User login
Middle East respiratory syndrome: SARS redux?
Middle East respiratory syndrome (MERS) is a potentially lethal illness caused by the Middle East respiratory syndrome coronavirus (MERS-CoV). The virus was first reported in 2012, when it was isolated from the sputum of a previously healthy man in Saudi Arabia who presented with acute pneumonia and subsequent renal failure with a fatal outcome.1 Retrospective studies subsequently identified an earlier outbreak that year involving 13 patients in Jordan, and since then cases have been reported in 25 countries across the Arabian Peninsula and in Asia, Europe, Africa, and the United States, with over 1,000 confirmed cases and 450 related deaths.2,3
At the time of this writing, two cases of MERS have been reported in the United States, both in May 2014. Both reported cases involved patients who had traveled from Saudi Arabia, and which did not result in secondary cases.4 Beginning in May 2015, the Republic of Korea had experienced the largest known outbreak of MERS outside the Arabian Peninsula, with over 100 cases.5
THE VIRUS
MERS-CoV is classified as a coronavirus, which is a family of single-stranded RNA viruses. In 2003, a previously unknown coronavirus (SARS-CoV) caused a global outbreak of pneumonia that resulted in approximately 800 deaths.6 The MERS-CoV virus attaches to dipeptidyl peptidase 4 to enter cells, and this receptor is believed to be critical for pathogenesis, as infection does not occur in its absence.7
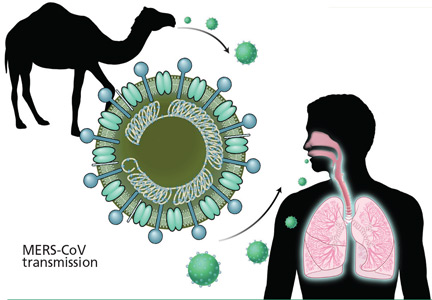
The source and mode of transmission to humans is not completely defined. Early reports suggested that MERS-CoV originated in bats, as RNA sequences related to MERS-CoV have been found in several bat species, but the virus itself has not been isolated from bats.8 Camels have been found to have a high rate of anti-MERS-CoV antibodies and to have the virus in nose swabs, and evidence for camel-to-human transmission has been presented.9–11 However, the precise role of camels and other animals as reservoirs or vectors of infection is still under investigation.
The incubation period from exposure to the development of clinical disease is estimated at 5 to 14 days.
For MERS-CoV, the basic reproduction ratio (R0), which measures the average number of secondary cases from each infected person, is estimated12 to be less than 0.7. In diseases in which the R0 is less than 1.0, infections occur in isolated clusters as limited chains of transmission, and thus the sustained transmission of MERS-CoV resulting in a large epidemic is thought to be unlikely. As a comparison, the median R0 value for seasonal influenza is estimated13 at 1.28. “Superspreading” may result in limited outbreaks of secondary cases; however, the continued epidemic spread of infection is thought to be unlikely.14 Nevertheless, viral adaptation with increased transmissibility remains a concern and a potential threat.
CLINICAL PRESENTATION
MERS most commonly presents as a respiratory illness, although asymptomatic infection occurs. The percentage of patients who experience asymptomatic infection is unknown. A recent survey of 255 patients with laboratory-confirmed MERS-CoV found that 64 (25.1%) were reported as asymptomatic at time of specimen collection. However, when 33 (52%) of those patients were interviewed, 26 (79%) reported at least one symptom that was consistent with a viral respiratory illness.15
For symptomatic patients, the initial complaints are nonspecific, beginning with fever, cough, sore throat, chills, and myalgia. Patients experiencing severe infection progress to dyspnea and pneumonia, with requirements for ventilatory support, vasopressors, and renal replacement therapy.16 Gastrointestinal symptoms such as vomiting and diarrhea have been reported in about one-third of patients.17
In a study of 47 patients with MERS-CoV, most of whom had underlying medical illnesses, 42 (89%) required intensive care and 34 (72%) required mechanical ventilation.17 The case-fatality rate in this study was 60%, but other studies have reported rates closer to 30%.15
Laboratory findings in patients with MERS-CoV infection usually include leukopenia and thrombocytopenia. Severely ill patients may have evidence of acute kidney injury.
Radiographic findings of MERS are those of viral pneumonitis and acute respiratory distress syndrome. Computed tomographic findings include ground-glass opacities, with peripheral lower-lobe preference.18
DIAGNOSIS
As MERS is a respiratory illness, sampling of respiratory secretions provides the highest yield for diagnosis. A study of 112 patients with MERS-CoV reported that polymerase chain reaction (PCR) testing of tracheal aspirates and bronchoalveolar lavage samples yielded significantly higher MERS-CoV loads than nasopharyngeal swab samples and sputum samples.19 However, upper respiratory tract testing is less invasive, and a positive nasopharyngeal swab result may obviate the need for further testing.
The US Centers for Disease Control and Prevention (CDC) recommends collecting multiple specimens from different sites at different times after the onset of symptoms in order to increase the diagnostic yield. Specifically, it recommends testing a lower respiratory specimen (eg, sputum, bronchoalveolar lavage fluid, tracheal aspirate), a nasopharyngeal and oropharyngeal swab, and serum, using the CDC MERS-CoV rRT-PCR assay. In addition, for patients whose symptoms began more than 14 days earlier, the CDC also recommends testing a serum specimen with the CDC MERS-CoV serologic assay. As these guidelines are updated frequently, clinicians are advised to check the CDC website for the most up-to-date information (www.cdc.gov/coronavirus/mers/guidelines-clinical-specimens.html).20 The identification of MERS-CoV by virus isolation in cell culture is not recommended and, if pursued, must be performed in a biosafety level 3 facility. (Level 3 is the second-highest level of biosafety. The highest, level 4, is reserved for extremely dangerous agents such as Ebola virus).20
Given the nonspecific clinical presentation of MERS-CoV, clinicians may consider testing for other respiratory pathogens. A recent review of 54 travelers to California from MERS-CoV-affected areas found that while none tested positive for MERS-CoV, 32 (62%) of 52 travelers had other respiratory viruses.21 When testing for alternative pathogens, clinicians should order molecular or antigen-based detection methods.
TREATMENT
Unfortunately, treatment for MERS is primarily supportive.
Ribavirin and interferon alfa-2b demonstrated activity in an animal model, but the regimen was ineffective when given a median of 19 (range 10–22) days after admission in 5 critically ill patients who subsequently died.22 A retrospective analysis comparing 20 patients with severe MERS-CoV who received ribavirin and interferon alfa-2a with 24 patients who did not reported that while survival was improved at 14 days, the mortality rates were similar at 28 days.23
A systematic review of treatments used for severe acute respiratory syndrome (SARS) reported that most studies investigating steroid use were inconclusive and some showed possible harm, suggesting that systemic steroids should be avoided in coronavirus infections.24
PREVENTION
Healthcare-associated outbreaks of MERS are well described, and thus recognition of potential cases and prompt institution of appropriate infection control measures are critical.15,25
Healthcare providers should ask patients about recent travel history and ascertain if they meet the CDC criteria for a “patient under investigation” (PUI), ie, if they have both clinical features and an epidemiologic risk of MERS (Table 1). However, these recommendations for identification will assuredly change as the outbreak matures, and healthcare providers should refer to the CDC website for the most up-to-date information.
Once a PUI is identified, standard, contact, and airborne precautions are advised. These measures include performing hand hygiene and donning personal protective equipment, including gloves, gowns, eye protection, and respiratory protection (ie, a respirator) that is at least as protective as a fit-tested National Institute for Occupational Safety and Health-certified N95 filtering face-piece respirator. In addition, a patient with possible MERS should be placed in an airborne infection isolation room.
Traveler’s advice
The CDC does not currently recommend that Americans change their travel plans because of MERS. Clinicians performing pretravel evaluations should advise patients of current information on MERS. Patients at risk for MERS who develop a respiratory illness within 14 days of return should seek medical attention and inform healthcare providers of their travel history.
SUMMARY
Recent experience with SARS, Ebola virus disease, and now MERS-CoV highlights the impact of global air travel as a vector for the rapid worldwide dissemination of communicable diseases. Healthcare providers should elicit a travel history in all patients presenting with a febrile illness, as an infection acquired in one continent may not become manifest until the patient presents in another.
The scope of the current MERS-CoV outbreak is still evolving, with concerns that viral evolution could result in a SARS-like outbreak, as experienced almost a decade ago.
Healthcare providers are advised to screen patients at risk for MERS-CoV for respiratory symptoms, and to institute appropriate infection control measures. Through recognition and isolation, healthcare providers are at the front line in limiting the spread of this potentially lethal virus.
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367:1814–1820.
- Al-Abdallat MM, Payne DC, Alqasrawi S, et al. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis 2014; 59:1225–1233.
- World Health Organization. Frequently asked questions on Middle East respiratory syndrome coronavirus (MERS-CoV). www.who.int/csr/disease/coronavirus_infections/faq/en/. Accessed July 29, 2015.
- Bialek SR, Allen D, Alvarado-Ramy F, et al; Centers for Disease Control and Prevention (CDC). First confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in the United States, updated information on the epidemiology of MERS-CoV infection, and guidance for the public, clinicians, and public health authorities—May 2014. MMWR Morb Mortal Wkly Rep 2014; 63:431–436.
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) – Republic of Korea. www.who.int/csr/don/12-june-2015-mers-korea/en/. Accessed July 29, 2015.
- Peiris JSM, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med 2004; 10:S88–S97.
- van Doremalen N, Miazqowicz KL, Milne-Price S, et al. Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4. J Virol 2014; 88:9220–9232.
- Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet 2015; S0140-6736(15)60454-604548 (Epub ahead of print).
- Meyer B, Muller MA, Corman WM, et al. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis 2014; 20:552–559.
- Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis 2014; 14:140–145.
- Azhar EI, El-Kafrawy SA, Farraj SA, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med 2014; 370:2499–2505.
- Chowell G, Blumberg S, Simonsen L, Miller MA, Viboud C. Synthesizing data and models for the spread of MERS-CoV, 2013: key role of index cases and hospital transmission. Epidemics 2014; 9:40–51.
- Biggerstaff M, Chauchemez S, Reed C, Gambhir M, Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis 2014: 14:480.
- Kucharski AJ, Althaus CL. The role of superspreading in Middle East respiratory syndrome coronavirus (MERS-CoV) transmission. Euro Surveill 2015; 20.
