User login
Breast cancer screening: Does tomosynthesis augment mammography?
Each year, millions of women undergo mammography in the hope of decreasing their risk of dying of breast cancer. The effectiveness of screening mammography, however, continues to be debated.
While most randomized controlled trials have demonstrated significantly lower mortality rates in women who undergo screening, not all trials have. Most experts agree that screening mammography programs decrease breast cancer mortality rates by 12% to 33%.1,2 But some point out that although mammography programs clearly detect more cases of breast cancer, some proportion of this detection may include “overdiagnosis” of cancers that would not have caused morbidity or mortality, including ductal carcinoma in situ. Also, although deaths from breast cancer have decreased in the United States, at least some of the decrease may be due to more effective treatment rather than early detection.
Moreover, screening has well-documented harms. False-positive results cause alarm and expose women to needless follow-up imaging and biopsies, with their attendant inconvenience, discomfort, risks, and costs. Conversely, false-negative results (especially common in women with dense breasts) lead to missed diagnosis and a false sense of security.
How could programs and technology be improved to make screening more beneficial, both for patients and for society as a whole? A major improvement would be if mammography could be made more sensitive and specific for detecting invasive cancers, with fewer false-positive results. Lower cost and less frequent screening would also be major improvements.
Digital breast tomosynthesis (DBT), also known as 3-dimensional (3D) mammography, may be a way to improve the value of breast cancer screening programs. In 2011, the US Food and Drug Administration (FDA) approved DBT for all mammographic indications, including screening.
WHAT IS TOMOSYNTHESIS?
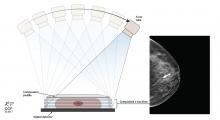
In DBT, the x-ray source is rotated in an arc around the patient’s breast (Figure 1), generating a 3D image.3 DBT is now routinely built into newer-generation mammography units. The 3D projections of DBT are obtained during the same breast compression required for standard 2D digital mammography. Thus, DBT requires minimal additional time on the part of the patient and the technologist.4
The 3D images are processed and sent to a viewing station, where a radiologist can interpret them next to 2D images. The radiologist has the ability to scroll through the DBT projections slice by slice, as in other cross-sectional imaging examinations. However, given the larger number of images compared with digital mammography, DBT requires more time for interpretation, interrupting the workflow. A population-based observational study suggested that combined digital mammography and DBT screening examinations take twice as long to interpret.5
The main advantage of DBT is that it can mitigate the problem of overlapping breast tissue on standard digital projections. These areas of focal asymmetry may represent suspicious masses—or merely overlapping breast parenchyma. When areas of focal asymmetry are found on 2D digital mammography without DBT, patients need to come back for further diagnostic imaging to resolve the finding.6 In addition, especially in women with dense breasts, areas of overlapping tissue can have a masking effect, obscuring small breast cancers.7
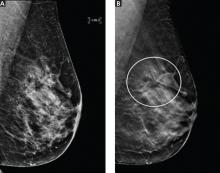
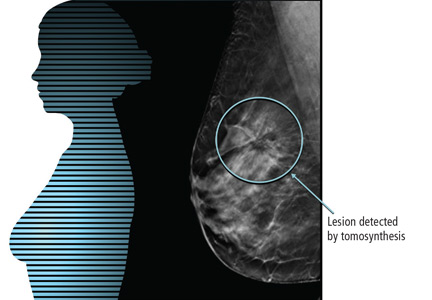
For breast cancer screening, DBT is read in conjunction with standard digital mammography. By allowing examination of the breast parenchyma in thin slices, DBT decreases the interpretive issue of overlapping breast parenchyma and the masking effect, potentially leading to fewer false-positive results and higher rates of cancer detection (Figure 2).
EFFECTIVENESS OF TOMOSYNTHESIS
There is limited evidence at this time to support the addition of DBT to digital mammography for primary breast cancer screening, with no published randomized trials that assessed outcomes. However, 2 population-based trials in Europe have prospectively assessed DBT plus digital mammography as a primary screening strategy: the Screening With Tomosynthesis or Standard Mammography (STORM) trial8 and the Oslo tomosynthesis screening trial.5 Only the STORM trial reported first-year interval cancer rates, from which the sensitivity and specificity of DBT plus 2D digital mammography could be calculated and compared with those of 2D digital mammography alone.8
The Oslo trial: Limited applicability in USA
In April 2013, the Oslo tomosynthesis screening trial published interim results of its prospective cohort study of 12,631 Norwegian women ages 50 to 69.5 Women were invited to participate based on the availability of technical staff and imaging systems at the time of screening, and all participants underwent digital mammography and DBT. Images were read independently by 4 radiologists using a double-reader protocol.
The interim results suggest that adding DBT to digital mammography increased cancer detection rates by 31% and decreased the false-positive rate by 13% compared with 2D digital mammography alone (Table 1). However, the double-reader protocol in this study differs from typical single-reader protocols in the United States, limiting the applicability of the findings.
