User login
Obesity: Are shared medical appointments part of the answer?
Obesity is a major health problem in the United States. The facts are well known:
- Its prevalence has almost tripled since the early 1960s1
- More than 35% of US adults are obese (body mass index [BMI] ≥ 30 kg/m2)2
- It increases the risk of comorbid conditions including type 2 diabetes mellitus, heart disease, hypertension, obstructive sleep apnea, certain cancers, asthma, and osteoarthritis3,4
- It decreases life expectancy5
- Medical costs are up to 6 times higher per patient.6
Moreover, obesity is often not appropriately managed, owing to a variety of factors. In this article, we describe use of shared medical appointments as a strategy to improve the efficiency and effectiveness of treating patients with obesity.
Big benefits from small changes in weight
As little as 3% to 5% weight loss is associated with significant clinical benefits, such as improved glycemic control, reduced blood pressure, and reduced cholesterol levels.7,8 However, many patients are unable to reach this modest goal using current approaches to obesity management.
This failure is partially related to the complexity and chronic nature of obesity, which requires continued medical management from a multidisciplinary team. We believe this is an area of care that can be appropriately addressed through shared medical appointments.
CURRENT APPROACHES
Interventions for obesity have increased along with the prevalence of the disease. Hundreds of diets, exercise plans, natural products, and behavioral interventions are marketed, all claiming to be successful. More-intense treatment options include antiobesity medications, intra-abdominal weight loss devices, and bariatric surgery. Despite the availability of treatments, rates of obesity have not declined.
Counseling is important, but underused
Lifestyle modifications that encompass nutrition, physical activity, and behavioral interventions are the mainstay of obesity treatment.
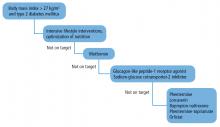
Intensive interventions work better than less-intensive ones. In large clinical trials in overweight patients with diabetes, those who received intensive lifestyle interventions lost 3 to 5 kg more (3% to 8% of body weight) than those who received brief diet and nutrition counseling, as is often performed in a physician’s office.9–12 The US Preventive Services Task Force recommends that patients whose BMI is 30 kg/m2 or higher be offered intensive lifestyle intervention consisting of at least 12 sessions in 1 year.13
But fewer than half of primary care practitioners consistently provide specific guidance on diet, exercise, or weight control to patients with obesity, including those with a weight-related comorbidity.14 The rate has decreased since the 1990s despite the increase in obesity.15
One reason for the underuse is that many primary care practitioners do not have the training or time to deliver the recommended high-intensity obesity treatment.14 Plus, evidence does not clearly show a weight loss benefit from low-intensity interventions. Even when patients lose weight, most regain it, and only 20% are able to maintain their weight loss 1 year after treatment ends.16
Drugs and surgery also underused
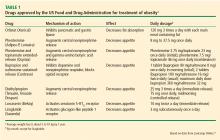
Antiobesity medications and bariatric surgery are effective when added to lifestyle interventions, but they are also underused.
Bariatric surgery provides the greatest and most durable weight loss—15% to 30% of body weight—along with improvement in comorbidities such as type 2 diabetes, and its benefits are sustained for at least 10 years.17 However, fewer than 1% of eligible patients undergo bariatric surgery because of its limited availability, invasive nature, potential complications, limited insurance coverage, and high cost.17
The story is similar for antiobesity drugs. They are useful adjuncts to lifestyle interventions, providing an additional 3% to 7% weight loss,18 but fewer than 2% of eligible patients receive them.19 This may be attributed to their modest effectiveness, weight regain after discontinuation, potential adverse effects, and expense due to lack of insurance coverage.
ARE SHARED MEDICAL APPOINTMEMNTS AN ANSWER?
Although treatments have shown some effectiveness at producing weight loss, none has had a widespread impact on obesity. Lifestyle interventions, drugs, and bariatric surgery continue to be underused. Current treatment models are not providing patients with the intensive interventions needed.
Providers often find themselves offering repetitive advice to patients with obesity regarding nutrition and exercise, while simultaneously trying to manage obesity-related comorbidities, all in a 20-minute appointment. Too often, a patient returns home with prescriptions for hypertension or diabetes but no clear plan for weight management.
What can a shared medical appointment do?
A shared medical appointment is a group medical visit in which several patients with a similar clinical diagnosis, such as obesity, see a multidisciplinary team of healthcare providers. Typically, 5 to 10 patients have consultations with providers during a 60- to 90-minute appointment.20
Part of the session is dedicated to education on the patients’ common medical condition with the goal of improving their self-management, but most of the time is spent addressing individual patient concerns.
Each patient takes a turn consulting with a provider, as in a traditional medical appointment, but in a group setting. This allows others in the group to observe and learn from their peers’ experiences. During this consultation, the patient’s concerns are addressed, medications are managed, necessary tests are ordered, and a treatment plan is made.
Patients can continue to receive follow-up care through shared medical appointments at predetermined times, instead of traditional individual medical appointments.
BENEFITS OF SHARED APPOINTMENTS
Shared medical appointments could improve patient access, clinical outcomes, and patient and provider satisfaction and decrease costs.20,21 Since being introduced in the 1990s, their use has dramatically increased. For example, in the first 2 years of conducting shared medical appointments at Cleveland Clinic (2002–2004), there were just 385 shared medical appointments,21 but in 2017 there were approximately 12,300. They are used in a variety of medical and surgical specialties, and have been studied most for treating diabetes.22–24
Increased face time and access
Individual patient follow-up visits typically last 15 to 20 minutes, limiting the provider to seeing a maximum of 6 patients in 90 minutes. In that same time in the setting of a shared appointment, a multidisciplinary team can see up to 10 patients, and the patients receive up to 90 minutes of time with multiple providers.
Additionally, shared medical appointments can improve patient access to timely appointments. In a busy bariatric surgery practice, implementing shared medical appointments reduced patients’ wait time for an appointment by more than half.25 This is particularly important for patients with obesity, who usually require 12 to 26 appointments per year.
Improved patient outcomes
Use of shared medical appointments has improved clinical outcomes compared with traditional care. Patients with type 2 diabetes who attend shared medical appointments are more likely to reach target hemoglobin A1c and blood pressure levels.22−24 These benefits may be attributed to increased access to care, improved self-management skills, more frequent visits, peer support of the group, and the synergistic knowledge of multiple providers on the shared medical appointment team.
Although some trials reported patient retention rates of 75% to 90% in shared medical appointments, many trials did not report their rates. It is likely that some patients declined randomization to avoid shared medical appointments, which could have led to potential attrition and selection biases.23
Increased patient and provider satisfaction
Both patients and providers report high satisfaction with shared medical appointments.22,26 Although patients may initially hesitate to participate, their opinions significantly improve after attending 1 session.26 From 85% to 90% of patients who attend a shared medical appointment schedule their next follow-up appointment as a shared appointment as well.21,25
In comparative studies, patients who attended shared medical appointments had satisfaction rates equal to or higher than rates in patients who participated in usual care,22 noting better access to care and more sensitivity to their needs.27 Providers report greater satisfaction from working more directly with a team of providers, clearing up a backlogged schedule, and adding variety to their practice.21,24
Decreased costs
Data on the cost-effectiveness of shared medical appointments are mixed; however, some studies have shown that they are associated with a decrease in hospital admissions and emergency department visits.22 It seems reasonable to assume that, in an appropriate patient population, shared medical appointments can be cost-effective owing to increased provider productivity, but more research is needed to verify this.
CHALLENGES TO STARTING SHARED APPOINTMENTS FOR OBESITY
Despite their potential to provide comprehensive care to patients, shared medical appointments have limitations. These need to be addressed before implementing a shared medical appointment program.
Adequate resources and staff training
To be successful, a shared medical appointment program needs to have intensive physical and staffing resources. You need a space large enough to accommodate the group and access to the necessary equipment (eg, projector, whiteboard) for educational sessions. Larger or armless chairs may better accommodate patients with obesity. Facilitators need training in how to lead the group sessions, including time management and handling conflicts between patients. Schedulers and clinical intake staff need training in answering patient questions regarding these appointments.
Maintaining patient attendance
The benefits of provider efficiency rest on having an adequate number of patients attend the shared appointments.21 Patient cancellations and no-shows decrease both the efficiency and cost-effectiveness of this model, and they detract from the peer support and group learning that occurs in the group dynamic. To help minimize patient dropout, a discussion of patient expectations should take place prior to enrollment in shared medical appointments. This should include information on the concept of shared appointments, frequency and duration of appointments, and realistic weight loss goals.
Logistical challenges
A shared medical appointment requires a longer patient time slot and is usually less flexible than an individual appointment. Not all patients can take the time for a prescheduled 60- to 90-minute appointment. However, reduced waiting-room time and increased face time with a provider offset some of these challenges.
Recruiting patients
A shared medical appointment is a novel experience for some, and concerns about it may make it a challenge to recruit patients. Patients might worry that the presence of the group will compromise the patient-doctor relationship. Other concerns include potential irrelevance of other patients’ medical issues and reluctance to participate because of body image and the stigma of obesity.
One solution is to select patients from your existing practice so that the individual patient-provider relationship is established before introducing the concept of shared appointments. You will need to explain how shared appointments work, discuss their pros and cons, stress your expectations about attendance and confidentiality, and address any concerns of the patient. It is also important to emphasize that nearly all patients find shared medical appointments useful.
Once a group is established, it may be a challenge to keep a constant group membership to promote positive group dynamics. In practice, patients may drop out or be added, and facilitators need to be able to integrate new members into the group. It is important to emphasize to the group that obesity is chronic and that patients at all stages and levels of treatment can contribute to group learning.
Despite the advantages of shared medical appointments, some patients may not find them useful, even after attending several sessions. These patients should be offered individual follow-up visits. Also, shared appointments may not be suitable for patients who cannot speak English very well, are hearing-impaired, have significant cognitive impairment, or have acute medical issues.
Maintaining patient confidentiality
Maintaining confidentiality of personal and health information in a shared medical appointment is an important concern for patients but can be appropriately managed. In a survey of patients attending pulmonary hypertension shared medical appointments, 24% had concerns about confidentiality before participating, but after a few sessions, this rate was cut in half.28
Patients have reported initially withholding some information, but over time, they usually become more comfortable with the group and disclose more helpful information.29 Strategies to ensure confidentiality include having patients sign a confidentiality agreement at each appointment, providing specific instruction on what characterizes confidentiality breaches, and allowing patients the opportunity to schedule individual appointments as needed.
Ensuring insurance coverage
A shared medical appointment should be billed as an individual medical appointment for level of care, rather than time spent with the provider. This ensures that insurance coverage and copayments are the same as for individual medical appointments.
Lack of insurance coverage is a major barrier to obesity treatment in general. The US Centers for Medicare and Medicaid Services reimburses intensive behavioral obesity treatment delivered by a primary care practitioner, but limits it to 1 year of treatment and requires patients to meet weight loss goals. Some individual and employer-based healthcare plans do not cover dietitian visits, weight management programs, or antiobesity prescriptions.
EVIDENCE OF EFFECTIVENESS IN OBESITY
Few studies have investigated the use of shared medical appointments in obesity treatment. In the pediatric population, these programs significantly decreased BMI and some other anthropometric measurements,30–32 but they did not consistently involve a prescribing provider. This means they did not manage medications or comorbidities as would be expected in a shared medical appointment.
In adults, reported effects have been encouraging, although the studies are not particularly robust. In a 2-year observational study of a single physician conducting biweekly weight management shared medical appointments, participants lost 1% of their baseline weight, while those continuing with usual care gained 0.8%, a statistically significant difference.33 However, participation rates were low, with patients attending an average of only 3 shared medical appointments during the study.
In a meta-analysis of 13 randomized controlled trials of shared medical appointments for patients with type 2 diabetes, only 3 studies reported weight outcomes.23 These results indicated a trend toward weight loss among patients attending shared appointments, but they were not statistically significant.
Positive results also were reported by the Veterans Administration’s MOVE! (Managing Overweight/obesity for Veterans Everywhere) program.34 Participants in shared medical appointments reported that they felt empowered to make positive lifestyle changes, gained knowledge about obesity, were held accountable by their peers, and appreciated the individualized care they received from the multidisciplinary healthcare teams.
A systematic review involving 336 participants in group-based obesity interventions found group treatment produced more robust weight loss than individual treatment.35 However, shared medical appointments are different from weight loss groups in that they combine an educational session and a medical appointment in a peer-group setting, which requires a provider with prescribing privileges to be present. Thus, shared medical appointments can manage medications as well as weight-related comorbidities such as diabetes, hypertension, polycystic ovarian syndrome, and hyperlipidemia.
One more point is that continued attendance at shared medical appointments, even after successful weight loss, may help to maintain the weight loss, which has otherwise been found to be extremely challenging using traditional medical approaches.
WHO SHOULD BE ON THE TEAM?
Because obesity is multifactorial, it requires a comprehensive treatment approach that can be difficult to deliver given the limited time of an individual appointment. In a shared appointment, providers across multiple specialties can meet with patients at the same time to coordinate approaches to obesity treatment.
A multidisciplinary team for shared medical appointments for obesity needs a physician or a nurse practitioner—or ideally, both— who specializes in obesity to facilitate the session. Other key providers include a registered dietitian, an exercise physiologist, a behavioral health specialist, a sleep specialist, and a social worker to participate as needed in the educational component of the appointment or act as outside consultants.
WHAT ARE REALISTIC TARGETS?
- Nutrition
- Physical activity
- Appetite control
- Sleep
- Stress and mood disorders.
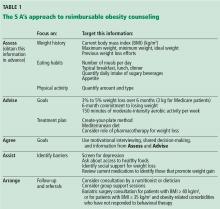
Nutrition
A calorie deficit of 500 to 750 calories per day is recommended for weight loss.7,8 Although there is no consensus on the best nutritional content of a diet, adherence to a diet is a significant predictor of weight loss.36 One reason diets fail to bring about weight loss is that patients tend to underestimate their caloric intake by almost 50%.37 Thus, they may benefit from a structured and supervised diet plan.
A dietitian can help patients develop an individualized diet plan that will promote adherence, which includes specific information on food choices, portion sizes, and timing of meals.
Physical activity
At least 150 minutes of physical activity per week is recommended for weight loss, and 200 to 300 minutes per week is recommended for long-term weight maintenance.7,8
An exercise physiologist can help patients design a personalized exercise plan to help achieve these goals. This plan should take into account the patient’s cardiac status, activity level, degree of mobility, and lifestyle.
Most patients are not able to achieve the recommended physical activity goals initially, and activity levels need to be gradually increased over a period of weeks to months. Patients who were previously inactive or have evidence of cardiovascular, renal, or metabolic disease may require a cardiopulmonary assessment, including an electrocardiogram and cardiac stress test, before starting an exercise program.
Appetite control
It is very difficult for patients to lose weight without appetite control. Weight loss that results from diet and exercise is often accompanied by a change in weight-regulating hormones (eg, leptin, ghrelin, peptide YY, and cholecystokinin) that promote weight regain.38 Thus, multiple compensatory mechanisms promote weight regain through increases in appetite and decreases in energy expenditure, resisting weight loss efforts.
Antiobesity drugs can help mitigate these adaptive weight-promoting responses through several mechanisms. They are indicated for use with lifestyle interventions for patients with a BMI of at least 30 mg/kg2 or a BMI of at least 27 kg/m2 with an obesity-related comorbidity.
These drugs promote an additional 3% to 7% weight loss when added to lifestyle interventions.18 But their effects are limited without appropriate lifestyle interventions.
Sleep
Adequate sleep is an often-overlooked component of obesity treatment. Inadequate sleep is associated with weight gain and an appetite-inducing hormone profile.39 Just 2 days of sleep deprivation in healthy normal-weight adult men was associated with a 70% increase in the ghrelin-to-leptin ratio, which showed a linear relationship with self-reported increased hunger.39 Sleep disorders, especially obstructive sleep apnea, are common in patients with obesity but are often underdiagnosed and undertreated.40
Healthy sleep habits and sleep quality should be addressed in shared medical appointments for obesity, as patients may be unaware of the impact that sleep may be having on their obesity treatment. The STOP-BANG questionnaire (snoring, tiredness, observed apnea, high blood pressure, BMI, age, neck circumference, and male sex) is a simple and reliable tool to screen for obstructive sleep apnea.41 Patients with symptoms of a sleep disorder should be referred to a sleep specialist for diagnosis and management.
Stress management and mood disorders
Stress and psychiatric disorders are underappreciated contributors to obesity. All patients receiving obesity treatment need to be screened for mood disorders and suicidal ideation.8
Chronic stress promotes weight gain through activation of the hypothalamic-pituitary-adrenocortical axis, whereby increased cortisol levels enhance appetite and accumulation of visceral fat.42 In addition, obesity is associated with a 25% increased risk of mood disorders, although the mechanism and direction of this association are unclear.43 Weight gain as a side effect of antidepressant or other psychiatric medications is another important consideration.
Management of stress and psychiatric disorders through goal-setting, self-monitoring, and patient education is vital to help patients fully participate in lifestyle changes and maximize weight loss. Patients participating in shared medical appointments usually benefit from consultations with psychiatrists or psychologists to manage psychiatric comorbidities and assist with adherence to behavior modification.
IN FAVOR OF SHARED MEDICAL APPOINTMENTS FOR OBESITY
Shared medical appointments can be an effective method of addressing the challenges of treating patients with obesity, using a multidisciplinary approach that combines nutrition, physical activity, appetite suppression, sleep improvement, and stress management. In addition, shared appointments allow practitioners to treat the primary problem of excess weight, rather than just its comorbidities, recognizing that obesity is a chronic disease that requires long-term, individualized treatment. Satisfaction rates are high for both patients and providers. Overall, education is essential to implementing and maintaining a successful shared medical appointment program.
- Ogden CL, Carroll MD. National Center for Health Statistics. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960-62 through 2007–2008. www.cdc.gov/nchs/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf. Accessed August 8, 2018.