- Oboho I, Tomczyk S, Al-Asmari A, et al. 2014 MERS-CoV outbreak in Jeddah—a link to health care facilities. N Engl J Med 2015; 372:846–854.
- Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med 2014; 160:389–397.
- Assiri A, Al-Tawfig JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013; 13:752–761.
- Das KM, Lee EY, Enani MA, et al. CT correlation with outcomes in 15 patients with acute Middle East respiratory syndrome coronavirus. AJR Am J Roentgenol 2015; 204:736–742.
- Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis 2014; 210:1590–1594.
- Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS). Interim guidelines for collecting, handling, and testing clinical specimens from patients under investigation (PUIs) for Middle East respiratory syndrome coronavirus (MERS-CoV)—version 2.1. www.cdc.gov/coronavirus/mers/guidelines-clinical-specimens.html. Accessed July 29, 2015.
- Shakhkarami M, Yen C, Glaser CA, Xia D, Watt J, Wadford DA. Laboratory testing for Middle East respiratory syndrome coronavirus, California, USA, 2013–2014. Emerg Infect Dis 2015; 21: E-pub ahead of print. wwwnc.cdc.gov/eid/article/21/9/15-0476_article. Accessed July 29, 2015.
- Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis 2014; 20:42–46.
- Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis 2014; 14:1090–1095.
- Stockman LJ, Bellamy R, Garner, P. SARS: systematic review of treatment effects. PLoS Med 2006; 3:e343.
- Assiri A, McGeer A, Perl TM, et al; KSA MERS-CoV Investigation Team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med 2013; 369:407–416.
Middle East respiratory syndrome (MERS) is a potentially lethal illness caused by the Middle East respiratory syndrome coronavirus (MERS-CoV). The virus was first reported in 2012, when it was isolated from the sputum of a previously healthy man in Saudi Arabia who presented with acute pneumonia and subsequent renal failure with a fatal outcome.1 Retrospective studies subsequently identified an earlier outbreak that year involving 13 patients in Jordan, and since then cases have been reported in 25 countries across the Arabian Peninsula and in Asia, Europe, Africa, and the United States, with over 1,000 confirmed cases and 450 related deaths.2,3
At the time of this writing, two cases of MERS have been reported in the United States, both in May 2014. Both reported cases involved patients who had traveled from Saudi Arabia, and which did not result in secondary cases.4 Beginning in May 2015, the Republic of Korea had experienced the largest known outbreak of MERS outside the Arabian Peninsula, with over 100 cases.5
THE VIRUS
MERS-CoV is classified as a coronavirus, which is a family of single-stranded RNA viruses. In 2003, a previously unknown coronavirus (SARS-CoV) caused a global outbreak of pneumonia that resulted in approximately 800 deaths.6 The MERS-CoV virus attaches to dipeptidyl peptidase 4 to enter cells, and this receptor is believed to be critical for pathogenesis, as infection does not occur in its absence.7
The source and mode of transmission to humans is not completely defined. Early reports suggested that MERS-CoV originated in bats, as RNA sequences related to MERS-CoV have been found in several bat species, but the virus itself has not been isolated from bats.8 Camels have been found to have a high rate of anti-MERS-CoV antibodies and to have the virus in nose swabs, and evidence for camel-to-human transmission has been presented.9–11 However, the precise role of camels and other animals as reservoirs or vectors of infection is still under investigation.
The incubation period from exposure to the development of clinical disease is estimated at 5 to 14 days.
For MERS-CoV, the basic reproduction ratio (R0), which measures the average number of secondary cases from each infected person, is estimated12 to be less than 0.7. In diseases in which the R0 is less than 1.0, infections occur in isolated clusters as limited chains of transmission, and thus the sustained transmission of MERS-CoV resulting in a large epidemic is thought to be unlikely. As a comparison, the median R0 value for seasonal influenza is estimated13 at 1.28. “Superspreading” may result in limited outbreaks of secondary cases; however, the continued epidemic spread of infection is thought to be unlikely.14 Nevertheless, viral adaptation with increased transmissibility remains a concern and a potential threat.
CLINICAL PRESENTATION
MERS most commonly presents as a respiratory illness, although asymptomatic infection occurs. The percentage of patients who experience asymptomatic infection is unknown. A recent survey of 255 patients with laboratory-confirmed MERS-CoV found that 64 (25.1%) were reported as asymptomatic at time of specimen collection. However, when 33 (52%) of those patients were interviewed, 26 (79%) reported at least one symptom that was consistent with a viral respiratory illness.15
For symptomatic patients, the initial complaints are nonspecific, beginning with fever, cough, sore throat, chills, and myalgia. Patients experiencing severe infection progress to dyspnea and pneumonia, with requirements for ventilatory support, vasopressors, and renal replacement therapy.16 Gastrointestinal symptoms such as vomiting and diarrhea have been reported in about one-third of patients.17
In a study of 47 patients with MERS-CoV, most of whom had underlying medical illnesses, 42 (89%) required intensive care and 34 (72%) required mechanical ventilation.17 The case-fatality rate in this study was 60%, but other studies have reported rates closer to 30%.15
Laboratory findings in patients with MERS-CoV infection usually include leukopenia and thrombocytopenia. Severely ill patients may have evidence of acute kidney injury.
Radiographic findings of MERS are those of viral pneumonitis and acute respiratory distress syndrome. Computed tomographic findings include ground-glass opacities, with peripheral lower-lobe preference.18
DIAGNOSIS
As MERS is a respiratory illness, sampling of respiratory secretions provides the highest yield for diagnosis. A study of 112 patients with MERS-CoV reported that polymerase chain reaction (PCR) testing of tracheal aspirates and bronchoalveolar lavage samples yielded significantly higher MERS-CoV loads than nasopharyngeal swab samples and sputum samples.19 However, upper respiratory tract testing is less invasive, and a positive nasopharyngeal swab result may obviate the need for further testing.
The US Centers for Disease Control and Prevention (CDC) recommends collecting multiple specimens from different sites at different times after the onset of symptoms in order to increase the diagnostic yield. Specifically, it recommends testing a lower respiratory specimen (eg, sputum, bronchoalveolar lavage fluid, tracheal aspirate), a nasopharyngeal and oropharyngeal swab, and serum, using the CDC MERS-CoV rRT-PCR assay. In addition, for patients whose symptoms began more than 14 days earlier, the CDC also recommends testing a serum specimen with the CDC MERS-CoV serologic assay. As these guidelines are updated frequently, clinicians are advised to check the CDC website for the most up-to-date information (www.cdc.gov/coronavirus/mers/guidelines-clinical-specimens.html).20 The identification of MERS-CoV by virus isolation in cell culture is not recommended and, if pursued, must be performed in a biosafety level 3 facility. (Level 3 is the second-highest level of biosafety. The highest, level 4, is reserved for extremely dangerous agents such as Ebola virus).20
Given the nonspecific clinical presentation of MERS-CoV, clinicians may consider testing for other respiratory pathogens. A recent review of 54 travelers to California from MERS-CoV-affected areas found that while none tested positive for MERS-CoV, 32 (62%) of 52 travelers had other respiratory viruses.21 When testing for alternative pathogens, clinicians should order molecular or antigen-based detection methods.
TREATMENT
Unfortunately, treatment for MERS is primarily supportive.
Ribavirin and interferon alfa-2b demonstrated activity in an animal model, but the regimen was ineffective when given a median of 19 (range 10–22) days after admission in 5 critically ill patients who subsequently died.22 A retrospective analysis comparing 20 patients with severe MERS-CoV who received ribavirin and interferon alfa-2a with 24 patients who did not reported that while survival was improved at 14 days, the mortality rates were similar at 28 days.23
A systematic review of treatments used for severe acute respiratory syndrome (SARS) reported that most studies investigating steroid use were inconclusive and some showed possible harm, suggesting that systemic steroids should be avoided in coronavirus infections.24
PREVENTION
Healthcare-associated outbreaks of MERS are well described, and thus recognition of potential cases and prompt institution of appropriate infection control measures are critical.15,25
Healthcare providers should ask patients about recent travel history and ascertain if they meet the CDC criteria for a “patient under investigation” (PUI), ie, if they have both clinical features and an epidemiologic risk of MERS (Table 1). However, these recommendations for identification will assuredly change as the outbreak matures, and healthcare providers should refer to the CDC website for the most up-to-date information.
Once a PUI is identified, standard, contact, and airborne precautions are advised. These measures include performing hand hygiene and donning personal protective equipment, including gloves, gowns, eye protection, and respiratory protection (ie, a respirator) that is at least as protective as a fit-tested National Institute for Occupational Safety and Health-certified N95 filtering face-piece respirator. In addition, a patient with possible MERS should be placed in an airborne infection isolation room.
Traveler’s advice
The CDC does not currently recommend that Americans change their travel plans because of MERS. Clinicians performing pretravel evaluations should advise patients of current information on MERS. Patients at risk for MERS who develop a respiratory illness within 14 days of return should seek medical attention and inform healthcare providers of their travel history.
SUMMARY
Recent experience with SARS, Ebola virus disease, and now MERS-CoV highlights the impact of global air travel as a vector for the rapid worldwide dissemination of communicable diseases. Healthcare providers should elicit a travel history in all patients presenting with a febrile illness, as an infection acquired in one continent may not become manifest until the patient presents in another.
The scope of the current MERS-CoV outbreak is still evolving, with concerns that viral evolution could result in a SARS-like outbreak, as experienced almost a decade ago.
Healthcare providers are advised to screen patients at risk for MERS-CoV for respiratory symptoms, and to institute appropriate infection control measures. Through recognition and isolation, healthcare providers are at the front line in limiting the spread of this potentially lethal virus.
Middle East respiratory syndrome (MERS) is a potentially lethal illness caused by the Middle East respiratory syndrome coronavirus (MERS-CoV). The virus was first reported in 2012, when it was isolated from the sputum of a previously healthy man in Saudi Arabia who presented with acute pneumonia and subsequent renal failure with a fatal outcome.1 Retrospective studies subsequently identified an earlier outbreak that year involving 13 patients in Jordan, and since then cases have been reported in 25 countries across the Arabian Peninsula and in Asia, Europe, Africa, and the United States, with over 1,000 confirmed cases and 450 related deaths.2,3
At the time of this writing, two cases of MERS have been reported in the United States, both in May 2014. Both reported cases involved patients who had traveled from Saudi Arabia, and which did not result in secondary cases.4 Beginning in May 2015, the Republic of Korea had experienced the largest known outbreak of MERS outside the Arabian Peninsula, with over 100 cases.5
THE VIRUS
MERS-CoV is classified as a coronavirus, which is a family of single-stranded RNA viruses. In 2003, a previously unknown coronavirus (SARS-CoV) caused a global outbreak of pneumonia that resulted in approximately 800 deaths.6 The MERS-CoV virus attaches to dipeptidyl peptidase 4 to enter cells, and this receptor is believed to be critical for pathogenesis, as infection does not occur in its absence.7
The source and mode of transmission to humans is not completely defined. Early reports suggested that MERS-CoV originated in bats, as RNA sequences related to MERS-CoV have been found in several bat species, but the virus itself has not been isolated from bats.8 Camels have been found to have a high rate of anti-MERS-CoV antibodies and to have the virus in nose swabs, and evidence for camel-to-human transmission has been presented.9–11 However, the precise role of camels and other animals as reservoirs or vectors of infection is still under investigation.
