User login
I have a dream … for psychiatry
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
From famous to infamous: Psychiatric aspects of the fall from grace
It’s an all-too-common news item: The crash and burn of yet another politician, celebrity, or prominent individual. It’s painful to watch someone who spent years to achieve the status of a household name suddenly, and often ignominiously, lose it all. This is the equivalent of a human train wreck.
Some adversaries (who doesn’t have a few?) will rejoice or express schadenfreude, but many people will experience some empathy or sorrow as they witness the implosion of a celebrity. Fans, followers, or voters may grieve as the object of their respect and adulation falls off the high pedestal of fame. What starts as a drip-drip of rumors and innuendos soon eventuates in a denouement. And with time, as additional public figures fall from grace, the previous casualties will become mere footnotes in the annals of human self-destruction. Their loss of face, shame, and wrenching emotional and financial toll will be forgotten from the public’s collective memory, but the embers of bitterness and regret will continue to smolder in the hearts and souls of those who inadvertently contributed to their own social or professional demise due to a mistake, error of judgement, or plain old-fashioned stupidity. For the fallen, forgiveness and redemption are hard to come by.
Oh, how the mighty have fallen over centuries, and they include historical figures such as kings, military leaders, religious leaders, and politicians. The fall from grace in the past often led to executions, excommunication, or persecution. In the contemporary era, the oppressive “cancel culture” will mercilessly discard anyone, regardless of stature, after only 1 “wrong” tweet. In the digital age of mass communication, being “cancelled” is a frequent fall from grace and is the equivalent of being ostracized from millions of denizens on social media, which can spell doom for one’s career and social interactions.
The list of those whose careers ended calamitously include many familiar names, but I will only cite their prominent roles (you can easily guess their names!):
- emperors, kings, presidents, prime ministers, and political demagogues
- congressmen, senators, governors, and mayors
- Nobel Laureates (a Medicine and Physiology winner went to prison for pedophilia, and a Peace Prize winner fell from grace for supporting a military dictatorship)
- Cardinals and bishops in various countries (for sexual or financial crimes)
- billionaires, often for erratic personal lives
- sport legends, including decorated athletes and coaches of college and professional teams
- world chess masters
- Wall Street moguls
- Hollywood celebrities, including actors and directors, some with Oscars and related recognitions
- television news anchors and commentators
- comedians of various stripes
- CEOs of major media companies
- talk show hosts watched by millions
- celebrated musicians (classical, pop, rap, or blues)
- university presidents
- others in esteemed positions (including some psychiatrists).
Why is this so common?
From a psychiatric perspective, the most compelling question is why is the fall from grace so common? What are the transgressions, flaws, and shortcomings of successful individuals whose reputations end up smeared or who lose everything they worked for? Why do high achievers, talented and successful, at the apogee of fame and fortune, lose it all with nary a chance for recovery
The answer is all too obvious: human frailties. Successful persons are by no means immune from poor judgment. They can be as error-prone as the rest of us mortals. Having robust cognitive intelligence can be undermined by stunted emotional intelligence or poor interpersonal or social judgment. In Freudian terms, famous people who crash and burn may have a “Swiss cheese superego” that allows their id to viciously weaken their ego. From a neuroscience perspective, their limbic system conquers their cortical circuitry with relentless innate forces, including:
- fervent sexual appetite, compounded by the cockiness that comes with fame
- felonious paraphilias, such as pedophilia or public indecency
- intense greed that clouds one’s judgment (a trait exhibited by some ultra-rich persons)
- narcissism, either inborn or acquired with unexpected success and power
- impulsivity and recklessness, with injurious words or actions.
- substance use.
Consideration should be given to psychopathology. Some may have a personality disorder. Others may be both blessed and cursed with hypomania that leads to high achievement but also to foolish and impulsive behavior.1 Some may have maladaptive social skills seen in autism spectrum disorder (recently, a very prominent and innovative billionaire casually announced that he has autistic traits). And others my have limited coping skills to deal with fame and fortune and unwittingly end up shooting themselves in both feet.
Continue to: But perhaps the most common thread...
But perhaps the most common thread across all the tragic cases of self-destruction is hubris. As humans become rich, famous, or powerful, they gradually develop the fallacious belief that they can get away with anything because they have masses of fans and followers who “love them no matter what.” This dangerous “acquired narcissism” is an unfortunate byproduct of success. Humility is rare among celebrities and powerful leaders. Modest celebrities almost never fall from grace and are endowed with an innate antidote to self-aggrandizement. A few years ago, I wrote an editorial in
In contemporary society, with the era of social media and toxic political zeitgeist, there are many inadvertent “opportunities” to stumble and ruin one’s career by uttering an “unacceptable” word or dispatching an “offensive tweet” or posting a politically incorrect photo. And even if one is currently careful, there are now social media detectives and fact-finding “archeologists” who can excavate and disseminate the faux pas, peccadillos, or misdeeds from a prominent person’s immature youth, which will destroy a famous person overnight. That can be a nightmare for anyone who becomes a bona fide celebrity after years of working hard to get there.
High achievers: Beware!
1. Gartner JD. The hypomanic edge: the link between (a little) craziness and (a lot of) success in America. Simon & Schuster; 2005.
2. Nasrallah HA. Should psychiatry list hubris in DSM-V? Current Psychiatry. 2008;7(12):14-15.
It’s an all-too-common news item: The crash and burn of yet another politician, celebrity, or prominent individual. It’s painful to watch someone who spent years to achieve the status of a household name suddenly, and often ignominiously, lose it all. This is the equivalent of a human train wreck.
Some adversaries (who doesn’t have a few?) will rejoice or express schadenfreude, but many people will experience some empathy or sorrow as they witness the implosion of a celebrity. Fans, followers, or voters may grieve as the object of their respect and adulation falls off the high pedestal of fame. What starts as a drip-drip of rumors and innuendos soon eventuates in a denouement. And with time, as additional public figures fall from grace, the previous casualties will become mere footnotes in the annals of human self-destruction. Their loss of face, shame, and wrenching emotional and financial toll will be forgotten from the public’s collective memory, but the embers of bitterness and regret will continue to smolder in the hearts and souls of those who inadvertently contributed to their own social or professional demise due to a mistake, error of judgement, or plain old-fashioned stupidity. For the fallen, forgiveness and redemption are hard to come by.
Oh, how the mighty have fallen over centuries, and they include historical figures such as kings, military leaders, religious leaders, and politicians. The fall from grace in the past often led to executions, excommunication, or persecution. In the contemporary era, the oppressive “cancel culture” will mercilessly discard anyone, regardless of stature, after only 1 “wrong” tweet. In the digital age of mass communication, being “cancelled” is a frequent fall from grace and is the equivalent of being ostracized from millions of denizens on social media, which can spell doom for one’s career and social interactions.
The list of those whose careers ended calamitously include many familiar names, but I will only cite their prominent roles (you can easily guess their names!):
- emperors, kings, presidents, prime ministers, and political demagogues
- congressmen, senators, governors, and mayors
- Nobel Laureates (a Medicine and Physiology winner went to prison for pedophilia, and a Peace Prize winner fell from grace for supporting a military dictatorship)
- Cardinals and bishops in various countries (for sexual or financial crimes)
- billionaires, often for erratic personal lives
- sport legends, including decorated athletes and coaches of college and professional teams
- world chess masters
- Wall Street moguls
- Hollywood celebrities, including actors and directors, some with Oscars and related recognitions
- television news anchors and commentators
- comedians of various stripes
- CEOs of major media companies
- talk show hosts watched by millions
- celebrated musicians (classical, pop, rap, or blues)
- university presidents
- others in esteemed positions (including some psychiatrists).
Why is this so common?
From a psychiatric perspective, the most compelling question is why is the fall from grace so common? What are the transgressions, flaws, and shortcomings of successful individuals whose reputations end up smeared or who lose everything they worked for? Why do high achievers, talented and successful, at the apogee of fame and fortune, lose it all with nary a chance for recovery
The answer is all too obvious: human frailties. Successful persons are by no means immune from poor judgment. They can be as error-prone as the rest of us mortals. Having robust cognitive intelligence can be undermined by stunted emotional intelligence or poor interpersonal or social judgment. In Freudian terms, famous people who crash and burn may have a “Swiss cheese superego” that allows their id to viciously weaken their ego. From a neuroscience perspective, their limbic system conquers their cortical circuitry with relentless innate forces, including:
- fervent sexual appetite, compounded by the cockiness that comes with fame
- felonious paraphilias, such as pedophilia or public indecency
- intense greed that clouds one’s judgment (a trait exhibited by some ultra-rich persons)
- narcissism, either inborn or acquired with unexpected success and power
- impulsivity and recklessness, with injurious words or actions.
- substance use.
Consideration should be given to psychopathology. Some may have a personality disorder. Others may be both blessed and cursed with hypomania that leads to high achievement but also to foolish and impulsive behavior.1 Some may have maladaptive social skills seen in autism spectrum disorder (recently, a very prominent and innovative billionaire casually announced that he has autistic traits). And others my have limited coping skills to deal with fame and fortune and unwittingly end up shooting themselves in both feet.
Continue to: But perhaps the most common thread...
But perhaps the most common thread across all the tragic cases of self-destruction is hubris. As humans become rich, famous, or powerful, they gradually develop the fallacious belief that they can get away with anything because they have masses of fans and followers who “love them no matter what.” This dangerous “acquired narcissism” is an unfortunate byproduct of success. Humility is rare among celebrities and powerful leaders. Modest celebrities almost never fall from grace and are endowed with an innate antidote to self-aggrandizement. A few years ago, I wrote an editorial in
In contemporary society, with the era of social media and toxic political zeitgeist, there are many inadvertent “opportunities” to stumble and ruin one’s career by uttering an “unacceptable” word or dispatching an “offensive tweet” or posting a politically incorrect photo. And even if one is currently careful, there are now social media detectives and fact-finding “archeologists” who can excavate and disseminate the faux pas, peccadillos, or misdeeds from a prominent person’s immature youth, which will destroy a famous person overnight. That can be a nightmare for anyone who becomes a bona fide celebrity after years of working hard to get there.
High achievers: Beware!
It’s an all-too-common news item: The crash and burn of yet another politician, celebrity, or prominent individual. It’s painful to watch someone who spent years to achieve the status of a household name suddenly, and often ignominiously, lose it all. This is the equivalent of a human train wreck.
Some adversaries (who doesn’t have a few?) will rejoice or express schadenfreude, but many people will experience some empathy or sorrow as they witness the implosion of a celebrity. Fans, followers, or voters may grieve as the object of their respect and adulation falls off the high pedestal of fame. What starts as a drip-drip of rumors and innuendos soon eventuates in a denouement. And with time, as additional public figures fall from grace, the previous casualties will become mere footnotes in the annals of human self-destruction. Their loss of face, shame, and wrenching emotional and financial toll will be forgotten from the public’s collective memory, but the embers of bitterness and regret will continue to smolder in the hearts and souls of those who inadvertently contributed to their own social or professional demise due to a mistake, error of judgement, or plain old-fashioned stupidity. For the fallen, forgiveness and redemption are hard to come by.
Oh, how the mighty have fallen over centuries, and they include historical figures such as kings, military leaders, religious leaders, and politicians. The fall from grace in the past often led to executions, excommunication, or persecution. In the contemporary era, the oppressive “cancel culture” will mercilessly discard anyone, regardless of stature, after only 1 “wrong” tweet. In the digital age of mass communication, being “cancelled” is a frequent fall from grace and is the equivalent of being ostracized from millions of denizens on social media, which can spell doom for one’s career and social interactions.
The list of those whose careers ended calamitously include many familiar names, but I will only cite their prominent roles (you can easily guess their names!):
- emperors, kings, presidents, prime ministers, and political demagogues
- congressmen, senators, governors, and mayors
- Nobel Laureates (a Medicine and Physiology winner went to prison for pedophilia, and a Peace Prize winner fell from grace for supporting a military dictatorship)
- Cardinals and bishops in various countries (for sexual or financial crimes)
- billionaires, often for erratic personal lives
- sport legends, including decorated athletes and coaches of college and professional teams
- world chess masters
- Wall Street moguls
- Hollywood celebrities, including actors and directors, some with Oscars and related recognitions
- television news anchors and commentators
- comedians of various stripes
- CEOs of major media companies
- talk show hosts watched by millions
- celebrated musicians (classical, pop, rap, or blues)
- university presidents
- others in esteemed positions (including some psychiatrists).
Why is this so common?
From a psychiatric perspective, the most compelling question is why is the fall from grace so common? What are the transgressions, flaws, and shortcomings of successful individuals whose reputations end up smeared or who lose everything they worked for? Why do high achievers, talented and successful, at the apogee of fame and fortune, lose it all with nary a chance for recovery
The answer is all too obvious: human frailties. Successful persons are by no means immune from poor judgment. They can be as error-prone as the rest of us mortals. Having robust cognitive intelligence can be undermined by stunted emotional intelligence or poor interpersonal or social judgment. In Freudian terms, famous people who crash and burn may have a “Swiss cheese superego” that allows their id to viciously weaken their ego. From a neuroscience perspective, their limbic system conquers their cortical circuitry with relentless innate forces, including:
- fervent sexual appetite, compounded by the cockiness that comes with fame
- felonious paraphilias, such as pedophilia or public indecency
- intense greed that clouds one’s judgment (a trait exhibited by some ultra-rich persons)
- narcissism, either inborn or acquired with unexpected success and power
- impulsivity and recklessness, with injurious words or actions.
- substance use.
Consideration should be given to psychopathology. Some may have a personality disorder. Others may be both blessed and cursed with hypomania that leads to high achievement but also to foolish and impulsive behavior.1 Some may have maladaptive social skills seen in autism spectrum disorder (recently, a very prominent and innovative billionaire casually announced that he has autistic traits). And others my have limited coping skills to deal with fame and fortune and unwittingly end up shooting themselves in both feet.
Continue to: But perhaps the most common thread...
But perhaps the most common thread across all the tragic cases of self-destruction is hubris. As humans become rich, famous, or powerful, they gradually develop the fallacious belief that they can get away with anything because they have masses of fans and followers who “love them no matter what.” This dangerous “acquired narcissism” is an unfortunate byproduct of success. Humility is rare among celebrities and powerful leaders. Modest celebrities almost never fall from grace and are endowed with an innate antidote to self-aggrandizement. A few years ago, I wrote an editorial in
In contemporary society, with the era of social media and toxic political zeitgeist, there are many inadvertent “opportunities” to stumble and ruin one’s career by uttering an “unacceptable” word or dispatching an “offensive tweet” or posting a politically incorrect photo. And even if one is currently careful, there are now social media detectives and fact-finding “archeologists” who can excavate and disseminate the faux pas, peccadillos, or misdeeds from a prominent person’s immature youth, which will destroy a famous person overnight. That can be a nightmare for anyone who becomes a bona fide celebrity after years of working hard to get there.
High achievers: Beware!
1. Gartner JD. The hypomanic edge: the link between (a little) craziness and (a lot of) success in America. Simon & Schuster; 2005.
2. Nasrallah HA. Should psychiatry list hubris in DSM-V? Current Psychiatry. 2008;7(12):14-15.
1. Gartner JD. The hypomanic edge: the link between (a little) craziness and (a lot of) success in America. Simon & Schuster; 2005.
2. Nasrallah HA. Should psychiatry list hubris in DSM-V? Current Psychiatry. 2008;7(12):14-15.
Beyond DSM symptoms: Behavioral clues to diagnosing bipolar II disorder
The diagnosis of bipolar II disorder is one of the most common challenges in psychiatric practice. Bipolar II disorder is frequently misdiagnosed as major depressive disorder (MDD) because symptoms of transient hypomanic episodes are either insufficiently probed or are rather vague. However, there are many valuable biographical clues that can expedite the diagnosis of bipolar II disorder.
The late Hagop S. Akiskal, MD, who passed away in January 2021, was an internationally recognized expert in mood disorders, and a dear friend for decades. He was a keen observer of human behavior who delved into the “life stories” of patients seeking help for depression. By thinking “outside the DSM box,” Dr. Akiskal was the first to recognize and codify a variety of behavioral and biographical clues for the bipolar spectrum (of which he was a pioneer) in patients presenting with a chief complaint of depression. He proposed a colorful set of behavioral stigmata in most patients with bipolar II disorder by carefully canvassing the life experiences of the patients he treated in the mood disorder clinic he established in the 1970s, which is believed to have been the first mood specialty clinic in the country.
Based on a review of >1,000 patients in his clinic who presented with depressive symptoms and were ultimately diagnosed as bipolar II disorder, Dr. Akiskal highlighted what he labeled as “behavioral activation, flamboyance and extravagance” among those patients. He referred to the cluster of those behaviors as “the soft spectrum” of bipolar disorder, which manifests in a set of distinctive behaviors in addition to depressive symptoms. He found that research tools such as the DSM-based Structured Clinical Interview often fail and frequently lead to a misdiagnosis of bipolar II disorder as MDD. This often condemns the patient to multiple failed trials of antidepressant monotherapy, and a delay in improvement, thus increasing the risk of job loss, disrupted relationships, and even suicide.
Over 3 decades, Dr. Akiskal developed the Mood Clinic Data Questionnaire (MCDQ) to systematize unstructured observations of patients presenting with a chief complaint of depression. His tool expedites the diagnosis of bipolar II disorder by understanding the patient as an individual, revealing personal and behavioral features consistent with what he labeled as episodic “hyperthymia” within the context of recurrent depression. This “social and behavioral phenotype,” as Dr. Akiskal called it, is rarely observed among patients with MDD.
By examining many patients with bipolar II disorder, Dr. Akiskal identified several “triads” of behavioral traits in the patients’ biographical history and in some of their close blood relatives as well. He also noticed that temperamentally, patients with bipolar II disorder thrive on “activity” and lovingly referred to themselves as “activity junkies.” Some of them may qualify as workaholics.
Biographical features that suggest bipolar II disorder
Here is a summary of the unique biographical features of patients with bipolar II disorder that Dr. Akiskal described:
Multilingual. Speaking ≥3 languages is unusual among individuals born in the United States, but often encountered among those with bipolar II disorder.
Continue to: Eminence
Eminence. Patients with bipolar II disorder, as well as their family members, tend to have leadership roles and prominence in journalism, media, and entertainment, fields that require interpersonal charm and eloquence. Those are common features of the “hyperthymic” temperament.
Creativity. Artists, poets, painters, and musicians who experience depression are more likely to have bipolar II disorder than MDD.
Biographical instability and/or excess. This is exemplified by going to 3 colleges and not necessarily obtaining a degree, or by frequently changing one’s line of work or city of residence. A classic example is a professor of medicine who also practices law and regularly sings in the opera, or a physician who is board-certified in 3 distinct specialties.
Activity junkies. Examples include a person with boundless energy, such as a novelist who writes 3 books a year or a professional who regularly works 12 hours a day without getting exhausted but seeks treatment for depressive episodes.
Multiple substances of abuse, such as nicotine, alcohol, stimulants, and opiates.
Continue to: Multiple psychiatric comorbidities
Multiple psychiatric comorbidities, such as having 3 types of anxiety (panic attacks, social phobia, and obsessive-compulsive disorder) or bulimia, seasonal depression, and anxiety.
Multiple pleasure-seeking or “outrageous” behaviors, such as compulsive gambling, sexual addiction, car racing, or skydiving. Another example is having a history of shoplifting, paraphilia, or arrest for participating in a riot, all of which are suggestive of antisocial traits in a patient seeking help for depression.
Sexual excesses, such as dating or having sex with ≥3 individuals concurrently, sometimes on the same day, or demanding sexual intercourse from a partner several times a day. Dr. Akiskal suggested that “sexual prowess” may represent an evolutionary advantage for the perpetuation of bipolar II disorder.
Marital history, such as a history of ≥3 marriages, or maintaining ≥2 families in different cities without being married.
Flamboyance and/or ornamentation. Examples might include wearing loud, colorful clothing (especially red), wearing ≥3 rings, or having piercings in ≥3 different body parts (tongue, nipples, navel, genitalia). Having elaborate tattoos across the body is no longer unique to “hyperthymic” persons with bipolar II disorder because tattoos have become far more common in the general population than they were in the 1970s. However, some take their tattoos to extremes.
Continue to: The above behaviors...
The above behaviors are condensed in a list that Dr. Akiskal called “the rule of 3” in patients with depression (Table1). Not all patients with bipolar II disorder will meet all the criteria of the rule of 3, but the first item in the mental status exam (appearance) alone may reflect the “soft bipolar spectrum,” such as garish clothing, red sneakers, multiple rings, bizarre hair coloring, and multiple piercings. This might prompt the clinician to ask further questions about hypomanic episodes as well as other personal behaviors related to the rule of 3.
