User login
Primary care pediatricians are often asked about manifestations of both common and rare dermatologic disorders. They frequently encounter pediatric skin conditions, making it important to stay abreast of new developments in diagnosis and treatment. Common pediatric dermatologic diagnoses include atopic dermatitis, allergic contact dermatitis, tinea capitis, infantile hemangioma, and alopecia areata. Less common conditions include epidermal nevi and tuberous sclerosis.
ATOPIC DERMATITIS (ECZEMA)
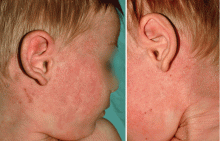
Atopic dermatitis, also referred to as eczema, is a common pediatric skin condition characterized by erythema, pruritus, scaling, lichenification, and papulovesicles (Figure 1). It affects up to 17% of children in the United States.1,2 A wide range of environmental factors, such as contact allergens, stress, food, skin flora, and humidity, affect the development and severity of atopic dermatitis. Studies also support a genetic basis; when both parents are atopic, their children have a 70% risk for developing atopic dermatitis.3
Approximately 50% of European whites have a filaggrin mutation thought to cause pediatric atopic dermatitis or eczema. The filaggrin gene (chromosome 1q21) is located in the epidermal differentiation complex and encodes profilaggrin. The defect leads to a poor protein-lipid cell envelope and a loss of the filaggrin hygroscopic amino acids that act as a natural barrier.4
Additionally, these patients have increased surface pH. This is of particular importance because at a certain pH, there is decreased inhibition of Staphylococcus aureus. Decreased activity of ceramide metabolism enzymes occurs, resulting in increased water loss along with increased entry of foreign substances.
The best treatment for atopic dermatitis is daily bathing with a mild, nondrying soap. Unfortunately, many parents purposely do not bathe their atopic children daily because they believe it is harmful. Many mild cleansers are available today that do not aggravate the condition.
Another effective treatment for eczema is to apply a moisturizer. Previously, thick ointments likely to occlude water loss were applied, but research on the pathophysiological process of atopic dermatitis has led to the development of moisturizers and topical skin products targeted to correct reduced amounts of ceramides and natural moisturizing factors in the skin with natural moisturizing factors, ceramides, and pseudoceramide products.5 A ceramide-containing moisturizer should be applied immediately after giving the child a bath. These moisturizers are available over-the-counter but can be relatively expensive. Some popular ceramide-containing skin lubricants include CeraVe cream, Cetaphil RestoraDerm cream, Aveeno eczema cream, and Eucerin eczema cream. If cost is an issue, a thick emollient such as petroleum jelly can be used.
Prevention
Several theories have been proposed to explain the development of eczema beyond genetic predisposition, leading to attempts to prevent its development. Theories include breastfeeding—both nursing and not nursing. While it seems that breastfeeding should be protective against atopic dermatitis, unfortunately there is no proof of this. Also, withholding certain foods during the introduction of solid foods (and not eating certain foods during nursing) will not decrease the risk of developing atopic dermatitis. Early exposure to farm animals has been debated as causative, and there is evidence that early exposure to antibiotics increases atopic dermatitis risk. Lastly, maternal fish oil or probiotic ingestion during pregnancy has not changed the rates of atopic dermatitis in infants.6,7 Therefore, the best course of action is to provide simple skin care guidelines and treat children as they present with atopic dermatitis.
ALLERGIC CONTACT DERMATITIS
Contrary to previously held beliefs, allergic contact dermatitis is not rare in children. In fact, rates are equal between children and adults. Allergic contact dermatitis or irritant contact dermatitis is also associated with skin barrier breakdown common among atopic dermatitis patients. Additionally, atopic dermatitis patients may exhibit allergic contact dermatitis due to exposure to various topical preparations.8
Toilet seats
Recently, allergic contact dermatitis has been reported in association with toilet seats,9 possibly due to polyurethane or polypropylene in the seat.10 Another possible reaction may be to chemicals in antiseptic wipes used on the toilet seat. The presentation of allergic contact dermatitis due to toilet seat contact is higher on the posterior thigh for older children compared to that seen in toddlers and younger children. It can be differentially diagnosed from atopic dermatitis because it is limited to the posterior leg.11
Baby wipes
Some baby-wipe brands contain the allergen methylisothiazolinone, alone or in combination with methylchloroisothiazolinone.12 Even expensive brands marketed as hypoallergenic may contain these chemicals or other types of preservatives. Interestingly, some parents continue to use baby wipes on their children’s faces and bodies as they grow up to provide a quick clean up. So, in children who are toddler age or older, allergic contact dermatitis to baby wipes may not be localized to the anogenital area. Consider this condition in the differential diagnoses with “lip licker” dermatitis.
Car seats
Car seats also are associated with contact dermatitis but the specific allergen is unknown. The presentation is similar to toilet seat dermatitis as the posterior leg is affected while the anterior leg remains clear. This type of contact dermatitis is more frequently reported with the use of car seats made of tightly woven, shiny material. A cotton cloth barrier can be placed over the car seat to prevent skin contact.13
Sports equipment
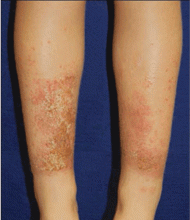
Allergic contact dermatitis may be associated with the use of shin guards and neoprene wetsuits, with p-tert butylphenol formaldehyde resin as the likely allergen.14 There is obvious delineation of the affected and nonaffected skin, which can help rule out atopic dermatitis (Figure 2). However, there is the potential for atopic dermatitis to develop with use of these items because the skin is occluded for long periods and will sweat, causing a reaction. Again, providing a barrier between the surface of the shin guard or wetsuit and the skin will help.