The incubation period from exposure to the development of clinical disease is estimated at 5 to 14 days.
For MERS-CoV, the basic reproduction ratio (R0), which measures the average number of secondary cases from each infected person, is estimated12 to be less than 0.7. In diseases in which the R0 is less than 1.0, infections occur in isolated clusters as limited chains of transmission, and thus the sustained transmission of MERS-CoV resulting in a large epidemic is thought to be unlikely. As a comparison, the median R0 value for seasonal influenza is estimated13 at 1.28. “Superspreading” may result in limited outbreaks of secondary cases; however, the continued epidemic spread of infection is thought to be unlikely.14 Nevertheless, viral adaptation with increased transmissibility remains a concern and a potential threat.
CLINICAL PRESENTATION
MERS most commonly presents as a respiratory illness, although asymptomatic infection occurs. The percentage of patients who experience asymptomatic infection is unknown. A recent survey of 255 patients with laboratory-confirmed MERS-CoV found that 64 (25.1%) were reported as asymptomatic at time of specimen collection. However, when 33 (52%) of those patients were interviewed, 26 (79%) reported at least one symptom that was consistent with a viral respiratory illness.15
For symptomatic patients, the initial complaints are nonspecific, beginning with fever, cough, sore throat, chills, and myalgia. Patients experiencing severe infection progress to dyspnea and pneumonia, with requirements for ventilatory support, vasopressors, and renal replacement therapy.16 Gastrointestinal symptoms such as vomiting and diarrhea have been reported in about one-third of patients.17
In a study of 47 patients with MERS-CoV, most of whom had underlying medical illnesses, 42 (89%) required intensive care and 34 (72%) required mechanical ventilation.17 The case-fatality rate in this study was 60%, but other studies have reported rates closer to 30%.15
Laboratory findings in patients with MERS-CoV infection usually include leukopenia and thrombocytopenia. Severely ill patients may have evidence of acute kidney injury.
Radiographic findings of MERS are those of viral pneumonitis and acute respiratory distress syndrome. Computed tomographic findings include ground-glass opacities, with peripheral lower-lobe preference.18
DIAGNOSIS
As MERS is a respiratory illness, sampling of respiratory secretions provides the highest yield for diagnosis. A study of 112 patients with MERS-CoV reported that polymerase chain reaction (PCR) testing of tracheal aspirates and bronchoalveolar lavage samples yielded significantly higher MERS-CoV loads than nasopharyngeal swab samples and sputum samples.19 However, upper respiratory tract testing is less invasive, and a positive nasopharyngeal swab result may obviate the need for further testing.
The US Centers for Disease Control and Prevention (CDC) recommends collecting multiple specimens from different sites at different times after the onset of symptoms in order to increase the diagnostic yield. Specifically, it recommends testing a lower respiratory specimen (eg, sputum, bronchoalveolar lavage fluid, tracheal aspirate), a nasopharyngeal and oropharyngeal swab, and serum, using the CDC MERS-CoV rRT-PCR assay. In addition, for patients whose symptoms began more than 14 days earlier, the CDC also recommends testing a serum specimen with the CDC MERS-CoV serologic assay. As these guidelines are updated frequently, clinicians are advised to check the CDC website for the most up-to-date information (www.cdc.gov/coronavirus/mers/guidelines-clinical-specimens.html).20 The identification of MERS-CoV by virus isolation in cell culture is not recommended and, if pursued, must be performed in a biosafety level 3 facility. (Level 3 is the second-highest level of biosafety. The highest, level 4, is reserved for extremely dangerous agents such as Ebola virus).20
Given the nonspecific clinical presentation of MERS-CoV, clinicians may consider testing for other respiratory pathogens. A recent review of 54 travelers to California from MERS-CoV-affected areas found that while none tested positive for MERS-CoV, 32 (62%) of 52 travelers had other respiratory viruses.21 When testing for alternative pathogens, clinicians should order molecular or antigen-based detection methods.
TREATMENT
Unfortunately, treatment for MERS is primarily supportive.
Ribavirin and interferon alfa-2b demonstrated activity in an animal model, but the regimen was ineffective when given a median of 19 (range 10–22) days after admission in 5 critically ill patients who subsequently died.22 A retrospective analysis comparing 20 patients with severe MERS-CoV who received ribavirin and interferon alfa-2a with 24 patients who did not reported that while survival was improved at 14 days, the mortality rates were similar at 28 days.23
A systematic review of treatments used for severe acute respiratory syndrome (SARS) reported that most studies investigating steroid use were inconclusive and some showed possible harm, suggesting that systemic steroids should be avoided in coronavirus infections.24
PREVENTION
Healthcare-associated outbreaks of MERS are well described, and thus recognition of potential cases and prompt institution of appropriate infection control measures are critical.15,25
Healthcare providers should ask patients about recent travel history and ascertain if they meet the CDC criteria for a “patient under investigation” (PUI), ie, if they have both clinical features and an epidemiologic risk of MERS (Table 1). However, these recommendations for identification will assuredly change as the outbreak matures, and healthcare providers should refer to the CDC website for the most up-to-date information.
Once a PUI is identified, standard, contact, and airborne precautions are advised. These measures include performing hand hygiene and donning personal protective equipment, including gloves, gowns, eye protection, and respiratory protection (ie, a respirator) that is at least as protective as a fit-tested National Institute for Occupational Safety and Health-certified N95 filtering face-piece respirator. In addition, a patient with possible MERS should be placed in an airborne infection isolation room.
Traveler’s advice
The CDC does not currently recommend that Americans change their travel plans because of MERS. Clinicians performing pretravel evaluations should advise patients of current information on MERS. Patients at risk for MERS who develop a respiratory illness within 14 days of return should seek medical attention and inform healthcare providers of their travel history.
SUMMARY
Recent experience with SARS, Ebola virus disease, and now MERS-CoV highlights the impact of global air travel as a vector for the rapid worldwide dissemination of communicable diseases. Healthcare providers should elicit a travel history in all patients presenting with a febrile illness, as an infection acquired in one continent may not become manifest until the patient presents in another.
The scope of the current MERS-CoV outbreak is still evolving, with concerns that viral evolution could result in a SARS-like outbreak, as experienced almost a decade ago.
Healthcare providers are advised to screen patients at risk for MERS-CoV for respiratory symptoms, and to institute appropriate infection control measures. Through recognition and isolation, healthcare providers are at the front line in limiting the spread of this potentially lethal virus.
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367:1814–1820.
- Al-Abdallat MM, Payne DC, Alqasrawi S, et al. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis 2014; 59:1225–1233.
- World Health Organization. Frequently asked questions on Middle East respiratory syndrome coronavirus (MERS-CoV). www.who.int/csr/disease/coronavirus_infections/faq/en/. Accessed July 29, 2015.
- Bialek SR, Allen D, Alvarado-Ramy F, et al; Centers for Disease Control and Prevention (CDC). First confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in the United States, updated information on the epidemiology of MERS-CoV infection, and guidance for the public, clinicians, and public health authorities—May 2014. MMWR Morb Mortal Wkly Rep 2014; 63:431–436.
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) – Republic of Korea. www.who.int/csr/don/12-june-2015-mers-korea/en/. Accessed July 29, 2015.
- Peiris JSM, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med 2004; 10:S88–S97.
- van Doremalen N, Miazqowicz KL, Milne-Price S, et al. Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4. J Virol 2014; 88:9220–9232.
- Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet 2015; S0140-6736(15)60454-604548 (Epub ahead of print).
- Meyer B, Muller MA, Corman WM, et al. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis 2014; 20:552–559.
- Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis 2014; 14:140–145.
- Azhar EI, El-Kafrawy SA, Farraj SA, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med 2014; 370:2499–2505.
- Chowell G, Blumberg S, Simonsen L, Miller MA, Viboud C. Synthesizing data and models for the spread of MERS-CoV, 2013: key role of index cases and hospital transmission. Epidemics 2014; 9:40–51.
- Biggerstaff M, Chauchemez S, Reed C, Gambhir M, Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis 2014: 14:480.
- Kucharski AJ, Althaus CL. The role of superspreading in Middle East respiratory syndrome coronavirus (MERS-CoV) transmission. Euro Surveill 2015; 20.
- Oboho I, Tomczyk S, Al-Asmari A, et al. 2014 MERS-CoV outbreak in Jeddah—a link to health care facilities. N Engl J Med 2015; 372:846–854.
- Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med 2014; 160:389–397.
- Assiri A, Al-Tawfig JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013; 13:752–761.
- Das KM, Lee EY, Enani MA, et al. CT correlation with outcomes in 15 patients with acute Middle East respiratory syndrome coronavirus. AJR Am J Roentgenol 2015; 204:736–742.
- Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis 2014; 210:1590–1594.
- Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS). Interim guidelines for collecting, handling, and testing clinical specimens from patients under investigation (PUIs) for Middle East respiratory syndrome coronavirus (MERS-CoV)—version 2.1. www.cdc.gov/coronavirus/mers/guidelines-clinical-specimens.html. Accessed July 29, 2015.
- Shakhkarami M, Yen C, Glaser CA, Xia D, Watt J, Wadford DA. Laboratory testing for Middle East respiratory syndrome coronavirus, California, USA, 2013–2014. Emerg Infect Dis 2015; 21: E-pub ahead of print. wwwnc.cdc.gov/eid/article/21/9/15-0476_article. Accessed July 29, 2015.
- Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis 2014; 20:42–46.
- Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis 2014; 14:1090–1095.
- Stockman LJ, Bellamy R, Garner, P. SARS: systematic review of treatment effects. PLoS Med 2006; 3:e343.
- Assiri A, McGeer A, Perl TM, et al; KSA MERS-CoV Investigation Team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med 2013; 369:407–416.
