User login
RSV infections: State of the art
Understanding of respiratory syncytial virus (RSV) infection has increased substantially since its discovery, but a curative treatment remains elusive. Insights into the impact of gestational age, epidemiologic patterns, virus incubation and proliferation, pathogenesis and pathophysiology, and host immune response have set the stage for preventive measures and effective therapy. In today’s clinical practice, a specific humanized antibody is administered to high-risk infants, which is safe and effective in preventing RSV-related acute and postacute symptoms.
VIRUS STRUCTURE AND CLASSIFICATION
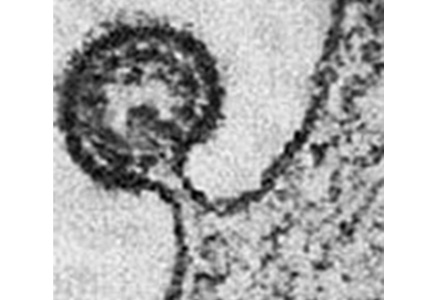
Knowledge of the molecular structure of RSV is important because it gives clues as to how it infects the human airways (Figure 1). The virus is made of a single strand of RNA contained in a nucleocapsid made of only 11 proteins and covered by a lipid envelope. Essential for the virulence of RSV are the surface glycoproteins G and F (fusion) that attach to the host airway epithelial cells and merge the viral envelope to the membranes of multiple adjacent cells, creating the “syncytia” that give RSV its name. G and F proteins are also the principal antigens exposed to the host immune system, and therefore the patient’s neutralizing antibodies are primarily directed against these targets.1
Human RSV is a Pneumovirus belonging to the Paramyxoviridae family. There are two strains of RSV (A and B) found in infants. Approximately 60% of all lower respiratory infections in preschool-aged children worldwide are caused by RSV (Figure 2).2 All the other viruses—including influenza, parainfluenza, metapneumovirus, and adenoviruses—as well as bacterial infections are much less common during the first years of life.
VIROLOGY
The burden of RSV continues to grow worldwide, particularly among the youngest segment of the population.2 During the first year of life, infants are not adequately protected from RSV by maternal immunoglobulins. Thus, approximately 80% of lower respiratory infections in children younger than 1 year are due to RSV with peak incidence occurring at 2 to 3 months of age. In the United States, bronchiolitis-associated hospitalizations in infants younger than 12 months more than doubled between 1980 and 1996, and in 1996, accounted for approximately 3% of all pediatric hospitalizations. Up to 120,000 RSV-related pediatric hospitalizations per year occur during a typical season.3
Mortality rates have improved significantly—at least in industrialized countries—and now are probably less than 1%.4 In the United States, RSV is estimated to cause fewer than 100 deaths annually, primarily because of better supportive care and the use of mechanical ventilation.
The seasonality of RSV varies by region. In the northern United States from October through March, RSV causes up to 90% of lower respiratory infection cases in infants. In the southern United States and farther into the southern hemisphere, the virus becomes endemic and can be present throughout the year with peak incidence occurring from August to September.2
RSV RISK FACTORS
The severity and frequency of RSV infection is influenced by well-known risk factors including environmental overcrowding, absence of breastfeeding, and immunosuppression. Children with chronic lung disease are predisposed to clinically significant RSV infection by their limited respiratory reserve, and 60% of those affected will be hospitalized. Hemodynamically significant congenital heart defects associated with higher pulmonary blood flow also increase risk for severe RSV disease, with more than 50% of children infected requiring hospitalization.4
Prematurity and increased RSV risk
Premature-born infants have a 10-fold increased risk of RSV infection and account for 25% to 30% of RSV hospitalizations annually.4 Prematurity as a risk factor for RSV is primarily tied to the physiology of placental immunoglobulin G (IgG) transfer. The human placenta is not permeable to IgG during the first half of pregnancy because of low expression of the Fc receptor needed to bind immunoglobulins and transfer them into the fetal circulation. Further, maternal IgG is recognized as a foreign protein by the newborn and is progressively removed from the circulation by the liver.5
Thus, the IgG decline continues postnatally, reaching the lowest concentration at 2 to 3 months of age because newborns are unable to synthesize their own antibodies. During this time, full-term born babies are at the highest risk for developing RSV infection. The risk is logically higher for premature infants who lack the full benefit of IgG transfer occurring during the last trimester, rendering antibody levels even lower at nadir. The greater the prematurity, the less the antibody protection and the more the predisposition to RSV infection.5
CLINICAL MANIFESTATIONS
The hallmark signs of RSV bronchiolitis are wheezing, cough, and increased work of breathing caused by infection of the bronchiolar airways. However, the nasal phase of the infection may cause irritation and trigeminal nerve activation associated with sneezing, congestion, and apnea. Indeed, approximately 20% of infants manifest an apnea episode as the first symptom of infection.6
The specific etiology of RSV can be confirmed by antigen detection tests, currently being replaced by more sensitive polymerase chain reaction (PCR)-based assays. Chest radiography reveals obvious signs of RSV lower respiratory infection, typically patch atelectasis, increased peribronchial markings, and bilateral hyperinflation with flattening of the diaphragm. Current guidelines indicate that the diagnosis of acute bronchiolitis should be based exclusively on the history and physical exam; it does not require radiographic or laboratory studies.
Correct etiologic diagnosis, however, is crucial to avoid unnecessary therapies and rule out rare conditions that could be worsened by therapies commonly used for bronchiolitis. For example, infants with dilated cardiomyopathy and congestive heart failure may present with symptoms of wheezing that mimic an acute respiratory infection, but these patients are at risk of developing supraventricular tachycardia and even cardiopulmonary collapse associated with administration of beta-agonist agents. In such cases, a chest radiograph will show cardiomegaly suggesting a different diagnosis and therapy, and thus avoid significant complications.
Recurrent wheezing
Children infected with RSV during their first year of life develop a higher risk of subsequent episodes of bronchial obstruction. Several retrospective studies conducted in the 1980s explored the incidence of lower respiratory symptoms continuing for years after initial RSV infection. Particularly unexpected was the finding that at least one-third of children hospitalized with RSV bronchiolitis will continue to wheeze beyond 6 to 8 years of age (Figure 3).7–9
More recent prospective studies of recurrent wheezing following RSV infection have been conducted. A Swedish study of 47 infants hospitalized with culture-proven RSV bronchiolitis showed significantly increased physician-diagnosed asthma (38%) compared with the 93 controls (2%) at age 7.5 years (P < .0001).10 Follow-up studies of the same cohort found increased risk for recurrent wheezing or asthma in the RSV group still present at age 13 years11 and 18 years12 compared with controls.
Asthma
History of RSV infection is important both as a trigger of asthma attacks and in the inception of asthma. Family history of asthma is the most important marker of a genetic predisposition to develop asthma. A multivariate analysis of the Swedish cohort discussed above showed that children with neither previous RSV infection nor family history of asthma had approximately a 5% risk of developing asthma by age 7.5 years. None of the children with a family history of asthma but no history of RSV infection developed asthma during follow-up. Children who had an RSV infection without a familial predisposition to asthma had approximately a 20% risk of asthma development. Children with both previous RSV infection and a family history of asthma had the highest (~40%) risk of developing asthma.10
These results provide important information about asthma pathogenesis in early life. The main implication is that even if a child has a genetic predisposition to asthma development, clinical manifestations will not develop without exposure to environmental agents that allow the actual expression of the predisposing genes, such as pollution, unbalanced diet, or early-life RSV infection. On the other hand, children without genetic predisposition can present with clinical manifestations undistinguishable from atopic asthma if their respiratory tract is exposed to specific viral pathogens during crucial developmental windows in early life. Finally, the combination of genetic predisposition and adverse environmental exposures in infancy carries the highest risk of asthma.
RSV MANAGEMENT
Ribavirin
The life cycle of RSV after the initial infection affects treatment strategies. RSV produces no symptoms for at least 3 to 5 days following infection (“eclipse phase”), during which time it reproduces exponentially and reaches the lungs, causing the first symptoms to be observable after 5 to 7 days. By the time the first symptoms emerge, the virus is already rapidly disappearing from the system.13 If ribavirin is administered at this point, the pediatrician has provided a virostatic agent against a virus that is no longer present, and therapy will not only be ineffective, but it may trigger bronchospasms.
The American Academy of Pediatrics (AAP) Committee on Infectious Diseases supported the use of ribavirin in 1993 guidelines,14 but changed their recommendation to “may be considered” in 1996.15 The only exception for ribavirin use is in the immunocompromised patient with RSV. In this scenario, the virus continues to replicate for months after the initial infection unopposed by the host defense mechanisms; therefore, aerosolized ribavirin therapy should be considered, either alone or in combination with humanized anti-RSV antibody.
Other treatments
Corticosteroids have not been shown to have a significant effect on RSV bronchiolitis.16 Alpha- and beta-adrenergic bronchodilators, such as epinephrine and albuterol, have shown very little or no effect on RSV symptoms in multiple controlled trials. The only effective treatment with proven efficacy is supportive therapy with adequate fluid intake and oxygen. Oral feeding should be withheld in patients with high respiratory rates to prevent aspiration.
PROPHYLAXIS
Vaccines
Vaccines have been ineffective against RSV and can be dangerous in children. RSV is not a strongly cytopathic virus; illness results primarily from the host immune response against the infection rather than from the virus itself.16 Therefore, any vaccine carries the risk of creating a stronger and potentially dangerous immune response to the next infection.
In the 1960s, a formalin-inactivated RSV vaccine was introduced with deleterious outcomes—it was minimally protective and was responsible for infant deaths. Because mortality was not immediate, the vaccine continued to be administered throughout the season. Unfortunately, with their immunity modified, vaccinated children became severely ill when they came in contact with the wild-type virus during the year following vaccination. The withdrawal of the formalin-inactivated vaccine still represents an important precedent that makes investigators and regulatory bodies very cautious about active immunization against this virus.
Palivizumab
Palivizumab is a 96% human monoclonal antibody targeting the RSV F protein, and it offers passive immunity for infants at risk for severe infection. The Impact-RSV clinical trial of palivizumab showed that five monthly intramuscular injections effectively reduced RSV hospitalizations by 78% in premature infants from 32 to 35 weeks gestation without bronchopulmonary dysplasia (BPD). Treatment offered only a 39% reduction in premature infants with BPD.17
The most recent AAP guidelines18 recommend palivizumab prophylaxis with a maximum of five monthly doses only in the first year of life for otherwise healthy infants born before 29 weeks gestation and for infants born before 32 weeks gestation with chronic lung disease of prematurity (CLD) defined as a requirement for supplemental oxygen for at least 28 days after birth. Prophylaxis is no longer recommended in the second year of life, except for infants with CLD still requiring oxygen, corticosteroids, or diuretics. Palivizumab prophylaxis should be discontinued if a breakthrough RSV hospitalization occurs because the likelihood of a second RSV hospitalization in the same season is low.
Palivizumab also should be considered for children with hemodynamically significant congenital heart defects, profound immunodeficiency, and pulmonary or neuromuscular pathologies impairing airway clearance; however, no formal recommendation was made for patients with Down syndrome or cystic fibrosis due to insufficient data.18 The protective effect of palivizumab appears to be cumulative, with almost half of the breakthrough infections observed in the clinical trials occurring after the first injection or immediately following the second.17
A recent randomized, double-blind, placebo-controlled trial has shown that palivizumab given to premature infants during the first year of life provides a 40% to 60% reduction of wheezing episodes.19 This trial confirms the results of previous retrospective studies; however, further large multicenter trials of palivizumab prophylaxis in both premature and full-term infants are needed to assess protection against recurrent wheezing and asthma, and to formulate evidence-based recommendations.
FUTURE DIRECTIONS
Nebulization of 3% saline improves mucociliary clearance and is increasingly being used in airway diseases involving mucus plugging (eg, cystic fibrosis). It also has been reported to reduce length of hospital stay and provide symptomatic relief in patients with bronchiolitis, but its use remains controversial. In particular, it has not been shown to be effective at reducing hospitalization when used in emergency settings. Therefore, based on current evidence, the administration of hypertonic saline for bronchiolitis should be limited to hospitalized infants.
Anti-leukotrienes used during the acute phase of RSV bronchiolitis have been shown to improve post-bronchiolitis respiratory symptoms, especially in younger patients with high urinary leukotriene E4 (LTE4). However, a large, multicenter, randomized, double-blind, placebo-controlled trial with montelukast failed to show statistically significant clinical improvement. A post-hoc data analysis revealed that children with persistent respiratory symptoms after the acute phase of the infection may benefit from montelukast, but the manufacturer (Merck) is no longer pursuing this indication.
There is not enough scientific evidence at present to support the use of DNAse or exogenous surfactant in the setting of acute bronchiolitis. New generations of specific antivirals and vaccines based on immunogens offer hope as new options for prophylaxis and/or management of RSV infection in the not too distant future.
Recent basic science investigations have provided new findings that could influence future therapies. Some of these studies have suggested that the tropism of RSV is not solely for the airway epithelium; rather, this virus can spread hematogenously to infect extrapulmonary targets, particularly the bone marrow stromal cells where RSV finds a sanctuary niche shielding it from immune protection and allowing subclinical latency. In one study, RSV was found in the bone marrow of every child and in about 80% of adults tested.20 Similar findings were replicated with other pathogens like Mycobacterium tuberculosis.21 This discovery could direct future treatment strategies for a number of viral, bacterial, and parasitic infections.
