User login
A Phase 3 Randomized, Double-blind, Vehicle-Controlled Trial of Azelaic Acid Foam 15% in the Treatment of Papulopustular Rosacea
Rosacea is a common dermatologic disorder that generally is characterized by erythema as well as papules and pustules on the cheeks, chin, forehead, and nose. Moreover, telangiectasia and burning or stinging sensations often occur.1,2 These clinical manifestations and other related ones frequently lead to the perception of “sensitive skin.” Rosacea patients often experience low self-esteem, anxiety, and social embarrassment.3 Reports of the gender distribution of the disease vary but often show female predominance.4 Although it also occurs in darker skin types, rosacea is more common in individuals with lighter skin.1
The etiology of rosacea is not yet fully understood, but the underlying pathology has been attributed to dysregulated immune responses. Although the flares of a typical fluctuating disease course often are caused by exogenous triggers, there is evidence that an underlying genetic component predisposes some individuals to pathologic changes associated with the condition.5 Augmented immune activity and proinflammatory signaling appear to induce the infiltration of inflammatory elements into affected areas.2 These regions show dilated vasculature and increased cutaneous blood flow secondary to inflammation. Systemic oxidative stress also may contribute to epidermal dysfunction, as the antioxidant capacity of the skin in patients with rosacea is depleted relative to that of healthy individuals. The biochemical and vascular changes characteristic of rosacea coincide with aberrant permeability of the stratum corneum.6 The resulting decreased hydration and water loss across the skin contribute to the sensitivity and irritation typical of the disease.2
Current guidelines for the optimal management of rosacea with papulopustular lesions recommend skin care, photoprotection, and topical therapy. Depending on the severity of disease and the likelihood of adherence to a topical regimen, use of oral agents may be warranted.7
Azelaic acid (AzA), an unbranched saturated dicarboxylic acid (1,7-heptanedicarboxylic acid) that occurs in plants, is one of several US Food and Drug Administration–approved topical agents for the treatment of inflammatory lesions in rosacea.8 Although the pathophysiology of rosacea is not yet fully understood, there is a growing consensus about the role of proinflammatory molecules (eg, kallikrein 5, cathelicidins) as well as reactive oxygen species (ROS).9 Azelaic acid has been demonstrated to modulate the inflammatory response in normal human keratinocytes through several pathways, including modulation of the signaling pathways of peroxisome proliferator-activated receptor g and nuclear factor kB, concurrent with the observed inhibition of proinflammatory cytokine secretion.10 Additionally, AzA can inhibit the release of ROS from neutrophils and also may reduce ROS by direct scavenging effects.11 Further, AzA shows direct inhibition of kallikrein 5 in human keratinocytes as well as a reduction of the expression of kallikrein 5 and cathelicidin in murine skin and the facial skin of patients with rosacea.12
In a series of randomized trials in patients with papulopustular rosacea (PPR), AzA has shown clinical efficacy and safety as a topical treatment.13-15 Based on these studies, a gel formulation of AzA with a 15% concentration has been approved for treating inflammatory papules and pustules of mild to moderate rosacea.16
Although AzA delivered in a gel matrix is an effective therapy, topical delivery of active pharmaceutical ingredients via foam is often preferred over traditional vehicles in patients with sensitive skin. Patient rationale for favoring foam includes improved appearance and ease of application, namely easier to spread with a reduced need to manipulate inflamed skin.17 Also, data reveal that patients may be more compliant with a treatment that meets their needs such as an optimized foam formulation.18 In addition, the lipid components of an optimized formulation are thought to contribute to an improved skin condition.19 The foam vehicle used in this study is a proprietary oil-in-water formulation that includes fatty alcohols and triglycerides. The novel delivery of AzA in a foam formulation will provide clinicians and patients with a new option for improved individualized care.
We report the primary results of a phase 3 study in patients with PPR comparing the efficacy and safety of twice-daily AzA foam 15% with vehicle foam. The phase 3 study builds on the results of a prior randomized double-blind trial (N=401) that demonstrated significant improvements relative to vehicle in therapeutic success rate (P=.017) and decreased inflammatory lesion count (ILC)(P<.001) among patients treated with AzA foam 15%.8
Methods
Study Design
This phase 3 randomized, double-blind, vehicle-controlled, parallel-group, multicenter study was conducted in patients with PPR according to Good Clinical Practice guidelines in 48 study centers in the United States. The objective was to evaluate a 12-week, twice-daily (morning and evening) course of AzA foam 15% versus vehicle.
Participants were men and women aged 18 years or older with moderate to severe PPR (as determined by investigator global assessment [IGA]) presenting with 12 to 50 papules and/or pustules and persistent erythema with or without telangiectasia. Informed consent was obtained from all participants before any study-related activities were carried out.
The study products were applied to the entire facial area each morning and evening at a dose of 0.5 g, thus administering 150 mg of AzA daily in the active arm of the trial (computerized randomization 1:1). The treatment period lasted 12 weeks, and participants were evaluated at baseline and weeks 4, 8, and 12. The follow-up period lasted 4 weeks following the end of treatment (EoT) and was concluded with one final end-of-study visit.
Efficacy Evaluations
There were 2 coprimary efficacy end points. Therapeutic success rate was evaluated using the IGA scale (clear, minimal, mild, moderate, or severe). Treatment success was defined as an IGA score of either clear or minimal (with at least a 2-step improvement) at EoT, whereas treatment failure was constituted by IGA scores of mild, moderate, or severe.
The second coprimary end point was the nominal change in ILC from baseline to EoT as determined by the total number of facial papules and pustules. Efficacy and safety parameters were evaluated at weeks 4, 8, and 12, as well as at the end of the 4-week follow-up period. Throughout the study, the investigator, participants, and all study personnel remained blinded.
Safety
Information about adverse events (AEs) was collected at each study visit, and AEs were graded according to seriousness (yes or no) and intensity (mild, moderate, or severe).
Statistical Analysis
Efficacy was confirmed by analysis of the treatment success rate at EoT with Cochran-Mantel-Haenszel test statistics, including a point estimate and 95% confidence interval (CI) for the odds ratio. Change in ILC at EoT was analyzed via an analysis of covariance model using treatment, center, and baseline lesion count as factors. (Additional methods can be found in the Appendix below.)
Results
Study Participants
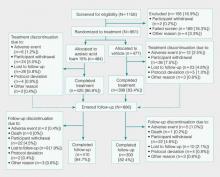
Of the 1156 patients who were screened for eligibility, 961 were randomized to treatment with AzA foam (n=484) or vehicle (n=477)(Figure 1). Sixty-four (13.2%) participants in the AzA foam group and 79 (16.6%) in the vehicle group discontinued treatment before completing the study. The most common reasons for discontinuation were participant withdrawal from the study and lost to follow-up. Six (1.2%) participants from the AzA foam group and 12 (2.5%) from the vehicle group discontinued because of AEs. All safety and efficacy data presented are based on the full analysis set, which consisted of the 961 participants randomized to treatment.
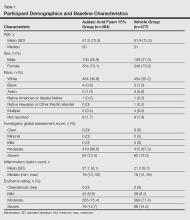
Demographic and baseline characteristics were balanced between the treatment groups (Table 1). The majority of participants were female (73.0%) and white (95.5%), reflecting the patient populations of independent studies that found a higher prevalence of rosacea in women and lighter skin types.4 There were no significant differences in baseline measures of PPR severity between the treatment groups. Participants in the AzA foam and vehicle groups had a mean ILC of 21.7 and 21.2, respectively, and 76.4% of participants had more than 14 lesions. All participants had an IGA score of moderate (86.8%) or severe (13.2%). Moderate or severe erythema was present in 91.5% of participants.