Dr. Akiskal’s contributions to psychiatry are legendary in their originality, creativity, and clinical relevance. The rule of 3 is but one of his clinical concepts that may help identify many individuals with bipolar II disorder who are misdiagnosed as having MDD and prescribed a treatment that does not help or may exacerbate their illness course and worsen their outcome.
Based on the referrals of patients who are “treatment-resistant” to our Resident Mood Clinic, there are numerous persons in the country with bipolar II disorder (possibly millions) who are mislabeled with MDD and receiving the wrong treatments, to which they failed to respond. Their lifestyles and behaviors can provide valuable clinical insights into their true psychopathology, and that will lead to developing the right treatment plan.
1. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the “red sign,” the “rule of three” and other biographic signs of temperamental extravagance, activation and hypomania. J Affect Disord. 2005;84(2-3):279-290.
The diagnosis of bipolar II disorder is one of the most common challenges in psychiatric practice. Bipolar II disorder is frequently misdiagnosed as major depressive disorder (MDD) because symptoms of transient hypomanic episodes are either insufficiently probed or are rather vague. However, there are many valuable biographical clues that can expedite the diagnosis of bipolar II disorder.
The late Hagop S. Akiskal, MD, who passed away in January 2021, was an internationally recognized expert in mood disorders, and a dear friend for decades. He was a keen observer of human behavior who delved into the “life stories” of patients seeking help for depression. By thinking “outside the DSM box,” Dr. Akiskal was the first to recognize and codify a variety of behavioral and biographical clues for the bipolar spectrum (of which he was a pioneer) in patients presenting with a chief complaint of depression. He proposed a colorful set of behavioral stigmata in most patients with bipolar II disorder by carefully canvassing the life experiences of the patients he treated in the mood disorder clinic he established in the 1970s, which is believed to have been the first mood specialty clinic in the country.
Based on a review of >1,000 patients in his clinic who presented with depressive symptoms and were ultimately diagnosed as bipolar II disorder, Dr. Akiskal highlighted what he labeled as “behavioral activation, flamboyance and extravagance” among those patients. He referred to the cluster of those behaviors as “the soft spectrum” of bipolar disorder, which manifests in a set of distinctive behaviors in addition to depressive symptoms. He found that research tools such as the DSM-based Structured Clinical Interview often fail and frequently lead to a misdiagnosis of bipolar II disorder as MDD. This often condemns the patient to multiple failed trials of antidepressant monotherapy, and a delay in improvement, thus increasing the risk of job loss, disrupted relationships, and even suicide.
Over 3 decades, Dr. Akiskal developed the Mood Clinic Data Questionnaire (MCDQ) to systematize unstructured observations of patients presenting with a chief complaint of depression. His tool expedites the diagnosis of bipolar II disorder by understanding the patient as an individual, revealing personal and behavioral features consistent with what he labeled as episodic “hyperthymia” within the context of recurrent depression. This “social and behavioral phenotype,” as Dr. Akiskal called it, is rarely observed among patients with MDD.
By examining many patients with bipolar II disorder, Dr. Akiskal identified several “triads” of behavioral traits in the patients’ biographical history and in some of their close blood relatives as well. He also noticed that temperamentally, patients with bipolar II disorder thrive on “activity” and lovingly referred to themselves as “activity junkies.” Some of them may qualify as workaholics.
Biographical features that suggest bipolar II disorder
Here is a summary of the unique biographical features of patients with bipolar II disorder that Dr. Akiskal described:
Multilingual. Speaking ≥3 languages is unusual among individuals born in the United States, but often encountered among those with bipolar II disorder.
Continue to: Eminence
Eminence. Patients with bipolar II disorder, as well as their family members, tend to have leadership roles and prominence in journalism, media, and entertainment, fields that require interpersonal charm and eloquence. Those are common features of the “hyperthymic” temperament.
Creativity. Artists, poets, painters, and musicians who experience depression are more likely to have bipolar II disorder than MDD.
Biographical instability and/or excess. This is exemplified by going to 3 colleges and not necessarily obtaining a degree, or by frequently changing one’s line of work or city of residence. A classic example is a professor of medicine who also practices law and regularly sings in the opera, or a physician who is board-certified in 3 distinct specialties.
Activity junkies. Examples include a person with boundless energy, such as a novelist who writes 3 books a year or a professional who regularly works 12 hours a day without getting exhausted but seeks treatment for depressive episodes.
Multiple substances of abuse, such as nicotine, alcohol, stimulants, and opiates.
Continue to: Multiple psychiatric comorbidities
Multiple psychiatric comorbidities, such as having 3 types of anxiety (panic attacks, social phobia, and obsessive-compulsive disorder) or bulimia, seasonal depression, and anxiety.
Multiple pleasure-seeking or “outrageous” behaviors, such as compulsive gambling, sexual addiction, car racing, or skydiving. Another example is having a history of shoplifting, paraphilia, or arrest for participating in a riot, all of which are suggestive of antisocial traits in a patient seeking help for depression.
Sexual excesses, such as dating or having sex with ≥3 individuals concurrently, sometimes on the same day, or demanding sexual intercourse from a partner several times a day. Dr. Akiskal suggested that “sexual prowess” may represent an evolutionary advantage for the perpetuation of bipolar II disorder.
Marital history, such as a history of ≥3 marriages, or maintaining ≥2 families in different cities without being married.
Flamboyance and/or ornamentation. Examples might include wearing loud, colorful clothing (especially red), wearing ≥3 rings, or having piercings in ≥3 different body parts (tongue, nipples, navel, genitalia). Having elaborate tattoos across the body is no longer unique to “hyperthymic” persons with bipolar II disorder because tattoos have become far more common in the general population than they were in the 1970s. However, some take their tattoos to extremes.
Continue to: The above behaviors...
The above behaviors are condensed in a list that Dr. Akiskal called “the rule of 3” in patients with depression (Table1). Not all patients with bipolar II disorder will meet all the criteria of the rule of 3, but the first item in the mental status exam (appearance) alone may reflect the “soft bipolar spectrum,” such as garish clothing, red sneakers, multiple rings, bizarre hair coloring, and multiple piercings. This might prompt the clinician to ask further questions about hypomanic episodes as well as other personal behaviors related to the rule of 3.
Dr. Akiskal’s contributions to psychiatry are legendary in their originality, creativity, and clinical relevance. The rule of 3 is but one of his clinical concepts that may help identify many individuals with bipolar II disorder who are misdiagnosed as having MDD and prescribed a treatment that does not help or may exacerbate their illness course and worsen their outcome.
Based on the referrals of patients who are “treatment-resistant” to our Resident Mood Clinic, there are numerous persons in the country with bipolar II disorder (possibly millions) who are mislabeled with MDD and receiving the wrong treatments, to which they failed to respond. Their lifestyles and behaviors can provide valuable clinical insights into their true psychopathology, and that will lead to developing the right treatment plan.
The diagnosis of bipolar II disorder is one of the most common challenges in psychiatric practice. Bipolar II disorder is frequently misdiagnosed as major depressive disorder (MDD) because symptoms of transient hypomanic episodes are either insufficiently probed or are rather vague. However, there are many valuable biographical clues that can expedite the diagnosis of bipolar II disorder.
The late Hagop S. Akiskal, MD, who passed away in January 2021, was an internationally recognized expert in mood disorders, and a dear friend for decades. He was a keen observer of human behavior who delved into the “life stories” of patients seeking help for depression. By thinking “outside the DSM box,” Dr. Akiskal was the first to recognize and codify a variety of behavioral and biographical clues for the bipolar spectrum (of which he was a pioneer) in patients presenting with a chief complaint of depression. He proposed a colorful set of behavioral stigmata in most patients with bipolar II disorder by carefully canvassing the life experiences of the patients he treated in the mood disorder clinic he established in the 1970s, which is believed to have been the first mood specialty clinic in the country.
Based on a review of >1,000 patients in his clinic who presented with depressive symptoms and were ultimately diagnosed as bipolar II disorder, Dr. Akiskal highlighted what he labeled as “behavioral activation, flamboyance and extravagance” among those patients. He referred to the cluster of those behaviors as “the soft spectrum” of bipolar disorder, which manifests in a set of distinctive behaviors in addition to depressive symptoms. He found that research tools such as the DSM-based Structured Clinical Interview often fail and frequently lead to a misdiagnosis of bipolar II disorder as MDD. This often condemns the patient to multiple failed trials of antidepressant monotherapy, and a delay in improvement, thus increasing the risk of job loss, disrupted relationships, and even suicide.
Over 3 decades, Dr. Akiskal developed the Mood Clinic Data Questionnaire (MCDQ) to systematize unstructured observations of patients presenting with a chief complaint of depression. His tool expedites the diagnosis of bipolar II disorder by understanding the patient as an individual, revealing personal and behavioral features consistent with what he labeled as episodic “hyperthymia” within the context of recurrent depression. This “social and behavioral phenotype,” as Dr. Akiskal called it, is rarely observed among patients with MDD.
By examining many patients with bipolar II disorder, Dr. Akiskal identified several “triads” of behavioral traits in the patients’ biographical history and in some of their close blood relatives as well. He also noticed that temperamentally, patients with bipolar II disorder thrive on “activity” and lovingly referred to themselves as “activity junkies.” Some of them may qualify as workaholics.
Biographical features that suggest bipolar II disorder
Here is a summary of the unique biographical features of patients with bipolar II disorder that Dr. Akiskal described:
Multilingual. Speaking ≥3 languages is unusual among individuals born in the United States, but often encountered among those with bipolar II disorder.
Continue to: Eminence
Eminence. Patients with bipolar II disorder, as well as their family members, tend to have leadership roles and prominence in journalism, media, and entertainment, fields that require interpersonal charm and eloquence. Those are common features of the “hyperthymic” temperament.
Creativity. Artists, poets, painters, and musicians who experience depression are more likely to have bipolar II disorder than MDD.
Biographical instability and/or excess. This is exemplified by going to 3 colleges and not necessarily obtaining a degree, or by frequently changing one’s line of work or city of residence. A classic example is a professor of medicine who also practices law and regularly sings in the opera, or a physician who is board-certified in 3 distinct specialties.
Activity junkies. Examples include a person with boundless energy, such as a novelist who writes 3 books a year or a professional who regularly works 12 hours a day without getting exhausted but seeks treatment for depressive episodes.
Multiple substances of abuse, such as nicotine, alcohol, stimulants, and opiates.
Continue to: Multiple psychiatric comorbidities
Multiple psychiatric comorbidities, such as having 3 types of anxiety (panic attacks, social phobia, and obsessive-compulsive disorder) or bulimia, seasonal depression, and anxiety.
Multiple pleasure-seeking or “outrageous” behaviors, such as compulsive gambling, sexual addiction, car racing, or skydiving. Another example is having a history of shoplifting, paraphilia, or arrest for participating in a riot, all of which are suggestive of antisocial traits in a patient seeking help for depression.
Sexual excesses, such as dating or having sex with ≥3 individuals concurrently, sometimes on the same day, or demanding sexual intercourse from a partner several times a day. Dr. Akiskal suggested that “sexual prowess” may represent an evolutionary advantage for the perpetuation of bipolar II disorder.
Marital history, such as a history of ≥3 marriages, or maintaining ≥2 families in different cities without being married.
Flamboyance and/or ornamentation. Examples might include wearing loud, colorful clothing (especially red), wearing ≥3 rings, or having piercings in ≥3 different body parts (tongue, nipples, navel, genitalia). Having elaborate tattoos across the body is no longer unique to “hyperthymic” persons with bipolar II disorder because tattoos have become far more common in the general population than they were in the 1970s. However, some take their tattoos to extremes.
Continue to: The above behaviors...
The above behaviors are condensed in a list that Dr. Akiskal called “the rule of 3” in patients with depression (Table1). Not all patients with bipolar II disorder will meet all the criteria of the rule of 3, but the first item in the mental status exam (appearance) alone may reflect the “soft bipolar spectrum,” such as garish clothing, red sneakers, multiple rings, bizarre hair coloring, and multiple piercings. This might prompt the clinician to ask further questions about hypomanic episodes as well as other personal behaviors related to the rule of 3.
Dr. Akiskal’s contributions to psychiatry are legendary in their originality, creativity, and clinical relevance. The rule of 3 is but one of his clinical concepts that may help identify many individuals with bipolar II disorder who are misdiagnosed as having MDD and prescribed a treatment that does not help or may exacerbate their illness course and worsen their outcome.
Based on the referrals of patients who are “treatment-resistant” to our Resident Mood Clinic, there are numerous persons in the country with bipolar II disorder (possibly millions) who are mislabeled with MDD and receiving the wrong treatments, to which they failed to respond. Their lifestyles and behaviors can provide valuable clinical insights into their true psychopathology, and that will lead to developing the right treatment plan.
1. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the “red sign,” the “rule of three” and other biographic signs of temperamental extravagance, activation and hypomania. J Affect Disord. 2005;84(2-3):279-290.
1. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the “red sign,” the “rule of three” and other biographic signs of temperamental extravagance, activation and hypomania. J Affect Disord. 2005;84(2-3):279-290.
Needed: More studies of CSF molecular biomarkers in psychiatric disorders
Psychiatry and neurology are the brain’s twin medical disciplines. Unlike neurologic brain disorders, where localizing the “lesion” is a primary objective, psychiatric brain disorders are much more subtle, with no “gross” lesions but numerous cellular and molecular pathologies within neural circuits.
Measuring the molecular components of the cerebrospinal fluid (CSF), the glorious “sewage system” of the brain, may help reveal granular clues to the neurobiology of psychiatric disorders.
Mental illnesses involve the disruption of brain structures and functions in a diffuse manner across the cortex. Abnormal neuroplasticity has been implicated in several major psychiatric disorders. Examples include hypoplasia of the hippocampus in major depressive disorder and cortical thinning/dysplasia in schizophrenia. Reductions of neurotropic factors such as nerve growth factor or brain-derived neurotropic factor have been reported in mood and psychotic disorders, and appear to correlate with neuroplasticity changes.
Recent advances in psychiatric neuroscience have provided many clues to the pathophysiology of psychopathological conditions, including neuroinflammation, oxidative stress, apoptosis, impaired energy metabolism, abnormal metabolomics and lipidomics, and hypo- and hyperfunction of various neurotransmitters systems (especially glutamate N-methyl-
Thus, psychiatric research should focus on exploring and detecting molecular signatures (ie, biomarkers) of psychiatric disorders, including biomarkers of axonal and synaptic damage, glial activation, and oxidative stress. This is especially critical given the extensive heterogeneity of schizophrenia and mood and anxiety disorders. The CSF is a vastly unexploited substrate for discovering molecular biomarkers that will pave the way to precision psychiatry, and possibly open the door for completely new therapeutic strategies to tackle the most challenging neuropsychiatric disorders.
A role for CSF analysis
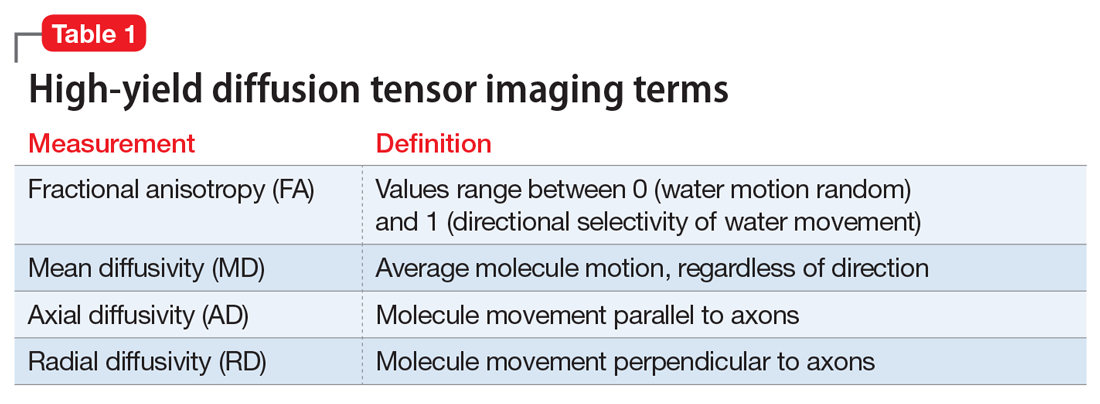
It’s quite puzzling why acute psychiatric episodes of schizophrenia, bipolar disorder, major depressive disorder, or panic attacks are not routinely assessed with a spinal tap, in conjunction with other brain measures such as neuroimaging (morphology, spectroscopy, cerebral blood flow, and diffusion tensor imaging) as well as a comprehensive neurocognitive examination and neurophysiological tests such as pre-pulse inhibition, mismatch negativity, and P-50, N-10, and P-300 evoked potentials. Combining CSF analysis with all those measures may help us stratify the spectra of psychosis, depression, and anxiety, as well as posttraumatic stress disorder and obsessive-compulsive disorder, into unique biotypes with overlapping clinical phenotypes and specific treatment approaches.
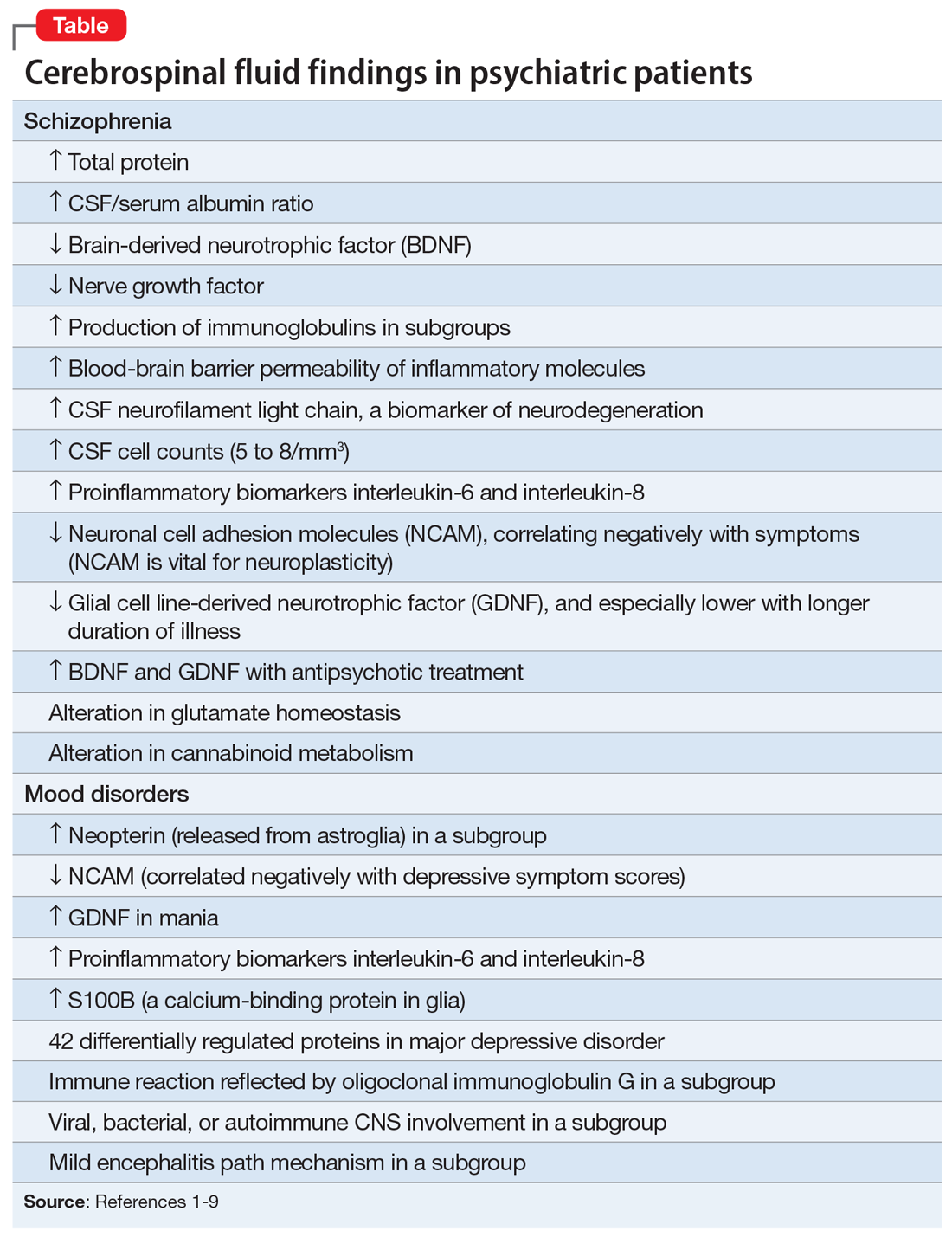
There are relatively few published CSF studies in psychiatric patients (mostly schizophrenia and bipolar and depressive disorders). The Table1-9 shows some of those findings. More than 365 biomarkers have been reported in schizophrenia, most of them in serum and tissue.10 However, none of them can be used for diagnostic purposes because schizophrenia is a syndrome comprised of several hundred different diseases (biotypes) that have similar clinical symptoms. Many of the serum and tissue biomarkers have not been studied in CSF, and they must if advances in the neurobiology and treatment of the psychotic and mood spectra are to be achieved. And adapting the CSF biomarkers described in neurologic disorders such as multiple sclerosis11 to schizophrenia and bipolar disorder (which also have well-established myelin pathologies) may yield a trove of neurobiologic findings.
