User login
When Reducing Low-Value Care in Hospital Medicine Saves Money, Who Benefits?
Physicians face growing pressure to reduce their use of “low value” care—services that provide either little to no benefit, little benefit relative to cost, or outsized potential harm compared to benefit. One emerging policy solution for deterring such services is to financially penalize physicians who prescribe them.1,2
Physicians’ willingness to support such policies may depend on who they believe benefits from reductions in low-value care. In previous studies of cancer screening, the more that primary care physicians felt that the money saved from cost-containment efforts went to insurance company profits rather than to patients, the less willing they were to use less expensive cancer screening approaches.3
Similarly, physicians may be more likely to support financial penalty policies if they perceive that the benefits from reducing low-value care accrue to patients (eg, lower out-of-pocket costs) rather than insurers or hospitals (eg, profits and salaries of their leaders). If present, such perceptions could inform incentive design. We explored the hypothesis that support of financial penalties
METHODS
Study Sample
By using a panel of internists maintained by the American College of Physicians, we conducted a randomized, web-based survey among 484 physicians who were either internal medicine residents or internal medicine physicians practicing hospital medicine.
Survey Instrument
Respondents used a 5-point scale (“strongly disagree” to “strongly agree”) to indicate their agreement with a policy that financially penalizes physicians for prescribing services that provide few benefits to patients. Respondents were asked to simultaneously consider the following hospital medicine services, deemed to be low value based on medical evidence and consensus guidelines4: (1) placing, and leaving in, urinary catheters for urine output monitoring in noncritically ill patients, (2) ordering continuous telemetry monitoring for nonintensive care unit patients without a protocol governing continuation, and (3) prescribing stress ulcer prophylaxis for medical patients not at a high risk for gastrointestinal complications. Policy support was defined as “somewhat” or “strongly” agreeing with the policy. As part of another study of this physician cohort, this question varied in how the harm of low-value services was framed: either as harm to patients, to society, or to hospitals and insurers as institutions. Respondent characteristics were balanced across survey versions, and for the current analysis, we pooled responses across all versions.
All other questions in the survey, described in detail elsewhere,5 were identical for all respondents. For this analysis, we focused on a question that asked physicians to assume that reducing these services saves money without harming the quality of care and to rate on a 4-point scale (“none” to “a lot”) how much of the money saved would ultimately go to the following 6 nonmutually exclusive areas: (a) other healthcare services for patients, (b) reduced charges to patients’ employers or insurers, (c) reduced out-of-pocket costs for patients, (d) salaries and bonuses for physicians, (e) salaries and profits for insurance companies and their leaders, and (f) salaries and profits for hospitals and/or health systems and their leaders.
Based on the positive correlation identified between the first 4 items (a to d) and negative correlation with the other 2 items (e and f), we reverse-coded the latter 2 and summed all 6 into a single-outcome scale, effectively representing the degree to which the money saved from reducing low-value services accrues generally to patients or physicians instead of to hospitals, insurance companies, and their leaders. The Cronbach alpha for the scale was 0.74, indicating acceptable reliability. Based on scale responses, we dichotomized respondents at the median into those who believe that the money saved from reducing low-value services would accrue as benefits to patients or physicians and those who believe benefits accrue to insurance companies or hospitals and/or health systems and their leaders. The protocol was exempted by the University of Pennsylvania Institutional Review Board.
Statistical Analysis
We used a χ2 test and multivariable logistic regression analysis to evaluate the association between policy support and physician beliefs about who benefits from reductions in low-value care. A χ2 test and a Kruskal-Wallis test were also used to evaluate the association between other respondent characteristics and beliefs about who benefits from reductions in low-value care. Analyses were performed by using Stata version 14.1 (StataCorp, College Station, TX). Tests of significance were 2-tailed at an alpha of .05.
RESULTS
Compared with nonrespondents, the 187 physicians who responded (39% response rate) were more likely to be female (30% vs 26%, P = 0.001), older (mean age 41 vs 36 years old, P < 0.001), and practicing clinicians rather than internal medicine residents (87% vs 69%, P < 0.001). Twenty-one percent reported that their personal compensation was tied to cost incentives.
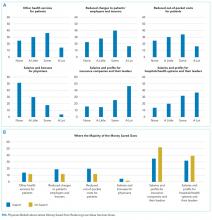
Overall, respondents believed that more of any money saved from reducing low-value services would go to profits and leadership salaries for insurance companies and hospitals and/or health systems rather than to patients (panel A of Figure). Few respondents felt that the money saved would ultimately go toward physician compensation.
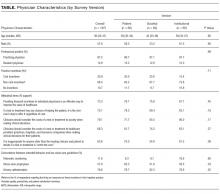
Sixty-six percent of physicians who supported penalties believed that benefits from reducing low-value care accrue to patients or physicians, compared to 39% among those not supporting penalties (P < 0.001). In multivariable analyses, policy support was associated with the belief that the money saved from reducing low-value services would accrue as benefits to patients or physicians rather than as salaries and profits for insurance companies or hospitals and/or health systems and their leaders (Table). There were no statistically significant associations between respondent age, gender, or professional status and beliefs about who benefits from reductions in low-value care.
DISCUSSION
Despite ongoing efforts to highlight how reducing low-value care benefits patients, physicians in our sample did not believe that much of the money saved would benefit patients.
This result may reflect that while some care patterns are considered low value because they provide little benefit at a high cost, others yield potential harm, regardless of cost. For example, limiting stress ulcer prophylaxis largely aims to avoid clinical harm (eg, adverse drug effects and nosocomial infections). Limiting telemetric monitoring largely aims to reduce costly care that provides only limited benefit. Therefore, the nature of potential benefit to patients is very different—improved clinical outcomes in the former and potential cost savings in the latter. Future studies could separately assess physician attitudes about these 2 different definitions of low-value services.
Our study also demonstrates that the more physicians believe that much of any money saved goes to the profits and salaries of insurance companies, hospitals and/or health systems, and their leaders rather than to patients, the less likely they are to support policies financially penalizing physicians for prescribing low-value services.
Our study does not address why physicians have the beliefs that they have, but a likely explanation, at least in part, is that financial flows in healthcare are complex and tangled. Indeed, a clear understanding of who actually benefits is so hard to determine that these stated beliefs may really derive from views of power or justice rather than from some understanding of funds flow. Whether or not ideological attitudes underlie these expressed beliefs, policymakers and healthcare institutions might be advised to increase transparency about how cost savings are realized and whom they benefit.
Our analysis has limitations. Although it provides insight into where physicians believe relative amounts of money saved go with respect to 6 common options, the study did not include an exhaustive list of possibilities. The response rate also limits the representativeness of our results. Additionally, the study design prevents conclusions about causality; we cannot determine whether the belief that savings go to insurance companies and their executives is what reduces physicians’ enthusiasm for penalties, whether the causal association is in the opposite direction, or whether the 2 factors are linked in another way.
Nonetheless, our findings are consistent with a sense of healthcare justice in which physicians support penalties imposed on themselves only if the resulting benefits accrue to patients rather than to corporate or organizational interests. Effective physician penalties will likely need to address the belief that insurers and provider organizations stand to gain more than patients when low-value care services are reduced.
Disclosure
Drs. Liao, Schapira, Mitra, and Weissman have no conflicts to disclose. Dr. Navathe serves as advisor to Navvis and Company, Navigant Inc., Lynx Medical, Indegene Inc., and Sutherland Global Services and receives an honorarium from Elsevier Press, none of which have relationship to this manuscript. Dr. Asch is a partner and partial owner of VAL Health, which has no relationship to this manuscript.
Funding
This work was supported by The Leonard Davis Institute of Health Economics at the University of Pennsylvania, which had no role in the study design, data collection, analysis, or interpretation of results.
1. Berwick DM. Avoiding overuse – the next quality frontier. Lancet. 2017;390(10090):102-104. PubMed
2. Centers for Medicare and Medicaid Services. CMS response to Public Comments on Non-Recommended PSA-Based Screening Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/eCQM-Development-and-Maintenance-for-Eligible-Professionals_CMS_PSA_Response_Public-Comment.pdf. Accessed September 18, 2017.
3. Asch DA, Jepson C, Hershey JC, Baron J, Ubel PA. When Money is Saved by Reducing Healthcare Costs, Where Do US Primary Care Physicians Think the Money Goes? Am J Manag Care. 2003;9(6):438-442. PubMed
4. Society of Hospital Medicine. Choosing Wisely. https://www.hospitalmedicine.org/choosingwisely. Accessed September 18, 2017.
5. Liao JM, Navathe AS, Schapira MS, Weissman A, Mitra N, Asch DAA. Penalizing Physicians for Low Value Care in Hospital Medicine: A Randomized Survey. J Hosp Med. 2017. (In press). PubMed
Physicians face growing pressure to reduce their use of “low value” care—services that provide either little to no benefit, little benefit relative to cost, or outsized potential harm compared to benefit. One emerging policy solution for deterring such services is to financially penalize physicians who prescribe them.1,2
Physicians’ willingness to support such policies may depend on who they believe benefits from reductions in low-value care. In previous studies of cancer screening, the more that primary care physicians felt that the money saved from cost-containment efforts went to insurance company profits rather than to patients, the less willing they were to use less expensive cancer screening approaches.3
Similarly, physicians may be more likely to support financial penalty policies if they perceive that the benefits from reducing low-value care accrue to patients (eg, lower out-of-pocket costs) rather than insurers or hospitals (eg, profits and salaries of their leaders). If present, such perceptions could inform incentive design. We explored the hypothesis that support of financial penalties
METHODS
Study Sample
By using a panel of internists maintained by the American College of Physicians, we conducted a randomized, web-based survey among 484 physicians who were either internal medicine residents or internal medicine physicians practicing hospital medicine.
Survey Instrument
Respondents used a 5-point scale (“strongly disagree” to “strongly agree”) to indicate their agreement with a policy that financially penalizes physicians for prescribing services that provide few benefits to patients. Respondents were asked to simultaneously consider the following hospital medicine services, deemed to be low value based on medical evidence and consensus guidelines4: (1) placing, and leaving in, urinary catheters for urine output monitoring in noncritically ill patients, (2) ordering continuous telemetry monitoring for nonintensive care unit patients without a protocol governing continuation, and (3) prescribing stress ulcer prophylaxis for medical patients not at a high risk for gastrointestinal complications. Policy support was defined as “somewhat” or “strongly” agreeing with the policy. As part of another study of this physician cohort, this question varied in how the harm of low-value services was framed: either as harm to patients, to society, or to hospitals and insurers as institutions. Respondent characteristics were balanced across survey versions, and for the current analysis, we pooled responses across all versions.
All other questions in the survey, described in detail elsewhere,5 were identical for all respondents. For this analysis, we focused on a question that asked physicians to assume that reducing these services saves money without harming the quality of care and to rate on a 4-point scale (“none” to “a lot”) how much of the money saved would ultimately go to the following 6 nonmutually exclusive areas: (a) other healthcare services for patients, (b) reduced charges to patients’ employers or insurers, (c) reduced out-of-pocket costs for patients, (d) salaries and bonuses for physicians, (e) salaries and profits for insurance companies and their leaders, and (f) salaries and profits for hospitals and/or health systems and their leaders.
Based on the positive correlation identified between the first 4 items (a to d) and negative correlation with the other 2 items (e and f), we reverse-coded the latter 2 and summed all 6 into a single-outcome scale, effectively representing the degree to which the money saved from reducing low-value services accrues generally to patients or physicians instead of to hospitals, insurance companies, and their leaders. The Cronbach alpha for the scale was 0.74, indicating acceptable reliability. Based on scale responses, we dichotomized respondents at the median into those who believe that the money saved from reducing low-value services would accrue as benefits to patients or physicians and those who believe benefits accrue to insurance companies or hospitals and/or health systems and their leaders. The protocol was exempted by the University of Pennsylvania Institutional Review Board.
Statistical Analysis
We used a χ2 test and multivariable logistic regression analysis to evaluate the association between policy support and physician beliefs about who benefits from reductions in low-value care. A χ2 test and a Kruskal-Wallis test were also used to evaluate the association between other respondent characteristics and beliefs about who benefits from reductions in low-value care. Analyses were performed by using Stata version 14.1 (StataCorp, College Station, TX). Tests of significance were 2-tailed at an alpha of .05.
RESULTS
Compared with nonrespondents, the 187 physicians who responded (39% response rate) were more likely to be female (30% vs 26%, P = 0.001), older (mean age 41 vs 36 years old, P < 0.001), and practicing clinicians rather than internal medicine residents (87% vs 69%, P < 0.001). Twenty-one percent reported that their personal compensation was tied to cost incentives.
Overall, respondents believed that more of any money saved from reducing low-value services would go to profits and leadership salaries for insurance companies and hospitals and/or health systems rather than to patients (panel A of Figure). Few respondents felt that the money saved would ultimately go toward physician compensation.
Sixty-six percent of physicians who supported penalties believed that benefits from reducing low-value care accrue to patients or physicians, compared to 39% among those not supporting penalties (P < 0.001). In multivariable analyses, policy support was associated with the belief that the money saved from reducing low-value services would accrue as benefits to patients or physicians rather than as salaries and profits for insurance companies or hospitals and/or health systems and their leaders (Table). There were no statistically significant associations between respondent age, gender, or professional status and beliefs about who benefits from reductions in low-value care.
DISCUSSION
Despite ongoing efforts to highlight how reducing low-value care benefits patients, physicians in our sample did not believe that much of the money saved would benefit patients.
This result may reflect that while some care patterns are considered low value because they provide little benefit at a high cost, others yield potential harm, regardless of cost. For example, limiting stress ulcer prophylaxis largely aims to avoid clinical harm (eg, adverse drug effects and nosocomial infections). Limiting telemetric monitoring largely aims to reduce costly care that provides only limited benefit. Therefore, the nature of potential benefit to patients is very different—improved clinical outcomes in the former and potential cost savings in the latter. Future studies could separately assess physician attitudes about these 2 different definitions of low-value services.
Our study also demonstrates that the more physicians believe that much of any money saved goes to the profits and salaries of insurance companies, hospitals and/or health systems, and their leaders rather than to patients, the less likely they are to support policies financially penalizing physicians for prescribing low-value services.
Our study does not address why physicians have the beliefs that they have, but a likely explanation, at least in part, is that financial flows in healthcare are complex and tangled. Indeed, a clear understanding of who actually benefits is so hard to determine that these stated beliefs may really derive from views of power or justice rather than from some understanding of funds flow. Whether or not ideological attitudes underlie these expressed beliefs, policymakers and healthcare institutions might be advised to increase transparency about how cost savings are realized and whom they benefit.
Our analysis has limitations. Although it provides insight into where physicians believe relative amounts of money saved go with respect to 6 common options, the study did not include an exhaustive list of possibilities. The response rate also limits the representativeness of our results. Additionally, the study design prevents conclusions about causality; we cannot determine whether the belief that savings go to insurance companies and their executives is what reduces physicians’ enthusiasm for penalties, whether the causal association is in the opposite direction, or whether the 2 factors are linked in another way.
Nonetheless, our findings are consistent with a sense of healthcare justice in which physicians support penalties imposed on themselves only if the resulting benefits accrue to patients rather than to corporate or organizational interests. Effective physician penalties will likely need to address the belief that insurers and provider organizations stand to gain more than patients when low-value care services are reduced.
Disclosure
Drs. Liao, Schapira, Mitra, and Weissman have no conflicts to disclose. Dr. Navathe serves as advisor to Navvis and Company, Navigant Inc., Lynx Medical, Indegene Inc., and Sutherland Global Services and receives an honorarium from Elsevier Press, none of which have relationship to this manuscript. Dr. Asch is a partner and partial owner of VAL Health, which has no relationship to this manuscript.
Funding
This work was supported by The Leonard Davis Institute of Health Economics at the University of Pennsylvania, which had no role in the study design, data collection, analysis, or interpretation of results.
Physicians face growing pressure to reduce their use of “low value” care—services that provide either little to no benefit, little benefit relative to cost, or outsized potential harm compared to benefit. One emerging policy solution for deterring such services is to financially penalize physicians who prescribe them.1,2
Physicians’ willingness to support such policies may depend on who they believe benefits from reductions in low-value care. In previous studies of cancer screening, the more that primary care physicians felt that the money saved from cost-containment efforts went to insurance company profits rather than to patients, the less willing they were to use less expensive cancer screening approaches.3
Similarly, physicians may be more likely to support financial penalty policies if they perceive that the benefits from reducing low-value care accrue to patients (eg, lower out-of-pocket costs) rather than insurers or hospitals (eg, profits and salaries of their leaders). If present, such perceptions could inform incentive design. We explored the hypothesis that support of financial penalties
METHODS
Study Sample
By using a panel of internists maintained by the American College of Physicians, we conducted a randomized, web-based survey among 484 physicians who were either internal medicine residents or internal medicine physicians practicing hospital medicine.
Survey Instrument
Respondents used a 5-point scale (“strongly disagree” to “strongly agree”) to indicate their agreement with a policy that financially penalizes physicians for prescribing services that provide few benefits to patients. Respondents were asked to simultaneously consider the following hospital medicine services, deemed to be low value based on medical evidence and consensus guidelines4: (1) placing, and leaving in, urinary catheters for urine output monitoring in noncritically ill patients, (2) ordering continuous telemetry monitoring for nonintensive care unit patients without a protocol governing continuation, and (3) prescribing stress ulcer prophylaxis for medical patients not at a high risk for gastrointestinal complications. Policy support was defined as “somewhat” or “strongly” agreeing with the policy. As part of another study of this physician cohort, this question varied in how the harm of low-value services was framed: either as harm to patients, to society, or to hospitals and insurers as institutions. Respondent characteristics were balanced across survey versions, and for the current analysis, we pooled responses across all versions.
All other questions in the survey, described in detail elsewhere,5 were identical for all respondents. For this analysis, we focused on a question that asked physicians to assume that reducing these services saves money without harming the quality of care and to rate on a 4-point scale (“none” to “a lot”) how much of the money saved would ultimately go to the following 6 nonmutually exclusive areas: (a) other healthcare services for patients, (b) reduced charges to patients’ employers or insurers, (c) reduced out-of-pocket costs for patients, (d) salaries and bonuses for physicians, (e) salaries and profits for insurance companies and their leaders, and (f) salaries and profits for hospitals and/or health systems and their leaders.
