User login
In the Literature
In This Edition
- Pharmacists and readmission rates.
- Geriatric discharge bundles and readmission rates.
- Medication reconciliation and risk of adverse drug events.
- End-of-life discussions and care outcomes.
- Effect of case management and housing for homeless adults.
- IV esomeprazole in bleeding ulcers.
- Causes of discharge delays.
- Methods to reduce ICU medication errors.
Addition of Pharmacists to Inpatient Teams Reduces Drug-Related Readmissions, Morbidity, and Costs for Elderly Patients
Clinical question: Would a ward-based pharmacist reduce morbidity, subsequent ED visits, and readmissions for elderly patients?
Background: Adverse drug events can cause significant drug-related morbidity and mortality, and lead to unnecessary healthcare costs. Elderly patients are more vulnerable to these effects given the polypharmacy often associated with their care. The effectiveness of a ward-based pharmacist intervention for elderly patients has not yet been studied.
Study design: Randomized controlled trial.
Setting: Two acute-care, internal-medicine wards at the University Hospital of Uppsala in Uppsala, Sweden.
Synopsis: Three hundred sixty-eight hospitalized patients ages 80 or older were randomized to control or intervention groups. The latter received enhanced services from a pharmacist who was integrated into the inpatient team. This individual performed medication reconciliation, reviewed the medication list, and advised the treating physician. The pharmacist educated and monitored patients during the hospitalization, counseled them at discharge, communicated pertinent medication information to the primary-care physicians (PCPs), and called the patients two months after discharge.
The primary outcome measure was the frequency of all hospital visits (ED visits plus hospital readmissions) during 12-month follow-up. The secondary outcome measure was the cost of hospital care.
The intervention group had a 16% reduction in all hospital visits and a 47% reduction in ED visits. There were five times as many drug-related readmissions in the control group compared with the intervention group, but the study did not have enough power to show a reduction in the total number of readmissions alone. The cost of hospital care minus the cost of the intervention resulted in a net savings of $230 per patient.
Bottom line: For elderly patients, adding a pharmacist to the inpatient team could lead to significant reductions in morbidity and, on a population basis, healthcare costs.
Citation: Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894-900.
Geriatric Care Coordination at Discharge Reduces Readmission Rates at 30 Days
Clinical question: Does a discharge planning service package affect readmission rates and ED visits?
Background: Elderly patients are at high risk for readmission after a hospitalization. Coordinated care packages, although effective in congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD) management, have yielded inconsistent results in terms of decreasing readmission rates for patients with a broader range of medical issues.
Study design: Randomized controlled pilot study.
Setting: A single large academic medical center in Texas.
Synopsis: Forty-one elderly patients at high risk for readmission—because of their age and comorbidities—were enrolled within 72 hours of admission. Patients randomized to the care bundle arm received daily education about medication reconciliation, health conditions, and self-care provided by study pharmacists and nurses. The intervention required approximately 40 minutes per day: 20 to 25 minutes for the care coordinators and 20 minutes for the pharmacists. A post-discharge phone call to review medication and confirm follow-up instructions was included.
Eight patients in the control group and two patients in the intervention arm were readmitted or seen in the ED within 30 days after discharge (38% vs. 10%, P=0.004). At 60 days, the difference between the two groups was no longer significant. The intervention group had a longer time interval before its first readmission (36.2 days vs. 15.7 days). The sample was too small to determine the effect on length of hospitalization.
Limitations of the study include its small sample size and unclear costs of the intervention.
Bottom line: Geriatric discharge bundles might decrease readmission and ED visits after discharge, but larger studies are needed to confirm this finding.
Citation: Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day post-discharge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211-218.
Computer-Assisted Medication Reconciliation Might Reduce Unintentional Drug Discrepancies with Potential for Harm
Clinical question: Does a computerized medication reconciliation intervention reduce unintentional medication discrepancies?
Background: Given the high prevalence of unintentional medication discrepancies in hospitalized patients and the potential for harm, medication reconciliation is a national patient safety goal. Little data exist on the efficacy of medication reconciliation interventions for reducing medication discrepancies.
Study design: Cluster-randomized controlled trial.
Setting: Two large academic hospitals in Boston.
Synopsis: Using 14 medical teams, the study enrolled and randomized 322 patients to a floor with intervention or to a floor with traditional care. The intervention teams utilized a computerized order entry application designed to facilitate medication reconciliation, as well as a process redesign for physicians, nurses, and pharmacists.
The primary outcome was the number of unintentional medication discrepancies with the potential for causing harm (PADEs) per patient.
Patients randomized to the intervention group had a 28% reduction in relative risk compared with the control group (1.05 PADEs vs. 1.44 PADEs; absolute relative risk 0.72 (0.52-0.99)). The absolute relative risk reduction between the two arms was 0.39 PADE per patient (NNT=2.6). The intervention was associated with a significant reduction in PADEs at discharge but not at admission. The effects of the intervention were greater in patients with a higher PADE score.
Bottom line: This computerized medication reconciliation program with process redesign was associated with reduced risk of unintentional medication discrepancies with potential for causing harms (PADEs).
Citation: Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med. 2009;169(8):771-780.
End-of-Life Discussions Associated with Lower Healthcare Costs
Clinical question: What is the impact of patient-physician discussions of end-of-life care on healthcare costs in the final week of a patient’s life?
Background: Life-sustaining medical care of patients with advanced cancer is costly, with disproportionate spending at the end of a patient’s life. The link between discussions of end-of-life care preferences and healthcare expenditure has not been studied thoroughly.
Study design: Prospective observational study.
Setting: Seven sites in Connecticut, Texas, New Hampshire, and Massachusetts.
Synopsis: More than 600 patients with advanced cancer were recruited from September 2002 through December 2007 as part of the Coping With Cancer study. The 188 patients (31%) who reported end-of-life discussions with their physicians at baseline were less likely to undergo mechanical ventilator use or resuscitation, or to be admitted or die in an intensive-care unit in the final week of life. They were more likely to receive outpatient hospice care and had less physical distress in the last week than those who did not.
The mean aggregate cost of care in this group was $1,876, which was 36% lower than in the group that did not discuss end-of-life care ($2,917), P=0.002. In addition, higher medical costs were associated with worse quality of death, as reported by caregivers.
This study is limited by its observational design.
Bottom line: Physician communication with patients regarding end-of-life care preferences is associated with lower costs in the final week of life.
Citation: Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480-488.
Reduction of ED Visits and Hospitalizations for Chronically Ill and Homeless Adults
Clinical question: Can a case management and housing program reduce the utilization of ED and hospital medical services among chronically ill homeless adults?
Background: Homeless adults have high rates of chronic illness, have poor access to uninterrupted primary healthcare, and frequently use costly medical services, including those provided by EDs and inpatient hospitalizations. Studies to determine the efficacy of housing and case management services in reducing hospital and ED utilization in this population are lacking.
Study design: Randomized controlled trial.
Setting: A public teaching hospital and a private nonprofit hospital in Chicago.
Synopsis: Four hundred seven chronically ill and homeless adults were randomized to receive a case management and housing intervention or traditional care following an index hospitalization. The intervention group received assistance with stable housing on discharge and biweekly case management services throughout the study period. Traditional care consisted of routine inpatient discharge planning and transportation to a shelter.
Patients were followed for 18 months for the primary outcomes: number of hospitalizations, total hospital days, and number of ED visits.
After adjusting for differences in baseline variables, the intervention group was found to have significantly lower rates of hospitalization (relative reduction 29%), total hospital days (29%), and ED visits (24%). The authors did not find a difference in mortality or quality of life between the two groups.
Limitations of this study include a small sample size, limited geographic distribution of subjects, and the lack of a cost-benefit analysis of the intervention.
Bottom line: Case management and housing interventions can decrease hospitalizations and ED visits among chronically ill homeless adults.
Citation: Sadowski LS, Kee RA, VanderWeele TJ, Buchanan D. Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults: a randomized trial. JAMA. 2009;301(17):1771-1778.
Intravenous Esomeprazole Reduces Recurrent Bleeding from Peptic Ulcers
Clinical question: Does intravenous esomeprazole prevent recurrent peptic ulcer bleeding, compared with placebo?
Background: U.S. hospitals admit more than 300,000 patients per year for peptic ulcer bleeding. Asian studies of proton pump inhibitors have demonstrated improved outcomes in patients with bleeding caused by peptic ulcers, but these results have not been consistently replicated in studies in Western Europe or North America.
Study design: Randomized, placebo-controlled, double-blind trial.
Setting: Ninety-one hospital EDs in 16 countries.
Synopsis: The study team randomized 764 adult patients with a single bleeding gastric or duodenal ulcer after successful endoscopic hemostasis. The study group received esomeprazole (80 mg bolus, given intravenously over 30 minutes, followed by an 8 mg/hour infusion for 71.5 hours). The second group received placebo. Each group subsequently received 40 mg/day of oral esomeprazole for 27 days.
Recurrent bleeding within 72 hours was reduced by nearly half in the intravenous esomeprazole arm compared with placebo (5.9% vs. 10.3%, P=0.026). This remained significant at seven and 30 days. Intravenous esomeprazole also reduced endoscopic retreatment (6.4% vs. 11.6%; P=0.012) and demonstrated a trend toward reduction in surgery (2.7% vs. 5.4%) and all-cause mortality (0.8% vs. 2.1%).
