User login
How Can Tumor Lysis Syndrome Be Prevented and Managed in Cancer Patients?
Case
A 25-year-old male with HIV/AIDS and a CD4 count of 65 cells/μL presents to the ED with intractable nausea and vomiting for one week. Laboratory evaluation revealed a white blood cell of 67,000 cells/mm3. An extended chemistry panel reveals creatinine 3.5 mg/dL, potassium 3.0 mmol/L, LDH 250 IU/L, and uric acid 5mg/dL. Calcium and phosphorus were both normal. The patient was admitted for further evaluation and management, and was later diagnosed with Burkitt’s lymphoma.
Overview
Tumor lysis syndrome (TLS) is an acute cell lysis of tumor cells with the release of cell content into circulation either spontaneously or in response to therapy, leading to hyperurecemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.1-3
TLS is one of the most common oncology emergencies encountered by hospitalists caring for patients with hematologic malignancies. The incidence and severity of TLS depend on the cell burden, cell proliferation rate, potential for cell lysis or chemo sensitivity, baseline clinical characteristics, and preventive measures taken (see Table 1).2,4
TLS is classified as laboratory or clinical. Laboratory TLS is described as the presence of two or more of the following serum abnormalities at the same time, present within three days before or seven days after the start of therapy.5
- Uric acid >8 mg/dL (475.8 micromole/L) or 25% increase;
- Potassium >6 mEq/L (6 mmol/L) or 25% increase;
- Phosphorus >6.5 mg/dL (2.1 mmol/L) for children or >4.5 mg/dl (1.45 mmol/L) for adults or 25% increase; and
- Calcium >7 mg/dL (1.75 mmol/L) or 25% increase.
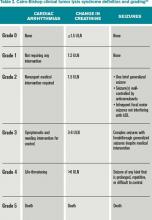
Clinical TLS is defined as laboratory TLS in association with increased creatinine levels, seizures, cardiac arrhythmias, or death (see Table 2).5
Pathogenesis
Tumor cell lysis releases DNA, cytokines, phosphate, and potassium. DNA is metabolized into adenosine and guanosine, which are then converted into xanthines. Xanthines are oxidized by xanthine oxidase into uric acid, which is then excreted through the kidneys.
TLS develops when the accumulation of xanthine, uric acid, potassium, and phosphorus exceeds the kidney’s capacity to excrete them. Cytokines cause hypotension, inflammation, and kidney injury, and worsen the kidney’s excretory capacity. Damage to the kidneys also occurs by renal precipitation of uric acid, xanthine, and calcium phosphate.4
Phosphorus concentrations in tumor cells are four times higher than in normal cells. When the calcium phosphorus product exceeds 60 mg2/dL2, there is an increased risk of calcium phosphate precipitation in the kidney tubules, which could lead to kidney failure. Accumulation of calcium phosphate product may also be cardiotoxic and can lead to cardiac arrhythmias. In addition, hyperphosphatemia can cause secondary hypocalcemia, which may lead to parasthesias, tetany, and cardiac arrhythmias.2,4
TLS is most common in tumors with high proliferative rates and high tumor burden, such as acute lymphoblastic leukemia and Burkitt’s lymphoma, but it can occur with other hematologic malignancies, such as T-cell precursor acute lymphocytic leukemia (ALL), B-cell precursor ALL, acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), anaplastic large cell lymphoma, and plasma cell disorders (e.g. multiple myeloma and plasmacytoma).6,7 TLS has also been reported with the treatment of solid organ nonhematologic tumors (see Table 3).
In hematologic tumors, TLS frequently is associated with cytotoxic chemotherapy, and less frequently with glucocorticoid treatment, monoclonal antibodies (eg, rituximab, bortezomab, imatinib), and radiation therapy.25-29
Patient factors, such as baseline kidney disease or lack of prophylactic/preventive measures for TLS, also increase the risk.4 TLS, however, can develop in patients classified as low risk (see Table 1.
TLS Prevention
Intravenous fluids. Every patient at intermediate or high risk of TLS should receive intravenous fluids (IVF) prior to cancer treatment; those at low risk may receive IVF based on the provider’s clinical judgment.30 The purpose of administering IVF is to generate high urine output to reduce the risk of precipitation of uric acid in the renal tubules.30 Both adults and children should receive approximately 2 to 3 L/m2 per day of IVF,30 and urine output should be maintained at 2 ml/kg/hr (or 4 to 6 ml/kg/hr for children <10kg).30 IVF should be cautiously administered in patients with renal insufficiency or heart failure, and diuretics may be used to maintain goal urine output. Recommended initial fluids are D51/4 normal saline, or normal saline for patients who are dehydrated or hyponatremic.30
Allopurinol. Allopurinol is usually also administered to patients at risk for developing TLS.30 Allopurinol inhibits the metabolism of hypoxanthine and xanthine to uric acid, which decreases the accumulation of uric acid in the renal tubules, thus preventing obstructive renal disease from precipitation of uric acid.4 The recommended dose of allopurinol is 100 mg/m2 every eight hours, and should not exceed 800 mg per day in adults. It should be started one to two days prior to induction chemotherapy and continued for three to seven days after the treatment and until uric acid levels and other electrolyte levels have returned to normal. The dose is adjusted to 50 mg/m2 every eight hours in patients with kidney failure.30
In some cases, allopurinol can lead to increased levels of xanthine crystals in the renal tubules, leading to acute kidney injury. Also, allopurinol does not have any effect on uric acid that has already been formed, so patients with elevated uric acid levels prior to the initiation of cancer therapy will not have any reduction in the levels of uric acid. Allopurinol reduces the degradation of other purines, so it can cause toxicity in patients on azathioprine and 6-mercaptopurine if the doses of these medications are not adjusted.
