User login
These days, an increasing number of people are receiving permanent pacemakers, implantable cardioverter-defibrillators, endovascular devices, and cardiac resynchronization therapy devices—collectively called cardiovascular implantable electronic devices (CIEDs). One reason for this upswing is that these devices have been approved for more indications, such as sick sinus syndrome, third-degree heart block, atrial fibrillation, life-threatening ventricular arrhythmias, survival of sudden cardiac death, and advanced congestive heart failure. Another reason is that the population is getting older, and therefore more people need these devices.
Although the use of a CIED is associated with a lower risk of death and a better quality of life, CIED-related infection can eclipse some of these benefits for their recipients. Historically reported rates of infections range from 0% to 19.9%.1 However, recent data point to a disturbing trend: infection rates are rising faster than implantation rates.2
Besides causing morbidity and even death, infection is also associated with significant financial cost for patients and third-party payers. The estimated average cost of combined medical and surgical treatment of CIED-related infection ranges from $25,000 for permanent pacemakers to $50,000 for implantable cardioverter-defibrillators.3,4
Although cardiologists and cardiac surgeons are the ones who implant these devices, most patients receive their routine outpatient care from a primary care physician, who can be a general internist, a family physician, or other specialist. Moreover, many patients with device infection are admitted to hospital internal medicine services for various diagnoses requiring inpatient care. Therefore, an internist, a family physician, or a hospitalist may be the first physician to respond to a suspected or confirmed device infection. Knowledge of the clinical manifestations and the initial steps in evaluation and management is essential for optimal care.
These complex infections pose challenges, which we will illustrate by presenting a case of CIED-related infection and reviewing key elements of diagnosis and management.
AN ILLUSTRATIVE CASE
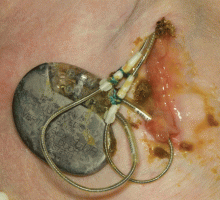
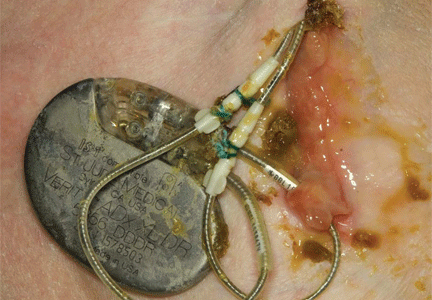
A 60-year-old man had a permanent pacemaker implanted 3 months ago because of third-degree heart block; he now presents to his primary care physician with increasing pain, swelling, and erythema at the site of his pacemaker pocket. He has a history of type 2 diabetes mellitus, stage 3 chronic kidney disease, and coronary artery disease.
The symptoms started 2 weeks ago and have slowly progressed, prompting him to seek medical care. He is quite anxious and wants to know if he needs to arrange an emergency consultation with his cardiologist.
IMPORTANT CLINICAL QUESTIONS
This presentation raises several important questions:
- What should be the next step in his evaluation?
- Which laboratory tests should be done?
- Should he be admitted to the hospital, or can he be managed as an outpatient?
- Should he be started empirically on antibiotics? If so, which antibiotics? Or is it better to wait?
- When should an infectious disease specialist be consulted?
- Should the device be removed, and if so, all of it or which components?
- How long should antibiotics be given?
We will provide evidence-based answers to these questions in the discussions below.
PATHOGENESIS AND RISK FACTORS FOR DEVICE INFECTION
The first step in understanding the clinical manifestations of CIED-related infections is to grasp their pathogenesis. Risk factors for device infection have been evaluated in several studies.1
Several factors interact in the inception and evolution of these infections, some related to the care in the perioperative period, some to the device, some to the host, and some to the causative microorganism.5 Although any one of these may play a predominant role in a given patient, most patients have a combination.
Perioperative factors that may contribute to a higher risk of infection include device revision; use of temporary pacing leads before placement of the permanent device; lack of antibiotic prophylaxis before implantation; longer operative time; operative inexperience; development of postoperative pocket hematoma; and factors such as diabetes mellitus and long-term use of corticosteroids and other immunosuppressive drugs that impair wound healing at the generator pocket.6–11
Device factors. Abdominal generator placement, use of epicardial leads, and complexity of the device play a significant role.6,12,13 In general, implantable cardioverter-defibrillators and cardiac resynchronization therapy devices have higher rates of infection than permanent pacemakers.2,14
Host factors. Diseases and conditions that predispose to bloodstream infection may result in hematogenous seeding of the device and its leads and are associated with a higher risk of late-onset infection. These include an implanted central venous catheter (for hemodialysis or other long-term access), a distant focus of primary infection (such as pneumonia and skin and soft-tissue infections), and invasive procedures unrelated to the CIED.10,15
In general, contamination at the time of surgery leads to early-onset infection (ie, within weeks to months of implantation), whereas hematogenous seeding is a predominant factor in most patients with late-onset infection.16
STAPHYLOCOCCI ARE THE MOST COMMON CAUSE
A key to making an accurate diagnosis and determining the appropriate empiric antibiotic therapy is to understand the microbiology of device infections.
Regardless of the clinical presentation, staphylococci are the predominant organisms responsible for both early- and late-onset infections.17,18 These include Staphylococcus aureus and coagulase-negative staphylococci. Depending on where the implanting hospital is located and where the organism was acquired (in the community or in the hospital), up to 50% of these staphylococci may be methicillin-resistant,17,18 a fact that necessitates using vancomycin for empiric coverage until the pathogen is identified and its susceptibility is known.
Gram-negative or polymicrobial CIED infections are infrequent. However, empiric gram-negative coverage should be considered for patients who present with systemic signs of infection, in whom delaying adequate coverage could jeopardize the successful outcome of infection treatment.
Fungal and mycobacterial infections of cardiac devices are exceedingly uncommon, mainly occurring in immunocompromised patients.
CLINICAL MANIFESTATIONS OF CARDIOVASCULAR DEVICE INFECTION
The clinical presentations of CIED-related infection can be broadly categorized into two groups: generator pocket infection and endovascular infection with an intact pocket.17,18
Generator pocket infection
Most patients with a pocket infection present with inflammatory changes at the device generator site. Usual signs and symptoms include pain, erythema, swelling, and serosanguinous or purulent drainage from the pocket.
Patients with a pocket infection generally present within weeks to months of implantation, as the predominant mechanism of pocket infection is contamination of the generator or leads during implantation. However, occasionally, pocket infection caused by indolent organisms such as Propionibacterium, Corynebacterium, and certain species of coagulase-negative staphylococci can present more than 1 year after implantation. Hematogenous seeding of the device pocket, as a result of bacteremia from a distant primary focus, is infrequent except in cases of S aureus bloodstream infection.19
Endovascular infection with an intact pocket
A subset of patients with CIED-related infections, mostly late-onset infections, present only with systemic signs and symptoms without inflammatory changes at the generator pocket.16–18 Most of these patients have multiple comorbid conditions and likely acquire the infection via hematogenous seeding of transvenous device leads from a distant focus of primary infection, such as a skin or soft-tissue infection, pneumonia, bacteremia arising from an implanted long-term central venous catheter, or bloodstream infection secondary to an invasive procedure unrelated to the CIED.
Most patients with an endovascular device infection have positive blood cultures at presentation. However, occasionally, blood cultures may be negative. The main reason for negative blood cultures in this setting is the use of empiric antibiotic therapy before blood cultures are drawn.
Endovascular device infections are further complicated by the formation of infected vegetations on the leads or cardiac valves in up to one-fourth of cases.16–18,20,21 This complication poses additional challenges in management, such as choosing the appropriate lead extraction technique, the waiting time before implanting a replacement device, and the optimal length of parenteral antimicrobial therapy. Many of these decisions are beyond the realm of internal medicine practice and are best managed by consultation with an infectious disease specialist and a cardiologist.
DIAGNOSIS OF INFECTION AND ASSOCIATED COMPLICATIONS
The clinical diagnosis of pocket infection is usually quite straightforward. However, occasionally, an early postoperative pocket hematoma can mimic pocket infection, and distinguishing these two may be difficult. Close collaboration between an internist, cardiologist, and infectious-disease specialist and careful observation of the patient may help to avoid a premature and incorrect diagnosis of pocket infection and unnecessary removal of the device in this scenario.
