User login
Gemcitabine-Induced Pseudocellulitis
To the Editor:
Gemcitabine is a nucleoside analogue used to treat a variety of solid and hematologic malignancies. Cutaneous toxicities include radiation recall dermatitis and erysipelaslike reactions that occur in areas not previously treated with radiation. Often referred to as pseudocellulitis, these reactions generally have been reported in areas of lymphedema in patients with solid malignancies.1-6 Herein, we report a rare case of gemcitabine-induced pseudocellulitis on the legs in a patient with a history of hematologic malignancy and total body irradiation (TBI).
A 61-year-old woman with history of peripheral T-cell lymphoma presented to the emergency department at our institution with acute-onset redness, tenderness, and swelling of the legs that was concerning for cellulitis. The patient’s history was notable for receiving gemcitabine 1000 mg/m2 for treatment of refractory lymphoma (12 and 4 days prior to presentation) as well as lymphedema of the legs. Her complete treatment course included multiple rounds of chemotherapy and matched unrelated donor nonmyeloablative allogeneic stem cell transplantation with a single dose of TBI at 200 cGy at our institution. Her transplant was complicated only by mild cutaneous graft-versus-host disease, which resolved with prednisone and tacrolimus.
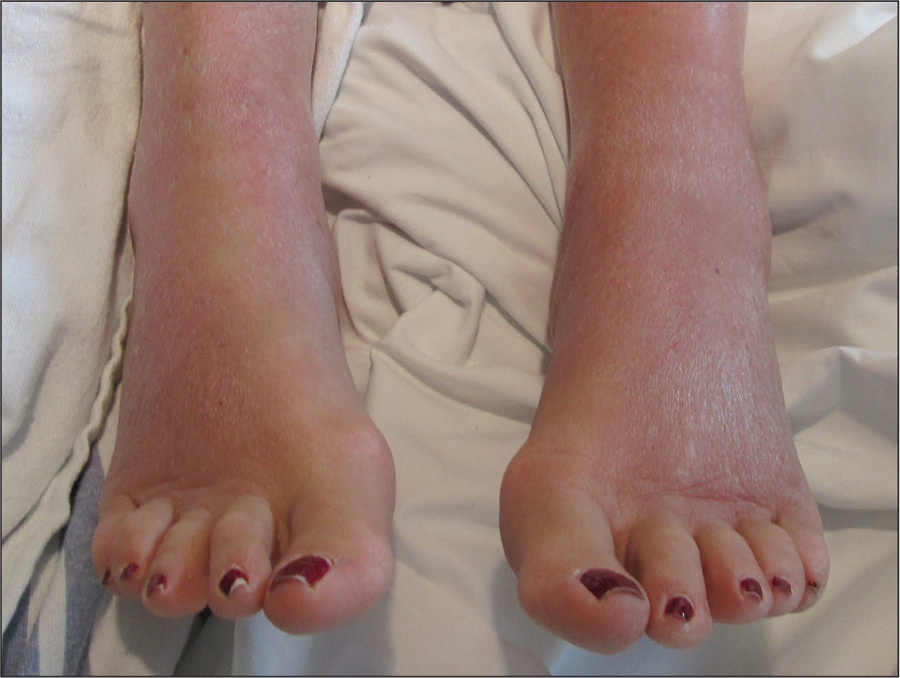
On physical examination, the patient was afebrile with symmetric erythema and induration extending from the bilateral knees to the dorsal feet. A complete blood cell count was notable for a white blood cell count of 5400/µL (reference range, 4500–11,000/µL) and a platelet count of 96,000/µL (reference range, 150,000–400,000/µL). Plain film radiographs of the bilateral ankles were remarkable only for moderate subcutaneous edema. She received vancomycin in the emergency department and was admitted to the oncology service. Blood cultures drawn on admission were negative. Dermatology was consulted on admission, and a diagnosis of pseudocellulitis was made in conjunction with oncology (Figure). Antibiotics were held, and the patient was treated symptomatically with ibuprofen and was discharged 1 day after admission. The reaction resolved after 1 week with the use of diphenhydramine, nonsteroidal anti-inflammatory drugs, and compression. The patient was not rechallenged with gemcitabine.
