User login
Deprescribing: A simple method for reducing polypharmacy
CASE An 82-year-old woman with a history of hypertension, diabetes, hyperlipidemia, stage 3 chronic kidney disease, anxiety, urge urinary incontinence, constipation, and bilateral knee osteoarthritis presents to her primary care physician’s office after a fall. She reports that she visited the emergency department (ED) a week ago after falling in the middle of the night on her way to the bathroom. This is the third fall she’s had this year. On chart review, she had a blood pressure (BP) of 112/60 mm Hg and a blood glucose level of 65 mg/dL in the ED. All other testing (head imaging, chest x-ray, urinalysis) was normal. The ED physician recommended that she stop taking her lisinopril-hydrochlorothiazide (HCTZ) and glipizide extended release (XL) until her follow-up appointment. Today, she asks about the need to restart these medications.
Polypharmacy is common among older adults due to a high prevalence of chronic conditions that often require multiple medications for optimal management. Cut points of 5 or 9 medications are frequently used to define polypharmacy. However, some define polypharmacy as taking a medication that lacks an indication, is ineffective, or is duplicating treatment provided by another medication.
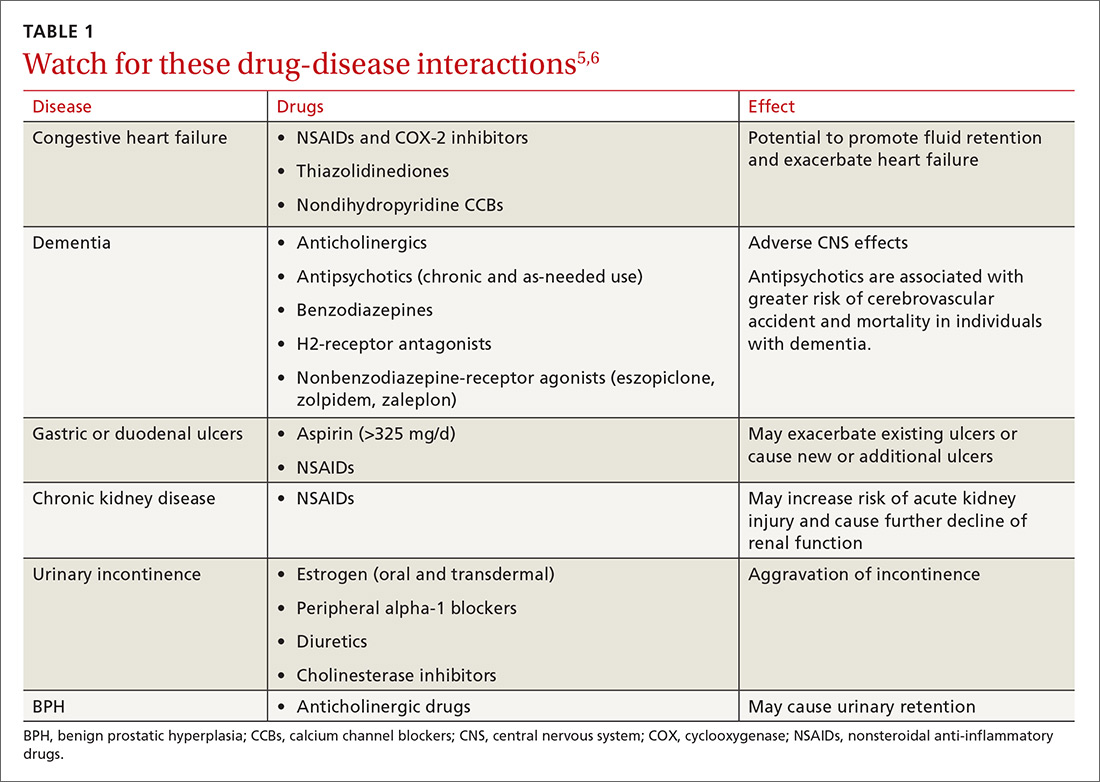
Either way, polypharmacy is associated with multiple negative consequences, including an increased risk for adverse drug events (ADEs),1-4 drug-drug and drug-disease interactions (TABLE 15,6),7 reduced functional capacity,8 multiple geriatric syndromes (TABLE 25,9-12), medication non-adherence,13 and increased mortality.14 Polypharmacy also contributes to increased health care costs for both the patient and the health care system.15
Taking a step back. Polypharmacy often results from prescribing cascades, which occur when an adverse drug effect is misinterpreted as a new medical problem, leading to the prescribing of more medication to treat the initial drug-induced symptom. Potentially inappropriate medications (PIMs), which are medications that should be avoided in older adults and in those with certain conditions, are also more likely to be prescribed in the setting of polypharmacy.16
Deprescribing is the process of identifying and discontinuing medications that are unnecessary, ineffective, and/or inappropriate in order to reduce polypharmacy and improve health outcomes. Deprescribing is a collaborative process that involves weighing the benefits and harms of medications in the context of a patient’s care goals, current level of functioning, life expectancy, values, and preferences. This article reviews polypharmacy and discusses safe and effective deprescribing strategies for older adults in the primary care setting.
[polldaddy:9781245]
How many people on how many meds?
According to a 2016 study, 36% of community-dwelling older adults (ages 62-85 years) were taking 5 or more prescription medications in 2010 to 2011—up from 31% in 2005 to 2006.17 When one narrows the population to older adults in the United States who are hospitalized, almost half (46%) take 7 or more medications.18 Among frail, older US veterans at hospital discharge, 40% were prescribed 9 or more medications, with 44% of these patients receiving at least one unnecessary drug.19
The challenges of multimorbidity
In the United States, 80% of those 65 and older have 2 or more chronic conditions, or multimorbidity.20 Clinical practice guidelines making recommendations for the management of single conditions, such as heart failure, hypertension, or diabetes, often suggest the use of 2 or more medications to achieve optimal management and fail to provide guidance in the setting of multimorbidity. Following treatment recommendations for multiple conditions predictably leads to polypharmacy, with complicated, costly, and burdensome regimens.
Further, the research contributing to the development of clinical practice guidelines frequently excludes older adults and those with multimorbidity, reducing applicability in this population. As a result, many treatment recommendations have uncertain benefit and may be harmful in the multimorbid older patient.21
CASE In addition to the patient’s multimorbidity, she had a stroke at age 73 and has some mild residual left-sided weakness. Functionally, she is independent and able to perform her activities of daily living and her instrumental activities of daily living. She lives alone, quit smoking at age 65, and has an occasional glass of wine during family parties. The patient’s daughter and granddaughter live 2 blocks away.
Her current medications include glipizide XL 10 mg/d and lisinopril-HCTZ 20-25 mg/d, which she has temporarily discontinued at the ED doctor’s recommendation, as well as: amlodipine 10 mg/d, metformin 1000 mg BID, senna 8.6 mg/d, docusate 100 mg BID, furosemide 40 mg/d, and ibuprofen 600 mg/d (for knee pain). She reports taking omeprazole 20 mg/d “for almost 20 years,” even though she has not had any reflux symptoms in recent memory. After her stroke, she began taking atorvastatin 10 mg/d, aspirin 81 mg/d, and clopidogrel 75 mg/d, which she continues to take today. About a year ago, she started oxybutynin 5 mg/d for urinary incontinence, but she has not noticed significant relief. Additionally, she takes lorazepam 1 mg for insomnia most nights of the week.
A review of systems reveals issues with chronic constipation and intermittent dizziness, but is otherwise negative. The physical examination reveals a well-appearing woman with a body mass index of 26. Her temperature is 98.5° F, her heart rate is 78 beats/min and regular, her respirations are 14 breaths/min, and her BP is 117/65 mm Hg. Orthostatic testing is negative. Her heart, lung, and abdominal exams are within normal limits. Her timed up and go test is 14 seconds. Her blood glucose level today in the office after eating breakfast 2 hours ago is 135 mg/dL (normal: <140 mg/dL). Laboratory tests performed at the time of the ED visit show a creatinine level of 1.2 mg/dL (normal range: 0.6 to 1.1 mg/dL), a glomerular filtration rate (GFR) of 44 units (normal range: >60 units), a hemoglobin level of 9.8 g/dL (normal range: 12-15.5 g/dL), and a thyroid stimulating hormone level of 1.4 mIU/L (normal range: 0.5-8.9 mIU/L). A recent hemoglobin A1C is 6.8% (normal: <5.7%), low-density lipoprotein (LDL) level is 103 mg/dL (optimal <100 mg/dL), and high-density lipoprotein (HDL) level is 65 mg/dL (optimal >60 mg/dL). An echocardiogram performed a year ago showed mild aortic stenosis with normal systolic and diastolic function.
Starting the deprescribing process: Several approaches to choose from
The goal of deprescribing is to reduce polypharmacy and improve health outcomes. It is a process defined as, “reviewing all current medications; identifying medications to be ceased, substituted, or reduced; planning a deprescribing regimen in partnership with the patient; and frequently reviewing and supporting the patient.”22 A medication review should include prescription, over-the-counter (OTC), and complementary/alternative medicine (CAM) agents.
Until recently, studies evaluating the process of deprescribing across drug classes and disease conditions were limited, but new research is beginning to show its potential impact. After deprescribing, patients experience fewer falls and show improvements in cognition.23 While there have not yet been large randomized trials to evaluate deprescribing, a recent systematic review and meta-analysis showed that use of patient-specific deprescribing interventions is associated with improved survival.24 Importantly, there have been no reported adverse drug withdrawal events or deaths associated with deprescribing.23
Smaller studies have reported additional benefits including decreases in health care costs, reductions in drug-drug interactions and PIMs, improvements in medication adherence, and increases in patient satisfaction.25 In addition, the removal of unnecessary medications may allow for increased consideration of prescribing appropriate medications with known benefit.25
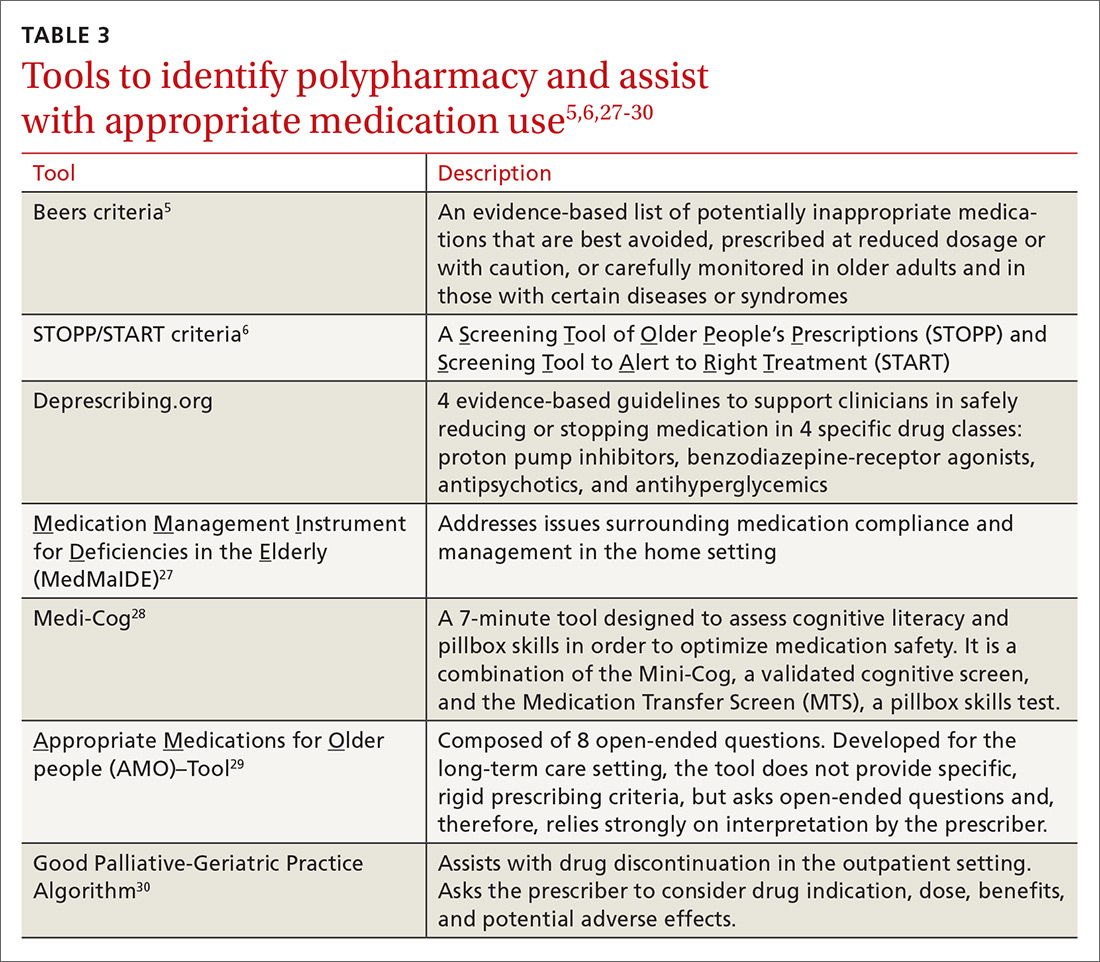
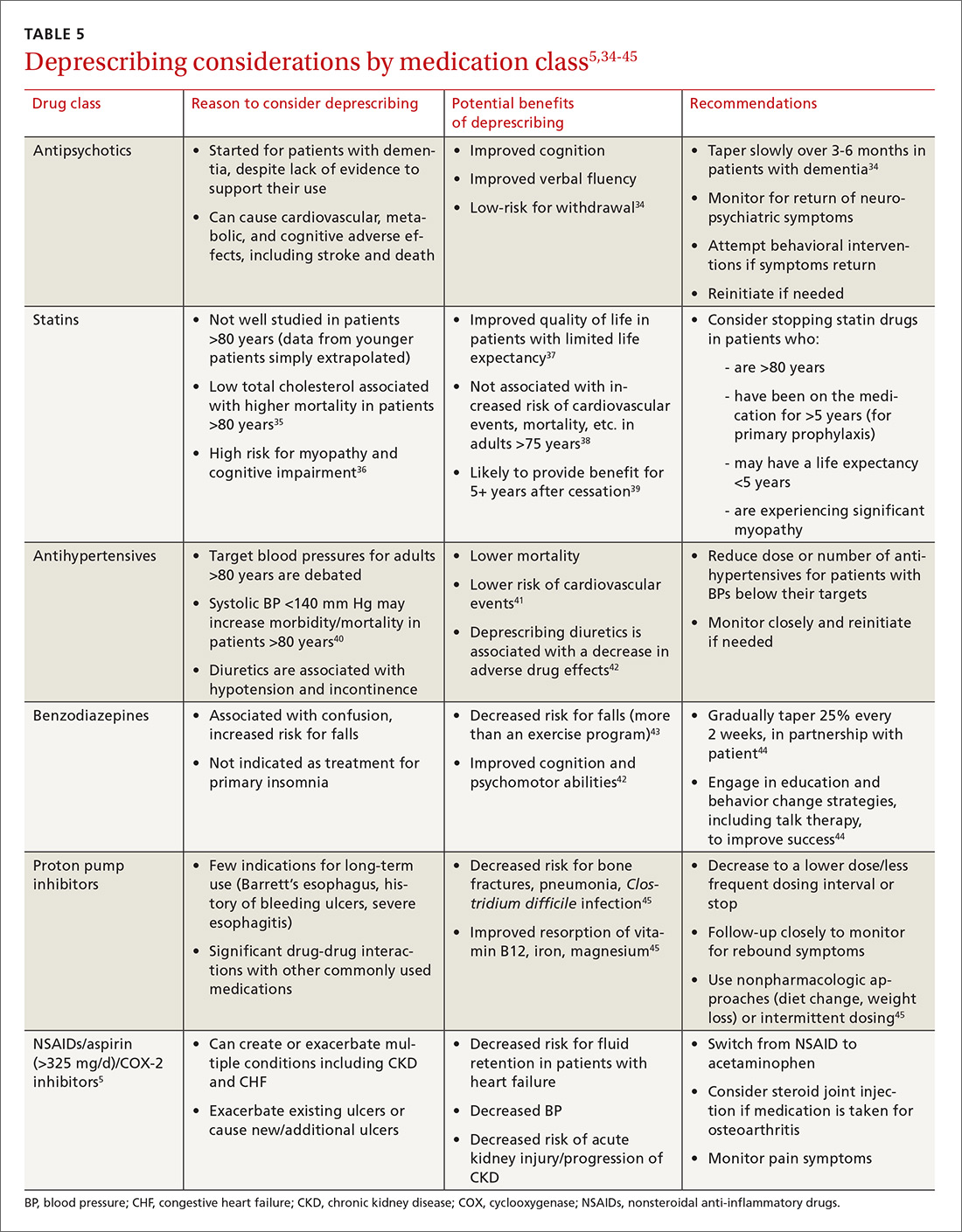
Practically speaking, every encounter between a patient and health care provider is an opportunity to reduce unnecessary medications. Electronic alert systems at pharmacies and those embedded within electronic health record (EHR) systems can also prompt a medication review and an effort to deprescribe.26 Evidence-based tools to identify polypharmacy and guide appropriate medication use are listed in TABLE 3.5,6,27-30 In addition, suggested approaches to beginning the deprescribing process are included in TABLE 4.5,31-33 And a medication class-based approach to deprescribing is provided in TABLE 5.5,34-45
Although no gold standard process exists for deprescribing, experts suggest that any deprescribing protocol should include the following steps:32,46
