User login
Intimate Partner Violence
The prevalence of intimate partner violence (IPV; defined as mental and/or physical violence directed from 1 person in an intimate relationship to the other) varies widely, depending on the population sampled and method of data collection. In the United States, IPV against women, occurring within the year prior to contact with a healthcare professional, ranges from 2% to 15% in surveys done by telephone, in primary care clinics, or in face‐to‐face home interviews19 and from 10% to 30% in surveys of patients visiting urgent care or emergency departments.1012 The prevalence of IPV occurring at any time during the life of the patient ranges from 18% in the aforementioned settings to as high as 88% in women applying for welfare.1, 2, 4, 5, 10, 1214
Although reports indicate that victims of IPV are more likely to be hospitalized,1517 the only study assessing the prevalence of IPV in hospitalized patients included women on medical, surgical, and obstetrical services and reported 1‐year and lifetime prevalences of only 5% and 23%, respectively.18
We hypothesized that the prevalence of IPV in hospitalized patients would be at least as high as that reported from emergency departments and sought to measure the 1‐year and lifetime prevalences of IPV in women admitted to a general internal medicine service. In addition, because studies done in various outpatient settings have reported that victims of IPV have a variety of somatic complaints and an increased prevalence of chronic and functional illnesses,1923 we also sought to determine whether women with a history of IPV and women without a history of IPV had different numbers or types of positive responses to questions asked on the review of systems.
PATIENTS AND METHODS
This study was approved by the Colorado Multiple Institution Review Board, and informed consent was obtained from all participants.
Women between the ages of 18 and 60 who were admitted to the internal medicine floor service of Denver Health Medical Center (a university‐affiliated public safety‐net hospital) between January 1 and February 28, 2004 and between October 1 and October 30, 2004 were approached to participate. These dates were selected on the basis of the availability of our interviewers. Patients older than 60 were excluded to avoid overlap between IPV and the problem of elder abuse. Women were excluded if they were unable to give informed consent, were pregnant, were incarcerated, were on contact precautions, or spoke a language other than English or Spanish. Although IPV is common in pregnant women and may occur in women who are incarcerated, these are considered vulnerable populations with respect to obtaining approval from internal review boards.
The questionnaire consisted of 23 review‐of‐systems questions,24 4 questions adapted from a previously validated screen for IPV11 (Table 1), and 1 question about attempts to seek help (Table 1). Women were considered to have experienced IPV if they gave positive responses to any of the 4 questions targeting IPV. According to patient preference, the combined questionnaire was either read and filled out by each subject independently or was read to her by a female interviewer who then recorded the subject's verbal responses. All interviewers were women with a shared common concern about, and interest in, IPV. Although none had advanced training in psychology, social work, or other formal discipline that involved interviewing skills, all interviews were scripted so that interactions with subjects and completion of the questionnaires would be uniform. Responses indicating sometimes were considered to be positive. Responses that were not answered, left blank, or marked as not applicable were considered to be negative.
| 1. Have you ever been hit, kicked, punched, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 2. Within the last year, have you been hit, kicked, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 3. Do you feel safe in your current relationship? |
| 4. Is there a partner from a previous relationship who is making you feel unsafe now? |
| 5. If you answered yes to any of the above, have you ever asked for help from police, shelter, counselor, physician? If so, how long ago? |
Each patient's medical record was reviewed to determine her age, race, number of previous hospital admissions, visits to the emergency department and walk‐in clinic, visits to primary care and subspecialty physicians, and whether the patient had been screened for IPV as recorded on the admission history and physical template. Admission diagnosis was obtained from the history and physical template, and the discharge diagnosis was obtained from the discharge paperwork. Functional diagnoses were considered to be symptoms (eg, shortness of breath) or problems (eg, constipation) that could not clearly be linked to a specific disease process. All participants were offered a card containing a list of resources for victims of IPV.
Data were analyzed with SAS 8.1 (SAS Institute, Cary, NC) and SPSS 11.5 (SPSS, Chicago, IL). The Student t test was used to compare continuous variables. Data are reported as means standard deviation. Chi‐square analysis was used to test associations between race, primary language, level of education, insurance status, admitting diagnosis, discharge diagnosis, number of previous hospital admissions, visit type, and the presence of IPV. For these, P < 0.05 was considered to be significant. The association of positive review‐of‐systems responses with the presence of IPV was also tested by chi‐square analysis, but P < 0.002 was considered to be significant on the basis of a Bonferroni adjustment for multiple comparisons. A receiver operating characteristic curve was used to assess the relationship between the number of positive responses to the questions included in the review of systems and a history of IPV. The odds ratio and confidence intervals were calculated to test the association between the number of positive responses to the review‐of‐systems questions and a lifetime history of IPV.
RESULTS
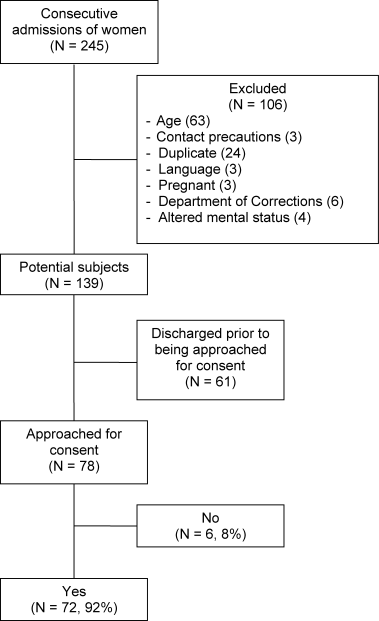
Throughout the dates of the study, 245 women were admitted to the internal medicine service, and 106 were excluded (Figure 1). Of the 139 eligible women, 78 were available to the interviewers and asked to participate, and 72 (92%) agreed. IPV occurring within the year prior to the interview or at any point in the patient's lifetime was reported by 16 (22%) and 44 (61%) subjects, respectively. No significant differences were seen in women who did or did not experience IPV at anytime in their life with respect to age, race, insurance status, education, number of scheduled outpatient, urgent, or emergent visits, or admission or discharge diagnosis even when the diagnoses were grouped into a functional category (although at best our study was powered to detect only >35% differences in prevalences; Tables 2 and 3). Of women reporting a lifetime history of IPV, 26 of 44 (59%) had previously sought help, and 9 of those 26 (35%) said that they sought help from a physician.
| IPV History | No IPV History | |
|---|---|---|
| ||
| Number (%) | 44 (61) | 28 (39) |
| Age (mean standard deviation) | 44 10 | 45 12 |
| Race [n, (%)] | ||
| Caucasian | 18 (41) | 6 (21) |
| Hispanic | 13 (30) | 15 (54) |
| African American | 12 (27) | 6 (21) |
| Other | 1 (2) | 1 (4) |
| Insurance status [n (%)] | ||
| Insured | 12 (27) | 5 (18) |
| Uninsured | 32 (73) | 23 (82) |
| Education [n (%)] | ||
| Grade school | 4 (9) | 3 (11) |
| Some high school | 13 (30) | 5 (18) |
| High school diploma | 15 (34) | 9 (32) |
| Some college | 9 (20) | 7 (25) |
| College degree | 2 (5) | 2 (7) |
| Postgraduate | 1 (2) | 2 (7) |
| Previous visit type (median, IQR) | ||
| Scheduled outpatient (includes primary care and subspecialty) | 2 (8) | 1.5 (7) |
| Emergency department and walk‐in clinic | 2 (3.5) | 1 (3) |
| Previous hospital admissions [n (%)] | ||
| 0 | 24 (55) | 16 (57) |
| 1 | 16 (36) | 4 (14) |
| 2 | 0 (0) | 4 (14) |
| 3 | 2 (5) | 2 (7) |
| >3 | 2 (5) | 2 (7) |
| Admission or Discharge Diagnosis | Admission | Discharge | ||
|---|---|---|---|---|
| IPV (n = 44) | No IPV (n = 28) | IPV (n = 44) | No IPV (n = 28) | |
| ||||
| Cardiovascular | ||||
| Chest pain (%)* | 8 (18) | 5 (18) | 6 (14) | 4 (14) |
| Cardiomyopathy | 0 | 0 | 1 | 0 |
| Cerebrovascular accident | 1 | 0 | 1 | 0 |
| Deep venous thrombosis | 0 | 0 | 1 | 0 |
| Hypertensive emergency | 0 | 0 | 1 | 0 |
| Palpitations* | 0 | 1 | 0 | 1 |
| Valvular disease | 0 | 0 | 1 | 0 |
| Venous stasis | 0 | 1 | 0 | 1 |
| Total (%) | 9 (20) | 7 (25) | 11 (25) | 6 (21) |
| Gastrointestinal | ||||
| Abdominal pain (%)* | 7 (16) | 4 (14) | 2 | 1 |
| Ascites | 0 | 1 | 0 | 0 |
| Constipation* | 0 | 0 | 1 | 0 |
| End‐stage liver disease | 1 | 1 | 1 | 2 |
| Esophagitis | 0 | 0 | 1 | 0 |
| Hepatitis | 1 | 0 | 1 | 0 |
| Nausea/vomiting* | 2 | 0 | 1 | 0 |
| Pancreatitis | 0 | 1 | 3 | 2 |
| Peptic ulcer disease | 1 | 0 | 1 | 0 |
| Upper gastrointestinal bleeding | 2 | 0 | 1 | 0 |
| Total (%) | 14 (32) | 7 (25) | 12 (27) | 5 (18) |
| Hematology/oncology | ||||
| Abdominal mass | 0 | 0 | 0 | 1 |
| Anemia | 1 | 0 | 1 | 0 |
| Breast cancer | 0 | 1 | 0 | 1 |
| Cervical cancer | 1 | 0 | 1 | 0 |
| Colon cancer | 0 | 1 | 0 | 1 |
| Sickle cell anemia | 1 | 0 | 1 | 0 |
| Thrombocytosis | 1 | 0 | 1 | 0 |
| Total (%) | 4 (9) | 2 (7) | 4 (9) | 3 (11) |
| Infectious disease | ||||
| Bacteremia/sepsis | 3 | 0 | 3 | 0 |
| Cellulitis | 1 | 0 | 1 | 1 |
| Cholangitis | 0 | 0 | 1 | 0 |
| Community‐acquired pneumonia | 2 | 2 | 2 | 1 |
| Endocarditis | 1 | 0 | 1 | 0 |
| Fever | 0 | 1 | 0 | 1 |
| Pelvic inflammatory disease | 0 | 0 | 0 | 1 |
| Urinary tract infection | 1 | 0 | 1 | 0 |
| Total (%) | 8 (18) | 3 (11) | 9 (20) | 4 (14) |
| Pulmonary | ||||
| Acute exacerbation of COPD | 0 | 0 | 1 | 0 |
| Asthma exacerbation | 1 | 1 | 1 | 2 |
| Pleuritic chest pain* | 0 | 0 | 1 | 0 |
| Pulmonary embolism | 0 | 0 | 1 | 0 |
| Shortness of breath* | 4 | 0 | 1 | 0 |
| Total (%) | 5 (11) | 1 (4) | 5 (11) | 2 (7) |
| Renal/genitourinary | ||||
| Acute renal failure | 0 | 1 | 0 | 1 |
| End‐stage renal disease | 1 | 2 | 1 | 2 |
| Nephrotic syndrome | 0 | 1 | 0 | 2 |
| Vaginal bleeding | 1 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 2 (5) | 5 (18) |
| Other | ||||
| Diabetic ketoacidosis | 0 | 1 | 0 | 1 |
| Extremity pain* | 0 | 1 | 0 | 0 |
| Mediastinal thickening | 0 | 0 | 0 | 1 |
| Hyponatremia | 0 | 1 | 0 | 1 |
| Lower extremity swelling | 2 | 1 | 0 | 0 |
| Somatization* | 0 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 1 (2) | 3 (11) |
| Total functional diagnoses (%) | 21 (48) | 11 (39) | 12 (27) | 6 (21) |
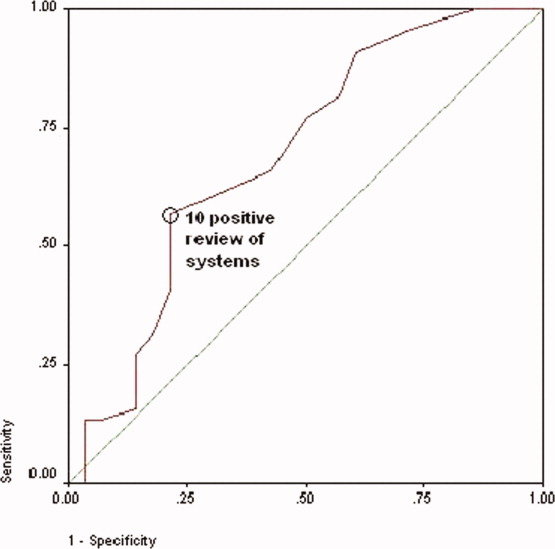
Women with a 1‐year history of IPV and women without a 1‐year history of IPV had 11.4 4.7 and 7.7 5.4 positive responses to the review of systems (P < 0.01), respectively. Women with a lifetime history of IPV and women without a lifetime history of IPV had 10.9 4.4 and 7.7 5.4 positive responses (P < 0.01), respectively. The receiver operating characteristic curve of the number of positive responses versus a lifetime history of IPV is presented in Figure 2. Subjects with 10 or more positive responses were 4.8 times more likely to report a lifetime history of IPV than subjects with 9 or fewer positive responses (confidence interval = 1.614.2, P = 0.003). The c‐statistic indicating the ability of the review of systems to properly classify cases when there were 10 or more positive responses was 0.692.