Treatment compliance, as measured by the percentage of expected doses that were actually administered, was 97.1% in the AzA foam group and 95.9% in the vehicle group.
Efficacy
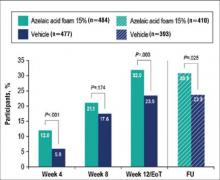
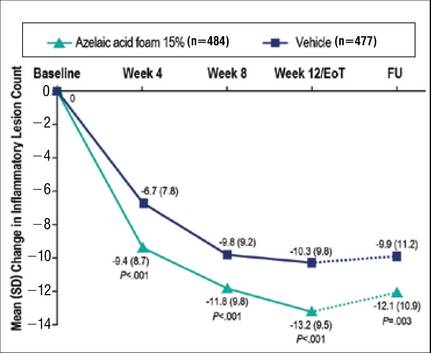
Results from both primary end points demonstrated superior efficacy of AzA foam over vehicle. The AzA foam group achieved a greater IGA success rate at EoT compared with the vehicle group (32.0% vs 23.5%; Cochran-Mantel-Haenszel test center-adjusted P<.001; odds ratio, 1.6; 95% CI, 1.2-2.2). Treatment success rate was higher in the AzA foam group than in the vehicle group at every time point past baseline (Figure 2). Similarly, the decrease in mean nominal ILC values was greater in the AzA foam group at every time point after baseline (Figure 3), and the treatment difference at EoT was statistically significant in favor of AzA foam (-2.7, F1,920=23.7, P<.001; 95% CI, -3.8 to -1.6). The divergence between treatment groups at week 4 reveals an onset of AzA effect early in the study.
 |
 |
Although the AzA foam group showed significantly better efficacy results than the vehicle group for the coprimary end points, participants in the vehicle group did show appreciable IGA success rates (23.5%) and changes in ILC (-10.3) at EoT (Figures 2 and 3).
Notably, the AzA foam group maintained better results than vehicle for both primary end points even at the end of the 4-week follow-up after EoT (Figures 2 and 3). Sensitivity analysis (data not shown) confirmed the findings from the full analysis set.
Safety
Adverse events were experienced by 149 (30.8%) participants in the AzA foam group and 119 (24.9%) in the vehicle group. The most common noncutaneous AEs (>1% of participants) reported during AzA foam treatment were nasopharyngitis, headache, upper respiratory tract infection, and influenza. In the vehicle group, the most common noncutaneous AEs reported were nasopharyngitis and headache. Drug-related AEs (relationship assessed by the investigator) were reported slightly more often in the AzA foam group (7.6%) than in the vehicle group (4.6%). Drug-related AEs were predominantly cutaneous and occurred at the site of application (Table 2). Drug-related cutaneous AEs were more common in the AzA foam group (7.0%) than in the vehicle group (4.4%). Although serious AEs were more common in the vehicle group, all were regarded as unrelated to the study medication. A single death occurred in the vehicle group due to an accident unrelated to the study drug.
The most frequent drug-related AEs in participants treated with AzA foam versus vehicle were application-site pain (3.5% vs 1.3%), application-site pruritus (1.4% vs 0.4%), and application-site dryness (1.0% vs 0.6%). The classical rosacea symptom of stinging is subsumed under the term application-site pain, according to MedDRA (Medical Dictionary for Regulatory Activities).
All other drug-related AEs occurred at a frequency of less than 1% in participants from both groups. Serious AEs were rare and unrelated to treatment, with 3 AEs reported in the AzA foam group and 4 in the vehicle group. Adverse events leading to study drug withdrawal occurred in less than 2% of participants and were more common in the vehicle group (2.5%) than in the AzA foam group (1.2%). Of the 3 drug-related AEs leading to withdrawal in the AzA foam group, 2 were due to cutaneous reaction and 1 was due to a burning sensation. The number of active drug-related cutaneous AEs was highest during the first 4 weeks of treatment and declined over the course of the study (eFigure).
More than 96% of AEs were resolved by the end of the study. Of the participants experiencing AEs that did not resolve during the course of the study, 16 were in the AzA foam group and 10 in the vehicle group. Six unresolved AEs were drug related, with 3 occurring in each treatment group. Unresolved drug-related cutaneous AEs in the AzA foam group were pain, pruritus, and dryness at the application site.
Comment
Overall, the results from this phase 3 trial demonstrate that the new foam formulation of AzA was efficacious and safe in a 12-week, twice-daily course of treatment for moderate to severe PPR. The AzA foam formulation was significantly superior to vehicle (P<.001) for both primary efficacy end points. Participants in the AzA foam group achieved therapeutic success at a higher rate than the vehicle group, and the change in nominal ILC at EoT was significantly greater for participants treated with AzA foam than for those treated with vehicle (P<.001). Differences between the 2 treatment groups for the coprimary end point measures arose early in the study, demonstrating that symptoms were rapidly controlled. Between weeks 8 and 12 (EoT), the rate of increase of beneficial effects in the AzA foam group remained high, while the vehicle group showed a notable slowing. There was no indication of any rebound effect in overall disease severity subsequent to EoT. After 4 weeks of follow-up, there was still a beneficial treatment effect present in favor of the AzA foam group, as indicated by the persistence of improvements in both coprimary end point measures throughout the follow-up period.
Analyses of alternative populations and secondary end points (data not shown) supported the efficacy results reported here. There was no indication of irregular study center effects, and the sensitivity analyses demonstrated robustness of the data for the observed treatment effects.
The use of vehicle foam alone appeared to be beneficial in reducing ILC and improving IGA rating, which suggests that the properties of the new foam formulation are favorable for the inflamed lesional skin of rosacea. Of note, other dermatology studies, including trials in rosacea, have reported therapeutic effects of vehicle treatment that may be attributable to the positive effects of skin care with certain formulations.20
Azelaic acid foam was well tolerated in the current study. More than 93% of AEs in either treatment group were of mild or moderate severity. The incidence of drug-related AEs was low in both groups and mainly occurred at the application site. There were no drug-related severe or serious AEs. The low incidence of reported drug-related noncutaneous AEs in the AzA foam group (dysgeusia in 1 patient and headache in 2 patients) supports the known favorable systemic tolerance profile of AzA.
Although most drug-related AEs occurred at the application site, they were generally transient, with the majority of events in the AzA foam group lasting no more than 1 hour. Most cutaneous AEs developed early in the study. In the AzA foam group, the prevalence of drug-related cutaneous AEs dropped at every time interval as the study progressed (eFigure). Very few AEs of any type persisted through the end of the study. These safety results were accompanied by a high compliance rate and a high participation rate throughout the course of the study. Taken together, the available data for this AzA foam formulation support a favorable tolerability profile. The results of this study are consistent with and expand on data from an earlier investigation of similar design.8
Conclusion
The development of an AzA foam formulation with higher lipid content was intended to expand the treatment options available to physicians and patients who are managing rosacea. Most topical dermatologic treatments are currently delivered in classical formulations such as creams or gels, but patients who use topical therapies have rated messiness and ease of application among the most important characteristics affecting quality of life.17,21 Foam formulations may offer improvements in this regard; ease of application may minimize unnecessary manipulation of inflamed skin and contribute to a high level of user satisfaction.22 However, the design of the current study was limited to evaluating only the AzA foam formulation versus a foam vehicle, and direct comparisons of clinical efficacy and tolerability to other AzA topical preparations were not performed. Nonetheless, patients have previously reported that they would be more likely to comply with a recommended course of dermatologic foam therapy than other topical formulations.18 The proposed foam formulation was designed to attend to the specific needs of the dry and sensitive skin in rosacea by combining the demonstrated efficacy properties exhibited by AzA gel 15% with the good tolerability and acceptability of a lipid-containing foam formulation. Development of this formulation was targeted to obtain a product that would be highly spreadable, dry quickly, and be easy to apply. The available data for this AzA foam formulation support the value of this option in the topical treatment of rosacea. The success in reduction of overall disease severity, lack of any rebound after EoT, and the observed tolerability and high adherence rates suggest that this novel formulation is a useful addition to current treatment options for rosacea.