Metals
Finally, nickel and other metals are known sources of allergic contact dermatitis.15
TINEA CAPITIS
The challenge for this dermatologic condition is finding an effective treatment without a prolonged course of medicine. If tinea capitis continues for too long, the inflammatory process can result in permanent alopecia. The condition is caused by fungus, most commonly Trycophyton tonsurans.16 Terbinafine (3–8 mg/kg/day for 2–4 weeks) is superior to griseofulvin for T tonsurans; however, griseofulvin is superior to terbinafine for treating Microsporum species.17 Health insurance coverage of terbinafine is a concern for some families. Finally, children with tinea capitis should use a sporicidal shampoo, selenium sulfide, or ketoconazole to decrease the spread of spores.
INFANTILE HEMANGIOMAS
Propranolol
The major breakthrough in pediatric dermatology of the past decade has been the use of propranolol to treat infantile hemangiomas. Propranolol hydrochloride (Hemangeol) received US Food and Drug Administration (FDA) approval in March 2014 for the treatment of proliferating infantile hemangioma. The proposed mechanism of action is suppression of vascular endothelial growth factor and basic fibroblast growth factor in in vitro hemangioma-derived stem cells.18
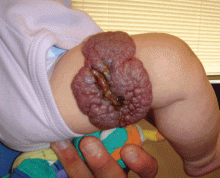
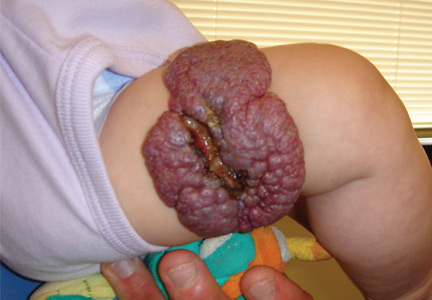
Propranolol is indicated for complex cutaneous, visceral, hepatic, and airway infantile hemangiomas. For most children, treatment with oral propranolol is preferable to prolonged treatment with systemic steroids. If propranolol is started early in the disease course, it can greatly reduce the need for extensive surgery, which is of particular importance when the nasal tip, oral mucosa, and face are affected. Children with large hemangiomas that are prone to ulceration also greatly benefit from propranolol treatment. Ulcerated hemangiomas are extremely painful and problematic, and can take months to heal (Figure 3).
Concomitant abnormalities and PHACE
A visceral evaluation is generally indicated for children with five or six lesions, but hemangioma size should also be considered when determining the need for additional tests. When a facial, scalp, or neck infantile hemangioma is larger than 5 cm, imaging studies are indicated to check for vascular anomalies in the brain and congenital heart defects (most commonly aortic problems).
A subgroup of these children have PHACE (posterior fossa anomalies, hemangioma, arterial lesions, cardiac abnormalities/aortic coarctation, and eye abnormalities) syndrome. A consensus guideline found that evidence supports treating these patients with propranolol.19 However, there have been isolated reports of acute ischemic stroke in PHACE syndrome patients on concomitant steroid therapy with severe arteriopathy. Therefore, before initiating propranolol therapy, infants with large facial hemangiomas who are at risk for PHACE should be evaluated with magnetic resonance angiography of the head and neck, and with cardiac imaging that includes the aortic arch.
Propranolol dosing
The FDA-approved dosing of propranolol hydrochloride is 1 to 3 mg/kg/day. Side effects include hypoglycemia, hypotension, exacerbation of asthma or respiratory infections, dental caries, cold extremities, and night terrors. These side effects should be closely monitored for, particularly in younger infants; however, propranolol has a relatively benign safety profile that should be taken into consideration in the risk/benefit analysis.
New pathogenesis insights
In addition to breakthrough treatment, a greater understanding of the pathogenesis of infantile hemangiomas has been attained. The most rapid lesion growth phase occurs at a younger age than was previously believed, and it is now thought to be greatest when patients are between 5.5 and 7.5 weeks old.20 Treatment initiation before the rapid growth period, rather than after, is crucial to successful outcomes.
Additionally, research has identified some of the clinical signs prior to ulceration of infantile hemangioma.21 A white central area occurring in a proliferating lesion is predictive of ulceration. This white coloration of the proliferation stage should not be mistaken for the graying of involution that occurs when infants are older than 3 months. Again, initiating early propranolol therapy should prevent some of the pain associated with hemangiomas that are likely to ulcerate.
ALOPECIA AREATA
Intralesional steroids have been the gold standard of treatment for localized alopecia areata. However, for children who cannot tolerate the injections, can topical steroids achieve enough penetrance to reduce lesion duration? A recent study compared twice-daily application of clobetasol propionate 0.05% cream or hydrocortisone 1% cream for 6 weeks on followed by 6 weeks off for 24 weeks in children with alopecia areata affecting at least 10% of scalp surface area. The clobetasol cream was superior in terms of decreasing alopetic surface area compared with hydrocortisone.22 In reference to the efficacy of clobetasol cream, only localized alopecia areata showed this response, and not alopecia totalis or universalis.
RELATIVELY RARE DIAGNOSES
Epidermal nevi
Epidermal nevi size and phenotype have guided treatment decisions when evaluating these lesions. It is believed that epidermal nevi are due to RAS/MAPK mutations, and further examination of genetic causes has become increasingly important. If the genetic mutation is known, clinical signs and genetic testing can be used to monitor children for conditions potentially related to this mosaic disorder, and to properly diagnose and treat the lesions.