Study limitations included a lack of standardization of endoscopic therapy across institutions.
Bottom line: Given after endoscopic hemostatis, intravenous esomeprazole followed by oral esomeprazole reduced recurrent bleeding in patients with a single duodenal or gastric ulcer.
Citation: Sung JJ, Barkun A, Kuipers EJ, et al. Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2009;150(7):455-464.
Discharge Time and Duration Affected by Several Factors During Hospitalization
Clinical question: What are the factors affecting late and long discharges in a general medical unit?
Background: The mismatch between admission and discharge times is a problem for many hospitals; admissions occur early in the day whereas, discharges occur later in the day. The reasons behind delays in discharge and prolongation of discharges are not fully understood.
Study design: Prospective cohort study.
Setting: A general medical unit without house staff coverage at an academic medical center in Baltimore.
Synopsis: Care providers completed surveys on 201 consecutive discharges from January to April 2005. Outcome variables included time of discharge and discharge duration.
Mean discharge time was 3:09 p.m. Delay in discharge was associated with a need for ambulance transportation (1.5 hours), need for prescriptions to be filled at the hospital (1.4 hours), and for patients whose final test was a procedure (1.2 hours) or consult (1.1 hours).
Median discharge time was 7.6 hours. Longer discharge duration was associated with discharge to a location other than home (28.9 hours), need for consultation (14.8 hours), or need for a procedure (13.4 hours) before discharge.
African-American race, gender, age, and comorbid psychiatric and substance abuse disorders were not associated with either late or prolonged discharges.
Bottom line: Final-day tests, procedures, and consults, as well as complex discharge arrangements, prolong and delay discharges more than the characteristics of patients themselves.
Citation: Chen LM, Freitag MH, Franco M, Sullivan CD, Dickson C, Brancati FL. Natural history of late discharges from a general medical ward. J Hosp Med. 2009;4(4):226-233.
Administration of Parenteral Medication a Common Point at Which Errors Occur in ICUs
Clinical question: To what extent are medication administration errors a problem across ICUs, and what are some ways to prevent them?
Background: High-acuity and complex systems increase the likelihood of medical errors in ICUs. The first multinational Sentinel Events Evaluation study reported an ICU medication error rate of 10.5 per 100 patient days at the prescription and administration stages of medication delivery.
Study design: Multinational observational, prospective, cross-sectional study.
Setting: One hundred thirteen ICUs in 27 countries on five continents.
Synopsis: This study addressed five types of medication error at the administration stage in the ICU in a 24-hour timeframe: wrong drug, wrong dose, wrong route, wrong time, and missed medication. The main outcome measures were the number and impact of administration errors, the distribution of error characteristics, and the distribution of contributing and preventive factors.
In the 1,328 critically ill patients included in the study, 861 medication errors were reported by structured questionnaire; 441 patients were affected by the errors. The prevalence was 74.5 errors per 100 patient days, and 12 patients (0.9%) suffered permanent harm or death. Most medication administration errors occurred during routine care, not during extraordinary situations. Most were omission errors.
This study is limited by its observational design and by the fact that self-reporting also carries the risk of under-reporting.
This study points out several ways to reduce medication errors. An independent predictor of decreased risk of medication errors of all types is an established incident reporting system. Routine checking of infusion pumps at every nursing shift change also reduced this risk.
Bottom line: This study confirmed that the administration of parenteral medications is a vulnerable point across many ICUs, and incident reporting systems and routine checks of infusion pumps are effective ways to reduce the risk of this type of error.
Citation: Valentin A, Capuzzo M, Guidet B, et al. Errors in administration of parenteral drugs in intensive care units: multinational prospective study. BMJ. 2009;338:b814. TH
In This Edition
- Pharmacists and readmission rates.
- Geriatric discharge bundles and readmission rates.
- Medication reconciliation and risk of adverse drug events.
- End-of-life discussions and care outcomes.
- Effect of case management and housing for homeless adults.
- IV esomeprazole in bleeding ulcers.
- Causes of discharge delays.
- Methods to reduce ICU medication errors.
Addition of Pharmacists to Inpatient Teams Reduces Drug-Related Readmissions, Morbidity, and Costs for Elderly Patients
Clinical question: Would a ward-based pharmacist reduce morbidity, subsequent ED visits, and readmissions for elderly patients?
Background: Adverse drug events can cause significant drug-related morbidity and mortality, and lead to unnecessary healthcare costs. Elderly patients are more vulnerable to these effects given the polypharmacy often associated with their care. The effectiveness of a ward-based pharmacist intervention for elderly patients has not yet been studied.
Study design: Randomized controlled trial.
Setting: Two acute-care, internal-medicine wards at the University Hospital of Uppsala in Uppsala, Sweden.
Synopsis: Three hundred sixty-eight hospitalized patients ages 80 or older were randomized to control or intervention groups. The latter received enhanced services from a pharmacist who was integrated into the inpatient team. This individual performed medication reconciliation, reviewed the medication list, and advised the treating physician. The pharmacist educated and monitored patients during the hospitalization, counseled them at discharge, communicated pertinent medication information to the primary-care physicians (PCPs), and called the patients two months after discharge.
The primary outcome measure was the frequency of all hospital visits (ED visits plus hospital readmissions) during 12-month follow-up. The secondary outcome measure was the cost of hospital care.
The intervention group had a 16% reduction in all hospital visits and a 47% reduction in ED visits. There were five times as many drug-related readmissions in the control group compared with the intervention group, but the study did not have enough power to show a reduction in the total number of readmissions alone. The cost of hospital care minus the cost of the intervention resulted in a net savings of $230 per patient.
Bottom line: For elderly patients, adding a pharmacist to the inpatient team could lead to significant reductions in morbidity and, on a population basis, healthcare costs.
Citation: Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894-900.
Geriatric Care Coordination at Discharge Reduces Readmission Rates at 30 Days
Clinical question: Does a discharge planning service package affect readmission rates and ED visits?
Background: Elderly patients are at high risk for readmission after a hospitalization. Coordinated care packages, although effective in congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD) management, have yielded inconsistent results in terms of decreasing readmission rates for patients with a broader range of medical issues.
Study design: Randomized controlled pilot study.
Setting: A single large academic medical center in Texas.
Synopsis: Forty-one elderly patients at high risk for readmission—because of their age and comorbidities—were enrolled within 72 hours of admission. Patients randomized to the care bundle arm received daily education about medication reconciliation, health conditions, and self-care provided by study pharmacists and nurses. The intervention required approximately 40 minutes per day: 20 to 25 minutes for the care coordinators and 20 minutes for the pharmacists. A post-discharge phone call to review medication and confirm follow-up instructions was included.
Eight patients in the control group and two patients in the intervention arm were readmitted or seen in the ED within 30 days after discharge (38% vs. 10%, P=0.004). At 60 days, the difference between the two groups was no longer significant. The intervention group had a longer time interval before its first readmission (36.2 days vs. 15.7 days). The sample was too small to determine the effect on length of hospitalization.
Limitations of the study include its small sample size and unclear costs of the intervention.
Bottom line: Geriatric discharge bundles might decrease readmission and ED visits after discharge, but larger studies are needed to confirm this finding.
Citation: Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day post-discharge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211-218.
Computer-Assisted Medication Reconciliation Might Reduce Unintentional Drug Discrepancies with Potential for Harm
Clinical question: Does a computerized medication reconciliation intervention reduce unintentional medication discrepancies?
Background: Given the high prevalence of unintentional medication discrepancies in hospitalized patients and the potential for harm, medication reconciliation is a national patient safety goal. Little data exist on the efficacy of medication reconciliation interventions for reducing medication discrepancies.
Study design: Cluster-randomized controlled trial.
Setting: Two large academic hospitals in Boston.
Synopsis: Using 14 medical teams, the study enrolled and randomized 322 patients to a floor with intervention or to a floor with traditional care. The intervention teams utilized a computerized order entry application designed to facilitate medication reconciliation, as well as a process redesign for physicians, nurses, and pharmacists.
The primary outcome was the number of unintentional medication discrepancies with the potential for causing harm (PADEs) per patient.
Patients randomized to the intervention group had a 28% reduction in relative risk compared with the control group (1.05 PADEs vs. 1.44 PADEs; absolute relative risk 0.72 (0.52-0.99)). The absolute relative risk reduction between the two arms was 0.39 PADE per patient (NNT=2.6). The intervention was associated with a significant reduction in PADEs at discharge but not at admission. The effects of the intervention were greater in patients with a higher PADE score.
Bottom line: This computerized medication reconciliation program with process redesign was associated with reduced risk of unintentional medication discrepancies with potential for causing harms (PADEs).
Citation: Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med. 2009;169(8):771-780.
End-of-Life Discussions Associated with Lower Healthcare Costs
Clinical question: What is the impact of patient-physician discussions of end-of-life care on healthcare costs in the final week of a patient’s life?
Background: Life-sustaining medical care of patients with advanced cancer is costly, with disproportionate spending at the end of a patient’s life. The link between discussions of end-of-life care preferences and healthcare expenditure has not been studied thoroughly.
Study design: Prospective observational study.
Setting: Seven sites in Connecticut, Texas, New Hampshire, and Massachusetts.
