonic acid is converted to prostaglandin H2 (PGH2) by cyclooxygenase‐1 (COX‐1; Fig. 2). Further metabolism of PGH2 by thromboxane synthase produces thromboxane A2, which induces vasoconstriction (Fig. 2). Fortunately, the ability of platelets to produce COX‐1 is limited, and irreversible inhibition of this enzyme can impair thromboxane A2 synthesis for approximately 10 days.

Aspirin is a potent COX‐1 inhibitor, whose effects are evident 1 hour after dosing (Fig. 2).4, 13 Aspirin effectively prevents fatal and nonfatal vascular events in healthy individuals and in patients who present with acute MI or ischemic stroke.13 Unfortunately, a proportion of patients are aspirin resistant. Recent studies have indicated that interactions with the nonsteroidal anti‐inflammatory drug (NSAID) ibuprofen may diminish the primary and secondary protective effects of aspirin and may contribute to aspirin resistance, although the origin of this remains unclear.
The results of a post hoc subgroup analysis of 22,071 apparently healthy male physicians randomized to take aspirin or placebo for 5 years indicated that individuals who used NSAIDs for at least 60 days/year increased their risk of MI by more than 2‐fold compared with those who did not use NSAIDs.14 A second study conducted in patients following a major adverse cardiovascular event showed that the combination of aspirin plus ibuprofen increased the adjusted relative risk of cardiovascular mortality over an 8‐year period compared with aspirin alone.15 However, the effects of NSAIDS on aspirin's ability to inhibit COX‐1 are reversible and only last for the dosing interval and body clearance time of the drug.16
Adeonsine diphosphate (ADP)dependent stimulation of the P2Y12 receptor is another target for antiplatelet therapy. On its release, ADP binds to the P2Y12 receptor on platelets, resulting in activation and aggregation (Fig. 2). Ticlopidine and clopidogrel are thienopyridines that may irreversibly modify the P2Y12 receptor (Fig. 2).13 Safety concerns associated with ticlopidine use, including severe neutropenia, have limited its administration. Conversely, clopidogrel is relatively well‐tolerated and can prevent cardiovascular events in patients with CAD, ischemic stroke, and PAD. This agent is an orally administered prodrug requiring activation by hepatic cytochrome P450 enzymes.13
Aspirin and thienopyridines do not inhibit platelet aggregation induced by the binding of fibrinogen to the platelet glycoprotein (GP) IIb/IIIa receptor (Fig. 2).4, 13 However, there are 3 commonly administered GP IIb/IIIa inhibitors: abciximab, eptifibatide, and tirofiban (Fig. 2).4 Abciximab is the fab fragment of the chimeric monoclonal antibody 7E3 and irreversibly inhibits the GP IIb/IIIa receptor. By contrast, eptifibatide is a cyclic heptapeptide, tirofiban is a nonpeptide, and both agents are reversible inhibitors. These agents are administered intravenously, and boluses are reserved for the short‐term treatment of atherothrombosis in patients undergoing percutaneous coronary intervention.13
CONCLUSIONS
Atherothrombosis is a systemic disease that often affects coronary, intracranial, and peripheral arterial beds concomitantly, which increases the probability of a thrombotic event. Aggressive treatments, including acute and long‐term antiplatelet therapies, are required to reduce the risks associated with atherothrombosis. This supplement reviews the evidence‐based approaches for managing atherothrombosis. It will provide hospitalists with the knowledge needed to treat patients with PAD, stroke, and acute coronary syndrome. First, the administration of antiplatelet therapies to patients with acute coronary syndrome will be described. Then, guidelines for the management of patients with acute ischemic stroke and the use of antiplatelet therapies to reduce mortality due to primary and secondary ischemic events will be reviewed. Finally, the role of the hospitalist in the diagnosis of PAD in asymptomatic patients and in those with confirmed atherothrombosis will be discussed.
Acute atherothrombotic events associated with ischemic heart disease and stroke are the first and third most common causes of death in the United States, respectively.1 Despite an overall decrease in age‐adjusted mortality since 1970 in the United States, the worldwide prevalence of these diseases is anticipated to sharply increase by 2020.1, 2 Caring for patients with atherothrombosis is now within the purview of hospitalists to a larger extent than ever before. In recognition of the expanding role of these health care professionals and to reduce the risk of adverse cardiovascular events in the outpatient setting, the Society of Hospital Medicine held a symposium during its 10th Annual Meeting.
Rules of Engagement: The Hospitalist and Atherothrombosis took place on May 24, 2007, in Dallas, Texas. This supplement summarizes the highlights from this symposium and reviews the causes and polyvascular nature of atherothrombosis. The role of the hospitalist in managing atherothrombotic disease and evidence‐based practices for the evaluation and treatment of patients with various manifestations of atherothrombotic disease are also discussed.
ARTERIAL THROMBOSIS AND ITS POLYVASCULAR NATURE
Atherothrombosis refers to the formation of large and occlusive mural thrombi that arise from the rupture of an atherosclerotic plaque. Myocardial infarction (MI), ischemic stroke, and acute limb ischemia are the most severe manifestations of this disease.3, 4 This process begins when denuded or inflamed endothelial cells develop properties that permit platelet adhesion. At the site of endothelial dysfunction, activation of adherent platelet results in the release of inflammatory and mitogenic factors. After a series of dynamic and repetitive processes including amplified platelet activation, monocyte chemotaxis, adhesion, transmigration, and lipoprotein retention, plaque formation occurs.5 Consequently, the rupture or erosion of an atherosclerotic plaque produces a higher degree of platelet adhesion, activation, and aggregation, causing the fibrotic organization of a mural thrombus.3
The number of persons with multiple, concomitant cardiovascular disease (CAD), cerebrovascular disease (CVD), and peripheral arterial disease (PAD) accentuates the polyvascular nature of atherothrombosis (Fig. 1). The international Reduction of Atherothrombosis for Continued Health (REACH) Registry demonstrated that 1‐year incidence rates of major cardiovascular events (eg, MI, stroke, death) were high in patients with an established atherothrombotic disease and increased with the number of concomitant vascular diseases.6 These data infer that the burden on the vascular system is considered extensive on diagnosis of a single atherothrombotic disease. Thus, aggressive therapies are needed to reduce the risk of recurrent or other cardiovascular events. The management of risk factors for atherothrombosis such as hypercholesterolemia, dyslipidemia, hypertension, and diabetes mellitus fall under specific disease‐specific guidelines for patients presenting with atherothrombotic diseases.712

ANTIPLATELET THERAPIES
Antiplatelet therapies are used for the acute and long‐term treatment of patients after a thrombic event. Antiplatelet agents target the molecular mechanisms responsible for platelet activation and aggregation, such as the synthesis of thromboxane A2. On platelet activation, free arachidonic acid is converted to prostaglandin H2 (PGH2) by cyclooxygenase‐1 (COX‐1; Fig. 2). Further metabolism of PGH2 by thromboxane synthase produces thromboxane A2, which induces vasoconstriction (Fig. 2). Fortunately, the ability of platelets to produce COX‐1 is limited, and irreversible inhibition of this enzyme can impair thromboxane A2 synthesis for approximately 10 days.

Aspirin is a potent COX‐1 inhibitor, whose effects are evident 1 hour after dosing (Fig. 2).4, 13 Aspirin effectively prevents fatal and nonfatal vascular events in healthy individuals and in patients who present with acute MI or ischemic stroke.13 Unfortunately, a proportion of patients are aspirin resistant. Recent studies have indicated that interactions with the nonsteroidal anti‐inflammatory drug (NSAID) ibuprofen may diminish the primary and secondary protective effects of aspirin and may contribute to aspirin resistance, although the origin of this remains unclear.