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016; 315(21):2284–2291. doi:10.1001/jama.2016.6458
- Pantalone KM, Hobbs TM, Chagin KM, et al. Prevalence and recognition of obesity and its associated comorbidities: cross-sectional analysis of electronic health record data from a large US integrated health system. BMJ Open 2017; 7(11):e017583. doi:10.1136/bmjopen-2017-017583
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. doi:10.1186/1471-2458-9-88
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA 2003; 289(2):187–193. pmid:12517229
- Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the United States: a quantitative systematic review. Int Assoc Study Obes Rev 2011; 12(1):50–61. doi:10.1111/j.1467-789X.2009.00708.x
- Jensen MD. Notice of duplicate publication of Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129(25 suppl 2):S102–S138. doi:10.1161/01.cir.0000437739.71477.ee. J Am Coll Cardiol 2014; 63(25 Pt B):2985–3023. doi:10.1016/j.jacc.2013.11.004
- Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity: executive summary. Endocr Pract 2016; 22(7):842–884. doi:10.4158/EP161356.ESGL
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346(6):393–403. doi:10.1056/NEJMoa012512
- Eriksson J, Lindstrom J, Valle T, et al. Prevention of type II diabetes in subjects with impaired glucose tolerance: The Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999; 42(7):793–801. pmid:10440120
- Look AHEAD Research Group; Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007; 30(6):1374–1383. doi:10.2337/dc07-0048
- Burguera B, Jesús Tur J, Escudero AJ, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015; 2015:194696. doi:10.1155/2015/194696
- Moyer VA; US Preventive Services Task Force. Screening for and management of obesity in adults: US Preventative Task Force Recommendation Statement. Ann Intern Med 2012; 157(5):373–378. doi:10.7326/0003-4819-157-5-201209040-00475
- Smith AW, Borowski LA, Liu B, et al. US primary care physicians’ diet-, physical activity-, and weight-related care of adult patients. Am J Prev Med 2011; 41(1):33–42. doi:10.1016/j.amepre.2011.03.017
- Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013; 51(2):186–192. doi:10.1097/MLR.0b013e3182726c33
- Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001; 21:323–341. doi:10.1146/annurev.nutr.21.1.323
- Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017; 14(3):160–169. doi:10.1038/nrgastro.2016.170
- Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014; 311(1):74–86. doi:10.1001/jama.2013.281361
- Xia Y, Kelton CM, Guo JJ, Bian B, Heaton PC. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity (Silver Spring) 2015; 23(8):1721–1728. doi:10.1002/oby.21136
- Ramdas K, Darzi A. Adopting innovations in care delivery—the care of shared medical appointments. N Engl J Med 2017; 376(12):1105–1107. doi:10.1056/NEJMp1612803
- Bronson DL, Maxwell RA. Shared medical appointments: increasing patient access without increasing physician hours. Cleve Clin J Med 2004; 71(5):369–377. pmid:15195773
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ 2013; 185(13):E635–E644. doi:10.1503/cmaj.130053
- Housden LM, Wong ST. Using group medical visits with those who have diabetes: examining the evidence. Curr Diab Rep 2016; 16(12):134. doi:10.1007/s11892-016-0817-4
- Kaidar-Person O, Swartz EW, Lefkowitz M, et al. Shared medical appointments: new concept for high-volume follow-up for bariatric patients. Surg Obes Relat Dis 2006; 2(5):509–512. doi:10.1016/j.soard.2006.05.010
- Seager MJ, Egan RJ, Meredith HE, Bates SE, Norton SA, Morgan JD. Shared medical appointments for bariatric surgery follow-up: a patient satisfaction questionnaire. Obes Surg 2012; 22(4):641–645. doi:10.1007/s11695-012-0603-6
- Heyworth L, Rozenblum R, Burgess JF Jr, et al. Influence of shared medical appointments on patient satisfaction: a retrospective 3-year study. Ann Fam Med 2014; 12(4):324–330. doi:10.1370/afm.1660
- Rahaghi FF, Chastain VL, Benavides R, et al. Shared medical appointments in pulmonary hypertension. Pulm Circ 2014; 4(1):53–60. doi:10.1086/674883
- Wong ST, Lavoie JG, Browne AJ, Macleod ML, Chongo M. Patient confidentiality within the context of group medical visits: Is there cause for concern? Health Expect 2015; 18(5):727–739. doi:10.1111/hex.12156
- Geller JS, Dube ET, Cruz GA, Stevens J, Keating Bench K. Pediatric Obesity Empowerment Model Group Medical Visits (POEM-GMV) as treatment for pediatric obesity in an underserved community. Child Obes 2015; 11(5):638–646. doi:10.1089/chi.2014.0163
- Weigel C, Kokocinski K, Lederer P, Dötsch J, Rascher W, Knerr I. Childhood obesity: concept, feasibility, and interim results of a local group-based, long-term treatment program. J Nutr Educ Behav 2008; 40(6):369–373. doi:10.1016/j.jneb.2007.07.009
- Hinchman J, Beno L, Mims A. Kaiser Permanente Georgia’s experience with operation zero: a group medical appointment to address pediatric overweight. Perm J 2006; 10(3):66–71. pmid:21519478
- Palaniappan LP, Muzaffar AL, Wang EJ, Wong EC, Orchard TJ, Mbbch M. Shared medical appointments: promoting weight loss in a clinical setting. J Am Board Fam Med 2011; 24(3):326–328. doi:10.3122/jabfm.2011.03.100220
- Cohen S, Hartley S, Mavi J, Vest B, Wilson M. Veteran experiences related to participation in shared medical appointments. Mil Med 2012; 177(11):1287–1292. pmid:23198503
- Paul-Ebhohimhen V, Avenell A. A systematic review of the effectiveness of group versus individual treatments for adult obesity. Obes Facts 2009; 2(1):17–24. doi:10.1159/000186144
- Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009; 360(9):859–873. doi:10.1056/NEJMoa0804748
- Lichtman SW, Pisarska K, Berman ER, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med 1992; 327(27):1893–1898. doi:10.1056/NEJM199212313272701
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011; 365(17):1597–1604. doi:10.1056/NEJMoa1105816
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141(11):846–850. pmid:15583226
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in US communities. Sleep Breath 2002; 6(2):49–54. doi:10.1007/s11325-002-0049-5
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108(5):812–821. doi:10.1097/ALN.0b013e31816d83e4
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 2005; 67:259–284. doi:10.1146/annurev.physiol.67.040403.120816
- Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry 2006; 63(7):824-830. doi:10.1001/archpsyc.63.7.824
Obesity is a major health problem in the United States. The facts are well known:
- Its prevalence has almost tripled since the early 1960s1
- More than 35% of US adults are obese (body mass index [BMI] ≥ 30 kg/m2)2
- It increases the risk of comorbid conditions including type 2 diabetes mellitus, heart disease, hypertension, obstructive sleep apnea, certain cancers, asthma, and osteoarthritis3,4
- It decreases life expectancy5
- Medical costs are up to 6 times higher per patient.6
Moreover, obesity is often not appropriately managed, owing to a variety of factors. In this article, we describe use of shared medical appointments as a strategy to improve the efficiency and effectiveness of treating patients with obesity.
Big benefits from small changes in weight
As little as 3% to 5% weight loss is associated with significant clinical benefits, such as improved glycemic control, reduced blood pressure, and reduced cholesterol levels.7,8 However, many patients are unable to reach this modest goal using current approaches to obesity management.
This failure is partially related to the complexity and chronic nature of obesity, which requires continued medical management from a multidisciplinary team. We believe this is an area of care that can be appropriately addressed through shared medical appointments.
CURRENT APPROACHES
Interventions for obesity have increased along with the prevalence of the disease. Hundreds of diets, exercise plans, natural products, and behavioral interventions are marketed, all claiming to be successful. More-intense treatment options include antiobesity medications, intra-abdominal weight loss devices, and bariatric surgery. Despite the availability of treatments, rates of obesity have not declined.
Counseling is important, but underused
Lifestyle modifications that encompass nutrition, physical activity, and behavioral interventions are the mainstay of obesity treatment.
Intensive interventions work better than less-intensive ones. In large clinical trials in overweight patients with diabetes, those who received intensive lifestyle interventions lost 3 to 5 kg more (3% to 8% of body weight) than those who received brief diet and nutrition counseling, as is often performed in a physician’s office.9–12 The US Preventive Services Task Force recommends that patients whose BMI is 30 kg/m2 or higher be offered intensive lifestyle intervention consisting of at least 12 sessions in 1 year.13
But fewer than half of primary care practitioners consistently provide specific guidance on diet, exercise, or weight control to patients with obesity, including those with a weight-related comorbidity.14 The rate has decreased since the 1990s despite the increase in obesity.15
One reason for the underuse is that many primary care practitioners do not have the training or time to deliver the recommended high-intensity obesity treatment.14 Plus, evidence does not clearly show a weight loss benefit from low-intensity interventions. Even when patients lose weight, most regain it, and only 20% are able to maintain their weight loss 1 year after treatment ends.16
Drugs and surgery also underused
Antiobesity medications and bariatric surgery are effective when added to lifestyle interventions, but they are also underused.
Bariatric surgery provides the greatest and most durable weight loss—15% to 30% of body weight—along with improvement in comorbidities such as type 2 diabetes, and its benefits are sustained for at least 10 years.17 However, fewer than 1% of eligible patients undergo bariatric surgery because of its limited availability, invasive nature, potential complications, limited insurance coverage, and high cost.17
The story is similar for antiobesity drugs. They are useful adjuncts to lifestyle interventions, providing an additional 3% to 7% weight loss,18 but fewer than 2% of eligible patients receive them.19 This may be attributed to their modest effectiveness, weight regain after discontinuation, potential adverse effects, and expense due to lack of insurance coverage.
ARE SHARED MEDICAL APPOINTMEMNTS AN ANSWER?
Although treatments have shown some effectiveness at producing weight loss, none has had a widespread impact on obesity. Lifestyle interventions, drugs, and bariatric surgery continue to be underused. Current treatment models are not providing patients with the intensive interventions needed.
Providers often find themselves offering repetitive advice to patients with obesity regarding nutrition and exercise, while simultaneously trying to manage obesity-related comorbidities, all in a 20-minute appointment. Too often, a patient returns home with prescriptions for hypertension or diabetes but no clear plan for weight management.
What can a shared medical appointment do?
A shared medical appointment is a group medical visit in which several patients with a similar clinical diagnosis, such as obesity, see a multidisciplinary team of healthcare providers. Typically, 5 to 10 patients have consultations with providers during a 60- to 90-minute appointment.20
Part of the session is dedicated to education on the patients’ common medical condition with the goal of improving their self-management, but most of the time is spent addressing individual patient concerns.
Each patient takes a turn consulting with a provider, as in a traditional medical appointment, but in a group setting. This allows others in the group to observe and learn from their peers’ experiences. During this consultation, the patient’s concerns are addressed, medications are managed, necessary tests are ordered, and a treatment plan is made.
Patients can continue to receive follow-up care through shared medical appointments at predetermined times, instead of traditional individual medical appointments.
BENEFITS OF SHARED APPOINTMENTS
Shared medical appointments could improve patient access, clinical outcomes, and patient and provider satisfaction and decrease costs.20,21 Since being introduced in the 1990s, their use has dramatically increased. For example, in the first 2 years of conducting shared medical appointments at Cleveland Clinic (2002–2004), there were just 385 shared medical appointments,21 but in 2017 there were approximately 12,300. They are used in a variety of medical and surgical specialties, and have been studied most for treating diabetes.22–24
Increased face time and access
Individual patient follow-up visits typically last 15 to 20 minutes, limiting the provider to seeing a maximum of 6 patients in 90 minutes. In that same time in the setting of a shared appointment, a multidisciplinary team can see up to 10 patients, and the patients receive up to 90 minutes of time with multiple providers.
Additionally, shared medical appointments can improve patient access to timely appointments. In a busy bariatric surgery practice, implementing shared medical appointments reduced patients’ wait time for an appointment by more than half.25 This is particularly important for patients with obesity, who usually require 12 to 26 appointments per year.
Improved patient outcomes
Use of shared medical appointments has improved clinical outcomes compared with traditional care. Patients with type 2 diabetes who attend shared medical appointments are more likely to reach target hemoglobin A1c and blood pressure levels.22−24 These benefits may be attributed to increased access to care, improved self-management skills, more frequent visits, peer support of the group, and the synergistic knowledge of multiple providers on the shared medical appointment team.
Although some trials reported patient retention rates of 75% to 90% in shared medical appointments, many trials did not report their rates. It is likely that some patients declined randomization to avoid shared medical appointments, which could have led to potential attrition and selection biases.23
Increased patient and provider satisfaction
Both patients and providers report high satisfaction with shared medical appointments.22,26 Although patients may initially hesitate to participate, their opinions significantly improve after attending 1 session.26 From 85% to 90% of patients who attend a shared medical appointment schedule their next follow-up appointment as a shared appointment as well.21,25
In comparative studies, patients who attended shared medical appointments had satisfaction rates equal to or higher than rates in patients who participated in usual care,22 noting better access to care and more sensitivity to their needs.27 Providers report greater satisfaction from working more directly with a team of providers, clearing up a backlogged schedule, and adding variety to their practice.21,24
Decreased costs
Data on the cost-effectiveness of shared medical appointments are mixed; however, some studies have shown that they are associated with a decrease in hospital admissions and emergency department visits.22 It seems reasonable to assume that, in an appropriate patient population, shared medical appointments can be cost-effective owing to increased provider productivity, but more research is needed to verify this.
CHALLENGES TO STARTING SHARED APPOINTMENTS FOR OBESITY
Despite their potential to provide comprehensive care to patients, shared medical appointments have limitations. These need to be addressed before implementing a shared medical appointment program.
Adequate resources and staff training
To be successful, a shared medical appointment program needs to have intensive physical and staffing resources. You need a space large enough to accommodate the group and access to the necessary equipment (eg, projector, whiteboard) for educational sessions. Larger or armless chairs may better accommodate patients with obesity. Facilitators need training in how to lead the group sessions, including time management and handling conflicts between patients. Schedulers and clinical intake staff need training in answering patient questions regarding these appointments.
Maintaining patient attendance
The benefits of provider efficiency rest on having an adequate number of patients attend the shared appointments.21 Patient cancellations and no-shows decrease both the efficiency and cost-effectiveness of this model, and they detract from the peer support and group learning that occurs in the group dynamic. To help minimize patient dropout, a discussion of patient expectations should take place prior to enrollment in shared medical appointments. This should include information on the concept of shared appointments, frequency and duration of appointments, and realistic weight loss goals.
Logistical challenges
A shared medical appointment requires a longer patient time slot and is usually less flexible than an individual appointment. Not all patients can take the time for a prescheduled 60- to 90-minute appointment. However, reduced waiting-room time and increased face time with a provider offset some of these challenges.
Recruiting patients
A shared medical appointment is a novel experience for some, and concerns about it may make it a challenge to recruit patients. Patients might worry that the presence of the group will compromise the patient-doctor relationship. Other concerns include potential irrelevance of other patients’ medical issues and reluctance to participate because of body image and the stigma of obesity.
One solution is to select patients from your existing practice so that the individual patient-provider relationship is established before introducing the concept of shared appointments. You will need to explain how shared appointments work, discuss their pros and cons, stress your expectations about attendance and confidentiality, and address any concerns of the patient. It is also important to emphasize that nearly all patients find shared medical appointments useful.
Once a group is established, it may be a challenge to keep a constant group membership to promote positive group dynamics. In practice, patients may drop out or be added, and facilitators need to be able to integrate new members into the group. It is important to emphasize to the group that obesity is chronic and that patients at all stages and levels of treatment can contribute to group learning.
Despite the advantages of shared medical appointments, some patients may not find them useful, even after attending several sessions. These patients should be offered individual follow-up visits. Also, shared appointments may not be suitable for patients who cannot speak English very well, are hearing-impaired, have significant cognitive impairment, or have acute medical issues.
Maintaining patient confidentiality
Maintaining confidentiality of personal and health information in a shared medical appointment is an important concern for patients but can be appropriately managed. In a survey of patients attending pulmonary hypertension shared medical appointments, 24% had concerns about confidentiality before participating, but after a few sessions, this rate was cut in half.28
Patients have reported initially withholding some information, but over time, they usually become more comfortable with the group and disclose more helpful information.29 Strategies to ensure confidentiality include having patients sign a confidentiality agreement at each appointment, providing specific instruction on what characterizes confidentiality breaches, and allowing patients the opportunity to schedule individual appointments as needed.
Ensuring insurance coverage
A shared medical appointment should be billed as an individual medical appointment for level of care, rather than time spent with the provider. This ensures that insurance coverage and copayments are the same as for individual medical appointments.
Lack of insurance coverage is a major barrier to obesity treatment in general. The US Centers for Medicare and Medicaid Services reimburses intensive behavioral obesity treatment delivered by a primary care practitioner, but limits it to 1 year of treatment and requires patients to meet weight loss goals. Some individual and employer-based healthcare plans do not cover dietitian visits, weight management programs, or antiobesity prescriptions.
EVIDENCE OF EFFECTIVENESS IN OBESITY
Few studies have investigated the use of shared medical appointments in obesity treatment. In the pediatric population, these programs significantly decreased BMI and some other anthropometric measurements,30–32 but they did not consistently involve a prescribing provider. This means they did not manage medications or comorbidities as would be expected in a shared medical appointment.
In adults, reported effects have been encouraging, although the studies are not particularly robust. In a 2-year observational study of a single physician conducting biweekly weight management shared medical appointments, participants lost 1% of their baseline weight, while those continuing with usual care gained 0.8%, a statistically significant difference.33 However, participation rates were low, with patients attending an average of only 3 shared medical appointments during the study.
In a meta-analysis of 13 randomized controlled trials of shared medical appointments for patients with type 2 diabetes, only 3 studies reported weight outcomes.23 These results indicated a trend toward weight loss among patients attending shared appointments, but they were not statistically significant.
Positive results also were reported by the Veterans Administration’s MOVE! (Managing Overweight/obesity for Veterans Everywhere) program.34 Participants in shared medical appointments reported that they felt empowered to make positive lifestyle changes, gained knowledge about obesity, were held accountable by their peers, and appreciated the individualized care they received from the multidisciplinary healthcare teams.
A systematic review involving 336 participants in group-based obesity interventions found group treatment produced more robust weight loss than individual treatment.35 However, shared medical appointments are different from weight loss groups in that they combine an educational session and a medical appointment in a peer-group setting, which requires a provider with prescribing privileges to be present. Thus, shared medical appointments can manage medications as well as weight-related comorbidities such as diabetes, hypertension, polycystic ovarian syndrome, and hyperlipidemia.
One more point is that continued attendance at shared medical appointments, even after successful weight loss, may help to maintain the weight loss, which has otherwise been found to be extremely challenging using traditional medical approaches.
WHO SHOULD BE ON THE TEAM?
Because obesity is multifactorial, it requires a comprehensive treatment approach that can be difficult to deliver given the limited time of an individual appointment. In a shared appointment, providers across multiple specialties can meet with patients at the same time to coordinate approaches to obesity treatment.
A multidisciplinary team for shared medical appointments for obesity needs a physician or a nurse practitioner—or ideally, both— who specializes in obesity to facilitate the session. Other key providers include a registered dietitian, an exercise physiologist, a behavioral health specialist, a sleep specialist, and a social worker to participate as needed in the educational component of the appointment or act as outside consultants.
WHAT ARE REALISTIC TARGETS?
- Nutrition
- Physical activity
- Appetite control
- Sleep
- Stress and mood disorders.
Nutrition
A calorie deficit of 500 to 750 calories per day is recommended for weight loss.7,8 Although there is no consensus on the best nutritional content of a diet, adherence to a diet is a significant predictor of weight loss.36 One reason diets fail to bring about weight loss is that patients tend to underestimate their caloric intake by almost 50%.37 Thus, they may benefit from a structured and supervised diet plan.
A dietitian can help patients develop an individualized diet plan that will promote adherence, which includes specific information on food choices, portion sizes, and timing of meals.
Physical activity
At least 150 minutes of physical activity per week is recommended for weight loss, and 200 to 300 minutes per week is recommended for long-term weight maintenance.7,8
An exercise physiologist can help patients design a personalized exercise plan to help achieve these goals. This plan should take into account the patient’s cardiac status, activity level, degree of mobility, and lifestyle.
Most patients are not able to achieve the recommended physical activity goals initially, and activity levels need to be gradually increased over a period of weeks to months. Patients who were previously inactive or have evidence of cardiovascular, renal, or metabolic disease may require a cardiopulmonary assessment, including an electrocardiogram and cardiac stress test, before starting an exercise program.
Appetite control
It is very difficult for patients to lose weight without appetite control. Weight loss that results from diet and exercise is often accompanied by a change in weight-regulating hormones (eg, leptin, ghrelin, peptide YY, and cholecystokinin) that promote weight regain.38 Thus, multiple compensatory mechanisms promote weight regain through increases in appetite and decreases in energy expenditure, resisting weight loss efforts.
Antiobesity drugs can help mitigate these adaptive weight-promoting responses through several mechanisms. They are indicated for use with lifestyle interventions for patients with a BMI of at least 30 mg/kg2 or a BMI of at least 27 kg/m2 with an obesity-related comorbidity.
These drugs promote an additional 3% to 7% weight loss when added to lifestyle interventions.18 But their effects are limited without appropriate lifestyle interventions.
Sleep
Adequate sleep is an often-overlooked component of obesity treatment. Inadequate sleep is associated with weight gain and an appetite-inducing hormone profile.39 Just 2 days of sleep deprivation in healthy normal-weight adult men was associated with a 70% increase in the ghrelin-to-leptin ratio, which showed a linear relationship with self-reported increased hunger.39 Sleep disorders, especially obstructive sleep apnea, are common in patients with obesity but are often underdiagnosed and undertreated.40
Healthy sleep habits and sleep quality should be addressed in shared medical appointments for obesity, as patients may be unaware of the impact that sleep may be having on their obesity treatment. The STOP-BANG questionnaire (snoring, tiredness, observed apnea, high blood pressure, BMI, age, neck circumference, and male sex) is a simple and reliable tool to screen for obstructive sleep apnea.41 Patients with symptoms of a sleep disorder should be referred to a sleep specialist for diagnosis and management.
Stress management and mood disorders
Stress and psychiatric disorders are underappreciated contributors to obesity. All patients receiving obesity treatment need to be screened for mood disorders and suicidal ideation.8
Chronic stress promotes weight gain through activation of the hypothalamic-pituitary-adrenocortical axis, whereby increased cortisol levels enhance appetite and accumulation of visceral fat.42 In addition, obesity is associated with a 25% increased risk of mood disorders, although the mechanism and direction of this association are unclear.43 Weight gain as a side effect of antidepressant or other psychiatric medications is another important consideration.