Based on the positive correlation identified between the first 4 items (a to d) and negative correlation with the other 2 items (e and f), we reverse-coded the latter 2 and summed all 6 into a single-outcome scale, effectively representing the degree to which the money saved from reducing low-value services accrues generally to patients or physicians instead of to hospitals, insurance companies, and their leaders. The Cronbach alpha for the scale was 0.74, indicating acceptable reliability. Based on scale responses, we dichotomized respondents at the median into those who believe that the money saved from reducing low-value services would accrue as benefits to patients or physicians and those who believe benefits accrue to insurance companies or hospitals and/or health systems and their leaders. The protocol was exempted by the University of Pennsylvania Institutional Review Board.
Statistical Analysis
We used a χ2 test and multivariable logistic regression analysis to evaluate the association between policy support and physician beliefs about who benefits from reductions in low-value care. A χ2 test and a Kruskal-Wallis test were also used to evaluate the association between other respondent characteristics and beliefs about who benefits from reductions in low-value care. Analyses were performed by using Stata version 14.1 (StataCorp, College Station, TX). Tests of significance were 2-tailed at an alpha of .05.
RESULTS
Compared with nonrespondents, the 187 physicians who responded (39% response rate) were more likely to be female (30% vs 26%, P = 0.001), older (mean age 41 vs 36 years old, P < 0.001), and practicing clinicians rather than internal medicine residents (87% vs 69%, P < 0.001). Twenty-one percent reported that their personal compensation was tied to cost incentives.
Overall, respondents believed that more of any money saved from reducing low-value services would go to profits and leadership salaries for insurance companies and hospitals and/or health systems rather than to patients (panel A of Figure). Few respondents felt that the money saved would ultimately go toward physician compensation.
Sixty-six percent of physicians who supported penalties believed that benefits from reducing low-value care accrue to patients or physicians, compared to 39% among those not supporting penalties (P < 0.001). In multivariable analyses, policy support was associated with the belief that the money saved from reducing low-value services would accrue as benefits to patients or physicians rather than as salaries and profits for insurance companies or hospitals and/or health systems and their leaders (Table). There were no statistically significant associations between respondent age, gender, or professional status and beliefs about who benefits from reductions in low-value care.
DISCUSSION
Despite ongoing efforts to highlight how reducing low-value care benefits patients, physicians in our sample did not believe that much of the money saved would benefit patients.
This result may reflect that while some care patterns are considered low value because they provide little benefit at a high cost, others yield potential harm, regardless of cost. For example, limiting stress ulcer prophylaxis largely aims to avoid clinical harm (eg, adverse drug effects and nosocomial infections). Limiting telemetric monitoring largely aims to reduce costly care that provides only limited benefit. Therefore, the nature of potential benefit to patients is very different—improved clinical outcomes in the former and potential cost savings in the latter. Future studies could separately assess physician attitudes about these 2 different definitions of low-value services.
Our study also demonstrates that the more physicians believe that much of any money saved goes to the profits and salaries of insurance companies, hospitals and/or health systems, and their leaders rather than to patients, the less likely they are to support policies financially penalizing physicians for prescribing low-value services.
Our study does not address why physicians have the beliefs that they have, but a likely explanation, at least in part, is that financial flows in healthcare are complex and tangled. Indeed, a clear understanding of who actually benefits is so hard to determine that these stated beliefs may really derive from views of power or justice rather than from some understanding of funds flow. Whether or not ideological attitudes underlie these expressed beliefs, policymakers and healthcare institutions might be advised to increase transparency about how cost savings are realized and whom they benefit.
Our analysis has limitations. Although it provides insight into where physicians believe relative amounts of money saved go with respect to 6 common options, the study did not include an exhaustive list of possibilities. The response rate also limits the representativeness of our results. Additionally, the study design prevents conclusions about causality; we cannot determine whether the belief that savings go to insurance companies and their executives is what reduces physicians’ enthusiasm for penalties, whether the causal association is in the opposite direction, or whether the 2 factors are linked in another way.
Nonetheless, our findings are consistent with a sense of healthcare justice in which physicians support penalties imposed on themselves only if the resulting benefits accrue to patients rather than to corporate or organizational interests. Effective physician penalties will likely need to address the belief that insurers and provider organizations stand to gain more than patients when low-value care services are reduced.
Disclosure
Drs. Liao, Schapira, Mitra, and Weissman have no conflicts to disclose. Dr. Navathe serves as advisor to Navvis and Company, Navigant Inc., Lynx Medical, Indegene Inc., and Sutherland Global Services and receives an honorarium from Elsevier Press, none of which have relationship to this manuscript. Dr. Asch is a partner and partial owner of VAL Health, which has no relationship to this manuscript.
Funding
This work was supported by The Leonard Davis Institute of Health Economics at the University of Pennsylvania, which had no role in the study design, data collection, analysis, or interpretation of results.
1. Berwick DM. Avoiding overuse – the next quality frontier. Lancet. 2017;390(10090):102-104. PubMed
2. Centers for Medicare and Medicaid Services. CMS response to Public Comments on Non-Recommended PSA-Based Screening Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/eCQM-Development-and-Maintenance-for-Eligible-Professionals_CMS_PSA_Response_Public-Comment.pdf. Accessed September 18, 2017.
3. Asch DA, Jepson C, Hershey JC, Baron J, Ubel PA. When Money is Saved by Reducing Healthcare Costs, Where Do US Primary Care Physicians Think the Money Goes? Am J Manag Care. 2003;9(6):438-442. PubMed
4. Society of Hospital Medicine. Choosing Wisely. https://www.hospitalmedicine.org/choosingwisely. Accessed September 18, 2017.
5. Liao JM, Navathe AS, Schapira MS, Weissman A, Mitra N, Asch DAA. Penalizing Physicians for Low Value Care in Hospital Medicine: A Randomized Survey. J Hosp Med. 2017. (In press). PubMed
1. Berwick DM. Avoiding overuse – the next quality frontier. Lancet. 2017;390(10090):102-104. PubMed
2. Centers for Medicare and Medicaid Services. CMS response to Public Comments on Non-Recommended PSA-Based Screening Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/eCQM-Development-and-Maintenance-for-Eligible-Professionals_CMS_PSA_Response_Public-Comment.pdf. Accessed September 18, 2017.
3. Asch DA, Jepson C, Hershey JC, Baron J, Ubel PA. When Money is Saved by Reducing Healthcare Costs, Where Do US Primary Care Physicians Think the Money Goes? Am J Manag Care. 2003;9(6):438-442. PubMed
4. Society of Hospital Medicine. Choosing Wisely. https://www.hospitalmedicine.org/choosingwisely. Accessed September 18, 2017.
5. Liao JM, Navathe AS, Schapira MS, Weissman A, Mitra N, Asch DAA. Penalizing Physicians for Low Value Care in Hospital Medicine: A Randomized Survey. J Hosp Med. 2017. (In press). PubMed
© 2018 Society of Hospital Medicine
Penalizing Physicians for Low-Value Care in Hospital Medicine: A Randomized Survey
Reducing low-value care—services for which there is little to no benefit, little benefit relative to cost, or outsized potential harm compared with benefit—is an essential step toward maintaining or improving quality while lowering cost. Unfortunately, low-value services persist widelydespite professional consensus, guidelines, and national campaigns aimed to reduce them.1-3 In turn, policy makers are beginning to consider financially penalizing physicians in order to deter low-value services.4,5 Physician support for such penalties remains unknown. In this study, we used a randomized survey experiment to evaluate how the framing of harms from low-value care—in terms of those to patients, healthcare institutions, or society—influenced physician support of financial penalties for low-value care services.
METHODS
Study Sample
By using a stratified random sample maintained by the American College of Physicians, we conducted a web-based survey among 484 physicians who were either internal medicine residents or internists practicing hospital medicine.
Instrument Design and Administration
Our study focused on 3 low-value services relevant to inpatient medicine: (1) placing, and leaving in, urinary catheters for urine output monitoring in noncritically ill patients; (2) ordering continuous telemetry monitoring for nonintensive care unit (non-ICU) patients without a protocol governing continuation; and (3) prescribing stress ulcer prophylaxis for medical patients not at a high risk for gastrointestinal (GI) complications. Although the nature and trade-offs between costs, harms, and benefits vary by individual service, all 3 are promulgated through the Choosing Wisely® guidelines as low value based on existing data and professional consensus from the Society of Hospital Medicine.6
To evaluate intended behavior related to these 3 low-value services, respondents were first presented with 3 clinical vignettes focused on the care of patients hospitalized for pneumonia, congestive heart failure, and alcohol withdrawal, which were selected to reflect common inpatient medicine scenarios. Respondents were asked to use a 4-point scale (very likely to very unlikely) to estimate how likely they were to recommend various tests or treatments, including the low-value services noted above. Respondents who were “somewhat unlikely” and “very unlikely” to recommend low-value services were considered concordant with low-value care guidelines.
Following the vignettes, respondents then used a 5-point scale (strongly agree to strongly disagree) to indicate their agreement with a policy that financially penalizes physicians for prescribing each service. Support was defined as “somewhat or strongly” agreeing with the policy. Respondents were randomized to receive 1 of 3 versions of this question (supplementary Appendix).
All versions stated that, “According to research and expert opinion, certain aspects of inpatient care provide little benefit to patients” and listed the 3 low-value services noted above. The “patient harm” version also described the harm of low-value care as costs to patients and risk for clinical harms and complications. The “societal harm” version described the harms as costs to society and utilization of limited healthcare resources. The “institutional harm” version described harms as costs to hospitals and insurers.
Other survey items were adapted from existing literature7-9 and evaluated respondent beliefs about the effectiveness of physician incentives in improving the value of care, as well as the appropriateness of including cost considerations in clinical decision-making.
The instrument was pilot tested among study team members and several independent internists affiliated with the University of Pennsylvania. After incorporating feedback into the final instrument, the web-based survey was distributed to eligible physicians via e-mail. Responses were anonymous and respondents received a $15 gift card for participation. The protocol was reviewed and deemed exempt by the University of Pennsylvania Institutional Review Board.
Statistical Analysis
Respondent characteristics (sociodemographic, intended clinical behavior, and cost control attitudes) were described by using percentages for categorical variables and medians and interquartile ranges for continuous variables. Balance in respondent characteristics across survey versions was evaluated using χ2 and Kruskal-Wallis tests. Multivariable logistic regression, adjusted for characteristics in the Table, was used to evaluate the association between survey version and policy support. All tests of significance were 2-tailed with significance level alpha = 0.05. Analyses were performed using STATA version 14.1 (StataCorp LLC, College Station, TX, http://www.stata.com).
RESULTS
Of 484 eligible respondents, 187 (39%) completed the survey. Compared with nonrespondents, respondents were more likely to be female (30% vs 26%, P = 0.001), older (mean age 41 vs 36 years, P < 0.001), and practicing clinicians rather than internal medicine residents (87% vs 69%, P < 0.001). Physician characteristics were similar across the 3 survey versions (Table). Most respondents agreed that financial incentives for individual physicians is an effective way to improve the value of healthcare (73.3%) and that physicians should consider the costs of a test or treatment to society when making clinical decisions for patients (79.1%). The majority also felt that clinicians have a duty to offer a test or treatment to a patient if it has any chance of helping them (70.1%) and that it is inappropriate for anyone beyond the clinician and patient to decide if a test or treatment is “worth the cost” (63.6%).
Overall, policy support rate was 39.6% and was the highest for the “societal harm” version (48.4%), followed by the “institutional harm” (36.9%) and “patient harm” (33.3%) versions. Compared with respondents receiving the “patient harm” version, those receiving the “societal harm” version (adjusted odds ratio [OR] 2.83; 95% confidence interval [CI], 1.20-6.69), but not the “institutional harm” framing (adjusted OR 1.53; 95% CI, 0.66-3.53), were more likely to report policy support. Policy support was also higher among those who agreed that providing financial incentives to individual physicians is an effective way to improve the value of healthcare (adjusted OR 4.61; 95% CI, 1.80-11.80).
DISCUSSION
To our knowledge, this study is the first to prospectively evaluate physician support of financial penalties for low-value services relevant to hospital medicine. It has 2 main findings.
First, although overall policy support was relatively low (39.6%), it varied significantly on the basis of how the harms of low-value care were framed. Support was highest in the “societal harm” version, suggesting that emphasizing these harms may increase acceptability of financial penalties among physicians and contribute to the larger effort to decrease low-value care in hospital settings. The comparatively low support for the “patient harm” version is somewhat surprising but may reflect variation in the nature of harm, benefit, and cost trade-offs for individual low-value services, as noted above, and physician belief that some low-value services do not in fact produce significant clinical harms.
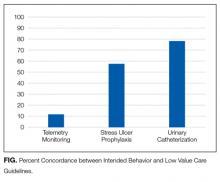
For example, whereas evidence demonstrates that stress ulcer prophylaxis in non-ICU patients can harm patients through nosocomial infections and adverse drug effects,10,11 the clinical harms of telemetry are less obvious. Telemetry’s low value derives more from its high cost relative to benefit, rather than its potential for clinical harm.6 The many paths to “low value” underscore the need to examine attitudes and uptake toward these services separately and may explain the wide range in concordance between intended clinical behavior and low-value care guidelines (11.8% to 78.6%).
Reinforcing policies could more effectively deter low-value care. For example, multiple forces, including Medicare payment reform and national accreditation policies,12,13 have converged to discourage low-value use of urinary catheters in hospitalized patients. In contrast, there has been little reinforcement beyond consensus guidelines to reduce low-value use of telemetric monitoring. Given questions about whether consensus methods alone can deter low-value care beyond obvious “low hanging fruit,”14 policy makers could coordinate policies to accelerate progress within other priority areas.
Broad policies should also be paired with local initiatives to influence physician behavior. For example, health systems have begun successfully leveraging the electronic medical record and utilizing behavioral economics principles to design interventions to reduce inappropriate overuse of antibiotics for upper respiratory infections in primary care clinics.15 Organizations are also redesigning care processes in response to resource utilization imperatives under ongoing value-based care payment reform. Care redesign and behavioral interventions embedded at the point of care can both help deter low-value services in inpatient settings.
Study limitations include a relatively low response rate, which limits generalizability. However, all 3 randomized groups were similar on measured characteristics, and experimental randomization reduces the nonresponse bias concerns accompanying descriptive surveys. Additionally, although we evaluated intended clinical behavior in a national sample, our results may not reflect actual behavior among all physicians practicing hospital medicine. Future work could include assessments of actual or self-reported practices or examine additional factors, including site, years of practice, knowledge about guidelines, and other possible determinants of guideline-concordant behaviors.
Despite these limitations, our study provides important early evidence about physician support of financial penalties for low-value care relevant to hospital medicine. As policy makers design and organizational leaders implement financial incentive policies, this information can help increase their acceptability among physicians and more effectively reduce low-value care within hospitals.
Disclosure
Drs. Liao, Schapira, Mitra, and Weissman have no conflicts to disclose. Dr. Navathe serves as advisor to Navvis and Company, Navigant Inc, Lynx Medical, Indegene Inc, and Sutherland Global Services and receives an honorarium from Elsevier Press, none of which have relationship to this manuscript. Dr. Asch is a partner and part owner of VAL Health, which has no relationship to this manuscript.
Funding
This work was supported by The Leonard Davis Institute of Health Economics at the University of Pennsylvania, which had no role in the study design, data collection, analysis, or interpretation of results.
1. The MedPAC blog. Use of low-value care in Medicare is substantial. http://www.medpac.gov/-blog-/medpacblog/2015/05/21/use-of-low-value-care-in-medicare-is-substantial. Accessed on September 18, 2017.
2. American Board of Internal Medicine Foundation. Choosing Wisely. http://www.choosingwisely.org/. Accessed on September 18, 2017.
3. Rosenberg A, Agiro A, Gottlieb M, et al. Early Trends Among Seven Recommendations From the Choosing Wisely Campaign. JAMA Intern Med. 2015;175(12):1913-1920. PubMed
4. Centers for Medicare & Medicaid Services. CMS Response to Public Comments on Non-Recommended PSA-Based Screening Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/eCQM-Development-and-Maintenance-for-Eligible-Professionals_CMS_PSA_Response_Public-Comment.pdf. Accessed September 18, 2017.
5. Berwick DM. Avoiding overuse-the next quality frontier. Lancet. 2017;390(10090):102-104. doi: 10.1016/S0140-6736(16)32570-3. PubMed
6. Society of Hospital Medicine. Choosing Wisely. https://www.hospitalmedicine.org/choosingwisely. Accessed on September 18, 2017.
7. Tilburt JC, Wynia MK, Sheeler RD, et al. Views of US Physicians About Controlling Health Care Costs. JAMA. 2013;310(4):380-388. PubMed
8. Ginsburg ME, Kravitz RL, Sandberg WA. A survey of physician attitudes and practices concerning cost-effectiveness in patient care. West J Med. 2000;173(6):309-394. PubMed
9. Colla CH, Kinsella EA, Morden NE, Meyers DJ, Rosenthal MB, Sequist TD. Physician perceptions of Choosing Wisely and drivers of overuse. Am J Manag Care. 2016;22(5):337-343. PubMed
10. Herzig SJ, Vaughn BP, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991-997. PubMed
11. Pappas M, Jolly S, Vijan S. Defining Appropriate Use of Proton-Pump Inhibitors Among Medical Inpatients. J Gen Intern Med. 2016;31(4):364-371. PubMed
12. Centers for Medicare & Medicaid Services. CMS’ Value-Based Programs. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/Value-Based-Programs.html. Accessed September 18, 2017.
13. The Joint Commission. Requirements for the Catheter-Associated Urinary Tract Infections (CAUTI) National Patient Safety Goal for Hospitals. https://www.jointcommission.org/assets/1/6/R3_Cauti_HAP.pdf. Accessed September 18, 2017 .
