User login
Differences in Underrepresented in Medicine Applicant Backgrounds and Outcomes in the 2020-2021 Dermatology Residency Match
Dermatology is one of the least diverse medical specialties with only 3% of dermatologists being Black and 4% Latinx.1 Leading dermatology organizations have called for specialty-wide efforts to improve diversity, with a particular focus on the resident selection process.2,3 Medical students who are underrepresented in medicine (UIM)(ie, those who identify as Black, Latinx, Native American, or Pacific Islander) face many potential barriers in applying to dermatology programs, including financial limitations, lack of support and mentorship, and less exposure to the specialty.1,2,4 The COVID-19 pandemic introduced additional challenges in the residency application process with limitations on clinical, research, and volunteer experiences; decreased opportunities for in-person mentorship and away rotations; and a shift to virtual recruitment. Although there has been increased emphasis on recruiting diverse candidates to dermatology, the COVID-19 pandemic may have exacerbated existing barriers for UIM applicants.
We surveyed dermatology residency program directors (PDs) and applicants to evaluate how UIM students approach and fare in the dermatology residency application process as well as the effects of COVID-19 on the most recent application cycle. Herein, we report the results of our surveys with a focus on racial differences in the application process.
Methods
We administered 2 anonymous online surveys—one to 115 PDs through the Association of Professors of Dermatology (APD) email listserve and another to applicants who participated in the 2020-2021 dermatology residency application cycle through the Dermatology Interest Group Association (DIGA) listserve. The surveys were distributed from March 29 through May 23, 2021. There was no way to determine the number of dermatology applicants on the DIGA listserve. The surveys were reviewed and approved by the University of Southern California (Los Angeles, California) institutional review board (approval #UP-21-00118).
Participants were not required to answer every survey question; response rates varied by question. Survey responses with less than 10% completion were excluded from analysis. Data were collected, analyzed, and stored using Qualtrics, a secure online survey platform. The test of equal or given proportions in R studio was used to determine statistically significant differences between variables (P<.05 indicated statistical significance).
Results
The PD survey received 79 complete responses (83.5% complete responses, 73.8% response rate) and the applicant survey received 232 complete responses (83.6% complete responses).
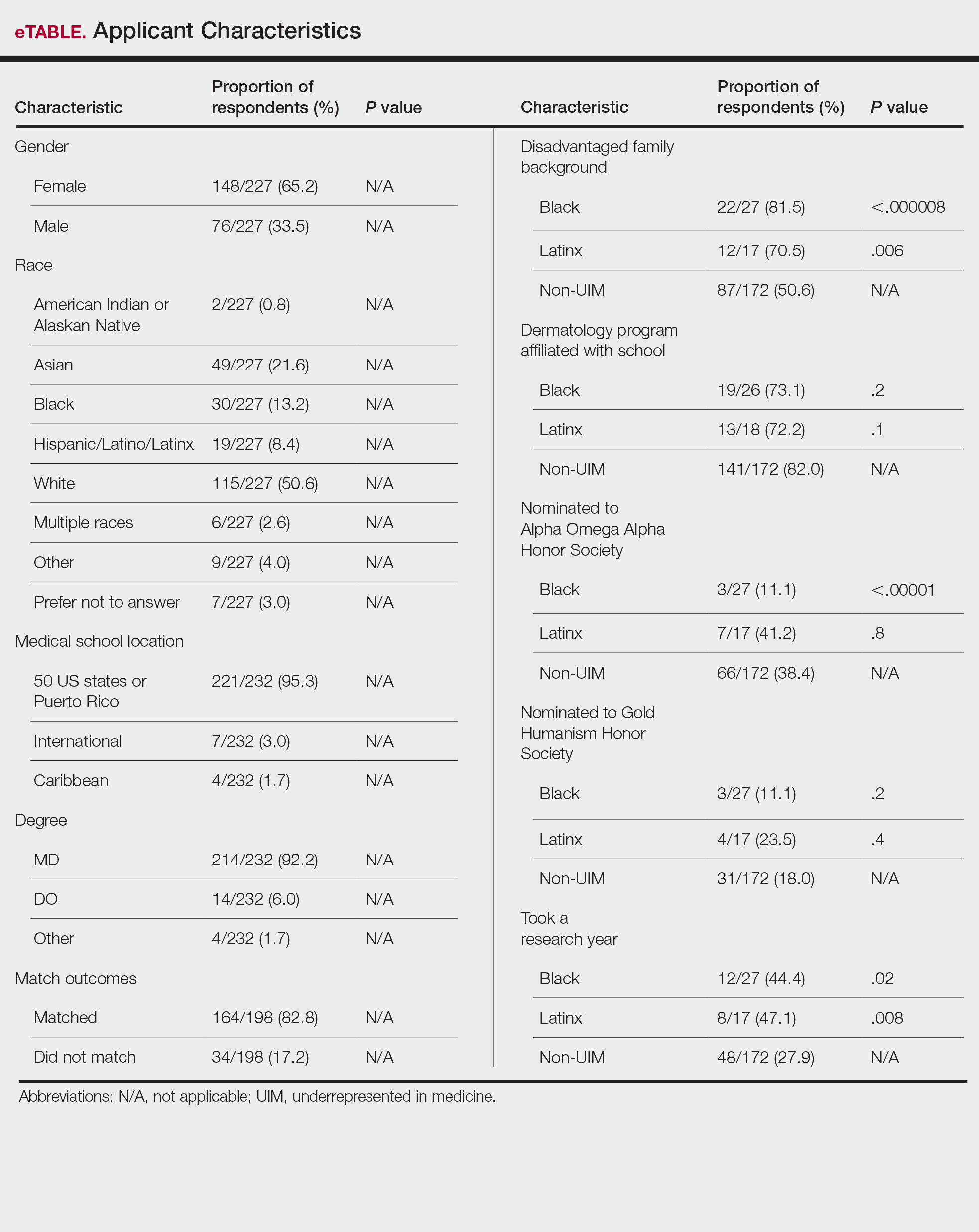
Applicant Characteristics—Applicant characteristics are provided in the eTable; 13.2% and 8.4% of participants were Black and Latinx (including those who identify as Hispanic/Latino), respectively. Only 0.8% of respondents identified as American Indian or Alaskan Native and were excluded from the analysis due to the limited sample size. Those who identified as White, Asian, multiple races, or other and those who preferred not to answer were considered non-UIM participants.
Differences in family background were observed in our cohort, with UIM candidates more likely to have experienced disadvantages, defined as being the first in their family to attend college/graduate school, growing up in a rural area, being a first-generation immigrant, or qualifying as low income. Underrepresented in medicine applicants also were less likely to have a dermatology program at their medical school (both Black and Latinx) and to have been elected to honor societies such as Alpha Omega Alpha and the Gold Humanism Honor Society (Black only).
Underrepresented in medicine applicants were more likely to complete a research gap year (eTable). Most applicants who took research years did so to improve their chances of matching, regardless of their race/ethnicity. For those who did not complete a research year, Black applicants (46.7%) were more likely to base that decision on financial limitations compared to non-UIMs (18.6%, P<.0001). Interestingly, in the PD survey, only 4.5% of respondents considered completion of a research year extremely or very important when compiling rank lists.
Application Process and Match Outcomes—The Table highlights differences in how UIM applicants approached the application process. Black but not Latinx applicants were less likely to be first-time applicants to dermatology compared to non-UIM applicants. Black applicants (8.3%) were significantly less likely to apply to more than 100 programs compared to non-UIM applicants (29.5%, P=.0002). Underrepresented in medicine applicants received greater numbers of interviews despite applying to fewer programs overall.
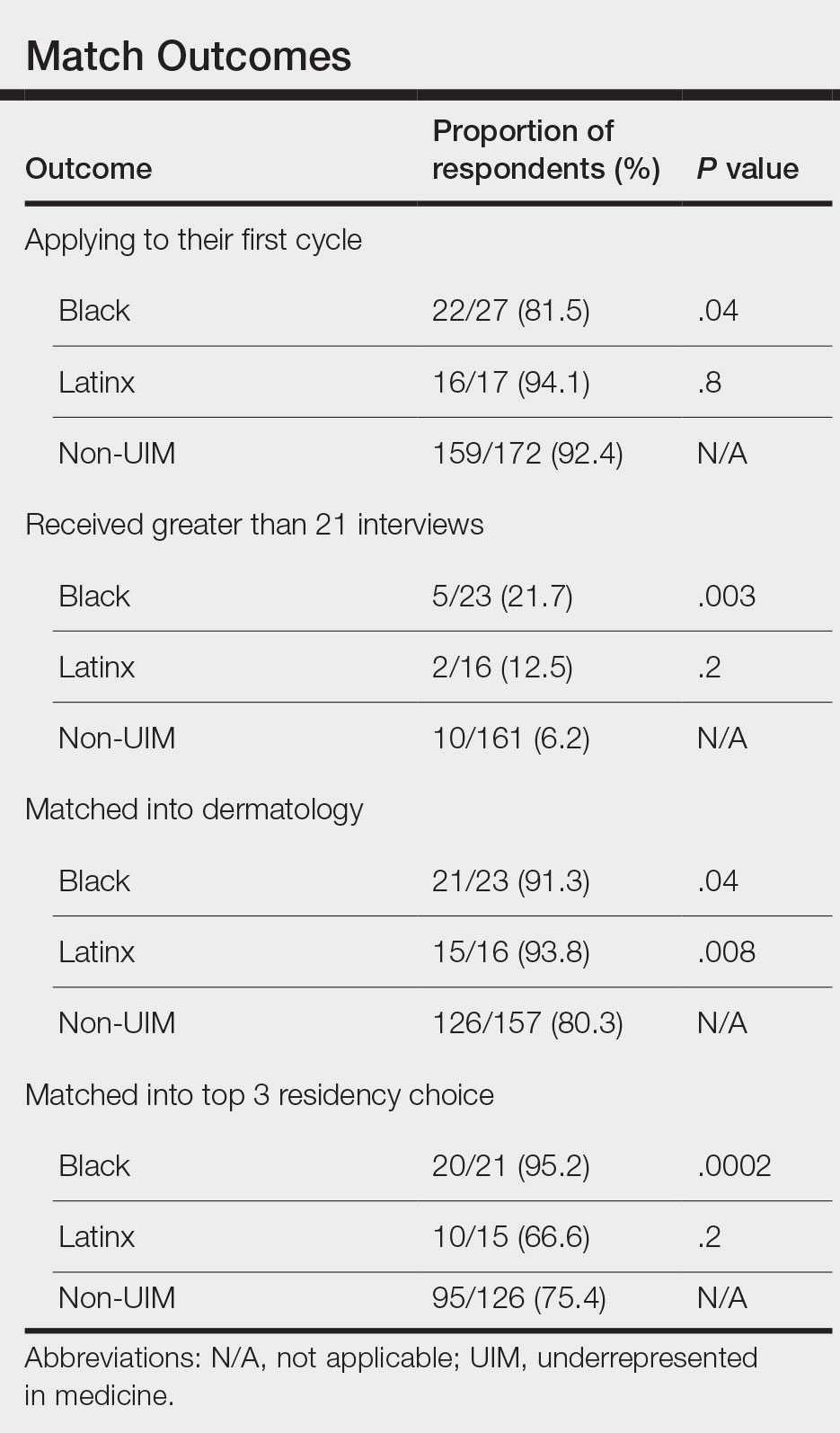
There also were differences in how UIM candidates approached their rank lists, with Black and Latinx applicants prioritizing diversity of patient populations and program faculty as well as program missions and values (Figure).