1. Start with a “brown bag” review of the patient’s medications.
Have the patient bring all of his/her medications in a bag to the visit; review them together or have the medication history taken by a pharmacist. Determine and discuss the indication for each medication and its effectiveness for that indication. Consider the potential benefits and harms of each medication in the context of the patient’s care goals and preferences. Assess whether the patient is taking all of the medications that have been prescribed, and identify any reasons for missed pills (eg, adverse effects, dosing regimens, understanding, cognitive issues).
2. Talk to the patient about the deprescribing process.
Talk with the patient about the risks and benefits of deprescribing, and prioritize which medications to address in the process. Prioritize the medications by balancing patient preferences with available pharmacologic evidence. If there is a lack of evidence supporting the benefits for a particular medication, consider known or suspected adverse effects, the ease or burden of the dosing regimen, the patient’s preferences and goals of care, remaining life expectancy, the time until drug benefit is appreciated, and the length of drug benefit after discontinuation.
3. Deprescribe medications.
If you are going to taper a medication, develop a schedule in partnership with the patient. Stop one medication at a time so that you can monitor for withdrawal symptoms or for the return of a condition.
Acknowledging potential barriers to deprescribing may help structure conversations and provide anticipatory guidance to patients and their families. Working to overcome these barriers will help maximize the benefits of deprescribing and help to build trust with patients.
Patient-driven barriers include fear of a condition worsening or returning, lack of a suitable alternative, lack of ongoing support to manage a particular condition, a previous bad experience with medication cessation, and influence from other care providers (eg, family, home caregivers, nurses, specialists, friends). Patients and family members sometimes cling to the hope of future effectiveness of a treatment, especially in the case of medications like donepezil for dementia.47 Utilizing a team-based and stepwise patient approach to deprescribing aims to provide hesitant patients with appropriate amounts of education and support to begin to reduce unnecessary medicines.
Provider-driven barriers include feeling uneasy about contradicting a specialist’s recommendations for initiation/continuation of specific medications, fear of causing withdrawal symptoms or disease relapse, and lack of specific data to adequately understand and assess benefits and harms in the older adult population. Primary care physicians have also acknowledged worry about discussing life expectancy and that patients will feel their care is being reduced or “downgraded.”48 Finally, there is limited time in which these complex shared decision-making conversations can take place. Thus, if medications are not causing a noticeable problem, it is often easier to just continue them.
One way to overcome some of these concerns is to consider working with a clinical pharmacist. By gaining information regarding medication-specific factors, such as half-life and expected withdrawal patterns, you can feel more confident deprescribing or continuing medications.
Additionally, communicating closely with specialists, ideally with the help of an integrated EHR, can allow you to discuss indications for particular medications or concerns about adverse effects, limited benefits, or difficulty with compliance, so that you can develop a collaborative, cohesive, and patient-centered plan. This, in turn, may improve patient understanding and compliance.
4. Create a follow-up plan.
At the time of deprescribing a medication, develop a plan with the patient for monitoring and assessment. Ensure that the patient understands which symptoms may occur in the event of drug withdrawal and which symptoms may suggest the return of a condition. Make sure that other supports are in place if needed (eg, cognitive behavioral therapy, physical therapy, social support or assistance) to help ensure that medication cessation is successful.
CASE During the office visit, you advise the patient that her BP looks normal, her blood sugar is within an appropriate range, and she is lucky to have not sustained any injuries after her most recent fall. In addition to discussing the benefits of some outpatient physical therapy to help with her balance, you ask if she would like to discuss reducing her medications. She is agreeable and asks for your recommendations.
You are aware of several resources that can help you with your recommendations, among them the STOPP/START6 and Beers criteria,5 as well as the Good Geriatric-Palliative Algorithm.30
If you were to use the STOPP/START and Beers criteria, you might consider stopping:
- lorazepam, which increases the risk of falls and confusion.
- ibuprofen, since this patient has only mild osteoarthritis pain, and ibuprofen has the potential for renal, cardiac, and gastrointestinal toxicities.
- oxybutynin, because it could be contributing to the patient’s constipation and cause confusion and falls.
- furosemide, since the patient has no clinical heart failure.
- omeprazole, since the indication is unknown and the patient has no history of ulceration, esophagitis, or symptomatic gastroesophageal reflux disease.
After reviewing the Good Geriatric-Palliative Algorithm,30 you might consider stopping:
- clopidogrel, as there is no clear indication for this medication in combination with aspirin in this patient.
- glipizide XL, as this patient’s A1c is below goal and this medication puts her at risk of hypoglycemia and its associated morbidities.
- metformin, as it increases her risk of lactic acidosis because her GFR is <45 units.
- docusate, as the evidence to show clear benefit in improving chronic constipation in older adults is lacking.
You tell your patient that there are multiple medications to consider stopping. In order to monitor any symptoms of withdrawal or return of a condition, it would be best to stop one at a time and follow-up closely. Since she has done well for the past week without the glipizide and lisinopril-HCTZ combination, she can remain off the glipizide and the HCTZ. Lisinopril, however, may provide renal protection in the setting of diabetes and will be continued at this time.
You ask her about adverse effects from her other medications. She indicates that the furosemide makes her run to the bathroom all the time, so she would like to try stopping it. You agree and make a plan for her to monitor her weight, watch for edema, and return in 4 weeks for a follow-up visit.
On follow-up, she is feeling well, has no edema on exam, and is happy to report her urinary incontinence has resolved. You therefore suggest her next deprescribing trial be discontinuation of her oxybutynin. She thanks you for your recommendations about her medications and heads off to her physical therapy appointment.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Division of Geriatric Medicine and Palliative Care, Thomas Jefferson University, 2422 S Broad St, 2nd Floor, Philadelphia, PA 19145; Kathryn.mcgrath@jefferson.edu.
1. Bourgeois FT, Shannon MW, Valim C, et al. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19:901-910.
2. Nair NP, Chalmers L, Peterson GM, et al. Hospitalization in older patients due to adverse drug reactions–the need for a prediction tool. Clin Interv Aging. 2016;11:497-506.
3. Nguyen JK, Fouts MM, Kotabe SE, et al. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother. 2006; 4:36-41.
4. Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38:666-671.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213-218.
7. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186.
8. Magaziner J, Cadigan DA, Fedder DO, et al. Medication use and functional decline among community-dwelling older women. J Aging Health. 1989;1:470-484.
9. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
10. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588-595.
11. Weiss BD. Diagnostic evaluation of urinary incontinence in geriatric patients. Am Fam Physician. 1998;57:2675-2694.
12. Syed Q, Hendler KT, Koncilja K. The impact of aging and medical status on dysgeusia. Am J Med. 2016;129:753, E1-E6.
13. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
14. Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: results from the Hispanic EPESE Study. J Gerontol A Biol Sci Med Sci. 2006;61:170-175.
15. Akazawa M, Imai H, Igarashi A, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010; 8:146-160.
16. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-1523.
17. Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473-482.
18. Flaherty JH, Perry HM 3rd, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55:554-559.
19. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
20. Gerteis J, Izrael D, Deitz D, et al. Multiple chronic conditions chartbook. Rockville, MD: Agency for Healthcare Research and Quality. 2014.
21. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60:E1-E25.
22. Woodward M. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33:323-328.
23. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
24. Page AT, Clifford RM, Potter K, et al. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82:583-623.
25. Reeve E, Shakib S, Hendrix I, et al. The benefits and harms of deprescribing. Med J Aust. 2014;201:386-389.
26. Walsh K, Kwan D, Marr P, et al. Deprescribing in a family health team: a study of chronic proton pump inhibitor use. J Prim Health Care. 2016;8:164-171.
27. Orwig D, Brandt N, Gruber-Baldini AL. Medication management assessment for older adults in the community. Gerontologist. 2006;46:661-668.
28. Anderson K, Jue SG, Madaras-Kelly KJ. Identifying patients at risk for medication mismanagement: using cognitive screens to predict a patient’s accuracy in filling a pillbox. Consult Pharm. 2008;23:459-472.
29. Lenaerts E, De Knijf F, Schoenmakers B. Appropriate prescribing for older people: a new tool for the general practitioner. J Frailty & Aging. 2013;2:8-14.
30. Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. IMAJ. 2007;9:430-434.
31. Holmes HM, Todd A. Evidence-based deprescribing of statins in patients with advanced illness. JAMA Intern Med. 2015;175:701-702.
32. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
33. Guirguis-Blake JM, Evans CV,Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
34. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
35. Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing. 2010;39:674-680.
36. Banach M, Serban MC. Discussion around statin discontinuation in older adults and patients with wasting diseases. J Cachexia Sarcopenia Muscle. 2016;7:396-399.
37. Goldstein MR, Mascitelli L, Pezzetta F. Statin therapy in the elderly: misconceptions. J Am Geriatr Soc. 2008;56:1365.
38. Han BH, Sutin D, Williamson JD, et al, for the ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT Randomized Clinical Trial. JAMA Intern Med. Published online May 22, 2017.
39. Sever PS, Chang CL, Gupta AK, et al. The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. Eur Heart J. 2011;32:2525-2532.
40. Denardo SJ, Gong Y, Nichols WW, et al. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719-726.
41. Ekbom T, Lindholm LH, Oden A, et al. A 5‐year prospective, observational study of the withdrawal of antihypertensive treatment in elderly people. J Intern Med. 1994;235:581-588.
42. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. Drugs Aging. 2008;25:1021-1031.
43. Campbell AJ, Robertson MC, Gardner MM, et al. Psychotropic medication withdrawal and a home‐based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47:850-853.
44. Pollmann AS, Murphy AL, Bergman JC, et al. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacol Toxicol. 2015;16:19.
45. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors. Can Fam Phys. 2017; 63:354-364.
46. Duncan P, Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24:37-42.
47. Schuling J, Gebben H, Veehof LJ, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56.
48. Scott I, Anderson K, Freeman CR, et al. First do no harm: a real need to deprescribe in older patients. Med J Aust. 2014;201:390-392.
CASE An 82-year-old woman with a history of hypertension, diabetes, hyperlipidemia, stage 3 chronic kidney disease, anxiety, urge urinary incontinence, constipation, and bilateral knee osteoarthritis presents to her primary care physician’s office after a fall. She reports that she visited the emergency department (ED) a week ago after falling in the middle of the night on her way to the bathroom. This is the third fall she’s had this year. On chart review, she had a blood pressure (BP) of 112/60 mm Hg and a blood glucose level of 65 mg/dL in the ED. All other testing (head imaging, chest x-ray, urinalysis) was normal. The ED physician recommended that she stop taking her lisinopril-hydrochlorothiazide (HCTZ) and glipizide extended release (XL) until her follow-up appointment. Today, she asks about the need to restart these medications.
Polypharmacy is common among older adults due to a high prevalence of chronic conditions that often require multiple medications for optimal management. Cut points of 5 or 9 medications are frequently used to define polypharmacy. However, some define polypharmacy as taking a medication that lacks an indication, is ineffective, or is duplicating treatment provided by another medication.