Rasburicase. Rasburicase is a recombinant urate oxidase, derived from aspergillus favus, which catalyzes the breakdown of uric acid to allantoin, which is a water-soluble product. Rasburicase is recommended as a first-line treatment for patients at high risk for clinical TLS.30 Rasburicase has an earlier onset than allopurinol and rapidly decreases serum levels of uric acid within four hours of administration.30,31 The recommended dose is 0.10 to 0.20 mg/kg once a day for five days in adults.30
A Phase III trial compared the efficiency and safety of rasburicase to rasburicase with allopurinol or allopurinol alone.32 A significantly higher normalization of uric acid was found in patients on rasburicase compared to allopurinol alone. The incidence of laboratory TLS was also significantly lower with rasburicase alone compared to allopurinol alone, and was even lower with allopurinol plus rasburicase. The incidence of acute kidney injury was the same with rasburicase alone or allopurinol alone but was higher with rasburicase plus allopurinol.
Serum uric acid, phosphorus, potassium, and calcium need to be monitored every four hours for 24 hours after the completion of chemotherapy in patients on rasburicase.4 The sample of blood drawn to check the uric acid levels has to be placed on ice and processed within four hours in order to avoid falsely lower levels of uric acid due to the conversion of uric acid to allantoin. Rasburicase is contraindicated in patients with G6PD deficiency and pregnant women, because one of the byproducts of uric acid breakdown is hydrogen peroxide, which can cause severe hemolysis and the formation of methemoglobin in these patients.30
Rasburicase has been approved for use in both children and adults, but there is more evidence for the use in children. Rasburicase has a black-box label for patients with anaphylaxis, methemoglobinemia, hemolysis, and hemoglobinuria, and there is a recommendation to check G6PD deficiency before use in high-risk patients.30
TLS Treatment
Alkalinization. Alkalinization of urine is controversial in the management of TLS. Urine alkalinization increases uric acid solubility but causes hyperphosphatemia and decreases calcium phosphate solubility, which can then deposit in the kidney once cancer treatment starts. Of note, hyperphosphatemia is much more difficult to correct than high levels of uric acid, and there are no clinical trials proving the superiority of urine alkalinization over normal saline.
Normalization of electrolytes. Electrolyte abnormalities should be corrected to avoid arrhythmias and seizures. Phosphorus levels >6.5 mg/dl (2.1 mmol/L) should be managed by restricting phosphorus intake, and by the use of phosphate binders (calcium acetate, calcium carbonate, sevelamer, lanthanum, or aluminum hydroxide). Aluminum hydroxide should be avoided in patients with renal insufficiency. In severe cases of hyperphosphatemia, dialysis should be considered.
Symptomatic hypocalcemia should be treated with calcium gluconate if changes are present on the electrocardiography (ECG). Hypocalcemia in the presence of hyperphosphatemia should be treated only in patients with tetany or cardiac arrhythmias; otherwise, hypocalcemia should not be treated until hyperphosphatemia has been corrected.
In cases of hyperkalemia, patients should be placed on a cardiac monitor and stabilized with calcium gluconate; kayexalate should be administered to reduce total body potassium. Other interventions, such as intravenous insulin given with dextrose, sodium bicarbonate, and albuterol, have a temporary effect on hyperkalemia and can be used as adjunct treatments in patients with severe hyperkalemia (>7). Hemodialysis should be strongly considered in severe cases of hyperkalemia, particularly in patients with persistently elevated potassium levels despite other treatments.
Back to the Case
Our patient was started on IVFs with close monitoring of his urine output. He was considered intermediate risk for developing TLS. Allopurinol, renally dosed, was administered for two days prior to initiating treatment with rituximab plus chemotherapy. His chemistry panel was monitored daily and he did not develop any form of TLS.
Bottom Line
TLS is a common oncology emergency in patients with hematologic malignancies. Preventative measures include starting IVF prior to cancer treatment, and administering allopurinol and/or rasburicase to patients at risk of developing TLS. Treatment should include normalizing electrolytes to avoid arrhythmias and seizures.
Dr. Akwe is assistant professor of medicine at the Emory University School of Medicine and a clinical instructor of medicine at the Morehouse School of Medicine, both in Atlanta. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory. Both work as hospitalists at the Atlanta VA Medical Center.
References
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55:Suppl 3:S1-S13.
- Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
- Gertz MA. Managing tumor lysis syndrome in 2010. Leuk Lymphoma. 2010;51:179-180.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3.
- Wössmann W, Schrappe M, Meyer U, et al. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82:160.
- Hussain K, Mazza JJ, Clouse LH. Tumor lysis syndrome (TLS) following fludarabine therapy Gemici C. Tumor lysis syndrome in solid tumors. J Clin Oncol. 2009;27:2738-2739
- Rostom AY, El-Hussainy G, Kandil A, Allam A. Tumor lysis syndrome following hemi-body irradiation for metastatic breast cancer. Ann Oncol. 2000;11:1349.
- Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in nonhematologic malignancies. Report of a case and review of the literature. Am J Clin Oncol. 1994;17:502.
- Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors—a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187.
- Kalemkerian GP, Darwish B, Varterasian ML. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med. 1997;103:363.
- Noh GY, Choe DH, Kim CH, Lee JC. Fatal tumor lysis syndrome during radiotherapy for non-small-cell lung cancer. J Clin Oncol. 2008;26:6005-6006.
- Pentheroudakis G, O’Neill VJ, Vasey P, Kaye SB. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Report of two cases. Support Care Cancer. 2001;9:554.
- Joshita S, Yoshizawa K, Sano K, et al., A patient with advanced hepatocellular carcinoma treated with sorafenib tosylate showed massive tumor lysis with avoidance of tumor lysis syndrome. Intern Med. 2010;49:991-994.
- Huang WS, Yang CH. Sorafenib-induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;15:4464-4466.
- Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol. 1993;21:521.
- Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol. 2005;23:6794.
- Godoy H, Kesterson JP, Lele S. Tumor lysis syndrome associated with carboplatin and paclitaxel in a woman with recurrent endometrial cancer. Int J Gynaecol Obstet. 2010;109:254.
- Shamseddine AI, Khalil AM, Wehbeh MH. Acute tumor lysis syndrome with squamous cell carcinoma of the vulva. Gynecol Oncol 1993;51:258
- Pinder EM, Atwal GS, Ayantunde AA, et al. Tumour lysis syndrome occurring in a patient with metastatic gastrointestinal stromal tumour treated with Glivec (imatinib mesylate, Gleevec, STI571). Sarcoma. 2007;2007:82012.
- Krishnan G, D’Silva K, Al-Janadi A. Cetuximab-related tumor lysis syndrome in metastatic colon carcinoma. J Clin Oncol. 2008;26:2406-2408.
- Oztop I, Demirkan B, Yaren A, et al. Rapid tumor lysis syndrome in a patient with metastatic colon cancer as a complication of treatment with 5-fluorouracil/leucoverin and irinotecan. Tumori. 2004;90:514.
- Lin CJ, Lim KH, Cheng YC, et al. Tumor lysis syndrome after treatment with gemcitabine for metastatic transitional cell carcinoma. Med Oncol. 2007;24:455.
- Malik IA, Abubakar S, Alam F, Khan A. Dexamethasone-induced tumor lysis syndrome in high-grade non-Hodgkin’s lymphoma. South Med J. 1994;87:409.
- Jabr FI. Acute tumor lysis syndrome induced by rituximab in diffuse large B-cell lymphoma. Int J Hematol. 2005;82:312.
- Sezer O, Vesole DH, Singhal S, et al. Bortezomib-induced tumor lysis syndrome in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:233.
- Jensen M, Winkler U, Manzke O, et al. Rapid tumor lysis in a patient with B-cell chronic lymphocytic leukemia and lymphocytosis treated with an anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab). Ann Hematol. 1998;77:89.
- Linck D, Basara N, Tran V, et al. Peracute onset of severe tumor lysis syndrome immediately after 4 Gy fractionated TBI as part of reduced intensity preparative regimen in a patient with T-ALL with high tumor burden. Bone Marrow Transplant. 2003;31:935.
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778. [Erratum, J Clin Oncol. 2010;28:708.]
- Cheuk DK, Chiang AK, Chan GC, Ha SY. Urate oxidase for the prevention and treatment of tumor lysis syndrome in children with cancer. Cochrane Database Syst Rev. 2010;(6):CD006945.
- Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28:4207.
Case
A 25-year-old male with HIV/AIDS and a CD4 count of 65 cells/μL presents to the ED with intractable nausea and vomiting for one week. Laboratory evaluation revealed a white blood cell of 67,000 cells/mm3. An extended chemistry panel reveals creatinine 3.5 mg/dL, potassium 3.0 mmol/L, LDH 250 IU/L, and uric acid 5mg/dL. Calcium and phosphorus were both normal. The patient was admitted for further evaluation and management, and was later diagnosed with Burkitt’s lymphoma.
Overview
Tumor lysis syndrome (TLS) is an acute cell lysis of tumor cells with the release of cell content into circulation either spontaneously or in response to therapy, leading to hyperurecemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.1-3
TLS is one of the most common oncology emergencies encountered by hospitalists caring for patients with hematologic malignancies. The incidence and severity of TLS depend on the cell burden, cell proliferation rate, potential for cell lysis or chemo sensitivity, baseline clinical characteristics, and preventive measures taken (see Table 1).2,4
TLS is classified as laboratory or clinical. Laboratory TLS is described as the presence of two or more of the following serum abnormalities at the same time, present within three days before or seven days after the start of therapy.5
- Uric acid >8 mg/dL (475.8 micromole/L) or 25% increase;
- Potassium >6 mEq/L (6 mmol/L) or 25% increase;
- Phosphorus >6.5 mg/dL (2.1 mmol/L) for children or >4.5 mg/dl (1.45 mmol/L) for adults or 25% increase; and
- Calcium >7 mg/dL (1.75 mmol/L) or 25% increase.