Management of stress and psychiatric disorders through goal-setting, self-monitoring, and patient education is vital to help patients fully participate in lifestyle changes and maximize weight loss. Patients participating in shared medical appointments usually benefit from consultations with psychiatrists or psychologists to manage psychiatric comorbidities and assist with adherence to behavior modification.
IN FAVOR OF SHARED MEDICAL APPOINTMENTS FOR OBESITY
Shared medical appointments can be an effective method of addressing the challenges of treating patients with obesity, using a multidisciplinary approach that combines nutrition, physical activity, appetite suppression, sleep improvement, and stress management. In addition, shared appointments allow practitioners to treat the primary problem of excess weight, rather than just its comorbidities, recognizing that obesity is a chronic disease that requires long-term, individualized treatment. Satisfaction rates are high for both patients and providers. Overall, education is essential to implementing and maintaining a successful shared medical appointment program.
Obesity is a major health problem in the United States. The facts are well known:
- Its prevalence has almost tripled since the early 1960s1
- More than 35% of US adults are obese (body mass index [BMI] ≥ 30 kg/m2)2
- It increases the risk of comorbid conditions including type 2 diabetes mellitus, heart disease, hypertension, obstructive sleep apnea, certain cancers, asthma, and osteoarthritis3,4
- It decreases life expectancy5
- Medical costs are up to 6 times higher per patient.6
Moreover, obesity is often not appropriately managed, owing to a variety of factors. In this article, we describe use of shared medical appointments as a strategy to improve the efficiency and effectiveness of treating patients with obesity.
Big benefits from small changes in weight
As little as 3% to 5% weight loss is associated with significant clinical benefits, such as improved glycemic control, reduced blood pressure, and reduced cholesterol levels.7,8 However, many patients are unable to reach this modest goal using current approaches to obesity management.
This failure is partially related to the complexity and chronic nature of obesity, which requires continued medical management from a multidisciplinary team. We believe this is an area of care that can be appropriately addressed through shared medical appointments.
CURRENT APPROACHES
Interventions for obesity have increased along with the prevalence of the disease. Hundreds of diets, exercise plans, natural products, and behavioral interventions are marketed, all claiming to be successful. More-intense treatment options include antiobesity medications, intra-abdominal weight loss devices, and bariatric surgery. Despite the availability of treatments, rates of obesity have not declined.
Counseling is important, but underused
Lifestyle modifications that encompass nutrition, physical activity, and behavioral interventions are the mainstay of obesity treatment.
Intensive interventions work better than less-intensive ones. In large clinical trials in overweight patients with diabetes, those who received intensive lifestyle interventions lost 3 to 5 kg more (3% to 8% of body weight) than those who received brief diet and nutrition counseling, as is often performed in a physician’s office.9–12 The US Preventive Services Task Force recommends that patients whose BMI is 30 kg/m2 or higher be offered intensive lifestyle intervention consisting of at least 12 sessions in 1 year.13
But fewer than half of primary care practitioners consistently provide specific guidance on diet, exercise, or weight control to patients with obesity, including those with a weight-related comorbidity.14 The rate has decreased since the 1990s despite the increase in obesity.15
One reason for the underuse is that many primary care practitioners do not have the training or time to deliver the recommended high-intensity obesity treatment.14 Plus, evidence does not clearly show a weight loss benefit from low-intensity interventions. Even when patients lose weight, most regain it, and only 20% are able to maintain their weight loss 1 year after treatment ends.16
Drugs and surgery also underused
Antiobesity medications and bariatric surgery are effective when added to lifestyle interventions, but they are also underused.
Bariatric surgery provides the greatest and most durable weight loss—15% to 30% of body weight—along with improvement in comorbidities such as type 2 diabetes, and its benefits are sustained for at least 10 years.17 However, fewer than 1% of eligible patients undergo bariatric surgery because of its limited availability, invasive nature, potential complications, limited insurance coverage, and high cost.17
The story is similar for antiobesity drugs. They are useful adjuncts to lifestyle interventions, providing an additional 3% to 7% weight loss,18 but fewer than 2% of eligible patients receive them.19 This may be attributed to their modest effectiveness, weight regain after discontinuation, potential adverse effects, and expense due to lack of insurance coverage.
ARE SHARED MEDICAL APPOINTMEMNTS AN ANSWER?
Although treatments have shown some effectiveness at producing weight loss, none has had a widespread impact on obesity. Lifestyle interventions, drugs, and bariatric surgery continue to be underused. Current treatment models are not providing patients with the intensive interventions needed.
Providers often find themselves offering repetitive advice to patients with obesity regarding nutrition and exercise, while simultaneously trying to manage obesity-related comorbidities, all in a 20-minute appointment. Too often, a patient returns home with prescriptions for hypertension or diabetes but no clear plan for weight management.
What can a shared medical appointment do?
A shared medical appointment is a group medical visit in which several patients with a similar clinical diagnosis, such as obesity, see a multidisciplinary team of healthcare providers. Typically, 5 to 10 patients have consultations with providers during a 60- to 90-minute appointment.20
Part of the session is dedicated to education on the patients’ common medical condition with the goal of improving their self-management, but most of the time is spent addressing individual patient concerns.
Each patient takes a turn consulting with a provider, as in a traditional medical appointment, but in a group setting. This allows others in the group to observe and learn from their peers’ experiences. During this consultation, the patient’s concerns are addressed, medications are managed, necessary tests are ordered, and a treatment plan is made.
Patients can continue to receive follow-up care through shared medical appointments at predetermined times, instead of traditional individual medical appointments.
BENEFITS OF SHARED APPOINTMENTS
Shared medical appointments could improve patient access, clinical outcomes, and patient and provider satisfaction and decrease costs.20,21 Since being introduced in the 1990s, their use has dramatically increased. For example, in the first 2 years of conducting shared medical appointments at Cleveland Clinic (2002–2004), there were just 385 shared medical appointments,21 but in 2017 there were approximately 12,300. They are used in a variety of medical and surgical specialties, and have been studied most for treating diabetes.22–24
Increased face time and access
Individual patient follow-up visits typically last 15 to 20 minutes, limiting the provider to seeing a maximum of 6 patients in 90 minutes. In that same time in the setting of a shared appointment, a multidisciplinary team can see up to 10 patients, and the patients receive up to 90 minutes of time with multiple providers.
Additionally, shared medical appointments can improve patient access to timely appointments. In a busy bariatric surgery practice, implementing shared medical appointments reduced patients’ wait time for an appointment by more than half.25 This is particularly important for patients with obesity, who usually require 12 to 26 appointments per year.
Improved patient outcomes
Use of shared medical appointments has improved clinical outcomes compared with traditional care. Patients with type 2 diabetes who attend shared medical appointments are more likely to reach target hemoglobin A1c and blood pressure levels.22−24 These benefits may be attributed to increased access to care, improved self-management skills, more frequent visits, peer support of the group, and the synergistic knowledge of multiple providers on the shared medical appointment team.
Although some trials reported patient retention rates of 75% to 90% in shared medical appointments, many trials did not report their rates. It is likely that some patients declined randomization to avoid shared medical appointments, which could have led to potential attrition and selection biases.23
Increased patient and provider satisfaction
Both patients and providers report high satisfaction with shared medical appointments.22,26 Although patients may initially hesitate to participate, their opinions significantly improve after attending 1 session.26 From 85% to 90% of patients who attend a shared medical appointment schedule their next follow-up appointment as a shared appointment as well.21,25
In comparative studies, patients who attended shared medical appointments had satisfaction rates equal to or higher than rates in patients who participated in usual care,22 noting better access to care and more sensitivity to their needs.27 Providers report greater satisfaction from working more directly with a team of providers, clearing up a backlogged schedule, and adding variety to their practice.21,24
Decreased costs
Data on the cost-effectiveness of shared medical appointments are mixed; however, some studies have shown that they are associated with a decrease in hospital admissions and emergency department visits.22 It seems reasonable to assume that, in an appropriate patient population, shared medical appointments can be cost-effective owing to increased provider productivity, but more research is needed to verify this.
CHALLENGES TO STARTING SHARED APPOINTMENTS FOR OBESITY
Despite their potential to provide comprehensive care to patients, shared medical appointments have limitations. These need to be addressed before implementing a shared medical appointment program.
Adequate resources and staff training
To be successful, a shared medical appointment program needs to have intensive physical and staffing resources. You need a space large enough to accommodate the group and access to the necessary equipment (eg, projector, whiteboard) for educational sessions. Larger or armless chairs may better accommodate patients with obesity. Facilitators need training in how to lead the group sessions, including time management and handling conflicts between patients. Schedulers and clinical intake staff need training in answering patient questions regarding these appointments.
Maintaining patient attendance
The benefits of provider efficiency rest on having an adequate number of patients attend the shared appointments.21 Patient cancellations and no-shows decrease both the efficiency and cost-effectiveness of this model, and they detract from the peer support and group learning that occurs in the group dynamic. To help minimize patient dropout, a discussion of patient expectations should take place prior to enrollment in shared medical appointments. This should include information on the concept of shared appointments, frequency and duration of appointments, and realistic weight loss goals.
Logistical challenges
A shared medical appointment requires a longer patient time slot and is usually less flexible than an individual appointment. Not all patients can take the time for a prescheduled 60- to 90-minute appointment. However, reduced waiting-room time and increased face time with a provider offset some of these challenges.
Recruiting patients
A shared medical appointment is a novel experience for some, and concerns about it may make it a challenge to recruit patients. Patients might worry that the presence of the group will compromise the patient-doctor relationship. Other concerns include potential irrelevance of other patients’ medical issues and reluctance to participate because of body image and the stigma of obesity.
One solution is to select patients from your existing practice so that the individual patient-provider relationship is established before introducing the concept of shared appointments. You will need to explain how shared appointments work, discuss their pros and cons, stress your expectations about attendance and confidentiality, and address any concerns of the patient. It is also important to emphasize that nearly all patients find shared medical appointments useful.
Once a group is established, it may be a challenge to keep a constant group membership to promote positive group dynamics. In practice, patients may drop out or be added, and facilitators need to be able to integrate new members into the group. It is important to emphasize to the group that obesity is chronic and that patients at all stages and levels of treatment can contribute to group learning.
Despite the advantages of shared medical appointments, some patients may not find them useful, even after attending several sessions. These patients should be offered individual follow-up visits. Also, shared appointments may not be suitable for patients who cannot speak English very well, are hearing-impaired, have significant cognitive impairment, or have acute medical issues.
Maintaining patient confidentiality
Maintaining confidentiality of personal and health information in a shared medical appointment is an important concern for patients but can be appropriately managed. In a survey of patients attending pulmonary hypertension shared medical appointments, 24% had concerns about confidentiality before participating, but after a few sessions, this rate was cut in half.28
Patients have reported initially withholding some information, but over time, they usually become more comfortable with the group and disclose more helpful information.29 Strategies to ensure confidentiality include having patients sign a confidentiality agreement at each appointment, providing specific instruction on what characterizes confidentiality breaches, and allowing patients the opportunity to schedule individual appointments as needed.
Ensuring insurance coverage
A shared medical appointment should be billed as an individual medical appointment for level of care, rather than time spent with the provider. This ensures that insurance coverage and copayments are the same as for individual medical appointments.
Lack of insurance coverage is a major barrier to obesity treatment in general. The US Centers for Medicare and Medicaid Services reimburses intensive behavioral obesity treatment delivered by a primary care practitioner, but limits it to 1 year of treatment and requires patients to meet weight loss goals. Some individual and employer-based healthcare plans do not cover dietitian visits, weight management programs, or antiobesity prescriptions.
EVIDENCE OF EFFECTIVENESS IN OBESITY
Few studies have investigated the use of shared medical appointments in obesity treatment. In the pediatric population, these programs significantly decreased BMI and some other anthropometric measurements,30–32 but they did not consistently involve a prescribing provider. This means they did not manage medications or comorbidities as would be expected in a shared medical appointment.
In adults, reported effects have been encouraging, although the studies are not particularly robust. In a 2-year observational study of a single physician conducting biweekly weight management shared medical appointments, participants lost 1% of their baseline weight, while those continuing with usual care gained 0.8%, a statistically significant difference.33 However, participation rates were low, with patients attending an average of only 3 shared medical appointments during the study.
In a meta-analysis of 13 randomized controlled trials of shared medical appointments for patients with type 2 diabetes, only 3 studies reported weight outcomes.23 These results indicated a trend toward weight loss among patients attending shared appointments, but they were not statistically significant.
Positive results also were reported by the Veterans Administration’s MOVE! (Managing Overweight/obesity for Veterans Everywhere) program.34 Participants in shared medical appointments reported that they felt empowered to make positive lifestyle changes, gained knowledge about obesity, were held accountable by their peers, and appreciated the individualized care they received from the multidisciplinary healthcare teams.
A systematic review involving 336 participants in group-based obesity interventions found group treatment produced more robust weight loss than individual treatment.35 However, shared medical appointments are different from weight loss groups in that they combine an educational session and a medical appointment in a peer-group setting, which requires a provider with prescribing privileges to be present. Thus, shared medical appointments can manage medications as well as weight-related comorbidities such as diabetes, hypertension, polycystic ovarian syndrome, and hyperlipidemia.
One more point is that continued attendance at shared medical appointments, even after successful weight loss, may help to maintain the weight loss, which has otherwise been found to be extremely challenging using traditional medical approaches.
WHO SHOULD BE ON THE TEAM?
Because obesity is multifactorial, it requires a comprehensive treatment approach that can be difficult to deliver given the limited time of an individual appointment. In a shared appointment, providers across multiple specialties can meet with patients at the same time to coordinate approaches to obesity treatment.
A multidisciplinary team for shared medical appointments for obesity needs a physician or a nurse practitioner—or ideally, both— who specializes in obesity to facilitate the session. Other key providers include a registered dietitian, an exercise physiologist, a behavioral health specialist, a sleep specialist, and a social worker to participate as needed in the educational component of the appointment or act as outside consultants.
WHAT ARE REALISTIC TARGETS?
- Nutrition
- Physical activity
- Appetite control
- Sleep
- Stress and mood disorders.
Nutrition
A calorie deficit of 500 to 750 calories per day is recommended for weight loss.7,8 Although there is no consensus on the best nutritional content of a diet, adherence to a diet is a significant predictor of weight loss.36 One reason diets fail to bring about weight loss is that patients tend to underestimate their caloric intake by almost 50%.37 Thus, they may benefit from a structured and supervised diet plan.
A dietitian can help patients develop an individualized diet plan that will promote adherence, which includes specific information on food choices, portion sizes, and timing of meals.
Physical activity
At least 150 minutes of physical activity per week is recommended for weight loss, and 200 to 300 minutes per week is recommended for long-term weight maintenance.7,8
An exercise physiologist can help patients design a personalized exercise plan to help achieve these goals. This plan should take into account the patient’s cardiac status, activity level, degree of mobility, and lifestyle.
Most patients are not able to achieve the recommended physical activity goals initially, and activity levels need to be gradually increased over a period of weeks to months. Patients who were previously inactive or have evidence of cardiovascular, renal, or metabolic disease may require a cardiopulmonary assessment, including an electrocardiogram and cardiac stress test, before starting an exercise program.
Appetite control
It is very difficult for patients to lose weight without appetite control. Weight loss that results from diet and exercise is often accompanied by a change in weight-regulating hormones (eg, leptin, ghrelin, peptide YY, and cholecystokinin) that promote weight regain.38 Thus, multiple compensatory mechanisms promote weight regain through increases in appetite and decreases in energy expenditure, resisting weight loss efforts.
Antiobesity drugs can help mitigate these adaptive weight-promoting responses through several mechanisms. They are indicated for use with lifestyle interventions for patients with a BMI of at least 30 mg/kg2 or a BMI of at least 27 kg/m2 with an obesity-related comorbidity.
These drugs promote an additional 3% to 7% weight loss when added to lifestyle interventions.18 But their effects are limited without appropriate lifestyle interventions.
Sleep
Adequate sleep is an often-overlooked component of obesity treatment. Inadequate sleep is associated with weight gain and an appetite-inducing hormone profile.39 Just 2 days of sleep deprivation in healthy normal-weight adult men was associated with a 70% increase in the ghrelin-to-leptin ratio, which showed a linear relationship with self-reported increased hunger.39 Sleep disorders, especially obstructive sleep apnea, are common in patients with obesity but are often underdiagnosed and undertreated.40
Healthy sleep habits and sleep quality should be addressed in shared medical appointments for obesity, as patients may be unaware of the impact that sleep may be having on their obesity treatment. The STOP-BANG questionnaire (snoring, tiredness, observed apnea, high blood pressure, BMI, age, neck circumference, and male sex) is a simple and reliable tool to screen for obstructive sleep apnea.41 Patients with symptoms of a sleep disorder should be referred to a sleep specialist for diagnosis and management.
Stress management and mood disorders
Stress and psychiatric disorders are underappreciated contributors to obesity. All patients receiving obesity treatment need to be screened for mood disorders and suicidal ideation.8
Chronic stress promotes weight gain through activation of the hypothalamic-pituitary-adrenocortical axis, whereby increased cortisol levels enhance appetite and accumulation of visceral fat.42 In addition, obesity is associated with a 25% increased risk of mood disorders, although the mechanism and direction of this association are unclear.43 Weight gain as a side effect of antidepressant or other psychiatric medications is another important consideration.
Management of stress and psychiatric disorders through goal-setting, self-monitoring, and patient education is vital to help patients fully participate in lifestyle changes and maximize weight loss. Patients participating in shared medical appointments usually benefit from consultations with psychiatrists or psychologists to manage psychiatric comorbidities and assist with adherence to behavior modification.
IN FAVOR OF SHARED MEDICAL APPOINTMENTS FOR OBESITY
Shared medical appointments can be an effective method of addressing the challenges of treating patients with obesity, using a multidisciplinary approach that combines nutrition, physical activity, appetite suppression, sleep improvement, and stress management. In addition, shared appointments allow practitioners to treat the primary problem of excess weight, rather than just its comorbidities, recognizing that obesity is a chronic disease that requires long-term, individualized treatment. Satisfaction rates are high for both patients and providers. Overall, education is essential to implementing and maintaining a successful shared medical appointment program.
- Ogden CL, Carroll MD. National Center for Health Statistics. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960-62 through 2007–2008. www.cdc.gov/nchs/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf. Accessed August 8, 2018.