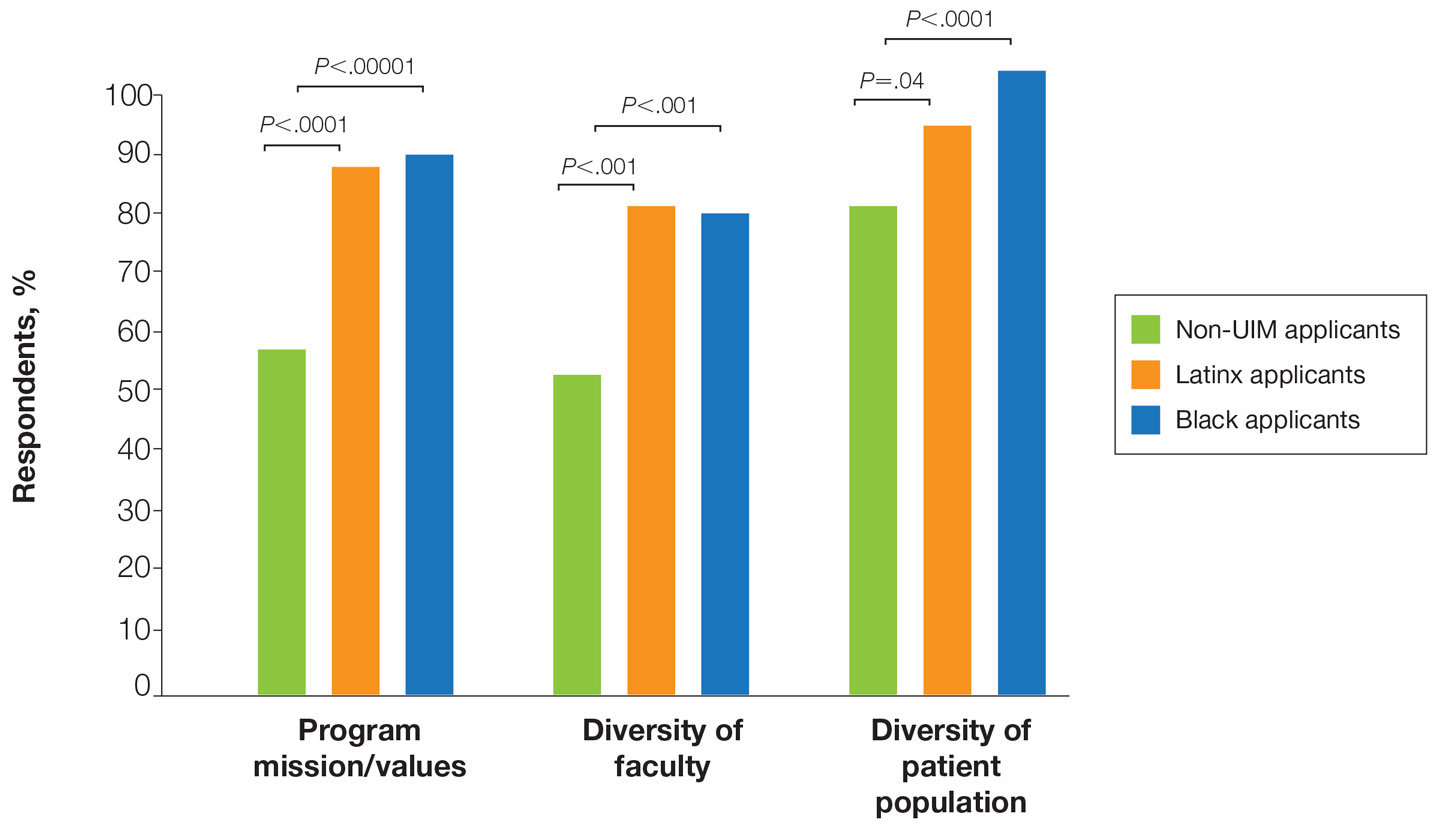
In our cohort, UIM candidates were more likely than non-UIM to match, and Black applicants were most likely to match at one of their top 3 choices (Table). In the PD survey, 77.6% of PDs considered contribution to diversity an important factor when compiling their rank lists.
Comment
Applicant Background—Dermatology is a competitive specialty with a challenging application process2 that has been further complicated by the COVID-19 pandemic. Our study elucidated how the 2020-2021 application cycle affected UIM dermatology applicants. Prior studies have found that UIM medical students were more likely to come from lower socioeconomic backgrounds; financial constraints pose a major barrier for UIM and low-income students interested in dermatology.4-6 We found this to be true in our cohort, as Black and Latinx applicants were significantly more likely to come from disadvantaged backgrounds (P<.000008 and P=.006, respectively). Additionally, we found that Black applicants were more likely than any other group to indicate financial concerns as their primary reason for not taking a research gap year.
Although most applicants who completed a research year did so to increase their chances of matching, a higher percentage of UIMs took research years compared to non-UIM applicants. This finding could indicate greater anxiety about matching among UIM applicants vs their non-UIM counterparts. Black students have faced discrimination in clinical grading,7 have perceived racial discrimination in residency interviews,8,9 and have shown to be less likely to be elected to medical school honor societies.10 We found that UIM applicants were more likely to pursue a research year compared to other applicants,11 possibly because they felt additional pressure to enhance their applications or because UIM candidates were less likely to have a home dermatology program. Expansion of mentorship programs, visiting student electives, and grants for UIMs may alleviate the need for these candidates to complete a research year and reduce disparities in the application process.
Factors Influencing Rank Lists for Applicants—In our cohort, UIMs were significantly more likely to rank diversity of patients (P<.0001 for Black applicants and P=.04 for Latinx applicants) and faculty (P<.001 for Black applicants and P<.001 for Latinx applicants) as important factors in choosing a residency program. Historically, dermatology has been disproportionately White in its physician workforce and patient population.1,12 Students with lower incomes or who identify as minorities cite the lack of diversity in dermatology as a considerable barrier to pursuing a career in the specialty.4,5 Service learning, pipeline programs aimed at early exposure to dermatology, and increased access to care for diverse patient populations are important measures to improve diversity in the dermatology workforce.13-15 Residency programs should consider how to incorporate these aspects into didactic and clinical curricula to better recruit diverse candidates to the field.
Equity in the Application Process—We found that Black applicants were more likely than non-UIM applicants to be reapplicants to dermatology; however, Black applicants in our study also were more likely to receive more interview invites, match into dermatology, and match into one of their top 3 programs. These findings are interesting, particularly given concerns about equity in the application process. It is possible that Black applicants who overcome barriers to applying to dermatology ultimately are more successful applicants. Recently, there has been an increased focus in the field on diversifying dermatology, which was further intensified last year.2,3 Indicative of this shift, our PD survey showed that most programs reported that applicants’ contributions to diversity were important factors in the application process. Additionally, an emphasis by PDs on a holistic review of applications coupled with direct advocacy for increased representation may have contributed to the increased match rates for UIM applicants reported in our survey.
Latinx Applicants—Our study showed differences in how Latinx candidates fared in the application process; although Latinx applicants were more likely than their non-Latinx counterparts to match into dermatology, they were less likely than non-Latinx applicants to match into one of their top 3 programs. Given that Latinx encompasses ethnicity, not race, there may be a difference in how intentional focus on and advocacy for increasing diversity in dermatology affected different UIM applicant groups. Both race and ethnicity are social constructs rather than scientific categorizations; thus, it is difficult in survey studies such as ours to capture the intersectionality present across and between groups. Lastly, it is possible that the respondents to our applicant survey are not representative of the full cohort of UIM applicants.
Study Limitations—A major limitation of our study was that we did not have a method of reaching all dermatology applicants. Although our study shows promising results suggestive of increased diversity in the last application cycle, release of the National Resident Matching Program results from 2020-2021 with racially stratified data will be imperative to assess equity in the match process for all specialties and to confirm the generalizability of our results.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001/jamadermatol.2016.4683
- American Academy of Dermatology Association. Diversity In Dermatology: Diversity Committee Approved Plan 2021-2023. Published January 26, 2021. Accessed July 26, 2022. https://assets.ctfassets.net/1ny4yoiyrqia/xQgnCE6ji5skUlcZQHS2b/65f0a9072811e11afcc33d043e02cd4d/DEI_Plan.pdf
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatologyresidency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Patel PM, et al. Considerations for dermatology residency applicants underrepresented in medicine amid the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E247.doi:10.1016/j.jaad.2020.05.141
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Grbic D, Jones DJ, Case ST. The role of socioeconomic status in medical school admissions: validation of a socioeconomic indicator for use in medical school admissions. Acad Med. 2015;90:953-960. doi:10.1097/ACM.0000000000000653
- Low D, Pollack SW, Liao ZC, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Ellis J, Otugo O, Landry A, et al. Interviewed while Black [published online November 11, 2020]. N Engl J Med. 2020;383:2401-2404. doi:10.1056/NEJMp2023999
- Anthony Douglas II, Hendrix J. Black medical student considerations in the era of virtual interviews. Ann Surg. 2021;274:232-233. doi:10.1097/SLA.0000000000004946
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha honor society. JAMA Intern Med. 2017;177:659. doi:10.1001/jamainternmed.2016.9623
- Runge M, Renati S, Helfrich Y. 16146 dermatology residency applicants: how many pursue a dedicated research year or dual-degree, and do their stats differ [published online December 1, 2020]? J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.304
- Stern RS. Dermatologists and office-based care of dermatologic disease in the 21st century. J Investig Dermatol Symp Proc. 2004;9:126-130. doi:10.1046/j.1087-0024.2003.09108.x
- Oyesanya T, Grossberg AL, Okoye GA. Increasing minority representation in the dermatology department: the Johns Hopkins experience. JAMA Dermatol. 2018;154:1133-1134. doi:10.1001/jamadermatol.2018.2018
- Humphrey VS, James AJ. The importance of service learning in dermatology residency: an actionable approach to improve resident education and skin health equity. Cutis. 2021;107:120-122. doi:10.12788/cutis.0199
Dermatology is one of the least diverse medical specialties with only 3% of dermatologists being Black and 4% Latinx.1 Leading dermatology organizations have called for specialty-wide efforts to improve diversity, with a particular focus on the resident selection process.2,3 Medical students who are underrepresented in medicine (UIM)(ie, those who identify as Black, Latinx, Native American, or Pacific Islander) face many potential barriers in applying to dermatology programs, including financial limitations, lack of support and mentorship, and less exposure to the specialty.1,2,4 The COVID-19 pandemic introduced additional challenges in the residency application process with limitations on clinical, research, and volunteer experiences; decreased opportunities for in-person mentorship and away rotations; and a shift to virtual recruitment. Although there has been increased emphasis on recruiting diverse candidates to dermatology, the COVID-19 pandemic may have exacerbated existing barriers for UIM applicants.
We surveyed dermatology residency program directors (PDs) and applicants to evaluate how UIM students approach and fare in the dermatology residency application process as well as the effects of COVID-19 on the most recent application cycle. Herein, we report the results of our surveys with a focus on racial differences in the application process.
Methods
We administered 2 anonymous online surveys—one to 115 PDs through the Association of Professors of Dermatology (APD) email listserve and another to applicants who participated in the 2020-2021 dermatology residency application cycle through the Dermatology Interest Group Association (DIGA) listserve. The surveys were distributed from March 29 through May 23, 2021. There was no way to determine the number of dermatology applicants on the DIGA listserve. The surveys were reviewed and approved by the University of Southern California (Los Angeles, California) institutional review board (approval #UP-21-00118).
Participants were not required to answer every survey question; response rates varied by question. Survey responses with less than 10% completion were excluded from analysis. Data were collected, analyzed, and stored using Qualtrics, a secure online survey platform. The test of equal or given proportions in R studio was used to determine statistically significant differences between variables (P<.05 indicated statistical significance).
Results
The PD survey received 79 complete responses (83.5% complete responses, 73.8% response rate) and the applicant survey received 232 complete responses (83.6% complete responses).
Applicant Characteristics—Applicant characteristics are provided in the eTable; 13.2% and 8.4% of participants were Black and Latinx (including those who identify as Hispanic/Latino), respectively. Only 0.8% of respondents identified as American Indian or Alaskan Native and were excluded from the analysis due to the limited sample size. Those who identified as White, Asian, multiple races, or other and those who preferred not to answer were considered non-UIM participants.
Differences in family background were observed in our cohort, with UIM candidates more likely to have experienced disadvantages, defined as being the first in their family to attend college/graduate school, growing up in a rural area, being a first-generation immigrant, or qualifying as low income. Underrepresented in medicine applicants also were less likely to have a dermatology program at their medical school (both Black and Latinx) and to have been elected to honor societies such as Alpha Omega Alpha and the Gold Humanism Honor Society (Black only).
Underrepresented in medicine applicants were more likely to complete a research gap year (eTable). Most applicants who took research years did so to improve their chances of matching, regardless of their race/ethnicity. For those who did not complete a research year, Black applicants (46.7%) were more likely to base that decision on financial limitations compared to non-UIMs (18.6%, P<.0001). Interestingly, in the PD survey, only 4.5% of respondents considered completion of a research year extremely or very important when compiling rank lists.

