User login
How Can Tumor Lysis Syndrome Be Prevented and Managed in Cancer Patients?
Case
A 25-year-old male with HIV/AIDS and a CD4 count of 65 cells/μL presents to the ED with intractable nausea and vomiting for one week. Laboratory evaluation revealed a white blood cell of 67,000 cells/mm3. An extended chemistry panel reveals creatinine 3.5 mg/dL, potassium 3.0 mmol/L, LDH 250 IU/L, and uric acid 5mg/dL. Calcium and phosphorus were both normal. The patient was admitted for further evaluation and management, and was later diagnosed with Burkitt’s lymphoma.
Overview
Tumor lysis syndrome (TLS) is an acute cell lysis of tumor cells with the release of cell content into circulation either spontaneously or in response to therapy, leading to hyperurecemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.1-3
TLS is one of the most common oncology emergencies encountered by hospitalists caring for patients with hematologic malignancies. The incidence and severity of TLS depend on the cell burden, cell proliferation rate, potential for cell lysis or chemo sensitivity, baseline clinical characteristics, and preventive measures taken (see Table 1).2,4
TLS is classified as laboratory or clinical. Laboratory TLS is described as the presence of two or more of the following serum abnormalities at the same time, present within three days before or seven days after the start of therapy.5
- Uric acid >8 mg/dL (475.8 micromole/L) or 25% increase;
- Potassium >6 mEq/L (6 mmol/L) or 25% increase;
- Phosphorus >6.5 mg/dL (2.1 mmol/L) for children or >4.5 mg/dl (1.45 mmol/L) for adults or 25% increase; and
- Calcium >7 mg/dL (1.75 mmol/L) or 25% increase.
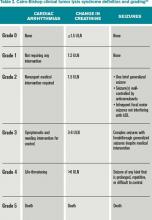
Clinical TLS is defined as laboratory TLS in association with increased creatinine levels, seizures, cardiac arrhythmias, or death (see Table 2).5
Pathogenesis
Tumor cell lysis releases DNA, cytokines, phosphate, and potassium. DNA is metabolized into adenosine and guanosine, which are then converted into xanthines. Xanthines are oxidized by xanthine oxidase into uric acid, which is then excreted through the kidneys.
TLS develops when the accumulation of xanthine, uric acid, potassium, and phosphorus exceeds the kidney’s capacity to excrete them. Cytokines cause hypotension, inflammation, and kidney injury, and worsen the kidney’s excretory capacity. Damage to the kidneys also occurs by renal precipitation of uric acid, xanthine, and calcium phosphate.4
Phosphorus concentrations in tumor cells are four times higher than in normal cells. When the calcium phosphorus product exceeds 60 mg2/dL2, there is an increased risk of calcium phosphate precipitation in the kidney tubules, which could lead to kidney failure. Accumulation of calcium phosphate product may also be cardiotoxic and can lead to cardiac arrhythmias. In addition, hyperphosphatemia can cause secondary hypocalcemia, which may lead to parasthesias, tetany, and cardiac arrhythmias.2,4
TLS is most common in tumors with high proliferative rates and high tumor burden, such as acute lymphoblastic leukemia and Burkitt’s lymphoma, but it can occur with other hematologic malignancies, such as T-cell precursor acute lymphocytic leukemia (ALL), B-cell precursor ALL, acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), anaplastic large cell lymphoma, and plasma cell disorders (e.g. multiple myeloma and plasmacytoma).6,7 TLS has also been reported with the treatment of solid organ nonhematologic tumors (see Table 3).
In hematologic tumors, TLS frequently is associated with cytotoxic chemotherapy, and less frequently with glucocorticoid treatment, monoclonal antibodies (eg, rituximab, bortezomab, imatinib), and radiation therapy.25-29
Patient factors, such as baseline kidney disease or lack of prophylactic/preventive measures for TLS, also increase the risk.4 TLS, however, can develop in patients classified as low risk (see Table 1.
TLS Prevention
Intravenous fluids. Every patient at intermediate or high risk of TLS should receive intravenous fluids (IVF) prior to cancer treatment; those at low risk may receive IVF based on the provider’s clinical judgment.30 The purpose of administering IVF is to generate high urine output to reduce the risk of precipitation of uric acid in the renal tubules.30 Both adults and children should receive approximately 2 to 3 L/m2 per day of IVF,30 and urine output should be maintained at 2 ml/kg/hr (or 4 to 6 ml/kg/hr for children <10kg).30 IVF should be cautiously administered in patients with renal insufficiency or heart failure, and diuretics may be used to maintain goal urine output. Recommended initial fluids are D51/4 normal saline, or normal saline for patients who are dehydrated or hyponatremic.30
Allopurinol. Allopurinol is usually also administered to patients at risk for developing TLS.30 Allopurinol inhibits the metabolism of hypoxanthine and xanthine to uric acid, which decreases the accumulation of uric acid in the renal tubules, thus preventing obstructive renal disease from precipitation of uric acid.4 The recommended dose of allopurinol is 100 mg/m2 every eight hours, and should not exceed 800 mg per day in adults. It should be started one to two days prior to induction chemotherapy and continued for three to seven days after the treatment and until uric acid levels and other electrolyte levels have returned to normal. The dose is adjusted to 50 mg/m2 every eight hours in patients with kidney failure.30
In some cases, allopurinol can lead to increased levels of xanthine crystals in the renal tubules, leading to acute kidney injury. Also, allopurinol does not have any effect on uric acid that has already been formed, so patients with elevated uric acid levels prior to the initiation of cancer therapy will not have any reduction in the levels of uric acid. Allopurinol reduces the degradation of other purines, so it can cause toxicity in patients on azathioprine and 6-mercaptopurine if the doses of these medications are not adjusted.
Rasburicase. Rasburicase is a recombinant urate oxidase, derived from aspergillus favus, which catalyzes the breakdown of uric acid to allantoin, which is a water-soluble product. Rasburicase is recommended as a first-line treatment for patients at high risk for clinical TLS.30 Rasburicase has an earlier onset than allopurinol and rapidly decreases serum levels of uric acid within four hours of administration.30,31 The recommended dose is 0.10 to 0.20 mg/kg once a day for five days in adults.30
A Phase III trial compared the efficiency and safety of rasburicase to rasburicase with allopurinol or allopurinol alone.32 A significantly higher normalization of uric acid was found in patients on rasburicase compared to allopurinol alone. The incidence of laboratory TLS was also significantly lower with rasburicase alone compared to allopurinol alone, and was even lower with allopurinol plus rasburicase. The incidence of acute kidney injury was the same with rasburicase alone or allopurinol alone but was higher with rasburicase plus allopurinol.
Serum uric acid, phosphorus, potassium, and calcium need to be monitored every four hours for 24 hours after the completion of chemotherapy in patients on rasburicase.4 The sample of blood drawn to check the uric acid levels has to be placed on ice and processed within four hours in order to avoid falsely lower levels of uric acid due to the conversion of uric acid to allantoin. Rasburicase is contraindicated in patients with G6PD deficiency and pregnant women, because one of the byproducts of uric acid breakdown is hydrogen peroxide, which can cause severe hemolysis and the formation of methemoglobin in these patients.30
Rasburicase has been approved for use in both children and adults, but there is more evidence for the use in children. Rasburicase has a black-box label for patients with anaphylaxis, methemoglobinemia, hemolysis, and hemoglobinuria, and there is a recommendation to check G6PD deficiency before use in high-risk patients.30
TLS Treatment
Alkalinization. Alkalinization of urine is controversial in the management of TLS. Urine alkalinization increases uric acid solubility but causes hyperphosphatemia and decreases calcium phosphate solubility, which can then deposit in the kidney once cancer treatment starts. Of note, hyperphosphatemia is much more difficult to correct than high levels of uric acid, and there are no clinical trials proving the superiority of urine alkalinization over normal saline.
Normalization of electrolytes. Electrolyte abnormalities should be corrected to avoid arrhythmias and seizures. Phosphorus levels >6.5 mg/dl (2.1 mmol/L) should be managed by restricting phosphorus intake, and by the use of phosphate binders (calcium acetate, calcium carbonate, sevelamer, lanthanum, or aluminum hydroxide). Aluminum hydroxide should be avoided in patients with renal insufficiency. In severe cases of hyperphosphatemia, dialysis should be considered.
Symptomatic hypocalcemia should be treated with calcium gluconate if changes are present on the electrocardiography (ECG). Hypocalcemia in the presence of hyperphosphatemia should be treated only in patients with tetany or cardiac arrhythmias; otherwise, hypocalcemia should not be treated until hyperphosphatemia has been corrected.
In cases of hyperkalemia, patients should be placed on a cardiac monitor and stabilized with calcium gluconate; kayexalate should be administered to reduce total body potassium. Other interventions, such as intravenous insulin given with dextrose, sodium bicarbonate, and albuterol, have a temporary effect on hyperkalemia and can be used as adjunct treatments in patients with severe hyperkalemia (>7). Hemodialysis should be strongly considered in severe cases of hyperkalemia, particularly in patients with persistently elevated potassium levels despite other treatments.
Back to the Case
Our patient was started on IVFs with close monitoring of his urine output. He was considered intermediate risk for developing TLS. Allopurinol, renally dosed, was administered for two days prior to initiating treatment with rituximab plus chemotherapy. His chemistry panel was monitored daily and he did not develop any form of TLS.
Bottom Line
TLS is a common oncology emergency in patients with hematologic malignancies. Preventative measures include starting IVF prior to cancer treatment, and administering allopurinol and/or rasburicase to patients at risk of developing TLS. Treatment should include normalizing electrolytes to avoid arrhythmias and seizures.
Dr. Akwe is assistant professor of medicine at the Emory University School of Medicine and a clinical instructor of medicine at the Morehouse School of Medicine, both in Atlanta. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory. Both work as hospitalists at the Atlanta VA Medical Center.
References
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55:Suppl 3:S1-S13.
- Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
- Gertz MA. Managing tumor lysis syndrome in 2010. Leuk Lymphoma. 2010;51:179-180.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3.
- Wössmann W, Schrappe M, Meyer U, et al. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82:160.