The STORM trial: Low sensitivity
The STORM trial is a prospective cohort study that included 7,292 women without symptoms, at average risk, age 48 and older, who participated in national breast cancer screening services in northern Italy. Each participant underwent digital mammography and DBT. The examinations were read sequentially (digital mammography first, then DBT plus digital mammography) either by a single radiologist, as is most common in the United States, or by 2 radiologists, as is standard in Europe.
Using the single-reader strategy, adding DBT significantly increased cancer detection rates and reduced the total recall rate (Table 1). Sensitivity was 85% vs 54%, and specificity was 97% vs 96%.8,9
Of note, the sensitivity of 54% for digital mammography in the STORM trial is substantially lower than the sensitivity of digital mammography reported in the United States.10
Friedewald et al confirmed Oslo and STORM
To date, the largest US study of DBT plus digital mammography for breast cancer screening was a multicenter retrospective cohort study by Friedewald et al in 2014.11 This study compared cancer detection and recall rates before and after the implementation of DBT at 13 breast centers and evaluated a total of 454,850 examinations (173,663 with DBT plus digital mammography and 281,187 with digital mammography only).
Overall, the recall rate decreased significantly after DBT was adopted and the cancer detection rate increased, findings consistent with those of the STORM and Oslo trials (Table 1). Adding DBT detected invasive cancers at a higher rate than 2D digital mammography alone (4.1/1000 vs 2.9/1,000), while there was no significant difference in ductal carcinoma in situ detection rates. This suggests that the additional cancers detected by DBT may be more clinically important. Nevertheless, the number of biopsies with negative results also increased, suggesting that adding DBT may pose potential harms.
In 2016, Rafferty et al12 published an additional analysis of the data from Friedewald et al, concluding that adding DBT to 2D digital mammography increased the cancer detection rate more in women with heterogeneously dense breasts than in those with either nondense breasts or extremely dense breasts.12 The reduction in recall rate was also greatest in the heterogeneously dense subgroup.
Insufficient evidence to recommend
Most other cohort studies comparing DBT and digital mammography have had findings similar to those of the European prospective studies and the large US retrospective cohort study, with the addition of DBT to mammography reducing recall rates and increasing cancer detection rates.13 However, many of these studies were subject to potential selection bias and did not provide information on the cancer risk of the participants. In addition, no studies have assessed clinical outcomes such as breast cancer stage at diagnosis or interval cancers, let alone breast cancer mortality.
Rigorous studies need to be done in the United States, using the standard single-reader protocol most often used in this country, to ascertain the clinical outcomes of DBT plus digital mammography for breast cancer screening for women at average risk. A 2016 review cited a dearth of high-quality US studies assessing the role of DBT in primary breast cancer.13
The US Preventive Services Task Force, in its 2016 guidelines for breast cancer screening, concluded that there was insufficient evidence to assess the harms and benefits of DBT as a method of breast cancer screening, including adjunctive screening in women with dense breasts.1
Similarly, the American College of Physicians has advised against screening average-risk women for breast cancer using DBT.14
APPROVAL, DISSEMINATION, COSTS, AND CHOICE FOR PATIENTS
Even with early promising data suggesting that DBT can increase cancer detection rates and decrease false-positive results when added to routine screening mammography, the rapid diffusion of DBT into clinical practice is outpacing evidence of its effectiveness.4 This adoption was spurred in January 2015 when the Centers for Medicare and Medicaid Services added a Current Procedural Terminology code for DBT, allowing for additional reimbursement for it for all mammography indications.15 Still, the use of DBT in community settings is inconsistent, given the significant up-front costs associated with equipment purchases and variable reimbursement by private insurers who consider the technology experimental.
For the US healthcare system as a whole, it is uncertain whether the purported benefits of DBT will outweigh the additional costs associated with its use. The average reimbursement for a routine digital mammography examination is $135; adding DBT adds an average of $56 to the cost.15
Using an established, discrete-event breast cancer simulation model, a team of investigators evaluated the cost-effectiveness of combined biennial digital mammography and DBT screening compared with biennial digital mammography screening alone in US women with dense breasts.16 They found that biennial combined screening is likely to be cost-effective in US women with dense breasts. They also found that for every 2,000 women screened from age 50 to age 74, adding DBT would prevent 1 breast cancer death and 810 false-positive screening examinations.16
In addition, some have expressed concern that adding DBT to standard digital mammography increases radiation exposure. In fact, the radiation dose with DBT is similar to that with standard 2D digital mammography. Thus, when acquired together, combined digital mammography and DBT screening leads to twice the radiation dose compared with digital mammography alone.17 Nevertheless, this increased dose remains well below the FDA limits for a screening examination. In addition, the FDA has approved software that allows reconstruction of 2D synthetic views from the 3D data set, which will eventually bring radiation dose levels down to levels comparable to those of conventional digital mammography.17
Given that DBT is built into newer mammography units and is available as an add-on feature for existing units, its use is likely to increase even faster than digital mammography did when it replaced screen-film mammography in the previous decade.4 Its adoption by screening facilities, however, remains variable, and patients wishing to obtain combined DBT and digital mammography screening may have to travel to a different facility from their usual place of screening.18
Moreover, not all insurance companies cover DBT, resulting in additional out-of-pocket costs to the patient. It is currently unclear how individual facilities are offering DBT services, including how patients are selected for additional DBT and if they are offered the choice to add or forego DBT screening in combination with standard digital mammography.