Application Process and Match Outcomes—The Table highlights differences in how UIM applicants approached the application process. Black but not Latinx applicants were less likely to be first-time applicants to dermatology compared to non-UIM applicants. Black applicants (8.3%) were significantly less likely to apply to more than 100 programs compared to non-UIM applicants (29.5%, P=.0002). Underrepresented in medicine applicants received greater numbers of interviews despite applying to fewer programs overall.

There also were differences in how UIM candidates approached their rank lists, with Black and Latinx applicants prioritizing diversity of patient populations and program faculty as well as program missions and values (Figure).

In our cohort, UIM candidates were more likely than non-UIM to match, and Black applicants were most likely to match at one of their top 3 choices (Table). In the PD survey, 77.6% of PDs considered contribution to diversity an important factor when compiling their rank lists.
Comment
Applicant Background—Dermatology is a competitive specialty with a challenging application process2 that has been further complicated by the COVID-19 pandemic. Our study elucidated how the 2020-2021 application cycle affected UIM dermatology applicants. Prior studies have found that UIM medical students were more likely to come from lower socioeconomic backgrounds; financial constraints pose a major barrier for UIM and low-income students interested in dermatology.4-6 We found this to be true in our cohort, as Black and Latinx applicants were significantly more likely to come from disadvantaged backgrounds (P<.000008 and P=.006, respectively). Additionally, we found that Black applicants were more likely than any other group to indicate financial concerns as their primary reason for not taking a research gap year.
Although most applicants who completed a research year did so to increase their chances of matching, a higher percentage of UIMs took research years compared to non-UIM applicants. This finding could indicate greater anxiety about matching among UIM applicants vs their non-UIM counterparts. Black students have faced discrimination in clinical grading,7 have perceived racial discrimination in residency interviews,8,9 and have shown to be less likely to be elected to medical school honor societies.10 We found that UIM applicants were more likely to pursue a research year compared to other applicants,11 possibly because they felt additional pressure to enhance their applications or because UIM candidates were less likely to have a home dermatology program. Expansion of mentorship programs, visiting student electives, and grants for UIMs may alleviate the need for these candidates to complete a research year and reduce disparities in the application process.
Factors Influencing Rank Lists for Applicants—In our cohort, UIMs were significantly more likely to rank diversity of patients (P<.0001 for Black applicants and P=.04 for Latinx applicants) and faculty (P<.001 for Black applicants and P<.001 for Latinx applicants) as important factors in choosing a residency program. Historically, dermatology has been disproportionately White in its physician workforce and patient population.1,12 Students with lower incomes or who identify as minorities cite the lack of diversity in dermatology as a considerable barrier to pursuing a career in the specialty.4,5 Service learning, pipeline programs aimed at early exposure to dermatology, and increased access to care for diverse patient populations are important measures to improve diversity in the dermatology workforce.13-15 Residency programs should consider how to incorporate these aspects into didactic and clinical curricula to better recruit diverse candidates to the field.
Equity in the Application Process—We found that Black applicants were more likely than non-UIM applicants to be reapplicants to dermatology; however, Black applicants in our study also were more likely to receive more interview invites, match into dermatology, and match into one of their top 3 programs. These findings are interesting, particularly given concerns about equity in the application process. It is possible that Black applicants who overcome barriers to applying to dermatology ultimately are more successful applicants. Recently, there has been an increased focus in the field on diversifying dermatology, which was further intensified last year.2,3 Indicative of this shift, our PD survey showed that most programs reported that applicants’ contributions to diversity were important factors in the application process. Additionally, an emphasis by PDs on a holistic review of applications coupled with direct advocacy for increased representation may have contributed to the increased match rates for UIM applicants reported in our survey.
Latinx Applicants—Our study showed differences in how Latinx candidates fared in the application process; although Latinx applicants were more likely than their non-Latinx counterparts to match into dermatology, they were less likely than non-Latinx applicants to match into one of their top 3 programs. Given that Latinx encompasses ethnicity, not race, there may be a difference in how intentional focus on and advocacy for increasing diversity in dermatology affected different UIM applicant groups. Both race and ethnicity are social constructs rather than scientific categorizations; thus, it is difficult in survey studies such as ours to capture the intersectionality present across and between groups. Lastly, it is possible that the respondents to our applicant survey are not representative of the full cohort of UIM applicants.
Study Limitations—A major limitation of our study was that we did not have a method of reaching all dermatology applicants. Although our study shows promising results suggestive of increased diversity in the last application cycle, release of the National Resident Matching Program results from 2020-2021 with racially stratified data will be imperative to assess equity in the match process for all specialties and to confirm the generalizability of our results.
Dermatology is one of the least diverse medical specialties with only 3% of dermatologists being Black and 4% Latinx.1 Leading dermatology organizations have called for specialty-wide efforts to improve diversity, with a particular focus on the resident selection process.2,3 Medical students who are underrepresented in medicine (UIM)(ie, those who identify as Black, Latinx, Native American, or Pacific Islander) face many potential barriers in applying to dermatology programs, including financial limitations, lack of support and mentorship, and less exposure to the specialty.1,2,4 The COVID-19 pandemic introduced additional challenges in the residency application process with limitations on clinical, research, and volunteer experiences; decreased opportunities for in-person mentorship and away rotations; and a shift to virtual recruitment. Although there has been increased emphasis on recruiting diverse candidates to dermatology, the COVID-19 pandemic may have exacerbated existing barriers for UIM applicants.
We surveyed dermatology residency program directors (PDs) and applicants to evaluate how UIM students approach and fare in the dermatology residency application process as well as the effects of COVID-19 on the most recent application cycle. Herein, we report the results of our surveys with a focus on racial differences in the application process.
Methods
We administered 2 anonymous online surveys—one to 115 PDs through the Association of Professors of Dermatology (APD) email listserve and another to applicants who participated in the 2020-2021 dermatology residency application cycle through the Dermatology Interest Group Association (DIGA) listserve. The surveys were distributed from March 29 through May 23, 2021. There was no way to determine the number of dermatology applicants on the DIGA listserve. The surveys were reviewed and approved by the University of Southern California (Los Angeles, California) institutional review board (approval #UP-21-00118).
Participants were not required to answer every survey question; response rates varied by question. Survey responses with less than 10% completion were excluded from analysis. Data were collected, analyzed, and stored using Qualtrics, a secure online survey platform. The test of equal or given proportions in R studio was used to determine statistically significant differences between variables (P<.05 indicated statistical significance).
Results
The PD survey received 79 complete responses (83.5% complete responses, 73.8% response rate) and the applicant survey received 232 complete responses (83.6% complete responses).
Applicant Characteristics—Applicant characteristics are provided in the eTable; 13.2% and 8.4% of participants were Black and Latinx (including those who identify as Hispanic/Latino), respectively. Only 0.8% of respondents identified as American Indian or Alaskan Native and were excluded from the analysis due to the limited sample size. Those who identified as White, Asian, multiple races, or other and those who preferred not to answer were considered non-UIM participants.
Differences in family background were observed in our cohort, with UIM candidates more likely to have experienced disadvantages, defined as being the first in their family to attend college/graduate school, growing up in a rural area, being a first-generation immigrant, or qualifying as low income. Underrepresented in medicine applicants also were less likely to have a dermatology program at their medical school (both Black and Latinx) and to have been elected to honor societies such as Alpha Omega Alpha and the Gold Humanism Honor Society (Black only).
Underrepresented in medicine applicants were more likely to complete a research gap year (eTable). Most applicants who took research years did so to improve their chances of matching, regardless of their race/ethnicity. For those who did not complete a research year, Black applicants (46.7%) were more likely to base that decision on financial limitations compared to non-UIMs (18.6%, P<.0001). Interestingly, in the PD survey, only 4.5% of respondents considered completion of a research year extremely or very important when compiling rank lists.

Application Process and Match Outcomes—The Table highlights differences in how UIM applicants approached the application process. Black but not Latinx applicants were less likely to be first-time applicants to dermatology compared to non-UIM applicants. Black applicants (8.3%) were significantly less likely to apply to more than 100 programs compared to non-UIM applicants (29.5%, P=.0002). Underrepresented in medicine applicants received greater numbers of interviews despite applying to fewer programs overall.

There also were differences in how UIM candidates approached their rank lists, with Black and Latinx applicants prioritizing diversity of patient populations and program faculty as well as program missions and values (Figure).

In our cohort, UIM candidates were more likely than non-UIM to match, and Black applicants were most likely to match at one of their top 3 choices (Table). In the PD survey, 77.6% of PDs considered contribution to diversity an important factor when compiling their rank lists.
Comment
Applicant Background—Dermatology is a competitive specialty with a challenging application process2 that has been further complicated by the COVID-19 pandemic. Our study elucidated how the 2020-2021 application cycle affected UIM dermatology applicants. Prior studies have found that UIM medical students were more likely to come from lower socioeconomic backgrounds; financial constraints pose a major barrier for UIM and low-income students interested in dermatology.4-6 We found this to be true in our cohort, as Black and Latinx applicants were significantly more likely to come from disadvantaged backgrounds (P<.000008 and P=.006, respectively). Additionally, we found that Black applicants were more likely than any other group to indicate financial concerns as their primary reason for not taking a research gap year.
Although most applicants who completed a research year did so to increase their chances of matching, a higher percentage of UIMs took research years compared to non-UIM applicants. This finding could indicate greater anxiety about matching among UIM applicants vs their non-UIM counterparts. Black students have faced discrimination in clinical grading,7 have perceived racial discrimination in residency interviews,8,9 and have shown to be less likely to be elected to medical school honor societies.10 We found that UIM applicants were more likely to pursue a research year compared to other applicants,11 possibly because they felt additional pressure to enhance their applications or because UIM candidates were less likely to have a home dermatology program. Expansion of mentorship programs, visiting student electives, and grants for UIMs may alleviate the need for these candidates to complete a research year and reduce disparities in the application process.
Factors Influencing Rank Lists for Applicants—In our cohort, UIMs were significantly more likely to rank diversity of patients (P<.0001 for Black applicants and P=.04 for Latinx applicants) and faculty (P<.001 for Black applicants and P<.001 for Latinx applicants) as important factors in choosing a residency program. Historically, dermatology has been disproportionately White in its physician workforce and patient population.1,12 Students with lower incomes or who identify as minorities cite the lack of diversity in dermatology as a considerable barrier to pursuing a career in the specialty.4,5 Service learning, pipeline programs aimed at early exposure to dermatology, and increased access to care for diverse patient populations are important measures to improve diversity in the dermatology workforce.13-15 Residency programs should consider how to incorporate these aspects into didactic and clinical curricula to better recruit diverse candidates to the field.
Equity in the Application Process—We found that Black applicants were more likely than non-UIM applicants to be reapplicants to dermatology; however, Black applicants in our study also were more likely to receive more interview invites, match into dermatology, and match into one of their top 3 programs. These findings are interesting, particularly given concerns about equity in the application process. It is possible that Black applicants who overcome barriers to applying to dermatology ultimately are more successful applicants. Recently, there has been an increased focus in the field on diversifying dermatology, which was further intensified last year.2,3 Indicative of this shift, our PD survey showed that most programs reported that applicants’ contributions to diversity were important factors in the application process. Additionally, an emphasis by PDs on a holistic review of applications coupled with direct advocacy for increased representation may have contributed to the increased match rates for UIM applicants reported in our survey.
Latinx Applicants—Our study showed differences in how Latinx candidates fared in the application process; although Latinx applicants were more likely than their non-Latinx counterparts to match into dermatology, they were less likely than non-Latinx applicants to match into one of their top 3 programs. Given that Latinx encompasses ethnicity, not race, there may be a difference in how intentional focus on and advocacy for increasing diversity in dermatology affected different UIM applicant groups. Both race and ethnicity are social constructs rather than scientific categorizations; thus, it is difficult in survey studies such as ours to capture the intersectionality present across and between groups. Lastly, it is possible that the respondents to our applicant survey are not representative of the full cohort of UIM applicants.