14. Beaudin-Seiler B, Ciarametaro M, Dubois R, Lee J, Fendrick AM. Reducing Low-Value Care. Health Affairs Blog. http://healthaffairs.org/blog/2016/09/20/reducing-low-value-care/. Accessed on September 18, 2017.
15. Meeker D, Linder JA, Fox CR, et al. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA. 2016;315(6):562-570. PubMed
Reducing low-value care—services for which there is little to no benefit, little benefit relative to cost, or outsized potential harm compared with benefit—is an essential step toward maintaining or improving quality while lowering cost. Unfortunately, low-value services persist widelydespite professional consensus, guidelines, and national campaigns aimed to reduce them.1-3 In turn, policy makers are beginning to consider financially penalizing physicians in order to deter low-value services.4,5 Physician support for such penalties remains unknown. In this study, we used a randomized survey experiment to evaluate how the framing of harms from low-value care—in terms of those to patients, healthcare institutions, or society—influenced physician support of financial penalties for low-value care services.
METHODS
Study Sample
By using a stratified random sample maintained by the American College of Physicians, we conducted a web-based survey among 484 physicians who were either internal medicine residents or internists practicing hospital medicine.
Instrument Design and Administration
Our study focused on 3 low-value services relevant to inpatient medicine: (1) placing, and leaving in, urinary catheters for urine output monitoring in noncritically ill patients; (2) ordering continuous telemetry monitoring for nonintensive care unit (non-ICU) patients without a protocol governing continuation; and (3) prescribing stress ulcer prophylaxis for medical patients not at a high risk for gastrointestinal (GI) complications. Although the nature and trade-offs between costs, harms, and benefits vary by individual service, all 3 are promulgated through the Choosing Wisely® guidelines as low value based on existing data and professional consensus from the Society of Hospital Medicine.6
To evaluate intended behavior related to these 3 low-value services, respondents were first presented with 3 clinical vignettes focused on the care of patients hospitalized for pneumonia, congestive heart failure, and alcohol withdrawal, which were selected to reflect common inpatient medicine scenarios. Respondents were asked to use a 4-point scale (very likely to very unlikely) to estimate how likely they were to recommend various tests or treatments, including the low-value services noted above. Respondents who were “somewhat unlikely” and “very unlikely” to recommend low-value services were considered concordant with low-value care guidelines.
Following the vignettes, respondents then used a 5-point scale (strongly agree to strongly disagree) to indicate their agreement with a policy that financially penalizes physicians for prescribing each service. Support was defined as “somewhat or strongly” agreeing with the policy. Respondents were randomized to receive 1 of 3 versions of this question (supplementary Appendix).
All versions stated that, “According to research and expert opinion, certain aspects of inpatient care provide little benefit to patients” and listed the 3 low-value services noted above. The “patient harm” version also described the harm of low-value care as costs to patients and risk for clinical harms and complications. The “societal harm” version described the harms as costs to society and utilization of limited healthcare resources. The “institutional harm” version described harms as costs to hospitals and insurers.
Other survey items were adapted from existing literature7-9 and evaluated respondent beliefs about the effectiveness of physician incentives in improving the value of care, as well as the appropriateness of including cost considerations in clinical decision-making.
The instrument was pilot tested among study team members and several independent internists affiliated with the University of Pennsylvania. After incorporating feedback into the final instrument, the web-based survey was distributed to eligible physicians via e-mail. Responses were anonymous and respondents received a $15 gift card for participation. The protocol was reviewed and deemed exempt by the University of Pennsylvania Institutional Review Board.
Statistical Analysis
Respondent characteristics (sociodemographic, intended clinical behavior, and cost control attitudes) were described by using percentages for categorical variables and medians and interquartile ranges for continuous variables. Balance in respondent characteristics across survey versions was evaluated using χ2 and Kruskal-Wallis tests. Multivariable logistic regression, adjusted for characteristics in the Table, was used to evaluate the association between survey version and policy support. All tests of significance were 2-tailed with significance level alpha = 0.05. Analyses were performed using STATA version 14.1 (StataCorp LLC, College Station, TX, http://www.stata.com).
RESULTS
Of 484 eligible respondents, 187 (39%) completed the survey. Compared with nonrespondents, respondents were more likely to be female (30% vs 26%, P = 0.001), older (mean age 41 vs 36 years, P < 0.001), and practicing clinicians rather than internal medicine residents (87% vs 69%, P < 0.001). Physician characteristics were similar across the 3 survey versions (Table). Most respondents agreed that financial incentives for individual physicians is an effective way to improve the value of healthcare (73.3%) and that physicians should consider the costs of a test or treatment to society when making clinical decisions for patients (79.1%). The majority also felt that clinicians have a duty to offer a test or treatment to a patient if it has any chance of helping them (70.1%) and that it is inappropriate for anyone beyond the clinician and patient to decide if a test or treatment is “worth the cost” (63.6%).
Overall, policy support rate was 39.6% and was the highest for the “societal harm” version (48.4%), followed by the “institutional harm” (36.9%) and “patient harm” (33.3%) versions. Compared with respondents receiving the “patient harm” version, those receiving the “societal harm” version (adjusted odds ratio [OR] 2.83; 95% confidence interval [CI], 1.20-6.69), but not the “institutional harm” framing (adjusted OR 1.53; 95% CI, 0.66-3.53), were more likely to report policy support. Policy support was also higher among those who agreed that providing financial incentives to individual physicians is an effective way to improve the value of healthcare (adjusted OR 4.61; 95% CI, 1.80-11.80).
DISCUSSION
To our knowledge, this study is the first to prospectively evaluate physician support of financial penalties for low-value services relevant to hospital medicine. It has 2 main findings.
First, although overall policy support was relatively low (39.6%), it varied significantly on the basis of how the harms of low-value care were framed. Support was highest in the “societal harm” version, suggesting that emphasizing these harms may increase acceptability of financial penalties among physicians and contribute to the larger effort to decrease low-value care in hospital settings. The comparatively low support for the “patient harm” version is somewhat surprising but may reflect variation in the nature of harm, benefit, and cost trade-offs for individual low-value services, as noted above, and physician belief that some low-value services do not in fact produce significant clinical harms.
For example, whereas evidence demonstrates that stress ulcer prophylaxis in non-ICU patients can harm patients through nosocomial infections and adverse drug effects,10,11 the clinical harms of telemetry are less obvious. Telemetry’s low value derives more from its high cost relative to benefit, rather than its potential for clinical harm.6 The many paths to “low value” underscore the need to examine attitudes and uptake toward these services separately and may explain the wide range in concordance between intended clinical behavior and low-value care guidelines (11.8% to 78.6%).
Reinforcing policies could more effectively deter low-value care. For example, multiple forces, including Medicare payment reform and national accreditation policies,12,13 have converged to discourage low-value use of urinary catheters in hospitalized patients. In contrast, there has been little reinforcement beyond consensus guidelines to reduce low-value use of telemetric monitoring. Given questions about whether consensus methods alone can deter low-value care beyond obvious “low hanging fruit,”14 policy makers could coordinate policies to accelerate progress within other priority areas.
Broad policies should also be paired with local initiatives to influence physician behavior. For example, health systems have begun successfully leveraging the electronic medical record and utilizing behavioral economics principles to design interventions to reduce inappropriate overuse of antibiotics for upper respiratory infections in primary care clinics.15 Organizations are also redesigning care processes in response to resource utilization imperatives under ongoing value-based care payment reform. Care redesign and behavioral interventions embedded at the point of care can both help deter low-value services in inpatient settings.
Study limitations include a relatively low response rate, which limits generalizability. However, all 3 randomized groups were similar on measured characteristics, and experimental randomization reduces the nonresponse bias concerns accompanying descriptive surveys. Additionally, although we evaluated intended clinical behavior in a national sample, our results may not reflect actual behavior among all physicians practicing hospital medicine. Future work could include assessments of actual or self-reported practices or examine additional factors, including site, years of practice, knowledge about guidelines, and other possible determinants of guideline-concordant behaviors.
Despite these limitations, our study provides important early evidence about physician support of financial penalties for low-value care relevant to hospital medicine. As policy makers design and organizational leaders implement financial incentive policies, this information can help increase their acceptability among physicians and more effectively reduce low-value care within hospitals.
Disclosure
Drs. Liao, Schapira, Mitra, and Weissman have no conflicts to disclose. Dr. Navathe serves as advisor to Navvis and Company, Navigant Inc, Lynx Medical, Indegene Inc, and Sutherland Global Services and receives an honorarium from Elsevier Press, none of which have relationship to this manuscript. Dr. Asch is a partner and part owner of VAL Health, which has no relationship to this manuscript.
Funding
This work was supported by The Leonard Davis Institute of Health Economics at the University of Pennsylvania, which had no role in the study design, data collection, analysis, or interpretation of results.
Reducing low-value care—services for which there is little to no benefit, little benefit relative to cost, or outsized potential harm compared with benefit—is an essential step toward maintaining or improving quality while lowering cost. Unfortunately, low-value services persist widelydespite professional consensus, guidelines, and national campaigns aimed to reduce them.1-3 In turn, policy makers are beginning to consider financially penalizing physicians in order to deter low-value services.4,5 Physician support for such penalties remains unknown. In this study, we used a randomized survey experiment to evaluate how the framing of harms from low-value care—in terms of those to patients, healthcare institutions, or society—influenced physician support of financial penalties for low-value care services.
METHODS
Study Sample
By using a stratified random sample maintained by the American College of Physicians, we conducted a web-based survey among 484 physicians who were either internal medicine residents or internists practicing hospital medicine.
Instrument Design and Administration
Our study focused on 3 low-value services relevant to inpatient medicine: (1) placing, and leaving in, urinary catheters for urine output monitoring in noncritically ill patients; (2) ordering continuous telemetry monitoring for nonintensive care unit (non-ICU) patients without a protocol governing continuation; and (3) prescribing stress ulcer prophylaxis for medical patients not at a high risk for gastrointestinal (GI) complications. Although the nature and trade-offs between costs, harms, and benefits vary by individual service, all 3 are promulgated through the Choosing Wisely® guidelines as low value based on existing data and professional consensus from the Society of Hospital Medicine.6
To evaluate intended behavior related to these 3 low-value services, respondents were first presented with 3 clinical vignettes focused on the care of patients hospitalized for pneumonia, congestive heart failure, and alcohol withdrawal, which were selected to reflect common inpatient medicine scenarios. Respondents were asked to use a 4-point scale (very likely to very unlikely) to estimate how likely they were to recommend various tests or treatments, including the low-value services noted above. Respondents who were “somewhat unlikely” and “very unlikely” to recommend low-value services were considered concordant with low-value care guidelines.
Following the vignettes, respondents then used a 5-point scale (strongly agree to strongly disagree) to indicate their agreement with a policy that financially penalizes physicians for prescribing each service. Support was defined as “somewhat or strongly” agreeing with the policy. Respondents were randomized to receive 1 of 3 versions of this question (supplementary Appendix).
All versions stated that, “According to research and expert opinion, certain aspects of inpatient care provide little benefit to patients” and listed the 3 low-value services noted above. The “patient harm” version also described the harm of low-value care as costs to patients and risk for clinical harms and complications. The “societal harm” version described the harms as costs to society and utilization of limited healthcare resources. The “institutional harm” version described harms as costs to hospitals and insurers.
Other survey items were adapted from existing literature7-9 and evaluated respondent beliefs about the effectiveness of physician incentives in improving the value of care, as well as the appropriateness of including cost considerations in clinical decision-making.
The instrument was pilot tested among study team members and several independent internists affiliated with the University of Pennsylvania. After incorporating feedback into the final instrument, the web-based survey was distributed to eligible physicians via e-mail. Responses were anonymous and respondents received a $15 gift card for participation. The protocol was reviewed and deemed exempt by the University of Pennsylvania Institutional Review Board.
Statistical Analysis
Respondent characteristics (sociodemographic, intended clinical behavior, and cost control attitudes) were described by using percentages for categorical variables and medians and interquartile ranges for continuous variables. Balance in respondent characteristics across survey versions was evaluated using χ2 and Kruskal-Wallis tests. Multivariable logistic regression, adjusted for characteristics in the Table, was used to evaluate the association between survey version and policy support. All tests of significance were 2-tailed with significance level alpha = 0.05. Analyses were performed using STATA version 14.1 (StataCorp LLC, College Station, TX, http://www.stata.com).
RESULTS
Of 484 eligible respondents, 187 (39%) completed the survey. Compared with nonrespondents, respondents were more likely to be female (30% vs 26%, P = 0.001), older (mean age 41 vs 36 years, P < 0.001), and practicing clinicians rather than internal medicine residents (87% vs 69%, P < 0.001). Physician characteristics were similar across the 3 survey versions (Table). Most respondents agreed that financial incentives for individual physicians is an effective way to improve the value of healthcare (73.3%) and that physicians should consider the costs of a test or treatment to society when making clinical decisions for patients (79.1%). The majority also felt that clinicians have a duty to offer a test or treatment to a patient if it has any chance of helping them (70.1%) and that it is inappropriate for anyone beyond the clinician and patient to decide if a test or treatment is “worth the cost” (63.6%).
Overall, policy support rate was 39.6% and was the highest for the “societal harm” version (48.4%), followed by the “institutional harm” (36.9%) and “patient harm” (33.3%) versions. Compared with respondents receiving the “patient harm” version, those receiving the “societal harm” version (adjusted odds ratio [OR] 2.83; 95% confidence interval [CI], 1.20-6.69), but not the “institutional harm” framing (adjusted OR 1.53; 95% CI, 0.66-3.53), were more likely to report policy support. Policy support was also higher among those who agreed that providing financial incentives to individual physicians is an effective way to improve the value of healthcare (adjusted OR 4.61; 95% CI, 1.80-11.80).
DISCUSSION
To our knowledge, this study is the first to prospectively evaluate physician support of financial penalties for low-value services relevant to hospital medicine. It has 2 main findings.
First, although overall policy support was relatively low (39.6%), it varied significantly on the basis of how the harms of low-value care were framed. Support was highest in the “societal harm” version, suggesting that emphasizing these harms may increase acceptability of financial penalties among physicians and contribute to the larger effort to decrease low-value care in hospital settings. The comparatively low support for the “patient harm” version is somewhat surprising but may reflect variation in the nature of harm, benefit, and cost trade-offs for individual low-value services, as noted above, and physician belief that some low-value services do not in fact produce significant clinical harms.
For example, whereas evidence demonstrates that stress ulcer prophylaxis in non-ICU patients can harm patients through nosocomial infections and adverse drug effects,10,11 the clinical harms of telemetry are less obvious. Telemetry’s low value derives more from its high cost relative to benefit, rather than its potential for clinical harm.6 The many paths to “low value” underscore the need to examine attitudes and uptake toward these services separately and may explain the wide range in concordance between intended clinical behavior and low-value care guidelines (11.8% to 78.6%).
Reinforcing policies could more effectively deter low-value care. For example, multiple forces, including Medicare payment reform and national accreditation policies,12,13 have converged to discourage low-value use of urinary catheters in hospitalized patients. In contrast, there has been little reinforcement beyond consensus guidelines to reduce low-value use of telemetric monitoring. Given questions about whether consensus methods alone can deter low-value care beyond obvious “low hanging fruit,”14 policy makers could coordinate policies to accelerate progress within other priority areas.
Broad policies should also be paired with local initiatives to influence physician behavior. For example, health systems have begun successfully leveraging the electronic medical record and utilizing behavioral economics principles to design interventions to reduce inappropriate overuse of antibiotics for upper respiratory infections in primary care clinics.15 Organizations are also redesigning care processes in response to resource utilization imperatives under ongoing value-based care payment reform. Care redesign and behavioral interventions embedded at the point of care can both help deter low-value services in inpatient settings.
Study limitations include a relatively low response rate, which limits generalizability. However, all 3 randomized groups were similar on measured characteristics, and experimental randomization reduces the nonresponse bias concerns accompanying descriptive surveys. Additionally, although we evaluated intended clinical behavior in a national sample, our results may not reflect actual behavior among all physicians practicing hospital medicine. Future work could include assessments of actual or self-reported practices or examine additional factors, including site, years of practice, knowledge about guidelines, and other possible determinants of guideline-concordant behaviors.
Despite these limitations, our study provides important early evidence about physician support of financial penalties for low-value care relevant to hospital medicine. As policy makers design and organizational leaders implement financial incentive policies, this information can help increase their acceptability among physicians and more effectively reduce low-value care within hospitals.
Disclosure
Drs. Liao, Schapira, Mitra, and Weissman have no conflicts to disclose. Dr. Navathe serves as advisor to Navvis and Company, Navigant Inc, Lynx Medical, Indegene Inc, and Sutherland Global Services and receives an honorarium from Elsevier Press, none of which have relationship to this manuscript. Dr. Asch is a partner and part owner of VAL Health, which has no relationship to this manuscript.
Funding
This work was supported by The Leonard Davis Institute of Health Economics at the University of Pennsylvania, which had no role in the study design, data collection, analysis, or interpretation of results.
1. The MedPAC blog. Use of low-value care in Medicare is substantial. http://www.medpac.gov/-blog-/medpacblog/2015/05/21/use-of-low-value-care-in-medicare-is-substantial. Accessed on September 18, 2017.
2. American Board of Internal Medicine Foundation. Choosing Wisely. http://www.choosingwisely.org/. Accessed on September 18, 2017.
3. Rosenberg A, Agiro A, Gottlieb M, et al. Early Trends Among Seven Recommendations From the Choosing Wisely Campaign. JAMA Intern Med. 2015;175(12):1913-1920. PubMed
4. Centers for Medicare & Medicaid Services. CMS Response to Public Comments on Non-Recommended PSA-Based Screening Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/eCQM-Development-and-Maintenance-for-Eligible-Professionals_CMS_PSA_Response_Public-Comment.pdf. Accessed September 18, 2017.
5. Berwick DM. Avoiding overuse-the next quality frontier. Lancet. 2017;390(10090):102-104. doi: 10.1016/S0140-6736(16)32570-3. PubMed
6. Society of Hospital Medicine. Choosing Wisely. https://www.hospitalmedicine.org/choosingwisely. Accessed on September 18, 2017.