Recently, researchers have been able to explain why some children with large epidermal nevi develop hypophosphatemic rickets and others do not. Mutations in HRAS or NRAS can lead to an increase in fibroblast growth factor-23, resulting in this bone abnormality.23
Tuberous sclerosis
Tuberous sclerosis (TS) is remarkable for noncancerous lesions occurring in skin, brain, kidneys, nervous system, heart, lungs, or retina. Skin presentations include patches of light-colored skin, thickened skin, growths under the nails, and facial lesions that resemble acne. Ash leaf or café au lait macules may occur, as well as large plaque angiofibromas.
While oral rapamycin has been used to treat internal tumors related to TS, a low-dose topical formula has been shown effective for treating facial angiofibromas of TS.24 This new therapy is important given the impact of facial anomalies on psychosocial development. In addition, topical rapamycin avoids the need for laser treatment, which is more painful and less effective.
SUMMARY
The understanding of common and rare skin conditions has increased during the past decade. Studies in dermatologic diagnoses and treatment have produced new insights into genetic mutations, including those involved in the development of epidermal nevi. Serendipitous findings, such as the use of propranolol for infantile hemangiomas, have also occurred. These advances have allowed for greater diagnostic accuracy and better treatments. Less invasive treatment strategies also have been developed, including clobetasol cream for localized alopecia areata and topical rapamycin for tuberous sclerosis, and these have led to greater compliance, fewer treatment-related adverse effects, and more efficacious outcomes.
- Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon school children. J Am Acad Dermatol 2000;43: 649–655.
- Hanifin JM, Reed ML; Eczema Prevalence and Impact Working Group. A population-based survey of eczema prevalence in the United States. Dermatitis 2007; 18:82–91.
- Ruzicka T. Atopic eczema between rationality and irrationality. Arch Dermatol 1998; 134:1462–1469.
- Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization through barrier-disrupted skin elicits a TH2-dominant cytokine response. Eur J Immunol 1998; 28:769–779.
- Hon KL, Leung AK, Barankin B. Barrier repair therapy in atopic dermatitis: an overview. Am J Clin Dermatol 2013; 14:389–399.
- Halken S. Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr Allergy Immunol 2004; 15(suppl 16):4–5, 9–32.
- Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years’ follow-up. Acta Paediatr Suppl 1996; 414:1–21.
- Admani S, Jacob SE. Allergic contact dermatitis in children: review of the past decade. Curr Allergy Asthma Rep 2014; 14:421.
- Holme SA, Stone NM, Mills CM. Toilet seat contact dermatitis. Pediatr Dermatol 2005; 22:344–345.
- Heilig S, Adams DR, Zaenglein AL. Persistent allergic contact dermatitis to plastic toilet seats. Pediatr Dermatol 2011; 28:587–590.
- Litvinov IV, Sugathan P, Cohen BA. Recognizing and treating toilet-seat contact dermatitis in children. Pediatrics 2010; 125:e419–e422.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis 2013; 24:2–6.
- Ghali FE. “Car seat dermatitis”: a newly described form of contact dermatitis. Pediatr Dermatol 2011; 28:321–326.
- Herro E, Jacob SE. p-tert-Butylphenol formaldehyde resin and its impact on children. Dermatitis 2012; 23:86–88.
- Malajian D, Belsito DV. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis. J Am Acad Dermatol 2013; 69:232–237.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol 2004; 50:748–752.
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol 2013; 30:1–6.
- Zhang L, Mai HM, Zheng J, et al. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol 2013; 7:48–55.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics 2013; 131:128–140.
- Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents’ photographs tell us. Pediatrics 2012; 130:e314–e320.
- Maguiness SM, Hoffman WY, McCalmont TH, Frieden IJ. Early white discoloration of infantile hemangioma: a sign of impending ulceration. Arch Dermatol 2010; 146:1235–1239.
- Lenane P, Macarthur C, Parkin PC, et al. Clobetasol propionate, 0.05%, vs hydrocortisone, 1%, for alopecia areata in children: a randomized clinical trial. JAMA Dermatol 2014; 150:47–50.
- Lim YH, Ovejero D, Sugarman JS, et al. Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Hum Mol Genet 2014; 23:397–407.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double-blind, randomized, controlled trial to evaluate the safety and efficacy of topically applied rapamycin. Drugs R D 2012; 12:121–126.
Primary care pediatricians are often asked about manifestations of both common and rare dermatologic disorders. They frequently encounter pediatric skin conditions, making it important to stay abreast of new developments in diagnosis and treatment. Common pediatric dermatologic diagnoses include atopic dermatitis, allergic contact dermatitis, tinea capitis, infantile hemangioma, and alopecia areata. Less common conditions include epidermal nevi and tuberous sclerosis.
ATOPIC DERMATITIS (ECZEMA)
Atopic dermatitis, also referred to as eczema, is a common pediatric skin condition characterized by erythema, pruritus, scaling, lichenification, and papulovesicles (Figure 1). It affects up to 17% of children in the United States.1,2 A wide range of environmental factors, such as contact allergens, stress, food, skin flora, and humidity, affect the development and severity of atopic dermatitis. Studies also support a genetic basis; when both parents are atopic, their children have a 70% risk for developing atopic dermatitis.3
Approximately 50% of European whites have a filaggrin mutation thought to cause pediatric atopic dermatitis or eczema. The filaggrin gene (chromosome 1q21) is located in the epidermal differentiation complex and encodes profilaggrin. The defect leads to a poor protein-lipid cell envelope and a loss of the filaggrin hygroscopic amino acids that act as a natural barrier.4
Additionally, these patients have increased surface pH. This is of particular importance because at a certain pH, there is decreased inhibition of Staphylococcus aureus. Decreased activity of ceramide metabolism enzymes occurs, resulting in increased water loss along with increased entry of foreign substances.