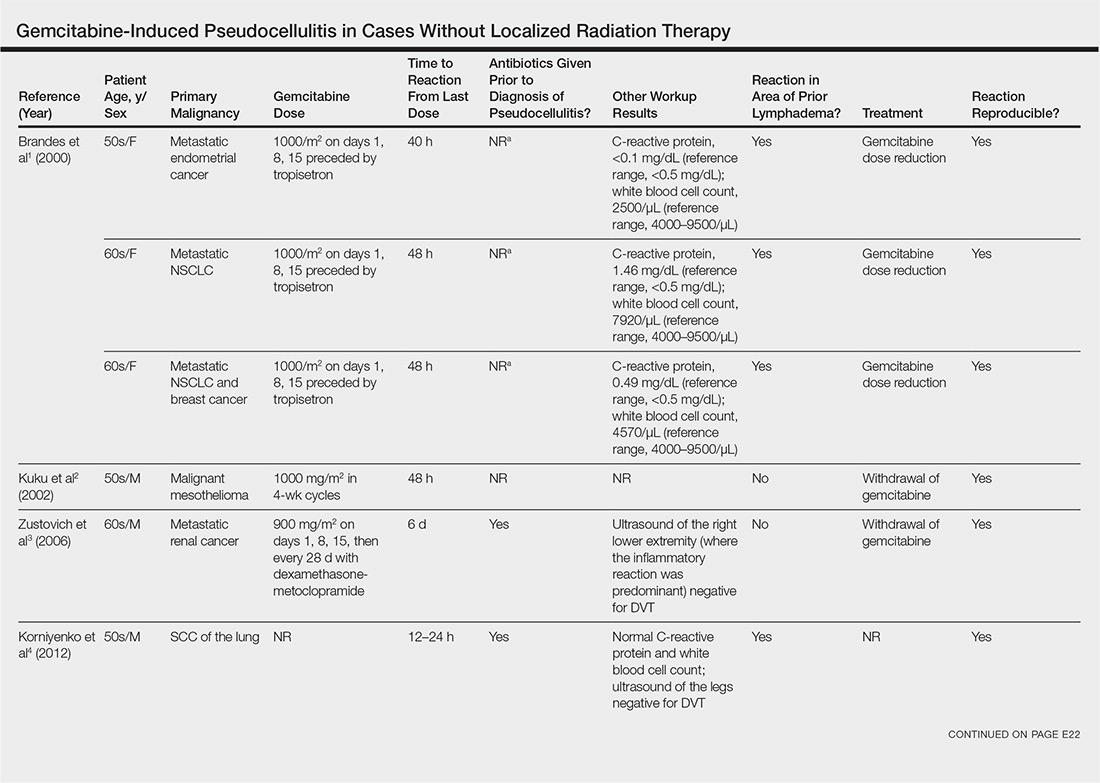
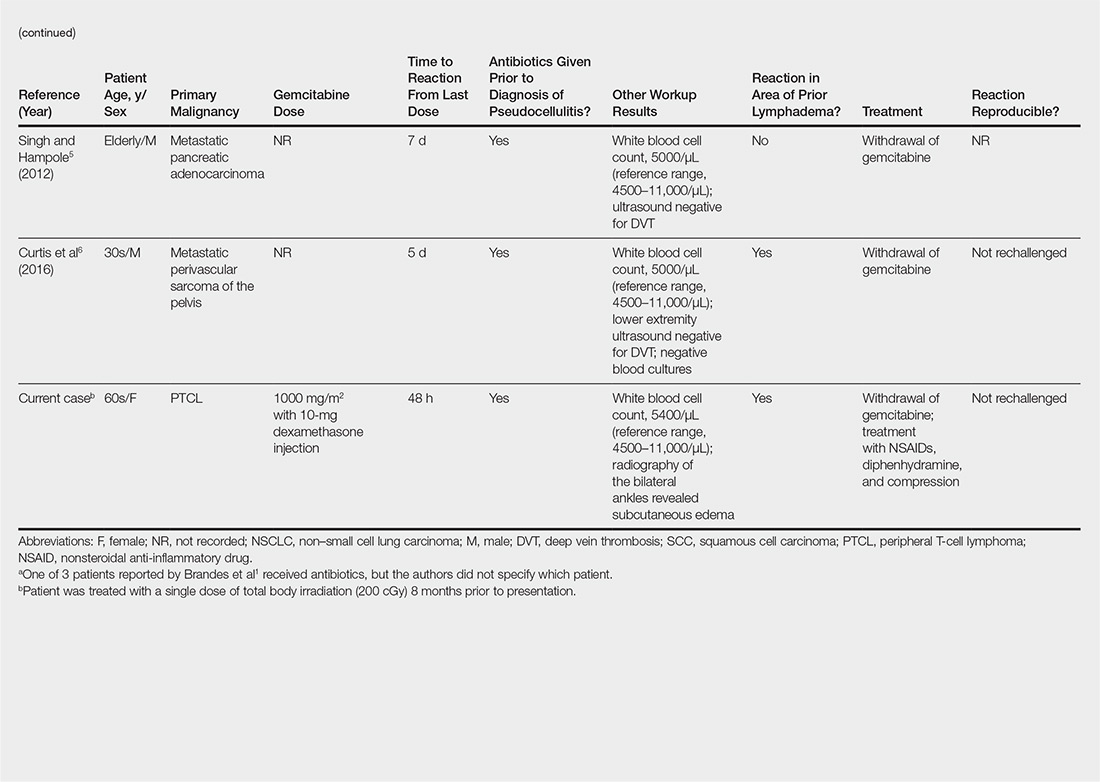
Gemcitabine-induced pseudocellulitis is a rare cutaneous side effect of gemcitabine therapy. Reported cases have suggested key characteristics of pseudocellulitis (Table). The reaction is characterized by localized erythema, edema, and tenderness of the skin, with onset generally 48 hours to 1 week after receiving gemcitabine.1-6 Lymphedema appears to be a risk factor.1,3-5 Six cases (including the current case) demonstrated confinement of these findings to areas of prior lymphedema.1,4,6 Infectious workup is negative, and rechallenging with gemcitabine likely will reproduce the reaction. Unlike radiation recall dermatitis, there is no prior localized radiation exposure.
Our patient had a history of hematologic malignancy and a one-time low-dose TBI of 200 cGy, unlike the other reported cases described in the Table. It is difficult to attribute our patient’s localized eruption to radiation recall given the history of TBI. The clinical examination, laboratory findings, and time frame of the reaction were consistent with gemcitabine-induced pseudocellulitis.
It is important to be aware of pseudocellulitis as a possible complication of gemcitabine therapy in patients without history of localized radiation. Early recognition of pseudocellulitis may prevent unnecessary exposure to broad-spectrum antibiotics. Patients’ temperature, white blood cell count, clinical examination, and potentially ancillary studies (eg, vascular studies, inflammatory markers) should be reviewed carefully to determine whether there is an infectious or alternate etiology. In patients with known prior lymphedema, it may be beneficial to educate clinicians and patients alike about this potential adverse effect of gemcitabine and the high likelihood of recurrence on re-exposure.
- Brandes A, Reichmann U, Plasswilm L, et al. Time- and dose-limiting erysipeloid rash confined to areas of lymphedema following treatment with gemcitabine—a report of three cases. Anticancer Drugs. 2000;11:15-17.