7. Tilburt JC, Wynia MK, Sheeler RD, et al. Views of US Physicians About Controlling Health Care Costs. JAMA. 2013;310(4):380-388. PubMed
8. Ginsburg ME, Kravitz RL, Sandberg WA. A survey of physician attitudes and practices concerning cost-effectiveness in patient care. West J Med. 2000;173(6):309-394. PubMed
9. Colla CH, Kinsella EA, Morden NE, Meyers DJ, Rosenthal MB, Sequist TD. Physician perceptions of Choosing Wisely and drivers of overuse. Am J Manag Care. 2016;22(5):337-343. PubMed
10. Herzig SJ, Vaughn BP, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991-997. PubMed
11. Pappas M, Jolly S, Vijan S. Defining Appropriate Use of Proton-Pump Inhibitors Among Medical Inpatients. J Gen Intern Med. 2016;31(4):364-371. PubMed
12. Centers for Medicare & Medicaid Services. CMS’ Value-Based Programs. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/Value-Based-Programs.html. Accessed September 18, 2017.
13. The Joint Commission. Requirements for the Catheter-Associated Urinary Tract Infections (CAUTI) National Patient Safety Goal for Hospitals. https://www.jointcommission.org/assets/1/6/R3_Cauti_HAP.pdf. Accessed September 18, 2017 .
14. Beaudin-Seiler B, Ciarametaro M, Dubois R, Lee J, Fendrick AM. Reducing Low-Value Care. Health Affairs Blog. http://healthaffairs.org/blog/2016/09/20/reducing-low-value-care/. Accessed on September 18, 2017.
15. Meeker D, Linder JA, Fox CR, et al. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA. 2016;315(6):562-570. PubMed
1. The MedPAC blog. Use of low-value care in Medicare is substantial. http://www.medpac.gov/-blog-/medpacblog/2015/05/21/use-of-low-value-care-in-medicare-is-substantial. Accessed on September 18, 2017.
2. American Board of Internal Medicine Foundation. Choosing Wisely. http://www.choosingwisely.org/. Accessed on September 18, 2017.
3. Rosenberg A, Agiro A, Gottlieb M, et al. Early Trends Among Seven Recommendations From the Choosing Wisely Campaign. JAMA Intern Med. 2015;175(12):1913-1920. PubMed
4. Centers for Medicare & Medicaid Services. CMS Response to Public Comments on Non-Recommended PSA-Based Screening Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/eCQM-Development-and-Maintenance-for-Eligible-Professionals_CMS_PSA_Response_Public-Comment.pdf. Accessed September 18, 2017.
5. Berwick DM. Avoiding overuse-the next quality frontier. Lancet. 2017;390(10090):102-104. doi: 10.1016/S0140-6736(16)32570-3. PubMed
6. Society of Hospital Medicine. Choosing Wisely. https://www.hospitalmedicine.org/choosingwisely. Accessed on September 18, 2017.
7. Tilburt JC, Wynia MK, Sheeler RD, et al. Views of US Physicians About Controlling Health Care Costs. JAMA. 2013;310(4):380-388. PubMed
8. Ginsburg ME, Kravitz RL, Sandberg WA. A survey of physician attitudes and practices concerning cost-effectiveness in patient care. West J Med. 2000;173(6):309-394. PubMed
9. Colla CH, Kinsella EA, Morden NE, Meyers DJ, Rosenthal MB, Sequist TD. Physician perceptions of Choosing Wisely and drivers of overuse. Am J Manag Care. 2016;22(5):337-343. PubMed
10. Herzig SJ, Vaughn BP, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991-997. PubMed
11. Pappas M, Jolly S, Vijan S. Defining Appropriate Use of Proton-Pump Inhibitors Among Medical Inpatients. J Gen Intern Med. 2016;31(4):364-371. PubMed
12. Centers for Medicare & Medicaid Services. CMS’ Value-Based Programs. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/Value-Based-Programs.html. Accessed September 18, 2017.
13. The Joint Commission. Requirements for the Catheter-Associated Urinary Tract Infections (CAUTI) National Patient Safety Goal for Hospitals. https://www.jointcommission.org/assets/1/6/R3_Cauti_HAP.pdf. Accessed September 18, 2017 .
14. Beaudin-Seiler B, Ciarametaro M, Dubois R, Lee J, Fendrick AM. Reducing Low-Value Care. Health Affairs Blog. http://healthaffairs.org/blog/2016/09/20/reducing-low-value-care/. Accessed on September 18, 2017.
15. Meeker D, Linder JA, Fox CR, et al. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA. 2016;315(6):562-570. PubMed
© 2018 Society of Hospital Medicine
Rapid-Cycle Innovation Testing of Text-Based Monitoring for Management of Postpartum Hypertension
From the Maternal and Child Health Research Program, Department of Obstetrics and Gynecology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Dr. Hirshberg, Dr. Srinivas); Hospital of the University of Pennsylvania, Department of Nursing, Department of Obstetrics and Gynecology, Philadelphia, PA (Ms. Bittle); Penn Medicine Center for Health Care Innovation, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Mr. Vandertuyn, Ms. Mahraj, Dr. Asch, Mr. Rosin); and the Department of Family Medicine, University of Washington, Seattle, WA (Dr. Bennett).
Abstract
- Objective: To investigate engagement with a bidirectional text messaging system as an alternative to in-person follow-up for postpartum women with hypertensive disorders.
- Methods: We utilized rapid-cycle innovation processes to implement postpartum SMS text messaging follow-up in women with hypertensive disorders who delivered between September–December 2014. Patients were given electronic blood pressure cuffs and education before discharge. Standard texts reminded patients to send blood pressures daily on each of the 7 days post discharge. The study obstetrician sent text message responses based on a pre-specified management algorithm. Ability to meet ACOG guidelines was defined as receiving at least 1 reading on post-discharge days 1 or 2 and days 5, 6, or 7.
- Results: We enrolled 32 patients. Six (19%) returned for usual care office blood pressure checks. We received at least 1 blood pressure from 27 (84%) participants. Nearly 20 (65%) texted readings on 5 of the 7 days. 27 (84%) texted at least one reading on day 1 or 2, and 21 (66%) texted at least one pressure on day 5, 6, or 7 (P = 0.001 vs. usual care). Two patients required medications and none were readmitted for hypertension. Patients reported preference for home testing and text messaging over return visits.
- Conclusion: Remote blood pressure monitoring via text messaging is a patient-centered method for postpartum hypertension surveillance. Further testing is needed prior to widespread adoption within the broader obstetric community.
Key words: postpartum hypertension, remote monitoring, text-based intervention.
Hypertensive disease is a leading cause of maternal morbidity and mortality [1,2] and the leading cause of obstetric readmissions, accounting for 27% of obstetric readmissions in the United States in 2009 [3]. The majority of patients readmitted with hypertension have a diagnosis of hypertensive disorder of pregnancy on initial admission for delivery, indicating that these readmissions are the result of disease persistence or progression in contrast to new-onset disease. Peak blood pressure in these patients usually occurs 3 to 6 days postpartum [4–6] and is typically unaccompanied by warning symptoms. For these reasons, identifying patients who are at risk for persistent disease and being proactive in their postpartum care may decrease postpartum stroke and seizure. The recent Hypertension in Pregnancy guidelines provided by the American College of Obstetricians and Gynecologists (ACOG) recommend monitoring blood pressure for at least 72 hours postpartum (inpatient or outpatient) and again 7 to 10 days after delivery in women in whom a hypertensive disease of pregnancy is diagnosed [6].
Although there is a clear need for effective and reliable blood pressure surveillance for high-risk women soon after delivery, significant obstacles exist. Our own high-risk blood pressure transition clinic, which occurred every other week and was staffed by maternal-fetal medicine specialists, had an average attendance of only 30% over a 2-year period. Moreover, all of the hypertension-related readmissions occurred in the first 7 days post discharge, which was before the scheduled clinic visit for approximately 50% of patients. Phone call reminders were also found to be an ineffective strategy, as the women did not answer or return voice messages left by the practice. In fact, a postpartum unit quality improvement project validated that follow-up phone calls after discharge from the postpartum unit were less effective than text messaging when reminding women of their blood pressure follow-up appointment at the clinic [7].
As an alternative to in-person visits or traditional voice telephonic communication, mobile phone “Short Message Service” (SMS) text messaging has been used successfully in health care for appointment reminders, result reporting, support of medication and treatment adherence, and dosage adjustment [8–13]. As of 2014, 90% of American adults own a cell phone and over 79% of those send and receive text messages [14]. Among a young population, which is at high risk for hypertensive disorders of pregnancy, data further reveals a preference for text messaging over live calls [15]. Among low-income women under age 30, the rates of cell phone use and text communication are very high [14,15], making text-based surveillance a promising and more patient-centered strategy for a broad population.
We report the results of rapid-cycle innovation and implementation of active, remote surveillance of hypertension with new text message communication strategies in the first 7 days post-discharge. We chose a Plan-Do-Study-Act cycle approach, in which small tests are performed and studied and changes made to accelerate improvement, in order to enhance our ability to acquire blood pressure data [16,17]. The goals of the work were to (1) assess patient engagement using a remote method of blood pressure monitoring, (2) increase ascertainment of postpartum blood pressure data and obtain at least once daily blood pressure readings on all patients on post discharge days 1–2 and 5–7, which is in accordance with the recommended guidelines [6] for blood pressure surveillance, and (3) address all “at risk” severe range blood pressure readings within a short time interval and prior to the need for readmission. We describe a program of remote blood pressure monitoring and communication via text message designed to increase patient engagement and participation, thereby having the potential to result in earlier interventions, reduce readmissions, and decrease overall morbidity.
Methods
We performed a series of 6 rapid-cycle innovation devel-opment and implementation interventions with a cohort of women with chronic hypertension (CHTN), gestational hypertension (GHTN), or preeclampsia (with and without severe features and superimposed) who delivered at our institution between 20 September 2014 and 14 December 2014. All patients were > 18 years old, able to speak and read English, had a hypertension diag-nosis listed above, and had access to a cell phone with unlimited text messaging capabilities. Patients received standard postpartum care and were continued or started on antihypertensive medications based on a standardized postpartum hypertension protocol previously developed at our institution (available on request). This project was undertaken as a quality improvement initiative and as such was exempt from formal review by our institutional review board. However, all patients signed a waiver acknowledging that SMS texting is not a secure communications technology. A single research telephone was used for physician-patient communication to further ensure privacy.
Patients who qualified for the intervention study were recruited on the postpartum unit following delivery. Those who agreed to participate were provided with electronic blood pressure monitors (CVS Pharmacy automatic blood pressure monitor and Omron 3 Series upper arm blood pressure monitor) prior to discharge and instructed on their use. Patients were told to expect their first text message reminder to send in their blood pressure the day after discharge; an example of a text reminder is “Good morning. Please send us a blood pressure reading by 12 pm.” Patients were enrolled for 7 days post discharge and were interviewed regarding their experience at the end of their 7-day enrollment. As this was primarily a feasibility and quality improvement study, patients were also instructed to continue to follow up with the standard of care at the hypertension clinic visit.
For each of the 7 days following discharge from the hospital, patients received a standard text message in the morning and afternoon reminding them to text their blood pressure to the research telephone by a specific time. Reported blood pressures were reviewed and a standard response was sent by the study obstetrician based on an algorithm consistent with the institution’s postpartum hypertension protocol. Patients were sent reminders at all time points whether or not they had texted any BPs.
The ACOG Hypertension in Pregnancy guidelines recommend monitoring blood pressure at 72 hours postpartum (inpatient or outpatient) and again 7 to 10 days postpartum in women diagnosed with a hypertensive disorder of pregnancy [6]. We measured our ability to meet these guidelines by identifying how many patients texted blood pressures on post-discharge days 1 or 2 and post-discharge days 5, 6, or 7, as most patients were discharged home on postpartum day 2 or 3.
Strategies to enhance patient engagement were modified based on patient interviews and results from the immediately preceding cycle (for example, Cycle 1 interview information and results were used to make changes in Cycle 2), as well as studies on telemonitoring adherence in other populations [18]. The program ended after 6 cycles, as the study team felt there was sufficient promise to design an expanded platform suitable for a larger study.
Results
Overall
At the patient level, we received at least 1 blood pressure during the requested time frame from 27 of the 32 patients enrolled (84%). Nearly 65% of patients (20/32) texted at least 1 blood pressure reading on at least 5 out of the 7 days enrolled.
By Cycle
Cycle 1 - Basic
Cycle 1 tested our basic hypothesis that patients would take their blood pressure at home and transmit the results by text message: 5 of 7 patients responded to our reminders, each transmitting blood pressures on at least 5 of the 7 days requested.
Four severe-range blood pressures, defined as systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 110 [6], were sent to the physician responder, two times each in 2 patients. All four “at risk” severe blood pressures were addressed within 24 hours of the text message. No medications were initiated, as elevated blood pressures were not persistent and patients were asymptomatic.
Cycle 2 - Education
Patients in Cycle 1 reported during their follow-up interview that they became more aware of the possible morbidity associated with persistent postpartum hypertension as the cycle progressed. Therefore, Cycle 2 tested our hypothesis that focused education would improve patient engagement.
All five patients in this cohort sent in at least one blood pressure during the cycle period. All transmitted at least one blood pressure text on post-discharge day 1 or 2. Four of the five patients (80%) also sent in at least one blood pressure on day 5, 6, or 7.
There were no significantly elevated blood pressures sent to the physician responder.
Cycle 3 - Personalization
Patients in Cycle 2 reported during their interview that they felt the text message responses from the provider were too automated. Cycle 3 tested our hypothesis that added personalization, with patient and infant names included in the messages, would improve engagement.
Three of five patients in this cohort sent at least one blood pressure text on post-discharge day 1 or 2 (60%). Only one patient (20%) also sent in at least one blood pressure on day 5, 6, or 7.
One significantly elevated blood pressure was sent to the physician responder. This blood pressure was addressed within 24 hours of the text message. No medications were initiated, as elevated blood pressures were not persistent and patients were asymptomatic.
Cycle 4 - Response Timing
Patients in Cycle 3 had lower response rates than previous cycles and noted that they wanted more flexibility in the time to respond, as their schedules were unpredictable with a newborn at home. Although they enjoyed the personalized aspect, they did not feel it influenced their responses, which is evidenced by the low response rate on days 5, 6, or 7. Therefore, Cycle 4 tested our hypothesis that allowing patients to commit to a time of their choice for receiving the reminder texts would improve their response rate.
All five patients enrolled in this cohort sent in at least one blood pressure. We received at least one blood pressure text on post-discharge day 1 or 2 from all five patients in this cycle (100%). Three of the five patients (60%) also sent in at least one blood pressure on day 5, 6, or 7.
Five severely elevated blood pressures were sent to the physician responder, all from a single patient. This patient had been discharged home on hydrochlorothiazide 12.5 mg for persistently elevated blood pressures while in the hospital after being diagnosed with severe preeclampsia. All five “at risk” blood pressures were addressed within 24 hours of the text message. On her fifth day of remote surveillance, 5 mg of amlodipine was added to her daily regimen for blood pressures ranging from 150–170/90–110 mm Hg. Her blood pressure at her 6-week postpartum visit was 120/60 mm Hg and she had seen her primary care doctor in the interim for further hypertension management.
Cycle 5 - Snooze and Countdown
Although most of the patients enrolled in Cycle 4 stated that they were very busy in the immediate postpartum period and not always able to respond in a timely fashion, allowing patients to receive the reminder text at their own designated convenient time did not increase engagement. Patients reported that while they always carried their cell phones, they did not always carry their blood pressure cuff, limiting their ability to send in a reading at the time of the reminder. Additionally, patients reported feeling less motivated to continue texting blood pressures towards the end of the cycle. Cycle 5 tested our hypothesis that patient engagement would improve if reminder text messages were sent closer to the morning or evening deadline. Patients were provided with the opportunity to request “snooze” response if they did have their cuff accessible. Additionally, standard responses were accompanied by a countdown message. For example, “Your blood pressure looks good. Four more days of checking your blood pressure to go.”
All five enrolled in this cohort sent in at least one blood pressure, and all (100%) transmitted at least one blood pressure text on post-discharge day 1 or 2 and on day 5, 6, or 7. Only two “snooze” requests were made over the course of the arm by a single patient, who responded both times after the additional reminder.
Four severely elevated blood pressures were sent to the physician responder, all from a single patient. This patient was diagnosed with preeclampsia with severe features on delivery admission, and her blood pressures normalized prior to discharge. All four “at risk” blood pressures were addressed within 24 hours of the text message. Due to persistently elevated diastolic blood pressures ranging from 110–120 mm Hg, she was started on hydrochlorothiazide 12.5 mg on day 6 of the cycle and monitored for additional days following cycle completion with improved blood pressures.
Cycle 6 - Snooze and Support Person
The patients in Cycle 5 were overall satisfied with their experience and did not provide any suggestions for change. However, we sought to see if integrating support persons into the protocol would affect engagement. Cycle 6 tested our hypothesis that patients would be more engaged if they had a self-identified support person reminding them to text their blood pressures. Patients provided the name of a support person to contact if a morning blood pressure was not received. Additionally, patients received the same “snooze” option as in Cycle 5. A total of five patients were enrolled in this cohort; one patient enrolled in the trial but did not send in any blood pressures despite daily reminders to both her and her support buddy. Only 2 additional buddy notifications were required in patients who did not send in a morning blood pressure reading and both times a subsequent blood pressure was sent. Two “snooze” requests were made over the course of the cycle by a single patient, who responded both times after the reminder.
Four of five patients in this cohort sent at least one blood pressure text on post-discharge day 1 or 2 (80%). Three patients (60%) also sent in at least one blood pressure on day 5, 6, or 7.
There were no significantly elevated blood pressures sent to the physician responder and no medications were initiated.