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016; 315(21):2284–2291. doi:10.1001/jama.2016.6458
- Pantalone KM, Hobbs TM, Chagin KM, et al. Prevalence and recognition of obesity and its associated comorbidities: cross-sectional analysis of electronic health record data from a large US integrated health system. BMJ Open 2017; 7(11):e017583. doi:10.1136/bmjopen-2017-017583
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. doi:10.1186/1471-2458-9-88
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA 2003; 289(2):187–193. pmid:12517229
- Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the United States: a quantitative systematic review. Int Assoc Study Obes Rev 2011; 12(1):50–61. doi:10.1111/j.1467-789X.2009.00708.x
- Jensen MD. Notice of duplicate publication of Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129(25 suppl 2):S102–S138. doi:10.1161/01.cir.0000437739.71477.ee. J Am Coll Cardiol 2014; 63(25 Pt B):2985–3023. doi:10.1016/j.jacc.2013.11.004
- Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity: executive summary. Endocr Pract 2016; 22(7):842–884. doi:10.4158/EP161356.ESGL
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346(6):393–403. doi:10.1056/NEJMoa012512
- Eriksson J, Lindstrom J, Valle T, et al. Prevention of type II diabetes in subjects with impaired glucose tolerance: The Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999; 42(7):793–801. pmid:10440120
- Look AHEAD Research Group; Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007; 30(6):1374–1383. doi:10.2337/dc07-0048
- Burguera B, Jesús Tur J, Escudero AJ, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015; 2015:194696. doi:10.1155/2015/194696
- Moyer VA; US Preventive Services Task Force. Screening for and management of obesity in adults: US Preventative Task Force Recommendation Statement. Ann Intern Med 2012; 157(5):373–378. doi:10.7326/0003-4819-157-5-201209040-00475
- Smith AW, Borowski LA, Liu B, et al. US primary care physicians’ diet-, physical activity-, and weight-related care of adult patients. Am J Prev Med 2011; 41(1):33–42. doi:10.1016/j.amepre.2011.03.017
- Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013; 51(2):186–192. doi:10.1097/MLR.0b013e3182726c33
- Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001; 21:323–341. doi:10.1146/annurev.nutr.21.1.323
- Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017; 14(3):160–169. doi:10.1038/nrgastro.2016.170
- Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014; 311(1):74–86. doi:10.1001/jama.2013.281361
- Xia Y, Kelton CM, Guo JJ, Bian B, Heaton PC. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity (Silver Spring) 2015; 23(8):1721–1728. doi:10.1002/oby.21136
- Ramdas K, Darzi A. Adopting innovations in care delivery—the care of shared medical appointments. N Engl J Med 2017; 376(12):1105–1107. doi:10.1056/NEJMp1612803
- Bronson DL, Maxwell RA. Shared medical appointments: increasing patient access without increasing physician hours. Cleve Clin J Med 2004; 71(5):369–377. pmid:15195773
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ 2013; 185(13):E635–E644. doi:10.1503/cmaj.130053
- Housden LM, Wong ST. Using group medical visits with those who have diabetes: examining the evidence. Curr Diab Rep 2016; 16(12):134. doi:10.1007/s11892-016-0817-4
- Kaidar-Person O, Swartz EW, Lefkowitz M, et al. Shared medical appointments: new concept for high-volume follow-up for bariatric patients. Surg Obes Relat Dis 2006; 2(5):509–512. doi:10.1016/j.soard.2006.05.010
- Seager MJ, Egan RJ, Meredith HE, Bates SE, Norton SA, Morgan JD. Shared medical appointments for bariatric surgery follow-up: a patient satisfaction questionnaire. Obes Surg 2012; 22(4):641–645. doi:10.1007/s11695-012-0603-6
- Heyworth L, Rozenblum R, Burgess JF Jr, et al. Influence of shared medical appointments on patient satisfaction: a retrospective 3-year study. Ann Fam Med 2014; 12(4):324–330. doi:10.1370/afm.1660
- Rahaghi FF, Chastain VL, Benavides R, et al. Shared medical appointments in pulmonary hypertension. Pulm Circ 2014; 4(1):53–60. doi:10.1086/674883
- Wong ST, Lavoie JG, Browne AJ, Macleod ML, Chongo M. Patient confidentiality within the context of group medical visits: Is there cause for concern? Health Expect 2015; 18(5):727–739. doi:10.1111/hex.12156
- Geller JS, Dube ET, Cruz GA, Stevens J, Keating Bench K. Pediatric Obesity Empowerment Model Group Medical Visits (POEM-GMV) as treatment for pediatric obesity in an underserved community. Child Obes 2015; 11(5):638–646. doi:10.1089/chi.2014.0163
- Weigel C, Kokocinski K, Lederer P, Dötsch J, Rascher W, Knerr I. Childhood obesity: concept, feasibility, and interim results of a local group-based, long-term treatment program. J Nutr Educ Behav 2008; 40(6):369–373. doi:10.1016/j.jneb.2007.07.009
- Hinchman J, Beno L, Mims A. Kaiser Permanente Georgia’s experience with operation zero: a group medical appointment to address pediatric overweight. Perm J 2006; 10(3):66–71. pmid:21519478
- Palaniappan LP, Muzaffar AL, Wang EJ, Wong EC, Orchard TJ, Mbbch M. Shared medical appointments: promoting weight loss in a clinical setting. J Am Board Fam Med 2011; 24(3):326–328. doi:10.3122/jabfm.2011.03.100220
- Cohen S, Hartley S, Mavi J, Vest B, Wilson M. Veteran experiences related to participation in shared medical appointments. Mil Med 2012; 177(11):1287–1292. pmid:23198503
- Paul-Ebhohimhen V, Avenell A. A systematic review of the effectiveness of group versus individual treatments for adult obesity. Obes Facts 2009; 2(1):17–24. doi:10.1159/000186144
- Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009; 360(9):859–873. doi:10.1056/NEJMoa0804748
- Lichtman SW, Pisarska K, Berman ER, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med 1992; 327(27):1893–1898. doi:10.1056/NEJM199212313272701
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011; 365(17):1597–1604. doi:10.1056/NEJMoa1105816
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141(11):846–850. pmid:15583226
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in US communities. Sleep Breath 2002; 6(2):49–54. doi:10.1007/s11325-002-0049-5
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108(5):812–821. doi:10.1097/ALN.0b013e31816d83e4
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 2005; 67:259–284. doi:10.1146/annurev.physiol.67.040403.120816
- Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry 2006; 63(7):824-830. doi:10.1001/archpsyc.63.7.824
- Ogden CL, Carroll MD. National Center for Health Statistics. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960-62 through 2007–2008. www.cdc.gov/nchs/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf. Accessed August 8, 2018.
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016; 315(21):2284–2291. doi:10.1001/jama.2016.6458
- Pantalone KM, Hobbs TM, Chagin KM, et al. Prevalence and recognition of obesity and its associated comorbidities: cross-sectional analysis of electronic health record data from a large US integrated health system. BMJ Open 2017; 7(11):e017583. doi:10.1136/bmjopen-2017-017583
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. doi:10.1186/1471-2458-9-88
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA 2003; 289(2):187–193. pmid:12517229
- Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the United States: a quantitative systematic review. Int Assoc Study Obes Rev 2011; 12(1):50–61. doi:10.1111/j.1467-789X.2009.00708.x
- Jensen MD. Notice of duplicate publication of Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129(25 suppl 2):S102–S138. doi:10.1161/01.cir.0000437739.71477.ee. J Am Coll Cardiol 2014; 63(25 Pt B):2985–3023. doi:10.1016/j.jacc.2013.11.004
- Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity: executive summary. Endocr Pract 2016; 22(7):842–884. doi:10.4158/EP161356.ESGL
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346(6):393–403. doi:10.1056/NEJMoa012512
- Eriksson J, Lindstrom J, Valle T, et al. Prevention of type II diabetes in subjects with impaired glucose tolerance: The Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999; 42(7):793–801. pmid:10440120
- Look AHEAD Research Group; Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007; 30(6):1374–1383. doi:10.2337/dc07-0048
- Burguera B, Jesús Tur J, Escudero AJ, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015; 2015:194696. doi:10.1155/2015/194696
- Moyer VA; US Preventive Services Task Force. Screening for and management of obesity in adults: US Preventative Task Force Recommendation Statement. Ann Intern Med 2012; 157(5):373–378. doi:10.7326/0003-4819-157-5-201209040-00475
- Smith AW, Borowski LA, Liu B, et al. US primary care physicians’ diet-, physical activity-, and weight-related care of adult patients. Am J Prev Med 2011; 41(1):33–42. doi:10.1016/j.amepre.2011.03.017
- Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013; 51(2):186–192. doi:10.1097/MLR.0b013e3182726c33
- Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001; 21:323–341. doi:10.1146/annurev.nutr.21.1.323
- Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017; 14(3):160–169. doi:10.1038/nrgastro.2016.170
- Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014; 311(1):74–86. doi:10.1001/jama.2013.281361
- Xia Y, Kelton CM, Guo JJ, Bian B, Heaton PC. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity (Silver Spring) 2015; 23(8):1721–1728. doi:10.1002/oby.21136
- Ramdas K, Darzi A. Adopting innovations in care delivery—the care of shared medical appointments. N Engl J Med 2017; 376(12):1105–1107. doi:10.1056/NEJMp1612803
- Bronson DL, Maxwell RA. Shared medical appointments: increasing patient access without increasing physician hours. Cleve Clin J Med 2004; 71(5):369–377. pmid:15195773
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ 2013; 185(13):E635–E644. doi:10.1503/cmaj.130053
- Housden LM, Wong ST. Using group medical visits with those who have diabetes: examining the evidence. Curr Diab Rep 2016; 16(12):134. doi:10.1007/s11892-016-0817-4
- Kaidar-Person O, Swartz EW, Lefkowitz M, et al. Shared medical appointments: new concept for high-volume follow-up for bariatric patients. Surg Obes Relat Dis 2006; 2(5):509–512. doi:10.1016/j.soard.2006.05.010
- Seager MJ, Egan RJ, Meredith HE, Bates SE, Norton SA, Morgan JD. Shared medical appointments for bariatric surgery follow-up: a patient satisfaction questionnaire. Obes Surg 2012; 22(4):641–645. doi:10.1007/s11695-012-0603-6
- Heyworth L, Rozenblum R, Burgess JF Jr, et al. Influence of shared medical appointments on patient satisfaction: a retrospective 3-year study. Ann Fam Med 2014; 12(4):324–330. doi:10.1370/afm.1660
- Rahaghi FF, Chastain VL, Benavides R, et al. Shared medical appointments in pulmonary hypertension. Pulm Circ 2014; 4(1):53–60. doi:10.1086/674883
- Wong ST, Lavoie JG, Browne AJ, Macleod ML, Chongo M. Patient confidentiality within the context of group medical visits: Is there cause for concern? Health Expect 2015; 18(5):727–739. doi:10.1111/hex.12156
- Geller JS, Dube ET, Cruz GA, Stevens J, Keating Bench K. Pediatric Obesity Empowerment Model Group Medical Visits (POEM-GMV) as treatment for pediatric obesity in an underserved community. Child Obes 2015; 11(5):638–646. doi:10.1089/chi.2014.0163
- Weigel C, Kokocinski K, Lederer P, Dötsch J, Rascher W, Knerr I. Childhood obesity: concept, feasibility, and interim results of a local group-based, long-term treatment program. J Nutr Educ Behav 2008; 40(6):369–373. doi:10.1016/j.jneb.2007.07.009
- Hinchman J, Beno L, Mims A. Kaiser Permanente Georgia’s experience with operation zero: a group medical appointment to address pediatric overweight. Perm J 2006; 10(3):66–71. pmid:21519478
- Palaniappan LP, Muzaffar AL, Wang EJ, Wong EC, Orchard TJ, Mbbch M. Shared medical appointments: promoting weight loss in a clinical setting. J Am Board Fam Med 2011; 24(3):326–328. doi:10.3122/jabfm.2011.03.100220
- Cohen S, Hartley S, Mavi J, Vest B, Wilson M. Veteran experiences related to participation in shared medical appointments. Mil Med 2012; 177(11):1287–1292. pmid:23198503
- Paul-Ebhohimhen V, Avenell A. A systematic review of the effectiveness of group versus individual treatments for adult obesity. Obes Facts 2009; 2(1):17–24. doi:10.1159/000186144
- Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009; 360(9):859–873. doi:10.1056/NEJMoa0804748
- Lichtman SW, Pisarska K, Berman ER, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med 1992; 327(27):1893–1898. doi:10.1056/NEJM199212313272701
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011; 365(17):1597–1604. doi:10.1056/NEJMoa1105816
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141(11):846–850. pmid:15583226
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in US communities. Sleep Breath 2002; 6(2):49–54. doi:10.1007/s11325-002-0049-5
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108(5):812–821. doi:10.1097/ALN.0b013e31816d83e4
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 2005; 67:259–284. doi:10.1146/annurev.physiol.67.040403.120816
- Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry 2006; 63(7):824-830. doi:10.1001/archpsyc.63.7.824
KEY POINTS
- Shared medical appointments have been shown to improve clinical outcomes and patient satisfaction compared with traditional care. However, they have not been well studied in patients with obesity.
- A shared medical appointment allows multiple patients to be medically managed by a multidisciplinary team, promoting more efficient delivery of care.
- Both patients and practitioners are satisfied with shared medical appointments and find them clinically useful.
Can effective obesity counseling fit into the 20-minute appointment?
Yes, by using a pre-visit questionnaire that zeroes in on weight history, eating habits, and level of physical activity. This information will lay the foundation for effective weight loss counseling and interventions consistent with intensive behavioral therapy for obesity, reimbursable by Medicare.1
More than one-third of US adults are obese.3 And even though the rate of obesity in adults has leveled off since 2009,3 more needs to be done to bend the arc of the national obesity trend. Clinicians tend to focus on the complications of obesity (coronary artery disease, type 2 diabetes, hypertension, hyperlipidemia) rather than on early identification and intervention of obesity itself.4–6 A national study of outpatient visits showed that only 29% of visits by patients who were obese according to their body mass index (BMI) had a documented diagnosis of obesity, suggesting a profound underdiagnosis of obesity.7 According to one study, primary care doctors lack the level of comfort and counseling experience needed to provide obesity and weight loss counseling.8 Yet recent changes to Medicare reimbursement encourage obesity screening and management by covering up to 20 visits for intensive behavioral therapy to treat obesity.1
We offer the following targeted approach to counseling, achievable within the context of a primary care visit and based on recent evidence, including the 2013 joint guidelines for the treatment of obesity of the American College of Cardiology, the American Heart Association Task Force on Practice Guidelines, and the Obesity Society.2
START WITH SCREENING
Measure the patient’s height and weight with the patient wearing light clothing and no shoes, and calculate the BMI as the weight in kilograms divided by the square of the height in meters. A BMI of 30 kg/m2 or greater defines obesity.
OBTAIN AN OBESITY HISTORY
According to the 2013 joint guidelines,2 when obtaining a thorough obesity history, the physician should do the following:
- Obtain information about weight the patient has gained and lost over time and previous weight loss efforts
- Ask the patient about eating habits, including number of meals per day, and the contents of a typical breakfast, lunch, and dinner; we recommend also asking about the number of daily beverages high in sugar
- Quantify the type and amount of physical activity performed within a specific time period.
This information can be obtained in advance of an office visit through either an electronic medical record portal or a pre-visit questionnaire (eg, http://onlinelibrary.wiley.com/doi/10.1038/oby.2002.205/full).
Also assess the patient’s risk of cardiovascular and obesity-related comorbidities. The waist circumference for patients with a BMI between 25 and 35 kg/m2 provides additional information on risk: eg, a waist circumference greater than 88 cm for women and greater than 102 cm for men indicates increased cardiometabolic risk.2
SUGGEST SPECIFIC GOALS
Use a shared decision-making process to arrive at a set of incremental goals centered around the following evidence-based targets2:
- Weight loss: 3% to 5% of baseline weight within 6 months
- 6-month commitment to a weight loss intervention
- Exercise: at least 150 minutes of moderate aerobic activity per week
- More vegetables, fewer carbohydrates, and less protein, according to the American Diabetes Association’s “Create your plate” plan9
- Mediterranean diet.10
Use motivational interviewing techniques along with the obesity history to negotiate goals. Exercise-related goals should consider the patient’s cardiovascular and musculoskeletal comorbidities.
CO-DEVELOP A TREATMENT PLAN AND ADDRESS POTENTIAL BARRIERS
The most effective weight loss treatment consists of in-person consultations in which comprehensive lifestyle interventions are included. The components of an effective intervention (Table 1) include a reduced-calorie diet, aerobic physical activity, and behavioral strategies to meaningfully support these changes.2
We recommend addressing potential barriers to initiating and maintaining weight-loss interventions, and revisiting them during follow-up visits. Barriers include the following:
Depression
Adults with depression are more likely to be obese than adults without depression, and the age-adjusted percentage of adults who are obese increases as depression severity increases.11
Access to healthy foods
Limited access to healthy food choices can lead to poor diets and higher levels of obesity.12 Local grocery store websites and nutrition specialists can help identify a range of healthy and affordable food to sustain a dietary intervention.
Medications associated with weight gain
Certain diabetic medications, contraceptives, tricyclic antidepressants, atypical antipsychotics, antiseizure drugs, and glucocorticoids promote weight gain and may have alternatives that do not promote weight gain.13
ARRANGE FOLLOW-UP AND REFERRALS
The literature supports frequent in-person sessions as the basis for a successful weight loss intervention (ie, ≥ 14 sessions in 6 months).2 Medicare beneficiaries are eligible for 14 covered visits in the first 6 months and become eligible for an additional monthly visit over the course of 6 subsequent months if a weight loss goal of 3 kg is met in the first 6-month period.
Nutritionists, dieticians, and behavioral psychologists are often instrumental in comprehensive weight loss interventions. Antiobesity drugs help curb appetite, promote weight loss, help enhance adherence to lifestyle modifications, and make it easier for patients to start a program of physical activity.14
The joint 2013 guidelines2 recommend referral for bariatric surgery for adults with a BMI 40 kg/m2 or higher, or for adults with a BMI 35 kg/m2 or higher and obesity-related comorbidities who have not responded to behavioral treatment (with or without pharmacotherapy).
A growing body of evidence promotes the use of group support sessions such as shared medical appointments to encourage healthy eating and physical activity.15
OBESITY COUNSELING IS ACHIEVABLE AND REIMBURSABLE
To receive reimbursement from Medicare for obesity counseling, the information listed under “assess” and “advise” in Table 1 should be obtained in the initial visit; and follow-up visits should be used to address items under “agree,” “assist,” and “arrange.” Up to 20 visits are eligible for reimbursement when patients meet the goal of a 3-kg weight loss in the first 6 months (or 14 visits).
- Centers for Medicare and Medicaid Services. Decision memo for intensive behavioral therapy for obesity (CAG-00423N). www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253. Accessed June 5, 2017.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol 2014; 63:2985–3023.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief 2013; 131:1–8.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001; 50:513–518.
- Galuska D, Will J, Serdula M, Ford E. Are health care professionals advising obese patients to lose weight? JAMA 1999; 282:1576–1578.
- Nawaz H, Adams ML, Katz DL. Weight loss counseling by health care providers. Am J Public Health 1999; 89:764–767.
- Ma J, Xiao L, Stafford R. Underdiagnosis of obesity in adults in US outpatient settings. Arch Intern Med 2009; 169:313–314.
- Huang J, Yu H, Marin E, Brock S, Carden D, Davis T. Physicians’ weight loss counseling in two public hospital primary care clinics. Acad Med 2004; 79:156–161.
- American Diabetes Association. Create your plate. www.diabetes.org/food-and-fitness/food/planning-meals/create-your-plate. Accessed May 19, 2017.
- Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev 2006; 64:S27–S47.
- Pratt LA, Brody DJ. Depression and obesity in the US adult household population, 2005-2010. NCHS Data Brief 2014; 167:1–8.
- Gordon-Larsen P. Food availability/convenience and obesity. Adv Nutr 2014; 5:809–817.
- Malone M. Medications associated with weight gain. Ann Pharmacother 2005; 39:2046–2055.
- Patel D. Pharmacotherapy for the management of obesity. Metabolism 2015; 64:1376–1385.
- Guthrie GE, Bogue RJ. Impact of a shared medical appointment lifestyle intervention on weight and lipid parameters in individuals with type 2 diabetes: a clinical pilot. J Am Coll Nutr 2015; 34:300–309.
Yes, by using a pre-visit questionnaire that zeroes in on weight history, eating habits, and level of physical activity. This information will lay the foundation for effective weight loss counseling and interventions consistent with intensive behavioral therapy for obesity, reimbursable by Medicare.1
More than one-third of US adults are obese.3 And even though the rate of obesity in adults has leveled off since 2009,3 more needs to be done to bend the arc of the national obesity trend. Clinicians tend to focus on the complications of obesity (coronary artery disease, type 2 diabetes, hypertension, hyperlipidemia) rather than on early identification and intervention of obesity itself.4–6 A national study of outpatient visits showed that only 29% of visits by patients who were obese according to their body mass index (BMI) had a documented diagnosis of obesity, suggesting a profound underdiagnosis of obesity.7 According to one study, primary care doctors lack the level of comfort and counseling experience needed to provide obesity and weight loss counseling.8 Yet recent changes to Medicare reimbursement encourage obesity screening and management by covering up to 20 visits for intensive behavioral therapy to treat obesity.1
We offer the following targeted approach to counseling, achievable within the context of a primary care visit and based on recent evidence, including the 2013 joint guidelines for the treatment of obesity of the American College of Cardiology, the American Heart Association Task Force on Practice Guidelines, and the Obesity Society.2
START WITH SCREENING
Measure the patient’s height and weight with the patient wearing light clothing and no shoes, and calculate the BMI as the weight in kilograms divided by the square of the height in meters. A BMI of 30 kg/m2 or greater defines obesity.