- Hussain K, Mazza JJ, Clouse LH. Tumor lysis syndrome (TLS) following fludarabine therapy Gemici C. Tumor lysis syndrome in solid tumors. J Clin Oncol. 2009;27:2738-2739
- Rostom AY, El-Hussainy G, Kandil A, Allam A. Tumor lysis syndrome following hemi-body irradiation for metastatic breast cancer. Ann Oncol. 2000;11:1349.
- Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in nonhematologic malignancies. Report of a case and review of the literature. Am J Clin Oncol. 1994;17:502.
- Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors—a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187.
- Kalemkerian GP, Darwish B, Varterasian ML. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med. 1997;103:363.
- Noh GY, Choe DH, Kim CH, Lee JC. Fatal tumor lysis syndrome during radiotherapy for non-small-cell lung cancer. J Clin Oncol. 2008;26:6005-6006.
- Pentheroudakis G, O’Neill VJ, Vasey P, Kaye SB. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Report of two cases. Support Care Cancer. 2001;9:554.
- Joshita S, Yoshizawa K, Sano K, et al., A patient with advanced hepatocellular carcinoma treated with sorafenib tosylate showed massive tumor lysis with avoidance of tumor lysis syndrome. Intern Med. 2010;49:991-994.
- Huang WS, Yang CH. Sorafenib-induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;15:4464-4466.
- Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol. 1993;21:521.
- Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol. 2005;23:6794.
- Godoy H, Kesterson JP, Lele S. Tumor lysis syndrome associated with carboplatin and paclitaxel in a woman with recurrent endometrial cancer. Int J Gynaecol Obstet. 2010;109:254.
- Shamseddine AI, Khalil AM, Wehbeh MH. Acute tumor lysis syndrome with squamous cell carcinoma of the vulva. Gynecol Oncol 1993;51:258
- Pinder EM, Atwal GS, Ayantunde AA, et al. Tumour lysis syndrome occurring in a patient with metastatic gastrointestinal stromal tumour treated with Glivec (imatinib mesylate, Gleevec, STI571). Sarcoma. 2007;2007:82012.
- Krishnan G, D’Silva K, Al-Janadi A. Cetuximab-related tumor lysis syndrome in metastatic colon carcinoma. J Clin Oncol. 2008;26:2406-2408.
- Oztop I, Demirkan B, Yaren A, et al. Rapid tumor lysis syndrome in a patient with metastatic colon cancer as a complication of treatment with 5-fluorouracil/leucoverin and irinotecan. Tumori. 2004;90:514.
- Lin CJ, Lim KH, Cheng YC, et al. Tumor lysis syndrome after treatment with gemcitabine for metastatic transitional cell carcinoma. Med Oncol. 2007;24:455.
- Malik IA, Abubakar S, Alam F, Khan A. Dexamethasone-induced tumor lysis syndrome in high-grade non-Hodgkin’s lymphoma. South Med J. 1994;87:409.
- Jabr FI. Acute tumor lysis syndrome induced by rituximab in diffuse large B-cell lymphoma. Int J Hematol. 2005;82:312.
- Sezer O, Vesole DH, Singhal S, et al. Bortezomib-induced tumor lysis syndrome in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:233.
- Jensen M, Winkler U, Manzke O, et al. Rapid tumor lysis in a patient with B-cell chronic lymphocytic leukemia and lymphocytosis treated with an anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab). Ann Hematol. 1998;77:89.
- Linck D, Basara N, Tran V, et al. Peracute onset of severe tumor lysis syndrome immediately after 4 Gy fractionated TBI as part of reduced intensity preparative regimen in a patient with T-ALL with high tumor burden. Bone Marrow Transplant. 2003;31:935.
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778. [Erratum, J Clin Oncol. 2010;28:708.]
- Cheuk DK, Chiang AK, Chan GC, Ha SY. Urate oxidase for the prevention and treatment of tumor lysis syndrome in children with cancer. Cochrane Database Syst Rev. 2010;(6):CD006945.
- Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28:4207.
Case
A 25-year-old male with HIV/AIDS and a CD4 count of 65 cells/μL presents to the ED with intractable nausea and vomiting for one week. Laboratory evaluation revealed a white blood cell of 67,000 cells/mm3. An extended chemistry panel reveals creatinine 3.5 mg/dL, potassium 3.0 mmol/L, LDH 250 IU/L, and uric acid 5mg/dL. Calcium and phosphorus were both normal. The patient was admitted for further evaluation and management, and was later diagnosed with Burkitt’s lymphoma.
Overview
Tumor lysis syndrome (TLS) is an acute cell lysis of tumor cells with the release of cell content into circulation either spontaneously or in response to therapy, leading to hyperurecemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.1-3
TLS is one of the most common oncology emergencies encountered by hospitalists caring for patients with hematologic malignancies. The incidence and severity of TLS depend on the cell burden, cell proliferation rate, potential for cell lysis or chemo sensitivity, baseline clinical characteristics, and preventive measures taken (see Table 1).2,4
TLS is classified as laboratory or clinical. Laboratory TLS is described as the presence of two or more of the following serum abnormalities at the same time, present within three days before or seven days after the start of therapy.5
- Uric acid >8 mg/dL (475.8 micromole/L) or 25% increase;
- Potassium >6 mEq/L (6 mmol/L) or 25% increase;
- Phosphorus >6.5 mg/dL (2.1 mmol/L) for children or >4.5 mg/dl (1.45 mmol/L) for adults or 25% increase; and
- Calcium >7 mg/dL (1.75 mmol/L) or 25% increase.
Clinical TLS is defined as laboratory TLS in association with increased creatinine levels, seizures, cardiac arrhythmias, or death (see Table 2).5
Pathogenesis
Tumor cell lysis releases DNA, cytokines, phosphate, and potassium. DNA is metabolized into adenosine and guanosine, which are then converted into xanthines. Xanthines are oxidized by xanthine oxidase into uric acid, which is then excreted through the kidneys.
TLS develops when the accumulation of xanthine, uric acid, potassium, and phosphorus exceeds the kidney’s capacity to excrete them. Cytokines cause hypotension, inflammation, and kidney injury, and worsen the kidney’s excretory capacity. Damage to the kidneys also occurs by renal precipitation of uric acid, xanthine, and calcium phosphate.4
Phosphorus concentrations in tumor cells are four times higher than in normal cells. When the calcium phosphorus product exceeds 60 mg2/dL2, there is an increased risk of calcium phosphate precipitation in the kidney tubules, which could lead to kidney failure. Accumulation of calcium phosphate product may also be cardiotoxic and can lead to cardiac arrhythmias. In addition, hyperphosphatemia can cause secondary hypocalcemia, which may lead to parasthesias, tetany, and cardiac arrhythmias.2,4
TLS is most common in tumors with high proliferative rates and high tumor burden, such as acute lymphoblastic leukemia and Burkitt’s lymphoma, but it can occur with other hematologic malignancies, such as T-cell precursor acute lymphocytic leukemia (ALL), B-cell precursor ALL, acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), anaplastic large cell lymphoma, and plasma cell disorders (e.g. multiple myeloma and plasmacytoma).6,7 TLS has also been reported with the treatment of solid organ nonhematologic tumors (see Table 3).
In hematologic tumors, TLS frequently is associated with cytotoxic chemotherapy, and less frequently with glucocorticoid treatment, monoclonal antibodies (eg, rituximab, bortezomab, imatinib), and radiation therapy.25-29
Patient factors, such as baseline kidney disease or lack of prophylactic/preventive measures for TLS, also increase the risk.4 TLS, however, can develop in patients classified as low risk (see Table 1.
TLS Prevention
Intravenous fluids. Every patient at intermediate or high risk of TLS should receive intravenous fluids (IVF) prior to cancer treatment; those at low risk may receive IVF based on the provider’s clinical judgment.30 The purpose of administering IVF is to generate high urine output to reduce the risk of precipitation of uric acid in the renal tubules.30 Both adults and children should receive approximately 2 to 3 L/m2 per day of IVF,30 and urine output should be maintained at 2 ml/kg/hr (or 4 to 6 ml/kg/hr for children <10kg).30 IVF should be cautiously administered in patients with renal insufficiency or heart failure, and diuretics may be used to maintain goal urine output. Recommended initial fluids are D51/4 normal saline, or normal saline for patients who are dehydrated or hyponatremic.30
Allopurinol. Allopurinol is usually also administered to patients at risk for developing TLS.30 Allopurinol inhibits the metabolism of hypoxanthine and xanthine to uric acid, which decreases the accumulation of uric acid in the renal tubules, thus preventing obstructive renal disease from precipitation of uric acid.4 The recommended dose of allopurinol is 100 mg/m2 every eight hours, and should not exceed 800 mg per day in adults. It should be started one to two days prior to induction chemotherapy and continued for three to seven days after the treatment and until uric acid levels and other electrolyte levels have returned to normal. The dose is adjusted to 50 mg/m2 every eight hours in patients with kidney failure.30
In some cases, allopurinol can lead to increased levels of xanthine crystals in the renal tubules, leading to acute kidney injury. Also, allopurinol does not have any effect on uric acid that has already been formed, so patients with elevated uric acid levels prior to the initiation of cancer therapy will not have any reduction in the levels of uric acid. Allopurinol reduces the degradation of other purines, so it can cause toxicity in patients on azathioprine and 6-mercaptopurine if the doses of these medications are not adjusted.
Rasburicase. Rasburicase is a recombinant urate oxidase, derived from aspergillus favus, which catalyzes the breakdown of uric acid to allantoin, which is a water-soluble product. Rasburicase is recommended as a first-line treatment for patients at high risk for clinical TLS.30 Rasburicase has an earlier onset than allopurinol and rapidly decreases serum levels of uric acid within four hours of administration.30,31 The recommended dose is 0.10 to 0.20 mg/kg once a day for five days in adults.30
A Phase III trial compared the efficiency and safety of rasburicase to rasburicase with allopurinol or allopurinol alone.32 A significantly higher normalization of uric acid was found in patients on rasburicase compared to allopurinol alone. The incidence of laboratory TLS was also significantly lower with rasburicase alone compared to allopurinol alone, and was even lower with allopurinol plus rasburicase. The incidence of acute kidney injury was the same with rasburicase alone or allopurinol alone but was higher with rasburicase plus allopurinol.