Synopsis: More than 600 patients with advanced cancer were recruited from September 2002 through December 2007 as part of the Coping With Cancer study. The 188 patients (31%) who reported end-of-life discussions with their physicians at baseline were less likely to undergo mechanical ventilator use or resuscitation, or to be admitted or die in an intensive-care unit in the final week of life. They were more likely to receive outpatient hospice care and had less physical distress in the last week than those who did not.
The mean aggregate cost of care in this group was $1,876, which was 36% lower than in the group that did not discuss end-of-life care ($2,917), P=0.002. In addition, higher medical costs were associated with worse quality of death, as reported by caregivers.
This study is limited by its observational design.
Bottom line: Physician communication with patients regarding end-of-life care preferences is associated with lower costs in the final week of life.
Citation: Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480-488.
Reduction of ED Visits and Hospitalizations for Chronically Ill and Homeless Adults
Clinical question: Can a case management and housing program reduce the utilization of ED and hospital medical services among chronically ill homeless adults?
Background: Homeless adults have high rates of chronic illness, have poor access to uninterrupted primary healthcare, and frequently use costly medical services, including those provided by EDs and inpatient hospitalizations. Studies to determine the efficacy of housing and case management services in reducing hospital and ED utilization in this population are lacking.
Study design: Randomized controlled trial.
Setting: A public teaching hospital and a private nonprofit hospital in Chicago.
Synopsis: Four hundred seven chronically ill and homeless adults were randomized to receive a case management and housing intervention or traditional care following an index hospitalization. The intervention group received assistance with stable housing on discharge and biweekly case management services throughout the study period. Traditional care consisted of routine inpatient discharge planning and transportation to a shelter.
Patients were followed for 18 months for the primary outcomes: number of hospitalizations, total hospital days, and number of ED visits.
After adjusting for differences in baseline variables, the intervention group was found to have significantly lower rates of hospitalization (relative reduction 29%), total hospital days (29%), and ED visits (24%). The authors did not find a difference in mortality or quality of life between the two groups.
Limitations of this study include a small sample size, limited geographic distribution of subjects, and the lack of a cost-benefit analysis of the intervention.
Bottom line: Case management and housing interventions can decrease hospitalizations and ED visits among chronically ill homeless adults.
Citation: Sadowski LS, Kee RA, VanderWeele TJ, Buchanan D. Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults: a randomized trial. JAMA. 2009;301(17):1771-1778.
Intravenous Esomeprazole Reduces Recurrent Bleeding from Peptic Ulcers
Clinical question: Does intravenous esomeprazole prevent recurrent peptic ulcer bleeding, compared with placebo?
Background: U.S. hospitals admit more than 300,000 patients per year for peptic ulcer bleeding. Asian studies of proton pump inhibitors have demonstrated improved outcomes in patients with bleeding caused by peptic ulcers, but these results have not been consistently replicated in studies in Western Europe or North America.
Study design: Randomized, placebo-controlled, double-blind trial.
Setting: Ninety-one hospital EDs in 16 countries.
Synopsis: The study team randomized 764 adult patients with a single bleeding gastric or duodenal ulcer after successful endoscopic hemostasis. The study group received esomeprazole (80 mg bolus, given intravenously over 30 minutes, followed by an 8 mg/hour infusion for 71.5 hours). The second group received placebo. Each group subsequently received 40 mg/day of oral esomeprazole for 27 days.
Recurrent bleeding within 72 hours was reduced by nearly half in the intravenous esomeprazole arm compared with placebo (5.9% vs. 10.3%, P=0.026). This remained significant at seven and 30 days. Intravenous esomeprazole also reduced endoscopic retreatment (6.4% vs. 11.6%; P=0.012) and demonstrated a trend toward reduction in surgery (2.7% vs. 5.4%) and all-cause mortality (0.8% vs. 2.1%).
Study limitations included a lack of standardization of endoscopic therapy across institutions.
Bottom line: Given after endoscopic hemostatis, intravenous esomeprazole followed by oral esomeprazole reduced recurrent bleeding in patients with a single duodenal or gastric ulcer.
Citation: Sung JJ, Barkun A, Kuipers EJ, et al. Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2009;150(7):455-464.
Discharge Time and Duration Affected by Several Factors During Hospitalization
Clinical question: What are the factors affecting late and long discharges in a general medical unit?
Background: The mismatch between admission and discharge times is a problem for many hospitals; admissions occur early in the day whereas, discharges occur later in the day. The reasons behind delays in discharge and prolongation of discharges are not fully understood.
Study design: Prospective cohort study.
Setting: A general medical unit without house staff coverage at an academic medical center in Baltimore.
Synopsis: Care providers completed surveys on 201 consecutive discharges from January to April 2005. Outcome variables included time of discharge and discharge duration.
Mean discharge time was 3:09 p.m. Delay in discharge was associated with a need for ambulance transportation (1.5 hours), need for prescriptions to be filled at the hospital (1.4 hours), and for patients whose final test was a procedure (1.2 hours) or consult (1.1 hours).
Median discharge time was 7.6 hours. Longer discharge duration was associated with discharge to a location other than home (28.9 hours), need for consultation (14.8 hours), or need for a procedure (13.4 hours) before discharge.
African-American race, gender, age, and comorbid psychiatric and substance abuse disorders were not associated with either late or prolonged discharges.
Bottom line: Final-day tests, procedures, and consults, as well as complex discharge arrangements, prolong and delay discharges more than the characteristics of patients themselves.
Citation: Chen LM, Freitag MH, Franco M, Sullivan CD, Dickson C, Brancati FL. Natural history of late discharges from a general medical ward. J Hosp Med. 2009;4(4):226-233.
Administration of Parenteral Medication a Common Point at Which Errors Occur in ICUs
Clinical question: To what extent are medication administration errors a problem across ICUs, and what are some ways to prevent them?
Background: High-acuity and complex systems increase the likelihood of medical errors in ICUs. The first multinational Sentinel Events Evaluation study reported an ICU medication error rate of 10.5 per 100 patient days at the prescription and administration stages of medication delivery.
Study design: Multinational observational, prospective, cross-sectional study.
Setting: One hundred thirteen ICUs in 27 countries on five continents.
Synopsis: This study addressed five types of medication error at the administration stage in the ICU in a 24-hour timeframe: wrong drug, wrong dose, wrong route, wrong time, and missed medication. The main outcome measures were the number and impact of administration errors, the distribution of error characteristics, and the distribution of contributing and preventive factors.
In the 1,328 critically ill patients included in the study, 861 medication errors were reported by structured questionnaire; 441 patients were affected by the errors. The prevalence was 74.5 errors per 100 patient days, and 12 patients (0.9%) suffered permanent harm or death. Most medication administration errors occurred during routine care, not during extraordinary situations. Most were omission errors.
This study is limited by its observational design and by the fact that self-reporting also carries the risk of under-reporting.
This study points out several ways to reduce medication errors. An independent predictor of decreased risk of medication errors of all types is an established incident reporting system. Routine checking of infusion pumps at every nursing shift change also reduced this risk.
Bottom line: This study confirmed that the administration of parenteral medications is a vulnerable point across many ICUs, and incident reporting systems and routine checks of infusion pumps are effective ways to reduce the risk of this type of error.
Citation: Valentin A, Capuzzo M, Guidet B, et al. Errors in administration of parenteral drugs in intensive care units: multinational prospective study. BMJ. 2009;338:b814. TH
In This Edition
- Pharmacists and readmission rates.
- Geriatric discharge bundles and readmission rates.
- Medication reconciliation and risk of adverse drug events.
- End-of-life discussions and care outcomes.
- Effect of case management and housing for homeless adults.
- IV esomeprazole in bleeding ulcers.
- Causes of discharge delays.
- Methods to reduce ICU medication errors.
Addition of Pharmacists to Inpatient Teams Reduces Drug-Related Readmissions, Morbidity, and Costs for Elderly Patients
Clinical question: Would a ward-based pharmacist reduce morbidity, subsequent ED visits, and readmissions for elderly patients?
Background: Adverse drug events can cause significant drug-related morbidity and mortality, and lead to unnecessary healthcare costs. Elderly patients are more vulnerable to these effects given the polypharmacy often associated with their care. The effectiveness of a ward-based pharmacist intervention for elderly patients has not yet been studied.
Study design: Randomized controlled trial.
Setting: Two acute-care, internal-medicine wards at the University Hospital of Uppsala in Uppsala, Sweden.
Synopsis: Three hundred sixty-eight hospitalized patients ages 80 or older were randomized to control or intervention groups. The latter received enhanced services from a pharmacist who was integrated into the inpatient team. This individual performed medication reconciliation, reviewed the medication list, and advised the treating physician. The pharmacist educated and monitored patients during the hospitalization, counseled them at discharge, communicated pertinent medication information to the primary-care physicians (PCPs), and called the patients two months after discharge.
The primary outcome measure was the frequency of all hospital visits (ED visits plus hospital readmissions) during 12-month follow-up. The secondary outcome measure was the cost of hospital care.
The intervention group had a 16% reduction in all hospital visits and a 47% reduction in ED visits. There were five times as many drug-related readmissions in the control group compared with the intervention group, but the study did not have enough power to show a reduction in the total number of readmissions alone. The cost of hospital care minus the cost of the intervention resulted in a net savings of $230 per patient.