SUMMARY: AN EMERGING TECHNOLOGY
DBT is an emerging imaging technology that allows the radiologist to view breast images in slices, as in computed tomography. DBT images can be obtained using the same breast compression that women already undergo for 2D digital mammography for breast cancer screening.
At this time, adding DBT to digital mammography screening nearly doubles the radiation exposure to the patient. However, new software is available that allows creation of synthetic 2D views from the 3D data set, resulting in radiation exposure that is similar to conventional digital mammography.
Although there are no published randomized controlled trials assessing the benefit of DBT over 2D digital mammography for breast cancer screening, prospective observational studies suggest that DBT may reduce false-positive recall rates and increase cancer detection rates when used in population-based screening programs. Assuming that additional breast cancer detection contributes to improved clinical outcomes, women with dense breasts may benefit more than women without dense breasts.
No national organizations currently recommend DBT for primary breast cancer screening. Ideally, future studies would determine whether DBT screening reduces breast cancer mortality. Since this research may not be feasible, surrogate clinical outcomes, such as a decrease in interval breast cancer rates and impact on stage at time of diagnosis, would allow us to more confidently recommend this new technology.
- Siu AL; US Preventive Services Task Force. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:279–296.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314:1599–1614.
- Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad Radiol 2011; 18:1298–1310.
- Lee CI, Lehman CD. Digital breast tomosynthesis and the challenges of implementing an emerging breast cancer screening technology into clinical practice. J Am Coll Radiol 2013; 10:913–917.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267:47–56.
- Helvie MA. Digital mammography imaging: breast tomosynthesis and advanced applications. Radiol Clin North Am 2010; 48:917–929.
- Gur D, Abrams GS, Chough DM, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol 2009; 193:586–591.
- Houssami N, Macaskill P, Bernardi D, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading—evidence to guide future screening strategies. Eur J Cancer 2014; 50:1799–1807.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14:583–589.
- Humphrey L, Chan BKS, Detlefsen S, Helfand M. Screening for Breast Cancer. Rockville, MD: Agency for Healthcare Research and Quality (US); 2002.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311:2499–2507.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315:1784–1786.
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164:268–278.
- Wilt TJ, Harris RP, Qaseem A; High Value Care Task Force of the American College of Physicians. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 2015; 162:718–725.
- American College of Radiology. CMS establishes breast tomosynthesis values in 2015 MPFS final rule. www.acr.org/News-Publications/News/News-Articles/2014/Economics/20141105-CMS-Establishes-Values-for-Breast-Tomosynthesis-in-2015-Final-Rule. Accessed June 14, 2017.
- Lee CI, Cevik M, Alagoz O, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology 2015; 274:772–780.
- Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015; 24:93–99.
- Lee CI, Bogart A, Hubbard RA, et al. Advanced breast imaging availability by screening facility characteristics. Acad Radiol 2015; 22:846–652.
Each year, millions of women undergo mammography in the hope of decreasing their risk of dying of breast cancer. The effectiveness of screening mammography, however, continues to be debated.
While most randomized controlled trials have demonstrated significantly lower mortality rates in women who undergo screening, not all trials have. Most experts agree that screening mammography programs decrease breast cancer mortality rates by 12% to 33%.1,2 But some point out that although mammography programs clearly detect more cases of breast cancer, some proportion of this detection may include “overdiagnosis” of cancers that would not have caused morbidity or mortality, including ductal carcinoma in situ. Also, although deaths from breast cancer have decreased in the United States, at least some of the decrease may be due to more effective treatment rather than early detection.
Moreover, screening has well-documented harms. False-positive results cause alarm and expose women to needless follow-up imaging and biopsies, with their attendant inconvenience, discomfort, risks, and costs. Conversely, false-negative results (especially common in women with dense breasts) lead to missed diagnosis and a false sense of security.
How could programs and technology be improved to make screening more beneficial, both for patients and for society as a whole? A major improvement would be if mammography could be made more sensitive and specific for detecting invasive cancers, with fewer false-positive results. Lower cost and less frequent screening would also be major improvements.
Digital breast tomosynthesis (DBT), also known as 3-dimensional (3D) mammography, may be a way to improve the value of breast cancer screening programs. In 2011, the US Food and Drug Administration (FDA) approved DBT for all mammographic indications, including screening.