Clinical TLS is defined as laboratory TLS in association with increased creatinine levels, seizures, cardiac arrhythmias, or death (see Table 2).5
Pathogenesis
Tumor cell lysis releases DNA, cytokines, phosphate, and potassium. DNA is metabolized into adenosine and guanosine, which are then converted into xanthines. Xanthines are oxidized by xanthine oxidase into uric acid, which is then excreted through the kidneys.
TLS develops when the accumulation of xanthine, uric acid, potassium, and phosphorus exceeds the kidney’s capacity to excrete them. Cytokines cause hypotension, inflammation, and kidney injury, and worsen the kidney’s excretory capacity. Damage to the kidneys also occurs by renal precipitation of uric acid, xanthine, and calcium phosphate.4
Phosphorus concentrations in tumor cells are four times higher than in normal cells. When the calcium phosphorus product exceeds 60 mg2/dL2, there is an increased risk of calcium phosphate precipitation in the kidney tubules, which could lead to kidney failure. Accumulation of calcium phosphate product may also be cardiotoxic and can lead to cardiac arrhythmias. In addition, hyperphosphatemia can cause secondary hypocalcemia, which may lead to parasthesias, tetany, and cardiac arrhythmias.2,4
TLS is most common in tumors with high proliferative rates and high tumor burden, such as acute lymphoblastic leukemia and Burkitt’s lymphoma, but it can occur with other hematologic malignancies, such as T-cell precursor acute lymphocytic leukemia (ALL), B-cell precursor ALL, acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), anaplastic large cell lymphoma, and plasma cell disorders (e.g. multiple myeloma and plasmacytoma).6,7 TLS has also been reported with the treatment of solid organ nonhematologic tumors (see Table 3).
In hematologic tumors, TLS frequently is associated with cytotoxic chemotherapy, and less frequently with glucocorticoid treatment, monoclonal antibodies (eg, rituximab, bortezomab, imatinib), and radiation therapy.25-29
Patient factors, such as baseline kidney disease or lack of prophylactic/preventive measures for TLS, also increase the risk.4 TLS, however, can develop in patients classified as low risk (see Table 1.
TLS Prevention
Intravenous fluids. Every patient at intermediate or high risk of TLS should receive intravenous fluids (IVF) prior to cancer treatment; those at low risk may receive IVF based on the provider’s clinical judgment.30 The purpose of administering IVF is to generate high urine output to reduce the risk of precipitation of uric acid in the renal tubules.30 Both adults and children should receive approximately 2 to 3 L/m2 per day of IVF,30 and urine output should be maintained at 2 ml/kg/hr (or 4 to 6 ml/kg/hr for children <10kg).30 IVF should be cautiously administered in patients with renal insufficiency or heart failure, and diuretics may be used to maintain goal urine output. Recommended initial fluids are D51/4 normal saline, or normal saline for patients who are dehydrated or hyponatremic.30
Allopurinol. Allopurinol is usually also administered to patients at risk for developing TLS.30 Allopurinol inhibits the metabolism of hypoxanthine and xanthine to uric acid, which decreases the accumulation of uric acid in the renal tubules, thus preventing obstructive renal disease from precipitation of uric acid.4 The recommended dose of allopurinol is 100 mg/m2 every eight hours, and should not exceed 800 mg per day in adults. It should be started one to two days prior to induction chemotherapy and continued for three to seven days after the treatment and until uric acid levels and other electrolyte levels have returned to normal. The dose is adjusted to 50 mg/m2 every eight hours in patients with kidney failure.30
In some cases, allopurinol can lead to increased levels of xanthine crystals in the renal tubules, leading to acute kidney injury. Also, allopurinol does not have any effect on uric acid that has already been formed, so patients with elevated uric acid levels prior to the initiation of cancer therapy will not have any reduction in the levels of uric acid. Allopurinol reduces the degradation of other purines, so it can cause toxicity in patients on azathioprine and 6-mercaptopurine if the doses of these medications are not adjusted.
Rasburicase. Rasburicase is a recombinant urate oxidase, derived from aspergillus favus, which catalyzes the breakdown of uric acid to allantoin, which is a water-soluble product. Rasburicase is recommended as a first-line treatment for patients at high risk for clinical TLS.30 Rasburicase has an earlier onset than allopurinol and rapidly decreases serum levels of uric acid within four hours of administration.30,31 The recommended dose is 0.10 to 0.20 mg/kg once a day for five days in adults.30
A Phase III trial compared the efficiency and safety of rasburicase to rasburicase with allopurinol or allopurinol alone.32 A significantly higher normalization of uric acid was found in patients on rasburicase compared to allopurinol alone. The incidence of laboratory TLS was also significantly lower with rasburicase alone compared to allopurinol alone, and was even lower with allopurinol plus rasburicase. The incidence of acute kidney injury was the same with rasburicase alone or allopurinol alone but was higher with rasburicase plus allopurinol.