Either way, polypharmacy is associated with multiple negative consequences, including an increased risk for adverse drug events (ADEs),1-4 drug-drug and drug-disease interactions (TABLE 15,6),7 reduced functional capacity,8 multiple geriatric syndromes (TABLE 25,9-12), medication non-adherence,13 and increased mortality.14 Polypharmacy also contributes to increased health care costs for both the patient and the health care system.15
Taking a step back. Polypharmacy often results from prescribing cascades, which occur when an adverse drug effect is misinterpreted as a new medical problem, leading to the prescribing of more medication to treat the initial drug-induced symptom. Potentially inappropriate medications (PIMs), which are medications that should be avoided in older adults and in those with certain conditions, are also more likely to be prescribed in the setting of polypharmacy.16
Deprescribing is the process of identifying and discontinuing medications that are unnecessary, ineffective, and/or inappropriate in order to reduce polypharmacy and improve health outcomes. Deprescribing is a collaborative process that involves weighing the benefits and harms of medications in the context of a patient’s care goals, current level of functioning, life expectancy, values, and preferences. This article reviews polypharmacy and discusses safe and effective deprescribing strategies for older adults in the primary care setting.
[polldaddy:9781245]
How many people on how many meds?
According to a 2016 study, 36% of community-dwelling older adults (ages 62-85 years) were taking 5 or more prescription medications in 2010 to 2011—up from 31% in 2005 to 2006.17 When one narrows the population to older adults in the United States who are hospitalized, almost half (46%) take 7 or more medications.18 Among frail, older US veterans at hospital discharge, 40% were prescribed 9 or more medications, with 44% of these patients receiving at least one unnecessary drug.19
The challenges of multimorbidity
In the United States, 80% of those 65 and older have 2 or more chronic conditions, or multimorbidity.20 Clinical practice guidelines making recommendations for the management of single conditions, such as heart failure, hypertension, or diabetes, often suggest the use of 2 or more medications to achieve optimal management and fail to provide guidance in the setting of multimorbidity. Following treatment recommendations for multiple conditions predictably leads to polypharmacy, with complicated, costly, and burdensome regimens.
Further, the research contributing to the development of clinical practice guidelines frequently excludes older adults and those with multimorbidity, reducing applicability in this population. As a result, many treatment recommendations have uncertain benefit and may be harmful in the multimorbid older patient.21
CASE In addition to the patient’s multimorbidity, she had a stroke at age 73 and has some mild residual left-sided weakness. Functionally, she is independent and able to perform her activities of daily living and her instrumental activities of daily living. She lives alone, quit smoking at age 65, and has an occasional glass of wine during family parties. The patient’s daughter and granddaughter live 2 blocks away.
Her current medications include glipizide XL 10 mg/d and lisinopril-HCTZ 20-25 mg/d, which she has temporarily discontinued at the ED doctor’s recommendation, as well as: amlodipine 10 mg/d, metformin 1000 mg BID, senna 8.6 mg/d, docusate 100 mg BID, furosemide 40 mg/d, and ibuprofen 600 mg/d (for knee pain). She reports taking omeprazole 20 mg/d “for almost 20 years,” even though she has not had any reflux symptoms in recent memory. After her stroke, she began taking atorvastatin 10 mg/d, aspirin 81 mg/d, and clopidogrel 75 mg/d, which she continues to take today. About a year ago, she started oxybutynin 5 mg/d for urinary incontinence, but she has not noticed significant relief. Additionally, she takes lorazepam 1 mg for insomnia most nights of the week.
A review of systems reveals issues with chronic constipation and intermittent dizziness, but is otherwise negative. The physical examination reveals a well-appearing woman with a body mass index of 26. Her temperature is 98.5° F, her heart rate is 78 beats/min and regular, her respirations are 14 breaths/min, and her BP is 117/65 mm Hg. Orthostatic testing is negative. Her heart, lung, and abdominal exams are within normal limits. Her timed up and go test is 14 seconds. Her blood glucose level today in the office after eating breakfast 2 hours ago is 135 mg/dL (normal: <140 mg/dL). Laboratory tests performed at the time of the ED visit show a creatinine level of 1.2 mg/dL (normal range: 0.6 to 1.1 mg/dL), a glomerular filtration rate (GFR) of 44 units (normal range: >60 units), a hemoglobin level of 9.8 g/dL (normal range: 12-15.5 g/dL), and a thyroid stimulating hormone level of 1.4 mIU/L (normal range: 0.5-8.9 mIU/L). A recent hemoglobin A1C is 6.8% (normal: <5.7%), low-density lipoprotein (LDL) level is 103 mg/dL (optimal <100 mg/dL), and high-density lipoprotein (HDL) level is 65 mg/dL (optimal >60 mg/dL). An echocardiogram performed a year ago showed mild aortic stenosis with normal systolic and diastolic function.
Starting the deprescribing process: Several approaches to choose from
The goal of deprescribing is to reduce polypharmacy and improve health outcomes. It is a process defined as, “reviewing all current medications; identifying medications to be ceased, substituted, or reduced; planning a deprescribing regimen in partnership with the patient; and frequently reviewing and supporting the patient.”22 A medication review should include prescription, over-the-counter (OTC), and complementary/alternative medicine (CAM) agents.
Until recently, studies evaluating the process of deprescribing across drug classes and disease conditions were limited, but new research is beginning to show its potential impact. After deprescribing, patients experience fewer falls and show improvements in cognition.23 While there have not yet been large randomized trials to evaluate deprescribing, a recent systematic review and meta-analysis showed that use of patient-specific deprescribing interventions is associated with improved survival.24 Importantly, there have been no reported adverse drug withdrawal events or deaths associated with deprescribing.23
Smaller studies have reported additional benefits including decreases in health care costs, reductions in drug-drug interactions and PIMs, improvements in medication adherence, and increases in patient satisfaction.25 In addition, the removal of unnecessary medications may allow for increased consideration of prescribing appropriate medications with known benefit.25
Practically speaking, every encounter between a patient and health care provider is an opportunity to reduce unnecessary medications. Electronic alert systems at pharmacies and those embedded within electronic health record (EHR) systems can also prompt a medication review and an effort to deprescribe.26 Evidence-based tools to identify polypharmacy and guide appropriate medication use are listed in TABLE 3.5,6,27-30 In addition, suggested approaches to beginning the deprescribing process are included in TABLE 4.5,31-33 And a medication class-based approach to deprescribing is provided in TABLE 5.5,34-45
Although no gold standard process exists for deprescribing, experts suggest that any deprescribing protocol should include the following steps:32,46
1. Start with a “brown bag” review of the patient’s medications.
Have the patient bring all of his/her medications in a bag to the visit; review them together or have the medication history taken by a pharmacist. Determine and discuss the indication for each medication and its effectiveness for that indication. Consider the potential benefits and harms of each medication in the context of the patient’s care goals and preferences. Assess whether the patient is taking all of the medications that have been prescribed, and identify any reasons for missed pills (eg, adverse effects, dosing regimens, understanding, cognitive issues).
2. Talk to the patient about the deprescribing process.
Talk with the patient about the risks and benefits of deprescribing, and prioritize which medications to address in the process. Prioritize the medications by balancing patient preferences with available pharmacologic evidence. If there is a lack of evidence supporting the benefits for a particular medication, consider known or suspected adverse effects, the ease or burden of the dosing regimen, the patient’s preferences and goals of care, remaining life expectancy, the time until drug benefit is appreciated, and the length of drug benefit after discontinuation.
3. Deprescribe medications.
If you are going to taper a medication, develop a schedule in partnership with the patient. Stop one medication at a time so that you can monitor for withdrawal symptoms or for the return of a condition.
Acknowledging potential barriers to deprescribing may help structure conversations and provide anticipatory guidance to patients and their families. Working to overcome these barriers will help maximize the benefits of deprescribing and help to build trust with patients.
Patient-driven barriers include fear of a condition worsening or returning, lack of a suitable alternative, lack of ongoing support to manage a particular condition, a previous bad experience with medication cessation, and influence from other care providers (eg, family, home caregivers, nurses, specialists, friends). Patients and family members sometimes cling to the hope of future effectiveness of a treatment, especially in the case of medications like donepezil for dementia.47 Utilizing a team-based and stepwise patient approach to deprescribing aims to provide hesitant patients with appropriate amounts of education and support to begin to reduce unnecessary medicines.
Provider-driven barriers include feeling uneasy about contradicting a specialist’s recommendations for initiation/continuation of specific medications, fear of causing withdrawal symptoms or disease relapse, and lack of specific data to adequately understand and assess benefits and harms in the older adult population. Primary care physicians have also acknowledged worry about discussing life expectancy and that patients will feel their care is being reduced or “downgraded.”48 Finally, there is limited time in which these complex shared decision-making conversations can take place. Thus, if medications are not causing a noticeable problem, it is often easier to just continue them.
One way to overcome some of these concerns is to consider working with a clinical pharmacist. By gaining information regarding medication-specific factors, such as half-life and expected withdrawal patterns, you can feel more confident deprescribing or continuing medications.
Additionally, communicating closely with specialists, ideally with the help of an integrated EHR, can allow you to discuss indications for particular medications or concerns about adverse effects, limited benefits, or difficulty with compliance, so that you can develop a collaborative, cohesive, and patient-centered plan. This, in turn, may improve patient understanding and compliance.
4. Create a follow-up plan.
At the time of deprescribing a medication, develop a plan with the patient for monitoring and assessment. Ensure that the patient understands which symptoms may occur in the event of drug withdrawal and which symptoms may suggest the return of a condition. Make sure that other supports are in place if needed (eg, cognitive behavioral therapy, physical therapy, social support or assistance) to help ensure that medication cessation is successful.
CASE During the office visit, you advise the patient that her BP looks normal, her blood sugar is within an appropriate range, and she is lucky to have not sustained any injuries after her most recent fall. In addition to discussing the benefits of some outpatient physical therapy to help with her balance, you ask if she would like to discuss reducing her medications. She is agreeable and asks for your recommendations.
You are aware of several resources that can help you with your recommendations, among them the STOPP/START6 and Beers criteria,5 as well as the Good Geriatric-Palliative Algorithm.30
If you were to use the STOPP/START and Beers criteria, you might consider stopping:
- lorazepam, which increases the risk of falls and confusion.
- ibuprofen, since this patient has only mild osteoarthritis pain, and ibuprofen has the potential for renal, cardiac, and gastrointestinal toxicities.
- oxybutynin, because it could be contributing to the patient’s constipation and cause confusion and falls.
- furosemide, since the patient has no clinical heart failure.
- omeprazole, since the indication is unknown and the patient has no history of ulceration, esophagitis, or symptomatic gastroesophageal reflux disease.
After reviewing the Good Geriatric-Palliative Algorithm,30 you might consider stopping:
- clopidogrel, as there is no clear indication for this medication in combination with aspirin in this patient.
- glipizide XL, as this patient’s A1c is below goal and this medication puts her at risk of hypoglycemia and its associated morbidities.
- metformin, as it increases her risk of lactic acidosis because her GFR is <45 units.
- docusate, as the evidence to show clear benefit in improving chronic constipation in older adults is lacking.
You tell your patient that there are multiple medications to consider stopping. In order to monitor any symptoms of withdrawal or return of a condition, it would be best to stop one at a time and follow-up closely. Since she has done well for the past week without the glipizide and lisinopril-HCTZ combination, she can remain off the glipizide and the HCTZ. Lisinopril, however, may provide renal protection in the setting of diabetes and will be continued at this time.
You ask her about adverse effects from her other medications. She indicates that the furosemide makes her run to the bathroom all the time, so she would like to try stopping it. You agree and make a plan for her to monitor her weight, watch for edema, and return in 4 weeks for a follow-up visit.
On follow-up, she is feeling well, has no edema on exam, and is happy to report her urinary incontinence has resolved. You therefore suggest her next deprescribing trial be discontinuation of her oxybutynin. She thanks you for your recommendations about her medications and heads off to her physical therapy appointment.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Division of Geriatric Medicine and Palliative Care, Thomas Jefferson University, 2422 S Broad St, 2nd Floor, Philadelphia, PA 19145; Kathryn.mcgrath@jefferson.edu.
CASE An 82-year-old woman with a history of hypertension, diabetes, hyperlipidemia, stage 3 chronic kidney disease, anxiety, urge urinary incontinence, constipation, and bilateral knee osteoarthritis presents to her primary care physician’s office after a fall. She reports that she visited the emergency department (ED) a week ago after falling in the middle of the night on her way to the bathroom. This is the third fall she’s had this year. On chart review, she had a blood pressure (BP) of 112/60 mm Hg and a blood glucose level of 65 mg/dL in the ED. All other testing (head imaging, chest x-ray, urinalysis) was normal. The ED physician recommended that she stop taking her lisinopril-hydrochlorothiazide (HCTZ) and glipizide extended release (XL) until her follow-up appointment. Today, she asks about the need to restart these medications.
Polypharmacy is common among older adults due to a high prevalence of chronic conditions that often require multiple medications for optimal management. Cut points of 5 or 9 medications are frequently used to define polypharmacy. However, some define polypharmacy as taking a medication that lacks an indication, is ineffective, or is duplicating treatment provided by another medication.
Either way, polypharmacy is associated with multiple negative consequences, including an increased risk for adverse drug events (ADEs),1-4 drug-drug and drug-disease interactions (TABLE 15,6),7 reduced functional capacity,8 multiple geriatric syndromes (TABLE 25,9-12), medication non-adherence,13 and increased mortality.14 Polypharmacy also contributes to increased health care costs for both the patient and the health care system.15
Taking a step back. Polypharmacy often results from prescribing cascades, which occur when an adverse drug effect is misinterpreted as a new medical problem, leading to the prescribing of more medication to treat the initial drug-induced symptom. Potentially inappropriate medications (PIMs), which are medications that should be avoided in older adults and in those with certain conditions, are also more likely to be prescribed in the setting of polypharmacy.16
Deprescribing is the process of identifying and discontinuing medications that are unnecessary, ineffective, and/or inappropriate in order to reduce polypharmacy and improve health outcomes. Deprescribing is a collaborative process that involves weighing the benefits and harms of medications in the context of a patient’s care goals, current level of functioning, life expectancy, values, and preferences. This article reviews polypharmacy and discusses safe and effective deprescribing strategies for older adults in the primary care setting.
[polldaddy:9781245]
How many people on how many meds?
According to a 2016 study, 36% of community-dwelling older adults (ages 62-85 years) were taking 5 or more prescription medications in 2010 to 2011—up from 31% in 2005 to 2006.17 When one narrows the population to older adults in the United States who are hospitalized, almost half (46%) take 7 or more medications.18 Among frail, older US veterans at hospital discharge, 40% were prescribed 9 or more medications, with 44% of these patients receiving at least one unnecessary drug.19
The challenges of multimorbidity
In the United States, 80% of those 65 and older have 2 or more chronic conditions, or multimorbidity.20 Clinical practice guidelines making recommendations for the management of single conditions, such as heart failure, hypertension, or diabetes, often suggest the use of 2 or more medications to achieve optimal management and fail to provide guidance in the setting of multimorbidity. Following treatment recommendations for multiple conditions predictably leads to polypharmacy, with complicated, costly, and burdensome regimens.