The results of a post hoc subgroup analysis of 22,071 apparently healthy male physicians randomized to take aspirin or placebo for 5 years indicated that individuals who used NSAIDs for at least 60 days/year increased their risk of MI by more than 2‐fold compared with those who did not use NSAIDs.14 A second study conducted in patients following a major adverse cardiovascular event showed that the combination of aspirin plus ibuprofen increased the adjusted relative risk of cardiovascular mortality over an 8‐year period compared with aspirin alone.15 However, the effects of NSAIDS on aspirin's ability to inhibit COX‐1 are reversible and only last for the dosing interval and body clearance time of the drug.16
Adeonsine diphosphate (ADP)dependent stimulation of the P2Y12 receptor is another target for antiplatelet therapy. On its release, ADP binds to the P2Y12 receptor on platelets, resulting in activation and aggregation (Fig. 2). Ticlopidine and clopidogrel are thienopyridines that may irreversibly modify the P2Y12 receptor (Fig. 2).13 Safety concerns associated with ticlopidine use, including severe neutropenia, have limited its administration. Conversely, clopidogrel is relatively well‐tolerated and can prevent cardiovascular events in patients with CAD, ischemic stroke, and PAD. This agent is an orally administered prodrug requiring activation by hepatic cytochrome P450 enzymes.13
Aspirin and thienopyridines do not inhibit platelet aggregation induced by the binding of fibrinogen to the platelet glycoprotein (GP) IIb/IIIa receptor (Fig. 2).4, 13 However, there are 3 commonly administered GP IIb/IIIa inhibitors: abciximab, eptifibatide, and tirofiban (Fig. 2).4 Abciximab is the fab fragment of the chimeric monoclonal antibody 7E3 and irreversibly inhibits the GP IIb/IIIa receptor. By contrast, eptifibatide is a cyclic heptapeptide, tirofiban is a nonpeptide, and both agents are reversible inhibitors. These agents are administered intravenously, and boluses are reserved for the short‐term treatment of atherothrombosis in patients undergoing percutaneous coronary intervention.13
CONCLUSIONS
Atherothrombosis is a systemic disease that often affects coronary, intracranial, and peripheral arterial beds concomitantly, which increases the probability of a thrombotic event. Aggressive treatments, including acute and long‐term antiplatelet therapies, are required to reduce the risks associated with atherothrombosis. This supplement reviews the evidence‐based approaches for managing atherothrombosis. It will provide hospitalists with the knowledge needed to treat patients with PAD, stroke, and acute coronary syndrome. First, the administration of antiplatelet therapies to patients with acute coronary syndrome will be described. Then, guidelines for the management of patients with acute ischemic stroke and the use of antiplatelet therapies to reduce mortality due to primary and secondary ischemic events will be reviewed. Finally, the role of the hospitalist in the diagnosis of PAD in asymptomatic patients and in those with confirmed atherothrombosis will be discussed.
- ,,,.Trends in the leading causes of death in the United States, 1970‐2002.JAMA.2005;294:1255–1259.
- ,.The global burden of disease, 1990‐2020.Nat Med.1998:4:1241–1243.
- ,,,.The pathogenesis of coronary artery disease and the acute coronary syndromes.N Engl J Med.1992;326:242–250.
- .Antiplatelet therapy.Am J Med.1996;101:199–209.
- ,,.Platelets in inflammation and atherogenesis.J Clin Invest.2005;115:3378–3384.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.ACC/AHA 2000 guidelines for management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2000;36:970–1062.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- ,,, et al.Guidelines for the prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:557–617.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines.Circulation.2006;113:463–654.
- ,,.Inflammation and atherosclerosis.Circulation.2002;105:1135–1143.
- ,,, et al.AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update.J Am Coll Cardiol.2006;47:2130–2139.
- ,,, et al.Platelet‐active drugs: the relationships among dose, effectiveness, and side effects.Chest.2001;119:39–63.
- ,,, et al.Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal anti‐inflammatory drugs.Circulation.2003;108:1191–1195.
- ,.The effect of ibuprofen on cardioprotective effects of aspirin.Lancet.2003;361:573–574.
- ,,, et al.Cyclooxygenase inhibitors and the antiplatelet effects of aspirin.N Engl J Med.2001;345:1809–1817.
- ,,,.Trends in the leading causes of death in the United States, 1970‐2002.JAMA.2005;294:1255–1259.
- ,.The global burden of disease, 1990‐2020.Nat Med.1998:4:1241–1243.
- ,,,.The pathogenesis of coronary artery disease and the acute coronary syndromes.N Engl J Med.1992;326:242–250.
- .Antiplatelet therapy.Am J Med.1996;101:199–209.
- ,,.Platelets in inflammation and atherogenesis.J Clin Invest.2005;115:3378–3384.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.ACC/AHA 2000 guidelines for management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2000;36:970–1062.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- ,,, et al.Guidelines for the prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:557–617.
- ,,, et al.ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines.Circulation.2006;113:463–654.
- ,,.Inflammation and atherosclerosis.Circulation.2002;105:1135–1143.
- ,,, et al.AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update.J Am Coll Cardiol.2006;47:2130–2139.
- ,,, et al.Platelet‐active drugs: the relationships among dose, effectiveness, and side effects.Chest.2001;119:39–63.
- ,,, et al.Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal anti‐inflammatory drugs.Circulation.2003;108:1191–1195.
- ,.The effect of ibuprofen on cardioprotective effects of aspirin.Lancet.2003;361:573–574.
- ,,, et al.Cyclooxygenase inhibitors and the antiplatelet effects of aspirin.N Engl J Med.2001;345:1809–1817.
Rules of Engagement
Atherothrombosis triggered by plaque rupture reduces arterial blood flow, leading to myocardial ischemia and/or necrosis, in which the extent of occlusion relates to the clinical presentations of acute coronary syndrome (ACS): unstable angina (UA), non‐ST‐segment and ST‐segment elevated myocardial infarction (NSTEMI and STEMI, respectively). Although UA and NSTEMI may be indistinguishable at the time of presentation, NSTEMI is defined by myocardial necrosis and is differentiated by release of cardiac enzymes.1 In UA, the myocardial ischemia is reversible, without necrosis. Typically, STEMI results from the total occlusion of a large epicardial infarct‐related artery and is diagnosed by electrocardiography (ECG) and the release of cardiac enzymes.2 Strategies employed by emergency physicians and hospitalists to treat the spectrum of symptoms caused by ACS include pharmacotherapy and revascularization procedures. Coordination of care between these 2 groups of physicians, and appropriate handoff of patients from the ED to hospitalists, utilizing guideline‐based care pathways and treatment protocols, will ensure maximization of outcomes in patients with ACS.
TREATMENT STRATEGIES FOR ACUTE CORONARY SYNDROME
The American College of Cardiology and American Heart Association (ACC/AHA) guidelines indicate the need for rapid triage and aggressive treatment when managing patients with ACS.1, 2 Those patients with NSTEMI are quickly differentiated from those with STEMI, who are evaluated rapidly for pharmacological and/or revascularization therapy.2 Patients with UA/NSTEMI are further stratified according to their risk of death or nonfatal MI, and appropriate care pathways are instituted as indicated by their risk for adverse outcomes. These care pathways must be guideline driven to assure maximal effectiveness.
Percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG) revascularization procedures have been highly successful with low complication rates in patients with ACS. Although revascularization in patients with UA/NSTEMI is determined in part by their risk stratification, those patients with STEMI are almost always candidates for PCI or CABG.