Study Limitations—A major limitation of our study was that we did not have a method of reaching all dermatology applicants. Although our study shows promising results suggestive of increased diversity in the last application cycle, release of the National Resident Matching Program results from 2020-2021 with racially stratified data will be imperative to assess equity in the match process for all specialties and to confirm the generalizability of our results.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001/jamadermatol.2016.4683
- American Academy of Dermatology Association. Diversity In Dermatology: Diversity Committee Approved Plan 2021-2023. Published January 26, 2021. Accessed July 26, 2022. https://assets.ctfassets.net/1ny4yoiyrqia/xQgnCE6ji5skUlcZQHS2b/65f0a9072811e11afcc33d043e02cd4d/DEI_Plan.pdf
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatologyresidency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Patel PM, et al. Considerations for dermatology residency applicants underrepresented in medicine amid the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E247.doi:10.1016/j.jaad.2020.05.141
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Grbic D, Jones DJ, Case ST. The role of socioeconomic status in medical school admissions: validation of a socioeconomic indicator for use in medical school admissions. Acad Med. 2015;90:953-960. doi:10.1097/ACM.0000000000000653
- Low D, Pollack SW, Liao ZC, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Ellis J, Otugo O, Landry A, et al. Interviewed while Black [published online November 11, 2020]. N Engl J Med. 2020;383:2401-2404. doi:10.1056/NEJMp2023999
- Anthony Douglas II, Hendrix J. Black medical student considerations in the era of virtual interviews. Ann Surg. 2021;274:232-233. doi:10.1097/SLA.0000000000004946
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha honor society. JAMA Intern Med. 2017;177:659. doi:10.1001/jamainternmed.2016.9623
- Runge M, Renati S, Helfrich Y. 16146 dermatology residency applicants: how many pursue a dedicated research year or dual-degree, and do their stats differ [published online December 1, 2020]? J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.304
- Stern RS. Dermatologists and office-based care of dermatologic disease in the 21st century. J Investig Dermatol Symp Proc. 2004;9:126-130. doi:10.1046/j.1087-0024.2003.09108.x
- Oyesanya T, Grossberg AL, Okoye GA. Increasing minority representation in the dermatology department: the Johns Hopkins experience. JAMA Dermatol. 2018;154:1133-1134. doi:10.1001/jamadermatol.2018.2018
- Humphrey VS, James AJ. The importance of service learning in dermatology residency: an actionable approach to improve resident education and skin health equity. Cutis. 2021;107:120-122. doi:10.12788/cutis.0199
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001/jamadermatol.2016.4683
- American Academy of Dermatology Association. Diversity In Dermatology: Diversity Committee Approved Plan 2021-2023. Published January 26, 2021. Accessed July 26, 2022. https://assets.ctfassets.net/1ny4yoiyrqia/xQgnCE6ji5skUlcZQHS2b/65f0a9072811e11afcc33d043e02cd4d/DEI_Plan.pdf
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatologyresidency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Patel PM, et al. Considerations for dermatology residency applicants underrepresented in medicine amid the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E247.doi:10.1016/j.jaad.2020.05.141
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Grbic D, Jones DJ, Case ST. The role of socioeconomic status in medical school admissions: validation of a socioeconomic indicator for use in medical school admissions. Acad Med. 2015;90:953-960. doi:10.1097/ACM.0000000000000653
- Low D, Pollack SW, Liao ZC, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Ellis J, Otugo O, Landry A, et al. Interviewed while Black [published online November 11, 2020]. N Engl J Med. 2020;383:2401-2404. doi:10.1056/NEJMp2023999
- Anthony Douglas II, Hendrix J. Black medical student considerations in the era of virtual interviews. Ann Surg. 2021;274:232-233. doi:10.1097/SLA.0000000000004946
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha honor society. JAMA Intern Med. 2017;177:659. doi:10.1001/jamainternmed.2016.9623
- Runge M, Renati S, Helfrich Y. 16146 dermatology residency applicants: how many pursue a dedicated research year or dual-degree, and do their stats differ [published online December 1, 2020]? J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.304
- Stern RS. Dermatologists and office-based care of dermatologic disease in the 21st century. J Investig Dermatol Symp Proc. 2004;9:126-130. doi:10.1046/j.1087-0024.2003.09108.x
- Oyesanya T, Grossberg AL, Okoye GA. Increasing minority representation in the dermatology department: the Johns Hopkins experience. JAMA Dermatol. 2018;154:1133-1134. doi:10.1001/jamadermatol.2018.2018
- Humphrey VS, James AJ. The importance of service learning in dermatology residency: an actionable approach to improve resident education and skin health equity. Cutis. 2021;107:120-122. doi:10.12788/cutis.0199
Practice Points
- Underrepresented in medicine (UIM) dermatology residency applicants (Black and Latinx) are more likely to come from disadvantaged backgrounds and to have financial concerns about the residency application process.
- When choosing a dermatology residency program, diversity of patients and faculty are more important to UIM dermatology residency applicants than to their non-UIM counterparts.
- Increased awareness of and focus on a holistic review process by dermatology residency programs may contribute to higher rates of matching among Black applicants in our study.
Pediatric Dermatology Emergencies
Many pediatric skin conditions can be safely monitored with minimal intervention, but certain skin conditions are emergent and require immediate attention and proper assessment of the neonate, infant, or child. The skin may provide the first presentation of a potentially fatal disease with serious sequelae. Cutaneous findings may indicate the need for further evaluation. Therefore, it is important to differentiate skin conditions with benign etiologies from those that require immediate diagnosis and treatment, as early intervention of some of these conditions can be lifesaving. Herein, we discuss pertinent pediatric dermatology emergencies that dermatologists should keep in mind so that these diagnoses are never missed.
Staphylococcal Scalded Skin Syndrome
Presentation
Staphylococcal scalded skin syndrome (SSSS), or Ritter disease, is a potentially fatal pediatric emergency, especially in newborns.1 The mortality rate for SSSS in the United States is 3.6% to 11% in children.2 It typically presents with a prodrome of tenderness, fever, and confluent erythematous patches on the folds of the skin such as the groin, axillae, nose, and ears, with eventual spread to the legs and trunk.1,2 Within 24 to 48 hours of symptom onset, blistering and fluid accumulation will appear diffusely. Bullae are flaccid, and tangential and gentle pressure on involved unblistered skin may lead to shearing of the epithelium, which is a positive Nikolsky sign.1,2
Causes
Staphylococcal scalded skin syndrome is caused by exfoliative toxins A and B, toxigenic strains of Staphylococcus aureus. Exfoliative toxins A and B are serine proteases that target and cleave desmoglein 1, which binds keratinocytes in the stratum granulosum.1,3 Exfoliative toxins disrupt the adhesion of keratinocytes, resulting in bullae formation and subsequently diffuse sheetlike desquamation.1,4,5 Although up to 30% of the human population are asymptomatically and permanently colonized with nasal S aureus,6 the exfoliative toxins are produced by only 5% of species.1
In neonates, the immune and renal systems are underdeveloped; therefore, patients are susceptible to SSSS due to lack of neutralizing antibodies and decreased renal toxin excretion.4 Potential complications of SSSS are deeper soft-tissue infection, septicemia (blood-borne infection), and fluid and electrolyte imbalance.1,4
Diagnosis and Treatment
The condition is diagnosed clinically based on the findings of tender erythroderma, bullae, and desquamation with a scalded appearance, especially in friction zones; periorificial crusting; positive Nikolsky sign; and lack of mucosal involvement (Figure 1).1 Histopathology can aid in complicated clinical scenarios as well as culture from affected areas, including the upper respiratory tract, diaper region, and umbilicus.1,4 Hospitalization is required for SSSS for intravenous antibiotics, fluids, and electrolyte repletion.
Differential Diagnosis
There are multiple diagnoses to consider in the setting of flaccid bullae in the pediatric population. Stevens-Johnson syndrome or toxic epidermal necrolysis also can present with fever and superficial desquamation or bullae; however, exposure to medications and mucosal involvement often are absent in SSSS (Figure 2).2 Pemphigus, particularly paraneoplastic pemphigus, also often includes mucosal involvement and scalding thermal burns that are often geometric or focal. Epidermolysis bullosa and toxic shock syndrome also should be considered.1
Impetigo
Presentation
Impetigo is the most common bacterial skin infection in children caused by S aureus or Streptococcus pyogenes.7-9 It begins as erythematous papules transitioning to thin-walled vesicles that rapidly rupture and result in honey-crusted papules.7,9,10 Individuals of any age can be affected by nonbullous impetigo, but it is the most common skin infection in children aged 2 to 5 years.7
Bullous impetigo primarily is seen in children, especially infants, and rarely can occur in teenagers or adults.7 It most commonly is caused by the exfoliative toxins of S aureus. Bullous impetigo presents as small vesicles that may converge into larger flaccid bullae or pustules.7-10 Once the bullae rupture, an erythematous base with a collarette of scale remains without the formation of a honey-colored crust.8 Bullous impetigo usually affects moist intertriginous areas such as the axillae, neck, and diaper area8,10 (Figure 3). Complications may result in cellulitis, septicemia, osteomyelitis, poststreptococcal glomerulonephritis associated with S pyogenes, and S aureus–induced SSSS.7-9
Diagnosis
Nonbullous and bullous impetigo are largely clinical diagnoses that can be confirmed by culture of a vesicle or pustular fluid.10 Treatment of impetigo includes topical or systemic antibiotics.7,10 Patients should be advised to keep lesions covered and avoid contact with others until all lesions resolve, as lesions are contagious.9
Eczema Herpeticum
Presentation
Eczema herpeticum (EH), also known as Kaposi varicelliform eruption, is a disseminated herpes simplex virus infection of impaired skin, most commonly in patients with atopic dermatitis (AD).11 Eczema herpeticum presents as a widespread eruption of erythematous monomorphic vesicles that progress to punched-out erosions with hemorrhagic crusting (Figure 4). Patients may have associated fever or lymphadenopathy.12,13
Causes
The number of children hospitalized annually for EH in the United States is approximately 4 to 7 cases per million children. Less than 3% of pediatric AD patients are affected, with a particularly increased risk in patients with severe and earlier-onset AD.12-15 Patients with AD have skin barrier defects, and decreased IFN-γ expression and cathelicidins predispose patients with AD to developing EH.12,16,17
Diagnosis
Viral polymerase chain reaction for herpes simplex virus types 1 and 2 is the standard for confirmatory diagnosis. Herpes simplex virus cultures from cutaneous scrapings, direct fluorescent antibody testing, or Tzanck test revealing multinucleated giant cells also may help establish the diagnosis.11,12,17
Management
Individuals with severe AD and other dermatologic conditions with cutaneous barrier compromise are at risk for developing EH, which is a medical emergency requiring hospitalization and prompt treatment with antiviral therapy such as acyclovir, often intravenously, as death can result if left untreated.11,17 Topical or systemic antibiotic therapy should be initiated if there is suspicion for secondary bacterial superinfection. Patients should be evaluated for multiorgan involvement such as keratoconjunctivitis, meningitis, encephalitis, and systemic viremia due to increased mortality, especially in infants.12,15,16
Langerhans Cell Histiocytosis
Presentation
Langerhans cell histiocytosis (LCH) has a variable clinical presentation and can involve a single or multiple organ systems, including the bones and skin. Cutaneous LCH can present as violaceous papules, nodules, or ulcerations and crusted erosions (Figure 5). The lymph nodes, liver, spleen, oral mucosa, and respiratory and central nervous systems also may be involved.
