User login
Syncope from a twiddled ICD
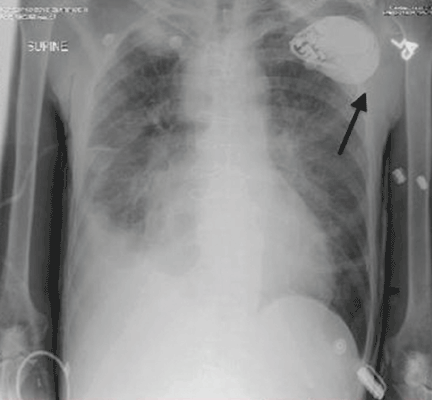
A 61-year-old man with ischemic cardiomyopathy underwent implantation of a cardioverter-defibrillator (ICD) in a left subpectoral pocket. Placement was confirmed with chest radiography (Figure 1). Six weeks later, he presented to the emergency department reporting two episodes of syncope within the previous 24 hours.
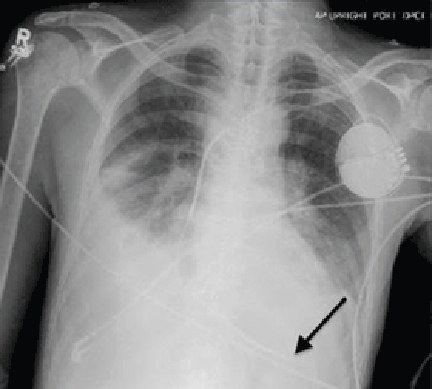
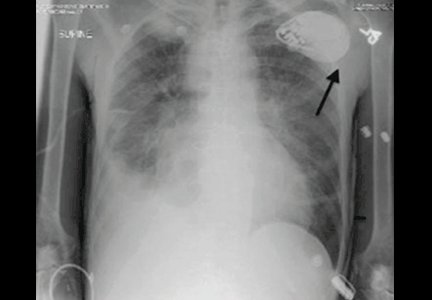
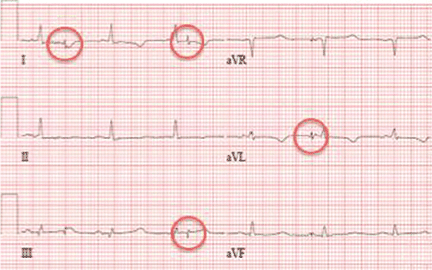
Electrocardiography at the time of presentation showed a normal sinus rhythm with inappropriate ICD discharges (Figure 2). This finding prompted chest radiography, which showed that the ICD had rotated 90 degrees from its original position, and that the lead had completely wrapped around the generator and thus was no longer correctly positioned (Figure 3). The patient admitted to frequently rubbing (ie, twiddling) the area, which led to twisting and dislocation of the device, a complication known as twiddler syndrome.
Twiddler syndrome is a rare complication of ICD placement, occurring in only about 0.1% of cases.1 It is usually a result of intentional manipulation (ie, twiddling) of the device by the patient, causing the ICD and the leads to dislodge, break, or retract. It is seen more often in women and in patients with cognitive dysfunction or psychiatric illness, in obese patients, and in patients with laxity of subcutaneous tissues, such as elderly people or people with accelerated weight loss. Placement of the ICD in an inappropriately large pocket predisposes to device rotation.2,3
Twiddler syndrome may cause mild discomfort or may even go unnoticed by the patient.4 In rare cases, it may lead to stimulation of the phrenic nerve, causing diaphragmatic pacing, or to stimulation of the brachial plexus, causing muscle twitching.2,3 However, malfunction of an ICD is life-threatening and requires immediate repair and replacement of the device. Educating the patient about the risks of twiddling the device site and the importance of periodic device checks is imperative to prevent this syndrome and ensure its early identification if it should occur. The patient should understand that dislodging the ICD can make it unable to sense abnormal rhythms and cause the device to deliver inappropriate shocks2,3,5 or stop delivering shocks altogether.
HOW IT IS TREATED
Treatment involves replacing the leads and affixing the device with sutures in the existing pocket or in a new pocket. The subpectoral position is preferred as it is more secure,3 although subcutaneous reimplantation has been successful.1 The device may also be placed in a fabric pouch to help lower the risk of migration or manipulation.3,6
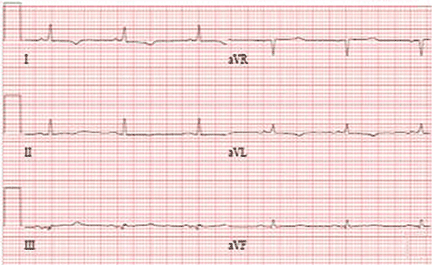
In this case, because of the patient’s slender body habitus (body mass index 13 kg/m2), the ICD was removed from the subpectoral pocket and a new ICD was sutured into a subcutaneous pocket. The single lead was secured to the sternum and fascia using nonabsorbable sutures and a tie-down sleeve, making the device less susceptible to dislodgement by twiddling. Electrocardiography after replacement showed a normal sinus rhythm (Figure 4).
- Constandse J, Smit JJ, Ramdat Misier AR, Elvan A, Delnoy PP. Unusual twiddler syndrome: movement ties the knot. Neth Heart J 2013; 21:253–254.
- Spencker S, Poppelbaum A, Müller D. An unusual cause of oversensing leading to inappropriate ICD discharges. Int J Cardiol 2008; 129:e24–e26.
- Benezet-Mazuecos J, Benezet J, Ortega-Carnicer J. Pacemaker twiddler syndrome. Eur Heart J 2007; 28:2000.
- Chemello D, Subramanian A, Cameron D. Twiddler syndrome with 180 degrees rotation of an implantable cardioverter defibrillator generator resulting in malfunction of one of the shocking coils. Europace 2009; 11:1259.
- Garweg C, Alzand BS, Willems R. Twiddler syndrome causing an inappropriate implantable cardioverter-defibrillator shock. Eur Heart J 2014; 35:516.
- Parsonnet V, Bernstein AD, Neglia D, Omar A. The usefulness of a stretch-polyester pouch to encase implanted pacemakers and defibrillators. Pacing Clin Electrophysiol 1994; 17:2274–2278.
A 61-year-old man with ischemic cardiomyopathy underwent implantation of a cardioverter-defibrillator (ICD) in a left subpectoral pocket. Placement was confirmed with chest radiography (Figure 1). Six weeks later, he presented to the emergency department reporting two episodes of syncope within the previous 24 hours.

Electrocardiography at the time of presentation showed a normal sinus rhythm with inappropriate ICD discharges (Figure 2). This finding prompted chest radiography, which showed that the ICD had rotated 90 degrees from its original position, and that the lead had completely wrapped around the generator and thus was no longer correctly positioned (Figure 3). The patient admitted to frequently rubbing (ie, twiddling) the area, which led to twisting and dislocation of the device, a complication known as twiddler syndrome.


Twiddler syndrome is a rare complication of ICD placement, occurring in only about 0.1% of cases.1 It is usually a result of intentional manipulation (ie, twiddling) of the device by the patient, causing the ICD and the leads to dislodge, break, or retract. It is seen more often in women and in patients with cognitive dysfunction or psychiatric illness, in obese patients, and in patients with laxity of subcutaneous tissues, such as elderly people or people with accelerated weight loss. Placement of the ICD in an inappropriately large pocket predisposes to device rotation.2,3
Twiddler syndrome may cause mild discomfort or may even go unnoticed by the patient.4 In rare cases, it may lead to stimulation of the phrenic nerve, causing diaphragmatic pacing, or to stimulation of the brachial plexus, causing muscle twitching.2,3 However, malfunction of an ICD is life-threatening and requires immediate repair and replacement of the device. Educating the patient about the risks of twiddling the device site and the importance of periodic device checks is imperative to prevent this syndrome and ensure its early identification if it should occur. The patient should understand that dislodging the ICD can make it unable to sense abnormal rhythms and cause the device to deliver inappropriate shocks2,3,5 or stop delivering shocks altogether.
HOW IT IS TREATED
Treatment involves replacing the leads and affixing the device with sutures in the existing pocket or in a new pocket. The subpectoral position is preferred as it is more secure,3 although subcutaneous reimplantation has been successful.1 The device may also be placed in a fabric pouch to help lower the risk of migration or manipulation.3,6
In this case, because of the patient’s slender body habitus (body mass index 13 kg/m2), the ICD was removed from the subpectoral pocket and a new ICD was sutured into a subcutaneous pocket. The single lead was secured to the sternum and fascia using nonabsorbable sutures and a tie-down sleeve, making the device less susceptible to dislodgement by twiddling. Electrocardiography after replacement showed a normal sinus rhythm (Figure 4).

A 61-year-old man with ischemic cardiomyopathy underwent implantation of a cardioverter-defibrillator (ICD) in a left subpectoral pocket. Placement was confirmed with chest radiography (Figure 1). Six weeks later, he presented to the emergency department reporting two episodes of syncope within the previous 24 hours.

Electrocardiography at the time of presentation showed a normal sinus rhythm with inappropriate ICD discharges (Figure 2). This finding prompted chest radiography, which showed that the ICD had rotated 90 degrees from its original position, and that the lead had completely wrapped around the generator and thus was no longer correctly positioned (Figure 3). The patient admitted to frequently rubbing (ie, twiddling) the area, which led to twisting and dislocation of the device, a complication known as twiddler syndrome.


Twiddler syndrome is a rare complication of ICD placement, occurring in only about 0.1% of cases.1 It is usually a result of intentional manipulation (ie, twiddling) of the device by the patient, causing the ICD and the leads to dislodge, break, or retract. It is seen more often in women and in patients with cognitive dysfunction or psychiatric illness, in obese patients, and in patients with laxity of subcutaneous tissues, such as elderly people or people with accelerated weight loss. Placement of the ICD in an inappropriately large pocket predisposes to device rotation.2,3
Twiddler syndrome may cause mild discomfort or may even go unnoticed by the patient.4 In rare cases, it may lead to stimulation of the phrenic nerve, causing diaphragmatic pacing, or to stimulation of the brachial plexus, causing muscle twitching.2,3 However, malfunction of an ICD is life-threatening and requires immediate repair and replacement of the device. Educating the patient about the risks of twiddling the device site and the importance of periodic device checks is imperative to prevent this syndrome and ensure its early identification if it should occur. The patient should understand that dislodging the ICD can make it unable to sense abnormal rhythms and cause the device to deliver inappropriate shocks2,3,5 or stop delivering shocks altogether.
HOW IT IS TREATED
Treatment involves replacing the leads and affixing the device with sutures in the existing pocket or in a new pocket. The subpectoral position is preferred as it is more secure,3 although subcutaneous reimplantation has been successful.1 The device may also be placed in a fabric pouch to help lower the risk of migration or manipulation.3,6
In this case, because of the patient’s slender body habitus (body mass index 13 kg/m2), the ICD was removed from the subpectoral pocket and a new ICD was sutured into a subcutaneous pocket. The single lead was secured to the sternum and fascia using nonabsorbable sutures and a tie-down sleeve, making the device less susceptible to dislodgement by twiddling. Electrocardiography after replacement showed a normal sinus rhythm (Figure 4).

- Constandse J, Smit JJ, Ramdat Misier AR, Elvan A, Delnoy PP. Unusual twiddler syndrome: movement ties the knot. Neth Heart J 2013; 21:253–254.
- Spencker S, Poppelbaum A, Müller D. An unusual cause of oversensing leading to inappropriate ICD discharges. Int J Cardiol 2008; 129:e24–e26.
- Benezet-Mazuecos J, Benezet J, Ortega-Carnicer J. Pacemaker twiddler syndrome. Eur Heart J 2007; 28:2000.
- Chemello D, Subramanian A, Cameron D. Twiddler syndrome with 180 degrees rotation of an implantable cardioverter defibrillator generator resulting in malfunction of one of the shocking coils. Europace 2009; 11:1259.
- Garweg C, Alzand BS, Willems R. Twiddler syndrome causing an inappropriate implantable cardioverter-defibrillator shock. Eur Heart J 2014; 35:516.
- Parsonnet V, Bernstein AD, Neglia D, Omar A. The usefulness of a stretch-polyester pouch to encase implanted pacemakers and defibrillators. Pacing Clin Electrophysiol 1994; 17:2274–2278.
- Constandse J, Smit JJ, Ramdat Misier AR, Elvan A, Delnoy PP. Unusual twiddler syndrome: movement ties the knot. Neth Heart J 2013; 21:253–254.
- Spencker S, Poppelbaum A, Müller D. An unusual cause of oversensing leading to inappropriate ICD discharges. Int J Cardiol 2008; 129:e24–e26.
- Benezet-Mazuecos J, Benezet J, Ortega-Carnicer J. Pacemaker twiddler syndrome. Eur Heart J 2007; 28:2000.
- Chemello D, Subramanian A, Cameron D. Twiddler syndrome with 180 degrees rotation of an implantable cardioverter defibrillator generator resulting in malfunction of one of the shocking coils. Europace 2009; 11:1259.
- Garweg C, Alzand BS, Willems R. Twiddler syndrome causing an inappropriate implantable cardioverter-defibrillator shock. Eur Heart J 2014; 35:516.
- Parsonnet V, Bernstein AD, Neglia D, Omar A. The usefulness of a stretch-polyester pouch to encase implanted pacemakers and defibrillators. Pacing Clin Electrophysiol 1994; 17:2274–2278.
Acute respiratory distress syndrome: Implications of recent studies
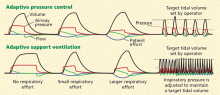
Continued progress in understanding the pathophysiology of acute respiratory distress syndrome (ARDS) is translating into changes in the way we diagnose and manage it. Over the past 20 years, low tidal volume,1 positive end-expiratory pressure (PEEP),2 and fluid restriction3 have become the standard of care. A multidisciplinary approach, including targeted use of sedatives, early mobilization, and protocols for weaning from the ventilator, has also brought about substantial changes in ARDS management and its outcomes.4–6
In this article, we review the most relevant articles about ARDS in the last 5 years. We include the new definition of ARDS and studies of ventilatory and nonventilatory therapies that have implications in managing patients with ARDS.
A STANDARDIZED APPROACH
ARDS is characterized by damage to the alveolar architecture, severe hypoxemia, and bilateral parenchymal opacities.
The working definition of ARDS developed in 1994 by the American-European Consensus Conference (AECC) was the basis for enrollment in most of the landmark trials and observational studies over the past 20 years.7,8 However, it was limited in its reliability and validity.
An updated definition
In 2011, the ARDS Definition Task Force, using a novel consensus process, updated the ARDS definition,9 focusing on its feasibility, reliability, and validity in predicting response to therapies and outcomes in ARDS. This new “Berlin” definition is not substantially different from the old, but defines the criteria more specifically:
- Bilateral opacities, unexplained by nodules, atelectasis, or effusion, on chest radiography or computed tomography
- New or worsening respiratory symptoms, or a clinical insult associated with ARDS within 7 days of diagnosis
- Objective assessment of cardiac function (eg, with echocardiography) to exclude cardiogenic pulmonary edema
- Hypoxemia, with a partial pressure of arterial oxygen divided by the percentage of inspired oxygen (PaO2/FiO2 ratio) of 300 mm Hg or less despite noninvasive or invasive mechanical ventilation with PEEP or continuous positive airway pressure (CPAP) of at least 5 cm H2O.
In addition, the new definition classifies the severity of disease on the basis of the degree of hypoxemia, ie, the PaO2/FiO2 ratio:
- Mild: PaO2/FiO2 ratio > 200 and ≤ 300 mm Hg
- Moderate: PaO2/FiO2 ratio > 100 and ≤ 200 mm Hg
- Severe: PaO2/FiO2 ratio ≤ 100 mm Hg.
The term “acute lung injury” has been eliminated, as has the previous criterion of a pulmonary artery wedge pressure of 18 mm Hg or less.
The panel also evaluated four ancillary variables for predicting outcomes in severe ARDS:
- Compliance of the respiratory system less than or equal to 40 mL/cm H2O
- Radiographic severity (involvement of three or four quadrants on chest radiography)
- PEEP of 10 cm H2O or greater
- Corrected expired volume 10 L/min or greater.
The task force evaluated the reliability and validity of this definition in a meta-analysis of 4,400 patients previously enrolled in randomized controlled trials or observational studies.
Findings. The Berlin definition predicted the risk of death better than the AECC definition. The mortality rate increased with the severity of ARDS, from 27% with mild disease to 32% with moderate disease to 45% with severe disease. The four ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition.
Thille et al10 retrospectively reviewed autopsy findings from 712 patients and found that the new definition identified a homogeneous group who had severe ARDS.10
Conclusions. The new definition may overcome some of the limitations of the old one, but it needs to be validated in clinical practice, especially its ability to predict death.
VENTILATORY SUPPORT
Prompt recognition, lung-protective ventilation, and a conservative fluid strategy remain the cornerstones of ARDS management. However, other strategies are being tested.
Prone-position ventilation in severe ARDS: The right therapy in a specific population
Prone-position ventilation was first described almost 30 years ago, but it has been used inconsistently in clinical practice.
Physiologic and observational studies indicated that prone positioning might improve survival in patients with ARDS, but several randomized trials failed to demonstrate any positive effect on outcomes.11,12 Some trials also reported a higher rate of complications with this intervention.13 However, meta-analyses suggested that prone-position ventilation might have a beneficial effect in patients with severe ARDS (defined as a PaO2/FiO2 ratio ≤ 100 mm Hg).14
In view of these findings, investigators conducted a trial of prone-position ventilation exclusively in patients with severe ARDS.
The PROSEVA study
The Proning Severe ARDS Patients (PROSEVA) study was a randomized controlled trial designed to determine whether prone-position ventilation, applied early, would improve outcomes in patients with severe ARDS.15
In PROSEVA, 466 patients with severe ARDS (defined as a PaO2/FiO2 ratio < 150 mm Hg, FiO2 ≥ 60%, and PEEP ≥ 5 cm H2O) underwent either at least 16 hours of prone positioning or were left in the supine position after 12 to 24 hours of initial conventional mechanical ventilation. The patients were recruited from centers in France and Spain where prone-position ventilation had been used in daily practice for more than 5 years.
The primary outcome studied was the rate of death at 28 days. The secondary end points were the death rate at day 90, rates of successful extubation, the length of stay in the intensive care unit, and complications.
Findings. At study entry, the patients in the supine group were sicker, more of them required a vasopressor, and fewer of them were receiving neuromuscular blocking agents than those in the prone group. These baseline differences may have influenced the outcomes; the unadjusted 28-day mortality rate was 16.0% in the prone group compared with 32.8% in the supine group (P < .001). However, the hazard ratio for death with prone positioning was 0.39 (95% confidence interval [CI] 0.25–0.63) even after adjusting for severity and the use of vasopressors and neuromuscular blocking agents. Prone-position ventilation was not associated with a higher incidence of complications, and the rate of successful extubation was higher.
Conclusions. In patients with severe ARDS, early use of prolonged prone positioning significantly decreased the 28-day and 90-day mortality rates. This trial has made prone positioning one of the strategies in managing patients with early severe ARDS. To minimize complications such as pressure ulcers and line or tube dislodgement, personnel caring for these patients must follow a protocol and undergo specific training.
These results were corroborated by a meta-analysis by Beitler et al16 that found a significant decrease in mortality rate with prone-position ventilation even in older studies when lung-protective ventilation strategies were separated from high-tidal-volume ventilation.
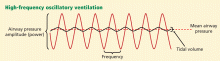
High-frequency oscillatory ventilation: No benefit in two trials
Observational data and experimental studies suggested that high-frequency oscillatory ventilation (HFOV) is superior to conventional mechanical ventilation in ARDS patients.17,18 However, outdated and cumbersome equipment, lack of protocols, and a lack of high-quality evidence led to limited and inconsistent use of HFOV, mainly as a rescue therapy in ARDS.19
Over the last few years, HFOV has been gaining acceptance, especially earlier in the course of ARDS.20 After preliminary clinical trials reported promising results, two trials conducted in Canada and the United Kingdom compared HFOV vs conventional mechanical ventilation in patients with ARDS.
The OSCAR study
The Oscillation in ARDS (OSCAR) study21 was a “pragmatic” trial22 (ie, it had minimal exclusion criteria) of the safety and effectiveness of HFOV as a primary ventilatory strategy for ARDS. It included 795 patients randomized to receive conventional ventilation (n = 397) or HFOV (n = 398). Research centers followed detailed algorithms for HFOV management and adopted their usual practice for conventional ventilation. Medical care was given according to the clinician’s judgment.
The primary outcome studied was survival at 30 days. The secondary outcomes were all-cause mortality in the intensive care unit and the hospital, duration of mechanical ventilation, and use of antimicrobial, sedative, vasoactive, and neuromuscular-blocking drugs.
Findings. The patient baseline characteristics were similar in both groups.
There was no significant difference in intensive care unit mortality rates, hospital mortality rates, or mortality rates at 30 days (41.7% in the HFOV group vs 41.1% in the conventional ventilation group; P = .85, 95% CI 6.1–7.5) even after adjustments for center or severity of illness.
The duration of mechanical ventilation was similar in both groups (14.9 ± 13.3 days in the HFOV group vs 14.1 ± 13.4 days in the conventional ventilation group, P = .41). However, sedatives and neuromuscular-blocking drugs were used more often and longer in the HFOV group than in the conventional ventilation group. There was no difference in the use of vasoactive or antimicrobial medications.
Conclusions. This multicenter randomized control trial did not demonstrate any benefit from using HFOV for routine management of ARDS. Its pragmatic design made it less likely to reach a firm conclusion,22 but it at least made a case against routinely using HFOV in patients with ARDS.
The OSCILLATE study
The Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) study23 assessed the safety and efficacy of HFOV as a treatment for early-onset moderate-to-severe ARDS.
The inclusion criteria were similar to those in the OSCAR trial except that pulmonary symptoms had to be present less than 2 weeks and ARDS assessment was done under standard ventilator settings. As this was an efficacy trial, it had more exclusion criteria than the OSCAR trial. A total of 548 patients were randomized to receive conventional ventilation (n = 273) or HFOV (n = 275). The baseline characteristics were similar between groups.
Conventional ventilation was given according to a protocol used in an earlier trial2 and included recruitment maneuvers. HFOV was given in centers that had experience in this treatment, and there were protocols for ventilation management, hemodynamic optimization, and weaning. All other care was left to the clinician’s choice.
The primary outcome studied was in-hospital mortality. The investigators also evaluated whether there were interactions between the treatment and baseline severity of lung injury and center experience with HFOV.
Findings. The trial was stopped after an interim analysis found that HFOV might be harmful, although the statistical threshold for stopping was not reached. The in-hospital mortality rate was 47% in the HFOV group and 35% in the control group (relative risk of death with HFOV 1.33, 95% CI 1.09–1.64, P = .005). HFOV was worse than conventional ventilation regardless of the severity of disease or center experience. The HFOV group had higher mean airway pressures but similar FiO2 compared with the conventional ventilation group.
The HFOV group received significantly more vasopressors, sedatives, and neuromuscular blockers. This group’s fluid balance was higher as well, but not significantly so. Refractory hypoxemia (defined as PaO2 < 60 mm Hg for 1 hour with an FiO2 of 1.0 and neuromuscular blockade) was more frequent in the conventional ventilation group, but the number of deaths in the subgroup with refractory hypoxemia was similar with either treatment.