Further, the research contributing to the development of clinical practice guidelines frequently excludes older adults and those with multimorbidity, reducing applicability in this population. As a result, many treatment recommendations have uncertain benefit and may be harmful in the multimorbid older patient.21
CASE In addition to the patient’s multimorbidity, she had a stroke at age 73 and has some mild residual left-sided weakness. Functionally, she is independent and able to perform her activities of daily living and her instrumental activities of daily living. She lives alone, quit smoking at age 65, and has an occasional glass of wine during family parties. The patient’s daughter and granddaughter live 2 blocks away.
Her current medications include glipizide XL 10 mg/d and lisinopril-HCTZ 20-25 mg/d, which she has temporarily discontinued at the ED doctor’s recommendation, as well as: amlodipine 10 mg/d, metformin 1000 mg BID, senna 8.6 mg/d, docusate 100 mg BID, furosemide 40 mg/d, and ibuprofen 600 mg/d (for knee pain). She reports taking omeprazole 20 mg/d “for almost 20 years,” even though she has not had any reflux symptoms in recent memory. After her stroke, she began taking atorvastatin 10 mg/d, aspirin 81 mg/d, and clopidogrel 75 mg/d, which she continues to take today. About a year ago, she started oxybutynin 5 mg/d for urinary incontinence, but she has not noticed significant relief. Additionally, she takes lorazepam 1 mg for insomnia most nights of the week.
A review of systems reveals issues with chronic constipation and intermittent dizziness, but is otherwise negative. The physical examination reveals a well-appearing woman with a body mass index of 26. Her temperature is 98.5° F, her heart rate is 78 beats/min and regular, her respirations are 14 breaths/min, and her BP is 117/65 mm Hg. Orthostatic testing is negative. Her heart, lung, and abdominal exams are within normal limits. Her timed up and go test is 14 seconds. Her blood glucose level today in the office after eating breakfast 2 hours ago is 135 mg/dL (normal: <140 mg/dL). Laboratory tests performed at the time of the ED visit show a creatinine level of 1.2 mg/dL (normal range: 0.6 to 1.1 mg/dL), a glomerular filtration rate (GFR) of 44 units (normal range: >60 units), a hemoglobin level of 9.8 g/dL (normal range: 12-15.5 g/dL), and a thyroid stimulating hormone level of 1.4 mIU/L (normal range: 0.5-8.9 mIU/L). A recent hemoglobin A1C is 6.8% (normal: <5.7%), low-density lipoprotein (LDL) level is 103 mg/dL (optimal <100 mg/dL), and high-density lipoprotein (HDL) level is 65 mg/dL (optimal >60 mg/dL). An echocardiogram performed a year ago showed mild aortic stenosis with normal systolic and diastolic function.
Starting the deprescribing process: Several approaches to choose from
The goal of deprescribing is to reduce polypharmacy and improve health outcomes. It is a process defined as, “reviewing all current medications; identifying medications to be ceased, substituted, or reduced; planning a deprescribing regimen in partnership with the patient; and frequently reviewing and supporting the patient.”22 A medication review should include prescription, over-the-counter (OTC), and complementary/alternative medicine (CAM) agents.
Until recently, studies evaluating the process of deprescribing across drug classes and disease conditions were limited, but new research is beginning to show its potential impact. After deprescribing, patients experience fewer falls and show improvements in cognition.23 While there have not yet been large randomized trials to evaluate deprescribing, a recent systematic review and meta-analysis showed that use of patient-specific deprescribing interventions is associated with improved survival.24 Importantly, there have been no reported adverse drug withdrawal events or deaths associated with deprescribing.23
Smaller studies have reported additional benefits including decreases in health care costs, reductions in drug-drug interactions and PIMs, improvements in medication adherence, and increases in patient satisfaction.25 In addition, the removal of unnecessary medications may allow for increased consideration of prescribing appropriate medications with known benefit.25
Practically speaking, every encounter between a patient and health care provider is an opportunity to reduce unnecessary medications. Electronic alert systems at pharmacies and those embedded within electronic health record (EHR) systems can also prompt a medication review and an effort to deprescribe.26 Evidence-based tools to identify polypharmacy and guide appropriate medication use are listed in TABLE 3.5,6,27-30 In addition, suggested approaches to beginning the deprescribing process are included in TABLE 4.5,31-33 And a medication class-based approach to deprescribing is provided in TABLE 5.5,34-45
Although no gold standard process exists for deprescribing, experts suggest that any deprescribing protocol should include the following steps:32,46
1. Start with a “brown bag” review of the patient’s medications.
Have the patient bring all of his/her medications in a bag to the visit; review them together or have the medication history taken by a pharmacist. Determine and discuss the indication for each medication and its effectiveness for that indication. Consider the potential benefits and harms of each medication in the context of the patient’s care goals and preferences. Assess whether the patient is taking all of the medications that have been prescribed, and identify any reasons for missed pills (eg, adverse effects, dosing regimens, understanding, cognitive issues).
2. Talk to the patient about the deprescribing process.
Talk with the patient about the risks and benefits of deprescribing, and prioritize which medications to address in the process. Prioritize the medications by balancing patient preferences with available pharmacologic evidence. If there is a lack of evidence supporting the benefits for a particular medication, consider known or suspected adverse effects, the ease or burden of the dosing regimen, the patient’s preferences and goals of care, remaining life expectancy, the time until drug benefit is appreciated, and the length of drug benefit after discontinuation.
3. Deprescribe medications.
If you are going to taper a medication, develop a schedule in partnership with the patient. Stop one medication at a time so that you can monitor for withdrawal symptoms or for the return of a condition.
Acknowledging potential barriers to deprescribing may help structure conversations and provide anticipatory guidance to patients and their families. Working to overcome these barriers will help maximize the benefits of deprescribing and help to build trust with patients.
Patient-driven barriers include fear of a condition worsening or returning, lack of a suitable alternative, lack of ongoing support to manage a particular condition, a previous bad experience with medication cessation, and influence from other care providers (eg, family, home caregivers, nurses, specialists, friends). Patients and family members sometimes cling to the hope of future effectiveness of a treatment, especially in the case of medications like donepezil for dementia.47 Utilizing a team-based and stepwise patient approach to deprescribing aims to provide hesitant patients with appropriate amounts of education and support to begin to reduce unnecessary medicines.
Provider-driven barriers include feeling uneasy about contradicting a specialist’s recommendations for initiation/continuation of specific medications, fear of causing withdrawal symptoms or disease relapse, and lack of specific data to adequately understand and assess benefits and harms in the older adult population. Primary care physicians have also acknowledged worry about discussing life expectancy and that patients will feel their care is being reduced or “downgraded.”48 Finally, there is limited time in which these complex shared decision-making conversations can take place. Thus, if medications are not causing a noticeable problem, it is often easier to just continue them.
One way to overcome some of these concerns is to consider working with a clinical pharmacist. By gaining information regarding medication-specific factors, such as half-life and expected withdrawal patterns, you can feel more confident deprescribing or continuing medications.
Additionally, communicating closely with specialists, ideally with the help of an integrated EHR, can allow you to discuss indications for particular medications or concerns about adverse effects, limited benefits, or difficulty with compliance, so that you can develop a collaborative, cohesive, and patient-centered plan. This, in turn, may improve patient understanding and compliance.
4. Create a follow-up plan.
At the time of deprescribing a medication, develop a plan with the patient for monitoring and assessment. Ensure that the patient understands which symptoms may occur in the event of drug withdrawal and which symptoms may suggest the return of a condition. Make sure that other supports are in place if needed (eg, cognitive behavioral therapy, physical therapy, social support or assistance) to help ensure that medication cessation is successful.
CASE During the office visit, you advise the patient that her BP looks normal, her blood sugar is within an appropriate range, and she is lucky to have not sustained any injuries after her most recent fall. In addition to discussing the benefits of some outpatient physical therapy to help with her balance, you ask if she would like to discuss reducing her medications. She is agreeable and asks for your recommendations.
You are aware of several resources that can help you with your recommendations, among them the STOPP/START6 and Beers criteria,5 as well as the Good Geriatric-Palliative Algorithm.30
If you were to use the STOPP/START and Beers criteria, you might consider stopping:
- lorazepam, which increases the risk of falls and confusion.
- ibuprofen, since this patient has only mild osteoarthritis pain, and ibuprofen has the potential for renal, cardiac, and gastrointestinal toxicities.
- oxybutynin, because it could be contributing to the patient’s constipation and cause confusion and falls.
- furosemide, since the patient has no clinical heart failure.
- omeprazole, since the indication is unknown and the patient has no history of ulceration, esophagitis, or symptomatic gastroesophageal reflux disease.
After reviewing the Good Geriatric-Palliative Algorithm,30 you might consider stopping:
- clopidogrel, as there is no clear indication for this medication in combination with aspirin in this patient.
- glipizide XL, as this patient’s A1c is below goal and this medication puts her at risk of hypoglycemia and its associated morbidities.
- metformin, as it increases her risk of lactic acidosis because her GFR is <45 units.
- docusate, as the evidence to show clear benefit in improving chronic constipation in older adults is lacking.
You tell your patient that there are multiple medications to consider stopping. In order to monitor any symptoms of withdrawal or return of a condition, it would be best to stop one at a time and follow-up closely. Since she has done well for the past week without the glipizide and lisinopril-HCTZ combination, she can remain off the glipizide and the HCTZ. Lisinopril, however, may provide renal protection in the setting of diabetes and will be continued at this time.
You ask her about adverse effects from her other medications. She indicates that the furosemide makes her run to the bathroom all the time, so she would like to try stopping it. You agree and make a plan for her to monitor her weight, watch for edema, and return in 4 weeks for a follow-up visit.
On follow-up, she is feeling well, has no edema on exam, and is happy to report her urinary incontinence has resolved. You therefore suggest her next deprescribing trial be discontinuation of her oxybutynin. She thanks you for your recommendations about her medications and heads off to her physical therapy appointment.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Division of Geriatric Medicine and Palliative Care, Thomas Jefferson University, 2422 S Broad St, 2nd Floor, Philadelphia, PA 19145; Kathryn.mcgrath@jefferson.edu.
1. Bourgeois FT, Shannon MW, Valim C, et al. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19:901-910.
2. Nair NP, Chalmers L, Peterson GM, et al. Hospitalization in older patients due to adverse drug reactions–the need for a prediction tool. Clin Interv Aging. 2016;11:497-506.
3. Nguyen JK, Fouts MM, Kotabe SE, et al. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother. 2006; 4:36-41.
4. Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38:666-671.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213-218.
7. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186.
8. Magaziner J, Cadigan DA, Fedder DO, et al. Medication use and functional decline among community-dwelling older women. J Aging Health. 1989;1:470-484.
9. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
10. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588-595.
11. Weiss BD. Diagnostic evaluation of urinary incontinence in geriatric patients. Am Fam Physician. 1998;57:2675-2694.
12. Syed Q, Hendler KT, Koncilja K. The impact of aging and medical status on dysgeusia. Am J Med. 2016;129:753, E1-E6.
13. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
14. Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: results from the Hispanic EPESE Study. J Gerontol A Biol Sci Med Sci. 2006;61:170-175.
15. Akazawa M, Imai H, Igarashi A, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010; 8:146-160.
16. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-1523.
17. Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473-482.
18. Flaherty JH, Perry HM 3rd, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55:554-559.
19. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
20. Gerteis J, Izrael D, Deitz D, et al. Multiple chronic conditions chartbook. Rockville, MD: Agency for Healthcare Research and Quality. 2014.
21. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60:E1-E25.
22. Woodward M. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33:323-328.
23. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
24. Page AT, Clifford RM, Potter K, et al. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82:583-623.
25. Reeve E, Shakib S, Hendrix I, et al. The benefits and harms of deprescribing. Med J Aust. 2014;201:386-389.
26. Walsh K, Kwan D, Marr P, et al. Deprescribing in a family health team: a study of chronic proton pump inhibitor use. J Prim Health Care. 2016;8:164-171.
27. Orwig D, Brandt N, Gruber-Baldini AL. Medication management assessment for older adults in the community. Gerontologist. 2006;46:661-668.
28. Anderson K, Jue SG, Madaras-Kelly KJ. Identifying patients at risk for medication mismanagement: using cognitive screens to predict a patient’s accuracy in filling a pillbox. Consult Pharm. 2008;23:459-472.
29. Lenaerts E, De Knijf F, Schoenmakers B. Appropriate prescribing for older people: a new tool for the general practitioner. J Frailty & Aging. 2013;2:8-14.
30. Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. IMAJ. 2007;9:430-434.
31. Holmes HM, Todd A. Evidence-based deprescribing of statins in patients with advanced illness. JAMA Intern Med. 2015;175:701-702.
32. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
33. Guirguis-Blake JM, Evans CV,Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
34. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
35. Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing. 2010;39:674-680.
36. Banach M, Serban MC. Discussion around statin discontinuation in older adults and patients with wasting diseases. J Cachexia Sarcopenia Muscle. 2016;7:396-399.
37. Goldstein MR, Mascitelli L, Pezzetta F. Statin therapy in the elderly: misconceptions. J Am Geriatr Soc. 2008;56:1365.
38. Han BH, Sutin D, Williamson JD, et al, for the ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT Randomized Clinical Trial. JAMA Intern Med. Published online May 22, 2017.
39. Sever PS, Chang CL, Gupta AK, et al. The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. Eur Heart J. 2011;32:2525-2532.
40. Denardo SJ, Gong Y, Nichols WW, et al. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719-726.
41. Ekbom T, Lindholm LH, Oden A, et al. A 5‐year prospective, observational study of the withdrawal of antihypertensive treatment in elderly people. J Intern Med. 1994;235:581-588.
42. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. Drugs Aging. 2008;25:1021-1031.
43. Campbell AJ, Robertson MC, Gardner MM, et al. Psychotropic medication withdrawal and a home‐based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47:850-853.
44. Pollmann AS, Murphy AL, Bergman JC, et al. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacol Toxicol. 2015;16:19.
45. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors. Can Fam Phys. 2017; 63:354-364.