OBTAIN AN OBESITY HISTORY
According to the 2013 joint guidelines,2 when obtaining a thorough obesity history, the physician should do the following:
- Obtain information about weight the patient has gained and lost over time and previous weight loss efforts
- Ask the patient about eating habits, including number of meals per day, and the contents of a typical breakfast, lunch, and dinner; we recommend also asking about the number of daily beverages high in sugar
- Quantify the type and amount of physical activity performed within a specific time period.
This information can be obtained in advance of an office visit through either an electronic medical record portal or a pre-visit questionnaire (eg, http://onlinelibrary.wiley.com/doi/10.1038/oby.2002.205/full).
Also assess the patient’s risk of cardiovascular and obesity-related comorbidities. The waist circumference for patients with a BMI between 25 and 35 kg/m2 provides additional information on risk: eg, a waist circumference greater than 88 cm for women and greater than 102 cm for men indicates increased cardiometabolic risk.2
SUGGEST SPECIFIC GOALS
Use a shared decision-making process to arrive at a set of incremental goals centered around the following evidence-based targets2:
- Weight loss: 3% to 5% of baseline weight within 6 months
- 6-month commitment to a weight loss intervention
- Exercise: at least 150 minutes of moderate aerobic activity per week
- More vegetables, fewer carbohydrates, and less protein, according to the American Diabetes Association’s “Create your plate” plan9
- Mediterranean diet.10
Use motivational interviewing techniques along with the obesity history to negotiate goals. Exercise-related goals should consider the patient’s cardiovascular and musculoskeletal comorbidities.
CO-DEVELOP A TREATMENT PLAN AND ADDRESS POTENTIAL BARRIERS
The most effective weight loss treatment consists of in-person consultations in which comprehensive lifestyle interventions are included. The components of an effective intervention (Table 1) include a reduced-calorie diet, aerobic physical activity, and behavioral strategies to meaningfully support these changes.2
We recommend addressing potential barriers to initiating and maintaining weight-loss interventions, and revisiting them during follow-up visits. Barriers include the following:
Depression
Adults with depression are more likely to be obese than adults without depression, and the age-adjusted percentage of adults who are obese increases as depression severity increases.11
Access to healthy foods
Limited access to healthy food choices can lead to poor diets and higher levels of obesity.12 Local grocery store websites and nutrition specialists can help identify a range of healthy and affordable food to sustain a dietary intervention.
Medications associated with weight gain
Certain diabetic medications, contraceptives, tricyclic antidepressants, atypical antipsychotics, antiseizure drugs, and glucocorticoids promote weight gain and may have alternatives that do not promote weight gain.13
ARRANGE FOLLOW-UP AND REFERRALS
The literature supports frequent in-person sessions as the basis for a successful weight loss intervention (ie, ≥ 14 sessions in 6 months).2 Medicare beneficiaries are eligible for 14 covered visits in the first 6 months and become eligible for an additional monthly visit over the course of 6 subsequent months if a weight loss goal of 3 kg is met in the first 6-month period.
Nutritionists, dieticians, and behavioral psychologists are often instrumental in comprehensive weight loss interventions. Antiobesity drugs help curb appetite, promote weight loss, help enhance adherence to lifestyle modifications, and make it easier for patients to start a program of physical activity.14
The joint 2013 guidelines2 recommend referral for bariatric surgery for adults with a BMI 40 kg/m2 or higher, or for adults with a BMI 35 kg/m2 or higher and obesity-related comorbidities who have not responded to behavioral treatment (with or without pharmacotherapy).
A growing body of evidence promotes the use of group support sessions such as shared medical appointments to encourage healthy eating and physical activity.15
OBESITY COUNSELING IS ACHIEVABLE AND REIMBURSABLE
To receive reimbursement from Medicare for obesity counseling, the information listed under “assess” and “advise” in Table 1 should be obtained in the initial visit; and follow-up visits should be used to address items under “agree,” “assist,” and “arrange.” Up to 20 visits are eligible for reimbursement when patients meet the goal of a 3-kg weight loss in the first 6 months (or 14 visits).
Yes, by using a pre-visit questionnaire that zeroes in on weight history, eating habits, and level of physical activity. This information will lay the foundation for effective weight loss counseling and interventions consistent with intensive behavioral therapy for obesity, reimbursable by Medicare.1
More than one-third of US adults are obese.3 And even though the rate of obesity in adults has leveled off since 2009,3 more needs to be done to bend the arc of the national obesity trend. Clinicians tend to focus on the complications of obesity (coronary artery disease, type 2 diabetes, hypertension, hyperlipidemia) rather than on early identification and intervention of obesity itself.4–6 A national study of outpatient visits showed that only 29% of visits by patients who were obese according to their body mass index (BMI) had a documented diagnosis of obesity, suggesting a profound underdiagnosis of obesity.7 According to one study, primary care doctors lack the level of comfort and counseling experience needed to provide obesity and weight loss counseling.8 Yet recent changes to Medicare reimbursement encourage obesity screening and management by covering up to 20 visits for intensive behavioral therapy to treat obesity.1
We offer the following targeted approach to counseling, achievable within the context of a primary care visit and based on recent evidence, including the 2013 joint guidelines for the treatment of obesity of the American College of Cardiology, the American Heart Association Task Force on Practice Guidelines, and the Obesity Society.2
START WITH SCREENING
Measure the patient’s height and weight with the patient wearing light clothing and no shoes, and calculate the BMI as the weight in kilograms divided by the square of the height in meters. A BMI of 30 kg/m2 or greater defines obesity.
OBTAIN AN OBESITY HISTORY
According to the 2013 joint guidelines,2 when obtaining a thorough obesity history, the physician should do the following:
- Obtain information about weight the patient has gained and lost over time and previous weight loss efforts
- Ask the patient about eating habits, including number of meals per day, and the contents of a typical breakfast, lunch, and dinner; we recommend also asking about the number of daily beverages high in sugar
- Quantify the type and amount of physical activity performed within a specific time period.
This information can be obtained in advance of an office visit through either an electronic medical record portal or a pre-visit questionnaire (eg, http://onlinelibrary.wiley.com/doi/10.1038/oby.2002.205/full).
Also assess the patient’s risk of cardiovascular and obesity-related comorbidities. The waist circumference for patients with a BMI between 25 and 35 kg/m2 provides additional information on risk: eg, a waist circumference greater than 88 cm for women and greater than 102 cm for men indicates increased cardiometabolic risk.2
SUGGEST SPECIFIC GOALS
Use a shared decision-making process to arrive at a set of incremental goals centered around the following evidence-based targets2:
- Weight loss: 3% to 5% of baseline weight within 6 months
- 6-month commitment to a weight loss intervention
- Exercise: at least 150 minutes of moderate aerobic activity per week
- More vegetables, fewer carbohydrates, and less protein, according to the American Diabetes Association’s “Create your plate” plan9
- Mediterranean diet.10
Use motivational interviewing techniques along with the obesity history to negotiate goals. Exercise-related goals should consider the patient’s cardiovascular and musculoskeletal comorbidities.
CO-DEVELOP A TREATMENT PLAN AND ADDRESS POTENTIAL BARRIERS
The most effective weight loss treatment consists of in-person consultations in which comprehensive lifestyle interventions are included. The components of an effective intervention (Table 1) include a reduced-calorie diet, aerobic physical activity, and behavioral strategies to meaningfully support these changes.2
We recommend addressing potential barriers to initiating and maintaining weight-loss interventions, and revisiting them during follow-up visits. Barriers include the following:
Depression
Adults with depression are more likely to be obese than adults without depression, and the age-adjusted percentage of adults who are obese increases as depression severity increases.11
Access to healthy foods
Limited access to healthy food choices can lead to poor diets and higher levels of obesity.12 Local grocery store websites and nutrition specialists can help identify a range of healthy and affordable food to sustain a dietary intervention.
Medications associated with weight gain
Certain diabetic medications, contraceptives, tricyclic antidepressants, atypical antipsychotics, antiseizure drugs, and glucocorticoids promote weight gain and may have alternatives that do not promote weight gain.13
ARRANGE FOLLOW-UP AND REFERRALS
The literature supports frequent in-person sessions as the basis for a successful weight loss intervention (ie, ≥ 14 sessions in 6 months).2 Medicare beneficiaries are eligible for 14 covered visits in the first 6 months and become eligible for an additional monthly visit over the course of 6 subsequent months if a weight loss goal of 3 kg is met in the first 6-month period.
Nutritionists, dieticians, and behavioral psychologists are often instrumental in comprehensive weight loss interventions. Antiobesity drugs help curb appetite, promote weight loss, help enhance adherence to lifestyle modifications, and make it easier for patients to start a program of physical activity.14
The joint 2013 guidelines2 recommend referral for bariatric surgery for adults with a BMI 40 kg/m2 or higher, or for adults with a BMI 35 kg/m2 or higher and obesity-related comorbidities who have not responded to behavioral treatment (with or without pharmacotherapy).
A growing body of evidence promotes the use of group support sessions such as shared medical appointments to encourage healthy eating and physical activity.15
OBESITY COUNSELING IS ACHIEVABLE AND REIMBURSABLE
To receive reimbursement from Medicare for obesity counseling, the information listed under “assess” and “advise” in Table 1 should be obtained in the initial visit; and follow-up visits should be used to address items under “agree,” “assist,” and “arrange.” Up to 20 visits are eligible for reimbursement when patients meet the goal of a 3-kg weight loss in the first 6 months (or 14 visits).
- Centers for Medicare and Medicaid Services. Decision memo for intensive behavioral therapy for obesity (CAG-00423N). www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253. Accessed June 5, 2017.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol 2014; 63:2985–3023.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief 2013; 131:1–8.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001; 50:513–518.
- Galuska D, Will J, Serdula M, Ford E. Are health care professionals advising obese patients to lose weight? JAMA 1999; 282:1576–1578.
- Nawaz H, Adams ML, Katz DL. Weight loss counseling by health care providers. Am J Public Health 1999; 89:764–767.
- Ma J, Xiao L, Stafford R. Underdiagnosis of obesity in adults in US outpatient settings. Arch Intern Med 2009; 169:313–314.
- Huang J, Yu H, Marin E, Brock S, Carden D, Davis T. Physicians’ weight loss counseling in two public hospital primary care clinics. Acad Med 2004; 79:156–161.
- American Diabetes Association. Create your plate. www.diabetes.org/food-and-fitness/food/planning-meals/create-your-plate. Accessed May 19, 2017.
- Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev 2006; 64:S27–S47.
- Pratt LA, Brody DJ. Depression and obesity in the US adult household population, 2005-2010. NCHS Data Brief 2014; 167:1–8.
- Gordon-Larsen P. Food availability/convenience and obesity. Adv Nutr 2014; 5:809–817.
- Malone M. Medications associated with weight gain. Ann Pharmacother 2005; 39:2046–2055.
- Patel D. Pharmacotherapy for the management of obesity. Metabolism 2015; 64:1376–1385.
- Guthrie GE, Bogue RJ. Impact of a shared medical appointment lifestyle intervention on weight and lipid parameters in individuals with type 2 diabetes: a clinical pilot. J Am Coll Nutr 2015; 34:300–309.
- Centers for Medicare and Medicaid Services. Decision memo for intensive behavioral therapy for obesity (CAG-00423N). www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253. Accessed June 5, 2017.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol 2014; 63:2985–3023.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief 2013; 131:1–8.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001; 50:513–518.
- Galuska D, Will J, Serdula M, Ford E. Are health care professionals advising obese patients to lose weight? JAMA 1999; 282:1576–1578.
- Nawaz H, Adams ML, Katz DL. Weight loss counseling by health care providers. Am J Public Health 1999; 89:764–767.
- Ma J, Xiao L, Stafford R. Underdiagnosis of obesity in adults in US outpatient settings. Arch Intern Med 2009; 169:313–314.
- Huang J, Yu H, Marin E, Brock S, Carden D, Davis T. Physicians’ weight loss counseling in two public hospital primary care clinics. Acad Med 2004; 79:156–161.
- American Diabetes Association. Create your plate. www.diabetes.org/food-and-fitness/food/planning-meals/create-your-plate. Accessed May 19, 2017.
- Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev 2006; 64:S27–S47.
- Pratt LA, Brody DJ. Depression and obesity in the US adult household population, 2005-2010. NCHS Data Brief 2014; 167:1–8.
- Gordon-Larsen P. Food availability/convenience and obesity. Adv Nutr 2014; 5:809–817.
- Malone M. Medications associated with weight gain. Ann Pharmacother 2005; 39:2046–2055.
- Patel D. Pharmacotherapy for the management of obesity. Metabolism 2015; 64:1376–1385.
- Guthrie GE, Bogue RJ. Impact of a shared medical appointment lifestyle intervention on weight and lipid parameters in individuals with type 2 diabetes: a clinical pilot. J Am Coll Nutr 2015; 34:300–309.
Antiobesity drugs in the management of type 2 diabetes: A shift in thinking?
Obesity is a leading public health concern, affecting nearly 60 million adult Americans.1 It is a major risk factor for the development of insulin resistance and type 2 diabetes mellitus (DM).2 More than 90% of patients with type 2 DM have obesity, and obesity is a major obstacle to achieving long-term glycemic control.3
Clinical studies have demonstrated that a 6- to 7-kg increase in body weight increases the risk of developing type 2 DM by 50%, while a 5-kg loss reduces the risk by a similar amount.4 As a result, most patients who have a body mass index greater than 40 kg/m2 suffer from type 2 DM.5 Strong evidence exists that bariatric surgery and its resulting weight loss has positive effects on fasting blood sugar, hemoglobin A1c (HbA1c), lipid profiles, and other metabolic variables.6
When combined, obesity and type 2 DM carry a significant burden of micro- and macrovascular complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disease. As a result, a high prevalence of morbidity and mortality is seen among patients with obesity and type 2 DM; those between the ages of 51 and 61 have a 7-times higher mortality rate compared with nonobese normoglycemic people, and patients with diabetes alone have a 2.6-times higher mortality rate.7
A DILEMMA IN THE CLINIC: FOCUS ON THE SUGAR OR THE WEIGHT?
Although type 2 DM and obesity go hand in hand, clinicians tend to focus on the sugar and neglect the weight, concentrating their efforts on improving blood glucose indices, and prescribing in many instances medications that cause weight gain. As a result, we are faced with a rising epidemic of obesity, perpetuating a preexisting epidemic of diabetes.
An optimal, comprehensive approach to managing patients with type 2 DM should encompass both the control of dysglycemia and its associated comorbidities, obesity being the key player.8 However, clinical practice is often misaligned with the evidence. For instance, many of our first-line oral treatments for type 2 DM (except for metformin) are associated with weight gain.9 With time, control of glycemia becomes more and more ineffective, at which point therapy is intensified with insulin, further exacerbating the weight gain.10
Therefore, it seems counterintuitive to treat a disease for which obesity is one of the main risk factors with medications that promote weight gain. Yet healthcare providers are faced with a therapeutic dilemma: should they focus their efforts on improving patients’ glycemic control, or should they invest in helping these patients lose weight? Although an ideal approach would incorporate both aspects, the reality is that it is far from practical.
A few issues impinge on integrating weight loss in the care of type 2 DM. Although the American Medical Association recognized obesity as a disease in 2013,11 some providers still perceive obesity as a self-inflicted condition that is due to bad lifestyle and behavior.11 Many clinicians may also have low expectations for patients’ success, and often lack the time and knowledge to intervene regarding nutrition, physical activity, and psychological issues pertinent to the management of obesity in type 2 DM. Therefore, in many cases, it seems less complicated and more rewarding for both patients and physicians to concentrate on improving the HbA1c value rather than investing efforts in weight loss. For diabetic patients with obesity, this could mean that clinicians may prescribe glucose-lowering therapies, such as insulin and sulfonylureas, at the expense of weight gain. Additionally, clinicians often experience the need to provide recommendations more aligned with metrics that dictate reimbursement (eg, HbA1c targets) within healthcare systems that still raise concerns regarding obesity visit reimbursements.
Lastly, the lack of trustworthy or pertinent evidence (lack of comparative effectiveness research) for antiobesity medications may limit their use in daily practice. Physicians have had little confidence in the efficacy of antiobesity drugs, and often raise significant safety concerns, especially after witnessing important fiascos in this field, eg, dexfenfluramine, rimonabant, and sibutramine.2,12,13
As a result, many of our patients with obesity and type 2 DM may not consider the need for weight loss, and may not even be aware that type 2 DM is caused by obesity and physical inactivity in the first place. Others have accumulated a significant degree of frustration, and have “thrown in the towel” already after unsuccessful weight-loss efforts, many of which were not medically supervised.
For all of the above reasons, both clinicians and patients often concentrate their efforts on treating blood glucose numbers rather than the “obesity-diabetes” as a whole.14 And as a result, our practices are slowly filling up with patients with obesity and type 2 DM who are treated primarily with insulin, resulting in a progressive (and untreated) obesity and diabetes epidemic.
DRUGS FOR TREATING OBESITY AND TYPE 2 DM
Orlistat
Orlistat (Xenical) is the only weight-loss drug approved by the US Food and Drug Administration (FDA) that acts outside the brain. It inhibits pancreatic lipases, resulting in up to 30% less fat absorption in the gut. Orlistat has been approved for long-term use by the FDA.
Benefits. In the XENical in the Prevention of Diabetes in Obese Subjects study, treatment with orlistat resulted in a significant reduction in the cumulative incidence of type 2 DM after 4 years of treatment (9.0% with placebo vs 6.2% with orlistat), corresponding to a risk reduction of 37.3%.16 Mean weight loss after 4 years was significantly greater in the orlistat group (5.8 vs 3.0 kg with placebo; P < .001).16 Other benefits of orlistat included a reduction in low-density lipoprotein cholesterol independent of that expected from change in body weight.16
Adverse effects include flatulence with discharge and fecal urgency after high-fat dietary indiscretions. Serum levels of fat-soluble vitamins (A, D, E, and K) were lower with orlistat than with placebo,16 and a fat-soluble vitamin supplement should be taken 2 hours before or after taking orlistat. Serious but very uncommon adverse events such as kidney damage have been reported.17 Kidney and liver function should be monitored while taking orlistat.
Phentermine
Phentermine (Adipex-P, Lomaira), a sympathomimetic amine, is the most commonly prescribed antiobesity drug in the United States. A schedule IV controlled substance, it is FDA-approved for short-term use (up to 12 weeks). Its primary mechanism of action is mediated by reduction in hunger perception. It was first developed in the 1970s and is available in doses ranging from 8 mg to 37.5 mg daily.18
Benefits. In a randomized trial, at 28 weeks, weight loss was 1.5 kg with placebo and 5.3 kg with phentermine.19 No long-term (> 1 year) randomized controlled trials of the effectiveness of phentermine monotherapy in weight loss have been conducted.
Adverse effects. Dizziness, dry mouth, insomnia, constipation, and increase in heart rate were most common.19
Phentermine is contraindicated in patients with coronary artery disease, congestive heart failure, stroke, and uncontrolled hypertension. Currently, no data exist on the long-term cardiovascular effects of phentermine. We believe phentermine, used in patients at low to intermediate cardiovascular risk, is a useful “jumpstart” tool, in combination with lifestyle changes, to achieve weight loss and improve metabolic values for those with type 2 DM and obesity.
Phentermine is a controlled substance per Ohio law. Patients must be seen once a month by the prescribing provider and prescriptions are limited to a 30-day supply, which must be filled within 7 days of the date of the prescription. Phentermine can only be prescribed for a maximum of 3 months and must be discontinued for 6 months before patients are eligible for a new prescription.
Phentermine and topiramate extended-release
Obesity is a product of complex interactions between several neurohormonal pathways. Approaches simultaneously targeting more than one regulatory pathway have become popular and quite efficient strategies in treating patients with obesity.20 Stemming from such approaches, antiobesity drug combinations such as phentermine and topiramate extended-release (Qsymia) have become increasingly recognized and used in clinical practice. The combination of these 2 medications has been approved for long-term use by the FDA.
Phentermine and topiramate extended-release is a fixed-dose combination that was approved for weight loss in 2012. Topiramate, an anticonvulsant, and phentermine exert their anorexigenic effects through regulating various brain neurotransmitters and result in more weight loss when used together than when either is used alone. Several clinical trials evaluated the efficacy of low doses of this combination in weight loss.