Bottom line: For elderly patients, adding a pharmacist to the inpatient team could lead to significant reductions in morbidity and, on a population basis, healthcare costs.
Citation: Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894-900.
Geriatric Care Coordination at Discharge Reduces Readmission Rates at 30 Days
Clinical question: Does a discharge planning service package affect readmission rates and ED visits?
Background: Elderly patients are at high risk for readmission after a hospitalization. Coordinated care packages, although effective in congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD) management, have yielded inconsistent results in terms of decreasing readmission rates for patients with a broader range of medical issues.
Study design: Randomized controlled pilot study.
Setting: A single large academic medical center in Texas.
Synopsis: Forty-one elderly patients at high risk for readmission—because of their age and comorbidities—were enrolled within 72 hours of admission. Patients randomized to the care bundle arm received daily education about medication reconciliation, health conditions, and self-care provided by study pharmacists and nurses. The intervention required approximately 40 minutes per day: 20 to 25 minutes for the care coordinators and 20 minutes for the pharmacists. A post-discharge phone call to review medication and confirm follow-up instructions was included.
Eight patients in the control group and two patients in the intervention arm were readmitted or seen in the ED within 30 days after discharge (38% vs. 10%, P=0.004). At 60 days, the difference between the two groups was no longer significant. The intervention group had a longer time interval before its first readmission (36.2 days vs. 15.7 days). The sample was too small to determine the effect on length of hospitalization.
Limitations of the study include its small sample size and unclear costs of the intervention.
Bottom line: Geriatric discharge bundles might decrease readmission and ED visits after discharge, but larger studies are needed to confirm this finding.
Citation: Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day post-discharge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211-218.
Computer-Assisted Medication Reconciliation Might Reduce Unintentional Drug Discrepancies with Potential for Harm
Clinical question: Does a computerized medication reconciliation intervention reduce unintentional medication discrepancies?
Background: Given the high prevalence of unintentional medication discrepancies in hospitalized patients and the potential for harm, medication reconciliation is a national patient safety goal. Little data exist on the efficacy of medication reconciliation interventions for reducing medication discrepancies.
Study design: Cluster-randomized controlled trial.
Setting: Two large academic hospitals in Boston.
Synopsis: Using 14 medical teams, the study enrolled and randomized 322 patients to a floor with intervention or to a floor with traditional care. The intervention teams utilized a computerized order entry application designed to facilitate medication reconciliation, as well as a process redesign for physicians, nurses, and pharmacists.
The primary outcome was the number of unintentional medication discrepancies with the potential for causing harm (PADEs) per patient.
Patients randomized to the intervention group had a 28% reduction in relative risk compared with the control group (1.05 PADEs vs. 1.44 PADEs; absolute relative risk 0.72 (0.52-0.99)). The absolute relative risk reduction between the two arms was 0.39 PADE per patient (NNT=2.6). The intervention was associated with a significant reduction in PADEs at discharge but not at admission. The effects of the intervention were greater in patients with a higher PADE score.
Bottom line: This computerized medication reconciliation program with process redesign was associated with reduced risk of unintentional medication discrepancies with potential for causing harms (PADEs).
Citation: Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med. 2009;169(8):771-780.
End-of-Life Discussions Associated with Lower Healthcare Costs
Clinical question: What is the impact of patient-physician discussions of end-of-life care on healthcare costs in the final week of a patient’s life?
Background: Life-sustaining medical care of patients with advanced cancer is costly, with disproportionate spending at the end of a patient’s life. The link between discussions of end-of-life care preferences and healthcare expenditure has not been studied thoroughly.
Study design: Prospective observational study.
Setting: Seven sites in Connecticut, Texas, New Hampshire, and Massachusetts.
Synopsis: More than 600 patients with advanced cancer were recruited from September 2002 through December 2007 as part of the Coping With Cancer study. The 188 patients (31%) who reported end-of-life discussions with their physicians at baseline were less likely to undergo mechanical ventilator use or resuscitation, or to be admitted or die in an intensive-care unit in the final week of life. They were more likely to receive outpatient hospice care and had less physical distress in the last week than those who did not.
The mean aggregate cost of care in this group was $1,876, which was 36% lower than in the group that did not discuss end-of-life care ($2,917), P=0.002. In addition, higher medical costs were associated with worse quality of death, as reported by caregivers.
This study is limited by its observational design.
Bottom line: Physician communication with patients regarding end-of-life care preferences is associated with lower costs in the final week of life.
Citation: Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480-488.
Reduction of ED Visits and Hospitalizations for Chronically Ill and Homeless Adults
Clinical question: Can a case management and housing program reduce the utilization of ED and hospital medical services among chronically ill homeless adults?
Background: Homeless adults have high rates of chronic illness, have poor access to uninterrupted primary healthcare, and frequently use costly medical services, including those provided by EDs and inpatient hospitalizations. Studies to determine the efficacy of housing and case management services in reducing hospital and ED utilization in this population are lacking.
Study design: Randomized controlled trial.
Setting: A public teaching hospital and a private nonprofit hospital in Chicago.
Synopsis: Four hundred seven chronically ill and homeless adults were randomized to receive a case management and housing intervention or traditional care following an index hospitalization. The intervention group received assistance with stable housing on discharge and biweekly case management services throughout the study period. Traditional care consisted of routine inpatient discharge planning and transportation to a shelter.
Patients were followed for 18 months for the primary outcomes: number of hospitalizations, total hospital days, and number of ED visits.
After adjusting for differences in baseline variables, the intervention group was found to have significantly lower rates of hospitalization (relative reduction 29%), total hospital days (29%), and ED visits (24%). The authors did not find a difference in mortality or quality of life between the two groups.
Limitations of this study include a small sample size, limited geographic distribution of subjects, and the lack of a cost-benefit analysis of the intervention.
Bottom line: Case management and housing interventions can decrease hospitalizations and ED visits among chronically ill homeless adults.
Citation: Sadowski LS, Kee RA, VanderWeele TJ, Buchanan D. Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults: a randomized trial. JAMA. 2009;301(17):1771-1778.
Intravenous Esomeprazole Reduces Recurrent Bleeding from Peptic Ulcers
Clinical question: Does intravenous esomeprazole prevent recurrent peptic ulcer bleeding, compared with placebo?
Background: U.S. hospitals admit more than 300,000 patients per year for peptic ulcer bleeding. Asian studies of proton pump inhibitors have demonstrated improved outcomes in patients with bleeding caused by peptic ulcers, but these results have not been consistently replicated in studies in Western Europe or North America.
Study design: Randomized, placebo-controlled, double-blind trial.
Setting: Ninety-one hospital EDs in 16 countries.
Synopsis: The study team randomized 764 adult patients with a single bleeding gastric or duodenal ulcer after successful endoscopic hemostasis. The study group received esomeprazole (80 mg bolus, given intravenously over 30 minutes, followed by an 8 mg/hour infusion for 71.5 hours). The second group received placebo. Each group subsequently received 40 mg/day of oral esomeprazole for 27 days.
Recurrent bleeding within 72 hours was reduced by nearly half in the intravenous esomeprazole arm compared with placebo (5.9% vs. 10.3%, P=0.026). This remained significant at seven and 30 days. Intravenous esomeprazole also reduced endoscopic retreatment (6.4% vs. 11.6%; P=0.012) and demonstrated a trend toward reduction in surgery (2.7% vs. 5.4%) and all-cause mortality (0.8% vs. 2.1%).
Study limitations included a lack of standardization of endoscopic therapy across institutions.
Bottom line: Given after endoscopic hemostatis, intravenous esomeprazole followed by oral esomeprazole reduced recurrent bleeding in patients with a single duodenal or gastric ulcer.
Citation: Sung JJ, Barkun A, Kuipers EJ, et al. Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2009;150(7):455-464.
Discharge Time and Duration Affected by Several Factors During Hospitalization
Clinical question: What are the factors affecting late and long discharges in a general medical unit?
Background: The mismatch between admission and discharge times is a problem for many hospitals; admissions occur early in the day whereas, discharges occur later in the day. The reasons behind delays in discharge and prolongation of discharges are not fully understood.
Study design: Prospective cohort study.
Setting: A general medical unit without house staff coverage at an academic medical center in Baltimore.
Synopsis: Care providers completed surveys on 201 consecutive discharges from January to April 2005. Outcome variables included time of discharge and discharge duration.
Mean discharge time was 3:09 p.m. Delay in discharge was associated with a need for ambulance transportation (1.5 hours), need for prescriptions to be filled at the hospital (1.4 hours), and for patients whose final test was a procedure (1.2 hours) or consult (1.1 hours).
Median discharge time was 7.6 hours. Longer discharge duration was associated with discharge to a location other than home (28.9 hours), need for consultation (14.8 hours), or need for a procedure (13.4 hours) before discharge.
African-American race, gender, age, and comorbid psychiatric and substance abuse disorders were not associated with either late or prolonged discharges.
Bottom line: Final-day tests, procedures, and consults, as well as complex discharge arrangements, prolong and delay discharges more than the characteristics of patients themselves.
Citation: Chen LM, Freitag MH, Franco M, Sullivan CD, Dickson C, Brancati FL. Natural history of late discharges from a general medical ward. J Hosp Med. 2009;4(4):226-233.