No differences were observed in the responses to the individual review of systems questions in women who did or did not have a lifetime history of IPV, with the exception that those with a positive history more commonly complained of difficulty sleeping and numbness and tingling in their hands or feet (although at best our study was sufficiently powered to detect only >20% differences in prevalences; Table 4). Although the sensitivity of having problems sleeping or experiencing numbness or tingling in patients with IPV was high, the specificity and positive and negative predictive values were not (Table 5).
| Review‐of‐Systems Questions | IPV History (n = 44) | No IPV History (n = 28) | P Value |
|---|---|---|---|
| |||
| 1. Shortness of breath | 25 (57) | 10 (36) | 0.081 |
| 2. Chest pain/pressure | 19 (43) | 9 (32) | 0.349 |
| 3. Abdominal pain | 17 (39) | 10 (36) | 0.803 |
| 4. Headaches | 24 (55) | 13 (46) | 0.502 |
| 5. Rashes | 15 (34) | 9 (32) | 0.864 |
| 6. Bruising | 32 (73) | 12 (43) | 0.011 |
| 7. Joint pain/stiffness | 27 (61) | 11 (39) | 0.067 |
| 8. Muscle pain/spasms | 22 (50) | 11 (39) | 0.374 |
| 9. Pain with intercourse | 8 (19) | 4 (14) | 0.753 |
| 10. Pelvic pain/cramps | 13 (30) | 5 (18) | 0.264 |
| 11. Nausea/vomiting | 19 (43) | 11 (39) | 0.744 |
| 12. Nervous/anxious | 28 (64) | 14 (50) | 0.253 |
| 13. Sad/crying | 21 (48) | 12 (43) | 0.686 |
| 14. Weight gain/loss | 26 (59) | 17 (61) | 0.891 |
| 15. Trouble sleeping | 37 (84) | 12 (43) | 0.000* |
| 16. Fever/chills | 19 (43) | 6 (21) | 0.059 |
| 17. Frequent/painful urination | 11 (25) | 6 (21) | 0.728 |
| 18. Pounding/emrregular heart beat | 14 (32) | 7 (25) | 0.535 |
| 19. Dizzy/passing out | 13 (30) | 7 (25) | 0.675 |
| 20. Memory problem | 19 (43) | 7 (25) | 0.117 |
| 21. Diarrhea/constipation | 27 (61) | 10 (36) | 0.034 |
| 22. Numbness/tingling | 35 (80) | 9 (32) | <0.0001* |
| 23. Pain chewing/swallowing | 8 (18) | 5 (18) | 0.972 |
| Trouble Sleeping | Numbness/Tingling | |
|---|---|---|
| Sensitivity (%) | 84 | 74 |
| Specificity (%) | 57 | 68 |
| Positive predictive value (%) | 76 | 78 |
| Negative predictive value (%) | 70 | 68 |
The admission history forms filled out by first‐year admitting residents showed that only 18 (25%) of the women were screened for IPV, even though the history and physical examination template used at Denver Health Medical Center includes a prompt in the social history section pertaining to a history of violence as a reminder.
DISCUSSION
The important findings of this study were that women admitted to the internal medicine service of a university‐affiliated public safety‐net hospital had a high prevalence of IPV (22% and 61% 1‐year and lifetime prevalences, respectively), that most women with a history of IPV had previously sought help for the problem, many from physicians, that women were more likely to have a history of IPV if they had >10 positive responses to questions asked in a routine review of systems (particularly problems sleeping and experiencing numbness or tingling in their extremities), and that routine screening for IPV was uncommon at the time of admission.
These conclusions should be interpreted with respect to a number of limitations in our study. First, although our study was designed to be a consecutive series, the interviewers did not have sufficient time to meet with and interview every woman admitted before they were discharged. This occurred in part because the interviewers were available only for a portion of each day, some patients were discharged within 24 hours of admission, and many were out of their rooms for ancillary testing. Within the interviewers' time constraints, however, all hospitalized women meeting entry criteria who were available were approached. Our data could, however, overrepresent the prevalence of IPV if hospitalized women with a history of IPV had longer hospital stays than those who did not or if those experiencing IPV were out of their rooms less frequently (eg, for diagnostic tests). On the other hand, our data could underrepresent the true prevalence of IPV if patients with a history of IPV had shorter hospital stays or if they received more ancillary testing that caused them to be out of their rooms more frequently. Second, none of our interviewers had specific training in interviewing techniques. Accordingly, our data could have underestimated the true prevalence of IPV if interviewers with advanced training in probing sensitive topics had more success in eliciting positive responses. Third, the relationship between a history of IPV and multiple positive responses to the review of systems may be confounded if some of these patients also had a history of adverse childhood experiences or other experiences resulting in posttraumatic stress disorder as these patients also have an increased prevalence of chronic and functional disorders.2527 Finally, as our numbers were small, we were not powered to detect clinically important differences in demographics or specific positive answers on the review of systems.
To the best of our knowledge, the only study presenting IPV prevalence data in patients hospitalized for other than psychiatric problems was performed by McKenzie and colleagues18 in 1997. In their group of 130 patients (61 on internal medicine, 59 on surgery, 7 on obstetrics, and 3 on psychiatry), the 1‐year and lifetime prevalences of IPV were only 5% and 26%, respectively. McKenzie and colleagues used only 1 question to screen for IPV, but that single question incorporated 2 of the 4 questions used in our survey. Forty‐three of our 44 patients (98%) with a history of IPV were discovered on the basis of these 2 questions. The hospitals in which the 2 studies were done were similar, as were the ages and levels of education of the 2 populations studied and the percentage of eligible patients who agreed to participate. The patients in the 2 studies were different with respect to race, language mix, and the percentage who were insured, but neither study found differences in the prevalence of IPV as a function of race or insurance (although others have found an association of IPV with being uninsured1, 3, 4, 12, 23). Our study was conducted in women admitted exclusively to an internal medicine service, whereas nearly half of the patients studied by McKenzie and colleagues were admitted to surgical, gynecologic, or psychiatric services. Although McKenzie and colleagues found no difference in the prevalence of IPV as a function of admitting service, others have suggested that the prevalence of IPV is higher in patients admitted for trauma or psychiatric problems.1517, 28 The percentage of patients who self‐administered the questionnaires was 57% in our study and 77% in the study by McKenzie and colleagues. Neither study, however, found a difference in the percentage of IPV in patients who self‐administered the survey versus those who were interviewed. Women may have become more comfortable discussing this issue in the 10‐year interval between these 2 studies, or the prevalence of IPV may have increased. The only other study of IPV in hospitalized patients of which we are aware reported a 90% 1‐year prevalence in suicidal women admitted to a psychiatric service.28
Several studies have reported that victims of IPV have multiple somatic complaints and an increased prevalence of chronic and functional illnesses.1923 We confirmed that women experiencing IPV have more positive responses to questions posed in a review of systems, but the low specificity and positive and negative predictive values of the responses make this association of little clinical utility.
For only 18 of the 72 patients (25%) in our study was there evidence that they were screened for a history of IPV by the admitting resident. If more women were screened without a response being recorded, or if women were screened only for a current history of violence, our data may not accurately reflect the true rate at which screening occurred; however, the rate of screening that we observed is consistent with a number of other studies.12, 22, 2931 Fourteen of 18 patients who were screened for IPV by the resident gave negative responses. Ten of these, however, gave positive responses to our interviewers. Accordingly, the sensitivity, specificity, and positive and negative predictive values of the information recorded by the admitting resident were 40%, 100%, 100%, and 57%, respectively (assuming that the responses given to the IPV survey represent the gold standard), and this confirms that routine screening underestimates the prevalence of this problem. Accordingly, we identified 2 problems pertaining to screening for IPV: (1) it is not routinely done at the time of hospital admission, and (2) responses reported during routine screening are frequently incorrect. A number of barriers to routine screening have been previously identified, as have interventions designed to increase screening.32 Providing specific screening questions increases the identification of victims of IPV, but simply educating healthcare providers does not.32 Our history and physical templates have a prompt for violence victim to facilitate the screening, but as a result of this study, we are changing our prompting question and indicating what should be done if the response is positive.