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367:1814–1820.
- Al-Abdallat MM, Payne DC, Alqasrawi S, et al. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis 2014; 59:1225–1233.
- World Health Organization. Frequently asked questions on Middle East respiratory syndrome coronavirus (MERS-CoV). www.who.int/csr/disease/coronavirus_infections/faq/en/. Accessed July 29, 2015.
- Bialek SR, Allen D, Alvarado-Ramy F, et al; Centers for Disease Control and Prevention (CDC). First confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in the United States, updated information on the epidemiology of MERS-CoV infection, and guidance for the public, clinicians, and public health authorities—May 2014. MMWR Morb Mortal Wkly Rep 2014; 63:431–436.
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) – Republic of Korea. www.who.int/csr/don/12-june-2015-mers-korea/en/. Accessed July 29, 2015.
- Peiris JSM, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med 2004; 10:S88–S97.
- van Doremalen N, Miazqowicz KL, Milne-Price S, et al. Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4. J Virol 2014; 88:9220–9232.
- Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet 2015; S0140-6736(15)60454-604548 (Epub ahead of print).
- Meyer B, Muller MA, Corman WM, et al. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis 2014; 20:552–559.
- Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis 2014; 14:140–145.
- Azhar EI, El-Kafrawy SA, Farraj SA, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med 2014; 370:2499–2505.
- Chowell G, Blumberg S, Simonsen L, Miller MA, Viboud C. Synthesizing data and models for the spread of MERS-CoV, 2013: key role of index cases and hospital transmission. Epidemics 2014; 9:40–51.
- Biggerstaff M, Chauchemez S, Reed C, Gambhir M, Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis 2014: 14:480.
- Kucharski AJ, Althaus CL. The role of superspreading in Middle East respiratory syndrome coronavirus (MERS-CoV) transmission. Euro Surveill 2015; 20.
- Oboho I, Tomczyk S, Al-Asmari A, et al. 2014 MERS-CoV outbreak in Jeddah—a link to health care facilities. N Engl J Med 2015; 372:846–854.
- Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med 2014; 160:389–397.
- Assiri A, Al-Tawfig JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013; 13:752–761.
- Das KM, Lee EY, Enani MA, et al. CT correlation with outcomes in 15 patients with acute Middle East respiratory syndrome coronavirus. AJR Am J Roentgenol 2015; 204:736–742.
- Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis 2014; 210:1590–1594.
- Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS). Interim guidelines for collecting, handling, and testing clinical specimens from patients under investigation (PUIs) for Middle East respiratory syndrome coronavirus (MERS-CoV)—version 2.1. www.cdc.gov/coronavirus/mers/guidelines-clinical-specimens.html. Accessed July 29, 2015.
- Shakhkarami M, Yen C, Glaser CA, Xia D, Watt J, Wadford DA. Laboratory testing for Middle East respiratory syndrome coronavirus, California, USA, 2013–2014. Emerg Infect Dis 2015; 21: E-pub ahead of print. wwwnc.cdc.gov/eid/article/21/9/15-0476_article. Accessed July 29, 2015.
- Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis 2014; 20:42–46.
- Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis 2014; 14:1090–1095.
- Stockman LJ, Bellamy R, Garner, P. SARS: systematic review of treatment effects. PLoS Med 2006; 3:e343.
- Assiri A, McGeer A, Perl TM, et al; KSA MERS-CoV Investigation Team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med 2013; 369:407–416.
KEY POINTS
- In MERS, initial complaints are of fever, cough, chills and myalgia. In a subset of patients, usually those with underlying illnesses, the disease can progress to fulminant sepsis with respiratory and renal failure and death.
- Healthcare providers should regularly visit the US Centers for Disease Control and Prevention website for current information on countries experiencing a MERS outbreak, and for advice on how to identify a potentially infected patient.
- MERS-CoV has caused several healthcare-related outbreaks, so prompt identification and isolation of infected patients is critical to limiting the spread of infection. A “patient under identification” (ie, a person who has both clinical features and an epidemiologic risk) should be cared for under standard, contact, and airborne precautions.
Vaccinating adults who are pregnant, older, or immunocompromised, or have chronic kidney disease
Most vaccinations are given during childhood, but some require boosting during adulthood or are indicated for specific patient populations such as international travelers or those with certain medical conditions. Although generally safe, some vaccines contain live, attenuated organisms that can cause disease in immunocompromised patients. Thus, knowledge of the indications for and contraindications to specific vaccinations is critical to protect adults in special circumstances who are at risk.
Vaccines have helped eliminate or significantly reduce the burden of more than a dozen illnesses.1–3 The Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) makes recommendations about vaccinations for normal adults and children as well as for certain groups at high risk of vaccine-preventable infections.4 Tables 1 and 2 summarize the recommendations for vaccination by medical condition.4 In addition, several applications are available online, including downloadable apps from the (www.cdc.gov/vaccines/schedules/Schedulers/adult-scheduler.html) and the American College of Physicians (http://immunization.acponline.org/app/).
HUMANITY’S GREATEST ADVANCES IN PREVENTING INFECTIOUS DISEASE
Immunization and improved sanitation are humanity’s greatest advances in preventing sickness and death from infectious diseases. Since Jenner’s discovery in 1796 that milkmaids who had contracted cowpox (vaccinia) were immune to smallpox, vaccination has eliminated smallpox, markedly decreased the incidence of many infectious diseases, and, most recently, shown efficacy in preventing cervical cancer (with the human papillomavirus vaccine) and hepatocellular cancer (with the hepatitis B vaccine).1–3
Unfortunately, vaccination rates remain low for most routine vaccinations indicated for adults. For example, about 60% of adults over age 65 receive pneumococcal vaccination, and fewer than 10% of black patients over age 60 receive zoster vaccination.5 Various factors may account for these low rates, including financial disincentives.6
Nevertheless, vaccination remains one of medicine’s most effective defenses against infectious diseases and is especially important in the special populations discussed below. By being steadfast proponents of vaccination, especially for the most vulnerable patients, physicians can help ensure the optimum protection for their patients.
VACCINATING PREGNANT PATIENTS
When considering vaccination during pregnancy, one must consider the risk and benefit of the vaccine and the risk of the disease in both the mother and the child.
In general, if a pregnant woman is at high risk of exposure to a particular infection, the benefits of vaccinating her against it outweigh the risks. Vaccinating the mother can also protect against certain infections in early infancy through transfer of vaccine-induced immunoglobin G (IgG) across the placenta.7 In general, inactivated vaccines are considered safe in pregnancy, while live-attenuated vaccines are contraindicated.4 Special considerations for pregnant women include:
Tetanus, diphtheria, and acellular pertussis (Tdap). One dose of Tdap vaccine should be given during each pregnancy, preferably at 27 to 36 weeks of gestation, regardless of when the patient received a previous dose.8
Inactivated influenza vaccine should be given as early as possible during the influenza season (October to March) to all pregnant women, regardless of trimester.
Inactivated polio vaccine may be considered for pregnant women with known exposure to polio or travel to endemic areas.
Hepatitis A, hepatitis B, pneumococcal polysaccharide, meningococcal conjugate, and meningococcal polysaccharide vaccines can be given to women at risk of these infections. If a pregnant patient requires pneumococcal polysaccharide vaccine, it should be given during the second or third trimester, as the safety of this vaccine during the first trimester has not been established.9
Smallpox, measles-mumps-rubella, and varicella-containing vaccines are contraindicated in pregnancy. Household contacts of a pregnant woman should not receive smallpox vaccine, as it is the only vaccine known to cause harm to the fetus.10
Human papillomavirus vaccination is not recommended during pregnancy.
Yellow fever live-attenuated vaccine. The safety of this vaccine during pregnancy has not been established, and it is in the US Food and Drug Administration (FDA) pregnancy category C. However, this vaccine is required for entry into certain countries, and it may be offered if the patient is truly at risk of contracting yellow fever. Because pregnancy may affect immunologic response, serologic testing is recommended to document an immune response. If the patient’s itinerary puts her at low risk of yellow fever, then writing her a vaccine waiver letter can be considered.11
VACCINATING IMMUNOCOMPROMISED PATIENTS (NON-HIV)
People who do not have human immunodeficiency virus (HIV) but have a condition such as functional asplenia (sickle cell disease), anatomic asplenia, or complement component deficiency are at higher risk of infection with the encapsulated bacteria Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b.
Corticosteroids, chemotherapy, radiation for hematologic or solid-organ malignancies, and immune modulators can alter the immune system and pose a risk with the use of live-attenuated vaccines. A corticosteroid dosage equivalent to 2 mg/kg of body weight per day or higher or 20 mg/day of prednisone or higher is generally considered immunosuppressive.
Candidates for organ transplantation should receive vaccinations as early as possible during the disease course leading to transplantation. Vaccinations should be given as soon as the decision is made that the patient is a candidate for transplantation, which could be years or months before the patient actually receives the transplant. In addition to reviewing previously administered vaccinations, pretransplant serologic testing for hepatitis B, varicella, measles, mumps, and rubella antibodies helps to evaluate the need for vaccination.12
Recipients of hematopoietic stem cell transplantation are at risk of infections with encapsulated bacteria and certain other vaccine-preventable infections. Antibody titers are significantly reduced after stem cell transplantation because of ablation of bone marrow, and thus certain vaccines should be readministered 3 to 6 months after transplantation (eg, influenza, pneumococcal, and H influenzae vaccines). If the recipient is presumed to be immunocompetent, then varicella or measles-mumps-rubella vaccine can be given 24 months after transplantation.13
Apart from adhering to the routine vaccination schedule and avoiding live-attenuated vaccines, specific recommendations apply to persons with immunocompromising conditions14:
Quadrivalent meningococcal conjugate vaccine should be given to adults of all ages with asplenia or complement component deficiency. The schedule includes two doses at least 2 months apart initially and then revaccination every 5 years.
H influenzae type b vaccine should be given to people with asplenia and recipients of hematopoietic stem cells. One dose is recommended for those with asplenia (functional, anatomic, or elective splenectomy) or sickle cell disease if they have not already received it. A three-dose schedule is considered for hematopoietic stem cell transplant recipients 6 to 12 months after successful transplantation.
Pneumococcal conjugate (PCV13) and pneumococcal polysaccharide (PPSV23) vaccinations are recommended for people who have immunocompromising conditions. PCV13, the newer pneumococcal vaccine, was approved by the FDA in 2010 for use in children and was recommended by the ACIP in 2012 for adults age 19 and older with immunocompromising conditions.
