User login
Proximal Periprosthetic Femur Fractures: Strategies for Internal Fixation
The rate of total hip arthroplasty (THA) is rising and demand is expected to increase by 174% to 572,000 by 2030.1 The rate of periprosthetic fracture around primary THA is frequently reported at around 1%,2-4 though a recent study of over 32,000 THAs quotes the 20-year probability of periprosthetic fracture at 3.5%.5 Revision THA is also increasing in frequency and associated rates of periprosthetic fracture range from 1.5% to 7.8% following revision THA,3,4,6 with the probability of fracture at 20 years of 11%.7 Projection models predict that the number of periprosthetic fractures will rise by 4.6% per decade over the next 30 years.8
Broadly, treatment options include open reduction internal fixation (ORIF), revision THA, and combined approaches. The Vancouver classification, based on fracture location, stem stability, and bone loss, is often used to guide fracture treatment, with stable implants treated with ORIF and unstable implants requiring revision arthroplasty.
Fixation strategies for treatment of periprosthetic fracture around a well-fixed arthroplasty stem have evolved over time, and there continue to be a variety of available internal fixation options with no clear consensus on the optimal strategy.9 Rates of reoperation following ORIF of periprosthetic femur fracture are reported from 13% to 23%,8,10-12 confirming that there remains room for improvement in management of these injuries.
Locking Plate Fixation
Early fixation strategies included allograft and cables alone as well as nonlocked plate and cerclage constructs. In response to the complication and reoperation rate for nonlocked plate constructs, reported at 33%,13 locking plates were introduced as a treatment option, allowing for both improved osseous vascularity and added screw options.14 When compared to the traditional nonlocked Ogden construct, locking plate constructs are more resistant to axial and torsional load.15 Clinically, the relative risk of nonunion after nonlocking plate fixation is reported at 11.9 times that of fixation with locking plate technology.16
Successful use of lateral locking plate fixation for treatment of this injury has been reported on in several clinical series.17-20 Froberg and colleagues12 evaluated 60 Vancouver B1 and C fractures treated by locking plate osteosynthesis and reported no nonunions, an improvement from previous constructs. However, 8 out of 60 patients with 2-year follow-up required reoperation—4 for infection, 3 for refracture, and 1 for stem loosening—making it clear that the locking plate alone was not a panacea.
With locking plate fixation a mainstay of modern treatment of periprosthetic femur fractures, many questions still remain.
Proximal Fixation
Even with the introduction of locked plates, treatment success after ORIF of Vancouver B1 fractures relies on adequate proximal fixation. Options for proximal fixation around the stem include cerclage wires or cables, unicortical locked screws, obliquely directed bicortical screws, and use of the locking attachment plate to insert bicortical locked screws. These strategies can be used in the presence of cemented or uncemented stems, with biomechanical evidence that screw fixation through the cement mantle does not cause failure.21
Several biomechanical studies address the stiffness and strength of varying proximal fixation strategies. While early fixation relied heavily on cables, the use of cables alone as proximal fixation has been linked to significantly higher rates of failure when compared to other constructs in a large clinical series.11 Multiple biomechanical studies have shown that newer methods of proximal fixation provide more rigid constructs.22,23
Unicortical locked screws appear to outperform cables biomechanically. The use of unicortical screws in lieu of or in addition to cables provides added resistance to lateral bending as well as torsion when compared to cables alone.24 A second group found that unicortical locked screws alone were superior to combined fixation with cerclage wires and unicortical locked screws.25
Added stability can be demonstrated by bicortical fixation strategies, which offer increased rigidity when compared to cables or unicortical screws.22 In vitro work has shown enhanced fixation stability with bicortical screw fixation using the locking attachment plate when compared to cerclage wires alone.23,26 Clinically, some authors have demonstrated success with the use of reversed distal femoral locking plates in order to enhance proximal locking options and allow for bicortical fixation around the stem.19 As noted above, the data favor the opinion that clinical failure rates with cerclage wires alone are high, and biomechanically, bicortical fixation around the femoral stem appears to be superior to unicortical locked screw fixation or cerclage wires. If rigid proximal fixation is desired, an effort should be made to obtain bicortical fixation around the femoral stem.
Allograft
Allograft strut, either alone or in addition to plate osteosynthesis, has long been used in treatment of periprosthetic fractures. Proponents of this technique cite improved biomechanical stability17 and allograft incorporation resulting in restoration of bone stock.
Early treatment of periprosthetic femur fractures consisted solely of allograft and cable fixation, but data on the technique is limited. A small series reported reasonable success, with only 2 out of 19 patients developing nonunion.27 More recently Haddad and colleagues28 reported malunions in 3 out of 19 patients treated with allograft and cables alone. Allograft alone has been largely abandoned in favor of plate fixation, and biomechanical evidence shows that plate and screw or cerclage constructs are more resistant to torsion and lateral bending than allograft with cables alone.29
However, the role of allograft in treatment of periprosthetic femur fractures is not clearly defined. Some authors advocate routinely supplementing plate fixation with allograft28,30 and others go as far as to suggest superior union rates of strut allograft augmented plate fixation when compared to plate fixation alone for periprosthetic fractures around a stable femoral stem.31 However, in that series, the failure rate of 5/11 patients treated with plate alone is higher than current series,12 and others have demonstrated good success without allograft, even with nonlocked plates.32
As recently as 2016, a lateral locking plate supplemented with allograft has been described as a successful technique, with no nonunions reported in a small series.30 However, without a comparison group, it is unclear what role the allograft plays in success in that construct.
Despite some proposed benefits, the additional soft tissue stripping required to place allograft has raised the question of delayed healing and increased infection rate as a result of this technique. A systematic review by Moore and colleagues33 looking at the use of allograft strut in Vancouver B1 fractures found increased time to union (4.4 vs 6.6 months) and deep infection rate (3.8% vs 8.3%) with the use of allograft strut, leading them to recommend cautious use of allograft when treating Vancouver B1 fractures.
With improved fixation strategies available, the role of allograft may be best reserved for patients with inadequate bone stock.
Dual Plate Fixation
Dual plate fixation has been proposed as one mechanism to increase construct strength. A periprosthetic fracture model has shown that, biomechanically, orthogonal plates have higher bending stiffness, torsional stiffness, cycles to failure, and load to failure when compared to a single lateral plate with use of a locking attachment plate proximally.34 Choi and colleagues35 compared lateral locking plates alone, lateral locking plates with allograft, and lateral locking plates with an orthogonal anterior plate and found the addition of an anterior plate resulted in the strongest construct.
Clinically, Müller and colleagues36 reported on a series of 10 patients treated with orthogonal (anterior and lateral) plating for periprosthetic femur fractures, including 3 nonunions. In their series, there was 1 plate failure and they conclude that dual plating is not associated with an increased risk of complications, and can also be used as a salvage procedure.
While the evidence for dual plating is limited, it may provide needed additional stability in certain cases without the added cost and exposure required for allograft.
Minimally Invasive Plate Osteosynthesis
Contrary to the extensive exposure required to place allograft, minimally invasive plate osteosynthesis (MIPO) of periprosthetic femur fractures is advocated by some authors.18,20 Ricci and colleagues18 reported no nonunions in 50 patients treated with indirect reduction techniques and laterally based plating alone without use of allograft. A combination of cables, locking, and nonlocking screws were used. Critical to their technique was preservation of the soft tissue envelope at the level of the fracture.
In further support of MIPO techniques, a systematic review of 1571 periprosthetic hip fractures reported significantly increased risk of nonunion with open approaches when compared to minimally invasive osteosynthesis,16 emphasizing the role of preservation of vascularity in treating these fractures.
Length of Fixation
For some time it was recommended that fixation of Vancouver B1 fractures end 2 cortical diameters below the level of the fracture.37,38 More recently there has been interest in the potential benefits of increased length of fixation.
A biomechanical study comparing long (20-hole) and short (12-hole) plates for periprosthetic fracture with regard to failure found no difference in failure rates between groups.39 While plate length did not appear to affect construct stiffness, the issue of subsequent fracture distal to the construct remains.
Moloney and colleagues40 proposed fixation of Vancouver B1 fractures using plates that span the length of the femur to the level of the femoral condyles to minimize peri-implant failures in osteoporotic patients. In 36 patients treated with standard-length plates, there were 2 fractures distal to the previous fixation compared to no subsequent fractures in 21 patients treated with spanning fixation.
Similarly, in Vancouver C fractures there is some evidence that fixation should span the femoral stem, regardless of available bone for fixation proximal to the fracture. Kubiak and colleagues41 found increasing load to failure and decreased cortical strain in a biomechanical model comparing plates that stop short of the femoral stem with those that span the stem.
Clinically, this concept is supported by Froberg and colleagues.12 In their series of 60 Vancouver B1 and C fractures treated with laterally based locked plating, 3 patients went on to refracture. All of these fractures occurred in patients with Vancouver C fractures treated with plates overlapping the preexisting stem by <50%. The fractures all occurred at the high stress area between the tip of the stem and the end of the plate.
Further support of extended plate length comes from Drew and colleagues,8 who demonstrated a significantly decreased risk of reoperation following ORIF of periprosthetic femur fracture when >75% of the length of the femur was spanned compared to <50%. Although in some settings short fixation may produce satisfactory results, consideration should be given to extending the length of fixation, especially in the osteoporotic population.
Interprosthetic Fractures
With a rising number of patients with ipsilateral hip and knee arthroplasty, the rate of interprosthetic fractures is rising. These fractures present additional challenges given preexisting implants above and below the level of the fracture. The use of a single precontoured laterally based locked plate has been reported with good union rates approaching 90%.42,43 In one series, all nonunions occurred in Vancouver B1 fractures,43 again bringing to light the challenging nature of the B1 fracture.
Nonunion
Success in treating periprosthetic femur fractures has improved with improved fixation methods and understanding of technique. However, current rates of nonunion are still reported up to 27% for B1 and C fractures.44
There is limited evidence on the treatment of periprosthetic femur fracture nonunion. However, treatment is difficult and complication rates are high. Crockarell and colleagues45 reported a 52% overall complication rate in their series of 23 periprosthetic femur fracture nonunions.
Nonunions of the femur near a prosthesis can be treated by revision of the fracture fixation using compression and grafting to achieve bone healing vs revision of the joint prosthesis to span the area of the nonunited bone. Case-by-case decision-making is based on the remaining bone stock and the type of revision prosthesis necessary to span the problem area. Given the challenges associated with their treatment, a focus on prevention of nonunion is of paramount importance.
Authors’ Preferred Treatment
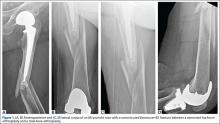
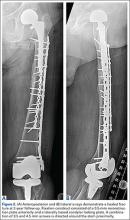
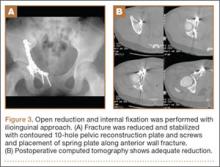
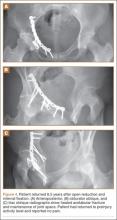
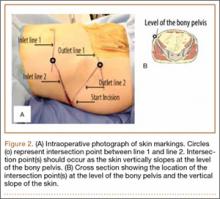
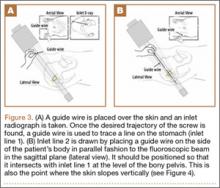
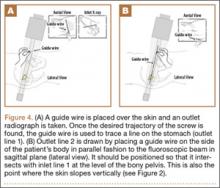
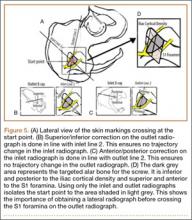
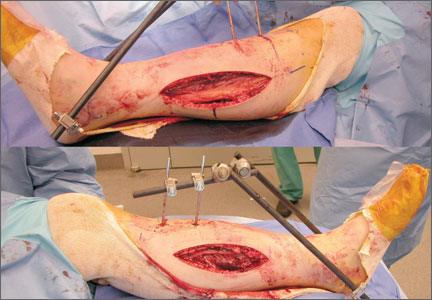
Our treatment of periprosthetic femur fractures with a well-fixed hip arthroplasty stem adheres to the principles supported in the literature (Figures 1A-1D and Figures 2A, 2B).
- Soft tissue friendly dissection with limited exposure at the fracture site is preferred as the fracture allows, particularly in cases with comminution where a direct assessment of the reduction is not available.
- Plate fixation strategy is dictated by the characteristics of the fracture. Fracture patterns amenable to anatomic reduction receive interfragmentary compression and absolute stability constructs. Highly comminuted fractures receive relatively stable bridging constructs to encourage callous.
- Locking screws are used rarely in diaphyseal fracture patterns, and when employed, are applied to only one side of the fracture to limit “over stiffening” the construct.
- Liberal use of dual plating, both as a method of maintaining fracture reduction while a structural plate is applied and increasing construct rigidity.
- Proximal fixation relies heavily on bicortical screws placed through the holes of the lateral plate. Cerclage wires and unicortical screws are rarely used in our practice. In the case of larger stems, a bicortical 3.5-mm screw can be placed through a 4.5-mm plate using a reduction washer.
Summary
Techniques for treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty stem are constantly evolving. Several principles have emerged to decrease rates of treatment failure and subsequent reoperation. While there are several methods to do so, it is critical to achieve stable proximal fixation. Long spanning fixation constructs are linked to lower failure and reoperation rates in both B1 and C type fractures. Additionally, the importance of soft tissue management and maintenance of local vascularity should not be underestimated.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Lewallen DG, Berry DJ. Periprosthetic fracture of the femur after total hip arthroplasty: treatment and results to date. Instr Course Lect. 1998;47:243-249.
3. Kavanagh BF. Femoral fractures associated with total hip arthroplasty. Orthop Clin North Am. 1992;23(2):249-257.
4. Meek RM, Norwood T, Smith R, Brenkel IJ, Howie CR. The risk of peri-prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg Br. 2011;93(1):96-101.
5. Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J. 2016;98-B(4):461-467.
6. Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999;30(2):183-190.
7. Abdel MP, Houdek MT, Watts CD, Lewallen DG, Berry DJ. Epidemiology of periprosthetic femoral fractures in 5417 revision total hip arthrolasties: a 40-year experience. Bone Joint J. 2016;98-B(4):468-474.
8. Drew JM, Griffin WL, Odum SM, Van Doren B, Weston BT, Stryker LS. Survivorship after periprosthetic femur fracture: factors affecting outcome. J Arthroplasty. 2015. [Epub ahead of print]
9. Dehghan N, McKee MD, Nauth A, Ristevski B, Schemitsch EH. Surgical fixation of Vancouver type B1 periprosthetic femur fractures: a systematic review. J Orthop Trauma. 2014;28(12):721-727.
10. Mukundan C, Rayan F, Kheir E, Macdonald D. Management of late periprosthetic femur fractures: a retrospective cohort of 72 patients. Int Orthop. 2010;34(4):485-489.
11. Lindahl H, Malchau H, Odén A, Garellick G. Risk factors for failure after treatment of a periprosthetic fracture of the femur. J Bone Joint Surg Br. 2006;88(1):26-30.
12. Froberg L, Troelsen A, Brix M. Periprosthetic Vancouver type B1 and C fractures treated by locking-plate osteosynthesis: fracture union and reoperations in 60 consecutive fractures. Acta Orthop. 2012;83(6):648-652.
13. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996(327):238-246.
14. Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84(8):1093-1110.
15. Fulkerson E, Koval K, Preston CF, Iesaka K, Kummer FJ, Egol KA. Fixation of periprosthetic femoral shaft fractures associated with cemented femoral stems: a biomechanical comparison of locked plating and conventional cable plates. J Orthop Trauma. 2006;20(2):89-93.
16. Stoffel K, Sommer C, Kalampoki V, Blumenthal A, Joeris A. The influence of the operation technique and implant used in the treatment of periprosthetic hip and interprosthetic femur fractures: a systematic literature review of 1571 cases. Arch Orthop Trauma Surg. 2016;136(4):553-561.
17. Fulkerson E, Tejwani N, Stuchin S, Egol K. Management of periprosthetic femur fractures with a first generation locking plate. Injury. 2007;38(8):965-972.
18. Ricci WM, Bolhofner BR, Loftus T, Cox C, Mitchell S, Borrelli J Jr. Indirect reduction and plate fixation, without grafting, for periprosthetic femoral shaft fractures about a stable intramedullary implant. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 2:275-282.
19. Ebraheim NA, Gomez C, Ramineni SK, Liu J. Fixation of periprosthetic femoral shaft fractures adjacent to a well-fixed femoral stem with reversed distal femoral locking plate. J Trauma. 2009;66(4):1152-1157.
20. Bryant GK, Morshed S, Agel J, et al. Isolated locked compression plating for Vancouver Type B1 periprosthetic femoral fractures. Injury. 2009;40(11):1180-1186.
21. Giesinger K, Ebneter L, Day RE, Stoffel KK, Yates PJ, Kuster MS. Can plate osteosynthesis of periprosthethic femoral fractures cause cement mantle failure around a stable hip stem? A biomechanical analysis. J Arthroplasty. 2014;29(6):1308-1312.
22. Lewis GS, Caroom CT, Wee H, et al. Tangential bicortical locked fixation improves stability in vancouver B1 periprosthetic femur fractures: a biomechanical study. J Orthop Trauma. 2015;29(10):e364-e370.
23. Lenz M, Perren SM, Gueorguiev B, et al. A biomechanical study on proximal plate fixation techniques in periprosthetic femur fractures. Injury. 2014;45 Suppl 1:S71-S75.
24. Dennis MG, Simon JA, Kummer FJ, Koval KJ, DiCesare PE. Fixation of periprosthetic femoral shaft fractures occurring at the tip of the stem: a biomechanical study of 5 techniques. J Arthroplasty. 2000;15(4):523-528.
25. Graham SM, Mak JH, Moazen M, et al. Periprosthetic femoral fracture fixation: a biomechanical comparison between proximal locking screws and cables. J Orthop Sci. 2015;20(5):875-880.
26. Griffiths JT, Taheri A, Day RE, Yates PJ. Better axial stiffness of a bicortical screw construct compared to a cable construct for comminuted Vancouver B1 proximal femoral fractures. J Arthroplasty. 2015;30(12):2333-2337.
27. Chandler HP, King D, Limbird R, et al. The use of cortical allograft struts for fixation of fractures associated with well-fixed total joint prostheses. Semin Arthroplasty. 1993;4(2):99-107.
28. Haddad FS, Duncan CP, Berry DJ, Lewallen DG, Gross AE, Chandler HP. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am. 2002;84-A(6):945-950.
29. Dennis MG, Simon JA, Kummer FJ, Koval KJ, Di Cesare PE. Fixation of periprosthetic femoral shaft fractures: a biomechanical comparison of two techniques. J Orthop Trauma. 2001;15(3):177-180.
30. Yeo I, Rhyu KH, Kim SM, Park YS, Lim SJ. High union rates of locking compression plating with cortical strut allograft for type B1 periprosthetic femoral fractures. Int Orthop. 2016. [Epub ahead of print]
31. Khashan M, Amar E, Drexler M, Chechik Ok, Cohen Z, Steinberg EL. Superior outcome of strut allograft-augmented plate fixation for the treatment of periprosthetic fractures around a stable femoral stem. Injury. 2013;44(11):1556-1560.
32. Old AB, McGrory BJ, White RR, Babikian GM. Fixation of Vancouver B1 peri-prosthetic fractures by broad metal plates without the application of strut allografts. J Bone Joint Surg Br. 2006;88(11):1425-1429.
33. Moore RE, Baldwin K, Austin MS, Mehta S. A systematic review of open reduction and internal fixation of periprosthetic femur fractures with or without allograft strut, cerclage, and locked plates. J Arthroplasty. 2014;29(5):872-876.
34. Lenz M, Stoffel K, Gueorguiev B, Klos K, Kielstein H, Hofmann GO. Enhancing fixation strength in periprosthetic femur fractures by orthogonal plating-a biomechanical study. J Orthop Res. 2016;34(4):591-596.
35. Choi JK, Gardner TR, Yoon E, Morrison TA, Macaulay WB, Geller JA. The effect of fixation technique on the stiffness of comminuted Vancouver B1 periprosthetic femur fractures. J Arthroplasty. 2010;25(6 Suppl):124-128.
36. Müller FJ, Galler M Füchtmeier B. Clinical and radiological results of patients treated with orthogonal double plating for periprosthetic femoral fractures. Int Orthop. 2014;38(12):2469-2472.
37. Pike J, Davidson D, Garbuz D, Duncan CP, O’Brien PJ, Masri BA. Principles of treatment for periprosthetic femoral shaft fractures around well-fixed total hip arthroplasty. J Am Acad Orthop Surg. 2009;17(11):677-688.
38. Serocki JH, Chandler RW, Dorr LD. Treatment of fractures about hip prostheses with compression plating. J Arthroplasty. 1992;7(2):129-135.
39. Pletka JD, Marsland D, Belkoff SM, Mears SC, Kates SL. Biomechanical comparison of 2 different locking plate fixation methods in vancouver b1 periprosthetic femur fractures. Geriatr Orthop Surg Rehabil. 2011;2(2):51-55.
40. Moloney GB, Westrick ER, Siska PA, Tarkin IS. Treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty implant: span the whole bone. Arch Orthop Trauma Surg. 2014;134(1):9-14.
41. Kubiak EN, Haller JM, Kemper DD, Presson AP, Higgins TF, Horowitz DS. Does the lateral plate need to overlap the stem to mitigate stress concentration when treating Vancouver C periprosthetic supracondylar femur fracture? J Arthroplasty. 2015;30(1):104-108.
42. Sah AP, Marshall A, Virkus WV, Estok DM 2nd, Della Valle CJ. Interprosthetic fractures of the femur: treatment with a single-locked plate. J Arthroplasty. 2010;25(2):280-286.
43. Hoffmann MF, Lotzien S, Schildhauer TA. Clinical outcome of interprosthetic femoral fractures treated with polyaxial locking plates. Injury. 2016. [Epub ahead of print]
44. Holder N, Papp S, Gofton W, Beaulé PE. Outcomes following surgical treatment of periprosthetic femur fractures: a single centre series. Can J Surg. 2014;57(3):209-213.
45. Crockarell JR Jr, Berry DJ, Lewallen DG. Nonunion after periprosthetic femoral fracture associated with total hip arthroplasty. J Bone Joint Surg Am. 1999;81(8):1073-1079.
The rate of total hip arthroplasty (THA) is rising and demand is expected to increase by 174% to 572,000 by 2030.1 The rate of periprosthetic fracture around primary THA is frequently reported at around 1%,2-4 though a recent study of over 32,000 THAs quotes the 20-year probability of periprosthetic fracture at 3.5%.5 Revision THA is also increasing in frequency and associated rates of periprosthetic fracture range from 1.5% to 7.8% following revision THA,3,4,6 with the probability of fracture at 20 years of 11%.7 Projection models predict that the number of periprosthetic fractures will rise by 4.6% per decade over the next 30 years.8
Broadly, treatment options include open reduction internal fixation (ORIF), revision THA, and combined approaches. The Vancouver classification, based on fracture location, stem stability, and bone loss, is often used to guide fracture treatment, with stable implants treated with ORIF and unstable implants requiring revision arthroplasty.
Fixation strategies for treatment of periprosthetic fracture around a well-fixed arthroplasty stem have evolved over time, and there continue to be a variety of available internal fixation options with no clear consensus on the optimal strategy.9 Rates of reoperation following ORIF of periprosthetic femur fracture are reported from 13% to 23%,8,10-12 confirming that there remains room for improvement in management of these injuries.
Locking Plate Fixation
Early fixation strategies included allograft and cables alone as well as nonlocked plate and cerclage constructs. In response to the complication and reoperation rate for nonlocked plate constructs, reported at 33%,13 locking plates were introduced as a treatment option, allowing for both improved osseous vascularity and added screw options.14 When compared to the traditional nonlocked Ogden construct, locking plate constructs are more resistant to axial and torsional load.15 Clinically, the relative risk of nonunion after nonlocking plate fixation is reported at 11.9 times that of fixation with locking plate technology.16
Successful use of lateral locking plate fixation for treatment of this injury has been reported on in several clinical series.17-20 Froberg and colleagues12 evaluated 60 Vancouver B1 and C fractures treated by locking plate osteosynthesis and reported no nonunions, an improvement from previous constructs. However, 8 out of 60 patients with 2-year follow-up required reoperation—4 for infection, 3 for refracture, and 1 for stem loosening—making it clear that the locking plate alone was not a panacea.
With locking plate fixation a mainstay of modern treatment of periprosthetic femur fractures, many questions still remain.
Proximal Fixation
Even with the introduction of locked plates, treatment success after ORIF of Vancouver B1 fractures relies on adequate proximal fixation. Options for proximal fixation around the stem include cerclage wires or cables, unicortical locked screws, obliquely directed bicortical screws, and use of the locking attachment plate to insert bicortical locked screws. These strategies can be used in the presence of cemented or uncemented stems, with biomechanical evidence that screw fixation through the cement mantle does not cause failure.21
Several biomechanical studies address the stiffness and strength of varying proximal fixation strategies. While early fixation relied heavily on cables, the use of cables alone as proximal fixation has been linked to significantly higher rates of failure when compared to other constructs in a large clinical series.11 Multiple biomechanical studies have shown that newer methods of proximal fixation provide more rigid constructs.22,23
Unicortical locked screws appear to outperform cables biomechanically. The use of unicortical screws in lieu of or in addition to cables provides added resistance to lateral bending as well as torsion when compared to cables alone.24 A second group found that unicortical locked screws alone were superior to combined fixation with cerclage wires and unicortical locked screws.25
Added stability can be demonstrated by bicortical fixation strategies, which offer increased rigidity when compared to cables or unicortical screws.22 In vitro work has shown enhanced fixation stability with bicortical screw fixation using the locking attachment plate when compared to cerclage wires alone.23,26 Clinically, some authors have demonstrated success with the use of reversed distal femoral locking plates in order to enhance proximal locking options and allow for bicortical fixation around the stem.19 As noted above, the data favor the opinion that clinical failure rates with cerclage wires alone are high, and biomechanically, bicortical fixation around the femoral stem appears to be superior to unicortical locked screw fixation or cerclage wires. If rigid proximal fixation is desired, an effort should be made to obtain bicortical fixation around the femoral stem.
Allograft
Allograft strut, either alone or in addition to plate osteosynthesis, has long been used in treatment of periprosthetic fractures. Proponents of this technique cite improved biomechanical stability17 and allograft incorporation resulting in restoration of bone stock.