If CSF studies eventually prove to be very useful for identifying subtypes for diagnosis and treatment, psychiatrists do not have to do the lumbar puncture themselves, but may refer patients to a “spinal tap” laboratory, just as they refer patients to a phlebotomy laboratory for routine blood tests. The adoption of CSF assessment in psychiatry will solidify its status as a clinical neuroscience, like its sister, neurology.
1. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262(5):375-391.
2. Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79-92.
3. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol. 2020;267(1):162-167.
4. Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321-330.
5. Hidese S, Hattori K, Sasayama D, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:12-18.
6. Tunca Z, Kıvırcık Akdede B, Özerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30(2):198-204.
7. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics. 2017;14(6):499-514.
8. Kroksmark H, Vinberg M. Does S100B have a potential role in affective disorders? A literature review. Nord J Psychiatry. 2018;72(7):462-470.
9. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869-887.
10. Nasrallah HA. Lab tests for psychiatric disorders: few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
11. Porter L, Shoushtarizadeh A, Jelinek GA, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133
Psychiatry and neurology are the brain’s twin medical disciplines. Unlike neurologic brain disorders, where localizing the “lesion” is a primary objective, psychiatric brain disorders are much more subtle, with no “gross” lesions but numerous cellular and molecular pathologies within neural circuits.
Measuring the molecular components of the cerebrospinal fluid (CSF), the glorious “sewage system” of the brain, may help reveal granular clues to the neurobiology of psychiatric disorders.
Mental illnesses involve the disruption of brain structures and functions in a diffuse manner across the cortex. Abnormal neuroplasticity has been implicated in several major psychiatric disorders. Examples include hypoplasia of the hippocampus in major depressive disorder and cortical thinning/dysplasia in schizophrenia. Reductions of neurotropic factors such as nerve growth factor or brain-derived neurotropic factor have been reported in mood and psychotic disorders, and appear to correlate with neuroplasticity changes.
Recent advances in psychiatric neuroscience have provided many clues to the pathophysiology of psychopathological conditions, including neuroinflammation, oxidative stress, apoptosis, impaired energy metabolism, abnormal metabolomics and lipidomics, and hypo- and hyperfunction of various neurotransmitters systems (especially glutamate N-methyl-
Thus, psychiatric research should focus on exploring and detecting molecular signatures (ie, biomarkers) of psychiatric disorders, including biomarkers of axonal and synaptic damage, glial activation, and oxidative stress. This is especially critical given the extensive heterogeneity of schizophrenia and mood and anxiety disorders. The CSF is a vastly unexploited substrate for discovering molecular biomarkers that will pave the way to precision psychiatry, and possibly open the door for completely new therapeutic strategies to tackle the most challenging neuropsychiatric disorders.
A role for CSF analysis
It’s quite puzzling why acute psychiatric episodes of schizophrenia, bipolar disorder, major depressive disorder, or panic attacks are not routinely assessed with a spinal tap, in conjunction with other brain measures such as neuroimaging (morphology, spectroscopy, cerebral blood flow, and diffusion tensor imaging) as well as a comprehensive neurocognitive examination and neurophysiological tests such as pre-pulse inhibition, mismatch negativity, and P-50, N-10, and P-300 evoked potentials. Combining CSF analysis with all those measures may help us stratify the spectra of psychosis, depression, and anxiety, as well as posttraumatic stress disorder and obsessive-compulsive disorder, into unique biotypes with overlapping clinical phenotypes and specific treatment approaches.
There are relatively few published CSF studies in psychiatric patients (mostly schizophrenia and bipolar and depressive disorders). The Table1-9 shows some of those findings. More than 365 biomarkers have been reported in schizophrenia, most of them in serum and tissue.10 However, none of them can be used for diagnostic purposes because schizophrenia is a syndrome comprised of several hundred different diseases (biotypes) that have similar clinical symptoms. Many of the serum and tissue biomarkers have not been studied in CSF, and they must if advances in the neurobiology and treatment of the psychotic and mood spectra are to be achieved. And adapting the CSF biomarkers described in neurologic disorders such as multiple sclerosis11 to schizophrenia and bipolar disorder (which also have well-established myelin pathologies) may yield a trove of neurobiologic findings.
If CSF studies eventually prove to be very useful for identifying subtypes for diagnosis and treatment, psychiatrists do not have to do the lumbar puncture themselves, but may refer patients to a “spinal tap” laboratory, just as they refer patients to a phlebotomy laboratory for routine blood tests. The adoption of CSF assessment in psychiatry will solidify its status as a clinical neuroscience, like its sister, neurology.
Psychiatry and neurology are the brain’s twin medical disciplines. Unlike neurologic brain disorders, where localizing the “lesion” is a primary objective, psychiatric brain disorders are much more subtle, with no “gross” lesions but numerous cellular and molecular pathologies within neural circuits.
Measuring the molecular components of the cerebrospinal fluid (CSF), the glorious “sewage system” of the brain, may help reveal granular clues to the neurobiology of psychiatric disorders.
Mental illnesses involve the disruption of brain structures and functions in a diffuse manner across the cortex. Abnormal neuroplasticity has been implicated in several major psychiatric disorders. Examples include hypoplasia of the hippocampus in major depressive disorder and cortical thinning/dysplasia in schizophrenia. Reductions of neurotropic factors such as nerve growth factor or brain-derived neurotropic factor have been reported in mood and psychotic disorders, and appear to correlate with neuroplasticity changes.
Recent advances in psychiatric neuroscience have provided many clues to the pathophysiology of psychopathological conditions, including neuroinflammation, oxidative stress, apoptosis, impaired energy metabolism, abnormal metabolomics and lipidomics, and hypo- and hyperfunction of various neurotransmitters systems (especially glutamate N-methyl-
Thus, psychiatric research should focus on exploring and detecting molecular signatures (ie, biomarkers) of psychiatric disorders, including biomarkers of axonal and synaptic damage, glial activation, and oxidative stress. This is especially critical given the extensive heterogeneity of schizophrenia and mood and anxiety disorders. The CSF is a vastly unexploited substrate for discovering molecular biomarkers that will pave the way to precision psychiatry, and possibly open the door for completely new therapeutic strategies to tackle the most challenging neuropsychiatric disorders.
A role for CSF analysis
It’s quite puzzling why acute psychiatric episodes of schizophrenia, bipolar disorder, major depressive disorder, or panic attacks are not routinely assessed with a spinal tap, in conjunction with other brain measures such as neuroimaging (morphology, spectroscopy, cerebral blood flow, and diffusion tensor imaging) as well as a comprehensive neurocognitive examination and neurophysiological tests such as pre-pulse inhibition, mismatch negativity, and P-50, N-10, and P-300 evoked potentials. Combining CSF analysis with all those measures may help us stratify the spectra of psychosis, depression, and anxiety, as well as posttraumatic stress disorder and obsessive-compulsive disorder, into unique biotypes with overlapping clinical phenotypes and specific treatment approaches.
There are relatively few published CSF studies in psychiatric patients (mostly schizophrenia and bipolar and depressive disorders). The Table1-9 shows some of those findings. More than 365 biomarkers have been reported in schizophrenia, most of them in serum and tissue.10 However, none of them can be used for diagnostic purposes because schizophrenia is a syndrome comprised of several hundred different diseases (biotypes) that have similar clinical symptoms. Many of the serum and tissue biomarkers have not been studied in CSF, and they must if advances in the neurobiology and treatment of the psychotic and mood spectra are to be achieved. And adapting the CSF biomarkers described in neurologic disorders such as multiple sclerosis11 to schizophrenia and bipolar disorder (which also have well-established myelin pathologies) may yield a trove of neurobiologic findings.
If CSF studies eventually prove to be very useful for identifying subtypes for diagnosis and treatment, psychiatrists do not have to do the lumbar puncture themselves, but may refer patients to a “spinal tap” laboratory, just as they refer patients to a phlebotomy laboratory for routine blood tests. The adoption of CSF assessment in psychiatry will solidify its status as a clinical neuroscience, like its sister, neurology.
1. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262(5):375-391.
2. Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79-92.
3. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol. 2020;267(1):162-167.
4. Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321-330.
5. Hidese S, Hattori K, Sasayama D, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:12-18.
6. Tunca Z, Kıvırcık Akdede B, Özerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30(2):198-204.
7. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics. 2017;14(6):499-514.
8. Kroksmark H, Vinberg M. Does S100B have a potential role in affective disorders? A literature review. Nord J Psychiatry. 2018;72(7):462-470.
9. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869-887.
10. Nasrallah HA. Lab tests for psychiatric disorders: few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
11. Porter L, Shoushtarizadeh A, Jelinek GA, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133
1. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262(5):375-391.
2. Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79-92.
3. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol. 2020;267(1):162-167.
4. Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321-330.
5. Hidese S, Hattori K, Sasayama D, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:12-18.
6. Tunca Z, Kıvırcık Akdede B, Özerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30(2):198-204.
7. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics. 2017;14(6):499-514.
8. Kroksmark H, Vinberg M. Does S100B have a potential role in affective disorders? A literature review. Nord J Psychiatry. 2018;72(7):462-470.
9. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869-887.
10. Nasrallah HA. Lab tests for psychiatric disorders: few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
11. Porter L, Shoushtarizadeh A, Jelinek GA, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133
From ideology to articles of faith: The ‘religification’ of political beliefs
Man is a political animal.
— Aristotle, Politics , Book 1, Section 1253a
Religion is the opium of the people.
— Karl Marx, A contribution to the critique of Hegel’s philosophy of right , introduction
Beliefs are at the core of psychiatric practice. Our patients are often shackled by their anomalous beliefs, which are not reality-based. These beliefs are often the primary targets of psychiatric treatment. Consider a day at the office of a psychiatrist who may see several patients impaired by false beliefs, such as:
- My neighbor is reading my mind remotely and is plotting to kill me
- If I ride on a plane, it will crash and I will die
- I am a failure, a worthless person, and a burden on my family
- I am hopeless and helpless; life is too painful and not worth living anymore
- I am a prophet with supernatural gifts, and I can predict the future
- Whenever I take this substance, I feel I can jump out of a window and fly
- If I do not shower 5 times in a row every night before going to bed, something terrible will happen to my family.
Patients with false beliefs obviously need psychiatric care. However, a large number of religious individuals harbor “unusual” beliefs involving angels and devils and hell and paradise after death. Those people of faith are not considered to have a DSM-5 psychiatric disorder. Billions of people around the world belong to one of the approximately 4,300 religions, which they celebrate using one of the more than 6,800 living languages. Psychiatrists encourage patients to have a faith because it can be quite comforting to its adherents, enhancing their social relations and providing them with hope and resilience during the darkest days of life. Regular attendance at a house of worship is a measure of the strong roots of one’s faith.
So why have there been so many religious wars over centuries of recorded history? Why have millions of people died during conflicts among religions? Why does one religious group adamantly believe that theirs is the real God, while the god of other religions is fake? And why have people who withdrew from or refused to adopt a certain religious belief been persecuted; labeled as “heretic,” “infidel,” “heathen,” or “apostate”; and burned at the stake or beheaded? Perhaps religion is not always a kinder, gentler belief system.
Continue to: Recent statistics...
Recent statistics show a precipitous decline in religious observance in the United States.1 So what happens to a society that gradually abandons its previously entrenched religious beliefs and becomes secular? This trend is spreading widely in Europe and North America. But widely held beliefs with powerful personal meaning don’t just fizzle away: they re-emerge in another form. The substantial energy of religious faith must be invested elsewhere and manifested in an alternative form with similar dynamics.
Enter politics!
It seems that humans’ need to uphold a strong belief is so powerful that they either incorporate political doctrines side-by-side with their religious beliefs (if the 2 are compatible) or adopt a strong political belief if they abandon their religion and become secular. This does not have to be an intellectually wrenching change because there are many similarities between hyper-religiosity and fanatic political beliefs (Table).
The toxic hyperpartisanship that has dominated the United States over the past several years may be the culmination of an intensified “religification” of politics. The incendiary mix of religious zealotry and political fanaticism is conducive to intensified loathing, hostility, and animus to those with an opposing political ideology.
So it all boils down to the human imperative of harboring a strong personal belief. What is the origin of beliefs, religious, political, or otherwise? Why does the human species have the overwhelming need to uphold a belief? Research suggests that it is the result of evolution and the phylogenetic enlargement of the brain, including the parietal and medial frontal cortex in humans.2 And according to many studies, abnormal and delusional beliefs encountered in psychiatric practice appear to be caused by altered perception and/or misattribution of aversive meaning.3 Lesions in the right hemisphere have been reported to play an important role in generating delusional beliefs.4 A healthy right hemisphere plays an important role in:
- pragmatic communications
- perceptual integration
- attentional surveillance and anomaly novelty detection
- belief updating.4
Right hemispheric pathology disrupts those functions and can lead to false beliefs such as delusions, or, on a milder scale, strongly held superstitions.
One wonders how the structure and function of the right hemisphere generates and perpetuates a belief in a religion or political ideology that ultimately shapes one’s life. Religiosity and politics are an inherent part of human nature, and they can replace each other or merge together. If one is to believe what Durkheim5 proposed more than a century ago, the existence of belief systems is essential for societal stability. He posited that the absence of stable belief systems can lead to what he labeled “anomie,” leading to a surge of suicide and crime. If that is true, then the coexistence of religious and political beliefs may have a significant upside, but also with a palpable downside when either or both of those belief systems become excessively antagonistic or extreme. Three cheers for religious and political moderation that allows them to peacefully coexist.
1. Jones JM. U.S. church membership falls below majority for first time. Gallup. March 29, 2021. Accessed June 7, 2021. https://news.gallup.com/poll/341963/church-membership-falls-below-majority-first-time.aspx
2. Seitz RJ, Angel HF. Belief formation—a driving force for brain evolution. Brain Cogn. 2020;140:105548. doi: 10.1016/j.bandc.2020.105548
3. Seitz RJ. Beliefs: a challenge in neuropsychological disorders. J Neuropsychol. 2021. doi: 10.1111/jnp.12249
4. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235. doi: 10.1176/appi.neuropsych.16060118
5. Durkheim E. Suicide: a study in sociology. The Free Press; 1951.
Man is a political animal.
— Aristotle, Politics , Book 1, Section 1253a
Religion is the opium of the people.
— Karl Marx, A contribution to the critique of Hegel’s philosophy of right , introduction
Beliefs are at the core of psychiatric practice. Our patients are often shackled by their anomalous beliefs, which are not reality-based. These beliefs are often the primary targets of psychiatric treatment. Consider a day at the office of a psychiatrist who may see several patients impaired by false beliefs, such as:
- My neighbor is reading my mind remotely and is plotting to kill me
- If I ride on a plane, it will crash and I will die
- I am a failure, a worthless person, and a burden on my family
- I am hopeless and helpless; life is too painful and not worth living anymore
- I am a prophet with supernatural gifts, and I can predict the future
- Whenever I take this substance, I feel I can jump out of a window and fly
- If I do not shower 5 times in a row every night before going to bed, something terrible will happen to my family.
Patients with false beliefs obviously need psychiatric care. However, a large number of religious individuals harbor “unusual” beliefs involving angels and devils and hell and paradise after death. Those people of faith are not considered to have a DSM-5 psychiatric disorder. Billions of people around the world belong to one of the approximately 4,300 religions, which they celebrate using one of the more than 6,800 living languages. Psychiatrists encourage patients to have a faith because it can be quite comforting to its adherents, enhancing their social relations and providing them with hope and resilience during the darkest days of life. Regular attendance at a house of worship is a measure of the strong roots of one’s faith.
So why have there been so many religious wars over centuries of recorded history? Why have millions of people died during conflicts among religions? Why does one religious group adamantly believe that theirs is the real God, while the god of other religions is fake? And why have people who withdrew from or refused to adopt a certain religious belief been persecuted; labeled as “heretic,” “infidel,” “heathen,” or “apostate”; and burned at the stake or beheaded? Perhaps religion is not always a kinder, gentler belief system.
Continue to: Recent statistics...
Recent statistics show a precipitous decline in religious observance in the United States.1 So what happens to a society that gradually abandons its previously entrenched religious beliefs and becomes secular? This trend is spreading widely in Europe and North America. But widely held beliefs with powerful personal meaning don’t just fizzle away: they re-emerge in another form. The substantial energy of religious faith must be invested elsewhere and manifested in an alternative form with similar dynamics.
Enter politics!
It seems that humans’ need to uphold a strong belief is so powerful that they either incorporate political doctrines side-by-side with their religious beliefs (if the 2 are compatible) or adopt a strong political belief if they abandon their religion and become secular. This does not have to be an intellectually wrenching change because there are many similarities between hyper-religiosity and fanatic political beliefs (Table).
The toxic hyperpartisanship that has dominated the United States over the past several years may be the culmination of an intensified “religification” of politics. The incendiary mix of religious zealotry and political fanaticism is conducive to intensified loathing, hostility, and animus to those with an opposing political ideology.
So it all boils down to the human imperative of harboring a strong personal belief. What is the origin of beliefs, religious, political, or otherwise? Why does the human species have the overwhelming need to uphold a belief? Research suggests that it is the result of evolution and the phylogenetic enlargement of the brain, including the parietal and medial frontal cortex in humans.2 And according to many studies, abnormal and delusional beliefs encountered in psychiatric practice appear to be caused by altered perception and/or misattribution of aversive meaning.3 Lesions in the right hemisphere have been reported to play an important role in generating delusional beliefs.4 A healthy right hemisphere plays an important role in:
- pragmatic communications
- perceptual integration
- attentional surveillance and anomaly novelty detection
- belief updating.4
Right hemispheric pathology disrupts those functions and can lead to false beliefs such as delusions, or, on a milder scale, strongly held superstitions.
One wonders how the structure and function of the right hemisphere generates and perpetuates a belief in a religion or political ideology that ultimately shapes one’s life. Religiosity and politics are an inherent part of human nature, and they can replace each other or merge together. If one is to believe what Durkheim5 proposed more than a century ago, the existence of belief systems is essential for societal stability. He posited that the absence of stable belief systems can lead to what he labeled “anomie,” leading to a surge of suicide and crime. If that is true, then the coexistence of religious and political beliefs may have a significant upside, but also with a palpable downside when either or both of those belief systems become excessively antagonistic or extreme. Three cheers for religious and political moderation that allows them to peacefully coexist.
Man is a political animal.
— Aristotle, Politics , Book 1, Section 1253a
Religion is the opium of the people.
— Karl Marx, A contribution to the critique of Hegel’s philosophy of right , introduction
Beliefs are at the core of psychiatric practice. Our patients are often shackled by their anomalous beliefs, which are not reality-based. These beliefs are often the primary targets of psychiatric treatment. Consider a day at the office of a psychiatrist who may see several patients impaired by false beliefs, such as:
- My neighbor is reading my mind remotely and is plotting to kill me
- If I ride on a plane, it will crash and I will die
- I am a failure, a worthless person, and a burden on my family
- I am hopeless and helpless; life is too painful and not worth living anymore
- I am a prophet with supernatural gifts, and I can predict the future
- Whenever I take this substance, I feel I can jump out of a window and fly
- If I do not shower 5 times in a row every night before going to bed, something terrible will happen to my family.
Patients with false beliefs obviously need psychiatric care. However, a large number of religious individuals harbor “unusual” beliefs involving angels and devils and hell and paradise after death. Those people of faith are not considered to have a DSM-5 psychiatric disorder. Billions of people around the world belong to one of the approximately 4,300 religions, which they celebrate using one of the more than 6,800 living languages. Psychiatrists encourage patients to have a faith because it can be quite comforting to its adherents, enhancing their social relations and providing them with hope and resilience during the darkest days of life. Regular attendance at a house of worship is a measure of the strong roots of one’s faith.