The US Preventive Services Task Force and the Canadian Task Force on Preventive Health Care both concluded that there was insufficient evidence to recommend for or against routine screening for IPV.3335 Their rationale was that trials assessing the effectiveness of screening have not been published, that studies designed to assess the effectiveness of any resulting intervention are few in number, focused on pregnant women, and limited by problems in study design, that no studies have determined the accuracy of the screening tools, and that none have addressed the potential harm of screening.3335 The US Preventive Services Task Force did recommend screening if providers were concerned about IPV.34 Our data would suggest that there is little in the admission history that distinguishes women who might be victims of IPV from those who might not. Guidelines published by the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists promote routine screening of all patients.3638 Janssen and colleagues39 support the importance of screening on the basis that IPV is associated with numerous physical and mental health problems (eg, arthritis, migraines and other types of headaches, vaginal bleeding, ulcers, spastic colon, chronic pain, substance abuse, depression, and suicide ideation) and that establishing the link between these conditions and IPV could be important with respect to developing appropriate diagnostic and therapeutic approaches to patients' complaints. Screening also allows physicians to become more knowledgeable about their patients' lives, facilitating their ability to provide a supportive relationship that, in turn, increases women's likelihood of using an intervention method.39 We did not confirm an increased prevalence of any of the complaints noted by Janssen and colleagues in the women experiencing a history of IPV, but we did find an increased prevalence of insomnia and extremity numbness in women admitting to IPV as well as an overall increase in the number of positive responses to the review of systems. Screening identifies women who should receive information about reporting IPV, obtaining available assistance, planning for personal safety, and formal counseling as these have all been shown to reduce the severity of IPV and to improve the quality of life in rather large, randomized controlled trials.4043
As previously observed by others,13, 22, 29, 4446 the large majority of women that we approached welcomed screening for IPV. Over half of those with a history of IPV had previously sought help for the problem, over one‐third of these sought help from physicians, and most took the resource card that we offered, regardless of whether they did or did not have a history of IPV (this suggests either that our data may actually underestimate the true prevalence of IPV or that patients taking the information knew of others experiencing this problem). Accordingly, regardless of whether physicians believe that routine screening is warranted, patients see physicians and other healthcare workers as a resource for this problem.
We have confirmed that a history of IPV is very common in women admitted to an internal medicine service of a university‐affiliated public hospital and that female victims of IPV have more positive responses on the review of systems (particularly difficulty sleeping and extremity numbness or tingling) than those who have not. Although we initially hypothesized that finding numerous somatic complaints might serve as a marker for IPV, thereby identifying patients for whom more careful screening should occur, finding such a high prevalence of IPV argues that screening should be a routine part of the history for all women admitted to internal medicine inpatient services.
Acknowledgements
The authors thank the patients who agreed to participate in this study during their hospitalization. They also thank Cheri Maestas and Debbie Rodriquez for their support and help in interviewing patients.
- ,,.Prevalence and determinants of intimate partner abuse among public hospital primary care patients.J Gen Intern Med.2000;15:811–817.
- ,,,.Women's experiences with violence: a national study.Womens Health Issues.2007;17:3–12.
- ,,,.Multistate analysis of factors associated with intimate partner violence.Am J Prev Med.2002;22:156–164.
- ,,,.Frequency and correlates of intimate partner violence by type: physical, sexual, and psychological battering.Am J Public Health.2000;90:553–559.
- ,,,,.Prevalence of domestic violence among patients in three ambulatory care internal medicine clinics.J Gen Intern Med.1991;6:317–322.
- ,,,.Prevalence of partner violence against 7,443 African American, White and Hispanic women receiving care at urban public primary care clinics.Public Health Nurs.2005;22:98–107.
- ,,,,.Evaluating domestic partner abuse in a family practice clinic.Fam Med.1997;29:492–495.
- ,.Prevalence and predictors of physical partner abuse among Mexican American women.Am J Public Health.2001;91:441–445.
- ,,.Rates of intimate partner violence in the United States.Am J Public Health.1998;88:1702–1704.
- ,,,.Domestic violence against women incidence and prevalence in an emergency department population.JAMA.1995;273:1763–1767.
- ,,, et al.Accuracy of 3 brief screening questions for detecting partner violence in the emergency department.JAMA.1997;277:1357–1361.
- ,,.A prevalence survey of abuse and screening for abuse in urgent care patients.Obstet Gynecol.1998;91:511–514.
- Morbidity and Mortality Weekly Report.Use of medical care, police assistance and restraining orders by women reporting intimate partner violence—Massachusetts, 1996–1997.JAMA.2000;284:558.
- ,,.Interpersonal violence among women seeking welfare: unraveling lives.Am J Public Health.2006;96:1409–1415.
- ,.A 5‐year follow‐up study of 117 battered women.Am J Public Health.1991;81:1486–1488.
- ,,.Rates and relative risk of hospital admission among women in violent intimate partner relationships.Am J Public Health.2000;90:1416–1420.
- ,,,.Intimate partner violence against women: do victims cost health plans more?J Fam Pract.1999;48:439–443.
- ,,,.Prevalence of domestic violence in an inpatient female population.J Gen Intern Med.1998;13:277–279.
- ,,, et al.Intimate partner violence and physical health consequences.Arch Intern Med.2002;162:1157–1163.
- ,,,,.Physical health consequences of physical and psychological intimate partner violence.Arch Fam Med.2000;9:451–457.
- ,,, et al.Sexual and physical abuse in women with functional or organic gastrointestinal disorders.Ann Intern Med.1990;113:828–833.
- ,,.Prevalence of intimate partner violence and health implications for women using emergency departments and primary care clinics.Womens Health Issues.2004;14:19–29.
- ,,, et al.The “battering syndrome”: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices.Ann Intern Med.1995;123:737–746.
- ,.DeGowin and DeGowin's Bedside Diagnostic Examination.5th ed.New York, NY:Macmillan Publishing;1987:18–29.
- ,,, et al.Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study.Am J Prev Med.1998;14:245–258.
- ,,,,,.Posttraumatic stress disorder and health status among female and male medical patients.J Trauma Stress.2004;17:1–9.
- ,,,,.Posttraumatic stress disorder and physical comorbidity among female children and adolescents: results from service‐use data.Pediatrics.2005:116;e767–e776.
- ,,,,.Prevalence and severity of intimate partner violence and associations with family functioning and alcohol abuse in psychiatric inpatients with suicidal intent.J Clin Psychiatry.2006;67:23–29.
- ,,.Intimate partner violence screening and intervention: data from eleven Pennsylvania and California community hospital emergency departments.J Emerg Nurs.2001;27:141–149.
- ,.Missed opportunities: emergency department visits by police‐identified victims of intimate partner violence.Emerg Med.2006;47:190–199.
- ,,, et al.Intimate partner violence and patient screening across medical specialties.Acad Emerg Med.2005;12:712–722.
- ,,,,.Screening for intimate partner violence by health care providers: barriers and interventions.Am J Prev Med.2000;19:230–237.
- ,,,.Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2004;140:387–396.
- U.S. Preventive Services Task Force.Screening for family and intimate partner violence: recommendation statement.Ann Intern Med.2004;140:382–386.
- ,.Interventions for violence against women: scientific review.JAMA.2003;289:589–600.
- American Medical Association. Policy H‐515.965: family and intimate partner violence. Available at: http://www.ama‐assn.org. Accessed May2007.
- American Academy of Family Physicians. Family and intimate partner violence and abuse. Available at: www.aafp.org/x16506.xml. Accessed May2007.
- Domestic Violence.Washington, DC:American College of Obstetrics and Gynecology;1999. Educational Bulletin Number; No. 257.
- ,,.Assessment for intimate partner violence: where do we stand?J Am Board Fam Med.2006;19:413–415.
- ,,,,,.What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors.JAMA.2003;58:76–81.
- ,,,,,.Assessing intimate partner violence in health care settings leads to women's receipt of interventions and improved health.Public Health Rep.2006;121:435–444.
- ,,.An evaluation of interventions to decrease intimate partner violence to pregnant women.Public Health Nurs.2000;17:443–451.
- ,.Reducing violence using community‐based advocacy for women with abusive partners.J Consult Clin Psychol.1999;67:43–53.
- ,,,,.Help‐seeking for intimate partner violence and forced sex in South Carolina.Am J Prev Med.2000;19:316–320.
- ,,, et al.Women's opinions about domestic violence screening and mandatory reporting.Am J Prev Med.2000;19:279–285.
- ,,,.The factors associated with disclosure of intimate partner abuse to clinicians.J Fam Pract.2001;50:338–344.
The prevalence of intimate partner violence (IPV; defined as mental and/or physical violence directed from 1 person in an intimate relationship to the other) varies widely, depending on the population sampled and method of data collection. In the United States, IPV against women, occurring within the year prior to contact with a healthcare professional, ranges from 2% to 15% in surveys done by telephone, in primary care clinics, or in face‐to‐face home interviews19 and from 10% to 30% in surveys of patients visiting urgent care or emergency departments.1012 The prevalence of IPV occurring at any time during the life of the patient ranges from 18% in the aforementioned settings to as high as 88% in women applying for welfare.1, 2, 4, 5, 10, 1214
Although reports indicate that victims of IPV are more likely to be hospitalized,1517 the only study assessing the prevalence of IPV in hospitalized patients included women on medical, surgical, and obstetrical services and reported 1‐year and lifetime prevalences of only 5% and 23%, respectively.18
We hypothesized that the prevalence of IPV in hospitalized patients would be at least as high as that reported from emergency departments and sought to measure the 1‐year and lifetime prevalences of IPV in women admitted to a general internal medicine service. In addition, because studies done in various outpatient settings have reported that victims of IPV have a variety of somatic complaints and an increased prevalence of chronic and functional illnesses,1923 we also sought to determine whether women with a history of IPV and women without a history of IPV had different numbers or types of positive responses to questions asked on the review of systems.
PATIENTS AND METHODS
This study was approved by the Colorado Multiple Institution Review Board, and informed consent was obtained from all participants.
Women between the ages of 18 and 60 who were admitted to the internal medicine floor service of Denver Health Medical Center (a university‐affiliated public safety‐net hospital) between January 1 and February 28, 2004 and between October 1 and October 30, 2004 were approached to participate. These dates were selected on the basis of the availability of our interviewers. Patients older than 60 were excluded to avoid overlap between IPV and the problem of elder abuse. Women were excluded if they were unable to give informed consent, were pregnant, were incarcerated, were on contact precautions, or spoke a language other than English or Spanish. Although IPV is common in pregnant women and may occur in women who are incarcerated, these are considered vulnerable populations with respect to obtaining approval from internal review boards.