Addendum
After release of the study data for unblinding and statistical evaluation, the following inconsistency regarding patient distribution was noted: 1 participant was incorrectly evaluated as part of the AzA foam analysis group when in fact this patient was randomized to vehicle and was treated throughout the study with vehicle. This participant did not experience any AE and did not show any IGA improvement at the EoT. As this single case did not have an impact on the statistical conclusions or interpretation of the results, the released study data have not been changed. This deviation was described as a database erratum in the study report.
Acknowledgement—Editorial support through inVentiv Medical Communications, New York, New York, was provided by Bayer HealthCare Pharmaceuticals Inc.
APPENDIX
Supplementary Methods
Supplementary Study Design
This study met all local legal and regulatory requirements and was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Before the start of the study and implementation, the protocol and all amendments were approved by the appropriate independent ethics committee or institutional review board at each study site. Two protocol amendments were implemented before the first participant visit.
Exclusion criteria included the presence of dermatoses that could interfere with rosacea diagnosis or evaluation, facial laser surgery or topical use of any medication to treat rosacea within 6 weeks before randomization, systemic use of any medications to treat rosacea, and known unresponsiveness to AzA treatment. Further standard exclusion criteria included alcohol or drug use or parallel participation in other clinical studies, which were necessary to exclude undue influence on study evaluations and/or participant safety. The study was conducted by qualified investigators at 48 centers in the United States.
The investigational product was filled in identical containers according to the randomization list generated by a computer program using blocks. Complete blocks of study medication were distributed to the centers. Eligible participants were randomized 1:1 into either AzA foam or vehicle treatment groups by assignment of a randomization number at baseline. A blind investigational product under the same randomization number was dispensed to and returned from participants by study personnel who were not involved in the assessments. Blinding was achieved by using labels on the investigational products that did not allow identification of the true medication.
Compliance was evaluated from participant diaries as well as the number of expected doses and actually applied doses.
Additional Efficacy Evaluations
A number of secondary variables (not reported here) were assessed, including changes in other manifestations of PPR, as well as participant assessments of treatment response, tolerability, cosmetic preferences, and quality of life.
Additional Safety
Investigators reported a yes or no response as to whether there was a reasonable causal relationship between AEs and treatment. Moreover, AEs that began at the start of or during treatment were considered treatment emergent. Cutaneous AEs were further assessed regarding location and duration. An AE was deemed local if it occurred at the application site and transient if it subsided within 60 minutes of onset.
Statistical Analysis
The primary efficacy analyses presented here were based on the full analysis set of participants who were randomized and had medication dispensed. For participants with no EoT value, the last nonmissing value was used including baseline (last-observation-carried-forward methodology). Participants who discontinued treatment prematurely because of lack of efficacy were considered to be treatment failures, regardless of the reported IGA score. Statistical significance was needed for both coprimary efficacy variables at a 1-sided 2.5% significance level to show confirmed superiority of AzA foam versus vehicle.
A number of sensitivity analyses were performed, including an analysis of the coprimary end points using observed data, analysis of the per-protocol population of participants who did not prematurely discontinue treatment and had no major protocol deviations, subgroup analyses, and the use of statistical methods to investigate the effect of missing observations. Analyses of success rate and nominal change in ILC were repeated for each postbaseline visit using χ² and t tests, respectively. All summary and statistical analyses were performed according to the study protocol (unchanged after the start of the study) using SAS version 9.2.
Results from a prior study provided the basis for the sample size, which was calculated to show a significant difference in both primary efficacy end points with a power of 90%.8 To allow for dropouts, 480 participants in each treatment group were to be randomized for a total of 960 participants.
1. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
2. Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
3. Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6:348-354.
4. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
5. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
6. Wollina U. Recent advances in the understanding and management of rosacea. F1000Prime Rep. 2014;6:50.
7. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis. 2014;93:134-138.
8. Draelos ZD, Elewski B, Staedtler G, et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306-317.
9. Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
10. Mastrofrancesco A, Ottaviani M, Aspite N, et al. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARg activation. Exp Dermatol. 2010;19:813-820.
11. Akamatsu H, Komura J, Asada Y, et al. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res. 1991;283:162-166.
12. Coda AB, Hata T, Miller J, et al. Cathelicidin, kalli-krein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol. 2013;69:570-577.
13. van Zuuren EJ, Kramer SF, Carter BR, et al. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760-781.
14. Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48:836-845.
15. Thiboutot DM, Fleischer AB Jr, Del Rosso JQ, et al. Azelaic acid 15% gel once daily versus twice daily in papulopustular rosacea. J Drugs Dermatol. 2008;7:541-546.
16. Finacea [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015.
17. Zhao Y, Jones SA, Brown MB. Dynamic foams in topical drug delivery. J Pharm Pharmacol. 2010;62:678-684.
18. Gottlieb AB, Ford RO, Spellman MC. The efficacy and tolerability of clobetasol propionate foam 0.05% in the treatment of mild to moderate plaque-type psoriasis of nonscalp regions. J Cutan Med Surg. 2003;7:185-192.
19. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771-788.
20. Jackson JM, Pelle M. Topical rosacea therapy: the importance of vehicles for efficacy, tolerability and compliance. J Drugs Dermatol. 2011;10:627-633.
21. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
22. Kircik LH, Bikowski JB. Vehicles matter: topical foam formulations. Practical Dermatology. January 2012(suppl):3-18.
Rosacea is a common dermatologic disorder that generally is characterized by erythema as well as papules and pustules on the cheeks, chin, forehead, and nose. Moreover, telangiectasia and burning or stinging sensations often occur.1,2 These clinical manifestations and other related ones frequently lead to the perception of “sensitive skin.” Rosacea patients often experience low self-esteem, anxiety, and social embarrassment.3 Reports of the gender distribution of the disease vary but often show female predominance.4 Although it also occurs in darker skin types, rosacea is more common in individuals with lighter skin.1
The etiology of rosacea is not yet fully understood, but the underlying pathology has been attributed to dysregulated immune responses. Although the flares of a typical fluctuating disease course often are caused by exogenous triggers, there is evidence that an underlying genetic component predisposes some individuals to pathologic changes associated with the condition.5 Augmented immune activity and proinflammatory signaling appear to induce the infiltration of inflammatory elements into affected areas.2 These regions show dilated vasculature and increased cutaneous blood flow secondary to inflammation. Systemic oxidative stress also may contribute to epidermal dysfunction, as the antioxidant capacity of the skin in patients with rosacea is depleted relative to that of healthy individuals. The biochemical and vascular changes characteristic of rosacea coincide with aberrant permeability of the stratum corneum.6 The resulting decreased hydration and water loss across the skin contribute to the sensitivity and irritation typical of the disease.2
Current guidelines for the optimal management of rosacea with papulopustular lesions recommend skin care, photoprotection, and topical therapy. Depending on the severity of disease and the likelihood of adherence to a topical regimen, use of oral agents may be warranted.7
Azelaic acid (AzA), an unbranched saturated dicarboxylic acid (1,7-heptanedicarboxylic acid) that occurs in plants, is one of several US Food and Drug Administration–approved topical agents for the treatment of inflammatory lesions in rosacea.8 Although the pathophysiology of rosacea is not yet fully understood, there is a growing consensus about the role of proinflammatory molecules (eg, kallikrein 5, cathelicidins) as well as reactive oxygen species (ROS).9 Azelaic acid has been demonstrated to modulate the inflammatory response in normal human keratinocytes through several pathways, including modulation of the signaling pathways of peroxisome proliferator-activated receptor g and nuclear factor kB, concurrent with the observed inhibition of proinflammatory cytokine secretion.10 Additionally, AzA can inhibit the release of ROS from neutrophils and also may reduce ROS by direct scavenging effects.11 Further, AzA shows direct inhibition of kallikrein 5 in human keratinocytes as well as a reduction of the expression of kallikrein 5 and cathelicidin in murine skin and the facial skin of patients with rosacea.12
In a series of randomized trials in patients with papulopustular rosacea (PPR), AzA has shown clinical efficacy and safety as a topical treatment.13-15 Based on these studies, a gel formulation of AzA with a 15% concentration has been approved for treating inflammatory papules and pustules of mild to moderate rosacea.16
Although AzA delivered in a gel matrix is an effective therapy, topical delivery of active pharmaceutical ingredients via foam is often preferred over traditional vehicles in patients with sensitive skin. Patient rationale for favoring foam includes improved appearance and ease of application, namely easier to spread with a reduced need to manipulate inflamed skin.17 Also, data reveal that patients may be more compliant with a treatment that meets their needs such as an optimized foam formulation.18 In addition, the lipid components of an optimized formulation are thought to contribute to an improved skin condition.19 The foam vehicle used in this study is a proprietary oil-in-water formulation that includes fatty alcohols and triglycerides. The novel delivery of AzA in a foam formulation will provide clinicians and patients with a new option for improved individualized care.