People who have not previously received either of these vaccines and are age 19 or older with immunocompromising conditions including asplenia, chronic renal failure, nephrotic syndrome, cerebrospinal fluid leakage, or cochlear implant should receive a single dose of PCV13 followed by a dose of PPSV23 at least 8 weeks later. One-time revaccination 5 years after the first dose of PPSV23 is recommended for patients with immunocompromising conditions.
For those who have previously been vaccinated with PPSV23, a dose of PCV13 can be given 1 or more years after the last dose of PPSV23. These dosing intervals are important, as lower opsonophagocytic antibody responses have been noted if repeat doses of either pneumococcal vaccine are given sooner than the recommended interval.15
Inactivated influenza vaccine is recommended annually, except for patients who are unlikely to respond or those who have received anti-B-cell antibodies within 6 months. Live-attenuated influenza vaccine should not be given to immunocompromised patients.
VACCINATING PATIENTS WHO HAVE HIV
People with HIV should be routinely screened for immunity against certain infections and should be offered vaccination if not immune. The response to vaccines may vary depending on the CD4 count, with a good response in patients whose infection is well controlled with antiretroviral agents and with a preserved CD4 count.16 Special considerations for HIV patients include the following:
Hepatitis A vaccine may be offered to all HIV patients who have no evidence of immunity against hepatitis A, with negative antihepatitis A total and IgG antibodies.
Human papillomavirus vaccine is recommended for men and women with HIV through age 26.
Varicella and measles-mumps-rubella are live-attenuated vaccines and may be considered in patients who are nonimmune and with CD4 counts of 200 cells/µL or higher. However, the ACIP does not make a recommendation regarding the zoster vaccine in HIV patients with CD4 cell counts of 200 cells/µL or higher. In general, live-attenuated vaccines should be avoided in patients with CD4 counts less than 200 or with severe immunocompromised status because of risk of acquiring severe, life-threatening infections.
Pneumococcal vaccine should be given to HIV patients if they have not received it before. The schedule is one dose of PCV13, followed by a dose of PPSV23 at least 8 weeks later. If a patient has been previously vaccinated with PPSV23, then PCV13 is recommended at least 1 year after PPSV23.
Inactivated influenza vaccine is recommended annually. Live-attenuated influenza vaccine should not be given.
Hepatitis B vaccine should be given to nonimmune patients without past or present hepatitis B infection. These patients require higher doses of hepatitis B vaccine (40 μg/mL) than immunocompetent patients, who receive 20 μg/mL. The options include Recombivax HB 40 μg/mL given on a three-dose schedule at 0, 1, and 6 months, and Engerix B, two 20-μg/mL injections given simultaneously on a four-dose schedule at 0, 1, 2, and 6 months.
Meningococcal vaccine. HIV infection is not an indication for meningococcal vaccine unless the patient has other risk factors, such as anatomic or functional asplenia, persistent complement component deficiency, occupational exposure, and travel to endemic areas.
VACCINATING PATIENTS WHO ARE OLDER THAN 60
The immune system deteriorates with age, as does immunity gained from previous vaccinations. Vaccination in this age group reduces the risk of illness and death.17
Zoster vaccine should be offered to people age 60 and older regardless of previous episodes of herpes zoster unless there is a contraindication such as severe immunodeficiency. The zoster vaccine can reduce the incidence of postherpetic neuralgia by 66.5% and herpes zoster by 51% in patients over age 60.18
Pneumococcal conjugate vaccine. PCV13 should be offered to all adults age 65 or older. If a person age 65 or older has not received any pneumococcal vaccine before then, PCV13 should be given first, followed by a dose of PPSV23 at least 6 to 12 months after PCV13.
Pneumococcal polysaccharide vaccine. If PPSV23 was given before age 65 for another indication, a dose of PCV 13 should be given at age 65 or later, as long as 6 to 12 months have passed since the previous dose of PPSV 23. The patient should receive the last dose of PPSV23 vaccine 5 years after the first dose of PPSV23.4
Influenza vaccine. People 65 or older are at higher risk of complications from influenza, and vaccine should be offered annually. High-dose inactivated influenza vaccine can be used in this age group.4
Tdap. If never given before, Tdap is recommended regardless of the interval since the most recent Td vaccination, followed by a Td booster every 10 years.
VACCINATING PATIENTS WHO HAVE CHRONIC KIDNEY DISEASE
Patients with chronic kidney disease are at risk of certain infections, so vaccination is an important preventive measure.19 Immunizations should be offered to all patients with chronic kidney disease regardless of the disease stage, but they are recommended during the early stages of progressive renal disease to increase the likelihood of vaccine-induced immunity.20
Pneumococcal conjugate vaccine. PCV13 is recommended for adults 19 or older with chronic renal disease or nephrotic syndrome. One dose of PCV13 should be given, followed by a dose of PPSV23 at least 8 weeks later. If the patient has been previously vaccinated with PPSV23, then PCV13 at least 1 year after PPSV23 is recommended.
Hepatitis B vaccine should be given to nonimmune patients without past or present hepatitis B infection. Adult patients on hemodialysis require higher doses of hepatitis B vaccine. The options include Recombivax HB 40 μg/mL given on a three-dose schedule at 0, 1, and 6 months, and Engerix B, two 20-μg/mL injections given simultaneously on a four-dose schedule at 0, 1, 2, and 6 months.
Influenza vaccine should be offered annually to patients with chronic kidney disease.
VACCINATING IMMUNOCOMPROMISED INTERNATIONAL TRAVELERS
International travel for business or pleasure is increasingly common, and immunocompromised patients require specific attention as they may face unanticipated pathogens or have special requirements. Transplant recipients should ideally receive routine and travel-related vaccines as early as possible before transplantation. Vaccination is generally avoided in the first 6 months after organ transplantation to avoid confusion with early graft dysfunction or rejection.21 However, it should be considered as soon as a patient develops an illness that might lead to transplantation.
Evaluation of patients for vaccination should include an assessment of the travel-specific epidemiologic risk, the nature of the vaccine (live-attenuated or other), and the immune status. As discussed above, live-attenuated vaccines should be avoided in immunocompromised patients, and thus the injectable typhoid vaccine should be given in lieu of the attenuated oral vaccine.
Yellow fever vaccine is required before entrance to certain countries but should not be given to immunocompromised patients, although it can probably be given to asymptomatic HIV-infected adults with a CD4 count higher than 200 cells/μL who are exposed to substantial risk.22 For patients who cannot receive the vaccine, some governments will accept a physician’s letter stating the patient has a contraindication to vaccination.
VACCINATING HOUSEHOLD MEMBERS OF IMMUNOCOMPROMISED PATIENTS
Protecting immunocompromised patients from infectious diseases involves vaccinating not only the patient but also household members so that they do not acquire infections and then bring them into the household. Immunocompetent members of a household can receive inactivated vaccines based on the recommended ACIP schedule.
Annual inactivated influenza vaccination is recommended, although the live-attenuated influenza virus vaccine can be substituted if the immunocompromised patient is not within 2 months of hematopoietic stem cell transplantation, does not have graft-vs-host disease, and does not have severe combined immune deficiency.
Other live-attenuated vaccines can usually be given if indicated, including measles-mumps-rubella vaccine, rotavirus vaccine in infants, varicella vaccine, and zoster vaccine.14
- Crosignani P, De Stefani A, Fara GM, et al. Towards the eradication of HPV infection through universal specific vaccination. BMC Public Health 2013;13:642.
- Plotkin SL, Plotkin SA. A short history of vaccination. In: Plotkin, SA, Orenstein W, Offit PA, editors. Vaccines, 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2008:1–16.
- Wong VW, Chan HL. Prevention of hepatocellular carcinoma: a concise review of contemporary issues. Ann Hepatol 2012; 11:284–293.
- Kim DK, Bridges CB, Harriman K; Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older: United States, 2015. Ann Intern Med 2015; 162:214–223.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults—United States, 2012. MMWR Morb Mortal Wkly Rep 2014; 63:95-102.
- Hurley LP, Bridges CB, Harpaz R, et al. US physicians’ perspective of adult vaccine delivery. Ann Intern Med 2014; 160:161.
- Lindsey B, Kampmann B, Jones C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr Opin Infect Dis 2013; 26:248–253.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013; 62:131–135.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:1–24.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52:1–16.
- Staples JE, Gershman M, Fischer M; Centers for Disease Control and Prevention (CDC). Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010; 59:1–27.
- Danziger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):311–317.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–64.
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA, Infectious Diseases Society of America. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1–10.
- Eilers R, Krabbe PF, van Essen TG, Suijkerbuijk A, van Lier A, de Melker HE. Assessment of vaccine candidates for persons aged 50 and older: a review. BMC Geriatr 2013; 13:32.
- Oxman MN, Levin MJ; Shingles Prevention Study Group. Vaccination against Herpes Zoster and Postherpetic Neuralgia. J Infect Dis 2008; 197(suppl 2):S228–S236.
- Soni R, Horowitz B, Unruh M. Immunization in end-stage renal disease: opportunity to improve outcomes. Semin Dial 2013; 26:416–426.
- Chi C, Patel P, Pilishvili T, Moore M, Murphy T, Strikas R. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. http://www.cdc.gov/vaccines/pubs/downloads/dialysis-guide-2012.pdf. Accessed March 31, 2015.
- Kotton CN, Ryan ET, Fishman JA. Prevention of infection in adult travelers after solid organ transplantation. Am J Transplant 2005; 5:8–14.
- Castelli F, Patroni A. The human immunodeficiency virus-infected traveler. Clin Infect Dis 2000; 31:1403–1408.
Most vaccinations are given during childhood, but some require boosting during adulthood or are indicated for specific patient populations such as international travelers or those with certain medical conditions. Although generally safe, some vaccines contain live, attenuated organisms that can cause disease in immunocompromised patients. Thus, knowledge of the indications for and contraindications to specific vaccinations is critical to protect adults in special circumstances who are at risk.
Vaccines have helped eliminate or significantly reduce the burden of more than a dozen illnesses.1–3 The Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) makes recommendations about vaccinations for normal adults and children as well as for certain groups at high risk of vaccine-preventable infections.4 Tables 1 and 2 summarize the recommendations for vaccination by medical condition.4 In addition, several applications are available online, including downloadable apps from the (www.cdc.gov/vaccines/schedules/Schedulers/adult-scheduler.html) and the American College of Physicians (http://immunization.acponline.org/app/).