Langerhans cell histiocytosis affects individuals of any age group but more often is seen in pediatric patients. The incidence of LCH is approximately 4.6 cases per million children.18 The pathogenesis is secondary to pathologic Langerhans cells, characterized as a clonal myeloid malignancy and dysregulation of the immune system.18,19
Diagnosis
A thorough physical examination is essential in patients with suspected LCH. Additionally, diagnosis of LCH is heavily based on histopathology of tissue from the involved organ system(s) with features of positive S-100 protein, CD1a, and CD207, and identification of Birbeck granules.20 Imaging and laboratory studies also are indicated and can include a skeletal survey (to assess osteolytic and organ involvement), a complete hematologic panel, coagulation studies, and liver function tests.18,21
Management
Management of LCH varies based on the organ system(s) involved along with the extent of the disease. Dermatology referral may be indicated in patients presenting with nonresolving cutaneous lesions as well as in severe cases. Single-organ and multisystem disease may require one treatment modality or a combination of chemotherapy, surgery, radiation, and/or immunotherapy.21
Infantile Hemangioma
Presentation
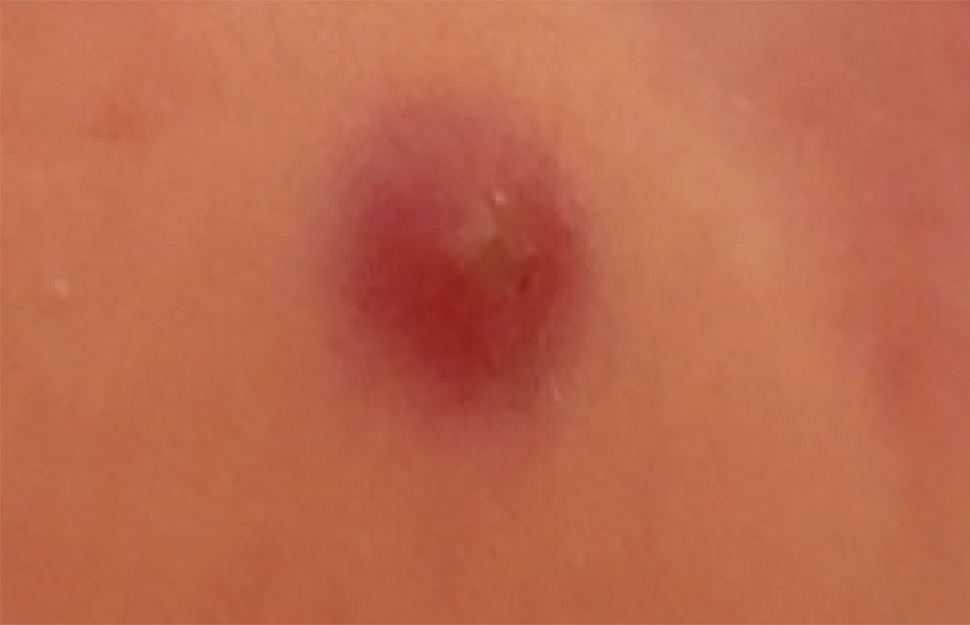
Infantile hemangioma (IH) is the most common benign tumor of infancy and usually is apparent a few weeks after birth. Lesions appear as bright red papules, nodules, or plaques. Deep or subcutaneous lesions present as raised, flesh-colored nodules with a blue hue and bruiselike appearance with or without a central patch of telangiectasia22-24 (Figure 6). Although all IHs eventually resolve, residual skin changes such as scarring, atrophy, and fibrosis can persist.24
The incidence of IH has been reported to occur in up to 4% to 5% of infants in the United States.23,25 Infantile hemangiomas also have been found to be more common among white, preterm, and multiple-gestation infants.25 The proposed pathogenesis of IHs includes angiogenic and vasogenic factors that cause rapid proliferation of blood vessels, likely driven by tissue hypoxia.23,26,27
Diagnosis
Infantile hemangioma is diagnosed clinically; however, immunohistochemical staining showing positivity for glucose transporter 1 also is helpful.26,27 Imaging modalities such as ultrasonography and magnetic resonance imaging also can be utilized to visualize the extent of lesions if necessary.25
Management
Around 15% to 25% of IHs are considered complicated and require intervention.25,27 Infantile hemangiomas can interfere with function depending on location or have potentially fatal complications. Based on the location and extent of involvement, these findings can include ulceration; hemorrhage; impairment of feeding, hearing, and/or vision; facial deformities; airway obstruction; hypothyroidism; and congestive heart failure.25,28 Early treatment with topical or oral beta-blockers is imperative for potentially life-threatening IHs, which can be seen due to large size or dangerous location.28,29 Because the rapid proliferative phase of IHs is thought to begin around 6 weeks of life, treatment should be initiated as early as possible. Initiation of beta-blocker therapy in the first few months of life can prevent functional impairment, ulceration, and permanent cosmetic changes. Additionally, surgery or pulsed dye laser treatment have been found to be effective for skin changes found after involution of IH.25,29
Differential Diagnosis
The differential diagnosis for IH includes vascular malformations, which are present at birth and do not undergo rapid proliferation; sarcoma; and kaposiform hemangioendothelioma, which causes the Kasabach-Merritt phenomenon secondary to platelet trapping. Careful attention to the history of the skin lesion provides good support for diagnosis of IH in most cases.
IgA Vasculitis
Presentation
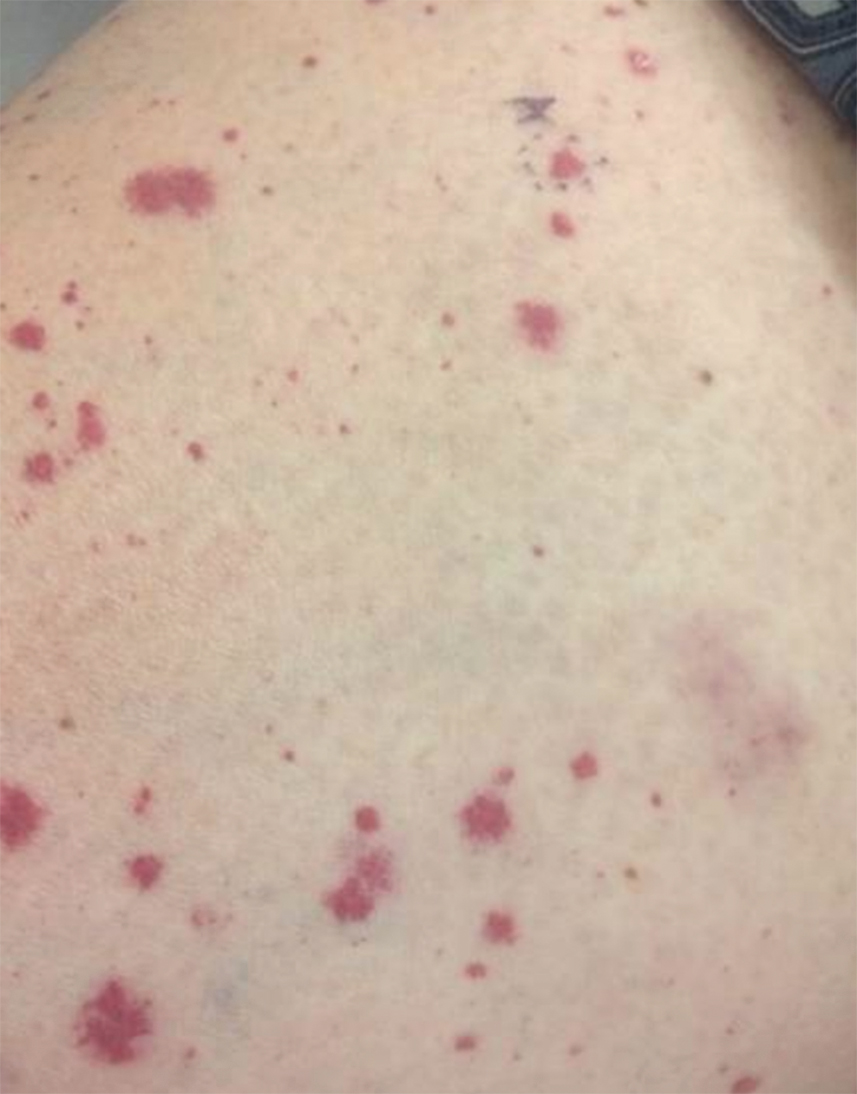
IgA vasculitis, or Henoch-Schönlein purpura, classically presents as a tetrad of palpable purpura, acute-onset arthritis or arthralgia, abdominal pain, and renal disease with proteinuria or hematuria.30 Skin involvement is seen in almost all cases and is essential for diagnosis of IgA vasculitis. The initial dermatosis may be pruritic and present as an erythematous macular or urticarial wheal that evolves into petechiae, along with palpable purpura that is most frequently located on the legs or buttocks (Figure 7).30-34
IgA vasculitis is an immune-mediated small vessel vasculitis with deposition of IgA in the small vessels. The underlying cause remains unknown, though infection, dietary allergens, drugs, vaccinations, and chemical triggers have been recognized in literature.32,35,36 IgA vasculitis is largely a pediatric diagnosis, with 90% of affected individuals younger than 10 years worldwide.37 In the pediatric population, the incidence has been reported to be 3 to 26.7 cases per 100,000 children.32
Diagnosis
Diagnosis is based on the clinical presentation and histopathology.30 On direct immunofluorescence, IgA deposition is seen in the vessel walls.35 Laboratory testing is not diagnostic, but urinalysis is mandatory to identify involvement of renal vasculature. Imaging studies may be used in patients with abdominal symptoms, as an ultrasound can be used to visualize bowel structure and abnormalities such as intussusception.33
Management
The majority of cases of IgA vasculitis recover spontaneously, with patients requiring hospital admission based on severity of symptoms.30 The primary approach to management involves providing supportive care including hydration, adequate rest, and symptomatic pain relief of the joints and abdomen with oral analgesics. Systemic corticosteroids or steroid-sparing agents such as dapsone or colchicine can be used to treat cutaneous manifestations in addition to severe pain symptoms.30,31 Patients with IgA vasculitis must be monitored for proteinuria or hematuria to assess the extent of renal involvement. Although much more common in adults, long-term renal impairment can result from childhood cases of IgA vasculitis.34
Final Thoughts
Pediatric dermatology emergencies can be difficult to detect and accurately diagnose. Many of these diseases are potential emergencies that that may result in delayed treatment and considerable morbidity and mortality if missed. Clinicians should be aware that timely recognition and diagnosis, along with possible referral to pediatric dermatology, are essential to avoid complications.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
- Davidson J, Polly S, Hayes P, et al. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7:E134-E137.
- Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150-159.
- Berk D. Staphylococcal scalded skin syndrome. Cancer Therapy Advisor website. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/pediatrics/staphylococcal-scalded-skin-syndrome/. Published 2017. Accessed February 19, 2020.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections [published online October 8, 2018]. Front Microbiol. 2018;9:2419.
- Pereira LB. Impetigo review. An Bras Dermatol. 2014;89:293-299.
- Nardi NM, Schaefer TJ. Impetigo. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK430974/. Accessed February 21, 2020.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
- Sommer LL, Reboli AC, Heymann WR. Bacterial diseases. In: Bolognia, JL Schaffer, JV Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1259-1295.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377:e9.
- Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients [published online November 16, 2019]. J Eur Acad Dermatology Venereol. doi:10.1111/jdv.16090.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93.
- Hsu DY, Shinkai K, Silverberg JI. Epidemiology of eczema herpeticum in hospitalized U.S. children: analysis of a nationwide cohort [published online September 17, 2018]. J Invest Dermatol. 2018;138:265-272.
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127:965-73.e1-5.
- Darji K, Frisch S, Adjei Boakye E, et al. Characterization of children with recurrent eczema herpeticum and response to treatment with interferon-gamma. Pediatr Dermatol. 2017;34:686-689.
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015;2015:565-570.
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184.
- Holland KE, Drolet BA. Infantile hemangioma [published online August 21, 2010]. Pediatr Clin North Am. 2010;57:1069-1083.
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108.
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18(suppl 1):S117-S120.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:786-791.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219-227.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:E259-E266.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas [published online January 2019]. Pediatrics. doi:10.1542/peds.2018-3475.
- Sohagia AB, Gunturu SG, Tong TR, et al. Henoch-Schönlein purpura—a case report and review of the literature [published online May 23, 2010]. Gastroenterol Res Pract. doi:10.1155/2010/597648.
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch–Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25:171-178.
- Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
- Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant. 2014;30:I94-I103.
- van Timmeren MM, Heeringa P, Kallenberg CG. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26:416-423.
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis [published online July 11, 2013]. Clin Exp Nephrol. 2013;17:607-610.
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387-1395.
Many pediatric skin conditions can be safely monitored with minimal intervention, but certain skin conditions are emergent and require immediate attention and proper assessment of the neonate, infant, or child. The skin may provide the first presentation of a potentially fatal disease with serious sequelae. Cutaneous findings may indicate the need for further evaluation. Therefore, it is important to differentiate skin conditions with benign etiologies from those that require immediate diagnosis and treatment, as early intervention of some of these conditions can be lifesaving. Herein, we discuss pertinent pediatric dermatology emergencies that dermatologists should keep in mind so that these diagnoses are never missed.