While diagnosing a pocket infection may be simple, an accurate and timely diagnosis of endovascular infection with an intact pocket can be challenging, especially if echocardiography shows no conclusive evidence of involvement of the device leads. Even when the infection is limited to the generator pocket, attempts to isolate causative pathogens may be hampered if empiric antibiotic therapy is started before culture samples are obtained from the pocket and from the blood.
Complete blood count with differential cell count.
Electrolyte and serum creatinine concentrations.
Inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein concentration.
Swabs for bacterial cultures should be sent if there is purulent drainage from the generator pocket. This can be done in the office before referral to the emergency department or a tertiary care center for inpatient admission. If the pocket appears swollen or fluctuant, needle aspiration should be avoided, as it can introduce organisms and cause contamination.5
Two sets of peripheral blood cultures should be obtained. If the patient has an implanted central venous catheter, blood cultures via each catheter port should also be obtained, as they may help to pinpoint the source of bloodstream infection in cases in which blood culture results are positive.
TEE should also be performed in patients with systemic signs and symptoms (such as fever, chills, malaise, dyspnea, hypotension, or peripheral stigmata of endocarditis) or abnormal test results (leukocytosis, elevated inflammatory markers, or evidence of pulmonary emboli on imaging), even if blood cultures are negative. Similarly, TEE should also be considered in patients in whom blood cultures may be negative as a result of previous antimicrobial therapy.
If a decision is made to remove the device (see below), intraoperative pocket tissue and lead-tip cultures should be sent for Gram staining and bacterial culture. Fungal and mycobacterial cultures may be necessary in immunocompromised hosts, or if Gram staining and bacterial cultures from pocket tissue samples are negative. Caution must be exercised when interpreting the results of lead-tip cultures, as lead tips may become contaminated while being pulled through an infected pocket during removal.20,22
This approach should lead to an accurate diagnosis of CIED-related infection and associated complications in most patients. However, the diagnosis may remain elusive if results of blood cultures are positive but the pocket is intact and there is no echocardiographic evidence of lead or valve involvement. This is especially true in cases of S aureus bacteremia, in which positive blood cultures may be the sole manifestation of underlying device infection.19,23 Factors associated with higher odds of underlying device infection in this scenario include bacteremia lasting more than 24 hours, prosthetic valves, bacteremia within 3 months of device implantation, and no alternative focus of bacteremia.12
Evidence is emerging that underlying device infection should also be considered in patients with bloodstream infection with coagulase-negative staphylococci in the setting of an implanted device.24 On the other hand, seeding of device leads with gram-negative organisms is infrequent, and routine imaging of intracardiac leads is not necessary in cases of gram-negative bacteremia.25
In our opinion, cases of bacteremia in which underlying occult device infection is a concern are best managed by consultation with an infectious disease specialist.
A STEPWISE APPROACH TO MANAGING DEVICE INFECTION
Should antibiotics be started empirically?
The first step in managing CIED-related infection is to decide whether empiric antibiotic therapy should be started immediately once infection is suspected or if it is prudent to wait until the culture results are available.
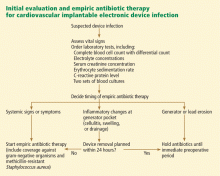
In our opinion, if the infection is limited to the generator pocket, it is reasonable to wait until immediately before surgery to maximize the culture yield from pocket tissue samples. An exception to this rule is when systemic signs or symptoms are present, in which case delaying antibiotic therapy could jeopardize the outcome (FIGURE 2). In such cases, empiric antibiotic therapy can be started once two sets of peripheral blood samples for cultures have been obtained.
Which antibiotics should be given empirically?
Because gram-positive organisms, namely coagulase-negative staphylococci and S aureus, are the causative pathogens in most cases of CIED-related infection, empiric antibiotic therapy should provide adequate coverage for these organisms. Because methicillin resistance is quite prevalent in staphylococci, we routinely use vancomycin (Vancocin) for empiric coverage. In patients who are allergic to vancomycin or cannot tolerate it, daptomycin (Cubicin) is an alternative.
Empiric gram-negative coverage is generally reserved for patients who present with systemic signs and symptoms, in whom delaying adequate coverage could have untoward consequences. We routinely use cefepime (Maxipime) for empiric gram-negative coverage in our institution. Other beta-lactam agents that provide coverage for gram-negative bacilli, especially Pseudomonas, are also appropriate in this setting.
Should the device be removed?
Superficial infection of the wound or incision site (eg, stitch abscess) early after implantation can be managed by conservative antibiotic therapy without removing the device. However, complete removal of the device system, including intracardiac leads, is necessary in all other presentations of device infection, even if the infection appears limited to the generator pocket.5,12 Leaving the device in place or removing parts of the device is associated with persistent or relapsed infection and is not advisable.17,26
Leaving the device in place may be necessary in extenuating circumstances, eg, if surgery would be too risky for the patient or if the patient refuses device removal or has a short life expectancy. In these cases, lifelong suppressive antibiotic therapy should be prescribed after an initial course of parenteral antibiotics.27 Antibiotic choices for long-term suppressive therapy should be guided by antimicrobial susceptibility testing and consultation with an infectious disease specialist.
How should the leads be removed?
Leads are extracted percutaneously in most cases. Percutaneous extraction is generally considered safe even in cases in which infection is complicated by lead vegetations, which raises concern about pulmonary embolization of detached vegetation fragments during extraction.5,20
Thoracotomy is generally reserved for patients who have cardiac complications (such as a cardiac abscess or the need to replace cardiac valves) or in whom attempts to extract the leads percutaneously are unsuccessful.
Details of the removal procedure and choice of extraction technique are beyond the scope of this paper and are best left to the discretion of the treating cardiologist or cardiac surgeon. Because of the potential for complications during percutaneous device removal, such as laceration of the superior vena cava or cardiac tamponade, the patient should be referred to a high-volume center where cardiothoracic intervention can be provided on an emergency basis if needed.
How long should antibiotic therapy go on?
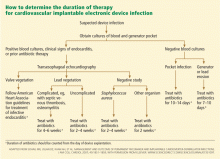
An algorithm for deciding the duration of antibiotic therapy is shown in Figure 3. These guidelines, first published in 2007,17 were adopted by the American Heart Association in its updated statement on the management of CIED-related infections.5 However, it should be noted that these guidelines are not based on randomized clinical trials; rather, they represent expert opinion based on published series of patients with CIED-related infections.
In general, cases of device erosion or pocket infection can be treated with 1 to 2 weeks of appropriate antibiotic therapy based on antimicrobial susceptibility testing. However, cases of bloodstream infection require 2 to 4 weeks of antibiotic therapy—or sometimes even longer if associated complications are present, such as septic thrombosis, endocarditis, or osteomyelitis.
We favor parenteral antibiotics for the entire course of treatment. However, patients can be discharged from the hospital once the bloodstream infection has cleared, and the antibiotic course can be completed on an outpatient basis.
Outpatient antimicrobial monitoring
We recommend adherence to the Infectious Diseases Society of America’s guidelines for monitoring outpatient parenteral antimicrobial therapy.28
At discharge from the hospital, patients should be instructed to promptly call their primary care physician if they have a fever or notice inflammatory changes at the pocket site. If the patient reports such symptoms, repeat blood cultures should be ordered, and the patient should be monitored closely for signs of a relapse of infection.
A routine follow-up visit should be arranged at 2 weeks and at the end of parenteral antibiotic therapy (for patients receiving therapy for 4 weeks or longer) to make sure the infection has resolved.
When should a new device be implanted?
Before deciding when a new device should be implanted, one should carefully assess whether the patient still needs one. Studies indicate that up to 30% of patients may no longer require a cardiac device.17,18
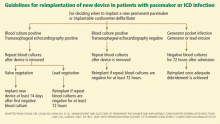
However, we believe that removal of drains and closure of the old pocket are not necessary before implanting a new device in a different location (usually the contralateral pectoral area). Exceptions to this general principle are cases of valvular endocarditis, in which a minimum of 2 weeks is recommended between removal of an infected device (plus clearance of bloodstream infection) and implantation of a new device.
OUTCOMES OF INFECTION
Despite improvements in our understanding of how to manage CIED-related infection, the rates of morbidity and death remain significant.
