User login
Does anticoagulation prevent thrombosis for persons with fractures distal to the hip?
Low-molecular-weight heparin (LMWH) prophylaxis significantly reduces the total incidence of deep venous thrombosis (DVT) for patients with lower-limb fractures managed with surgical fixation and cast immobilization (strength of recommendation [SOR]: A, based on multiple randomized controlled studies [RCTs]). Evidence is insufficient to show whether LMWH specifically reduces the risk of clinically significant DVTs, and recommendations on its use are conflicting (SOR: C, based on expert opinion). Evidence is insufficient to recommend for or against warfarin prophylaxis for DVT in fractures distal to the hip (SOR: C, based on expert opinion).
Evidence summary
Thrombotic complications are common in lowerlimb fractures. In 1968, a prospective observational study evaluated the natural history of DVT and pulmonary embolism (PE) in tibial fractures treated with open reduction and internal fixation with early mobilization. Seventy-six consecutive patients with 79 tibial fractures were evaluated with venograms, most within 1 month of injury. The overall incidence of thrombosis was 45%. Half were minor, involving 1 to 3 of the paired deep venous trunks of the lower leg without clinical signs of embolism. Twelve patients (16%) had extensive thrombosis, involving 4 to 6 of the deep venous trunks. Three of these had nonfatal PE diagnosed clinically, and 1 had a fatal PE confirmed at autopsy. The mean age of those with extensive thrombosis or PE was 54 years, and these events were uncommon below age 25 years.1
Incidence of DVT and PE was also evaluated in a cohort of 102 unselected patients who underwent operative fixation for lower-limb fractures, excluding patella, ankle, and foot fractures. All underwent venography approximately 9 days after fixation and were followed clinically for 6 weeks. The overall incidence of DVT was 28% (40% with femoral shaft, 43% with tibial plateau, 22% with tibial shaft, and 12% with tibial plafond [distal articular tibia]). Four developed clinical evidence of PE during hospitalization but only 1 had objective confirmation. None of the patients showed clinical evidence of PE as outpatients.2
LMWH prophylaxis significantly reduced thrombosis in patients with lower-limb fractures in 3 out of 4 RCTs. The first RCT evaluated 253 patients with lower-limb fractures immobilized in plaster casts after surgical fixation. Half the patients received subcutaneous LMWH (nadroparin [Fraxiparin], a European LMWH similar to enoxaparin), and half received no thrombosis prophylaxis. Based on compression ultrasound at the time of cast removal (17 days postinjury, on average), the overall DVT incidence was 11%. Six patients (5%) receiving LMWH had DVTs vs 21 (17%) in the control group (number needed to treat [NNT]=8 to prevent 1 DVT detectible by compression ultrasound). Two thirds of patients with DVT were asymptomatic. One third had clinical signs of DVT, including 1 patient diagnosed with PE on clinical grounds. There was no difference in bleeding complications between the treatment groups.3
A second RCT evaluated LMWH (Mono-Embolex, a European LMWH) prophylaxis in 328 outpatients with lower limb injuries, which included fractures, severe contusions, and ligamentous injuries. All were treated nonsurgically with cast immobilization (mean=18.8 days, range=2–72 days) and 176 patients used daily LMWH injections. All underwent Doppler evaluation for leg thromboses after cast removal, and positive results were confirmed with venograms. Overall, there were no DVTs among the LMWH prophylaxis group and 7 DVTs (4.3%) in the group without LMWH prophylaxis (P<.006). Among those with fractures, the untreated DVT rate was 5.9% (vs 0% with LMWH prophylaxis). Those over age 40 who did not use LMWH had a DVT rate of 11.4% (vs 1.7% in younger patients). Without LMWH prophylaxis, casting for more than 10 days approximately doubled the risk of DVT compared with less than 10 days (6.1% vs 3.1%). This study did not report on the anatomic location of DVTs or if they were clinically evident.4
The third RCT evaluated reviparin (another European LMWH) vs placebo in 440 outpatients with lower limb injuries, of whom 293 had fractures. About half had surgical management and all were treated with a plaster cast or brace for an average of 44 days. Most were ambulatory with crutches. All underwent venography within a week of cast removal. The DVT rate for fracture patients using reviparin was 10.4%, vs 18.2% among those without LMWH prophylaxis (absolute risk reduction=7.8%; NNT=12.8). Three fourths of the DVTs were in distal veins, and 21% of the DVTs in the LMWH patients occurred in deep veins compared with 34% in patients without. Two pulmonary emboli occurred, both in patients without LMWH prophylaxis.5
The final RCT evaluated tinzaparin (yet another European LMWH) in 300 adult outpatients immobilized in plaster for at least 3 weeks. Most patients (205 out of 300) underwent venography, and the overall DVT rate was 10% (tinzaparin) vs 17% (controls). Among the 150 fracture patients who underwent venography, the DVT rate was 11% (tinzaparin) vs 13% (controls). This difference was not significant, probably due to insufficient numbers. None of the DVTs was clinically detectable.6
In hip fracture and hip arthroplasty, warfarin and LMWH are both effective in preventing thrombosis. No studies have specifically evaluated warfarin prophylaxis in lower extremity fractures or compared it with LMWH.
Recommendations from others
The American College of Chest Physicians (ACCP) says that LMWH prophylaxis reduces the risk of asymptomatic DVTs and is standard of care in Europe. The ACCP does not recommend thromboprophylaxis for isolated lower extremity fractures in the US because of cost and insufficient evidence of clinically important reduction in venous thromboembolism (VTE). However, ACCP lists unspecified “lower extremity or pelvic fracture” as a risk factor for VTE, and does recommend that trauma patients with at least 1 risk factor for VTE receive thromboprophylaxis. They make no recommendation about the use of warfarin.7
Although LMWH costs more than daily warfarin, it has fewer complications
Dana Nadalo, MHS, PA-C
Patricia Janki, MD, PA
Houston, Tex
LMWH has largely replaced warfarin for DVT prevention in lower extremity fractures in our clinic. Subsequently, screening for warfarin’s drug-drug interactions and measuring the PT/INR levels to adjust patient doses are no longer needed. LMHW provides effective DVT prevention without laboratory monitoring. Even though LMWH costs significantly more than daily warfarin, the complications associated with warfarin use, or no prophylaxis therapy at all, could be substantially greater. We do not typically use prophylactic anticoagulation on ankle fractures, but we do routinely put high-risk patients with tibia, fibula, and femur fractures on aspirin and LMWH. In our experience, we have not had a patient develop a DVT while on LMWH prophylaxis.
1. Hjelmstedt A, Bergvall U. Incidence of thrombosis in patients with tibial fractures. Acta Chir Scand 1968;134:209-218.
2. Abelseth G, Buckley RE, Pineo GE, Hull R, Rose MS. Incidence of deep-vein thrombosis in patients with fracture of the lower extremity distal to the hip. J Orthop Trauma 1996;10:230-235.
3. Kujath P, Spannagel U, Habscheid W. Incidence and prophylaxis of deep venous thrombosis in outpatients with injury of the lower limb. Haemostasis 1993;23 Suppl 1:20-26.
4. Kock HJ, Schmit-Neuerburg KP, Hanke J, Rudofsky G, Hirche H. Thromboprophylaxis with low-molecular-weight- heparin in out-patients with plaster-cast immobilization of the leg. Lancet 1995;346:459-461.
5. Lassen MR, Borris LC, Nakov RL. Use of the low-molecular-weight heparin reviparin to prevent deep-vein thrombosis after leg injury requiring immobilization. N Engl J Med 2002;347:726-730.
6. Jorgensen PS, Warming T, Hansen K, et al. Low molecular weight heparin (Innohep) as thromboprophylaxis in outpatients with a plaster cast: a venografic controlled study. Thrombosis Research 2002;105:477-480.
7. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous throm-boembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:338S-400S.
Low-molecular-weight heparin (LMWH) prophylaxis significantly reduces the total incidence of deep venous thrombosis (DVT) for patients with lower-limb fractures managed with surgical fixation and cast immobilization (strength of recommendation [SOR]: A, based on multiple randomized controlled studies [RCTs]). Evidence is insufficient to show whether LMWH specifically reduces the risk of clinically significant DVTs, and recommendations on its use are conflicting (SOR: C, based on expert opinion). Evidence is insufficient to recommend for or against warfarin prophylaxis for DVT in fractures distal to the hip (SOR: C, based on expert opinion).
Evidence summary
Thrombotic complications are common in lowerlimb fractures. In 1968, a prospective observational study evaluated the natural history of DVT and pulmonary embolism (PE) in tibial fractures treated with open reduction and internal fixation with early mobilization. Seventy-six consecutive patients with 79 tibial fractures were evaluated with venograms, most within 1 month of injury. The overall incidence of thrombosis was 45%. Half were minor, involving 1 to 3 of the paired deep venous trunks of the lower leg without clinical signs of embolism. Twelve patients (16%) had extensive thrombosis, involving 4 to 6 of the deep venous trunks. Three of these had nonfatal PE diagnosed clinically, and 1 had a fatal PE confirmed at autopsy. The mean age of those with extensive thrombosis or PE was 54 years, and these events were uncommon below age 25 years.1
Incidence of DVT and PE was also evaluated in a cohort of 102 unselected patients who underwent operative fixation for lower-limb fractures, excluding patella, ankle, and foot fractures. All underwent venography approximately 9 days after fixation and were followed clinically for 6 weeks. The overall incidence of DVT was 28% (40% with femoral shaft, 43% with tibial plateau, 22% with tibial shaft, and 12% with tibial plafond [distal articular tibia]). Four developed clinical evidence of PE during hospitalization but only 1 had objective confirmation. None of the patients showed clinical evidence of PE as outpatients.2
LMWH prophylaxis significantly reduced thrombosis in patients with lower-limb fractures in 3 out of 4 RCTs. The first RCT evaluated 253 patients with lower-limb fractures immobilized in plaster casts after surgical fixation. Half the patients received subcutaneous LMWH (nadroparin [Fraxiparin], a European LMWH similar to enoxaparin), and half received no thrombosis prophylaxis. Based on compression ultrasound at the time of cast removal (17 days postinjury, on average), the overall DVT incidence was 11%. Six patients (5%) receiving LMWH had DVTs vs 21 (17%) in the control group (number needed to treat [NNT]=8 to prevent 1 DVT detectible by compression ultrasound). Two thirds of patients with DVT were asymptomatic. One third had clinical signs of DVT, including 1 patient diagnosed with PE on clinical grounds. There was no difference in bleeding complications between the treatment groups.3
A second RCT evaluated LMWH (Mono-Embolex, a European LMWH) prophylaxis in 328 outpatients with lower limb injuries, which included fractures, severe contusions, and ligamentous injuries. All were treated nonsurgically with cast immobilization (mean=18.8 days, range=2–72 days) and 176 patients used daily LMWH injections. All underwent Doppler evaluation for leg thromboses after cast removal, and positive results were confirmed with venograms. Overall, there were no DVTs among the LMWH prophylaxis group and 7 DVTs (4.3%) in the group without LMWH prophylaxis (P<.006). Among those with fractures, the untreated DVT rate was 5.9% (vs 0% with LMWH prophylaxis). Those over age 40 who did not use LMWH had a DVT rate of 11.4% (vs 1.7% in younger patients). Without LMWH prophylaxis, casting for more than 10 days approximately doubled the risk of DVT compared with less than 10 days (6.1% vs 3.1%). This study did not report on the anatomic location of DVTs or if they were clinically evident.4
The third RCT evaluated reviparin (another European LMWH) vs placebo in 440 outpatients with lower limb injuries, of whom 293 had fractures. About half had surgical management and all were treated with a plaster cast or brace for an average of 44 days. Most were ambulatory with crutches. All underwent venography within a week of cast removal. The DVT rate for fracture patients using reviparin was 10.4%, vs 18.2% among those without LMWH prophylaxis (absolute risk reduction=7.8%; NNT=12.8). Three fourths of the DVTs were in distal veins, and 21% of the DVTs in the LMWH patients occurred in deep veins compared with 34% in patients without. Two pulmonary emboli occurred, both in patients without LMWH prophylaxis.5
The final RCT evaluated tinzaparin (yet another European LMWH) in 300 adult outpatients immobilized in plaster for at least 3 weeks. Most patients (205 out of 300) underwent venography, and the overall DVT rate was 10% (tinzaparin) vs 17% (controls). Among the 150 fracture patients who underwent venography, the DVT rate was 11% (tinzaparin) vs 13% (controls). This difference was not significant, probably due to insufficient numbers. None of the DVTs was clinically detectable.6
In hip fracture and hip arthroplasty, warfarin and LMWH are both effective in preventing thrombosis. No studies have specifically evaluated warfarin prophylaxis in lower extremity fractures or compared it with LMWH.
Recommendations from others
The American College of Chest Physicians (ACCP) says that LMWH prophylaxis reduces the risk of asymptomatic DVTs and is standard of care in Europe. The ACCP does not recommend thromboprophylaxis for isolated lower extremity fractures in the US because of cost and insufficient evidence of clinically important reduction in venous thromboembolism (VTE). However, ACCP lists unspecified “lower extremity or pelvic fracture” as a risk factor for VTE, and does recommend that trauma patients with at least 1 risk factor for VTE receive thromboprophylaxis. They make no recommendation about the use of warfarin.7
Although LMWH costs more than daily warfarin, it has fewer complications
Dana Nadalo, MHS, PA-C
Patricia Janki, MD, PA
Houston, Tex
LMWH has largely replaced warfarin for DVT prevention in lower extremity fractures in our clinic. Subsequently, screening for warfarin’s drug-drug interactions and measuring the PT/INR levels to adjust patient doses are no longer needed. LMHW provides effective DVT prevention without laboratory monitoring. Even though LMWH costs significantly more than daily warfarin, the complications associated with warfarin use, or no prophylaxis therapy at all, could be substantially greater. We do not typically use prophylactic anticoagulation on ankle fractures, but we do routinely put high-risk patients with tibia, fibula, and femur fractures on aspirin and LMWH. In our experience, we have not had a patient develop a DVT while on LMWH prophylaxis.
Low-molecular-weight heparin (LMWH) prophylaxis significantly reduces the total incidence of deep venous thrombosis (DVT) for patients with lower-limb fractures managed with surgical fixation and cast immobilization (strength of recommendation [SOR]: A, based on multiple randomized controlled studies [RCTs]). Evidence is insufficient to show whether LMWH specifically reduces the risk of clinically significant DVTs, and recommendations on its use are conflicting (SOR: C, based on expert opinion). Evidence is insufficient to recommend for or against warfarin prophylaxis for DVT in fractures distal to the hip (SOR: C, based on expert opinion).
Evidence summary
Thrombotic complications are common in lowerlimb fractures. In 1968, a prospective observational study evaluated the natural history of DVT and pulmonary embolism (PE) in tibial fractures treated with open reduction and internal fixation with early mobilization. Seventy-six consecutive patients with 79 tibial fractures were evaluated with venograms, most within 1 month of injury. The overall incidence of thrombosis was 45%. Half were minor, involving 1 to 3 of the paired deep venous trunks of the lower leg without clinical signs of embolism. Twelve patients (16%) had extensive thrombosis, involving 4 to 6 of the deep venous trunks. Three of these had nonfatal PE diagnosed clinically, and 1 had a fatal PE confirmed at autopsy. The mean age of those with extensive thrombosis or PE was 54 years, and these events were uncommon below age 25 years.1
Incidence of DVT and PE was also evaluated in a cohort of 102 unselected patients who underwent operative fixation for lower-limb fractures, excluding patella, ankle, and foot fractures. All underwent venography approximately 9 days after fixation and were followed clinically for 6 weeks. The overall incidence of DVT was 28% (40% with femoral shaft, 43% with tibial plateau, 22% with tibial shaft, and 12% with tibial plafond [distal articular tibia]). Four developed clinical evidence of PE during hospitalization but only 1 had objective confirmation. None of the patients showed clinical evidence of PE as outpatients.2
LMWH prophylaxis significantly reduced thrombosis in patients with lower-limb fractures in 3 out of 4 RCTs. The first RCT evaluated 253 patients with lower-limb fractures immobilized in plaster casts after surgical fixation. Half the patients received subcutaneous LMWH (nadroparin [Fraxiparin], a European LMWH similar to enoxaparin), and half received no thrombosis prophylaxis. Based on compression ultrasound at the time of cast removal (17 days postinjury, on average), the overall DVT incidence was 11%. Six patients (5%) receiving LMWH had DVTs vs 21 (17%) in the control group (number needed to treat [NNT]=8 to prevent 1 DVT detectible by compression ultrasound). Two thirds of patients with DVT were asymptomatic. One third had clinical signs of DVT, including 1 patient diagnosed with PE on clinical grounds. There was no difference in bleeding complications between the treatment groups.3
A second RCT evaluated LMWH (Mono-Embolex, a European LMWH) prophylaxis in 328 outpatients with lower limb injuries, which included fractures, severe contusions, and ligamentous injuries. All were treated nonsurgically with cast immobilization (mean=18.8 days, range=2–72 days) and 176 patients used daily LMWH injections. All underwent Doppler evaluation for leg thromboses after cast removal, and positive results were confirmed with venograms. Overall, there were no DVTs among the LMWH prophylaxis group and 7 DVTs (4.3%) in the group without LMWH prophylaxis (P<.006). Among those with fractures, the untreated DVT rate was 5.9% (vs 0% with LMWH prophylaxis). Those over age 40 who did not use LMWH had a DVT rate of 11.4% (vs 1.7% in younger patients). Without LMWH prophylaxis, casting for more than 10 days approximately doubled the risk of DVT compared with less than 10 days (6.1% vs 3.1%). This study did not report on the anatomic location of DVTs or if they were clinically evident.4
The third RCT evaluated reviparin (another European LMWH) vs placebo in 440 outpatients with lower limb injuries, of whom 293 had fractures. About half had surgical management and all were treated with a plaster cast or brace for an average of 44 days. Most were ambulatory with crutches. All underwent venography within a week of cast removal. The DVT rate for fracture patients using reviparin was 10.4%, vs 18.2% among those without LMWH prophylaxis (absolute risk reduction=7.8%; NNT=12.8). Three fourths of the DVTs were in distal veins, and 21% of the DVTs in the LMWH patients occurred in deep veins compared with 34% in patients without. Two pulmonary emboli occurred, both in patients without LMWH prophylaxis.5
The final RCT evaluated tinzaparin (yet another European LMWH) in 300 adult outpatients immobilized in plaster for at least 3 weeks. Most patients (205 out of 300) underwent venography, and the overall DVT rate was 10% (tinzaparin) vs 17% (controls). Among the 150 fracture patients who underwent venography, the DVT rate was 11% (tinzaparin) vs 13% (controls). This difference was not significant, probably due to insufficient numbers. None of the DVTs was clinically detectable.6
In hip fracture and hip arthroplasty, warfarin and LMWH are both effective in preventing thrombosis. No studies have specifically evaluated warfarin prophylaxis in lower extremity fractures or compared it with LMWH.
Recommendations from others
The American College of Chest Physicians (ACCP) says that LMWH prophylaxis reduces the risk of asymptomatic DVTs and is standard of care in Europe. The ACCP does not recommend thromboprophylaxis for isolated lower extremity fractures in the US because of cost and insufficient evidence of clinically important reduction in venous thromboembolism (VTE). However, ACCP lists unspecified “lower extremity or pelvic fracture” as a risk factor for VTE, and does recommend that trauma patients with at least 1 risk factor for VTE receive thromboprophylaxis. They make no recommendation about the use of warfarin.7
Although LMWH costs more than daily warfarin, it has fewer complications
Dana Nadalo, MHS, PA-C
Patricia Janki, MD, PA
Houston, Tex
LMWH has largely replaced warfarin for DVT prevention in lower extremity fractures in our clinic. Subsequently, screening for warfarin’s drug-drug interactions and measuring the PT/INR levels to adjust patient doses are no longer needed. LMHW provides effective DVT prevention without laboratory monitoring. Even though LMWH costs significantly more than daily warfarin, the complications associated with warfarin use, or no prophylaxis therapy at all, could be substantially greater. We do not typically use prophylactic anticoagulation on ankle fractures, but we do routinely put high-risk patients with tibia, fibula, and femur fractures on aspirin and LMWH. In our experience, we have not had a patient develop a DVT while on LMWH prophylaxis.
1. Hjelmstedt A, Bergvall U. Incidence of thrombosis in patients with tibial fractures. Acta Chir Scand 1968;134:209-218.
2. Abelseth G, Buckley RE, Pineo GE, Hull R, Rose MS. Incidence of deep-vein thrombosis in patients with fracture of the lower extremity distal to the hip. J Orthop Trauma 1996;10:230-235.
3. Kujath P, Spannagel U, Habscheid W. Incidence and prophylaxis of deep venous thrombosis in outpatients with injury of the lower limb. Haemostasis 1993;23 Suppl 1:20-26.
4. Kock HJ, Schmit-Neuerburg KP, Hanke J, Rudofsky G, Hirche H. Thromboprophylaxis with low-molecular-weight- heparin in out-patients with plaster-cast immobilization of the leg. Lancet 1995;346:459-461.
5. Lassen MR, Borris LC, Nakov RL. Use of the low-molecular-weight heparin reviparin to prevent deep-vein thrombosis after leg injury requiring immobilization. N Engl J Med 2002;347:726-730.
6. Jorgensen PS, Warming T, Hansen K, et al. Low molecular weight heparin (Innohep) as thromboprophylaxis in outpatients with a plaster cast: a venografic controlled study. Thrombosis Research 2002;105:477-480.
7. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous throm-boembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:338S-400S.
1. Hjelmstedt A, Bergvall U. Incidence of thrombosis in patients with tibial fractures. Acta Chir Scand 1968;134:209-218.
2. Abelseth G, Buckley RE, Pineo GE, Hull R, Rose MS. Incidence of deep-vein thrombosis in patients with fracture of the lower extremity distal to the hip. J Orthop Trauma 1996;10:230-235.
3. Kujath P, Spannagel U, Habscheid W. Incidence and prophylaxis of deep venous thrombosis in outpatients with injury of the lower limb. Haemostasis 1993;23 Suppl 1:20-26.
4. Kock HJ, Schmit-Neuerburg KP, Hanke J, Rudofsky G, Hirche H. Thromboprophylaxis with low-molecular-weight- heparin in out-patients with plaster-cast immobilization of the leg. Lancet 1995;346:459-461.
5. Lassen MR, Borris LC, Nakov RL. Use of the low-molecular-weight heparin reviparin to prevent deep-vein thrombosis after leg injury requiring immobilization. N Engl J Med 2002;347:726-730.
6. Jorgensen PS, Warming T, Hansen K, et al. Low molecular weight heparin (Innohep) as thromboprophylaxis in outpatients with a plaster cast: a venografic controlled study. Thrombosis Research 2002;105:477-480.
7. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous throm-boembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:338S-400S.
Evidence-based answers from the Family Physicians Inquiries Network
Can type 2 diabetes be prevented through diet and exercise?
Diets that result in long-term weight loss of 5% to 7%, along with moderate-intensity exercise for more than 150 minutes per week, reduce the incidence of type 2 diabetes for patients with impaired glucose tolerance (IGT) (strength of recommendation [SOR]: A, based on multiple randomized controlled trials [RCTs]). Each of the trials demonstrating this finding included fairly intensive counseling as part of the successful intervention. Diet and exercise reduce the incidence of diabetes in both lean (body mass index [BMI] <25) and overweight patients with IGT (SOR: B, based on a single, large RCT).
Evidence summary
Three large prospective RCTs evaluated the effect of dietary and exercise interventions in populations at risk for developing diabetes.
The Diabetes Prevention Program Research Group1 randomized 3234 patients age >24 years without diabetes but with IGT and a BMI >24 to 1 of 3 groups: intensive lifestyle modification, metformin, or control; they then compared the incidence of diabetes over 3 years. Patients were men and women from primary care populations and represented diverse ethnic backgrounds. Investigators defined IGT as plasma glucose of 140 to 200 mg/dL 2 hours after a 75-g glucose bolus when the fasting glucose was <140 mg/dL. Intensive lifestyle intervention comprised individual training sessions on a low-calorie, low-fat diet, aerobic exercise (such as brisk walking), and behavior modification. Case managers met with each participant for at least 16 sessions during the first 24 weeks and at least monthly thereafter. The control group received lifestyle change recommendations without individualized attention.
After 24 weeks, 50% of the lifestyle group met the 7% weight loss goal and 74% were exercising at least 150 minutes per week. At the final visit, 38% maintained their target weight and 58% met their exercise goal. Lifestyle intervention produced greater weight reduction and increased activity compared with the metformin and control groups, with a corresponding decreased incidence of diabetes (TABLE). Subgroup analysis found that lifestyle intervention produced the greatest reduction in diabetes (71%) for patients aged >60 years.
The Finnish Diabetes Prevention Study2 similarly randomized 522 patients, aged 40 to 65 years, with IGT and obesity (mean BMI=31) to either intensive lifestyle intervention or control and followed them for 3.2 years. The lifestyle intervention included moderate exercise for at least 150 minutes per week and weight loss of at least 5%. Patients were offered an individualized exercise plan with supervised aerobic exercise plus circuit-type resistance sessions 3 times a week. Nutritionists met with patients 7 times in the first year and every 3 months after that. Patients were counseled to increase fiber intake, reduce total fat below 30% of total calories, and reduce saturated fat below 10%. The control group was given general information on diet and exercise without individualized programs. Most patients (86%) in the intervention group met their exercise goal, and 25% met the fiber requirement.
Compared with the control group, the intervention group had greater success rate for each category. Intensive lifestyle intervention reduced the incidence of diabetes by 58% (number needed to treat=5 for 5 years; (see TABLE).
The Da Qing IGT and Diabetes Study3 divided 577 patients with IGT into 1 control and 3 intervention groups: diet, aerobic exercise, and combined diet plus aerobic exercise. Patients in this study had the lowest average BMI (25.8) of the 3 studies. The intervention group received individual and group counseling sessions at weekly intervals for 1 month, then monthly for 3 months, and then every 3 months. The control group received generalized information on IGT and diabetes but individual or group instruction was not included.
At the 6-year follow-up, the quantity of exercise was significantly higher in the exercise intervention groups, but no significant difference in caloric intake was seen among all 4 groups. The incidence of diabetes in the exercise intervention group was approximately half that in the control group overall (TABLE 1). Exercise was more effective in reducing diabetes in lean patients (BMI <25), but both lean and overweight patients benefited. The combination of diet plus exercise and diet changes also significantly reduced diabetes, although to a lesser degree.