We report the primary results of a phase 3 study in patients with PPR comparing the efficacy and safety of twice-daily AzA foam 15% with vehicle foam. The phase 3 study builds on the results of a prior randomized double-blind trial (N=401) that demonstrated significant improvements relative to vehicle in therapeutic success rate (P=.017) and decreased inflammatory lesion count (ILC)(P<.001) among patients treated with AzA foam 15%.8
Methods
Study Design
This phase 3 randomized, double-blind, vehicle-controlled, parallel-group, multicenter study was conducted in patients with PPR according to Good Clinical Practice guidelines in 48 study centers in the United States. The objective was to evaluate a 12-week, twice-daily (morning and evening) course of AzA foam 15% versus vehicle.
Participants were men and women aged 18 years or older with moderate to severe PPR (as determined by investigator global assessment [IGA]) presenting with 12 to 50 papules and/or pustules and persistent erythema with or without telangiectasia. Informed consent was obtained from all participants before any study-related activities were carried out.
The study products were applied to the entire facial area each morning and evening at a dose of 0.5 g, thus administering 150 mg of AzA daily in the active arm of the trial (computerized randomization 1:1). The treatment period lasted 12 weeks, and participants were evaluated at baseline and weeks 4, 8, and 12. The follow-up period lasted 4 weeks following the end of treatment (EoT) and was concluded with one final end-of-study visit.
Efficacy Evaluations
There were 2 coprimary efficacy end points. Therapeutic success rate was evaluated using the IGA scale (clear, minimal, mild, moderate, or severe). Treatment success was defined as an IGA score of either clear or minimal (with at least a 2-step improvement) at EoT, whereas treatment failure was constituted by IGA scores of mild, moderate, or severe.
The second coprimary end point was the nominal change in ILC from baseline to EoT as determined by the total number of facial papules and pustules. Efficacy and safety parameters were evaluated at weeks 4, 8, and 12, as well as at the end of the 4-week follow-up period. Throughout the study, the investigator, participants, and all study personnel remained blinded.
Safety
Information about adverse events (AEs) was collected at each study visit, and AEs were graded according to seriousness (yes or no) and intensity (mild, moderate, or severe).
Statistical Analysis
Efficacy was confirmed by analysis of the treatment success rate at EoT with Cochran-Mantel-Haenszel test statistics, including a point estimate and 95% confidence interval (CI) for the odds ratio. Change in ILC at EoT was analyzed via an analysis of covariance model using treatment, center, and baseline lesion count as factors. (Additional methods can be found in the Appendix below.)
Results
Study Participants
Of the 1156 patients who were screened for eligibility, 961 were randomized to treatment with AzA foam (n=484) or vehicle (n=477)(Figure 1). Sixty-four (13.2%) participants in the AzA foam group and 79 (16.6%) in the vehicle group discontinued treatment before completing the study. The most common reasons for discontinuation were participant withdrawal from the study and lost to follow-up. Six (1.2%) participants from the AzA foam group and 12 (2.5%) from the vehicle group discontinued because of AEs. All safety and efficacy data presented are based on the full analysis set, which consisted of the 961 participants randomized to treatment.
Demographic and baseline characteristics were balanced between the treatment groups (Table 1). The majority of participants were female (73.0%) and white (95.5%), reflecting the patient populations of independent studies that found a higher prevalence of rosacea in women and lighter skin types.4 There were no significant differences in baseline measures of PPR severity between the treatment groups. Participants in the AzA foam and vehicle groups had a mean ILC of 21.7 and 21.2, respectively, and 76.4% of participants had more than 14 lesions. All participants had an IGA score of moderate (86.8%) or severe (13.2%). Moderate or severe erythema was present in 91.5% of participants.
Treatment compliance, as measured by the percentage of expected doses that were actually administered, was 97.1% in the AzA foam group and 95.9% in the vehicle group.
Efficacy
Results from both primary end points demonstrated superior efficacy of AzA foam over vehicle. The AzA foam group achieved a greater IGA success rate at EoT compared with the vehicle group (32.0% vs 23.5%; Cochran-Mantel-Haenszel test center-adjusted P<.001; odds ratio, 1.6; 95% CI, 1.2-2.2). Treatment success rate was higher in the AzA foam group than in the vehicle group at every time point past baseline (Figure 2). Similarly, the decrease in mean nominal ILC values was greater in the AzA foam group at every time point after baseline (Figure 3), and the treatment difference at EoT was statistically significant in favor of AzA foam (-2.7, F1,920=23.7, P<.001; 95% CI, -3.8 to -1.6). The divergence between treatment groups at week 4 reveals an onset of AzA effect early in the study.
 |
 |
Although the AzA foam group showed significantly better efficacy results than the vehicle group for the coprimary end points, participants in the vehicle group did show appreciable IGA success rates (23.5%) and changes in ILC (-10.3) at EoT (Figures 2 and 3).
Notably, the AzA foam group maintained better results than vehicle for both primary end points even at the end of the 4-week follow-up after EoT (Figures 2 and 3). Sensitivity analysis (data not shown) confirmed the findings from the full analysis set.
Safety
Adverse events were experienced by 149 (30.8%) participants in the AzA foam group and 119 (24.9%) in the vehicle group. The most common noncutaneous AEs (>1% of participants) reported during AzA foam treatment were nasopharyngitis, headache, upper respiratory tract infection, and influenza. In the vehicle group, the most common noncutaneous AEs reported were nasopharyngitis and headache. Drug-related AEs (relationship assessed by the investigator) were reported slightly more often in the AzA foam group (7.6%) than in the vehicle group (4.6%). Drug-related AEs were predominantly cutaneous and occurred at the site of application (Table 2). Drug-related cutaneous AEs were more common in the AzA foam group (7.0%) than in the vehicle group (4.4%). Although serious AEs were more common in the vehicle group, all were regarded as unrelated to the study medication. A single death occurred in the vehicle group due to an accident unrelated to the study drug.