So why have there been so many religious wars over centuries of recorded history? Why have millions of people died during conflicts among religions? Why does one religious group adamantly believe that theirs is the real God, while the god of other religions is fake? And why have people who withdrew from or refused to adopt a certain religious belief been persecuted; labeled as “heretic,” “infidel,” “heathen,” or “apostate”; and burned at the stake or beheaded? Perhaps religion is not always a kinder, gentler belief system.
Continue to: Recent statistics...
Recent statistics show a precipitous decline in religious observance in the United States.1 So what happens to a society that gradually abandons its previously entrenched religious beliefs and becomes secular? This trend is spreading widely in Europe and North America. But widely held beliefs with powerful personal meaning don’t just fizzle away: they re-emerge in another form. The substantial energy of religious faith must be invested elsewhere and manifested in an alternative form with similar dynamics.
Enter politics!
It seems that humans’ need to uphold a strong belief is so powerful that they either incorporate political doctrines side-by-side with their religious beliefs (if the 2 are compatible) or adopt a strong political belief if they abandon their religion and become secular. This does not have to be an intellectually wrenching change because there are many similarities between hyper-religiosity and fanatic political beliefs (Table).
The toxic hyperpartisanship that has dominated the United States over the past several years may be the culmination of an intensified “religification” of politics. The incendiary mix of religious zealotry and political fanaticism is conducive to intensified loathing, hostility, and animus to those with an opposing political ideology.
So it all boils down to the human imperative of harboring a strong personal belief. What is the origin of beliefs, religious, political, or otherwise? Why does the human species have the overwhelming need to uphold a belief? Research suggests that it is the result of evolution and the phylogenetic enlargement of the brain, including the parietal and medial frontal cortex in humans.2 And according to many studies, abnormal and delusional beliefs encountered in psychiatric practice appear to be caused by altered perception and/or misattribution of aversive meaning.3 Lesions in the right hemisphere have been reported to play an important role in generating delusional beliefs.4 A healthy right hemisphere plays an important role in:
- pragmatic communications
- perceptual integration
- attentional surveillance and anomaly novelty detection
- belief updating.4
Right hemispheric pathology disrupts those functions and can lead to false beliefs such as delusions, or, on a milder scale, strongly held superstitions.
One wonders how the structure and function of the right hemisphere generates and perpetuates a belief in a religion or political ideology that ultimately shapes one’s life. Religiosity and politics are an inherent part of human nature, and they can replace each other or merge together. If one is to believe what Durkheim5 proposed more than a century ago, the existence of belief systems is essential for societal stability. He posited that the absence of stable belief systems can lead to what he labeled “anomie,” leading to a surge of suicide and crime. If that is true, then the coexistence of religious and political beliefs may have a significant upside, but also with a palpable downside when either or both of those belief systems become excessively antagonistic or extreme. Three cheers for religious and political moderation that allows them to peacefully coexist.
1. Jones JM. U.S. church membership falls below majority for first time. Gallup. March 29, 2021. Accessed June 7, 2021. https://news.gallup.com/poll/341963/church-membership-falls-below-majority-first-time.aspx
2. Seitz RJ, Angel HF. Belief formation—a driving force for brain evolution. Brain Cogn. 2020;140:105548. doi: 10.1016/j.bandc.2020.105548
3. Seitz RJ. Beliefs: a challenge in neuropsychological disorders. J Neuropsychol. 2021. doi: 10.1111/jnp.12249
4. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235. doi: 10.1176/appi.neuropsych.16060118
5. Durkheim E. Suicide: a study in sociology. The Free Press; 1951.
1. Jones JM. U.S. church membership falls below majority for first time. Gallup. March 29, 2021. Accessed June 7, 2021. https://news.gallup.com/poll/341963/church-membership-falls-below-majority-first-time.aspx
2. Seitz RJ, Angel HF. Belief formation—a driving force for brain evolution. Brain Cogn. 2020;140:105548. doi: 10.1016/j.bandc.2020.105548
3. Seitz RJ. Beliefs: a challenge in neuropsychological disorders. J Neuropsychol. 2021. doi: 10.1111/jnp.12249
4. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235. doi: 10.1176/appi.neuropsych.16060118
5. Durkheim E. Suicide: a study in sociology. The Free Press; 1951.
More on long-acting injectable antipsychotics
Benefits of early LAI use
I want to thank Dr. Nasrallah for his editorial calling for more frequent and earlier use of long-acting injectable antipsychotics (LAIs) in schizophrenia (From the Editor,
In addition to the neuroprotective biologic effects of early LAI usage, I’ve found that many of my FEP patients find great psychological comfort from incorporating LAIs into their treatment plan. The first psychotic break is generally when a person (and their family) feels the most afraid about the future and is in desperate need of hope that they can have a full life—with educational opportunities, sustained employment, meaningful relationships, and more. Just as society has seen the COVID-19 vaccines as a symbol of hope and the first step in overcoming the oppression of living in fear of an uncertain future, we need to help people experiencing FEP find hope in a needle.
Craig Chepke, MD, FAPA
Excel Psychiatric Associates
Huntersville, North Carolina
Reference
1. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
Dr. Nasrallah responds
Thank you, Dr. Chepke, for your letter confirming full support for using LAIs in schizophrenia. I like the phrase you coined: “hope in a needle.” The early use of LAIs in schizophrenia can provide the same type of hope that the vaccines against the life-threatening COVID-19 virus have generated in our society. Based on my direct observations, I also agree with you that the longer patients with schizophrenia remain on LAIs, the more engaged and happy they are with their progress and the quality of their lives. It is tragic that many patients never had the opportunity to return to their baseline with the early use of LAIs immediately following their first psychotic episode, instead of relapsing again and again due to their inability to adhere completely to their oral medications.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: LAIs as the standard of care
LAIs as the standard of care
Thank you, Dr. Nasrallah, for reiterating the importance of compliance with pharmacologic management of schizophrenia after FEP (From the Editor,
As you point out in your editorial, the facts are powerful, well-known, undisputed, and yet not adopted in the United States, when in other countries LAIs are first-line care. Yes, LAIs are expensive, but not nearly as expensive as the disabilities caused by noncompliance are to society.
Why isn’t LAI use the standard of care here in the United States? In the United States, there is advocacy for treatment because there’s money in it. There is no good advocacy for preventive care because there’s no immediate money in it. We have another good example of this in the United States: private, for-profit prisons. They have a vested interest in keeping prisons full and building new ones. Patients with FEP are most often treated in the hospital, where a standard of care could easily be established that mandates LAIs as first-tier care. Why is that not so? Who is pushing for it? Who is resisting?
Your editorial inspired me to advocate more strongly. Do you have advice about how to effect policy change? I know administrators respond when we talk dollars and cents, not quality of care. What is the dollar cost of not using LAIs as the standard of care after FEP? Who cares? Who would listen to the numbers?
Edward A. Major, MD, LFAPA
Clinical Professor of Psychiatry
Upstate Medical Center
Syracuse, New York
Dr. Nasrallah responds
Dr. Major, thanks for your message. Establishing a standard of care for the use of LAIs (or any other therapy) is not that simple. It requires well-coordinated collaboration among several stakeholders (clinicians, researchers, payors, advocacy groups, and a national organization such as the American Psychiatric Association). The cost issue is certainly powerful, but the equation works in favor of LAIs because 1 psychiatric hospitalization due to a psychotic relapse costs up to 3 times the annual cost of an LAI medication that can prevent that rehospitalization. In addition, disability comprises the lion’s share of the large indirect costs of schizophrenia (disability payments, lifetime room and board, incarceration and legal costs, and loss of work and generation of taxes). LAIs can save both lives and expenditures, and a lot of suffering by patients and their families. I, too, long to see the emergence of a rational standard of care for schizophrenia using LAIs right after the initial psychotic episode. Oncology and cardiology have standards of care, so why not psychiatry?
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: Psychosis and epilepsy
Psychosis and epilepsy
I just read your editorial regarding the devastating consequences of psychotic relapses (From the Editor,
I work in the spheres of psychiatry, epileptology, and whole genome sequencing, and have experienced a psychotic episode myself (in 2013, after temporal lobe resection and overdose). I now consider myself even more lucky to be out the other side! As Governor for South London and Maudsley NHS Foundation Trust (SLaM) and Trustee for Epilepsy Action, many of our patients have psychosis. Some patients with epilepsy even experience postictal psychosis. Just yesterday, we had a call at SLaM regarding patients from a secure unit, and a psychiatric nurse spoke about patients at risk to themselves and others because of their psychotic illness, and how crucial effective long-term care was.
Torie Robinson
CEO, Epilepsy Sparks
Dr. Nasrallah responds
Ms. Robinson, thank you for sharing your story. It is important to note that the neurobiology of the psychosis that may occur with epilepsy may not be as neurodegenerative as the psychosis of schizophrenia. Many neurologic conditions can be associated with psychotic episodes, not only epilepsy. I am glad you overcame your post-temporal lobectomy psychotic episode and have had a very good outcome with high functioning.
Henry A. Nasrallah, MD
Editor-In-Chief
Disclosures
Dr. Chepke is a consultant to and speaker for Janssen Pharmaceuticals, Otsuka Pharmaceuticals, and Alkermes. The other authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
Benefits of early LAI use
I want to thank Dr. Nasrallah for his editorial calling for more frequent and earlier use of long-acting injectable antipsychotics (LAIs) in schizophrenia (From the Editor,
In addition to the neuroprotective biologic effects of early LAI usage, I’ve found that many of my FEP patients find great psychological comfort from incorporating LAIs into their treatment plan. The first psychotic break is generally when a person (and their family) feels the most afraid about the future and is in desperate need of hope that they can have a full life—with educational opportunities, sustained employment, meaningful relationships, and more. Just as society has seen the COVID-19 vaccines as a symbol of hope and the first step in overcoming the oppression of living in fear of an uncertain future, we need to help people experiencing FEP find hope in a needle.
Craig Chepke, MD, FAPA
Excel Psychiatric Associates
Huntersville, North Carolina
Reference
1. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
Dr. Nasrallah responds
Thank you, Dr. Chepke, for your letter confirming full support for using LAIs in schizophrenia. I like the phrase you coined: “hope in a needle.” The early use of LAIs in schizophrenia can provide the same type of hope that the vaccines against the life-threatening COVID-19 virus have generated in our society. Based on my direct observations, I also agree with you that the longer patients with schizophrenia remain on LAIs, the more engaged and happy they are with their progress and the quality of their lives. It is tragic that many patients never had the opportunity to return to their baseline with the early use of LAIs immediately following their first psychotic episode, instead of relapsing again and again due to their inability to adhere completely to their oral medications.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: LAIs as the standard of care
LAIs as the standard of care
Thank you, Dr. Nasrallah, for reiterating the importance of compliance with pharmacologic management of schizophrenia after FEP (From the Editor,
As you point out in your editorial, the facts are powerful, well-known, undisputed, and yet not adopted in the United States, when in other countries LAIs are first-line care. Yes, LAIs are expensive, but not nearly as expensive as the disabilities caused by noncompliance are to society.
Why isn’t LAI use the standard of care here in the United States? In the United States, there is advocacy for treatment because there’s money in it. There is no good advocacy for preventive care because there’s no immediate money in it. We have another good example of this in the United States: private, for-profit prisons. They have a vested interest in keeping prisons full and building new ones. Patients with FEP are most often treated in the hospital, where a standard of care could easily be established that mandates LAIs as first-tier care. Why is that not so? Who is pushing for it? Who is resisting?
Your editorial inspired me to advocate more strongly. Do you have advice about how to effect policy change? I know administrators respond when we talk dollars and cents, not quality of care. What is the dollar cost of not using LAIs as the standard of care after FEP? Who cares? Who would listen to the numbers?
Edward A. Major, MD, LFAPA
Clinical Professor of Psychiatry
Upstate Medical Center
Syracuse, New York
Dr. Nasrallah responds
Dr. Major, thanks for your message. Establishing a standard of care for the use of LAIs (or any other therapy) is not that simple. It requires well-coordinated collaboration among several stakeholders (clinicians, researchers, payors, advocacy groups, and a national organization such as the American Psychiatric Association). The cost issue is certainly powerful, but the equation works in favor of LAIs because 1 psychiatric hospitalization due to a psychotic relapse costs up to 3 times the annual cost of an LAI medication that can prevent that rehospitalization. In addition, disability comprises the lion’s share of the large indirect costs of schizophrenia (disability payments, lifetime room and board, incarceration and legal costs, and loss of work and generation of taxes). LAIs can save both lives and expenditures, and a lot of suffering by patients and their families. I, too, long to see the emergence of a rational standard of care for schizophrenia using LAIs right after the initial psychotic episode. Oncology and cardiology have standards of care, so why not psychiatry?
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: Psychosis and epilepsy
Psychosis and epilepsy
I just read your editorial regarding the devastating consequences of psychotic relapses (From the Editor,
I work in the spheres of psychiatry, epileptology, and whole genome sequencing, and have experienced a psychotic episode myself (in 2013, after temporal lobe resection and overdose). I now consider myself even more lucky to be out the other side! As Governor for South London and Maudsley NHS Foundation Trust (SLaM) and Trustee for Epilepsy Action, many of our patients have psychosis. Some patients with epilepsy even experience postictal psychosis. Just yesterday, we had a call at SLaM regarding patients from a secure unit, and a psychiatric nurse spoke about patients at risk to themselves and others because of their psychotic illness, and how crucial effective long-term care was.
Torie Robinson
CEO, Epilepsy Sparks
Dr. Nasrallah responds
Ms. Robinson, thank you for sharing your story. It is important to note that the neurobiology of the psychosis that may occur with epilepsy may not be as neurodegenerative as the psychosis of schizophrenia. Many neurologic conditions can be associated with psychotic episodes, not only epilepsy. I am glad you overcame your post-temporal lobectomy psychotic episode and have had a very good outcome with high functioning.
Henry A. Nasrallah, MD
Editor-In-Chief
Disclosures
Dr. Chepke is a consultant to and speaker for Janssen Pharmaceuticals, Otsuka Pharmaceuticals, and Alkermes. The other authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
Benefits of early LAI use
I want to thank Dr. Nasrallah for his editorial calling for more frequent and earlier use of long-acting injectable antipsychotics (LAIs) in schizophrenia (From the Editor,
In addition to the neuroprotective biologic effects of early LAI usage, I’ve found that many of my FEP patients find great psychological comfort from incorporating LAIs into their treatment plan. The first psychotic break is generally when a person (and their family) feels the most afraid about the future and is in desperate need of hope that they can have a full life—with educational opportunities, sustained employment, meaningful relationships, and more. Just as society has seen the COVID-19 vaccines as a symbol of hope and the first step in overcoming the oppression of living in fear of an uncertain future, we need to help people experiencing FEP find hope in a needle.
Craig Chepke, MD, FAPA
Excel Psychiatric Associates
Huntersville, North Carolina
Reference
1. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
Dr. Nasrallah responds
Thank you, Dr. Chepke, for your letter confirming full support for using LAIs in schizophrenia. I like the phrase you coined: “hope in a needle.” The early use of LAIs in schizophrenia can provide the same type of hope that the vaccines against the life-threatening COVID-19 virus have generated in our society. Based on my direct observations, I also agree with you that the longer patients with schizophrenia remain on LAIs, the more engaged and happy they are with their progress and the quality of their lives. It is tragic that many patients never had the opportunity to return to their baseline with the early use of LAIs immediately following their first psychotic episode, instead of relapsing again and again due to their inability to adhere completely to their oral medications.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: LAIs as the standard of care
LAIs as the standard of care
Thank you, Dr. Nasrallah, for reiterating the importance of compliance with pharmacologic management of schizophrenia after FEP (From the Editor,
As you point out in your editorial, the facts are powerful, well-known, undisputed, and yet not adopted in the United States, when in other countries LAIs are first-line care. Yes, LAIs are expensive, but not nearly as expensive as the disabilities caused by noncompliance are to society.
Why isn’t LAI use the standard of care here in the United States? In the United States, there is advocacy for treatment because there’s money in it. There is no good advocacy for preventive care because there’s no immediate money in it. We have another good example of this in the United States: private, for-profit prisons. They have a vested interest in keeping prisons full and building new ones. Patients with FEP are most often treated in the hospital, where a standard of care could easily be established that mandates LAIs as first-tier care. Why is that not so? Who is pushing for it? Who is resisting?
Your editorial inspired me to advocate more strongly. Do you have advice about how to effect policy change? I know administrators respond when we talk dollars and cents, not quality of care. What is the dollar cost of not using LAIs as the standard of care after FEP? Who cares? Who would listen to the numbers?
Edward A. Major, MD, LFAPA
Clinical Professor of Psychiatry
Upstate Medical Center
Syracuse, New York
Dr. Nasrallah responds
Dr. Major, thanks for your message. Establishing a standard of care for the use of LAIs (or any other therapy) is not that simple. It requires well-coordinated collaboration among several stakeholders (clinicians, researchers, payors, advocacy groups, and a national organization such as the American Psychiatric Association). The cost issue is certainly powerful, but the equation works in favor of LAIs because 1 psychiatric hospitalization due to a psychotic relapse costs up to 3 times the annual cost of an LAI medication that can prevent that rehospitalization. In addition, disability comprises the lion’s share of the large indirect costs of schizophrenia (disability payments, lifetime room and board, incarceration and legal costs, and loss of work and generation of taxes). LAIs can save both lives and expenditures, and a lot of suffering by patients and their families. I, too, long to see the emergence of a rational standard of care for schizophrenia using LAIs right after the initial psychotic episode. Oncology and cardiology have standards of care, so why not psychiatry?
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: Psychosis and epilepsy
Psychosis and epilepsy
I just read your editorial regarding the devastating consequences of psychotic relapses (From the Editor,
I work in the spheres of psychiatry, epileptology, and whole genome sequencing, and have experienced a psychotic episode myself (in 2013, after temporal lobe resection and overdose). I now consider myself even more lucky to be out the other side! As Governor for South London and Maudsley NHS Foundation Trust (SLaM) and Trustee for Epilepsy Action, many of our patients have psychosis. Some patients with epilepsy even experience postictal psychosis. Just yesterday, we had a call at SLaM regarding patients from a secure unit, and a psychiatric nurse spoke about patients at risk to themselves and others because of their psychotic illness, and how crucial effective long-term care was.
Torie Robinson
CEO, Epilepsy Sparks
Dr. Nasrallah responds
Ms. Robinson, thank you for sharing your story. It is important to note that the neurobiology of the psychosis that may occur with epilepsy may not be as neurodegenerative as the psychosis of schizophrenia. Many neurologic conditions can be associated with psychotic episodes, not only epilepsy. I am glad you overcame your post-temporal lobectomy psychotic episode and have had a very good outcome with high functioning.
Henry A. Nasrallah, MD
Editor-In-Chief
Disclosures
Dr. Chepke is a consultant to and speaker for Janssen Pharmaceuticals, Otsuka Pharmaceuticals, and Alkermes. The other authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
Psychiatry is Neurology: White matter pathology permeates psychiatric disorders
Ask neurologists or psychiatrists to name a white matter (WM) brain disease and they are very likely to say multiple sclerosis (MS), a demyelinating brain disorder caused by immune-mediated destruction of oligodendrocytes, the glial cells that manufacture myelin without which brain communications would come to a standstill.
MS is often associated with mood or psychotic disorders, yet it is regarded as a neurologic illness, not a psychiatric disorder.
Many neurologists and psychiatrists may not be aware that during the past few years, multiple diffusion tensor imaging (DTI) studies have revealed that many psychiatric disorders are associated with WM pathology.1
Most people think that the brain is composed mostly of neurons, but in fact the bulk of brain volume (60%) is comprised of WM and only 40% is gray matter, which includes both neurons and glial cells (astroglia, microglia, and oligodendroglia). WM includes >137,000 km of myelinated fibers, an extensive network that connects all brain regions and integrates its complex, multifaceted functions, culminating in a unified sense of self and agency.
The role of the corpus callosum
Early in my research career, I became interested in the corpus callosum, the largest interhemispheric WM commissure connecting homologous areas across the 2 cerebral hemispheres. It is comprised of 200 million fibers of various diameters. Reasons for my fascination with the corpus callosum were:
The studies of Roger Sperry, the 1981 Nobel Laureate who led the team that was awarded the prize for split-brain research, which involved patients whose corpus callosum was cut to prevent the transfer of intractable epilepsy from 1 hemisphere to the other. Using a tachistoscope that he designed, Sperry discovered that the right and left hemispheres are 2 independent spheres of consciousness (ie, 2 individuals) with different skills.2 Cerebral dominance (laterality) fully integrates the 2 hemispheres via the corpus callosum, with a verbal hemisphere (the left, in 90% of people) dominating the other hemisphere and serving as the “spokesman self.” Thus, we all have 2 persons in our brain completely integrated into 1 “self.”2 This led me to wonder about the effects of an impaired corpus callosum on the “unified self.”