TABLE
Incidence of diabetes among patients with impaired glucose tolerance participating in diet and exercise programs
| Study population | Mean BMI | Intervention | Diabetes incidence* | RRR | NNT |
|---|---|---|---|---|---|
| Diabetes Prevention Program1(3234 primary care patients, men and women, mixed ethnic backgrounds, various ages) | 34 | Control | 11.0 | Baseline | Baseline |
| Metformin | 7.8 | 31% | 14 (over 7 years) | ||
| Intensive lifestyle modification | 4.8 | 58% | 7 (over 7 years) | ||
| Finnish Diabetes PreventionStudy2 (522 patients) | 31 | Control | 23 | Baseline | Baseline |
| Intensive lifestyle modification | 11 | 58% | 5 (over 5 years) | ||
| Da Qing IGTand Diabetes Study3 (577 primary care patients, men and women aged >25 years) | 25.8 | Control | 15 | Baseline | Baseline (all over 6 years) |
| Diet | 10 | 31% | 17 | ||
| Exercise | 8 | 46% | 14 | ||
| Diet and exercise | 9.5 | 42% | 16 | ||
| *Incidence of diabetes per 100 person-years. | |||||
| IGT, intensive glucose control; BMI, body mass index; RRR, relative risk reduction; NNT, number needed to treat. | |||||
Recommendations from others
The American Diabetes Association recommends structured programs that emphasize lifestyle changes, including education, reduced fat and energy intake, regular physical activity, and regular participant contact. These changes can produce long-term weight loss of 5% to 7% of starting weight and reduce the risk for developing diabetes.4 They also stress the importance of promoting exercise as a vital component of the prevention as well as management of type 2 diabetes. The benefit of exercise in improving the metabolic abnormalities of type 2 diabetes is probably greatest when it is used early in its progression from insulin resistance to impaired glucose tolerance to overt hyperglycemia.5 The Exercise was more effective in reducing diabetes in lean patients, but overweight patients also benefited World Health Organization states that increased physical activity and maintaining a healthy weight play critical roles in the prevention and treatment of diabetes.6
Encourage patients to exercise and eat well, and see a dietician if they are willing
Julia Fashner, MD
St. Joseph Family Medicine Residency, South Bend, Ind
Diet and exercise are important components in the management of patients at risk for diabetes; the challenge revolves around the time and money commitment necessary for these interventions. A physician in a typical office setting has limited time to implement the interventions used in these trials. Referral to other health professionals (dietician, exercise physiatrist, etc) for counseling or individual guidance may be prohibitively costly, as these services are often not covered by insurance, and patients may not be willing to pay.
Bottom line—at every office visit, encourage patients to increase their exercise and watch what they eat as part of prevention. If they are willing to see a dietician, by all means send them.
1. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403.
2. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-1350.
3. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIIDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537-544.
4. American Diabetes Association. Evidence based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 2002;25(Suppl 1):S50-S60.
5. American Diabetes Association. Diabetes mellitus and exercise. Diabetes Care 2002;25(Suppl 1):S64-S68.
6. World Health Organization. Diet, nutrition and the prevention of chronic diseases: report of the joint WHO/FAO expert consultation. WHO Technical Report Series No. 916 (TRS 916), 2002.
Diets that result in long-term weight loss of 5% to 7%, along with moderate-intensity exercise for more than 150 minutes per week, reduce the incidence of type 2 diabetes for patients with impaired glucose tolerance (IGT) (strength of recommendation [SOR]: A, based on multiple randomized controlled trials [RCTs]). Each of the trials demonstrating this finding included fairly intensive counseling as part of the successful intervention. Diet and exercise reduce the incidence of diabetes in both lean (body mass index [BMI] <25) and overweight patients with IGT (SOR: B, based on a single, large RCT).
Evidence summary
Three large prospective RCTs evaluated the effect of dietary and exercise interventions in populations at risk for developing diabetes.
The Diabetes Prevention Program Research Group1 randomized 3234 patients age >24 years without diabetes but with IGT and a BMI >24 to 1 of 3 groups: intensive lifestyle modification, metformin, or control; they then compared the incidence of diabetes over 3 years. Patients were men and women from primary care populations and represented diverse ethnic backgrounds. Investigators defined IGT as plasma glucose of 140 to 200 mg/dL 2 hours after a 75-g glucose bolus when the fasting glucose was <140 mg/dL. Intensive lifestyle intervention comprised individual training sessions on a low-calorie, low-fat diet, aerobic exercise (such as brisk walking), and behavior modification. Case managers met with each participant for at least 16 sessions during the first 24 weeks and at least monthly thereafter. The control group received lifestyle change recommendations without individualized attention.
After 24 weeks, 50% of the lifestyle group met the 7% weight loss goal and 74% were exercising at least 150 minutes per week. At the final visit, 38% maintained their target weight and 58% met their exercise goal. Lifestyle intervention produced greater weight reduction and increased activity compared with the metformin and control groups, with a corresponding decreased incidence of diabetes (TABLE). Subgroup analysis found that lifestyle intervention produced the greatest reduction in diabetes (71%) for patients aged >60 years.
The Finnish Diabetes Prevention Study2 similarly randomized 522 patients, aged 40 to 65 years, with IGT and obesity (mean BMI=31) to either intensive lifestyle intervention or control and followed them for 3.2 years. The lifestyle intervention included moderate exercise for at least 150 minutes per week and weight loss of at least 5%. Patients were offered an individualized exercise plan with supervised aerobic exercise plus circuit-type resistance sessions 3 times a week. Nutritionists met with patients 7 times in the first year and every 3 months after that. Patients were counseled to increase fiber intake, reduce total fat below 30% of total calories, and reduce saturated fat below 10%. The control group was given general information on diet and exercise without individualized programs. Most patients (86%) in the intervention group met their exercise goal, and 25% met the fiber requirement.
Compared with the control group, the intervention group had greater success rate for each category. Intensive lifestyle intervention reduced the incidence of diabetes by 58% (number needed to treat=5 for 5 years; (see TABLE).
The Da Qing IGT and Diabetes Study3 divided 577 patients with IGT into 1 control and 3 intervention groups: diet, aerobic exercise, and combined diet plus aerobic exercise. Patients in this study had the lowest average BMI (25.8) of the 3 studies. The intervention group received individual and group counseling sessions at weekly intervals for 1 month, then monthly for 3 months, and then every 3 months. The control group received generalized information on IGT and diabetes but individual or group instruction was not included.
At the 6-year follow-up, the quantity of exercise was significantly higher in the exercise intervention groups, but no significant difference in caloric intake was seen among all 4 groups. The incidence of diabetes in the exercise intervention group was approximately half that in the control group overall (TABLE 1). Exercise was more effective in reducing diabetes in lean patients (BMI <25), but both lean and overweight patients benefited. The combination of diet plus exercise and diet changes also significantly reduced diabetes, although to a lesser degree.
TABLE
Incidence of diabetes among patients with impaired glucose tolerance participating in diet and exercise programs
| Study population | Mean BMI | Intervention | Diabetes incidence* | RRR | NNT |
|---|---|---|---|---|---|
| Diabetes Prevention Program1(3234 primary care patients, men and women, mixed ethnic backgrounds, various ages) | 34 | Control | 11.0 | Baseline | Baseline |
| Metformin | 7.8 | 31% | 14 (over 7 years) | ||
| Intensive lifestyle modification | 4.8 | 58% | 7 (over 7 years) | ||
| Finnish Diabetes PreventionStudy2 (522 patients) | 31 | Control | 23 | Baseline | Baseline |
| Intensive lifestyle modification | 11 | 58% | 5 (over 5 years) | ||
| Da Qing IGTand Diabetes Study3 (577 primary care patients, men and women aged >25 years) | 25.8 | Control | 15 | Baseline | Baseline (all over 6 years) |
| Diet | 10 | 31% | 17 | ||
| Exercise | 8 | 46% | 14 | ||
| Diet and exercise | 9.5 | 42% | 16 | ||
| *Incidence of diabetes per 100 person-years. | |||||
| IGT, intensive glucose control; BMI, body mass index; RRR, relative risk reduction; NNT, number needed to treat. | |||||
Recommendations from others
The American Diabetes Association recommends structured programs that emphasize lifestyle changes, including education, reduced fat and energy intake, regular physical activity, and regular participant contact. These changes can produce long-term weight loss of 5% to 7% of starting weight and reduce the risk for developing diabetes.4 They also stress the importance of promoting exercise as a vital component of the prevention as well as management of type 2 diabetes. The benefit of exercise in improving the metabolic abnormalities of type 2 diabetes is probably greatest when it is used early in its progression from insulin resistance to impaired glucose tolerance to overt hyperglycemia.5 The Exercise was more effective in reducing diabetes in lean patients, but overweight patients also benefited World Health Organization states that increased physical activity and maintaining a healthy weight play critical roles in the prevention and treatment of diabetes.6
Encourage patients to exercise and eat well, and see a dietician if they are willing
Julia Fashner, MD
St. Joseph Family Medicine Residency, South Bend, Ind
Diet and exercise are important components in the management of patients at risk for diabetes; the challenge revolves around the time and money commitment necessary for these interventions. A physician in a typical office setting has limited time to implement the interventions used in these trials. Referral to other health professionals (dietician, exercise physiatrist, etc) for counseling or individual guidance may be prohibitively costly, as these services are often not covered by insurance, and patients may not be willing to pay.
Bottom line—at every office visit, encourage patients to increase their exercise and watch what they eat as part of prevention. If they are willing to see a dietician, by all means send them.
Diets that result in long-term weight loss of 5% to 7%, along with moderate-intensity exercise for more than 150 minutes per week, reduce the incidence of type 2 diabetes for patients with impaired glucose tolerance (IGT) (strength of recommendation [SOR]: A, based on multiple randomized controlled trials [RCTs]). Each of the trials demonstrating this finding included fairly intensive counseling as part of the successful intervention. Diet and exercise reduce the incidence of diabetes in both lean (body mass index [BMI] <25) and overweight patients with IGT (SOR: B, based on a single, large RCT).
Evidence summary
Three large prospective RCTs evaluated the effect of dietary and exercise interventions in populations at risk for developing diabetes.
The Diabetes Prevention Program Research Group1 randomized 3234 patients age >24 years without diabetes but with IGT and a BMI >24 to 1 of 3 groups: intensive lifestyle modification, metformin, or control; they then compared the incidence of diabetes over 3 years. Patients were men and women from primary care populations and represented diverse ethnic backgrounds. Investigators defined IGT as plasma glucose of 140 to 200 mg/dL 2 hours after a 75-g glucose bolus when the fasting glucose was <140 mg/dL. Intensive lifestyle intervention comprised individual training sessions on a low-calorie, low-fat diet, aerobic exercise (such as brisk walking), and behavior modification. Case managers met with each participant for at least 16 sessions during the first 24 weeks and at least monthly thereafter. The control group received lifestyle change recommendations without individualized attention.
After 24 weeks, 50% of the lifestyle group met the 7% weight loss goal and 74% were exercising at least 150 minutes per week. At the final visit, 38% maintained their target weight and 58% met their exercise goal. Lifestyle intervention produced greater weight reduction and increased activity compared with the metformin and control groups, with a corresponding decreased incidence of diabetes (TABLE). Subgroup analysis found that lifestyle intervention produced the greatest reduction in diabetes (71%) for patients aged >60 years.
The Finnish Diabetes Prevention Study2 similarly randomized 522 patients, aged 40 to 65 years, with IGT and obesity (mean BMI=31) to either intensive lifestyle intervention or control and followed them for 3.2 years. The lifestyle intervention included moderate exercise for at least 150 minutes per week and weight loss of at least 5%. Patients were offered an individualized exercise plan with supervised aerobic exercise plus circuit-type resistance sessions 3 times a week. Nutritionists met with patients 7 times in the first year and every 3 months after that. Patients were counseled to increase fiber intake, reduce total fat below 30% of total calories, and reduce saturated fat below 10%. The control group was given general information on diet and exercise without individualized programs. Most patients (86%) in the intervention group met their exercise goal, and 25% met the fiber requirement.
Compared with the control group, the intervention group had greater success rate for each category. Intensive lifestyle intervention reduced the incidence of diabetes by 58% (number needed to treat=5 for 5 years; (see TABLE).
The Da Qing IGT and Diabetes Study3 divided 577 patients with IGT into 1 control and 3 intervention groups: diet, aerobic exercise, and combined diet plus aerobic exercise. Patients in this study had the lowest average BMI (25.8) of the 3 studies. The intervention group received individual and group counseling sessions at weekly intervals for 1 month, then monthly for 3 months, and then every 3 months. The control group received generalized information on IGT and diabetes but individual or group instruction was not included.
At the 6-year follow-up, the quantity of exercise was significantly higher in the exercise intervention groups, but no significant difference in caloric intake was seen among all 4 groups. The incidence of diabetes in the exercise intervention group was approximately half that in the control group overall (TABLE 1). Exercise was more effective in reducing diabetes in lean patients (BMI <25), but both lean and overweight patients benefited. The combination of diet plus exercise and diet changes also significantly reduced diabetes, although to a lesser degree.
TABLE
Incidence of diabetes among patients with impaired glucose tolerance participating in diet and exercise programs
| Study population | Mean BMI | Intervention | Diabetes incidence* | RRR | NNT |
|---|---|---|---|---|---|
| Diabetes Prevention Program1(3234 primary care patients, men and women, mixed ethnic backgrounds, various ages) | 34 | Control | 11.0 | Baseline | Baseline |
| Metformin | 7.8 | 31% | 14 (over 7 years) | ||
| Intensive lifestyle modification | 4.8 | 58% | 7 (over 7 years) | ||
| Finnish Diabetes PreventionStudy2 (522 patients) | 31 | Control | 23 | Baseline | Baseline |
| Intensive lifestyle modification | 11 | 58% | 5 (over 5 years) | ||
| Da Qing IGTand Diabetes Study3 (577 primary care patients, men and women aged >25 years) | 25.8 | Control | 15 | Baseline | Baseline (all over 6 years) |
| Diet | 10 | 31% | 17 | ||
| Exercise | 8 | 46% | 14 | ||
| Diet and exercise | 9.5 | 42% | 16 | ||
| *Incidence of diabetes per 100 person-years. | |||||
| IGT, intensive glucose control; BMI, body mass index; RRR, relative risk reduction; NNT, number needed to treat. | |||||
Recommendations from others
The American Diabetes Association recommends structured programs that emphasize lifestyle changes, including education, reduced fat and energy intake, regular physical activity, and regular participant contact. These changes can produce long-term weight loss of 5% to 7% of starting weight and reduce the risk for developing diabetes.4 They also stress the importance of promoting exercise as a vital component of the prevention as well as management of type 2 diabetes. The benefit of exercise in improving the metabolic abnormalities of type 2 diabetes is probably greatest when it is used early in its progression from insulin resistance to impaired glucose tolerance to overt hyperglycemia.5 The Exercise was more effective in reducing diabetes in lean patients, but overweight patients also benefited World Health Organization states that increased physical activity and maintaining a healthy weight play critical roles in the prevention and treatment of diabetes.6
Encourage patients to exercise and eat well, and see a dietician if they are willing
Julia Fashner, MD
St. Joseph Family Medicine Residency, South Bend, Ind
Diet and exercise are important components in the management of patients at risk for diabetes; the challenge revolves around the time and money commitment necessary for these interventions. A physician in a typical office setting has limited time to implement the interventions used in these trials. Referral to other health professionals (dietician, exercise physiatrist, etc) for counseling or individual guidance may be prohibitively costly, as these services are often not covered by insurance, and patients may not be willing to pay.
Bottom line—at every office visit, encourage patients to increase their exercise and watch what they eat as part of prevention. If they are willing to see a dietician, by all means send them.
1. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403.
2. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-1350.
3. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIIDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537-544.
4. American Diabetes Association. Evidence based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 2002;25(Suppl 1):S50-S60.
5. American Diabetes Association. Diabetes mellitus and exercise. Diabetes Care 2002;25(Suppl 1):S64-S68.
6. World Health Organization. Diet, nutrition and the prevention of chronic diseases: report of the joint WHO/FAO expert consultation. WHO Technical Report Series No. 916 (TRS 916), 2002.
1. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403.
2. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-1350.
3. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIIDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537-544.
4. American Diabetes Association. Evidence based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 2002;25(Suppl 1):S50-S60.
5. American Diabetes Association. Diabetes mellitus and exercise. Diabetes Care 2002;25(Suppl 1):S64-S68.
6. World Health Organization. Diet, nutrition and the prevention of chronic diseases: report of the joint WHO/FAO expert consultation. WHO Technical Report Series No. 916 (TRS 916), 2002.
Evidence-based answers from the Family Physicians Inquiries Network
Is antibiotic prophylaxis effective for recurrent acute otitis media?
For children who have recurrent episodes of clinically diagnosed acute otitis media (AOM), antibiotic prophylaxis significantly reduces recurrence, although the effect is not large (strength of recommendation: A–, based on 1 systematic review of randomized controlled trials [RCTs] with below-average quality and 1 subsequent RCT with conflicting results). Evidence is insufficient to suggest which antibiotic is most appropriate, the optimal length of prophylaxis, or the number of episodes of AOM needed to justify prophylactic treatment. Possible harms of antibiotics include vomiting, diarrhea, rash, and infection with antibiotic-resistant organisms.
Evidence summary
A systematic review of antibiotic prophylaxis for recurrent AOM examined 9 RCTs with a total of 958 children. Recurrent AOM was defined as 3 or more episodes per 6 to 18 months. The studies were low to moderate in quality (mean methodologic quality score of 11.8 out of 29 possible points). The most commonly used antibiotics were amoxicillin, cotrimoxazole, and sulfamethoxazole, given for 3 to 24 months (dosing not reported).
Children taking antibiotics had 0.11 (95% confidence interval [CI], 0.03–0.19) fewer episodes of recurrent AOM per patient-month than those taking placebo. The rate in the control group was 0.19 (95% CI, 0.13–0.26). Nine children would have to be treated per month to prevent 1 ear infection (NNT=9; 95% CI, 5–33). Only 2 of the 9 studies had statistically significant results; both used sulfisoxazole for 10 to 12 weeks and were of similar methodologic quality (12.5 out 29 points).
A trend towards a better outcome in studies that used sulfisoxazole did not reach significance compared with those using other medications (ie, ampicillin, amoxicillin, cotrimoxazole). Shorter treatment intervals (<6 months) trended toward being more effective than longer intervals, but this also did not reach significance. Children with more frequent episodes of AOM did no better than those with less frequent episodes.1
Since that review was published, another study of prophylaxis for ear infections had been published. This randomized, double blind, placebo-controlled study enrolled 194 children aged 3 months to 6 years with at least 3 documented AOM episodes in the preceding 6 months. The children were given amoxicillin (20 mg/kg/d) either once daily (n=55) or divided twice daily (n=44) or placebo (n=59). Excluding 36 noncompliant subjects, the percentages without a recurrent episode were 63% for the placebo group, 64% for the once-daily amoxicillin group, and 61% for the twice-daily amoxicillin group. There was no significant difference in the incidence of new AOM episodes among the children in the 3 groups.2
A review article states: “Many children with acute otitis media do not benefit from antimicrobial therapy because the cause of their illness is not bacterial or the infection is cleared by the immune system without use of a drug. At present, we do not have clinical criteria for distinguishing which children are in need of antibiotic therapy for AOM.”3 The lack of criteria for determining which children need antibiotic therapy for AOM makes it more difficult to select children for antibiotic prophylaxis against recurrent AOM.
Recommendations from others
The American Academy of Pediatrics and the American Academy of Family Physicians do not address antibiotic prophylaxis for recurrent episodes of otitis media in their guidelines. Both groups recommend modification of risk factors to decrease recurrent AOM, including promoting breastfeeding during the first 6 months, avoiding bottle-propping, reducing or eliminating pacifier use in the second 6 months of life, and eliminating exposure to secondhand smoke.
They also recommend pneumococcal conjugate vaccine to reduce vaccine-serotype pneumococcal otitis and live-attenuated influenza vaccine during respiratory virus season for children aged >2 years.
Treatment options include observation, antibiotic prophylaxis, tympanostomy tubes; no option is ideal for all
Alex Krist, MD
Fairfax Family Practice Residency, Virginia Commonwealth University, Fairfax, Va
Treatment options for children with recurrent acute otitis media include observation with treatment of recurrences, antibiotic prophylaxis, or tympanostomy tubes. No option is ideal for all children.
Multiple factors can be weighed to choose more or less aggressive treatment including frequency and severity of infections, exposure to secondhand smoke, day care enrollment, sibling history, parental comfort and anxiety, presence of serous otitis media between episodes, time of year, and effect on overall hearing. Measures to prevent otitis media and reserving the diagnosis of acute otitis media for “true” purulent infections can help limit the number of children diagnosed with recurrent disease.
1. Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA 1993;270:1344-1351.
2. Roark R, Berman S. Continuous twice daily or once daily amoxicillin prophylaxis compared with placebo for children with recurrent acute otitis media. Pediatr Infect Dis 1997;16:376-381.
3. Pichichero ME. Acute otitis media: part II. Treatment in an era of increasing antibiotic resistance. Am Fam Physician 2000;61:2410-2416.
4. American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics 2004;113:1451-1465.
For children who have recurrent episodes of clinically diagnosed acute otitis media (AOM), antibiotic prophylaxis significantly reduces recurrence, although the effect is not large (strength of recommendation: A–, based on 1 systematic review of randomized controlled trials [RCTs] with below-average quality and 1 subsequent RCT with conflicting results). Evidence is insufficient to suggest which antibiotic is most appropriate, the optimal length of prophylaxis, or the number of episodes of AOM needed to justify prophylactic treatment. Possible harms of antibiotics include vomiting, diarrhea, rash, and infection with antibiotic-resistant organisms.
Evidence summary
A systematic review of antibiotic prophylaxis for recurrent AOM examined 9 RCTs with a total of 958 children. Recurrent AOM was defined as 3 or more episodes per 6 to 18 months. The studies were low to moderate in quality (mean methodologic quality score of 11.8 out of 29 possible points). The most commonly used antibiotics were amoxicillin, cotrimoxazole, and sulfamethoxazole, given for 3 to 24 months (dosing not reported).
Children taking antibiotics had 0.11 (95% confidence interval [CI], 0.03–0.19) fewer episodes of recurrent AOM per patient-month than those taking placebo. The rate in the control group was 0.19 (95% CI, 0.13–0.26). Nine children would have to be treated per month to prevent 1 ear infection (NNT=9; 95% CI, 5–33). Only 2 of the 9 studies had statistically significant results; both used sulfisoxazole for 10 to 12 weeks and were of similar methodologic quality (12.5 out 29 points).
A trend towards a better outcome in studies that used sulfisoxazole did not reach significance compared with those using other medications (ie, ampicillin, amoxicillin, cotrimoxazole). Shorter treatment intervals (<6 months) trended toward being more effective than longer intervals, but this also did not reach significance. Children with more frequent episodes of AOM did no better than those with less frequent episodes.1
Since that review was published, another study of prophylaxis for ear infections had been published. This randomized, double blind, placebo-controlled study enrolled 194 children aged 3 months to 6 years with at least 3 documented AOM episodes in the preceding 6 months. The children were given amoxicillin (20 mg/kg/d) either once daily (n=55) or divided twice daily (n=44) or placebo (n=59). Excluding 36 noncompliant subjects, the percentages without a recurrent episode were 63% for the placebo group, 64% for the once-daily amoxicillin group, and 61% for the twice-daily amoxicillin group. There was no significant difference in the incidence of new AOM episodes among the children in the 3 groups.2
A review article states: “Many children with acute otitis media do not benefit from antimicrobial therapy because the cause of their illness is not bacterial or the infection is cleared by the immune system without use of a drug. At present, we do not have clinical criteria for distinguishing which children are in need of antibiotic therapy for AOM.”3 The lack of criteria for determining which children need antibiotic therapy for AOM makes it more difficult to select children for antibiotic prophylaxis against recurrent AOM.
Recommendations from others
The American Academy of Pediatrics and the American Academy of Family Physicians do not address antibiotic prophylaxis for recurrent episodes of otitis media in their guidelines. Both groups recommend modification of risk factors to decrease recurrent AOM, including promoting breastfeeding during the first 6 months, avoiding bottle-propping, reducing or eliminating pacifier use in the second 6 months of life, and eliminating exposure to secondhand smoke.
They also recommend pneumococcal conjugate vaccine to reduce vaccine-serotype pneumococcal otitis and live-attenuated influenza vaccine during respiratory virus season for children aged >2 years.
Treatment options include observation, antibiotic prophylaxis, tympanostomy tubes; no option is ideal for all
Alex Krist, MD
Fairfax Family Practice Residency, Virginia Commonwealth University, Fairfax, Va
Treatment options for children with recurrent acute otitis media include observation with treatment of recurrences, antibiotic prophylaxis, or tympanostomy tubes. No option is ideal for all children.
Multiple factors can be weighed to choose more or less aggressive treatment including frequency and severity of infections, exposure to secondhand smoke, day care enrollment, sibling history, parental comfort and anxiety, presence of serous otitis media between episodes, time of year, and effect on overall hearing. Measures to prevent otitis media and reserving the diagnosis of acute otitis media for “true” purulent infections can help limit the number of children diagnosed with recurrent disease.
For children who have recurrent episodes of clinically diagnosed acute otitis media (AOM), antibiotic prophylaxis significantly reduces recurrence, although the effect is not large (strength of recommendation: A–, based on 1 systematic review of randomized controlled trials [RCTs] with below-average quality and 1 subsequent RCT with conflicting results). Evidence is insufficient to suggest which antibiotic is most appropriate, the optimal length of prophylaxis, or the number of episodes of AOM needed to justify prophylactic treatment. Possible harms of antibiotics include vomiting, diarrhea, rash, and infection with antibiotic-resistant organisms.
Evidence summary
A systematic review of antibiotic prophylaxis for recurrent AOM examined 9 RCTs with a total of 958 children. Recurrent AOM was defined as 3 or more episodes per 6 to 18 months. The studies were low to moderate in quality (mean methodologic quality score of 11.8 out of 29 possible points). The most commonly used antibiotics were amoxicillin, cotrimoxazole, and sulfamethoxazole, given for 3 to 24 months (dosing not reported).
Children taking antibiotics had 0.11 (95% confidence interval [CI], 0.03–0.19) fewer episodes of recurrent AOM per patient-month than those taking placebo. The rate in the control group was 0.19 (95% CI, 0.13–0.26). Nine children would have to be treated per month to prevent 1 ear infection (NNT=9; 95% CI, 5–33). Only 2 of the 9 studies had statistically significant results; both used sulfisoxazole for 10 to 12 weeks and were of similar methodologic quality (12.5 out 29 points).
A trend towards a better outcome in studies that used sulfisoxazole did not reach significance compared with those using other medications (ie, ampicillin, amoxicillin, cotrimoxazole). Shorter treatment intervals (<6 months) trended toward being more effective than longer intervals, but this also did not reach significance. Children with more frequent episodes of AOM did no better than those with less frequent episodes.1
Since that review was published, another study of prophylaxis for ear infections had been published. This randomized, double blind, placebo-controlled study enrolled 194 children aged 3 months to 6 years with at least 3 documented AOM episodes in the preceding 6 months. The children were given amoxicillin (20 mg/kg/d) either once daily (n=55) or divided twice daily (n=44) or placebo (n=59). Excluding 36 noncompliant subjects, the percentages without a recurrent episode were 63% for the placebo group, 64% for the once-daily amoxicillin group, and 61% for the twice-daily amoxicillin group. There was no significant difference in the incidence of new AOM episodes among the children in the 3 groups.2
A review article states: “Many children with acute otitis media do not benefit from antimicrobial therapy because the cause of their illness is not bacterial or the infection is cleared by the immune system without use of a drug. At present, we do not have clinical criteria for distinguishing which children are in need of antibiotic therapy for AOM.”3 The lack of criteria for determining which children need antibiotic therapy for AOM makes it more difficult to select children for antibiotic prophylaxis against recurrent AOM.