The outcome, in part, depends on the clinical presentation and the patient’s comorbid conditions. In general, the death rate in patients with a pocket infection is less than 5%. However, in patients with endovascular infection, it may be as high as 20%.16–18 Other factors that affect the outcome include complications such as septic thrombosis, valvular endocarditis, or osteomyelitis; complications during device extraction; the need for open heart surgery; and the overall health of the patient.
Complete removal of the device system is a requisite for successful outcome, and the risk of death tends to be higher if only part of the infected CIED system is extracted.26
STRATEGIES TO PREVENT DEVICE INFECTION
Preventive efforts should focus on strategies to minimize the chances of contamination of the generator, leads, and pocket during implantation.29 Patients who are known to be colonized with methicillin-resistant S aureus may benefit from decolonization programs, which should include nasal application of mupirocin (Bactroban) ointment preoperatively.30 In addition, use of chlorhexidine for surgical-site antisepsis has been shown to reduce the risk of surgical site infection.31
Moreover, all patients should receive antibiotic prophylaxis before implantation of a CIED.32,33 Most institutions use a first-generation cephalosporin, such as cefazolin (Ancef), for this purpose.34 However, the increasing rate of methicillin resistance in staphylococci has led to the routine use of vancomycin for preoperative prophylaxis at some centers.18
Regardless of the antibiotic chosen for prophylaxis, protocols that ensure that all patients receive an appropriate antibiotic at the appropriate time are a key determinant in the success of these infection-control programs.
- Sohail MR, Wilson WR, Baddour LM. Infections of nonvalvular cardiovascular devices. In:Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia: Churchill Livingstone/Elsevier; 2010:1127–1142.
- Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol 2006; 48:590–591.
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med 2004; 350:1422–1429.
- Ferguson TB, Ferguson CL, Crites K, Crimmins-Reda P. The additional hospital costs generated in the management of complications of pacemaker and defibrillator implantations. J Thorac Cardiovasc Surg 1996; 111:742–751.
- Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477.
- Klug D, Balde M, Pavin D, et al; PEOPLE Study Group. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116:1349–1355.
- Sohail MR, Hussain S, Dib C, et al. Risk factor analysis of implantable cardioverter-defibrillator infections. Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). Boston, MA, Sept. 12–15, 2010.
- Lai KK, Fontecchio SA. Infections associated with implantable cardioverter defibrillators placed transvenously and via thoracotomies: epidemiology, infection control, and management. Clin Infect Dis 1998; 27:265–269.
- Mela T, McGovern BA, Garan H, et al. Long-term infection rates associated with the pectoral versus abdominal approach to cardioverter-defibrillator implants. Am J Cardiol 2001; 88:750–753.
- Al-Khatib SM, Lucas FL, Jollis JG, Malenka DJ, Wennberg DE. The relation between patients’ outcomes and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating Medicare beneficiaries. J Am Coll Cardiol 2005; 46:1536–1540.
- Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart 2009; 95:715–720.
- Sohail MR, Sultan OW, Raza SS. Contemporary management of cardiovascular implantable electronic device infections. Expert Rev Anti Infect Ther 2010; 8:831–839.
- Sohail MR, Uslan DZ, Khan AH, et al. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis 2007; 45:166–173.
- Uslan DZ, Sohail MR, St Sauver JL, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 2007; 167:669–675.
- Bloom H, Heeke B, Leon A, et al. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol 2006; 29:142–145.
- Le KY, Sohail MR, Friedman PA, et al for the Mayo Cardiovascular Infections Study Group. Clinical predictors of cardiovascular implantable electronic device-related infective endocarditis. Pacing Clin Electrophysiol2911; 34:450–459.
- Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007; 49:1851–1859.
- Tarakji KG, Chan EJ, Cantillon DJ, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm 2010; 7:1043–1047.
- Chamis AL, Peterson GE, Cabell CH, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 2001; 104:1029–1033.
- Sohail MR, Uslan DZ, Khan AH, et al. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc 2008; 83:46–53.
- Arber N, Pras E, Copperman Y, et al. Pacemaker endocarditis. Report of 44 cases and review of the literature. Medicine (Baltimore) 1994; 73:299–305.
- Sohail MR. Concerning diagnosis and management of pacemaker endocarditis [letter]. Pacing Clin Electrophysiol 2007; 30:829.
- Uslan DZ, Dowsley TF, Sohail MR, et al. Cardiovascular implantable electronic device infection in patients with Staphylococcus aureus bacteremia. Pacing Clin Electrophysiol 2009; 33:407–413.
- Madhavan M, Sohail MR, Friedman PA, et al. Outcomes in patients with cardiovascular implantable electronic devices and bacteremia due to Gram-positive cocci other than Staphylococcus aureus. Circ Arrhythm Electrophysiol 2010; 3:639–645.
- Uslan DZ, Sohail MR, Friedman PA, et al. Frequency of permanent pacemaker or implantable cardioverter-defibrillator infection in patients with gram-negative bacteremia. Clin Infect Dis 2006; 43:731–736.
- Margey R, McCann H, Blake G, et al. Contemporary management of and outcomes from cardiac device related infections. Europace 2010; 12:64–70.
- Baddour LM. Long-term suppressive antimicrobial therapy for intravascular device-related infections. Am J Med Sci 2001; 322:209–212.
- Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 2004; 38:1651–1672.
- Wenzel RP. Minimizing surgical-site infections. N Engl J Med 2010; 362:75–77.
- Bode LGM, Kluytmans JAJW, Wertheim HFL, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010; 362:9–17.
- Darouiche RO, Wall MJ, Itani KMF, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 2010; 362:18–26.
- Da Costa A, Kirkorian G, Cucherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation 1998; 97:1796–1801.
- de Oliveira JC, Martinelli M, Nishioka SA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, doubleblinded, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009; 2:29–34.
- Bertaglia E, Zerbo F, Zardo S, Barzan D, Zoppo F, Pascotto P. Antibiotic prophylaxis with a single dose of cefazolin during pacemaker implantation: incidence of long-term infective complications. Pacing Clin Electrophysiol 2006; 29:29–33.
These days, an increasing number of people are receiving permanent pacemakers, implantable cardioverter-defibrillators, endovascular devices, and cardiac resynchronization therapy devices—collectively called cardiovascular implantable electronic devices (CIEDs). One reason for this upswing is that these devices have been approved for more indications, such as sick sinus syndrome, third-degree heart block, atrial fibrillation, life-threatening ventricular arrhythmias, survival of sudden cardiac death, and advanced congestive heart failure. Another reason is that the population is getting older, and therefore more people need these devices.
Although the use of a CIED is associated with a lower risk of death and a better quality of life, CIED-related infection can eclipse some of these benefits for their recipients. Historically reported rates of infections range from 0% to 19.9%.1 However, recent data point to a disturbing trend: infection rates are rising faster than implantation rates.2
Besides causing morbidity and even death, infection is also associated with significant financial cost for patients and third-party payers. The estimated average cost of combined medical and surgical treatment of CIED-related infection ranges from $25,000 for permanent pacemakers to $50,000 for implantable cardioverter-defibrillators.3,4
Although cardiologists and cardiac surgeons are the ones who implant these devices, most patients receive their routine outpatient care from a primary care physician, who can be a general internist, a family physician, or other specialist. Moreover, many patients with device infection are admitted to hospital internal medicine services for various diagnoses requiring inpatient care. Therefore, an internist, a family physician, or a hospitalist may be the first physician to respond to a suspected or confirmed device infection. Knowledge of the clinical manifestations and the initial steps in evaluation and management is essential for optimal care.
These complex infections pose challenges, which we will illustrate by presenting a case of CIED-related infection and reviewing key elements of diagnosis and management.
AN ILLUSTRATIVE CASE
A 60-year-old man had a permanent pacemaker implanted 3 months ago because of third-degree heart block; he now presents to his primary care physician with increasing pain, swelling, and erythema at the site of his pacemaker pocket. He has a history of type 2 diabetes mellitus, stage 3 chronic kidney disease, and coronary artery disease.
The symptoms started 2 weeks ago and have slowly progressed, prompting him to seek medical care. He is quite anxious and wants to know if he needs to arrange an emergency consultation with his cardiologist.
IMPORTANT CLINICAL QUESTIONS
This presentation raises several important questions:
- What should be the next step in his evaluation?