Postmortem and MRI studies conducted by our research group showed a significant difference in the thickness of the corpus callosum in a group of patients with schizophrenia vs healthy controls, which implied abnormal connectivity across the left and right hemispheres.3
Continue to: I then conducted a clinical study
I then conducted a clinical study examining patients with tumors impinging on the corpus callosum, which revealed that they developed psychotic symptoms (delusions and hallucinations).4 This study suggested that disrupting the integrity of the callosal inter-hemispheric fibers can trigger fixed false beliefs and perceptual anomalies.4
A ‘dysconnection’ between hemispheres
I translated those observations about the corpus callosum into a published hypothesis5 in which I proposed that Schneider’s First-Rank Symptoms of schizophrenia of thought insertion, thought withdrawal, and thought broadcasting—as well as delusional experiences of “external control”—may be due to a neurobiologic abnormality in the corpus callosum that disrupts the flow of ongoing bits of information transmitted from the left to the right hemisphere, and vice versa. I proposed in my model that this disruption leads to the verbal left hemisphere of a psychotic patient to describe having thoughts inserted into it from an alien source, failing to recognize that the thoughts it is receiving are being transmitted from the disconnected right hemisphere, which is no longer part of the “self.” Similarly, impulses from the right hemispheric consciousness are now perceived by the patient’s verbal left hemisphere (which talks to the examining physician) as “external control.” Thus, I postulated that an abnormal corpus callosum structure would lead to a “dysconnection” (not “disconnection”) between the 2 hemispheres, and that anomalous dysconnectivity may generate both delusions and hallucinations. 6
Two decades later, my assumptions were vindicated when DTI was invented, enabling the measurement of WM integrity, including the corpus callosum, the largest body of WM in the brain. Table 1 defines the main parameters of WM integrity, anisotropy and diffusivity, which measure water flow inside WM fibers.
During the past 15 years, many studies have confirmed the presence of significant abnormalities in the myelinated fibers of the corpus callosum in schizophrenia, which can be considered a validation of my hypothesis that the corpus callosum becomes a dysfunctional channel of communications between the right and left hemisphere. Subsequently, DTI studies have reported a spectrum of WM pathologies in various other cerebral bundles and not only in schizophrenia, but also in other major psychiatric disorders (Table 27-19).
The pathophysiology of WM pathology in many psychiatric disorders may include neurodevelopmental aberrations (genetic, environmental, or both, which may alter WM structure and/or myelination), neuroinflammation, or oxidative stress (free radicals), which can cause disintegration of the vital myelin sheaths, leading to disruption of brain connectivity.6,7 Researchers now consider the brain’s WM network dysconnectivity as generating a variety of psychiatric symptoms, including psychosis, depression, mania, anxiety, autism, aggression, impulsivity, psychopathy, and cognitive impairments.
It is not surprising that WM repair has become a therapeutic target in psychiatry and neurology. Among the strategies being investigated are inhibiting the Nogo-A signaling pathways20 or modulating the Lingo-1 signaling.21 However, the most well-established myelin repair pathway is prolactin, a neuroprotective hormone with several beneficial effects on the brain (Table 322,23), including the proliferation of oligodendroglia, the main source of myelin (and the number of which declines in schizophrenia). Antipsychotics that increase prolactin have been shown to increase WM volume.24,25 It has even been proposed that a decline in oligodendrocytes and low myelin synthesis may be one of the neurobiologic pathologies in schizophrenia.26 One of the 24 neuroprotective properties of the second-generation antipsychotics (SGAs) is the restoration of WM integrity.27 It’s worth noting that WM pathology has been found to be present at the onset of schizophrenia before treatment, and that SGAs have been reported to correct it.28
Continue to: In conclusion...
In conclusion, psychiatric disorders, usually referred to as “mental illnesses,” are unquestionably neurologic disorders. Similarly, all neurologic disorders are associated with psychiatric manifestations. WM pathology is only 1 of numerous structural brain abnormalities that have been documented across psychiatric disorders, which proves that psychiatry is a clinical neuroscience, just like neurology. I strongly advocate that psychiatry and neurology reunite into a single medical specialty. Both focus on disorders of brain structure and/or function, and these disorders also share much more than WM pathology.29
1. Sagarwala R and Nasrallah HA. White matter pathology is shared across multiple psychiatric brain disorders: Is abnormal diffusivity a transdiagnostic biomarker for psychopathology? Biomarkers in Neuropsychiatry. 2020;2:00010. https://doi.org/10.1016/j.bionps.2019.100010
2. Pearce JMS; FRCP. The “split brain” and Roger Wolcott Sperry (1913-1994). Rev Neurol (Paris). 2019;175(4):217-220.
3. Nasrallah HA, Andreasen NC, Coffman JA, et al. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry. 1986;21(3):274-282.
4. Nasrallah HA, McChesney CM. Psychopathology of corpus callosum tumors. Biol Psychiatry. 1981;16(7):663-669.
5. Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985;26(3):273-282.
6. Friston K, Brown HR, Siemerkus J, et al. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83-94.
7. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
8. Benedetti F, Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):338-341.
9. Zheng H, Bergamino M, Ford BN, et al; Tulsa 1000 Investigators. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46(5):928-938.
10. Sagarwala R, Nasrallah HA. A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Ann Clin Psychiatry. 2020;32(1):42-47.
11. Lee KS, Lee SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. 2020;1191:61-70.
12. Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33.
13. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288-298.
14. Waller R, Dotterer HL, Murray L, et al. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. Neuroimage Clin. 2017;14:201-215.
15. Wolf RC, Pujara MS, Motzkin JC, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36(10):4202-4209.
16. Puzzo I, Seunarine K, Sully K, et al. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J Abnorm Child Psychol. 2018;46(7):1451-1466.
17. Finger EC, Marsh A, Blair KS, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202(3):239-244.
18. Li C, Dong M, Womer FY, et al. Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Hum Brain Mapp. 2021;42(4):1182-1196.
19. Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Transl Psychiatry. 2019;9(1):251.
20. Petratos S, Theotokis P, Kim MJ, et al. That’s a wrap! Molecular drivers governing neuronal nogo receptor-dependent myelin plasticity and integrity. Front Cell Neurosci. 2020;14:227
21. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4(1):e348. doi: 10.1038/tp.2013.121
22. Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2-3):322-331.
23. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011 Oct;132(1):35-41
24. Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462.
25. Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol. 2013;16(3):691-700.
26. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272-281.
27. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7
28. Sagarwala R, Nasrallah HA. (In press.) The effect of antipsychotic medications on white matter integrity in first-episode drug naïve patients with psychosis. Asian Journal of Psychiatry.
29. Nasrallah HA. Let’s tear down the silos and reunify psychiatry and neurology. Current Psychiatry. 2013;12(8):9-10.
Ask neurologists or psychiatrists to name a white matter (WM) brain disease and they are very likely to say multiple sclerosis (MS), a demyelinating brain disorder caused by immune-mediated destruction of oligodendrocytes, the glial cells that manufacture myelin without which brain communications would come to a standstill.
MS is often associated with mood or psychotic disorders, yet it is regarded as a neurologic illness, not a psychiatric disorder.
Many neurologists and psychiatrists may not be aware that during the past few years, multiple diffusion tensor imaging (DTI) studies have revealed that many psychiatric disorders are associated with WM pathology.1
Most people think that the brain is composed mostly of neurons, but in fact the bulk of brain volume (60%) is comprised of WM and only 40% is gray matter, which includes both neurons and glial cells (astroglia, microglia, and oligodendroglia). WM includes >137,000 km of myelinated fibers, an extensive network that connects all brain regions and integrates its complex, multifaceted functions, culminating in a unified sense of self and agency.
The role of the corpus callosum
Early in my research career, I became interested in the corpus callosum, the largest interhemispheric WM commissure connecting homologous areas across the 2 cerebral hemispheres. It is comprised of 200 million fibers of various diameters. Reasons for my fascination with the corpus callosum were:
The studies of Roger Sperry, the 1981 Nobel Laureate who led the team that was awarded the prize for split-brain research, which involved patients whose corpus callosum was cut to prevent the transfer of intractable epilepsy from 1 hemisphere to the other. Using a tachistoscope that he designed, Sperry discovered that the right and left hemispheres are 2 independent spheres of consciousness (ie, 2 individuals) with different skills.2 Cerebral dominance (laterality) fully integrates the 2 hemispheres via the corpus callosum, with a verbal hemisphere (the left, in 90% of people) dominating the other hemisphere and serving as the “spokesman self.” Thus, we all have 2 persons in our brain completely integrated into 1 “self.”2 This led me to wonder about the effects of an impaired corpus callosum on the “unified self.”
Postmortem and MRI studies conducted by our research group showed a significant difference in the thickness of the corpus callosum in a group of patients with schizophrenia vs healthy controls, which implied abnormal connectivity across the left and right hemispheres.3
Continue to: I then conducted a clinical study
I then conducted a clinical study examining patients with tumors impinging on the corpus callosum, which revealed that they developed psychotic symptoms (delusions and hallucinations).4 This study suggested that disrupting the integrity of the callosal inter-hemispheric fibers can trigger fixed false beliefs and perceptual anomalies.4
A ‘dysconnection’ between hemispheres
I translated those observations about the corpus callosum into a published hypothesis5 in which I proposed that Schneider’s First-Rank Symptoms of schizophrenia of thought insertion, thought withdrawal, and thought broadcasting—as well as delusional experiences of “external control”—may be due to a neurobiologic abnormality in the corpus callosum that disrupts the flow of ongoing bits of information transmitted from the left to the right hemisphere, and vice versa. I proposed in my model that this disruption leads to the verbal left hemisphere of a psychotic patient to describe having thoughts inserted into it from an alien source, failing to recognize that the thoughts it is receiving are being transmitted from the disconnected right hemisphere, which is no longer part of the “self.” Similarly, impulses from the right hemispheric consciousness are now perceived by the patient’s verbal left hemisphere (which talks to the examining physician) as “external control.” Thus, I postulated that an abnormal corpus callosum structure would lead to a “dysconnection” (not “disconnection”) between the 2 hemispheres, and that anomalous dysconnectivity may generate both delusions and hallucinations. 6
Two decades later, my assumptions were vindicated when DTI was invented, enabling the measurement of WM integrity, including the corpus callosum, the largest body of WM in the brain. Table 1 defines the main parameters of WM integrity, anisotropy and diffusivity, which measure water flow inside WM fibers.
During the past 15 years, many studies have confirmed the presence of significant abnormalities in the myelinated fibers of the corpus callosum in schizophrenia, which can be considered a validation of my hypothesis that the corpus callosum becomes a dysfunctional channel of communications between the right and left hemisphere. Subsequently, DTI studies have reported a spectrum of WM pathologies in various other cerebral bundles and not only in schizophrenia, but also in other major psychiatric disorders (Table 27-19).
The pathophysiology of WM pathology in many psychiatric disorders may include neurodevelopmental aberrations (genetic, environmental, or both, which may alter WM structure and/or myelination), neuroinflammation, or oxidative stress (free radicals), which can cause disintegration of the vital myelin sheaths, leading to disruption of brain connectivity.6,7 Researchers now consider the brain’s WM network dysconnectivity as generating a variety of psychiatric symptoms, including psychosis, depression, mania, anxiety, autism, aggression, impulsivity, psychopathy, and cognitive impairments.
It is not surprising that WM repair has become a therapeutic target in psychiatry and neurology. Among the strategies being investigated are inhibiting the Nogo-A signaling pathways20 or modulating the Lingo-1 signaling.21 However, the most well-established myelin repair pathway is prolactin, a neuroprotective hormone with several beneficial effects on the brain (Table 322,23), including the proliferation of oligodendroglia, the main source of myelin (and the number of which declines in schizophrenia). Antipsychotics that increase prolactin have been shown to increase WM volume.24,25 It has even been proposed that a decline in oligodendrocytes and low myelin synthesis may be one of the neurobiologic pathologies in schizophrenia.26 One of the 24 neuroprotective properties of the second-generation antipsychotics (SGAs) is the restoration of WM integrity.27 It’s worth noting that WM pathology has been found to be present at the onset of schizophrenia before treatment, and that SGAs have been reported to correct it.28
Continue to: In conclusion...
In conclusion, psychiatric disorders, usually referred to as “mental illnesses,” are unquestionably neurologic disorders. Similarly, all neurologic disorders are associated with psychiatric manifestations. WM pathology is only 1 of numerous structural brain abnormalities that have been documented across psychiatric disorders, which proves that psychiatry is a clinical neuroscience, just like neurology. I strongly advocate that psychiatry and neurology reunite into a single medical specialty. Both focus on disorders of brain structure and/or function, and these disorders also share much more than WM pathology.29
Ask neurologists or psychiatrists to name a white matter (WM) brain disease and they are very likely to say multiple sclerosis (MS), a demyelinating brain disorder caused by immune-mediated destruction of oligodendrocytes, the glial cells that manufacture myelin without which brain communications would come to a standstill.
MS is often associated with mood or psychotic disorders, yet it is regarded as a neurologic illness, not a psychiatric disorder.
Many neurologists and psychiatrists may not be aware that during the past few years, multiple diffusion tensor imaging (DTI) studies have revealed that many psychiatric disorders are associated with WM pathology.1
Most people think that the brain is composed mostly of neurons, but in fact the bulk of brain volume (60%) is comprised of WM and only 40% is gray matter, which includes both neurons and glial cells (astroglia, microglia, and oligodendroglia). WM includes >137,000 km of myelinated fibers, an extensive network that connects all brain regions and integrates its complex, multifaceted functions, culminating in a unified sense of self and agency.
The role of the corpus callosum
Early in my research career, I became interested in the corpus callosum, the largest interhemispheric WM commissure connecting homologous areas across the 2 cerebral hemispheres. It is comprised of 200 million fibers of various diameters. Reasons for my fascination with the corpus callosum were:
The studies of Roger Sperry, the 1981 Nobel Laureate who led the team that was awarded the prize for split-brain research, which involved patients whose corpus callosum was cut to prevent the transfer of intractable epilepsy from 1 hemisphere to the other. Using a tachistoscope that he designed, Sperry discovered that the right and left hemispheres are 2 independent spheres of consciousness (ie, 2 individuals) with different skills.2 Cerebral dominance (laterality) fully integrates the 2 hemispheres via the corpus callosum, with a verbal hemisphere (the left, in 90% of people) dominating the other hemisphere and serving as the “spokesman self.” Thus, we all have 2 persons in our brain completely integrated into 1 “self.”2 This led me to wonder about the effects of an impaired corpus callosum on the “unified self.”
Postmortem and MRI studies conducted by our research group showed a significant difference in the thickness of the corpus callosum in a group of patients with schizophrenia vs healthy controls, which implied abnormal connectivity across the left and right hemispheres.3
Continue to: I then conducted a clinical study
I then conducted a clinical study examining patients with tumors impinging on the corpus callosum, which revealed that they developed psychotic symptoms (delusions and hallucinations).4 This study suggested that disrupting the integrity of the callosal inter-hemispheric fibers can trigger fixed false beliefs and perceptual anomalies.4
A ‘dysconnection’ between hemispheres
I translated those observations about the corpus callosum into a published hypothesis5 in which I proposed that Schneider’s First-Rank Symptoms of schizophrenia of thought insertion, thought withdrawal, and thought broadcasting—as well as delusional experiences of “external control”—may be due to a neurobiologic abnormality in the corpus callosum that disrupts the flow of ongoing bits of information transmitted from the left to the right hemisphere, and vice versa. I proposed in my model that this disruption leads to the verbal left hemisphere of a psychotic patient to describe having thoughts inserted into it from an alien source, failing to recognize that the thoughts it is receiving are being transmitted from the disconnected right hemisphere, which is no longer part of the “self.” Similarly, impulses from the right hemispheric consciousness are now perceived by the patient’s verbal left hemisphere (which talks to the examining physician) as “external control.” Thus, I postulated that an abnormal corpus callosum structure would lead to a “dysconnection” (not “disconnection”) between the 2 hemispheres, and that anomalous dysconnectivity may generate both delusions and hallucinations. 6
Two decades later, my assumptions were vindicated when DTI was invented, enabling the measurement of WM integrity, including the corpus callosum, the largest body of WM in the brain. Table 1 defines the main parameters of WM integrity, anisotropy and diffusivity, which measure water flow inside WM fibers.
During the past 15 years, many studies have confirmed the presence of significant abnormalities in the myelinated fibers of the corpus callosum in schizophrenia, which can be considered a validation of my hypothesis that the corpus callosum becomes a dysfunctional channel of communications between the right and left hemisphere. Subsequently, DTI studies have reported a spectrum of WM pathologies in various other cerebral bundles and not only in schizophrenia, but also in other major psychiatric disorders (Table 27-19).
The pathophysiology of WM pathology in many psychiatric disorders may include neurodevelopmental aberrations (genetic, environmental, or both, which may alter WM structure and/or myelination), neuroinflammation, or oxidative stress (free radicals), which can cause disintegration of the vital myelin sheaths, leading to disruption of brain connectivity.6,7 Researchers now consider the brain’s WM network dysconnectivity as generating a variety of psychiatric symptoms, including psychosis, depression, mania, anxiety, autism, aggression, impulsivity, psychopathy, and cognitive impairments.
It is not surprising that WM repair has become a therapeutic target in psychiatry and neurology. Among the strategies being investigated are inhibiting the Nogo-A signaling pathways20 or modulating the Lingo-1 signaling.21 However, the most well-established myelin repair pathway is prolactin, a neuroprotective hormone with several beneficial effects on the brain (Table 322,23), including the proliferation of oligodendroglia, the main source of myelin (and the number of which declines in schizophrenia). Antipsychotics that increase prolactin have been shown to increase WM volume.24,25 It has even been proposed that a decline in oligodendrocytes and low myelin synthesis may be one of the neurobiologic pathologies in schizophrenia.26 One of the 24 neuroprotective properties of the second-generation antipsychotics (SGAs) is the restoration of WM integrity.27 It’s worth noting that WM pathology has been found to be present at the onset of schizophrenia before treatment, and that SGAs have been reported to correct it.28
Continue to: In conclusion...
In conclusion, psychiatric disorders, usually referred to as “mental illnesses,” are unquestionably neurologic disorders. Similarly, all neurologic disorders are associated with psychiatric manifestations. WM pathology is only 1 of numerous structural brain abnormalities that have been documented across psychiatric disorders, which proves that psychiatry is a clinical neuroscience, just like neurology. I strongly advocate that psychiatry and neurology reunite into a single medical specialty. Both focus on disorders of brain structure and/or function, and these disorders also share much more than WM pathology.29
1. Sagarwala R and Nasrallah HA. White matter pathology is shared across multiple psychiatric brain disorders: Is abnormal diffusivity a transdiagnostic biomarker for psychopathology? Biomarkers in Neuropsychiatry. 2020;2:00010. https://doi.org/10.1016/j.bionps.2019.100010
2. Pearce JMS; FRCP. The “split brain” and Roger Wolcott Sperry (1913-1994). Rev Neurol (Paris). 2019;175(4):217-220.
3. Nasrallah HA, Andreasen NC, Coffman JA, et al. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry. 1986;21(3):274-282.
4. Nasrallah HA, McChesney CM. Psychopathology of corpus callosum tumors. Biol Psychiatry. 1981;16(7):663-669.
5. Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985;26(3):273-282.
6. Friston K, Brown HR, Siemerkus J, et al. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83-94.
7. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
8. Benedetti F, Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):338-341.
9. Zheng H, Bergamino M, Ford BN, et al; Tulsa 1000 Investigators. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46(5):928-938.
10. Sagarwala R, Nasrallah HA. A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Ann Clin Psychiatry. 2020;32(1):42-47.
11. Lee KS, Lee SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. 2020;1191:61-70.
12. Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33.
13. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288-298.
14. Waller R, Dotterer HL, Murray L, et al. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. Neuroimage Clin. 2017;14:201-215.
15. Wolf RC, Pujara MS, Motzkin JC, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36(10):4202-4209.
16. Puzzo I, Seunarine K, Sully K, et al. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J Abnorm Child Psychol. 2018;46(7):1451-1466.
17. Finger EC, Marsh A, Blair KS, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202(3):239-244.
18. Li C, Dong M, Womer FY, et al. Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Hum Brain Mapp. 2021;42(4):1182-1196.
19. Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Transl Psychiatry. 2019;9(1):251.
20. Petratos S, Theotokis P, Kim MJ, et al. That’s a wrap! Molecular drivers governing neuronal nogo receptor-dependent myelin plasticity and integrity. Front Cell Neurosci. 2020;14:227
21. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4(1):e348. doi: 10.1038/tp.2013.121
22. Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2-3):322-331.
23. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011 Oct;132(1):35-41
24. Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462.
25. Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol. 2013;16(3):691-700.
26. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272-281.
27. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7
28. Sagarwala R, Nasrallah HA. (In press.) The effect of antipsychotic medications on white matter integrity in first-episode drug naïve patients with psychosis. Asian Journal of Psychiatry.
29. Nasrallah HA. Let’s tear down the silos and reunify psychiatry and neurology. Current Psychiatry. 2013;12(8):9-10.
1. Sagarwala R and Nasrallah HA. White matter pathology is shared across multiple psychiatric brain disorders: Is abnormal diffusivity a transdiagnostic biomarker for psychopathology? Biomarkers in Neuropsychiatry. 2020;2:00010. https://doi.org/10.1016/j.bionps.2019.100010
2. Pearce JMS; FRCP. The “split brain” and Roger Wolcott Sperry (1913-1994). Rev Neurol (Paris). 2019;175(4):217-220.
3. Nasrallah HA, Andreasen NC, Coffman JA, et al. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry. 1986;21(3):274-282.
4. Nasrallah HA, McChesney CM. Psychopathology of corpus callosum tumors. Biol Psychiatry. 1981;16(7):663-669.
5. Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985;26(3):273-282.
6. Friston K, Brown HR, Siemerkus J, et al. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83-94.
7. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
8. Benedetti F, Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):338-341.
9. Zheng H, Bergamino M, Ford BN, et al; Tulsa 1000 Investigators. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46(5):928-938.
10. Sagarwala R, Nasrallah HA. A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Ann Clin Psychiatry. 2020;32(1):42-47.
11. Lee KS, Lee SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. 2020;1191:61-70.
12. Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33.
13. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288-298.
14. Waller R, Dotterer HL, Murray L, et al. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. Neuroimage Clin. 2017;14:201-215.
15. Wolf RC, Pujara MS, Motzkin JC, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36(10):4202-4209.
16. Puzzo I, Seunarine K, Sully K, et al. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J Abnorm Child Psychol. 2018;46(7):1451-1466.
17. Finger EC, Marsh A, Blair KS, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202(3):239-244.
18. Li C, Dong M, Womer FY, et al. Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Hum Brain Mapp. 2021;42(4):1182-1196.
19. Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Transl Psychiatry. 2019;9(1):251.
20. Petratos S, Theotokis P, Kim MJ, et al. That’s a wrap! Molecular drivers governing neuronal nogo receptor-dependent myelin plasticity and integrity. Front Cell Neurosci. 2020;14:227
21. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4(1):e348. doi: 10.1038/tp.2013.121
22. Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2-3):322-331.
23. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011 Oct;132(1):35-41
24. Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462.
25. Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol. 2013;16(3):691-700.
26. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272-281.
27. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7
28. Sagarwala R, Nasrallah HA. (In press.) The effect of antipsychotic medications on white matter integrity in first-episode drug naïve patients with psychosis. Asian Journal of Psychiatry.
29. Nasrallah HA. Let’s tear down the silos and reunify psychiatry and neurology. Current Psychiatry. 2013;12(8):9-10.
10 devastating consequences of psychotic relapses
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
Today’s psychiatric neuroscience advances were science fiction during my residency
During my residency training years, I had many rosy and bold dreams about the future of psychiatry, hoping for many breakthroughs.
Early on, I decided to pursue an academic career, and specifically to focus on the neurobiology of schizophrenia, bipolar disorder, and other psychoses. I secured a neuroscience mentor, conducted a research project, and presented my findings at the American Psychiatric Association Annual Meeting. Although at the time everyone used the term “functional” to describe mental illnesses, I was convinced that they were all neurologic conditions, with prominent psychiatric manifestations. And I have been proven right.
After my residency, I eagerly pursued a neuroscience fellowship at the National Institutes of Health. My fantasy was that during my career as a psychiatric neuroscientist, brain exploration would uncover the many mysteries of psychiatric disorders. I was insightful enough to recognize that what I envisioned for the future of psychiatry qualified as science fiction, but I never stopped dreaming.
Today, the advances in psychiatric neuroscience that were unimaginable during my residency have become dazzling discoveries. My journey as a psychiatric neuroscientist has been more thrilling than I ever imagined. I recall doing postmortem research on the brains of hundreds of deceased psychiatric patients, noticing sulci widening and ventricular dilatation, and wondering whether one day we would be able to detect those atrophic changes while the patients were alive. Although I measured those changes in postmortem brains, I was cognizant that due to preservation artifacts, such measurements were less reliable than measurements of living brains.
And then the advent of neuroimaging fulfilled my fantasies. This began towards the end of my fellowship, and has exploded with neurobiologic findings throughout my academic career. Then came dramatic methodologies to probe brain molecular and cellular pathologies, followed by breakthrough clinical advances. Entirely new vistas of research into psychiatric brain disorders are opening every day. The exhilaration will never end!
From science fiction to clinical reality
Here is a quick outline of some of the “science fiction” of psychiatry that has come true since my training days. Back then, these discoveries were completely absent from the radar screen of psychiatry, when it was still a fledgling medical specialty struggling to emerge from the dominant yet nonempirical era of psychoanalysis.
Brain exploration methods. Unprecedented breakthroughs in computer technology have allowed psychiatric neuroscientists to create a new field of neuroimaging research that includes:
- cerebral blood flow (CBF)
- position emission tomography (PET)
- single photon emission computed tomography (SPECT).
Continue to: These functional neuroimaging...
These functional neuroimaging methods (using ionizing radiation) have enabled clinicians to see abnormal blood flow patterns in the brains of living patients. One of the earliest findings was hypofrontality in patients with schizophrenia, implicating frontal pathology in this severe brain disorder. PET was also used for dopamine and serotonin receptor imaging.
Computerized axia tomography. Compared with skull X-rays, CT (“CAT”) scans provided a more detailed view of brain tissue, and began a structural neuroimaging revolution that enriched psychiatric research, but also was applied to organs other than the brain.
Magnetic resonance imaging (MRI) became the “big kahuna” of neuroimaging when arrived in the early 1980s and quickly supplanted CT research because it is safer (no ionizing radiation, and it can be repeated multiple times with or without tasks). It also provided exquisite neuroanatomical details of brain tissue with stunning fidelity. Subsequently, several MRI techniques/software programs were developed that advanced research in psychiatry to multiple new frontiers, including:
- Morphological neuroimaging with MRI
- Magnetic resonance spectroscopy (MRS), which acts like a living, noninvasive biopsy of several chemicals (such as choline, lactate, glutamine, adenosine triphosphate, and the neuronal marker N-acetylcysteine) in a small volume (≤1 cc) of neural tissue in various regions
- Functional MRI (fMRI), which measures blood flow changes during actual or imagined tasks in the brains of patients vs healthy controls
- Diffusion tensor imaging (DTI), which evaluates the integrity of white matter (60% of brain volume, including 137,000 miles of myelinated fibers) by measuring the flow of water inside myelinated fibers (anisotropy and diffusivity). DTI of the corpus callosum, the largest brain commissure that is comprised of 200 million interhemispheric fibers, has revealed many abnormalities. This was one of the structures I investigated during my fellowship, including a histopathological study.1
All 4 of these neuroimaging techniques continue to generate a wealth of data about brain structure and function in psychosis, mood disorders, anxiety disorders, borderline personality disorder, obsessive-compulsive disorder, eating disorders, and substance use disorders. All these discoveries were utterly impossible to predict during my residency. I am proud to have published the first reports in the literature of ventricular enlargement in patients with bipolar disorder,2 cortical atrophy in schizophrenia and mania,3 reductions of hippocampal volume in patients with schizophrenia using MRS,4 and progressive brain atrophy in patients with schizophrenia.5 It is especially gratifying that I played a small role in translating my science fiction fantasies into clinical reality!
Other breakthrough methodologies that are advancing psychiatric neuroscience today but were science fiction during my residency days include:
- Pluripotent stem cells, which enable the de-differentiation of adult skin cells and then re-differentiating them into any type of cell, including neurons. This allows researchers to conduct studies on any patient’s brain cells without needing to do an invasive, high-risk brain biopsy. As a young resident, I would never have predicted that this virtual brain biopsy would be possible!
- Optogenetics, which enables controlling cell behavior using light and genetically encoded light-sensitive proteins. This triggered a cornucopia of neuroscience discoveries by using optogenetics to modulate cell-signaling cascades to understand cellular biology. Halorhodopsin and bacteriorhodopsin are used as tools to turn neurons off or on rapidly and safely.
- Genome-wide association studies (GWAS) have revolutionized the field of molecular neurogenetics and are enabling clinicians to detect risk genes by comparing the DNA samples of thousands of psychiatric patients with thousands of healthy controls. This is how several hundred risk genes have been identified for schizophrenia, bipolar disorder, autism spectrum disorder, and more to come.
- Clustered regularly interspaced short palindromic repeats (CRISPR) is a remarkable genetic “scissors” (that earned its inventors the 2020 Nobel Prize) that allows splicing out a disease gene and splicing in a normal gene. This will have an enormous future application in preventing an adulthood illness at its roots during fetal life. The future medical implications for psychiatric disorders are prodigious!
Continue to: Clinical advances
Clinical advances. Many therapies or approaches that did not exist during my residency (and how I dreamed about them back then!) are available to today’s clinicians. These include:
- Rapid-acting antidepressants that reverse severe and chronic depression and suicidal urges within a few hours or a couple of days. As a resident, I waited for weeks or months to see patients with depression reach the full remission that is now achieved practically the same day with IV ketamine, intranasal esketamine, IV scopolamine, and inhalable nitrous oxide. During my residency, the closest thing we had to a rapid-acting treatment for depression was electroconvulsive therapy (ECT), but that usually took 2 to 3 weeks. Psychiatric clinicians should never cease to appreciate how an intractable, treatment-refractory depression can rapidly be turned off like a light switch, restoring normal mood to desperately ill persons.
- Neuromodulation techniques are flourishing. Beyond ECT, transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), transcranial direct current stimulation (tDCS), deep brain stimulation (DBS), low field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near-infrared radiation (NIR), and focused ultrasound (FUS) are approved or under development, offering millions of patients with various neuropsychiatric disorders potential recovery not with pharmacotherapy, but via a brain-targeted approach.
- Telepsychiatry. Now taken for granted during the COVID-19 pandemic, telepsychiatry was completely unimaginable during my residency. Yes, we had phones, but not smartphones! The only “zoom” we knew was the furious sound of a sports car engine! To be able to see and evaluate a patient literally anywhere in the world was science fiction personified! Increased remote access to psychiatric care by patients everywhere is a truly remarkable advance that helped avoid a disastrous lack of psychiatric treatment during the current pandemic that brought in-person interactions between psychiatric physicians and their patients to a screeching halt.
- Neurobiologic effects of psychotherapy. Viewing psychotherapy as a neurobiologic treatment was totally unknown and unimaginable during my residency. I was heavily trained in various types of psychotherapies, but not once did any of my supervisors mention experiential neuroplasticity as a brain-altering process, or that psychotherapy changes brain structure, induces experimental neuroplasticity, and induces billions of dendritic spines in patients’ cortex and limbic structures, helping them connect the dots and develop new insights. No one knew that psychotherapy can mimic the neural effects of pharmacotherapy.
- Immunomodulatory effects of psychotherapy. It was completely unknown that psychotherapies such as cognitive-behavioral therapy can lower levels of inflammatory biomarkers in patients’ CSF and serum. Back then, no one imagined that psychotherapy had immunomodulatory effects. These discoveries are revolutionary for us psychiatrists and confirm the neurobiologic mechanisms of psychotherapy for every patient we treat.
- Epigenetics. This was rarely, if ever, mentioned when I was a resident. We knew from clinical studies that children who were abused or neglected often develop severe mood or psychotic disorders in adulthood. But we did not know that trauma modifies some genes via under- or overexpression, and that such epigenetic changes alter brain development towards psychopathology. The mysteries of psychiatric brain disorders generated by childhood trauma have been clarified by advances in epigenetics.
Aspirational, futuristic therapies. Even now, as a seasoned psychiatric neuroscientist, I continue to dream. Research is providing many clues for potentially radical psychiatric treatments that go beyond standard antipsychotics, antidepressants, mood stabilizers, or anxiolytics. But today, I fully expect that scientific dreams eventually come true through research. For example, the following neuroscientific therapeutics strategies may someday become routine in clinical practice:
- microglia inhibition
- mitochondria repair
- anti-apoptotic therapy
- white matter connectivity restoration
- neuroprotection (enhancing neurogenesis, increasing neurotropic factors, and enhancing synaptogenesis)
- reverse glutamate N-methyl-
d -aspartate hypofunction - prevent amyloid formation.
Data analysis breakthroughs. Side-by-side with the explosion of new findings and amassing mountains of data in psychiatric neuroscience, unprecedented and revolutionary data-management techniques have emerged to facilitate the herculean task of data analysis to extract the mythical needle in a haystack and derive the overall impact of masses of data. These techniques, whose names were not in our vocabulary during my residency days, include:
- machine learning
- artificial intelligence
- deep learning
- big data.
With the help of powerful computers and ingenious software, discovering critical nuggets of knowledge about the brain and predicting the best approaches to healing dysfunctional brains are now possible. Those powerful methods of analyzing massive data are the vehicles for transforming science fiction to reality by assembling the jigsaw puzzle(s) of the human brain, arguably the last frontier in medical science.
My life experiences as a psychiatric neuroscientist have convinced me that nothing is beyond the reach of scientific research. Unraveling the divine brain’s complexities will eventually become reality. So, let us never stop dreaming and fantasizing!
1. Nasrallah HA, McCalley-Whitters M, Bigelow LB, et al. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res. 1983;8(4):251-260.
2. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affect Disord. 1982;4(1):15-19.
3. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cortical atrophy in schizophrenia and mania: a comparative CT study. J Clin Psychiatry. 1982;43(11):439-441.
4. Nasrallah HA, Skinner TE, Schmalbrock P, et al. Proton magnetic resonance spectroscopy (1H MRS) of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry. 1994;165(4):481-485.
5. Nasrallah HA, Olson SC, McCalley-Whitters M, et al. Cerebral ventricular enlargement in schizophrenia. A preliminary follow-up study. Arch Gen Psychiatry. 1986;43(2):157-159.
During my residency training years, I had many rosy and bold dreams about the future of psychiatry, hoping for many breakthroughs.
Early on, I decided to pursue an academic career, and specifically to focus on the neurobiology of schizophrenia, bipolar disorder, and other psychoses. I secured a neuroscience mentor, conducted a research project, and presented my findings at the American Psychiatric Association Annual Meeting. Although at the time everyone used the term “functional” to describe mental illnesses, I was convinced that they were all neurologic conditions, with prominent psychiatric manifestations. And I have been proven right.
After my residency, I eagerly pursued a neuroscience fellowship at the National Institutes of Health. My fantasy was that during my career as a psychiatric neuroscientist, brain exploration would uncover the many mysteries of psychiatric disorders. I was insightful enough to recognize that what I envisioned for the future of psychiatry qualified as science fiction, but I never stopped dreaming.
Today, the advances in psychiatric neuroscience that were unimaginable during my residency have become dazzling discoveries. My journey as a psychiatric neuroscientist has been more thrilling than I ever imagined. I recall doing postmortem research on the brains of hundreds of deceased psychiatric patients, noticing sulci widening and ventricular dilatation, and wondering whether one day we would be able to detect those atrophic changes while the patients were alive. Although I measured those changes in postmortem brains, I was cognizant that due to preservation artifacts, such measurements were less reliable than measurements of living brains.
And then the advent of neuroimaging fulfilled my fantasies. This began towards the end of my fellowship, and has exploded with neurobiologic findings throughout my academic career. Then came dramatic methodologies to probe brain molecular and cellular pathologies, followed by breakthrough clinical advances. Entirely new vistas of research into psychiatric brain disorders are opening every day. The exhilaration will never end!
From science fiction to clinical reality
Here is a quick outline of some of the “science fiction” of psychiatry that has come true since my training days. Back then, these discoveries were completely absent from the radar screen of psychiatry, when it was still a fledgling medical specialty struggling to emerge from the dominant yet nonempirical era of psychoanalysis.
Brain exploration methods. Unprecedented breakthroughs in computer technology have allowed psychiatric neuroscientists to create a new field of neuroimaging research that includes:
- cerebral blood flow (CBF)
- position emission tomography (PET)
- single photon emission computed tomography (SPECT).
Continue to: These functional neuroimaging...
These functional neuroimaging methods (using ionizing radiation) have enabled clinicians to see abnormal blood flow patterns in the brains of living patients. One of the earliest findings was hypofrontality in patients with schizophrenia, implicating frontal pathology in this severe brain disorder. PET was also used for dopamine and serotonin receptor imaging.
Computerized axia tomography. Compared with skull X-rays, CT (“CAT”) scans provided a more detailed view of brain tissue, and began a structural neuroimaging revolution that enriched psychiatric research, but also was applied to organs other than the brain.
Magnetic resonance imaging (MRI) became the “big kahuna” of neuroimaging when arrived in the early 1980s and quickly supplanted CT research because it is safer (no ionizing radiation, and it can be repeated multiple times with or without tasks). It also provided exquisite neuroanatomical details of brain tissue with stunning fidelity. Subsequently, several MRI techniques/software programs were developed that advanced research in psychiatry to multiple new frontiers, including:
- Morphological neuroimaging with MRI
- Magnetic resonance spectroscopy (MRS), which acts like a living, noninvasive biopsy of several chemicals (such as choline, lactate, glutamine, adenosine triphosphate, and the neuronal marker N-acetylcysteine) in a small volume (≤1 cc) of neural tissue in various regions
- Functional MRI (fMRI), which measures blood flow changes during actual or imagined tasks in the brains of patients vs healthy controls
- Diffusion tensor imaging (DTI), which evaluates the integrity of white matter (60% of brain volume, including 137,000 miles of myelinated fibers) by measuring the flow of water inside myelinated fibers (anisotropy and diffusivity). DTI of the corpus callosum, the largest brain commissure that is comprised of 200 million interhemispheric fibers, has revealed many abnormalities. This was one of the structures I investigated during my fellowship, including a histopathological study.1
All 4 of these neuroimaging techniques continue to generate a wealth of data about brain structure and function in psychosis, mood disorders, anxiety disorders, borderline personality disorder, obsessive-compulsive disorder, eating disorders, and substance use disorders. All these discoveries were utterly impossible to predict during my residency. I am proud to have published the first reports in the literature of ventricular enlargement in patients with bipolar disorder,2 cortical atrophy in schizophrenia and mania,3 reductions of hippocampal volume in patients with schizophrenia using MRS,4 and progressive brain atrophy in patients with schizophrenia.5 It is especially gratifying that I played a small role in translating my science fiction fantasies into clinical reality!
Other breakthrough methodologies that are advancing psychiatric neuroscience today but were science fiction during my residency days include:
- Pluripotent stem cells, which enable the de-differentiation of adult skin cells and then re-differentiating them into any type of cell, including neurons. This allows researchers to conduct studies on any patient’s brain cells without needing to do an invasive, high-risk brain biopsy. As a young resident, I would never have predicted that this virtual brain biopsy would be possible!
- Optogenetics, which enables controlling cell behavior using light and genetically encoded light-sensitive proteins. This triggered a cornucopia of neuroscience discoveries by using optogenetics to modulate cell-signaling cascades to understand cellular biology. Halorhodopsin and bacteriorhodopsin are used as tools to turn neurons off or on rapidly and safely.
- Genome-wide association studies (GWAS) have revolutionized the field of molecular neurogenetics and are enabling clinicians to detect risk genes by comparing the DNA samples of thousands of psychiatric patients with thousands of healthy controls. This is how several hundred risk genes have been identified for schizophrenia, bipolar disorder, autism spectrum disorder, and more to come.
- Clustered regularly interspaced short palindromic repeats (CRISPR) is a remarkable genetic “scissors” (that earned its inventors the 2020 Nobel Prize) that allows splicing out a disease gene and splicing in a normal gene. This will have an enormous future application in preventing an adulthood illness at its roots during fetal life. The future medical implications for psychiatric disorders are prodigious!