The most frequent drug-related AEs in participants treated with AzA foam versus vehicle were application-site pain (3.5% vs 1.3%), application-site pruritus (1.4% vs 0.4%), and application-site dryness (1.0% vs 0.6%). The classical rosacea symptom of stinging is subsumed under the term application-site pain, according to MedDRA (Medical Dictionary for Regulatory Activities).
All other drug-related AEs occurred at a frequency of less than 1% in participants from both groups. Serious AEs were rare and unrelated to treatment, with 3 AEs reported in the AzA foam group and 4 in the vehicle group. Adverse events leading to study drug withdrawal occurred in less than 2% of participants and were more common in the vehicle group (2.5%) than in the AzA foam group (1.2%). Of the 3 drug-related AEs leading to withdrawal in the AzA foam group, 2 were due to cutaneous reaction and 1 was due to a burning sensation. The number of active drug-related cutaneous AEs was highest during the first 4 weeks of treatment and declined over the course of the study (eFigure).
More than 96% of AEs were resolved by the end of the study. Of the participants experiencing AEs that did not resolve during the course of the study, 16 were in the AzA foam group and 10 in the vehicle group. Six unresolved AEs were drug related, with 3 occurring in each treatment group. Unresolved drug-related cutaneous AEs in the AzA foam group were pain, pruritus, and dryness at the application site.
Comment
Overall, the results from this phase 3 trial demonstrate that the new foam formulation of AzA was efficacious and safe in a 12-week, twice-daily course of treatment for moderate to severe PPR. The AzA foam formulation was significantly superior to vehicle (P<.001) for both primary efficacy end points. Participants in the AzA foam group achieved therapeutic success at a higher rate than the vehicle group, and the change in nominal ILC at EoT was significantly greater for participants treated with AzA foam than for those treated with vehicle (P<.001). Differences between the 2 treatment groups for the coprimary end point measures arose early in the study, demonstrating that symptoms were rapidly controlled. Between weeks 8 and 12 (EoT), the rate of increase of beneficial effects in the AzA foam group remained high, while the vehicle group showed a notable slowing. There was no indication of any rebound effect in overall disease severity subsequent to EoT. After 4 weeks of follow-up, there was still a beneficial treatment effect present in favor of the AzA foam group, as indicated by the persistence of improvements in both coprimary end point measures throughout the follow-up period.
Analyses of alternative populations and secondary end points (data not shown) supported the efficacy results reported here. There was no indication of irregular study center effects, and the sensitivity analyses demonstrated robustness of the data for the observed treatment effects.
The use of vehicle foam alone appeared to be beneficial in reducing ILC and improving IGA rating, which suggests that the properties of the new foam formulation are favorable for the inflamed lesional skin of rosacea. Of note, other dermatology studies, including trials in rosacea, have reported therapeutic effects of vehicle treatment that may be attributable to the positive effects of skin care with certain formulations.20
Azelaic acid foam was well tolerated in the current study. More than 93% of AEs in either treatment group were of mild or moderate severity. The incidence of drug-related AEs was low in both groups and mainly occurred at the application site. There were no drug-related severe or serious AEs. The low incidence of reported drug-related noncutaneous AEs in the AzA foam group (dysgeusia in 1 patient and headache in 2 patients) supports the known favorable systemic tolerance profile of AzA.
Although most drug-related AEs occurred at the application site, they were generally transient, with the majority of events in the AzA foam group lasting no more than 1 hour. Most cutaneous AEs developed early in the study. In the AzA foam group, the prevalence of drug-related cutaneous AEs dropped at every time interval as the study progressed (eFigure). Very few AEs of any type persisted through the end of the study. These safety results were accompanied by a high compliance rate and a high participation rate throughout the course of the study. Taken together, the available data for this AzA foam formulation support a favorable tolerability profile. The results of this study are consistent with and expand on data from an earlier investigation of similar design.8
Conclusion
The development of an AzA foam formulation with higher lipid content was intended to expand the treatment options available to physicians and patients who are managing rosacea. Most topical dermatologic treatments are currently delivered in classical formulations such as creams or gels, but patients who use topical therapies have rated messiness and ease of application among the most important characteristics affecting quality of life.17,21 Foam formulations may offer improvements in this regard; ease of application may minimize unnecessary manipulation of inflamed skin and contribute to a high level of user satisfaction.22 However, the design of the current study was limited to evaluating only the AzA foam formulation versus a foam vehicle, and direct comparisons of clinical efficacy and tolerability to other AzA topical preparations were not performed. Nonetheless, patients have previously reported that they would be more likely to comply with a recommended course of dermatologic foam therapy than other topical formulations.18 The proposed foam formulation was designed to attend to the specific needs of the dry and sensitive skin in rosacea by combining the demonstrated efficacy properties exhibited by AzA gel 15% with the good tolerability and acceptability of a lipid-containing foam formulation. Development of this formulation was targeted to obtain a product that would be highly spreadable, dry quickly, and be easy to apply. The available data for this AzA foam formulation support the value of this option in the topical treatment of rosacea. The success in reduction of overall disease severity, lack of any rebound after EoT, and the observed tolerability and high adherence rates suggest that this novel formulation is a useful addition to current treatment options for rosacea.
Addendum
After release of the study data for unblinding and statistical evaluation, the following inconsistency regarding patient distribution was noted: 1 participant was incorrectly evaluated as part of the AzA foam analysis group when in fact this patient was randomized to vehicle and was treated throughout the study with vehicle. This participant did not experience any AE and did not show any IGA improvement at the EoT. As this single case did not have an impact on the statistical conclusions or interpretation of the results, the released study data have not been changed. This deviation was described as a database erratum in the study report.
Acknowledgement—Editorial support through inVentiv Medical Communications, New York, New York, was provided by Bayer HealthCare Pharmaceuticals Inc.
APPENDIX
Supplementary Methods
Supplementary Study Design
This study met all local legal and regulatory requirements and was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Before the start of the study and implementation, the protocol and all amendments were approved by the appropriate independent ethics committee or institutional review board at each study site. Two protocol amendments were implemented before the first participant visit.
Exclusion criteria included the presence of dermatoses that could interfere with rosacea diagnosis or evaluation, facial laser surgery or topical use of any medication to treat rosacea within 6 weeks before randomization, systemic use of any medications to treat rosacea, and known unresponsiveness to AzA treatment. Further standard exclusion criteria included alcohol or drug use or parallel participation in other clinical studies, which were necessary to exclude undue influence on study evaluations and/or participant safety. The study was conducted by qualified investigators at 48 centers in the United States.
The investigational product was filled in identical containers according to the randomization list generated by a computer program using blocks. Complete blocks of study medication were distributed to the centers. Eligible participants were randomized 1:1 into either AzA foam or vehicle treatment groups by assignment of a randomization number at baseline. A blind investigational product under the same randomization number was dispensed to and returned from participants by study personnel who were not involved in the assessments. Blinding was achieved by using labels on the investigational products that did not allow identification of the true medication.
Compliance was evaluated from participant diaries as well as the number of expected doses and actually applied doses.
Additional Efficacy Evaluations
A number of secondary variables (not reported here) were assessed, including changes in other manifestations of PPR, as well as participant assessments of treatment response, tolerability, cosmetic preferences, and quality of life.
Additional Safety
Investigators reported a yes or no response as to whether there was a reasonable causal relationship between AEs and treatment. Moreover, AEs that began at the start of or during treatment were considered treatment emergent. Cutaneous AEs were further assessed regarding location and duration. An AE was deemed local if it occurred at the application site and transient if it subsided within 60 minutes of onset.