Conclusions. This multicenter randomized controlled trial demonstrated that HFOV was harmful when used routinely to manage ARDS. The trial’s protocol was based on the results of a pilot study carried out by the same investigators, which provided the best evidence available regarding the safety of HFOV at that time.
The results of the OSCAR and OSCILLATE trials have quelled enthusiasm for early, routine use of HFOV in ARDS. Although there are concerns that the protocol (ie, the way HFOV was implemented) rather than HFOV itself may have led to worse outcomes, there is no signal to support its routine use. We need further studies to define if it remains a viable rescue therapy.
Extracorporeal membrane oxygenation: Is it a viable option in severe ARDS?
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to provide gas exchange. In patients with severe hypoxemia, ECMO can ensure adequate oxygenation and ventilation while ensuring the optimization of lung-protective ventilation. But ECMO was never as successful in adults with ARDS as it was in children and neonates.24
The first two trials of ECMO in ARDS24,25 reported equal or worse survival rates compared with conventional ventilation, and the overall mortality rate in these studies was staggeringly high. However, these studies were carried out before the era of lung-protective ventilation and at a time when ECMO technology was relatively primitive.
With new technology such as venovenous circuits and smaller cannulas, ECMO has gained more acceptance. It was used in patients with severe or refractory hypoxemia associated with ARDS during the H1N1 pandemic.26,27
The CESAR trial
The Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial28 assessed the safety, clinical efficacy, and cost-effectiveness of ECMO in managing severe ARDS. It compared best standard practice vs a protocol that included ECMO. The trial was conducted from 2001 to 2006.
Patients with severe ARDS, as defined by a Murray score29 greater than 3 or uncompensated hypercapnea, were prospectively randomized and recruited from an ECMO center and 148 tertiary intensive care units and referral hospitals in England. This was a pragmatic trial, with minimal exclusion criteria (essentially, mechanical ventilation with high pressures and high FiO2 for more than 7 days, intracranial bleeding, or contraindication to heparinization).
A total of 180 patients were randomized in a one-to-one ratio to receive ECMO or conventional management. The ventilator management in the conventional treatment group was not done according to a protocol but in general was low-volume and low-pressure. All patients randomized to ECMO were transferred to the ECMO center and treated according to a standardized ventilation protocol. After 12 hours, if predefined goals were not reached, venovenous ECMO was started. Patients assigned to conventional management could not cross over to ECMO.
The primary outcomes were death or severe disability at 6 months after randomization, and cost-effectiveness. The secondary outcomes were hospital resource use (eg, rescue techniques, length of stay, duration of ECMO) and health status after 6 months.
Findings. The groups were similar at baseline. Sixty-eight (75%) of the 90 patients randomized to receive ECMO actually received it. Of the 22 patients who did not receive ECMO, 16 (18% of the 90) improved on conventional therapy, 5 (6%) died during or before transfer, and 1 could not receive heparin.
Two patients had severe complications in the ECMO group: one had an arterial puncture, and one had an oxygen delivery failure during transport. In each case, these events contributed to the death of the patient.
More patients in the ECMO group received lung-protective ventilation, 84 (93%) vs 63 (70%).
The primary outcome, ie, death or severe disability at 6 months, occurred in 33 (37%) of the 90 patients in the ECMO group and in 46 (53%) of the patients in the conventional management group (relative risk 0.69, 95% CI 0.05–0.97, P = .03). More patients in the ECMO group survived, but the difference was not statistically significant (relative risk of death 0.73, 95% CI 0.52–1.03, P = .07). The most common cause of death in the ECMO group was multiorgan failure (42%), whereas in the conventional management group, the most common cause of death was respiratory failure (60%).
Length of stay in the hospital and in the critical care unit and health care costs were double for patients in the ECMO group. There was no difference in quality-of-life markers at 6 months in the survivors.
Conclusions. This pragmatic trial demonstrated that a protocol that includes ECMO could improve survival rates in ARDS.
Of note, the ECMO group got care in regional centers that used protocols. Therefore, in interpreting the results of this trial, we have to consider that being in a center with protocol-specified care for ARDS could drive some of the difference in mortality rates.
Regardless, this trial demonstrated that ECMO is feasible and led to better outcomes than expected. The findings were encouraging, and spurred the use of ECMO in severe ARDS during the 2009 H1N1 pandemic. Two propensity-matched studies and a number of case series reported a survival benefit associated with the use of ECMO in patients with severe ARDS.27,30
A recent meta-analysis also reported that ECMO might lower the mortality rate in ARDS; however, the patients in the H1N1 pandemic were younger and usually had isolated respiratory failure.31
The success of ECMO has opened new possibilities in the management of ARDS. As the technology improves and our experience increases, ECMO will likely gain more acceptance as a treatment for severe ARDS.
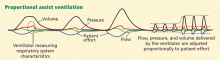
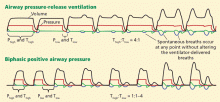
Airway pressure release ventilation
The use of airway pressure release ventilation and other ventilator modalities in ARDS is not supported by current evidence, though results of clinical trials may influence our practice in the future.
PHARMACOTHERAPY IN ARDS
The pathogenesis of ARDS includes damage to the alveolar-capillary membrane, with leakage of protein-rich edema fluid into alveoli. This damage is propagated by a complex inflammatory response including but not limited to neutrophil activation, free-radical formation, dysregulation of the coagulation system, and extensive release of inflammatory mediators.32,33 As a consequence, there are multiple potential targets for pharmacologic therapy in ARDS.
A variety of drugs, including corticosteroids, anti-inflammatory agents, immune-modulating agents, pulmonary vasodilators, antioxidants, and surfactants, have been studied in patients with ARDS.34 But effective pharmacotherapy for ARDS remains extremely limited.
Neuromuscular blockade in early severe ARDS
Mechanical ventilation can result in injurious stretching of the lung parenchyma, either from alveolar overdistention (volutrauma) or from continual recruitment and derecruitment of unstable lung units during the ventilator cycle (atelectrauma).35 Ventilator-induced lung injury can be exacerbated by asynchronous breathing.
In theory, neuromuscular blockers could minimize patient-ventilator asynchrony and provide much better control of tidal volume and pressure in patients with ARDS. This may result in less volutrauma and atelectrauma associated with asynchronous breathing. Data also suggest that cisatracurium (Nimbex), a neuromuscular blocking agent, may have a direct effect on the amount of inflammation in lungs with ARDS.36
The ACURASYS study
The ARDS et Curarisation Systématique (ACURASYS) study37 was a randomized trial in 340 patients undergoing mechanical ventilation for severe ARDS to evaluate the impact of neuromuscular blockade within the first 48 hours in this population.
The primary outcome was the mortality rate before hospital discharge or within 90 days of study entry. Secondary outcomes included the 28-day mortality rate, the rate of intensive care unit-acquired paresis, and the number of ventilator-free days. To be included, patients had to have been mechanically ventilated for less than 48 hours and to meet the AECC criteria for severe ARDS, with a PaO2/FiO2 ratio less than 150 mm Hg.
The intervention group received a continuous infusion of cisatracurium for 48 hours, while the control patients received placebo. Muscle strength was evaluated by clinical scoring of strength in different muscle groups.
Findings. The study groups were similar at baseline.
The crude 90-day mortality rate was lower in the cisatracurium group (31.6% vs 40.7%, P = .08). Regression analysis showed an improved 90-day survival rate with the use of this neuromuscular blocker after adjustment for severity of illness and the severity of ARDS (based on degree of hypoxemia and plateau pressures) (hazard ratio for death at 90 days 0.68; 95% CI 0.48–0.98; P = .04). The rate of paresis acquired in the intensive care unit did not differ significantly between the two groups.
Conclusion. In patients with severe ARDS, giving a neuromuscular blocking agent early improved the survival rate and increased the time off the ventilator without increasing muscle weakness.
These data are in line with similar findings from two other studies published by the same group.38,39 A meta-analysis of 432 patients showed that the use of neuromuscular blockade in early severe ARDS is associated with a statistically significant effect on early mortality (relative risk 0.66, 95% CI 0.50–0.87).40 The pooled analysis of these trials did not show any statistically significant critical-illness polyneuropathy.
These results need to be interpreted carefully, as we have inadequate data to see if they generalize to different intensive care units, and the evaluation and categorization of critical-illness polyneuropathy remains to be defined.
Cisatracurium is a promising treatment for moderate to severe ARDS and merits investigation in a large confirmatory randomized controlled trial.
Other pharmacologic agents
A number of other drugs have been studied in ARDS patients, including both inhaled and intravenous beta agonists,41,42 statins,43 and nutritional supplements.44 But as with other drugs previously studied in ARDS such as corticosteroids, N-acetylcysteine, and surfactant,34 these agents showed no effect on outcomes. In fact, a recent trial of intravenous salbutamol in ARDS patients was stopped after an interim analysis because of a higher incidence of arrhythmias and lactic acidosis with this agent.42
These findings reaffirm that pharmacologic therapy needs to be carefully considered, and potential harms associated with these therapies need to be addressed before they are introduced in the care of critically ill patients.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308.
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582.
- ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533.
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767.
- Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345:568–573.
- Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302:1977–1984.
- Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173:1233–1239.
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599.
- Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168.
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014; 40:332–341.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008; 36:1043–1048.
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 2005; 33:479–486.
- Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009; 62:464–475.
- Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009; 302:1888–1895.
- Pham T, Combes A, Rozé H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Zampieri FG, Mendes PV, Ranzani OT, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care 2013; 28:998–1005.
- Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008; 15:1911–1924.
- Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004; 3:307–328.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; 4:CD004477.
- Terragni PP, Rosboch GL, Lisi A, Viale AG, Ranieri VM. How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients. Eur Respir J Suppl 2003; 42:15s–21s.
- Forel JM, Roch A, Papazian L. Paralytics in critical care: not always the bad guy. Curr Opin Crit Care 2009; 15:59–66.
- Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116.
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004; 32:113–19.
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006; 34:2749–2757.
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013; 17:R43.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–568.
- Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012; 379:229–235.
- Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307:795–803.
Continued progress in understanding the pathophysiology of acute respiratory distress syndrome (ARDS) is translating into changes in the way we diagnose and manage it. Over the past 20 years, low tidal volume,1 positive end-expiratory pressure (PEEP),2 and fluid restriction3 have become the standard of care. A multidisciplinary approach, including targeted use of sedatives, early mobilization, and protocols for weaning from the ventilator, has also brought about substantial changes in ARDS management and its outcomes.4–6
In this article, we review the most relevant articles about ARDS in the last 5 years. We include the new definition of ARDS and studies of ventilatory and nonventilatory therapies that have implications in managing patients with ARDS.
A STANDARDIZED APPROACH
ARDS is characterized by damage to the alveolar architecture, severe hypoxemia, and bilateral parenchymal opacities.
The working definition of ARDS developed in 1994 by the American-European Consensus Conference (AECC) was the basis for enrollment in most of the landmark trials and observational studies over the past 20 years.7,8 However, it was limited in its reliability and validity.
An updated definition
In 2011, the ARDS Definition Task Force, using a novel consensus process, updated the ARDS definition,9 focusing on its feasibility, reliability, and validity in predicting response to therapies and outcomes in ARDS. This new “Berlin” definition is not substantially different from the old, but defines the criteria more specifically:
- Bilateral opacities, unexplained by nodules, atelectasis, or effusion, on chest radiography or computed tomography
- New or worsening respiratory symptoms, or a clinical insult associated with ARDS within 7 days of diagnosis
- Objective assessment of cardiac function (eg, with echocardiography) to exclude cardiogenic pulmonary edema
- Hypoxemia, with a partial pressure of arterial oxygen divided by the percentage of inspired oxygen (PaO2/FiO2 ratio) of 300 mm Hg or less despite noninvasive or invasive mechanical ventilation with PEEP or continuous positive airway pressure (CPAP) of at least 5 cm H2O.
In addition, the new definition classifies the severity of disease on the basis of the degree of hypoxemia, ie, the PaO2/FiO2 ratio:
- Mild: PaO2/FiO2 ratio > 200 and ≤ 300 mm Hg
- Moderate: PaO2/FiO2 ratio > 100 and ≤ 200 mm Hg
- Severe: PaO2/FiO2 ratio ≤ 100 mm Hg.
The term “acute lung injury” has been eliminated, as has the previous criterion of a pulmonary artery wedge pressure of 18 mm Hg or less.
The panel also evaluated four ancillary variables for predicting outcomes in severe ARDS:
- Compliance of the respiratory system less than or equal to 40 mL/cm H2O
- Radiographic severity (involvement of three or four quadrants on chest radiography)
- PEEP of 10 cm H2O or greater
- Corrected expired volume 10 L/min or greater.
The task force evaluated the reliability and validity of this definition in a meta-analysis of 4,400 patients previously enrolled in randomized controlled trials or observational studies.
Findings. The Berlin definition predicted the risk of death better than the AECC definition. The mortality rate increased with the severity of ARDS, from 27% with mild disease to 32% with moderate disease to 45% with severe disease. The four ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition.
Thille et al10 retrospectively reviewed autopsy findings from 712 patients and found that the new definition identified a homogeneous group who had severe ARDS.10
Conclusions. The new definition may overcome some of the limitations of the old one, but it needs to be validated in clinical practice, especially its ability to predict death.
VENTILATORY SUPPORT
Prompt recognition, lung-protective ventilation, and a conservative fluid strategy remain the cornerstones of ARDS management. However, other strategies are being tested.
Prone-position ventilation in severe ARDS: The right therapy in a specific population
Prone-position ventilation was first described almost 30 years ago, but it has been used inconsistently in clinical practice.
Physiologic and observational studies indicated that prone positioning might improve survival in patients with ARDS, but several randomized trials failed to demonstrate any positive effect on outcomes.11,12 Some trials also reported a higher rate of complications with this intervention.13 However, meta-analyses suggested that prone-position ventilation might have a beneficial effect in patients with severe ARDS (defined as a PaO2/FiO2 ratio ≤ 100 mm Hg).14
In view of these findings, investigators conducted a trial of prone-position ventilation exclusively in patients with severe ARDS.
The PROSEVA study
The Proning Severe ARDS Patients (PROSEVA) study was a randomized controlled trial designed to determine whether prone-position ventilation, applied early, would improve outcomes in patients with severe ARDS.15
In PROSEVA, 466 patients with severe ARDS (defined as a PaO2/FiO2 ratio < 150 mm Hg, FiO2 ≥ 60%, and PEEP ≥ 5 cm H2O) underwent either at least 16 hours of prone positioning or were left in the supine position after 12 to 24 hours of initial conventional mechanical ventilation. The patients were recruited from centers in France and Spain where prone-position ventilation had been used in daily practice for more than 5 years.
The primary outcome studied was the rate of death at 28 days. The secondary end points were the death rate at day 90, rates of successful extubation, the length of stay in the intensive care unit, and complications.
Findings. At study entry, the patients in the supine group were sicker, more of them required a vasopressor, and fewer of them were receiving neuromuscular blocking agents than those in the prone group. These baseline differences may have influenced the outcomes; the unadjusted 28-day mortality rate was 16.0% in the prone group compared with 32.8% in the supine group (P < .001). However, the hazard ratio for death with prone positioning was 0.39 (95% confidence interval [CI] 0.25–0.63) even after adjusting for severity and the use of vasopressors and neuromuscular blocking agents. Prone-position ventilation was not associated with a higher incidence of complications, and the rate of successful extubation was higher.
Conclusions. In patients with severe ARDS, early use of prolonged prone positioning significantly decreased the 28-day and 90-day mortality rates. This trial has made prone positioning one of the strategies in managing patients with early severe ARDS. To minimize complications such as pressure ulcers and line or tube dislodgement, personnel caring for these patients must follow a protocol and undergo specific training.
These results were corroborated by a meta-analysis by Beitler et al16 that found a significant decrease in mortality rate with prone-position ventilation even in older studies when lung-protective ventilation strategies were separated from high-tidal-volume ventilation.
High-frequency oscillatory ventilation: No benefit in two trials
Observational data and experimental studies suggested that high-frequency oscillatory ventilation (HFOV) is superior to conventional mechanical ventilation in ARDS patients.17,18 However, outdated and cumbersome equipment, lack of protocols, and a lack of high-quality evidence led to limited and inconsistent use of HFOV, mainly as a rescue therapy in ARDS.19
Over the last few years, HFOV has been gaining acceptance, especially earlier in the course of ARDS.20 After preliminary clinical trials reported promising results, two trials conducted in Canada and the United Kingdom compared HFOV vs conventional mechanical ventilation in patients with ARDS.
The OSCAR study
The Oscillation in ARDS (OSCAR) study21 was a “pragmatic” trial22 (ie, it had minimal exclusion criteria) of the safety and effectiveness of HFOV as a primary ventilatory strategy for ARDS. It included 795 patients randomized to receive conventional ventilation (n = 397) or HFOV (n = 398). Research centers followed detailed algorithms for HFOV management and adopted their usual practice for conventional ventilation. Medical care was given according to the clinician’s judgment.
The primary outcome studied was survival at 30 days. The secondary outcomes were all-cause mortality in the intensive care unit and the hospital, duration of mechanical ventilation, and use of antimicrobial, sedative, vasoactive, and neuromuscular-blocking drugs.
Findings. The patient baseline characteristics were similar in both groups.
There was no significant difference in intensive care unit mortality rates, hospital mortality rates, or mortality rates at 30 days (41.7% in the HFOV group vs 41.1% in the conventional ventilation group; P = .85, 95% CI 6.1–7.5) even after adjustments for center or severity of illness.
The duration of mechanical ventilation was similar in both groups (14.9 ± 13.3 days in the HFOV group vs 14.1 ± 13.4 days in the conventional ventilation group, P = .41). However, sedatives and neuromuscular-blocking drugs were used more often and longer in the HFOV group than in the conventional ventilation group. There was no difference in the use of vasoactive or antimicrobial medications.
Conclusions. This multicenter randomized control trial did not demonstrate any benefit from using HFOV for routine management of ARDS. Its pragmatic design made it less likely to reach a firm conclusion,22 but it at least made a case against routinely using HFOV in patients with ARDS.
The OSCILLATE study
The Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) study23 assessed the safety and efficacy of HFOV as a treatment for early-onset moderate-to-severe ARDS.
The inclusion criteria were similar to those in the OSCAR trial except that pulmonary symptoms had to be present less than 2 weeks and ARDS assessment was done under standard ventilator settings. As this was an efficacy trial, it had more exclusion criteria than the OSCAR trial. A total of 548 patients were randomized to receive conventional ventilation (n = 273) or HFOV (n = 275). The baseline characteristics were similar between groups.
Conventional ventilation was given according to a protocol used in an earlier trial2 and included recruitment maneuvers. HFOV was given in centers that had experience in this treatment, and there were protocols for ventilation management, hemodynamic optimization, and weaning. All other care was left to the clinician’s choice.
The primary outcome studied was in-hospital mortality. The investigators also evaluated whether there were interactions between the treatment and baseline severity of lung injury and center experience with HFOV.
Findings. The trial was stopped after an interim analysis found that HFOV might be harmful, although the statistical threshold for stopping was not reached. The in-hospital mortality rate was 47% in the HFOV group and 35% in the control group (relative risk of death with HFOV 1.33, 95% CI 1.09–1.64, P = .005). HFOV was worse than conventional ventilation regardless of the severity of disease or center experience. The HFOV group had higher mean airway pressures but similar FiO2 compared with the conventional ventilation group.
The HFOV group received significantly more vasopressors, sedatives, and neuromuscular blockers. This group’s fluid balance was higher as well, but not significantly so. Refractory hypoxemia (defined as PaO2 < 60 mm Hg for 1 hour with an FiO2 of 1.0 and neuromuscular blockade) was more frequent in the conventional ventilation group, but the number of deaths in the subgroup with refractory hypoxemia was similar with either treatment.
Conclusions. This multicenter randomized controlled trial demonstrated that HFOV was harmful when used routinely to manage ARDS. The trial’s protocol was based on the results of a pilot study carried out by the same investigators, which provided the best evidence available regarding the safety of HFOV at that time.
The results of the OSCAR and OSCILLATE trials have quelled enthusiasm for early, routine use of HFOV in ARDS. Although there are concerns that the protocol (ie, the way HFOV was implemented) rather than HFOV itself may have led to worse outcomes, there is no signal to support its routine use. We need further studies to define if it remains a viable rescue therapy.
Extracorporeal membrane oxygenation: Is it a viable option in severe ARDS?
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to provide gas exchange. In patients with severe hypoxemia, ECMO can ensure adequate oxygenation and ventilation while ensuring the optimization of lung-protective ventilation. But ECMO was never as successful in adults with ARDS as it was in children and neonates.24
The first two trials of ECMO in ARDS24,25 reported equal or worse survival rates compared with conventional ventilation, and the overall mortality rate in these studies was staggeringly high. However, these studies were carried out before the era of lung-protective ventilation and at a time when ECMO technology was relatively primitive.
With new technology such as venovenous circuits and smaller cannulas, ECMO has gained more acceptance. It was used in patients with severe or refractory hypoxemia associated with ARDS during the H1N1 pandemic.26,27
The CESAR trial
The Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial28 assessed the safety, clinical efficacy, and cost-effectiveness of ECMO in managing severe ARDS. It compared best standard practice vs a protocol that included ECMO. The trial was conducted from 2001 to 2006.
Patients with severe ARDS, as defined by a Murray score29 greater than 3 or uncompensated hypercapnea, were prospectively randomized and recruited from an ECMO center and 148 tertiary intensive care units and referral hospitals in England. This was a pragmatic trial, with minimal exclusion criteria (essentially, mechanical ventilation with high pressures and high FiO2 for more than 7 days, intracranial bleeding, or contraindication to heparinization).
A total of 180 patients were randomized in a one-to-one ratio to receive ECMO or conventional management. The ventilator management in the conventional treatment group was not done according to a protocol but in general was low-volume and low-pressure. All patients randomized to ECMO were transferred to the ECMO center and treated according to a standardized ventilation protocol. After 12 hours, if predefined goals were not reached, venovenous ECMO was started. Patients assigned to conventional management could not cross over to ECMO.
The primary outcomes were death or severe disability at 6 months after randomization, and cost-effectiveness. The secondary outcomes were hospital resource use (eg, rescue techniques, length of stay, duration of ECMO) and health status after 6 months.
Findings. The groups were similar at baseline. Sixty-eight (75%) of the 90 patients randomized to receive ECMO actually received it. Of the 22 patients who did not receive ECMO, 16 (18% of the 90) improved on conventional therapy, 5 (6%) died during or before transfer, and 1 could not receive heparin.
Two patients had severe complications in the ECMO group: one had an arterial puncture, and one had an oxygen delivery failure during transport. In each case, these events contributed to the death of the patient.
More patients in the ECMO group received lung-protective ventilation, 84 (93%) vs 63 (70%).
The primary outcome, ie, death or severe disability at 6 months, occurred in 33 (37%) of the 90 patients in the ECMO group and in 46 (53%) of the patients in the conventional management group (relative risk 0.69, 95% CI 0.05–0.97, P = .03). More patients in the ECMO group survived, but the difference was not statistically significant (relative risk of death 0.73, 95% CI 0.52–1.03, P = .07). The most common cause of death in the ECMO group was multiorgan failure (42%), whereas in the conventional management group, the most common cause of death was respiratory failure (60%).
Length of stay in the hospital and in the critical care unit and health care costs were double for patients in the ECMO group. There was no difference in quality-of-life markers at 6 months in the survivors.
Conclusions. This pragmatic trial demonstrated that a protocol that includes ECMO could improve survival rates in ARDS.