HUMANITY’S GREATEST ADVANCES IN PREVENTING INFECTIOUS DISEASE
Immunization and improved sanitation are humanity’s greatest advances in preventing sickness and death from infectious diseases. Since Jenner’s discovery in 1796 that milkmaids who had contracted cowpox (vaccinia) were immune to smallpox, vaccination has eliminated smallpox, markedly decreased the incidence of many infectious diseases, and, most recently, shown efficacy in preventing cervical cancer (with the human papillomavirus vaccine) and hepatocellular cancer (with the hepatitis B vaccine).1–3
Unfortunately, vaccination rates remain low for most routine vaccinations indicated for adults. For example, about 60% of adults over age 65 receive pneumococcal vaccination, and fewer than 10% of black patients over age 60 receive zoster vaccination.5 Various factors may account for these low rates, including financial disincentives.6
Nevertheless, vaccination remains one of medicine’s most effective defenses against infectious diseases and is especially important in the special populations discussed below. By being steadfast proponents of vaccination, especially for the most vulnerable patients, physicians can help ensure the optimum protection for their patients.
VACCINATING PREGNANT PATIENTS
When considering vaccination during pregnancy, one must consider the risk and benefit of the vaccine and the risk of the disease in both the mother and the child.
In general, if a pregnant woman is at high risk of exposure to a particular infection, the benefits of vaccinating her against it outweigh the risks. Vaccinating the mother can also protect against certain infections in early infancy through transfer of vaccine-induced immunoglobin G (IgG) across the placenta.7 In general, inactivated vaccines are considered safe in pregnancy, while live-attenuated vaccines are contraindicated.4 Special considerations for pregnant women include:
Tetanus, diphtheria, and acellular pertussis (Tdap). One dose of Tdap vaccine should be given during each pregnancy, preferably at 27 to 36 weeks of gestation, regardless of when the patient received a previous dose.8
Inactivated influenza vaccine should be given as early as possible during the influenza season (October to March) to all pregnant women, regardless of trimester.
Inactivated polio vaccine may be considered for pregnant women with known exposure to polio or travel to endemic areas.
Hepatitis A, hepatitis B, pneumococcal polysaccharide, meningococcal conjugate, and meningococcal polysaccharide vaccines can be given to women at risk of these infections. If a pregnant patient requires pneumococcal polysaccharide vaccine, it should be given during the second or third trimester, as the safety of this vaccine during the first trimester has not been established.9
Smallpox, measles-mumps-rubella, and varicella-containing vaccines are contraindicated in pregnancy. Household contacts of a pregnant woman should not receive smallpox vaccine, as it is the only vaccine known to cause harm to the fetus.10
Human papillomavirus vaccination is not recommended during pregnancy.
Yellow fever live-attenuated vaccine. The safety of this vaccine during pregnancy has not been established, and it is in the US Food and Drug Administration (FDA) pregnancy category C. However, this vaccine is required for entry into certain countries, and it may be offered if the patient is truly at risk of contracting yellow fever. Because pregnancy may affect immunologic response, serologic testing is recommended to document an immune response. If the patient’s itinerary puts her at low risk of yellow fever, then writing her a vaccine waiver letter can be considered.11
VACCINATING IMMUNOCOMPROMISED PATIENTS (NON-HIV)
People who do not have human immunodeficiency virus (HIV) but have a condition such as functional asplenia (sickle cell disease), anatomic asplenia, or complement component deficiency are at higher risk of infection with the encapsulated bacteria Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b.
Corticosteroids, chemotherapy, radiation for hematologic or solid-organ malignancies, and immune modulators can alter the immune system and pose a risk with the use of live-attenuated vaccines. A corticosteroid dosage equivalent to 2 mg/kg of body weight per day or higher or 20 mg/day of prednisone or higher is generally considered immunosuppressive.
Candidates for organ transplantation should receive vaccinations as early as possible during the disease course leading to transplantation. Vaccinations should be given as soon as the decision is made that the patient is a candidate for transplantation, which could be years or months before the patient actually receives the transplant. In addition to reviewing previously administered vaccinations, pretransplant serologic testing for hepatitis B, varicella, measles, mumps, and rubella antibodies helps to evaluate the need for vaccination.12
Recipients of hematopoietic stem cell transplantation are at risk of infections with encapsulated bacteria and certain other vaccine-preventable infections. Antibody titers are significantly reduced after stem cell transplantation because of ablation of bone marrow, and thus certain vaccines should be readministered 3 to 6 months after transplantation (eg, influenza, pneumococcal, and H influenzae vaccines). If the recipient is presumed to be immunocompetent, then varicella or measles-mumps-rubella vaccine can be given 24 months after transplantation.13
Apart from adhering to the routine vaccination schedule and avoiding live-attenuated vaccines, specific recommendations apply to persons with immunocompromising conditions14:
Quadrivalent meningococcal conjugate vaccine should be given to adults of all ages with asplenia or complement component deficiency. The schedule includes two doses at least 2 months apart initially and then revaccination every 5 years.
H influenzae type b vaccine should be given to people with asplenia and recipients of hematopoietic stem cells. One dose is recommended for those with asplenia (functional, anatomic, or elective splenectomy) or sickle cell disease if they have not already received it. A three-dose schedule is considered for hematopoietic stem cell transplant recipients 6 to 12 months after successful transplantation.
Pneumococcal conjugate (PCV13) and pneumococcal polysaccharide (PPSV23) vaccinations are recommended for people who have immunocompromising conditions. PCV13, the newer pneumococcal vaccine, was approved by the FDA in 2010 for use in children and was recommended by the ACIP in 2012 for adults age 19 and older with immunocompromising conditions.
People who have not previously received either of these vaccines and are age 19 or older with immunocompromising conditions including asplenia, chronic renal failure, nephrotic syndrome, cerebrospinal fluid leakage, or cochlear implant should receive a single dose of PCV13 followed by a dose of PPSV23 at least 8 weeks later. One-time revaccination 5 years after the first dose of PPSV23 is recommended for patients with immunocompromising conditions.
For those who have previously been vaccinated with PPSV23, a dose of PCV13 can be given 1 or more years after the last dose of PPSV23. These dosing intervals are important, as lower opsonophagocytic antibody responses have been noted if repeat doses of either pneumococcal vaccine are given sooner than the recommended interval.15
Inactivated influenza vaccine is recommended annually, except for patients who are unlikely to respond or those who have received anti-B-cell antibodies within 6 months. Live-attenuated influenza vaccine should not be given to immunocompromised patients.
VACCINATING PATIENTS WHO HAVE HIV
People with HIV should be routinely screened for immunity against certain infections and should be offered vaccination if not immune. The response to vaccines may vary depending on the CD4 count, with a good response in patients whose infection is well controlled with antiretroviral agents and with a preserved CD4 count.16 Special considerations for HIV patients include the following:
Hepatitis A vaccine may be offered to all HIV patients who have no evidence of immunity against hepatitis A, with negative antihepatitis A total and IgG antibodies.
Human papillomavirus vaccine is recommended for men and women with HIV through age 26.
Varicella and measles-mumps-rubella are live-attenuated vaccines and may be considered in patients who are nonimmune and with CD4 counts of 200 cells/µL or higher. However, the ACIP does not make a recommendation regarding the zoster vaccine in HIV patients with CD4 cell counts of 200 cells/µL or higher. In general, live-attenuated vaccines should be avoided in patients with CD4 counts less than 200 or with severe immunocompromised status because of risk of acquiring severe, life-threatening infections.
Pneumococcal vaccine should be given to HIV patients if they have not received it before. The schedule is one dose of PCV13, followed by a dose of PPSV23 at least 8 weeks later. If a patient has been previously vaccinated with PPSV23, then PCV13 is recommended at least 1 year after PPSV23.
Inactivated influenza vaccine is recommended annually. Live-attenuated influenza vaccine should not be given.
Hepatitis B vaccine should be given to nonimmune patients without past or present hepatitis B infection. These patients require higher doses of hepatitis B vaccine (40 μg/mL) than immunocompetent patients, who receive 20 μg/mL. The options include Recombivax HB 40 μg/mL given on a three-dose schedule at 0, 1, and 6 months, and Engerix B, two 20-μg/mL injections given simultaneously on a four-dose schedule at 0, 1, 2, and 6 months.
Meningococcal vaccine. HIV infection is not an indication for meningococcal vaccine unless the patient has other risk factors, such as anatomic or functional asplenia, persistent complement component deficiency, occupational exposure, and travel to endemic areas.
VACCINATING PATIENTS WHO ARE OLDER THAN 60
The immune system deteriorates with age, as does immunity gained from previous vaccinations. Vaccination in this age group reduces the risk of illness and death.17
Zoster vaccine should be offered to people age 60 and older regardless of previous episodes of herpes zoster unless there is a contraindication such as severe immunodeficiency. The zoster vaccine can reduce the incidence of postherpetic neuralgia by 66.5% and herpes zoster by 51% in patients over age 60.18
Pneumococcal conjugate vaccine. PCV13 should be offered to all adults age 65 or older. If a person age 65 or older has not received any pneumococcal vaccine before then, PCV13 should be given first, followed by a dose of PPSV23 at least 6 to 12 months after PCV13.
Pneumococcal polysaccharide vaccine. If PPSV23 was given before age 65 for another indication, a dose of PCV 13 should be given at age 65 or later, as long as 6 to 12 months have passed since the previous dose of PPSV 23. The patient should receive the last dose of PPSV23 vaccine 5 years after the first dose of PPSV23.4
Influenza vaccine. People 65 or older are at higher risk of complications from influenza, and vaccine should be offered annually. High-dose inactivated influenza vaccine can be used in this age group.4
Tdap. If never given before, Tdap is recommended regardless of the interval since the most recent Td vaccination, followed by a Td booster every 10 years.
VACCINATING PATIENTS WHO HAVE CHRONIC KIDNEY DISEASE
Patients with chronic kidney disease are at risk of certain infections, so vaccination is an important preventive measure.19 Immunizations should be offered to all patients with chronic kidney disease regardless of the disease stage, but they are recommended during the early stages of progressive renal disease to increase the likelihood of vaccine-induced immunity.20
Pneumococcal conjugate vaccine. PCV13 is recommended for adults 19 or older with chronic renal disease or nephrotic syndrome. One dose of PCV13 should be given, followed by a dose of PPSV23 at least 8 weeks later. If the patient has been previously vaccinated with PPSV23, then PCV13 at least 1 year after PPSV23 is recommended.