The questionnaire consisted of 23 review‐of‐systems questions,24 4 questions adapted from a previously validated screen for IPV11 (Table 1), and 1 question about attempts to seek help (Table 1). Women were considered to have experienced IPV if they gave positive responses to any of the 4 questions targeting IPV. According to patient preference, the combined questionnaire was either read and filled out by each subject independently or was read to her by a female interviewer who then recorded the subject's verbal responses. All interviewers were women with a shared common concern about, and interest in, IPV. Although none had advanced training in psychology, social work, or other formal discipline that involved interviewing skills, all interviews were scripted so that interactions with subjects and completion of the questionnaires would be uniform. Responses indicating sometimes were considered to be positive. Responses that were not answered, left blank, or marked as not applicable were considered to be negative.
| 1. Have you ever been hit, kicked, punched, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 2. Within the last year, have you been hit, kicked, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 3. Do you feel safe in your current relationship? |
| 4. Is there a partner from a previous relationship who is making you feel unsafe now? |
| 5. If you answered yes to any of the above, have you ever asked for help from police, shelter, counselor, physician? If so, how long ago? |
Each patient's medical record was reviewed to determine her age, race, number of previous hospital admissions, visits to the emergency department and walk‐in clinic, visits to primary care and subspecialty physicians, and whether the patient had been screened for IPV as recorded on the admission history and physical template. Admission diagnosis was obtained from the history and physical template, and the discharge diagnosis was obtained from the discharge paperwork. Functional diagnoses were considered to be symptoms (eg, shortness of breath) or problems (eg, constipation) that could not clearly be linked to a specific disease process. All participants were offered a card containing a list of resources for victims of IPV.
Data were analyzed with SAS 8.1 (SAS Institute, Cary, NC) and SPSS 11.5 (SPSS, Chicago, IL). The Student t test was used to compare continuous variables. Data are reported as means standard deviation. Chi‐square analysis was used to test associations between race, primary language, level of education, insurance status, admitting diagnosis, discharge diagnosis, number of previous hospital admissions, visit type, and the presence of IPV. For these, P < 0.05 was considered to be significant. The association of positive review‐of‐systems responses with the presence of IPV was also tested by chi‐square analysis, but P < 0.002 was considered to be significant on the basis of a Bonferroni adjustment for multiple comparisons. A receiver operating characteristic curve was used to assess the relationship between the number of positive responses to the questions included in the review of systems and a history of IPV. The odds ratio and confidence intervals were calculated to test the association between the number of positive responses to the review‐of‐systems questions and a lifetime history of IPV.
RESULTS
Throughout the dates of the study, 245 women were admitted to the internal medicine service, and 106 were excluded (Figure 1). Of the 139 eligible women, 78 were available to the interviewers and asked to participate, and 72 (92%) agreed. IPV occurring within the year prior to the interview or at any point in the patient's lifetime was reported by 16 (22%) and 44 (61%) subjects, respectively. No significant differences were seen in women who did or did not experience IPV at anytime in their life with respect to age, race, insurance status, education, number of scheduled outpatient, urgent, or emergent visits, or admission or discharge diagnosis even when the diagnoses were grouped into a functional category (although at best our study was powered to detect only >35% differences in prevalences; Tables 2 and 3). Of women reporting a lifetime history of IPV, 26 of 44 (59%) had previously sought help, and 9 of those 26 (35%) said that they sought help from a physician.

| IPV History | No IPV History | |
|---|---|---|
| ||
| Number (%) | 44 (61) | 28 (39) |
| Age (mean standard deviation) | 44 10 | 45 12 |
| Race [n, (%)] | ||
| Caucasian | 18 (41) | 6 (21) |
| Hispanic | 13 (30) | 15 (54) |
| African American | 12 (27) | 6 (21) |
| Other | 1 (2) | 1 (4) |
| Insurance status [n (%)] | ||
| Insured | 12 (27) | 5 (18) |
| Uninsured | 32 (73) | 23 (82) |
| Education [n (%)] | ||
| Grade school | 4 (9) | 3 (11) |
| Some high school | 13 (30) | 5 (18) |
| High school diploma | 15 (34) | 9 (32) |
| Some college | 9 (20) | 7 (25) |
| College degree | 2 (5) | 2 (7) |
| Postgraduate | 1 (2) | 2 (7) |
| Previous visit type (median, IQR) | ||
| Scheduled outpatient (includes primary care and subspecialty) | 2 (8) | 1.5 (7) |
| Emergency department and walk‐in clinic | 2 (3.5) | 1 (3) |
| Previous hospital admissions [n (%)] | ||
| 0 | 24 (55) | 16 (57) |
| 1 | 16 (36) | 4 (14) |
| 2 | 0 (0) | 4 (14) |
| 3 | 2 (5) | 2 (7) |
| >3 | 2 (5) | 2 (7) |
| Admission or Discharge Diagnosis | Admission | Discharge | ||
|---|---|---|---|---|
| IPV (n = 44) | No IPV (n = 28) | IPV (n = 44) | No IPV (n = 28) | |
| ||||
| Cardiovascular | ||||
| Chest pain (%)* | 8 (18) | 5 (18) | 6 (14) | 4 (14) |
| Cardiomyopathy | 0 | 0 | 1 | 0 |
| Cerebrovascular accident | 1 | 0 | 1 | 0 |
| Deep venous thrombosis | 0 | 0 | 1 | 0 |
| Hypertensive emergency | 0 | 0 | 1 | 0 |
| Palpitations* | 0 | 1 | 0 | 1 |
| Valvular disease | 0 | 0 | 1 | 0 |
| Venous stasis | 0 | 1 | 0 | 1 |
| Total (%) | 9 (20) | 7 (25) | 11 (25) | 6 (21) |
| Gastrointestinal | ||||
| Abdominal pain (%)* | 7 (16) | 4 (14) | 2 | 1 |
| Ascites | 0 | 1 | 0 | 0 |
| Constipation* | 0 | 0 | 1 | 0 |
| End‐stage liver disease | 1 | 1 | 1 | 2 |
| Esophagitis | 0 | 0 | 1 | 0 |
| Hepatitis | 1 | 0 | 1 | 0 |
| Nausea/vomiting* | 2 | 0 | 1 | 0 |
| Pancreatitis | 0 | 1 | 3 | 2 |
| Peptic ulcer disease | 1 | 0 | 1 | 0 |
| Upper gastrointestinal bleeding | 2 | 0 | 1 | 0 |
| Total (%) | 14 (32) | 7 (25) | 12 (27) | 5 (18) |
| Hematology/oncology | ||||
| Abdominal mass | 0 | 0 | 0 | 1 |
| Anemia | 1 | 0 | 1 | 0 |
| Breast cancer | 0 | 1 | 0 | 1 |
| Cervical cancer | 1 | 0 | 1 | 0 |
| Colon cancer | 0 | 1 | 0 | 1 |
| Sickle cell anemia | 1 | 0 | 1 | 0 |
| Thrombocytosis | 1 | 0 | 1 | 0 |
| Total (%) | 4 (9) | 2 (7) | 4 (9) | 3 (11) |
| Infectious disease | ||||
| Bacteremia/sepsis | 3 | 0 | 3 | 0 |
| Cellulitis | 1 | 0 | 1 | 1 |
| Cholangitis | 0 | 0 | 1 | 0 |
| Community‐acquired pneumonia | 2 | 2 | 2 | 1 |
| Endocarditis | 1 | 0 | 1 | 0 |
| Fever | 0 | 1 | 0 | 1 |
| Pelvic inflammatory disease | 0 | 0 | 0 | 1 |
| Urinary tract infection | 1 | 0 | 1 | 0 |
| Total (%) | 8 (18) | 3 (11) | 9 (20) | 4 (14) |
| Pulmonary | ||||
| Acute exacerbation of COPD | 0 | 0 | 1 | 0 |
| Asthma exacerbation | 1 | 1 | 1 | 2 |
| Pleuritic chest pain* | 0 | 0 | 1 | 0 |
| Pulmonary embolism | 0 | 0 | 1 | 0 |
| Shortness of breath* | 4 | 0 | 1 | 0 |
| Total (%) | 5 (11) | 1 (4) | 5 (11) | 2 (7) |
| Renal/genitourinary | ||||
| Acute renal failure | 0 | 1 | 0 | 1 |
| End‐stage renal disease | 1 | 2 | 1 | 2 |
| Nephrotic syndrome | 0 | 1 | 0 | 2 |
| Vaginal bleeding | 1 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 2 (5) | 5 (18) |
| Other | ||||
| Diabetic ketoacidosis | 0 | 1 | 0 | 1 |
| Extremity pain* | 0 | 1 | 0 | 0 |
| Mediastinal thickening | 0 | 0 | 0 | 1 |
| Hyponatremia | 0 | 1 | 0 | 1 |
| Lower extremity swelling | 2 | 1 | 0 | 0 |
| Somatization* | 0 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 1 (2) | 3 (11) |
| Total functional diagnoses (%) | 21 (48) | 11 (39) | 12 (27) | 6 (21) |
Women with a 1‐year history of IPV and women without a 1‐year history of IPV had 11.4 4.7 and 7.7 5.4 positive responses to the review of systems (P < 0.01), respectively. Women with a lifetime history of IPV and women without a lifetime history of IPV had 10.9 4.4 and 7.7 5.4 positive responses (P < 0.01), respectively. The receiver operating characteristic curve of the number of positive responses versus a lifetime history of IPV is presented in Figure 2. Subjects with 10 or more positive responses were 4.8 times more likely to report a lifetime history of IPV than subjects with 9 or fewer positive responses (confidence interval = 1.614.2, P = 0.003). The c‐statistic indicating the ability of the review of systems to properly classify cases when there were 10 or more positive responses was 0.692.

No differences were observed in the responses to the individual review of systems questions in women who did or did not have a lifetime history of IPV, with the exception that those with a positive history more commonly complained of difficulty sleeping and numbness and tingling in their hands or feet (although at best our study was sufficiently powered to detect only >20% differences in prevalences; Table 4). Although the sensitivity of having problems sleeping or experiencing numbness or tingling in patients with IPV was high, the specificity and positive and negative predictive values were not (Table 5).
| Review‐of‐Systems Questions | IPV History (n = 44) | No IPV History (n = 28) | P Value |
|---|---|---|---|
| |||
| 1. Shortness of breath | 25 (57) | 10 (36) | 0.081 |
| 2. Chest pain/pressure | 19 (43) | 9 (32) | 0.349 |
| 3. Abdominal pain | 17 (39) | 10 (36) | 0.803 |
| 4. Headaches | 24 (55) | 13 (46) | 0.502 |
| 5. Rashes | 15 (34) | 9 (32) | 0.864 |
| 6. Bruising | 32 (73) | 12 (43) | 0.011 |
| 7. Joint pain/stiffness | 27 (61) | 11 (39) | 0.067 |
| 8. Muscle pain/spasms | 22 (50) | 11 (39) | 0.374 |
| 9. Pain with intercourse | 8 (19) | 4 (14) | 0.753 |
| 10. Pelvic pain/cramps | 13 (30) | 5 (18) | 0.264 |
| 11. Nausea/vomiting | 19 (43) | 11 (39) | 0.744 |
| 12. Nervous/anxious | 28 (64) | 14 (50) | 0.253 |
| 13. Sad/crying | 21 (48) | 12 (43) | 0.686 |
| 14. Weight gain/loss | 26 (59) | 17 (61) | 0.891 |
| 15. Trouble sleeping | 37 (84) | 12 (43) | 0.000* |
| 16. Fever/chills | 19 (43) | 6 (21) | 0.059 |
| 17. Frequent/painful urination | 11 (25) | 6 (21) | 0.728 |
| 18. Pounding/emrregular heart beat | 14 (32) | 7 (25) | 0.535 |
| 19. Dizzy/passing out | 13 (30) | 7 (25) | 0.675 |
| 20. Memory problem | 19 (43) | 7 (25) | 0.117 |
| 21. Diarrhea/constipation | 27 (61) | 10 (36) | 0.034 |
| 22. Numbness/tingling | 35 (80) | 9 (32) | <0.0001* |
| 23. Pain chewing/swallowing | 8 (18) | 5 (18) | 0.972 |
| Trouble Sleeping | Numbness/Tingling | |
|---|---|---|
| Sensitivity (%) | 84 | 74 |
| Specificity (%) | 57 | 68 |
| Positive predictive value (%) | 76 | 78 |
| Negative predictive value (%) | 70 | 68 |
The admission history forms filled out by first‐year admitting residents showed that only 18 (25%) of the women were screened for IPV, even though the history and physical examination template used at Denver Health Medical Center includes a prompt in the social history section pertaining to a history of violence as a reminder.