Serum uric acid, phosphorus, potassium, and calcium need to be monitored every four hours for 24 hours after the completion of chemotherapy in patients on rasburicase.4 The sample of blood drawn to check the uric acid levels has to be placed on ice and processed within four hours in order to avoid falsely lower levels of uric acid due to the conversion of uric acid to allantoin. Rasburicase is contraindicated in patients with G6PD deficiency and pregnant women, because one of the byproducts of uric acid breakdown is hydrogen peroxide, which can cause severe hemolysis and the formation of methemoglobin in these patients.30
Rasburicase has been approved for use in both children and adults, but there is more evidence for the use in children. Rasburicase has a black-box label for patients with anaphylaxis, methemoglobinemia, hemolysis, and hemoglobinuria, and there is a recommendation to check G6PD deficiency before use in high-risk patients.30
TLS Treatment
Alkalinization. Alkalinization of urine is controversial in the management of TLS. Urine alkalinization increases uric acid solubility but causes hyperphosphatemia and decreases calcium phosphate solubility, which can then deposit in the kidney once cancer treatment starts. Of note, hyperphosphatemia is much more difficult to correct than high levels of uric acid, and there are no clinical trials proving the superiority of urine alkalinization over normal saline.
Normalization of electrolytes. Electrolyte abnormalities should be corrected to avoid arrhythmias and seizures. Phosphorus levels >6.5 mg/dl (2.1 mmol/L) should be managed by restricting phosphorus intake, and by the use of phosphate binders (calcium acetate, calcium carbonate, sevelamer, lanthanum, or aluminum hydroxide). Aluminum hydroxide should be avoided in patients with renal insufficiency. In severe cases of hyperphosphatemia, dialysis should be considered.
Symptomatic hypocalcemia should be treated with calcium gluconate if changes are present on the electrocardiography (ECG). Hypocalcemia in the presence of hyperphosphatemia should be treated only in patients with tetany or cardiac arrhythmias; otherwise, hypocalcemia should not be treated until hyperphosphatemia has been corrected.
In cases of hyperkalemia, patients should be placed on a cardiac monitor and stabilized with calcium gluconate; kayexalate should be administered to reduce total body potassium. Other interventions, such as intravenous insulin given with dextrose, sodium bicarbonate, and albuterol, have a temporary effect on hyperkalemia and can be used as adjunct treatments in patients with severe hyperkalemia (>7). Hemodialysis should be strongly considered in severe cases of hyperkalemia, particularly in patients with persistently elevated potassium levels despite other treatments.
Back to the Case
Our patient was started on IVFs with close monitoring of his urine output. He was considered intermediate risk for developing TLS. Allopurinol, renally dosed, was administered for two days prior to initiating treatment with rituximab plus chemotherapy. His chemistry panel was monitored daily and he did not develop any form of TLS.
Bottom Line
TLS is a common oncology emergency in patients with hematologic malignancies. Preventative measures include starting IVF prior to cancer treatment, and administering allopurinol and/or rasburicase to patients at risk of developing TLS. Treatment should include normalizing electrolytes to avoid arrhythmias and seizures.
Dr. Akwe is assistant professor of medicine at the Emory University School of Medicine and a clinical instructor of medicine at the Morehouse School of Medicine, both in Atlanta. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory. Both work as hospitalists at the Atlanta VA Medical Center.
References
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55:Suppl 3:S1-S13.
- Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
- Gertz MA. Managing tumor lysis syndrome in 2010. Leuk Lymphoma. 2010;51:179-180.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3.
- Wössmann W, Schrappe M, Meyer U, et al. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82:160.
- Hussain K, Mazza JJ, Clouse LH. Tumor lysis syndrome (TLS) following fludarabine therapy Gemici C. Tumor lysis syndrome in solid tumors. J Clin Oncol. 2009;27:2738-2739
- Rostom AY, El-Hussainy G, Kandil A, Allam A. Tumor lysis syndrome following hemi-body irradiation for metastatic breast cancer. Ann Oncol. 2000;11:1349.
- Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in nonhematologic malignancies. Report of a case and review of the literature. Am J Clin Oncol. 1994;17:502.
- Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors—a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187.
- Kalemkerian GP, Darwish B, Varterasian ML. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med. 1997;103:363.
- Noh GY, Choe DH, Kim CH, Lee JC. Fatal tumor lysis syndrome during radiotherapy for non-small-cell lung cancer. J Clin Oncol. 2008;26:6005-6006.
- Pentheroudakis G, O’Neill VJ, Vasey P, Kaye SB. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Report of two cases. Support Care Cancer. 2001;9:554.
- Joshita S, Yoshizawa K, Sano K, et al., A patient with advanced hepatocellular carcinoma treated with sorafenib tosylate showed massive tumor lysis with avoidance of tumor lysis syndrome. Intern Med. 2010;49:991-994.
- Huang WS, Yang CH. Sorafenib-induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;15:4464-4466.
- Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol. 1993;21:521.
- Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol. 2005;23:6794.
- Godoy H, Kesterson JP, Lele S. Tumor lysis syndrome associated with carboplatin and paclitaxel in a woman with recurrent endometrial cancer. Int J Gynaecol Obstet. 2010;109:254.
- Shamseddine AI, Khalil AM, Wehbeh MH. Acute tumor lysis syndrome with squamous cell carcinoma of the vulva. Gynecol Oncol 1993;51:258
- Pinder EM, Atwal GS, Ayantunde AA, et al. Tumour lysis syndrome occurring in a patient with metastatic gastrointestinal stromal tumour treated with Glivec (imatinib mesylate, Gleevec, STI571). Sarcoma. 2007;2007:82012.
- Krishnan G, D’Silva K, Al-Janadi A. Cetuximab-related tumor lysis syndrome in metastatic colon carcinoma. J Clin Oncol. 2008;26:2406-2408.
- Oztop I, Demirkan B, Yaren A, et al. Rapid tumor lysis syndrome in a patient with metastatic colon cancer as a complication of treatment with 5-fluorouracil/leucoverin and irinotecan. Tumori. 2004;90:514.
- Lin CJ, Lim KH, Cheng YC, et al. Tumor lysis syndrome after treatment with gemcitabine for metastatic transitional cell carcinoma. Med Oncol. 2007;24:455.
- Malik IA, Abubakar S, Alam F, Khan A. Dexamethasone-induced tumor lysis syndrome in high-grade non-Hodgkin’s lymphoma. South Med J. 1994;87:409.
- Jabr FI. Acute tumor lysis syndrome induced by rituximab in diffuse large B-cell lymphoma. Int J Hematol. 2005;82:312.
- Sezer O, Vesole DH, Singhal S, et al. Bortezomib-induced tumor lysis syndrome in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:233.
- Jensen M, Winkler U, Manzke O, et al. Rapid tumor lysis in a patient with B-cell chronic lymphocytic leukemia and lymphocytosis treated with an anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab). Ann Hematol. 1998;77:89.
- Linck D, Basara N, Tran V, et al. Peracute onset of severe tumor lysis syndrome immediately after 4 Gy fractionated TBI as part of reduced intensity preparative regimen in a patient with T-ALL with high tumor burden. Bone Marrow Transplant. 2003;31:935.
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778. [Erratum, J Clin Oncol. 2010;28:708.]
- Cheuk DK, Chiang AK, Chan GC, Ha SY. Urate oxidase for the prevention and treatment of tumor lysis syndrome in children with cancer. Cochrane Database Syst Rev. 2010;(6):CD006945.
- Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28:4207.
Case
A 25-year-old male with HIV/AIDS and a CD4 count of 65 cells/μL presents to the ED with intractable nausea and vomiting for one week. Laboratory evaluation revealed a white blood cell of 67,000 cells/mm3. An extended chemistry panel reveals creatinine 3.5 mg/dL, potassium 3.0 mmol/L, LDH 250 IU/L, and uric acid 5mg/dL. Calcium and phosphorus were both normal. The patient was admitted for further evaluation and management, and was later diagnosed with Burkitt’s lymphoma.
Overview
Tumor lysis syndrome (TLS) is an acute cell lysis of tumor cells with the release of cell content into circulation either spontaneously or in response to therapy, leading to hyperurecemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.1-3
TLS is one of the most common oncology emergencies encountered by hospitalists caring for patients with hematologic malignancies. The incidence and severity of TLS depend on the cell burden, cell proliferation rate, potential for cell lysis or chemo sensitivity, baseline clinical characteristics, and preventive measures taken (see Table 1).2,4
TLS is classified as laboratory or clinical. Laboratory TLS is described as the presence of two or more of the following serum abnormalities at the same time, present within three days before or seven days after the start of therapy.5
- Uric acid >8 mg/dL (475.8 micromole/L) or 25% increase;
- Potassium >6 mEq/L (6 mmol/L) or 25% increase;
- Phosphorus >6.5 mg/dL (2.1 mmol/L) for children or >4.5 mg/dl (1.45 mmol/L) for adults or 25% increase; and
- Calcium >7 mg/dL (1.75 mmol/L) or 25% increase.
Clinical TLS is defined as laboratory TLS in association with increased creatinine levels, seizures, cardiac arrhythmias, or death (see Table 2).5
Pathogenesis
Tumor cell lysis releases DNA, cytokines, phosphate, and potassium. DNA is metabolized into adenosine and guanosine, which are then converted into xanthines. Xanthines are oxidized by xanthine oxidase into uric acid, which is then excreted through the kidneys.
TLS develops when the accumulation of xanthine, uric acid, potassium, and phosphorus exceeds the kidney’s capacity to excrete them. Cytokines cause hypotension, inflammation, and kidney injury, and worsen the kidney’s excretory capacity. Damage to the kidneys also occurs by renal precipitation of uric acid, xanthine, and calcium phosphate.4
Phosphorus concentrations in tumor cells are four times higher than in normal cells. When the calcium phosphorus product exceeds 60 mg2/dL2, there is an increased risk of calcium phosphate precipitation in the kidney tubules, which could lead to kidney failure. Accumulation of calcium phosphate product may also be cardiotoxic and can lead to cardiac arrhythmias. In addition, hyperphosphatemia can cause secondary hypocalcemia, which may lead to parasthesias, tetany, and cardiac arrhythmias.2,4
TLS is most common in tumors with high proliferative rates and high tumor burden, such as acute lymphoblastic leukemia and Burkitt’s lymphoma, but it can occur with other hematologic malignancies, such as T-cell precursor acute lymphocytic leukemia (ALL), B-cell precursor ALL, acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), anaplastic large cell lymphoma, and plasma cell disorders (e.g. multiple myeloma and plasmacytoma).6,7 TLS has also been reported with the treatment of solid organ nonhematologic tumors (see Table 3).