46. Duncan P, Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24:37-42.
47. Schuling J, Gebben H, Veehof LJ, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56.
48. Scott I, Anderson K, Freeman CR, et al. First do no harm: a real need to deprescribe in older patients. Med J Aust. 2014;201:390-392.
1. Bourgeois FT, Shannon MW, Valim C, et al. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19:901-910.
2. Nair NP, Chalmers L, Peterson GM, et al. Hospitalization in older patients due to adverse drug reactions–the need for a prediction tool. Clin Interv Aging. 2016;11:497-506.
3. Nguyen JK, Fouts MM, Kotabe SE, et al. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother. 2006; 4:36-41.
4. Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38:666-671.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213-218.
7. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186.
8. Magaziner J, Cadigan DA, Fedder DO, et al. Medication use and functional decline among community-dwelling older women. J Aging Health. 1989;1:470-484.
9. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
10. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588-595.
11. Weiss BD. Diagnostic evaluation of urinary incontinence in geriatric patients. Am Fam Physician. 1998;57:2675-2694.
12. Syed Q, Hendler KT, Koncilja K. The impact of aging and medical status on dysgeusia. Am J Med. 2016;129:753, E1-E6.
13. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
14. Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: results from the Hispanic EPESE Study. J Gerontol A Biol Sci Med Sci. 2006;61:170-175.
15. Akazawa M, Imai H, Igarashi A, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010; 8:146-160.
16. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-1523.
17. Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473-482.
18. Flaherty JH, Perry HM 3rd, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55:554-559.
19. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
20. Gerteis J, Izrael D, Deitz D, et al. Multiple chronic conditions chartbook. Rockville, MD: Agency for Healthcare Research and Quality. 2014.
21. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60:E1-E25.
22. Woodward M. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33:323-328.
23. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
24. Page AT, Clifford RM, Potter K, et al. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82:583-623.
25. Reeve E, Shakib S, Hendrix I, et al. The benefits and harms of deprescribing. Med J Aust. 2014;201:386-389.
26. Walsh K, Kwan D, Marr P, et al. Deprescribing in a family health team: a study of chronic proton pump inhibitor use. J Prim Health Care. 2016;8:164-171.
27. Orwig D, Brandt N, Gruber-Baldini AL. Medication management assessment for older adults in the community. Gerontologist. 2006;46:661-668.
28. Anderson K, Jue SG, Madaras-Kelly KJ. Identifying patients at risk for medication mismanagement: using cognitive screens to predict a patient’s accuracy in filling a pillbox. Consult Pharm. 2008;23:459-472.
29. Lenaerts E, De Knijf F, Schoenmakers B. Appropriate prescribing for older people: a new tool for the general practitioner. J Frailty & Aging. 2013;2:8-14.
30. Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. IMAJ. 2007;9:430-434.
31. Holmes HM, Todd A. Evidence-based deprescribing of statins in patients with advanced illness. JAMA Intern Med. 2015;175:701-702.
32. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
33. Guirguis-Blake JM, Evans CV,Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
34. Declercq T, Petrovic M, Azermai M, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2013;3:CD007726.
35. Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing. 2010;39:674-680.
36. Banach M, Serban MC. Discussion around statin discontinuation in older adults and patients with wasting diseases. J Cachexia Sarcopenia Muscle. 2016;7:396-399.
37. Goldstein MR, Mascitelli L, Pezzetta F. Statin therapy in the elderly: misconceptions. J Am Geriatr Soc. 2008;56:1365.
38. Han BH, Sutin D, Williamson JD, et al, for the ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT Randomized Clinical Trial. JAMA Intern Med. Published online May 22, 2017.
39. Sever PS, Chang CL, Gupta AK, et al. The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. Eur Heart J. 2011;32:2525-2532.
40. Denardo SJ, Gong Y, Nichols WW, et al. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719-726.
41. Ekbom T, Lindholm LH, Oden A, et al. A 5‐year prospective, observational study of the withdrawal of antihypertensive treatment in elderly people. J Intern Med. 1994;235:581-588.
42. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. Drugs Aging. 2008;25:1021-1031.
43. Campbell AJ, Robertson MC, Gardner MM, et al. Psychotropic medication withdrawal and a home‐based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47:850-853.
44. Pollmann AS, Murphy AL, Bergman JC, et al. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: a scoping review. BMC Pharmacol Toxicol. 2015;16:19.
45. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors. Can Fam Phys. 2017; 63:354-364.
46. Duncan P, Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24:37-42.
47. Schuling J, Gebben H, Veehof LJ, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56.
48. Scott I, Anderson K, Freeman CR, et al. First do no harm: a real need to deprescribe in older patients. Med J Aust. 2014;201:390-392.
From The Journal of Family Practice | 2017;66(7):436-445.
PRACTICE RECOMMENDATIONS
› Avoid medications that are inappropriate for older adults because of adverse effects, lack of efficacy, and/or potential for interactions. A
› Discontinue medications when the harms outweigh the benefits in the context of the patient’s care goals, life expectancy, and/or preferences. C
› Utilize resources such as the STOPP/START and Beers criteria to help you decide where to begin the deprescribing process. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Strategies to help reduce hospital readmissions
› Use risk stratification methods such as the Probability of Repeated Admission (Pra) or the LACE index to identify patients at high risk for readmission. B
› Take steps to ensure that follow-up appointments are made within the first one to 2 weeks of discharge, depending on the patient’s risk of readmission. C
› Reconcile preadmission and postdischarge medications to identify discrepancies and possible interactions. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Charles T, age 74, has a 3-year history of myocardial infarction (MI) and congestive heart failure (CHF) and a 10-year his-tory of type 2 diabetes with retinopathy. You have cared for him in the outpatient setting for 8 years. You are notified that he is in the emergency department (ED) and being admitted to the hospital, again. This is his third ED visit in the past 3 months; he was hospitalized for 6 days during his last admission 3 weeks ago.
What should you do with this information? How can you best communicate with the admitting team?
Hospital readmissions are widespread, costly, and often avoidable. Nearly 20% of Medicare beneficiaries discharged from hospitals are rehospitalized within 30 days, and 34% are rehospitalized within 90 days.1 For patients with conditions like CHF, the rate of readmission within 30 days approaches 25%.2 The estimated cost to Medicare for unplanned rehospitalizations in 2004 was $17.4 billion.1 The Centers for Medicare and Medicaid Services penalizes hospitals for high rates of readmission within 30 days of discharge for patients with CHF, MI, and pneumonia.
“Avoidable” hospitalizations are those that may be prevented by effective outpatient management and improved care coordination. Although efforts to reduce readmissions have focused on improving the discharge process, family physicians (FPs) can play a central role in reducing readmissions. This article describes key approaches that FPs can take to address this important issue. Because patients ages ≥65 years consistently have the highest rate of hospital readmissions,1 we will focus on this population.
Multiple complex factors are associated with hospital readmissions
Characteristics of the patient, physician, and health care setting contribute to potentially avoidable readmissions (TABLE 1).3,4
Medical conditions and comorbidities associated with high rates of rehospitalization include CHF, acute MI, pneumonia, diabetes, and chronic obstructive pulmonary disease. However, a recent study found that a diverse range of conditions, frequently differing from the index cause of hospitalization, were responsible for 30-day readmissions of Medicare patients.5
Identifying those at high risk: Why and how
Determining which patients are at highest risk for readmission enables health care teams to match the intensity of interventions to the individual’s likelihood of readmission. However, current readmission risk prediction models remain a work in progress6 and few models have been tested in the outpatient setting. Despite numerous limitations, it’s still important to focus resources more efficiently. Thus, we recommend using risk stratification tools to identify patients at high risk for readmission.
Many risk stratification methods use data from electronic medical records (EMRs) and administrative databases or self-reported data from patients.7 Risk prediction tools that are relatively simple and easy to administer or generate through EMRs—such as the Probability of Repeated Admission (Pra),8 the LACE (Length of stay, acuity of the admission, comorbidities, ED visits in the previous 6 months) index,9 or the Community Assessment Risk Screen (CARS)10—may be best for use in the primary care setting. These tools generally identify key risk factors, such as prior health care utilization, presence of specific conditions such as heart disease or cognitive impairment, self-reported health status, absence of a caregiver, and/or need for assistance with daily routines.
Many of these tools have been used to identify high-risk older adults and may not be appropriate for patients who are likely to be readmitted for different reasons, such as mental illness, substance abuse, or chronic pain. Therefore, it is important to use a risk stratification method that captures the issues most likely to cause readmissions in your patient population, or to consider using a variety of methods.
The American Academy of Family Physicians (AAFP) offers resources to help FPs design methods for determining a patient’s health risk status and linking higher levels of risk to increasing care management at http://www.aafp.org/practice-management/pcmh/initiatives/cpci/rscm.html.
CASE › Mr. T has been admitted to the hospital 3 times in the past 3 months, so you use the lace index to evaluate his risk. You determine that Mr. T’s score is 15, which means his expected risk of death or unplanned readmission is 26.6% (TABLE 2).8,11 What are your next steps?
Foster communication between the hospital and outpatient office
Patients are particularly vulnerable during the transition from hospital to home. Delayed or inaccurate information adversely affects continuity of care, patient safety and satisfaction, and efficient use of resources.12 Discharge summaries are the main method of communication between providers, but their content, timeliness, availability, and quality frequently are lacking.13 Discharge summaries are available at only 12% to 34% of first postdischarge visits, and these summaries often lack important information such as diagnostic test results (33%-63%) or discharge medications (2%-40%).12 Although researchers have not consistently found that transferring a discharge summary to an outpatient physician reduces readmission rates, it is likely that direct communication can improve the handoff process independent of its effects on readmissions.12,14
Timely follow-up appointments are essential
Many factors influence the need for rapid follow-up, including disease severity, management complexity, ability of the patient to provide sufficient self-care, and adequacy of social supports.15,16 Studies have found that discharged patients who receive timely outpatient follow-up are less likely to be readmitted.1,17 While the optimal time interval between discharge and the first follow-up appointment is unknown, some literature supports follow-up within 4 weeks.15,18 However, because readmissions often cluster in the first several days or week following discharge,18 follow-up within the first 2 weeks (and within the first week for higher-risk patients) may be appropriate.19 Ideally, follow-up appointments should be scheduled before the patient is discharged. Patients who schedule a follow-up appointment before they are discharged are more likely to make their follow-up visit than those who are asked to call after discharge and schedule their own appointment.12
Employ outpatient follow-up alternatives
Follow-up telephone calls to patients after discharge help patients understand and adhere to discharge instructions and troubleshoot problems. Clinicians who use scripted telephone calls can evaluate symptoms related to the index hospitalization, provide patient education, schedule relevant appointments or testing, and, most importantly, initiate medication reconciliation, which is described at right.20 The FIGURE includes the script we use at our practice.
Home visits may be appropriate for certain patients, including the frail elderly. Home visits allow clinicians to evaluate the patient’s environmental safety, social sup port, and medication adherence.12 Preventive home visits generally have not been found to reduce hospital readmissions, but do enhance patient satisfaction with care.21
Bundled interventions, such as alternating home visits and follow-up telephone calls, may be more effective than individual interventions in reducing readmission.22
Reconciling medications may have far-reaching benefits
Medication discrepancies are observed in up to 70% of all patients at admission or discharge and are associated with adverse drug events (ADEs).23 To prevent ADEs and possibly readmission, take the following steps to reconcile a patient’s medications23:
Obtain a complete list of current medications. Information on all of the patient’s prescription and nonprescription medications should be collected from the patient/caregiver, the discharge summary, prescription bottles, home visits, and pharmacies.12,24
Reconcile preadmission and postdischarge medications. Clarify any discrepancies, review all medications for safety and appropriateness, and, when appropriate, resume any held medications and/or discontinue unnecessary ones.
Research shows that patients who received a phone call from a pharmacist within 3 to 7 days of discharge had lower readmission rates.Enlist pharmacy support. Pharmacists are uniquely positioned to review indications as well as potential duplication and interactions of a patient’s medications. Inpatient studies have demonstrated that partnering with pharmacists results in fewer ADEs.12,25 One study showed that patients at high risk for readmission who received a phone call from a pharmacist 3 to 7 days after discharge had lower readmission rates.26 The pharmacist reconciled the patients’ medications and ensured that patients had a clear understanding of each medication, its common safety concerns, and how often they were supposed to take it.26
Make medication adherence as easy as possible
As many as half of all patients don’t take their medications as prescribed.27 There is limited data on health outcomes associated with medication nonadherence, and existing data frequently are contradictory—some studies have found that as many as 11% of hospital admissions are attributed to nonadherence, while others show no association.28
Factors that affect adherence include psychiatric or cognitive impairment, limited insight into disease process or lack of belief in benefit of treatment, medication cost or adverse effect profile, poor provider-patient relationship, limited access to care or medication, or complexity of treatment.29 To promote medication adherence, consider the following educational and behavioral strategies30:
Identify patients at risk for nonadherence. This includes those with complex regimens and/or uncontrolled disease states or symptoms.
Increase patient communication and counseling. Patient education, particularly on the importance of adherence, is one of the few solo interventions that can improve compliance.31 Involving caregivers and using both verbal and written materials provides additional benefit.31,32
Simplify dosing schedules. Simple, convenient medication regimens may im- prove adherence. For example, adjusting dosing from 3 times a day to once a day can increase adherence from 59% to 83%.33 Aids such as pillboxes to organize medications may be of benefit.29,32
Ensure consistent follow-up. Patients who miss appointments are more likely to be nonadherent. They may benefit from easy access, help with scheduling, and frequent visits.32
Be mindful of patients’ out-of-pocket expenses. Reducing copayments improves adherence rates.30
Minimize polypharmacy. Polypharmacy has been independently associated with nonadherence and increased risk for ADEs.34
Identify patients who have limited health literacy. Limited health literacy may be linked to increased medication errors and nonadherence.12,35 Patients with low health literacy may be unable to identify medications recorded in their medical record. TABLE W336-41 outlines strategies for identifying patients with low health literacy and improving communication with them.
CASE › By speaking with hospital staff before Mr. T is discharged, you are able to confirm that he has scheduled a follow-up visit with you for one week after discharge, and that a discharge summary will be available for him to bring to that visit. Mr. T brings his discharge summary with him to your office, and you reconcile his medication list. Because he is your last patient of the day, you have some time to sit with him and his wife to explore his goals of care.