Benefits. In a randomized trial in patients with obesity and cardiometabolic diseases, at 56 weeks, the mean weight loss was:
- 1.2% in the placebo group
- 7.8% in the group receiving phentermine 7.5 mg and topiramate 46 mg
- 9.8% in the group receiving phentermine 15 mg and topiramate 92 mg.21
Patients in the active treatment groups also had significant improvements in cardiovascular and metabolic risk factors such as waist circumference, systolic blood pressure, and total cholesterol/high-density lipoprotein cholesterol ratio. At 56 weeks, patients with diabetes and prediabetes taking this preparation had greater reductions in HbA1c values, and fewer prediabetes patients progressed to type 2 DM.21
Adverse effects most commonly seen were dry mouth, paresthesia, and constipation.21
This combination is contraindicated in pregnancy, patients with recent stroke, uncontrolled hypertension, coronary artery disease, glaucoma, hyperthyroidism, or in patients taking monoamine oxidase inhibitors. Women of childbearing age should be tested for pregnancy before starting therapy, and monthly thereafter, and also be advised to use effective methods of contraception while taking the medication. Topiramate has been associated with the development of renal stones and thus should be used with caution in patients with a history of kidney stones.
Bupropion and naltrexone sustained-release
Bupropion and naltrexone sustained-release (Contrave) is another FDA-approved combination drug for chronic weight management. Bupropion is a dopamine and norepinephrine reuptake inhibitor approved for depression and smoking cessation, and naltrexone is an opioid receptor antagonist approved for treating alcohol and opioid dependence. The combination of these 2 medications has been approved for long-term use by the FDA.
Benefits. In a randomized trial in patients with obesity and type 2 DM, weight loss at 56 weeks was:
- 1.8% with placebo
- 5.0% with naltrexone 32 mg and bupropion 360 mg daily.
Absolute reductions in HbA1c were:
- 0.1% with placebo
- 0.6% with naltrexone-bupropion.
Improvements were also seen in other cardiometabolic risk factors such as triglyceride and high-density lipoprotein cholesterol levels.22
Adverse effects. The most common adverse effect leading to drug discontinuation was nausea. Other adverse effects reported were constipation, headache, vomiting, and dizziness.22
Naltrexone-bupropion is contraindicated in patients with a history of seizure disorder or a diagnosis of anorexia nervosa or bulimia, or who are on chronic opioid therapy.
Diethylpropion
Diethylpropion (Tenuate, Tenuate Dospan) is a central nervous system stimulant similar to bupropion in its structure. It was approved by the FDA for treating obesity in 1959. It should be used as part of a short-term weight-loss plan, along with a low-calorie diet. Diethylpropion is also a controlled substance and, as with phentermine therapy, patients are required to be seen once a month by their prescriber. Diethylpropion cannot be prescribed for more than 3 months.
Benefits. Weight loss in a randomized trial at 6 months:
- 3.2% with placebo
- 9.8% with diethylpropion 50 mg twice a day.23
After 6 months, all participants received diethylpropion in an open-label extension for an additional 6 months. At 12 months, the mean weight loss produced by diethylpropion was 10.6%.23 No differences in heart rate, blood pressure, electrocardiographic results, or psychiatric evaluations were observed.
Adverse effects. As with phentermine, common side effects of diethylpropion include insomnia, dry mouth, dizziness, headache, mild increases in blood pressure, and palpitations.23
Lorcaserin
Lorcaserin (Belviq) was approved by the FDA for chronic weight management in June 2012. It exerts its effects through binding selectively to central 5-HT2C serotonin receptors, with poor affinity for 5-HT2A and 5-HT2B receptors. Nonselective serotoninergic agents, including fenfluramine and dexfenfluramine, were withdrawn from the market in 1997 after being reported to be associated with valvular heart abnormalities.24 Lorcaserin has been approved for long-term use by the FDA.
Benefits. Mean weight loss at 1 year in the Behavioral Modification and Lorcaserin for Overweight and Obesity Management in Diabetes Mellitus trial25 was:
- 1.5% with placebo
- 5.0% with lorcaserin 10 mg once daily
- 4.5% with lorcaserin 10 mg twice daily.
Absolute reductions in HbA1c values were:
- 0.4% with placebo
- 0.9% with lorcaserin 10 mg once daily
- 1.0% with lorcaserin 10 mg twice daily.
Absolute reductions in fasting plasma glucose values were:
- 11.9 mg/dL with placebo
- 27.4 mg/dL with lorcaserin 10 mg once daily
- 28.4 mg/dL with lorcaserin 10 mg twice daily.25
Adverse effects. The most common adverse effects were headache, dizziness, and fatigue. There was no significant increase in valvulopathy on echocardiography of participants receiving lorcaserin compared with placebo.25
Liraglutide
Liraglutide (Saxenda, Victoza) is a glucagon-like peptide-1 (GLP-1) receptor agonist. Native GLP-1 is a hormone secreted by intestinal L cells in response to consumption of fat and carbohydrate-rich foods. It stimulates the release of insulin and suppresses any inappropriately elevated postprandial glucagon levels. In addition to its effect on glucose metabolism, GLP-1 also reduces appetite and delays gastric emptying in humans.26 Unlike the extremely short half-life of native GLP-1 (estimated at 1 to 2 minutes), liraglutide has a half-life of 13 hours, allowing it to be given once daily.26 Liraglutide medication has been approved for long-term use by the FDA.
Benefits. The Liraglutide Effect and Action in Diabetes 1–5 studies compared the effects of liraglutide monotherapy with antidiabetic oral medications or insulin, as well as in combination with antidiabetic oral agents. Liraglutide (Victoza) at doses approved for type 2 DM of 1.2 mg and 1.8 mg daily had significant effects in reducing HbA1c by 0.48% to 1.84% and weight by 2.5 kg to 4 kg.27,28 At a dose of 3.0 mg, liraglutide (Saxenda) is approved for chronic weight management. This dose of liraglutide has been shown to be effective and safe in patients with type 2 DM and obesity.
In the 56-week SCALE Diabetes trial,29 liraglutide at a dose of 3.0 mg resulted in 6.0% weight reduction, compared with 2.0% in the placebo group. Of participants receiving 3.0 mg of liraglutide, 54.3% achieved more than 5% weight loss at 56 weeks compared with 21.4% with placebo. Liraglutide also resulted in significant improvements in HbA1c (mean change −1.3% vs −0.3% with placebo), fasting and postprandial glucose levels, and fasting glucagon levels.29
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial has shown liraglutide to significantly reduce rates of major cardiovascular events (first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in patients with elevated cardiovascular risk factors.30 These findings make liraglutide a favorable choice for high-risk patients with type 2 DM, obesity, and cardiovascular disease.
It is important to indicate that if a 5% weight loss is not achieved by 3 months with any of these weight-loss medications, it would be reasonable to stop the medication and consider switching to a different medication. These medications work best when combined with diet and increased physical activity. Weight-loss medications should never be used during pregnancy.
Women of childbearing age should be advised to use effective contraception methods while taking any of the above antiobesity medications.
Diabetes medications associated with weight loss: Metformin and SGLT-2 inhibitors
Although not FDA-approved for weight management, metformin has anorexigenic effects that aid in weight loss. It also inhibits hepatic glucose production and improves peripheral insulin sensitivity, making it a useful agent in patients with type 2 DM and obesity.
A meta-analysis of 31 trials showed that metformin reduced body mass index by 5.3% compared with placebo.31 Metformin should be considered as a first-line agent in obese patients with type 2 DM.
In healthy people, nearly all glucose is filtered in the glomerulus, but then 98% of it is reabsorbed in the proximal tubule by sodium-glucose cotransporter-2 (SGLT-2). Drugs that inhibit SGLT-2 increase urinary glucose excretion and, as a result, help control hyperglycemia. Another, off-label effect of excreting more glucose is weight loss: a sustained weight loss of about 3 kg to 5 kg in clinical studies.32 Although they can be used as monotherapies, SGLT-2 inhibitors are usually used as add-on therapies in patients with type 2 DM.
AN ALGORITHM FOR TREATMENT
First, we believe that lifestyle interventions by optimization of nutrition and physical activity should be the cornerstone therapy in the management plan of any patient with type 2 DM and obesity. These interventions are best implemented through a comprehensive, multidisciplinary approach that integrates the care of dietitians, physical therapists, exercise physiologists, psychologists, and social workers.33 Patients need also to be seen frequently, ie, at least once every 3 months. The possibility of seeing patients in group-shared medical appointments on a monthly basis could also be considered.
We also believe that metformin should be added early in the course of treatment for its known benefits of improving insulin sensitivity and suppressing appetite. Target HbA1c goals and body weight in patients with type 2 diabetes and obesity should be tailored to the individual based on age, general health status, risk of hypoglycemia, capacity to do physical activity, and associated comorbidities. If no improvements are seen (HbA1c > 7% and < 3 % weight loss) despite lifestyle changes and the addition of metformin, the possibility of adding a GLP-1 receptor agonist or an SGLT-2 inhibitor as a second-line therapy should be considered. Both classes of medications aid in lowering HbA1c and promote further weight loss.
If no clinical progress is achieved at 3 months, the possibility of adding an FDA-approved weight-loss medication, as discussed above, should be strongly considered. Of note, this algorithm targets different endogenous pathways for weight loss and thus minimizes weight regain through compensatory mechanisms.
THE NEED FOR PATIENT-CENTERED WEIGHT-LOSS CONVERSATIONS
Patient-centered care has become a core quality measure in our healthcare systems and a key to our patients’ success. The decision to start an antiobesity drug should therefore reflect careful consideration of medical and personal patient issues, all of which are valued differently by patients.34
Individualized therapy is even more relevant among patients suffering from a significant burden of disease. About 80% of patients with diabetes live with at least 1 other medical condition,35 and each of these patients spends over 2 hours a day, on average, following doctors’ recommendations.36 If antiobesity medications are prescribed without careful consideration of the patient’s preexisting workload, they will be destined to fail. Therefore, it becomes crucial to first account for the patient’s ability to cope with therapy intensification. This requires careful deliberation between healthcare providers and patients, in aims of targeting a weight-loss plan that fits patients’ goals and is aligned with providers’ expectations.
Healthcare systems also play a key role in supporting better conversations about obesity in type 2 DM patients. They could implement multifaceted initiatives to promote shared decision-making and the use of decision aids to advance patient-centered obesity practices.37 Policymakers could redesign quality measures aimed at capturing the quality of obesity conversations, and develop policies that support better education for clinicians regarding the importance of addressing obesity with adequate communication and patient-centered skills. Guidelines are often too disease-specific and do not consider comorbidities in their context when providing recommendations.38 Thus, diabetes societies should respond to the need to guide care for patients with diabetes and its comorbidities, particularly obesity.
CONCLUSIONS
Obesity is a serious global health issue and a leading risk factor for type 2 DM. Lifestyle measures are the cornerstone of preventing and treating obesity and type 2 DM. Emerging data support the effectiveness of intensive, interdisciplinary weight-loss programs in patients with diabetes. The use of antiobesity drugs should be considered in patients who have not achieved adequate responses to lifestyle interventions. Medications should be tailored to the individual’s health risks and metabolic and psychobehavioral characteristics. In many cases, the addition of weight-loss drugs will help accomplish and maintain the recommended 10% weight reduction, resulting in improvement in glycemic control and significant reduction in cardiovascular risk factors. New studies combining antiobesity and antidiabetes medications in the context of lifestyle interventions will help define the optimal therapeutic approach for patients with type 2 DM and obesity.
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015; (219):1–8.
- Lyznicki JM, Young DC, Riggs JA, Davis RM; for the Council on Scientific Affairs, American Medical Association. Obesity: assessment and management in primary care. Am Fam Physician 2001; 63:2185–2196.
- World Health Organization (WHO). Obesity and overweight fact sheet. www.who.int/mediacentre/factsheets/fs311/en. Updated June 2016. Accessed June 22, 2017.
- Daniels J. Obesity: America’s epidemic. Am J Nurs 2006; 106:40–49.
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995; 122:481–486.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes-5-year outcomes. N Engl J Med 2017; 376:641–651.
- Oldridge NB, Stump TE, Nothwehr FK, Clark DO. Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle- and older-age adults. J Clin Epidemiol 2001; 54:928–934.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocr Pract 2016; 22:84–113.
- McFarlane SI. Antidiabetic medications and weight gain: implications for the practicing physician. Curr Diab Rep 2009; 9:249–254.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Beal E. The pros and cons of designating obesity a disease: the new AMA designation stirs debate. Am J Nurs 2013; 113:18–19.
- Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013; 51:186–192.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001; 50:513–518.
- Pappachan JM, Viswanath AK. Medical management of diabesity: do we have realistic targets? Curr Diab Rep 2017; 17:4.
- Lexicomp Online, Lexi-Drugs, Hudson, OH: Wolters Kluwer Clinical Drug Information, Inc., 2017.
- Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004; 27:155–161.
- Buysschaert B, Aydin S, Morelle J, Hermans MP, Jadoul M, Demoulin N. Weight loss at a high cost: orlistat-induced late-onset severe kidney disease. Diabetes Metab 2016; 42:62–64.
- Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med 2005; 143:380–385.
- Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013; 21:2163–2171.
- Solas M, Milagro FI, Martínez-Urbistondo D, Ramirez MJ, Martínez JA. Precision obesity treatments including pharmacogenetic and nutrigenetic approaches. Trends Pharmacol Sci 2016; 37:575–593.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:1341–1352.
- Hollander P, Gupta AK, Plodkowski R, et al; for the COR-Diabetes Study Group. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013; 36:4022–4029.
- Cercato C, Roizenblatt VA, Leança CC, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes (Lond) 2009; 33:857–865.
- Gardin JM, Schumacher D, Constantine G, Davis KD, Leung C, Reid CL. Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine. JAMA 2000; 283:1703–1709.
- O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012; 20:1426–1436.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studies. Diabetes Obes Metab 2009; 11(suppl 3):26–34.
- Buse JB, Rosenstock J, Sesti G, et al; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374:39–47.
- Davies MJ, Bergenstal R, Bode B, et al; for the NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015; 314:687–699.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; for the LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375:311–322.
- Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med 2008; 121:149–157.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Burguera B, Jesus Tur J; Escudero AJ, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015; 2015:194696.
- Hargraves I, LeBlanc A, Shah ND, Montori VM. Shared decision making: the need for patient-clinician conversation, not just information. Health Aff (Millwood) 2016; 35:627–629.
- Lin P-J, Kent DM, Winn AN, Cohen JT, Neumann PJ. Multiple chronic conditions in type 2 diabetes mellitus: prevalence and consequences. Am J Manag Care 2015; 21:e23–e34.
- Russell LB, Suh D-C, Safford MA. Time requirements for diabetes self-management: too much for many? J Fam Pract 2005; 54:52–56.
- Serrano V, Rodriguez-Gutierrez R, Hargraves I, Gionfriddo MR, Tamhane S, Montori VM. Shared decision-making in the care of individuals with diabetes. Diabet Med 2016; 33:742–751.
- Wyatt KD, Stuart LM, Brito JP, et al. Out of context: clinical practice guidelines and patients with multiple chronic conditions: a systematic review. Med Care 2014; 52(suppl 3):S92–S100.
Obesity is a leading public health concern, affecting nearly 60 million adult Americans.1 It is a major risk factor for the development of insulin resistance and type 2 diabetes mellitus (DM).2 More than 90% of patients with type 2 DM have obesity, and obesity is a major obstacle to achieving long-term glycemic control.3
Clinical studies have demonstrated that a 6- to 7-kg increase in body weight increases the risk of developing type 2 DM by 50%, while a 5-kg loss reduces the risk by a similar amount.4 As a result, most patients who have a body mass index greater than 40 kg/m2 suffer from type 2 DM.5 Strong evidence exists that bariatric surgery and its resulting weight loss has positive effects on fasting blood sugar, hemoglobin A1c (HbA1c), lipid profiles, and other metabolic variables.6
When combined, obesity and type 2 DM carry a significant burden of micro- and macrovascular complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disease. As a result, a high prevalence of morbidity and mortality is seen among patients with obesity and type 2 DM; those between the ages of 51 and 61 have a 7-times higher mortality rate compared with nonobese normoglycemic people, and patients with diabetes alone have a 2.6-times higher mortality rate.7
A DILEMMA IN THE CLINIC: FOCUS ON THE SUGAR OR THE WEIGHT?
Although type 2 DM and obesity go hand in hand, clinicians tend to focus on the sugar and neglect the weight, concentrating their efforts on improving blood glucose indices, and prescribing in many instances medications that cause weight gain. As a result, we are faced with a rising epidemic of obesity, perpetuating a preexisting epidemic of diabetes.
An optimal, comprehensive approach to managing patients with type 2 DM should encompass both the control of dysglycemia and its associated comorbidities, obesity being the key player.8 However, clinical practice is often misaligned with the evidence. For instance, many of our first-line oral treatments for type 2 DM (except for metformin) are associated with weight gain.9 With time, control of glycemia becomes more and more ineffective, at which point therapy is intensified with insulin, further exacerbating the weight gain.10
Therefore, it seems counterintuitive to treat a disease for which obesity is one of the main risk factors with medications that promote weight gain. Yet healthcare providers are faced with a therapeutic dilemma: should they focus their efforts on improving patients’ glycemic control, or should they invest in helping these patients lose weight? Although an ideal approach would incorporate both aspects, the reality is that it is far from practical.
A few issues impinge on integrating weight loss in the care of type 2 DM. Although the American Medical Association recognized obesity as a disease in 2013,11 some providers still perceive obesity as a self-inflicted condition that is due to bad lifestyle and behavior.11 Many clinicians may also have low expectations for patients’ success, and often lack the time and knowledge to intervene regarding nutrition, physical activity, and psychological issues pertinent to the management of obesity in type 2 DM. Therefore, in many cases, it seems less complicated and more rewarding for both patients and physicians to concentrate on improving the HbA1c value rather than investing efforts in weight loss. For diabetic patients with obesity, this could mean that clinicians may prescribe glucose-lowering therapies, such as insulin and sulfonylureas, at the expense of weight gain. Additionally, clinicians often experience the need to provide recommendations more aligned with metrics that dictate reimbursement (eg, HbA1c targets) within healthcare systems that still raise concerns regarding obesity visit reimbursements.
Lastly, the lack of trustworthy or pertinent evidence (lack of comparative effectiveness research) for antiobesity medications may limit their use in daily practice. Physicians have had little confidence in the efficacy of antiobesity drugs, and often raise significant safety concerns, especially after witnessing important fiascos in this field, eg, dexfenfluramine, rimonabant, and sibutramine.2,12,13
As a result, many of our patients with obesity and type 2 DM may not consider the need for weight loss, and may not even be aware that type 2 DM is caused by obesity and physical inactivity in the first place. Others have accumulated a significant degree of frustration, and have “thrown in the towel” already after unsuccessful weight-loss efforts, many of which were not medically supervised.
For all of the above reasons, both clinicians and patients often concentrate their efforts on treating blood glucose numbers rather than the “obesity-diabetes” as a whole.14 And as a result, our practices are slowly filling up with patients with obesity and type 2 DM who are treated primarily with insulin, resulting in a progressive (and untreated) obesity and diabetes epidemic.
DRUGS FOR TREATING OBESITY AND TYPE 2 DM
Orlistat
Orlistat (Xenical) is the only weight-loss drug approved by the US Food and Drug Administration (FDA) that acts outside the brain. It inhibits pancreatic lipases, resulting in up to 30% less fat absorption in the gut. Orlistat has been approved for long-term use by the FDA.
Benefits. In the XENical in the Prevention of Diabetes in Obese Subjects study, treatment with orlistat resulted in a significant reduction in the cumulative incidence of type 2 DM after 4 years of treatment (9.0% with placebo vs 6.2% with orlistat), corresponding to a risk reduction of 37.3%.16 Mean weight loss after 4 years was significantly greater in the orlistat group (5.8 vs 3.0 kg with placebo; P < .001).16 Other benefits of orlistat included a reduction in low-density lipoprotein cholesterol independent of that expected from change in body weight.16
Adverse effects include flatulence with discharge and fecal urgency after high-fat dietary indiscretions. Serum levels of fat-soluble vitamins (A, D, E, and K) were lower with orlistat than with placebo,16 and a fat-soluble vitamin supplement should be taken 2 hours before or after taking orlistat. Serious but very uncommon adverse events such as kidney damage have been reported.17 Kidney and liver function should be monitored while taking orlistat.