Recommendations from others
The American Academy of Pediatrics and the American Academy of Family Physicians do not address antibiotic prophylaxis for recurrent episodes of otitis media in their guidelines. Both groups recommend modification of risk factors to decrease recurrent AOM, including promoting breastfeeding during the first 6 months, avoiding bottle-propping, reducing or eliminating pacifier use in the second 6 months of life, and eliminating exposure to secondhand smoke.
They also recommend pneumococcal conjugate vaccine to reduce vaccine-serotype pneumococcal otitis and live-attenuated influenza vaccine during respiratory virus season for children aged >2 years.
Treatment options include observation, antibiotic prophylaxis, tympanostomy tubes; no option is ideal for all
Alex Krist, MD
Fairfax Family Practice Residency, Virginia Commonwealth University, Fairfax, Va
Treatment options for children with recurrent acute otitis media include observation with treatment of recurrences, antibiotic prophylaxis, or tympanostomy tubes. No option is ideal for all children.
Multiple factors can be weighed to choose more or less aggressive treatment including frequency and severity of infections, exposure to secondhand smoke, day care enrollment, sibling history, parental comfort and anxiety, presence of serous otitis media between episodes, time of year, and effect on overall hearing. Measures to prevent otitis media and reserving the diagnosis of acute otitis media for “true” purulent infections can help limit the number of children diagnosed with recurrent disease.
1. Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA 1993;270:1344-1351.
2. Roark R, Berman S. Continuous twice daily or once daily amoxicillin prophylaxis compared with placebo for children with recurrent acute otitis media. Pediatr Infect Dis 1997;16:376-381.
3. Pichichero ME. Acute otitis media: part II. Treatment in an era of increasing antibiotic resistance. Am Fam Physician 2000;61:2410-2416.
4. American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics 2004;113:1451-1465.
1. Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA 1993;270:1344-1351.
2. Roark R, Berman S. Continuous twice daily or once daily amoxicillin prophylaxis compared with placebo for children with recurrent acute otitis media. Pediatr Infect Dis 1997;16:376-381.
3. Pichichero ME. Acute otitis media: part II. Treatment in an era of increasing antibiotic resistance. Am Fam Physician 2000;61:2410-2416.
4. American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics 2004;113:1451-1465.
Evidence-based answers from the Family Physicians Inquiries Network
How effective is gastric bypass for weight loss?
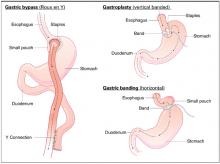
Gastric bypass results in weight loss of approximately 33% at 2 years and 25% at 8 years (strength of recommendation [SOR]: B, based on a cohort study). Gastric bypass is one type of bariatric surgery, which also includes gastroplasty and gastric banding procedures ( Figure 1 ). These procedures all can produce enough weight loss to measurably improve health, but they differ in the amount of long-term weight loss, as well as side effects, which can be serious.
Gastric bypass is more effective than gastroplasty for weight loss and is associated with fewer revisions, but it has more side effects (SOR: A, based on a systematic review). Limited evidence suggests that gastric bypass produces more weight loss than gastric banding (SOR: B, based on a cohort study).
Bariatric surgery, including gastric bypass, improves conditions comorbid with obesity, including diabetes, abnormal lipid profiles, and low quality-of-life scores. It decreases the incidence of hypertension at 2 years after surgery, but whether this effect is sustained is unclear (SOR: B, based on a cohort study and multiple case series). Bariatric surgery also improves obstructive sleep apnea, obesity hypoventilation syndrome, menstrual irregularity, and female urinary stress incontinence (SOR: C, based on multiple case series). Bariatric surgery has a complication rate of 13% and a mortality rate of 0.2% (SOR: B, based on 1 cohort study).
FIGURE 1
Bariatric surgical techniques for weight loss
Evidence summary
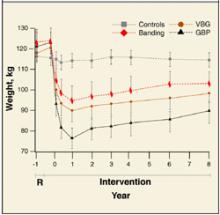
A systematic review comparing bariatric surgery with conventional medical therapy for obesity included 1 randomized controlled trial and the Swedish Obesity Study, a large cohort study with matched controls. Surgery produced 23 to 28 kg more weight loss at 2 years.1 The study demonstrated 33% ± 10% weight loss for gastric bypass and 0% for medical therapy (not described) at 2 years,2 and 25% ± 6% loss vs 0.9% gain at 8 years.3 Among bariatric surgical techniques, patients undergoing gastric bypass lost more weight than those with gastroplasty (using staples to partition the stomach, either horizontally or vertically ( Figure 1 ) (P=.057, not significant) or gastric banding (placing a constricting ring around the stomach) (P<.05) at 8 years.3
The same systematic review assessed multiple randomized controlled trials comparing gastric bypass with gastroplasty and found greater weight loss, fewer revisions, and more side effects from gastric bypass ( Figure 2 ).1 Five trials comparing gastric bypass with horizontal gastroplasty demonstrated significantly greater weight loss from gastric bypass. Five other trials comparing weight loss from gastric bypass with vertical gastroplasty produced mixed results, with 3 trials favoring gastric bypass and 2 showing no difference.1 Fewer patients required revision after gastric bypass (0%–4%) compared with vertical gastroplasty (9%) or horizontal gastroplasty (19%–40%). One included trial found that postoperative dumping syndrome (28% vs 0%, P<0.05) and heartburn (59% vs 32%, P<.05) were more common with gastric bypass than with gastroplasty.1
Bariatric surgery, including gastric bypass, improves a variety of obesity-related comorbid conditions. Diabetes prevalence decreased among gastric bypass patients at 2 years (0.0% vs 4.7%, P<0.005) and 8 years (3.6% vs 18.5%, P<.0005) compared with those receiving medical therapy.2,3 In a case series involving 154 diabetic gastric bypass patients, diabetes resolved for 83% by 1 year, and for 86% at 5 to 7 years.4 In several case series, most patients became euglycemic and discontinued insulin or oral agents.
In the Swedish Obesity Study, hypertriglyceridemia decreased postoperatively but hypercholesterolemia did not.5 In a case series, bariatric surgery reduced triglycerides (50%) as well as total cholesterol (15%) (P<.05 for both) at 6 months and significantly increased high-density lipoprotein cholesterol levels at 1 and 5 years.6
Bariatric surgery significantly lowered the incidence of hypertension at 2 years (3.2%) compared with conventional treatment (9.9%), but after 8 years this difference disappeared.2,3,5 However, in multiple large case series with morbidly obese patients, hypertension resolved or improved. The largest study showed resolution of hypertension for 69% at 1 to 2 years (91% follow-up), 66% at 5 to 7 years (50% follow-up), and 51% at 10 to 12 years (37% follow-up).4
Bariatric surgery improved obstructive sleep apnea and obesity hypoventilation syndrome in 2 case series. In one, Epworth Sleepiness Scale scores, minimum O2 saturation, and other measures improved significantly (P<.001) by 3 to 21 months after surgery.7
In another case series, menstrual irregularities decreased from 40.4% to 4.6% following surgery (P<.001) among women who lost 50% of their excess weight.8 The incidence of urinary stress incontinence also decreased significantly (61.2% to 11.6%, P<.001 in this study8 ). The Swedish Obesity Study found significant improvements in Health-Related Quality of Life scores at 2 years with surgery vs conventional treatment.9
Bariatric surgery, including gastric bypass, has significant postoperative morbidity and mortality. Thirteen percent of patients in the Swedish Obesity Study experienced peri-operative complications, including pulmonary symptoms (6.2%), abdominal infection (2.1%), wound complications (1.8%), bleeding (0.9%), thromboembolic events (0.8%), and other miscellaneous complications (4.8%). Postoperative complications required reoperation for 2.2% of surgical patients, and there were 4 postoperative deaths (0.2% of the operative patients; 3 due to leakage, and 1 due to a technical laparoscopic error).2
Nutritional and vitamin deficiencies are common following gastric bypass, including deficiencies of vitamin B12, iron, folate, and calcium. Lifelong nutritional supplementation is generally necessary following this procedure.10
FIGURE 2
Long-term weight loss with bariatric surgery
Long-term weight loss with bariatric surgery: comparison of controls, horizontal gastric banding (Banding), vertical band-ed gastroplasty (VPG), and gastric bypass (GBP). Source: Sjostrom et al 2000. 3
Recommendations from others
A 1991 National Institutes of Health consensus conference suggested consideration of obesity surgery for patients with a body-mass index ≥40, or ≥35 plus severe obesity-related medical comorbidities (such as severe sleep apnea, obesity hypoventilation syndrome, obesity-related cardiomyopathy, or severe diabetes) who have not been successfully treated with non-surgical attempts at weight reduction.
Selected patients should be well-informed and motivated, with acceptable operative risk. A multidisciplinary team with medical, surgical, psychiatric, and nutritional expertise should evaluate patients who are candidates for surgery. An experienced surgeon, working in a clinical setting with adequate support for all aspects of management and assessment, should perform the surgery.
Lifelong medical surveillance is necessary after surgery, and patients should be selected who are likely to comply with this.11
Bariatric surgery is an important option for select patients
Tim Mott, MD
Family Practice Staff, Navy Hospital, Pensacola, Fla
The lack of successful interventions for obesity is frustrating. This is accentuated as obesity is increasingly recognized as the proverbial forest in which we find ourselves hacking at the “trees” of diabetes, hypertension, dyslipidemia, and many other diseases. As we focus on this, the second-leading preventable cause of death, we find ourselves uniquely skilled as family physicians to offer balanced advice and advocacy.12
Bariatric surgery is an important option for select patients. For such a patient, I continuously advocate for lifestyle changes, document all non-surgical measures pursued (important for third-party review), discuss realistic expectations and risks, and direct the patient to a trusted bariatric surgery center. For the postsurgical patient, I reinforce the lifestyle commitments, ensure ongoing vitamin and mineral supplementation, and help monitor for possible complications.
1. Colquitt J, Clegg A, Sidhu M, Royle P. Surgery for morbid obesity (Cochrane Review). In: The Cochrane Library, Issue 4, 2003; Chichester, UK: John Wiley & Sons, Ltd.
2. Torgerson JS, Sjostrom L. The Swedish Obese Subjects (SOS) study—rationale and results. Int J Obes Relat Metab Disord 2001;25 Supp1:S2-S4.
3. Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 2000;36:20-25.
4. Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003;237:751-758.
5. Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension, and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 1999;7:477-484.
6. Brolin RE, Bradley LJ, Wilson AC, Cody RP. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J Gastrointest Surg 2000;4:464-469.
7. Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg 2003;13:58-61.
8. Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr 1988;7:147-153.
9. Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS)- an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord 1998;22:113-126.
10. Kushner R. Managing the obese patient after bariatric surgery: A case report of severe malnutrition and review of the literature. J Parenteral Enteral Nutrition 2000;24:126-132.
11. NIH conference: Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956-961.
12. Flegal K, Carroll M, Ogden C, et al. Prevalence trends in obesity among US adults, 1999–2000. JAMA 2002;288:1723-1727.
Gastric bypass results in weight loss of approximately 33% at 2 years and 25% at 8 years (strength of recommendation [SOR]: B, based on a cohort study). Gastric bypass is one type of bariatric surgery, which also includes gastroplasty and gastric banding procedures ( Figure 1 ). These procedures all can produce enough weight loss to measurably improve health, but they differ in the amount of long-term weight loss, as well as side effects, which can be serious.
Gastric bypass is more effective than gastroplasty for weight loss and is associated with fewer revisions, but it has more side effects (SOR: A, based on a systematic review). Limited evidence suggests that gastric bypass produces more weight loss than gastric banding (SOR: B, based on a cohort study).
Bariatric surgery, including gastric bypass, improves conditions comorbid with obesity, including diabetes, abnormal lipid profiles, and low quality-of-life scores. It decreases the incidence of hypertension at 2 years after surgery, but whether this effect is sustained is unclear (SOR: B, based on a cohort study and multiple case series). Bariatric surgery also improves obstructive sleep apnea, obesity hypoventilation syndrome, menstrual irregularity, and female urinary stress incontinence (SOR: C, based on multiple case series). Bariatric surgery has a complication rate of 13% and a mortality rate of 0.2% (SOR: B, based on 1 cohort study).
FIGURE 1
Bariatric surgical techniques for weight loss
Evidence summary
A systematic review comparing bariatric surgery with conventional medical therapy for obesity included 1 randomized controlled trial and the Swedish Obesity Study, a large cohort study with matched controls. Surgery produced 23 to 28 kg more weight loss at 2 years.1 The study demonstrated 33% ± 10% weight loss for gastric bypass and 0% for medical therapy (not described) at 2 years,2 and 25% ± 6% loss vs 0.9% gain at 8 years.3 Among bariatric surgical techniques, patients undergoing gastric bypass lost more weight than those with gastroplasty (using staples to partition the stomach, either horizontally or vertically ( Figure 1 ) (P=.057, not significant) or gastric banding (placing a constricting ring around the stomach) (P<.05) at 8 years.3
The same systematic review assessed multiple randomized controlled trials comparing gastric bypass with gastroplasty and found greater weight loss, fewer revisions, and more side effects from gastric bypass ( Figure 2 ).1 Five trials comparing gastric bypass with horizontal gastroplasty demonstrated significantly greater weight loss from gastric bypass. Five other trials comparing weight loss from gastric bypass with vertical gastroplasty produced mixed results, with 3 trials favoring gastric bypass and 2 showing no difference.1 Fewer patients required revision after gastric bypass (0%–4%) compared with vertical gastroplasty (9%) or horizontal gastroplasty (19%–40%). One included trial found that postoperative dumping syndrome (28% vs 0%, P<0.05) and heartburn (59% vs 32%, P<.05) were more common with gastric bypass than with gastroplasty.1
Bariatric surgery, including gastric bypass, improves a variety of obesity-related comorbid conditions. Diabetes prevalence decreased among gastric bypass patients at 2 years (0.0% vs 4.7%, P<0.005) and 8 years (3.6% vs 18.5%, P<.0005) compared with those receiving medical therapy.2,3 In a case series involving 154 diabetic gastric bypass patients, diabetes resolved for 83% by 1 year, and for 86% at 5 to 7 years.4 In several case series, most patients became euglycemic and discontinued insulin or oral agents.
In the Swedish Obesity Study, hypertriglyceridemia decreased postoperatively but hypercholesterolemia did not.5 In a case series, bariatric surgery reduced triglycerides (50%) as well as total cholesterol (15%) (P<.05 for both) at 6 months and significantly increased high-density lipoprotein cholesterol levels at 1 and 5 years.6
Bariatric surgery significantly lowered the incidence of hypertension at 2 years (3.2%) compared with conventional treatment (9.9%), but after 8 years this difference disappeared.2,3,5 However, in multiple large case series with morbidly obese patients, hypertension resolved or improved. The largest study showed resolution of hypertension for 69% at 1 to 2 years (91% follow-up), 66% at 5 to 7 years (50% follow-up), and 51% at 10 to 12 years (37% follow-up).4
Bariatric surgery improved obstructive sleep apnea and obesity hypoventilation syndrome in 2 case series. In one, Epworth Sleepiness Scale scores, minimum O2 saturation, and other measures improved significantly (P<.001) by 3 to 21 months after surgery.7
In another case series, menstrual irregularities decreased from 40.4% to 4.6% following surgery (P<.001) among women who lost 50% of their excess weight.8 The incidence of urinary stress incontinence also decreased significantly (61.2% to 11.6%, P<.001 in this study8 ). The Swedish Obesity Study found significant improvements in Health-Related Quality of Life scores at 2 years with surgery vs conventional treatment.9
Bariatric surgery, including gastric bypass, has significant postoperative morbidity and mortality. Thirteen percent of patients in the Swedish Obesity Study experienced peri-operative complications, including pulmonary symptoms (6.2%), abdominal infection (2.1%), wound complications (1.8%), bleeding (0.9%), thromboembolic events (0.8%), and other miscellaneous complications (4.8%). Postoperative complications required reoperation for 2.2% of surgical patients, and there were 4 postoperative deaths (0.2% of the operative patients; 3 due to leakage, and 1 due to a technical laparoscopic error).2
Nutritional and vitamin deficiencies are common following gastric bypass, including deficiencies of vitamin B12, iron, folate, and calcium. Lifelong nutritional supplementation is generally necessary following this procedure.10
FIGURE 2
Long-term weight loss with bariatric surgery
Long-term weight loss with bariatric surgery: comparison of controls, horizontal gastric banding (Banding), vertical band-ed gastroplasty (VPG), and gastric bypass (GBP). Source: Sjostrom et al 2000. 3
Recommendations from others
A 1991 National Institutes of Health consensus conference suggested consideration of obesity surgery for patients with a body-mass index ≥40, or ≥35 plus severe obesity-related medical comorbidities (such as severe sleep apnea, obesity hypoventilation syndrome, obesity-related cardiomyopathy, or severe diabetes) who have not been successfully treated with non-surgical attempts at weight reduction.
Selected patients should be well-informed and motivated, with acceptable operative risk. A multidisciplinary team with medical, surgical, psychiatric, and nutritional expertise should evaluate patients who are candidates for surgery. An experienced surgeon, working in a clinical setting with adequate support for all aspects of management and assessment, should perform the surgery.
Lifelong medical surveillance is necessary after surgery, and patients should be selected who are likely to comply with this.11
Bariatric surgery is an important option for select patients
Tim Mott, MD
Family Practice Staff, Navy Hospital, Pensacola, Fla
The lack of successful interventions for obesity is frustrating. This is accentuated as obesity is increasingly recognized as the proverbial forest in which we find ourselves hacking at the “trees” of diabetes, hypertension, dyslipidemia, and many other diseases. As we focus on this, the second-leading preventable cause of death, we find ourselves uniquely skilled as family physicians to offer balanced advice and advocacy.12
Bariatric surgery is an important option for select patients. For such a patient, I continuously advocate for lifestyle changes, document all non-surgical measures pursued (important for third-party review), discuss realistic expectations and risks, and direct the patient to a trusted bariatric surgery center. For the postsurgical patient, I reinforce the lifestyle commitments, ensure ongoing vitamin and mineral supplementation, and help monitor for possible complications.
Gastric bypass results in weight loss of approximately 33% at 2 years and 25% at 8 years (strength of recommendation [SOR]: B, based on a cohort study). Gastric bypass is one type of bariatric surgery, which also includes gastroplasty and gastric banding procedures ( Figure 1 ). These procedures all can produce enough weight loss to measurably improve health, but they differ in the amount of long-term weight loss, as well as side effects, which can be serious.
Gastric bypass is more effective than gastroplasty for weight loss and is associated with fewer revisions, but it has more side effects (SOR: A, based on a systematic review). Limited evidence suggests that gastric bypass produces more weight loss than gastric banding (SOR: B, based on a cohort study).
Bariatric surgery, including gastric bypass, improves conditions comorbid with obesity, including diabetes, abnormal lipid profiles, and low quality-of-life scores. It decreases the incidence of hypertension at 2 years after surgery, but whether this effect is sustained is unclear (SOR: B, based on a cohort study and multiple case series). Bariatric surgery also improves obstructive sleep apnea, obesity hypoventilation syndrome, menstrual irregularity, and female urinary stress incontinence (SOR: C, based on multiple case series). Bariatric surgery has a complication rate of 13% and a mortality rate of 0.2% (SOR: B, based on 1 cohort study).
FIGURE 1
Bariatric surgical techniques for weight loss
Evidence summary
A systematic review comparing bariatric surgery with conventional medical therapy for obesity included 1 randomized controlled trial and the Swedish Obesity Study, a large cohort study with matched controls. Surgery produced 23 to 28 kg more weight loss at 2 years.1 The study demonstrated 33% ± 10% weight loss for gastric bypass and 0% for medical therapy (not described) at 2 years,2 and 25% ± 6% loss vs 0.9% gain at 8 years.3 Among bariatric surgical techniques, patients undergoing gastric bypass lost more weight than those with gastroplasty (using staples to partition the stomach, either horizontally or vertically ( Figure 1 ) (P=.057, not significant) or gastric banding (placing a constricting ring around the stomach) (P<.05) at 8 years.3
The same systematic review assessed multiple randomized controlled trials comparing gastric bypass with gastroplasty and found greater weight loss, fewer revisions, and more side effects from gastric bypass ( Figure 2 ).1 Five trials comparing gastric bypass with horizontal gastroplasty demonstrated significantly greater weight loss from gastric bypass. Five other trials comparing weight loss from gastric bypass with vertical gastroplasty produced mixed results, with 3 trials favoring gastric bypass and 2 showing no difference.1 Fewer patients required revision after gastric bypass (0%–4%) compared with vertical gastroplasty (9%) or horizontal gastroplasty (19%–40%). One included trial found that postoperative dumping syndrome (28% vs 0%, P<0.05) and heartburn (59% vs 32%, P<.05) were more common with gastric bypass than with gastroplasty.1
Bariatric surgery, including gastric bypass, improves a variety of obesity-related comorbid conditions. Diabetes prevalence decreased among gastric bypass patients at 2 years (0.0% vs 4.7%, P<0.005) and 8 years (3.6% vs 18.5%, P<.0005) compared with those receiving medical therapy.2,3 In a case series involving 154 diabetic gastric bypass patients, diabetes resolved for 83% by 1 year, and for 86% at 5 to 7 years.4 In several case series, most patients became euglycemic and discontinued insulin or oral agents.
In the Swedish Obesity Study, hypertriglyceridemia decreased postoperatively but hypercholesterolemia did not.5 In a case series, bariatric surgery reduced triglycerides (50%) as well as total cholesterol (15%) (P<.05 for both) at 6 months and significantly increased high-density lipoprotein cholesterol levels at 1 and 5 years.6
Bariatric surgery significantly lowered the incidence of hypertension at 2 years (3.2%) compared with conventional treatment (9.9%), but after 8 years this difference disappeared.2,3,5 However, in multiple large case series with morbidly obese patients, hypertension resolved or improved. The largest study showed resolution of hypertension for 69% at 1 to 2 years (91% follow-up), 66% at 5 to 7 years (50% follow-up), and 51% at 10 to 12 years (37% follow-up).4
Bariatric surgery improved obstructive sleep apnea and obesity hypoventilation syndrome in 2 case series. In one, Epworth Sleepiness Scale scores, minimum O2 saturation, and other measures improved significantly (P<.001) by 3 to 21 months after surgery.7
In another case series, menstrual irregularities decreased from 40.4% to 4.6% following surgery (P<.001) among women who lost 50% of their excess weight.8 The incidence of urinary stress incontinence also decreased significantly (61.2% to 11.6%, P<.001 in this study8 ). The Swedish Obesity Study found significant improvements in Health-Related Quality of Life scores at 2 years with surgery vs conventional treatment.9
Bariatric surgery, including gastric bypass, has significant postoperative morbidity and mortality. Thirteen percent of patients in the Swedish Obesity Study experienced peri-operative complications, including pulmonary symptoms (6.2%), abdominal infection (2.1%), wound complications (1.8%), bleeding (0.9%), thromboembolic events (0.8%), and other miscellaneous complications (4.8%). Postoperative complications required reoperation for 2.2% of surgical patients, and there were 4 postoperative deaths (0.2% of the operative patients; 3 due to leakage, and 1 due to a technical laparoscopic error).2
Nutritional and vitamin deficiencies are common following gastric bypass, including deficiencies of vitamin B12, iron, folate, and calcium. Lifelong nutritional supplementation is generally necessary following this procedure.10
FIGURE 2
Long-term weight loss with bariatric surgery
Long-term weight loss with bariatric surgery: comparison of controls, horizontal gastric banding (Banding), vertical band-ed gastroplasty (VPG), and gastric bypass (GBP). Source: Sjostrom et al 2000. 3
Recommendations from others
A 1991 National Institutes of Health consensus conference suggested consideration of obesity surgery for patients with a body-mass index ≥40, or ≥35 plus severe obesity-related medical comorbidities (such as severe sleep apnea, obesity hypoventilation syndrome, obesity-related cardiomyopathy, or severe diabetes) who have not been successfully treated with non-surgical attempts at weight reduction.
Selected patients should be well-informed and motivated, with acceptable operative risk. A multidisciplinary team with medical, surgical, psychiatric, and nutritional expertise should evaluate patients who are candidates for surgery. An experienced surgeon, working in a clinical setting with adequate support for all aspects of management and assessment, should perform the surgery.
Lifelong medical surveillance is necessary after surgery, and patients should be selected who are likely to comply with this.11
Bariatric surgery is an important option for select patients
Tim Mott, MD
Family Practice Staff, Navy Hospital, Pensacola, Fla
The lack of successful interventions for obesity is frustrating. This is accentuated as obesity is increasingly recognized as the proverbial forest in which we find ourselves hacking at the “trees” of diabetes, hypertension, dyslipidemia, and many other diseases. As we focus on this, the second-leading preventable cause of death, we find ourselves uniquely skilled as family physicians to offer balanced advice and advocacy.12
Bariatric surgery is an important option for select patients. For such a patient, I continuously advocate for lifestyle changes, document all non-surgical measures pursued (important for third-party review), discuss realistic expectations and risks, and direct the patient to a trusted bariatric surgery center. For the postsurgical patient, I reinforce the lifestyle commitments, ensure ongoing vitamin and mineral supplementation, and help monitor for possible complications.
1. Colquitt J, Clegg A, Sidhu M, Royle P. Surgery for morbid obesity (Cochrane Review). In: The Cochrane Library, Issue 4, 2003; Chichester, UK: John Wiley & Sons, Ltd.
2. Torgerson JS, Sjostrom L. The Swedish Obese Subjects (SOS) study—rationale and results. Int J Obes Relat Metab Disord 2001;25 Supp1:S2-S4.
3. Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 2000;36:20-25.
4. Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003;237:751-758.
5. Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension, and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 1999;7:477-484.
6. Brolin RE, Bradley LJ, Wilson AC, Cody RP. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J Gastrointest Surg 2000;4:464-469.
7. Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg 2003;13:58-61.
8. Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr 1988;7:147-153.
9. Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS)- an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord 1998;22:113-126.
10. Kushner R. Managing the obese patient after bariatric surgery: A case report of severe malnutrition and review of the literature. J Parenteral Enteral Nutrition 2000;24:126-132.
11. NIH conference: Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956-961.
12. Flegal K, Carroll M, Ogden C, et al. Prevalence trends in obesity among US adults, 1999–2000. JAMA 2002;288:1723-1727.
1. Colquitt J, Clegg A, Sidhu M, Royle P. Surgery for morbid obesity (Cochrane Review). In: The Cochrane Library, Issue 4, 2003; Chichester, UK: John Wiley & Sons, Ltd.
2. Torgerson JS, Sjostrom L. The Swedish Obese Subjects (SOS) study—rationale and results. Int J Obes Relat Metab Disord 2001;25 Supp1:S2-S4.
3. Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 2000;36:20-25.
4. Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003;237:751-758.
5. Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension, and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 1999;7:477-484.
6. Brolin RE, Bradley LJ, Wilson AC, Cody RP. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J Gastrointest Surg 2000;4:464-469.
7. Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg 2003;13:58-61.
8. Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr 1988;7:147-153.
9. Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS)- an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord 1998;22:113-126.
10. Kushner R. Managing the obese patient after bariatric surgery: A case report of severe malnutrition and review of the literature. J Parenteral Enteral Nutrition 2000;24:126-132.
11. NIH conference: Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956-961.
12. Flegal K, Carroll M, Ogden C, et al. Prevalence trends in obesity among US adults, 1999–2000. JAMA 2002;288:1723-1727.
Evidence-based answers from the Family Physicians Inquiries Network
What is the most effective diagnostic evaluation of streptococcal pharyngitis?
Standardized clinical decision rules, such as the Centor criteria, can identify patients with low likelihood of group A beta-hemolytic streptococ-cal (GABHS) pharyngitis who require no further evaluation or antibiotics (strength of recommendation [SOR]: A, based on validated cohort studies). For patients at intermediate and higher risk by clinical prediction rules, a positive rapid anti-gen detection (RAD) test is highly specific for GABHS (SOR: A, based on systematic reviews of diagnostic trials).
A negative RAD test result, using the best technique, approaches the sensitivity of throat culture (SOR: B, based on retrospective cohort studies). In children and populations with an increased prevalence of GABHS and GABHS complications, adding a backup throat culture reduces the risk of missing GABHS due to false-negative RAD results (SOR: C, based on expert opinion).
Evidence summary
In the US, GABHS is the cause of acute pharyn-gitis in 5% to 10% of adults and 15% to 30% of children. It is the only commonly occurring cause of pharyngitis with an indication for antibiotic therapy.1 The main benefit of antibiotic treatment in adults is earlier symptom relief—1 fewer day of fever and pain if antibiotics are begun within 3 days of onset.
Antibiotic treatment also reduces the incidence of acute rheumatic fever, which complicates 1 case per 100,000 in most of the US and Europe (relative risk reduction [RRR]=0.28).2 The risk of acute rheumatic fever is higher in some populaHawaiians (13–45 per 100,000).3 Treatment may also reduce suppurative complications (peritonsil-tions, particularly Native Americans and lar or retropharyngeal abscess), which occur in 1 case out of 1000.2,4
A systematic review of the diagnosis of GABHS evaluated the accuracy of history and physical exam elements.5 Clinical prediction rules based on selected symptoms and signs can identify patients at low risk for GABHS. The 4 Centor criteria (history of fever, anterior cervical adenopathy, tonsillar exudates, absence of cough) are well validated in adult populations ( Table 1 ), while other clinical prediction rules (such as McIssac) are validated in populations with children and adults ( Table 2 ). The number of criteria present determines the likelihood ratio (LR), with which to calculate the posttest probability of GABHS.
The usefulness of clinical prediction rules depends on knowing how prevalent GABHS is among cases of pharyngitis in a particular community. In a typical US adult population, GABHS comprises 5% to 10% of cases. The presence of only 1 Centor criterion would reduce the probability of GABHS pharyngitis to 2% to 3%, while meeting all 4 criteria would raise the probability to 25% to 40%, an intermediate value ( Table 1 ). If the prevalence of GABHS pharyngitis were 50%, as in some Native communities in Alaska, meeting all 4 criteria would predict an 86% probability of pharyngitis due to GABHS. Performing additional testing for patients with intermediate or high probability based on clinical prediction rules reduces the likelihood of unnecessary antibiotic treatment.1
A systematic review6 of RAD testing demonstrates that the newer techniques (optical immunoassay, chemiluminescent DNA probes) have a sensitivity of 80% to 90%, which compares closely with that of throat culture (90%–95%). Both have a specificity greater than 95%, so false-positive test results are uncommon (LR+ =16–19). Treatment based on a positive RAD test would result in few unnecessary antibiotic prescriptions.1
A retrospective outcome study4 reviewed the frequency of suppurative complications of GABHS among 30,036 patients with pharyngitis diagnosed with either RAD testing or throat culture. Patients included adults and children in a primary care setting. Complication rates were identical. A prospective study of 465 suburban outpatients with pharyngitis assessed the accuracy of RAD diagnosis using throat culture as a reference. The RAD accuracy was 93% for pediatric patients and 97% for adults.5 In another retrospective review of RAD testing, investigators performed 11,427 RAD tests over 3 years in a private pediatric group. There were 8385 negative tests, among which follow-up cultures detected 200 (2.4%) that were positive for GABHS. In the second half of the study, a newer RAD test produced a false-negative rate of 1.4%.7 Because of the possibility of higher false-negative RAD test rates in some settings, unless the physician has ascertained that RAD testing is comparable to throat culture in their own setting, expert opinion recommends confirming a negative RAD test in children or adolescents with a throat culture.1 Patients at higher risk of GABHS or GABHS complications may also warrant throat culture back up of RAD testing.1
TABLE 1
Centor clinical prediction rules for diagnosis of GABHS (for adults)
| One point for each: History of fever, anterior cervical adenopathy, tonsillar exudates, absence of cough | |||||
| Points | LR+ | Pretest prevalence of GABHS (%) | |||
| 5 | 10 | 25 | 50 | ||
| Post-test probability of GABHS (%) | |||||
| 0 | 0.16 | 1 | 2 | 5 | 14 |
| 1 | 0.3 | 2 | 3 | 9 | 23 |
| 2 | 0.75 | 4 | 8 | 20 | 43 |
| 3 | 2.1 | 10 | 19 | 41 | 68 |
| 4 | 6.3 | 25 | 41 | 68 | 86 |
| GABHS, group A beta-hemolytic streptococcus; LR+, positive likelihood ratio. | |||||
| Adapted from data in Ebell et al 2000.5 | |||||
TABLE 2
McIssac clinical prediction rules for diagnosis of GABHS (for adults and children)
| One point for each: History of fever (or measured temperature >38°C), absence of cough, tender anterior cervical adenopathy, tonsillar swelling or exudates, age <15. Subtract 1 point if age 45 or more | |||||
| Points | LR+ | Pretest prevalence of GABHS (%) | |||
| 5 | 10 | 25 | 50 | ||
| Post-test probability of GABHS (%) | |||||
| –1 or 0 | 0.05 | <1 | 1 | 2 | 5 |
| 1 | 0.52 | 3 | 5 | 15 | 33 |
| 2 | 0.95 | 5 | 10 | 24 | 47 |
| 3 | 2.5 | 12 | 22 | 45 | 56 |
| 4 or 5 | 4.9 | 20 | 35 | 62 | 71 |
| GABHS, group A beta-hemolytic streptococcus; LR+, positive likelihood ratio. | |||||
| Adapted from data in Ebell et al 2000.5 | |||||
Recommendations from others
The Infectious Diseases Society of America recommends that if the physician is unable to exclude the diagnosis of GABHS on epidemiological or clinical grounds, either RAD testing or throat culture should be done. A positive result warrants treatment for patients with signs and symptoms of acute pharyngitis. A negative RAD result for a child or adolescent should be confirmed by throat culture unless the physician has ascertained that the sensitivity of RAD testing and throat culture are comparable in his or her practice setting.1
The American Academy of Pediatrics also recommends laboratory confirmation of GABHS pharyngitis in children with throat culture or RAD testing. If a patient suspected clinically of GABHS has a negative RAD test, a throat culture should be done. Since some experts believe RAD tests using optical immunoassay are sufficiently sensitive to be used without throat culture backup, physicians who wish to use them should validate them by comparison to throat culture in their practice.8
The RAD test helps to avoid overprescribing antibiotics
Peter Danis, MD
St. John’s Mercy Medical Center, St. Louis, Mo
The patient with a sore throat presents a diagnostic dilemma at 8:00 in the evening or on a Sunday morning. Patients (or parents) want something done, and frequently request antibiotics. Most of the time, they appreciate accurate information on the likelihood of a sore throat being a “strep throat” and the benefit or lack of benefit of antibiotics. The “in-between” cases are the toughest to manage, and the RAD test gives us the additional information needed to avoid overprescribing antibiotics. Empathetic reassurance and symptomatic treatment still suffice in most cases.
1. Bisno AL, Gerber MA, Gwaltney JM, Kaplan EL, Schwartz RH. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis 2002;35:113-125.
2. Cooper RJ, Hoffman JR, Bartlett JG, et al. Special report: CDC principles of judicious antibiotics use. Ann Emerg Med 2001;37:711-719.
3. Needham CA, McPherson KA, Webb KH. Streptococcal pharyngitis: impact of a high-sensitivity antigen test on physician outcome. J Clin Microbiol 1998;36:3468-3473.
4. Webb KH, Needham CA, Kurtz SR. Use of a high-sensitivity rapid strep test without culture confirmation of negative results: 2 years’ experience. J Fam Pract 2000;49:34-43.
5. Ebell MH, Smith MA, Barry HC, Ives K, Carey M. The rational clinical examination. Does this patient have strep throat? JAMA 2000;284:2912-2918.
6. Stewart MH, Siff JE, Cydulka RK. Evaluation of the patient with sore throat, earache, and sinusitis: an evidence-based approach. Emerg Med Clin North Am 1999;17:153-187.
7. Mayes T, Pichichero ME. Are follow-up throat cultures necessary when rapid antigen detection test are negative for group A streptococci?. Clin Pediatr 2001;40:191-195.
8. American Academy of Pediatrics. Group A streptococcal infections. In: Pickering LK, ed. Red Book: 2003 Report of the committee on infectious diseases. 26th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 2003;573-584.
Standardized clinical decision rules, such as the Centor criteria, can identify patients with low likelihood of group A beta-hemolytic streptococ-cal (GABHS) pharyngitis who require no further evaluation or antibiotics (strength of recommendation [SOR]: A, based on validated cohort studies). For patients at intermediate and higher risk by clinical prediction rules, a positive rapid anti-gen detection (RAD) test is highly specific for GABHS (SOR: A, based on systematic reviews of diagnostic trials).
A negative RAD test result, using the best technique, approaches the sensitivity of throat culture (SOR: B, based on retrospective cohort studies). In children and populations with an increased prevalence of GABHS and GABHS complications, adding a backup throat culture reduces the risk of missing GABHS due to false-negative RAD results (SOR: C, based on expert opinion).
Evidence summary
In the US, GABHS is the cause of acute pharyn-gitis in 5% to 10% of adults and 15% to 30% of children. It is the only commonly occurring cause of pharyngitis with an indication for antibiotic therapy.1 The main benefit of antibiotic treatment in adults is earlier symptom relief—1 fewer day of fever and pain if antibiotics are begun within 3 days of onset.
Antibiotic treatment also reduces the incidence of acute rheumatic fever, which complicates 1 case per 100,000 in most of the US and Europe (relative risk reduction [RRR]=0.28).2 The risk of acute rheumatic fever is higher in some populaHawaiians (13–45 per 100,000).3 Treatment may also reduce suppurative complications (peritonsil-tions, particularly Native Americans and lar or retropharyngeal abscess), which occur in 1 case out of 1000.2,4
A systematic review of the diagnosis of GABHS evaluated the accuracy of history and physical exam elements.5 Clinical prediction rules based on selected symptoms and signs can identify patients at low risk for GABHS. The 4 Centor criteria (history of fever, anterior cervical adenopathy, tonsillar exudates, absence of cough) are well validated in adult populations ( Table 1 ), while other clinical prediction rules (such as McIssac) are validated in populations with children and adults ( Table 2 ). The number of criteria present determines the likelihood ratio (LR), with which to calculate the posttest probability of GABHS.
The usefulness of clinical prediction rules depends on knowing how prevalent GABHS is among cases of pharyngitis in a particular community. In a typical US adult population, GABHS comprises 5% to 10% of cases. The presence of only 1 Centor criterion would reduce the probability of GABHS pharyngitis to 2% to 3%, while meeting all 4 criteria would raise the probability to 25% to 40%, an intermediate value ( Table 1 ). If the prevalence of GABHS pharyngitis were 50%, as in some Native communities in Alaska, meeting all 4 criteria would predict an 86% probability of pharyngitis due to GABHS. Performing additional testing for patients with intermediate or high probability based on clinical prediction rules reduces the likelihood of unnecessary antibiotic treatment.1
A systematic review6 of RAD testing demonstrates that the newer techniques (optical immunoassay, chemiluminescent DNA probes) have a sensitivity of 80% to 90%, which compares closely with that of throat culture (90%–95%). Both have a specificity greater than 95%, so false-positive test results are uncommon (LR+ =16–19). Treatment based on a positive RAD test would result in few unnecessary antibiotic prescriptions.1
A retrospective outcome study4 reviewed the frequency of suppurative complications of GABHS among 30,036 patients with pharyngitis diagnosed with either RAD testing or throat culture. Patients included adults and children in a primary care setting. Complication rates were identical. A prospective study of 465 suburban outpatients with pharyngitis assessed the accuracy of RAD diagnosis using throat culture as a reference. The RAD accuracy was 93% for pediatric patients and 97% for adults.5 In another retrospective review of RAD testing, investigators performed 11,427 RAD tests over 3 years in a private pediatric group. There were 8385 negative tests, among which follow-up cultures detected 200 (2.4%) that were positive for GABHS. In the second half of the study, a newer RAD test produced a false-negative rate of 1.4%.7 Because of the possibility of higher false-negative RAD test rates in some settings, unless the physician has ascertained that RAD testing is comparable to throat culture in their own setting, expert opinion recommends confirming a negative RAD test in children or adolescents with a throat culture.1 Patients at higher risk of GABHS or GABHS complications may also warrant throat culture back up of RAD testing.1
TABLE 1
Centor clinical prediction rules for diagnosis of GABHS (for adults)
| One point for each: History of fever, anterior cervical adenopathy, tonsillar exudates, absence of cough | |||||
| Points | LR+ | Pretest prevalence of GABHS (%) | |||
| 5 | 10 | 25 | 50 | ||
| Post-test probability of GABHS (%) | |||||
| 0 | 0.16 | 1 | 2 | 5 | 14 |
| 1 | 0.3 | 2 | 3 | 9 | 23 |
| 2 | 0.75 | 4 | 8 | 20 | 43 |
| 3 | 2.1 | 10 | 19 | 41 | 68 |
| 4 | 6.3 | 25 | 41 | 68 | 86 |
| GABHS, group A beta-hemolytic streptococcus; LR+, positive likelihood ratio. | |||||
| Adapted from data in Ebell et al 2000.5 | |||||
TABLE 2
McIssac clinical prediction rules for diagnosis of GABHS (for adults and children)
| One point for each: History of fever (or measured temperature >38°C), absence of cough, tender anterior cervical adenopathy, tonsillar swelling or exudates, age <15. Subtract 1 point if age 45 or more | |||||
| Points | LR+ | Pretest prevalence of GABHS (%) | |||
| 5 | 10 | 25 | 50 | ||
| Post-test probability of GABHS (%) | |||||
| –1 or 0 | 0.05 | <1 | 1 | 2 | 5 |
| 1 | 0.52 | 3 | 5 | 15 | 33 |
| 2 | 0.95 | 5 | 10 | 24 | 47 |
| 3 | 2.5 | 12 | 22 | 45 | 56 |
| 4 or 5 | 4.9 | 20 | 35 | 62 | 71 |
| GABHS, group A beta-hemolytic streptococcus; LR+, positive likelihood ratio. | |||||
| Adapted from data in Ebell et al 2000.5 | |||||
Recommendations from others
The Infectious Diseases Society of America recommends that if the physician is unable to exclude the diagnosis of GABHS on epidemiological or clinical grounds, either RAD testing or throat culture should be done. A positive result warrants treatment for patients with signs and symptoms of acute pharyngitis. A negative RAD result for a child or adolescent should be confirmed by throat culture unless the physician has ascertained that the sensitivity of RAD testing and throat culture are comparable in his or her practice setting.1
The American Academy of Pediatrics also recommends laboratory confirmation of GABHS pharyngitis in children with throat culture or RAD testing. If a patient suspected clinically of GABHS has a negative RAD test, a throat culture should be done. Since some experts believe RAD tests using optical immunoassay are sufficiently sensitive to be used without throat culture backup, physicians who wish to use them should validate them by comparison to throat culture in their practice.8
The RAD test helps to avoid overprescribing antibiotics
Peter Danis, MD
St. John’s Mercy Medical Center, St. Louis, Mo
The patient with a sore throat presents a diagnostic dilemma at 8:00 in the evening or on a Sunday morning. Patients (or parents) want something done, and frequently request antibiotics. Most of the time, they appreciate accurate information on the likelihood of a sore throat being a “strep throat” and the benefit or lack of benefit of antibiotics. The “in-between” cases are the toughest to manage, and the RAD test gives us the additional information needed to avoid overprescribing antibiotics. Empathetic reassurance and symptomatic treatment still suffice in most cases.
Standardized clinical decision rules, such as the Centor criteria, can identify patients with low likelihood of group A beta-hemolytic streptococ-cal (GABHS) pharyngitis who require no further evaluation or antibiotics (strength of recommendation [SOR]: A, based on validated cohort studies). For patients at intermediate and higher risk by clinical prediction rules, a positive rapid anti-gen detection (RAD) test is highly specific for GABHS (SOR: A, based on systematic reviews of diagnostic trials).
A negative RAD test result, using the best technique, approaches the sensitivity of throat culture (SOR: B, based on retrospective cohort studies). In children and populations with an increased prevalence of GABHS and GABHS complications, adding a backup throat culture reduces the risk of missing GABHS due to false-negative RAD results (SOR: C, based on expert opinion).
Evidence summary
In the US, GABHS is the cause of acute pharyn-gitis in 5% to 10% of adults and 15% to 30% of children. It is the only commonly occurring cause of pharyngitis with an indication for antibiotic therapy.1 The main benefit of antibiotic treatment in adults is earlier symptom relief—1 fewer day of fever and pain if antibiotics are begun within 3 days of onset.
Antibiotic treatment also reduces the incidence of acute rheumatic fever, which complicates 1 case per 100,000 in most of the US and Europe (relative risk reduction [RRR]=0.28).2 The risk of acute rheumatic fever is higher in some populaHawaiians (13–45 per 100,000).3 Treatment may also reduce suppurative complications (peritonsil-tions, particularly Native Americans and lar or retropharyngeal abscess), which occur in 1 case out of 1000.2,4
A systematic review of the diagnosis of GABHS evaluated the accuracy of history and physical exam elements.5 Clinical prediction rules based on selected symptoms and signs can identify patients at low risk for GABHS. The 4 Centor criteria (history of fever, anterior cervical adenopathy, tonsillar exudates, absence of cough) are well validated in adult populations ( Table 1 ), while other clinical prediction rules (such as McIssac) are validated in populations with children and adults ( Table 2 ). The number of criteria present determines the likelihood ratio (LR), with which to calculate the posttest probability of GABHS.
The usefulness of clinical prediction rules depends on knowing how prevalent GABHS is among cases of pharyngitis in a particular community. In a typical US adult population, GABHS comprises 5% to 10% of cases. The presence of only 1 Centor criterion would reduce the probability of GABHS pharyngitis to 2% to 3%, while meeting all 4 criteria would raise the probability to 25% to 40%, an intermediate value ( Table 1 ). If the prevalence of GABHS pharyngitis were 50%, as in some Native communities in Alaska, meeting all 4 criteria would predict an 86% probability of pharyngitis due to GABHS. Performing additional testing for patients with intermediate or high probability based on clinical prediction rules reduces the likelihood of unnecessary antibiotic treatment.1
A systematic review6 of RAD testing demonstrates that the newer techniques (optical immunoassay, chemiluminescent DNA probes) have a sensitivity of 80% to 90%, which compares closely with that of throat culture (90%–95%). Both have a specificity greater than 95%, so false-positive test results are uncommon (LR+ =16–19). Treatment based on a positive RAD test would result in few unnecessary antibiotic prescriptions.1
A retrospective outcome study4 reviewed the frequency of suppurative complications of GABHS among 30,036 patients with pharyngitis diagnosed with either RAD testing or throat culture. Patients included adults and children in a primary care setting. Complication rates were identical. A prospective study of 465 suburban outpatients with pharyngitis assessed the accuracy of RAD diagnosis using throat culture as a reference. The RAD accuracy was 93% for pediatric patients and 97% for adults.5 In another retrospective review of RAD testing, investigators performed 11,427 RAD tests over 3 years in a private pediatric group. There were 8385 negative tests, among which follow-up cultures detected 200 (2.4%) that were positive for GABHS. In the second half of the study, a newer RAD test produced a false-negative rate of 1.4%.7 Because of the possibility of higher false-negative RAD test rates in some settings, unless the physician has ascertained that RAD testing is comparable to throat culture in their own setting, expert opinion recommends confirming a negative RAD test in children or adolescents with a throat culture.1 Patients at higher risk of GABHS or GABHS complications may also warrant throat culture back up of RAD testing.1
TABLE 1
Centor clinical prediction rules for diagnosis of GABHS (for adults)
| One point for each: History of fever, anterior cervical adenopathy, tonsillar exudates, absence of cough | |||||
| Points | LR+ | Pretest prevalence of GABHS (%) | |||
| 5 | 10 | 25 | 50 | ||
| Post-test probability of GABHS (%) | |||||
| 0 | 0.16 | 1 | 2 | 5 | 14 |
| 1 | 0.3 | 2 | 3 | 9 | 23 |
| 2 | 0.75 | 4 | 8 | 20 | 43 |
| 3 | 2.1 | 10 | 19 | 41 | 68 |
| 4 | 6.3 | 25 | 41 | 68 | 86 |
| GABHS, group A beta-hemolytic streptococcus; LR+, positive likelihood ratio. | |||||
| Adapted from data in Ebell et al 2000.5 | |||||
TABLE 2
McIssac clinical prediction rules for diagnosis of GABHS (for adults and children)
| One point for each: History of fever (or measured temperature >38°C), absence of cough, tender anterior cervical adenopathy, tonsillar swelling or exudates, age <15. Subtract 1 point if age 45 or more | |||||
| Points | LR+ | Pretest prevalence of GABHS (%) | |||
| 5 | 10 | 25 | 50 | ||
| Post-test probability of GABHS (%) | |||||
| –1 or 0 | 0.05 | <1 | 1 | 2 | 5 |
| 1 | 0.52 | 3 | 5 | 15 | 33 |
| 2 | 0.95 | 5 | 10 | 24 | 47 |
| 3 | 2.5 | 12 | 22 | 45 | 56 |
| 4 or 5 | 4.9 | 20 | 35 | 62 | 71 |
| GABHS, group A beta-hemolytic streptococcus; LR+, positive likelihood ratio. | |||||
| Adapted from data in Ebell et al 2000.5 | |||||
Recommendations from others
The Infectious Diseases Society of America recommends that if the physician is unable to exclude the diagnosis of GABHS on epidemiological or clinical grounds, either RAD testing or throat culture should be done. A positive result warrants treatment for patients with signs and symptoms of acute pharyngitis. A negative RAD result for a child or adolescent should be confirmed by throat culture unless the physician has ascertained that the sensitivity of RAD testing and throat culture are comparable in his or her practice setting.1
The American Academy of Pediatrics also recommends laboratory confirmation of GABHS pharyngitis in children with throat culture or RAD testing. If a patient suspected clinically of GABHS has a negative RAD test, a throat culture should be done. Since some experts believe RAD tests using optical immunoassay are sufficiently sensitive to be used without throat culture backup, physicians who wish to use them should validate them by comparison to throat culture in their practice.8
The RAD test helps to avoid overprescribing antibiotics
Peter Danis, MD
St. John’s Mercy Medical Center, St. Louis, Mo
The patient with a sore throat presents a diagnostic dilemma at 8:00 in the evening or on a Sunday morning. Patients (or parents) want something done, and frequently request antibiotics. Most of the time, they appreciate accurate information on the likelihood of a sore throat being a “strep throat” and the benefit or lack of benefit of antibiotics. The “in-between” cases are the toughest to manage, and the RAD test gives us the additional information needed to avoid overprescribing antibiotics. Empathetic reassurance and symptomatic treatment still suffice in most cases.
1. Bisno AL, Gerber MA, Gwaltney JM, Kaplan EL, Schwartz RH. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis 2002;35:113-125.
2. Cooper RJ, Hoffman JR, Bartlett JG, et al. Special report: CDC principles of judicious antibiotics use. Ann Emerg Med 2001;37:711-719.
3. Needham CA, McPherson KA, Webb KH. Streptococcal pharyngitis: impact of a high-sensitivity antigen test on physician outcome. J Clin Microbiol 1998;36:3468-3473.
4. Webb KH, Needham CA, Kurtz SR. Use of a high-sensitivity rapid strep test without culture confirmation of negative results: 2 years’ experience. J Fam Pract 2000;49:34-43.
5. Ebell MH, Smith MA, Barry HC, Ives K, Carey M. The rational clinical examination. Does this patient have strep throat? JAMA 2000;284:2912-2918.
6. Stewart MH, Siff JE, Cydulka RK. Evaluation of the patient with sore throat, earache, and sinusitis: an evidence-based approach. Emerg Med Clin North Am 1999;17:153-187.
7. Mayes T, Pichichero ME. Are follow-up throat cultures necessary when rapid antigen detection test are negative for group A streptococci?. Clin Pediatr 2001;40:191-195.
8. American Academy of Pediatrics. Group A streptococcal infections. In: Pickering LK, ed. Red Book: 2003 Report of the committee on infectious diseases. 26th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 2003;573-584.
1. Bisno AL, Gerber MA, Gwaltney JM, Kaplan EL, Schwartz RH. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis 2002;35:113-125.
2. Cooper RJ, Hoffman JR, Bartlett JG, et al. Special report: CDC principles of judicious antibiotics use. Ann Emerg Med 2001;37:711-719.
3. Needham CA, McPherson KA, Webb KH. Streptococcal pharyngitis: impact of a high-sensitivity antigen test on physician outcome. J Clin Microbiol 1998;36:3468-3473.
4. Webb KH, Needham CA, Kurtz SR. Use of a high-sensitivity rapid strep test without culture confirmation of negative results: 2 years’ experience. J Fam Pract 2000;49:34-43.
5. Ebell MH, Smith MA, Barry HC, Ives K, Carey M. The rational clinical examination. Does this patient have strep throat? JAMA 2000;284:2912-2918.
6. Stewart MH, Siff JE, Cydulka RK. Evaluation of the patient with sore throat, earache, and sinusitis: an evidence-based approach. Emerg Med Clin North Am 1999;17:153-187.
7. Mayes T, Pichichero ME. Are follow-up throat cultures necessary when rapid antigen detection test are negative for group A streptococci?. Clin Pediatr 2001;40:191-195.
8. American Academy of Pediatrics. Group A streptococcal infections. In: Pickering LK, ed. Red Book: 2003 Report of the committee on infectious diseases. 26th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 2003;573-584.
Evidence-based answers from the Family Physicians Inquiries Network
Should we screen women for hypothyroidism?