Hepatitis B vaccine should be given to nonimmune patients without past or present hepatitis B infection. Adult patients on hemodialysis require higher doses of hepatitis B vaccine. The options include Recombivax HB 40 μg/mL given on a three-dose schedule at 0, 1, and 6 months, and Engerix B, two 20-μg/mL injections given simultaneously on a four-dose schedule at 0, 1, 2, and 6 months.
Influenza vaccine should be offered annually to patients with chronic kidney disease.
VACCINATING IMMUNOCOMPROMISED INTERNATIONAL TRAVELERS
International travel for business or pleasure is increasingly common, and immunocompromised patients require specific attention as they may face unanticipated pathogens or have special requirements. Transplant recipients should ideally receive routine and travel-related vaccines as early as possible before transplantation. Vaccination is generally avoided in the first 6 months after organ transplantation to avoid confusion with early graft dysfunction or rejection.21 However, it should be considered as soon as a patient develops an illness that might lead to transplantation.
Evaluation of patients for vaccination should include an assessment of the travel-specific epidemiologic risk, the nature of the vaccine (live-attenuated or other), and the immune status. As discussed above, live-attenuated vaccines should be avoided in immunocompromised patients, and thus the injectable typhoid vaccine should be given in lieu of the attenuated oral vaccine.
Yellow fever vaccine is required before entrance to certain countries but should not be given to immunocompromised patients, although it can probably be given to asymptomatic HIV-infected adults with a CD4 count higher than 200 cells/μL who are exposed to substantial risk.22 For patients who cannot receive the vaccine, some governments will accept a physician’s letter stating the patient has a contraindication to vaccination.
VACCINATING HOUSEHOLD MEMBERS OF IMMUNOCOMPROMISED PATIENTS
Protecting immunocompromised patients from infectious diseases involves vaccinating not only the patient but also household members so that they do not acquire infections and then bring them into the household. Immunocompetent members of a household can receive inactivated vaccines based on the recommended ACIP schedule.
Annual inactivated influenza vaccination is recommended, although the live-attenuated influenza virus vaccine can be substituted if the immunocompromised patient is not within 2 months of hematopoietic stem cell transplantation, does not have graft-vs-host disease, and does not have severe combined immune deficiency.
Other live-attenuated vaccines can usually be given if indicated, including measles-mumps-rubella vaccine, rotavirus vaccine in infants, varicella vaccine, and zoster vaccine.14
Most vaccinations are given during childhood, but some require boosting during adulthood or are indicated for specific patient populations such as international travelers or those with certain medical conditions. Although generally safe, some vaccines contain live, attenuated organisms that can cause disease in immunocompromised patients. Thus, knowledge of the indications for and contraindications to specific vaccinations is critical to protect adults in special circumstances who are at risk.
Vaccines have helped eliminate or significantly reduce the burden of more than a dozen illnesses.1–3 The Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) makes recommendations about vaccinations for normal adults and children as well as for certain groups at high risk of vaccine-preventable infections.4 Tables 1 and 2 summarize the recommendations for vaccination by medical condition.4 In addition, several applications are available online, including downloadable apps from the (www.cdc.gov/vaccines/schedules/Schedulers/adult-scheduler.html) and the American College of Physicians (http://immunization.acponline.org/app/).
HUMANITY’S GREATEST ADVANCES IN PREVENTING INFECTIOUS DISEASE
Immunization and improved sanitation are humanity’s greatest advances in preventing sickness and death from infectious diseases. Since Jenner’s discovery in 1796 that milkmaids who had contracted cowpox (vaccinia) were immune to smallpox, vaccination has eliminated smallpox, markedly decreased the incidence of many infectious diseases, and, most recently, shown efficacy in preventing cervical cancer (with the human papillomavirus vaccine) and hepatocellular cancer (with the hepatitis B vaccine).1–3
Unfortunately, vaccination rates remain low for most routine vaccinations indicated for adults. For example, about 60% of adults over age 65 receive pneumococcal vaccination, and fewer than 10% of black patients over age 60 receive zoster vaccination.5 Various factors may account for these low rates, including financial disincentives.6
Nevertheless, vaccination remains one of medicine’s most effective defenses against infectious diseases and is especially important in the special populations discussed below. By being steadfast proponents of vaccination, especially for the most vulnerable patients, physicians can help ensure the optimum protection for their patients.
VACCINATING PREGNANT PATIENTS
When considering vaccination during pregnancy, one must consider the risk and benefit of the vaccine and the risk of the disease in both the mother and the child.
In general, if a pregnant woman is at high risk of exposure to a particular infection, the benefits of vaccinating her against it outweigh the risks. Vaccinating the mother can also protect against certain infections in early infancy through transfer of vaccine-induced immunoglobin G (IgG) across the placenta.7 In general, inactivated vaccines are considered safe in pregnancy, while live-attenuated vaccines are contraindicated.4 Special considerations for pregnant women include:
Tetanus, diphtheria, and acellular pertussis (Tdap). One dose of Tdap vaccine should be given during each pregnancy, preferably at 27 to 36 weeks of gestation, regardless of when the patient received a previous dose.8
Inactivated influenza vaccine should be given as early as possible during the influenza season (October to March) to all pregnant women, regardless of trimester.
Inactivated polio vaccine may be considered for pregnant women with known exposure to polio or travel to endemic areas.
Hepatitis A, hepatitis B, pneumococcal polysaccharide, meningococcal conjugate, and meningococcal polysaccharide vaccines can be given to women at risk of these infections. If a pregnant patient requires pneumococcal polysaccharide vaccine, it should be given during the second or third trimester, as the safety of this vaccine during the first trimester has not been established.9
Smallpox, measles-mumps-rubella, and varicella-containing vaccines are contraindicated in pregnancy. Household contacts of a pregnant woman should not receive smallpox vaccine, as it is the only vaccine known to cause harm to the fetus.10
Human papillomavirus vaccination is not recommended during pregnancy.
Yellow fever live-attenuated vaccine. The safety of this vaccine during pregnancy has not been established, and it is in the US Food and Drug Administration (FDA) pregnancy category C. However, this vaccine is required for entry into certain countries, and it may be offered if the patient is truly at risk of contracting yellow fever. Because pregnancy may affect immunologic response, serologic testing is recommended to document an immune response. If the patient’s itinerary puts her at low risk of yellow fever, then writing her a vaccine waiver letter can be considered.11
VACCINATING IMMUNOCOMPROMISED PATIENTS (NON-HIV)
People who do not have human immunodeficiency virus (HIV) but have a condition such as functional asplenia (sickle cell disease), anatomic asplenia, or complement component deficiency are at higher risk of infection with the encapsulated bacteria Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b.
Corticosteroids, chemotherapy, radiation for hematologic or solid-organ malignancies, and immune modulators can alter the immune system and pose a risk with the use of live-attenuated vaccines. A corticosteroid dosage equivalent to 2 mg/kg of body weight per day or higher or 20 mg/day of prednisone or higher is generally considered immunosuppressive.
Candidates for organ transplantation should receive vaccinations as early as possible during the disease course leading to transplantation. Vaccinations should be given as soon as the decision is made that the patient is a candidate for transplantation, which could be years or months before the patient actually receives the transplant. In addition to reviewing previously administered vaccinations, pretransplant serologic testing for hepatitis B, varicella, measles, mumps, and rubella antibodies helps to evaluate the need for vaccination.12
Recipients of hematopoietic stem cell transplantation are at risk of infections with encapsulated bacteria and certain other vaccine-preventable infections. Antibody titers are significantly reduced after stem cell transplantation because of ablation of bone marrow, and thus certain vaccines should be readministered 3 to 6 months after transplantation (eg, influenza, pneumococcal, and H influenzae vaccines). If the recipient is presumed to be immunocompetent, then varicella or measles-mumps-rubella vaccine can be given 24 months after transplantation.13
Apart from adhering to the routine vaccination schedule and avoiding live-attenuated vaccines, specific recommendations apply to persons with immunocompromising conditions14:
Quadrivalent meningococcal conjugate vaccine should be given to adults of all ages with asplenia or complement component deficiency. The schedule includes two doses at least 2 months apart initially and then revaccination every 5 years.
H influenzae type b vaccine should be given to people with asplenia and recipients of hematopoietic stem cells. One dose is recommended for those with asplenia (functional, anatomic, or elective splenectomy) or sickle cell disease if they have not already received it. A three-dose schedule is considered for hematopoietic stem cell transplant recipients 6 to 12 months after successful transplantation.
Pneumococcal conjugate (PCV13) and pneumococcal polysaccharide (PPSV23) vaccinations are recommended for people who have immunocompromising conditions. PCV13, the newer pneumococcal vaccine, was approved by the FDA in 2010 for use in children and was recommended by the ACIP in 2012 for adults age 19 and older with immunocompromising conditions.
People who have not previously received either of these vaccines and are age 19 or older with immunocompromising conditions including asplenia, chronic renal failure, nephrotic syndrome, cerebrospinal fluid leakage, or cochlear implant should receive a single dose of PCV13 followed by a dose of PPSV23 at least 8 weeks later. One-time revaccination 5 years after the first dose of PPSV23 is recommended for patients with immunocompromising conditions.
For those who have previously been vaccinated with PPSV23, a dose of PCV13 can be given 1 or more years after the last dose of PPSV23. These dosing intervals are important, as lower opsonophagocytic antibody responses have been noted if repeat doses of either pneumococcal vaccine are given sooner than the recommended interval.15
Inactivated influenza vaccine is recommended annually, except for patients who are unlikely to respond or those who have received anti-B-cell antibodies within 6 months. Live-attenuated influenza vaccine should not be given to immunocompromised patients.
VACCINATING PATIENTS WHO HAVE HIV
People with HIV should be routinely screened for immunity against certain infections and should be offered vaccination if not immune. The response to vaccines may vary depending on the CD4 count, with a good response in patients whose infection is well controlled with antiretroviral agents and with a preserved CD4 count.16 Special considerations for HIV patients include the following:
Hepatitis A vaccine may be offered to all HIV patients who have no evidence of immunity against hepatitis A, with negative antihepatitis A total and IgG antibodies.
Human papillomavirus vaccine is recommended for men and women with HIV through age 26.
Varicella and measles-mumps-rubella are live-attenuated vaccines and may be considered in patients who are nonimmune and with CD4 counts of 200 cells/µL or higher. However, the ACIP does not make a recommendation regarding the zoster vaccine in HIV patients with CD4 cell counts of 200 cells/µL or higher. In general, live-attenuated vaccines should be avoided in patients with CD4 counts less than 200 or with severe immunocompromised status because of risk of acquiring severe, life-threatening infections.