- Which laboratory tests should be done?
- Should he be admitted to the hospital, or can he be managed as an outpatient?
- Should he be started empirically on antibiotics? If so, which antibiotics? Or is it better to wait?
- When should an infectious disease specialist be consulted?
- Should the device be removed, and if so, all of it or which components?
- How long should antibiotics be given?
We will provide evidence-based answers to these questions in the discussions below.
PATHOGENESIS AND RISK FACTORS FOR DEVICE INFECTION
The first step in understanding the clinical manifestations of CIED-related infections is to grasp their pathogenesis. Risk factors for device infection have been evaluated in several studies.1
Several factors interact in the inception and evolution of these infections, some related to the care in the perioperative period, some to the device, some to the host, and some to the causative microorganism.5 Although any one of these may play a predominant role in a given patient, most patients have a combination.
Perioperative factors that may contribute to a higher risk of infection include device revision; use of temporary pacing leads before placement of the permanent device; lack of antibiotic prophylaxis before implantation; longer operative time; operative inexperience; development of postoperative pocket hematoma; and factors such as diabetes mellitus and long-term use of corticosteroids and other immunosuppressive drugs that impair wound healing at the generator pocket.6–11
Device factors. Abdominal generator placement, use of epicardial leads, and complexity of the device play a significant role.6,12,13 In general, implantable cardioverter-defibrillators and cardiac resynchronization therapy devices have higher rates of infection than permanent pacemakers.2,14
Host factors. Diseases and conditions that predispose to bloodstream infection may result in hematogenous seeding of the device and its leads and are associated with a higher risk of late-onset infection. These include an implanted central venous catheter (for hemodialysis or other long-term access), a distant focus of primary infection (such as pneumonia and skin and soft-tissue infections), and invasive procedures unrelated to the CIED.10,15
In general, contamination at the time of surgery leads to early-onset infection (ie, within weeks to months of implantation), whereas hematogenous seeding is a predominant factor in most patients with late-onset infection.16
STAPHYLOCOCCI ARE THE MOST COMMON CAUSE
A key to making an accurate diagnosis and determining the appropriate empiric antibiotic therapy is to understand the microbiology of device infections.
Regardless of the clinical presentation, staphylococci are the predominant organisms responsible for both early- and late-onset infections.17,18 These include Staphylococcus aureus and coagulase-negative staphylococci. Depending on where the implanting hospital is located and where the organism was acquired (in the community or in the hospital), up to 50% of these staphylococci may be methicillin-resistant,17,18 a fact that necessitates using vancomycin for empiric coverage until the pathogen is identified and its susceptibility is known.
Gram-negative or polymicrobial CIED infections are infrequent. However, empiric gram-negative coverage should be considered for patients who present with systemic signs of infection, in whom delaying adequate coverage could jeopardize the successful outcome of infection treatment.
Fungal and mycobacterial infections of cardiac devices are exceedingly uncommon, mainly occurring in immunocompromised patients.
CLINICAL MANIFESTATIONS OF CARDIOVASCULAR DEVICE INFECTION
The clinical presentations of CIED-related infection can be broadly categorized into two groups: generator pocket infection and endovascular infection with an intact pocket.17,18
Generator pocket infection
Most patients with a pocket infection present with inflammatory changes at the device generator site. Usual signs and symptoms include pain, erythema, swelling, and serosanguinous or purulent drainage from the pocket.
Patients with a pocket infection generally present within weeks to months of implantation, as the predominant mechanism of pocket infection is contamination of the generator or leads during implantation. However, occasionally, pocket infection caused by indolent organisms such as Propionibacterium, Corynebacterium, and certain species of coagulase-negative staphylococci can present more than 1 year after implantation. Hematogenous seeding of the device pocket, as a result of bacteremia from a distant primary focus, is infrequent except in cases of S aureus bloodstream infection.19
Endovascular infection with an intact pocket
A subset of patients with CIED-related infections, mostly late-onset infections, present only with systemic signs and symptoms without inflammatory changes at the generator pocket.16–18 Most of these patients have multiple comorbid conditions and likely acquire the infection via hematogenous seeding of transvenous device leads from a distant focus of primary infection, such as a skin or soft-tissue infection, pneumonia, bacteremia arising from an implanted long-term central venous catheter, or bloodstream infection secondary to an invasive procedure unrelated to the CIED.
Most patients with an endovascular device infection have positive blood cultures at presentation. However, occasionally, blood cultures may be negative. The main reason for negative blood cultures in this setting is the use of empiric antibiotic therapy before blood cultures are drawn.
Endovascular device infections are further complicated by the formation of infected vegetations on the leads or cardiac valves in up to one-fourth of cases.16–18,20,21 This complication poses additional challenges in management, such as choosing the appropriate lead extraction technique, the waiting time before implanting a replacement device, and the optimal length of parenteral antimicrobial therapy. Many of these decisions are beyond the realm of internal medicine practice and are best managed by consultation with an infectious disease specialist and a cardiologist.
DIAGNOSIS OF INFECTION AND ASSOCIATED COMPLICATIONS
The clinical diagnosis of pocket infection is usually quite straightforward. However, occasionally, an early postoperative pocket hematoma can mimic pocket infection, and distinguishing these two may be difficult. Close collaboration between an internist, cardiologist, and infectious-disease specialist and careful observation of the patient may help to avoid a premature and incorrect diagnosis of pocket infection and unnecessary removal of the device in this scenario.
While diagnosing a pocket infection may be simple, an accurate and timely diagnosis of endovascular infection with an intact pocket can be challenging, especially if echocardiography shows no conclusive evidence of involvement of the device leads. Even when the infection is limited to the generator pocket, attempts to isolate causative pathogens may be hampered if empiric antibiotic therapy is started before culture samples are obtained from the pocket and from the blood.
Complete blood count with differential cell count.
Electrolyte and serum creatinine concentrations.
Inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein concentration.
Swabs for bacterial cultures should be sent if there is purulent drainage from the generator pocket. This can be done in the office before referral to the emergency department or a tertiary care center for inpatient admission. If the pocket appears swollen or fluctuant, needle aspiration should be avoided, as it can introduce organisms and cause contamination.5
Two sets of peripheral blood cultures should be obtained. If the patient has an implanted central venous catheter, blood cultures via each catheter port should also be obtained, as they may help to pinpoint the source of bloodstream infection in cases in which blood culture results are positive.
TEE should also be performed in patients with systemic signs and symptoms (such as fever, chills, malaise, dyspnea, hypotension, or peripheral stigmata of endocarditis) or abnormal test results (leukocytosis, elevated inflammatory markers, or evidence of pulmonary emboli on imaging), even if blood cultures are negative. Similarly, TEE should also be considered in patients in whom blood cultures may be negative as a result of previous antimicrobial therapy.
If a decision is made to remove the device (see below), intraoperative pocket tissue and lead-tip cultures should be sent for Gram staining and bacterial culture. Fungal and mycobacterial cultures may be necessary in immunocompromised hosts, or if Gram staining and bacterial cultures from pocket tissue samples are negative. Caution must be exercised when interpreting the results of lead-tip cultures, as lead tips may become contaminated while being pulled through an infected pocket during removal.20,22
This approach should lead to an accurate diagnosis of CIED-related infection and associated complications in most patients. However, the diagnosis may remain elusive if results of blood cultures are positive but the pocket is intact and there is no echocardiographic evidence of lead or valve involvement. This is especially true in cases of S aureus bacteremia, in which positive blood cultures may be the sole manifestation of underlying device infection.19,23 Factors associated with higher odds of underlying device infection in this scenario include bacteremia lasting more than 24 hours, prosthetic valves, bacteremia within 3 months of device implantation, and no alternative focus of bacteremia.12
Evidence is emerging that underlying device infection should also be considered in patients with bloodstream infection with coagulase-negative staphylococci in the setting of an implanted device.24 On the other hand, seeding of device leads with gram-negative organisms is infrequent, and routine imaging of intracardiac leads is not necessary in cases of gram-negative bacteremia.25
In our opinion, cases of bacteremia in which underlying occult device infection is a concern are best managed by consultation with an infectious disease specialist.
A STEPWISE APPROACH TO MANAGING DEVICE INFECTION
Should antibiotics be started empirically?