Statistical Analysis
The primary efficacy analyses presented here were based on the full analysis set of participants who were randomized and had medication dispensed. For participants with no EoT value, the last nonmissing value was used including baseline (last-observation-carried-forward methodology). Participants who discontinued treatment prematurely because of lack of efficacy were considered to be treatment failures, regardless of the reported IGA score. Statistical significance was needed for both coprimary efficacy variables at a 1-sided 2.5% significance level to show confirmed superiority of AzA foam versus vehicle.
A number of sensitivity analyses were performed, including an analysis of the coprimary end points using observed data, analysis of the per-protocol population of participants who did not prematurely discontinue treatment and had no major protocol deviations, subgroup analyses, and the use of statistical methods to investigate the effect of missing observations. Analyses of success rate and nominal change in ILC were repeated for each postbaseline visit using χ² and t tests, respectively. All summary and statistical analyses were performed according to the study protocol (unchanged after the start of the study) using SAS version 9.2.
Results from a prior study provided the basis for the sample size, which was calculated to show a significant difference in both primary efficacy end points with a power of 90%.8 To allow for dropouts, 480 participants in each treatment group were to be randomized for a total of 960 participants.
Rosacea is a common dermatologic disorder that generally is characterized by erythema as well as papules and pustules on the cheeks, chin, forehead, and nose. Moreover, telangiectasia and burning or stinging sensations often occur.1,2 These clinical manifestations and other related ones frequently lead to the perception of “sensitive skin.” Rosacea patients often experience low self-esteem, anxiety, and social embarrassment.3 Reports of the gender distribution of the disease vary but often show female predominance.4 Although it also occurs in darker skin types, rosacea is more common in individuals with lighter skin.1
The etiology of rosacea is not yet fully understood, but the underlying pathology has been attributed to dysregulated immune responses. Although the flares of a typical fluctuating disease course often are caused by exogenous triggers, there is evidence that an underlying genetic component predisposes some individuals to pathologic changes associated with the condition.5 Augmented immune activity and proinflammatory signaling appear to induce the infiltration of inflammatory elements into affected areas.2 These regions show dilated vasculature and increased cutaneous blood flow secondary to inflammation. Systemic oxidative stress also may contribute to epidermal dysfunction, as the antioxidant capacity of the skin in patients with rosacea is depleted relative to that of healthy individuals. The biochemical and vascular changes characteristic of rosacea coincide with aberrant permeability of the stratum corneum.6 The resulting decreased hydration and water loss across the skin contribute to the sensitivity and irritation typical of the disease.2
Current guidelines for the optimal management of rosacea with papulopustular lesions recommend skin care, photoprotection, and topical therapy. Depending on the severity of disease and the likelihood of adherence to a topical regimen, use of oral agents may be warranted.7
Azelaic acid (AzA), an unbranched saturated dicarboxylic acid (1,7-heptanedicarboxylic acid) that occurs in plants, is one of several US Food and Drug Administration–approved topical agents for the treatment of inflammatory lesions in rosacea.8 Although the pathophysiology of rosacea is not yet fully understood, there is a growing consensus about the role of proinflammatory molecules (eg, kallikrein 5, cathelicidins) as well as reactive oxygen species (ROS).9 Azelaic acid has been demonstrated to modulate the inflammatory response in normal human keratinocytes through several pathways, including modulation of the signaling pathways of peroxisome proliferator-activated receptor g and nuclear factor kB, concurrent with the observed inhibition of proinflammatory cytokine secretion.10 Additionally, AzA can inhibit the release of ROS from neutrophils and also may reduce ROS by direct scavenging effects.11 Further, AzA shows direct inhibition of kallikrein 5 in human keratinocytes as well as a reduction of the expression of kallikrein 5 and cathelicidin in murine skin and the facial skin of patients with rosacea.12
In a series of randomized trials in patients with papulopustular rosacea (PPR), AzA has shown clinical efficacy and safety as a topical treatment.13-15 Based on these studies, a gel formulation of AzA with a 15% concentration has been approved for treating inflammatory papules and pustules of mild to moderate rosacea.16
Although AzA delivered in a gel matrix is an effective therapy, topical delivery of active pharmaceutical ingredients via foam is often preferred over traditional vehicles in patients with sensitive skin. Patient rationale for favoring foam includes improved appearance and ease of application, namely easier to spread with a reduced need to manipulate inflamed skin.17 Also, data reveal that patients may be more compliant with a treatment that meets their needs such as an optimized foam formulation.18 In addition, the lipid components of an optimized formulation are thought to contribute to an improved skin condition.19 The foam vehicle used in this study is a proprietary oil-in-water formulation that includes fatty alcohols and triglycerides. The novel delivery of AzA in a foam formulation will provide clinicians and patients with a new option for improved individualized care.
We report the primary results of a phase 3 study in patients with PPR comparing the efficacy and safety of twice-daily AzA foam 15% with vehicle foam. The phase 3 study builds on the results of a prior randomized double-blind trial (N=401) that demonstrated significant improvements relative to vehicle in therapeutic success rate (P=.017) and decreased inflammatory lesion count (ILC)(P<.001) among patients treated with AzA foam 15%.8
Methods
Study Design
This phase 3 randomized, double-blind, vehicle-controlled, parallel-group, multicenter study was conducted in patients with PPR according to Good Clinical Practice guidelines in 48 study centers in the United States. The objective was to evaluate a 12-week, twice-daily (morning and evening) course of AzA foam 15% versus vehicle.
Participants were men and women aged 18 years or older with moderate to severe PPR (as determined by investigator global assessment [IGA]) presenting with 12 to 50 papules and/or pustules and persistent erythema with or without telangiectasia. Informed consent was obtained from all participants before any study-related activities were carried out.
The study products were applied to the entire facial area each morning and evening at a dose of 0.5 g, thus administering 150 mg of AzA daily in the active arm of the trial (computerized randomization 1:1). The treatment period lasted 12 weeks, and participants were evaluated at baseline and weeks 4, 8, and 12. The follow-up period lasted 4 weeks following the end of treatment (EoT) and was concluded with one final end-of-study visit.
Efficacy Evaluations
There were 2 coprimary efficacy end points. Therapeutic success rate was evaluated using the IGA scale (clear, minimal, mild, moderate, or severe). Treatment success was defined as an IGA score of either clear or minimal (with at least a 2-step improvement) at EoT, whereas treatment failure was constituted by IGA scores of mild, moderate, or severe.
The second coprimary end point was the nominal change in ILC from baseline to EoT as determined by the total number of facial papules and pustules. Efficacy and safety parameters were evaluated at weeks 4, 8, and 12, as well as at the end of the 4-week follow-up period. Throughout the study, the investigator, participants, and all study personnel remained blinded.
Safety
Information about adverse events (AEs) was collected at each study visit, and AEs were graded according to seriousness (yes or no) and intensity (mild, moderate, or severe).
Statistical Analysis
Efficacy was confirmed by analysis of the treatment success rate at EoT with Cochran-Mantel-Haenszel test statistics, including a point estimate and 95% confidence interval (CI) for the odds ratio. Change in ILC at EoT was analyzed via an analysis of covariance model using treatment, center, and baseline lesion count as factors. (Additional methods can be found in the Appendix below.)
Results
Study Participants
Of the 1156 patients who were screened for eligibility, 961 were randomized to treatment with AzA foam (n=484) or vehicle (n=477)(Figure 1). Sixty-four (13.2%) participants in the AzA foam group and 79 (16.6%) in the vehicle group discontinued treatment before completing the study. The most common reasons for discontinuation were participant withdrawal from the study and lost to follow-up. Six (1.2%) participants from the AzA foam group and 12 (2.5%) from the vehicle group discontinued because of AEs. All safety and efficacy data presented are based on the full analysis set, which consisted of the 961 participants randomized to treatment.