Post-Cycle Interviews
Overall, patients reported satisfaction with the text messaging system in their post-cycle interviews. The convenience of the intervention was acknowledged by many, including one patient who commented that “this was a lot better than having to pay for the bus and waiting for hours in some waiting room.” One patient also reported that the increased awareness was important, stating that “when [she] got home and realized that [her blood pressure] was still high, [she] did her own research and learned more about hypertension and preeclampsia.” Others reported that they still checked their blood pressure after the cycle, and “would have went longer than a week if they had asked me to.”
Discussions
Our results suggest that remote blood pressure monitoring via text message communication engages patients and shows promise as a convenient and effective means of hypertension surveillance in the immediate postpartum period, in accordance to ACOG guidelines. Additionally, we were able to test this monitoring system using inexpensive, rapid-cycle validation techniques. Although these techniques are insufficiently controlled and of inadequate statistical power for definitive results, they were able to provide quick evidence toward a pragmatic and workable solution to an important clinical problem within the specific clinical context of our practice, though the results are likely to generalize to other settings. We found varied compliance based on the different engagement strategies, and although no single cycle proved superior, overall patient participation was good and provides a basis for different texting options in future work. Developing a method that both engages patients and is streamlined for providers is critical to our ability to translate this recommendation into practice. Although we did not specifically test how the system works from a provider’s point of view, the study obstetricians believe that this would help and can be fit within the existing workflows of the practices at most institutions.
This rapid-cycle intervention study provides several additional lessons, as we were able to rapidly implement this on our unit and test several hypotheses related to patient engagement. Most patients found the text messaging system to be a convenient way to communicate with their obstetrician. Even when patients had prenatal care at other institutions and delivered at our hospital without a prior patient/physician relationship (n = 5), we were able to engage them in text messaging. However, there was some evidence of patient drop out over the course of the week, as patients were more likely to text in blood pressure in the first few days of the cycle than the last few days (Figure 2).
Other telemedicine interventions have been studied in maternity care and have had inconsistent results. The Cochrane review on telephone support for women during pregnancy and up to 6 weeks after birth found that interventions were mainly aimed at smoking cessation, breastfeeding continuation, preterm birth, and postpartum depression [19]. To date, none of the randomized trials in pregnancy or the postpartum period have focused on postpartum hypertension. The results of our interventions are encouraging and support the use of text messaging in obstetrical care, particularly in the postpartum period. While text messaging cannot provide all the information that can be obtained in a doctor’s visit, such as physical exam, urine dipsticks, and review of symptoms, it can identify the minority of patients that may need to be seen in the office based on the severity of their blood pressures.
While some cases of postpartum preeclampsia occur in the absence of peripartum disease, most readmitted patients are diagnosed with preeclampsia prior to delivery and readmission is due to worsening or persistence of disease and therefore, potentially preventable. These patients are the primary target of our intervention, as remote hypertension surveillance provides an opportunity to start or adjust medications and minimize both patient inconvenience and hospital cost of a readmission.
However, our feasibility study has some limitations. Despite overall patient satisfaction, acceptability, and compliance with text message monitoring of hypertension, the small sample size and qualitative nature of our cycles merits further pursuit and follow-up studies prior to implementation. Overall, we had only a small number of elevated blood pressures requiring intervention; however, this underscores the need to identify patients most at risk for persistent or delayed hypertension and the importance of developing a method of follow-up that engages all patients. Additionally, as patients were asked to both text in blood pressure values and also present for office visits, and therefore acted as their own control, it is not surprising that more patients were compliant with the simple texting method than standard of care; however, even when comparing texting compliance to historical attendance in our clinic of only 30%, our results remain promising.
While our results are encouraging, we believe it is important to test text messaging surveillance and patient compliance in a larger trial prior to implementing within the broader community. This study provides critical data to support the development of a HIPAA-compliant, automated monitoring system that can provide timely responses to patient texts using a provider derived response to blood pressure values. Future work includes the development of an automated hypertension tool as well as a randomized controlled trial to more rigorously compare office blood pressure visits to remote text message surveillance. If effective, use of text messaging technology may allow for an improved patient partnership and more robust follow-up data, especially in patients with less than optimal compliance, as well as the ability to improve maternal care and decrease morbidity and mortality.
Corresponding author: Adi Hirshberg, MD, Dept. of Maternal-Fetal Medicine, 2 Silverstein, 3400 Spruce St., Philadelphia, PA 19104, adi.hirshberg@uphs.upenn.edu.
Funding/support: Supported by a Penn Medicine Innovation Accelerator grant.
Financial disclosures. None reported.
1. Creanga AA, Berg CJ, Syverson C, et al. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol 2015;125:5–12.
2. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:S1–S22.
3. Muri JH, Crawford N, Jellen BC. Reducing avoidable obstetrical and neonatal readmissions. American Hospital Association. Accessed 20 Sep 2016 at www.aha.org/content/11/PerinatalReadmissionscall1.pdf.
4. Walters BN, Walters T. Hypertension in the puerperium. Lancet 1987;2:330.
5. Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol 2012;206:470–5.
6. Executive summary: Hypertension in pregnancy. American College of Obstetricians and Gynecologists. Obstet Gynecol 2013;122:1122–31.
7. Scalise LF, Stringer M. Follow-up text messages for patients at high risk for postpartum hypertension. J Obstet Gynecol Neonatal Nurs 2015;44:S6.
8. Using health text messages to improve consumer health knowledge, behaviors, and outcomes: an environmental scan. Rockville, MD: U.S. Department of Health and Human Services; 2014.
9. Gurol-Urganci I, de Jongh T, Vodopivec-Jamsek V, et al. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database Syst Rev 2013;5;12:CD007458.
10. Saffari M, Ghanizadeh G, Koenig HG. Health education via mobile text messaging for glycemic control in adults with type 2 diabetes: a systematic review and meta-analysis. Prim Care Diabetes 2014;8:275–85.
11. Tran N, Coffma JM, Sumino K, Cabana MD. Patient reminder systems and asthma medication adherence: a systematic review. J Asthma 2014;51:536–43.
12. Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev 2012;3:CD009756.
13. Kannisto KA, Koivunen MF, Valimaki MA. Use of mobile phone text message reminders in health care services: a narrative literature review. J Med Internet Res 2010;16:e222.
14. Pew Research Center. Mobile technology fact sheet. Accessed 17 Dec 2014 at www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/.
15. Duggan M. Cell phone activities 2013. Pew Research Center’s Internet and American Life Project. Available at www.pewinternet.org/Reports/2013/Cell-Activities.aspx.
16. Langley G, Nolan K, Nolan T, et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco: Jossey-Bass; 1996.
17. Brown P, Hare D. Rapid cycle improvement: controlling change. J Ark Med Soc 2003;99:320–1.
18. Aikens JE, Trivedi R, Aron DC, Piette JD. Integrating support persons into diabetes telemonitoring to improve self-management and medication adherence. J Gen Intern Med 2015;30:319–26.
19. Lavender T, Richens Y, Milan SJ, et al. Telephone support for women during pregnancy and the first six weeks postpartum. Cochrane Database Syst Rev 2013;7:CD009338.
From the Maternal and Child Health Research Program, Department of Obstetrics and Gynecology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Dr. Hirshberg, Dr. Srinivas); Hospital of the University of Pennsylvania, Department of Nursing, Department of Obstetrics and Gynecology, Philadelphia, PA (Ms. Bittle); Penn Medicine Center for Health Care Innovation, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Mr. Vandertuyn, Ms. Mahraj, Dr. Asch, Mr. Rosin); and the Department of Family Medicine, University of Washington, Seattle, WA (Dr. Bennett).
Abstract
- Objective: To investigate engagement with a bidirectional text messaging system as an alternative to in-person follow-up for postpartum women with hypertensive disorders.
- Methods: We utilized rapid-cycle innovation processes to implement postpartum SMS text messaging follow-up in women with hypertensive disorders who delivered between September–December 2014. Patients were given electronic blood pressure cuffs and education before discharge. Standard texts reminded patients to send blood pressures daily on each of the 7 days post discharge. The study obstetrician sent text message responses based on a pre-specified management algorithm. Ability to meet ACOG guidelines was defined as receiving at least 1 reading on post-discharge days 1 or 2 and days 5, 6, or 7.
- Results: We enrolled 32 patients. Six (19%) returned for usual care office blood pressure checks. We received at least 1 blood pressure from 27 (84%) participants. Nearly 20 (65%) texted readings on 5 of the 7 days. 27 (84%) texted at least one reading on day 1 or 2, and 21 (66%) texted at least one pressure on day 5, 6, or 7 (P = 0.001 vs. usual care). Two patients required medications and none were readmitted for hypertension. Patients reported preference for home testing and text messaging over return visits.
- Conclusion: Remote blood pressure monitoring via text messaging is a patient-centered method for postpartum hypertension surveillance. Further testing is needed prior to widespread adoption within the broader obstetric community.
Key words: postpartum hypertension, remote monitoring, text-based intervention.
Hypertensive disease is a leading cause of maternal morbidity and mortality [1,2] and the leading cause of obstetric readmissions, accounting for 27% of obstetric readmissions in the United States in 2009 [3]. The majority of patients readmitted with hypertension have a diagnosis of hypertensive disorder of pregnancy on initial admission for delivery, indicating that these readmissions are the result of disease persistence or progression in contrast to new-onset disease. Peak blood pressure in these patients usually occurs 3 to 6 days postpartum [4–6] and is typically unaccompanied by warning symptoms. For these reasons, identifying patients who are at risk for persistent disease and being proactive in their postpartum care may decrease postpartum stroke and seizure. The recent Hypertension in Pregnancy guidelines provided by the American College of Obstetricians and Gynecologists (ACOG) recommend monitoring blood pressure for at least 72 hours postpartum (inpatient or outpatient) and again 7 to 10 days after delivery in women in whom a hypertensive disease of pregnancy is diagnosed [6].
Although there is a clear need for effective and reliable blood pressure surveillance for high-risk women soon after delivery, significant obstacles exist. Our own high-risk blood pressure transition clinic, which occurred every other week and was staffed by maternal-fetal medicine specialists, had an average attendance of only 30% over a 2-year period. Moreover, all of the hypertension-related readmissions occurred in the first 7 days post discharge, which was before the scheduled clinic visit for approximately 50% of patients. Phone call reminders were also found to be an ineffective strategy, as the women did not answer or return voice messages left by the practice. In fact, a postpartum unit quality improvement project validated that follow-up phone calls after discharge from the postpartum unit were less effective than text messaging when reminding women of their blood pressure follow-up appointment at the clinic [7].
As an alternative to in-person visits or traditional voice telephonic communication, mobile phone “Short Message Service” (SMS) text messaging has been used successfully in health care for appointment reminders, result reporting, support of medication and treatment adherence, and dosage adjustment [8–13]. As of 2014, 90% of American adults own a cell phone and over 79% of those send and receive text messages [14]. Among a young population, which is at high risk for hypertensive disorders of pregnancy, data further reveals a preference for text messaging over live calls [15]. Among low-income women under age 30, the rates of cell phone use and text communication are very high [14,15], making text-based surveillance a promising and more patient-centered strategy for a broad population.
We report the results of rapid-cycle innovation and implementation of active, remote surveillance of hypertension with new text message communication strategies in the first 7 days post-discharge. We chose a Plan-Do-Study-Act cycle approach, in which small tests are performed and studied and changes made to accelerate improvement, in order to enhance our ability to acquire blood pressure data [16,17]. The goals of the work were to (1) assess patient engagement using a remote method of blood pressure monitoring, (2) increase ascertainment of postpartum blood pressure data and obtain at least once daily blood pressure readings on all patients on post discharge days 1–2 and 5–7, which is in accordance with the recommended guidelines [6] for blood pressure surveillance, and (3) address all “at risk” severe range blood pressure readings within a short time interval and prior to the need for readmission. We describe a program of remote blood pressure monitoring and communication via text message designed to increase patient engagement and participation, thereby having the potential to result in earlier interventions, reduce readmissions, and decrease overall morbidity.
Methods
We performed a series of 6 rapid-cycle innovation devel-opment and implementation interventions with a cohort of women with chronic hypertension (CHTN), gestational hypertension (GHTN), or preeclampsia (with and without severe features and superimposed) who delivered at our institution between 20 September 2014 and 14 December 2014. All patients were > 18 years old, able to speak and read English, had a hypertension diag-nosis listed above, and had access to a cell phone with unlimited text messaging capabilities. Patients received standard postpartum care and were continued or started on antihypertensive medications based on a standardized postpartum hypertension protocol previously developed at our institution (available on request). This project was undertaken as a quality improvement initiative and as such was exempt from formal review by our institutional review board. However, all patients signed a waiver acknowledging that SMS texting is not a secure communications technology. A single research telephone was used for physician-patient communication to further ensure privacy.
Patients who qualified for the intervention study were recruited on the postpartum unit following delivery. Those who agreed to participate were provided with electronic blood pressure monitors (CVS Pharmacy automatic blood pressure monitor and Omron 3 Series upper arm blood pressure monitor) prior to discharge and instructed on their use. Patients were told to expect their first text message reminder to send in their blood pressure the day after discharge; an example of a text reminder is “Good morning. Please send us a blood pressure reading by 12 pm.” Patients were enrolled for 7 days post discharge and were interviewed regarding their experience at the end of their 7-day enrollment. As this was primarily a feasibility and quality improvement study, patients were also instructed to continue to follow up with the standard of care at the hypertension clinic visit.
For each of the 7 days following discharge from the hospital, patients received a standard text message in the morning and afternoon reminding them to text their blood pressure to the research telephone by a specific time. Reported blood pressures were reviewed and a standard response was sent by the study obstetrician based on an algorithm consistent with the institution’s postpartum hypertension protocol. Patients were sent reminders at all time points whether or not they had texted any BPs.
The ACOG Hypertension in Pregnancy guidelines recommend monitoring blood pressure at 72 hours postpartum (inpatient or outpatient) and again 7 to 10 days postpartum in women diagnosed with a hypertensive disorder of pregnancy [6]. We measured our ability to meet these guidelines by identifying how many patients texted blood pressures on post-discharge days 1 or 2 and post-discharge days 5, 6, or 7, as most patients were discharged home on postpartum day 2 or 3.
Strategies to enhance patient engagement were modified based on patient interviews and results from the immediately preceding cycle (for example, Cycle 1 interview information and results were used to make changes in Cycle 2), as well as studies on telemonitoring adherence in other populations [18]. The program ended after 6 cycles, as the study team felt there was sufficient promise to design an expanded platform suitable for a larger study.
Results
Overall
At the patient level, we received at least 1 blood pressure during the requested time frame from 27 of the 32 patients enrolled (84%). Nearly 65% of patients (20/32) texted at least 1 blood pressure reading on at least 5 out of the 7 days enrolled.
By Cycle
Cycle 1 - Basic
Cycle 1 tested our basic hypothesis that patients would take their blood pressure at home and transmit the results by text message: 5 of 7 patients responded to our reminders, each transmitting blood pressures on at least 5 of the 7 days requested.
Four severe-range blood pressures, defined as systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 110 [6], were sent to the physician responder, two times each in 2 patients. All four “at risk” severe blood pressures were addressed within 24 hours of the text message. No medications were initiated, as elevated blood pressures were not persistent and patients were asymptomatic.
Cycle 2 - Education
Patients in Cycle 1 reported during their follow-up interview that they became more aware of the possible morbidity associated with persistent postpartum hypertension as the cycle progressed. Therefore, Cycle 2 tested our hypothesis that focused education would improve patient engagement.
All five patients in this cohort sent in at least one blood pressure during the cycle period. All transmitted at least one blood pressure text on post-discharge day 1 or 2. Four of the five patients (80%) also sent in at least one blood pressure on day 5, 6, or 7.
There were no significantly elevated blood pressures sent to the physician responder.
Cycle 3 - Personalization
Patients in Cycle 2 reported during their interview that they felt the text message responses from the provider were too automated. Cycle 3 tested our hypothesis that added personalization, with patient and infant names included in the messages, would improve engagement.
Three of five patients in this cohort sent at least one blood pressure text on post-discharge day 1 or 2 (60%). Only one patient (20%) also sent in at least one blood pressure on day 5, 6, or 7.
One significantly elevated blood pressure was sent to the physician responder. This blood pressure was addressed within 24 hours of the text message. No medications were initiated, as elevated blood pressures were not persistent and patients were asymptomatic.
Cycle 4 - Response Timing
Patients in Cycle 3 had lower response rates than previous cycles and noted that they wanted more flexibility in the time to respond, as their schedules were unpredictable with a newborn at home. Although they enjoyed the personalized aspect, they did not feel it influenced their responses, which is evidenced by the low response rate on days 5, 6, or 7. Therefore, Cycle 4 tested our hypothesis that allowing patients to commit to a time of their choice for receiving the reminder texts would improve their response rate.
All five patients enrolled in this cohort sent in at least one blood pressure. We received at least one blood pressure text on post-discharge day 1 or 2 from all five patients in this cycle (100%). Three of the five patients (60%) also sent in at least one blood pressure on day 5, 6, or 7.
Five severely elevated blood pressures were sent to the physician responder, all from a single patient. This patient had been discharged home on hydrochlorothiazide 12.5 mg for persistently elevated blood pressures while in the hospital after being diagnosed with severe preeclampsia. All five “at risk” blood pressures were addressed within 24 hours of the text message. On her fifth day of remote surveillance, 5 mg of amlodipine was added to her daily regimen for blood pressures ranging from 150–170/90–110 mm Hg. Her blood pressure at her 6-week postpartum visit was 120/60 mm Hg and she had seen her primary care doctor in the interim for further hypertension management.
Cycle 5 - Snooze and Countdown
Although most of the patients enrolled in Cycle 4 stated that they were very busy in the immediate postpartum period and not always able to respond in a timely fashion, allowing patients to receive the reminder text at their own designated convenient time did not increase engagement. Patients reported that while they always carried their cell phones, they did not always carry their blood pressure cuff, limiting their ability to send in a reading at the time of the reminder. Additionally, patients reported feeling less motivated to continue texting blood pressures towards the end of the cycle. Cycle 5 tested our hypothesis that patient engagement would improve if reminder text messages were sent closer to the morning or evening deadline. Patients were provided with the opportunity to request “snooze” response if they did have their cuff accessible. Additionally, standard responses were accompanied by a countdown message. For example, “Your blood pressure looks good. Four more days of checking your blood pressure to go.”