The first step in managing CIED-related infection is to decide whether empiric antibiotic therapy should be started immediately once infection is suspected or if it is prudent to wait until the culture results are available.
In our opinion, if the infection is limited to the generator pocket, it is reasonable to wait until immediately before surgery to maximize the culture yield from pocket tissue samples. An exception to this rule is when systemic signs or symptoms are present, in which case delaying antibiotic therapy could jeopardize the outcome (FIGURE 2). In such cases, empiric antibiotic therapy can be started once two sets of peripheral blood samples for cultures have been obtained.
Which antibiotics should be given empirically?
Because gram-positive organisms, namely coagulase-negative staphylococci and S aureus, are the causative pathogens in most cases of CIED-related infection, empiric antibiotic therapy should provide adequate coverage for these organisms. Because methicillin resistance is quite prevalent in staphylococci, we routinely use vancomycin (Vancocin) for empiric coverage. In patients who are allergic to vancomycin or cannot tolerate it, daptomycin (Cubicin) is an alternative.
Empiric gram-negative coverage is generally reserved for patients who present with systemic signs and symptoms, in whom delaying adequate coverage could have untoward consequences. We routinely use cefepime (Maxipime) for empiric gram-negative coverage in our institution. Other beta-lactam agents that provide coverage for gram-negative bacilli, especially Pseudomonas, are also appropriate in this setting.
Should the device be removed?
Superficial infection of the wound or incision site (eg, stitch abscess) early after implantation can be managed by conservative antibiotic therapy without removing the device. However, complete removal of the device system, including intracardiac leads, is necessary in all other presentations of device infection, even if the infection appears limited to the generator pocket.5,12 Leaving the device in place or removing parts of the device is associated with persistent or relapsed infection and is not advisable.17,26
Leaving the device in place may be necessary in extenuating circumstances, eg, if surgery would be too risky for the patient or if the patient refuses device removal or has a short life expectancy. In these cases, lifelong suppressive antibiotic therapy should be prescribed after an initial course of parenteral antibiotics.27 Antibiotic choices for long-term suppressive therapy should be guided by antimicrobial susceptibility testing and consultation with an infectious disease specialist.
How should the leads be removed?
Leads are extracted percutaneously in most cases. Percutaneous extraction is generally considered safe even in cases in which infection is complicated by lead vegetations, which raises concern about pulmonary embolization of detached vegetation fragments during extraction.5,20
Thoracotomy is generally reserved for patients who have cardiac complications (such as a cardiac abscess or the need to replace cardiac valves) or in whom attempts to extract the leads percutaneously are unsuccessful.
Details of the removal procedure and choice of extraction technique are beyond the scope of this paper and are best left to the discretion of the treating cardiologist or cardiac surgeon. Because of the potential for complications during percutaneous device removal, such as laceration of the superior vena cava or cardiac tamponade, the patient should be referred to a high-volume center where cardiothoracic intervention can be provided on an emergency basis if needed.
How long should antibiotic therapy go on?
An algorithm for deciding the duration of antibiotic therapy is shown in Figure 3. These guidelines, first published in 2007,17 were adopted by the American Heart Association in its updated statement on the management of CIED-related infections.5 However, it should be noted that these guidelines are not based on randomized clinical trials; rather, they represent expert opinion based on published series of patients with CIED-related infections.
In general, cases of device erosion or pocket infection can be treated with 1 to 2 weeks of appropriate antibiotic therapy based on antimicrobial susceptibility testing. However, cases of bloodstream infection require 2 to 4 weeks of antibiotic therapy—or sometimes even longer if associated complications are present, such as septic thrombosis, endocarditis, or osteomyelitis.
We favor parenteral antibiotics for the entire course of treatment. However, patients can be discharged from the hospital once the bloodstream infection has cleared, and the antibiotic course can be completed on an outpatient basis.
Outpatient antimicrobial monitoring
We recommend adherence to the Infectious Diseases Society of America’s guidelines for monitoring outpatient parenteral antimicrobial therapy.28
At discharge from the hospital, patients should be instructed to promptly call their primary care physician if they have a fever or notice inflammatory changes at the pocket site. If the patient reports such symptoms, repeat blood cultures should be ordered, and the patient should be monitored closely for signs of a relapse of infection.
A routine follow-up visit should be arranged at 2 weeks and at the end of parenteral antibiotic therapy (for patients receiving therapy for 4 weeks or longer) to make sure the infection has resolved.
When should a new device be implanted?
Before deciding when a new device should be implanted, one should carefully assess whether the patient still needs one. Studies indicate that up to 30% of patients may no longer require a cardiac device.17,18
However, we believe that removal of drains and closure of the old pocket are not necessary before implanting a new device in a different location (usually the contralateral pectoral area). Exceptions to this general principle are cases of valvular endocarditis, in which a minimum of 2 weeks is recommended between removal of an infected device (plus clearance of bloodstream infection) and implantation of a new device.
OUTCOMES OF INFECTION
Despite improvements in our understanding of how to manage CIED-related infection, the rates of morbidity and death remain significant.
The outcome, in part, depends on the clinical presentation and the patient’s comorbid conditions. In general, the death rate in patients with a pocket infection is less than 5%. However, in patients with endovascular infection, it may be as high as 20%.16–18 Other factors that affect the outcome include complications such as septic thrombosis, valvular endocarditis, or osteomyelitis; complications during device extraction; the need for open heart surgery; and the overall health of the patient.
Complete removal of the device system is a requisite for successful outcome, and the risk of death tends to be higher if only part of the infected CIED system is extracted.26
STRATEGIES TO PREVENT DEVICE INFECTION
Preventive efforts should focus on strategies to minimize the chances of contamination of the generator, leads, and pocket during implantation.29 Patients who are known to be colonized with methicillin-resistant S aureus may benefit from decolonization programs, which should include nasal application of mupirocin (Bactroban) ointment preoperatively.30 In addition, use of chlorhexidine for surgical-site antisepsis has been shown to reduce the risk of surgical site infection.31
Moreover, all patients should receive antibiotic prophylaxis before implantation of a CIED.32,33 Most institutions use a first-generation cephalosporin, such as cefazolin (Ancef), for this purpose.34 However, the increasing rate of methicillin resistance in staphylococci has led to the routine use of vancomycin for preoperative prophylaxis at some centers.18
Regardless of the antibiotic chosen for prophylaxis, protocols that ensure that all patients receive an appropriate antibiotic at the appropriate time are a key determinant in the success of these infection-control programs.
These days, an increasing number of people are receiving permanent pacemakers, implantable cardioverter-defibrillators, endovascular devices, and cardiac resynchronization therapy devices—collectively called cardiovascular implantable electronic devices (CIEDs). One reason for this upswing is that these devices have been approved for more indications, such as sick sinus syndrome, third-degree heart block, atrial fibrillation, life-threatening ventricular arrhythmias, survival of sudden cardiac death, and advanced congestive heart failure. Another reason is that the population is getting older, and therefore more people need these devices.
Although the use of a CIED is associated with a lower risk of death and a better quality of life, CIED-related infection can eclipse some of these benefits for their recipients. Historically reported rates of infections range from 0% to 19.9%.1 However, recent data point to a disturbing trend: infection rates are rising faster than implantation rates.2
Besides causing morbidity and even death, infection is also associated with significant financial cost for patients and third-party payers. The estimated average cost of combined medical and surgical treatment of CIED-related infection ranges from $25,000 for permanent pacemakers to $50,000 for implantable cardioverter-defibrillators.3,4
Although cardiologists and cardiac surgeons are the ones who implant these devices, most patients receive their routine outpatient care from a primary care physician, who can be a general internist, a family physician, or other specialist. Moreover, many patients with device infection are admitted to hospital internal medicine services for various diagnoses requiring inpatient care. Therefore, an internist, a family physician, or a hospitalist may be the first physician to respond to a suspected or confirmed device infection. Knowledge of the clinical manifestations and the initial steps in evaluation and management is essential for optimal care.
These complex infections pose challenges, which we will illustrate by presenting a case of CIED-related infection and reviewing key elements of diagnosis and management.
AN ILLUSTRATIVE CASE
A 60-year-old man had a permanent pacemaker implanted 3 months ago because of third-degree heart block; he now presents to his primary care physician with increasing pain, swelling, and erythema at the site of his pacemaker pocket. He has a history of type 2 diabetes mellitus, stage 3 chronic kidney disease, and coronary artery disease.