In another study, transplacental transmission of RSV from the mother’s airways to fetal lung tissues was shown for the first time in an animal model of infection. When the virus enters fetal respiratory cells, it persists and induces the expression of neurotrophic factors and receptors resulting in postnatal airways with dramatically increased parasympathetic innervation and methacholine reactivity. These structural and functional changes provide a suitable model to explain the development of long-term bronchial hyperreactivity following re-exposure to RSV in early life.22
In conclusion, the search for a cure for the most common respiratory infection in children has humbled several generations of investigators through the more than 50 years since RSV was discovered. Nevertheless, the rapid evolution in the fields of virology and immunology and recent breakthrough discoveries promise to deliver new strategies that may make a difference in the management of this common infection and its long-term sequelae.
- Peters TR, Crowe JE Jr. Respiratory syncytial virus. In: Long SS, Pickering LK, Prober CG, eds. Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2008.
- Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 2014; 35:519–530.
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999; 282:1440–1446.
- Simoes EA, Rieger CHL. RSV infection in developed and developing countries. Infect Med 1999; 16:S11–S17.
- Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1,500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res 1986; 20:899 –904.
- Sabogal C, Auais A, Napchan G, et al. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res 2005; 57:819–825.
- Hall CB, Hall WJ, Gala CL, MaGill FB, Leddy JP. Long-term prospective study in children after respiratory syncytial virus infection. J Pediatr 1984; 105:358–364.
- Henry RL, Hodges IGC, Milner AD, Stokes GM. Respiratory problems 2 years after acute bronchiolitis in infancy. Arch Dis Child 1983; 58:713–716.
- Webb MSC, Henry RL, Milner AD, Stokes GM, Swarbrick AS. Continuing respiratory problems three and a half years after acute viral bronchiolitis. Arch Dis Child 1985; 60:1064–1067.
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161:1501–1507.
- Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005; 171:137–141.
- Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65:1045–1052.
- McIntosh K, Chanock RM. Respiratory syncytial virus. In: Fields BN, Knipe DM, eds. Virology. New York: Raven Press; 1990:1045.
- Committee on Infectious Diseases. American Academy of Pediatrics: use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics 1993; 92:501–504.
- Committee on Infectious Diseases. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 1996; 97:137–140.
- Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol 2011; 46:324–347.
- The Impact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory synctial virus infection in high-risk infants. Pediatrics 1998; 102(3 Pt 1):531–537.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134:e1474–e1502.
- Blanken MO, Rovers MM, Molenaar JM, et al; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–1799.
- Rezaee F, Gibson LF, Piktel D, Othumpangat S, Piedimonte G. Respiratory syncytial virus infection in human bone marrow stromal cells. Am J Respir Cell Mol Biol 2011; 45:277–286.
- Das B, Kashino SS, Pulu I, et al. CD271+ bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med 2013; 5:170ra13.
- Piedimonte G, Perez MK. Alternative mechanisms for respiratory syncytial virus (RSV) infection and persistence: could RSV be transmitted through the placenta and persist into developing fetal lungs? Curr Opin Pharmacol 2014; 16:82–88.
Understanding of respiratory syncytial virus (RSV) infection has increased substantially since its discovery, but a curative treatment remains elusive. Insights into the impact of gestational age, epidemiologic patterns, virus incubation and proliferation, pathogenesis and pathophysiology, and host immune response have set the stage for preventive measures and effective therapy. In today’s clinical practice, a specific humanized antibody is administered to high-risk infants, which is safe and effective in preventing RSV-related acute and postacute symptoms.
VIRUS STRUCTURE AND CLASSIFICATION
Knowledge of the molecular structure of RSV is important because it gives clues as to how it infects the human airways (Figure 1). The virus is made of a single strand of RNA contained in a nucleocapsid made of only 11 proteins and covered by a lipid envelope. Essential for the virulence of RSV are the surface glycoproteins G and F (fusion) that attach to the host airway epithelial cells and merge the viral envelope to the membranes of multiple adjacent cells, creating the “syncytia” that give RSV its name. G and F proteins are also the principal antigens exposed to the host immune system, and therefore the patient’s neutralizing antibodies are primarily directed against these targets.1
Human RSV is a Pneumovirus belonging to the Paramyxoviridae family. There are two strains of RSV (A and B) found in infants. Approximately 60% of all lower respiratory infections in preschool-aged children worldwide are caused by RSV (Figure 2).2 All the other viruses—including influenza, parainfluenza, metapneumovirus, and adenoviruses—as well as bacterial infections are much less common during the first years of life.
VIROLOGY
The burden of RSV continues to grow worldwide, particularly among the youngest segment of the population.2 During the first year of life, infants are not adequately protected from RSV by maternal immunoglobulins. Thus, approximately 80% of lower respiratory infections in children younger than 1 year are due to RSV with peak incidence occurring at 2 to 3 months of age. In the United States, bronchiolitis-associated hospitalizations in infants younger than 12 months more than doubled between 1980 and 1996, and in 1996, accounted for approximately 3% of all pediatric hospitalizations. Up to 120,000 RSV-related pediatric hospitalizations per year occur during a typical season.3
Mortality rates have improved significantly—at least in industrialized countries—and now are probably less than 1%.4 In the United States, RSV is estimated to cause fewer than 100 deaths annually, primarily because of better supportive care and the use of mechanical ventilation.
The seasonality of RSV varies by region. In the northern United States from October through March, RSV causes up to 90% of lower respiratory infection cases in infants. In the southern United States and farther into the southern hemisphere, the virus becomes endemic and can be present throughout the year with peak incidence occurring from August to September.2
RSV RISK FACTORS
The severity and frequency of RSV infection is influenced by well-known risk factors including environmental overcrowding, absence of breastfeeding, and immunosuppression. Children with chronic lung disease are predisposed to clinically significant RSV infection by their limited respiratory reserve, and 60% of those affected will be hospitalized. Hemodynamically significant congenital heart defects associated with higher pulmonary blood flow also increase risk for severe RSV disease, with more than 50% of children infected requiring hospitalization.4
Prematurity and increased RSV risk
Premature-born infants have a 10-fold increased risk of RSV infection and account for 25% to 30% of RSV hospitalizations annually.4 Prematurity as a risk factor for RSV is primarily tied to the physiology of placental immunoglobulin G (IgG) transfer. The human placenta is not permeable to IgG during the first half of pregnancy because of low expression of the Fc receptor needed to bind immunoglobulins and transfer them into the fetal circulation. Further, maternal IgG is recognized as a foreign protein by the newborn and is progressively removed from the circulation by the liver.5
Thus, the IgG decline continues postnatally, reaching the lowest concentration at 2 to 3 months of age because newborns are unable to synthesize their own antibodies. During this time, full-term born babies are at the highest risk for developing RSV infection. The risk is logically higher for premature infants who lack the full benefit of IgG transfer occurring during the last trimester, rendering antibody levels even lower at nadir. The greater the prematurity, the less the antibody protection and the more the predisposition to RSV infection.5
CLINICAL MANIFESTATIONS
The hallmark signs of RSV bronchiolitis are wheezing, cough, and increased work of breathing caused by infection of the bronchiolar airways. However, the nasal phase of the infection may cause irritation and trigeminal nerve activation associated with sneezing, congestion, and apnea. Indeed, approximately 20% of infants manifest an apnea episode as the first symptom of infection.6
The specific etiology of RSV can be confirmed by antigen detection tests, currently being replaced by more sensitive polymerase chain reaction (PCR)-based assays. Chest radiography reveals obvious signs of RSV lower respiratory infection, typically patch atelectasis, increased peribronchial markings, and bilateral hyperinflation with flattening of the diaphragm. Current guidelines indicate that the diagnosis of acute bronchiolitis should be based exclusively on the history and physical exam; it does not require radiographic or laboratory studies.
Correct etiologic diagnosis, however, is crucial to avoid unnecessary therapies and rule out rare conditions that could be worsened by therapies commonly used for bronchiolitis. For example, infants with dilated cardiomyopathy and congestive heart failure may present with symptoms of wheezing that mimic an acute respiratory infection, but these patients are at risk of developing supraventricular tachycardia and even cardiopulmonary collapse associated with administration of beta-agonist agents. In such cases, a chest radiograph will show cardiomegaly suggesting a different diagnosis and therapy, and thus avoid significant complications.
Recurrent wheezing
Children infected with RSV during their first year of life develop a higher risk of subsequent episodes of bronchial obstruction. Several retrospective studies conducted in the 1980s explored the incidence of lower respiratory symptoms continuing for years after initial RSV infection. Particularly unexpected was the finding that at least one-third of children hospitalized with RSV bronchiolitis will continue to wheeze beyond 6 to 8 years of age (Figure 3).7–9
More recent prospective studies of recurrent wheezing following RSV infection have been conducted. A Swedish study of 47 infants hospitalized with culture-proven RSV bronchiolitis showed significantly increased physician-diagnosed asthma (38%) compared with the 93 controls (2%) at age 7.5 years (P < .0001).10 Follow-up studies of the same cohort found increased risk for recurrent wheezing or asthma in the RSV group still present at age 13 years11 and 18 years12 compared with controls.
Asthma
History of RSV infection is important both as a trigger of asthma attacks and in the inception of asthma. Family history of asthma is the most important marker of a genetic predisposition to develop asthma. A multivariate analysis of the Swedish cohort discussed above showed that children with neither previous RSV infection nor family history of asthma had approximately a 5% risk of developing asthma by age 7.5 years. None of the children with a family history of asthma but no history of RSV infection developed asthma during follow-up. Children who had an RSV infection without a familial predisposition to asthma had approximately a 20% risk of asthma development. Children with both previous RSV infection and a family history of asthma had the highest (~40%) risk of developing asthma.10
These results provide important information about asthma pathogenesis in early life. The main implication is that even if a child has a genetic predisposition to asthma development, clinical manifestations will not develop without exposure to environmental agents that allow the actual expression of the predisposing genes, such as pollution, unbalanced diet, or early-life RSV infection. On the other hand, children without genetic predisposition can present with clinical manifestations undistinguishable from atopic asthma if their respiratory tract is exposed to specific viral pathogens during crucial developmental windows in early life. Finally, the combination of genetic predisposition and adverse environmental exposures in infancy carries the highest risk of asthma.
RSV MANAGEMENT
Ribavirin
The life cycle of RSV after the initial infection affects treatment strategies. RSV produces no symptoms for at least 3 to 5 days following infection (“eclipse phase”), during which time it reproduces exponentially and reaches the lungs, causing the first symptoms to be observable after 5 to 7 days. By the time the first symptoms emerge, the virus is already rapidly disappearing from the system.13 If ribavirin is administered at this point, the pediatrician has provided a virostatic agent against a virus that is no longer present, and therapy will not only be ineffective, but it may trigger bronchospasms.
The American Academy of Pediatrics (AAP) Committee on Infectious Diseases supported the use of ribavirin in 1993 guidelines,14 but changed their recommendation to “may be considered” in 1996.15 The only exception for ribavirin use is in the immunocompromised patient with RSV. In this scenario, the virus continues to replicate for months after the initial infection unopposed by the host defense mechanisms; therefore, aerosolized ribavirin therapy should be considered, either alone or in combination with humanized anti-RSV antibody.
Other treatments
Corticosteroids have not been shown to have a significant effect on RSV bronchiolitis.16 Alpha- and beta-adrenergic bronchodilators, such as epinephrine and albuterol, have shown very little or no effect on RSV symptoms in multiple controlled trials. The only effective treatment with proven efficacy is supportive therapy with adequate fluid intake and oxygen. Oral feeding should be withheld in patients with high respiratory rates to prevent aspiration.
PROPHYLAXIS
Vaccines
Vaccines have been ineffective against RSV and can be dangerous in children. RSV is not a strongly cytopathic virus; illness results primarily from the host immune response against the infection rather than from the virus itself.16 Therefore, any vaccine carries the risk of creating a stronger and potentially dangerous immune response to the next infection.
In the 1960s, a formalin-inactivated RSV vaccine was introduced with deleterious outcomes—it was minimally protective and was responsible for infant deaths. Because mortality was not immediate, the vaccine continued to be administered throughout the season. Unfortunately, with their immunity modified, vaccinated children became severely ill when they came in contact with the wild-type virus during the year following vaccination. The withdrawal of the formalin-inactivated vaccine still represents an important precedent that makes investigators and regulatory bodies very cautious about active immunization against this virus.