Though evidence is insufficient to recommend screening all women for hypothyroidism, women aged >50 years are at increased risk. Screening is most likely to detect subclinical hypothyroidism. Studies evaluating treatment of subclinical hypothyroidism in women aged >50 years offer a mix of potential benefits and harms but without long-term outcome information. No studies address asymptomatic women aged <50.
Testing for thyroid-stimulating hormone (TSH) finds more cases of unrecognized hypothyroidism than history and physical examination (strength of recommendation [SOR]: A, based on cohort studies). Women with an initial screening TSH >10 mU/L are more likely to develop complications of hypothyroidism and to benefit from treatment (SOR: A, based on prospective cohort studies).
Treating women who have asymptomatic hypothyroidism and a screening TSH >10 mU/L prevents progression to symptomatic overt disease (SOR: A, based on prospective cohort studies) and reduces serum lipid levels (SOR: A, based on randomized controlled trials).
Treating women who have subclinical hypothyroidism found by screening does not reduce symptoms (SOR: A, small randomized controlled trials), and its effect on cardiac disease remains controversial. Treatment may increase bone loss in premenopausal women (SOR: A, based on randomized controlled trials and controlled cross-sectional studies), and it may cause symptoms in certain individuals (SOR: C, based on observational studies).
Evidence Summary
Screening for hypothyroidism is more likely to detect the elevated TSH and normal free thyroxine level (FT4) of subclinical hypothyroidism than it is to detect overt hypothyroidism with a high TSH and a low FT4. We reviewed the accuracy of detection, natural history, and benefits and harms of treating subclinical hypothyroidism.
Detection. Subclinical hypothyroidism is found in 7% to 26% of women (with increasing prevalence as women reach age 60 and 70 years); overt hypothyroidism occurs in approximately 5%.1-4 Two studies assessed the ability of the history and physical to detect hypothyroidism.
The first study evaluated 1154 women (aged 50–72) in a primary care setting using both history and physical and TSH testing. TSH testing found 3 women with overt hypothyroidism not identified by history and physical. History and physical identified 286 women with indications for TSH testing, 2 of whom had mild hypothyroidism and 1 with mild hyperthyroidism.5
In the second study, 2000 adults from a primary care population underwent history and physical and TSH testing. The TSH screen identified 19 cases of hypothyroidism not found by history and physical, while the history and physical prompted evaluation of 35 patients without abnormal TSH.6
Natural history of subclinical hypothyroidism. Among 1210 primary care patients (700 women) aged >60 years, 73 women with subclinical hypothyroidism were identified and followed for 12 months. Of these, 13 (18%) progressed to overt disease.7 Another prospective study of 30 patients (6 women) with subclinical hypothyroidism found 16 (3 women) who progressed to overt disease within 24 months. The remaining patients maintained normal FT4 levels for at least 15 years despite persistently elevated TSH.8 A third study followed 2779 adults (1494 women) with all types of thyroid disease for 20 years and found that 55% of women with TSH >6 and a positive antibody test developed overt hypothyroidism. Ninety percent of patients with an initial TSH >10 eventually progressed to overt disease.2
Serum lipid reduction. A retrospective study of 709 women referred to an endocrine clinic for evaluation of abnormal lipoprotein levels identified 34 (4.8%) with undiagnosed hypothyroidism. Thyroid hormone treatment significantly reduced total cholesterol and low-density lipoprotein (LDL) cholesterol in patients with initial TSH >10, but not in those with a TSH <10.9
A randomized trial involving 42 women with subclinical hypothyroidism measured lipid levels before and after 6 months of levothyroxine treatment. Levothyroxine reduced total cholesterol and LDL significantly compared with placebo. Additionally, the subclinical hypothyroidism patients had higher baseline lipid levels when compared with 27 euthyroid controls.10
A meta-analysis combined 13 studies, involving 247 patients with subclinical hypothyroidism, all of whom were given thyroid replacement. All studies reported a decrease in total cholesterol (mean –7.9 mg/dL), and 9 reported a decrease in LDL (mean –10 mg/dL).11 A second meta-analysis with 278 hypothyroid patients given thyroid replacement also found a reduction of total cholesterol (mean –15 mg/dL). LDL effects were not reported.12 The clinical significance of lipid changes in these circumstances is unknown.
Symptom relief. Four small randomized controlled trials used symptom-rating scales to measure symptom relief with treatment of subclinical hypothyroidism. One study involved patients found by screening; the other 3 did not indicate means of diagnosis. Three studies found no significant improvement.13-15 The most recent, involving 33 unblinded patients, found that those taking thyroid replacement had lower symptom scores (number needed to treat [NNT]=3.5).16
Cardiac manifestations. Subclinical hypothyroidism may be associated with ventricular dysfunction, myocardial infarction, and atherosclerosis.18-20 A randomized controlled trial of 20 people with subclinical hypothyroidism found significantly improved left ventricular function assessed by echocardiography after 6 months of treatment with levothyroxine vs placebo.18 Whether treatment prevents myocardial infarction and atherosclerosis is unknown.19,20 A cohort study, involving 2779 adults studied aged >20 years, did not find an association between subclinical hypothyroidism and ischemic heart disease.2
Risks of replacement. A meta-analysis of 41 controlled, cross-sectional studies involving 1250 women treated with thyroid replacement for all causes (ie, not specifically subclinical hypothyroidism) found that replacement therapy (mean duration of treatment, 7 to 9 years) was associated with bone loss in premenopausal women, but not in postmenopausal women.17
A randomized trial of 37 patients over 55 with subclinical hypothyroidism (28 of whom were women), found that thyroid hormone reduced bone mineral density, as assessed by dual-energy x-ray absorptiometry (DEXA) scans over a 10-month period.14 In several trials, patients withdrew due to adverse effects. Two of 37 patients receiving L-thyroxine in 1 study withdrew because of new atrial fibrillation and worsened angina, and 2 of 20 patients in another study withdrew because of nervousness and palpitations.13,14
Recommendations from others
The US Preventive Services Task Force concluded the evidence is insufficient to recommend for or against routine screening for thyroid disease in adults. The yield of screening is greater in high-risk groups such as postpartum women, people with Down syndrome, and the elderly; however, there is poor evidence that screening these groups leads to clinically important benefits.21
The American Thyroid Association recommends screening men and women beginning at age 35 and every 5 years thereafter.22 The American Academy of Family Physicians recommends screening for men and women over age 60.23 The American College of Physicians states screening may be indicated in women over age 50.24
Consider screening all female patients, particularly those over age 50
Julian T. Hsu, MD
A.F. Williams Family Medicine Center, University of Colorado Health Sciences Center, Denver
In my practice, there recently seems to be increased pressure from patients to screen for hypothyroidism, perhaps based on media or Internet information. I have used an individual “risk factor” approach when patients ask me for testing, based on their age, family history, and current symptoms. Based on the data, using the history and physical examination to tailor screening is an ineffective method of detecting hypothyroidism.
Until we have more evidence, I believe a reasonable approach is to offer screening to all of our female patients, particularly those over age 50, along with a careful acknowledgment of the lack of data for or against screening.
1. Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community: the Whickham Survey. Clin Endocrinol (Oxf) 1977;7:481-493.
2. Vanderpump MP, Turnbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55-68.
3. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000;160:526-534.
4. Sawin CT, Chopra D, Azizi F, Mannix JE, Bacharach P. The aging thyroid.Increasing prevalence of elevated serum thyrotropin levels in the elderly. JAMA 1979;242:247-250.
5. Petersen K, Lindstedt G, Lundberg PA, Bengtsson C, Lapidus L, Nystrom E. Thyroid disease in middle-aged and elderly Swedish women: thyroid-related hormones, thyroid dysfunction and goitre in relation to age and smoking. J Intern Med 1991;229:407-413.
6. Eggertsen R, Petersen K, Lundberg PA, Nystorm E, Lindstedt G. Screening for thyroid disease in a primary care unit with a thyroid stimulating hormone assay with a low detection limit. BMJ 1988;297:1586-1592.
7. Parle JV, Franklyn JA, Cross KW, Jones SC, Sheppard MC. Prevalence and follow up of abnormal thyrotropin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol (Oxf) 1991;34:77-83.
8. Kabadi UM. ‘Subclinical hypothyroidism’. Natural course of the syndrome during a prolonged follow-up study. Arch Intern Med 1993;153:957-961.
9. Diekman T, Lansberg PJ, Kastelein JJ, Wiersinga WM. Prevalence and correction of hypothyroidism in a large cohort of patients referred for dyslipidemia. Arch Intern Med 1995;155:1490-1495.
10. Caraccio N, Ferrannini E, Monzani F. Lipoprotein profile in subclinical hypothyroidism: response to levothyroxine replacement, a randomized placebo-controlled study. J Clin Endocrinol Metab 2002;87:1533-1538.
11. Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab 2000;85:2993-3001.
12. Tanis BC, Westerndorp GJ, Smelt HM. Effect of thyroid substitution on hypercholesterolaemia in patients with subclinical hypothyroidism: a reanalysis of intervention studies. Clin Endocrinol (Oxf) 1996;44:643-649.
13. Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt G. A double–blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988;29:63-76.
14. Jaeschke R, Guyatt G, Gerstein H, et al. Does treatment with L-thyroxine influence health status in middle-aged and older adults with subclinical hypothyroidism? J Gen Intern Med 1996;11:744-749.
15. Kong WM, Sheikh MH, Lumb PJ, et al. A 6-month randomized trial of thyroxine treatment in women with mild subclinical hypothyroidism. Am J Med 2002;112:348-354.
16. Cooper DS, Halpern R, Wood LC, Levin AA, Ridgeway EC. L-thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med 1984;101:18-24.
17. Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY. Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab 1996;81:4278-4289.
18. Monzani F, Di Bello V, Caraccio N, et al. Effects of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double-blind, placebo-controlled study. J Clin Endocrinol Metab 2001;86:1110-1115.
19. Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med 2002;137:904-914.
20. Hak AE, Pols HAP, Visser TJ, et al. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med 2000;132:270-278.
21. U. S. Preventive Services Task Force. Guide to Clinical Preventive Services: report of the US Preventive Services Task Force. Screening for thyroid disease, January 2004.
22. Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000;160:1573-1575.
23. Recommendations for Periodic Health Examinations. Revision 5.4. Leawood, Kansas: American Academy of Family Physicians, 2003. Available at: www.aafp.org/x24973.xml. Accessed on July 8, 2004.
24. Helfand M, Redfern CC. Clinical guidelines, part 2. Screening for thyroid disease: an update. American College of Physicians. Ann Intern Med 1998;129:144-158.
Though evidence is insufficient to recommend screening all women for hypothyroidism, women aged >50 years are at increased risk. Screening is most likely to detect subclinical hypothyroidism. Studies evaluating treatment of subclinical hypothyroidism in women aged >50 years offer a mix of potential benefits and harms but without long-term outcome information. No studies address asymptomatic women aged <50.
Testing for thyroid-stimulating hormone (TSH) finds more cases of unrecognized hypothyroidism than history and physical examination (strength of recommendation [SOR]: A, based on cohort studies). Women with an initial screening TSH >10 mU/L are more likely to develop complications of hypothyroidism and to benefit from treatment (SOR: A, based on prospective cohort studies).
Treating women who have asymptomatic hypothyroidism and a screening TSH >10 mU/L prevents progression to symptomatic overt disease (SOR: A, based on prospective cohort studies) and reduces serum lipid levels (SOR: A, based on randomized controlled trials).
Treating women who have subclinical hypothyroidism found by screening does not reduce symptoms (SOR: A, small randomized controlled trials), and its effect on cardiac disease remains controversial. Treatment may increase bone loss in premenopausal women (SOR: A, based on randomized controlled trials and controlled cross-sectional studies), and it may cause symptoms in certain individuals (SOR: C, based on observational studies).
Evidence Summary
Screening for hypothyroidism is more likely to detect the elevated TSH and normal free thyroxine level (FT4) of subclinical hypothyroidism than it is to detect overt hypothyroidism with a high TSH and a low FT4. We reviewed the accuracy of detection, natural history, and benefits and harms of treating subclinical hypothyroidism.
Detection. Subclinical hypothyroidism is found in 7% to 26% of women (with increasing prevalence as women reach age 60 and 70 years); overt hypothyroidism occurs in approximately 5%.1-4 Two studies assessed the ability of the history and physical to detect hypothyroidism.
The first study evaluated 1154 women (aged 50–72) in a primary care setting using both history and physical and TSH testing. TSH testing found 3 women with overt hypothyroidism not identified by history and physical. History and physical identified 286 women with indications for TSH testing, 2 of whom had mild hypothyroidism and 1 with mild hyperthyroidism.5
In the second study, 2000 adults from a primary care population underwent history and physical and TSH testing. The TSH screen identified 19 cases of hypothyroidism not found by history and physical, while the history and physical prompted evaluation of 35 patients without abnormal TSH.6
Natural history of subclinical hypothyroidism. Among 1210 primary care patients (700 women) aged >60 years, 73 women with subclinical hypothyroidism were identified and followed for 12 months. Of these, 13 (18%) progressed to overt disease.7 Another prospective study of 30 patients (6 women) with subclinical hypothyroidism found 16 (3 women) who progressed to overt disease within 24 months. The remaining patients maintained normal FT4 levels for at least 15 years despite persistently elevated TSH.8 A third study followed 2779 adults (1494 women) with all types of thyroid disease for 20 years and found that 55% of women with TSH >6 and a positive antibody test developed overt hypothyroidism. Ninety percent of patients with an initial TSH >10 eventually progressed to overt disease.2
Serum lipid reduction. A retrospective study of 709 women referred to an endocrine clinic for evaluation of abnormal lipoprotein levels identified 34 (4.8%) with undiagnosed hypothyroidism. Thyroid hormone treatment significantly reduced total cholesterol and low-density lipoprotein (LDL) cholesterol in patients with initial TSH >10, but not in those with a TSH <10.9
A randomized trial involving 42 women with subclinical hypothyroidism measured lipid levels before and after 6 months of levothyroxine treatment. Levothyroxine reduced total cholesterol and LDL significantly compared with placebo. Additionally, the subclinical hypothyroidism patients had higher baseline lipid levels when compared with 27 euthyroid controls.10
A meta-analysis combined 13 studies, involving 247 patients with subclinical hypothyroidism, all of whom were given thyroid replacement. All studies reported a decrease in total cholesterol (mean –7.9 mg/dL), and 9 reported a decrease in LDL (mean –10 mg/dL).11 A second meta-analysis with 278 hypothyroid patients given thyroid replacement also found a reduction of total cholesterol (mean –15 mg/dL). LDL effects were not reported.12 The clinical significance of lipid changes in these circumstances is unknown.
Symptom relief. Four small randomized controlled trials used symptom-rating scales to measure symptom relief with treatment of subclinical hypothyroidism. One study involved patients found by screening; the other 3 did not indicate means of diagnosis. Three studies found no significant improvement.13-15 The most recent, involving 33 unblinded patients, found that those taking thyroid replacement had lower symptom scores (number needed to treat [NNT]=3.5).16
Cardiac manifestations. Subclinical hypothyroidism may be associated with ventricular dysfunction, myocardial infarction, and atherosclerosis.18-20 A randomized controlled trial of 20 people with subclinical hypothyroidism found significantly improved left ventricular function assessed by echocardiography after 6 months of treatment with levothyroxine vs placebo.18 Whether treatment prevents myocardial infarction and atherosclerosis is unknown.19,20 A cohort study, involving 2779 adults studied aged >20 years, did not find an association between subclinical hypothyroidism and ischemic heart disease.2
Risks of replacement. A meta-analysis of 41 controlled, cross-sectional studies involving 1250 women treated with thyroid replacement for all causes (ie, not specifically subclinical hypothyroidism) found that replacement therapy (mean duration of treatment, 7 to 9 years) was associated with bone loss in premenopausal women, but not in postmenopausal women.17
A randomized trial of 37 patients over 55 with subclinical hypothyroidism (28 of whom were women), found that thyroid hormone reduced bone mineral density, as assessed by dual-energy x-ray absorptiometry (DEXA) scans over a 10-month period.14 In several trials, patients withdrew due to adverse effects. Two of 37 patients receiving L-thyroxine in 1 study withdrew because of new atrial fibrillation and worsened angina, and 2 of 20 patients in another study withdrew because of nervousness and palpitations.13,14
Recommendations from others
The US Preventive Services Task Force concluded the evidence is insufficient to recommend for or against routine screening for thyroid disease in adults. The yield of screening is greater in high-risk groups such as postpartum women, people with Down syndrome, and the elderly; however, there is poor evidence that screening these groups leads to clinically important benefits.21
The American Thyroid Association recommends screening men and women beginning at age 35 and every 5 years thereafter.22 The American Academy of Family Physicians recommends screening for men and women over age 60.23 The American College of Physicians states screening may be indicated in women over age 50.24
Consider screening all female patients, particularly those over age 50
Julian T. Hsu, MD
A.F. Williams Family Medicine Center, University of Colorado Health Sciences Center, Denver
In my practice, there recently seems to be increased pressure from patients to screen for hypothyroidism, perhaps based on media or Internet information. I have used an individual “risk factor” approach when patients ask me for testing, based on their age, family history, and current symptoms. Based on the data, using the history and physical examination to tailor screening is an ineffective method of detecting hypothyroidism.
Until we have more evidence, I believe a reasonable approach is to offer screening to all of our female patients, particularly those over age 50, along with a careful acknowledgment of the lack of data for or against screening.
Though evidence is insufficient to recommend screening all women for hypothyroidism, women aged >50 years are at increased risk. Screening is most likely to detect subclinical hypothyroidism. Studies evaluating treatment of subclinical hypothyroidism in women aged >50 years offer a mix of potential benefits and harms but without long-term outcome information. No studies address asymptomatic women aged <50.
Testing for thyroid-stimulating hormone (TSH) finds more cases of unrecognized hypothyroidism than history and physical examination (strength of recommendation [SOR]: A, based on cohort studies). Women with an initial screening TSH >10 mU/L are more likely to develop complications of hypothyroidism and to benefit from treatment (SOR: A, based on prospective cohort studies).
Treating women who have asymptomatic hypothyroidism and a screening TSH >10 mU/L prevents progression to symptomatic overt disease (SOR: A, based on prospective cohort studies) and reduces serum lipid levels (SOR: A, based on randomized controlled trials).
Treating women who have subclinical hypothyroidism found by screening does not reduce symptoms (SOR: A, small randomized controlled trials), and its effect on cardiac disease remains controversial. Treatment may increase bone loss in premenopausal women (SOR: A, based on randomized controlled trials and controlled cross-sectional studies), and it may cause symptoms in certain individuals (SOR: C, based on observational studies).
Evidence Summary
Screening for hypothyroidism is more likely to detect the elevated TSH and normal free thyroxine level (FT4) of subclinical hypothyroidism than it is to detect overt hypothyroidism with a high TSH and a low FT4. We reviewed the accuracy of detection, natural history, and benefits and harms of treating subclinical hypothyroidism.
Detection. Subclinical hypothyroidism is found in 7% to 26% of women (with increasing prevalence as women reach age 60 and 70 years); overt hypothyroidism occurs in approximately 5%.1-4 Two studies assessed the ability of the history and physical to detect hypothyroidism.
The first study evaluated 1154 women (aged 50–72) in a primary care setting using both history and physical and TSH testing. TSH testing found 3 women with overt hypothyroidism not identified by history and physical. History and physical identified 286 women with indications for TSH testing, 2 of whom had mild hypothyroidism and 1 with mild hyperthyroidism.5
In the second study, 2000 adults from a primary care population underwent history and physical and TSH testing. The TSH screen identified 19 cases of hypothyroidism not found by history and physical, while the history and physical prompted evaluation of 35 patients without abnormal TSH.6
Natural history of subclinical hypothyroidism. Among 1210 primary care patients (700 women) aged >60 years, 73 women with subclinical hypothyroidism were identified and followed for 12 months. Of these, 13 (18%) progressed to overt disease.7 Another prospective study of 30 patients (6 women) with subclinical hypothyroidism found 16 (3 women) who progressed to overt disease within 24 months. The remaining patients maintained normal FT4 levels for at least 15 years despite persistently elevated TSH.8 A third study followed 2779 adults (1494 women) with all types of thyroid disease for 20 years and found that 55% of women with TSH >6 and a positive antibody test developed overt hypothyroidism. Ninety percent of patients with an initial TSH >10 eventually progressed to overt disease.2
Serum lipid reduction. A retrospective study of 709 women referred to an endocrine clinic for evaluation of abnormal lipoprotein levels identified 34 (4.8%) with undiagnosed hypothyroidism. Thyroid hormone treatment significantly reduced total cholesterol and low-density lipoprotein (LDL) cholesterol in patients with initial TSH >10, but not in those with a TSH <10.9
A randomized trial involving 42 women with subclinical hypothyroidism measured lipid levels before and after 6 months of levothyroxine treatment. Levothyroxine reduced total cholesterol and LDL significantly compared with placebo. Additionally, the subclinical hypothyroidism patients had higher baseline lipid levels when compared with 27 euthyroid controls.10
A meta-analysis combined 13 studies, involving 247 patients with subclinical hypothyroidism, all of whom were given thyroid replacement. All studies reported a decrease in total cholesterol (mean –7.9 mg/dL), and 9 reported a decrease in LDL (mean –10 mg/dL).11 A second meta-analysis with 278 hypothyroid patients given thyroid replacement also found a reduction of total cholesterol (mean –15 mg/dL). LDL effects were not reported.12 The clinical significance of lipid changes in these circumstances is unknown.
Symptom relief. Four small randomized controlled trials used symptom-rating scales to measure symptom relief with treatment of subclinical hypothyroidism. One study involved patients found by screening; the other 3 did not indicate means of diagnosis. Three studies found no significant improvement.13-15 The most recent, involving 33 unblinded patients, found that those taking thyroid replacement had lower symptom scores (number needed to treat [NNT]=3.5).16
Cardiac manifestations. Subclinical hypothyroidism may be associated with ventricular dysfunction, myocardial infarction, and atherosclerosis.18-20 A randomized controlled trial of 20 people with subclinical hypothyroidism found significantly improved left ventricular function assessed by echocardiography after 6 months of treatment with levothyroxine vs placebo.18 Whether treatment prevents myocardial infarction and atherosclerosis is unknown.19,20 A cohort study, involving 2779 adults studied aged >20 years, did not find an association between subclinical hypothyroidism and ischemic heart disease.2
Risks of replacement. A meta-analysis of 41 controlled, cross-sectional studies involving 1250 women treated with thyroid replacement for all causes (ie, not specifically subclinical hypothyroidism) found that replacement therapy (mean duration of treatment, 7 to 9 years) was associated with bone loss in premenopausal women, but not in postmenopausal women.17
A randomized trial of 37 patients over 55 with subclinical hypothyroidism (28 of whom were women), found that thyroid hormone reduced bone mineral density, as assessed by dual-energy x-ray absorptiometry (DEXA) scans over a 10-month period.14 In several trials, patients withdrew due to adverse effects. Two of 37 patients receiving L-thyroxine in 1 study withdrew because of new atrial fibrillation and worsened angina, and 2 of 20 patients in another study withdrew because of nervousness and palpitations.13,14
Recommendations from others
The US Preventive Services Task Force concluded the evidence is insufficient to recommend for or against routine screening for thyroid disease in adults. The yield of screening is greater in high-risk groups such as postpartum women, people with Down syndrome, and the elderly; however, there is poor evidence that screening these groups leads to clinically important benefits.21
The American Thyroid Association recommends screening men and women beginning at age 35 and every 5 years thereafter.22 The American Academy of Family Physicians recommends screening for men and women over age 60.23 The American College of Physicians states screening may be indicated in women over age 50.24
Consider screening all female patients, particularly those over age 50
Julian T. Hsu, MD
A.F. Williams Family Medicine Center, University of Colorado Health Sciences Center, Denver
In my practice, there recently seems to be increased pressure from patients to screen for hypothyroidism, perhaps based on media or Internet information. I have used an individual “risk factor” approach when patients ask me for testing, based on their age, family history, and current symptoms. Based on the data, using the history and physical examination to tailor screening is an ineffective method of detecting hypothyroidism.
Until we have more evidence, I believe a reasonable approach is to offer screening to all of our female patients, particularly those over age 50, along with a careful acknowledgment of the lack of data for or against screening.
1. Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community: the Whickham Survey. Clin Endocrinol (Oxf) 1977;7:481-493.
2. Vanderpump MP, Turnbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55-68.
3. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000;160:526-534.
4. Sawin CT, Chopra D, Azizi F, Mannix JE, Bacharach P. The aging thyroid.Increasing prevalence of elevated serum thyrotropin levels in the elderly. JAMA 1979;242:247-250.
5. Petersen K, Lindstedt G, Lundberg PA, Bengtsson C, Lapidus L, Nystrom E. Thyroid disease in middle-aged and elderly Swedish women: thyroid-related hormones, thyroid dysfunction and goitre in relation to age and smoking. J Intern Med 1991;229:407-413.
6. Eggertsen R, Petersen K, Lundberg PA, Nystorm E, Lindstedt G. Screening for thyroid disease in a primary care unit with a thyroid stimulating hormone assay with a low detection limit. BMJ 1988;297:1586-1592.
7. Parle JV, Franklyn JA, Cross KW, Jones SC, Sheppard MC. Prevalence and follow up of abnormal thyrotropin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol (Oxf) 1991;34:77-83.
8. Kabadi UM. ‘Subclinical hypothyroidism’. Natural course of the syndrome during a prolonged follow-up study. Arch Intern Med 1993;153:957-961.
9. Diekman T, Lansberg PJ, Kastelein JJ, Wiersinga WM. Prevalence and correction of hypothyroidism in a large cohort of patients referred for dyslipidemia. Arch Intern Med 1995;155:1490-1495.
10. Caraccio N, Ferrannini E, Monzani F. Lipoprotein profile in subclinical hypothyroidism: response to levothyroxine replacement, a randomized placebo-controlled study. J Clin Endocrinol Metab 2002;87:1533-1538.
11. Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab 2000;85:2993-3001.
12. Tanis BC, Westerndorp GJ, Smelt HM. Effect of thyroid substitution on hypercholesterolaemia in patients with subclinical hypothyroidism: a reanalysis of intervention studies. Clin Endocrinol (Oxf) 1996;44:643-649.
13. Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt G. A double–blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988;29:63-76.
14. Jaeschke R, Guyatt G, Gerstein H, et al. Does treatment with L-thyroxine influence health status in middle-aged and older adults with subclinical hypothyroidism? J Gen Intern Med 1996;11:744-749.
15. Kong WM, Sheikh MH, Lumb PJ, et al. A 6-month randomized trial of thyroxine treatment in women with mild subclinical hypothyroidism. Am J Med 2002;112:348-354.
16. Cooper DS, Halpern R, Wood LC, Levin AA, Ridgeway EC. L-thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med 1984;101:18-24.
17. Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY. Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab 1996;81:4278-4289.
18. Monzani F, Di Bello V, Caraccio N, et al. Effects of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double-blind, placebo-controlled study. J Clin Endocrinol Metab 2001;86:1110-1115.
19. Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med 2002;137:904-914.