The symptoms started 2 weeks ago and have slowly progressed, prompting him to seek medical care. He is quite anxious and wants to know if he needs to arrange an emergency consultation with his cardiologist.
IMPORTANT CLINICAL QUESTIONS
This presentation raises several important questions:
- What should be the next step in his evaluation?
- Which laboratory tests should be done?
- Should he be admitted to the hospital, or can he be managed as an outpatient?
- Should he be started empirically on antibiotics? If so, which antibiotics? Or is it better to wait?
- When should an infectious disease specialist be consulted?
- Should the device be removed, and if so, all of it or which components?
- How long should antibiotics be given?
We will provide evidence-based answers to these questions in the discussions below.
PATHOGENESIS AND RISK FACTORS FOR DEVICE INFECTION
The first step in understanding the clinical manifestations of CIED-related infections is to grasp their pathogenesis. Risk factors for device infection have been evaluated in several studies.1
Several factors interact in the inception and evolution of these infections, some related to the care in the perioperative period, some to the device, some to the host, and some to the causative microorganism.5 Although any one of these may play a predominant role in a given patient, most patients have a combination.
Perioperative factors that may contribute to a higher risk of infection include device revision; use of temporary pacing leads before placement of the permanent device; lack of antibiotic prophylaxis before implantation; longer operative time; operative inexperience; development of postoperative pocket hematoma; and factors such as diabetes mellitus and long-term use of corticosteroids and other immunosuppressive drugs that impair wound healing at the generator pocket.6–11
Device factors. Abdominal generator placement, use of epicardial leads, and complexity of the device play a significant role.6,12,13 In general, implantable cardioverter-defibrillators and cardiac resynchronization therapy devices have higher rates of infection than permanent pacemakers.2,14
Host factors. Diseases and conditions that predispose to bloodstream infection may result in hematogenous seeding of the device and its leads and are associated with a higher risk of late-onset infection. These include an implanted central venous catheter (for hemodialysis or other long-term access), a distant focus of primary infection (such as pneumonia and skin and soft-tissue infections), and invasive procedures unrelated to the CIED.10,15
In general, contamination at the time of surgery leads to early-onset infection (ie, within weeks to months of implantation), whereas hematogenous seeding is a predominant factor in most patients with late-onset infection.16
STAPHYLOCOCCI ARE THE MOST COMMON CAUSE
A key to making an accurate diagnosis and determining the appropriate empiric antibiotic therapy is to understand the microbiology of device infections.
Regardless of the clinical presentation, staphylococci are the predominant organisms responsible for both early- and late-onset infections.17,18 These include Staphylococcus aureus and coagulase-negative staphylococci. Depending on where the implanting hospital is located and where the organism was acquired (in the community or in the hospital), up to 50% of these staphylococci may be methicillin-resistant,17,18 a fact that necessitates using vancomycin for empiric coverage until the pathogen is identified and its susceptibility is known.
Gram-negative or polymicrobial CIED infections are infrequent. However, empiric gram-negative coverage should be considered for patients who present with systemic signs of infection, in whom delaying adequate coverage could jeopardize the successful outcome of infection treatment.
Fungal and mycobacterial infections of cardiac devices are exceedingly uncommon, mainly occurring in immunocompromised patients.
CLINICAL MANIFESTATIONS OF CARDIOVASCULAR DEVICE INFECTION
The clinical presentations of CIED-related infection can be broadly categorized into two groups: generator pocket infection and endovascular infection with an intact pocket.17,18
Generator pocket infection
Most patients with a pocket infection present with inflammatory changes at the device generator site. Usual signs and symptoms include pain, erythema, swelling, and serosanguinous or purulent drainage from the pocket.
Patients with a pocket infection generally present within weeks to months of implantation, as the predominant mechanism of pocket infection is contamination of the generator or leads during implantation. However, occasionally, pocket infection caused by indolent organisms such as Propionibacterium, Corynebacterium, and certain species of coagulase-negative staphylococci can present more than 1 year after implantation. Hematogenous seeding of the device pocket, as a result of bacteremia from a distant primary focus, is infrequent except in cases of S aureus bloodstream infection.19
Endovascular infection with an intact pocket
A subset of patients with CIED-related infections, mostly late-onset infections, present only with systemic signs and symptoms without inflammatory changes at the generator pocket.16–18 Most of these patients have multiple comorbid conditions and likely acquire the infection via hematogenous seeding of transvenous device leads from a distant focus of primary infection, such as a skin or soft-tissue infection, pneumonia, bacteremia arising from an implanted long-term central venous catheter, or bloodstream infection secondary to an invasive procedure unrelated to the CIED.
Most patients with an endovascular device infection have positive blood cultures at presentation. However, occasionally, blood cultures may be negative. The main reason for negative blood cultures in this setting is the use of empiric antibiotic therapy before blood cultures are drawn.
Endovascular device infections are further complicated by the formation of infected vegetations on the leads or cardiac valves in up to one-fourth of cases.16–18,20,21 This complication poses additional challenges in management, such as choosing the appropriate lead extraction technique, the waiting time before implanting a replacement device, and the optimal length of parenteral antimicrobial therapy. Many of these decisions are beyond the realm of internal medicine practice and are best managed by consultation with an infectious disease specialist and a cardiologist.
DIAGNOSIS OF INFECTION AND ASSOCIATED COMPLICATIONS
The clinical diagnosis of pocket infection is usually quite straightforward. However, occasionally, an early postoperative pocket hematoma can mimic pocket infection, and distinguishing these two may be difficult. Close collaboration between an internist, cardiologist, and infectious-disease specialist and careful observation of the patient may help to avoid a premature and incorrect diagnosis of pocket infection and unnecessary removal of the device in this scenario.
While diagnosing a pocket infection may be simple, an accurate and timely diagnosis of endovascular infection with an intact pocket can be challenging, especially if echocardiography shows no conclusive evidence of involvement of the device leads. Even when the infection is limited to the generator pocket, attempts to isolate causative pathogens may be hampered if empiric antibiotic therapy is started before culture samples are obtained from the pocket and from the blood.
Complete blood count with differential cell count.
Electrolyte and serum creatinine concentrations.
Inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein concentration.
Swabs for bacterial cultures should be sent if there is purulent drainage from the generator pocket. This can be done in the office before referral to the emergency department or a tertiary care center for inpatient admission. If the pocket appears swollen or fluctuant, needle aspiration should be avoided, as it can introduce organisms and cause contamination.5
Two sets of peripheral blood cultures should be obtained. If the patient has an implanted central venous catheter, blood cultures via each catheter port should also be obtained, as they may help to pinpoint the source of bloodstream infection in cases in which blood culture results are positive.
TEE should also be performed in patients with systemic signs and symptoms (such as fever, chills, malaise, dyspnea, hypotension, or peripheral stigmata of endocarditis) or abnormal test results (leukocytosis, elevated inflammatory markers, or evidence of pulmonary emboli on imaging), even if blood cultures are negative. Similarly, TEE should also be considered in patients in whom blood cultures may be negative as a result of previous antimicrobial therapy.
If a decision is made to remove the device (see below), intraoperative pocket tissue and lead-tip cultures should be sent for Gram staining and bacterial culture. Fungal and mycobacterial cultures may be necessary in immunocompromised hosts, or if Gram staining and bacterial cultures from pocket tissue samples are negative. Caution must be exercised when interpreting the results of lead-tip cultures, as lead tips may become contaminated while being pulled through an infected pocket during removal.20,22
This approach should lead to an accurate diagnosis of CIED-related infection and associated complications in most patients. However, the diagnosis may remain elusive if results of blood cultures are positive but the pocket is intact and there is no echocardiographic evidence of lead or valve involvement. This is especially true in cases of S aureus bacteremia, in which positive blood cultures may be the sole manifestation of underlying device infection.19,23 Factors associated with higher odds of underlying device infection in this scenario include bacteremia lasting more than 24 hours, prosthetic valves, bacteremia within 3 months of device implantation, and no alternative focus of bacteremia.12
Evidence is emerging that underlying device infection should also be considered in patients with bloodstream infection with coagulase-negative staphylococci in the setting of an implanted device.24 On the other hand, seeding of device leads with gram-negative organisms is infrequent, and routine imaging of intracardiac leads is not necessary in cases of gram-negative bacteremia.25
In our opinion, cases of bacteremia in which underlying occult device infection is a concern are best managed by consultation with an infectious disease specialist.