Of note, the ECMO group got care in regional centers that used protocols. Therefore, in interpreting the results of this trial, we have to consider that being in a center with protocol-specified care for ARDS could drive some of the difference in mortality rates.
Regardless, this trial demonstrated that ECMO is feasible and led to better outcomes than expected. The findings were encouraging, and spurred the use of ECMO in severe ARDS during the 2009 H1N1 pandemic. Two propensity-matched studies and a number of case series reported a survival benefit associated with the use of ECMO in patients with severe ARDS.27,30
A recent meta-analysis also reported that ECMO might lower the mortality rate in ARDS; however, the patients in the H1N1 pandemic were younger and usually had isolated respiratory failure.31
The success of ECMO has opened new possibilities in the management of ARDS. As the technology improves and our experience increases, ECMO will likely gain more acceptance as a treatment for severe ARDS.
Airway pressure release ventilation
The use of airway pressure release ventilation and other ventilator modalities in ARDS is not supported by current evidence, though results of clinical trials may influence our practice in the future.
PHARMACOTHERAPY IN ARDS
The pathogenesis of ARDS includes damage to the alveolar-capillary membrane, with leakage of protein-rich edema fluid into alveoli. This damage is propagated by a complex inflammatory response including but not limited to neutrophil activation, free-radical formation, dysregulation of the coagulation system, and extensive release of inflammatory mediators.32,33 As a consequence, there are multiple potential targets for pharmacologic therapy in ARDS.
A variety of drugs, including corticosteroids, anti-inflammatory agents, immune-modulating agents, pulmonary vasodilators, antioxidants, and surfactants, have been studied in patients with ARDS.34 But effective pharmacotherapy for ARDS remains extremely limited.
Neuromuscular blockade in early severe ARDS
Mechanical ventilation can result in injurious stretching of the lung parenchyma, either from alveolar overdistention (volutrauma) or from continual recruitment and derecruitment of unstable lung units during the ventilator cycle (atelectrauma).35 Ventilator-induced lung injury can be exacerbated by asynchronous breathing.
In theory, neuromuscular blockers could minimize patient-ventilator asynchrony and provide much better control of tidal volume and pressure in patients with ARDS. This may result in less volutrauma and atelectrauma associated with asynchronous breathing. Data also suggest that cisatracurium (Nimbex), a neuromuscular blocking agent, may have a direct effect on the amount of inflammation in lungs with ARDS.36
The ACURASYS study
The ARDS et Curarisation Systématique (ACURASYS) study37 was a randomized trial in 340 patients undergoing mechanical ventilation for severe ARDS to evaluate the impact of neuromuscular blockade within the first 48 hours in this population.
The primary outcome was the mortality rate before hospital discharge or within 90 days of study entry. Secondary outcomes included the 28-day mortality rate, the rate of intensive care unit-acquired paresis, and the number of ventilator-free days. To be included, patients had to have been mechanically ventilated for less than 48 hours and to meet the AECC criteria for severe ARDS, with a PaO2/FiO2 ratio less than 150 mm Hg.
The intervention group received a continuous infusion of cisatracurium for 48 hours, while the control patients received placebo. Muscle strength was evaluated by clinical scoring of strength in different muscle groups.
Findings. The study groups were similar at baseline.
The crude 90-day mortality rate was lower in the cisatracurium group (31.6% vs 40.7%, P = .08). Regression analysis showed an improved 90-day survival rate with the use of this neuromuscular blocker after adjustment for severity of illness and the severity of ARDS (based on degree of hypoxemia and plateau pressures) (hazard ratio for death at 90 days 0.68; 95% CI 0.48–0.98; P = .04). The rate of paresis acquired in the intensive care unit did not differ significantly between the two groups.
Conclusion. In patients with severe ARDS, giving a neuromuscular blocking agent early improved the survival rate and increased the time off the ventilator without increasing muscle weakness.
These data are in line with similar findings from two other studies published by the same group.38,39 A meta-analysis of 432 patients showed that the use of neuromuscular blockade in early severe ARDS is associated with a statistically significant effect on early mortality (relative risk 0.66, 95% CI 0.50–0.87).40 The pooled analysis of these trials did not show any statistically significant critical-illness polyneuropathy.
These results need to be interpreted carefully, as we have inadequate data to see if they generalize to different intensive care units, and the evaluation and categorization of critical-illness polyneuropathy remains to be defined.
Cisatracurium is a promising treatment for moderate to severe ARDS and merits investigation in a large confirmatory randomized controlled trial.
Other pharmacologic agents
A number of other drugs have been studied in ARDS patients, including both inhaled and intravenous beta agonists,41,42 statins,43 and nutritional supplements.44 But as with other drugs previously studied in ARDS such as corticosteroids, N-acetylcysteine, and surfactant,34 these agents showed no effect on outcomes. In fact, a recent trial of intravenous salbutamol in ARDS patients was stopped after an interim analysis because of a higher incidence of arrhythmias and lactic acidosis with this agent.42
These findings reaffirm that pharmacologic therapy needs to be carefully considered, and potential harms associated with these therapies need to be addressed before they are introduced in the care of critically ill patients.
Continued progress in understanding the pathophysiology of acute respiratory distress syndrome (ARDS) is translating into changes in the way we diagnose and manage it. Over the past 20 years, low tidal volume,1 positive end-expiratory pressure (PEEP),2 and fluid restriction3 have become the standard of care. A multidisciplinary approach, including targeted use of sedatives, early mobilization, and protocols for weaning from the ventilator, has also brought about substantial changes in ARDS management and its outcomes.4–6
In this article, we review the most relevant articles about ARDS in the last 5 years. We include the new definition of ARDS and studies of ventilatory and nonventilatory therapies that have implications in managing patients with ARDS.
A STANDARDIZED APPROACH
ARDS is characterized by damage to the alveolar architecture, severe hypoxemia, and bilateral parenchymal opacities.
The working definition of ARDS developed in 1994 by the American-European Consensus Conference (AECC) was the basis for enrollment in most of the landmark trials and observational studies over the past 20 years.7,8 However, it was limited in its reliability and validity.
An updated definition
In 2011, the ARDS Definition Task Force, using a novel consensus process, updated the ARDS definition,9 focusing on its feasibility, reliability, and validity in predicting response to therapies and outcomes in ARDS. This new “Berlin” definition is not substantially different from the old, but defines the criteria more specifically:
- Bilateral opacities, unexplained by nodules, atelectasis, or effusion, on chest radiography or computed tomography
- New or worsening respiratory symptoms, or a clinical insult associated with ARDS within 7 days of diagnosis
- Objective assessment of cardiac function (eg, with echocardiography) to exclude cardiogenic pulmonary edema
- Hypoxemia, with a partial pressure of arterial oxygen divided by the percentage of inspired oxygen (PaO2/FiO2 ratio) of 300 mm Hg or less despite noninvasive or invasive mechanical ventilation with PEEP or continuous positive airway pressure (CPAP) of at least 5 cm H2O.
In addition, the new definition classifies the severity of disease on the basis of the degree of hypoxemia, ie, the PaO2/FiO2 ratio:
- Mild: PaO2/FiO2 ratio > 200 and ≤ 300 mm Hg
- Moderate: PaO2/FiO2 ratio > 100 and ≤ 200 mm Hg
- Severe: PaO2/FiO2 ratio ≤ 100 mm Hg.
The term “acute lung injury” has been eliminated, as has the previous criterion of a pulmonary artery wedge pressure of 18 mm Hg or less.
The panel also evaluated four ancillary variables for predicting outcomes in severe ARDS:
- Compliance of the respiratory system less than or equal to 40 mL/cm H2O
- Radiographic severity (involvement of three or four quadrants on chest radiography)
- PEEP of 10 cm H2O or greater
- Corrected expired volume 10 L/min or greater.
The task force evaluated the reliability and validity of this definition in a meta-analysis of 4,400 patients previously enrolled in randomized controlled trials or observational studies.
Findings. The Berlin definition predicted the risk of death better than the AECC definition. The mortality rate increased with the severity of ARDS, from 27% with mild disease to 32% with moderate disease to 45% with severe disease. The four ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition.
Thille et al10 retrospectively reviewed autopsy findings from 712 patients and found that the new definition identified a homogeneous group who had severe ARDS.10
Conclusions. The new definition may overcome some of the limitations of the old one, but it needs to be validated in clinical practice, especially its ability to predict death.
VENTILATORY SUPPORT
Prompt recognition, lung-protective ventilation, and a conservative fluid strategy remain the cornerstones of ARDS management. However, other strategies are being tested.
Prone-position ventilation in severe ARDS: The right therapy in a specific population
Prone-position ventilation was first described almost 30 years ago, but it has been used inconsistently in clinical practice.
Physiologic and observational studies indicated that prone positioning might improve survival in patients with ARDS, but several randomized trials failed to demonstrate any positive effect on outcomes.11,12 Some trials also reported a higher rate of complications with this intervention.13 However, meta-analyses suggested that prone-position ventilation might have a beneficial effect in patients with severe ARDS (defined as a PaO2/FiO2 ratio ≤ 100 mm Hg).14
In view of these findings, investigators conducted a trial of prone-position ventilation exclusively in patients with severe ARDS.
The PROSEVA study
The Proning Severe ARDS Patients (PROSEVA) study was a randomized controlled trial designed to determine whether prone-position ventilation, applied early, would improve outcomes in patients with severe ARDS.15
In PROSEVA, 466 patients with severe ARDS (defined as a PaO2/FiO2 ratio < 150 mm Hg, FiO2 ≥ 60%, and PEEP ≥ 5 cm H2O) underwent either at least 16 hours of prone positioning or were left in the supine position after 12 to 24 hours of initial conventional mechanical ventilation. The patients were recruited from centers in France and Spain where prone-position ventilation had been used in daily practice for more than 5 years.
The primary outcome studied was the rate of death at 28 days. The secondary end points were the death rate at day 90, rates of successful extubation, the length of stay in the intensive care unit, and complications.
Findings. At study entry, the patients in the supine group were sicker, more of them required a vasopressor, and fewer of them were receiving neuromuscular blocking agents than those in the prone group. These baseline differences may have influenced the outcomes; the unadjusted 28-day mortality rate was 16.0% in the prone group compared with 32.8% in the supine group (P < .001). However, the hazard ratio for death with prone positioning was 0.39 (95% confidence interval [CI] 0.25–0.63) even after adjusting for severity and the use of vasopressors and neuromuscular blocking agents. Prone-position ventilation was not associated with a higher incidence of complications, and the rate of successful extubation was higher.
Conclusions. In patients with severe ARDS, early use of prolonged prone positioning significantly decreased the 28-day and 90-day mortality rates. This trial has made prone positioning one of the strategies in managing patients with early severe ARDS. To minimize complications such as pressure ulcers and line or tube dislodgement, personnel caring for these patients must follow a protocol and undergo specific training.
These results were corroborated by a meta-analysis by Beitler et al16 that found a significant decrease in mortality rate with prone-position ventilation even in older studies when lung-protective ventilation strategies were separated from high-tidal-volume ventilation.
High-frequency oscillatory ventilation: No benefit in two trials
Observational data and experimental studies suggested that high-frequency oscillatory ventilation (HFOV) is superior to conventional mechanical ventilation in ARDS patients.17,18 However, outdated and cumbersome equipment, lack of protocols, and a lack of high-quality evidence led to limited and inconsistent use of HFOV, mainly as a rescue therapy in ARDS.19
Over the last few years, HFOV has been gaining acceptance, especially earlier in the course of ARDS.20 After preliminary clinical trials reported promising results, two trials conducted in Canada and the United Kingdom compared HFOV vs conventional mechanical ventilation in patients with ARDS.
The OSCAR study
The Oscillation in ARDS (OSCAR) study21 was a “pragmatic” trial22 (ie, it had minimal exclusion criteria) of the safety and effectiveness of HFOV as a primary ventilatory strategy for ARDS. It included 795 patients randomized to receive conventional ventilation (n = 397) or HFOV (n = 398). Research centers followed detailed algorithms for HFOV management and adopted their usual practice for conventional ventilation. Medical care was given according to the clinician’s judgment.
The primary outcome studied was survival at 30 days. The secondary outcomes were all-cause mortality in the intensive care unit and the hospital, duration of mechanical ventilation, and use of antimicrobial, sedative, vasoactive, and neuromuscular-blocking drugs.
Findings. The patient baseline characteristics were similar in both groups.
There was no significant difference in intensive care unit mortality rates, hospital mortality rates, or mortality rates at 30 days (41.7% in the HFOV group vs 41.1% in the conventional ventilation group; P = .85, 95% CI 6.1–7.5) even after adjustments for center or severity of illness.
The duration of mechanical ventilation was similar in both groups (14.9 ± 13.3 days in the HFOV group vs 14.1 ± 13.4 days in the conventional ventilation group, P = .41). However, sedatives and neuromuscular-blocking drugs were used more often and longer in the HFOV group than in the conventional ventilation group. There was no difference in the use of vasoactive or antimicrobial medications.
Conclusions. This multicenter randomized control trial did not demonstrate any benefit from using HFOV for routine management of ARDS. Its pragmatic design made it less likely to reach a firm conclusion,22 but it at least made a case against routinely using HFOV in patients with ARDS.
The OSCILLATE study
The Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) study23 assessed the safety and efficacy of HFOV as a treatment for early-onset moderate-to-severe ARDS.
The inclusion criteria were similar to those in the OSCAR trial except that pulmonary symptoms had to be present less than 2 weeks and ARDS assessment was done under standard ventilator settings. As this was an efficacy trial, it had more exclusion criteria than the OSCAR trial. A total of 548 patients were randomized to receive conventional ventilation (n = 273) or HFOV (n = 275). The baseline characteristics were similar between groups.
Conventional ventilation was given according to a protocol used in an earlier trial2 and included recruitment maneuvers. HFOV was given in centers that had experience in this treatment, and there were protocols for ventilation management, hemodynamic optimization, and weaning. All other care was left to the clinician’s choice.
The primary outcome studied was in-hospital mortality. The investigators also evaluated whether there were interactions between the treatment and baseline severity of lung injury and center experience with HFOV.
Findings. The trial was stopped after an interim analysis found that HFOV might be harmful, although the statistical threshold for stopping was not reached. The in-hospital mortality rate was 47% in the HFOV group and 35% in the control group (relative risk of death with HFOV 1.33, 95% CI 1.09–1.64, P = .005). HFOV was worse than conventional ventilation regardless of the severity of disease or center experience. The HFOV group had higher mean airway pressures but similar FiO2 compared with the conventional ventilation group.
The HFOV group received significantly more vasopressors, sedatives, and neuromuscular blockers. This group’s fluid balance was higher as well, but not significantly so. Refractory hypoxemia (defined as PaO2 < 60 mm Hg for 1 hour with an FiO2 of 1.0 and neuromuscular blockade) was more frequent in the conventional ventilation group, but the number of deaths in the subgroup with refractory hypoxemia was similar with either treatment.
Conclusions. This multicenter randomized controlled trial demonstrated that HFOV was harmful when used routinely to manage ARDS. The trial’s protocol was based on the results of a pilot study carried out by the same investigators, which provided the best evidence available regarding the safety of HFOV at that time.
The results of the OSCAR and OSCILLATE trials have quelled enthusiasm for early, routine use of HFOV in ARDS. Although there are concerns that the protocol (ie, the way HFOV was implemented) rather than HFOV itself may have led to worse outcomes, there is no signal to support its routine use. We need further studies to define if it remains a viable rescue therapy.
Extracorporeal membrane oxygenation: Is it a viable option in severe ARDS?
Extracorporeal membrane oxygenation (ECMO) uses cardiopulmonary bypass technology to provide gas exchange. In patients with severe hypoxemia, ECMO can ensure adequate oxygenation and ventilation while ensuring the optimization of lung-protective ventilation. But ECMO was never as successful in adults with ARDS as it was in children and neonates.24
The first two trials of ECMO in ARDS24,25 reported equal or worse survival rates compared with conventional ventilation, and the overall mortality rate in these studies was staggeringly high. However, these studies were carried out before the era of lung-protective ventilation and at a time when ECMO technology was relatively primitive.
With new technology such as venovenous circuits and smaller cannulas, ECMO has gained more acceptance. It was used in patients with severe or refractory hypoxemia associated with ARDS during the H1N1 pandemic.26,27
The CESAR trial
The Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial28 assessed the safety, clinical efficacy, and cost-effectiveness of ECMO in managing severe ARDS. It compared best standard practice vs a protocol that included ECMO. The trial was conducted from 2001 to 2006.
Patients with severe ARDS, as defined by a Murray score29 greater than 3 or uncompensated hypercapnea, were prospectively randomized and recruited from an ECMO center and 148 tertiary intensive care units and referral hospitals in England. This was a pragmatic trial, with minimal exclusion criteria (essentially, mechanical ventilation with high pressures and high FiO2 for more than 7 days, intracranial bleeding, or contraindication to heparinization).
A total of 180 patients were randomized in a one-to-one ratio to receive ECMO or conventional management. The ventilator management in the conventional treatment group was not done according to a protocol but in general was low-volume and low-pressure. All patients randomized to ECMO were transferred to the ECMO center and treated according to a standardized ventilation protocol. After 12 hours, if predefined goals were not reached, venovenous ECMO was started. Patients assigned to conventional management could not cross over to ECMO.
The primary outcomes were death or severe disability at 6 months after randomization, and cost-effectiveness. The secondary outcomes were hospital resource use (eg, rescue techniques, length of stay, duration of ECMO) and health status after 6 months.
Findings. The groups were similar at baseline. Sixty-eight (75%) of the 90 patients randomized to receive ECMO actually received it. Of the 22 patients who did not receive ECMO, 16 (18% of the 90) improved on conventional therapy, 5 (6%) died during or before transfer, and 1 could not receive heparin.
Two patients had severe complications in the ECMO group: one had an arterial puncture, and one had an oxygen delivery failure during transport. In each case, these events contributed to the death of the patient.
More patients in the ECMO group received lung-protective ventilation, 84 (93%) vs 63 (70%).
The primary outcome, ie, death or severe disability at 6 months, occurred in 33 (37%) of the 90 patients in the ECMO group and in 46 (53%) of the patients in the conventional management group (relative risk 0.69, 95% CI 0.05–0.97, P = .03). More patients in the ECMO group survived, but the difference was not statistically significant (relative risk of death 0.73, 95% CI 0.52–1.03, P = .07). The most common cause of death in the ECMO group was multiorgan failure (42%), whereas in the conventional management group, the most common cause of death was respiratory failure (60%).
Length of stay in the hospital and in the critical care unit and health care costs were double for patients in the ECMO group. There was no difference in quality-of-life markers at 6 months in the survivors.
Conclusions. This pragmatic trial demonstrated that a protocol that includes ECMO could improve survival rates in ARDS.
Of note, the ECMO group got care in regional centers that used protocols. Therefore, in interpreting the results of this trial, we have to consider that being in a center with protocol-specified care for ARDS could drive some of the difference in mortality rates.
Regardless, this trial demonstrated that ECMO is feasible and led to better outcomes than expected. The findings were encouraging, and spurred the use of ECMO in severe ARDS during the 2009 H1N1 pandemic. Two propensity-matched studies and a number of case series reported a survival benefit associated with the use of ECMO in patients with severe ARDS.27,30
A recent meta-analysis also reported that ECMO might lower the mortality rate in ARDS; however, the patients in the H1N1 pandemic were younger and usually had isolated respiratory failure.31
The success of ECMO has opened new possibilities in the management of ARDS. As the technology improves and our experience increases, ECMO will likely gain more acceptance as a treatment for severe ARDS.
Airway pressure release ventilation
The use of airway pressure release ventilation and other ventilator modalities in ARDS is not supported by current evidence, though results of clinical trials may influence our practice in the future.
PHARMACOTHERAPY IN ARDS
The pathogenesis of ARDS includes damage to the alveolar-capillary membrane, with leakage of protein-rich edema fluid into alveoli. This damage is propagated by a complex inflammatory response including but not limited to neutrophil activation, free-radical formation, dysregulation of the coagulation system, and extensive release of inflammatory mediators.32,33 As a consequence, there are multiple potential targets for pharmacologic therapy in ARDS.
A variety of drugs, including corticosteroids, anti-inflammatory agents, immune-modulating agents, pulmonary vasodilators, antioxidants, and surfactants, have been studied in patients with ARDS.34 But effective pharmacotherapy for ARDS remains extremely limited.
Neuromuscular blockade in early severe ARDS
Mechanical ventilation can result in injurious stretching of the lung parenchyma, either from alveolar overdistention (volutrauma) or from continual recruitment and derecruitment of unstable lung units during the ventilator cycle (atelectrauma).35 Ventilator-induced lung injury can be exacerbated by asynchronous breathing.
In theory, neuromuscular blockers could minimize patient-ventilator asynchrony and provide much better control of tidal volume and pressure in patients with ARDS. This may result in less volutrauma and atelectrauma associated with asynchronous breathing. Data also suggest that cisatracurium (Nimbex), a neuromuscular blocking agent, may have a direct effect on the amount of inflammation in lungs with ARDS.36
The ACURASYS study
The ARDS et Curarisation Systématique (ACURASYS) study37 was a randomized trial in 340 patients undergoing mechanical ventilation for severe ARDS to evaluate the impact of neuromuscular blockade within the first 48 hours in this population.
The primary outcome was the mortality rate before hospital discharge or within 90 days of study entry. Secondary outcomes included the 28-day mortality rate, the rate of intensive care unit-acquired paresis, and the number of ventilator-free days. To be included, patients had to have been mechanically ventilated for less than 48 hours and to meet the AECC criteria for severe ARDS, with a PaO2/FiO2 ratio less than 150 mm Hg.
The intervention group received a continuous infusion of cisatracurium for 48 hours, while the control patients received placebo. Muscle strength was evaluated by clinical scoring of strength in different muscle groups.
Findings. The study groups were similar at baseline.
The crude 90-day mortality rate was lower in the cisatracurium group (31.6% vs 40.7%, P = .08). Regression analysis showed an improved 90-day survival rate with the use of this neuromuscular blocker after adjustment for severity of illness and the severity of ARDS (based on degree of hypoxemia and plateau pressures) (hazard ratio for death at 90 days 0.68; 95% CI 0.48–0.98; P = .04). The rate of paresis acquired in the intensive care unit did not differ significantly between the two groups.
Conclusion. In patients with severe ARDS, giving a neuromuscular blocking agent early improved the survival rate and increased the time off the ventilator without increasing muscle weakness.
These data are in line with similar findings from two other studies published by the same group.38,39 A meta-analysis of 432 patients showed that the use of neuromuscular blockade in early severe ARDS is associated with a statistically significant effect on early mortality (relative risk 0.66, 95% CI 0.50–0.87).40 The pooled analysis of these trials did not show any statistically significant critical-illness polyneuropathy.
These results need to be interpreted carefully, as we have inadequate data to see if they generalize to different intensive care units, and the evaluation and categorization of critical-illness polyneuropathy remains to be defined.
Cisatracurium is a promising treatment for moderate to severe ARDS and merits investigation in a large confirmatory randomized controlled trial.
Other pharmacologic agents
A number of other drugs have been studied in ARDS patients, including both inhaled and intravenous beta agonists,41,42 statins,43 and nutritional supplements.44 But as with other drugs previously studied in ARDS such as corticosteroids, N-acetylcysteine, and surfactant,34 these agents showed no effect on outcomes. In fact, a recent trial of intravenous salbutamol in ARDS patients was stopped after an interim analysis because of a higher incidence of arrhythmias and lactic acidosis with this agent.42
These findings reaffirm that pharmacologic therapy needs to be carefully considered, and potential harms associated with these therapies need to be addressed before they are introduced in the care of critically ill patients.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308.