- Kuku I, Kaya E, Sevinc A, et al. Gemcitabine-induced erysipeloid skin lesions in a patient with malignant mesothelioma. J Eur Acad Dermatol Venereol. 2002;16:271-272.
- Zustovich F, Pavei P, Cartei G. Erysipeloid skin toxicity induced by gemcitabine. J Eur Acad Dermatol Venereol. 2006;20:757-758.
- Korniyenko A, Lozada J, Ranade A, et al. Recurrent lower extremity pseudocellulitis. Am J Ther. 2012;19:e141-e142.
- Singh A, Hampole H. Gemcitabine associated pseudocellulitis [published online June 14, 2012]. J Gen Intern Med. 2012;27:1721.
- Curtis S, Hong S, Gucalp R, et al. Gemcitabine-induced pseudocellulitis in a patient with recurrent lymphedema: a case report and review of the current literature. Am J Ther. 2016;23:e321-323.
To the Editor:
Gemcitabine is a nucleoside analogue used to treat a variety of solid and hematologic malignancies. Cutaneous toxicities include radiation recall dermatitis and erysipelaslike reactions that occur in areas not previously treated with radiation. Often referred to as pseudocellulitis, these reactions generally have been reported in areas of lymphedema in patients with solid malignancies.1-6 Herein, we report a rare case of gemcitabine-induced pseudocellulitis on the legs in a patient with a history of hematologic malignancy and total body irradiation (TBI).
A 61-year-old woman with history of peripheral T-cell lymphoma presented to the emergency department at our institution with acute-onset redness, tenderness, and swelling of the legs that was concerning for cellulitis. The patient’s history was notable for receiving gemcitabine 1000 mg/m2 for treatment of refractory lymphoma (12 and 4 days prior to presentation) as well as lymphedema of the legs. Her complete treatment course included multiple rounds of chemotherapy and matched unrelated donor nonmyeloablative allogeneic stem cell transplantation with a single dose of TBI at 200 cGy at our institution. Her transplant was complicated only by mild cutaneous graft-versus-host disease, which resolved with prednisone and tacrolimus.