Serum uric acid, phosphorus, potassium, and calcium need to be monitored every four hours for 24 hours after the completion of chemotherapy in patients on rasburicase.4 The sample of blood drawn to check the uric acid levels has to be placed on ice and processed within four hours in order to avoid falsely lower levels of uric acid due to the conversion of uric acid to allantoin. Rasburicase is contraindicated in patients with G6PD deficiency and pregnant women, because one of the byproducts of uric acid breakdown is hydrogen peroxide, which can cause severe hemolysis and the formation of methemoglobin in these patients.30
Rasburicase has been approved for use in both children and adults, but there is more evidence for the use in children. Rasburicase has a black-box label for patients with anaphylaxis, methemoglobinemia, hemolysis, and hemoglobinuria, and there is a recommendation to check G6PD deficiency before use in high-risk patients.30
TLS Treatment
Alkalinization. Alkalinization of urine is controversial in the management of TLS. Urine alkalinization increases uric acid solubility but causes hyperphosphatemia and decreases calcium phosphate solubility, which can then deposit in the kidney once cancer treatment starts. Of note, hyperphosphatemia is much more difficult to correct than high levels of uric acid, and there are no clinical trials proving the superiority of urine alkalinization over normal saline.
Normalization of electrolytes. Electrolyte abnormalities should be corrected to avoid arrhythmias and seizures. Phosphorus levels >6.5 mg/dl (2.1 mmol/L) should be managed by restricting phosphorus intake, and by the use of phosphate binders (calcium acetate, calcium carbonate, sevelamer, lanthanum, or aluminum hydroxide). Aluminum hydroxide should be avoided in patients with renal insufficiency. In severe cases of hyperphosphatemia, dialysis should be considered.
Symptomatic hypocalcemia should be treated with calcium gluconate if changes are present on the electrocardiography (ECG). Hypocalcemia in the presence of hyperphosphatemia should be treated only in patients with tetany or cardiac arrhythmias; otherwise, hypocalcemia should not be treated until hyperphosphatemia has been corrected.
In cases of hyperkalemia, patients should be placed on a cardiac monitor and stabilized with calcium gluconate; kayexalate should be administered to reduce total body potassium. Other interventions, such as intravenous insulin given with dextrose, sodium bicarbonate, and albuterol, have a temporary effect on hyperkalemia and can be used as adjunct treatments in patients with severe hyperkalemia (>7). Hemodialysis should be strongly considered in severe cases of hyperkalemia, particularly in patients with persistently elevated potassium levels despite other treatments.
Back to the Case
Our patient was started on IVFs with close monitoring of his urine output. He was considered intermediate risk for developing TLS. Allopurinol, renally dosed, was administered for two days prior to initiating treatment with rituximab plus chemotherapy. His chemistry panel was monitored daily and he did not develop any form of TLS.
Bottom Line
TLS is a common oncology emergency in patients with hematologic malignancies. Preventative measures include starting IVF prior to cancer treatment, and administering allopurinol and/or rasburicase to patients at risk of developing TLS. Treatment should include normalizing electrolytes to avoid arrhythmias and seizures.
Dr. Akwe is assistant professor of medicine at the Emory University School of Medicine and a clinical instructor of medicine at the Morehouse School of Medicine, both in Atlanta. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory. Both work as hospitalists at the Atlanta VA Medical Center.
References
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55:Suppl 3:S1-S13.
- Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
- Gertz MA. Managing tumor lysis syndrome in 2010. Leuk Lymphoma. 2010;51:179-180.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3.
- Wössmann W, Schrappe M, Meyer U, et al. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82:160.
- Hussain K, Mazza JJ, Clouse LH. Tumor lysis syndrome (TLS) following fludarabine therapy Gemici C. Tumor lysis syndrome in solid tumors. J Clin Oncol. 2009;27:2738-2739
- Rostom AY, El-Hussainy G, Kandil A, Allam A. Tumor lysis syndrome following hemi-body irradiation for metastatic breast cancer. Ann Oncol. 2000;11:1349.
- Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in nonhematologic malignancies. Report of a case and review of the literature. Am J Clin Oncol. 1994;17:502.
- Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors—a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187.
- Kalemkerian GP, Darwish B, Varterasian ML. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med. 1997;103:363.
- Noh GY, Choe DH, Kim CH, Lee JC. Fatal tumor lysis syndrome during radiotherapy for non-small-cell lung cancer. J Clin Oncol. 2008;26:6005-6006.
- Pentheroudakis G, O’Neill VJ, Vasey P, Kaye SB. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Report of two cases. Support Care Cancer. 2001;9:554.
- Joshita S, Yoshizawa K, Sano K, et al., A patient with advanced hepatocellular carcinoma treated with sorafenib tosylate showed massive tumor lysis with avoidance of tumor lysis syndrome. Intern Med. 2010;49:991-994.
- Huang WS, Yang CH. Sorafenib-induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;15:4464-4466.
- Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol. 1993;21:521.
- Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol. 2005;23:6794.
- Godoy H, Kesterson JP, Lele S. Tumor lysis syndrome associated with carboplatin and paclitaxel in a woman with recurrent endometrial cancer. Int J Gynaecol Obstet. 2010;109:254.
- Shamseddine AI, Khalil AM, Wehbeh MH. Acute tumor lysis syndrome with squamous cell carcinoma of the vulva. Gynecol Oncol 1993;51:258
- Pinder EM, Atwal GS, Ayantunde AA, et al. Tumour lysis syndrome occurring in a patient with metastatic gastrointestinal stromal tumour treated with Glivec (imatinib mesylate, Gleevec, STI571). Sarcoma. 2007;2007:82012.