All five enrolled in this cohort sent in at least one blood pressure, and all (100%) transmitted at least one blood pressure text on post-discharge day 1 or 2 and on day 5, 6, or 7. Only two “snooze” requests were made over the course of the arm by a single patient, who responded both times after the additional reminder.
Four severely elevated blood pressures were sent to the physician responder, all from a single patient. This patient was diagnosed with preeclampsia with severe features on delivery admission, and her blood pressures normalized prior to discharge. All four “at risk” blood pressures were addressed within 24 hours of the text message. Due to persistently elevated diastolic blood pressures ranging from 110–120 mm Hg, she was started on hydrochlorothiazide 12.5 mg on day 6 of the cycle and monitored for additional days following cycle completion with improved blood pressures.
Cycle 6 - Snooze and Support Person
The patients in Cycle 5 were overall satisfied with their experience and did not provide any suggestions for change. However, we sought to see if integrating support persons into the protocol would affect engagement. Cycle 6 tested our hypothesis that patients would be more engaged if they had a self-identified support person reminding them to text their blood pressures. Patients provided the name of a support person to contact if a morning blood pressure was not received. Additionally, patients received the same “snooze” option as in Cycle 5. A total of five patients were enrolled in this cohort; one patient enrolled in the trial but did not send in any blood pressures despite daily reminders to both her and her support buddy. Only 2 additional buddy notifications were required in patients who did not send in a morning blood pressure reading and both times a subsequent blood pressure was sent. Two “snooze” requests were made over the course of the cycle by a single patient, who responded both times after the reminder.
Four of five patients in this cohort sent at least one blood pressure text on post-discharge day 1 or 2 (80%). Three patients (60%) also sent in at least one blood pressure on day 5, 6, or 7.
There were no significantly elevated blood pressures sent to the physician responder and no medications were initiated.
Post-Cycle Interviews
Overall, patients reported satisfaction with the text messaging system in their post-cycle interviews. The convenience of the intervention was acknowledged by many, including one patient who commented that “this was a lot better than having to pay for the bus and waiting for hours in some waiting room.” One patient also reported that the increased awareness was important, stating that “when [she] got home and realized that [her blood pressure] was still high, [she] did her own research and learned more about hypertension and preeclampsia.” Others reported that they still checked their blood pressure after the cycle, and “would have went longer than a week if they had asked me to.”
Discussions
Our results suggest that remote blood pressure monitoring via text message communication engages patients and shows promise as a convenient and effective means of hypertension surveillance in the immediate postpartum period, in accordance to ACOG guidelines. Additionally, we were able to test this monitoring system using inexpensive, rapid-cycle validation techniques. Although these techniques are insufficiently controlled and of inadequate statistical power for definitive results, they were able to provide quick evidence toward a pragmatic and workable solution to an important clinical problem within the specific clinical context of our practice, though the results are likely to generalize to other settings. We found varied compliance based on the different engagement strategies, and although no single cycle proved superior, overall patient participation was good and provides a basis for different texting options in future work. Developing a method that both engages patients and is streamlined for providers is critical to our ability to translate this recommendation into practice. Although we did not specifically test how the system works from a provider’s point of view, the study obstetricians believe that this would help and can be fit within the existing workflows of the practices at most institutions.
This rapid-cycle intervention study provides several additional lessons, as we were able to rapidly implement this on our unit and test several hypotheses related to patient engagement. Most patients found the text messaging system to be a convenient way to communicate with their obstetrician. Even when patients had prenatal care at other institutions and delivered at our hospital without a prior patient/physician relationship (n = 5), we were able to engage them in text messaging. However, there was some evidence of patient drop out over the course of the week, as patients were more likely to text in blood pressure in the first few days of the cycle than the last few days (Figure 2).
Other telemedicine interventions have been studied in maternity care and have had inconsistent results. The Cochrane review on telephone support for women during pregnancy and up to 6 weeks after birth found that interventions were mainly aimed at smoking cessation, breastfeeding continuation, preterm birth, and postpartum depression [19]. To date, none of the randomized trials in pregnancy or the postpartum period have focused on postpartum hypertension. The results of our interventions are encouraging and support the use of text messaging in obstetrical care, particularly in the postpartum period. While text messaging cannot provide all the information that can be obtained in a doctor’s visit, such as physical exam, urine dipsticks, and review of symptoms, it can identify the minority of patients that may need to be seen in the office based on the severity of their blood pressures.
While some cases of postpartum preeclampsia occur in the absence of peripartum disease, most readmitted patients are diagnosed with preeclampsia prior to delivery and readmission is due to worsening or persistence of disease and therefore, potentially preventable. These patients are the primary target of our intervention, as remote hypertension surveillance provides an opportunity to start or adjust medications and minimize both patient inconvenience and hospital cost of a readmission.
However, our feasibility study has some limitations. Despite overall patient satisfaction, acceptability, and compliance with text message monitoring of hypertension, the small sample size and qualitative nature of our cycles merits further pursuit and follow-up studies prior to implementation. Overall, we had only a small number of elevated blood pressures requiring intervention; however, this underscores the need to identify patients most at risk for persistent or delayed hypertension and the importance of developing a method of follow-up that engages all patients. Additionally, as patients were asked to both text in blood pressure values and also present for office visits, and therefore acted as their own control, it is not surprising that more patients were compliant with the simple texting method than standard of care; however, even when comparing texting compliance to historical attendance in our clinic of only 30%, our results remain promising.
While our results are encouraging, we believe it is important to test text messaging surveillance and patient compliance in a larger trial prior to implementing within the broader community. This study provides critical data to support the development of a HIPAA-compliant, automated monitoring system that can provide timely responses to patient texts using a provider derived response to blood pressure values. Future work includes the development of an automated hypertension tool as well as a randomized controlled trial to more rigorously compare office blood pressure visits to remote text message surveillance. If effective, use of text messaging technology may allow for an improved patient partnership and more robust follow-up data, especially in patients with less than optimal compliance, as well as the ability to improve maternal care and decrease morbidity and mortality.
Corresponding author: Adi Hirshberg, MD, Dept. of Maternal-Fetal Medicine, 2 Silverstein, 3400 Spruce St., Philadelphia, PA 19104, adi.hirshberg@uphs.upenn.edu.
Funding/support: Supported by a Penn Medicine Innovation Accelerator grant.
Financial disclosures. None reported.
From the Maternal and Child Health Research Program, Department of Obstetrics and Gynecology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Dr. Hirshberg, Dr. Srinivas); Hospital of the University of Pennsylvania, Department of Nursing, Department of Obstetrics and Gynecology, Philadelphia, PA (Ms. Bittle); Penn Medicine Center for Health Care Innovation, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Mr. Vandertuyn, Ms. Mahraj, Dr. Asch, Mr. Rosin); and the Department of Family Medicine, University of Washington, Seattle, WA (Dr. Bennett).
Abstract
- Objective: To investigate engagement with a bidirectional text messaging system as an alternative to in-person follow-up for postpartum women with hypertensive disorders.
- Methods: We utilized rapid-cycle innovation processes to implement postpartum SMS text messaging follow-up in women with hypertensive disorders who delivered between September–December 2014. Patients were given electronic blood pressure cuffs and education before discharge. Standard texts reminded patients to send blood pressures daily on each of the 7 days post discharge. The study obstetrician sent text message responses based on a pre-specified management algorithm. Ability to meet ACOG guidelines was defined as receiving at least 1 reading on post-discharge days 1 or 2 and days 5, 6, or 7.
- Results: We enrolled 32 patients. Six (19%) returned for usual care office blood pressure checks. We received at least 1 blood pressure from 27 (84%) participants. Nearly 20 (65%) texted readings on 5 of the 7 days. 27 (84%) texted at least one reading on day 1 or 2, and 21 (66%) texted at least one pressure on day 5, 6, or 7 (P = 0.001 vs. usual care). Two patients required medications and none were readmitted for hypertension. Patients reported preference for home testing and text messaging over return visits.
- Conclusion: Remote blood pressure monitoring via text messaging is a patient-centered method for postpartum hypertension surveillance. Further testing is needed prior to widespread adoption within the broader obstetric community.
Key words: postpartum hypertension, remote monitoring, text-based intervention.
Hypertensive disease is a leading cause of maternal morbidity and mortality [1,2] and the leading cause of obstetric readmissions, accounting for 27% of obstetric readmissions in the United States in 2009 [3]. The majority of patients readmitted with hypertension have a diagnosis of hypertensive disorder of pregnancy on initial admission for delivery, indicating that these readmissions are the result of disease persistence or progression in contrast to new-onset disease. Peak blood pressure in these patients usually occurs 3 to 6 days postpartum [4–6] and is typically unaccompanied by warning symptoms. For these reasons, identifying patients who are at risk for persistent disease and being proactive in their postpartum care may decrease postpartum stroke and seizure. The recent Hypertension in Pregnancy guidelines provided by the American College of Obstetricians and Gynecologists (ACOG) recommend monitoring blood pressure for at least 72 hours postpartum (inpatient or outpatient) and again 7 to 10 days after delivery in women in whom a hypertensive disease of pregnancy is diagnosed [6].
Although there is a clear need for effective and reliable blood pressure surveillance for high-risk women soon after delivery, significant obstacles exist. Our own high-risk blood pressure transition clinic, which occurred every other week and was staffed by maternal-fetal medicine specialists, had an average attendance of only 30% over a 2-year period. Moreover, all of the hypertension-related readmissions occurred in the first 7 days post discharge, which was before the scheduled clinic visit for approximately 50% of patients. Phone call reminders were also found to be an ineffective strategy, as the women did not answer or return voice messages left by the practice. In fact, a postpartum unit quality improvement project validated that follow-up phone calls after discharge from the postpartum unit were less effective than text messaging when reminding women of their blood pressure follow-up appointment at the clinic [7].
As an alternative to in-person visits or traditional voice telephonic communication, mobile phone “Short Message Service” (SMS) text messaging has been used successfully in health care for appointment reminders, result reporting, support of medication and treatment adherence, and dosage adjustment [8–13]. As of 2014, 90% of American adults own a cell phone and over 79% of those send and receive text messages [14]. Among a young population, which is at high risk for hypertensive disorders of pregnancy, data further reveals a preference for text messaging over live calls [15]. Among low-income women under age 30, the rates of cell phone use and text communication are very high [14,15], making text-based surveillance a promising and more patient-centered strategy for a broad population.
We report the results of rapid-cycle innovation and implementation of active, remote surveillance of hypertension with new text message communication strategies in the first 7 days post-discharge. We chose a Plan-Do-Study-Act cycle approach, in which small tests are performed and studied and changes made to accelerate improvement, in order to enhance our ability to acquire blood pressure data [16,17]. The goals of the work were to (1) assess patient engagement using a remote method of blood pressure monitoring, (2) increase ascertainment of postpartum blood pressure data and obtain at least once daily blood pressure readings on all patients on post discharge days 1–2 and 5–7, which is in accordance with the recommended guidelines [6] for blood pressure surveillance, and (3) address all “at risk” severe range blood pressure readings within a short time interval and prior to the need for readmission. We describe a program of remote blood pressure monitoring and communication via text message designed to increase patient engagement and participation, thereby having the potential to result in earlier interventions, reduce readmissions, and decrease overall morbidity.
Methods
We performed a series of 6 rapid-cycle innovation devel-opment and implementation interventions with a cohort of women with chronic hypertension (CHTN), gestational hypertension (GHTN), or preeclampsia (with and without severe features and superimposed) who delivered at our institution between 20 September 2014 and 14 December 2014. All patients were > 18 years old, able to speak and read English, had a hypertension diag-nosis listed above, and had access to a cell phone with unlimited text messaging capabilities. Patients received standard postpartum care and were continued or started on antihypertensive medications based on a standardized postpartum hypertension protocol previously developed at our institution (available on request). This project was undertaken as a quality improvement initiative and as such was exempt from formal review by our institutional review board. However, all patients signed a waiver acknowledging that SMS texting is not a secure communications technology. A single research telephone was used for physician-patient communication to further ensure privacy.
Patients who qualified for the intervention study were recruited on the postpartum unit following delivery. Those who agreed to participate were provided with electronic blood pressure monitors (CVS Pharmacy automatic blood pressure monitor and Omron 3 Series upper arm blood pressure monitor) prior to discharge and instructed on their use. Patients were told to expect their first text message reminder to send in their blood pressure the day after discharge; an example of a text reminder is “Good morning. Please send us a blood pressure reading by 12 pm.” Patients were enrolled for 7 days post discharge and were interviewed regarding their experience at the end of their 7-day enrollment. As this was primarily a feasibility and quality improvement study, patients were also instructed to continue to follow up with the standard of care at the hypertension clinic visit.
For each of the 7 days following discharge from the hospital, patients received a standard text message in the morning and afternoon reminding them to text their blood pressure to the research telephone by a specific time. Reported blood pressures were reviewed and a standard response was sent by the study obstetrician based on an algorithm consistent with the institution’s postpartum hypertension protocol. Patients were sent reminders at all time points whether or not they had texted any BPs.
The ACOG Hypertension in Pregnancy guidelines recommend monitoring blood pressure at 72 hours postpartum (inpatient or outpatient) and again 7 to 10 days postpartum in women diagnosed with a hypertensive disorder of pregnancy [6]. We measured our ability to meet these guidelines by identifying how many patients texted blood pressures on post-discharge days 1 or 2 and post-discharge days 5, 6, or 7, as most patients were discharged home on postpartum day 2 or 3.
Strategies to enhance patient engagement were modified based on patient interviews and results from the immediately preceding cycle (for example, Cycle 1 interview information and results were used to make changes in Cycle 2), as well as studies on telemonitoring adherence in other populations [18]. The program ended after 6 cycles, as the study team felt there was sufficient promise to design an expanded platform suitable for a larger study.
Results
Overall
At the patient level, we received at least 1 blood pressure during the requested time frame from 27 of the 32 patients enrolled (84%). Nearly 65% of patients (20/32) texted at least 1 blood pressure reading on at least 5 out of the 7 days enrolled.
By Cycle
Cycle 1 - Basic
Cycle 1 tested our basic hypothesis that patients would take their blood pressure at home and transmit the results by text message: 5 of 7 patients responded to our reminders, each transmitting blood pressures on at least 5 of the 7 days requested.
Four severe-range blood pressures, defined as systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 110 [6], were sent to the physician responder, two times each in 2 patients. All four “at risk” severe blood pressures were addressed within 24 hours of the text message. No medications were initiated, as elevated blood pressures were not persistent and patients were asymptomatic.
Cycle 2 - Education
Patients in Cycle 1 reported during their follow-up interview that they became more aware of the possible morbidity associated with persistent postpartum hypertension as the cycle progressed. Therefore, Cycle 2 tested our hypothesis that focused education would improve patient engagement.
All five patients in this cohort sent in at least one blood pressure during the cycle period. All transmitted at least one blood pressure text on post-discharge day 1 or 2. Four of the five patients (80%) also sent in at least one blood pressure on day 5, 6, or 7.
There were no significantly elevated blood pressures sent to the physician responder.
Cycle 3 - Personalization
Patients in Cycle 2 reported during their interview that they felt the text message responses from the provider were too automated. Cycle 3 tested our hypothesis that added personalization, with patient and infant names included in the messages, would improve engagement.
Three of five patients in this cohort sent at least one blood pressure text on post-discharge day 1 or 2 (60%). Only one patient (20%) also sent in at least one blood pressure on day 5, 6, or 7.
One significantly elevated blood pressure was sent to the physician responder. This blood pressure was addressed within 24 hours of the text message. No medications were initiated, as elevated blood pressures were not persistent and patients were asymptomatic.
Cycle 4 - Response Timing
Patients in Cycle 3 had lower response rates than previous cycles and noted that they wanted more flexibility in the time to respond, as their schedules were unpredictable with a newborn at home. Although they enjoyed the personalized aspect, they did not feel it influenced their responses, which is evidenced by the low response rate on days 5, 6, or 7. Therefore, Cycle 4 tested our hypothesis that allowing patients to commit to a time of their choice for receiving the reminder texts would improve their response rate.
All five patients enrolled in this cohort sent in at least one blood pressure. We received at least one blood pressure text on post-discharge day 1 or 2 from all five patients in this cycle (100%). Three of the five patients (60%) also sent in at least one blood pressure on day 5, 6, or 7.
Five severely elevated blood pressures were sent to the physician responder, all from a single patient. This patient had been discharged home on hydrochlorothiazide 12.5 mg for persistently elevated blood pressures while in the hospital after being diagnosed with severe preeclampsia. All five “at risk” blood pressures were addressed within 24 hours of the text message. On her fifth day of remote surveillance, 5 mg of amlodipine was added to her daily regimen for blood pressures ranging from 150–170/90–110 mm Hg. Her blood pressure at her 6-week postpartum visit was 120/60 mm Hg and she had seen her primary care doctor in the interim for further hypertension management.
Cycle 5 - Snooze and Countdown
Although most of the patients enrolled in Cycle 4 stated that they were very busy in the immediate postpartum period and not always able to respond in a timely fashion, allowing patients to receive the reminder text at their own designated convenient time did not increase engagement. Patients reported that while they always carried their cell phones, they did not always carry their blood pressure cuff, limiting their ability to send in a reading at the time of the reminder. Additionally, patients reported feeling less motivated to continue texting blood pressures towards the end of the cycle. Cycle 5 tested our hypothesis that patient engagement would improve if reminder text messages were sent closer to the morning or evening deadline. Patients were provided with the opportunity to request “snooze” response if they did have their cuff accessible. Additionally, standard responses were accompanied by a countdown message. For example, “Your blood pressure looks good. Four more days of checking your blood pressure to go.”
All five enrolled in this cohort sent in at least one blood pressure, and all (100%) transmitted at least one blood pressure text on post-discharge day 1 or 2 and on day 5, 6, or 7. Only two “snooze” requests were made over the course of the arm by a single patient, who responded both times after the additional reminder.