WHAT IS TOMOSYNTHESIS?
In DBT, the x-ray source is rotated in an arc around the patient’s breast (Figure 1), generating a 3D image.3 DBT is now routinely built into newer-generation mammography units. The 3D projections of DBT are obtained during the same breast compression required for standard 2D digital mammography. Thus, DBT requires minimal additional time on the part of the patient and the technologist.4
The 3D images are processed and sent to a viewing station, where a radiologist can interpret them next to 2D images. The radiologist has the ability to scroll through the DBT projections slice by slice, as in other cross-sectional imaging examinations. However, given the larger number of images compared with digital mammography, DBT requires more time for interpretation, interrupting the workflow. A population-based observational study suggested that combined digital mammography and DBT screening examinations take twice as long to interpret.5
The main advantage of DBT is that it can mitigate the problem of overlapping breast tissue on standard digital projections. These areas of focal asymmetry may represent suspicious masses—or merely overlapping breast parenchyma. When areas of focal asymmetry are found on 2D digital mammography without DBT, patients need to come back for further diagnostic imaging to resolve the finding.6 In addition, especially in women with dense breasts, areas of overlapping tissue can have a masking effect, obscuring small breast cancers.7
For breast cancer screening, DBT is read in conjunction with standard digital mammography. By allowing examination of the breast parenchyma in thin slices, DBT decreases the interpretive issue of overlapping breast parenchyma and the masking effect, potentially leading to fewer false-positive results and higher rates of cancer detection (Figure 2).
EFFECTIVENESS OF TOMOSYNTHESIS
There is limited evidence at this time to support the addition of DBT to digital mammography for primary breast cancer screening, with no published randomized trials that assessed outcomes. However, 2 population-based trials in Europe have prospectively assessed DBT plus digital mammography as a primary screening strategy: the Screening With Tomosynthesis or Standard Mammography (STORM) trial8 and the Oslo tomosynthesis screening trial.5 Only the STORM trial reported first-year interval cancer rates, from which the sensitivity and specificity of DBT plus 2D digital mammography could be calculated and compared with those of 2D digital mammography alone.8
The Oslo trial: Limited applicability in USA
In April 2013, the Oslo tomosynthesis screening trial published interim results of its prospective cohort study of 12,631 Norwegian women ages 50 to 69.5 Women were invited to participate based on the availability of technical staff and imaging systems at the time of screening, and all participants underwent digital mammography and DBT. Images were read independently by 4 radiologists using a double-reader protocol.
The interim results suggest that adding DBT to digital mammography increased cancer detection rates by 31% and decreased the false-positive rate by 13% compared with 2D digital mammography alone (Table 1). However, the double-reader protocol in this study differs from typical single-reader protocols in the United States, limiting the applicability of the findings.
The STORM trial: Low sensitivity
The STORM trial is a prospective cohort study that included 7,292 women without symptoms, at average risk, age 48 and older, who participated in national breast cancer screening services in northern Italy. Each participant underwent digital mammography and DBT. The examinations were read sequentially (digital mammography first, then DBT plus digital mammography) either by a single radiologist, as is most common in the United States, or by 2 radiologists, as is standard in Europe.
Using the single-reader strategy, adding DBT significantly increased cancer detection rates and reduced the total recall rate (Table 1). Sensitivity was 85% vs 54%, and specificity was 97% vs 96%.8,9
Of note, the sensitivity of 54% for digital mammography in the STORM trial is substantially lower than the sensitivity of digital mammography reported in the United States.10
Friedewald et al confirmed Oslo and STORM
To date, the largest US study of DBT plus digital mammography for breast cancer screening was a multicenter retrospective cohort study by Friedewald et al in 2014.11 This study compared cancer detection and recall rates before and after the implementation of DBT at 13 breast centers and evaluated a total of 454,850 examinations (173,663 with DBT plus digital mammography and 281,187 with digital mammography only).
Overall, the recall rate decreased significantly after DBT was adopted and the cancer detection rate increased, findings consistent with those of the STORM and Oslo trials (Table 1). Adding DBT detected invasive cancers at a higher rate than 2D digital mammography alone (4.1/1000 vs 2.9/1,000), while there was no significant difference in ductal carcinoma in situ detection rates. This suggests that the additional cancers detected by DBT may be more clinically important. Nevertheless, the number of biopsies with negative results also increased, suggesting that adding DBT may pose potential harms.
In 2016, Rafferty et al12 published an additional analysis of the data from Friedewald et al, concluding that adding DBT to 2D digital mammography increased the cancer detection rate more in women with heterogeneously dense breasts than in those with either nondense breasts or extremely dense breasts.12 The reduction in recall rate was also greatest in the heterogeneously dense subgroup.