Unfortunately, vascular flow is not completely restored in many patients after undergoing revascularization, both STEMI and UA/NSTEMI. This may be attributed to microvascular damage caused by the formation of microemboli during the initial atherothrombotic event or revascularization.1, 2 Given that percutaneous catheters are incapable of restoring microvasculature patency, pharmacotherapy is the only alternative for this cohort of patients. Thus, acute antithrombotic therapies (antiplatelet agents and anticoagulants) are administered to maintain vascular flow in patients with ACS before and after revascularization. Maximization of the effectiveness of this antithrombotic therapy in the precatheterization period will decrease the occurrence of ischemia and the extent of infarction.
Patients with ACS often have multiple vulnerable plaques in addition to the culprit lesion responsible for the initial bout of ischemia. The presence of multiple vulnerable plaques increases the risk of secondary vascular events such as stroke, myocardial infarction (MI), or vascular death in patients with ACS.3, 4 Thus, ongoing anti‐inflammatory and antiplatelet therapies are needed to stabilize vulnerable plaques and prevent secondary vascular events.5 The ACC/AHA guidelines recommend sustained antiplatelet and antithrombin therapies to improve long‐term outcomes in patients with ACS.1
ASPIRIN THERAPY FOR ACUTE CORONARY SYNDROME
Aspirin effectively reduces the short‐term risk of myocardial ischemic events in patients with ACS. The RISC and ISIS‐2 studies showed a clinically relevant reduction in risk of MI or death after short durations of aspirin therapy in patients with UA (3 months) and suspected acute MI (5 weeks), respectively.6, 7 Although long‐term therapy with aspirin has not been tested among individual ACS subgroups, clinical studies have demonstrated that aspirin therapy effectively prevents MI, stroke, and vascular death in patients with prior MI or other vascular events. A meta‐analysis of 65 trials in patients with atherothrombosis showed that aspirin therapy reduces the odds of vascular events by 23% 2%.8 Moreover, aspirin has a class IA recommendation from the ACC/AHA for the management of patients with UA/NSTEMI or STEMI; in the absence of contraindications, it should be initiated as soon as possible and continued indefinitely.1, 2 On initial presentation of ACS, high‐dose aspirin is recommended (162‐325 mg), and lower doses (75‐162 mg), which minimize bleeding or gastrointestinal side effects, are indicated thereafter.1, 2
SUSTAINED ANTIPLATELET THERAPIES FORUA/NSTEMI
Clinical data have indicated that long‐term therapies may be more beneficial to patients with UA/NSTEMI than to those with STEMI. Antiplatelet therapy reduces the risk of vascular events in patients with UA/NSTEMI; benefits are noticeable soon after therapy is initiated. The sustained use of clopidogrel, an inhibitor of ADP‐dependent platelet activation, is at least as effective as aspirin in reducing long‐term vascular events in patients with atherothrombotic diseases, and the ACC/AHA recommends this agent be used when aspirin is contraindicated.1, 9
Recently, the Clopidogrel in Unstable Angina Recurrent Events (CURE) study demonstrated that the combination of clopidogrel and aspirin is superior to either agent alone in preventing vascular events in patients with UA/NSTEMI.10 Patients (N = 12,562) were randomized within 24 hours of UA/NSTEMI presentation to receive aspirin (75‐325 mg/day), an immediate loading dose of clopidogrel (300 mg) followed by once‐daily clopidogrel (75 mg), or a matching placebo for 3‐12 months.10 At the treating physicians' discretion, patients were treated with anticoagulants, revascularization procedures, or GP IIb/IIIa inhibitors after randomization. When used in combination with aspirin, clopidogrel reduced the risk of a major cardiovascular event by 20% (relative risk RR, 0.80; 95% CI, 0.72‐0.90; P < .001) compared with aspirin alone.10
A retrospective subgroup analysis revealed that the therapeutic benefits of this drug combination increases as the risk of a vascular event increases in patients with UA/NSTEMI.11 In addition, the effects of clopidogrel and aspirin were apparent early after randomization (Fig. 1); a 34% reduction in the risk of major cardiovascular events (RR, 0.66; 95% CI, 0.51‐0.86; P < .003) was observed within 24 hours postrandomization.12 Thirty days after randomization, the relative risk reduction in the composite end point of cardiovascular death and nonfatal MI, stroke, and refractory ischemia was 17% (RR, 0.83; 95% CI, 0.73‐0.93; P < .002) for clopidogrel with aspirin.
The combination of clopidogrel and aspirin was associated with a 38% (RR, 1.38; 95% CI, 1.13‐1.67; P = .001) increase in major bleeding episodes compared with patients taking aspirin alone. Conversely, the incidence of bleeding requiring surgical intervention, hemorrhagic stroke, and fatal hemorrhage between treatment groups did not differ significantly.10 The clinical benefits of clopidogrel and aspirin outweighed the risk of life‐threatening bleeding (RR, 0.84; 95% CI, 0.76‐0.93), even when the number of deaths and the number of life‐threatening bleeds were taken into account in the efficacy‐safety analysis.13 As a result, the ACC/AHA guidelines for the management of patients with UA/NSTEMI recommend that clopidogrel and aspirin should be administered to hospitalized patients as soon as possible and should be continued for 1 month and up to 12 months after the initial presentation of symptoms.1 The new ACC/AHA guidelines recommend upstream clopidogrel as a class IA treatment for UA/non‐ST‐elevation MI in patients who are managed either invasively (catheterization within 6‐24 hours) or conservatively (selectively invasive medical management, followed by catheterization if needed). This latter recommendation is much stronger than the prior guidelines and illustrates the importance of antiplatelet therapy as a part of medical management for intermediate‐ to high‐risk ACS.