Early treatment of periprosthetic femur fractures consisted solely of allograft and cable fixation, but data on the technique is limited. A small series reported reasonable success, with only 2 out of 19 patients developing nonunion.27 More recently Haddad and colleagues28 reported malunions in 3 out of 19 patients treated with allograft and cables alone. Allograft alone has been largely abandoned in favor of plate fixation, and biomechanical evidence shows that plate and screw or cerclage constructs are more resistant to torsion and lateral bending than allograft with cables alone.29
However, the role of allograft in treatment of periprosthetic femur fractures is not clearly defined. Some authors advocate routinely supplementing plate fixation with allograft28,30 and others go as far as to suggest superior union rates of strut allograft augmented plate fixation when compared to plate fixation alone for periprosthetic fractures around a stable femoral stem.31 However, in that series, the failure rate of 5/11 patients treated with plate alone is higher than current series,12 and others have demonstrated good success without allograft, even with nonlocked plates.32
As recently as 2016, a lateral locking plate supplemented with allograft has been described as a successful technique, with no nonunions reported in a small series.30 However, without a comparison group, it is unclear what role the allograft plays in success in that construct.
Despite some proposed benefits, the additional soft tissue stripping required to place allograft has raised the question of delayed healing and increased infection rate as a result of this technique. A systematic review by Moore and colleagues33 looking at the use of allograft strut in Vancouver B1 fractures found increased time to union (4.4 vs 6.6 months) and deep infection rate (3.8% vs 8.3%) with the use of allograft strut, leading them to recommend cautious use of allograft when treating Vancouver B1 fractures.
With improved fixation strategies available, the role of allograft may be best reserved for patients with inadequate bone stock.
Dual Plate Fixation
Dual plate fixation has been proposed as one mechanism to increase construct strength. A periprosthetic fracture model has shown that, biomechanically, orthogonal plates have higher bending stiffness, torsional stiffness, cycles to failure, and load to failure when compared to a single lateral plate with use of a locking attachment plate proximally.34 Choi and colleagues35 compared lateral locking plates alone, lateral locking plates with allograft, and lateral locking plates with an orthogonal anterior plate and found the addition of an anterior plate resulted in the strongest construct.
Clinically, Müller and colleagues36 reported on a series of 10 patients treated with orthogonal (anterior and lateral) plating for periprosthetic femur fractures, including 3 nonunions. In their series, there was 1 plate failure and they conclude that dual plating is not associated with an increased risk of complications, and can also be used as a salvage procedure.
While the evidence for dual plating is limited, it may provide needed additional stability in certain cases without the added cost and exposure required for allograft.
Minimally Invasive Plate Osteosynthesis
Contrary to the extensive exposure required to place allograft, minimally invasive plate osteosynthesis (MIPO) of periprosthetic femur fractures is advocated by some authors.18,20 Ricci and colleagues18 reported no nonunions in 50 patients treated with indirect reduction techniques and laterally based plating alone without use of allograft. A combination of cables, locking, and nonlocking screws were used. Critical to their technique was preservation of the soft tissue envelope at the level of the fracture.
In further support of MIPO techniques, a systematic review of 1571 periprosthetic hip fractures reported significantly increased risk of nonunion with open approaches when compared to minimally invasive osteosynthesis,16 emphasizing the role of preservation of vascularity in treating these fractures.
Length of Fixation
For some time it was recommended that fixation of Vancouver B1 fractures end 2 cortical diameters below the level of the fracture.37,38 More recently there has been interest in the potential benefits of increased length of fixation.
A biomechanical study comparing long (20-hole) and short (12-hole) plates for periprosthetic fracture with regard to failure found no difference in failure rates between groups.39 While plate length did not appear to affect construct stiffness, the issue of subsequent fracture distal to the construct remains.
Moloney and colleagues40 proposed fixation of Vancouver B1 fractures using plates that span the length of the femur to the level of the femoral condyles to minimize peri-implant failures in osteoporotic patients. In 36 patients treated with standard-length plates, there were 2 fractures distal to the previous fixation compared to no subsequent fractures in 21 patients treated with spanning fixation.
Similarly, in Vancouver C fractures there is some evidence that fixation should span the femoral stem, regardless of available bone for fixation proximal to the fracture. Kubiak and colleagues41 found increasing load to failure and decreased cortical strain in a biomechanical model comparing plates that stop short of the femoral stem with those that span the stem.
Clinically, this concept is supported by Froberg and colleagues.12 In their series of 60 Vancouver B1 and C fractures treated with laterally based locked plating, 3 patients went on to refracture. All of these fractures occurred in patients with Vancouver C fractures treated with plates overlapping the preexisting stem by <50%. The fractures all occurred at the high stress area between the tip of the stem and the end of the plate.
Further support of extended plate length comes from Drew and colleagues,8 who demonstrated a significantly decreased risk of reoperation following ORIF of periprosthetic femur fracture when >75% of the length of the femur was spanned compared to <50%. Although in some settings short fixation may produce satisfactory results, consideration should be given to extending the length of fixation, especially in the osteoporotic population.
Interprosthetic Fractures
With a rising number of patients with ipsilateral hip and knee arthroplasty, the rate of interprosthetic fractures is rising. These fractures present additional challenges given preexisting implants above and below the level of the fracture. The use of a single precontoured laterally based locked plate has been reported with good union rates approaching 90%.42,43 In one series, all nonunions occurred in Vancouver B1 fractures,43 again bringing to light the challenging nature of the B1 fracture.
Nonunion
Success in treating periprosthetic femur fractures has improved with improved fixation methods and understanding of technique. However, current rates of nonunion are still reported up to 27% for B1 and C fractures.44
There is limited evidence on the treatment of periprosthetic femur fracture nonunion. However, treatment is difficult and complication rates are high. Crockarell and colleagues45 reported a 52% overall complication rate in their series of 23 periprosthetic femur fracture nonunions.
Nonunions of the femur near a prosthesis can be treated by revision of the fracture fixation using compression and grafting to achieve bone healing vs revision of the joint prosthesis to span the area of the nonunited bone. Case-by-case decision-making is based on the remaining bone stock and the type of revision prosthesis necessary to span the problem area. Given the challenges associated with their treatment, a focus on prevention of nonunion is of paramount importance.
Authors’ Preferred Treatment
Our treatment of periprosthetic femur fractures with a well-fixed hip arthroplasty stem adheres to the principles supported in the literature (Figures 1A-1D and Figures 2A, 2B).
- Soft tissue friendly dissection with limited exposure at the fracture site is preferred as the fracture allows, particularly in cases with comminution where a direct assessment of the reduction is not available.
- Plate fixation strategy is dictated by the characteristics of the fracture. Fracture patterns amenable to anatomic reduction receive interfragmentary compression and absolute stability constructs. Highly comminuted fractures receive relatively stable bridging constructs to encourage callous.
- Locking screws are used rarely in diaphyseal fracture patterns, and when employed, are applied to only one side of the fracture to limit “over stiffening” the construct.
- Liberal use of dual plating, both as a method of maintaining fracture reduction while a structural plate is applied and increasing construct rigidity.
- Proximal fixation relies heavily on bicortical screws placed through the holes of the lateral plate. Cerclage wires and unicortical screws are rarely used in our practice. In the case of larger stems, a bicortical 3.5-mm screw can be placed through a 4.5-mm plate using a reduction washer.
Summary
Techniques for treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty stem are constantly evolving. Several principles have emerged to decrease rates of treatment failure and subsequent reoperation. While there are several methods to do so, it is critical to achieve stable proximal fixation. Long spanning fixation constructs are linked to lower failure and reoperation rates in both B1 and C type fractures. Additionally, the importance of soft tissue management and maintenance of local vascularity should not be underestimated.
The rate of total hip arthroplasty (THA) is rising and demand is expected to increase by 174% to 572,000 by 2030.1 The rate of periprosthetic fracture around primary THA is frequently reported at around 1%,2-4 though a recent study of over 32,000 THAs quotes the 20-year probability of periprosthetic fracture at 3.5%.5 Revision THA is also increasing in frequency and associated rates of periprosthetic fracture range from 1.5% to 7.8% following revision THA,3,4,6 with the probability of fracture at 20 years of 11%.7 Projection models predict that the number of periprosthetic fractures will rise by 4.6% per decade over the next 30 years.8
Broadly, treatment options include open reduction internal fixation (ORIF), revision THA, and combined approaches. The Vancouver classification, based on fracture location, stem stability, and bone loss, is often used to guide fracture treatment, with stable implants treated with ORIF and unstable implants requiring revision arthroplasty.
Fixation strategies for treatment of periprosthetic fracture around a well-fixed arthroplasty stem have evolved over time, and there continue to be a variety of available internal fixation options with no clear consensus on the optimal strategy.9 Rates of reoperation following ORIF of periprosthetic femur fracture are reported from 13% to 23%,8,10-12 confirming that there remains room for improvement in management of these injuries.
Locking Plate Fixation
Early fixation strategies included allograft and cables alone as well as nonlocked plate and cerclage constructs. In response to the complication and reoperation rate for nonlocked plate constructs, reported at 33%,13 locking plates were introduced as a treatment option, allowing for both improved osseous vascularity and added screw options.14 When compared to the traditional nonlocked Ogden construct, locking plate constructs are more resistant to axial and torsional load.15 Clinically, the relative risk of nonunion after nonlocking plate fixation is reported at 11.9 times that of fixation with locking plate technology.16
Successful use of lateral locking plate fixation for treatment of this injury has been reported on in several clinical series.17-20 Froberg and colleagues12 evaluated 60 Vancouver B1 and C fractures treated by locking plate osteosynthesis and reported no nonunions, an improvement from previous constructs. However, 8 out of 60 patients with 2-year follow-up required reoperation—4 for infection, 3 for refracture, and 1 for stem loosening—making it clear that the locking plate alone was not a panacea.
With locking plate fixation a mainstay of modern treatment of periprosthetic femur fractures, many questions still remain.
Proximal Fixation
Even with the introduction of locked plates, treatment success after ORIF of Vancouver B1 fractures relies on adequate proximal fixation. Options for proximal fixation around the stem include cerclage wires or cables, unicortical locked screws, obliquely directed bicortical screws, and use of the locking attachment plate to insert bicortical locked screws. These strategies can be used in the presence of cemented or uncemented stems, with biomechanical evidence that screw fixation through the cement mantle does not cause failure.21
Several biomechanical studies address the stiffness and strength of varying proximal fixation strategies. While early fixation relied heavily on cables, the use of cables alone as proximal fixation has been linked to significantly higher rates of failure when compared to other constructs in a large clinical series.11 Multiple biomechanical studies have shown that newer methods of proximal fixation provide more rigid constructs.22,23
Unicortical locked screws appear to outperform cables biomechanically. The use of unicortical screws in lieu of or in addition to cables provides added resistance to lateral bending as well as torsion when compared to cables alone.24 A second group found that unicortical locked screws alone were superior to combined fixation with cerclage wires and unicortical locked screws.25
Added stability can be demonstrated by bicortical fixation strategies, which offer increased rigidity when compared to cables or unicortical screws.22 In vitro work has shown enhanced fixation stability with bicortical screw fixation using the locking attachment plate when compared to cerclage wires alone.23,26 Clinically, some authors have demonstrated success with the use of reversed distal femoral locking plates in order to enhance proximal locking options and allow for bicortical fixation around the stem.19 As noted above, the data favor the opinion that clinical failure rates with cerclage wires alone are high, and biomechanically, bicortical fixation around the femoral stem appears to be superior to unicortical locked screw fixation or cerclage wires. If rigid proximal fixation is desired, an effort should be made to obtain bicortical fixation around the femoral stem.
Allograft
Allograft strut, either alone or in addition to plate osteosynthesis, has long been used in treatment of periprosthetic fractures. Proponents of this technique cite improved biomechanical stability17 and allograft incorporation resulting in restoration of bone stock.
Early treatment of periprosthetic femur fractures consisted solely of allograft and cable fixation, but data on the technique is limited. A small series reported reasonable success, with only 2 out of 19 patients developing nonunion.27 More recently Haddad and colleagues28 reported malunions in 3 out of 19 patients treated with allograft and cables alone. Allograft alone has been largely abandoned in favor of plate fixation, and biomechanical evidence shows that plate and screw or cerclage constructs are more resistant to torsion and lateral bending than allograft with cables alone.29
However, the role of allograft in treatment of periprosthetic femur fractures is not clearly defined. Some authors advocate routinely supplementing plate fixation with allograft28,30 and others go as far as to suggest superior union rates of strut allograft augmented plate fixation when compared to plate fixation alone for periprosthetic fractures around a stable femoral stem.31 However, in that series, the failure rate of 5/11 patients treated with plate alone is higher than current series,12 and others have demonstrated good success without allograft, even with nonlocked plates.32
As recently as 2016, a lateral locking plate supplemented with allograft has been described as a successful technique, with no nonunions reported in a small series.30 However, without a comparison group, it is unclear what role the allograft plays in success in that construct.
Despite some proposed benefits, the additional soft tissue stripping required to place allograft has raised the question of delayed healing and increased infection rate as a result of this technique. A systematic review by Moore and colleagues33 looking at the use of allograft strut in Vancouver B1 fractures found increased time to union (4.4 vs 6.6 months) and deep infection rate (3.8% vs 8.3%) with the use of allograft strut, leading them to recommend cautious use of allograft when treating Vancouver B1 fractures.
With improved fixation strategies available, the role of allograft may be best reserved for patients with inadequate bone stock.
Dual Plate Fixation
Dual plate fixation has been proposed as one mechanism to increase construct strength. A periprosthetic fracture model has shown that, biomechanically, orthogonal plates have higher bending stiffness, torsional stiffness, cycles to failure, and load to failure when compared to a single lateral plate with use of a locking attachment plate proximally.34 Choi and colleagues35 compared lateral locking plates alone, lateral locking plates with allograft, and lateral locking plates with an orthogonal anterior plate and found the addition of an anterior plate resulted in the strongest construct.
Clinically, Müller and colleagues36 reported on a series of 10 patients treated with orthogonal (anterior and lateral) plating for periprosthetic femur fractures, including 3 nonunions. In their series, there was 1 plate failure and they conclude that dual plating is not associated with an increased risk of complications, and can also be used as a salvage procedure.
While the evidence for dual plating is limited, it may provide needed additional stability in certain cases without the added cost and exposure required for allograft.
Minimally Invasive Plate Osteosynthesis
Contrary to the extensive exposure required to place allograft, minimally invasive plate osteosynthesis (MIPO) of periprosthetic femur fractures is advocated by some authors.18,20 Ricci and colleagues18 reported no nonunions in 50 patients treated with indirect reduction techniques and laterally based plating alone without use of allograft. A combination of cables, locking, and nonlocking screws were used. Critical to their technique was preservation of the soft tissue envelope at the level of the fracture.
In further support of MIPO techniques, a systematic review of 1571 periprosthetic hip fractures reported significantly increased risk of nonunion with open approaches when compared to minimally invasive osteosynthesis,16 emphasizing the role of preservation of vascularity in treating these fractures.
Length of Fixation
For some time it was recommended that fixation of Vancouver B1 fractures end 2 cortical diameters below the level of the fracture.37,38 More recently there has been interest in the potential benefits of increased length of fixation.
A biomechanical study comparing long (20-hole) and short (12-hole) plates for periprosthetic fracture with regard to failure found no difference in failure rates between groups.39 While plate length did not appear to affect construct stiffness, the issue of subsequent fracture distal to the construct remains.
Moloney and colleagues40 proposed fixation of Vancouver B1 fractures using plates that span the length of the femur to the level of the femoral condyles to minimize peri-implant failures in osteoporotic patients. In 36 patients treated with standard-length plates, there were 2 fractures distal to the previous fixation compared to no subsequent fractures in 21 patients treated with spanning fixation.
Similarly, in Vancouver C fractures there is some evidence that fixation should span the femoral stem, regardless of available bone for fixation proximal to the fracture. Kubiak and colleagues41 found increasing load to failure and decreased cortical strain in a biomechanical model comparing plates that stop short of the femoral stem with those that span the stem.
Clinically, this concept is supported by Froberg and colleagues.12 In their series of 60 Vancouver B1 and C fractures treated with laterally based locked plating, 3 patients went on to refracture. All of these fractures occurred in patients with Vancouver C fractures treated with plates overlapping the preexisting stem by <50%. The fractures all occurred at the high stress area between the tip of the stem and the end of the plate.
Further support of extended plate length comes from Drew and colleagues,8 who demonstrated a significantly decreased risk of reoperation following ORIF of periprosthetic femur fracture when >75% of the length of the femur was spanned compared to <50%. Although in some settings short fixation may produce satisfactory results, consideration should be given to extending the length of fixation, especially in the osteoporotic population.
Interprosthetic Fractures
With a rising number of patients with ipsilateral hip and knee arthroplasty, the rate of interprosthetic fractures is rising. These fractures present additional challenges given preexisting implants above and below the level of the fracture. The use of a single precontoured laterally based locked plate has been reported with good union rates approaching 90%.42,43 In one series, all nonunions occurred in Vancouver B1 fractures,43 again bringing to light the challenging nature of the B1 fracture.
Nonunion
Success in treating periprosthetic femur fractures has improved with improved fixation methods and understanding of technique. However, current rates of nonunion are still reported up to 27% for B1 and C fractures.44
There is limited evidence on the treatment of periprosthetic femur fracture nonunion. However, treatment is difficult and complication rates are high. Crockarell and colleagues45 reported a 52% overall complication rate in their series of 23 periprosthetic femur fracture nonunions.
Nonunions of the femur near a prosthesis can be treated by revision of the fracture fixation using compression and grafting to achieve bone healing vs revision of the joint prosthesis to span the area of the nonunited bone. Case-by-case decision-making is based on the remaining bone stock and the type of revision prosthesis necessary to span the problem area. Given the challenges associated with their treatment, a focus on prevention of nonunion is of paramount importance.
Authors’ Preferred Treatment
Our treatment of periprosthetic femur fractures with a well-fixed hip arthroplasty stem adheres to the principles supported in the literature (Figures 1A-1D and Figures 2A, 2B).
- Soft tissue friendly dissection with limited exposure at the fracture site is preferred as the fracture allows, particularly in cases with comminution where a direct assessment of the reduction is not available.
- Plate fixation strategy is dictated by the characteristics of the fracture. Fracture patterns amenable to anatomic reduction receive interfragmentary compression and absolute stability constructs. Highly comminuted fractures receive relatively stable bridging constructs to encourage callous.
- Locking screws are used rarely in diaphyseal fracture patterns, and when employed, are applied to only one side of the fracture to limit “over stiffening” the construct.
- Liberal use of dual plating, both as a method of maintaining fracture reduction while a structural plate is applied and increasing construct rigidity.
- Proximal fixation relies heavily on bicortical screws placed through the holes of the lateral plate. Cerclage wires and unicortical screws are rarely used in our practice. In the case of larger stems, a bicortical 3.5-mm screw can be placed through a 4.5-mm plate using a reduction washer.
Summary
Techniques for treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty stem are constantly evolving. Several principles have emerged to decrease rates of treatment failure and subsequent reoperation. While there are several methods to do so, it is critical to achieve stable proximal fixation. Long spanning fixation constructs are linked to lower failure and reoperation rates in both B1 and C type fractures. Additionally, the importance of soft tissue management and maintenance of local vascularity should not be underestimated.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Lewallen DG, Berry DJ. Periprosthetic fracture of the femur after total hip arthroplasty: treatment and results to date. Instr Course Lect. 1998;47:243-249.
3. Kavanagh BF. Femoral fractures associated with total hip arthroplasty. Orthop Clin North Am. 1992;23(2):249-257.
4. Meek RM, Norwood T, Smith R, Brenkel IJ, Howie CR. The risk of peri-prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg Br. 2011;93(1):96-101.
5. Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J. 2016;98-B(4):461-467.
6. Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999;30(2):183-190.
7. Abdel MP, Houdek MT, Watts CD, Lewallen DG, Berry DJ. Epidemiology of periprosthetic femoral fractures in 5417 revision total hip arthrolasties: a 40-year experience. Bone Joint J. 2016;98-B(4):468-474.
8. Drew JM, Griffin WL, Odum SM, Van Doren B, Weston BT, Stryker LS. Survivorship after periprosthetic femur fracture: factors affecting outcome. J Arthroplasty. 2015. [Epub ahead of print]
9. Dehghan N, McKee MD, Nauth A, Ristevski B, Schemitsch EH. Surgical fixation of Vancouver type B1 periprosthetic femur fractures: a systematic review. J Orthop Trauma. 2014;28(12):721-727.
10. Mukundan C, Rayan F, Kheir E, Macdonald D. Management of late periprosthetic femur fractures: a retrospective cohort of 72 patients. Int Orthop. 2010;34(4):485-489.
11. Lindahl H, Malchau H, Odén A, Garellick G. Risk factors for failure after treatment of a periprosthetic fracture of the femur. J Bone Joint Surg Br. 2006;88(1):26-30.
12. Froberg L, Troelsen A, Brix M. Periprosthetic Vancouver type B1 and C fractures treated by locking-plate osteosynthesis: fracture union and reoperations in 60 consecutive fractures. Acta Orthop. 2012;83(6):648-652.
13. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996(327):238-246.
14. Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84(8):1093-1110.
15. Fulkerson E, Koval K, Preston CF, Iesaka K, Kummer FJ, Egol KA. Fixation of periprosthetic femoral shaft fractures associated with cemented femoral stems: a biomechanical comparison of locked plating and conventional cable plates. J Orthop Trauma. 2006;20(2):89-93.
16. Stoffel K, Sommer C, Kalampoki V, Blumenthal A, Joeris A. The influence of the operation technique and implant used in the treatment of periprosthetic hip and interprosthetic femur fractures: a systematic literature review of 1571 cases. Arch Orthop Trauma Surg. 2016;136(4):553-561.
17. Fulkerson E, Tejwani N, Stuchin S, Egol K. Management of periprosthetic femur fractures with a first generation locking plate. Injury. 2007;38(8):965-972.
18. Ricci WM, Bolhofner BR, Loftus T, Cox C, Mitchell S, Borrelli J Jr. Indirect reduction and plate fixation, without grafting, for periprosthetic femoral shaft fractures about a stable intramedullary implant. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 2:275-282.
19. Ebraheim NA, Gomez C, Ramineni SK, Liu J. Fixation of periprosthetic femoral shaft fractures adjacent to a well-fixed femoral stem with reversed distal femoral locking plate. J Trauma. 2009;66(4):1152-1157.
20. Bryant GK, Morshed S, Agel J, et al. Isolated locked compression plating for Vancouver Type B1 periprosthetic femoral fractures. Injury. 2009;40(11):1180-1186.
21. Giesinger K, Ebneter L, Day RE, Stoffel KK, Yates PJ, Kuster MS. Can plate osteosynthesis of periprosthethic femoral fractures cause cement mantle failure around a stable hip stem? A biomechanical analysis. J Arthroplasty. 2014;29(6):1308-1312.
22. Lewis GS, Caroom CT, Wee H, et al. Tangential bicortical locked fixation improves stability in vancouver B1 periprosthetic femur fractures: a biomechanical study. J Orthop Trauma. 2015;29(10):e364-e370.
23. Lenz M, Perren SM, Gueorguiev B, et al. A biomechanical study on proximal plate fixation techniques in periprosthetic femur fractures. Injury. 2014;45 Suppl 1:S71-S75.
24. Dennis MG, Simon JA, Kummer FJ, Koval KJ, DiCesare PE. Fixation of periprosthetic femoral shaft fractures occurring at the tip of the stem: a biomechanical study of 5 techniques. J Arthroplasty. 2000;15(4):523-528.
25. Graham SM, Mak JH, Moazen M, et al. Periprosthetic femoral fracture fixation: a biomechanical comparison between proximal locking screws and cables. J Orthop Sci. 2015;20(5):875-880.
26. Griffiths JT, Taheri A, Day RE, Yates PJ. Better axial stiffness of a bicortical screw construct compared to a cable construct for comminuted Vancouver B1 proximal femoral fractures. J Arthroplasty. 2015;30(12):2333-2337.
27. Chandler HP, King D, Limbird R, et al. The use of cortical allograft struts for fixation of fractures associated with well-fixed total joint prostheses. Semin Arthroplasty. 1993;4(2):99-107.
28. Haddad FS, Duncan CP, Berry DJ, Lewallen DG, Gross AE, Chandler HP. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am. 2002;84-A(6):945-950.
29. Dennis MG, Simon JA, Kummer FJ, Koval KJ, Di Cesare PE. Fixation of periprosthetic femoral shaft fractures: a biomechanical comparison of two techniques. J Orthop Trauma. 2001;15(3):177-180.
30. Yeo I, Rhyu KH, Kim SM, Park YS, Lim SJ. High union rates of locking compression plating with cortical strut allograft for type B1 periprosthetic femoral fractures. Int Orthop. 2016. [Epub ahead of print]
31. Khashan M, Amar E, Drexler M, Chechik Ok, Cohen Z, Steinberg EL. Superior outcome of strut allograft-augmented plate fixation for the treatment of periprosthetic fractures around a stable femoral stem. Injury. 2013;44(11):1556-1560.
32. Old AB, McGrory BJ, White RR, Babikian GM. Fixation of Vancouver B1 peri-prosthetic fractures by broad metal plates without the application of strut allografts. J Bone Joint Surg Br. 2006;88(11):1425-1429.
33. Moore RE, Baldwin K, Austin MS, Mehta S. A systematic review of open reduction and internal fixation of periprosthetic femur fractures with or without allograft strut, cerclage, and locked plates. J Arthroplasty. 2014;29(5):872-876.
34. Lenz M, Stoffel K, Gueorguiev B, Klos K, Kielstein H, Hofmann GO. Enhancing fixation strength in periprosthetic femur fractures by orthogonal plating-a biomechanical study. J Orthop Res. 2016;34(4):591-596.
35. Choi JK, Gardner TR, Yoon E, Morrison TA, Macaulay WB, Geller JA. The effect of fixation technique on the stiffness of comminuted Vancouver B1 periprosthetic femur fractures. J Arthroplasty. 2010;25(6 Suppl):124-128.
36. Müller FJ, Galler M Füchtmeier B. Clinical and radiological results of patients treated with orthogonal double plating for periprosthetic femoral fractures. Int Orthop. 2014;38(12):2469-2472.
37. Pike J, Davidson D, Garbuz D, Duncan CP, O’Brien PJ, Masri BA. Principles of treatment for periprosthetic femoral shaft fractures around well-fixed total hip arthroplasty. J Am Acad Orthop Surg. 2009;17(11):677-688.
38. Serocki JH, Chandler RW, Dorr LD. Treatment of fractures about hip prostheses with compression plating. J Arthroplasty. 1992;7(2):129-135.
39. Pletka JD, Marsland D, Belkoff SM, Mears SC, Kates SL. Biomechanical comparison of 2 different locking plate fixation methods in vancouver b1 periprosthetic femur fractures. Geriatr Orthop Surg Rehabil. 2011;2(2):51-55.
40. Moloney GB, Westrick ER, Siska PA, Tarkin IS. Treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty implant: span the whole bone. Arch Orthop Trauma Surg. 2014;134(1):9-14.