DISCUSSION
The important findings of this study were that women admitted to the internal medicine service of a university‐affiliated public safety‐net hospital had a high prevalence of IPV (22% and 61% 1‐year and lifetime prevalences, respectively), that most women with a history of IPV had previously sought help for the problem, many from physicians, that women were more likely to have a history of IPV if they had >10 positive responses to questions asked in a routine review of systems (particularly problems sleeping and experiencing numbness or tingling in their extremities), and that routine screening for IPV was uncommon at the time of admission.
These conclusions should be interpreted with respect to a number of limitations in our study. First, although our study was designed to be a consecutive series, the interviewers did not have sufficient time to meet with and interview every woman admitted before they were discharged. This occurred in part because the interviewers were available only for a portion of each day, some patients were discharged within 24 hours of admission, and many were out of their rooms for ancillary testing. Within the interviewers' time constraints, however, all hospitalized women meeting entry criteria who were available were approached. Our data could, however, overrepresent the prevalence of IPV if hospitalized women with a history of IPV had longer hospital stays than those who did not or if those experiencing IPV were out of their rooms less frequently (eg, for diagnostic tests). On the other hand, our data could underrepresent the true prevalence of IPV if patients with a history of IPV had shorter hospital stays or if they received more ancillary testing that caused them to be out of their rooms more frequently. Second, none of our interviewers had specific training in interviewing techniques. Accordingly, our data could have underestimated the true prevalence of IPV if interviewers with advanced training in probing sensitive topics had more success in eliciting positive responses. Third, the relationship between a history of IPV and multiple positive responses to the review of systems may be confounded if some of these patients also had a history of adverse childhood experiences or other experiences resulting in posttraumatic stress disorder as these patients also have an increased prevalence of chronic and functional disorders.2527 Finally, as our numbers were small, we were not powered to detect clinically important differences in demographics or specific positive answers on the review of systems.
To the best of our knowledge, the only study presenting IPV prevalence data in patients hospitalized for other than psychiatric problems was performed by McKenzie and colleagues18 in 1997. In their group of 130 patients (61 on internal medicine, 59 on surgery, 7 on obstetrics, and 3 on psychiatry), the 1‐year and lifetime prevalences of IPV were only 5% and 26%, respectively. McKenzie and colleagues used only 1 question to screen for IPV, but that single question incorporated 2 of the 4 questions used in our survey. Forty‐three of our 44 patients (98%) with a history of IPV were discovered on the basis of these 2 questions. The hospitals in which the 2 studies were done were similar, as were the ages and levels of education of the 2 populations studied and the percentage of eligible patients who agreed to participate. The patients in the 2 studies were different with respect to race, language mix, and the percentage who were insured, but neither study found differences in the prevalence of IPV as a function of race or insurance (although others have found an association of IPV with being uninsured1, 3, 4, 12, 23). Our study was conducted in women admitted exclusively to an internal medicine service, whereas nearly half of the patients studied by McKenzie and colleagues were admitted to surgical, gynecologic, or psychiatric services. Although McKenzie and colleagues found no difference in the prevalence of IPV as a function of admitting service, others have suggested that the prevalence of IPV is higher in patients admitted for trauma or psychiatric problems.1517, 28 The percentage of patients who self‐administered the questionnaires was 57% in our study and 77% in the study by McKenzie and colleagues. Neither study, however, found a difference in the percentage of IPV in patients who self‐administered the survey versus those who were interviewed. Women may have become more comfortable discussing this issue in the 10‐year interval between these 2 studies, or the prevalence of IPV may have increased. The only other study of IPV in hospitalized patients of which we are aware reported a 90% 1‐year prevalence in suicidal women admitted to a psychiatric service.28
Several studies have reported that victims of IPV have multiple somatic complaints and an increased prevalence of chronic and functional illnesses.1923 We confirmed that women experiencing IPV have more positive responses to questions posed in a review of systems, but the low specificity and positive and negative predictive values of the responses make this association of little clinical utility.
For only 18 of the 72 patients (25%) in our study was there evidence that they were screened for a history of IPV by the admitting resident. If more women were screened without a response being recorded, or if women were screened only for a current history of violence, our data may not accurately reflect the true rate at which screening occurred; however, the rate of screening that we observed is consistent with a number of other studies.12, 22, 2931 Fourteen of 18 patients who were screened for IPV by the resident gave negative responses. Ten of these, however, gave positive responses to our interviewers. Accordingly, the sensitivity, specificity, and positive and negative predictive values of the information recorded by the admitting resident were 40%, 100%, 100%, and 57%, respectively (assuming that the responses given to the IPV survey represent the gold standard), and this confirms that routine screening underestimates the prevalence of this problem. Accordingly, we identified 2 problems pertaining to screening for IPV: (1) it is not routinely done at the time of hospital admission, and (2) responses reported during routine screening are frequently incorrect. A number of barriers to routine screening have been previously identified, as have interventions designed to increase screening.32 Providing specific screening questions increases the identification of victims of IPV, but simply educating healthcare providers does not.32 Our history and physical templates have a prompt for violence victim to facilitate the screening, but as a result of this study, we are changing our prompting question and indicating what should be done if the response is positive.
The US Preventive Services Task Force and the Canadian Task Force on Preventive Health Care both concluded that there was insufficient evidence to recommend for or against routine screening for IPV.3335 Their rationale was that trials assessing the effectiveness of screening have not been published, that studies designed to assess the effectiveness of any resulting intervention are few in number, focused on pregnant women, and limited by problems in study design, that no studies have determined the accuracy of the screening tools, and that none have addressed the potential harm of screening.3335 The US Preventive Services Task Force did recommend screening if providers were concerned about IPV.34 Our data would suggest that there is little in the admission history that distinguishes women who might be victims of IPV from those who might not. Guidelines published by the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists promote routine screening of all patients.3638 Janssen and colleagues39 support the importance of screening on the basis that IPV is associated with numerous physical and mental health problems (eg, arthritis, migraines and other types of headaches, vaginal bleeding, ulcers, spastic colon, chronic pain, substance abuse, depression, and suicide ideation) and that establishing the link between these conditions and IPV could be important with respect to developing appropriate diagnostic and therapeutic approaches to patients' complaints. Screening also allows physicians to become more knowledgeable about their patients' lives, facilitating their ability to provide a supportive relationship that, in turn, increases women's likelihood of using an intervention method.39 We did not confirm an increased prevalence of any of the complaints noted by Janssen and colleagues in the women experiencing a history of IPV, but we did find an increased prevalence of insomnia and extremity numbness in women admitting to IPV as well as an overall increase in the number of positive responses to the review of systems. Screening identifies women who should receive information about reporting IPV, obtaining available assistance, planning for personal safety, and formal counseling as these have all been shown to reduce the severity of IPV and to improve the quality of life in rather large, randomized controlled trials.4043
As previously observed by others,13, 22, 29, 4446 the large majority of women that we approached welcomed screening for IPV. Over half of those with a history of IPV had previously sought help for the problem, over one‐third of these sought help from physicians, and most took the resource card that we offered, regardless of whether they did or did not have a history of IPV (this suggests either that our data may actually underestimate the true prevalence of IPV or that patients taking the information knew of others experiencing this problem). Accordingly, regardless of whether physicians believe that routine screening is warranted, patients see physicians and other healthcare workers as a resource for this problem.
We have confirmed that a history of IPV is very common in women admitted to an internal medicine service of a university‐affiliated public hospital and that female victims of IPV have more positive responses on the review of systems (particularly difficulty sleeping and extremity numbness or tingling) than those who have not. Although we initially hypothesized that finding numerous somatic complaints might serve as a marker for IPV, thereby identifying patients for whom more careful screening should occur, finding such a high prevalence of IPV argues that screening should be a routine part of the history for all women admitted to internal medicine inpatient services.
Acknowledgements
The authors thank the patients who agreed to participate in this study during their hospitalization. They also thank Cheri Maestas and Debbie Rodriquez for their support and help in interviewing patients.
The prevalence of intimate partner violence (IPV; defined as mental and/or physical violence directed from 1 person in an intimate relationship to the other) varies widely, depending on the population sampled and method of data collection. In the United States, IPV against women, occurring within the year prior to contact with a healthcare professional, ranges from 2% to 15% in surveys done by telephone, in primary care clinics, or in face‐to‐face home interviews19 and from 10% to 30% in surveys of patients visiting urgent care or emergency departments.1012 The prevalence of IPV occurring at any time during the life of the patient ranges from 18% in the aforementioned settings to as high as 88% in women applying for welfare.1, 2, 4, 5, 10, 1214
Although reports indicate that victims of IPV are more likely to be hospitalized,1517 the only study assessing the prevalence of IPV in hospitalized patients included women on medical, surgical, and obstetrical services and reported 1‐year and lifetime prevalences of only 5% and 23%, respectively.18
We hypothesized that the prevalence of IPV in hospitalized patients would be at least as high as that reported from emergency departments and sought to measure the 1‐year and lifetime prevalences of IPV in women admitted to a general internal medicine service. In addition, because studies done in various outpatient settings have reported that victims of IPV have a variety of somatic complaints and an increased prevalence of chronic and functional illnesses,1923 we also sought to determine whether women with a history of IPV and women without a history of IPV had different numbers or types of positive responses to questions asked on the review of systems.
PATIENTS AND METHODS
This study was approved by the Colorado Multiple Institution Review Board, and informed consent was obtained from all participants.