In hematologic tumors, TLS frequently is associated with cytotoxic chemotherapy, and less frequently with glucocorticoid treatment, monoclonal antibodies (eg, rituximab, bortezomab, imatinib), and radiation therapy.25-29
Patient factors, such as baseline kidney disease or lack of prophylactic/preventive measures for TLS, also increase the risk.4 TLS, however, can develop in patients classified as low risk (see Table 1.
TLS Prevention
Intravenous fluids. Every patient at intermediate or high risk of TLS should receive intravenous fluids (IVF) prior to cancer treatment; those at low risk may receive IVF based on the provider’s clinical judgment.30 The purpose of administering IVF is to generate high urine output to reduce the risk of precipitation of uric acid in the renal tubules.30 Both adults and children should receive approximately 2 to 3 L/m2 per day of IVF,30 and urine output should be maintained at 2 ml/kg/hr (or 4 to 6 ml/kg/hr for children <10kg).30 IVF should be cautiously administered in patients with renal insufficiency or heart failure, and diuretics may be used to maintain goal urine output. Recommended initial fluids are D51/4 normal saline, or normal saline for patients who are dehydrated or hyponatremic.30
Allopurinol. Allopurinol is usually also administered to patients at risk for developing TLS.30 Allopurinol inhibits the metabolism of hypoxanthine and xanthine to uric acid, which decreases the accumulation of uric acid in the renal tubules, thus preventing obstructive renal disease from precipitation of uric acid.4 The recommended dose of allopurinol is 100 mg/m2 every eight hours, and should not exceed 800 mg per day in adults. It should be started one to two days prior to induction chemotherapy and continued for three to seven days after the treatment and until uric acid levels and other electrolyte levels have returned to normal. The dose is adjusted to 50 mg/m2 every eight hours in patients with kidney failure.30
In some cases, allopurinol can lead to increased levels of xanthine crystals in the renal tubules, leading to acute kidney injury. Also, allopurinol does not have any effect on uric acid that has already been formed, so patients with elevated uric acid levels prior to the initiation of cancer therapy will not have any reduction in the levels of uric acid. Allopurinol reduces the degradation of other purines, so it can cause toxicity in patients on azathioprine and 6-mercaptopurine if the doses of these medications are not adjusted.
Rasburicase. Rasburicase is a recombinant urate oxidase, derived from aspergillus favus, which catalyzes the breakdown of uric acid to allantoin, which is a water-soluble product. Rasburicase is recommended as a first-line treatment for patients at high risk for clinical TLS.30 Rasburicase has an earlier onset than allopurinol and rapidly decreases serum levels of uric acid within four hours of administration.30,31 The recommended dose is 0.10 to 0.20 mg/kg once a day for five days in adults.30
A Phase III trial compared the efficiency and safety of rasburicase to rasburicase with allopurinol or allopurinol alone.32 A significantly higher normalization of uric acid was found in patients on rasburicase compared to allopurinol alone. The incidence of laboratory TLS was also significantly lower with rasburicase alone compared to allopurinol alone, and was even lower with allopurinol plus rasburicase. The incidence of acute kidney injury was the same with rasburicase alone or allopurinol alone but was higher with rasburicase plus allopurinol.
Serum uric acid, phosphorus, potassium, and calcium need to be monitored every four hours for 24 hours after the completion of chemotherapy in patients on rasburicase.4 The sample of blood drawn to check the uric acid levels has to be placed on ice and processed within four hours in order to avoid falsely lower levels of uric acid due to the conversion of uric acid to allantoin. Rasburicase is contraindicated in patients with G6PD deficiency and pregnant women, because one of the byproducts of uric acid breakdown is hydrogen peroxide, which can cause severe hemolysis and the formation of methemoglobin in these patients.30
Rasburicase has been approved for use in both children and adults, but there is more evidence for the use in children. Rasburicase has a black-box label for patients with anaphylaxis, methemoglobinemia, hemolysis, and hemoglobinuria, and there is a recommendation to check G6PD deficiency before use in high-risk patients.30
TLS Treatment
Alkalinization. Alkalinization of urine is controversial in the management of TLS. Urine alkalinization increases uric acid solubility but causes hyperphosphatemia and decreases calcium phosphate solubility, which can then deposit in the kidney once cancer treatment starts. Of note, hyperphosphatemia is much more difficult to correct than high levels of uric acid, and there are no clinical trials proving the superiority of urine alkalinization over normal saline.
Normalization of electrolytes. Electrolyte abnormalities should be corrected to avoid arrhythmias and seizures. Phosphorus levels >6.5 mg/dl (2.1 mmol/L) should be managed by restricting phosphorus intake, and by the use of phosphate binders (calcium acetate, calcium carbonate, sevelamer, lanthanum, or aluminum hydroxide). Aluminum hydroxide should be avoided in patients with renal insufficiency. In severe cases of hyperphosphatemia, dialysis should be considered.
Symptomatic hypocalcemia should be treated with calcium gluconate if changes are present on the electrocardiography (ECG). Hypocalcemia in the presence of hyperphosphatemia should be treated only in patients with tetany or cardiac arrhythmias; otherwise, hypocalcemia should not be treated until hyperphosphatemia has been corrected.
In cases of hyperkalemia, patients should be placed on a cardiac monitor and stabilized with calcium gluconate; kayexalate should be administered to reduce total body potassium. Other interventions, such as intravenous insulin given with dextrose, sodium bicarbonate, and albuterol, have a temporary effect on hyperkalemia and can be used as adjunct treatments in patients with severe hyperkalemia (>7). Hemodialysis should be strongly considered in severe cases of hyperkalemia, particularly in patients with persistently elevated potassium levels despite other treatments.
Back to the Case
Our patient was started on IVFs with close monitoring of his urine output. He was considered intermediate risk for developing TLS. Allopurinol, renally dosed, was administered for two days prior to initiating treatment with rituximab plus chemotherapy. His chemistry panel was monitored daily and he did not develop any form of TLS.
Bottom Line
TLS is a common oncology emergency in patients with hematologic malignancies. Preventative measures include starting IVF prior to cancer treatment, and administering allopurinol and/or rasburicase to patients at risk of developing TLS. Treatment should include normalizing electrolytes to avoid arrhythmias and seizures.
Dr. Akwe is assistant professor of medicine at the Emory University School of Medicine and a clinical instructor of medicine at the Morehouse School of Medicine, both in Atlanta. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory. Both work as hospitalists at the Atlanta VA Medical Center.
References
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55:Suppl 3:S1-S13.
- Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
- Gertz MA. Managing tumor lysis syndrome in 2010. Leuk Lymphoma. 2010;51:179-180.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3.
- Wössmann W, Schrappe M, Meyer U, et al. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82:160.
- Hussain K, Mazza JJ, Clouse LH. Tumor lysis syndrome (TLS) following fludarabine therapy Gemici C. Tumor lysis syndrome in solid tumors. J Clin Oncol. 2009;27:2738-2739
- Rostom AY, El-Hussainy G, Kandil A, Allam A. Tumor lysis syndrome following hemi-body irradiation for metastatic breast cancer. Ann Oncol. 2000;11:1349.
- Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in nonhematologic malignancies. Report of a case and review of the literature. Am J Clin Oncol. 1994;17:502.
- Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors—a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187.
- Kalemkerian GP, Darwish B, Varterasian ML. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med. 1997;103:363.
- Noh GY, Choe DH, Kim CH, Lee JC. Fatal tumor lysis syndrome during radiotherapy for non-small-cell lung cancer. J Clin Oncol. 2008;26:6005-6006.
- Pentheroudakis G, O’Neill VJ, Vasey P, Kaye SB. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Report of two cases. Support Care Cancer. 2001;9:554.
- Joshita S, Yoshizawa K, Sano K, et al., A patient with advanced hepatocellular carcinoma treated with sorafenib tosylate showed massive tumor lysis with avoidance of tumor lysis syndrome. Intern Med. 2010;49:991-994.
- Huang WS, Yang CH. Sorafenib-induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;15:4464-4466.
- Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol. 1993;21:521.
- Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol. 2005;23:6794.
- Godoy H, Kesterson JP, Lele S. Tumor lysis syndrome associated with carboplatin and paclitaxel in a woman with recurrent endometrial cancer. Int J Gynaecol Obstet. 2010;109:254.
- Shamseddine AI, Khalil AM, Wehbeh MH. Acute tumor lysis syndrome with squamous cell carcinoma of the vulva. Gynecol Oncol 1993;51:258
- Pinder EM, Atwal GS, Ayantunde AA, et al. Tumour lysis syndrome occurring in a patient with metastatic gastrointestinal stromal tumour treated with Glivec (imatinib mesylate, Gleevec, STI571). Sarcoma. 2007;2007:82012.
- Krishnan G, D’Silva K, Al-Janadi A. Cetuximab-related tumor lysis syndrome in metastatic colon carcinoma. J Clin Oncol. 2008;26:2406-2408.
- Oztop I, Demirkan B, Yaren A, et al. Rapid tumor lysis syndrome in a patient with metastatic colon cancer as a complication of treatment with 5-fluorouracil/leucoverin and irinotecan. Tumori. 2004;90:514.
- Lin CJ, Lim KH, Cheng YC, et al. Tumor lysis syndrome after treatment with gemcitabine for metastatic transitional cell carcinoma. Med Oncol. 2007;24:455.
- Malik IA, Abubakar S, Alam F, Khan A. Dexamethasone-induced tumor lysis syndrome in high-grade non-Hodgkin’s lymphoma. South Med J. 1994;87:409.
- Jabr FI. Acute tumor lysis syndrome induced by rituximab in diffuse large B-cell lymphoma. Int J Hematol. 2005;82:312.