41. Kubiak EN, Haller JM, Kemper DD, Presson AP, Higgins TF, Horowitz DS. Does the lateral plate need to overlap the stem to mitigate stress concentration when treating Vancouver C periprosthetic supracondylar femur fracture? J Arthroplasty. 2015;30(1):104-108.
42. Sah AP, Marshall A, Virkus WV, Estok DM 2nd, Della Valle CJ. Interprosthetic fractures of the femur: treatment with a single-locked plate. J Arthroplasty. 2010;25(2):280-286.
43. Hoffmann MF, Lotzien S, Schildhauer TA. Clinical outcome of interprosthetic femoral fractures treated with polyaxial locking plates. Injury. 2016. [Epub ahead of print]
44. Holder N, Papp S, Gofton W, Beaulé PE. Outcomes following surgical treatment of periprosthetic femur fractures: a single centre series. Can J Surg. 2014;57(3):209-213.
45. Crockarell JR Jr, Berry DJ, Lewallen DG. Nonunion after periprosthetic femoral fracture associated with total hip arthroplasty. J Bone Joint Surg Am. 1999;81(8):1073-1079.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Lewallen DG, Berry DJ. Periprosthetic fracture of the femur after total hip arthroplasty: treatment and results to date. Instr Course Lect. 1998;47:243-249.
3. Kavanagh BF. Femoral fractures associated with total hip arthroplasty. Orthop Clin North Am. 1992;23(2):249-257.
4. Meek RM, Norwood T, Smith R, Brenkel IJ, Howie CR. The risk of peri-prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg Br. 2011;93(1):96-101.
5. Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J. 2016;98-B(4):461-467.
6. Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999;30(2):183-190.
7. Abdel MP, Houdek MT, Watts CD, Lewallen DG, Berry DJ. Epidemiology of periprosthetic femoral fractures in 5417 revision total hip arthrolasties: a 40-year experience. Bone Joint J. 2016;98-B(4):468-474.
8. Drew JM, Griffin WL, Odum SM, Van Doren B, Weston BT, Stryker LS. Survivorship after periprosthetic femur fracture: factors affecting outcome. J Arthroplasty. 2015. [Epub ahead of print]
9. Dehghan N, McKee MD, Nauth A, Ristevski B, Schemitsch EH. Surgical fixation of Vancouver type B1 periprosthetic femur fractures: a systematic review. J Orthop Trauma. 2014;28(12):721-727.
10. Mukundan C, Rayan F, Kheir E, Macdonald D. Management of late periprosthetic femur fractures: a retrospective cohort of 72 patients. Int Orthop. 2010;34(4):485-489.
11. Lindahl H, Malchau H, Odén A, Garellick G. Risk factors for failure after treatment of a periprosthetic fracture of the femur. J Bone Joint Surg Br. 2006;88(1):26-30.
12. Froberg L, Troelsen A, Brix M. Periprosthetic Vancouver type B1 and C fractures treated by locking-plate osteosynthesis: fracture union and reoperations in 60 consecutive fractures. Acta Orthop. 2012;83(6):648-652.
13. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996(327):238-246.
14. Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84(8):1093-1110.
15. Fulkerson E, Koval K, Preston CF, Iesaka K, Kummer FJ, Egol KA. Fixation of periprosthetic femoral shaft fractures associated with cemented femoral stems: a biomechanical comparison of locked plating and conventional cable plates. J Orthop Trauma. 2006;20(2):89-93.
16. Stoffel K, Sommer C, Kalampoki V, Blumenthal A, Joeris A. The influence of the operation technique and implant used in the treatment of periprosthetic hip and interprosthetic femur fractures: a systematic literature review of 1571 cases. Arch Orthop Trauma Surg. 2016;136(4):553-561.
17. Fulkerson E, Tejwani N, Stuchin S, Egol K. Management of periprosthetic femur fractures with a first generation locking plate. Injury. 2007;38(8):965-972.
18. Ricci WM, Bolhofner BR, Loftus T, Cox C, Mitchell S, Borrelli J Jr. Indirect reduction and plate fixation, without grafting, for periprosthetic femoral shaft fractures about a stable intramedullary implant. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 2:275-282.
19. Ebraheim NA, Gomez C, Ramineni SK, Liu J. Fixation of periprosthetic femoral shaft fractures adjacent to a well-fixed femoral stem with reversed distal femoral locking plate. J Trauma. 2009;66(4):1152-1157.
20. Bryant GK, Morshed S, Agel J, et al. Isolated locked compression plating for Vancouver Type B1 periprosthetic femoral fractures. Injury. 2009;40(11):1180-1186.
21. Giesinger K, Ebneter L, Day RE, Stoffel KK, Yates PJ, Kuster MS. Can plate osteosynthesis of periprosthethic femoral fractures cause cement mantle failure around a stable hip stem? A biomechanical analysis. J Arthroplasty. 2014;29(6):1308-1312.
22. Lewis GS, Caroom CT, Wee H, et al. Tangential bicortical locked fixation improves stability in vancouver B1 periprosthetic femur fractures: a biomechanical study. J Orthop Trauma. 2015;29(10):e364-e370.
23. Lenz M, Perren SM, Gueorguiev B, et al. A biomechanical study on proximal plate fixation techniques in periprosthetic femur fractures. Injury. 2014;45 Suppl 1:S71-S75.
24. Dennis MG, Simon JA, Kummer FJ, Koval KJ, DiCesare PE. Fixation of periprosthetic femoral shaft fractures occurring at the tip of the stem: a biomechanical study of 5 techniques. J Arthroplasty. 2000;15(4):523-528.
25. Graham SM, Mak JH, Moazen M, et al. Periprosthetic femoral fracture fixation: a biomechanical comparison between proximal locking screws and cables. J Orthop Sci. 2015;20(5):875-880.
26. Griffiths JT, Taheri A, Day RE, Yates PJ. Better axial stiffness of a bicortical screw construct compared to a cable construct for comminuted Vancouver B1 proximal femoral fractures. J Arthroplasty. 2015;30(12):2333-2337.
27. Chandler HP, King D, Limbird R, et al. The use of cortical allograft struts for fixation of fractures associated with well-fixed total joint prostheses. Semin Arthroplasty. 1993;4(2):99-107.
28. Haddad FS, Duncan CP, Berry DJ, Lewallen DG, Gross AE, Chandler HP. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am. 2002;84-A(6):945-950.
29. Dennis MG, Simon JA, Kummer FJ, Koval KJ, Di Cesare PE. Fixation of periprosthetic femoral shaft fractures: a biomechanical comparison of two techniques. J Orthop Trauma. 2001;15(3):177-180.
30. Yeo I, Rhyu KH, Kim SM, Park YS, Lim SJ. High union rates of locking compression plating with cortical strut allograft for type B1 periprosthetic femoral fractures. Int Orthop. 2016. [Epub ahead of print]
31. Khashan M, Amar E, Drexler M, Chechik Ok, Cohen Z, Steinberg EL. Superior outcome of strut allograft-augmented plate fixation for the treatment of periprosthetic fractures around a stable femoral stem. Injury. 2013;44(11):1556-1560.
32. Old AB, McGrory BJ, White RR, Babikian GM. Fixation of Vancouver B1 peri-prosthetic fractures by broad metal plates without the application of strut allografts. J Bone Joint Surg Br. 2006;88(11):1425-1429.
33. Moore RE, Baldwin K, Austin MS, Mehta S. A systematic review of open reduction and internal fixation of periprosthetic femur fractures with or without allograft strut, cerclage, and locked plates. J Arthroplasty. 2014;29(5):872-876.
34. Lenz M, Stoffel K, Gueorguiev B, Klos K, Kielstein H, Hofmann GO. Enhancing fixation strength in periprosthetic femur fractures by orthogonal plating-a biomechanical study. J Orthop Res. 2016;34(4):591-596.
35. Choi JK, Gardner TR, Yoon E, Morrison TA, Macaulay WB, Geller JA. The effect of fixation technique on the stiffness of comminuted Vancouver B1 periprosthetic femur fractures. J Arthroplasty. 2010;25(6 Suppl):124-128.
36. Müller FJ, Galler M Füchtmeier B. Clinical and radiological results of patients treated with orthogonal double plating for periprosthetic femoral fractures. Int Orthop. 2014;38(12):2469-2472.
37. Pike J, Davidson D, Garbuz D, Duncan CP, O’Brien PJ, Masri BA. Principles of treatment for periprosthetic femoral shaft fractures around well-fixed total hip arthroplasty. J Am Acad Orthop Surg. 2009;17(11):677-688.
38. Serocki JH, Chandler RW, Dorr LD. Treatment of fractures about hip prostheses with compression plating. J Arthroplasty. 1992;7(2):129-135.
39. Pletka JD, Marsland D, Belkoff SM, Mears SC, Kates SL. Biomechanical comparison of 2 different locking plate fixation methods in vancouver b1 periprosthetic femur fractures. Geriatr Orthop Surg Rehabil. 2011;2(2):51-55.
40. Moloney GB, Westrick ER, Siska PA, Tarkin IS. Treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty implant: span the whole bone. Arch Orthop Trauma Surg. 2014;134(1):9-14.
41. Kubiak EN, Haller JM, Kemper DD, Presson AP, Higgins TF, Horowitz DS. Does the lateral plate need to overlap the stem to mitigate stress concentration when treating Vancouver C periprosthetic supracondylar femur fracture? J Arthroplasty. 2015;30(1):104-108.
42. Sah AP, Marshall A, Virkus WV, Estok DM 2nd, Della Valle CJ. Interprosthetic fractures of the femur: treatment with a single-locked plate. J Arthroplasty. 2010;25(2):280-286.
43. Hoffmann MF, Lotzien S, Schildhauer TA. Clinical outcome of interprosthetic femoral fractures treated with polyaxial locking plates. Injury. 2016. [Epub ahead of print]
44. Holder N, Papp S, Gofton W, Beaulé PE. Outcomes following surgical treatment of periprosthetic femur fractures: a single centre series. Can J Surg. 2014;57(3):209-213.
45. Crockarell JR Jr, Berry DJ, Lewallen DG. Nonunion after periprosthetic femoral fracture associated with total hip arthroplasty. J Bone Joint Surg Am. 1999;81(8):1073-1079.
Using 3-Dimensional Fluoroscopy to Assess Acute Clavicle Fracture Displacement: A Radiographic Study
Clavicle fractures are common injuries, accounting for 2.6% to 5% of all adult fractures.1,2 Most clavicle fractures (69%-82%) occur in the middle third or midshaft.3,4 Midshaft clavicle fractures are often treated successfully with nonoperative means consisting of shoulder immobilization with either a sling or a figure-of-8 brace. Operative indications historically have been limited to open or impending open injuries and to patients with underlying neurovascular compromise. However, recent clinical studies have found that fractures with particular characteristics may benefit from surgical fixation. Important relative indications for open reduction and internal fixation of midshaft clavicle fractures are complete fracture fragment displacement with no cortical contact, and fractures with axial shortening of more than 20 mm.5,6
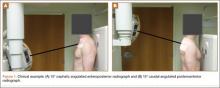
Accurately determining the extent of displacement and shortening can therefore be important in guiding treatment recommendations. The standard radiographic view for a clavicle fracture is upright or supine anteroposterior (AP). Typically, an AP radiograph with cephalic tilt of about 20° is obtained as well. On occasion, other supplemental radiographs, such as a 45° angulated view, as originally described by Quesada,7 are obtained. To our knowledge, the literature includes only 2 reports of studies that have compared different radiographic views and their accuracy in measuring fracture shortening8,9; no study has determined the best radiographic view for evaluating fracture displacement.
We conducted a study to determine which radiographic view best captures the most fracture fragment displacement. Acute midshaft clavicle fractures were assessed with simulated angled radiographs created from preoperative upright 3-dimensional (3-D) fluoroscopy scans. Our hypothesis was that a radiographic view with 20° of cephalic tilt would most often detect the most fracture displacement. In addition, we retrospectively reviewed our study patients’ initial AP injury radiographs to determine if obtaining a different view at maximum displacement would have helped identify a larger number of completely displaced midshaft clavicle fractures.
Patients and Methods
Institutional review board approval was obtained. Using our institution’s trauma registry database, we retrospectively identified 10 cases of patients who had undergone preoperative 3-D fluoroscopy for midshaft clavicle fractures. Study inclusion criteria were age 18 years or older, acute midshaft clavicle fracture, and preoperative 3-D fluoroscopy scan of clavicle available. Pediatric patients, nonacute injuries, and clavicle fractures of the lateral or medial third were excluded.
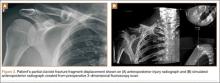
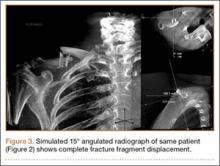
Three-dimensional fluoroscopy was used when the treating surgeon deemed it necessary for preoperative planning. All imaging was performed with a Philips MultiDiagnost Eleva 3-D fluoroscopy imager with patients in the upright standing position. (Informed patient consent was obtained.) Software bundled with the imager was used to create representative radiographs of differing angulation.
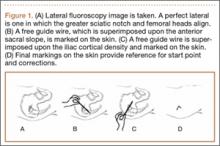
The common practice at most institutions is to obtain 2 radiographic views as part of a standard clavicle series. The additional AP angulated radiograph typically is obtained with 20° to 45° cephalic tilt from the horizontal axis. Therefore, simulated radiographs ranging from 15° to 50° of angulation in 5° increments were created, and the amount of superior displacement of the medial fragment was measured. As the simulated views were constructed from a 3-D composite image, there was none of the magnification error that occurs with AP or posteroanterior (PA) views. The stated degree of angulation mimics a radiograph’s AP cephalic tilt or PA caudal tilt (Figures 1A, 1B). For all radiographic images, displacement between fracture fragments was determined by measuring the distance between the superior cortices at the fracture site of the medial and lateral fragments. Each simulated radiograph was measured by 2 readers using standard computerized radiographic measurement tools. Final displacement was taken as the mean of the 2 measurements.
After determining which radiographic angulation demonstrated the largest number of maximally displaced fractures, we compared the simulated radiographs at that angulation with the injury AP images for all patients. Total number of patients with a completely displaced midshaft clavicle fracture and no cortical contact was recorded for the 2 radiographic views.
The Orthopaedic Trauma Association classification system8 was used to classify the clavicle fractures. Statistical analysis was performed with the Fisher exact test and a regression model, using SPSS Version 19.0 (IBM SPSS Statistics).
Results
Ten patients met the study inclusion criteria. Mean age was 32.9 years (range, 18-65 years). Seven of the 10 patients were male. Six patients had right-side clavicle fractures. Of the 10 patients, 5 had the comminuted wedge fracture pattern (15-B2.3), 2 had the simple spiral pattern (15-B1.1), 2 had the spiral wedge pattern (15-B2.1), and 1 had the oblique pattern (15-B1.2).
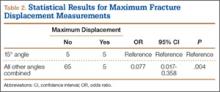
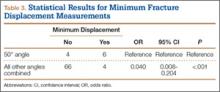
Table 1 summarizes the fracture displacement measurements obtained with the different radiographic views. Of the 10 cases, 5 showed the most displacement with the 15° tilted view (P = .004), and the other 5 showed maximum displacement with different radiographic angulations. In addition, 6 patients showed the least displacement with the 50° angulated view (P < .001). Results of the regression analysis are summarized in Tables 2 and 3.
Initial horizontal AP imaging showed completely displaced midshaft clavicle fractures in 9 of the 10 patients, and 15° simulated radiographs showed completely displaced fractures in all 10 patients (P = .50).
Discussion
Our study results demonstrated that an upright 15° radiographic tilt (AP cephalad or PA caudal) identified the most fracture displacement in the most patients with acute midshaft clavicle fractures. To our knowledge, this is the first study to identify the radiographic angulation that best shows the most clavicle fracture fragment displacement.
Other investigators have studied the accuracy of different radiographic views in the assessment of midshaft clavicle fractures, but they concentrated on fracture shortening. Smekal and colleagues9 used computed tomography (CT) and 3 different radiographic views to evaluate malunited midshaft clavicle fractures. Comparing the horizontal clavicular length measurements obtained with radiographs and CT scans, they determined that PA thoracic radiographs were in highest agreement with the CT scans. The results, however, were not statistically significant. In their study, supine CT was successful because the fractures were healed, and the displacement and shortening amounts were not affected by patient position. Sharr and Mohammed10 studied the accuracy of different views in the assessment of clavicle length in an articulated cadaver specimen. They obtained multiple AP and PA radiographs of different horizontal (medial, lateral) and vertical (cephalad, caudal) angulations. Actual clavicle length was then directly measured and compared with the length measured on the different views. The authors concluded that a PA 15° caudal radiograph was most accurate in assessing clavicular length. Both Smekal and colleagues9 and Sharr and Mohammed10 recommended the PA radiograph because it decreases the degree of magnification on AP radiographs by minimizing the film-to-object distance.
Our findings are important because more accurate determination of fracture displacement in patients with midshaft clavicle fractures may change clinical management. Nowak and colleagues11 investigated various patient and clavicle fracture characteristics that were predictive of a higher rate of long-term sequelae. They found that complete fracture displacement was the strongest radiographic predictor of patients’ beliefs that they were fully recovered from injury at final follow-up. The authors concluded that fractures with no bony contact should receive more “active” management. Robinson and colleagues12 studied a cohort of patients with nonoperatively managed midshaft clavicle fractures and concluded that complete fracture displacement significantly increased risk for nonunion (this risk was 2.3 times higher in patients with displaced fractures than in patients with nondisplaced fractures). Last, McKee and colleagues13 found that shoulder strength and endurance were significantly decreased in nonoperatively treated displaced midshaft clavicle fractures than in the same patients’ uninjured shoulders.
Extending the results of these studies, recent prospective randomized control trials and a meta-analysis have compared the clinical outcomes of nonoperatively and operatively managed displaced midshaft clavicle fractures.14-18 With few exceptions, these studies found improved clinical results with operative fixation. In one such study, the Canadian Orthopaedic Trauma Society14 randomized patients with displaced midshaft clavicle fractures to either operative plate fixation or sling immobilization. The operative group was found to have improved Disability of the Arm, Shoulder, and Hand scores, improved Constant shoulder scores, increased patient satisfaction, faster mean time to bony fracture union, higher satisfaction with shoulder appearance, and lower rates of nonunion and malunion. Given the results of these studies, accurate identification of a displaced midshaft clavicle fracture with no cortical contact is fundamental in deciding whether to recommend operative fixation.
Retrospective review of our cohort’s initial radiographs revealed 1 case in which the patient’s completely displaced midshaft clavicle fracture would not have been diagnosed solely with an AP horizontal image. Cortical contact was seen on a standard AP clavicle radiograph (Figures 2A, 2B), and a 15° tilt radiograph created from 3-D fluoroscopy scan showed complete fracture fragment displacement (Figure 3). A change in fracture classification from partially displaced to fully displaced could alter the type of management used by a treating surgeon.
There were obvious weaknesses to this study. First, its sample size was small (10 patients). Nevertheless, we had sufficient numbers to find a statistically significant angulation. Second, a wider range of radiographic angles could have been studied. Our intent, however, was to investigate the accuracy of the 2 most common supplementary clavicle views (20° and 45° cephalic tilt). Therefore, we selected a range of simulated radiographs that began 5° outside these angulations. Third, we measured only the degree of fracture displacement; we were unable to accurately access fracture shortening, as the 3-D fluoroscopic images were limited to the injured clavicles. A potential solution to this problem is to widen the exposure field in order to include the entire chest and allow clavicular length comparison against the uninjured side. Doing this would have been possible, but at the expense of increasing the patient’s radiation exposure.
This innovative study used 3-D fluoroscopy to capture clavicle fracture images with patients in an upright position. Unlike standard CT, in which patients are supine, this 3-D imaging technology better emulates the patient positioning used for upright radiographs, thereby avoiding potential fracture fragment alignment changes caused by shifts in body position. In addition, 3-D fluoroscopy allows us to create multiple precise simulated radiographic angulations without the magnification error of AP radiographs and, to a lesser extent, PA radiographs. Having a standing PA 15° caudal tilt radiograph obviates the need for CT with 3-D reconstruction. More fine detail may be revealed by CT with 3-D reconstruction than by a standing PA 15° caudal tilt radiograph, but the patient faces less radiation risk and cost with the radiograph.
There is no consensus as to what constitutes the standard radiographic series for clavicle fractures. Radiographic technique can vary with respect to supplemental view angulation, supine or upright patient positioning, and AP or PA radiographic views. Although our study did not address the effect of supine versus upright patient positioning on acute midshaft clavicle fracture displacement, we think that, for all clinical and research purposes, upright 15° caudal PA radiographs should be obtained for patients with acute midshaft clavicle fractures.
Conclusion
Our retrospective study of 10 patients with acute midshaft clavicle fractures and preoperative upright 3-D fluoroscopy scans found that a 15° angulated radiograph most often demonstrated the most fracture fragment displacement. Given these findings, we recommend obtaining an additional PA 15° caudal radiograph in the upright position for patients with midshaft clavicle fractures to best assess the extent of fracture displacement. Accurately identifying the degree of fracture displacement is important, as operative management of completely displaced fractures has been shown to improve clinical outcomes.
1. Postacchini F, Gumina S, De Santis P, Albo F. Epidemiology of clavicle fractures. J Shoulder Elbow Surg. 2002;11(5):452-456.
2. Nordqvist A, Petersson C. The incidence of fractures of the clavicle. Clin Orthop Relat Res. 1994;(300):127-132.
3. Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. J Bone Joint Surg Br. 1998;80(3):476-484.
4. Rowe CR. An atlas of anatomy and treatment of midclavicular fractures. Clin Orthop Relat Res. 1968;(58):29-42.
5. Jeray KJ. Acute midshaft clavicular fracture. J Am Acad Orthop Surg. 2007;15(4):239-248.
6. Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. J Bone Joint Surg Am. 2009;91(2):447-460.
7. Quesada F. Technique for the roentgen diagnosis of fractures of the clavicle. Surg Gynecol Obstet. 1926;42:424-428.
8. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association Classification, Database and Outcomes Committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
9. Smekal V, Deml C, Irenberger A, et al. Length determination in midshaft clavicle fractures: validation of measurement. J Orthop Trauma. 2008;22(7):458-462.
10. Sharr JR, Mohammed KD. Optimizing the radiographic technique in clavicular fractures. J Shoulder Elbow Surg. 2003;12(2):170-172.
11. Nowak J, Holgersson M, Larsson S. Can we predict long-term sequelae after fractures of the clavicle based on initial findings? A prospective study with nine to ten years of follow-up. J Shoulder Elbow Surg. 2004;13(5):479-486.
12. Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am. 2004;86(7):1359-1365.
13. McKee MD, Pedersen EM, Jones C, et al. Deficits following nonoperative treatment of displaced midshaft clavicular fractures. J Bone Joint Surg Am. 2006;88(1):35-40.
14. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
15. Judd DB, Pallis MP, Smith E, Bottoni CR. Acute operative stabilization versus nonoperative management of clavicle fractures. Am J Orthop. 2009;38(7):341-345.
16. Smekal V, Irenberger A, Struve P, Wambacher M, Krappinger D, Kralinger FS. Elastic stable intramedullary nailing versus nonoperative treatment of displaced midshaft clavicular fractures—a randomized, controlled, clinical trial. J Orthop Trauma. 2009;23(2):106-112.
17. Witzel K. Intramedullary osteosynthesis in fractures of the mid-third of the clavicle in sports traumatology [in German]. Z Orthop Unfall. 2007;145(5):639-642.
18. McKee RC, Whelan DB, Schemitsch EH, McKee MD. Operative versus nonoperative care of displaced midshaft clavicular fractures: a meta-analysis of randomized clinical trials. J Bone Joint Surg Am. 2012;94(8):675-684.
Clavicle fractures are common injuries, accounting for 2.6% to 5% of all adult fractures.1,2 Most clavicle fractures (69%-82%) occur in the middle third or midshaft.3,4 Midshaft clavicle fractures are often treated successfully with nonoperative means consisting of shoulder immobilization with either a sling or a figure-of-8 brace. Operative indications historically have been limited to open or impending open injuries and to patients with underlying neurovascular compromise. However, recent clinical studies have found that fractures with particular characteristics may benefit from surgical fixation. Important relative indications for open reduction and internal fixation of midshaft clavicle fractures are complete fracture fragment displacement with no cortical contact, and fractures with axial shortening of more than 20 mm.5,6
Accurately determining the extent of displacement and shortening can therefore be important in guiding treatment recommendations. The standard radiographic view for a clavicle fracture is upright or supine anteroposterior (AP). Typically, an AP radiograph with cephalic tilt of about 20° is obtained as well. On occasion, other supplemental radiographs, such as a 45° angulated view, as originally described by Quesada,7 are obtained. To our knowledge, the literature includes only 2 reports of studies that have compared different radiographic views and their accuracy in measuring fracture shortening8,9; no study has determined the best radiographic view for evaluating fracture displacement.
We conducted a study to determine which radiographic view best captures the most fracture fragment displacement. Acute midshaft clavicle fractures were assessed with simulated angled radiographs created from preoperative upright 3-dimensional (3-D) fluoroscopy scans. Our hypothesis was that a radiographic view with 20° of cephalic tilt would most often detect the most fracture displacement. In addition, we retrospectively reviewed our study patients’ initial AP injury radiographs to determine if obtaining a different view at maximum displacement would have helped identify a larger number of completely displaced midshaft clavicle fractures.
Patients and Methods
Institutional review board approval was obtained. Using our institution’s trauma registry database, we retrospectively identified 10 cases of patients who had undergone preoperative 3-D fluoroscopy for midshaft clavicle fractures. Study inclusion criteria were age 18 years or older, acute midshaft clavicle fracture, and preoperative 3-D fluoroscopy scan of clavicle available. Pediatric patients, nonacute injuries, and clavicle fractures of the lateral or medial third were excluded.
Three-dimensional fluoroscopy was used when the treating surgeon deemed it necessary for preoperative planning. All imaging was performed with a Philips MultiDiagnost Eleva 3-D fluoroscopy imager with patients in the upright standing position. (Informed patient consent was obtained.) Software bundled with the imager was used to create representative radiographs of differing angulation.
The common practice at most institutions is to obtain 2 radiographic views as part of a standard clavicle series. The additional AP angulated radiograph typically is obtained with 20° to 45° cephalic tilt from the horizontal axis. Therefore, simulated radiographs ranging from 15° to 50° of angulation in 5° increments were created, and the amount of superior displacement of the medial fragment was measured. As the simulated views were constructed from a 3-D composite image, there was none of the magnification error that occurs with AP or posteroanterior (PA) views. The stated degree of angulation mimics a radiograph’s AP cephalic tilt or PA caudal tilt (Figures 1A, 1B). For all radiographic images, displacement between fracture fragments was determined by measuring the distance between the superior cortices at the fracture site of the medial and lateral fragments. Each simulated radiograph was measured by 2 readers using standard computerized radiographic measurement tools. Final displacement was taken as the mean of the 2 measurements.