The best treatment for atopic dermatitis is daily bathing with a mild, nondrying soap. Unfortunately, many parents purposely do not bathe their atopic children daily because they believe it is harmful. Many mild cleansers are available today that do not aggravate the condition.
Another effective treatment for eczema is to apply a moisturizer. Previously, thick ointments likely to occlude water loss were applied, but research on the pathophysiological process of atopic dermatitis has led to the development of moisturizers and topical skin products targeted to correct reduced amounts of ceramides and natural moisturizing factors in the skin with natural moisturizing factors, ceramides, and pseudoceramide products.5 A ceramide-containing moisturizer should be applied immediately after giving the child a bath. These moisturizers are available over-the-counter but can be relatively expensive. Some popular ceramide-containing skin lubricants include CeraVe cream, Cetaphil RestoraDerm cream, Aveeno eczema cream, and Eucerin eczema cream. If cost is an issue, a thick emollient such as petroleum jelly can be used.
Prevention
Several theories have been proposed to explain the development of eczema beyond genetic predisposition, leading to attempts to prevent its development. Theories include breastfeeding—both nursing and not nursing. While it seems that breastfeeding should be protective against atopic dermatitis, unfortunately there is no proof of this. Also, withholding certain foods during the introduction of solid foods (and not eating certain foods during nursing) will not decrease the risk of developing atopic dermatitis. Early exposure to farm animals has been debated as causative, and there is evidence that early exposure to antibiotics increases atopic dermatitis risk. Lastly, maternal fish oil or probiotic ingestion during pregnancy has not changed the rates of atopic dermatitis in infants.6,7 Therefore, the best course of action is to provide simple skin care guidelines and treat children as they present with atopic dermatitis.
ALLERGIC CONTACT DERMATITIS
Contrary to previously held beliefs, allergic contact dermatitis is not rare in children. In fact, rates are equal between children and adults. Allergic contact dermatitis or irritant contact dermatitis is also associated with skin barrier breakdown common among atopic dermatitis patients. Additionally, atopic dermatitis patients may exhibit allergic contact dermatitis due to exposure to various topical preparations.8
Toilet seats
Recently, allergic contact dermatitis has been reported in association with toilet seats,9 possibly due to polyurethane or polypropylene in the seat.10 Another possible reaction may be to chemicals in antiseptic wipes used on the toilet seat. The presentation of allergic contact dermatitis due to toilet seat contact is higher on the posterior thigh for older children compared to that seen in toddlers and younger children. It can be differentially diagnosed from atopic dermatitis because it is limited to the posterior leg.11
Baby wipes
Some baby-wipe brands contain the allergen methylisothiazolinone, alone or in combination with methylchloroisothiazolinone.12 Even expensive brands marketed as hypoallergenic may contain these chemicals or other types of preservatives. Interestingly, some parents continue to use baby wipes on their children’s faces and bodies as they grow up to provide a quick clean up. So, in children who are toddler age or older, allergic contact dermatitis to baby wipes may not be localized to the anogenital area. Consider this condition in the differential diagnoses with “lip licker” dermatitis.
Car seats
Car seats also are associated with contact dermatitis but the specific allergen is unknown. The presentation is similar to toilet seat dermatitis as the posterior leg is affected while the anterior leg remains clear. This type of contact dermatitis is more frequently reported with the use of car seats made of tightly woven, shiny material. A cotton cloth barrier can be placed over the car seat to prevent skin contact.13
Sports equipment
Allergic contact dermatitis may be associated with the use of shin guards and neoprene wetsuits, with p-tert butylphenol formaldehyde resin as the likely allergen.14 There is obvious delineation of the affected and nonaffected skin, which can help rule out atopic dermatitis (Figure 2). However, there is the potential for atopic dermatitis to develop with use of these items because the skin is occluded for long periods and will sweat, causing a reaction. Again, providing a barrier between the surface of the shin guard or wetsuit and the skin will help.
Metals
Finally, nickel and other metals are known sources of allergic contact dermatitis.15
TINEA CAPITIS
The challenge for this dermatologic condition is finding an effective treatment without a prolonged course of medicine. If tinea capitis continues for too long, the inflammatory process can result in permanent alopecia. The condition is caused by fungus, most commonly Trycophyton tonsurans.16 Terbinafine (3–8 mg/kg/day for 2–4 weeks) is superior to griseofulvin for T tonsurans; however, griseofulvin is superior to terbinafine for treating Microsporum species.17 Health insurance coverage of terbinafine is a concern for some families. Finally, children with tinea capitis should use a sporicidal shampoo, selenium sulfide, or ketoconazole to decrease the spread of spores.
INFANTILE HEMANGIOMAS
Propranolol
The major breakthrough in pediatric dermatology of the past decade has been the use of propranolol to treat infantile hemangiomas. Propranolol hydrochloride (Hemangeol) received US Food and Drug Administration (FDA) approval in March 2014 for the treatment of proliferating infantile hemangioma. The proposed mechanism of action is suppression of vascular endothelial growth factor and basic fibroblast growth factor in in vitro hemangioma-derived stem cells.18
Propranolol is indicated for complex cutaneous, visceral, hepatic, and airway infantile hemangiomas. For most children, treatment with oral propranolol is preferable to prolonged treatment with systemic steroids. If propranolol is started early in the disease course, it can greatly reduce the need for extensive surgery, which is of particular importance when the nasal tip, oral mucosa, and face are affected. Children with large hemangiomas that are prone to ulceration also greatly benefit from propranolol treatment. Ulcerated hemangiomas are extremely painful and problematic, and can take months to heal (Figure 3).