20. Hak AE, Pols HAP, Visser TJ, et al. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med 2000;132:270-278.
21. U. S. Preventive Services Task Force. Guide to Clinical Preventive Services: report of the US Preventive Services Task Force. Screening for thyroid disease, January 2004.
22. Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000;160:1573-1575.
23. Recommendations for Periodic Health Examinations. Revision 5.4. Leawood, Kansas: American Academy of Family Physicians, 2003. Available at: www.aafp.org/x24973.xml. Accessed on July 8, 2004.
24. Helfand M, Redfern CC. Clinical guidelines, part 2. Screening for thyroid disease: an update. American College of Physicians. Ann Intern Med 1998;129:144-158.
1. Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community: the Whickham Survey. Clin Endocrinol (Oxf) 1977;7:481-493.
2. Vanderpump MP, Turnbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55-68.
3. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000;160:526-534.
4. Sawin CT, Chopra D, Azizi F, Mannix JE, Bacharach P. The aging thyroid.Increasing prevalence of elevated serum thyrotropin levels in the elderly. JAMA 1979;242:247-250.
5. Petersen K, Lindstedt G, Lundberg PA, Bengtsson C, Lapidus L, Nystrom E. Thyroid disease in middle-aged and elderly Swedish women: thyroid-related hormones, thyroid dysfunction and goitre in relation to age and smoking. J Intern Med 1991;229:407-413.
6. Eggertsen R, Petersen K, Lundberg PA, Nystorm E, Lindstedt G. Screening for thyroid disease in a primary care unit with a thyroid stimulating hormone assay with a low detection limit. BMJ 1988;297:1586-1592.
7. Parle JV, Franklyn JA, Cross KW, Jones SC, Sheppard MC. Prevalence and follow up of abnormal thyrotropin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol (Oxf) 1991;34:77-83.
8. Kabadi UM. ‘Subclinical hypothyroidism’. Natural course of the syndrome during a prolonged follow-up study. Arch Intern Med 1993;153:957-961.
9. Diekman T, Lansberg PJ, Kastelein JJ, Wiersinga WM. Prevalence and correction of hypothyroidism in a large cohort of patients referred for dyslipidemia. Arch Intern Med 1995;155:1490-1495.
10. Caraccio N, Ferrannini E, Monzani F. Lipoprotein profile in subclinical hypothyroidism: response to levothyroxine replacement, a randomized placebo-controlled study. J Clin Endocrinol Metab 2002;87:1533-1538.
11. Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab 2000;85:2993-3001.
12. Tanis BC, Westerndorp GJ, Smelt HM. Effect of thyroid substitution on hypercholesterolaemia in patients with subclinical hypothyroidism: a reanalysis of intervention studies. Clin Endocrinol (Oxf) 1996;44:643-649.
13. Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt G. A double–blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988;29:63-76.
14. Jaeschke R, Guyatt G, Gerstein H, et al. Does treatment with L-thyroxine influence health status in middle-aged and older adults with subclinical hypothyroidism? J Gen Intern Med 1996;11:744-749.
15. Kong WM, Sheikh MH, Lumb PJ, et al. A 6-month randomized trial of thyroxine treatment in women with mild subclinical hypothyroidism. Am J Med 2002;112:348-354.
16. Cooper DS, Halpern R, Wood LC, Levin AA, Ridgeway EC. L-thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med 1984;101:18-24.
17. Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY. Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab 1996;81:4278-4289.
18. Monzani F, Di Bello V, Caraccio N, et al. Effects of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double-blind, placebo-controlled study. J Clin Endocrinol Metab 2001;86:1110-1115.
19. Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med 2002;137:904-914.
20. Hak AE, Pols HAP, Visser TJ, et al. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med 2000;132:270-278.
21. U. S. Preventive Services Task Force. Guide to Clinical Preventive Services: report of the US Preventive Services Task Force. Screening for thyroid disease, January 2004.
22. Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000;160:1573-1575.
23. Recommendations for Periodic Health Examinations. Revision 5.4. Leawood, Kansas: American Academy of Family Physicians, 2003. Available at: www.aafp.org/x24973.xml. Accessed on July 8, 2004.
24. Helfand M, Redfern CC. Clinical guidelines, part 2. Screening for thyroid disease: an update. American College of Physicians. Ann Intern Med 1998;129:144-158.
Evidence-based answers from the Family Physicians Inquiries Network
Does moderate exercise prevent MI for patients with coronary heart disease?
Moderate exercise reduces mortality for patients with known coronary heart disease but does not significantly decrease the risk of recurrent nonfatal myocardial infarction (MI) (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials). Exercise-based cardiac rehabilitation also reduces all-cause mortality (SOR: A, based on systematic review).
For patients with stable angina, a daily exercise program is more effective than percutaneous transluminal coronary angioplasty (PTCA) with stenting in preventing major cardiovascular events (number needed to treat [NNT]=5.5; SOR: A, based on a single randomized controlled trial).
Evidence summary
A systematic review of cardiac rehabilitation programs evaluated 14 randomized controlled trials with exercise-based interventions.1 An updated review added 5 more for a total of 2984 patients with coronary heart disease.2 Patients with coronary heart disease comprised those with prior MI, prior coronary artery bypass graft surgery, or PTCA, and those with angina pectoris and angiographically confirmed coronary heart disease.
Exercise-based cardiac rehabilitation significantly reduced all-cause mortality (relative risk [RR]=0.76; 95% confidence interval [CI], 0.59–0.98) compared with usual care (NNT=66; 95% CI, 35–273). Cardiac mortality also decreased significantly with exercise (RR=0.73; 95% CI, 0.56–0.96) compared with usual care (NNT=49; 95% CI, 26–120).
Six studies showed particularly significant improvement in total cardiac mortality.3-8 Exercise was variably defined. Training sessions lasted 30 minutes and occurred on 2 to 5 days per week. Intensity was typically 75% to 85% of a maximum work capacity determined on an exercise test before initiating the training sessions. The type of exercise ranged from cycling alone to circuit training with 6 stationary devices. Patients were trained with supervision 1 to 36 months and followed for a mean of 24 months (range, 6–60 months).
A trend was observed toward decreased recurrence of nonfatal MI with exercise-based cardiac rehabilitation, which did not reach significance (RR=0.78; 95% CI, 0.59–1.03). An inadequate number of subjects is the most likely reason; however, other possibilities include an increase in the frequency of nonfatal MI after rehabilitation, or an increased rate of survival after MI for patients undergoing exercise-based rehabilitation.
The studies included in these reviews had several limitations. The population appears skewed in age (mean=54 years, with patients aged >65 years excluded from most studies) and gender (4.9% female); ethnicity was rarely reported. The adequacy of randomization was poor or unclear in 71% of studies, and only 4 trials reported blind assessment of outcomes. Finally, in 34% of studies the loss of participants to follow-up was more than 20%.
A well-done study randomized 101 male patients (age <70 years) with stable angina to either a daily exercise program or standard PTCA with stenting.9 After 12 months, event-free survival was significantly greater among patients randomized to exercise than in those randomized to PTCA with stenting (88% vs 70%; P=.023; NNT=5.5). Cardiovascular events were defined as percutaneous interventions, hospitalizations, acute MI, cerebrovascular accidents, coronary artery bypass graft operation, and death.
Recommendation from others
The American Heart Association (AHA) supports aggressive risk factor management for patients with coronary heart disease, and recommends a minimum of 30 minutes of exercise 3 to 4 days per week as well as an increase in daily lifestyle activities.10 The American College of Cardiology endorses the position of the AHA.
Add exercise to routine post-MI treatment
Bill Kerns, MD
Shenandoah Valley Family Practice Residency, Virginia Commonwealth University/Medical College of Virginia, Winchester
We should add exercise to routine post-MI treatment checklists, along with aspirin, beta-blockers, statins, angiotensin-converting enzyme inhibitors, and so on. Precise exercise prescribing requires a stress test because, as the adage goes, “If we don’t do an exercise test with monitoring, the patient will eventually do one unmonitored at home.”
Medicare pays for cardiac rehabilitation for acute MI (within 6 months), coronary artery bypass (within a year), and stable angina. Other insurance reimbursement varies.
The evidence isn’t the quality I would like, and for women and minorities it is lacking. However, evidence sticklers like USPSTF11 state that exercise reduces morbidity and mortality for (almost) everyone. The question is how to make exercise happen; people with CHD can often be motivated.
Drug brand names
- Amiodarone • Cordarone
- Atenolol • Tenormin
- Atorvastatin • Lipitor
- Diclofenac • Cataflam, Voltaren
- Disopyramide • Norpace
- Dofetilide • Tikosyn
- Enoxaparin • Lovenox
- Flecainide • Tambocor
- Metoprolol • Lopressor
- Propafenone • Rythmol
- Simvastatin • Zocor
- Solatol • Betapace
- Warfarin • Coumadin
1. Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease (Cochrane Review). In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
2. Brown A, Noorani H, Taylor R, Stone J, Skidmore B. A clinical and economic review of exercise-based cardiac rehabilitation programs for coronary artery disease. Technology overview no. 11. Ottawa: Canadian Coordinating Office for Health Technology Assessment; 2003.
3. Carson P, Phillips R, Lloyd M, et al. Exercise after myocardial infarction: a controlled trial. J R Coll Physicians Lond 1982;16:147-151.
4. Kentala E. Physical fitness and feasibility of physical rehabilitation after myocardial infarction in men of working age. Ann Clin Res 1972;4 Suppl 9:1-84.
5. Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction. The National Exercise and Heart Disease Project. Am J Cardiol 1981;48:39-46.
6. Specchia G, DeServi S, Scire A, et al. Interaction between exercise training and ejection fraction in predicting prognosis after a first myocardial infarction. Circulation 1996;94:978-982.
7. Sanne H. Exercise tolerance and physical training of non-selected patients after myocardial infarction. Acta Med Scan Suppl 1973;551:1-124.
8. Wilhelmsen L, Sanne H, Elmfeldt D, Grimby G, Tibblin G, Wedel H. A controlled trial of physical training after myocardial infarction. Effects on risk factors, nonfatal reinfarction an death. Prev Med 1975;4:491-508.
9. Hambrecht R, Walther C, Möbius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation 2004;109:1371-1378.
10. Smith SC, Blair SN, Bonow RO, et al. AHA/ACC Scientific Statement: AHA/ACC guidelines for preventing heart attack and death in patients with atherosclerotic cardiovascular disease: 2001 update. A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation 2001;104:1577-
11. US Preventive Services Task Force. Behavioral counseling in primary care to promote physical activity: recommendation and rationale. Ann Intern Med 2002;137:205-207.
Moderate exercise reduces mortality for patients with known coronary heart disease but does not significantly decrease the risk of recurrent nonfatal myocardial infarction (MI) (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials). Exercise-based cardiac rehabilitation also reduces all-cause mortality (SOR: A, based on systematic review).
For patients with stable angina, a daily exercise program is more effective than percutaneous transluminal coronary angioplasty (PTCA) with stenting in preventing major cardiovascular events (number needed to treat [NNT]=5.5; SOR: A, based on a single randomized controlled trial).
Evidence summary
A systematic review of cardiac rehabilitation programs evaluated 14 randomized controlled trials with exercise-based interventions.1 An updated review added 5 more for a total of 2984 patients with coronary heart disease.2 Patients with coronary heart disease comprised those with prior MI, prior coronary artery bypass graft surgery, or PTCA, and those with angina pectoris and angiographically confirmed coronary heart disease.
Exercise-based cardiac rehabilitation significantly reduced all-cause mortality (relative risk [RR]=0.76; 95% confidence interval [CI], 0.59–0.98) compared with usual care (NNT=66; 95% CI, 35–273). Cardiac mortality also decreased significantly with exercise (RR=0.73; 95% CI, 0.56–0.96) compared with usual care (NNT=49; 95% CI, 26–120).
Six studies showed particularly significant improvement in total cardiac mortality.3-8 Exercise was variably defined. Training sessions lasted 30 minutes and occurred on 2 to 5 days per week. Intensity was typically 75% to 85% of a maximum work capacity determined on an exercise test before initiating the training sessions. The type of exercise ranged from cycling alone to circuit training with 6 stationary devices. Patients were trained with supervision 1 to 36 months and followed for a mean of 24 months (range, 6–60 months).
A trend was observed toward decreased recurrence of nonfatal MI with exercise-based cardiac rehabilitation, which did not reach significance (RR=0.78; 95% CI, 0.59–1.03). An inadequate number of subjects is the most likely reason; however, other possibilities include an increase in the frequency of nonfatal MI after rehabilitation, or an increased rate of survival after MI for patients undergoing exercise-based rehabilitation.
The studies included in these reviews had several limitations. The population appears skewed in age (mean=54 years, with patients aged >65 years excluded from most studies) and gender (4.9% female); ethnicity was rarely reported. The adequacy of randomization was poor or unclear in 71% of studies, and only 4 trials reported blind assessment of outcomes. Finally, in 34% of studies the loss of participants to follow-up was more than 20%.
A well-done study randomized 101 male patients (age <70 years) with stable angina to either a daily exercise program or standard PTCA with stenting.9 After 12 months, event-free survival was significantly greater among patients randomized to exercise than in those randomized to PTCA with stenting (88% vs 70%; P=.023; NNT=5.5). Cardiovascular events were defined as percutaneous interventions, hospitalizations, acute MI, cerebrovascular accidents, coronary artery bypass graft operation, and death.
Recommendation from others
The American Heart Association (AHA) supports aggressive risk factor management for patients with coronary heart disease, and recommends a minimum of 30 minutes of exercise 3 to 4 days per week as well as an increase in daily lifestyle activities.10 The American College of Cardiology endorses the position of the AHA.
Add exercise to routine post-MI treatment
Bill Kerns, MD
Shenandoah Valley Family Practice Residency, Virginia Commonwealth University/Medical College of Virginia, Winchester
We should add exercise to routine post-MI treatment checklists, along with aspirin, beta-blockers, statins, angiotensin-converting enzyme inhibitors, and so on. Precise exercise prescribing requires a stress test because, as the adage goes, “If we don’t do an exercise test with monitoring, the patient will eventually do one unmonitored at home.”
Medicare pays for cardiac rehabilitation for acute MI (within 6 months), coronary artery bypass (within a year), and stable angina. Other insurance reimbursement varies.
The evidence isn’t the quality I would like, and for women and minorities it is lacking. However, evidence sticklers like USPSTF11 state that exercise reduces morbidity and mortality for (almost) everyone. The question is how to make exercise happen; people with CHD can often be motivated.
Drug brand names
- Amiodarone • Cordarone
- Atenolol • Tenormin
- Atorvastatin • Lipitor
- Diclofenac • Cataflam, Voltaren
- Disopyramide • Norpace
- Dofetilide • Tikosyn
- Enoxaparin • Lovenox
- Flecainide • Tambocor
- Metoprolol • Lopressor
- Propafenone • Rythmol
- Simvastatin • Zocor
- Solatol • Betapace
- Warfarin • Coumadin
Moderate exercise reduces mortality for patients with known coronary heart disease but does not significantly decrease the risk of recurrent nonfatal myocardial infarction (MI) (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials). Exercise-based cardiac rehabilitation also reduces all-cause mortality (SOR: A, based on systematic review).
For patients with stable angina, a daily exercise program is more effective than percutaneous transluminal coronary angioplasty (PTCA) with stenting in preventing major cardiovascular events (number needed to treat [NNT]=5.5; SOR: A, based on a single randomized controlled trial).
Evidence summary
A systematic review of cardiac rehabilitation programs evaluated 14 randomized controlled trials with exercise-based interventions.1 An updated review added 5 more for a total of 2984 patients with coronary heart disease.2 Patients with coronary heart disease comprised those with prior MI, prior coronary artery bypass graft surgery, or PTCA, and those with angina pectoris and angiographically confirmed coronary heart disease.
Exercise-based cardiac rehabilitation significantly reduced all-cause mortality (relative risk [RR]=0.76; 95% confidence interval [CI], 0.59–0.98) compared with usual care (NNT=66; 95% CI, 35–273). Cardiac mortality also decreased significantly with exercise (RR=0.73; 95% CI, 0.56–0.96) compared with usual care (NNT=49; 95% CI, 26–120).
Six studies showed particularly significant improvement in total cardiac mortality.3-8 Exercise was variably defined. Training sessions lasted 30 minutes and occurred on 2 to 5 days per week. Intensity was typically 75% to 85% of a maximum work capacity determined on an exercise test before initiating the training sessions. The type of exercise ranged from cycling alone to circuit training with 6 stationary devices. Patients were trained with supervision 1 to 36 months and followed for a mean of 24 months (range, 6–60 months).
A trend was observed toward decreased recurrence of nonfatal MI with exercise-based cardiac rehabilitation, which did not reach significance (RR=0.78; 95% CI, 0.59–1.03). An inadequate number of subjects is the most likely reason; however, other possibilities include an increase in the frequency of nonfatal MI after rehabilitation, or an increased rate of survival after MI for patients undergoing exercise-based rehabilitation.
The studies included in these reviews had several limitations. The population appears skewed in age (mean=54 years, with patients aged >65 years excluded from most studies) and gender (4.9% female); ethnicity was rarely reported. The adequacy of randomization was poor or unclear in 71% of studies, and only 4 trials reported blind assessment of outcomes. Finally, in 34% of studies the loss of participants to follow-up was more than 20%.
A well-done study randomized 101 male patients (age <70 years) with stable angina to either a daily exercise program or standard PTCA with stenting.9 After 12 months, event-free survival was significantly greater among patients randomized to exercise than in those randomized to PTCA with stenting (88% vs 70%; P=.023; NNT=5.5). Cardiovascular events were defined as percutaneous interventions, hospitalizations, acute MI, cerebrovascular accidents, coronary artery bypass graft operation, and death.
Recommendation from others
The American Heart Association (AHA) supports aggressive risk factor management for patients with coronary heart disease, and recommends a minimum of 30 minutes of exercise 3 to 4 days per week as well as an increase in daily lifestyle activities.10 The American College of Cardiology endorses the position of the AHA.
Add exercise to routine post-MI treatment
Bill Kerns, MD
Shenandoah Valley Family Practice Residency, Virginia Commonwealth University/Medical College of Virginia, Winchester
We should add exercise to routine post-MI treatment checklists, along with aspirin, beta-blockers, statins, angiotensin-converting enzyme inhibitors, and so on. Precise exercise prescribing requires a stress test because, as the adage goes, “If we don’t do an exercise test with monitoring, the patient will eventually do one unmonitored at home.”
Medicare pays for cardiac rehabilitation for acute MI (within 6 months), coronary artery bypass (within a year), and stable angina. Other insurance reimbursement varies.
The evidence isn’t the quality I would like, and for women and minorities it is lacking. However, evidence sticklers like USPSTF11 state that exercise reduces morbidity and mortality for (almost) everyone. The question is how to make exercise happen; people with CHD can often be motivated.
Drug brand names
- Amiodarone • Cordarone
- Atenolol • Tenormin
- Atorvastatin • Lipitor
- Diclofenac • Cataflam, Voltaren
- Disopyramide • Norpace
- Dofetilide • Tikosyn
- Enoxaparin • Lovenox
- Flecainide • Tambocor
- Metoprolol • Lopressor
- Propafenone • Rythmol
- Simvastatin • Zocor
- Solatol • Betapace
- Warfarin • Coumadin
1. Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease (Cochrane Review). In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
2. Brown A, Noorani H, Taylor R, Stone J, Skidmore B. A clinical and economic review of exercise-based cardiac rehabilitation programs for coronary artery disease. Technology overview no. 11. Ottawa: Canadian Coordinating Office for Health Technology Assessment; 2003.
3. Carson P, Phillips R, Lloyd M, et al. Exercise after myocardial infarction: a controlled trial. J R Coll Physicians Lond 1982;16:147-151.
4. Kentala E. Physical fitness and feasibility of physical rehabilitation after myocardial infarction in men of working age. Ann Clin Res 1972;4 Suppl 9:1-84.
5. Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction. The National Exercise and Heart Disease Project. Am J Cardiol 1981;48:39-46.
6. Specchia G, DeServi S, Scire A, et al. Interaction between exercise training and ejection fraction in predicting prognosis after a first myocardial infarction. Circulation 1996;94:978-982.
7. Sanne H. Exercise tolerance and physical training of non-selected patients after myocardial infarction. Acta Med Scan Suppl 1973;551:1-124.
8. Wilhelmsen L, Sanne H, Elmfeldt D, Grimby G, Tibblin G, Wedel H. A controlled trial of physical training after myocardial infarction. Effects on risk factors, nonfatal reinfarction an death. Prev Med 1975;4:491-508.
9. Hambrecht R, Walther C, Möbius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation 2004;109:1371-1378.
10. Smith SC, Blair SN, Bonow RO, et al. AHA/ACC Scientific Statement: AHA/ACC guidelines for preventing heart attack and death in patients with atherosclerotic cardiovascular disease: 2001 update. A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation 2001;104:1577-
11. US Preventive Services Task Force. Behavioral counseling in primary care to promote physical activity: recommendation and rationale. Ann Intern Med 2002;137:205-207.
1. Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease (Cochrane Review). In: The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons, Ltd.
2. Brown A, Noorani H, Taylor R, Stone J, Skidmore B. A clinical and economic review of exercise-based cardiac rehabilitation programs for coronary artery disease. Technology overview no. 11. Ottawa: Canadian Coordinating Office for Health Technology Assessment; 2003.
3. Carson P, Phillips R, Lloyd M, et al. Exercise after myocardial infarction: a controlled trial. J R Coll Physicians Lond 1982;16:147-151.
4. Kentala E. Physical fitness and feasibility of physical rehabilitation after myocardial infarction in men of working age. Ann Clin Res 1972;4 Suppl 9:1-84.
5. Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction. The National Exercise and Heart Disease Project. Am J Cardiol 1981;48:39-46.
6. Specchia G, DeServi S, Scire A, et al. Interaction between exercise training and ejection fraction in predicting prognosis after a first myocardial infarction. Circulation 1996;94:978-982.
7. Sanne H. Exercise tolerance and physical training of non-selected patients after myocardial infarction. Acta Med Scan Suppl 1973;551:1-124.
8. Wilhelmsen L, Sanne H, Elmfeldt D, Grimby G, Tibblin G, Wedel H. A controlled trial of physical training after myocardial infarction. Effects on risk factors, nonfatal reinfarction an death. Prev Med 1975;4:491-508.
9. Hambrecht R, Walther C, Möbius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation 2004;109:1371-1378.
10. Smith SC, Blair SN, Bonow RO, et al. AHA/ACC Scientific Statement: AHA/ACC guidelines for preventing heart attack and death in patients with atherosclerotic cardiovascular disease: 2001 update. A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation 2001;104:1577-
11. US Preventive Services Task Force. Behavioral counseling in primary care to promote physical activity: recommendation and rationale. Ann Intern Med 2002;137:205-207.
Evidence-based answers from the Family Physicians Inquiries Network
Do statins reduce the risk of stroke?
HMG Co-A reductase inhibitors (statins) are effective for primary prevention of ischemic stroke in people who have a history of occlusive artery disease, coronary artery disease, or diabetes without history of cerebrovascular disease (strength of recommendation [SOR]: A, based on 1 randomized controlled trial [RCT]).
Statins reduce the risk of ischemic stroke in hypertensive patients with multiple cardiovascular risk factors and nonfasting total cholesterol <250 mg/dL (SOR: A, based on RCT). Statins also reduce the risk of ischemic stroke for patients with coronary disease or equivalents (such as diabetes or peripheral artery disease), including patients who have a normal fasting lipid profile (SOR: A, based on RCT). For patients with ischemic stroke who have coronary disease, statins prevent recurrent ischemic stroke; evidence is conflicting about whether this benefit is proportional to initial cholesterol levels (SOR: A, systematic review). Statins do not prevent hemorrhagic stroke (SOR: A, based on RCTs).
Evidence summary
We found no studies evaluating statins for the primary prevention of stroke. An observational study of 433 patients with ischemic stroke found that patients who were taking statins before hospital admission more often had better outcomes (51%) than those who were not taking statins (38%). However, the groups differed in many respects.1 Many coronary event prevention and treatment trials using statins include the risk of primary and recurrent ischemic stroke as secondary endpoints for patients with high cardiac risk.
Primary prevention of stroke in vascular disease. The Heart Protection Study followed 20,536 patients in the United Kingdom (aged 40–80 years), 3280 with a history of cerebrovascular disease (defined as nondisabling stroke, transient cerebral ischemic attack, or carotid endarterectomy or angioplasty) and 17,256 with other occlusive arterial disease, coronary artery disease, or diabetes. Patients were randomized to receive either simvastatin 40 mg or placebo for an average of 5 years. The endpoint was major vascular events: myocardial infarction, stroke of any type, and revascularization procedure.
Simvastatin reduced the combined risk of non-fatal or fatal ischemic stroke for patients with no history of cerebrovascular disease (3.2% for simvastatin vs 4.8% with placebo; relative risk reduction=33%, number needed to treat [NNT]=63; P=.0001).2 As noted in other well-done studies, the Heart Protection Study showed no difference in the number of hemorrhagic strokes between treatment and placebo groups. There were 3500 subjects with pretreatment low-density lipoprotein (LDL) cholesterol <100 mg/dL; lowering LDL to 65 mg/dL reduced major vascular event risk by about 25%.3
Hypertension with multiple cardiovascular risk factors and cholesterol <250 mg/dL. The ASCOT-LLA study compared atorvastatin with placebo in 10,305 hypertensive Caucasian patients with multiple cardiovascular risk factors and a total nonfasting cholesterol of 250 mg/dL (6.5 mmol/L) or less. Patients were aged 40 to 79 years and had at least 3 other cardiovascular risk factors (left ventricular hypertrophy, abnormal electrocardiogram, type 2 diabetes, peripheral artery disease, stroke or transient ischemic attack, male sex, age >55 years, proteinuria or microalbuminuria, smoking, family history of premature coronary heart disease). The study was stopped early at a median of 3.3 years because atorvastatin significantly reduced cardiac events. Atorvastatin also significantly reduced ischemic strokes when compared with placebo (relative risk [RR]=0.73, 95% confidence interval [CI], 0.56–0.96; P=.024). This study did not differentiate between first or second stroke. The NNT was 155.4
Ischemic stroke and coronary disease. The LIPID trial randomized 9014 patients with a history of acute coronary syndromes and total cholesterol of 150 to 270 mg/dL (4 to 7 mmol/L) to either pravastatin or placebo and followed them for 6 years. Among the 350 patients with prior ischemic stroke, there were 388 new ischemic stokes over the course of the study. When adjusted for risk factors (atrial fibrillation, history of cerebrovascular accident, diabetes, hypertension, cigarette smoking, body mass index, and male sex), pravastatin reduced recurrent ischemic stroke by 21% relative to placebo (P=.024). The reduction was not modified by baseline lipid level.5
A meta-analysis of 15 randomized placebo-controlled trials using various statins (32,684 participants) assessed the risk of strokes for patients with a history of coronary disease. Among patients who had cerebrovascular disease, statins significantly reduced recurrent ischemic stroke (RR=0.74; 95% CI, 0.64–0.86). One recurrence of ischemic stroke would be prevented for every 110 coronary disease patients treated with a statin. Achieving final total cholesterol <232 mg/dL correlated with reduced risk of recurrent stroke.6 Three of the studies evaluated primary prevention of stroke and did not show a significant risk reduction (RR=0.85; P=.4). Statins did not reduce the rate of hemorrhagic stroke or fatal strokes.