Women between the ages of 18 and 60 who were admitted to the internal medicine floor service of Denver Health Medical Center (a university‐affiliated public safety‐net hospital) between January 1 and February 28, 2004 and between October 1 and October 30, 2004 were approached to participate. These dates were selected on the basis of the availability of our interviewers. Patients older than 60 were excluded to avoid overlap between IPV and the problem of elder abuse. Women were excluded if they were unable to give informed consent, were pregnant, were incarcerated, were on contact precautions, or spoke a language other than English or Spanish. Although IPV is common in pregnant women and may occur in women who are incarcerated, these are considered vulnerable populations with respect to obtaining approval from internal review boards.
The questionnaire consisted of 23 review‐of‐systems questions,24 4 questions adapted from a previously validated screen for IPV11 (Table 1), and 1 question about attempts to seek help (Table 1). Women were considered to have experienced IPV if they gave positive responses to any of the 4 questions targeting IPV. According to patient preference, the combined questionnaire was either read and filled out by each subject independently or was read to her by a female interviewer who then recorded the subject's verbal responses. All interviewers were women with a shared common concern about, and interest in, IPV. Although none had advanced training in psychology, social work, or other formal discipline that involved interviewing skills, all interviews were scripted so that interactions with subjects and completion of the questionnaires would be uniform. Responses indicating sometimes were considered to be positive. Responses that were not answered, left blank, or marked as not applicable were considered to be negative.
| 1. Have you ever been hit, kicked, punched, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 2. Within the last year, have you been hit, kicked, or otherwise hurt by someone? If so, by whom? Friend, boyfriend, girlfriend, husband, family member, somebody you do not know, other |
| 3. Do you feel safe in your current relationship? |
| 4. Is there a partner from a previous relationship who is making you feel unsafe now? |
| 5. If you answered yes to any of the above, have you ever asked for help from police, shelter, counselor, physician? If so, how long ago? |
Each patient's medical record was reviewed to determine her age, race, number of previous hospital admissions, visits to the emergency department and walk‐in clinic, visits to primary care and subspecialty physicians, and whether the patient had been screened for IPV as recorded on the admission history and physical template. Admission diagnosis was obtained from the history and physical template, and the discharge diagnosis was obtained from the discharge paperwork. Functional diagnoses were considered to be symptoms (eg, shortness of breath) or problems (eg, constipation) that could not clearly be linked to a specific disease process. All participants were offered a card containing a list of resources for victims of IPV.
Data were analyzed with SAS 8.1 (SAS Institute, Cary, NC) and SPSS 11.5 (SPSS, Chicago, IL). The Student t test was used to compare continuous variables. Data are reported as means standard deviation. Chi‐square analysis was used to test associations between race, primary language, level of education, insurance status, admitting diagnosis, discharge diagnosis, number of previous hospital admissions, visit type, and the presence of IPV. For these, P < 0.05 was considered to be significant. The association of positive review‐of‐systems responses with the presence of IPV was also tested by chi‐square analysis, but P < 0.002 was considered to be significant on the basis of a Bonferroni adjustment for multiple comparisons. A receiver operating characteristic curve was used to assess the relationship between the number of positive responses to the questions included in the review of systems and a history of IPV. The odds ratio and confidence intervals were calculated to test the association between the number of positive responses to the review‐of‐systems questions and a lifetime history of IPV.
RESULTS
Throughout the dates of the study, 245 women were admitted to the internal medicine service, and 106 were excluded (Figure 1). Of the 139 eligible women, 78 were available to the interviewers and asked to participate, and 72 (92%) agreed. IPV occurring within the year prior to the interview or at any point in the patient's lifetime was reported by 16 (22%) and 44 (61%) subjects, respectively. No significant differences were seen in women who did or did not experience IPV at anytime in their life with respect to age, race, insurance status, education, number of scheduled outpatient, urgent, or emergent visits, or admission or discharge diagnosis even when the diagnoses were grouped into a functional category (although at best our study was powered to detect only >35% differences in prevalences; Tables 2 and 3). Of women reporting a lifetime history of IPV, 26 of 44 (59%) had previously sought help, and 9 of those 26 (35%) said that they sought help from a physician.

| IPV History | No IPV History | |
|---|---|---|
| ||
| Number (%) | 44 (61) | 28 (39) |
| Age (mean standard deviation) | 44 10 | 45 12 |
| Race [n, (%)] | ||
| Caucasian | 18 (41) | 6 (21) |
| Hispanic | 13 (30) | 15 (54) |
| African American | 12 (27) | 6 (21) |
| Other | 1 (2) | 1 (4) |
| Insurance status [n (%)] | ||
| Insured | 12 (27) | 5 (18) |
| Uninsured | 32 (73) | 23 (82) |
| Education [n (%)] | ||
| Grade school | 4 (9) | 3 (11) |
| Some high school | 13 (30) | 5 (18) |
| High school diploma | 15 (34) | 9 (32) |
| Some college | 9 (20) | 7 (25) |
| College degree | 2 (5) | 2 (7) |
| Postgraduate | 1 (2) | 2 (7) |
| Previous visit type (median, IQR) | ||
| Scheduled outpatient (includes primary care and subspecialty) | 2 (8) | 1.5 (7) |
| Emergency department and walk‐in clinic | 2 (3.5) | 1 (3) |
| Previous hospital admissions [n (%)] | ||
| 0 | 24 (55) | 16 (57) |
| 1 | 16 (36) | 4 (14) |
| 2 | 0 (0) | 4 (14) |
| 3 | 2 (5) | 2 (7) |
| >3 | 2 (5) | 2 (7) |
| Admission or Discharge Diagnosis | Admission | Discharge | ||
|---|---|---|---|---|
| IPV (n = 44) | No IPV (n = 28) | IPV (n = 44) | No IPV (n = 28) | |
| ||||
| Cardiovascular | ||||
| Chest pain (%)* | 8 (18) | 5 (18) | 6 (14) | 4 (14) |
| Cardiomyopathy | 0 | 0 | 1 | 0 |
| Cerebrovascular accident | 1 | 0 | 1 | 0 |
| Deep venous thrombosis | 0 | 0 | 1 | 0 |
| Hypertensive emergency | 0 | 0 | 1 | 0 |
| Palpitations* | 0 | 1 | 0 | 1 |
| Valvular disease | 0 | 0 | 1 | 0 |
| Venous stasis | 0 | 1 | 0 | 1 |
| Total (%) | 9 (20) | 7 (25) | 11 (25) | 6 (21) |
| Gastrointestinal | ||||
| Abdominal pain (%)* | 7 (16) | 4 (14) | 2 | 1 |
| Ascites | 0 | 1 | 0 | 0 |
| Constipation* | 0 | 0 | 1 | 0 |
| End‐stage liver disease | 1 | 1 | 1 | 2 |
| Esophagitis | 0 | 0 | 1 | 0 |
| Hepatitis | 1 | 0 | 1 | 0 |
| Nausea/vomiting* | 2 | 0 | 1 | 0 |
| Pancreatitis | 0 | 1 | 3 | 2 |
| Peptic ulcer disease | 1 | 0 | 1 | 0 |
| Upper gastrointestinal bleeding | 2 | 0 | 1 | 0 |
| Total (%) | 14 (32) | 7 (25) | 12 (27) | 5 (18) |
| Hematology/oncology | ||||
| Abdominal mass | 0 | 0 | 0 | 1 |
| Anemia | 1 | 0 | 1 | 0 |
| Breast cancer | 0 | 1 | 0 | 1 |
| Cervical cancer | 1 | 0 | 1 | 0 |
| Colon cancer | 0 | 1 | 0 | 1 |
| Sickle cell anemia | 1 | 0 | 1 | 0 |
| Thrombocytosis | 1 | 0 | 1 | 0 |
| Total (%) | 4 (9) | 2 (7) | 4 (9) | 3 (11) |
| Infectious disease | ||||
| Bacteremia/sepsis | 3 | 0 | 3 | 0 |
| Cellulitis | 1 | 0 | 1 | 1 |
| Cholangitis | 0 | 0 | 1 | 0 |
| Community‐acquired pneumonia | 2 | 2 | 2 | 1 |
| Endocarditis | 1 | 0 | 1 | 0 |
| Fever | 0 | 1 | 0 | 1 |
| Pelvic inflammatory disease | 0 | 0 | 0 | 1 |
| Urinary tract infection | 1 | 0 | 1 | 0 |
| Total (%) | 8 (18) | 3 (11) | 9 (20) | 4 (14) |
| Pulmonary | ||||
| Acute exacerbation of COPD | 0 | 0 | 1 | 0 |
| Asthma exacerbation | 1 | 1 | 1 | 2 |
| Pleuritic chest pain* | 0 | 0 | 1 | 0 |
| Pulmonary embolism | 0 | 0 | 1 | 0 |
| Shortness of breath* | 4 | 0 | 1 | 0 |
| Total (%) | 5 (11) | 1 (4) | 5 (11) | 2 (7) |
| Renal/genitourinary | ||||
| Acute renal failure | 0 | 1 | 0 | 1 |
| End‐stage renal disease | 1 | 2 | 1 | 2 |
| Nephrotic syndrome | 0 | 1 | 0 | 2 |
| Vaginal bleeding | 1 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 2 (5) | 5 (18) |
| Other | ||||
| Diabetic ketoacidosis | 0 | 1 | 0 | 1 |
| Extremity pain* | 0 | 1 | 0 | 0 |
| Mediastinal thickening | 0 | 0 | 0 | 1 |
| Hyponatremia | 0 | 1 | 0 | 1 |
| Lower extremity swelling | 2 | 1 | 0 | 0 |
| Somatization* | 0 | 0 | 1 | 0 |
| Total (%) | 2 (5) | 4 (14) | 1 (2) | 3 (11) |
| Total functional diagnoses (%) | 21 (48) | 11 (39) | 12 (27) | 6 (21) |
Women with a 1‐year history of IPV and women without a 1‐year history of IPV had 11.4 4.7 and 7.7 5.4 positive responses to the review of systems (P < 0.01), respectively. Women with a lifetime history of IPV and women without a lifetime history of IPV had 10.9 4.4 and 7.7 5.4 positive responses (P < 0.01), respectively. The receiver operating characteristic curve of the number of positive responses versus a lifetime history of IPV is presented in Figure 2. Subjects with 10 or more positive responses were 4.8 times more likely to report a lifetime history of IPV than subjects with 9 or fewer positive responses (confidence interval = 1.614.2, P = 0.003). The c‐statistic indicating the ability of the review of systems to properly classify cases when there were 10 or more positive responses was 0.692.