- Krishnan G, D’Silva K, Al-Janadi A. Cetuximab-related tumor lysis syndrome in metastatic colon carcinoma. J Clin Oncol. 2008;26:2406-2408.
- Oztop I, Demirkan B, Yaren A, et al. Rapid tumor lysis syndrome in a patient with metastatic colon cancer as a complication of treatment with 5-fluorouracil/leucoverin and irinotecan. Tumori. 2004;90:514.
- Lin CJ, Lim KH, Cheng YC, et al. Tumor lysis syndrome after treatment with gemcitabine for metastatic transitional cell carcinoma. Med Oncol. 2007;24:455.
- Malik IA, Abubakar S, Alam F, Khan A. Dexamethasone-induced tumor lysis syndrome in high-grade non-Hodgkin’s lymphoma. South Med J. 1994;87:409.
- Jabr FI. Acute tumor lysis syndrome induced by rituximab in diffuse large B-cell lymphoma. Int J Hematol. 2005;82:312.
- Sezer O, Vesole DH, Singhal S, et al. Bortezomib-induced tumor lysis syndrome in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:233.
- Jensen M, Winkler U, Manzke O, et al. Rapid tumor lysis in a patient with B-cell chronic lymphocytic leukemia and lymphocytosis treated with an anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab). Ann Hematol. 1998;77:89.
- Linck D, Basara N, Tran V, et al. Peracute onset of severe tumor lysis syndrome immediately after 4 Gy fractionated TBI as part of reduced intensity preparative regimen in a patient with T-ALL with high tumor burden. Bone Marrow Transplant. 2003;31:935.
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778. [Erratum, J Clin Oncol. 2010;28:708.]
- Cheuk DK, Chiang AK, Chan GC, Ha SY. Urate oxidase for the prevention and treatment of tumor lysis syndrome in children with cancer. Cochrane Database Syst Rev. 2010;(6):CD006945.
- Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28:4207.
Case
A 25-year-old male with HIV/AIDS and a CD4 count of 65 cells/μL presents to the ED with intractable nausea and vomiting for one week. Laboratory evaluation revealed a white blood cell of 67,000 cells/mm3. An extended chemistry panel reveals creatinine 3.5 mg/dL, potassium 3.0 mmol/L, LDH 250 IU/L, and uric acid 5mg/dL. Calcium and phosphorus were both normal. The patient was admitted for further evaluation and management, and was later diagnosed with Burkitt’s lymphoma.
Overview
Tumor lysis syndrome (TLS) is an acute cell lysis of tumor cells with the release of cell content into circulation either spontaneously or in response to therapy, leading to hyperurecemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.1-3
TLS is one of the most common oncology emergencies encountered by hospitalists caring for patients with hematologic malignancies. The incidence and severity of TLS depend on the cell burden, cell proliferation rate, potential for cell lysis or chemo sensitivity, baseline clinical characteristics, and preventive measures taken (see Table 1).2,4
TLS is classified as laboratory or clinical. Laboratory TLS is described as the presence of two or more of the following serum abnormalities at the same time, present within three days before or seven days after the start of therapy.5
- Uric acid >8 mg/dL (475.8 micromole/L) or 25% increase;
- Potassium >6 mEq/L (6 mmol/L) or 25% increase;
- Phosphorus >6.5 mg/dL (2.1 mmol/L) for children or >4.5 mg/dl (1.45 mmol/L) for adults or 25% increase; and
- Calcium >7 mg/dL (1.75 mmol/L) or 25% increase.
Clinical TLS is defined as laboratory TLS in association with increased creatinine levels, seizures, cardiac arrhythmias, or death (see Table 2).5
Pathogenesis
Tumor cell lysis releases DNA, cytokines, phosphate, and potassium. DNA is metabolized into adenosine and guanosine, which are then converted into xanthines. Xanthines are oxidized by xanthine oxidase into uric acid, which is then excreted through the kidneys.
TLS develops when the accumulation of xanthine, uric acid, potassium, and phosphorus exceeds the kidney’s capacity to excrete them. Cytokines cause hypotension, inflammation, and kidney injury, and worsen the kidney’s excretory capacity. Damage to the kidneys also occurs by renal precipitation of uric acid, xanthine, and calcium phosphate.4
Phosphorus concentrations in tumor cells are four times higher than in normal cells. When the calcium phosphorus product exceeds 60 mg2/dL2, there is an increased risk of calcium phosphate precipitation in the kidney tubules, which could lead to kidney failure. Accumulation of calcium phosphate product may also be cardiotoxic and can lead to cardiac arrhythmias. In addition, hyperphosphatemia can cause secondary hypocalcemia, which may lead to parasthesias, tetany, and cardiac arrhythmias.2,4
TLS is most common in tumors with high proliferative rates and high tumor burden, such as acute lymphoblastic leukemia and Burkitt’s lymphoma, but it can occur with other hematologic malignancies, such as T-cell precursor acute lymphocytic leukemia (ALL), B-cell precursor ALL, acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), anaplastic large cell lymphoma, and plasma cell disorders (e.g. multiple myeloma and plasmacytoma).6,7 TLS has also been reported with the treatment of solid organ nonhematologic tumors (see Table 3).