Continue to: Clinical advances
Clinical advances. Many therapies or approaches that did not exist during my residency (and how I dreamed about them back then!) are available to today’s clinicians. These include:
- Rapid-acting antidepressants that reverse severe and chronic depression and suicidal urges within a few hours or a couple of days. As a resident, I waited for weeks or months to see patients with depression reach the full remission that is now achieved practically the same day with IV ketamine, intranasal esketamine, IV scopolamine, and inhalable nitrous oxide. During my residency, the closest thing we had to a rapid-acting treatment for depression was electroconvulsive therapy (ECT), but that usually took 2 to 3 weeks. Psychiatric clinicians should never cease to appreciate how an intractable, treatment-refractory depression can rapidly be turned off like a light switch, restoring normal mood to desperately ill persons.
- Neuromodulation techniques are flourishing. Beyond ECT, transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), transcranial direct current stimulation (tDCS), deep brain stimulation (DBS), low field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near-infrared radiation (NIR), and focused ultrasound (FUS) are approved or under development, offering millions of patients with various neuropsychiatric disorders potential recovery not with pharmacotherapy, but via a brain-targeted approach.
- Telepsychiatry. Now taken for granted during the COVID-19 pandemic, telepsychiatry was completely unimaginable during my residency. Yes, we had phones, but not smartphones! The only “zoom” we knew was the furious sound of a sports car engine! To be able to see and evaluate a patient literally anywhere in the world was science fiction personified! Increased remote access to psychiatric care by patients everywhere is a truly remarkable advance that helped avoid a disastrous lack of psychiatric treatment during the current pandemic that brought in-person interactions between psychiatric physicians and their patients to a screeching halt.
- Neurobiologic effects of psychotherapy. Viewing psychotherapy as a neurobiologic treatment was totally unknown and unimaginable during my residency. I was heavily trained in various types of psychotherapies, but not once did any of my supervisors mention experiential neuroplasticity as a brain-altering process, or that psychotherapy changes brain structure, induces experimental neuroplasticity, and induces billions of dendritic spines in patients’ cortex and limbic structures, helping them connect the dots and develop new insights. No one knew that psychotherapy can mimic the neural effects of pharmacotherapy.
- Immunomodulatory effects of psychotherapy. It was completely unknown that psychotherapies such as cognitive-behavioral therapy can lower levels of inflammatory biomarkers in patients’ CSF and serum. Back then, no one imagined that psychotherapy had immunomodulatory effects. These discoveries are revolutionary for us psychiatrists and confirm the neurobiologic mechanisms of psychotherapy for every patient we treat.
- Epigenetics. This was rarely, if ever, mentioned when I was a resident. We knew from clinical studies that children who were abused or neglected often develop severe mood or psychotic disorders in adulthood. But we did not know that trauma modifies some genes via under- or overexpression, and that such epigenetic changes alter brain development towards psychopathology. The mysteries of psychiatric brain disorders generated by childhood trauma have been clarified by advances in epigenetics.
Aspirational, futuristic therapies. Even now, as a seasoned psychiatric neuroscientist, I continue to dream. Research is providing many clues for potentially radical psychiatric treatments that go beyond standard antipsychotics, antidepressants, mood stabilizers, or anxiolytics. But today, I fully expect that scientific dreams eventually come true through research. For example, the following neuroscientific therapeutics strategies may someday become routine in clinical practice:
- microglia inhibition
- mitochondria repair
- anti-apoptotic therapy
- white matter connectivity restoration
- neuroprotection (enhancing neurogenesis, increasing neurotropic factors, and enhancing synaptogenesis)
- reverse glutamate N-methyl-
d -aspartate hypofunction - prevent amyloid formation.
Data analysis breakthroughs. Side-by-side with the explosion of new findings and amassing mountains of data in psychiatric neuroscience, unprecedented and revolutionary data-management techniques have emerged to facilitate the herculean task of data analysis to extract the mythical needle in a haystack and derive the overall impact of masses of data. These techniques, whose names were not in our vocabulary during my residency days, include:
- machine learning
- artificial intelligence
- deep learning
- big data.
With the help of powerful computers and ingenious software, discovering critical nuggets of knowledge about the brain and predicting the best approaches to healing dysfunctional brains are now possible. Those powerful methods of analyzing massive data are the vehicles for transforming science fiction to reality by assembling the jigsaw puzzle(s) of the human brain, arguably the last frontier in medical science.
My life experiences as a psychiatric neuroscientist have convinced me that nothing is beyond the reach of scientific research. Unraveling the divine brain’s complexities will eventually become reality. So, let us never stop dreaming and fantasizing!
During my residency training years, I had many rosy and bold dreams about the future of psychiatry, hoping for many breakthroughs.
Early on, I decided to pursue an academic career, and specifically to focus on the neurobiology of schizophrenia, bipolar disorder, and other psychoses. I secured a neuroscience mentor, conducted a research project, and presented my findings at the American Psychiatric Association Annual Meeting. Although at the time everyone used the term “functional” to describe mental illnesses, I was convinced that they were all neurologic conditions, with prominent psychiatric manifestations. And I have been proven right.
After my residency, I eagerly pursued a neuroscience fellowship at the National Institutes of Health. My fantasy was that during my career as a psychiatric neuroscientist, brain exploration would uncover the many mysteries of psychiatric disorders. I was insightful enough to recognize that what I envisioned for the future of psychiatry qualified as science fiction, but I never stopped dreaming.
Today, the advances in psychiatric neuroscience that were unimaginable during my residency have become dazzling discoveries. My journey as a psychiatric neuroscientist has been more thrilling than I ever imagined. I recall doing postmortem research on the brains of hundreds of deceased psychiatric patients, noticing sulci widening and ventricular dilatation, and wondering whether one day we would be able to detect those atrophic changes while the patients were alive. Although I measured those changes in postmortem brains, I was cognizant that due to preservation artifacts, such measurements were less reliable than measurements of living brains.
And then the advent of neuroimaging fulfilled my fantasies. This began towards the end of my fellowship, and has exploded with neurobiologic findings throughout my academic career. Then came dramatic methodologies to probe brain molecular and cellular pathologies, followed by breakthrough clinical advances. Entirely new vistas of research into psychiatric brain disorders are opening every day. The exhilaration will never end!
From science fiction to clinical reality
Here is a quick outline of some of the “science fiction” of psychiatry that has come true since my training days. Back then, these discoveries were completely absent from the radar screen of psychiatry, when it was still a fledgling medical specialty struggling to emerge from the dominant yet nonempirical era of psychoanalysis.
Brain exploration methods. Unprecedented breakthroughs in computer technology have allowed psychiatric neuroscientists to create a new field of neuroimaging research that includes:
- cerebral blood flow (CBF)
- position emission tomography (PET)
- single photon emission computed tomography (SPECT).
Continue to: These functional neuroimaging...
These functional neuroimaging methods (using ionizing radiation) have enabled clinicians to see abnormal blood flow patterns in the brains of living patients. One of the earliest findings was hypofrontality in patients with schizophrenia, implicating frontal pathology in this severe brain disorder. PET was also used for dopamine and serotonin receptor imaging.
Computerized axia tomography. Compared with skull X-rays, CT (“CAT”) scans provided a more detailed view of brain tissue, and began a structural neuroimaging revolution that enriched psychiatric research, but also was applied to organs other than the brain.
Magnetic resonance imaging (MRI) became the “big kahuna” of neuroimaging when arrived in the early 1980s and quickly supplanted CT research because it is safer (no ionizing radiation, and it can be repeated multiple times with or without tasks). It also provided exquisite neuroanatomical details of brain tissue with stunning fidelity. Subsequently, several MRI techniques/software programs were developed that advanced research in psychiatry to multiple new frontiers, including:
- Morphological neuroimaging with MRI
- Magnetic resonance spectroscopy (MRS), which acts like a living, noninvasive biopsy of several chemicals (such as choline, lactate, glutamine, adenosine triphosphate, and the neuronal marker N-acetylcysteine) in a small volume (≤1 cc) of neural tissue in various regions
- Functional MRI (fMRI), which measures blood flow changes during actual or imagined tasks in the brains of patients vs healthy controls
- Diffusion tensor imaging (DTI), which evaluates the integrity of white matter (60% of brain volume, including 137,000 miles of myelinated fibers) by measuring the flow of water inside myelinated fibers (anisotropy and diffusivity). DTI of the corpus callosum, the largest brain commissure that is comprised of 200 million interhemispheric fibers, has revealed many abnormalities. This was one of the structures I investigated during my fellowship, including a histopathological study.1
All 4 of these neuroimaging techniques continue to generate a wealth of data about brain structure and function in psychosis, mood disorders, anxiety disorders, borderline personality disorder, obsessive-compulsive disorder, eating disorders, and substance use disorders. All these discoveries were utterly impossible to predict during my residency. I am proud to have published the first reports in the literature of ventricular enlargement in patients with bipolar disorder,2 cortical atrophy in schizophrenia and mania,3 reductions of hippocampal volume in patients with schizophrenia using MRS,4 and progressive brain atrophy in patients with schizophrenia.5 It is especially gratifying that I played a small role in translating my science fiction fantasies into clinical reality!
Other breakthrough methodologies that are advancing psychiatric neuroscience today but were science fiction during my residency days include:
- Pluripotent stem cells, which enable the de-differentiation of adult skin cells and then re-differentiating them into any type of cell, including neurons. This allows researchers to conduct studies on any patient’s brain cells without needing to do an invasive, high-risk brain biopsy. As a young resident, I would never have predicted that this virtual brain biopsy would be possible!
- Optogenetics, which enables controlling cell behavior using light and genetically encoded light-sensitive proteins. This triggered a cornucopia of neuroscience discoveries by using optogenetics to modulate cell-signaling cascades to understand cellular biology. Halorhodopsin and bacteriorhodopsin are used as tools to turn neurons off or on rapidly and safely.
- Genome-wide association studies (GWAS) have revolutionized the field of molecular neurogenetics and are enabling clinicians to detect risk genes by comparing the DNA samples of thousands of psychiatric patients with thousands of healthy controls. This is how several hundred risk genes have been identified for schizophrenia, bipolar disorder, autism spectrum disorder, and more to come.
- Clustered regularly interspaced short palindromic repeats (CRISPR) is a remarkable genetic “scissors” (that earned its inventors the 2020 Nobel Prize) that allows splicing out a disease gene and splicing in a normal gene. This will have an enormous future application in preventing an adulthood illness at its roots during fetal life. The future medical implications for psychiatric disorders are prodigious!
Continue to: Clinical advances
Clinical advances. Many therapies or approaches that did not exist during my residency (and how I dreamed about them back then!) are available to today’s clinicians. These include:
- Rapid-acting antidepressants that reverse severe and chronic depression and suicidal urges within a few hours or a couple of days. As a resident, I waited for weeks or months to see patients with depression reach the full remission that is now achieved practically the same day with IV ketamine, intranasal esketamine, IV scopolamine, and inhalable nitrous oxide. During my residency, the closest thing we had to a rapid-acting treatment for depression was electroconvulsive therapy (ECT), but that usually took 2 to 3 weeks. Psychiatric clinicians should never cease to appreciate how an intractable, treatment-refractory depression can rapidly be turned off like a light switch, restoring normal mood to desperately ill persons.
- Neuromodulation techniques are flourishing. Beyond ECT, transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), transcranial direct current stimulation (tDCS), deep brain stimulation (DBS), low field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near-infrared radiation (NIR), and focused ultrasound (FUS) are approved or under development, offering millions of patients with various neuropsychiatric disorders potential recovery not with pharmacotherapy, but via a brain-targeted approach.
- Telepsychiatry. Now taken for granted during the COVID-19 pandemic, telepsychiatry was completely unimaginable during my residency. Yes, we had phones, but not smartphones! The only “zoom” we knew was the furious sound of a sports car engine! To be able to see and evaluate a patient literally anywhere in the world was science fiction personified! Increased remote access to psychiatric care by patients everywhere is a truly remarkable advance that helped avoid a disastrous lack of psychiatric treatment during the current pandemic that brought in-person interactions between psychiatric physicians and their patients to a screeching halt.
- Neurobiologic effects of psychotherapy. Viewing psychotherapy as a neurobiologic treatment was totally unknown and unimaginable during my residency. I was heavily trained in various types of psychotherapies, but not once did any of my supervisors mention experiential neuroplasticity as a brain-altering process, or that psychotherapy changes brain structure, induces experimental neuroplasticity, and induces billions of dendritic spines in patients’ cortex and limbic structures, helping them connect the dots and develop new insights. No one knew that psychotherapy can mimic the neural effects of pharmacotherapy.
- Immunomodulatory effects of psychotherapy. It was completely unknown that psychotherapies such as cognitive-behavioral therapy can lower levels of inflammatory biomarkers in patients’ CSF and serum. Back then, no one imagined that psychotherapy had immunomodulatory effects. These discoveries are revolutionary for us psychiatrists and confirm the neurobiologic mechanisms of psychotherapy for every patient we treat.
- Epigenetics. This was rarely, if ever, mentioned when I was a resident. We knew from clinical studies that children who were abused or neglected often develop severe mood or psychotic disorders in adulthood. But we did not know that trauma modifies some genes via under- or overexpression, and that such epigenetic changes alter brain development towards psychopathology. The mysteries of psychiatric brain disorders generated by childhood trauma have been clarified by advances in epigenetics.
Aspirational, futuristic therapies. Even now, as a seasoned psychiatric neuroscientist, I continue to dream. Research is providing many clues for potentially radical psychiatric treatments that go beyond standard antipsychotics, antidepressants, mood stabilizers, or anxiolytics. But today, I fully expect that scientific dreams eventually come true through research. For example, the following neuroscientific therapeutics strategies may someday become routine in clinical practice:
- microglia inhibition
- mitochondria repair
- anti-apoptotic therapy
- white matter connectivity restoration
- neuroprotection (enhancing neurogenesis, increasing neurotropic factors, and enhancing synaptogenesis)
- reverse glutamate N-methyl-
d -aspartate hypofunction - prevent amyloid formation.
Data analysis breakthroughs. Side-by-side with the explosion of new findings and amassing mountains of data in psychiatric neuroscience, unprecedented and revolutionary data-management techniques have emerged to facilitate the herculean task of data analysis to extract the mythical needle in a haystack and derive the overall impact of masses of data. These techniques, whose names were not in our vocabulary during my residency days, include:
- machine learning
- artificial intelligence
- deep learning
- big data.
With the help of powerful computers and ingenious software, discovering critical nuggets of knowledge about the brain and predicting the best approaches to healing dysfunctional brains are now possible. Those powerful methods of analyzing massive data are the vehicles for transforming science fiction to reality by assembling the jigsaw puzzle(s) of the human brain, arguably the last frontier in medical science.
My life experiences as a psychiatric neuroscientist have convinced me that nothing is beyond the reach of scientific research. Unraveling the divine brain’s complexities will eventually become reality. So, let us never stop dreaming and fantasizing!
1. Nasrallah HA, McCalley-Whitters M, Bigelow LB, et al. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res. 1983;8(4):251-260.
2. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affect Disord. 1982;4(1):15-19.
3. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cortical atrophy in schizophrenia and mania: a comparative CT study. J Clin Psychiatry. 1982;43(11):439-441.
4. Nasrallah HA, Skinner TE, Schmalbrock P, et al. Proton magnetic resonance spectroscopy (1H MRS) of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry. 1994;165(4):481-485.
5. Nasrallah HA, Olson SC, McCalley-Whitters M, et al. Cerebral ventricular enlargement in schizophrenia. A preliminary follow-up study. Arch Gen Psychiatry. 1986;43(2):157-159.
1. Nasrallah HA, McCalley-Whitters M, Bigelow LB, et al. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res. 1983;8(4):251-260.
2. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affect Disord. 1982;4(1):15-19.
3. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cortical atrophy in schizophrenia and mania: a comparative CT study. J Clin Psychiatry. 1982;43(11):439-441.
4. Nasrallah HA, Skinner TE, Schmalbrock P, et al. Proton magnetic resonance spectroscopy (1H MRS) of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry. 1994;165(4):481-485.
5. Nasrallah HA, Olson SC, McCalley-Whitters M, et al. Cerebral ventricular enlargement in schizophrenia. A preliminary follow-up study. Arch Gen Psychiatry. 1986;43(2):157-159.
Treatment resistance is a myth!
For millennia, serious psychiatric brain disorders (aka mental illnesses, melancholia, madness, insanity) were written off as incurable, permanent afflictions. It’s no wonder that they were engulfed with the stigma of hopelessness.
But then came the era of serendipitous discoveries in the mid-20th century, with the felicitous arrival of antipsychotics, antidepressants, and lithium. The dogma of untreatability was shattered, but in its wake, the notion of treatment resistance emerged, and promptly became the bane of psychiatric clinicians and the practice of psychopharmacology.
Many patients with mood and psychotic disorders responded to the medications that were introduced in the 1950s and 1960s, but some either derived partial benefit or did not improve at all. These partial or poor responders were labeled “treatment-resistant,” and caring for them became a major challenge for psychiatric physicians that continues to this day. However, rapid advances in understanding the many etiologies and subtypes of the heterogeneous mood and psychotic disorders are invalidating the notion of treatment resistance, showing it is a fallacy and a misnomer. Let’s examine why.
Treatment-resistant depression (TRD)
Psychiatric clinics and hospitals are clogged with patients who do not respond to ≥2 evidence-based antidepressants and carry the disparaging label of “TRD.” But a patient manifesting what appears to be major depressive disorder (MDD) may actually have one of several types of depression that are unlikely to respond to an antidepressant, including:
- iatrogenic depression due to a prescription medication
- depression secondary to recreational drug use
- depressive symptoms secondary to a general medical condition
- bipolar depression.
Thus, a significant proportion of patients diagnosed with MDD are labeled TRD because they do not respond to standard antidepressants, when in fact they have been misdiagnosed and need a different treatment.
Even when the diagnosis of MDD is accurate, psychiatric neuroscience advances have informed us that MDD is a heterogeneous syndrome with multiple “biotypes” that share a similar phenotype.1,2 In the past, TRD has been defined as a failure to respond to ≥2 adequate trials (8 to 12 weeks at a maximum tolerated dose) of antidepressants from different classes (such as tricyclic or heterocyclic antidepressants, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors). For decades, patients with TRD have been referred to electroconvulsive therapy (ECT), and have experienced an excellent response rate. So TRD is in fact an artificial concept and term, applied to a subtype of MDD that does not respond to standard antidepressants, but often responds very well to neurostimulation (ECT and transcranial magnetic stimulation [TMS]).
When an antidepressant is approved by the FDA based on “successful” placebo-controlled double-blind trials, there is always a subset of patients who do not respond. However, the success of a controlled clinical trial is based on a decline in overall mean depression rating scale score in the antidepressant group compared with the placebo group. Not a single antidepressant has ever exerted full efficacy in 100% of patients who received it in an FDA trial because the sample is always a heterogeneous mix of patients with various depression biotypes who meet the DSM clinical diagnosis of MDD. Most often, only approximately 50% do, which is enough to be statistically significantly better than the roughly 30% response rate in the placebo group. It is impossible for a heterogeneous syndrome comprised of biologically different “diseases” to respond to any single medication! Patients who do not respond to an antidepressant medication that works in other patients represent a different subtype of depression that is not TRD. Biotypes of the depression syndrome have different neurochemical underpinnings and may respond to different mechanisms of therapeutic action, yet to be discovered.
Continue to: A very common...
A very common clinical mistake occurs when patients with bipolar depression are misdiagnosed as having MDD because most of them experience depression as their initial mood episode. These patients often end up being classified as having TRD because bipolar depression very frequently fails to respond to several of the antidepressants that are FDA-approved for MDD. When these patients are correctly diagnosed, many will respond to one of the medications specifically approved for bipolar depression that were launched over the past 15 years (quetiapine, lurasidone, and cariprazine). However, bipolar disorder is also a heterogeneous spectrum, and some patients with bipolar depression may fail to respond to any of these 3 medications and are promptly regarded as TRD. Such patients often respond to neuromodulation (TMS, ECT, or vagus nerve stimulation [VNS]), indicating that they may have a different type of bipolar depression, such as bipolar type II.
A more recent example of the falsehood of TRD as a spurious diagnosis is the dramatic and rapid response of patients who are chronically depressed (both those with MDD and those with bipolar depression) to ketamine infusions.3,4 Responders to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, prove that nonresponders to monoamine reuptake inhibitors must not be falsely labeled as having TRD. They have a different subtype within the depression syndrome that is mediated by glutamatergic pathways, instead of monoamines such as serotonin, norepinephrine, or dopamine. In addition, unlike monoaminergic antidepressants, NMDA antagonists rapidly reverse suicidal urges, above and beyond rapidly reversing chronic, so-called TRD.