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582.
- ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533.
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767.
- Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345:568–573.
- Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302:1977–1984.
- Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173:1233–1239.
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599.
- Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168.
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014; 40:332–341.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008; 36:1043–1048.
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 2005; 33:479–486.
- Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009; 62:464–475.
- Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009; 302:1888–1895.
- Pham T, Combes A, Rozé H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Zampieri FG, Mendes PV, Ranzani OT, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care 2013; 28:998–1005.
- Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008; 15:1911–1924.
- Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004; 3:307–328.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; 4:CD004477.
- Terragni PP, Rosboch GL, Lisi A, Viale AG, Ranieri VM. How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients. Eur Respir J Suppl 2003; 42:15s–21s.
- Forel JM, Roch A, Papazian L. Paralytics in critical care: not always the bad guy. Curr Opin Crit Care 2009; 15:59–66.
- Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116.
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004; 32:113–19.
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006; 34:2749–2757.
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013; 17:R43.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–568.
- Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012; 379:229–235.
- Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307:795–803.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308.
- Meade MO, Cook DJ, Guyatt GH, et al; Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008; 299:637–645.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371:126–134.
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373:1874–1882.
- Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869.
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38:1573–1582.
- ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533.
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013; 187:761–767.
- Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345:568–573.
- Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009; 302:1977–1984.
- Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173:1233–1239.
- Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36:585–599.
- Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168.
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014; 40:332–341.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008; 36:1043–1048.
- Mehta S, Granton J, MacDonald RJ, et al. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Ferguson ND, Chiche JD, Kacmarek RM, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 2005; 33:479–486.
- Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813.
- Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009; 62:464–475.
- Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805.
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149:295–305.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979; 242:2193–2196.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009; 302:1888–1895.
- Pham T, Combes A, Rozé H, et al; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013; 187:276–285.
- Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374:1351–1363.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723.
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306:1659–1668.
- Zampieri FG, Mendes PV, Ranzani OT, et al. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. J Crit Care 2013; 28:998–1005.
- Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Pharmacotherapy of acute lung injury and acute respiratory distress syndrome. Curr Med Chem 2008; 15:1911–1924.
- Adhikari N, Burns KE, Meade MO. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 2004; 3:307–328.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; 4:CD004477.
- Terragni PP, Rosboch GL, Lisi A, Viale AG, Ranieri VM. How respiratory system mechanics may help in minimising ventilator-induced lung injury in ARDS patients. Eur Respir J Suppl 2003; 42:15s–21s.
- Forel JM, Roch A, Papazian L. Paralytics in critical care: not always the bad guy. Curr Opin Crit Care 2009; 15:59–66.
- Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116.
- Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 2004; 32:113–19.
- Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 2006; 34:2749–2757.
- Alhazzani W, Alshahrani M, Jaeschke R, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care 2013; 17:R43.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011; 184:561–568.
- Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012; 379:229–235.
- Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 2012; 307:795–803.
KEY POINTS
- The new definition of ARDS categorizes it as mild, moderate, or severe on the basis of oxygenation, specifically, the PaO2/FiO2 ratio.
- Neuromuscular blockade and prone positioning, used early in moderate or severe cases of ARDS, have shown some promise in trials, but questions remain about their application in critically ill patients.
- Based on two large trials, HFOV is no longer recommended as a primary therapy for ARDS, but it may still be considered as a rescue therapy in patients with refractory hypoxemia.
- In light of observational studies and randomized trials, ECMO should be considered an option in cases of refractory hypoxemia.
Changes to practice may help avoid ‘double trouble’
Large-volume thoracentesis is defined as the drainage of more than 1 L of fluid. Inherent in this procedure is the removal of a large amount of fluid from a cavity with a rigid wall, which leads to changes in pleural pressure and to expansion of the lung. Two specific complications occur, pneumothorax and reexpansion pulmonary edema. The images submitted for the Clinical Picture article by Drs. Apter and Aronowitz in this issue of the Journal highlight these complications.
Retrospective studies have found an association between the amount of fluid drained and the incidence of pneumothorax.1,2 Although technical issues may account for it (eg, needle injury to the lung that leads to postprocedural pneumothorax), the available evidence suggests that it has more to do with the drainage of larger volumes than the lung can expand to fill.3,4 That is, the patient’s lung cannot expand,5 so drainage creates a vacuum, and air enters the pleural space3 through the lung parenchyma, or perhaps from around the drainage catheter.
In a series of patients who underwent therapeutic thoracentesis,3 23 (8.7%) of 265 patients had pneumothorax. Interestingly, some patients had only symptoms, some had only excessively negative pressures (< 25 cm H2O), some had both, and some had neither. Thus, there does not seem to be a reliable sign or symptom of an unexpanding lung, but pleural manometry may help increase its detection.6 This technique, however, is rarely used in clinical practice.
Another consequence of therapeutic thoracentesis is reexpansion pulmonary edema. This rare condition occurs only after large-volume thoracentesis or evacuation of a moderate to large pneumothorax.7 The pathophysiology behind this is controversial.8 As with pneumothorax, a large case series did not find a correlation between volume removed or pleural pressures and reexpansion pulmonary edema.7 Experimental data and analysis of case series8–10 suggest that the duration of lung collapse and the speed of drainage and negative pressure applied contribute to the development of edema. Vacuum bottles are often used to speed drainage and to contain the large amount of fluid drained. These bottles have an initial negative pressure of about −723 mm Hg (personal communication with Baxter Healthcare Product information line), which may lead to rapid changes in lung volume and perhaps to higher negative pleural pressures.
Given the risks discussed above, we believe it is appropriate to avoid vacuum bottles and instead to use the syringe and one-way valve supplied in most thoracentesis kits. Further, pleural manometry to detect changes in pressure that suggest an unexpandable lung may lead to the appropriate early termination of a planned large-volume thoracentesis.3 The complications reported by Drs. Apter and Aronowitz are relatively rare and, at this point, unpredictable; therefore, generating high-quality evidence for prediction or management will be difficult. In the meantime, understanding the physiologic changes in the lung and the pleural space when draining large effusions from the chest may help avoid double trouble.
- Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol 2009; 50:42–47.
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170:332–339.
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130:1173–1184.
- Huggins JT, Sahn SA, Heidecker J, Ravenel JG, Doelken P. Characteristics of trapped lung: pleural fluid analysis, manometry, and air-contrast chest CT. Chest 2007; 131:206–213.
- Woodring JH, Baker MD, Stark P. Pneumothorax ex vacuo. Chest 1996; 110:1102–1105.
- Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulmon Med 2007; 13:312–318.
- Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg 2007; 84:1656–1661.
- Tarver RD, Broderick LS, Conces DJ, Jr. Reexpansion pulmonary edema. J Thorac Imag 1996; 11:198–209.
- Murphy K, Tomlanovich MC. Unilateral pulmonary edema after drainage of a spontaneous pneumothorax: case report and review of the world literature. J Emerg Med 1983; 1:29–36.
- Pavlin J, Cheney FW Unilateral pulmonary edema in rabbits after reexpansion of collapsed lung. J Appl Physiol Respir Environ Exerc Physiol 1979; 46:31–35.
Large-volume thoracentesis is defined as the drainage of more than 1 L of fluid. Inherent in this procedure is the removal of a large amount of fluid from a cavity with a rigid wall, which leads to changes in pleural pressure and to expansion of the lung. Two specific complications occur, pneumothorax and reexpansion pulmonary edema. The images submitted for the Clinical Picture article by Drs. Apter and Aronowitz in this issue of the Journal highlight these complications.
Retrospective studies have found an association between the amount of fluid drained and the incidence of pneumothorax.1,2 Although technical issues may account for it (eg, needle injury to the lung that leads to postprocedural pneumothorax), the available evidence suggests that it has more to do with the drainage of larger volumes than the lung can expand to fill.3,4 That is, the patient’s lung cannot expand,5 so drainage creates a vacuum, and air enters the pleural space3 through the lung parenchyma, or perhaps from around the drainage catheter.
In a series of patients who underwent therapeutic thoracentesis,3 23 (8.7%) of 265 patients had pneumothorax. Interestingly, some patients had only symptoms, some had only excessively negative pressures (< 25 cm H2O), some had both, and some had neither. Thus, there does not seem to be a reliable sign or symptom of an unexpanding lung, but pleural manometry may help increase its detection.6 This technique, however, is rarely used in clinical practice.
Another consequence of therapeutic thoracentesis is reexpansion pulmonary edema. This rare condition occurs only after large-volume thoracentesis or evacuation of a moderate to large pneumothorax.7 The pathophysiology behind this is controversial.8 As with pneumothorax, a large case series did not find a correlation between volume removed or pleural pressures and reexpansion pulmonary edema.7 Experimental data and analysis of case series8–10 suggest that the duration of lung collapse and the speed of drainage and negative pressure applied contribute to the development of edema. Vacuum bottles are often used to speed drainage and to contain the large amount of fluid drained. These bottles have an initial negative pressure of about −723 mm Hg (personal communication with Baxter Healthcare Product information line), which may lead to rapid changes in lung volume and perhaps to higher negative pleural pressures.
Given the risks discussed above, we believe it is appropriate to avoid vacuum bottles and instead to use the syringe and one-way valve supplied in most thoracentesis kits. Further, pleural manometry to detect changes in pressure that suggest an unexpandable lung may lead to the appropriate early termination of a planned large-volume thoracentesis.3 The complications reported by Drs. Apter and Aronowitz are relatively rare and, at this point, unpredictable; therefore, generating high-quality evidence for prediction or management will be difficult. In the meantime, understanding the physiologic changes in the lung and the pleural space when draining large effusions from the chest may help avoid double trouble.
Large-volume thoracentesis is defined as the drainage of more than 1 L of fluid. Inherent in this procedure is the removal of a large amount of fluid from a cavity with a rigid wall, which leads to changes in pleural pressure and to expansion of the lung. Two specific complications occur, pneumothorax and reexpansion pulmonary edema. The images submitted for the Clinical Picture article by Drs. Apter and Aronowitz in this issue of the Journal highlight these complications.
Retrospective studies have found an association between the amount of fluid drained and the incidence of pneumothorax.1,2 Although technical issues may account for it (eg, needle injury to the lung that leads to postprocedural pneumothorax), the available evidence suggests that it has more to do with the drainage of larger volumes than the lung can expand to fill.3,4 That is, the patient’s lung cannot expand,5 so drainage creates a vacuum, and air enters the pleural space3 through the lung parenchyma, or perhaps from around the drainage catheter.
In a series of patients who underwent therapeutic thoracentesis,3 23 (8.7%) of 265 patients had pneumothorax. Interestingly, some patients had only symptoms, some had only excessively negative pressures (< 25 cm H2O), some had both, and some had neither. Thus, there does not seem to be a reliable sign or symptom of an unexpanding lung, but pleural manometry may help increase its detection.6 This technique, however, is rarely used in clinical practice.
Another consequence of therapeutic thoracentesis is reexpansion pulmonary edema. This rare condition occurs only after large-volume thoracentesis or evacuation of a moderate to large pneumothorax.7 The pathophysiology behind this is controversial.8 As with pneumothorax, a large case series did not find a correlation between volume removed or pleural pressures and reexpansion pulmonary edema.7 Experimental data and analysis of case series8–10 suggest that the duration of lung collapse and the speed of drainage and negative pressure applied contribute to the development of edema. Vacuum bottles are often used to speed drainage and to contain the large amount of fluid drained. These bottles have an initial negative pressure of about −723 mm Hg (personal communication with Baxter Healthcare Product information line), which may lead to rapid changes in lung volume and perhaps to higher negative pleural pressures.
Given the risks discussed above, we believe it is appropriate to avoid vacuum bottles and instead to use the syringe and one-way valve supplied in most thoracentesis kits. Further, pleural manometry to detect changes in pressure that suggest an unexpandable lung may lead to the appropriate early termination of a planned large-volume thoracentesis.3 The complications reported by Drs. Apter and Aronowitz are relatively rare and, at this point, unpredictable; therefore, generating high-quality evidence for prediction or management will be difficult. In the meantime, understanding the physiologic changes in the lung and the pleural space when draining large effusions from the chest may help avoid double trouble.
- Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol 2009; 50:42–47.
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170:332–339.
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130:1173–1184.
- Huggins JT, Sahn SA, Heidecker J, Ravenel JG, Doelken P. Characteristics of trapped lung: pleural fluid analysis, manometry, and air-contrast chest CT. Chest 2007; 131:206–213.
- Woodring JH, Baker MD, Stark P. Pneumothorax ex vacuo. Chest 1996; 110:1102–1105.
- Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulmon Med 2007; 13:312–318.
- Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg 2007; 84:1656–1661.
- Tarver RD, Broderick LS, Conces DJ, Jr. Reexpansion pulmonary edema. J Thorac Imag 1996; 11:198–209.
- Murphy K, Tomlanovich MC. Unilateral pulmonary edema after drainage of a spontaneous pneumothorax: case report and review of the world literature. J Emerg Med 1983; 1:29–36.
- Pavlin J, Cheney FW Unilateral pulmonary edema in rabbits after reexpansion of collapsed lung. J Appl Physiol Respir Environ Exerc Physiol 1979; 46:31–35.
- Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol 2009; 50:42–47.
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170:332–339.
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130:1173–1184.
- Huggins JT, Sahn SA, Heidecker J, Ravenel JG, Doelken P. Characteristics of trapped lung: pleural fluid analysis, manometry, and air-contrast chest CT. Chest 2007; 131:206–213.
- Woodring JH, Baker MD, Stark P. Pneumothorax ex vacuo. Chest 1996; 110:1102–1105.
- Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulmon Med 2007; 13:312–318.
- Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg 2007; 84:1656–1661.
- Tarver RD, Broderick LS, Conces DJ, Jr. Reexpansion pulmonary edema. J Thorac Imag 1996; 11:198–209.
- Murphy K, Tomlanovich MC. Unilateral pulmonary edema after drainage of a spontaneous pneumothorax: case report and review of the world literature. J Emerg Med 1983; 1:29–36.
- Pavlin J, Cheney FW Unilateral pulmonary edema in rabbits after reexpansion of collapsed lung. J Appl Physiol Respir Environ Exerc Physiol 1979; 46:31–35.
Alternative modes of mechanical ventilation: A review for the hospitalist
Technologic advances and computerized control of mechanical ventilators have made it possible to deliver ventilatory assistance in new modes. Driving these innovations is the desire to prevent ventilator-induced lung injury, improve patient comfort, and liberate the patient from mechanical ventilation as soon as possible.
We call these innovations “alternative” modes to differentiate them from the plain volume-control and pressure-control modes. Some clinicians rarely use these new modes, but in some medical centers they have become the most common ones used, or are being used unknowingly (the operator misunderstands the mode name). The information we provide on these modes of ventilation is by no means an endorsement of their use, but rather a tool to help the clinician understand their physiologic, theoretical, and clinical effects.
We focused on two goals:
- Explain what the mode does
- Briefly review the theoretical benefits and the actual evidence supporting these alternative modes of ventilation.
STANDARD NOMENCLATURE NEEDED
Since its invention, mechanical ventilation has been plagued by multiple names being used to describe the same things. For example, volume-control ventilation is also called volume-cycled ventilation, assist-control ventilation, volume-limited ventilation, and controlled mechanical ventilation. Similarly, multiple abbreviations are used, each depending on the brand of ventilator, and new acronyms have been added in recent years as new modes have been developed. The vast number of names and modes can confuse even the most seasoned critical care physician.
Efforts to establish a common nomenclature are under way.1
WHAT IS A MODE?
A mode of mechanical ventilation has three essential components:
- The control variable
- The breath sequence
- The targeting scheme.
Similar modes may require more detailed descriptions to distinguish them, but the basic function can be explained by these three components.
The control variable
In general, inspiration is an active process, driven by the patient’s effort, the ventilator, or both, while expiration is passive. For simplicity, in this article a mechanical breath means the inspiratory phase of the breath.
The machine can only control the volume (and flow) or the pressure given. The breaths can be further described on the basis of what triggers the breath, what limits it (the maximum value of a control variable), and what ends (cycles) it.
The breath sequence
There are three possible breath sequences:
- Intermittent mandatory ventilation, in which the patient can take spontaneous breaths between mandatory breaths
- Continuous spontaneous ventilation, in which all breaths are spontaneous (Table 1).
The targeting scheme
The targeting or feedback scheme refers to the ventilator settings and programming that dictate its response to the patient’s lung compliance, lung resistance, and respiratory effort. The regulation can be as simple as controlling the pressure in pressure-control mode, or it can be based on a complicated algorithm.
ADAPTIVE PRESSURE CONTROL
Other names for adaptive pressure control
- Pressure Regulated Volume Control (Maquet Servo-i, Rastatt, Germany)
- AutoFlow (Dräger Medical AG, Lübeck, Germany)
- Adaptive Pressure Ventilation (Hamilton Galileo, Hamilton Medical AG, Bonaduz, Switzerland)
- Volume Control+ (Puritan Bennett, Tyco Healthcare; Mansfield, MA)
- Volume Targeted Pressure Control, Pressure Controlled Volume Guaranteed (Engström, General Electric, Madison, WI).
What does adaptive pressure control do?
The APC mode delivers pressure-controlled breaths with an adaptive targeting scheme (Table 2).
In pressure-control ventilation, tidal volumes depend on the lung’s physiologic mechanics (compliance and resistance) and patient effort (Figure 1). Therefore, the tidal volume varies with changes in lung physiology (ie, larger or smaller tidal volumes than targeted).
To overcome this effect, a machine in APC mode adjusts the inspiratory pressure to deliver the set minimal target tidal volume. If tidal volume increases, the machine decreases the inspiratory pressure, and if tidal volume decreases, the machine increases the inspiratory pressure. However, if the patient effort is large enough, the tidal volume will increase in spite of decreasing the inspiratory pressure (Figure 2). The adjustments to the inspiratory pressure occur after the tidal volume is off-target in a number of breaths.
Common sources of confusion with adaptive pressure control
First, APC is not a volume-control mode. In volume control, the tidal volume does not change; in APC the tidal volume can increase or decrease, and the ventilator will adjust the inflation pressure to achieve the target volume. Thus, APC guarantees an average minimum tidal volume but not a maximum tidal volume.
Second, a characteristic of pressure control (and hence, APC) is that the flow of gas varies to maintain constant airway pressure (ie, maintain the set inspiratory pressure). This characteristic allows a patient who generates an inspiratory effort to receive flow as demanded, which is likely more comfortable. This is essentially different from volume control, in which flow is set by the operator and hence is fixed. Thus, if the patient effort is strong enough (Figure 1), this leads to what is called flow asynchrony, in which the patient does not get the flow asked for in a breath.
Ventilator settings in adaptive pressure control
Ventilator settings in APC are:
- Tidal volume
- Time spent in inspiration (inspiratory time)
- Frequency
- Fraction of inspired oxygen (Fio2)
- Positive end-expiratory pressure (PEEP).
Some ventilators also require setting the speed to reach the peak pressure (also known as slope percent or inspiratory rise time).
Clinical applications of adaptive pressure control
This mode is designed to maintain a consistent tidal volume during pressure-control ventilation and to promote inspiratory flow synchrony. It is a means of automatically reducing ventilatory support (ie, weaning) as the patient’s inspiratory effort becomes stronger, as in awakening from anesthesia.
APC may not be ideal for patients who have an inappropriately increased respiratory drive (eg, in severe metabolic acidosis), since the inspiratory pressure will decrease to maintain the targeted average tidal volume, inappropriately shifting the work of breathing onto the patient.
Theoretical benefits of adaptive pressure control
APC guarantees a minimum average tidal volume (unless the pressure alarm threshold is set too low, so that the target tidal volume is not delivered). Other theoretical benefits are flow synchrony, less ventilator manipulation by the operator, and automatic weaning of ventilator support.
Evidence of benefit of adaptive pressure control
Physiologic benefits. This mode has lower peak inspiratory pressures than does volume-control ventilation,3,4 which is often reported as a positive finding. However, in volume-control mode (the usual comparator), the peak inspiratory pressure is a manifestation of both resistance and compliance. Hence, peak inspiratory pressure is expected to be higher but does not reflect actual lung-distending pressures. It is the plateau pressure, a manifestation of lung compliance, that is related to lung injury.
Patient comfort. APC may increase the work of breathing when using low tidal volume ventilation and when there is increased respiratory effort (drive).5 Interestingly, APC was less comfortable than pressure support ventilation in a small trial.6
Outcomes have not been studied.7
Adaptive pressure control: Bottom line
APC is widely available and widely used, sometimes unknowingly (eg, if the operator thinks it is volume control). It is relatively easy to use and to set; however, evidence of its benefit is scant.
ADAPTIVE SUPPORT VENTILATION
Adaptive support ventilation (ASV) evolved as a form of mandatory minute ventilation implemented with adaptive pressure control. Mandatory minute ventilation is a mode that allows the operator to preset a target minute ventilation, and the ventilator then supplies mandatory breaths, either volume- or pressure-controlled, if the patient’s spontaneous breaths generate a lower minute ventilation.
ASV automatically selects the appropriate tidal volume and frequency for mandatory breaths and the appropriate tidal volume for spontaneous breaths on the basis of the respiratory system mechanics and target minute alveolar ventilation.
Described in 1994 by Laubscher et al,8,9 ASV became commercially available in 1998 in Europe and in 2007 in the United States (Hamilton Galileo ventilator, Hamilton Medical AG). This is the first commercially available ventilator that uses an “optimal” targeting scheme (see below).
What does adaptive support ventilation do?
ASV delivers pressure-controlled breaths using an adaptive (optimal) scheme (Table 2). “Optimal,” in this context, means minimizing the mechanical work of breathing: the machine selects a tidal volume and frequency that the patient’s brain would presumably select if the patient were not connected to a ventilator. This pattern is assumed to encourage the patient to generate spontaneous breaths.
The ventilator calculates the normal required minute ventilation based on the patient’s ideal weight and estimated dead space volume (ie, 2.2 mL/kg). This calculation represents 100% of minute ventilation. The clinician at the bedside sets a target percent of minute ventilation that the ventilator will support—higher than 100% if the patient has increased requirements due, eg, to sepsis or increased dead space, or less than 100% during weaning.
The ventilator initially delivers test breaths, in which it measures the expiratory time constant for the respiratory system and then uses this along with the estimated dead space and normal minute ventilation to calculate an optimal breathing frequency in terms of mechanical work.
The optimal or target tidal volume is calculated as the normal minute ventilation divided by the optimal frequency. The target tidal volume is achieved by the use of APC (see above) (Figure 2). This means that the pressure limit is automatically adjusted to achieve an average delivered tidal volume equal to the target. The ventilator continuously monitors the respiratory system mechanics and adjusts its settings accordingly.
The ventilator adjusts its breaths to avoid air trapping by allowing enough time to exhale, to avoid hypoventilation by delivering tidal volume greater than the dead space, and to avoid volutrauma by avoiding large tidal volumes.
Ventilator settings in adaptive support ventilation
Ventilator settings in ASV are:
- Patient height (to calculate the ideal body weight)
- Sex
- Percent of normal predicted minute ventilation goal
- Fio2
- PEEP.
Clinical applications of adaptive support ventilation
ASV is intended as a sole mode of ventilation, from initial support to weaning.