ANTIPLATELET THERAPIES WITH REVASCULARIZATION PROCEDURES IN UA/NSTEMI
The efficacy of clopidogrel and aspirin has been examined in patients enrolled in the CURE study who underwent revascularization procedures (eg, PCI, CABG). These agents reduced the relative risk of cardiovascular death or nonfatal MI by 31% (RR, 0.69; 95% CI, 0.54‐0.87; P = .002) in patients who underwent PCI.14 Subgroup analysis of PCI timing relative to randomization showed that the risk of vascular events increased with time to revascularization. However, this relationship was not evident in patients treated with aspirin alone (Fig. 2).14 There was no significant difference in the incidence of major bleeding (RR, 1.2; 95% CI, 0.70‐1.78; P = .64) between the treatment groups from PCI to follow‐up.14, 15 Similar results have been reported elsewhere.16 As a result, ACC/AHA guidelines recommend the early administration of clopidogrel and aspirin to patients undergoing planned PCI, and should be continued up to 12 months following the procedure, unless it is contraindicated.1
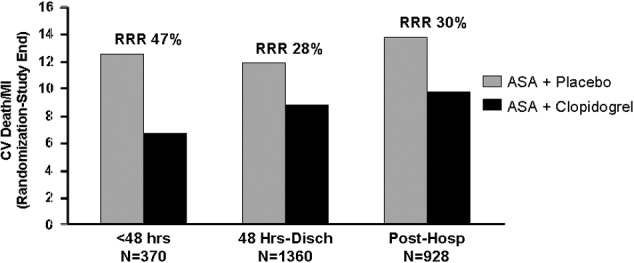
Clopidogrel and aspirin also reduced the risk of vascular death by 11% (RR, 0.89; 95% CI, 0.71‐1.11) in patients who underwent CABG.13 Study medications were stopped prior to the procedure. Major bleeding was not observed in patients who stopped medication at least 5 days prior to surgery and did not differ significantly from those who stopped therapy less than 5 days before CABG.13 Thus, the ACC/AHA recommends withholding clopidogrel therapy for 5‐7 days prior to elective CABG.1
Platelet glycoprotein (GP) IIb/IIIa inhibitors are another class of antiplatelet therapy that can be beneficial to patients with UA/NSTEMI undergoing PCI. Analysis of the CAPTURE, PURSUIT, and PRISM‐PLUS trials of the GP IIb/IIIa inhibitors abciximab, eptifibatide, and tirofiban, respectively, showed they effectively reduce the rates of death and/or MI (odds ratio [OR], 0.66; 95% CI, 0.54‐0.81) in patients with UA/STEMI prior to PCI, which was more pronounced (OR, 0.59; 95% CI, 0.44‐0.81) when outcomes were measured 48 hours after revascularization.17
The ISAR‐REACT 2 study examined the efficacy of abciximab with clopidogrel and aspirin in patients with NSTEMI prior to PCI.18 The primary end point was a composite of death, MI, and secondary urgent target revascularization 30 days after randomization. The addition of abciximab to 600 mg of the clopidogrel loading dose and aspirin reduced the risk of major adverse cardiovascular events by 25% (RR, 0.75; 95% CI, 0.58‐0.97; P = .03) 30 days after the initiation of therapy. However, subgroup analysis revealed that this therapeutic benefit was confined to patients with elevated troponin levels.18 The ACC/AHA also recommends the concomitant administration of GP IIb/IIIa inhibitors to patients receiving heparin, aspirin, and clopidogrel and undergoing planned PCI.1 The guideline recommendation is for either clopidogrel or a GP IIb/IIIa inhibitor in the invasive pathway (class IA), but both are recommended in patients with elevated troponin, recurrent ischemia, or delay to catheterization (class IIaB). This triple antiplatelet therapy is considered advantageous in the highest=risk NSTEMI patients. The short‐term and long‐term benefits of antiplatelet therapies are consistent across the UA/NSTEMI‐risk spectrum and galvanize the ACC/AHA recommendations for antithrombotic therapy in patients with UA/NSTEMI (Fig. 3).1
ANTIPLATELET THERAPIES FOR STEMI
The ACC/AHA has made several recommendations regarding the administration of clopidogrel in patients with STEMI. Clopidogrel should be administered to patients with contraindications to aspirin. After placement of a bare metal or drug‐eluting stent, this agent should be administered at least 1 month and less than 12 months after surgery, respectively. It should also be withheld at least 5‐7 days prior to CABG.2 These guidelines are largely based on clinical trials in patients with UA/STEMI or ACS.9, 15 Fortunately, several more recent studies have examined the use of antiplatelet therapy in patients with STEMI.
In the Clopidogrel as Adjunctive Reperfusion TherapyThrombolysis in Myocardial Infarction (CLARITY‐TIMI) 28 study, 3491 patients with STEMI were randomized to receive clopidogrel (300 mg loading dose, then 75 mg/day) or placebo. Patients were also treated with a fibrinolytic agent, aspirin, and unfractionated heparin and underwent angiography 48‐192 hours after randomization. Clopidogrel reduced the risk of detecting an occluded infarct‐related artery by angiography or recurrent MI/death prior to angiography by 36% (OR, 0.64; 95% CI, 0.53‐0.76; P < .001) compared with placebo.19 It also reduced the risk of major adverse cardiovascular events 30 days after randomization by 20% (OR, 0.80; 95% CI, 0.65‐0.97; P = .03) compared with placebo, with no significant difference in the risk of bleeding between the treatment groups.
The PCI‐CLARITY study examined the efficacy of clopidogrel in patients undergoing PCI during the CLARITY‐TIMI 28 trial. Clopidogrel reduced the rate of major adverse cardiovascular events by 46% (OR, 0.54; 95% CI, 0.35‐0.85; P = .008) after PCI and 30 days after randomization, with no excess in major bleeding.20 Although the use of this agent along with contemporary reperfusion therapies in patients with STEMI is supported, further research into the sustained use of clopidogrel in STEMI is needed.
CONCLUSIONS
Patients with ACS require aggressive diagnosis and acute treatment. However, long‐term therapies are also needed to improve outcomes. Antiplatelet therapies are a key component of the treatment of ACS. The benefits of aspirin and clopidogrel combination therapy are evident early, and their sustained use improves the outcome of patients who receive medical therapy and/or revascularization procedures. Early initiation of antiplatelet therapy in patients with ACS is best accomplished with care pathways or ACS protocols that are guideline driven. Initiation of these protocols in the ED, with appropriate handoff to hospitalists, will ensure maximization of antiplatelet therapy for patients throughout the precatheterization medical management period. Although antiplatelet agents may be associated with an increased risk of bleeding in some patients, these risks can be minimized and are outweighed by the benefits of clopidogrel and aspirin.
- ,,, et al.ACC/AHA guidelines for the management of patients with unstable angina/non‐ST‐segment elevation myocardial infarction: executive summary.J Am Coll Cardiol.2007;50:e1–e157. Available at: http://www.acc.org.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association.Circulation.2006;113:873–823.
- The RISC Group.Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease.Lancet.1990;336:827–830.
- ISIS‐2 Collaborative Group.Randomized trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction: ISIS‐2.Lancet.1988;2:349–360.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- CAPRIE steering committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- CURE Trial Investigators.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med.2001;345:494–502.
- ,,, et al.Benefit of clopidogrel in patients with acute coronary syndromes without ST‐segment elevation in various risk groups.Circulation.2002;106:1622–1626.
- ,,, et al.Early and late effects of clopidogrel in patients with acute coronary syndromes.Circulation.2003;107:966–972.
- ,,, et al.Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non‐ST‐elevation acute coronary syndrome: The clopidogrel in unstable angina to prevent recurrent ischemic events (CURE) trial.Circulation.2004;110:1202–1208.
- ,,, et al.Benefit of clopidogrel according to timing of percutaneous coronary intervention in patients with acute coronary syndromes: Further results from the clopidogrel in unstable angina to prevent recurrent events (CURE) study.Am Heart J.2005;150:1177–1184.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet.2001;358:527–533.
- ,,, et al.Early and sustained dual oral antiplatelet therapy following percutaneous intervention. A randomized trial.JAMA.2002;288:2411–2420.
- ,,,,,.Platelet glycoprotein IIb/IIIa receptor inhibition in non‐ST‐elevation acute coronary syndromes: early benefit during medical treatment only, with additional protection during percutaneous coronary intervention.Circulation.1999;100:2045–2048.
- ,,, et al.Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment. The ISAR‐REACT2 randomized trial.JAMA.2006;295:1531–1538.
- ,,, et al.Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation.N Engl J Med.2005;352:1179–1189.
- ,,, et al.Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST‐elevation myocardial infarction treated with fibrinolytics. The PCI‐CLARITY study.JAMA.2005;294:1224–1232.
Atherothrombosis triggered by plaque rupture reduces arterial blood flow, leading to myocardial ischemia and/or necrosis, in which the extent of occlusion relates to the clinical presentations of acute coronary syndrome (ACS): unstable angina (UA), non‐ST‐segment and ST‐segment elevated myocardial infarction (NSTEMI and STEMI, respectively). Although UA and NSTEMI may be indistinguishable at the time of presentation, NSTEMI is defined by myocardial necrosis and is differentiated by release of cardiac enzymes.1 In UA, the myocardial ischemia is reversible, without necrosis. Typically, STEMI results from the total occlusion of a large epicardial infarct‐related artery and is diagnosed by electrocardiography (ECG) and the release of cardiac enzymes.2 Strategies employed by emergency physicians and hospitalists to treat the spectrum of symptoms caused by ACS include pharmacotherapy and revascularization procedures. Coordination of care between these 2 groups of physicians, and appropriate handoff of patients from the ED to hospitalists, utilizing guideline‐based care pathways and treatment protocols, will ensure maximization of outcomes in patients with ACS.