Administration of Parenteral Medication a Common Point at Which Errors Occur in ICUs
Clinical question: To what extent are medication administration errors a problem across ICUs, and what are some ways to prevent them?
Background: High-acuity and complex systems increase the likelihood of medical errors in ICUs. The first multinational Sentinel Events Evaluation study reported an ICU medication error rate of 10.5 per 100 patient days at the prescription and administration stages of medication delivery.
Study design: Multinational observational, prospective, cross-sectional study.
Setting: One hundred thirteen ICUs in 27 countries on five continents.
Synopsis: This study addressed five types of medication error at the administration stage in the ICU in a 24-hour timeframe: wrong drug, wrong dose, wrong route, wrong time, and missed medication. The main outcome measures were the number and impact of administration errors, the distribution of error characteristics, and the distribution of contributing and preventive factors.
In the 1,328 critically ill patients included in the study, 861 medication errors were reported by structured questionnaire; 441 patients were affected by the errors. The prevalence was 74.5 errors per 100 patient days, and 12 patients (0.9%) suffered permanent harm or death. Most medication administration errors occurred during routine care, not during extraordinary situations. Most were omission errors.
This study is limited by its observational design and by the fact that self-reporting also carries the risk of under-reporting.
This study points out several ways to reduce medication errors. An independent predictor of decreased risk of medication errors of all types is an established incident reporting system. Routine checking of infusion pumps at every nursing shift change also reduced this risk.
Bottom line: This study confirmed that the administration of parenteral medications is a vulnerable point across many ICUs, and incident reporting systems and routine checks of infusion pumps are effective ways to reduce the risk of this type of error.
Citation: Valentin A, Capuzzo M, Guidet B, et al. Errors in administration of parenteral drugs in intensive care units: multinational prospective study. BMJ. 2009;338:b814. TH
When should lipid-lowering therapy be started in the hospitalized patient?
Case
A 52-year-old man with no medical history other than a transient ischemic attack (TIA) three months ago presents to the emergency department (ED) following multiple episodes of substernal (ST) chest pressure. He takes no medication. His electrocardiogram (ECG) revealed lateral ST segment depressions, and his cardiac biomarkers were elevated. He underwent cardiac catheterization, and a single drug-eluting stent was successfully placed to a culprit left circumflex lesion. He is now stable less than 24 hours following his initial presentation, without any evidence of heart failure. His providers prescribe aspirin, clopidogrel, metoprolol, and lisinopril. His fasting LDL level is 92 mg/dL.
What, if any, is the role for lipid-lowering therapy at this time?
Overview
Long-term therapy with HMG CoA reductase inhibitors (statins) has been shown through several large, randomized, controlled trials to reduce the risk for death, myocardial infarction (MI), and stroke in patients with established coronary disease. The most significant effects were evident after approximately two years of treatment.1,2,3,4
Subsequent trials have shown earlier and more significant reductions in the rates of recurrent ischemic cardiovascular events following acute coronary syndromes (ACS) when statins are administered early—within days of the initial event. This is a window of time in which most patients still are hospitalized.4,5,6,7
In addition to this data regarding statin use following ACS, a large, randomized, controlled trial demonstrated similar reductions in the incidence of strokes and cardiovascular events when high-dose atorvastatin was administered within one to six months following TIA or stroke in patients without established coronary disease.8 There is growing data supporting the hypothesis that statins have pleiotropic (non cholesterol-lowering), neuroprotective, properties that may improve patient outcomes following cerebrovascular events.9,10,11 There are ongoing trials investing the role of statins in the acute management of stroke.12,13
Hospitalists frequently manage patients in the stages immediately following ACS and stroke. Based on the large and evolving volume of data regarding the use of statins following these events, when and how should a statin be started in the hospital?
Review of the Data
Following Acute Coronary Syndrome: Death and recurrent ischemic events following ACS are most likely to occur in the early phase of recovery. Based on this observation and evidence supporting the early (in some cases within hours of administration) ‘pleiotropic’ or non-cholesterol lowering effects of statins, including improvement in endothelial function and decreases in platelet aggregation, thrombus deposition, and vascular inflammation, the MIRACL study was designed to answer the question of whether the initiation of treatment with a statin within 24 to 96 hours following ACS would reduce the occurrence of death and recurrent ischemia.4,7,14 Investigators randomized 3,086 patients within 24-96 hours (mean 63 hours) following admission for non-ST segment myocardial infarction (NSTEMI) or unstable angina (UA) to receive either atorvastatin 80 mg/d or placebo.
Investigators monitored patients for the primary end points of ischemic events (death, non-fatal MI, cardiac arrest with resuscitation, symptomatic myocardial ischemia with objective evidence) during a 16-week period. In the treatment arm, the risk of the primary combined end point was significantly reduced—relative risk (RR) 0.84; 95% confidence interval (CI), 0.70-1.00; p=0.048. (See Figure 1, pg. 39)
No significant differences were found between atorvastatin and placebo in the risk of death, non-fatal MI, or cardiac arrest with resuscitation. There was, however, a significantly lower risk of recurrent symptomatic myocardial ischemia with objective evidence requiring emergent re-hospitalization in the treatment arm (RR, 0.74; 95% CI, 0.57-0.95; p=0.02). The mean baseline LDL level in the treatment arm was 124 mg/dL, a value that may represent, in part, suppression of the LDL level in the setting of acute ACS. This is a phenomenon previously described in an analysis of the LUNAR trial.15
Suppression of LDL level after ACS appeared to be minimal, however, and is unlikely to be clinically significant. The benefits of atorvastatin in the MIRACL trial did not appear to depend on baseline LDL level—suggesting the decision to initiate statin therapy after ACS should not be influenced by LDL level at the time of the event.
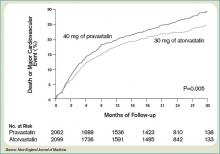
Only one dose of statin was used in the MIRACL trial, and the investigators commented they were unable to determine if a lower dose of atorvastatin or a gradual dose titration to a predetermined LDL target would have achieved similar benefits. The PROVE IT-TIMI 22 trial was designed to compare the reductions in death and major cardiovascular events following ACS between LDL lowering to approximately 100 mg/dL using 40 mg/d of pravastatin, and more intensive LDL lowering to approximately 70 mg/dL using 80 mg/d of atorvastatin.5 Investigators enrolled 4,162 patients for a median of seven days following ACS (STEMI, NSTEMI, or UA) to the two treatment arms. Investigators observed patients for a period of 18 to 36 months for the primary end points of death, MI, UA, revascularization, and stroke. The median LDL level at the time of enrollment was 106 mg/dL in both treatment arms. During follow up, the median LDL levels achieved were 95 mg/dL in the pravastatin group and 62 mg/dL in the atorvastatin group. After two years, a 16% reduction in the hazard ratio for any primary end point was seen favoring 80 mg/d of atorvastatin—p=0.005; 95% CI=5-26%. (See Figure 2, pg. 39) The benefit of high-dose atorvastatin was seen as early as 30 days after randomization and was sustained throughout the trial.
While the PROVE IT-TIMI 22 trial supported a specified dosing strategy for statin use following ACS, Phase Z of the A-to-Z trial was designed to evaluate the early initiation of intensive lipid lowering following ACS, as compared to a delayed and less-intensive strategy.5,6 Investigators randomized 4,497 patients (a mean of 3.7 days following either NSTEMI or STEMI) to receive either placebo for four months followed by simvastatin 20 mg/d or simvastatin 40 mg/d for one month followed by simvastatin 80 mg/d. They followed patients for 24 months for the primary end points of cardiovascular death, MI, readmission for ACS, or stroke. The primary end point occurred in 16.7% of the delayed, lower-intensity treatment group and in 14.4% of the early, higher-intensity treatment group (95% CI 0.76-1.04; p=0.14). Despite the lack of a significant difference in the composite primary end point between the two treatment arms, a significant reduction in the secondary end points of cardiovascular mortality (absolute risk reduction (ARR 1.3%; P=0.05) and congestive heart failure (ARR 1.3%; P=0.04) was evident favoring the early, intensive treatment strategy. These differences were not evident until at least four months after randomization. The A-to-Z trial investigators offered several possible explanations for the delay in evident clinical benefits in their trial when compared against the strong trend toward clinical benefit seen with 30 days following the early initiation of high-dose atorvastatin following ACS in the PROVE IT-TIMI 22 trial. In the PROVE IT trial, patients were enrolled an average of seven days after their index event, and as a result, 69% had undergone revascularization by this time. In the A-to-Z trial, patients were enrolled an average of three to four days earlier, and, therefore, were less likely to have undergone a revascularization procedure by the time of enrollment—and may have continued on with active thrombotic processes relatively less responsive to statin therapy.6 Another notable difference between PROVE IT and A-to-Z subjects was the C-reactive protein (CRP) concentrations in the A-to-Z subjects did not differ between treatment groups within 30 days despite significant differences in their LDL levels.16 This lack of a concurrent, pleiotropic, anti-inflammatory effect in the A-to-Z trial aggressive treatment arm may also have contributed to the delayed treatment effect.
In conclusion, the A-to-Z investigators suggest more intensive statin therapy (than the 40 mg Simvastatin in their intensive treatment arm) may be required to derive the most rapid and maximal clinical benefits during the highest risk period immediately following ACS.