After determining which radiographic angulation demonstrated the largest number of maximally displaced fractures, we compared the simulated radiographs at that angulation with the injury AP images for all patients. Total number of patients with a completely displaced midshaft clavicle fracture and no cortical contact was recorded for the 2 radiographic views.
The Orthopaedic Trauma Association classification system8 was used to classify the clavicle fractures. Statistical analysis was performed with the Fisher exact test and a regression model, using SPSS Version 19.0 (IBM SPSS Statistics).
Results
Ten patients met the study inclusion criteria. Mean age was 32.9 years (range, 18-65 years). Seven of the 10 patients were male. Six patients had right-side clavicle fractures. Of the 10 patients, 5 had the comminuted wedge fracture pattern (15-B2.3), 2 had the simple spiral pattern (15-B1.1), 2 had the spiral wedge pattern (15-B2.1), and 1 had the oblique pattern (15-B1.2).
Table 1 summarizes the fracture displacement measurements obtained with the different radiographic views. Of the 10 cases, 5 showed the most displacement with the 15° tilted view (P = .004), and the other 5 showed maximum displacement with different radiographic angulations. In addition, 6 patients showed the least displacement with the 50° angulated view (P < .001). Results of the regression analysis are summarized in Tables 2 and 3.
Initial horizontal AP imaging showed completely displaced midshaft clavicle fractures in 9 of the 10 patients, and 15° simulated radiographs showed completely displaced fractures in all 10 patients (P = .50).
Discussion
Our study results demonstrated that an upright 15° radiographic tilt (AP cephalad or PA caudal) identified the most fracture displacement in the most patients with acute midshaft clavicle fractures. To our knowledge, this is the first study to identify the radiographic angulation that best shows the most clavicle fracture fragment displacement.
Other investigators have studied the accuracy of different radiographic views in the assessment of midshaft clavicle fractures, but they concentrated on fracture shortening. Smekal and colleagues9 used computed tomography (CT) and 3 different radiographic views to evaluate malunited midshaft clavicle fractures. Comparing the horizontal clavicular length measurements obtained with radiographs and CT scans, they determined that PA thoracic radiographs were in highest agreement with the CT scans. The results, however, were not statistically significant. In their study, supine CT was successful because the fractures were healed, and the displacement and shortening amounts were not affected by patient position. Sharr and Mohammed10 studied the accuracy of different views in the assessment of clavicle length in an articulated cadaver specimen. They obtained multiple AP and PA radiographs of different horizontal (medial, lateral) and vertical (cephalad, caudal) angulations. Actual clavicle length was then directly measured and compared with the length measured on the different views. The authors concluded that a PA 15° caudal radiograph was most accurate in assessing clavicular length. Both Smekal and colleagues9 and Sharr and Mohammed10 recommended the PA radiograph because it decreases the degree of magnification on AP radiographs by minimizing the film-to-object distance.
Our findings are important because more accurate determination of fracture displacement in patients with midshaft clavicle fractures may change clinical management. Nowak and colleagues11 investigated various patient and clavicle fracture characteristics that were predictive of a higher rate of long-term sequelae. They found that complete fracture displacement was the strongest radiographic predictor of patients’ beliefs that they were fully recovered from injury at final follow-up. The authors concluded that fractures with no bony contact should receive more “active” management. Robinson and colleagues12 studied a cohort of patients with nonoperatively managed midshaft clavicle fractures and concluded that complete fracture displacement significantly increased risk for nonunion (this risk was 2.3 times higher in patients with displaced fractures than in patients with nondisplaced fractures). Last, McKee and colleagues13 found that shoulder strength and endurance were significantly decreased in nonoperatively treated displaced midshaft clavicle fractures than in the same patients’ uninjured shoulders.
Extending the results of these studies, recent prospective randomized control trials and a meta-analysis have compared the clinical outcomes of nonoperatively and operatively managed displaced midshaft clavicle fractures.14-18 With few exceptions, these studies found improved clinical results with operative fixation. In one such study, the Canadian Orthopaedic Trauma Society14 randomized patients with displaced midshaft clavicle fractures to either operative plate fixation or sling immobilization. The operative group was found to have improved Disability of the Arm, Shoulder, and Hand scores, improved Constant shoulder scores, increased patient satisfaction, faster mean time to bony fracture union, higher satisfaction with shoulder appearance, and lower rates of nonunion and malunion. Given the results of these studies, accurate identification of a displaced midshaft clavicle fracture with no cortical contact is fundamental in deciding whether to recommend operative fixation.
Retrospective review of our cohort’s initial radiographs revealed 1 case in which the patient’s completely displaced midshaft clavicle fracture would not have been diagnosed solely with an AP horizontal image. Cortical contact was seen on a standard AP clavicle radiograph (Figures 2A, 2B), and a 15° tilt radiograph created from 3-D fluoroscopy scan showed complete fracture fragment displacement (Figure 3). A change in fracture classification from partially displaced to fully displaced could alter the type of management used by a treating surgeon.
There were obvious weaknesses to this study. First, its sample size was small (10 patients). Nevertheless, we had sufficient numbers to find a statistically significant angulation. Second, a wider range of radiographic angles could have been studied. Our intent, however, was to investigate the accuracy of the 2 most common supplementary clavicle views (20° and 45° cephalic tilt). Therefore, we selected a range of simulated radiographs that began 5° outside these angulations. Third, we measured only the degree of fracture displacement; we were unable to accurately access fracture shortening, as the 3-D fluoroscopic images were limited to the injured clavicles. A potential solution to this problem is to widen the exposure field in order to include the entire chest and allow clavicular length comparison against the uninjured side. Doing this would have been possible, but at the expense of increasing the patient’s radiation exposure.
This innovative study used 3-D fluoroscopy to capture clavicle fracture images with patients in an upright position. Unlike standard CT, in which patients are supine, this 3-D imaging technology better emulates the patient positioning used for upright radiographs, thereby avoiding potential fracture fragment alignment changes caused by shifts in body position. In addition, 3-D fluoroscopy allows us to create multiple precise simulated radiographic angulations without the magnification error of AP radiographs and, to a lesser extent, PA radiographs. Having a standing PA 15° caudal tilt radiograph obviates the need for CT with 3-D reconstruction. More fine detail may be revealed by CT with 3-D reconstruction than by a standing PA 15° caudal tilt radiograph, but the patient faces less radiation risk and cost with the radiograph.
There is no consensus as to what constitutes the standard radiographic series for clavicle fractures. Radiographic technique can vary with respect to supplemental view angulation, supine or upright patient positioning, and AP or PA radiographic views. Although our study did not address the effect of supine versus upright patient positioning on acute midshaft clavicle fracture displacement, we think that, for all clinical and research purposes, upright 15° caudal PA radiographs should be obtained for patients with acute midshaft clavicle fractures.
Conclusion
Our retrospective study of 10 patients with acute midshaft clavicle fractures and preoperative upright 3-D fluoroscopy scans found that a 15° angulated radiograph most often demonstrated the most fracture fragment displacement. Given these findings, we recommend obtaining an additional PA 15° caudal radiograph in the upright position for patients with midshaft clavicle fractures to best assess the extent of fracture displacement. Accurately identifying the degree of fracture displacement is important, as operative management of completely displaced fractures has been shown to improve clinical outcomes.
Clavicle fractures are common injuries, accounting for 2.6% to 5% of all adult fractures.1,2 Most clavicle fractures (69%-82%) occur in the middle third or midshaft.3,4 Midshaft clavicle fractures are often treated successfully with nonoperative means consisting of shoulder immobilization with either a sling or a figure-of-8 brace. Operative indications historically have been limited to open or impending open injuries and to patients with underlying neurovascular compromise. However, recent clinical studies have found that fractures with particular characteristics may benefit from surgical fixation. Important relative indications for open reduction and internal fixation of midshaft clavicle fractures are complete fracture fragment displacement with no cortical contact, and fractures with axial shortening of more than 20 mm.5,6
Accurately determining the extent of displacement and shortening can therefore be important in guiding treatment recommendations. The standard radiographic view for a clavicle fracture is upright or supine anteroposterior (AP). Typically, an AP radiograph with cephalic tilt of about 20° is obtained as well. On occasion, other supplemental radiographs, such as a 45° angulated view, as originally described by Quesada,7 are obtained. To our knowledge, the literature includes only 2 reports of studies that have compared different radiographic views and their accuracy in measuring fracture shortening8,9; no study has determined the best radiographic view for evaluating fracture displacement.
We conducted a study to determine which radiographic view best captures the most fracture fragment displacement. Acute midshaft clavicle fractures were assessed with simulated angled radiographs created from preoperative upright 3-dimensional (3-D) fluoroscopy scans. Our hypothesis was that a radiographic view with 20° of cephalic tilt would most often detect the most fracture displacement. In addition, we retrospectively reviewed our study patients’ initial AP injury radiographs to determine if obtaining a different view at maximum displacement would have helped identify a larger number of completely displaced midshaft clavicle fractures.
Patients and Methods
Institutional review board approval was obtained. Using our institution’s trauma registry database, we retrospectively identified 10 cases of patients who had undergone preoperative 3-D fluoroscopy for midshaft clavicle fractures. Study inclusion criteria were age 18 years or older, acute midshaft clavicle fracture, and preoperative 3-D fluoroscopy scan of clavicle available. Pediatric patients, nonacute injuries, and clavicle fractures of the lateral or medial third were excluded.
Three-dimensional fluoroscopy was used when the treating surgeon deemed it necessary for preoperative planning. All imaging was performed with a Philips MultiDiagnost Eleva 3-D fluoroscopy imager with patients in the upright standing position. (Informed patient consent was obtained.) Software bundled with the imager was used to create representative radiographs of differing angulation.
The common practice at most institutions is to obtain 2 radiographic views as part of a standard clavicle series. The additional AP angulated radiograph typically is obtained with 20° to 45° cephalic tilt from the horizontal axis. Therefore, simulated radiographs ranging from 15° to 50° of angulation in 5° increments were created, and the amount of superior displacement of the medial fragment was measured. As the simulated views were constructed from a 3-D composite image, there was none of the magnification error that occurs with AP or posteroanterior (PA) views. The stated degree of angulation mimics a radiograph’s AP cephalic tilt or PA caudal tilt (Figures 1A, 1B). For all radiographic images, displacement between fracture fragments was determined by measuring the distance between the superior cortices at the fracture site of the medial and lateral fragments. Each simulated radiograph was measured by 2 readers using standard computerized radiographic measurement tools. Final displacement was taken as the mean of the 2 measurements.
After determining which radiographic angulation demonstrated the largest number of maximally displaced fractures, we compared the simulated radiographs at that angulation with the injury AP images for all patients. Total number of patients with a completely displaced midshaft clavicle fracture and no cortical contact was recorded for the 2 radiographic views.
The Orthopaedic Trauma Association classification system8 was used to classify the clavicle fractures. Statistical analysis was performed with the Fisher exact test and a regression model, using SPSS Version 19.0 (IBM SPSS Statistics).
Results
Ten patients met the study inclusion criteria. Mean age was 32.9 years (range, 18-65 years). Seven of the 10 patients were male. Six patients had right-side clavicle fractures. Of the 10 patients, 5 had the comminuted wedge fracture pattern (15-B2.3), 2 had the simple spiral pattern (15-B1.1), 2 had the spiral wedge pattern (15-B2.1), and 1 had the oblique pattern (15-B1.2).
Table 1 summarizes the fracture displacement measurements obtained with the different radiographic views. Of the 10 cases, 5 showed the most displacement with the 15° tilted view (P = .004), and the other 5 showed maximum displacement with different radiographic angulations. In addition, 6 patients showed the least displacement with the 50° angulated view (P < .001). Results of the regression analysis are summarized in Tables 2 and 3.
Initial horizontal AP imaging showed completely displaced midshaft clavicle fractures in 9 of the 10 patients, and 15° simulated radiographs showed completely displaced fractures in all 10 patients (P = .50).
Discussion
Our study results demonstrated that an upright 15° radiographic tilt (AP cephalad or PA caudal) identified the most fracture displacement in the most patients with acute midshaft clavicle fractures. To our knowledge, this is the first study to identify the radiographic angulation that best shows the most clavicle fracture fragment displacement.
Other investigators have studied the accuracy of different radiographic views in the assessment of midshaft clavicle fractures, but they concentrated on fracture shortening. Smekal and colleagues9 used computed tomography (CT) and 3 different radiographic views to evaluate malunited midshaft clavicle fractures. Comparing the horizontal clavicular length measurements obtained with radiographs and CT scans, they determined that PA thoracic radiographs were in highest agreement with the CT scans. The results, however, were not statistically significant. In their study, supine CT was successful because the fractures were healed, and the displacement and shortening amounts were not affected by patient position. Sharr and Mohammed10 studied the accuracy of different views in the assessment of clavicle length in an articulated cadaver specimen. They obtained multiple AP and PA radiographs of different horizontal (medial, lateral) and vertical (cephalad, caudal) angulations. Actual clavicle length was then directly measured and compared with the length measured on the different views. The authors concluded that a PA 15° caudal radiograph was most accurate in assessing clavicular length. Both Smekal and colleagues9 and Sharr and Mohammed10 recommended the PA radiograph because it decreases the degree of magnification on AP radiographs by minimizing the film-to-object distance.
Our findings are important because more accurate determination of fracture displacement in patients with midshaft clavicle fractures may change clinical management. Nowak and colleagues11 investigated various patient and clavicle fracture characteristics that were predictive of a higher rate of long-term sequelae. They found that complete fracture displacement was the strongest radiographic predictor of patients’ beliefs that they were fully recovered from injury at final follow-up. The authors concluded that fractures with no bony contact should receive more “active” management. Robinson and colleagues12 studied a cohort of patients with nonoperatively managed midshaft clavicle fractures and concluded that complete fracture displacement significantly increased risk for nonunion (this risk was 2.3 times higher in patients with displaced fractures than in patients with nondisplaced fractures). Last, McKee and colleagues13 found that shoulder strength and endurance were significantly decreased in nonoperatively treated displaced midshaft clavicle fractures than in the same patients’ uninjured shoulders.
Extending the results of these studies, recent prospective randomized control trials and a meta-analysis have compared the clinical outcomes of nonoperatively and operatively managed displaced midshaft clavicle fractures.14-18 With few exceptions, these studies found improved clinical results with operative fixation. In one such study, the Canadian Orthopaedic Trauma Society14 randomized patients with displaced midshaft clavicle fractures to either operative plate fixation or sling immobilization. The operative group was found to have improved Disability of the Arm, Shoulder, and Hand scores, improved Constant shoulder scores, increased patient satisfaction, faster mean time to bony fracture union, higher satisfaction with shoulder appearance, and lower rates of nonunion and malunion. Given the results of these studies, accurate identification of a displaced midshaft clavicle fracture with no cortical contact is fundamental in deciding whether to recommend operative fixation.
Retrospective review of our cohort’s initial radiographs revealed 1 case in which the patient’s completely displaced midshaft clavicle fracture would not have been diagnosed solely with an AP horizontal image. Cortical contact was seen on a standard AP clavicle radiograph (Figures 2A, 2B), and a 15° tilt radiograph created from 3-D fluoroscopy scan showed complete fracture fragment displacement (Figure 3). A change in fracture classification from partially displaced to fully displaced could alter the type of management used by a treating surgeon.
There were obvious weaknesses to this study. First, its sample size was small (10 patients). Nevertheless, we had sufficient numbers to find a statistically significant angulation. Second, a wider range of radiographic angles could have been studied. Our intent, however, was to investigate the accuracy of the 2 most common supplementary clavicle views (20° and 45° cephalic tilt). Therefore, we selected a range of simulated radiographs that began 5° outside these angulations. Third, we measured only the degree of fracture displacement; we were unable to accurately access fracture shortening, as the 3-D fluoroscopic images were limited to the injured clavicles. A potential solution to this problem is to widen the exposure field in order to include the entire chest and allow clavicular length comparison against the uninjured side. Doing this would have been possible, but at the expense of increasing the patient’s radiation exposure.
This innovative study used 3-D fluoroscopy to capture clavicle fracture images with patients in an upright position. Unlike standard CT, in which patients are supine, this 3-D imaging technology better emulates the patient positioning used for upright radiographs, thereby avoiding potential fracture fragment alignment changes caused by shifts in body position. In addition, 3-D fluoroscopy allows us to create multiple precise simulated radiographic angulations without the magnification error of AP radiographs and, to a lesser extent, PA radiographs. Having a standing PA 15° caudal tilt radiograph obviates the need for CT with 3-D reconstruction. More fine detail may be revealed by CT with 3-D reconstruction than by a standing PA 15° caudal tilt radiograph, but the patient faces less radiation risk and cost with the radiograph.
There is no consensus as to what constitutes the standard radiographic series for clavicle fractures. Radiographic technique can vary with respect to supplemental view angulation, supine or upright patient positioning, and AP or PA radiographic views. Although our study did not address the effect of supine versus upright patient positioning on acute midshaft clavicle fracture displacement, we think that, for all clinical and research purposes, upright 15° caudal PA radiographs should be obtained for patients with acute midshaft clavicle fractures.
Conclusion
Our retrospective study of 10 patients with acute midshaft clavicle fractures and preoperative upright 3-D fluoroscopy scans found that a 15° angulated radiograph most often demonstrated the most fracture fragment displacement. Given these findings, we recommend obtaining an additional PA 15° caudal radiograph in the upright position for patients with midshaft clavicle fractures to best assess the extent of fracture displacement. Accurately identifying the degree of fracture displacement is important, as operative management of completely displaced fractures has been shown to improve clinical outcomes.
1. Postacchini F, Gumina S, De Santis P, Albo F. Epidemiology of clavicle fractures. J Shoulder Elbow Surg. 2002;11(5):452-456.
2. Nordqvist A, Petersson C. The incidence of fractures of the clavicle. Clin Orthop Relat Res. 1994;(300):127-132.
3. Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. J Bone Joint Surg Br. 1998;80(3):476-484.
4. Rowe CR. An atlas of anatomy and treatment of midclavicular fractures. Clin Orthop Relat Res. 1968;(58):29-42.
5. Jeray KJ. Acute midshaft clavicular fracture. J Am Acad Orthop Surg. 2007;15(4):239-248.
6. Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. J Bone Joint Surg Am. 2009;91(2):447-460.
7. Quesada F. Technique for the roentgen diagnosis of fractures of the clavicle. Surg Gynecol Obstet. 1926;42:424-428.
8. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association Classification, Database and Outcomes Committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
9. Smekal V, Deml C, Irenberger A, et al. Length determination in midshaft clavicle fractures: validation of measurement. J Orthop Trauma. 2008;22(7):458-462.
10. Sharr JR, Mohammed KD. Optimizing the radiographic technique in clavicular fractures. J Shoulder Elbow Surg. 2003;12(2):170-172.
11. Nowak J, Holgersson M, Larsson S. Can we predict long-term sequelae after fractures of the clavicle based on initial findings? A prospective study with nine to ten years of follow-up. J Shoulder Elbow Surg. 2004;13(5):479-486.
12. Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am. 2004;86(7):1359-1365.
13. McKee MD, Pedersen EM, Jones C, et al. Deficits following nonoperative treatment of displaced midshaft clavicular fractures. J Bone Joint Surg Am. 2006;88(1):35-40.
14. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
15. Judd DB, Pallis MP, Smith E, Bottoni CR. Acute operative stabilization versus nonoperative management of clavicle fractures. Am J Orthop. 2009;38(7):341-345.
16. Smekal V, Irenberger A, Struve P, Wambacher M, Krappinger D, Kralinger FS. Elastic stable intramedullary nailing versus nonoperative treatment of displaced midshaft clavicular fractures—a randomized, controlled, clinical trial. J Orthop Trauma. 2009;23(2):106-112.
17. Witzel K. Intramedullary osteosynthesis in fractures of the mid-third of the clavicle in sports traumatology [in German]. Z Orthop Unfall. 2007;145(5):639-642.
18. McKee RC, Whelan DB, Schemitsch EH, McKee MD. Operative versus nonoperative care of displaced midshaft clavicular fractures: a meta-analysis of randomized clinical trials. J Bone Joint Surg Am. 2012;94(8):675-684.
1. Postacchini F, Gumina S, De Santis P, Albo F. Epidemiology of clavicle fractures. J Shoulder Elbow Surg. 2002;11(5):452-456.
2. Nordqvist A, Petersson C. The incidence of fractures of the clavicle. Clin Orthop Relat Res. 1994;(300):127-132.
3. Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. J Bone Joint Surg Br. 1998;80(3):476-484.
4. Rowe CR. An atlas of anatomy and treatment of midclavicular fractures. Clin Orthop Relat Res. 1968;(58):29-42.
5. Jeray KJ. Acute midshaft clavicular fracture. J Am Acad Orthop Surg. 2007;15(4):239-248.
6. Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. J Bone Joint Surg Am. 2009;91(2):447-460.
7. Quesada F. Technique for the roentgen diagnosis of fractures of the clavicle. Surg Gynecol Obstet. 1926;42:424-428.
8. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association Classification, Database and Outcomes Committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
9. Smekal V, Deml C, Irenberger A, et al. Length determination in midshaft clavicle fractures: validation of measurement. J Orthop Trauma. 2008;22(7):458-462.
10. Sharr JR, Mohammed KD. Optimizing the radiographic technique in clavicular fractures. J Shoulder Elbow Surg. 2003;12(2):170-172.
11. Nowak J, Holgersson M, Larsson S. Can we predict long-term sequelae after fractures of the clavicle based on initial findings? A prospective study with nine to ten years of follow-up. J Shoulder Elbow Surg. 2004;13(5):479-486.
12. Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am. 2004;86(7):1359-1365.
13. McKee MD, Pedersen EM, Jones C, et al. Deficits following nonoperative treatment of displaced midshaft clavicular fractures. J Bone Joint Surg Am. 2006;88(1):35-40.
14. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
15. Judd DB, Pallis MP, Smith E, Bottoni CR. Acute operative stabilization versus nonoperative management of clavicle fractures. Am J Orthop. 2009;38(7):341-345.
16. Smekal V, Irenberger A, Struve P, Wambacher M, Krappinger D, Kralinger FS. Elastic stable intramedullary nailing versus nonoperative treatment of displaced midshaft clavicular fractures—a randomized, controlled, clinical trial. J Orthop Trauma. 2009;23(2):106-112.
17. Witzel K. Intramedullary osteosynthesis in fractures of the mid-third of the clavicle in sports traumatology [in German]. Z Orthop Unfall. 2007;145(5):639-642.
18. McKee RC, Whelan DB, Schemitsch EH, McKee MD. Operative versus nonoperative care of displaced midshaft clavicular fractures: a meta-analysis of randomized clinical trials. J Bone Joint Surg Am. 2012;94(8):675-684.
Treatment of Acetabular Fractures in Adolescents
In children, pelvic fractures are uncommon, with an incidence ranging from 1% to 4.6% of all pediatric fractures,1-4 and acetabular fractures make up only 0.8% to 15% of pelvic fractures.1,3,5,6 Acetabular fractures are so uncommon in children partly because of the cartilaginous nature of the immature acetabulum. The increased cartilage volume relative to adults provides greater capacity for energy absorption, resulting in greater elastic and plastic deformation before fracture occurrence. More force is therefore required to cause a fracture, and associated visceral injuries, head injuries, and long-bone fractures are common.3,7,8
The impact of acetabular fractures on adolescents warrants special attention because any resulting disability will affect them during their most productive years. Both avascular necrosis (AVN) and degenerative arthritis are particularly devastating complications in this age group. Complications such as premature physeal closure9-15 are unique to adolescents, and there is little information available on how injury in older children affects growth in this area.
There have been very few studies of the outcomes of these injuries in children. Mostly, there have been case reports and small series primarily dealing with nonoperative management of acetabular fractures in adolescents.3,10,11,16-20 By contrast, operative treatment of acetabular fractures in adults has been well described, and outcomes widely reported. As a result, much of our knowledge about managing these injuries is extrapolated from the adult literature. Although treatment of acetabular fractures in adults has evolved substantially, treatment of these injuries in adolescents remains primarily nonoperative. We conducted a study to evaluate outcomes of treatment of adolescent acetabular fractures.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the cases of all adolescent patients admitted with a diagnosis of acetabular fracture to 2 academic institutions between 1991 and 2003. Thirty-eight patients (28 males, 10 females) were identified. Mean age at time of injury was 15 years (range, 11-18 years). Mean follow-up was 3.2 years (range, 5-180 months).
Data on fracture types, treatment methods, associated injuries, complications, union rates, pain, and return to normal activities were collected. Acetabular fractures were classified according to the system of Letournel and Judet.21 There were 20 elementary and 18 associated fractures.
Of the 38 patients, 30 sustained high-energy trauma in motor vehicle accidents (25) or in falls from significant heights (5). The other 8 patients injured themselves playing sports (4 had severe traumatic brain injury, 2 had labial wounds, and 2 had injuries involving the abdominal viscera). Twelve patients had associated pelvic ring injuries, 18 had femoral head dislocations, 2 had femoral head fractures, and 13 had evidence of impaction injury to the femoral head articular cartilage. Twelve patients had marginal impaction of the acetabular wall. Fifteen patients had open triradiate physes at time of injury (Table 1).
Thirty-seven of the 38 patients were treated with open reduction and internal fixation (ORIF) by an experienced orthopedic trauma surgeon; 1 patient with a stable posterior wall fracture was treated nonoperatively. Surgical indications were articular displacement of more than 1 mm, hip joint instability, irreducible hip dislocation, and intra-articular fracture fragments. In the 37 surgically treated cases, the approaches used were Kocher-Langenbeck (22), ilioinguinal (8), combined Kocher-Langenbeck/ilioinguinal (5), and triradiate (2).
Immediate postoperative radiographs were evaluated by 3 orthopedic surgeons blinded to the patients’ clinical outcomes. Displacement was evaluated on anteroposterior (AP) and Judet views of the pelvis, as described by Matta,22 and reductions were classified as anatomical (0-1 mm of displacement), imperfect (>1 to 3 mm), poor (>3 mm), or surgical secondary congruence (Table 2).
Results
Thirty-seven patients underwent acetabular fracture ORIF. Immediate postoperative radiographs showed 30 anatomical reductions and 7 imperfect reductions. One patient had surgical secondary congruence and developed AVN of the hip. We could not identify an association between the quality of the reduction and the outcome with respect to pain or return to activity. However, no patient had a poor reduction. An illustrative case is presented in Figures 1 to 4.
All acetabular fractures united within 4.5 months (range, 3.0-8.0 months) after the index procedure. Early postoperative complications included 3 cases of meralgia paresthetica and 13 cases of abductor weakness. Meralgia paresthetica resolved spontaneously in all 3 patients. Of the 13 patients with abductor weakness, 11 improved with physical therapy, 1 was limited by the head injury, and 1 subsequently underwent hip fusion. One patient had a deep vein thrombosis (DVT) that was identified before surgery and managed with warfarin.