TREATMENT STRATEGIES FOR ACUTE CORONARY SYNDROME
The American College of Cardiology and American Heart Association (ACC/AHA) guidelines indicate the need for rapid triage and aggressive treatment when managing patients with ACS.1, 2 Those patients with NSTEMI are quickly differentiated from those with STEMI, who are evaluated rapidly for pharmacological and/or revascularization therapy.2 Patients with UA/NSTEMI are further stratified according to their risk of death or nonfatal MI, and appropriate care pathways are instituted as indicated by their risk for adverse outcomes. These care pathways must be guideline driven to assure maximal effectiveness.
Percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG) revascularization procedures have been highly successful with low complication rates in patients with ACS. Although revascularization in patients with UA/NSTEMI is determined in part by their risk stratification, those patients with STEMI are almost always candidates for PCI or CABG.
Unfortunately, vascular flow is not completely restored in many patients after undergoing revascularization, both STEMI and UA/NSTEMI. This may be attributed to microvascular damage caused by the formation of microemboli during the initial atherothrombotic event or revascularization.1, 2 Given that percutaneous catheters are incapable of restoring microvasculature patency, pharmacotherapy is the only alternative for this cohort of patients. Thus, acute antithrombotic therapies (antiplatelet agents and anticoagulants) are administered to maintain vascular flow in patients with ACS before and after revascularization. Maximization of the effectiveness of this antithrombotic therapy in the precatheterization period will decrease the occurrence of ischemia and the extent of infarction.
Patients with ACS often have multiple vulnerable plaques in addition to the culprit lesion responsible for the initial bout of ischemia. The presence of multiple vulnerable plaques increases the risk of secondary vascular events such as stroke, myocardial infarction (MI), or vascular death in patients with ACS.3, 4 Thus, ongoing anti‐inflammatory and antiplatelet therapies are needed to stabilize vulnerable plaques and prevent secondary vascular events.5 The ACC/AHA guidelines recommend sustained antiplatelet and antithrombin therapies to improve long‐term outcomes in patients with ACS.1
ASPIRIN THERAPY FOR ACUTE CORONARY SYNDROME
Aspirin effectively reduces the short‐term risk of myocardial ischemic events in patients with ACS. The RISC and ISIS‐2 studies showed a clinically relevant reduction in risk of MI or death after short durations of aspirin therapy in patients with UA (3 months) and suspected acute MI (5 weeks), respectively.6, 7 Although long‐term therapy with aspirin has not been tested among individual ACS subgroups, clinical studies have demonstrated that aspirin therapy effectively prevents MI, stroke, and vascular death in patients with prior MI or other vascular events. A meta‐analysis of 65 trials in patients with atherothrombosis showed that aspirin therapy reduces the odds of vascular events by 23% 2%.8 Moreover, aspirin has a class IA recommendation from the ACC/AHA for the management of patients with UA/NSTEMI or STEMI; in the absence of contraindications, it should be initiated as soon as possible and continued indefinitely.1, 2 On initial presentation of ACS, high‐dose aspirin is recommended (162‐325 mg), and lower doses (75‐162 mg), which minimize bleeding or gastrointestinal side effects, are indicated thereafter.1, 2
SUSTAINED ANTIPLATELET THERAPIES FORUA/NSTEMI
Clinical data have indicated that long‐term therapies may be more beneficial to patients with UA/NSTEMI than to those with STEMI. Antiplatelet therapy reduces the risk of vascular events in patients with UA/NSTEMI; benefits are noticeable soon after therapy is initiated. The sustained use of clopidogrel, an inhibitor of ADP‐dependent platelet activation, is at least as effective as aspirin in reducing long‐term vascular events in patients with atherothrombotic diseases, and the ACC/AHA recommends this agent be used when aspirin is contraindicated.1, 9
Recently, the Clopidogrel in Unstable Angina Recurrent Events (CURE) study demonstrated that the combination of clopidogrel and aspirin is superior to either agent alone in preventing vascular events in patients with UA/NSTEMI.10 Patients (N = 12,562) were randomized within 24 hours of UA/NSTEMI presentation to receive aspirin (75‐325 mg/day), an immediate loading dose of clopidogrel (300 mg) followed by once‐daily clopidogrel (75 mg), or a matching placebo for 3‐12 months.10 At the treating physicians' discretion, patients were treated with anticoagulants, revascularization procedures, or GP IIb/IIIa inhibitors after randomization. When used in combination with aspirin, clopidogrel reduced the risk of a major cardiovascular event by 20% (relative risk RR, 0.80; 95% CI, 0.72‐0.90; P < .001) compared with aspirin alone.10
A retrospective subgroup analysis revealed that the therapeutic benefits of this drug combination increases as the risk of a vascular event increases in patients with UA/NSTEMI.11 In addition, the effects of clopidogrel and aspirin were apparent early after randomization (Fig. 1); a 34% reduction in the risk of major cardiovascular events (RR, 0.66; 95% CI, 0.51‐0.86; P < .003) was observed within 24 hours postrandomization.12 Thirty days after randomization, the relative risk reduction in the composite end point of cardiovascular death and nonfatal MI, stroke, and refractory ischemia was 17% (RR, 0.83; 95% CI, 0.73‐0.93; P < .002) for clopidogrel with aspirin.

The combination of clopidogrel and aspirin was associated with a 38% (RR, 1.38; 95% CI, 1.13‐1.67; P = .001) increase in major bleeding episodes compared with patients taking aspirin alone. Conversely, the incidence of bleeding requiring surgical intervention, hemorrhagic stroke, and fatal hemorrhage between treatment groups did not differ significantly.10 The clinical benefits of clopidogrel and aspirin outweighed the risk of life‐threatening bleeding (RR, 0.84; 95% CI, 0.76‐0.93), even when the number of deaths and the number of life‐threatening bleeds were taken into account in the efficacy‐safety analysis.13 As a result, the ACC/AHA guidelines for the management of patients with UA/NSTEMI recommend that clopidogrel and aspirin should be administered to hospitalized patients as soon as possible and should be continued for 1 month and up to 12 months after the initial presentation of symptoms.1 The new ACC/AHA guidelines recommend upstream clopidogrel as a class IA treatment for UA/non‐ST‐elevation MI in patients who are managed either invasively (catheterization within 6‐24 hours) or conservatively (selectively invasive medical management, followed by catheterization if needed). This latter recommendation is much stronger than the prior guidelines and illustrates the importance of antiplatelet therapy as a part of medical management for intermediate‐ to high‐risk ACS.