Concomitant abnormalities and PHACE
A visceral evaluation is generally indicated for children with five or six lesions, but hemangioma size should also be considered when determining the need for additional tests. When a facial, scalp, or neck infantile hemangioma is larger than 5 cm, imaging studies are indicated to check for vascular anomalies in the brain and congenital heart defects (most commonly aortic problems).
A subgroup of these children have PHACE (posterior fossa anomalies, hemangioma, arterial lesions, cardiac abnormalities/aortic coarctation, and eye abnormalities) syndrome. A consensus guideline found that evidence supports treating these patients with propranolol.19 However, there have been isolated reports of acute ischemic stroke in PHACE syndrome patients on concomitant steroid therapy with severe arteriopathy. Therefore, before initiating propranolol therapy, infants with large facial hemangiomas who are at risk for PHACE should be evaluated with magnetic resonance angiography of the head and neck, and with cardiac imaging that includes the aortic arch.
Propranolol dosing
The FDA-approved dosing of propranolol hydrochloride is 1 to 3 mg/kg/day. Side effects include hypoglycemia, hypotension, exacerbation of asthma or respiratory infections, dental caries, cold extremities, and night terrors. These side effects should be closely monitored for, particularly in younger infants; however, propranolol has a relatively benign safety profile that should be taken into consideration in the risk/benefit analysis.
New pathogenesis insights
In addition to breakthrough treatment, a greater understanding of the pathogenesis of infantile hemangiomas has been attained. The most rapid lesion growth phase occurs at a younger age than was previously believed, and it is now thought to be greatest when patients are between 5.5 and 7.5 weeks old.20 Treatment initiation before the rapid growth period, rather than after, is crucial to successful outcomes.
Additionally, research has identified some of the clinical signs prior to ulceration of infantile hemangioma.21 A white central area occurring in a proliferating lesion is predictive of ulceration. This white coloration of the proliferation stage should not be mistaken for the graying of involution that occurs when infants are older than 3 months. Again, initiating early propranolol therapy should prevent some of the pain associated with hemangiomas that are likely to ulcerate.
ALOPECIA AREATA
Intralesional steroids have been the gold standard of treatment for localized alopecia areata. However, for children who cannot tolerate the injections, can topical steroids achieve enough penetrance to reduce lesion duration? A recent study compared twice-daily application of clobetasol propionate 0.05% cream or hydrocortisone 1% cream for 6 weeks on followed by 6 weeks off for 24 weeks in children with alopecia areata affecting at least 10% of scalp surface area. The clobetasol cream was superior in terms of decreasing alopetic surface area compared with hydrocortisone.22 In reference to the efficacy of clobetasol cream, only localized alopecia areata showed this response, and not alopecia totalis or universalis.
RELATIVELY RARE DIAGNOSES
Epidermal nevi
Epidermal nevi size and phenotype have guided treatment decisions when evaluating these lesions. It is believed that epidermal nevi are due to RAS/MAPK mutations, and further examination of genetic causes has become increasingly important. If the genetic mutation is known, clinical signs and genetic testing can be used to monitor children for conditions potentially related to this mosaic disorder, and to properly diagnose and treat the lesions.
Recently, researchers have been able to explain why some children with large epidermal nevi develop hypophosphatemic rickets and others do not. Mutations in HRAS or NRAS can lead to an increase in fibroblast growth factor-23, resulting in this bone abnormality.23
Tuberous sclerosis
Tuberous sclerosis (TS) is remarkable for noncancerous lesions occurring in skin, brain, kidneys, nervous system, heart, lungs, or retina. Skin presentations include patches of light-colored skin, thickened skin, growths under the nails, and facial lesions that resemble acne. Ash leaf or café au lait macules may occur, as well as large plaque angiofibromas.
While oral rapamycin has been used to treat internal tumors related to TS, a low-dose topical formula has been shown effective for treating facial angiofibromas of TS.24 This new therapy is important given the impact of facial anomalies on psychosocial development. In addition, topical rapamycin avoids the need for laser treatment, which is more painful and less effective.
SUMMARY
The understanding of common and rare skin conditions has increased during the past decade. Studies in dermatologic diagnoses and treatment have produced new insights into genetic mutations, including those involved in the development of epidermal nevi. Serendipitous findings, such as the use of propranolol for infantile hemangiomas, have also occurred. These advances have allowed for greater diagnostic accuracy and better treatments. Less invasive treatment strategies also have been developed, including clobetasol cream for localized alopecia areata and topical rapamycin for tuberous sclerosis, and these have led to greater compliance, fewer treatment-related adverse effects, and more efficacious outcomes.
Primary care pediatricians are often asked about manifestations of both common and rare dermatologic disorders. They frequently encounter pediatric skin conditions, making it important to stay abreast of new developments in diagnosis and treatment. Common pediatric dermatologic diagnoses include atopic dermatitis, allergic contact dermatitis, tinea capitis, infantile hemangioma, and alopecia areata. Less common conditions include epidermal nevi and tuberous sclerosis.