Theoretical benefits of adaptive support ventilation
In theory, ASV offers automatic selection of ventilator settings, automatic adaptation to changing patient lung mechanics, less need for human manipulation of the machine, improved synchrony, and automatic weaning.
Evidence of benefit of adaptive support ventilation
Physiologic benefits. Ventilator settings are adjusted automatically. ASV selects different tidal volume-respiratory rate combinations based on respiratory mechanics in passive and paralyzed patients.10–12 In actively breathing patients, there was no difference in the ventilator settings chosen by ASV for different clinical scenarios (and lung physiology).10 Compared with pressure-controlled intermittent mandatory ventilation, with ASV, the inspiratory load is less and patient-ventilator interaction is better.13
Patient-ventilator synchrony and comfort have not been studied.
Outcomes. Two trials suggest that ASV may decrease time on mechanical ventilation.14,15 However, in another trial,16 compared with a standard protocol, ASV led to fewer ventilator adjustments but achieved similar postsurgical weaning outcomes. The effect of this mode on the death rate has not been examined.17,18
Adaptive support ventilation: Bottom line
ASV is the first commercially available mode that automatically selects all the ventilator settings except PEEP and Fio2. These seem appropriate for different clinical scenarios in patients with poor respiratory effort or in paralyzed patients. Evidence of the effect in actively breathing patients and on outcomes such as length of stay or death is still lacking.
PROPORTIONAL ASSIST VENTILATION
Patients who have normal respiratory drive but who have difficulty sustaining adequate spontaneous ventilation are often subjected to pressure support ventilation (PSV), in which the ventilator generates a constant pressure throughout inspiration regardless of the intensity of the patient’s effort.
In 1992, Younes and colleagues19,20 developed proportional assist ventilation (PAV) as an alternative in which the ventilator generates pressure in proportion to the patient’s effort. PAV became commercially available in Europe in 1999 and was approved in the United States in 2006, available on the Puritan Bennett 840 ventilator (Puritan Bennett Co, Boulder, CO). PAV has also been used for noninvasive ventilation, but this is not available in the United States.
Other names for proportional assist ventilation
Proportional Pressure Support (Dräger Medical; not yet available in the United States).
What does proportional assist ventilation do?
This mode delivers pressure-controlled breaths with a servo control scheme (Table 2).
To better understand PAV, we can compare it with PSV. With PSV, the pressure applied by the ventilator rises to a preset level that is held constant (a set-point scheme) until a cycling criterion (a percent of the maximum inspiratory flow value) is reached. The inspiratory flow and tidal volume are the result of the patient’s inspiratory effort, the level of pressure applied, and the respiratory system mechanics.
In PAV, as in PSV, all breaths are spontaneous (Table 1). The patient controls the timing and size of the breath. There are no preset pressure, flow, or volume goals, but safety limits on the volume and pressure delivered can be set.
Ventilator settings in proportional assist ventilation
Ventilator settings in PAV are:
- Airway type (endotracheal tube, tracheostomy)
- Airway size (inner diameter)
- Percentage of work supported (assist range 5%–95%)
- Tidal volume limit
- Pressure limit
- Expiratory sensitivity (normally, as inspiration ends, flow should stop; this parameter tells the ventilator at what flow to end inspiration).
Caution when assessing the literature. Earlier ventilator versions, ie, Dräger and Manitoba (University of Manitoba, Winnipeg, MB, Canada), which are not available in the United States, required the repeated calculation of the respiratory system mechanics and the manual setting of flow and volume assists (amplification factors) independently. To overcome this limitation, new software automatically adjusts the flow and volume amplification to support the loads imposed by the automatically measured values of resistance and elastance (inverse of compliance) of the respiratory system.21 This software is included in the model (Puritan Bennett) available in the United States.
Clinical applications of proportional assist ventilation
The PAV mode is indicated for maximizing ventilator patient synchrony for assisted spontaneous ventilation.
PAV is contraindicated in patients with respiratory depression (bradypnea) or large air leaks (eg, bronchopleural fistulas). It should be used with caution in patients with severe hyperinflation, in which the patient may still be exhaling but the ventilator doesn’t recognize it. Another group in which PAV should be used with caution is those with high ventilatory drives, in which the ventilator overestimates respiratory system mechanics. This situation can lead to overassistance due to the “runaway phenomenon,” in which the ventilator continues to provide support even if the patient has stopped inspiration.22
Theoretical benefits of proportional assist ventilation
In theory, PAV should reduce the work of breathing, improve synchrony, automatically adapt to changing patient lung mechanics and effort, decrease the need for ventilator intervention and manipulation, decrease the need for sedation, and improve sleep.
Evidence of benefit of proportional assist ventilation
Physiologic benefits. PAV reduces the work of breathing better than PSV,21 even in the face of changing respiratory mechanics or increased respiratory demand (hypercapnia).23–25 The hemodynamic profile is similar to that in PSV. Tidal volumes are variable; however, in recent reports the tidal volumes were within the lung-protective range (6–8 mL/kg, plateau pressure < 30 cm H20).26,27
Comfort. PAV entails less patient effort and discomfort that PSV does.23,25 PAV significantly reduces asynchrony,27 which in turn may favorably affect sleep in critically ill patients. 28
Outcomes. The probability of spontaneous breathing without assistance was significantly better in critically ill patients ventilated with PAV than with PSV. No trial has reported the effect of PAV on deaths.27,29
Proportional assist ventilation: Bottom line
Extensive basic research has been done with PAV in different forms of respiratory failure, such as obstructive lung disease, acute respiratory distress syndrome (ARDS), and chronic respiratory failure. It fulfills its main goal, which is to improve patient-ventilator synchrony. Clinical experience with PAV in the United States is limited, as it was only recently approved.
AIRWAY PRESSURE-RELEASE VENTILATION AND BIPHASIC POSITIVE AIRWAY PRESSURE
Airway pressure-release ventilation (APRV) was described in 1987 by Stock et al30 as a mode for delivering ventilation in acute lung injury while avoiding high airway pressures. APRV combines high constant positive airway pressure (improving oxygenation and promoting alveolar recruitment) with intermittent releases (causing exhalation).
Other names for biphasic positive airway pressure
Other names for biphasic positive airway pressure are:
- BiLevel (Puritan Bennett)
- BIPAP (Dräger Europe)
- Bi Vent (Siemens)
- BiPhasic (Avea, Cardinal Health, Inc, Dublin, OH)
- PCV+ (Dräger Medical)
- DuoPAP (Hamilton).
Caution—name confusion. In North America, BiPAP (Respironics, Murrysville, PA) and BiLevel are used to refer to noninvasive modes of ventilation.
APRV has no other name.
What do these modes do?
These modes deliver pressure-controlled, time-triggered, and time-cycled breaths using a set-point targeting scheme (Table 2). This means that the ventilator maintains a constant pressure (set point) even in the face of spontaneous breaths.
Caution—source of confusion. The term continuous positive airway pressure (CPAP) is often used to describe this mode. However, CPAP is pressure that is applied continuously at the same level; the patient generates all the work to maintain ventilation (“pressure-controlled continuous spontaneous ventilation” in the current nomenclature). In APRV, the airway pressure is intermittently released and reapplied, generating a tidal volume that supports ventilation. In other words, this is a pressure-controlled breath with a very prolonged inspiratory time and a short expiratory time in which spontaneous ventilation is possible at any point (“pressure-controlled intermittent mandatory ventilation” in the current nomenclature).
How these modes are set in the ventilator may also be a source of confusion. To describe the time spent in high and low airway pressures, we use the terms Thigh and Tlow, respectively. By convention, the difference between APRV and biphasic mode is the duration of Tlow (< 1.5 sec for APRV).
Similarly, Phigh and Plow are used to describe the high and low airway pressure. To better understand this concept, you can create the same mode in conventional pressure-control ventilation by thinking of the Thigh as the inspiratory time, the Tlow as the expiratory time, the Phigh as inspiratory pressure, and the Plow as PEEP.
Hence, APRV is an extreme form of inverse ratio ventilation, with an inspiration-to-expiration ratio of 4:1. This means a patient spends most of the time in Phigh and Thigh, and exhalations are short (Tlow and Plow). In contrast, the biphasic mode uses conventional inspiration-expiration ratios (Figure 4).
As with any form of pressure control, the tidal volume is generated by airway pressure rising above baseline (ie, the end-expiratory value). Hence, to ensure an increase in minute ventilation, the mandatory breath rate must be increased (ie, decreasing Thigh, Tlow, or both) or the tidal volume must be increased (ie, increasing the difference between Phigh and Plow). This means that in APRV the Tlow has to happen more often (by increasing the number of breaths) or be more prolonged (allowing more air to exhale). Because unrestricted spontaneous breaths are permitted at any point of the cycle, the patient contributes to the total minute ventilation (usually 10%–40%).
In APRV and biphasic mode, the operator’s set time and pressure in inspiration and expiration will be delivered regardless of the patient’s breathing efforts—the patient’s spontaneous breath does not trigger a mechanical breath. Some ventilators have automatic adjustments to improve the trigger synchrony.
Ventilator settings in APRV and biphasic mode
These modes require the setting of two pressure levels (Phigh and Plow) and two time durations (Thigh and Tlow). One can add pressure support or automatic tube compensation to assist spontaneous breaths. The difference in Tlow generates differences in the Thigh:Tlow ratio: APRV has a short Tlow (an inspiration-expiration ratio of 4:1). Biphasic mode has a conventional inspiration-expiration ratio of 1:1 to 1:4.
Clinical applications
APRV is used in acute lung injury and ARDS. This mode should be used with caution or not at all in patients with obstructive lung disease or inappropriately increased respiratory drive.32–35
Biphasic mode is intended for both ventilation and weaning. In a patient who has poor respiratory effort or who is paralyzed, biphasic is identical to pressure-control/continuous mandatory ventilation.
Theoretical benefits of APRV and biphasic mode
Multiple benefits have been ascribed to these modes. In theory, APRV will maximize and maintain alveolar recruitment, improve oxygenation, lower inflation pressures, and decrease overinflation. Both APRV and biphasic, by preserving spontaneous breathing, will improve ventilation-perfusion matching and gas diffusion, improve the hemodynamic profile (less need for vasopressors, higher cardiac output, reduced ventricular workload, improved organ perfusion), and improve synchrony (decrease the work of breathing and the need for sedation).
Evidence of benefit of APRV and biphasic mode
APRV and biphasic are different modes. However studies evaluating their effects are combined. This is in part the result of the nomenclature confusion and different practice in different countries.36
Physiologic benefits. In studies, spontaneous breaths contributed to 10% to 40% of minute ventilation,37,38 improved ventilation of dependent areas of the lung, improved ventilation-perfusion match and recruitment,39 and improved hemodynamic profile.40
Patient comfort. These modes are thought to decrease the need for analgesia and sedation,38 but a recent trial showed no difference with pressure-controlled intermittent mandatory ventilation.41 Patient ventilator synchrony and comfort have not been studied.32,42
Outcomes. In small trials, these modes made no difference in terms of deaths, but they may decrease the length of mechanical ventilation.38,41,43,44
APRV and biphasic mode: Bottom line
Maintaining spontaneous breathing while on mechanical ventilation has hemodynamic and ventilatory benefits.
APRV and biphasic mode are not the same thing. APRV’s main goal is to maximize mean airway pressure and, hence, lung recruitment, whereas the main goal of the biphasic mode is synchrony.
There is a plethora of ventilator settings and questions related to physiologic effects.33,34,36
Although these modes are widely used in some centers, there is no evidence yet that they are superior to conventional volume- or pressure-control ventilation with low tidal volume for ARDS and acute lung injury. There is no conclusive evidence that these modes improve synchrony, time to weaning, or patient comfort.
HIGH-FREQUENCY OSCILLATORY VENTILATION
High-frequency oscillatory ventilation (HFOV) was first described and patented in 1952 by Emerson and was clinically developed in the early 1970s by Lunkenheimer.45
The goal of HFOV is to minimize lung injury; its characteristics (discussed below) make it useful in patients with severe ARDS. The US Food and Drug Administration approved it for infants in 1991 and for children in 1995. The adult model has been available since 1993, but it was not approved until 2001 (SensorMedics 3100B, Cardinal Health, Inc).
Other names for high-frequency oscillatory ventilation
While HFOV has no alternative names, the following acronyms describe similar modes:
- HFPPV (high-frequency positive pressure ventilation)
- HFJV (high-frequency jet ventilation)
- HFFI (high-frequency flow interruption)
- HFPV (high-frequency percussive ventilation)
- HFCWO (high-frequency chest wall oscillation).
All of these modes require different specialized ventilators.
What does high-frequency oscillatory ventilation do?
Conceptually, HFOV is a form of pressure-controlled intermittent mandatory ventilation with a set-point control scheme. In contrast to conventional pressure-controlled intermittent mandatory ventilation, in which relatively small spontaneous breaths may be superimposed on relatively large mandatory breaths, HFOV superimposes very small mandatory breaths (oscillations) on top of spontaneous breaths.
Adult patients are usually paralyzed or deeply sedated, since deep spontaneous breathing will trigger alarms and affect ventilator performance.
To manage ventilation (CO2 clearance), one or several of the following maneuvers can be done: decrease the oscillation frequency, increase the amplitude of the oscillations, increase the inspiratory time, or increase bias flow (while allowing an endotracheal tube cuff leak). Oxygenation adjustments are controlled by manipulating the mean airway pressure and the Fio2.
Ventilator settings in high-frequency oscillatory ventilation
Ventilator settings in HFOV are46:
- Airway pressure amplitude (delta P or power)
- Mean airway pressure
- Percent inspiration
- Inspiratory bias flow
- Fio2.
Clinical applications of high-frequency oscillatory ventilation
This mode is usually reserved for ARDS patients for whom conventional ventilation is failing. A recently published protocol46 suggests considering HFOV when there is oxygenation failure (Fio2 ≥ 0.7 and PEEP ≥ 14 cm H2O) or ventilation failure (pH < 7.25 with tidal volume ≥ 6 mL/kg predicted body weight and plateau airway pressure ≥ 30 cm H2O).
This mode is contraindicated when there is known severe airflow obstruction or intracranial hypertension.
Theoretical benefits of high-frequency oscillatory ventilation
Conceptually, HFOV can provide the highest mean airway pressure paired with the lowest tidal volume of any mode. These benefits might make HFOV the ideal lung-protective ventilation strategy.
Evidence of benefit of high-frequency oscillatory ventilation
Physiologic benefits. Animal models have shown less histologic damage and lung inflammation with HFOV than with high-tidal-volume conventional ventilation47,48 and low-tidal-volume conventional ventilation.49
Patient comfort has not been studied. However, current technology does impose undue work of breathing in spontaneously breathing patients.50
Outcomes. Several retrospective case series have described better oxygenation with HFOV as rescue therapy for severe ARDS than with conventional mechanical ventilation. Two randomized controlled trials have studied HFOV vs high-tidal-volume conventional mechanical ventilation for early severe ARDS; HFOV was safe but made no difference in terms of deaths.42,51–54
High-frequency oscillatory ventilation: Bottom line
In theory, HFOV provides all the benefits of an ideal lung-protective strategy, at least for paralyzed or deeply sedated patients. Animal studies support these concepts. In human adults, HFOV has been shown to be safe and to provide better oxygenation but no improvement in death rates compared with conventional mechanical ventilation. Currently, HFOV is better reserved for patients with severe ARDS for whom conventional mechanical ventilation is failing.
- Chatburn RL. Classification of ventilator modes: update and proposal for implementation. Respir Care 2007; 52:301–323.
- Chatburn RL. Computer control of mechanical ventilation. Respir Care 2004; 49:507–517.
- Alvarez A, Subirana M, Benito S. Decelerating flow ventilation effects in acute respiratory failure. J Crit Care 1998; 13:21–25.
- Guldager H, Nielsen SL, Carl P, Soerensen MB. A comparison of volume control and pressure regulated volume control ventilation in acute respiratory failure. Crit Care 1997; 1:75–77.
- Kallet RH, Campbell AR, Dicker RA, Katz JA, Mackersie RC. Work of breathing during lung protective ventilation in patients with acute lung injury and acute respiratory distress syndrome: a comparison between volume and pressure regulated breathing modes. Respir Care 2005; 50:1623–1631.
- Betensley AD, Khalid I, Crawford J, Pensler RA, DiGiovine B. Patient comfort during pressure support and volume controlled continuous mandatory ventilation. Respir Care 2008; 53:897–902.
- Branson RD, Chatburn RL. Controversies in the critical care setting. Should adaptive pressure control modes be utilized for virtually all patients receiving mechanical ventilation? Respir Care 2007; 52:478–485.
- Laubscher TP, Frutiger A, Fanconi S, Jutzi H, Brunner JX. Automatic selection of tidal volume, respiratory frequency and minute ventilation in intubated ICU patients as start up procedure for closed-loop controlled ventilation. Int J Clin Monit Comput 1994; 11:19–30.
- Laubscher TP, Heinrichs W, Weiler N, Hartmann G, Brunner JX. An adaptive lung ventilation controller. IEEE Trans Biomed Eng 1994; 41:51–59.
- Arnal JM, Wysocki M, Nafati C, et al. Automatic selection of breathing pattern using adaptive support ventilation. Intensive Care Med 2008; 34:75–81.
- Campbell RS, Sinamban RP, Johannigman JA, et al. Clinical evaluation of a new closed loop ventilation mode: adaptive supportive ventilation (ASV). Crit Care 1999; 3( suppl 1):083.
- Belliato M, Palo A, Pasero D, Iotti GA, Mojoli F, Braschi A. Evaluation of adaptive support ventilation in paralysed patients and in a physical lung model. Int J Artif Organs 2004; 27:709–716.
- Tassaux D, Dalmas E, Gratadour P, Jolliet P. Patient ventilator interactions during partial ventilatory support: a preliminary study comparing the effects of adaptive support ventilation with synchronized intermittent mandatory ventilation plus inspiratory pressure support. Crit Care Med 2002; 30:801–807.
- Gruber PC, Gomersall CD, Leung P, et al. Randomized controlled trial comparing adaptive-support ventilation with pressure-regulated volume-controlled ventilation with automode in weaning patients after cardiac surgery. Anesthesiology 2008; 109:81–87.
- Sulzer CF, Chiolero R, Chassot PG, et al. Adaptive support ventilation for fast tracheal extubation after cardiac surgery: a randomized controlled study. Anesthesiology 2001; 95:1339–1345.
- Petter AH, Chiolèro RL, Cassina T, Chassot PG, Müller XM, Revelly JP. Automatic “respirator/weaning” with adaptive support ventilation: the effect on duration of endotracheal intubation and patient management. Anesth Analg 2003; 97:1743–1750.
- Brunner JX, Iotti GA. Adaptive support ventilation (ASV). Minerva Anestesiol 2002; 68:365–368.
- Campbell RS, Branson RD, Johannigman JA. Adaptive support ventilation. Respir Care Clin North Am 2001; 7:425–440.
- Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis 1992; 145:114–120.
- Younes M, Puddy A, Roberts D, et al. Proportional assist ventilation. Results of an initial clinical trial. Am Rev Respir Dis 1992; 145:121–129.
- Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Respiratory load compensation during mechanical ventilatio—proportional assist ventilation with load-adjustable gain factors versus pressure support. Intensive Care Med 2006; 32:692–699.
- Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Effect of different levels of pressure support and proportional assist ventilation on breathing pattern, work of breathing and gas exchange in mechanically ventilated hypercapnic COPD patients with acute respiratory failure. Respiration 2003; 70:355–361.
- Grasso S, Puntillo F, Mascia L, et al. Compensation for increase in respiratory workload during mechanical ventilation. Pressure support versus proportional assist ventilation. Am J Respir Crit Care Med 2000; 161:819–826.
- Wrigge H, Golisch W, Zinserling J, Sydow M, Almeling G, Burchardi H. Proportional assist versus pressure support ventilation: effects on breathing pattern and respiratory work of patients with chronic obstructive pulmonary disease. Intensive Care Med 1999; 25:790–798.
- Ranieri VM, Giuliani R, Mascia L, et al. Patient ventilator interaction during acute hypercapnia: pressure support vs. proportional assist ventilation. J Appl Physiol 1996; 81:426–436.
- Kondili E, Xirouchaki N, Vaporidi K, Klimathianaki M, Georgopoulos D. Short-term cardiorespiratory effects of proportional assist and pressure support ventilation in patients with acute lung injury/acute respiratory distress syndrome. Anesthesiology 2006; 105:703–708.
- Xirouchaki N, Kondili E, Vaporidi K, et al. Proportional assist ventilation with load-adjustable gain factors in critically ill patients: comparison with pressure support. Intensive Care Med 2008; 34:2026–2034.
- Bosma K, Ferreyra G, Ambrogio C, et al. Patient ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med 2007; 35:1048–1054.
- Sinderby C, Beck J. Proportional assist ventilation and neurally adjusted ventilatory assist—better approaches to patient ventilator synchrony? Clin Chest Med 2008; 29:329–342.
- Stock MC, Downs JB, Frolicher DA. Airway pressure release ventilation. Crit Care Med 1987; 15:462–466.
- Baum M, Benzer H, Putensen C, Koller W, Putz G. [Biphasic positive airway pressure (BIPAP)—a new form of augmented ventilation]. Anaesthesist 1989; 38:452–458.
- Seymour CW, Frazer M, Reilly PM, Fuchs BD. Airway pressure release and biphasic intermittent positive airway pressure ventilation: are they ready for prime time? J Trauma 2007; 62:1298–1308.
- Myers TR, MacIntyre NR. Respiratory controversies in the critical care setting. Does airway pressure release ventilation offer important new advantages in mechanical ventilator support? Respir Care 2007; 52:452–458.
- Neumann P, Golisch W, Strohmeyer A, Buscher H, Burchardi H, Sydow M. Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive Care Med 2002; 28:1742–1749.
- Calzia E, Lindner KH, Witt S, et al. Pressure-time product and work of breathing during biphasic continuous positive airway pressure and assisted spontaneous breathing. Am J Respir Crit Care Med 1994; 150:904–910.
- Rose L, Hawkins M. Airway pressure release ventilation and biphasic positive airway pressure: a systematic review of definitional criteria. Intensive Care Med 2008; 34:1766–1773.
- Sydow M, Burchardi H, Ephraim E, Zielmann S, Crozier TA. Longterm effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med 1994; 149:1550–1556.
- Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 2001; 164:43–49.
- Davis K, Johnson DJ, Branson RD, Campbell RS, Johannigman JA, Porembka D. Airway pressure release ventilation. Arch Surg 1993; 128:1348–1352.
- Kaplan LJ, Bailey H, Formosa V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care 2001; 5:221–226.
- Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettilä VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand 2004; 48:722–731.
- Siau C, Stewart TE. Current role of high frequency oscillatory ventilation and airway pressure release ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med 2008; 29:265–275.
- Rathgeber J, Schorn B, Falk V, Kazmaier S, Spiegel T, Burchardi H. The influence of controlled mandatory ventilation (CMV), intermittent mandatory ventilation (IMV) and biphasic intermittent positive airway pressure (BIPAP) on duration of intubation and consumption of analgesics and sedatives. A prospective analysis in 596 patients following adult cardiac surgery. Eur J Anaesthesiol 1997; 14:576–582.
- Habashi NM. Other approaches to open lung ventilation: airway pressure release ventilation. Crit Care Med 2005; 33 suppl 3:S228–S240.
- Hess D, Mason S, Branson R. High-frequency ventilation design and equipment issues. Respir Care Clin North Am 2001; 7:577–598.