- Sezer O, Vesole DH, Singhal S, et al. Bortezomib-induced tumor lysis syndrome in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:233.
- Jensen M, Winkler U, Manzke O, et al. Rapid tumor lysis in a patient with B-cell chronic lymphocytic leukemia and lymphocytosis treated with an anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab). Ann Hematol. 1998;77:89.
- Linck D, Basara N, Tran V, et al. Peracute onset of severe tumor lysis syndrome immediately after 4 Gy fractionated TBI as part of reduced intensity preparative regimen in a patient with T-ALL with high tumor burden. Bone Marrow Transplant. 2003;31:935.
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778. [Erratum, J Clin Oncol. 2010;28:708.]
- Cheuk DK, Chiang AK, Chan GC, Ha SY. Urate oxidase for the prevention and treatment of tumor lysis syndrome in children with cancer. Cochrane Database Syst Rev. 2010;(6):CD006945.
- Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28:4207.
Conference highlights growing HAI concerns
The Fifth Decennial International Conference on Healthcare-Associated Infections 2010, held in March in Atlanta, featured experts from several different fields discussing the significant prevalence of healthcare-associated infections (HAIs) and strategies that may be implemented to reduce their occurrence.
HAIs precipitated by the use of such devices as central venous catheters (CVCs), mechanical ventilators, and indwelling urinary catheters received special emphasis as important sources of patient morbidity and mortality.
Naomi O’Grady of the National Institutes of Health (NIH) summarized the current available knowledge regarding the prevention of central-line-associated bloodstream infections (CLABSIs). Strategies targeting appropriate line maintenance include:
- Chlorhexidine sponge dressings at the CVC insertion site in patients with short-term catheters;
- Cleanse catheter hubs and connectors with alcoholic-chlorhexidine (rather than alcohol alone) after each use; and
- Consider daily bathing of patients with chlorhexidine soap.
Speakers stressed that novel technologies, such as antimicrobial lock solutions and antiseptic- or antibiotic-impregnated catheters, should be considered when CLABSI rates remain high. Mark Shelly, MD, of Rochester, N.Y., emphasized awareness that CLABSIs occur frequently outside the ICU. “If you are only looking for CLABSI in the ICU, then you are missing more than half of the story,” Dr. Shelly said. Researchers from the National Health Safety Network (NHSN) provided more information about the substantial numbers of CLABSIs that occur on general medical wards.
Carolyn Gould, MD, MS, of the Centers for Disease Control and Prevention (CDC) confirmed that catheter-associated urinary tract infections (CAUTIs) are the most common type of HAI. CAUTIs occur at a frequency of >560,000 infections per year and cost as much as $500 million per year, she explained. Strategies to prevent CAUTIs include inserting urinary catheters only for appropriate indications and leaving them in place for the shortest possible duration.
In recent years, concern has grown about the prevalence of healthcare-associated Clostridium difficile infection (HA-CDI), which can lead to uncomplicated diarrhea, sepsis, or even death. Several speakers described strategies that reduce HA-CDI development, including the identification and removal of environmental sources of C. diff, accommodating CDI patients in a private room with contact precautions, and minimizing both the frequency and duration of antimicrobial therapy.
Uncertainty about the most reliable tests to confirm CDI was a topic of focus. Enzyme immunoassay (EIA) testing, cell cytotoxin assays, and polymerase chain reaction (PCR) testing are readily available in most U.S. hospitals; however, PCR testing might prove to be the most advantageous since it is rapid, sensitive, and specific.
Neil Fishman, MD, of the University of Pennsylvania School of Medicine in Philadelphia was one of several speakers to address the important role of antimicrobial stewardship program (ASP) development. According to Dr. Fishman, ASP goals should be to “ensure the proper use of antimicrobials” and to “promote cost-effectiveness.” By taking actions that promote the appropriate use of antimicrobials, the following positive consequences can be anticipated:
- Improved clinical outcomes;
- Reduced risk of adverse drug effects; and
- A reduction in, or stabilization of, the rate of antimicrobial resistance.
Multidrug-resistant (MDR) gram-negative Bacillus is a major challenge for hospitals worldwide. The CDC offers two guidelines for the optimal management and isolation of MDR organisms (MDRO): HICPAC 2006 (a management guideline) and HICPAC 2007 (MDRO isolation precaution guidelines). Consistent utilization of these guidelines is crucial to control the spread of MDRO.
The CDC’s Alexander Killen, MD, discussed the increasing proportion of MDR Acinetobacter and Enterobacteriaceae. Emerging issues among these organisms include the development of highly resistant strains, the incidence of which is increasing in nonacute-care settings.
The CDC’s Karen Anderson reported laboratory data on carbapenem-resistant Enterobacteriaceae (CRE) in a long-term-care facility. Her team demonstrated that CRE colonization can persist for up to six months. She speculated that the transfer of resistance between different species occurs, as does patient-to-patient transmission.
The CDC recommends the use of surveillance cultures as part of enhanced precautions. Surveillance is to continue until no new cases are detected.
Karen Clarke, MD, MS, MPH
Ketino Kobaidze, MD, PhD
Mohamad Moussa, MD
Sheri Tejedor, MD
Emory University
School of Medicine, Atlanta
The Fifth Decennial International Conference on Healthcare-Associated Infections 2010, held in March in Atlanta, featured experts from several different fields discussing the significant prevalence of healthcare-associated infections (HAIs) and strategies that may be implemented to reduce their occurrence.
HAIs precipitated by the use of such devices as central venous catheters (CVCs), mechanical ventilators, and indwelling urinary catheters received special emphasis as important sources of patient morbidity and mortality.
Naomi O’Grady of the National Institutes of Health (NIH) summarized the current available knowledge regarding the prevention of central-line-associated bloodstream infections (CLABSIs). Strategies targeting appropriate line maintenance include:
- Chlorhexidine sponge dressings at the CVC insertion site in patients with short-term catheters;
- Cleanse catheter hubs and connectors with alcoholic-chlorhexidine (rather than alcohol alone) after each use; and
- Consider daily bathing of patients with chlorhexidine soap.
Speakers stressed that novel technologies, such as antimicrobial lock solutions and antiseptic- or antibiotic-impregnated catheters, should be considered when CLABSI rates remain high. Mark Shelly, MD, of Rochester, N.Y., emphasized awareness that CLABSIs occur frequently outside the ICU. “If you are only looking for CLABSI in the ICU, then you are missing more than half of the story,” Dr. Shelly said. Researchers from the National Health Safety Network (NHSN) provided more information about the substantial numbers of CLABSIs that occur on general medical wards.
Carolyn Gould, MD, MS, of the Centers for Disease Control and Prevention (CDC) confirmed that catheter-associated urinary tract infections (CAUTIs) are the most common type of HAI. CAUTIs occur at a frequency of >560,000 infections per year and cost as much as $500 million per year, she explained. Strategies to prevent CAUTIs include inserting urinary catheters only for appropriate indications and leaving them in place for the shortest possible duration.
In recent years, concern has grown about the prevalence of healthcare-associated Clostridium difficile infection (HA-CDI), which can lead to uncomplicated diarrhea, sepsis, or even death. Several speakers described strategies that reduce HA-CDI development, including the identification and removal of environmental sources of C. diff, accommodating CDI patients in a private room with contact precautions, and minimizing both the frequency and duration of antimicrobial therapy.
Uncertainty about the most reliable tests to confirm CDI was a topic of focus. Enzyme immunoassay (EIA) testing, cell cytotoxin assays, and polymerase chain reaction (PCR) testing are readily available in most U.S. hospitals; however, PCR testing might prove to be the most advantageous since it is rapid, sensitive, and specific.
Neil Fishman, MD, of the University of Pennsylvania School of Medicine in Philadelphia was one of several speakers to address the important role of antimicrobial stewardship program (ASP) development. According to Dr. Fishman, ASP goals should be to “ensure the proper use of antimicrobials” and to “promote cost-effectiveness.” By taking actions that promote the appropriate use of antimicrobials, the following positive consequences can be anticipated:
- Improved clinical outcomes;
- Reduced risk of adverse drug effects; and
- A reduction in, or stabilization of, the rate of antimicrobial resistance.
Multidrug-resistant (MDR) gram-negative Bacillus is a major challenge for hospitals worldwide. The CDC offers two guidelines for the optimal management and isolation of MDR organisms (MDRO): HICPAC 2006 (a management guideline) and HICPAC 2007 (MDRO isolation precaution guidelines). Consistent utilization of these guidelines is crucial to control the spread of MDRO.
The CDC’s Alexander Killen, MD, discussed the increasing proportion of MDR Acinetobacter and Enterobacteriaceae. Emerging issues among these organisms include the development of highly resistant strains, the incidence of which is increasing in nonacute-care settings.
The CDC’s Karen Anderson reported laboratory data on carbapenem-resistant Enterobacteriaceae (CRE) in a long-term-care facility. Her team demonstrated that CRE colonization can persist for up to six months. She speculated that the transfer of resistance between different species occurs, as does patient-to-patient transmission.
The CDC recommends the use of surveillance cultures as part of enhanced precautions. Surveillance is to continue until no new cases are detected.
Karen Clarke, MD, MS, MPH
Ketino Kobaidze, MD, PhD
Mohamad Moussa, MD
Sheri Tejedor, MD
Emory University
School of Medicine, Atlanta
The Fifth Decennial International Conference on Healthcare-Associated Infections 2010, held in March in Atlanta, featured experts from several different fields discussing the significant prevalence of healthcare-associated infections (HAIs) and strategies that may be implemented to reduce their occurrence.
HAIs precipitated by the use of such devices as central venous catheters (CVCs), mechanical ventilators, and indwelling urinary catheters received special emphasis as important sources of patient morbidity and mortality.
Naomi O’Grady of the National Institutes of Health (NIH) summarized the current available knowledge regarding the prevention of central-line-associated bloodstream infections (CLABSIs). Strategies targeting appropriate line maintenance include:
- Chlorhexidine sponge dressings at the CVC insertion site in patients with short-term catheters;
- Cleanse catheter hubs and connectors with alcoholic-chlorhexidine (rather than alcohol alone) after each use; and
- Consider daily bathing of patients with chlorhexidine soap.