Staphylococcal Scalded Skin Syndrome
Presentation
Staphylococcal scalded skin syndrome (SSSS), or Ritter disease, is a potentially fatal pediatric emergency, especially in newborns.1 The mortality rate for SSSS in the United States is 3.6% to 11% in children.2 It typically presents with a prodrome of tenderness, fever, and confluent erythematous patches on the folds of the skin such as the groin, axillae, nose, and ears, with eventual spread to the legs and trunk.1,2 Within 24 to 48 hours of symptom onset, blistering and fluid accumulation will appear diffusely. Bullae are flaccid, and tangential and gentle pressure on involved unblistered skin may lead to shearing of the epithelium, which is a positive Nikolsky sign.1,2
Causes
Staphylococcal scalded skin syndrome is caused by exfoliative toxins A and B, toxigenic strains of Staphylococcus aureus. Exfoliative toxins A and B are serine proteases that target and cleave desmoglein 1, which binds keratinocytes in the stratum granulosum.1,3 Exfoliative toxins disrupt the adhesion of keratinocytes, resulting in bullae formation and subsequently diffuse sheetlike desquamation.1,4,5 Although up to 30% of the human population are asymptomatically and permanently colonized with nasal S aureus,6 the exfoliative toxins are produced by only 5% of species.1
In neonates, the immune and renal systems are underdeveloped; therefore, patients are susceptible to SSSS due to lack of neutralizing antibodies and decreased renal toxin excretion.4 Potential complications of SSSS are deeper soft-tissue infection, septicemia (blood-borne infection), and fluid and electrolyte imbalance.1,4
Diagnosis and Treatment
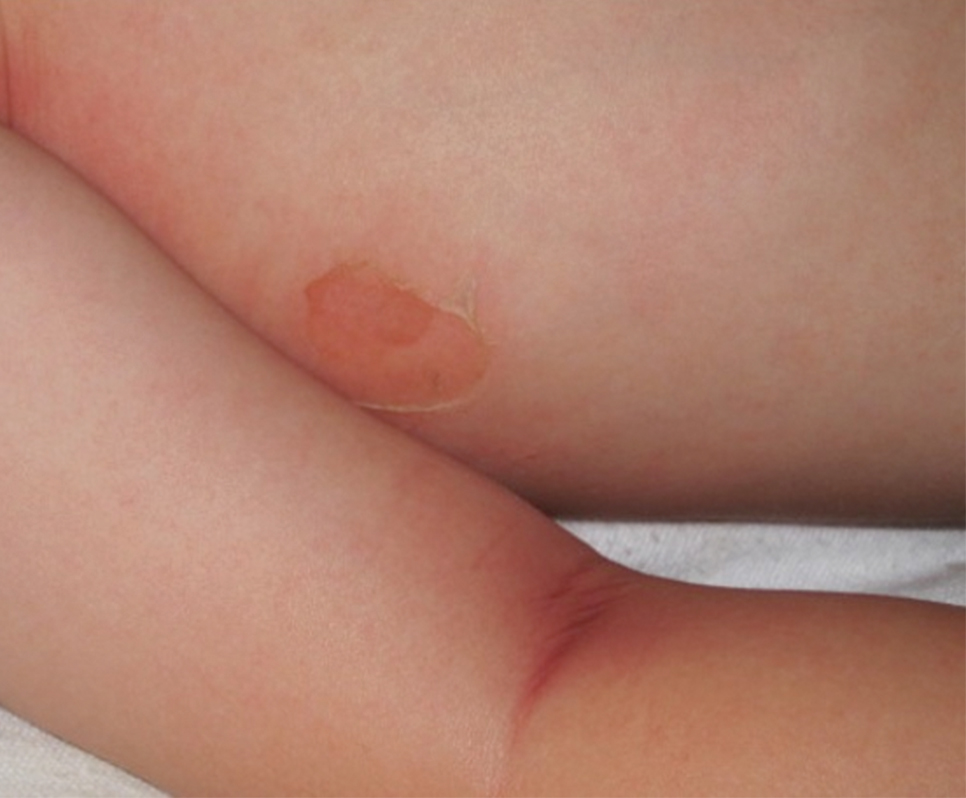
The condition is diagnosed clinically based on the findings of tender erythroderma, bullae, and desquamation with a scalded appearance, especially in friction zones; periorificial crusting; positive Nikolsky sign; and lack of mucosal involvement (Figure 1).1 Histopathology can aid in complicated clinical scenarios as well as culture from affected areas, including the upper respiratory tract, diaper region, and umbilicus.1,4 Hospitalization is required for SSSS for intravenous antibiotics, fluids, and electrolyte repletion.
Differential Diagnosis
There are multiple diagnoses to consider in the setting of flaccid bullae in the pediatric population. Stevens-Johnson syndrome or toxic epidermal necrolysis also can present with fever and superficial desquamation or bullae; however, exposure to medications and mucosal involvement often are absent in SSSS (Figure 2).2 Pemphigus, particularly paraneoplastic pemphigus, also often includes mucosal involvement and scalding thermal burns that are often geometric or focal. Epidermolysis bullosa and toxic shock syndrome also should be considered.1
Impetigo
Presentation
Impetigo is the most common bacterial skin infection in children caused by S aureus or Streptococcus pyogenes.7-9 It begins as erythematous papules transitioning to thin-walled vesicles that rapidly rupture and result in honey-crusted papules.7,9,10 Individuals of any age can be affected by nonbullous impetigo, but it is the most common skin infection in children aged 2 to 5 years.7
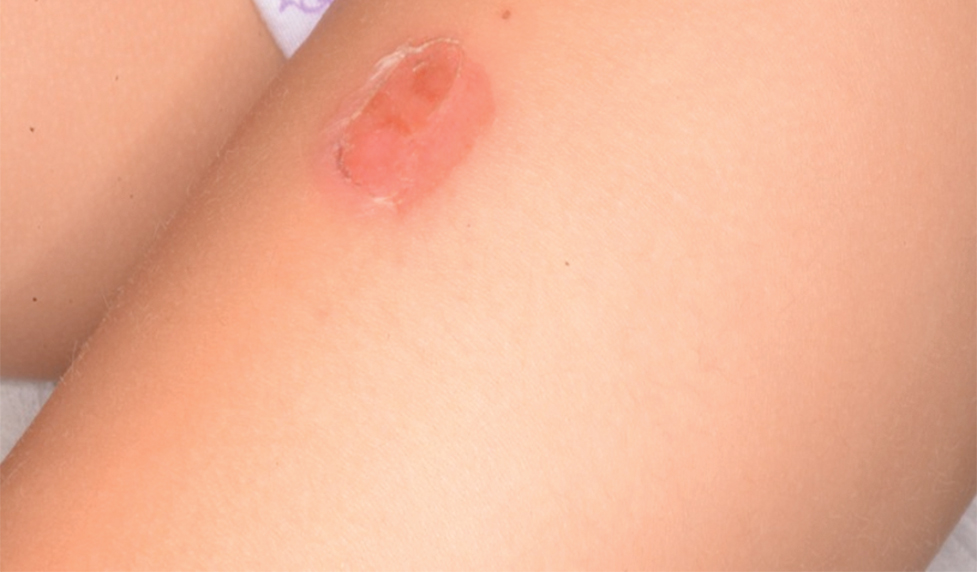
Bullous impetigo primarily is seen in children, especially infants, and rarely can occur in teenagers or adults.7 It most commonly is caused by the exfoliative toxins of S aureus. Bullous impetigo presents as small vesicles that may converge into larger flaccid bullae or pustules.7-10 Once the bullae rupture, an erythematous base with a collarette of scale remains without the formation of a honey-colored crust.8 Bullous impetigo usually affects moist intertriginous areas such as the axillae, neck, and diaper area8,10 (Figure 3). Complications may result in cellulitis, septicemia, osteomyelitis, poststreptococcal glomerulonephritis associated with S pyogenes, and S aureus–induced SSSS.7-9
Diagnosis
Nonbullous and bullous impetigo are largely clinical diagnoses that can be confirmed by culture of a vesicle or pustular fluid.10 Treatment of impetigo includes topical or systemic antibiotics.7,10 Patients should be advised to keep lesions covered and avoid contact with others until all lesions resolve, as lesions are contagious.9
Eczema Herpeticum
Presentation
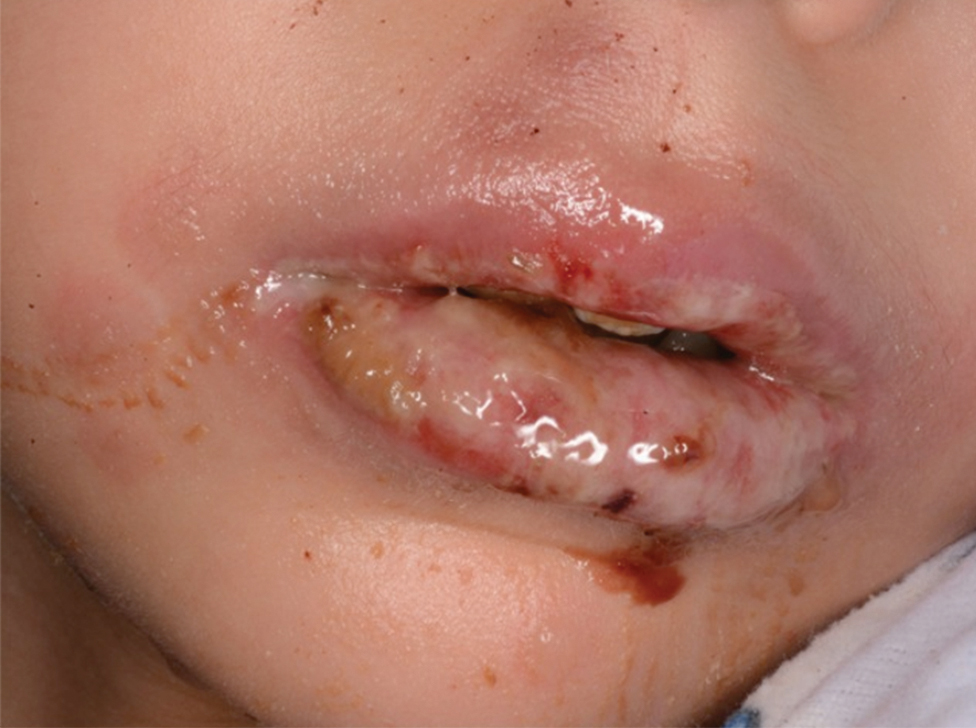
Eczema herpeticum (EH), also known as Kaposi varicelliform eruption, is a disseminated herpes simplex virus infection of impaired skin, most commonly in patients with atopic dermatitis (AD).11 Eczema herpeticum presents as a widespread eruption of erythematous monomorphic vesicles that progress to punched-out erosions with hemorrhagic crusting (Figure 4). Patients may have associated fever or lymphadenopathy.12,13
Causes
The number of children hospitalized annually for EH in the United States is approximately 4 to 7 cases per million children. Less than 3% of pediatric AD patients are affected, with a particularly increased risk in patients with severe and earlier-onset AD.12-15 Patients with AD have skin barrier defects, and decreased IFN-γ expression and cathelicidins predispose patients with AD to developing EH.12,16,17
Diagnosis
Viral polymerase chain reaction for herpes simplex virus types 1 and 2 is the standard for confirmatory diagnosis. Herpes simplex virus cultures from cutaneous scrapings, direct fluorescent antibody testing, or Tzanck test revealing multinucleated giant cells also may help establish the diagnosis.11,12,17
Management
Individuals with severe AD and other dermatologic conditions with cutaneous barrier compromise are at risk for developing EH, which is a medical emergency requiring hospitalization and prompt treatment with antiviral therapy such as acyclovir, often intravenously, as death can result if left untreated.11,17 Topical or systemic antibiotic therapy should be initiated if there is suspicion for secondary bacterial superinfection. Patients should be evaluated for multiorgan involvement such as keratoconjunctivitis, meningitis, encephalitis, and systemic viremia due to increased mortality, especially in infants.12,15,16
Langerhans Cell Histiocytosis
Presentation
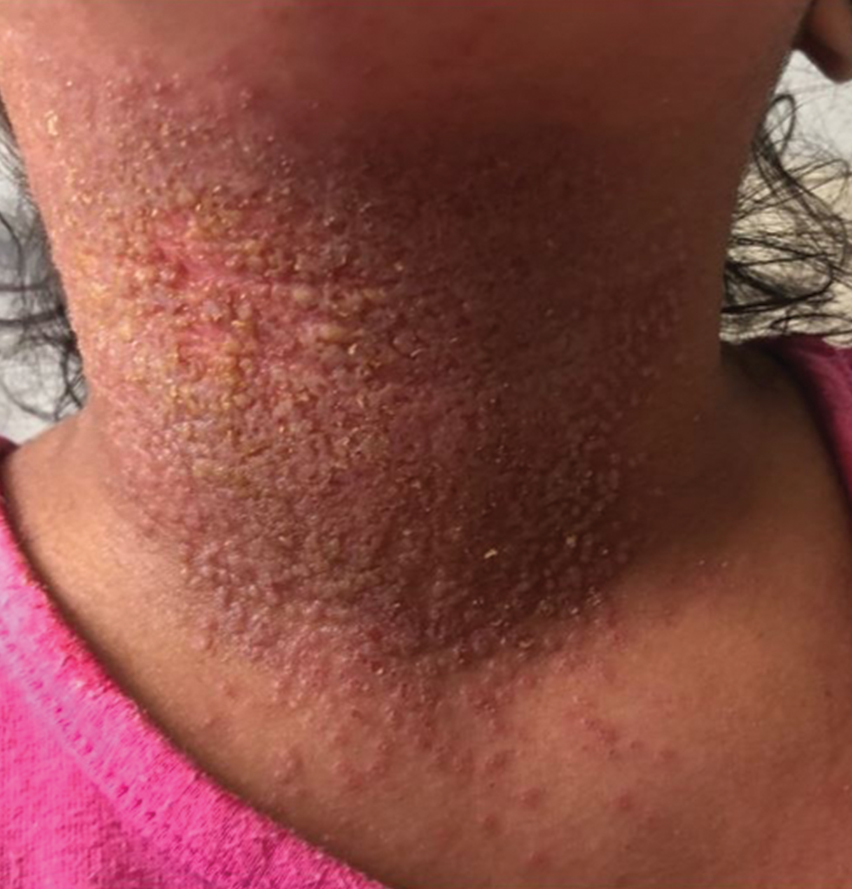
Langerhans cell histiocytosis (LCH) has a variable clinical presentation and can involve a single or multiple organ systems, including the bones and skin. Cutaneous LCH can present as violaceous papules, nodules, or ulcerations and crusted erosions (Figure 5). The lymph nodes, liver, spleen, oral mucosa, and respiratory and central nervous systems also may be involved.