Pneumococcal vaccine should be given to HIV patients if they have not received it before. The schedule is one dose of PCV13, followed by a dose of PPSV23 at least 8 weeks later. If a patient has been previously vaccinated with PPSV23, then PCV13 is recommended at least 1 year after PPSV23.
Inactivated influenza vaccine is recommended annually. Live-attenuated influenza vaccine should not be given.
Hepatitis B vaccine should be given to nonimmune patients without past or present hepatitis B infection. These patients require higher doses of hepatitis B vaccine (40 μg/mL) than immunocompetent patients, who receive 20 μg/mL. The options include Recombivax HB 40 μg/mL given on a three-dose schedule at 0, 1, and 6 months, and Engerix B, two 20-μg/mL injections given simultaneously on a four-dose schedule at 0, 1, 2, and 6 months.
Meningococcal vaccine. HIV infection is not an indication for meningococcal vaccine unless the patient has other risk factors, such as anatomic or functional asplenia, persistent complement component deficiency, occupational exposure, and travel to endemic areas.
VACCINATING PATIENTS WHO ARE OLDER THAN 60
The immune system deteriorates with age, as does immunity gained from previous vaccinations. Vaccination in this age group reduces the risk of illness and death.17
Zoster vaccine should be offered to people age 60 and older regardless of previous episodes of herpes zoster unless there is a contraindication such as severe immunodeficiency. The zoster vaccine can reduce the incidence of postherpetic neuralgia by 66.5% and herpes zoster by 51% in patients over age 60.18
Pneumococcal conjugate vaccine. PCV13 should be offered to all adults age 65 or older. If a person age 65 or older has not received any pneumococcal vaccine before then, PCV13 should be given first, followed by a dose of PPSV23 at least 6 to 12 months after PCV13.
Pneumococcal polysaccharide vaccine. If PPSV23 was given before age 65 for another indication, a dose of PCV 13 should be given at age 65 or later, as long as 6 to 12 months have passed since the previous dose of PPSV 23. The patient should receive the last dose of PPSV23 vaccine 5 years after the first dose of PPSV23.4
Influenza vaccine. People 65 or older are at higher risk of complications from influenza, and vaccine should be offered annually. High-dose inactivated influenza vaccine can be used in this age group.4
Tdap. If never given before, Tdap is recommended regardless of the interval since the most recent Td vaccination, followed by a Td booster every 10 years.
VACCINATING PATIENTS WHO HAVE CHRONIC KIDNEY DISEASE
Patients with chronic kidney disease are at risk of certain infections, so vaccination is an important preventive measure.19 Immunizations should be offered to all patients with chronic kidney disease regardless of the disease stage, but they are recommended during the early stages of progressive renal disease to increase the likelihood of vaccine-induced immunity.20
Pneumococcal conjugate vaccine. PCV13 is recommended for adults 19 or older with chronic renal disease or nephrotic syndrome. One dose of PCV13 should be given, followed by a dose of PPSV23 at least 8 weeks later. If the patient has been previously vaccinated with PPSV23, then PCV13 at least 1 year after PPSV23 is recommended.
Hepatitis B vaccine should be given to nonimmune patients without past or present hepatitis B infection. Adult patients on hemodialysis require higher doses of hepatitis B vaccine. The options include Recombivax HB 40 μg/mL given on a three-dose schedule at 0, 1, and 6 months, and Engerix B, two 20-μg/mL injections given simultaneously on a four-dose schedule at 0, 1, 2, and 6 months.
Influenza vaccine should be offered annually to patients with chronic kidney disease.
VACCINATING IMMUNOCOMPROMISED INTERNATIONAL TRAVELERS
International travel for business or pleasure is increasingly common, and immunocompromised patients require specific attention as they may face unanticipated pathogens or have special requirements. Transplant recipients should ideally receive routine and travel-related vaccines as early as possible before transplantation. Vaccination is generally avoided in the first 6 months after organ transplantation to avoid confusion with early graft dysfunction or rejection.21 However, it should be considered as soon as a patient develops an illness that might lead to transplantation.
Evaluation of patients for vaccination should include an assessment of the travel-specific epidemiologic risk, the nature of the vaccine (live-attenuated or other), and the immune status. As discussed above, live-attenuated vaccines should be avoided in immunocompromised patients, and thus the injectable typhoid vaccine should be given in lieu of the attenuated oral vaccine.
Yellow fever vaccine is required before entrance to certain countries but should not be given to immunocompromised patients, although it can probably be given to asymptomatic HIV-infected adults with a CD4 count higher than 200 cells/μL who are exposed to substantial risk.22 For patients who cannot receive the vaccine, some governments will accept a physician’s letter stating the patient has a contraindication to vaccination.
VACCINATING HOUSEHOLD MEMBERS OF IMMUNOCOMPROMISED PATIENTS
Protecting immunocompromised patients from infectious diseases involves vaccinating not only the patient but also household members so that they do not acquire infections and then bring them into the household. Immunocompetent members of a household can receive inactivated vaccines based on the recommended ACIP schedule.
Annual inactivated influenza vaccination is recommended, although the live-attenuated influenza virus vaccine can be substituted if the immunocompromised patient is not within 2 months of hematopoietic stem cell transplantation, does not have graft-vs-host disease, and does not have severe combined immune deficiency.
Other live-attenuated vaccines can usually be given if indicated, including measles-mumps-rubella vaccine, rotavirus vaccine in infants, varicella vaccine, and zoster vaccine.14
- Crosignani P, De Stefani A, Fara GM, et al. Towards the eradication of HPV infection through universal specific vaccination. BMC Public Health 2013;13:642.
- Plotkin SL, Plotkin SA. A short history of vaccination. In: Plotkin, SA, Orenstein W, Offit PA, editors. Vaccines, 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2008:1–16.
- Wong VW, Chan HL. Prevention of hepatocellular carcinoma: a concise review of contemporary issues. Ann Hepatol 2012; 11:284–293.
- Kim DK, Bridges CB, Harriman K; Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older: United States, 2015. Ann Intern Med 2015; 162:214–223.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults—United States, 2012. MMWR Morb Mortal Wkly Rep 2014; 63:95-102.
- Hurley LP, Bridges CB, Harpaz R, et al. US physicians’ perspective of adult vaccine delivery. Ann Intern Med 2014; 160:161.
- Lindsey B, Kampmann B, Jones C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr Opin Infect Dis 2013; 26:248–253.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013; 62:131–135.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:1–24.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52:1–16.
- Staples JE, Gershman M, Fischer M; Centers for Disease Control and Prevention (CDC). Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010; 59:1–27.
- Danziger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):311–317.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–64.
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA, Infectious Diseases Society of America. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1–10.
- Eilers R, Krabbe PF, van Essen TG, Suijkerbuijk A, van Lier A, de Melker HE. Assessment of vaccine candidates for persons aged 50 and older: a review. BMC Geriatr 2013; 13:32.
- Oxman MN, Levin MJ; Shingles Prevention Study Group. Vaccination against Herpes Zoster and Postherpetic Neuralgia. J Infect Dis 2008; 197(suppl 2):S228–S236.
- Soni R, Horowitz B, Unruh M. Immunization in end-stage renal disease: opportunity to improve outcomes. Semin Dial 2013; 26:416–426.
- Chi C, Patel P, Pilishvili T, Moore M, Murphy T, Strikas R. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. http://www.cdc.gov/vaccines/pubs/downloads/dialysis-guide-2012.pdf. Accessed March 31, 2015.
- Kotton CN, Ryan ET, Fishman JA. Prevention of infection in adult travelers after solid organ transplantation. Am J Transplant 2005; 5:8–14.
- Castelli F, Patroni A. The human immunodeficiency virus-infected traveler. Clin Infect Dis 2000; 31:1403–1408.
- Crosignani P, De Stefani A, Fara GM, et al. Towards the eradication of HPV infection through universal specific vaccination. BMC Public Health 2013;13:642.
- Plotkin SL, Plotkin SA. A short history of vaccination. In: Plotkin, SA, Orenstein W, Offit PA, editors. Vaccines, 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2008:1–16.
- Wong VW, Chan HL. Prevention of hepatocellular carcinoma: a concise review of contemporary issues. Ann Hepatol 2012; 11:284–293.
- Kim DK, Bridges CB, Harriman K; Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older: United States, 2015. Ann Intern Med 2015; 162:214–223.
- Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults—United States, 2012. MMWR Morb Mortal Wkly Rep 2014; 63:95-102.
- Hurley LP, Bridges CB, Harpaz R, et al. US physicians’ perspective of adult vaccine delivery. Ann Intern Med 2014; 160:161.
- Lindsey B, Kampmann B, Jones C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr Opin Infect Dis 2013; 26:248–253.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013; 62:131–135.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:1–24.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52:1–16.
- Staples JE, Gershman M, Fischer M; Centers for Disease Control and Prevention (CDC). Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010; 59:1–27.
- Danziger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):311–317.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–64.
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:309–318.
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819.
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA, Infectious Diseases Society of America. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1–10.
- Eilers R, Krabbe PF, van Essen TG, Suijkerbuijk A, van Lier A, de Melker HE. Assessment of vaccine candidates for persons aged 50 and older: a review. BMC Geriatr 2013; 13:32.
- Oxman MN, Levin MJ; Shingles Prevention Study Group. Vaccination against Herpes Zoster and Postherpetic Neuralgia. J Infect Dis 2008; 197(suppl 2):S228–S236.
- Soni R, Horowitz B, Unruh M. Immunization in end-stage renal disease: opportunity to improve outcomes. Semin Dial 2013; 26:416–426.
- Chi C, Patel P, Pilishvili T, Moore M, Murphy T, Strikas R. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. http://www.cdc.gov/vaccines/pubs/downloads/dialysis-guide-2012.pdf. Accessed March 31, 2015.
- Kotton CN, Ryan ET, Fishman JA. Prevention of infection in adult travelers after solid organ transplantation. Am J Transplant 2005; 5:8–14.
- Castelli F, Patroni A. The human immunodeficiency virus-infected traveler. Clin Infect Dis 2000; 31:1403–1408.
KEY POINTS
- Avoid live-attenuated vaccines (influenza, varicella, zoster, measles-mumps-rubella, and yellow fever) in immunocompromised patients.
- Tetanus, diphtheria, and acellular pertussis (Tdap) vaccine is now recommended for pregnant women during each pregnancy, preferably at 27 to 36 weeks of gestation.
- Zoster vaccine is recommended for patients age 60 and older, regardless of earlier episodes of herpes zoster.