- Fessler HE, Derdak S, Ferguson ND, et al. A protocol for high frequency oscillatory ventilation in adults: results from a roundtable discussion. Crit Care Med 2007; 35:1649–1654.
- Hamilton PP, Onayemi A, Smyth JA, et al. Comparison of conventional and high-frequency ventilation: oxygenation and lung pathology. J Appl Physiol 1983; 55:131–138.
- Sedeek KA, Takeuchi M, Suchodolski K, et al. Open-lung protective ventilation with pressure control ventilation, high-frequency oscillation, and intratracheal pulmonary ventilation results in similar gas exchange, hemodynamics, and lung mechanics. Anesthesiology 2003; 99:1102–1111.
- Imai Y, Nakagawa S, Ito Y, Kawano T, Slutsky AS, Miyasaka K. Comparison of lung protection strategies using conventional and high-frequency oscillatory ventilation. J Appl Physiol 2001; 91:1836–1844.
- van Heerde M, Roubik K, Kopelent V, Plötz FB, Markhorst DG. Unloading work of breathing during high-frequency oscillatory ventilation: a bench study. Crit Care 2006; 10:R103.
- Derdak S, Mehta S, Stewart TE, et al., Multicenter Oscillatory Ventilation For Acute Respiratory Distress Syndrome Trial (MOAT) Study Investigators. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med 2002; 166:801–808.
- Bollen CW, van Well GT, Sherry T, et al. High-frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care 2005; 9:R430–R439.
- Mehta S, Granton J, MacDonald RJ, et al. High frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
Technologic advances and computerized control of mechanical ventilators have made it possible to deliver ventilatory assistance in new modes. Driving these innovations is the desire to prevent ventilator-induced lung injury, improve patient comfort, and liberate the patient from mechanical ventilation as soon as possible.
We call these innovations “alternative” modes to differentiate them from the plain volume-control and pressure-control modes. Some clinicians rarely use these new modes, but in some medical centers they have become the most common ones used, or are being used unknowingly (the operator misunderstands the mode name). The information we provide on these modes of ventilation is by no means an endorsement of their use, but rather a tool to help the clinician understand their physiologic, theoretical, and clinical effects.
We focused on two goals:
- Explain what the mode does
- Briefly review the theoretical benefits and the actual evidence supporting these alternative modes of ventilation.

STANDARD NOMENCLATURE NEEDED
Since its invention, mechanical ventilation has been plagued by multiple names being used to describe the same things. For example, volume-control ventilation is also called volume-cycled ventilation, assist-control ventilation, volume-limited ventilation, and controlled mechanical ventilation. Similarly, multiple abbreviations are used, each depending on the brand of ventilator, and new acronyms have been added in recent years as new modes have been developed. The vast number of names and modes can confuse even the most seasoned critical care physician.
Efforts to establish a common nomenclature are under way.1
WHAT IS A MODE?
A mode of mechanical ventilation has three essential components:
- The control variable
- The breath sequence
- The targeting scheme.
Similar modes may require more detailed descriptions to distinguish them, but the basic function can be explained by these three components.
The control variable
In general, inspiration is an active process, driven by the patient’s effort, the ventilator, or both, while expiration is passive. For simplicity, in this article a mechanical breath means the inspiratory phase of the breath.
The machine can only control the volume (and flow) or the pressure given. The breaths can be further described on the basis of what triggers the breath, what limits it (the maximum value of a control variable), and what ends (cycles) it.
The breath sequence
There are three possible breath sequences:
- Intermittent mandatory ventilation, in which the patient can take spontaneous breaths between mandatory breaths
- Continuous spontaneous ventilation, in which all breaths are spontaneous (Table 1).
The targeting scheme
The targeting or feedback scheme refers to the ventilator settings and programming that dictate its response to the patient’s lung compliance, lung resistance, and respiratory effort. The regulation can be as simple as controlling the pressure in pressure-control mode, or it can be based on a complicated algorithm.
ADAPTIVE PRESSURE CONTROL
Other names for adaptive pressure control
- Pressure Regulated Volume Control (Maquet Servo-i, Rastatt, Germany)
- AutoFlow (Dräger Medical AG, Lübeck, Germany)
- Adaptive Pressure Ventilation (Hamilton Galileo, Hamilton Medical AG, Bonaduz, Switzerland)
- Volume Control+ (Puritan Bennett, Tyco Healthcare; Mansfield, MA)
- Volume Targeted Pressure Control, Pressure Controlled Volume Guaranteed (Engström, General Electric, Madison, WI).
What does adaptive pressure control do?
The APC mode delivers pressure-controlled breaths with an adaptive targeting scheme (Table 2).
In pressure-control ventilation, tidal volumes depend on the lung’s physiologic mechanics (compliance and resistance) and patient effort (Figure 1). Therefore, the tidal volume varies with changes in lung physiology (ie, larger or smaller tidal volumes than targeted).
To overcome this effect, a machine in APC mode adjusts the inspiratory pressure to deliver the set minimal target tidal volume. If tidal volume increases, the machine decreases the inspiratory pressure, and if tidal volume decreases, the machine increases the inspiratory pressure. However, if the patient effort is large enough, the tidal volume will increase in spite of decreasing the inspiratory pressure (Figure 2). The adjustments to the inspiratory pressure occur after the tidal volume is off-target in a number of breaths.
Common sources of confusion with adaptive pressure control
First, APC is not a volume-control mode. In volume control, the tidal volume does not change; in APC the tidal volume can increase or decrease, and the ventilator will adjust the inflation pressure to achieve the target volume. Thus, APC guarantees an average minimum tidal volume but not a maximum tidal volume.
Second, a characteristic of pressure control (and hence, APC) is that the flow of gas varies to maintain constant airway pressure (ie, maintain the set inspiratory pressure). This characteristic allows a patient who generates an inspiratory effort to receive flow as demanded, which is likely more comfortable. This is essentially different from volume control, in which flow is set by the operator and hence is fixed. Thus, if the patient effort is strong enough (Figure 1), this leads to what is called flow asynchrony, in which the patient does not get the flow asked for in a breath.
Ventilator settings in adaptive pressure control
Ventilator settings in APC are:
- Tidal volume
- Time spent in inspiration (inspiratory time)
- Frequency
- Fraction of inspired oxygen (Fio2)
- Positive end-expiratory pressure (PEEP).
Some ventilators also require setting the speed to reach the peak pressure (also known as slope percent or inspiratory rise time).
Clinical applications of adaptive pressure control
This mode is designed to maintain a consistent tidal volume during pressure-control ventilation and to promote inspiratory flow synchrony. It is a means of automatically reducing ventilatory support (ie, weaning) as the patient’s inspiratory effort becomes stronger, as in awakening from anesthesia.
APC may not be ideal for patients who have an inappropriately increased respiratory drive (eg, in severe metabolic acidosis), since the inspiratory pressure will decrease to maintain the targeted average tidal volume, inappropriately shifting the work of breathing onto the patient.
Theoretical benefits of adaptive pressure control
APC guarantees a minimum average tidal volume (unless the pressure alarm threshold is set too low, so that the target tidal volume is not delivered). Other theoretical benefits are flow synchrony, less ventilator manipulation by the operator, and automatic weaning of ventilator support.
Evidence of benefit of adaptive pressure control
Physiologic benefits. This mode has lower peak inspiratory pressures than does volume-control ventilation,3,4 which is often reported as a positive finding. However, in volume-control mode (the usual comparator), the peak inspiratory pressure is a manifestation of both resistance and compliance. Hence, peak inspiratory pressure is expected to be higher but does not reflect actual lung-distending pressures. It is the plateau pressure, a manifestation of lung compliance, that is related to lung injury.
Patient comfort. APC may increase the work of breathing when using low tidal volume ventilation and when there is increased respiratory effort (drive).5 Interestingly, APC was less comfortable than pressure support ventilation in a small trial.6
Outcomes have not been studied.7
Adaptive pressure control: Bottom line
APC is widely available and widely used, sometimes unknowingly (eg, if the operator thinks it is volume control). It is relatively easy to use and to set; however, evidence of its benefit is scant.
ADAPTIVE SUPPORT VENTILATION
Adaptive support ventilation (ASV) evolved as a form of mandatory minute ventilation implemented with adaptive pressure control. Mandatory minute ventilation is a mode that allows the operator to preset a target minute ventilation, and the ventilator then supplies mandatory breaths, either volume- or pressure-controlled, if the patient’s spontaneous breaths generate a lower minute ventilation.
ASV automatically selects the appropriate tidal volume and frequency for mandatory breaths and the appropriate tidal volume for spontaneous breaths on the basis of the respiratory system mechanics and target minute alveolar ventilation.
Described in 1994 by Laubscher et al,8,9 ASV became commercially available in 1998 in Europe and in 2007 in the United States (Hamilton Galileo ventilator, Hamilton Medical AG). This is the first commercially available ventilator that uses an “optimal” targeting scheme (see below).
What does adaptive support ventilation do?
ASV delivers pressure-controlled breaths using an adaptive (optimal) scheme (Table 2). “Optimal,” in this context, means minimizing the mechanical work of breathing: the machine selects a tidal volume and frequency that the patient’s brain would presumably select if the patient were not connected to a ventilator. This pattern is assumed to encourage the patient to generate spontaneous breaths.
The ventilator calculates the normal required minute ventilation based on the patient’s ideal weight and estimated dead space volume (ie, 2.2 mL/kg). This calculation represents 100% of minute ventilation. The clinician at the bedside sets a target percent of minute ventilation that the ventilator will support—higher than 100% if the patient has increased requirements due, eg, to sepsis or increased dead space, or less than 100% during weaning.
The ventilator initially delivers test breaths, in which it measures the expiratory time constant for the respiratory system and then uses this along with the estimated dead space and normal minute ventilation to calculate an optimal breathing frequency in terms of mechanical work.
The optimal or target tidal volume is calculated as the normal minute ventilation divided by the optimal frequency. The target tidal volume is achieved by the use of APC (see above) (Figure 2). This means that the pressure limit is automatically adjusted to achieve an average delivered tidal volume equal to the target. The ventilator continuously monitors the respiratory system mechanics and adjusts its settings accordingly.
The ventilator adjusts its breaths to avoid air trapping by allowing enough time to exhale, to avoid hypoventilation by delivering tidal volume greater than the dead space, and to avoid volutrauma by avoiding large tidal volumes.
Ventilator settings in adaptive support ventilation
Ventilator settings in ASV are:
- Patient height (to calculate the ideal body weight)
- Sex
- Percent of normal predicted minute ventilation goal
- Fio2
- PEEP.
Clinical applications of adaptive support ventilation
ASV is intended as a sole mode of ventilation, from initial support to weaning.
Theoretical benefits of adaptive support ventilation
In theory, ASV offers automatic selection of ventilator settings, automatic adaptation to changing patient lung mechanics, less need for human manipulation of the machine, improved synchrony, and automatic weaning.
Evidence of benefit of adaptive support ventilation
Physiologic benefits. Ventilator settings are adjusted automatically. ASV selects different tidal volume-respiratory rate combinations based on respiratory mechanics in passive and paralyzed patients.10–12 In actively breathing patients, there was no difference in the ventilator settings chosen by ASV for different clinical scenarios (and lung physiology).10 Compared with pressure-controlled intermittent mandatory ventilation, with ASV, the inspiratory load is less and patient-ventilator interaction is better.13
Patient-ventilator synchrony and comfort have not been studied.
Outcomes. Two trials suggest that ASV may decrease time on mechanical ventilation.14,15 However, in another trial,16 compared with a standard protocol, ASV led to fewer ventilator adjustments but achieved similar postsurgical weaning outcomes. The effect of this mode on the death rate has not been examined.17,18
Adaptive support ventilation: Bottom line
ASV is the first commercially available mode that automatically selects all the ventilator settings except PEEP and Fio2. These seem appropriate for different clinical scenarios in patients with poor respiratory effort or in paralyzed patients. Evidence of the effect in actively breathing patients and on outcomes such as length of stay or death is still lacking.
PROPORTIONAL ASSIST VENTILATION
Patients who have normal respiratory drive but who have difficulty sustaining adequate spontaneous ventilation are often subjected to pressure support ventilation (PSV), in which the ventilator generates a constant pressure throughout inspiration regardless of the intensity of the patient’s effort.
In 1992, Younes and colleagues19,20 developed proportional assist ventilation (PAV) as an alternative in which the ventilator generates pressure in proportion to the patient’s effort. PAV became commercially available in Europe in 1999 and was approved in the United States in 2006, available on the Puritan Bennett 840 ventilator (Puritan Bennett Co, Boulder, CO). PAV has also been used for noninvasive ventilation, but this is not available in the United States.
Other names for proportional assist ventilation
Proportional Pressure Support (Dräger Medical; not yet available in the United States).
What does proportional assist ventilation do?
This mode delivers pressure-controlled breaths with a servo control scheme (Table 2).
To better understand PAV, we can compare it with PSV. With PSV, the pressure applied by the ventilator rises to a preset level that is held constant (a set-point scheme) until a cycling criterion (a percent of the maximum inspiratory flow value) is reached. The inspiratory flow and tidal volume are the result of the patient’s inspiratory effort, the level of pressure applied, and the respiratory system mechanics.
In PAV, as in PSV, all breaths are spontaneous (Table 1). The patient controls the timing and size of the breath. There are no preset pressure, flow, or volume goals, but safety limits on the volume and pressure delivered can be set.
Ventilator settings in proportional assist ventilation
Ventilator settings in PAV are:
- Airway type (endotracheal tube, tracheostomy)
- Airway size (inner diameter)
- Percentage of work supported (assist range 5%–95%)
- Tidal volume limit
- Pressure limit
- Expiratory sensitivity (normally, as inspiration ends, flow should stop; this parameter tells the ventilator at what flow to end inspiration).
Caution when assessing the literature. Earlier ventilator versions, ie, Dräger and Manitoba (University of Manitoba, Winnipeg, MB, Canada), which are not available in the United States, required the repeated calculation of the respiratory system mechanics and the manual setting of flow and volume assists (amplification factors) independently. To overcome this limitation, new software automatically adjusts the flow and volume amplification to support the loads imposed by the automatically measured values of resistance and elastance (inverse of compliance) of the respiratory system.21 This software is included in the model (Puritan Bennett) available in the United States.
Clinical applications of proportional assist ventilation
The PAV mode is indicated for maximizing ventilator patient synchrony for assisted spontaneous ventilation.
PAV is contraindicated in patients with respiratory depression (bradypnea) or large air leaks (eg, bronchopleural fistulas). It should be used with caution in patients with severe hyperinflation, in which the patient may still be exhaling but the ventilator doesn’t recognize it. Another group in which PAV should be used with caution is those with high ventilatory drives, in which the ventilator overestimates respiratory system mechanics. This situation can lead to overassistance due to the “runaway phenomenon,” in which the ventilator continues to provide support even if the patient has stopped inspiration.22
Theoretical benefits of proportional assist ventilation
In theory, PAV should reduce the work of breathing, improve synchrony, automatically adapt to changing patient lung mechanics and effort, decrease the need for ventilator intervention and manipulation, decrease the need for sedation, and improve sleep.
Evidence of benefit of proportional assist ventilation
Physiologic benefits. PAV reduces the work of breathing better than PSV,21 even in the face of changing respiratory mechanics or increased respiratory demand (hypercapnia).23–25 The hemodynamic profile is similar to that in PSV. Tidal volumes are variable; however, in recent reports the tidal volumes were within the lung-protective range (6–8 mL/kg, plateau pressure < 30 cm H20).26,27
Comfort. PAV entails less patient effort and discomfort that PSV does.23,25 PAV significantly reduces asynchrony,27 which in turn may favorably affect sleep in critically ill patients. 28
Outcomes. The probability of spontaneous breathing without assistance was significantly better in critically ill patients ventilated with PAV than with PSV. No trial has reported the effect of PAV on deaths.27,29
Proportional assist ventilation: Bottom line
Extensive basic research has been done with PAV in different forms of respiratory failure, such as obstructive lung disease, acute respiratory distress syndrome (ARDS), and chronic respiratory failure. It fulfills its main goal, which is to improve patient-ventilator synchrony. Clinical experience with PAV in the United States is limited, as it was only recently approved.
AIRWAY PRESSURE-RELEASE VENTILATION AND BIPHASIC POSITIVE AIRWAY PRESSURE
Airway pressure-release ventilation (APRV) was described in 1987 by Stock et al30 as a mode for delivering ventilation in acute lung injury while avoiding high airway pressures. APRV combines high constant positive airway pressure (improving oxygenation and promoting alveolar recruitment) with intermittent releases (causing exhalation).
Other names for biphasic positive airway pressure
Other names for biphasic positive airway pressure are:
- BiLevel (Puritan Bennett)
- BIPAP (Dräger Europe)
- Bi Vent (Siemens)
- BiPhasic (Avea, Cardinal Health, Inc, Dublin, OH)
- PCV+ (Dräger Medical)
- DuoPAP (Hamilton).
Caution—name confusion. In North America, BiPAP (Respironics, Murrysville, PA) and BiLevel are used to refer to noninvasive modes of ventilation.
APRV has no other name.
What do these modes do?
These modes deliver pressure-controlled, time-triggered, and time-cycled breaths using a set-point targeting scheme (Table 2). This means that the ventilator maintains a constant pressure (set point) even in the face of spontaneous breaths.
Caution—source of confusion. The term continuous positive airway pressure (CPAP) is often used to describe this mode. However, CPAP is pressure that is applied continuously at the same level; the patient generates all the work to maintain ventilation (“pressure-controlled continuous spontaneous ventilation” in the current nomenclature). In APRV, the airway pressure is intermittently released and reapplied, generating a tidal volume that supports ventilation. In other words, this is a pressure-controlled breath with a very prolonged inspiratory time and a short expiratory time in which spontaneous ventilation is possible at any point (“pressure-controlled intermittent mandatory ventilation” in the current nomenclature).
How these modes are set in the ventilator may also be a source of confusion. To describe the time spent in high and low airway pressures, we use the terms Thigh and Tlow, respectively. By convention, the difference between APRV and biphasic mode is the duration of Tlow (< 1.5 sec for APRV).
Similarly, Phigh and Plow are used to describe the high and low airway pressure. To better understand this concept, you can create the same mode in conventional pressure-control ventilation by thinking of the Thigh as the inspiratory time, the Tlow as the expiratory time, the Phigh as inspiratory pressure, and the Plow as PEEP.
Hence, APRV is an extreme form of inverse ratio ventilation, with an inspiration-to-expiration ratio of 4:1. This means a patient spends most of the time in Phigh and Thigh, and exhalations are short (Tlow and Plow). In contrast, the biphasic mode uses conventional inspiration-expiration ratios (Figure 4).
As with any form of pressure control, the tidal volume is generated by airway pressure rising above baseline (ie, the end-expiratory value). Hence, to ensure an increase in minute ventilation, the mandatory breath rate must be increased (ie, decreasing Thigh, Tlow, or both) or the tidal volume must be increased (ie, increasing the difference between Phigh and Plow). This means that in APRV the Tlow has to happen more often (by increasing the number of breaths) or be more prolonged (allowing more air to exhale). Because unrestricted spontaneous breaths are permitted at any point of the cycle, the patient contributes to the total minute ventilation (usually 10%–40%).
In APRV and biphasic mode, the operator’s set time and pressure in inspiration and expiration will be delivered regardless of the patient’s breathing efforts—the patient’s spontaneous breath does not trigger a mechanical breath. Some ventilators have automatic adjustments to improve the trigger synchrony.
Ventilator settings in APRV and biphasic mode
These modes require the setting of two pressure levels (Phigh and Plow) and two time durations (Thigh and Tlow). One can add pressure support or automatic tube compensation to assist spontaneous breaths. The difference in Tlow generates differences in the Thigh:Tlow ratio: APRV has a short Tlow (an inspiration-expiration ratio of 4:1). Biphasic mode has a conventional inspiration-expiration ratio of 1:1 to 1:4.
Clinical applications
APRV is used in acute lung injury and ARDS. This mode should be used with caution or not at all in patients with obstructive lung disease or inappropriately increased respiratory drive.32–35
Biphasic mode is intended for both ventilation and weaning. In a patient who has poor respiratory effort or who is paralyzed, biphasic is identical to pressure-control/continuous mandatory ventilation.
Theoretical benefits of APRV and biphasic mode
Multiple benefits have been ascribed to these modes. In theory, APRV will maximize and maintain alveolar recruitment, improve oxygenation, lower inflation pressures, and decrease overinflation. Both APRV and biphasic, by preserving spontaneous breathing, will improve ventilation-perfusion matching and gas diffusion, improve the hemodynamic profile (less need for vasopressors, higher cardiac output, reduced ventricular workload, improved organ perfusion), and improve synchrony (decrease the work of breathing and the need for sedation).
Evidence of benefit of APRV and biphasic mode
APRV and biphasic are different modes. However studies evaluating their effects are combined. This is in part the result of the nomenclature confusion and different practice in different countries.36
Physiologic benefits. In studies, spontaneous breaths contributed to 10% to 40% of minute ventilation,37,38 improved ventilation of dependent areas of the lung, improved ventilation-perfusion match and recruitment,39 and improved hemodynamic profile.40
Patient comfort. These modes are thought to decrease the need for analgesia and sedation,38 but a recent trial showed no difference with pressure-controlled intermittent mandatory ventilation.41 Patient ventilator synchrony and comfort have not been studied.32,42
Outcomes. In small trials, these modes made no difference in terms of deaths, but they may decrease the length of mechanical ventilation.38,41,43,44
APRV and biphasic mode: Bottom line
Maintaining spontaneous breathing while on mechanical ventilation has hemodynamic and ventilatory benefits.
APRV and biphasic mode are not the same thing. APRV’s main goal is to maximize mean airway pressure and, hence, lung recruitment, whereas the main goal of the biphasic mode is synchrony.
There is a plethora of ventilator settings and questions related to physiologic effects.33,34,36
Although these modes are widely used in some centers, there is no evidence yet that they are superior to conventional volume- or pressure-control ventilation with low tidal volume for ARDS and acute lung injury. There is no conclusive evidence that these modes improve synchrony, time to weaning, or patient comfort.
HIGH-FREQUENCY OSCILLATORY VENTILATION
High-frequency oscillatory ventilation (HFOV) was first described and patented in 1952 by Emerson and was clinically developed in the early 1970s by Lunkenheimer.45
The goal of HFOV is to minimize lung injury; its characteristics (discussed below) make it useful in patients with severe ARDS. The US Food and Drug Administration approved it for infants in 1991 and for children in 1995. The adult model has been available since 1993, but it was not approved until 2001 (SensorMedics 3100B, Cardinal Health, Inc).
Other names for high-frequency oscillatory ventilation
While HFOV has no alternative names, the following acronyms describe similar modes:
- HFPPV (high-frequency positive pressure ventilation)
- HFJV (high-frequency jet ventilation)
- HFFI (high-frequency flow interruption)
- HFPV (high-frequency percussive ventilation)
- HFCWO (high-frequency chest wall oscillation).
All of these modes require different specialized ventilators.
What does high-frequency oscillatory ventilation do?
Conceptually, HFOV is a form of pressure-controlled intermittent mandatory ventilation with a set-point control scheme. In contrast to conventional pressure-controlled intermittent mandatory ventilation, in which relatively small spontaneous breaths may be superimposed on relatively large mandatory breaths, HFOV superimposes very small mandatory breaths (oscillations) on top of spontaneous breaths.