Insufficient evidence to recommend
Most other cohort studies comparing DBT and digital mammography have had findings similar to those of the European prospective studies and the large US retrospective cohort study, with the addition of DBT to mammography reducing recall rates and increasing cancer detection rates.13 However, many of these studies were subject to potential selection bias and did not provide information on the cancer risk of the participants. In addition, no studies have assessed clinical outcomes such as breast cancer stage at diagnosis or interval cancers, let alone breast cancer mortality.
Rigorous studies need to be done in the United States, using the standard single-reader protocol most often used in this country, to ascertain the clinical outcomes of DBT plus digital mammography for breast cancer screening for women at average risk. A 2016 review cited a dearth of high-quality US studies assessing the role of DBT in primary breast cancer.13
The US Preventive Services Task Force, in its 2016 guidelines for breast cancer screening, concluded that there was insufficient evidence to assess the harms and benefits of DBT as a method of breast cancer screening, including adjunctive screening in women with dense breasts.1
Similarly, the American College of Physicians has advised against screening average-risk women for breast cancer using DBT.14
APPROVAL, DISSEMINATION, COSTS, AND CHOICE FOR PATIENTS
Even with early promising data suggesting that DBT can increase cancer detection rates and decrease false-positive results when added to routine screening mammography, the rapid diffusion of DBT into clinical practice is outpacing evidence of its effectiveness.4 This adoption was spurred in January 2015 when the Centers for Medicare and Medicaid Services added a Current Procedural Terminology code for DBT, allowing for additional reimbursement for it for all mammography indications.15 Still, the use of DBT in community settings is inconsistent, given the significant up-front costs associated with equipment purchases and variable reimbursement by private insurers who consider the technology experimental.
For the US healthcare system as a whole, it is uncertain whether the purported benefits of DBT will outweigh the additional costs associated with its use. The average reimbursement for a routine digital mammography examination is $135; adding DBT adds an average of $56 to the cost.15
Using an established, discrete-event breast cancer simulation model, a team of investigators evaluated the cost-effectiveness of combined biennial digital mammography and DBT screening compared with biennial digital mammography screening alone in US women with dense breasts.16 They found that biennial combined screening is likely to be cost-effective in US women with dense breasts. They also found that for every 2,000 women screened from age 50 to age 74, adding DBT would prevent 1 breast cancer death and 810 false-positive screening examinations.16
In addition, some have expressed concern that adding DBT to standard digital mammography increases radiation exposure. In fact, the radiation dose with DBT is similar to that with standard 2D digital mammography. Thus, when acquired together, combined digital mammography and DBT screening leads to twice the radiation dose compared with digital mammography alone.17 Nevertheless, this increased dose remains well below the FDA limits for a screening examination. In addition, the FDA has approved software that allows reconstruction of 2D synthetic views from the 3D data set, which will eventually bring radiation dose levels down to levels comparable to those of conventional digital mammography.17
Given that DBT is built into newer mammography units and is available as an add-on feature for existing units, its use is likely to increase even faster than digital mammography did when it replaced screen-film mammography in the previous decade.4 Its adoption by screening facilities, however, remains variable, and patients wishing to obtain combined DBT and digital mammography screening may have to travel to a different facility from their usual place of screening.18
Moreover, not all insurance companies cover DBT, resulting in additional out-of-pocket costs to the patient. It is currently unclear how individual facilities are offering DBT services, including how patients are selected for additional DBT and if they are offered the choice to add or forego DBT screening in combination with standard digital mammography.
SUMMARY: AN EMERGING TECHNOLOGY
DBT is an emerging imaging technology that allows the radiologist to view breast images in slices, as in computed tomography. DBT images can be obtained using the same breast compression that women already undergo for 2D digital mammography for breast cancer screening.
At this time, adding DBT to digital mammography screening nearly doubles the radiation exposure to the patient. However, new software is available that allows creation of synthetic 2D views from the 3D data set, resulting in radiation exposure that is similar to conventional digital mammography.
Although there are no published randomized controlled trials assessing the benefit of DBT over 2D digital mammography for breast cancer screening, prospective observational studies suggest that DBT may reduce false-positive recall rates and increase cancer detection rates when used in population-based screening programs. Assuming that additional breast cancer detection contributes to improved clinical outcomes, women with dense breasts may benefit more than women without dense breasts.
No national organizations currently recommend DBT for primary breast cancer screening. Ideally, future studies would determine whether DBT screening reduces breast cancer mortality. Since this research may not be feasible, surrogate clinical outcomes, such as a decrease in interval breast cancer rates and impact on stage at time of diagnosis, would allow us to more confidently recommend this new technology.
Each year, millions of women undergo mammography in the hope of decreasing their risk of dying of breast cancer. The effectiveness of screening mammography, however, continues to be debated.