Palivizumab
Palivizumab is a 96% human monoclonal antibody targeting the RSV F protein, and it offers passive immunity for infants at risk for severe infection. The Impact-RSV clinical trial of palivizumab showed that five monthly intramuscular injections effectively reduced RSV hospitalizations by 78% in premature infants from 32 to 35 weeks gestation without bronchopulmonary dysplasia (BPD). Treatment offered only a 39% reduction in premature infants with BPD.17
The most recent AAP guidelines18 recommend palivizumab prophylaxis with a maximum of five monthly doses only in the first year of life for otherwise healthy infants born before 29 weeks gestation and for infants born before 32 weeks gestation with chronic lung disease of prematurity (CLD) defined as a requirement for supplemental oxygen for at least 28 days after birth. Prophylaxis is no longer recommended in the second year of life, except for infants with CLD still requiring oxygen, corticosteroids, or diuretics. Palivizumab prophylaxis should be discontinued if a breakthrough RSV hospitalization occurs because the likelihood of a second RSV hospitalization in the same season is low.
Palivizumab also should be considered for children with hemodynamically significant congenital heart defects, profound immunodeficiency, and pulmonary or neuromuscular pathologies impairing airway clearance; however, no formal recommendation was made for patients with Down syndrome or cystic fibrosis due to insufficient data.18 The protective effect of palivizumab appears to be cumulative, with almost half of the breakthrough infections observed in the clinical trials occurring after the first injection or immediately following the second.17
A recent randomized, double-blind, placebo-controlled trial has shown that palivizumab given to premature infants during the first year of life provides a 40% to 60% reduction of wheezing episodes.19 This trial confirms the results of previous retrospective studies; however, further large multicenter trials of palivizumab prophylaxis in both premature and full-term infants are needed to assess protection against recurrent wheezing and asthma, and to formulate evidence-based recommendations.
FUTURE DIRECTIONS
Nebulization of 3% saline improves mucociliary clearance and is increasingly being used in airway diseases involving mucus plugging (eg, cystic fibrosis). It also has been reported to reduce length of hospital stay and provide symptomatic relief in patients with bronchiolitis, but its use remains controversial. In particular, it has not been shown to be effective at reducing hospitalization when used in emergency settings. Therefore, based on current evidence, the administration of hypertonic saline for bronchiolitis should be limited to hospitalized infants.
Anti-leukotrienes used during the acute phase of RSV bronchiolitis have been shown to improve post-bronchiolitis respiratory symptoms, especially in younger patients with high urinary leukotriene E4 (LTE4). However, a large, multicenter, randomized, double-blind, placebo-controlled trial with montelukast failed to show statistically significant clinical improvement. A post-hoc data analysis revealed that children with persistent respiratory symptoms after the acute phase of the infection may benefit from montelukast, but the manufacturer (Merck) is no longer pursuing this indication.
There is not enough scientific evidence at present to support the use of DNAse or exogenous surfactant in the setting of acute bronchiolitis. New generations of specific antivirals and vaccines based on immunogens offer hope as new options for prophylaxis and/or management of RSV infection in the not too distant future.
Recent basic science investigations have provided new findings that could influence future therapies. Some of these studies have suggested that the tropism of RSV is not solely for the airway epithelium; rather, this virus can spread hematogenously to infect extrapulmonary targets, particularly the bone marrow stromal cells where RSV finds a sanctuary niche shielding it from immune protection and allowing subclinical latency. In one study, RSV was found in the bone marrow of every child and in about 80% of adults tested.20 Similar findings were replicated with other pathogens like Mycobacterium tuberculosis.21 This discovery could direct future treatment strategies for a number of viral, bacterial, and parasitic infections.
In another study, transplacental transmission of RSV from the mother’s airways to fetal lung tissues was shown for the first time in an animal model of infection. When the virus enters fetal respiratory cells, it persists and induces the expression of neurotrophic factors and receptors resulting in postnatal airways with dramatically increased parasympathetic innervation and methacholine reactivity. These structural and functional changes provide a suitable model to explain the development of long-term bronchial hyperreactivity following re-exposure to RSV in early life.22
In conclusion, the search for a cure for the most common respiratory infection in children has humbled several generations of investigators through the more than 50 years since RSV was discovered. Nevertheless, the rapid evolution in the fields of virology and immunology and recent breakthrough discoveries promise to deliver new strategies that may make a difference in the management of this common infection and its long-term sequelae.
Understanding of respiratory syncytial virus (RSV) infection has increased substantially since its discovery, but a curative treatment remains elusive. Insights into the impact of gestational age, epidemiologic patterns, virus incubation and proliferation, pathogenesis and pathophysiology, and host immune response have set the stage for preventive measures and effective therapy. In today’s clinical practice, a specific humanized antibody is administered to high-risk infants, which is safe and effective in preventing RSV-related acute and postacute symptoms.
VIRUS STRUCTURE AND CLASSIFICATION
Knowledge of the molecular structure of RSV is important because it gives clues as to how it infects the human airways (Figure 1). The virus is made of a single strand of RNA contained in a nucleocapsid made of only 11 proteins and covered by a lipid envelope. Essential for the virulence of RSV are the surface glycoproteins G and F (fusion) that attach to the host airway epithelial cells and merge the viral envelope to the membranes of multiple adjacent cells, creating the “syncytia” that give RSV its name. G and F proteins are also the principal antigens exposed to the host immune system, and therefore the patient’s neutralizing antibodies are primarily directed against these targets.1
Human RSV is a Pneumovirus belonging to the Paramyxoviridae family. There are two strains of RSV (A and B) found in infants. Approximately 60% of all lower respiratory infections in preschool-aged children worldwide are caused by RSV (Figure 2).2 All the other viruses—including influenza, parainfluenza, metapneumovirus, and adenoviruses—as well as bacterial infections are much less common during the first years of life.
VIROLOGY
The burden of RSV continues to grow worldwide, particularly among the youngest segment of the population.2 During the first year of life, infants are not adequately protected from RSV by maternal immunoglobulins. Thus, approximately 80% of lower respiratory infections in children younger than 1 year are due to RSV with peak incidence occurring at 2 to 3 months of age. In the United States, bronchiolitis-associated hospitalizations in infants younger than 12 months more than doubled between 1980 and 1996, and in 1996, accounted for approximately 3% of all pediatric hospitalizations. Up to 120,000 RSV-related pediatric hospitalizations per year occur during a typical season.3
Mortality rates have improved significantly—at least in industrialized countries—and now are probably less than 1%.4 In the United States, RSV is estimated to cause fewer than 100 deaths annually, primarily because of better supportive care and the use of mechanical ventilation.
The seasonality of RSV varies by region. In the northern United States from October through March, RSV causes up to 90% of lower respiratory infection cases in infants. In the southern United States and farther into the southern hemisphere, the virus becomes endemic and can be present throughout the year with peak incidence occurring from August to September.2
RSV RISK FACTORS
The severity and frequency of RSV infection is influenced by well-known risk factors including environmental overcrowding, absence of breastfeeding, and immunosuppression. Children with chronic lung disease are predisposed to clinically significant RSV infection by their limited respiratory reserve, and 60% of those affected will be hospitalized. Hemodynamically significant congenital heart defects associated with higher pulmonary blood flow also increase risk for severe RSV disease, with more than 50% of children infected requiring hospitalization.4
Prematurity and increased RSV risk
Premature-born infants have a 10-fold increased risk of RSV infection and account for 25% to 30% of RSV hospitalizations annually.4 Prematurity as a risk factor for RSV is primarily tied to the physiology of placental immunoglobulin G (IgG) transfer. The human placenta is not permeable to IgG during the first half of pregnancy because of low expression of the Fc receptor needed to bind immunoglobulins and transfer them into the fetal circulation. Further, maternal IgG is recognized as a foreign protein by the newborn and is progressively removed from the circulation by the liver.5
Thus, the IgG decline continues postnatally, reaching the lowest concentration at 2 to 3 months of age because newborns are unable to synthesize their own antibodies. During this time, full-term born babies are at the highest risk for developing RSV infection. The risk is logically higher for premature infants who lack the full benefit of IgG transfer occurring during the last trimester, rendering antibody levels even lower at nadir. The greater the prematurity, the less the antibody protection and the more the predisposition to RSV infection.5
CLINICAL MANIFESTATIONS
The hallmark signs of RSV bronchiolitis are wheezing, cough, and increased work of breathing caused by infection of the bronchiolar airways. However, the nasal phase of the infection may cause irritation and trigeminal nerve activation associated with sneezing, congestion, and apnea. Indeed, approximately 20% of infants manifest an apnea episode as the first symptom of infection.6
The specific etiology of RSV can be confirmed by antigen detection tests, currently being replaced by more sensitive polymerase chain reaction (PCR)-based assays. Chest radiography reveals obvious signs of RSV lower respiratory infection, typically patch atelectasis, increased peribronchial markings, and bilateral hyperinflation with flattening of the diaphragm. Current guidelines indicate that the diagnosis of acute bronchiolitis should be based exclusively on the history and physical exam; it does not require radiographic or laboratory studies.
Correct etiologic diagnosis, however, is crucial to avoid unnecessary therapies and rule out rare conditions that could be worsened by therapies commonly used for bronchiolitis. For example, infants with dilated cardiomyopathy and congestive heart failure may present with symptoms of wheezing that mimic an acute respiratory infection, but these patients are at risk of developing supraventricular tachycardia and even cardiopulmonary collapse associated with administration of beta-agonist agents. In such cases, a chest radiograph will show cardiomegaly suggesting a different diagnosis and therapy, and thus avoid significant complications.
Recurrent wheezing
Children infected with RSV during their first year of life develop a higher risk of subsequent episodes of bronchial obstruction. Several retrospective studies conducted in the 1980s explored the incidence of lower respiratory symptoms continuing for years after initial RSV infection. Particularly unexpected was the finding that at least one-third of children hospitalized with RSV bronchiolitis will continue to wheeze beyond 6 to 8 years of age (Figure 3).7–9
More recent prospective studies of recurrent wheezing following RSV infection have been conducted. A Swedish study of 47 infants hospitalized with culture-proven RSV bronchiolitis showed significantly increased physician-diagnosed asthma (38%) compared with the 93 controls (2%) at age 7.5 years (P < .0001).10 Follow-up studies of the same cohort found increased risk for recurrent wheezing or asthma in the RSV group still present at age 13 years11 and 18 years12 compared with controls.
Asthma
History of RSV infection is important both as a trigger of asthma attacks and in the inception of asthma. Family history of asthma is the most important marker of a genetic predisposition to develop asthma. A multivariate analysis of the Swedish cohort discussed above showed that children with neither previous RSV infection nor family history of asthma had approximately a 5% risk of developing asthma by age 7.5 years. None of the children with a family history of asthma but no history of RSV infection developed asthma during follow-up. Children who had an RSV infection without a familial predisposition to asthma had approximately a 20% risk of asthma development. Children with both previous RSV infection and a family history of asthma had the highest (~40%) risk of developing asthma.10
These results provide important information about asthma pathogenesis in early life. The main implication is that even if a child has a genetic predisposition to asthma development, clinical manifestations will not develop without exposure to environmental agents that allow the actual expression of the predisposing genes, such as pollution, unbalanced diet, or early-life RSV infection. On the other hand, children without genetic predisposition can present with clinical manifestations undistinguishable from atopic asthma if their respiratory tract is exposed to specific viral pathogens during crucial developmental windows in early life. Finally, the combination of genetic predisposition and adverse environmental exposures in infancy carries the highest risk of asthma.
RSV MANAGEMENT
Ribavirin
The life cycle of RSV after the initial infection affects treatment strategies. RSV produces no symptoms for at least 3 to 5 days following infection (“eclipse phase”), during which time it reproduces exponentially and reaches the lungs, causing the first symptoms to be observable after 5 to 7 days. By the time the first symptoms emerge, the virus is already rapidly disappearing from the system.13 If ribavirin is administered at this point, the pediatrician has provided a virostatic agent against a virus that is no longer present, and therapy will not only be ineffective, but it may trigger bronchospasms.
The American Academy of Pediatrics (AAP) Committee on Infectious Diseases supported the use of ribavirin in 1993 guidelines,14 but changed their recommendation to “may be considered” in 1996.15 The only exception for ribavirin use is in the immunocompromised patient with RSV. In this scenario, the virus continues to replicate for months after the initial infection unopposed by the host defense mechanisms; therefore, aerosolized ribavirin therapy should be considered, either alone or in combination with humanized anti-RSV antibody.
Other treatments
Corticosteroids have not been shown to have a significant effect on RSV bronchiolitis.16 Alpha- and beta-adrenergic bronchodilators, such as epinephrine and albuterol, have shown very little or no effect on RSV symptoms in multiple controlled trials. The only effective treatment with proven efficacy is supportive therapy with adequate fluid intake and oxygen. Oral feeding should be withheld in patients with high respiratory rates to prevent aspiration.
PROPHYLAXIS
Vaccines
Vaccines have been ineffective against RSV and can be dangerous in children. RSV is not a strongly cytopathic virus; illness results primarily from the host immune response against the infection rather than from the virus itself.16 Therefore, any vaccine carries the risk of creating a stronger and potentially dangerous immune response to the next infection.
In the 1960s, a formalin-inactivated RSV vaccine was introduced with deleterious outcomes—it was minimally protective and was responsible for infant deaths. Because mortality was not immediate, the vaccine continued to be administered throughout the season. Unfortunately, with their immunity modified, vaccinated children became severely ill when they came in contact with the wild-type virus during the year following vaccination. The withdrawal of the formalin-inactivated vaccine still represents an important precedent that makes investigators and regulatory bodies very cautious about active immunization against this virus.