Risks of statins. In 1 study involving 35,000 participants and 158,000 person-years of observation, there were 8 cases of rhabdomyolysis in the treatment groups vs 5 in the placebo groups.7 Forty-three deaths attributed to statin therapy have been reported to the Food and Drug Administration from 1987 to 2001, or 1 per million person-years of use. The Heart Protection Study found simvastatin and placebo users reported myopathy or muscle pain at the same annual rate of 0.01%.
Recommendations from others
We found no recommendations specifically regarding the use of statins to prevent stroke. However, the Third Report of the National Cholesterol Education Program, Adult Treatment Panel III (NCEP-ATP III) describes symptomatic carotid artery disease as a coronary heart disease risk equivalent and recommends therapy to reduce the LDL below 100 mg/dL.8
Statins prevent cerebrovascular accidents and have low adverse event rates
Alex Krist, MD
Fairfax Family Practice Residency, Virginia Commonwealth University, Fairfax
Statins are effective for primary and tertiary cardiovascular disease prevention. For those with vascular disease or significant risks, statins prevent cerebrovascular accidents and have low adverse event rates.
While no evidence is available about primary prevention of cerebrovascular accidents for those at lower risk, in practice statins are often appropriately initiated. NCEP-ATP III,8 the key guideline on when to start statins, is based more on cardiac benefits. Most studies evaluating statins use a triple outcome of mortality, myocardial infarction, or cerebrovascular accident. Since myocardial infarction is more common than the other adverse endpoints, there is a greater demonstrated cardioprotective effect (prevention of myocardial infarction: NNT=95; prevention of cerebrovascular accidents: NNT=735).9 However, regardless of whether the benefits are cardiac or cerebrovascular, statins will prevent disease for many patients.
1. Yoon SS, Dambrosia J, Chalela J, Ezzeddine M, Warach S, Haymore J, Davis L, Baird AE. Rising statin use and effect on ischemic stroke outcome. BMC Med 2004;2:4.-Available at: www.biomedcentral.com/1741-7015/2/4. Accessed on April 8, 2004.
2. Collins R, Armitage J, Parish S, Sleight P, Peto R. Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004;363:757-767.
3. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7-22.
4. Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149-1158.
5. West MJ, White HD, Simes RJ, Kirby A, Watson JD, Anderson NE, et al. Risk factors for non-haemorrhagic stroke in patients with coronary heart disease and the effect of lipid-modifying therapy with pravastatin. J Hypertens 2002;20:2513-2517.
6. Corvol JC, Bouzamondo A, Sirol M, Hulot JS, Sanchez P, Lechat P. Differential effects of lipid-lowering therapies on stroke prevention: a meta-analysis of randomized trials. Arch Intern Med 2003;163:669-676.
7. Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low-density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326:1423-1427.
8. National Cholesterol Education Program. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. NIH Pub. No. 02-5215. Bethesda, Md: National Heart, Lung, and Blood Institute; 2002.
9. Corvol JC, Bouzamondo A, Sirol M, Hulot JS, Sanchez P, Lechat P. Differential effects of lipid-lowering therapies on stroke prevention: a meta-analysis of randomized trials. Arch Intern Med 2003;163:669-676.
HMG Co-A reductase inhibitors (statins) are effective for primary prevention of ischemic stroke in people who have a history of occlusive artery disease, coronary artery disease, or diabetes without history of cerebrovascular disease (strength of recommendation [SOR]: A, based on 1 randomized controlled trial [RCT]).
Statins reduce the risk of ischemic stroke in hypertensive patients with multiple cardiovascular risk factors and nonfasting total cholesterol <250 mg/dL (SOR: A, based on RCT). Statins also reduce the risk of ischemic stroke for patients with coronary disease or equivalents (such as diabetes or peripheral artery disease), including patients who have a normal fasting lipid profile (SOR: A, based on RCT). For patients with ischemic stroke who have coronary disease, statins prevent recurrent ischemic stroke; evidence is conflicting about whether this benefit is proportional to initial cholesterol levels (SOR: A, systematic review). Statins do not prevent hemorrhagic stroke (SOR: A, based on RCTs).
Evidence summary
We found no studies evaluating statins for the primary prevention of stroke. An observational study of 433 patients with ischemic stroke found that patients who were taking statins before hospital admission more often had better outcomes (51%) than those who were not taking statins (38%). However, the groups differed in many respects.1 Many coronary event prevention and treatment trials using statins include the risk of primary and recurrent ischemic stroke as secondary endpoints for patients with high cardiac risk.
Primary prevention of stroke in vascular disease. The Heart Protection Study followed 20,536 patients in the United Kingdom (aged 40–80 years), 3280 with a history of cerebrovascular disease (defined as nondisabling stroke, transient cerebral ischemic attack, or carotid endarterectomy or angioplasty) and 17,256 with other occlusive arterial disease, coronary artery disease, or diabetes. Patients were randomized to receive either simvastatin 40 mg or placebo for an average of 5 years. The endpoint was major vascular events: myocardial infarction, stroke of any type, and revascularization procedure.
Simvastatin reduced the combined risk of non-fatal or fatal ischemic stroke for patients with no history of cerebrovascular disease (3.2% for simvastatin vs 4.8% with placebo; relative risk reduction=33%, number needed to treat [NNT]=63; P=.0001).2 As noted in other well-done studies, the Heart Protection Study showed no difference in the number of hemorrhagic strokes between treatment and placebo groups. There were 3500 subjects with pretreatment low-density lipoprotein (LDL) cholesterol <100 mg/dL; lowering LDL to 65 mg/dL reduced major vascular event risk by about 25%.3
Hypertension with multiple cardiovascular risk factors and cholesterol <250 mg/dL. The ASCOT-LLA study compared atorvastatin with placebo in 10,305 hypertensive Caucasian patients with multiple cardiovascular risk factors and a total nonfasting cholesterol of 250 mg/dL (6.5 mmol/L) or less. Patients were aged 40 to 79 years and had at least 3 other cardiovascular risk factors (left ventricular hypertrophy, abnormal electrocardiogram, type 2 diabetes, peripheral artery disease, stroke or transient ischemic attack, male sex, age >55 years, proteinuria or microalbuminuria, smoking, family history of premature coronary heart disease). The study was stopped early at a median of 3.3 years because atorvastatin significantly reduced cardiac events. Atorvastatin also significantly reduced ischemic strokes when compared with placebo (relative risk [RR]=0.73, 95% confidence interval [CI], 0.56–0.96; P=.024). This study did not differentiate between first or second stroke. The NNT was 155.4
Ischemic stroke and coronary disease. The LIPID trial randomized 9014 patients with a history of acute coronary syndromes and total cholesterol of 150 to 270 mg/dL (4 to 7 mmol/L) to either pravastatin or placebo and followed them for 6 years. Among the 350 patients with prior ischemic stroke, there were 388 new ischemic stokes over the course of the study. When adjusted for risk factors (atrial fibrillation, history of cerebrovascular accident, diabetes, hypertension, cigarette smoking, body mass index, and male sex), pravastatin reduced recurrent ischemic stroke by 21% relative to placebo (P=.024). The reduction was not modified by baseline lipid level.5
A meta-analysis of 15 randomized placebo-controlled trials using various statins (32,684 participants) assessed the risk of strokes for patients with a history of coronary disease. Among patients who had cerebrovascular disease, statins significantly reduced recurrent ischemic stroke (RR=0.74; 95% CI, 0.64–0.86). One recurrence of ischemic stroke would be prevented for every 110 coronary disease patients treated with a statin. Achieving final total cholesterol <232 mg/dL correlated with reduced risk of recurrent stroke.6 Three of the studies evaluated primary prevention of stroke and did not show a significant risk reduction (RR=0.85; P=.4). Statins did not reduce the rate of hemorrhagic stroke or fatal strokes.
Risks of statins. In 1 study involving 35,000 participants and 158,000 person-years of observation, there were 8 cases of rhabdomyolysis in the treatment groups vs 5 in the placebo groups.7 Forty-three deaths attributed to statin therapy have been reported to the Food and Drug Administration from 1987 to 2001, or 1 per million person-years of use. The Heart Protection Study found simvastatin and placebo users reported myopathy or muscle pain at the same annual rate of 0.01%.
Recommendations from others
We found no recommendations specifically regarding the use of statins to prevent stroke. However, the Third Report of the National Cholesterol Education Program, Adult Treatment Panel III (NCEP-ATP III) describes symptomatic carotid artery disease as a coronary heart disease risk equivalent and recommends therapy to reduce the LDL below 100 mg/dL.8
Statins prevent cerebrovascular accidents and have low adverse event rates
Alex Krist, MD
Fairfax Family Practice Residency, Virginia Commonwealth University, Fairfax
Statins are effective for primary and tertiary cardiovascular disease prevention. For those with vascular disease or significant risks, statins prevent cerebrovascular accidents and have low adverse event rates.
While no evidence is available about primary prevention of cerebrovascular accidents for those at lower risk, in practice statins are often appropriately initiated. NCEP-ATP III,8 the key guideline on when to start statins, is based more on cardiac benefits. Most studies evaluating statins use a triple outcome of mortality, myocardial infarction, or cerebrovascular accident. Since myocardial infarction is more common than the other adverse endpoints, there is a greater demonstrated cardioprotective effect (prevention of myocardial infarction: NNT=95; prevention of cerebrovascular accidents: NNT=735).9 However, regardless of whether the benefits are cardiac or cerebrovascular, statins will prevent disease for many patients.
HMG Co-A reductase inhibitors (statins) are effective for primary prevention of ischemic stroke in people who have a history of occlusive artery disease, coronary artery disease, or diabetes without history of cerebrovascular disease (strength of recommendation [SOR]: A, based on 1 randomized controlled trial [RCT]).
Statins reduce the risk of ischemic stroke in hypertensive patients with multiple cardiovascular risk factors and nonfasting total cholesterol <250 mg/dL (SOR: A, based on RCT). Statins also reduce the risk of ischemic stroke for patients with coronary disease or equivalents (such as diabetes or peripheral artery disease), including patients who have a normal fasting lipid profile (SOR: A, based on RCT). For patients with ischemic stroke who have coronary disease, statins prevent recurrent ischemic stroke; evidence is conflicting about whether this benefit is proportional to initial cholesterol levels (SOR: A, systematic review). Statins do not prevent hemorrhagic stroke (SOR: A, based on RCTs).
Evidence summary
We found no studies evaluating statins for the primary prevention of stroke. An observational study of 433 patients with ischemic stroke found that patients who were taking statins before hospital admission more often had better outcomes (51%) than those who were not taking statins (38%). However, the groups differed in many respects.1 Many coronary event prevention and treatment trials using statins include the risk of primary and recurrent ischemic stroke as secondary endpoints for patients with high cardiac risk.
Primary prevention of stroke in vascular disease. The Heart Protection Study followed 20,536 patients in the United Kingdom (aged 40–80 years), 3280 with a history of cerebrovascular disease (defined as nondisabling stroke, transient cerebral ischemic attack, or carotid endarterectomy or angioplasty) and 17,256 with other occlusive arterial disease, coronary artery disease, or diabetes. Patients were randomized to receive either simvastatin 40 mg or placebo for an average of 5 years. The endpoint was major vascular events: myocardial infarction, stroke of any type, and revascularization procedure.
Simvastatin reduced the combined risk of non-fatal or fatal ischemic stroke for patients with no history of cerebrovascular disease (3.2% for simvastatin vs 4.8% with placebo; relative risk reduction=33%, number needed to treat [NNT]=63; P=.0001).2 As noted in other well-done studies, the Heart Protection Study showed no difference in the number of hemorrhagic strokes between treatment and placebo groups. There were 3500 subjects with pretreatment low-density lipoprotein (LDL) cholesterol <100 mg/dL; lowering LDL to 65 mg/dL reduced major vascular event risk by about 25%.3
Hypertension with multiple cardiovascular risk factors and cholesterol <250 mg/dL. The ASCOT-LLA study compared atorvastatin with placebo in 10,305 hypertensive Caucasian patients with multiple cardiovascular risk factors and a total nonfasting cholesterol of 250 mg/dL (6.5 mmol/L) or less. Patients were aged 40 to 79 years and had at least 3 other cardiovascular risk factors (left ventricular hypertrophy, abnormal electrocardiogram, type 2 diabetes, peripheral artery disease, stroke or transient ischemic attack, male sex, age >55 years, proteinuria or microalbuminuria, smoking, family history of premature coronary heart disease). The study was stopped early at a median of 3.3 years because atorvastatin significantly reduced cardiac events. Atorvastatin also significantly reduced ischemic strokes when compared with placebo (relative risk [RR]=0.73, 95% confidence interval [CI], 0.56–0.96; P=.024). This study did not differentiate between first or second stroke. The NNT was 155.4
Ischemic stroke and coronary disease. The LIPID trial randomized 9014 patients with a history of acute coronary syndromes and total cholesterol of 150 to 270 mg/dL (4 to 7 mmol/L) to either pravastatin or placebo and followed them for 6 years. Among the 350 patients with prior ischemic stroke, there were 388 new ischemic stokes over the course of the study. When adjusted for risk factors (atrial fibrillation, history of cerebrovascular accident, diabetes, hypertension, cigarette smoking, body mass index, and male sex), pravastatin reduced recurrent ischemic stroke by 21% relative to placebo (P=.024). The reduction was not modified by baseline lipid level.5
A meta-analysis of 15 randomized placebo-controlled trials using various statins (32,684 participants) assessed the risk of strokes for patients with a history of coronary disease. Among patients who had cerebrovascular disease, statins significantly reduced recurrent ischemic stroke (RR=0.74; 95% CI, 0.64–0.86). One recurrence of ischemic stroke would be prevented for every 110 coronary disease patients treated with a statin. Achieving final total cholesterol <232 mg/dL correlated with reduced risk of recurrent stroke.6 Three of the studies evaluated primary prevention of stroke and did not show a significant risk reduction (RR=0.85; P=.4). Statins did not reduce the rate of hemorrhagic stroke or fatal strokes.
Risks of statins. In 1 study involving 35,000 participants and 158,000 person-years of observation, there were 8 cases of rhabdomyolysis in the treatment groups vs 5 in the placebo groups.7 Forty-three deaths attributed to statin therapy have been reported to the Food and Drug Administration from 1987 to 2001, or 1 per million person-years of use. The Heart Protection Study found simvastatin and placebo users reported myopathy or muscle pain at the same annual rate of 0.01%.
Recommendations from others
We found no recommendations specifically regarding the use of statins to prevent stroke. However, the Third Report of the National Cholesterol Education Program, Adult Treatment Panel III (NCEP-ATP III) describes symptomatic carotid artery disease as a coronary heart disease risk equivalent and recommends therapy to reduce the LDL below 100 mg/dL.8
Statins prevent cerebrovascular accidents and have low adverse event rates
Alex Krist, MD
Fairfax Family Practice Residency, Virginia Commonwealth University, Fairfax
Statins are effective for primary and tertiary cardiovascular disease prevention. For those with vascular disease or significant risks, statins prevent cerebrovascular accidents and have low adverse event rates.
While no evidence is available about primary prevention of cerebrovascular accidents for those at lower risk, in practice statins are often appropriately initiated. NCEP-ATP III,8 the key guideline on when to start statins, is based more on cardiac benefits. Most studies evaluating statins use a triple outcome of mortality, myocardial infarction, or cerebrovascular accident. Since myocardial infarction is more common than the other adverse endpoints, there is a greater demonstrated cardioprotective effect (prevention of myocardial infarction: NNT=95; prevention of cerebrovascular accidents: NNT=735).9 However, regardless of whether the benefits are cardiac or cerebrovascular, statins will prevent disease for many patients.
1. Yoon SS, Dambrosia J, Chalela J, Ezzeddine M, Warach S, Haymore J, Davis L, Baird AE. Rising statin use and effect on ischemic stroke outcome. BMC Med 2004;2:4.-Available at: www.biomedcentral.com/1741-7015/2/4. Accessed on April 8, 2004.
2. Collins R, Armitage J, Parish S, Sleight P, Peto R. Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004;363:757-767.
3. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7-22.
4. Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149-1158.
5. West MJ, White HD, Simes RJ, Kirby A, Watson JD, Anderson NE, et al. Risk factors for non-haemorrhagic stroke in patients with coronary heart disease and the effect of lipid-modifying therapy with pravastatin. J Hypertens 2002;20:2513-2517.
6. Corvol JC, Bouzamondo A, Sirol M, Hulot JS, Sanchez P, Lechat P. Differential effects of lipid-lowering therapies on stroke prevention: a meta-analysis of randomized trials. Arch Intern Med 2003;163:669-676.
7. Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low-density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326:1423-1427.
8. National Cholesterol Education Program. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. NIH Pub. No. 02-5215. Bethesda, Md: National Heart, Lung, and Blood Institute; 2002.
9. Corvol JC, Bouzamondo A, Sirol M, Hulot JS, Sanchez P, Lechat P. Differential effects of lipid-lowering therapies on stroke prevention: a meta-analysis of randomized trials. Arch Intern Med 2003;163:669-676.
1. Yoon SS, Dambrosia J, Chalela J, Ezzeddine M, Warach S, Haymore J, Davis L, Baird AE. Rising statin use and effect on ischemic stroke outcome. BMC Med 2004;2:4.-Available at: www.biomedcentral.com/1741-7015/2/4. Accessed on April 8, 2004.
2. Collins R, Armitage J, Parish S, Sleight P, Peto R. Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004;363:757-767.
3. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7-22.
4. Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149-1158.
5. West MJ, White HD, Simes RJ, Kirby A, Watson JD, Anderson NE, et al. Risk factors for non-haemorrhagic stroke in patients with coronary heart disease and the effect of lipid-modifying therapy with pravastatin. J Hypertens 2002;20:2513-2517.
6. Corvol JC, Bouzamondo A, Sirol M, Hulot JS, Sanchez P, Lechat P. Differential effects of lipid-lowering therapies on stroke prevention: a meta-analysis of randomized trials. Arch Intern Med 2003;163:669-676.
7. Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low-density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326:1423-1427.
8. National Cholesterol Education Program. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. NIH Pub. No. 02-5215. Bethesda, Md: National Heart, Lung, and Blood Institute; 2002.
9. Corvol JC, Bouzamondo A, Sirol M, Hulot JS, Sanchez P, Lechat P. Differential effects of lipid-lowering therapies on stroke prevention: a meta-analysis of randomized trials. Arch Intern Med 2003;163:669-676.
Evidence-based answers from the Family Physicians Inquiries Network
Which healthy adults should take aspirin?
In adults with no history of cardiovascular disease, aspirin reduces the risk of nonfatal myocardial infarction (MI). Aspirin prophylaxis does not decrease all-cause mortality, risk of fatal coronary heart disease, or risk of first stroke (strength of recommendation [SOR]: A–, based on multiple randomized controlled trials).
The benefits of aspirin use must be weighed against its potential risks, primarily gastrointestinal bleeding and cerebral hemorrhage. The benefit of aspirin increases with higher levels of cardiovascular risk, while the potential for harm remains relatively constant. Adults with a calculated 5-year coronary heart disease (CHD) event risk of 3% or greater should receive prophylaxis (SOR: A, based on multiple randomized controlled trials). The ideal dose of aspirin for prophylaxis is unknown, but it appears that low doses (75–81 mg/d) are as effective as higher doses.
Evidence summary
The leading cause of morbidity and mortality in the United States is cardiovascular disease (ischemic CHD, stroke, peripheral vascular disease).1 A meta-analysis of 5 placebo-controlled randomized controlled trials involving more than 50,000 patients free of CHD and stroke evaluated aspirin for primary prevention of cardiovascular disease. Since 3 of the trials excluded women, only 20% of the participants were female. The mean age of participants was 57 years.
The treatment groups took aspirin 75 to 500 mg/d for 3 to 7 years. The meta-analysis found that compared with placebo, aspirin significantly reduced total CHD events (odds ratio [OR]=0.72; 95% confidence interval [CI], 0.60–0.87).2 Aspirin did not reduce coronary disease mortality (OR=0.87; 95% CI, 0.70–1.09); however, results from 1 study did achieve statistical significance (OR=0.64; 95% CI, 0.42–0.99).3 No differences were found between aspirin-treated and control groups for all-cause mortality or ischemic stroke reduction.
Aspirin increased the risk of major gastrointestinal bleeding events by almost twofold (OR=1.70; 95% CI, 1.4–2.1). Three of the 5 trials showed no significant increase of intracranial hemorrhage event rates (OR=1.4; 95% CI, 0.9–2.0). Based on combined primary and secondary prevention trials, the risk of intracranial bleeding with aspirin is estimated at 0 to 2 events per 1000 patients per year.2
Although the ideal aspirin dosage is uncertain, lower dosages (75–81 mg/d) have been shown to be as beneficial as higher dosages, and may have fewer bleeding complications. Buffered and entericcoated formulations are no more protective than plain aspirin.4
In patients with no known cardiovascular disease, aspirin chemoprevention has been shown to decrease the risk of nonfatal MI and fatal CHD by 28%. At a 5-year CHD risk of 3%, the benefits of prophylaxis outweigh the harms (see Table ) by 2 to 1—assuming the events of stroke, MI, and bleeding are considered roughly equivalent in severity. (A different threshold may be appropriate for patients that perceive 1 of these events as significantly more serious than the others.) Typical patients at a 3% or greater risk for cardiovascular disease include men aged >40 years, post-menopausal women, and younger persons with risk factors for CHD. Physicians determine cardiovascular risk from the presence and severity of risk factors: gender, age, blood pressure, lipid status, diabetes, and smoking status.
Simple risk-assessment tools based on Framingham data are available for computers and palmtop devices (eg, Heart to Heart CV Risk Assessment Calculator, www.meddecisions.com; National Institutes of Health, www.nhlbi.nih.gov/health/prof/heart/). Because only 2 trials included women, it is less clear whether both sexes benefit equally from aspirin prophylaxis.1
TABLE
Net benefits and harms of aspirin prophylaxis, per 1000 patients
| Outcome | Estimated 5-year risk for CHD event | ||
|---|---|---|---|
| 1% | 3% | 5% | |
| All-cause mortality | NS | NS | NS |
| CHD events avoided | 3 | 8 | 14 |
| Ischemic strokes avoided | NS | NS | NS |
| Hemorrhagic strokes | 1 | 1 | 1 |
| Major gastrointestinal bleeding | 3 | 3 | 3 |
| NS, not significant | |||
Recommendations from others
The US Preventive Services Task Force recommends that clinicians discuss aspirin prophylaxis with adults at increased risk for CHD (defined as a 5-year risk of 3% or more). Discussion should include the potential benefits and harms of aspirin therapy.5
The American Heart Association recommends low-dose aspirin in people at higher risk of coronary heart disease (especially those with a 10-year CHD risk of 10% or greater).6 The European Society of Cardiology says there is evidence that low-dose aspirin can reduce the risk of cardiovascular events in asymptomatic high-risk people, such as those with diabetes or well-controlled hypertension, and in men at high multifactorial risk of cardiovascular disease.7
Aspirin: effective, safe, inexpensive—and it may prevent heart attacks
Paul V. Aitken, Jr, MD, MPH
Residency in Family Medicine, University of North Carolina at Chapel Hill; New Hanover Regional Medical Center, Wilmington, NC
Acetylsalicylic acid was first compounded in Germany by chemist Felix Hoffman in 1897. According to information from the Bayer Company, aspirin’s cardioprotective effect was first recognized by Dr Lawrence Craven, a California general practitioner. He noted a decreased rate of heart attacks in patients taking this medication.
We now have evidence supporting Dr Craven’s astute clinical observation. In adults with no history of cardiovascular disease, aspirin reduces the risk of nonfatal MI. For an individual at a 5-year CHD risk as low as 3%, the benefits of prophylaxis outweigh the harms. The leading cause of morbidity and mortality in the US is still cardiovascular disease. A simple, effective, safe, and inexpensive preventive measure like recommending aspirin has the potential to prevent heart attacks on a grand scale. A low-dose aspirin per day should be recommended for patients at risk for cardiovascular disease, including men aged >40 years, postmenopausal women, and younger persons with risk factors for CHD. As a 40-something male with a family history of cardiovascular disease reviewing this Clinical Inquiry, I will be taking my aspirin a day.
1. Hoyert DL, Arias E, Smith BL, Murphy SL, Kochanek KD. Deaths: final data for 1999. Natl Vital Stat Rep. 2001;49:1-113.
2. Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;136:161-172.
3. Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989;321:129-135.
4. Hart RG, Halperin JL, McBride R, Benavente O, Man-Son-Hing M, Kronmal RA. Aspirin for the primary prevention of stroke and other major vascular events; meta-analysis and hypotheses. Arch Neurol 2000;57:326-332.
5. US Preventive Services Task Force. Aspirin for the primary prevention of cardiovascular events: chemoprevention. January 2002. Available at www.ahrq.gov/clinic/uspstf/uspsasmi.htm. Accessed on January 6, 2004.
6. American Heart Association. Primary prevention in the adult. 2003. Available at: www.americanheart.org/presen-ter.jhtml?identifier=4704. Accessed on January 6, 2004.
7. De Backer G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2003;24:1601-1610.
In adults with no history of cardiovascular disease, aspirin reduces the risk of nonfatal myocardial infarction (MI). Aspirin prophylaxis does not decrease all-cause mortality, risk of fatal coronary heart disease, or risk of first stroke (strength of recommendation [SOR]: A–, based on multiple randomized controlled trials).
The benefits of aspirin use must be weighed against its potential risks, primarily gastrointestinal bleeding and cerebral hemorrhage. The benefit of aspirin increases with higher levels of cardiovascular risk, while the potential for harm remains relatively constant. Adults with a calculated 5-year coronary heart disease (CHD) event risk of 3% or greater should receive prophylaxis (SOR: A, based on multiple randomized controlled trials). The ideal dose of aspirin for prophylaxis is unknown, but it appears that low doses (75–81 mg/d) are as effective as higher doses.
Evidence summary
The leading cause of morbidity and mortality in the United States is cardiovascular disease (ischemic CHD, stroke, peripheral vascular disease).1 A meta-analysis of 5 placebo-controlled randomized controlled trials involving more than 50,000 patients free of CHD and stroke evaluated aspirin for primary prevention of cardiovascular disease. Since 3 of the trials excluded women, only 20% of the participants were female. The mean age of participants was 57 years.
The treatment groups took aspirin 75 to 500 mg/d for 3 to 7 years. The meta-analysis found that compared with placebo, aspirin significantly reduced total CHD events (odds ratio [OR]=0.72; 95% confidence interval [CI], 0.60–0.87).2 Aspirin did not reduce coronary disease mortality (OR=0.87; 95% CI, 0.70–1.09); however, results from 1 study did achieve statistical significance (OR=0.64; 95% CI, 0.42–0.99).3 No differences were found between aspirin-treated and control groups for all-cause mortality or ischemic stroke reduction.