On physical examination, the patient was afebrile with symmetric erythema and induration extending from the bilateral knees to the dorsal feet. A complete blood cell count was notable for a white blood cell count of 5400/µL (reference range, 4500–11,000/µL) and a platelet count of 96,000/µL (reference range, 150,000–400,000/µL). Plain film radiographs of the bilateral ankles were remarkable only for moderate subcutaneous edema. She received vancomycin in the emergency department and was admitted to the oncology service. Blood cultures drawn on admission were negative. Dermatology was consulted on admission, and a diagnosis of pseudocellulitis was made in conjunction with oncology (Figure). Antibiotics were held, and the patient was treated symptomatically with ibuprofen and was discharged 1 day after admission. The reaction resolved after 1 week with the use of diphenhydramine, nonsteroidal anti-inflammatory drugs, and compression. The patient was not rechallenged with gemcitabine.
Gemcitabine-induced pseudocellulitis is a rare cutaneous side effect of gemcitabine therapy. Reported cases have suggested key characteristics of pseudocellulitis (Table). The reaction is characterized by localized erythema, edema, and tenderness of the skin, with onset generally 48 hours to 1 week after receiving gemcitabine.1-6 Lymphedema appears to be a risk factor.1,3-5 Six cases (including the current case) demonstrated confinement of these findings to areas of prior lymphedema.1,4,6 Infectious workup is negative, and rechallenging with gemcitabine likely will reproduce the reaction. Unlike radiation recall dermatitis, there is no prior localized radiation exposure.
Our patient had a history of hematologic malignancy and a one-time low-dose TBI of 200 cGy, unlike the other reported cases described in the Table. It is difficult to attribute our patient’s localized eruption to radiation recall given the history of TBI. The clinical examination, laboratory findings, and time frame of the reaction were consistent with gemcitabine-induced pseudocellulitis.
It is important to be aware of pseudocellulitis as a possible complication of gemcitabine therapy in patients without history of localized radiation. Early recognition of pseudocellulitis may prevent unnecessary exposure to broad-spectrum antibiotics. Patients’ temperature, white blood cell count, clinical examination, and potentially ancillary studies (eg, vascular studies, inflammatory markers) should be reviewed carefully to determine whether there is an infectious or alternate etiology. In patients with known prior lymphedema, it may be beneficial to educate clinicians and patients alike about this potential adverse effect of gemcitabine and the high likelihood of recurrence on re-exposure.
To the Editor:
Gemcitabine is a nucleoside analogue used to treat a variety of solid and hematologic malignancies. Cutaneous toxicities include radiation recall dermatitis and erysipelaslike reactions that occur in areas not previously treated with radiation. Often referred to as pseudocellulitis, these reactions generally have been reported in areas of lymphedema in patients with solid malignancies.1-6 Herein, we report a rare case of gemcitabine-induced pseudocellulitis on the legs in a patient with a history of hematologic malignancy and total body irradiation (TBI).
A 61-year-old woman with history of peripheral T-cell lymphoma presented to the emergency department at our institution with acute-onset redness, tenderness, and swelling of the legs that was concerning for cellulitis. The patient’s history was notable for receiving gemcitabine 1000 mg/m2 for treatment of refractory lymphoma (12 and 4 days prior to presentation) as well as lymphedema of the legs. Her complete treatment course included multiple rounds of chemotherapy and matched unrelated donor nonmyeloablative allogeneic stem cell transplantation with a single dose of TBI at 200 cGy at our institution. Her transplant was complicated only by mild cutaneous graft-versus-host disease, which resolved with prednisone and tacrolimus.
On physical examination, the patient was afebrile with symmetric erythema and induration extending from the bilateral knees to the dorsal feet. A complete blood cell count was notable for a white blood cell count of 5400/µL (reference range, 4500–11,000/µL) and a platelet count of 96,000/µL (reference range, 150,000–400,000/µL). Plain film radiographs of the bilateral ankles were remarkable only for moderate subcutaneous edema. She received vancomycin in the emergency department and was admitted to the oncology service. Blood cultures drawn on admission were negative. Dermatology was consulted on admission, and a diagnosis of pseudocellulitis was made in conjunction with oncology (Figure). Antibiotics were held, and the patient was treated symptomatically with ibuprofen and was discharged 1 day after admission. The reaction resolved after 1 week with the use of diphenhydramine, nonsteroidal anti-inflammatory drugs, and compression. The patient was not rechallenged with gemcitabine.