Improve care—and possibly reduce readmissions—through goal setting
Goal setting is an important element of postdischarge follow-up, particularly for elderly patients and those with progressive or end-stage diseases. Goal setting can improve patient care by linking care plans with desired outcomes and keeping diagnostic and therapeutic interventions relevant to the patient.42 A patient who understands the purpose of a recommendation—especially when directly linked to a patient-derived goal—may be more likely to adhere to the plan of care.
Asking patients to articulate their goals of care using “Ask-Tell-Ask” framework described in TABLE W336-41 will allow you to deliver the prognosis, reinforce treatment options to achieve patient-specific goals, empower patients to assert their preferences, and develop a follow-up plan to see if treatment is successful.
Empowering patients
Consider using both verbal and written approaches when educating patients about self-care behaviors such as monitoring symptoms and adhering to dietary/behavior restrictions and medication instructions. One study showed that a brief one-on-one patient education session decreased readmissions in patients with heart failure,43 although another study found that patient education alone yielded a nonsignificant decrease.44
Providing caregivers with education and support is a critical and perhaps overlooked opportunity to reduce readmissions.45 Involving key family members in discharge planning, preparation, follow-up, and ongoing management is essential in caring for patients with functional deficits and/or complex care needs. Educating caregivers can help them feel more prepared and effective in their roles.
Establish an “action plan.” For patients with chronic, periodically symptomatic diseases such as asthma and heart failure, action planning can be useful. Action plans should include information that reinforces patients’ daily self-care behaviors and instructions for what to do if symptoms get worse. Action planning also might include simple if-then plans (“if x happens, then I will do y”), which can help with problem solving for common scenarios. Action plans have been shown to reduce admissions for children with asthma46 and adults with heart failure when coupled with home monitoring or telephone support from a registered nurse.16,47
Generate an individualized care plan for each patient, taking into account your patient’s health literacy, goals of care, and level of social support. This care plan may include educational and behavioral interventions, action planning, and follow-up plans. Most successful approaches to reducing readmissions have included both system-level and patient-level interventions that use an interdisciplinary team of providers.48
Make the most of follow-up visits. The traditional 15-minute FP visit can make it challenging to provide the level of care necessary for recently discharged patients. Multiple models of team-based care have been proposed to improve this situation, including using the “teamlet” model, which may include a clinician and one or 2 health coaches.49 During each visit, the health coaches—often medical assistants trained in chronic disease self-management skills—see patients before and after the physician. They also contact patients be- tween visits to facilitate action planning and to promote self-management.
Palliative care programs: A resource for FPs
The growth of palliative care programs in US hospitals has helped increase the emphasis on establishing goals of care. Inpatient-based palliative care consultation programs work with patients and families to establish goals. However, after discharge, many of these goals and plans begin to unravel due to gaps in the current health care model, including lack of follow-up and support.50 Outpatient palliative care programs have begun to address these gaps in care.50 Comprehensive palliative care programs are quickly becoming an important resource for FPs to help address transitional care issues.
CASE › When you ask Mr. and Mrs. T about his goals for treatment, they say are getting tired of the “back and forth” to the hospital. After discussing his lengthy history of worsening CHF and diabetes, you raise the idea of palliative care, including hospice, with the couple. They acknowledge that they have had family members get hospice care, and they are open to it—just not yet. The 3 of you craft an “if-then” plan of care to use at home. You schedule a 2-week follow-up visit and remind Mr. T and his wife of your office’s 24-hour on-call service.
CORRESPONDENCE
Danielle Snyderman, MD, Department of Family and Community Medicine, Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; danielle.snyderman@jefferson.edu
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428
2. O’Connor CM, Miller AB, Blair JE, et al; Efficacy of Vasopressin Antagonism in heart Failure Outcome Study with Tolvaptan (EVEREST) investigators. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduce left ventricular ejection fraction; results from EVEREST program. Am Heart J. 2010;159:841-849.e1.
3. Garrison GM, Mansukhani MP, Bohn B. Predictors of thirty-day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71-77.
4. Boult C, Dowd B, McCaffrey D, et al. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41:811-817.
5. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
6. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688-1698.
7. Haas LR, Takahashi PY, Shah ND, et al. Risk-stratification methods for identifying patients for care coordination. Am J Manag Care. 2013;19:725-732.
8. Wallace E, Hinchey T, Dimitrov BD, et al. A systematic review of the probability of repeated admission score in community-dwelling adults. J Am Geriatr Soc. 2013;61:357-364.
9. Cotter PE, Bhalla VK, Wallis SJ, et al. Predicting readmissions: poor performance of the LACE index in an older UK population. Age Ageing. 2012;41:784-789.
10. Shelton P, Sager MA, Schraeder C. The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit. Am J Manag Care. 2000;6:925-933.
11. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373-383.
12. Kripalani S, Jackson AT, Schnipper JL, et al. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314-323.
13. Kim CS, Flanders SA. In the clinic. Transitions of care. Ann Intern Med. 2013;158(5 pt 1):ITC3-1.
14. Hansen LO, Strater A, Smith L, et al. Hospital discharge documentation and risk of rehospitalisation. BMJ Qual Saf. 2011;20:773-778.
15. Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345-346.
16. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520-528.
17. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397.
18. van Walraven C, Jennings A, Taljaard M, et al. Incidence of potentially avoidable urgent readmissions and their relation to all-cause urgent readmissions. CMAJ. 2011;183:E1067-E1072.
19. Tang, N. A primary care physician’s ideal transitions of care—where’s the evidence? J Hosp Med. 2013;8:472-477.
20. Crocker JB, Crocker JT, Greenwald JL. Telephone follow-up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125:915-921.
21. Wong FK, Chow S, Chung L, et al. Can home visits help reduce hospital readmissions? Randomized controlled trial. J Adv Nurs. 2008;62:585-595.
22. Wong FK, Chow SK, Chan TM, et al. Comparison of effects between home visits with telephone calls and telephone calls only for transitional discharge support: a randomised controlled trial. Age Ageing. 2014;43:91-97.
23. Mueller SK, Sponsler KC, Kripalani S, et al. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172:1057-1069.
24. Glintborg B, Andersen SE, Dalhoff K. Insufficient communication about medication use at the interface between hospital and primary care. Qual Saf Health Care. 2007;16:34-39.
25. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
26. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc (2003). 2013;53:78-84.
27. Vermeire E, Hearnshaw H, Van Royen P, et al. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331-342.
28. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
29. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
30. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157:785-795.
31. McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868-2879.
32. Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540-550.
33. Eisen SA, Miller DK, Woodward RS, et al. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150:1881-1884.
34. Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629-1634.
35. Persell SD, Osborn CY, Richard R, et al. Limited health literacy is a barrier to medication reconciliation in ambulatory care. J Gen Intern Med. 2007;22:1523-1526.
36. Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. Manual for Clinicians. Chicago, IL: American Medical Association Foundation; 2007.
37. Chew LD, Bradley KA, Bokyo EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588-594.
38. Wallace LS, Rogers ES, Roskos SE, et al. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877.
39. Doak CC, Doak LG, Root JH. Teaching Patients with Low Literacy Skills. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1996.
40. Back AL, Arnold RM, Baile WF, et al. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55: 164-177.
41. Doak LG, Doak CC, eds. Pfizer Principles for Clear Health Communication: A Handbook for Creating Patient Education Materials that Enhance Understanding and Promote Health Outcomes. 2nd ed. New York, NY: Pfizer; 2004.
42. Bradley EH, Bogardus ST Jr, Tinetti M, et al. Goal-setting in clinical medicine. Soc Sci Med. 1999;49:267-278.
43. Koelling TM, Johnson ML, Cody RJ, et al. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179-185.
44. Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83-89.
45. Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med. 2013;173:695-698.
46. Kessler KR. Relationship between the use of asthma action plans and asthma exacerbations in children with asthma: A systematic review. J Asthma Allergy Educators. 2011;2:11-21.
47. Maric B, Kaan A, Ignaszewski A, et al. A systematic review of telemonitoring technologies in heart failure. Eur J Heart Fail. 2009;11:506-517.
48. Boutwell A, Hwu S. Effective Interventions to Reduce Rehospitalizations: A Survey of the Published Evidence. Cambridge, MA: Institute for Healthcare Improvement; 2009.
49. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
50. Meier D, Beresford L. Outpatient clinics are a new frontier for palliative care. J Pall Med. 2008;11:823-828.
› Use risk stratification methods such as the Probability of Repeated Admission (Pra) or the LACE index to identify patients at high risk for readmission. B
› Take steps to ensure that follow-up appointments are made within the first one to 2 weeks of discharge, depending on the patient’s risk of readmission. C
› Reconcile preadmission and postdischarge medications to identify discrepancies and possible interactions. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Charles T, age 74, has a 3-year history of myocardial infarction (MI) and congestive heart failure (CHF) and a 10-year his-tory of type 2 diabetes with retinopathy. You have cared for him in the outpatient setting for 8 years. You are notified that he is in the emergency department (ED) and being admitted to the hospital, again. This is his third ED visit in the past 3 months; he was hospitalized for 6 days during his last admission 3 weeks ago.
What should you do with this information? How can you best communicate with the admitting team?
Hospital readmissions are widespread, costly, and often avoidable. Nearly 20% of Medicare beneficiaries discharged from hospitals are rehospitalized within 30 days, and 34% are rehospitalized within 90 days.1 For patients with conditions like CHF, the rate of readmission within 30 days approaches 25%.2 The estimated cost to Medicare for unplanned rehospitalizations in 2004 was $17.4 billion.1 The Centers for Medicare and Medicaid Services penalizes hospitals for high rates of readmission within 30 days of discharge for patients with CHF, MI, and pneumonia.
“Avoidable” hospitalizations are those that may be prevented by effective outpatient management and improved care coordination. Although efforts to reduce readmissions have focused on improving the discharge process, family physicians (FPs) can play a central role in reducing readmissions. This article describes key approaches that FPs can take to address this important issue. Because patients ages ≥65 years consistently have the highest rate of hospital readmissions,1 we will focus on this population.
Multiple complex factors are associated with hospital readmissions
Characteristics of the patient, physician, and health care setting contribute to potentially avoidable readmissions (TABLE 1).3,4
Medical conditions and comorbidities associated with high rates of rehospitalization include CHF, acute MI, pneumonia, diabetes, and chronic obstructive pulmonary disease. However, a recent study found that a diverse range of conditions, frequently differing from the index cause of hospitalization, were responsible for 30-day readmissions of Medicare patients.5
Identifying those at high risk: Why and how
Determining which patients are at highest risk for readmission enables health care teams to match the intensity of interventions to the individual’s likelihood of readmission. However, current readmission risk prediction models remain a work in progress6 and few models have been tested in the outpatient setting. Despite numerous limitations, it’s still important to focus resources more efficiently. Thus, we recommend using risk stratification tools to identify patients at high risk for readmission.
Many risk stratification methods use data from electronic medical records (EMRs) and administrative databases or self-reported data from patients.7 Risk prediction tools that are relatively simple and easy to administer or generate through EMRs—such as the Probability of Repeated Admission (Pra),8 the LACE (Length of stay, acuity of the admission, comorbidities, ED visits in the previous 6 months) index,9 or the Community Assessment Risk Screen (CARS)10—may be best for use in the primary care setting. These tools generally identify key risk factors, such as prior health care utilization, presence of specific conditions such as heart disease or cognitive impairment, self-reported health status, absence of a caregiver, and/or need for assistance with daily routines.
Many of these tools have been used to identify high-risk older adults and may not be appropriate for patients who are likely to be readmitted for different reasons, such as mental illness, substance abuse, or chronic pain. Therefore, it is important to use a risk stratification method that captures the issues most likely to cause readmissions in your patient population, or to consider using a variety of methods.
The American Academy of Family Physicians (AAFP) offers resources to help FPs design methods for determining a patient’s health risk status and linking higher levels of risk to increasing care management at http://www.aafp.org/practice-management/pcmh/initiatives/cpci/rscm.html.
CASE › Mr. T has been admitted to the hospital 3 times in the past 3 months, so you use the lace index to evaluate his risk. You determine that Mr. T’s score is 15, which means his expected risk of death or unplanned readmission is 26.6% (TABLE 2).8,11 What are your next steps?
Foster communication between the hospital and outpatient office
Patients are particularly vulnerable during the transition from hospital to home. Delayed or inaccurate information adversely affects continuity of care, patient safety and satisfaction, and efficient use of resources.12 Discharge summaries are the main method of communication between providers, but their content, timeliness, availability, and quality frequently are lacking.13 Discharge summaries are available at only 12% to 34% of first postdischarge visits, and these summaries often lack important information such as diagnostic test results (33%-63%) or discharge medications (2%-40%).12 Although researchers have not consistently found that transferring a discharge summary to an outpatient physician reduces readmission rates, it is likely that direct communication can improve the handoff process independent of its effects on readmissions.12,14
Timely follow-up appointments are essential
Many factors influence the need for rapid follow-up, including disease severity, management complexity, ability of the patient to provide sufficient self-care, and adequacy of social supports.15,16 Studies have found that discharged patients who receive timely outpatient follow-up are less likely to be readmitted.1,17 While the optimal time interval between discharge and the first follow-up appointment is unknown, some literature supports follow-up within 4 weeks.15,18 However, because readmissions often cluster in the first several days or week following discharge,18 follow-up within the first 2 weeks (and within the first week for higher-risk patients) may be appropriate.19 Ideally, follow-up appointments should be scheduled before the patient is discharged. Patients who schedule a follow-up appointment before they are discharged are more likely to make their follow-up visit than those who are asked to call after discharge and schedule their own appointment.12
Employ outpatient follow-up alternatives
Follow-up telephone calls to patients after discharge help patients understand and adhere to discharge instructions and troubleshoot problems. Clinicians who use scripted telephone calls can evaluate symptoms related to the index hospitalization, provide patient education, schedule relevant appointments or testing, and, most importantly, initiate medication reconciliation, which is described at right.20 The FIGURE includes the script we use at our practice.
Home visits may be appropriate for certain patients, including the frail elderly. Home visits allow clinicians to evaluate the patient’s environmental safety, social sup port, and medication adherence.12 Preventive home visits generally have not been found to reduce hospital readmissions, but do enhance patient satisfaction with care.21
Bundled interventions, such as alternating home visits and follow-up telephone calls, may be more effective than individual interventions in reducing readmission.22
Reconciling medications may have far-reaching benefits
Medication discrepancies are observed in up to 70% of all patients at admission or discharge and are associated with adverse drug events (ADEs).23 To prevent ADEs and possibly readmission, take the following steps to reconcile a patient’s medications23:
Obtain a complete list of current medications. Information on all of the patient’s prescription and nonprescription medications should be collected from the patient/caregiver, the discharge summary, prescription bottles, home visits, and pharmacies.12,24
Reconcile preadmission and postdischarge medications. Clarify any discrepancies, review all medications for safety and appropriateness, and, when appropriate, resume any held medications and/or discontinue unnecessary ones.