ANTIPLATELET THERAPIES WITH REVASCULARIZATION PROCEDURES IN UA/NSTEMI
The efficacy of clopidogrel and aspirin has been examined in patients enrolled in the CURE study who underwent revascularization procedures (eg, PCI, CABG). These agents reduced the relative risk of cardiovascular death or nonfatal MI by 31% (RR, 0.69; 95% CI, 0.54‐0.87; P = .002) in patients who underwent PCI.14 Subgroup analysis of PCI timing relative to randomization showed that the risk of vascular events increased with time to revascularization. However, this relationship was not evident in patients treated with aspirin alone (Fig. 2).14 There was no significant difference in the incidence of major bleeding (RR, 1.2; 95% CI, 0.70‐1.78; P = .64) between the treatment groups from PCI to follow‐up.14, 15 Similar results have been reported elsewhere.16 As a result, ACC/AHA guidelines recommend the early administration of clopidogrel and aspirin to patients undergoing planned PCI, and should be continued up to 12 months following the procedure, unless it is contraindicated.1

Clopidogrel and aspirin also reduced the risk of vascular death by 11% (RR, 0.89; 95% CI, 0.71‐1.11) in patients who underwent CABG.13 Study medications were stopped prior to the procedure. Major bleeding was not observed in patients who stopped medication at least 5 days prior to surgery and did not differ significantly from those who stopped therapy less than 5 days before CABG.13 Thus, the ACC/AHA recommends withholding clopidogrel therapy for 5‐7 days prior to elective CABG.1
Platelet glycoprotein (GP) IIb/IIIa inhibitors are another class of antiplatelet therapy that can be beneficial to patients with UA/NSTEMI undergoing PCI. Analysis of the CAPTURE, PURSUIT, and PRISM‐PLUS trials of the GP IIb/IIIa inhibitors abciximab, eptifibatide, and tirofiban, respectively, showed they effectively reduce the rates of death and/or MI (odds ratio [OR], 0.66; 95% CI, 0.54‐0.81) in patients with UA/STEMI prior to PCI, which was more pronounced (OR, 0.59; 95% CI, 0.44‐0.81) when outcomes were measured 48 hours after revascularization.17
The ISAR‐REACT 2 study examined the efficacy of abciximab with clopidogrel and aspirin in patients with NSTEMI prior to PCI.18 The primary end point was a composite of death, MI, and secondary urgent target revascularization 30 days after randomization. The addition of abciximab to 600 mg of the clopidogrel loading dose and aspirin reduced the risk of major adverse cardiovascular events by 25% (RR, 0.75; 95% CI, 0.58‐0.97; P = .03) 30 days after the initiation of therapy. However, subgroup analysis revealed that this therapeutic benefit was confined to patients with elevated troponin levels.18 The ACC/AHA also recommends the concomitant administration of GP IIb/IIIa inhibitors to patients receiving heparin, aspirin, and clopidogrel and undergoing planned PCI.1 The guideline recommendation is for either clopidogrel or a GP IIb/IIIa inhibitor in the invasive pathway (class IA), but both are recommended in patients with elevated troponin, recurrent ischemia, or delay to catheterization (class IIaB). This triple antiplatelet therapy is considered advantageous in the highest=risk NSTEMI patients. The short‐term and long‐term benefits of antiplatelet therapies are consistent across the UA/NSTEMI‐risk spectrum and galvanize the ACC/AHA recommendations for antithrombotic therapy in patients with UA/NSTEMI (Fig. 3).1

ANTIPLATELET THERAPIES FOR STEMI
The ACC/AHA has made several recommendations regarding the administration of clopidogrel in patients with STEMI. Clopidogrel should be administered to patients with contraindications to aspirin. After placement of a bare metal or drug‐eluting stent, this agent should be administered at least 1 month and less than 12 months after surgery, respectively. It should also be withheld at least 5‐7 days prior to CABG.2 These guidelines are largely based on clinical trials in patients with UA/STEMI or ACS.9, 15 Fortunately, several more recent studies have examined the use of antiplatelet therapy in patients with STEMI.
In the Clopidogrel as Adjunctive Reperfusion TherapyThrombolysis in Myocardial Infarction (CLARITY‐TIMI) 28 study, 3491 patients with STEMI were randomized to receive clopidogrel (300 mg loading dose, then 75 mg/day) or placebo. Patients were also treated with a fibrinolytic agent, aspirin, and unfractionated heparin and underwent angiography 48‐192 hours after randomization. Clopidogrel reduced the risk of detecting an occluded infarct‐related artery by angiography or recurrent MI/death prior to angiography by 36% (OR, 0.64; 95% CI, 0.53‐0.76; P < .001) compared with placebo.19 It also reduced the risk of major adverse cardiovascular events 30 days after randomization by 20% (OR, 0.80; 95% CI, 0.65‐0.97; P = .03) compared with placebo, with no significant difference in the risk of bleeding between the treatment groups.
The PCI‐CLARITY study examined the efficacy of clopidogrel in patients undergoing PCI during the CLARITY‐TIMI 28 trial. Clopidogrel reduced the rate of major adverse cardiovascular events by 46% (OR, 0.54; 95% CI, 0.35‐0.85; P = .008) after PCI and 30 days after randomization, with no excess in major bleeding.20 Although the use of this agent along with contemporary reperfusion therapies in patients with STEMI is supported, further research into the sustained use of clopidogrel in STEMI is needed.
CONCLUSIONS
Patients with ACS require aggressive diagnosis and acute treatment. However, long‐term therapies are also needed to improve outcomes. Antiplatelet therapies are a key component of the treatment of ACS. The benefits of aspirin and clopidogrel combination therapy are evident early, and their sustained use improves the outcome of patients who receive medical therapy and/or revascularization procedures. Early initiation of antiplatelet therapy in patients with ACS is best accomplished with care pathways or ACS protocols that are guideline driven. Initiation of these protocols in the ED, with appropriate handoff to hospitalists, will ensure maximization of antiplatelet therapy for patients throughout the precatheterization medical management period. Although antiplatelet agents may be associated with an increased risk of bleeding in some patients, these risks can be minimized and are outweighed by the benefits of clopidogrel and aspirin.
Atherothrombosis triggered by plaque rupture reduces arterial blood flow, leading to myocardial ischemia and/or necrosis, in which the extent of occlusion relates to the clinical presentations of acute coronary syndrome (ACS): unstable angina (UA), non‐ST‐segment and ST‐segment elevated myocardial infarction (NSTEMI and STEMI, respectively). Although UA and NSTEMI may be indistinguishable at the time of presentation, NSTEMI is defined by myocardial necrosis and is differentiated by release of cardiac enzymes.1 In UA, the myocardial ischemia is reversible, without necrosis. Typically, STEMI results from the total occlusion of a large epicardial infarct‐related artery and is diagnosed by electrocardiography (ECG) and the release of cardiac enzymes.2 Strategies employed by emergency physicians and hospitalists to treat the spectrum of symptoms caused by ACS include pharmacotherapy and revascularization procedures. Coordination of care between these 2 groups of physicians, and appropriate handoff of patients from the ED to hospitalists, utilizing guideline‐based care pathways and treatment protocols, will ensure maximization of outcomes in patients with ACS.
TREATMENT STRATEGIES FOR ACUTE CORONARY SYNDROME
The American College of Cardiology and American Heart Association (ACC/AHA) guidelines indicate the need for rapid triage and aggressive treatment when managing patients with ACS.1, 2 Those patients with NSTEMI are quickly differentiated from those with STEMI, who are evaluated rapidly for pharmacological and/or revascularization therapy.2 Patients with UA/NSTEMI are further stratified according to their risk of death or nonfatal MI, and appropriate care pathways are instituted as indicated by their risk for adverse outcomes. These care pathways must be guideline driven to assure maximal effectiveness.
Percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG) revascularization procedures have been highly successful with low complication rates in patients with ACS. Although revascularization in patients with UA/NSTEMI is determined in part by their risk stratification, those patients with STEMI are almost always candidates for PCI or CABG.
Unfortunately, vascular flow is not completely restored in many patients after undergoing revascularization, both STEMI and UA/NSTEMI. This may be attributed to microvascular damage caused by the formation of microemboli during the initial atherothrombotic event or revascularization.1, 2 Given that percutaneous catheters are incapable of restoring microvasculature patency, pharmacotherapy is the only alternative for this cohort of patients. Thus, acute antithrombotic therapies (antiplatelet agents and anticoagulants) are administered to maintain vascular flow in patients with ACS before and after revascularization. Maximization of the effectiveness of this antithrombotic therapy in the precatheterization period will decrease the occurrence of ischemia and the extent of infarction.