Speakers stressed that novel technologies, such as antimicrobial lock solutions and antiseptic- or antibiotic-impregnated catheters, should be considered when CLABSI rates remain high. Mark Shelly, MD, of Rochester, N.Y., emphasized awareness that CLABSIs occur frequently outside the ICU. “If you are only looking for CLABSI in the ICU, then you are missing more than half of the story,” Dr. Shelly said. Researchers from the National Health Safety Network (NHSN) provided more information about the substantial numbers of CLABSIs that occur on general medical wards.
Carolyn Gould, MD, MS, of the Centers for Disease Control and Prevention (CDC) confirmed that catheter-associated urinary tract infections (CAUTIs) are the most common type of HAI. CAUTIs occur at a frequency of >560,000 infections per year and cost as much as $500 million per year, she explained. Strategies to prevent CAUTIs include inserting urinary catheters only for appropriate indications and leaving them in place for the shortest possible duration.
In recent years, concern has grown about the prevalence of healthcare-associated Clostridium difficile infection (HA-CDI), which can lead to uncomplicated diarrhea, sepsis, or even death. Several speakers described strategies that reduce HA-CDI development, including the identification and removal of environmental sources of C. diff, accommodating CDI patients in a private room with contact precautions, and minimizing both the frequency and duration of antimicrobial therapy.
Uncertainty about the most reliable tests to confirm CDI was a topic of focus. Enzyme immunoassay (EIA) testing, cell cytotoxin assays, and polymerase chain reaction (PCR) testing are readily available in most U.S. hospitals; however, PCR testing might prove to be the most advantageous since it is rapid, sensitive, and specific.
Neil Fishman, MD, of the University of Pennsylvania School of Medicine in Philadelphia was one of several speakers to address the important role of antimicrobial stewardship program (ASP) development. According to Dr. Fishman, ASP goals should be to “ensure the proper use of antimicrobials” and to “promote cost-effectiveness.” By taking actions that promote the appropriate use of antimicrobials, the following positive consequences can be anticipated:
- Improved clinical outcomes;
- Reduced risk of adverse drug effects; and
- A reduction in, or stabilization of, the rate of antimicrobial resistance.
Multidrug-resistant (MDR) gram-negative Bacillus is a major challenge for hospitals worldwide. The CDC offers two guidelines for the optimal management and isolation of MDR organisms (MDRO): HICPAC 2006 (a management guideline) and HICPAC 2007 (MDRO isolation precaution guidelines). Consistent utilization of these guidelines is crucial to control the spread of MDRO.
The CDC’s Alexander Killen, MD, discussed the increasing proportion of MDR Acinetobacter and Enterobacteriaceae. Emerging issues among these organisms include the development of highly resistant strains, the incidence of which is increasing in nonacute-care settings.
The CDC’s Karen Anderson reported laboratory data on carbapenem-resistant Enterobacteriaceae (CRE) in a long-term-care facility. Her team demonstrated that CRE colonization can persist for up to six months. She speculated that the transfer of resistance between different species occurs, as does patient-to-patient transmission.
The CDC recommends the use of surveillance cultures as part of enhanced precautions. Surveillance is to continue until no new cases are detected.
Karen Clarke, MD, MS, MPH
Ketino Kobaidze, MD, PhD
Mohamad Moussa, MD
Sheri Tejedor, MD
Emory University
School of Medicine, Atlanta
How should a patient with a new-onset seizure be managed?
Case
A 42-year-old man is brought to the hospital by his family after a reported seizure. The patient was found on the floor, unresponsive, and suffering convulsions lasting less than a minute. He suffered no apparent trauma before or during the event. He has no history of seizures. His mental status quickly improved; he experienced oriented lucidity with slight drowsiness. His neurological exam is nonfocal, and his vital signs and laboratory values are normal. A noncontrast head computed tomogram (CT) is normal.
What is the appropriate approach to diagnosis and management for this patient with a new-onset seizure?
Overview
A patient with a first seizure presents a dilemma. Underlying causes for seizure are potentially life-threatening, and must be identified if present. A patient whose first seizure is unprovoked is at risk for future seizures (i.e., epilepsy). However, long-term therapy with anticonvulsant medication has morbidity, side effects, and expense. Advising a patient on whether to drive has public safety and legal implications, as well as major lifestyle changes for the patient.
Seizures may be focal (limited to one area of the brain) or generalized (involving both hemispheres). For the most part, focal (also known as partial) seizures do not impair consciousness; generalized seizures do. Approximately 70% of first seizures are partial focal seizures.1 Such provoking causes as head trauma, stroke, alcohol withdrawal, brain tumors, and infections can be identified in about one-third of cases.1
Electroencephalogram (EEG) and computed tomogram (CT) of the brain should be obtained, but insufficient evidence exists to recommend other testing, which should be pursued according to the clinical context.2
Unprovoked seizures recur in about 25% to 50% of patients, resulting in a diagnosis of epilepsy.1,2,4-7
Therapy is unnecessary in patients whose seizures will not recur, but reliably identifying these patients is a challenge. Whether antiepileptic drug (AED) therapy should be initiated in patients with a first unprovoked seizure is controversial and will be reviewed below.
Review of the Data
History: No test or finding can reliably differentiate unwitnessed seizures from other events (e.g., syncope).2 History from a reliable observer often is necessary to determine whether the event actually was a seizure.2 In as many as 50% of patients with a “first” seizure, thorough history will likely reveal previously unrecognized seizures.1 Although most epilepsy syndromes begin in childhood or adolescence, a significant number of patients will experience their first seizure in adulthood.2
A thorough neurologic examination should be performed. In a minority of cases, an exam will suggest a focal lesion. An impaired level of consciousness might represent a post-ictal state or delirium.2
Diagnostic evaluation: If the history suggests a seizure, an EEG should be obtained. Although the EEG will be normal in 50% of patients following a first seizure, an abnormal EEG provides useful information about seizure type and the likelihood of recurrence.2 In nearly a quarter of patients, the EEG will show epileptiform abnormalities that predict future seizures.2
Generally, an EEG should be obtained as soon as feasible, once a seizure is suspected. Some evidence in children suggests that EEG yield is higher in the 24 hours after a first seizure.
A noncontrast head CT or magnetic resonance imaging (MRI) reveals a significant abnormality about 10% of the time.2 A CT or MRI should be obtained. Few studies have compared CT to MRI in terms of yield in determining first seizure etiology, and those that do compare the two suffer from selection bias.2 Although CT or MRI are appropriate in evaluating a patient with a first seizure, the MRI’s greater resolution might provide a higher diagnostic yield in terms of seizure etiology, and, therefore, some experts recommend MRI over CT in nonemergent cases.2
Insufficient data exist to support or refute diagnostic testing beyond brain imaging and EEG. Although electrolyte abnormalities, hypoglycemia, and infections might infrequently cause seizures, such routine blood tests as complete blood count (CBC) and chemistry panels are rarely helpful.
As many as 15% of patients with a seizure will have minor abnormalities on routine lab tests, but the abnormalities do not appear to be the cause of the seizure.2
Lumbar puncture (LP) is categorically recommended only in patients in whom there is a clinical suspicion for infection as a seizure etiology. Reviews suggest that signs and symptoms of infection are typically present in patients with meningitis or another infectious cause for seizure; LP generally has limited utility in other noninfectious causes of seizure.2
The utility of toxicology testing in a first seizure has not been studied widely. Testing urine or blood for the presence of alcohol, cocaine, methamphetamines, benzodiazepines, or drug metabolites could be useful in the appropriate clinical setting.2
It is unclear whether a patient with a first seizure requires hospitalization. If initial testing in the ED rules out serious causes of seizures, the yield for hospitalization is likely to be low. In clinical practice, however, hospitalization is common and often necessary to complete such diagnostic testing as EEG and MRI.
Medical therapy: Patients with suspected epilepsy (e.g., those whose presenting seizure is, in retrospect, not their first seizure) should begin antiepileptic drug therapy (AED).1
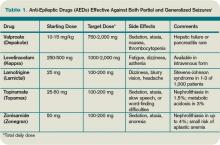
Typically, a broad-spectrum AED—one that is effective against both partial and generalized seizures—should be used as initial therapy for epilepsy. These include valproate, lamotrigine, topiramate, zonisamide, and levetiracetam (see Table 1, above). Valproate has the longest history of effectiveness; levetiracetam has fewer drug interactions, and randomized trials support its efficacy.1
Checking blood electrolytes and liver enzymes is recommended before beginning AED treatment. Significant hepatic or renal dysfunction might necessitate dosing adjustments in many AEDs.2
Inpatient consultation with a neurologist might be helpful, although insufficient evidence exists that such consultation improves patient outcomes or makes care more cost-efficient. A neurologist should follow up on patients with a first seizure after hospital discharge.2
Patients with a first seizure that likely was provoked by a reversible condition (e.g., hypotension, hypoglycemia, infection) should generally not begin AED therapy. This also includes patients with multiple seizures in a brief period of time (less than 24 hours), all attributed to the same reversible cause.1
The decision to begin AED therapy after a first unprovoked seizure is controversial. Estimates of the likelihood of seizure recurrence range from 25% at two years to 50% at one year (in the absence of AED therapy).1-2,4-7 The decision to start AED therapy after a first seizure must therefore be individualized for each patient.
Patients at high risk for recurrent seizures should begin AED therapy.1 However, no test or prognostic tool reliably identifies these patients, and initiating therapy carries side effects and places psychological, financial, and social burdens on the patient. The prevailing clinical practice, therefore, has been watchful waiting, with a second seizure constituting proof of high risk for recurrence—and need for AED therapy. Three-quarters of patients with two or more unprovoked seizures likely will go on to have recurrent seizures.6
On the other hand, in patients believed to be at high risk for seizure recurrence, a more aggressive approach of initiating AED therapy after the first seizure is reasonable. A number of risk factors increasing risk for seizure recurrence have been identified (see Table 2, left).1,2 It is justified to initiate AED therapy if any of these factors are present, even after a single seizure. Still, it’s important to note that most people with risk factors will not benefit from AEDs, as only about 40% will have a seizure in the following two years.1
Early initiation of AED therapy might be appropriate for patients with occupations or hobbies in which seizures could be life-threatening (e.g., scuba divers, truck drivers).2
Low-risk patients still have a roughly 20% to 30% risk of seizure recurrence within three years.1 A second seizure that occurs while driving or while engaged in any hazardous activity could lead to serious injury.