In hematologic tumors, TLS frequently is associated with cytotoxic chemotherapy, and less frequently with glucocorticoid treatment, monoclonal antibodies (eg, rituximab, bortezomab, imatinib), and radiation therapy.25-29
Patient factors, such as baseline kidney disease or lack of prophylactic/preventive measures for TLS, also increase the risk.4 TLS, however, can develop in patients classified as low risk (see Table 1.
TLS Prevention
Intravenous fluids. Every patient at intermediate or high risk of TLS should receive intravenous fluids (IVF) prior to cancer treatment; those at low risk may receive IVF based on the provider’s clinical judgment.30 The purpose of administering IVF is to generate high urine output to reduce the risk of precipitation of uric acid in the renal tubules.30 Both adults and children should receive approximately 2 to 3 L/m2 per day of IVF,30 and urine output should be maintained at 2 ml/kg/hr (or 4 to 6 ml/kg/hr for children <10kg).30 IVF should be cautiously administered in patients with renal insufficiency or heart failure, and diuretics may be used to maintain goal urine output. Recommended initial fluids are D51/4 normal saline, or normal saline for patients who are dehydrated or hyponatremic.30
Allopurinol. Allopurinol is usually also administered to patients at risk for developing TLS.30 Allopurinol inhibits the metabolism of hypoxanthine and xanthine to uric acid, which decreases the accumulation of uric acid in the renal tubules, thus preventing obstructive renal disease from precipitation of uric acid.4 The recommended dose of allopurinol is 100 mg/m2 every eight hours, and should not exceed 800 mg per day in adults. It should be started one to two days prior to induction chemotherapy and continued for three to seven days after the treatment and until uric acid levels and other electrolyte levels have returned to normal. The dose is adjusted to 50 mg/m2 every eight hours in patients with kidney failure.30
In some cases, allopurinol can lead to increased levels of xanthine crystals in the renal tubules, leading to acute kidney injury. Also, allopurinol does not have any effect on uric acid that has already been formed, so patients with elevated uric acid levels prior to the initiation of cancer therapy will not have any reduction in the levels of uric acid. Allopurinol reduces the degradation of other purines, so it can cause toxicity in patients on azathioprine and 6-mercaptopurine if the doses of these medications are not adjusted.
Rasburicase. Rasburicase is a recombinant urate oxidase, derived from aspergillus favus, which catalyzes the breakdown of uric acid to allantoin, which is a water-soluble product. Rasburicase is recommended as a first-line treatment for patients at high risk for clinical TLS.30 Rasburicase has an earlier onset than allopurinol and rapidly decreases serum levels of uric acid within four hours of administration.30,31 The recommended dose is 0.10 to 0.20 mg/kg once a day for five days in adults.30
A Phase III trial compared the efficiency and safety of rasburicase to rasburicase with allopurinol or allopurinol alone.32 A significantly higher normalization of uric acid was found in patients on rasburicase compared to allopurinol alone. The incidence of laboratory TLS was also significantly lower with rasburicase alone compared to allopurinol alone, and was even lower with allopurinol plus rasburicase. The incidence of acute kidney injury was the same with rasburicase alone or allopurinol alone but was higher with rasburicase plus allopurinol.
Serum uric acid, phosphorus, potassium, and calcium need to be monitored every four hours for 24 hours after the completion of chemotherapy in patients on rasburicase.4 The sample of blood drawn to check the uric acid levels has to be placed on ice and processed within four hours in order to avoid falsely lower levels of uric acid due to the conversion of uric acid to allantoin. Rasburicase is contraindicated in patients with G6PD deficiency and pregnant women, because one of the byproducts of uric acid breakdown is hydrogen peroxide, which can cause severe hemolysis and the formation of methemoglobin in these patients.30
Rasburicase has been approved for use in both children and adults, but there is more evidence for the use in children. Rasburicase has a black-box label for patients with anaphylaxis, methemoglobinemia, hemolysis, and hemoglobinuria, and there is a recommendation to check G6PD deficiency before use in high-risk patients.30
TLS Treatment
Alkalinization. Alkalinization of urine is controversial in the management of TLS. Urine alkalinization increases uric acid solubility but causes hyperphosphatemia and decreases calcium phosphate solubility, which can then deposit in the kidney once cancer treatment starts. Of note, hyperphosphatemia is much more difficult to correct than high levels of uric acid, and there are no clinical trials proving the superiority of urine alkalinization over normal saline.
Normalization of electrolytes. Electrolyte abnormalities should be corrected to avoid arrhythmias and seizures. Phosphorus levels >6.5 mg/dl (2.1 mmol/L) should be managed by restricting phosphorus intake, and by the use of phosphate binders (calcium acetate, calcium carbonate, sevelamer, lanthanum, or aluminum hydroxide). Aluminum hydroxide should be avoided in patients with renal insufficiency. In severe cases of hyperphosphatemia, dialysis should be considered.
Symptomatic hypocalcemia should be treated with calcium gluconate if changes are present on the electrocardiography (ECG). Hypocalcemia in the presence of hyperphosphatemia should be treated only in patients with tetany or cardiac arrhythmias; otherwise, hypocalcemia should not be treated until hyperphosphatemia has been corrected.
In cases of hyperkalemia, patients should be placed on a cardiac monitor and stabilized with calcium gluconate; kayexalate should be administered to reduce total body potassium. Other interventions, such as intravenous insulin given with dextrose, sodium bicarbonate, and albuterol, have a temporary effect on hyperkalemia and can be used as adjunct treatments in patients with severe hyperkalemia (>7). Hemodialysis should be strongly considered in severe cases of hyperkalemia, particularly in patients with persistently elevated potassium levels despite other treatments.