Following stroke: Although there is more robust data supporting the benefits of early, intensive, statin therapy following ACS, there also is established and emerging data supporting similar treatment approaches following stroke.
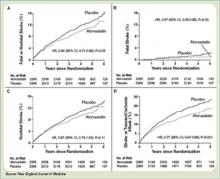
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial was designed to determine whether or not atorvastatin 80 mg daily would reduce the risk of stroke in patients without known coronary heart disease who had suffered a TIA or stroke within the preceding six months.8 Patients who experienced a hemorrhagic or ischemic TIA or stroke between one to six months before study entry were randomized to receive either atorvastatin 80 mg/d or placebo.
Investigators followed patients for a mean period of 4.9 years for the primary end point of time to non-fatal or fatal stroke. Secondary composite end points included stroke or TIA, and any coronary or peripheral arterial event, including death from cardiac causes, non-fatal MI, ACS, revascularization (coronary, carotid, peripheral), and death from any cause. No difference in mean baseline LDL levels was witnessed between the treatment and placebo arms (132.7 and 133.7 mg/dL, respectively). Atorvastatin was associated with a 16% relative reduction in the risk of stroke—hazard ratio, 0.84; 95% CI 0.71–0.99; p=0.03. This was found despite an increase in hemorrhagic stroke in the atorvastatin group—a finding that supports an epidemiologic association between low cholesterol levels and brain hemorrhage. The risk of cardiovascular events also was significantly reduced, however, no significant difference in overall mortality was observed between the two groups.
In conclusion, the authors recommend the initiation of high-dose atorvastatin “soon” after stroke or TIA. One can only conclude, based on these data, statin therapy should be initiated within six months of TIA or stroke, in accordance with the study design. There is retrospective data suggesting benefit to statin therapy initiated within four weeks following ischemic stroke, and there are prospective trials in process evaluating the potential benefits of statins initiated within 24 hours following ischemic stroke, however, no large, randomized, controlled trial can demonstrate the effect of statins when used as acute stroke therapy.9,12,13,17
Back to the Case
The patient described in our case has a history of TIA and experienced an acute coronary syndrome (NSTEMI) within the preceding 24 hours. He underwent a revascularization procedure (PCI with stent), and is on appropriate therapy, including dual anti-platelet therapy with aspirin and clopidogrel, a beta-blocker, and an angiotensin-converting enzyme inhibitor. Based on the data and conclusions of the MIRACL, PROVE IT-TIMI 22, and SPARCL trials, high-dose statin therapy with atorvastatin 80 mg/d should be initiated immediately in the patient in order to significantly reduce his risk of recurrent ischemic cardiovascular events and stroke following his acute coronary syndrome and TIA.
Bottom Line
Following ACS, high-dose statin therapy with 80 mg of atorvastatin per day should be initiated when the patient is still in the hospital, irrespective of baseline LDL level. Statin therapy should also strongly be considered for secondary stroke prevention in most patients with a history of stroke or transient ischemic attack. TH
Caleb Hale, MD, is a hospitalist at Beth Israel Deaconess Medical Center in Boston. Joseph Ming Wah Li is director of the hospital medicine program and associate chief, division of general medicine and primary care at Beth Israel Deaconess Medical Center in Boston, and assistant professor of Medicine at Harvard Medical School.
References
1. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
2. Sacks RM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-1009.
3. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349-1357.
4. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711-1718.
5. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
6. Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs. a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
7. Waters D, Schwartz GG, Olsson AG. The myocardial ischemia reduction with acute cholesterol lowering (MIRACL) trial: a new frontier for statins? Curr Control Trials Cardiovasc Med. 2001;2;111-114.
8. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
9. Moonis M, Kane K, Schwiderski U, Sandage BW, Fisher M. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke. 2005;36:1298-1300.
10. Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology. 2005;65:253-258.
11. Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969-1973.
12. Elkind MS, Sacco RL, MacArthur RB, et al. The neuroprotection with statin therapy for acute recovery trial (NeuSTART): an adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke. Int J Stroke. 2008;3:210-218.
13. Montaner J, Chacon P, Krupinski J, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J of Neurol. 2008;15:82-90.
14. Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20-28.
15. Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. JACC. 2008;51:1440-1445.
16. Wiviott SD, de Lemos JA, Cannon CP, et al. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials of A to Z and PROVE IT-TIMI 22. Circulation. 2006;113:1406-1414.
17. Elking MS. Statins as acute-stroke treatment. Int J Stroke. 2006;1:224-225.
Case
A 52-year-old man with no medical history other than a transient ischemic attack (TIA) three months ago presents to the emergency department (ED) following multiple episodes of substernal (ST) chest pressure. He takes no medication. His electrocardiogram (ECG) revealed lateral ST segment depressions, and his cardiac biomarkers were elevated. He underwent cardiac catheterization, and a single drug-eluting stent was successfully placed to a culprit left circumflex lesion. He is now stable less than 24 hours following his initial presentation, without any evidence of heart failure. His providers prescribe aspirin, clopidogrel, metoprolol, and lisinopril. His fasting LDL level is 92 mg/dL.
What, if any, is the role for lipid-lowering therapy at this time?
Overview
Long-term therapy with HMG CoA reductase inhibitors (statins) has been shown through several large, randomized, controlled trials to reduce the risk for death, myocardial infarction (MI), and stroke in patients with established coronary disease. The most significant effects were evident after approximately two years of treatment.1,2,3,4
Subsequent trials have shown earlier and more significant reductions in the rates of recurrent ischemic cardiovascular events following acute coronary syndromes (ACS) when statins are administered early—within days of the initial event. This is a window of time in which most patients still are hospitalized.4,5,6,7
In addition to this data regarding statin use following ACS, a large, randomized, controlled trial demonstrated similar reductions in the incidence of strokes and cardiovascular events when high-dose atorvastatin was administered within one to six months following TIA or stroke in patients without established coronary disease.8 There is growing data supporting the hypothesis that statins have pleiotropic (non cholesterol-lowering), neuroprotective, properties that may improve patient outcomes following cerebrovascular events.9,10,11 There are ongoing trials investing the role of statins in the acute management of stroke.12,13
Hospitalists frequently manage patients in the stages immediately following ACS and stroke. Based on the large and evolving volume of data regarding the use of statins following these events, when and how should a statin be started in the hospital?
Review of the Data
Following Acute Coronary Syndrome: Death and recurrent ischemic events following ACS are most likely to occur in the early phase of recovery. Based on this observation and evidence supporting the early (in some cases within hours of administration) ‘pleiotropic’ or non-cholesterol lowering effects of statins, including improvement in endothelial function and decreases in platelet aggregation, thrombus deposition, and vascular inflammation, the MIRACL study was designed to answer the question of whether the initiation of treatment with a statin within 24 to 96 hours following ACS would reduce the occurrence of death and recurrent ischemia.4,7,14 Investigators randomized 3,086 patients within 24-96 hours (mean 63 hours) following admission for non-ST segment myocardial infarction (NSTEMI) or unstable angina (UA) to receive either atorvastatin 80 mg/d or placebo.
Investigators monitored patients for the primary end points of ischemic events (death, non-fatal MI, cardiac arrest with resuscitation, symptomatic myocardial ischemia with objective evidence) during a 16-week period. In the treatment arm, the risk of the primary combined end point was significantly reduced—relative risk (RR) 0.84; 95% confidence interval (CI), 0.70-1.00; p=0.048. (See Figure 1, pg. 39)
No significant differences were found between atorvastatin and placebo in the risk of death, non-fatal MI, or cardiac arrest with resuscitation. There was, however, a significantly lower risk of recurrent symptomatic myocardial ischemia with objective evidence requiring emergent re-hospitalization in the treatment arm (RR, 0.74; 95% CI, 0.57-0.95; p=0.02). The mean baseline LDL level in the treatment arm was 124 mg/dL, a value that may represent, in part, suppression of the LDL level in the setting of acute ACS. This is a phenomenon previously described in an analysis of the LUNAR trial.15
Suppression of LDL level after ACS appeared to be minimal, however, and is unlikely to be clinically significant. The benefits of atorvastatin in the MIRACL trial did not appear to depend on baseline LDL level—suggesting the decision to initiate statin therapy after ACS should not be influenced by LDL level at the time of the event.
Only one dose of statin was used in the MIRACL trial, and the investigators commented they were unable to determine if a lower dose of atorvastatin or a gradual dose titration to a predetermined LDL target would have achieved similar benefits. The PROVE IT-TIMI 22 trial was designed to compare the reductions in death and major cardiovascular events following ACS between LDL lowering to approximately 100 mg/dL using 40 mg/d of pravastatin, and more intensive LDL lowering to approximately 70 mg/dL using 80 mg/d of atorvastatin.5 Investigators enrolled 4,162 patients for a median of seven days following ACS (STEMI, NSTEMI, or UA) to the two treatment arms. Investigators observed patients for a period of 18 to 36 months for the primary end points of death, MI, UA, revascularization, and stroke. The median LDL level at the time of enrollment was 106 mg/dL in both treatment arms. During follow up, the median LDL levels achieved were 95 mg/dL in the pravastatin group and 62 mg/dL in the atorvastatin group. After two years, a 16% reduction in the hazard ratio for any primary end point was seen favoring 80 mg/d of atorvastatin—p=0.005; 95% CI=5-26%. (See Figure 2, pg. 39) The benefit of high-dose atorvastatin was seen as early as 30 days after randomization and was sustained throughout the trial.