Demographic and baseline characteristics were balanced between the treatment groups (Table 1). The majority of participants were female (73.0%) and white (95.5%), reflecting the patient populations of independent studies that found a higher prevalence of rosacea in women and lighter skin types.4 There were no significant differences in baseline measures of PPR severity between the treatment groups. Participants in the AzA foam and vehicle groups had a mean ILC of 21.7 and 21.2, respectively, and 76.4% of participants had more than 14 lesions. All participants had an IGA score of moderate (86.8%) or severe (13.2%). Moderate or severe erythema was present in 91.5% of participants.
Treatment compliance, as measured by the percentage of expected doses that were actually administered, was 97.1% in the AzA foam group and 95.9% in the vehicle group.
Efficacy
Results from both primary end points demonstrated superior efficacy of AzA foam over vehicle. The AzA foam group achieved a greater IGA success rate at EoT compared with the vehicle group (32.0% vs 23.5%; Cochran-Mantel-Haenszel test center-adjusted P<.001; odds ratio, 1.6; 95% CI, 1.2-2.2). Treatment success rate was higher in the AzA foam group than in the vehicle group at every time point past baseline (Figure 2). Similarly, the decrease in mean nominal ILC values was greater in the AzA foam group at every time point after baseline (Figure 3), and the treatment difference at EoT was statistically significant in favor of AzA foam (-2.7, F1,920=23.7, P<.001; 95% CI, -3.8 to -1.6). The divergence between treatment groups at week 4 reveals an onset of AzA effect early in the study.
 |
 |
Although the AzA foam group showed significantly better efficacy results than the vehicle group for the coprimary end points, participants in the vehicle group did show appreciable IGA success rates (23.5%) and changes in ILC (-10.3) at EoT (Figures 2 and 3).
Notably, the AzA foam group maintained better results than vehicle for both primary end points even at the end of the 4-week follow-up after EoT (Figures 2 and 3). Sensitivity analysis (data not shown) confirmed the findings from the full analysis set.
Safety
Adverse events were experienced by 149 (30.8%) participants in the AzA foam group and 119 (24.9%) in the vehicle group. The most common noncutaneous AEs (>1% of participants) reported during AzA foam treatment were nasopharyngitis, headache, upper respiratory tract infection, and influenza. In the vehicle group, the most common noncutaneous AEs reported were nasopharyngitis and headache. Drug-related AEs (relationship assessed by the investigator) were reported slightly more often in the AzA foam group (7.6%) than in the vehicle group (4.6%). Drug-related AEs were predominantly cutaneous and occurred at the site of application (Table 2). Drug-related cutaneous AEs were more common in the AzA foam group (7.0%) than in the vehicle group (4.4%). Although serious AEs were more common in the vehicle group, all were regarded as unrelated to the study medication. A single death occurred in the vehicle group due to an accident unrelated to the study drug.
The most frequent drug-related AEs in participants treated with AzA foam versus vehicle were application-site pain (3.5% vs 1.3%), application-site pruritus (1.4% vs 0.4%), and application-site dryness (1.0% vs 0.6%). The classical rosacea symptom of stinging is subsumed under the term application-site pain, according to MedDRA (Medical Dictionary for Regulatory Activities).
All other drug-related AEs occurred at a frequency of less than 1% in participants from both groups. Serious AEs were rare and unrelated to treatment, with 3 AEs reported in the AzA foam group and 4 in the vehicle group. Adverse events leading to study drug withdrawal occurred in less than 2% of participants and were more common in the vehicle group (2.5%) than in the AzA foam group (1.2%). Of the 3 drug-related AEs leading to withdrawal in the AzA foam group, 2 were due to cutaneous reaction and 1 was due to a burning sensation. The number of active drug-related cutaneous AEs was highest during the first 4 weeks of treatment and declined over the course of the study (eFigure).
More than 96% of AEs were resolved by the end of the study. Of the participants experiencing AEs that did not resolve during the course of the study, 16 were in the AzA foam group and 10 in the vehicle group. Six unresolved AEs were drug related, with 3 occurring in each treatment group. Unresolved drug-related cutaneous AEs in the AzA foam group were pain, pruritus, and dryness at the application site.
Comment
Overall, the results from this phase 3 trial demonstrate that the new foam formulation of AzA was efficacious and safe in a 12-week, twice-daily course of treatment for moderate to severe PPR. The AzA foam formulation was significantly superior to vehicle (P<.001) for both primary efficacy end points. Participants in the AzA foam group achieved therapeutic success at a higher rate than the vehicle group, and the change in nominal ILC at EoT was significantly greater for participants treated with AzA foam than for those treated with vehicle (P<.001). Differences between the 2 treatment groups for the coprimary end point measures arose early in the study, demonstrating that symptoms were rapidly controlled. Between weeks 8 and 12 (EoT), the rate of increase of beneficial effects in the AzA foam group remained high, while the vehicle group showed a notable slowing. There was no indication of any rebound effect in overall disease severity subsequent to EoT. After 4 weeks of follow-up, there was still a beneficial treatment effect present in favor of the AzA foam group, as indicated by the persistence of improvements in both coprimary end point measures throughout the follow-up period.
Analyses of alternative populations and secondary end points (data not shown) supported the efficacy results reported here. There was no indication of irregular study center effects, and the sensitivity analyses demonstrated robustness of the data for the observed treatment effects.
The use of vehicle foam alone appeared to be beneficial in reducing ILC and improving IGA rating, which suggests that the properties of the new foam formulation are favorable for the inflamed lesional skin of rosacea. Of note, other dermatology studies, including trials in rosacea, have reported therapeutic effects of vehicle treatment that may be attributable to the positive effects of skin care with certain formulations.20
Azelaic acid foam was well tolerated in the current study. More than 93% of AEs in either treatment group were of mild or moderate severity. The incidence of drug-related AEs was low in both groups and mainly occurred at the application site. There were no drug-related severe or serious AEs. The low incidence of reported drug-related noncutaneous AEs in the AzA foam group (dysgeusia in 1 patient and headache in 2 patients) supports the known favorable systemic tolerance profile of AzA.
Although most drug-related AEs occurred at the application site, they were generally transient, with the majority of events in the AzA foam group lasting no more than 1 hour. Most cutaneous AEs developed early in the study. In the AzA foam group, the prevalence of drug-related cutaneous AEs dropped at every time interval as the study progressed (eFigure). Very few AEs of any type persisted through the end of the study. These safety results were accompanied by a high compliance rate and a high participation rate throughout the course of the study. Taken together, the available data for this AzA foam formulation support a favorable tolerability profile. The results of this study are consistent with and expand on data from an earlier investigation of similar design.8
Conclusion
The development of an AzA foam formulation with higher lipid content was intended to expand the treatment options available to physicians and patients who are managing rosacea. Most topical dermatologic treatments are currently delivered in classical formulations such as creams or gels, but patients who use topical therapies have rated messiness and ease of application among the most important characteristics affecting quality of life.17,21 Foam formulations may offer improvements in this regard; ease of application may minimize unnecessary manipulation of inflamed skin and contribute to a high level of user satisfaction.22 However, the design of the current study was limited to evaluating only the AzA foam formulation versus a foam vehicle, and direct comparisons of clinical efficacy and tolerability to other AzA topical preparations were not performed. Nonetheless, patients have previously reported that they would be more likely to comply with a recommended course of dermatologic foam therapy than other topical formulations.18 The proposed foam formulation was designed to attend to the specific needs of the dry and sensitive skin in rosacea by combining the demonstrated efficacy properties exhibited by AzA gel 15% with the good tolerability and acceptability of a lipid-containing foam formulation. Development of this formulation was targeted to obtain a product that would be highly spreadable, dry quickly, and be easy to apply. The available data for this AzA foam formulation support the value of this option in the topical treatment of rosacea. The success in reduction of overall disease severity, lack of any rebound after EoT, and the observed tolerability and high adherence rates suggest that this novel formulation is a useful addition to current treatment options for rosacea.