Research shows that patients who received a phone call from a pharmacist within 3 to 7 days of discharge had lower readmission rates.Enlist pharmacy support. Pharmacists are uniquely positioned to review indications as well as potential duplication and interactions of a patient’s medications. Inpatient studies have demonstrated that partnering with pharmacists results in fewer ADEs.12,25 One study showed that patients at high risk for readmission who received a phone call from a pharmacist 3 to 7 days after discharge had lower readmission rates.26 The pharmacist reconciled the patients’ medications and ensured that patients had a clear understanding of each medication, its common safety concerns, and how often they were supposed to take it.26
Make medication adherence as easy as possible
As many as half of all patients don’t take their medications as prescribed.27 There is limited data on health outcomes associated with medication nonadherence, and existing data frequently are contradictory—some studies have found that as many as 11% of hospital admissions are attributed to nonadherence, while others show no association.28
Factors that affect adherence include psychiatric or cognitive impairment, limited insight into disease process or lack of belief in benefit of treatment, medication cost or adverse effect profile, poor provider-patient relationship, limited access to care or medication, or complexity of treatment.29 To promote medication adherence, consider the following educational and behavioral strategies30:
Identify patients at risk for nonadherence. This includes those with complex regimens and/or uncontrolled disease states or symptoms.
Increase patient communication and counseling. Patient education, particularly on the importance of adherence, is one of the few solo interventions that can improve compliance.31 Involving caregivers and using both verbal and written materials provides additional benefit.31,32
Simplify dosing schedules. Simple, convenient medication regimens may im- prove adherence. For example, adjusting dosing from 3 times a day to once a day can increase adherence from 59% to 83%.33 Aids such as pillboxes to organize medications may be of benefit.29,32
Ensure consistent follow-up. Patients who miss appointments are more likely to be nonadherent. They may benefit from easy access, help with scheduling, and frequent visits.32
Be mindful of patients’ out-of-pocket expenses. Reducing copayments improves adherence rates.30
Minimize polypharmacy. Polypharmacy has been independently associated with nonadherence and increased risk for ADEs.34
Identify patients who have limited health literacy. Limited health literacy may be linked to increased medication errors and nonadherence.12,35 Patients with low health literacy may be unable to identify medications recorded in their medical record. TABLE W336-41 outlines strategies for identifying patients with low health literacy and improving communication with them.
CASE › By speaking with hospital staff before Mr. T is discharged, you are able to confirm that he has scheduled a follow-up visit with you for one week after discharge, and that a discharge summary will be available for him to bring to that visit. Mr. T brings his discharge summary with him to your office, and you reconcile his medication list. Because he is your last patient of the day, you have some time to sit with him and his wife to explore his goals of care.
Improve care—and possibly reduce readmissions—through goal setting
Goal setting is an important element of postdischarge follow-up, particularly for elderly patients and those with progressive or end-stage diseases. Goal setting can improve patient care by linking care plans with desired outcomes and keeping diagnostic and therapeutic interventions relevant to the patient.42 A patient who understands the purpose of a recommendation—especially when directly linked to a patient-derived goal—may be more likely to adhere to the plan of care.
Asking patients to articulate their goals of care using “Ask-Tell-Ask” framework described in TABLE W336-41 will allow you to deliver the prognosis, reinforce treatment options to achieve patient-specific goals, empower patients to assert their preferences, and develop a follow-up plan to see if treatment is successful.
Empowering patients
Consider using both verbal and written approaches when educating patients about self-care behaviors such as monitoring symptoms and adhering to dietary/behavior restrictions and medication instructions. One study showed that a brief one-on-one patient education session decreased readmissions in patients with heart failure,43 although another study found that patient education alone yielded a nonsignificant decrease.44
Providing caregivers with education and support is a critical and perhaps overlooked opportunity to reduce readmissions.45 Involving key family members in discharge planning, preparation, follow-up, and ongoing management is essential in caring for patients with functional deficits and/or complex care needs. Educating caregivers can help them feel more prepared and effective in their roles.
Establish an “action plan.” For patients with chronic, periodically symptomatic diseases such as asthma and heart failure, action planning can be useful. Action plans should include information that reinforces patients’ daily self-care behaviors and instructions for what to do if symptoms get worse. Action planning also might include simple if-then plans (“if x happens, then I will do y”), which can help with problem solving for common scenarios. Action plans have been shown to reduce admissions for children with asthma46 and adults with heart failure when coupled with home monitoring or telephone support from a registered nurse.16,47
Generate an individualized care plan for each patient, taking into account your patient’s health literacy, goals of care, and level of social support. This care plan may include educational and behavioral interventions, action planning, and follow-up plans. Most successful approaches to reducing readmissions have included both system-level and patient-level interventions that use an interdisciplinary team of providers.48
Make the most of follow-up visits. The traditional 15-minute FP visit can make it challenging to provide the level of care necessary for recently discharged patients. Multiple models of team-based care have been proposed to improve this situation, including using the “teamlet” model, which may include a clinician and one or 2 health coaches.49 During each visit, the health coaches—often medical assistants trained in chronic disease self-management skills—see patients before and after the physician. They also contact patients be- tween visits to facilitate action planning and to promote self-management.
Palliative care programs: A resource for FPs
The growth of palliative care programs in US hospitals has helped increase the emphasis on establishing goals of care. Inpatient-based palliative care consultation programs work with patients and families to establish goals. However, after discharge, many of these goals and plans begin to unravel due to gaps in the current health care model, including lack of follow-up and support.50 Outpatient palliative care programs have begun to address these gaps in care.50 Comprehensive palliative care programs are quickly becoming an important resource for FPs to help address transitional care issues.
CASE › When you ask Mr. and Mrs. T about his goals for treatment, they say are getting tired of the “back and forth” to the hospital. After discussing his lengthy history of worsening CHF and diabetes, you raise the idea of palliative care, including hospice, with the couple. They acknowledge that they have had family members get hospice care, and they are open to it—just not yet. The 3 of you craft an “if-then” plan of care to use at home. You schedule a 2-week follow-up visit and remind Mr. T and his wife of your office’s 24-hour on-call service.
CORRESPONDENCE
Danielle Snyderman, MD, Department of Family and Community Medicine, Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; danielle.snyderman@jefferson.edu
› Use risk stratification methods such as the Probability of Repeated Admission (Pra) or the LACE index to identify patients at high risk for readmission. B
› Take steps to ensure that follow-up appointments are made within the first one to 2 weeks of discharge, depending on the patient’s risk of readmission. C
› Reconcile preadmission and postdischarge medications to identify discrepancies and possible interactions. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Charles T, age 74, has a 3-year history of myocardial infarction (MI) and congestive heart failure (CHF) and a 10-year his-tory of type 2 diabetes with retinopathy. You have cared for him in the outpatient setting for 8 years. You are notified that he is in the emergency department (ED) and being admitted to the hospital, again. This is his third ED visit in the past 3 months; he was hospitalized for 6 days during his last admission 3 weeks ago.
What should you do with this information? How can you best communicate with the admitting team?
Hospital readmissions are widespread, costly, and often avoidable. Nearly 20% of Medicare beneficiaries discharged from hospitals are rehospitalized within 30 days, and 34% are rehospitalized within 90 days.1 For patients with conditions like CHF, the rate of readmission within 30 days approaches 25%.2 The estimated cost to Medicare for unplanned rehospitalizations in 2004 was $17.4 billion.1 The Centers for Medicare and Medicaid Services penalizes hospitals for high rates of readmission within 30 days of discharge for patients with CHF, MI, and pneumonia.
“Avoidable” hospitalizations are those that may be prevented by effective outpatient management and improved care coordination. Although efforts to reduce readmissions have focused on improving the discharge process, family physicians (FPs) can play a central role in reducing readmissions. This article describes key approaches that FPs can take to address this important issue. Because patients ages ≥65 years consistently have the highest rate of hospital readmissions,1 we will focus on this population.
Multiple complex factors are associated with hospital readmissions
Characteristics of the patient, physician, and health care setting contribute to potentially avoidable readmissions (TABLE 1).3,4
Medical conditions and comorbidities associated with high rates of rehospitalization include CHF, acute MI, pneumonia, diabetes, and chronic obstructive pulmonary disease. However, a recent study found that a diverse range of conditions, frequently differing from the index cause of hospitalization, were responsible for 30-day readmissions of Medicare patients.5
Identifying those at high risk: Why and how
Determining which patients are at highest risk for readmission enables health care teams to match the intensity of interventions to the individual’s likelihood of readmission. However, current readmission risk prediction models remain a work in progress6 and few models have been tested in the outpatient setting. Despite numerous limitations, it’s still important to focus resources more efficiently. Thus, we recommend using risk stratification tools to identify patients at high risk for readmission.
Many risk stratification methods use data from electronic medical records (EMRs) and administrative databases or self-reported data from patients.7 Risk prediction tools that are relatively simple and easy to administer or generate through EMRs—such as the Probability of Repeated Admission (Pra),8 the LACE (Length of stay, acuity of the admission, comorbidities, ED visits in the previous 6 months) index,9 or the Community Assessment Risk Screen (CARS)10—may be best for use in the primary care setting. These tools generally identify key risk factors, such as prior health care utilization, presence of specific conditions such as heart disease or cognitive impairment, self-reported health status, absence of a caregiver, and/or need for assistance with daily routines.
Many of these tools have been used to identify high-risk older adults and may not be appropriate for patients who are likely to be readmitted for different reasons, such as mental illness, substance abuse, or chronic pain. Therefore, it is important to use a risk stratification method that captures the issues most likely to cause readmissions in your patient population, or to consider using a variety of methods.
The American Academy of Family Physicians (AAFP) offers resources to help FPs design methods for determining a patient’s health risk status and linking higher levels of risk to increasing care management at http://www.aafp.org/practice-management/pcmh/initiatives/cpci/rscm.html.
CASE › Mr. T has been admitted to the hospital 3 times in the past 3 months, so you use the lace index to evaluate his risk. You determine that Mr. T’s score is 15, which means his expected risk of death or unplanned readmission is 26.6% (TABLE 2).8,11 What are your next steps?
Foster communication between the hospital and outpatient office
Patients are particularly vulnerable during the transition from hospital to home. Delayed or inaccurate information adversely affects continuity of care, patient safety and satisfaction, and efficient use of resources.12 Discharge summaries are the main method of communication between providers, but their content, timeliness, availability, and quality frequently are lacking.13 Discharge summaries are available at only 12% to 34% of first postdischarge visits, and these summaries often lack important information such as diagnostic test results (33%-63%) or discharge medications (2%-40%).12 Although researchers have not consistently found that transferring a discharge summary to an outpatient physician reduces readmission rates, it is likely that direct communication can improve the handoff process independent of its effects on readmissions.12,14
Timely follow-up appointments are essential
Many factors influence the need for rapid follow-up, including disease severity, management complexity, ability of the patient to provide sufficient self-care, and adequacy of social supports.15,16 Studies have found that discharged patients who receive timely outpatient follow-up are less likely to be readmitted.1,17 While the optimal time interval between discharge and the first follow-up appointment is unknown, some literature supports follow-up within 4 weeks.15,18 However, because readmissions often cluster in the first several days or week following discharge,18 follow-up within the first 2 weeks (and within the first week for higher-risk patients) may be appropriate.19 Ideally, follow-up appointments should be scheduled before the patient is discharged. Patients who schedule a follow-up appointment before they are discharged are more likely to make their follow-up visit than those who are asked to call after discharge and schedule their own appointment.12
Employ outpatient follow-up alternatives
Follow-up telephone calls to patients after discharge help patients understand and adhere to discharge instructions and troubleshoot problems. Clinicians who use scripted telephone calls can evaluate symptoms related to the index hospitalization, provide patient education, schedule relevant appointments or testing, and, most importantly, initiate medication reconciliation, which is described at right.20 The FIGURE includes the script we use at our practice.
Home visits may be appropriate for certain patients, including the frail elderly. Home visits allow clinicians to evaluate the patient’s environmental safety, social sup port, and medication adherence.12 Preventive home visits generally have not been found to reduce hospital readmissions, but do enhance patient satisfaction with care.21
Bundled interventions, such as alternating home visits and follow-up telephone calls, may be more effective than individual interventions in reducing readmission.22
Reconciling medications may have far-reaching benefits
Medication discrepancies are observed in up to 70% of all patients at admission or discharge and are associated with adverse drug events (ADEs).23 To prevent ADEs and possibly readmission, take the following steps to reconcile a patient’s medications23:
Obtain a complete list of current medications. Information on all of the patient’s prescription and nonprescription medications should be collected from the patient/caregiver, the discharge summary, prescription bottles, home visits, and pharmacies.12,24
Reconcile preadmission and postdischarge medications. Clarify any discrepancies, review all medications for safety and appropriateness, and, when appropriate, resume any held medications and/or discontinue unnecessary ones.
Research shows that patients who received a phone call from a pharmacist within 3 to 7 days of discharge had lower readmission rates.Enlist pharmacy support. Pharmacists are uniquely positioned to review indications as well as potential duplication and interactions of a patient’s medications. Inpatient studies have demonstrated that partnering with pharmacists results in fewer ADEs.12,25 One study showed that patients at high risk for readmission who received a phone call from a pharmacist 3 to 7 days after discharge had lower readmission rates.26 The pharmacist reconciled the patients’ medications and ensured that patients had a clear understanding of each medication, its common safety concerns, and how often they were supposed to take it.26
Make medication adherence as easy as possible
As many as half of all patients don’t take their medications as prescribed.27 There is limited data on health outcomes associated with medication nonadherence, and existing data frequently are contradictory—some studies have found that as many as 11% of hospital admissions are attributed to nonadherence, while others show no association.28
Factors that affect adherence include psychiatric or cognitive impairment, limited insight into disease process or lack of belief in benefit of treatment, medication cost or adverse effect profile, poor provider-patient relationship, limited access to care or medication, or complexity of treatment.29 To promote medication adherence, consider the following educational and behavioral strategies30:
Identify patients at risk for nonadherence. This includes those with complex regimens and/or uncontrolled disease states or symptoms.