Patients with ACS often have multiple vulnerable plaques in addition to the culprit lesion responsible for the initial bout of ischemia. The presence of multiple vulnerable plaques increases the risk of secondary vascular events such as stroke, myocardial infarction (MI), or vascular death in patients with ACS.3, 4 Thus, ongoing anti‐inflammatory and antiplatelet therapies are needed to stabilize vulnerable plaques and prevent secondary vascular events.5 The ACC/AHA guidelines recommend sustained antiplatelet and antithrombin therapies to improve long‐term outcomes in patients with ACS.1
ASPIRIN THERAPY FOR ACUTE CORONARY SYNDROME
Aspirin effectively reduces the short‐term risk of myocardial ischemic events in patients with ACS. The RISC and ISIS‐2 studies showed a clinically relevant reduction in risk of MI or death after short durations of aspirin therapy in patients with UA (3 months) and suspected acute MI (5 weeks), respectively.6, 7 Although long‐term therapy with aspirin has not been tested among individual ACS subgroups, clinical studies have demonstrated that aspirin therapy effectively prevents MI, stroke, and vascular death in patients with prior MI or other vascular events. A meta‐analysis of 65 trials in patients with atherothrombosis showed that aspirin therapy reduces the odds of vascular events by 23% 2%.8 Moreover, aspirin has a class IA recommendation from the ACC/AHA for the management of patients with UA/NSTEMI or STEMI; in the absence of contraindications, it should be initiated as soon as possible and continued indefinitely.1, 2 On initial presentation of ACS, high‐dose aspirin is recommended (162‐325 mg), and lower doses (75‐162 mg), which minimize bleeding or gastrointestinal side effects, are indicated thereafter.1, 2
SUSTAINED ANTIPLATELET THERAPIES FORUA/NSTEMI
Clinical data have indicated that long‐term therapies may be more beneficial to patients with UA/NSTEMI than to those with STEMI. Antiplatelet therapy reduces the risk of vascular events in patients with UA/NSTEMI; benefits are noticeable soon after therapy is initiated. The sustained use of clopidogrel, an inhibitor of ADP‐dependent platelet activation, is at least as effective as aspirin in reducing long‐term vascular events in patients with atherothrombotic diseases, and the ACC/AHA recommends this agent be used when aspirin is contraindicated.1, 9
Recently, the Clopidogrel in Unstable Angina Recurrent Events (CURE) study demonstrated that the combination of clopidogrel and aspirin is superior to either agent alone in preventing vascular events in patients with UA/NSTEMI.10 Patients (N = 12,562) were randomized within 24 hours of UA/NSTEMI presentation to receive aspirin (75‐325 mg/day), an immediate loading dose of clopidogrel (300 mg) followed by once‐daily clopidogrel (75 mg), or a matching placebo for 3‐12 months.10 At the treating physicians' discretion, patients were treated with anticoagulants, revascularization procedures, or GP IIb/IIIa inhibitors after randomization. When used in combination with aspirin, clopidogrel reduced the risk of a major cardiovascular event by 20% (relative risk RR, 0.80; 95% CI, 0.72‐0.90; P < .001) compared with aspirin alone.10
A retrospective subgroup analysis revealed that the therapeutic benefits of this drug combination increases as the risk of a vascular event increases in patients with UA/NSTEMI.11 In addition, the effects of clopidogrel and aspirin were apparent early after randomization (Fig. 1); a 34% reduction in the risk of major cardiovascular events (RR, 0.66; 95% CI, 0.51‐0.86; P < .003) was observed within 24 hours postrandomization.12 Thirty days after randomization, the relative risk reduction in the composite end point of cardiovascular death and nonfatal MI, stroke, and refractory ischemia was 17% (RR, 0.83; 95% CI, 0.73‐0.93; P < .002) for clopidogrel with aspirin.

The combination of clopidogrel and aspirin was associated with a 38% (RR, 1.38; 95% CI, 1.13‐1.67; P = .001) increase in major bleeding episodes compared with patients taking aspirin alone. Conversely, the incidence of bleeding requiring surgical intervention, hemorrhagic stroke, and fatal hemorrhage between treatment groups did not differ significantly.10 The clinical benefits of clopidogrel and aspirin outweighed the risk of life‐threatening bleeding (RR, 0.84; 95% CI, 0.76‐0.93), even when the number of deaths and the number of life‐threatening bleeds were taken into account in the efficacy‐safety analysis.13 As a result, the ACC/AHA guidelines for the management of patients with UA/NSTEMI recommend that clopidogrel and aspirin should be administered to hospitalized patients as soon as possible and should be continued for 1 month and up to 12 months after the initial presentation of symptoms.1 The new ACC/AHA guidelines recommend upstream clopidogrel as a class IA treatment for UA/non‐ST‐elevation MI in patients who are managed either invasively (catheterization within 6‐24 hours) or conservatively (selectively invasive medical management, followed by catheterization if needed). This latter recommendation is much stronger than the prior guidelines and illustrates the importance of antiplatelet therapy as a part of medical management for intermediate‐ to high‐risk ACS.
ANTIPLATELET THERAPIES WITH REVASCULARIZATION PROCEDURES IN UA/NSTEMI
The efficacy of clopidogrel and aspirin has been examined in patients enrolled in the CURE study who underwent revascularization procedures (eg, PCI, CABG). These agents reduced the relative risk of cardiovascular death or nonfatal MI by 31% (RR, 0.69; 95% CI, 0.54‐0.87; P = .002) in patients who underwent PCI.14 Subgroup analysis of PCI timing relative to randomization showed that the risk of vascular events increased with time to revascularization. However, this relationship was not evident in patients treated with aspirin alone (Fig. 2).14 There was no significant difference in the incidence of major bleeding (RR, 1.2; 95% CI, 0.70‐1.78; P = .64) between the treatment groups from PCI to follow‐up.14, 15 Similar results have been reported elsewhere.16 As a result, ACC/AHA guidelines recommend the early administration of clopidogrel and aspirin to patients undergoing planned PCI, and should be continued up to 12 months following the procedure, unless it is contraindicated.1

Clopidogrel and aspirin also reduced the risk of vascular death by 11% (RR, 0.89; 95% CI, 0.71‐1.11) in patients who underwent CABG.13 Study medications were stopped prior to the procedure. Major bleeding was not observed in patients who stopped medication at least 5 days prior to surgery and did not differ significantly from those who stopped therapy less than 5 days before CABG.13 Thus, the ACC/AHA recommends withholding clopidogrel therapy for 5‐7 days prior to elective CABG.1
Platelet glycoprotein (GP) IIb/IIIa inhibitors are another class of antiplatelet therapy that can be beneficial to patients with UA/NSTEMI undergoing PCI. Analysis of the CAPTURE, PURSUIT, and PRISM‐PLUS trials of the GP IIb/IIIa inhibitors abciximab, eptifibatide, and tirofiban, respectively, showed they effectively reduce the rates of death and/or MI (odds ratio [OR], 0.66; 95% CI, 0.54‐0.81) in patients with UA/STEMI prior to PCI, which was more pronounced (OR, 0.59; 95% CI, 0.44‐0.81) when outcomes were measured 48 hours after revascularization.17
The ISAR‐REACT 2 study examined the efficacy of abciximab with clopidogrel and aspirin in patients with NSTEMI prior to PCI.18 The primary end point was a composite of death, MI, and secondary urgent target revascularization 30 days after randomization. The addition of abciximab to 600 mg of the clopidogrel loading dose and aspirin reduced the risk of major adverse cardiovascular events by 25% (RR, 0.75; 95% CI, 0.58‐0.97; P = .03) 30 days after the initiation of therapy. However, subgroup analysis revealed that this therapeutic benefit was confined to patients with elevated troponin levels.18 The ACC/AHA also recommends the concomitant administration of GP IIb/IIIa inhibitors to patients receiving heparin, aspirin, and clopidogrel and undergoing planned PCI.1 The guideline recommendation is for either clopidogrel or a GP IIb/IIIa inhibitor in the invasive pathway (class IA), but both are recommended in patients with elevated troponin, recurrent ischemia, or delay to catheterization (class IIaB). This triple antiplatelet therapy is considered advantageous in the highest=risk NSTEMI patients. The short‐term and long‐term benefits of antiplatelet therapies are consistent across the UA/NSTEMI‐risk spectrum and galvanize the ACC/AHA recommendations for antithrombotic therapy in patients with UA/NSTEMI (Fig. 3).1

ANTIPLATELET THERAPIES FOR STEMI
The ACC/AHA has made several recommendations regarding the administration of clopidogrel in patients with STEMI. Clopidogrel should be administered to patients with contraindications to aspirin. After placement of a bare metal or drug‐eluting stent, this agent should be administered at least 1 month and less than 12 months after surgery, respectively. It should also be withheld at least 5‐7 days prior to CABG.2 These guidelines are largely based on clinical trials in patients with UA/STEMI or ACS.9, 15 Fortunately, several more recent studies have examined the use of antiplatelet therapy in patients with STEMI.