Palivizumab
Palivizumab is a 96% human monoclonal antibody targeting the RSV F protein, and it offers passive immunity for infants at risk for severe infection. The Impact-RSV clinical trial of palivizumab showed that five monthly intramuscular injections effectively reduced RSV hospitalizations by 78% in premature infants from 32 to 35 weeks gestation without bronchopulmonary dysplasia (BPD). Treatment offered only a 39% reduction in premature infants with BPD.17
The most recent AAP guidelines18 recommend palivizumab prophylaxis with a maximum of five monthly doses only in the first year of life for otherwise healthy infants born before 29 weeks gestation and for infants born before 32 weeks gestation with chronic lung disease of prematurity (CLD) defined as a requirement for supplemental oxygen for at least 28 days after birth. Prophylaxis is no longer recommended in the second year of life, except for infants with CLD still requiring oxygen, corticosteroids, or diuretics. Palivizumab prophylaxis should be discontinued if a breakthrough RSV hospitalization occurs because the likelihood of a second RSV hospitalization in the same season is low.
Palivizumab also should be considered for children with hemodynamically significant congenital heart defects, profound immunodeficiency, and pulmonary or neuromuscular pathologies impairing airway clearance; however, no formal recommendation was made for patients with Down syndrome or cystic fibrosis due to insufficient data.18 The protective effect of palivizumab appears to be cumulative, with almost half of the breakthrough infections observed in the clinical trials occurring after the first injection or immediately following the second.17
A recent randomized, double-blind, placebo-controlled trial has shown that palivizumab given to premature infants during the first year of life provides a 40% to 60% reduction of wheezing episodes.19 This trial confirms the results of previous retrospective studies; however, further large multicenter trials of palivizumab prophylaxis in both premature and full-term infants are needed to assess protection against recurrent wheezing and asthma, and to formulate evidence-based recommendations.
FUTURE DIRECTIONS
Nebulization of 3% saline improves mucociliary clearance and is increasingly being used in airway diseases involving mucus plugging (eg, cystic fibrosis). It also has been reported to reduce length of hospital stay and provide symptomatic relief in patients with bronchiolitis, but its use remains controversial. In particular, it has not been shown to be effective at reducing hospitalization when used in emergency settings. Therefore, based on current evidence, the administration of hypertonic saline for bronchiolitis should be limited to hospitalized infants.
Anti-leukotrienes used during the acute phase of RSV bronchiolitis have been shown to improve post-bronchiolitis respiratory symptoms, especially in younger patients with high urinary leukotriene E4 (LTE4). However, a large, multicenter, randomized, double-blind, placebo-controlled trial with montelukast failed to show statistically significant clinical improvement. A post-hoc data analysis revealed that children with persistent respiratory symptoms after the acute phase of the infection may benefit from montelukast, but the manufacturer (Merck) is no longer pursuing this indication.
There is not enough scientific evidence at present to support the use of DNAse or exogenous surfactant in the setting of acute bronchiolitis. New generations of specific antivirals and vaccines based on immunogens offer hope as new options for prophylaxis and/or management of RSV infection in the not too distant future.
Recent basic science investigations have provided new findings that could influence future therapies. Some of these studies have suggested that the tropism of RSV is not solely for the airway epithelium; rather, this virus can spread hematogenously to infect extrapulmonary targets, particularly the bone marrow stromal cells where RSV finds a sanctuary niche shielding it from immune protection and allowing subclinical latency. In one study, RSV was found in the bone marrow of every child and in about 80% of adults tested.20 Similar findings were replicated with other pathogens like Mycobacterium tuberculosis.21 This discovery could direct future treatment strategies for a number of viral, bacterial, and parasitic infections.
In another study, transplacental transmission of RSV from the mother’s airways to fetal lung tissues was shown for the first time in an animal model of infection. When the virus enters fetal respiratory cells, it persists and induces the expression of neurotrophic factors and receptors resulting in postnatal airways with dramatically increased parasympathetic innervation and methacholine reactivity. These structural and functional changes provide a suitable model to explain the development of long-term bronchial hyperreactivity following re-exposure to RSV in early life.22
In conclusion, the search for a cure for the most common respiratory infection in children has humbled several generations of investigators through the more than 50 years since RSV was discovered. Nevertheless, the rapid evolution in the fields of virology and immunology and recent breakthrough discoveries promise to deliver new strategies that may make a difference in the management of this common infection and its long-term sequelae.
- Peters TR, Crowe JE Jr. Respiratory syncytial virus. In: Long SS, Pickering LK, Prober CG, eds. Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2008.
- Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 2014; 35:519–530.
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999; 282:1440–1446.
- Simoes EA, Rieger CHL. RSV infection in developed and developing countries. Infect Med 1999; 16:S11–S17.
- Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1,500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res 1986; 20:899 –904.
- Sabogal C, Auais A, Napchan G, et al. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res 2005; 57:819–825.
- Hall CB, Hall WJ, Gala CL, MaGill FB, Leddy JP. Long-term prospective study in children after respiratory syncytial virus infection. J Pediatr 1984; 105:358–364.
- Henry RL, Hodges IGC, Milner AD, Stokes GM. Respiratory problems 2 years after acute bronchiolitis in infancy. Arch Dis Child 1983; 58:713–716.
- Webb MSC, Henry RL, Milner AD, Stokes GM, Swarbrick AS. Continuing respiratory problems three and a half years after acute viral bronchiolitis. Arch Dis Child 1985; 60:1064–1067.
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161:1501–1507.
- Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005; 171:137–141.
- Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65:1045–1052.
- McIntosh K, Chanock RM. Respiratory syncytial virus. In: Fields BN, Knipe DM, eds. Virology. New York: Raven Press; 1990:1045.
- Committee on Infectious Diseases. American Academy of Pediatrics: use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics 1993; 92:501–504.
- Committee on Infectious Diseases. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 1996; 97:137–140.
- Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol 2011; 46:324–347.
- The Impact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory synctial virus infection in high-risk infants. Pediatrics 1998; 102(3 Pt 1):531–537.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134:e1474–e1502.
- Blanken MO, Rovers MM, Molenaar JM, et al; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–1799.
- Rezaee F, Gibson LF, Piktel D, Othumpangat S, Piedimonte G. Respiratory syncytial virus infection in human bone marrow stromal cells. Am J Respir Cell Mol Biol 2011; 45:277–286.
- Das B, Kashino SS, Pulu I, et al. CD271+ bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med 2013; 5:170ra13.
- Piedimonte G, Perez MK. Alternative mechanisms for respiratory syncytial virus (RSV) infection and persistence: could RSV be transmitted through the placenta and persist into developing fetal lungs? Curr Opin Pharmacol 2014; 16:82–88.
- Peters TR, Crowe JE Jr. Respiratory syncytial virus. In: Long SS, Pickering LK, Prober CG, eds. Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2008.
- Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 2014; 35:519–530.
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999; 282:1440–1446.
- Simoes EA, Rieger CHL. RSV infection in developed and developing countries. Infect Med 1999; 16:S11–S17.
- Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1,500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res 1986; 20:899 –904.
- Sabogal C, Auais A, Napchan G, et al. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res 2005; 57:819–825.
- Hall CB, Hall WJ, Gala CL, MaGill FB, Leddy JP. Long-term prospective study in children after respiratory syncytial virus infection. J Pediatr 1984; 105:358–364.
- Henry RL, Hodges IGC, Milner AD, Stokes GM. Respiratory problems 2 years after acute bronchiolitis in infancy. Arch Dis Child 1983; 58:713–716.
- Webb MSC, Henry RL, Milner AD, Stokes GM, Swarbrick AS. Continuing respiratory problems three and a half years after acute viral bronchiolitis. Arch Dis Child 1985; 60:1064–1067.
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161:1501–1507.
- Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005; 171:137–141.
- Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65:1045–1052.
- McIntosh K, Chanock RM. Respiratory syncytial virus. In: Fields BN, Knipe DM, eds. Virology. New York: Raven Press; 1990:1045.
- Committee on Infectious Diseases. American Academy of Pediatrics: use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics 1993; 92:501–504.
- Committee on Infectious Diseases. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 1996; 97:137–140.
- Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol 2011; 46:324–347.
- The Impact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory synctial virus infection in high-risk infants. Pediatrics 1998; 102(3 Pt 1):531–537.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134:e1474–e1502.
- Blanken MO, Rovers MM, Molenaar JM, et al; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–1799.
- Rezaee F, Gibson LF, Piktel D, Othumpangat S, Piedimonte G. Respiratory syncytial virus infection in human bone marrow stromal cells. Am J Respir Cell Mol Biol 2011; 45:277–286.
- Das B, Kashino SS, Pulu I, et al. CD271+ bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med 2013; 5:170ra13.
- Piedimonte G, Perez MK. Alternative mechanisms for respiratory syncytial virus (RSV) infection and persistence: could RSV be transmitted through the placenta and persist into developing fetal lungs? Curr Opin Pharmacol 2014; 16:82–88.
Enterovirus D68: A clinically important respiratory enterovirus
In the fall of 2014, the United States experienced an outbreak of severe respiratory illness due to a virus of emerging importance, enterovirus D68 (EV-D68). Here, we review the features of this virus and related viruses, the clinical syndromes this virus causes, the epidemiology of the recent outbreak, and its diagnosis and treatment.
THE ENTEROVIRUSES: AN OVERVIEW
Originally identified in 1962 from the throat swab of a child with pneumonia, human EV-D68 has unique genetic and clinical features that blur the typical division between human enteroviruses and rhinoviruses.1–4 Enteroviruses and rhinoviruses are closely related species within the Picornaviridae family that are now classified together within the genus Enterovirus.5 Picornaviruses are small, nonenveloped, positive-stranded RNA viruses of medical significance.
Poliovirus: The first enterovirus discovered
The first human enterovirus to be discovered was poliovirus.6 Although sporadic cases of “infantile paralysis” occurred before the late 19th century, epidemic poliomyelitis abruptly appeared in Europe and the United States beginning around 1880. Before the introduction in 1955 of the inactivated poliovirus vaccine and then the oral poliovirus vaccine, polio was one of the most feared illnesses in the developed world. Outbreaks occurred primarily in cities during summer months. At its peak, epidemic polio killed or paralyzed more than half a million people a year.
One hypothesis to explain the sudden emergence of epidemic polio is that improved personal hygiene and public sanitation delayed the age at which children acquired this enteric infection.7 Infections acquired after infancy occurred in the absence of maternal antibodies that may have protected against the virus’s propensity to invade the nervous system.
Nonpolio human enteroviruses
In the decades since poliovirus was discovered, more than 100 nonpolio human enteroviruses have been recognized.8 This group includes the coxsackieviruses, echoviruses, and the newer numbered nonpolio human enteroviruses classified into four species, designated Human enterovirus A, B, C, and D. The last of these, Human enterovirus D, includes three serotypes known to cause disease in humans: EV-D68, EV-D70, and EV-D94.9
As with poliovirus infection, most people infected with a nonpolio human enterovirus have a mild illness without distinctive features.5 In temperate climates, enteroviral infections are most common during the summer and fall and are an important cause of the “summer cold.” In tropical climates, the seasonal pattern is absent, and infections may occur throughout the year.
The clinical syndromes associated with a nonpolio human enterovirus can include nonspecific febrile illness; upper respiratory tract infection; pharyngitis; herpangina; hand, foot, and mouth syndrome; various skin exanthems; bronchiolitis; asthma exacerbation; gastrointestinal manifestations such as diarrhea and vomiting (which are especially common); more serious clinical syndromes such as hepatitis, pancreatitis, and cardiomyopathy; and neurologic illness, including aseptic meningitis, encephalitis, and polio-like paralytic disease.
Outbreaks caused by nonpolio human enteroviruses occur on a regular basis, may vary by strain from year to year, and often occur within a geographic region; multiple strains may circulate simultaneously. Occasionally, as with EV-D68 in August 2014 in the United States, epidemics can emerge suddenly and spread rapidly across the world, causing disease in hundreds or thousands of people, demonstrating the breadth of illness associated with particular strains.10
ENTEROVIRUS D68: AN EMERGING PATHOGEN
EV-D68 was first isolated in the United States from four children in Berkeley, California, who had lower respiratory tract symptoms (bronchiolitis and pneumonia) in 1962. The finding was published in the medical literature in 1967.1 Since its initial identification, EV-D68 was infrequently reported as a cause of human disease, with the US Centers for Disease Control and Prevention (CDC) listing only 26 cases in the 36 years from 1970 through 2005.11
However, the past decade has seen EV-D68 emerge as a significant respiratory pathogen, with more reports of acute respiratory illness associated with it in North America, Europe, and Asia, especially in children.12–17 A seasonal pattern may exist; a longitudinal survey of samples collected from New York City detected a focal outbreak in the fall of 2009.18
The observation that recent EV-D68 outbreaks have primarily been in children suggests that most adults have immunity to it. In this regard, seroepidemiologic studies from Finland demonstrated that most adults have neutralizing antibodies from previous infection.9
The blurred line between enteroviruses and rhinoviruses
Enteroviruses and rhinoviruses are typically distinguished on the basis of the temperature at which they grow best (rhinoviruses grow better at lower temperatures, allowing them to replicate in the nose) and their sensitivity to acidity (enteroviruses are more resistant, enabling them to survive in the stomach).