ATOPIC DERMATITIS (ECZEMA)
Atopic dermatitis, also referred to as eczema, is a common pediatric skin condition characterized by erythema, pruritus, scaling, lichenification, and papulovesicles (Figure 1). It affects up to 17% of children in the United States.1,2 A wide range of environmental factors, such as contact allergens, stress, food, skin flora, and humidity, affect the development and severity of atopic dermatitis. Studies also support a genetic basis; when both parents are atopic, their children have a 70% risk for developing atopic dermatitis.3
Approximately 50% of European whites have a filaggrin mutation thought to cause pediatric atopic dermatitis or eczema. The filaggrin gene (chromosome 1q21) is located in the epidermal differentiation complex and encodes profilaggrin. The defect leads to a poor protein-lipid cell envelope and a loss of the filaggrin hygroscopic amino acids that act as a natural barrier.4
Additionally, these patients have increased surface pH. This is of particular importance because at a certain pH, there is decreased inhibition of Staphylococcus aureus. Decreased activity of ceramide metabolism enzymes occurs, resulting in increased water loss along with increased entry of foreign substances.
The best treatment for atopic dermatitis is daily bathing with a mild, nondrying soap. Unfortunately, many parents purposely do not bathe their atopic children daily because they believe it is harmful. Many mild cleansers are available today that do not aggravate the condition.
Another effective treatment for eczema is to apply a moisturizer. Previously, thick ointments likely to occlude water loss were applied, but research on the pathophysiological process of atopic dermatitis has led to the development of moisturizers and topical skin products targeted to correct reduced amounts of ceramides and natural moisturizing factors in the skin with natural moisturizing factors, ceramides, and pseudoceramide products.5 A ceramide-containing moisturizer should be applied immediately after giving the child a bath. These moisturizers are available over-the-counter but can be relatively expensive. Some popular ceramide-containing skin lubricants include CeraVe cream, Cetaphil RestoraDerm cream, Aveeno eczema cream, and Eucerin eczema cream. If cost is an issue, a thick emollient such as petroleum jelly can be used.
Prevention
Several theories have been proposed to explain the development of eczema beyond genetic predisposition, leading to attempts to prevent its development. Theories include breastfeeding—both nursing and not nursing. While it seems that breastfeeding should be protective against atopic dermatitis, unfortunately there is no proof of this. Also, withholding certain foods during the introduction of solid foods (and not eating certain foods during nursing) will not decrease the risk of developing atopic dermatitis. Early exposure to farm animals has been debated as causative, and there is evidence that early exposure to antibiotics increases atopic dermatitis risk. Lastly, maternal fish oil or probiotic ingestion during pregnancy has not changed the rates of atopic dermatitis in infants.6,7 Therefore, the best course of action is to provide simple skin care guidelines and treat children as they present with atopic dermatitis.
ALLERGIC CONTACT DERMATITIS
Contrary to previously held beliefs, allergic contact dermatitis is not rare in children. In fact, rates are equal between children and adults. Allergic contact dermatitis or irritant contact dermatitis is also associated with skin barrier breakdown common among atopic dermatitis patients. Additionally, atopic dermatitis patients may exhibit allergic contact dermatitis due to exposure to various topical preparations.8
Toilet seats
Recently, allergic contact dermatitis has been reported in association with toilet seats,9 possibly due to polyurethane or polypropylene in the seat.10 Another possible reaction may be to chemicals in antiseptic wipes used on the toilet seat. The presentation of allergic contact dermatitis due to toilet seat contact is higher on the posterior thigh for older children compared to that seen in toddlers and younger children. It can be differentially diagnosed from atopic dermatitis because it is limited to the posterior leg.11
Baby wipes
Some baby-wipe brands contain the allergen methylisothiazolinone, alone or in combination with methylchloroisothiazolinone.12 Even expensive brands marketed as hypoallergenic may contain these chemicals or other types of preservatives. Interestingly, some parents continue to use baby wipes on their children’s faces and bodies as they grow up to provide a quick clean up. So, in children who are toddler age or older, allergic contact dermatitis to baby wipes may not be localized to the anogenital area. Consider this condition in the differential diagnoses with “lip licker” dermatitis.
Car seats
Car seats also are associated with contact dermatitis but the specific allergen is unknown. The presentation is similar to toilet seat dermatitis as the posterior leg is affected while the anterior leg remains clear. This type of contact dermatitis is more frequently reported with the use of car seats made of tightly woven, shiny material. A cotton cloth barrier can be placed over the car seat to prevent skin contact.13
Sports equipment
Allergic contact dermatitis may be associated with the use of shin guards and neoprene wetsuits, with p-tert butylphenol formaldehyde resin as the likely allergen.14 There is obvious delineation of the affected and nonaffected skin, which can help rule out atopic dermatitis (Figure 2). However, there is the potential for atopic dermatitis to develop with use of these items because the skin is occluded for long periods and will sweat, causing a reaction. Again, providing a barrier between the surface of the shin guard or wetsuit and the skin will help.
Metals
Finally, nickel and other metals are known sources of allergic contact dermatitis.15
TINEA CAPITIS
The challenge for this dermatologic condition is finding an effective treatment without a prolonged course of medicine. If tinea capitis continues for too long, the inflammatory process can result in permanent alopecia. The condition is caused by fungus, most commonly Trycophyton tonsurans.16 Terbinafine (3–8 mg/kg/day for 2–4 weeks) is superior to griseofulvin for T tonsurans; however, griseofulvin is superior to terbinafine for treating Microsporum species.17 Health insurance coverage of terbinafine is a concern for some families. Finally, children with tinea capitis should use a sporicidal shampoo, selenium sulfide, or ketoconazole to decrease the spread of spores.