Four severely elevated blood pressures were sent to the physician responder, all from a single patient. This patient was diagnosed with preeclampsia with severe features on delivery admission, and her blood pressures normalized prior to discharge. All four “at risk” blood pressures were addressed within 24 hours of the text message. Due to persistently elevated diastolic blood pressures ranging from 110–120 mm Hg, she was started on hydrochlorothiazide 12.5 mg on day 6 of the cycle and monitored for additional days following cycle completion with improved blood pressures.
Cycle 6 - Snooze and Support Person
The patients in Cycle 5 were overall satisfied with their experience and did not provide any suggestions for change. However, we sought to see if integrating support persons into the protocol would affect engagement. Cycle 6 tested our hypothesis that patients would be more engaged if they had a self-identified support person reminding them to text their blood pressures. Patients provided the name of a support person to contact if a morning blood pressure was not received. Additionally, patients received the same “snooze” option as in Cycle 5. A total of five patients were enrolled in this cohort; one patient enrolled in the trial but did not send in any blood pressures despite daily reminders to both her and her support buddy. Only 2 additional buddy notifications were required in patients who did not send in a morning blood pressure reading and both times a subsequent blood pressure was sent. Two “snooze” requests were made over the course of the cycle by a single patient, who responded both times after the reminder.
Four of five patients in this cohort sent at least one blood pressure text on post-discharge day 1 or 2 (80%). Three patients (60%) also sent in at least one blood pressure on day 5, 6, or 7.
There were no significantly elevated blood pressures sent to the physician responder and no medications were initiated.
Post-Cycle Interviews
Overall, patients reported satisfaction with the text messaging system in their post-cycle interviews. The convenience of the intervention was acknowledged by many, including one patient who commented that “this was a lot better than having to pay for the bus and waiting for hours in some waiting room.” One patient also reported that the increased awareness was important, stating that “when [she] got home and realized that [her blood pressure] was still high, [she] did her own research and learned more about hypertension and preeclampsia.” Others reported that they still checked their blood pressure after the cycle, and “would have went longer than a week if they had asked me to.”
Discussions
Our results suggest that remote blood pressure monitoring via text message communication engages patients and shows promise as a convenient and effective means of hypertension surveillance in the immediate postpartum period, in accordance to ACOG guidelines. Additionally, we were able to test this monitoring system using inexpensive, rapid-cycle validation techniques. Although these techniques are insufficiently controlled and of inadequate statistical power for definitive results, they were able to provide quick evidence toward a pragmatic and workable solution to an important clinical problem within the specific clinical context of our practice, though the results are likely to generalize to other settings. We found varied compliance based on the different engagement strategies, and although no single cycle proved superior, overall patient participation was good and provides a basis for different texting options in future work. Developing a method that both engages patients and is streamlined for providers is critical to our ability to translate this recommendation into practice. Although we did not specifically test how the system works from a provider’s point of view, the study obstetricians believe that this would help and can be fit within the existing workflows of the practices at most institutions.
This rapid-cycle intervention study provides several additional lessons, as we were able to rapidly implement this on our unit and test several hypotheses related to patient engagement. Most patients found the text messaging system to be a convenient way to communicate with their obstetrician. Even when patients had prenatal care at other institutions and delivered at our hospital without a prior patient/physician relationship (n = 5), we were able to engage them in text messaging. However, there was some evidence of patient drop out over the course of the week, as patients were more likely to text in blood pressure in the first few days of the cycle than the last few days (Figure 2).
Other telemedicine interventions have been studied in maternity care and have had inconsistent results. The Cochrane review on telephone support for women during pregnancy and up to 6 weeks after birth found that interventions were mainly aimed at smoking cessation, breastfeeding continuation, preterm birth, and postpartum depression [19]. To date, none of the randomized trials in pregnancy or the postpartum period have focused on postpartum hypertension. The results of our interventions are encouraging and support the use of text messaging in obstetrical care, particularly in the postpartum period. While text messaging cannot provide all the information that can be obtained in a doctor’s visit, such as physical exam, urine dipsticks, and review of symptoms, it can identify the minority of patients that may need to be seen in the office based on the severity of their blood pressures.
While some cases of postpartum preeclampsia occur in the absence of peripartum disease, most readmitted patients are diagnosed with preeclampsia prior to delivery and readmission is due to worsening or persistence of disease and therefore, potentially preventable. These patients are the primary target of our intervention, as remote hypertension surveillance provides an opportunity to start or adjust medications and minimize both patient inconvenience and hospital cost of a readmission.
However, our feasibility study has some limitations. Despite overall patient satisfaction, acceptability, and compliance with text message monitoring of hypertension, the small sample size and qualitative nature of our cycles merits further pursuit and follow-up studies prior to implementation. Overall, we had only a small number of elevated blood pressures requiring intervention; however, this underscores the need to identify patients most at risk for persistent or delayed hypertension and the importance of developing a method of follow-up that engages all patients. Additionally, as patients were asked to both text in blood pressure values and also present for office visits, and therefore acted as their own control, it is not surprising that more patients were compliant with the simple texting method than standard of care; however, even when comparing texting compliance to historical attendance in our clinic of only 30%, our results remain promising.
While our results are encouraging, we believe it is important to test text messaging surveillance and patient compliance in a larger trial prior to implementing within the broader community. This study provides critical data to support the development of a HIPAA-compliant, automated monitoring system that can provide timely responses to patient texts using a provider derived response to blood pressure values. Future work includes the development of an automated hypertension tool as well as a randomized controlled trial to more rigorously compare office blood pressure visits to remote text message surveillance. If effective, use of text messaging technology may allow for an improved patient partnership and more robust follow-up data, especially in patients with less than optimal compliance, as well as the ability to improve maternal care and decrease morbidity and mortality.
Corresponding author: Adi Hirshberg, MD, Dept. of Maternal-Fetal Medicine, 2 Silverstein, 3400 Spruce St., Philadelphia, PA 19104, adi.hirshberg@uphs.upenn.edu.
Funding/support: Supported by a Penn Medicine Innovation Accelerator grant.
Financial disclosures. None reported.
1. Creanga AA, Berg CJ, Syverson C, et al. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol 2015;125:5–12.
2. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:S1–S22.
3. Muri JH, Crawford N, Jellen BC. Reducing avoidable obstetrical and neonatal readmissions. American Hospital Association. Accessed 20 Sep 2016 at www.aha.org/content/11/PerinatalReadmissionscall1.pdf.
4. Walters BN, Walters T. Hypertension in the puerperium. Lancet 1987;2:330.
5. Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol 2012;206:470–5.
6. Executive summary: Hypertension in pregnancy. American College of Obstetricians and Gynecologists. Obstet Gynecol 2013;122:1122–31.
7. Scalise LF, Stringer M. Follow-up text messages for patients at high risk for postpartum hypertension. J Obstet Gynecol Neonatal Nurs 2015;44:S6.
8. Using health text messages to improve consumer health knowledge, behaviors, and outcomes: an environmental scan. Rockville, MD: U.S. Department of Health and Human Services; 2014.
9. Gurol-Urganci I, de Jongh T, Vodopivec-Jamsek V, et al. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database Syst Rev 2013;5;12:CD007458.
10. Saffari M, Ghanizadeh G, Koenig HG. Health education via mobile text messaging for glycemic control in adults with type 2 diabetes: a systematic review and meta-analysis. Prim Care Diabetes 2014;8:275–85.
11. Tran N, Coffma JM, Sumino K, Cabana MD. Patient reminder systems and asthma medication adherence: a systematic review. J Asthma 2014;51:536–43.
12. Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev 2012;3:CD009756.
13. Kannisto KA, Koivunen MF, Valimaki MA. Use of mobile phone text message reminders in health care services: a narrative literature review. J Med Internet Res 2010;16:e222.
14. Pew Research Center. Mobile technology fact sheet. Accessed 17 Dec 2014 at www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/.
15. Duggan M. Cell phone activities 2013. Pew Research Center’s Internet and American Life Project. Available at www.pewinternet.org/Reports/2013/Cell-Activities.aspx.
16. Langley G, Nolan K, Nolan T, et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco: Jossey-Bass; 1996.
17. Brown P, Hare D. Rapid cycle improvement: controlling change. J Ark Med Soc 2003;99:320–1.
18. Aikens JE, Trivedi R, Aron DC, Piette JD. Integrating support persons into diabetes telemonitoring to improve self-management and medication adherence. J Gen Intern Med 2015;30:319–26.
19. Lavender T, Richens Y, Milan SJ, et al. Telephone support for women during pregnancy and the first six weeks postpartum. Cochrane Database Syst Rev 2013;7:CD009338.
1. Creanga AA, Berg CJ, Syverson C, et al. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol 2015;125:5–12.
2. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:S1–S22.
3. Muri JH, Crawford N, Jellen BC. Reducing avoidable obstetrical and neonatal readmissions. American Hospital Association. Accessed 20 Sep 2016 at www.aha.org/content/11/PerinatalReadmissionscall1.pdf.
4. Walters BN, Walters T. Hypertension in the puerperium. Lancet 1987;2:330.
5. Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol 2012;206:470–5.
6. Executive summary: Hypertension in pregnancy. American College of Obstetricians and Gynecologists. Obstet Gynecol 2013;122:1122–31.
7. Scalise LF, Stringer M. Follow-up text messages for patients at high risk for postpartum hypertension. J Obstet Gynecol Neonatal Nurs 2015;44:S6.
8. Using health text messages to improve consumer health knowledge, behaviors, and outcomes: an environmental scan. Rockville, MD: U.S. Department of Health and Human Services; 2014.
9. Gurol-Urganci I, de Jongh T, Vodopivec-Jamsek V, et al. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database Syst Rev 2013;5;12:CD007458.
10. Saffari M, Ghanizadeh G, Koenig HG. Health education via mobile text messaging for glycemic control in adults with type 2 diabetes: a systematic review and meta-analysis. Prim Care Diabetes 2014;8:275–85.
11. Tran N, Coffma JM, Sumino K, Cabana MD. Patient reminder systems and asthma medication adherence: a systematic review. J Asthma 2014;51:536–43.
12. Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev 2012;3:CD009756.
13. Kannisto KA, Koivunen MF, Valimaki MA. Use of mobile phone text message reminders in health care services: a narrative literature review. J Med Internet Res 2010;16:e222.
14. Pew Research Center. Mobile technology fact sheet. Accessed 17 Dec 2014 at www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/.
15. Duggan M. Cell phone activities 2013. Pew Research Center’s Internet and American Life Project. Available at www.pewinternet.org/Reports/2013/Cell-Activities.aspx.
16. Langley G, Nolan K, Nolan T, et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco: Jossey-Bass; 1996.
17. Brown P, Hare D. Rapid cycle improvement: controlling change. J Ark Med Soc 2003;99:320–1.
18. Aikens JE, Trivedi R, Aron DC, Piette JD. Integrating support persons into diabetes telemonitoring to improve self-management and medication adherence. J Gen Intern Med 2015;30:319–26.
19. Lavender T, Richens Y, Milan SJ, et al. Telephone support for women during pregnancy and the first six weeks postpartum. Cochrane Database Syst Rev 2013;7:CD009338.
When are effective medications just too expensive?
The era of all-oral agents for hepatitis C virus infection has begun. Previous treatments for this disease included pegylated interferon and ribavirin, which had limited effectiveness and side effects severe enough to reduce adherence and quality of life. Recent trials have documented the effectiveness of the new direct-acting antiviral agents.1 These new drugs work better and offer the promise of an all-oral treatment regimen that avoids pegylated interferon.
But they cost a lot. Prices of more than $50,000 are estimated for a 2-to-3-month course of treatment.2 These new medications reflect the kind of societal advances that justify a long-term investment in basic and clinical research. But do we value advances at any cost?
DOES COST MATTER?
Leaving aside the question of whether these particular drugs are too expensive, the general question remains whether effective therapies can ever be so expensive that we should not use them.
Does cost matter? Well, we all know that it does. We pay attention to cost in our individual purchasing and in how we think about business and government spending. And yet, while everyone agrees that we shouldn’t pay for care that provides no benefit, many of us stop at just that line, and think or act as if we can’t put a price on those elements of health care that offer some potential to save lives. It’s a comfortable position, because in going after pure waste we feel like fiscally temperate guardians of societal resources without feeling responsible for heart-rending choices about overspending on things that do work. Yet that spending threatens societal resources just as much as useless therapies.
In the end, though, it is an illogical position. The illogic is easy to understand once you walk it through: if you are unwilling to put a price on life, then you are saying that there is no price too high for any potential health benefit, no matter how small. That means you commit all your resources to health and you go bankrupt.
So, implicitly or explicitly (our society does so implicitly—and inconsistently, at that), you have to put a maximum price on life. But at that point, you are (again, implicitly) saying that when there are treatments that cost more, you shouldn’t buy them.3 Admittedly, it doesn’t sound good, and in health care, which touches us so intimately, it doesn’t feel good either.
SHOULD PHYSICIANS CARE ABOUT COST?
Many of us were taught in medical school that it isn’t the doctor’s job to think about cost. Physicians are to be clinical advocates for their patients without consideration of cost—but that can’t be right, and it isn’t right.
First, even if physicians are patient advocates first, they ought to consider cost when the patient is paying. The rise in the use of high-deductible health insurance plans has expanded the financial risk that individual patients face in their own health care decisions. Physicians may be unprepared to help patients with those decisions, but it seems like a service they ought to provide.
Second, the line between cost to the individual and cost to society is blurred at best. Our societal health care spending is nothing more than the aggregation of our individual health care spending. Even if we don’t want physicians to focus on cost when with an individual patient at the bedside or at the examination table, don’t we want societal cost to be at least in their peripheral vision?
Many obstacles impede this view. Even if physicians can keep societal costs in their peripheral vision, they certainly can’t see to the edges of the broad canvas that all of health care represents, and they have no easy decision rules for how to turn what vision they have into a decision for a particular patient.
A variety of stakeholders have succeeded in turning what might have been seen as socially responsible thinking into a dirty word. The same politicians who use the term “stewardship” when they are in favor of considering societal implications call it “rationing” when they feel the other way. As a result, some of our most important institutions—eg, Medicare—are prohibited from considering price. Commercial insurers, still smarting from the managed-care backlash of the 1990s, have limited ability to effectively manage costs while maintaining quality. In some sense, this vacuum creates an opportunity for physician leadership.
COST-EFFECTIVENESS ANALYSIS AND ITS LIMITATIONS
Cost-effectiveness analysis, which represents the health care value of a therapy as the ratio of its financial cost to its benefit (eg, cost per quality-adjusted life-year), offers a disciplined approach to these conflicts between individual good and social good.4
The long-term costs of hepatitis C are substantial and include multiple diagnostic tests, hospitalization, surgery, and death. A major treatment for both liver failure and hepatocellular cancer is liver transplantation, which can entail hundreds of thousands of dollars in cost for the surgery and ongoing care. Preventing just one transplant can provide enormous savings, in addition to freeing up cadaveric organs for another patient. A careful cost-effectiveness analysis could tell us whether the new direct-acting antiviral agents are worth their cost.
These analyses are appealing because they are formal and disciplined, but it turns out that they are far from value-free. Their methodology is complicated and is sensitive to subjective modeling assumptions whose implications are often not straightforward, are hard to report in the compact methods sections of manuscripts, and are harder still to interpret by most readers of these articles.
Further, these models focus exclusively on economic efficiency, so even the most carefully constructed cost-effectiveness analyses need to be tempered by a sense of social equity not captured in these models. For example, an emphasis on increasing quality-adjusted life-years will naturally lead to policy decisions that favor groups that have more life-years remaining. That may sound fine if we are comfortable with the idea that, in general, we should target our resources toward younger people rather than older people. But the same thinking means we should target our resources away from men (who don’t live as long as women) or away from members of racial minority groups (who don’t live as long as whites).
Finally, although some throw about numbers like $50,000 to $100,000 per quality-adjusted life-year as a guide, the price thresholds revealed by our current practices and policies are inconsistent. Hemodialysis is funded through Medicare by a federal mandate, but more cost-effective vaccines and preventive care are not covered to the same degree. Cost-effectiveness analyses are essential to establish a quantitative sense about the efficient use of resources, but they need to be interpreted alongside other considerations we also value. Cost-effectiveness analyses don’t take us all the way to the decision line by themselves.
WHY ARE NEW DRUGS SO EXPENSIVE?
The high cost of the new direct-acting antivirals for just months of therapy seems excessive on its face. Even though most patients will not pay these costs directly, they are borne by society through higher taxes or premiums for commercial insurance, which are paid out-of-pocket by those who purchase individual insurance, or substitute for wages in employment-based health insurance.
We know that the actual cost to manufacture these drugs is significantly less than the prices charged by pharmaceutical companies5 and that the government subsidizes both the research and the reimbursement for certain therapies. However, the companies need to cover the long-term costs of research and development not only for these drugs but for other drugs that did not make it through the pipeline but might have.6
There are at least two sides to this economy. First, the more we are willing to pay for successful drugs that go to market, the more the developers of those drugs will be willing to invest in finding new ones. If we were to pay less for individual successes, we would in the end have fewer trials and fewer overall successes.
Second, pharmaceutical companies hire economists to do their own cost-effectiveness calculations. One reason it should be no surprise that new drugs often arrive on the market at prices that are pretty close to commonly accepted thresholds for cost-effectiveness is that this is partly how they were priced in the first place. Pharmaceutical companies naturally want to price their products as high as they can. Since there is a limit to what people are willing to pay for the benefit they get in return, determining that limit and setting the price at that point helps firms extract as much of the surplus as possible.
AN OPPORTUNITY FOR LEADERSHIP
A disciplined analysis of the costs and benefits of new drug therapies is critical to any medical policy decision, rather than cost alone. There will always be a point where new treatments are too expensive—a point not based on absolute cost, but on cost relative to what is gained over and above the next best alternative.7 However, we should acknowledge that these analyses are based on estimates that may change over time, that they require modeling assumptions that are often subjective and opaque, and that the interpretation and implementation of these policies within their social context is just as important as the analysis of their economic efficiency.