Other complications included 1 case of deep infection of the surgical wound. This infection presented 4 months after surgery and was treated with débridement, hardware removal, and a 3-month course of antibiotics. Two patients who sustained hip dislocations at time of injury developed AVN of the femoral head. Both developed osteoarthritis, and 1 underwent hip fusion. Eight patients developed heterotopic ossification on the side of the acetabular fracture; 4 of them underwent surgical excision. Four patients required a separate operation for hardware removal. Four patients with triradiate cartilage involvement went on to premature closure. No patient had any leg-length discrepancy or dysplasia at time of follow-up.
Thirty-four of the 38 patients returned to their regular activities. For these patients, mean time to return to full activity was 7.0 months (range, 3-30 months); there was no difference in mean time to return to full activity between skeletally mature and skeletally immature patients (6.6 vs 7.4 months; P = .57). Of the other 4 patients, 1 had permanent cognitive and physical disability with an ataxic gait as a result of a traumatic brain injury, 2 were limited by AVN (1 underwent hip fusion), and 1 was limited by an ipsilateral knee injury.
Of the 38 patients, 29 were pain-free; 6 had occasional, intermittent mild pain that did not limit their activities; and 3 had severe, activity-limiting pain. Of the 6 patients with mild pain, 2 had femoral impaction injuries, and 4 had marginal impaction injuries. Of the 3 patients with severe pain, 2 developed femoral head AVN, and 1 had multiple ipsilateral extremity injuries involving the femur, knee, and tibia.
Discussion
The traditional treatment for acetabular fractures in children has been nonoperative,8,10 and there are few specific treatment guidelines.13 Recent recommendations are nonoperative treatment for minimally displaced fractures (<1 mm) and acetabular fracture ORIF for fractures displaced more than 2 mm.11 No clear consensus exists on management for fractures displaced 1 to 2 mm. Few studies have investigated the outcomes of operative management of these fractures in the pediatric or adolescent population.
In our series of adolescent acetabular fractures, we examined unions, complications, and return to activity. Of 38 patients with acetabular fractures, 37 were treated with ORIF. Anatomical reduction was achieved in the majority of patients. Posterior wall fractures were by far the most common fracture type, which is consistent with previous reports.10,11 All acetabular fractures united, and most patients were pain-free at latest follow-up. There was a low incidence of major complications in our patient population. One major complication was a DVT in a 14-year-old boy who was in a motor vehicle accident and sustained a T-type fracture of the right acetabulum with contralateral femoral shaft and ankle fractures. The DVT was in the right internal iliac and common femoral veins and was diagnosed on magnetic resonance venography. The patient was treated with warfarin for 3 months without incident.
Two patients developed AVN of the femoral head. One of these patients was an 11-year-old girl who was in a motor vehicle accident and sustained a T-type fracture with marginal impaction of the posterior wall, posterior hip dislocation, and a pelvic ring injury. She was treated with ORIF through combined Kocher-Langenbeck/ilioinguinal approaches. By 4 months after surgery, the acetabular fracture was united. Nine months after surgery, she still had pain (activity-limiting) and a 35° flexion contracture of the hip, and she was ambulating with a cane. The diagnosis was AVN of the hip. The patient underwent hip fusion 1 year after surgery.
The second patient with femoral head AVN was a 12-year-old boy who fell while skiing and sustained a fracture of the posterior wall and a hip dislocation with impaction of the femoral head. Initial treatment at an outside institution consisted of open reduction of the hip and excision of a “loose body” from the joint. Eight weeks after surgery, the patient continued to have pain and was referred to our institution. A second operation was performed. Findings included a defect involving 40% of the posterior wall, and signs that the posterior wall had been excised during the initial operation. The patient eventually developed AVN of the hip. This patient was also diagnosed with a deep wound infection 4 months after surgery. He presented with pain and a fluid collection around the hip. The infection was not confirmed through fluid culture, and, as he eventually developed AVN of the hip, his symptoms may have been the result of chondrolysis or AVN rather than infection.
There were no cases of nonunion or malunion, leg-length discrepancy, or permanent sciatic nerve palsy. Although there were a few cases of premature closure of the triradiate cartilage, no acetabular dysplasia was seen at latest follow-up, likely because of the relative maturity of our pediatric group (age range, 11-18 years). Age at time of injury is thought to be the most important factor influencing growth and development of the acetabulum.9,13 In addition, previous studies have demonstrated a tendency toward acetabular fractures in patients with mature triradiate cartilage—versus pelvic ring injuries in patients with immature triradiate cartilage.8,11 This may also account for the older age of our study group.
Minor complications (eg, meralgia paresthetica) resolved spontaneously. The most common complications were abductor weakness and heterotopic ossification. In only 4 cases was a secondary procedure for excision of the heterotopic bone required. Abductor weakness, more commonly associated with a Kocher-Langenbeck approach to the hip, resolved with therapy in almost all cases. Only 4 of our patients required removal of hardware from the acetabulum.
Although the majority of acetabular fractures resulted from high-energy trauma, 8 cases were sports-related. Six of these involved posterior wall fractures, suggesting the injury resulted from a fall on flexed knee and hip. This was not known to be a common mechanism of injury in this age group.3,7 An additional concern was how to size the posterior wall fragment when not ossified. At one center, preoperative magnetic resonance imaging (MRI) was effectively used to size the osteochondral posterior wall fragment as standard protocol for patients with posterior wall fractures in this age group—resulting in better decisions regarding the need for ORIF. At the other institution, preoperative MRI was not performed routinely for this subset of patients.
Thirty-four of our 38 patients returned to their normal activities. Of the other 4 patients, 1 was permanently disabled secondary to traumatic brain injury, 1 had other ipsilateral extremity injuries that limited his mobility, and 2 developed AVN of the femoral head. Both patients with AVN had hip dislocations. Four of the 6 patients who were symptomatic during activity sustained impaction injuries of the femoral head or posterior wall. This suggests that poorer outcomes may be associated with dislocation or with articular injuries—similar to what has been reported in the adult literature.
This study had several limitations. First, it was a retrospective case series, so there was no control group for comparison. Second, the relatively short follow-up did not allow evaluation of the incidence of degenerative arthritis secondary to articular injury, the symptoms of which may develop 1 to 2 decades after injury.13 This phenomenon was well described by Letournel and Judet21 in the adult population, and there is no reason to presume the adolescent population is any different. Third, our sample was small and unlikely to represent a uniform sampling of the general pediatric population. Fourth, it was not possible to draw detailed conclusions about the outcome of ORIF for a particular type of acetabular fracture. Fifth, we did not see as many of the associated visceral injuries that are so prevalent in the literature. This may reflect improvement in safety specifications for automobiles, or our group may not have had the most severe or high-energy injuries. Here our population sample may have skewed our results, leading to better than expected outcomes.
One last study limitation, a major one, was the age of our population, 11 to 18 years, which makes it difficult to extrapolate results to the entire pediatric population. On one hand, a more immature skeleton has a higher chance of remodeling and is more forgiving of deformities and small amounts of displacement. On the other hand, injury and premature triradiate cartilage fusion in a younger patient can lead to significant deformity and acetabular dysplasia.9 Whether ORIF of these fractures would alter the outcome of an injury to the triradiate cartilage is yet to be determined.
Conclusion
In agreement with earlier studies,10,11,15,18 the good outcomes in our series correlated with congruence of reduction. Outcome predictors such as dislocation, femoral head injury, and marginal impaction are similar to those described in the adult literature. Although our study did not have a nonoperative group for comparison, the favorable outcomes of ORIF of acetabular fractures suggest that a more aggressive approach to treatment should be considered. Given the added benefits of early, pain-free mobilization, we think that only stable, undisplaced fractures (<1 mm) should be managed nonoperatively. In the adolescent population, we recommend ORIF for optimal management of unstable acetabular fractures, fractures with any hip subluxation, and fractures displaced more than 1 mm.
1. Canale ST, Beaty JH. Fractures of the pelvis. In: Beaty JH, Kassler JR, eds. Rockwood and Wilkin’s Fractures in Children. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:883-991.
2. Demetriades D, Karaiskakis M, Velmahos GC, Alo K, Murray J, Chan L. Pelvic fractures in pediatric and adult trauma patients: are they different injuries? J Trauma. 2003;54(6):1146-1151.
3. Grisoni N, Connor S, Marsh E, Thompson GH, Cooperman DR, Blakemore LC. Pelvic fractures in a pediatric level I trauma center. J Orthop Trauma. 2002;16(7):458-463.
4. Ismail N, Bellemare JF, Mollitt DL, Di Scala C, Koeppel B, Tepas JJ. Death from pelvic fracture: children are different. J Pediatr Surg. 1996;31(1):82-85.
5. Schlickwei W, Keck T. Pelvic and acetabular fractures in childhood. Injury. 2005;36(suppl 1):A57-A63.
6. Swiontkowski MF. Fractures and dislocations about the hip and pelvis. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. Philadelphia, PA: Saunders; 2003:371-406.
7. Silber JS, Flynn JM, Koffler KM, Dormans JP, Drummond DS. Analysis of the cause, classification, and associated injuries of 166 consecutive pediatric pelvic fractures. J Pediatr Orthop. 2001;21(4):446-450.
8. Silber JS, Flynn JM. Changing patterns of pediatric pelvic fractures with skeletal maturation: implications for classification and management. J Pediatr Orthop. 2002;22(1):22-26.
9. Bucholz RW, Ezaki M, Ogden JA. Injury to the acetabular triradiate physeal cartilage. J Bone Joint Surg Am. 1982;64(4):600-609.
10. Heeg M, Klasen HJ, Visser JD. Acetabular fractures in children and adolescents. J Bone Joint Surg Br. 1989;71(3):418-421.
11. Heeg M, de Ridder VA, Tornetta P, de Lange S, Klasen HJ. Acetabular fractures in children and adolescents. Clin Orthop Relat Res. 2000;(376):80-86.
12. Heeg M, Visser JD, Oostvogel HJ. Injuries of the acetabular triradiate cartilage and sacroiliac joint. J Bone Joint Surg Br. 1988;70(1):34-37.
13. Liporace FA, Ong B, Mohaideen A, Ong A, Koval KJ. Development and injury of the triradiate cartilage with its effects on acetabular development: review of the literature. J Trauma. 2003;54(6):1245-1249.
14. Rodrigues KF. Injury of the acetabular epiphysis. Injury. 1973;4(3):258-260.
15. Trousdale RT, Ganz R. Posttraumatic acetabular dysplasia. Clin Orthop Relat Res. 1994;(305):124-132.
16. Brooks E, Rosman M. Central fracture-dislocation of the hip in a child. J Trauma. 1988;28(11):1590-1592.
17. Habacker TA, Heinrich SD, Dehne R. Fracture of the superior pelvic quadrant in a child. J Pediatr Orthop. 1995;15(1):69-72.
18. Karunakar MA, Goulet JA, Mueller KL, Bedi A, Le TT. Operative treatment of unstable pediatric pelvis and acetabular fractures. J Pediatr Orthop. 2005;25(1):34-38.
19. Rieger H, Brug E. Fractures of the pelvis in children. Clin Orthop Relat Res. 1997;(336);226-239.
20. Torode I, Zieg D. Pelvic fractures in children. J Pediatr Orthop. 1985;5(1):76-84.
21. Letournel E, Judet R. Fractures of the Acetabulum. 2nd ed. New York, NY: Springer-Verlag; 1993.
22. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks of the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645.
In children, pelvic fractures are uncommon, with an incidence ranging from 1% to 4.6% of all pediatric fractures,1-4 and acetabular fractures make up only 0.8% to 15% of pelvic fractures.1,3,5,6 Acetabular fractures are so uncommon in children partly because of the cartilaginous nature of the immature acetabulum. The increased cartilage volume relative to adults provides greater capacity for energy absorption, resulting in greater elastic and plastic deformation before fracture occurrence. More force is therefore required to cause a fracture, and associated visceral injuries, head injuries, and long-bone fractures are common.3,7,8
The impact of acetabular fractures on adolescents warrants special attention because any resulting disability will affect them during their most productive years. Both avascular necrosis (AVN) and degenerative arthritis are particularly devastating complications in this age group. Complications such as premature physeal closure9-15 are unique to adolescents, and there is little information available on how injury in older children affects growth in this area.
There have been very few studies of the outcomes of these injuries in children. Mostly, there have been case reports and small series primarily dealing with nonoperative management of acetabular fractures in adolescents.3,10,11,16-20 By contrast, operative treatment of acetabular fractures in adults has been well described, and outcomes widely reported. As a result, much of our knowledge about managing these injuries is extrapolated from the adult literature. Although treatment of acetabular fractures in adults has evolved substantially, treatment of these injuries in adolescents remains primarily nonoperative. We conducted a study to evaluate outcomes of treatment of adolescent acetabular fractures.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the cases of all adolescent patients admitted with a diagnosis of acetabular fracture to 2 academic institutions between 1991 and 2003. Thirty-eight patients (28 males, 10 females) were identified. Mean age at time of injury was 15 years (range, 11-18 years). Mean follow-up was 3.2 years (range, 5-180 months).
Data on fracture types, treatment methods, associated injuries, complications, union rates, pain, and return to normal activities were collected. Acetabular fractures were classified according to the system of Letournel and Judet.21 There were 20 elementary and 18 associated fractures.
Of the 38 patients, 30 sustained high-energy trauma in motor vehicle accidents (25) or in falls from significant heights (5). The other 8 patients injured themselves playing sports (4 had severe traumatic brain injury, 2 had labial wounds, and 2 had injuries involving the abdominal viscera). Twelve patients had associated pelvic ring injuries, 18 had femoral head dislocations, 2 had femoral head fractures, and 13 had evidence of impaction injury to the femoral head articular cartilage. Twelve patients had marginal impaction of the acetabular wall. Fifteen patients had open triradiate physes at time of injury (Table 1).
Thirty-seven of the 38 patients were treated with open reduction and internal fixation (ORIF) by an experienced orthopedic trauma surgeon; 1 patient with a stable posterior wall fracture was treated nonoperatively. Surgical indications were articular displacement of more than 1 mm, hip joint instability, irreducible hip dislocation, and intra-articular fracture fragments. In the 37 surgically treated cases, the approaches used were Kocher-Langenbeck (22), ilioinguinal (8), combined Kocher-Langenbeck/ilioinguinal (5), and triradiate (2).
Immediate postoperative radiographs were evaluated by 3 orthopedic surgeons blinded to the patients’ clinical outcomes. Displacement was evaluated on anteroposterior (AP) and Judet views of the pelvis, as described by Matta,22 and reductions were classified as anatomical (0-1 mm of displacement), imperfect (>1 to 3 mm), poor (>3 mm), or surgical secondary congruence (Table 2).
Results
Thirty-seven patients underwent acetabular fracture ORIF. Immediate postoperative radiographs showed 30 anatomical reductions and 7 imperfect reductions. One patient had surgical secondary congruence and developed AVN of the hip. We could not identify an association between the quality of the reduction and the outcome with respect to pain or return to activity. However, no patient had a poor reduction. An illustrative case is presented in Figures 1 to 4.
All acetabular fractures united within 4.5 months (range, 3.0-8.0 months) after the index procedure. Early postoperative complications included 3 cases of meralgia paresthetica and 13 cases of abductor weakness. Meralgia paresthetica resolved spontaneously in all 3 patients. Of the 13 patients with abductor weakness, 11 improved with physical therapy, 1 was limited by the head injury, and 1 subsequently underwent hip fusion. One patient had a deep vein thrombosis (DVT) that was identified before surgery and managed with warfarin.
Other complications included 1 case of deep infection of the surgical wound. This infection presented 4 months after surgery and was treated with débridement, hardware removal, and a 3-month course of antibiotics. Two patients who sustained hip dislocations at time of injury developed AVN of the femoral head. Both developed osteoarthritis, and 1 underwent hip fusion. Eight patients developed heterotopic ossification on the side of the acetabular fracture; 4 of them underwent surgical excision. Four patients required a separate operation for hardware removal. Four patients with triradiate cartilage involvement went on to premature closure. No patient had any leg-length discrepancy or dysplasia at time of follow-up.
Thirty-four of the 38 patients returned to their regular activities. For these patients, mean time to return to full activity was 7.0 months (range, 3-30 months); there was no difference in mean time to return to full activity between skeletally mature and skeletally immature patients (6.6 vs 7.4 months; P = .57). Of the other 4 patients, 1 had permanent cognitive and physical disability with an ataxic gait as a result of a traumatic brain injury, 2 were limited by AVN (1 underwent hip fusion), and 1 was limited by an ipsilateral knee injury.
Of the 38 patients, 29 were pain-free; 6 had occasional, intermittent mild pain that did not limit their activities; and 3 had severe, activity-limiting pain. Of the 6 patients with mild pain, 2 had femoral impaction injuries, and 4 had marginal impaction injuries. Of the 3 patients with severe pain, 2 developed femoral head AVN, and 1 had multiple ipsilateral extremity injuries involving the femur, knee, and tibia.
Discussion
The traditional treatment for acetabular fractures in children has been nonoperative,8,10 and there are few specific treatment guidelines.13 Recent recommendations are nonoperative treatment for minimally displaced fractures (<1 mm) and acetabular fracture ORIF for fractures displaced more than 2 mm.11 No clear consensus exists on management for fractures displaced 1 to 2 mm. Few studies have investigated the outcomes of operative management of these fractures in the pediatric or adolescent population.
In our series of adolescent acetabular fractures, we examined unions, complications, and return to activity. Of 38 patients with acetabular fractures, 37 were treated with ORIF. Anatomical reduction was achieved in the majority of patients. Posterior wall fractures were by far the most common fracture type, which is consistent with previous reports.10,11 All acetabular fractures united, and most patients were pain-free at latest follow-up. There was a low incidence of major complications in our patient population. One major complication was a DVT in a 14-year-old boy who was in a motor vehicle accident and sustained a T-type fracture of the right acetabulum with contralateral femoral shaft and ankle fractures. The DVT was in the right internal iliac and common femoral veins and was diagnosed on magnetic resonance venography. The patient was treated with warfarin for 3 months without incident.
Two patients developed AVN of the femoral head. One of these patients was an 11-year-old girl who was in a motor vehicle accident and sustained a T-type fracture with marginal impaction of the posterior wall, posterior hip dislocation, and a pelvic ring injury. She was treated with ORIF through combined Kocher-Langenbeck/ilioinguinal approaches. By 4 months after surgery, the acetabular fracture was united. Nine months after surgery, she still had pain (activity-limiting) and a 35° flexion contracture of the hip, and she was ambulating with a cane. The diagnosis was AVN of the hip. The patient underwent hip fusion 1 year after surgery.
The second patient with femoral head AVN was a 12-year-old boy who fell while skiing and sustained a fracture of the posterior wall and a hip dislocation with impaction of the femoral head. Initial treatment at an outside institution consisted of open reduction of the hip and excision of a “loose body” from the joint. Eight weeks after surgery, the patient continued to have pain and was referred to our institution. A second operation was performed. Findings included a defect involving 40% of the posterior wall, and signs that the posterior wall had been excised during the initial operation. The patient eventually developed AVN of the hip. This patient was also diagnosed with a deep wound infection 4 months after surgery. He presented with pain and a fluid collection around the hip. The infection was not confirmed through fluid culture, and, as he eventually developed AVN of the hip, his symptoms may have been the result of chondrolysis or AVN rather than infection.
There were no cases of nonunion or malunion, leg-length discrepancy, or permanent sciatic nerve palsy. Although there were a few cases of premature closure of the triradiate cartilage, no acetabular dysplasia was seen at latest follow-up, likely because of the relative maturity of our pediatric group (age range, 11-18 years). Age at time of injury is thought to be the most important factor influencing growth and development of the acetabulum.9,13 In addition, previous studies have demonstrated a tendency toward acetabular fractures in patients with mature triradiate cartilage—versus pelvic ring injuries in patients with immature triradiate cartilage.8,11 This may also account for the older age of our study group.
Minor complications (eg, meralgia paresthetica) resolved spontaneously. The most common complications were abductor weakness and heterotopic ossification. In only 4 cases was a secondary procedure for excision of the heterotopic bone required. Abductor weakness, more commonly associated with a Kocher-Langenbeck approach to the hip, resolved with therapy in almost all cases. Only 4 of our patients required removal of hardware from the acetabulum.
Although the majority of acetabular fractures resulted from high-energy trauma, 8 cases were sports-related. Six of these involved posterior wall fractures, suggesting the injury resulted from a fall on flexed knee and hip. This was not known to be a common mechanism of injury in this age group.3,7 An additional concern was how to size the posterior wall fragment when not ossified. At one center, preoperative magnetic resonance imaging (MRI) was effectively used to size the osteochondral posterior wall fragment as standard protocol for patients with posterior wall fractures in this age group—resulting in better decisions regarding the need for ORIF. At the other institution, preoperative MRI was not performed routinely for this subset of patients.
Thirty-four of our 38 patients returned to their normal activities. Of the other 4 patients, 1 was permanently disabled secondary to traumatic brain injury, 1 had other ipsilateral extremity injuries that limited his mobility, and 2 developed AVN of the femoral head. Both patients with AVN had hip dislocations. Four of the 6 patients who were symptomatic during activity sustained impaction injuries of the femoral head or posterior wall. This suggests that poorer outcomes may be associated with dislocation or with articular injuries—similar to what has been reported in the adult literature.
This study had several limitations. First, it was a retrospective case series, so there was no control group for comparison. Second, the relatively short follow-up did not allow evaluation of the incidence of degenerative arthritis secondary to articular injury, the symptoms of which may develop 1 to 2 decades after injury.13 This phenomenon was well described by Letournel and Judet21 in the adult population, and there is no reason to presume the adolescent population is any different. Third, our sample was small and unlikely to represent a uniform sampling of the general pediatric population. Fourth, it was not possible to draw detailed conclusions about the outcome of ORIF for a particular type of acetabular fracture. Fifth, we did not see as many of the associated visceral injuries that are so prevalent in the literature. This may reflect improvement in safety specifications for automobiles, or our group may not have had the most severe or high-energy injuries. Here our population sample may have skewed our results, leading to better than expected outcomes.
One last study limitation, a major one, was the age of our population, 11 to 18 years, which makes it difficult to extrapolate results to the entire pediatric population. On one hand, a more immature skeleton has a higher chance of remodeling and is more forgiving of deformities and small amounts of displacement. On the other hand, injury and premature triradiate cartilage fusion in a younger patient can lead to significant deformity and acetabular dysplasia.9 Whether ORIF of these fractures would alter the outcome of an injury to the triradiate cartilage is yet to be determined.
Conclusion
In agreement with earlier studies,10,11,15,18 the good outcomes in our series correlated with congruence of reduction. Outcome predictors such as dislocation, femoral head injury, and marginal impaction are similar to those described in the adult literature. Although our study did not have a nonoperative group for comparison, the favorable outcomes of ORIF of acetabular fractures suggest that a more aggressive approach to treatment should be considered. Given the added benefits of early, pain-free mobilization, we think that only stable, undisplaced fractures (<1 mm) should be managed nonoperatively. In the adolescent population, we recommend ORIF for optimal management of unstable acetabular fractures, fractures with any hip subluxation, and fractures displaced more than 1 mm.
In children, pelvic fractures are uncommon, with an incidence ranging from 1% to 4.6% of all pediatric fractures,1-4 and acetabular fractures make up only 0.8% to 15% of pelvic fractures.1,3,5,6 Acetabular fractures are so uncommon in children partly because of the cartilaginous nature of the immature acetabulum. The increased cartilage volume relative to adults provides greater capacity for energy absorption, resulting in greater elastic and plastic deformation before fracture occurrence. More force is therefore required to cause a fracture, and associated visceral injuries, head injuries, and long-bone fractures are common.3,7,8
The impact of acetabular fractures on adolescents warrants special attention because any resulting disability will affect them during their most productive years. Both avascular necrosis (AVN) and degenerative arthritis are particularly devastating complications in this age group. Complications such as premature physeal closure9-15 are unique to adolescents, and there is little information available on how injury in older children affects growth in this area.
There have been very few studies of the outcomes of these injuries in children. Mostly, there have been case reports and small series primarily dealing with nonoperative management of acetabular fractures in adolescents.3,10,11,16-20 By contrast, operative treatment of acetabular fractures in adults has been well described, and outcomes widely reported. As a result, much of our knowledge about managing these injuries is extrapolated from the adult literature. Although treatment of acetabular fractures in adults has evolved substantially, treatment of these injuries in adolescents remains primarily nonoperative. We conducted a study to evaluate outcomes of treatment of adolescent acetabular fractures.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the cases of all adolescent patients admitted with a diagnosis of acetabular fracture to 2 academic institutions between 1991 and 2003. Thirty-eight patients (28 males, 10 females) were identified. Mean age at time of injury was 15 years (range, 11-18 years). Mean follow-up was 3.2 years (range, 5-180 months).
Data on fracture types, treatment methods, associated injuries, complications, union rates, pain, and return to normal activities were collected. Acetabular fractures were classified according to the system of Letournel and Judet.21 There were 20 elementary and 18 associated fractures.
Of the 38 patients, 30 sustained high-energy trauma in motor vehicle accidents (25) or in falls from significant heights (5). The other 8 patients injured themselves playing sports (4 had severe traumatic brain injury, 2 had labial wounds, and 2 had injuries involving the abdominal viscera). Twelve patients had associated pelvic ring injuries, 18 had femoral head dislocations, 2 had femoral head fractures, and 13 had evidence of impaction injury to the femoral head articular cartilage. Twelve patients had marginal impaction of the acetabular wall. Fifteen patients had open triradiate physes at time of injury (Table 1).
Thirty-seven of the 38 patients were treated with open reduction and internal fixation (ORIF) by an experienced orthopedic trauma surgeon; 1 patient with a stable posterior wall fracture was treated nonoperatively. Surgical indications were articular displacement of more than 1 mm, hip joint instability, irreducible hip dislocation, and intra-articular fracture fragments. In the 37 surgically treated cases, the approaches used were Kocher-Langenbeck (22), ilioinguinal (8), combined Kocher-Langenbeck/ilioinguinal (5), and triradiate (2).
Immediate postoperative radiographs were evaluated by 3 orthopedic surgeons blinded to the patients’ clinical outcomes. Displacement was evaluated on anteroposterior (AP) and Judet views of the pelvis, as described by Matta,22 and reductions were classified as anatomical (0-1 mm of displacement), imperfect (>1 to 3 mm), poor (>3 mm), or surgical secondary congruence (Table 2).