A STEPWISE APPROACH TO MANAGING DEVICE INFECTION
Should antibiotics be started empirically?
The first step in managing CIED-related infection is to decide whether empiric antibiotic therapy should be started immediately once infection is suspected or if it is prudent to wait until the culture results are available.
In our opinion, if the infection is limited to the generator pocket, it is reasonable to wait until immediately before surgery to maximize the culture yield from pocket tissue samples. An exception to this rule is when systemic signs or symptoms are present, in which case delaying antibiotic therapy could jeopardize the outcome (FIGURE 2). In such cases, empiric antibiotic therapy can be started once two sets of peripheral blood samples for cultures have been obtained.
Which antibiotics should be given empirically?
Because gram-positive organisms, namely coagulase-negative staphylococci and S aureus, are the causative pathogens in most cases of CIED-related infection, empiric antibiotic therapy should provide adequate coverage for these organisms. Because methicillin resistance is quite prevalent in staphylococci, we routinely use vancomycin (Vancocin) for empiric coverage. In patients who are allergic to vancomycin or cannot tolerate it, daptomycin (Cubicin) is an alternative.
Empiric gram-negative coverage is generally reserved for patients who present with systemic signs and symptoms, in whom delaying adequate coverage could have untoward consequences. We routinely use cefepime (Maxipime) for empiric gram-negative coverage in our institution. Other beta-lactam agents that provide coverage for gram-negative bacilli, especially Pseudomonas, are also appropriate in this setting.
Should the device be removed?
Superficial infection of the wound or incision site (eg, stitch abscess) early after implantation can be managed by conservative antibiotic therapy without removing the device. However, complete removal of the device system, including intracardiac leads, is necessary in all other presentations of device infection, even if the infection appears limited to the generator pocket.5,12 Leaving the device in place or removing parts of the device is associated with persistent or relapsed infection and is not advisable.17,26
Leaving the device in place may be necessary in extenuating circumstances, eg, if surgery would be too risky for the patient or if the patient refuses device removal or has a short life expectancy. In these cases, lifelong suppressive antibiotic therapy should be prescribed after an initial course of parenteral antibiotics.27 Antibiotic choices for long-term suppressive therapy should be guided by antimicrobial susceptibility testing and consultation with an infectious disease specialist.
How should the leads be removed?
Leads are extracted percutaneously in most cases. Percutaneous extraction is generally considered safe even in cases in which infection is complicated by lead vegetations, which raises concern about pulmonary embolization of detached vegetation fragments during extraction.5,20
Thoracotomy is generally reserved for patients who have cardiac complications (such as a cardiac abscess or the need to replace cardiac valves) or in whom attempts to extract the leads percutaneously are unsuccessful.
Details of the removal procedure and choice of extraction technique are beyond the scope of this paper and are best left to the discretion of the treating cardiologist or cardiac surgeon. Because of the potential for complications during percutaneous device removal, such as laceration of the superior vena cava or cardiac tamponade, the patient should be referred to a high-volume center where cardiothoracic intervention can be provided on an emergency basis if needed.
How long should antibiotic therapy go on?
An algorithm for deciding the duration of antibiotic therapy is shown in Figure 3. These guidelines, first published in 2007,17 were adopted by the American Heart Association in its updated statement on the management of CIED-related infections.5 However, it should be noted that these guidelines are not based on randomized clinical trials; rather, they represent expert opinion based on published series of patients with CIED-related infections.
In general, cases of device erosion or pocket infection can be treated with 1 to 2 weeks of appropriate antibiotic therapy based on antimicrobial susceptibility testing. However, cases of bloodstream infection require 2 to 4 weeks of antibiotic therapy—or sometimes even longer if associated complications are present, such as septic thrombosis, endocarditis, or osteomyelitis.
We favor parenteral antibiotics for the entire course of treatment. However, patients can be discharged from the hospital once the bloodstream infection has cleared, and the antibiotic course can be completed on an outpatient basis.
Outpatient antimicrobial monitoring
We recommend adherence to the Infectious Diseases Society of America’s guidelines for monitoring outpatient parenteral antimicrobial therapy.28
At discharge from the hospital, patients should be instructed to promptly call their primary care physician if they have a fever or notice inflammatory changes at the pocket site. If the patient reports such symptoms, repeat blood cultures should be ordered, and the patient should be monitored closely for signs of a relapse of infection.
A routine follow-up visit should be arranged at 2 weeks and at the end of parenteral antibiotic therapy (for patients receiving therapy for 4 weeks or longer) to make sure the infection has resolved.
When should a new device be implanted?
Before deciding when a new device should be implanted, one should carefully assess whether the patient still needs one. Studies indicate that up to 30% of patients may no longer require a cardiac device.17,18
However, we believe that removal of drains and closure of the old pocket are not necessary before implanting a new device in a different location (usually the contralateral pectoral area). Exceptions to this general principle are cases of valvular endocarditis, in which a minimum of 2 weeks is recommended between removal of an infected device (plus clearance of bloodstream infection) and implantation of a new device.
OUTCOMES OF INFECTION
Despite improvements in our understanding of how to manage CIED-related infection, the rates of morbidity and death remain significant.
The outcome, in part, depends on the clinical presentation and the patient’s comorbid conditions. In general, the death rate in patients with a pocket infection is less than 5%. However, in patients with endovascular infection, it may be as high as 20%.16–18 Other factors that affect the outcome include complications such as septic thrombosis, valvular endocarditis, or osteomyelitis; complications during device extraction; the need for open heart surgery; and the overall health of the patient.
Complete removal of the device system is a requisite for successful outcome, and the risk of death tends to be higher if only part of the infected CIED system is extracted.26
STRATEGIES TO PREVENT DEVICE INFECTION
Preventive efforts should focus on strategies to minimize the chances of contamination of the generator, leads, and pocket during implantation.29 Patients who are known to be colonized with methicillin-resistant S aureus may benefit from decolonization programs, which should include nasal application of mupirocin (Bactroban) ointment preoperatively.30 In addition, use of chlorhexidine for surgical-site antisepsis has been shown to reduce the risk of surgical site infection.31
Moreover, all patients should receive antibiotic prophylaxis before implantation of a CIED.32,33 Most institutions use a first-generation cephalosporin, such as cefazolin (Ancef), for this purpose.34 However, the increasing rate of methicillin resistance in staphylococci has led to the routine use of vancomycin for preoperative prophylaxis at some centers.18
Regardless of the antibiotic chosen for prophylaxis, protocols that ensure that all patients receive an appropriate antibiotic at the appropriate time are a key determinant in the success of these infection-control programs.
- Sohail MR, Wilson WR, Baddour LM. Infections of nonvalvular cardiovascular devices. In:Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia: Churchill Livingstone/Elsevier; 2010:1127–1142.
- Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol 2006; 48:590–591.
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med 2004; 350:1422–1429.
- Ferguson TB, Ferguson CL, Crites K, Crimmins-Reda P. The additional hospital costs generated in the management of complications of pacemaker and defibrillator implantations. J Thorac Cardiovasc Surg 1996; 111:742–751.
- Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477.
- Klug D, Balde M, Pavin D, et al; PEOPLE Study Group. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116:1349–1355.
- Sohail MR, Hussain S, Dib C, et al. Risk factor analysis of implantable cardioverter-defibrillator infections. Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). Boston, MA, Sept. 12–15, 2010.
- Lai KK, Fontecchio SA. Infections associated with implantable cardioverter defibrillators placed transvenously and via thoracotomies: epidemiology, infection control, and management. Clin Infect Dis 1998; 27:265–269.
- Mela T, McGovern BA, Garan H, et al. Long-term infection rates associated with the pectoral versus abdominal approach to cardioverter-defibrillator implants. Am J Cardiol 2001; 88:750–753.
- Al-Khatib SM, Lucas FL, Jollis JG, Malenka DJ, Wennberg DE. The relation between patients’ outcomes and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating Medicare beneficiaries. J Am Coll Cardiol 2005; 46:1536–1540.
- Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart 2009; 95:715–720.