INFANTILE HEMANGIOMAS
Propranolol
The major breakthrough in pediatric dermatology of the past decade has been the use of propranolol to treat infantile hemangiomas. Propranolol hydrochloride (Hemangeol) received US Food and Drug Administration (FDA) approval in March 2014 for the treatment of proliferating infantile hemangioma. The proposed mechanism of action is suppression of vascular endothelial growth factor and basic fibroblast growth factor in in vitro hemangioma-derived stem cells.18
Propranolol is indicated for complex cutaneous, visceral, hepatic, and airway infantile hemangiomas. For most children, treatment with oral propranolol is preferable to prolonged treatment with systemic steroids. If propranolol is started early in the disease course, it can greatly reduce the need for extensive surgery, which is of particular importance when the nasal tip, oral mucosa, and face are affected. Children with large hemangiomas that are prone to ulceration also greatly benefit from propranolol treatment. Ulcerated hemangiomas are extremely painful and problematic, and can take months to heal (Figure 3).
Concomitant abnormalities and PHACE
A visceral evaluation is generally indicated for children with five or six lesions, but hemangioma size should also be considered when determining the need for additional tests. When a facial, scalp, or neck infantile hemangioma is larger than 5 cm, imaging studies are indicated to check for vascular anomalies in the brain and congenital heart defects (most commonly aortic problems).
A subgroup of these children have PHACE (posterior fossa anomalies, hemangioma, arterial lesions, cardiac abnormalities/aortic coarctation, and eye abnormalities) syndrome. A consensus guideline found that evidence supports treating these patients with propranolol.19 However, there have been isolated reports of acute ischemic stroke in PHACE syndrome patients on concomitant steroid therapy with severe arteriopathy. Therefore, before initiating propranolol therapy, infants with large facial hemangiomas who are at risk for PHACE should be evaluated with magnetic resonance angiography of the head and neck, and with cardiac imaging that includes the aortic arch.
Propranolol dosing
The FDA-approved dosing of propranolol hydrochloride is 1 to 3 mg/kg/day. Side effects include hypoglycemia, hypotension, exacerbation of asthma or respiratory infections, dental caries, cold extremities, and night terrors. These side effects should be closely monitored for, particularly in younger infants; however, propranolol has a relatively benign safety profile that should be taken into consideration in the risk/benefit analysis.
New pathogenesis insights
In addition to breakthrough treatment, a greater understanding of the pathogenesis of infantile hemangiomas has been attained. The most rapid lesion growth phase occurs at a younger age than was previously believed, and it is now thought to be greatest when patients are between 5.5 and 7.5 weeks old.20 Treatment initiation before the rapid growth period, rather than after, is crucial to successful outcomes.
Additionally, research has identified some of the clinical signs prior to ulceration of infantile hemangioma.21 A white central area occurring in a proliferating lesion is predictive of ulceration. This white coloration of the proliferation stage should not be mistaken for the graying of involution that occurs when infants are older than 3 months. Again, initiating early propranolol therapy should prevent some of the pain associated with hemangiomas that are likely to ulcerate.
ALOPECIA AREATA
Intralesional steroids have been the gold standard of treatment for localized alopecia areata. However, for children who cannot tolerate the injections, can topical steroids achieve enough penetrance to reduce lesion duration? A recent study compared twice-daily application of clobetasol propionate 0.05% cream or hydrocortisone 1% cream for 6 weeks on followed by 6 weeks off for 24 weeks in children with alopecia areata affecting at least 10% of scalp surface area. The clobetasol cream was superior in terms of decreasing alopetic surface area compared with hydrocortisone.22 In reference to the efficacy of clobetasol cream, only localized alopecia areata showed this response, and not alopecia totalis or universalis.
RELATIVELY RARE DIAGNOSES
Epidermal nevi
Epidermal nevi size and phenotype have guided treatment decisions when evaluating these lesions. It is believed that epidermal nevi are due to RAS/MAPK mutations, and further examination of genetic causes has become increasingly important. If the genetic mutation is known, clinical signs and genetic testing can be used to monitor children for conditions potentially related to this mosaic disorder, and to properly diagnose and treat the lesions.
Recently, researchers have been able to explain why some children with large epidermal nevi develop hypophosphatemic rickets and others do not. Mutations in HRAS or NRAS can lead to an increase in fibroblast growth factor-23, resulting in this bone abnormality.23
Tuberous sclerosis
Tuberous sclerosis (TS) is remarkable for noncancerous lesions occurring in skin, brain, kidneys, nervous system, heart, lungs, or retina. Skin presentations include patches of light-colored skin, thickened skin, growths under the nails, and facial lesions that resemble acne. Ash leaf or café au lait macules may occur, as well as large plaque angiofibromas.
While oral rapamycin has been used to treat internal tumors related to TS, a low-dose topical formula has been shown effective for treating facial angiofibromas of TS.24 This new therapy is important given the impact of facial anomalies on psychosocial development. In addition, topical rapamycin avoids the need for laser treatment, which is more painful and less effective.
SUMMARY
The understanding of common and rare skin conditions has increased during the past decade. Studies in dermatologic diagnoses and treatment have produced new insights into genetic mutations, including those involved in the development of epidermal nevi. Serendipitous findings, such as the use of propranolol for infantile hemangiomas, have also occurred. These advances have allowed for greater diagnostic accuracy and better treatments. Less invasive treatment strategies also have been developed, including clobetasol cream for localized alopecia areata and topical rapamycin for tuberous sclerosis, and these have led to greater compliance, fewer treatment-related adverse effects, and more efficacious outcomes.
- Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon school children. J Am Acad Dermatol 2000;43: 649–655.
- Hanifin JM, Reed ML; Eczema Prevalence and Impact Working Group. A population-based survey of eczema prevalence in the United States. Dermatitis 2007; 18:82–91.