No differences were observed in the responses to the individual review of systems questions in women who did or did not have a lifetime history of IPV, with the exception that those with a positive history more commonly complained of difficulty sleeping and numbness and tingling in their hands or feet (although at best our study was sufficiently powered to detect only >20% differences in prevalences; Table 4). Although the sensitivity of having problems sleeping or experiencing numbness or tingling in patients with IPV was high, the specificity and positive and negative predictive values were not (Table 5).
| Review‐of‐Systems Questions | IPV History (n = 44) | No IPV History (n = 28) | P Value |
|---|---|---|---|
| |||
| 1. Shortness of breath | 25 (57) | 10 (36) | 0.081 |
| 2. Chest pain/pressure | 19 (43) | 9 (32) | 0.349 |
| 3. Abdominal pain | 17 (39) | 10 (36) | 0.803 |
| 4. Headaches | 24 (55) | 13 (46) | 0.502 |
| 5. Rashes | 15 (34) | 9 (32) | 0.864 |
| 6. Bruising | 32 (73) | 12 (43) | 0.011 |
| 7. Joint pain/stiffness | 27 (61) | 11 (39) | 0.067 |
| 8. Muscle pain/spasms | 22 (50) | 11 (39) | 0.374 |
| 9. Pain with intercourse | 8 (19) | 4 (14) | 0.753 |
| 10. Pelvic pain/cramps | 13 (30) | 5 (18) | 0.264 |
| 11. Nausea/vomiting | 19 (43) | 11 (39) | 0.744 |
| 12. Nervous/anxious | 28 (64) | 14 (50) | 0.253 |
| 13. Sad/crying | 21 (48) | 12 (43) | 0.686 |
| 14. Weight gain/loss | 26 (59) | 17 (61) | 0.891 |
| 15. Trouble sleeping | 37 (84) | 12 (43) | 0.000* |
| 16. Fever/chills | 19 (43) | 6 (21) | 0.059 |
| 17. Frequent/painful urination | 11 (25) | 6 (21) | 0.728 |
| 18. Pounding/emrregular heart beat | 14 (32) | 7 (25) | 0.535 |
| 19. Dizzy/passing out | 13 (30) | 7 (25) | 0.675 |
| 20. Memory problem | 19 (43) | 7 (25) | 0.117 |
| 21. Diarrhea/constipation | 27 (61) | 10 (36) | 0.034 |
| 22. Numbness/tingling | 35 (80) | 9 (32) | <0.0001* |
| 23. Pain chewing/swallowing | 8 (18) | 5 (18) | 0.972 |
| Trouble Sleeping | Numbness/Tingling | |
|---|---|---|
| Sensitivity (%) | 84 | 74 |
| Specificity (%) | 57 | 68 |
| Positive predictive value (%) | 76 | 78 |
| Negative predictive value (%) | 70 | 68 |
The admission history forms filled out by first‐year admitting residents showed that only 18 (25%) of the women were screened for IPV, even though the history and physical examination template used at Denver Health Medical Center includes a prompt in the social history section pertaining to a history of violence as a reminder.
DISCUSSION
The important findings of this study were that women admitted to the internal medicine service of a university‐affiliated public safety‐net hospital had a high prevalence of IPV (22% and 61% 1‐year and lifetime prevalences, respectively), that most women with a history of IPV had previously sought help for the problem, many from physicians, that women were more likely to have a history of IPV if they had >10 positive responses to questions asked in a routine review of systems (particularly problems sleeping and experiencing numbness or tingling in their extremities), and that routine screening for IPV was uncommon at the time of admission.
These conclusions should be interpreted with respect to a number of limitations in our study. First, although our study was designed to be a consecutive series, the interviewers did not have sufficient time to meet with and interview every woman admitted before they were discharged. This occurred in part because the interviewers were available only for a portion of each day, some patients were discharged within 24 hours of admission, and many were out of their rooms for ancillary testing. Within the interviewers' time constraints, however, all hospitalized women meeting entry criteria who were available were approached. Our data could, however, overrepresent the prevalence of IPV if hospitalized women with a history of IPV had longer hospital stays than those who did not or if those experiencing IPV were out of their rooms less frequently (eg, for diagnostic tests). On the other hand, our data could underrepresent the true prevalence of IPV if patients with a history of IPV had shorter hospital stays or if they received more ancillary testing that caused them to be out of their rooms more frequently. Second, none of our interviewers had specific training in interviewing techniques. Accordingly, our data could have underestimated the true prevalence of IPV if interviewers with advanced training in probing sensitive topics had more success in eliciting positive responses. Third, the relationship between a history of IPV and multiple positive responses to the review of systems may be confounded if some of these patients also had a history of adverse childhood experiences or other experiences resulting in posttraumatic stress disorder as these patients also have an increased prevalence of chronic and functional disorders.2527 Finally, as our numbers were small, we were not powered to detect clinically important differences in demographics or specific positive answers on the review of systems.
To the best of our knowledge, the only study presenting IPV prevalence data in patients hospitalized for other than psychiatric problems was performed by McKenzie and colleagues18 in 1997. In their group of 130 patients (61 on internal medicine, 59 on surgery, 7 on obstetrics, and 3 on psychiatry), the 1‐year and lifetime prevalences of IPV were only 5% and 26%, respectively. McKenzie and colleagues used only 1 question to screen for IPV, but that single question incorporated 2 of the 4 questions used in our survey. Forty‐three of our 44 patients (98%) with a history of IPV were discovered on the basis of these 2 questions. The hospitals in which the 2 studies were done were similar, as were the ages and levels of education of the 2 populations studied and the percentage of eligible patients who agreed to participate. The patients in the 2 studies were different with respect to race, language mix, and the percentage who were insured, but neither study found differences in the prevalence of IPV as a function of race or insurance (although others have found an association of IPV with being uninsured1, 3, 4, 12, 23). Our study was conducted in women admitted exclusively to an internal medicine service, whereas nearly half of the patients studied by McKenzie and colleagues were admitted to surgical, gynecologic, or psychiatric services. Although McKenzie and colleagues found no difference in the prevalence of IPV as a function of admitting service, others have suggested that the prevalence of IPV is higher in patients admitted for trauma or psychiatric problems.1517, 28 The percentage of patients who self‐administered the questionnaires was 57% in our study and 77% in the study by McKenzie and colleagues. Neither study, however, found a difference in the percentage of IPV in patients who self‐administered the survey versus those who were interviewed. Women may have become more comfortable discussing this issue in the 10‐year interval between these 2 studies, or the prevalence of IPV may have increased. The only other study of IPV in hospitalized patients of which we are aware reported a 90% 1‐year prevalence in suicidal women admitted to a psychiatric service.28
Several studies have reported that victims of IPV have multiple somatic complaints and an increased prevalence of chronic and functional illnesses.1923 We confirmed that women experiencing IPV have more positive responses to questions posed in a review of systems, but the low specificity and positive and negative predictive values of the responses make this association of little clinical utility.
For only 18 of the 72 patients (25%) in our study was there evidence that they were screened for a history of IPV by the admitting resident. If more women were screened without a response being recorded, or if women were screened only for a current history of violence, our data may not accurately reflect the true rate at which screening occurred; however, the rate of screening that we observed is consistent with a number of other studies.12, 22, 2931 Fourteen of 18 patients who were screened for IPV by the resident gave negative responses. Ten of these, however, gave positive responses to our interviewers. Accordingly, the sensitivity, specificity, and positive and negative predictive values of the information recorded by the admitting resident were 40%, 100%, 100%, and 57%, respectively (assuming that the responses given to the IPV survey represent the gold standard), and this confirms that routine screening underestimates the prevalence of this problem. Accordingly, we identified 2 problems pertaining to screening for IPV: (1) it is not routinely done at the time of hospital admission, and (2) responses reported during routine screening are frequently incorrect. A number of barriers to routine screening have been previously identified, as have interventions designed to increase screening.32 Providing specific screening questions increases the identification of victims of IPV, but simply educating healthcare providers does not.32 Our history and physical templates have a prompt for violence victim to facilitate the screening, but as a result of this study, we are changing our prompting question and indicating what should be done if the response is positive.
The US Preventive Services Task Force and the Canadian Task Force on Preventive Health Care both concluded that there was insufficient evidence to recommend for or against routine screening for IPV.3335 Their rationale was that trials assessing the effectiveness of screening have not been published, that studies designed to assess the effectiveness of any resulting intervention are few in number, focused on pregnant women, and limited by problems in study design, that no studies have determined the accuracy of the screening tools, and that none have addressed the potential harm of screening.3335 The US Preventive Services Task Force did recommend screening if providers were concerned about IPV.34 Our data would suggest that there is little in the admission history that distinguishes women who might be victims of IPV from those who might not. Guidelines published by the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists promote routine screening of all patients.3638 Janssen and colleagues39 support the importance of screening on the basis that IPV is associated with numerous physical and mental health problems (eg, arthritis, migraines and other types of headaches, vaginal bleeding, ulcers, spastic colon, chronic pain, substance abuse, depression, and suicide ideation) and that establishing the link between these conditions and IPV could be important with respect to developing appropriate diagnostic and therapeutic approaches to patients' complaints. Screening also allows physicians to become more knowledgeable about their patients' lives, facilitating their ability to provide a supportive relationship that, in turn, increases women's likelihood of using an intervention method.39 We did not confirm an increased prevalence of any of the complaints noted by Janssen and colleagues in the women experiencing a history of IPV, but we did find an increased prevalence of insomnia and extremity numbness in women admitting to IPV as well as an overall increase in the number of positive responses to the review of systems. Screening identifies women who should receive information about reporting IPV, obtaining available assistance, planning for personal safety, and formal counseling as these have all been shown to reduce the severity of IPV and to improve the quality of life in rather large, randomized controlled trials.4043
As previously observed by others,13, 22, 29, 4446 the large majority of women that we approached welcomed screening for IPV. Over half of those with a history of IPV had previously sought help for the problem, over one‐third of these sought help from physicians, and most took the resource card that we offered, regardless of whether they did or did not have a history of IPV (this suggests either that our data may actually underestimate the true prevalence of IPV or that patients taking the information knew of others experiencing this problem). Accordingly, regardless of whether physicians believe that routine screening is warranted, patients see physicians and other healthcare workers as a resource for this problem.
We have confirmed that a history of IPV is very common in women admitted to an internal medicine service of a university‐affiliated public hospital and that female victims of IPV have more positive responses on the review of systems (particularly difficulty sleeping and extremity numbness or tingling) than those who have not. Although we initially hypothesized that finding numerous somatic complaints might serve as a marker for IPV, thereby identifying patients for whom more careful screening should occur, finding such a high prevalence of IPV argues that screening should be a routine part of the history for all women admitted to internal medicine inpatient services.
Acknowledgements
The authors thank the patients who agreed to participate in this study during their hospitalization. They also thank Cheri Maestas and Debbie Rodriquez for their support and help in interviewing patients.
- ,,.Prevalence and determinants of intimate partner abuse among public hospital primary care patients.J Gen Intern Med.2000;15:811–817.
- ,,,.Women's experiences with violence: a national study.Womens Health Issues.2007;17:3–12.
- ,,,.Multistate analysis of factors associated with intimate partner violence.Am J Prev Med.2002;22:156–164.
- ,,,.Frequency and correlates of intimate partner violence by type: physical, sexual, and psychological battering.Am J Public Health.2000;90:553–559.