Adult patients are usually paralyzed or deeply sedated, since deep spontaneous breathing will trigger alarms and affect ventilator performance.
To manage ventilation (CO2 clearance), one or several of the following maneuvers can be done: decrease the oscillation frequency, increase the amplitude of the oscillations, increase the inspiratory time, or increase bias flow (while allowing an endotracheal tube cuff leak). Oxygenation adjustments are controlled by manipulating the mean airway pressure and the Fio2.
Ventilator settings in high-frequency oscillatory ventilation
Ventilator settings in HFOV are46:
- Airway pressure amplitude (delta P or power)
- Mean airway pressure
- Percent inspiration
- Inspiratory bias flow
- Fio2.
Clinical applications of high-frequency oscillatory ventilation
This mode is usually reserved for ARDS patients for whom conventional ventilation is failing. A recently published protocol46 suggests considering HFOV when there is oxygenation failure (Fio2 ≥ 0.7 and PEEP ≥ 14 cm H2O) or ventilation failure (pH < 7.25 with tidal volume ≥ 6 mL/kg predicted body weight and plateau airway pressure ≥ 30 cm H2O).
This mode is contraindicated when there is known severe airflow obstruction or intracranial hypertension.
Theoretical benefits of high-frequency oscillatory ventilation
Conceptually, HFOV can provide the highest mean airway pressure paired with the lowest tidal volume of any mode. These benefits might make HFOV the ideal lung-protective ventilation strategy.
Evidence of benefit of high-frequency oscillatory ventilation
Physiologic benefits. Animal models have shown less histologic damage and lung inflammation with HFOV than with high-tidal-volume conventional ventilation47,48 and low-tidal-volume conventional ventilation.49
Patient comfort has not been studied. However, current technology does impose undue work of breathing in spontaneously breathing patients.50
Outcomes. Several retrospective case series have described better oxygenation with HFOV as rescue therapy for severe ARDS than with conventional mechanical ventilation. Two randomized controlled trials have studied HFOV vs high-tidal-volume conventional mechanical ventilation for early severe ARDS; HFOV was safe but made no difference in terms of deaths.42,51–54
High-frequency oscillatory ventilation: Bottom line
In theory, HFOV provides all the benefits of an ideal lung-protective strategy, at least for paralyzed or deeply sedated patients. Animal studies support these concepts. In human adults, HFOV has been shown to be safe and to provide better oxygenation but no improvement in death rates compared with conventional mechanical ventilation. Currently, HFOV is better reserved for patients with severe ARDS for whom conventional mechanical ventilation is failing.
Technologic advances and computerized control of mechanical ventilators have made it possible to deliver ventilatory assistance in new modes. Driving these innovations is the desire to prevent ventilator-induced lung injury, improve patient comfort, and liberate the patient from mechanical ventilation as soon as possible.
We call these innovations “alternative” modes to differentiate them from the plain volume-control and pressure-control modes. Some clinicians rarely use these new modes, but in some medical centers they have become the most common ones used, or are being used unknowingly (the operator misunderstands the mode name). The information we provide on these modes of ventilation is by no means an endorsement of their use, but rather a tool to help the clinician understand their physiologic, theoretical, and clinical effects.
We focused on two goals:
- Explain what the mode does
- Briefly review the theoretical benefits and the actual evidence supporting these alternative modes of ventilation.

STANDARD NOMENCLATURE NEEDED
Since its invention, mechanical ventilation has been plagued by multiple names being used to describe the same things. For example, volume-control ventilation is also called volume-cycled ventilation, assist-control ventilation, volume-limited ventilation, and controlled mechanical ventilation. Similarly, multiple abbreviations are used, each depending on the brand of ventilator, and new acronyms have been added in recent years as new modes have been developed. The vast number of names and modes can confuse even the most seasoned critical care physician.
Efforts to establish a common nomenclature are under way.1
WHAT IS A MODE?
A mode of mechanical ventilation has three essential components:
- The control variable
- The breath sequence
- The targeting scheme.
Similar modes may require more detailed descriptions to distinguish them, but the basic function can be explained by these three components.
The control variable
In general, inspiration is an active process, driven by the patient’s effort, the ventilator, or both, while expiration is passive. For simplicity, in this article a mechanical breath means the inspiratory phase of the breath.
The machine can only control the volume (and flow) or the pressure given. The breaths can be further described on the basis of what triggers the breath, what limits it (the maximum value of a control variable), and what ends (cycles) it.
The breath sequence
There are three possible breath sequences:
- Intermittent mandatory ventilation, in which the patient can take spontaneous breaths between mandatory breaths
- Continuous spontaneous ventilation, in which all breaths are spontaneous (Table 1).
The targeting scheme
The targeting or feedback scheme refers to the ventilator settings and programming that dictate its response to the patient’s lung compliance, lung resistance, and respiratory effort. The regulation can be as simple as controlling the pressure in pressure-control mode, or it can be based on a complicated algorithm.
ADAPTIVE PRESSURE CONTROL
Other names for adaptive pressure control
- Pressure Regulated Volume Control (Maquet Servo-i, Rastatt, Germany)
- AutoFlow (Dräger Medical AG, Lübeck, Germany)
- Adaptive Pressure Ventilation (Hamilton Galileo, Hamilton Medical AG, Bonaduz, Switzerland)
- Volume Control+ (Puritan Bennett, Tyco Healthcare; Mansfield, MA)
- Volume Targeted Pressure Control, Pressure Controlled Volume Guaranteed (Engström, General Electric, Madison, WI).
What does adaptive pressure control do?
The APC mode delivers pressure-controlled breaths with an adaptive targeting scheme (Table 2).
In pressure-control ventilation, tidal volumes depend on the lung’s physiologic mechanics (compliance and resistance) and patient effort (Figure 1). Therefore, the tidal volume varies with changes in lung physiology (ie, larger or smaller tidal volumes than targeted).
To overcome this effect, a machine in APC mode adjusts the inspiratory pressure to deliver the set minimal target tidal volume. If tidal volume increases, the machine decreases the inspiratory pressure, and if tidal volume decreases, the machine increases the inspiratory pressure. However, if the patient effort is large enough, the tidal volume will increase in spite of decreasing the inspiratory pressure (Figure 2). The adjustments to the inspiratory pressure occur after the tidal volume is off-target in a number of breaths.
Common sources of confusion with adaptive pressure control
First, APC is not a volume-control mode. In volume control, the tidal volume does not change; in APC the tidal volume can increase or decrease, and the ventilator will adjust the inflation pressure to achieve the target volume. Thus, APC guarantees an average minimum tidal volume but not a maximum tidal volume.
Second, a characteristic of pressure control (and hence, APC) is that the flow of gas varies to maintain constant airway pressure (ie, maintain the set inspiratory pressure). This characteristic allows a patient who generates an inspiratory effort to receive flow as demanded, which is likely more comfortable. This is essentially different from volume control, in which flow is set by the operator and hence is fixed. Thus, if the patient effort is strong enough (Figure 1), this leads to what is called flow asynchrony, in which the patient does not get the flow asked for in a breath.
Ventilator settings in adaptive pressure control
Ventilator settings in APC are:
- Tidal volume
- Time spent in inspiration (inspiratory time)
- Frequency
- Fraction of inspired oxygen (Fio2)
- Positive end-expiratory pressure (PEEP).
Some ventilators also require setting the speed to reach the peak pressure (also known as slope percent or inspiratory rise time).
Clinical applications of adaptive pressure control
This mode is designed to maintain a consistent tidal volume during pressure-control ventilation and to promote inspiratory flow synchrony. It is a means of automatically reducing ventilatory support (ie, weaning) as the patient’s inspiratory effort becomes stronger, as in awakening from anesthesia.
APC may not be ideal for patients who have an inappropriately increased respiratory drive (eg, in severe metabolic acidosis), since the inspiratory pressure will decrease to maintain the targeted average tidal volume, inappropriately shifting the work of breathing onto the patient.
Theoretical benefits of adaptive pressure control
APC guarantees a minimum average tidal volume (unless the pressure alarm threshold is set too low, so that the target tidal volume is not delivered). Other theoretical benefits are flow synchrony, less ventilator manipulation by the operator, and automatic weaning of ventilator support.
Evidence of benefit of adaptive pressure control
Physiologic benefits. This mode has lower peak inspiratory pressures than does volume-control ventilation,3,4 which is often reported as a positive finding. However, in volume-control mode (the usual comparator), the peak inspiratory pressure is a manifestation of both resistance and compliance. Hence, peak inspiratory pressure is expected to be higher but does not reflect actual lung-distending pressures. It is the plateau pressure, a manifestation of lung compliance, that is related to lung injury.
Patient comfort. APC may increase the work of breathing when using low tidal volume ventilation and when there is increased respiratory effort (drive).5 Interestingly, APC was less comfortable than pressure support ventilation in a small trial.6
Outcomes have not been studied.7
Adaptive pressure control: Bottom line
APC is widely available and widely used, sometimes unknowingly (eg, if the operator thinks it is volume control). It is relatively easy to use and to set; however, evidence of its benefit is scant.
ADAPTIVE SUPPORT VENTILATION
Adaptive support ventilation (ASV) evolved as a form of mandatory minute ventilation implemented with adaptive pressure control. Mandatory minute ventilation is a mode that allows the operator to preset a target minute ventilation, and the ventilator then supplies mandatory breaths, either volume- or pressure-controlled, if the patient’s spontaneous breaths generate a lower minute ventilation.
ASV automatically selects the appropriate tidal volume and frequency for mandatory breaths and the appropriate tidal volume for spontaneous breaths on the basis of the respiratory system mechanics and target minute alveolar ventilation.
Described in 1994 by Laubscher et al,8,9 ASV became commercially available in 1998 in Europe and in 2007 in the United States (Hamilton Galileo ventilator, Hamilton Medical AG). This is the first commercially available ventilator that uses an “optimal” targeting scheme (see below).
What does adaptive support ventilation do?
ASV delivers pressure-controlled breaths using an adaptive (optimal) scheme (Table 2). “Optimal,” in this context, means minimizing the mechanical work of breathing: the machine selects a tidal volume and frequency that the patient’s brain would presumably select if the patient were not connected to a ventilator. This pattern is assumed to encourage the patient to generate spontaneous breaths.
The ventilator calculates the normal required minute ventilation based on the patient’s ideal weight and estimated dead space volume (ie, 2.2 mL/kg). This calculation represents 100% of minute ventilation. The clinician at the bedside sets a target percent of minute ventilation that the ventilator will support—higher than 100% if the patient has increased requirements due, eg, to sepsis or increased dead space, or less than 100% during weaning.
The ventilator initially delivers test breaths, in which it measures the expiratory time constant for the respiratory system and then uses this along with the estimated dead space and normal minute ventilation to calculate an optimal breathing frequency in terms of mechanical work.
The optimal or target tidal volume is calculated as the normal minute ventilation divided by the optimal frequency. The target tidal volume is achieved by the use of APC (see above) (Figure 2). This means that the pressure limit is automatically adjusted to achieve an average delivered tidal volume equal to the target. The ventilator continuously monitors the respiratory system mechanics and adjusts its settings accordingly.
The ventilator adjusts its breaths to avoid air trapping by allowing enough time to exhale, to avoid hypoventilation by delivering tidal volume greater than the dead space, and to avoid volutrauma by avoiding large tidal volumes.
Ventilator settings in adaptive support ventilation
Ventilator settings in ASV are:
- Patient height (to calculate the ideal body weight)
- Sex
- Percent of normal predicted minute ventilation goal
- Fio2
- PEEP.
Clinical applications of adaptive support ventilation
ASV is intended as a sole mode of ventilation, from initial support to weaning.
Theoretical benefits of adaptive support ventilation
In theory, ASV offers automatic selection of ventilator settings, automatic adaptation to changing patient lung mechanics, less need for human manipulation of the machine, improved synchrony, and automatic weaning.
Evidence of benefit of adaptive support ventilation
Physiologic benefits. Ventilator settings are adjusted automatically. ASV selects different tidal volume-respiratory rate combinations based on respiratory mechanics in passive and paralyzed patients.10–12 In actively breathing patients, there was no difference in the ventilator settings chosen by ASV for different clinical scenarios (and lung physiology).10 Compared with pressure-controlled intermittent mandatory ventilation, with ASV, the inspiratory load is less and patient-ventilator interaction is better.13
Patient-ventilator synchrony and comfort have not been studied.
Outcomes. Two trials suggest that ASV may decrease time on mechanical ventilation.14,15 However, in another trial,16 compared with a standard protocol, ASV led to fewer ventilator adjustments but achieved similar postsurgical weaning outcomes. The effect of this mode on the death rate has not been examined.17,18
Adaptive support ventilation: Bottom line
ASV is the first commercially available mode that automatically selects all the ventilator settings except PEEP and Fio2. These seem appropriate for different clinical scenarios in patients with poor respiratory effort or in paralyzed patients. Evidence of the effect in actively breathing patients and on outcomes such as length of stay or death is still lacking.
PROPORTIONAL ASSIST VENTILATION
Patients who have normal respiratory drive but who have difficulty sustaining adequate spontaneous ventilation are often subjected to pressure support ventilation (PSV), in which the ventilator generates a constant pressure throughout inspiration regardless of the intensity of the patient’s effort.
In 1992, Younes and colleagues19,20 developed proportional assist ventilation (PAV) as an alternative in which the ventilator generates pressure in proportion to the patient’s effort. PAV became commercially available in Europe in 1999 and was approved in the United States in 2006, available on the Puritan Bennett 840 ventilator (Puritan Bennett Co, Boulder, CO). PAV has also been used for noninvasive ventilation, but this is not available in the United States.
Other names for proportional assist ventilation
Proportional Pressure Support (Dräger Medical; not yet available in the United States).
What does proportional assist ventilation do?
This mode delivers pressure-controlled breaths with a servo control scheme (Table 2).
To better understand PAV, we can compare it with PSV. With PSV, the pressure applied by the ventilator rises to a preset level that is held constant (a set-point scheme) until a cycling criterion (a percent of the maximum inspiratory flow value) is reached. The inspiratory flow and tidal volume are the result of the patient’s inspiratory effort, the level of pressure applied, and the respiratory system mechanics.
In PAV, as in PSV, all breaths are spontaneous (Table 1). The patient controls the timing and size of the breath. There are no preset pressure, flow, or volume goals, but safety limits on the volume and pressure delivered can be set.
Ventilator settings in proportional assist ventilation
Ventilator settings in PAV are:
- Airway type (endotracheal tube, tracheostomy)
- Airway size (inner diameter)
- Percentage of work supported (assist range 5%–95%)
- Tidal volume limit
- Pressure limit
- Expiratory sensitivity (normally, as inspiration ends, flow should stop; this parameter tells the ventilator at what flow to end inspiration).
Caution when assessing the literature. Earlier ventilator versions, ie, Dräger and Manitoba (University of Manitoba, Winnipeg, MB, Canada), which are not available in the United States, required the repeated calculation of the respiratory system mechanics and the manual setting of flow and volume assists (amplification factors) independently. To overcome this limitation, new software automatically adjusts the flow and volume amplification to support the loads imposed by the automatically measured values of resistance and elastance (inverse of compliance) of the respiratory system.21 This software is included in the model (Puritan Bennett) available in the United States.
Clinical applications of proportional assist ventilation
The PAV mode is indicated for maximizing ventilator patient synchrony for assisted spontaneous ventilation.
PAV is contraindicated in patients with respiratory depression (bradypnea) or large air leaks (eg, bronchopleural fistulas). It should be used with caution in patients with severe hyperinflation, in which the patient may still be exhaling but the ventilator doesn’t recognize it. Another group in which PAV should be used with caution is those with high ventilatory drives, in which the ventilator overestimates respiratory system mechanics. This situation can lead to overassistance due to the “runaway phenomenon,” in which the ventilator continues to provide support even if the patient has stopped inspiration.22
Theoretical benefits of proportional assist ventilation
In theory, PAV should reduce the work of breathing, improve synchrony, automatically adapt to changing patient lung mechanics and effort, decrease the need for ventilator intervention and manipulation, decrease the need for sedation, and improve sleep.
Evidence of benefit of proportional assist ventilation
Physiologic benefits. PAV reduces the work of breathing better than PSV,21 even in the face of changing respiratory mechanics or increased respiratory demand (hypercapnia).23–25 The hemodynamic profile is similar to that in PSV. Tidal volumes are variable; however, in recent reports the tidal volumes were within the lung-protective range (6–8 mL/kg, plateau pressure < 30 cm H20).26,27
Comfort. PAV entails less patient effort and discomfort that PSV does.23,25 PAV significantly reduces asynchrony,27 which in turn may favorably affect sleep in critically ill patients. 28
Outcomes. The probability of spontaneous breathing without assistance was significantly better in critically ill patients ventilated with PAV than with PSV. No trial has reported the effect of PAV on deaths.27,29
Proportional assist ventilation: Bottom line
Extensive basic research has been done with PAV in different forms of respiratory failure, such as obstructive lung disease, acute respiratory distress syndrome (ARDS), and chronic respiratory failure. It fulfills its main goal, which is to improve patient-ventilator synchrony. Clinical experience with PAV in the United States is limited, as it was only recently approved.
AIRWAY PRESSURE-RELEASE VENTILATION AND BIPHASIC POSITIVE AIRWAY PRESSURE
Airway pressure-release ventilation (APRV) was described in 1987 by Stock et al30 as a mode for delivering ventilation in acute lung injury while avoiding high airway pressures. APRV combines high constant positive airway pressure (improving oxygenation and promoting alveolar recruitment) with intermittent releases (causing exhalation).
Other names for biphasic positive airway pressure
Other names for biphasic positive airway pressure are:
- BiLevel (Puritan Bennett)
- BIPAP (Dräger Europe)
- Bi Vent (Siemens)
- BiPhasic (Avea, Cardinal Health, Inc, Dublin, OH)
- PCV+ (Dräger Medical)
- DuoPAP (Hamilton).
Caution—name confusion. In North America, BiPAP (Respironics, Murrysville, PA) and BiLevel are used to refer to noninvasive modes of ventilation.
APRV has no other name.
What do these modes do?
These modes deliver pressure-controlled, time-triggered, and time-cycled breaths using a set-point targeting scheme (Table 2). This means that the ventilator maintains a constant pressure (set point) even in the face of spontaneous breaths.
Caution—source of confusion. The term continuous positive airway pressure (CPAP) is often used to describe this mode. However, CPAP is pressure that is applied continuously at the same level; the patient generates all the work to maintain ventilation (“pressure-controlled continuous spontaneous ventilation” in the current nomenclature). In APRV, the airway pressure is intermittently released and reapplied, generating a tidal volume that supports ventilation. In other words, this is a pressure-controlled breath with a very prolonged inspiratory time and a short expiratory time in which spontaneous ventilation is possible at any point (“pressure-controlled intermittent mandatory ventilation” in the current nomenclature).
How these modes are set in the ventilator may also be a source of confusion. To describe the time spent in high and low airway pressures, we use the terms Thigh and Tlow, respectively. By convention, the difference between APRV and biphasic mode is the duration of Tlow (< 1.5 sec for APRV).
Similarly, Phigh and Plow are used to describe the high and low airway pressure. To better understand this concept, you can create the same mode in conventional pressure-control ventilation by thinking of the Thigh as the inspiratory time, the Tlow as the expiratory time, the Phigh as inspiratory pressure, and the Plow as PEEP.
Hence, APRV is an extreme form of inverse ratio ventilation, with an inspiration-to-expiration ratio of 4:1. This means a patient spends most of the time in Phigh and Thigh, and exhalations are short (Tlow and Plow). In contrast, the biphasic mode uses conventional inspiration-expiration ratios (Figure 4).
As with any form of pressure control, the tidal volume is generated by airway pressure rising above baseline (ie, the end-expiratory value). Hence, to ensure an increase in minute ventilation, the mandatory breath rate must be increased (ie, decreasing Thigh, Tlow, or both) or the tidal volume must be increased (ie, increasing the difference between Phigh and Plow). This means that in APRV the Tlow has to happen more often (by increasing the number of breaths) or be more prolonged (allowing more air to exhale). Because unrestricted spontaneous breaths are permitted at any point of the cycle, the patient contributes to the total minute ventilation (usually 10%–40%).
In APRV and biphasic mode, the operator’s set time and pressure in inspiration and expiration will be delivered regardless of the patient’s breathing efforts—the patient’s spontaneous breath does not trigger a mechanical breath. Some ventilators have automatic adjustments to improve the trigger synchrony.
Ventilator settings in APRV and biphasic mode
These modes require the setting of two pressure levels (Phigh and Plow) and two time durations (Thigh and Tlow). One can add pressure support or automatic tube compensation to assist spontaneous breaths. The difference in Tlow generates differences in the Thigh:Tlow ratio: APRV has a short Tlow (an inspiration-expiration ratio of 4:1). Biphasic mode has a conventional inspiration-expiration ratio of 1:1 to 1:4.
Clinical applications
APRV is used in acute lung injury and ARDS. This mode should be used with caution or not at all in patients with obstructive lung disease or inappropriately increased respiratory drive.32–35
Biphasic mode is intended for both ventilation and weaning. In a patient who has poor respiratory effort or who is paralyzed, biphasic is identical to pressure-control/continuous mandatory ventilation.
Theoretical benefits of APRV and biphasic mode
Multiple benefits have been ascribed to these modes. In theory, APRV will maximize and maintain alveolar recruitment, improve oxygenation, lower inflation pressures, and decrease overinflation. Both APRV and biphasic, by preserving spontaneous breathing, will improve ventilation-perfusion matching and gas diffusion, improve the hemodynamic profile (less need for vasopressors, higher cardiac output, reduced ventricular workload, improved organ perfusion), and improve synchrony (decrease the work of breathing and the need for sedation).
Evidence of benefit of APRV and biphasic mode
APRV and biphasic are different modes. However studies evaluating their effects are combined. This is in part the result of the nomenclature confusion and different practice in different countries.36
Physiologic benefits. In studies, spontaneous breaths contributed to 10% to 40% of minute ventilation,37,38 improved ventilation of dependent areas of the lung, improved ventilation-perfusion match and recruitment,39 and improved hemodynamic profile.40
Patient comfort. These modes are thought to decrease the need for analgesia and sedation,38 but a recent trial showed no difference with pressure-controlled intermittent mandatory ventilation.41 Patient ventilator synchrony and comfort have not been studied.32,42
Outcomes. In small trials, these modes made no difference in terms of deaths, but they may decrease the length of mechanical ventilation.38,41,43,44
APRV and biphasic mode: Bottom line
Maintaining spontaneous breathing while on mechanical ventilation has hemodynamic and ventilatory benefits.
APRV and biphasic mode are not the same thing. APRV’s main goal is to maximize mean airway pressure and, hence, lung recruitment, whereas the main goal of the biphasic mode is synchrony.
There is a plethora of ventilator settings and questions related to physiologic effects.33,34,36
Although these modes are widely used in some centers, there is no evidence yet that they are superior to conventional volume- or pressure-control ventilation with low tidal volume for ARDS and acute lung injury. There is no conclusive evidence that these modes improve synchrony, time to weaning, or patient comfort.
HIGH-FREQUENCY OSCILLATORY VENTILATION
High-frequency oscillatory ventilation (HFOV) was first described and patented in 1952 by Emerson and was clinically developed in the early 1970s by Lunkenheimer.45
The goal of HFOV is to minimize lung injury; its characteristics (discussed below) make it useful in patients with severe ARDS. The US Food and Drug Administration approved it for infants in 1991 and for children in 1995. The adult model has been available since 1993, but it was not approved until 2001 (SensorMedics 3100B, Cardinal Health, Inc).