Results
Thirty-seven patients underwent acetabular fracture ORIF. Immediate postoperative radiographs showed 30 anatomical reductions and 7 imperfect reductions. One patient had surgical secondary congruence and developed AVN of the hip. We could not identify an association between the quality of the reduction and the outcome with respect to pain or return to activity. However, no patient had a poor reduction. An illustrative case is presented in Figures 1 to 4.
All acetabular fractures united within 4.5 months (range, 3.0-8.0 months) after the index procedure. Early postoperative complications included 3 cases of meralgia paresthetica and 13 cases of abductor weakness. Meralgia paresthetica resolved spontaneously in all 3 patients. Of the 13 patients with abductor weakness, 11 improved with physical therapy, 1 was limited by the head injury, and 1 subsequently underwent hip fusion. One patient had a deep vein thrombosis (DVT) that was identified before surgery and managed with warfarin.
Other complications included 1 case of deep infection of the surgical wound. This infection presented 4 months after surgery and was treated with débridement, hardware removal, and a 3-month course of antibiotics. Two patients who sustained hip dislocations at time of injury developed AVN of the femoral head. Both developed osteoarthritis, and 1 underwent hip fusion. Eight patients developed heterotopic ossification on the side of the acetabular fracture; 4 of them underwent surgical excision. Four patients required a separate operation for hardware removal. Four patients with triradiate cartilage involvement went on to premature closure. No patient had any leg-length discrepancy or dysplasia at time of follow-up.
Thirty-four of the 38 patients returned to their regular activities. For these patients, mean time to return to full activity was 7.0 months (range, 3-30 months); there was no difference in mean time to return to full activity between skeletally mature and skeletally immature patients (6.6 vs 7.4 months; P = .57). Of the other 4 patients, 1 had permanent cognitive and physical disability with an ataxic gait as a result of a traumatic brain injury, 2 were limited by AVN (1 underwent hip fusion), and 1 was limited by an ipsilateral knee injury.
Of the 38 patients, 29 were pain-free; 6 had occasional, intermittent mild pain that did not limit their activities; and 3 had severe, activity-limiting pain. Of the 6 patients with mild pain, 2 had femoral impaction injuries, and 4 had marginal impaction injuries. Of the 3 patients with severe pain, 2 developed femoral head AVN, and 1 had multiple ipsilateral extremity injuries involving the femur, knee, and tibia.
Discussion
The traditional treatment for acetabular fractures in children has been nonoperative,8,10 and there are few specific treatment guidelines.13 Recent recommendations are nonoperative treatment for minimally displaced fractures (<1 mm) and acetabular fracture ORIF for fractures displaced more than 2 mm.11 No clear consensus exists on management for fractures displaced 1 to 2 mm. Few studies have investigated the outcomes of operative management of these fractures in the pediatric or adolescent population.
In our series of adolescent acetabular fractures, we examined unions, complications, and return to activity. Of 38 patients with acetabular fractures, 37 were treated with ORIF. Anatomical reduction was achieved in the majority of patients. Posterior wall fractures were by far the most common fracture type, which is consistent with previous reports.10,11 All acetabular fractures united, and most patients were pain-free at latest follow-up. There was a low incidence of major complications in our patient population. One major complication was a DVT in a 14-year-old boy who was in a motor vehicle accident and sustained a T-type fracture of the right acetabulum with contralateral femoral shaft and ankle fractures. The DVT was in the right internal iliac and common femoral veins and was diagnosed on magnetic resonance venography. The patient was treated with warfarin for 3 months without incident.
Two patients developed AVN of the femoral head. One of these patients was an 11-year-old girl who was in a motor vehicle accident and sustained a T-type fracture with marginal impaction of the posterior wall, posterior hip dislocation, and a pelvic ring injury. She was treated with ORIF through combined Kocher-Langenbeck/ilioinguinal approaches. By 4 months after surgery, the acetabular fracture was united. Nine months after surgery, she still had pain (activity-limiting) and a 35° flexion contracture of the hip, and she was ambulating with a cane. The diagnosis was AVN of the hip. The patient underwent hip fusion 1 year after surgery.
The second patient with femoral head AVN was a 12-year-old boy who fell while skiing and sustained a fracture of the posterior wall and a hip dislocation with impaction of the femoral head. Initial treatment at an outside institution consisted of open reduction of the hip and excision of a “loose body” from the joint. Eight weeks after surgery, the patient continued to have pain and was referred to our institution. A second operation was performed. Findings included a defect involving 40% of the posterior wall, and signs that the posterior wall had been excised during the initial operation. The patient eventually developed AVN of the hip. This patient was also diagnosed with a deep wound infection 4 months after surgery. He presented with pain and a fluid collection around the hip. The infection was not confirmed through fluid culture, and, as he eventually developed AVN of the hip, his symptoms may have been the result of chondrolysis or AVN rather than infection.
There were no cases of nonunion or malunion, leg-length discrepancy, or permanent sciatic nerve palsy. Although there were a few cases of premature closure of the triradiate cartilage, no acetabular dysplasia was seen at latest follow-up, likely because of the relative maturity of our pediatric group (age range, 11-18 years). Age at time of injury is thought to be the most important factor influencing growth and development of the acetabulum.9,13 In addition, previous studies have demonstrated a tendency toward acetabular fractures in patients with mature triradiate cartilage—versus pelvic ring injuries in patients with immature triradiate cartilage.8,11 This may also account for the older age of our study group.
Minor complications (eg, meralgia paresthetica) resolved spontaneously. The most common complications were abductor weakness and heterotopic ossification. In only 4 cases was a secondary procedure for excision of the heterotopic bone required. Abductor weakness, more commonly associated with a Kocher-Langenbeck approach to the hip, resolved with therapy in almost all cases. Only 4 of our patients required removal of hardware from the acetabulum.
Although the majority of acetabular fractures resulted from high-energy trauma, 8 cases were sports-related. Six of these involved posterior wall fractures, suggesting the injury resulted from a fall on flexed knee and hip. This was not known to be a common mechanism of injury in this age group.3,7 An additional concern was how to size the posterior wall fragment when not ossified. At one center, preoperative magnetic resonance imaging (MRI) was effectively used to size the osteochondral posterior wall fragment as standard protocol for patients with posterior wall fractures in this age group—resulting in better decisions regarding the need for ORIF. At the other institution, preoperative MRI was not performed routinely for this subset of patients.
Thirty-four of our 38 patients returned to their normal activities. Of the other 4 patients, 1 was permanently disabled secondary to traumatic brain injury, 1 had other ipsilateral extremity injuries that limited his mobility, and 2 developed AVN of the femoral head. Both patients with AVN had hip dislocations. Four of the 6 patients who were symptomatic during activity sustained impaction injuries of the femoral head or posterior wall. This suggests that poorer outcomes may be associated with dislocation or with articular injuries—similar to what has been reported in the adult literature.
This study had several limitations. First, it was a retrospective case series, so there was no control group for comparison. Second, the relatively short follow-up did not allow evaluation of the incidence of degenerative arthritis secondary to articular injury, the symptoms of which may develop 1 to 2 decades after injury.13 This phenomenon was well described by Letournel and Judet21 in the adult population, and there is no reason to presume the adolescent population is any different. Third, our sample was small and unlikely to represent a uniform sampling of the general pediatric population. Fourth, it was not possible to draw detailed conclusions about the outcome of ORIF for a particular type of acetabular fracture. Fifth, we did not see as many of the associated visceral injuries that are so prevalent in the literature. This may reflect improvement in safety specifications for automobiles, or our group may not have had the most severe or high-energy injuries. Here our population sample may have skewed our results, leading to better than expected outcomes.
One last study limitation, a major one, was the age of our population, 11 to 18 years, which makes it difficult to extrapolate results to the entire pediatric population. On one hand, a more immature skeleton has a higher chance of remodeling and is more forgiving of deformities and small amounts of displacement. On the other hand, injury and premature triradiate cartilage fusion in a younger patient can lead to significant deformity and acetabular dysplasia.9 Whether ORIF of these fractures would alter the outcome of an injury to the triradiate cartilage is yet to be determined.
Conclusion
In agreement with earlier studies,10,11,15,18 the good outcomes in our series correlated with congruence of reduction. Outcome predictors such as dislocation, femoral head injury, and marginal impaction are similar to those described in the adult literature. Although our study did not have a nonoperative group for comparison, the favorable outcomes of ORIF of acetabular fractures suggest that a more aggressive approach to treatment should be considered. Given the added benefits of early, pain-free mobilization, we think that only stable, undisplaced fractures (<1 mm) should be managed nonoperatively. In the adolescent population, we recommend ORIF for optimal management of unstable acetabular fractures, fractures with any hip subluxation, and fractures displaced more than 1 mm.
1. Canale ST, Beaty JH. Fractures of the pelvis. In: Beaty JH, Kassler JR, eds. Rockwood and Wilkin’s Fractures in Children. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:883-991.
2. Demetriades D, Karaiskakis M, Velmahos GC, Alo K, Murray J, Chan L. Pelvic fractures in pediatric and adult trauma patients: are they different injuries? J Trauma. 2003;54(6):1146-1151.
3. Grisoni N, Connor S, Marsh E, Thompson GH, Cooperman DR, Blakemore LC. Pelvic fractures in a pediatric level I trauma center. J Orthop Trauma. 2002;16(7):458-463.
4. Ismail N, Bellemare JF, Mollitt DL, Di Scala C, Koeppel B, Tepas JJ. Death from pelvic fracture: children are different. J Pediatr Surg. 1996;31(1):82-85.
5. Schlickwei W, Keck T. Pelvic and acetabular fractures in childhood. Injury. 2005;36(suppl 1):A57-A63.
6. Swiontkowski MF. Fractures and dislocations about the hip and pelvis. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. Philadelphia, PA: Saunders; 2003:371-406.
7. Silber JS, Flynn JM, Koffler KM, Dormans JP, Drummond DS. Analysis of the cause, classification, and associated injuries of 166 consecutive pediatric pelvic fractures. J Pediatr Orthop. 2001;21(4):446-450.
8. Silber JS, Flynn JM. Changing patterns of pediatric pelvic fractures with skeletal maturation: implications for classification and management. J Pediatr Orthop. 2002;22(1):22-26.
9. Bucholz RW, Ezaki M, Ogden JA. Injury to the acetabular triradiate physeal cartilage. J Bone Joint Surg Am. 1982;64(4):600-609.
10. Heeg M, Klasen HJ, Visser JD. Acetabular fractures in children and adolescents. J Bone Joint Surg Br. 1989;71(3):418-421.
11. Heeg M, de Ridder VA, Tornetta P, de Lange S, Klasen HJ. Acetabular fractures in children and adolescents. Clin Orthop Relat Res. 2000;(376):80-86.
12. Heeg M, Visser JD, Oostvogel HJ. Injuries of the acetabular triradiate cartilage and sacroiliac joint. J Bone Joint Surg Br. 1988;70(1):34-37.
13. Liporace FA, Ong B, Mohaideen A, Ong A, Koval KJ. Development and injury of the triradiate cartilage with its effects on acetabular development: review of the literature. J Trauma. 2003;54(6):1245-1249.
14. Rodrigues KF. Injury of the acetabular epiphysis. Injury. 1973;4(3):258-260.
15. Trousdale RT, Ganz R. Posttraumatic acetabular dysplasia. Clin Orthop Relat Res. 1994;(305):124-132.
16. Brooks E, Rosman M. Central fracture-dislocation of the hip in a child. J Trauma. 1988;28(11):1590-1592.
17. Habacker TA, Heinrich SD, Dehne R. Fracture of the superior pelvic quadrant in a child. J Pediatr Orthop. 1995;15(1):69-72.
18. Karunakar MA, Goulet JA, Mueller KL, Bedi A, Le TT. Operative treatment of unstable pediatric pelvis and acetabular fractures. J Pediatr Orthop. 2005;25(1):34-38.
19. Rieger H, Brug E. Fractures of the pelvis in children. Clin Orthop Relat Res. 1997;(336);226-239.
20. Torode I, Zieg D. Pelvic fractures in children. J Pediatr Orthop. 1985;5(1):76-84.
21. Letournel E, Judet R. Fractures of the Acetabulum. 2nd ed. New York, NY: Springer-Verlag; 1993.
22. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks of the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645.
1. Canale ST, Beaty JH. Fractures of the pelvis. In: Beaty JH, Kassler JR, eds. Rockwood and Wilkin’s Fractures in Children. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:883-991.
2. Demetriades D, Karaiskakis M, Velmahos GC, Alo K, Murray J, Chan L. Pelvic fractures in pediatric and adult trauma patients: are they different injuries? J Trauma. 2003;54(6):1146-1151.
3. Grisoni N, Connor S, Marsh E, Thompson GH, Cooperman DR, Blakemore LC. Pelvic fractures in a pediatric level I trauma center. J Orthop Trauma. 2002;16(7):458-463.
4. Ismail N, Bellemare JF, Mollitt DL, Di Scala C, Koeppel B, Tepas JJ. Death from pelvic fracture: children are different. J Pediatr Surg. 1996;31(1):82-85.
5. Schlickwei W, Keck T. Pelvic and acetabular fractures in childhood. Injury. 2005;36(suppl 1):A57-A63.
6. Swiontkowski MF. Fractures and dislocations about the hip and pelvis. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. Philadelphia, PA: Saunders; 2003:371-406.
7. Silber JS, Flynn JM, Koffler KM, Dormans JP, Drummond DS. Analysis of the cause, classification, and associated injuries of 166 consecutive pediatric pelvic fractures. J Pediatr Orthop. 2001;21(4):446-450.
8. Silber JS, Flynn JM. Changing patterns of pediatric pelvic fractures with skeletal maturation: implications for classification and management. J Pediatr Orthop. 2002;22(1):22-26.
9. Bucholz RW, Ezaki M, Ogden JA. Injury to the acetabular triradiate physeal cartilage. J Bone Joint Surg Am. 1982;64(4):600-609.
10. Heeg M, Klasen HJ, Visser JD. Acetabular fractures in children and adolescents. J Bone Joint Surg Br. 1989;71(3):418-421.
11. Heeg M, de Ridder VA, Tornetta P, de Lange S, Klasen HJ. Acetabular fractures in children and adolescents. Clin Orthop Relat Res. 2000;(376):80-86.
12. Heeg M, Visser JD, Oostvogel HJ. Injuries of the acetabular triradiate cartilage and sacroiliac joint. J Bone Joint Surg Br. 1988;70(1):34-37.
13. Liporace FA, Ong B, Mohaideen A, Ong A, Koval KJ. Development and injury of the triradiate cartilage with its effects on acetabular development: review of the literature. J Trauma. 2003;54(6):1245-1249.
14. Rodrigues KF. Injury of the acetabular epiphysis. Injury. 1973;4(3):258-260.
15. Trousdale RT, Ganz R. Posttraumatic acetabular dysplasia. Clin Orthop Relat Res. 1994;(305):124-132.
16. Brooks E, Rosman M. Central fracture-dislocation of the hip in a child. J Trauma. 1988;28(11):1590-1592.
17. Habacker TA, Heinrich SD, Dehne R. Fracture of the superior pelvic quadrant in a child. J Pediatr Orthop. 1995;15(1):69-72.
18. Karunakar MA, Goulet JA, Mueller KL, Bedi A, Le TT. Operative treatment of unstable pediatric pelvis and acetabular fractures. J Pediatr Orthop. 2005;25(1):34-38.
19. Rieger H, Brug E. Fractures of the pelvis in children. Clin Orthop Relat Res. 1997;(336);226-239.
20. Torode I, Zieg D. Pelvic fractures in children. J Pediatr Orthop. 1985;5(1):76-84.
21. Letournel E, Judet R. Fractures of the Acetabulum. 2nd ed. New York, NY: Springer-Verlag; 1993.
22. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks of the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645.
Image-Based Techniques for Percutaneous Iliosacral Screw Start-Site Localization
Iliosacral (SI) screws remain the standard of care for the vast majority of posterior pelvic ring disruptions.1,2 However, despite their routine use, the procedure remains technically demanding with repeated cases of aberrant screw placement and complications.3,4 Sacral morphology is extremely variable within a patient population and affects accurate placement and trajectory of percutaneous screws.5 Classically, it is taught that the external starting position/landmark is at an intersection point of the greater trochanter and the anterior superior iliac spine (ASIS). While this “one size fits all” approach will certainly help to coordinate a start position, it is our experience that multiple stab incisions are necessary to find the optimal start site. To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side, guided by the lateral image.5 This article provides a novel image-based technique to be used with, or as a replacement for, the traditional technique.
Techniques
The patient is brought to the operating room and placed supine on a radiolucent operating table. If the closed reduction of the pelvic ring is successful or can be achieved via anterior manipulation/traction, posterior percutaneous pinning is planned. Either a rolled towel or a bag of saline is used as a bolster and placed midline underneath the sacrum and lumbar spine to help “bump” the pelvis and improve the range of motion for the surgeon’s drill. The patient is brought to the edge of the table when possible (ie, a posterior ring injury requiring fixation from only 1 side) to further enhance drill motion. If bilateral screws are planned, surgeons must be careful not to position 1 side at the expense of screw placement on the contralateral side. Nitrous-based anesthetic agents are avoided, because they may collect in the bowel and obscure good radiographic visualization. Arms are placed perpendicular to the body to facilitate the inlet view. Pre-preparation anteroposterior pelvis, inlet, and outlet views are obtained to assure ability to accurately and safely assess landmarks on all projections, and to mark the C-arm position and angles. This process helps decrease “useless” radiographs obtained during the procedure. Acceptable inlet radiographs show the anterior cortex of the S1 body superimposed on the S2 body. Acceptable outlet radiographs show the superior pubic symphysis at the level of the S2 foramen and visualization of the S1/S2 sacral foramen.6 The patient is then prepared in the standard fashion. Reduction maneuvers are performed and, if acceptable alignment is achieved, posterior percutaneous screw placement begins.
Technique 1
To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side using the lateral image. The fluoroscopic machine is set up in a lateral position.5 A free guide wire is superimposed upon the iliac cortical density and anterior sacral slope, which is marked on the skin (Figure 1). The superior portion of S1, as well as the posterior sacral slope, can be marked as well. This process has outlined the sacrum and provides an external landmark for the “safe zone” for screw placement. The operation proceeds in the standard fashion using inlet, outlet, and lateral radiographs. However, the externally drawn sacrum can aid as a reference during guide-pin placement.
Technique 2
This technique takes into account bone anatomy and soft-tissue coverage. It is helpful to think of the abdomen/pelvis as a box. The anterior abdomen represents the top of the box and the lateral buttock represents the side of the box. The corner of the imaginary box is where the abdomen begins to slope down and transitions laterally to become the buttock. This will be referenced as the “down-sloping point” and typically corresponds to the level of the iliac crest (Figure 2).
To begin, a standard cannulated screw guide wire is placed flush on the skin of the abdomen. An inlet fluoroscopy image is taken with the guide pin on the abdomen. Imagine that the resulting image represents the planned screw trajectory (Figure 3A). When the position of the guide wire is deemed adequate, a line is marked on the abdomen, using a pen, directly adjacent to the guide wire. This line represents inlet line 1 (Figure 2). The line must continue laterally until the down-sloping point. The sagittal angle of the imaginary inlet fluoroscopic beam is noted, and a guide wire is placed in the same sagittal orientation flush with the skin on the lateral buttock (Figure 3B). The guide wire must be placed so that it intersects with the first line at the down-sloping point. The skin on the lateral aspect of buttock is marked with a second line, which represents inlet line 2 (Figure 2).
The same process is repeated using an outlet view to create outlet lines 1 and 2 (Figures 4A, 4B). At this point there are 4 lines drawn on the patient (Figure 2). A stab incision is made at the intersection of the 2 lines drawn on the lateral buttock; this represents the skin start point, labeled “start incision” (Figure 2). The procedure continues in standard fashion.
The 4 external reference lines serve multiple purposes. First, the lines mark the true lateral start point for the pin at the level of the skin. This contrasts with the standard technique in which bony landmarks are marked on the skin and the surgeon must estimate a point on the skin that will provide an appropriate trajectory to the bony start point on the ilium. Further, the lines can also be used to reorient the cartesian plane so that adjustments can be isolated to a single plane, ensuring movements only alter the position on a single radiographic view (Figure 5).
Discussion
Despite the widespread use of percutaneous screw placement for posterior pelvic ring injuries, this remains a technically demanding surgery. Recent data suggest patient pelvic anatomy is extremely varied, especially the sacrum.7 Further, screw trajectories vary depending on surgical goals, fracture pattern, and number of screws. Taken together, this implies that there is no perfect universal starting site along the external ilium. Therefore, while classic teaching states to begin screw insertion within the vicinity of the intersection of the greater trochanter and the ASIS, it is our experience that this location is often not ideal.
The inlet, outlet, and lateral radiographs are all vital to assess correct trajectory of the guide pin and drill prior to final screw insertion, but the start site remains a critical step to assure a successful surgical outcome. We present 2 techniques, used together or separately, that allow the surgeon to place the initial guide pin more accurately for percutanous iliosacral screws. Though not specifically examined in this study, we think technique 2 has the potential to save operative time and use less fluoroscopic imaging because a lateral image is not required until later in the case. Technique 2 identifies the start point at the level of the skin. This is in contrast to technique 1, which identifies the desired sacral target and requires a surgeon to select a skin start site that will provide an optimal trajectory towards the desired target. Judging trajectory can be difficult, particularly in obese patients, and technique 2 eliminates this extra variable.
It is also important to consider that criteria-based nonorthogonal imaging is required for percutaneous screw placement. In these cases, it is more difficult to judge trajectory corrections because the fluoroscopic beam cannot guide perpendicular corrections as it can in operations that use orthogonal imaging. Adjustments made perpendicular to the fluoroscopic beam will change trajectory in multiple planes.8 Moreover, because the standard cartesian frame of reference is rotated, understanding the location of the sacrum in space can be especially challenging. When using the first technique, sacral landmarks are delineated, and a virtual sacrum drawn on the patient’s exterior helps with orientation. In the second technique, the ideal pin placement is mapped, and the external reference lines guide uniplanar changes. For example, the line drawn co-planar with the inlet view is essentially marking the sacral slope. Therefore, by following this line, uniplanar changes in the cranial and caudal direction are achieved on the outlet view (Figure 5). Because this line is also in reference to the already known ideal pin placement, ideal pin placement can be maintained in 1 radiographic projection while changing the start site in the appropriate direction. In a similar fashion, the co-planar line identified on the inlet view can be used on the outlet image to affect uniplanar changes in the anteroposterior direction. This technique effectively minimizes disorientation when placing percutaneous SI screws. This can be particularly beneficial when placing screws in the prone position.
Conclusion
We have shown 2 techniques that are routinely used at our institution to help identify an accurate starting position for percutaneous screw placement in posterior pelvic ring injuries. Even experienced traumatologists can more quickly and accurately identify the correct stab incisions leading to more confidently placed screws. Further, we believe understanding the usage of fluoroscopy and the concepts involved in drawing the lines enhance trainees’ comprehension of the complex anatomy of the sacrum.
1. Matta JM, Saucedo T. Internal fixation of pelvic ring fractures. Clin Orthop Relat Res. 1989;242:83-97.
2. Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207-214.
3. Sagi HC, Lindvall EM. Inadvertent intraforaminal iliosacral screw placement despite apparent appropriate positioning on intraoperative fluoroscopy.
J Orthop Trauma. 2005;19(2):130-133.
4. Routt ML Jr, Simonian PT, Mills WJ. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11(8):584-589.
5. Routt ML Jr, Simonian PT, Agnew SG, Mann FA. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma. 1996;10(3):171-177.
6. Gardner MJ, Ferrell ED, Nork SE, Segina DN, Routt ML Jr. Percutaneous placement of iliosacral screws without electrodiagnostic monitoring. J Trauma. 2009;66(5):1411-1415.
7. Miller AN, Routt ML Jr. Variations in sacral morphology and implications for iliosacral screw fixation. J Am Acad Orthop Surg. 2012;20(1):8-16.
8. Graves ML, Routt ML. Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Orthop Trauma. 2011;71(1):204-208.
Iliosacral (SI) screws remain the standard of care for the vast majority of posterior pelvic ring disruptions.1,2 However, despite their routine use, the procedure remains technically demanding with repeated cases of aberrant screw placement and complications.3,4 Sacral morphology is extremely variable within a patient population and affects accurate placement and trajectory of percutaneous screws.5 Classically, it is taught that the external starting position/landmark is at an intersection point of the greater trochanter and the anterior superior iliac spine (ASIS). While this “one size fits all” approach will certainly help to coordinate a start position, it is our experience that multiple stab incisions are necessary to find the optimal start site. To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side, guided by the lateral image.5 This article provides a novel image-based technique to be used with, or as a replacement for, the traditional technique.
Techniques
The patient is brought to the operating room and placed supine on a radiolucent operating table. If the closed reduction of the pelvic ring is successful or can be achieved via anterior manipulation/traction, posterior percutaneous pinning is planned. Either a rolled towel or a bag of saline is used as a bolster and placed midline underneath the sacrum and lumbar spine to help “bump” the pelvis and improve the range of motion for the surgeon’s drill. The patient is brought to the edge of the table when possible (ie, a posterior ring injury requiring fixation from only 1 side) to further enhance drill motion. If bilateral screws are planned, surgeons must be careful not to position 1 side at the expense of screw placement on the contralateral side. Nitrous-based anesthetic agents are avoided, because they may collect in the bowel and obscure good radiographic visualization. Arms are placed perpendicular to the body to facilitate the inlet view. Pre-preparation anteroposterior pelvis, inlet, and outlet views are obtained to assure ability to accurately and safely assess landmarks on all projections, and to mark the C-arm position and angles. This process helps decrease “useless” radiographs obtained during the procedure. Acceptable inlet radiographs show the anterior cortex of the S1 body superimposed on the S2 body. Acceptable outlet radiographs show the superior pubic symphysis at the level of the S2 foramen and visualization of the S1/S2 sacral foramen.6 The patient is then prepared in the standard fashion. Reduction maneuvers are performed and, if acceptable alignment is achieved, posterior percutaneous screw placement begins.
Technique 1
To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side using the lateral image. The fluoroscopic machine is set up in a lateral position.5 A free guide wire is superimposed upon the iliac cortical density and anterior sacral slope, which is marked on the skin (Figure 1). The superior portion of S1, as well as the posterior sacral slope, can be marked as well. This process has outlined the sacrum and provides an external landmark for the “safe zone” for screw placement. The operation proceeds in the standard fashion using inlet, outlet, and lateral radiographs. However, the externally drawn sacrum can aid as a reference during guide-pin placement.
Technique 2
This technique takes into account bone anatomy and soft-tissue coverage. It is helpful to think of the abdomen/pelvis as a box. The anterior abdomen represents the top of the box and the lateral buttock represents the side of the box. The corner of the imaginary box is where the abdomen begins to slope down and transitions laterally to become the buttock. This will be referenced as the “down-sloping point” and typically corresponds to the level of the iliac crest (Figure 2).