While the PROVE IT-TIMI 22 trial supported a specified dosing strategy for statin use following ACS, Phase Z of the A-to-Z trial was designed to evaluate the early initiation of intensive lipid lowering following ACS, as compared to a delayed and less-intensive strategy.5,6 Investigators randomized 4,497 patients (a mean of 3.7 days following either NSTEMI or STEMI) to receive either placebo for four months followed by simvastatin 20 mg/d or simvastatin 40 mg/d for one month followed by simvastatin 80 mg/d. They followed patients for 24 months for the primary end points of cardiovascular death, MI, readmission for ACS, or stroke. The primary end point occurred in 16.7% of the delayed, lower-intensity treatment group and in 14.4% of the early, higher-intensity treatment group (95% CI 0.76-1.04; p=0.14). Despite the lack of a significant difference in the composite primary end point between the two treatment arms, a significant reduction in the secondary end points of cardiovascular mortality (absolute risk reduction (ARR 1.3%; P=0.05) and congestive heart failure (ARR 1.3%; P=0.04) was evident favoring the early, intensive treatment strategy. These differences were not evident until at least four months after randomization. The A-to-Z trial investigators offered several possible explanations for the delay in evident clinical benefits in their trial when compared against the strong trend toward clinical benefit seen with 30 days following the early initiation of high-dose atorvastatin following ACS in the PROVE IT-TIMI 22 trial. In the PROVE IT trial, patients were enrolled an average of seven days after their index event, and as a result, 69% had undergone revascularization by this time. In the A-to-Z trial, patients were enrolled an average of three to four days earlier, and, therefore, were less likely to have undergone a revascularization procedure by the time of enrollment—and may have continued on with active thrombotic processes relatively less responsive to statin therapy.6 Another notable difference between PROVE IT and A-to-Z subjects was the C-reactive protein (CRP) concentrations in the A-to-Z subjects did not differ between treatment groups within 30 days despite significant differences in their LDL levels.16 This lack of a concurrent, pleiotropic, anti-inflammatory effect in the A-to-Z trial aggressive treatment arm may also have contributed to the delayed treatment effect.
In conclusion, the A-to-Z investigators suggest more intensive statin therapy (than the 40 mg Simvastatin in their intensive treatment arm) may be required to derive the most rapid and maximal clinical benefits during the highest risk period immediately following ACS.
Following stroke: Although there is more robust data supporting the benefits of early, intensive, statin therapy following ACS, there also is established and emerging data supporting similar treatment approaches following stroke.
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial was designed to determine whether or not atorvastatin 80 mg daily would reduce the risk of stroke in patients without known coronary heart disease who had suffered a TIA or stroke within the preceding six months.8 Patients who experienced a hemorrhagic or ischemic TIA or stroke between one to six months before study entry were randomized to receive either atorvastatin 80 mg/d or placebo.
Investigators followed patients for a mean period of 4.9 years for the primary end point of time to non-fatal or fatal stroke. Secondary composite end points included stroke or TIA, and any coronary or peripheral arterial event, including death from cardiac causes, non-fatal MI, ACS, revascularization (coronary, carotid, peripheral), and death from any cause. No difference in mean baseline LDL levels was witnessed between the treatment and placebo arms (132.7 and 133.7 mg/dL, respectively). Atorvastatin was associated with a 16% relative reduction in the risk of stroke—hazard ratio, 0.84; 95% CI 0.71–0.99; p=0.03. This was found despite an increase in hemorrhagic stroke in the atorvastatin group—a finding that supports an epidemiologic association between low cholesterol levels and brain hemorrhage. The risk of cardiovascular events also was significantly reduced, however, no significant difference in overall mortality was observed between the two groups.
In conclusion, the authors recommend the initiation of high-dose atorvastatin “soon” after stroke or TIA. One can only conclude, based on these data, statin therapy should be initiated within six months of TIA or stroke, in accordance with the study design. There is retrospective data suggesting benefit to statin therapy initiated within four weeks following ischemic stroke, and there are prospective trials in process evaluating the potential benefits of statins initiated within 24 hours following ischemic stroke, however, no large, randomized, controlled trial can demonstrate the effect of statins when used as acute stroke therapy.9,12,13,17
Back to the Case
The patient described in our case has a history of TIA and experienced an acute coronary syndrome (NSTEMI) within the preceding 24 hours. He underwent a revascularization procedure (PCI with stent), and is on appropriate therapy, including dual anti-platelet therapy with aspirin and clopidogrel, a beta-blocker, and an angiotensin-converting enzyme inhibitor. Based on the data and conclusions of the MIRACL, PROVE IT-TIMI 22, and SPARCL trials, high-dose statin therapy with atorvastatin 80 mg/d should be initiated immediately in the patient in order to significantly reduce his risk of recurrent ischemic cardiovascular events and stroke following his acute coronary syndrome and TIA.
Bottom Line
Following ACS, high-dose statin therapy with 80 mg of atorvastatin per day should be initiated when the patient is still in the hospital, irrespective of baseline LDL level. Statin therapy should also strongly be considered for secondary stroke prevention in most patients with a history of stroke or transient ischemic attack. TH
Caleb Hale, MD, is a hospitalist at Beth Israel Deaconess Medical Center in Boston. Joseph Ming Wah Li is director of the hospital medicine program and associate chief, division of general medicine and primary care at Beth Israel Deaconess Medical Center in Boston, and assistant professor of Medicine at Harvard Medical School.
References
1. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
2. Sacks RM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-1009.
3. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349-1357.
4. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711-1718.
5. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
6. Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs. a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
7. Waters D, Schwartz GG, Olsson AG. The myocardial ischemia reduction with acute cholesterol lowering (MIRACL) trial: a new frontier for statins? Curr Control Trials Cardiovasc Med. 2001;2;111-114.
8. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
9. Moonis M, Kane K, Schwiderski U, Sandage BW, Fisher M. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke. 2005;36:1298-1300.
10. Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology. 2005;65:253-258.
11. Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969-1973.
12. Elkind MS, Sacco RL, MacArthur RB, et al. The neuroprotection with statin therapy for acute recovery trial (NeuSTART): an adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke. Int J Stroke. 2008;3:210-218.
13. Montaner J, Chacon P, Krupinski J, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J of Neurol. 2008;15:82-90.
14. Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20-28.
15. Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. JACC. 2008;51:1440-1445.
16. Wiviott SD, de Lemos JA, Cannon CP, et al. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials of A to Z and PROVE IT-TIMI 22. Circulation. 2006;113:1406-1414.
17. Elking MS. Statins as acute-stroke treatment. Int J Stroke. 2006;1:224-225.
Case
A 52-year-old man with no medical history other than a transient ischemic attack (TIA) three months ago presents to the emergency department (ED) following multiple episodes of substernal (ST) chest pressure. He takes no medication. His electrocardiogram (ECG) revealed lateral ST segment depressions, and his cardiac biomarkers were elevated. He underwent cardiac catheterization, and a single drug-eluting stent was successfully placed to a culprit left circumflex lesion. He is now stable less than 24 hours following his initial presentation, without any evidence of heart failure. His providers prescribe aspirin, clopidogrel, metoprolol, and lisinopril. His fasting LDL level is 92 mg/dL.
What, if any, is the role for lipid-lowering therapy at this time?
Overview
Long-term therapy with HMG CoA reductase inhibitors (statins) has been shown through several large, randomized, controlled trials to reduce the risk for death, myocardial infarction (MI), and stroke in patients with established coronary disease. The most significant effects were evident after approximately two years of treatment.1,2,3,4
Subsequent trials have shown earlier and more significant reductions in the rates of recurrent ischemic cardiovascular events following acute coronary syndromes (ACS) when statins are administered early—within days of the initial event. This is a window of time in which most patients still are hospitalized.4,5,6,7
In addition to this data regarding statin use following ACS, a large, randomized, controlled trial demonstrated similar reductions in the incidence of strokes and cardiovascular events when high-dose atorvastatin was administered within one to six months following TIA or stroke in patients without established coronary disease.8 There is growing data supporting the hypothesis that statins have pleiotropic (non cholesterol-lowering), neuroprotective, properties that may improve patient outcomes following cerebrovascular events.9,10,11 There are ongoing trials investing the role of statins in the acute management of stroke.12,13
Hospitalists frequently manage patients in the stages immediately following ACS and stroke. Based on the large and evolving volume of data regarding the use of statins following these events, when and how should a statin be started in the hospital?
Review of the Data
Following Acute Coronary Syndrome: Death and recurrent ischemic events following ACS are most likely to occur in the early phase of recovery. Based on this observation and evidence supporting the early (in some cases within hours of administration) ‘pleiotropic’ or non-cholesterol lowering effects of statins, including improvement in endothelial function and decreases in platelet aggregation, thrombus deposition, and vascular inflammation, the MIRACL study was designed to answer the question of whether the initiation of treatment with a statin within 24 to 96 hours following ACS would reduce the occurrence of death and recurrent ischemia.4,7,14 Investigators randomized 3,086 patients within 24-96 hours (mean 63 hours) following admission for non-ST segment myocardial infarction (NSTEMI) or unstable angina (UA) to receive either atorvastatin 80 mg/d or placebo.