While most randomized controlled trials have demonstrated significantly lower mortality rates in women who undergo screening, not all trials have. Most experts agree that screening mammography programs decrease breast cancer mortality rates by 12% to 33%.1,2 But some point out that although mammography programs clearly detect more cases of breast cancer, some proportion of this detection may include “overdiagnosis” of cancers that would not have caused morbidity or mortality, including ductal carcinoma in situ. Also, although deaths from breast cancer have decreased in the United States, at least some of the decrease may be due to more effective treatment rather than early detection.
Moreover, screening has well-documented harms. False-positive results cause alarm and expose women to needless follow-up imaging and biopsies, with their attendant inconvenience, discomfort, risks, and costs. Conversely, false-negative results (especially common in women with dense breasts) lead to missed diagnosis and a false sense of security.
How could programs and technology be improved to make screening more beneficial, both for patients and for society as a whole? A major improvement would be if mammography could be made more sensitive and specific for detecting invasive cancers, with fewer false-positive results. Lower cost and less frequent screening would also be major improvements.
Digital breast tomosynthesis (DBT), also known as 3-dimensional (3D) mammography, may be a way to improve the value of breast cancer screening programs. In 2011, the US Food and Drug Administration (FDA) approved DBT for all mammographic indications, including screening.
WHAT IS TOMOSYNTHESIS?
In DBT, the x-ray source is rotated in an arc around the patient’s breast (Figure 1), generating a 3D image.3 DBT is now routinely built into newer-generation mammography units. The 3D projections of DBT are obtained during the same breast compression required for standard 2D digital mammography. Thus, DBT requires minimal additional time on the part of the patient and the technologist.4
The 3D images are processed and sent to a viewing station, where a radiologist can interpret them next to 2D images. The radiologist has the ability to scroll through the DBT projections slice by slice, as in other cross-sectional imaging examinations. However, given the larger number of images compared with digital mammography, DBT requires more time for interpretation, interrupting the workflow. A population-based observational study suggested that combined digital mammography and DBT screening examinations take twice as long to interpret.5
The main advantage of DBT is that it can mitigate the problem of overlapping breast tissue on standard digital projections. These areas of focal asymmetry may represent suspicious masses—or merely overlapping breast parenchyma. When areas of focal asymmetry are found on 2D digital mammography without DBT, patients need to come back for further diagnostic imaging to resolve the finding.6 In addition, especially in women with dense breasts, areas of overlapping tissue can have a masking effect, obscuring small breast cancers.7
For breast cancer screening, DBT is read in conjunction with standard digital mammography. By allowing examination of the breast parenchyma in thin slices, DBT decreases the interpretive issue of overlapping breast parenchyma and the masking effect, potentially leading to fewer false-positive results and higher rates of cancer detection (Figure 2).
EFFECTIVENESS OF TOMOSYNTHESIS
There is limited evidence at this time to support the addition of DBT to digital mammography for primary breast cancer screening, with no published randomized trials that assessed outcomes. However, 2 population-based trials in Europe have prospectively assessed DBT plus digital mammography as a primary screening strategy: the Screening With Tomosynthesis or Standard Mammography (STORM) trial8 and the Oslo tomosynthesis screening trial.5 Only the STORM trial reported first-year interval cancer rates, from which the sensitivity and specificity of DBT plus 2D digital mammography could be calculated and compared with those of 2D digital mammography alone.8
The Oslo trial: Limited applicability in USA
In April 2013, the Oslo tomosynthesis screening trial published interim results of its prospective cohort study of 12,631 Norwegian women ages 50 to 69.5 Women were invited to participate based on the availability of technical staff and imaging systems at the time of screening, and all participants underwent digital mammography and DBT. Images were read independently by 4 radiologists using a double-reader protocol.
The interim results suggest that adding DBT to digital mammography increased cancer detection rates by 31% and decreased the false-positive rate by 13% compared with 2D digital mammography alone (Table 1). However, the double-reader protocol in this study differs from typical single-reader protocols in the United States, limiting the applicability of the findings.
The STORM trial: Low sensitivity
The STORM trial is a prospective cohort study that included 7,292 women without symptoms, at average risk, age 48 and older, who participated in national breast cancer screening services in northern Italy. Each participant underwent digital mammography and DBT. The examinations were read sequentially (digital mammography first, then DBT plus digital mammography) either by a single radiologist, as is most common in the United States, or by 2 radiologists, as is standard in Europe.
Using the single-reader strategy, adding DBT significantly increased cancer detection rates and reduced the total recall rate (Table 1). Sensitivity was 85% vs 54%, and specificity was 97% vs 96%.8,9
Of note, the sensitivity of 54% for digital mammography in the STORM trial is substantially lower than the sensitivity of digital mammography reported in the United States.10
Friedewald et al confirmed Oslo and STORM
To date, the largest US study of DBT plus digital mammography for breast cancer screening was a multicenter retrospective cohort study by Friedewald et al in 2014.11 This study compared cancer detection and recall rates before and after the implementation of DBT at 13 breast centers and evaluated a total of 454,850 examinations (173,663 with DBT plus digital mammography and 281,187 with digital mammography only).