To begin, a standard cannulated screw guide wire is placed flush on the skin of the abdomen. An inlet fluoroscopy image is taken with the guide pin on the abdomen. Imagine that the resulting image represents the planned screw trajectory (Figure 3A). When the position of the guide wire is deemed adequate, a line is marked on the abdomen, using a pen, directly adjacent to the guide wire. This line represents inlet line 1 (Figure 2). The line must continue laterally until the down-sloping point. The sagittal angle of the imaginary inlet fluoroscopic beam is noted, and a guide wire is placed in the same sagittal orientation flush with the skin on the lateral buttock (Figure 3B). The guide wire must be placed so that it intersects with the first line at the down-sloping point. The skin on the lateral aspect of buttock is marked with a second line, which represents inlet line 2 (Figure 2).
The same process is repeated using an outlet view to create outlet lines 1 and 2 (Figures 4A, 4B). At this point there are 4 lines drawn on the patient (Figure 2). A stab incision is made at the intersection of the 2 lines drawn on the lateral buttock; this represents the skin start point, labeled “start incision” (Figure 2). The procedure continues in standard fashion.
The 4 external reference lines serve multiple purposes. First, the lines mark the true lateral start point for the pin at the level of the skin. This contrasts with the standard technique in which bony landmarks are marked on the skin and the surgeon must estimate a point on the skin that will provide an appropriate trajectory to the bony start point on the ilium. Further, the lines can also be used to reorient the cartesian plane so that adjustments can be isolated to a single plane, ensuring movements only alter the position on a single radiographic view (Figure 5).
Discussion
Despite the widespread use of percutaneous screw placement for posterior pelvic ring injuries, this remains a technically demanding surgery. Recent data suggest patient pelvic anatomy is extremely varied, especially the sacrum.7 Further, screw trajectories vary depending on surgical goals, fracture pattern, and number of screws. Taken together, this implies that there is no perfect universal starting site along the external ilium. Therefore, while classic teaching states to begin screw insertion within the vicinity of the intersection of the greater trochanter and the ASIS, it is our experience that this location is often not ideal.
The inlet, outlet, and lateral radiographs are all vital to assess correct trajectory of the guide pin and drill prior to final screw insertion, but the start site remains a critical step to assure a successful surgical outcome. We present 2 techniques, used together or separately, that allow the surgeon to place the initial guide pin more accurately for percutanous iliosacral screws. Though not specifically examined in this study, we think technique 2 has the potential to save operative time and use less fluoroscopic imaging because a lateral image is not required until later in the case. Technique 2 identifies the start point at the level of the skin. This is in contrast to technique 1, which identifies the desired sacral target and requires a surgeon to select a skin start site that will provide an optimal trajectory towards the desired target. Judging trajectory can be difficult, particularly in obese patients, and technique 2 eliminates this extra variable.
It is also important to consider that criteria-based nonorthogonal imaging is required for percutaneous screw placement. In these cases, it is more difficult to judge trajectory corrections because the fluoroscopic beam cannot guide perpendicular corrections as it can in operations that use orthogonal imaging. Adjustments made perpendicular to the fluoroscopic beam will change trajectory in multiple planes.8 Moreover, because the standard cartesian frame of reference is rotated, understanding the location of the sacrum in space can be especially challenging. When using the first technique, sacral landmarks are delineated, and a virtual sacrum drawn on the patient’s exterior helps with orientation. In the second technique, the ideal pin placement is mapped, and the external reference lines guide uniplanar changes. For example, the line drawn co-planar with the inlet view is essentially marking the sacral slope. Therefore, by following this line, uniplanar changes in the cranial and caudal direction are achieved on the outlet view (Figure 5). Because this line is also in reference to the already known ideal pin placement, ideal pin placement can be maintained in 1 radiographic projection while changing the start site in the appropriate direction. In a similar fashion, the co-planar line identified on the inlet view can be used on the outlet image to affect uniplanar changes in the anteroposterior direction. This technique effectively minimizes disorientation when placing percutaneous SI screws. This can be particularly beneficial when placing screws in the prone position.
Conclusion
We have shown 2 techniques that are routinely used at our institution to help identify an accurate starting position for percutaneous screw placement in posterior pelvic ring injuries. Even experienced traumatologists can more quickly and accurately identify the correct stab incisions leading to more confidently placed screws. Further, we believe understanding the usage of fluoroscopy and the concepts involved in drawing the lines enhance trainees’ comprehension of the complex anatomy of the sacrum.
Iliosacral (SI) screws remain the standard of care for the vast majority of posterior pelvic ring disruptions.1,2 However, despite their routine use, the procedure remains technically demanding with repeated cases of aberrant screw placement and complications.3,4 Sacral morphology is extremely variable within a patient population and affects accurate placement and trajectory of percutaneous screws.5 Classically, it is taught that the external starting position/landmark is at an intersection point of the greater trochanter and the anterior superior iliac spine (ASIS). While this “one size fits all” approach will certainly help to coordinate a start position, it is our experience that multiple stab incisions are necessary to find the optimal start site. To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side, guided by the lateral image.5 This article provides a novel image-based technique to be used with, or as a replacement for, the traditional technique.
Techniques
The patient is brought to the operating room and placed supine on a radiolucent operating table. If the closed reduction of the pelvic ring is successful or can be achieved via anterior manipulation/traction, posterior percutaneous pinning is planned. Either a rolled towel or a bag of saline is used as a bolster and placed midline underneath the sacrum and lumbar spine to help “bump” the pelvis and improve the range of motion for the surgeon’s drill. The patient is brought to the edge of the table when possible (ie, a posterior ring injury requiring fixation from only 1 side) to further enhance drill motion. If bilateral screws are planned, surgeons must be careful not to position 1 side at the expense of screw placement on the contralateral side. Nitrous-based anesthetic agents are avoided, because they may collect in the bowel and obscure good radiographic visualization. Arms are placed perpendicular to the body to facilitate the inlet view. Pre-preparation anteroposterior pelvis, inlet, and outlet views are obtained to assure ability to accurately and safely assess landmarks on all projections, and to mark the C-arm position and angles. This process helps decrease “useless” radiographs obtained during the procedure. Acceptable inlet radiographs show the anterior cortex of the S1 body superimposed on the S2 body. Acceptable outlet radiographs show the superior pubic symphysis at the level of the S2 foramen and visualization of the S1/S2 sacral foramen.6 The patient is then prepared in the standard fashion. Reduction maneuvers are performed and, if acceptable alignment is achieved, posterior percutaneous screw placement begins.
Technique 1
To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side using the lateral image. The fluoroscopic machine is set up in a lateral position.5 A free guide wire is superimposed upon the iliac cortical density and anterior sacral slope, which is marked on the skin (Figure 1). The superior portion of S1, as well as the posterior sacral slope, can be marked as well. This process has outlined the sacrum and provides an external landmark for the “safe zone” for screw placement. The operation proceeds in the standard fashion using inlet, outlet, and lateral radiographs. However, the externally drawn sacrum can aid as a reference during guide-pin placement.
Technique 2
This technique takes into account bone anatomy and soft-tissue coverage. It is helpful to think of the abdomen/pelvis as a box. The anterior abdomen represents the top of the box and the lateral buttock represents the side of the box. The corner of the imaginary box is where the abdomen begins to slope down and transitions laterally to become the buttock. This will be referenced as the “down-sloping point” and typically corresponds to the level of the iliac crest (Figure 2).
To begin, a standard cannulated screw guide wire is placed flush on the skin of the abdomen. An inlet fluoroscopy image is taken with the guide pin on the abdomen. Imagine that the resulting image represents the planned screw trajectory (Figure 3A). When the position of the guide wire is deemed adequate, a line is marked on the abdomen, using a pen, directly adjacent to the guide wire. This line represents inlet line 1 (Figure 2). The line must continue laterally until the down-sloping point. The sagittal angle of the imaginary inlet fluoroscopic beam is noted, and a guide wire is placed in the same sagittal orientation flush with the skin on the lateral buttock (Figure 3B). The guide wire must be placed so that it intersects with the first line at the down-sloping point. The skin on the lateral aspect of buttock is marked with a second line, which represents inlet line 2 (Figure 2).
The same process is repeated using an outlet view to create outlet lines 1 and 2 (Figures 4A, 4B). At this point there are 4 lines drawn on the patient (Figure 2). A stab incision is made at the intersection of the 2 lines drawn on the lateral buttock; this represents the skin start point, labeled “start incision” (Figure 2). The procedure continues in standard fashion.
The 4 external reference lines serve multiple purposes. First, the lines mark the true lateral start point for the pin at the level of the skin. This contrasts with the standard technique in which bony landmarks are marked on the skin and the surgeon must estimate a point on the skin that will provide an appropriate trajectory to the bony start point on the ilium. Further, the lines can also be used to reorient the cartesian plane so that adjustments can be isolated to a single plane, ensuring movements only alter the position on a single radiographic view (Figure 5).
Discussion
Despite the widespread use of percutaneous screw placement for posterior pelvic ring injuries, this remains a technically demanding surgery. Recent data suggest patient pelvic anatomy is extremely varied, especially the sacrum.7 Further, screw trajectories vary depending on surgical goals, fracture pattern, and number of screws. Taken together, this implies that there is no perfect universal starting site along the external ilium. Therefore, while classic teaching states to begin screw insertion within the vicinity of the intersection of the greater trochanter and the ASIS, it is our experience that this location is often not ideal.
The inlet, outlet, and lateral radiographs are all vital to assess correct trajectory of the guide pin and drill prior to final screw insertion, but the start site remains a critical step to assure a successful surgical outcome. We present 2 techniques, used together or separately, that allow the surgeon to place the initial guide pin more accurately for percutanous iliosacral screws. Though not specifically examined in this study, we think technique 2 has the potential to save operative time and use less fluoroscopic imaging because a lateral image is not required until later in the case. Technique 2 identifies the start point at the level of the skin. This is in contrast to technique 1, which identifies the desired sacral target and requires a surgeon to select a skin start site that will provide an optimal trajectory towards the desired target. Judging trajectory can be difficult, particularly in obese patients, and technique 2 eliminates this extra variable.
It is also important to consider that criteria-based nonorthogonal imaging is required for percutaneous screw placement. In these cases, it is more difficult to judge trajectory corrections because the fluoroscopic beam cannot guide perpendicular corrections as it can in operations that use orthogonal imaging. Adjustments made perpendicular to the fluoroscopic beam will change trajectory in multiple planes.8 Moreover, because the standard cartesian frame of reference is rotated, understanding the location of the sacrum in space can be especially challenging. When using the first technique, sacral landmarks are delineated, and a virtual sacrum drawn on the patient’s exterior helps with orientation. In the second technique, the ideal pin placement is mapped, and the external reference lines guide uniplanar changes. For example, the line drawn co-planar with the inlet view is essentially marking the sacral slope. Therefore, by following this line, uniplanar changes in the cranial and caudal direction are achieved on the outlet view (Figure 5). Because this line is also in reference to the already known ideal pin placement, ideal pin placement can be maintained in 1 radiographic projection while changing the start site in the appropriate direction. In a similar fashion, the co-planar line identified on the inlet view can be used on the outlet image to affect uniplanar changes in the anteroposterior direction. This technique effectively minimizes disorientation when placing percutaneous SI screws. This can be particularly beneficial when placing screws in the prone position.
Conclusion
We have shown 2 techniques that are routinely used at our institution to help identify an accurate starting position for percutaneous screw placement in posterior pelvic ring injuries. Even experienced traumatologists can more quickly and accurately identify the correct stab incisions leading to more confidently placed screws. Further, we believe understanding the usage of fluoroscopy and the concepts involved in drawing the lines enhance trainees’ comprehension of the complex anatomy of the sacrum.
1. Matta JM, Saucedo T. Internal fixation of pelvic ring fractures. Clin Orthop Relat Res. 1989;242:83-97.
2. Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207-214.
3. Sagi HC, Lindvall EM. Inadvertent intraforaminal iliosacral screw placement despite apparent appropriate positioning on intraoperative fluoroscopy.
J Orthop Trauma. 2005;19(2):130-133.
4. Routt ML Jr, Simonian PT, Mills WJ. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11(8):584-589.
5. Routt ML Jr, Simonian PT, Agnew SG, Mann FA. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma. 1996;10(3):171-177.
6. Gardner MJ, Ferrell ED, Nork SE, Segina DN, Routt ML Jr. Percutaneous placement of iliosacral screws without electrodiagnostic monitoring. J Trauma. 2009;66(5):1411-1415.
7. Miller AN, Routt ML Jr. Variations in sacral morphology and implications for iliosacral screw fixation. J Am Acad Orthop Surg. 2012;20(1):8-16.
8. Graves ML, Routt ML. Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Orthop Trauma. 2011;71(1):204-208.
1. Matta JM, Saucedo T. Internal fixation of pelvic ring fractures. Clin Orthop Relat Res. 1989;242:83-97.
2. Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207-214.
3. Sagi HC, Lindvall EM. Inadvertent intraforaminal iliosacral screw placement despite apparent appropriate positioning on intraoperative fluoroscopy.
J Orthop Trauma. 2005;19(2):130-133.
4. Routt ML Jr, Simonian PT, Mills WJ. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11(8):584-589.
5. Routt ML Jr, Simonian PT, Agnew SG, Mann FA. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma. 1996;10(3):171-177.
6. Gardner MJ, Ferrell ED, Nork SE, Segina DN, Routt ML Jr. Percutaneous placement of iliosacral screws without electrodiagnostic monitoring. J Trauma. 2009;66(5):1411-1415.
7. Miller AN, Routt ML Jr. Variations in sacral morphology and implications for iliosacral screw fixation. J Am Acad Orthop Surg. 2012;20(1):8-16.
8. Graves ML, Routt ML. Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Orthop Trauma. 2011;71(1):204-208.
Big Data: The Paradigm Shift Needed to Revolutionize Musculoskeletal Clinical Research
One year ago, we wrote an editorial in The American Journal of Orthopedics on missing data.1 This year, data is once again the focus of our editorial but from a
different perspective. Rather than focus on the problems of incomplete data, we want to talk about the possibilities of collecting all data through advanced technology, a phenomenon better known as “Big Data.”
New Technology
The factors driving Big Data’s ascendency are the digitalization of useful data, increased means to gather digitalized data, and cheaper analytic power.2 Computer behemoth IBM claims that 90% of the data in the world today has been created in the last 2 years alone.3 Big Data is not just an industry buzzword; it is already an industry in itself. Revenue from Big Data reached $18 billion in 2013 and is predicted to rise to $50 billion in the next 5 years.4 While it is easy to see how Internet companies like Amazon can both collect and use all of the data they receive from customers (to suggest their next purchase, for example), it might be less easy to see how Big Data concepts can be applied to clinical research.
Health Care
Electronic data records are propelling the development of pools of information in health care. Almost half of all hospitals in the United States are participating in health information exchanges (HIEs).5 When these sources of data pools are integrated, the information collected can be used in a powerful way. For example, the health maintenance organization Kaiser Permanente uses a new computer system that drives data exchange between medical facilities. Patient benefits include improved outcomes of cardiovascular disease, and an estimated $1 billion has been saved due to reduced office visits and laboratory tests.5
Contemporary Studies
Let’s quickly consider how we currently conduct clinical studies. Because we do not usually collect data from the entire population, contemporary clinical studies offer only a snapshot of a subsection of patients. The results from this sample are then usually extrapolated to the general population. This was fine when there were insurmountable technological and logistical issues. So instead of trying to
collect data from everyone in the population of interest, we select a sample of patients and expend our energy on controlling for the suboptimal methods we currently employ, techniques which are the best ones available to us.
What are the consequences of all this? Those of us in clinical research are usually very concerned about dealing with confounding factors: selection bias, adjusting for missing data, controlling for errors, and so on. We can also see how imprecise our current methods are by how often a scientific manuscript ends with a call for larger-scale research. Indeed, a scientific research paper that does not list the study’s limitations is often regarded with suspicion, a telling indictment of the problems we expect to encounter in clinical research.
So what has historically been the best current solution to overcome these challenges? A meta-analysis of randomized controlled trials sits atop the evidence pyramid as being the best level of evidence. However, even the use of meta-analyses can be problematic. One group of researchers found that in 2005 and 2008, respectively, 18% and 30% of orthopedic meta-analyses had major to extensive flaws in their methodology.6 Indeed, implicit in the use of a meta-analysis is a criticism that our current studies with their limited sample sizes do not tell the whole story.
Paradigm Shift
We are in the middle of a paradigm shift in the way we can collect and analyze data. Our focus until now has been on identifying a causal relationship in our studies. New technology which allows for large-scale data collection and analysis means that we can now collect ALL patient data, in other words N = all. When you can collect all data, the why (causality) something is happening becomes less
important than the what (correlation) is happening.7 Studies will therefore begin to focus on effectiveness in the real world as opposed to measurements taken under the ideal (or nearly ideal) conditions of efficacy.
All of this is going to have implications, the greatest of which is the change in mindset that we are going to have to go through. How we conduct our studies and what their focus is will both change and expand. For example, the Mini-Sentinel project uses preexisting electronic health care data from multiple sources to monitor the safety of medical products that are regulated by the US Food and
Drug Administration (FDA). This FDA-sponsored initiative, which only began in 2008, had already collected data on 178 million individuals by July 2014.8
Since we cannot ignore Big Data, we must do what we can to ensure that its potential is harnessed to reduce costs and improve patient outcomes. Given the potential of using electronic clinical data, it is also necessary to strike a note of caution. We have to keep uppermost in mind that new technologies like Big Data can unsettle a lot of people. A central tenet of clinical research is that patient data belong to the patient. Robust and transparent processes need to be developed to ensure that patients do not feel compromised in any way by their data being used in such new and widespread methods. The need to rethink and implement safeguards is already being addressed. For example, the university-associated Regenstrief Institute does not pass along even deidentified data to their Big Data industry partner.9
However, we need to also be cognizant of the fact that society is changing in the way people use and regard their own information. Patient-reported data is already being shared among patients online, for both common and rare diseases. The data are also richer and can go beyond the usual outcomes that are recorded to give a bigger picture, eg, why patients are not adhering to treatment regimens.10
In summary, it is our earnest belief that if the health care industry can embrace the concept of Big Data and utilize it properly, our patients and medical practices will be all the better for it.
References
1. Helfet DL, Hanson BP, De Faoite D. Publish or perish; but what, when,
and how? Am J Orthop. 2013;42(9):399-400.
2. Nash DB. Harnessing the power of big data in healthcare. Am Health
Drug Benefits. 2014;7(2):69-70.
3. What is big data? IBM website. http://www-01.ibm.com/software/data/
bigdata/what-is-big-data.html. Accessed July 22, 2014.
4. Upbin B. Visualizing the big data industrial complex. Forbes website.
http://www.forbes.com/sites/bruceupbin/2013/08/30/visualizing-thebig-
data-industrial-complex-infographic/. Published August 30, 2013. Accessed July 22, 2014.
5. Kayyali B, Knott D, Van Kuiken S. The big-data revolution in US health
care: accelerating value and innovation. McKinsey & Company website.
http://www.mckinsey.com/insights/health_systems_and_services/
the_big-data_revolution_in_us_health_care. Published January 2013.
Accessed July 22, 2014.
6. Dijkman BG, Abouali JA, Kooistra BW, et al. Twenty years of meta-analyses
in orthopaedic surgery: has quality kept up with quantity? J Bone Joint Surg Am. 2010;92(1):48-57.
7. Cukier K, Mayer-Schonberger V. Big Data: A Revolution That Will Transform
How We Live, Work, and Think. New York, NY: Eamon Dolan/Houghton Mifflin Harcourt; 2013.
8. Mini-Sentinel distributed data “at a glance.” Mini-Sentinel website.
http://www.mini-sentinel.org/about_us/MSDD_At-a-Glance.aspx. Accessed July 22, 2014.
9. Jain SH, Rosenblatt M, Duke J. Is big data the new frontier for academic-
industry collaboration? JAMA. 2014;311(21):2171-2172.
10. Okun S, McGraw D, Stang P, et al. Making the case for continuous
learning from routinely collected data. Institute of Medicine website.
http://www.iom.edu/Global/Perspectives/2013/~/media/Files/
Perspectives-Files/2013/Discussion-Papers/VSRT-MakingtheCase.pdf. Published April 15, 2013. Accessed July 22, 2014.
One year ago, we wrote an editorial in The American Journal of Orthopedics on missing data.1 This year, data is once again the focus of our editorial but from a
different perspective. Rather than focus on the problems of incomplete data, we want to talk about the possibilities of collecting all data through advanced technology, a phenomenon better known as “Big Data.”
New Technology
The factors driving Big Data’s ascendency are the digitalization of useful data, increased means to gather digitalized data, and cheaper analytic power.2 Computer behemoth IBM claims that 90% of the data in the world today has been created in the last 2 years alone.3 Big Data is not just an industry buzzword; it is already an industry in itself. Revenue from Big Data reached $18 billion in 2013 and is predicted to rise to $50 billion in the next 5 years.4 While it is easy to see how Internet companies like Amazon can both collect and use all of the data they receive from customers (to suggest their next purchase, for example), it might be less easy to see how Big Data concepts can be applied to clinical research.
Health Care
Electronic data records are propelling the development of pools of information in health care. Almost half of all hospitals in the United States are participating in health information exchanges (HIEs).5 When these sources of data pools are integrated, the information collected can be used in a powerful way. For example, the health maintenance organization Kaiser Permanente uses a new computer system that drives data exchange between medical facilities. Patient benefits include improved outcomes of cardiovascular disease, and an estimated $1 billion has been saved due to reduced office visits and laboratory tests.5
Contemporary Studies
Let’s quickly consider how we currently conduct clinical studies. Because we do not usually collect data from the entire population, contemporary clinical studies offer only a snapshot of a subsection of patients. The results from this sample are then usually extrapolated to the general population. This was fine when there were insurmountable technological and logistical issues. So instead of trying to
collect data from everyone in the population of interest, we select a sample of patients and expend our energy on controlling for the suboptimal methods we currently employ, techniques which are the best ones available to us.
What are the consequences of all this? Those of us in clinical research are usually very concerned about dealing with confounding factors: selection bias, adjusting for missing data, controlling for errors, and so on. We can also see how imprecise our current methods are by how often a scientific manuscript ends with a call for larger-scale research. Indeed, a scientific research paper that does not list the study’s limitations is often regarded with suspicion, a telling indictment of the problems we expect to encounter in clinical research.
So what has historically been the best current solution to overcome these challenges? A meta-analysis of randomized controlled trials sits atop the evidence pyramid as being the best level of evidence. However, even the use of meta-analyses can be problematic. One group of researchers found that in 2005 and 2008, respectively, 18% and 30% of orthopedic meta-analyses had major to extensive flaws in their methodology.6 Indeed, implicit in the use of a meta-analysis is a criticism that our current studies with their limited sample sizes do not tell the whole story.
Paradigm Shift
We are in the middle of a paradigm shift in the way we can collect and analyze data. Our focus until now has been on identifying a causal relationship in our studies. New technology which allows for large-scale data collection and analysis means that we can now collect ALL patient data, in other words N = all. When you can collect all data, the why (causality) something is happening becomes less
important than the what (correlation) is happening.7 Studies will therefore begin to focus on effectiveness in the real world as opposed to measurements taken under the ideal (or nearly ideal) conditions of efficacy.
All of this is going to have implications, the greatest of which is the change in mindset that we are going to have to go through. How we conduct our studies and what their focus is will both change and expand. For example, the Mini-Sentinel project uses preexisting electronic health care data from multiple sources to monitor the safety of medical products that are regulated by the US Food and
Drug Administration (FDA). This FDA-sponsored initiative, which only began in 2008, had already collected data on 178 million individuals by July 2014.8
Since we cannot ignore Big Data, we must do what we can to ensure that its potential is harnessed to reduce costs and improve patient outcomes. Given the potential of using electronic clinical data, it is also necessary to strike a note of caution. We have to keep uppermost in mind that new technologies like Big Data can unsettle a lot of people. A central tenet of clinical research is that patient data belong to the patient. Robust and transparent processes need to be developed to ensure that patients do not feel compromised in any way by their data being used in such new and widespread methods. The need to rethink and implement safeguards is already being addressed. For example, the university-associated Regenstrief Institute does not pass along even deidentified data to their Big Data industry partner.9
However, we need to also be cognizant of the fact that society is changing in the way people use and regard their own information. Patient-reported data is already being shared among patients online, for both common and rare diseases. The data are also richer and can go beyond the usual outcomes that are recorded to give a bigger picture, eg, why patients are not adhering to treatment regimens.10
In summary, it is our earnest belief that if the health care industry can embrace the concept of Big Data and utilize it properly, our patients and medical practices will be all the better for it.
References
1. Helfet DL, Hanson BP, De Faoite D. Publish or perish; but what, when,
and how? Am J Orthop. 2013;42(9):399-400.
2. Nash DB. Harnessing the power of big data in healthcare. Am Health
Drug Benefits. 2014;7(2):69-70.
3. What is big data? IBM website. http://www-01.ibm.com/software/data/
bigdata/what-is-big-data.html. Accessed July 22, 2014.
4. Upbin B. Visualizing the big data industrial complex. Forbes website.
http://www.forbes.com/sites/bruceupbin/2013/08/30/visualizing-thebig-
data-industrial-complex-infographic/. Published August 30, 2013. Accessed July 22, 2014.
5. Kayyali B, Knott D, Van Kuiken S. The big-data revolution in US health
care: accelerating value and innovation. McKinsey & Company website.
http://www.mckinsey.com/insights/health_systems_and_services/
the_big-data_revolution_in_us_health_care. Published January 2013.
Accessed July 22, 2014.
6. Dijkman BG, Abouali JA, Kooistra BW, et al. Twenty years of meta-analyses
in orthopaedic surgery: has quality kept up with quantity? J Bone Joint Surg Am. 2010;92(1):48-57.
7. Cukier K, Mayer-Schonberger V. Big Data: A Revolution That Will Transform
How We Live, Work, and Think. New York, NY: Eamon Dolan/Houghton Mifflin Harcourt; 2013.
8. Mini-Sentinel distributed data “at a glance.” Mini-Sentinel website.
http://www.mini-sentinel.org/about_us/MSDD_At-a-Glance.aspx. Accessed July 22, 2014.
9. Jain SH, Rosenblatt M, Duke J. Is big data the new frontier for academic-
industry collaboration? JAMA. 2014;311(21):2171-2172.
10. Okun S, McGraw D, Stang P, et al. Making the case for continuous
learning from routinely collected data. Institute of Medicine website.
http://www.iom.edu/Global/Perspectives/2013/~/media/Files/
Perspectives-Files/2013/Discussion-Papers/VSRT-MakingtheCase.pdf. Published April 15, 2013. Accessed July 22, 2014.