The original (“Fermon”) strain of EV-D68 isolated in 1962 was first classified as an enterovirus because it was resistant to low pH.1 However, when molecular sequencing became available, EV-D68 was found to be identical to human rhinovirus 87 (HRV87), a phylogenetic outlier among the rhinoviruses that binds to cells at a receptor site distinct from that of other human rhinoviruses.19
Thereafter, further testing showed that both EV-D68 and HRV87 isolates were sensitive to acid treatment by two different methods.4 Moreover, unlike most enteroviruses, EV-D68 behaves like a rhinovirus and grows preferentially at 33°C, the temperature of the nose.2
How enterovirus D68 enters cells
Viral surface proteins, including hemagglutinin, from certain respiratory viruses have the ability to bind sugars on cells in the nose and lungs, which facilitates viral entry and replication. EV-D68 binds specifically to alpha 2-6 sialic acid, the predominant sialic acid found in the human upper respiratory tract.19,20 The absence of EV-D68 binding affinity for alpha 2-3 sialic acid, present in ciliated epithelial cells of the lower tract, suggests that alternative mechanisms may be responsible for the severe lower respiratory disease associated with this virus.
Entry of EV-D68 into cells requires additional mediators. EV-D70 belongs to the same genetic cluster as EV-D68 and enters HeLa cells using decay-accelerating factor (DAF).21 Evidence that EV-D68 also uses DAF for cell entry comes from experiments showing that monoclonal antibodies against DAF inhibit the cytopathic effects of this virus.4 Virus-receptor interactions have been more thoroughly characterized for other enteroviruses.22 In this regard, coxsackieviruses of group B use DAF as a coreceptor. Since DAF is expressed at high levels in both epithelial and endothelial cells, it may play an important role in the induction of the viremia that precedes the infection of specific tissues such as the heart or pancreas.
Different strains exist
EV-D68 strains can be divided into three genetic groups based on the sequence of the capsid-coding VP1 region, the most variable genome region of enteroviruses.23
Investigators have explored whether emergent EV-D68 strains differ in their anti-
genicity and receptor-binding properties in comparison to the Fermon strain isolated in 1962.20 Using antisera generated from various strains of EV-D68, significant differences were observed in terms of hemagglutination inhibition and neutralization titers both between emergent strains and the original Fermon strain and among the emergent strains.
Viremia in systemic disease
Like other enteroviruses, EV-D68 has the ability to infect lymphocytes.9 This may provide a mechanism by which the virus is transported during the viremic phase to secondary target organs. Indeed, EV-D68 was detected in the serum of 12 (43%) of 28 pediatric patients with pneumonia and positive nasopharyngeal swabs.24
Interestingly, whether EV-D68 was detected in the serum varied with age. Viremia was not detected in the serum of children younger than 1 year, an observation suggesting that maternal antibodies protect against viremia.
The role of viremia in systemic disease associated with EV-D68 is intriguing, especially since delayed acquisition of polio infection beyond infancy is hypothesized to have contributed to disease severity.7
ENTEROVIRUS D68 CAUSES SEVERE LOWER RESPIRATORY DISEASE
While identification of large numbers of patients with respiratory illnesses due to EV-D68 in a single season is unique to 2014, clusters of EV-D68-related respiratory illnesses have previously been recognized.25,26
As with EV-D68 outbreaks in other parts of the world, the outbreak in the US Midwest in August 2014 primarily involved children, many of whom needed to be admitted to the hospital because of severe lower respiratory symptoms.10 In the 30 children admitted to two children’s hospitals described in the initial report, difficulty breathing, hypoxemia, and wheezing were common. A minority of patients (23%) presented with fever. Of hospitalized children, 67% required admission to the intensive care unit. Two patients required intubation, including one who required extracorporeal membrane oxygenation. Six required bilevel positive airway pressure therapy.
Cleveland Clinic experience
At Cleveland Clinic during the same time, nearly 45% of patients identified with a respiratory enterovirus infection required intensive care.
For patients previously diagnosed with asthma, chronic lung disease, or wheezing, essential supportive care measures included continuing the inhaled steroids the patients were already taking, early use of short-acting beta agonists, and, in those with previously diagnosed asthma, consideration of a systemic steroid. Many of our patients with previously diagnosed asthma had an unusually long prodrome of an increase in mild symptoms, followed by a rapid and severe decline in respiratory status.
At the later phase, supportive care measures that were needed included maintenance of hydration and monitoring of oxyhemoglobin saturation with use of supplemental oxygen as necessary, as well as close observation of clinical indicators of respiratory distress, such as development of crackles, asymmetric air exchange, and progression in wheezing or in use of accessory muscles. In an attempt to avoid invasive ventilatory support in patients with asthma or other comorbid conditions, some patients were treated with aerosolized epinephrine, ipratropium, heliox, and noninvasive positive pressure ventilatory support.
NEUROLOGIC DISEASE: ACUTE FLACCID PARALYSIS
Although EV-D68 causes primarily respiratory illness, systemic disease occurs, especially neurologic involvement.
Before the recent outbreak of EV-D68, two cases of neurologic involvement from EV-D68 were reported. The first of these, mentioned in a 2006 enterovirus surveillance report issued by the CDC, was in a young adult with acute flaccid paralysis and EV-D68 isolated from the cerebral spinal fluid.11 In the second case, from 2010, a 5-year-old boy developed fatal meningomyeloencephalitis. The child had presented with pneumonia and acute flaccid paralysis. EV-D68 was identified in his cerebral spinal fluid by polymerase chain reaction (PCR), and histopathologic study of the meninges, cerebellum, midbrain, pons, medulla, and cervical cord demonstrated extensive T-cell lymphocytic meningomyelitis and encephalitis, characterized by prominent neuronophagia in motor nuclei.27
At the same time as the recent outbreak of EV-D68 respiratory disease, neurologists throughout the United States observed an increase in the number of children with polio-like acute flaccid paralysis. On September 26, 2014, the CDC issued an alert describing acute neurologic illness with focal limb weakness of unknown etiology in children, possibly associated with EV-D68.28 The report described nine cases of an acute neurologic illness in children ages 1 through 18 years (median age, 10) hospitalized in Colorado between August 9 and September 17, 2014. Common clinical features included acute focal limb weakness and paralysis and acute cranial nerve dysfunction, with no altered mental status or seizures. Pain before the onset of weakness was also identified as a common complaint.
Specific findings on magnetic resonance imaging of the spinal cord consisted of nonenhancing lesions largely restricted to the gray matter and in most cases spanning more than one level of the spinal cord. In patients with cranial nerve dysfunction, correlating nonenhancing brainstem lesions were observed.
Most children experienced a febrile respiratory illness in the 2 weeks preceding the onset of neurologic symptoms. In most cases, cerebrospinal fluid analyses demonstrated mild or moderate pleocytosis consistent with an inflammatory or infectious process, with normal to mildly elevated protein and normal glucose levels. In six of the eight patients tested, nasopharyngeal specimens were positive for rhinovirus-enterovirus. Of the six positive specimens, at least four were typed as EV-D68.
The CDC also reported a second cluster of cases of acute flaccid paralysis with anterior myelitis on magnetic resonance imaging, in 23 children (mean age 10 years) in California from June 2012 to June 2014.29 No common cause was identified, although clinical and laboratory findings supported a viral etiology. Two patients tested positive for EV-D68 from upper respiratory tract specimens. Common features among the clinical presentations included an upper respiratory or gastrointestinal prodrome less than 10 days before the onset of the paralysis (83%), cerebrospinal fluid pleocytosis (83%), and absence of sensory deficits (78%). Ten patients (43%) also had concomitant mental status changes, and eight (34%) had cranial nerve abnormalities.
Details regarding outcomes from these paralytic illnesses remain unclear, although it would appear that time to recovery has been prolonged in many cases, and the degree of recovery remains uncertain.
TREATMENT IS SUPPORTIVE
The treatment of EV-D68 infection is mainly supportive, as no specific antiviral therapy is currently available for any of the enteroviruses. Critically ill patients require organ-specific supportive care.
Potential targets for novel antienteroviral therapies exist; some of the experimental compounds were initially evaluated for their activity against polioviruses or rhinoviruses.30
TESTING MAY HAVE A ROLE
In general, testing does not play a role in the management of patients with mild disease, but it may be indicated for epidemiologic purposes or for specific diagnosis in critically ill patients. Molecular techniques are commonly used to detect respiratory viruses from clinical samples, either as discrete tests or as a multiplex viral panel.
Since patients with EV-D68 infection typically have respiratory symptoms, the virus is generally tested for in nasal wash samples. However, depending on the clinical presentation, it may be appropriate to attempt to detect the virus from other sites using either PCR or culture.
Many clinical laboratories use real-time PCR assays designed to detect both rhinoviruses and enteroviruses, but these tests do not distinguish between the species. While more specific real-time PCR assays are available that generally distinguish rhinoviruses from enteroviruses,31 during the recent outbreak our laboratory observed that confirmed EV-D68 samples cross-reacted with rhinovirus. Most clinical laboratories do not routinely perform viral sequence analysis to specifically identify EV-D68, but this test may be obtained through state health departments and the CDC on a case-by-case basis.
Recently, the CDC’s enterovirus laboratory announced the development of a real-time PCR assay specifically for EV-D68, which may make specific detection more readily available.
INFECTION PREVENTION
The routes by which EV-D68 is transmitted are not fully understood. In contrast to most enteroviruses, which are spread in a fecal-oral manner, it is possible that EV-D68 is also spread through close respiratory or mucous contact.
For this reason, interim infection prevention guidelines issued by the CDC recommend that hospitals use droplet precautions along with contact or standard precautions, depending on the scenario.32 In our children’s hospital, we use droplet and contact precautions for hospitalized patients.
- Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol 1967; 85:297–310.
- Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–2584.
- Ishiko H, Miura R, Shimada Y, et al. Human rhinovirus 87 identified as human enterovirus 68 by VP4-based molecular diagnosis. Intervirology 2002; 45:136–141.
- Blomqvist S, Savolainen C, Raman L, Roivainen M, Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J Clin Microbiol 2002; 40:4218–4223.
- Cherry JD, Krogstad P. Enterovirus, parechoviruses, and Saffold viruses. In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hoetez PJ, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Vol 2. Seventh ed. Philadelphia: Elsevier Saunders; 2014:2051–2109.
- Rotbart HA. Enteroviral infections of the central nervous system. Clin Infect Dis 1995; 20:971–981.
- Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol 2010; 172:1213–1229.
- Santti J, Vainionpää R, Hyypiä T. Molecular detection and typing of human picornaviruses. Virus Res 1999; 62:177–183.
- Smura T, Ylipaasto P, Klemola P, et al. Cellular tropism of human enterovirus D species serotypes EV-94, EV-70, and EV-68 in vitro: implications for pathogenesis. J Med Virol 2010; 82:1940–1949.
- Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus d68 - Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:798–799.
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ 2006; 55:1–20.
- Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 2012; 93:1952–1958.
- Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. MMWR Morb Mortal Wkly Rep 2011; 60:1301–1304.
- Lauinger IL, Bible JM, Halligan EP, Aarons EJ, MacMahon E, Tong CY. Lineages, sub-lineages and variants of enterovirus 68 in recent outbreaks. PLoS One 2012; 7:e36005.
- Lu QB, Wo Y, Wang HY, et al. Detection of enterovirus 68 as one of the commonest types of enterovirus found in patients with acute respiratory tract infection in China. J Med Microbiol 2014; 63:408–414.
- Meijer A, van der Sanden S, Snijders BE, et al. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in The Netherlands in 2010. Virology 2012; 423:49–57.
- Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol 2011; 52:103–106.
- Tokarz R, Kapoor V, Wu W, et al. Longitudinal molecular microbial analysis of influenza-like illness in New York City, May 2009 through May 2010. Virol J 2011; 8:288.
- Uncapher CR, DeWitt CM, Colonno RJ. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 1991; 180:814–817.
- Imamura T, Okamoto M, Nakakita S, et al. Antigenic and receptor binding properties of enterovirus 68. J Virol 2014; 88:2374–2384.
- Karnauchow TM, Tolson DL, Harrison BA, Altman E, Lublin DM, Dimock K. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J Virol 1996; 70:5143–5152.
- Selinka HC, Wolde A, Sauter M, Kandolf R, Klingel K. Virus-receptor interactions of coxsackie B viruses and their putative influence on cardiotropism. Med Microbiol Immunol 2004; 193:127–131.
- Piralla A, Girello A, Grignani M, et al. Phylogenetic characterization of enterovirus 68 strains in patients with respiratory syndromes in Italy. J Med Virol 2014; 86:1590–1593.
- Imamura T, Suzuki A, Lupisan S, et al. Detection of enterovirus 68 in serum from pediatric patients with pneumonia and their clinical outcomes. Influenza Other Respir Viruses 2014; 8:21–24.
- Imamura T, Fuji N, Suzuki A, et al. Enterovirus 68 among children with severe acute respiratory infection, the Philippines. Emerg Infect Dis 2011; 17:1430–1435.