Overall, the recall rate decreased significantly after DBT was adopted and the cancer detection rate increased, findings consistent with those of the STORM and Oslo trials (Table 1). Adding DBT detected invasive cancers at a higher rate than 2D digital mammography alone (4.1/1000 vs 2.9/1,000), while there was no significant difference in ductal carcinoma in situ detection rates. This suggests that the additional cancers detected by DBT may be more clinically important. Nevertheless, the number of biopsies with negative results also increased, suggesting that adding DBT may pose potential harms.
In 2016, Rafferty et al12 published an additional analysis of the data from Friedewald et al, concluding that adding DBT to 2D digital mammography increased the cancer detection rate more in women with heterogeneously dense breasts than in those with either nondense breasts or extremely dense breasts.12 The reduction in recall rate was also greatest in the heterogeneously dense subgroup.
Insufficient evidence to recommend
Most other cohort studies comparing DBT and digital mammography have had findings similar to those of the European prospective studies and the large US retrospective cohort study, with the addition of DBT to mammography reducing recall rates and increasing cancer detection rates.13 However, many of these studies were subject to potential selection bias and did not provide information on the cancer risk of the participants. In addition, no studies have assessed clinical outcomes such as breast cancer stage at diagnosis or interval cancers, let alone breast cancer mortality.
Rigorous studies need to be done in the United States, using the standard single-reader protocol most often used in this country, to ascertain the clinical outcomes of DBT plus digital mammography for breast cancer screening for women at average risk. A 2016 review cited a dearth of high-quality US studies assessing the role of DBT in primary breast cancer.13
The US Preventive Services Task Force, in its 2016 guidelines for breast cancer screening, concluded that there was insufficient evidence to assess the harms and benefits of DBT as a method of breast cancer screening, including adjunctive screening in women with dense breasts.1
Similarly, the American College of Physicians has advised against screening average-risk women for breast cancer using DBT.14
APPROVAL, DISSEMINATION, COSTS, AND CHOICE FOR PATIENTS
Even with early promising data suggesting that DBT can increase cancer detection rates and decrease false-positive results when added to routine screening mammography, the rapid diffusion of DBT into clinical practice is outpacing evidence of its effectiveness.4 This adoption was spurred in January 2015 when the Centers for Medicare and Medicaid Services added a Current Procedural Terminology code for DBT, allowing for additional reimbursement for it for all mammography indications.15 Still, the use of DBT in community settings is inconsistent, given the significant up-front costs associated with equipment purchases and variable reimbursement by private insurers who consider the technology experimental.
For the US healthcare system as a whole, it is uncertain whether the purported benefits of DBT will outweigh the additional costs associated with its use. The average reimbursement for a routine digital mammography examination is $135; adding DBT adds an average of $56 to the cost.15
Using an established, discrete-event breast cancer simulation model, a team of investigators evaluated the cost-effectiveness of combined biennial digital mammography and DBT screening compared with biennial digital mammography screening alone in US women with dense breasts.16 They found that biennial combined screening is likely to be cost-effective in US women with dense breasts. They also found that for every 2,000 women screened from age 50 to age 74, adding DBT would prevent 1 breast cancer death and 810 false-positive screening examinations.16
In addition, some have expressed concern that adding DBT to standard digital mammography increases radiation exposure. In fact, the radiation dose with DBT is similar to that with standard 2D digital mammography. Thus, when acquired together, combined digital mammography and DBT screening leads to twice the radiation dose compared with digital mammography alone.17 Nevertheless, this increased dose remains well below the FDA limits for a screening examination. In addition, the FDA has approved software that allows reconstruction of 2D synthetic views from the 3D data set, which will eventually bring radiation dose levels down to levels comparable to those of conventional digital mammography.17
Given that DBT is built into newer mammography units and is available as an add-on feature for existing units, its use is likely to increase even faster than digital mammography did when it replaced screen-film mammography in the previous decade.4 Its adoption by screening facilities, however, remains variable, and patients wishing to obtain combined DBT and digital mammography screening may have to travel to a different facility from their usual place of screening.18
Moreover, not all insurance companies cover DBT, resulting in additional out-of-pocket costs to the patient. It is currently unclear how individual facilities are offering DBT services, including how patients are selected for additional DBT and if they are offered the choice to add or forego DBT screening in combination with standard digital mammography.
SUMMARY: AN EMERGING TECHNOLOGY
DBT is an emerging imaging technology that allows the radiologist to view breast images in slices, as in computed tomography. DBT images can be obtained using the same breast compression that women already undergo for 2D digital mammography for breast cancer screening.
At this time, adding DBT to digital mammography screening nearly doubles the radiation exposure to the patient. However, new software is available that allows creation of synthetic 2D views from the 3D data set, resulting in radiation exposure that is similar to conventional digital mammography.