Phentermine
Phentermine (Adipex-P, Lomaira), a sympathomimetic amine, is the most commonly prescribed antiobesity drug in the United States. A schedule IV controlled substance, it is FDA-approved for short-term use (up to 12 weeks). Its primary mechanism of action is mediated by reduction in hunger perception. It was first developed in the 1970s and is available in doses ranging from 8 mg to 37.5 mg daily.18
Benefits. In a randomized trial, at 28 weeks, weight loss was 1.5 kg with placebo and 5.3 kg with phentermine.19 No long-term (> 1 year) randomized controlled trials of the effectiveness of phentermine monotherapy in weight loss have been conducted.
Adverse effects. Dizziness, dry mouth, insomnia, constipation, and increase in heart rate were most common.19
Phentermine is contraindicated in patients with coronary artery disease, congestive heart failure, stroke, and uncontrolled hypertension. Currently, no data exist on the long-term cardiovascular effects of phentermine. We believe phentermine, used in patients at low to intermediate cardiovascular risk, is a useful “jumpstart” tool, in combination with lifestyle changes, to achieve weight loss and improve metabolic values for those with type 2 DM and obesity.
Phentermine is a controlled substance per Ohio law. Patients must be seen once a month by the prescribing provider and prescriptions are limited to a 30-day supply, which must be filled within 7 days of the date of the prescription. Phentermine can only be prescribed for a maximum of 3 months and must be discontinued for 6 months before patients are eligible for a new prescription.
Phentermine and topiramate extended-release
Obesity is a product of complex interactions between several neurohormonal pathways. Approaches simultaneously targeting more than one regulatory pathway have become popular and quite efficient strategies in treating patients with obesity.20 Stemming from such approaches, antiobesity drug combinations such as phentermine and topiramate extended-release (Qsymia) have become increasingly recognized and used in clinical practice. The combination of these 2 medications has been approved for long-term use by the FDA.
Phentermine and topiramate extended-release is a fixed-dose combination that was approved for weight loss in 2012. Topiramate, an anticonvulsant, and phentermine exert their anorexigenic effects through regulating various brain neurotransmitters and result in more weight loss when used together than when either is used alone. Several clinical trials evaluated the efficacy of low doses of this combination in weight loss.
Benefits. In a randomized trial in patients with obesity and cardiometabolic diseases, at 56 weeks, the mean weight loss was:
- 1.2% in the placebo group
- 7.8% in the group receiving phentermine 7.5 mg and topiramate 46 mg
- 9.8% in the group receiving phentermine 15 mg and topiramate 92 mg.21
Patients in the active treatment groups also had significant improvements in cardiovascular and metabolic risk factors such as waist circumference, systolic blood pressure, and total cholesterol/high-density lipoprotein cholesterol ratio. At 56 weeks, patients with diabetes and prediabetes taking this preparation had greater reductions in HbA1c values, and fewer prediabetes patients progressed to type 2 DM.21
Adverse effects most commonly seen were dry mouth, paresthesia, and constipation.21
This combination is contraindicated in pregnancy, patients with recent stroke, uncontrolled hypertension, coronary artery disease, glaucoma, hyperthyroidism, or in patients taking monoamine oxidase inhibitors. Women of childbearing age should be tested for pregnancy before starting therapy, and monthly thereafter, and also be advised to use effective methods of contraception while taking the medication. Topiramate has been associated with the development of renal stones and thus should be used with caution in patients with a history of kidney stones.
Bupropion and naltrexone sustained-release
Bupropion and naltrexone sustained-release (Contrave) is another FDA-approved combination drug for chronic weight management. Bupropion is a dopamine and norepinephrine reuptake inhibitor approved for depression and smoking cessation, and naltrexone is an opioid receptor antagonist approved for treating alcohol and opioid dependence. The combination of these 2 medications has been approved for long-term use by the FDA.
Benefits. In a randomized trial in patients with obesity and type 2 DM, weight loss at 56 weeks was:
- 1.8% with placebo
- 5.0% with naltrexone 32 mg and bupropion 360 mg daily.
Absolute reductions in HbA1c were:
- 0.1% with placebo
- 0.6% with naltrexone-bupropion.
Improvements were also seen in other cardiometabolic risk factors such as triglyceride and high-density lipoprotein cholesterol levels.22
Adverse effects. The most common adverse effect leading to drug discontinuation was nausea. Other adverse effects reported were constipation, headache, vomiting, and dizziness.22
Naltrexone-bupropion is contraindicated in patients with a history of seizure disorder or a diagnosis of anorexia nervosa or bulimia, or who are on chronic opioid therapy.
Diethylpropion
Diethylpropion (Tenuate, Tenuate Dospan) is a central nervous system stimulant similar to bupropion in its structure. It was approved by the FDA for treating obesity in 1959. It should be used as part of a short-term weight-loss plan, along with a low-calorie diet. Diethylpropion is also a controlled substance and, as with phentermine therapy, patients are required to be seen once a month by their prescriber. Diethylpropion cannot be prescribed for more than 3 months.
Benefits. Weight loss in a randomized trial at 6 months:
- 3.2% with placebo
- 9.8% with diethylpropion 50 mg twice a day.23
After 6 months, all participants received diethylpropion in an open-label extension for an additional 6 months. At 12 months, the mean weight loss produced by diethylpropion was 10.6%.23 No differences in heart rate, blood pressure, electrocardiographic results, or psychiatric evaluations were observed.
Adverse effects. As with phentermine, common side effects of diethylpropion include insomnia, dry mouth, dizziness, headache, mild increases in blood pressure, and palpitations.23
Lorcaserin
Lorcaserin (Belviq) was approved by the FDA for chronic weight management in June 2012. It exerts its effects through binding selectively to central 5-HT2C serotonin receptors, with poor affinity for 5-HT2A and 5-HT2B receptors. Nonselective serotoninergic agents, including fenfluramine and dexfenfluramine, were withdrawn from the market in 1997 after being reported to be associated with valvular heart abnormalities.24 Lorcaserin has been approved for long-term use by the FDA.
Benefits. Mean weight loss at 1 year in the Behavioral Modification and Lorcaserin for Overweight and Obesity Management in Diabetes Mellitus trial25 was:
- 1.5% with placebo
- 5.0% with lorcaserin 10 mg once daily
- 4.5% with lorcaserin 10 mg twice daily.
Absolute reductions in HbA1c values were:
- 0.4% with placebo
- 0.9% with lorcaserin 10 mg once daily
- 1.0% with lorcaserin 10 mg twice daily.
Absolute reductions in fasting plasma glucose values were:
- 11.9 mg/dL with placebo
- 27.4 mg/dL with lorcaserin 10 mg once daily
- 28.4 mg/dL with lorcaserin 10 mg twice daily.25
Adverse effects. The most common adverse effects were headache, dizziness, and fatigue. There was no significant increase in valvulopathy on echocardiography of participants receiving lorcaserin compared with placebo.25
Liraglutide
Liraglutide (Saxenda, Victoza) is a glucagon-like peptide-1 (GLP-1) receptor agonist. Native GLP-1 is a hormone secreted by intestinal L cells in response to consumption of fat and carbohydrate-rich foods. It stimulates the release of insulin and suppresses any inappropriately elevated postprandial glucagon levels. In addition to its effect on glucose metabolism, GLP-1 also reduces appetite and delays gastric emptying in humans.26 Unlike the extremely short half-life of native GLP-1 (estimated at 1 to 2 minutes), liraglutide has a half-life of 13 hours, allowing it to be given once daily.26 Liraglutide medication has been approved for long-term use by the FDA.
Benefits. The Liraglutide Effect and Action in Diabetes 1–5 studies compared the effects of liraglutide monotherapy with antidiabetic oral medications or insulin, as well as in combination with antidiabetic oral agents. Liraglutide (Victoza) at doses approved for type 2 DM of 1.2 mg and 1.8 mg daily had significant effects in reducing HbA1c by 0.48% to 1.84% and weight by 2.5 kg to 4 kg.27,28 At a dose of 3.0 mg, liraglutide (Saxenda) is approved for chronic weight management. This dose of liraglutide has been shown to be effective and safe in patients with type 2 DM and obesity.
In the 56-week SCALE Diabetes trial,29 liraglutide at a dose of 3.0 mg resulted in 6.0% weight reduction, compared with 2.0% in the placebo group. Of participants receiving 3.0 mg of liraglutide, 54.3% achieved more than 5% weight loss at 56 weeks compared with 21.4% with placebo. Liraglutide also resulted in significant improvements in HbA1c (mean change −1.3% vs −0.3% with placebo), fasting and postprandial glucose levels, and fasting glucagon levels.29
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial has shown liraglutide to significantly reduce rates of major cardiovascular events (first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in patients with elevated cardiovascular risk factors.30 These findings make liraglutide a favorable choice for high-risk patients with type 2 DM, obesity, and cardiovascular disease.
It is important to indicate that if a 5% weight loss is not achieved by 3 months with any of these weight-loss medications, it would be reasonable to stop the medication and consider switching to a different medication. These medications work best when combined with diet and increased physical activity. Weight-loss medications should never be used during pregnancy.
Women of childbearing age should be advised to use effective contraception methods while taking any of the above antiobesity medications.
Diabetes medications associated with weight loss: Metformin and SGLT-2 inhibitors
Although not FDA-approved for weight management, metformin has anorexigenic effects that aid in weight loss. It also inhibits hepatic glucose production and improves peripheral insulin sensitivity, making it a useful agent in patients with type 2 DM and obesity.
A meta-analysis of 31 trials showed that metformin reduced body mass index by 5.3% compared with placebo.31 Metformin should be considered as a first-line agent in obese patients with type 2 DM.
In healthy people, nearly all glucose is filtered in the glomerulus, but then 98% of it is reabsorbed in the proximal tubule by sodium-glucose cotransporter-2 (SGLT-2). Drugs that inhibit SGLT-2 increase urinary glucose excretion and, as a result, help control hyperglycemia. Another, off-label effect of excreting more glucose is weight loss: a sustained weight loss of about 3 kg to 5 kg in clinical studies.32 Although they can be used as monotherapies, SGLT-2 inhibitors are usually used as add-on therapies in patients with type 2 DM.
AN ALGORITHM FOR TREATMENT
First, we believe that lifestyle interventions by optimization of nutrition and physical activity should be the cornerstone therapy in the management plan of any patient with type 2 DM and obesity. These interventions are best implemented through a comprehensive, multidisciplinary approach that integrates the care of dietitians, physical therapists, exercise physiologists, psychologists, and social workers.33 Patients need also to be seen frequently, ie, at least once every 3 months. The possibility of seeing patients in group-shared medical appointments on a monthly basis could also be considered.
We also believe that metformin should be added early in the course of treatment for its known benefits of improving insulin sensitivity and suppressing appetite. Target HbA1c goals and body weight in patients with type 2 diabetes and obesity should be tailored to the individual based on age, general health status, risk of hypoglycemia, capacity to do physical activity, and associated comorbidities. If no improvements are seen (HbA1c > 7% and < 3 % weight loss) despite lifestyle changes and the addition of metformin, the possibility of adding a GLP-1 receptor agonist or an SGLT-2 inhibitor as a second-line therapy should be considered. Both classes of medications aid in lowering HbA1c and promote further weight loss.
If no clinical progress is achieved at 3 months, the possibility of adding an FDA-approved weight-loss medication, as discussed above, should be strongly considered. Of note, this algorithm targets different endogenous pathways for weight loss and thus minimizes weight regain through compensatory mechanisms.
THE NEED FOR PATIENT-CENTERED WEIGHT-LOSS CONVERSATIONS
Patient-centered care has become a core quality measure in our healthcare systems and a key to our patients’ success. The decision to start an antiobesity drug should therefore reflect careful consideration of medical and personal patient issues, all of which are valued differently by patients.34
Individualized therapy is even more relevant among patients suffering from a significant burden of disease. About 80% of patients with diabetes live with at least 1 other medical condition,35 and each of these patients spends over 2 hours a day, on average, following doctors’ recommendations.36 If antiobesity medications are prescribed without careful consideration of the patient’s preexisting workload, they will be destined to fail. Therefore, it becomes crucial to first account for the patient’s ability to cope with therapy intensification. This requires careful deliberation between healthcare providers and patients, in aims of targeting a weight-loss plan that fits patients’ goals and is aligned with providers’ expectations.
Healthcare systems also play a key role in supporting better conversations about obesity in type 2 DM patients. They could implement multifaceted initiatives to promote shared decision-making and the use of decision aids to advance patient-centered obesity practices.37 Policymakers could redesign quality measures aimed at capturing the quality of obesity conversations, and develop policies that support better education for clinicians regarding the importance of addressing obesity with adequate communication and patient-centered skills. Guidelines are often too disease-specific and do not consider comorbidities in their context when providing recommendations.38 Thus, diabetes societies should respond to the need to guide care for patients with diabetes and its comorbidities, particularly obesity.
CONCLUSIONS
Obesity is a serious global health issue and a leading risk factor for type 2 DM. Lifestyle measures are the cornerstone of preventing and treating obesity and type 2 DM. Emerging data support the effectiveness of intensive, interdisciplinary weight-loss programs in patients with diabetes. The use of antiobesity drugs should be considered in patients who have not achieved adequate responses to lifestyle interventions. Medications should be tailored to the individual’s health risks and metabolic and psychobehavioral characteristics. In many cases, the addition of weight-loss drugs will help accomplish and maintain the recommended 10% weight reduction, resulting in improvement in glycemic control and significant reduction in cardiovascular risk factors. New studies combining antiobesity and antidiabetes medications in the context of lifestyle interventions will help define the optimal therapeutic approach for patients with type 2 DM and obesity.
Obesity is a leading public health concern, affecting nearly 60 million adult Americans.1 It is a major risk factor for the development of insulin resistance and type 2 diabetes mellitus (DM).2 More than 90% of patients with type 2 DM have obesity, and obesity is a major obstacle to achieving long-term glycemic control.3
Clinical studies have demonstrated that a 6- to 7-kg increase in body weight increases the risk of developing type 2 DM by 50%, while a 5-kg loss reduces the risk by a similar amount.4 As a result, most patients who have a body mass index greater than 40 kg/m2 suffer from type 2 DM.5 Strong evidence exists that bariatric surgery and its resulting weight loss has positive effects on fasting blood sugar, hemoglobin A1c (HbA1c), lipid profiles, and other metabolic variables.6
When combined, obesity and type 2 DM carry a significant burden of micro- and macrovascular complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disease. As a result, a high prevalence of morbidity and mortality is seen among patients with obesity and type 2 DM; those between the ages of 51 and 61 have a 7-times higher mortality rate compared with nonobese normoglycemic people, and patients with diabetes alone have a 2.6-times higher mortality rate.7
A DILEMMA IN THE CLINIC: FOCUS ON THE SUGAR OR THE WEIGHT?
Although type 2 DM and obesity go hand in hand, clinicians tend to focus on the sugar and neglect the weight, concentrating their efforts on improving blood glucose indices, and prescribing in many instances medications that cause weight gain. As a result, we are faced with a rising epidemic of obesity, perpetuating a preexisting epidemic of diabetes.
An optimal, comprehensive approach to managing patients with type 2 DM should encompass both the control of dysglycemia and its associated comorbidities, obesity being the key player.8 However, clinical practice is often misaligned with the evidence. For instance, many of our first-line oral treatments for type 2 DM (except for metformin) are associated with weight gain.9 With time, control of glycemia becomes more and more ineffective, at which point therapy is intensified with insulin, further exacerbating the weight gain.10
Therefore, it seems counterintuitive to treat a disease for which obesity is one of the main risk factors with medications that promote weight gain. Yet healthcare providers are faced with a therapeutic dilemma: should they focus their efforts on improving patients’ glycemic control, or should they invest in helping these patients lose weight? Although an ideal approach would incorporate both aspects, the reality is that it is far from practical.
A few issues impinge on integrating weight loss in the care of type 2 DM. Although the American Medical Association recognized obesity as a disease in 2013,11 some providers still perceive obesity as a self-inflicted condition that is due to bad lifestyle and behavior.11 Many clinicians may also have low expectations for patients’ success, and often lack the time and knowledge to intervene regarding nutrition, physical activity, and psychological issues pertinent to the management of obesity in type 2 DM. Therefore, in many cases, it seems less complicated and more rewarding for both patients and physicians to concentrate on improving the HbA1c value rather than investing efforts in weight loss. For diabetic patients with obesity, this could mean that clinicians may prescribe glucose-lowering therapies, such as insulin and sulfonylureas, at the expense of weight gain. Additionally, clinicians often experience the need to provide recommendations more aligned with metrics that dictate reimbursement (eg, HbA1c targets) within healthcare systems that still raise concerns regarding obesity visit reimbursements.
Lastly, the lack of trustworthy or pertinent evidence (lack of comparative effectiveness research) for antiobesity medications may limit their use in daily practice. Physicians have had little confidence in the efficacy of antiobesity drugs, and often raise significant safety concerns, especially after witnessing important fiascos in this field, eg, dexfenfluramine, rimonabant, and sibutramine.2,12,13
As a result, many of our patients with obesity and type 2 DM may not consider the need for weight loss, and may not even be aware that type 2 DM is caused by obesity and physical inactivity in the first place. Others have accumulated a significant degree of frustration, and have “thrown in the towel” already after unsuccessful weight-loss efforts, many of which were not medically supervised.
For all of the above reasons, both clinicians and patients often concentrate their efforts on treating blood glucose numbers rather than the “obesity-diabetes” as a whole.14 And as a result, our practices are slowly filling up with patients with obesity and type 2 DM who are treated primarily with insulin, resulting in a progressive (and untreated) obesity and diabetes epidemic.
DRUGS FOR TREATING OBESITY AND TYPE 2 DM
Orlistat
Orlistat (Xenical) is the only weight-loss drug approved by the US Food and Drug Administration (FDA) that acts outside the brain. It inhibits pancreatic lipases, resulting in up to 30% less fat absorption in the gut. Orlistat has been approved for long-term use by the FDA.
Benefits. In the XENical in the Prevention of Diabetes in Obese Subjects study, treatment with orlistat resulted in a significant reduction in the cumulative incidence of type 2 DM after 4 years of treatment (9.0% with placebo vs 6.2% with orlistat), corresponding to a risk reduction of 37.3%.16 Mean weight loss after 4 years was significantly greater in the orlistat group (5.8 vs 3.0 kg with placebo; P < .001).16 Other benefits of orlistat included a reduction in low-density lipoprotein cholesterol independent of that expected from change in body weight.16
Adverse effects include flatulence with discharge and fecal urgency after high-fat dietary indiscretions. Serum levels of fat-soluble vitamins (A, D, E, and K) were lower with orlistat than with placebo,16 and a fat-soluble vitamin supplement should be taken 2 hours before or after taking orlistat. Serious but very uncommon adverse events such as kidney damage have been reported.17 Kidney and liver function should be monitored while taking orlistat.
Phentermine
Phentermine (Adipex-P, Lomaira), a sympathomimetic amine, is the most commonly prescribed antiobesity drug in the United States. A schedule IV controlled substance, it is FDA-approved for short-term use (up to 12 weeks). Its primary mechanism of action is mediated by reduction in hunger perception. It was first developed in the 1970s and is available in doses ranging from 8 mg to 37.5 mg daily.18
Benefits. In a randomized trial, at 28 weeks, weight loss was 1.5 kg with placebo and 5.3 kg with phentermine.19 No long-term (> 1 year) randomized controlled trials of the effectiveness of phentermine monotherapy in weight loss have been conducted.
Adverse effects. Dizziness, dry mouth, insomnia, constipation, and increase in heart rate were most common.19
Phentermine is contraindicated in patients with coronary artery disease, congestive heart failure, stroke, and uncontrolled hypertension. Currently, no data exist on the long-term cardiovascular effects of phentermine. We believe phentermine, used in patients at low to intermediate cardiovascular risk, is a useful “jumpstart” tool, in combination with lifestyle changes, to achieve weight loss and improve metabolic values for those with type 2 DM and obesity.
Phentermine is a controlled substance per Ohio law. Patients must be seen once a month by the prescribing provider and prescriptions are limited to a 30-day supply, which must be filled within 7 days of the date of the prescription. Phentermine can only be prescribed for a maximum of 3 months and must be discontinued for 6 months before patients are eligible for a new prescription.