Addendum
After release of the study data for unblinding and statistical evaluation, the following inconsistency regarding patient distribution was noted: 1 participant was incorrectly evaluated as part of the AzA foam analysis group when in fact this patient was randomized to vehicle and was treated throughout the study with vehicle. This participant did not experience any AE and did not show any IGA improvement at the EoT. As this single case did not have an impact on the statistical conclusions or interpretation of the results, the released study data have not been changed. This deviation was described as a database erratum in the study report.
Acknowledgement—Editorial support through inVentiv Medical Communications, New York, New York, was provided by Bayer HealthCare Pharmaceuticals Inc.
APPENDIX
Supplementary Methods
Supplementary Study Design
This study met all local legal and regulatory requirements and was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Before the start of the study and implementation, the protocol and all amendments were approved by the appropriate independent ethics committee or institutional review board at each study site. Two protocol amendments were implemented before the first participant visit.
Exclusion criteria included the presence of dermatoses that could interfere with rosacea diagnosis or evaluation, facial laser surgery or topical use of any medication to treat rosacea within 6 weeks before randomization, systemic use of any medications to treat rosacea, and known unresponsiveness to AzA treatment. Further standard exclusion criteria included alcohol or drug use or parallel participation in other clinical studies, which were necessary to exclude undue influence on study evaluations and/or participant safety. The study was conducted by qualified investigators at 48 centers in the United States.
The investigational product was filled in identical containers according to the randomization list generated by a computer program using blocks. Complete blocks of study medication were distributed to the centers. Eligible participants were randomized 1:1 into either AzA foam or vehicle treatment groups by assignment of a randomization number at baseline. A blind investigational product under the same randomization number was dispensed to and returned from participants by study personnel who were not involved in the assessments. Blinding was achieved by using labels on the investigational products that did not allow identification of the true medication.
Compliance was evaluated from participant diaries as well as the number of expected doses and actually applied doses.
Additional Efficacy Evaluations
A number of secondary variables (not reported here) were assessed, including changes in other manifestations of PPR, as well as participant assessments of treatment response, tolerability, cosmetic preferences, and quality of life.
Additional Safety
Investigators reported a yes or no response as to whether there was a reasonable causal relationship between AEs and treatment. Moreover, AEs that began at the start of or during treatment were considered treatment emergent. Cutaneous AEs were further assessed regarding location and duration. An AE was deemed local if it occurred at the application site and transient if it subsided within 60 minutes of onset.
Statistical Analysis
The primary efficacy analyses presented here were based on the full analysis set of participants who were randomized and had medication dispensed. For participants with no EoT value, the last nonmissing value was used including baseline (last-observation-carried-forward methodology). Participants who discontinued treatment prematurely because of lack of efficacy were considered to be treatment failures, regardless of the reported IGA score. Statistical significance was needed for both coprimary efficacy variables at a 1-sided 2.5% significance level to show confirmed superiority of AzA foam versus vehicle.
A number of sensitivity analyses were performed, including an analysis of the coprimary end points using observed data, analysis of the per-protocol population of participants who did not prematurely discontinue treatment and had no major protocol deviations, subgroup analyses, and the use of statistical methods to investigate the effect of missing observations. Analyses of success rate and nominal change in ILC were repeated for each postbaseline visit using χ² and t tests, respectively. All summary and statistical analyses were performed according to the study protocol (unchanged after the start of the study) using SAS version 9.2.
Results from a prior study provided the basis for the sample size, which was calculated to show a significant difference in both primary efficacy end points with a power of 90%.8 To allow for dropouts, 480 participants in each treatment group were to be randomized for a total of 960 participants.
1. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
2. Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
3. Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6:348-354.
4. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
5. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
6. Wollina U. Recent advances in the understanding and management of rosacea. F1000Prime Rep. 2014;6:50.
7. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis. 2014;93:134-138.
8. Draelos ZD, Elewski B, Staedtler G, et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306-317.
9. Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
10. Mastrofrancesco A, Ottaviani M, Aspite N, et al. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARg activation. Exp Dermatol. 2010;19:813-820.
11. Akamatsu H, Komura J, Asada Y, et al. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res. 1991;283:162-166.
12. Coda AB, Hata T, Miller J, et al. Cathelicidin, kalli-krein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol. 2013;69:570-577.
13. van Zuuren EJ, Kramer SF, Carter BR, et al. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760-781.
14. Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48:836-845.
15. Thiboutot DM, Fleischer AB Jr, Del Rosso JQ, et al. Azelaic acid 15% gel once daily versus twice daily in papulopustular rosacea. J Drugs Dermatol. 2008;7:541-546.
16. Finacea [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015.
17. Zhao Y, Jones SA, Brown MB. Dynamic foams in topical drug delivery. J Pharm Pharmacol. 2010;62:678-684.
18. Gottlieb AB, Ford RO, Spellman MC. The efficacy and tolerability of clobetasol propionate foam 0.05% in the treatment of mild to moderate plaque-type psoriasis of nonscalp regions. J Cutan Med Surg. 2003;7:185-192.
19. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771-788.
20. Jackson JM, Pelle M. Topical rosacea therapy: the importance of vehicles for efficacy, tolerability and compliance. J Drugs Dermatol. 2011;10:627-633.
21. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
22. Kircik LH, Bikowski JB. Vehicles matter: topical foam formulations. Practical Dermatology. January 2012(suppl):3-18.
1. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
2. Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
3. Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6:348-354.
4. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
5. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
6. Wollina U. Recent advances in the understanding and management of rosacea. F1000Prime Rep. 2014;6:50.
7. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis. 2014;93:134-138.
8. Draelos ZD, Elewski B, Staedtler G, et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306-317.
9. Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
10. Mastrofrancesco A, Ottaviani M, Aspite N, et al. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARg activation. Exp Dermatol. 2010;19:813-820.
11. Akamatsu H, Komura J, Asada Y, et al. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res. 1991;283:162-166.
12. Coda AB, Hata T, Miller J, et al. Cathelicidin, kalli-krein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol. 2013;69:570-577.
13. van Zuuren EJ, Kramer SF, Carter BR, et al. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760-781.
14. Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48:836-845.
15. Thiboutot DM, Fleischer AB Jr, Del Rosso JQ, et al. Azelaic acid 15% gel once daily versus twice daily in papulopustular rosacea. J Drugs Dermatol. 2008;7:541-546.
16. Finacea [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015.
17. Zhao Y, Jones SA, Brown MB. Dynamic foams in topical drug delivery. J Pharm Pharmacol. 2010;62:678-684.
18. Gottlieb AB, Ford RO, Spellman MC. The efficacy and tolerability of clobetasol propionate foam 0.05% in the treatment of mild to moderate plaque-type psoriasis of nonscalp regions. J Cutan Med Surg. 2003;7:185-192.
19. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771-788.
20. Jackson JM, Pelle M. Topical rosacea therapy: the importance of vehicles for efficacy, tolerability and compliance. J Drugs Dermatol. 2011;10:627-633.
21. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
22. Kircik LH, Bikowski JB. Vehicles matter: topical foam formulations. Practical Dermatology. January 2012(suppl):3-18.