Back to the Case
Our patient was started on IVFs with close monitoring of his urine output. He was considered intermediate risk for developing TLS. Allopurinol, renally dosed, was administered for two days prior to initiating treatment with rituximab plus chemotherapy. His chemistry panel was monitored daily and he did not develop any form of TLS.
Bottom Line
TLS is a common oncology emergency in patients with hematologic malignancies. Preventative measures include starting IVF prior to cancer treatment, and administering allopurinol and/or rasburicase to patients at risk of developing TLS. Treatment should include normalizing electrolytes to avoid arrhythmias and seizures.
Dr. Akwe is assistant professor of medicine at the Emory University School of Medicine and a clinical instructor of medicine at the Morehouse School of Medicine, both in Atlanta. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory. Both work as hospitalists at the Atlanta VA Medical Center.
References
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55:Suppl 3:S1-S13.
- Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
- Gertz MA. Managing tumor lysis syndrome in 2010. Leuk Lymphoma. 2010;51:179-180.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3.
- Wössmann W, Schrappe M, Meyer U, et al. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82:160.
- Hussain K, Mazza JJ, Clouse LH. Tumor lysis syndrome (TLS) following fludarabine therapy Gemici C. Tumor lysis syndrome in solid tumors. J Clin Oncol. 2009;27:2738-2739
- Rostom AY, El-Hussainy G, Kandil A, Allam A. Tumor lysis syndrome following hemi-body irradiation for metastatic breast cancer. Ann Oncol. 2000;11:1349.
- Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in nonhematologic malignancies. Report of a case and review of the literature. Am J Clin Oncol. 1994;17:502.
- Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors—a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187.
- Kalemkerian GP, Darwish B, Varterasian ML. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med. 1997;103:363.
- Noh GY, Choe DH, Kim CH, Lee JC. Fatal tumor lysis syndrome during radiotherapy for non-small-cell lung cancer. J Clin Oncol. 2008;26:6005-6006.
- Pentheroudakis G, O’Neill VJ, Vasey P, Kaye SB. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Report of two cases. Support Care Cancer. 2001;9:554.
- Joshita S, Yoshizawa K, Sano K, et al., A patient with advanced hepatocellular carcinoma treated with sorafenib tosylate showed massive tumor lysis with avoidance of tumor lysis syndrome. Intern Med. 2010;49:991-994.
- Huang WS, Yang CH. Sorafenib-induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;15:4464-4466.
- Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol. 1993;21:521.
- Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol. 2005;23:6794.
- Godoy H, Kesterson JP, Lele S. Tumor lysis syndrome associated with carboplatin and paclitaxel in a woman with recurrent endometrial cancer. Int J Gynaecol Obstet. 2010;109:254.
- Shamseddine AI, Khalil AM, Wehbeh MH. Acute tumor lysis syndrome with squamous cell carcinoma of the vulva. Gynecol Oncol 1993;51:258
- Pinder EM, Atwal GS, Ayantunde AA, et al. Tumour lysis syndrome occurring in a patient with metastatic gastrointestinal stromal tumour treated with Glivec (imatinib mesylate, Gleevec, STI571). Sarcoma. 2007;2007:82012.
- Krishnan G, D’Silva K, Al-Janadi A. Cetuximab-related tumor lysis syndrome in metastatic colon carcinoma. J Clin Oncol. 2008;26:2406-2408.
- Oztop I, Demirkan B, Yaren A, et al. Rapid tumor lysis syndrome in a patient with metastatic colon cancer as a complication of treatment with 5-fluorouracil/leucoverin and irinotecan. Tumori. 2004;90:514.
- Lin CJ, Lim KH, Cheng YC, et al. Tumor lysis syndrome after treatment with gemcitabine for metastatic transitional cell carcinoma. Med Oncol. 2007;24:455.
- Malik IA, Abubakar S, Alam F, Khan A. Dexamethasone-induced tumor lysis syndrome in high-grade non-Hodgkin’s lymphoma. South Med J. 1994;87:409.
- Jabr FI. Acute tumor lysis syndrome induced by rituximab in diffuse large B-cell lymphoma. Int J Hematol. 2005;82:312.
- Sezer O, Vesole DH, Singhal S, et al. Bortezomib-induced tumor lysis syndrome in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:233.
- Jensen M, Winkler U, Manzke O, et al. Rapid tumor lysis in a patient with B-cell chronic lymphocytic leukemia and lymphocytosis treated with an anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab). Ann Hematol. 1998;77:89.
- Linck D, Basara N, Tran V, et al. Peracute onset of severe tumor lysis syndrome immediately after 4 Gy fractionated TBI as part of reduced intensity preparative regimen in a patient with T-ALL with high tumor burden. Bone Marrow Transplant. 2003;31:935.
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778. [Erratum, J Clin Oncol. 2010;28:708.]
- Cheuk DK, Chiang AK, Chan GC, Ha SY. Urate oxidase for the prevention and treatment of tumor lysis syndrome in children with cancer. Cochrane Database Syst Rev. 2010;(6):CD006945.
- Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28:4207.