Other names for high-frequency oscillatory ventilation
While HFOV has no alternative names, the following acronyms describe similar modes:
- HFPPV (high-frequency positive pressure ventilation)
- HFJV (high-frequency jet ventilation)
- HFFI (high-frequency flow interruption)
- HFPV (high-frequency percussive ventilation)
- HFCWO (high-frequency chest wall oscillation).
All of these modes require different specialized ventilators.
What does high-frequency oscillatory ventilation do?
Conceptually, HFOV is a form of pressure-controlled intermittent mandatory ventilation with a set-point control scheme. In contrast to conventional pressure-controlled intermittent mandatory ventilation, in which relatively small spontaneous breaths may be superimposed on relatively large mandatory breaths, HFOV superimposes very small mandatory breaths (oscillations) on top of spontaneous breaths.
Adult patients are usually paralyzed or deeply sedated, since deep spontaneous breathing will trigger alarms and affect ventilator performance.
To manage ventilation (CO2 clearance), one or several of the following maneuvers can be done: decrease the oscillation frequency, increase the amplitude of the oscillations, increase the inspiratory time, or increase bias flow (while allowing an endotracheal tube cuff leak). Oxygenation adjustments are controlled by manipulating the mean airway pressure and the Fio2.
Ventilator settings in high-frequency oscillatory ventilation
Ventilator settings in HFOV are46:
- Airway pressure amplitude (delta P or power)
- Mean airway pressure
- Percent inspiration
- Inspiratory bias flow
- Fio2.
Clinical applications of high-frequency oscillatory ventilation
This mode is usually reserved for ARDS patients for whom conventional ventilation is failing. A recently published protocol46 suggests considering HFOV when there is oxygenation failure (Fio2 ≥ 0.7 and PEEP ≥ 14 cm H2O) or ventilation failure (pH < 7.25 with tidal volume ≥ 6 mL/kg predicted body weight and plateau airway pressure ≥ 30 cm H2O).
This mode is contraindicated when there is known severe airflow obstruction or intracranial hypertension.
Theoretical benefits of high-frequency oscillatory ventilation
Conceptually, HFOV can provide the highest mean airway pressure paired with the lowest tidal volume of any mode. These benefits might make HFOV the ideal lung-protective ventilation strategy.
Evidence of benefit of high-frequency oscillatory ventilation
Physiologic benefits. Animal models have shown less histologic damage and lung inflammation with HFOV than with high-tidal-volume conventional ventilation47,48 and low-tidal-volume conventional ventilation.49
Patient comfort has not been studied. However, current technology does impose undue work of breathing in spontaneously breathing patients.50
Outcomes. Several retrospective case series have described better oxygenation with HFOV as rescue therapy for severe ARDS than with conventional mechanical ventilation. Two randomized controlled trials have studied HFOV vs high-tidal-volume conventional mechanical ventilation for early severe ARDS; HFOV was safe but made no difference in terms of deaths.42,51–54
High-frequency oscillatory ventilation: Bottom line
In theory, HFOV provides all the benefits of an ideal lung-protective strategy, at least for paralyzed or deeply sedated patients. Animal studies support these concepts. In human adults, HFOV has been shown to be safe and to provide better oxygenation but no improvement in death rates compared with conventional mechanical ventilation. Currently, HFOV is better reserved for patients with severe ARDS for whom conventional mechanical ventilation is failing.
- Chatburn RL. Classification of ventilator modes: update and proposal for implementation. Respir Care 2007; 52:301–323.
- Chatburn RL. Computer control of mechanical ventilation. Respir Care 2004; 49:507–517.
- Alvarez A, Subirana M, Benito S. Decelerating flow ventilation effects in acute respiratory failure. J Crit Care 1998; 13:21–25.
- Guldager H, Nielsen SL, Carl P, Soerensen MB. A comparison of volume control and pressure regulated volume control ventilation in acute respiratory failure. Crit Care 1997; 1:75–77.
- Kallet RH, Campbell AR, Dicker RA, Katz JA, Mackersie RC. Work of breathing during lung protective ventilation in patients with acute lung injury and acute respiratory distress syndrome: a comparison between volume and pressure regulated breathing modes. Respir Care 2005; 50:1623–1631.
- Betensley AD, Khalid I, Crawford J, Pensler RA, DiGiovine B. Patient comfort during pressure support and volume controlled continuous mandatory ventilation. Respir Care 2008; 53:897–902.
- Branson RD, Chatburn RL. Controversies in the critical care setting. Should adaptive pressure control modes be utilized for virtually all patients receiving mechanical ventilation? Respir Care 2007; 52:478–485.
- Laubscher TP, Frutiger A, Fanconi S, Jutzi H, Brunner JX. Automatic selection of tidal volume, respiratory frequency and minute ventilation in intubated ICU patients as start up procedure for closed-loop controlled ventilation. Int J Clin Monit Comput 1994; 11:19–30.
- Laubscher TP, Heinrichs W, Weiler N, Hartmann G, Brunner JX. An adaptive lung ventilation controller. IEEE Trans Biomed Eng 1994; 41:51–59.
- Arnal JM, Wysocki M, Nafati C, et al. Automatic selection of breathing pattern using adaptive support ventilation. Intensive Care Med 2008; 34:75–81.
- Campbell RS, Sinamban RP, Johannigman JA, et al. Clinical evaluation of a new closed loop ventilation mode: adaptive supportive ventilation (ASV). Crit Care 1999; 3( suppl 1):083.
- Belliato M, Palo A, Pasero D, Iotti GA, Mojoli F, Braschi A. Evaluation of adaptive support ventilation in paralysed patients and in a physical lung model. Int J Artif Organs 2004; 27:709–716.
- Tassaux D, Dalmas E, Gratadour P, Jolliet P. Patient ventilator interactions during partial ventilatory support: a preliminary study comparing the effects of adaptive support ventilation with synchronized intermittent mandatory ventilation plus inspiratory pressure support. Crit Care Med 2002; 30:801–807.
- Gruber PC, Gomersall CD, Leung P, et al. Randomized controlled trial comparing adaptive-support ventilation with pressure-regulated volume-controlled ventilation with automode in weaning patients after cardiac surgery. Anesthesiology 2008; 109:81–87.
- Sulzer CF, Chiolero R, Chassot PG, et al. Adaptive support ventilation for fast tracheal extubation after cardiac surgery: a randomized controlled study. Anesthesiology 2001; 95:1339–1345.
- Petter AH, Chiolèro RL, Cassina T, Chassot PG, Müller XM, Revelly JP. Automatic “respirator/weaning” with adaptive support ventilation: the effect on duration of endotracheal intubation and patient management. Anesth Analg 2003; 97:1743–1750.
- Brunner JX, Iotti GA. Adaptive support ventilation (ASV). Minerva Anestesiol 2002; 68:365–368.
- Campbell RS, Branson RD, Johannigman JA. Adaptive support ventilation. Respir Care Clin North Am 2001; 7:425–440.
- Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis 1992; 145:114–120.
- Younes M, Puddy A, Roberts D, et al. Proportional assist ventilation. Results of an initial clinical trial. Am Rev Respir Dis 1992; 145:121–129.
- Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Respiratory load compensation during mechanical ventilatio—proportional assist ventilation with load-adjustable gain factors versus pressure support. Intensive Care Med 2006; 32:692–699.
- Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Effect of different levels of pressure support and proportional assist ventilation on breathing pattern, work of breathing and gas exchange in mechanically ventilated hypercapnic COPD patients with acute respiratory failure. Respiration 2003; 70:355–361.
- Grasso S, Puntillo F, Mascia L, et al. Compensation for increase in respiratory workload during mechanical ventilation. Pressure support versus proportional assist ventilation. Am J Respir Crit Care Med 2000; 161:819–826.
- Wrigge H, Golisch W, Zinserling J, Sydow M, Almeling G, Burchardi H. Proportional assist versus pressure support ventilation: effects on breathing pattern and respiratory work of patients with chronic obstructive pulmonary disease. Intensive Care Med 1999; 25:790–798.
- Ranieri VM, Giuliani R, Mascia L, et al. Patient ventilator interaction during acute hypercapnia: pressure support vs. proportional assist ventilation. J Appl Physiol 1996; 81:426–436.
- Kondili E, Xirouchaki N, Vaporidi K, Klimathianaki M, Georgopoulos D. Short-term cardiorespiratory effects of proportional assist and pressure support ventilation in patients with acute lung injury/acute respiratory distress syndrome. Anesthesiology 2006; 105:703–708.
- Xirouchaki N, Kondili E, Vaporidi K, et al. Proportional assist ventilation with load-adjustable gain factors in critically ill patients: comparison with pressure support. Intensive Care Med 2008; 34:2026–2034.
- Bosma K, Ferreyra G, Ambrogio C, et al. Patient ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med 2007; 35:1048–1054.
- Sinderby C, Beck J. Proportional assist ventilation and neurally adjusted ventilatory assist—better approaches to patient ventilator synchrony? Clin Chest Med 2008; 29:329–342.
- Stock MC, Downs JB, Frolicher DA. Airway pressure release ventilation. Crit Care Med 1987; 15:462–466.
- Baum M, Benzer H, Putensen C, Koller W, Putz G. [Biphasic positive airway pressure (BIPAP)—a new form of augmented ventilation]. Anaesthesist 1989; 38:452–458.
- Seymour CW, Frazer M, Reilly PM, Fuchs BD. Airway pressure release and biphasic intermittent positive airway pressure ventilation: are they ready for prime time? J Trauma 2007; 62:1298–1308.
- Myers TR, MacIntyre NR. Respiratory controversies in the critical care setting. Does airway pressure release ventilation offer important new advantages in mechanical ventilator support? Respir Care 2007; 52:452–458.
- Neumann P, Golisch W, Strohmeyer A, Buscher H, Burchardi H, Sydow M. Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive Care Med 2002; 28:1742–1749.
- Calzia E, Lindner KH, Witt S, et al. Pressure-time product and work of breathing during biphasic continuous positive airway pressure and assisted spontaneous breathing. Am J Respir Crit Care Med 1994; 150:904–910.
- Rose L, Hawkins M. Airway pressure release ventilation and biphasic positive airway pressure: a systematic review of definitional criteria. Intensive Care Med 2008; 34:1766–1773.
- Sydow M, Burchardi H, Ephraim E, Zielmann S, Crozier TA. Longterm effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med 1994; 149:1550–1556.
- Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 2001; 164:43–49.
- Davis K, Johnson DJ, Branson RD, Campbell RS, Johannigman JA, Porembka D. Airway pressure release ventilation. Arch Surg 1993; 128:1348–1352.
- Kaplan LJ, Bailey H, Formosa V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care 2001; 5:221–226.
- Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettilä VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand 2004; 48:722–731.
- Siau C, Stewart TE. Current role of high frequency oscillatory ventilation and airway pressure release ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med 2008; 29:265–275.
- Rathgeber J, Schorn B, Falk V, Kazmaier S, Spiegel T, Burchardi H. The influence of controlled mandatory ventilation (CMV), intermittent mandatory ventilation (IMV) and biphasic intermittent positive airway pressure (BIPAP) on duration of intubation and consumption of analgesics and sedatives. A prospective analysis in 596 patients following adult cardiac surgery. Eur J Anaesthesiol 1997; 14:576–582.
- Habashi NM. Other approaches to open lung ventilation: airway pressure release ventilation. Crit Care Med 2005; 33 suppl 3:S228–S240.
- Hess D, Mason S, Branson R. High-frequency ventilation design and equipment issues. Respir Care Clin North Am 2001; 7:577–598.
- Fessler HE, Derdak S, Ferguson ND, et al. A protocol for high frequency oscillatory ventilation in adults: results from a roundtable discussion. Crit Care Med 2007; 35:1649–1654.
- Hamilton PP, Onayemi A, Smyth JA, et al. Comparison of conventional and high-frequency ventilation: oxygenation and lung pathology. J Appl Physiol 1983; 55:131–138.
- Sedeek KA, Takeuchi M, Suchodolski K, et al. Open-lung protective ventilation with pressure control ventilation, high-frequency oscillation, and intratracheal pulmonary ventilation results in similar gas exchange, hemodynamics, and lung mechanics. Anesthesiology 2003; 99:1102–1111.
- Imai Y, Nakagawa S, Ito Y, Kawano T, Slutsky AS, Miyasaka K. Comparison of lung protection strategies using conventional and high-frequency oscillatory ventilation. J Appl Physiol 2001; 91:1836–1844.
- van Heerde M, Roubik K, Kopelent V, Plötz FB, Markhorst DG. Unloading work of breathing during high-frequency oscillatory ventilation: a bench study. Crit Care 2006; 10:R103.
- Derdak S, Mehta S, Stewart TE, et al., Multicenter Oscillatory Ventilation For Acute Respiratory Distress Syndrome Trial (MOAT) Study Investigators. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med 2002; 166:801–808.
- Bollen CW, van Well GT, Sherry T, et al. High-frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care 2005; 9:R430–R439.
- Mehta S, Granton J, MacDonald RJ, et al. High frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
- Chatburn RL. Classification of ventilator modes: update and proposal for implementation. Respir Care 2007; 52:301–323.
- Chatburn RL. Computer control of mechanical ventilation. Respir Care 2004; 49:507–517.
- Alvarez A, Subirana M, Benito S. Decelerating flow ventilation effects in acute respiratory failure. J Crit Care 1998; 13:21–25.
- Guldager H, Nielsen SL, Carl P, Soerensen MB. A comparison of volume control and pressure regulated volume control ventilation in acute respiratory failure. Crit Care 1997; 1:75–77.
- Kallet RH, Campbell AR, Dicker RA, Katz JA, Mackersie RC. Work of breathing during lung protective ventilation in patients with acute lung injury and acute respiratory distress syndrome: a comparison between volume and pressure regulated breathing modes. Respir Care 2005; 50:1623–1631.
- Betensley AD, Khalid I, Crawford J, Pensler RA, DiGiovine B. Patient comfort during pressure support and volume controlled continuous mandatory ventilation. Respir Care 2008; 53:897–902.
- Branson RD, Chatburn RL. Controversies in the critical care setting. Should adaptive pressure control modes be utilized for virtually all patients receiving mechanical ventilation? Respir Care 2007; 52:478–485.
- Laubscher TP, Frutiger A, Fanconi S, Jutzi H, Brunner JX. Automatic selection of tidal volume, respiratory frequency and minute ventilation in intubated ICU patients as start up procedure for closed-loop controlled ventilation. Int J Clin Monit Comput 1994; 11:19–30.
- Laubscher TP, Heinrichs W, Weiler N, Hartmann G, Brunner JX. An adaptive lung ventilation controller. IEEE Trans Biomed Eng 1994; 41:51–59.
- Arnal JM, Wysocki M, Nafati C, et al. Automatic selection of breathing pattern using adaptive support ventilation. Intensive Care Med 2008; 34:75–81.
- Campbell RS, Sinamban RP, Johannigman JA, et al. Clinical evaluation of a new closed loop ventilation mode: adaptive supportive ventilation (ASV). Crit Care 1999; 3( suppl 1):083.
- Belliato M, Palo A, Pasero D, Iotti GA, Mojoli F, Braschi A. Evaluation of adaptive support ventilation in paralysed patients and in a physical lung model. Int J Artif Organs 2004; 27:709–716.
- Tassaux D, Dalmas E, Gratadour P, Jolliet P. Patient ventilator interactions during partial ventilatory support: a preliminary study comparing the effects of adaptive support ventilation with synchronized intermittent mandatory ventilation plus inspiratory pressure support. Crit Care Med 2002; 30:801–807.
- Gruber PC, Gomersall CD, Leung P, et al. Randomized controlled trial comparing adaptive-support ventilation with pressure-regulated volume-controlled ventilation with automode in weaning patients after cardiac surgery. Anesthesiology 2008; 109:81–87.
- Sulzer CF, Chiolero R, Chassot PG, et al. Adaptive support ventilation for fast tracheal extubation after cardiac surgery: a randomized controlled study. Anesthesiology 2001; 95:1339–1345.
- Petter AH, Chiolèro RL, Cassina T, Chassot PG, Müller XM, Revelly JP. Automatic “respirator/weaning” with adaptive support ventilation: the effect on duration of endotracheal intubation and patient management. Anesth Analg 2003; 97:1743–1750.
- Brunner JX, Iotti GA. Adaptive support ventilation (ASV). Minerva Anestesiol 2002; 68:365–368.
- Campbell RS, Branson RD, Johannigman JA. Adaptive support ventilation. Respir Care Clin North Am 2001; 7:425–440.
- Younes M. Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis 1992; 145:114–120.
- Younes M, Puddy A, Roberts D, et al. Proportional assist ventilation. Results of an initial clinical trial. Am Rev Respir Dis 1992; 145:121–129.
- Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Respiratory load compensation during mechanical ventilatio—proportional assist ventilation with load-adjustable gain factors versus pressure support. Intensive Care Med 2006; 32:692–699.
- Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Effect of different levels of pressure support and proportional assist ventilation on breathing pattern, work of breathing and gas exchange in mechanically ventilated hypercapnic COPD patients with acute respiratory failure. Respiration 2003; 70:355–361.
- Grasso S, Puntillo F, Mascia L, et al. Compensation for increase in respiratory workload during mechanical ventilation. Pressure support versus proportional assist ventilation. Am J Respir Crit Care Med 2000; 161:819–826.
- Wrigge H, Golisch W, Zinserling J, Sydow M, Almeling G, Burchardi H. Proportional assist versus pressure support ventilation: effects on breathing pattern and respiratory work of patients with chronic obstructive pulmonary disease. Intensive Care Med 1999; 25:790–798.
- Ranieri VM, Giuliani R, Mascia L, et al. Patient ventilator interaction during acute hypercapnia: pressure support vs. proportional assist ventilation. J Appl Physiol 1996; 81:426–436.
- Kondili E, Xirouchaki N, Vaporidi K, Klimathianaki M, Georgopoulos D. Short-term cardiorespiratory effects of proportional assist and pressure support ventilation in patients with acute lung injury/acute respiratory distress syndrome. Anesthesiology 2006; 105:703–708.
- Xirouchaki N, Kondili E, Vaporidi K, et al. Proportional assist ventilation with load-adjustable gain factors in critically ill patients: comparison with pressure support. Intensive Care Med 2008; 34:2026–2034.
- Bosma K, Ferreyra G, Ambrogio C, et al. Patient ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med 2007; 35:1048–1054.
- Sinderby C, Beck J. Proportional assist ventilation and neurally adjusted ventilatory assist—better approaches to patient ventilator synchrony? Clin Chest Med 2008; 29:329–342.
- Stock MC, Downs JB, Frolicher DA. Airway pressure release ventilation. Crit Care Med 1987; 15:462–466.
- Baum M, Benzer H, Putensen C, Koller W, Putz G. [Biphasic positive airway pressure (BIPAP)—a new form of augmented ventilation]. Anaesthesist 1989; 38:452–458.
- Seymour CW, Frazer M, Reilly PM, Fuchs BD. Airway pressure release and biphasic intermittent positive airway pressure ventilation: are they ready for prime time? J Trauma 2007; 62:1298–1308.
- Myers TR, MacIntyre NR. Respiratory controversies in the critical care setting. Does airway pressure release ventilation offer important new advantages in mechanical ventilator support? Respir Care 2007; 52:452–458.
- Neumann P, Golisch W, Strohmeyer A, Buscher H, Burchardi H, Sydow M. Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive Care Med 2002; 28:1742–1749.
- Calzia E, Lindner KH, Witt S, et al. Pressure-time product and work of breathing during biphasic continuous positive airway pressure and assisted spontaneous breathing. Am J Respir Crit Care Med 1994; 150:904–910.
- Rose L, Hawkins M. Airway pressure release ventilation and biphasic positive airway pressure: a systematic review of definitional criteria. Intensive Care Med 2008; 34:1766–1773.
- Sydow M, Burchardi H, Ephraim E, Zielmann S, Crozier TA. Longterm effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med 1994; 149:1550–1556.
- Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 2001; 164:43–49.
- Davis K, Johnson DJ, Branson RD, Campbell RS, Johannigman JA, Porembka D. Airway pressure release ventilation. Arch Surg 1993; 128:1348–1352.
- Kaplan LJ, Bailey H, Formosa V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care 2001; 5:221–226.
- Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettilä VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand 2004; 48:722–731.
- Siau C, Stewart TE. Current role of high frequency oscillatory ventilation and airway pressure release ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med 2008; 29:265–275.
- Rathgeber J, Schorn B, Falk V, Kazmaier S, Spiegel T, Burchardi H. The influence of controlled mandatory ventilation (CMV), intermittent mandatory ventilation (IMV) and biphasic intermittent positive airway pressure (BIPAP) on duration of intubation and consumption of analgesics and sedatives. A prospective analysis in 596 patients following adult cardiac surgery. Eur J Anaesthesiol 1997; 14:576–582.
- Habashi NM. Other approaches to open lung ventilation: airway pressure release ventilation. Crit Care Med 2005; 33 suppl 3:S228–S240.
- Hess D, Mason S, Branson R. High-frequency ventilation design and equipment issues. Respir Care Clin North Am 2001; 7:577–598.
- Fessler HE, Derdak S, Ferguson ND, et al. A protocol for high frequency oscillatory ventilation in adults: results from a roundtable discussion. Crit Care Med 2007; 35:1649–1654.
- Hamilton PP, Onayemi A, Smyth JA, et al. Comparison of conventional and high-frequency ventilation: oxygenation and lung pathology. J Appl Physiol 1983; 55:131–138.
- Sedeek KA, Takeuchi M, Suchodolski K, et al. Open-lung protective ventilation with pressure control ventilation, high-frequency oscillation, and intratracheal pulmonary ventilation results in similar gas exchange, hemodynamics, and lung mechanics. Anesthesiology 2003; 99:1102–1111.
- Imai Y, Nakagawa S, Ito Y, Kawano T, Slutsky AS, Miyasaka K. Comparison of lung protection strategies using conventional and high-frequency oscillatory ventilation. J Appl Physiol 2001; 91:1836–1844.
- van Heerde M, Roubik K, Kopelent V, Plötz FB, Markhorst DG. Unloading work of breathing during high-frequency oscillatory ventilation: a bench study. Crit Care 2006; 10:R103.
- Derdak S, Mehta S, Stewart TE, et al., Multicenter Oscillatory Ventilation For Acute Respiratory Distress Syndrome Trial (MOAT) Study Investigators. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med 2002; 166:801–808.
- Bollen CW, van Well GT, Sherry T, et al. High-frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care 2005; 9:R430–R439.
- Mehta S, Granton J, MacDonald RJ, et al. High frequency oscillatory ventilation in adults: the Toronto experience. Chest 2004; 126:518–527.
- Chan KP, Stewart TE, Mehta S. High-frequency oscillatory ventilation for adult patients with ARDS. Chest 2007; 131:1907–1916.
KEY POINTS
- The alternative modes of ventilation were developed to prevent lung injury and asynchrony, promote better oxygenation and faster weaning, and be easier to use. However, evidence of their benefit is scant.
- Until now, we have lacked a standard nomenclature for mechanical ventilation, leading to confusion.
- Regardless of the mode used, the goals are to avoid lung injury, keep the patient comfortable, and wean the patient from mechanical ventilation as soon as possible.