One year ago, we wrote an editorial in The American Journal of Orthopedics on missing data.1 This year, data is once again the focus of our editorial but from a
different perspective. Rather than focus on the problems of incomplete data, we want to talk about the possibilities of collecting all data through advanced technology, a phenomenon better known as “Big Data.”
New Technology
The factors driving Big Data’s ascendency are the digitalization of useful data, increased means to gather digitalized data, and cheaper analytic power.2 Computer behemoth IBM claims that 90% of the data in the world today has been created in the last 2 years alone.3 Big Data is not just an industry buzzword; it is already an industry in itself. Revenue from Big Data reached $18 billion in 2013 and is predicted to rise to $50 billion in the next 5 years.4 While it is easy to see how Internet companies like Amazon can both collect and use all of the data they receive from customers (to suggest their next purchase, for example), it might be less easy to see how Big Data concepts can be applied to clinical research.
Health Care
Electronic data records are propelling the development of pools of information in health care. Almost half of all hospitals in the United States are participating in health information exchanges (HIEs).5 When these sources of data pools are integrated, the information collected can be used in a powerful way. For example, the health maintenance organization Kaiser Permanente uses a new computer system that drives data exchange between medical facilities. Patient benefits include improved outcomes of cardiovascular disease, and an estimated $1 billion has been saved due to reduced office visits and laboratory tests.5
Contemporary Studies
Let’s quickly consider how we currently conduct clinical studies. Because we do not usually collect data from the entire population, contemporary clinical studies offer only a snapshot of a subsection of patients. The results from this sample are then usually extrapolated to the general population. This was fine when there were insurmountable technological and logistical issues. So instead of trying to
collect data from everyone in the population of interest, we select a sample of patients and expend our energy on controlling for the suboptimal methods we currently employ, techniques which are the best ones available to us.
What are the consequences of all this? Those of us in clinical research are usually very concerned about dealing with confounding factors: selection bias, adjusting for missing data, controlling for errors, and so on. We can also see how imprecise our current methods are by how often a scientific manuscript ends with a call for larger-scale research. Indeed, a scientific research paper that does not list the study’s limitations is often regarded with suspicion, a telling indictment of the problems we expect to encounter in clinical research.
So what has historically been the best current solution to overcome these challenges? A meta-analysis of randomized controlled trials sits atop the evidence pyramid as being the best level of evidence. However, even the use of meta-analyses can be problematic. One group of researchers found that in 2005 and 2008, respectively, 18% and 30% of orthopedic meta-analyses had major to extensive flaws in their methodology.6 Indeed, implicit in the use of a meta-analysis is a criticism that our current studies with their limited sample sizes do not tell the whole story.
Paradigm Shift
We are in the middle of a paradigm shift in the way we can collect and analyze data. Our focus until now has been on identifying a causal relationship in our studies. New technology which allows for large-scale data collection and analysis means that we can now collect ALL patient data, in other words N = all. When you can collect all data, the why (causality) something is happening becomes less
important than the what (correlation) is happening.7 Studies will therefore begin to focus on effectiveness in the real world as opposed to measurements taken under the ideal (or nearly ideal) conditions of efficacy.
All of this is going to have implications, the greatest of which is the change in mindset that we are going to have to go through. How we conduct our studies and what their focus is will both change and expand. For example, the Mini-Sentinel project uses preexisting electronic health care data from multiple sources to monitor the safety of medical products that are regulated by the US Food and
Drug Administration (FDA). This FDA-sponsored initiative, which only began in 2008, had already collected data on 178 million individuals by July 2014.8
Since we cannot ignore Big Data, we must do what we can to ensure that its potential is harnessed to reduce costs and improve patient outcomes. Given the potential of using electronic clinical data, it is also necessary to strike a note of caution. We have to keep uppermost in mind that new technologies like Big Data can unsettle a lot of people. A central tenet of clinical research is that patient data belong to the patient. Robust and transparent processes need to be developed to ensure that patients do not feel compromised in any way by their data being used in such new and widespread methods. The need to rethink and implement safeguards is already being addressed. For example, the university-associated Regenstrief Institute does not pass along even deidentified data to their Big Data industry partner.9
However, we need to also be cognizant of the fact that society is changing in the way people use and regard their own information. Patient-reported data is already being shared among patients online, for both common and rare diseases. The data are also richer and can go beyond the usual outcomes that are recorded to give a bigger picture, eg, why patients are not adhering to treatment regimens.10
In summary, it is our earnest belief that if the health care industry can embrace the concept of Big Data and utilize it properly, our patients and medical practices will be all the better for it.
References
1. Helfet DL, Hanson BP, De Faoite D. Publish or perish; but what, when,
and how? Am J Orthop. 2013;42(9):399-400.
2. Nash DB. Harnessing the power of big data in healthcare. Am Health
Drug Benefits. 2014;7(2):69-70.
3. What is big data? IBM website. http://www-01.ibm.com/software/data/
bigdata/what-is-big-data.html. Accessed July 22, 2014.
4. Upbin B. Visualizing the big data industrial complex. Forbes website.
http://www.forbes.com/sites/bruceupbin/2013/08/30/visualizing-thebig-
data-industrial-complex-infographic/. Published August 30, 2013. Accessed July 22, 2014.
5. Kayyali B, Knott D, Van Kuiken S. The big-data revolution in US health
care: accelerating value and innovation. McKinsey & Company website.
http://www.mckinsey.com/insights/health_systems_and_services/
the_big-data_revolution_in_us_health_care. Published January 2013.
Accessed July 22, 2014.
6. Dijkman BG, Abouali JA, Kooistra BW, et al. Twenty years of meta-analyses
in orthopaedic surgery: has quality kept up with quantity? J Bone Joint Surg Am. 2010;92(1):48-57.
7. Cukier K, Mayer-Schonberger V. Big Data: A Revolution That Will Transform
How We Live, Work, and Think. New York, NY: Eamon Dolan/Houghton Mifflin Harcourt; 2013.
8. Mini-Sentinel distributed data “at a glance.” Mini-Sentinel website.
http://www.mini-sentinel.org/about_us/MSDD_At-a-Glance.aspx. Accessed July 22, 2014.
9. Jain SH, Rosenblatt M, Duke J. Is big data the new frontier for academic-
industry collaboration? JAMA. 2014;311(21):2171-2172.
10. Okun S, McGraw D, Stang P, et al. Making the case for continuous
learning from routinely collected data. Institute of Medicine website.
http://www.iom.edu/Global/Perspectives/2013/~/media/Files/
Perspectives-Files/2013/Discussion-Papers/VSRT-MakingtheCase.pdf. Published April 15, 2013. Accessed July 22, 2014.
Acute Compartment Syndrome in Patients With Tibia Fractures Transferred for Definitive Fracture Care
An Isolated Iliac Wing Stress Fracture in a Marathon Runner
Symptomatic Hip Impingement Due to Exostosis Associated With Supra-Acetabular Pelvic External Fixator Pin
Publish or Perish; But What, When, and How?
If we were to try to identify a Zeitgeist (spirit of the time) in society, one possible answer would be data. In the field of clinical research this could mean data that is collected, not collected, public, hidden from view, published, not published—the list of issues connected to data is almost endless.
In this editorial, we would like to examine clinical research data from 3 different perspectives. What happens when there is no data available? Or when only incomplete data can be accessed? Or when all of the data is in the public realm but is uncritically taken at face value?
There is currently a groundswell of opinion that the subject of transparency of clinical trial data needs to be tackled. This campaign is particularly strong in the United Kingdom where the British Medical Journal and advocacy groups like www.alltrials.net have gained prominence. Ben Goldacre, author of the recent Bad Pharma book, goes so far as to say, “The problem of missing trials is one of the greatest ethical and practical problems facing medicine today.”1
Here in the United States we also have issues with data. One study from 2009 found that the results of only 44% of trials conducted in the United States and Canada is published in the medical literature.2 However, this study was on general medicine, how are we faring in orthopedics? A study from 2011 targeted orthopedic trauma trials registered on www.clinicaltrials.gov and followed them up to see if they were published within a reasonable timeframe.3 The result? Only 43.2% of the orthopedic trauma trials studied resulted in a publication—a figure that almost exactly mirrors the findings from the general medicine study.
Data that is not released obviously skews the evidence available to us as clinicians and researchers. More insidious still is incomplete data as it gives a false picture to anyone reading the original study or to a researcher who wants to include the study in a meta-analysis. We are all aware of the difficulty of having complete patient follow-up because, ironically, we as surgeons have enabled our patients to walk away from the study. How should we best deal with these gaps in our knowledge? Some statistical techniques have been developed to deal with just this problem.
One set of researchers looked at how missing data was dealt with in an intention-to-treat analysis in orthopedic randomized clinical trials.4 They took 1 published study and recalculated the way patients on a displaced midshaft clavicular fracture trial who were lost to follow-up are handled. These researchers used the Last Observation Carried Forward technique and compared this to the original method, which was exclusion from the analysis. This change in approach changed the significance of the nonunion and overall complication results. However, the use of these various methods to deal with missing data in intention-to-treat analysis is in itself the subject of some controversy in orthopedic clinical research.5
There is more than merely anecdotal evidence that uncritical acceptance of research findings could harm patients. We are all familiar with the recent metal-on-metal hip implant controversy when promising early results were not borne out by later experience. One study, which found combined clinical and radiographic failure rates of 28% among large diameter metal-on-metal articulations in total hip arthroplasty, notes that, “adequate preclinical trials may have identified some of the shortcomings of this class of implants before the marketing and widespread use of these implants ensued.”6
Is this volte-face in the evidence released a rare occurrence? Perhaps not. A well-known review of 49 studies from 2005 found that 45 claimed the intervention was effective.7 Subsequent investigations contradicted the findings of 7 of the original studies with positive results (16%), and a further 7 of these studies (16%) reported effects stronger than those of any of the follow-up studies, studies which were larger or better controlled. The evidence for almost one-third of the positive result studies was therefore changed, either wholly or partly. Keep in mind that this figure does not take into account the 11 positive result studies which were not replicated at all.
In all of this, we have to accept that things are rarely black and white. When is the best time to release information? For example, the conclusion for a closed fracture treatment subgroup in the study to prospectively evaluate reamed intramedullary (IM) nails in tibial fractures (SPRINT) changed only after 800 patients had been enrolled. A smaller trial would have led to an incorrect conclusion for this subgroup.8 As you can see, deciding on when to release data is a delicate subject and is influenced by many factors, not least time and costs. Many contemporary clinical researchers also operate under publication pressures.9 And all of us are aware of the kudos that accrue from being first-in-manuscript authors!
Unfortunately, knowing how to identify good and bad (and premature) information, and how to filter out relevant information in today’s flood of publications in the field of medicine is likely to remain an intractable problem for all of us involved in conducting or assessing clinical research for the foreseeable future. This is why the critical appraisal techniques of evidence-based medicine are invaluable.
Starr10 in writing about the advances in fracture repair achieved by the AO (Arbeitsgemeinschaft für Osteosynthesefragen/Association for the Study of Internal Fixation), says that, “Fortunately, the surgical pioneers who described early use of these techniques were harsh critics of their own work. The need for better methods and implants was evident.” From its founding, the AO inculcated a culture in which data, positive or negative, was shared.
Perhaps the ‘Golden Age of Orthopedic Surgery’ has already passed. But even with all of the advances in today’s operating room, we should continue to strive to improve what it is we do, even if it is only incrementally. As this editorial has illustrated, complacency about clinical research data presents a challenge to better patient care. We need to continue to be inquisitive and questioning in our quest to be better!
Dr. Helfet is Associate Editor of Trauma of this journal; Professor, Department of Orthopedic Surgery, Cornell University Medical College; and Director of the Orthopaedic Trauma Service, at the Hospital for Special Surgery and New York–Presbyterian Hospital, New York, New York. Dr. Hanson is Director and Mr. De Faoite is Education Manager, AO (Arbeitsgemeinschaft für Osteosynthesefragen/Association for the Study of Internal Fixation) Clinical Investigation and Documentation (AOCID), Dübendorf, Switzerland.
Authors’ Disclosure Statement: The authors report no actual or potential conflict of interest in relation to this article.
Am J Orthop. 2013;42(9):399-400. Copyright Frontline Medical Communications Inc. 2013. All rights reserved.
1. Davies E. The shifting debate on trial data transparency. BMJ. 2013;347:f4485.
2. Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.Gov: a cross-sectional analysis. PLoS Med. 2009;6(9):e1000144.
3. Gandhi R, Jan M, Smith HN, Mahomed NN, Bhandari M. Comparison of published orthopaedic trauma trials following registration in Clinicaltrials.gov. BMC Musculoskelet Disord. 2011;12:278.
4. Herman A, Botser IB, Tenenbaum S, Chechick A. Intention-to-treat analysis and accounting for missing data in orthopaedic randomized clinical trials. J Bone Joint Surg Am. 2009;91(9):2137-2143.
5. Scharfstein DO, Hogan J, Herman A. On the prevention and analysis of missing data in randomized clinical trials: the state of the art. J Bone Joint Surg Am. 2012;94 suppl 1:80-84.
6. Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6 suppl):14-18.
7. Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294(2):218-228.
8. Slobogean GP, Sprague S, Bhandari M. The tactics of large randomized trials. J Bone Joint Surg Am. 2012;94 suppl 1:19-23.
9. Duvivier R, Crocker-Buqué T, Stull MJ. Young doctors and the pressure of publication. Lancet. 2013;381(9876):e10.
10. Starr AJ. Fracture repair: successful advances, persistent problems, and the psychological burden of trauma. J Bone Joint Surg Am. 2008;90 suppl 1:132-137.
If we were to try to identify a Zeitgeist (spirit of the time) in society, one possible answer would be data. In the field of clinical research this could mean data that is collected, not collected, public, hidden from view, published, not published—the list of issues connected to data is almost endless.
In this editorial, we would like to examine clinical research data from 3 different perspectives. What happens when there is no data available? Or when only incomplete data can be accessed? Or when all of the data is in the public realm but is uncritically taken at face value?
There is currently a groundswell of opinion that the subject of transparency of clinical trial data needs to be tackled. This campaign is particularly strong in the United Kingdom where the British Medical Journal and advocacy groups like www.alltrials.net have gained prominence. Ben Goldacre, author of the recent Bad Pharma book, goes so far as to say, “The problem of missing trials is one of the greatest ethical and practical problems facing medicine today.”1
Here in the United States we also have issues with data. One study from 2009 found that the results of only 44% of trials conducted in the United States and Canada is published in the medical literature.2 However, this study was on general medicine, how are we faring in orthopedics? A study from 2011 targeted orthopedic trauma trials registered on www.clinicaltrials.gov and followed them up to see if they were published within a reasonable timeframe.3 The result? Only 43.2% of the orthopedic trauma trials studied resulted in a publication—a figure that almost exactly mirrors the findings from the general medicine study.
Data that is not released obviously skews the evidence available to us as clinicians and researchers. More insidious still is incomplete data as it gives a false picture to anyone reading the original study or to a researcher who wants to include the study in a meta-analysis. We are all aware of the difficulty of having complete patient follow-up because, ironically, we as surgeons have enabled our patients to walk away from the study. How should we best deal with these gaps in our knowledge? Some statistical techniques have been developed to deal with just this problem.
One set of researchers looked at how missing data was dealt with in an intention-to-treat analysis in orthopedic randomized clinical trials.4 They took 1 published study and recalculated the way patients on a displaced midshaft clavicular fracture trial who were lost to follow-up are handled. These researchers used the Last Observation Carried Forward technique and compared this to the original method, which was exclusion from the analysis. This change in approach changed the significance of the nonunion and overall complication results. However, the use of these various methods to deal with missing data in intention-to-treat analysis is in itself the subject of some controversy in orthopedic clinical research.5
There is more than merely anecdotal evidence that uncritical acceptance of research findings could harm patients. We are all familiar with the recent metal-on-metal hip implant controversy when promising early results were not borne out by later experience. One study, which found combined clinical and radiographic failure rates of 28% among large diameter metal-on-metal articulations in total hip arthroplasty, notes that, “adequate preclinical trials may have identified some of the shortcomings of this class of implants before the marketing and widespread use of these implants ensued.”6
Is this volte-face in the evidence released a rare occurrence? Perhaps not. A well-known review of 49 studies from 2005 found that 45 claimed the intervention was effective.7 Subsequent investigations contradicted the findings of 7 of the original studies with positive results (16%), and a further 7 of these studies (16%) reported effects stronger than those of any of the follow-up studies, studies which were larger or better controlled. The evidence for almost one-third of the positive result studies was therefore changed, either wholly or partly. Keep in mind that this figure does not take into account the 11 positive result studies which were not replicated at all.
In all of this, we have to accept that things are rarely black and white. When is the best time to release information? For example, the conclusion for a closed fracture treatment subgroup in the study to prospectively evaluate reamed intramedullary (IM) nails in tibial fractures (SPRINT) changed only after 800 patients had been enrolled. A smaller trial would have led to an incorrect conclusion for this subgroup.8 As you can see, deciding on when to release data is a delicate subject and is influenced by many factors, not least time and costs. Many contemporary clinical researchers also operate under publication pressures.9 And all of us are aware of the kudos that accrue from being first-in-manuscript authors!
Unfortunately, knowing how to identify good and bad (and premature) information, and how to filter out relevant information in today’s flood of publications in the field of medicine is likely to remain an intractable problem for all of us involved in conducting or assessing clinical research for the foreseeable future. This is why the critical appraisal techniques of evidence-based medicine are invaluable.
Starr10 in writing about the advances in fracture repair achieved by the AO (Arbeitsgemeinschaft für Osteosynthesefragen/Association for the Study of Internal Fixation), says that, “Fortunately, the surgical pioneers who described early use of these techniques were harsh critics of their own work. The need for better methods and implants was evident.” From its founding, the AO inculcated a culture in which data, positive or negative, was shared.
Perhaps the ‘Golden Age of Orthopedic Surgery’ has already passed. But even with all of the advances in today’s operating room, we should continue to strive to improve what it is we do, even if it is only incrementally. As this editorial has illustrated, complacency about clinical research data presents a challenge to better patient care. We need to continue to be inquisitive and questioning in our quest to be better!
Dr. Helfet is Associate Editor of Trauma of this journal; Professor, Department of Orthopedic Surgery, Cornell University Medical College; and Director of the Orthopaedic Trauma Service, at the Hospital for Special Surgery and New York–Presbyterian Hospital, New York, New York. Dr. Hanson is Director and Mr. De Faoite is Education Manager, AO (Arbeitsgemeinschaft für Osteosynthesefragen/Association for the Study of Internal Fixation) Clinical Investigation and Documentation (AOCID), Dübendorf, Switzerland.
Authors’ Disclosure Statement: The authors report no actual or potential conflict of interest in relation to this article.
Am J Orthop. 2013;42(9):399-400. Copyright Frontline Medical Communications Inc. 2013. All rights reserved.
If we were to try to identify a Zeitgeist (spirit of the time) in society, one possible answer would be data. In the field of clinical research this could mean data that is collected, not collected, public, hidden from view, published, not published—the list of issues connected to data is almost endless.
In this editorial, we would like to examine clinical research data from 3 different perspectives. What happens when there is no data available? Or when only incomplete data can be accessed? Or when all of the data is in the public realm but is uncritically taken at face value?
There is currently a groundswell of opinion that the subject of transparency of clinical trial data needs to be tackled. This campaign is particularly strong in the United Kingdom where the British Medical Journal and advocacy groups like www.alltrials.net have gained prominence. Ben Goldacre, author of the recent Bad Pharma book, goes so far as to say, “The problem of missing trials is one of the greatest ethical and practical problems facing medicine today.”1
Here in the United States we also have issues with data. One study from 2009 found that the results of only 44% of trials conducted in the United States and Canada is published in the medical literature.2 However, this study was on general medicine, how are we faring in orthopedics? A study from 2011 targeted orthopedic trauma trials registered on www.clinicaltrials.gov and followed them up to see if they were published within a reasonable timeframe.3 The result? Only 43.2% of the orthopedic trauma trials studied resulted in a publication—a figure that almost exactly mirrors the findings from the general medicine study.
Data that is not released obviously skews the evidence available to us as clinicians and researchers. More insidious still is incomplete data as it gives a false picture to anyone reading the original study or to a researcher who wants to include the study in a meta-analysis. We are all aware of the difficulty of having complete patient follow-up because, ironically, we as surgeons have enabled our patients to walk away from the study. How should we best deal with these gaps in our knowledge? Some statistical techniques have been developed to deal with just this problem.
One set of researchers looked at how missing data was dealt with in an intention-to-treat analysis in orthopedic randomized clinical trials.4 They took 1 published study and recalculated the way patients on a displaced midshaft clavicular fracture trial who were lost to follow-up are handled. These researchers used the Last Observation Carried Forward technique and compared this to the original method, which was exclusion from the analysis. This change in approach changed the significance of the nonunion and overall complication results. However, the use of these various methods to deal with missing data in intention-to-treat analysis is in itself the subject of some controversy in orthopedic clinical research.5
There is more than merely anecdotal evidence that uncritical acceptance of research findings could harm patients. We are all familiar with the recent metal-on-metal hip implant controversy when promising early results were not borne out by later experience. One study, which found combined clinical and radiographic failure rates of 28% among large diameter metal-on-metal articulations in total hip arthroplasty, notes that, “adequate preclinical trials may have identified some of the shortcomings of this class of implants before the marketing and widespread use of these implants ensued.”6
Is this volte-face in the evidence released a rare occurrence? Perhaps not. A well-known review of 49 studies from 2005 found that 45 claimed the intervention was effective.7 Subsequent investigations contradicted the findings of 7 of the original studies with positive results (16%), and a further 7 of these studies (16%) reported effects stronger than those of any of the follow-up studies, studies which were larger or better controlled. The evidence for almost one-third of the positive result studies was therefore changed, either wholly or partly. Keep in mind that this figure does not take into account the 11 positive result studies which were not replicated at all.
In all of this, we have to accept that things are rarely black and white. When is the best time to release information? For example, the conclusion for a closed fracture treatment subgroup in the study to prospectively evaluate reamed intramedullary (IM) nails in tibial fractures (SPRINT) changed only after 800 patients had been enrolled. A smaller trial would have led to an incorrect conclusion for this subgroup.8 As you can see, deciding on when to release data is a delicate subject and is influenced by many factors, not least time and costs. Many contemporary clinical researchers also operate under publication pressures.9 And all of us are aware of the kudos that accrue from being first-in-manuscript authors!
Unfortunately, knowing how to identify good and bad (and premature) information, and how to filter out relevant information in today’s flood of publications in the field of medicine is likely to remain an intractable problem for all of us involved in conducting or assessing clinical research for the foreseeable future. This is why the critical appraisal techniques of evidence-based medicine are invaluable.
Starr10 in writing about the advances in fracture repair achieved by the AO (Arbeitsgemeinschaft für Osteosynthesefragen/Association for the Study of Internal Fixation), says that, “Fortunately, the surgical pioneers who described early use of these techniques were harsh critics of their own work. The need for better methods and implants was evident.” From its founding, the AO inculcated a culture in which data, positive or negative, was shared.
Perhaps the ‘Golden Age of Orthopedic Surgery’ has already passed. But even with all of the advances in today’s operating room, we should continue to strive to improve what it is we do, even if it is only incrementally. As this editorial has illustrated, complacency about clinical research data presents a challenge to better patient care. We need to continue to be inquisitive and questioning in our quest to be better!
Dr. Helfet is Associate Editor of Trauma of this journal; Professor, Department of Orthopedic Surgery, Cornell University Medical College; and Director of the Orthopaedic Trauma Service, at the Hospital for Special Surgery and New York–Presbyterian Hospital, New York, New York. Dr. Hanson is Director and Mr. De Faoite is Education Manager, AO (Arbeitsgemeinschaft für Osteosynthesefragen/Association for the Study of Internal Fixation) Clinical Investigation and Documentation (AOCID), Dübendorf, Switzerland.
Authors’ Disclosure Statement: The authors report no actual or potential conflict of interest in relation to this article.
Am J Orthop. 2013;42(9):399-400. Copyright Frontline Medical Communications Inc. 2013. All rights reserved.
1. Davies E. The shifting debate on trial data transparency. BMJ. 2013;347:f4485.
2. Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.Gov: a cross-sectional analysis. PLoS Med. 2009;6(9):e1000144.
3. Gandhi R, Jan M, Smith HN, Mahomed NN, Bhandari M. Comparison of published orthopaedic trauma trials following registration in Clinicaltrials.gov. BMC Musculoskelet Disord. 2011;12:278.
4. Herman A, Botser IB, Tenenbaum S, Chechick A. Intention-to-treat analysis and accounting for missing data in orthopaedic randomized clinical trials. J Bone Joint Surg Am. 2009;91(9):2137-2143.
5. Scharfstein DO, Hogan J, Herman A. On the prevention and analysis of missing data in randomized clinical trials: the state of the art. J Bone Joint Surg Am. 2012;94 suppl 1:80-84.
6. Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6 suppl):14-18.
7. Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294(2):218-228.
8. Slobogean GP, Sprague S, Bhandari M. The tactics of large randomized trials. J Bone Joint Surg Am. 2012;94 suppl 1:19-23.
9. Duvivier R, Crocker-Buqué T, Stull MJ. Young doctors and the pressure of publication. Lancet. 2013;381(9876):e10.
10. Starr AJ. Fracture repair: successful advances, persistent problems, and the psychological burden of trauma. J Bone Joint Surg Am. 2008;90 suppl 1:132-137.
1. Davies E. The shifting debate on trial data transparency. BMJ. 2013;347:f4485.
2. Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.Gov: a cross-sectional analysis. PLoS Med. 2009;6(9):e1000144.
3. Gandhi R, Jan M, Smith HN, Mahomed NN, Bhandari M. Comparison of published orthopaedic trauma trials following registration in Clinicaltrials.gov. BMC Musculoskelet Disord. 2011;12:278.
4. Herman A, Botser IB, Tenenbaum S, Chechick A. Intention-to-treat analysis and accounting for missing data in orthopaedic randomized clinical trials. J Bone Joint Surg Am. 2009;91(9):2137-2143.
5. Scharfstein DO, Hogan J, Herman A. On the prevention and analysis of missing data in randomized clinical trials: the state of the art. J Bone Joint Surg Am. 2012;94 suppl 1:80-84.
6. Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6 suppl):14-18.
7. Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294(2):218-228.
8. Slobogean GP, Sprague S, Bhandari M. The tactics of large randomized trials. J Bone Joint Surg Am. 2012;94 suppl 1:19-23.
9. Duvivier R, Crocker-Buqué T, Stull MJ. Young doctors and the pressure of publication. Lancet. 2013;381(9876):e10.
10. Starr AJ. Fracture repair: successful advances, persistent problems, and the psychological burden of trauma. J Bone Joint Surg Am. 2008;90 suppl 1:132-137.