Patients should be advised of this small but inescapable risk and instructed to contact their department of motor vehicles for specific legal restrictions, which vary by state. Once three seizure-free years have passed after a patient’s initial seizure, the chance of a recurrence falls to around 10% to 20%.6-7
Back to the Case
Our 42-year-old patient with a first seizure had normal findings on examination, laboratory studies, and brain imaging. An EEG showed epileptiform discharges in a spike and wave pattern. The attending hospitalist counseled him on his elevated risk of future seizures; the patient then elected to begin AED therapy, citing a fear of losing his driving privileges. Levetiracetam was started, which he tolerated despite mild sedation.
A year later, he suffered another seizure at his home. With regular followup and titration of his AED, he remained seizure-free for the next five years.
Bottom Line
Most patients with a single unprovoked seizure can be managed with watchful waiting, counseling, and neurological followup. Initiation of AED therapy is appropriate for patients with a high risk of seizure recurrence, or for whom another seizure could pose personal or social harm. TH
Dr. Hoffman is a hospitalist at Emory University School of Medicine in Atlanta.
References
- French JA, Pedley TA. Clinical practice: Initial management of epilepsy. N Engl J Med. 2008;359:166-176.
- Krumholz A, Wiebe S, Gronseth G, et al. Practice Parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2007;69:1996-2007.
- Schachter SC. Antiepileptic drug therapy: general treatment principles and application for special patient populations. Epilepsia. 1999;40(9):S20-25.
- Hauser WA, Rich SS, Annegers JF, et al. Seizure recurrence after a first unprovoked seizure: an extended follow-up. Neurology. 1990;40:1163-1170.
- Marson A, Jacoby A, Johnson A, et al. Immediate versus deferred antiepileptic drug treatment for epilepsy and single seizures: a randomized controlled trial. Lancet. 2005;365: 2007-2013.
- Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med. 1998;338:429-434.
- Berg AT. Risk of recurrence after a first unprovoked seizure. Epilepsia. 2008;49:S13-18.
- Kim LG, Johnson TL, Marson AG, et al. Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial. Lancet Neurology. 2006;5(4):317-322.
Case
A 42-year-old man is brought to the hospital by his family after a reported seizure. The patient was found on the floor, unresponsive, and suffering convulsions lasting less than a minute. He suffered no apparent trauma before or during the event. He has no history of seizures. His mental status quickly improved; he experienced oriented lucidity with slight drowsiness. His neurological exam is nonfocal, and his vital signs and laboratory values are normal. A noncontrast head computed tomogram (CT) is normal.
What is the appropriate approach to diagnosis and management for this patient with a new-onset seizure?
Overview
A patient with a first seizure presents a dilemma. Underlying causes for seizure are potentially life-threatening, and must be identified if present. A patient whose first seizure is unprovoked is at risk for future seizures (i.e., epilepsy). However, long-term therapy with anticonvulsant medication has morbidity, side effects, and expense. Advising a patient on whether to drive has public safety and legal implications, as well as major lifestyle changes for the patient.
Seizures may be focal (limited to one area of the brain) or generalized (involving both hemispheres). For the most part, focal (also known as partial) seizures do not impair consciousness; generalized seizures do. Approximately 70% of first seizures are partial focal seizures.1 Such provoking causes as head trauma, stroke, alcohol withdrawal, brain tumors, and infections can be identified in about one-third of cases.1
Electroencephalogram (EEG) and computed tomogram (CT) of the brain should be obtained, but insufficient evidence exists to recommend other testing, which should be pursued according to the clinical context.2
Unprovoked seizures recur in about 25% to 50% of patients, resulting in a diagnosis of epilepsy.1,2,4-7
Therapy is unnecessary in patients whose seizures will not recur, but reliably identifying these patients is a challenge. Whether antiepileptic drug (AED) therapy should be initiated in patients with a first unprovoked seizure is controversial and will be reviewed below.
Review of the Data
History: No test or finding can reliably differentiate unwitnessed seizures from other events (e.g., syncope).2 History from a reliable observer often is necessary to determine whether the event actually was a seizure.2 In as many as 50% of patients with a “first” seizure, thorough history will likely reveal previously unrecognized seizures.1 Although most epilepsy syndromes begin in childhood or adolescence, a significant number of patients will experience their first seizure in adulthood.2
A thorough neurologic examination should be performed. In a minority of cases, an exam will suggest a focal lesion. An impaired level of consciousness might represent a post-ictal state or delirium.2
Diagnostic evaluation: If the history suggests a seizure, an EEG should be obtained. Although the EEG will be normal in 50% of patients following a first seizure, an abnormal EEG provides useful information about seizure type and the likelihood of recurrence.2 In nearly a quarter of patients, the EEG will show epileptiform abnormalities that predict future seizures.2
Generally, an EEG should be obtained as soon as feasible, once a seizure is suspected. Some evidence in children suggests that EEG yield is higher in the 24 hours after a first seizure.
A noncontrast head CT or magnetic resonance imaging (MRI) reveals a significant abnormality about 10% of the time.2 A CT or MRI should be obtained. Few studies have compared CT to MRI in terms of yield in determining first seizure etiology, and those that do compare the two suffer from selection bias.2 Although CT or MRI are appropriate in evaluating a patient with a first seizure, the MRI’s greater resolution might provide a higher diagnostic yield in terms of seizure etiology, and, therefore, some experts recommend MRI over CT in nonemergent cases.2
Insufficient data exist to support or refute diagnostic testing beyond brain imaging and EEG. Although electrolyte abnormalities, hypoglycemia, and infections might infrequently cause seizures, such routine blood tests as complete blood count (CBC) and chemistry panels are rarely helpful.
As many as 15% of patients with a seizure will have minor abnormalities on routine lab tests, but the abnormalities do not appear to be the cause of the seizure.2
Lumbar puncture (LP) is categorically recommended only in patients in whom there is a clinical suspicion for infection as a seizure etiology. Reviews suggest that signs and symptoms of infection are typically present in patients with meningitis or another infectious cause for seizure; LP generally has limited utility in other noninfectious causes of seizure.2
The utility of toxicology testing in a first seizure has not been studied widely. Testing urine or blood for the presence of alcohol, cocaine, methamphetamines, benzodiazepines, or drug metabolites could be useful in the appropriate clinical setting.2
It is unclear whether a patient with a first seizure requires hospitalization. If initial testing in the ED rules out serious causes of seizures, the yield for hospitalization is likely to be low. In clinical practice, however, hospitalization is common and often necessary to complete such diagnostic testing as EEG and MRI.
Medical therapy: Patients with suspected epilepsy (e.g., those whose presenting seizure is, in retrospect, not their first seizure) should begin antiepileptic drug therapy (AED).1
Typically, a broad-spectrum AED—one that is effective against both partial and generalized seizures—should be used as initial therapy for epilepsy. These include valproate, lamotrigine, topiramate, zonisamide, and levetiracetam (see Table 1, above). Valproate has the longest history of effectiveness; levetiracetam has fewer drug interactions, and randomized trials support its efficacy.1
Checking blood electrolytes and liver enzymes is recommended before beginning AED treatment. Significant hepatic or renal dysfunction might necessitate dosing adjustments in many AEDs.2
Inpatient consultation with a neurologist might be helpful, although insufficient evidence exists that such consultation improves patient outcomes or makes care more cost-efficient. A neurologist should follow up on patients with a first seizure after hospital discharge.2
Patients with a first seizure that likely was provoked by a reversible condition (e.g., hypotension, hypoglycemia, infection) should generally not begin AED therapy. This also includes patients with multiple seizures in a brief period of time (less than 24 hours), all attributed to the same reversible cause.1
The decision to begin AED therapy after a first unprovoked seizure is controversial. Estimates of the likelihood of seizure recurrence range from 25% at two years to 50% at one year (in the absence of AED therapy).1-2,4-7 The decision to start AED therapy after a first seizure must therefore be individualized for each patient.
Patients at high risk for recurrent seizures should begin AED therapy.1 However, no test or prognostic tool reliably identifies these patients, and initiating therapy carries side effects and places psychological, financial, and social burdens on the patient. The prevailing clinical practice, therefore, has been watchful waiting, with a second seizure constituting proof of high risk for recurrence—and need for AED therapy. Three-quarters of patients with two or more unprovoked seizures likely will go on to have recurrent seizures.6
On the other hand, in patients believed to be at high risk for seizure recurrence, a more aggressive approach of initiating AED therapy after the first seizure is reasonable. A number of risk factors increasing risk for seizure recurrence have been identified (see Table 2, left).1,2 It is justified to initiate AED therapy if any of these factors are present, even after a single seizure. Still, it’s important to note that most people with risk factors will not benefit from AEDs, as only about 40% will have a seizure in the following two years.1
Early initiation of AED therapy might be appropriate for patients with occupations or hobbies in which seizures could be life-threatening (e.g., scuba divers, truck drivers).2
Low-risk patients still have a roughly 20% to 30% risk of seizure recurrence within three years.1 A second seizure that occurs while driving or while engaged in any hazardous activity could lead to serious injury.
Patients should be advised of this small but inescapable risk and instructed to contact their department of motor vehicles for specific legal restrictions, which vary by state. Once three seizure-free years have passed after a patient’s initial seizure, the chance of a recurrence falls to around 10% to 20%.6-7
Back to the Case
Our 42-year-old patient with a first seizure had normal findings on examination, laboratory studies, and brain imaging. An EEG showed epileptiform discharges in a spike and wave pattern. The attending hospitalist counseled him on his elevated risk of future seizures; the patient then elected to begin AED therapy, citing a fear of losing his driving privileges. Levetiracetam was started, which he tolerated despite mild sedation.
A year later, he suffered another seizure at his home. With regular followup and titration of his AED, he remained seizure-free for the next five years.
Bottom Line
Most patients with a single unprovoked seizure can be managed with watchful waiting, counseling, and neurological followup. Initiation of AED therapy is appropriate for patients with a high risk of seizure recurrence, or for whom another seizure could pose personal or social harm. TH
Dr. Hoffman is a hospitalist at Emory University School of Medicine in Atlanta.