Investigators monitored patients for the primary end points of ischemic events (death, non-fatal MI, cardiac arrest with resuscitation, symptomatic myocardial ischemia with objective evidence) during a 16-week period. In the treatment arm, the risk of the primary combined end point was significantly reduced—relative risk (RR) 0.84; 95% confidence interval (CI), 0.70-1.00; p=0.048. (See Figure 1, pg. 39)
No significant differences were found between atorvastatin and placebo in the risk of death, non-fatal MI, or cardiac arrest with resuscitation. There was, however, a significantly lower risk of recurrent symptomatic myocardial ischemia with objective evidence requiring emergent re-hospitalization in the treatment arm (RR, 0.74; 95% CI, 0.57-0.95; p=0.02). The mean baseline LDL level in the treatment arm was 124 mg/dL, a value that may represent, in part, suppression of the LDL level in the setting of acute ACS. This is a phenomenon previously described in an analysis of the LUNAR trial.15
Suppression of LDL level after ACS appeared to be minimal, however, and is unlikely to be clinically significant. The benefits of atorvastatin in the MIRACL trial did not appear to depend on baseline LDL level—suggesting the decision to initiate statin therapy after ACS should not be influenced by LDL level at the time of the event.
Only one dose of statin was used in the MIRACL trial, and the investigators commented they were unable to determine if a lower dose of atorvastatin or a gradual dose titration to a predetermined LDL target would have achieved similar benefits. The PROVE IT-TIMI 22 trial was designed to compare the reductions in death and major cardiovascular events following ACS between LDL lowering to approximately 100 mg/dL using 40 mg/d of pravastatin, and more intensive LDL lowering to approximately 70 mg/dL using 80 mg/d of atorvastatin.5 Investigators enrolled 4,162 patients for a median of seven days following ACS (STEMI, NSTEMI, or UA) to the two treatment arms. Investigators observed patients for a period of 18 to 36 months for the primary end points of death, MI, UA, revascularization, and stroke. The median LDL level at the time of enrollment was 106 mg/dL in both treatment arms. During follow up, the median LDL levels achieved were 95 mg/dL in the pravastatin group and 62 mg/dL in the atorvastatin group. After two years, a 16% reduction in the hazard ratio for any primary end point was seen favoring 80 mg/d of atorvastatin—p=0.005; 95% CI=5-26%. (See Figure 2, pg. 39) The benefit of high-dose atorvastatin was seen as early as 30 days after randomization and was sustained throughout the trial.
While the PROVE IT-TIMI 22 trial supported a specified dosing strategy for statin use following ACS, Phase Z of the A-to-Z trial was designed to evaluate the early initiation of intensive lipid lowering following ACS, as compared to a delayed and less-intensive strategy.5,6 Investigators randomized 4,497 patients (a mean of 3.7 days following either NSTEMI or STEMI) to receive either placebo for four months followed by simvastatin 20 mg/d or simvastatin 40 mg/d for one month followed by simvastatin 80 mg/d. They followed patients for 24 months for the primary end points of cardiovascular death, MI, readmission for ACS, or stroke. The primary end point occurred in 16.7% of the delayed, lower-intensity treatment group and in 14.4% of the early, higher-intensity treatment group (95% CI 0.76-1.04; p=0.14). Despite the lack of a significant difference in the composite primary end point between the two treatment arms, a significant reduction in the secondary end points of cardiovascular mortality (absolute risk reduction (ARR 1.3%; P=0.05) and congestive heart failure (ARR 1.3%; P=0.04) was evident favoring the early, intensive treatment strategy. These differences were not evident until at least four months after randomization. The A-to-Z trial investigators offered several possible explanations for the delay in evident clinical benefits in their trial when compared against the strong trend toward clinical benefit seen with 30 days following the early initiation of high-dose atorvastatin following ACS in the PROVE IT-TIMI 22 trial. In the PROVE IT trial, patients were enrolled an average of seven days after their index event, and as a result, 69% had undergone revascularization by this time. In the A-to-Z trial, patients were enrolled an average of three to four days earlier, and, therefore, were less likely to have undergone a revascularization procedure by the time of enrollment—and may have continued on with active thrombotic processes relatively less responsive to statin therapy.6 Another notable difference between PROVE IT and A-to-Z subjects was the C-reactive protein (CRP) concentrations in the A-to-Z subjects did not differ between treatment groups within 30 days despite significant differences in their LDL levels.16 This lack of a concurrent, pleiotropic, anti-inflammatory effect in the A-to-Z trial aggressive treatment arm may also have contributed to the delayed treatment effect.
In conclusion, the A-to-Z investigators suggest more intensive statin therapy (than the 40 mg Simvastatin in their intensive treatment arm) may be required to derive the most rapid and maximal clinical benefits during the highest risk period immediately following ACS.
Following stroke: Although there is more robust data supporting the benefits of early, intensive, statin therapy following ACS, there also is established and emerging data supporting similar treatment approaches following stroke.
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial was designed to determine whether or not atorvastatin 80 mg daily would reduce the risk of stroke in patients without known coronary heart disease who had suffered a TIA or stroke within the preceding six months.8 Patients who experienced a hemorrhagic or ischemic TIA or stroke between one to six months before study entry were randomized to receive either atorvastatin 80 mg/d or placebo.
Investigators followed patients for a mean period of 4.9 years for the primary end point of time to non-fatal or fatal stroke. Secondary composite end points included stroke or TIA, and any coronary or peripheral arterial event, including death from cardiac causes, non-fatal MI, ACS, revascularization (coronary, carotid, peripheral), and death from any cause. No difference in mean baseline LDL levels was witnessed between the treatment and placebo arms (132.7 and 133.7 mg/dL, respectively). Atorvastatin was associated with a 16% relative reduction in the risk of stroke—hazard ratio, 0.84; 95% CI 0.71–0.99; p=0.03. This was found despite an increase in hemorrhagic stroke in the atorvastatin group—a finding that supports an epidemiologic association between low cholesterol levels and brain hemorrhage. The risk of cardiovascular events also was significantly reduced, however, no significant difference in overall mortality was observed between the two groups.
In conclusion, the authors recommend the initiation of high-dose atorvastatin “soon” after stroke or TIA. One can only conclude, based on these data, statin therapy should be initiated within six months of TIA or stroke, in accordance with the study design. There is retrospective data suggesting benefit to statin therapy initiated within four weeks following ischemic stroke, and there are prospective trials in process evaluating the potential benefits of statins initiated within 24 hours following ischemic stroke, however, no large, randomized, controlled trial can demonstrate the effect of statins when used as acute stroke therapy.9,12,13,17
Back to the Case
The patient described in our case has a history of TIA and experienced an acute coronary syndrome (NSTEMI) within the preceding 24 hours. He underwent a revascularization procedure (PCI with stent), and is on appropriate therapy, including dual anti-platelet therapy with aspirin and clopidogrel, a beta-blocker, and an angiotensin-converting enzyme inhibitor. Based on the data and conclusions of the MIRACL, PROVE IT-TIMI 22, and SPARCL trials, high-dose statin therapy with atorvastatin 80 mg/d should be initiated immediately in the patient in order to significantly reduce his risk of recurrent ischemic cardiovascular events and stroke following his acute coronary syndrome and TIA.
Bottom Line
Following ACS, high-dose statin therapy with 80 mg of atorvastatin per day should be initiated when the patient is still in the hospital, irrespective of baseline LDL level. Statin therapy should also strongly be considered for secondary stroke prevention in most patients with a history of stroke or transient ischemic attack. TH
Caleb Hale, MD, is a hospitalist at Beth Israel Deaconess Medical Center in Boston. Joseph Ming Wah Li is director of the hospital medicine program and associate chief, division of general medicine and primary care at Beth Israel Deaconess Medical Center in Boston, and assistant professor of Medicine at Harvard Medical School.
References
1. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
2. Sacks RM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-1009.
3. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349-1357.
4. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711-1718.
5. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
6. Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs. a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
7. Waters D, Schwartz GG, Olsson AG. The myocardial ischemia reduction with acute cholesterol lowering (MIRACL) trial: a new frontier for statins? Curr Control Trials Cardiovasc Med. 2001;2;111-114.
8. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
9. Moonis M, Kane K, Schwiderski U, Sandage BW, Fisher M. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke. 2005;36:1298-1300.
10. Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology. 2005;65:253-258.
11. Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969-1973.
12. Elkind MS, Sacco RL, MacArthur RB, et al. The neuroprotection with statin therapy for acute recovery trial (NeuSTART): an adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke. Int J Stroke. 2008;3:210-218.
13. Montaner J, Chacon P, Krupinski J, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J of Neurol. 2008;15:82-90.
14. Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20-28.
15. Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. JACC. 2008;51:1440-1445.
16. Wiviott SD, de Lemos JA, Cannon CP, et al. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials of A to Z and PROVE IT-TIMI 22. Circulation. 2006;113:1406-1414.
17. Elking MS. Statins as acute-stroke treatment. Int J Stroke. 2006;1:224-225.