In the same vein, numerous reports have shown that buprenorphine has significant efficacy in TRD (and suicide urges, as does ketamine), which implicates opioid pathways as mediating some subtypes of TRD.5 The monoamine model of depression, which dominated the field and dragged on for half a century, has distracted psychiatric researchers from exploring and recognizing the multiple neurochemical and neuroplastic pathways of the depression syndrome, thus falsely assuming that depression is a monolithic disorder that responds to elevating the activity of brain monoamines. This major blind spot led to the ersatz concept of TRD.
Treatment-resistant schizophrenia (TRS)
Since the discovery of chlorpromazine and other antipsychotics in the 1950s, it became apparent that a subset of patients with schizophrenia do not respond to medications that block dopamine D2 receptors. Partial responders were labeled as having TRS, and complete nonresponse was called refractory schizophrenia. Many patients with severe and persistent delusions and hallucinations were permanently hospitalized, and unable to live in the community like those who responded to dopamine antagonism.
In the late 1980s, the discovery that clozapine has significant efficacy in TRS and refractory schizophrenia provided the first insight that TRS and refractory schizophrenia represent different neurobiologic subtypes of schizophrenia.6,7 The extensive heterogeneity of schizophrenia (with hundreds of genetic and nongenetic etiologies) is now widely accepted.8 Patients with schizophrenia who do not respond to dopamine receptor antagonism should not be labeled TRS, because they can respond to a different antipsychotic agent, such as clozapine, which is believed to exert its efficacy via glutamate pathways.
Continue to: But what about the 50%...
But what about the 50% of patients with TRS or refractory schizophrenia who do not respond to clozapine?9 They do not have TRS, either, but represent different schizophrenia biotypes that may respond to other medications with different mechanisms of action, such as lamotrigine,10 which is a glutamate modulator; pimavanserin,11 which is an inverse agonist of the serotonin 5HT-2A receptor; allopurinol,12,13 an adenosine modulator; or estrogen,14 a neurosteroid. Future research will continue to unravel the many biotypes of the highly heterogeneous schizophrenia syndrome that are “nondopaminergic” and do not respond to the standard class of dopamine antagonists (previously called neuroleptics and now known as antipsychotics).15 Future treatments for schizophrenia may depart from modulating various neurotransmitter receptors to targeting entirely different neurobiologic processes, such as correcting mitochondria pathology, inhibiting microglia activation, repairing white matter, reversing apoptosis pathways, inducing neuroplasticity, arresting oxidative stress and inflammation, and other neuroprotective mechanisms.
The rapid growth of biomarkers in psychiatry16 will usher in an era of precision psychiatry17 that will eliminate the term “treatment resistance.” Our psychiatric practice will then benefit from “canceling” this demoralizing and clinically unjustified term that has needlessly fostered therapeutic nihilism among psychiatric physicians.
1. Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolism depression. Biol Psychiatry. 2020;88(5):369-380.
2. Akiskal HS, McKinney WT Jr. Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975;32(3):285-305.
3. Zarate CA Jr. Ketamine: a new chapter in antidepressant development. Brazilian J Psychiatry. 2020;42(6):581-582.
4. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
5. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8):2410.
6. Potkin SG, Kane JM, Correll CU, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1.
7. Campana M, Falkai P, Siskind D, et al. Characteristics and definitions of ultra-treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
8. Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in treatment-resistant schizophrenia (TRS). Front Psychiatry. 2019;9:757.
9. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777.
10. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
11. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
12. Linden N, Onwuanibe A, Sandson N. Rapid resolution of psychotic symptoms in a patient with schizophrenia using allopurinol as an adjuvant: a case report. Clin Schizophr Relat Psychoses. 2014;7(4):231-234.
13 Lintunen J, Lähteenvuo M, Tiihonen J, et al. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021;7(1):1.
14. Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002
15. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present and future. Schizophr Res. 2010;122(1-3):1-23.
16. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi: 10.1016/j.bionps.2019.100001
17. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
For millennia, serious psychiatric brain disorders (aka mental illnesses, melancholia, madness, insanity) were written off as incurable, permanent afflictions. It’s no wonder that they were engulfed with the stigma of hopelessness.
But then came the era of serendipitous discoveries in the mid-20th century, with the felicitous arrival of antipsychotics, antidepressants, and lithium. The dogma of untreatability was shattered, but in its wake, the notion of treatment resistance emerged, and promptly became the bane of psychiatric clinicians and the practice of psychopharmacology.
Many patients with mood and psychotic disorders responded to the medications that were introduced in the 1950s and 1960s, but some either derived partial benefit or did not improve at all. These partial or poor responders were labeled “treatment-resistant,” and caring for them became a major challenge for psychiatric physicians that continues to this day. However, rapid advances in understanding the many etiologies and subtypes of the heterogeneous mood and psychotic disorders are invalidating the notion of treatment resistance, showing it is a fallacy and a misnomer. Let’s examine why.
Treatment-resistant depression (TRD)
Psychiatric clinics and hospitals are clogged with patients who do not respond to ≥2 evidence-based antidepressants and carry the disparaging label of “TRD.” But a patient manifesting what appears to be major depressive disorder (MDD) may actually have one of several types of depression that are unlikely to respond to an antidepressant, including:
- iatrogenic depression due to a prescription medication
- depression secondary to recreational drug use
- depressive symptoms secondary to a general medical condition
- bipolar depression.
Thus, a significant proportion of patients diagnosed with MDD are labeled TRD because they do not respond to standard antidepressants, when in fact they have been misdiagnosed and need a different treatment.
Even when the diagnosis of MDD is accurate, psychiatric neuroscience advances have informed us that MDD is a heterogeneous syndrome with multiple “biotypes” that share a similar phenotype.1,2 In the past, TRD has been defined as a failure to respond to ≥2 adequate trials (8 to 12 weeks at a maximum tolerated dose) of antidepressants from different classes (such as tricyclic or heterocyclic antidepressants, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors). For decades, patients with TRD have been referred to electroconvulsive therapy (ECT), and have experienced an excellent response rate. So TRD is in fact an artificial concept and term, applied to a subtype of MDD that does not respond to standard antidepressants, but often responds very well to neurostimulation (ECT and transcranial magnetic stimulation [TMS]).
When an antidepressant is approved by the FDA based on “successful” placebo-controlled double-blind trials, there is always a subset of patients who do not respond. However, the success of a controlled clinical trial is based on a decline in overall mean depression rating scale score in the antidepressant group compared with the placebo group. Not a single antidepressant has ever exerted full efficacy in 100% of patients who received it in an FDA trial because the sample is always a heterogeneous mix of patients with various depression biotypes who meet the DSM clinical diagnosis of MDD. Most often, only approximately 50% do, which is enough to be statistically significantly better than the roughly 30% response rate in the placebo group. It is impossible for a heterogeneous syndrome comprised of biologically different “diseases” to respond to any single medication! Patients who do not respond to an antidepressant medication that works in other patients represent a different subtype of depression that is not TRD. Biotypes of the depression syndrome have different neurochemical underpinnings and may respond to different mechanisms of therapeutic action, yet to be discovered.
Continue to: A very common...
A very common clinical mistake occurs when patients with bipolar depression are misdiagnosed as having MDD because most of them experience depression as their initial mood episode. These patients often end up being classified as having TRD because bipolar depression very frequently fails to respond to several of the antidepressants that are FDA-approved for MDD. When these patients are correctly diagnosed, many will respond to one of the medications specifically approved for bipolar depression that were launched over the past 15 years (quetiapine, lurasidone, and cariprazine). However, bipolar disorder is also a heterogeneous spectrum, and some patients with bipolar depression may fail to respond to any of these 3 medications and are promptly regarded as TRD. Such patients often respond to neuromodulation (TMS, ECT, or vagus nerve stimulation [VNS]), indicating that they may have a different type of bipolar depression, such as bipolar type II.
A more recent example of the falsehood of TRD as a spurious diagnosis is the dramatic and rapid response of patients who are chronically depressed (both those with MDD and those with bipolar depression) to ketamine infusions.3,4 Responders to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, prove that nonresponders to monoamine reuptake inhibitors must not be falsely labeled as having TRD. They have a different subtype within the depression syndrome that is mediated by glutamatergic pathways, instead of monoamines such as serotonin, norepinephrine, or dopamine. In addition, unlike monoaminergic antidepressants, NMDA antagonists rapidly reverse suicidal urges, above and beyond rapidly reversing chronic, so-called TRD.
In the same vein, numerous reports have shown that buprenorphine has significant efficacy in TRD (and suicide urges, as does ketamine), which implicates opioid pathways as mediating some subtypes of TRD.5 The monoamine model of depression, which dominated the field and dragged on for half a century, has distracted psychiatric researchers from exploring and recognizing the multiple neurochemical and neuroplastic pathways of the depression syndrome, thus falsely assuming that depression is a monolithic disorder that responds to elevating the activity of brain monoamines. This major blind spot led to the ersatz concept of TRD.
Treatment-resistant schizophrenia (TRS)
Since the discovery of chlorpromazine and other antipsychotics in the 1950s, it became apparent that a subset of patients with schizophrenia do not respond to medications that block dopamine D2 receptors. Partial responders were labeled as having TRS, and complete nonresponse was called refractory schizophrenia. Many patients with severe and persistent delusions and hallucinations were permanently hospitalized, and unable to live in the community like those who responded to dopamine antagonism.
In the late 1980s, the discovery that clozapine has significant efficacy in TRS and refractory schizophrenia provided the first insight that TRS and refractory schizophrenia represent different neurobiologic subtypes of schizophrenia.6,7 The extensive heterogeneity of schizophrenia (with hundreds of genetic and nongenetic etiologies) is now widely accepted.8 Patients with schizophrenia who do not respond to dopamine receptor antagonism should not be labeled TRS, because they can respond to a different antipsychotic agent, such as clozapine, which is believed to exert its efficacy via glutamate pathways.
Continue to: But what about the 50%...
But what about the 50% of patients with TRS or refractory schizophrenia who do not respond to clozapine?9 They do not have TRS, either, but represent different schizophrenia biotypes that may respond to other medications with different mechanisms of action, such as lamotrigine,10 which is a glutamate modulator; pimavanserin,11 which is an inverse agonist of the serotonin 5HT-2A receptor; allopurinol,12,13 an adenosine modulator; or estrogen,14 a neurosteroid. Future research will continue to unravel the many biotypes of the highly heterogeneous schizophrenia syndrome that are “nondopaminergic” and do not respond to the standard class of dopamine antagonists (previously called neuroleptics and now known as antipsychotics).15 Future treatments for schizophrenia may depart from modulating various neurotransmitter receptors to targeting entirely different neurobiologic processes, such as correcting mitochondria pathology, inhibiting microglia activation, repairing white matter, reversing apoptosis pathways, inducing neuroplasticity, arresting oxidative stress and inflammation, and other neuroprotective mechanisms.
The rapid growth of biomarkers in psychiatry16 will usher in an era of precision psychiatry17 that will eliminate the term “treatment resistance.” Our psychiatric practice will then benefit from “canceling” this demoralizing and clinically unjustified term that has needlessly fostered therapeutic nihilism among psychiatric physicians.
For millennia, serious psychiatric brain disorders (aka mental illnesses, melancholia, madness, insanity) were written off as incurable, permanent afflictions. It’s no wonder that they were engulfed with the stigma of hopelessness.
But then came the era of serendipitous discoveries in the mid-20th century, with the felicitous arrival of antipsychotics, antidepressants, and lithium. The dogma of untreatability was shattered, but in its wake, the notion of treatment resistance emerged, and promptly became the bane of psychiatric clinicians and the practice of psychopharmacology.
Many patients with mood and psychotic disorders responded to the medications that were introduced in the 1950s and 1960s, but some either derived partial benefit or did not improve at all. These partial or poor responders were labeled “treatment-resistant,” and caring for them became a major challenge for psychiatric physicians that continues to this day. However, rapid advances in understanding the many etiologies and subtypes of the heterogeneous mood and psychotic disorders are invalidating the notion of treatment resistance, showing it is a fallacy and a misnomer. Let’s examine why.
Treatment-resistant depression (TRD)
Psychiatric clinics and hospitals are clogged with patients who do not respond to ≥2 evidence-based antidepressants and carry the disparaging label of “TRD.” But a patient manifesting what appears to be major depressive disorder (MDD) may actually have one of several types of depression that are unlikely to respond to an antidepressant, including:
- iatrogenic depression due to a prescription medication
- depression secondary to recreational drug use
- depressive symptoms secondary to a general medical condition
- bipolar depression.
Thus, a significant proportion of patients diagnosed with MDD are labeled TRD because they do not respond to standard antidepressants, when in fact they have been misdiagnosed and need a different treatment.
Even when the diagnosis of MDD is accurate, psychiatric neuroscience advances have informed us that MDD is a heterogeneous syndrome with multiple “biotypes” that share a similar phenotype.1,2 In the past, TRD has been defined as a failure to respond to ≥2 adequate trials (8 to 12 weeks at a maximum tolerated dose) of antidepressants from different classes (such as tricyclic or heterocyclic antidepressants, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors). For decades, patients with TRD have been referred to electroconvulsive therapy (ECT), and have experienced an excellent response rate. So TRD is in fact an artificial concept and term, applied to a subtype of MDD that does not respond to standard antidepressants, but often responds very well to neurostimulation (ECT and transcranial magnetic stimulation [TMS]).
When an antidepressant is approved by the FDA based on “successful” placebo-controlled double-blind trials, there is always a subset of patients who do not respond. However, the success of a controlled clinical trial is based on a decline in overall mean depression rating scale score in the antidepressant group compared with the placebo group. Not a single antidepressant has ever exerted full efficacy in 100% of patients who received it in an FDA trial because the sample is always a heterogeneous mix of patients with various depression biotypes who meet the DSM clinical diagnosis of MDD. Most often, only approximately 50% do, which is enough to be statistically significantly better than the roughly 30% response rate in the placebo group. It is impossible for a heterogeneous syndrome comprised of biologically different “diseases” to respond to any single medication! Patients who do not respond to an antidepressant medication that works in other patients represent a different subtype of depression that is not TRD. Biotypes of the depression syndrome have different neurochemical underpinnings and may respond to different mechanisms of therapeutic action, yet to be discovered.
Continue to: A very common...
A very common clinical mistake occurs when patients with bipolar depression are misdiagnosed as having MDD because most of them experience depression as their initial mood episode. These patients often end up being classified as having TRD because bipolar depression very frequently fails to respond to several of the antidepressants that are FDA-approved for MDD. When these patients are correctly diagnosed, many will respond to one of the medications specifically approved for bipolar depression that were launched over the past 15 years (quetiapine, lurasidone, and cariprazine). However, bipolar disorder is also a heterogeneous spectrum, and some patients with bipolar depression may fail to respond to any of these 3 medications and are promptly regarded as TRD. Such patients often respond to neuromodulation (TMS, ECT, or vagus nerve stimulation [VNS]), indicating that they may have a different type of bipolar depression, such as bipolar type II.
A more recent example of the falsehood of TRD as a spurious diagnosis is the dramatic and rapid response of patients who are chronically depressed (both those with MDD and those with bipolar depression) to ketamine infusions.3,4 Responders to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, prove that nonresponders to monoamine reuptake inhibitors must not be falsely labeled as having TRD. They have a different subtype within the depression syndrome that is mediated by glutamatergic pathways, instead of monoamines such as serotonin, norepinephrine, or dopamine. In addition, unlike monoaminergic antidepressants, NMDA antagonists rapidly reverse suicidal urges, above and beyond rapidly reversing chronic, so-called TRD.
In the same vein, numerous reports have shown that buprenorphine has significant efficacy in TRD (and suicide urges, as does ketamine), which implicates opioid pathways as mediating some subtypes of TRD.5 The monoamine model of depression, which dominated the field and dragged on for half a century, has distracted psychiatric researchers from exploring and recognizing the multiple neurochemical and neuroplastic pathways of the depression syndrome, thus falsely assuming that depression is a monolithic disorder that responds to elevating the activity of brain monoamines. This major blind spot led to the ersatz concept of TRD.
Treatment-resistant schizophrenia (TRS)
Since the discovery of chlorpromazine and other antipsychotics in the 1950s, it became apparent that a subset of patients with schizophrenia do not respond to medications that block dopamine D2 receptors. Partial responders were labeled as having TRS, and complete nonresponse was called refractory schizophrenia. Many patients with severe and persistent delusions and hallucinations were permanently hospitalized, and unable to live in the community like those who responded to dopamine antagonism.
In the late 1980s, the discovery that clozapine has significant efficacy in TRS and refractory schizophrenia provided the first insight that TRS and refractory schizophrenia represent different neurobiologic subtypes of schizophrenia.6,7 The extensive heterogeneity of schizophrenia (with hundreds of genetic and nongenetic etiologies) is now widely accepted.8 Patients with schizophrenia who do not respond to dopamine receptor antagonism should not be labeled TRS, because they can respond to a different antipsychotic agent, such as clozapine, which is believed to exert its efficacy via glutamate pathways.
Continue to: But what about the 50%...
But what about the 50% of patients with TRS or refractory schizophrenia who do not respond to clozapine?9 They do not have TRS, either, but represent different schizophrenia biotypes that may respond to other medications with different mechanisms of action, such as lamotrigine,10 which is a glutamate modulator; pimavanserin,11 which is an inverse agonist of the serotonin 5HT-2A receptor; allopurinol,12,13 an adenosine modulator; or estrogen,14 a neurosteroid. Future research will continue to unravel the many biotypes of the highly heterogeneous schizophrenia syndrome that are “nondopaminergic” and do not respond to the standard class of dopamine antagonists (previously called neuroleptics and now known as antipsychotics).15 Future treatments for schizophrenia may depart from modulating various neurotransmitter receptors to targeting entirely different neurobiologic processes, such as correcting mitochondria pathology, inhibiting microglia activation, repairing white matter, reversing apoptosis pathways, inducing neuroplasticity, arresting oxidative stress and inflammation, and other neuroprotective mechanisms.
The rapid growth of biomarkers in psychiatry16 will usher in an era of precision psychiatry17 that will eliminate the term “treatment resistance.” Our psychiatric practice will then benefit from “canceling” this demoralizing and clinically unjustified term that has needlessly fostered therapeutic nihilism among psychiatric physicians.
1. Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolism depression. Biol Psychiatry. 2020;88(5):369-380.
2. Akiskal HS, McKinney WT Jr. Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975;32(3):285-305.
3. Zarate CA Jr. Ketamine: a new chapter in antidepressant development. Brazilian J Psychiatry. 2020;42(6):581-582.
4. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
5. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8):2410.
6. Potkin SG, Kane JM, Correll CU, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1.
7. Campana M, Falkai P, Siskind D, et al. Characteristics and definitions of ultra-treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
8. Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in treatment-resistant schizophrenia (TRS). Front Psychiatry. 2019;9:757.
9. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777.
10. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
11. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
12. Linden N, Onwuanibe A, Sandson N. Rapid resolution of psychotic symptoms in a patient with schizophrenia using allopurinol as an adjuvant: a case report. Clin Schizophr Relat Psychoses. 2014;7(4):231-234.
13 Lintunen J, Lähteenvuo M, Tiihonen J, et al. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021;7(1):1.
14. Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002
15. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present and future. Schizophr Res. 2010;122(1-3):1-23.
16. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi: 10.1016/j.bionps.2019.100001
17. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
1. Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolism depression. Biol Psychiatry. 2020;88(5):369-380.
2. Akiskal HS, McKinney WT Jr. Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975;32(3):285-305.
3. Zarate CA Jr. Ketamine: a new chapter in antidepressant development. Brazilian J Psychiatry. 2020;42(6):581-582.
4. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
5. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8):2410.
6. Potkin SG, Kane JM, Correll CU, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1.
7. Campana M, Falkai P, Siskind D, et al. Characteristics and definitions of ultra-treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
8. Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in treatment-resistant schizophrenia (TRS). Front Psychiatry. 2019;9:757.
9. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777.
10. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
11. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
12. Linden N, Onwuanibe A, Sandson N. Rapid resolution of psychotic symptoms in a patient with schizophrenia using allopurinol as an adjuvant: a case report. Clin Schizophr Relat Psychoses. 2014;7(4):231-234.
13 Lintunen J, Lähteenvuo M, Tiihonen J, et al. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021;7(1):1.
14. Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002
15. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present and future. Schizophr Res. 2010;122(1-3):1-23.
16. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi: 10.1016/j.bionps.2019.100001
17. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.