References
- French JA, Pedley TA. Clinical practice: Initial management of epilepsy. N Engl J Med. 2008;359:166-176.
- Krumholz A, Wiebe S, Gronseth G, et al. Practice Parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2007;69:1996-2007.
- Schachter SC. Antiepileptic drug therapy: general treatment principles and application for special patient populations. Epilepsia. 1999;40(9):S20-25.
- Hauser WA, Rich SS, Annegers JF, et al. Seizure recurrence after a first unprovoked seizure: an extended follow-up. Neurology. 1990;40:1163-1170.
- Marson A, Jacoby A, Johnson A, et al. Immediate versus deferred antiepileptic drug treatment for epilepsy and single seizures: a randomized controlled trial. Lancet. 2005;365: 2007-2013.
- Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med. 1998;338:429-434.
- Berg AT. Risk of recurrence after a first unprovoked seizure. Epilepsia. 2008;49:S13-18.
- Kim LG, Johnson TL, Marson AG, et al. Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial. Lancet Neurology. 2006;5(4):317-322.
Case
A 42-year-old man is brought to the hospital by his family after a reported seizure. The patient was found on the floor, unresponsive, and suffering convulsions lasting less than a minute. He suffered no apparent trauma before or during the event. He has no history of seizures. His mental status quickly improved; he experienced oriented lucidity with slight drowsiness. His neurological exam is nonfocal, and his vital signs and laboratory values are normal. A noncontrast head computed tomogram (CT) is normal.
What is the appropriate approach to diagnosis and management for this patient with a new-onset seizure?
Overview
A patient with a first seizure presents a dilemma. Underlying causes for seizure are potentially life-threatening, and must be identified if present. A patient whose first seizure is unprovoked is at risk for future seizures (i.e., epilepsy). However, long-term therapy with anticonvulsant medication has morbidity, side effects, and expense. Advising a patient on whether to drive has public safety and legal implications, as well as major lifestyle changes for the patient.
Seizures may be focal (limited to one area of the brain) or generalized (involving both hemispheres). For the most part, focal (also known as partial) seizures do not impair consciousness; generalized seizures do. Approximately 70% of first seizures are partial focal seizures.1 Such provoking causes as head trauma, stroke, alcohol withdrawal, brain tumors, and infections can be identified in about one-third of cases.1
Electroencephalogram (EEG) and computed tomogram (CT) of the brain should be obtained, but insufficient evidence exists to recommend other testing, which should be pursued according to the clinical context.2
Unprovoked seizures recur in about 25% to 50% of patients, resulting in a diagnosis of epilepsy.1,2,4-7
Therapy is unnecessary in patients whose seizures will not recur, but reliably identifying these patients is a challenge. Whether antiepileptic drug (AED) therapy should be initiated in patients with a first unprovoked seizure is controversial and will be reviewed below.
Review of the Data
History: No test or finding can reliably differentiate unwitnessed seizures from other events (e.g., syncope).2 History from a reliable observer often is necessary to determine whether the event actually was a seizure.2 In as many as 50% of patients with a “first” seizure, thorough history will likely reveal previously unrecognized seizures.1 Although most epilepsy syndromes begin in childhood or adolescence, a significant number of patients will experience their first seizure in adulthood.2
A thorough neurologic examination should be performed. In a minority of cases, an exam will suggest a focal lesion. An impaired level of consciousness might represent a post-ictal state or delirium.2
Diagnostic evaluation: If the history suggests a seizure, an EEG should be obtained. Although the EEG will be normal in 50% of patients following a first seizure, an abnormal EEG provides useful information about seizure type and the likelihood of recurrence.2 In nearly a quarter of patients, the EEG will show epileptiform abnormalities that predict future seizures.2
Generally, an EEG should be obtained as soon as feasible, once a seizure is suspected. Some evidence in children suggests that EEG yield is higher in the 24 hours after a first seizure.
A noncontrast head CT or magnetic resonance imaging (MRI) reveals a significant abnormality about 10% of the time.2 A CT or MRI should be obtained. Few studies have compared CT to MRI in terms of yield in determining first seizure etiology, and those that do compare the two suffer from selection bias.2 Although CT or MRI are appropriate in evaluating a patient with a first seizure, the MRI’s greater resolution might provide a higher diagnostic yield in terms of seizure etiology, and, therefore, some experts recommend MRI over CT in nonemergent cases.2
Insufficient data exist to support or refute diagnostic testing beyond brain imaging and EEG. Although electrolyte abnormalities, hypoglycemia, and infections might infrequently cause seizures, such routine blood tests as complete blood count (CBC) and chemistry panels are rarely helpful.
As many as 15% of patients with a seizure will have minor abnormalities on routine lab tests, but the abnormalities do not appear to be the cause of the seizure.2
Lumbar puncture (LP) is categorically recommended only in patients in whom there is a clinical suspicion for infection as a seizure etiology. Reviews suggest that signs and symptoms of infection are typically present in patients with meningitis or another infectious cause for seizure; LP generally has limited utility in other noninfectious causes of seizure.2
The utility of toxicology testing in a first seizure has not been studied widely. Testing urine or blood for the presence of alcohol, cocaine, methamphetamines, benzodiazepines, or drug metabolites could be useful in the appropriate clinical setting.2
It is unclear whether a patient with a first seizure requires hospitalization. If initial testing in the ED rules out serious causes of seizures, the yield for hospitalization is likely to be low. In clinical practice, however, hospitalization is common and often necessary to complete such diagnostic testing as EEG and MRI.
Medical therapy: Patients with suspected epilepsy (e.g., those whose presenting seizure is, in retrospect, not their first seizure) should begin antiepileptic drug therapy (AED).1
Typically, a broad-spectrum AED—one that is effective against both partial and generalized seizures—should be used as initial therapy for epilepsy. These include valproate, lamotrigine, topiramate, zonisamide, and levetiracetam (see Table 1, above). Valproate has the longest history of effectiveness; levetiracetam has fewer drug interactions, and randomized trials support its efficacy.1
Checking blood electrolytes and liver enzymes is recommended before beginning AED treatment. Significant hepatic or renal dysfunction might necessitate dosing adjustments in many AEDs.2
Inpatient consultation with a neurologist might be helpful, although insufficient evidence exists that such consultation improves patient outcomes or makes care more cost-efficient. A neurologist should follow up on patients with a first seizure after hospital discharge.2
Patients with a first seizure that likely was provoked by a reversible condition (e.g., hypotension, hypoglycemia, infection) should generally not begin AED therapy. This also includes patients with multiple seizures in a brief period of time (less than 24 hours), all attributed to the same reversible cause.1
The decision to begin AED therapy after a first unprovoked seizure is controversial. Estimates of the likelihood of seizure recurrence range from 25% at two years to 50% at one year (in the absence of AED therapy).1-2,4-7 The decision to start AED therapy after a first seizure must therefore be individualized for each patient.
Patients at high risk for recurrent seizures should begin AED therapy.1 However, no test or prognostic tool reliably identifies these patients, and initiating therapy carries side effects and places psychological, financial, and social burdens on the patient. The prevailing clinical practice, therefore, has been watchful waiting, with a second seizure constituting proof of high risk for recurrence—and need for AED therapy. Three-quarters of patients with two or more unprovoked seizures likely will go on to have recurrent seizures.6
On the other hand, in patients believed to be at high risk for seizure recurrence, a more aggressive approach of initiating AED therapy after the first seizure is reasonable. A number of risk factors increasing risk for seizure recurrence have been identified (see Table 2, left).1,2 It is justified to initiate AED therapy if any of these factors are present, even after a single seizure. Still, it’s important to note that most people with risk factors will not benefit from AEDs, as only about 40% will have a seizure in the following two years.1
Early initiation of AED therapy might be appropriate for patients with occupations or hobbies in which seizures could be life-threatening (e.g., scuba divers, truck drivers).2
Low-risk patients still have a roughly 20% to 30% risk of seizure recurrence within three years.1 A second seizure that occurs while driving or while engaged in any hazardous activity could lead to serious injury.
Patients should be advised of this small but inescapable risk and instructed to contact their department of motor vehicles for specific legal restrictions, which vary by state. Once three seizure-free years have passed after a patient’s initial seizure, the chance of a recurrence falls to around 10% to 20%.6-7
Back to the Case
Our 42-year-old patient with a first seizure had normal findings on examination, laboratory studies, and brain imaging. An EEG showed epileptiform discharges in a spike and wave pattern. The attending hospitalist counseled him on his elevated risk of future seizures; the patient then elected to begin AED therapy, citing a fear of losing his driving privileges. Levetiracetam was started, which he tolerated despite mild sedation.
A year later, he suffered another seizure at his home. With regular followup and titration of his AED, he remained seizure-free for the next five years.
Bottom Line
Most patients with a single unprovoked seizure can be managed with watchful waiting, counseling, and neurological followup. Initiation of AED therapy is appropriate for patients with a high risk of seizure recurrence, or for whom another seizure could pose personal or social harm. TH
Dr. Hoffman is a hospitalist at Emory University School of Medicine in Atlanta.
References
- French JA, Pedley TA. Clinical practice: Initial management of epilepsy. N Engl J Med. 2008;359:166-176.
- Krumholz A, Wiebe S, Gronseth G, et al. Practice Parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2007;69:1996-2007.
- Schachter SC. Antiepileptic drug therapy: general treatment principles and application for special patient populations. Epilepsia. 1999;40(9):S20-25.
- Hauser WA, Rich SS, Annegers JF, et al. Seizure recurrence after a first unprovoked seizure: an extended follow-up. Neurology. 1990;40:1163-1170.
- Marson A, Jacoby A, Johnson A, et al. Immediate versus deferred antiepileptic drug treatment for epilepsy and single seizures: a randomized controlled trial. Lancet. 2005;365: 2007-2013.
- Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med. 1998;338:429-434.
- Berg AT. Risk of recurrence after a first unprovoked seizure. Epilepsia. 2008;49:S13-18.
- Kim LG, Johnson TL, Marson AG, et al. Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial. Lancet Neurology. 2006;5(4):317-322.