Langerhans cell histiocytosis affects individuals of any age group but more often is seen in pediatric patients. The incidence of LCH is approximately 4.6 cases per million children.18 The pathogenesis is secondary to pathologic Langerhans cells, characterized as a clonal myeloid malignancy and dysregulation of the immune system.18,19
Diagnosis
A thorough physical examination is essential in patients with suspected LCH. Additionally, diagnosis of LCH is heavily based on histopathology of tissue from the involved organ system(s) with features of positive S-100 protein, CD1a, and CD207, and identification of Birbeck granules.20 Imaging and laboratory studies also are indicated and can include a skeletal survey (to assess osteolytic and organ involvement), a complete hematologic panel, coagulation studies, and liver function tests.18,21
Management
Management of LCH varies based on the organ system(s) involved along with the extent of the disease. Dermatology referral may be indicated in patients presenting with nonresolving cutaneous lesions as well as in severe cases. Single-organ and multisystem disease may require one treatment modality or a combination of chemotherapy, surgery, radiation, and/or immunotherapy.21
Infantile Hemangioma
Presentation
Infantile hemangioma (IH) is the most common benign tumor of infancy and usually is apparent a few weeks after birth. Lesions appear as bright red papules, nodules, or plaques. Deep or subcutaneous lesions present as raised, flesh-colored nodules with a blue hue and bruiselike appearance with or without a central patch of telangiectasia22-24 (Figure 6). Although all IHs eventually resolve, residual skin changes such as scarring, atrophy, and fibrosis can persist.24
The incidence of IH has been reported to occur in up to 4% to 5% of infants in the United States.23,25 Infantile hemangiomas also have been found to be more common among white, preterm, and multiple-gestation infants.25 The proposed pathogenesis of IHs includes angiogenic and vasogenic factors that cause rapid proliferation of blood vessels, likely driven by tissue hypoxia.23,26,27
Diagnosis
Infantile hemangioma is diagnosed clinically; however, immunohistochemical staining showing positivity for glucose transporter 1 also is helpful.26,27 Imaging modalities such as ultrasonography and magnetic resonance imaging also can be utilized to visualize the extent of lesions if necessary.25
Management
Around 15% to 25% of IHs are considered complicated and require intervention.25,27 Infantile hemangiomas can interfere with function depending on location or have potentially fatal complications. Based on the location and extent of involvement, these findings can include ulceration; hemorrhage; impairment of feeding, hearing, and/or vision; facial deformities; airway obstruction; hypothyroidism; and congestive heart failure.25,28 Early treatment with topical or oral beta-blockers is imperative for potentially life-threatening IHs, which can be seen due to large size or dangerous location.28,29 Because the rapid proliferative phase of IHs is thought to begin around 6 weeks of life, treatment should be initiated as early as possible. Initiation of beta-blocker therapy in the first few months of life can prevent functional impairment, ulceration, and permanent cosmetic changes. Additionally, surgery or pulsed dye laser treatment have been found to be effective for skin changes found after involution of IH.25,29
Differential Diagnosis
The differential diagnosis for IH includes vascular malformations, which are present at birth and do not undergo rapid proliferation; sarcoma; and kaposiform hemangioendothelioma, which causes the Kasabach-Merritt phenomenon secondary to platelet trapping. Careful attention to the history of the skin lesion provides good support for diagnosis of IH in most cases.
IgA Vasculitis
Presentation
IgA vasculitis, or Henoch-Schönlein purpura, classically presents as a tetrad of palpable purpura, acute-onset arthritis or arthralgia, abdominal pain, and renal disease with proteinuria or hematuria.30 Skin involvement is seen in almost all cases and is essential for diagnosis of IgA vasculitis. The initial dermatosis may be pruritic and present as an erythematous macular or urticarial wheal that evolves into petechiae, along with palpable purpura that is most frequently located on the legs or buttocks (Figure 7).30-34
IgA vasculitis is an immune-mediated small vessel vasculitis with deposition of IgA in the small vessels. The underlying cause remains unknown, though infection, dietary allergens, drugs, vaccinations, and chemical triggers have been recognized in literature.32,35,36 IgA vasculitis is largely a pediatric diagnosis, with 90% of affected individuals younger than 10 years worldwide.37 In the pediatric population, the incidence has been reported to be 3 to 26.7 cases per 100,000 children.32
Diagnosis
Diagnosis is based on the clinical presentation and histopathology.30 On direct immunofluorescence, IgA deposition is seen in the vessel walls.35 Laboratory testing is not diagnostic, but urinalysis is mandatory to identify involvement of renal vasculature. Imaging studies may be used in patients with abdominal symptoms, as an ultrasound can be used to visualize bowel structure and abnormalities such as intussusception.33
Management
The majority of cases of IgA vasculitis recover spontaneously, with patients requiring hospital admission based on severity of symptoms.30 The primary approach to management involves providing supportive care including hydration, adequate rest, and symptomatic pain relief of the joints and abdomen with oral analgesics. Systemic corticosteroids or steroid-sparing agents such as dapsone or colchicine can be used to treat cutaneous manifestations in addition to severe pain symptoms.30,31 Patients with IgA vasculitis must be monitored for proteinuria or hematuria to assess the extent of renal involvement. Although much more common in adults, long-term renal impairment can result from childhood cases of IgA vasculitis.34
Final Thoughts
Pediatric dermatology emergencies can be difficult to detect and accurately diagnose. Many of these diseases are potential emergencies that that may result in delayed treatment and considerable morbidity and mortality if missed. Clinicians should be aware that timely recognition and diagnosis, along with possible referral to pediatric dermatology, are essential to avoid complications.
Many pediatric skin conditions can be safely monitored with minimal intervention, but certain skin conditions are emergent and require immediate attention and proper assessment of the neonate, infant, or child. The skin may provide the first presentation of a potentially fatal disease with serious sequelae. Cutaneous findings may indicate the need for further evaluation. Therefore, it is important to differentiate skin conditions with benign etiologies from those that require immediate diagnosis and treatment, as early intervention of some of these conditions can be lifesaving. Herein, we discuss pertinent pediatric dermatology emergencies that dermatologists should keep in mind so that these diagnoses are never missed.
Staphylococcal Scalded Skin Syndrome
Presentation
Staphylococcal scalded skin syndrome (SSSS), or Ritter disease, is a potentially fatal pediatric emergency, especially in newborns.1 The mortality rate for SSSS in the United States is 3.6% to 11% in children.2 It typically presents with a prodrome of tenderness, fever, and confluent erythematous patches on the folds of the skin such as the groin, axillae, nose, and ears, with eventual spread to the legs and trunk.1,2 Within 24 to 48 hours of symptom onset, blistering and fluid accumulation will appear diffusely. Bullae are flaccid, and tangential and gentle pressure on involved unblistered skin may lead to shearing of the epithelium, which is a positive Nikolsky sign.1,2
Causes
Staphylococcal scalded skin syndrome is caused by exfoliative toxins A and B, toxigenic strains of Staphylococcus aureus. Exfoliative toxins A and B are serine proteases that target and cleave desmoglein 1, which binds keratinocytes in the stratum granulosum.1,3 Exfoliative toxins disrupt the adhesion of keratinocytes, resulting in bullae formation and subsequently diffuse sheetlike desquamation.1,4,5 Although up to 30% of the human population are asymptomatically and permanently colonized with nasal S aureus,6 the exfoliative toxins are produced by only 5% of species.1
In neonates, the immune and renal systems are underdeveloped; therefore, patients are susceptible to SSSS due to lack of neutralizing antibodies and decreased renal toxin excretion.4 Potential complications of SSSS are deeper soft-tissue infection, septicemia (blood-borne infection), and fluid and electrolyte imbalance.1,4
Diagnosis and Treatment
The condition is diagnosed clinically based on the findings of tender erythroderma, bullae, and desquamation with a scalded appearance, especially in friction zones; periorificial crusting; positive Nikolsky sign; and lack of mucosal involvement (Figure 1).1 Histopathology can aid in complicated clinical scenarios as well as culture from affected areas, including the upper respiratory tract, diaper region, and umbilicus.1,4 Hospitalization is required for SSSS for intravenous antibiotics, fluids, and electrolyte repletion.