- Kaida A, Kubo H, Sekiguchi J, et al. Enterovirus 68 in children with acute respiratory tract infections, Osaka, Japan. Emerg Infect Dis 2011; 17:1494–1497.
- Kreuter JD, Barnes A, McCarthy JE, et al. A fatal central nervous system enterovirus 68 infection. Arch Pathol Lab Med 2011; 135:793–796.
- Pastula DM, Aliabadi N, Haynes AK, et al. Acute neurologic illness of unknown etiology in children—Colorado, August-September 2014. MMWR Morb Mortal Wkly Rep 2014; 63:901–902.
- Ayscue P, Haren KV, Sheriff H, et al. Acute flaccid paralysis with anterior myelitis—California, June 2012–June 2014. MMWR Morb Mortal Wkly Rep 2014; 63:903–906.
- Abzug MJ. The enteroviruses: problems in need of treatments. J Infect 2014; 68(suppl 1):S108–S114.
- Pierce VM, Hodinka RL. Comparison of the GenMark Diagnostics eSensor respiratory viral panel to real-time PCR for detection of respiratory viruses in children. J Clin Microbiol 2012; 50:3458–3465.
- Non-polio enterovirus infection: enterovirus D68 (EV-D68)-CDC. 2014; www.cdc.gov/non-polio-enterovirus/about/ev-d68.html. Accessed November 21, 2014.
In the fall of 2014, the United States experienced an outbreak of severe respiratory illness due to a virus of emerging importance, enterovirus D68 (EV-D68). Here, we review the features of this virus and related viruses, the clinical syndromes this virus causes, the epidemiology of the recent outbreak, and its diagnosis and treatment.
THE ENTEROVIRUSES: AN OVERVIEW
Originally identified in 1962 from the throat swab of a child with pneumonia, human EV-D68 has unique genetic and clinical features that blur the typical division between human enteroviruses and rhinoviruses.1–4 Enteroviruses and rhinoviruses are closely related species within the Picornaviridae family that are now classified together within the genus Enterovirus.5 Picornaviruses are small, nonenveloped, positive-stranded RNA viruses of medical significance.
Poliovirus: The first enterovirus discovered
The first human enterovirus to be discovered was poliovirus.6 Although sporadic cases of “infantile paralysis” occurred before the late 19th century, epidemic poliomyelitis abruptly appeared in Europe and the United States beginning around 1880. Before the introduction in 1955 of the inactivated poliovirus vaccine and then the oral poliovirus vaccine, polio was one of the most feared illnesses in the developed world. Outbreaks occurred primarily in cities during summer months. At its peak, epidemic polio killed or paralyzed more than half a million people a year.
One hypothesis to explain the sudden emergence of epidemic polio is that improved personal hygiene and public sanitation delayed the age at which children acquired this enteric infection.7 Infections acquired after infancy occurred in the absence of maternal antibodies that may have protected against the virus’s propensity to invade the nervous system.
Nonpolio human enteroviruses
In the decades since poliovirus was discovered, more than 100 nonpolio human enteroviruses have been recognized.8 This group includes the coxsackieviruses, echoviruses, and the newer numbered nonpolio human enteroviruses classified into four species, designated Human enterovirus A, B, C, and D. The last of these, Human enterovirus D, includes three serotypes known to cause disease in humans: EV-D68, EV-D70, and EV-D94.9
As with poliovirus infection, most people infected with a nonpolio human enterovirus have a mild illness without distinctive features.5 In temperate climates, enteroviral infections are most common during the summer and fall and are an important cause of the “summer cold.” In tropical climates, the seasonal pattern is absent, and infections may occur throughout the year.
The clinical syndromes associated with a nonpolio human enterovirus can include nonspecific febrile illness; upper respiratory tract infection; pharyngitis; herpangina; hand, foot, and mouth syndrome; various skin exanthems; bronchiolitis; asthma exacerbation; gastrointestinal manifestations such as diarrhea and vomiting (which are especially common); more serious clinical syndromes such as hepatitis, pancreatitis, and cardiomyopathy; and neurologic illness, including aseptic meningitis, encephalitis, and polio-like paralytic disease.
Outbreaks caused by nonpolio human enteroviruses occur on a regular basis, may vary by strain from year to year, and often occur within a geographic region; multiple strains may circulate simultaneously. Occasionally, as with EV-D68 in August 2014 in the United States, epidemics can emerge suddenly and spread rapidly across the world, causing disease in hundreds or thousands of people, demonstrating the breadth of illness associated with particular strains.10
ENTEROVIRUS D68: AN EMERGING PATHOGEN
EV-D68 was first isolated in the United States from four children in Berkeley, California, who had lower respiratory tract symptoms (bronchiolitis and pneumonia) in 1962. The finding was published in the medical literature in 1967.1 Since its initial identification, EV-D68 was infrequently reported as a cause of human disease, with the US Centers for Disease Control and Prevention (CDC) listing only 26 cases in the 36 years from 1970 through 2005.11
However, the past decade has seen EV-D68 emerge as a significant respiratory pathogen, with more reports of acute respiratory illness associated with it in North America, Europe, and Asia, especially in children.12–17 A seasonal pattern may exist; a longitudinal survey of samples collected from New York City detected a focal outbreak in the fall of 2009.18
The observation that recent EV-D68 outbreaks have primarily been in children suggests that most adults have immunity to it. In this regard, seroepidemiologic studies from Finland demonstrated that most adults have neutralizing antibodies from previous infection.9
The blurred line between enteroviruses and rhinoviruses
Enteroviruses and rhinoviruses are typically distinguished on the basis of the temperature at which they grow best (rhinoviruses grow better at lower temperatures, allowing them to replicate in the nose) and their sensitivity to acidity (enteroviruses are more resistant, enabling them to survive in the stomach).
The original (“Fermon”) strain of EV-D68 isolated in 1962 was first classified as an enterovirus because it was resistant to low pH.1 However, when molecular sequencing became available, EV-D68 was found to be identical to human rhinovirus 87 (HRV87), a phylogenetic outlier among the rhinoviruses that binds to cells at a receptor site distinct from that of other human rhinoviruses.19
Thereafter, further testing showed that both EV-D68 and HRV87 isolates were sensitive to acid treatment by two different methods.4 Moreover, unlike most enteroviruses, EV-D68 behaves like a rhinovirus and grows preferentially at 33°C, the temperature of the nose.2
How enterovirus D68 enters cells
Viral surface proteins, including hemagglutinin, from certain respiratory viruses have the ability to bind sugars on cells in the nose and lungs, which facilitates viral entry and replication. EV-D68 binds specifically to alpha 2-6 sialic acid, the predominant sialic acid found in the human upper respiratory tract.19,20 The absence of EV-D68 binding affinity for alpha 2-3 sialic acid, present in ciliated epithelial cells of the lower tract, suggests that alternative mechanisms may be responsible for the severe lower respiratory disease associated with this virus.
Entry of EV-D68 into cells requires additional mediators. EV-D70 belongs to the same genetic cluster as EV-D68 and enters HeLa cells using decay-accelerating factor (DAF).21 Evidence that EV-D68 also uses DAF for cell entry comes from experiments showing that monoclonal antibodies against DAF inhibit the cytopathic effects of this virus.4 Virus-receptor interactions have been more thoroughly characterized for other enteroviruses.22 In this regard, coxsackieviruses of group B use DAF as a coreceptor. Since DAF is expressed at high levels in both epithelial and endothelial cells, it may play an important role in the induction of the viremia that precedes the infection of specific tissues such as the heart or pancreas.
Different strains exist
EV-D68 strains can be divided into three genetic groups based on the sequence of the capsid-coding VP1 region, the most variable genome region of enteroviruses.23
Investigators have explored whether emergent EV-D68 strains differ in their anti-
genicity and receptor-binding properties in comparison to the Fermon strain isolated in 1962.20 Using antisera generated from various strains of EV-D68, significant differences were observed in terms of hemagglutination inhibition and neutralization titers both between emergent strains and the original Fermon strain and among the emergent strains.
Viremia in systemic disease
Like other enteroviruses, EV-D68 has the ability to infect lymphocytes.9 This may provide a mechanism by which the virus is transported during the viremic phase to secondary target organs. Indeed, EV-D68 was detected in the serum of 12 (43%) of 28 pediatric patients with pneumonia and positive nasopharyngeal swabs.24
Interestingly, whether EV-D68 was detected in the serum varied with age. Viremia was not detected in the serum of children younger than 1 year, an observation suggesting that maternal antibodies protect against viremia.
The role of viremia in systemic disease associated with EV-D68 is intriguing, especially since delayed acquisition of polio infection beyond infancy is hypothesized to have contributed to disease severity.7
ENTEROVIRUS D68 CAUSES SEVERE LOWER RESPIRATORY DISEASE
While identification of large numbers of patients with respiratory illnesses due to EV-D68 in a single season is unique to 2014, clusters of EV-D68-related respiratory illnesses have previously been recognized.25,26
As with EV-D68 outbreaks in other parts of the world, the outbreak in the US Midwest in August 2014 primarily involved children, many of whom needed to be admitted to the hospital because of severe lower respiratory symptoms.10 In the 30 children admitted to two children’s hospitals described in the initial report, difficulty breathing, hypoxemia, and wheezing were common. A minority of patients (23%) presented with fever. Of hospitalized children, 67% required admission to the intensive care unit. Two patients required intubation, including one who required extracorporeal membrane oxygenation. Six required bilevel positive airway pressure therapy.
Cleveland Clinic experience
At Cleveland Clinic during the same time, nearly 45% of patients identified with a respiratory enterovirus infection required intensive care.
For patients previously diagnosed with asthma, chronic lung disease, or wheezing, essential supportive care measures included continuing the inhaled steroids the patients were already taking, early use of short-acting beta agonists, and, in those with previously diagnosed asthma, consideration of a systemic steroid. Many of our patients with previously diagnosed asthma had an unusually long prodrome of an increase in mild symptoms, followed by a rapid and severe decline in respiratory status.
At the later phase, supportive care measures that were needed included maintenance of hydration and monitoring of oxyhemoglobin saturation with use of supplemental oxygen as necessary, as well as close observation of clinical indicators of respiratory distress, such as development of crackles, asymmetric air exchange, and progression in wheezing or in use of accessory muscles. In an attempt to avoid invasive ventilatory support in patients with asthma or other comorbid conditions, some patients were treated with aerosolized epinephrine, ipratropium, heliox, and noninvasive positive pressure ventilatory support.
NEUROLOGIC DISEASE: ACUTE FLACCID PARALYSIS
Although EV-D68 causes primarily respiratory illness, systemic disease occurs, especially neurologic involvement.
Before the recent outbreak of EV-D68, two cases of neurologic involvement from EV-D68 were reported. The first of these, mentioned in a 2006 enterovirus surveillance report issued by the CDC, was in a young adult with acute flaccid paralysis and EV-D68 isolated from the cerebral spinal fluid.11 In the second case, from 2010, a 5-year-old boy developed fatal meningomyeloencephalitis. The child had presented with pneumonia and acute flaccid paralysis. EV-D68 was identified in his cerebral spinal fluid by polymerase chain reaction (PCR), and histopathologic study of the meninges, cerebellum, midbrain, pons, medulla, and cervical cord demonstrated extensive T-cell lymphocytic meningomyelitis and encephalitis, characterized by prominent neuronophagia in motor nuclei.27
At the same time as the recent outbreak of EV-D68 respiratory disease, neurologists throughout the United States observed an increase in the number of children with polio-like acute flaccid paralysis. On September 26, 2014, the CDC issued an alert describing acute neurologic illness with focal limb weakness of unknown etiology in children, possibly associated with EV-D68.28 The report described nine cases of an acute neurologic illness in children ages 1 through 18 years (median age, 10) hospitalized in Colorado between August 9 and September 17, 2014. Common clinical features included acute focal limb weakness and paralysis and acute cranial nerve dysfunction, with no altered mental status or seizures. Pain before the onset of weakness was also identified as a common complaint.
Specific findings on magnetic resonance imaging of the spinal cord consisted of nonenhancing lesions largely restricted to the gray matter and in most cases spanning more than one level of the spinal cord. In patients with cranial nerve dysfunction, correlating nonenhancing brainstem lesions were observed.
Most children experienced a febrile respiratory illness in the 2 weeks preceding the onset of neurologic symptoms. In most cases, cerebrospinal fluid analyses demonstrated mild or moderate pleocytosis consistent with an inflammatory or infectious process, with normal to mildly elevated protein and normal glucose levels. In six of the eight patients tested, nasopharyngeal specimens were positive for rhinovirus-enterovirus. Of the six positive specimens, at least four were typed as EV-D68.
The CDC also reported a second cluster of cases of acute flaccid paralysis with anterior myelitis on magnetic resonance imaging, in 23 children (mean age 10 years) in California from June 2012 to June 2014.29 No common cause was identified, although clinical and laboratory findings supported a viral etiology. Two patients tested positive for EV-D68 from upper respiratory tract specimens. Common features among the clinical presentations included an upper respiratory or gastrointestinal prodrome less than 10 days before the onset of the paralysis (83%), cerebrospinal fluid pleocytosis (83%), and absence of sensory deficits (78%). Ten patients (43%) also had concomitant mental status changes, and eight (34%) had cranial nerve abnormalities.