As challenging as these decisions are, they offer an opportunity for leadership from medicine. Some organizations have already taken a stance on eliminating waste—through their participation in the Choosing Wisely initiative led by the American Board of Internal Medicine8 or through stands against the use of drugs and procedures that offer no benefit over cheaper alternatives.9 As these decisions get harder and as we aim to reduce not just zero-value care, but also low-value care, physicians have an enormous amount to contribute.
- Dugum M, O’Shea R. Hepatitis C virus: here comes alloral treatment. Cleve Clin J Med 2014; 81:159–172.
- Soriano V, Vispo E, de Mendoza C, et al. Hepatitis C therapy with HCV NS5B polymerase inhibitors. Expert Opin Pharmacother 2013; 14:1161–1170.
- Asch DA. Basic lessons in resource allocation: sharing, setting limits, and being fair. Pharos Alpha Omega Alpha Honor Med Soc 1995; 58:33–34.
- Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med 1977; 296:716–721.
- Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct acting antivirals, for use in large-scale treatment access programs in developing countries. Clin Infect Dis 2014; Jan 6 [Epub ahead of print].
- Adams CP, Brantner VV. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff (Millwood) 2006; 25:420–428.
- Eisenberg JM. Clinical economics. A guide to the economic analysis of clinical practices. JAMA 1989; 262:2879–2886.
- Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012; 307:1801–1802.
- Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters. New York Times. October 15, 2012:A25.
The era of all-oral agents for hepatitis C virus infection has begun. Previous treatments for this disease included pegylated interferon and ribavirin, which had limited effectiveness and side effects severe enough to reduce adherence and quality of life. Recent trials have documented the effectiveness of the new direct-acting antiviral agents.1 These new drugs work better and offer the promise of an all-oral treatment regimen that avoids pegylated interferon.
But they cost a lot. Prices of more than $50,000 are estimated for a 2-to-3-month course of treatment.2 These new medications reflect the kind of societal advances that justify a long-term investment in basic and clinical research. But do we value advances at any cost?
DOES COST MATTER?
Leaving aside the question of whether these particular drugs are too expensive, the general question remains whether effective therapies can ever be so expensive that we should not use them.
Does cost matter? Well, we all know that it does. We pay attention to cost in our individual purchasing and in how we think about business and government spending. And yet, while everyone agrees that we shouldn’t pay for care that provides no benefit, many of us stop at just that line, and think or act as if we can’t put a price on those elements of health care that offer some potential to save lives. It’s a comfortable position, because in going after pure waste we feel like fiscally temperate guardians of societal resources without feeling responsible for heart-rending choices about overspending on things that do work. Yet that spending threatens societal resources just as much as useless therapies.
In the end, though, it is an illogical position. The illogic is easy to understand once you walk it through: if you are unwilling to put a price on life, then you are saying that there is no price too high for any potential health benefit, no matter how small. That means you commit all your resources to health and you go bankrupt.
So, implicitly or explicitly (our society does so implicitly—and inconsistently, at that), you have to put a maximum price on life. But at that point, you are (again, implicitly) saying that when there are treatments that cost more, you shouldn’t buy them.3 Admittedly, it doesn’t sound good, and in health care, which touches us so intimately, it doesn’t feel good either.
SHOULD PHYSICIANS CARE ABOUT COST?
Many of us were taught in medical school that it isn’t the doctor’s job to think about cost. Physicians are to be clinical advocates for their patients without consideration of cost—but that can’t be right, and it isn’t right.
First, even if physicians are patient advocates first, they ought to consider cost when the patient is paying. The rise in the use of high-deductible health insurance plans has expanded the financial risk that individual patients face in their own health care decisions. Physicians may be unprepared to help patients with those decisions, but it seems like a service they ought to provide.
Second, the line between cost to the individual and cost to society is blurred at best. Our societal health care spending is nothing more than the aggregation of our individual health care spending. Even if we don’t want physicians to focus on cost when with an individual patient at the bedside or at the examination table, don’t we want societal cost to be at least in their peripheral vision?
Many obstacles impede this view. Even if physicians can keep societal costs in their peripheral vision, they certainly can’t see to the edges of the broad canvas that all of health care represents, and they have no easy decision rules for how to turn what vision they have into a decision for a particular patient.
A variety of stakeholders have succeeded in turning what might have been seen as socially responsible thinking into a dirty word. The same politicians who use the term “stewardship” when they are in favor of considering societal implications call it “rationing” when they feel the other way. As a result, some of our most important institutions—eg, Medicare—are prohibited from considering price. Commercial insurers, still smarting from the managed-care backlash of the 1990s, have limited ability to effectively manage costs while maintaining quality. In some sense, this vacuum creates an opportunity for physician leadership.
COST-EFFECTIVENESS ANALYSIS AND ITS LIMITATIONS
Cost-effectiveness analysis, which represents the health care value of a therapy as the ratio of its financial cost to its benefit (eg, cost per quality-adjusted life-year), offers a disciplined approach to these conflicts between individual good and social good.4
The long-term costs of hepatitis C are substantial and include multiple diagnostic tests, hospitalization, surgery, and death. A major treatment for both liver failure and hepatocellular cancer is liver transplantation, which can entail hundreds of thousands of dollars in cost for the surgery and ongoing care. Preventing just one transplant can provide enormous savings, in addition to freeing up cadaveric organs for another patient. A careful cost-effectiveness analysis could tell us whether the new direct-acting antiviral agents are worth their cost.
These analyses are appealing because they are formal and disciplined, but it turns out that they are far from value-free. Their methodology is complicated and is sensitive to subjective modeling assumptions whose implications are often not straightforward, are hard to report in the compact methods sections of manuscripts, and are harder still to interpret by most readers of these articles.
Further, these models focus exclusively on economic efficiency, so even the most carefully constructed cost-effectiveness analyses need to be tempered by a sense of social equity not captured in these models. For example, an emphasis on increasing quality-adjusted life-years will naturally lead to policy decisions that favor groups that have more life-years remaining. That may sound fine if we are comfortable with the idea that, in general, we should target our resources toward younger people rather than older people. But the same thinking means we should target our resources away from men (who don’t live as long as women) or away from members of racial minority groups (who don’t live as long as whites).
Finally, although some throw about numbers like $50,000 to $100,000 per quality-adjusted life-year as a guide, the price thresholds revealed by our current practices and policies are inconsistent. Hemodialysis is funded through Medicare by a federal mandate, but more cost-effective vaccines and preventive care are not covered to the same degree. Cost-effectiveness analyses are essential to establish a quantitative sense about the efficient use of resources, but they need to be interpreted alongside other considerations we also value. Cost-effectiveness analyses don’t take us all the way to the decision line by themselves.
WHY ARE NEW DRUGS SO EXPENSIVE?
The high cost of the new direct-acting antivirals for just months of therapy seems excessive on its face. Even though most patients will not pay these costs directly, they are borne by society through higher taxes or premiums for commercial insurance, which are paid out-of-pocket by those who purchase individual insurance, or substitute for wages in employment-based health insurance.
We know that the actual cost to manufacture these drugs is significantly less than the prices charged by pharmaceutical companies5 and that the government subsidizes both the research and the reimbursement for certain therapies. However, the companies need to cover the long-term costs of research and development not only for these drugs but for other drugs that did not make it through the pipeline but might have.6
There are at least two sides to this economy. First, the more we are willing to pay for successful drugs that go to market, the more the developers of those drugs will be willing to invest in finding new ones. If we were to pay less for individual successes, we would in the end have fewer trials and fewer overall successes.
Second, pharmaceutical companies hire economists to do their own cost-effectiveness calculations. One reason it should be no surprise that new drugs often arrive on the market at prices that are pretty close to commonly accepted thresholds for cost-effectiveness is that this is partly how they were priced in the first place. Pharmaceutical companies naturally want to price their products as high as they can. Since there is a limit to what people are willing to pay for the benefit they get in return, determining that limit and setting the price at that point helps firms extract as much of the surplus as possible.
AN OPPORTUNITY FOR LEADERSHIP
A disciplined analysis of the costs and benefits of new drug therapies is critical to any medical policy decision, rather than cost alone. There will always be a point where new treatments are too expensive—a point not based on absolute cost, but on cost relative to what is gained over and above the next best alternative.7 However, we should acknowledge that these analyses are based on estimates that may change over time, that they require modeling assumptions that are often subjective and opaque, and that the interpretation and implementation of these policies within their social context is just as important as the analysis of their economic efficiency.
As challenging as these decisions are, they offer an opportunity for leadership from medicine. Some organizations have already taken a stance on eliminating waste—through their participation in the Choosing Wisely initiative led by the American Board of Internal Medicine8 or through stands against the use of drugs and procedures that offer no benefit over cheaper alternatives.9 As these decisions get harder and as we aim to reduce not just zero-value care, but also low-value care, physicians have an enormous amount to contribute.
The era of all-oral agents for hepatitis C virus infection has begun. Previous treatments for this disease included pegylated interferon and ribavirin, which had limited effectiveness and side effects severe enough to reduce adherence and quality of life. Recent trials have documented the effectiveness of the new direct-acting antiviral agents.1 These new drugs work better and offer the promise of an all-oral treatment regimen that avoids pegylated interferon.
But they cost a lot. Prices of more than $50,000 are estimated for a 2-to-3-month course of treatment.2 These new medications reflect the kind of societal advances that justify a long-term investment in basic and clinical research. But do we value advances at any cost?
DOES COST MATTER?
Leaving aside the question of whether these particular drugs are too expensive, the general question remains whether effective therapies can ever be so expensive that we should not use them.
Does cost matter? Well, we all know that it does. We pay attention to cost in our individual purchasing and in how we think about business and government spending. And yet, while everyone agrees that we shouldn’t pay for care that provides no benefit, many of us stop at just that line, and think or act as if we can’t put a price on those elements of health care that offer some potential to save lives. It’s a comfortable position, because in going after pure waste we feel like fiscally temperate guardians of societal resources without feeling responsible for heart-rending choices about overspending on things that do work. Yet that spending threatens societal resources just as much as useless therapies.
In the end, though, it is an illogical position. The illogic is easy to understand once you walk it through: if you are unwilling to put a price on life, then you are saying that there is no price too high for any potential health benefit, no matter how small. That means you commit all your resources to health and you go bankrupt.
So, implicitly or explicitly (our society does so implicitly—and inconsistently, at that), you have to put a maximum price on life. But at that point, you are (again, implicitly) saying that when there are treatments that cost more, you shouldn’t buy them.3 Admittedly, it doesn’t sound good, and in health care, which touches us so intimately, it doesn’t feel good either.
SHOULD PHYSICIANS CARE ABOUT COST?
Many of us were taught in medical school that it isn’t the doctor’s job to think about cost. Physicians are to be clinical advocates for their patients without consideration of cost—but that can’t be right, and it isn’t right.
First, even if physicians are patient advocates first, they ought to consider cost when the patient is paying. The rise in the use of high-deductible health insurance plans has expanded the financial risk that individual patients face in their own health care decisions. Physicians may be unprepared to help patients with those decisions, but it seems like a service they ought to provide.
Second, the line between cost to the individual and cost to society is blurred at best. Our societal health care spending is nothing more than the aggregation of our individual health care spending. Even if we don’t want physicians to focus on cost when with an individual patient at the bedside or at the examination table, don’t we want societal cost to be at least in their peripheral vision?
Many obstacles impede this view. Even if physicians can keep societal costs in their peripheral vision, they certainly can’t see to the edges of the broad canvas that all of health care represents, and they have no easy decision rules for how to turn what vision they have into a decision for a particular patient.
A variety of stakeholders have succeeded in turning what might have been seen as socially responsible thinking into a dirty word. The same politicians who use the term “stewardship” when they are in favor of considering societal implications call it “rationing” when they feel the other way. As a result, some of our most important institutions—eg, Medicare—are prohibited from considering price. Commercial insurers, still smarting from the managed-care backlash of the 1990s, have limited ability to effectively manage costs while maintaining quality. In some sense, this vacuum creates an opportunity for physician leadership.
COST-EFFECTIVENESS ANALYSIS AND ITS LIMITATIONS
Cost-effectiveness analysis, which represents the health care value of a therapy as the ratio of its financial cost to its benefit (eg, cost per quality-adjusted life-year), offers a disciplined approach to these conflicts between individual good and social good.4
The long-term costs of hepatitis C are substantial and include multiple diagnostic tests, hospitalization, surgery, and death. A major treatment for both liver failure and hepatocellular cancer is liver transplantation, which can entail hundreds of thousands of dollars in cost for the surgery and ongoing care. Preventing just one transplant can provide enormous savings, in addition to freeing up cadaveric organs for another patient. A careful cost-effectiveness analysis could tell us whether the new direct-acting antiviral agents are worth their cost.
These analyses are appealing because they are formal and disciplined, but it turns out that they are far from value-free. Their methodology is complicated and is sensitive to subjective modeling assumptions whose implications are often not straightforward, are hard to report in the compact methods sections of manuscripts, and are harder still to interpret by most readers of these articles.
Further, these models focus exclusively on economic efficiency, so even the most carefully constructed cost-effectiveness analyses need to be tempered by a sense of social equity not captured in these models. For example, an emphasis on increasing quality-adjusted life-years will naturally lead to policy decisions that favor groups that have more life-years remaining. That may sound fine if we are comfortable with the idea that, in general, we should target our resources toward younger people rather than older people. But the same thinking means we should target our resources away from men (who don’t live as long as women) or away from members of racial minority groups (who don’t live as long as whites).
Finally, although some throw about numbers like $50,000 to $100,000 per quality-adjusted life-year as a guide, the price thresholds revealed by our current practices and policies are inconsistent. Hemodialysis is funded through Medicare by a federal mandate, but more cost-effective vaccines and preventive care are not covered to the same degree. Cost-effectiveness analyses are essential to establish a quantitative sense about the efficient use of resources, but they need to be interpreted alongside other considerations we also value. Cost-effectiveness analyses don’t take us all the way to the decision line by themselves.
WHY ARE NEW DRUGS SO EXPENSIVE?
The high cost of the new direct-acting antivirals for just months of therapy seems excessive on its face. Even though most patients will not pay these costs directly, they are borne by society through higher taxes or premiums for commercial insurance, which are paid out-of-pocket by those who purchase individual insurance, or substitute for wages in employment-based health insurance.
We know that the actual cost to manufacture these drugs is significantly less than the prices charged by pharmaceutical companies5 and that the government subsidizes both the research and the reimbursement for certain therapies. However, the companies need to cover the long-term costs of research and development not only for these drugs but for other drugs that did not make it through the pipeline but might have.6
There are at least two sides to this economy. First, the more we are willing to pay for successful drugs that go to market, the more the developers of those drugs will be willing to invest in finding new ones. If we were to pay less for individual successes, we would in the end have fewer trials and fewer overall successes.
Second, pharmaceutical companies hire economists to do their own cost-effectiveness calculations. One reason it should be no surprise that new drugs often arrive on the market at prices that are pretty close to commonly accepted thresholds for cost-effectiveness is that this is partly how they were priced in the first place. Pharmaceutical companies naturally want to price their products as high as they can. Since there is a limit to what people are willing to pay for the benefit they get in return, determining that limit and setting the price at that point helps firms extract as much of the surplus as possible.
AN OPPORTUNITY FOR LEADERSHIP
A disciplined analysis of the costs and benefits of new drug therapies is critical to any medical policy decision, rather than cost alone. There will always be a point where new treatments are too expensive—a point not based on absolute cost, but on cost relative to what is gained over and above the next best alternative.7 However, we should acknowledge that these analyses are based on estimates that may change over time, that they require modeling assumptions that are often subjective and opaque, and that the interpretation and implementation of these policies within their social context is just as important as the analysis of their economic efficiency.
As challenging as these decisions are, they offer an opportunity for leadership from medicine. Some organizations have already taken a stance on eliminating waste—through their participation in the Choosing Wisely initiative led by the American Board of Internal Medicine8 or through stands against the use of drugs and procedures that offer no benefit over cheaper alternatives.9 As these decisions get harder and as we aim to reduce not just zero-value care, but also low-value care, physicians have an enormous amount to contribute.
- Dugum M, O’Shea R. Hepatitis C virus: here comes alloral treatment. Cleve Clin J Med 2014; 81:159–172.
- Soriano V, Vispo E, de Mendoza C, et al. Hepatitis C therapy with HCV NS5B polymerase inhibitors. Expert Opin Pharmacother 2013; 14:1161–1170.
- Asch DA. Basic lessons in resource allocation: sharing, setting limits, and being fair. Pharos Alpha Omega Alpha Honor Med Soc 1995; 58:33–34.
- Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med 1977; 296:716–721.
- Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct acting antivirals, for use in large-scale treatment access programs in developing countries. Clin Infect Dis 2014; Jan 6 [Epub ahead of print].
- Adams CP, Brantner VV. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff (Millwood) 2006; 25:420–428.
- Eisenberg JM. Clinical economics. A guide to the economic analysis of clinical practices. JAMA 1989; 262:2879–2886.
- Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012; 307:1801–1802.
- Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters. New York Times. October 15, 2012:A25.
- Dugum M, O’Shea R. Hepatitis C virus: here comes alloral treatment. Cleve Clin J Med 2014; 81:159–172.
- Soriano V, Vispo E, de Mendoza C, et al. Hepatitis C therapy with HCV NS5B polymerase inhibitors. Expert Opin Pharmacother 2013; 14:1161–1170.
- Asch DA. Basic lessons in resource allocation: sharing, setting limits, and being fair. Pharos Alpha Omega Alpha Honor Med Soc 1995; 58:33–34.
- Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med 1977; 296:716–721.
- Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct acting antivirals, for use in large-scale treatment access programs in developing countries. Clin Infect Dis 2014; Jan 6 [Epub ahead of print].
- Adams CP, Brantner VV. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff (Millwood) 2006; 25:420–428.
- Eisenberg JM. Clinical economics. A guide to the economic analysis of clinical practices. JAMA 1989; 262:2879–2886.
- Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012; 307:1801–1802.
- Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters. New York Times. October 15, 2012:A25.