- Ruzicka T. Atopic eczema between rationality and irrationality. Arch Dermatol 1998; 134:1462–1469.
- Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization through barrier-disrupted skin elicits a TH2-dominant cytokine response. Eur J Immunol 1998; 28:769–779.
- Hon KL, Leung AK, Barankin B. Barrier repair therapy in atopic dermatitis: an overview. Am J Clin Dermatol 2013; 14:389–399.
- Halken S. Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr Allergy Immunol 2004; 15(suppl 16):4–5, 9–32.
- Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years’ follow-up. Acta Paediatr Suppl 1996; 414:1–21.
- Admani S, Jacob SE. Allergic contact dermatitis in children: review of the past decade. Curr Allergy Asthma Rep 2014; 14:421.
- Holme SA, Stone NM, Mills CM. Toilet seat contact dermatitis. Pediatr Dermatol 2005; 22:344–345.
- Heilig S, Adams DR, Zaenglein AL. Persistent allergic contact dermatitis to plastic toilet seats. Pediatr Dermatol 2011; 28:587–590.
- Litvinov IV, Sugathan P, Cohen BA. Recognizing and treating toilet-seat contact dermatitis in children. Pediatrics 2010; 125:e419–e422.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis 2013; 24:2–6.
- Ghali FE. “Car seat dermatitis”: a newly described form of contact dermatitis. Pediatr Dermatol 2011; 28:321–326.
- Herro E, Jacob SE. p-tert-Butylphenol formaldehyde resin and its impact on children. Dermatitis 2012; 23:86–88.
- Malajian D, Belsito DV. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis. J Am Acad Dermatol 2013; 69:232–237.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol 2004; 50:748–752.
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol 2013; 30:1–6.
- Zhang L, Mai HM, Zheng J, et al. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol 2013; 7:48–55.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics 2013; 131:128–140.
- Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents’ photographs tell us. Pediatrics 2012; 130:e314–e320.
- Maguiness SM, Hoffman WY, McCalmont TH, Frieden IJ. Early white discoloration of infantile hemangioma: a sign of impending ulceration. Arch Dermatol 2010; 146:1235–1239.
- Lenane P, Macarthur C, Parkin PC, et al. Clobetasol propionate, 0.05%, vs hydrocortisone, 1%, for alopecia areata in children: a randomized clinical trial. JAMA Dermatol 2014; 150:47–50.
- Lim YH, Ovejero D, Sugarman JS, et al. Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Hum Mol Genet 2014; 23:397–407.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double-blind, randomized, controlled trial to evaluate the safety and efficacy of topically applied rapamycin. Drugs R D 2012; 12:121–126.
- Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon school children. J Am Acad Dermatol 2000;43: 649–655.
- Hanifin JM, Reed ML; Eczema Prevalence and Impact Working Group. A population-based survey of eczema prevalence in the United States. Dermatitis 2007; 18:82–91.
- Ruzicka T. Atopic eczema between rationality and irrationality. Arch Dermatol 1998; 134:1462–1469.
- Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization through barrier-disrupted skin elicits a TH2-dominant cytokine response. Eur J Immunol 1998; 28:769–779.
- Hon KL, Leung AK, Barankin B. Barrier repair therapy in atopic dermatitis: an overview. Am J Clin Dermatol 2013; 14:389–399.
- Halken S. Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr Allergy Immunol 2004; 15(suppl 16):4–5, 9–32.
- Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years’ follow-up. Acta Paediatr Suppl 1996; 414:1–21.
- Admani S, Jacob SE. Allergic contact dermatitis in children: review of the past decade. Curr Allergy Asthma Rep 2014; 14:421.
- Holme SA, Stone NM, Mills CM. Toilet seat contact dermatitis. Pediatr Dermatol 2005; 22:344–345.
- Heilig S, Adams DR, Zaenglein AL. Persistent allergic contact dermatitis to plastic toilet seats. Pediatr Dermatol 2011; 28:587–590.
- Litvinov IV, Sugathan P, Cohen BA. Recognizing and treating toilet-seat contact dermatitis in children. Pediatrics 2010; 125:e419–e422.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis 2013; 24:2–6.
- Ghali FE. “Car seat dermatitis”: a newly described form of contact dermatitis. Pediatr Dermatol 2011; 28:321–326.
- Herro E, Jacob SE. p-tert-Butylphenol formaldehyde resin and its impact on children. Dermatitis 2012; 23:86–88.
- Malajian D, Belsito DV. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis. J Am Acad Dermatol 2013; 69:232–237.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol 2004; 50:748–752.
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol 2013; 30:1–6.
- Zhang L, Mai HM, Zheng J, et al. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol 2013; 7:48–55.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics 2013; 131:128–140.
- Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents’ photographs tell us. Pediatrics 2012; 130:e314–e320.
- Maguiness SM, Hoffman WY, McCalmont TH, Frieden IJ. Early white discoloration of infantile hemangioma: a sign of impending ulceration. Arch Dermatol 2010; 146:1235–1239.
- Lenane P, Macarthur C, Parkin PC, et al. Clobetasol propionate, 0.05%, vs hydrocortisone, 1%, for alopecia areata in children: a randomized clinical trial. JAMA Dermatol 2014; 150:47–50.
- Lim YH, Ovejero D, Sugarman JS, et al. Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Hum Mol Genet 2014; 23:397–407.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double-blind, randomized, controlled trial to evaluate the safety and efficacy of topically applied rapamycin. Drugs R D 2012; 12:121–126.