Details regarding outcomes from these paralytic illnesses remain unclear, although it would appear that time to recovery has been prolonged in many cases, and the degree of recovery remains uncertain.
TREATMENT IS SUPPORTIVE
The treatment of EV-D68 infection is mainly supportive, as no specific antiviral therapy is currently available for any of the enteroviruses. Critically ill patients require organ-specific supportive care.
Potential targets for novel antienteroviral therapies exist; some of the experimental compounds were initially evaluated for their activity against polioviruses or rhinoviruses.30
TESTING MAY HAVE A ROLE
In general, testing does not play a role in the management of patients with mild disease, but it may be indicated for epidemiologic purposes or for specific diagnosis in critically ill patients. Molecular techniques are commonly used to detect respiratory viruses from clinical samples, either as discrete tests or as a multiplex viral panel.
Since patients with EV-D68 infection typically have respiratory symptoms, the virus is generally tested for in nasal wash samples. However, depending on the clinical presentation, it may be appropriate to attempt to detect the virus from other sites using either PCR or culture.
Many clinical laboratories use real-time PCR assays designed to detect both rhinoviruses and enteroviruses, but these tests do not distinguish between the species. While more specific real-time PCR assays are available that generally distinguish rhinoviruses from enteroviruses,31 during the recent outbreak our laboratory observed that confirmed EV-D68 samples cross-reacted with rhinovirus. Most clinical laboratories do not routinely perform viral sequence analysis to specifically identify EV-D68, but this test may be obtained through state health departments and the CDC on a case-by-case basis.
Recently, the CDC’s enterovirus laboratory announced the development of a real-time PCR assay specifically for EV-D68, which may make specific detection more readily available.
INFECTION PREVENTION
The routes by which EV-D68 is transmitted are not fully understood. In contrast to most enteroviruses, which are spread in a fecal-oral manner, it is possible that EV-D68 is also spread through close respiratory or mucous contact.
For this reason, interim infection prevention guidelines issued by the CDC recommend that hospitals use droplet precautions along with contact or standard precautions, depending on the scenario.32 In our children’s hospital, we use droplet and contact precautions for hospitalized patients.
In the fall of 2014, the United States experienced an outbreak of severe respiratory illness due to a virus of emerging importance, enterovirus D68 (EV-D68). Here, we review the features of this virus and related viruses, the clinical syndromes this virus causes, the epidemiology of the recent outbreak, and its diagnosis and treatment.
THE ENTEROVIRUSES: AN OVERVIEW
Originally identified in 1962 from the throat swab of a child with pneumonia, human EV-D68 has unique genetic and clinical features that blur the typical division between human enteroviruses and rhinoviruses.1–4 Enteroviruses and rhinoviruses are closely related species within the Picornaviridae family that are now classified together within the genus Enterovirus.5 Picornaviruses are small, nonenveloped, positive-stranded RNA viruses of medical significance.
Poliovirus: The first enterovirus discovered
The first human enterovirus to be discovered was poliovirus.6 Although sporadic cases of “infantile paralysis” occurred before the late 19th century, epidemic poliomyelitis abruptly appeared in Europe and the United States beginning around 1880. Before the introduction in 1955 of the inactivated poliovirus vaccine and then the oral poliovirus vaccine, polio was one of the most feared illnesses in the developed world. Outbreaks occurred primarily in cities during summer months. At its peak, epidemic polio killed or paralyzed more than half a million people a year.
One hypothesis to explain the sudden emergence of epidemic polio is that improved personal hygiene and public sanitation delayed the age at which children acquired this enteric infection.7 Infections acquired after infancy occurred in the absence of maternal antibodies that may have protected against the virus’s propensity to invade the nervous system.
Nonpolio human enteroviruses
In the decades since poliovirus was discovered, more than 100 nonpolio human enteroviruses have been recognized.8 This group includes the coxsackieviruses, echoviruses, and the newer numbered nonpolio human enteroviruses classified into four species, designated Human enterovirus A, B, C, and D. The last of these, Human enterovirus D, includes three serotypes known to cause disease in humans: EV-D68, EV-D70, and EV-D94.9
As with poliovirus infection, most people infected with a nonpolio human enterovirus have a mild illness without distinctive features.5 In temperate climates, enteroviral infections are most common during the summer and fall and are an important cause of the “summer cold.” In tropical climates, the seasonal pattern is absent, and infections may occur throughout the year.
The clinical syndromes associated with a nonpolio human enterovirus can include nonspecific febrile illness; upper respiratory tract infection; pharyngitis; herpangina; hand, foot, and mouth syndrome; various skin exanthems; bronchiolitis; asthma exacerbation; gastrointestinal manifestations such as diarrhea and vomiting (which are especially common); more serious clinical syndromes such as hepatitis, pancreatitis, and cardiomyopathy; and neurologic illness, including aseptic meningitis, encephalitis, and polio-like paralytic disease.
Outbreaks caused by nonpolio human enteroviruses occur on a regular basis, may vary by strain from year to year, and often occur within a geographic region; multiple strains may circulate simultaneously. Occasionally, as with EV-D68 in August 2014 in the United States, epidemics can emerge suddenly and spread rapidly across the world, causing disease in hundreds or thousands of people, demonstrating the breadth of illness associated with particular strains.10
ENTEROVIRUS D68: AN EMERGING PATHOGEN
EV-D68 was first isolated in the United States from four children in Berkeley, California, who had lower respiratory tract symptoms (bronchiolitis and pneumonia) in 1962. The finding was published in the medical literature in 1967.1 Since its initial identification, EV-D68 was infrequently reported as a cause of human disease, with the US Centers for Disease Control and Prevention (CDC) listing only 26 cases in the 36 years from 1970 through 2005.11
However, the past decade has seen EV-D68 emerge as a significant respiratory pathogen, with more reports of acute respiratory illness associated with it in North America, Europe, and Asia, especially in children.12–17 A seasonal pattern may exist; a longitudinal survey of samples collected from New York City detected a focal outbreak in the fall of 2009.18
The observation that recent EV-D68 outbreaks have primarily been in children suggests that most adults have immunity to it. In this regard, seroepidemiologic studies from Finland demonstrated that most adults have neutralizing antibodies from previous infection.9
The blurred line between enteroviruses and rhinoviruses
Enteroviruses and rhinoviruses are typically distinguished on the basis of the temperature at which they grow best (rhinoviruses grow better at lower temperatures, allowing them to replicate in the nose) and their sensitivity to acidity (enteroviruses are more resistant, enabling them to survive in the stomach).
The original (“Fermon”) strain of EV-D68 isolated in 1962 was first classified as an enterovirus because it was resistant to low pH.1 However, when molecular sequencing became available, EV-D68 was found to be identical to human rhinovirus 87 (HRV87), a phylogenetic outlier among the rhinoviruses that binds to cells at a receptor site distinct from that of other human rhinoviruses.19
Thereafter, further testing showed that both EV-D68 and HRV87 isolates were sensitive to acid treatment by two different methods.4 Moreover, unlike most enteroviruses, EV-D68 behaves like a rhinovirus and grows preferentially at 33°C, the temperature of the nose.2
How enterovirus D68 enters cells
Viral surface proteins, including hemagglutinin, from certain respiratory viruses have the ability to bind sugars on cells in the nose and lungs, which facilitates viral entry and replication. EV-D68 binds specifically to alpha 2-6 sialic acid, the predominant sialic acid found in the human upper respiratory tract.19,20 The absence of EV-D68 binding affinity for alpha 2-3 sialic acid, present in ciliated epithelial cells of the lower tract, suggests that alternative mechanisms may be responsible for the severe lower respiratory disease associated with this virus.
Entry of EV-D68 into cells requires additional mediators. EV-D70 belongs to the same genetic cluster as EV-D68 and enters HeLa cells using decay-accelerating factor (DAF).21 Evidence that EV-D68 also uses DAF for cell entry comes from experiments showing that monoclonal antibodies against DAF inhibit the cytopathic effects of this virus.4 Virus-receptor interactions have been more thoroughly characterized for other enteroviruses.22 In this regard, coxsackieviruses of group B use DAF as a coreceptor. Since DAF is expressed at high levels in both epithelial and endothelial cells, it may play an important role in the induction of the viremia that precedes the infection of specific tissues such as the heart or pancreas.
Different strains exist
EV-D68 strains can be divided into three genetic groups based on the sequence of the capsid-coding VP1 region, the most variable genome region of enteroviruses.23
Investigators have explored whether emergent EV-D68 strains differ in their anti-
genicity and receptor-binding properties in comparison to the Fermon strain isolated in 1962.20 Using antisera generated from various strains of EV-D68, significant differences were observed in terms of hemagglutination inhibition and neutralization titers both between emergent strains and the original Fermon strain and among the emergent strains.
Viremia in systemic disease
Like other enteroviruses, EV-D68 has the ability to infect lymphocytes.9 This may provide a mechanism by which the virus is transported during the viremic phase to secondary target organs. Indeed, EV-D68 was detected in the serum of 12 (43%) of 28 pediatric patients with pneumonia and positive nasopharyngeal swabs.24
Interestingly, whether EV-D68 was detected in the serum varied with age. Viremia was not detected in the serum of children younger than 1 year, an observation suggesting that maternal antibodies protect against viremia.
The role of viremia in systemic disease associated with EV-D68 is intriguing, especially since delayed acquisition of polio infection beyond infancy is hypothesized to have contributed to disease severity.7
ENTEROVIRUS D68 CAUSES SEVERE LOWER RESPIRATORY DISEASE
While identification of large numbers of patients with respiratory illnesses due to EV-D68 in a single season is unique to 2014, clusters of EV-D68-related respiratory illnesses have previously been recognized.25,26
As with EV-D68 outbreaks in other parts of the world, the outbreak in the US Midwest in August 2014 primarily involved children, many of whom needed to be admitted to the hospital because of severe lower respiratory symptoms.10 In the 30 children admitted to two children’s hospitals described in the initial report, difficulty breathing, hypoxemia, and wheezing were common. A minority of patients (23%) presented with fever. Of hospitalized children, 67% required admission to the intensive care unit. Two patients required intubation, including one who required extracorporeal membrane oxygenation. Six required bilevel positive airway pressure therapy.
Cleveland Clinic experience
At Cleveland Clinic during the same time, nearly 45% of patients identified with a respiratory enterovirus infection required intensive care.
For patients previously diagnosed with asthma, chronic lung disease, or wheezing, essential supportive care measures included continuing the inhaled steroids the patients were already taking, early use of short-acting beta agonists, and, in those with previously diagnosed asthma, consideration of a systemic steroid. Many of our patients with previously diagnosed asthma had an unusually long prodrome of an increase in mild symptoms, followed by a rapid and severe decline in respiratory status.
At the later phase, supportive care measures that were needed included maintenance of hydration and monitoring of oxyhemoglobin saturation with use of supplemental oxygen as necessary, as well as close observation of clinical indicators of respiratory distress, such as development of crackles, asymmetric air exchange, and progression in wheezing or in use of accessory muscles. In an attempt to avoid invasive ventilatory support in patients with asthma or other comorbid conditions, some patients were treated with aerosolized epinephrine, ipratropium, heliox, and noninvasive positive pressure ventilatory support.
NEUROLOGIC DISEASE: ACUTE FLACCID PARALYSIS
Although EV-D68 causes primarily respiratory illness, systemic disease occurs, especially neurologic involvement.
Before the recent outbreak of EV-D68, two cases of neurologic involvement from EV-D68 were reported. The first of these, mentioned in a 2006 enterovirus surveillance report issued by the CDC, was in a young adult with acute flaccid paralysis and EV-D68 isolated from the cerebral spinal fluid.11 In the second case, from 2010, a 5-year-old boy developed fatal meningomyeloencephalitis. The child had presented with pneumonia and acute flaccid paralysis. EV-D68 was identified in his cerebral spinal fluid by polymerase chain reaction (PCR), and histopathologic study of the meninges, cerebellum, midbrain, pons, medulla, and cervical cord demonstrated extensive T-cell lymphocytic meningomyelitis and encephalitis, characterized by prominent neuronophagia in motor nuclei.27
At the same time as the recent outbreak of EV-D68 respiratory disease, neurologists throughout the United States observed an increase in the number of children with polio-like acute flaccid paralysis. On September 26, 2014, the CDC issued an alert describing acute neurologic illness with focal limb weakness of unknown etiology in children, possibly associated with EV-D68.28 The report described nine cases of an acute neurologic illness in children ages 1 through 18 years (median age, 10) hospitalized in Colorado between August 9 and September 17, 2014. Common clinical features included acute focal limb weakness and paralysis and acute cranial nerve dysfunction, with no altered mental status or seizures. Pain before the onset of weakness was also identified as a common complaint.
Specific findings on magnetic resonance imaging of the spinal cord consisted of nonenhancing lesions largely restricted to the gray matter and in most cases spanning more than one level of the spinal cord. In patients with cranial nerve dysfunction, correlating nonenhancing brainstem lesions were observed.