Increase patient communication and counseling. Patient education, particularly on the importance of adherence, is one of the few solo interventions that can improve compliance.31 Involving caregivers and using both verbal and written materials provides additional benefit.31,32
Simplify dosing schedules. Simple, convenient medication regimens may im- prove adherence. For example, adjusting dosing from 3 times a day to once a day can increase adherence from 59% to 83%.33 Aids such as pillboxes to organize medications may be of benefit.29,32
Ensure consistent follow-up. Patients who miss appointments are more likely to be nonadherent. They may benefit from easy access, help with scheduling, and frequent visits.32
Be mindful of patients’ out-of-pocket expenses. Reducing copayments improves adherence rates.30
Minimize polypharmacy. Polypharmacy has been independently associated with nonadherence and increased risk for ADEs.34
Identify patients who have limited health literacy. Limited health literacy may be linked to increased medication errors and nonadherence.12,35 Patients with low health literacy may be unable to identify medications recorded in their medical record. TABLE W336-41 outlines strategies for identifying patients with low health literacy and improving communication with them.
CASE › By speaking with hospital staff before Mr. T is discharged, you are able to confirm that he has scheduled a follow-up visit with you for one week after discharge, and that a discharge summary will be available for him to bring to that visit. Mr. T brings his discharge summary with him to your office, and you reconcile his medication list. Because he is your last patient of the day, you have some time to sit with him and his wife to explore his goals of care.
Improve care—and possibly reduce readmissions—through goal setting
Goal setting is an important element of postdischarge follow-up, particularly for elderly patients and those with progressive or end-stage diseases. Goal setting can improve patient care by linking care plans with desired outcomes and keeping diagnostic and therapeutic interventions relevant to the patient.42 A patient who understands the purpose of a recommendation—especially when directly linked to a patient-derived goal—may be more likely to adhere to the plan of care.
Asking patients to articulate their goals of care using “Ask-Tell-Ask” framework described in TABLE W336-41 will allow you to deliver the prognosis, reinforce treatment options to achieve patient-specific goals, empower patients to assert their preferences, and develop a follow-up plan to see if treatment is successful.
Empowering patients
Consider using both verbal and written approaches when educating patients about self-care behaviors such as monitoring symptoms and adhering to dietary/behavior restrictions and medication instructions. One study showed that a brief one-on-one patient education session decreased readmissions in patients with heart failure,43 although another study found that patient education alone yielded a nonsignificant decrease.44
Providing caregivers with education and support is a critical and perhaps overlooked opportunity to reduce readmissions.45 Involving key family members in discharge planning, preparation, follow-up, and ongoing management is essential in caring for patients with functional deficits and/or complex care needs. Educating caregivers can help them feel more prepared and effective in their roles.
Establish an “action plan.” For patients with chronic, periodically symptomatic diseases such as asthma and heart failure, action planning can be useful. Action plans should include information that reinforces patients’ daily self-care behaviors and instructions for what to do if symptoms get worse. Action planning also might include simple if-then plans (“if x happens, then I will do y”), which can help with problem solving for common scenarios. Action plans have been shown to reduce admissions for children with asthma46 and adults with heart failure when coupled with home monitoring or telephone support from a registered nurse.16,47
Generate an individualized care plan for each patient, taking into account your patient’s health literacy, goals of care, and level of social support. This care plan may include educational and behavioral interventions, action planning, and follow-up plans. Most successful approaches to reducing readmissions have included both system-level and patient-level interventions that use an interdisciplinary team of providers.48
Make the most of follow-up visits. The traditional 15-minute FP visit can make it challenging to provide the level of care necessary for recently discharged patients. Multiple models of team-based care have been proposed to improve this situation, including using the “teamlet” model, which may include a clinician and one or 2 health coaches.49 During each visit, the health coaches—often medical assistants trained in chronic disease self-management skills—see patients before and after the physician. They also contact patients be- tween visits to facilitate action planning and to promote self-management.
Palliative care programs: A resource for FPs
The growth of palliative care programs in US hospitals has helped increase the emphasis on establishing goals of care. Inpatient-based palliative care consultation programs work with patients and families to establish goals. However, after discharge, many of these goals and plans begin to unravel due to gaps in the current health care model, including lack of follow-up and support.50 Outpatient palliative care programs have begun to address these gaps in care.50 Comprehensive palliative care programs are quickly becoming an important resource for FPs to help address transitional care issues.
CASE › When you ask Mr. and Mrs. T about his goals for treatment, they say are getting tired of the “back and forth” to the hospital. After discussing his lengthy history of worsening CHF and diabetes, you raise the idea of palliative care, including hospice, with the couple. They acknowledge that they have had family members get hospice care, and they are open to it—just not yet. The 3 of you craft an “if-then” plan of care to use at home. You schedule a 2-week follow-up visit and remind Mr. T and his wife of your office’s 24-hour on-call service.
CORRESPONDENCE
Danielle Snyderman, MD, Department of Family and Community Medicine, Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; danielle.snyderman@jefferson.edu
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428
2. O’Connor CM, Miller AB, Blair JE, et al; Efficacy of Vasopressin Antagonism in heart Failure Outcome Study with Tolvaptan (EVEREST) investigators. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduce left ventricular ejection fraction; results from EVEREST program. Am Heart J. 2010;159:841-849.e1.
3. Garrison GM, Mansukhani MP, Bohn B. Predictors of thirty-day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71-77.
4. Boult C, Dowd B, McCaffrey D, et al. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41:811-817.
5. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
6. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688-1698.
7. Haas LR, Takahashi PY, Shah ND, et al. Risk-stratification methods for identifying patients for care coordination. Am J Manag Care. 2013;19:725-732.
8. Wallace E, Hinchey T, Dimitrov BD, et al. A systematic review of the probability of repeated admission score in community-dwelling adults. J Am Geriatr Soc. 2013;61:357-364.
9. Cotter PE, Bhalla VK, Wallis SJ, et al. Predicting readmissions: poor performance of the LACE index in an older UK population. Age Ageing. 2012;41:784-789.
10. Shelton P, Sager MA, Schraeder C. The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit. Am J Manag Care. 2000;6:925-933.
11. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373-383.
12. Kripalani S, Jackson AT, Schnipper JL, et al. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314-323.
13. Kim CS, Flanders SA. In the clinic. Transitions of care. Ann Intern Med. 2013;158(5 pt 1):ITC3-1.
14. Hansen LO, Strater A, Smith L, et al. Hospital discharge documentation and risk of rehospitalisation. BMJ Qual Saf. 2011;20:773-778.
15. Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345-346.
16. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520-528.
17. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397.
18. van Walraven C, Jennings A, Taljaard M, et al. Incidence of potentially avoidable urgent readmissions and their relation to all-cause urgent readmissions. CMAJ. 2011;183:E1067-E1072.
19. Tang, N. A primary care physician’s ideal transitions of care—where’s the evidence? J Hosp Med. 2013;8:472-477.
20. Crocker JB, Crocker JT, Greenwald JL. Telephone follow-up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125:915-921.
21. Wong FK, Chow S, Chung L, et al. Can home visits help reduce hospital readmissions? Randomized controlled trial. J Adv Nurs. 2008;62:585-595.
22. Wong FK, Chow SK, Chan TM, et al. Comparison of effects between home visits with telephone calls and telephone calls only for transitional discharge support: a randomised controlled trial. Age Ageing. 2014;43:91-97.
23. Mueller SK, Sponsler KC, Kripalani S, et al. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172:1057-1069.
24. Glintborg B, Andersen SE, Dalhoff K. Insufficient communication about medication use at the interface between hospital and primary care. Qual Saf Health Care. 2007;16:34-39.
25. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
26. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc (2003). 2013;53:78-84.
27. Vermeire E, Hearnshaw H, Van Royen P, et al. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331-342.
28. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
29. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
30. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157:785-795.
31. McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868-2879.
32. Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540-550.
33. Eisen SA, Miller DK, Woodward RS, et al. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150:1881-1884.
34. Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629-1634.
35. Persell SD, Osborn CY, Richard R, et al. Limited health literacy is a barrier to medication reconciliation in ambulatory care. J Gen Intern Med. 2007;22:1523-1526.
36. Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. Manual for Clinicians. Chicago, IL: American Medical Association Foundation; 2007.
37. Chew LD, Bradley KA, Bokyo EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588-594.
38. Wallace LS, Rogers ES, Roskos SE, et al. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877.
39. Doak CC, Doak LG, Root JH. Teaching Patients with Low Literacy Skills. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1996.
40. Back AL, Arnold RM, Baile WF, et al. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55: 164-177.
41. Doak LG, Doak CC, eds. Pfizer Principles for Clear Health Communication: A Handbook for Creating Patient Education Materials that Enhance Understanding and Promote Health Outcomes. 2nd ed. New York, NY: Pfizer; 2004.
42. Bradley EH, Bogardus ST Jr, Tinetti M, et al. Goal-setting in clinical medicine. Soc Sci Med. 1999;49:267-278.
43. Koelling TM, Johnson ML, Cody RJ, et al. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179-185.
44. Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83-89.
45. Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med. 2013;173:695-698.
46. Kessler KR. Relationship between the use of asthma action plans and asthma exacerbations in children with asthma: A systematic review. J Asthma Allergy Educators. 2011;2:11-21.
47. Maric B, Kaan A, Ignaszewski A, et al. A systematic review of telemonitoring technologies in heart failure. Eur J Heart Fail. 2009;11:506-517.
48. Boutwell A, Hwu S. Effective Interventions to Reduce Rehospitalizations: A Survey of the Published Evidence. Cambridge, MA: Institute for Healthcare Improvement; 2009.
49. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
50. Meier D, Beresford L. Outpatient clinics are a new frontier for palliative care. J Pall Med. 2008;11:823-828.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428
2. O’Connor CM, Miller AB, Blair JE, et al; Efficacy of Vasopressin Antagonism in heart Failure Outcome Study with Tolvaptan (EVEREST) investigators. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduce left ventricular ejection fraction; results from EVEREST program. Am Heart J. 2010;159:841-849.e1.
3. Garrison GM, Mansukhani MP, Bohn B. Predictors of thirty-day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71-77.
4. Boult C, Dowd B, McCaffrey D, et al. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41:811-817.
5. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
6. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688-1698.
7. Haas LR, Takahashi PY, Shah ND, et al. Risk-stratification methods for identifying patients for care coordination. Am J Manag Care. 2013;19:725-732.
8. Wallace E, Hinchey T, Dimitrov BD, et al. A systematic review of the probability of repeated admission score in community-dwelling adults. J Am Geriatr Soc. 2013;61:357-364.
9. Cotter PE, Bhalla VK, Wallis SJ, et al. Predicting readmissions: poor performance of the LACE index in an older UK population. Age Ageing. 2012;41:784-789.
10. Shelton P, Sager MA, Schraeder C. The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit. Am J Manag Care. 2000;6:925-933.
11. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373-383.
12. Kripalani S, Jackson AT, Schnipper JL, et al. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314-323.
13. Kim CS, Flanders SA. In the clinic. Transitions of care. Ann Intern Med. 2013;158(5 pt 1):ITC3-1.
14. Hansen LO, Strater A, Smith L, et al. Hospital discharge documentation and risk of rehospitalisation. BMJ Qual Saf. 2011;20:773-778.
15. Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345-346.
16. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520-528.
17. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397.
18. van Walraven C, Jennings A, Taljaard M, et al. Incidence of potentially avoidable urgent readmissions and their relation to all-cause urgent readmissions. CMAJ. 2011;183:E1067-E1072.
19. Tang, N. A primary care physician’s ideal transitions of care—where’s the evidence? J Hosp Med. 2013;8:472-477.
20. Crocker JB, Crocker JT, Greenwald JL. Telephone follow-up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125:915-921.
21. Wong FK, Chow S, Chung L, et al. Can home visits help reduce hospital readmissions? Randomized controlled trial. J Adv Nurs. 2008;62:585-595.
22. Wong FK, Chow SK, Chan TM, et al. Comparison of effects between home visits with telephone calls and telephone calls only for transitional discharge support: a randomised controlled trial. Age Ageing. 2014;43:91-97.
23. Mueller SK, Sponsler KC, Kripalani S, et al. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172:1057-1069.
24. Glintborg B, Andersen SE, Dalhoff K. Insufficient communication about medication use at the interface between hospital and primary care. Qual Saf Health Care. 2007;16:34-39.
25. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
26. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc (2003). 2013;53:78-84.
27. Vermeire E, Hearnshaw H, Van Royen P, et al. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331-342.
28. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
29. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
30. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157:785-795.
31. McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868-2879.
32. Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540-550.
33. Eisen SA, Miller DK, Woodward RS, et al. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150:1881-1884.
34. Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629-1634.
35. Persell SD, Osborn CY, Richard R, et al. Limited health literacy is a barrier to medication reconciliation in ambulatory care. J Gen Intern Med. 2007;22:1523-1526.
36. Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. Manual for Clinicians. Chicago, IL: American Medical Association Foundation; 2007.
37. Chew LD, Bradley KA, Bokyo EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588-594.
38. Wallace LS, Rogers ES, Roskos SE, et al. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877.
39. Doak CC, Doak LG, Root JH. Teaching Patients with Low Literacy Skills. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1996.
40. Back AL, Arnold RM, Baile WF, et al. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55: 164-177.
41. Doak LG, Doak CC, eds. Pfizer Principles for Clear Health Communication: A Handbook for Creating Patient Education Materials that Enhance Understanding and Promote Health Outcomes. 2nd ed. New York, NY: Pfizer; 2004.
42. Bradley EH, Bogardus ST Jr, Tinetti M, et al. Goal-setting in clinical medicine. Soc Sci Med. 1999;49:267-278.
43. Koelling TM, Johnson ML, Cody RJ, et al. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179-185.
44. Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83-89.
45. Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med. 2013;173:695-698.
46. Kessler KR. Relationship between the use of asthma action plans and asthma exacerbations in children with asthma: A systematic review. J Asthma Allergy Educators. 2011;2:11-21.
47. Maric B, Kaan A, Ignaszewski A, et al. A systematic review of telemonitoring technologies in heart failure. Eur J Heart Fail. 2009;11:506-517.
48. Boutwell A, Hwu S. Effective Interventions to Reduce Rehospitalizations: A Survey of the Published Evidence. Cambridge, MA: Institute for Healthcare Improvement; 2009.
49. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
50. Meier D, Beresford L. Outpatient clinics are a new frontier for palliative care. J Pall Med. 2008;11:823-828.