- ,,,,.Prevalence of domestic violence among patients in three ambulatory care internal medicine clinics.J Gen Intern Med.1991;6:317–322.
- ,,,.Prevalence of partner violence against 7,443 African American, White and Hispanic women receiving care at urban public primary care clinics.Public Health Nurs.2005;22:98–107.
- ,,,,.Evaluating domestic partner abuse in a family practice clinic.Fam Med.1997;29:492–495.
- ,.Prevalence and predictors of physical partner abuse among Mexican American women.Am J Public Health.2001;91:441–445.
- ,,.Rates of intimate partner violence in the United States.Am J Public Health.1998;88:1702–1704.
- ,,,.Domestic violence against women incidence and prevalence in an emergency department population.JAMA.1995;273:1763–1767.
- ,,, et al.Accuracy of 3 brief screening questions for detecting partner violence in the emergency department.JAMA.1997;277:1357–1361.
- ,,.A prevalence survey of abuse and screening for abuse in urgent care patients.Obstet Gynecol.1998;91:511–514.
- Morbidity and Mortality Weekly Report.Use of medical care, police assistance and restraining orders by women reporting intimate partner violence—Massachusetts, 1996–1997.JAMA.2000;284:558.
- ,,.Interpersonal violence among women seeking welfare: unraveling lives.Am J Public Health.2006;96:1409–1415.
- ,.A 5‐year follow‐up study of 117 battered women.Am J Public Health.1991;81:1486–1488.
- ,,.Rates and relative risk of hospital admission among women in violent intimate partner relationships.Am J Public Health.2000;90:1416–1420.
- ,,,.Intimate partner violence against women: do victims cost health plans more?J Fam Pract.1999;48:439–443.
- ,,,.Prevalence of domestic violence in an inpatient female population.J Gen Intern Med.1998;13:277–279.
- ,,, et al.Intimate partner violence and physical health consequences.Arch Intern Med.2002;162:1157–1163.
- ,,,,.Physical health consequences of physical and psychological intimate partner violence.Arch Fam Med.2000;9:451–457.
- ,,, et al.Sexual and physical abuse in women with functional or organic gastrointestinal disorders.Ann Intern Med.1990;113:828–833.
- ,,.Prevalence of intimate partner violence and health implications for women using emergency departments and primary care clinics.Womens Health Issues.2004;14:19–29.
- ,,, et al.The “battering syndrome”: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices.Ann Intern Med.1995;123:737–746.
- ,.DeGowin and DeGowin's Bedside Diagnostic Examination.5th ed.New York, NY:Macmillan Publishing;1987:18–29.
- ,,, et al.Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study.Am J Prev Med.1998;14:245–258.
- ,,,,,.Posttraumatic stress disorder and health status among female and male medical patients.J Trauma Stress.2004;17:1–9.
- ,,,,.Posttraumatic stress disorder and physical comorbidity among female children and adolescents: results from service‐use data.Pediatrics.2005:116;e767–e776.
- ,,,,.Prevalence and severity of intimate partner violence and associations with family functioning and alcohol abuse in psychiatric inpatients with suicidal intent.J Clin Psychiatry.2006;67:23–29.
- ,,.Intimate partner violence screening and intervention: data from eleven Pennsylvania and California community hospital emergency departments.J Emerg Nurs.2001;27:141–149.
- ,.Missed opportunities: emergency department visits by police‐identified victims of intimate partner violence.Emerg Med.2006;47:190–199.
- ,,, et al.Intimate partner violence and patient screening across medical specialties.Acad Emerg Med.2005;12:712–722.
- ,,,,.Screening for intimate partner violence by health care providers: barriers and interventions.Am J Prev Med.2000;19:230–237.
- ,,,.Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2004;140:387–396.
- U.S. Preventive Services Task Force.Screening for family and intimate partner violence: recommendation statement.Ann Intern Med.2004;140:382–386.
- ,.Interventions for violence against women: scientific review.JAMA.2003;289:589–600.
- American Medical Association. Policy H‐515.965: family and intimate partner violence. Available at: http://www.ama‐assn.org. Accessed May2007.
- American Academy of Family Physicians. Family and intimate partner violence and abuse. Available at: www.aafp.org/x16506.xml. Accessed May2007.
- Domestic Violence.Washington, DC:American College of Obstetrics and Gynecology;1999. Educational Bulletin Number; No. 257.
- ,,.Assessment for intimate partner violence: where do we stand?J Am Board Fam Med.2006;19:413–415.
- ,,,,,.What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors.JAMA.2003;58:76–81.
- ,,,,,.Assessing intimate partner violence in health care settings leads to women's receipt of interventions and improved health.Public Health Rep.2006;121:435–444.
- ,,.An evaluation of interventions to decrease intimate partner violence to pregnant women.Public Health Nurs.2000;17:443–451.
- ,.Reducing violence using community‐based advocacy for women with abusive partners.J Consult Clin Psychol.1999;67:43–53.
- ,,,,.Help‐seeking for intimate partner violence and forced sex in South Carolina.Am J Prev Med.2000;19:316–320.
- ,,, et al.Women's opinions about domestic violence screening and mandatory reporting.Am J Prev Med.2000;19:279–285.
- ,,,.The factors associated with disclosure of intimate partner abuse to clinicians.J Fam Pract.2001;50:338–344.
- ,,.Prevalence and determinants of intimate partner abuse among public hospital primary care patients.J Gen Intern Med.2000;15:811–817.
- ,,,.Women's experiences with violence: a national study.Womens Health Issues.2007;17:3–12.
- ,,,.Multistate analysis of factors associated with intimate partner violence.Am J Prev Med.2002;22:156–164.
- ,,,.Frequency and correlates of intimate partner violence by type: physical, sexual, and psychological battering.Am J Public Health.2000;90:553–559.
- ,,,,.Prevalence of domestic violence among patients in three ambulatory care internal medicine clinics.J Gen Intern Med.1991;6:317–322.
- ,,,.Prevalence of partner violence against 7,443 African American, White and Hispanic women receiving care at urban public primary care clinics.Public Health Nurs.2005;22:98–107.
- ,,,,.Evaluating domestic partner abuse in a family practice clinic.Fam Med.1997;29:492–495.
- ,.Prevalence and predictors of physical partner abuse among Mexican American women.Am J Public Health.2001;91:441–445.
- ,,.Rates of intimate partner violence in the United States.Am J Public Health.1998;88:1702–1704.
- ,,,.Domestic violence against women incidence and prevalence in an emergency department population.JAMA.1995;273:1763–1767.
- ,,, et al.Accuracy of 3 brief screening questions for detecting partner violence in the emergency department.JAMA.1997;277:1357–1361.
- ,,.A prevalence survey of abuse and screening for abuse in urgent care patients.Obstet Gynecol.1998;91:511–514.
- Morbidity and Mortality Weekly Report.Use of medical care, police assistance and restraining orders by women reporting intimate partner violence—Massachusetts, 1996–1997.JAMA.2000;284:558.
- ,,.Interpersonal violence among women seeking welfare: unraveling lives.Am J Public Health.2006;96:1409–1415.
- ,.A 5‐year follow‐up study of 117 battered women.Am J Public Health.1991;81:1486–1488.
- ,,.Rates and relative risk of hospital admission among women in violent intimate partner relationships.Am J Public Health.2000;90:1416–1420.
- ,,,.Intimate partner violence against women: do victims cost health plans more?J Fam Pract.1999;48:439–443.
- ,,,.Prevalence of domestic violence in an inpatient female population.J Gen Intern Med.1998;13:277–279.
- ,,, et al.Intimate partner violence and physical health consequences.Arch Intern Med.2002;162:1157–1163.
- ,,,,.Physical health consequences of physical and psychological intimate partner violence.Arch Fam Med.2000;9:451–457.
- ,,, et al.Sexual and physical abuse in women with functional or organic gastrointestinal disorders.Ann Intern Med.1990;113:828–833.
- ,,.Prevalence of intimate partner violence and health implications for women using emergency departments and primary care clinics.Womens Health Issues.2004;14:19–29.
- ,,, et al.The “battering syndrome”: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices.Ann Intern Med.1995;123:737–746.
- ,.DeGowin and DeGowin's Bedside Diagnostic Examination.5th ed.New York, NY:Macmillan Publishing;1987:18–29.
- ,,, et al.Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study.Am J Prev Med.1998;14:245–258.
- ,,,,,.Posttraumatic stress disorder and health status among female and male medical patients.J Trauma Stress.2004;17:1–9.
- ,,,,.Posttraumatic stress disorder and physical comorbidity among female children and adolescents: results from service‐use data.Pediatrics.2005:116;e767–e776.
- ,,,,.Prevalence and severity of intimate partner violence and associations with family functioning and alcohol abuse in psychiatric inpatients with suicidal intent.J Clin Psychiatry.2006;67:23–29.
- ,,.Intimate partner violence screening and intervention: data from eleven Pennsylvania and California community hospital emergency departments.J Emerg Nurs.2001;27:141–149.
- ,.Missed opportunities: emergency department visits by police‐identified victims of intimate partner violence.Emerg Med.2006;47:190–199.
- ,,, et al.Intimate partner violence and patient screening across medical specialties.Acad Emerg Med.2005;12:712–722.
- ,,,,.Screening for intimate partner violence by health care providers: barriers and interventions.Am J Prev Med.2000;19:230–237.
- ,,,.Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2004;140:387–396.
- U.S. Preventive Services Task Force.Screening for family and intimate partner violence: recommendation statement.Ann Intern Med.2004;140:382–386.
- ,.Interventions for violence against women: scientific review.JAMA.2003;289:589–600.
- American Medical Association. Policy H‐515.965: family and intimate partner violence. Available at: http://www.ama‐assn.org. Accessed May2007.
- American Academy of Family Physicians. Family and intimate partner violence and abuse. Available at: www.aafp.org/x16506.xml. Accessed May2007.
- Domestic Violence.Washington, DC:American College of Obstetrics and Gynecology;1999. Educational Bulletin Number; No. 257.
- ,,.Assessment for intimate partner violence: where do we stand?J Am Board Fam Med.2006;19:413–415.
- ,,,,,.What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors.JAMA.2003;58:76–81.
- ,,,,,.Assessing intimate partner violence in health care settings leads to women's receipt of interventions and improved health.Public Health Rep.2006;121:435–444.
- ,,.An evaluation of interventions to decrease intimate partner violence to pregnant women.Public Health Nurs.2000;17:443–451.
- ,.Reducing violence using community‐based advocacy for women with abusive partners.J Consult Clin Psychol.1999;67:43–53.
- ,,,,.Help‐seeking for intimate partner violence and forced sex in South Carolina.Am J Prev Med.2000;19:316–320.
- ,,, et al.Women's opinions about domestic violence screening and mandatory reporting.Am J Prev Med.2000;19:279–285.
- ,,,.The factors associated with disclosure of intimate partner abuse to clinicians.J Fam Pract.2001;50:338–344.
Copyright © 2008 Society of Hospital Medicine