Phentermine and topiramate extended-release
Obesity is a product of complex interactions between several neurohormonal pathways. Approaches simultaneously targeting more than one regulatory pathway have become popular and quite efficient strategies in treating patients with obesity.20 Stemming from such approaches, antiobesity drug combinations such as phentermine and topiramate extended-release (Qsymia) have become increasingly recognized and used in clinical practice. The combination of these 2 medications has been approved for long-term use by the FDA.
Phentermine and topiramate extended-release is a fixed-dose combination that was approved for weight loss in 2012. Topiramate, an anticonvulsant, and phentermine exert their anorexigenic effects through regulating various brain neurotransmitters and result in more weight loss when used together than when either is used alone. Several clinical trials evaluated the efficacy of low doses of this combination in weight loss.
Benefits. In a randomized trial in patients with obesity and cardiometabolic diseases, at 56 weeks, the mean weight loss was:
- 1.2% in the placebo group
- 7.8% in the group receiving phentermine 7.5 mg and topiramate 46 mg
- 9.8% in the group receiving phentermine 15 mg and topiramate 92 mg.21
Patients in the active treatment groups also had significant improvements in cardiovascular and metabolic risk factors such as waist circumference, systolic blood pressure, and total cholesterol/high-density lipoprotein cholesterol ratio. At 56 weeks, patients with diabetes and prediabetes taking this preparation had greater reductions in HbA1c values, and fewer prediabetes patients progressed to type 2 DM.21
Adverse effects most commonly seen were dry mouth, paresthesia, and constipation.21
This combination is contraindicated in pregnancy, patients with recent stroke, uncontrolled hypertension, coronary artery disease, glaucoma, hyperthyroidism, or in patients taking monoamine oxidase inhibitors. Women of childbearing age should be tested for pregnancy before starting therapy, and monthly thereafter, and also be advised to use effective methods of contraception while taking the medication. Topiramate has been associated with the development of renal stones and thus should be used with caution in patients with a history of kidney stones.
Bupropion and naltrexone sustained-release
Bupropion and naltrexone sustained-release (Contrave) is another FDA-approved combination drug for chronic weight management. Bupropion is a dopamine and norepinephrine reuptake inhibitor approved for depression and smoking cessation, and naltrexone is an opioid receptor antagonist approved for treating alcohol and opioid dependence. The combination of these 2 medications has been approved for long-term use by the FDA.
Benefits. In a randomized trial in patients with obesity and type 2 DM, weight loss at 56 weeks was:
- 1.8% with placebo
- 5.0% with naltrexone 32 mg and bupropion 360 mg daily.
Absolute reductions in HbA1c were:
- 0.1% with placebo
- 0.6% with naltrexone-bupropion.
Improvements were also seen in other cardiometabolic risk factors such as triglyceride and high-density lipoprotein cholesterol levels.22
Adverse effects. The most common adverse effect leading to drug discontinuation was nausea. Other adverse effects reported were constipation, headache, vomiting, and dizziness.22
Naltrexone-bupropion is contraindicated in patients with a history of seizure disorder or a diagnosis of anorexia nervosa or bulimia, or who are on chronic opioid therapy.
Diethylpropion
Diethylpropion (Tenuate, Tenuate Dospan) is a central nervous system stimulant similar to bupropion in its structure. It was approved by the FDA for treating obesity in 1959. It should be used as part of a short-term weight-loss plan, along with a low-calorie diet. Diethylpropion is also a controlled substance and, as with phentermine therapy, patients are required to be seen once a month by their prescriber. Diethylpropion cannot be prescribed for more than 3 months.
Benefits. Weight loss in a randomized trial at 6 months:
- 3.2% with placebo
- 9.8% with diethylpropion 50 mg twice a day.23
After 6 months, all participants received diethylpropion in an open-label extension for an additional 6 months. At 12 months, the mean weight loss produced by diethylpropion was 10.6%.23 No differences in heart rate, blood pressure, electrocardiographic results, or psychiatric evaluations were observed.
Adverse effects. As with phentermine, common side effects of diethylpropion include insomnia, dry mouth, dizziness, headache, mild increases in blood pressure, and palpitations.23
Lorcaserin
Lorcaserin (Belviq) was approved by the FDA for chronic weight management in June 2012. It exerts its effects through binding selectively to central 5-HT2C serotonin receptors, with poor affinity for 5-HT2A and 5-HT2B receptors. Nonselective serotoninergic agents, including fenfluramine and dexfenfluramine, were withdrawn from the market in 1997 after being reported to be associated with valvular heart abnormalities.24 Lorcaserin has been approved for long-term use by the FDA.
Benefits. Mean weight loss at 1 year in the Behavioral Modification and Lorcaserin for Overweight and Obesity Management in Diabetes Mellitus trial25 was:
- 1.5% with placebo
- 5.0% with lorcaserin 10 mg once daily
- 4.5% with lorcaserin 10 mg twice daily.
Absolute reductions in HbA1c values were:
- 0.4% with placebo
- 0.9% with lorcaserin 10 mg once daily
- 1.0% with lorcaserin 10 mg twice daily.
Absolute reductions in fasting plasma glucose values were:
- 11.9 mg/dL with placebo
- 27.4 mg/dL with lorcaserin 10 mg once daily
- 28.4 mg/dL with lorcaserin 10 mg twice daily.25
Adverse effects. The most common adverse effects were headache, dizziness, and fatigue. There was no significant increase in valvulopathy on echocardiography of participants receiving lorcaserin compared with placebo.25
Liraglutide
Liraglutide (Saxenda, Victoza) is a glucagon-like peptide-1 (GLP-1) receptor agonist. Native GLP-1 is a hormone secreted by intestinal L cells in response to consumption of fat and carbohydrate-rich foods. It stimulates the release of insulin and suppresses any inappropriately elevated postprandial glucagon levels. In addition to its effect on glucose metabolism, GLP-1 also reduces appetite and delays gastric emptying in humans.26 Unlike the extremely short half-life of native GLP-1 (estimated at 1 to 2 minutes), liraglutide has a half-life of 13 hours, allowing it to be given once daily.26 Liraglutide medication has been approved for long-term use by the FDA.
Benefits. The Liraglutide Effect and Action in Diabetes 1–5 studies compared the effects of liraglutide monotherapy with antidiabetic oral medications or insulin, as well as in combination with antidiabetic oral agents. Liraglutide (Victoza) at doses approved for type 2 DM of 1.2 mg and 1.8 mg daily had significant effects in reducing HbA1c by 0.48% to 1.84% and weight by 2.5 kg to 4 kg.27,28 At a dose of 3.0 mg, liraglutide (Saxenda) is approved for chronic weight management. This dose of liraglutide has been shown to be effective and safe in patients with type 2 DM and obesity.
In the 56-week SCALE Diabetes trial,29 liraglutide at a dose of 3.0 mg resulted in 6.0% weight reduction, compared with 2.0% in the placebo group. Of participants receiving 3.0 mg of liraglutide, 54.3% achieved more than 5% weight loss at 56 weeks compared with 21.4% with placebo. Liraglutide also resulted in significant improvements in HbA1c (mean change −1.3% vs −0.3% with placebo), fasting and postprandial glucose levels, and fasting glucagon levels.29
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial has shown liraglutide to significantly reduce rates of major cardiovascular events (first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in patients with elevated cardiovascular risk factors.30 These findings make liraglutide a favorable choice for high-risk patients with type 2 DM, obesity, and cardiovascular disease.
It is important to indicate that if a 5% weight loss is not achieved by 3 months with any of these weight-loss medications, it would be reasonable to stop the medication and consider switching to a different medication. These medications work best when combined with diet and increased physical activity. Weight-loss medications should never be used during pregnancy.
Women of childbearing age should be advised to use effective contraception methods while taking any of the above antiobesity medications.
Diabetes medications associated with weight loss: Metformin and SGLT-2 inhibitors
Although not FDA-approved for weight management, metformin has anorexigenic effects that aid in weight loss. It also inhibits hepatic glucose production and improves peripheral insulin sensitivity, making it a useful agent in patients with type 2 DM and obesity.
A meta-analysis of 31 trials showed that metformin reduced body mass index by 5.3% compared with placebo.31 Metformin should be considered as a first-line agent in obese patients with type 2 DM.
In healthy people, nearly all glucose is filtered in the glomerulus, but then 98% of it is reabsorbed in the proximal tubule by sodium-glucose cotransporter-2 (SGLT-2). Drugs that inhibit SGLT-2 increase urinary glucose excretion and, as a result, help control hyperglycemia. Another, off-label effect of excreting more glucose is weight loss: a sustained weight loss of about 3 kg to 5 kg in clinical studies.32 Although they can be used as monotherapies, SGLT-2 inhibitors are usually used as add-on therapies in patients with type 2 DM.
AN ALGORITHM FOR TREATMENT
First, we believe that lifestyle interventions by optimization of nutrition and physical activity should be the cornerstone therapy in the management plan of any patient with type 2 DM and obesity. These interventions are best implemented through a comprehensive, multidisciplinary approach that integrates the care of dietitians, physical therapists, exercise physiologists, psychologists, and social workers.33 Patients need also to be seen frequently, ie, at least once every 3 months. The possibility of seeing patients in group-shared medical appointments on a monthly basis could also be considered.
We also believe that metformin should be added early in the course of treatment for its known benefits of improving insulin sensitivity and suppressing appetite. Target HbA1c goals and body weight in patients with type 2 diabetes and obesity should be tailored to the individual based on age, general health status, risk of hypoglycemia, capacity to do physical activity, and associated comorbidities. If no improvements are seen (HbA1c > 7% and < 3 % weight loss) despite lifestyle changes and the addition of metformin, the possibility of adding a GLP-1 receptor agonist or an SGLT-2 inhibitor as a second-line therapy should be considered. Both classes of medications aid in lowering HbA1c and promote further weight loss.
If no clinical progress is achieved at 3 months, the possibility of adding an FDA-approved weight-loss medication, as discussed above, should be strongly considered. Of note, this algorithm targets different endogenous pathways for weight loss and thus minimizes weight regain through compensatory mechanisms.
THE NEED FOR PATIENT-CENTERED WEIGHT-LOSS CONVERSATIONS
Patient-centered care has become a core quality measure in our healthcare systems and a key to our patients’ success. The decision to start an antiobesity drug should therefore reflect careful consideration of medical and personal patient issues, all of which are valued differently by patients.34
Individualized therapy is even more relevant among patients suffering from a significant burden of disease. About 80% of patients with diabetes live with at least 1 other medical condition,35 and each of these patients spends over 2 hours a day, on average, following doctors’ recommendations.36 If antiobesity medications are prescribed without careful consideration of the patient’s preexisting workload, they will be destined to fail. Therefore, it becomes crucial to first account for the patient’s ability to cope with therapy intensification. This requires careful deliberation between healthcare providers and patients, in aims of targeting a weight-loss plan that fits patients’ goals and is aligned with providers’ expectations.
Healthcare systems also play a key role in supporting better conversations about obesity in type 2 DM patients. They could implement multifaceted initiatives to promote shared decision-making and the use of decision aids to advance patient-centered obesity practices.37 Policymakers could redesign quality measures aimed at capturing the quality of obesity conversations, and develop policies that support better education for clinicians regarding the importance of addressing obesity with adequate communication and patient-centered skills. Guidelines are often too disease-specific and do not consider comorbidities in their context when providing recommendations.38 Thus, diabetes societies should respond to the need to guide care for patients with diabetes and its comorbidities, particularly obesity.
CONCLUSIONS
Obesity is a serious global health issue and a leading risk factor for type 2 DM. Lifestyle measures are the cornerstone of preventing and treating obesity and type 2 DM. Emerging data support the effectiveness of intensive, interdisciplinary weight-loss programs in patients with diabetes. The use of antiobesity drugs should be considered in patients who have not achieved adequate responses to lifestyle interventions. Medications should be tailored to the individual’s health risks and metabolic and psychobehavioral characteristics. In many cases, the addition of weight-loss drugs will help accomplish and maintain the recommended 10% weight reduction, resulting in improvement in glycemic control and significant reduction in cardiovascular risk factors. New studies combining antiobesity and antidiabetes medications in the context of lifestyle interventions will help define the optimal therapeutic approach for patients with type 2 DM and obesity.
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015; (219):1–8.
- Lyznicki JM, Young DC, Riggs JA, Davis RM; for the Council on Scientific Affairs, American Medical Association. Obesity: assessment and management in primary care. Am Fam Physician 2001; 63:2185–2196.
- World Health Organization (WHO). Obesity and overweight fact sheet. www.who.int/mediacentre/factsheets/fs311/en. Updated June 2016. Accessed June 22, 2017.
- Daniels J. Obesity: America’s epidemic. Am J Nurs 2006; 106:40–49.
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995; 122:481–486.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes-5-year outcomes. N Engl J Med 2017; 376:641–651.
- Oldridge NB, Stump TE, Nothwehr FK, Clark DO. Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle- and older-age adults. J Clin Epidemiol 2001; 54:928–934.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocr Pract 2016; 22:84–113.
- McFarlane SI. Antidiabetic medications and weight gain: implications for the practicing physician. Curr Diab Rep 2009; 9:249–254.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Beal E. The pros and cons of designating obesity a disease: the new AMA designation stirs debate. Am J Nurs 2013; 113:18–19.
- Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013; 51:186–192.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001; 50:513–518.
- Pappachan JM, Viswanath AK. Medical management of diabesity: do we have realistic targets? Curr Diab Rep 2017; 17:4.
- Lexicomp Online, Lexi-Drugs, Hudson, OH: Wolters Kluwer Clinical Drug Information, Inc., 2017.
- Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004; 27:155–161.
- Buysschaert B, Aydin S, Morelle J, Hermans MP, Jadoul M, Demoulin N. Weight loss at a high cost: orlistat-induced late-onset severe kidney disease. Diabetes Metab 2016; 42:62–64.
- Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med 2005; 143:380–385.
- Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013; 21:2163–2171.
- Solas M, Milagro FI, Martínez-Urbistondo D, Ramirez MJ, Martínez JA. Precision obesity treatments including pharmacogenetic and nutrigenetic approaches. Trends Pharmacol Sci 2016; 37:575–593.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:1341–1352.
- Hollander P, Gupta AK, Plodkowski R, et al; for the COR-Diabetes Study Group. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013; 36:4022–4029.
- Cercato C, Roizenblatt VA, Leança CC, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes (Lond) 2009; 33:857–865.
- Gardin JM, Schumacher D, Constantine G, Davis KD, Leung C, Reid CL. Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine. JAMA 2000; 283:1703–1709.
- O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012; 20:1426–1436.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studies. Diabetes Obes Metab 2009; 11(suppl 3):26–34.
- Buse JB, Rosenstock J, Sesti G, et al; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374:39–47.
- Davies MJ, Bergenstal R, Bode B, et al; for the NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015; 314:687–699.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; for the LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375:311–322.
- Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med 2008; 121:149–157.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Burguera B, Jesus Tur J; Escudero AJ, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015; 2015:194696.
- Hargraves I, LeBlanc A, Shah ND, Montori VM. Shared decision making: the need for patient-clinician conversation, not just information. Health Aff (Millwood) 2016; 35:627–629.
- Lin P-J, Kent DM, Winn AN, Cohen JT, Neumann PJ. Multiple chronic conditions in type 2 diabetes mellitus: prevalence and consequences. Am J Manag Care 2015; 21:e23–e34.
- Russell LB, Suh D-C, Safford MA. Time requirements for diabetes self-management: too much for many? J Fam Pract 2005; 54:52–56.
- Serrano V, Rodriguez-Gutierrez R, Hargraves I, Gionfriddo MR, Tamhane S, Montori VM. Shared decision-making in the care of individuals with diabetes. Diabet Med 2016; 33:742–751.
- Wyatt KD, Stuart LM, Brito JP, et al. Out of context: clinical practice guidelines and patients with multiple chronic conditions: a systematic review. Med Care 2014; 52(suppl 3):S92–S100.
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015; (219):1–8.
- Lyznicki JM, Young DC, Riggs JA, Davis RM; for the Council on Scientific Affairs, American Medical Association. Obesity: assessment and management in primary care. Am Fam Physician 2001; 63:2185–2196.
- World Health Organization (WHO). Obesity and overweight fact sheet. www.who.int/mediacentre/factsheets/fs311/en. Updated June 2016. Accessed June 22, 2017.
- Daniels J. Obesity: America’s epidemic. Am J Nurs 2006; 106:40–49.
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995; 122:481–486.
- Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes-5-year outcomes. N Engl J Med 2017; 376:641–651.
- Oldridge NB, Stump TE, Nothwehr FK, Clark DO. Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle- and older-age adults. J Clin Epidemiol 2001; 54:928–934.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocr Pract 2016; 22:84–113.
- McFarlane SI. Antidiabetic medications and weight gain: implications for the practicing physician. Curr Diab Rep 2009; 9:249–254.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Beal E. The pros and cons of designating obesity a disease: the new AMA designation stirs debate. Am J Nurs 2013; 113:18–19.
- Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013; 51:186–192.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001; 50:513–518.
- Pappachan JM, Viswanath AK. Medical management of diabesity: do we have realistic targets? Curr Diab Rep 2017; 17:4.
- Lexicomp Online, Lexi-Drugs, Hudson, OH: Wolters Kluwer Clinical Drug Information, Inc., 2017.
- Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004; 27:155–161.
- Buysschaert B, Aydin S, Morelle J, Hermans MP, Jadoul M, Demoulin N. Weight loss at a high cost: orlistat-induced late-onset severe kidney disease. Diabetes Metab 2016; 42:62–64.
- Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med 2005; 143:380–385.
- Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013; 21:2163–2171.
- Solas M, Milagro FI, Martínez-Urbistondo D, Ramirez MJ, Martínez JA. Precision obesity treatments including pharmacogenetic and nutrigenetic approaches. Trends Pharmacol Sci 2016; 37:575–593.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:1341–1352.
- Hollander P, Gupta AK, Plodkowski R, et al; for the COR-Diabetes Study Group. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013; 36:4022–4029.
- Cercato C, Roizenblatt VA, Leança CC, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes (Lond) 2009; 33:857–865.
- Gardin JM, Schumacher D, Constantine G, Davis KD, Leung C, Reid CL. Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine. JAMA 2000; 283:1703–1709.
- O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012; 20:1426–1436.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studies. Diabetes Obes Metab 2009; 11(suppl 3):26–34.
- Buse JB, Rosenstock J, Sesti G, et al; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374:39–47.
- Davies MJ, Bergenstal R, Bode B, et al; for the NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015; 314:687–699.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; for the LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375:311–322.
- Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med 2008; 121:149–157.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Burguera B, Jesus Tur J; Escudero AJ, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015; 2015:194696.
- Hargraves I, LeBlanc A, Shah ND, Montori VM. Shared decision making: the need for patient-clinician conversation, not just information. Health Aff (Millwood) 2016; 35:627–629.
- Lin P-J, Kent DM, Winn AN, Cohen JT, Neumann PJ. Multiple chronic conditions in type 2 diabetes mellitus: prevalence and consequences. Am J Manag Care 2015; 21:e23–e34.
- Russell LB, Suh D-C, Safford MA. Time requirements for diabetes self-management: too much for many? J Fam Pract 2005; 54:52–56.
- Serrano V, Rodriguez-Gutierrez R, Hargraves I, Gionfriddo MR, Tamhane S, Montori VM. Shared decision-making in the care of individuals with diabetes. Diabet Med 2016; 33:742–751.
- Wyatt KD, Stuart LM, Brito JP, et al. Out of context: clinical practice guidelines and patients with multiple chronic conditions: a systematic review. Med Care 2014; 52(suppl 3):S92–S100.
KEY POINTS
- Obesity contributes to type 2 DM and worsens its control. Yet insulin therapy and most first-line diabetes drugs cause weight gain as a side effect.
- We believe that physicians should include body weight along with blood glucose levels as targets of therapy in patients with type 2 DM.
- Several drugs are approved for weight loss, and although their effect on weight tends to be moderate, some have been shown to reduce the incidence of type 2 DM and improve diabetic control.
- A stepwise approach to managing type 2 DM and obesity starts with lifestyle interventions and advances to adding (1) metformin, (2) a glucagon-like peptide-1 receptor agonist or a sodium-glucose cotransporter-2 inhibitor, and (3) one of the approved weight-loss drugs.