In the Clopidogrel as Adjunctive Reperfusion TherapyThrombolysis in Myocardial Infarction (CLARITY‐TIMI) 28 study, 3491 patients with STEMI were randomized to receive clopidogrel (300 mg loading dose, then 75 mg/day) or placebo. Patients were also treated with a fibrinolytic agent, aspirin, and unfractionated heparin and underwent angiography 48‐192 hours after randomization. Clopidogrel reduced the risk of detecting an occluded infarct‐related artery by angiography or recurrent MI/death prior to angiography by 36% (OR, 0.64; 95% CI, 0.53‐0.76; P < .001) compared with placebo.19 It also reduced the risk of major adverse cardiovascular events 30 days after randomization by 20% (OR, 0.80; 95% CI, 0.65‐0.97; P = .03) compared with placebo, with no significant difference in the risk of bleeding between the treatment groups.
The PCI‐CLARITY study examined the efficacy of clopidogrel in patients undergoing PCI during the CLARITY‐TIMI 28 trial. Clopidogrel reduced the rate of major adverse cardiovascular events by 46% (OR, 0.54; 95% CI, 0.35‐0.85; P = .008) after PCI and 30 days after randomization, with no excess in major bleeding.20 Although the use of this agent along with contemporary reperfusion therapies in patients with STEMI is supported, further research into the sustained use of clopidogrel in STEMI is needed.
CONCLUSIONS
Patients with ACS require aggressive diagnosis and acute treatment. However, long‐term therapies are also needed to improve outcomes. Antiplatelet therapies are a key component of the treatment of ACS. The benefits of aspirin and clopidogrel combination therapy are evident early, and their sustained use improves the outcome of patients who receive medical therapy and/or revascularization procedures. Early initiation of antiplatelet therapy in patients with ACS is best accomplished with care pathways or ACS protocols that are guideline driven. Initiation of these protocols in the ED, with appropriate handoff to hospitalists, will ensure maximization of antiplatelet therapy for patients throughout the precatheterization medical management period. Although antiplatelet agents may be associated with an increased risk of bleeding in some patients, these risks can be minimized and are outweighed by the benefits of clopidogrel and aspirin.
- ,,, et al.ACC/AHA guidelines for the management of patients with unstable angina/non‐ST‐segment elevation myocardial infarction: executive summary.J Am Coll Cardiol.2007;50:e1–e157. Available at: http://www.acc.org.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association.Circulation.2006;113:873–823.
- The RISC Group.Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease.Lancet.1990;336:827–830.
- ISIS‐2 Collaborative Group.Randomized trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction: ISIS‐2.Lancet.1988;2:349–360.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- CAPRIE steering committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- CURE Trial Investigators.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med.2001;345:494–502.
- ,,, et al.Benefit of clopidogrel in patients with acute coronary syndromes without ST‐segment elevation in various risk groups.Circulation.2002;106:1622–1626.
- ,,, et al.Early and late effects of clopidogrel in patients with acute coronary syndromes.Circulation.2003;107:966–972.
- ,,, et al.Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non‐ST‐elevation acute coronary syndrome: The clopidogrel in unstable angina to prevent recurrent ischemic events (CURE) trial.Circulation.2004;110:1202–1208.
- ,,, et al.Benefit of clopidogrel according to timing of percutaneous coronary intervention in patients with acute coronary syndromes: Further results from the clopidogrel in unstable angina to prevent recurrent events (CURE) study.Am Heart J.2005;150:1177–1184.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet.2001;358:527–533.
- ,,, et al.Early and sustained dual oral antiplatelet therapy following percutaneous intervention. A randomized trial.JAMA.2002;288:2411–2420.
- ,,,,,.Platelet glycoprotein IIb/IIIa receptor inhibition in non‐ST‐elevation acute coronary syndromes: early benefit during medical treatment only, with additional protection during percutaneous coronary intervention.Circulation.1999;100:2045–2048.
- ,,, et al.Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment. The ISAR‐REACT2 randomized trial.JAMA.2006;295:1531–1538.
- ,,, et al.Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation.N Engl J Med.2005;352:1179–1189.
- ,,, et al.Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST‐elevation myocardial infarction treated with fibrinolytics. The PCI‐CLARITY study.JAMA.2005;294:1224–1232.
- ,,, et al.ACC/AHA guidelines for the management of patients with unstable angina/non‐ST‐segment elevation myocardial infarction: executive summary.J Am Coll Cardiol.2007;50:e1–e157. Available at: http://www.acc.org.
- ,,, et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction.Circulation.2004;110:82–292.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomized trials of antiplatelet therapy. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- ,,, et al.One‐year cardiovascular event rates in outpatients with atherothrombosis.JAMA.2007;297:1197–1206.
- ,,, et al.Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association.Circulation.2006;113:873–823.
- The RISC Group.Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease.Lancet.1990;336:827–830.
- ISIS‐2 Collaborative Group.Randomized trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction: ISIS‐2.Lancet.1988;2:349–360.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- CAPRIE steering committee.A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE).Lancet.1996;348:1329–1339.
- CURE Trial Investigators.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med.2001;345:494–502.
- ,,, et al.Benefit of clopidogrel in patients with acute coronary syndromes without ST‐segment elevation in various risk groups.Circulation.2002;106:1622–1626.
- ,,, et al.Early and late effects of clopidogrel in patients with acute coronary syndromes.Circulation.2003;107:966–972.
- ,,, et al.Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non‐ST‐elevation acute coronary syndrome: The clopidogrel in unstable angina to prevent recurrent ischemic events (CURE) trial.Circulation.2004;110:1202–1208.
- ,,, et al.Benefit of clopidogrel according to timing of percutaneous coronary intervention in patients with acute coronary syndromes: Further results from the clopidogrel in unstable angina to prevent recurrent events (CURE) study.Am Heart J.2005;150:1177–1184.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet.2001;358:527–533.
- ,,, et al.Early and sustained dual oral antiplatelet therapy following percutaneous intervention. A randomized trial.JAMA.2002;288:2411–2420.
- ,,,,,.Platelet glycoprotein IIb/IIIa receptor inhibition in non‐ST‐elevation acute coronary syndromes: early benefit during medical treatment only, with additional protection during percutaneous coronary intervention.Circulation.1999;100:2045–2048.
- ,,, et al.Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment. The ISAR‐REACT2 randomized trial.JAMA.2006;295:1531–1538.
- ,,, et al.Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation.N Engl J Med.2005;352:1179–1189.
- ,,, et al.Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST‐elevation myocardial infarction treated with fibrinolytics. The PCI‐CLARITY study.JAMA.2005;294:1224–1232.