Although there are no published randomized controlled trials assessing the benefit of DBT over 2D digital mammography for breast cancer screening, prospective observational studies suggest that DBT may reduce false-positive recall rates and increase cancer detection rates when used in population-based screening programs. Assuming that additional breast cancer detection contributes to improved clinical outcomes, women with dense breasts may benefit more than women without dense breasts.
No national organizations currently recommend DBT for primary breast cancer screening. Ideally, future studies would determine whether DBT screening reduces breast cancer mortality. Since this research may not be feasible, surrogate clinical outcomes, such as a decrease in interval breast cancer rates and impact on stage at time of diagnosis, would allow us to more confidently recommend this new technology.
- Siu AL; US Preventive Services Task Force. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:279–296.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314:1599–1614.
- Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad Radiol 2011; 18:1298–1310.
- Lee CI, Lehman CD. Digital breast tomosynthesis and the challenges of implementing an emerging breast cancer screening technology into clinical practice. J Am Coll Radiol 2013; 10:913–917.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267:47–56.
- Helvie MA. Digital mammography imaging: breast tomosynthesis and advanced applications. Radiol Clin North Am 2010; 48:917–929.
- Gur D, Abrams GS, Chough DM, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol 2009; 193:586–591.
- Houssami N, Macaskill P, Bernardi D, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading—evidence to guide future screening strategies. Eur J Cancer 2014; 50:1799–1807.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14:583–589.
- Humphrey L, Chan BKS, Detlefsen S, Helfand M. Screening for Breast Cancer. Rockville, MD: Agency for Healthcare Research and Quality (US); 2002.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311:2499–2507.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315:1784–1786.
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164:268–278.
- Wilt TJ, Harris RP, Qaseem A; High Value Care Task Force of the American College of Physicians. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 2015; 162:718–725.
- American College of Radiology. CMS establishes breast tomosynthesis values in 2015 MPFS final rule. www.acr.org/News-Publications/News/News-Articles/2014/Economics/20141105-CMS-Establishes-Values-for-Breast-Tomosynthesis-in-2015-Final-Rule. Accessed June 14, 2017.
- Lee CI, Cevik M, Alagoz O, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology 2015; 274:772–780.
- Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015; 24:93–99.
- Lee CI, Bogart A, Hubbard RA, et al. Advanced breast imaging availability by screening facility characteristics. Acad Radiol 2015; 22:846–652.
- Siu AL; US Preventive Services Task Force. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164:279–296.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314:1599–1614.
- Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad Radiol 2011; 18:1298–1310.
- Lee CI, Lehman CD. Digital breast tomosynthesis and the challenges of implementing an emerging breast cancer screening technology into clinical practice. J Am Coll Radiol 2013; 10:913–917.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267:47–56.
- Helvie MA. Digital mammography imaging: breast tomosynthesis and advanced applications. Radiol Clin North Am 2010; 48:917–929.
- Gur D, Abrams GS, Chough DM, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol 2009; 193:586–591.
- Houssami N, Macaskill P, Bernardi D, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading—evidence to guide future screening strategies. Eur J Cancer 2014; 50:1799–1807.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14:583–589.
- Humphrey L, Chan BKS, Detlefsen S, Helfand M. Screening for Breast Cancer. Rockville, MD: Agency for Healthcare Research and Quality (US); 2002.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311:2499–2507.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315:1784–1786.
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164:268–278.
- Wilt TJ, Harris RP, Qaseem A; High Value Care Task Force of the American College of Physicians. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 2015; 162:718–725.
- American College of Radiology. CMS establishes breast tomosynthesis values in 2015 MPFS final rule. www.acr.org/News-Publications/News/News-Articles/2014/Economics/20141105-CMS-Establishes-Values-for-Breast-Tomosynthesis-in-2015-Final-Rule. Accessed June 14, 2017.
- Lee CI, Cevik M, Alagoz O, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology 2015; 274:772–780.
- Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015; 24:93–99.
- Lee CI, Bogart A, Hubbard RA, et al. Advanced breast imaging availability by screening facility characteristics. Acad Radiol 2015; 22:846–652.
KEY POINTS
- DBT creates 3-dimensional images of the breast that the radiologist can view slice by slice, as in other cross-sectional imaging examinations.
- Initial studies suggest that, when used in conjunction with standard 2-dimensional digital mammography as a screening test, DBT can reduce recall rates and increase cancer detection rates, but its impact on breast cancer mortality rates and cancer stage at diagnosis is not known.
- Drawbacks of DBT: it exposes the patient to more radiation, takes the radiologist longer to interpret, and costs more than standard digital mammography alone.
- Not all insurance companies cover DBT for breast cancer screening.
- Dr. Lee has received research grant funding from GE Healthcare. Dr. Lee’s time is supported in part by the American Cancer Society (126947-MRSG-14-160-01-CPHPS).
- The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the University of Washington, Seattle.