Most children experienced a febrile respiratory illness in the 2 weeks preceding the onset of neurologic symptoms. In most cases, cerebrospinal fluid analyses demonstrated mild or moderate pleocytosis consistent with an inflammatory or infectious process, with normal to mildly elevated protein and normal glucose levels. In six of the eight patients tested, nasopharyngeal specimens were positive for rhinovirus-enterovirus. Of the six positive specimens, at least four were typed as EV-D68.
The CDC also reported a second cluster of cases of acute flaccid paralysis with anterior myelitis on magnetic resonance imaging, in 23 children (mean age 10 years) in California from June 2012 to June 2014.29 No common cause was identified, although clinical and laboratory findings supported a viral etiology. Two patients tested positive for EV-D68 from upper respiratory tract specimens. Common features among the clinical presentations included an upper respiratory or gastrointestinal prodrome less than 10 days before the onset of the paralysis (83%), cerebrospinal fluid pleocytosis (83%), and absence of sensory deficits (78%). Ten patients (43%) also had concomitant mental status changes, and eight (34%) had cranial nerve abnormalities.
Details regarding outcomes from these paralytic illnesses remain unclear, although it would appear that time to recovery has been prolonged in many cases, and the degree of recovery remains uncertain.
TREATMENT IS SUPPORTIVE
The treatment of EV-D68 infection is mainly supportive, as no specific antiviral therapy is currently available for any of the enteroviruses. Critically ill patients require organ-specific supportive care.
Potential targets for novel antienteroviral therapies exist; some of the experimental compounds were initially evaluated for their activity against polioviruses or rhinoviruses.30
TESTING MAY HAVE A ROLE
In general, testing does not play a role in the management of patients with mild disease, but it may be indicated for epidemiologic purposes or for specific diagnosis in critically ill patients. Molecular techniques are commonly used to detect respiratory viruses from clinical samples, either as discrete tests or as a multiplex viral panel.
Since patients with EV-D68 infection typically have respiratory symptoms, the virus is generally tested for in nasal wash samples. However, depending on the clinical presentation, it may be appropriate to attempt to detect the virus from other sites using either PCR or culture.
Many clinical laboratories use real-time PCR assays designed to detect both rhinoviruses and enteroviruses, but these tests do not distinguish between the species. While more specific real-time PCR assays are available that generally distinguish rhinoviruses from enteroviruses,31 during the recent outbreak our laboratory observed that confirmed EV-D68 samples cross-reacted with rhinovirus. Most clinical laboratories do not routinely perform viral sequence analysis to specifically identify EV-D68, but this test may be obtained through state health departments and the CDC on a case-by-case basis.
Recently, the CDC’s enterovirus laboratory announced the development of a real-time PCR assay specifically for EV-D68, which may make specific detection more readily available.
INFECTION PREVENTION
The routes by which EV-D68 is transmitted are not fully understood. In contrast to most enteroviruses, which are spread in a fecal-oral manner, it is possible that EV-D68 is also spread through close respiratory or mucous contact.
For this reason, interim infection prevention guidelines issued by the CDC recommend that hospitals use droplet precautions along with contact or standard precautions, depending on the scenario.32 In our children’s hospital, we use droplet and contact precautions for hospitalized patients.
- Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol 1967; 85:297–310.
- Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–2584.
- Ishiko H, Miura R, Shimada Y, et al. Human rhinovirus 87 identified as human enterovirus 68 by VP4-based molecular diagnosis. Intervirology 2002; 45:136–141.
- Blomqvist S, Savolainen C, Raman L, Roivainen M, Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J Clin Microbiol 2002; 40:4218–4223.
- Cherry JD, Krogstad P. Enterovirus, parechoviruses, and Saffold viruses. In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hoetez PJ, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Vol 2. Seventh ed. Philadelphia: Elsevier Saunders; 2014:2051–2109.
- Rotbart HA. Enteroviral infections of the central nervous system. Clin Infect Dis 1995; 20:971–981.
- Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol 2010; 172:1213–1229.
- Santti J, Vainionpää R, Hyypiä T. Molecular detection and typing of human picornaviruses. Virus Res 1999; 62:177–183.
- Smura T, Ylipaasto P, Klemola P, et al. Cellular tropism of human enterovirus D species serotypes EV-94, EV-70, and EV-68 in vitro: implications for pathogenesis. J Med Virol 2010; 82:1940–1949.
- Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus d68 - Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:798–799.
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ 2006; 55:1–20.
- Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 2012; 93:1952–1958.
- Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. MMWR Morb Mortal Wkly Rep 2011; 60:1301–1304.
- Lauinger IL, Bible JM, Halligan EP, Aarons EJ, MacMahon E, Tong CY. Lineages, sub-lineages and variants of enterovirus 68 in recent outbreaks. PLoS One 2012; 7:e36005.
- Lu QB, Wo Y, Wang HY, et al. Detection of enterovirus 68 as one of the commonest types of enterovirus found in patients with acute respiratory tract infection in China. J Med Microbiol 2014; 63:408–414.
- Meijer A, van der Sanden S, Snijders BE, et al. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in The Netherlands in 2010. Virology 2012; 423:49–57.
- Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol 2011; 52:103–106.
- Tokarz R, Kapoor V, Wu W, et al. Longitudinal molecular microbial analysis of influenza-like illness in New York City, May 2009 through May 2010. Virol J 2011; 8:288.
- Uncapher CR, DeWitt CM, Colonno RJ. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 1991; 180:814–817.
- Imamura T, Okamoto M, Nakakita S, et al. Antigenic and receptor binding properties of enterovirus 68. J Virol 2014; 88:2374–2384.
- Karnauchow TM, Tolson DL, Harrison BA, Altman E, Lublin DM, Dimock K. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J Virol 1996; 70:5143–5152.
- Selinka HC, Wolde A, Sauter M, Kandolf R, Klingel K. Virus-receptor interactions of coxsackie B viruses and their putative influence on cardiotropism. Med Microbiol Immunol 2004; 193:127–131.
- Piralla A, Girello A, Grignani M, et al. Phylogenetic characterization of enterovirus 68 strains in patients with respiratory syndromes in Italy. J Med Virol 2014; 86:1590–1593.
- Imamura T, Suzuki A, Lupisan S, et al. Detection of enterovirus 68 in serum from pediatric patients with pneumonia and their clinical outcomes. Influenza Other Respir Viruses 2014; 8:21–24.
- Imamura T, Fuji N, Suzuki A, et al. Enterovirus 68 among children with severe acute respiratory infection, the Philippines. Emerg Infect Dis 2011; 17:1430–1435.
- Kaida A, Kubo H, Sekiguchi J, et al. Enterovirus 68 in children with acute respiratory tract infections, Osaka, Japan. Emerg Infect Dis 2011; 17:1494–1497.
- Kreuter JD, Barnes A, McCarthy JE, et al. A fatal central nervous system enterovirus 68 infection. Arch Pathol Lab Med 2011; 135:793–796.
- Pastula DM, Aliabadi N, Haynes AK, et al. Acute neurologic illness of unknown etiology in children—Colorado, August-September 2014. MMWR Morb Mortal Wkly Rep 2014; 63:901–902.
- Ayscue P, Haren KV, Sheriff H, et al. Acute flaccid paralysis with anterior myelitis—California, June 2012–June 2014. MMWR Morb Mortal Wkly Rep 2014; 63:903–906.
- Abzug MJ. The enteroviruses: problems in need of treatments. J Infect 2014; 68(suppl 1):S108–S114.
- Pierce VM, Hodinka RL. Comparison of the GenMark Diagnostics eSensor respiratory viral panel to real-time PCR for detection of respiratory viruses in children. J Clin Microbiol 2012; 50:3458–3465.
- Non-polio enterovirus infection: enterovirus D68 (EV-D68)-CDC. 2014; www.cdc.gov/non-polio-enterovirus/about/ev-d68.html. Accessed November 21, 2014.
- Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol 1967; 85:297–310.
- Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–2584.
- Ishiko H, Miura R, Shimada Y, et al. Human rhinovirus 87 identified as human enterovirus 68 by VP4-based molecular diagnosis. Intervirology 2002; 45:136–141.
- Blomqvist S, Savolainen C, Raman L, Roivainen M, Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J Clin Microbiol 2002; 40:4218–4223.
- Cherry JD, Krogstad P. Enterovirus, parechoviruses, and Saffold viruses. In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hoetez PJ, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Vol 2. Seventh ed. Philadelphia: Elsevier Saunders; 2014:2051–2109.
- Rotbart HA. Enteroviral infections of the central nervous system. Clin Infect Dis 1995; 20:971–981.
- Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol 2010; 172:1213–1229.
- Santti J, Vainionpää R, Hyypiä T. Molecular detection and typing of human picornaviruses. Virus Res 1999; 62:177–183.
- Smura T, Ylipaasto P, Klemola P, et al. Cellular tropism of human enterovirus D species serotypes EV-94, EV-70, and EV-68 in vitro: implications for pathogenesis. J Med Virol 2010; 82:1940–1949.
- Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus d68 - Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:798–799.
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ 2006; 55:1–20.
- Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 2012; 93:1952–1958.
- Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. MMWR Morb Mortal Wkly Rep 2011; 60:1301–1304.
- Lauinger IL, Bible JM, Halligan EP, Aarons EJ, MacMahon E, Tong CY. Lineages, sub-lineages and variants of enterovirus 68 in recent outbreaks. PLoS One 2012; 7:e36005.
- Lu QB, Wo Y, Wang HY, et al. Detection of enterovirus 68 as one of the commonest types of enterovirus found in patients with acute respiratory tract infection in China. J Med Microbiol 2014; 63:408–414.
- Meijer A, van der Sanden S, Snijders BE, et al. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in The Netherlands in 2010. Virology 2012; 423:49–57.
- Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol 2011; 52:103–106.
- Tokarz R, Kapoor V, Wu W, et al. Longitudinal molecular microbial analysis of influenza-like illness in New York City, May 2009 through May 2010. Virol J 2011; 8:288.
- Uncapher CR, DeWitt CM, Colonno RJ. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 1991; 180:814–817.
- Imamura T, Okamoto M, Nakakita S, et al. Antigenic and receptor binding properties of enterovirus 68. J Virol 2014; 88:2374–2384.
- Karnauchow TM, Tolson DL, Harrison BA, Altman E, Lublin DM, Dimock K. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J Virol 1996; 70:5143–5152.
- Selinka HC, Wolde A, Sauter M, Kandolf R, Klingel K. Virus-receptor interactions of coxsackie B viruses and their putative influence on cardiotropism. Med Microbiol Immunol 2004; 193:127–131.
- Piralla A, Girello A, Grignani M, et al. Phylogenetic characterization of enterovirus 68 strains in patients with respiratory syndromes in Italy. J Med Virol 2014; 86:1590–1593.
- Imamura T, Suzuki A, Lupisan S, et al. Detection of enterovirus 68 in serum from pediatric patients with pneumonia and their clinical outcomes. Influenza Other Respir Viruses 2014; 8:21–24.
- Imamura T, Fuji N, Suzuki A, et al. Enterovirus 68 among children with severe acute respiratory infection, the Philippines. Emerg Infect Dis 2011; 17:1430–1435.
- Kaida A, Kubo H, Sekiguchi J, et al. Enterovirus 68 in children with acute respiratory tract infections, Osaka, Japan. Emerg Infect Dis 2011; 17:1494–1497.
- Kreuter JD, Barnes A, McCarthy JE, et al. A fatal central nervous system enterovirus 68 infection. Arch Pathol Lab Med 2011; 135:793–796.
- Pastula DM, Aliabadi N, Haynes AK, et al. Acute neurologic illness of unknown etiology in children—Colorado, August-September 2014. MMWR Morb Mortal Wkly Rep 2014; 63:901–902.
- Ayscue P, Haren KV, Sheriff H, et al. Acute flaccid paralysis with anterior myelitis—California, June 2012–June 2014. MMWR Morb Mortal Wkly Rep 2014; 63:903–906.
- Abzug MJ. The enteroviruses: problems in need of treatments. J Infect 2014; 68(suppl 1):S108–S114.
- Pierce VM, Hodinka RL. Comparison of the GenMark Diagnostics eSensor respiratory viral panel to real-time PCR for detection of respiratory viruses in children. J Clin Microbiol 2012; 50:3458–3465.
- Non-polio enterovirus infection: enterovirus D68 (EV-D68)-CDC. 2014; www.cdc.gov/non-polio-enterovirus/about/ev-d68.html. Accessed November 21, 2014.
KEY POINTS
- EV-D68 is a respiratory virus that has genetic and biologic features that blur the distinction between the rhinoviruses and enteroviruses.
- Recognition of EV-D68 as an important cause of viral lower respiratory tract illness in children underscores the role of specific strain typing in advancing our understanding of the epidemiology of respiratory virus infections.
- Given the inability of commonly used clinical tests for rhinovirus to distinguish EV-D68 in the absence of strain-specific sequence data, caution needs to be used in attributing severe or acute lower respiratory illness to rhinovirus and in interpreting epidemiologic associations between asthma and rhinovirus.
- Emerging data suggest that, in addition to its important role in pediatric respiratory illness, EV-D68 may cause systemic disease, especially acute neurologic disease.