Aspirin increased the risk of major gastrointestinal bleeding events by almost twofold (OR=1.70; 95% CI, 1.4–2.1). Three of the 5 trials showed no significant increase of intracranial hemorrhage event rates (OR=1.4; 95% CI, 0.9–2.0). Based on combined primary and secondary prevention trials, the risk of intracranial bleeding with aspirin is estimated at 0 to 2 events per 1000 patients per year.2
Although the ideal aspirin dosage is uncertain, lower dosages (75–81 mg/d) have been shown to be as beneficial as higher dosages, and may have fewer bleeding complications. Buffered and entericcoated formulations are no more protective than plain aspirin.4
In patients with no known cardiovascular disease, aspirin chemoprevention has been shown to decrease the risk of nonfatal MI and fatal CHD by 28%. At a 5-year CHD risk of 3%, the benefits of prophylaxis outweigh the harms (see Table ) by 2 to 1—assuming the events of stroke, MI, and bleeding are considered roughly equivalent in severity. (A different threshold may be appropriate for patients that perceive 1 of these events as significantly more serious than the others.) Typical patients at a 3% or greater risk for cardiovascular disease include men aged >40 years, post-menopausal women, and younger persons with risk factors for CHD. Physicians determine cardiovascular risk from the presence and severity of risk factors: gender, age, blood pressure, lipid status, diabetes, and smoking status.
Simple risk-assessment tools based on Framingham data are available for computers and palmtop devices (eg, Heart to Heart CV Risk Assessment Calculator, www.meddecisions.com; National Institutes of Health, www.nhlbi.nih.gov/health/prof/heart/). Because only 2 trials included women, it is less clear whether both sexes benefit equally from aspirin prophylaxis.1
TABLE
Net benefits and harms of aspirin prophylaxis, per 1000 patients
| Outcome | Estimated 5-year risk for CHD event | ||
|---|---|---|---|
| 1% | 3% | 5% | |
| All-cause mortality | NS | NS | NS |
| CHD events avoided | 3 | 8 | 14 |
| Ischemic strokes avoided | NS | NS | NS |
| Hemorrhagic strokes | 1 | 1 | 1 |
| Major gastrointestinal bleeding | 3 | 3 | 3 |
| NS, not significant | |||
Recommendations from others
The US Preventive Services Task Force recommends that clinicians discuss aspirin prophylaxis with adults at increased risk for CHD (defined as a 5-year risk of 3% or more). Discussion should include the potential benefits and harms of aspirin therapy.5
The American Heart Association recommends low-dose aspirin in people at higher risk of coronary heart disease (especially those with a 10-year CHD risk of 10% or greater).6 The European Society of Cardiology says there is evidence that low-dose aspirin can reduce the risk of cardiovascular events in asymptomatic high-risk people, such as those with diabetes or well-controlled hypertension, and in men at high multifactorial risk of cardiovascular disease.7
Aspirin: effective, safe, inexpensive—and it may prevent heart attacks
Paul V. Aitken, Jr, MD, MPH
Residency in Family Medicine, University of North Carolina at Chapel Hill; New Hanover Regional Medical Center, Wilmington, NC
Acetylsalicylic acid was first compounded in Germany by chemist Felix Hoffman in 1897. According to information from the Bayer Company, aspirin’s cardioprotective effect was first recognized by Dr Lawrence Craven, a California general practitioner. He noted a decreased rate of heart attacks in patients taking this medication.
We now have evidence supporting Dr Craven’s astute clinical observation. In adults with no history of cardiovascular disease, aspirin reduces the risk of nonfatal MI. For an individual at a 5-year CHD risk as low as 3%, the benefits of prophylaxis outweigh the harms. The leading cause of morbidity and mortality in the US is still cardiovascular disease. A simple, effective, safe, and inexpensive preventive measure like recommending aspirin has the potential to prevent heart attacks on a grand scale. A low-dose aspirin per day should be recommended for patients at risk for cardiovascular disease, including men aged >40 years, postmenopausal women, and younger persons with risk factors for CHD. As a 40-something male with a family history of cardiovascular disease reviewing this Clinical Inquiry, I will be taking my aspirin a day.
In adults with no history of cardiovascular disease, aspirin reduces the risk of nonfatal myocardial infarction (MI). Aspirin prophylaxis does not decrease all-cause mortality, risk of fatal coronary heart disease, or risk of first stroke (strength of recommendation [SOR]: A–, based on multiple randomized controlled trials).
The benefits of aspirin use must be weighed against its potential risks, primarily gastrointestinal bleeding and cerebral hemorrhage. The benefit of aspirin increases with higher levels of cardiovascular risk, while the potential for harm remains relatively constant. Adults with a calculated 5-year coronary heart disease (CHD) event risk of 3% or greater should receive prophylaxis (SOR: A, based on multiple randomized controlled trials). The ideal dose of aspirin for prophylaxis is unknown, but it appears that low doses (75–81 mg/d) are as effective as higher doses.
Evidence summary
The leading cause of morbidity and mortality in the United States is cardiovascular disease (ischemic CHD, stroke, peripheral vascular disease).1 A meta-analysis of 5 placebo-controlled randomized controlled trials involving more than 50,000 patients free of CHD and stroke evaluated aspirin for primary prevention of cardiovascular disease. Since 3 of the trials excluded women, only 20% of the participants were female. The mean age of participants was 57 years.
The treatment groups took aspirin 75 to 500 mg/d for 3 to 7 years. The meta-analysis found that compared with placebo, aspirin significantly reduced total CHD events (odds ratio [OR]=0.72; 95% confidence interval [CI], 0.60–0.87).2 Aspirin did not reduce coronary disease mortality (OR=0.87; 95% CI, 0.70–1.09); however, results from 1 study did achieve statistical significance (OR=0.64; 95% CI, 0.42–0.99).3 No differences were found between aspirin-treated and control groups for all-cause mortality or ischemic stroke reduction.
Aspirin increased the risk of major gastrointestinal bleeding events by almost twofold (OR=1.70; 95% CI, 1.4–2.1). Three of the 5 trials showed no significant increase of intracranial hemorrhage event rates (OR=1.4; 95% CI, 0.9–2.0). Based on combined primary and secondary prevention trials, the risk of intracranial bleeding with aspirin is estimated at 0 to 2 events per 1000 patients per year.2
Although the ideal aspirin dosage is uncertain, lower dosages (75–81 mg/d) have been shown to be as beneficial as higher dosages, and may have fewer bleeding complications. Buffered and entericcoated formulations are no more protective than plain aspirin.4
In patients with no known cardiovascular disease, aspirin chemoprevention has been shown to decrease the risk of nonfatal MI and fatal CHD by 28%. At a 5-year CHD risk of 3%, the benefits of prophylaxis outweigh the harms (see Table ) by 2 to 1—assuming the events of stroke, MI, and bleeding are considered roughly equivalent in severity. (A different threshold may be appropriate for patients that perceive 1 of these events as significantly more serious than the others.) Typical patients at a 3% or greater risk for cardiovascular disease include men aged >40 years, post-menopausal women, and younger persons with risk factors for CHD. Physicians determine cardiovascular risk from the presence and severity of risk factors: gender, age, blood pressure, lipid status, diabetes, and smoking status.
Simple risk-assessment tools based on Framingham data are available for computers and palmtop devices (eg, Heart to Heart CV Risk Assessment Calculator, www.meddecisions.com; National Institutes of Health, www.nhlbi.nih.gov/health/prof/heart/). Because only 2 trials included women, it is less clear whether both sexes benefit equally from aspirin prophylaxis.1
TABLE
Net benefits and harms of aspirin prophylaxis, per 1000 patients
| Outcome | Estimated 5-year risk for CHD event | ||
|---|---|---|---|
| 1% | 3% | 5% | |
| All-cause mortality | NS | NS | NS |
| CHD events avoided | 3 | 8 | 14 |
| Ischemic strokes avoided | NS | NS | NS |
| Hemorrhagic strokes | 1 | 1 | 1 |
| Major gastrointestinal bleeding | 3 | 3 | 3 |
| NS, not significant | |||
Recommendations from others
The US Preventive Services Task Force recommends that clinicians discuss aspirin prophylaxis with adults at increased risk for CHD (defined as a 5-year risk of 3% or more). Discussion should include the potential benefits and harms of aspirin therapy.5
The American Heart Association recommends low-dose aspirin in people at higher risk of coronary heart disease (especially those with a 10-year CHD risk of 10% or greater).6 The European Society of Cardiology says there is evidence that low-dose aspirin can reduce the risk of cardiovascular events in asymptomatic high-risk people, such as those with diabetes or well-controlled hypertension, and in men at high multifactorial risk of cardiovascular disease.7
Aspirin: effective, safe, inexpensive—and it may prevent heart attacks
Paul V. Aitken, Jr, MD, MPH
Residency in Family Medicine, University of North Carolina at Chapel Hill; New Hanover Regional Medical Center, Wilmington, NC
Acetylsalicylic acid was first compounded in Germany by chemist Felix Hoffman in 1897. According to information from the Bayer Company, aspirin’s cardioprotective effect was first recognized by Dr Lawrence Craven, a California general practitioner. He noted a decreased rate of heart attacks in patients taking this medication.
We now have evidence supporting Dr Craven’s astute clinical observation. In adults with no history of cardiovascular disease, aspirin reduces the risk of nonfatal MI. For an individual at a 5-year CHD risk as low as 3%, the benefits of prophylaxis outweigh the harms. The leading cause of morbidity and mortality in the US is still cardiovascular disease. A simple, effective, safe, and inexpensive preventive measure like recommending aspirin has the potential to prevent heart attacks on a grand scale. A low-dose aspirin per day should be recommended for patients at risk for cardiovascular disease, including men aged >40 years, postmenopausal women, and younger persons with risk factors for CHD. As a 40-something male with a family history of cardiovascular disease reviewing this Clinical Inquiry, I will be taking my aspirin a day.
1. Hoyert DL, Arias E, Smith BL, Murphy SL, Kochanek KD. Deaths: final data for 1999. Natl Vital Stat Rep. 2001;49:1-113.
2. Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;136:161-172.
3. Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989;321:129-135.
4. Hart RG, Halperin JL, McBride R, Benavente O, Man-Son-Hing M, Kronmal RA. Aspirin for the primary prevention of stroke and other major vascular events; meta-analysis and hypotheses. Arch Neurol 2000;57:326-332.
5. US Preventive Services Task Force. Aspirin for the primary prevention of cardiovascular events: chemoprevention. January 2002. Available at www.ahrq.gov/clinic/uspstf/uspsasmi.htm. Accessed on January 6, 2004.
6. American Heart Association. Primary prevention in the adult. 2003. Available at: www.americanheart.org/presen-ter.jhtml?identifier=4704. Accessed on January 6, 2004.
7. De Backer G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2003;24:1601-1610.
1. Hoyert DL, Arias E, Smith BL, Murphy SL, Kochanek KD. Deaths: final data for 1999. Natl Vital Stat Rep. 2001;49:1-113.
2. Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;136:161-172.
3. Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989;321:129-135.
4. Hart RG, Halperin JL, McBride R, Benavente O, Man-Son-Hing M, Kronmal RA. Aspirin for the primary prevention of stroke and other major vascular events; meta-analysis and hypotheses. Arch Neurol 2000;57:326-332.
5. US Preventive Services Task Force. Aspirin for the primary prevention of cardiovascular events: chemoprevention. January 2002. Available at www.ahrq.gov/clinic/uspstf/uspsasmi.htm. Accessed on January 6, 2004.
6. American Heart Association. Primary prevention in the adult. 2003. Available at: www.americanheart.org/presen-ter.jhtml?identifier=4704. Accessed on January 6, 2004.
7. De Backer G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2003;24:1601-1610.
Evidence-based answers from the Family Physicians Inquiries Network
Should we screen adults for asymptomatic microhematuria?
Screening patients for asymptomatic microhematuria does not appear to improve outcomes, since screening does not identify a population with increased prevalence of urologic malignancy (strength of recommendation [SOR]: A, based on prospective cohort studies) or the presence of urologic disease of any type (SOR: B, based on 1 cohort study). Asymptomatic microhematuria is sometimes associated with urologic disease that requires intervention to prevent death or disability (SOR: B, based on cohort studies). However, no studies demonstrate improved outcomes from screening for asymptomatic microhematuria.
Evidence summary
Asymptomatic microhematuria is common in adult primary care populations, with a prevalence ranging from 2.5% to 4.3% in 3 studies.1-3 It is variably associated with urologic disease.
A retrospective cohort study of 2005 British men aged >40 years found 85 (4%) with asymptomatic microhematuria. Subsequent evaluation including intravenous pyelogram and cystoscopy found 2 men with infections—1 with bladder cancer and 1 with polycystic kidneys. Benign prostatic hypertrophy, prostatitis, anatomic abnormalities, and stones accounted for the rest.3
A prospective cohort study similarly evaluated 1034 patients with asymptomatic micro-hematuria found through annual health screening of Japanese adults; 471 (45%) had some urologic diagnosis, including 30 (2.9%) with serious disease (urologic malignancies or progressive glomerulopathy), 195 (18.9%) with moderate disease (such as stones, infection, stable glomerulopathy), and the remainder with less serious disease.4
However, it is unclear whether asymptomatic microhematuria is a useful marker for detecting urologic disease. Two retrospective cohort studies assessed the prevalence of urologic disease in patients with asymptomatic microhematuria compared with those without. Of 501 male steel-workers—an occupation believed to have a higher risk for urologic malignancy—57 men had urologic disease of any type. Six men with urolog-ic disease had asymptomatic microhematuria, while 51 men with urologic disease did not. The correlation between asymptomatic microhema-turia and the presence of urologic disease was not significant (P>.05). There were 3 cases of urolog-ic cancer in the study, all diagnosed in men without asymptomatic microhematuria.5
Among 20,751 California HMO patients who had a periodic health appraisal, screening identified 598 patients with asymptomatic microhematuria (prevalence=2.9%). The medical records for all patients were reviewed for the year prior to screening to find pre-existing urologic disease and then reviewed for new diagnoses over the next 6 years. Three cases of urologic cancer occurred in the group of patients with asymptomatic microhematuria (incidence=0.5%) and 102 cancer cases among the 20,153 patients without asymptomatic microhematuria (incidence=0.5%). Its presence was not significantly associated with either uro-logic cancers or other serious urologic disease.2
No studies demonstrate improved outcomes from screening for asymptomatic microhematuria. Earlier discovery of serious diseases would not often change patient outcome, according to expert opinion.6,7 Invasive studies, such as intravenous pyelogram and cystoscopy, used to evaluate asymptomatic microhematuria have a rate of serious complications approaching 0.3% (number needed to harm=333).7
Recommendations from others
The American Urological Association recommends that all patients with asymptomatic microhematuria be evaluated. However, they do not recommend routine screening for asympto-matic microhematuria to detect urologic malig-nancy.8 The US Preventive Services Task Force does not recommend routine screening for bladder cancer by any means, including screening for hematuria.9
This poor screening measure is not helpful
Dan DePietropaolo, MD
Director, Family Practice Residency Program; Medical Director, Heartland Hospice, Christianacare Health System, Wilmington, Del
A fairly sensitive and specific way to screen for urological malignancies would certainly be worthwhile, but, as this inquiry points out, none exists. The presence of asymptomatic microhematuria in the adult population does not aid in detecting urologic malignancies or any other serious pathology. The incidence of serious disease in the control group is just as high as in the patients with a positive screen for hematuria. A poor screening measure like this one not only is not helpful but also holds the potential to harm patients because of false positive results and the ensuing invasive workups. The USPSTF does not recommend this screening measure.
1. Ritchie CD, Bevan EA, Collier SJ. Importance of occult haematuria found at screening. Brit Med J (Clin Res Ed) 1986;292:681-683.
2. Hiatt RA, Ordenez JD. Dipstick urinalysis screening, asymptomatic microhematuria, and subsequent urological cancers in a population-based sample. Cancer Epidemiol Biomarkers Prev 1994;3:439-443.
3. Thompson IM. The evaluation of microscopic hematuria: a population-based study. J Urol 1987;138:1189-1190.
4. Murakami S, Igarashi T, Hara S, Shimazaki J. Strategies for asymptomatic microscopic hematuria: a prospective study of 1,034 patients. J Urol 1990;144:99-101.
5. Choi BC, Farmilo JA. Microscopic haematuria as a predictor of urological diseases among steel workers. J Soc Occup Med 1990;40:47-52.
6. Mohr DN, Offord KP, Owen RA, Melton LJ 3rd. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224-229.
7. Froom P, Froom J, Ribak J. Asymptomatic microscopic hematuria—is investigation necessary? J Clin Epidemiol 1997;50:1197-1200.
8. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence and etiology. Urology 2001;57:559-603.
9. USPSTF website Screening: bladder cancer. Last updated 1996. Available at www.ahrq.gov/clinic/uspstf/uspsblad.htm. Accessed on January 6, 2004.
Screening patients for asymptomatic microhematuria does not appear to improve outcomes, since screening does not identify a population with increased prevalence of urologic malignancy (strength of recommendation [SOR]: A, based on prospective cohort studies) or the presence of urologic disease of any type (SOR: B, based on 1 cohort study). Asymptomatic microhematuria is sometimes associated with urologic disease that requires intervention to prevent death or disability (SOR: B, based on cohort studies). However, no studies demonstrate improved outcomes from screening for asymptomatic microhematuria.
Evidence summary
Asymptomatic microhematuria is common in adult primary care populations, with a prevalence ranging from 2.5% to 4.3% in 3 studies.1-3 It is variably associated with urologic disease.
A retrospective cohort study of 2005 British men aged >40 years found 85 (4%) with asymptomatic microhematuria. Subsequent evaluation including intravenous pyelogram and cystoscopy found 2 men with infections—1 with bladder cancer and 1 with polycystic kidneys. Benign prostatic hypertrophy, prostatitis, anatomic abnormalities, and stones accounted for the rest.3
A prospective cohort study similarly evaluated 1034 patients with asymptomatic micro-hematuria found through annual health screening of Japanese adults; 471 (45%) had some urologic diagnosis, including 30 (2.9%) with serious disease (urologic malignancies or progressive glomerulopathy), 195 (18.9%) with moderate disease (such as stones, infection, stable glomerulopathy), and the remainder with less serious disease.4
However, it is unclear whether asymptomatic microhematuria is a useful marker for detecting urologic disease. Two retrospective cohort studies assessed the prevalence of urologic disease in patients with asymptomatic microhematuria compared with those without. Of 501 male steel-workers—an occupation believed to have a higher risk for urologic malignancy—57 men had urologic disease of any type. Six men with urolog-ic disease had asymptomatic microhematuria, while 51 men with urologic disease did not. The correlation between asymptomatic microhema-turia and the presence of urologic disease was not significant (P>.05). There were 3 cases of urolog-ic cancer in the study, all diagnosed in men without asymptomatic microhematuria.5
Among 20,751 California HMO patients who had a periodic health appraisal, screening identified 598 patients with asymptomatic microhematuria (prevalence=2.9%). The medical records for all patients were reviewed for the year prior to screening to find pre-existing urologic disease and then reviewed for new diagnoses over the next 6 years. Three cases of urologic cancer occurred in the group of patients with asymptomatic microhematuria (incidence=0.5%) and 102 cancer cases among the 20,153 patients without asymptomatic microhematuria (incidence=0.5%). Its presence was not significantly associated with either uro-logic cancers or other serious urologic disease.2
No studies demonstrate improved outcomes from screening for asymptomatic microhematuria. Earlier discovery of serious diseases would not often change patient outcome, according to expert opinion.6,7 Invasive studies, such as intravenous pyelogram and cystoscopy, used to evaluate asymptomatic microhematuria have a rate of serious complications approaching 0.3% (number needed to harm=333).7
Recommendations from others
The American Urological Association recommends that all patients with asymptomatic microhematuria be evaluated. However, they do not recommend routine screening for asympto-matic microhematuria to detect urologic malig-nancy.8 The US Preventive Services Task Force does not recommend routine screening for bladder cancer by any means, including screening for hematuria.9
This poor screening measure is not helpful
Dan DePietropaolo, MD
Director, Family Practice Residency Program; Medical Director, Heartland Hospice, Christianacare Health System, Wilmington, Del
A fairly sensitive and specific way to screen for urological malignancies would certainly be worthwhile, but, as this inquiry points out, none exists. The presence of asymptomatic microhematuria in the adult population does not aid in detecting urologic malignancies or any other serious pathology. The incidence of serious disease in the control group is just as high as in the patients with a positive screen for hematuria. A poor screening measure like this one not only is not helpful but also holds the potential to harm patients because of false positive results and the ensuing invasive workups. The USPSTF does not recommend this screening measure.
Screening patients for asymptomatic microhematuria does not appear to improve outcomes, since screening does not identify a population with increased prevalence of urologic malignancy (strength of recommendation [SOR]: A, based on prospective cohort studies) or the presence of urologic disease of any type (SOR: B, based on 1 cohort study). Asymptomatic microhematuria is sometimes associated with urologic disease that requires intervention to prevent death or disability (SOR: B, based on cohort studies). However, no studies demonstrate improved outcomes from screening for asymptomatic microhematuria.
Evidence summary
Asymptomatic microhematuria is common in adult primary care populations, with a prevalence ranging from 2.5% to 4.3% in 3 studies.1-3 It is variably associated with urologic disease.
A retrospective cohort study of 2005 British men aged >40 years found 85 (4%) with asymptomatic microhematuria. Subsequent evaluation including intravenous pyelogram and cystoscopy found 2 men with infections—1 with bladder cancer and 1 with polycystic kidneys. Benign prostatic hypertrophy, prostatitis, anatomic abnormalities, and stones accounted for the rest.3
A prospective cohort study similarly evaluated 1034 patients with asymptomatic micro-hematuria found through annual health screening of Japanese adults; 471 (45%) had some urologic diagnosis, including 30 (2.9%) with serious disease (urologic malignancies or progressive glomerulopathy), 195 (18.9%) with moderate disease (such as stones, infection, stable glomerulopathy), and the remainder with less serious disease.4
However, it is unclear whether asymptomatic microhematuria is a useful marker for detecting urologic disease. Two retrospective cohort studies assessed the prevalence of urologic disease in patients with asymptomatic microhematuria compared with those without. Of 501 male steel-workers—an occupation believed to have a higher risk for urologic malignancy—57 men had urologic disease of any type. Six men with urolog-ic disease had asymptomatic microhematuria, while 51 men with urologic disease did not. The correlation between asymptomatic microhema-turia and the presence of urologic disease was not significant (P>.05). There were 3 cases of urolog-ic cancer in the study, all diagnosed in men without asymptomatic microhematuria.5
Among 20,751 California HMO patients who had a periodic health appraisal, screening identified 598 patients with asymptomatic microhematuria (prevalence=2.9%). The medical records for all patients were reviewed for the year prior to screening to find pre-existing urologic disease and then reviewed for new diagnoses over the next 6 years. Three cases of urologic cancer occurred in the group of patients with asymptomatic microhematuria (incidence=0.5%) and 102 cancer cases among the 20,153 patients without asymptomatic microhematuria (incidence=0.5%). Its presence was not significantly associated with either uro-logic cancers or other serious urologic disease.2
No studies demonstrate improved outcomes from screening for asymptomatic microhematuria. Earlier discovery of serious diseases would not often change patient outcome, according to expert opinion.6,7 Invasive studies, such as intravenous pyelogram and cystoscopy, used to evaluate asymptomatic microhematuria have a rate of serious complications approaching 0.3% (number needed to harm=333).7
Recommendations from others
The American Urological Association recommends that all patients with asymptomatic microhematuria be evaluated. However, they do not recommend routine screening for asympto-matic microhematuria to detect urologic malig-nancy.8 The US Preventive Services Task Force does not recommend routine screening for bladder cancer by any means, including screening for hematuria.9
This poor screening measure is not helpful
Dan DePietropaolo, MD
Director, Family Practice Residency Program; Medical Director, Heartland Hospice, Christianacare Health System, Wilmington, Del
A fairly sensitive and specific way to screen for urological malignancies would certainly be worthwhile, but, as this inquiry points out, none exists. The presence of asymptomatic microhematuria in the adult population does not aid in detecting urologic malignancies or any other serious pathology. The incidence of serious disease in the control group is just as high as in the patients with a positive screen for hematuria. A poor screening measure like this one not only is not helpful but also holds the potential to harm patients because of false positive results and the ensuing invasive workups. The USPSTF does not recommend this screening measure.
1. Ritchie CD, Bevan EA, Collier SJ. Importance of occult haematuria found at screening. Brit Med J (Clin Res Ed) 1986;292:681-683.
2. Hiatt RA, Ordenez JD. Dipstick urinalysis screening, asymptomatic microhematuria, and subsequent urological cancers in a population-based sample. Cancer Epidemiol Biomarkers Prev 1994;3:439-443.
3. Thompson IM. The evaluation of microscopic hematuria: a population-based study. J Urol 1987;138:1189-1190.
4. Murakami S, Igarashi T, Hara S, Shimazaki J. Strategies for asymptomatic microscopic hematuria: a prospective study of 1,034 patients. J Urol 1990;144:99-101.
5. Choi BC, Farmilo JA. Microscopic haematuria as a predictor of urological diseases among steel workers. J Soc Occup Med 1990;40:47-52.
6. Mohr DN, Offord KP, Owen RA, Melton LJ 3rd. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224-229.
7. Froom P, Froom J, Ribak J. Asymptomatic microscopic hematuria—is investigation necessary? J Clin Epidemiol 1997;50:1197-1200.
8. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence and etiology. Urology 2001;57:559-603.
9. USPSTF website Screening: bladder cancer. Last updated 1996. Available at www.ahrq.gov/clinic/uspstf/uspsblad.htm. Accessed on January 6, 2004.
1. Ritchie CD, Bevan EA, Collier SJ. Importance of occult haematuria found at screening. Brit Med J (Clin Res Ed) 1986;292:681-683.
2. Hiatt RA, Ordenez JD. Dipstick urinalysis screening, asymptomatic microhematuria, and subsequent urological cancers in a population-based sample. Cancer Epidemiol Biomarkers Prev 1994;3:439-443.
3. Thompson IM. The evaluation of microscopic hematuria: a population-based study. J Urol 1987;138:1189-1190.
4. Murakami S, Igarashi T, Hara S, Shimazaki J. Strategies for asymptomatic microscopic hematuria: a prospective study of 1,034 patients. J Urol 1990;144:99-101.
5. Choi BC, Farmilo JA. Microscopic haematuria as a predictor of urological diseases among steel workers. J Soc Occup Med 1990;40:47-52.
6. Mohr DN, Offord KP, Owen RA, Melton LJ 3rd. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224-229.
7. Froom P, Froom J, Ribak J. Asymptomatic microscopic hematuria—is investigation necessary? J Clin Epidemiol 1997;50:1197-1200.
8. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence and etiology. Urology 2001;57:559-603.
9. USPSTF website Screening: bladder cancer. Last updated 1996. Available at www.ahrq.gov/clinic/uspstf/uspsblad.htm. Accessed on January 6, 2004.
Evidence-based answers from the Family Physicians Inquiries Network