Differential Diagnosis
There are multiple diagnoses to consider in the setting of flaccid bullae in the pediatric population. Stevens-Johnson syndrome or toxic epidermal necrolysis also can present with fever and superficial desquamation or bullae; however, exposure to medications and mucosal involvement often are absent in SSSS (Figure 2).2 Pemphigus, particularly paraneoplastic pemphigus, also often includes mucosal involvement and scalding thermal burns that are often geometric or focal. Epidermolysis bullosa and toxic shock syndrome also should be considered.1
Impetigo
Presentation
Impetigo is the most common bacterial skin infection in children caused by S aureus or Streptococcus pyogenes.7-9 It begins as erythematous papules transitioning to thin-walled vesicles that rapidly rupture and result in honey-crusted papules.7,9,10 Individuals of any age can be affected by nonbullous impetigo, but it is the most common skin infection in children aged 2 to 5 years.7
Bullous impetigo primarily is seen in children, especially infants, and rarely can occur in teenagers or adults.7 It most commonly is caused by the exfoliative toxins of S aureus. Bullous impetigo presents as small vesicles that may converge into larger flaccid bullae or pustules.7-10 Once the bullae rupture, an erythematous base with a collarette of scale remains without the formation of a honey-colored crust.8 Bullous impetigo usually affects moist intertriginous areas such as the axillae, neck, and diaper area8,10 (Figure 3). Complications may result in cellulitis, septicemia, osteomyelitis, poststreptococcal glomerulonephritis associated with S pyogenes, and S aureus–induced SSSS.7-9
Diagnosis
Nonbullous and bullous impetigo are largely clinical diagnoses that can be confirmed by culture of a vesicle or pustular fluid.10 Treatment of impetigo includes topical or systemic antibiotics.7,10 Patients should be advised to keep lesions covered and avoid contact with others until all lesions resolve, as lesions are contagious.9
Eczema Herpeticum
Presentation
Eczema herpeticum (EH), also known as Kaposi varicelliform eruption, is a disseminated herpes simplex virus infection of impaired skin, most commonly in patients with atopic dermatitis (AD).11 Eczema herpeticum presents as a widespread eruption of erythematous monomorphic vesicles that progress to punched-out erosions with hemorrhagic crusting (Figure 4). Patients may have associated fever or lymphadenopathy.12,13
Causes
The number of children hospitalized annually for EH in the United States is approximately 4 to 7 cases per million children. Less than 3% of pediatric AD patients are affected, with a particularly increased risk in patients with severe and earlier-onset AD.12-15 Patients with AD have skin barrier defects, and decreased IFN-γ expression and cathelicidins predispose patients with AD to developing EH.12,16,17
Diagnosis
Viral polymerase chain reaction for herpes simplex virus types 1 and 2 is the standard for confirmatory diagnosis. Herpes simplex virus cultures from cutaneous scrapings, direct fluorescent antibody testing, or Tzanck test revealing multinucleated giant cells also may help establish the diagnosis.11,12,17
Management
Individuals with severe AD and other dermatologic conditions with cutaneous barrier compromise are at risk for developing EH, which is a medical emergency requiring hospitalization and prompt treatment with antiviral therapy such as acyclovir, often intravenously, as death can result if left untreated.11,17 Topical or systemic antibiotic therapy should be initiated if there is suspicion for secondary bacterial superinfection. Patients should be evaluated for multiorgan involvement such as keratoconjunctivitis, meningitis, encephalitis, and systemic viremia due to increased mortality, especially in infants.12,15,16
Langerhans Cell Histiocytosis
Presentation
Langerhans cell histiocytosis (LCH) has a variable clinical presentation and can involve a single or multiple organ systems, including the bones and skin. Cutaneous LCH can present as violaceous papules, nodules, or ulcerations and crusted erosions (Figure 5). The lymph nodes, liver, spleen, oral mucosa, and respiratory and central nervous systems also may be involved.
Langerhans cell histiocytosis affects individuals of any age group but more often is seen in pediatric patients. The incidence of LCH is approximately 4.6 cases per million children.18 The pathogenesis is secondary to pathologic Langerhans cells, characterized as a clonal myeloid malignancy and dysregulation of the immune system.18,19
Diagnosis
A thorough physical examination is essential in patients with suspected LCH. Additionally, diagnosis of LCH is heavily based on histopathology of tissue from the involved organ system(s) with features of positive S-100 protein, CD1a, and CD207, and identification of Birbeck granules.20 Imaging and laboratory studies also are indicated and can include a skeletal survey (to assess osteolytic and organ involvement), a complete hematologic panel, coagulation studies, and liver function tests.18,21
Management
Management of LCH varies based on the organ system(s) involved along with the extent of the disease. Dermatology referral may be indicated in patients presenting with nonresolving cutaneous lesions as well as in severe cases. Single-organ and multisystem disease may require one treatment modality or a combination of chemotherapy, surgery, radiation, and/or immunotherapy.21
Infantile Hemangioma
Presentation
Infantile hemangioma (IH) is the most common benign tumor of infancy and usually is apparent a few weeks after birth. Lesions appear as bright red papules, nodules, or plaques. Deep or subcutaneous lesions present as raised, flesh-colored nodules with a blue hue and bruiselike appearance with or without a central patch of telangiectasia22-24 (Figure 6). Although all IHs eventually resolve, residual skin changes such as scarring, atrophy, and fibrosis can persist.24
The incidence of IH has been reported to occur in up to 4% to 5% of infants in the United States.23,25 Infantile hemangiomas also have been found to be more common among white, preterm, and multiple-gestation infants.25 The proposed pathogenesis of IHs includes angiogenic and vasogenic factors that cause rapid proliferation of blood vessels, likely driven by tissue hypoxia.23,26,27
Diagnosis
Infantile hemangioma is diagnosed clinically; however, immunohistochemical staining showing positivity for glucose transporter 1 also is helpful.26,27 Imaging modalities such as ultrasonography and magnetic resonance imaging also can be utilized to visualize the extent of lesions if necessary.25
Management
Around 15% to 25% of IHs are considered complicated and require intervention.25,27 Infantile hemangiomas can interfere with function depending on location or have potentially fatal complications. Based on the location and extent of involvement, these findings can include ulceration; hemorrhage; impairment of feeding, hearing, and/or vision; facial deformities; airway obstruction; hypothyroidism; and congestive heart failure.25,28 Early treatment with topical or oral beta-blockers is imperative for potentially life-threatening IHs, which can be seen due to large size or dangerous location.28,29 Because the rapid proliferative phase of IHs is thought to begin around 6 weeks of life, treatment should be initiated as early as possible. Initiation of beta-blocker therapy in the first few months of life can prevent functional impairment, ulceration, and permanent cosmetic changes. Additionally, surgery or pulsed dye laser treatment have been found to be effective for skin changes found after involution of IH.25,29
Differential Diagnosis
The differential diagnosis for IH includes vascular malformations, which are present at birth and do not undergo rapid proliferation; sarcoma; and kaposiform hemangioendothelioma, which causes the Kasabach-Merritt phenomenon secondary to platelet trapping. Careful attention to the history of the skin lesion provides good support for diagnosis of IH in most cases.
IgA Vasculitis
Presentation
IgA vasculitis, or Henoch-Schönlein purpura, classically presents as a tetrad of palpable purpura, acute-onset arthritis or arthralgia, abdominal pain, and renal disease with proteinuria or hematuria.30 Skin involvement is seen in almost all cases and is essential for diagnosis of IgA vasculitis. The initial dermatosis may be pruritic and present as an erythematous macular or urticarial wheal that evolves into petechiae, along with palpable purpura that is most frequently located on the legs or buttocks (Figure 7).30-34
IgA vasculitis is an immune-mediated small vessel vasculitis with deposition of IgA in the small vessels. The underlying cause remains unknown, though infection, dietary allergens, drugs, vaccinations, and chemical triggers have been recognized in literature.32,35,36 IgA vasculitis is largely a pediatric diagnosis, with 90% of affected individuals younger than 10 years worldwide.37 In the pediatric population, the incidence has been reported to be 3 to 26.7 cases per 100,000 children.32
Diagnosis
Diagnosis is based on the clinical presentation and histopathology.30 On direct immunofluorescence, IgA deposition is seen in the vessel walls.35 Laboratory testing is not diagnostic, but urinalysis is mandatory to identify involvement of renal vasculature. Imaging studies may be used in patients with abdominal symptoms, as an ultrasound can be used to visualize bowel structure and abnormalities such as intussusception.33
Management
The majority of cases of IgA vasculitis recover spontaneously, with patients requiring hospital admission based on severity of symptoms.30 The primary approach to management involves providing supportive care including hydration, adequate rest, and symptomatic pain relief of the joints and abdomen with oral analgesics. Systemic corticosteroids or steroid-sparing agents such as dapsone or colchicine can be used to treat cutaneous manifestations in addition to severe pain symptoms.30,31 Patients with IgA vasculitis must be monitored for proteinuria or hematuria to assess the extent of renal involvement. Although much more common in adults, long-term renal impairment can result from childhood cases of IgA vasculitis.34
Final Thoughts
Pediatric dermatology emergencies can be difficult to detect and accurately diagnose. Many of these diseases are potential emergencies that that may result in delayed treatment and considerable morbidity and mortality if missed. Clinicians should be aware that timely recognition and diagnosis, along with possible referral to pediatric dermatology, are essential to avoid complications.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
- Davidson J, Polly S, Hayes P, et al. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7:E134-E137.
- Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150-159.
- Berk D. Staphylococcal scalded skin syndrome. Cancer Therapy Advisor website. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/pediatrics/staphylococcal-scalded-skin-syndrome/. Published 2017. Accessed February 19, 2020.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections [published online October 8, 2018]. Front Microbiol. 2018;9:2419.
- Pereira LB. Impetigo review. An Bras Dermatol. 2014;89:293-299.
- Nardi NM, Schaefer TJ. Impetigo. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK430974/. Accessed February 21, 2020.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
- Sommer LL, Reboli AC, Heymann WR. Bacterial diseases. In: Bolognia, JL Schaffer, JV Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1259-1295.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377:e9.
- Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients [published online November 16, 2019]. J Eur Acad Dermatology Venereol. doi:10.1111/jdv.16090.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93.
- Hsu DY, Shinkai K, Silverberg JI. Epidemiology of eczema herpeticum in hospitalized U.S. children: analysis of a nationwide cohort [published online September 17, 2018]. J Invest Dermatol. 2018;138:265-272.
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127:965-73.e1-5.
- Darji K, Frisch S, Adjei Boakye E, et al. Characterization of children with recurrent eczema herpeticum and response to treatment with interferon-gamma. Pediatr Dermatol. 2017;34:686-689.
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015;2015:565-570.
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184.
- Holland KE, Drolet BA. Infantile hemangioma [published online August 21, 2010]. Pediatr Clin North Am. 2010;57:1069-1083.
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108.
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18(suppl 1):S117-S120.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:786-791.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219-227.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:E259-E266.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas [published online January 2019]. Pediatrics. doi:10.1542/peds.2018-3475.
- Sohagia AB, Gunturu SG, Tong TR, et al. Henoch-Schönlein purpura—a case report and review of the literature [published online May 23, 2010]. Gastroenterol Res Pract. doi:10.1155/2010/597648.
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch–Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25:171-178.
- Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
- Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant. 2014;30:I94-I103.
- van Timmeren MM, Heeringa P, Kallenberg CG. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26:416-423.
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis [published online July 11, 2013]. Clin Exp Nephrol. 2013;17:607-610.
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387-1395.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
- Davidson J, Polly S, Hayes P, et al. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7:E134-E137.
- Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150-159.
- Berk D. Staphylococcal scalded skin syndrome. Cancer Therapy Advisor website. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/pediatrics/staphylococcal-scalded-skin-syndrome/. Published 2017. Accessed February 19, 2020.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections [published online October 8, 2018]. Front Microbiol. 2018;9:2419.
- Pereira LB. Impetigo review. An Bras Dermatol. 2014;89:293-299.
- Nardi NM, Schaefer TJ. Impetigo. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK430974/. Accessed February 21, 2020.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
- Sommer LL, Reboli AC, Heymann WR. Bacterial diseases. In: Bolognia, JL Schaffer, JV Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1259-1295.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377:e9.
- Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients [published online November 16, 2019]. J Eur Acad Dermatology Venereol. doi:10.1111/jdv.16090.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93.
- Hsu DY, Shinkai K, Silverberg JI. Epidemiology of eczema herpeticum in hospitalized U.S. children: analysis of a nationwide cohort [published online September 17, 2018]. J Invest Dermatol. 2018;138:265-272.
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127:965-73.e1-5.
- Darji K, Frisch S, Adjei Boakye E, et al. Characterization of children with recurrent eczema herpeticum and response to treatment with interferon-gamma. Pediatr Dermatol. 2017;34:686-689.
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015;2015:565-570.
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184.
- Holland KE, Drolet BA. Infantile hemangioma [published online August 21, 2010]. Pediatr Clin North Am. 2010;57:1069-1083.
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108.
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18(suppl 1):S117-S120.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:786-791.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219-227.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:E259-E266.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas [published online January 2019]. Pediatrics. doi:10.1542/peds.2018-3475.
- Sohagia AB, Gunturu SG, Tong TR, et al. Henoch-Schönlein purpura—a case report and review of the literature [published online May 23, 2010]. Gastroenterol Res Pract. doi:10.1155/2010/597648.
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch–Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25:171-178.
- Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
- Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant. 2014;30:I94-I103.
- van Timmeren MM, Heeringa P, Kallenberg CG. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26:416-423.
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis [published online July 11, 2013]. Clin Exp Nephrol. 2013;17:607-610.
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387-1395.
Practice Points
- Staphylococcal scalded skin syndrome, impetigo, eczema herpeticum, Langerhans cell histiocytosis, infantile hemangiomas, and IgA vasculitis all present potential emergencies in pediatric patients in dermatologic settings.
- Early and accurate identification and management of these entities is critical to avoid short-term and long-term negative sequalae.