- Sohail MR, Sultan OW, Raza SS. Contemporary management of cardiovascular implantable electronic device infections. Expert Rev Anti Infect Ther 2010; 8:831–839.
- Sohail MR, Uslan DZ, Khan AH, et al. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis 2007; 45:166–173.
- Uslan DZ, Sohail MR, St Sauver JL, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 2007; 167:669–675.
- Bloom H, Heeke B, Leon A, et al. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol 2006; 29:142–145.
- Le KY, Sohail MR, Friedman PA, et al for the Mayo Cardiovascular Infections Study Group. Clinical predictors of cardiovascular implantable electronic device-related infective endocarditis. Pacing Clin Electrophysiol2911; 34:450–459.
- Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007; 49:1851–1859.
- Tarakji KG, Chan EJ, Cantillon DJ, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm 2010; 7:1043–1047.
- Chamis AL, Peterson GE, Cabell CH, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 2001; 104:1029–1033.
- Sohail MR, Uslan DZ, Khan AH, et al. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc 2008; 83:46–53.
- Arber N, Pras E, Copperman Y, et al. Pacemaker endocarditis. Report of 44 cases and review of the literature. Medicine (Baltimore) 1994; 73:299–305.
- Sohail MR. Concerning diagnosis and management of pacemaker endocarditis [letter]. Pacing Clin Electrophysiol 2007; 30:829.
- Uslan DZ, Dowsley TF, Sohail MR, et al. Cardiovascular implantable electronic device infection in patients with Staphylococcus aureus bacteremia. Pacing Clin Electrophysiol 2009; 33:407–413.
- Madhavan M, Sohail MR, Friedman PA, et al. Outcomes in patients with cardiovascular implantable electronic devices and bacteremia due to Gram-positive cocci other than Staphylococcus aureus. Circ Arrhythm Electrophysiol 2010; 3:639–645.
- Uslan DZ, Sohail MR, Friedman PA, et al. Frequency of permanent pacemaker or implantable cardioverter-defibrillator infection in patients with gram-negative bacteremia. Clin Infect Dis 2006; 43:731–736.
- Margey R, McCann H, Blake G, et al. Contemporary management of and outcomes from cardiac device related infections. Europace 2010; 12:64–70.
- Baddour LM. Long-term suppressive antimicrobial therapy for intravascular device-related infections. Am J Med Sci 2001; 322:209–212.
- Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 2004; 38:1651–1672.
- Wenzel RP. Minimizing surgical-site infections. N Engl J Med 2010; 362:75–77.
- Bode LGM, Kluytmans JAJW, Wertheim HFL, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010; 362:9–17.
- Darouiche RO, Wall MJ, Itani KMF, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 2010; 362:18–26.
- Da Costa A, Kirkorian G, Cucherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation 1998; 97:1796–1801.
- de Oliveira JC, Martinelli M, Nishioka SA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, doubleblinded, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009; 2:29–34.
- Bertaglia E, Zerbo F, Zardo S, Barzan D, Zoppo F, Pascotto P. Antibiotic prophylaxis with a single dose of cefazolin during pacemaker implantation: incidence of long-term infective complications. Pacing Clin Electrophysiol 2006; 29:29–33.
- Sohail MR, Wilson WR, Baddour LM. Infections of nonvalvular cardiovascular devices. In:Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia: Churchill Livingstone/Elsevier; 2010:1127–1142.
- Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol 2006; 48:590–591.
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med 2004; 350:1422–1429.
- Ferguson TB, Ferguson CL, Crites K, Crimmins-Reda P. The additional hospital costs generated in the management of complications of pacemaker and defibrillator implantations. J Thorac Cardiovasc Surg 1996; 111:742–751.
- Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477.
- Klug D, Balde M, Pavin D, et al; PEOPLE Study Group. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116:1349–1355.
- Sohail MR, Hussain S, Dib C, et al. Risk factor analysis of implantable cardioverter-defibrillator infections. Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). Boston, MA, Sept. 12–15, 2010.
- Lai KK, Fontecchio SA. Infections associated with implantable cardioverter defibrillators placed transvenously and via thoracotomies: epidemiology, infection control, and management. Clin Infect Dis 1998; 27:265–269.
- Mela T, McGovern BA, Garan H, et al. Long-term infection rates associated with the pectoral versus abdominal approach to cardioverter-defibrillator implants. Am J Cardiol 2001; 88:750–753.
- Al-Khatib SM, Lucas FL, Jollis JG, Malenka DJ, Wennberg DE. The relation between patients’ outcomes and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating Medicare beneficiaries. J Am Coll Cardiol 2005; 46:1536–1540.
- Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart 2009; 95:715–720.
- Sohail MR, Sultan OW, Raza SS. Contemporary management of cardiovascular implantable electronic device infections. Expert Rev Anti Infect Ther 2010; 8:831–839.
- Sohail MR, Uslan DZ, Khan AH, et al. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis 2007; 45:166–173.
- Uslan DZ, Sohail MR, St Sauver JL, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 2007; 167:669–675.
- Bloom H, Heeke B, Leon A, et al. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol 2006; 29:142–145.
- Le KY, Sohail MR, Friedman PA, et al for the Mayo Cardiovascular Infections Study Group. Clinical predictors of cardiovascular implantable electronic device-related infective endocarditis. Pacing Clin Electrophysiol2911; 34:450–459.
- Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007; 49:1851–1859.
- Tarakji KG, Chan EJ, Cantillon DJ, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm 2010; 7:1043–1047.
- Chamis AL, Peterson GE, Cabell CH, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 2001; 104:1029–1033.
- Sohail MR, Uslan DZ, Khan AH, et al. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc 2008; 83:46–53.
- Arber N, Pras E, Copperman Y, et al. Pacemaker endocarditis. Report of 44 cases and review of the literature. Medicine (Baltimore) 1994; 73:299–305.
- Sohail MR. Concerning diagnosis and management of pacemaker endocarditis [letter]. Pacing Clin Electrophysiol 2007; 30:829.
- Uslan DZ, Dowsley TF, Sohail MR, et al. Cardiovascular implantable electronic device infection in patients with Staphylococcus aureus bacteremia. Pacing Clin Electrophysiol 2009; 33:407–413.
- Madhavan M, Sohail MR, Friedman PA, et al. Outcomes in patients with cardiovascular implantable electronic devices and bacteremia due to Gram-positive cocci other than Staphylococcus aureus. Circ Arrhythm Electrophysiol 2010; 3:639–645.
- Uslan DZ, Sohail MR, Friedman PA, et al. Frequency of permanent pacemaker or implantable cardioverter-defibrillator infection in patients with gram-negative bacteremia. Clin Infect Dis 2006; 43:731–736.
- Margey R, McCann H, Blake G, et al. Contemporary management of and outcomes from cardiac device related infections. Europace 2010; 12:64–70.
- Baddour LM. Long-term suppressive antimicrobial therapy for intravascular device-related infections. Am J Med Sci 2001; 322:209–212.
- Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 2004; 38:1651–1672.
- Wenzel RP. Minimizing surgical-site infections. N Engl J Med 2010; 362:75–77.
- Bode LGM, Kluytmans JAJW, Wertheim HFL, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010; 362:9–17.
- Darouiche RO, Wall MJ, Itani KMF, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 2010; 362:18–26.
- Da Costa A, Kirkorian G, Cucherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation 1998; 97:1796–1801.
- de Oliveira JC, Martinelli M, Nishioka SA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, doubleblinded, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009; 2:29–34.
- Bertaglia E, Zerbo F, Zardo S, Barzan D, Zoppo F, Pascotto P. Antibiotic prophylaxis with a single dose of cefazolin during pacemaker implantation: incidence of long-term infective complications. Pacing Clin Electrophysiol 2006; 29:29–33.
KEY POINTS
- Although inflammatory signs at the generator pocket are the most common presentation of an infection occurring soon after the device is implanted, positive blood cultures may be the sole manifestation of a late-onset endovascular infection.
- Staphylococci are the most common pathogens in both pocket infections and endovascular infections.
- Two sets of blood cultures should be obtained in all patients suspected of having a cardiac device infection.
- Transesophageal echocardiography should be ordered in all patients with suspected cardiac device infection who have positive blood cultures, as it can identify intracardiac complications of infection and assess for evidence of cardiac valve involvement.