Gemcitabine-induced pseudocellulitis is a rare cutaneous side effect of gemcitabine therapy. Reported cases have suggested key characteristics of pseudocellulitis (Table). The reaction is characterized by localized erythema, edema, and tenderness of the skin, with onset generally 48 hours to 1 week after receiving gemcitabine.1-6 Lymphedema appears to be a risk factor.1,3-5 Six cases (including the current case) demonstrated confinement of these findings to areas of prior lymphedema.1,4,6 Infectious workup is negative, and rechallenging with gemcitabine likely will reproduce the reaction. Unlike radiation recall dermatitis, there is no prior localized radiation exposure.
Our patient had a history of hematologic malignancy and a one-time low-dose TBI of 200 cGy, unlike the other reported cases described in the Table. It is difficult to attribute our patient’s localized eruption to radiation recall given the history of TBI. The clinical examination, laboratory findings, and time frame of the reaction were consistent with gemcitabine-induced pseudocellulitis.
It is important to be aware of pseudocellulitis as a possible complication of gemcitabine therapy in patients without history of localized radiation. Early recognition of pseudocellulitis may prevent unnecessary exposure to broad-spectrum antibiotics. Patients’ temperature, white blood cell count, clinical examination, and potentially ancillary studies (eg, vascular studies, inflammatory markers) should be reviewed carefully to determine whether there is an infectious or alternate etiology. In patients with known prior lymphedema, it may be beneficial to educate clinicians and patients alike about this potential adverse effect of gemcitabine and the high likelihood of recurrence on re-exposure.
- Brandes A, Reichmann U, Plasswilm L, et al. Time- and dose-limiting erysipeloid rash confined to areas of lymphedema following treatment with gemcitabine—a report of three cases. Anticancer Drugs. 2000;11:15-17.
- Kuku I, Kaya E, Sevinc A, et al. Gemcitabine-induced erysipeloid skin lesions in a patient with malignant mesothelioma. J Eur Acad Dermatol Venereol. 2002;16:271-272.
- Zustovich F, Pavei P, Cartei G. Erysipeloid skin toxicity induced by gemcitabine. J Eur Acad Dermatol Venereol. 2006;20:757-758.
- Korniyenko A, Lozada J, Ranade A, et al. Recurrent lower extremity pseudocellulitis. Am J Ther. 2012;19:e141-e142.
- Singh A, Hampole H. Gemcitabine associated pseudocellulitis [published online June 14, 2012]. J Gen Intern Med. 2012;27:1721.
- Curtis S, Hong S, Gucalp R, et al. Gemcitabine-induced pseudocellulitis in a patient with recurrent lymphedema: a case report and review of the current literature. Am J Ther. 2016;23:e321-323.
- Brandes A, Reichmann U, Plasswilm L, et al. Time- and dose-limiting erysipeloid rash confined to areas of lymphedema following treatment with gemcitabine—a report of three cases. Anticancer Drugs. 2000;11:15-17.
- Kuku I, Kaya E, Sevinc A, et al. Gemcitabine-induced erysipeloid skin lesions in a patient with malignant mesothelioma. J Eur Acad Dermatol Venereol. 2002;16:271-272.
- Zustovich F, Pavei P, Cartei G. Erysipeloid skin toxicity induced by gemcitabine. J Eur Acad Dermatol Venereol. 2006;20:757-758.
- Korniyenko A, Lozada J, Ranade A, et al. Recurrent lower extremity pseudocellulitis. Am J Ther. 2012;19:e141-e142.
- Singh A, Hampole H. Gemcitabine associated pseudocellulitis [published online June 14, 2012]. J Gen Intern Med. 2012;27:1721.
- Curtis S, Hong S, Gucalp R, et al. Gemcitabine-induced pseudocellulitis in a patient with recurrent lymphedema: a case report and review of the current literature. Am J Ther. 2016;23:e321-323.
Practice Points
- Gemcitabine is a nucleoside analogue used to treat a variety of solid and hematologic malignancies.
- Gemcitabine-induced pseudocellulitis is a rare cutaneous side effect of gemcitabine therapy.
- Early recognition of pseudocellulitis may prevent unnecessary exposure to broad-spectrum antibiotics.